Patents
Literature
53 results about "Cyclic guanosine monophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclic guanosine monophosphate (cGMP) is a cyclic nucleotide derived from guanosine triphosphate (GTP). cGMP acts as a second messenger much like cyclic AMP. Its most likely mechanism of action is activation of intracellular protein kinases in response to the binding of membrane-impermeable peptide hormones to the external cell surface.
Soluble guanylate cyclase activators
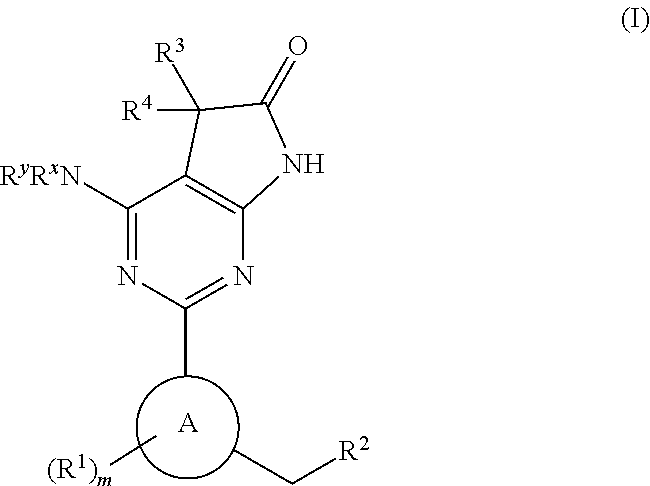
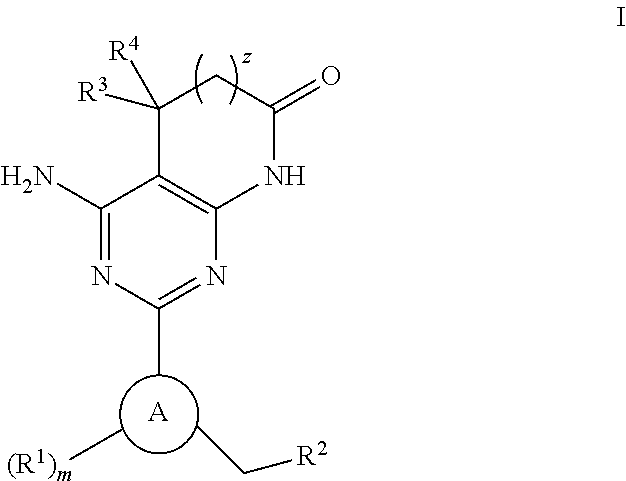
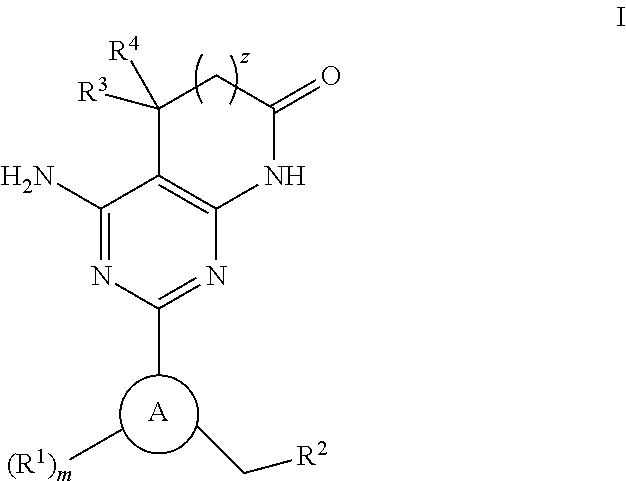
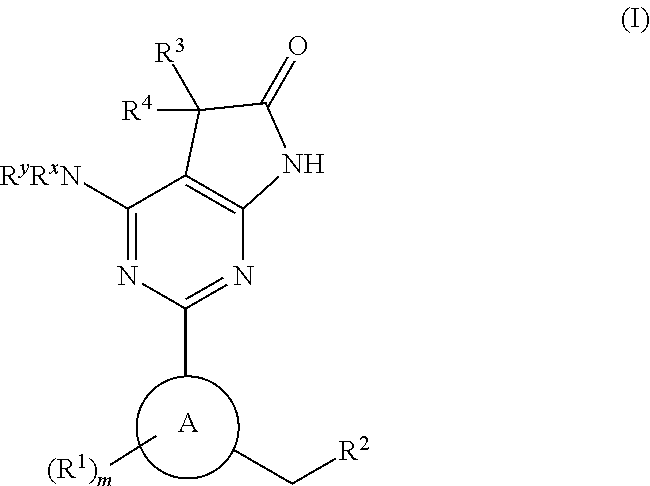
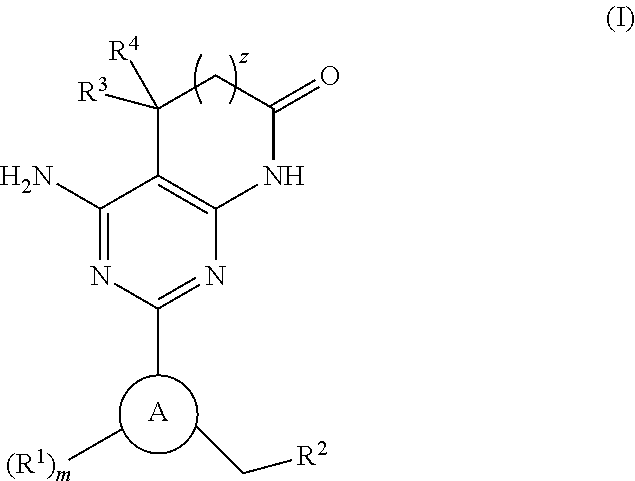
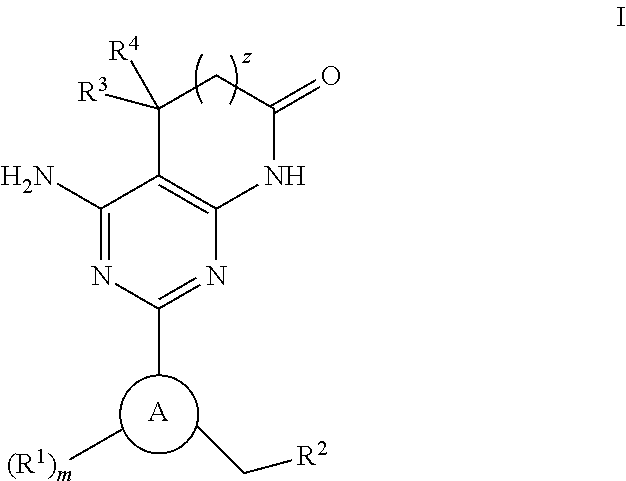
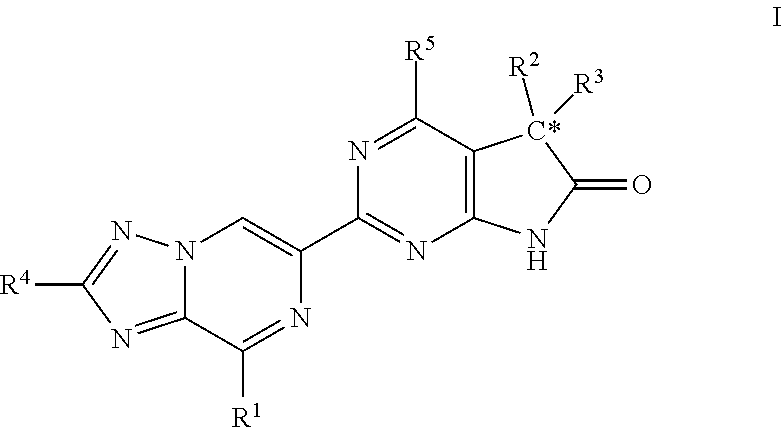
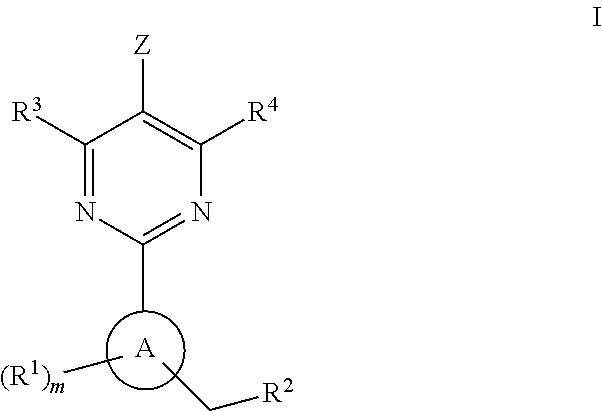
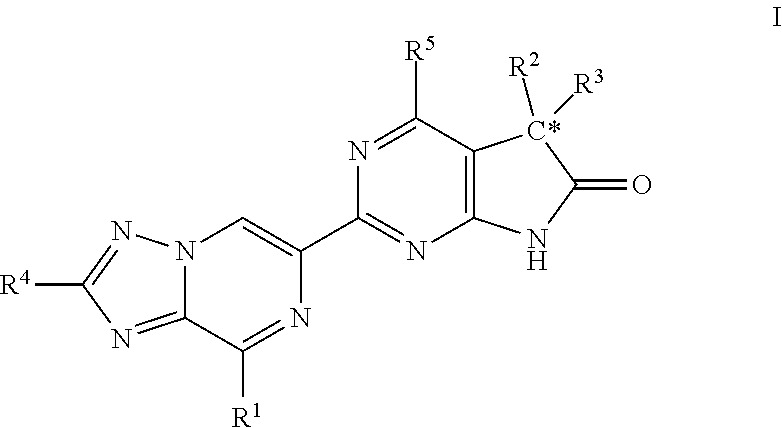
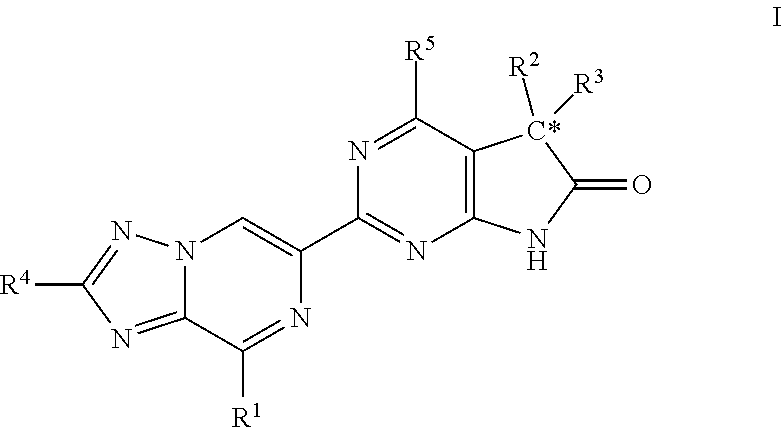
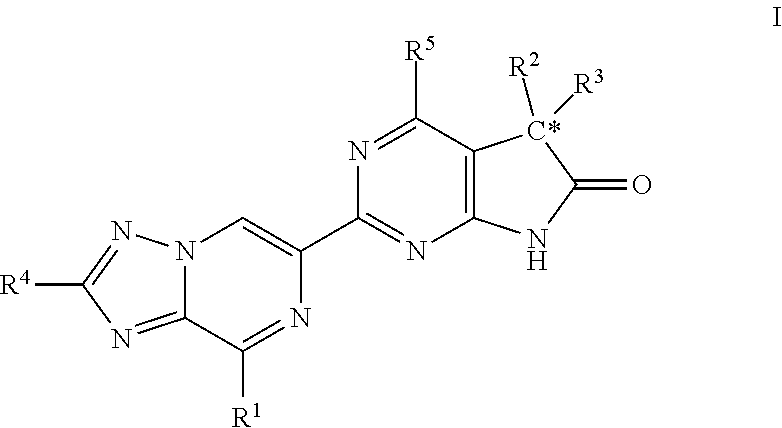
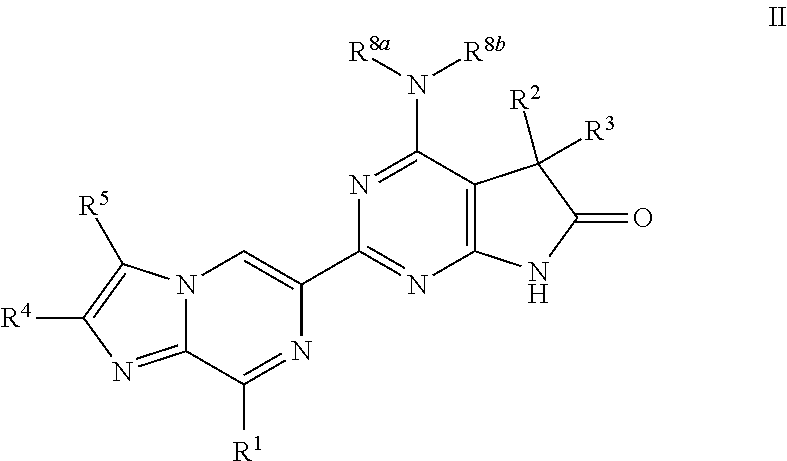
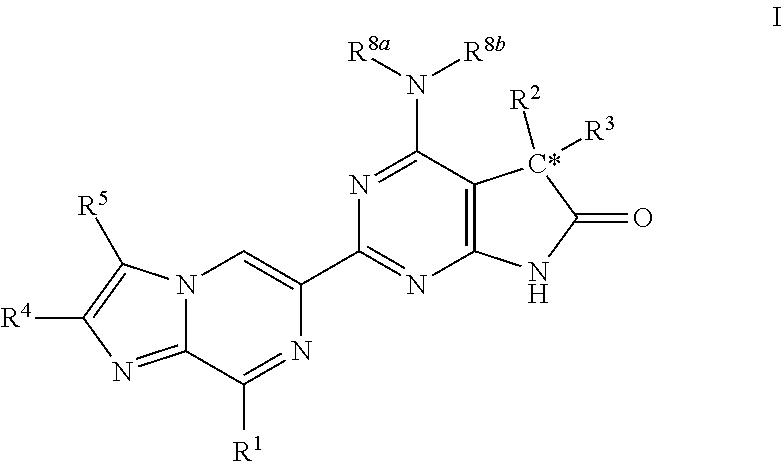
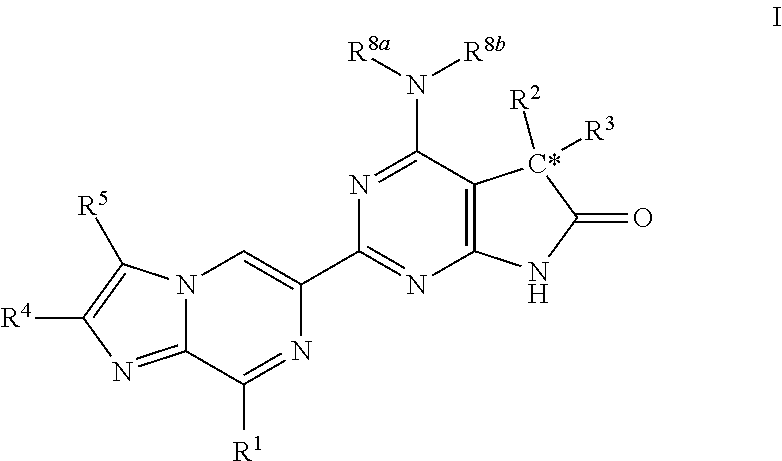
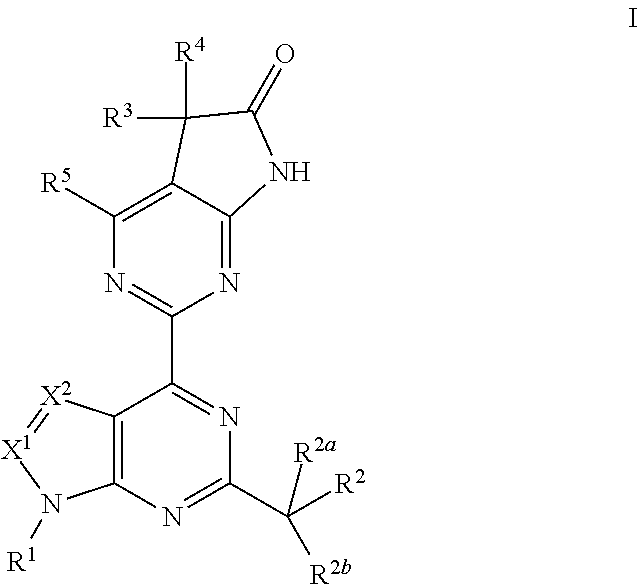
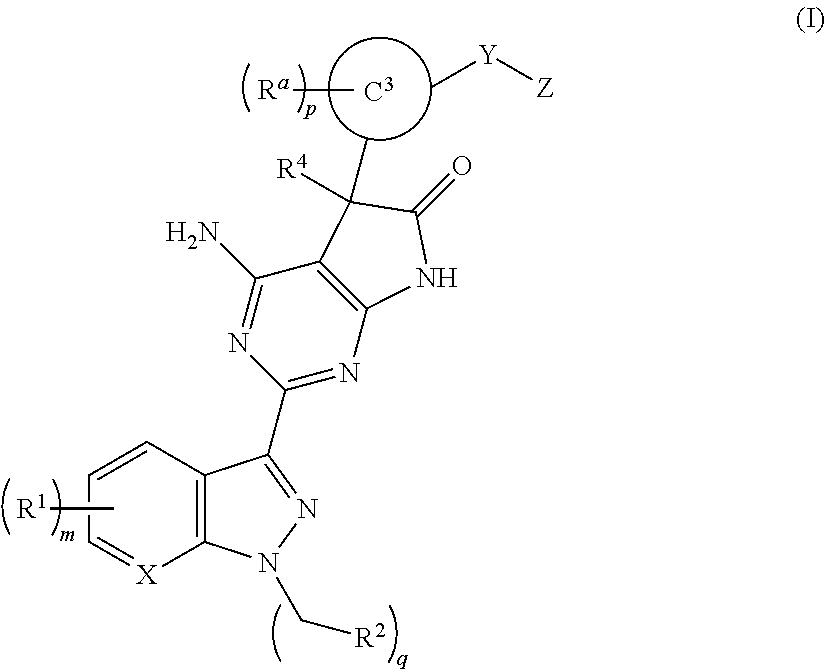
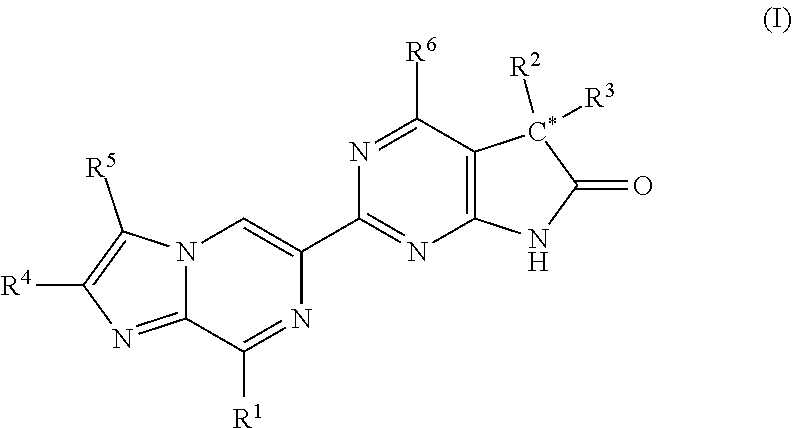
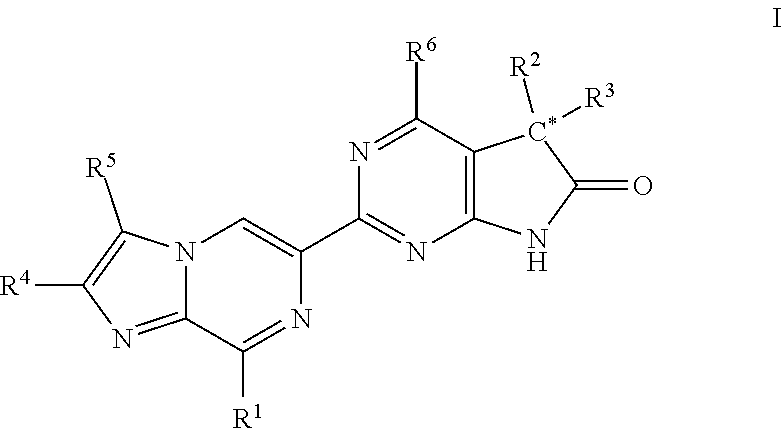
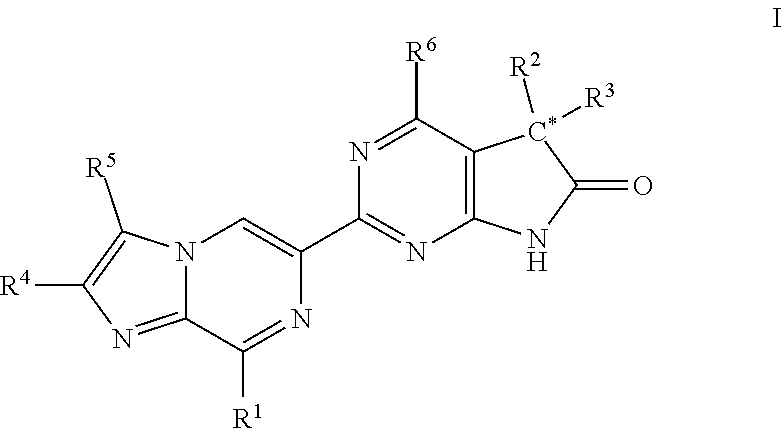
A compound of Formula (I): or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
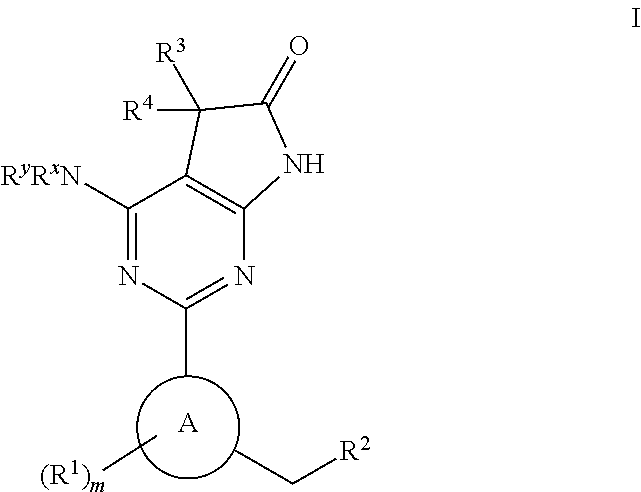
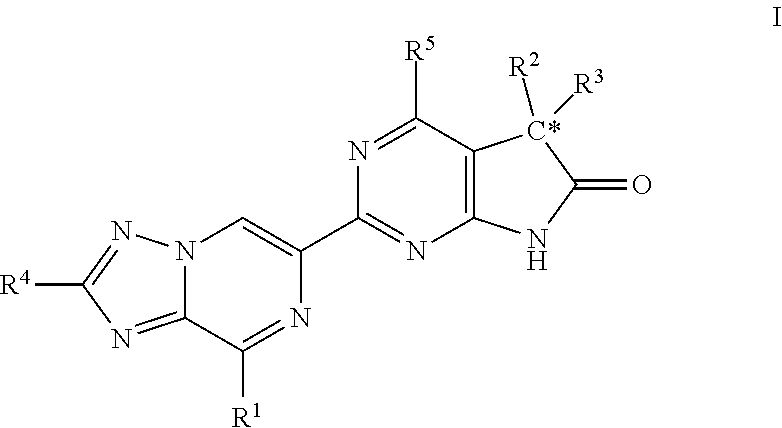
Compounds of Formula I are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of the Formula I, to their use for the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of the Formula I.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
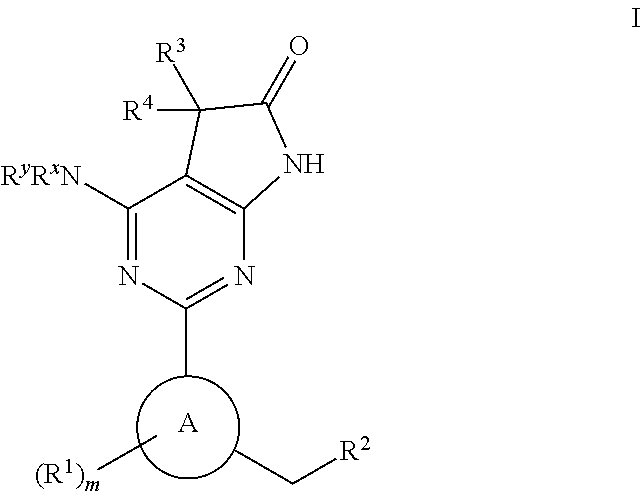
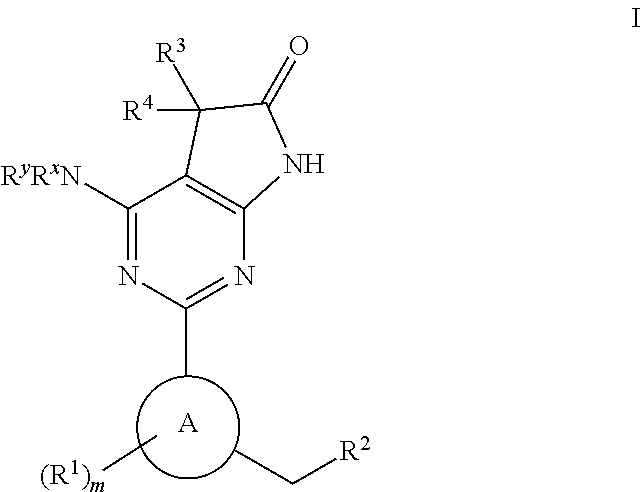
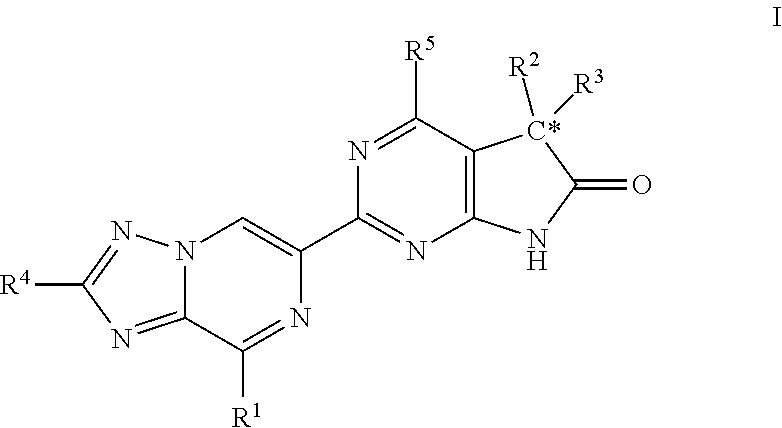
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Medicinal agent for treating erectile dysfunction
A medicament based on antibodies contains an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to endothelial nitric oxide synthase (NO synthase), the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to the homeopathic technology. A method of treating erectile dysfunctions and vegetative disturbances of male climax by regulating the level of cyclic guanosine monophosphate (cGMP) in the cavernous bodies on sexual stimulation, the method being characterized by the use of activated forms of ultra-low doses of antibodies to the entire molecule of the endothelial NO synthase or to its polypeptide fragments, activated forms being prepared by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Method and apparatus for electromagnetic enhancement of biochemical signaling pathways for therapeutics and prophylaxis in plants, animals and humans
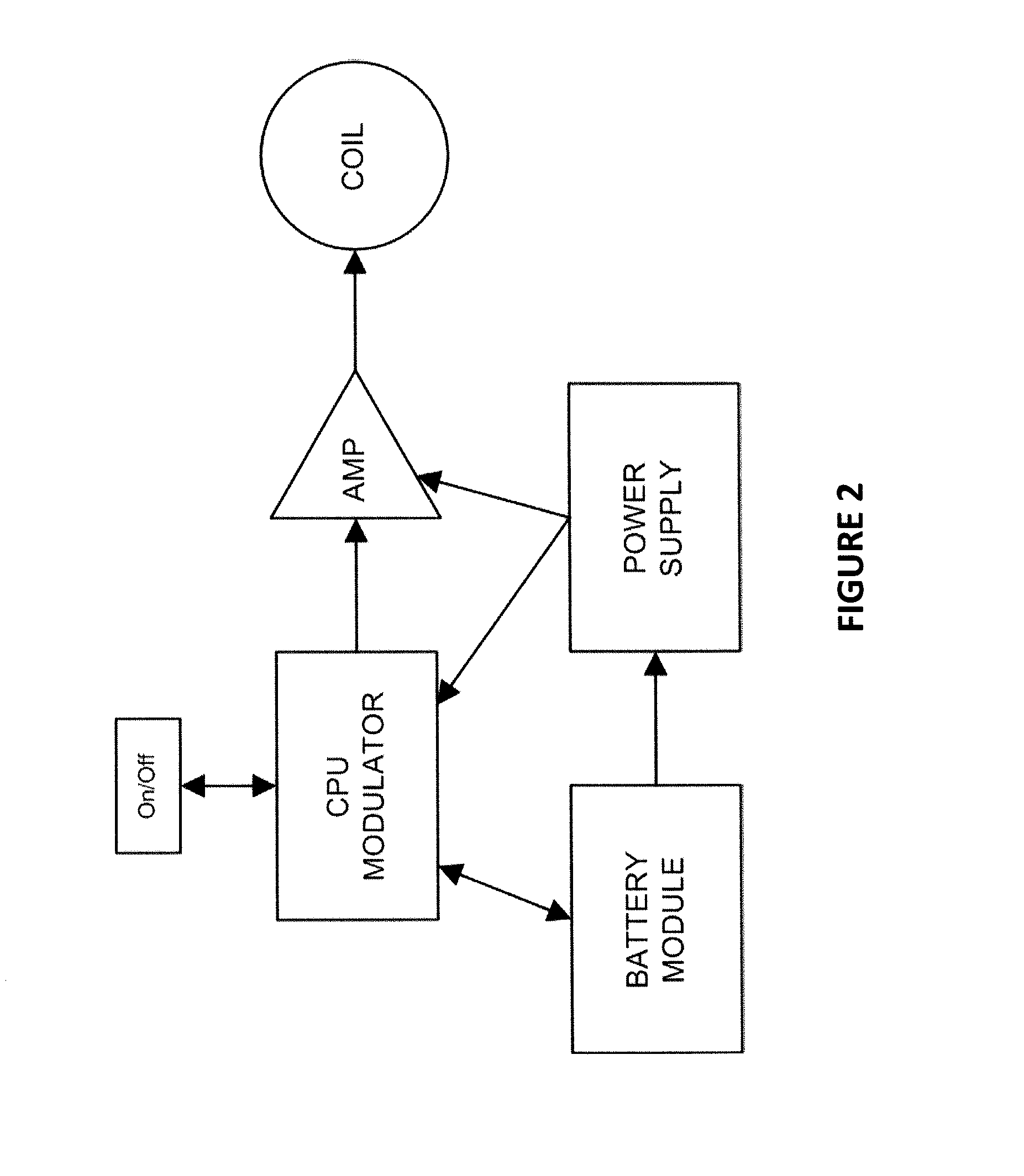
ActiveUS20120089201A1Enhance immune responseEnhanced signalElectrotherapyMagnetotherapy using coils/electromagnetsLiving systemsBiological body
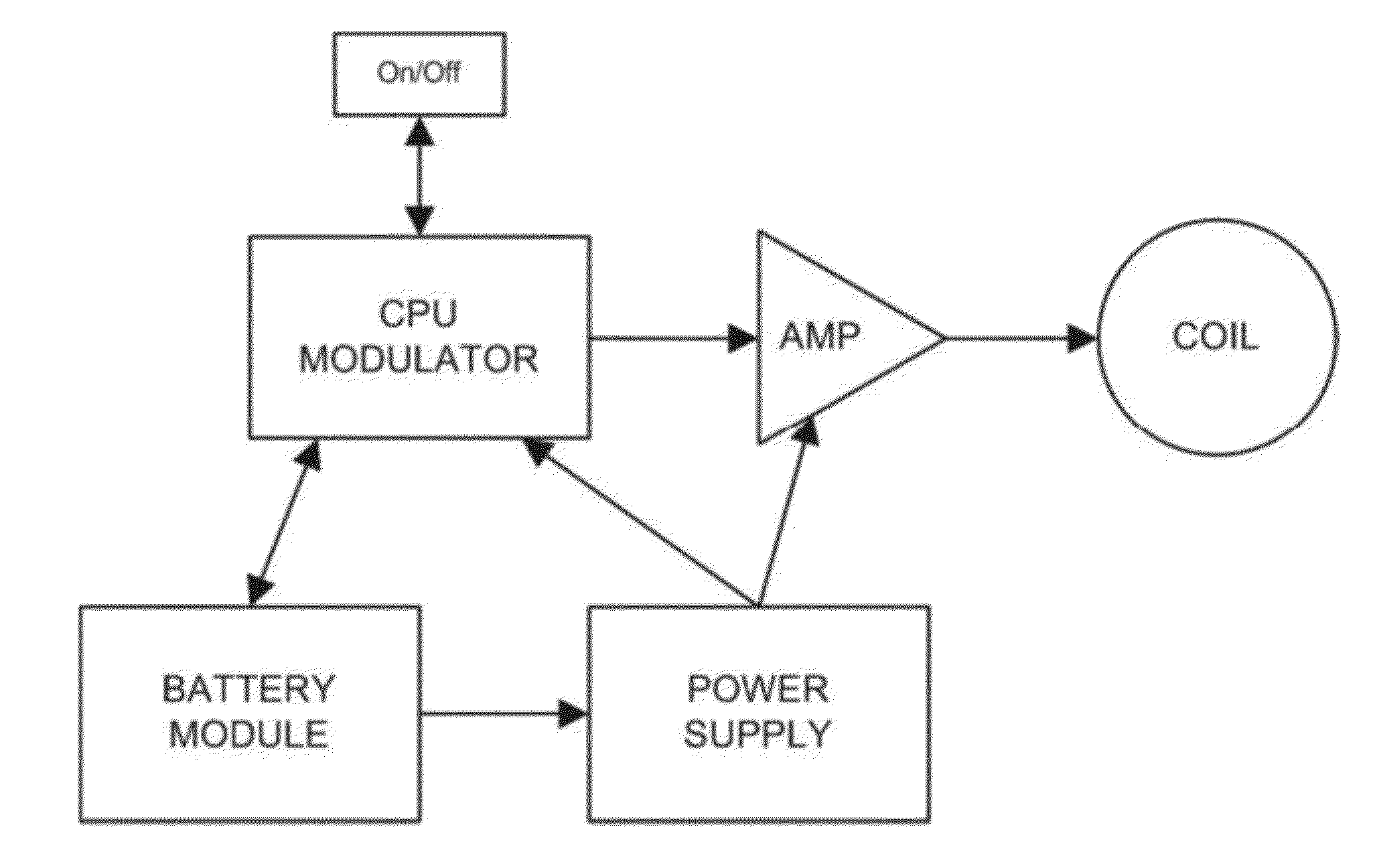
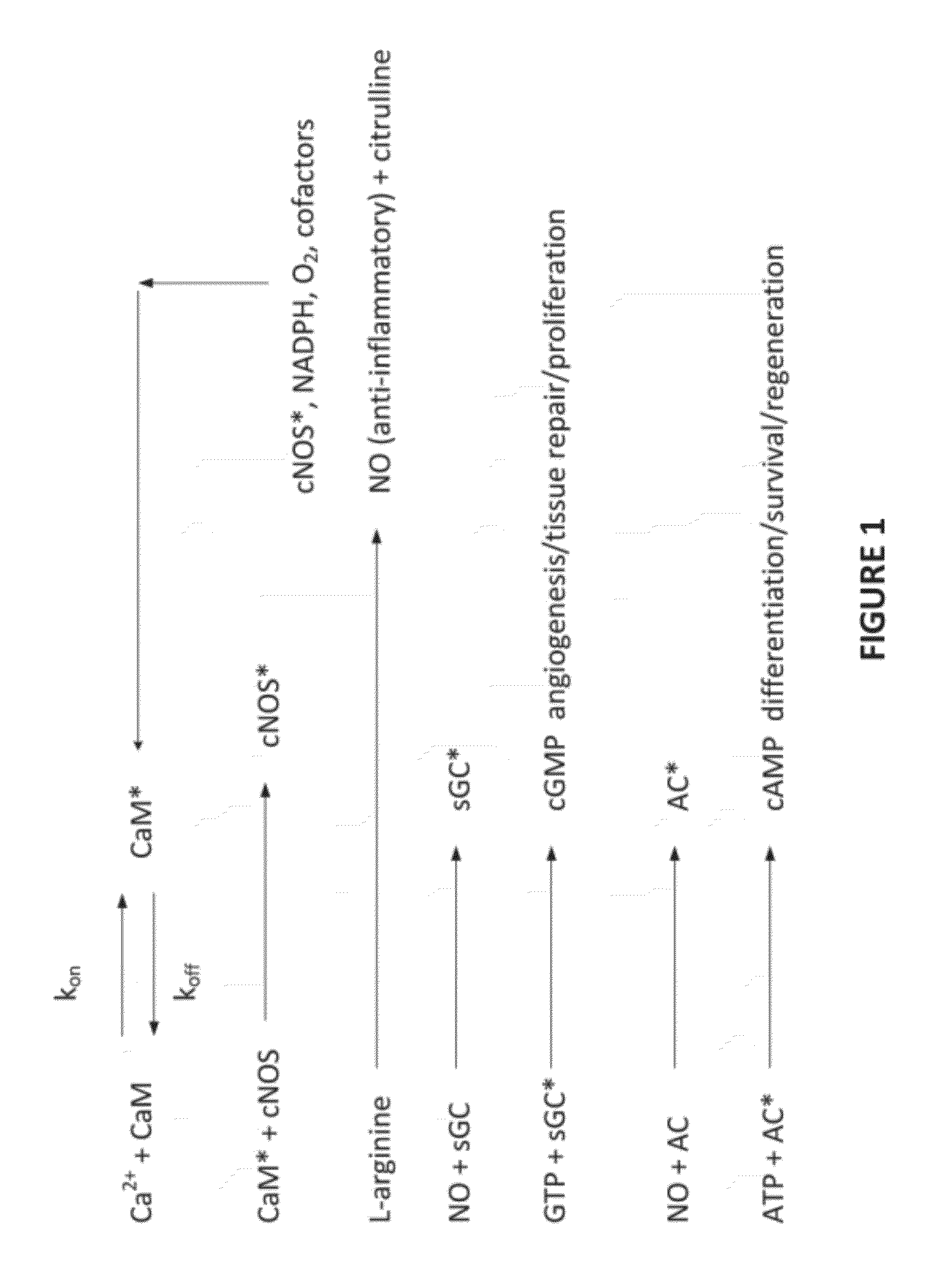
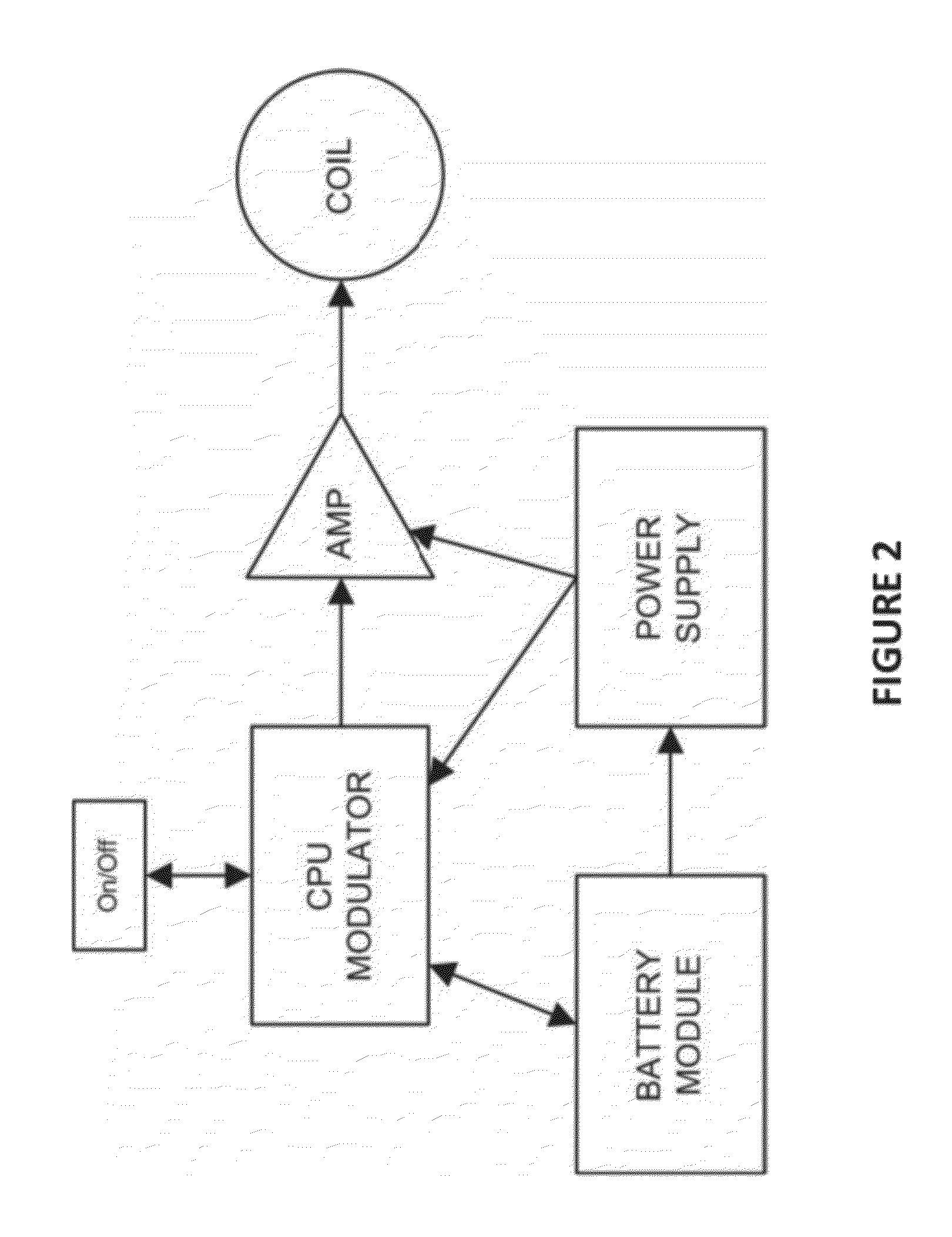
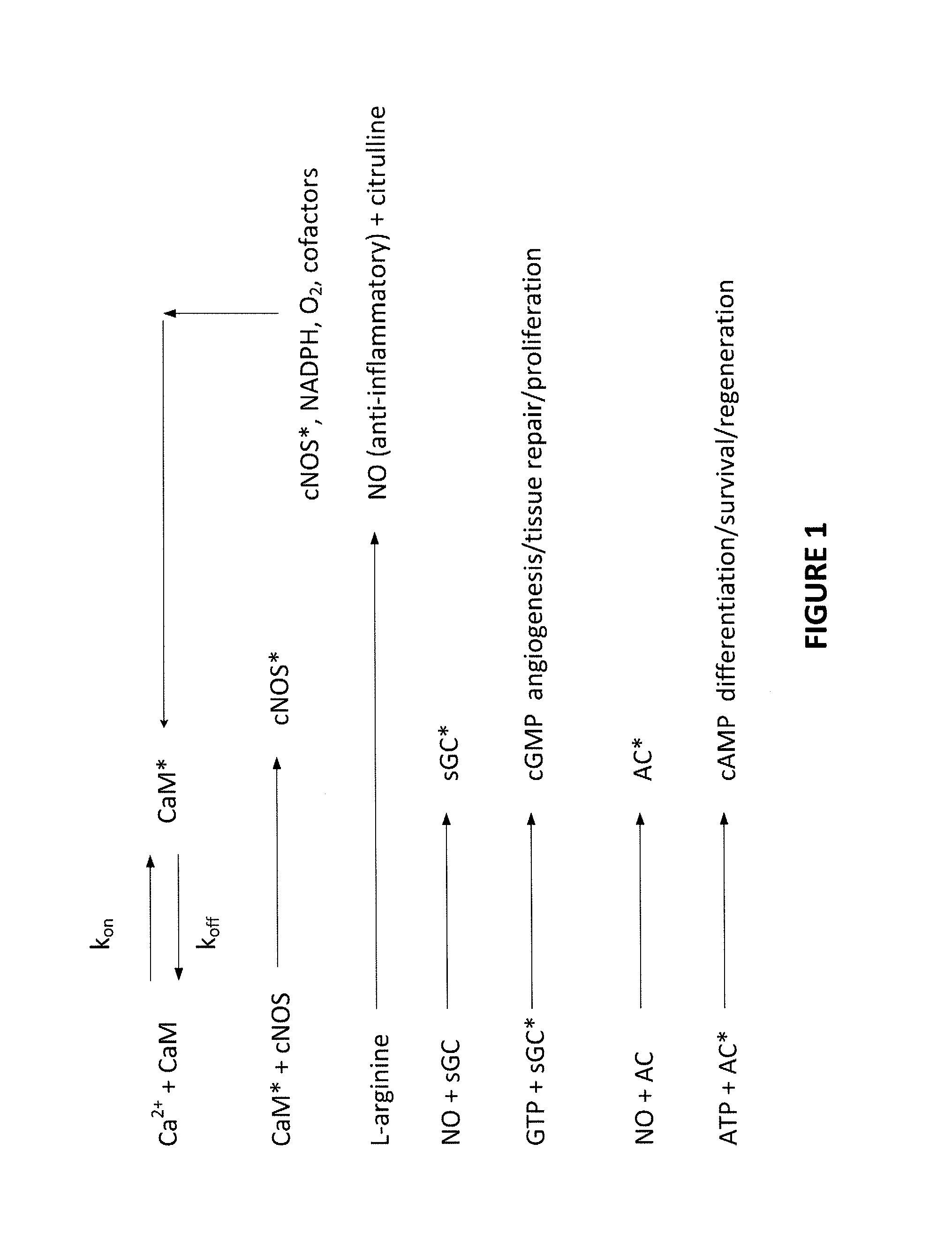
Apparatus and methods for delivering electromagnetic signals configured specifically to accelerate the asymmetrical kinetics of the binding of intracellular ions to their respective intracellular buffers, to enhance the biochemical signaling pathways plant animal and human molecules, cells, tissues, organs, portions of entire organisms and entire organisms employ for growth, repair and maintenance. Described herein are devices and methods that utilize repetitive bursts of waveforms configured to maximize the bound concentration of intracellular ions at their associated molecular buffers to enhance the biochemical signaling pathways living systems employ for growth, repair and maintenance. For example the systems and methods described herein may drive the binding of calcium to calmodulin (CaM), thereby enhancing the CaM-dependent nitric oxide (NO) / cyclic guanosine monophosphate (cGMP) signaling pathway.
Owner:ENDONOVO THERAPEUTICS INC
Medicinal agent and method for curing erectile dysfunction
A medicament based on antibodies contains an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to endothelial nitric oxide synthase (NO synthase), the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to the homeopathic technology. A method of treating erectile dysfunctions and vegetative disturbances of male climax by regulating the level of cyclic guanosine monophosphate (cGMP) in the cavernous bodies on sexual stimulation, the method being characterized by the use of activated forms of ultra-low doses of antibodies to the entire molecule of the endothelial NO synthase or to its polypeptide fragments, activated forms being prepared by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Soluble guanylate cyclase activators
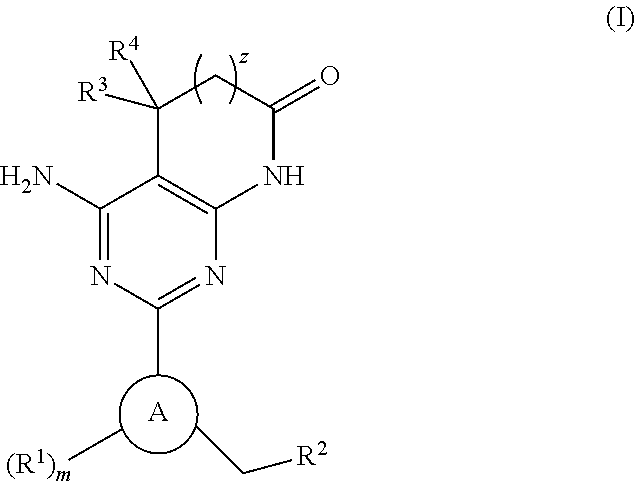
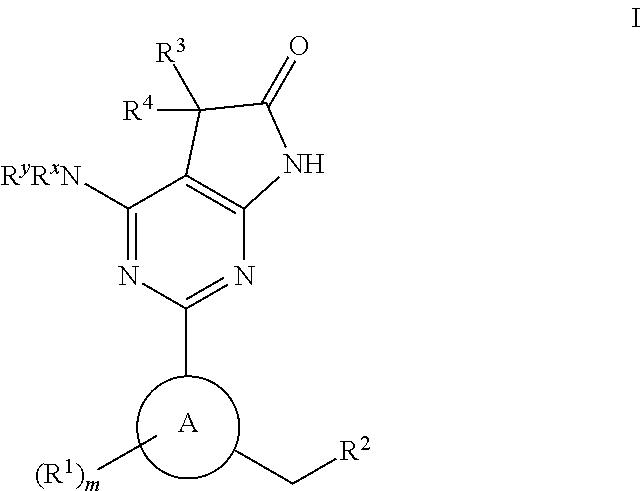
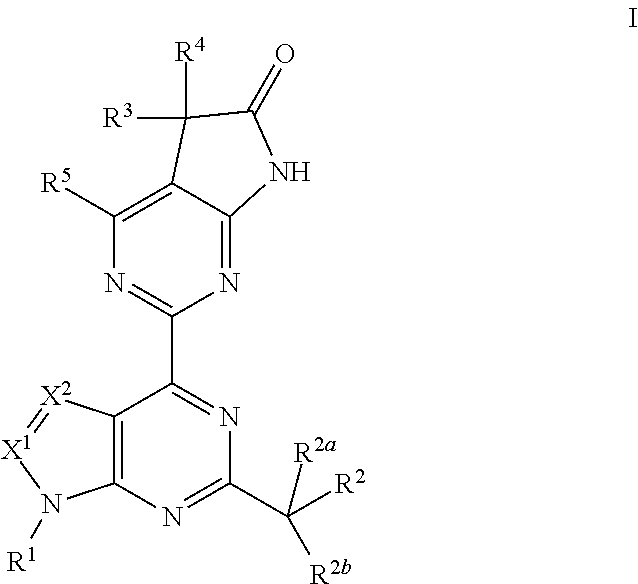
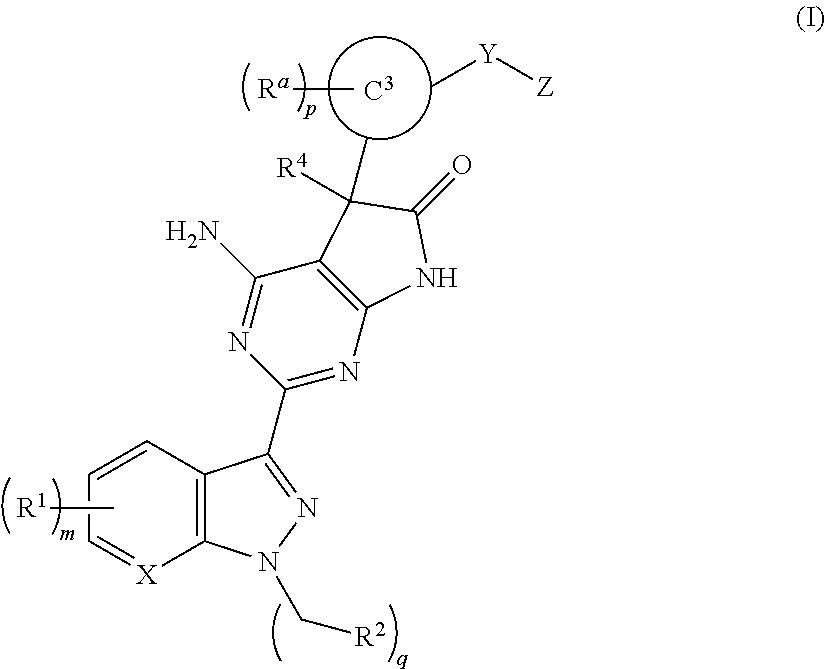
A compound of Formula (I): or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
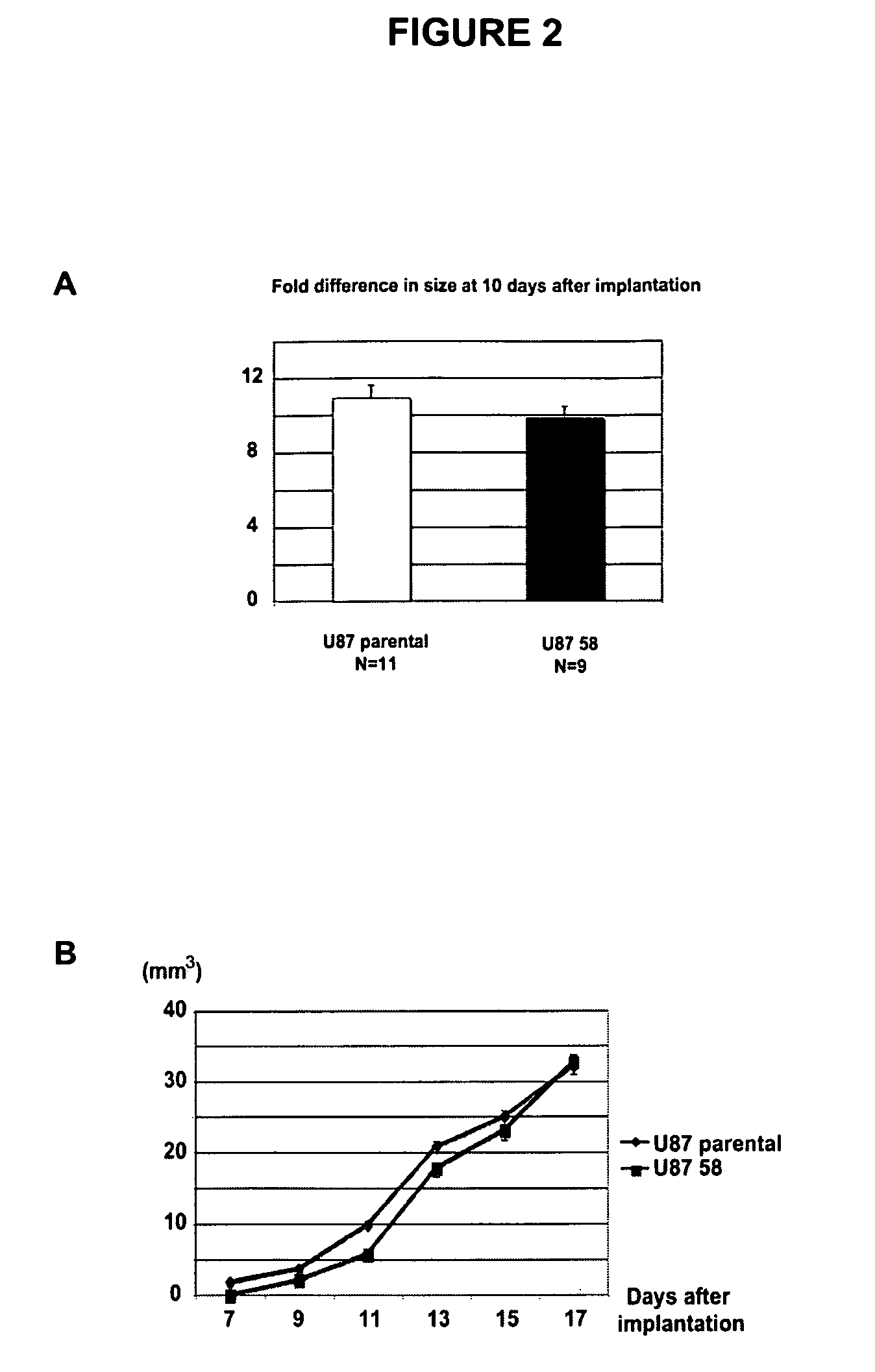
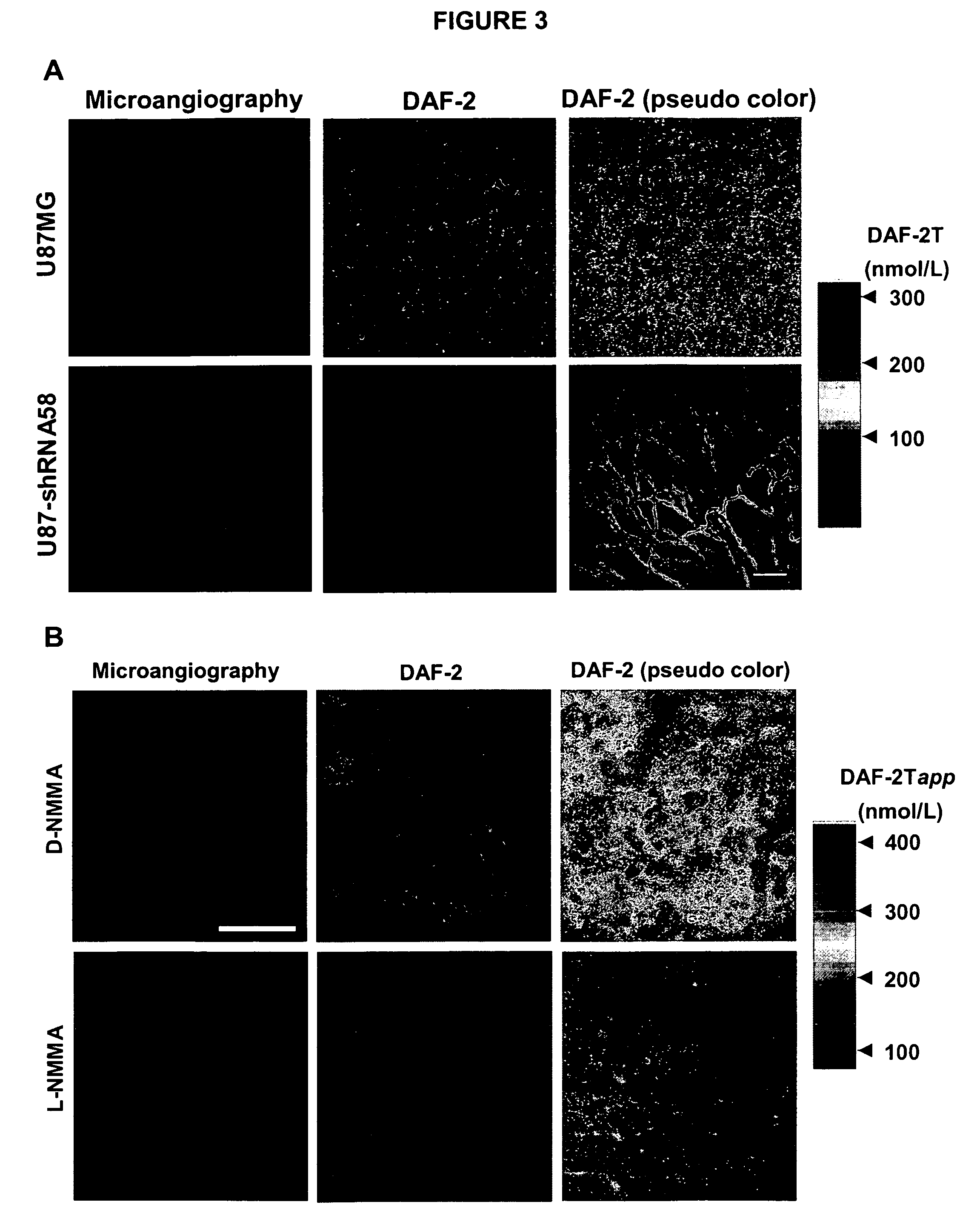
Modulation of nitric oxide signaling to normalize tumor vasculature
InactiveUS20100087370A1Increase productionHigh expressionKallidin/bradykinin ingredientsGenetic material ingredientsTumor therapyTumor vessel
The instant invention provides methods for treating a solid tumor in a subject comprising modulating nitric oxide production in the tumor to normalize tumor vasculature and administering an anti-tumor therapy to the subject. The invention further provides methods of treating a solid tumor in a subject comprising selectively increasing cyclic guanosine monophosphate (cGMP) or cGMP dependent protein kinase G production in the tumor vasculature to an amount effective to normalize tumor vasculature and administering an anti-tumor therapy to the subject.
Owner:THE GENERAL HOSPITAL CORP
Soluble guanylate cyclase activators
Compounds of Formula I are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of the Formula I, to their use for the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of the Formula I.
Owner:MERCK SHARP & DOHME LLC
Method and apparatus for electromagnetic enhancement of biochemical signaling pathways for therapeutics and prophylaxis in plants, animals and humans
InactiveUS20150217126A1Enhance CaM-dependent signalingIncrease blood flowElectrotherapySeed and root treatmentBiological bodyLiving systems
Apparatus and methods for delivering electromagnetic signals configured specifically to accelerate the asymmetrical kinetics of the binding of intracellular ions to their respective intracellular buffers, to enhance the biochemical signaling pathways plant animal and human molecules, cells, tissues, organs, portions of entire organisms and entire organisms employ for growth, repair and maintenance. Described herein are devices and methods that utilize repetitive bursts of waveforms configured to maximize the bound concentration of intracellular ions at their associated molecular buffers to enhance the biochemical signaling pathways living systems employ for growth, repair and maintenance. For example the systems and methods described herein may drive the binding of calcium to calmodulin (CaM), thereby enhancing the CaM-dependent nitric oxide (NO) / cyclic guanosine monophosphate (cGMP) signaling pathway.
Owner:ENDONOVO THERAPEUTICS INC
Triazolo-pyrazinyl derivatives useful as soluble guanylate cyclase activators
A compound of Formula Ior a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical compositions which comprise compounds of Formula I or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
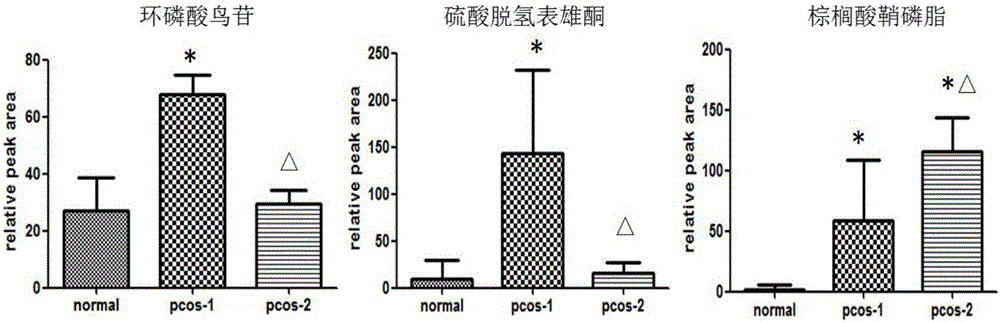
Diagnosis and/or typing marker for PCOS (polycystic ovarian syndrome) and application of preparation reagent
ActiveCN106442764AEasy diagnosisSolve complexityComponent separationDehydroepiandrosterone sulfateIndex system
The invention discloses a diagnosis and / or typing marker for the PCOS (polycystic ovarian syndrome) and an application of the preparation reagent and particularly relates to an application of cyclic guanosine monophosphate, dehydroepiandrosterone sulfate, palm sphingomyelin combined HDL-C (high-density lipoprotein cholesterol) and the left follicle number as the diagnosis and / or typing marker for the PCOS. Compared with existing PCOS diagnosis clinical indexes, the marker can realize the effect of distinguishing a PCOS subgroup I and a PCOS subgroup II through combined diagnosis of cyclic guanosine monophosphate, dehydroepiandrosterone sulfate and palm sphingomyelin. In combination of combined diagnosis of HDL-C (high-density lipoprotein cholesterol) and the left follicle number, the very high diagnosis accuracy is realized for a normal group, the PCOS subgroup I and the PCOS subgroup II, and accurate and effective index systems are provided for clinical disease diagnosis.
Owner:王义明
Triazolo-pyrazinyl derivatives useful as soluble guanylate cyclase activators
A compound of Formula Ior a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical compositions which comprise compounds of Formula I or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
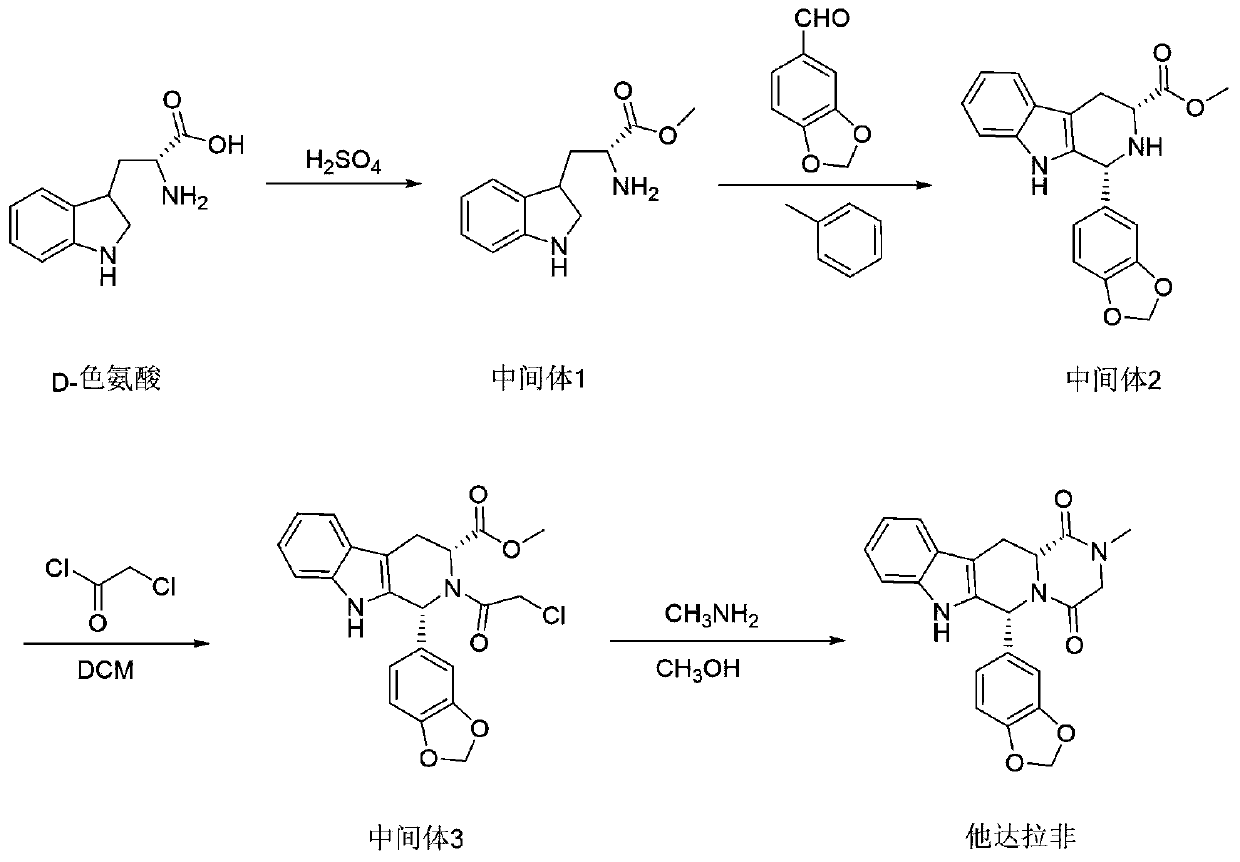
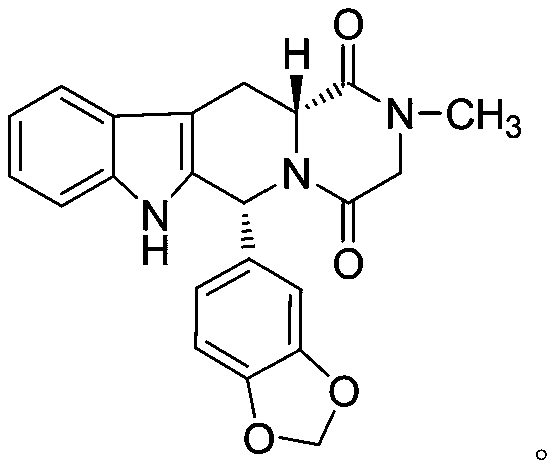
Preparation method of tadalafil and intermediate of tadalafil
ActiveCN110437228AAvoid it happening againReduce lossesOrganic chemistry methodsPhosphodiesteraseTadalafil
The invention relates to a preparation method of a selective and reversible inhibitor tadalafil of cyclic guanosine monophosphate (cGMP) specific phosphodiesterase 5 (PDE5). The method comprises the following steps: carrying out an esterification reaction on D-tryptophan as an initial raw material and methanol under catalysis of sulfuric acid to generate D-tryptophan methyl ester (an intermediate1); carrying out a Pictet-Spengler (P-S) reaction on the D-tryptophan methyl ester and heliotropin to prepare (1R,3R)-1-(1,3-benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester hydrochloride (an intermediate 2); carrying out an amidation reaction on the (1R,3R)-1-(1,3-benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester hydrochloride and chloroacetyl chloride to prepare (1R,3R)-1-(1,3-benzodioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester (an intermediate 3); andfinally carrying out a cyclization reaction on the (1R,3R)-1-(1,3-benzodioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester and a methylamine alcohol solution to obtain the tadalafil. The method provided by the invention has the advantages of easily available raw materials, simple operation, greenness, environmental protection and low costs, andis suitable for industrial production.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES +1
Imidazo-pyrazine derivatives useful as soluble guanylate cyclase activators
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
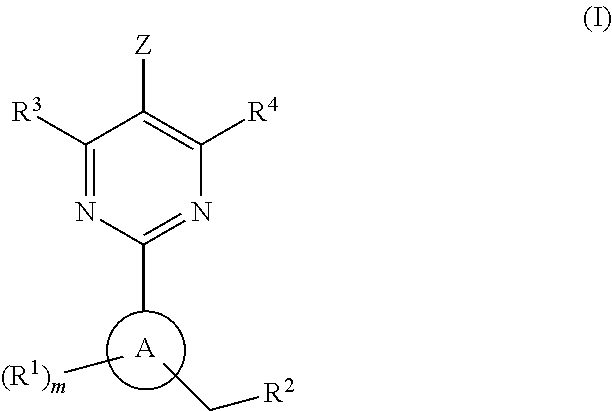
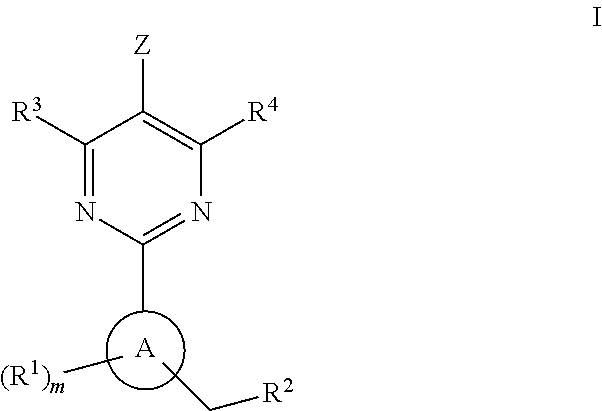
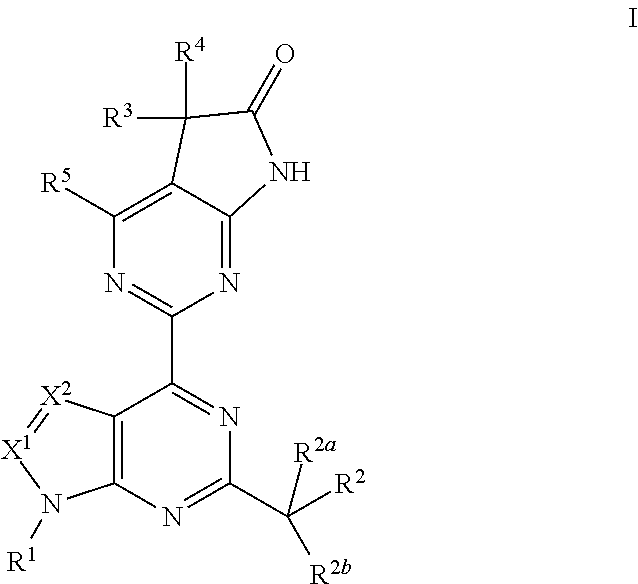
A compound of Formula I or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of Formula I or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
A compound of Formula I or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical preparations which comprise compounds of Formula I or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase stimulators
ActiveUS20170174693A1Organic active ingredientsOrganic chemistryGuanylate Cyclase StimulatorsCyclase
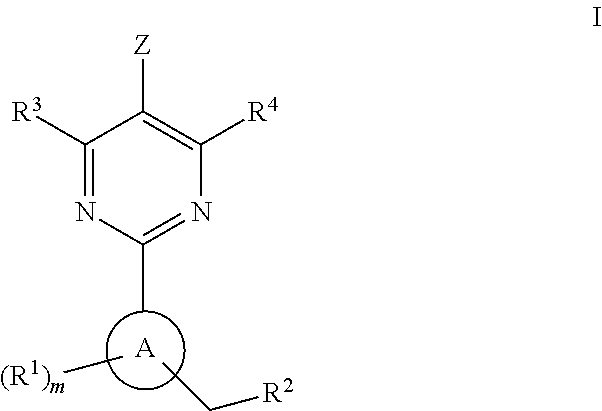
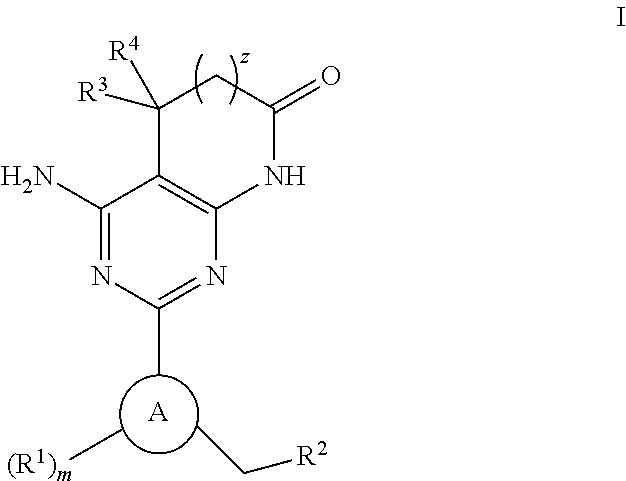
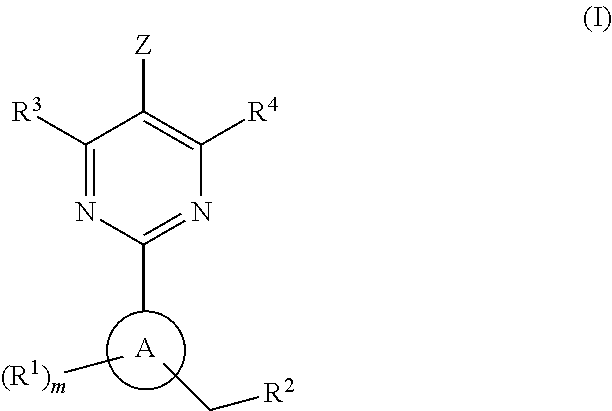
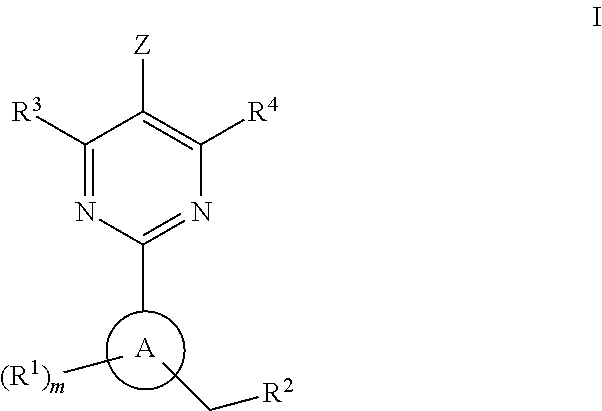
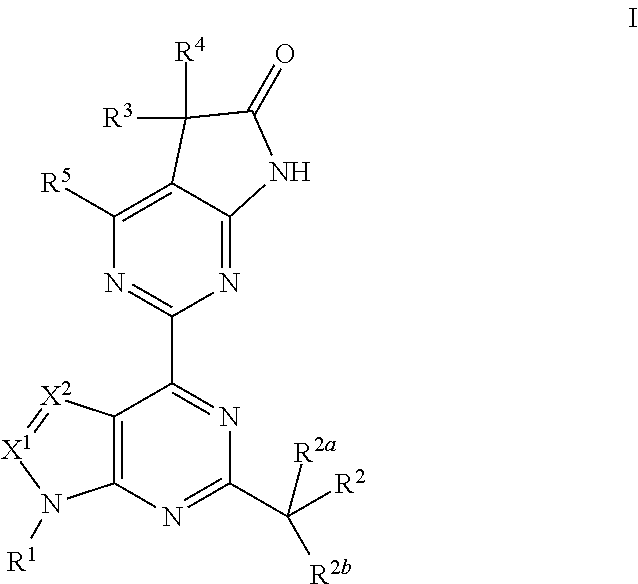
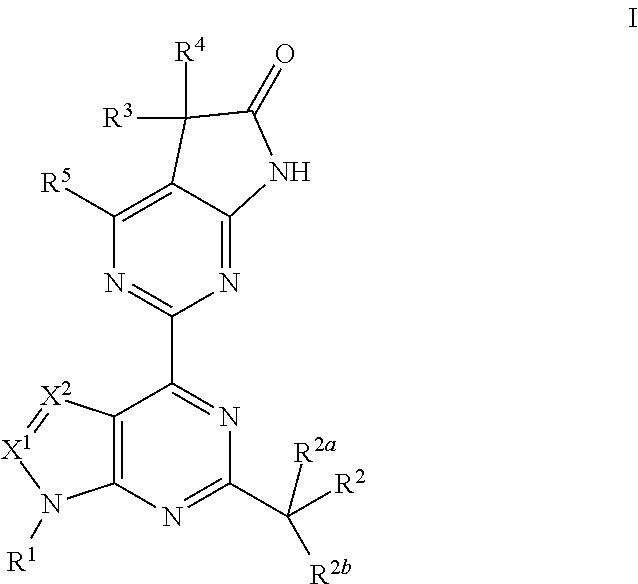
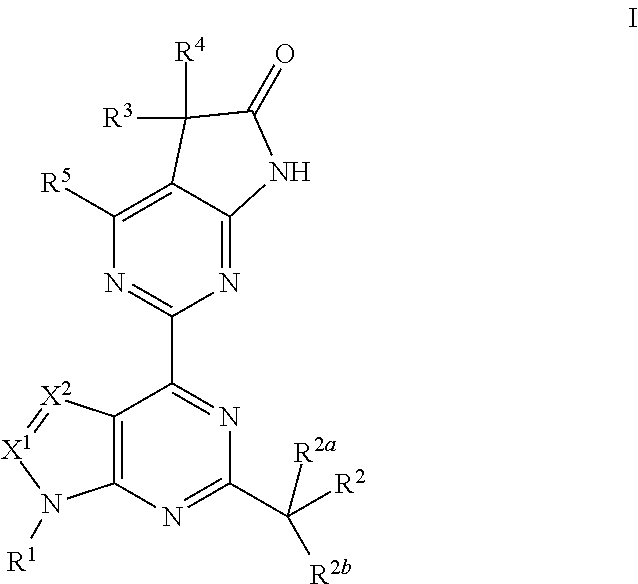
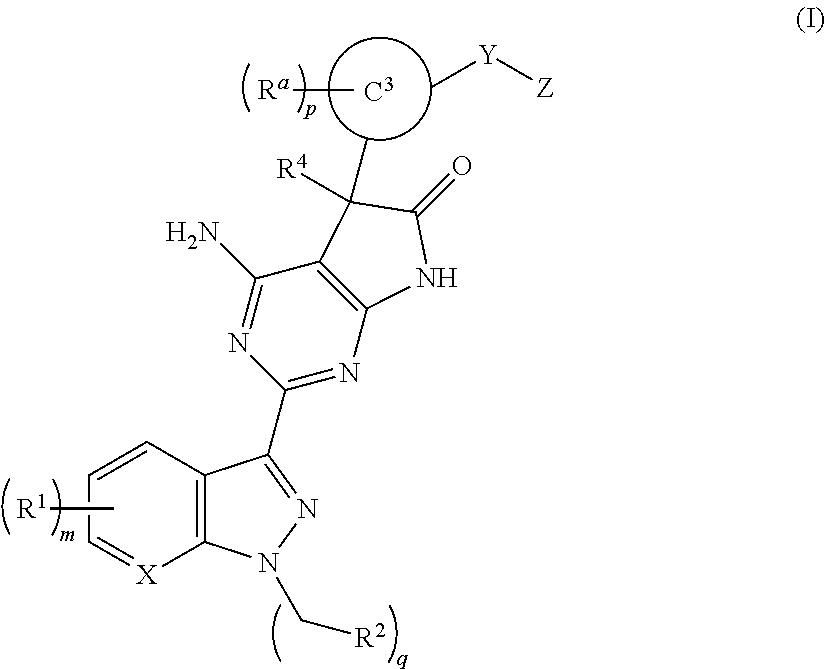
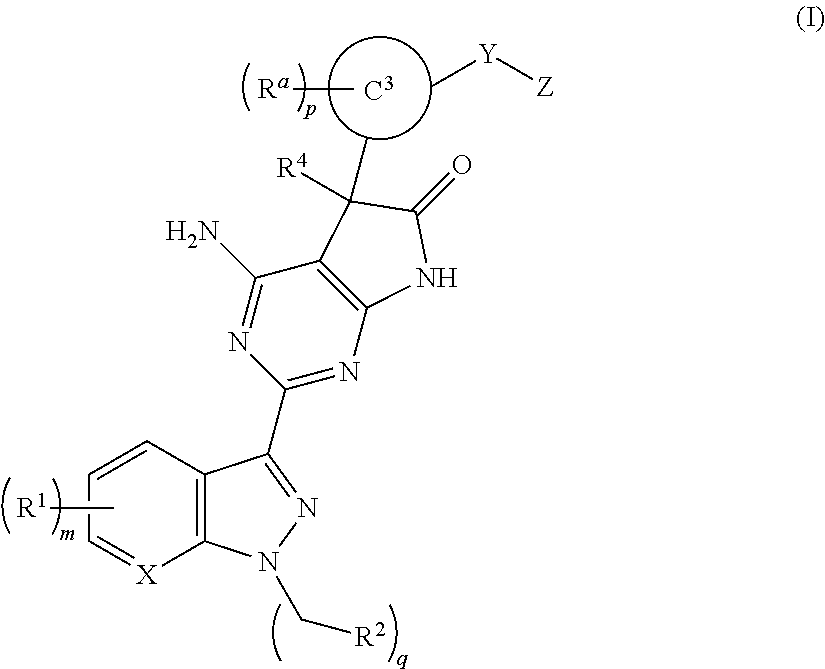
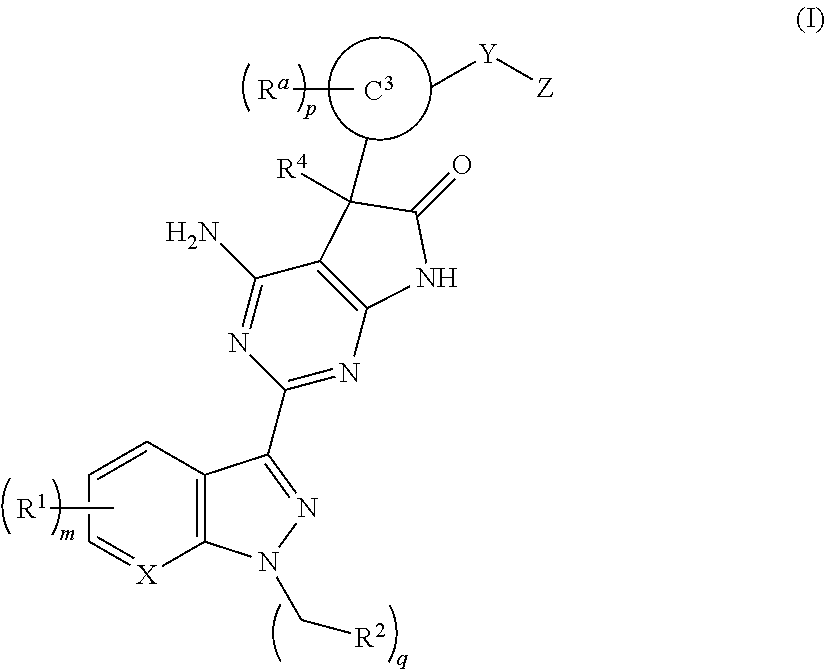
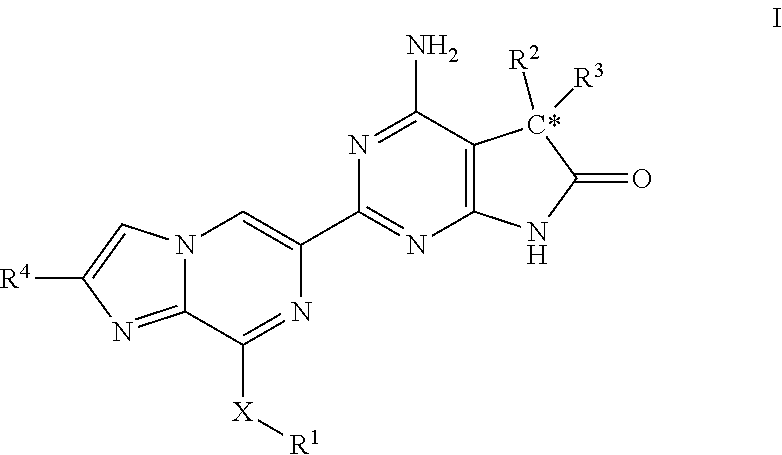
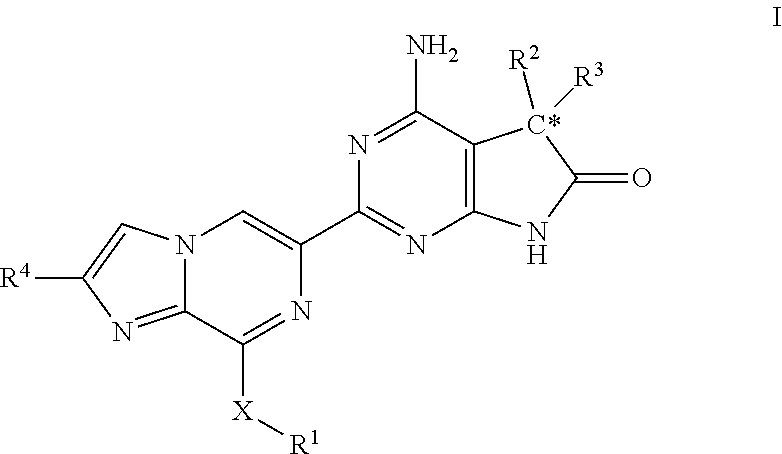
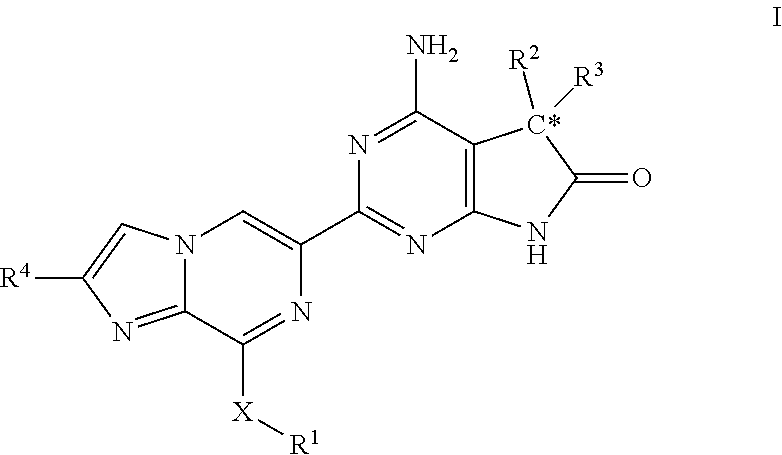
The invention provides compounds of the Formula (I)or a pharmaceutically acceptable salts thereof, wherein X, Y, Z, R1, R2, R4, Ra, and the subscripts m, p, and q are as described herein. The compounds or their pharmaceutically acceptable salts can modulate the body's production of cyclic guanosine monophosphate (“cGMP”), and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention also provides pharmaceutical compositions which comprise compounds of Formula (I) or pharmaceutically acceptable salts thereof. The invention also relates to methods for use of the compounds or their pharmaceutically acceptable salts in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose.
Owner:MERCK SHARP & DOHME CORP
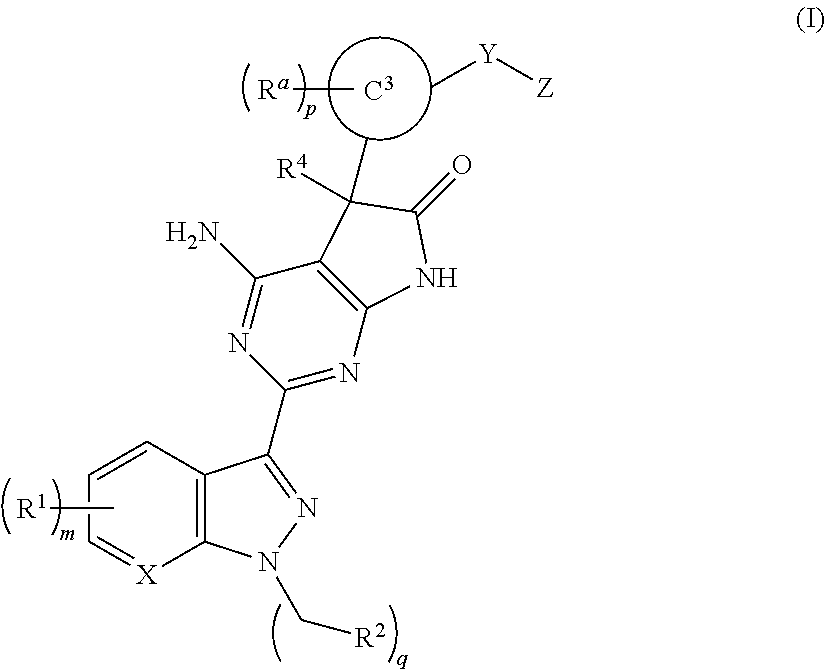
Soluble guanylate cyclase stimulators
The invention provides compounds of the Formula (I)or a pharmaceutically acceptable salts thereof, wherein X, Y, Z, R1, R2, R4, Ra, and the subscripts m, p, and q are as described herein. The compounds or their pharmaceutically acceptable salts can modulate the body's production of cyclic guanosine monophosphate (“cGMP”), and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention also provides pharmaceutical compositions which comprise compounds of Formula (I) or pharmaceutically acceptable salts thereof. The invention also relates to methods for use of the compounds or their pharmaceutically acceptable salts in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose.
Owner:MERCK SHARP & DOHME CORP
Red date-flavored short biscuit and preparation method thereof
InactiveCN108935584AAdd new usesBreak the monotonyDough treatmentModified nutritive productsSodium bicarbonateVegetable oil
The present invention relates to the technical field of leisure foods, and specifically discloses a red date-flavored short biscuit and a preparation method thereof. The red date-flavored short biscuit disclosed by the invention is prepared from the following raw materials in parts by weight: 18 to 22 parts of skim milk powder, 28 to 32 parts of red date powder, 20 to 30 parts of wheat flour, 32 to 36 parts of white sugar powder, 24 to 26 parts of edible vegetable oil, 0.3 to 0.5 part of lecithin, 0.1 to 0.3 part of sodium bicarbonate, 0.015 to 0.025 part of edible salt and 0.75 to 0.85 part of water. With scientific and reasonable material selection and production, the red date-flavored short biscuit provided by the invention has the advantages: the biscuit provided has a strong natural flavor, the monotonicity of traditional biscuits and the homogenization characteristics of biscuit products on the market are broken, and the new application of the biscuit is expanded; when matching with yoghurt or milk with other flavors, the biscuit can improve the taste, improve the added value of the product and attract the eyeball of consumers; and the red date powder contains a plurality ofbioactive substances such as jujube polysaccharides, flavonoids, saponins, triterpenes, alkaloids, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), and has a pluralityof health-care and treating effects on human body.
Owner:焦作荣利达食品有限公司
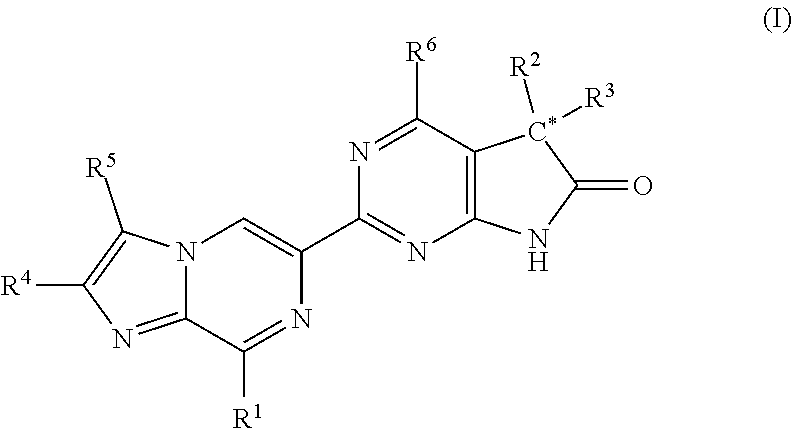
Imidazo-pyrazinyl derivatives useful as soluble guanylate cyclase activators
ActiveUS20180193343A1Organic active ingredientsOrganic chemistryMedicineGuanylate Cyclase Activators
A compound of Formula I or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or a pharmaceutically acceptable salt thereof, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical compositions which comprise compounds of Formula I or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME LLC
Formula of anther induction medium for cultivation of capsicum annuum variety
ActiveCN105638480ASpeed up the breeding processImprove cultivation efficiencyPlant tissue cultureHorticulture methodsVitamin CPollen
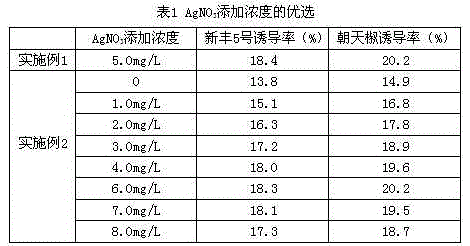
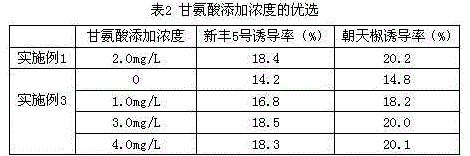
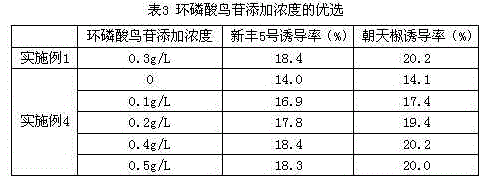
The invention provides a formula of an anther induction medium for cultivation of the capsicum annuum variety. The formula of the medium comprises KNO3, NH4NO3, KH2PO4, MgSO4*H2O, CaCl2*2H2O, Na2-EDTA (ethylene diamine tetraacetic acid), FeSO4*7H2O, MnSO4*H2O, ZnSO4*7H2O, H3BO3, KI, CuSO4*5H2O, CoCl*6H2O, NaMoO4*2H2O, AgNO3, inositol, vitamin B1, vitamin B6, nicotinic acid, glycine, folic acid, cyclic guanosine monophosphate, maltose, cane sugar, phytagel, 2,4-D, kinetin, polyphthalein nucleic acid, biotin, vitamin C, activated carbon and hydrolyzed protein. The medium has the advantages that capsicum annuum anther is efficiently induced to produce pollen callus, the capsicum annuum anther cultivation efficiency can be remarkably improved, and the fine variety cultivation process of capsicum annuum is accelerated.
Owner:新沂市芭缇雅商贸有限公司
Method for inducing anther calluses of sainfoin and special culture medium of method
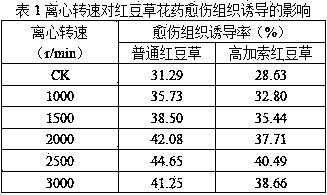
InactiveCN110214700APromote formationHigh induction rateHorticulture methodsPlant tissue culturePlant hormoneSucrose
The invention provides a method for inducing anther calluses of sainfoin and a special culture medium of the method, and belongs to the technical field of pasture biology. The method includes, firstly, carrying out low-temperature and centrifugal pretreatment on the screened sainfoin inflorescence, which is beneficial to promoting the formation of anther calluses; and then taking off the anthers and inoculating on the callus induction culture medium for culture to obtain calluses. The callus induction culture medium is optimized on the basis of a basic culture medium, appropriately improves the concentrations of KNO3, KH2PO4, vitamins, sucrose and the like, adjusts the proportion of conventional plant hormones, and adds L-proline, gamma-aminobutyric acid, cyclic guanosine phosphate, brassinolide, N-methylmorpholine, fulvic acid, butyl hydroxy anisole, dithiothreitol and an astragalus sinicus extract, and thus the induction rate of the sainfoin anther calluses is effectively improved, so that the sainfoin anther culture efficiency is improved.
Owner:郭根霞
Wild jujube beer and preparation method thereof
InactiveCN104073390AFragrant flavorSweet and sour tasteBeer brewingBiotechnologyBULK ACTIVE INGREDIENT
The invention relates to a wild jujube beer and a preparation method thereof. Wort is prepared according to the production process of common beer, a wild jujube extract is added when the wort is fermented, and the wild jujube beer is obtained through filtration, filling and sterilization. The beer is added with the fragrance of wild jujube on the basis of original flavor, and contains a large amount of active ingredients such as polysaccharides, flavonoids, saponins, triterpenes, alkaloids, cyclic adenosine monophosphate, cyclic guanosine monophosphate and the like, so that the beer has the effects of tonifying deficiency and qi, nourishing blood for tranquillization, strengthening the spleen and stomach and the like.
Owner:NANJING ZELANG AGRI DEV
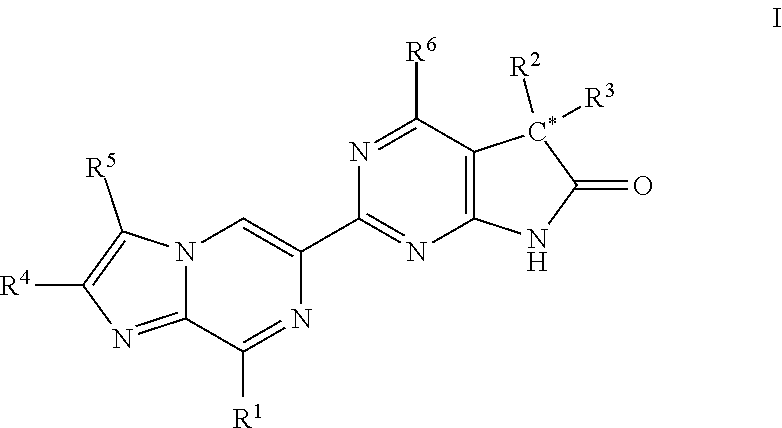
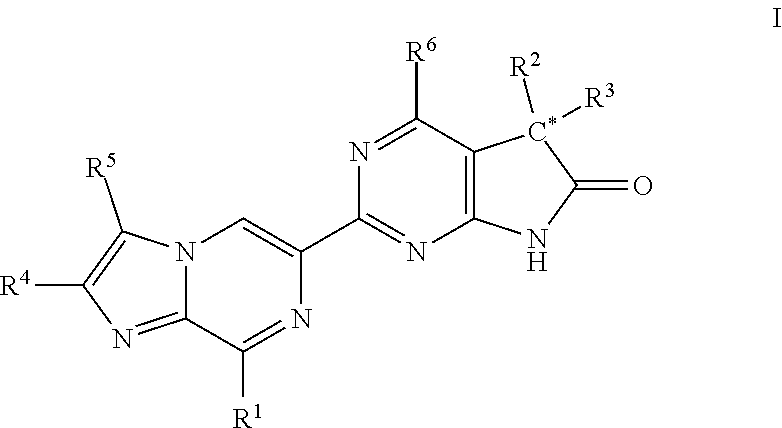
Imidazo-pyrazinyl derivatives useful as soluble guanylate cyclase activators
Owner:MERCK SHARP & DOHME LLC
Imidazo-pyrazinyl derivatives useful as soluble guanylate cyclase activators
ActiveUS20180147208A1Organic active ingredientsOrganic chemistryMedicineGuanylate Cyclase Activators
A compound of Formula I or a pharmaceutically acceptable salt thereof, are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The invention furthermore relates to processes for preparing compounds of Formula I, or pharmaceutically acceptable salt thereofs, for their use in the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and to pharmaceutical compositions which comprise compounds of Formula I or pharmaceutically acceptable salts thereof.
Owner:MERCK SHARP & DOHME LLC
Drugs with improved pharmacokinetic properties
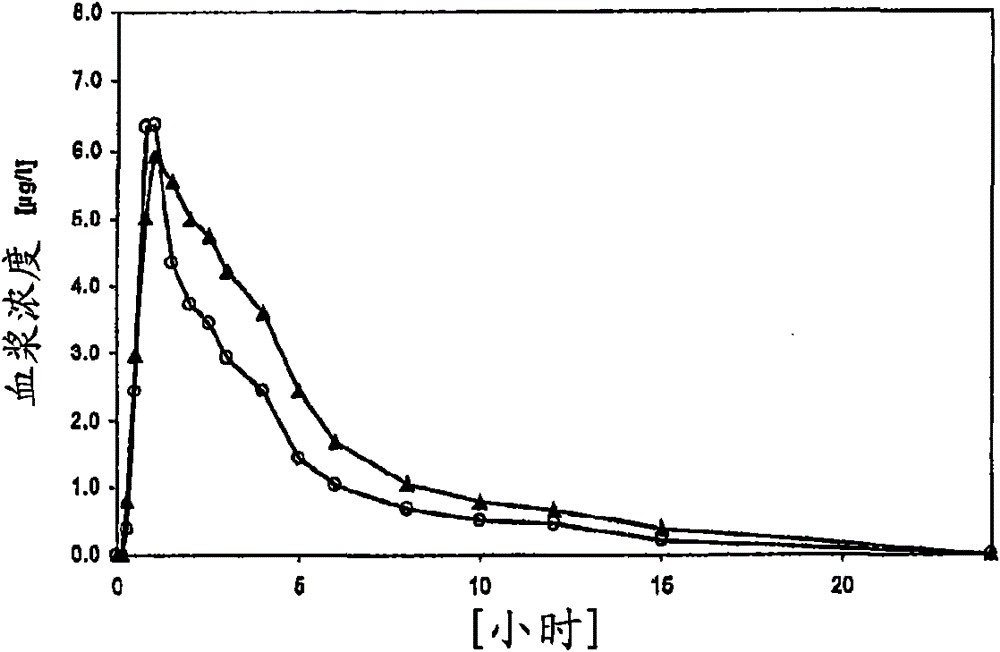
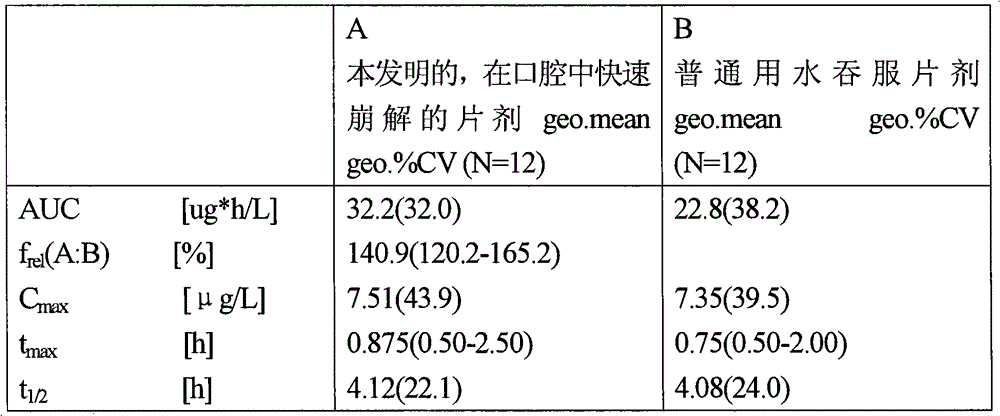
The application relates to a novel vardenafil pharmaceutical preparation, which disintegrates rapidly in the oral cavity, improves bioavailability, and causes a plateau-like plasma concentration change process, and a preparation method thereof.
Owner:BAYER IP GMBH
Finasteride and sildenafil compositions and applications
InactiveUS20190282591A1Dissolve fastOrganic active ingredientsPharmaceutical delivery mechanismPhosphodiesteraseHuman body
The human body is mediated by a large number of chemicals and chemical processes where imbalances can result in an abnormal condition that affects part or all of the human body. Amongst these conditions are hair loss and male impotence or erectile dysfunction, both of which can have psychological consequences for the patient and others as they can be tied to relationship difficulties and self-image. 5α-reductase Type II inhibitors prevent DHT production and reduce androgen activity in key tissues such as prostate and scalp. Similarly, an inhibitor of cyclic guanosine monophosphate (cGMP) specific phosphodiesterase type 5 (PDE5) can lead to improved vasodilation and blood flow. It would be beneficial to provide patients with either of these treatments within a topical cream form allowing localized targeted delivery.
Owner:BLUE GOOSE DRUGS INC
Microdose therapy
InactiveUS7393825B2Restores normal vascular toneBiocideNitro compound active ingredientsCyclasePhosphodiesterase
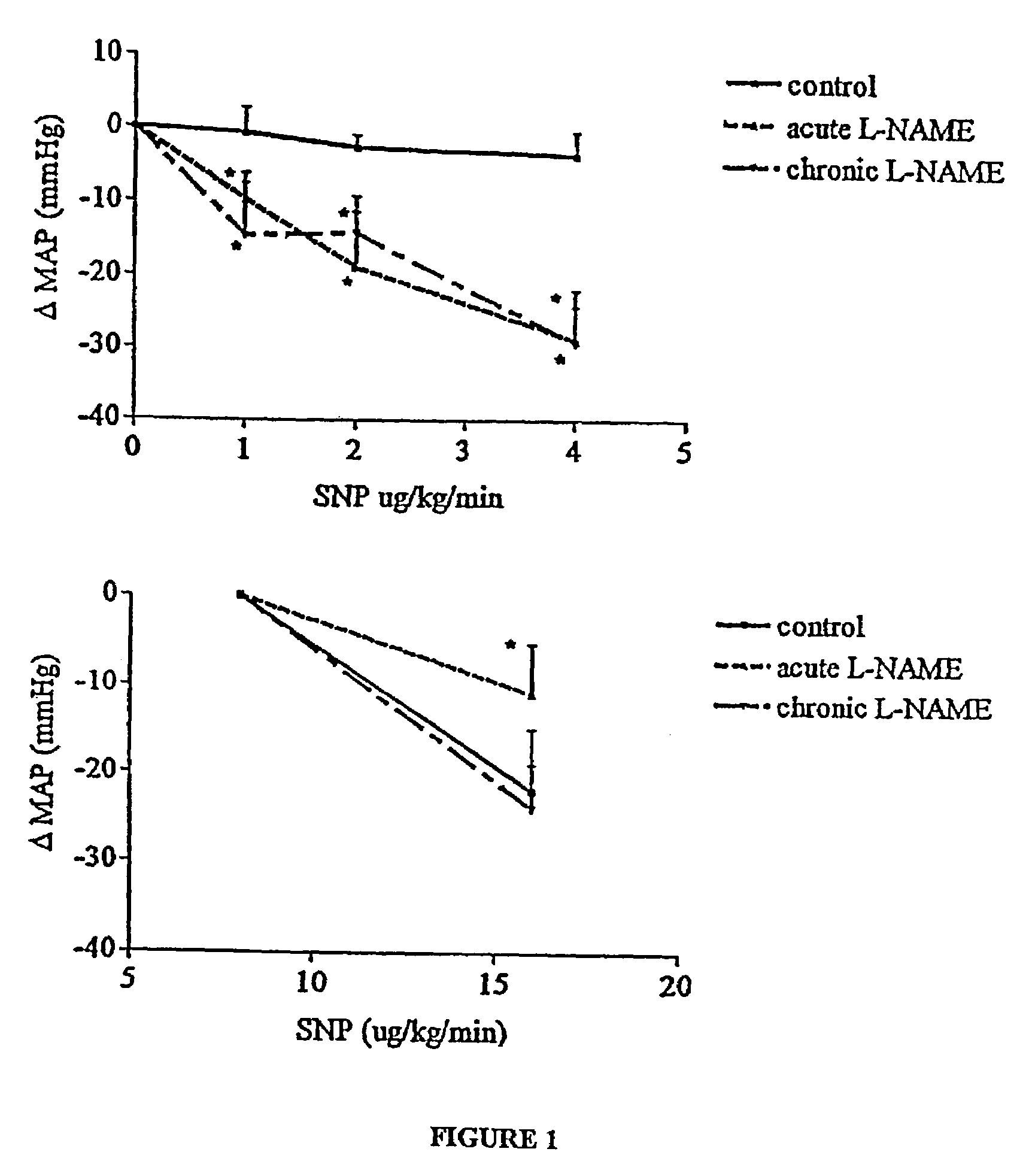
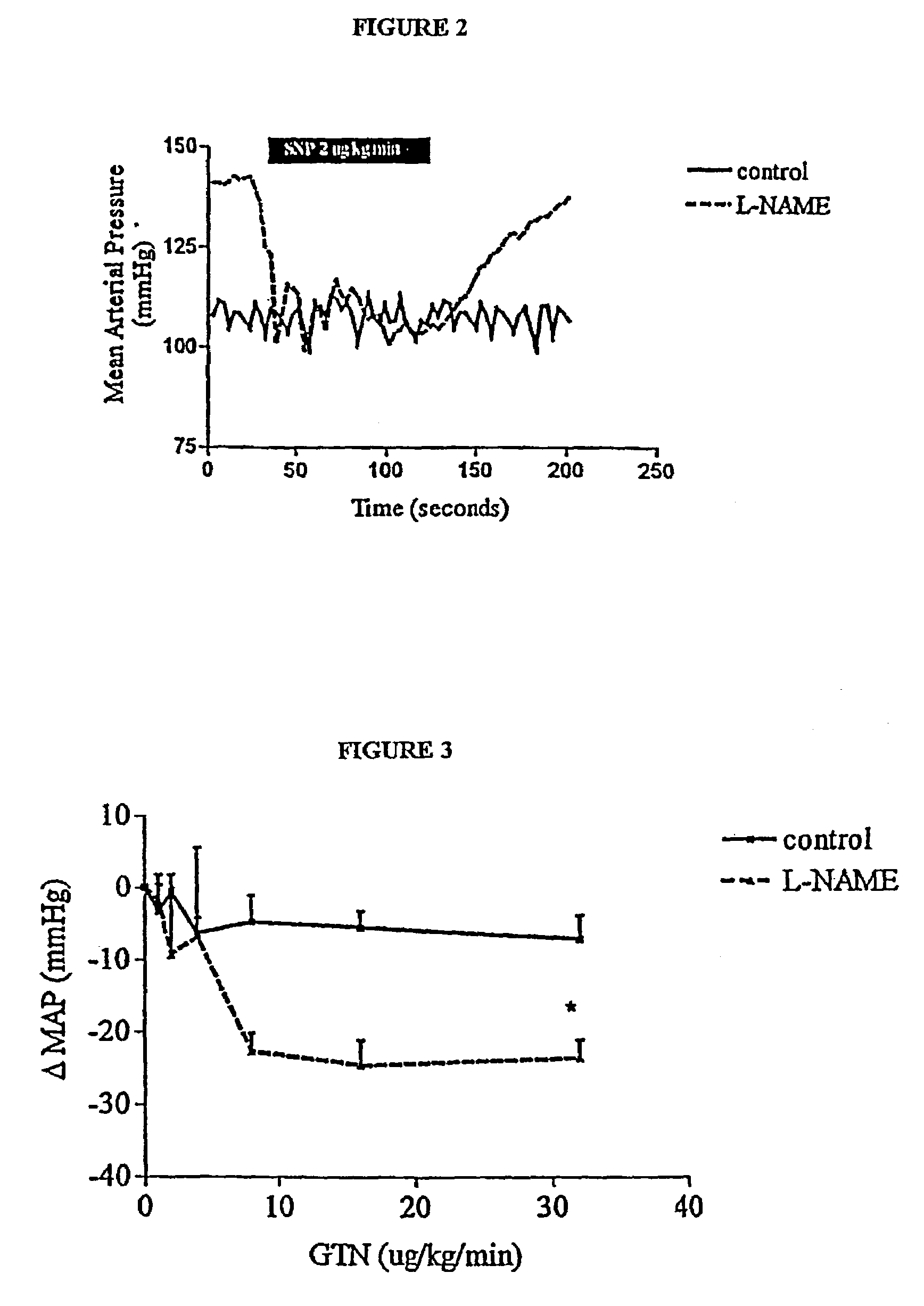
Methods for treating vascular conditions associated with localized imbalance in vascular tone, which are hypothesized to be largely due to elevated endothelin (ET) are provided. The methods involve administration of nitric oxide (NO), agents which are able to provide NO, such as NO donors, agents which activate guanyl cyclase, such as YC-1, or agents which prolong the actions of endogenous NO or cyclic guanosine monophosphate (cGMP; a 2nd messenger molecule), such as phosphodiesterase (PDE) inhibitors. According to the invention, such agents are administered in minimal doses or microdoses by any route known in the art, so as to provide dosages which are about one half to about one twentieth (½ to 1 / 20) of those known to induce vasodilation in “normal” circulations. The low doses of these agents effectively alleviate vascular conditions associated with a reduction in NO production or an attenuation of NO effect, by restoring balance in vascular tone while exerting almost no systemic effect in normal vasculature.
Owner:QUEENS UNIV OF KINGSTON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com