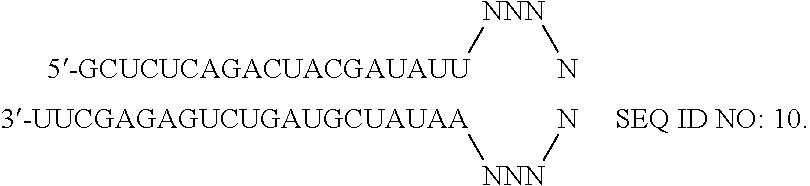Patents
Literature
43 results about "Tumor necrosis factor α" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
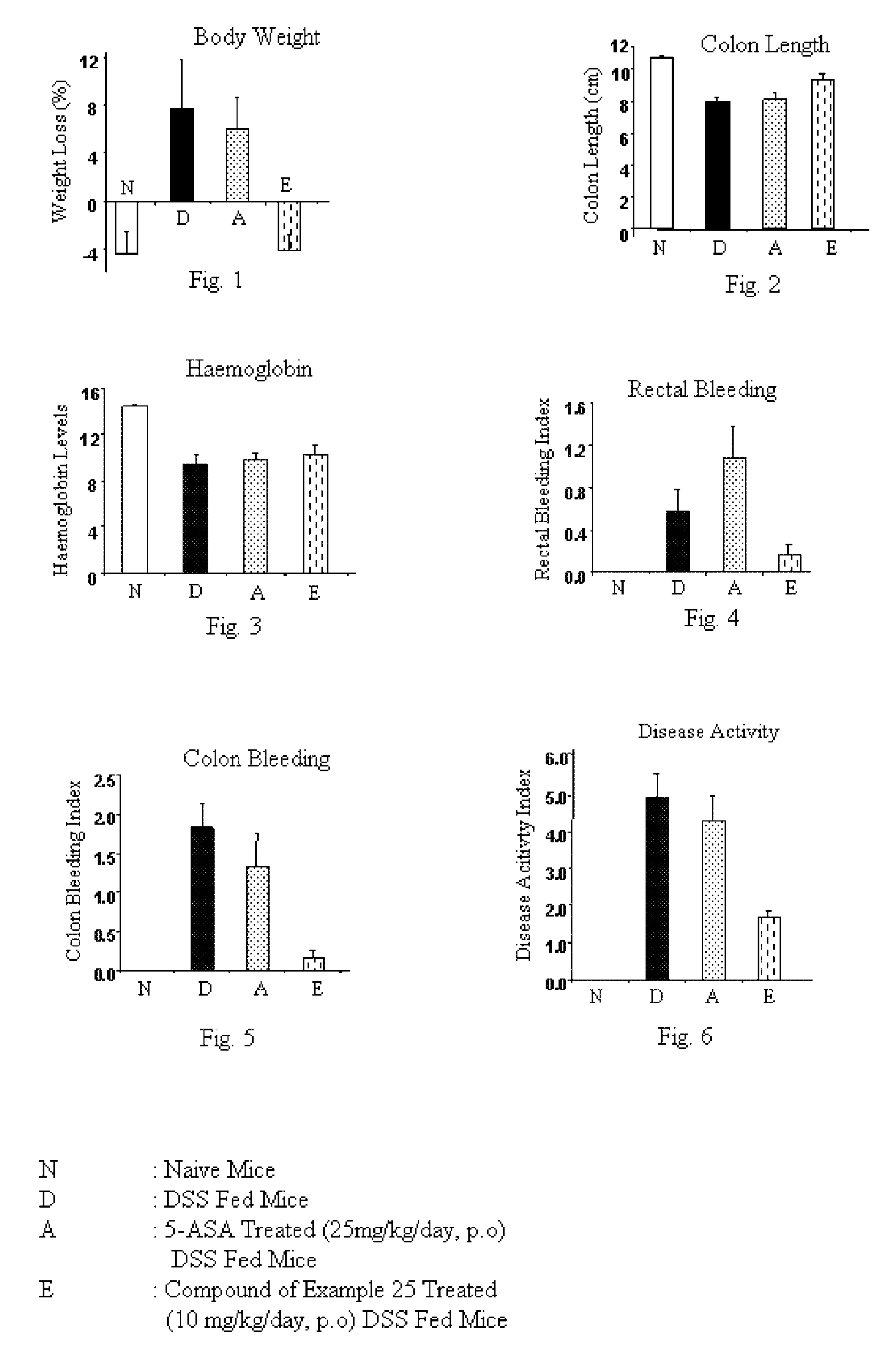
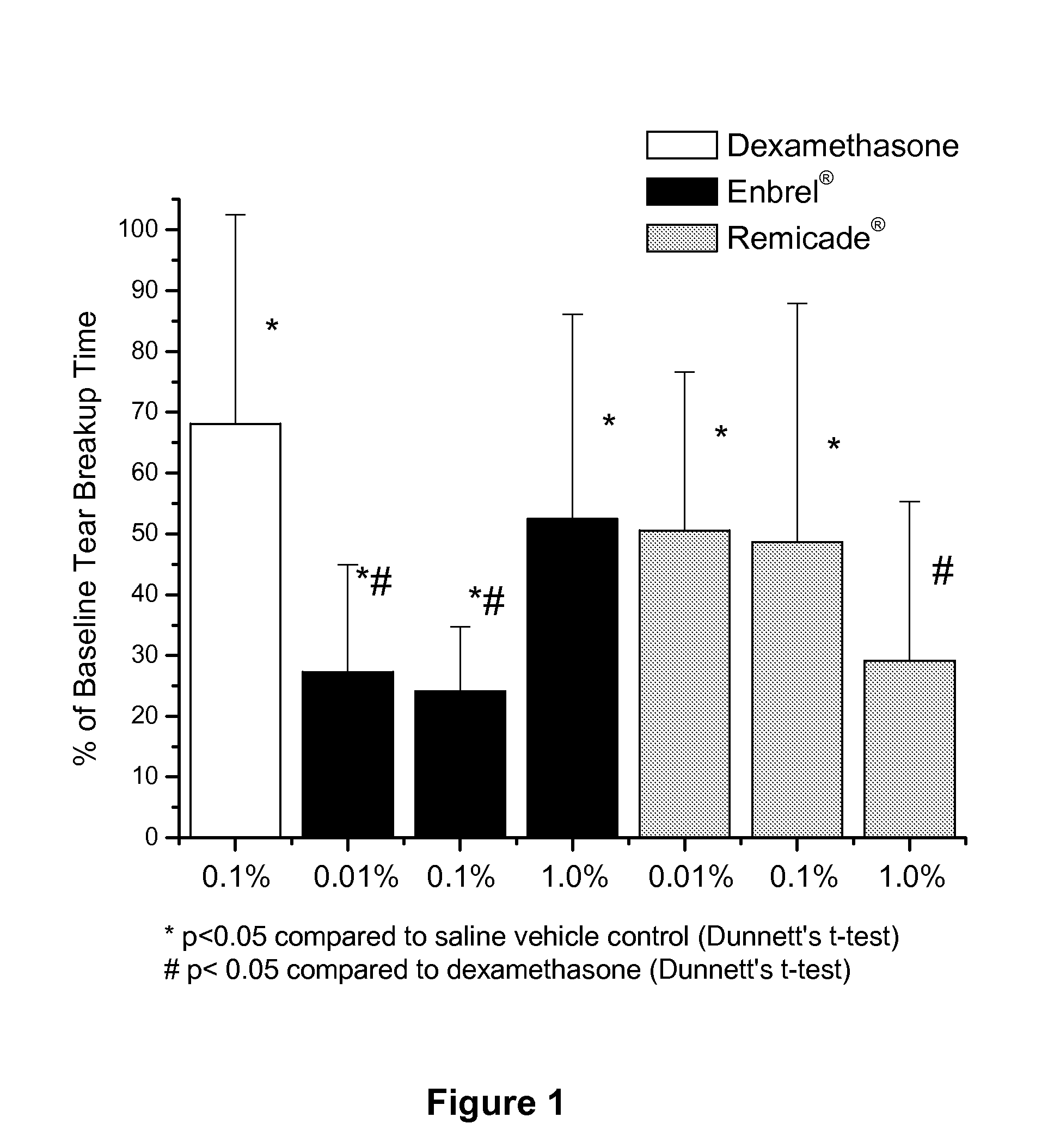
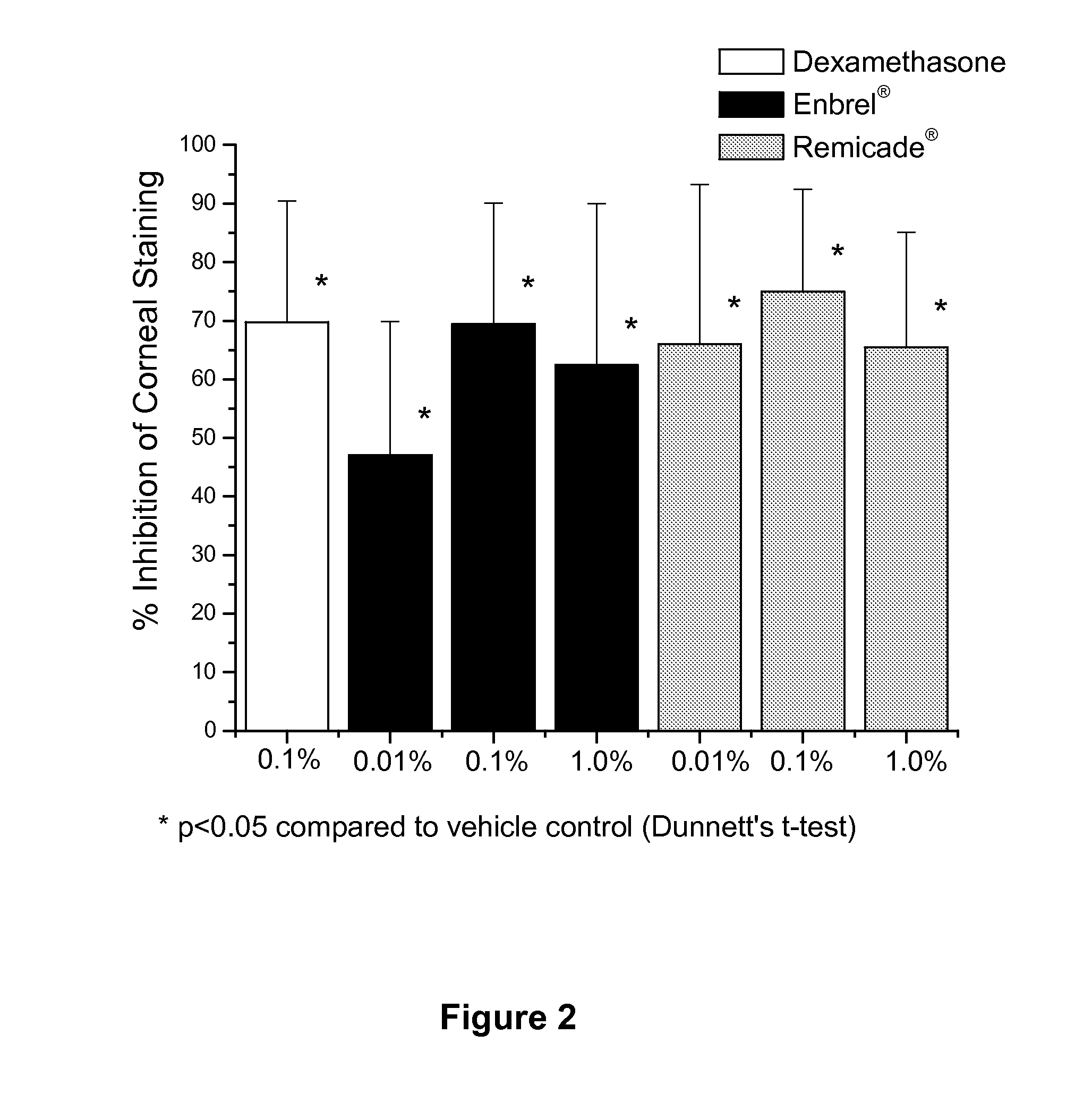
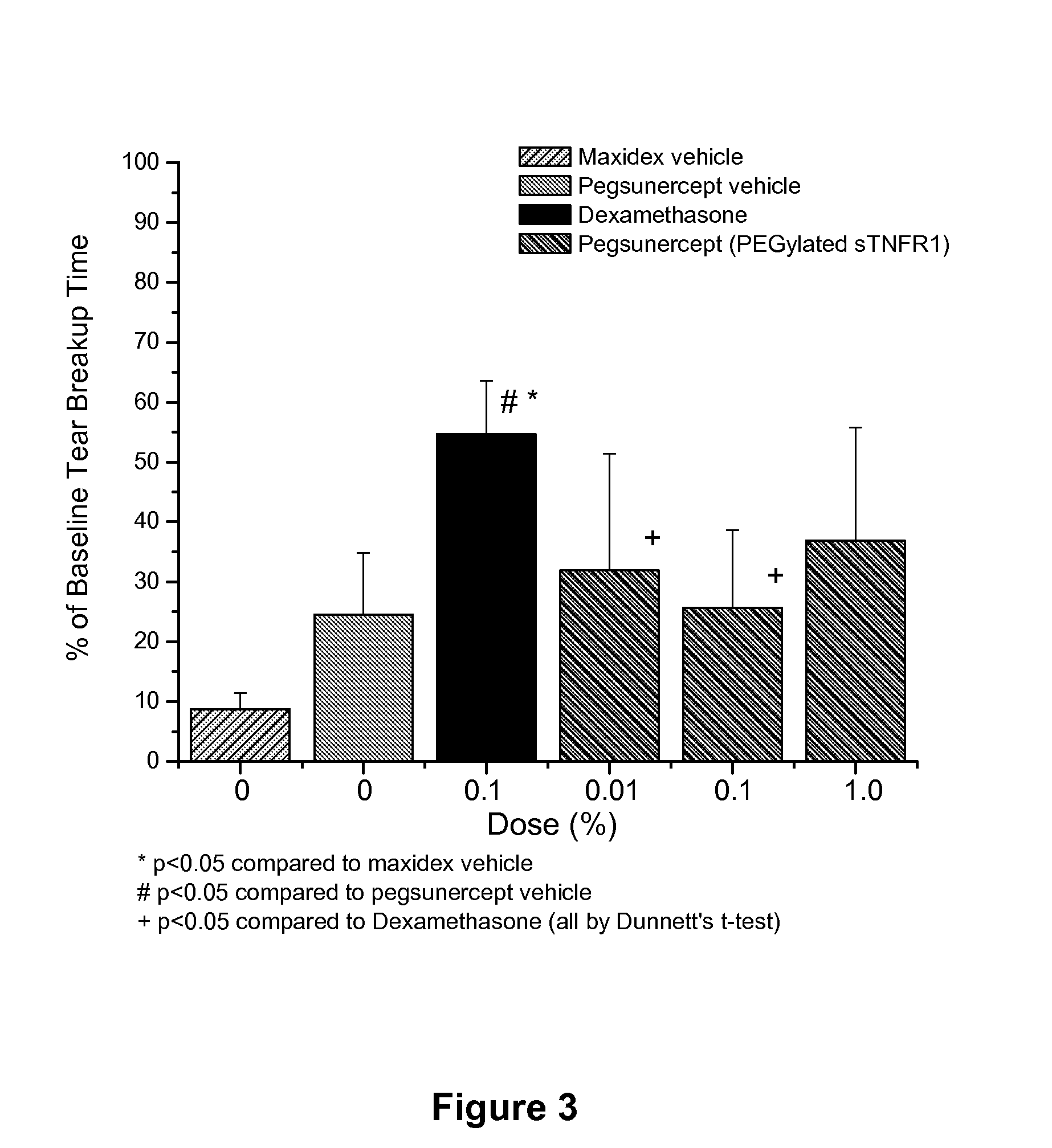
Use of TNF receptor antagonists for treating dry eye
Methods of treating dry eye by administering inhibitors of tumor necrosis factor α (TNFα) are disclosed.
Owner:ALCON RES LTD
Methods and compositions for the prevention and treatment of atherosclerosis, restenosis and related disorders
Methods and compositions for the prevention and treatment of all forms of atherosclerosis are described. Administration of compounds such as thalidomide, its analogs, hydrolysis products, metabolites, derivatives and precursors as well as additional compounds capable of inhibiting tumor necrosis factor α(TNF-α) are used in the invention. Also disclosed is the coating of prosthetic devices, such as stents, with the compounds of the invention for the prevention and / or treatment of restenosis.
Owner:CELGENE CORP
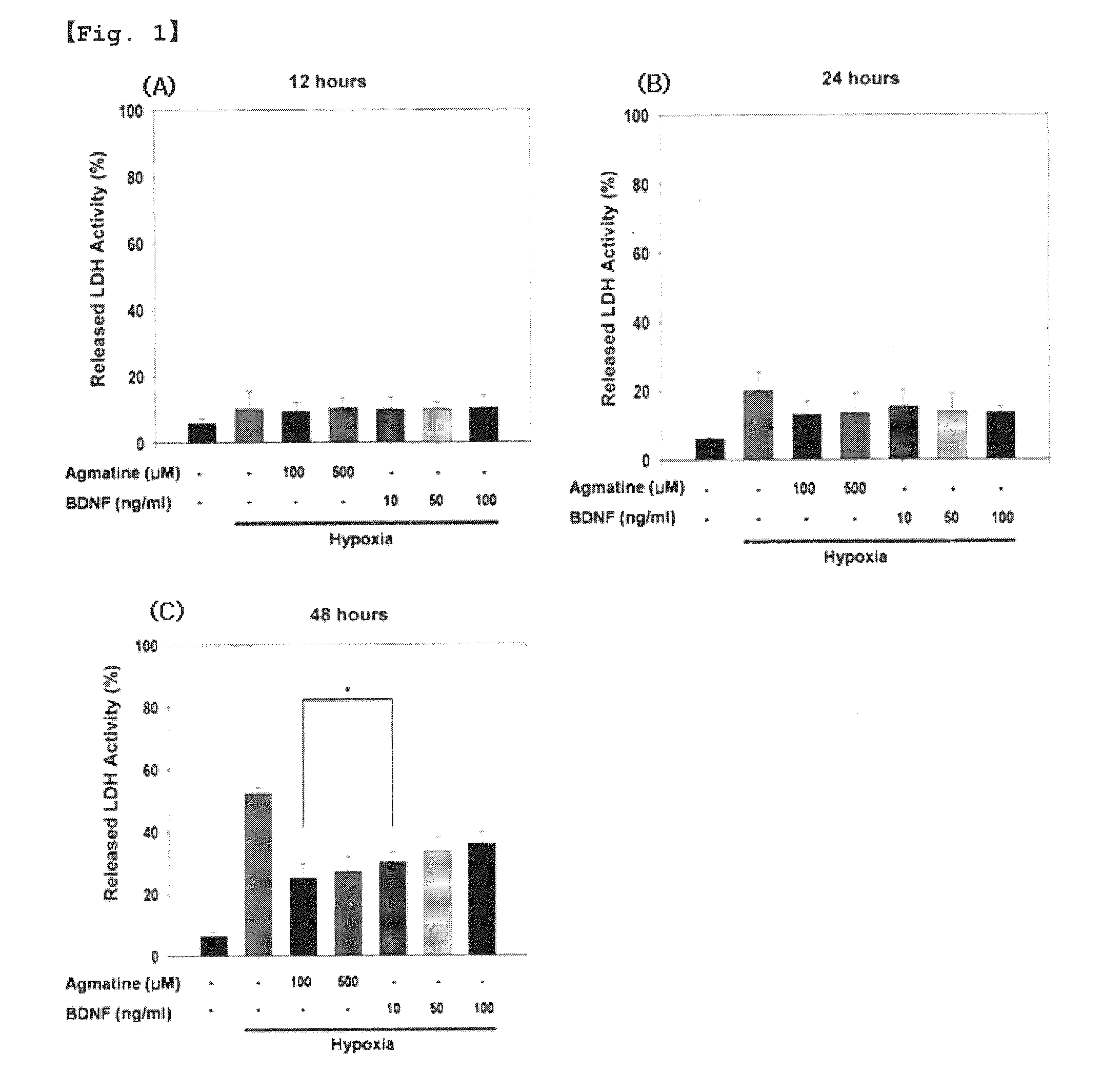
Use of agmatine for protection of retinal ganglion cells
ActiveUS20100130783A1Curing and preventing eye diseaseBiocideOrganic active ingredientsApoptosisFactor ii
A use method of agmatine or a pharmaceutically allowable salt thereof, and a pharmaceutical composition comprising the same are disclosed. The method and pharmaceutical composition of the present invention can effectively cure or prevent eye diseases preferably including glaucoma, retinopathy, and optic neuropathy associated with apoptosis in retinal ganglion cells (RGCs), particularly hypoxia-induced or tumor necrosis factor-α (TNF-α)-induced apoptosis.
Owner:SEONG GONG JE +3
Treatment of diseases with alpha-7nACh receptor full agonists
The present invention relates to compositions and methods for treating related diseases or conditions by reducing tumor necrosis factor-alpha levels and / or by stimulating angiogenesis using alpha-7 nicotinic acetylcholine receptor (AChR) full agonists.
Owner:PHARMACIA & UPJOHN CO
Decoy compositions for treating and preventing brain diseases and disorders
InactiveUS20080207552A1Inhibit expressionAvoid damageSsRNA viruses negative-senseOrganic active ingredientsDiseaseStaining
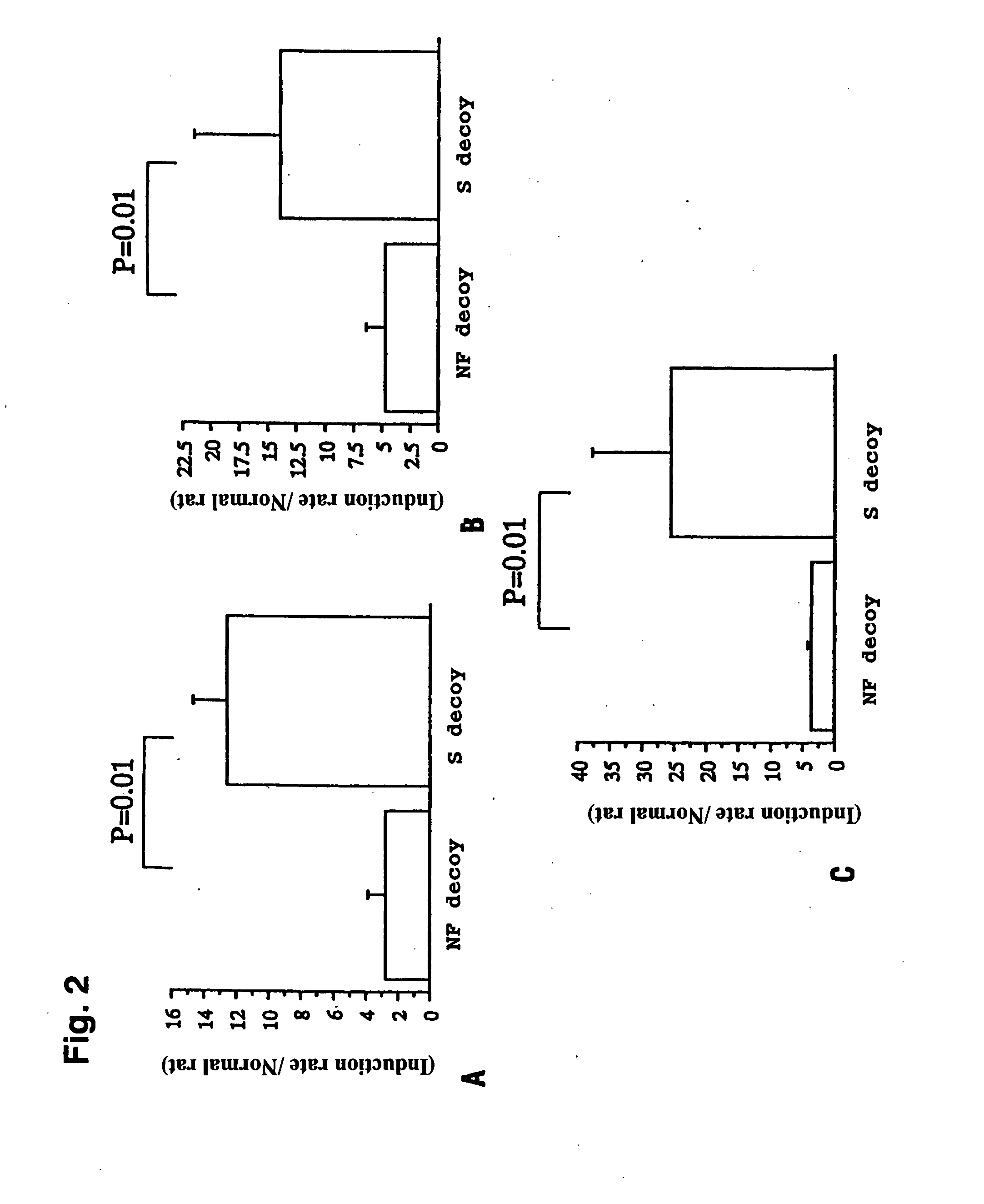
The present invention provides introduction of NF-κB decoy oligodeoxynucleotide into rat cranial nerve through a carotid artery during global brain ischemia. Polymerase chain reaction demonstrated that one hour after global brain ischemia, transfected NF-κB decoy oligodeoxynucleotide effectively suppressed expression of tumor necrosis factor α, interleukin 1β and intracellular adhesion molecule 1 messenger RNAs. Terminal deoxynucleotidyl transferase-mediated deoxyuridine nick-end labeling staining and immunohistochemistry using microtubule-associated protein 2 demonstrated that transfected NF-κB decoy oligodeoxynucleotide significantly attenuated neuronal damage seven days after global brain ischemia. Therapeutic transfection of NF-κB decoy oligodeoxynucleotide during brain ischemia may be effective for attenuation of neuronal damage, suggesting a strategy for protecting the cerebrum from global ischemia.
Owner:ANGES MG INC
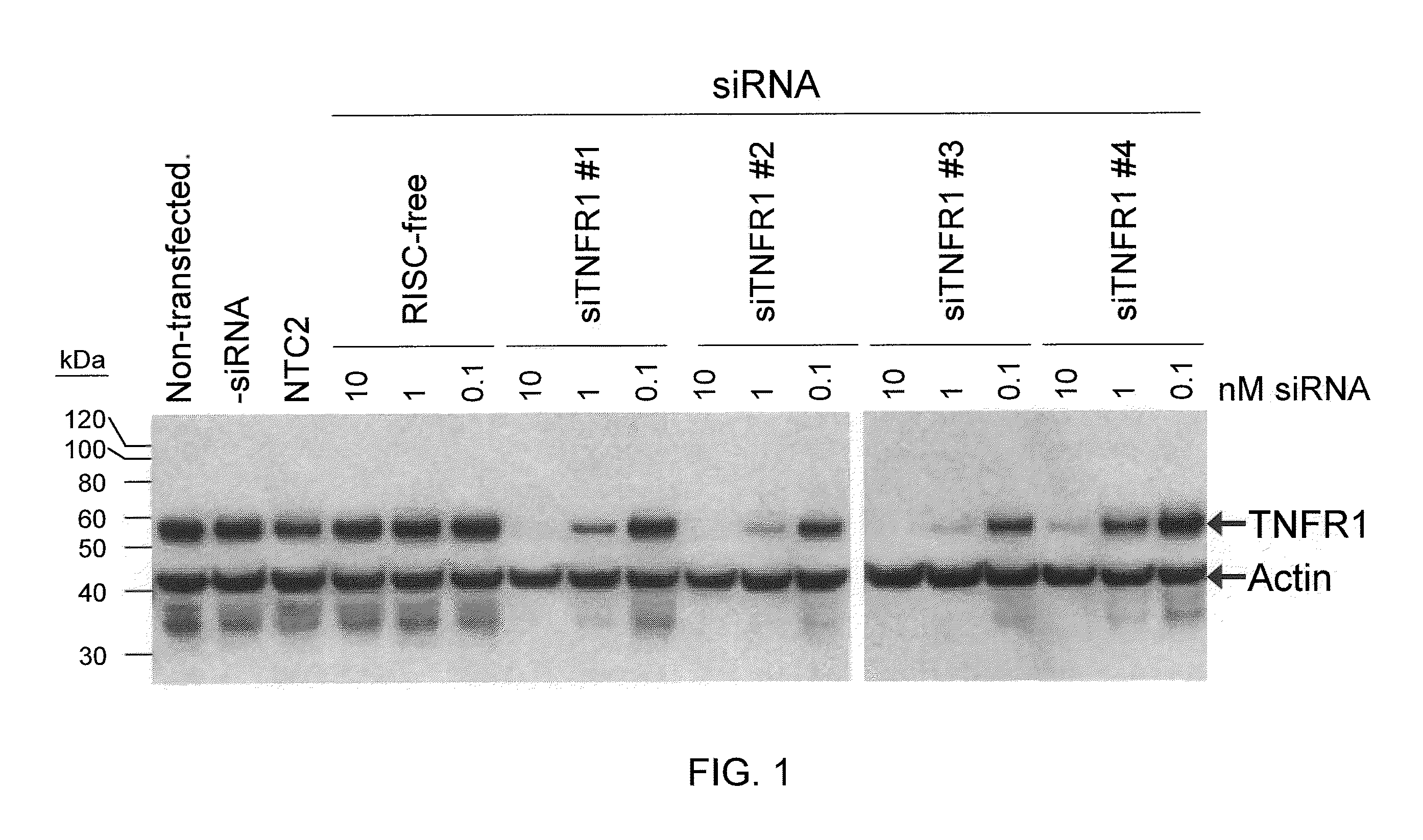
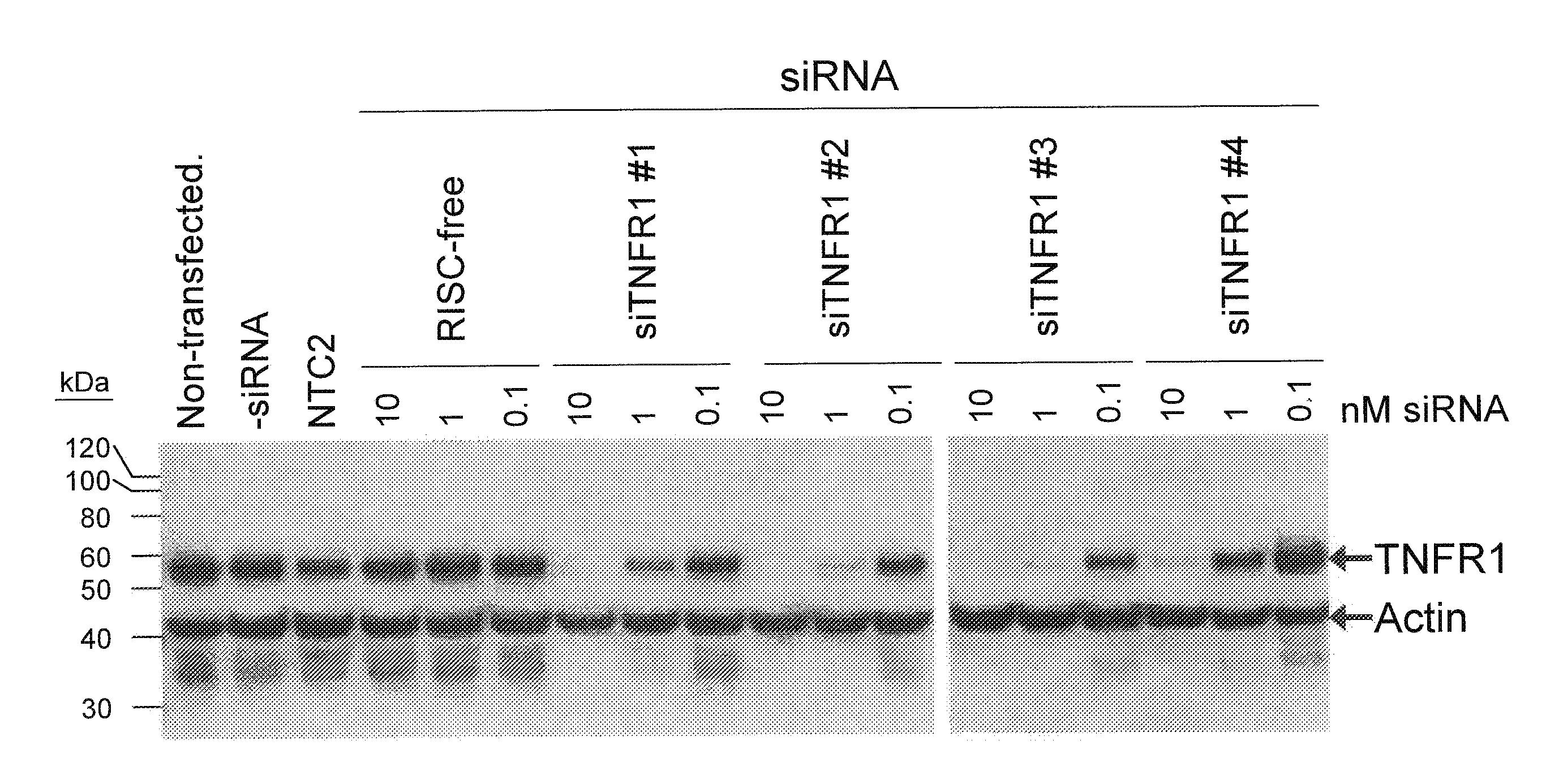
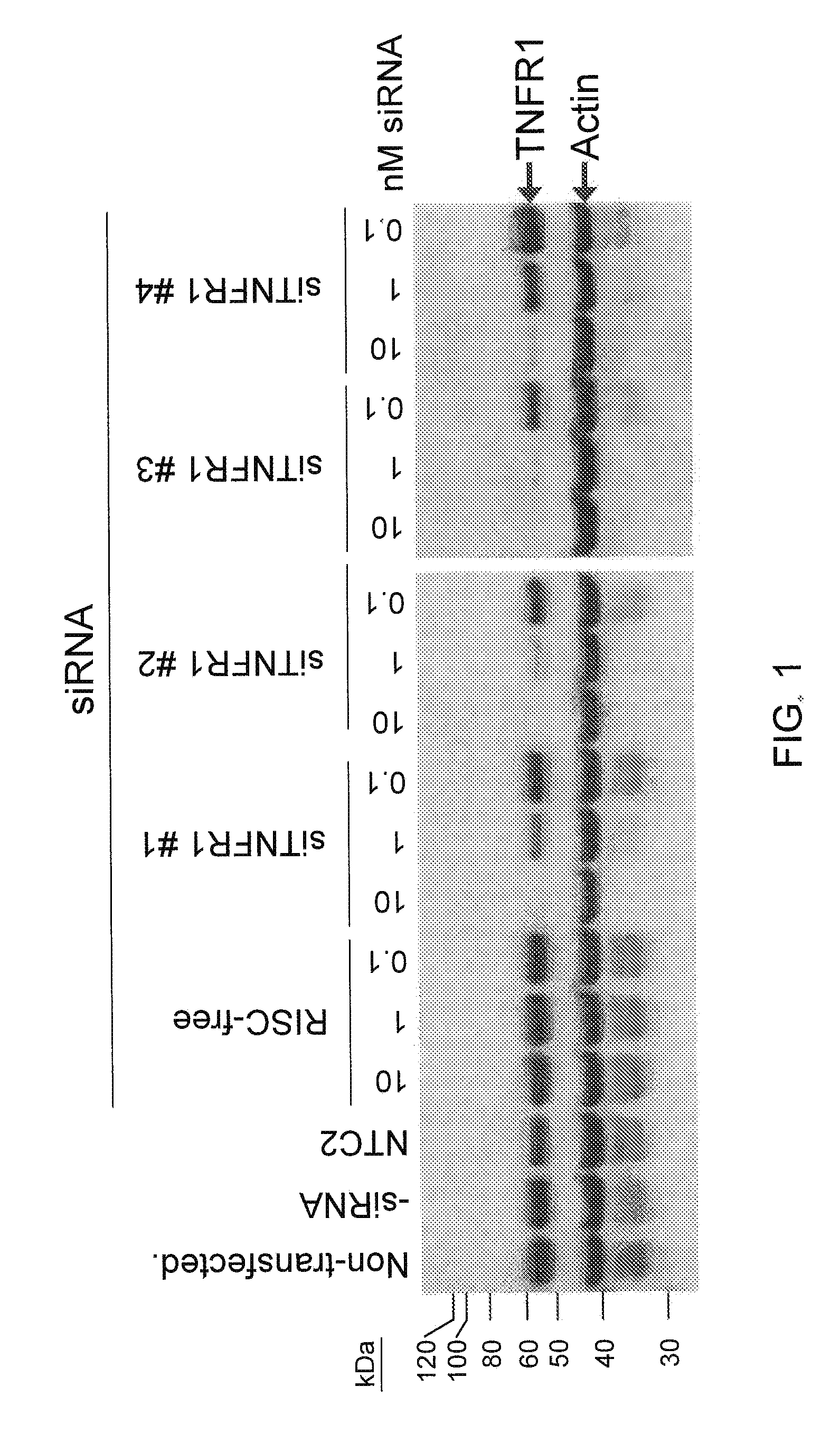
RNAi-RELATED INHIBITION OF TNFa SIGNALING PATHWAY FOR TREATMENT OF GLAUCOMA
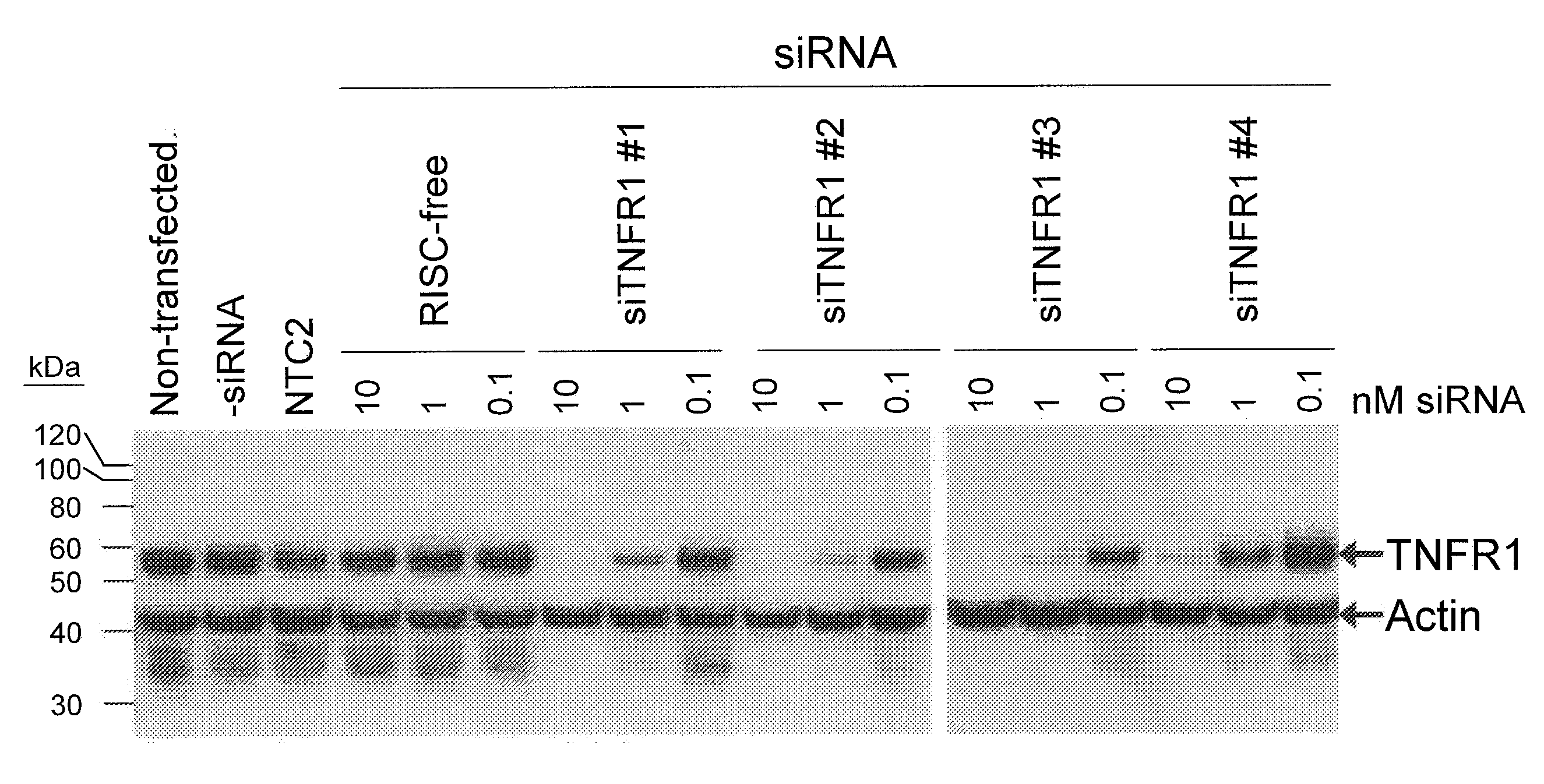
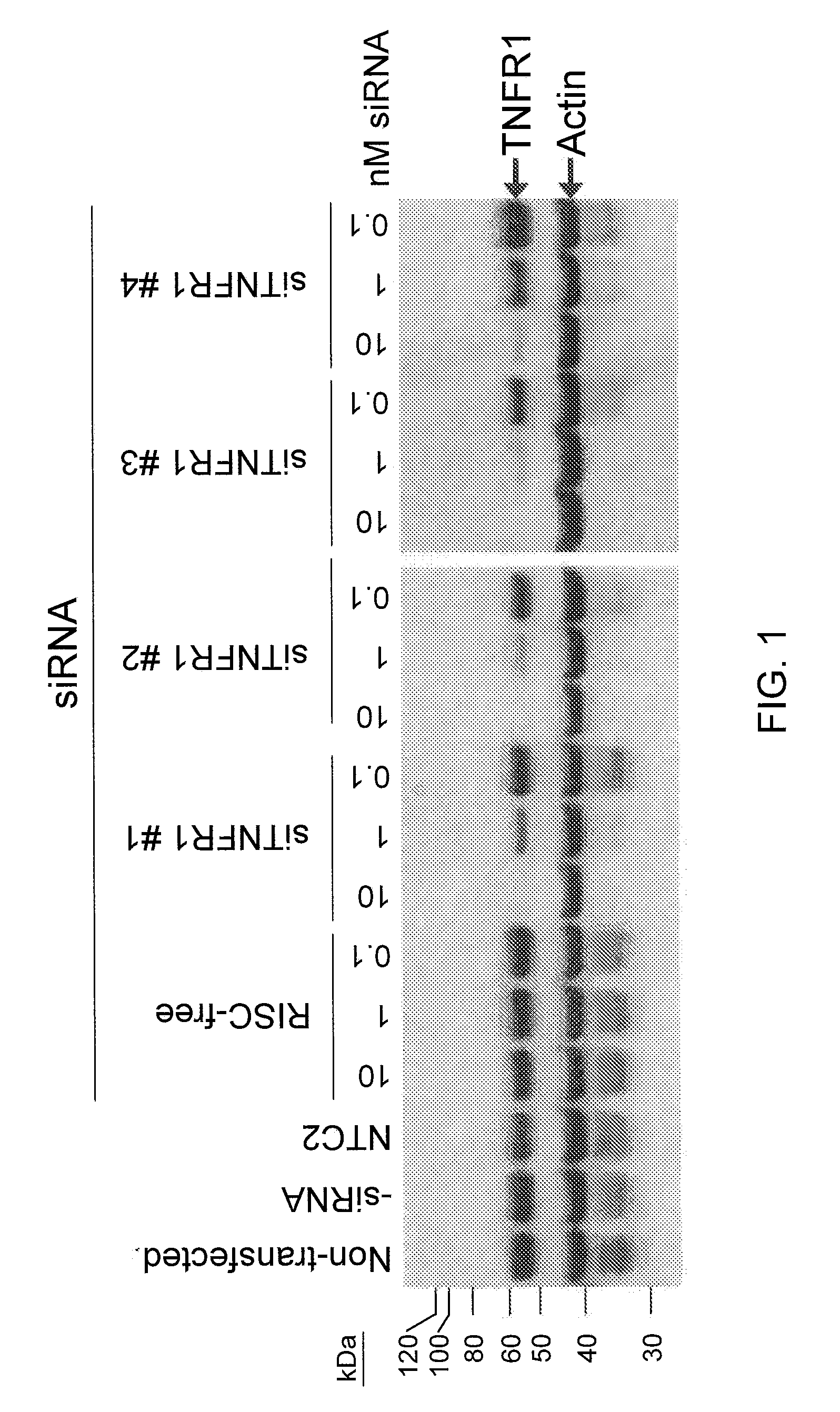
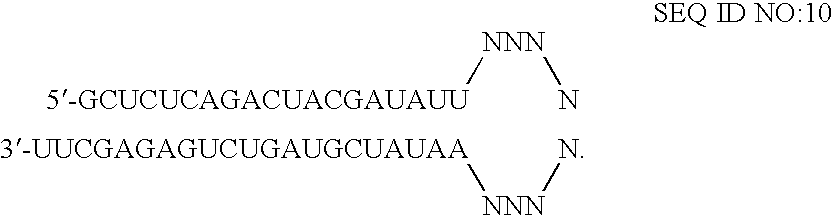
RNA interference is provided for inhibition of tumor necrosis factor α (TNFα) by silencing TNFα cell surface receptor TNF receptor-1 (TNFR1) mRNA expression, or by silencing TNFα converting enzyme (TACE / ADAM17) mRNA expression. Silencing such TNFα targets, in particular, is useful for treating patients having a TNFα-related condition or at risk of developing a TNFα-related condition such as the ocular conditions associated with elevated intraocular pressure (IOP), including glaucoma and ocular hypertension.
Owner:ARROWHEAD RES CORP
Immunotherapeutic agents
Cyano and carboxy derivatives of substituted styrenes are inhibitors of tumor necrosis factor α, nuclear factor κB, and phosphodiesterase and can be used to combat cachexia, endotoxic shock, retrovirus replication, asthma, and inflammatory conditions. A typical embodiment is 3,3-bis-(3,4-dimethoxyphenyl)acrylonitrile.
Owner:CELGENE CORP
RNAi-RELATED INHIBITION OF TNFalpha SIGNALING PATHWAY FOR TREATMENT OF OCULAR ANGIOGENESIS
InactiveUS20090036396A1Organic active ingredientsSenses disorderReceptor degradationTnfα converting enzyme
RNA interference is provided for inhibition of tumor necrosis factor α (TNFα) by silencing TNFα cell surface receptor TNF receptor-1 (TNFR1) mRNA expression, or by silencing TNFα converting enzyme (TACE / ADAM17) mRNA expression. Silencing such TNFα targets, in particular, is useful for treating patients having a TNFα-related condition or at risk of developing a TNFα-related condition, such as ocular angiogenesis, retinal ischemia, and diabetic retinopathy.
Owner:ARROWHEAD RES CORP
Compositions and methods of using a soluble TNF-alpha receptor modified for increased half-life
InactiveUS20180028612A1Effective preventionEffective treatmentOrganic active ingredientsPeptide/protein ingredientsDiabetic retinopathyEnprofylline
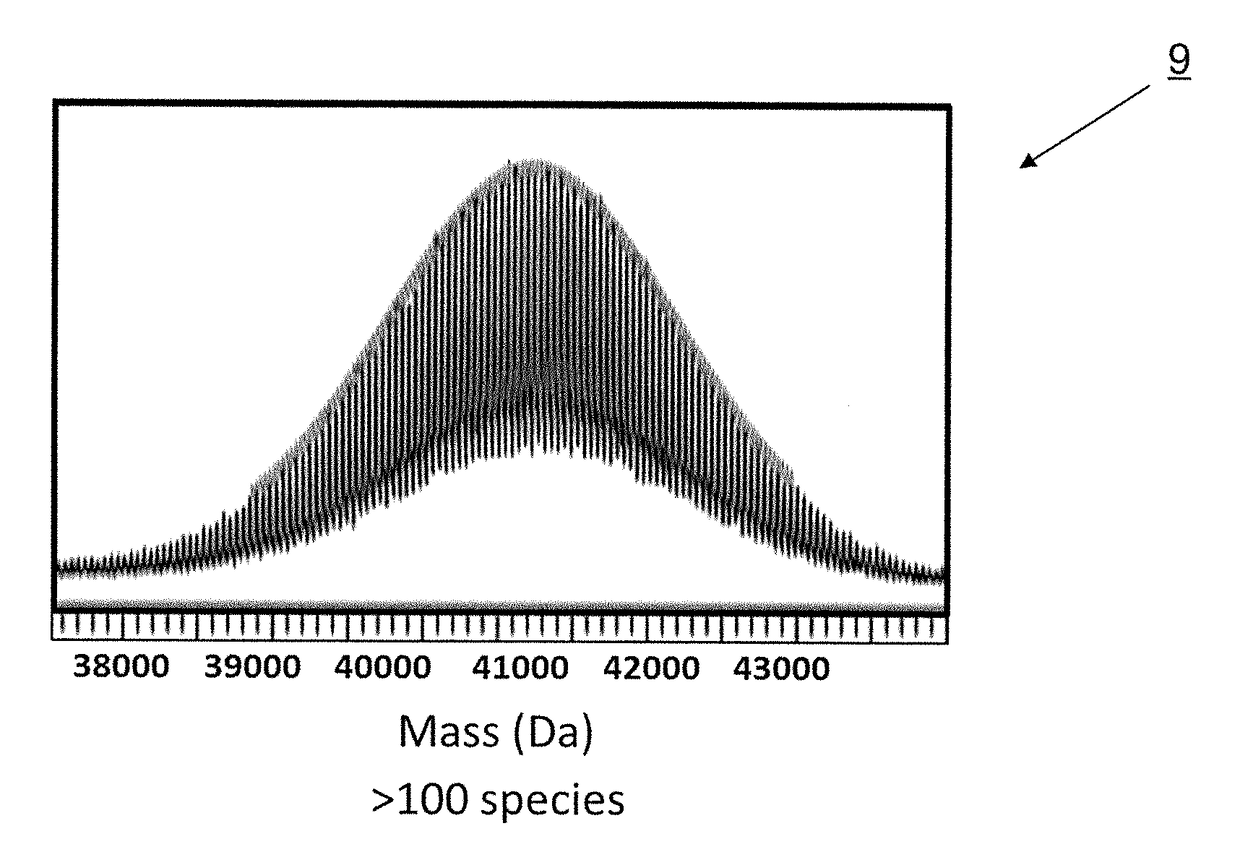
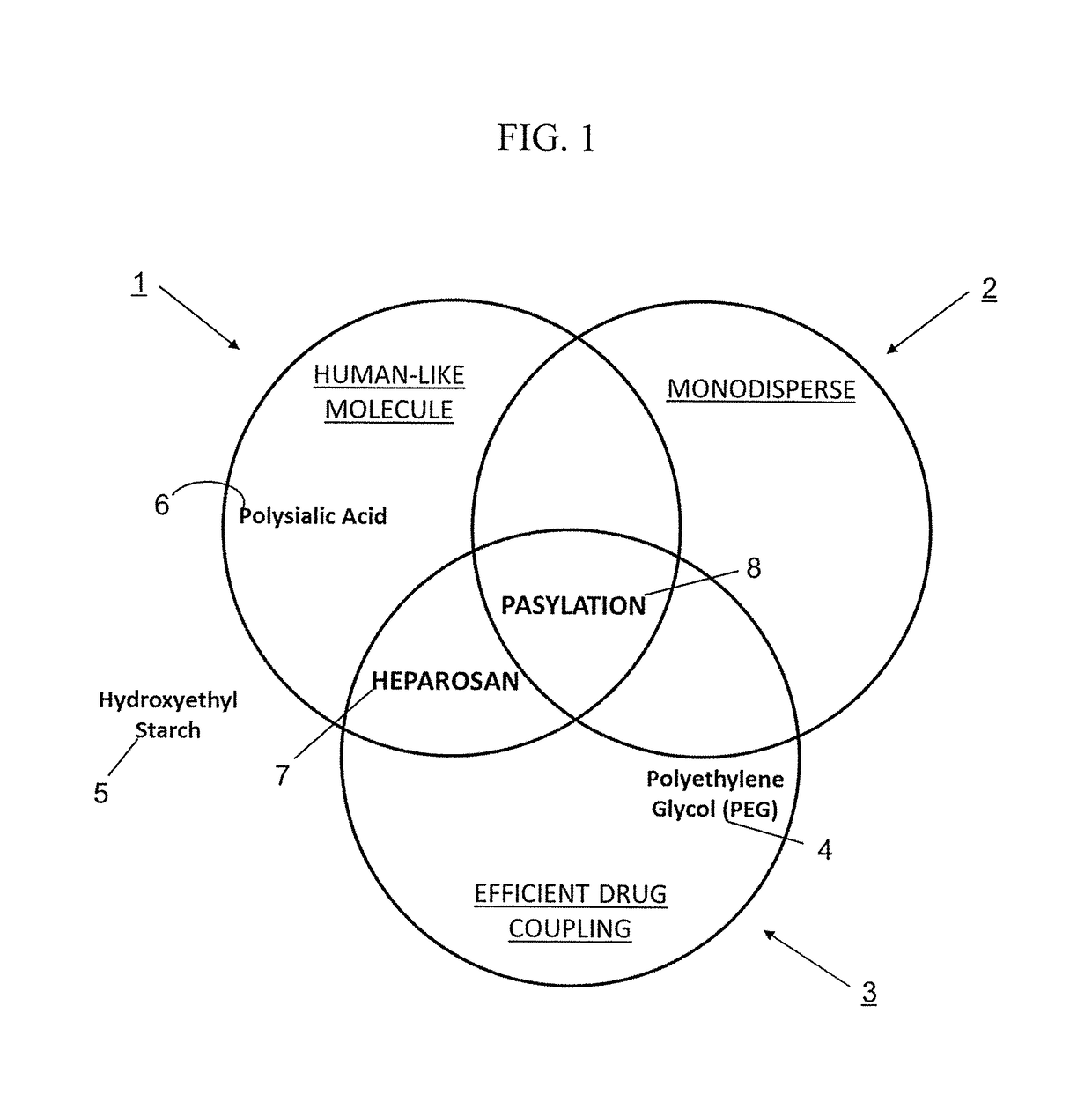
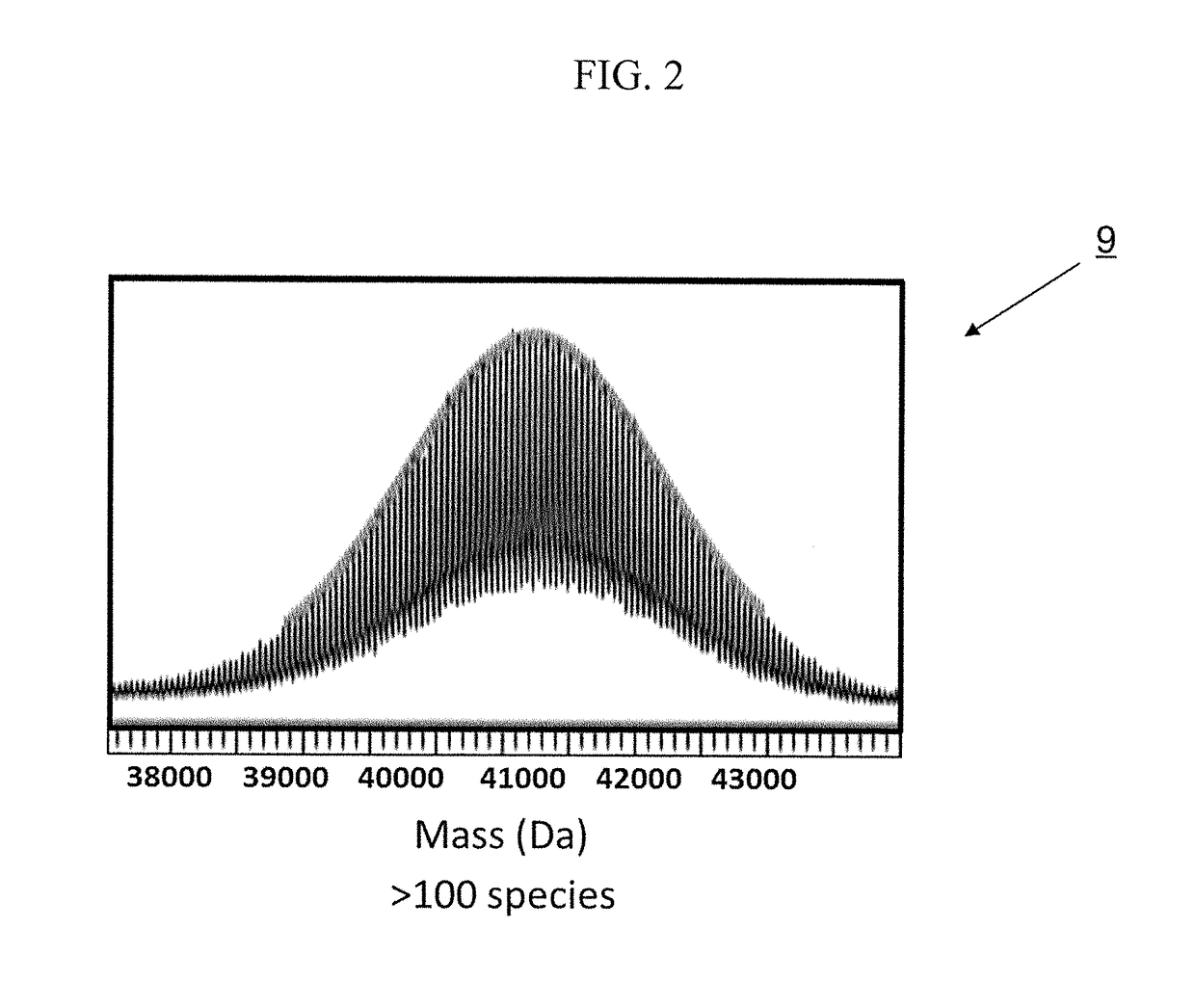
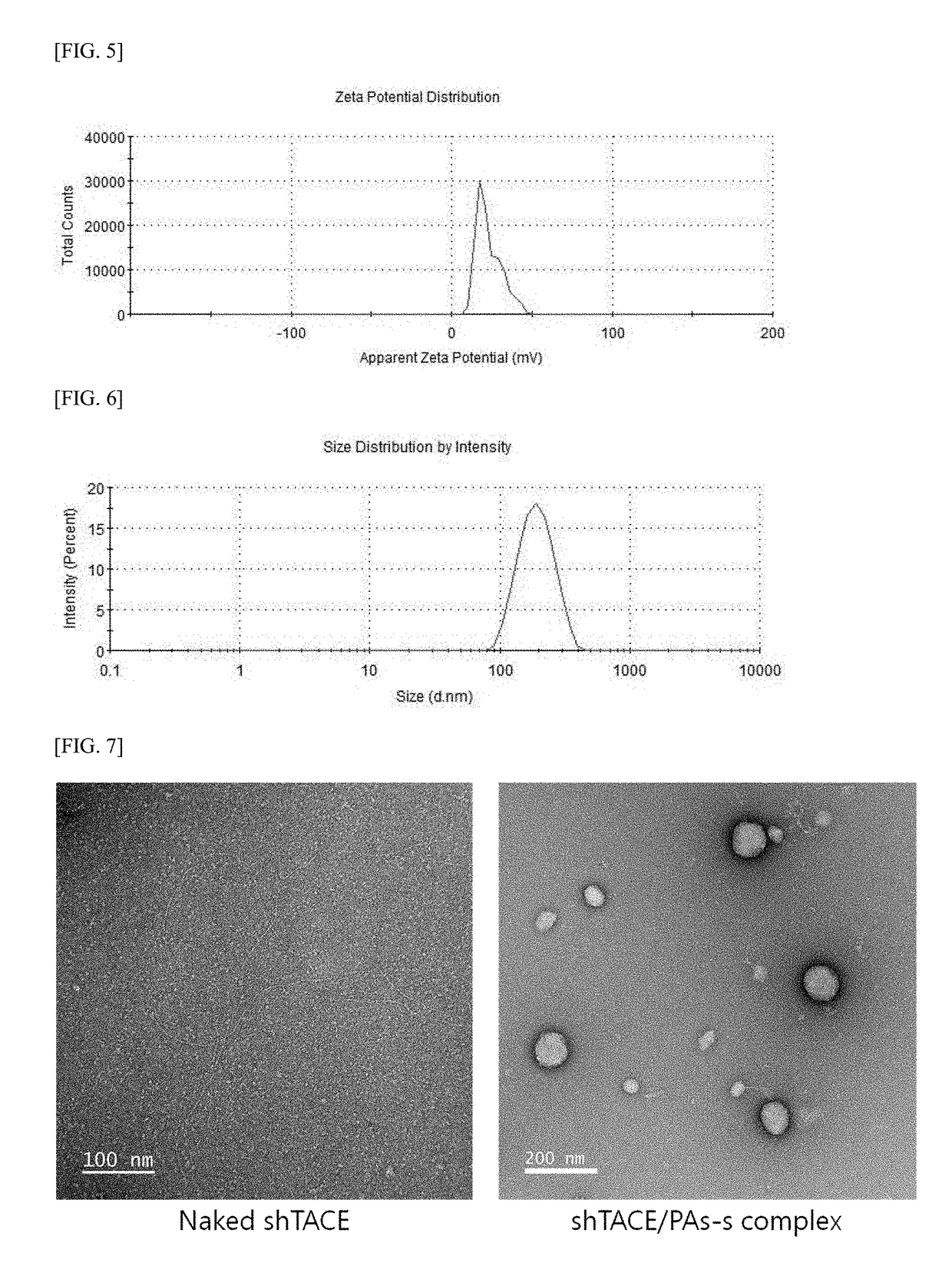
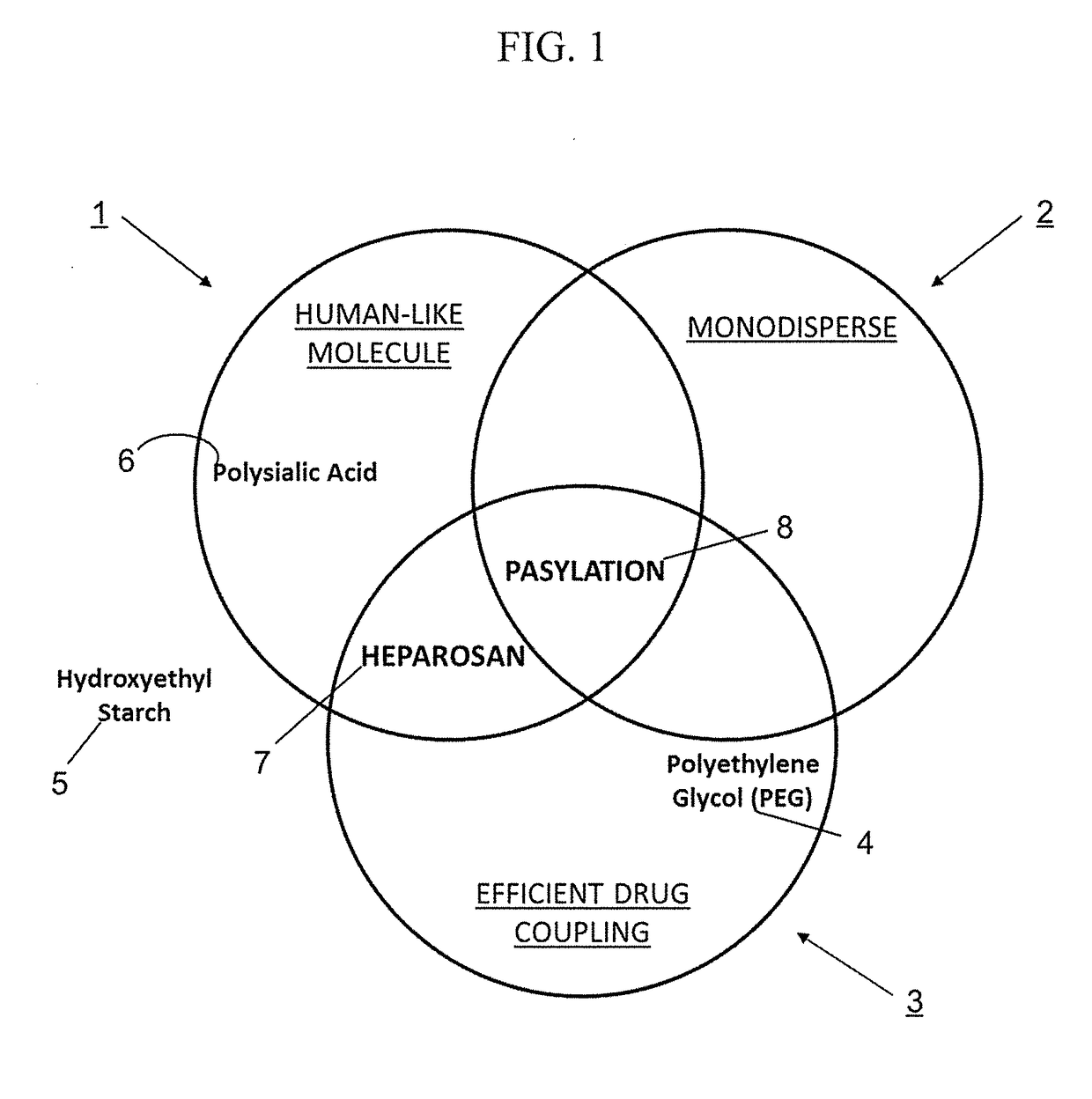
Methods and pharmaceutical compositions for preventing and / or treating acute and chronic inflammation and autoimmune diseases are provided herein. Tumor necrosis factor-α (TNFα) promotes an inflammatory response, which causes clinical problems associated with inflammation and autoimmune disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa, and refractory asthma. TNFα is also implicated in promoting pathogenesis of diabetic retinopathy leading to loss of retinal microvascular cells. Methods herein contain the step of administering a prophylactic and / or therapeutic formulation of a pharmaceutical composition containing a recombinant soluble human TNF receptor or portions thereof which are TNFα inhibitors. These pharmaceutic compositions have been modified by conjugating natural amino acids such as proline and alanine, and / or serine (PA / S) via PASylation® to create a linear polypeptide that possesses fewer of the processing, preparation, formulation, cost, and other long-term issues of administering PEGylated drugs.
Owner:DNX BIOTECH LLC
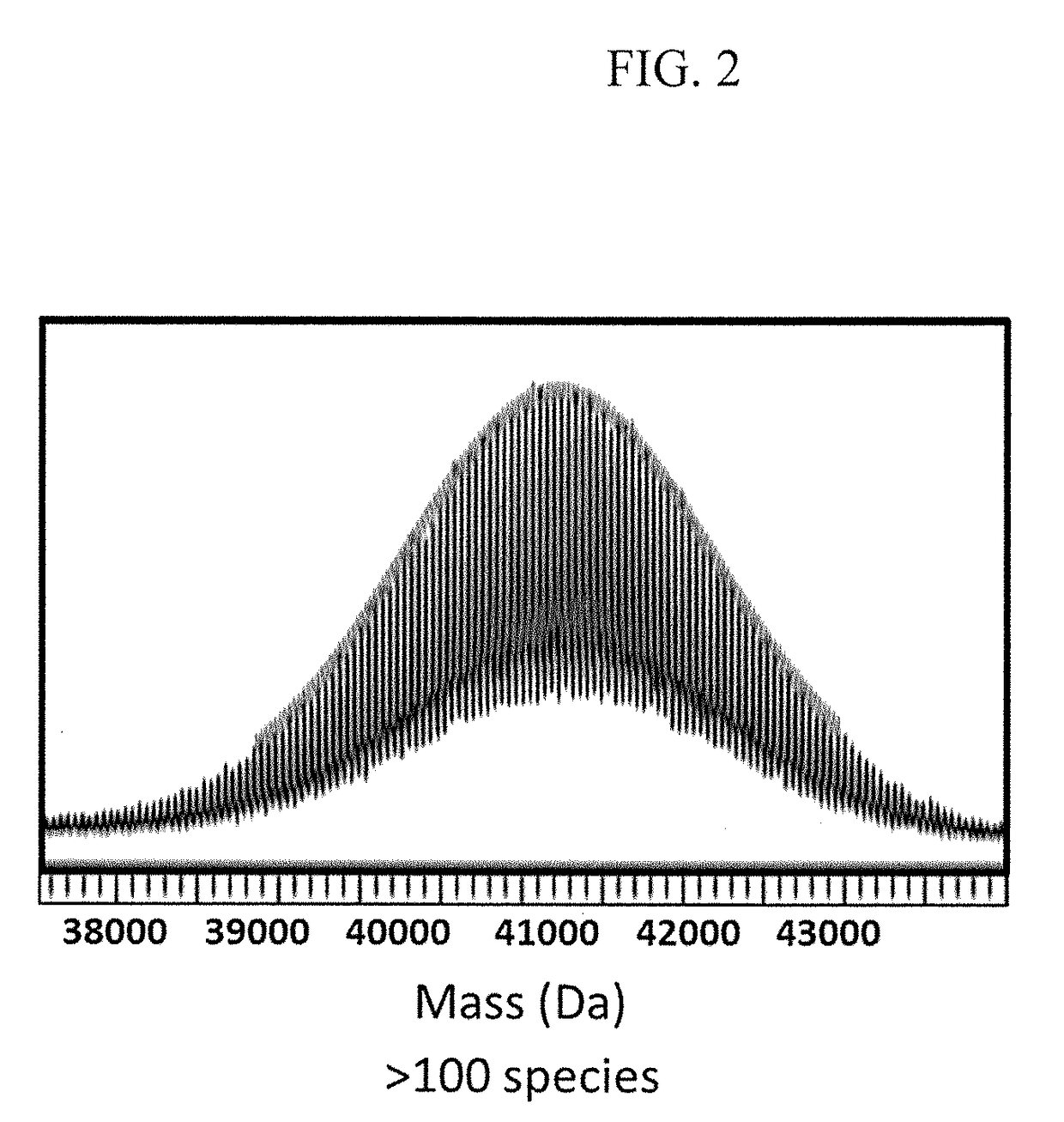
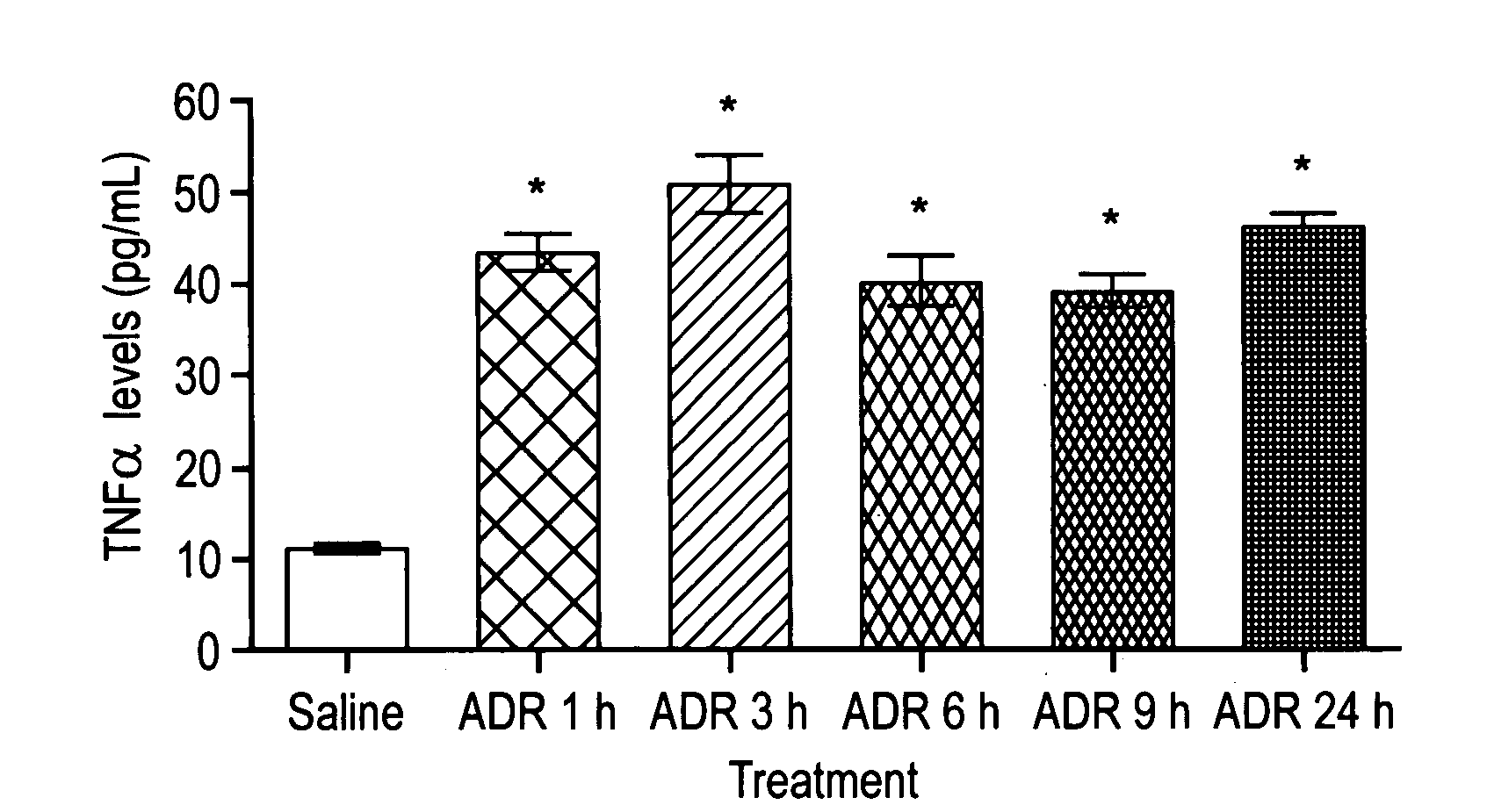
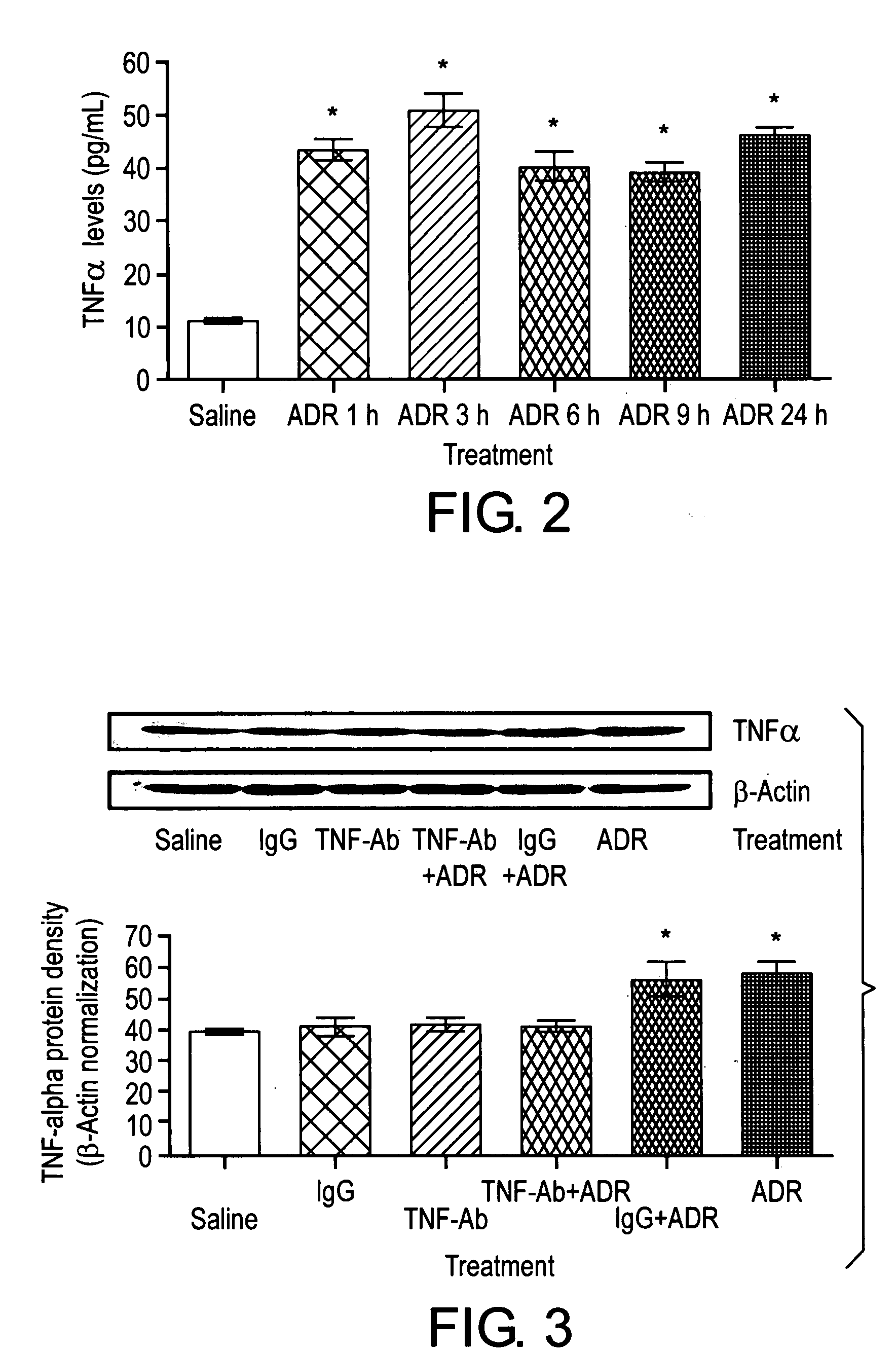
Method of treating the chemotoxic effects of chemotherapeutic drugs on brain tissue
This invention is related to a novel method of treating the chemotoxic effects on the central nervous system of chemotherapeutic drugs, namely Adriamycin, by administering a therapeutically effective amount of anti-Tumor Necrosis Factor-α antibody to a patient to alleviate the symptoms of the effects. The antibody operates to decrease the serum level of Tumor Necrosis Factor-α, prevents the p53 translocation to mitochondria and prevents the decline in mitochondrial respiration of brain tissues and thereby relieves the symptoms of somnolence caused by chemotherapeutic drugs.
Owner:UNIV OF KENTUCKY RES FOUND
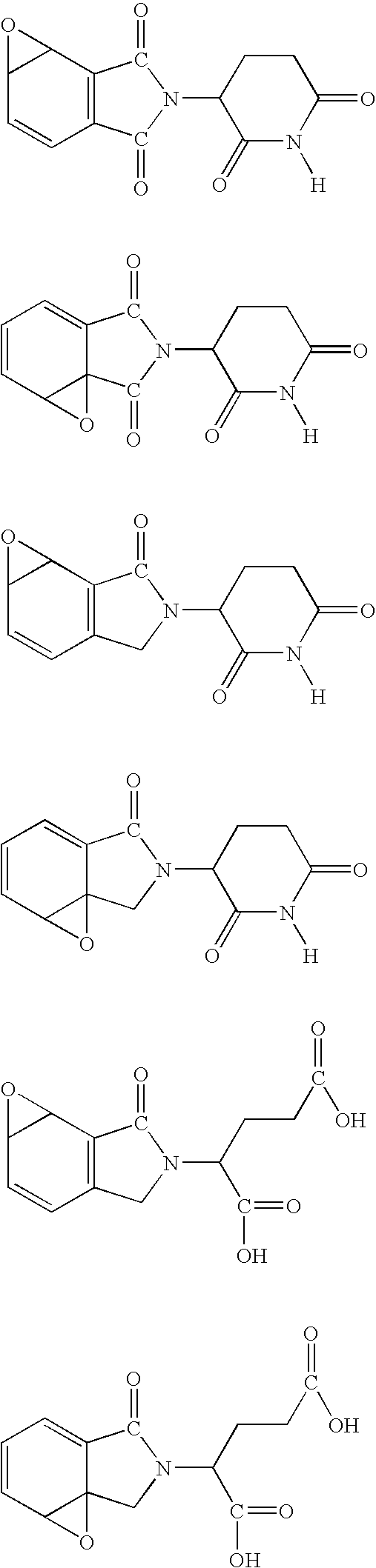
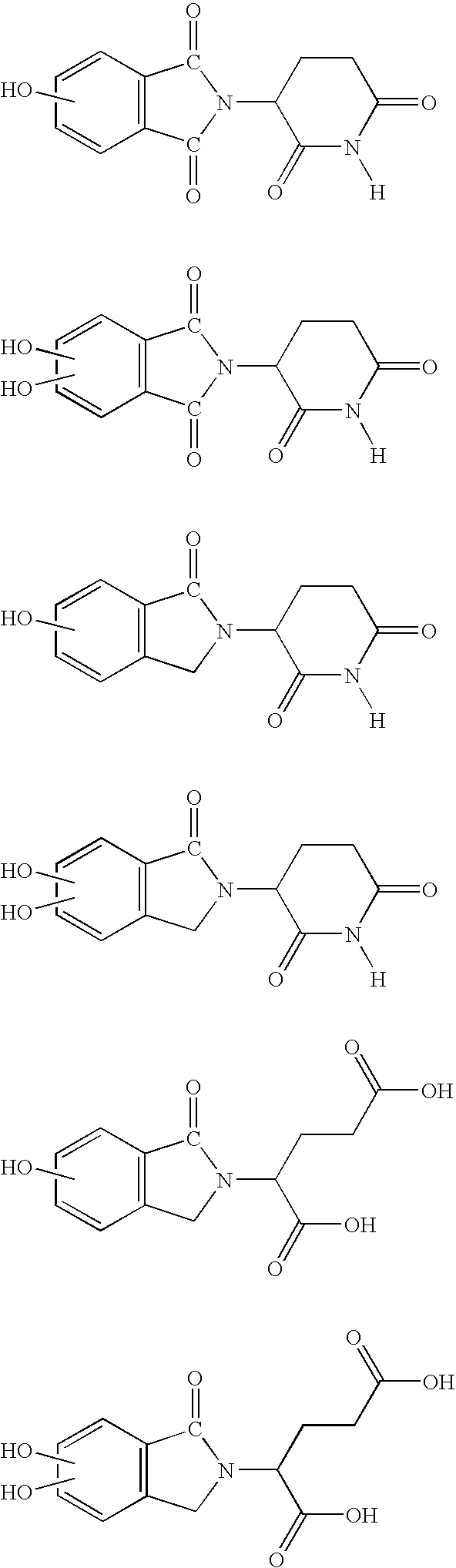
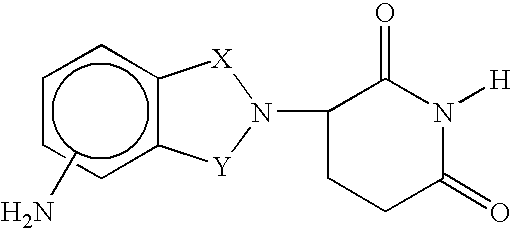
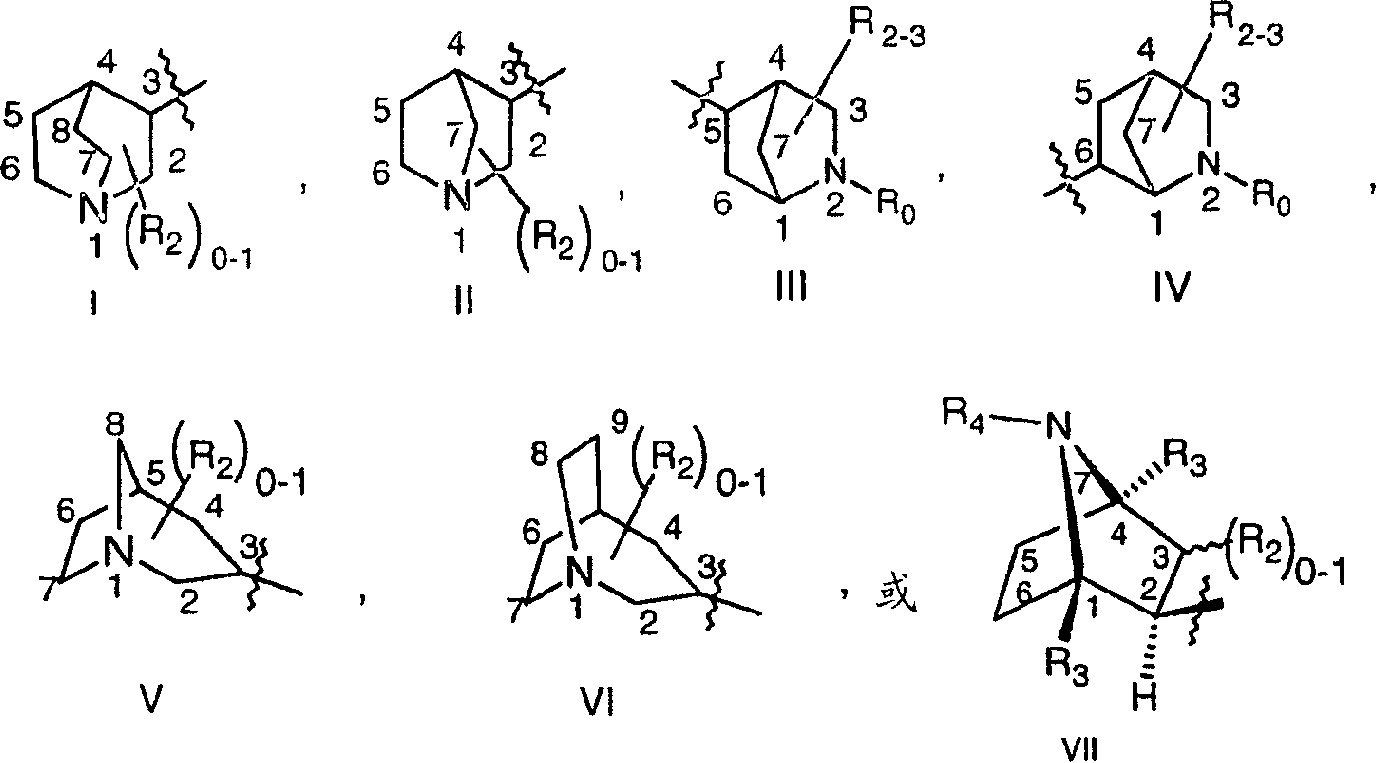
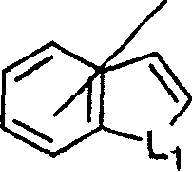
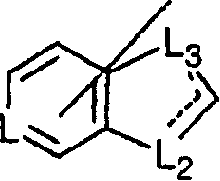
Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation
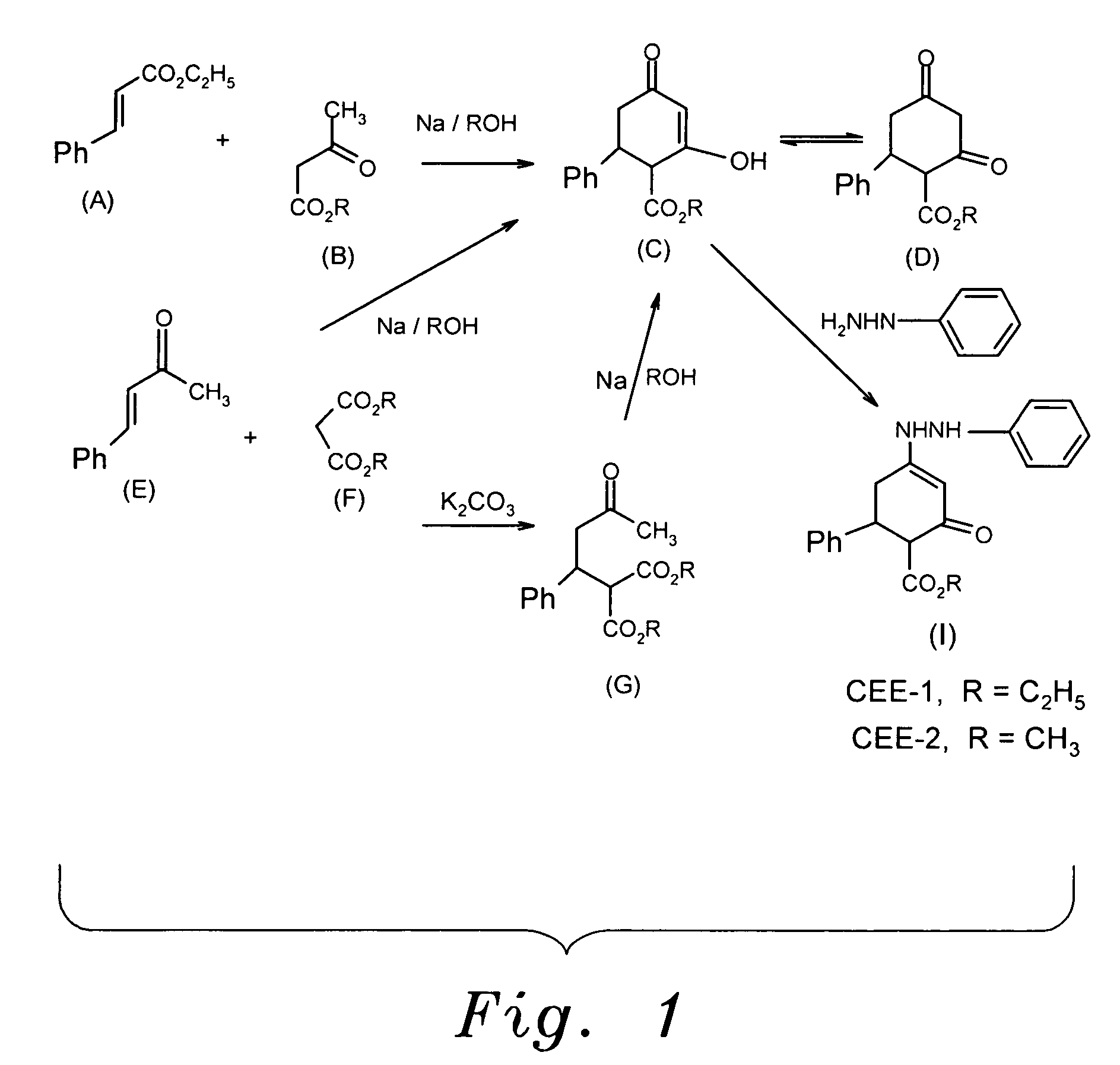
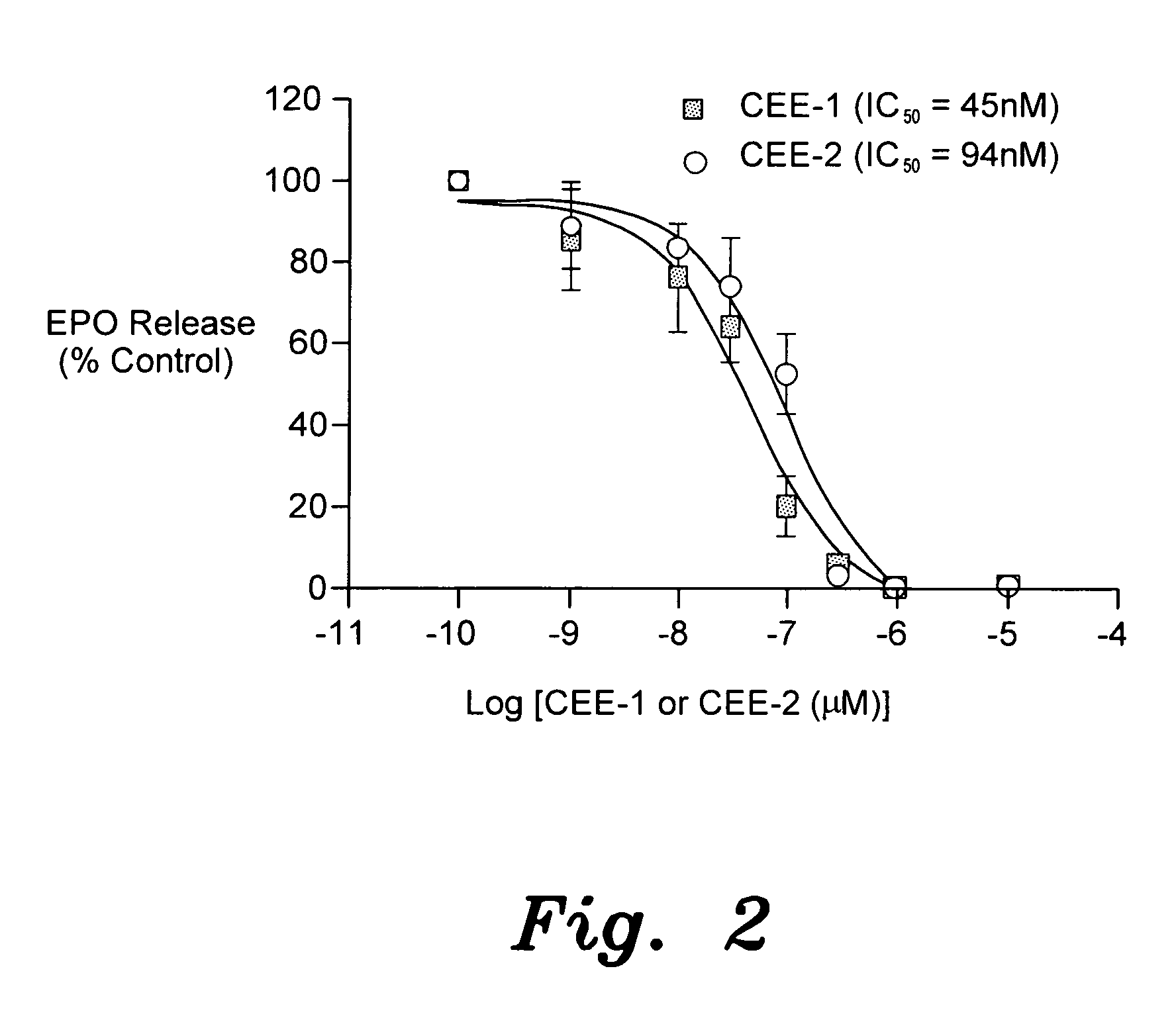
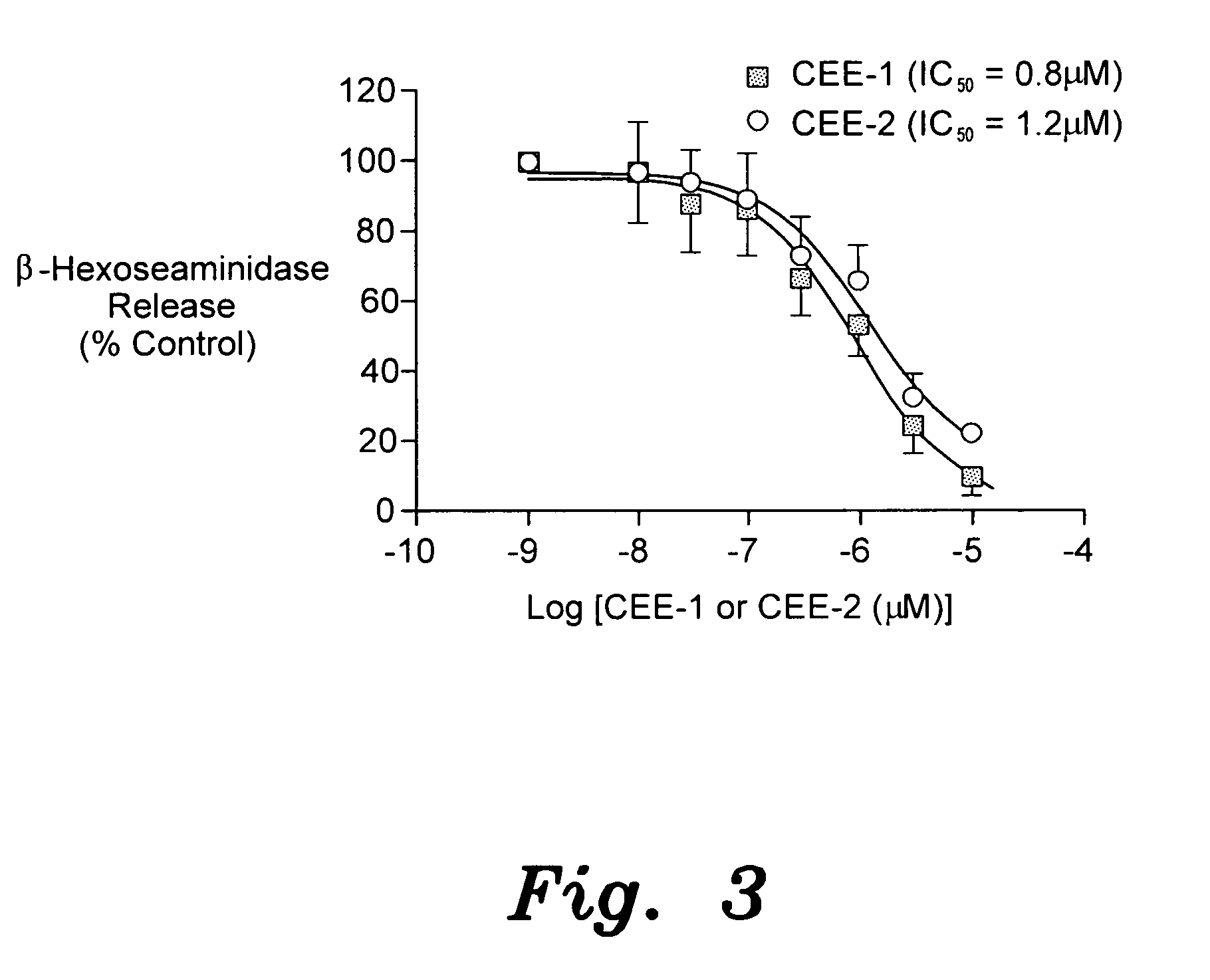
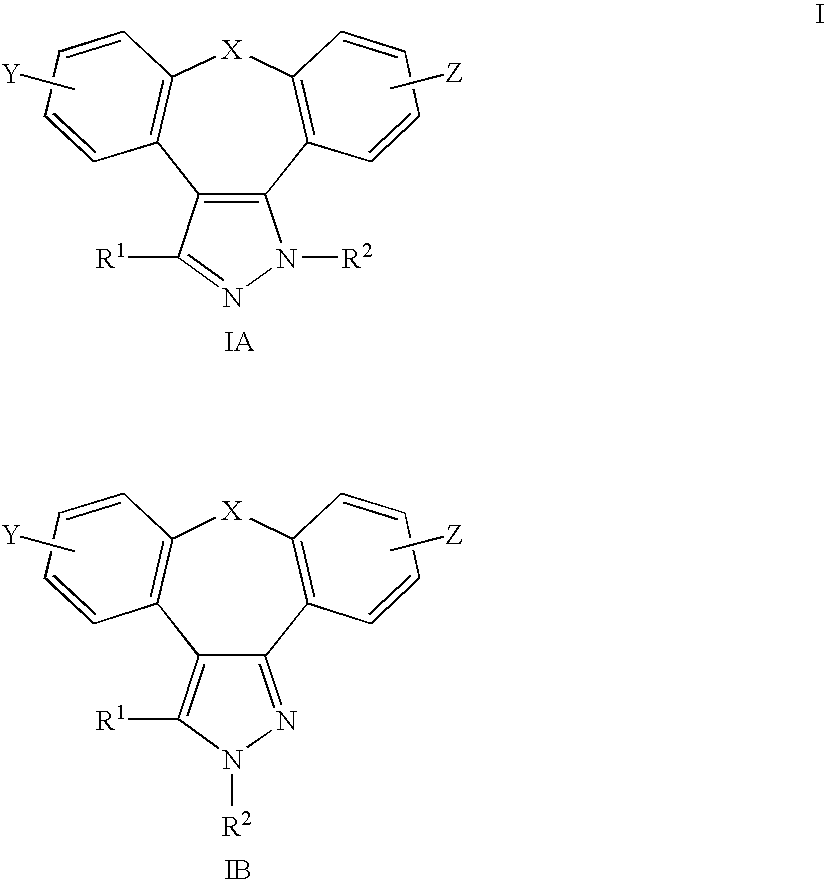
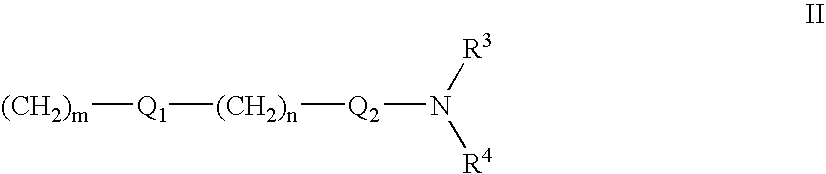
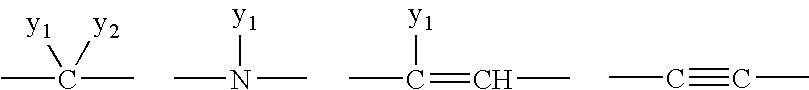
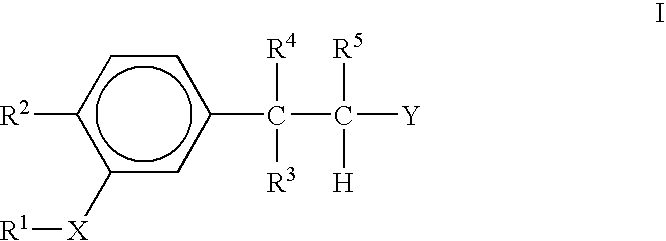
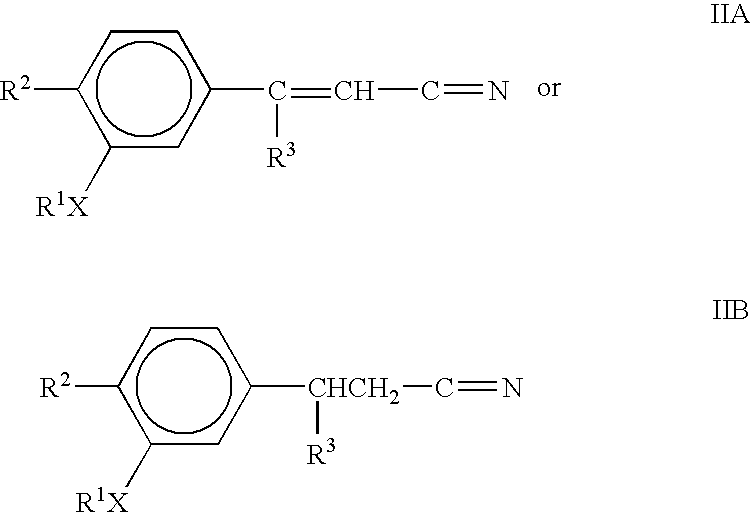
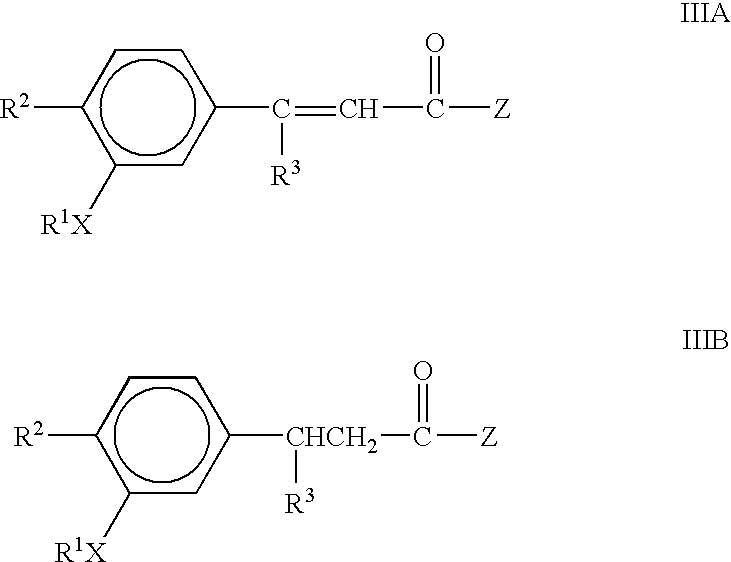
The present invention provides the compounds of formula (I):The present invention relates to imidazo[4,5-c]quinoline derivatives of formula (I), process for their preparation, pharmaceutical compositions containing them and their use in the treatment of diseases mediated by phosphatidylinositol-3-kinase (PBK) and / or mammalian target of rapamycin (mTOR) and / or tumor necrosis factor-α (TNF-oc) and / or interleukin-6 (IL-6), particularly in the treatment of cancer and inflammation.
Owner:PIRAMAL ENTERPRISES LTD
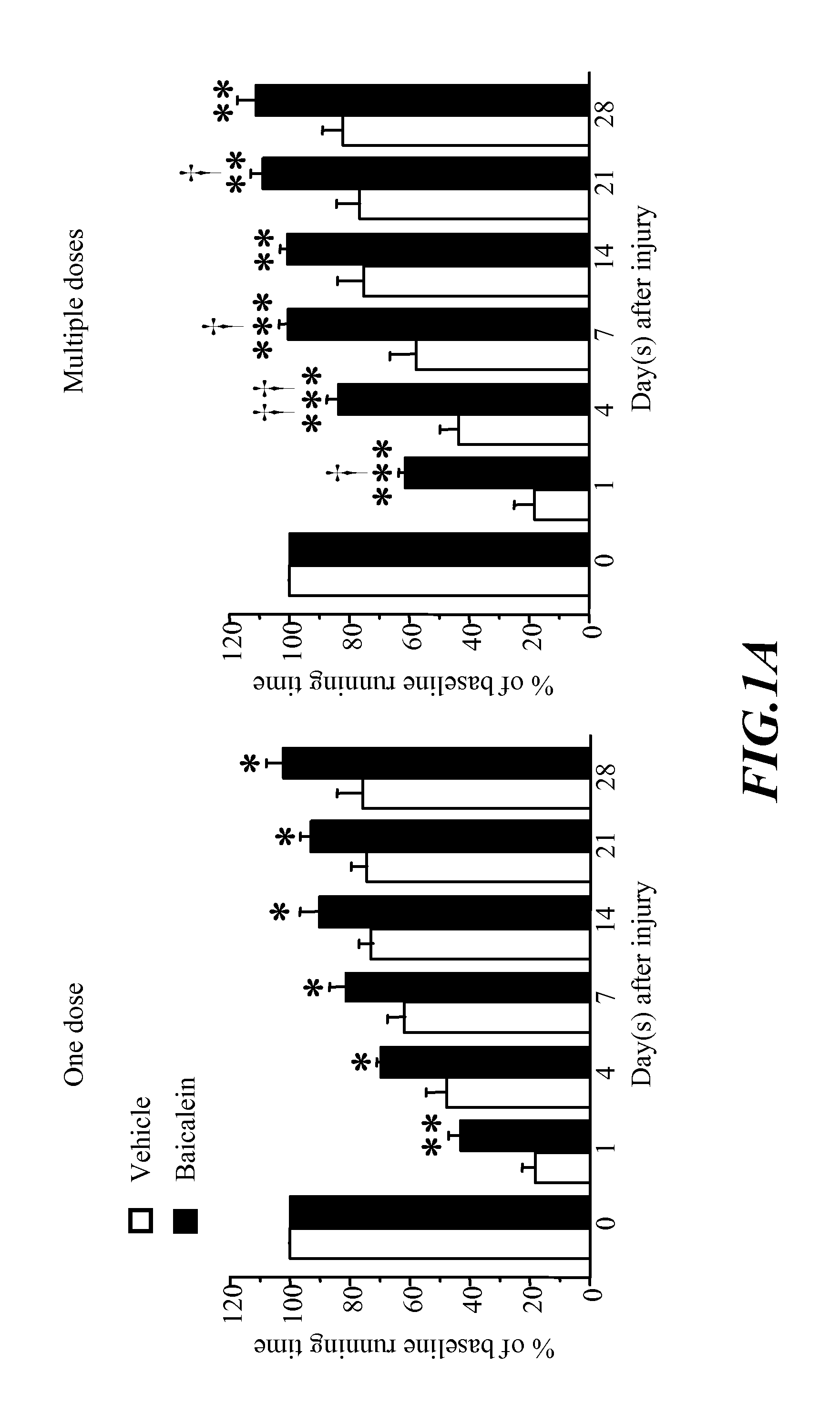
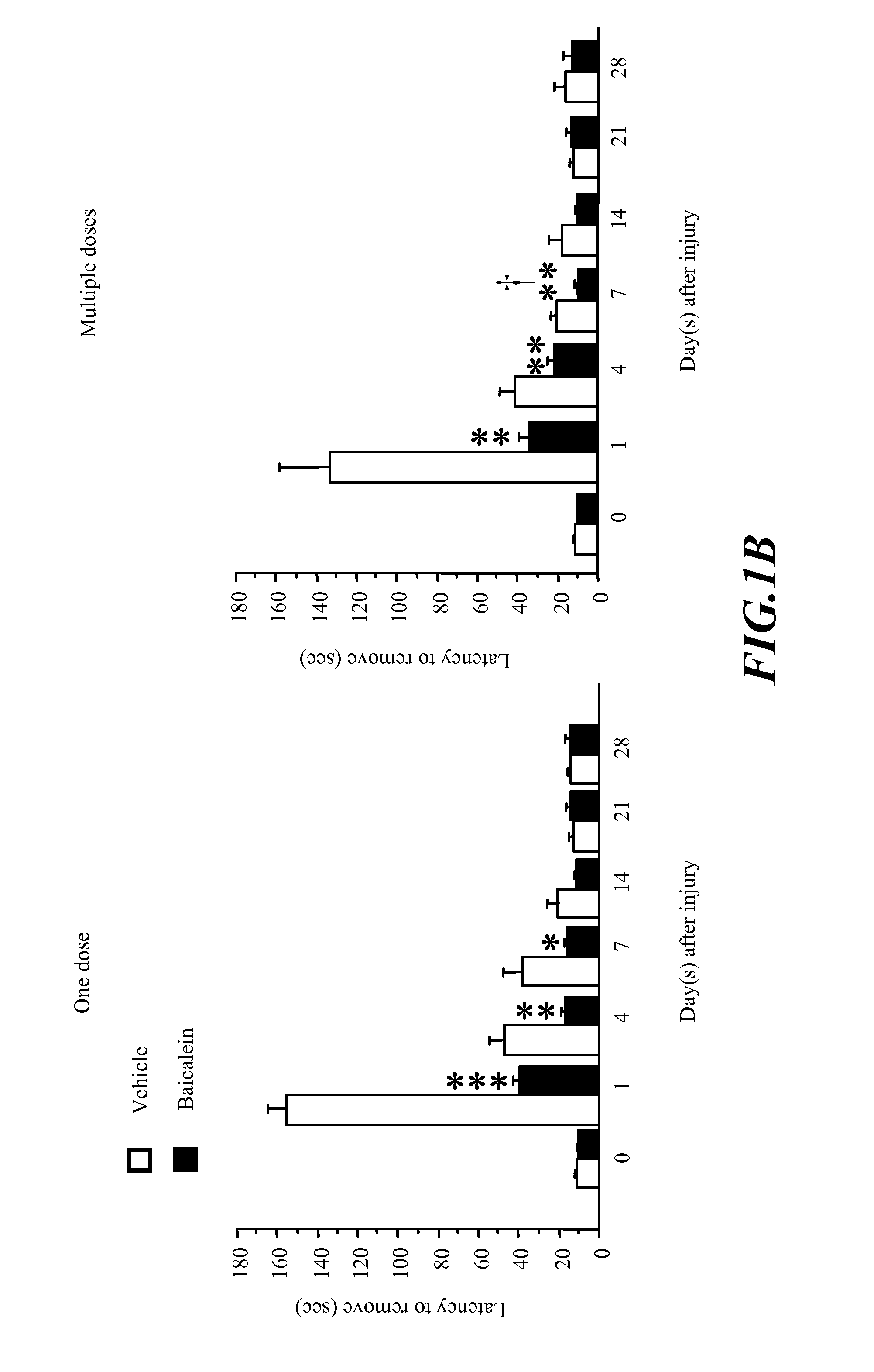
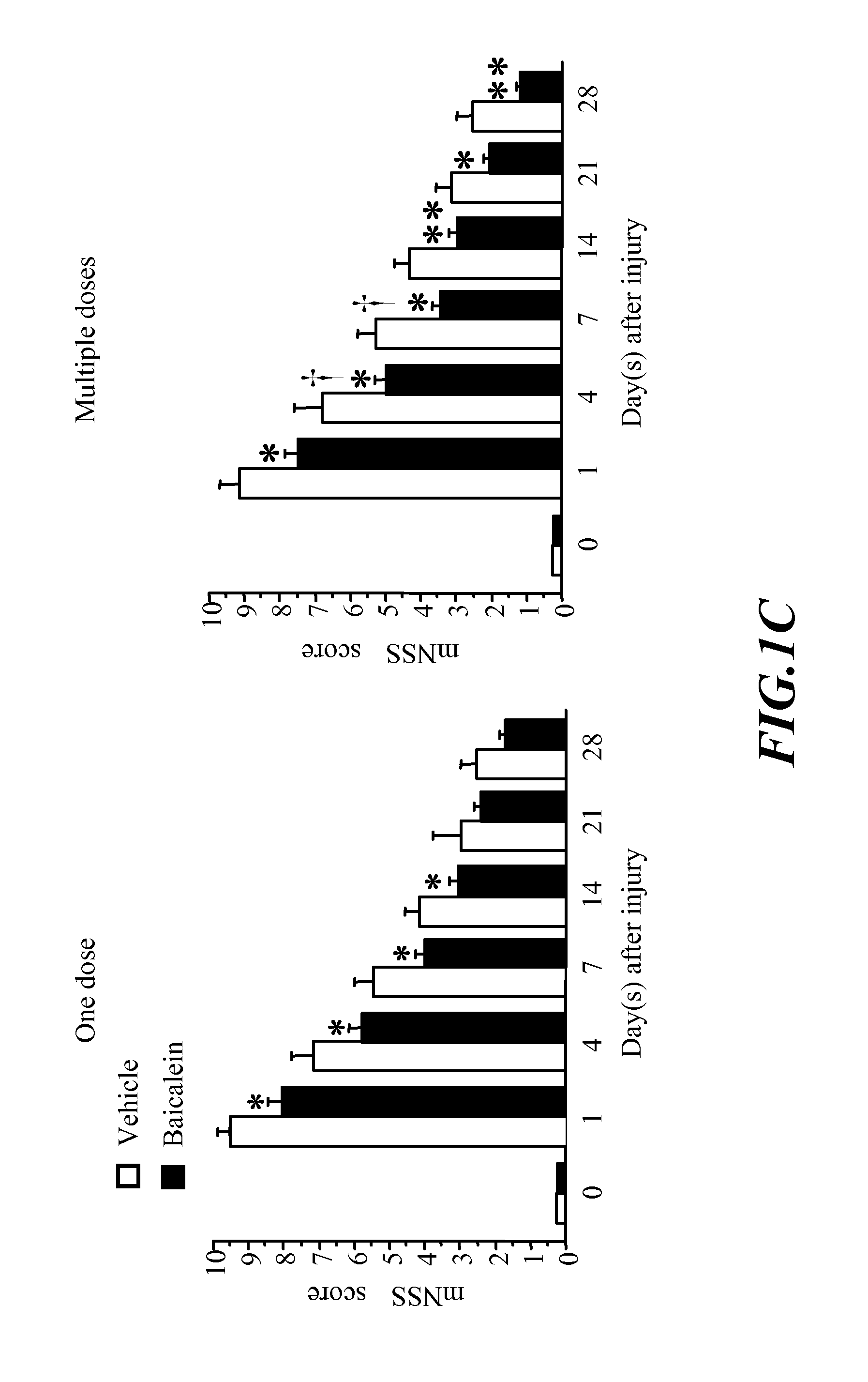
Methods of treating traumatic brain injury by administering baicalein preparation
InactiveUS20110124721A1Improve functional defectsLower the volumeBiocideNervous disorderInterleukin 6Neuronal degeneration
The invention provides a novel medical use of Baicalein, and in particular, a use of baicalein in the preparation of a pharmaceutical composition useful for treating traumatic brain injury. The baicalein pharmaceutical composition in this invention includes a treating effective amount of baicalein and a suitable pharmaceutically acceptable excipient or carrier. The baicalein pharmaceutical composition is applicable for improving the behaviour function deficit in traumatic brain injury, reducing the contusion volume of traumatic brain injury, improving the brain neuronal degeneration in traumatic brain injury, reducing proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) or other relative inflammation factor induced in traumatic brain injury.
Owner:NAT DEFENSE MEDICAL CENT
Use of long-acting recombinant human soluble tumor necrosis factor alpha receptor in manufacture of a medicament for the treatment and/or prophylaxis of hepatic failure
ActiveUS20090176702A1Decreases IL- levelPrevention of acutePeptide/protein ingredientsMetabolism disorderTreatment effectHalf-life
The present invention belongs to the field of the application of genetic engineering and gene function, and it is directed to a new medical use of the gene encoding the recombinant soluble tumor necrosis factor α receptor (HusTNFR). The present invention made intervention to fulminant hepatic failure in mice by use of the long-acting recombinant human soluble tumor necrosis factor α receptor and the classic animal models of acute and sub-acute hepatic failure. The results showed that the long-acting soluble tumor necrosis factor αreceptor of the present invention has a half-life extended more than 10 times, and it significantly decreased the mortality of model animals and has superior therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in model animals. These receptors have a noticeable therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in comparison with the non-long-acting HusTNFR.
Owner:LI ZHENYI
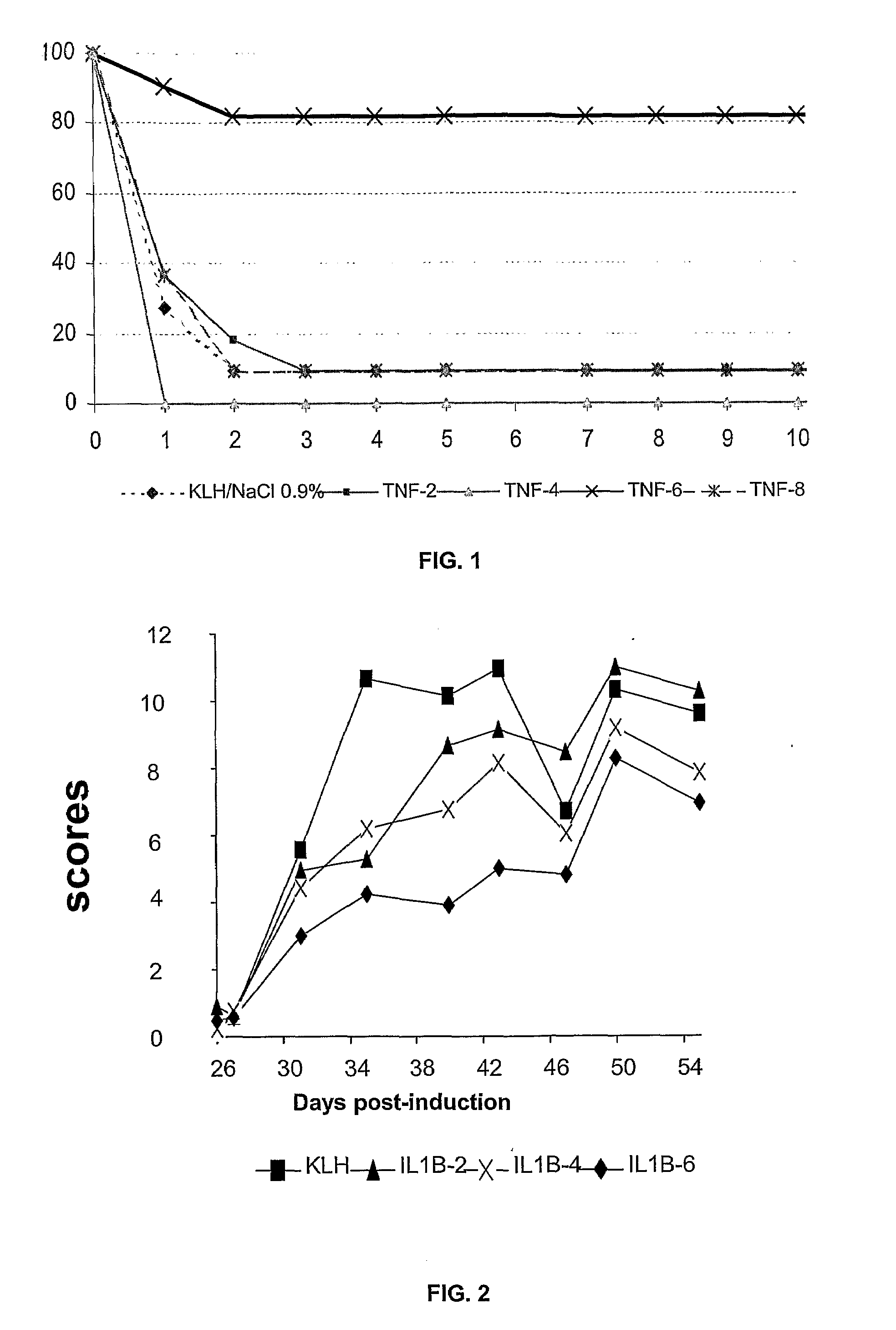
Peptides of Il1 Beta and Tnf Alpha and Method Treatment
InactiveUS20080305108A1Prevent and treat diseasePeptide/protein ingredientsAntipyreticAutoimmune diabetesAutoimmunity
The present invention relates to peptides derived from the proinflammatory cytokines, interleukin-1β, (IL1β) and tumor necrosis factor α, (TNFα), and their use in human or veterinary therapy, such as to generally treat diseases linked to the overproduction of IL1β or TNFα as well as acute or chronic inflammatory diseases, rheumatoid arthritis, septic shock, autoimmune diabetes, graft rejection in the host, etc.
Owner:VAXCONSULTING
Increasing the half-life of a full-length or a functional fragment of variant anti-human TNF-alpha antibody
InactiveUS20180066049A1Low immunogenicityExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeInflammatory Bowel Diseases
Tumor Necrosis Factor-α (TNFα) promotes an inflammatory response resulting in many clinical problems associated with autoimmune disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa, and refractory asthma. Dysregulation of TNF production is implicated in a variety of human diseases including Alzheimer's disease, cancer, major depression, and inflammatory bowel disease. These disorders are treated with a TNFα inhibitor. Embodiments herein provide methods of preventing and / or treating acute and chronic inflammation, and autoimmune diseases by administering a prophylactic and / or therapeutic formulation containing an antibody fragment (Fab or F(ab′)2) of adalimumab modified by conjugation of natural amino acids such as proline, alanine and / or serine (PA / S) by PASylation®, and / or unnatural amino acids such as cysteine and other derivatives, thereby creating a polypeptide possessing none of the processing, preparation, formulation, cost, clinical performance, and other long-term issues of administering PEGylated drugs.
Owner:DNX BIOTECH LLC
Crystalline TNF-α-converting enzyme and uses thereof
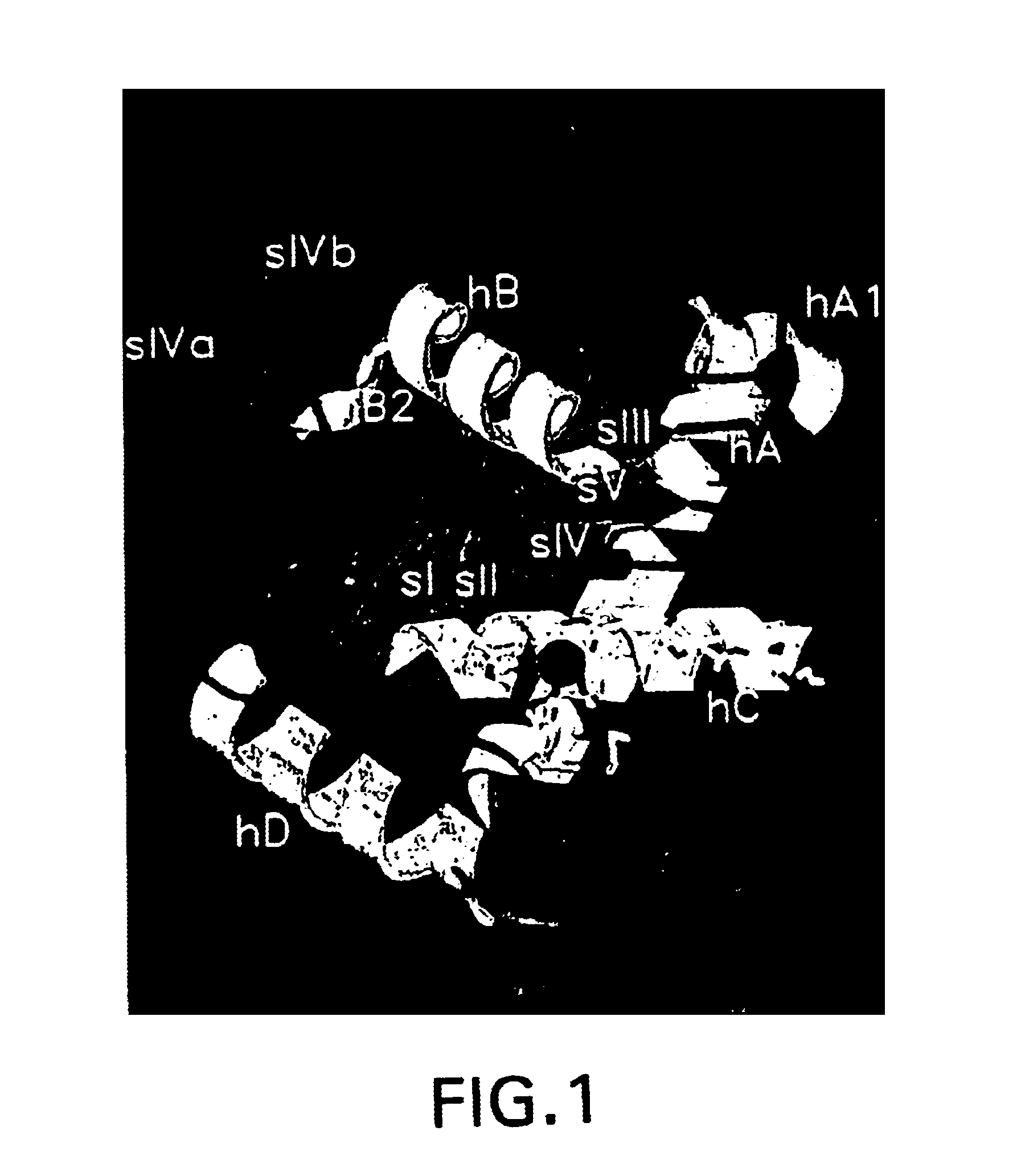
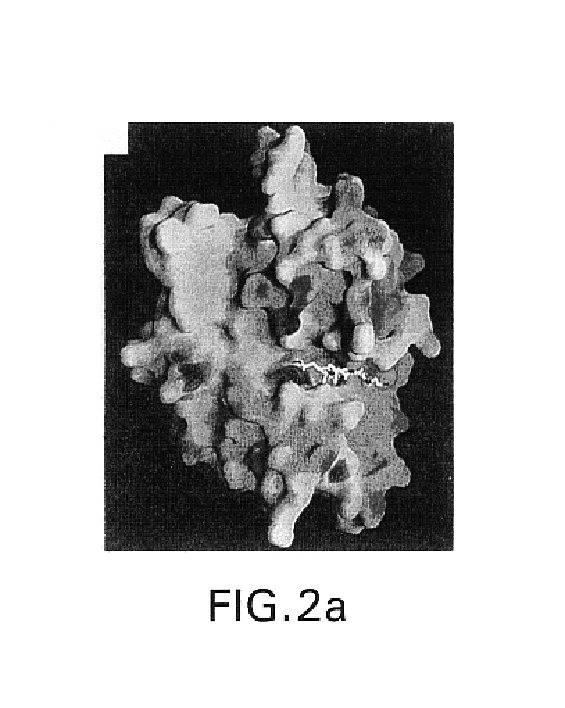
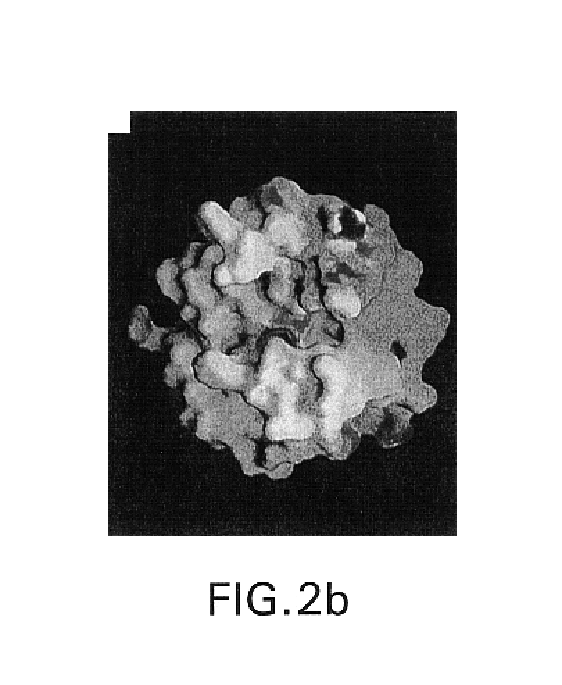
A tumor necrosis factor-α converting enzyme (TACE) is produced, purified, and crystallized. The three-dimensional coordinates of the crystal are obtained by X-ray diffraction. The coordinates can be recorded on a computer readable medium, or are part of a video memory, where they can be used as part of a system for studying for studying TACE. The coordinates are also used in designing, screening, and developing compounds that associate with TACE.
Owner:WYETH +2
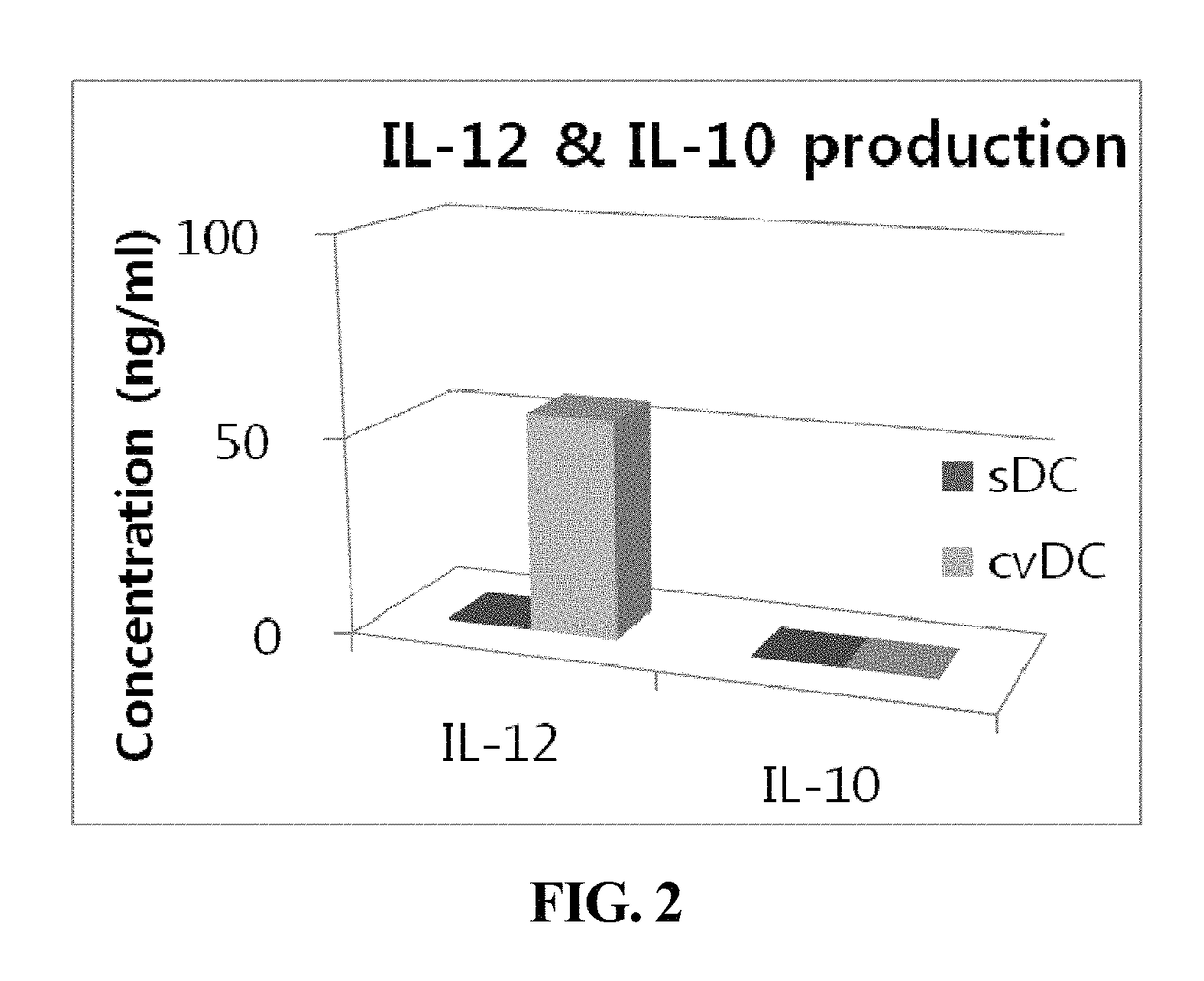
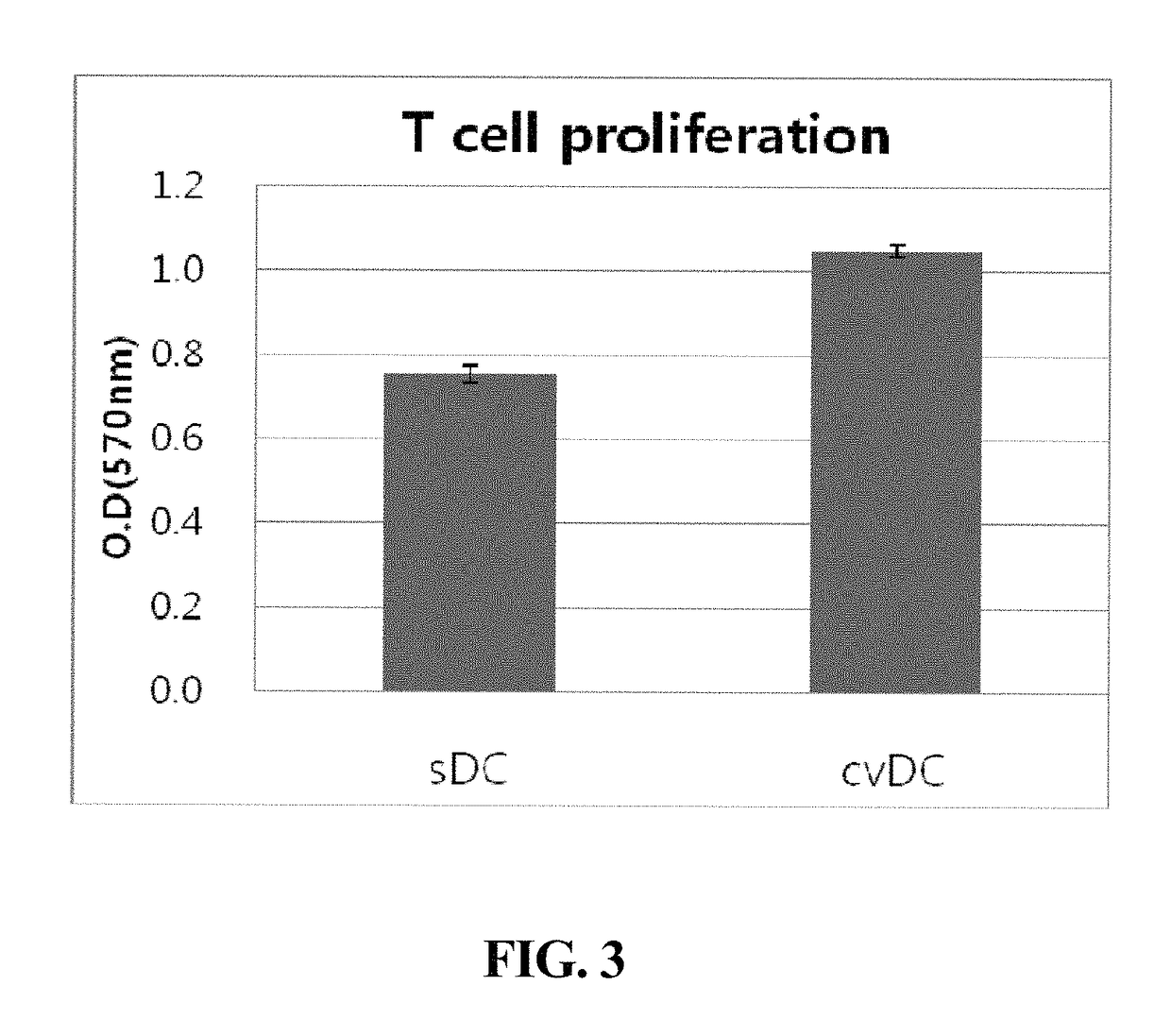
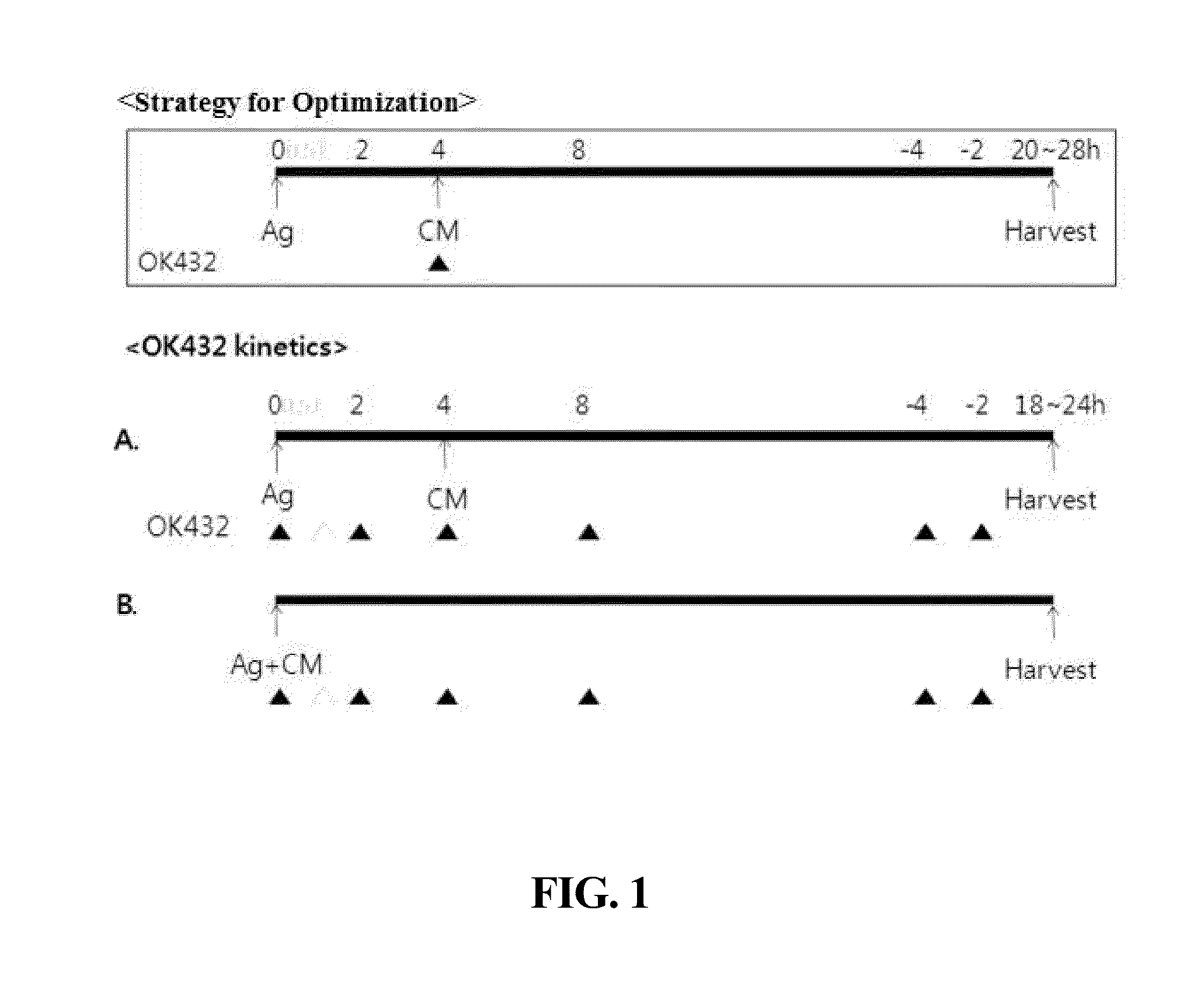
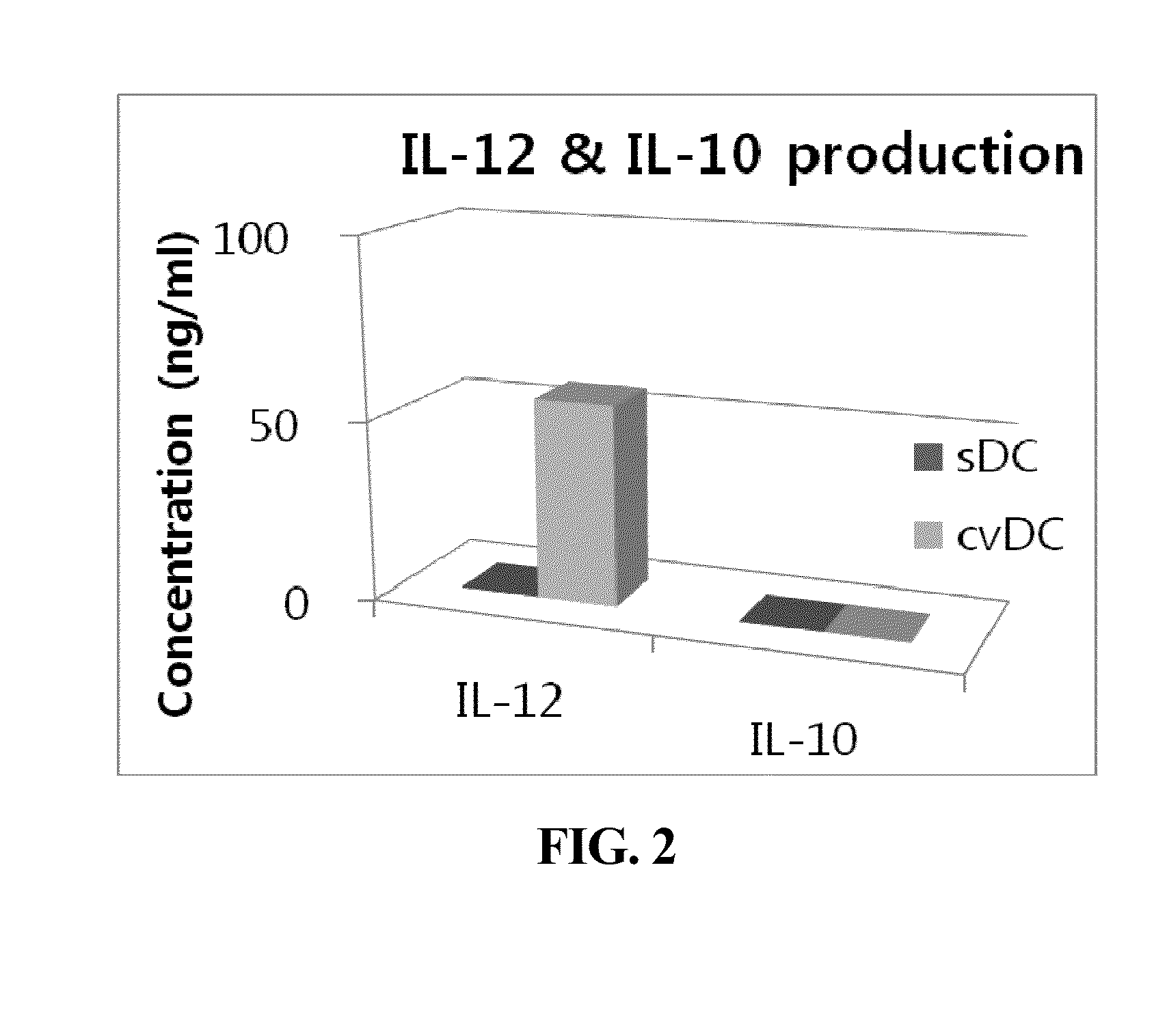
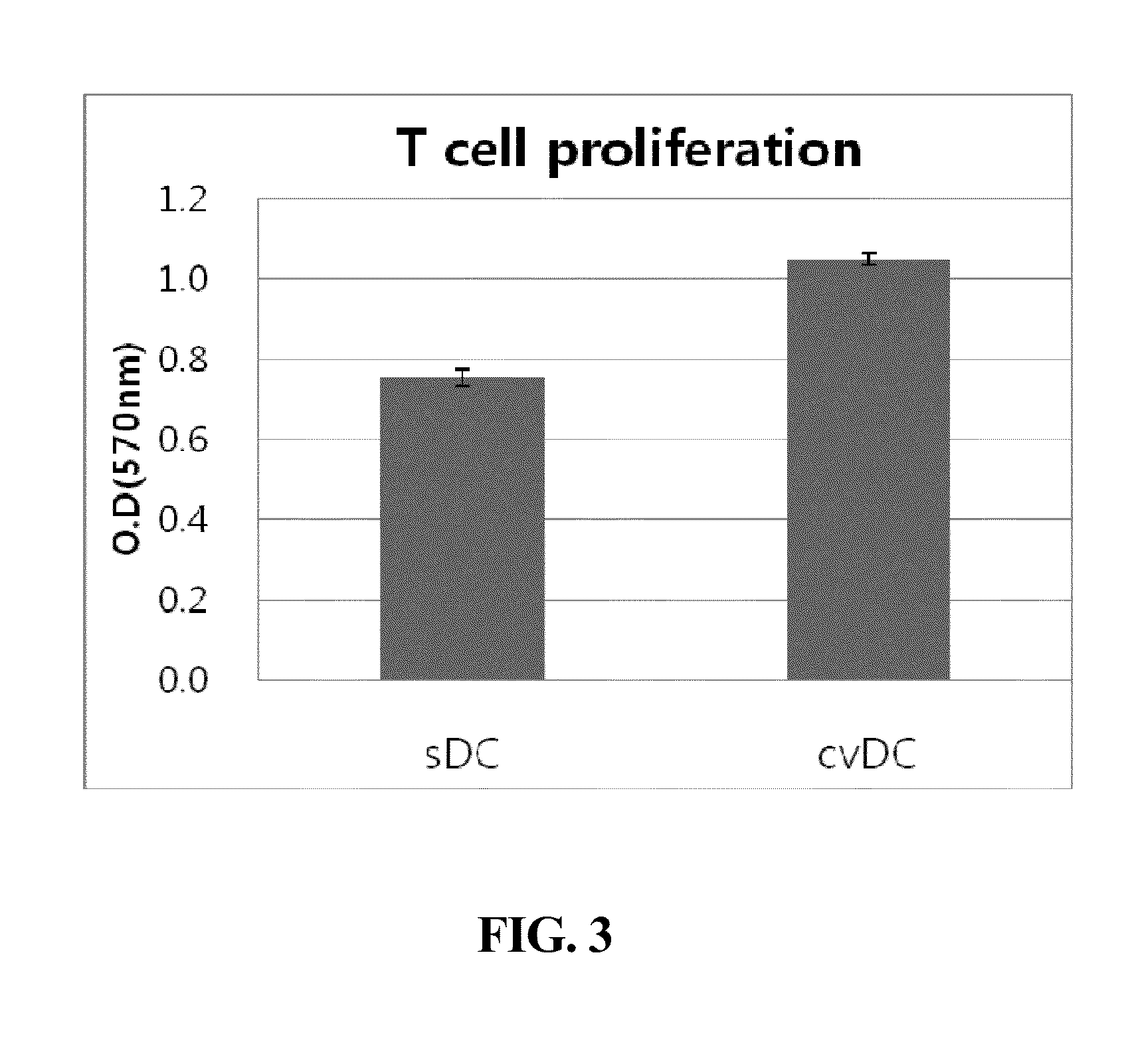
Composition for maturing dendritic cells, and method for preparing antigen-specific dendritic cells using same
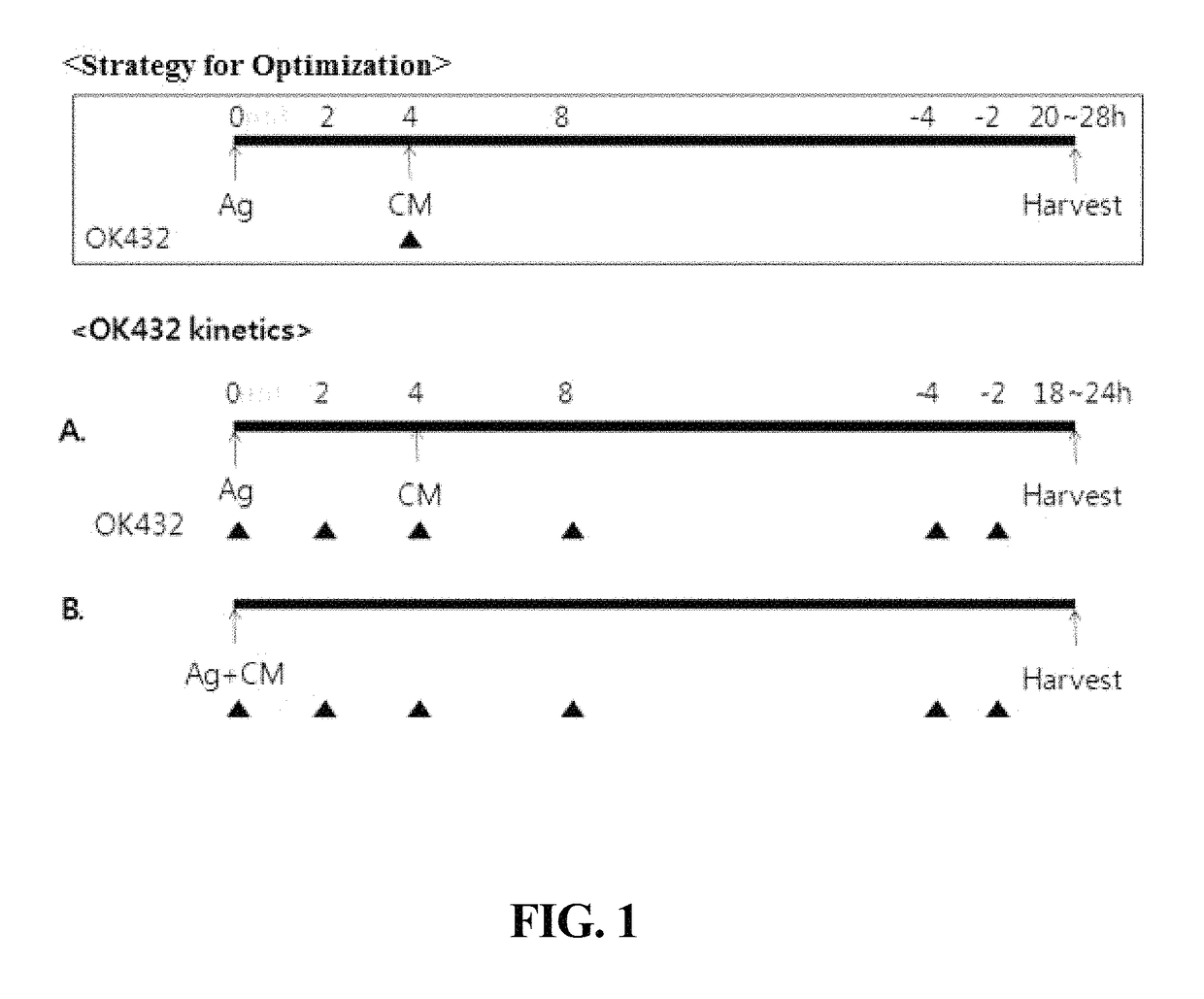
ActiveUS9701942B2Improve abilitiesDecrease antigen non-specific immune responseCulture processBlood/immune system cellsInterleukin 6Dendritic cell
The present invention relates to a composition for maturing dendritic cells, comprising, as a maturation-promoting factor, Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α), Interferon-γ (IFN-γ), Prostaglandin E2 (PGE2), Picibanil (OK432) and / or Poly IC. The composition for maturing dendritic cells of the present invention may have the effects of not only improving the ability of dendritic cells to induce an immune response, but also of decreasing the antigen non-specific immune response of dendritic cells and increasing antigen-specific immune response of dendritic cells, thus maximizing the effects of immunotherapy.
Owner:JW CREAGENE
T cell activation
A method for antigen independent activation of T cells comprising contacting T cells with a combination of cytokines such as two or more of interleukin-2, interleukin-6 and tumor necrosis factor α.
Owner:CHIRON SPA
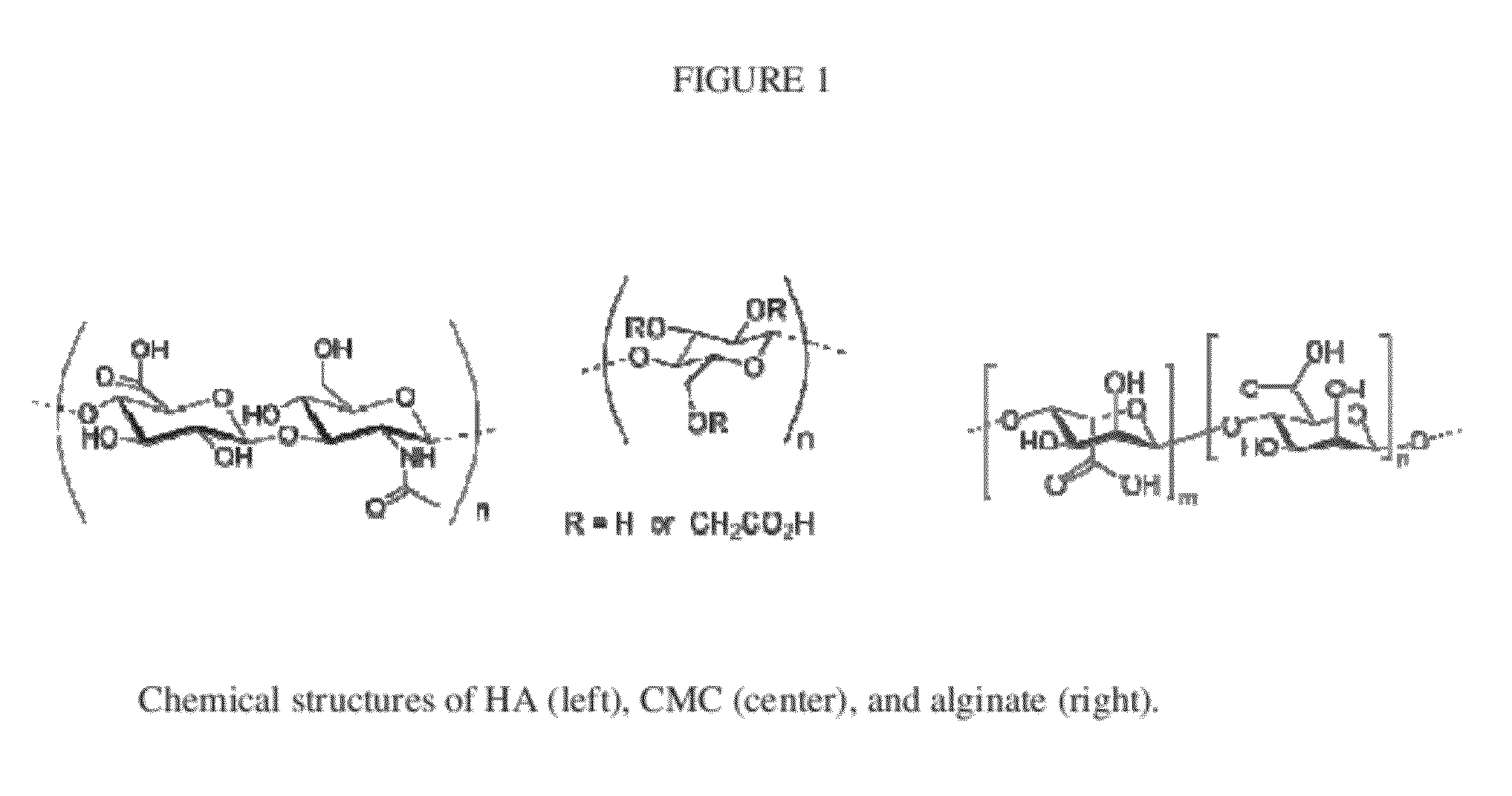
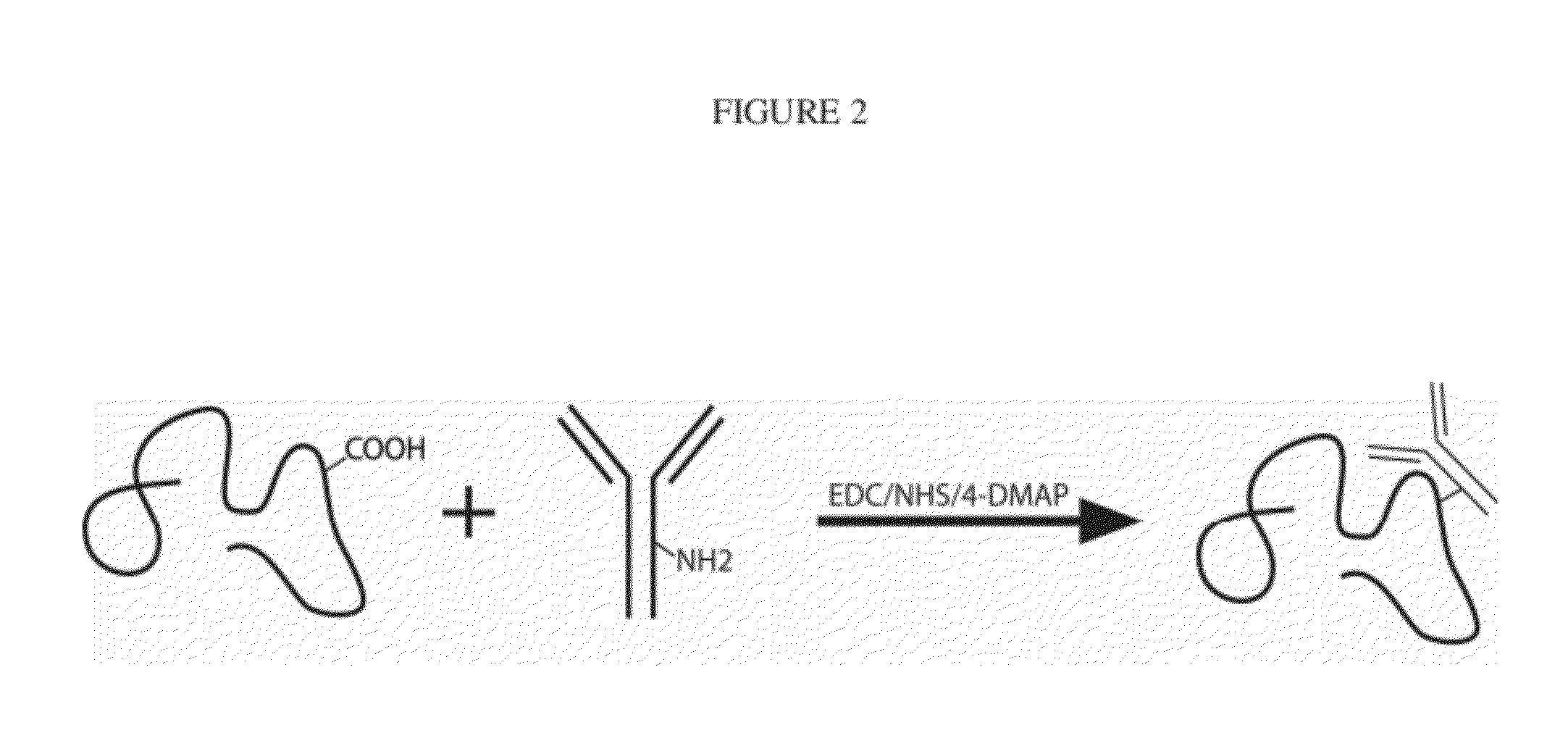
Enhanced Binding of Pro-Inflammatory Cytokines by Polysaccharide-Antibody Conjugates
InactiveUS20120064097A1High binding affinityReduced binding affinityAntipyreticAnalgesicsAntibody conjugateNeutralizing antibody
We provide monoclonal antibodies against interleukin-1β and tumor necrosis factor-α that remain biologically active in vitro when conjugated to high molecular weight polysaccharides. We report enhanced binding of these cytokines when their monoclonal antibodies are conjugated to alginate compared to non-conjugated monoclonal antibodies. In cell assays, polysaccharide-antibody constructs of the invention inhibited cytokine signaling to comparable levels as that of unmodified antibodies. Conjugation of cytokine-neutralizing antibodies to high molecular weight polymers enhances the affinities cytokine-binding moieties used as anti-inflammatory therapeutics.
Owner:WASHBURN THERAPEUTICS
Composition for maturing dendritic cells, and method for preparing antigen-specific dendritic cells using same
ActiveUS20150125956A1Improve abilitiesIncrease heightCulture processBlood/immune system cellsInterleukin 6Dendritic cell
The present invention relates to a composition for maturing dendritic cells, comprising, as a maturation-promoting factor, Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α), Interferon-γ (IFN-γ), Prostaglandin E2 (PGE2), Picibanil (OK432) and / or Poly IC. The composition for maturing dendritic cells of the present invention may have the effects of not only improving the ability of dendritic cells to induce an immune response, but also of decreasing the antigen non-specific immune response of dendritic cells and increasing antigen-specific immune response of dendritic cells, thus maximizing the effects of immunotherapy.
Owner:JW CREAGENE
Method for diagnosing sleep apnea
ActiveUS20170059587A1Readily apparentDisease diagnosisBiological testingBetatrophinBrain-derived neurotrophic factor
The method for diagnosing sleep apnea includes measuring concentrations of biomarkers in a patient's bodily sample. To determine whether a patient suffers from sleep apnea, or has a predisposition for developing sleep apnea, a sample from the patient is analyzed. If one or more of the following biomarker concentrations are found in the patient's sample, then the patient may be diagnosed as suffering from sleep apnea or having a predisposition for developing sleep apnea: between approximately 992.8 pg / mL and approximately 1309.6 pg / mL of adipsin; between approximately 1,640 pg / mL and approximately 2,900 pg / mL of betatrophin; between approximately 8,090.82 pg / mL and approximately 11,829.07 pg / mL of brain-derived neurotrophic factor (BDNF); between approximately 11.82 pg / mL and approximately 88.26 pg / mL of interleukin-13 (IL-13); between approximately 49.45 pg / mL and approximately 103.29 pg / mL of tumor necrosis factor-α (TNF-α); and between approximately 16.55 pg / mL and approximately 29.76 pg / mL of the protein encoded by Human DNAJC27.
Owner:ALTERKI ABDULMOHSEN EBRAHIM
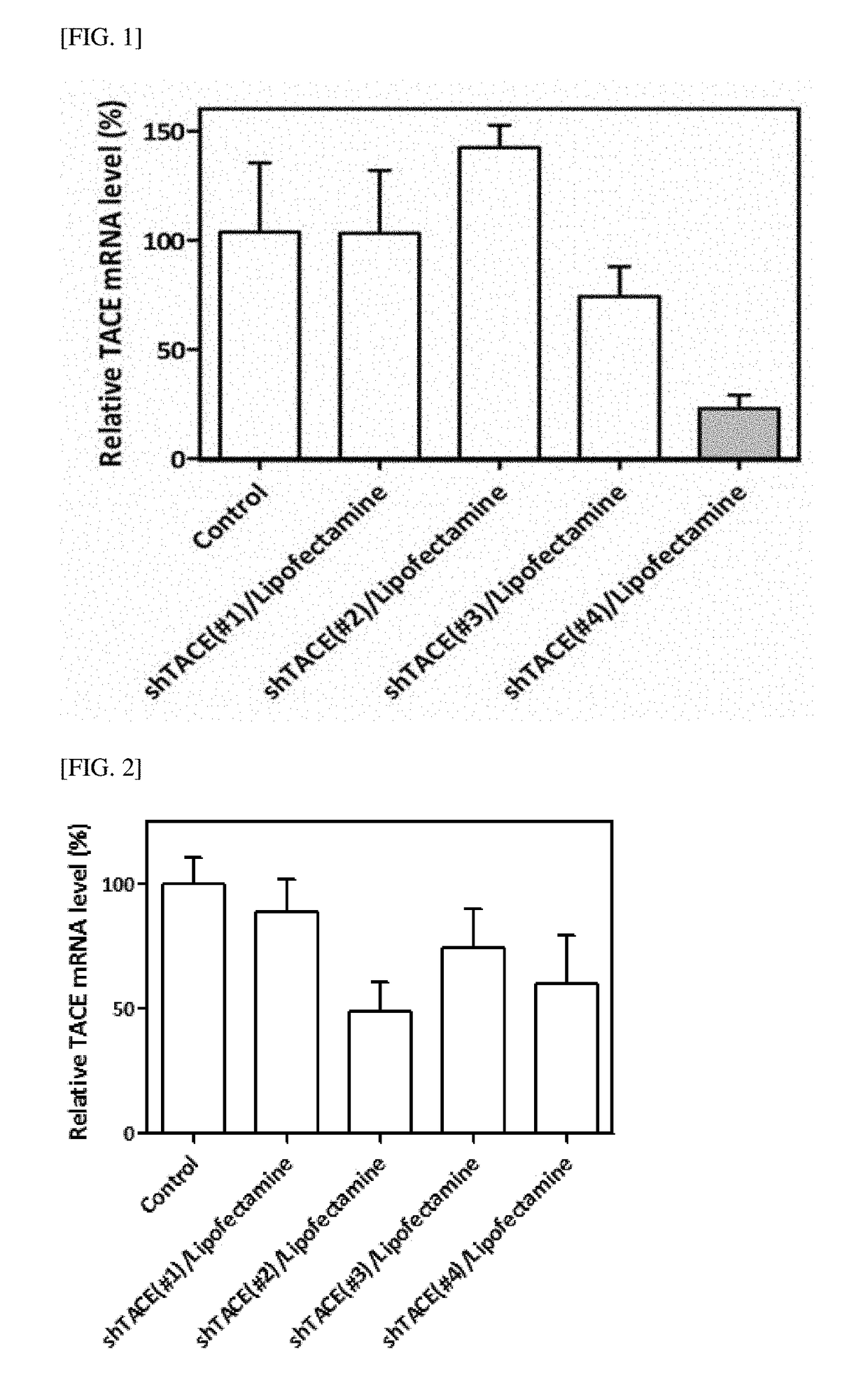
Gene/carrier complex for preventing or treating inflammatory diseases
Disclosed is a gene / carrier complex for preventing or treating inflammatory diseases, including tumor necrosis factor-α converting enzyme (TNF-α converting enzyme, TACE) shRNA and a nonviral gene carrier, wherein the nonviral gene carrier includes an acetate of disulfide-linked poly(oligo-arginine) or a TFA salt of poly(oligo-aspartic acid)poly(oligo-arginine).
Owner:IUCF HYU (IND UNIV COOP FOUND HANYANG UNIV) +1
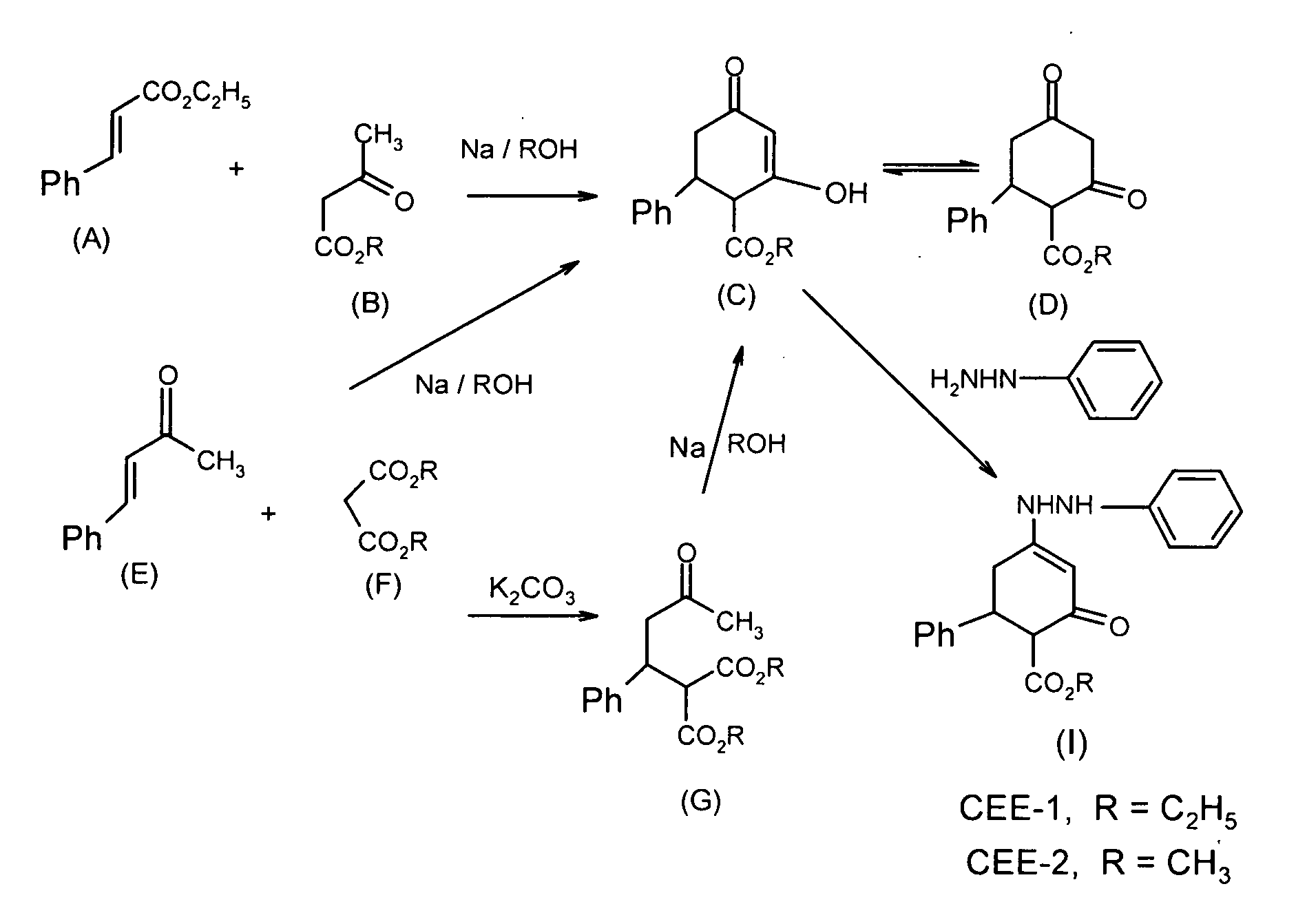
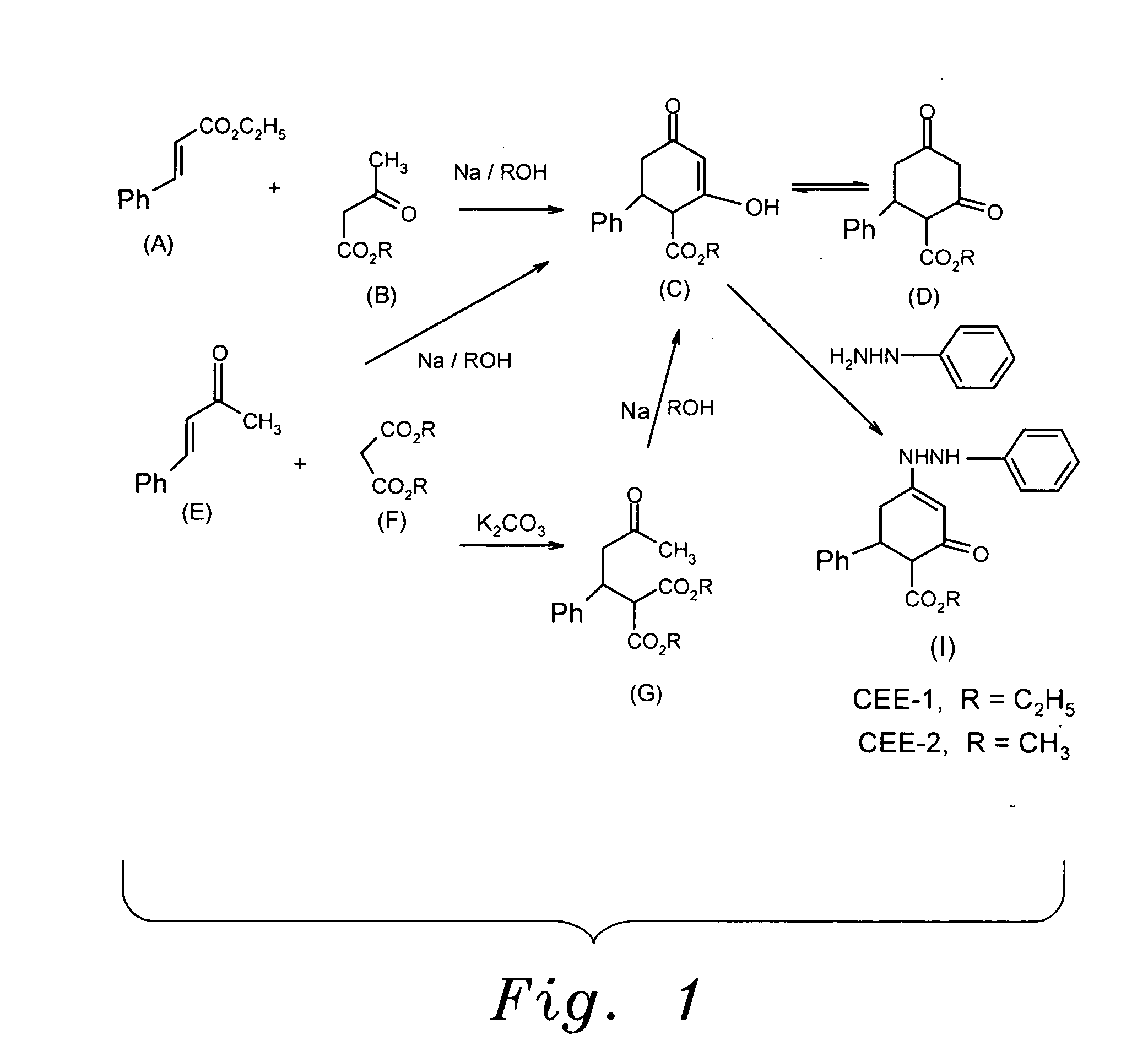
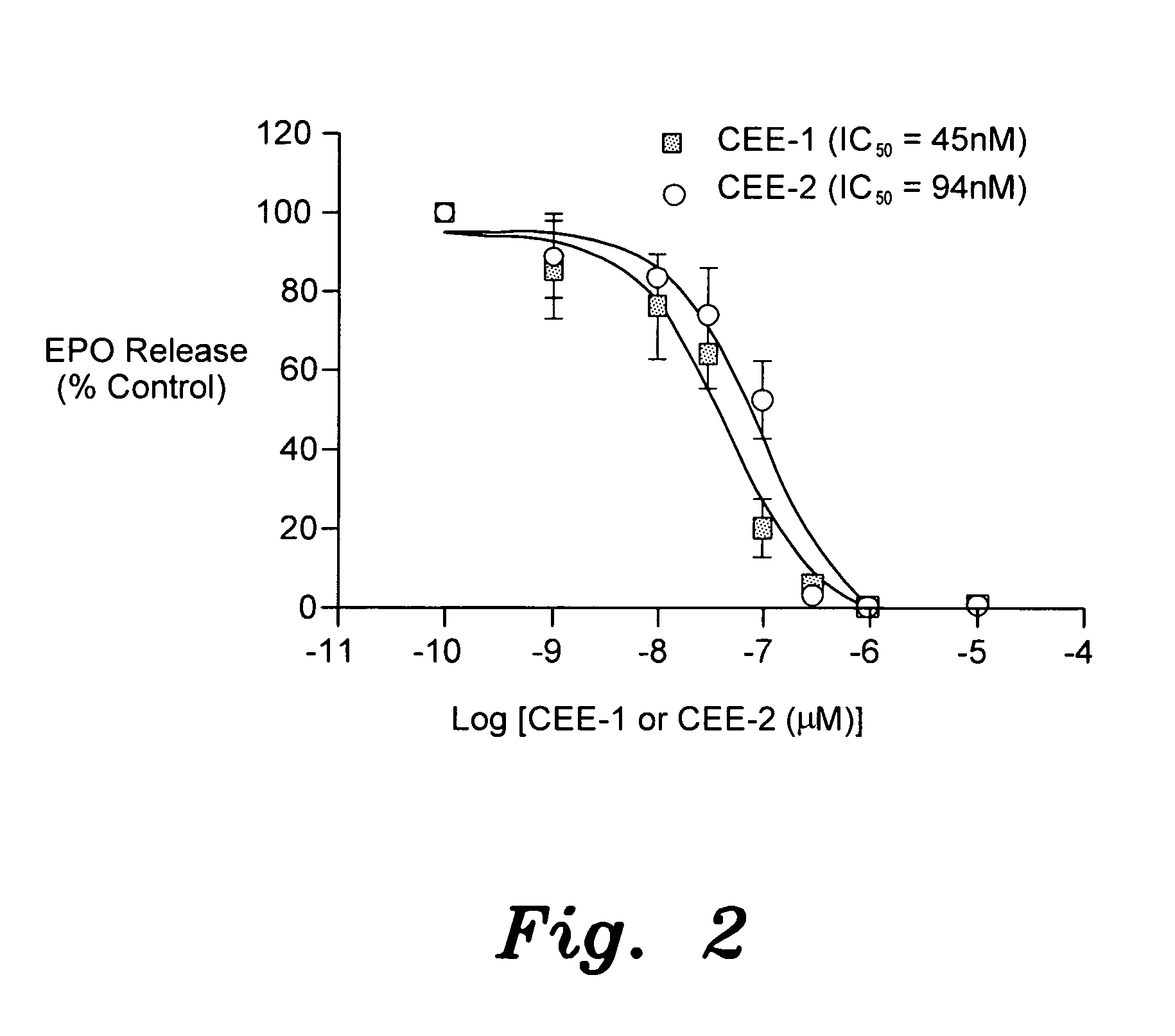
Enhydrazone esters for treating asthma, allergy and inflammation
Owner:KUWAIT UNIV OF
Orally administrable immunostimulant product for aquaculture
InactiveCN102209530BMaintain propertiesKeep alivePeptide/protein ingredientsAccessory food factorsMicroorganismWilms' tumor
The present invention relates to an orally administrable immunostimulatory product comprising microencapsulated cytokines, which are cells of fish, molluscs or crustaceans, and an enteroprotective polymer for protecting said cytokines Factors, preferably recombinant cytokines, such as tumor necrosis factor alpha (TNFα) overexpressed in the host microorganism.
Owner:PROBELTE PHARMA
Method of preventing acute or sub-acute hepatic failure in a subject by administering a soluble human tumor necrosis factor alpha receptor fusion protein
ActiveUS8227404B2Good effectEfficiently preventBiocideOrganic active ingredientsHuman tumorAcute hepatic failure
The present invention belongs to the field of the application of genetic engineering and gene function, and it is directed to a new medical use of the gene encoding the recombinant soluble tumor necrosis factor α receptor (HusTNFR). The present invention made intervention to fulminant hepatic failure in mice by use of the long-acting recombinant human soluble tumor necrosis factor α receptor and the classic animal models of acute and sub-acute hepatic failure. The results showed that the long-acting soluble tumor necrosis factor α receptor of the present invention has a half-life extended more than 10 times, and it significantly decreased the mortality of model animals and has superior therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in model animals. These receptors have a noticeable therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in comparison with the non-long-acting HusTNFR.
Owner:LI ZHENYI
1,2-Diaza-dibenzoazulenes as inhibitors of tumour necrosis factor production and intermediates for the preparation thereof
Owner:GLAXOSMITHKLINE ISTRAZIVACKI CENTAR ZAGREB D O O
Imidazopyridine derivatives
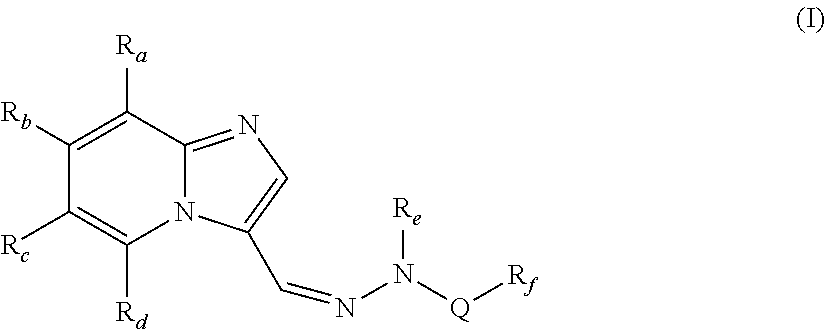
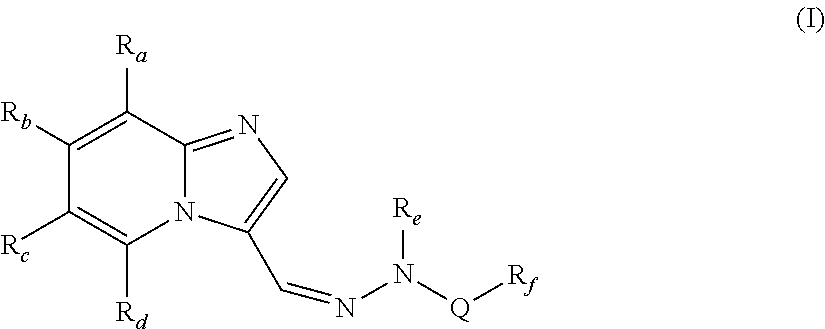
The present invention relates to compounds of formula (I),wherein Ra, Rb, Rc, Rd, Re and Rf are as defined in the specification, processes for their preparation, pharmaceutical compositions containing them and their use in the treatment of diseases mediated by phosphatidylinositol-3-kinase (PI3K), mammalian target of rapamycin (mTOR), Signal transducer and activator of transcription 3 (STAT 3), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) or a combination thereof particularly in the treatment of cancer and inflammation.
Owner:PIRAMAL ENTERPRISES LTD
Enhydrazone esters for treating asthma, allergy and inflammation
InactiveUS20100216881A1Inhibit inflammationUseful in treatmentBiocideOrganic chemistryMast cellAnaphylaxis
The enhydrazone esters for treating asthma, allergy, and inflammation relate to the use of two cyclohexenone derivatives and pharmaceutically acceptable salts thereof in the treatment of asthma, allergies, and inflammation. The esters have the formula:wherein R is ethyl or methyl. Administration of the esters inhibits the degranulation of eosinophils, the degranulation of mast cells, and the release or expression of tumor necrosis factor-α, thereby alleviating the debilitating symptoms of asthma, allergic reactions, and inflammatory responses.
Owner:KUWAIT UNIV OF
RNAi-RELATED INHIBITION OF TNFa SIGNALING PATHWAY FOR TREATMENT OF GLAUCOMA
RNA interference is provided for inhibition of tumor necrosis factor α (TNFα) by silencing TNFα cell surface receptor TNF receptor-1 (TNFR1) mRNA expression, or by silencing TNFα converting enzyme (TACE / ADAM17) mRNA expression. Silencing such TNFα targets, in particular, is useful for treating patients having a TNFα-related condition or at risk of developing a TNFα-related condition such as the ocular conditions associated with elevated intraocular pressure (IOP), including glaucoma and ocular hypertension.
Owner:ARROWHEAD RES CORP
Method for diagnosing sleep apnea by measuring adipsin and betatrophin levels
The method for diagnosing sleep apnea includes measuring concentrations of biomarkers in a patient's bodily sample. To determine whether a patient suffers from sleep apnea, or has a predisposition for developing sleep apnea, a sample from the patient is analyzed. If one or more of the following biomarker concentrations are found in the patient's sample, then the patient may be diagnosed as suffering from sleep apnea or having a predisposition for developing sleep apnea: between approximately 992.8 pg / mL and approximately 1309.6 pg / mL of adipsin; between approximately 1,640 pg / mL and approximately 2,900 pg / mL of betatrophin; between approximately 8,090.82 pg / mL and approximately 11,829.07 pg / mL of brain-derived neurotrophic factor (BDNF); between approximately 11.82 pg / mL and approximately 88.26 pg / mL of interleukin-13 (IL-13); between approximately 49.45 pg / mL and approximately 103.29 pg / mL of tumor necrosis factor-α (TNF-α); and between approximately 16.55 pg / mL and approximately 29.76 pg / mL of the protein encoded by Human DNAJC27.
Owner:ALTERKI ABDULMOHSEN EBRAHIM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
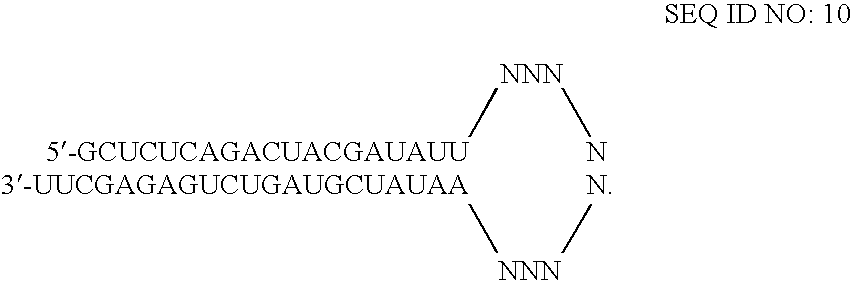
![Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation](https://images-eureka.patsnap.com/patent_img/82bd8510-afc7-4d5b-ae3e-387be6c3bc72/US08637670-20140128-C00001.png)
![Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation](https://images-eureka.patsnap.com/patent_img/82bd8510-afc7-4d5b-ae3e-387be6c3bc72/US08637670-20140128-C00002.png)
![Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation Imidazo [4,5-C]quinoline derivatives and their use in the treatment of tumors and/or inflammation](https://images-eureka.patsnap.com/patent_img/82bd8510-afc7-4d5b-ae3e-387be6c3bc72/US08637670-20140128-C00003.png)