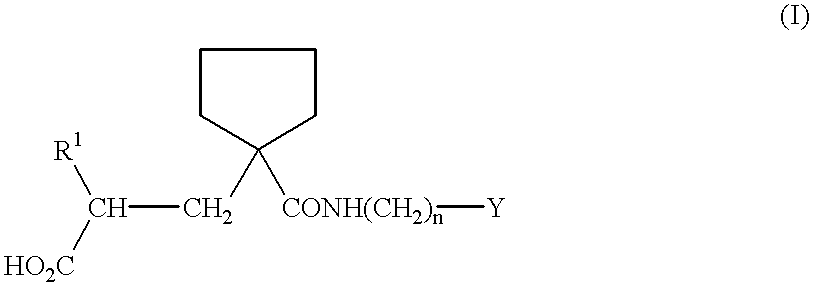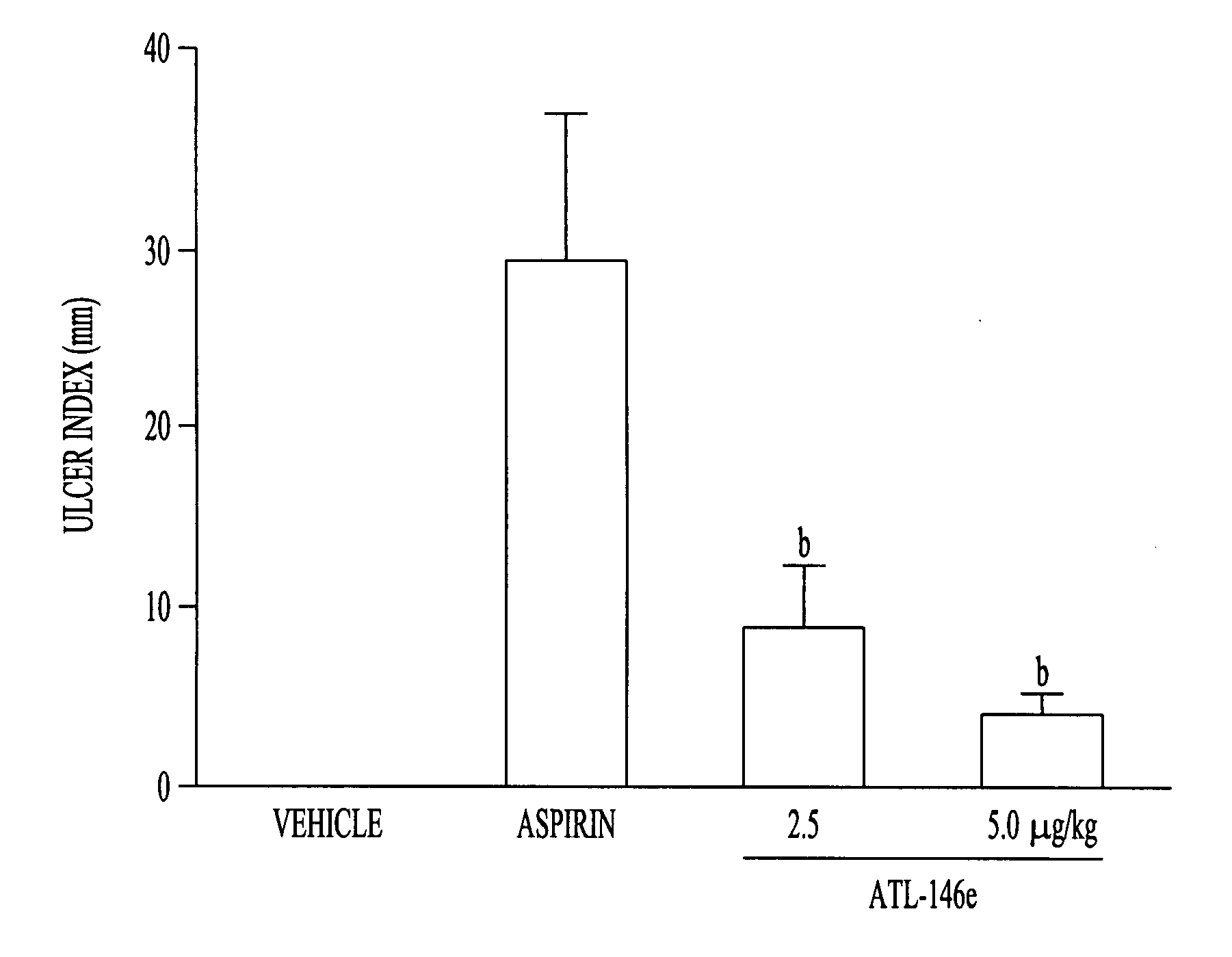Patents
Literature
449 results about "Phosphodiesterase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, phosphodiesterase refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases (which all break the phosphodiester backbone of DNA or RNA), as well as numerous less-well-characterized small-molecule phosphodiesterases.
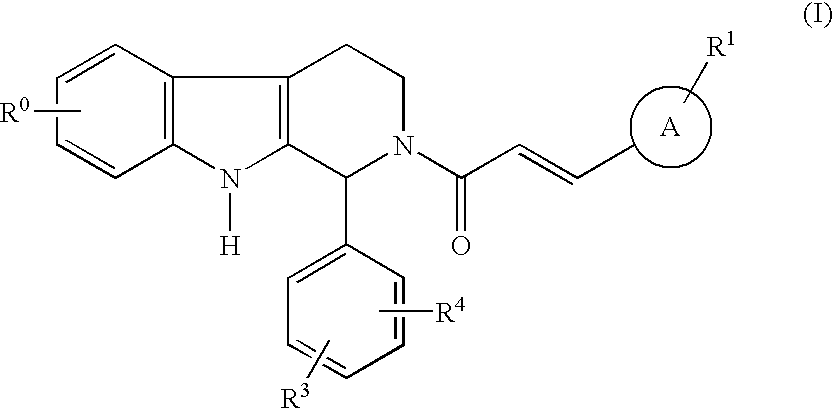
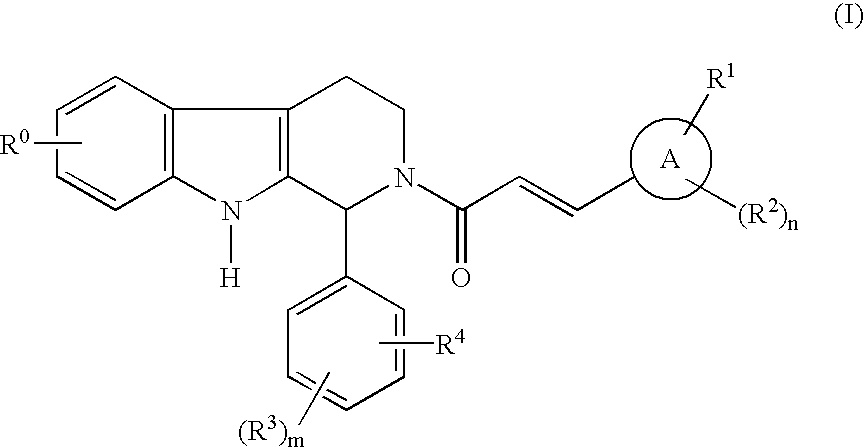
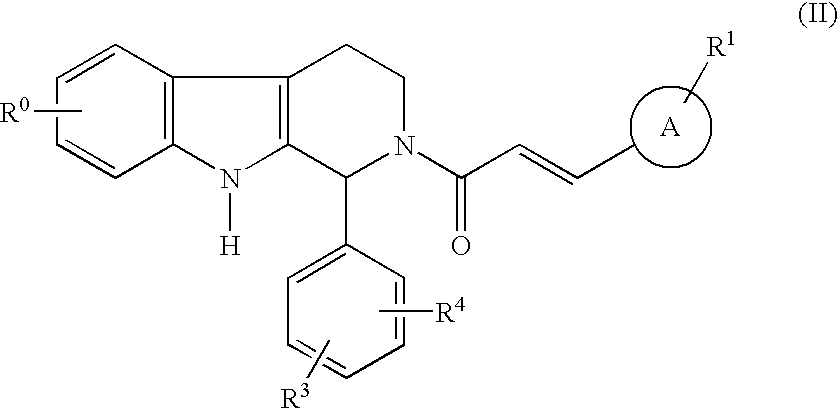
Carboline derivatives
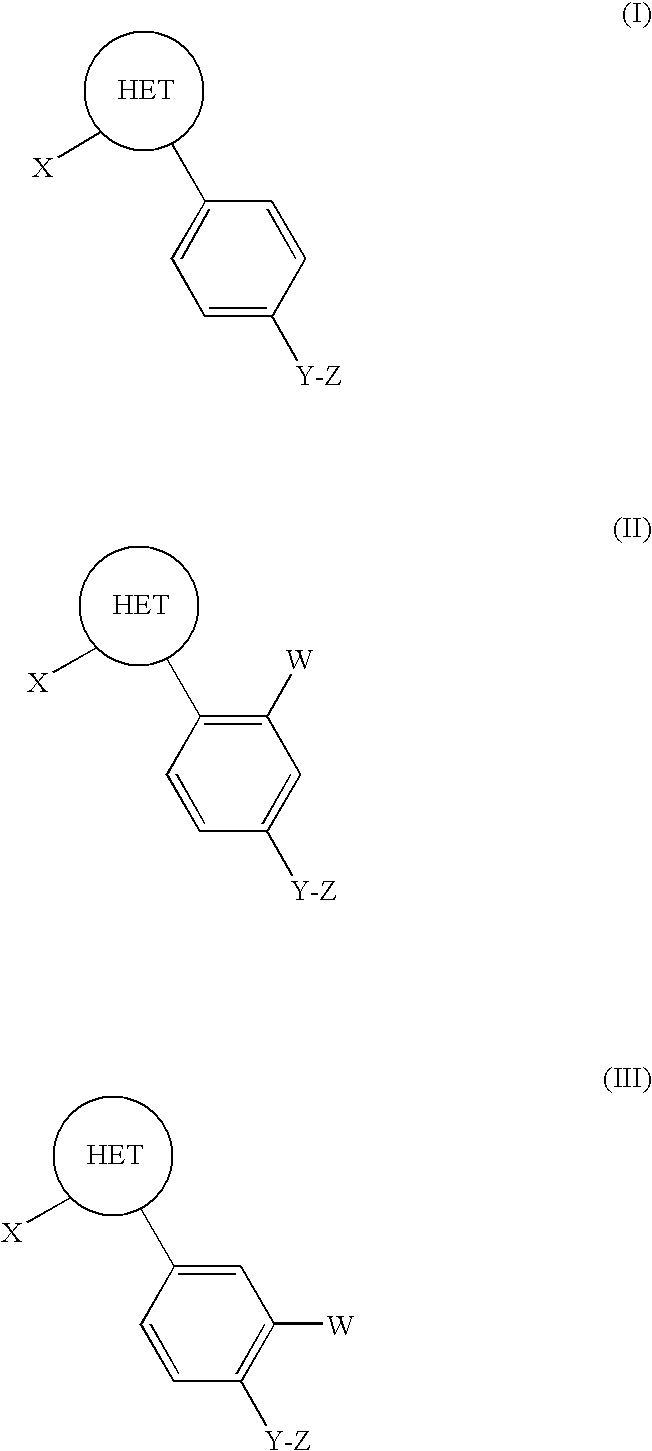
Carboline derivatives of formula (I) are potent and selective inhibitors of cyclic guanosine 3',5'-monophosphate specific phosphodiesterase (cGMP-specific PDE) and have utility in a variety of therapeutic areas where such inhibition is thought to be beneficial, including the treatment of cardiovascular disorders and erectile dysfunction.
Owner:ICOS CORP
Topical treatments for pain
InactiveUS20130029989A1Increase blood flowGood analgesic effectBiocideNervous disorderPhosphodiesteraseNitric oxide
Owner:MCGILL UNIV
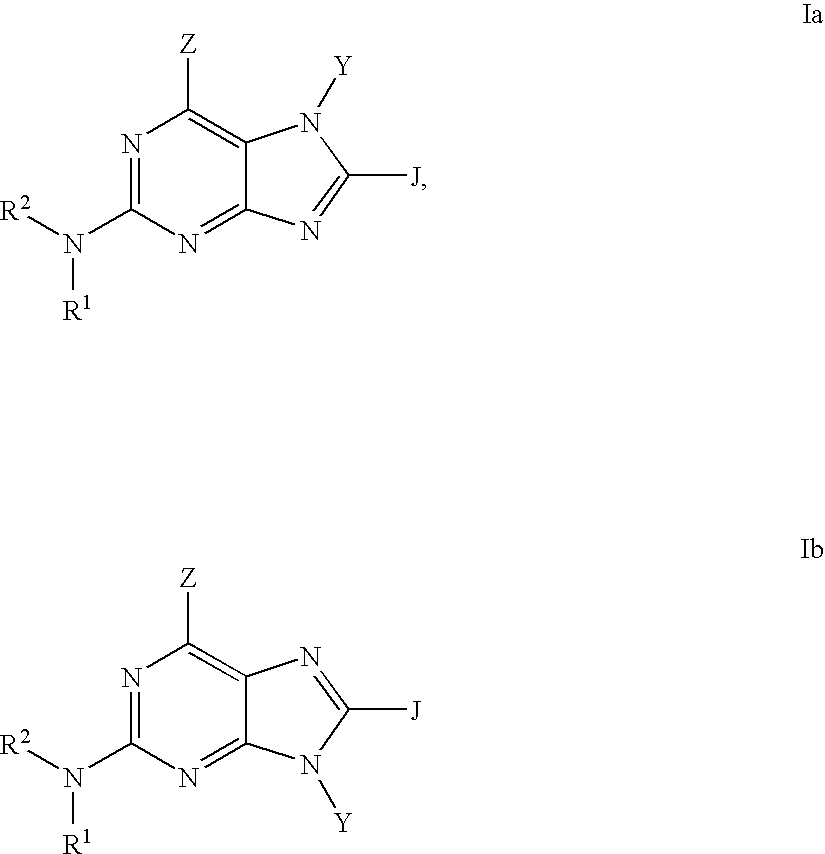
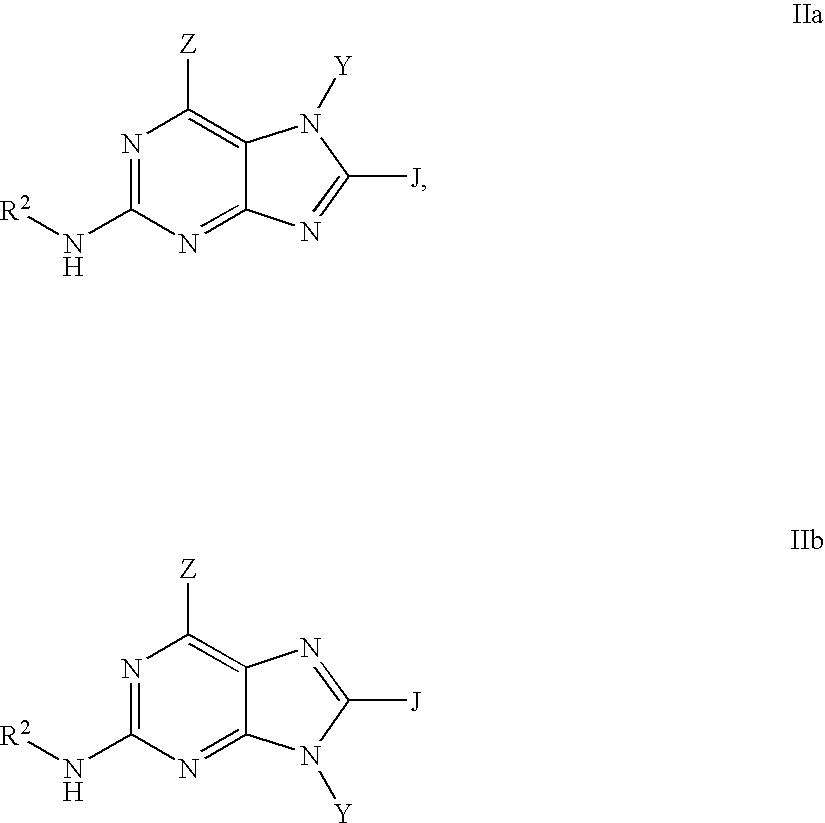
Purine inhibitors of phosphodiesterase (PDE) 7
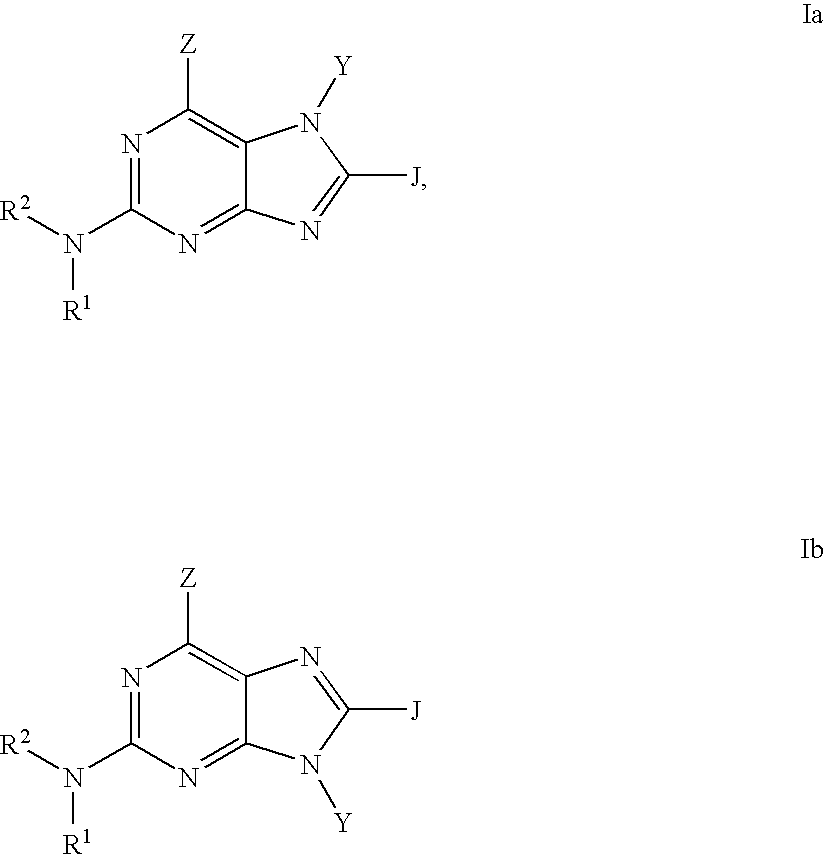
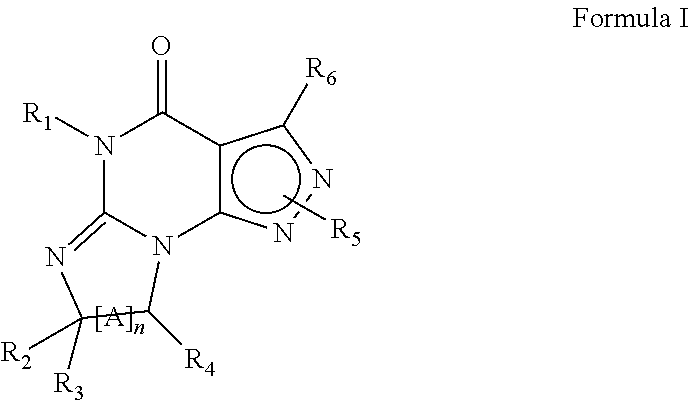
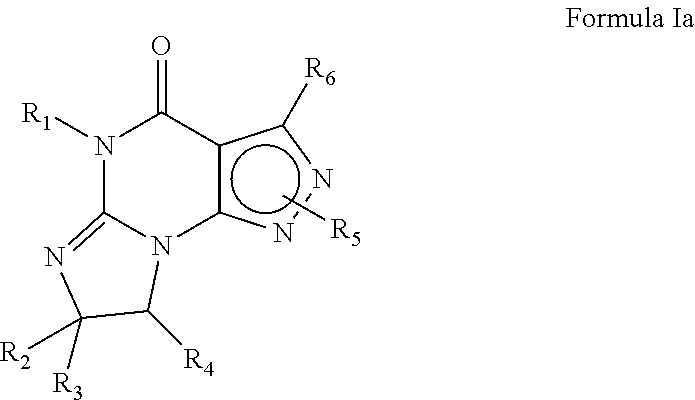
Purine phosphodiesterase 7 (PDE 7) inhibitors of the following formulas wherein R1, Z, Y and J are described herein, and analogs thereof are provided which are useful in treated T-cell mediated diseases. R2 is defined by the following(a) heteroaryl, or heterocyclo, either of which may be optionally substituted with one to three groups T1, T2, T3;(b) aryl substituted with one to three groups T1, T2, T3 provided that at least one of T1, T2, T3 is other than H; or(c) aryl fused to a heteroaryl or heterocyclo ring wherein the combined ring system may be optionally substituted with one to three groups T1, T2, T3.
Owner:BRISTOL MYERS SQUIBB CO
Benzenesulfonamide inhibitors of PDE-IV and their therapeutic use
InactiveUS6162830ASimple structureSynthetic is simpleBiocideNervous disorderDiseasePhosphodiesterase
PCT No. PCT / US98 / 23482 Sec. 371 Date Feb. 7, 2000 Sec. 102(e) Date Feb. 7, 2000 PCT Filed Nov. 4, 1998 PCT Pub. No. WO99 / 26616 PCT Pub. Date Jun. 3, 1999The present invention provides compounds and pharmaceutical compositions thereof, and methods of using same in the treatment of diseases whose treatment benefits from the inhibition of phosphodiesterase (PDE-IV) or Tumor Necrosis Factor (TNF) including asthma, allergic diseases, rheumatoid arthritis, osteoarthritis, septic shock. The compounds provided by this invention have formula (I) wherein R1, R2, R3, R4 are as defined herein.
Owner:WARNER-LAMBERT CO
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
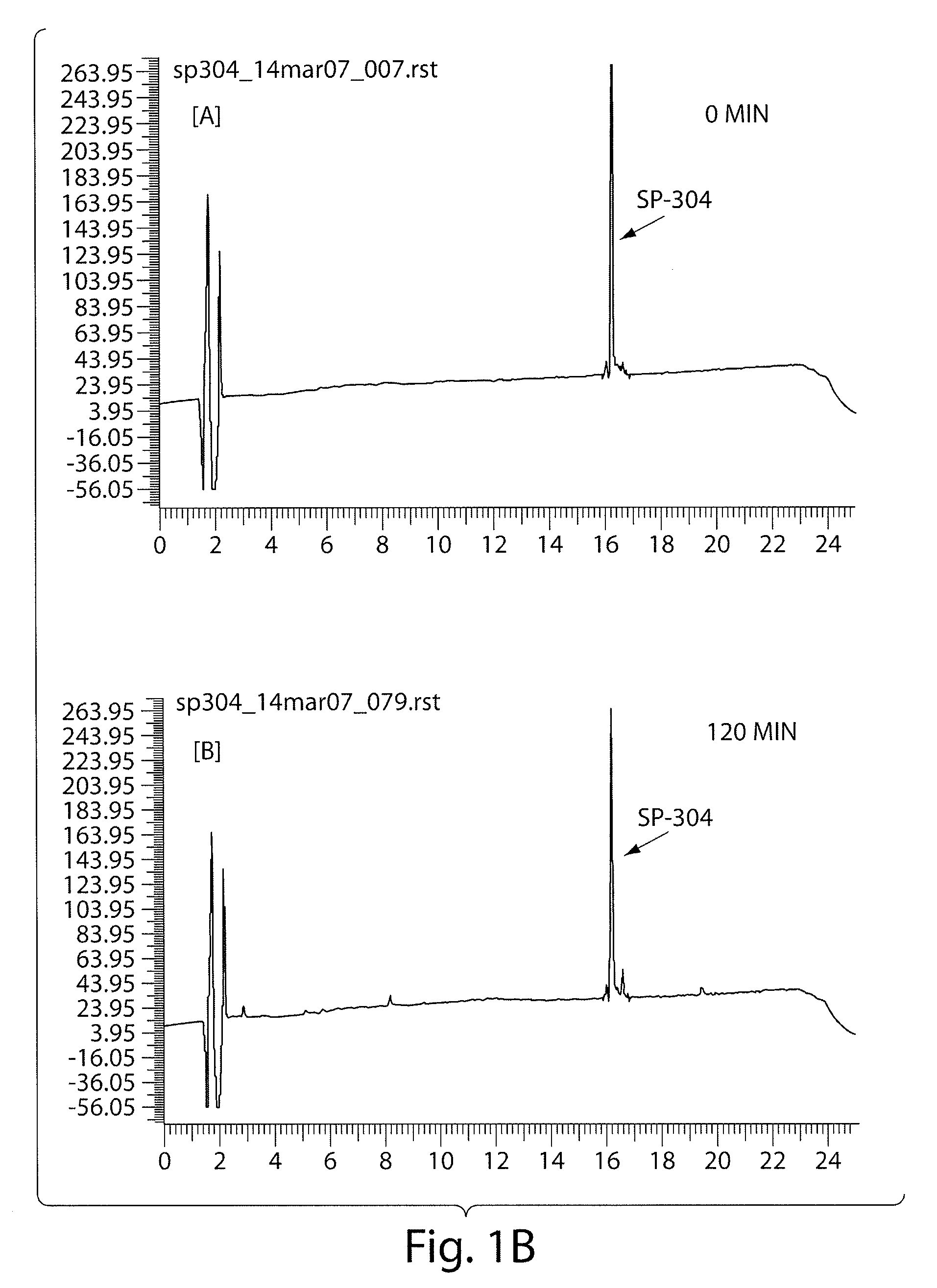
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Organic compounds
ActiveUS20120053190A1Useful in treatmentInhibitory activityBiocideOrganic active ingredientsPhosphodiesteraseBipolar mood disorder
The present invention relates to a new use of phosphodiesterase 1 (PDE1) inhibitors for the treatment of psychosis, schizophrenia, schizoaffective disorder, schizophreniform disorder, psychotic disorder, delusional disorder, mania, or bipolar disorder.
Owner:INTRA CELLULAR THERAPIES INC
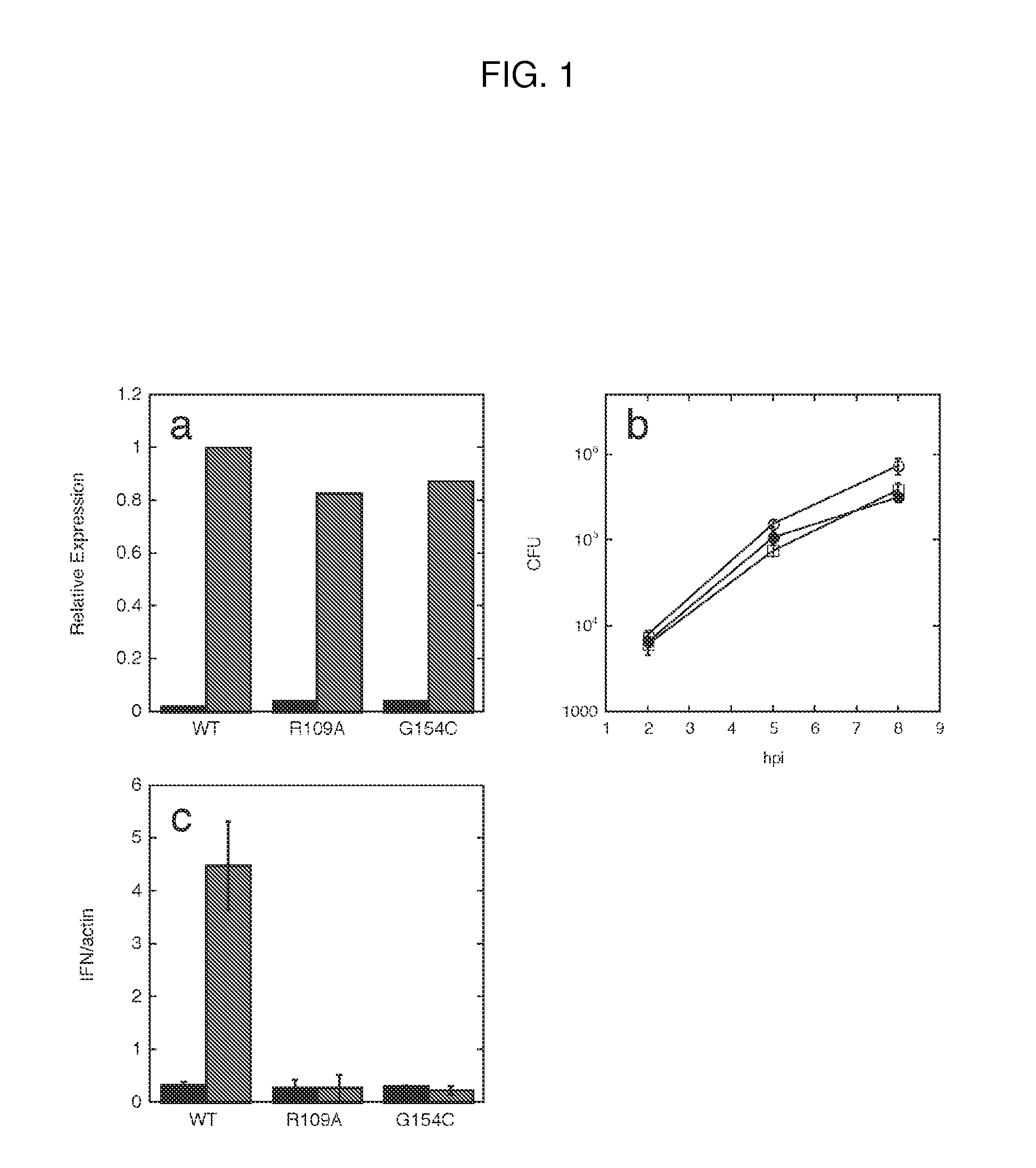
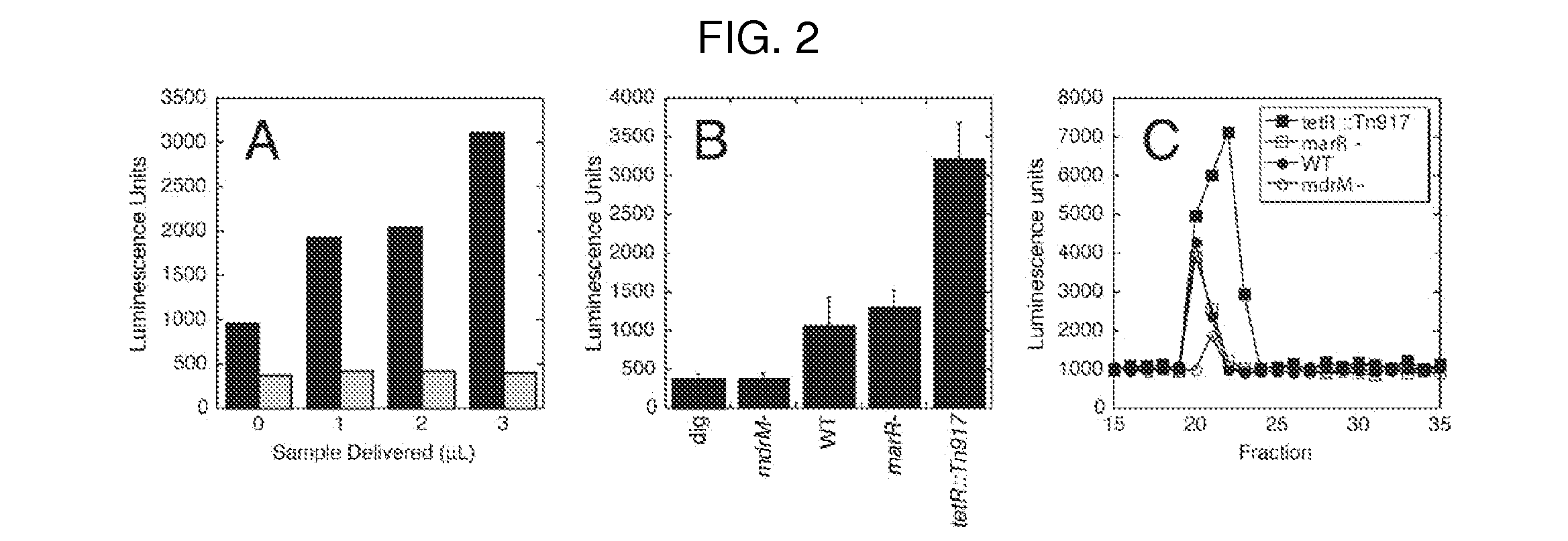
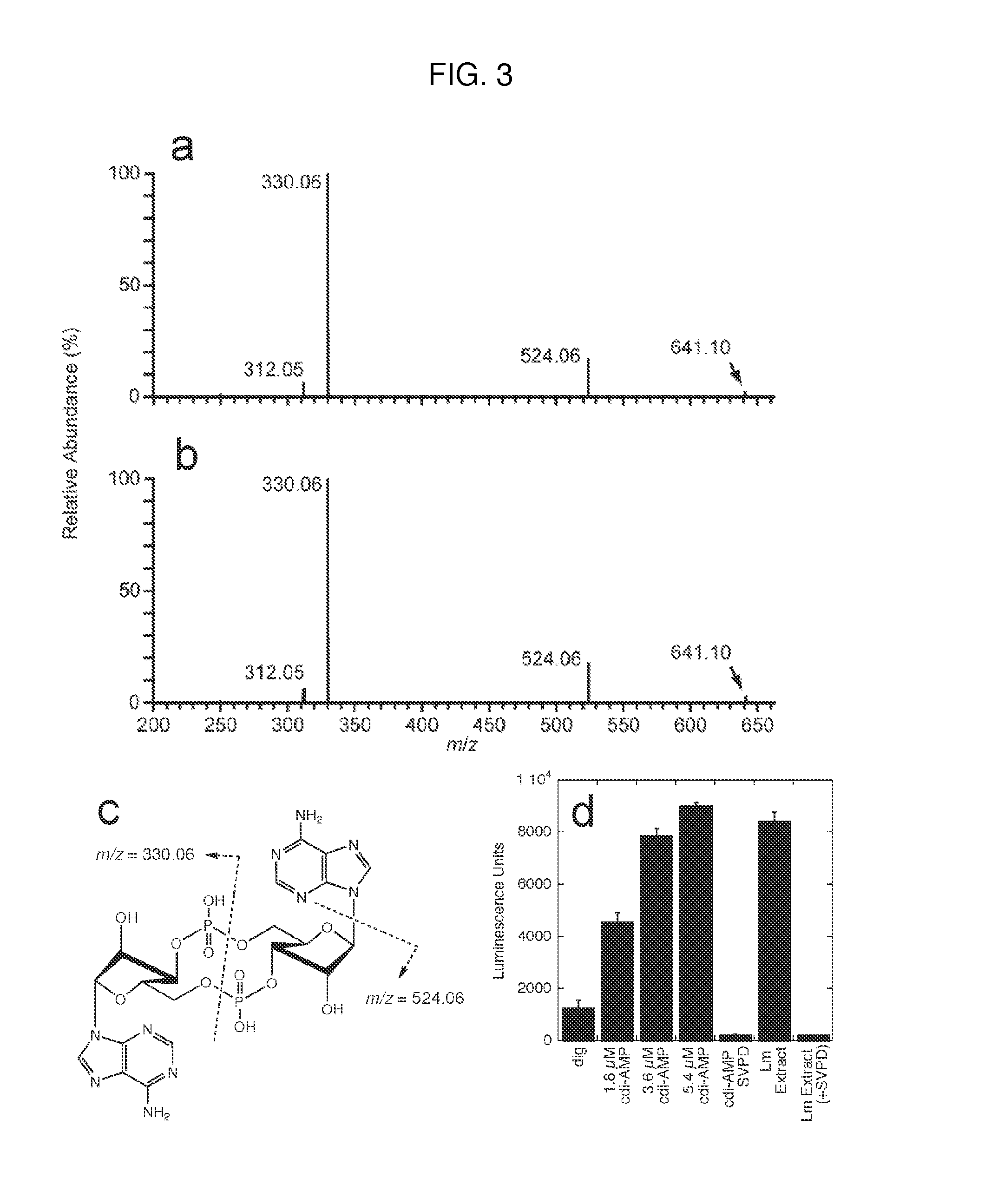
Cyclic di-amp induction of type i interferon
InactiveUS20120164107A1Increase secretionDecrease c-di-AMP phosphodiesterase activityBiocideBacteriaCyclaseProtein composition
Methods of modulating type-I interferon production in a cell are provided. Aspects of the methods include modulating cytosolic cyclic di-adenosine monophosphate (c-di-AMP) activity in the cell in a manner sufficient to modulate type-I interferon production in the cell. Additional aspects of the invention include c-di-AMP activity modulatory compositions, e.g., c-di-AMP, mutant Listeria bacteria, cyclase and / or phosphodiesterase nucleic acid or protein compositions, etc. The subject methods and compositions find use in a variety of applications, including therapeutic applications.
Owner:RGT UNIV OF CALIFORNIA
Agonists of Guanylate Cyclase UseFul For The Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100093635A1Reduce inflammationPositive therapeutic effectAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Agonists of Guanylate Cyclase Useful for the Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100069306A1Maintain good propertiesIncrease resistancePeptide/protein ingredientsAntipyreticPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
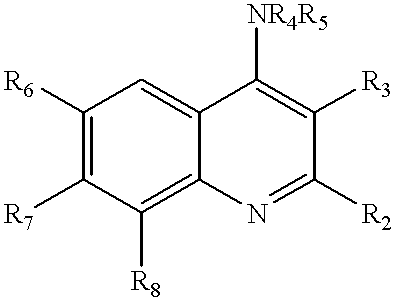
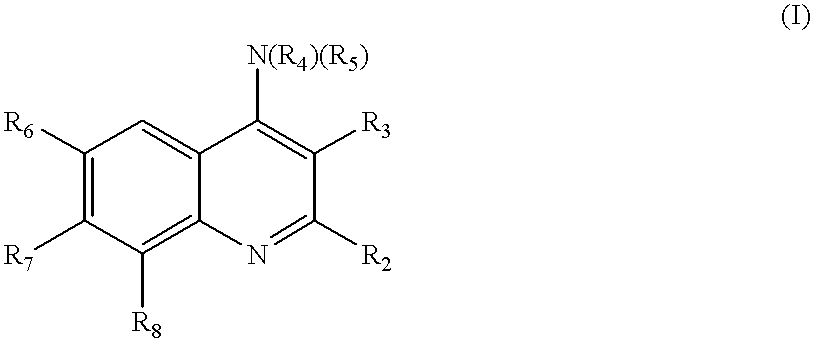
Quinoline inhibitors of cGMP phosphodiesterase
Owner:BRISTOL MYERS SQUIBB CO
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS7879802B2Excellent propertyIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
Owner:BAUSCH HEALTH IRELAND LTD
Oligonucleotide compositions and methods for treating disease including inflammatory conditions
InactiveUS20050153919A1Reduced activityReduce expressionAntibacterial agentsSenses disorderPhosphodiesteraseAllograft rejection
The invention relates to therapeutic antisense oligonucleotides directed against genes coding for phosphodiesterase (PDEs) and the use of these in combination. These antisense oligonucleotides may be used as analytical tools and / or as therapeutic agents in the treatment of disease associated with reduced cellular cAMP in a patient, such as inflammatory diseases of the respiratory tract including, for example, asthma, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome, bronchitis, chronic bronchitis, silicosis, pulmonary fibrosis, lung allograft rejection, allergic rhinitis and chronic sinusitis as well as other conditions in which an increase in cyclic AMP or a decrease in PDE levels is beneficial.
Owner:TOPIGEN PHARMA
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS8034782B2Excellent propertyIncrease resistanceAntipyreticAnalgesicsPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
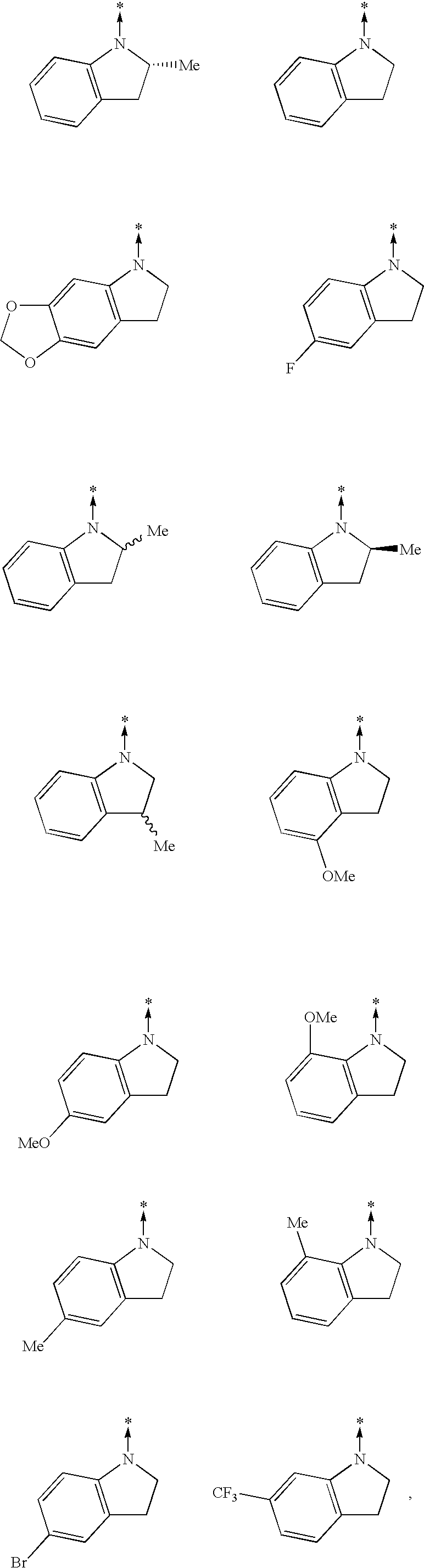
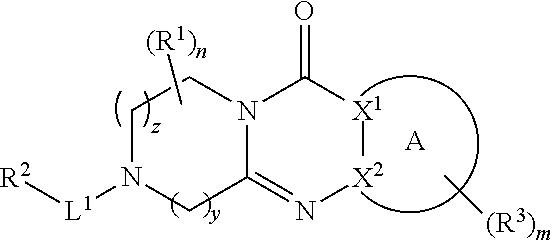
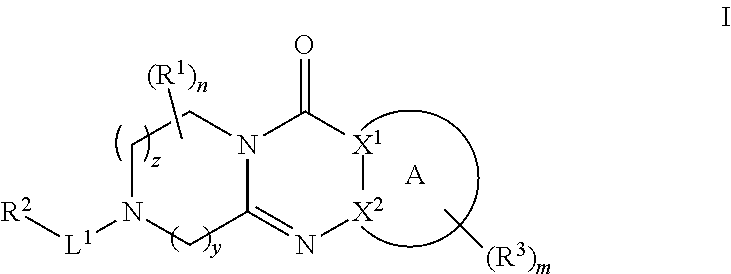
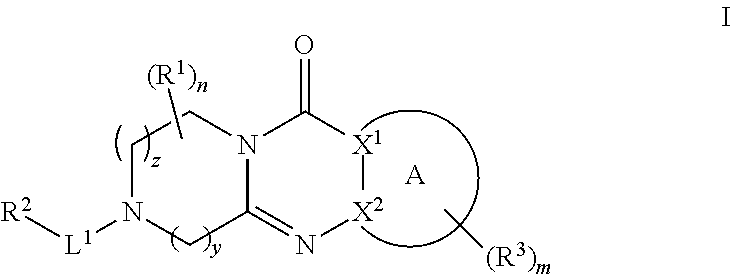
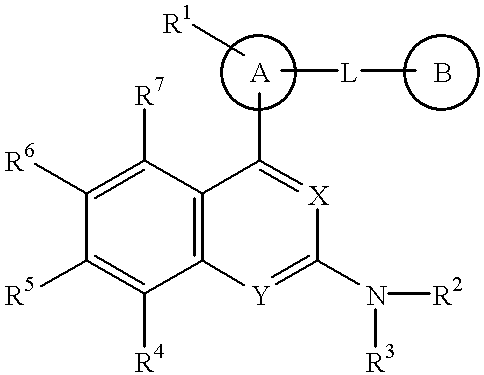
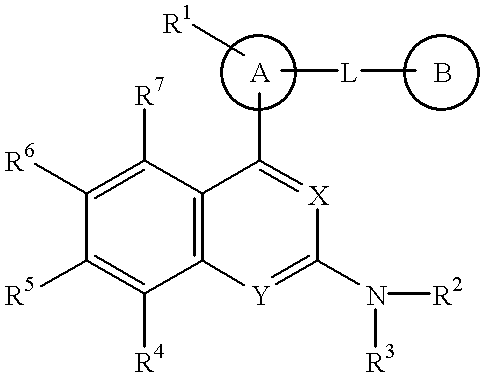
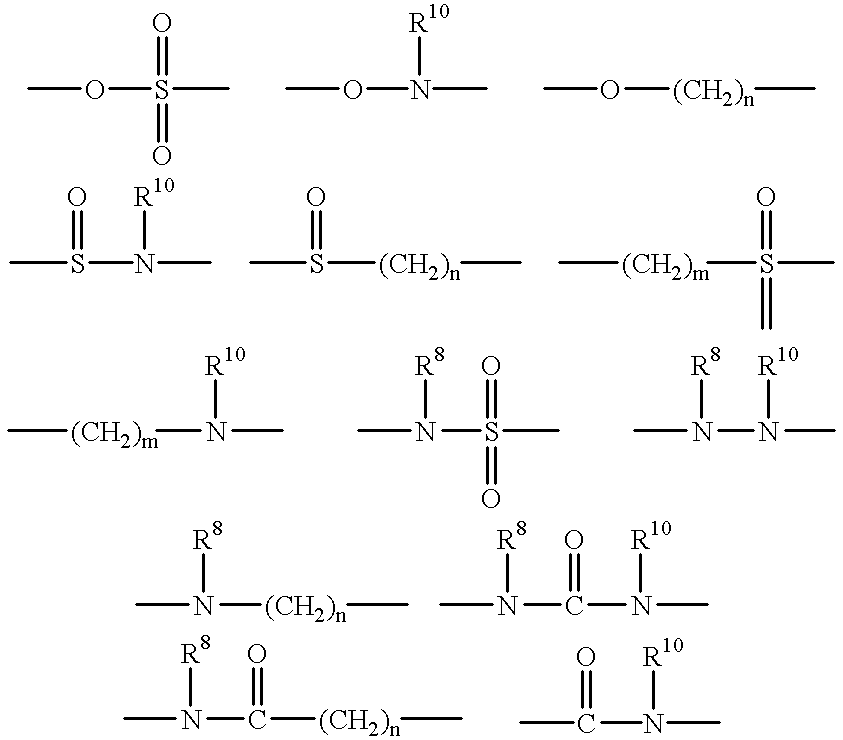
Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7
Owner:BRISTOL MYERS SQUIBB CO
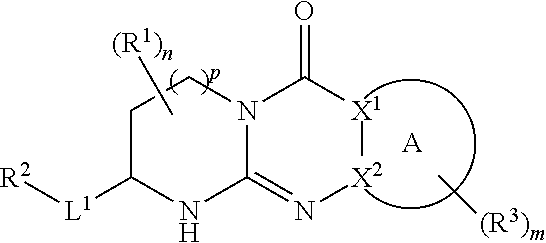
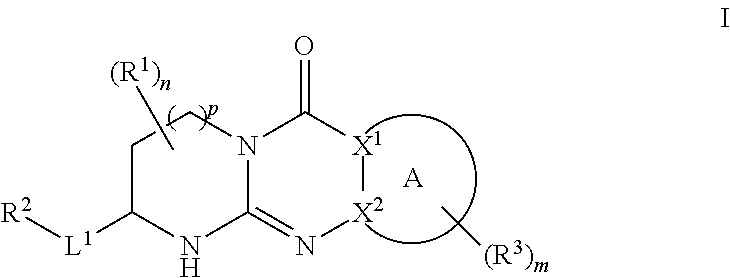
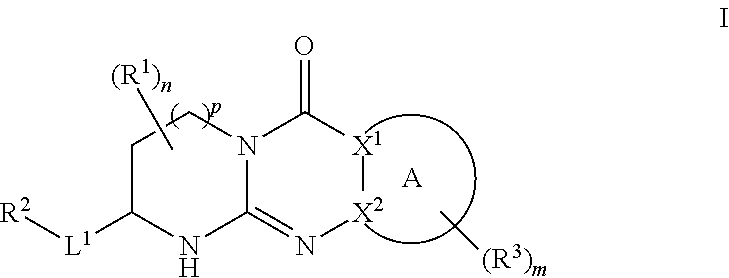
Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7
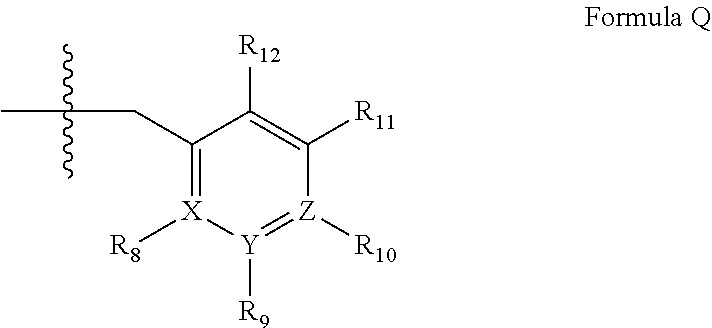
Quinazoline and pyrido[2,3-d]pyrimidine phosphodiesterase 7 (PDE 7) inhibitors of the following formula wherein R1, R2, L, Y1, Y2, Y3 and Z are as described herein, are provided which are useful in treating T-cell mediated diseases.
Owner:BRISTOL MYERS SQUIBB CO
Treatment of male sexual dysfunction
InactiveUS20020028799A1Increase heightSpeed up the processBiocideOrganic chemistryPhosphodiesterasePeptidase Inhibitors
The present invention relates to the use of neutral endopeptidase inhibitors (NEPi) and a combination of NEPi and phosphodiesterase type 5 (PDE5) inhibitor for the treatment of male sexual dysfunction, in particular MED.
Owner:PFIZER INC
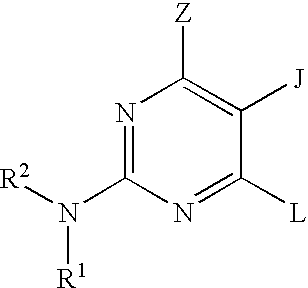
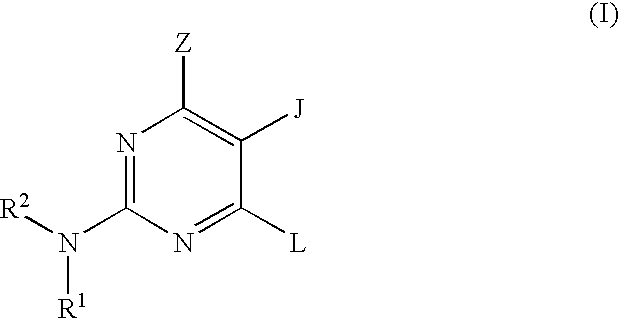
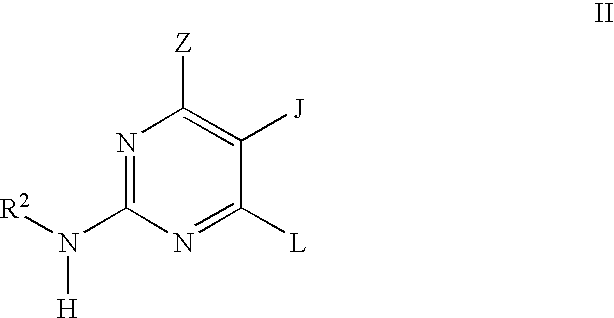
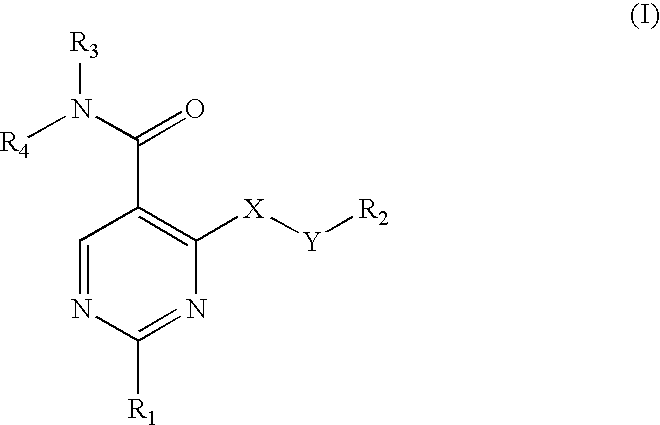
Pyrimidine inhibitors of phosphodiesterase (PDE) 7
Pyrimidine phosphodiesterase 7 (PDE 7) inhibitors of the following formulawherein R1, R2, Z, J and L are described herein, and analogs thereof are provided which are useful in treating T-cell mediated diseases.
Owner:BRISTOL MYERS SQUIBB CO
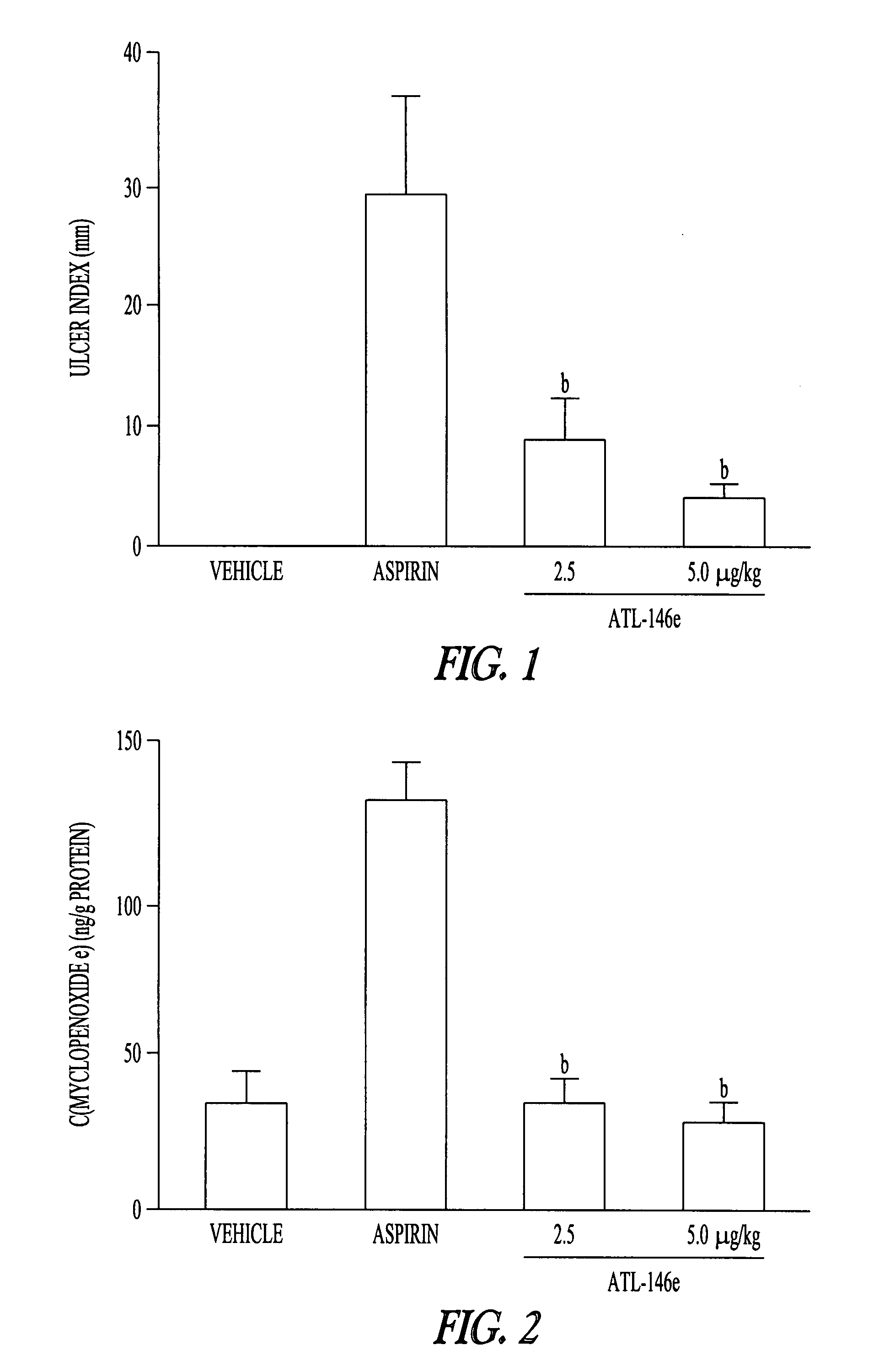
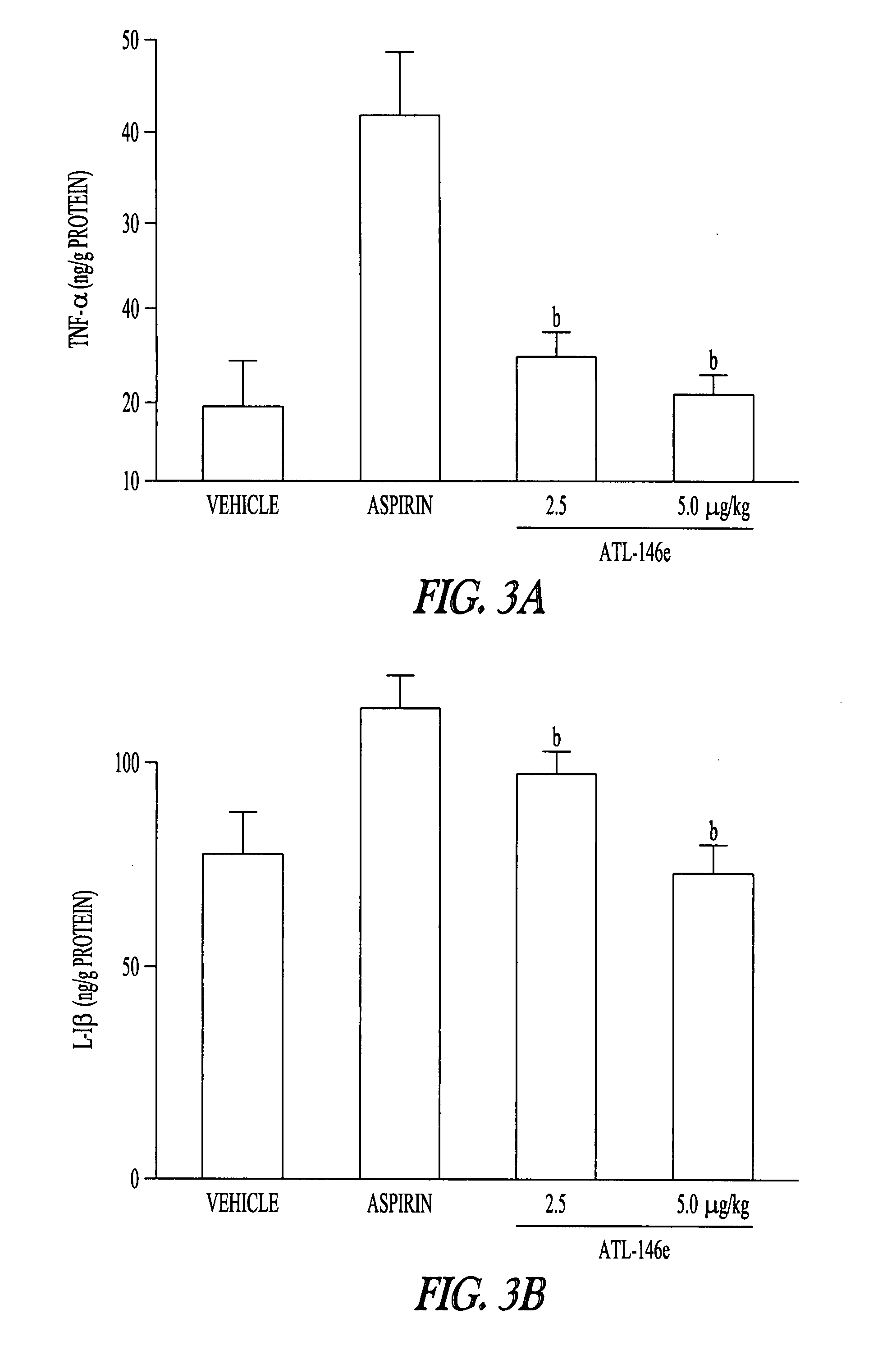
Method to treat gastric lesions
InactiveUS20080027022A1Inhibit inflammationReduced responseBiocideDigestive systemAspirinPhosphodiesterase
The present invention provides a therapeutic method for treating gastric lesions, including administration to a patient in need thereof of an effective amount of an A2A adenosine receptor agonist. The A2A adenosine receptor agonist can be a compound of formula (I) as disclosed herein. The invention further provides a therapeutic method for treating the patient with an A2A adenosine receptor agonist, optionally, in combination with a Type IV phosphodiesterase (PDE) inhibitor. In one embodiment, the gastric lesions are caused by, or aggravated by, the use of NSAIDS such as, for example, aspirin.
Owner:AKITA UNIV SCHOOL OF MEDICINE +1
Carboline derivatives as cGMP phosphodiesterase inhibitors
Compounds of general structural formula (I) wherein A represents a 5- or 6-membered heteroaryl group containing at least one heteroatom selected from the group consisting of oxygen, nitrogen, and sulfur, and use of the compounds, and salts and solvates thereof, as therapeutic agents, are disclosed.
Owner:ICOS CORP
Tricyclic guanidine derivative
InactiveUS20160083400A1Effective inhibitorBiocideOrganic chemistryGuanidine derivativesPhosphodiesterase
Disclosed are compounds useful as inhibitors of Phosphodiesterase 1 (PDE1), compositions thereof, and methods of using the same.
Owner:SUNOVION PHARMA INC +1
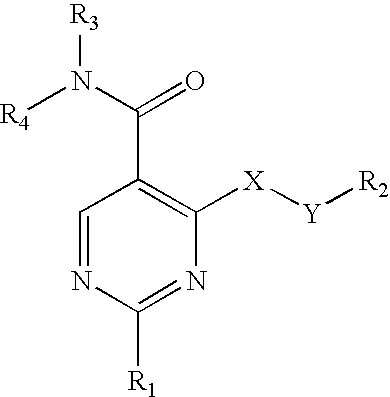
Pyrimidine 5-carboxamide compounds, process for producing the same and use thereof
Compounds of the formulawherein R1, R2, R3, R4, X and Y are as defined, which have a superior cGMP specific phosphodiesterase (PDE) inhibitory activity, and can be used as an agent for the treatment of cardiovascular diseases such as angina pectoris, heart failure, cardiac infarction, hypertension, arteriosclerosis, and the like; allergic diseases such as asthma, or disorders of male or female genital function and the like.
Owner:TAKEDA PHARMA CO LTD
Tricyclic piperazine derivative
Disclosed are compounds useful as inhibitors of Phosphodiesterase 1 (PDE1), compositions thereof, and methods of using the same.
Owner:SUNOVION PHARMA INC +1
Nitrogenous heterocyclic derivatives and medicine thereof
InactiveUS6352989B1High activityInhibitor of production of TNF.alphaAntibacterial agentsBiocidePhosphodiesteraseNitrogenous heterocyclic compound
The present invention provides a novel nitrogen-containing heterocyclic compound useful as a phosphodiesterase-4 inhibitor, and a medicament comprising the same. Further, the present invention provides a nitrogen-containing heterocyclic compound represented by the following formula, its salt or hydrates thereof, and a medicament comprising the same.wherein the ring A is an aromatic hydrocarbon ring which may have a heteroatom, the ring B represents (a) a saturated hydrocarbon ring, (b) an unsaturated hydrocarbon ring, (c) a saturated heterocyclic ring or (d) an unsaturated heterocyclic ring, all of which may have a substituent group.
Owner:EISIA R&D MANAGEMENT CO LTD
Method for preparing mixed type nucleotide feed addictive from yeast cell and application
InactiveCN101480220AHigh purityPromote growthAnimal feeding stuffAccessory food factorsAnti stressPhosphodiesterase
The invention relates to a method for preparing a mixed-type nucleotide feed additive from yeast cells and applications of the feed additive, which belong to the technical field of the preparation of nucleotide feed additives from yeast cells. The method comprises the steps: using yeast cells as raw materials and combining a homogenization method and a salt method to break yeast cell walls ; separating the yeast cell walls, protein and other insoluble substances by centrifugation and fractional isoelectric crystallization; specially collecting RNA precipitates at special isoelectric points to obtain wet and crude nucleic acid with the purity of 70 percent; dissolving the wet and crude nucleic acid in water, using high-activity phosphodiesterase from malt roots to carry out a high-efficiency directional enzymatic reaction; directly spraying, drying and crushing the solution after the enzymatic reaction to obtain the mixed-type nucleotide feed additive product comprising the protein content lower than 10 percent and four monosomic nucleotides (AMP, GMP, CMP and UMP) larger than 60 percent. After the feed additive is applied to animal feed, the intestinal absorption function of animals can be notably improved, the growth of the animals is stimulated, and the immune function, the antiviral capability and the anti-stress capability of the animals are improved.
Owner:江苏省苏微微生物研究有限公司 +2
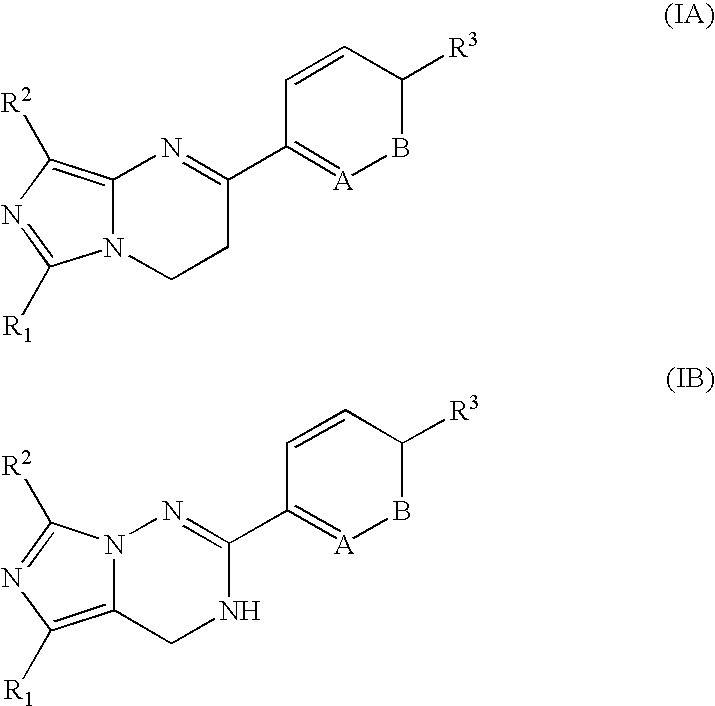
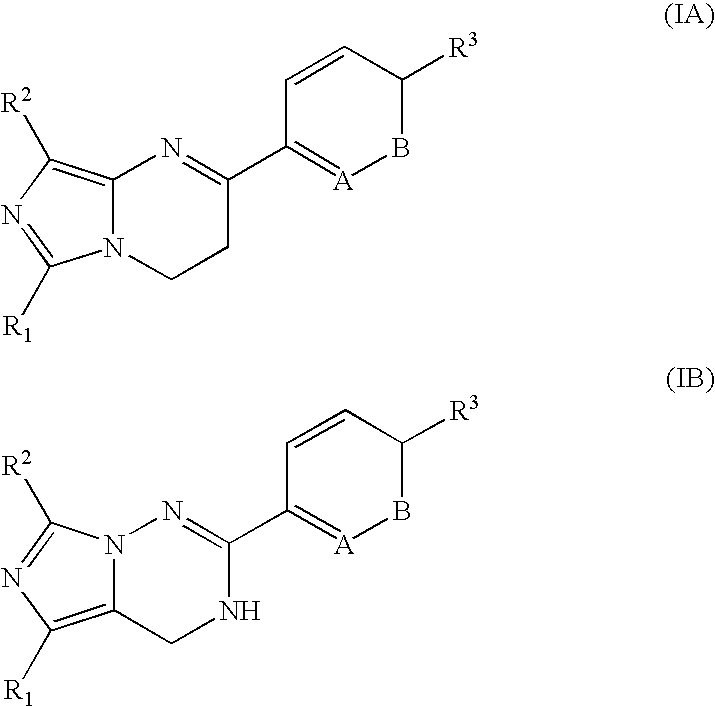
Imidazotriazinone derivatives as pde 7 (phosphodiesterase 7) inhibitors
InactiveUS20060128707A1Potent and selective PDE inhibiting effectAntibacterial agentsBiocideMethyl groupDisease cause
The present invention provides the compounds inhibiting PDE 7 selectively, and therefore, enhances cellular cAMP level. Consequently, the compound is useful for treating various kinds of disease such as allergic disease, inflammatory disease or immunologic disease. The compound is imidazotirazinone compound represented by the following formula (IA) or (IB): especially, R1 is cyclohexyl group, R2 is methyl group; R3 is a hydrogen atom; nitro group; cyano group; a halogen atom; heteroaryl group; substituted or unsubstituted C1-C6 alkyl group; substituted or unsubstituted C2-C6 alkenyl group; saturated or unsaturated heterocycloalkyl group which is substituted or unsubstituted; a group: —NR5R6, —C(O)R7, —SO2R7, —OR8, —NR8COR7, —NR8S02R7; A is CR4; and B is CH.
Owner:ASUBIO PHARMA
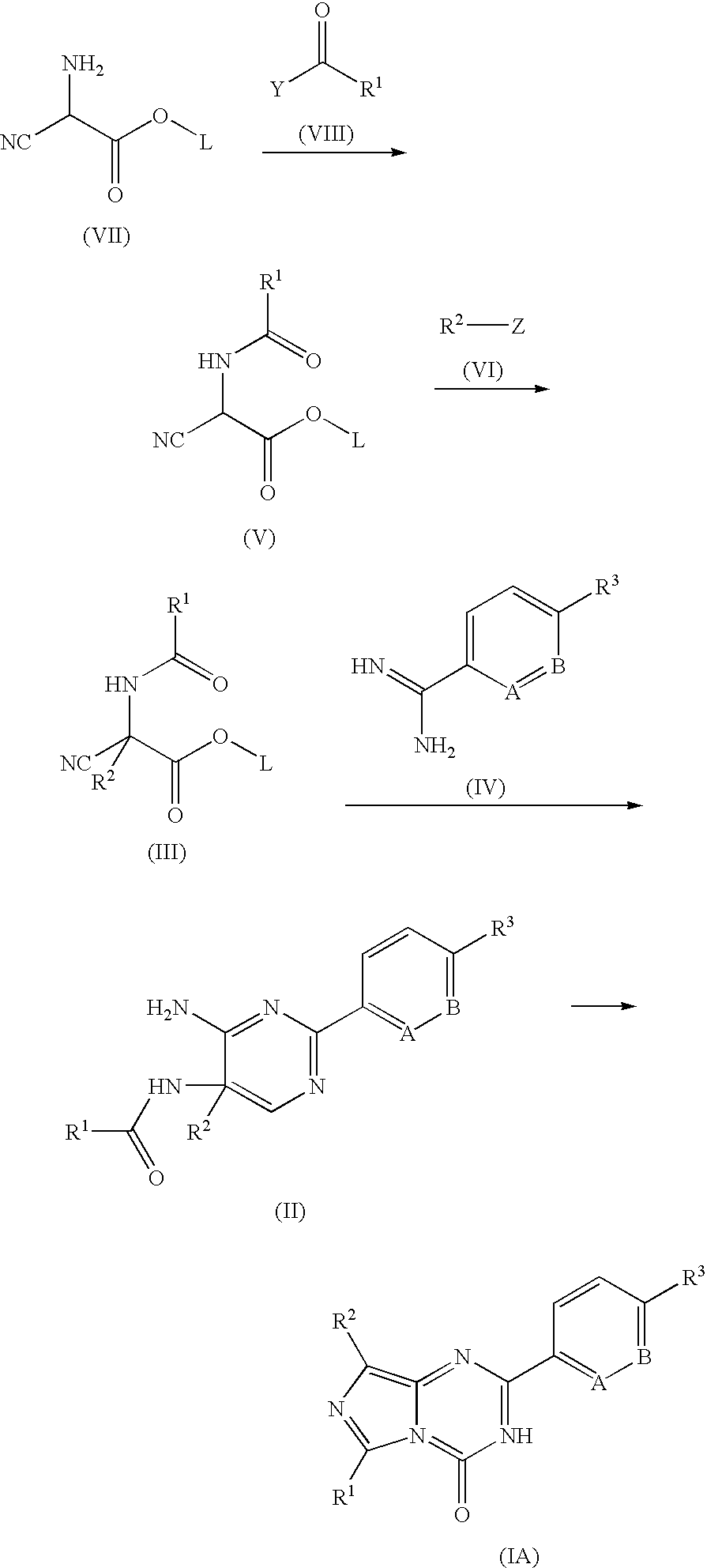
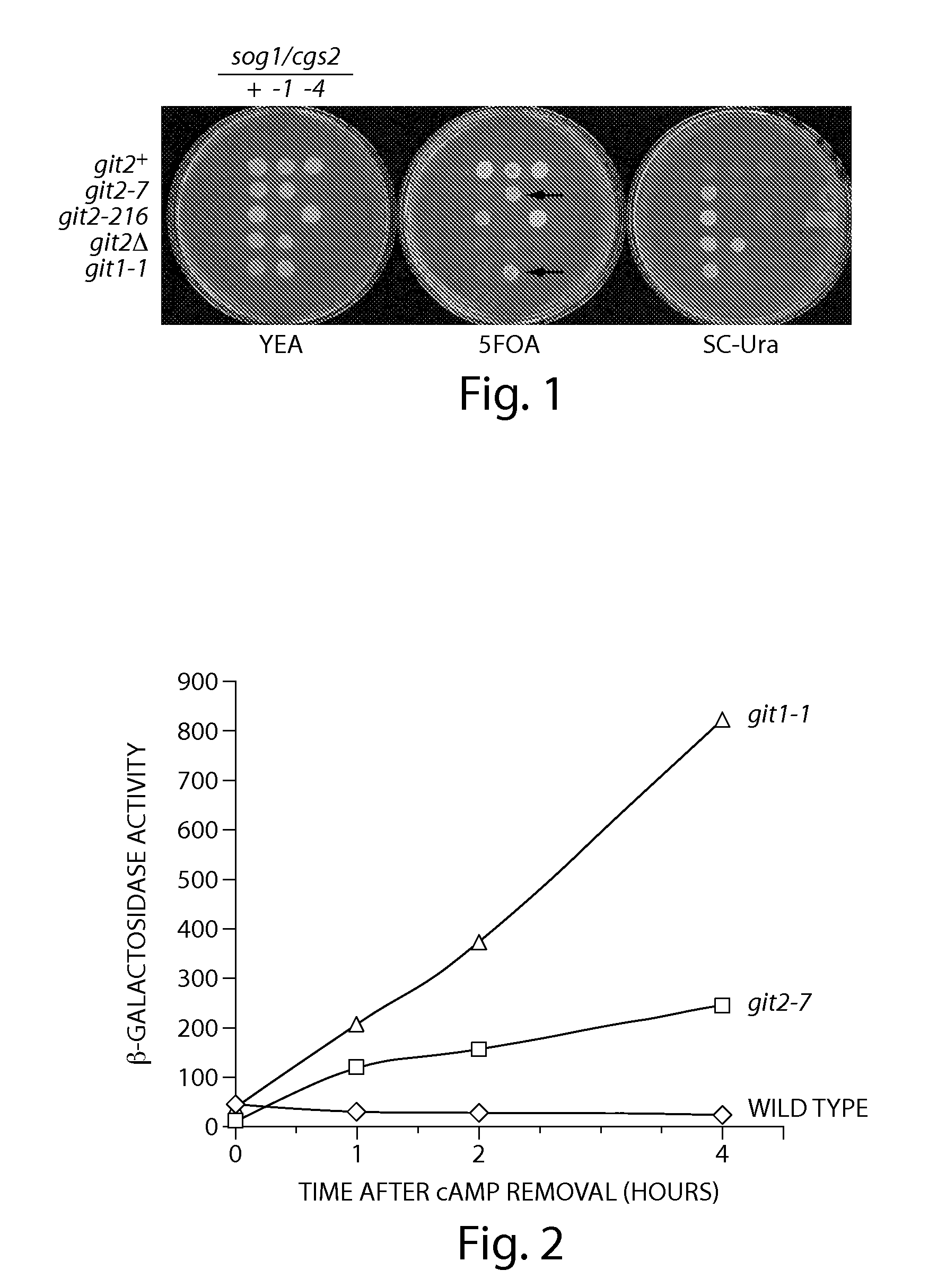
Inhibitors of cyclic amp phosphodiesterases
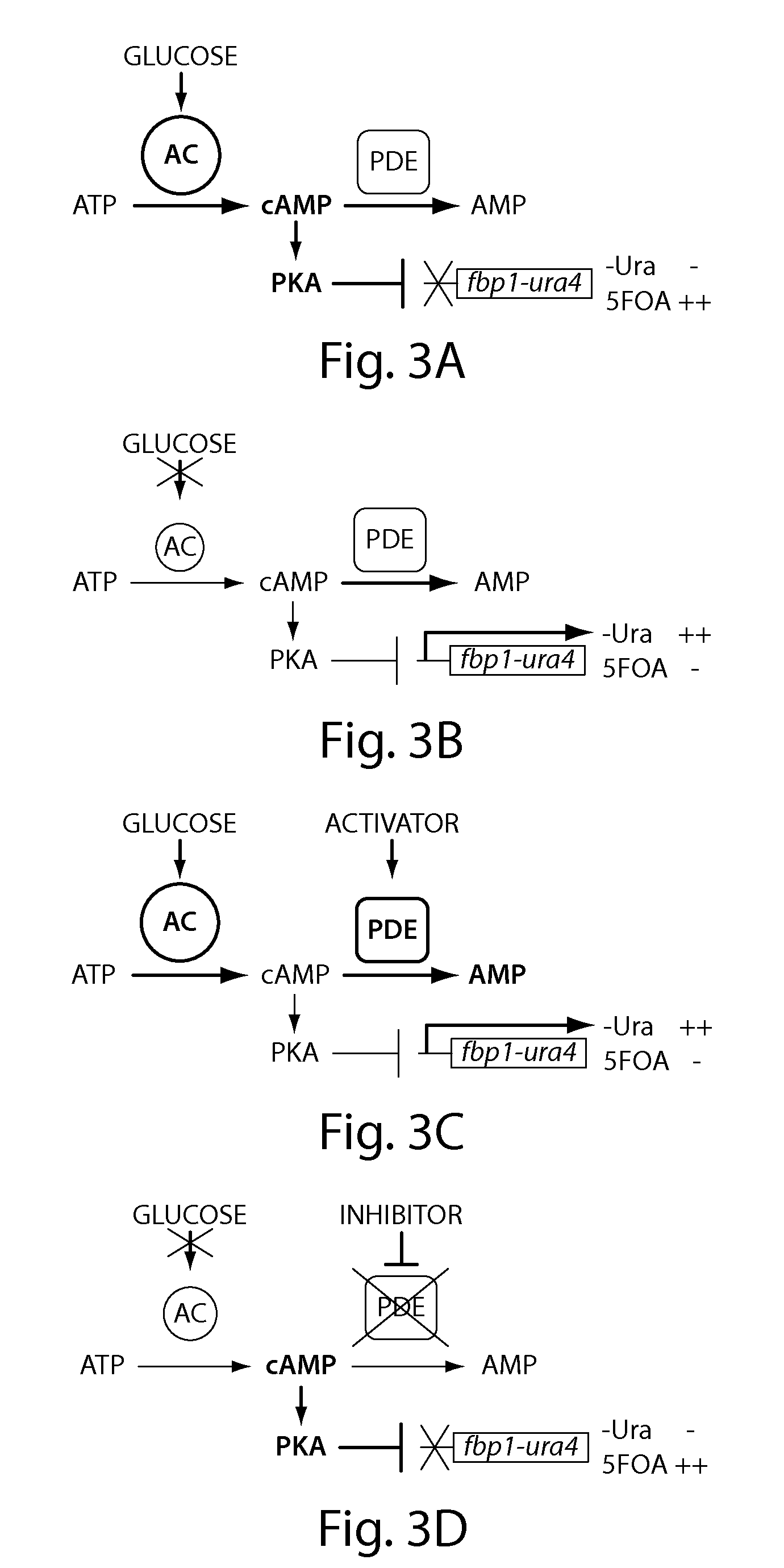
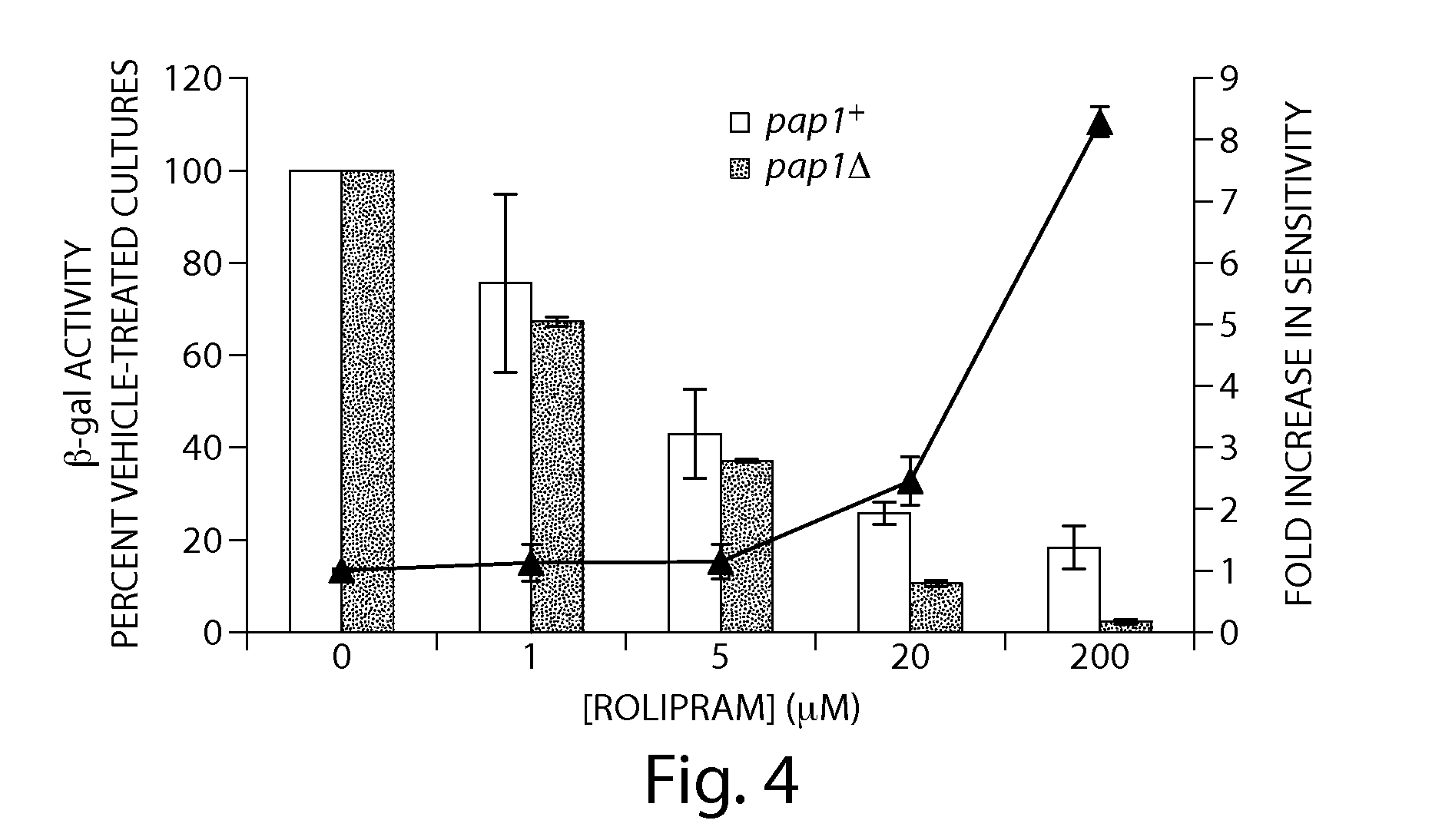
Recombinant fission yeast cells and methods of using them are described, which provide for identification of chemical and biological inhibitors or activators of a target exogenous phosphodiesterase (PDE). The invention provides, in some aspects, compounds that inhibit cAMP PDE activity and compositions that include such compounds. The invention, in part, also includes methods of using cAMP PDE-inhibiting compounds in the treatment of cAMP PDE-associated diseases and / or disorders.
Owner:BOSTON COLLEGE
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS8207295B2Excellent propertyIncrease resistancePeptide/protein ingredientsAntipyreticPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Pyridocarbazole derivatives having cGMP-PDE inhibitory activity
InactiveUS6018046ALow selectivityEasy to useBiocideOrganic chemistryIschemic Heart DiseasesActive ingredient
PCT No. PCT / JP97 / 01829 Sec. 371 Date Jan. 29, 1998 Sec. 102(e) Date Jan. 29, 1998 PCT Filed May 29, 1997 PCT Pub. No. WO97 / 45427 PCT Pub. Date Dec. 4, 1997The invention relates to novel pyridocarbazole derivatives having highly selective action in inhibiting cyclic GMP-phosphodiesterase (hereinafter abbreviated as cGMP-PDE), processes for producing such derivatives, agents containing at least one of such derivatives as an active ingredient for preventing and / or treating pulmonary hypertension, ischemic heart diseases or diseases against which the cGMP-PDE inhibitory action is effective, and intermediates useful for the production of pyridocarbazole derivatives.
Owner:MOCHIDA PHARM CO LTD
1,2-Disubstituted Heterocyclic Compounds
InactiveUS20100137317A1Improvement in side-effect profileLow dosing requirementBiocideNervous disorderPhosphodiesteraseMammal
1,2-disubstituted heterocyclic compounds which are inhibitors of phosphodiesterase 10 are described. Also described are processes, pharmaceutical compositions, pharmaceutical preparations and pharmaceutical use of the compounds in the treatment of mammals, including human(s) for central nervous system (CNS) disorders and other disorders which may affect CNS function. Among the disorders which may be treated are neurological, neurodegenerative and psychiatric disorders including, but not limited to, those associated with cognitive deficits or schizophrenic symptoms.
Owner:FORUM PHARMA
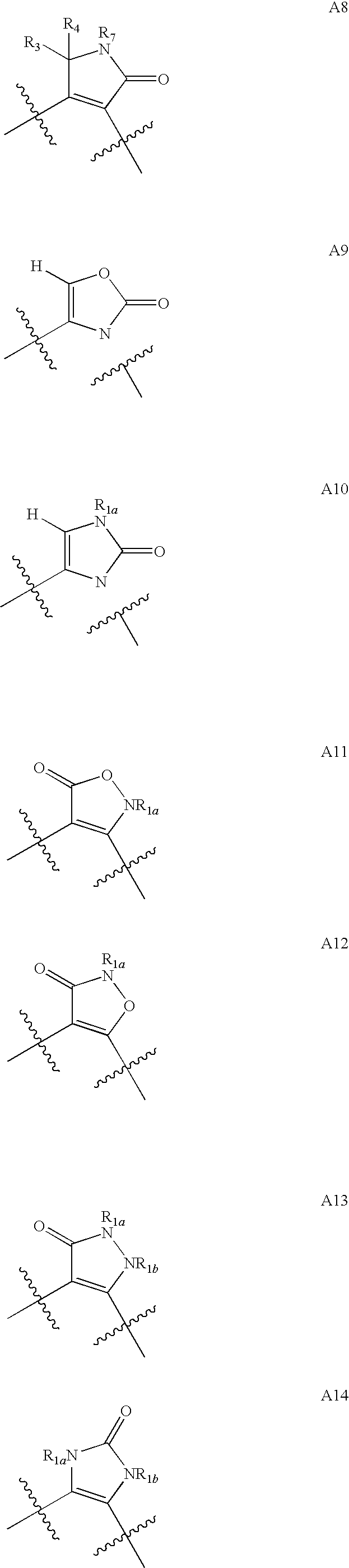
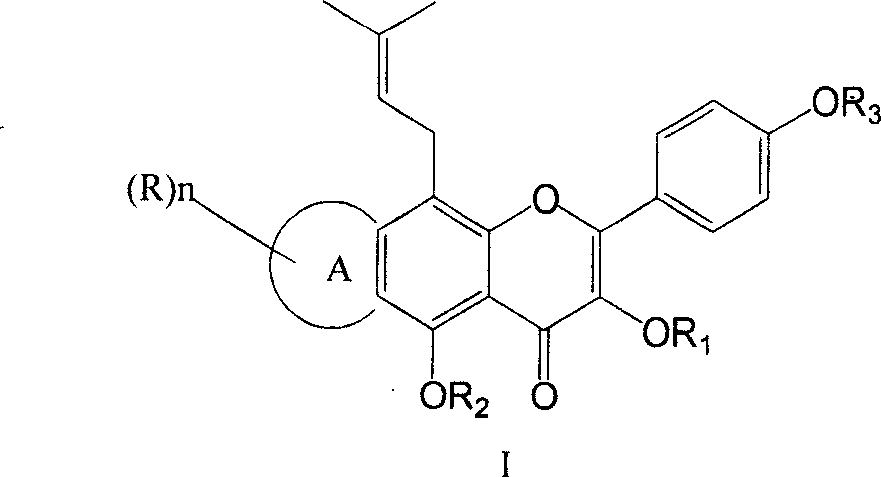
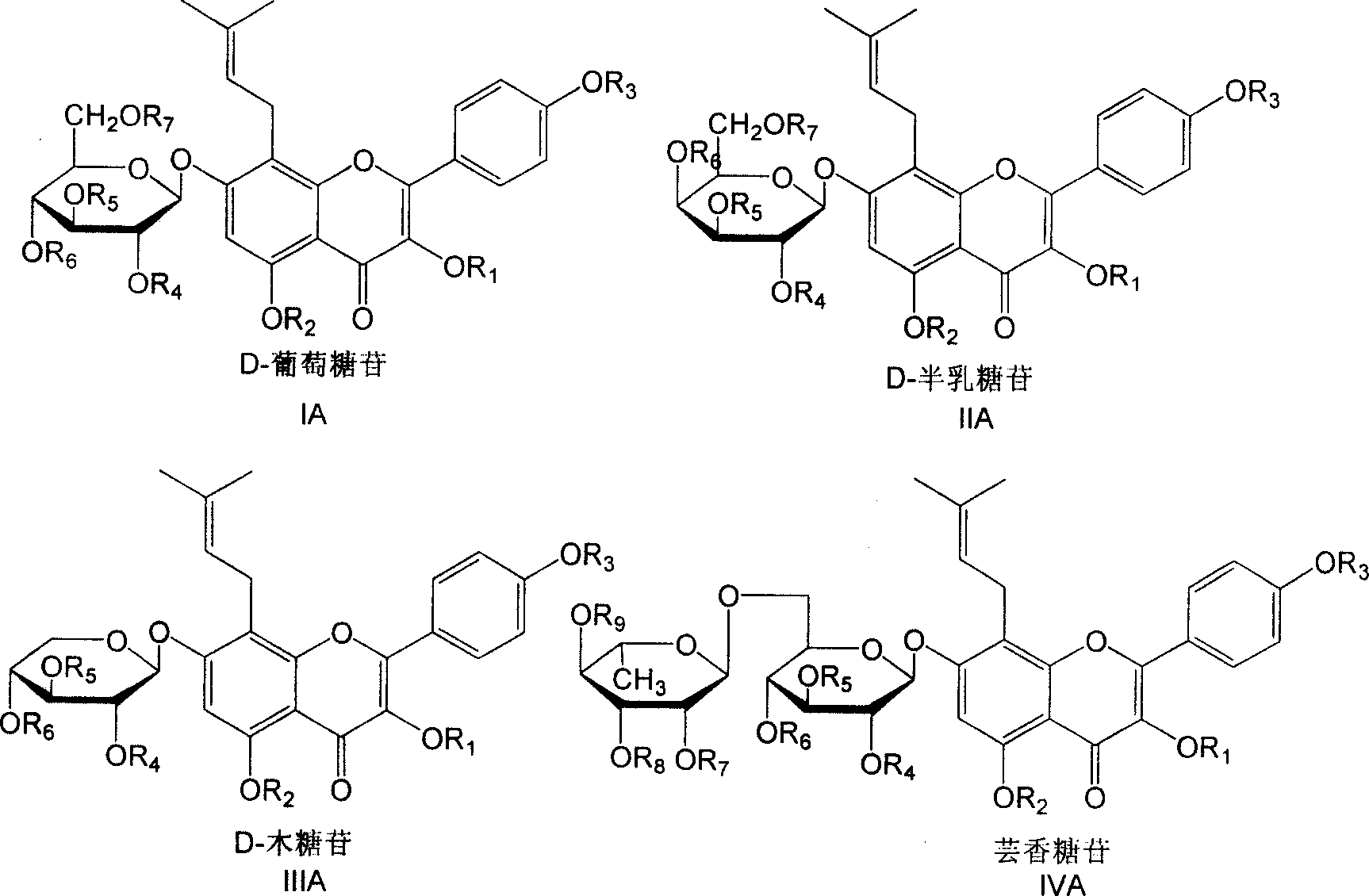
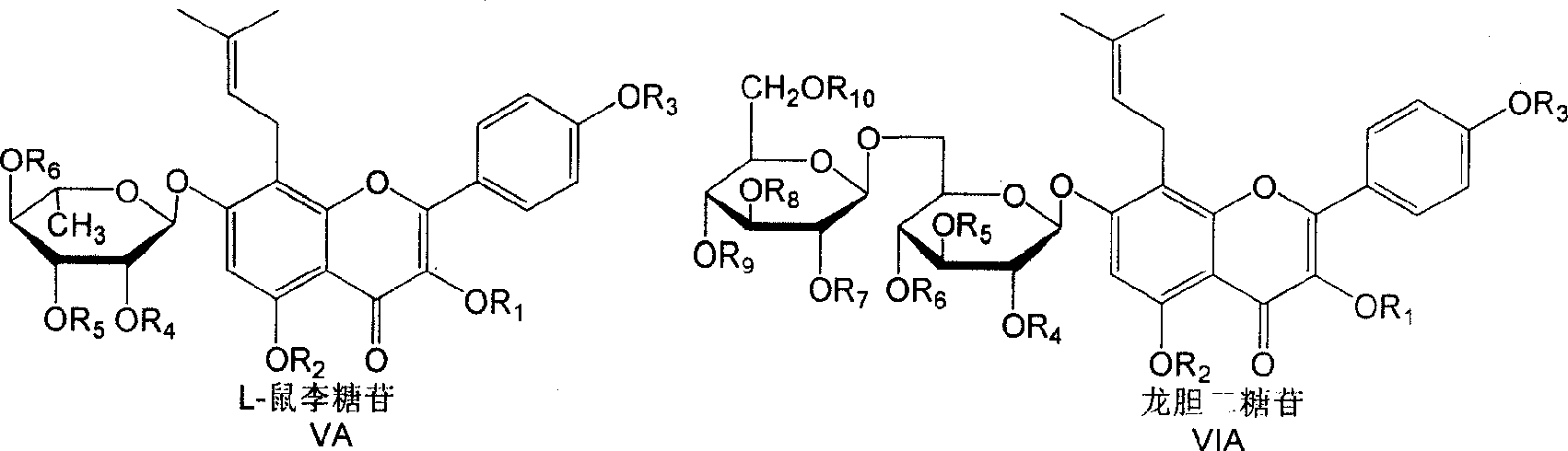
Isoamylene radical chromocor derivative, preparation method and uses thereof
InactiveCN101113157AIncreased ICP/BPIncrease ICP/BP valueOrganic active ingredientsSenses disorderSexual functionPhosphodiesterase
The invention relates to a series of derivatives (I) of isopentenyl flavonoid and preparation methods and uses thereof. The pharmacology experiments show that: the compounds can inhibit cGMP specific phosphodiesterase, in particular to selectively inhibiting phosphodiesterase V (PDE5), and have wide application in the treatment and prevention of sexual function trouble and the improvement of vascoconstriction related diseases.
Owner:TOPHARMAN SHANGHAI +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka.patsnap.com/patent_img/4ed49fc3-cda5-4b77-abdb-a8271bf83186/US20060116516A1-20060601-C00001.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka.patsnap.com/patent_img/4ed49fc3-cda5-4b77-abdb-a8271bf83186/US20060116516A1-20060601-C00002.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka.patsnap.com/patent_img/4ed49fc3-cda5-4b77-abdb-a8271bf83186/US20060116516A1-20060601-C00003.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka.patsnap.com/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00001.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka.patsnap.com/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00002.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka.patsnap.com/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00003.png)