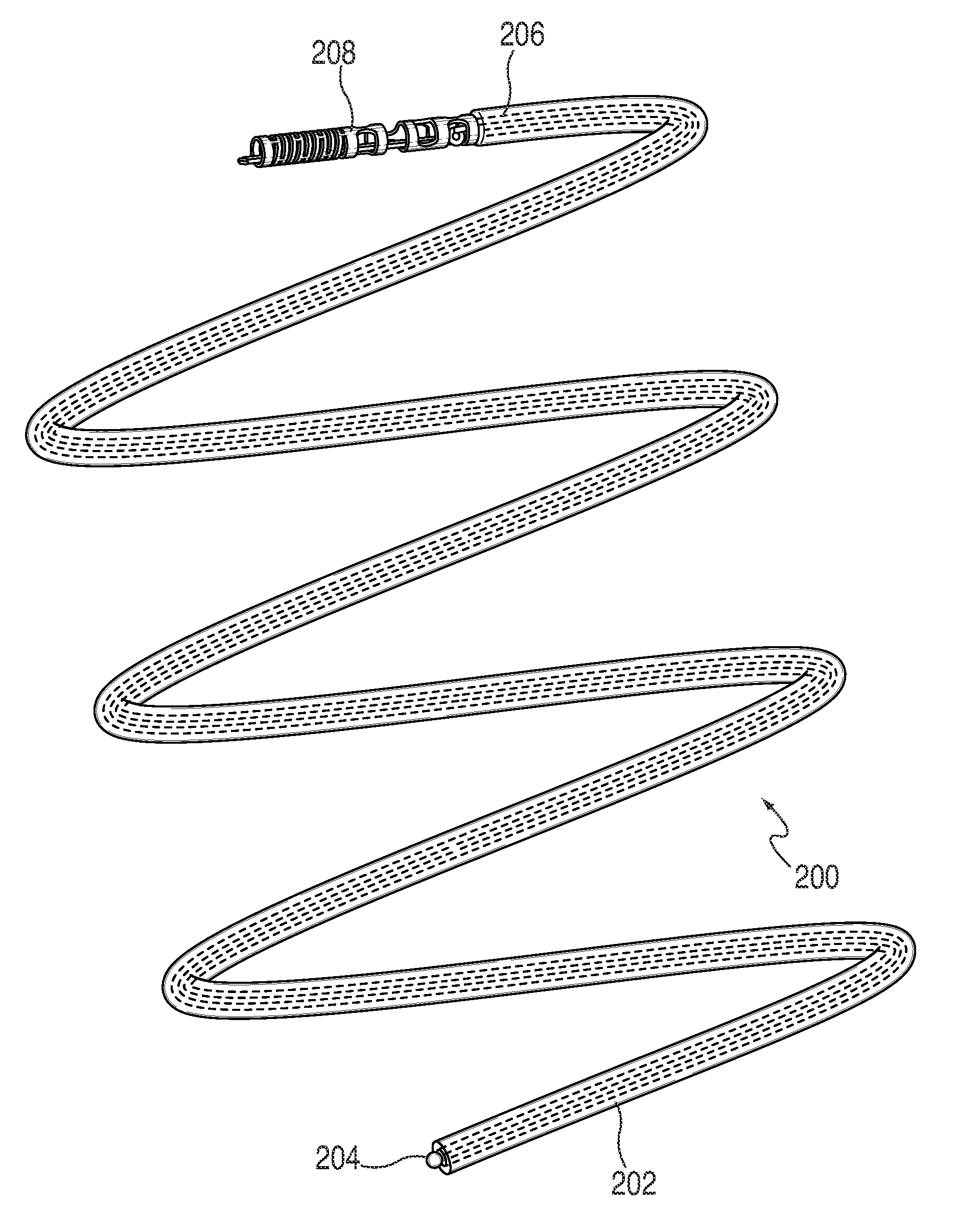
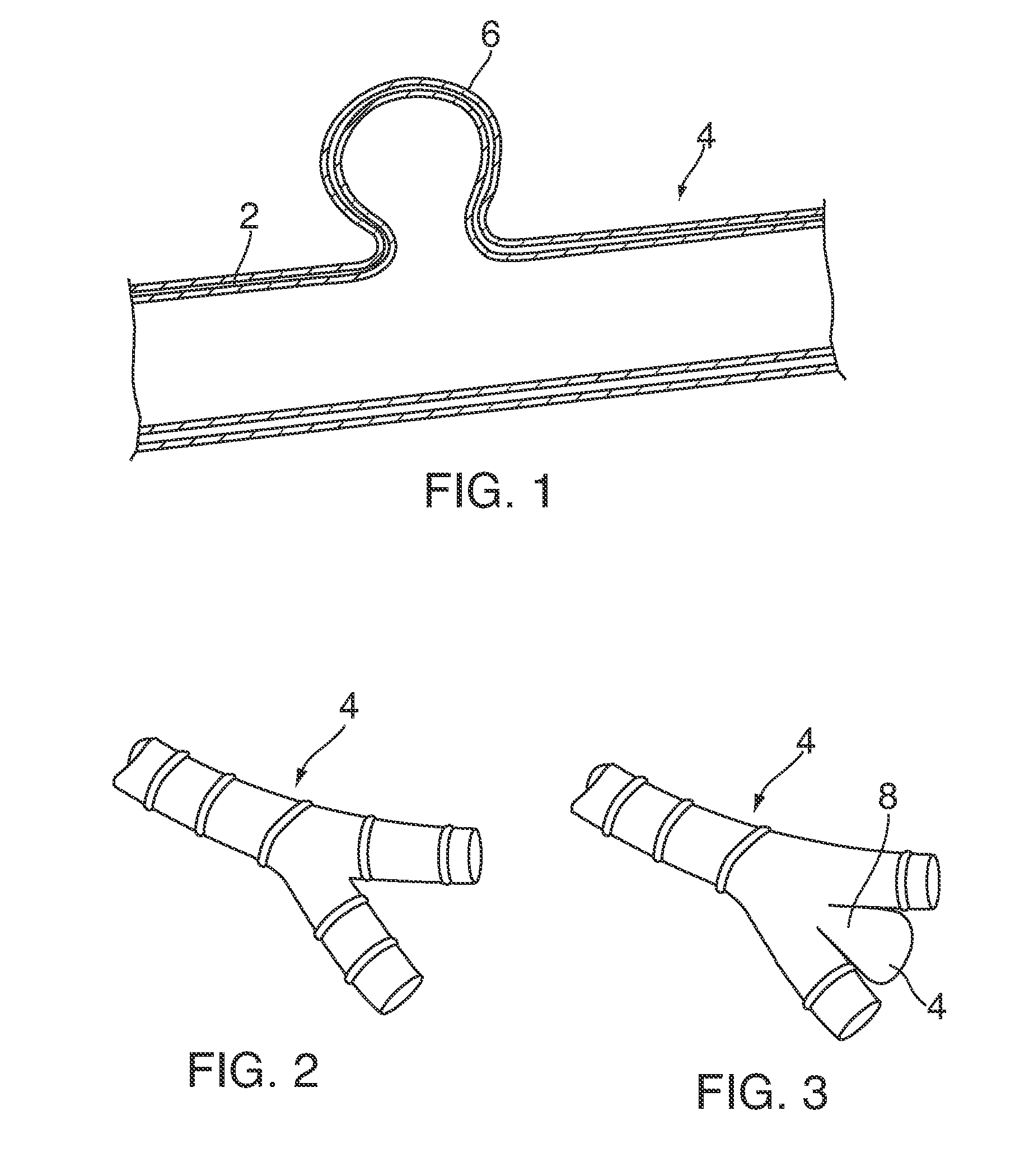
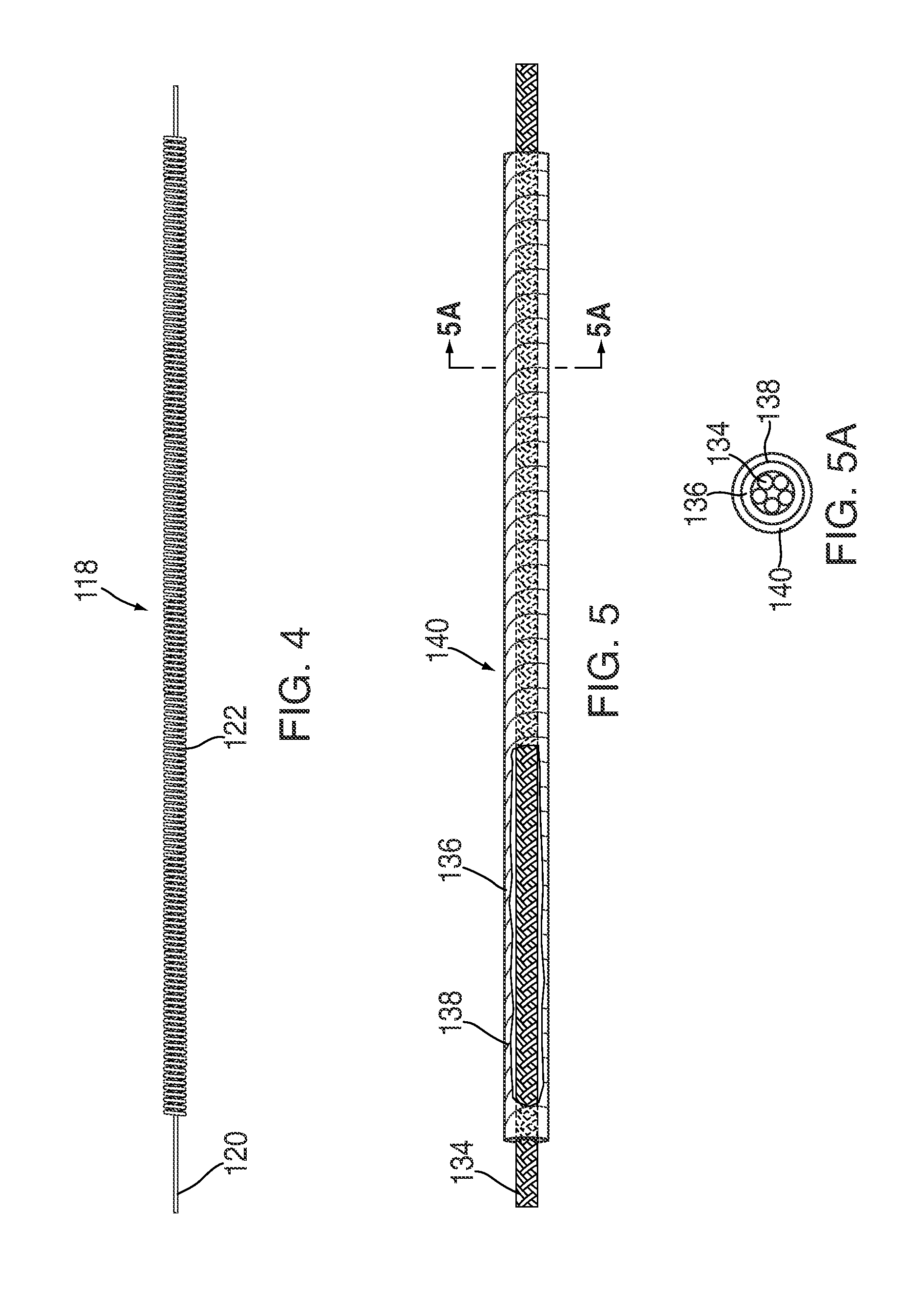
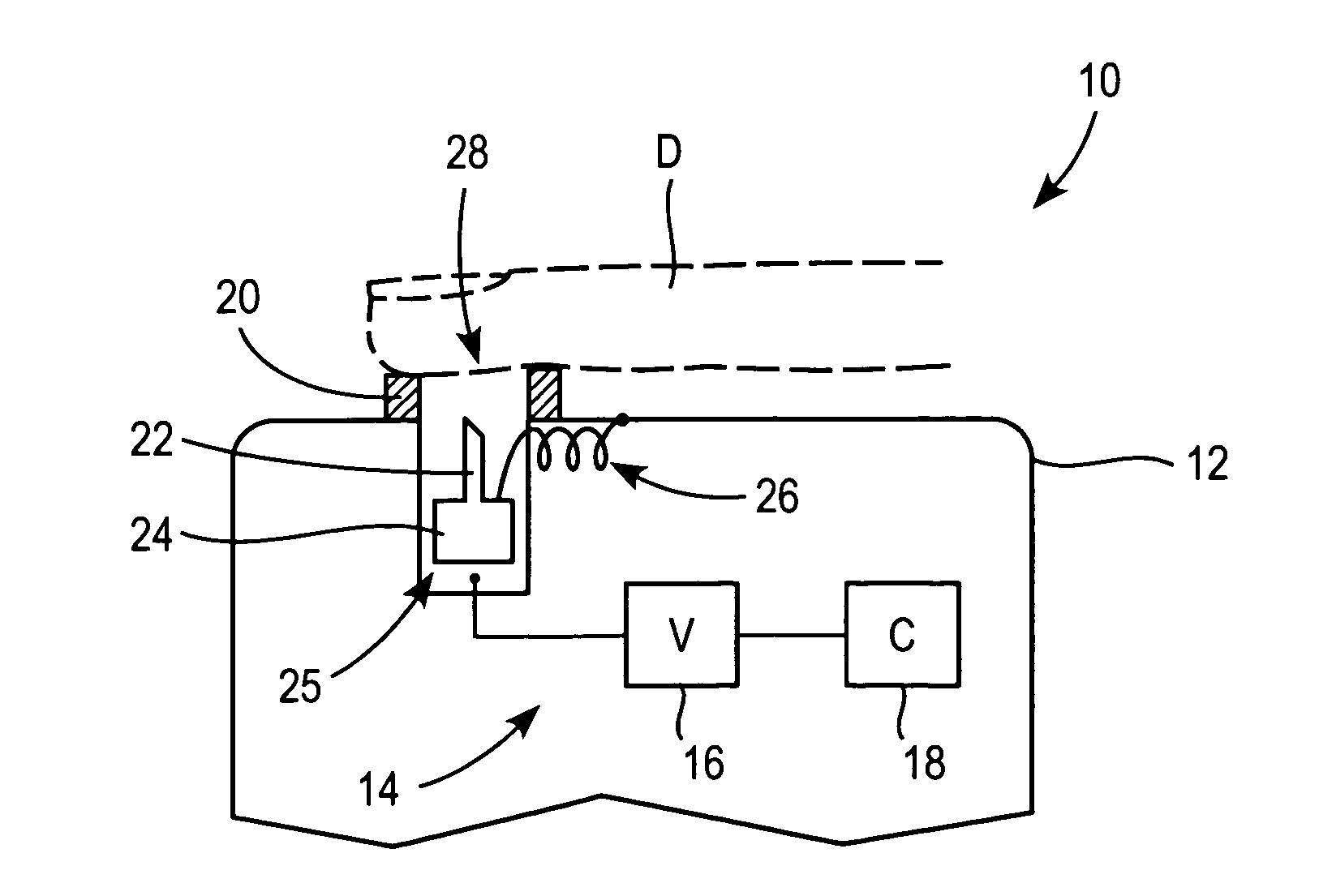
Patents
Literature
1673 results about "Lumen intima" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
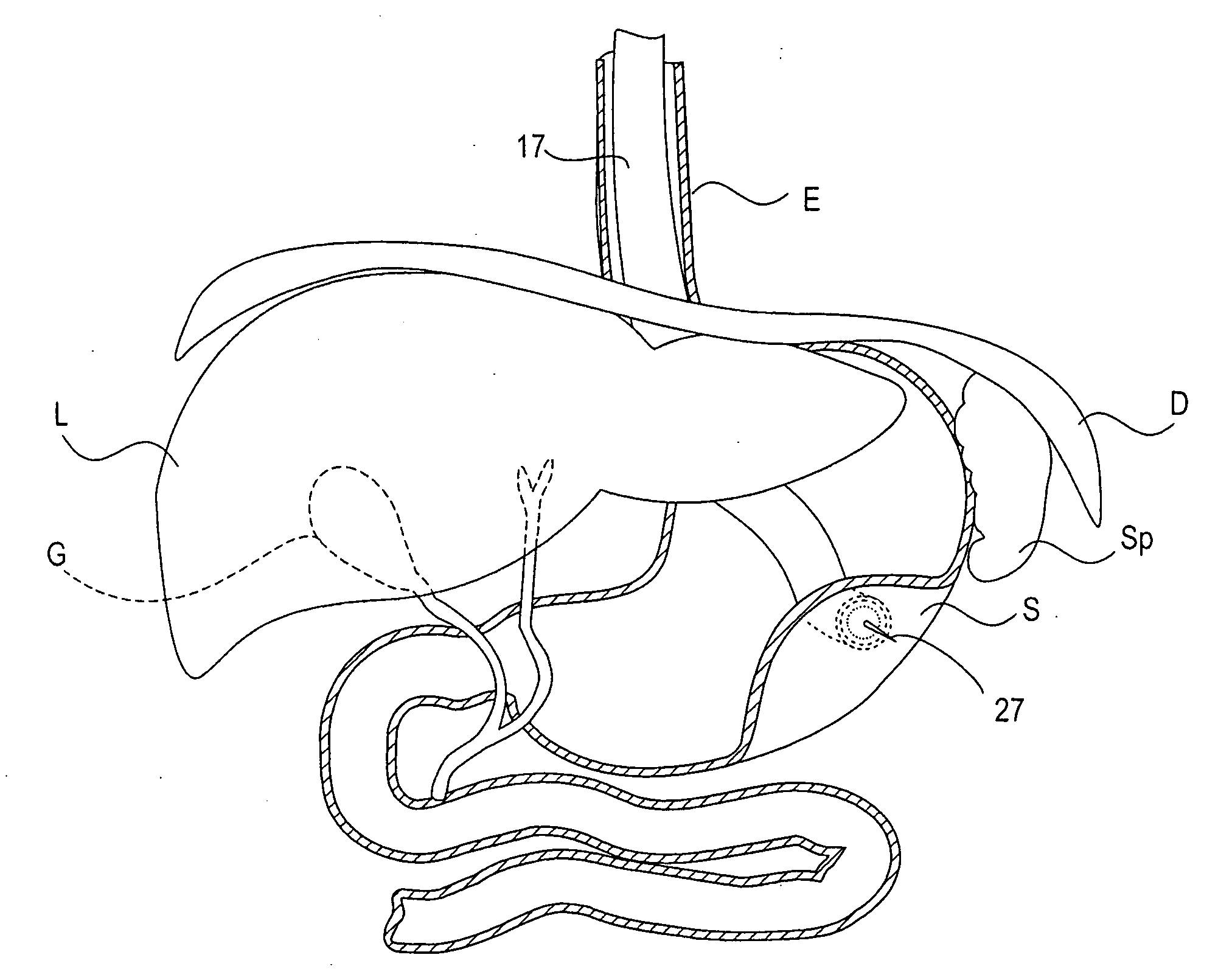
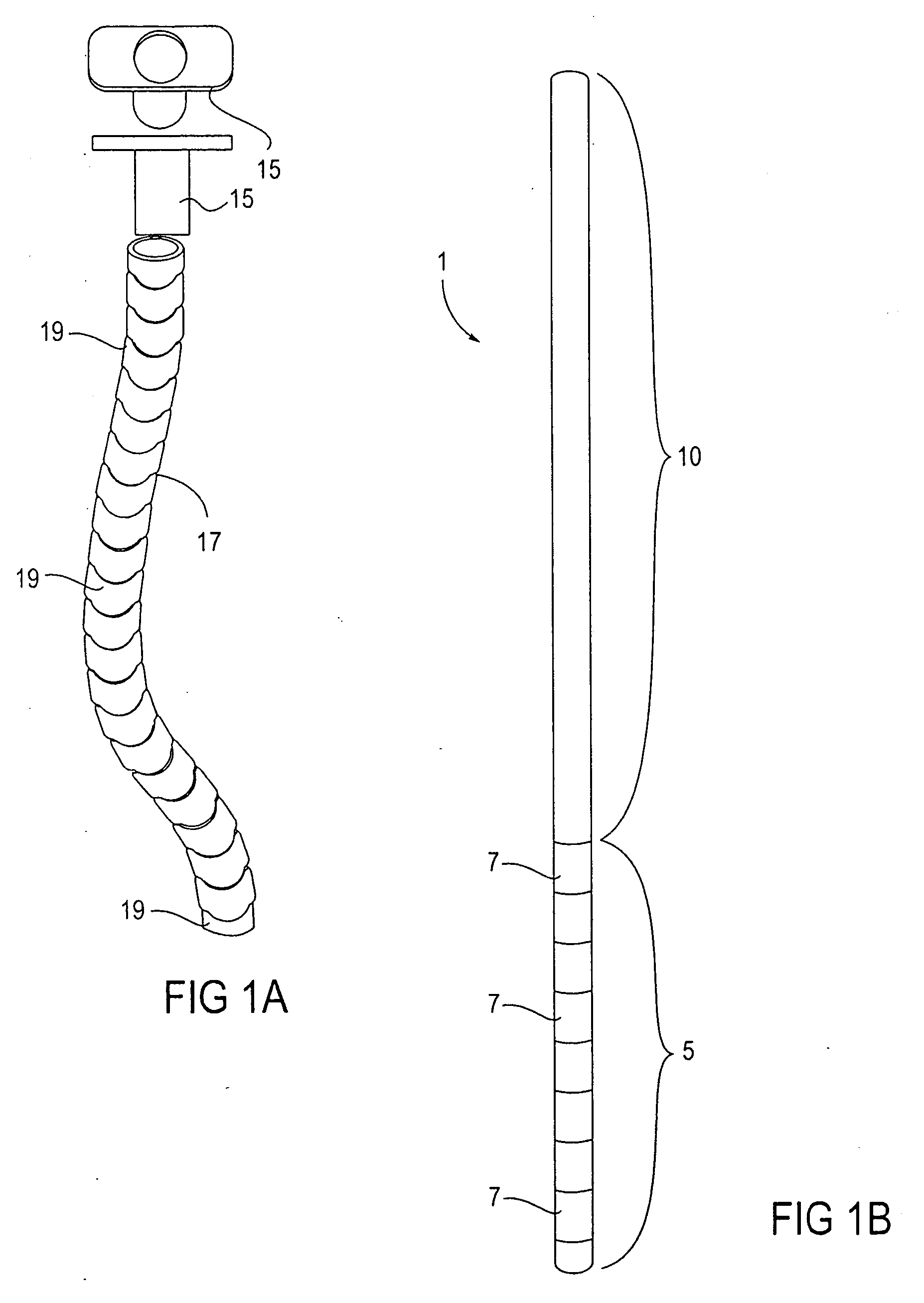
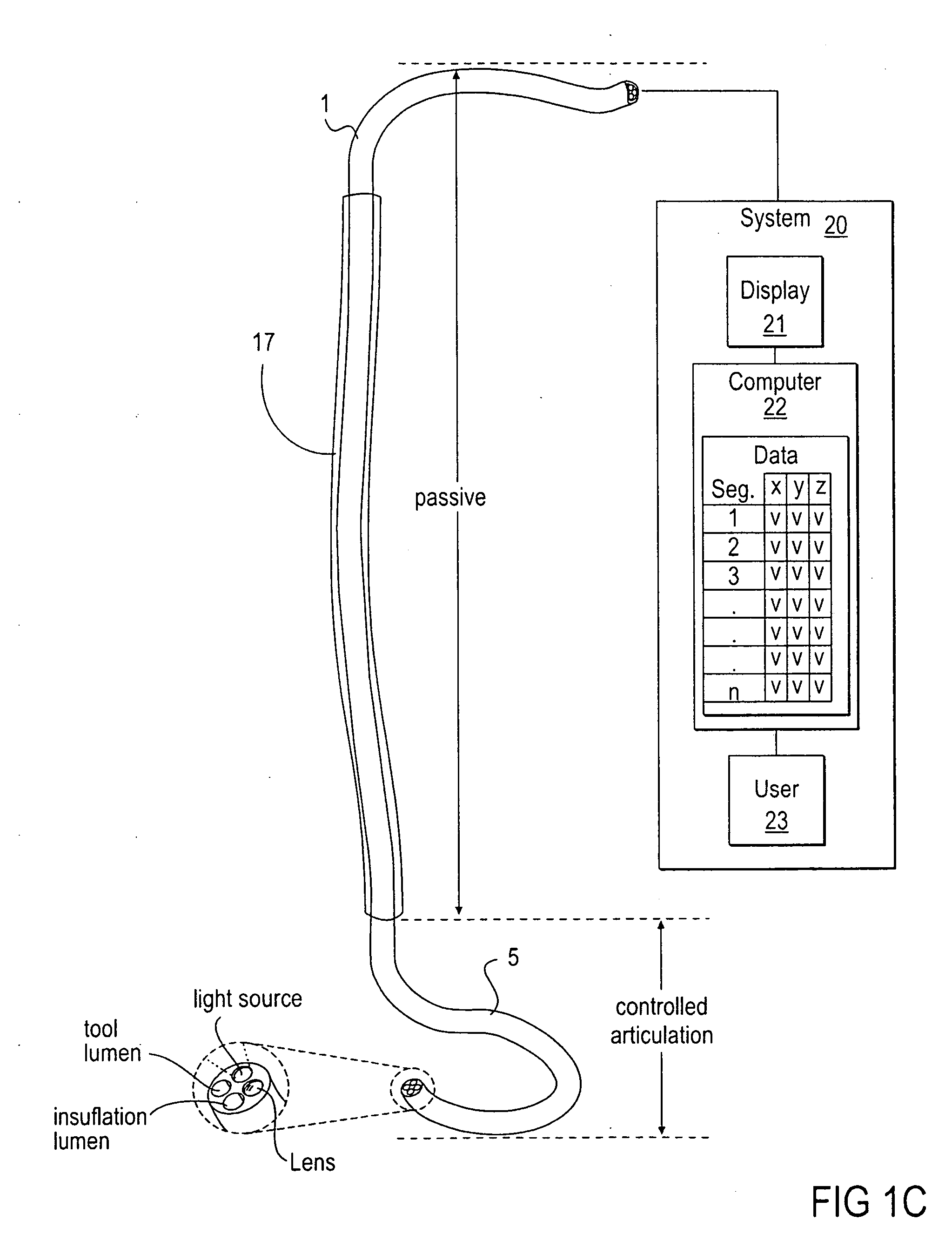
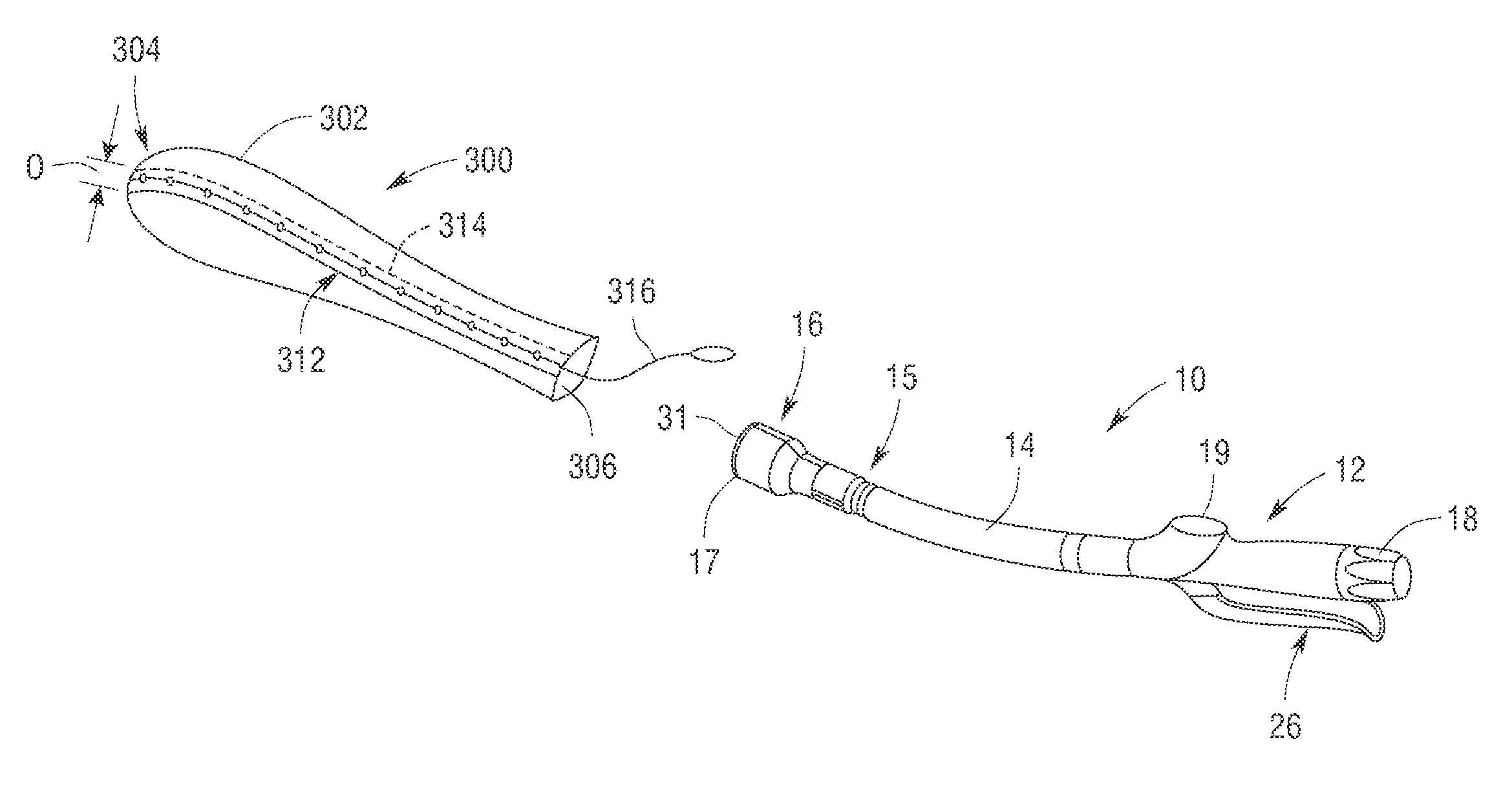
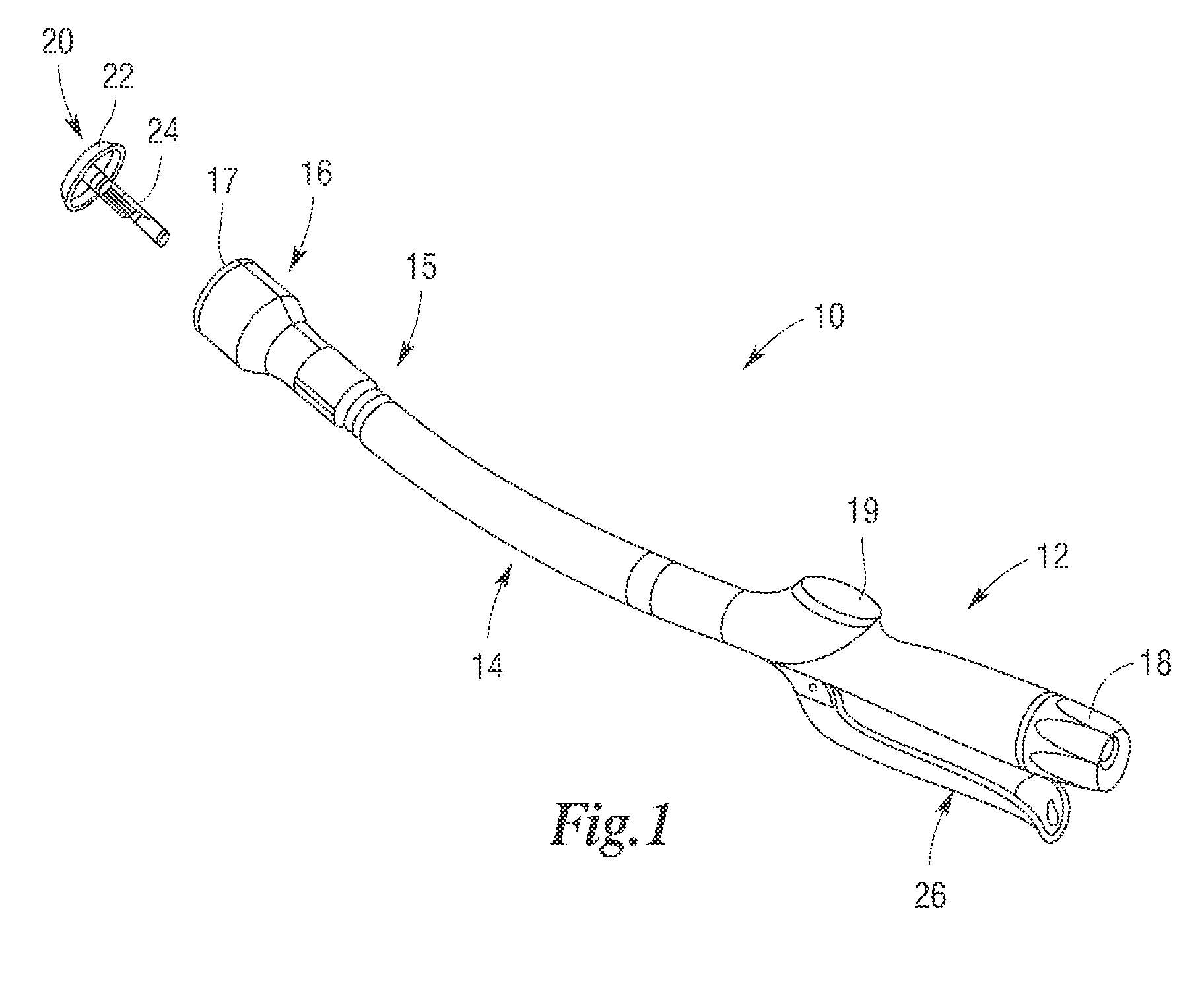
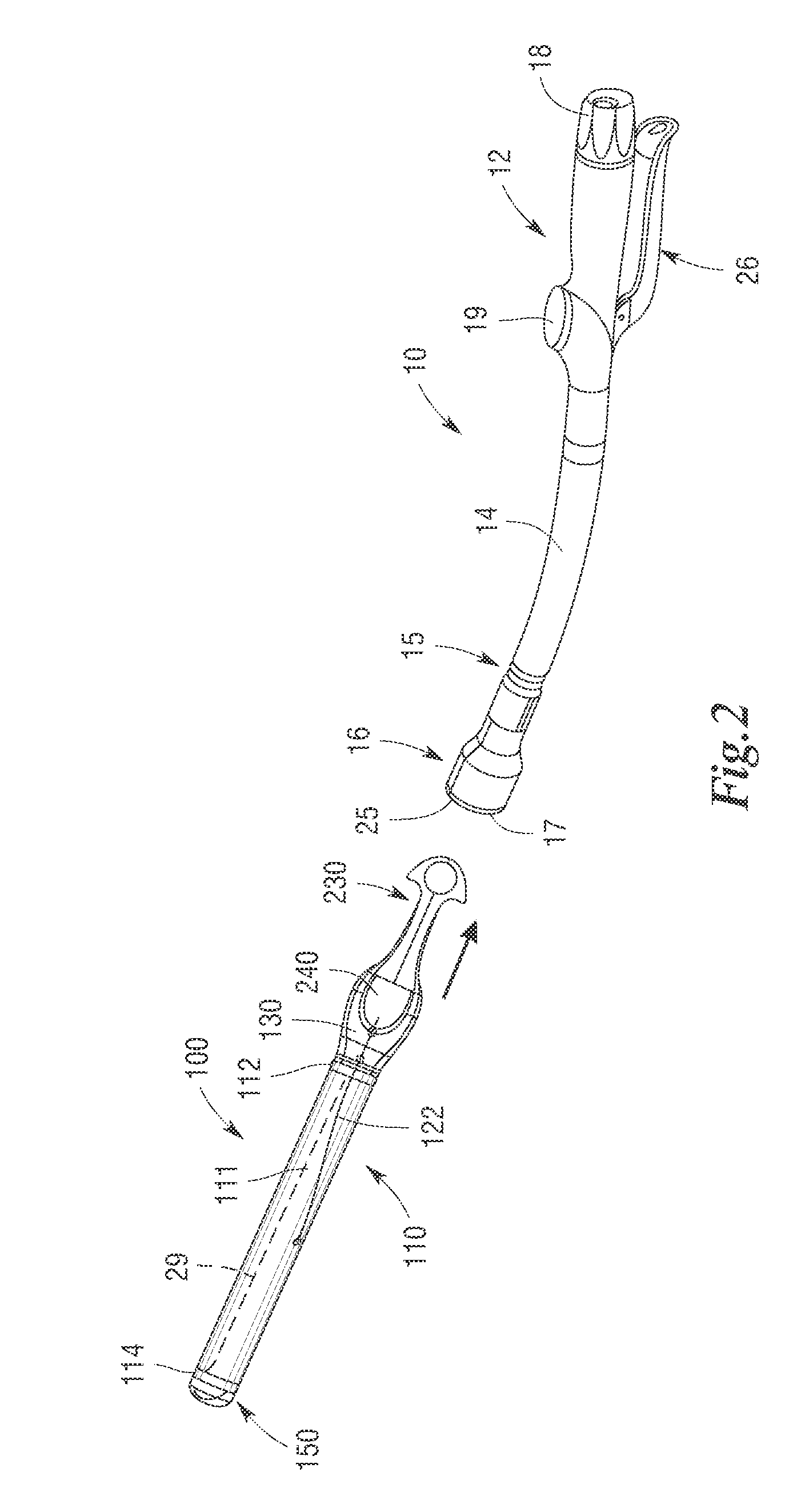
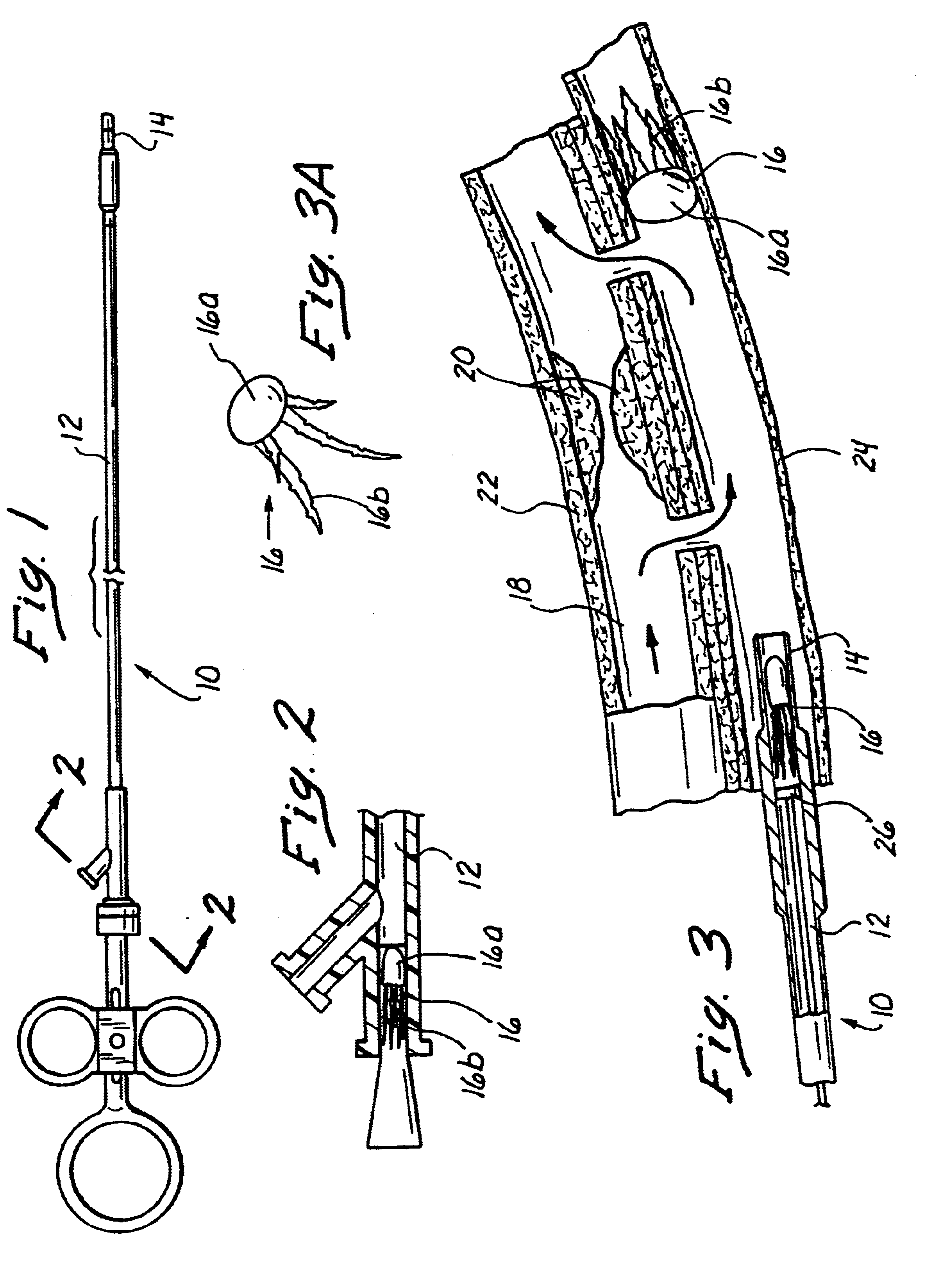
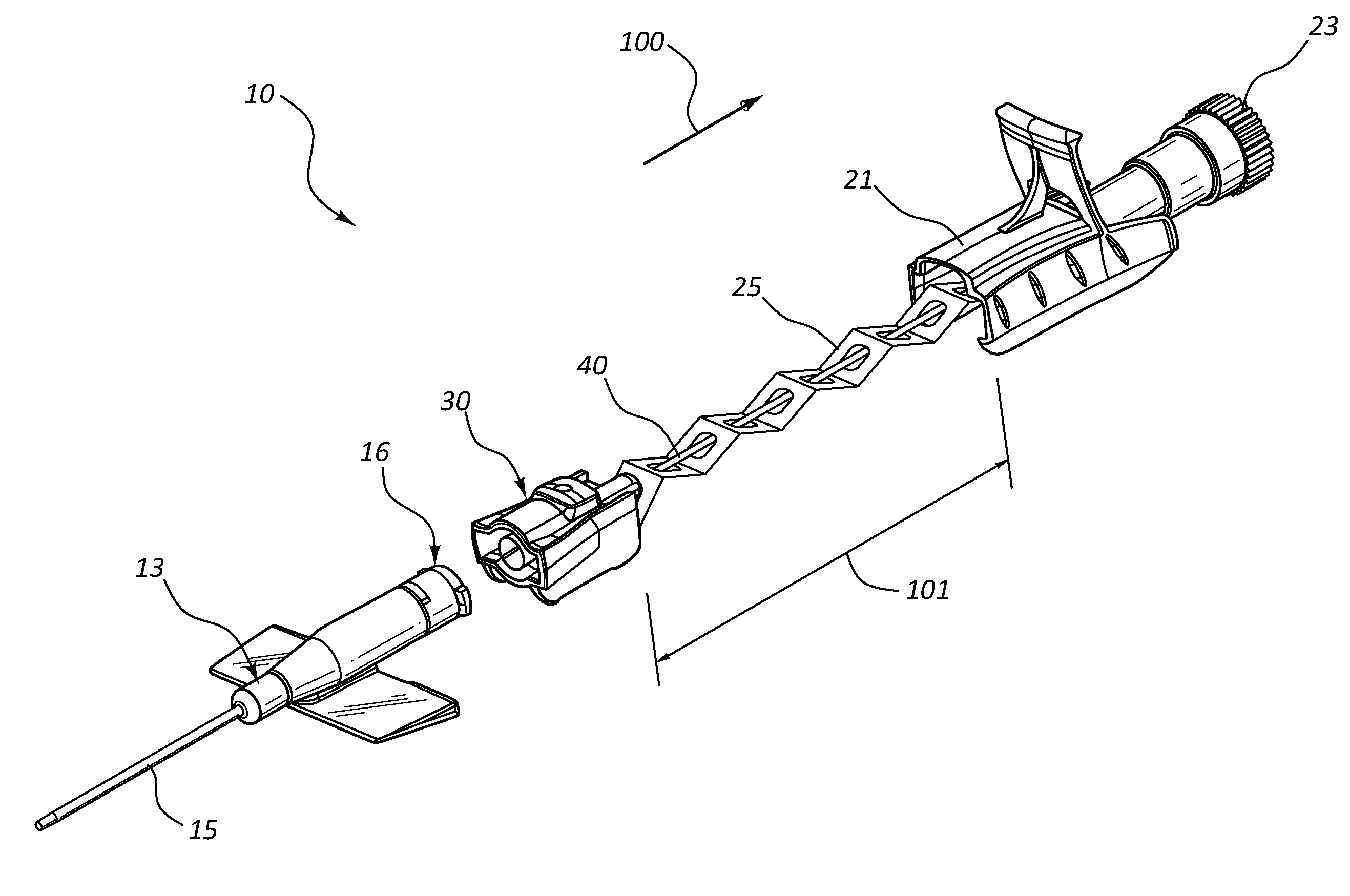
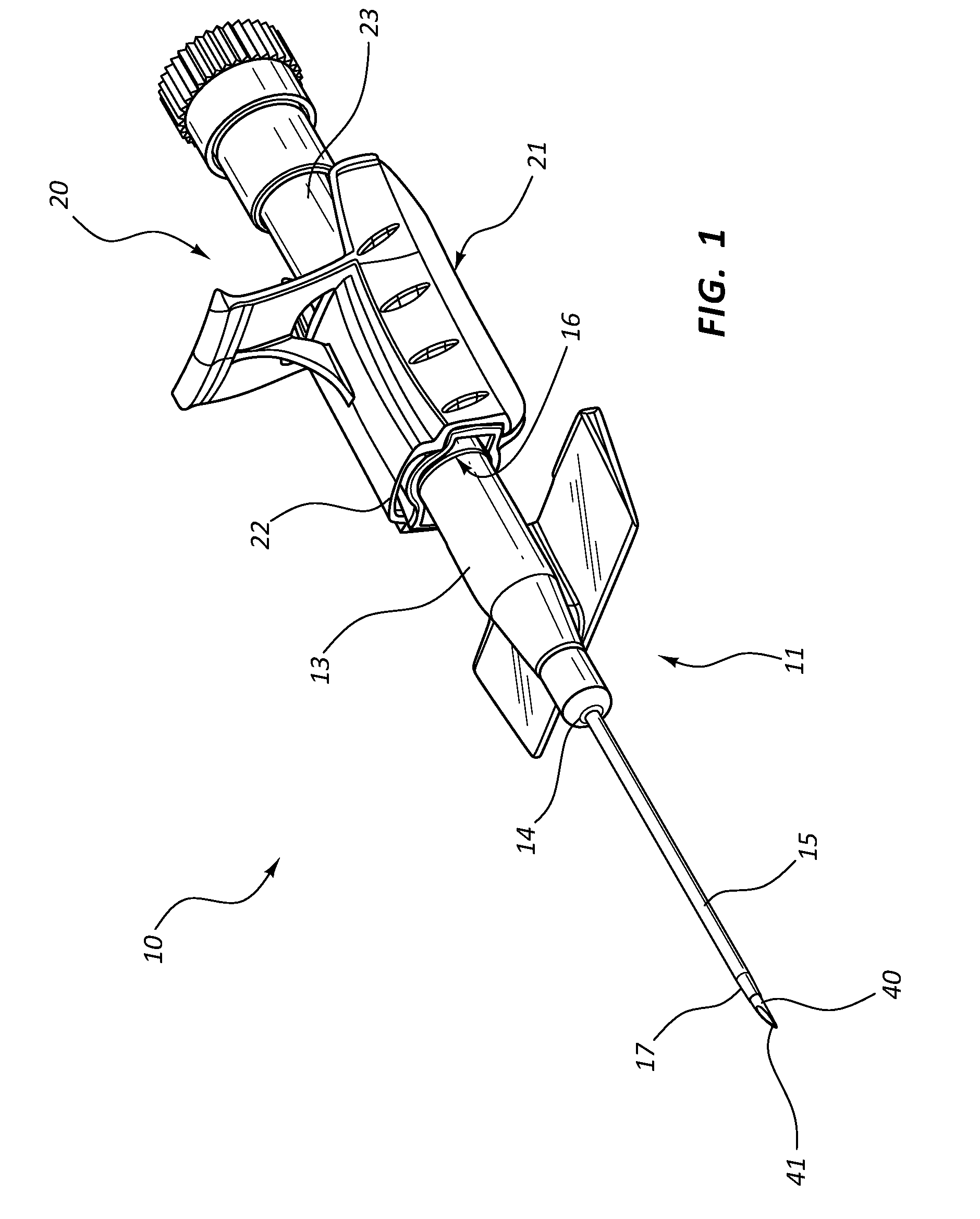
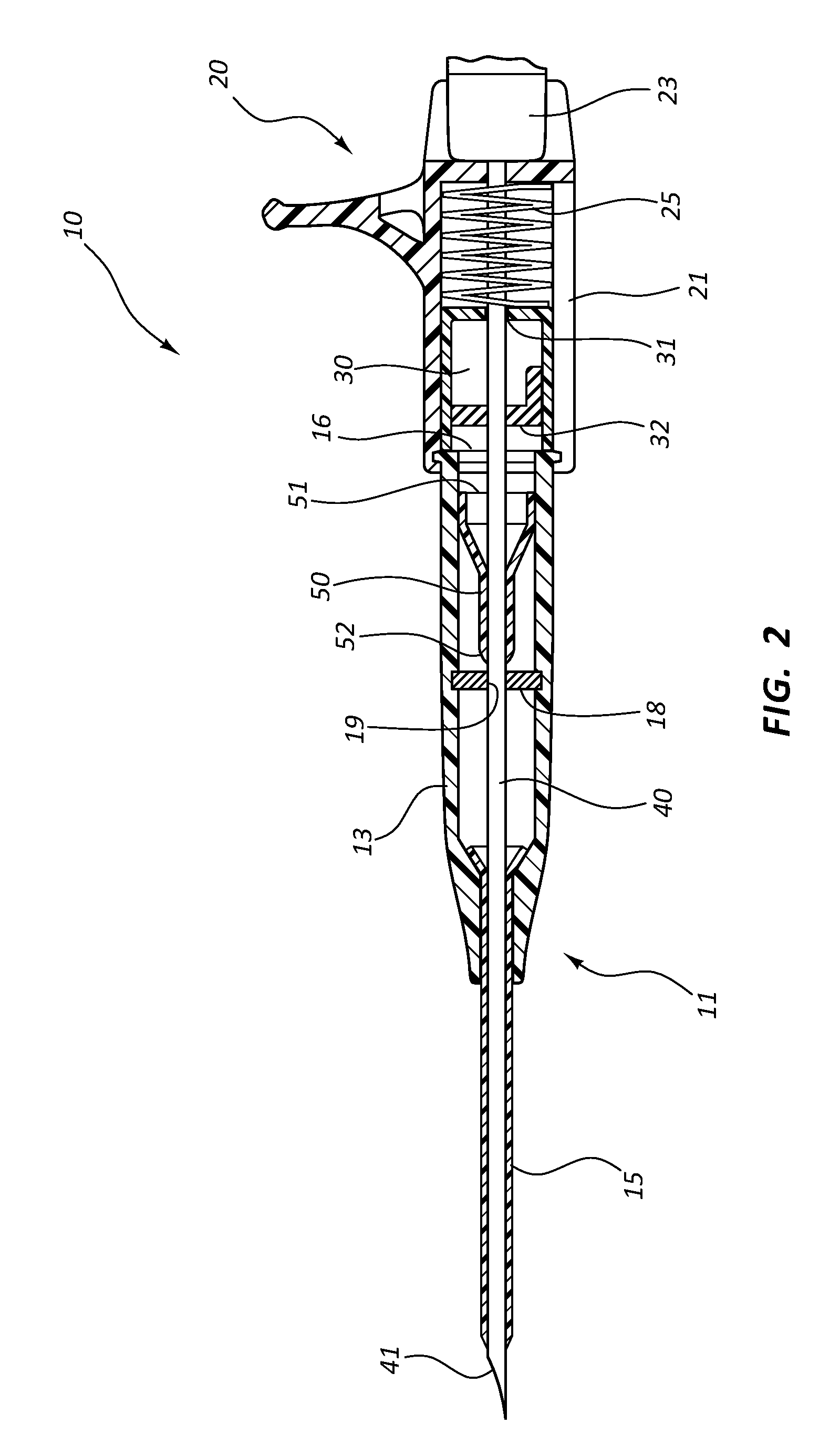
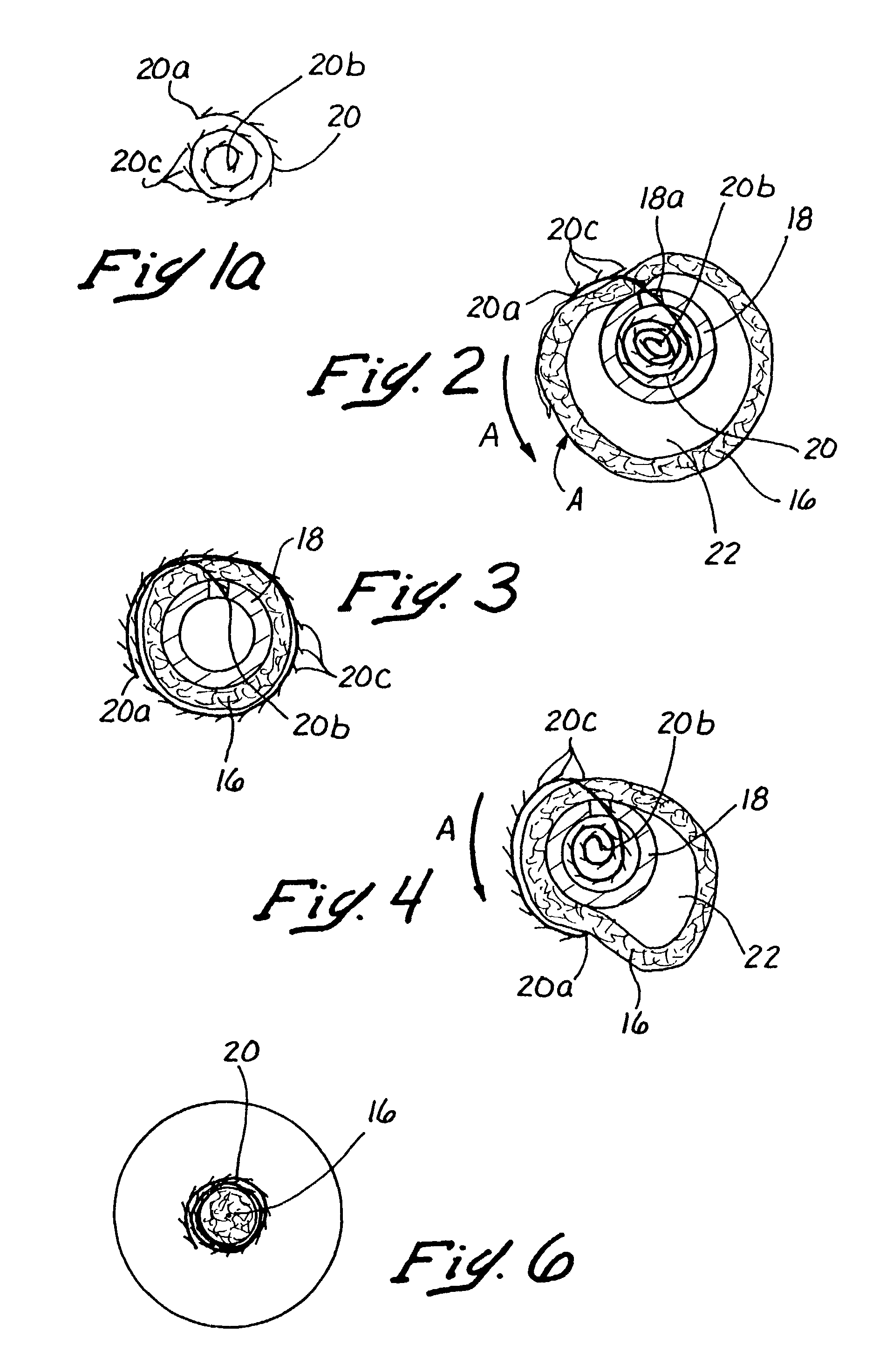
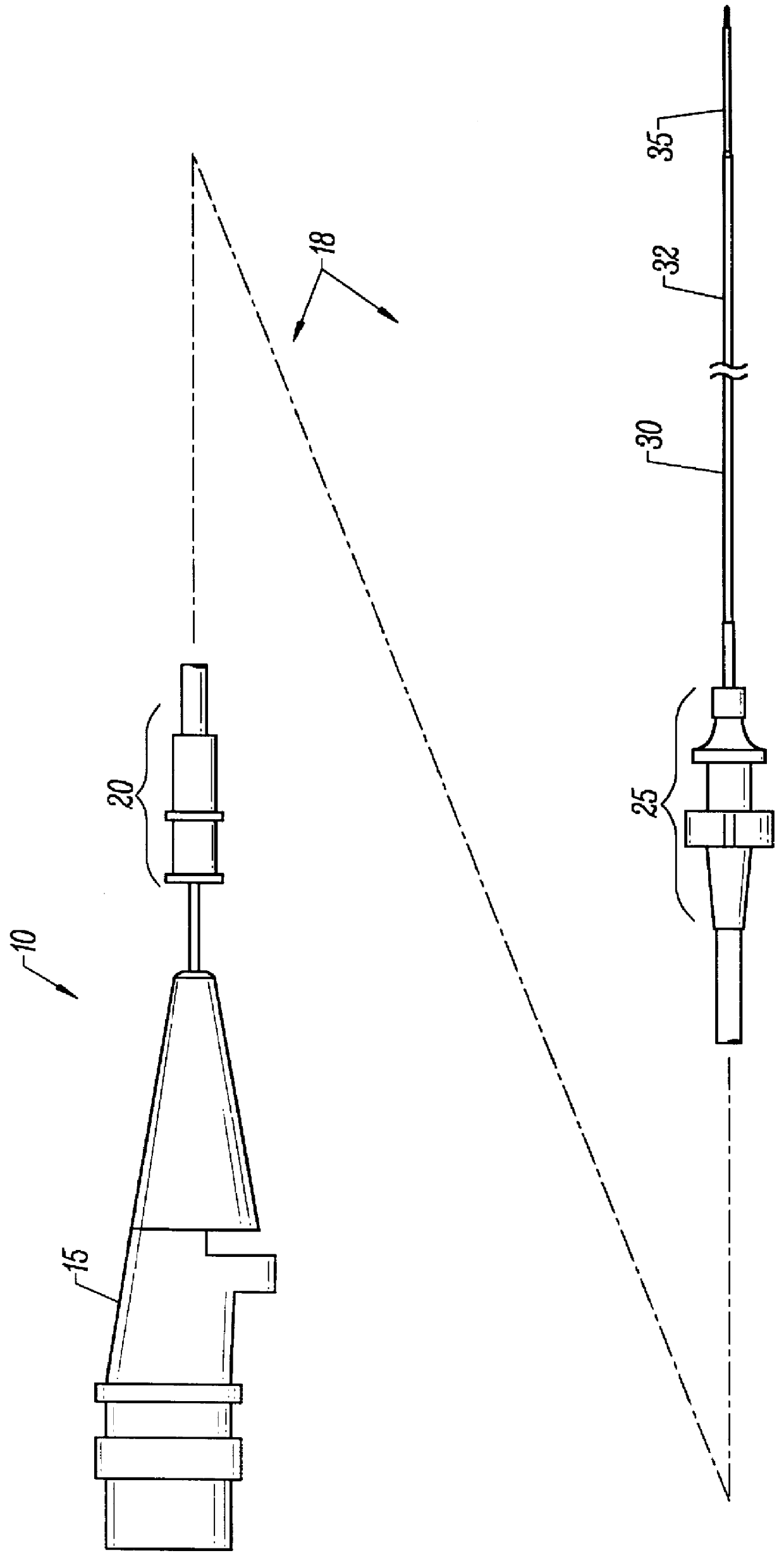
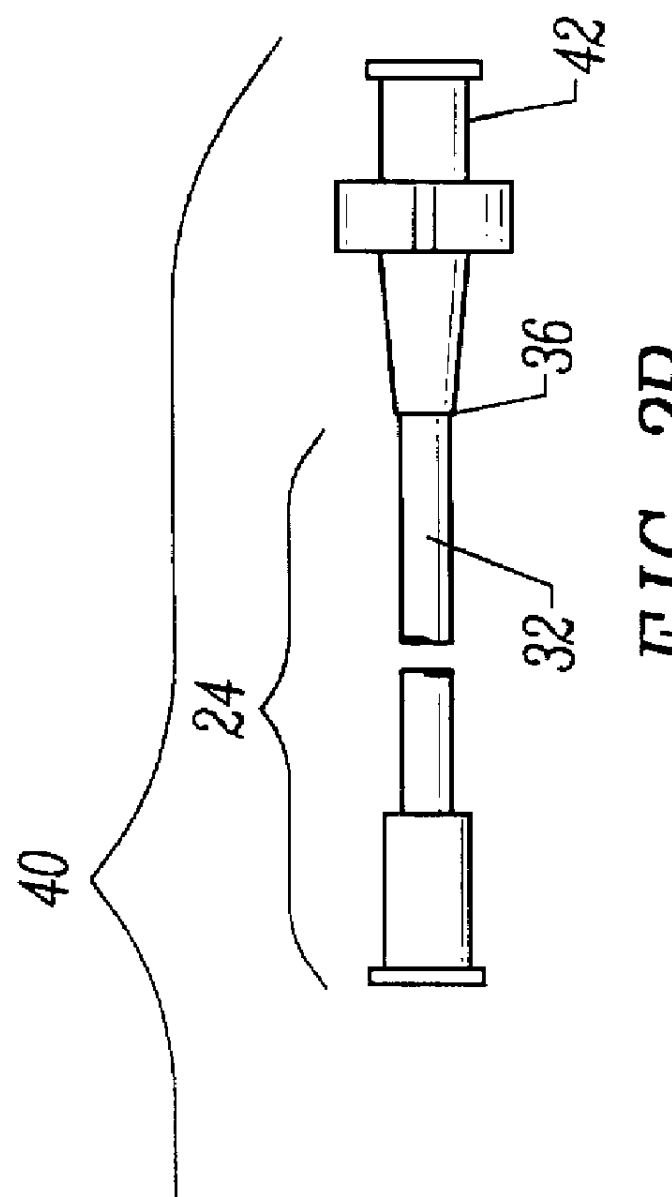
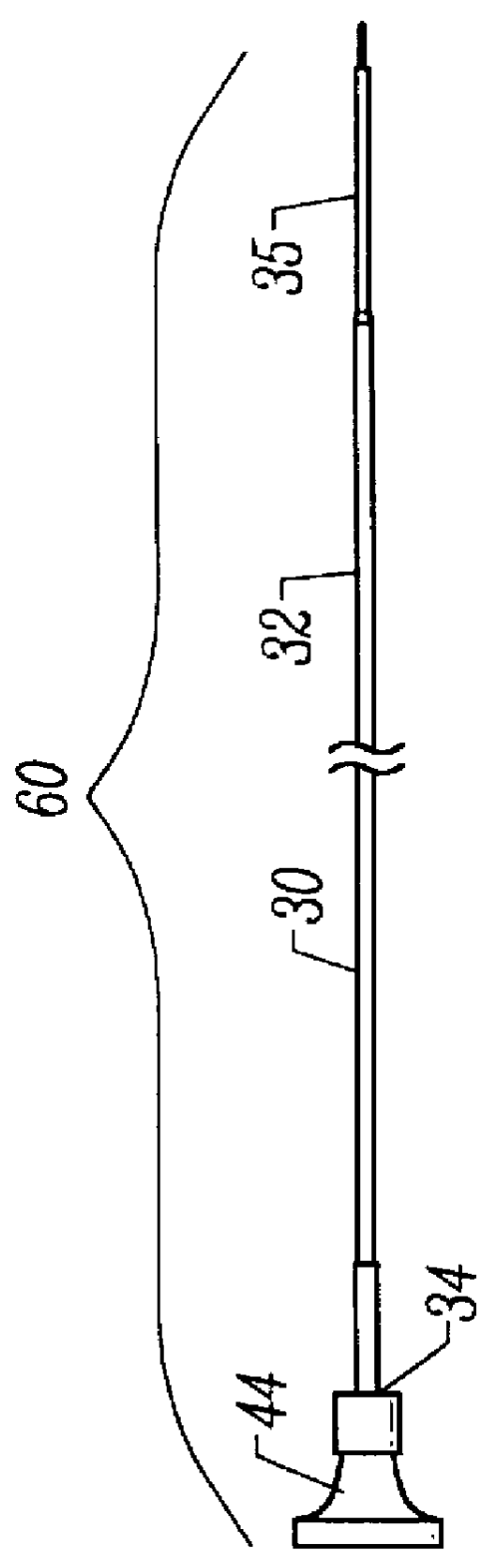
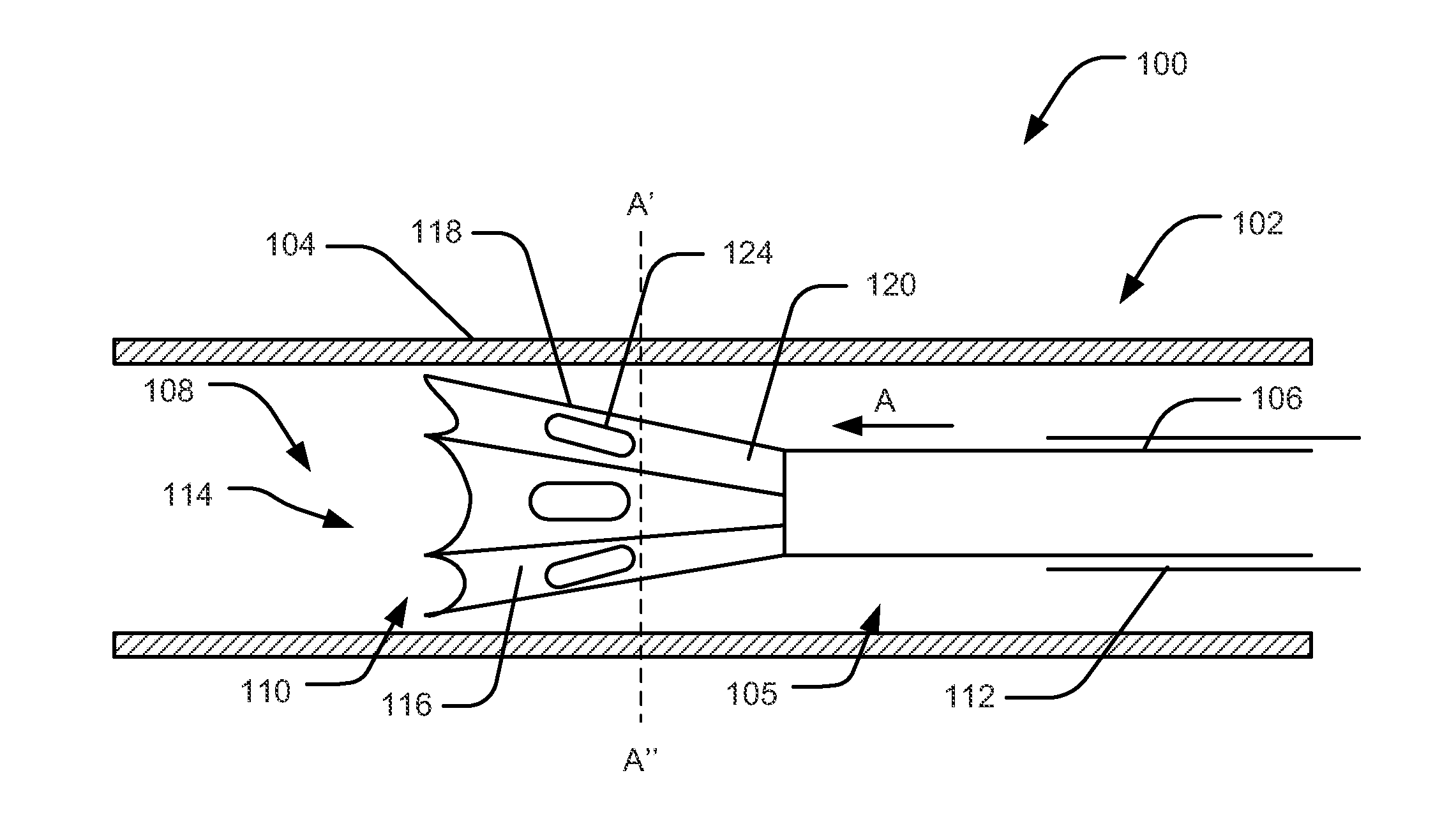
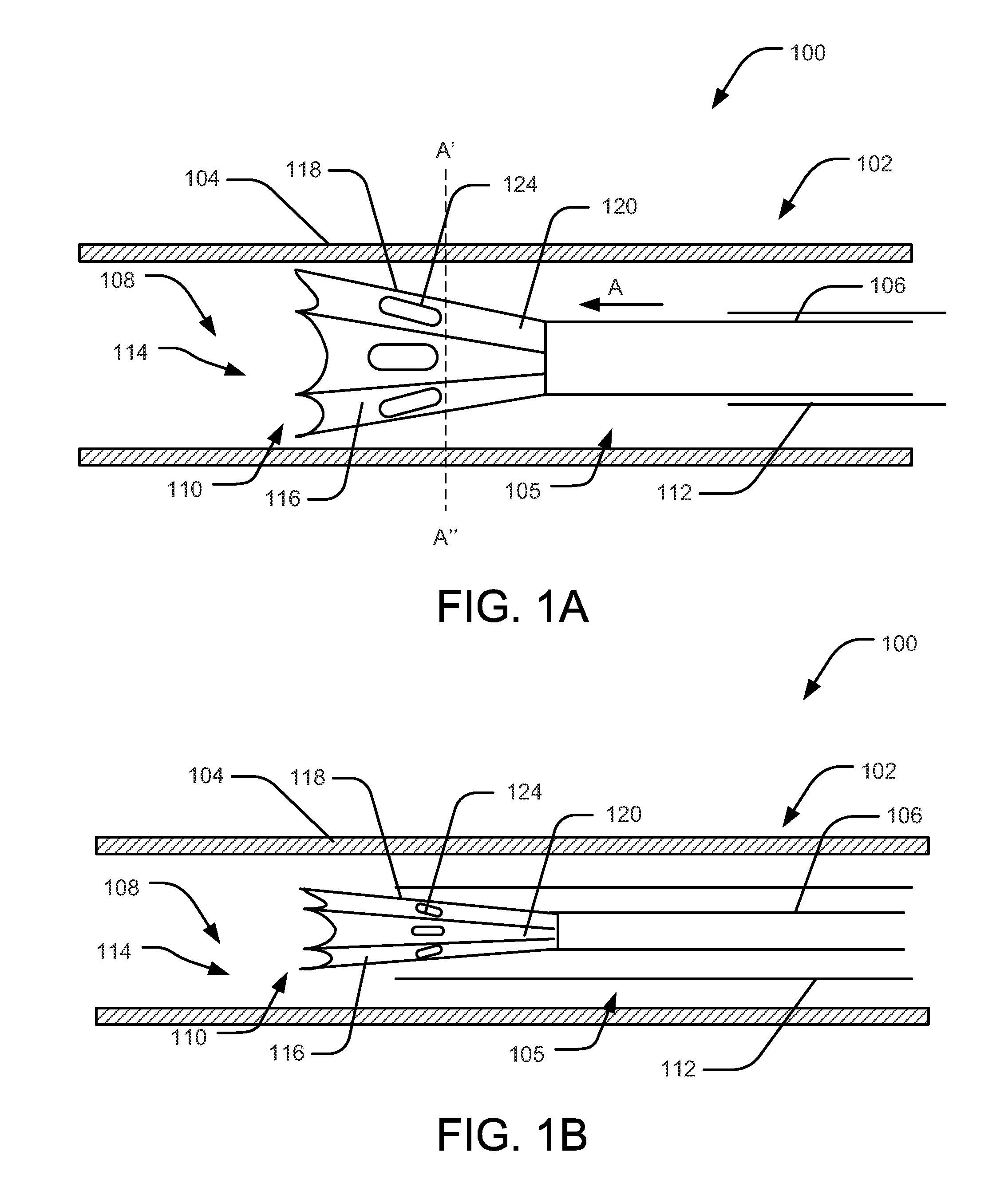
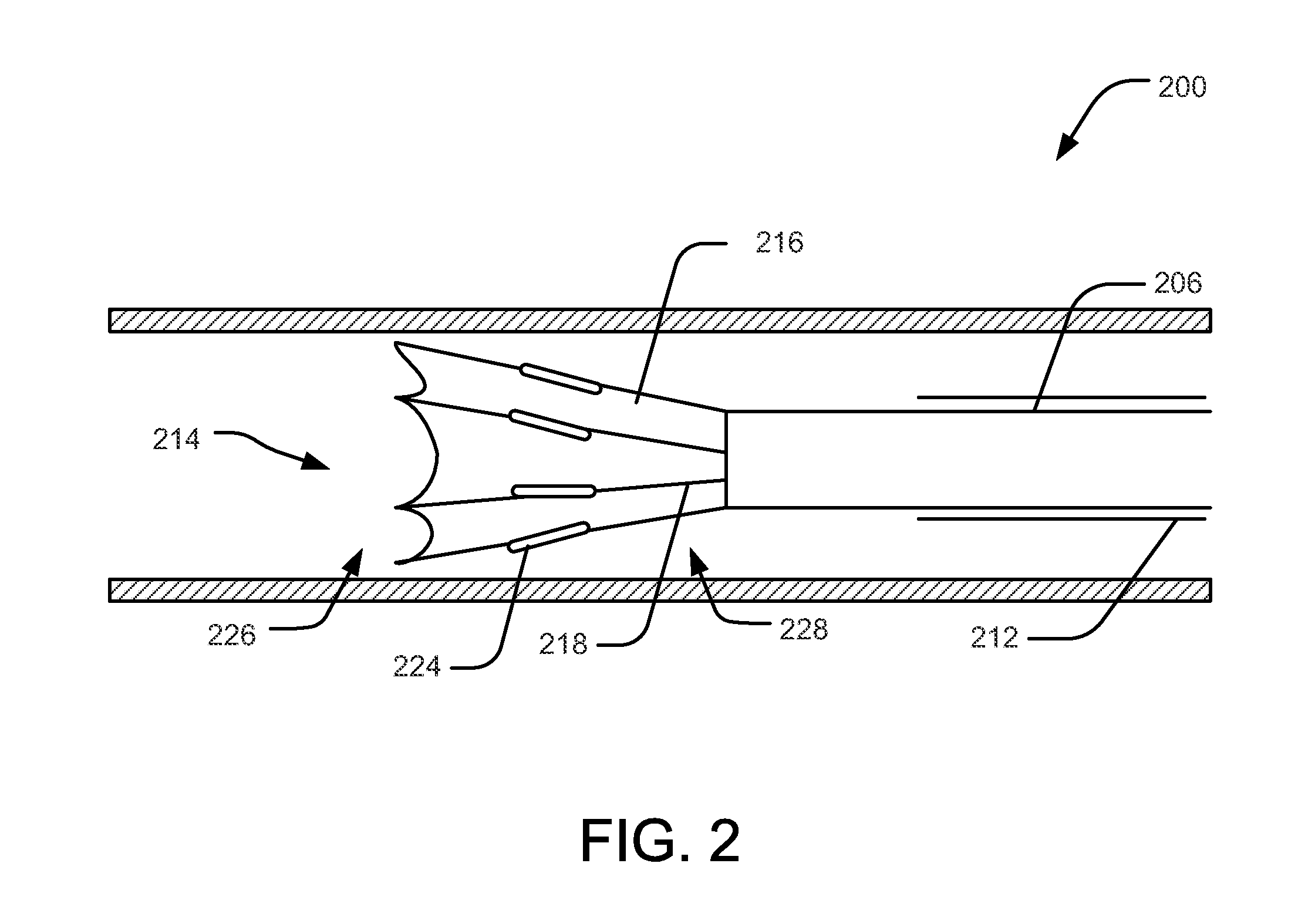
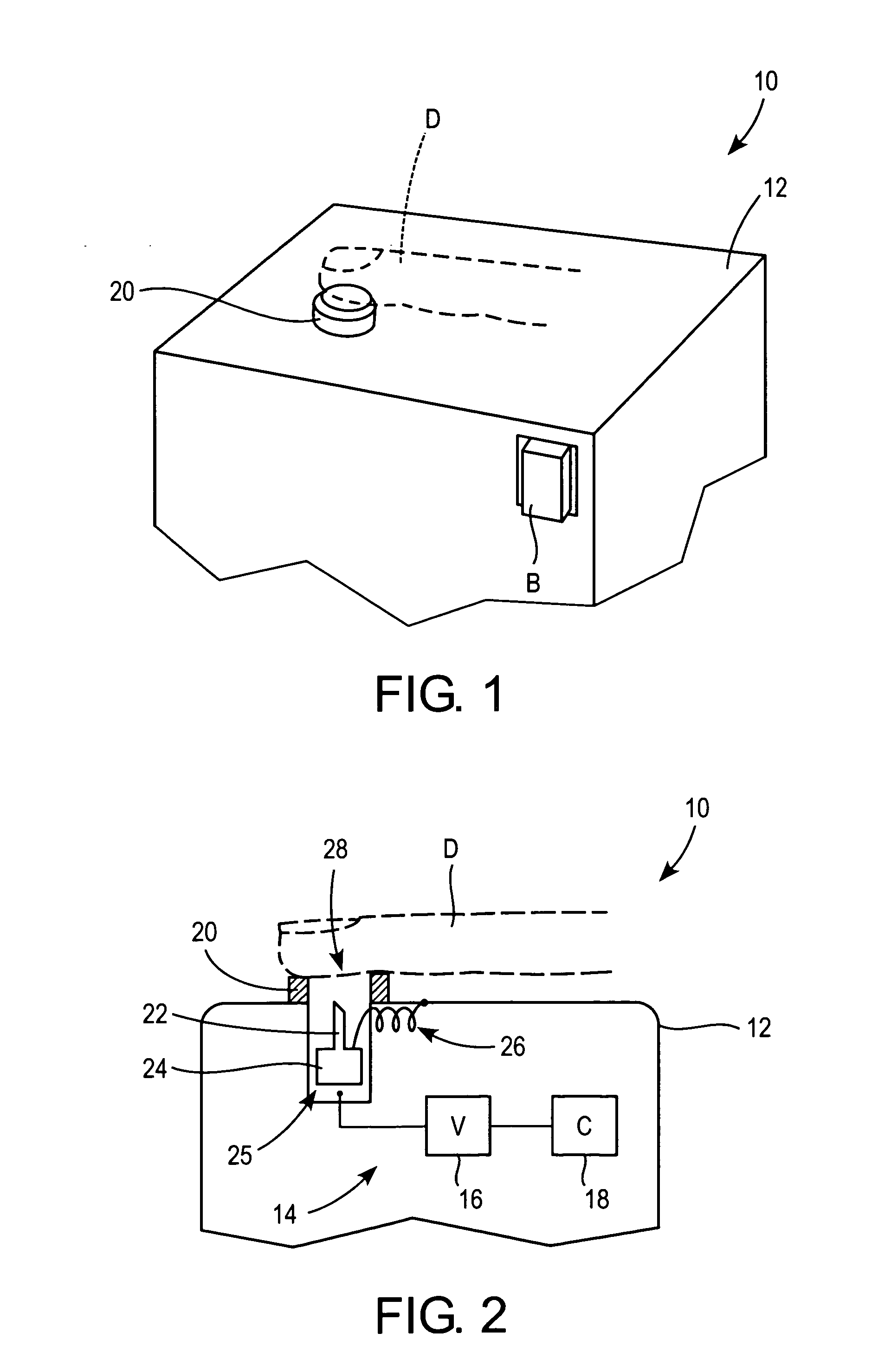
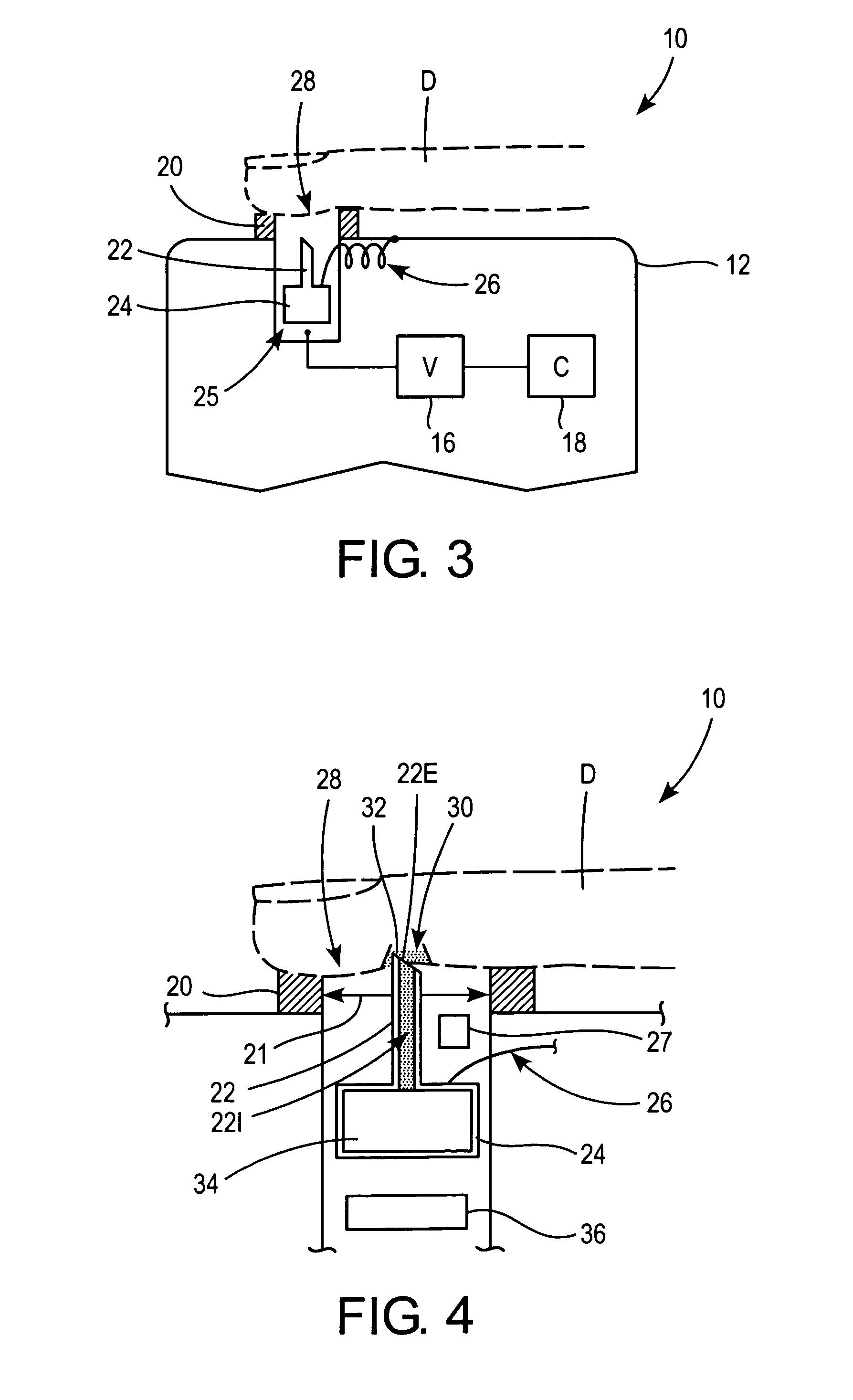
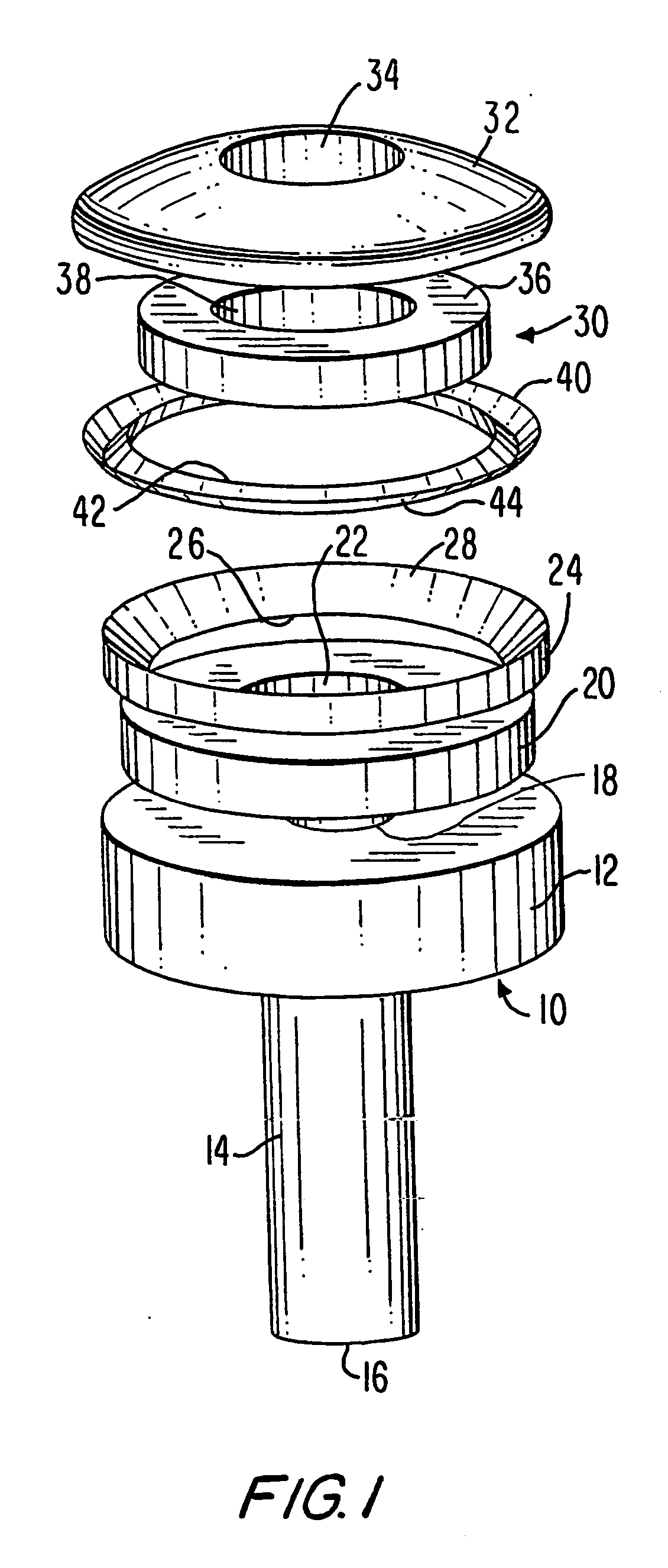
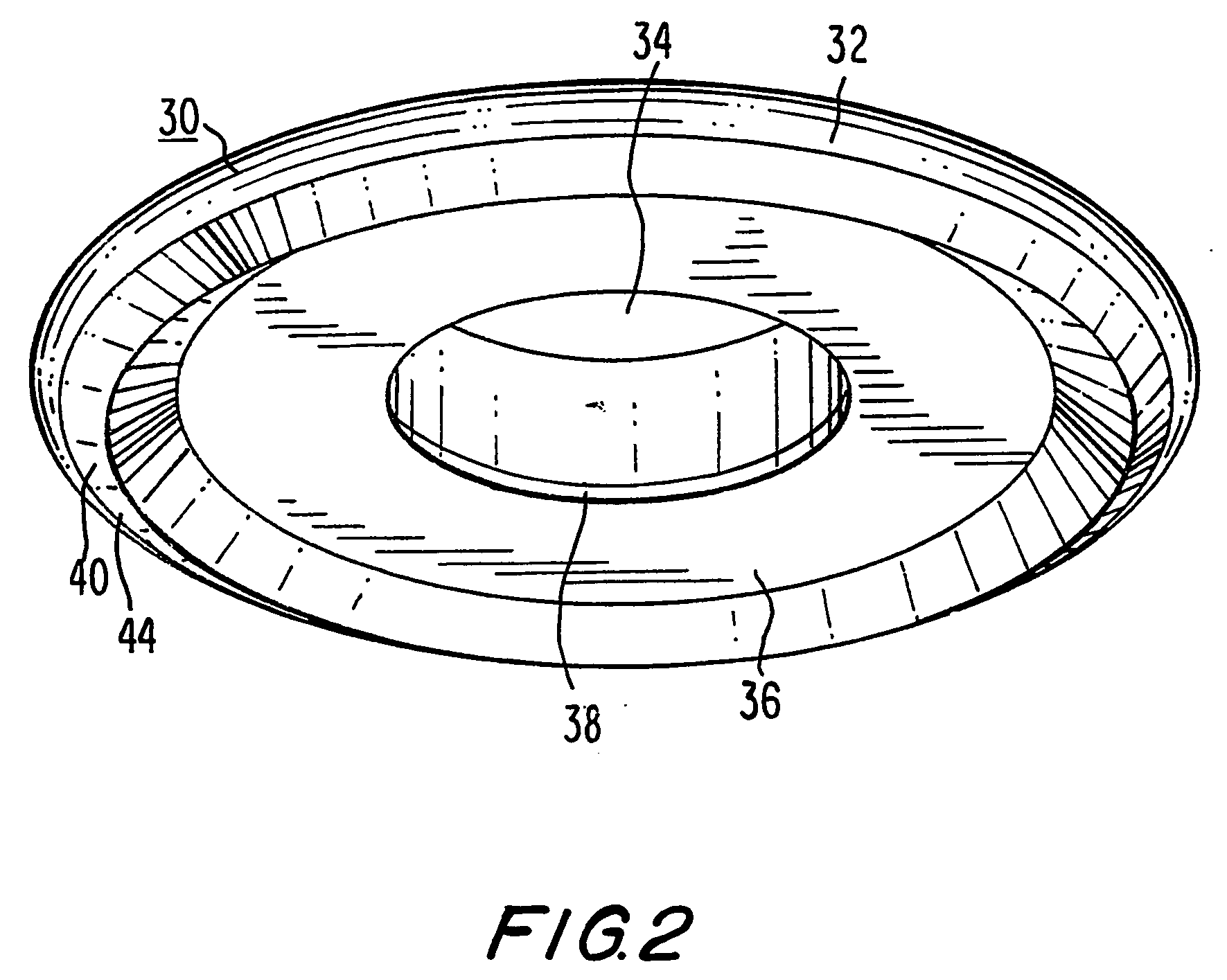
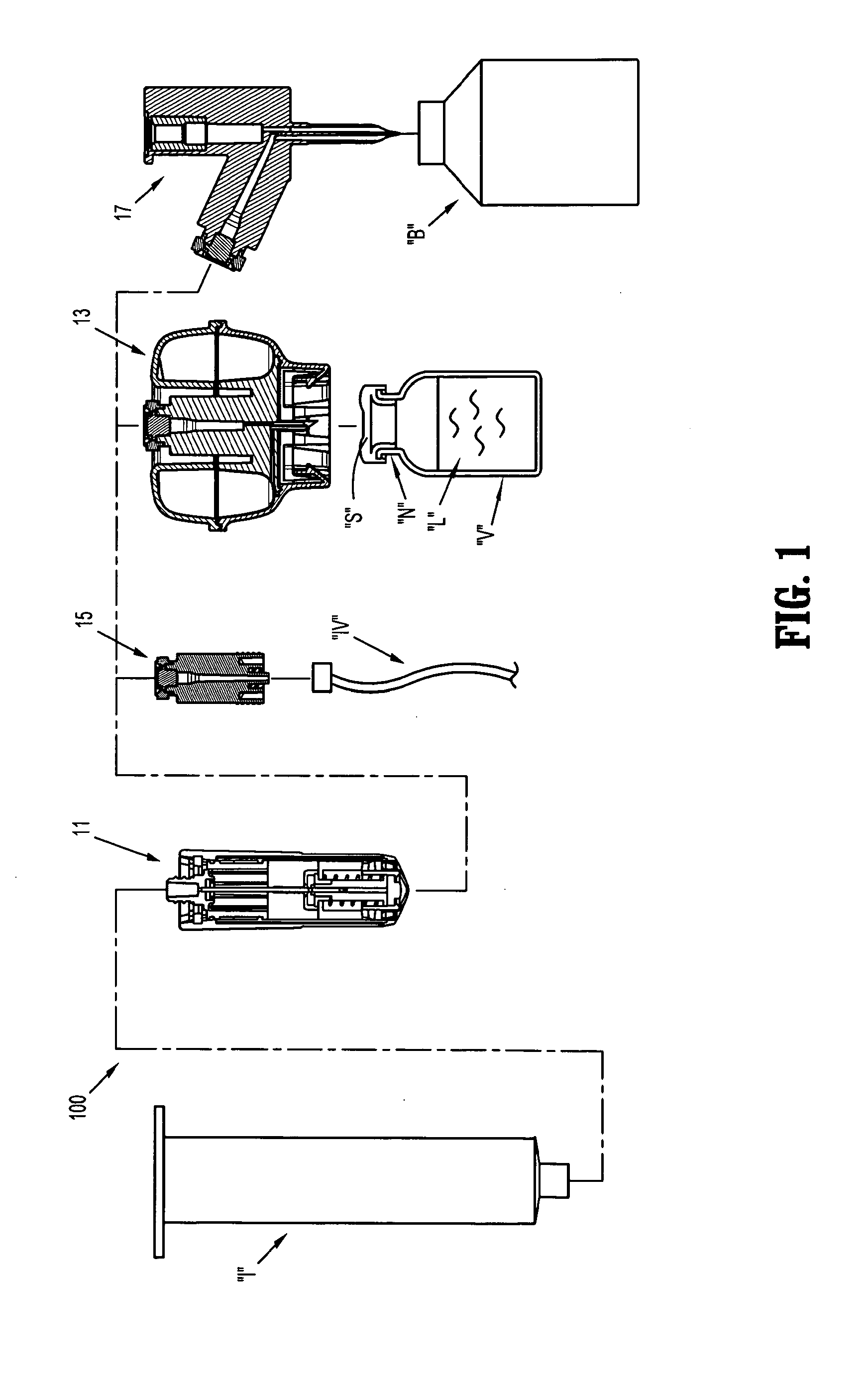
Methods and apparatus for performing transluminal and other procedures
InactiveUS20070135803A1Prevent overflowPrevent leakageSuture equipmentsEar treatmentSurgeryInstrumentation
Owner:INTUITIVE SURGICAL OPERATIONS INC
Circular stapler introducer with multi-lumen sheath
Owner:CILAG GMBH INTERNATIONAL +1
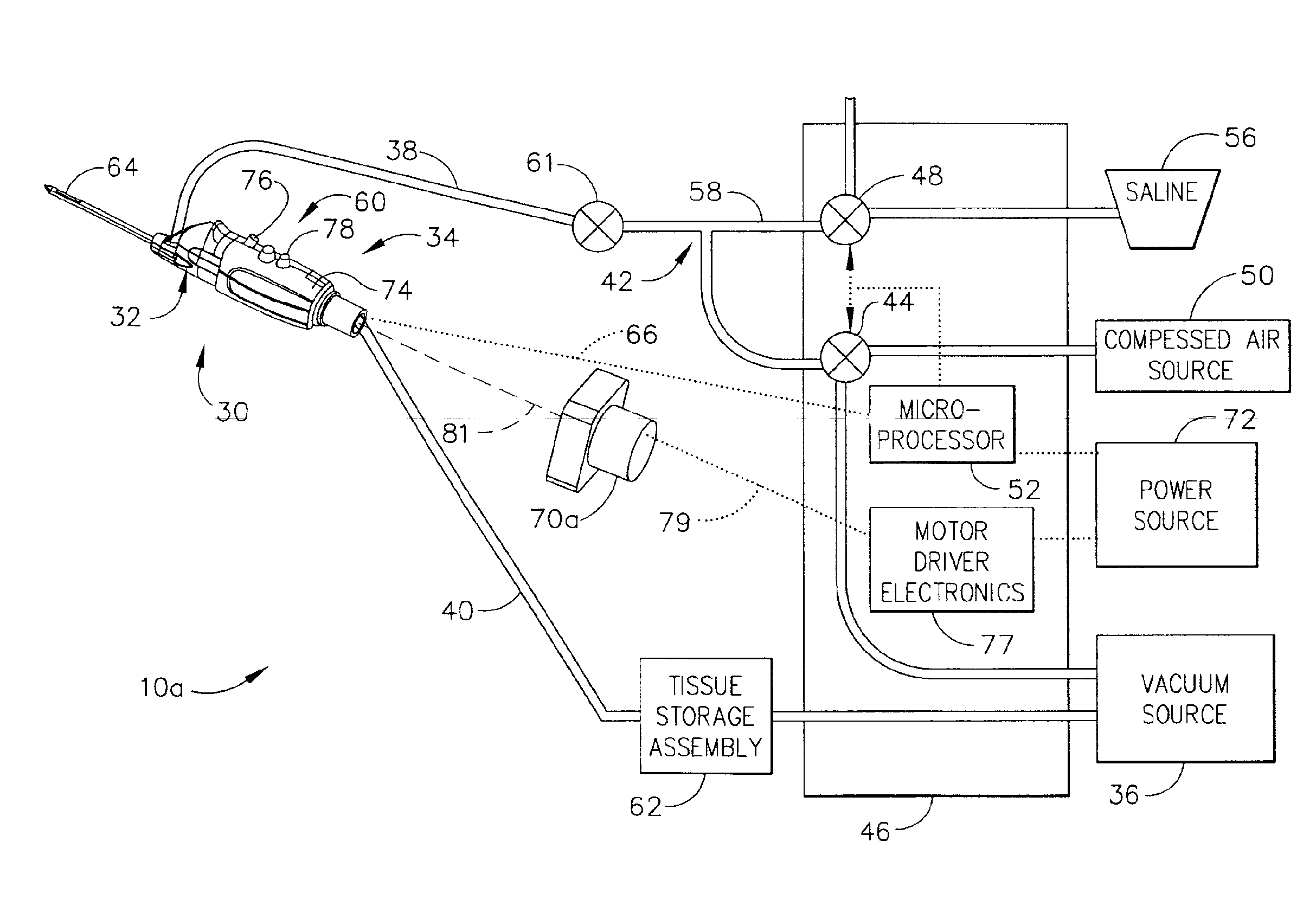
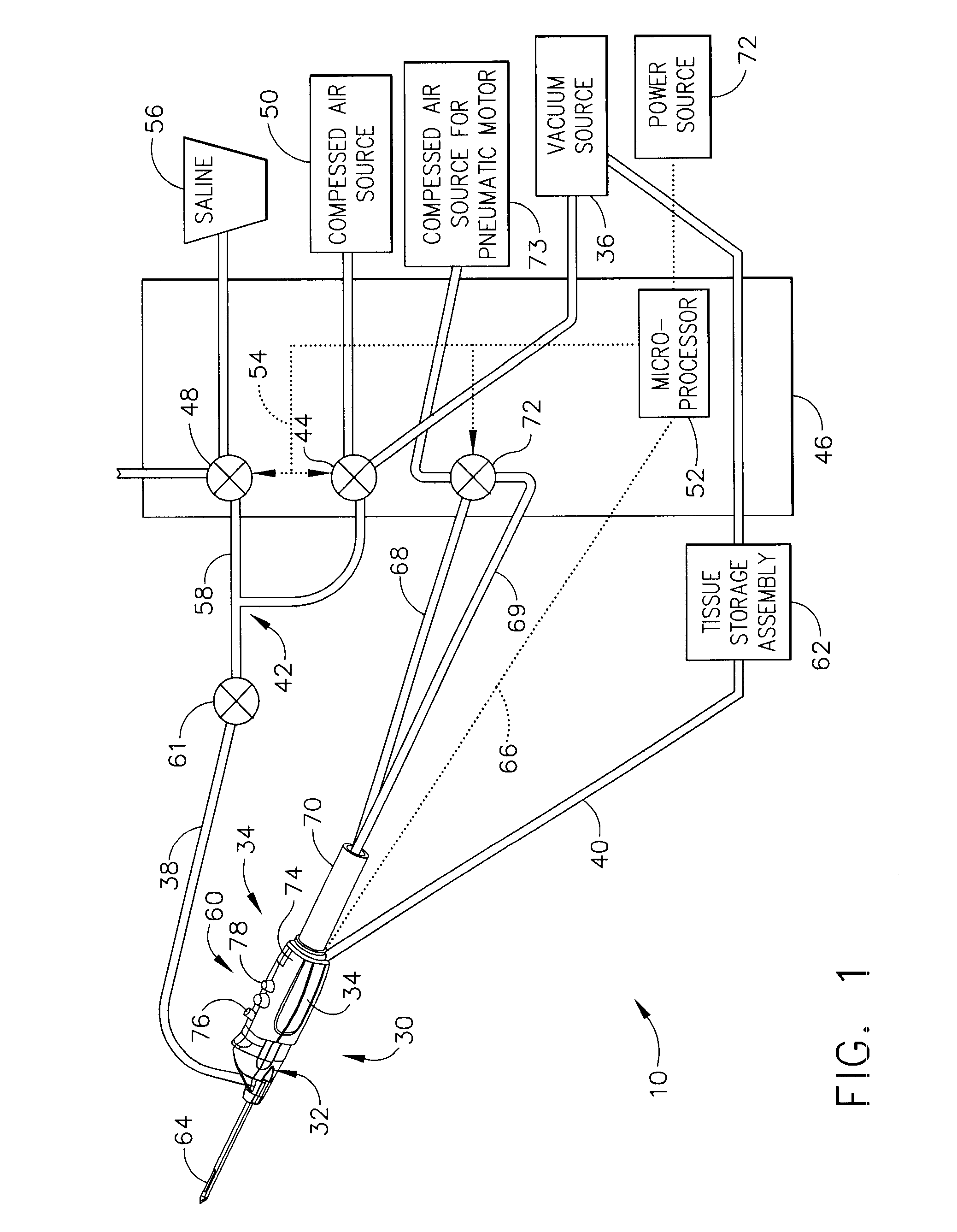
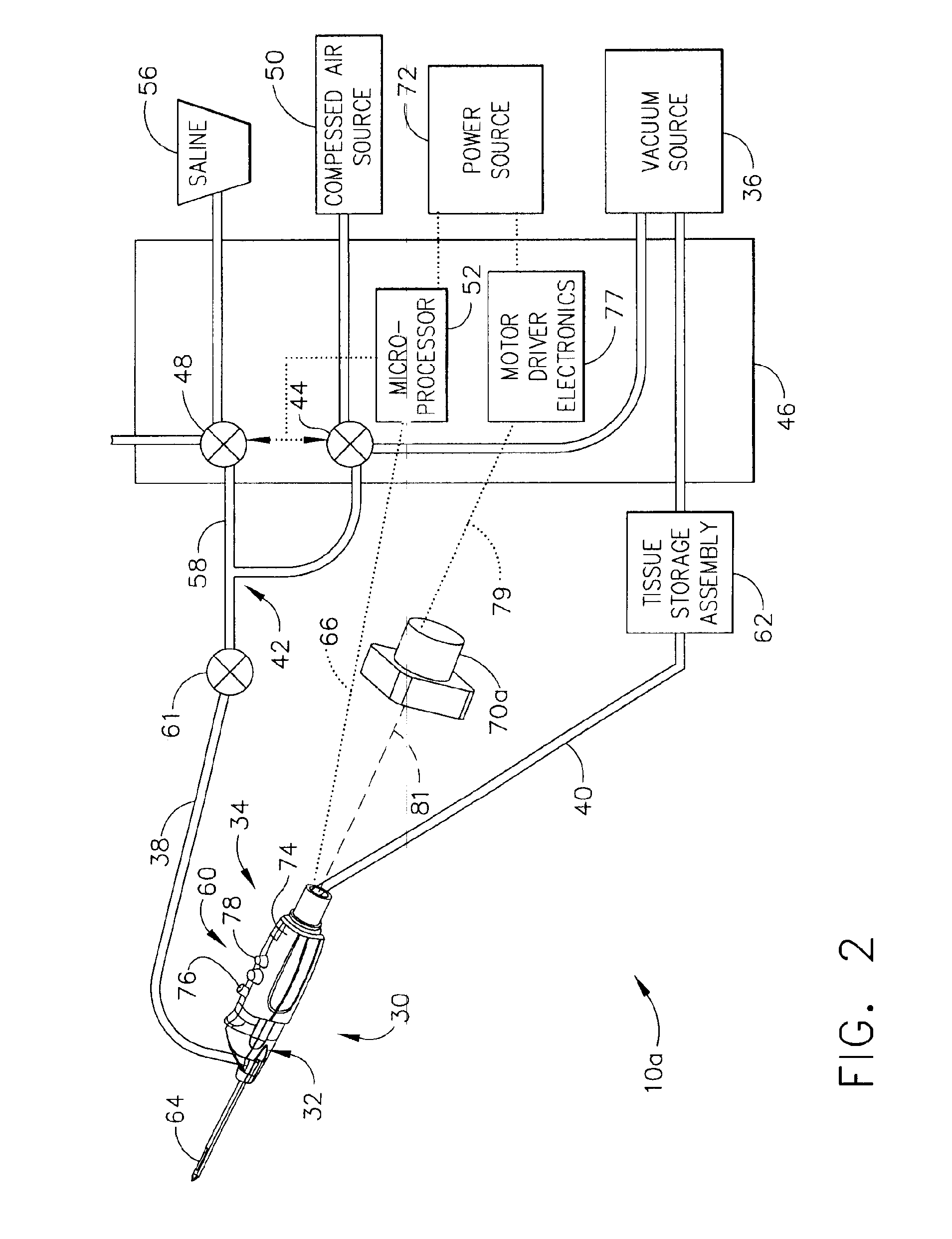
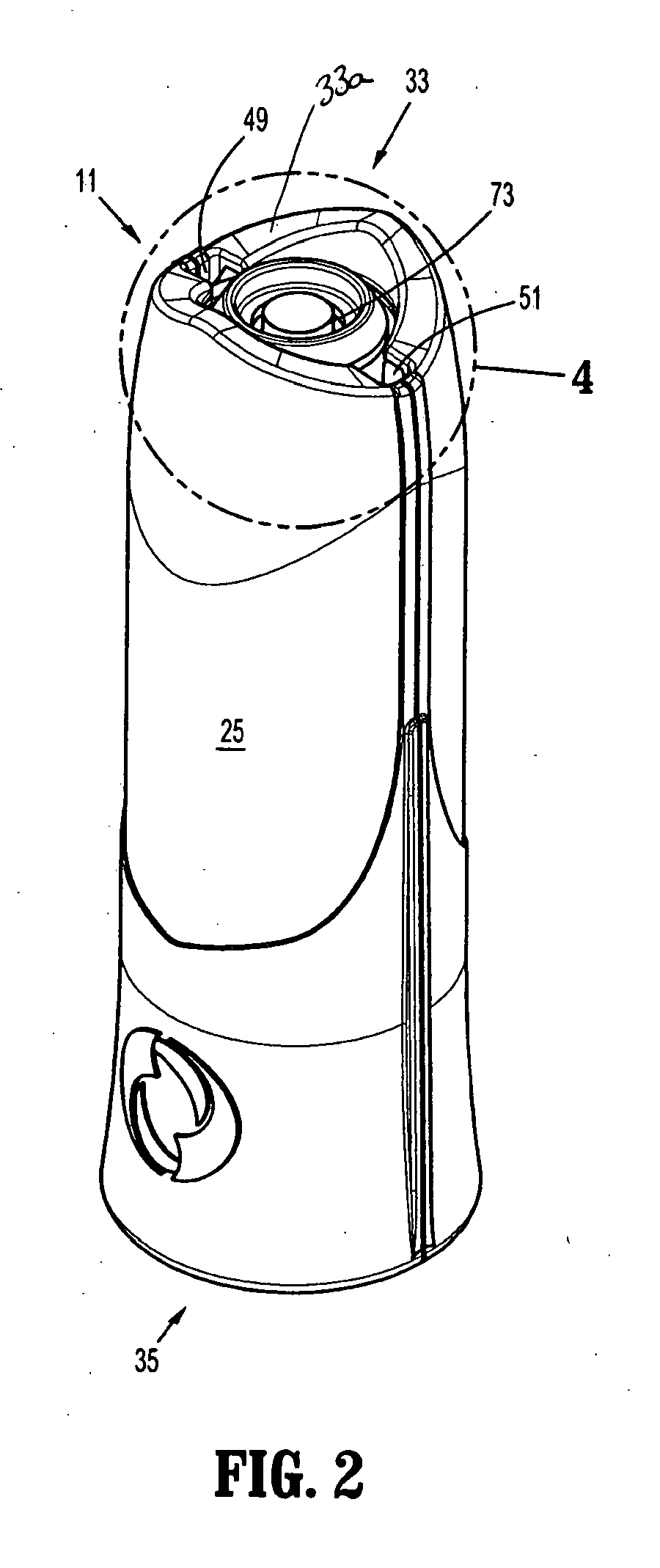
Core sampling biopsy device with short coupled MRI-compatible driver
A core sampling biopsy device is compatible with use in a Magnetic Resonance Imaging (MRI) environment by being driven by either a pneumatic rotary motor or a piezoelectric drive motor. The core sampling biopsy device obtains a tissue sample, such as a breast tissue biopsy sample, for diagnostic or therapeutic purposes. The biopsy device may include an outer cannula having a distal piercing tip, a cutter lumen, a side tissue port communicating with the cutter lumen, and at least one fluid passageway disposed distally of the side tissue port. The inner cutter may be advanced in the cutter lumen past the side tissue port to sever a tissue sample. A cutter drive assembly maintains a fixed gear ratio relationship between a cutter rotation speed and translation speed of the inner cutter regardless of the density of the tissue encountered to yield consistent sample size.
Owner:DEVICOR MEDICAL PROD
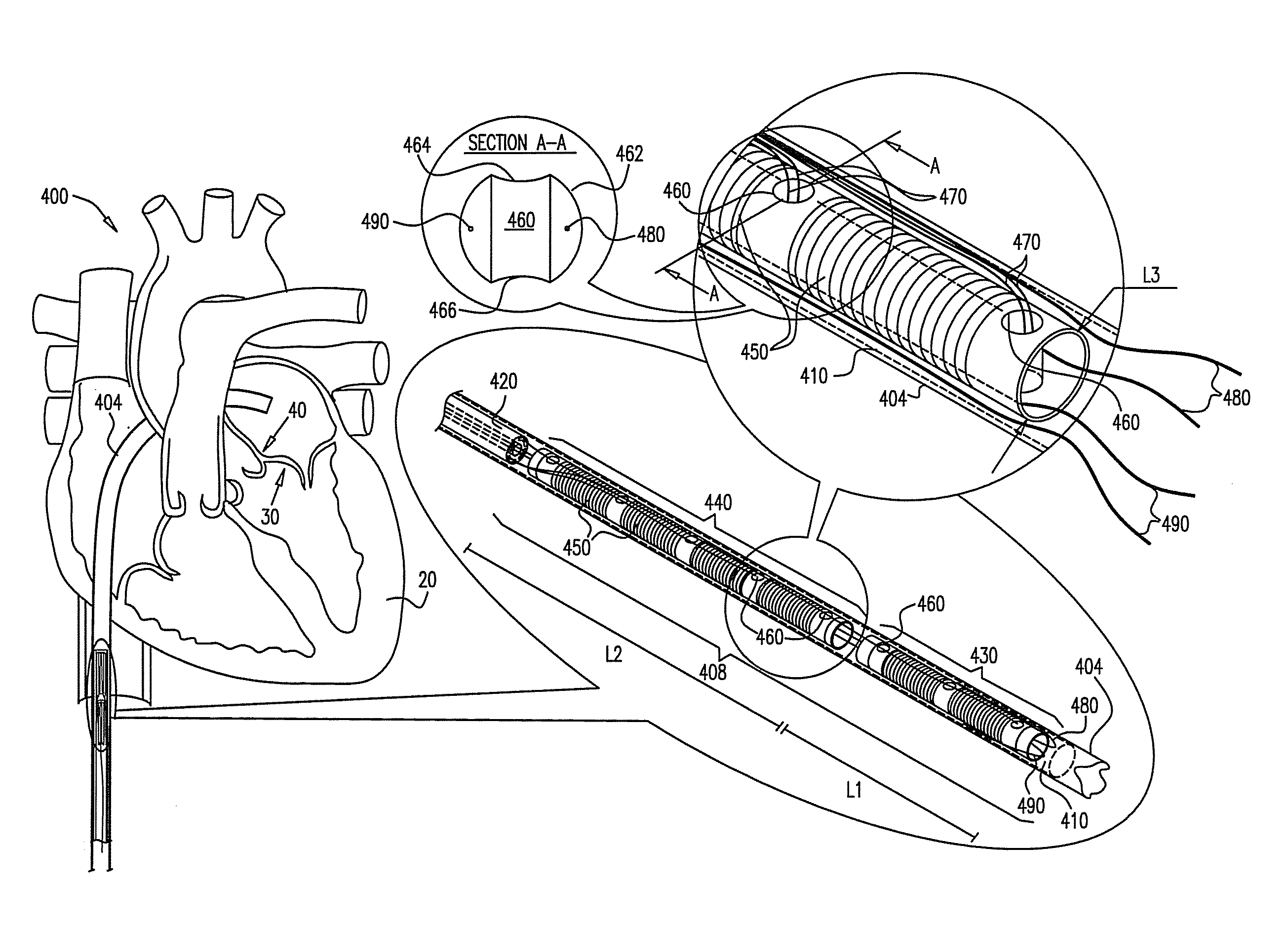
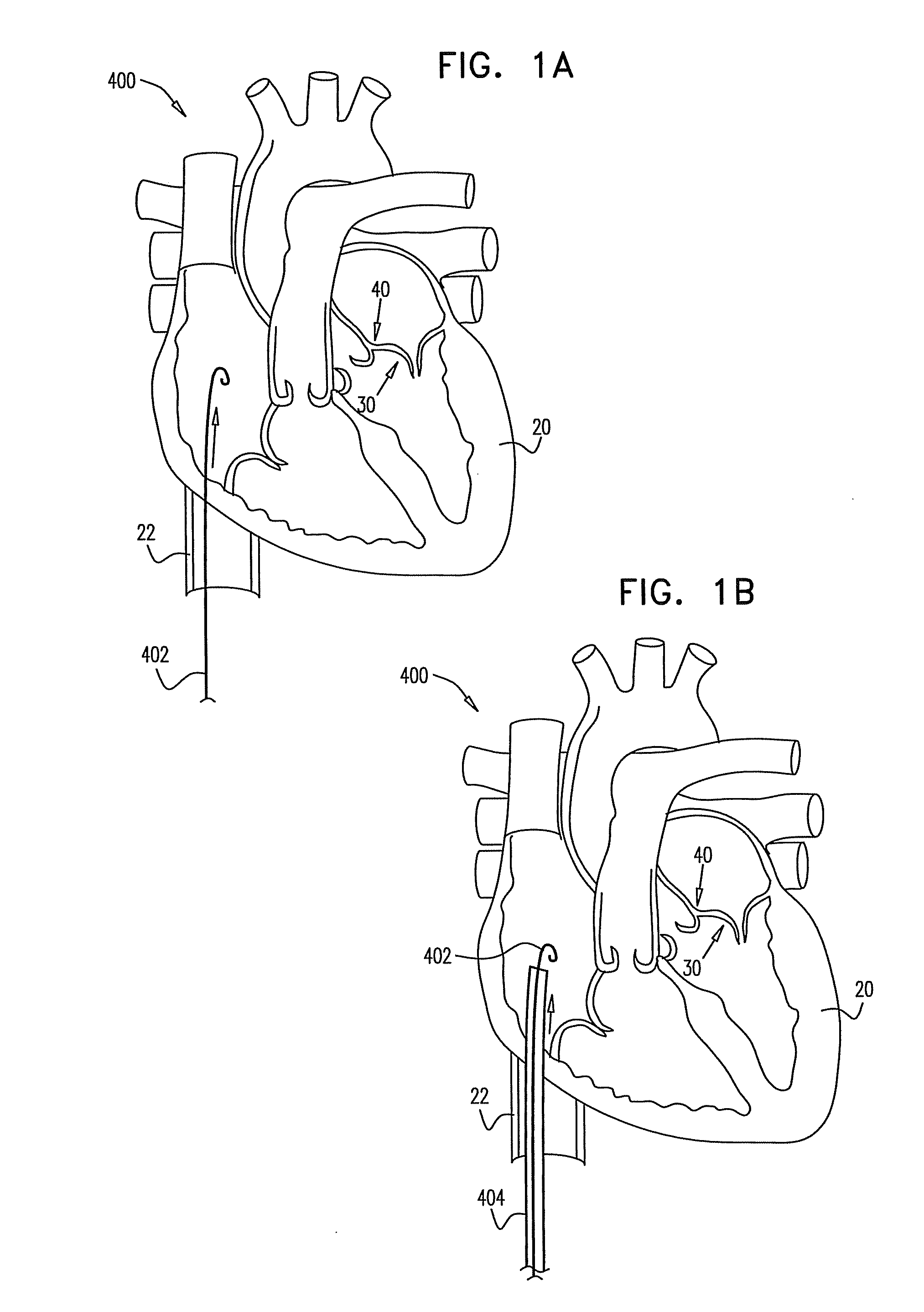
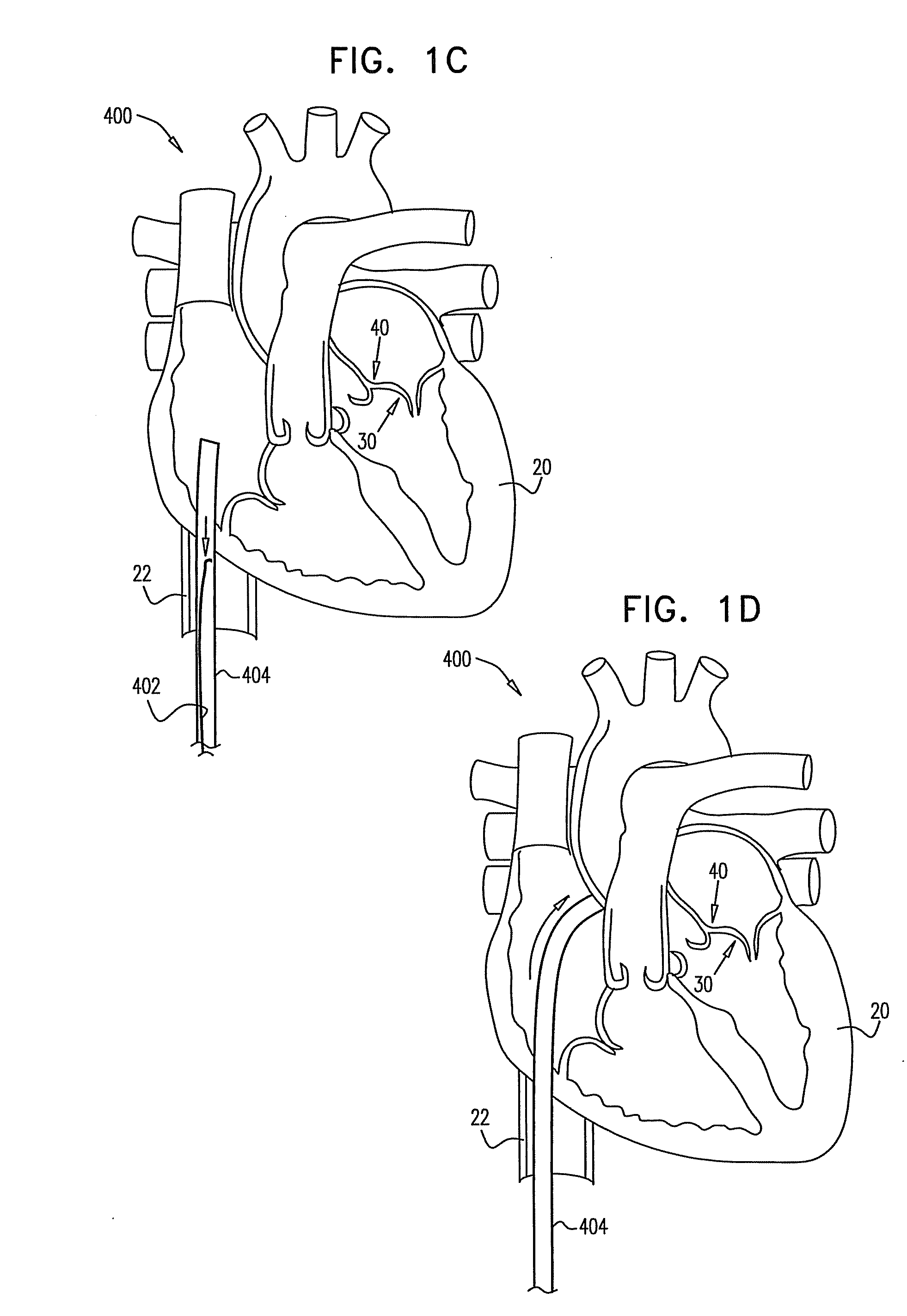
Devices and methods for transluminal or transthoracic interstitial electrode placement
ActiveUS7191015B2Epicardial electrodesTransvascular endocardial electrodesElectrode placementDevice implant
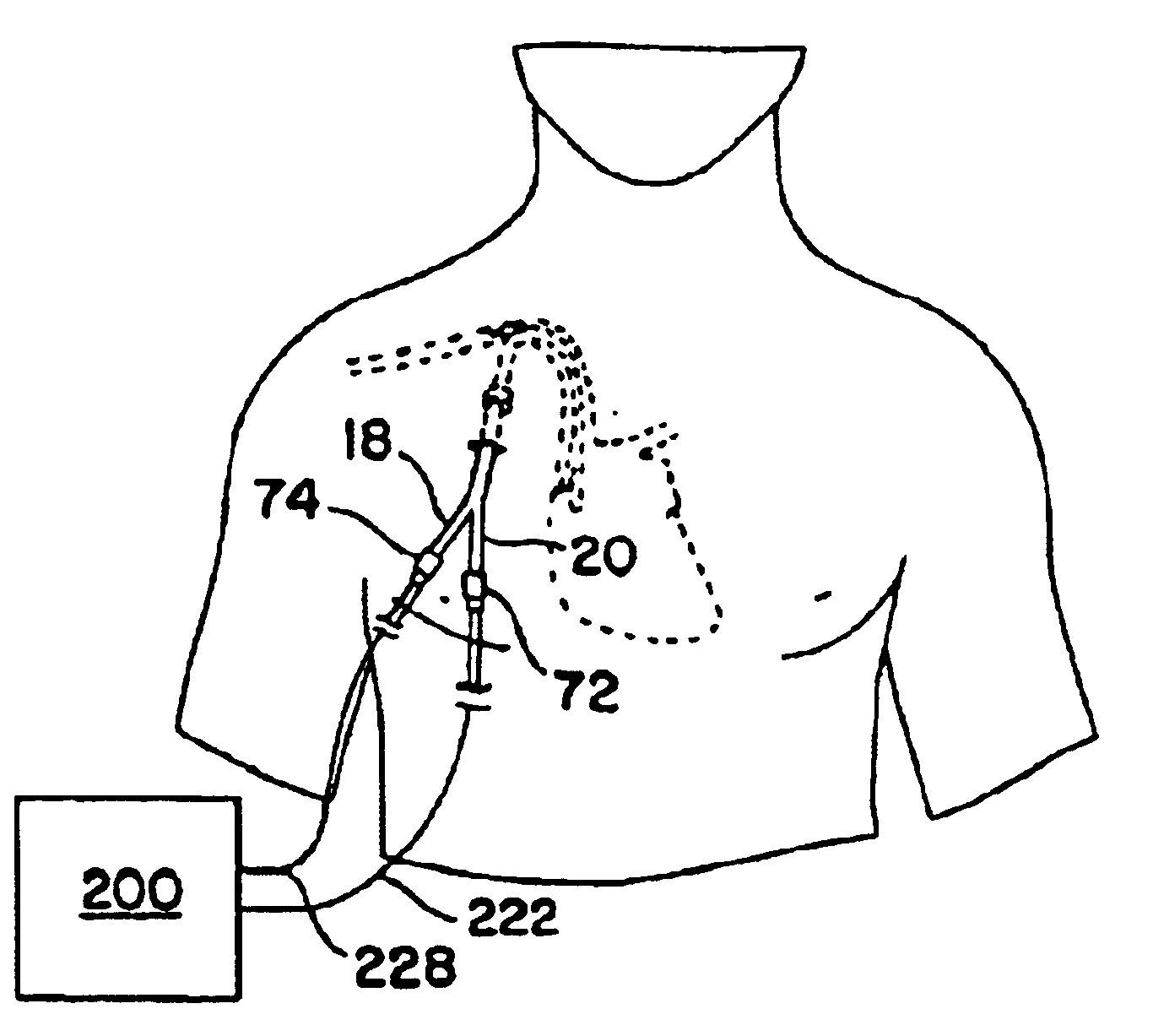
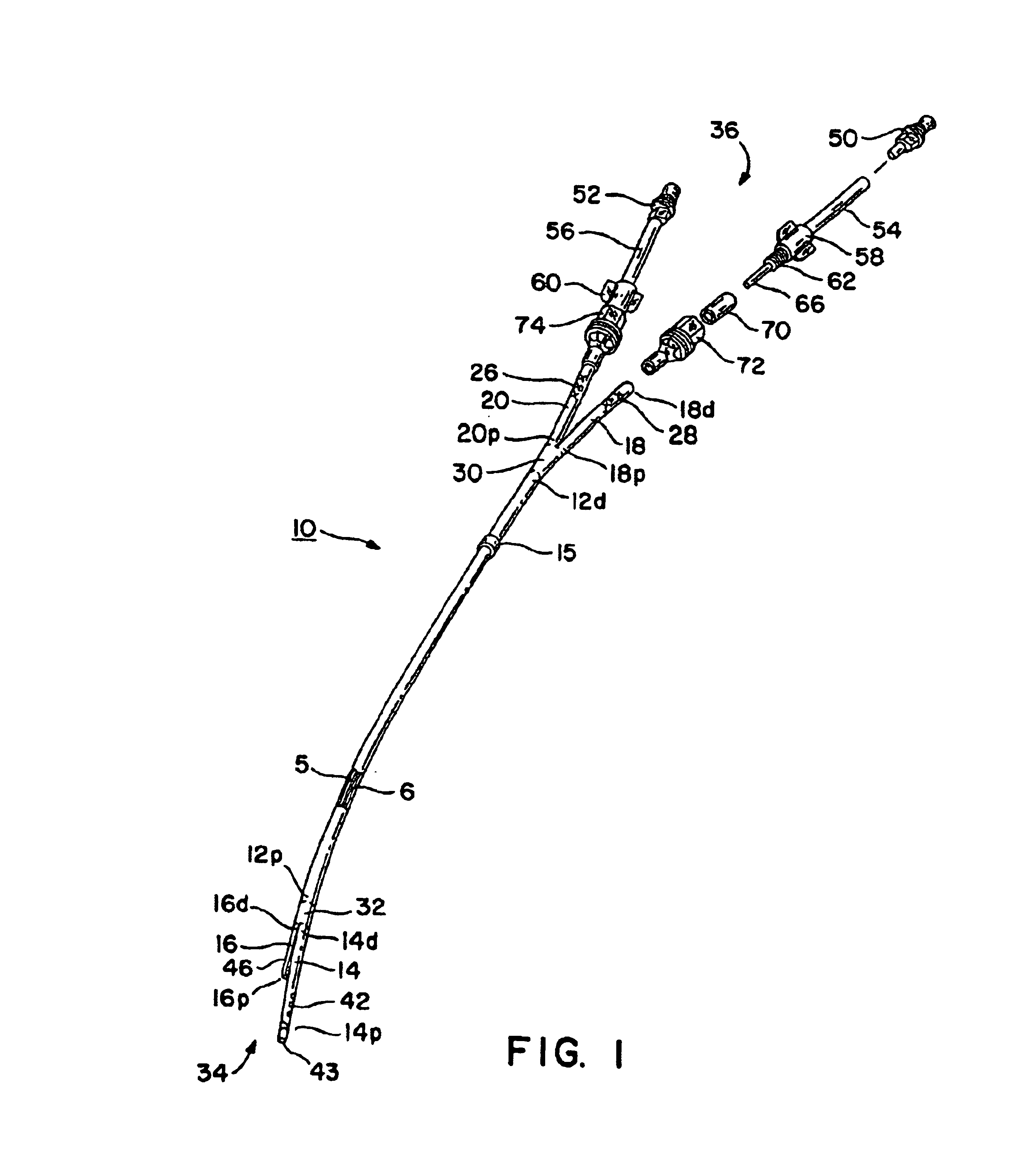
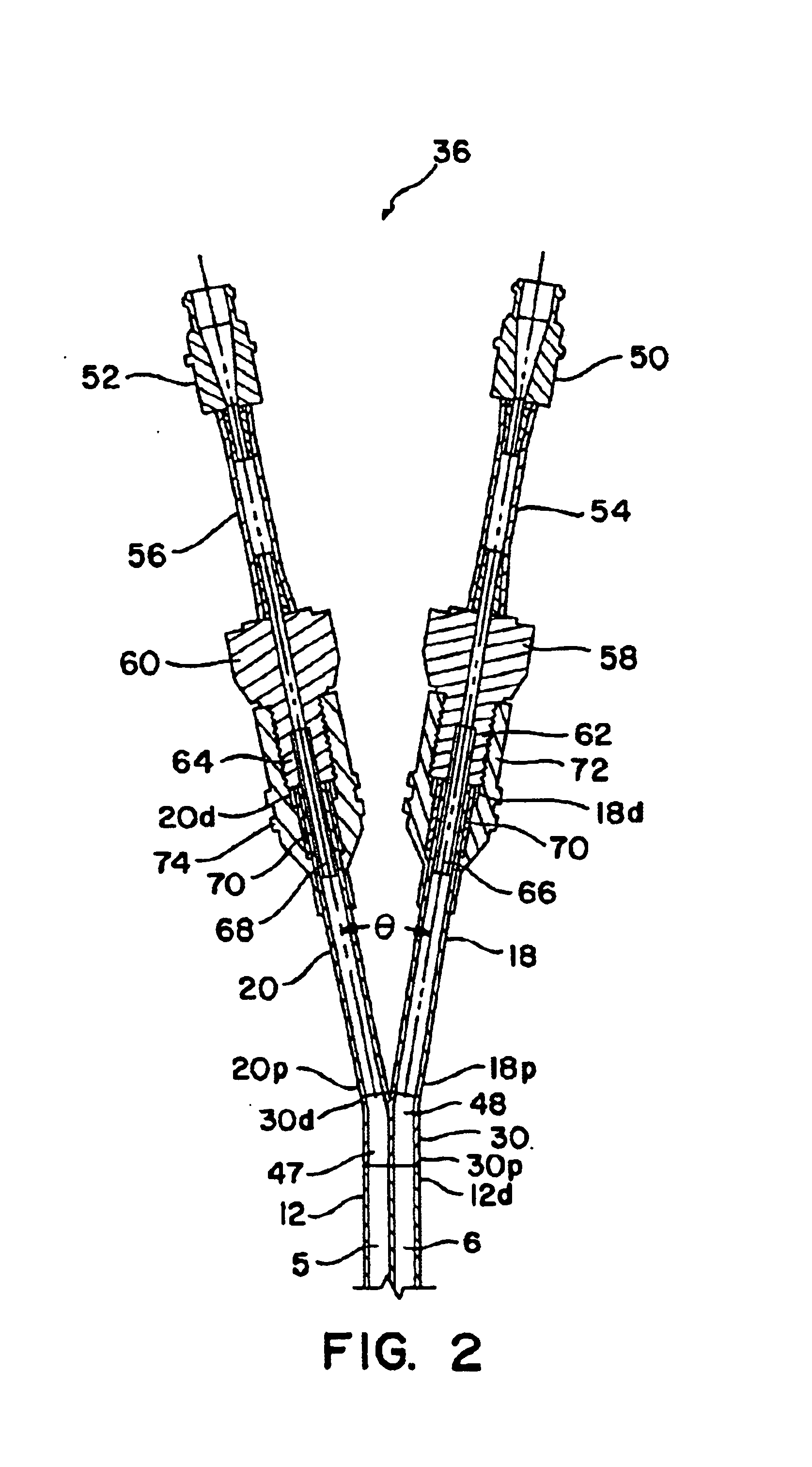
Methods and devices for implanting pacing electrodes or other apparatus, or for delivering substances, to the heart of other tissues within the body. A guided tissue penetrating catheter is inserted into a body lumen (e.g., blood vessel) or into a body cavity or space (e.g., the pericardial space) and a penetrator is advanced from the catheter to a target location. In some embodiments, a substance or an apparatus (such as an electrode) may be delivered through a lumen in the penetrator. In other embodiments, a guidewire may be advanced through the penetrator, the penetrating catheter may then be removed and an apparatus (e.g., electrode) may then be advanced over that guidewire. Also disclosed are various implantable electrodes and electrode anchoring apparatus.
Owner:MEDTRONIC VASCULAR INC
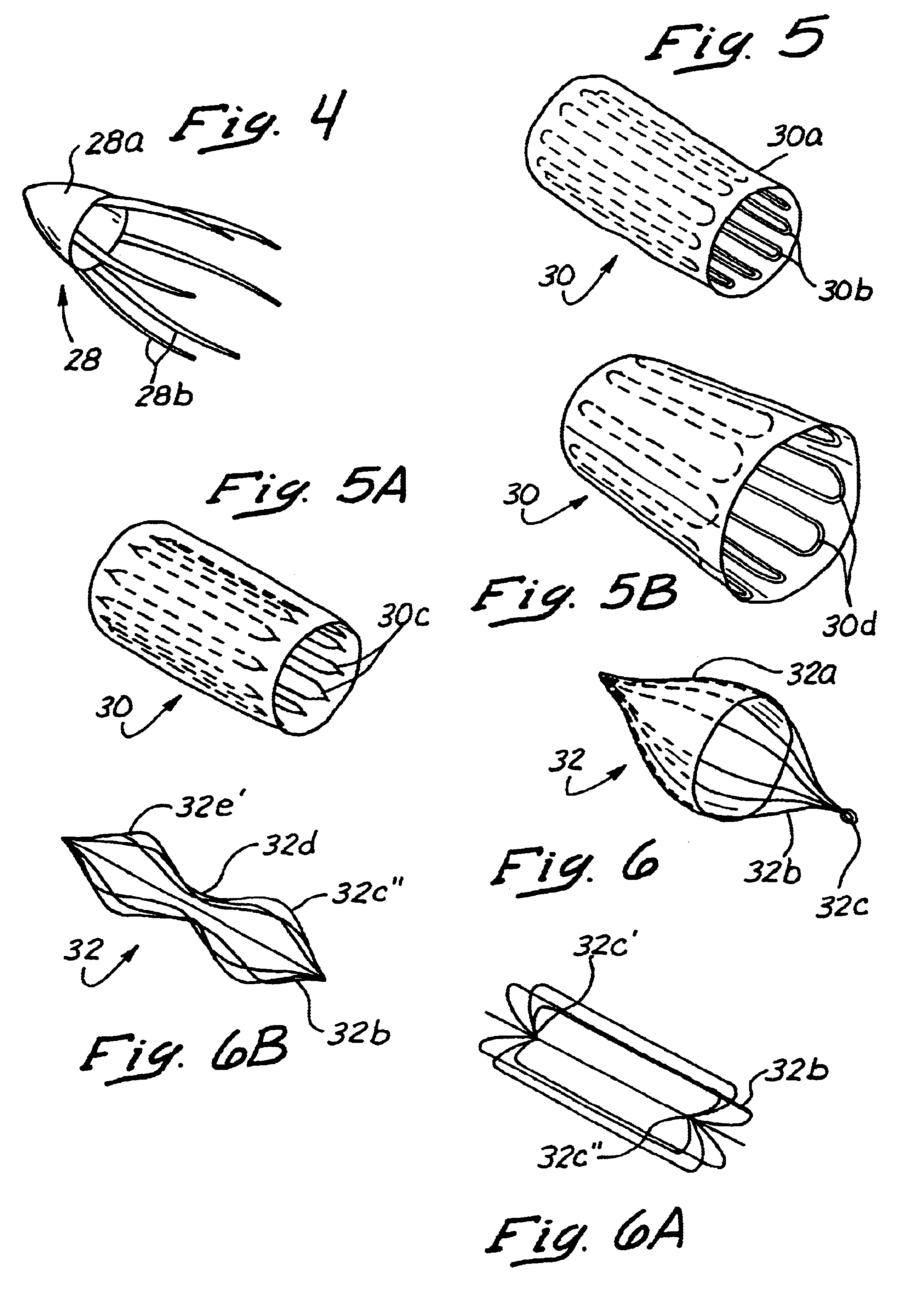
Methods and apparatus for blocking flow through blood vessels
This invention is methods and apparatus for occluding blood flow within a blood vessel (22). In a first series of embodiments, the present invention comprises a plurality of embolic devices (16) deployable through the lumen (12) of a conventional catheter (10) such that when deployed, said embolic devices (16) remain resident and occlude blood flow at a specific site within the lumen of the blood vessel (22). Such embolic devices (16) comprise either mechanical embolic devices that become embedded within or compress against the lumen of the vessel or chemical vaso occlusive agents that seal off blood flow at a given site. A second embodiment of the present invention comprises utilization of a vacuum / cauterizing device capable of sucking in the lumen of the vessel about the device to maintain the vessel in a closed condition where there is then applied a sufficient amount of energy to cause the tissue collapsed about the device to denature into a closure. In a third series of embodiments, the present invention comprises the combination of an embolization facilitator coupled with the application of an energy force to form an intraluminal closure at a specified site within a vessel.
Owner:MEDTRONIC VASCULAR INC
Segmented ring placement
Owner:VALTECH CARDIO LTD
Deflectable catheter assembly and method of making same
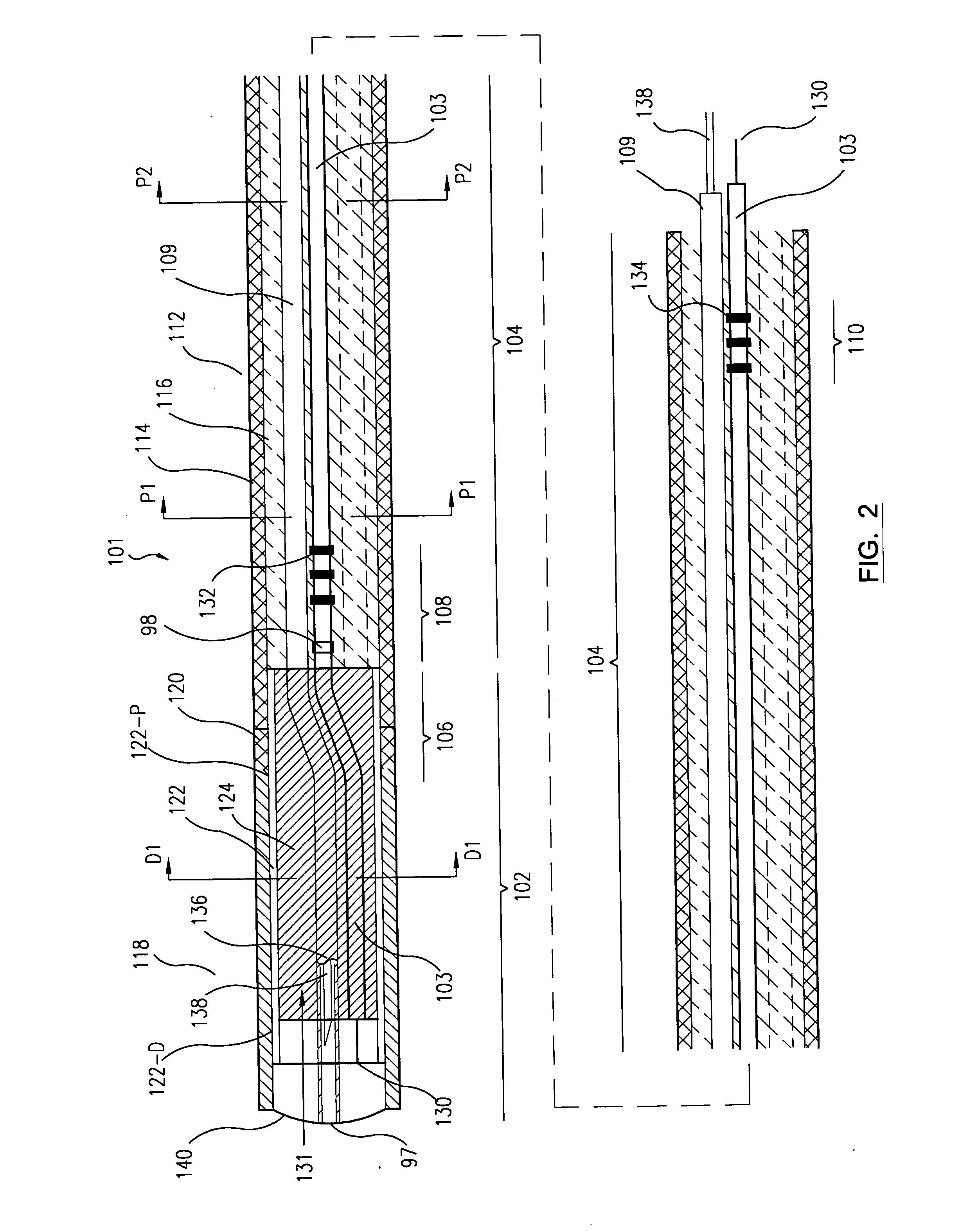
ActiveUS20050070844A1Balance of powerMulti-lumen catheterMedical devicesCatheterBiomedical engineering
A deflectable catheter assembly is disclosed. The assembly comprises a catheter shaft having a catheter proximal section and a catheter distal section, and at least one lumen extending therethrough. The catheter distal section is more flexible than the catheter proximal section. A tendon is disposed within a first lumen of said catheter shaft. The first lumen is approximately centrally located within the catheter shaft at the catheter proximal section. The first lumen is located off-center of the catheter shaft at the catheter distal section. The tendon is able to deflect the catheter distal section when being pulled on. A catheter handle is coupled to the catheter shaft at the catheter proximal section, the catheter handle includes a control mechanism to control the tendon.
Owner:ABBOTT CARDIOVASCULAR
Biopsy device with selectable tissue receiving aperture orientation and site illumination
The invention is directed to a system and device for separating and collecting a tissue specimen from a target site within a patient. The device includes a probe component with an elongated tubular section, a penetrating distal tip and a tissue receiving aperture in the distal end of the tubular section proximal to the distal tip, and a tissue cutting member which is slidably disposed within the probe member to cut a tissue specimen drawn into the interior of the device through the aperture by applying a vacuum to the inner lumen of the tissue cutting member. The device also has a driver component to which the probe component is releasably secured. The driver has a drive member for adjusting the orientation of the tubular section and thus the aperture therein and one or more drive members for moving the tissue cutting member within the tubular section to sever a tissue specimen from tissue extending into the interior of the tubular section through the aperture. The motion imparted to the tissue cutter is at least longitudinal and preferably is also oscillation and / or rotational to effectively separate a tissue specimen from tissue extending through the aperture in the tubular section.
Owner:SENORX
Ported iv catheter having external needle shield and internal blood control septum
An extravascular system is provided which includes a catheter adapter having a blood control septum configured to control flow of a fluid through the catheter adapter, the catheter adapter further having a catheter configured for intravenous insertion. The extravascular system further includes a septum activator slidably inserted within the catheter adapter and configured for advancement through the blood control septum to provide a fluid pathway through the blood control septum. Further still, the extravascular system includes an external safety mechanism comprising a needle hub and a needle shield interconnected via a tether, wherein the needle shield includes a safety clip that is configured to retain a sharpened end of the introducer needle within the needle shield. Some implementations further comprise an access port forming a portion of the catheter adapter and providing selective access to the lumen of the catheter adapter.
Owner:BECTON DICKINSON & CO
Stent system for preventing restenosis
A system for treating a body lumen is disclosed. The system comprises an outer stent and an inner stent disposed within the lumen of the outer stent. At least one end of the inner stent extends outside of the lumen of the outer stent, so that the end of the inner stent contacts and conforms to the body lumen wall that is adjacent the end of the outer stent. A coating can be disposed on a surface, preferably the outer surface, of the inner stent. The coating contains a therapeutic substance that may be released into the body lumen wall to help in preventing restenosis. Also disclosed is a stent having a balloon-expandable portion connected to a self-expanding portion. Methods for deploying the system and the stent are also disclosed.
Owner:BOSTON SCI SCIMED INC
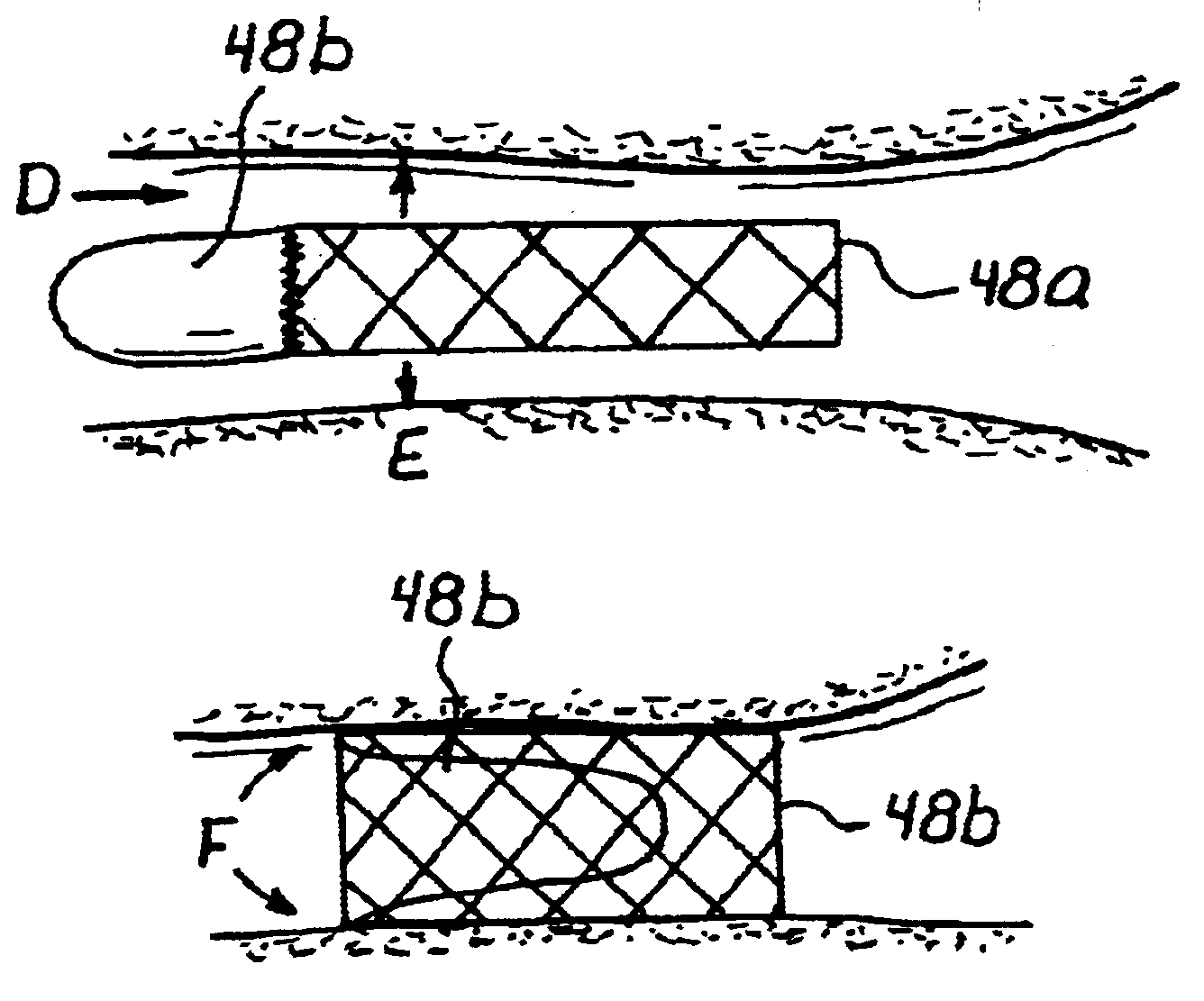
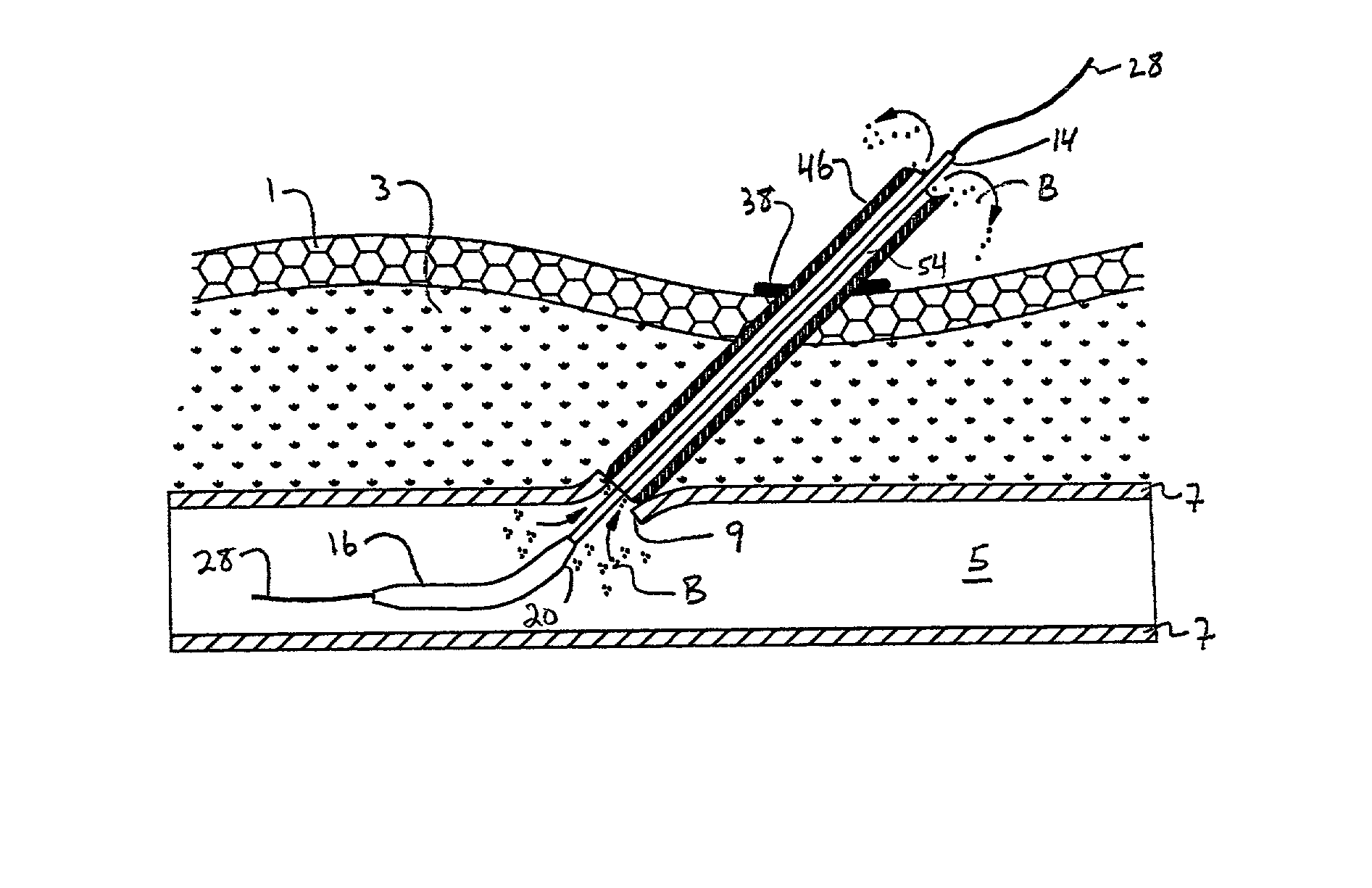
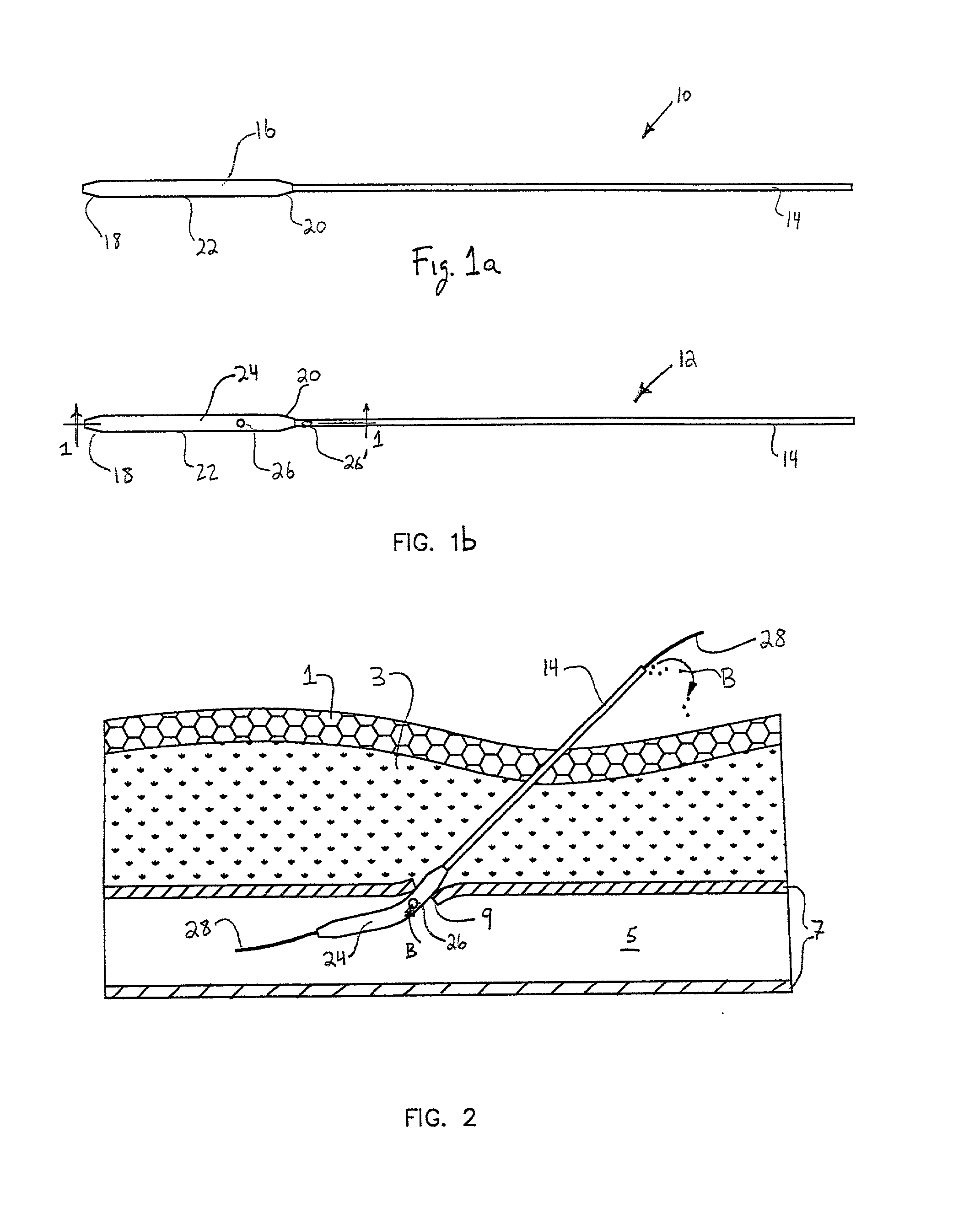
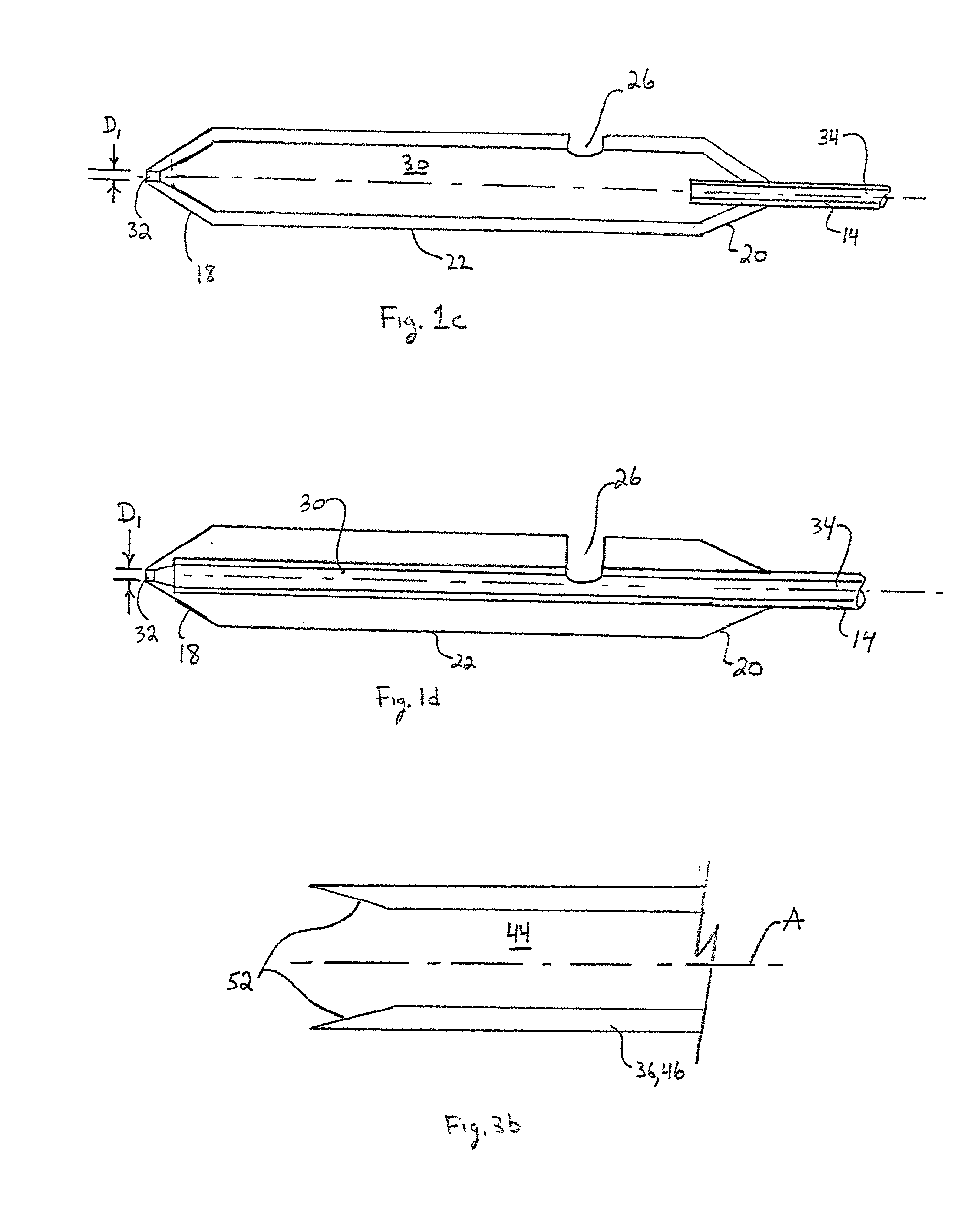
Transluminal methods and devices for closing, forming attachments to, and/or forming anastomotic junctions in, luminal anatomical structures
InactiveUS7056325B1Increase engagementImprove gripSuture equipmentsSurgical needlesAnatomical structuresAnatomical conduit
Methods and apparatus for passing attachment apparatus (e.g., connector devices, staples, etc.) or connector material (e.g., suture thread, wire, cord, filament, monofilament, etc.) into or through the wall of a luminal anatomical structure (e.g., a blood vessel or other anatomical conduit) for the purpose of; i) closing the lumen of the anatomical structure, ii) forming an anastomotic junction between separate anatomical structures (or between approximated segments of the same anatomical structure), and / or iii) attaching an article (e.g., an endoluminal, extraluminal or transluminal graft) or other apparatus to the wall of the anatomical structure.
Owner:MEDTRONIC VASCULAR INC
Catheher system having connectable distal and proximal portions
InactiveUS6050949AEnhanced hoop strengthBending stiffnessUltrasonic/sonic/infrasonic diagnosticsSurgeryPlastic materialsUltrasonic imaging
A vascular catheter system comprises a catheter body having a proximal and distal portion and a single common lumen therebetween. The catheter body includes a first connector secured to the distal end of the proximal portion and a second connector secured to the proximal end of the distal portion. The connectors can be selectively connected to each other to join the lumens of the proximal and distal portions together in a continuous, axially fixed relationship. Disposed within the lumens, when the proximal and distal portions are joined together, is a drive cable. The drive cable may be movably, rotatable about its own longitudinal axis and carries at its distal end, a work element, which is typically an ultrasonic imaging transducer or interventional device. The lumen carrying the cable will be sufficiently large along a proximal portion to permit preferential collapse of the cable should rotation of the distal end become impeded. The catheter body may be made of a polymer or plastic material, such as polyetheretherketone (PEEK), which provides adequate bonding stiffness and resistance to kinking from large hoop stresses.
Owner:BOSTON SCI SCIMED INC
Double-y-shaped multi-lumen catheter with selectively attachable hubs
A multi-lumen catheter and method for inserting same in a patient is disclosed. The catheter includes an elongated, central, multi-lumen tube portion having a proximal end and a distal end. The central tube portion has a substantially cylindrical outer shape and is internally segmented into a plurality of lumens. A distal branch portion includes a plurality of single-lumen distal extension tubes. A proximal branch portion includes a plurality of single-lumen proximal extension tubes. Each proximal extension tube has a distal first end and a proximal second end. The distal first end of each proximal extension tube is connected to the proximal end of the central tube portion such that the single lumen of each distal extension tube is in fluid communication with one of the plurality of lumens of the central tube portion. Each lumen of the central tube portion and the lumens of the distal and proximal extension tubes in fluid communication therewith define a flow path through the catheter. Selectively attachable hub connectors are provided for selective attachment to the distal extension tubes and connection of the catheter to a fluid exchange device.
Owner:ARROW INT INC
Sheath Capture Device for Stent Graft Delivery System and Method for Operating Same
InactiveUS20080264102A1Reduce the possibilityIncreases blood-tight vascular connectionStentsJewelleryStent graftingExternal catheter
A delivery system for delivering and deploying stent grafts having a proximal stent includes a first lumen and a stent capture device including a capture portion fixedly connected adjacent a first lumen distal end. An outer catheter has a catheter distal end and a catheter inner diameter. A second lumen having a second distal end is slidably disposed about the first lumen and within the outer catheter. A stent graft sheath has a sheath proximal end connected to the second distal end and disposed about the first lumen. The sheath has a sheath distal end and a sheath inner diameter greater than the catheter inner diameter for holding a compressed stent graft. A distal nose cone has a cone proximal end connected to either the capture portion or the first distal end. The nose cone and the capture portion are movably adjustable to selectively capture the sheath distal end therebetween.
Owner:BOLTON MEDICAL INC
Thrombectomy System and Method
Disclosed is a clot and foreign body removal system, including a catheter with at least one lumen. Located within the catheter is a clot capture wire that is connected to a hub at the proximal end. In one embodiment, the clot capture wire includes a coil made out of an elastic or superelastic material, preferably nitinol. The elasticity or superelasticity of the coil allows it to be deformed within the catheter and to then reform its original coil configuration when the coil is moved outside of the catheter lumen. In another embodiment the coil is a biphasic, shape memory coil, which changes shape upon heating, energy application, or passing an electric current. Once the coil configuration has been established, the coil can be used to ensnare and corkscrew a clot or blockage in a vessel. A clot is extracted from the vessel by moving the clot capture coil and catheter proximally until the clot can be removed completely or released into a different vessel that does not perfuse a critical organ. Foreign bodies are similarly captured by deploying the coil distal to the foreign body and moving the clot capture coil proximally until the foreign body is trapped within the coil. By removing the device from the body, the foreign material is also removed.
Owner:TYCO HEALTHCARE GRP LP
Depth and puncture control for blood vessel hemostasis system
A depth and puncture control system for a blood vessel hemostasis system includes a blood vessel puncture control tip which, when positioned in the lumen of a blood vessel, can inhibit the flow of blood out of the puncture site. When used together with a pledget delivery cannula and a pledget pusher, the control tip and the delivery catheter can both inhibit blood loss out the puncture site and inhibit the introduction of pledget material and tissue fragments into the blood vessel. The system also includes a handle which releasably connects together the control tip, pusher, and delivery cannula to permit limited longitudinal motion between the control tip and the delivery cannula, and between the pusher and the delivery cannula.
Owner:BOSTON SCI CORP +11
Ablative catheter with electrode cooling and related methods of use
InactiveUS20130096550A1Increase blood flowCatheterSurgical instruments for heatingMembrane configurationBiomedical engineering
Medical devices and methods for making and using medical devices are disclosed. An example medical device may include an ablative catheter system including an elongate member having a proximal end, a distal end, and a lumen extending there between. An end-effector may be disposed at the distal end of the elongate member. The end-effector may include an expandable frame. A membrane may be supported on the frame. The membrane may be configured to partially occlude fluid flow upon frame expansion. One or more electrodes may be placed on the end-effector. The system may also include a control member that is configured to shift the end-effector between a collapsed state and the frame expansion state.
Owner:BOSTON SCI SCIMED INC
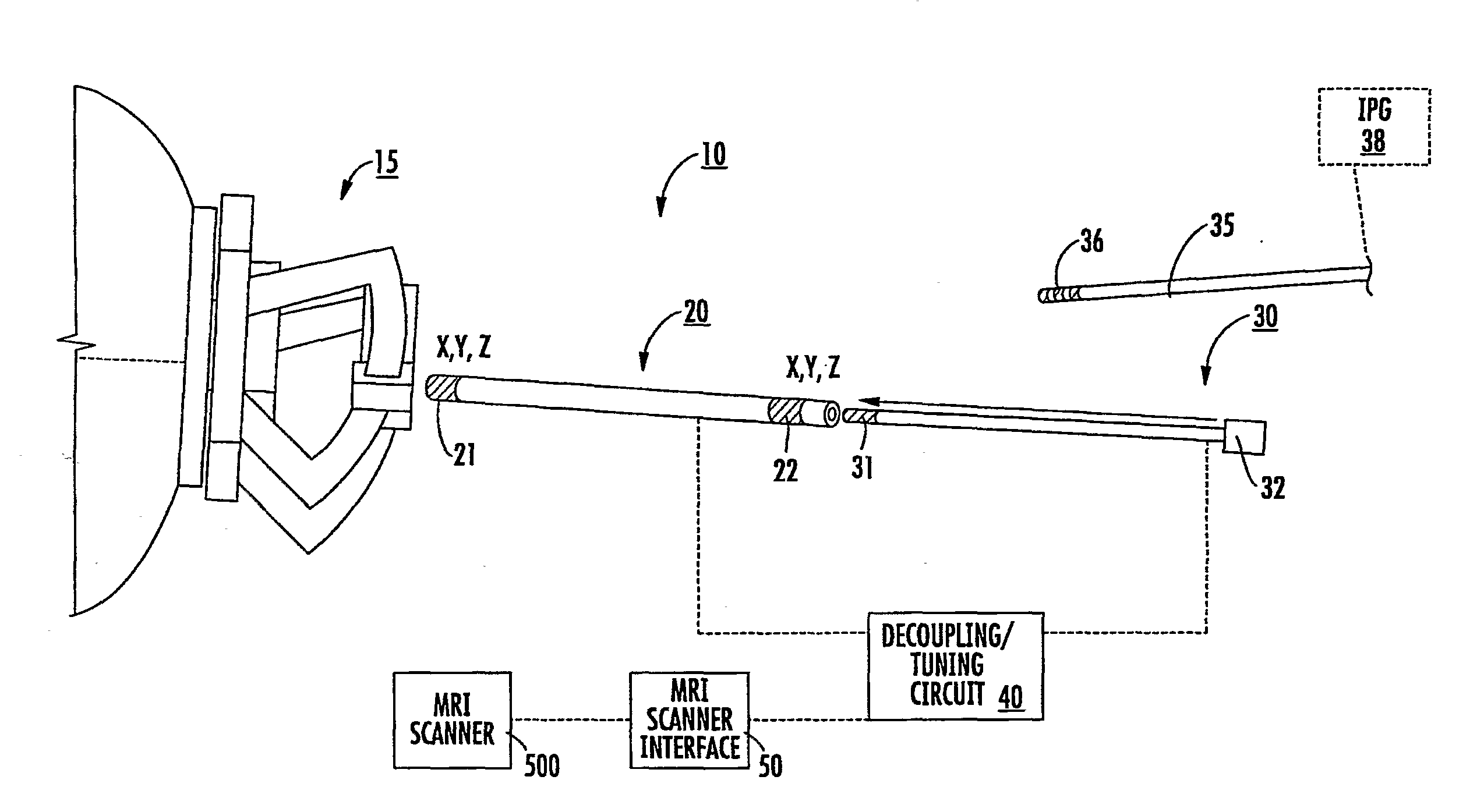
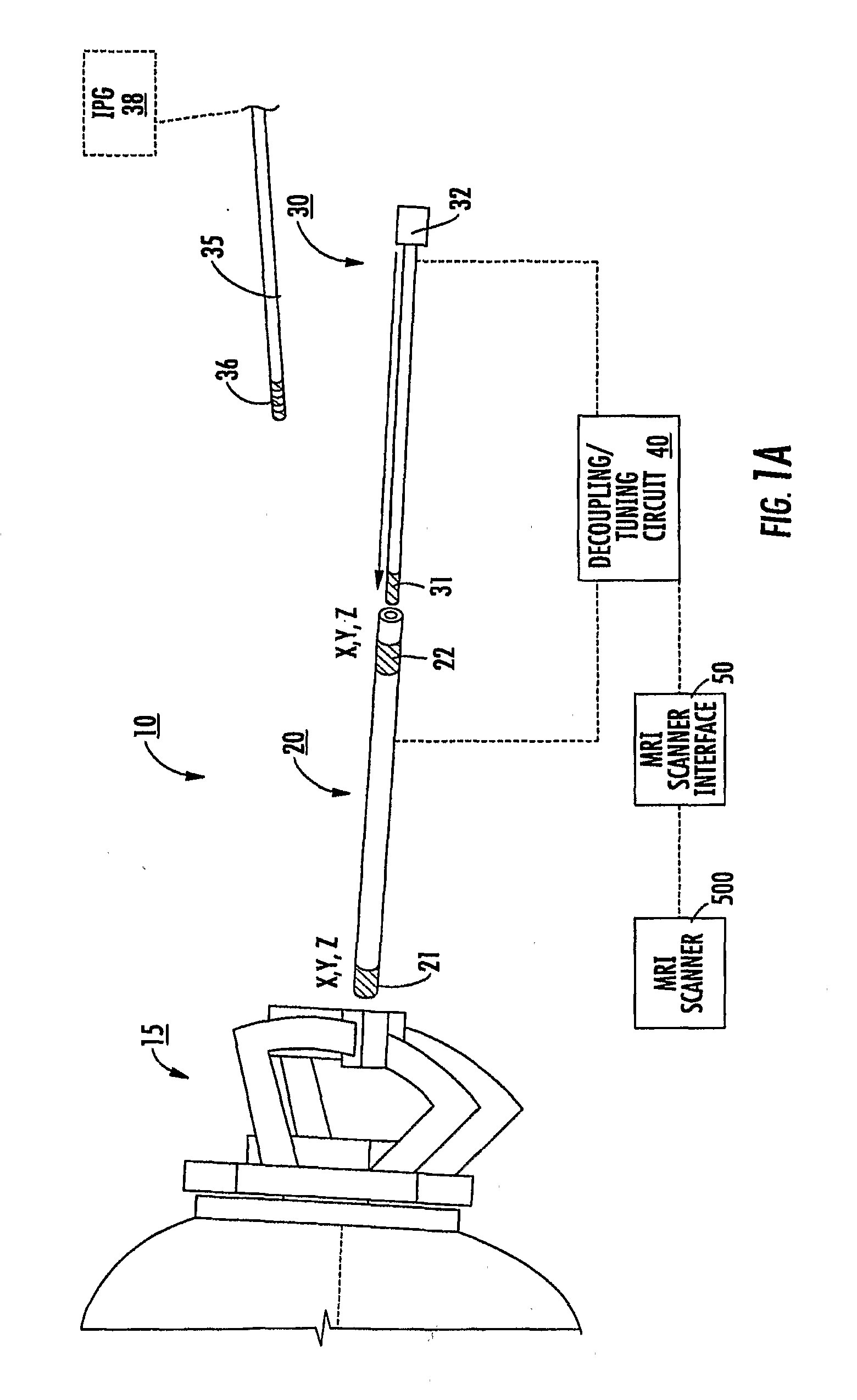
Mri-guided localization and/or lead placement systems, related methods, devices and computer program products
MRI compatible localization and / or guidance systems for facilitating placement of an interventional therapy and / or device in vivo include: (a) a mount adapted for fixation to a patient; (b) a targeting cannula with a lumen configured to attach to the mount so as to be able to controllably translate in at least three dimensions; and (c) an elongate probe configured to snugly slidably advance and retract in the targeting cannula lumen, the elongate probe comprising at least one of a stimulation or recording electrode. In operation, the targeting cannula can be aligned with a first trajectory and positionally adjusted to provide a desired internal access path to a target location with a corresponding trajectory for the elongate probe. Automated systems for determining an MR scan plane associated with a trajectory and for determining mount adjustments are also described.
Owner:CLEARPOINT NEURO INC
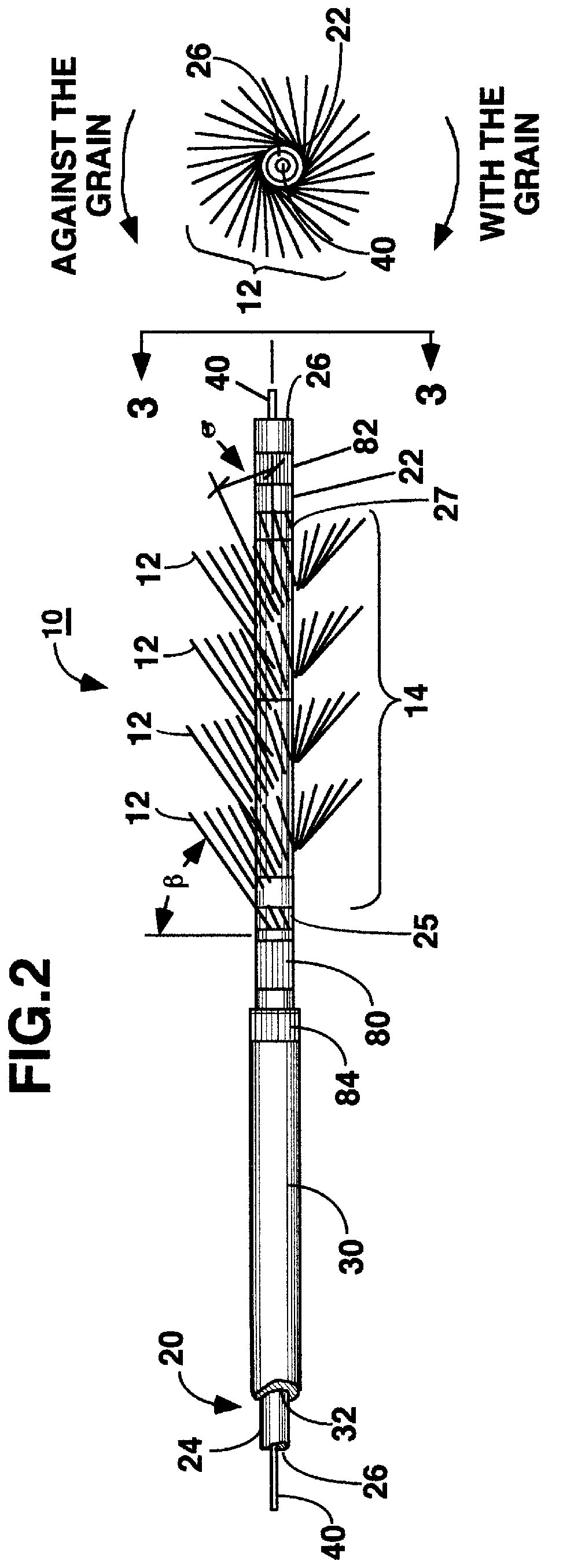
Miniaturized medical brush
A miniaturized brush particularly adapted for medical use formed at the distal end of an elongated brush drive shaft having a hollow lumen formed therein for introduction over a guidewire. The brush drive shaft is enclosed in the lumen of a brush delivery catheter and other components of a brush sub-assembly adapted to deliver infusate through the catheter lumen and to be coupled to a drive motor unit for rotating the brush drive shaft and brush. The brush bristles of the distal brush are adapted to be garaged in a distal end section of the brush delivery catheter lumen during introduction through a body lumen. The brush bristles are formed of a thin sheet of rigid plastic material that is shaped to have a plurality of fringe elements extending in parallel from a mounting web. The mounting web is wound about and attached to a distal end section of the drive shaft outer circumference so that the fringe elements extend outward from the drive shaft surface and obliquely of the drive shaft axis. Preferably, proximal and distal spiral brush sections are formed in this manner so that fluids are impelled distally and proximally, respectively, between the proximal and distal brush sections as infusate is delivered.
Owner:TYCO HEALTHCARE GRP LP
Trocar assembly with pneumatic sealing
A trocar assembly for creating a pneumatic seal during a minimally-invasive surgical procedure. The trocar assembly including an elongated body having a lumen extending therethrough. The proximal end portion of the body defining a housing. A fluid supply plenum is defined in the housing configured to deliver pressurized insufflation fluid to a nozzle. The nozzle configured for directing pressurized fluid into the lumen and creating a pneumatic seal. A fluid return plenum is defined in the housing configured to collect spent insufflation fluid from as patient's abdominal cavity. The fluid return plenum including a plurality of axially and radially oriented elongate vanes configured to permit spent insufflation fluid to proceed between the vanes and direct spent insufflation fluid back to the fluid return plenum.
Owner:SURGIQUEST
Vascular occlusion devices and methods
A device for in situ treatment of vascular or cerebral aneurysms comprises an occlusion device having a flexible, longitudinally extending elastomeric matrix member that assumes a non-linear shape to conformally fill a targeted site. The occlusion device comprises a flexible, longitudinally extending elastomeric matrix member, wherein the device assumes a non-linear shape capable of fully, substantially, or partially conformally filling a targeted vascular site. In one embodiment the vascular occlusion device comprises a first longitudinally extending structural element having a longitudinally extending lumen and an outer surface; a second longitudinally extending structural element extending through the lumen; and an elastomeric matrix member surrounding the outer surface, wherein the second structural member does not engage or attach to the first structural element or the elastomeric matrix.
Owner:BIOMERIX CORP
Catalysts for body fluid sample extraction
ActiveUS20070083131A1Material analysis by observing effect on chemical indicatorWithdrawing sample devicesPtru catalystSurgery
An arrangement for producing a sample of body fluid from a wound opening created in a skin surface at a sampling site includes at least one skin-penetration member having a first end configured to pierce the surface of the skin, and a inner lumen in communication with the first end; at least one actuator operatively associated with the at least one skin-penetration member; and at least one catalyst device configured to cause perfusion of body fluid at the sampling site; wherein the at least one actuator is configured to locate the at least one skin-penetration member so as to obstruct the wound opening while transporting body fluid through the inner lumen. Associated methods are also described.
Owner:INTUITY MEDICAL INC
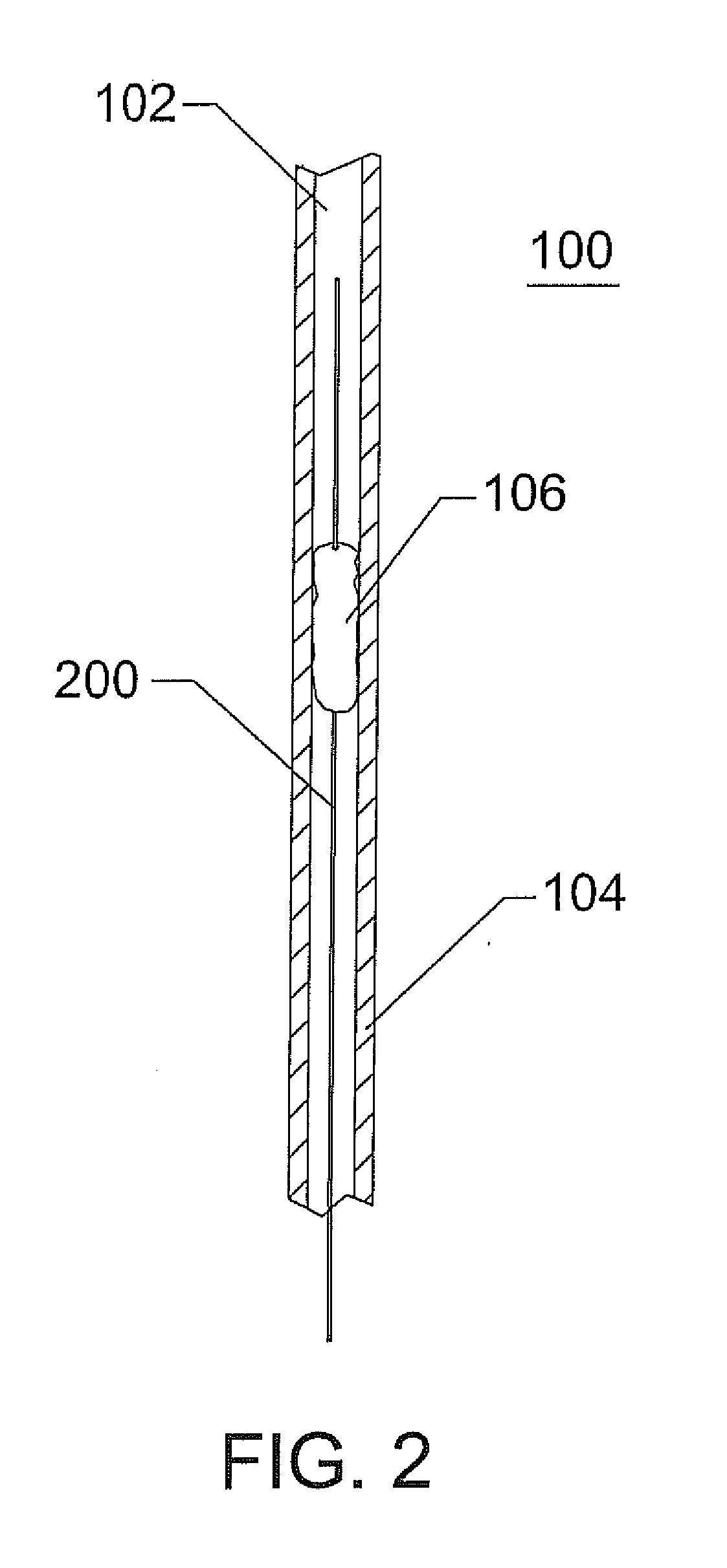
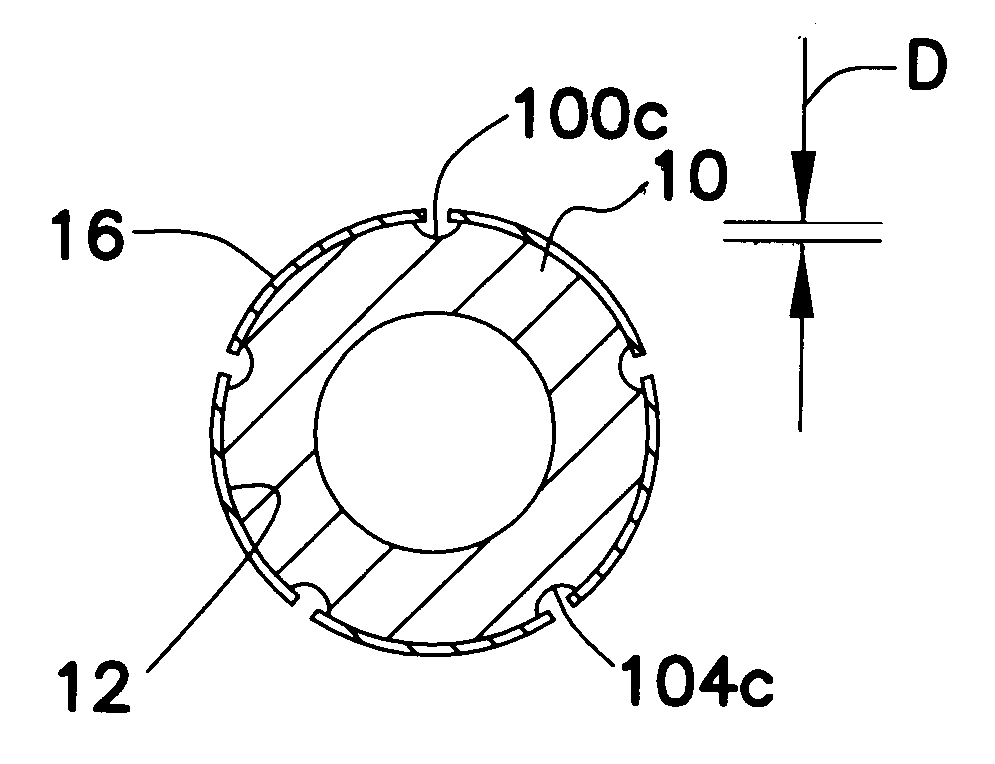
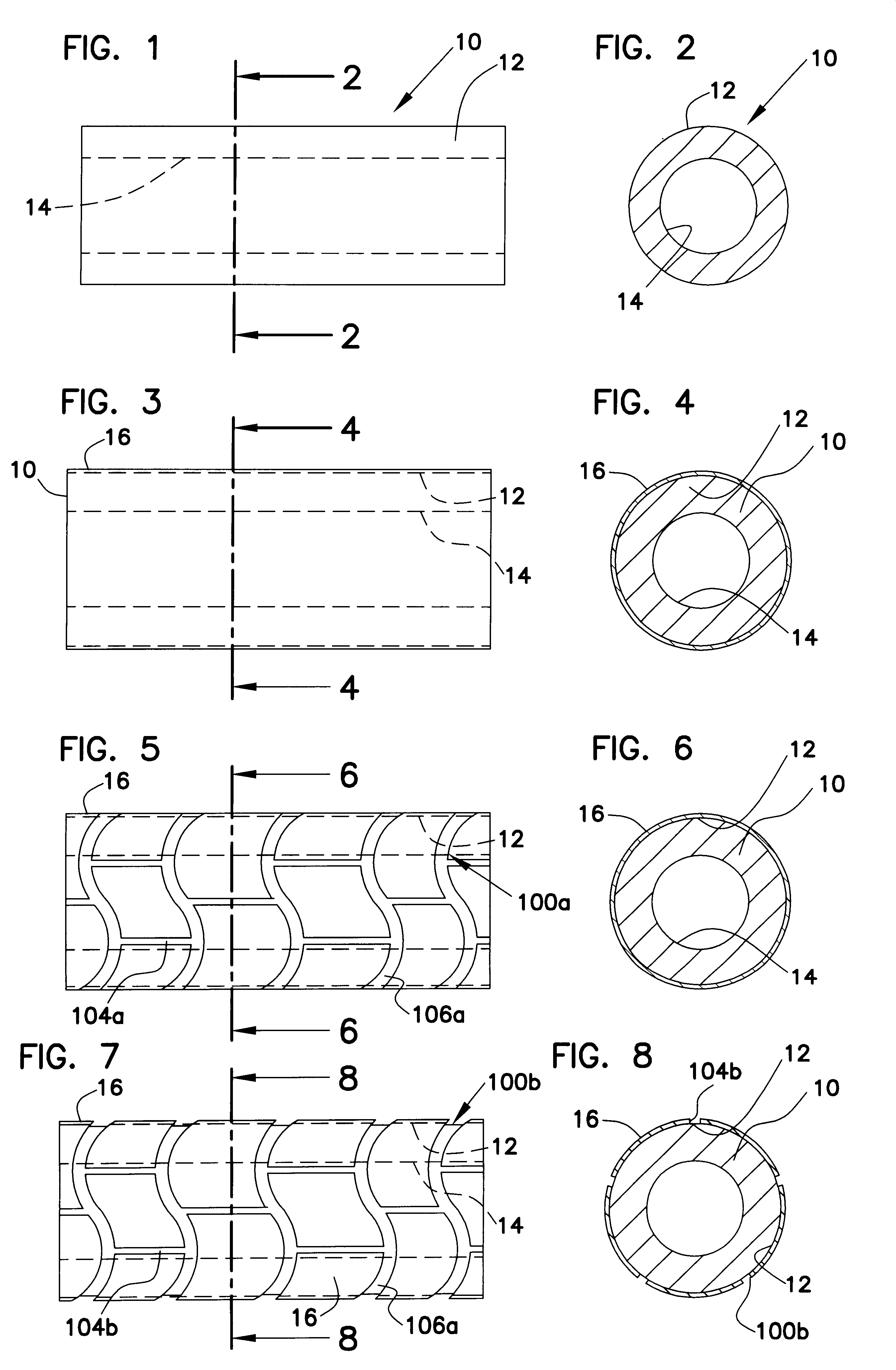
Method for manufacturing intraluminal device
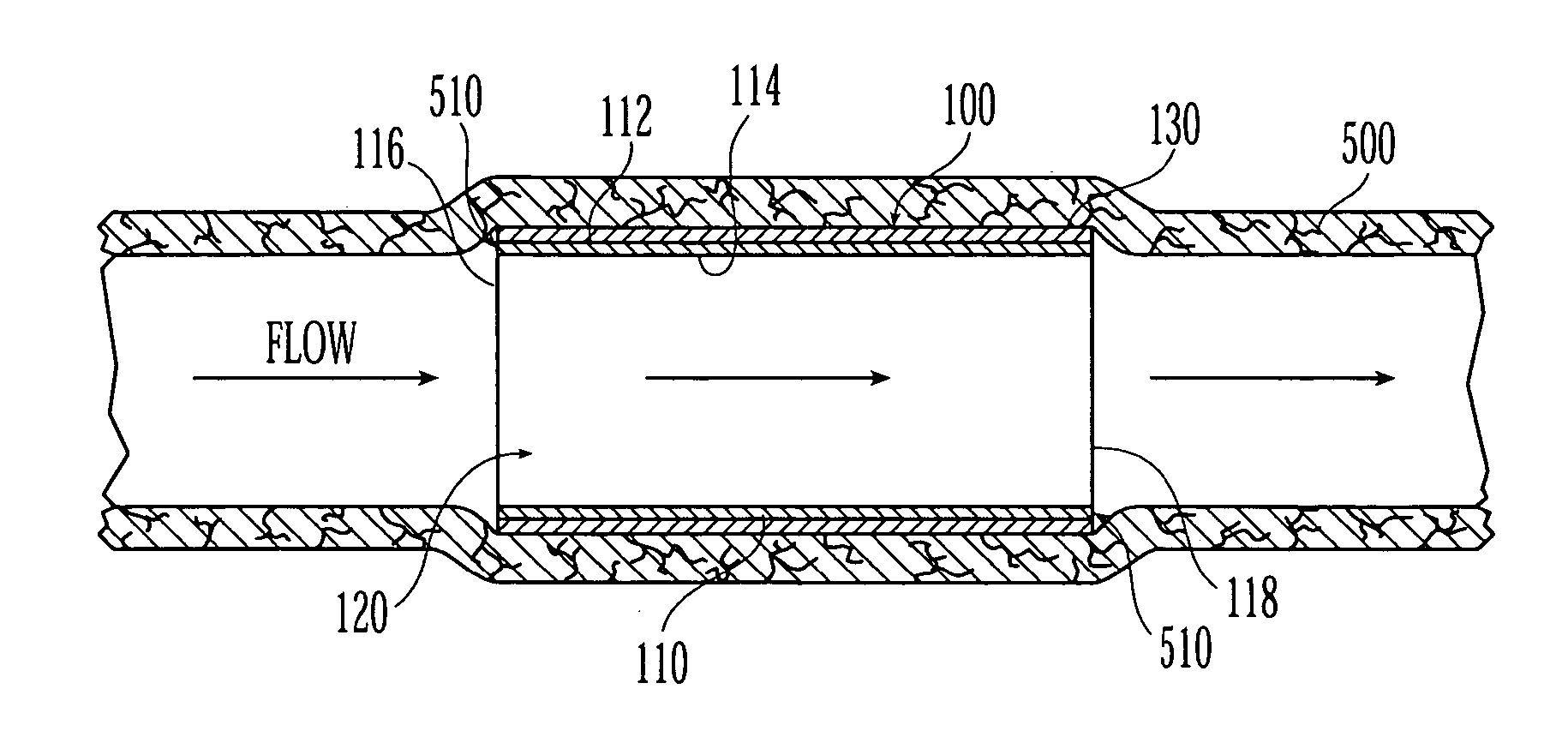
A generally tubular device (e.g., a stent or catheter) for placement in a lumen of a patient's body is made by forming a depressed pattern in an external surface of a mold. The depressed pattern corresponds to a desired shape of the generally tubular device. A material is deposited in the depressed pattern for the material to form the generally tubular device conforming to the depressed pattern. The generally tubular device is separated from the mold.
Owner:EV3 PERIPHERAL +1
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS8734429B2Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsDrugs preparations
Owner:RANI THERAPEUTICS
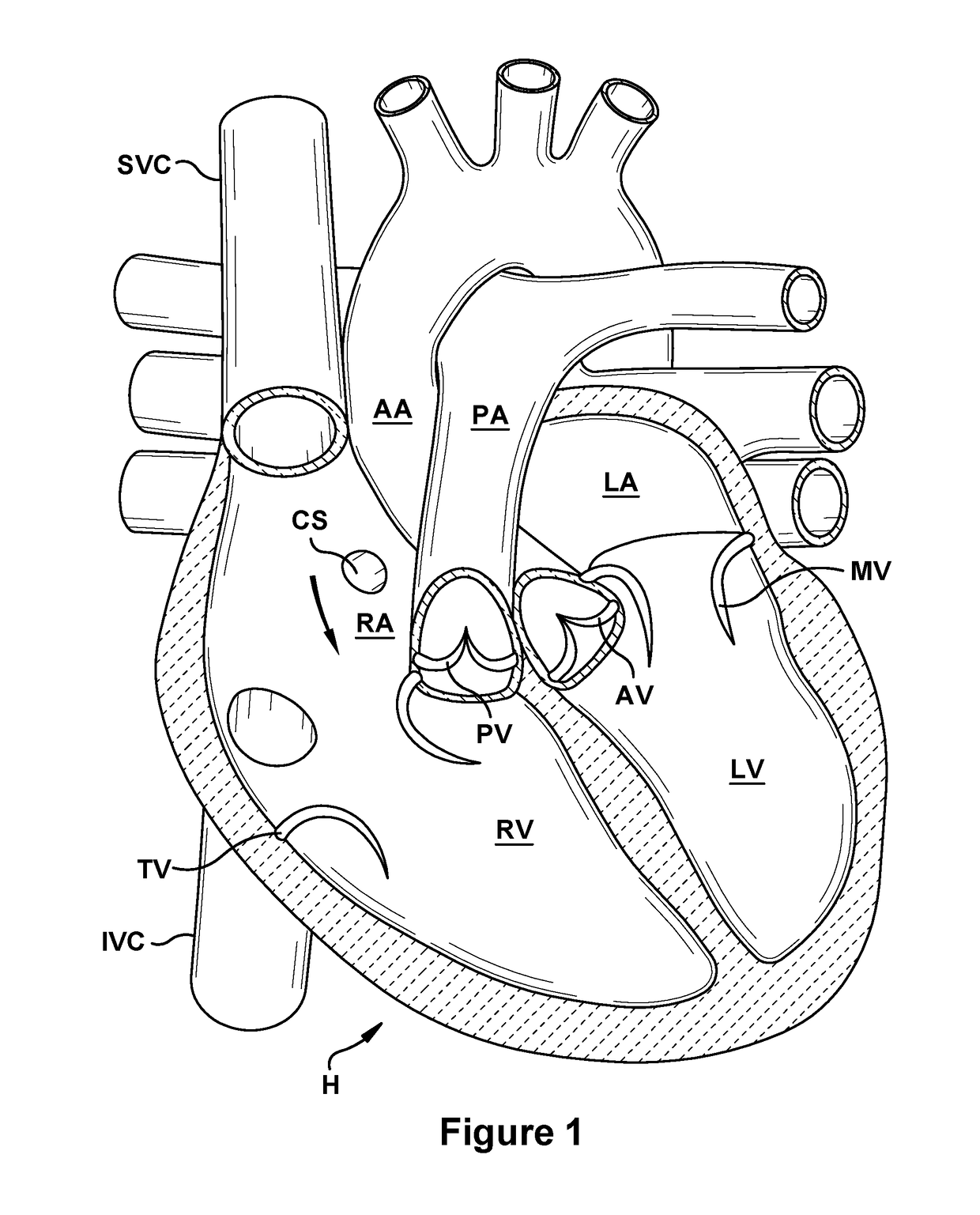
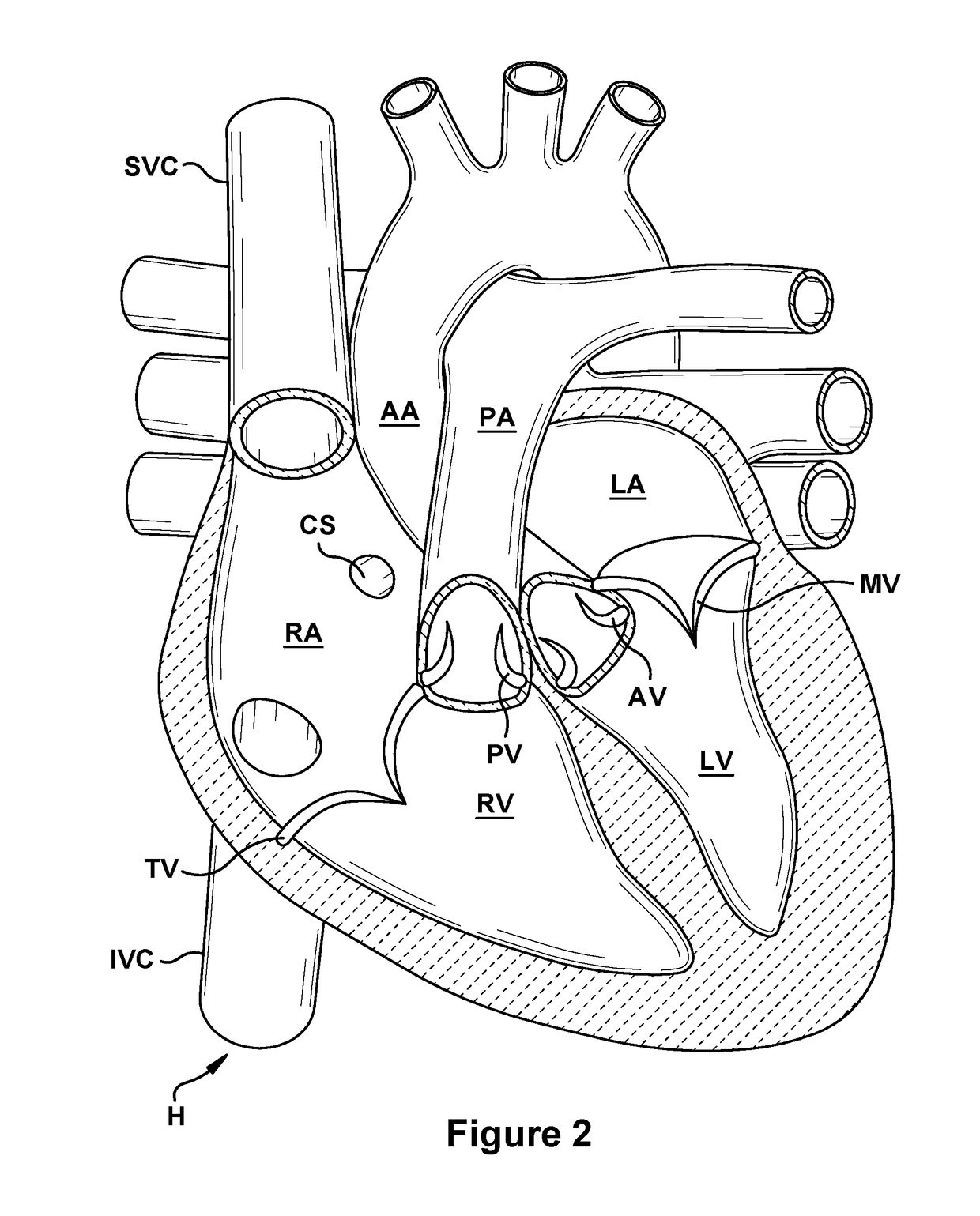
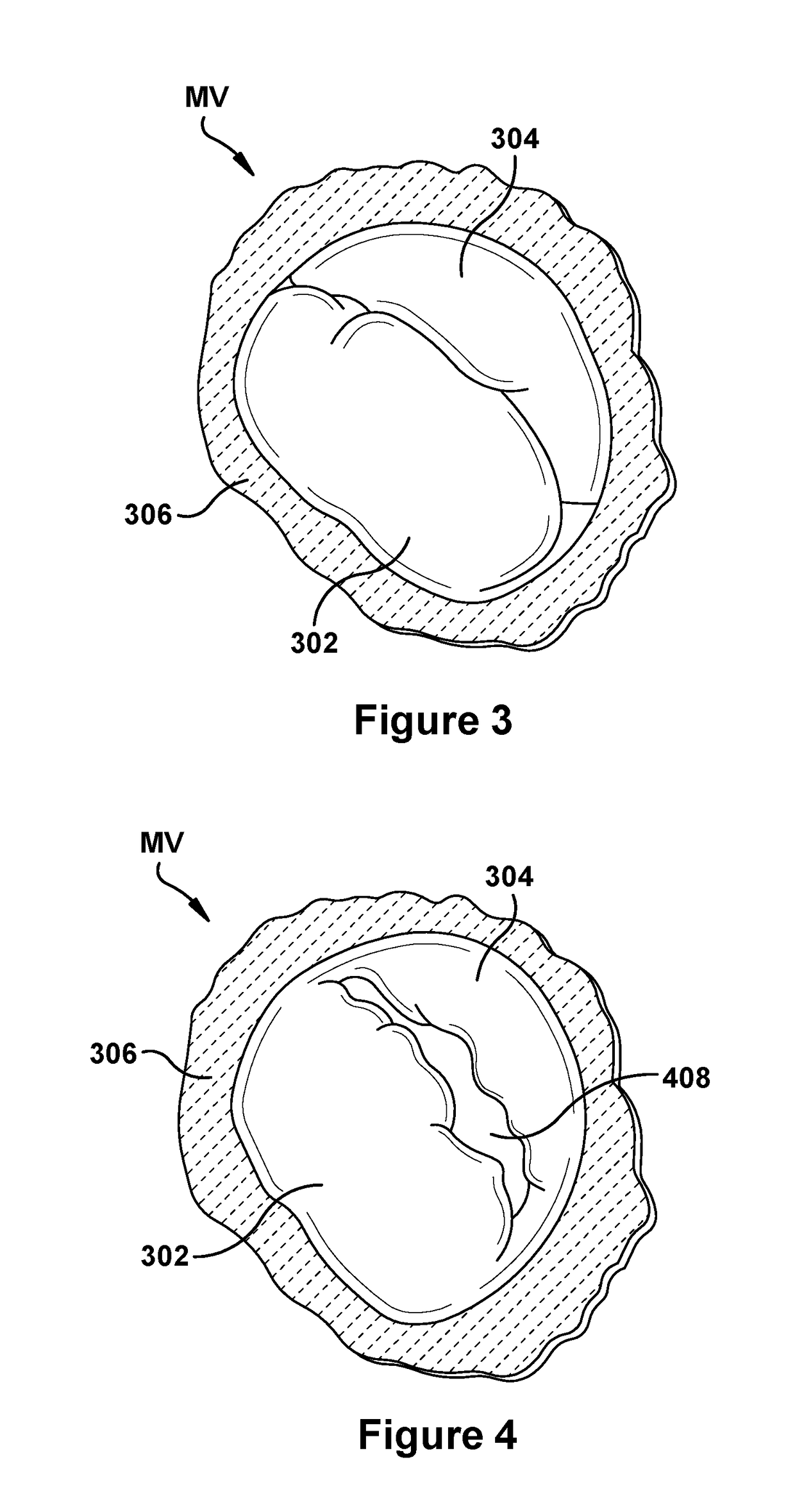
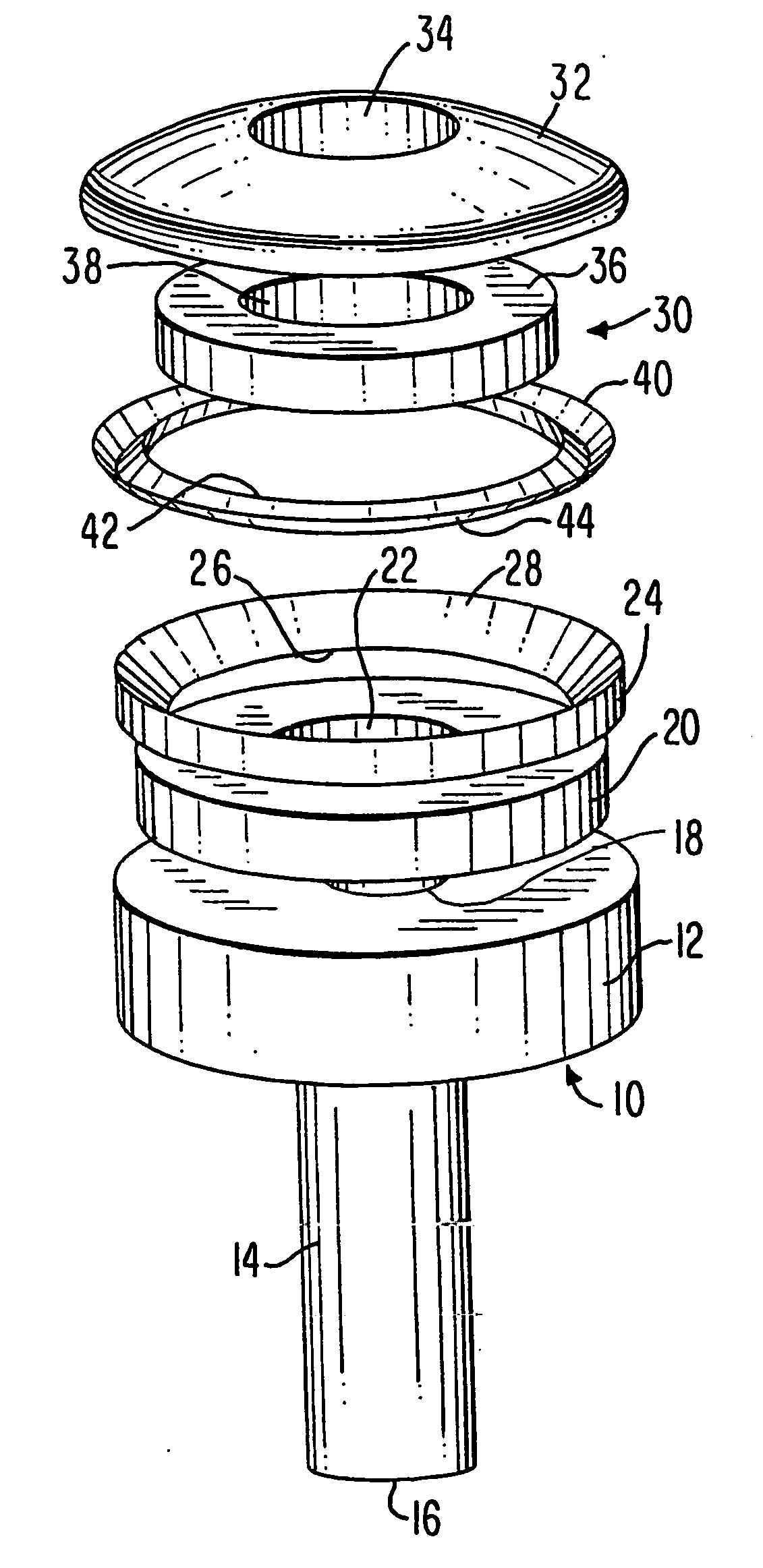
Native valve repair devices and procedures
A system for implanting a repair device onto a native valve of a natural heart to repairing the native valve of a patient during a non-open-heart procedure. The system includes a surgical delivery instrument, a valve repair device, and a gripper control mechanism. The surgical delivery instrument has at least one lumen. The valve repair device is configured to be delivered through the lumen of the surgical delivery instrument and to be attached to a native valve of a patient. The valve repair device has a pair of paddles, and first and second gripping members. The paddles are movable between an open position and a closed position. The paddles and the first and second gripping members are configured to attach to the native valve of the patient. The gripper control mechanisms each include a suture and a wire having a loop at a distal end. The suture extends from the surgical delivery instrument and through the loop of the wire. The suture is removably attached to the gripping member. The loop of the wire is configured to engage the gripping member to move the gripping member between one or more positions.
Owner:EDWARDS LIFESCIENCES CORP
Magnetic devices and applications for medical/surgical procedures and methods for using same
InactiveUS20070142780A1Facilitate manipulationEasy alignmentCannulasInfusion syringesEngineeringReducer
One embodiment of the invention comprises a trocar and a reducer cap that magnetically attaches to the trocar. The trocar and the cap each include a magnetic member, at least one of which is a first magnet, and the other of which is either a second magnet or a non-magnetized magnetically permeable member. Including a magnet of sufficient strength in the trocar and / or the cap will create a magnetic field that automatically holds a surgical instrument having a magnetically permeable member at its tip in axial alignment with the cap or trocar lumen. Introduction of the surgical instrument into the lumen can be further facilitated by providing the trocar or cap lumen with a funnel-shaped opening. A lumen seal can be provided by one or more compliant toroidal seal members that expand radially inwardly when compressed axially by the magnetic attraction between the cap and trocar. The alignment feature is particularly advantageous when incorporated in a mini-trocar having a lumen on the order of 1-3 mm in diameter. In that case, a trocar cap can be a small disc magnetically attracted to the trocar to cover the lumen. Magnetic aligning devices according to the invention can be used internally of a patient or transdermally. Another embodiment of the invention is an ostium plug with a lumen therethrough that can be used in tubal sterilization. The plug is permanently implanted in the patient, but a cap is coupled magnetically to the proximal end of the plug to permit reopening of the lumen when desired.
Owner:VAN LUE VETERINARY SURGICAL
Portable wound treatment apparatus having pressure feedback capabilities
InactiveUS20070167927A1Readily remove and replaceIncrease speedSurgeryWound drainsPressure feedbackIntensive care medicine
A reduced pressure treatment apparatus includes a drape for positioning over the wound site to create and maintain a substantially air-tight cavity between the wound site and the drape. A multi-lumen suction tube is provided to be attached to a reduced pressure source. The multi-lumen suction tube includes a center lumen and at least one outer lumen and is configured to deliver reduced pressure beneath the drape to the substantially air-tight cavity. The multi-lumen suction tube is adapted to allow fluid to be drawn from the wound site through the center lumen and pressure to be monitored at the wound site through the at least one outer lumen.
Owner:KCI LICENSING INC
Devices and methods for facilitating fluid transport
ActiveUS20070078358A1Easy to transportShorten the timeDiagnostic recording/measuringSensorsFluid transportEngineering
Arrangements are provided including a base having a bore disposed therein extending from a first surface of the base through a second surface of the base, a fluid transport tube having a first end, a second end opposite the first end, and a lumen having an inner diameter, at least the second end of the tube being received within the bore of the base, and at least one fluid transport enhancing groove having at least a first section disposed in the second surface of the base and in fluid communication with the bore.
Owner:INTUITY MEDICAL INC
Interventional catheters incorporating an active aspiration system
ActiveUS20110118660A1Reduce amountHigh and consistent aspiration pressureGuide wiresExcision instrumentsCatheterTarget site
An interventional catheter assembly comprises an operating head for removing obstructive material from a target site in a body lumen or cavity and at least one aspiration port located proximal to the operating head and penetrating the catheter assembly, the aspiration port being in communication with a sealed lumen that communicates with a vacuum system for withdrawing aspirate fluid and obstructive material from the target site. The interventional catheter incorporates an elongated guidewire lumen bushing extending proximally from a distal region of the operating head to reduce the clearance between the guidewire and the internal surface of the guidewire lumen during operation of the interventional catheter. This feature restricts entry of fluid and debris into the guidewire lumen and promotes maintenance of consistent and high aspiration pressure and volume during operation of the aspiration system and interventional catheter assembly.
Owner:BOSTON SCI LTD
Closed fluid transfer system
A closed fluid transfer system for fluidly interconnecting a syringe to any one of a patient I.V. set, a vial and an I.V. bag, is provided and includes a first adapter defining a lumen and supporting a seal across a first end of its lumen, the first adapter supporting a rear end of a needle within the lumen, wherein the seal is movable relative to the needle such that a tip of the needle penetrates through the seal; and a second adapter defining a lumen and supporting a seal across a first end of its lumen; wherein when the second adapter is coupled to the first adapter, the second adapter seal abuts the first adapter seal and moves the first adapter seal relative to the tip of the needle such that the tip of the needle penetrates through the abutting first adapter seal and second adapter seal.
Owner:CORVIDA MEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com