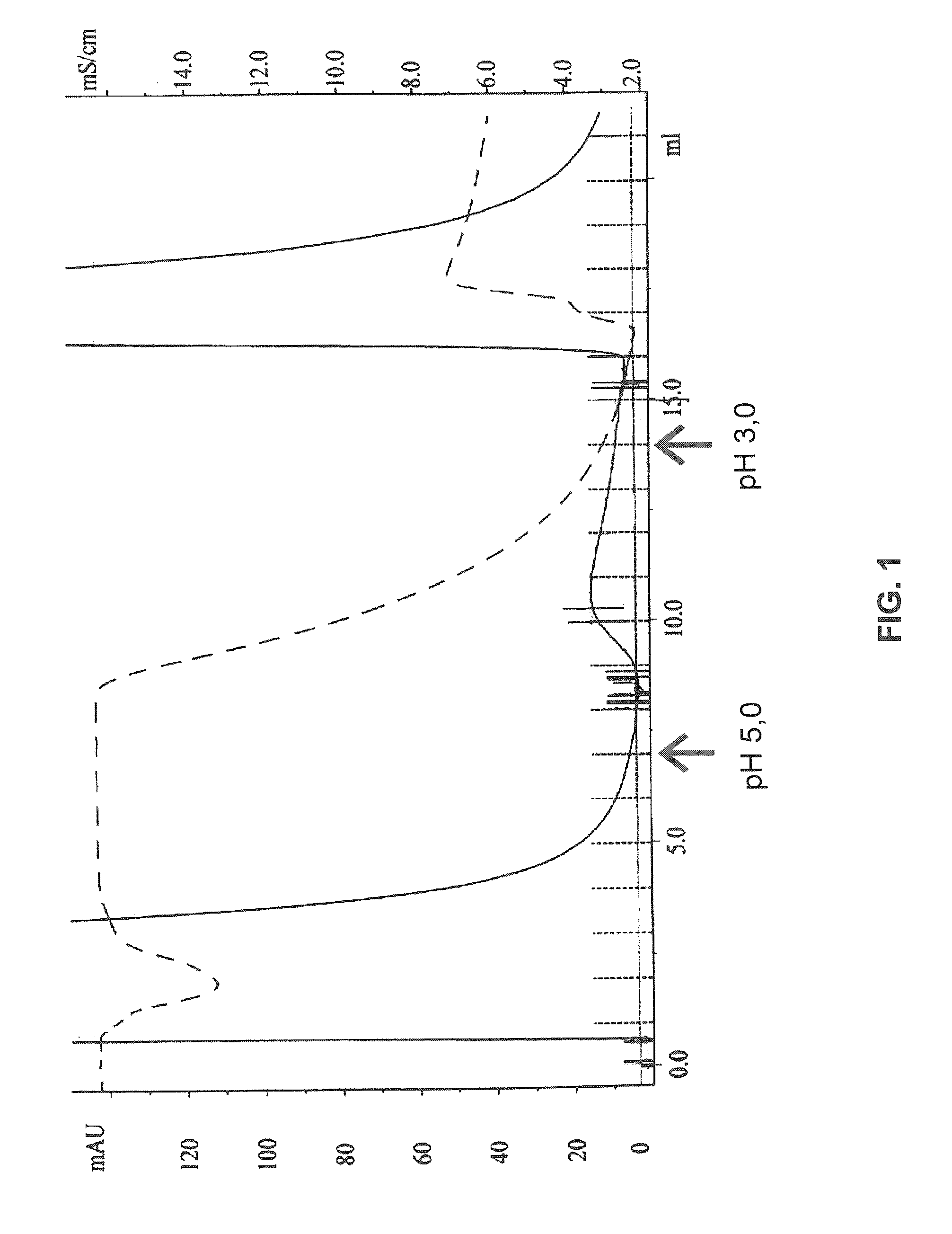
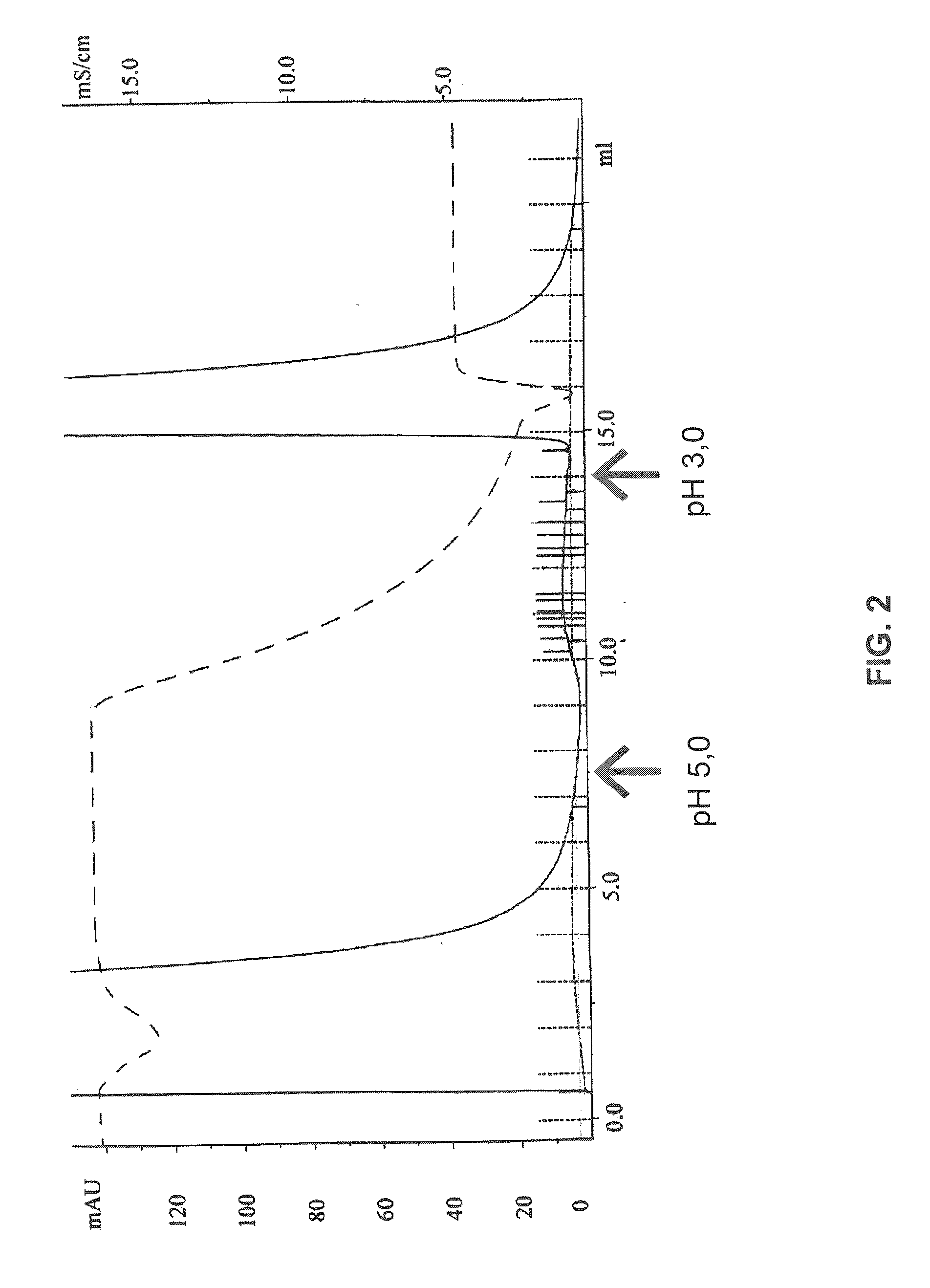
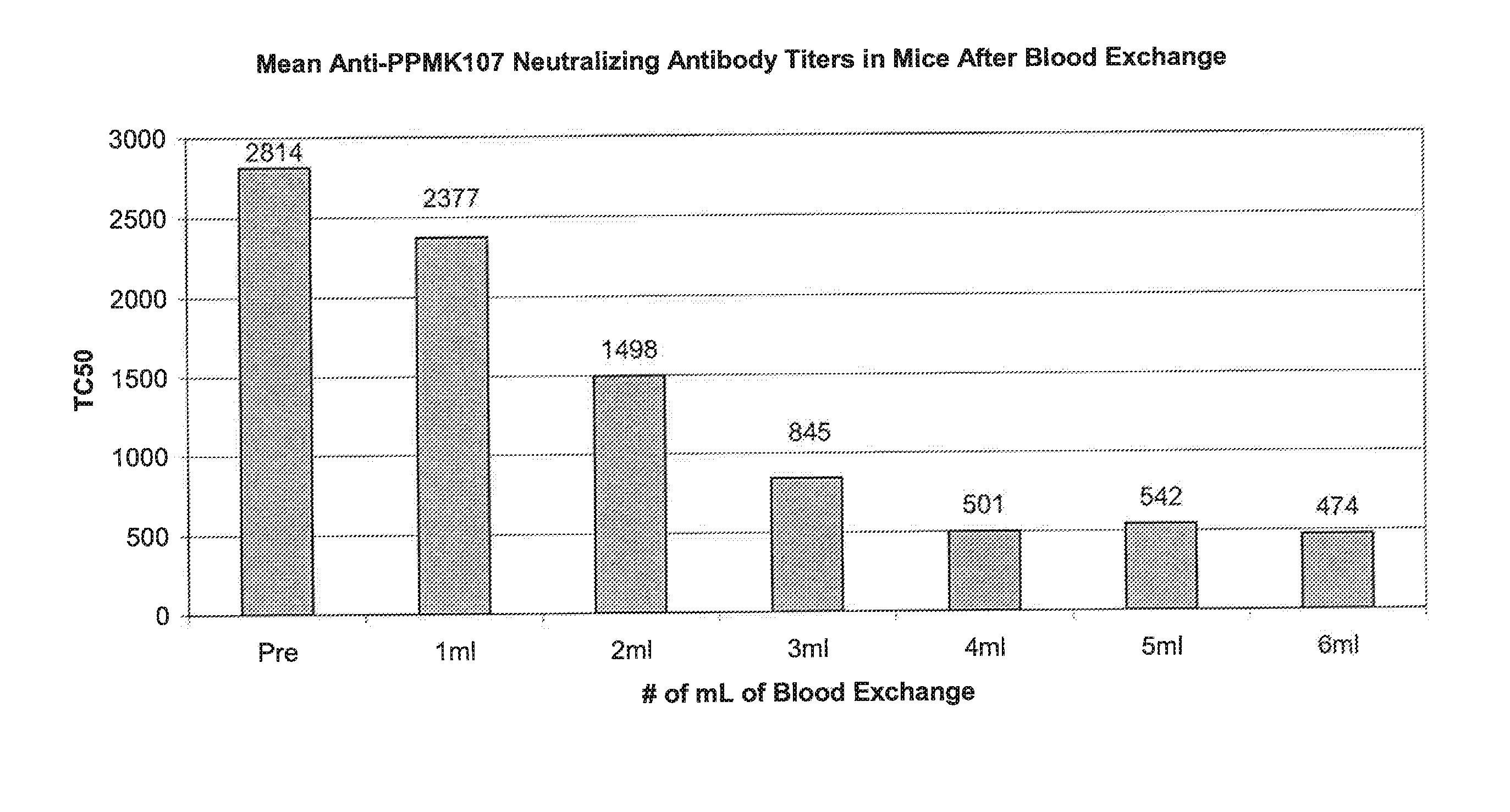
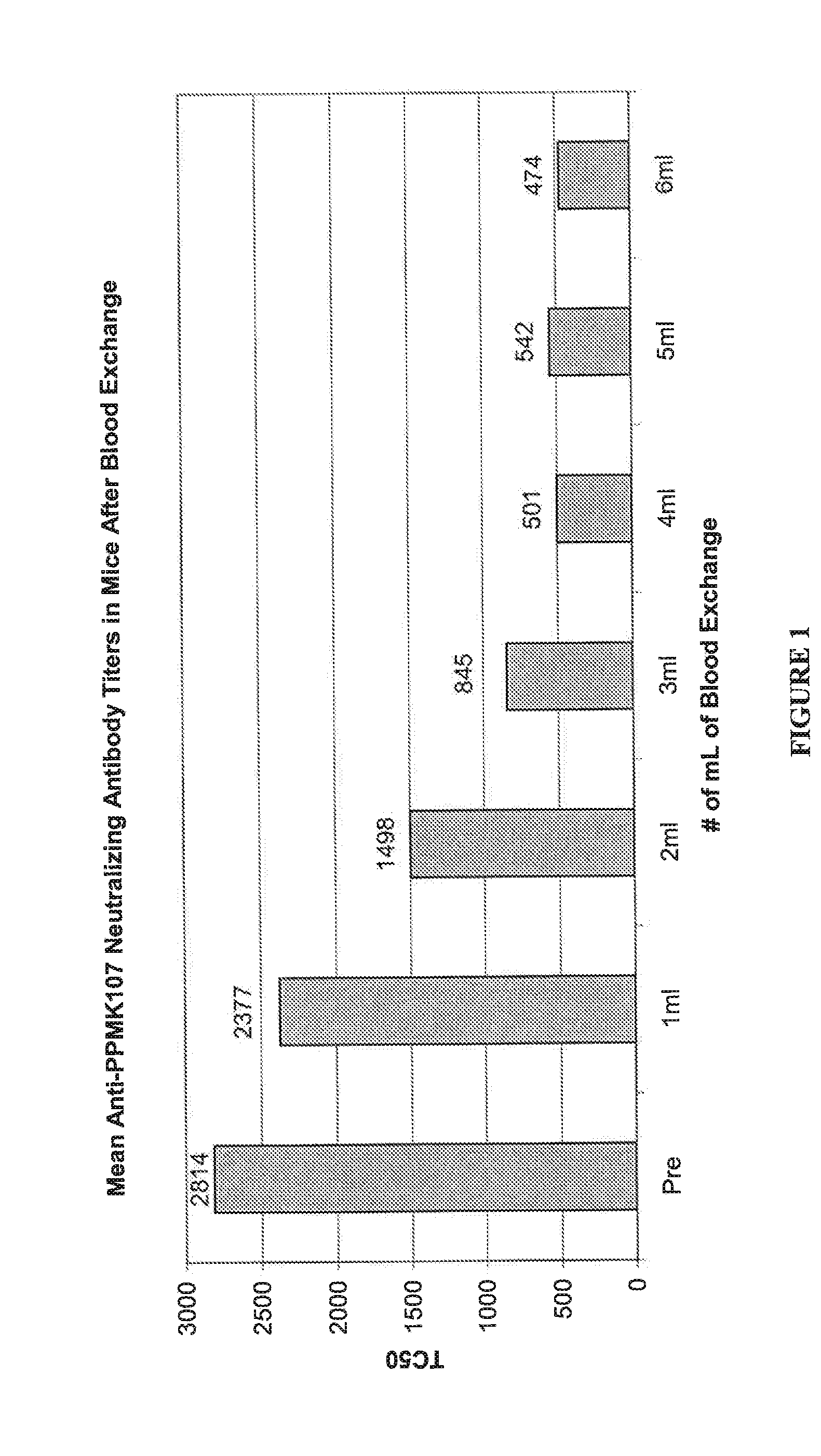
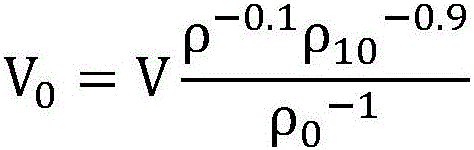Patents
Literature
56 results about "Plasmapheresis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasmapheresis (from the Greek πλάσμα—plasma, something molded, and ἀφαίρεσις—aphairesis, taking away) is the removal, treatment, and return or exchange of blood plasma or components thereof from and to the blood circulation. It is thus an extracorporeal therapy (a medical procedure performed outside the body).
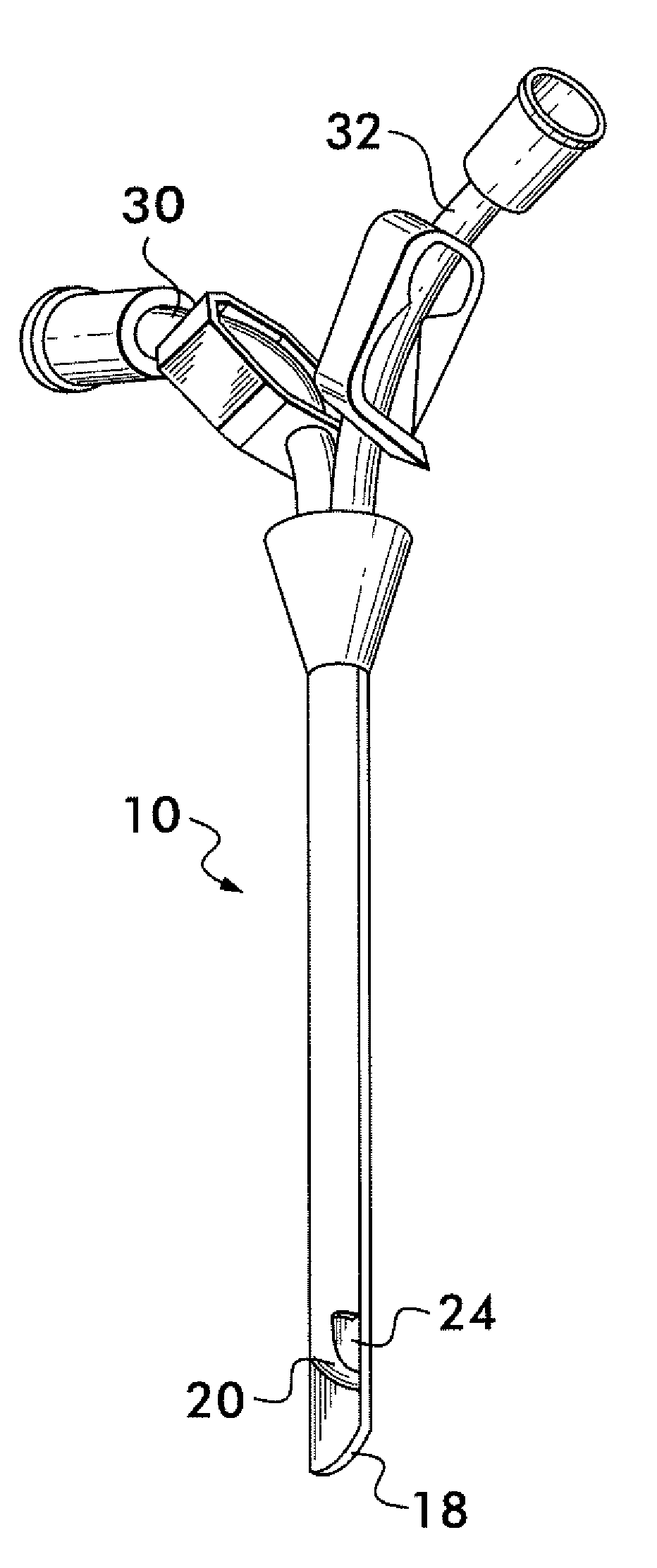
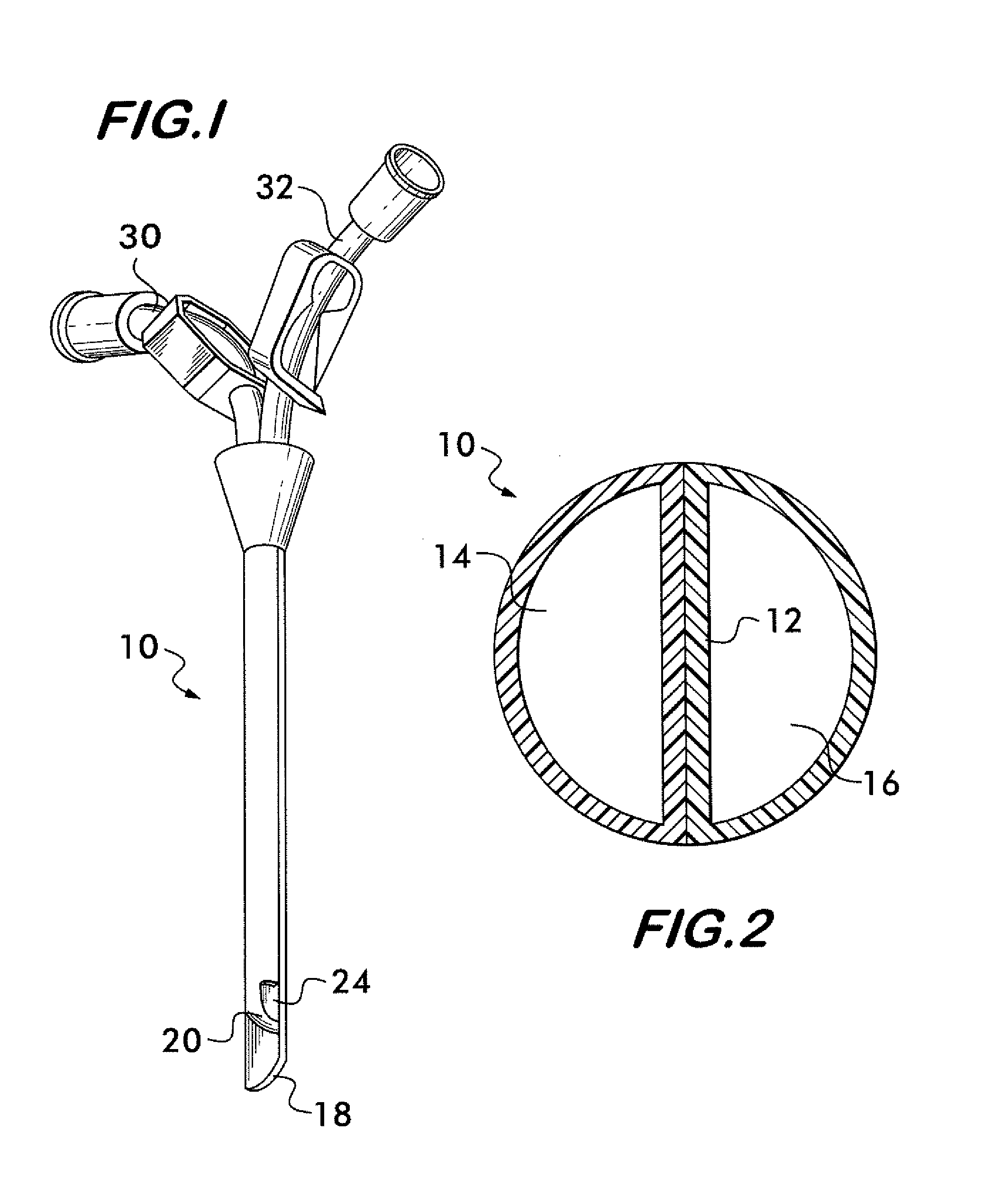
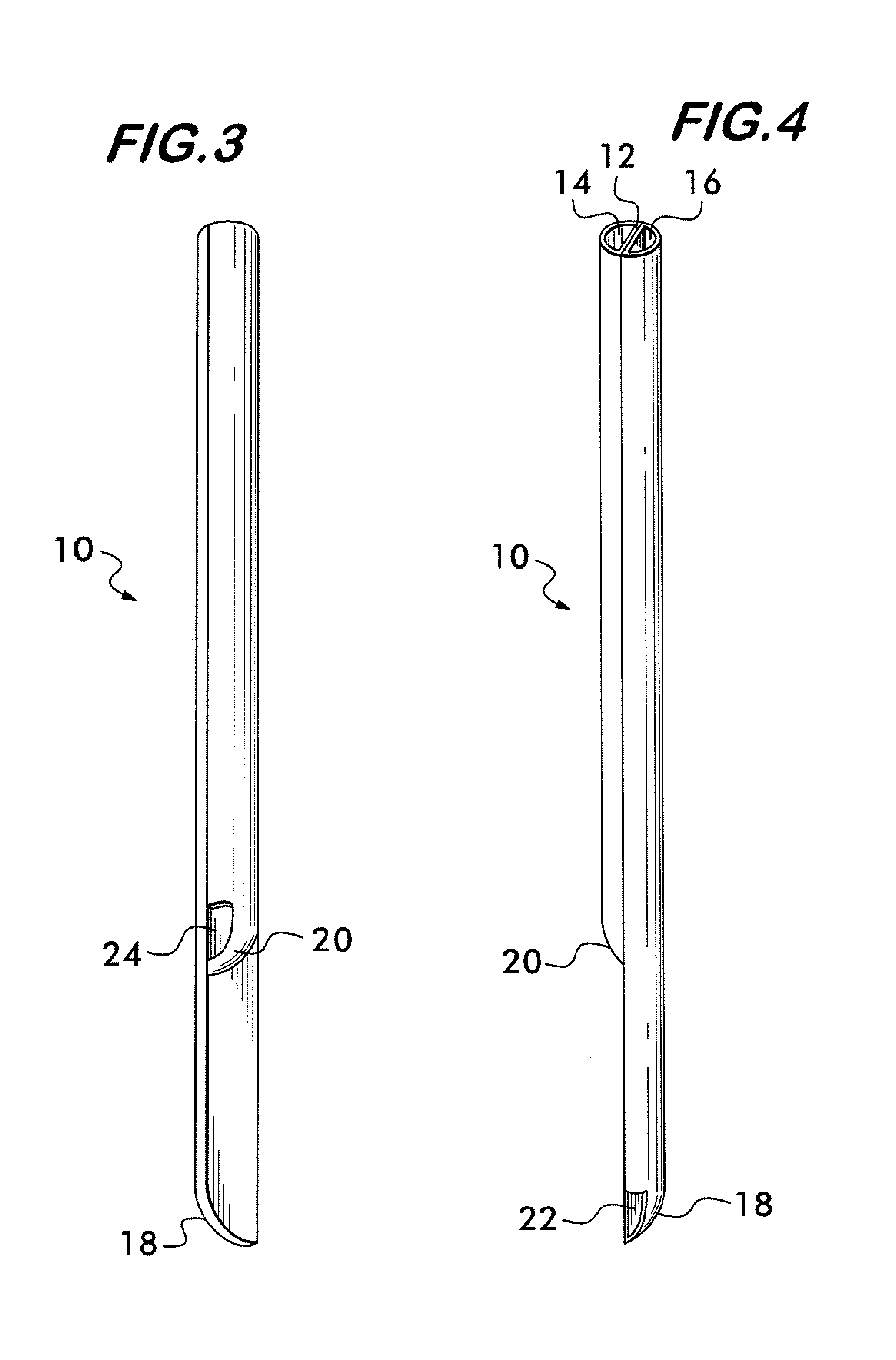
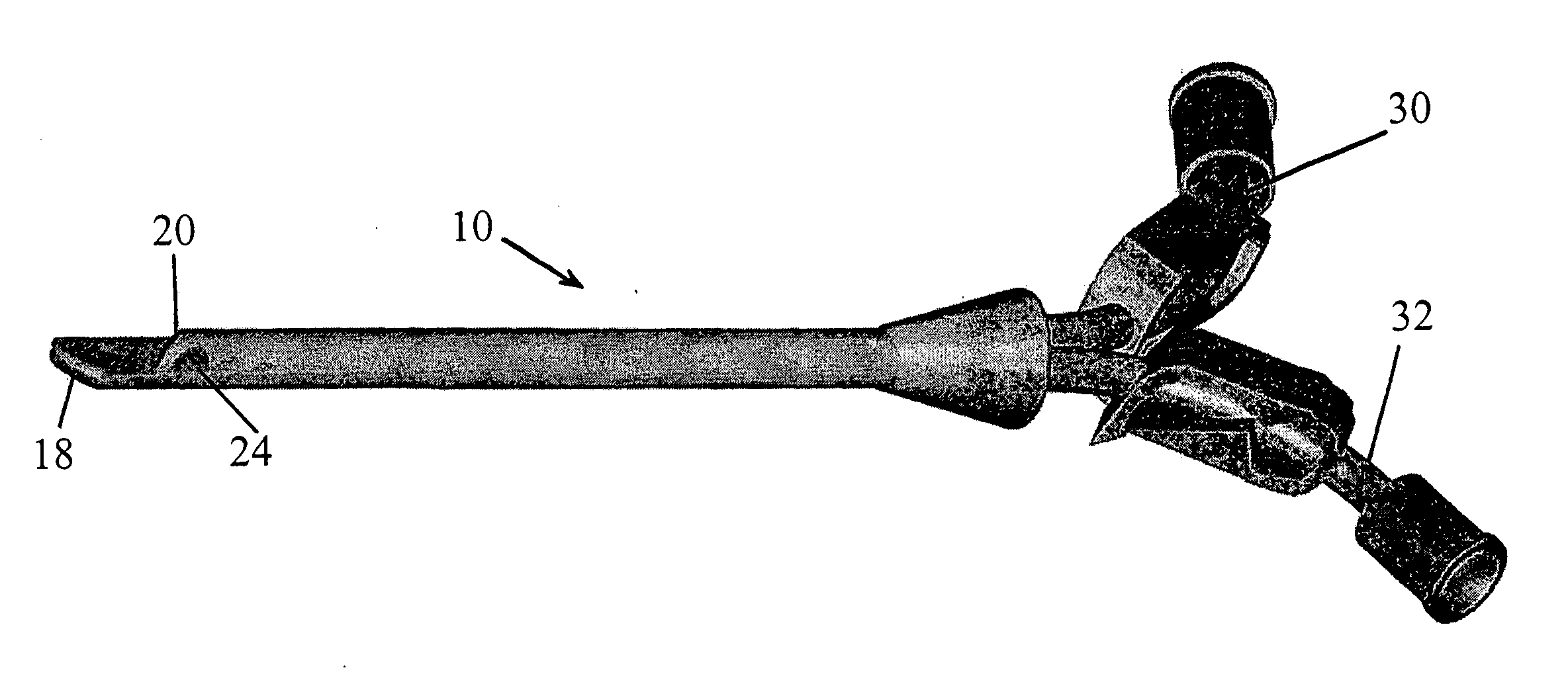
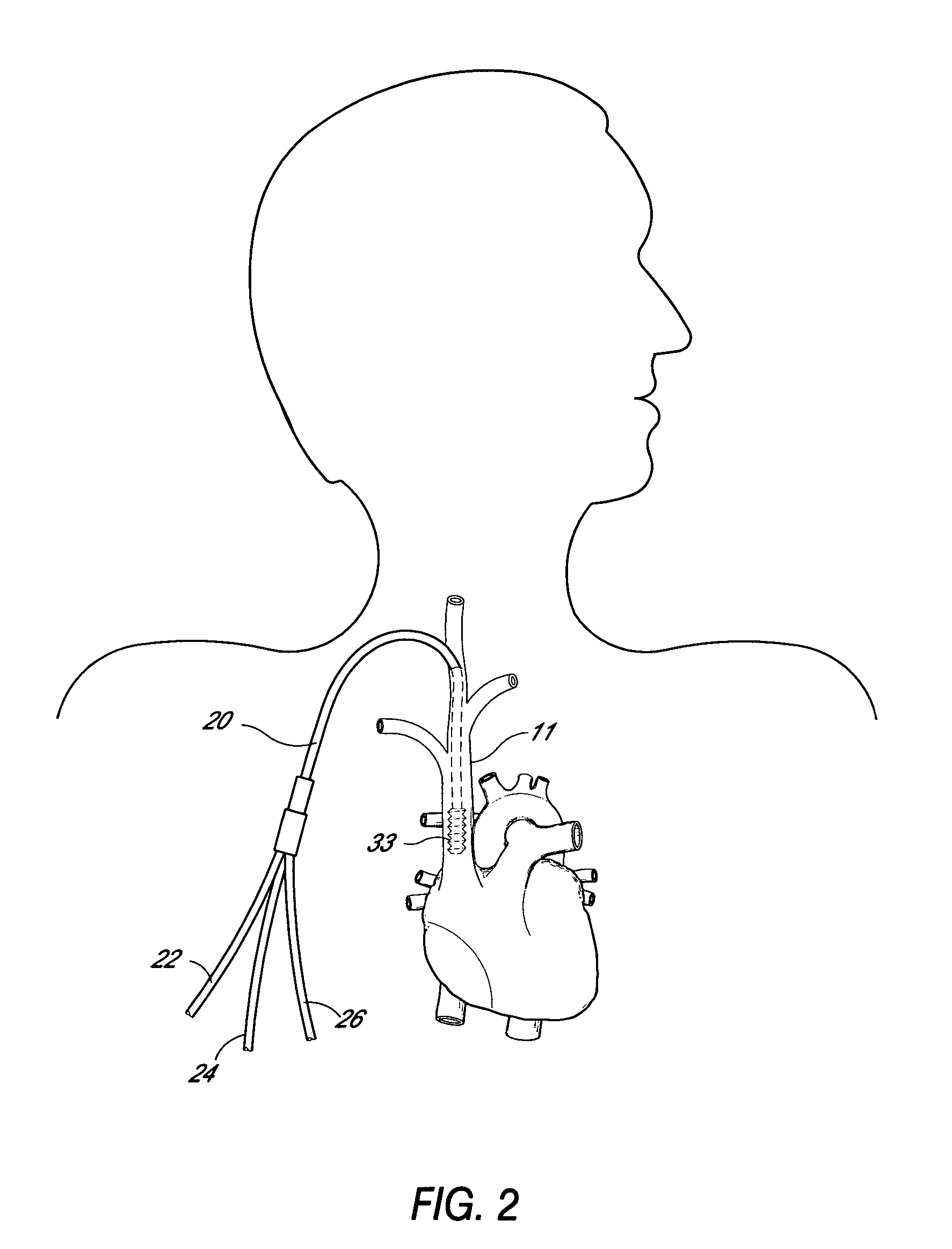
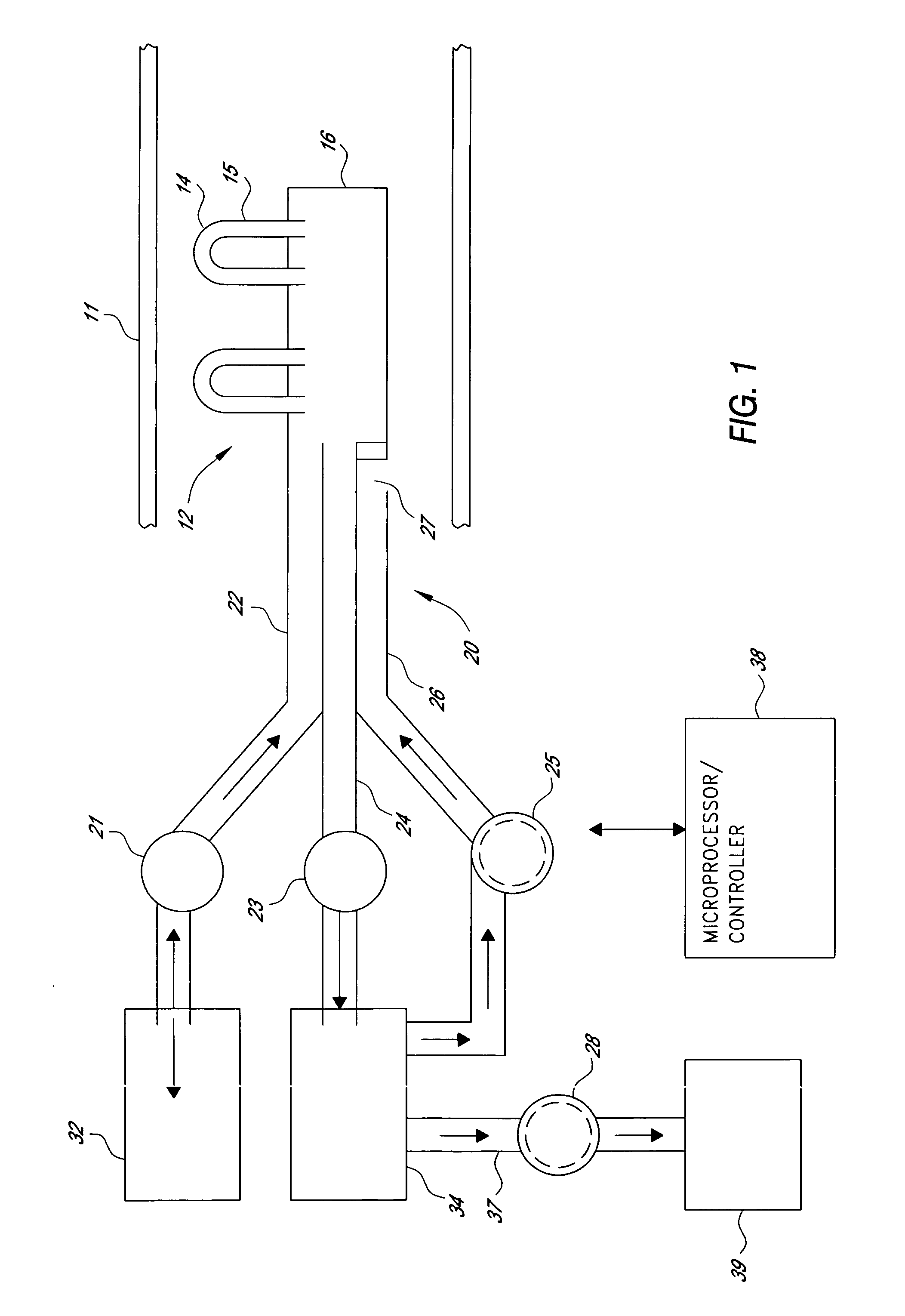
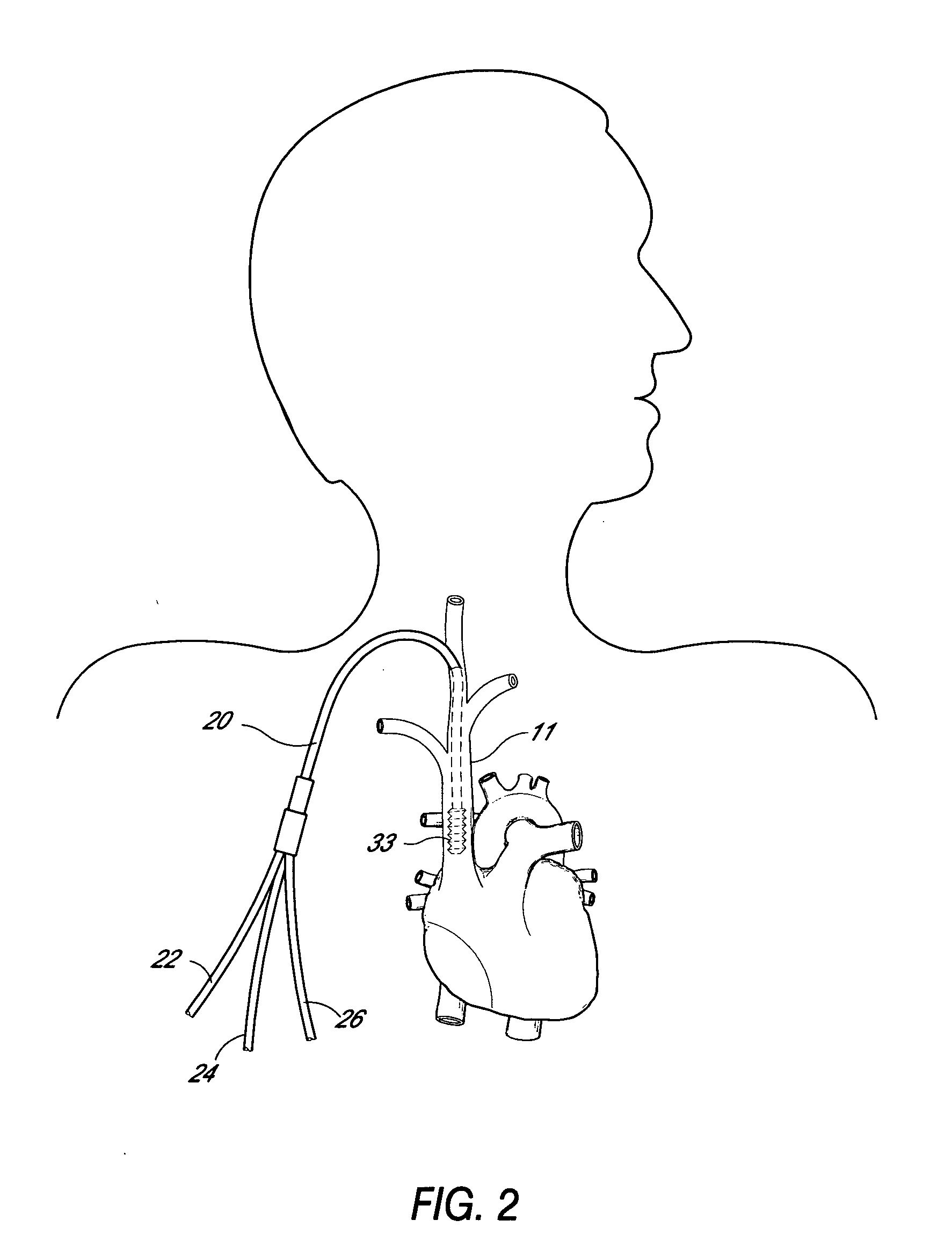
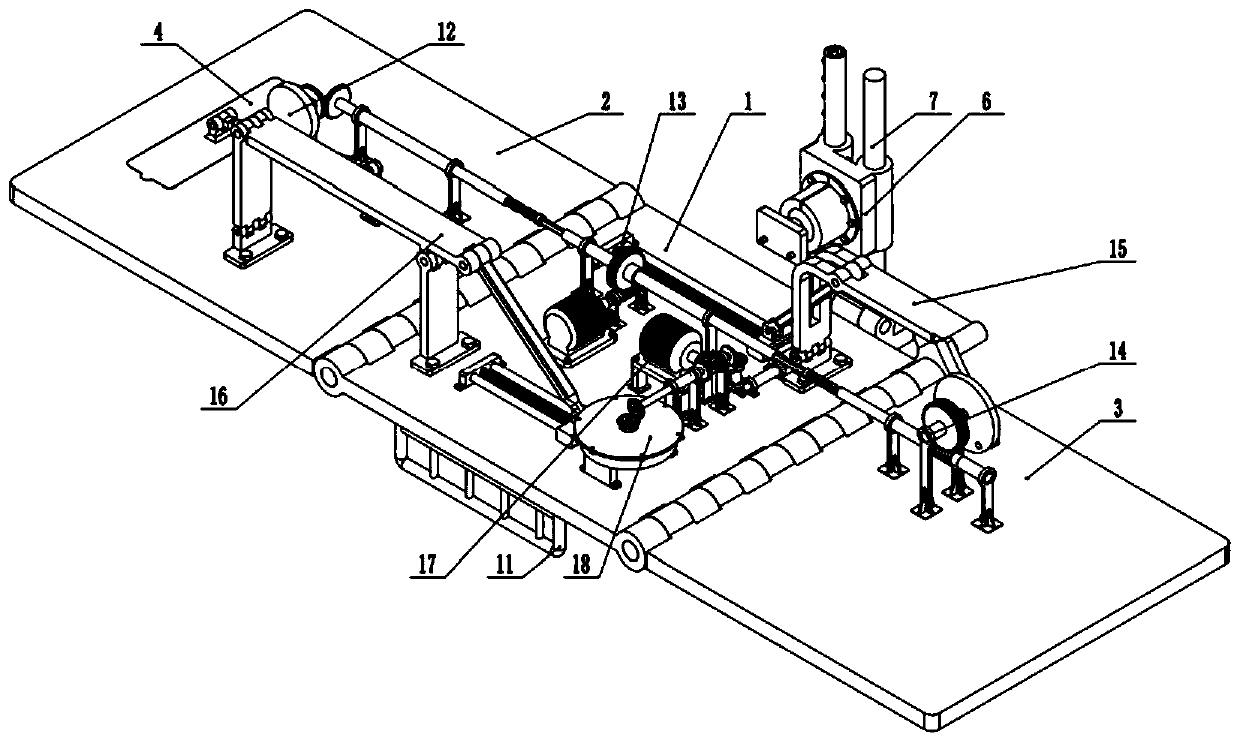
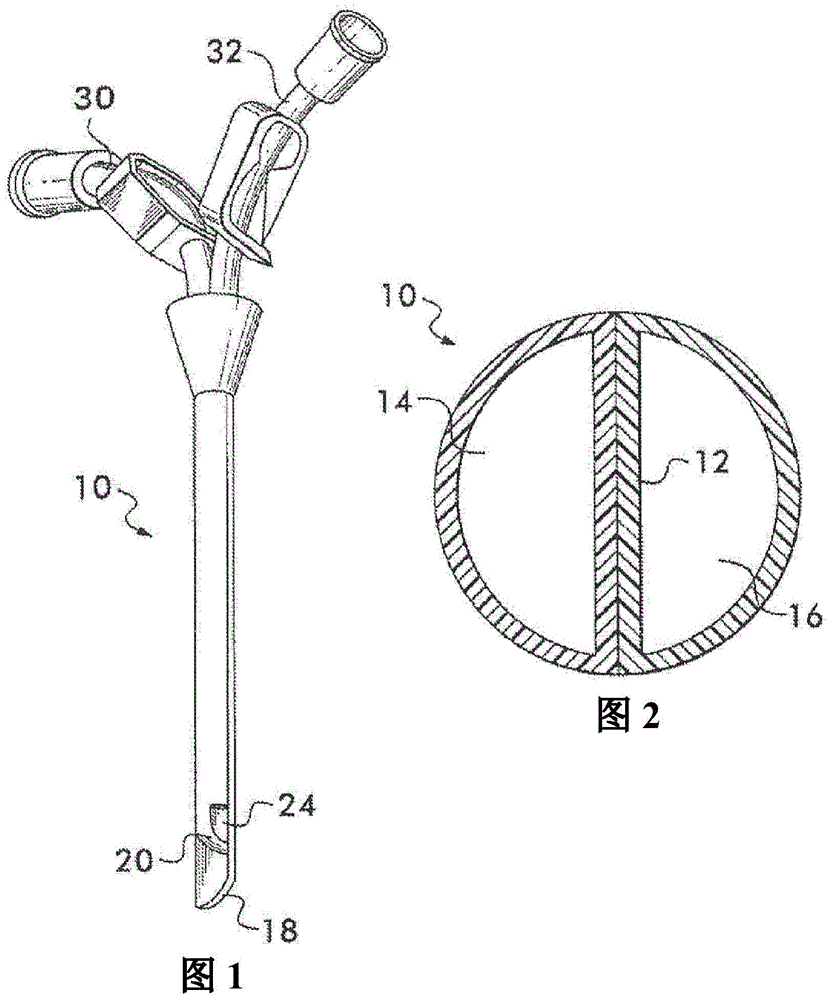
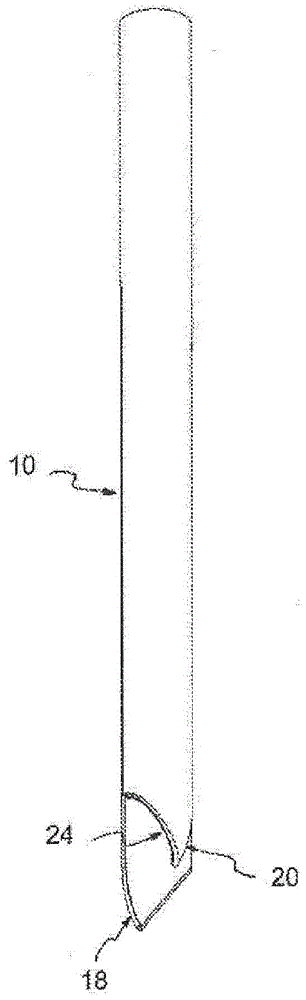
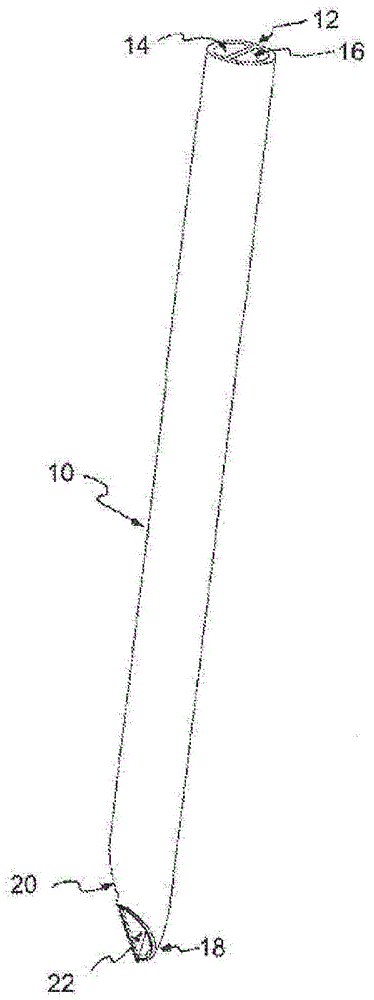
Multi-lumen catheter
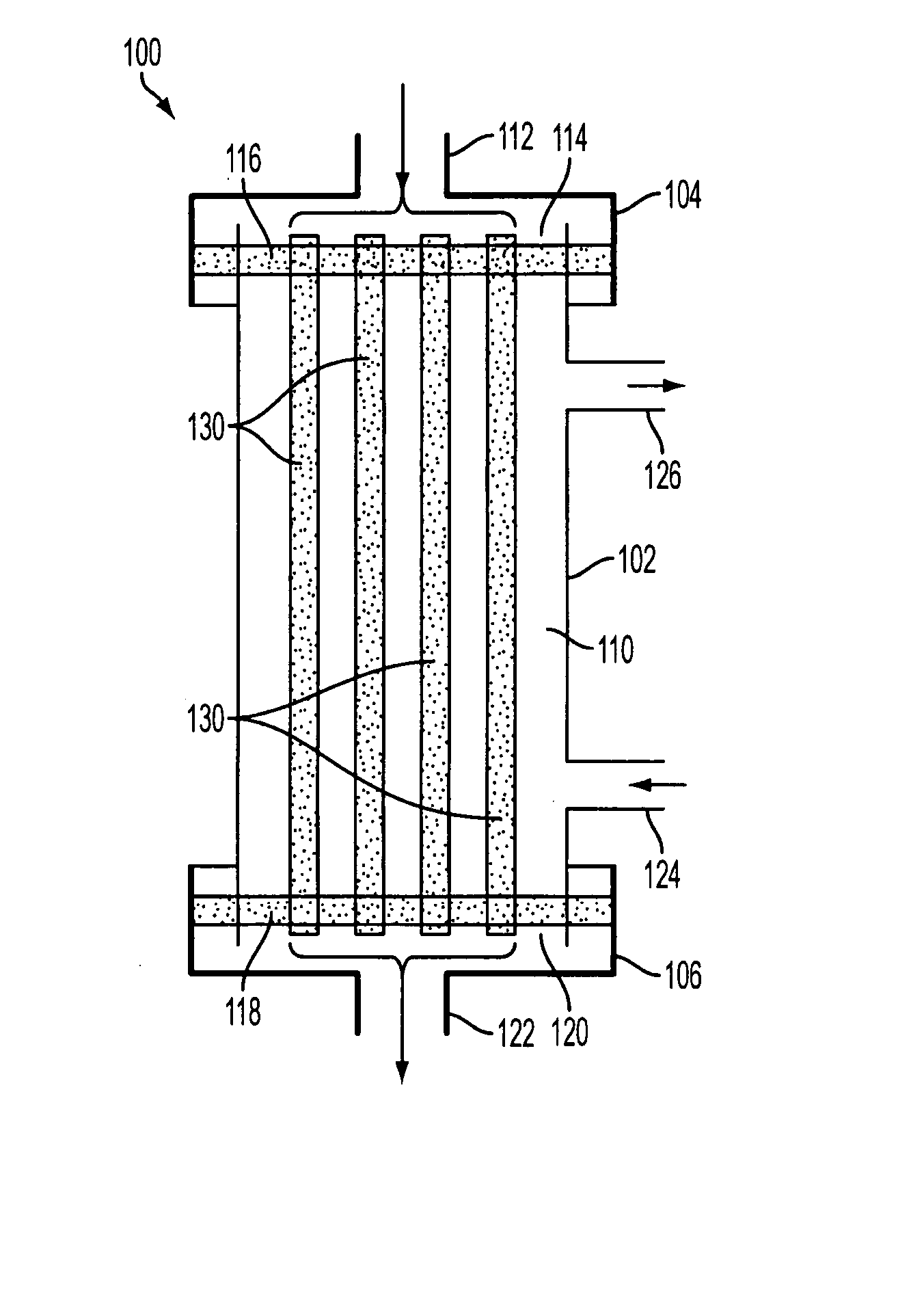
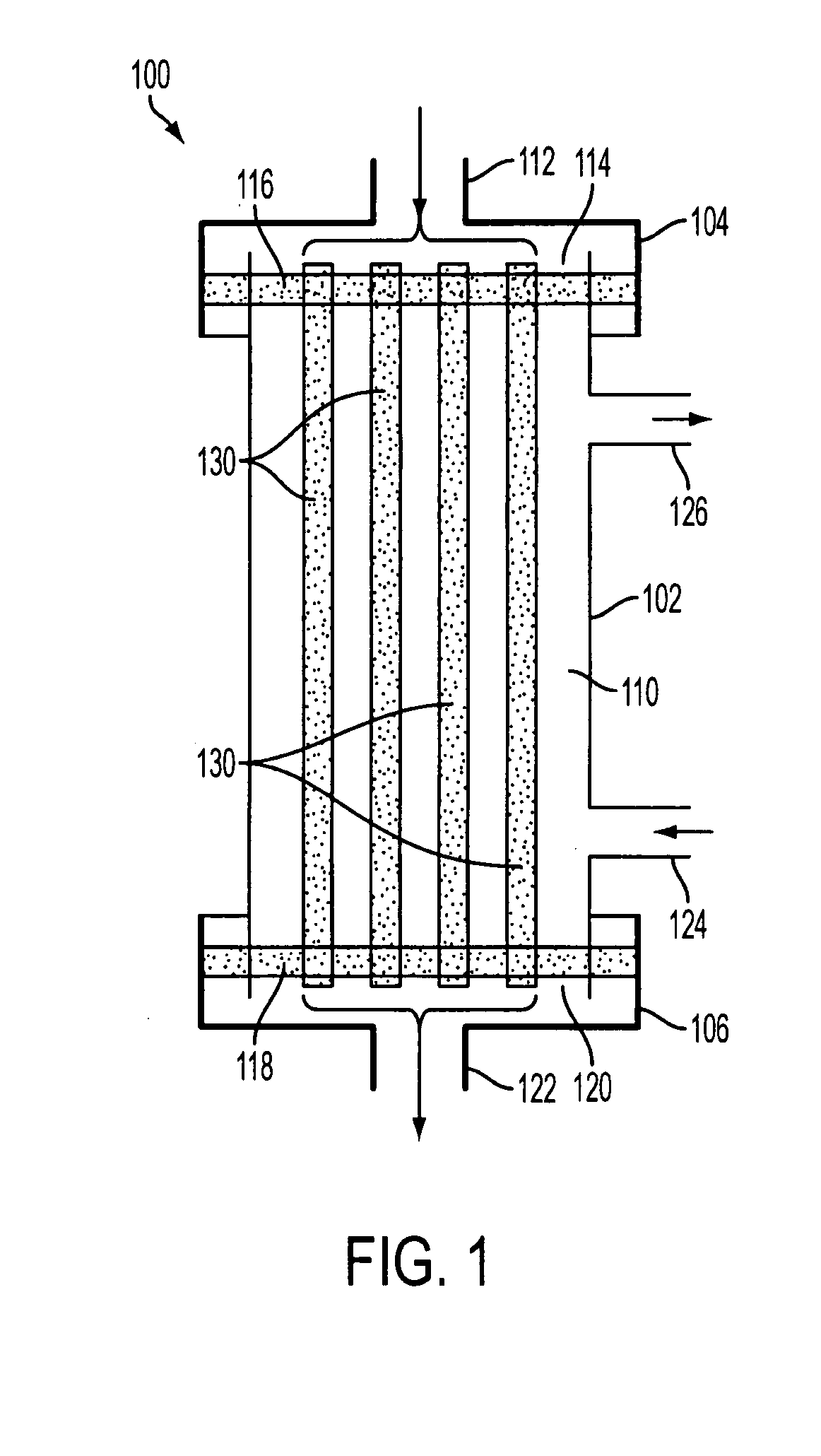
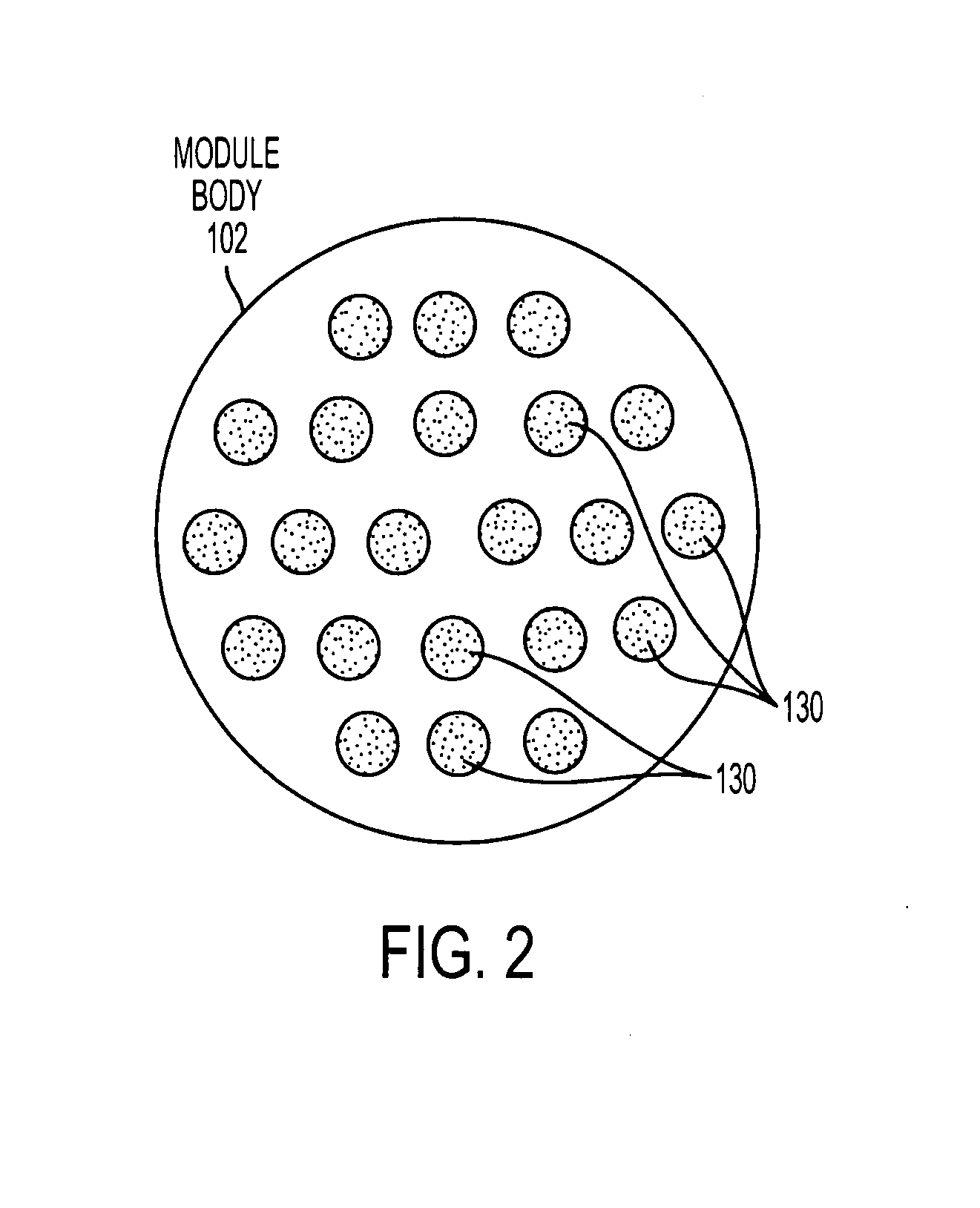
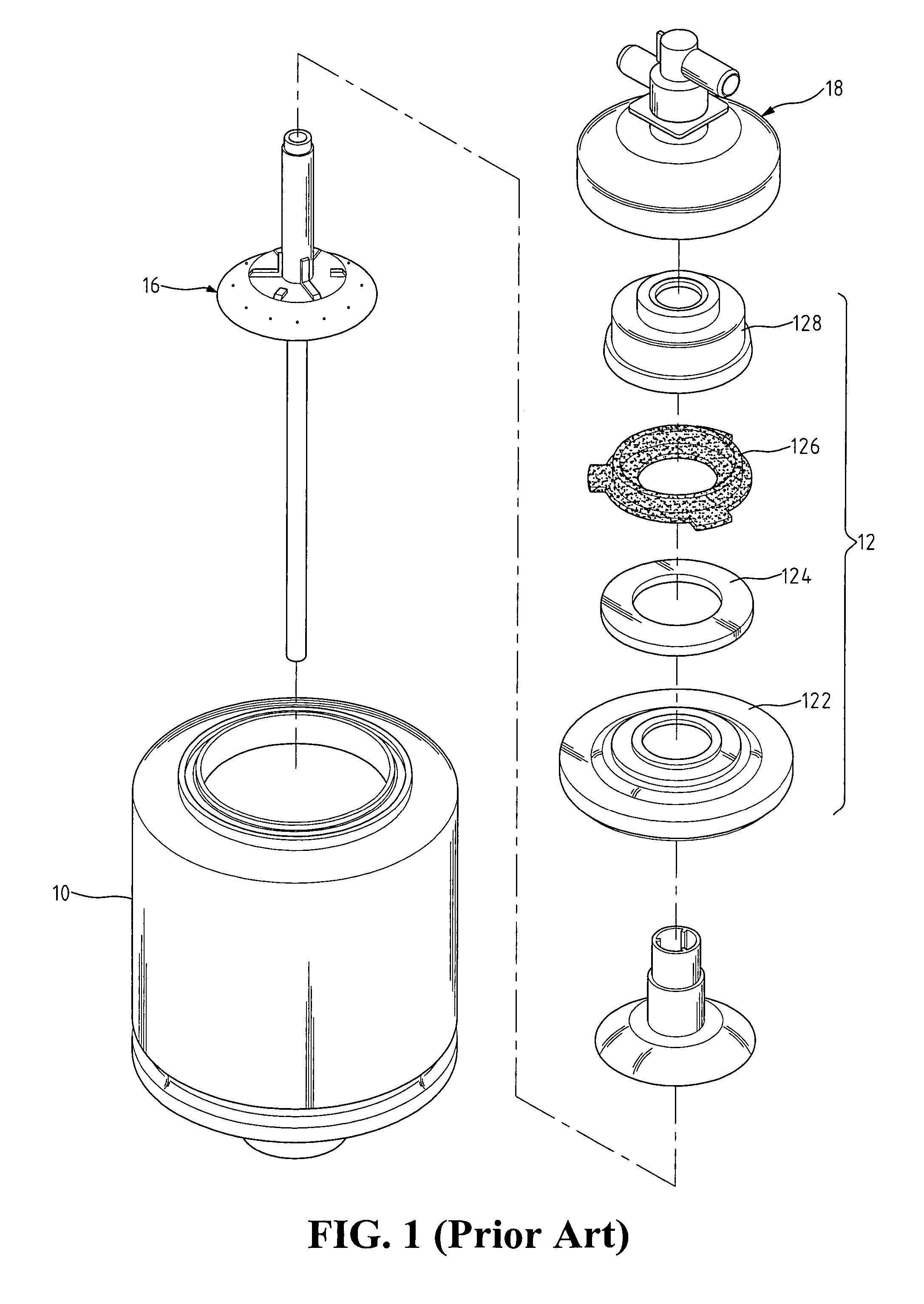
ActiveUS7569029B2Increase flow rateReduce traumaMulti-lumen catheterOther blood circulation devicesHemodialysisHaemodialysis machine
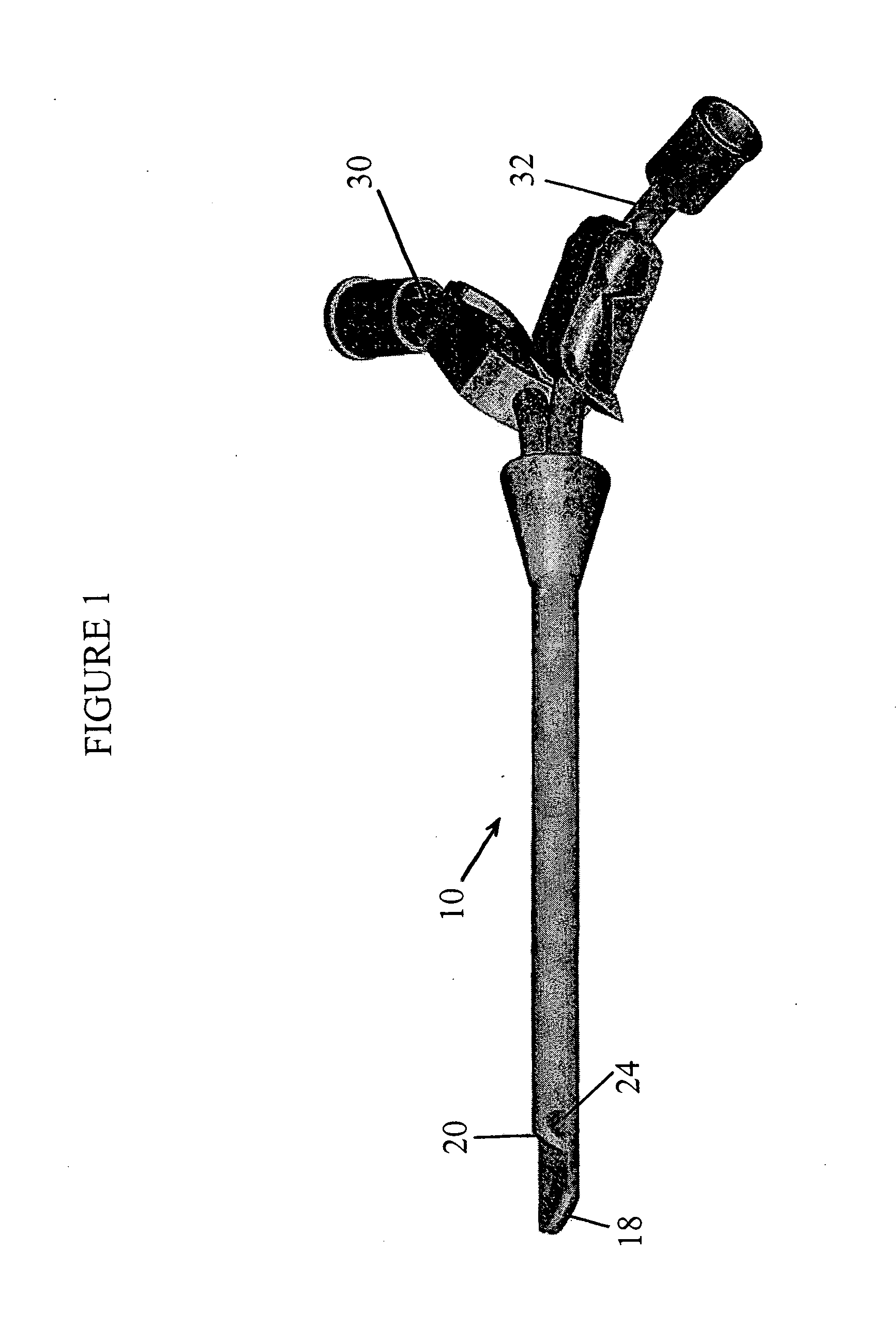
The invention provides a catheter for placement within a vessel of a patient. The catheter comprises an elongated catheter body, a septum extending longitudinally through the interior of the catheter body from the dividing the interior of the catheter body into a first lumen and a second lumen. Each lumen has curved or angled internal walls at the distal end of the catheter that terminate at ports located on opposing sides of the catheter body. The invention also provides a method for exchanging fluids in a patient comprising the step of positioning the catheter of the present invention in communication with a fluid-containing vessel of a patient. The method is particularly well-suited for hemodialysis, plasmapheresis, and other therapies which require removal and return of blood from a patient.
Owner:AEGIS MEDICAL TECH
Multi-lumen catheter
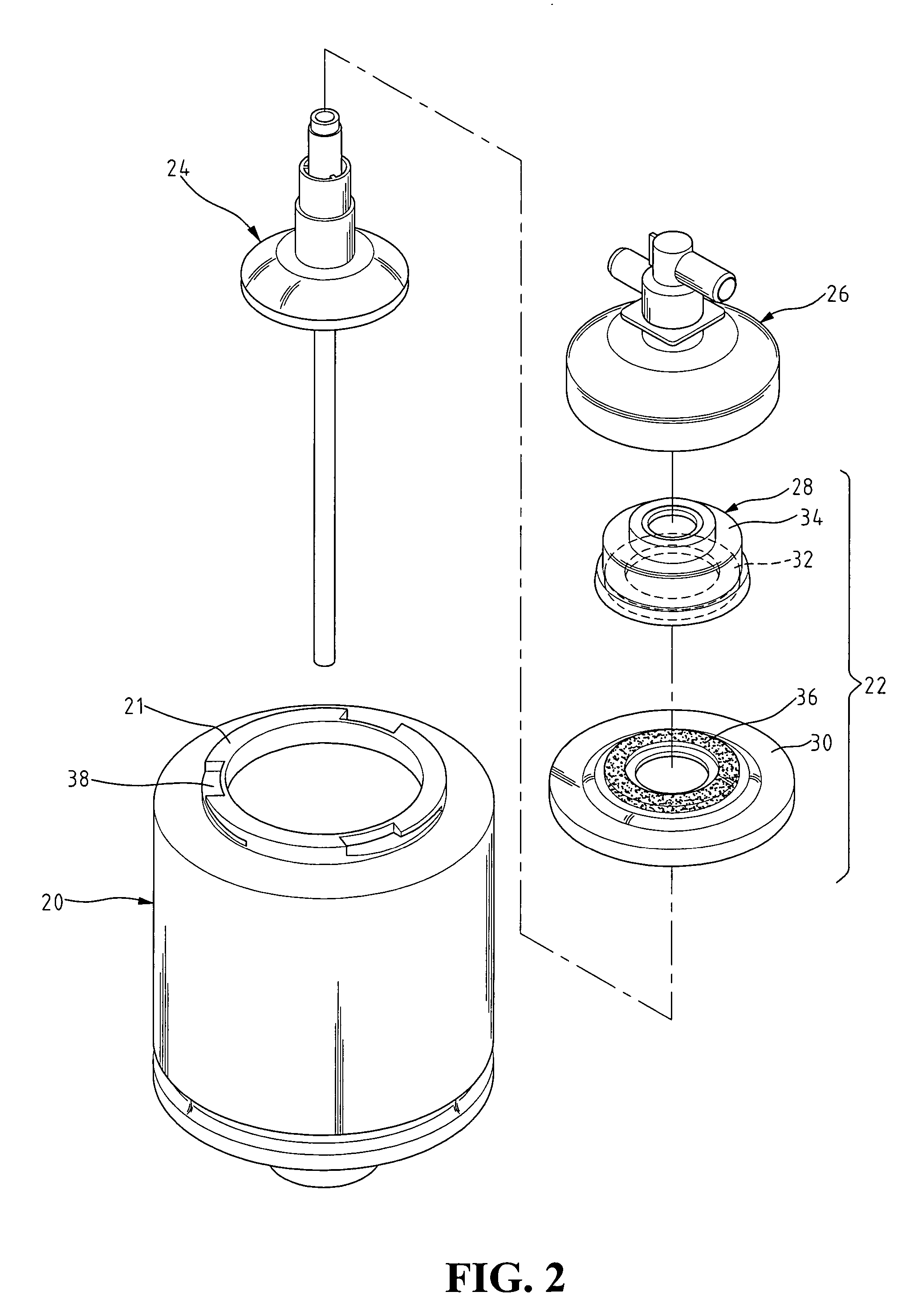
ActiveUS20050228339A1Efficient exchangeMinimize recirculationMulti-lumen catheterOther blood circulation devicesHemodialysisHaemodialysis machine
The invention provides a catheter for placement within a vessel of a patient. The catheter comprises an elongated catheter body, a septum extending longitudinally through the interior of the catheter body from the dividing the interior of the catheter body into a first lumen and a second lumen. Each lumen has curved or angled internal walls at the distal end of the catheter that terminate at ports located on opposing sides of the catheter body. The invention also provides a method for exchanging fluids in a patient comprising the step of positioning the catheter of the present invention in communication with a fluid-containing vessel of a patient. The method is particularly well-suited for hemodialysis, plasmapheresis, and other therapies which require removal and return of blood from a patient.
Owner:AEGIS MEDICAL TECH
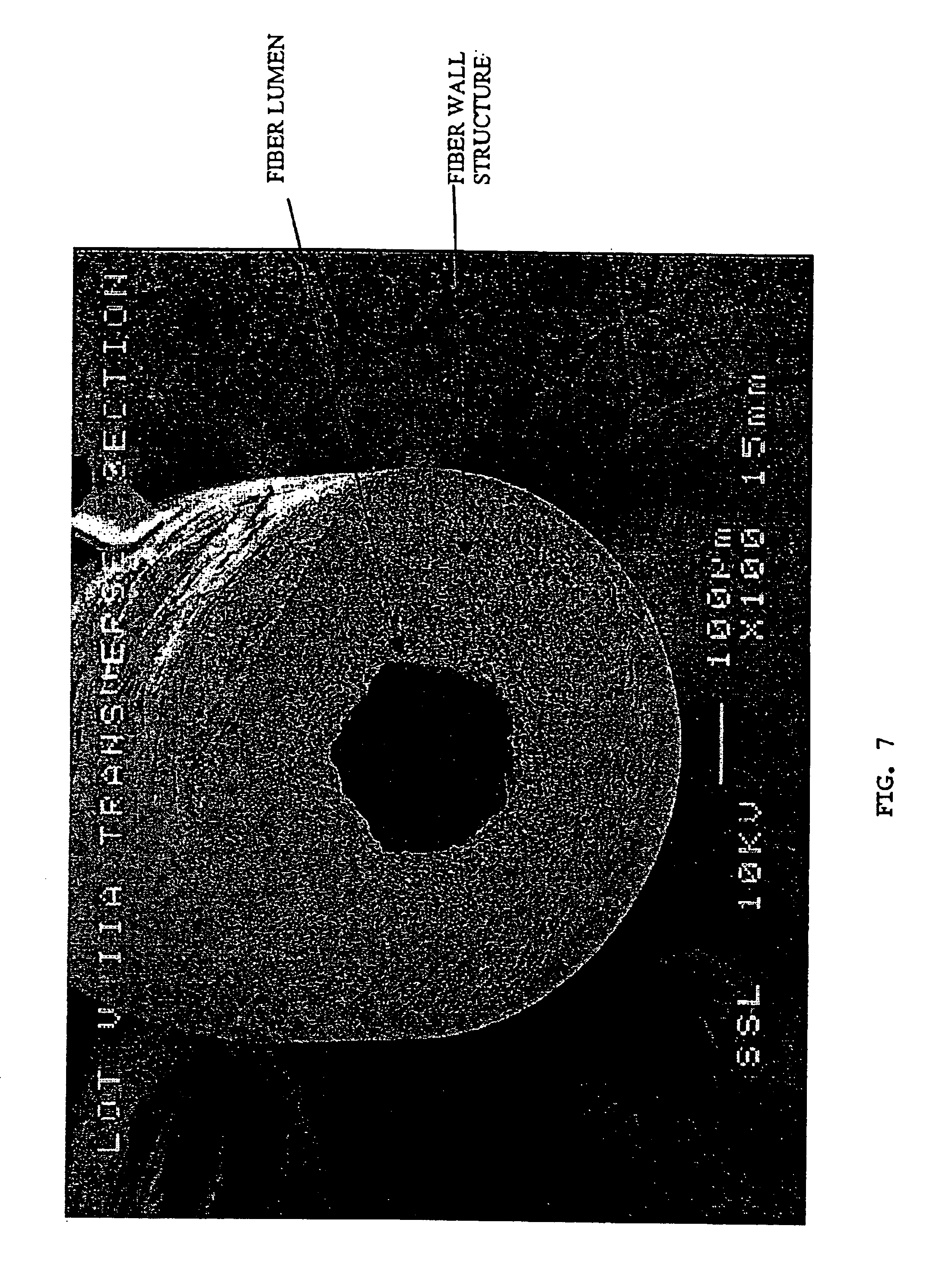
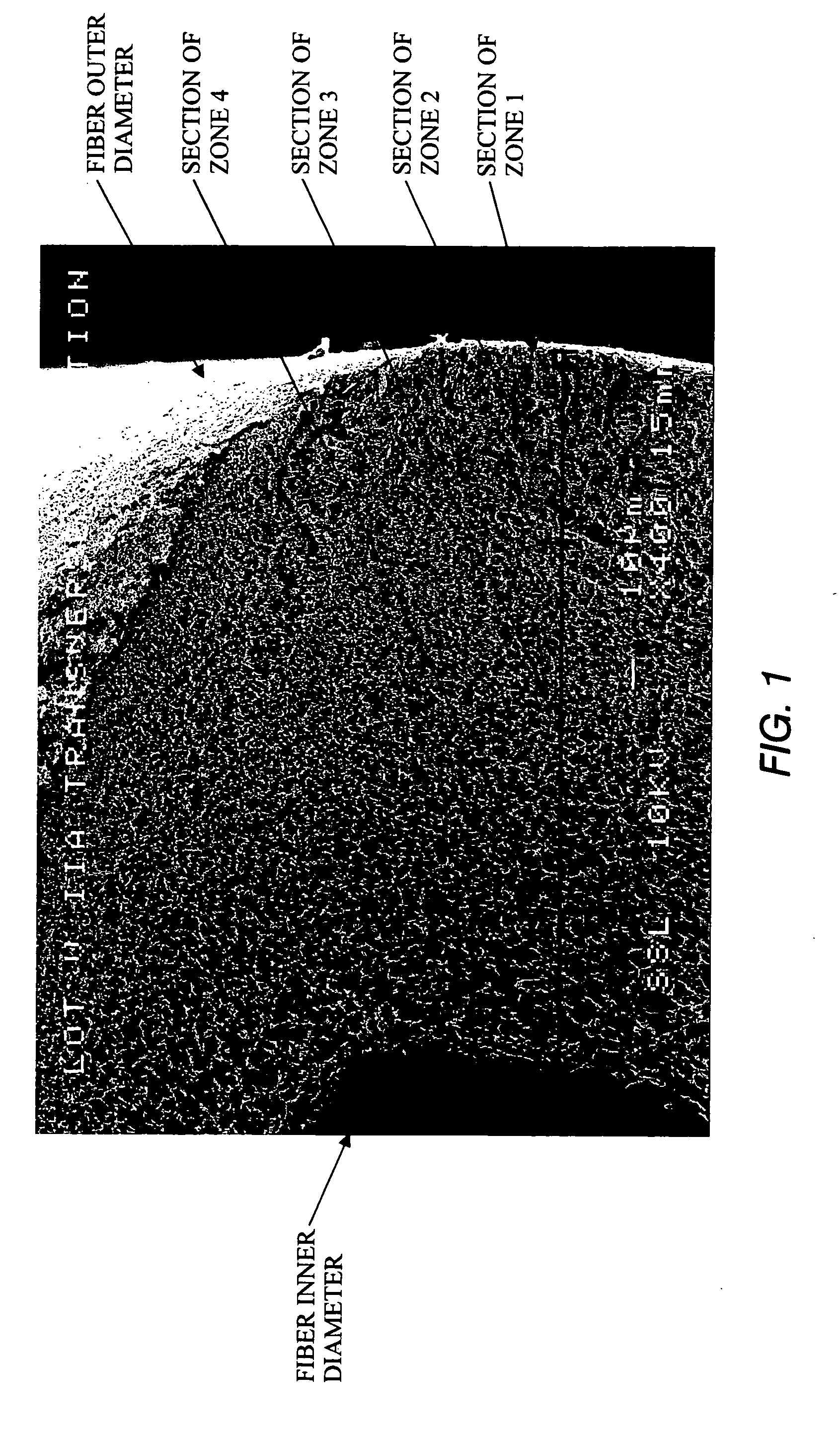
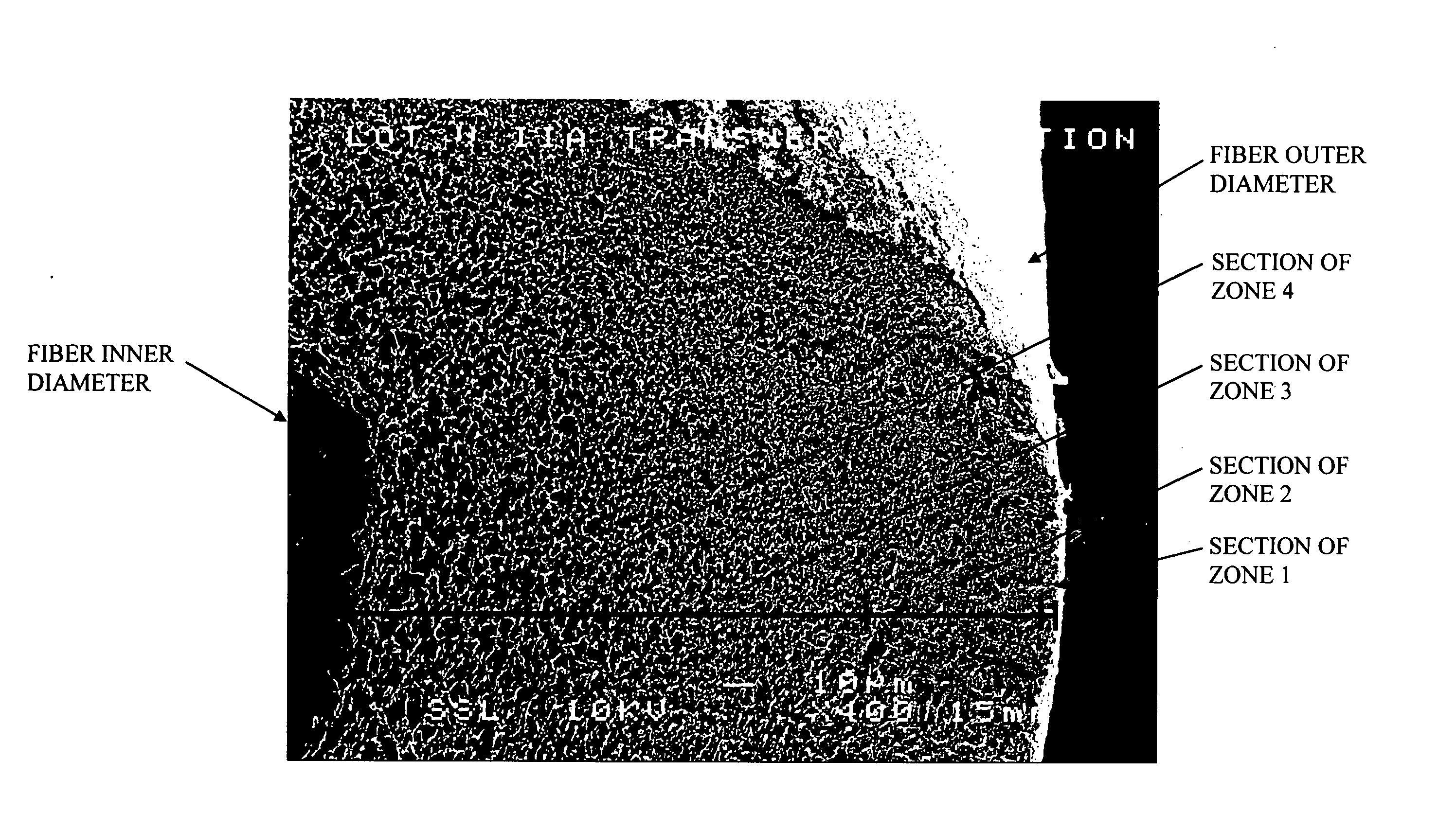
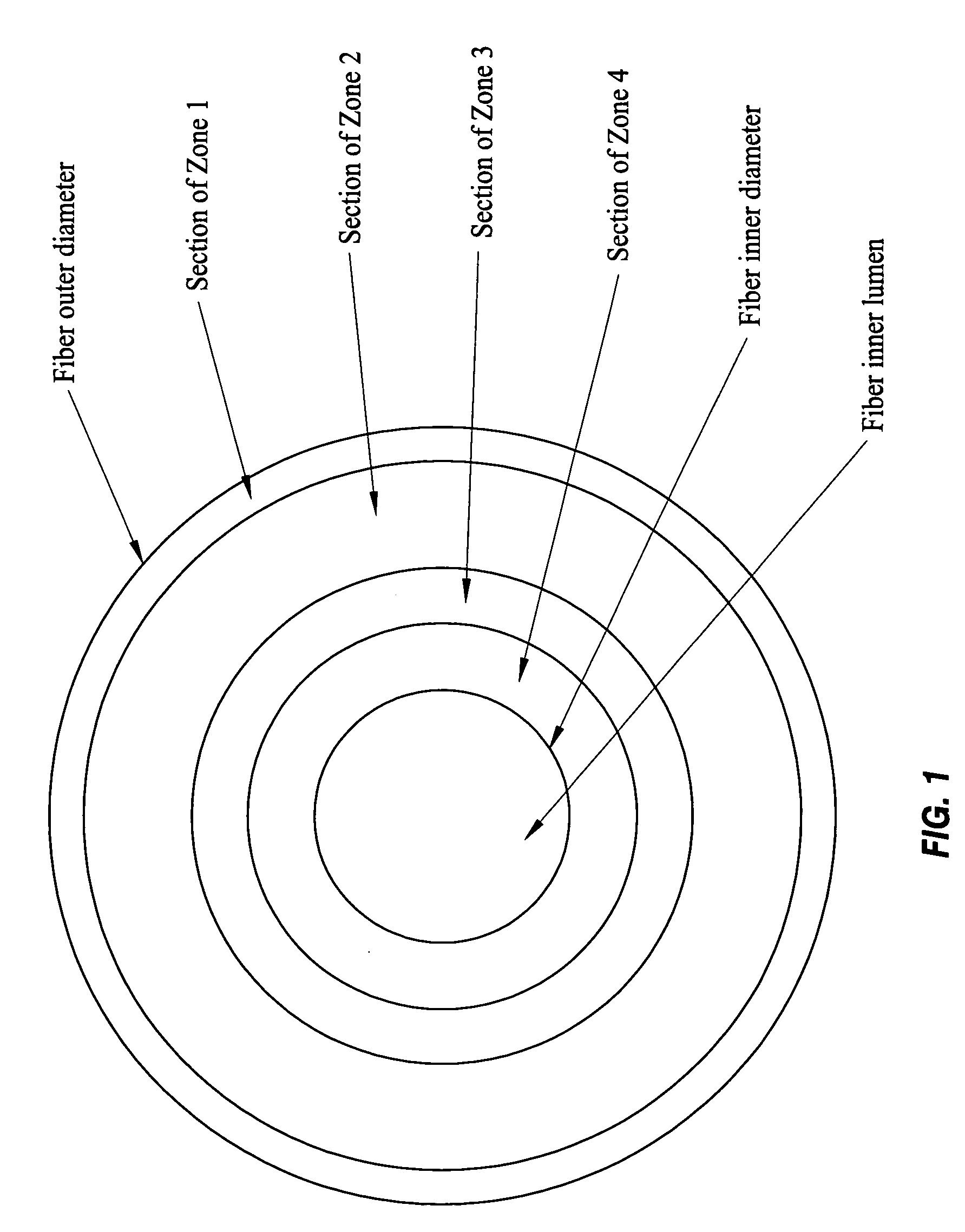
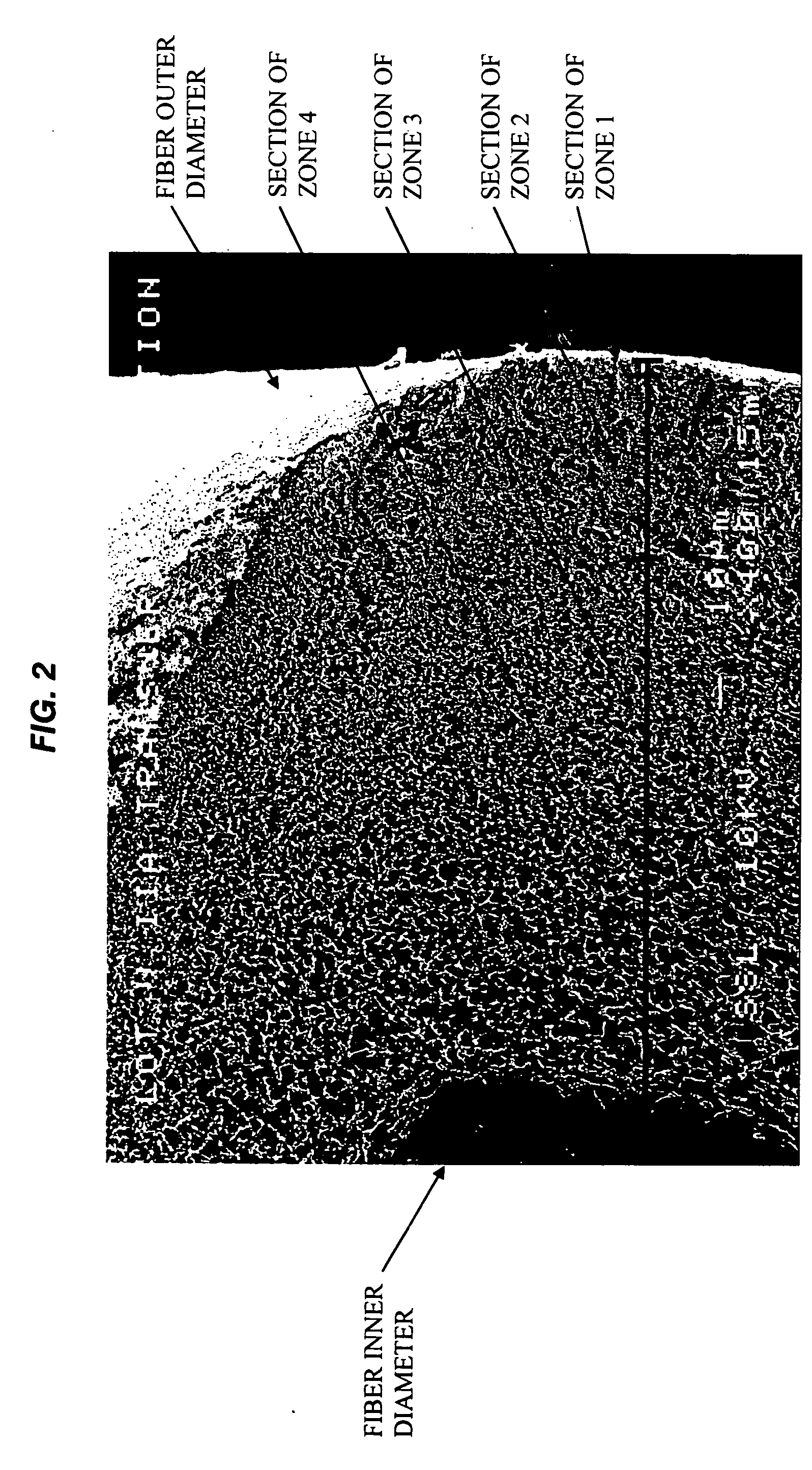
Specialized hollow fiber membranes for plasmapheresis and ultrafiltration
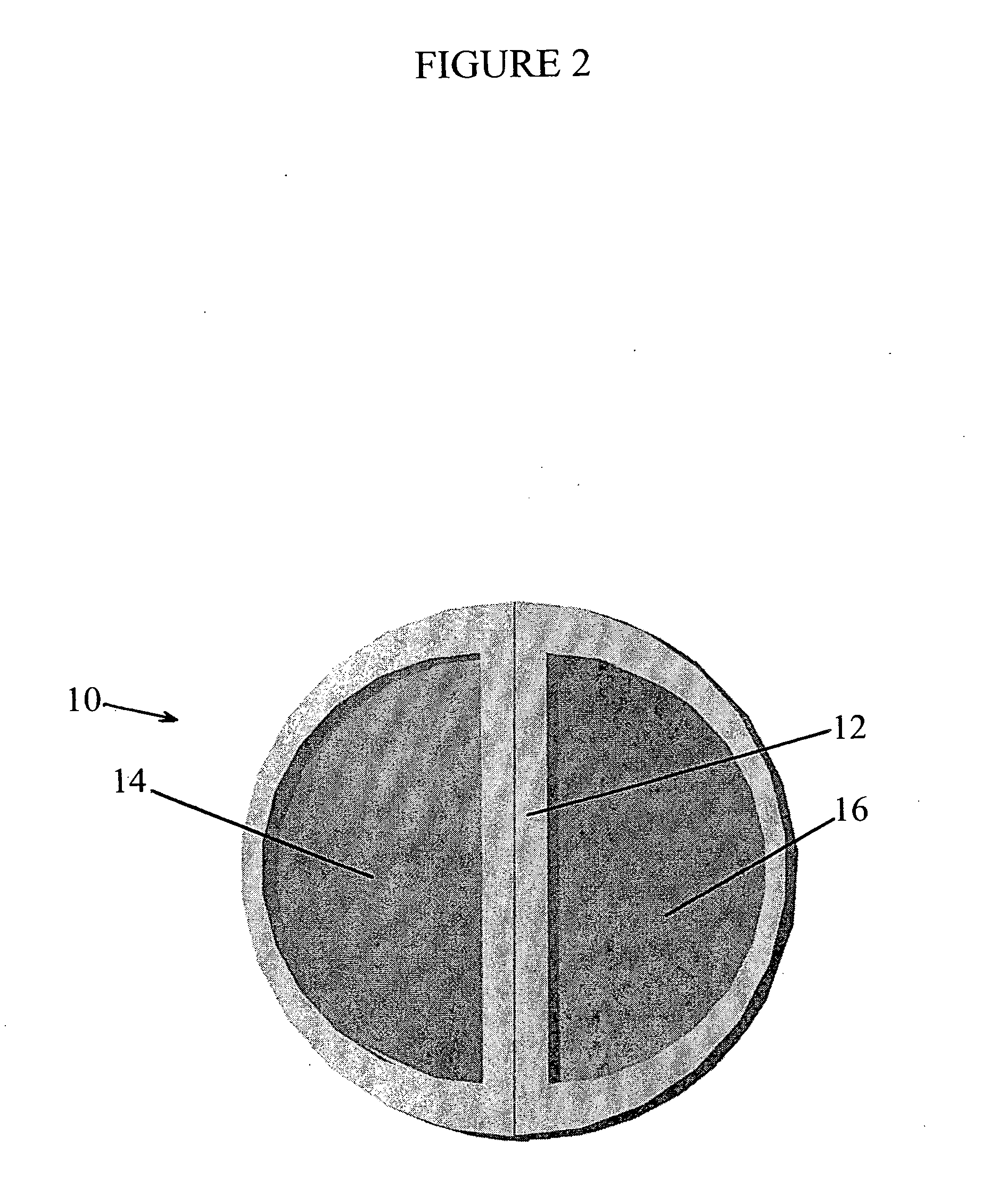
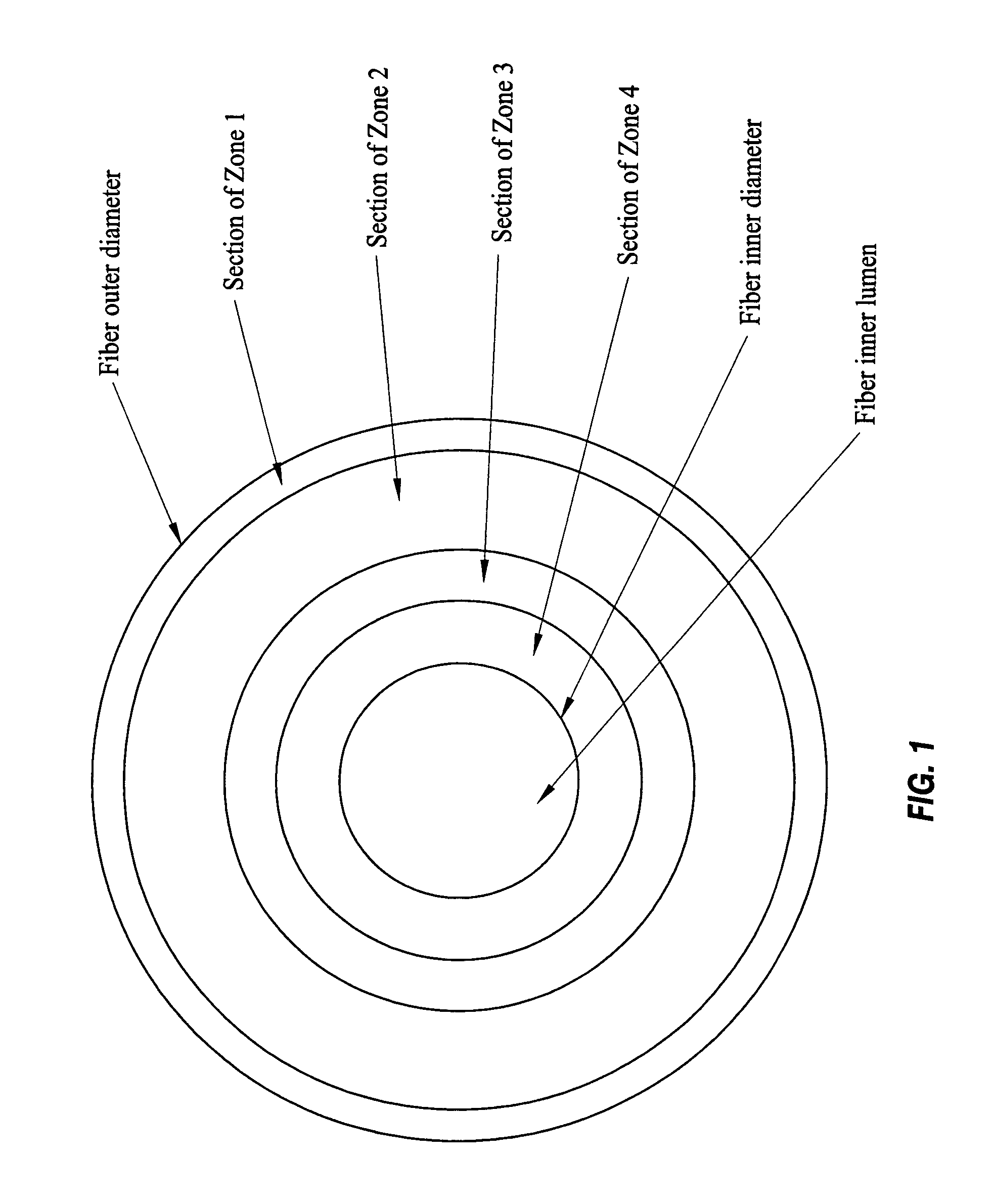
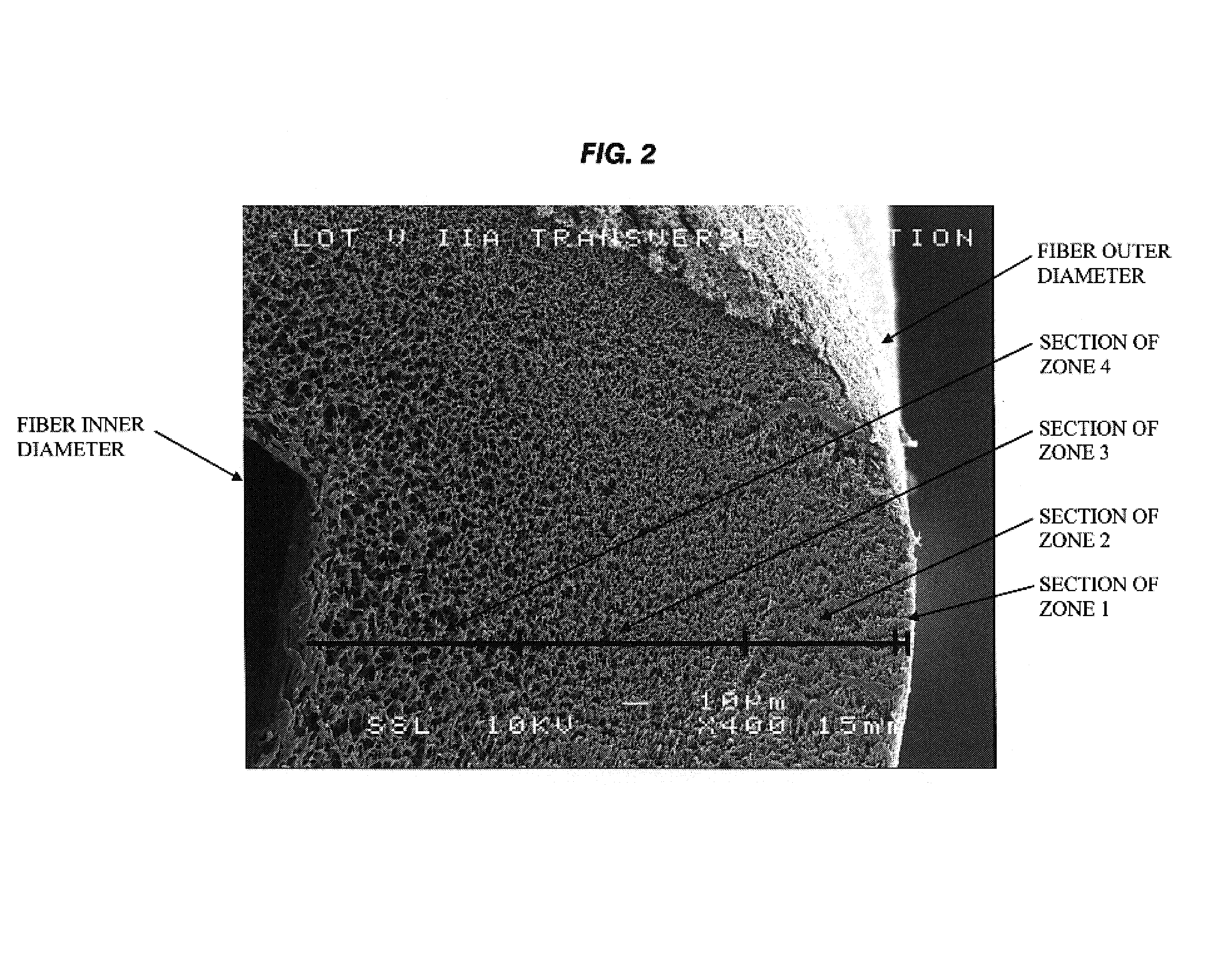
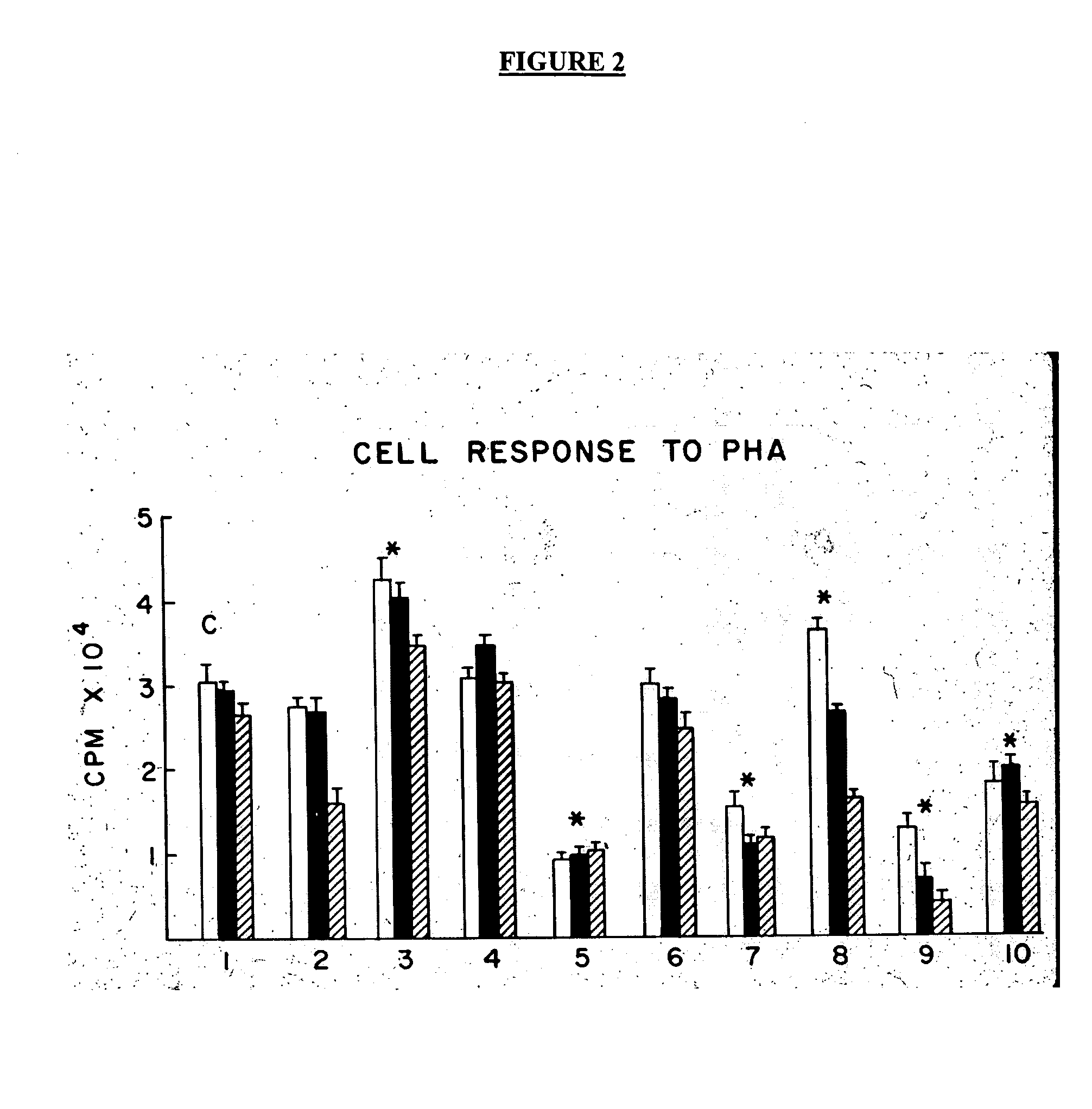
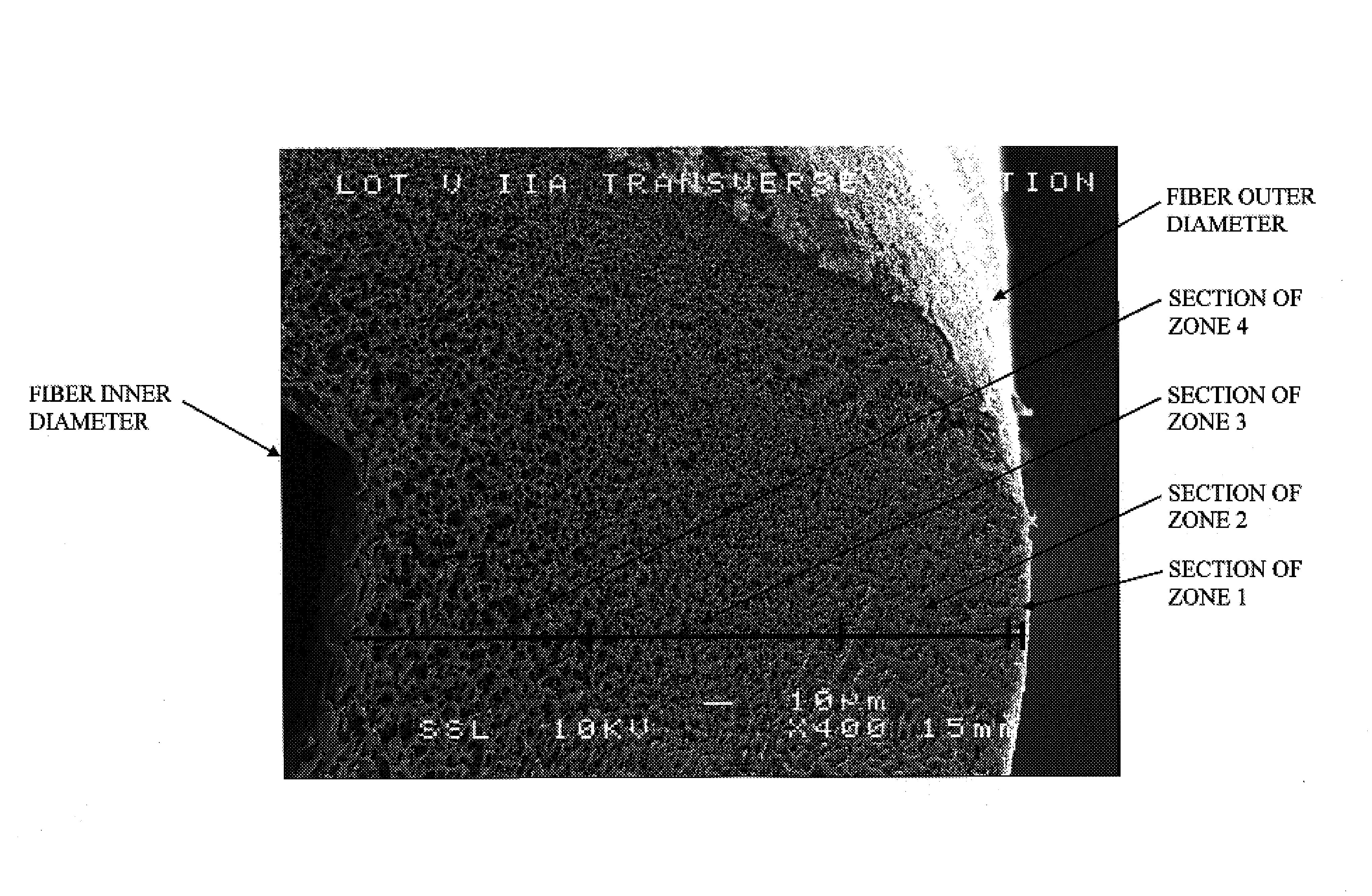
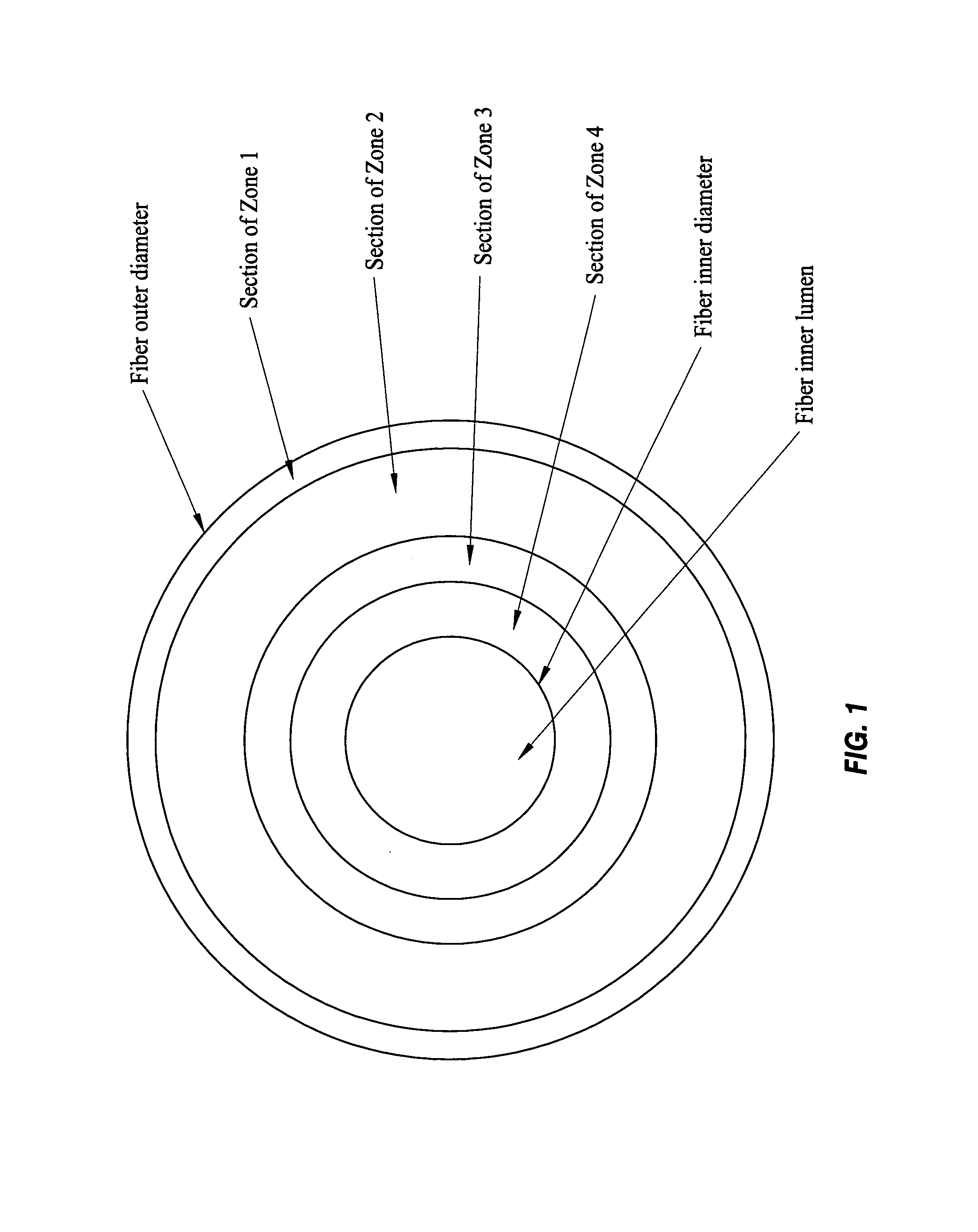
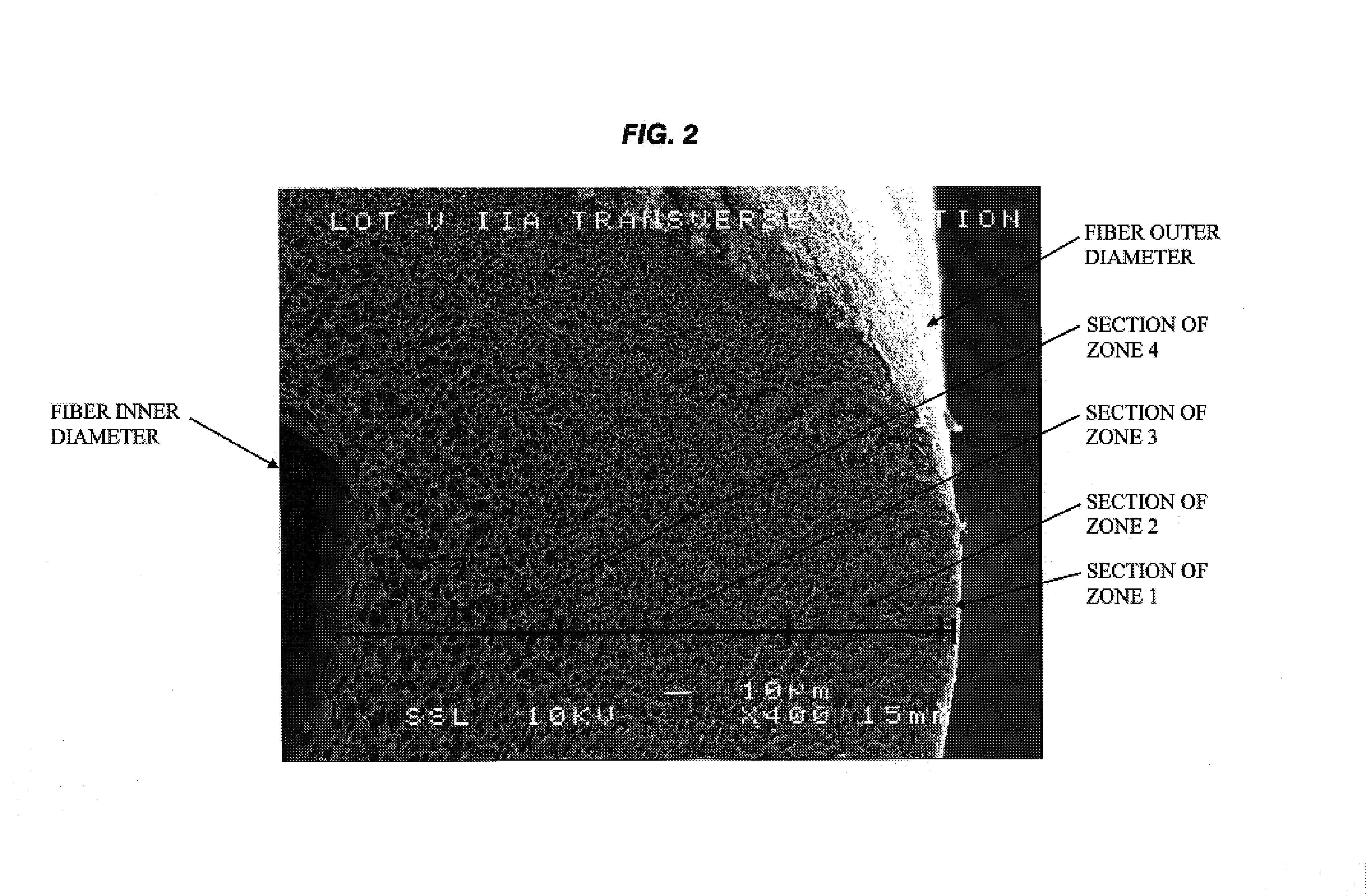
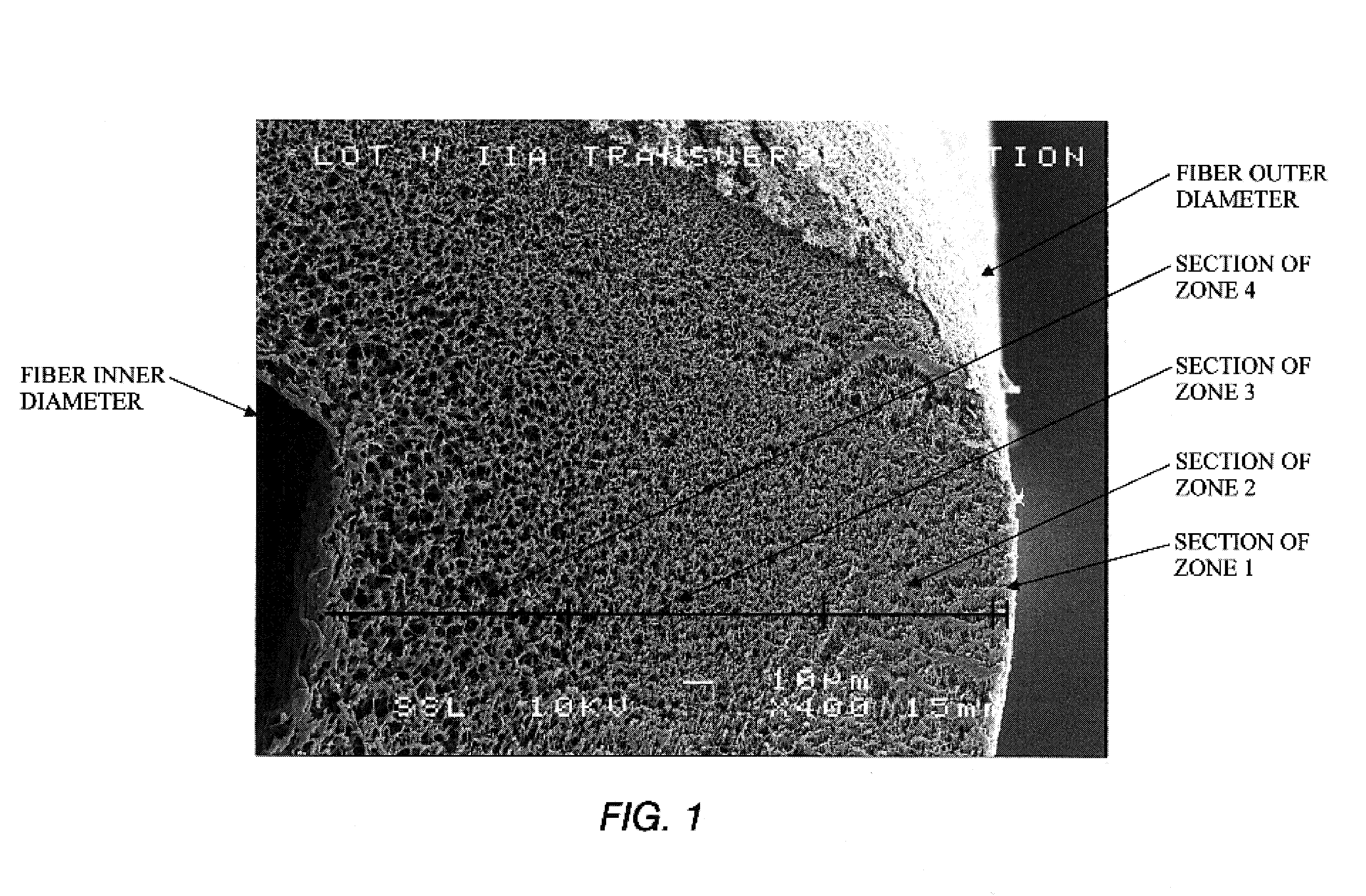
A plasmapheresis and / or ultrafiltration membrane comprises elongated hollow fibers each fiber having an interior lumen extending along the fiber length, the fiber wall having a plurality of zones between the inner and outer wall surfaces, each of the zones having a mass density different than the mass density of an adjacent zone. The fiber wall is characterized by having a lower mass density zone at the inner wall surface and a higher mass density zone at the outer wall surface. The fiber is further characterized by having an average elongation breaking force strength of at least about 0.2 lbs. and an average elongation of at least about 45%.
Owner:TRANSVIVO
Apparatus for enhanced plasmapheresis and methods thereof
InactiveUS6960178B2Easy to separateEnhanced continuous plasmapheresisWater/sewage treatment by centrifugal separationSemi-permeable membranesRed blood cellPlasma flow
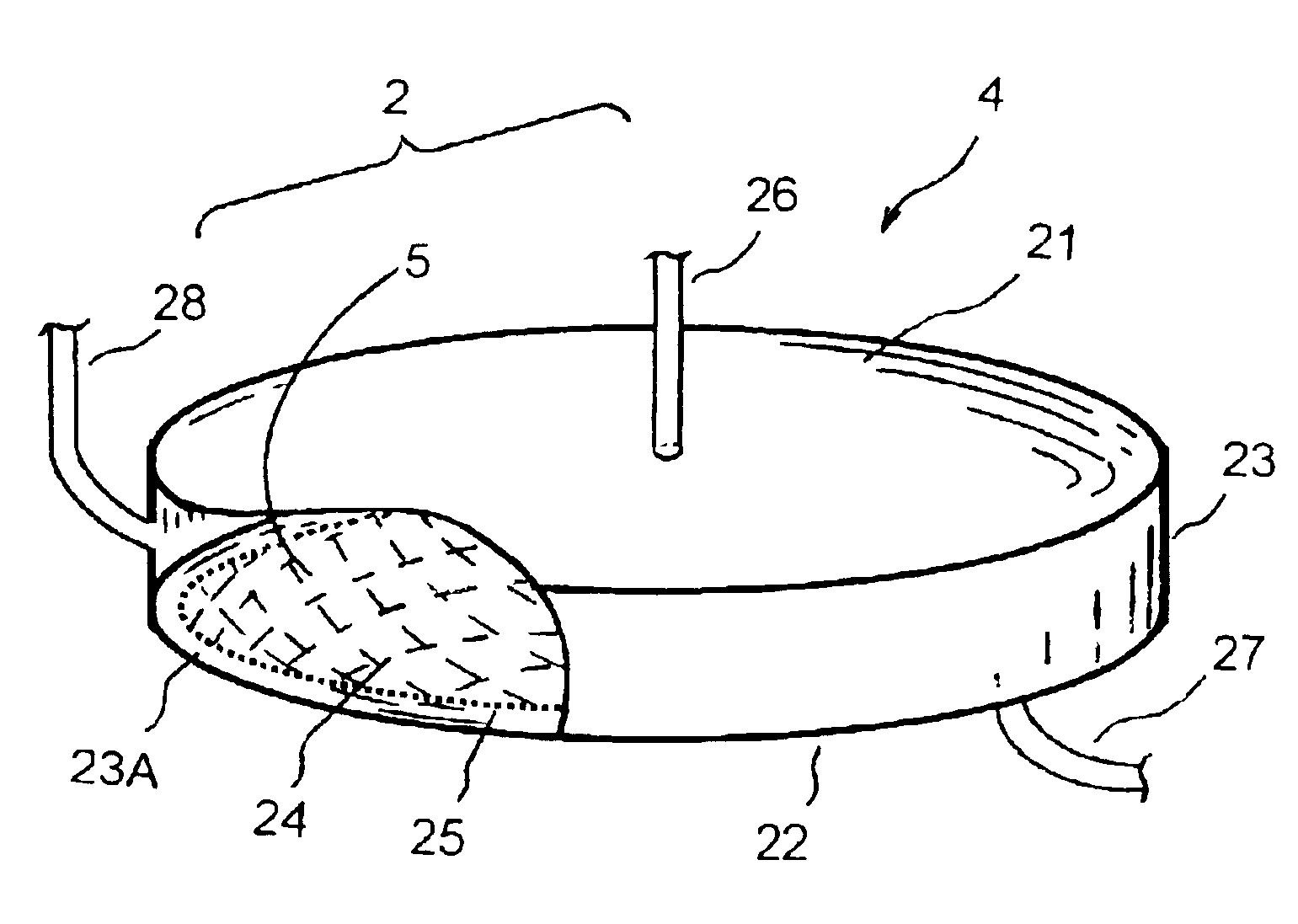
An apparatus and methods for enhanced plasmapheresis comprising a filter membrane under an orbital motion or movement that has optimal local shear forces and maximum plasma flow output. The methods for biological separation and therapies comprise platelet collection, viral particle removal, cell washing and processing for stem cell selection, bone marrow purging, red blood cell collection, auto-transfusion, auto-immune disease treatment, selective macro-molecule removal, toxin removal, LDL removal, extracorporeal plasma delipidation, and the like.
Owner:XEPMED INC (US)
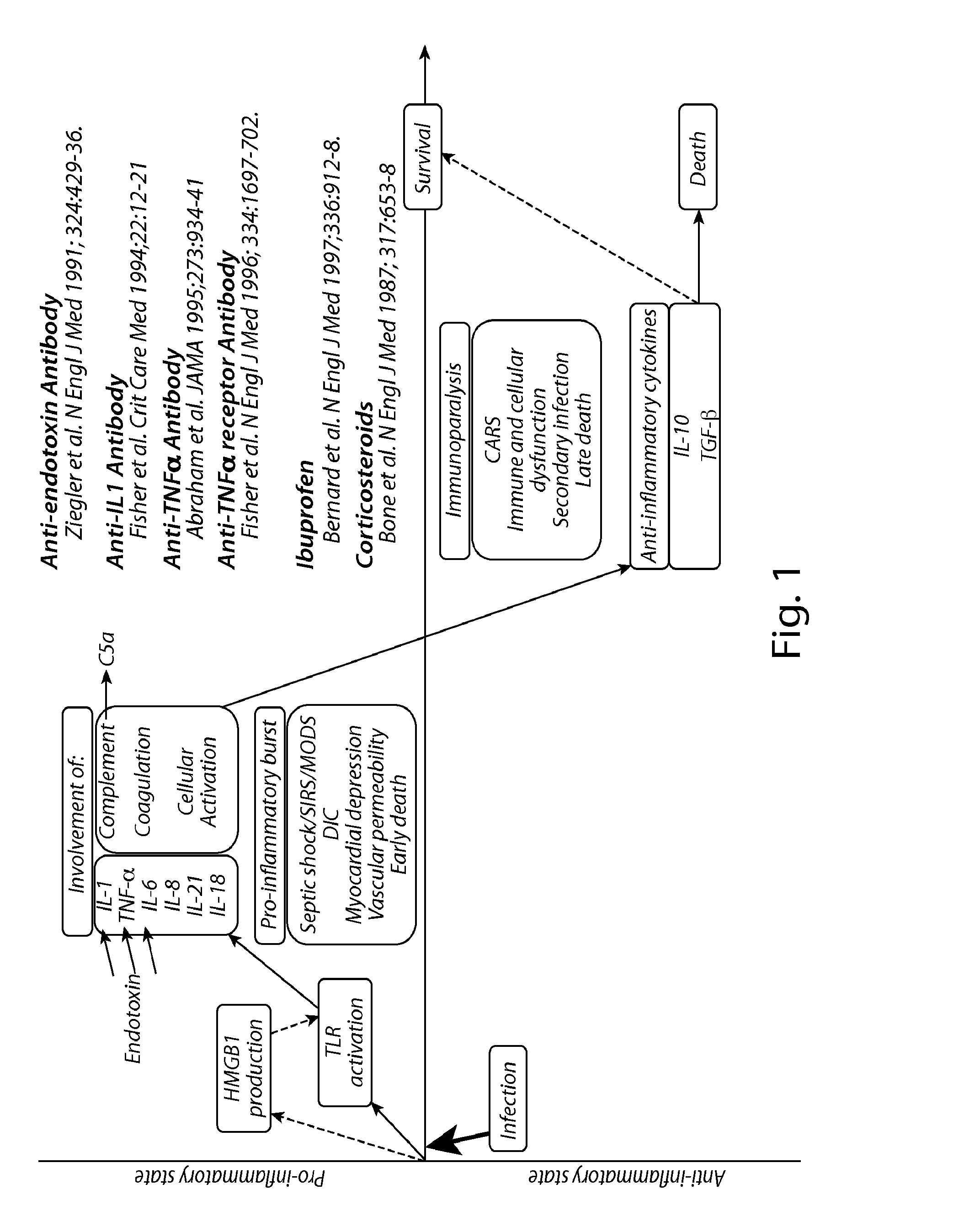
Treatment of inflammatory, non-infectious, autoimmune, vasculitic, degenerative vascular, host-v-graft diseases, Alzheimers disease, and amyloidosis using mammalian, dsDNA vaccination
The present invention relates generally to compositions and methods using mammalian, dsDNA (Double Stranded Deoxyribonucleic Acid) vaccination for the induction and maintenance of regulator suppressor T cells resulting in suppression of non infectious, and post infectious, inflammatory, allergic, auto-immune, vasculitic, certain degenerative vascular, and graft versus host diseases, with or without the use of IL-10, and with or without the use or TGFβ, with or without the use of anti-IL 6 receptor antibody, anti TNF antibody and or Plasmapheresis, IVIG, Corticosteroids, Methotrexate, Bromocriptine, and or vitamin D analogues.
Owner:LAWLESS OLIVER J
Substrate having an electron donating surface with metal particles comprising palladium on said surface
There is disclosed a substrate with an electron donating surface, characterized in having metal particles on said surface, said metal particles comprising palladium and at least one metal selected from the group consisting of gold, ruthenium, rhodium, osmium, iridium, and platinum, wherein the amount of said metal particles is from about 0.001 to about 8 μg / cm2. Examples of coated objects include contact lenses, pacemakers, pacemaker electrodes, stents, dental implants, rupture nets, rupture mesh, blood centrifuge equipment, surgical instruments, gloves, blood bags, artificial heart valves, central venous catheters, peripheral venous catheters, vascular ports, haemodialysis equipment, peritoneal dialysis equipment, plasmapheresis devices, inhalation drug delivery devices, vascular grafts, arterial grafts, cardiac assist devices, wound dressings, intermittent catheters, ECG electrodes, peripheral stents, bone replacing implants, orthopaedic implants, orthopaedic devices, tissue replacing implants, intraocular lenses, sutures, needles, drug delivery devices, endotracheal tubes, shunts, drains, suction devices, hearing aid devices, urethral medical devices, and artificial blood vessels.
Owner:BACTIGUARD AB
Specialized hollow fiber membranes for in-vivo plasmapheresis and ultrafiltration
An in-vivo plasmapheresis and / or in-vivo ultrafiltration membrane comprises elongated hollow fibers each fiber having an interior lumen extending along the fiber length, the fiber wall having a plurality of zones between the inner and outer wall surfaces, each of the zones having a mass density different than the mass density of an adjacent zone. The fiber wall is characterized by having a lower mass density zone at the inner wall surface and a higher mass density zone at the outer wall surface. The fiber is further characterized by having an average elongation breaking force strength of at least about 0.2 lbs. and an average elongation of at least about 45%.
Owner:TRANSVIVO
Apparatus and Method for Filtering Fluids
InactiveUS20080035568A1Easily areaIncrease packing densityMembranesSolvent extractionBlood componentEngineering
A filter module utilizing a nano-porous ceramic membrane is provided for various applications including, but not limited to, enhanced hemodialysis performance, the removal (or separation) of cryoprotectant from biological materials, the separation of blood components (e.g., plasmapheresis), and controlling the concentration of cells in a biological fluid solution.
Owner:HUANG ZHONGPING +2
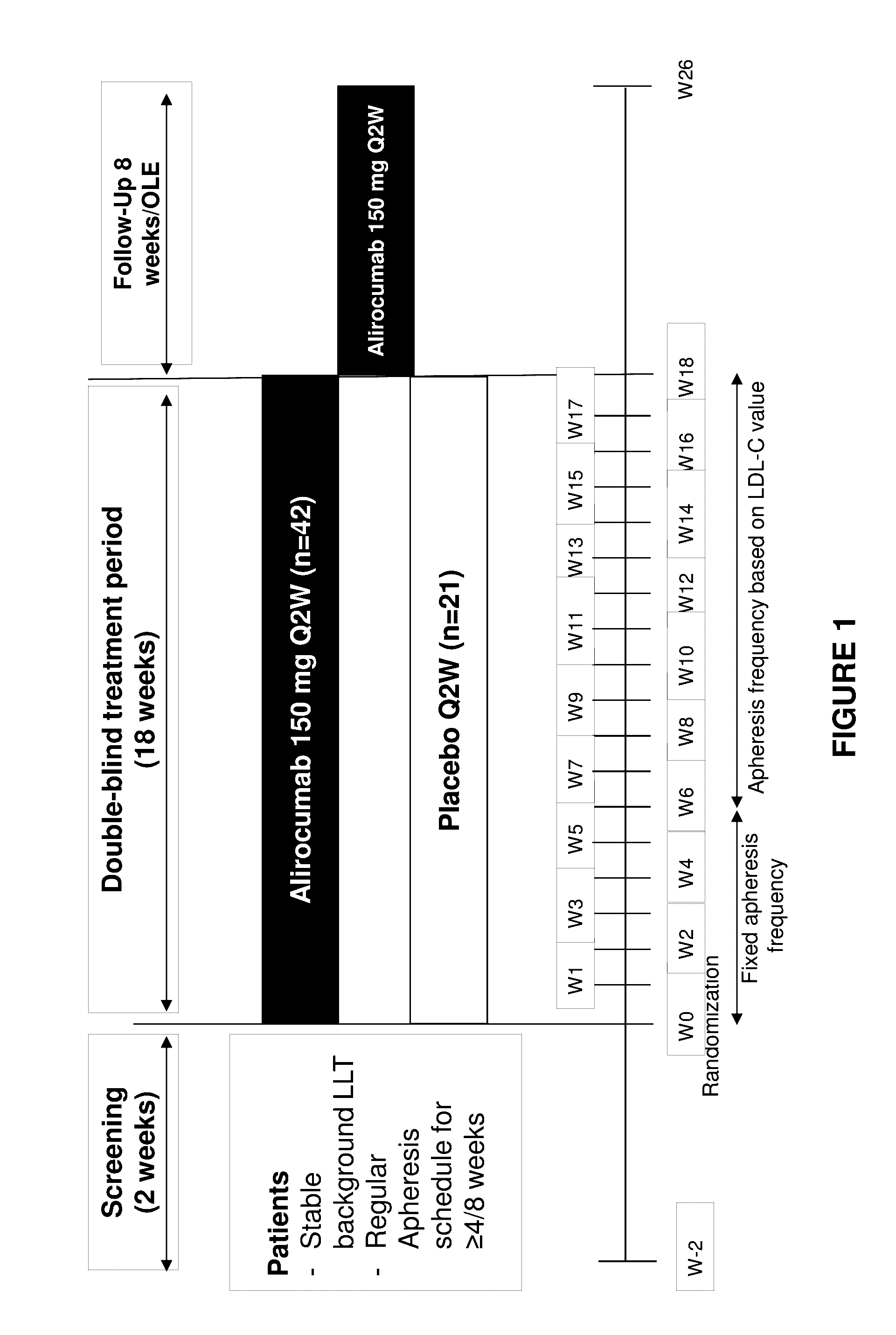
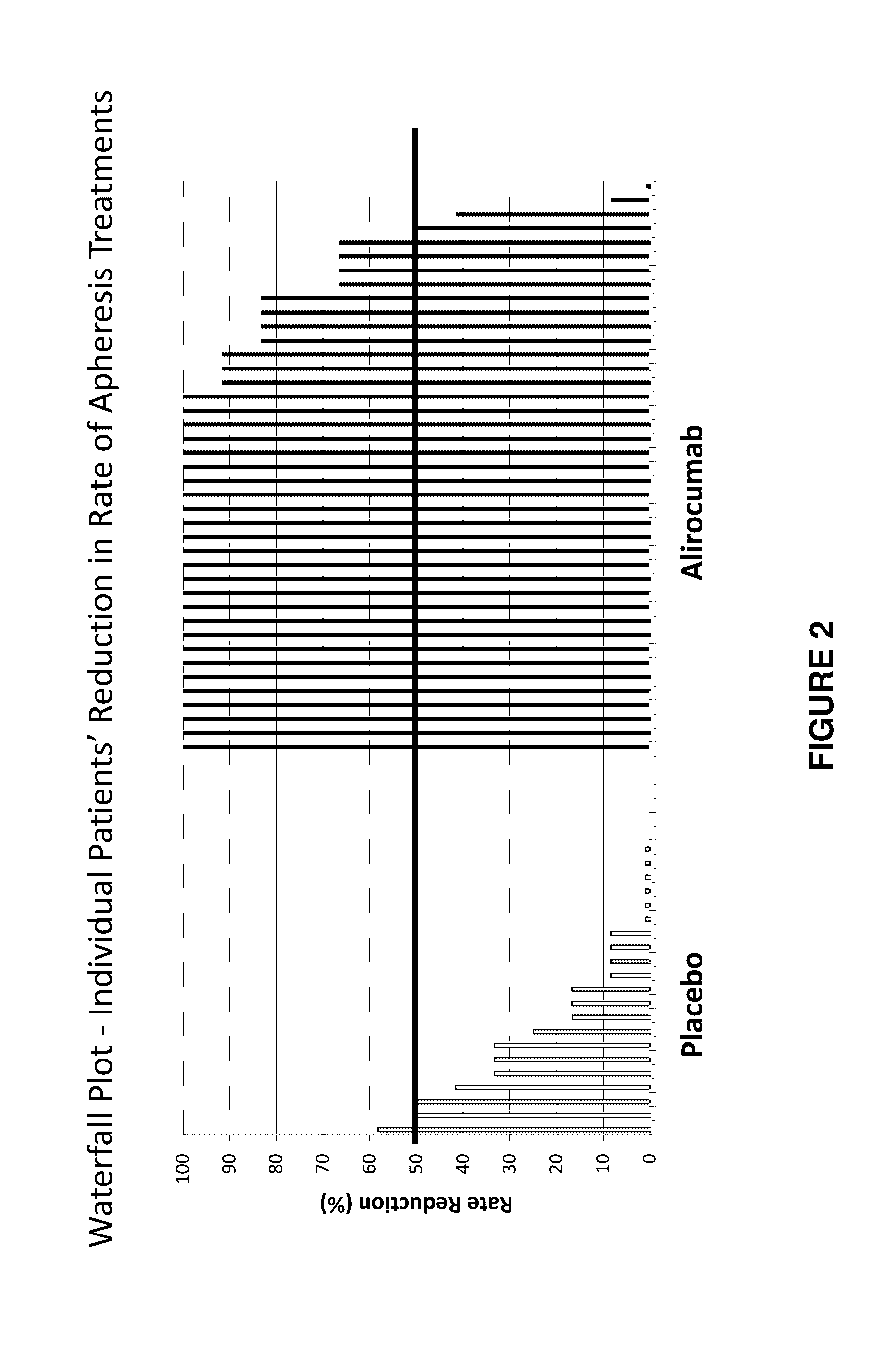
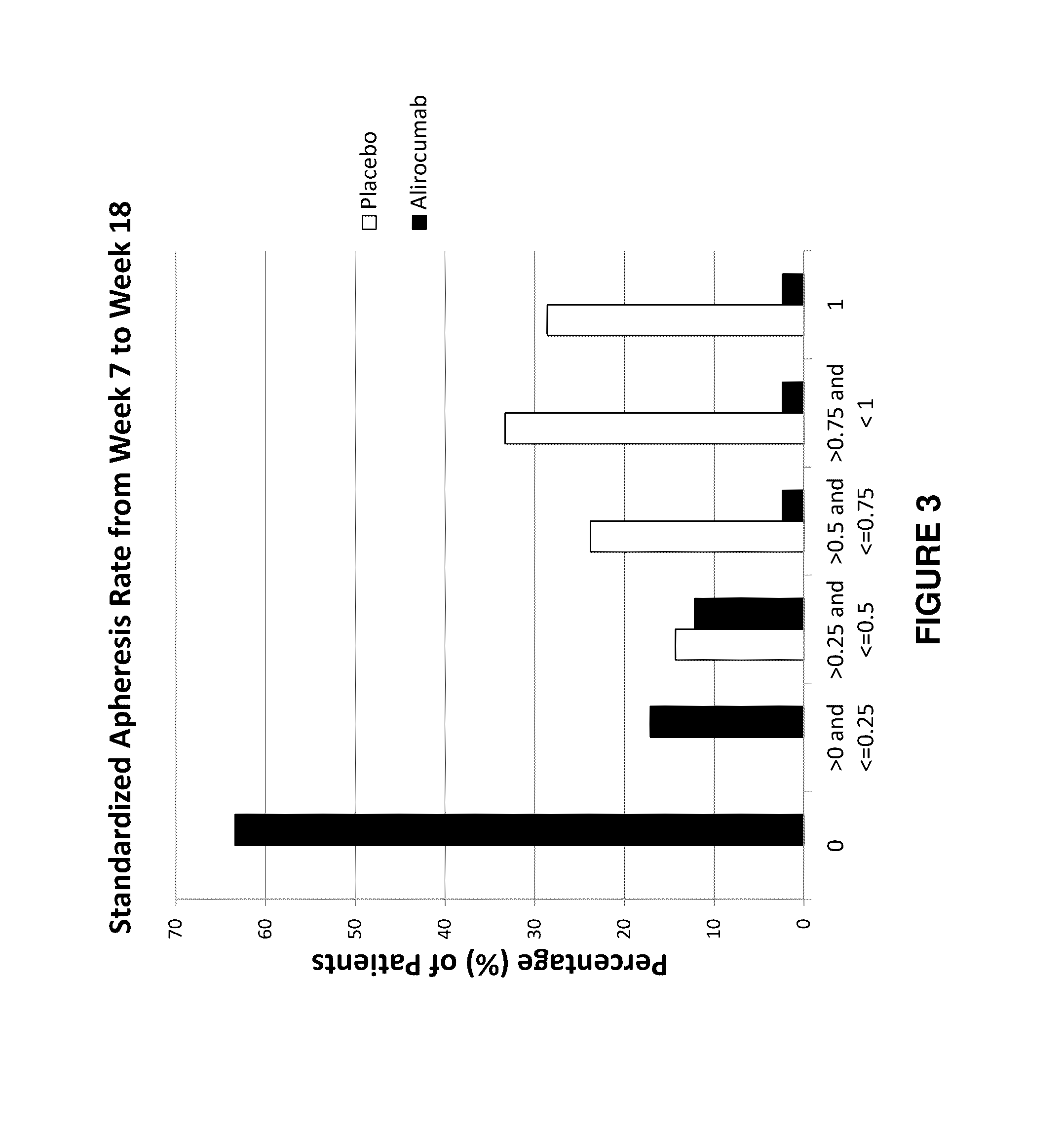
Methods for reducing or eliminating the need for lipoprotein apheresis in patients with hyperlipidemia by administering a pcsk9 inhibitor
ActiveUS20170049886A1Lowering of serum lipoprotein levelReduce and eliminate needOrganic active ingredientsMetabolism disorderSecondary hyperlipidemiaLDL apheresis
The present invention provides methods for reducing or eliminating a patient's need for lipoprotein apheresis therapy. The methods of the present invention comprise administering to a patient a pharmaceutical composition comprising a PCSK9 inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody. The methods of the present invention are useful for treating patients with hyperlipidemia and related conditions who are currently being treated with a therapeutic regimen comprising lipoprotein apheresis (e.g., LDL apheresis or Lp(a) apheresis).
Owner:REGENERON PHARM INC
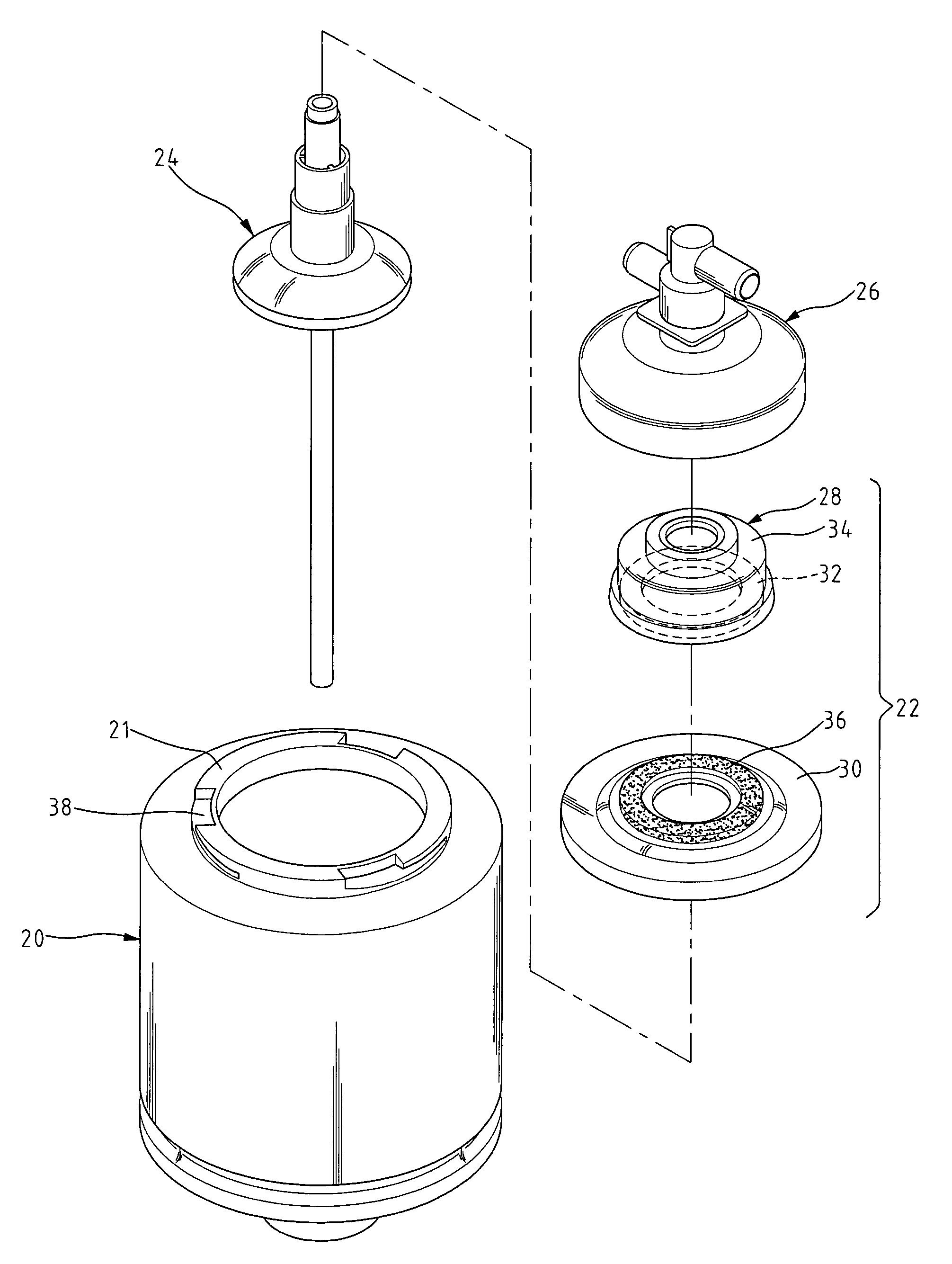
Plasmapheresis centrifuge bowl
InactiveUS20060199720A1Reduce heatReduce the heat generated by the rotating frictionPharmaceutical containersMedical packagingShape dynamicsMechanical engineering
A plasmapheresis centrifuge bowl comprises a ring-shaped dynamic sealing assembly having a ring-shaped static frictional assembly and a ring-shaped rotary frictional upper cover, both of which have a sealing surface. A dynamic seal can be provided between the contacting portions of the sealing surfaces of the ring-shaped static frictional assembly and the ring-shaped rotary frictional upper cover. The ring-shaped static frictional assembly comprises a ring-shaped ceramic piece. A ring-shaped frictional area is provided on the ring-shaped rotary frictional upper cover, and the heat transfer rate of the ring-shaped frictional area is less than the heat transfer rate of the ring-shaped ceramic piece. When the plasmapheresis centrifuge bowl of the present invention is operated to treat the blood, the heat generated by the friction can be conducted and dissipated into the air via the ring-shaped ceramic piece. As a result, the rise in the temperature of the plasmapheresis due to the frictional heat can be effectively avoided.
Owner:JUAN TIEN CHU
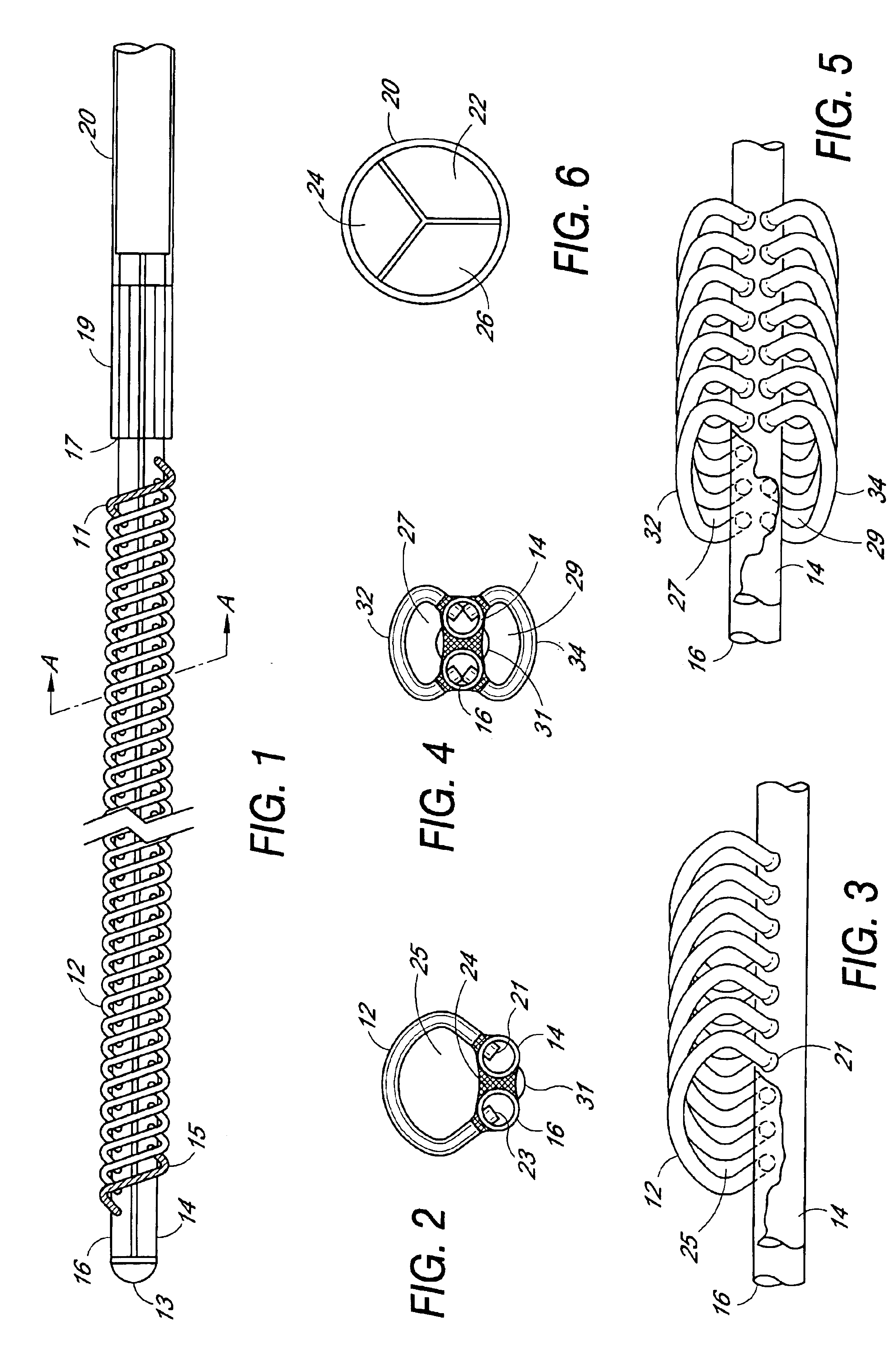
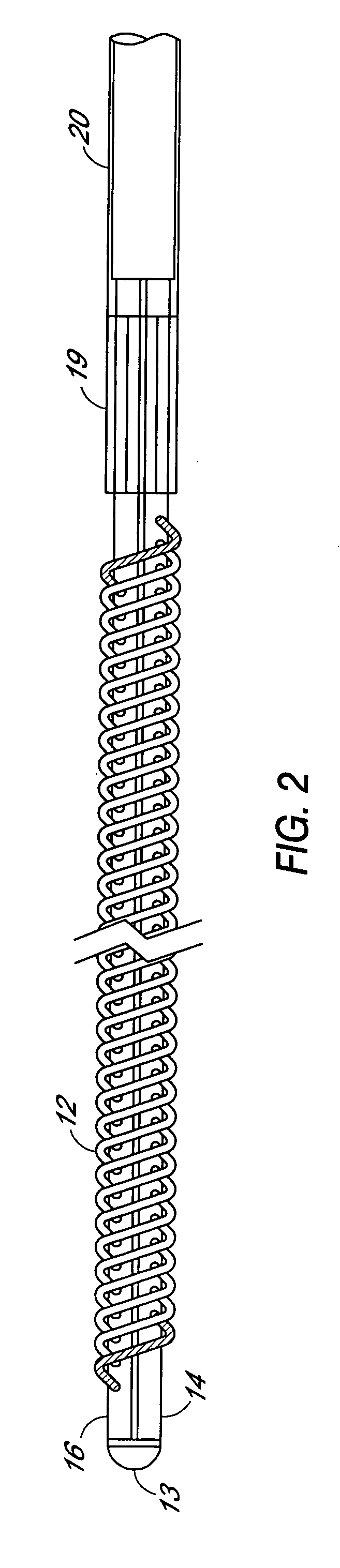
Plasmapheresis filter device and catheter assembly
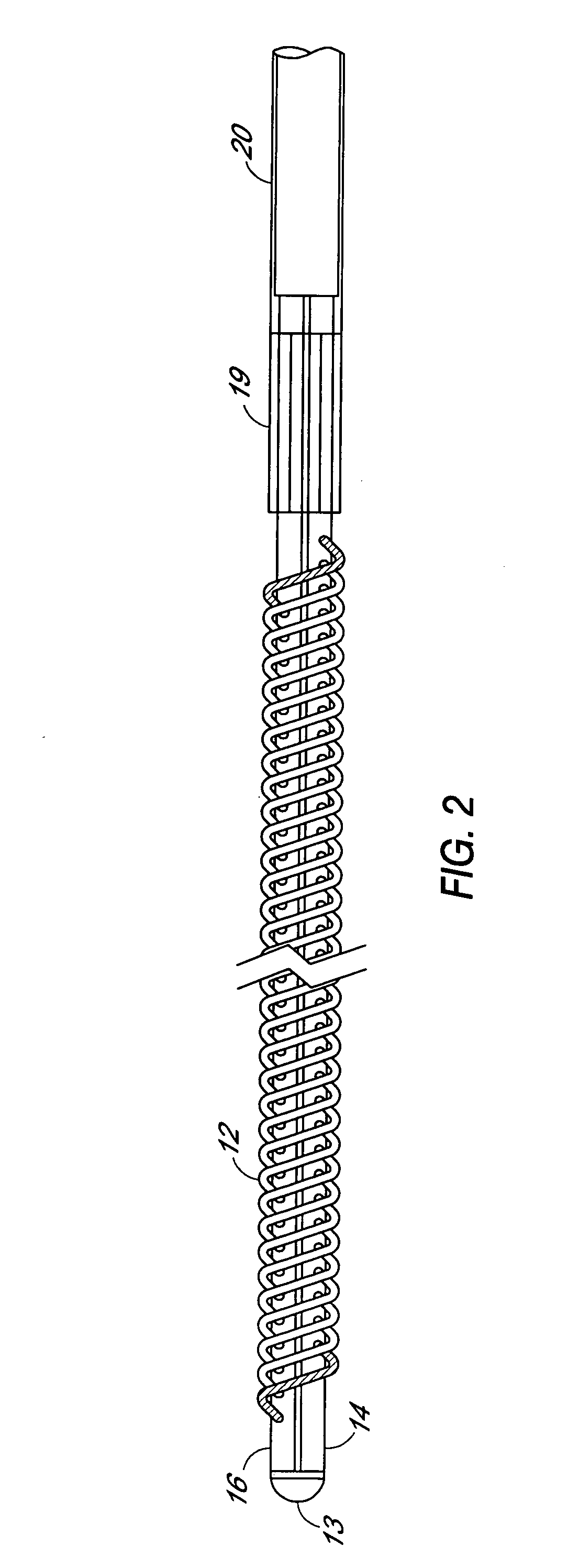
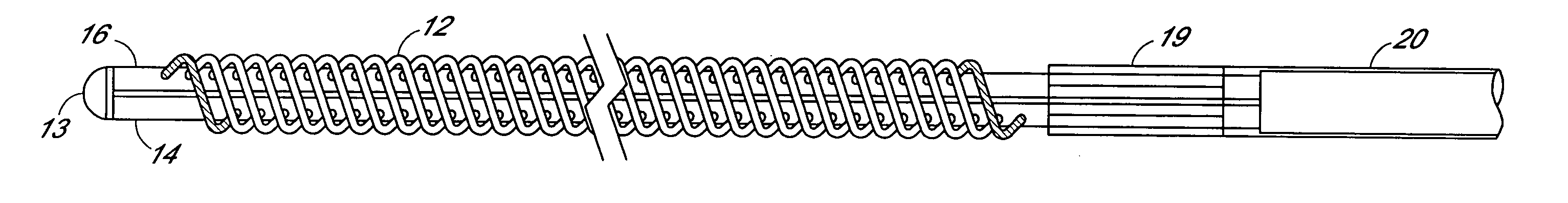
A filter device for being implanted in a blood vessel for carrying out in-vivo plasma separation comprises one or more elongated hollow tubes and a plurality of elongated hollow microporous fibers, each fiber having a first and second end secured to one or more of the elongated hollow tubes with the interior lumen of each of the fibers communicating with the interior of the one or more of the hollow tubes, and wherein the fiber wall has a higher mass density zone adjacent to the outer wall surface and a lower mass density zone adjacent to the inner wall surface.
Owner:TRANSVIVO
Anticoagulant and thrombo-resistant hollow fiber membranes for in-vivo plasmapheresis and ultrafiltration
An in-vivo plasmapheresis or in-vivo ultrafiltration membrane comprises a plurality of elongated hollow microporous fibers each fiber having an outer wall, an inner wall and an interior lumen extending along the length thereof and defined by an inner wall surface, and wherein the fiber wall structure is a substantially continuous change in mass density from the outer wall to the inner wall and comprises a continuum of voids bounded by solid frames, the fiber wall having an asymmetrical pore size and asymmetrical mass density between said inner wall surface and the outer wall surface with a higher mass density adjacent to the outer wall and a lower mass density adjacent to said inner wall, and characterized by a nitric oxide donor composition capable of producing or releasing nitric oxide or inducing nitric oxide production or release in-vivo with a blood vessel, distributed on or in the fiber wall.
Owner:TRANSVIVO
Preparation method of quality control material of glycosylated hemoglobin and quality control material thereof
ActiveCN106771261ASimple methodHigh measurement accuracyBiological testingReference solutionsDiabetes mellitusCentrifugation
The invention provides a preparation method of a quality control material of glycosylated hemoglobin, comprising the following steps: collecting a blood sample of a nondiabetic patient, performing centrifugation and plasmapheresis to obtain red blood cells; adding red blood cell preserving fluid to the red blood cells to obtain a red blood cell sample; adding the red blood cell sample slowly into separating fluid and centrifuging to obtain separated red blood cells, wherein red blood cells adaptive to the cell separation fluid are dispersed in the cell separation fluid of each density; separately charging the separated red blood cells into different test tubes, adding physiological saline to each test tube to obtain a red blood cell suspension, regulating the hemoglobin concentration of the red blood cell suspensions to 120g / L, and screening out red blood cell suspensions with the HbA1c value of 3-5% to obtain the quality control material of glycosylated hemoglobin. The invention also provides the quality control material. The preparation method provided by the invention can perform mass production of low-value quality control materials of glycosylated hemoglobin.
Owner:深圳市雷诺华科技实业有限公司
Anticoagulant and thrombo-resistant hollow fiber membranes for in-vivo plasmapheresis and ultrafiltration
InactiveUS20060124530A1Reduces thrombosis formationReduces pore foulingSemi-permeable membranesMembranesFiberHollow fibre membrane
An in-vivo plasmapheresis or in-vivo ultrafiltration membrane comprises a plurality of elongated hollow microporous fibers each fiber having an outer wall, an inner wall and an interior lumen extending along the length thereof and defined by an inner wall surface, and wherein the fiber wall structure is a substantially continuous change in mass density from the outer wall to the inner wall and comprises a continuum of voids bounded by solid frames, the fiber wall having an asymmetrical pore size and asymmetrical mass density between said inner wall surface and the outer wall surface with a higher mass density adjacent to the outer wall and a lower mass density adjacent to said inner wall, and characterized by a nitric oxide donor composition capable of producing or releasing nitric oxide or inducing nitric oxide production or release in-vivo with a blood vessel, distributed on or in the fiber wall.
Owner:TRANSVIVO
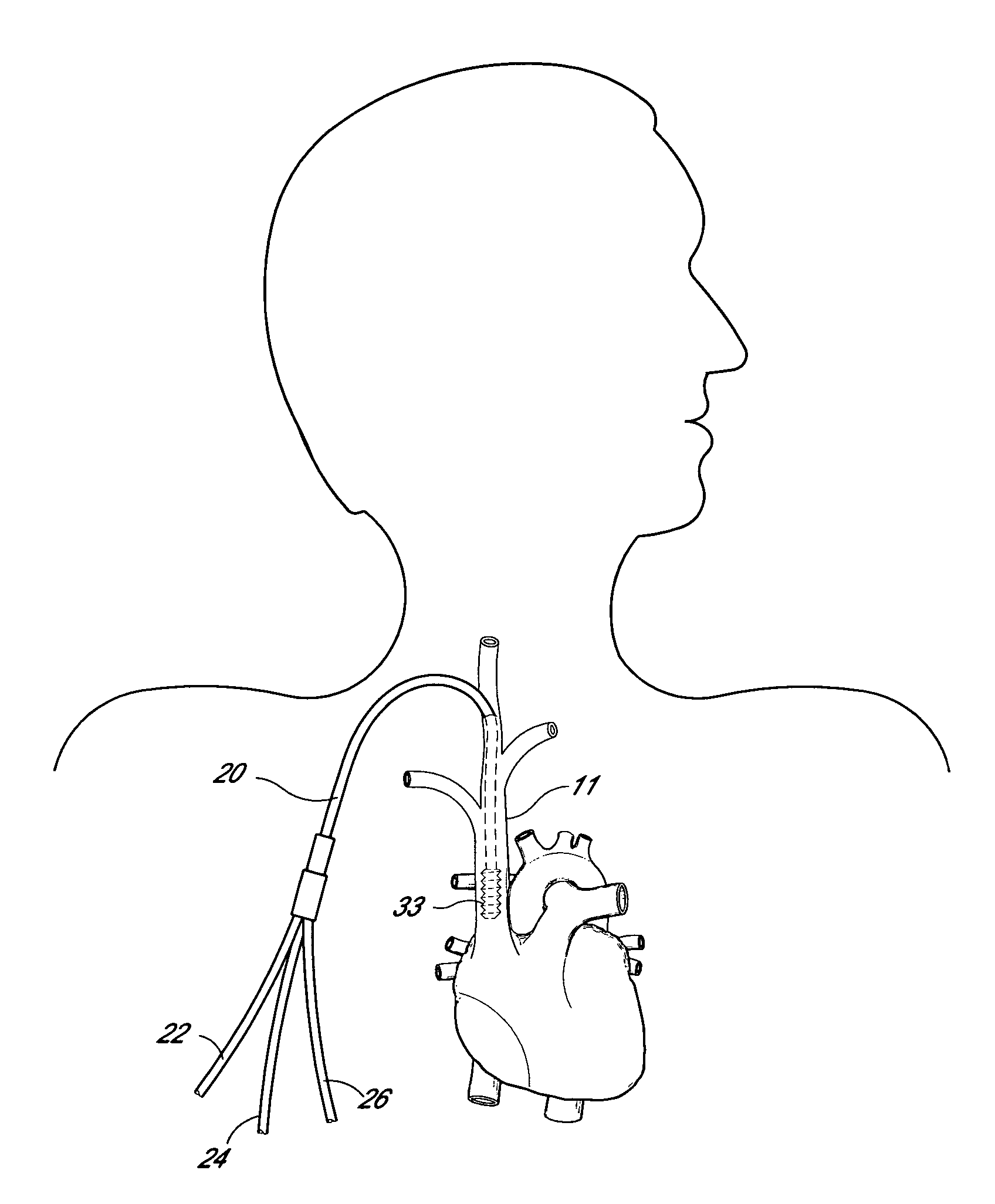
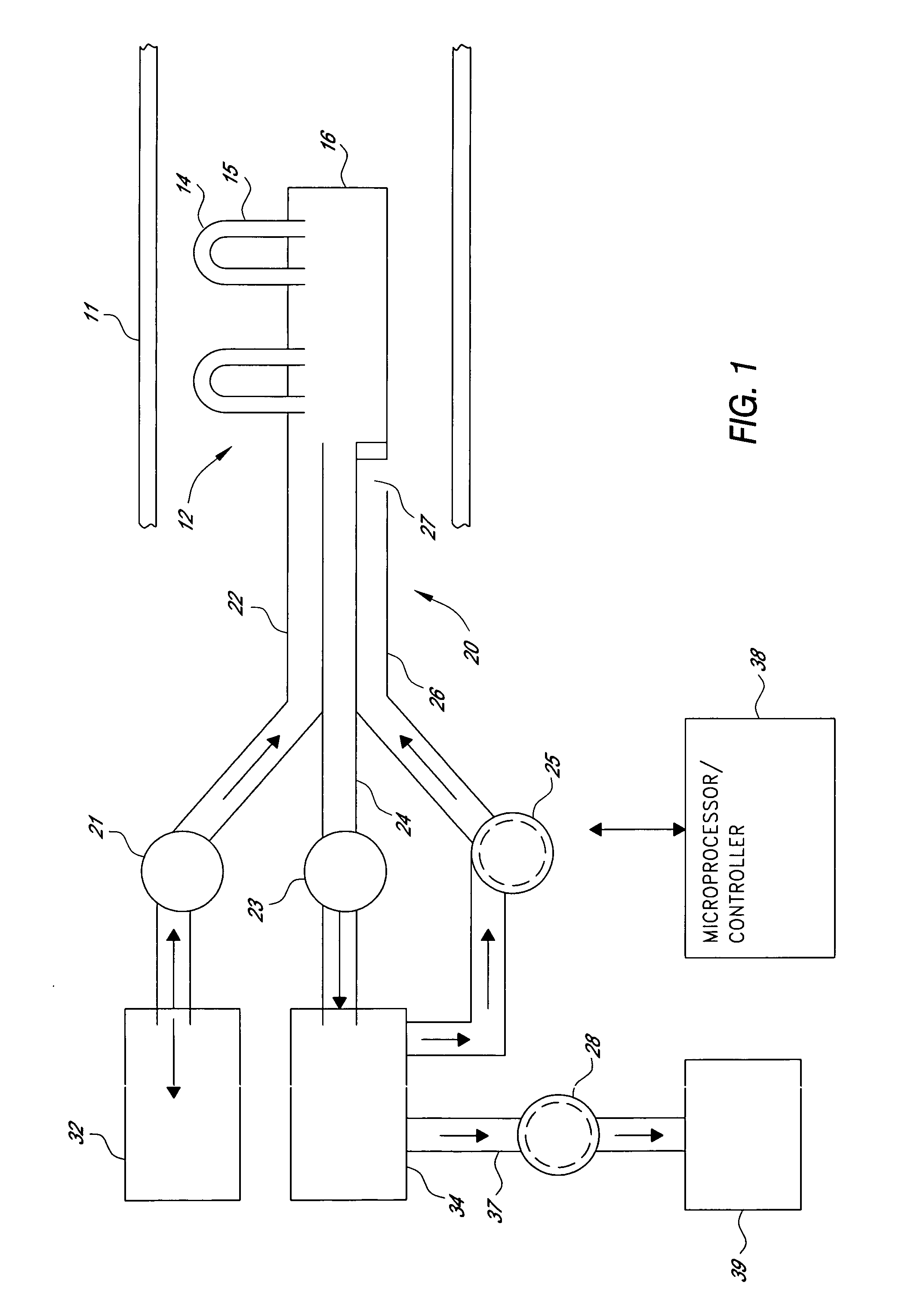
Apparatus and method for in-vivo plasmapheresis using periodic backflush containing anticoagulant
Method for in-vivo plasmapheresis utilizing a plurality of elongated hollow microporous filter fibers periodically interrupt diffusion of blood plasma from a patient, and, for a selected time, directing backflush fluid into the fibers at a pressure and interval sufficient to cleanse the fiber pores, after which plasma diffusion is resumed. The backflush fluid, preferably a normal saline solution, may contain an anticoagulant such as heparin, citrate or NO donor in suitable concentration for systemic anti-coagulation or for treating the fiber for thromboresistance.
Owner:TRANSVIVO
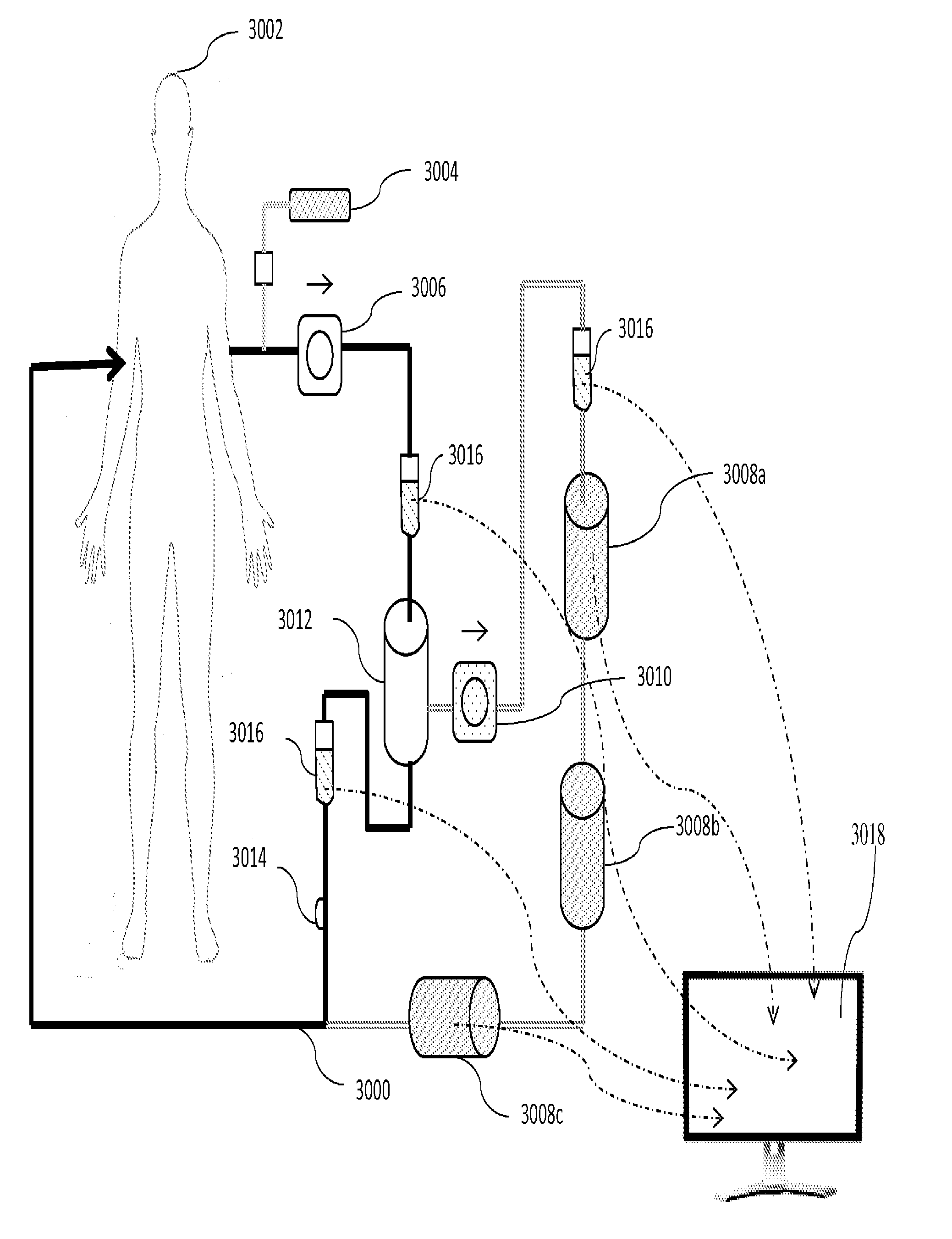
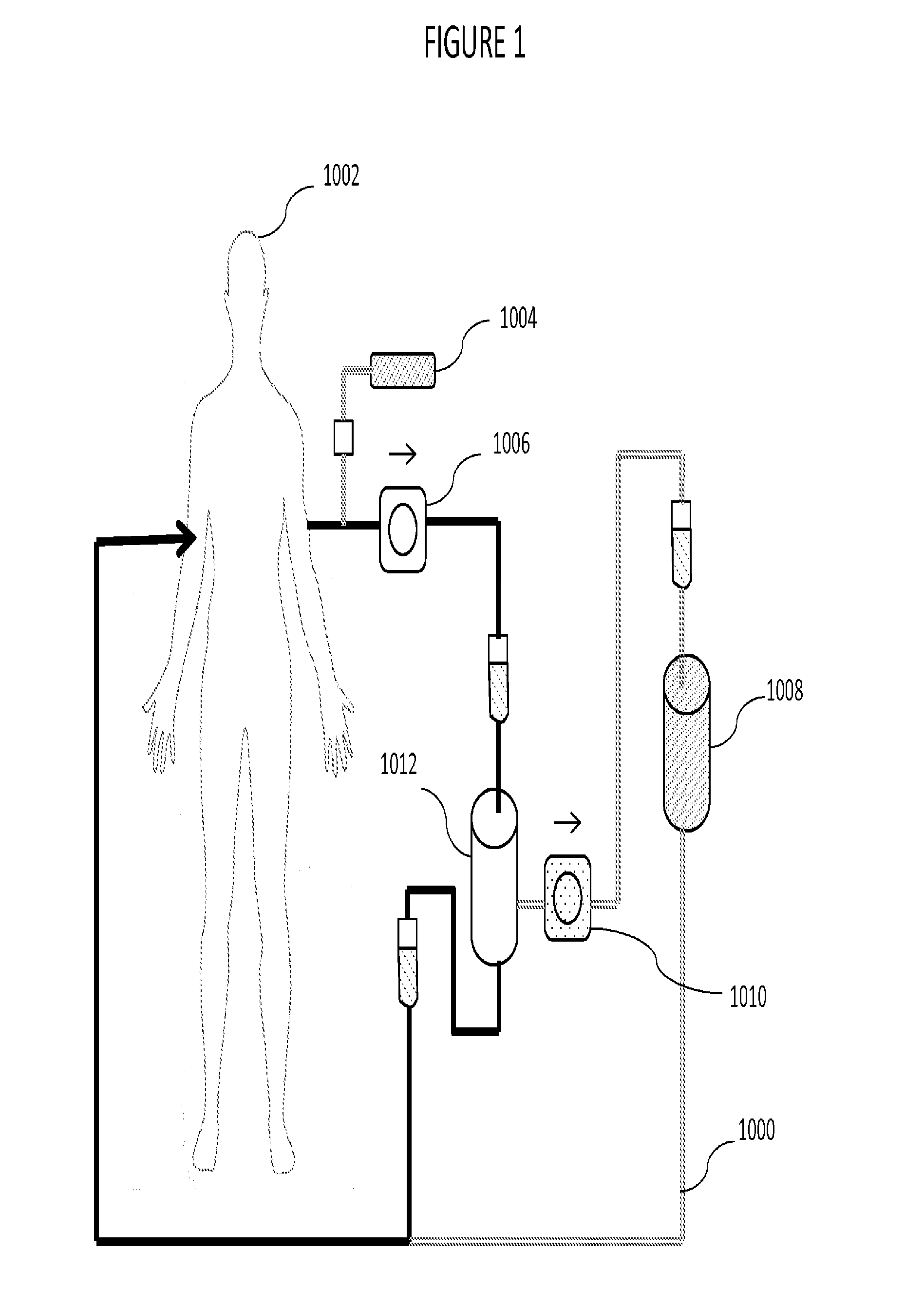
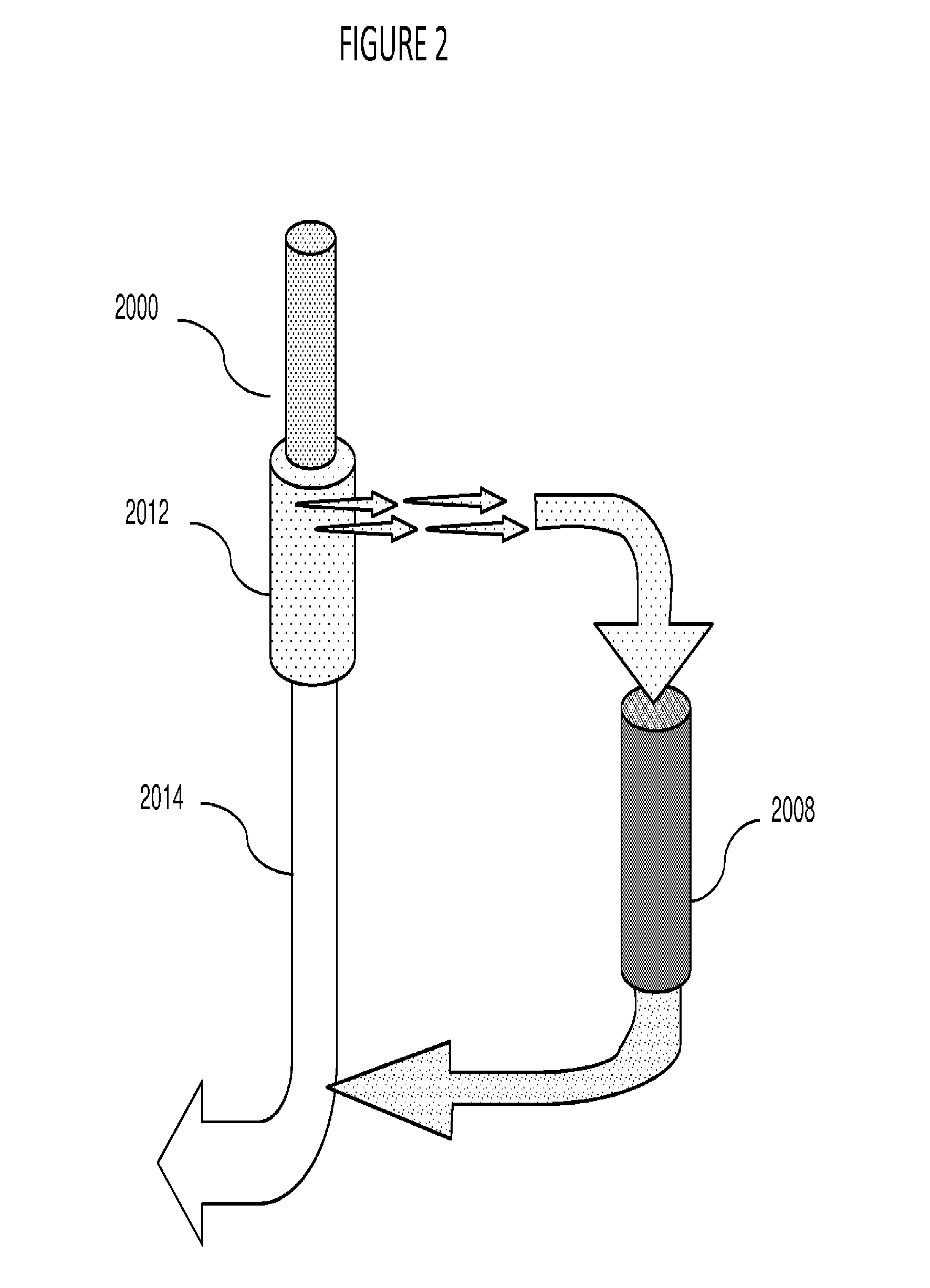
Plasmapheresis device
ActiveUS20160317734A1Reduce riskConvenient treatmentAntibacterial agentsAntimycoticsCellular componentBlood plasma
A plasmapheresis device includes a column or other flow mechanism in which plasma flows following separation of the plasma from cellular components like blood cells, platelets and the like. The column includes a moiety, such as an antibody, which selectively binds to galectin-3. By removing galectin-3 from the blood stream of a mammal by at least 10%, improvements in the treatment of inflammation, suppression of the formation of fibroses, and a variety of cancer treatments can be effected or improved. The device provides for multiple columns to remove a variety of elements but includes one which selectively removes galectin-3 from the blood flow. Other agents may be added to the plasma before recombination with the cellular components of the blood, and before returning the recombined flow to the patient.
Owner:ELIAZ THERAPEUTICS INC
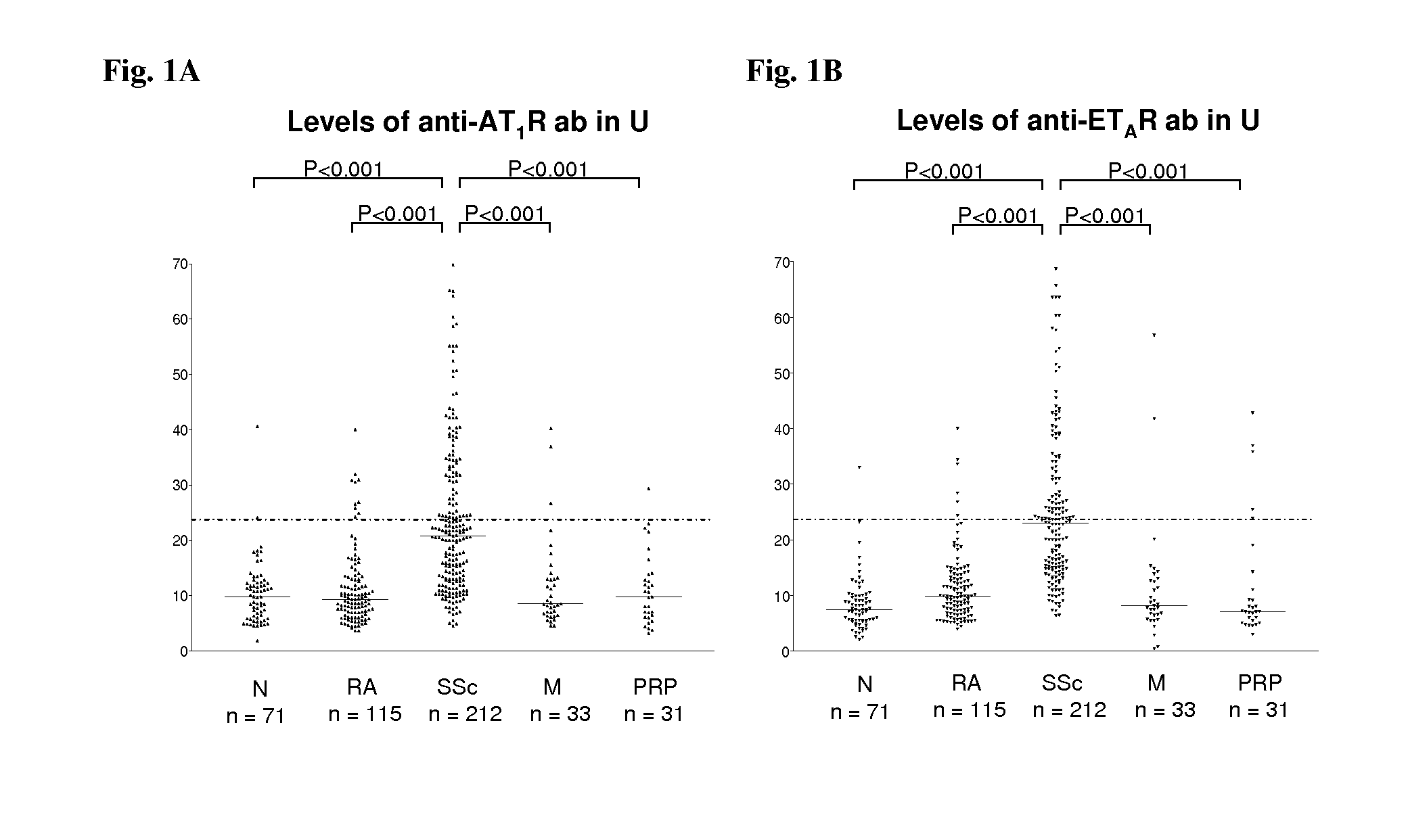
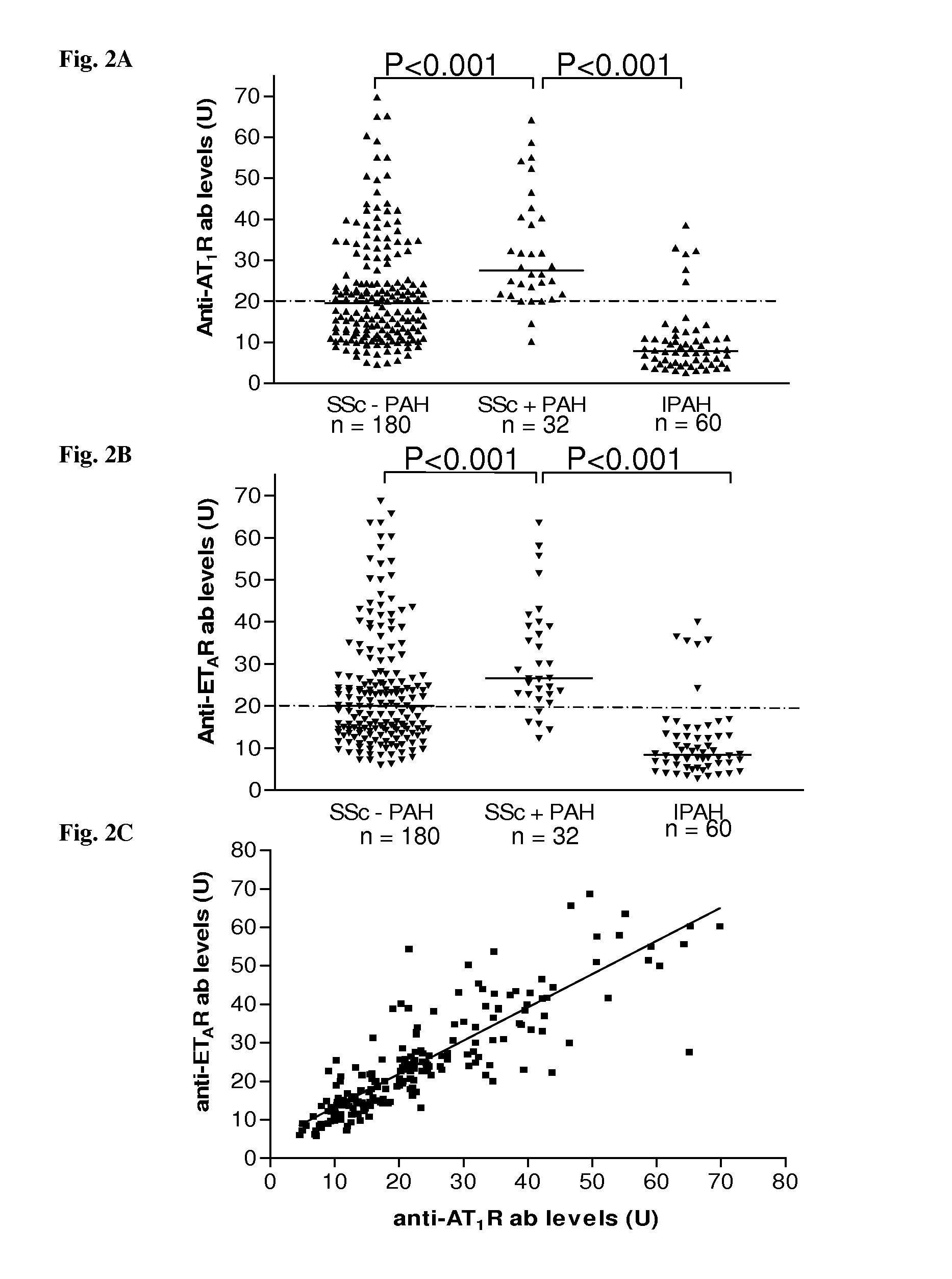
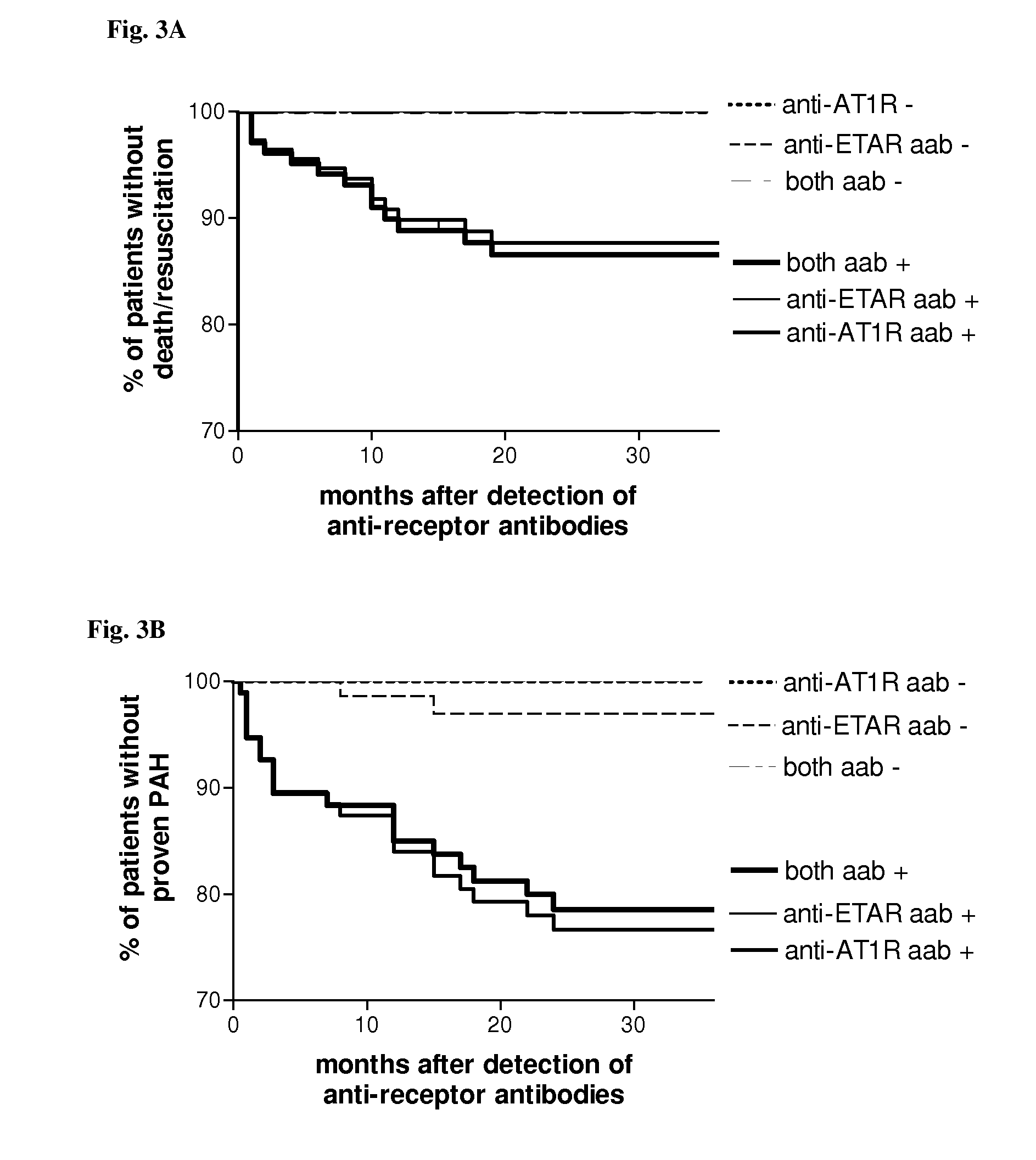
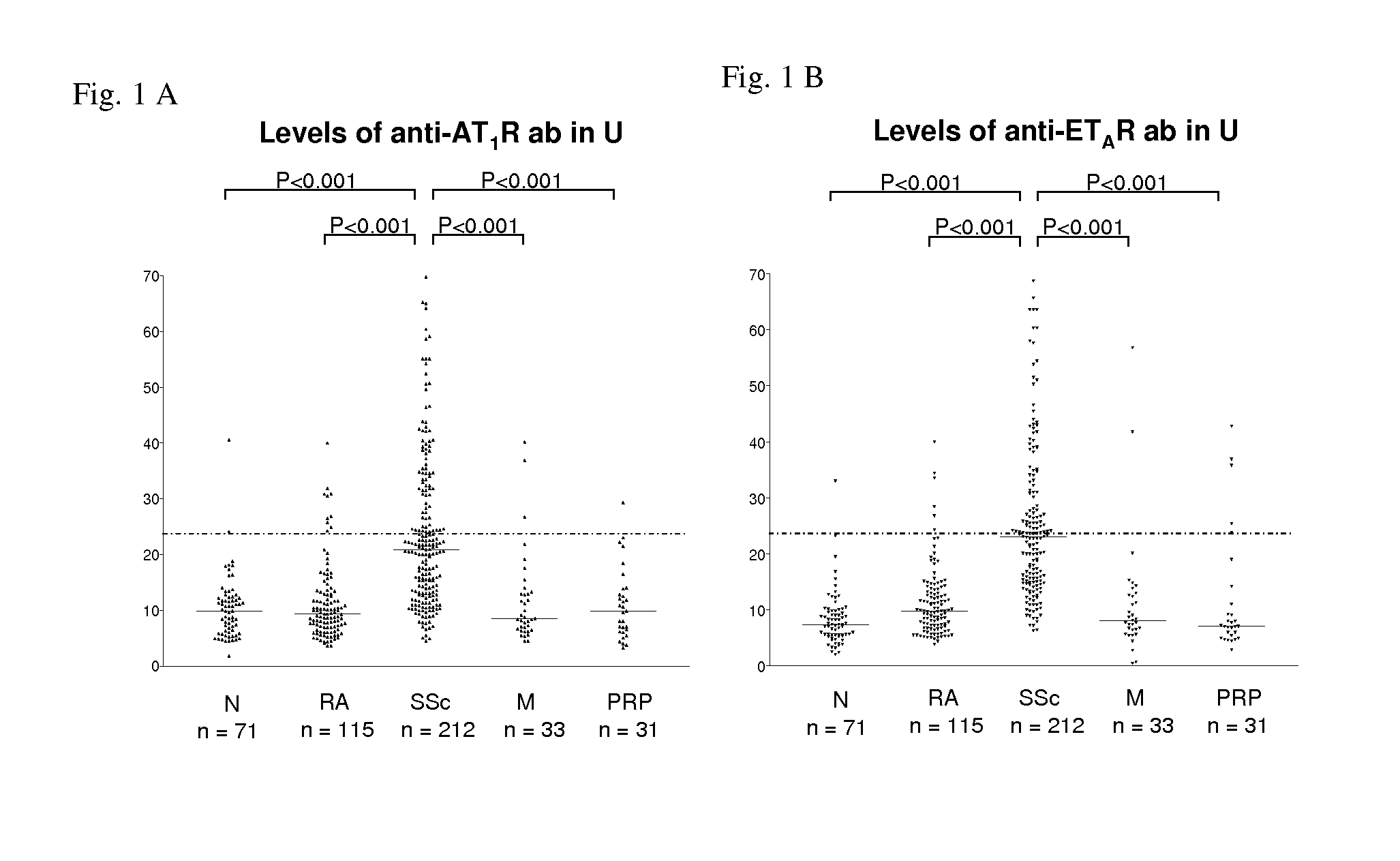
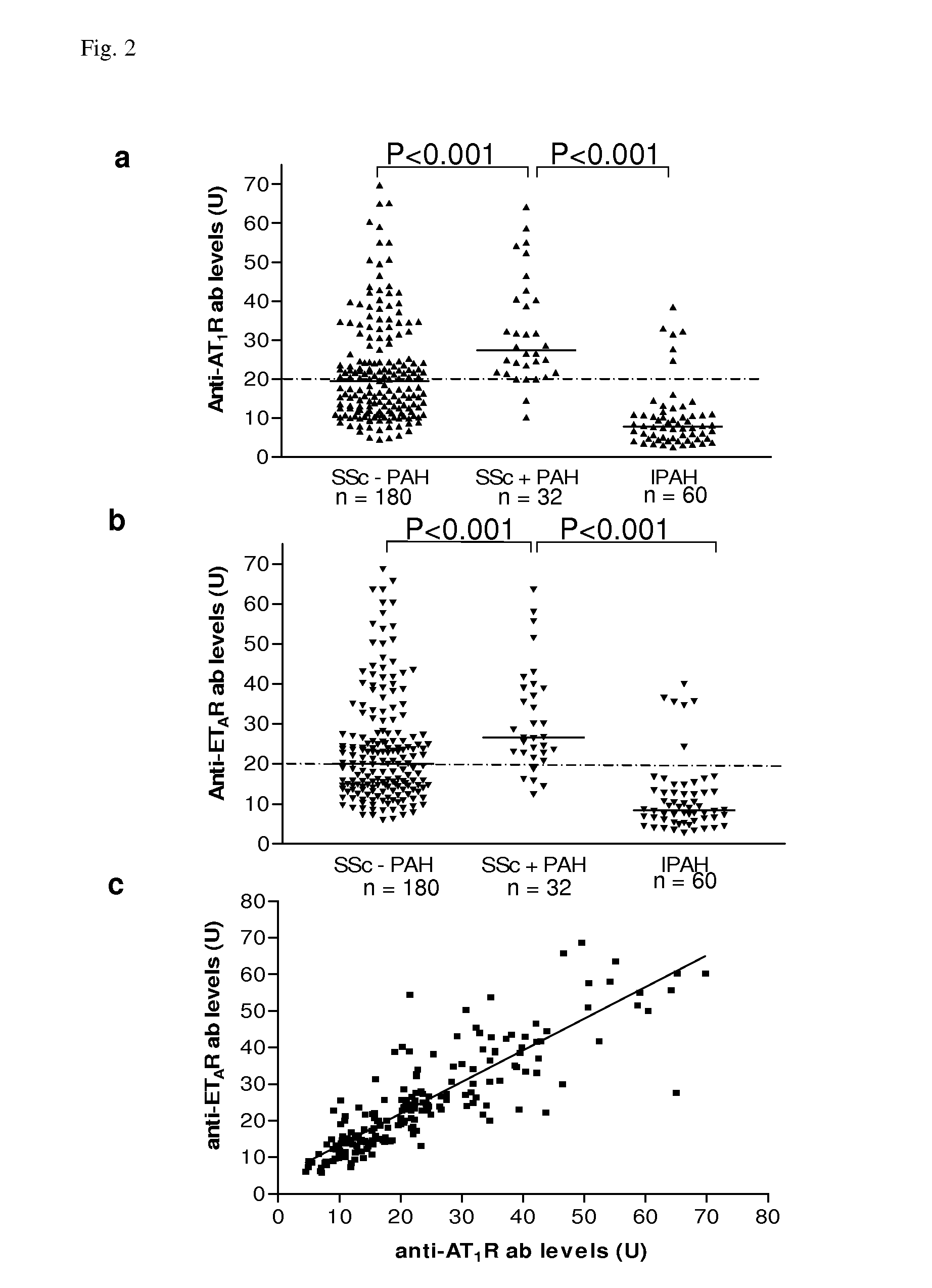
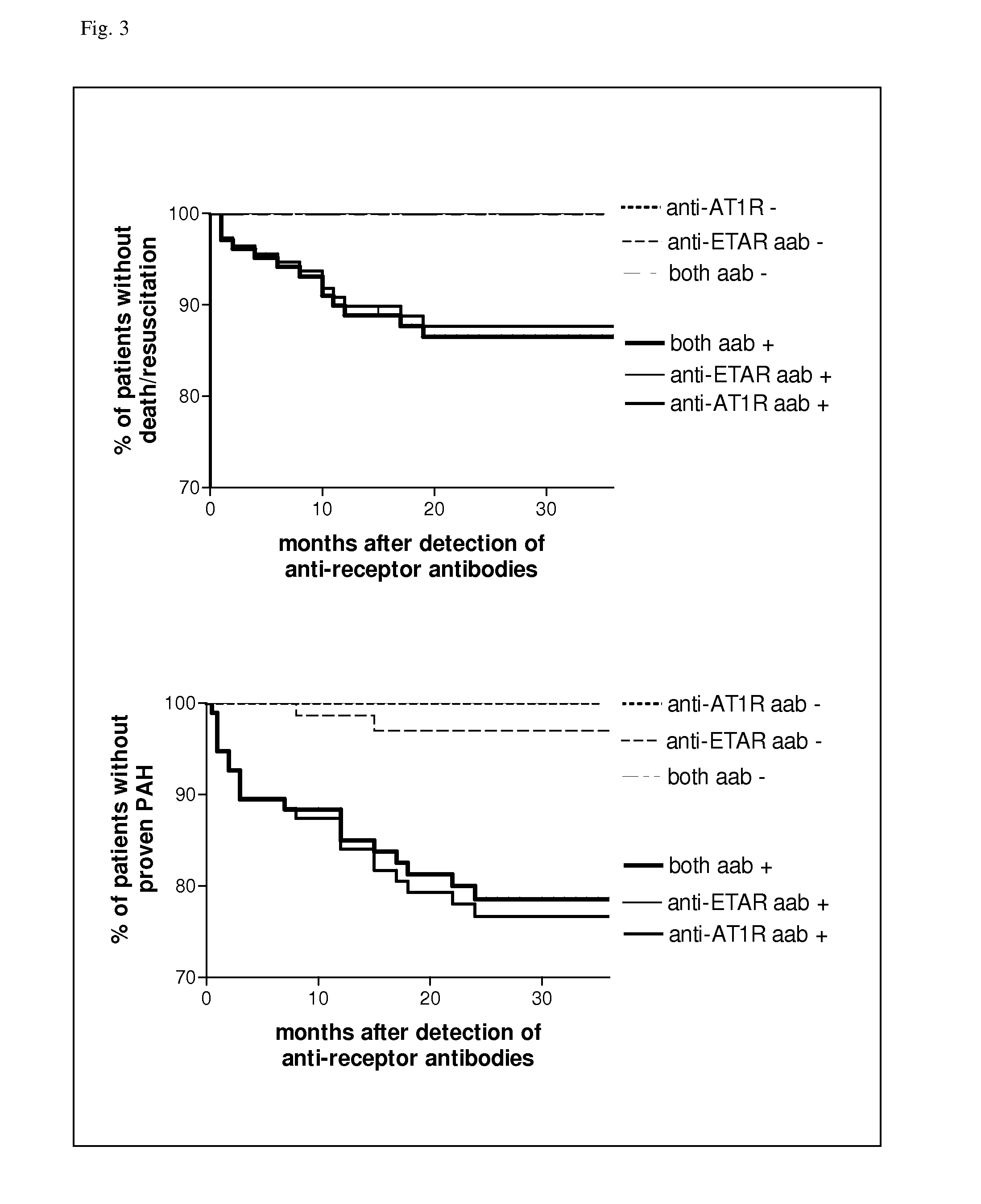
Method for diagnosis of a disease involving an Anti-at1-receptor antibody
The present invention relates to a method for diagnosis of a disease selected from the group of diabetes, vasculitis, collagenosis, an inflammatory rheumatic disease and arteriosclerosis wherein, presence or absence of an anti-AT1-receptor antibody is determined in a sample from a patient to be diagnosed and wherein, the presence of an anti-AT1-receptor antibody is indicative of the disease. The invention further relates to the use of an inhibitor of an anti-AT1-receptor antibody or an inhibitor of an AT1-receptor for the production of a medicament as well as plasmapheresis of blood for the removal of anti-AT1-receptor antibodies.
Owner:CELLTREND
Treatment of inflammatory, autoimmune, or other disorders, using agents that reduce the sequestering of zinc by calprotectin
InactiveUS20070275095A1Reduce activity levelReduce concentrationBiocideNervous disorderDiseaseWhole body
Treatments are disclosed for inflammatory, autoimmune, or other disorders characterized by excessive activity of calprotectin, a protein that normally defends against microbial infections by sequestering available zinc, at a site of infection. Excessive calprotectin activity, which can cause zinc deficiencies in localized tissues, can create or aggravate various disorders. However, ingestion of systemic (oral) zinc supplements tends to activate offsetting mechanisms, and such supplements therefore usually are ineffective. Accordingly, targeted treatments are disclosed herein for suppressing and controlling excessive calprotectin activity, in local tissues. Such methods include targeted injections of zinc solutions, and plasmapheresis treatment. Screening tests also are described for identifying non-protein drugs that can either (i) bind specifically to the zinc-binding sites of calprotectin, or (ii) suppress the release of calprotectin by neutrophil cells.
Owner:KOSSOR DAVID C
Specialized hollow fiber membranes for in-vivo plasmapheresis and ultrafiltration
InactiveUS20050121384A1Facilitate in-vivo plasmapheresisFacilitate ultrafiltrationSemi-permeable membranesMembranesHollow fibreUltrafiltration
An in-vivo plasmapheresis and / or in-vivo ultrafiltration membrane comprises elongated hollow fibers each fiber having an interior lumen extending along the fiber length, the fiber wall having a plurality of zones between the inner and outer wall surfaces, each of the zones having a mass density different than the mass density of an adjacent zone. The fiber wall is characterized by having a lower mass density zone at the inner wall surface and a higher mass density zone at the outer wall surface. The fiber is further characterized by having an average elongation breaking force strength of at least about 0.2 lbs. and an average elongation of at least about 45%.
Owner:TRANSVIVO
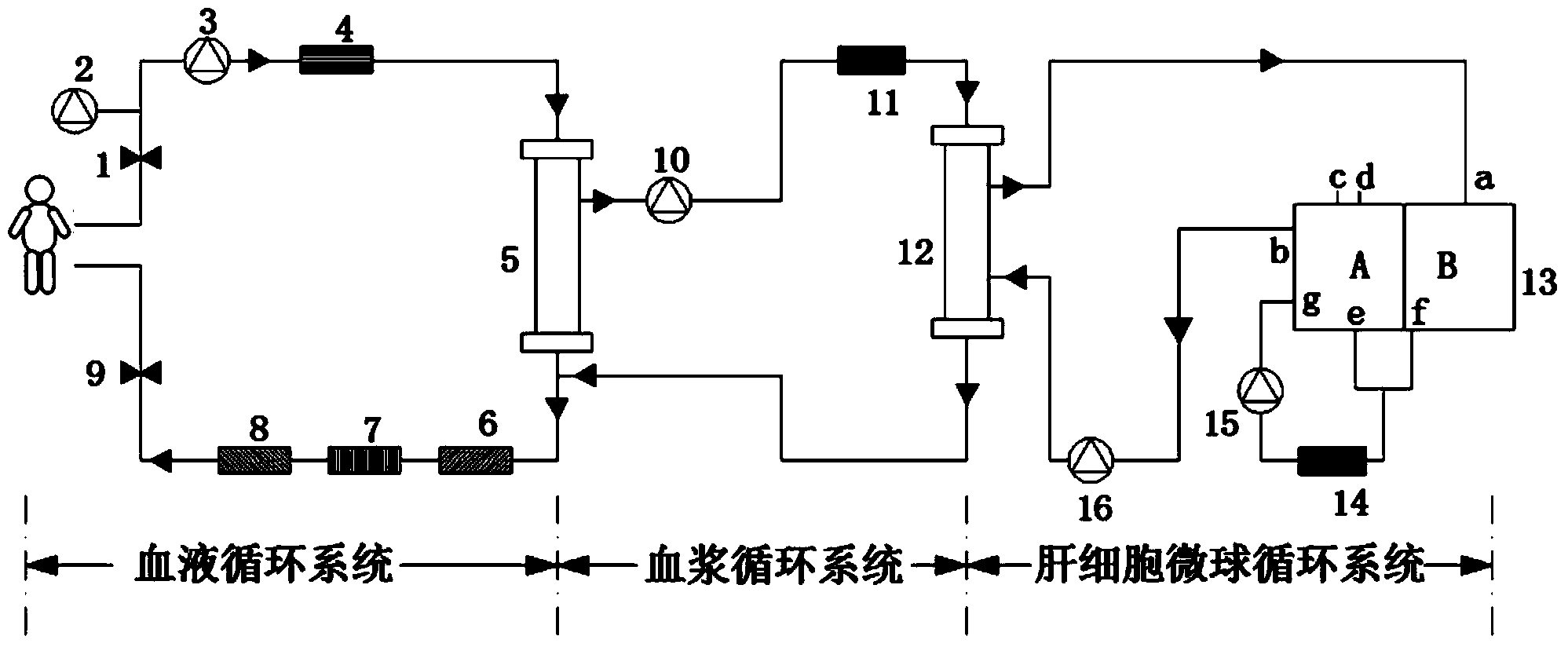
Hepatocyte microsphere bioartificial liver supporting system
ActiveCN104225698AEasy to handleImprove transmission performanceDialysis systemsCulture fluidMicrosphere
The invention relates to a bioartificial liver supporting system which comprises a blood circulation system, a plasmapheresis circulation system and a hepatocyte microsphere circulation system. An integrated blood oxygen, pH, blood pressure and temperature sensor is adopted for not only monitoring various physiological parameters in the system in real time, but also reducing the complication degree of the whole system, and facilitating data management; furthermore, a hepatocyte microsphere culture fluid storage device and a recycling method are adopted, so that not only can nutrient substances and the oxygen transmitting capacity be improved and can durable functions and activity of hepatocytes be maintained, but also full contact between the hepatocyte and plasma is facilitated, the bioartificial liver supporting system is capable of substituting the liver function of a patient with hepatic failure in a long time; furthermore, the hepatocyte microsphere culture fluid storage device is provided with a culture fluid extraction port and a culture fluid adding port, so that under the condition of not influencing normal operation of the system, cell samples can be extracted, the cell activity is detected and a fresh culture fluid containing hepatocyte microspheres is added.
Owner:XI AN JIAOTONG UNIV
Apparatus and method for in-vivo plasmapheresis using periodic backflush containing anticoagulant
Method for in-vivo plasmapheresis utilizing a plurality of elongated hollow microporous filter fibers periodically interrupt diffusion of blood plasma from a patient, and, for a selected time, directing backflush fluid into the fibers at a pressure and interval sufficient to cleanse the fiber pores, after which plasma diffusion is resumed. The backflush fluid, preferably a normal saline solution, may contain an anticoagulant such as heparin, citrate or NO donor in suitable concentration for systemic anti-coagulation or for treating the fiber for thromboresistance.
Owner:TRANSVIVO
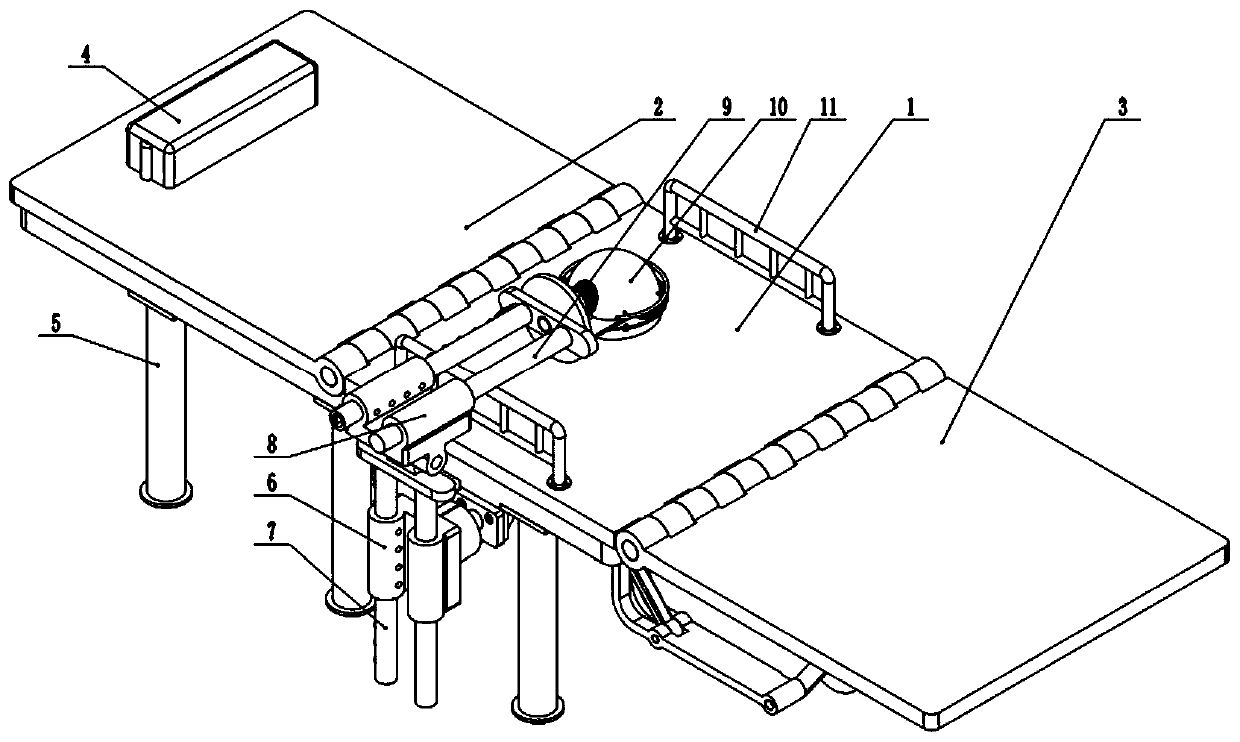
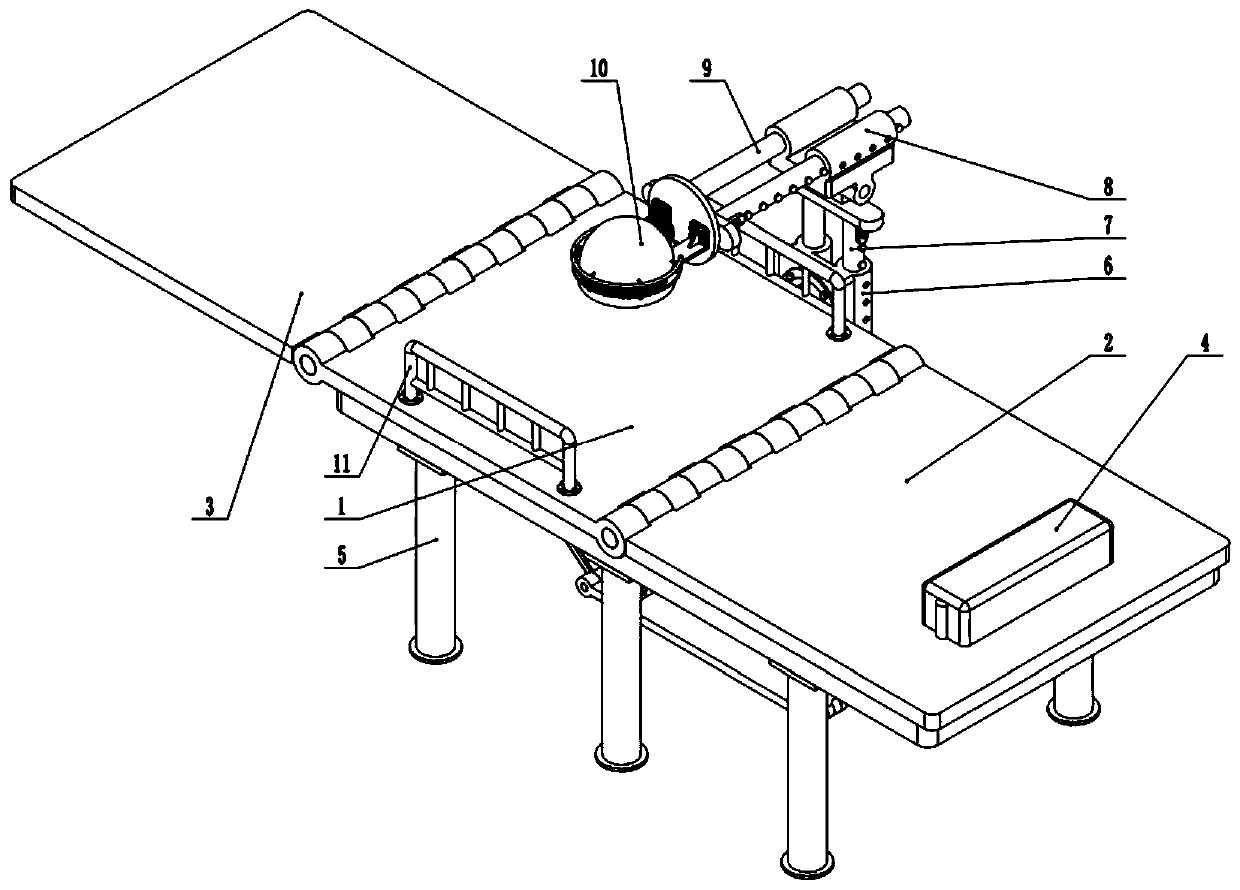
Plasmapheresis assisting device
ActiveCN110236858ADisplacement stabilizationShorten the timeOther blood circulation devicesOperating tablesMassageEngineering
The invention provides a plasmapheresis assisting device. The plasmapheresis assisting device comprises a frame body, wherein the top end of the frame body is fixedly connected with a middle plate, the front end of the middle plate is hinged with a leg plate capable of swinging up and down, the rear end of the middle plate is hinged with a back plate capable of swinging up and down, the rear end of the back plate is provided with a pillow plate capable of moving up and down, the side end of the frame body is provided with a universal seat capable of rotating in multiple angles, the universal seat is slidably connected with a lifting rod capable of moving up and down, the top end of the lifting rod is hinged with an overturning seat capable of swinging left and right, the overturning seat is slidably connected with a transverse rod, the left end of the transverse rod is provided with a massage disc, the bottom end of the massage disc is provided with a massage planet carrier capable of horizontally rotating, the bottom end of the massage planet carrier is provided with a pressing ball capable of reciprocating up and down, the bottom end of the massage planet carrier is provided with a rotatable massage rod, the bottom end of the massage planet carrier is provided with a rotating plate, and the bottom end face of the rotating plate is provided with a plurality of massage wheels. The plasmapheresis assisting device effectively solves the problem that the medical staff quickly and accurately alleviate or eliminate the adverse reaction difficultly when a patient has symptoms such as hypotension, cramps and abdominal pain during existing plasmapheresis.
Owner:NANYANG CITY CENT HOSPITAL
Multi-lumen catheter
The invention provides a catheter for placement within a vessel of a patient. The catheter comprises an elongated catheter body, a septum extending longitudinally through the interior of the catheter body from the dividing the interior of the catheter body into a first lumen and a second lumen. Each lumen has curved or angled internal walls at the distal end of the catheter that terminate at ports located on opposing sides of the catheter body. The invention also provides a method for exchanging fluids in a patient comprising the step of positioning the catheter of the present invention in communication with a fluid-containing vessel of a patient. The method is particularly well-suited for hemodialysis, plasmapheresis, and other therapies which require removal and return of blood from a patient.
Owner:AEGIS MEDICAL TECH
Compositions, Methods for Treatment, and Diagnoses of Autoimmunity-Related Disorders and Methods for Making Such Compositions
InactiveUS20110097344A1Disturb balanceNervous disorderComponent separationDiseaseAutoimmune responses
The present invention provides compositions and methods useful in the diagnosis and treatment of autoimmunity-related disorders, including cancers and other disorders involving angiogenesis, as well as non-cancer disorders involving a dysfunction in the immune system. In some embodiments, the invention described a plasma assay. In other embodiments, urine assay. In certain other embodiments, the invention provides therapeutic methods comprising removing toxic autoantibodies from the circulation of a patient, e.g., via plasmapheresis, and subsequently infusing the patient with one or more immunoglobulins or immunoglobulin complexes to restore the immune system of the patient to a baseline status whereby the patient's restored immune system either eliminates the source of the disorder (e.g., in the case of cancers) or no longer causes the disease or disorder (e.g., in the case of autoimmune disorders such as multiple sclerosis, psoriasis, latent autoimmune type 1 diabetes in adults (LADA) and the like). Methods of making the high activity IVIG preparation are also provided.
Owner:EIGER HEALTH PARTNERS
Immunogenic agent therapy using plasmapheresis
InactiveUS20070258991A1Lower immune responseLower antibody levelsBacterial antigen ingredientsViral antigen ingredientsEpitopeImmunogenicity
Lowering the level of antibody or complement in the blood of a subject by plasmapheresis or exchange transfusion prior to administering an immunogenic therapeutic agent containing a foreign epitope reduces the immune response of the subject to the therapeutic agent.
Owner:WELLSTAT BIOLOGICS CORP
Human blood-derived products having decreased fibrinolytic activity and uses thereof in hemostatic disorders
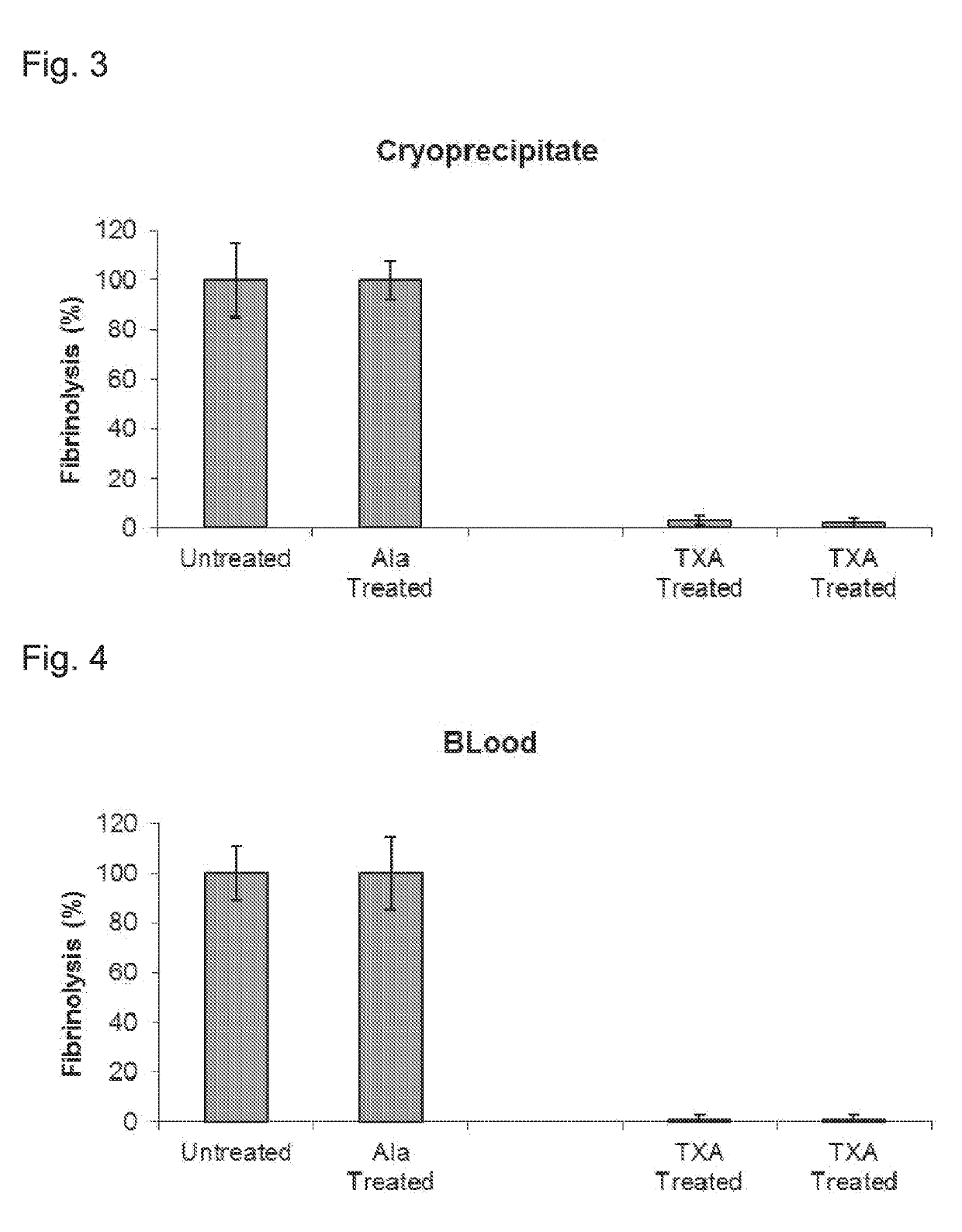
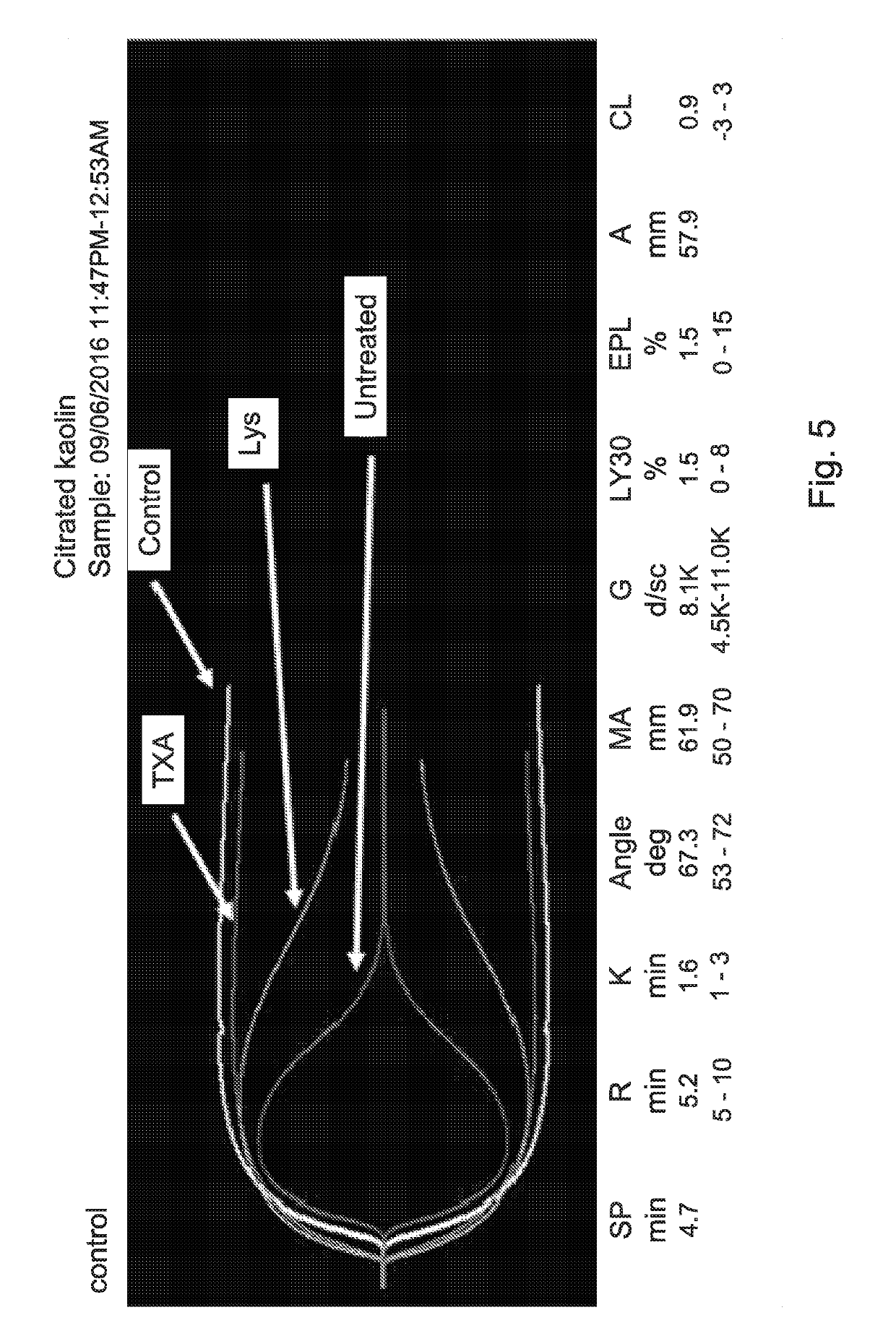
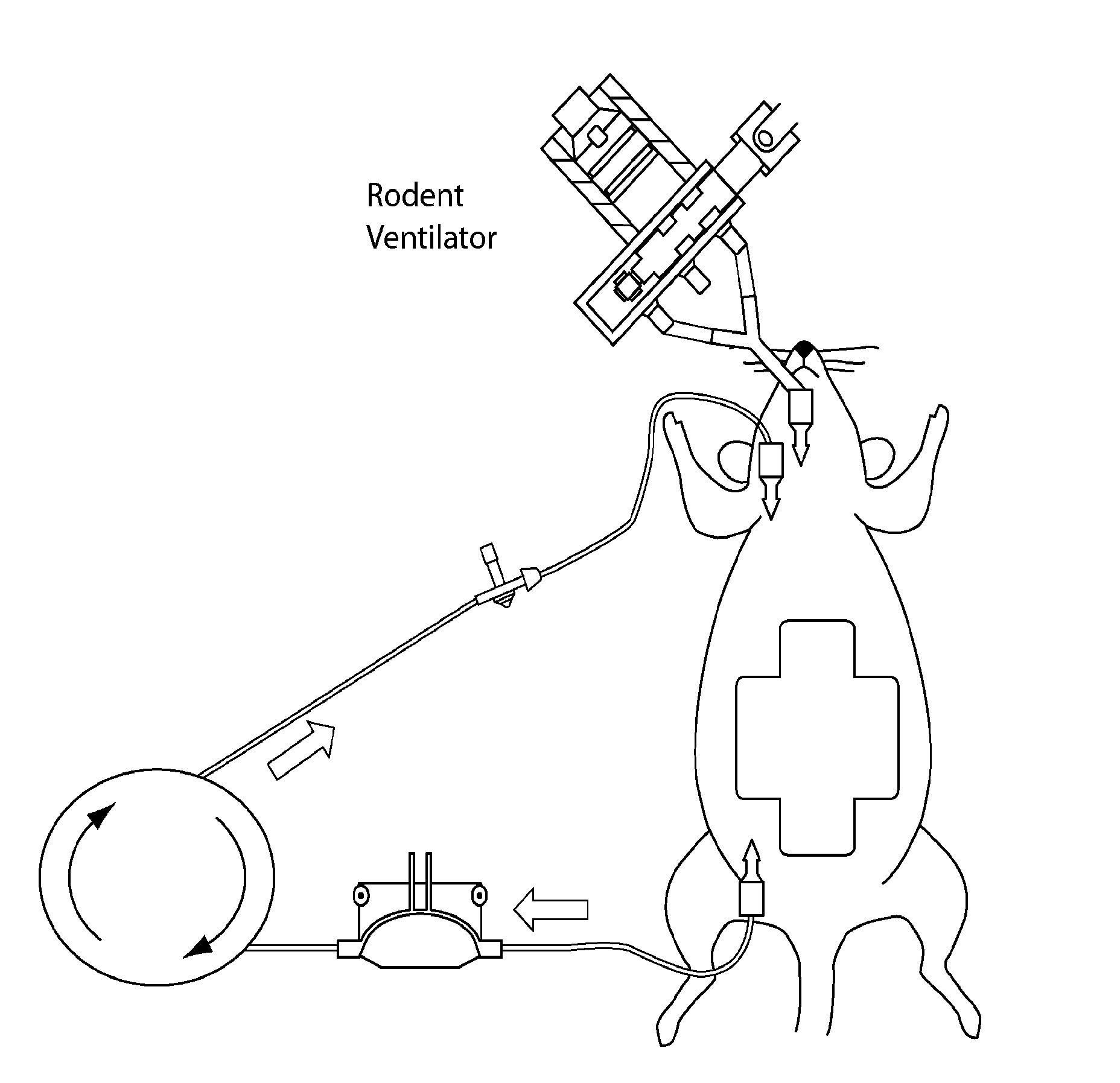
ActiveUS20190192564A1Reduced fibrinolytic activityAvoid delayPeptide/protein ingredientsMammal material medical ingredientsHemostatic DisordersPlasmin
The present invention provides therapeutic products with decreased fibrinolytic activity of t-PA-deficient and / or plasminogen-deficient blood products, as well as compositions, kits and methods using the same in treating bleeding associated with hereditary or acquired bleeding disorders. The invention further provides extracorporeal apparatus for blood or blood products Plasmapheresis aimed to prevent or treat bleeding disorders.
Owner:PLAS FREE LTD
Systems and methods for extracorporeal blood modification
The present invention generally relates to systems and methods for targeted removal of a substance or biomolecule such as a protein from a biological fluid, such as blood. In some cases, the blood may be withdrawn from a subject, treated, and returned to the subject. Previous techniques for removal of biological materials from blood, such as hemodialysis and plasmapheresis, were generally non-specific (i.e., they removed a multitude of proteins / toxins from the blood). By contrast, novel methods and devices described herein are capable of removing specific or single substances such as proteins from biological fluids such as blood in a specific manner. Such highly specific protein removal has a broad array of clinical applications, including treatment of inflammatory conditions and autoimmune diseases.
Owner:CHILDRENS MEDICAL CENT CORP
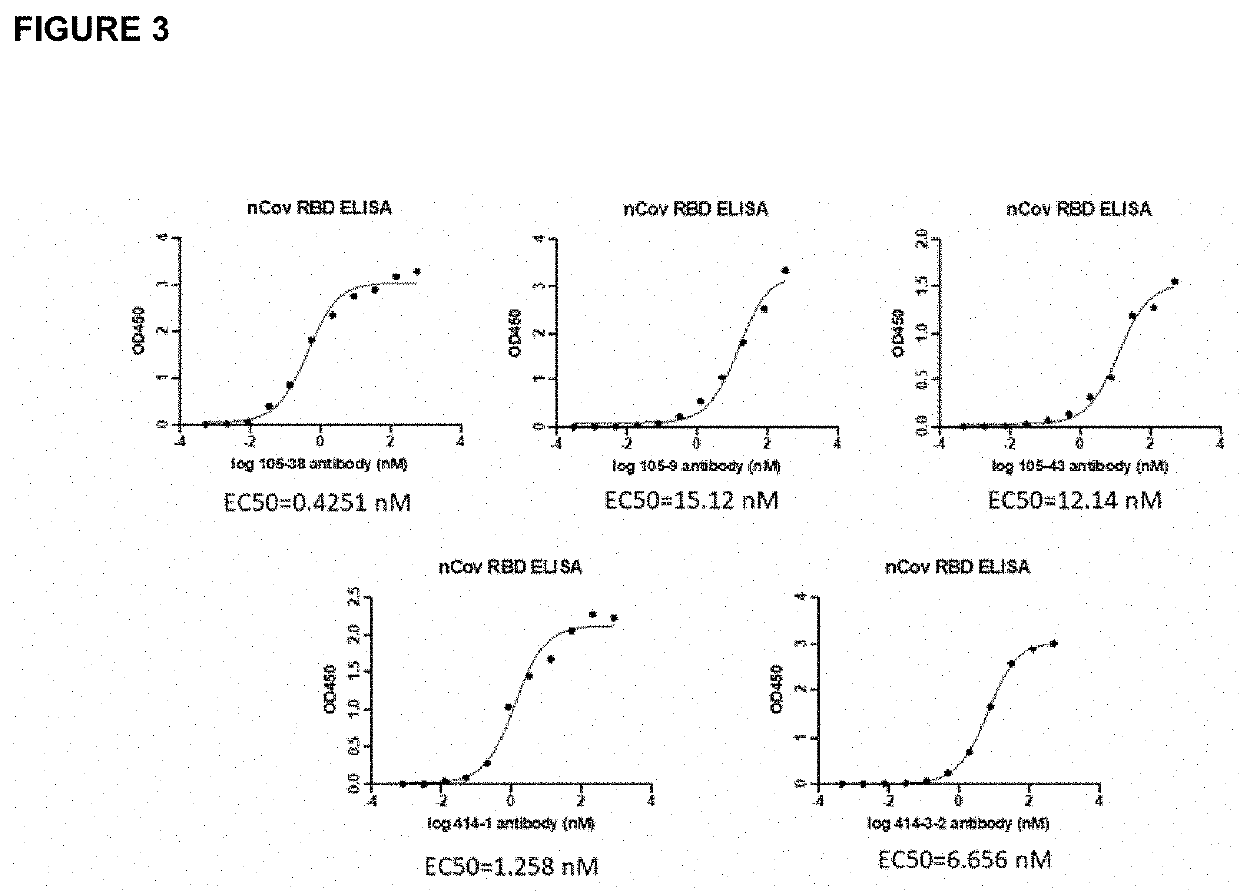
Antibodies to sars-coronavirus (covid-19) s1 spike protein
The present invention provides recombinant monoclonal antibodies that bind to the Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2 or COVID-19) spike protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to SARS-CoV-2 spike protein. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing SARS-CoV-2 activity, thus providing a means of treating or preventing COVID-19 infection in humans. In some embodiments, the invention provides for a combination of one or more antibodies that bind to the SARS-CoV-2 spike protein for use in treating COVID-19 infection. In certain embodiments, the one or more antibodies bind to distinct non-competing epitopes comprised in the receptor binding domain of the SARS-CoV-2 spike protein. In certain embodiments, the antibodies of the invention can be used to make an in vitro diagnostic for detection of COVID-19 in human and non-human animal samples In still other embodiments the antibodies of the present invention can be used to remove virus from patient plasma via plasmapheresis.
Owner:ACTIVE MOTIF SHANGHAI LTD +2
Method for diagnosis of a disease involving an Anti-endothelin-receptor antibody
The invention relates to a method for diagnosis of a disease, wherein presence or absence of an anti-endothelin-receptor antibody is determined in a sample from a patient to be diagnosed more in particular an anti-endothelin-receptor-A antibody. The disease according to the invention is in particular selected from diabetes, preferably type I diabetes, graft rejection, pre-eclampsia, hypertension, vasculitis, collagenosis, Raynaud-Syndrom (Morbus Raynaud), and inflammatory rheumatic disease and arteriosclerosis. The invention further relates to the use of an inhibitor of an anti-endothelin-receptor antibody or an inhibitor of an endothelin-receptor for the production of a medicament as well as a method for removing anti-endothelin-receptor antibodies from isolated blood by means of plasmapheresis.
Owner:CELLTREND
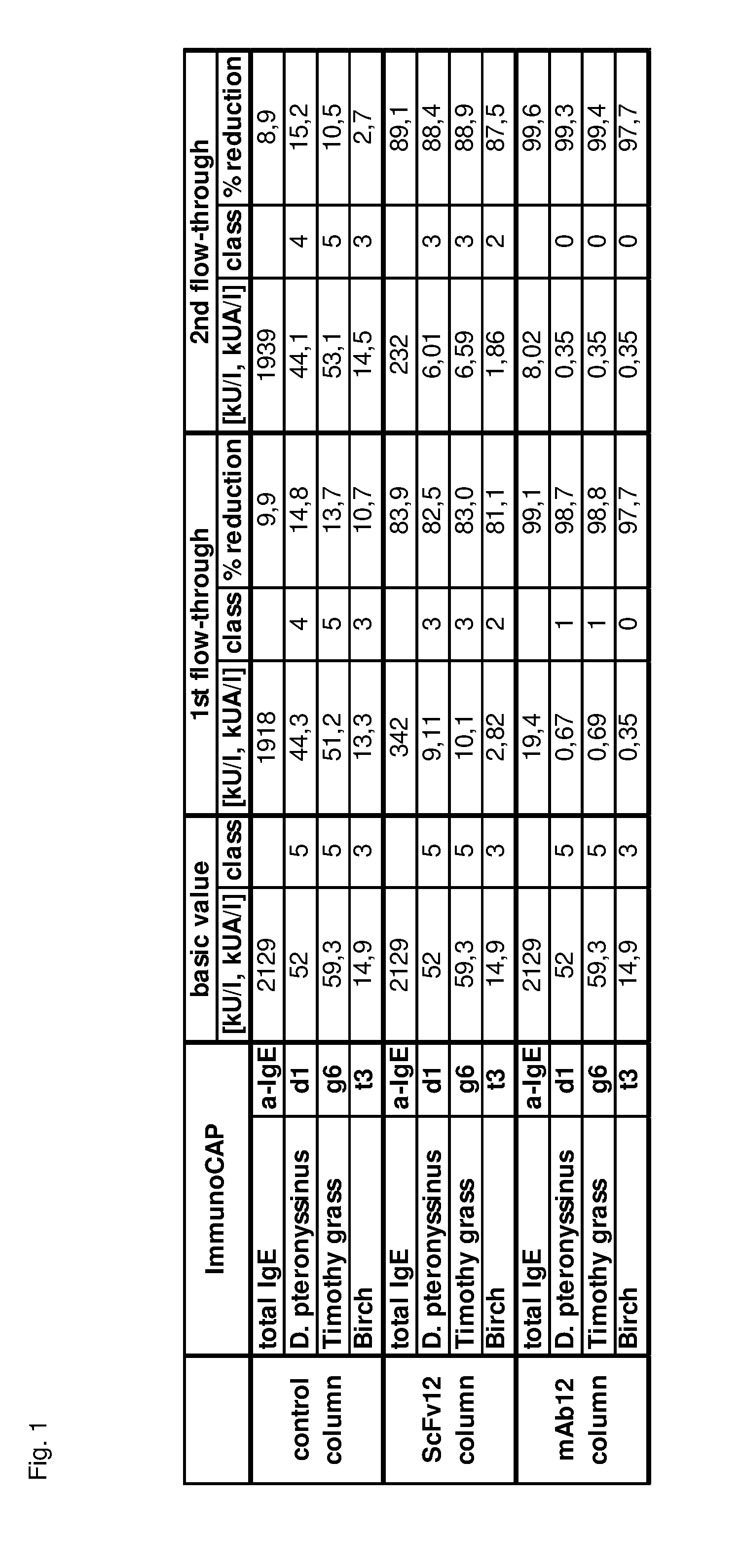
IMMUNOAFFINITY SEPARATION MATERIALS COMPRISING ANTI-IgE ANTIBODY DERIVATIVES
InactiveUS20140124448A1HaemofiltrationSolid sorbent liquid separationSingle-Chain AntibodiesAntibody
The present invention provides an immunoaffinity separation material, comprising an antibody derivative having high specificity for soluble and cell bound IgE, an apheresis device comprising said material and its use for apheresis, specifically for plasmapheresis. It further provides a recombinant single chain antibody fragment with high specificity for soluble and cell bound IgE that is free of any tag sequences as well as the method for its production.
Owner:BIOMAY AG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com