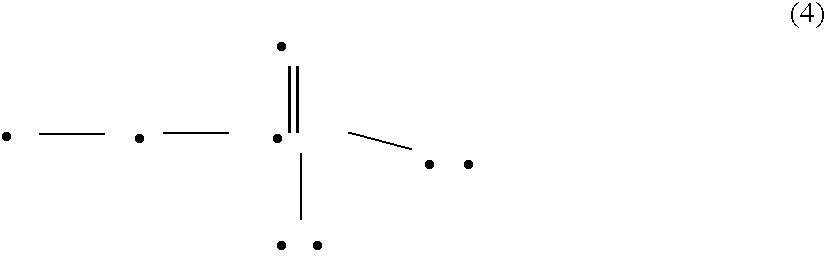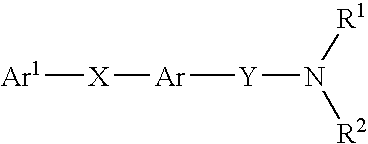Patents
Literature
4122 results about "Drugs preparations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
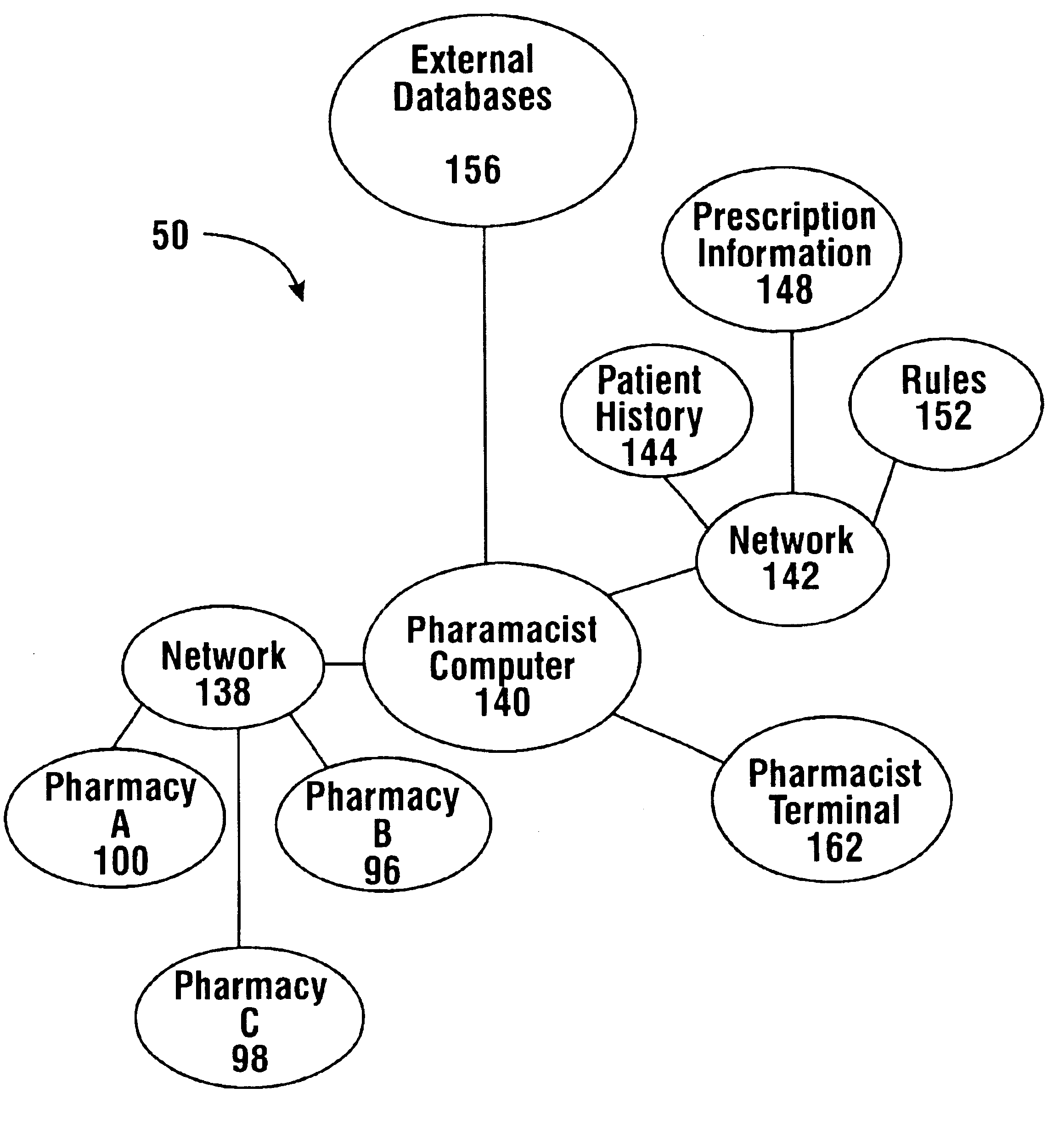
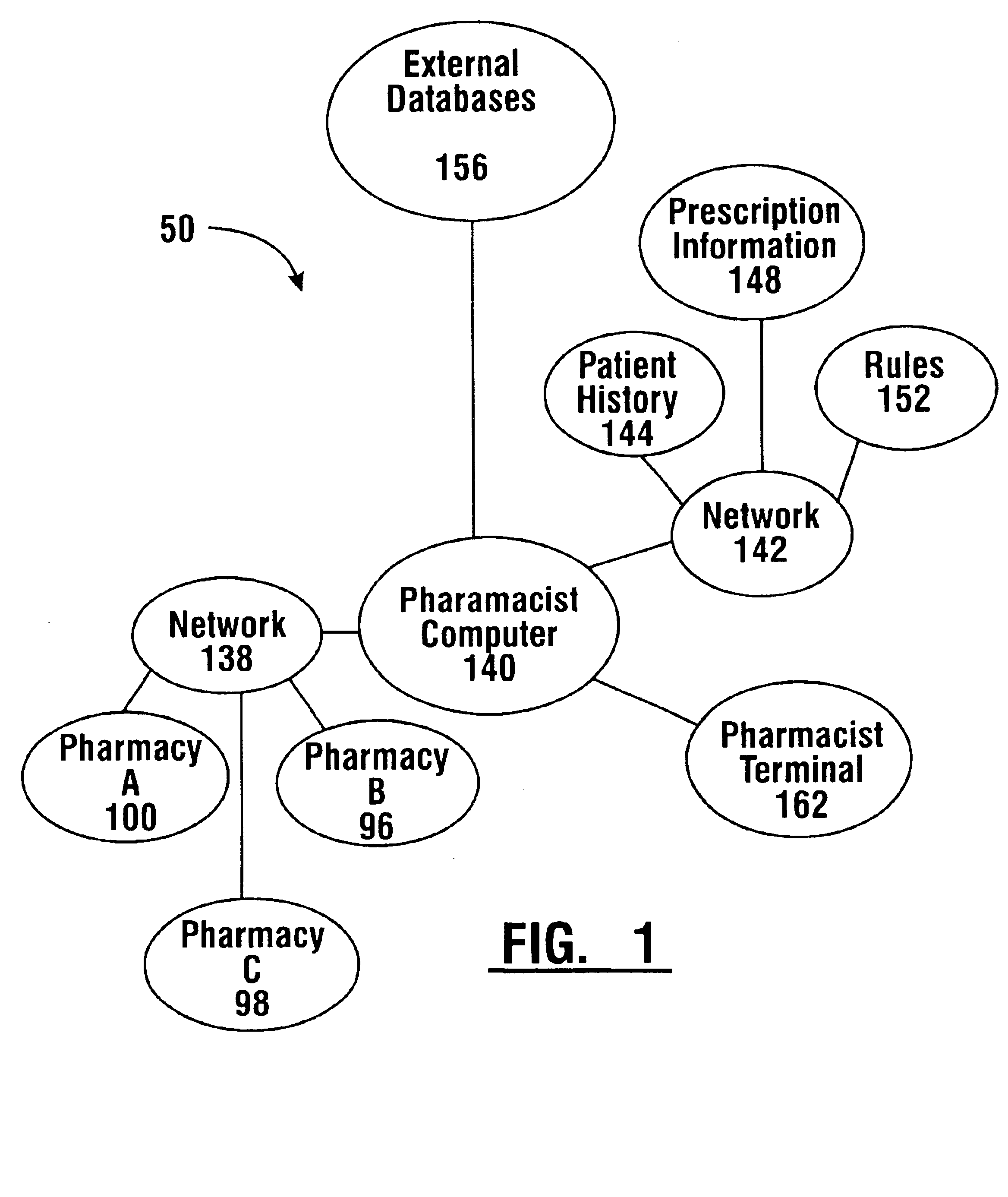
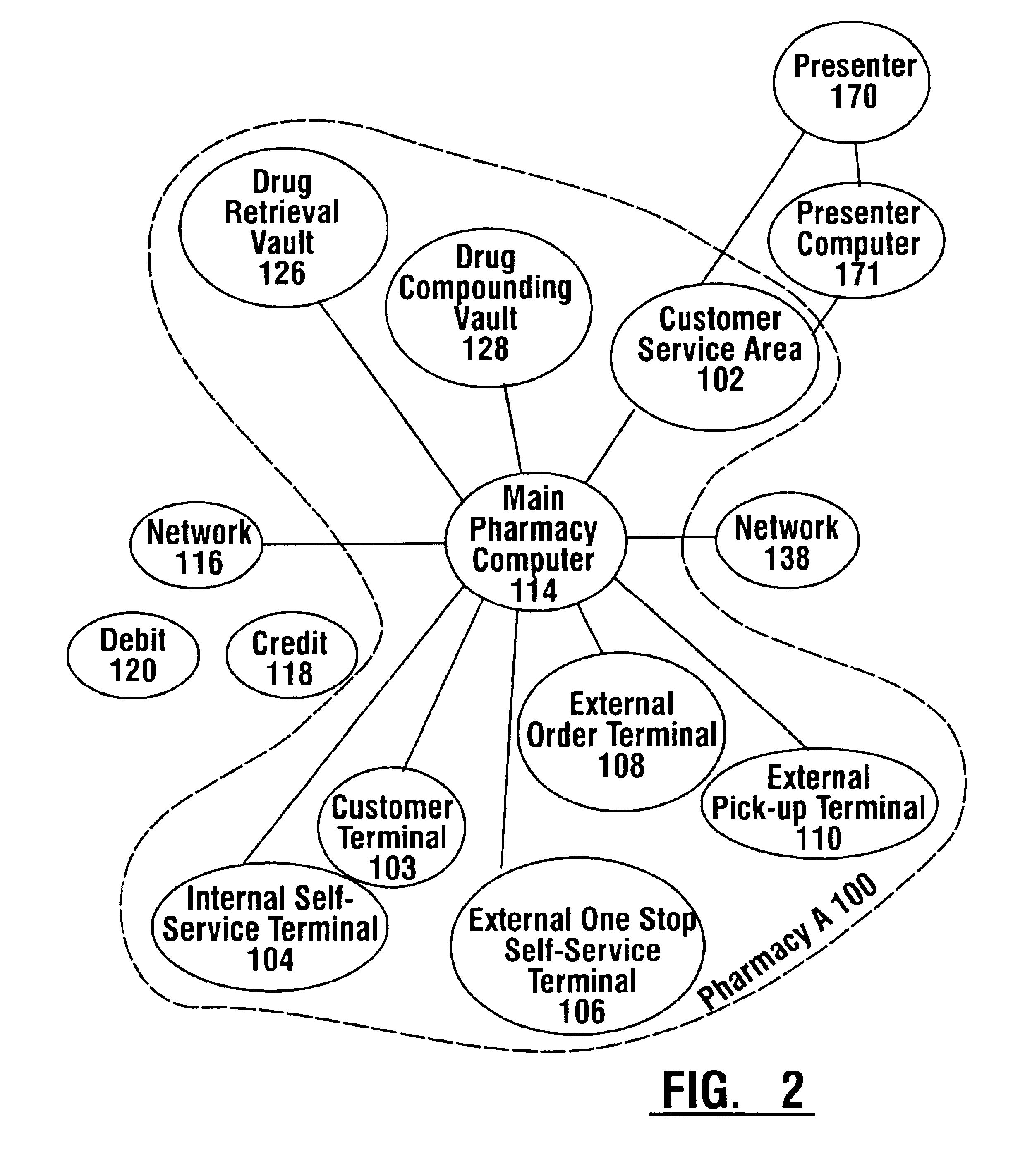
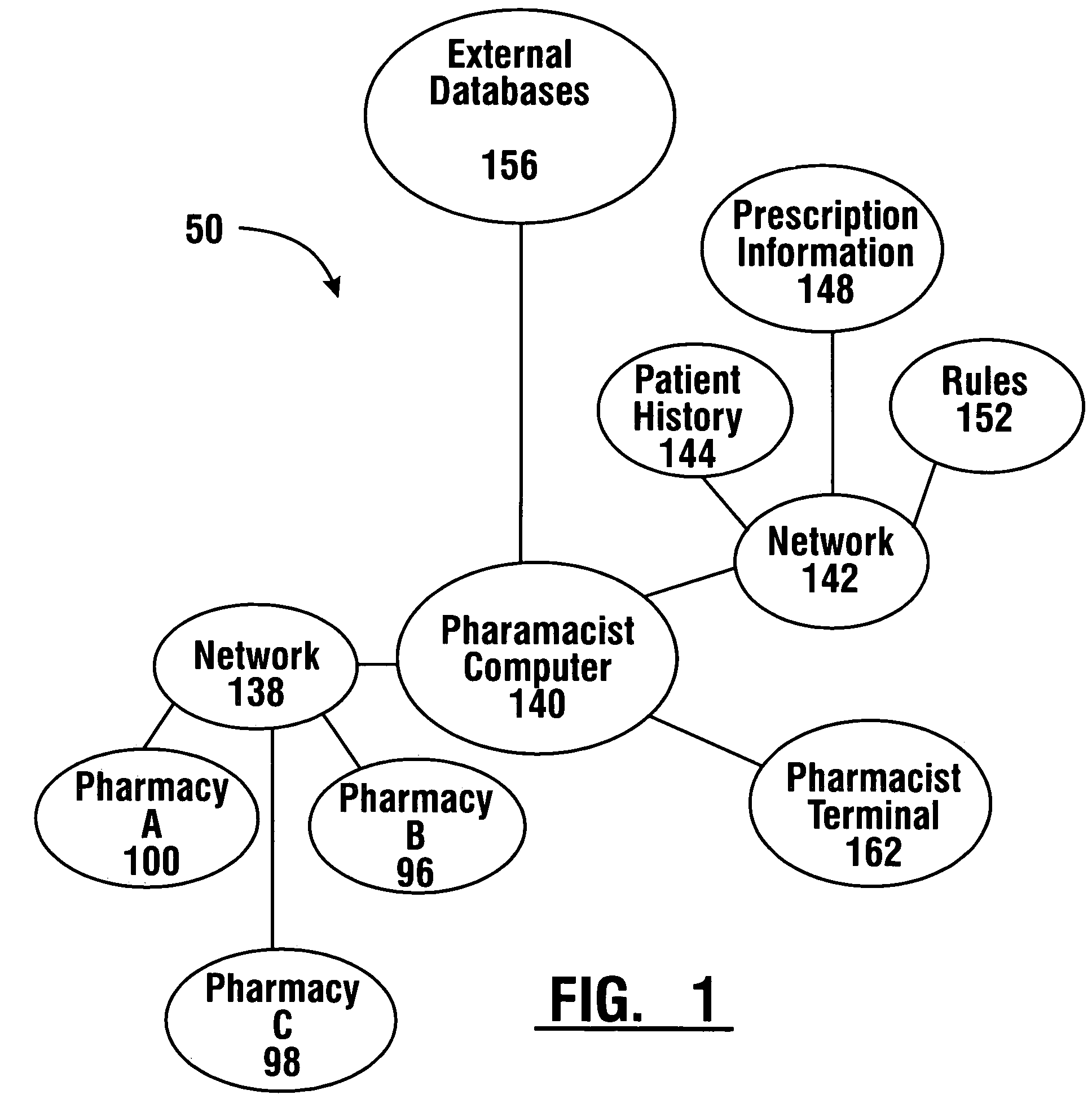
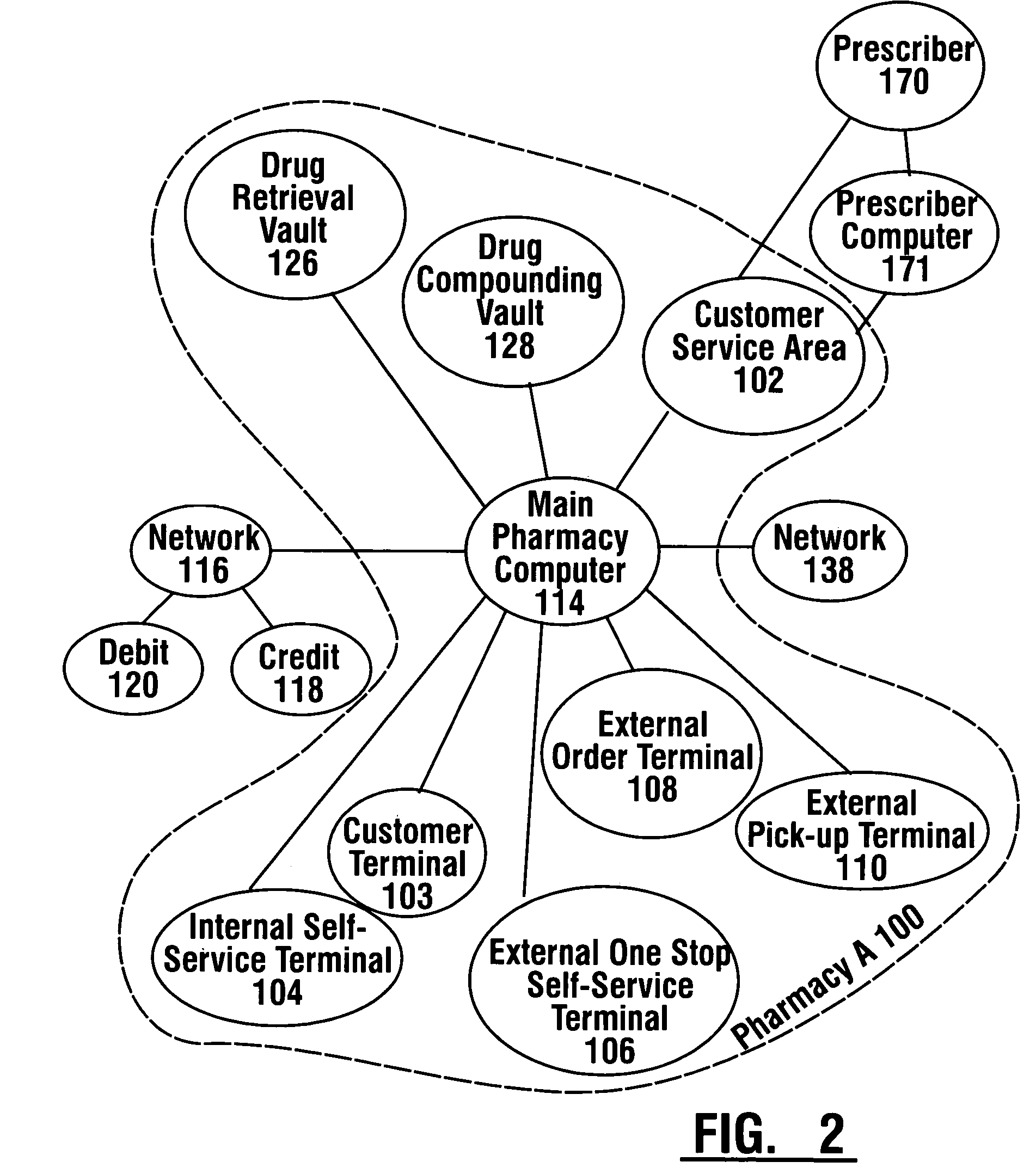
Pharmaceutical system in which pharmaceutical care is provided by a remote professional serving multiple pharmacies
InactiveUS6711460B1More accuracyGuaranteed growthFinanceDigital data processing detailsElectronic networkDrugs preparations
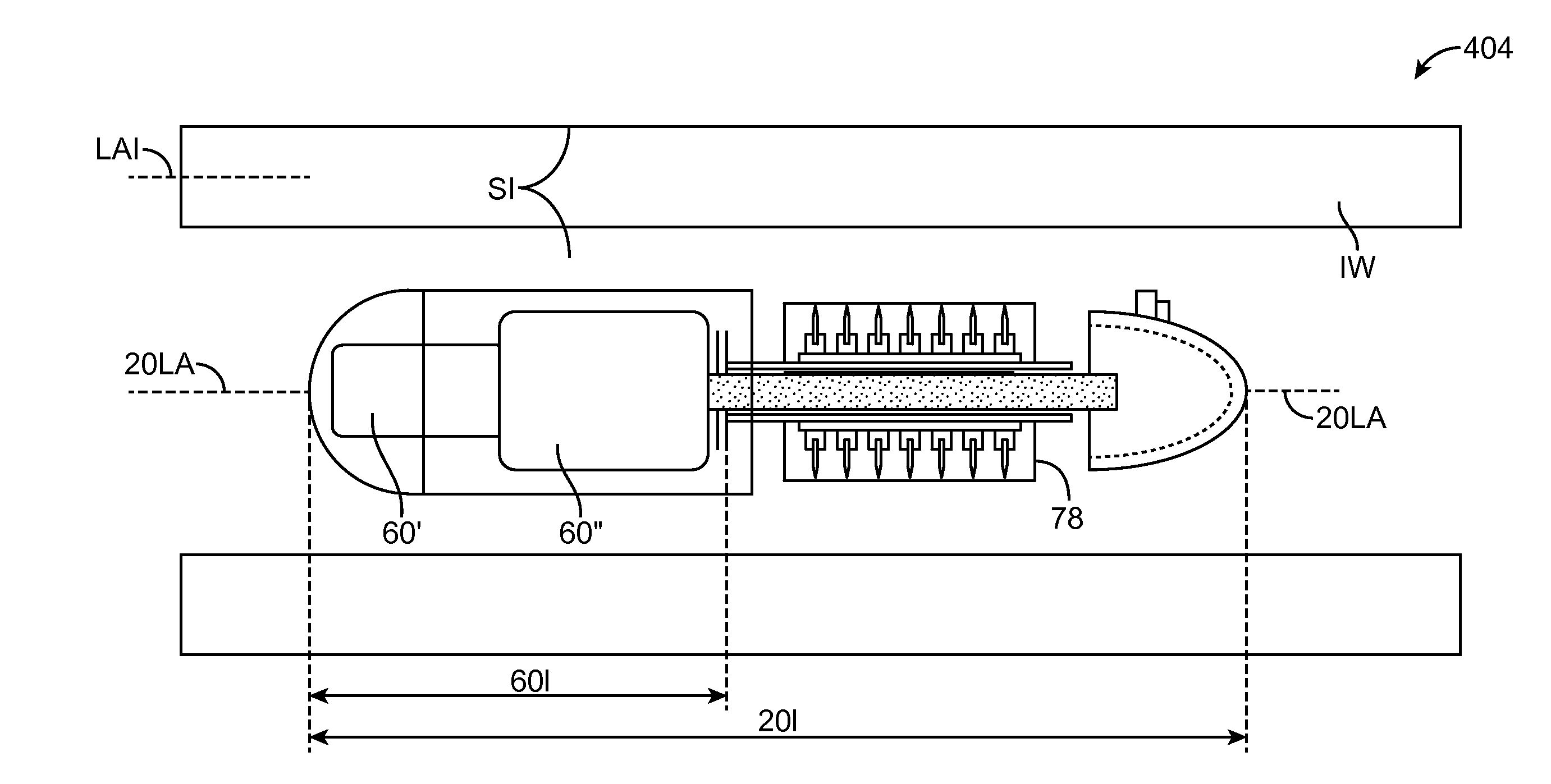
A pharmaceutical system and method of operation in which a single remote professional provides pharmaceutical care and oversight of multiple local pharmacies. A control location is connected through an electronic network to one or more individual pharmacies, each of which may be located at a different physical site. Each individual pharmacy includes one or more drug preparation areas, and one or more self-service or staffed customer terminals. A drug preparation area includes a robot, which is adapted to prepare prescriptions or other items, and which is connected by a pneumatic delivery system to one or more customer terminals within the pharmacy.
Owner:DIEBOLD NIXDORF
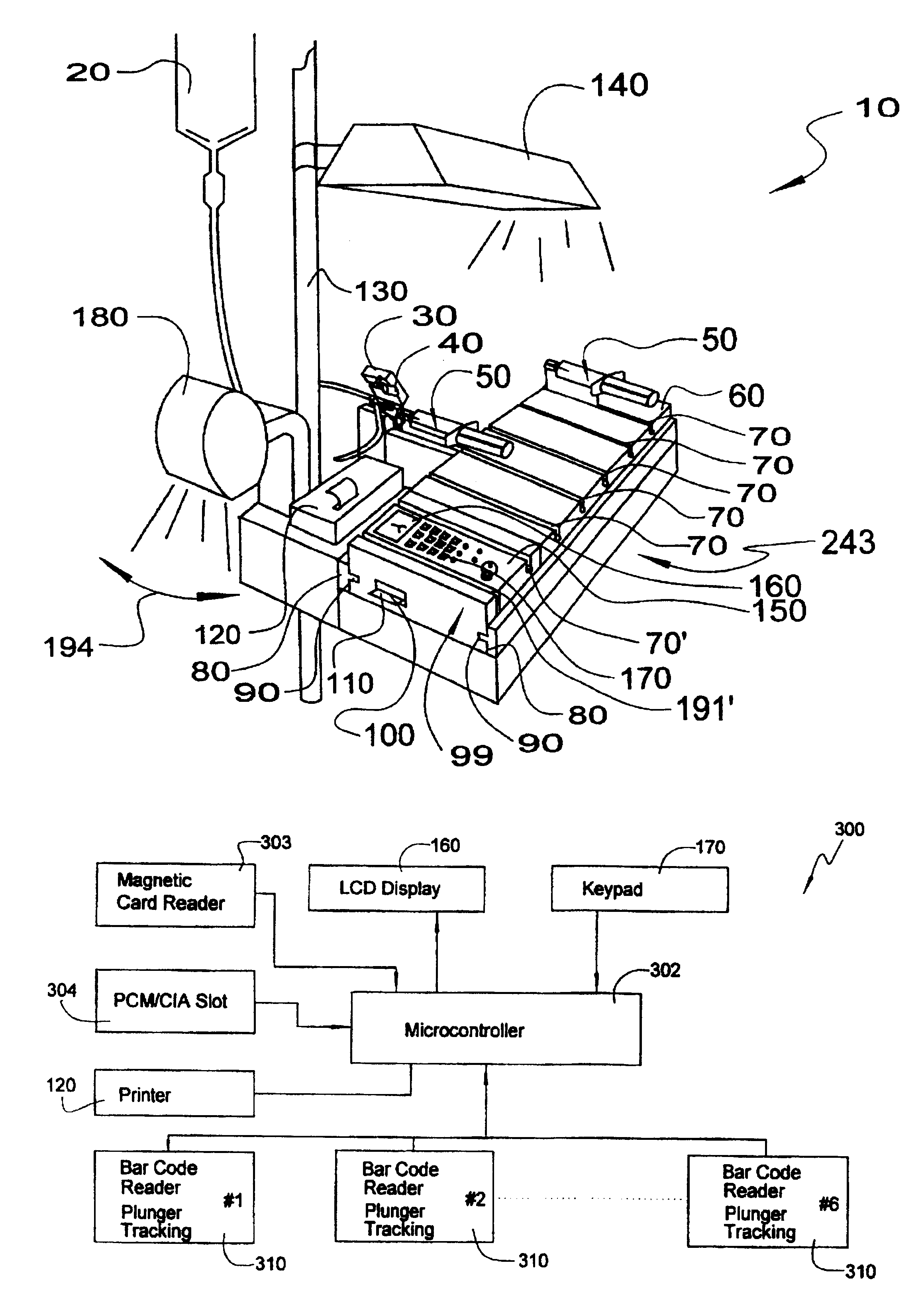
Medication delivery and monitoring system and methods
InactiveUSRE38189E1Low costLimited accessMedical devicesPressure infusionOperational systemDrugs preparations
A medication delivery and monitoring system and methods whereby drugs are safely delivered to a patient, monitored in real-time during delivery and crucial events are recorded during delivery to provide real-time, on-line information and detail for an audit trail. A novel safety label cradle unit is disclosed. Safety label cradles (SLC's) are provided in a plurality of sizes to match varying sizes of syringes which are disposed on a cradle of the SLC to provide a constant needle height on the SLC unit independent of syringe volume (barrel diameter). A selected SLC is securely affixed to a syringe by an adhesively backed label wrapping. The label is preprinted to provide drug identification indicia and drug preparation information. The information is automatically read into the system from the label. A novel delivery station of the system monitors drug delivery as a plunger of the syringe is pushed to deliver a drug to a patient. A smart tray in cooperation with a slider portion of the SLC is used to selectively deliver drugs to a port in the IV set. The smart tray comprises a first portion for carrying SLC units, an attachable second portion having a control panel for operating the system and a cover for lockably affixing the SLC units to the tray.
Owner:IBM CORP
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS8734429B2Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsDrugs preparations
Owner:RANI THERAPEUTICS
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS9149617B2Rapid drug releasePoor absorptionPeptide/protein ingredientsMedical devicesIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS20130165859A1Rapid drug releasePoor absorptionPeptide/protein ingredientsSurgeryIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Pharmaceutical system in which pharmaceutical care is provided by a remote professional serving multiple pharmacies
InactiveUS7630788B1More accuracyGuaranteed growthFinanceDrug and medicationsElectronic networkDrugs preparations
A pharmaceutical system and method of operation in which a single remote professional provides pharmaceutical care and oversight of multiple local pharmacies. A control location is connected through an electronic network to one or more individual pharmacies, each of which may be located at a different physical site. Each individual pharmacy includes one or more drug preparation areas, and one or more self-service or staffed customer terminals. A drug preparation area includes a robot, which is adapted to prepare prescriptions or other items, and which is connected by a pneumatic delivery system to one or more customer terminals within the pharmacy.
Owner:DIEBOLD NIXDORF
Curing method for pathologic syndrome and medicinal preparation
A method of treating a pathological syndrome includes administration of an activated form of ultra-low doses of antibodies to an antigen, wherein said activated form is obtained by repeated consecutive dilution combined with external impact, and the antigen is a substance or a pharmaceutical agent exerting influence upon the mechanisms of formation of this particular pathological syndrome. Pharmaceutical agent for treating a pathological syndrome contains activated form of ultra-low doses of monoclonal, polyclonal or natural antibodies to an antigen, wherein said activated form is prepared by means of repeated consecutive dilution and external treatment, predominantly based on homeopathic technology, and said antigen is a substance or a drug acting as a direct cause of the pathological syndrome or involved in regulation of mechanisms of its formation. At that, activated forms of ultra-low doses of antibodies are raised against antigens of exogenous or endogenous origin, against autologous antigens, fetal antigens; anti-idiotypic antibodies are used too.
Owner:EPSHTEIN OLEG I
Cosmetic and/or pharmaceutical preparations
Cosmetic and / or pharmaceutical compositions which contain: (a) a Brassicaceae extract; and (b) one or more compounds selected from the group consisting of oil components, emulsifiers, antioxidants and UV / IR protection factors are described, along with methods for preparing and using the same.
Owner:COGNIS FRANCE SA
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug for treating sexual dysfunction dispersed within a polymer matrix and methods and treatments using said compositions.
Owner:GLYCOBIOSCI +1
Pharmaceutical Preparations For And Treatment Of Ocular Surface and Other Disorders
InactiveUS20070280924A1Significant positive effectOrganic active ingredientsBiocidePharmaceutical formulationDisease
A pharmaceutical preparation suitable for use in the eye, which comprises: (i) a pharmaceutically acceptable carrier suitable for use in the eye; (ii) one or more ingredients selected from factors and agents that promote any one or more of survival, health, cell attachment and normal differentiation of ocular surface epithelial cells and optionally factors and agents to prevent squamous metaplasia; (iii) one or more agents capable of altering the fluid properties of a tear film including at least one agent capable of establishing and / or maintaining a stable tear film and optionally one or more agents selected from opthalmological lubricating agents, viscosity enhancing agents and agents capable of reducing tear film evaporation; the factors and agents in component (ii) and (iii) being synthetic or recombinant or licensed for pharmaceutical use.
Owner:INST OF OPTHALMOLOGY OF UNIV COLLEGE LONDON
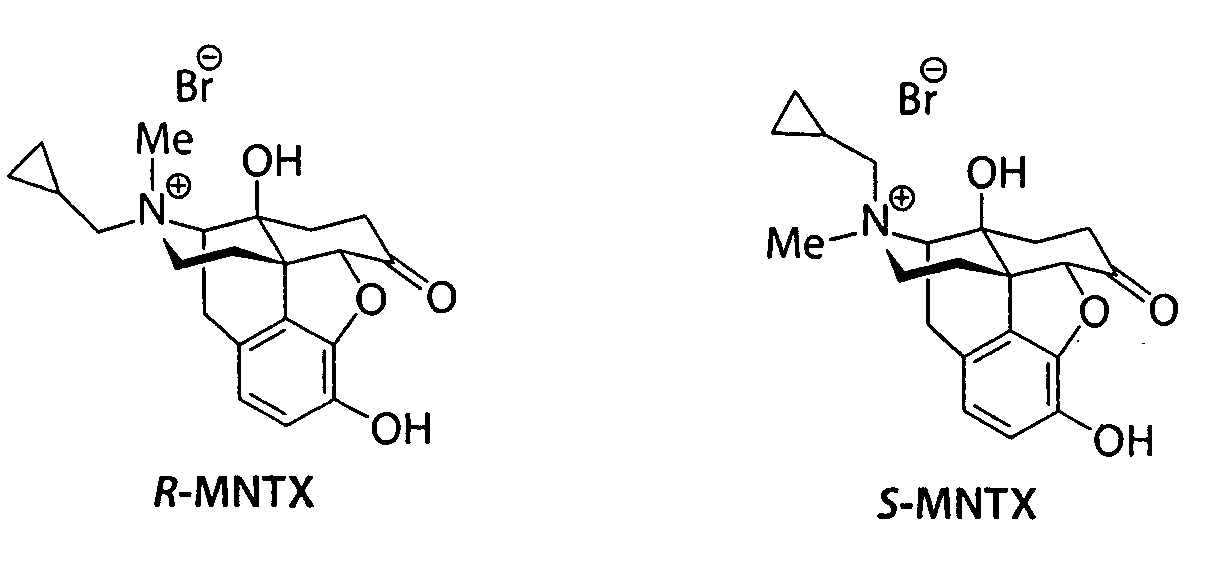
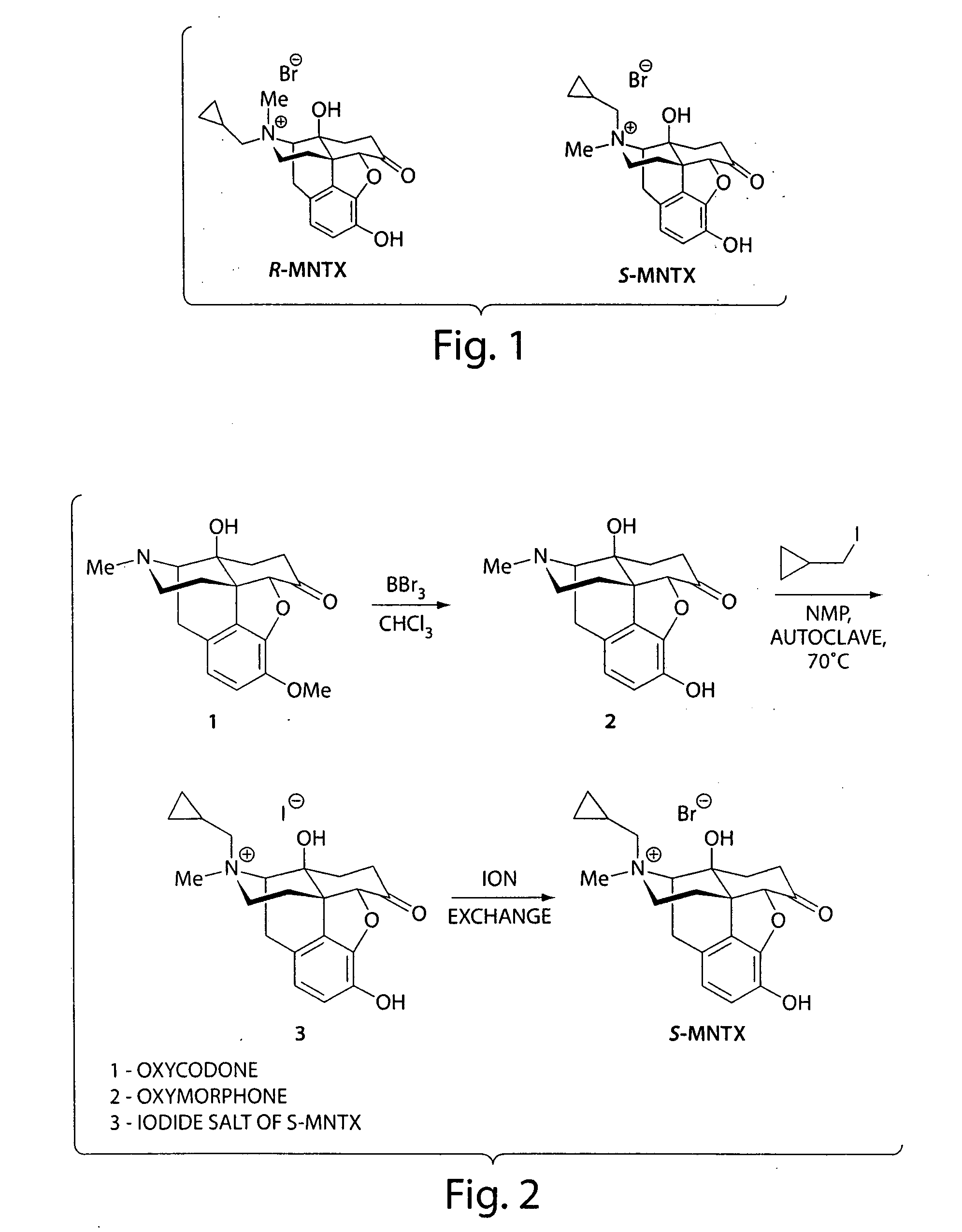
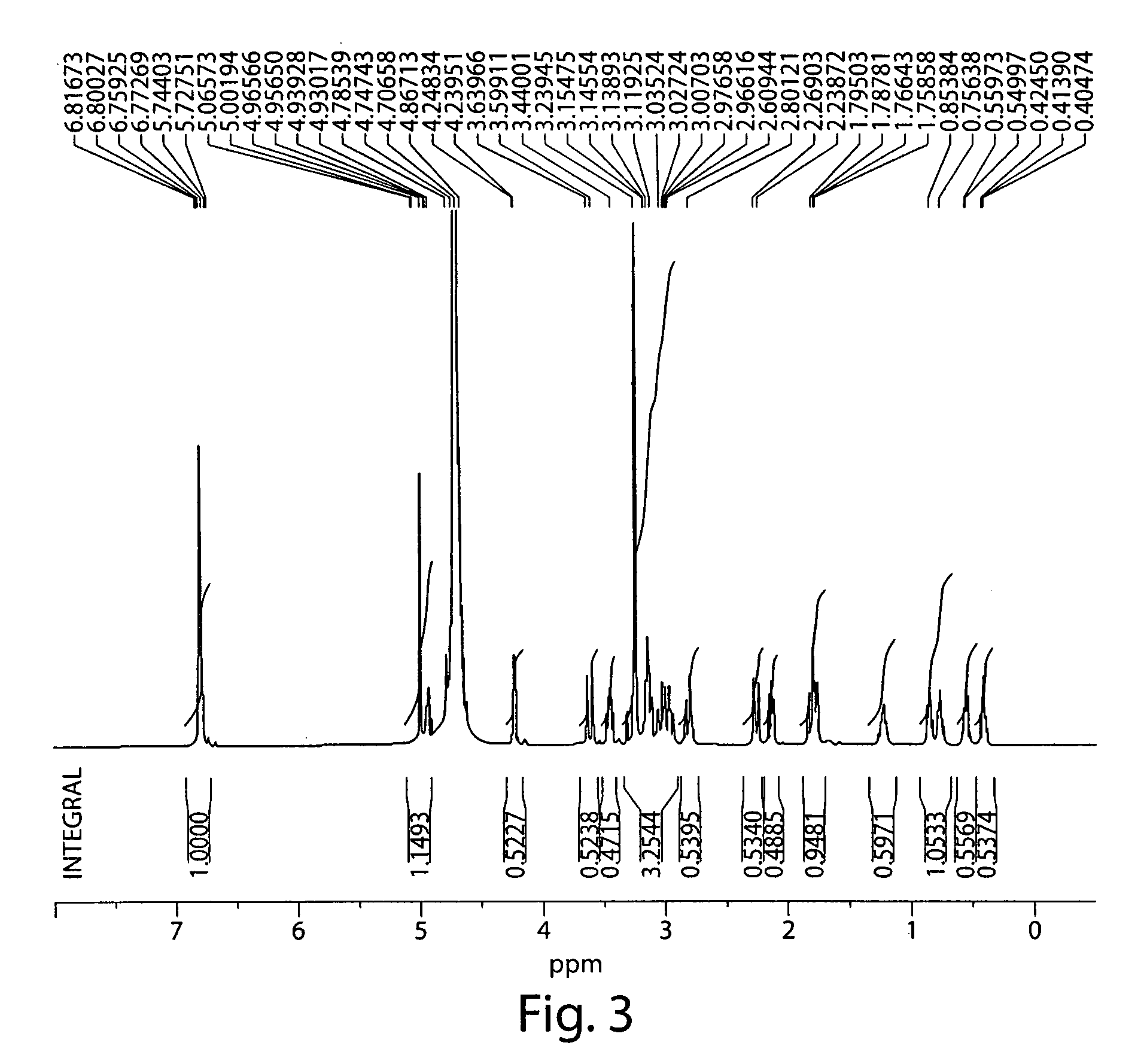
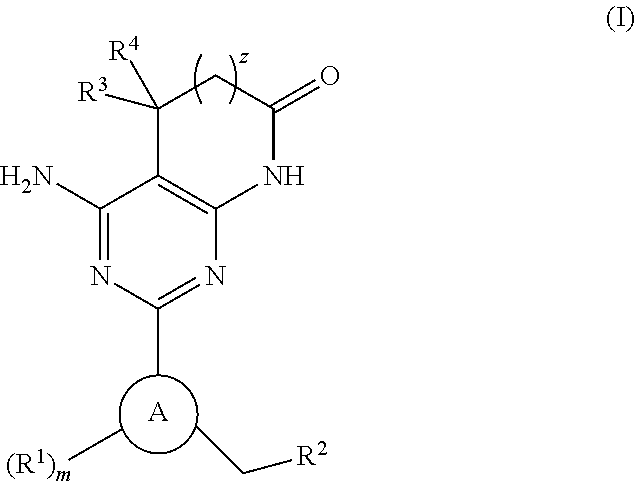
(S)-N-methylnaltrexone
Owner:PROGENICS PHARMA INC
Soluble guanylate cyclase activators
Compounds of Formula I are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of the Formula I, to their use for the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of the Formula I.
Owner:MERCK SHARP & DOHME LLC
Aggregates with increased deformability, comprising at least three amphipats, for improved transport through semi-permeable barriers and for the non-invasive drug application in vivo, especially through the skin
InactiveUS20040105881A1Increase drug concentrationImprove distributionOrganic active ingredientsAntipyreticBiological bodyDrug carrier
Owner:IDEA AG
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug which causes vasodilation, and optionally prostaglandin E1, dispersed within a polymer matrix, and methods of treating sexual dysfunction, including both male and female sexual dysfunction, using said compositions.
Owner:L A M PHARMA +1
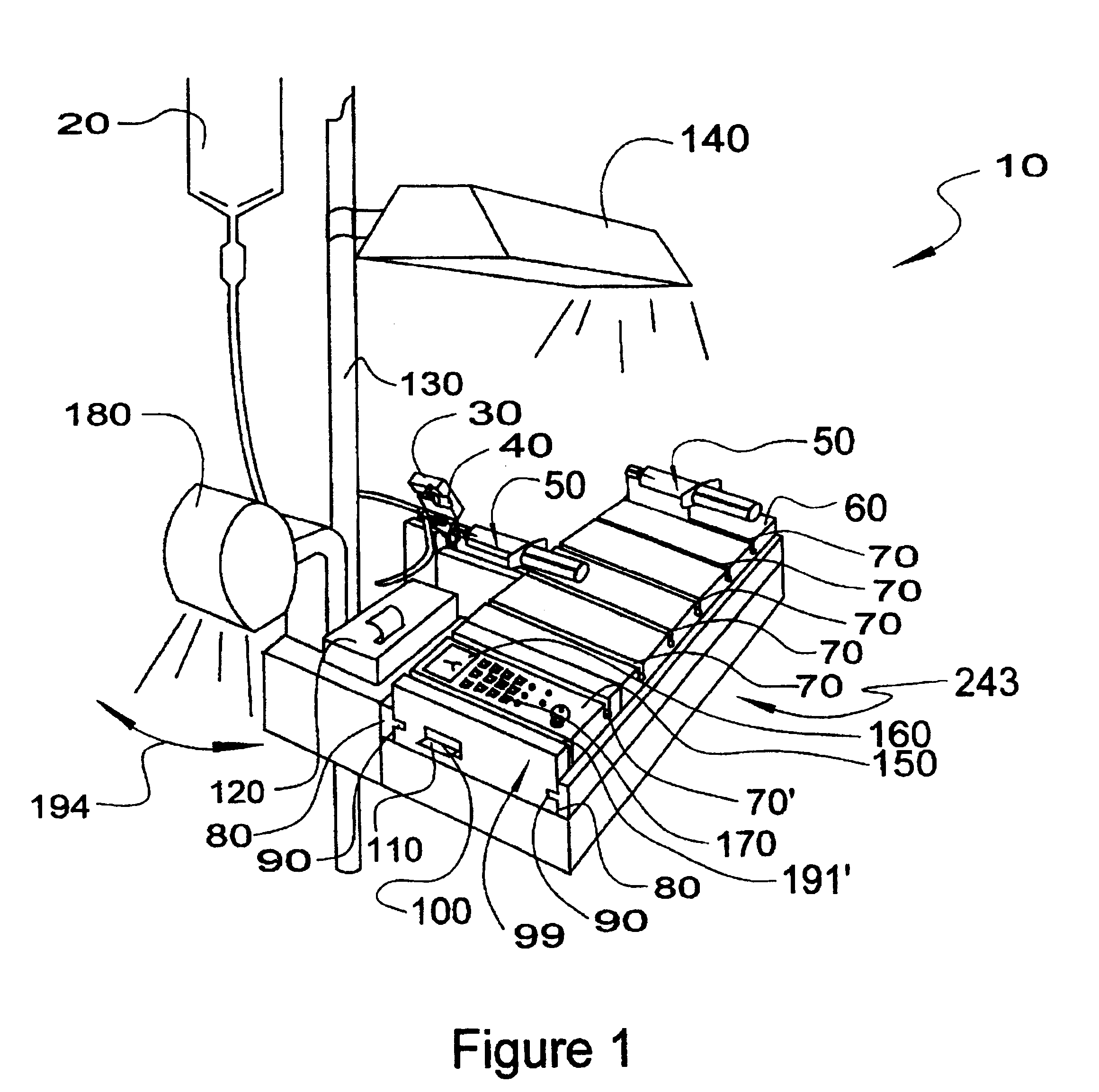
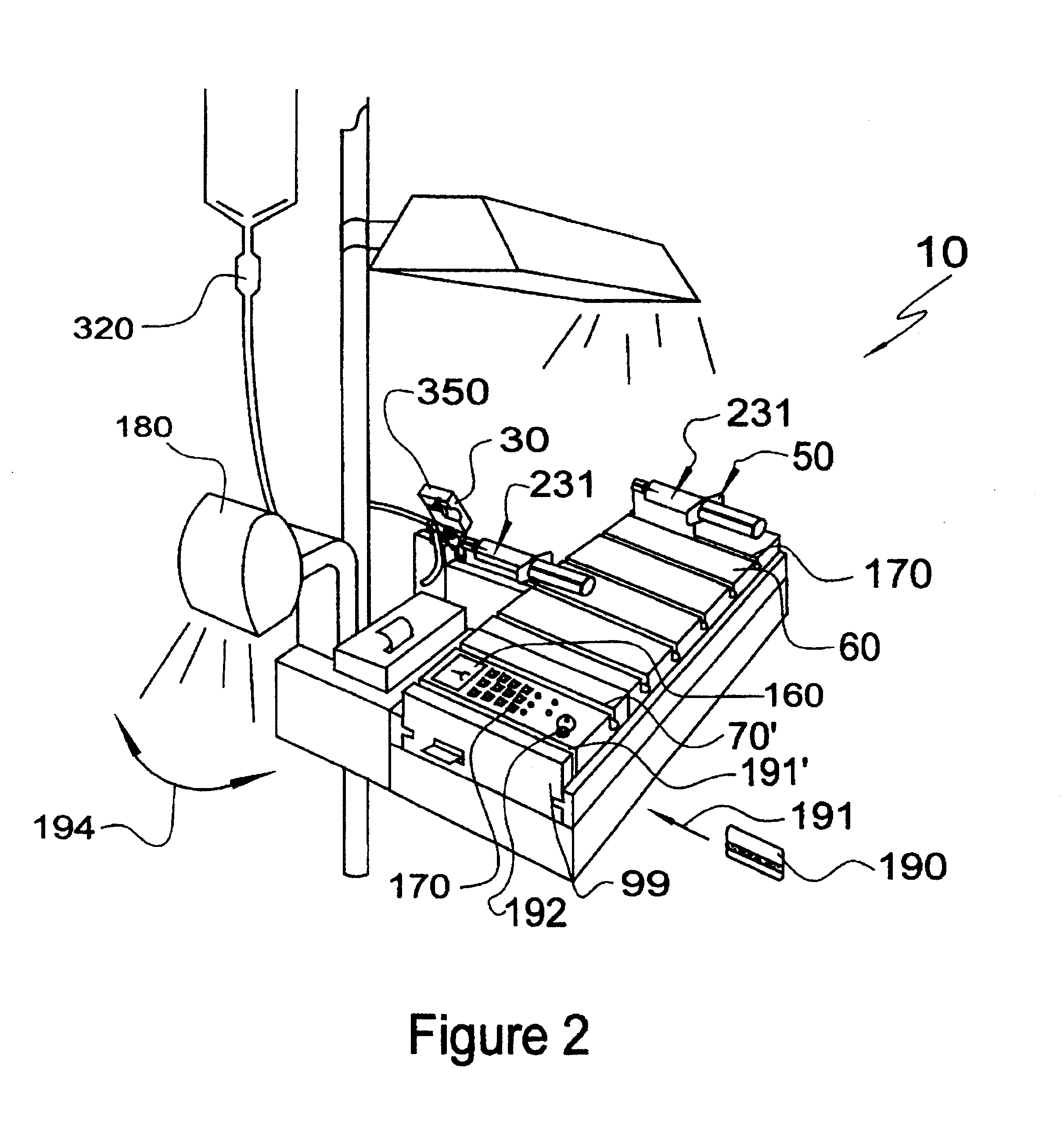
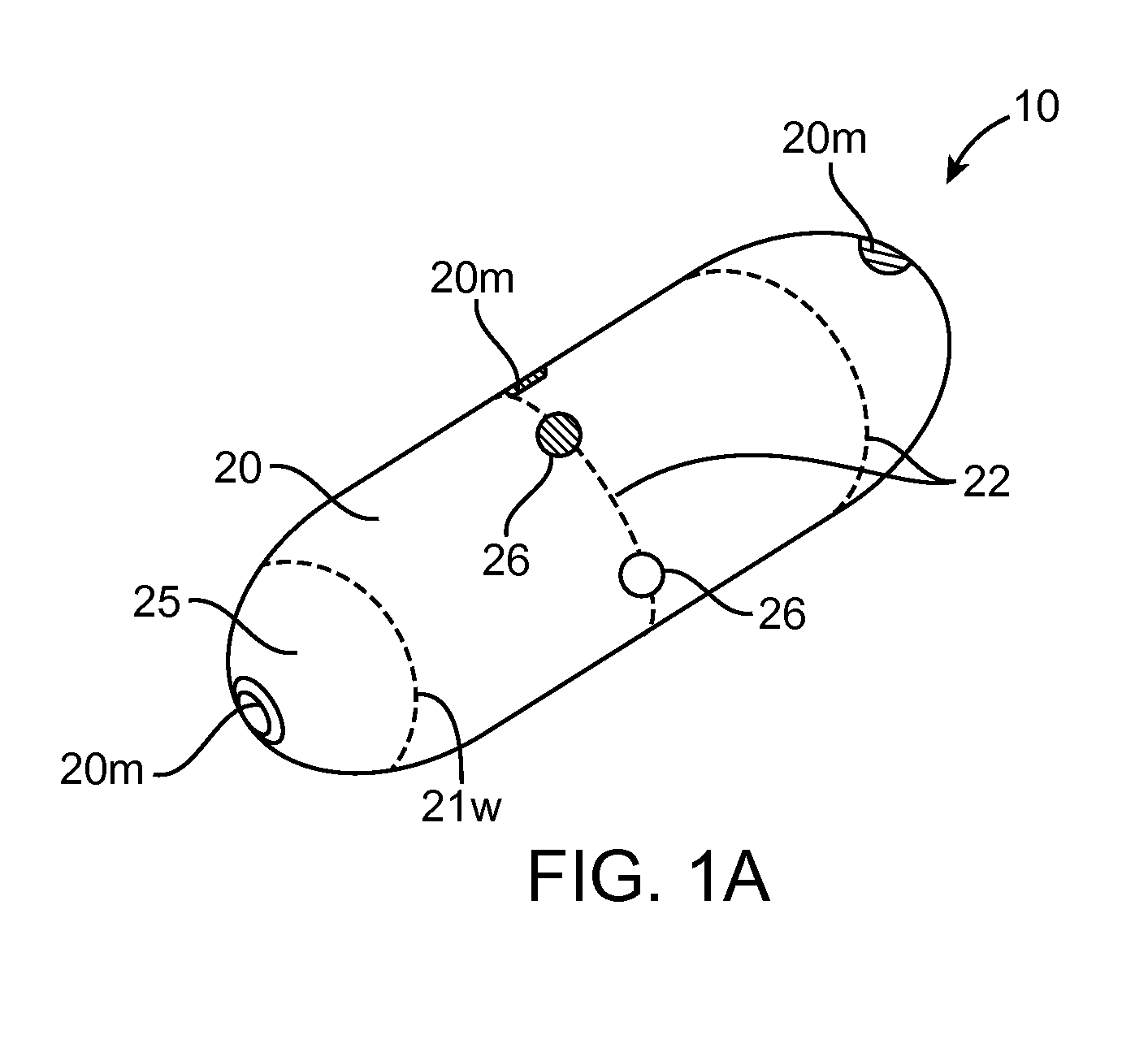
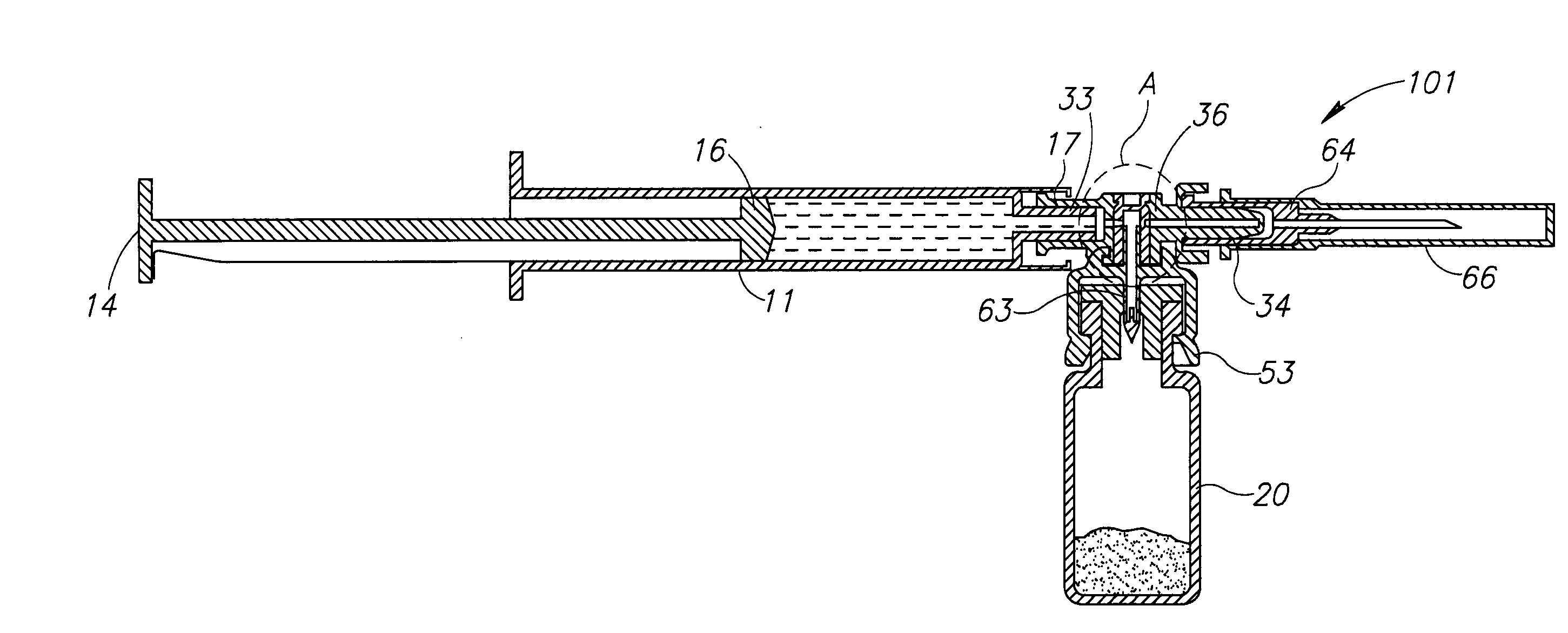
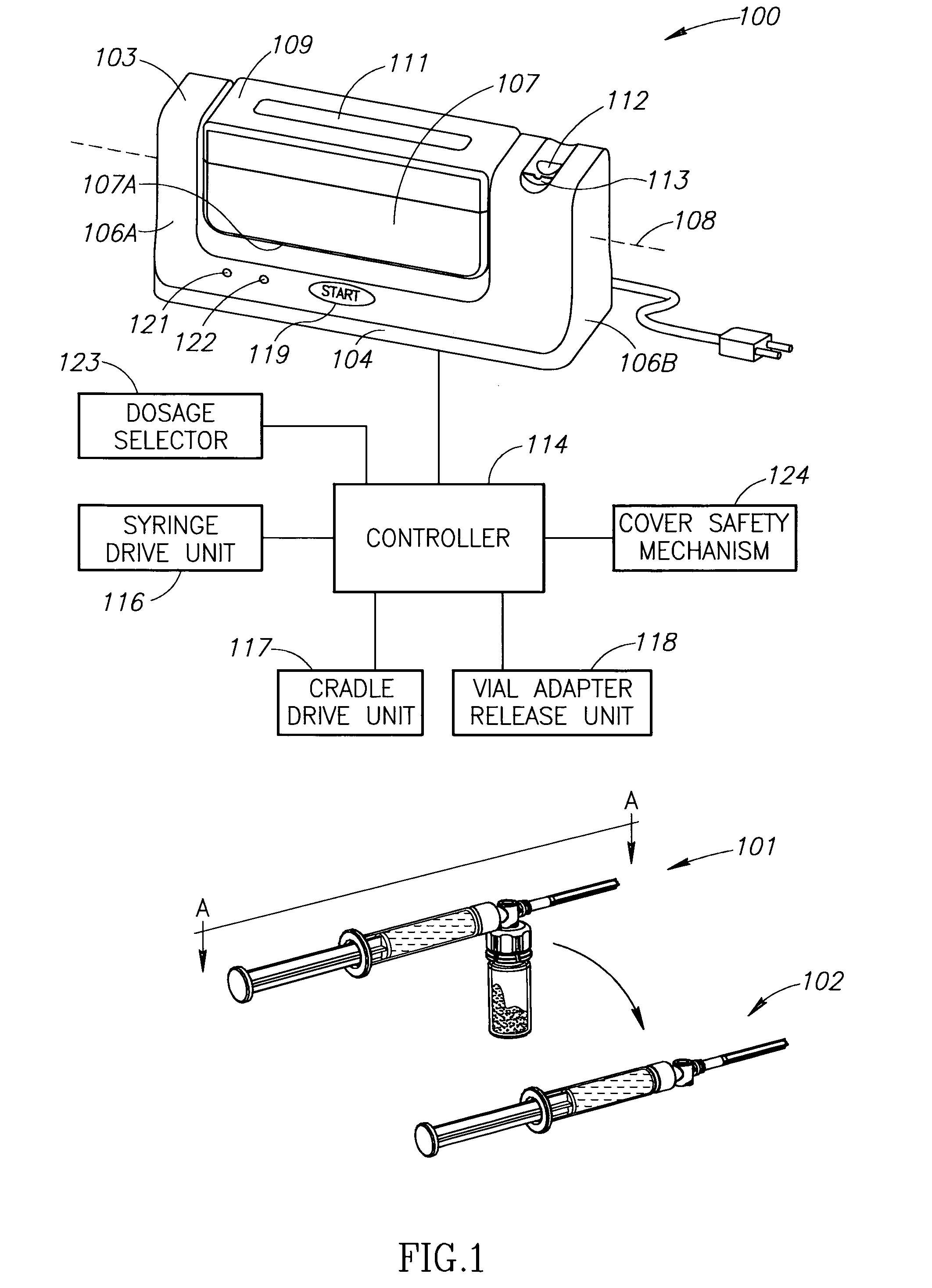
Automatic Liquid Drug Preparation Apparatus for the Preparation of a Predetermined Dosage of Liquid Drug
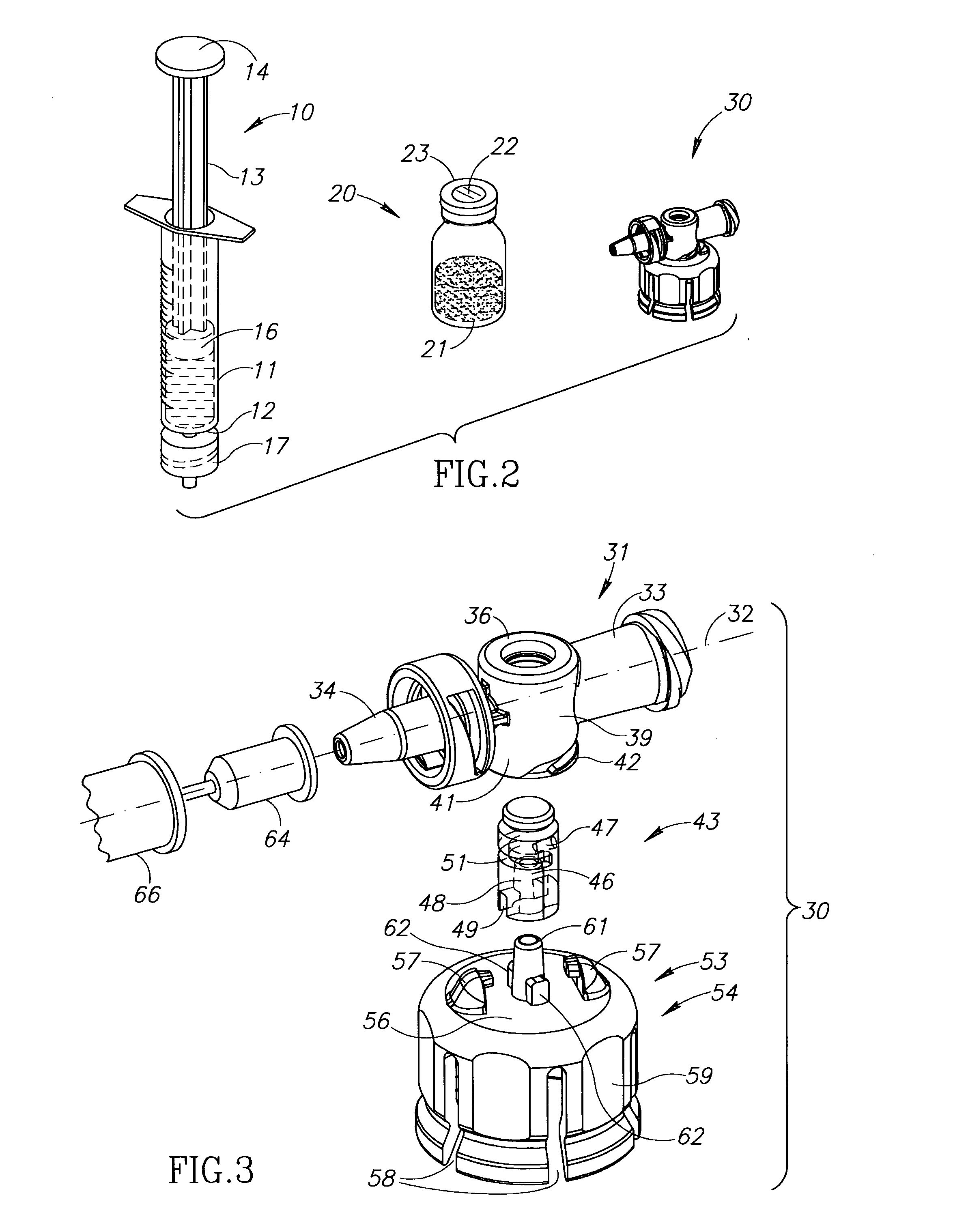
Automatic liquid drug preparation apparatus for preparing a liquid drug assemblage with a predetermined dosage of liquid drug. The liquid drug assemblage is prepared from a preparatory assemblage including a fluid control device having a body member with a syringe port, a vial adapter, and an administration port, a vial pre-filled with powder or liquid medicament, and a syringe pre-filled with diluent for mixing with the medicament. The automatic liquid drug preparation apparatus includes a housing, a cradle for receiving the preparatory assemblage, and a controller for controlling a motorized syringe drive unit for selectively transferring liquid contents between the syringe and the vial, a motorized cradle drive unit for selectively rotating the cradle relative to the housing, and a motorized vial adapter release unit for selectively detaching the vial adapter with its attached spent vial from the body member to form the liquid drug assemblage.
Owner:MEDIMOP MEDICAL PROJECTS
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS20140221927A1Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Novel pharmaceutical compositions administering n-0923
The invention relates to a pharmaceutical composition for administering the dopamine agonist N-0923 in depot form. The invention makes available for the first time a depot form of N-0923, which achieves a therapeutically significant plasma level over a period of at least 24 hours after administration to a patient. As a result of poor oral bio-availability and the short plasma half-life, N-0923 was previously administered either by an intravenous drip or by transdermal systems. Preferred embodiments of said invention are oily suspensions, containing the active ingredient N-0923 in a solid phase, in addition to anhydrous pharmaceutical preparations of N-0923.
Owner:UCB SA
Active substances for use in cosmetic and/or pharmaceutical products, obtainable from the fermentation of plant components and/or plant extracts
Processes for producing cosmetic and / or pharmaceutical active components which comprise: (a) providing a fermentation broth comprising a plant component selected from the group consisting of plant constituents, plant extracts and mixtures thereof; (b) inoculating the fermentation broth with a microorganism; and (c) fermenting the microorganism-containing fermentation broth to produce an active component; are described along with cosmetic and / or pharmaceutical preparations containing such active components and methods of using the same to treat the skin and / or hair.
Owner:COGNIS FRANCE SA
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS20130274659A1Rapid drug releasePoor absorptionSurgeryMedical devicesIntestinal wallsChemical compound
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
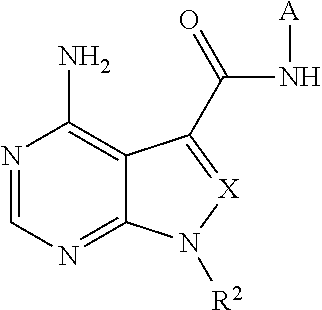
New carboxamide compounds having melanin concentrating hormone antagonistic activity, pharmaceutical preparations comprising these compounds and process for their manufacture
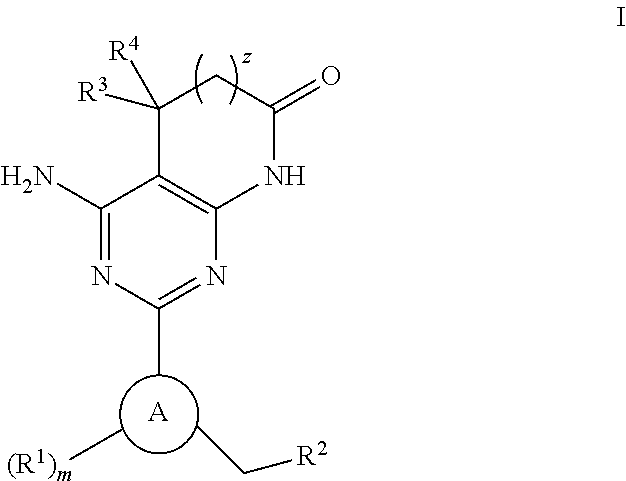
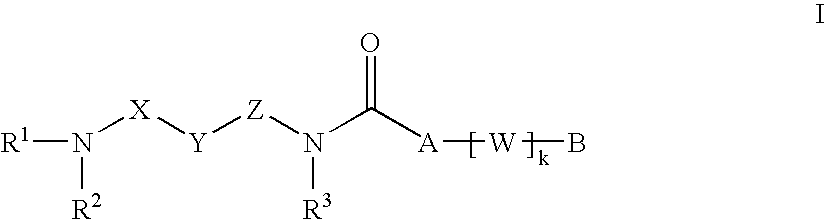
The present invention relates to carboxamide compounds of general formula I wherein the groups and residues A, B, W, X, Y, Z, R<1>, R<2>, R<3 >and k have the meanings given in claim 1. Moreover the invention relates to process for preparing the above mentioned carboxamides as well as pharmaceutical compositions containing at least one carboxamide according to the invention. In view of their MCH-receptor antagonistic activity the pharmaceutical compositions according to the invention are suitable for the treatment of metabolic disorders and / or eating disorders, particularly obesity, bulimia, anorexia, hyperphagia and diabetes.
Owner:BOEHRINGER INGELHEIM INT GMBH
Chinese medicine bone-setting ointment and preparing method
ActiveCN1814247AOvercoming the defects in the traditional methodGood curative effectHydroxy compound active ingredientsAerosol deliveryMedicinal herbsDisease
The invention discloses a Chinese medicinal plaster for curing orthopaedics disease and the preparing method thereof, characterized by that it uses several ones of black-bone chicken, Chinese Angelica, Notoginseng powder,Myrrha, Rhizoma Drynariae, Dragon's Blood, Radix Curcumae, Common Burreed Rhizome, Zedoary, Giant Knotweed Rhizome, Ground Beeltle, Pyrite, and Frankincense as principal drugs and selects parts of 97 Chinese drugs such as Long-nosed Pit Viper, Earthworm, Dragon's Blood, Prepared ChuanWu, Pangolin Scales, Doubleteeth Pubesscent Angelica Root, Rhizoma Corydalis, Himalayan Teasel Root, Cortex Cinnamomi Cassiae, Flos Caryophylli, and Borneol as adjuvant drugs, according to different properties of Chinese drugs, fries partial medicinal materials, decocts partial light and strongly fibrous medicinal materials with water for three times, concentrates the decoction into extract, dries and crushes the extract into fine powder, and adds the fine powder into medicinal oil added with pills; and finally adds in powder of partial fine materials. Its formula and preparing method are peculiar and it is an ideal traditional external drug preparation for curing orthopaedics disease.
Owner:田茂宁
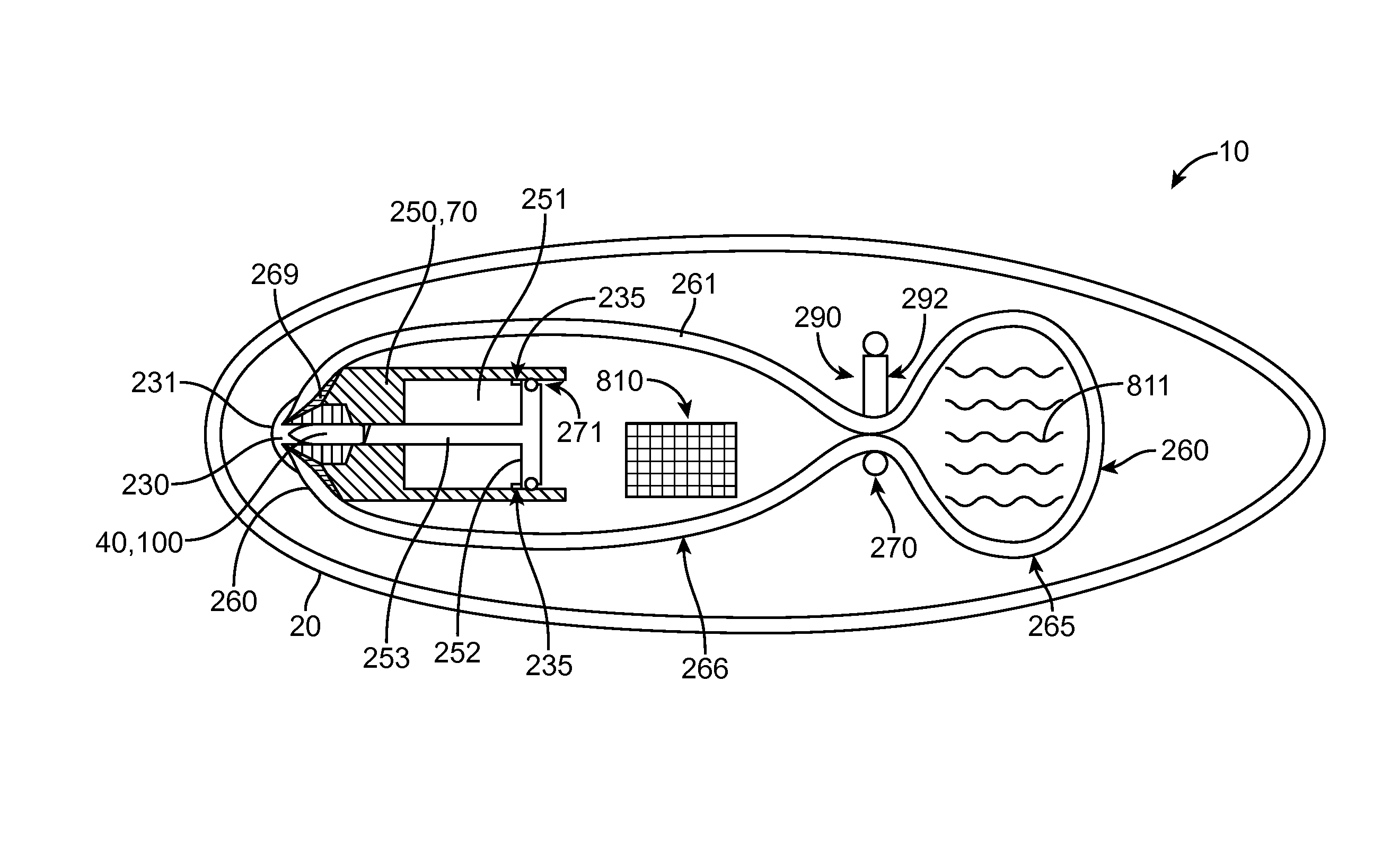
Device and method for delivery of two or more drug agents
ActiveCN102740907AEasy dose settingEase of dosingAmpoule syringesAutomatic syringesDose profileMechanical drive
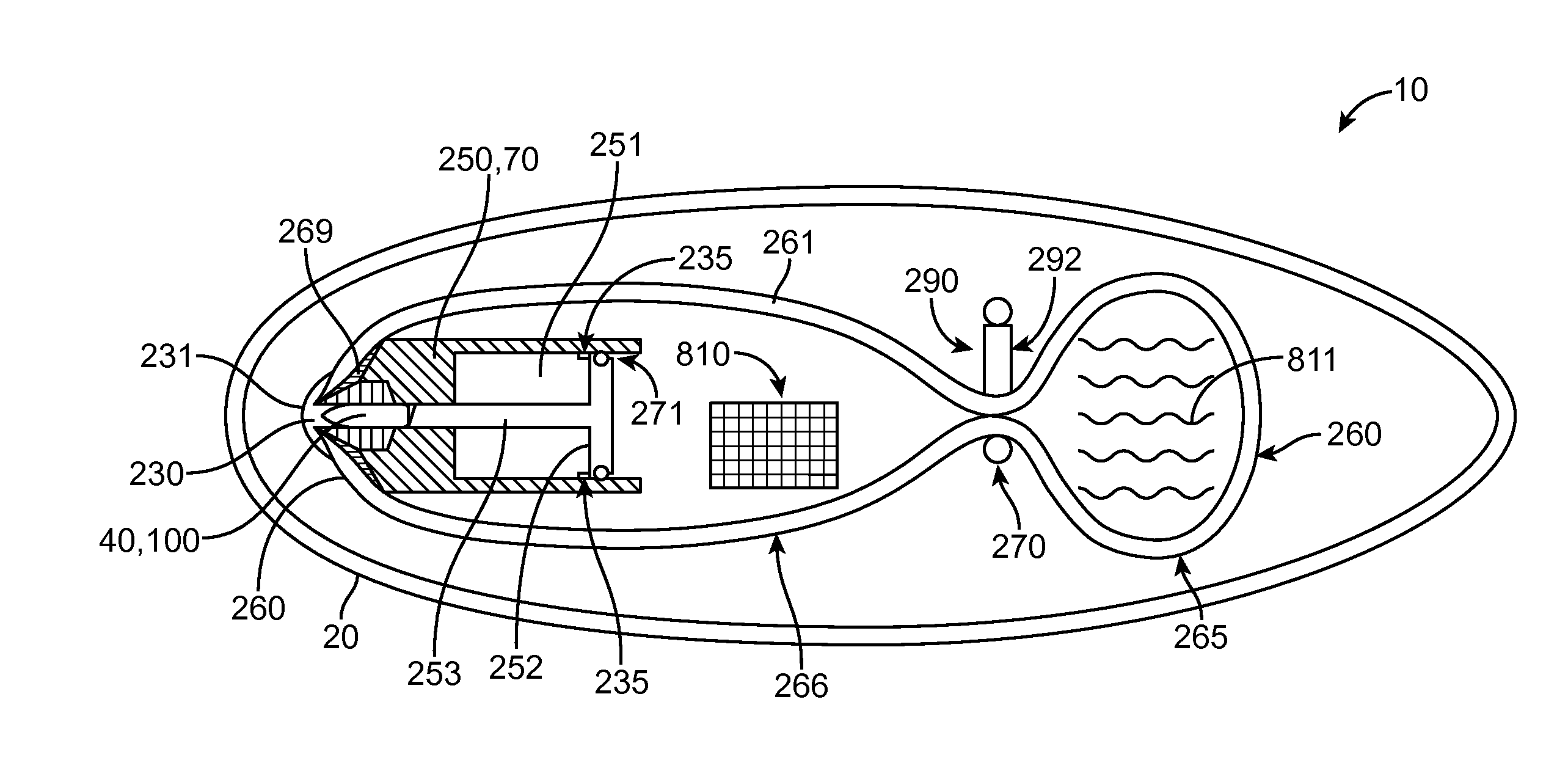
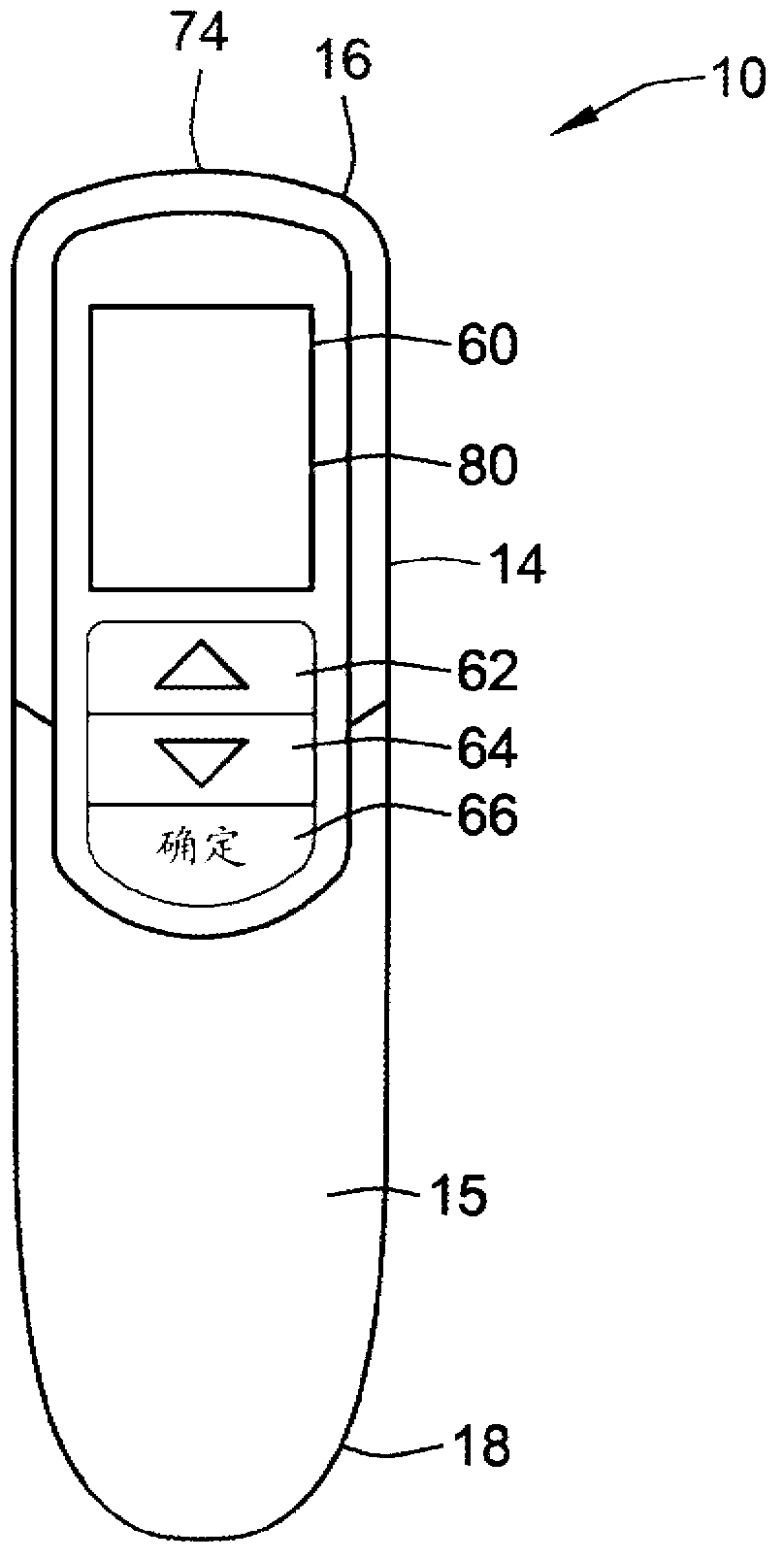
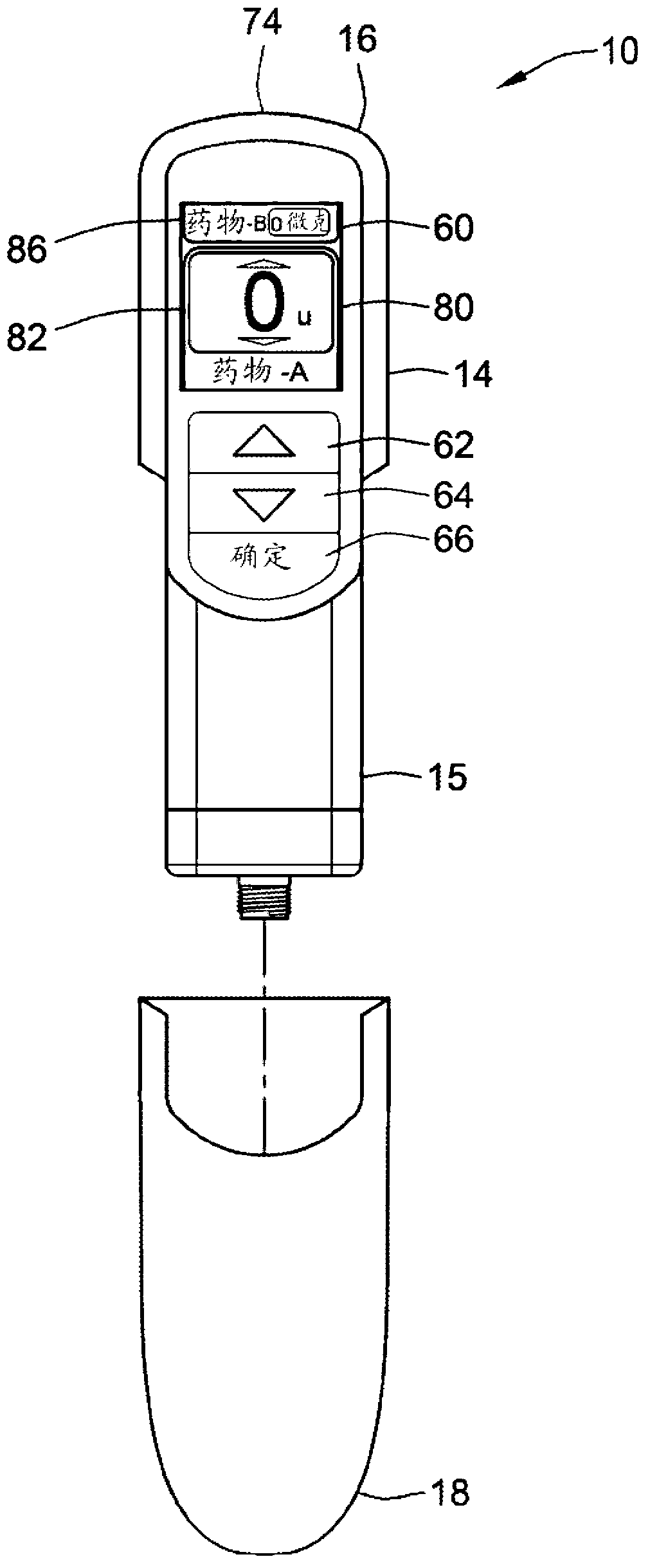
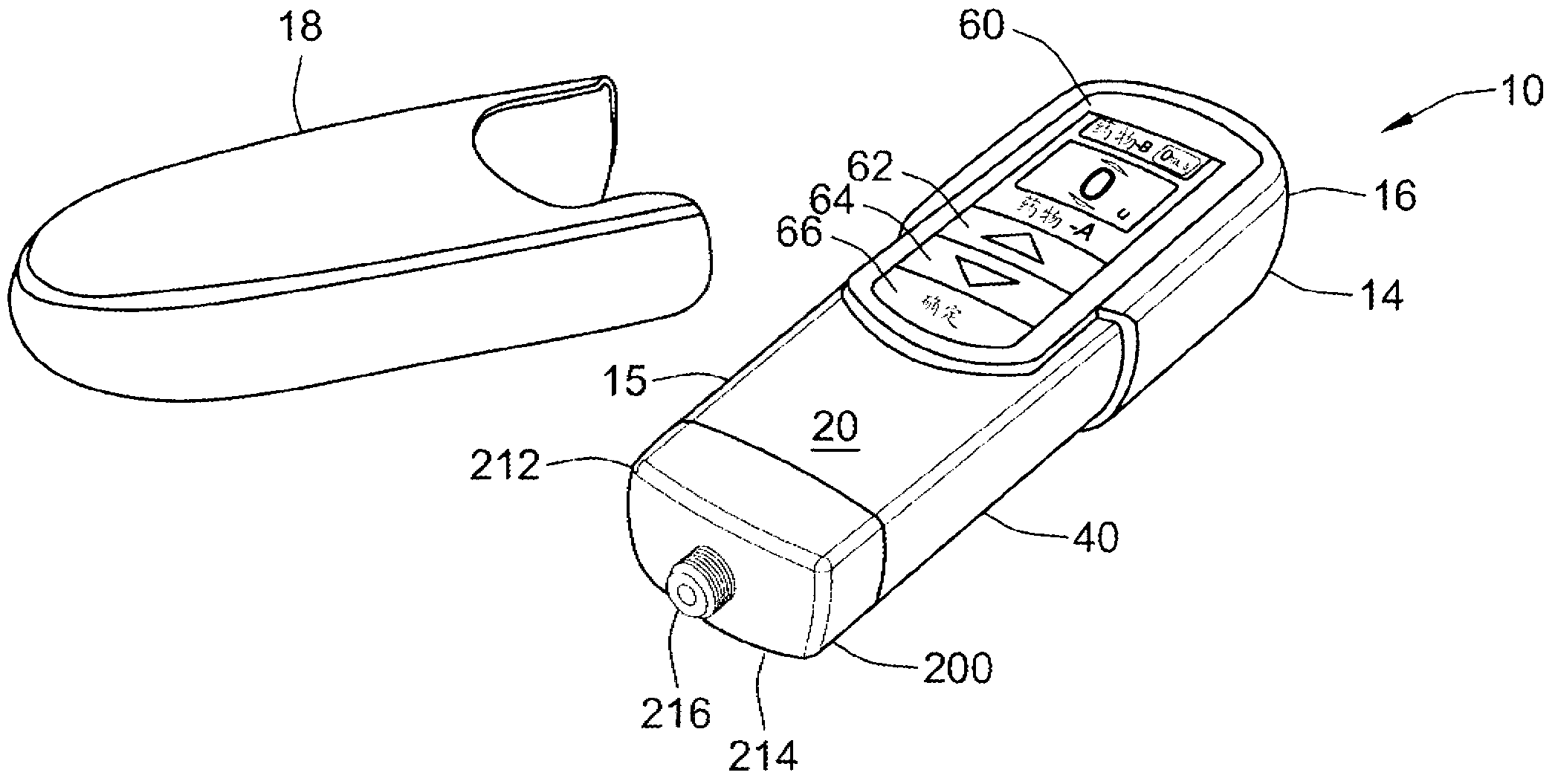
A computerized electro-mechanical drug delivery device (10) configured to deliver at least one dose of two or more medicaments. The device comprises a control unit (300). An electro-mechanical drive unit (500, 600) is operably coupled to the control unit and a primary reservoir (90) for a first medicament and a secondary reservoir (100) for a fluid agent, e.g. a second medicament. An operator interface (60) is in communication with the control unit (300). A single dispense assembly (200) is configured for fluid communication with the primary (90) and the secondary (100) reservoir. Activation of the operator panel sets a first dose from the primary reservoir and based on the first dose and a therapeutic dose profile (860), the control unit (300) is configured to determine a dose or range of the fluid agent. Alternatively, the control unit determines or calculates a dose or range of a third medicament.
Owner:SANOFI AVENTIS DEUT GMBH
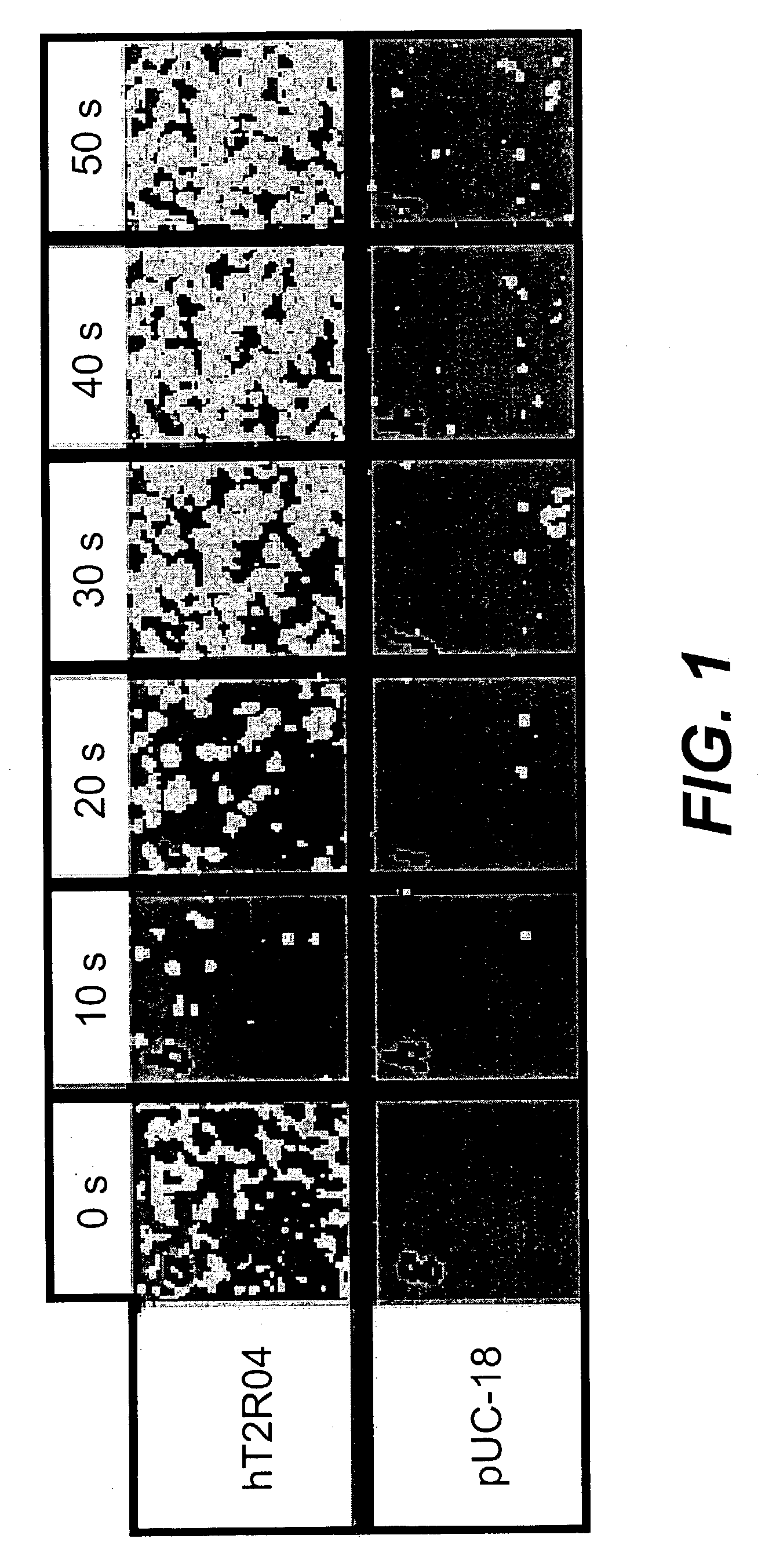
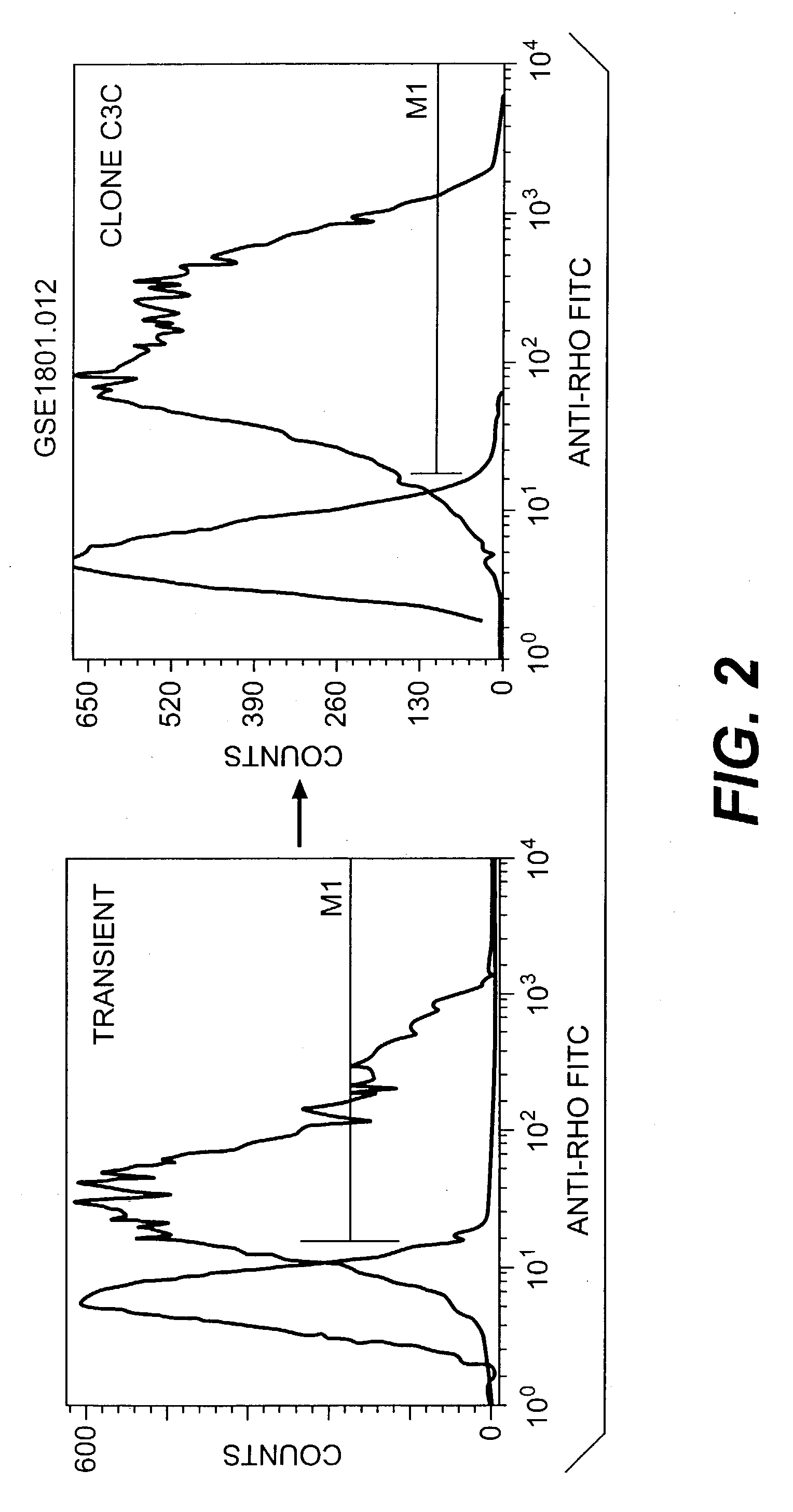
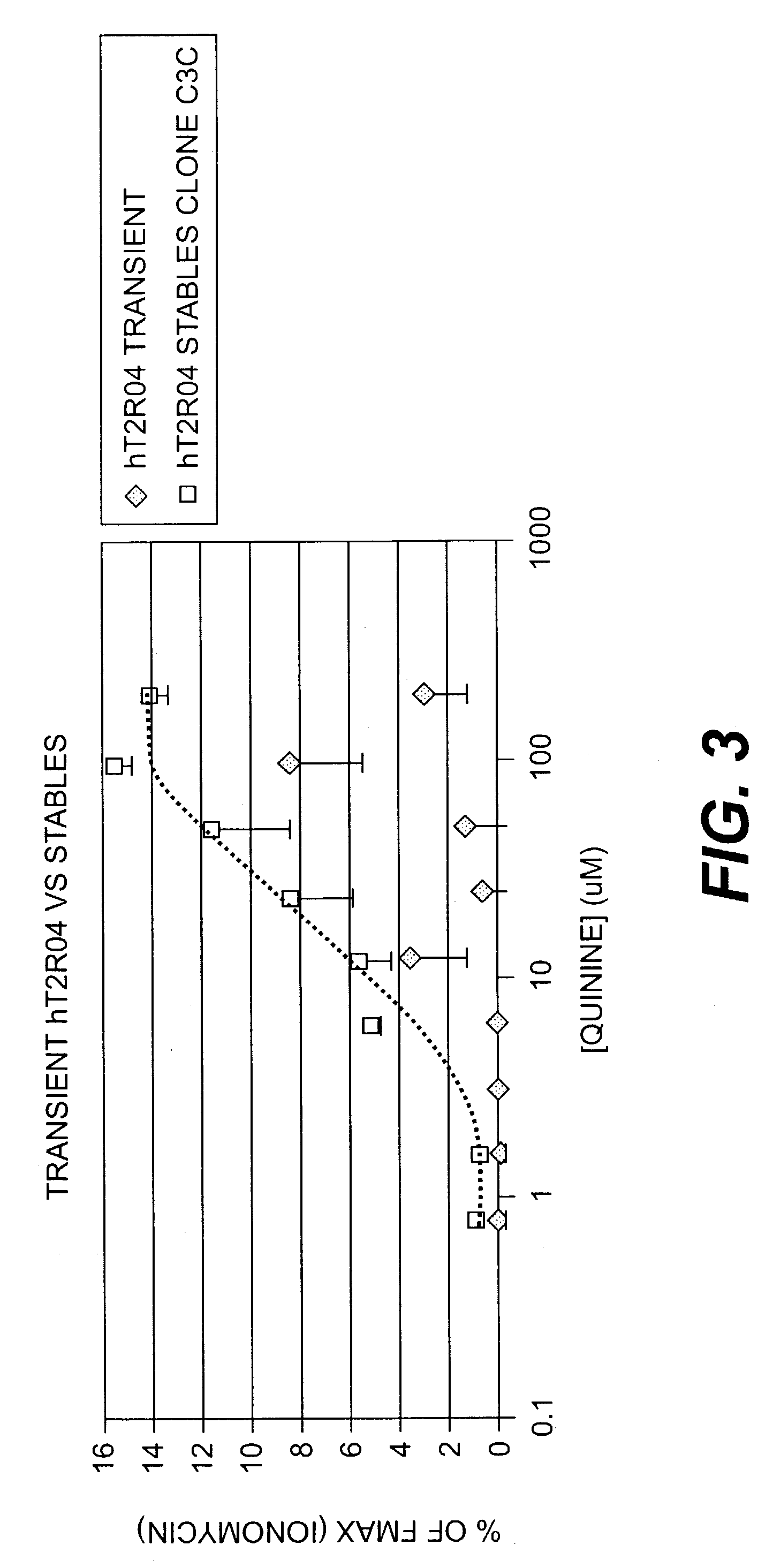
Use of specific T2R taste receptors to identify compounds that block bitter taste
ActiveUS20030170608A1Block bitter tasteInhibit activationCompound screeningApoptosis detectionDrug6-nitrosaccharin
Assays for identifying compounds that modulate, preferably inhibit bitter taste associated with the activation of hT2R4, hT2R44 and / or hT2R61 are provided. The compounds identified according to these assays should modulate, e.g., inhibit bitter taste associated with bitter tasting compounds including quinine, 6-nitrosaccharin, saccharin and / or denatonium. These compounds are useful additives for foods, beverages or medicinal preparationshaving a bitter taste.
Owner:SENOMYX INC
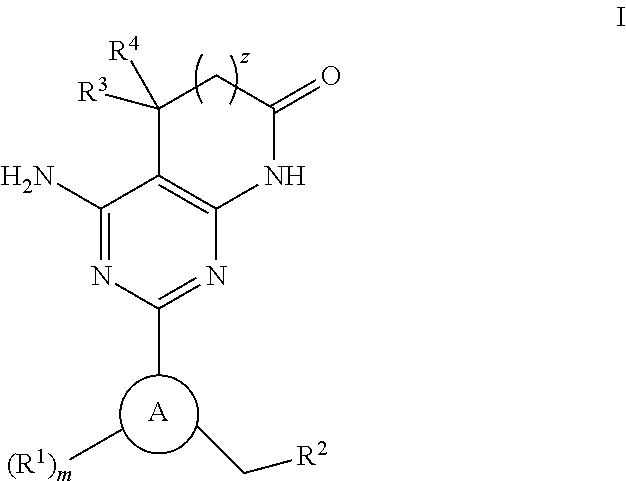
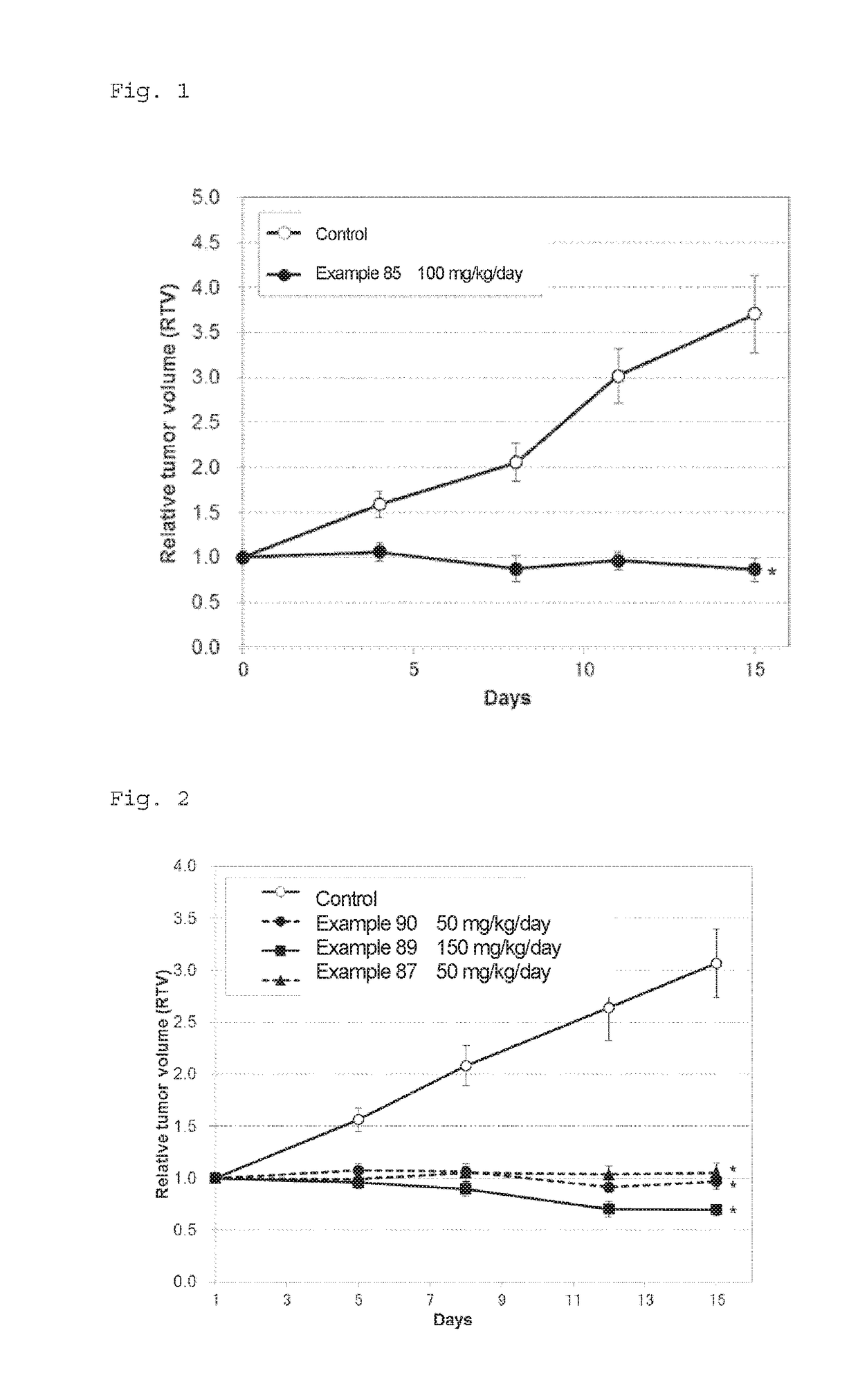
Novel fused pyrimidine compound or salt thereof
ActiveUS20180009818A1Excellent inhibitory activity against RETOrganic active ingredientsOrganic chemistryDiseaseDrugs preparations
The problem to be solved by the present invention is to provide a novel compound having RET inhibitory activity. The present invention also provides a pharmaceutical preparation that is useful for the prevention and / or treatment of RET-related diseases, particularly cancer, based on RET inhibitory activity. The present invention provides a compound represented by Formula (I):wherein A, R2, and X are as defined in the specification; or a salt thereof.
Owner:TAIHO PHARMA CO LTD
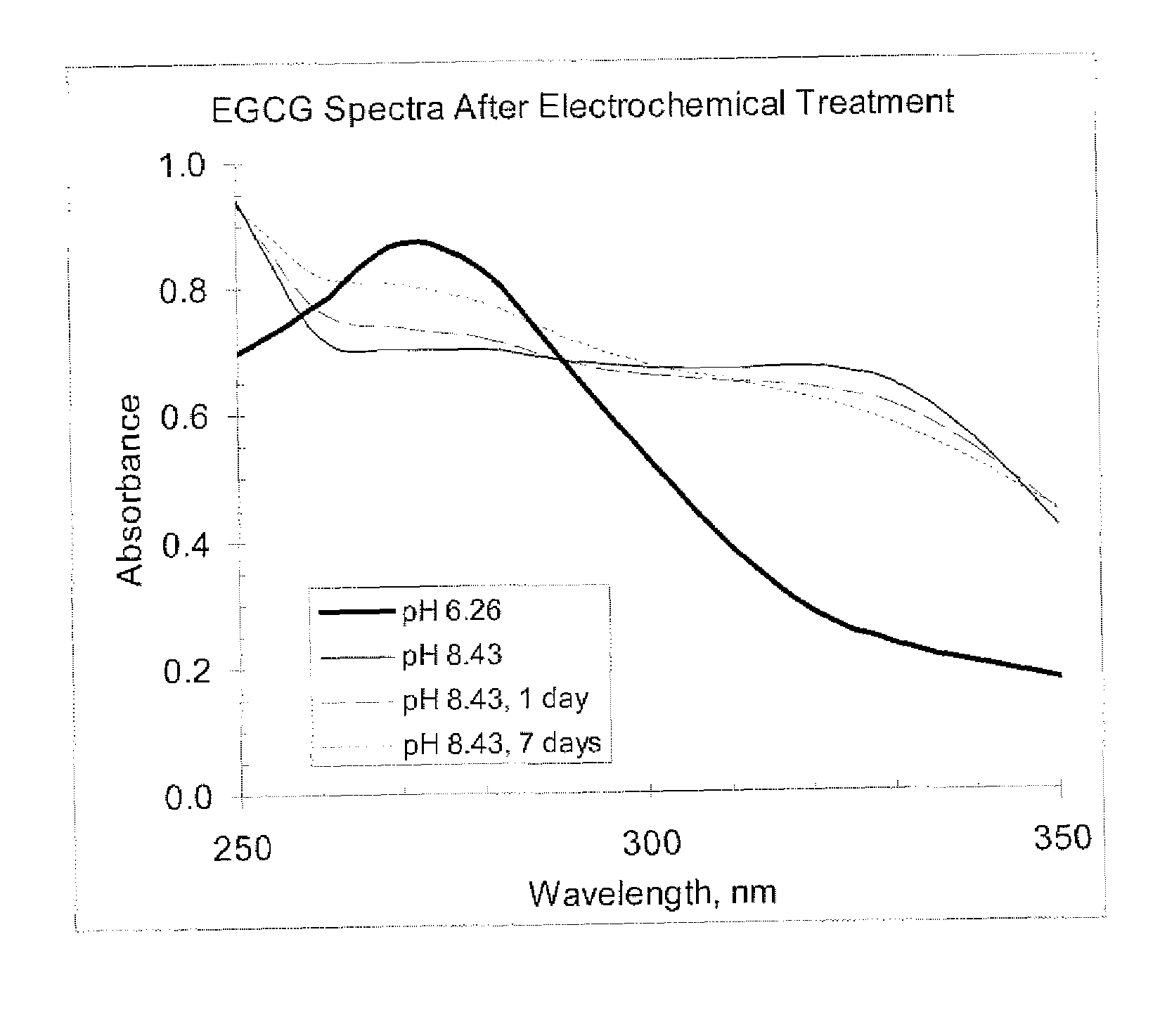
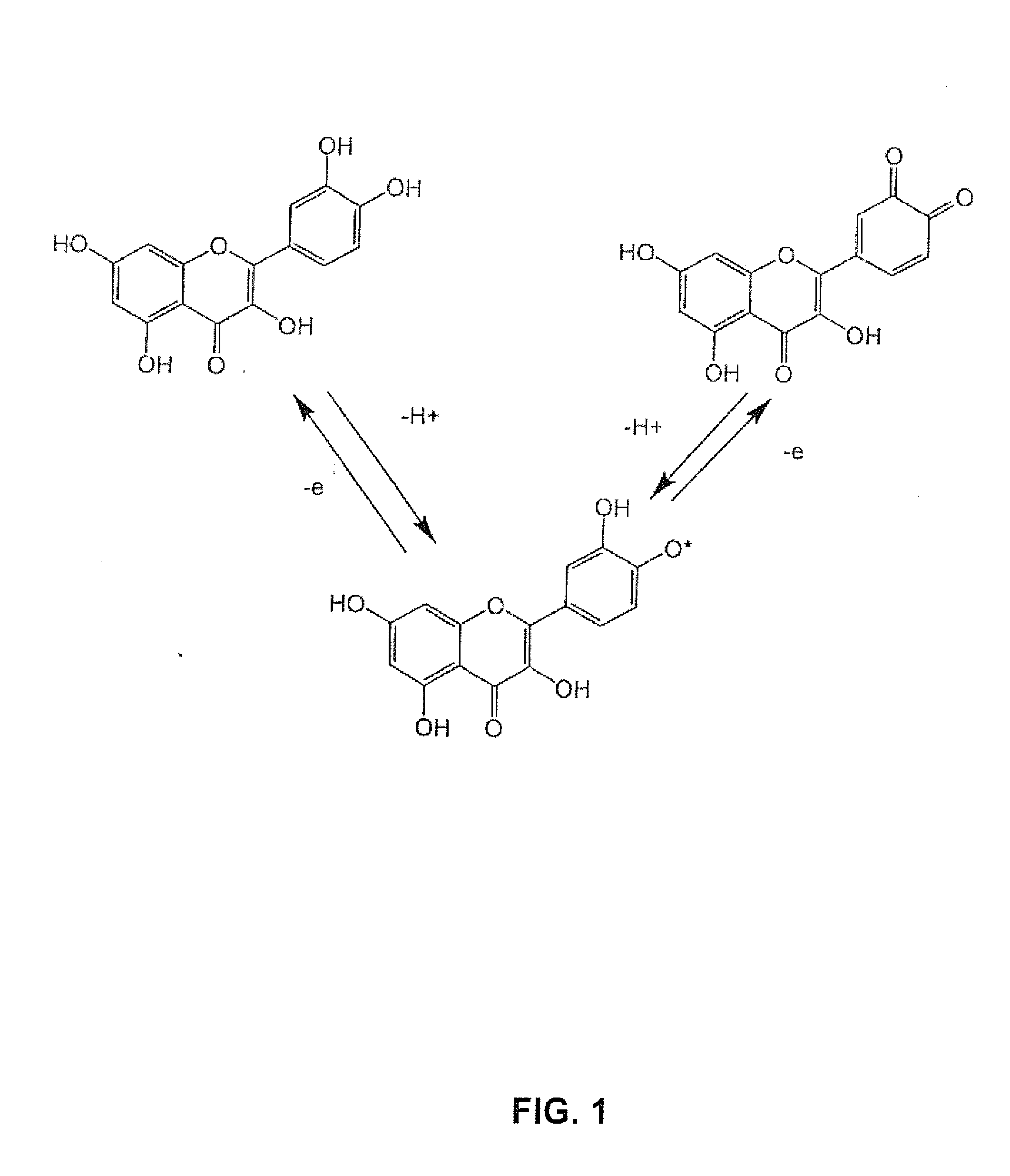
Electrochemical methods for redox control to preserve, stabilize and activate compounds
To maximize and maintain the antioxidant or pro-oxidant state for foods, beverages, personal care products, cosmetics, nutritional supplements, reagents, analytical standards, medical device formulations, pharmaceutical preparations or drags, the present invention discloses methods and devices to control redox equilibrium of such preparations throughout the processing steps and storage prior to, or at, the time of administration or use. The preparations (solid or liquid form) can then be stored in a redox-controlled container, package or applicator as described in the specification. Foods, beverages, personal care products, cosmetics, nutritional supplements, reagents, analytical standards, medical device formulations, pharmaceutical preparations and drugs stored in reactive oxidation states can be activated and stabilized by electrical voltages applied with a small battery and electrodes designed into the applicator, container or package.
Owner:CURAPHARM
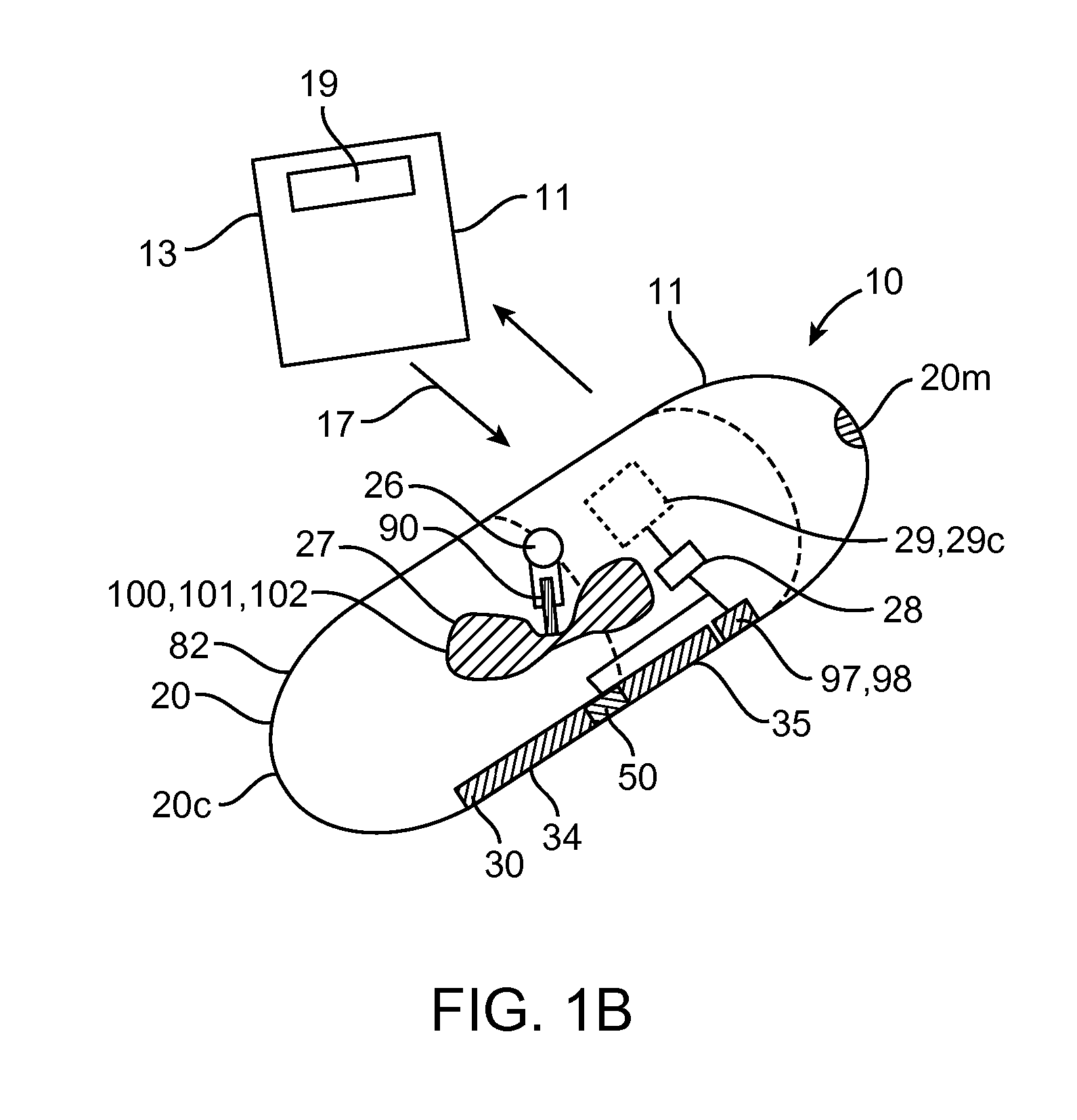
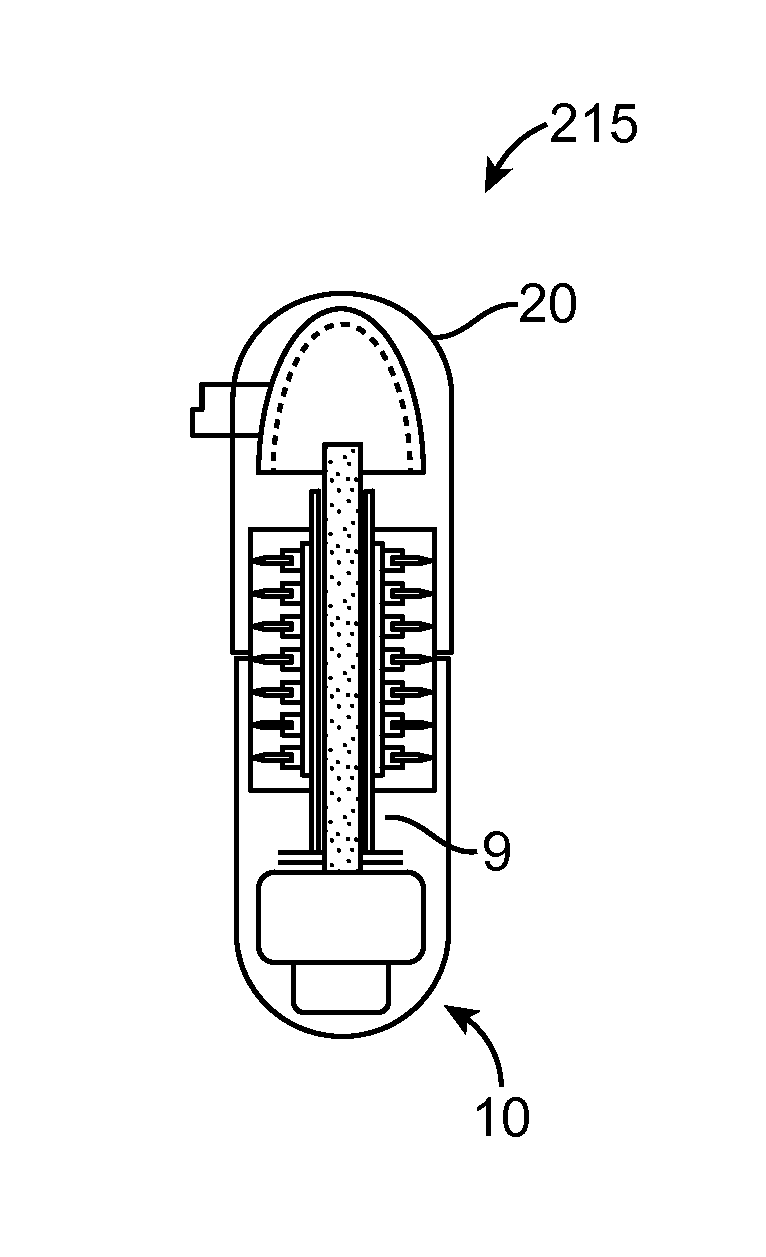
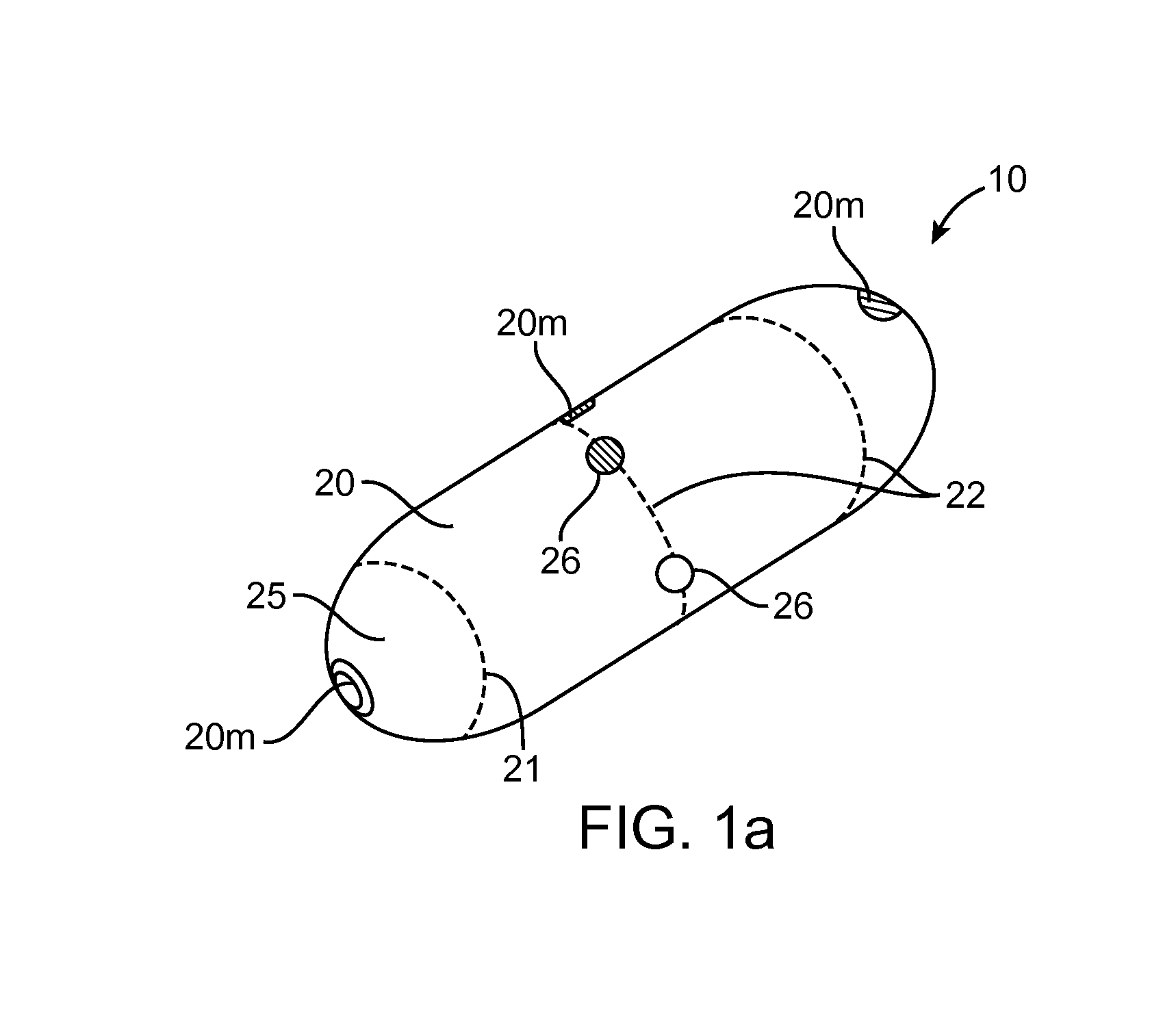
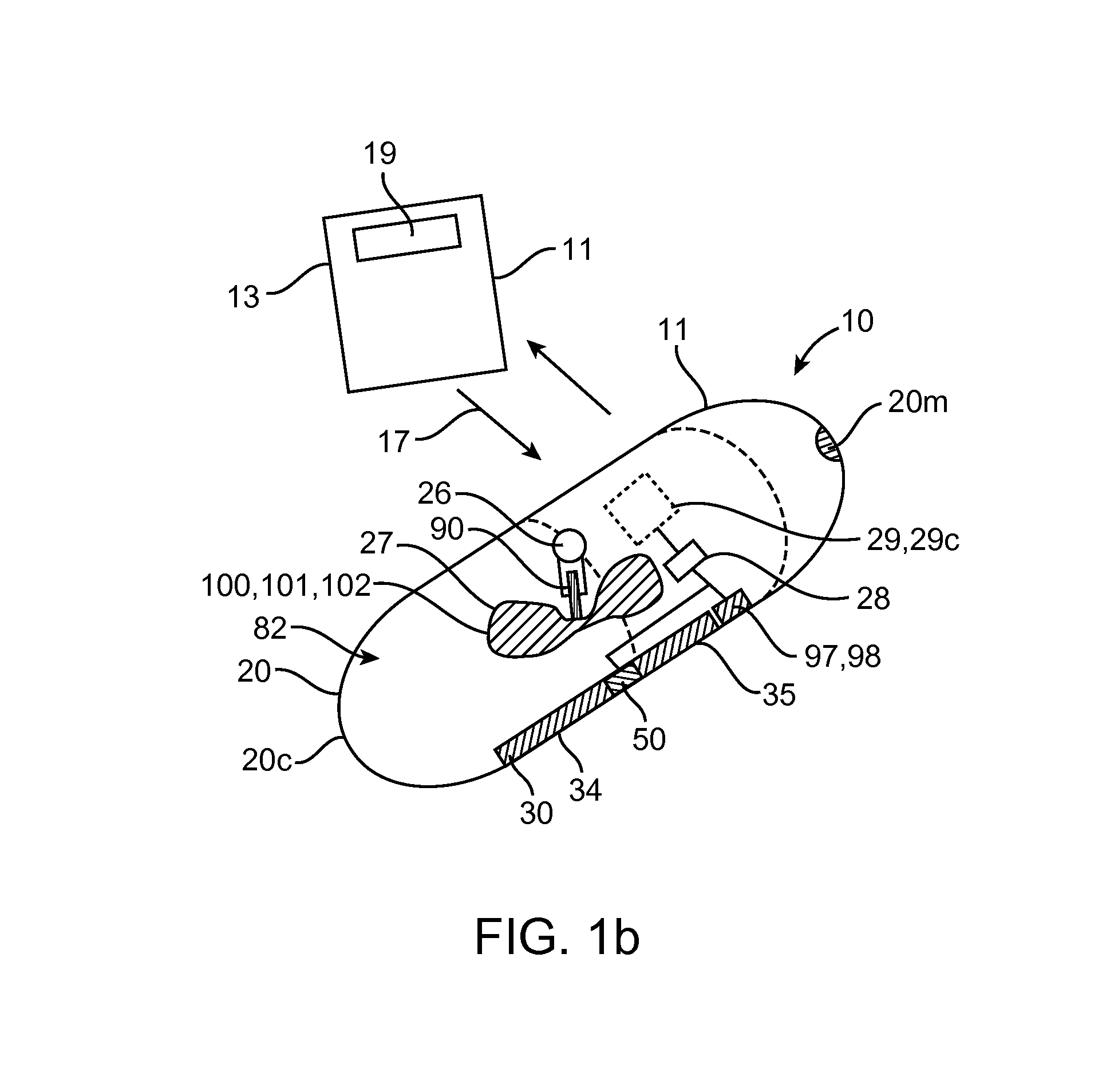
Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device
ActiveCN103025319AImprove pharmacokineticsGood curative effectAntibacterial agentsPeptide/protein ingredientsTolerabilityIntestinal walls
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Many embodiments provide a swallowable device for delivering the agents. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to an actuator having a first configuration where the preparation is contained in the capsule and a second configuration where the preparation is advanced out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Cosmetic, dermatological or pharmaceutical preparations of lipid/wax mixtures containing gases
ActiveUS20060147390A1Protects against oxidative stressCosmetic preparationsHair removalDERMATOLOGY/SKINDrugs preparations
Owner:BEIERSDORF AG
Polymorphs of abiraterone acetate and preparation method thereof
The invention discloses polymorphs A, B, C and D of abiraterone acetate. A preparation method of the polymorphs comprises the step of re-crystallizing the abiraterone acetate subjected to the separation and the purification of a chromatographic column in different solvents. Through stable investigation, four polymorphs have favorable stability and flowability, can be used as raw materials for storage and transportation and are suitably applied to antitumor medicinal preparations.
Owner:深圳万乐药业有限公司
Derivatives of pyridone and the use of them
Owner:CATALYST BIOSCIENCES INC
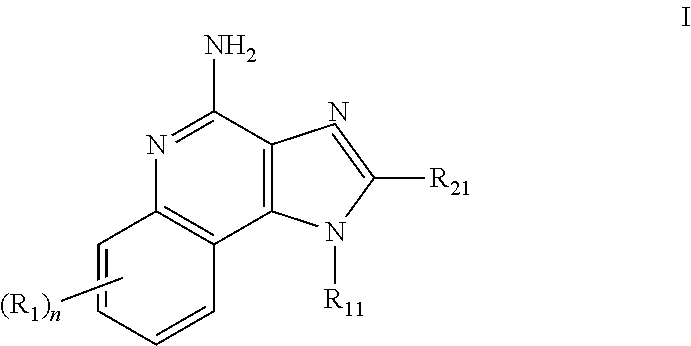
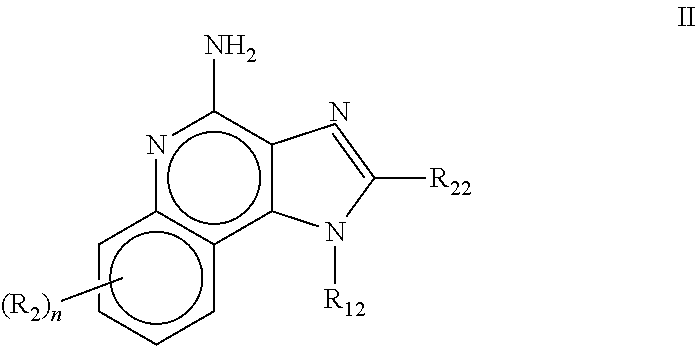
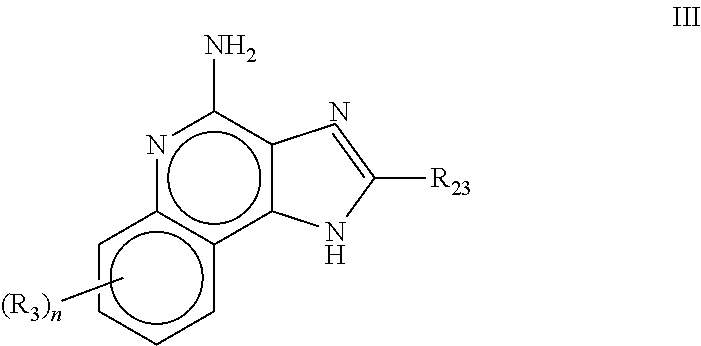
Pharmaceutical formulations comprising an immune response modifier
Pharmaceutical formulations comprising an immune response modifier (IRM) chosen from imidazoquinoline amines, imidazotetrahydroquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, 1,2-bridged imidazoquinoline amines, thiazolo-quinolineamines, oxazolo-quinolinamines, thiazolo-pyridinamines, oxazolo-pyridinamines, imidazonaphthyridine amines, tetrahydroimidazonaphthyridine amines, and thiazolonaphthyridine amines; a fatty acid; and a hydrophobic, aprotic component miscible with the fatty acid are useful for the treatment of dermal associated conditions. Novel topical formulations are provided. In one embodiment, the topical formulations are advantageous for treatment of actinic keratosis, postsurgical scars, basal cell carcinoma, atopic dermatitis, and warts.
Owner:3M INNOVATIVE PROPERTIES CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com