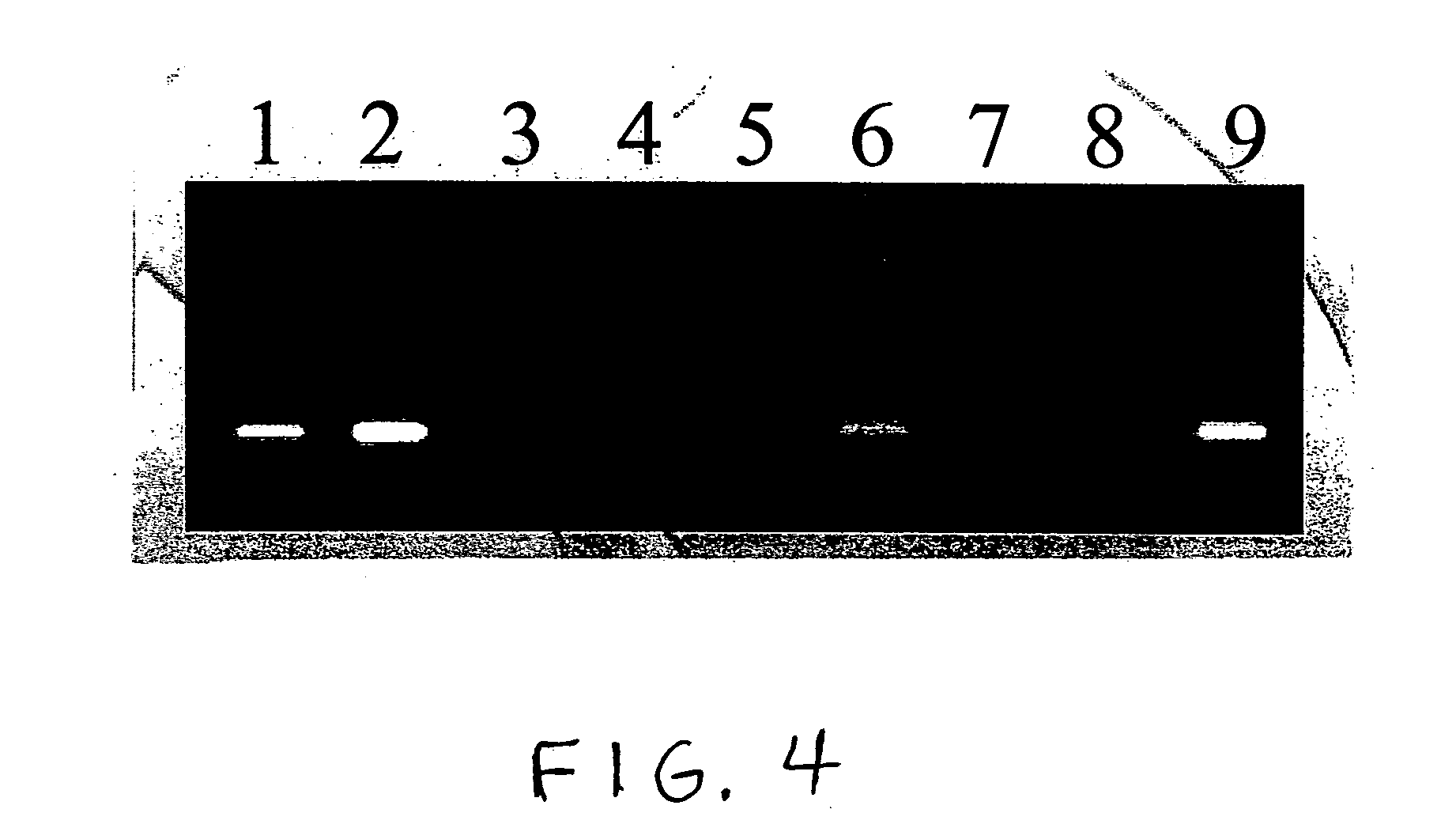
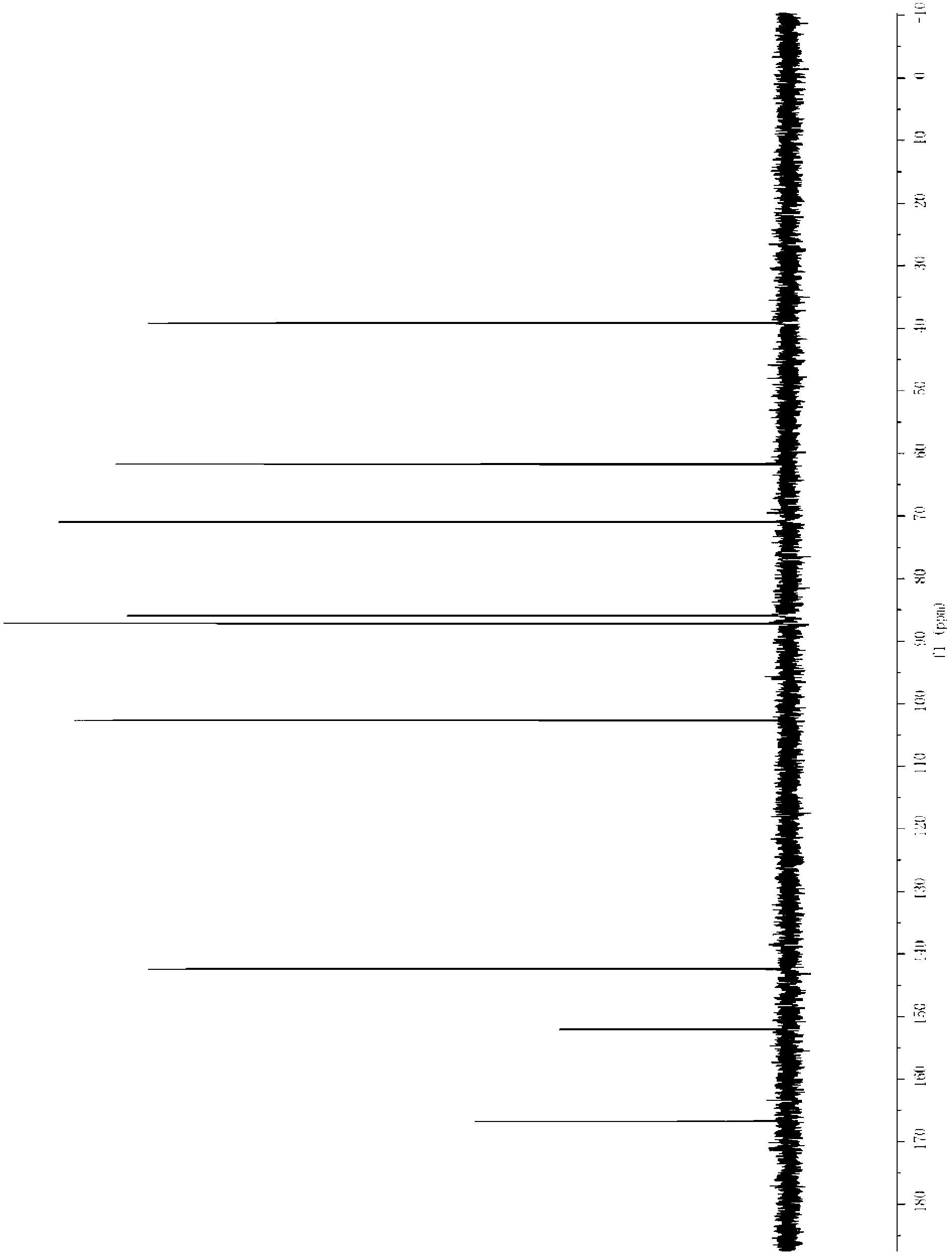
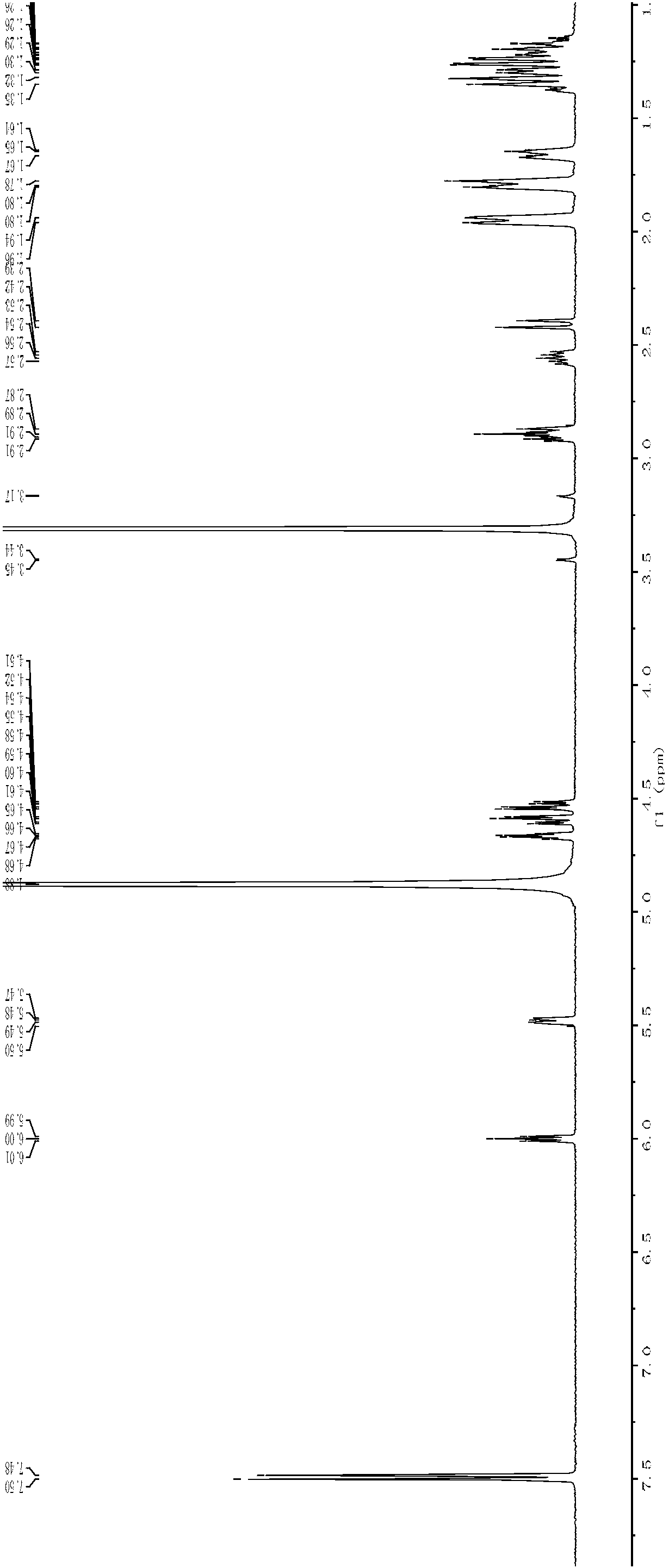
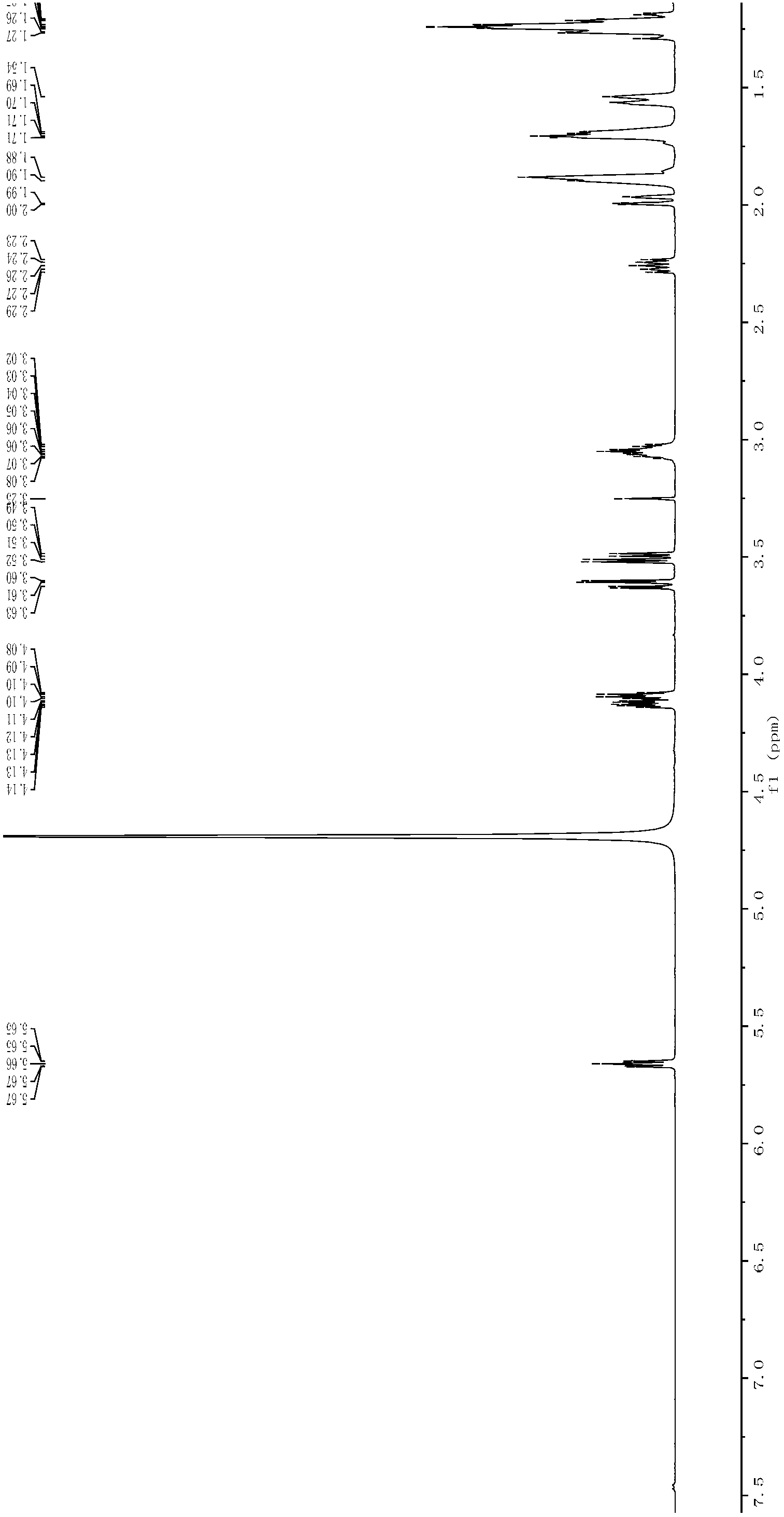
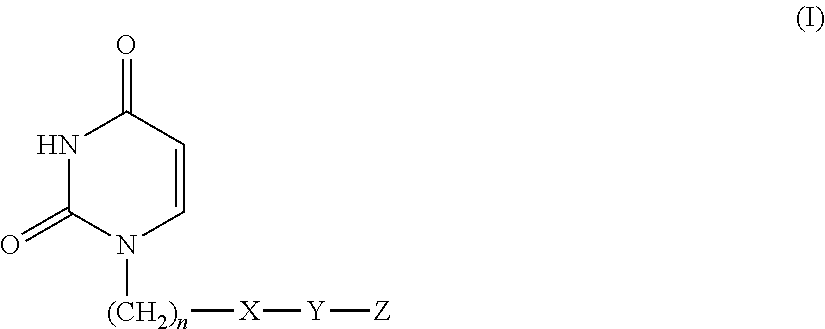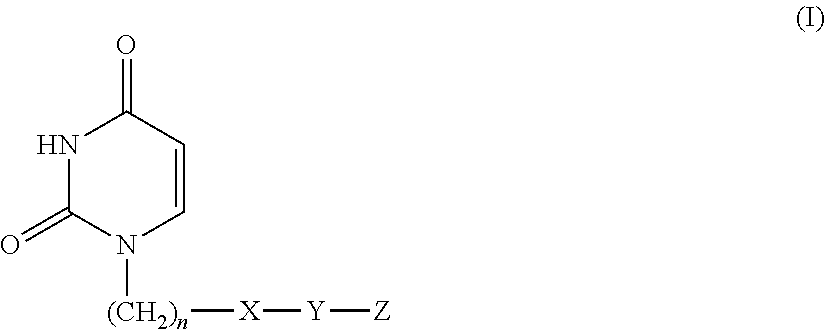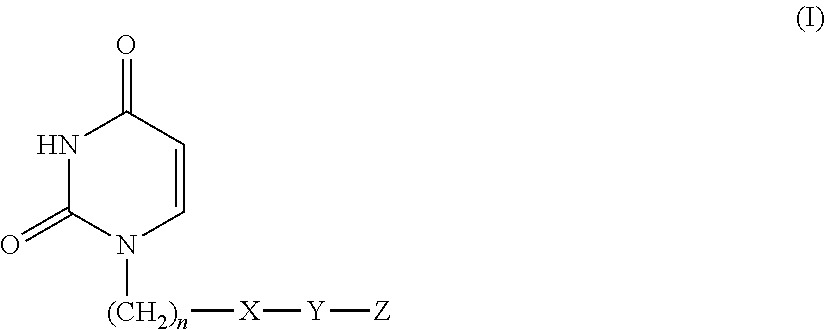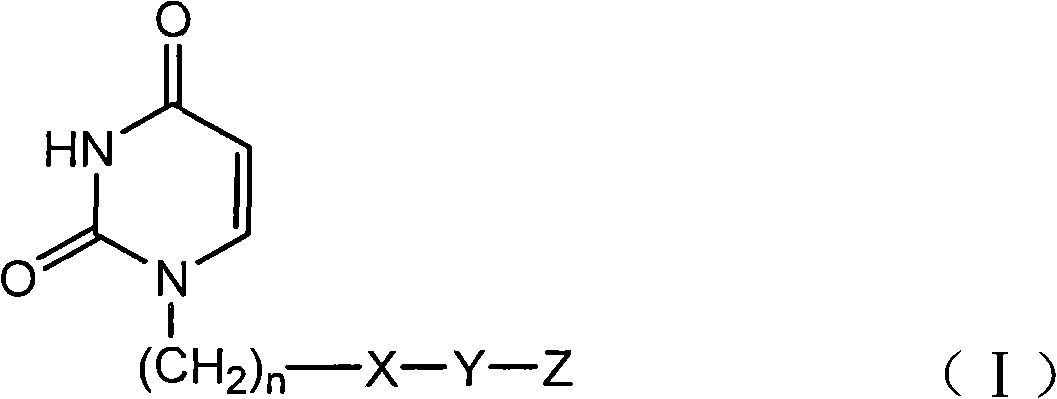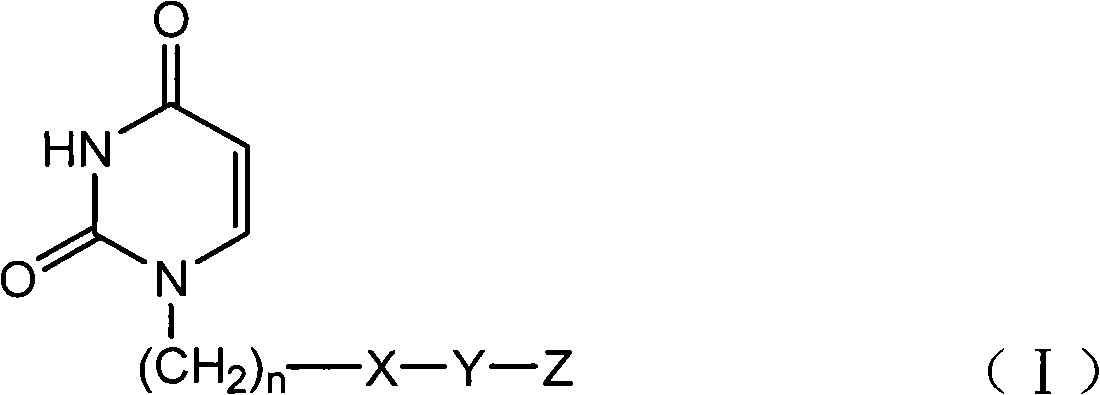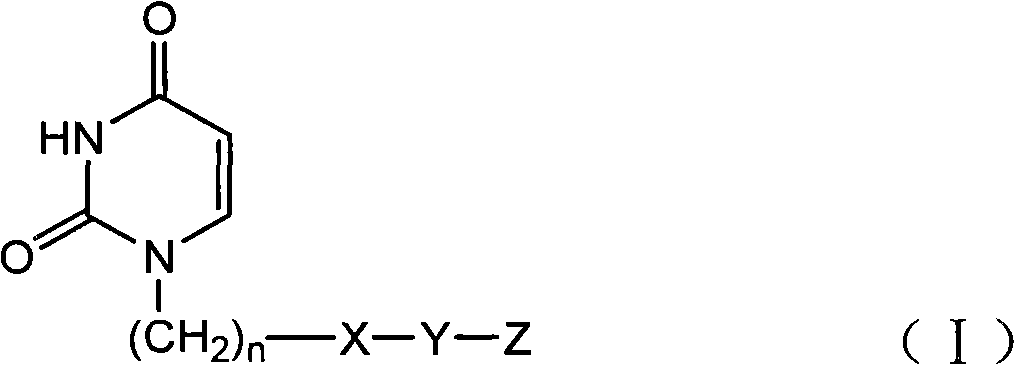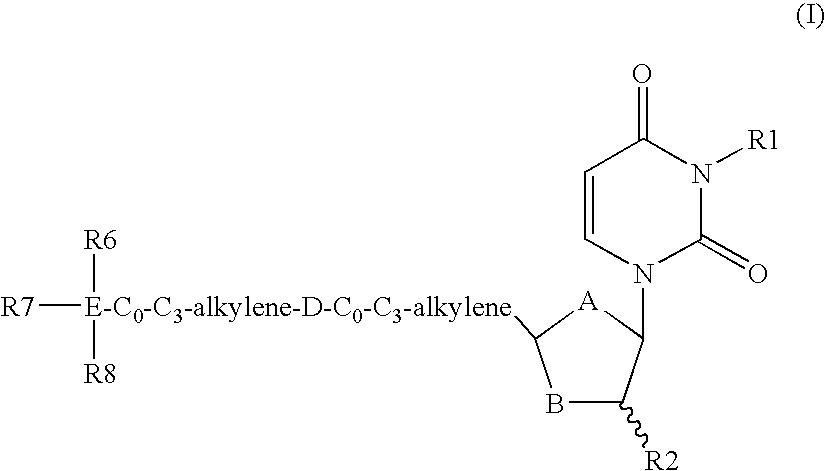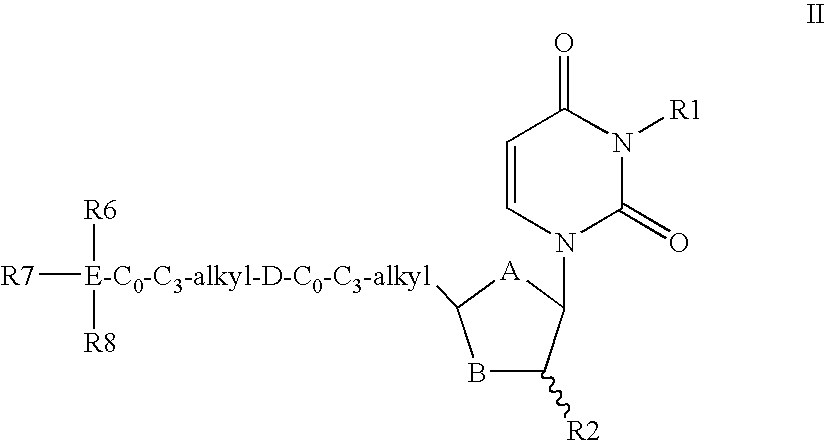Patents
Literature
111 results about "Deoxyuridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
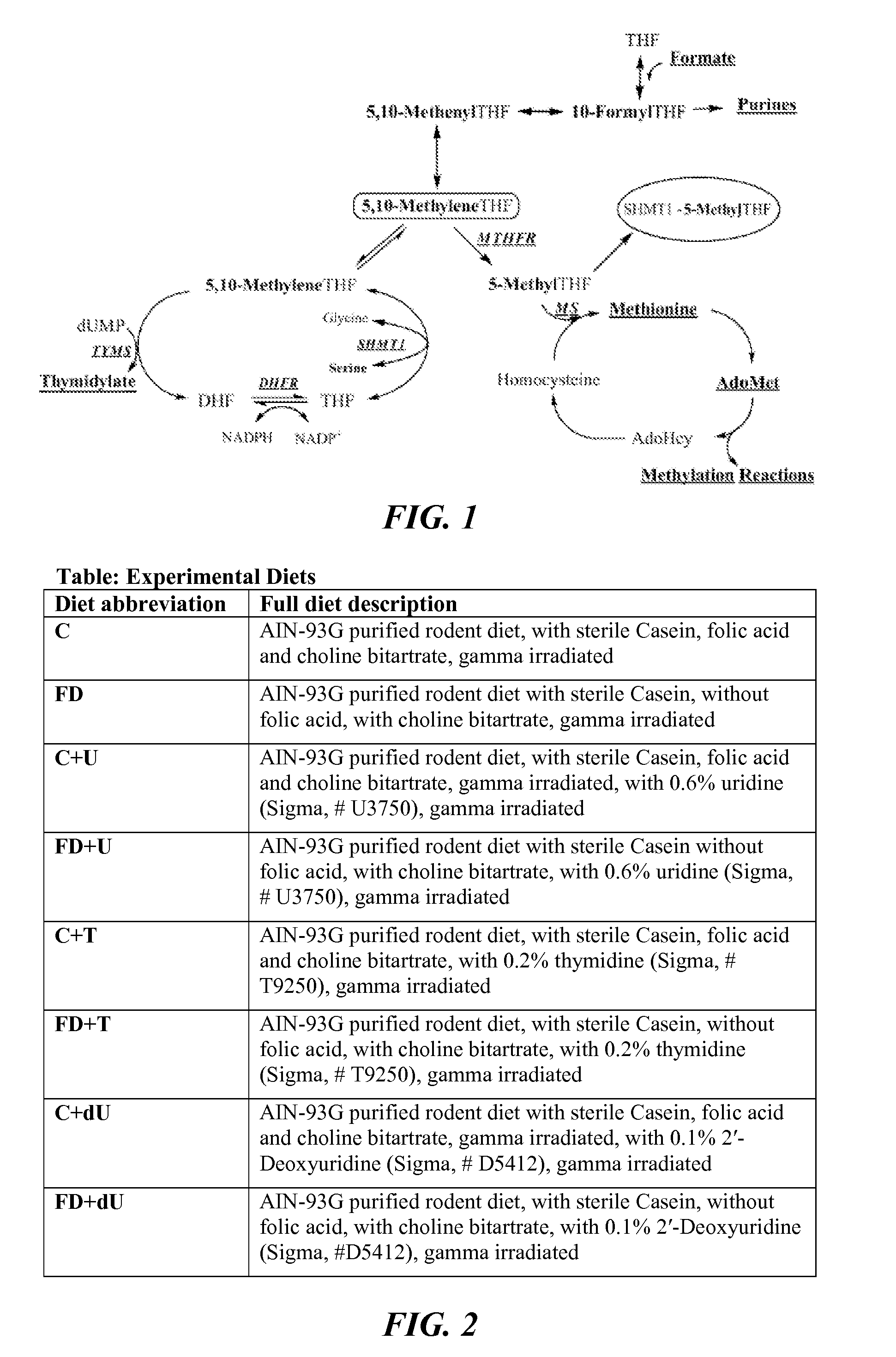
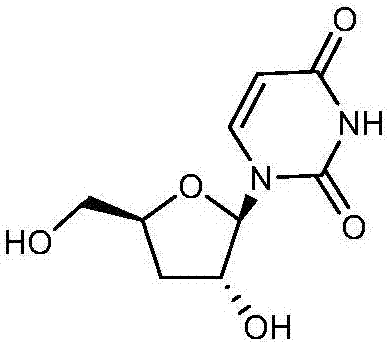
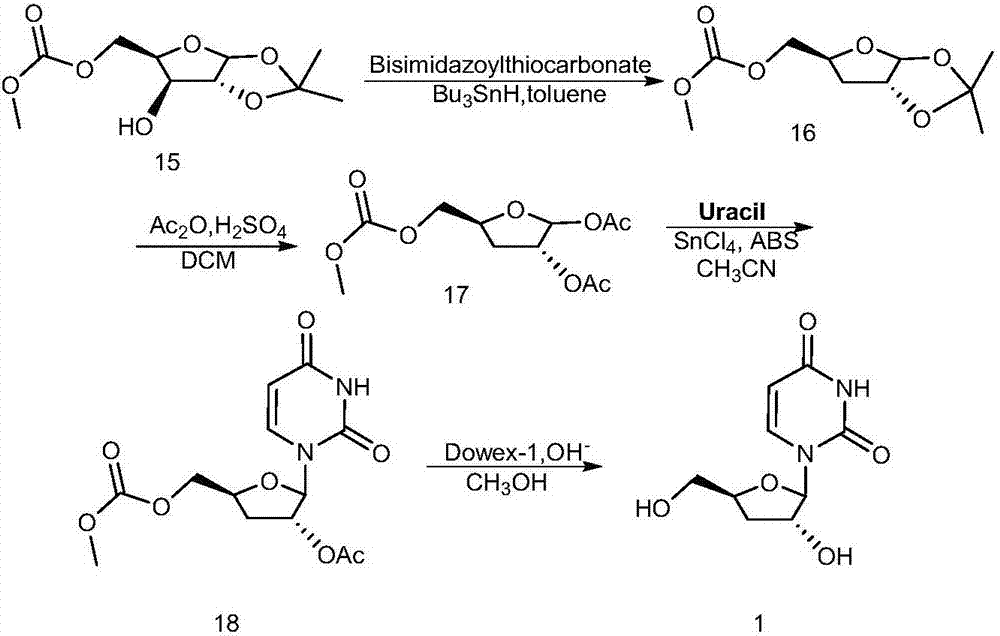
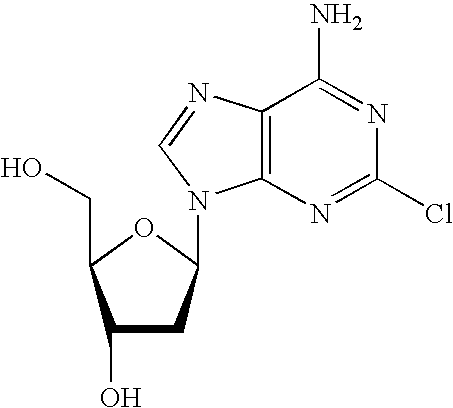
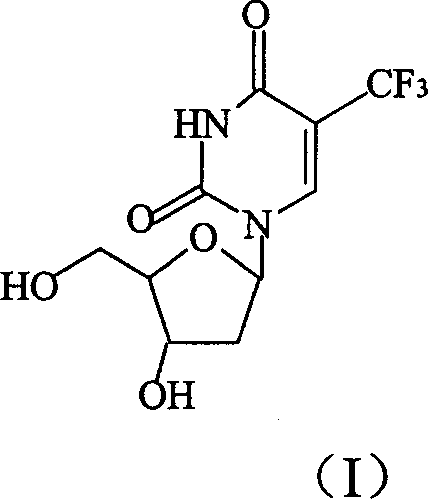
Deoxyuridine (dU) is a compound and a nucleoside. It is similar in chemical structure to uridine, but without the 2'-hydroxyl group. Idoxuridine and Trifluridine are variants of deoxyuridine used as antiviral drugs. They are similar enough to be incorporated as part of DNA replication, but they possess side groups on the uracil component (an iodine and a CF₃ group, respectively), that prevent base pairing.
Novel uracil compound or salt thereof having human deoxyuridine triphosphatase inhibitory activity
ActiveUS20110082163A1Potent human dUTPase inhibitory activityAntibacterial agentsBiocideDeoxyuridinePharmaceutical Substances
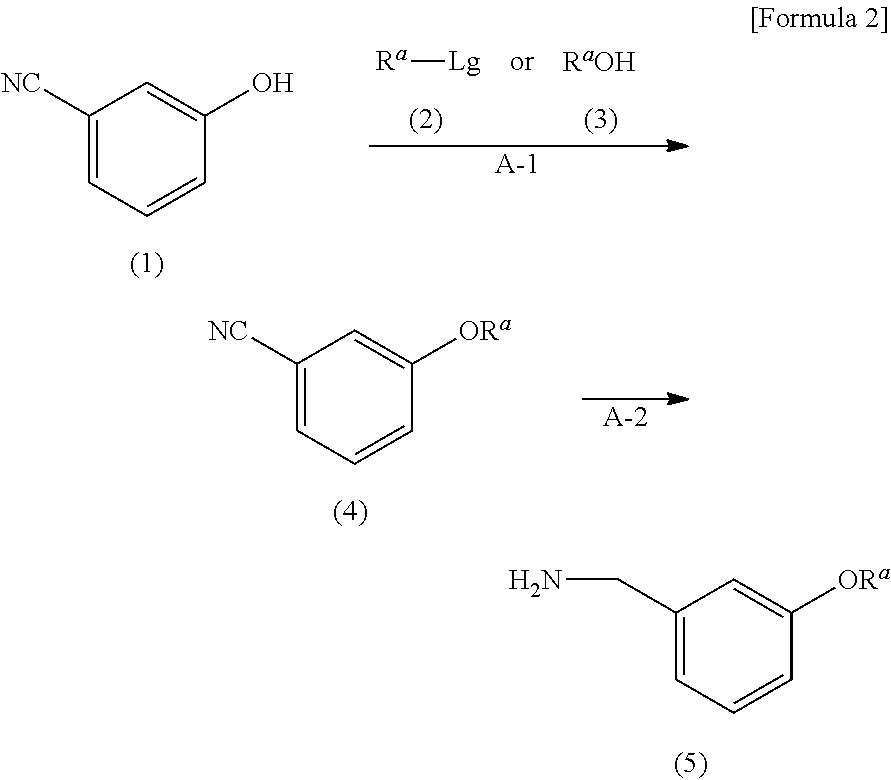
Provided is a uracil compound or a salt thereof, which has potent human dUTPase inhibitory activity and is useful as, for example, an antitumor drug.A uracil compound represented by the general formula (I) or a salt thereof:wherein n represents an integer of 1 to 3; X represents a bond, an oxygen atom, a sulfur atom, or the like; Y represents a linear or branched alkylene group having 1 to 8 carbon atoms, or the like; and Z represents —SO2NR1R2 or —NR3SO2—R4, wherein R1 and R2 each represent an alkyl group having 1 to 6 carbon atoms, an aralkyl group which is optionally substituted, or the like; R3 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R4 represents an aromatic hydrocarbon group, an unsaturated heterocyclic group, or the like.
Owner:TAIHO PHARMA CO LTD
Uracil compound or salt thereof having human deoxyuridine triphosphatase inhibitory activity
ActiveUS8530490B2Potent human dUTPase inhibitory activityAntibacterial agentsOrganic active ingredientsDeoxyuridinePharmaceutical Substances
Provided is a uracil compound or a salt thereof, which has potent human dUTPase inhibitory activity and is useful as, for example, an antitumor drug.A uracil compound represented by the general formula (I) or a salt thereof:wherein n represents an integer of 1 to 3; X represents a bond, an oxygen atom, a sulfur atom, or the like; Y represents a linear or branched alkylene group having 1 to 8 carbon atoms, or the like; and Z represents —SO2NR1R2 or —NR3SO2—R4, wherein R1 and R2 each represent an alkyl group having 1 to 6 carbon atoms, an aralkyl group which is optionally substituted, or the like; R3 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R4 represents an aromatic hydrocarbon group, an unsaturated heterocyclic group, or the like.
Owner:TAIHO PHARMA CO LTD
Novel uracil compound having inhibitory activity on human deoxyuridine triphosphatase or salt thereof
ActiveCN102056905AExcellent human dUTPase inhibitory activityAntibacterial agentsOrganic active ingredientsUracilChemical compound
Disclosed is an uracil compound which has an excellent inhibitory activity on human dUTPase and is useful as an anti-tumor agent or the like or a salt of the uracil compound. Specifically disclosed is an uracil compound represented by general formula (I) or a salt thereof. [In general formula (I), n represents a number of 1 to 3; X represents a bond, an oxygen atom, a sulfur atom, or the like; Y represents a linear or branched alkylene group having 1 to 8 carbon atoms, or the like; Z represents -SO2NR1R2 or -NR3SO2-R4; R1 and R2 independently represent an alkyl group having 1 to 6 carbon atoms, an aralkyl group which may have a substituent, or the like; R3 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R4 represents an aromatic hydrocarbon group, an unsaturated heterocyclic group, or the like].
Owner:TAIHO PHARMA CO LTD
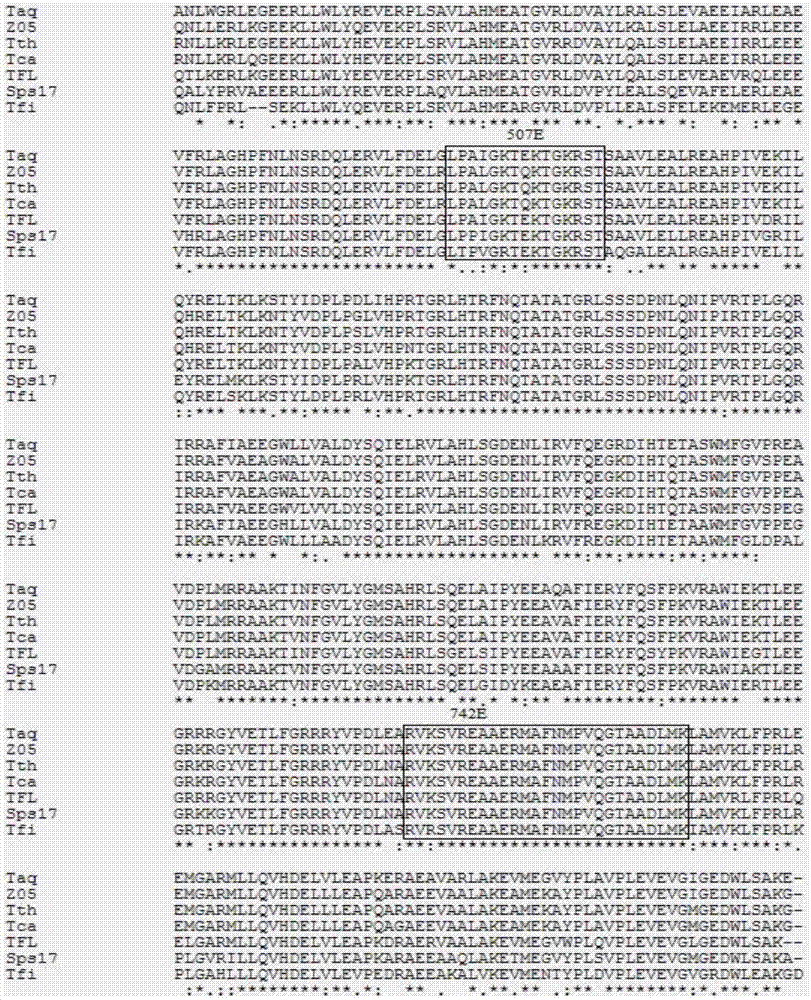
Mutant A type DNA (deoxyribonucleic acid) polymerase, and encoding gene and application of mutant A type DNA polymerase
ActiveCN107299091AIncorporation efficiency increasedNo loss of amplification efficiencyTransferasesFermentationBiotechnologyPolymerase L
The invention relates to the field of molecular biology and discloses mutant A type DNA (deoxyribonucleic acid) polymerase, and an encoding gene and an application of the mutant A type DNA polymerase. The mutant A type DNA polymerase is generated by amino acid site mutation of conservational motif of A type DNA polymerase in a dNTP (deoxyribonucleoside triphosphate) bond zone. Compared with the A type DNA polymerase not modified or mutated, the mutant A type DNA polymerase has increased dUTP (deoxyuridine triphosphokinase) doping speed; a dUTP doping effect of the mutant A type DNA polymerase is obviously better than control A type DNA polymerase; therefore, the mutant A type DNA polymerase is more applicable to some nucleic acid amplification systems using dUTP to substitute dTTP (deoxy- thymidine triphosphate) and allows the system to avoid nucleic acid amplification product contamination and not to lose amplification efficiency of a target product at the same time; and the mutant A type DNA polymerase meets application requirements of multiple PCR (polymerase chain reaction) fields of food, animal quarantine, human disease screening and the like, a forensic medicine field and a scientific research.
Owner:SUZHOU NUHIGH BIOTECH
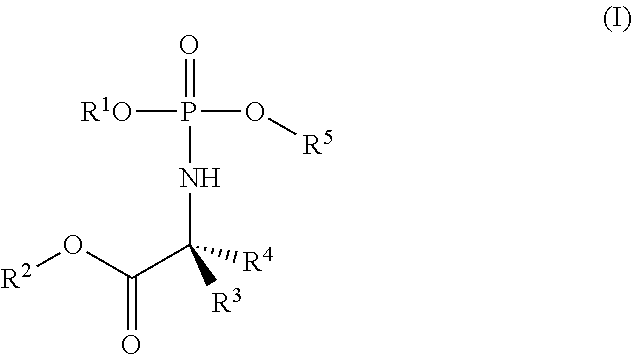
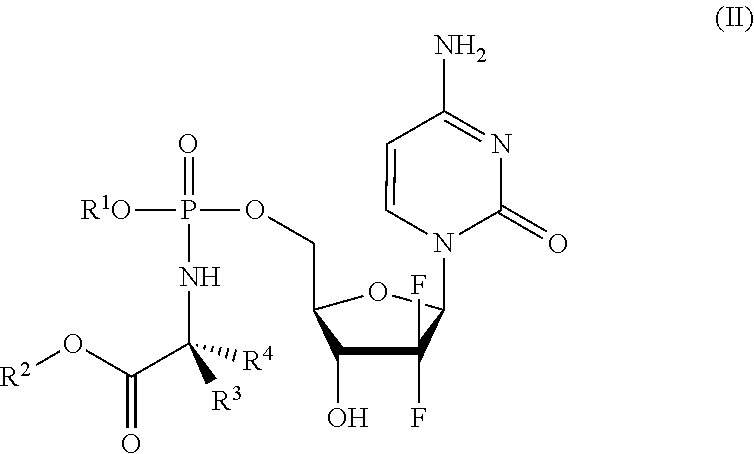
Formulations of phosphoramidate derivatives of nucleoside drugs
InactiveUS20180369266A1Mitigates risk of precipitationDirect contact guaranteeOrganic active ingredientsPharmaceutical delivery mechanismPhosphateDeoxyuridine
This invention relates to pharmaceutical formulations and formulation strategies of protides (phosphoramidate derivatives of nucleosides) and, in particular, protides useful in the treatment of cancer, such as NUC-3373 (5-fluoro-2′-deoxyuridine-5′-O-[1-naphthyl (benzoxy-L-alaninyl)] phosphate) and NUC-7738 (3′-deoxyadenosine-5′-O-[phenyl(benzyloxy-L-alaninyl)] phosphate). In particular, the invention relates to formulations that comprise a polar aprotic solvent, for example, dimethyl acetamide (DMA).
Owner:NUCANA PLC
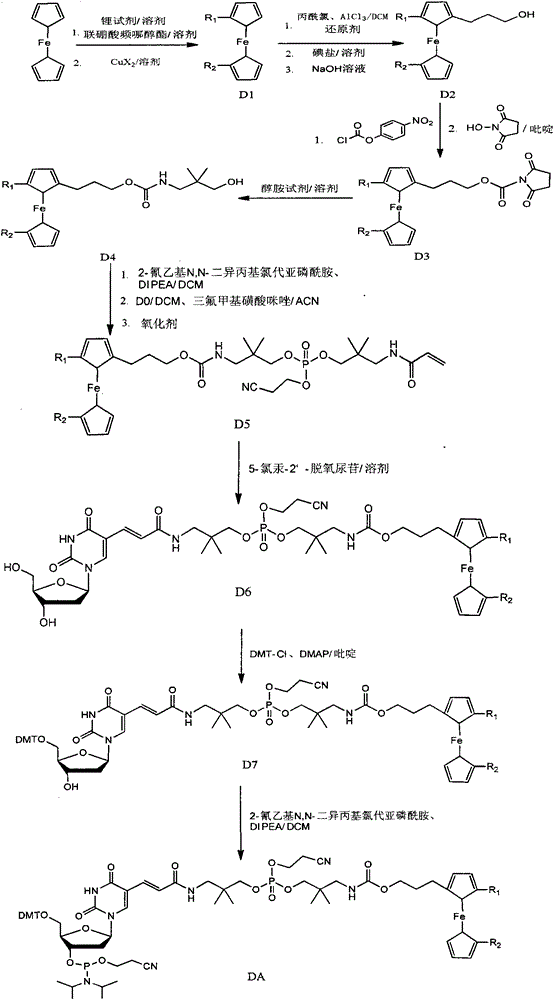
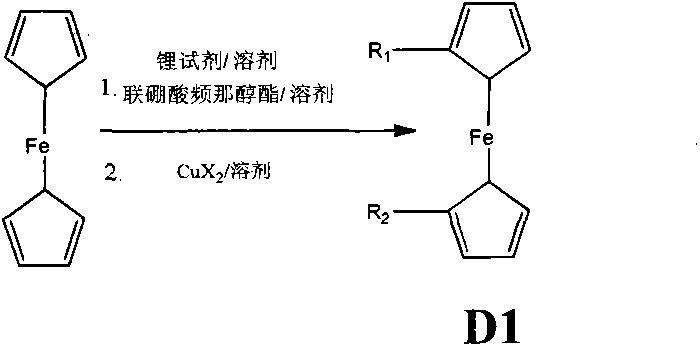
Synthetic method of ferrocene derivatives
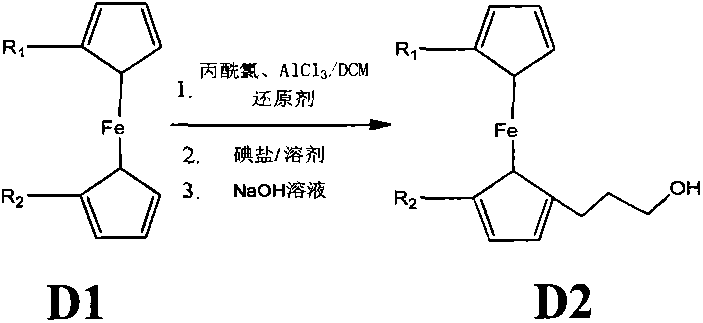
The invention relates to a synthetic method of ferrocene derivatives. Ferrocene is used as a raw material for the ferrocene derivatives. The ferrocene derivatives which can be used for labeling special nucleotides are prepared by steps of: treating the ferrocene with a lithium reagent and bis(pinacolato)diboron to obtain a halogenated ferrocene; performing Friedel-Crafts acylation, Clemmensen reduction and hydrolysis under an alkaline condition to obtain a halogenated ferrocene alkyl alcohol; reacting with nitrophenyl chloroformate and N-hydroxy succinimide to obtain ferrocene succinimide carbonate; condensing with an alcohol amine reagent to obtain a ferrocene alkylolamide carbonate; performing substitution, oxidation, and other reactions by reacting with a phosphoramidite reagent to obtain a phosphorous ferrocene reagent; reacting with 5-chloromercuri-2'-deoxyuridine to obtain a deoxyuridine phosphorous ferrocene reagent; modifying the 5' end of the deoxyuridine through DMT-C1 to obtain a DMT-modified deoxyuridine phosphorous ferrocene reagent; and finally performing substitution by reacting with the phosphoramidite reagent. The method has operation safety, convenience, high product purity and high yield. The method has a wider application scope than other synthetic methods.
Owner:DAAN GENE CO LTD
Biocatalytic synthesis of aminodeoxy purine N9-beta-D-nucleosides containing 3-amino-3-deoxy-beta-D-ribofuranose, 3-amino-2,3-dideoxy-beta-D-ribofuranose, and 2-amino-2-deoxy-beta-D-ribofuranose as sugar moieties
Purine N9-β-D-nucleosides containing 3-amino-3-deoxy-β-D-ribofaranose, 3-amino-2,3-dideoxy-β-D-ribofuranose, and 2-amino-2-deoxy-β-D-ribofuranose as sugar moieties are synthesized by biocatalytic transglycosylation of purine bases and the respective 3′-amino-3′-deoxyuridine, 3′-amino-3′-deoxythymidine and 2′-amino-2′-deoxyuridine as donors of the carbohydrate moiety, and the cells of Escherichia coli as a biocatalyst or glutaraldehyde (GA) treated cells of Escherichia coli as a biocatalyst or a mixture of thymidine (uridine) phosphorylase and purine nucleoside phosphorylase.
Owner:METKINEN
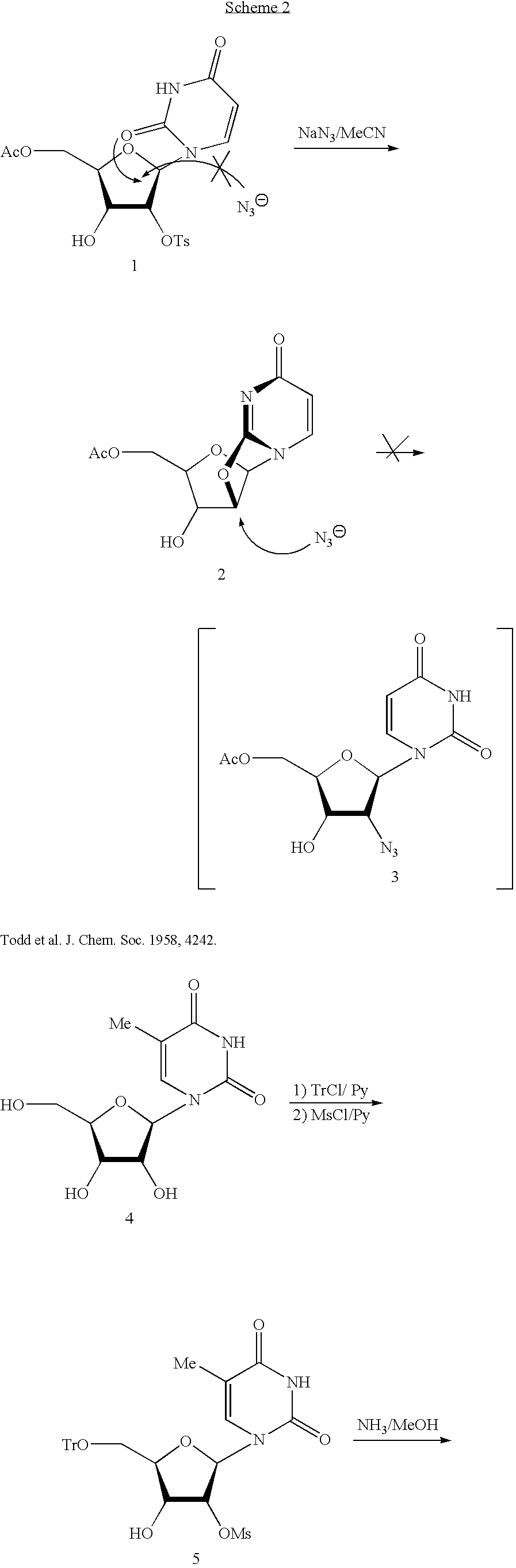
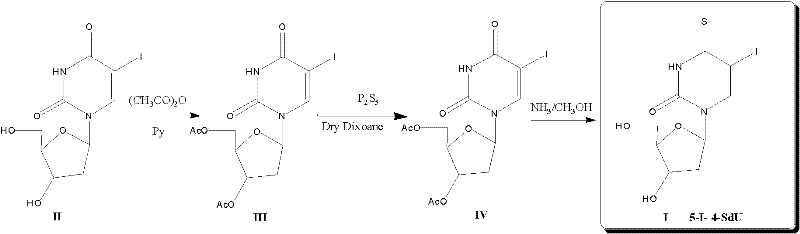
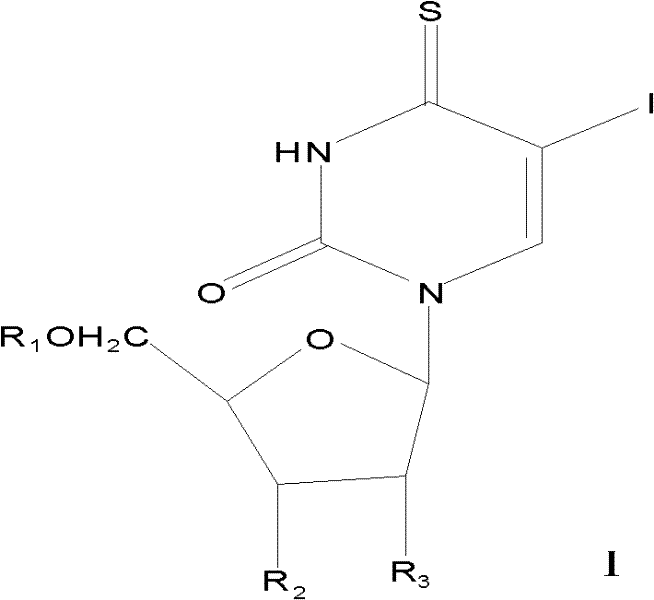
5-iodine-4-sulfur-2'-deoxyuridine, and derivatives and synthetic method thereof
ActiveCN102675389AIncreased sensitivityOvercoming the inability to act selectively on cancer cellsSugar derivativesSugar derivatives preparationPhosphorus pentasulfideAcetic anhydride
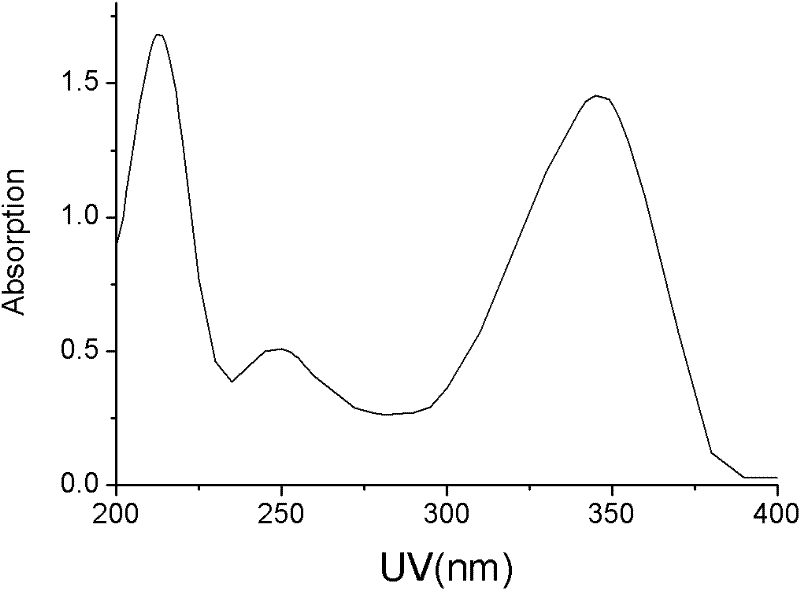
The invention discloses 5-iodine-4-sulfur-2'-deoxyuridine, and derivatives and a synthetic method of the 5-iodine-4-sulfur-2'-deoxyuridine. The synthetic method comprises the steps of using 5-iodine-2'-deoxyuridine as main raw material, treating by using acetic anhydride under a certain conditions to obtain 3', 5'-O-dioxo acetyl-5-iodine-2'-deoxyuridine, reacting under the action of phosphorus pentasulfide, then, introducing ammonia to saturated methanol solution, stirring and deprotecting at the room temperature to obtain 5-iodine-4-sulfur-2'-deoxyuridine. According to the invention, the compound and the derivatives have greatest absorption with the ultraviolet spectrum at 345 nm and stronger UVA photosensitivity. Therefore, the compound provided by the invention is a new basic base sulfur-containing nucleosides compound, can alternatively acts on cancer cells and has potential medical value; besides, the synthetic method provided by the invention has the advantages of simple reaction conditions, accessible raw material, low cost, high product yield, high purity and high efficiency.
Owner:DALIAN UNIV
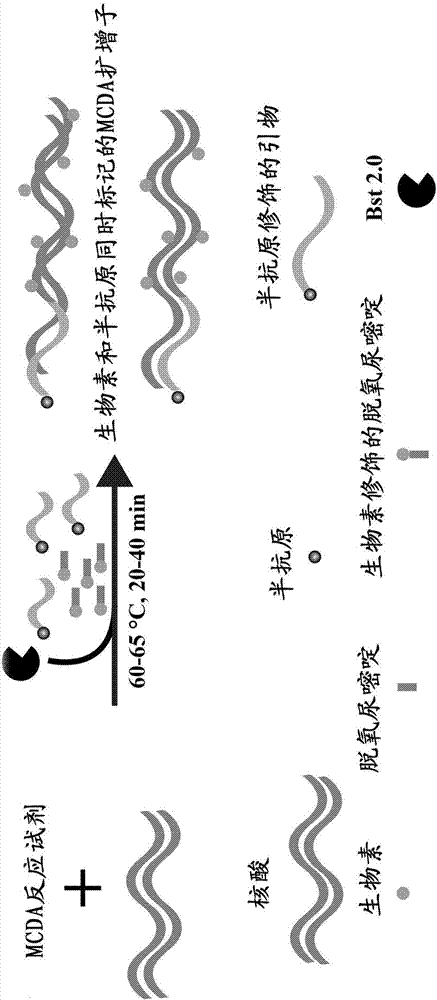
AUDG (antarctic thermal sensitive uracil deoxyribonucleic acid glycosylase) mediated multiple cross displacement amplification and biosensing combined nucleic acid testing technique
ActiveCN107164541AEliminate effectiveStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAntigenNano biosensor
The invention discloses a multiple cross isothermal amplification and macromolecular nano biosensing combined target gene testing method. According to the method, a half antigen is marked at a 5' terminal of a cross primer CP1 or CP2 in multiple cross displacement amplification, and antarctic thermal sensitive uracil deoxyribonucleic acid glycosylase and biotinylated deoxyuridine are introduced into an amplification system to test amplification products on the basis of multiple cross displacement amplification combined with macromolecular nano biosensing. According to the method, amplification products of HPV16 type E7 gene or HPV18 type L1 gene can be visually tested by a macromolecular nano biosensor. The method is convenient, fast, sensitive, specific and suitable for testing of various nucleotide fragments.
Owner:ICDC CHINA CDC
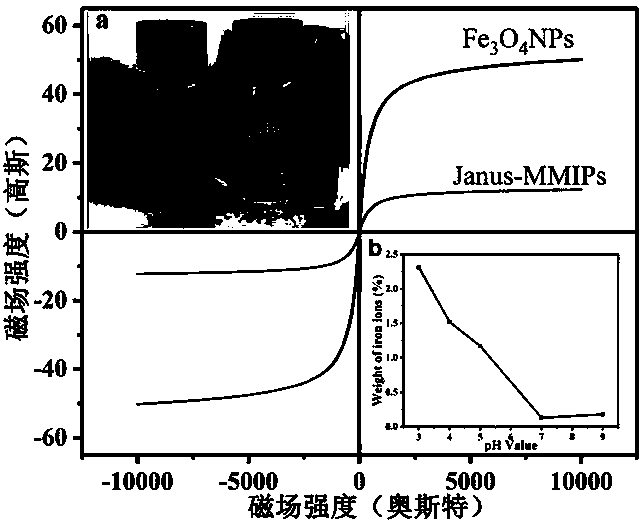
Janus magnetic imprinted nanosheet, preparation method and application thereof
ActiveCN109718745AImprove specific recognition abilityLarge and fast separationOther chemical processesAlkali metal oxides/hydroxidesFunctional monomerMicrosphere
Belonging to the technical field of functional materials, the invention relates to a Janus magnetic molecularly imprinted nanosheet, a preparation method and application thereof. The method includes:preparing Janus hollow microspheres by sol-gel method, and conducting ultrasonic breaking to obtain a Janus nanosheet containing amino on one side and containing chlorine element on the other side; then taking surface chlorine element as the initiator of atom transfer radical polymerization, adopting dA as the template molecule, using 5-(2-methoxy vinyl)-2'-deoxyuridine as the functional monomer,and grafting a dA molecularly imprinted polymer to the hydrophobic surface of the Janus nanosheet; and then combining oleic acid coated Fe3O4 particles to the hydrophobic surface of Janus through thebonding effect between amino and carboxyl to prepare a Janus magnetic molecularly imprinted nanosheet adsorbent, and applying the obtained material to adsorption and separation of dA. The imprinted adsorption material constructed by the invention has the advantages of good selectivity, large adsorptive capacity to target dA and rapid separation.
Owner:江苏艾科姆检测有限公司
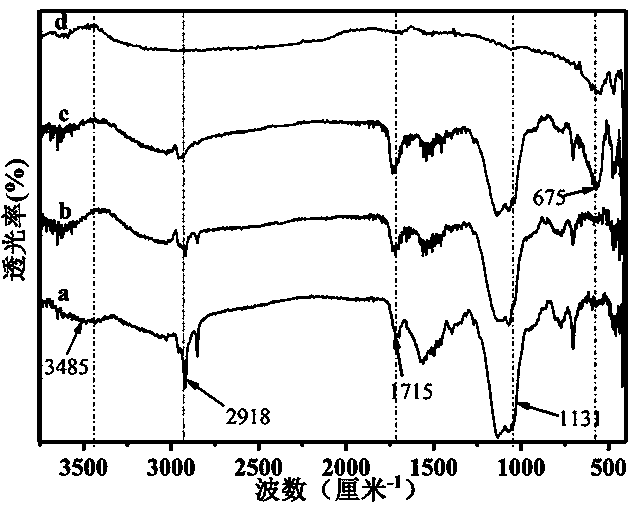
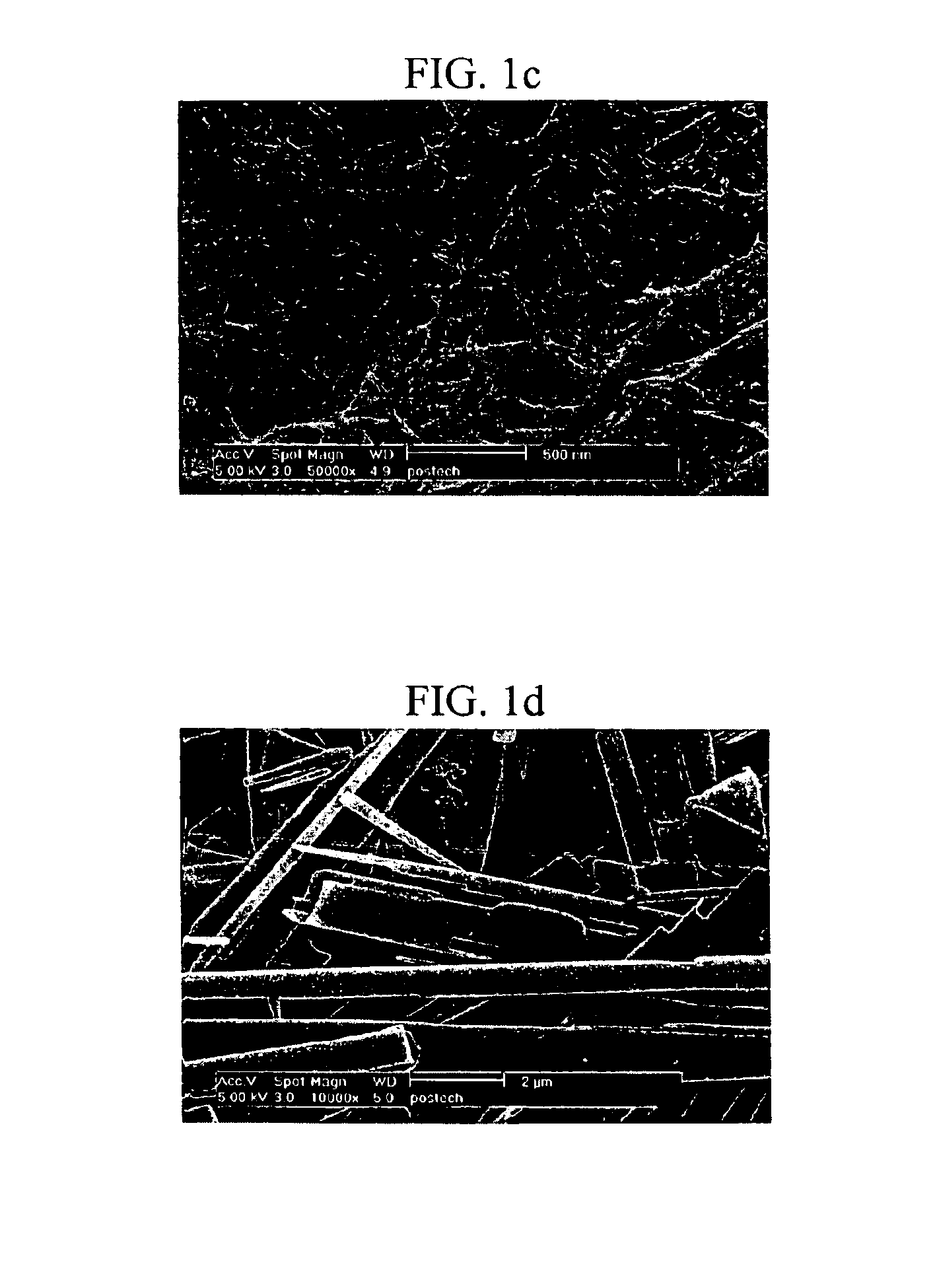
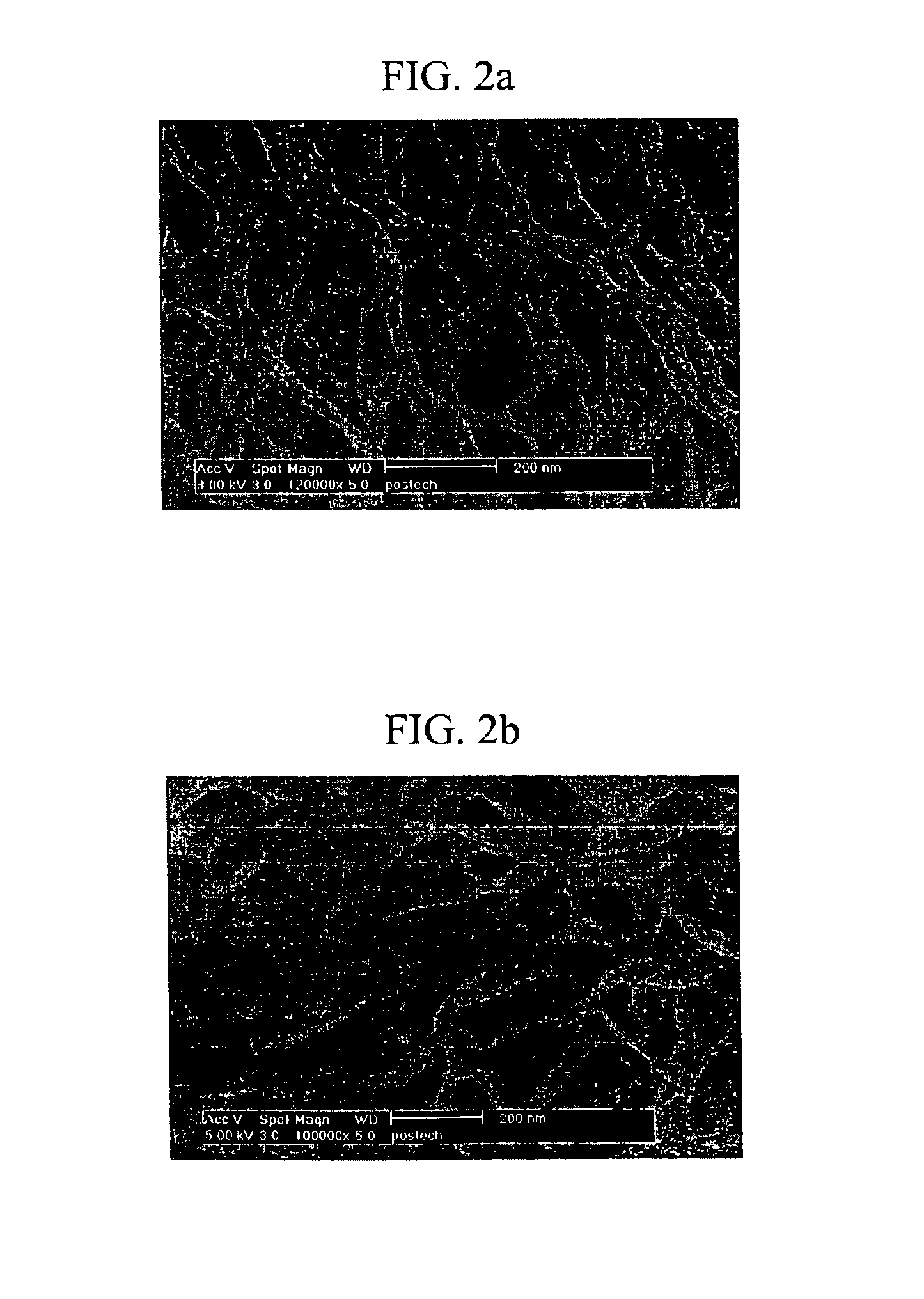
2'-deoxyuridine derivatives and hydrogels formed therewith
InactiveUS6884882B1Reduce molecular weightImprove thermal stabilityOrganic active ingredientsSugar derivativesDeoxyuridineThermal stability
A 2′-deoxyuridine derivative of formula I is bioavailable and thermally stable and forms a gel in water at a low concentration; and, can be employed as a drug delivery vehicle:
Owner:POSTECH ACAD IND FOUND
Activity-based assay for ricin-like toxins
InactiveUS20060057596A1Sensitive highEasy to adaptSugar derivativesMicrobiological testing/measurementPositive controlRicin
A method of detecting N-glycosylase activity in a sample involves incubating an oligodeoxyribonucleotide substrate containing a deoxyadenosine or deoxyuridine residue with the sample to be tested such that the N-glycosylase, if present, hydrolyzes the deoxyadenosine or deoxyuridine residue to result in an N-glycosylase product having an abasic site. A primer is annealed to the N-glycosylase product, and the primer is extended with a DNA polymerase, such as Taq DNA polymerase, that pauses at abasic sites. The resulting extension products are melted from the N-glycosylase product, allowed to form hairpins due to self-complementarity, and further extended in the presence of labeled precursors to result in labeled products. Extension products synthesized from undigested substrate as template do not result in labeled products. Thus, detection of labeled products results in detection of N-glycosylase activity. Oligodeoxyribonucleotide substrates, primer, and positive controls and a kit for N-glycosylase assay are also disclosed.
Owner:BATTELLE ENERGY ALLIANCE LLC
Method for preparing 2'-deoxyuridine by chemical-biological enzyme method in combination
ActiveCN102827902AHigh yieldImprove conversion rateChemical recyclingFermentationUracil2-deoxy-alpha-D-ribose 1-phosphate
The invention provides a method for preparing 2'-deoxyuridine by a chemical-biological enzyme method in combination. A 'crystallization induction asymmetric transformation' technology is adopted to synthetize and obtain single alpha-configuration nucleoside analogues medicine intermediate 2-deoxy-alpha-D-ribose-1-phosphate; the intermediate is utilized as a substrate; uracil is added, and 2'-deoxyuridine is synthetized and obtained by biotransformation under the action of uridine phosphorylase. By applying the chemical-biological combination technology disclosed by the invention, the advantages of a chemical method and a biological method are combined; the defects of the chemical method and the biological method are avoided; the yield is high; the method is suitable for industrial production; enough supply can be obtained by the 'crystallization induction asymmetric transformation' technology; 2-deoxy-alpha-D-ribose-1-phosphate intermediate with single configuration does not need to be separated and purified; the biotransformation process is finished by catalysis with efficient and specific uridine phosphorylase in one step; the specificity is strong; the conversion rate is high; the condition is mild and the environment is friendly.
Owner:ZHEJIANG UNIV OF TECH
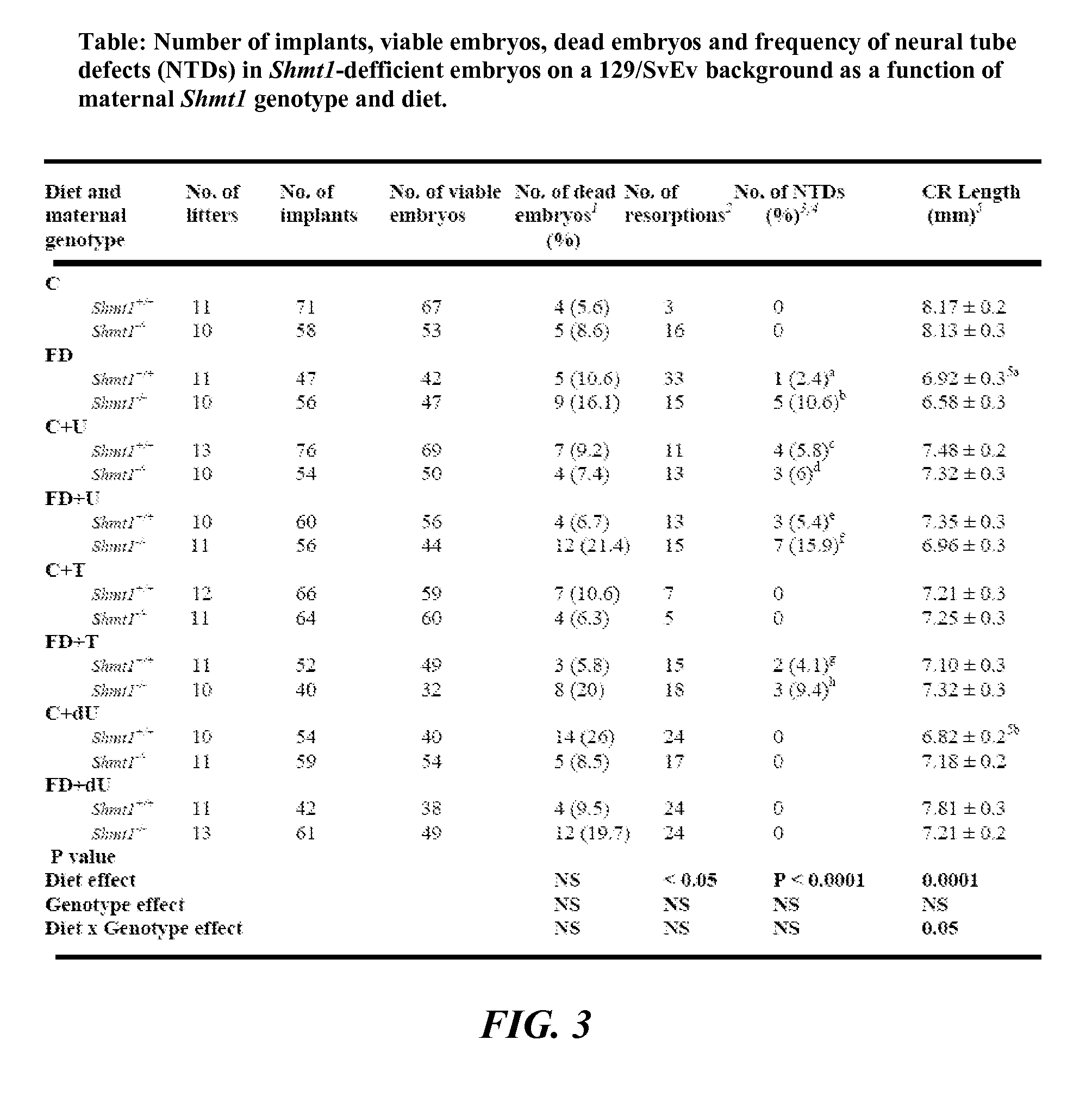
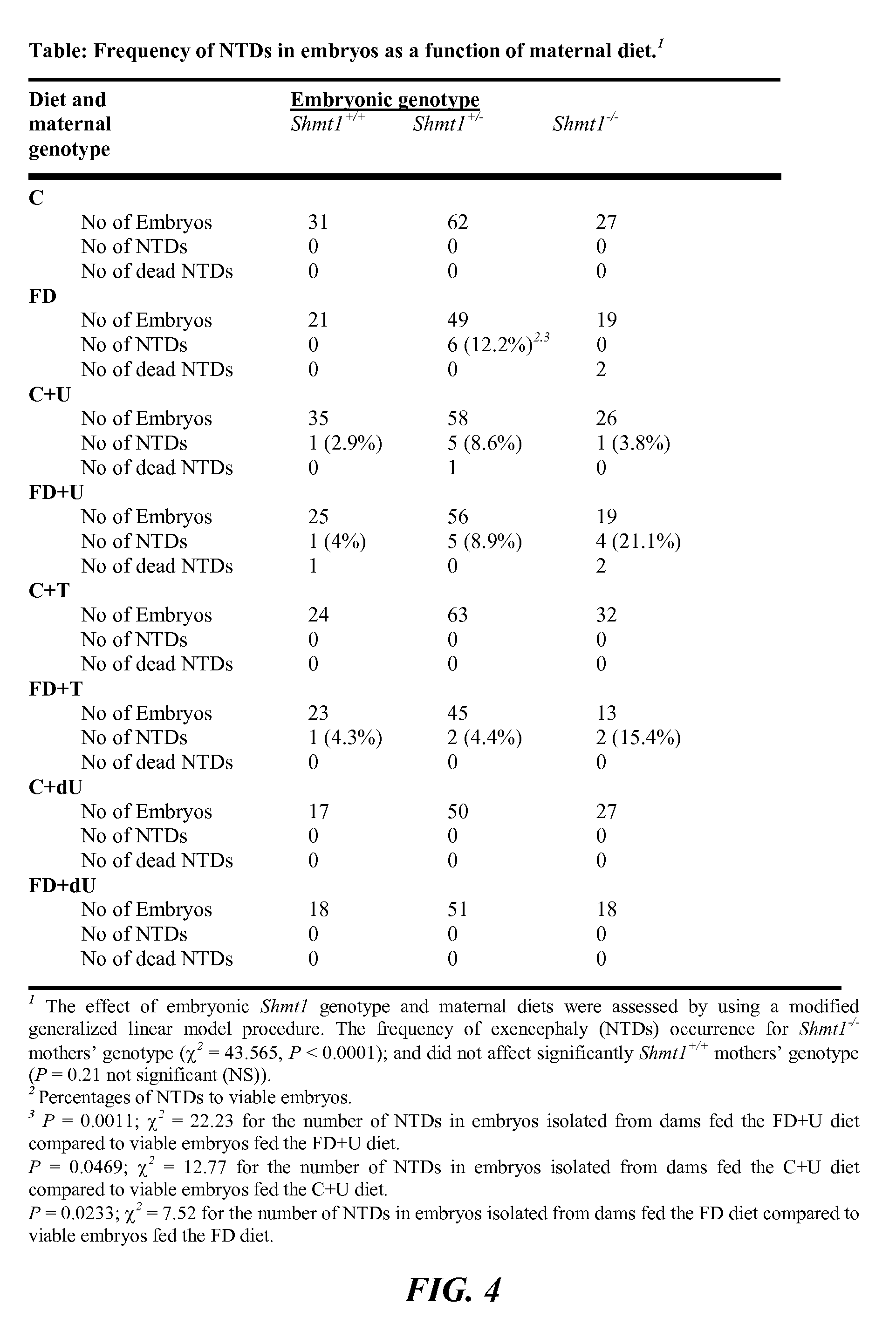
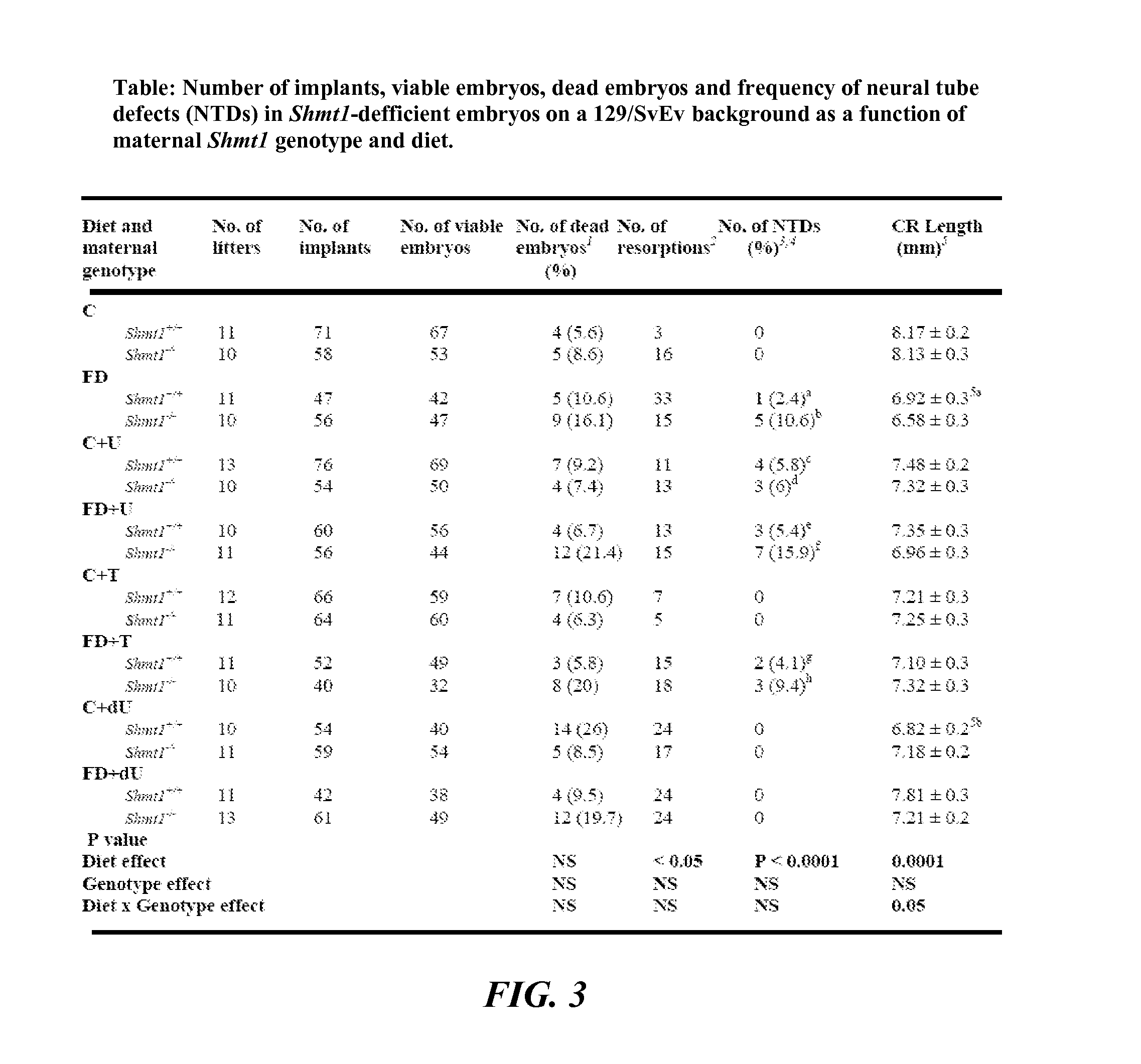
Use of uridine and deoxyuridine to treat folate-responsive pathologies
ActiveUS9579337B2Increased riskReduce the numberOrganic active ingredientsAntineoplastic agentsMedicineDietary supplement
The present invention relates to a pharmaceutical or dietary composition comprising deoxyuridine and a pharmaceutically or dietetically suitable carrier. Another aspect of the present invention relates to a method of supplementing the dietary needs of a subject. This method includes administering to the subject a dietary supplementing effective amount of deoxyuridine. Yet another aspect of the present invention relates to a method of treating cancer in a subject. This method includes selecting a subject having cancer and administering to the selected subject a therapeutically effective amount of uridine, thereby treating the cancer in the selected subject.
Owner:CORNELL UNIVERSITY
Fluorescence detection method of five-position aldehyde-group deoxidizing uridine
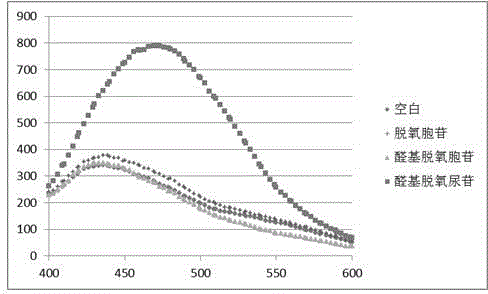
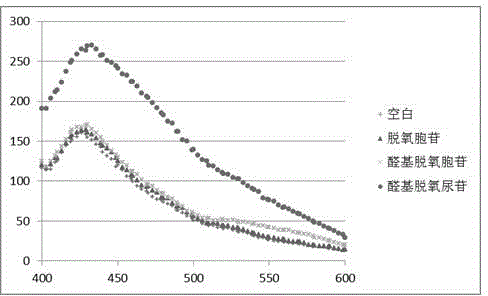
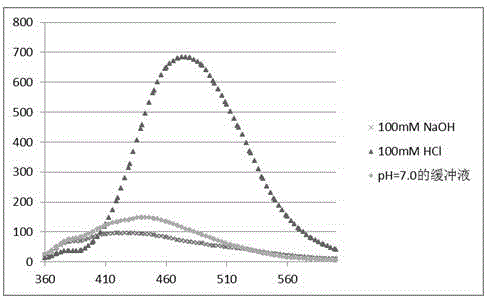
The invention relates to a chemical method for detecting how deoxythymidine is oxidized and mutated into five-position aldehyde-group deoxidizing uridine, which includes: using 4-methoxy group o-phenylenediamine or o-phenylenediamine as a detection reagent, using dithiothreitol as an antioxidant reagent, and dissolving the detection reagent and the antioxidant reagent into 10mM of acetic acid buffer liquid with the potential of hydrogen (pH) of 4.5 to form a detection sample; and leading the detection sample to react for 3 hours under aerobic room temperature condition and using a fluorophotometric detector to detect a blank sample and the detection sample, wherein through the detection, users can find out that when the 4-methoxy group o-phenylenediamine is used as the detection reagent, the peak value shifts to 475nm, and when the o-phenylenediamine is used as the detection reagent, the peak value shifts to 430nm. The fluorescence detection method generates compounds capable of generating fluorescence by using the 4-methoxy group o-phenylenediamine or the o-phenylenediamine and 5-aldehyde group deoxyuridine which are easy to achieve and are arranged in acetic acid buffer liquid so that existence of oxidation and mutation of the deoxythymidine can be detected in fluorescence mode.
Owner:WUHAN UNIV
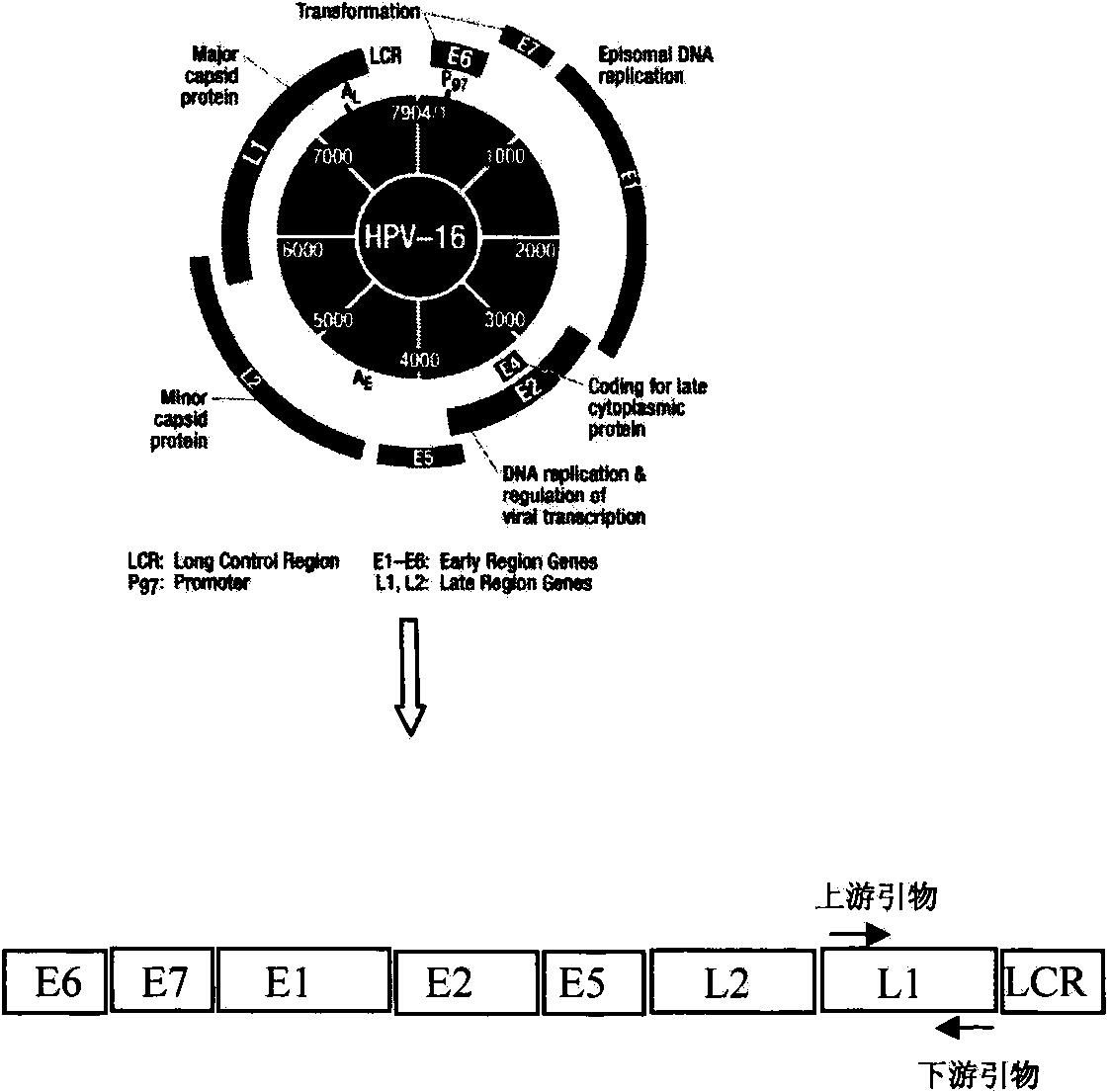
Broad-spectrum, efficient and economical PCR (Polymerase Chain Reaction) detection method for high-risk human papilloma virus
InactiveCN103409560AMicrobiological testing/measurementDeoxyuridine TriphosphatePolymerase chain reaction
The invention discloses a broad-spectrum, efficient and economical PCR (Polymerase Chain Reaction) detection method for high-risk human papilloma virus. The detection method comprises the following steps of (1) designing primers aiming at 12 high-risk HPV (human papilloma virus) subtype L1 genes, wherein the melt points of the all genes are 60 DEG C, and the gas chromatography (GC) percents are 50%, so that the primers can amplify virus gene sequences in the same PCR tube under the same temperature, so as to save time and money; (2) taking glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a positive contrast, so as to avoid a false negative result; (3) avoiding a false positive result by utilizing an anti-pollution warm-start PCR system containing deoxyuridine triphosphate (dUTP) and uracil-N-glycosylase (UNG). According to the detection method, a PCR system is optimized based on the embodiment, and the specificity and the accuracy of a result are guaranteed, and according to the detection method, time saving and economy are realized, efficient and low-cost screening work is carried out on HPV viruses easily by a base hygiene department, and a purpose of preventing the cervical cancer is achieved.
Owner:潘晓静
Decoy compositions for treating and preventing brain diseases and disorders
InactiveUS20080207552A1Inhibit expressionAvoid damageSsRNA viruses negative-senseOrganic active ingredientsDiseaseStaining
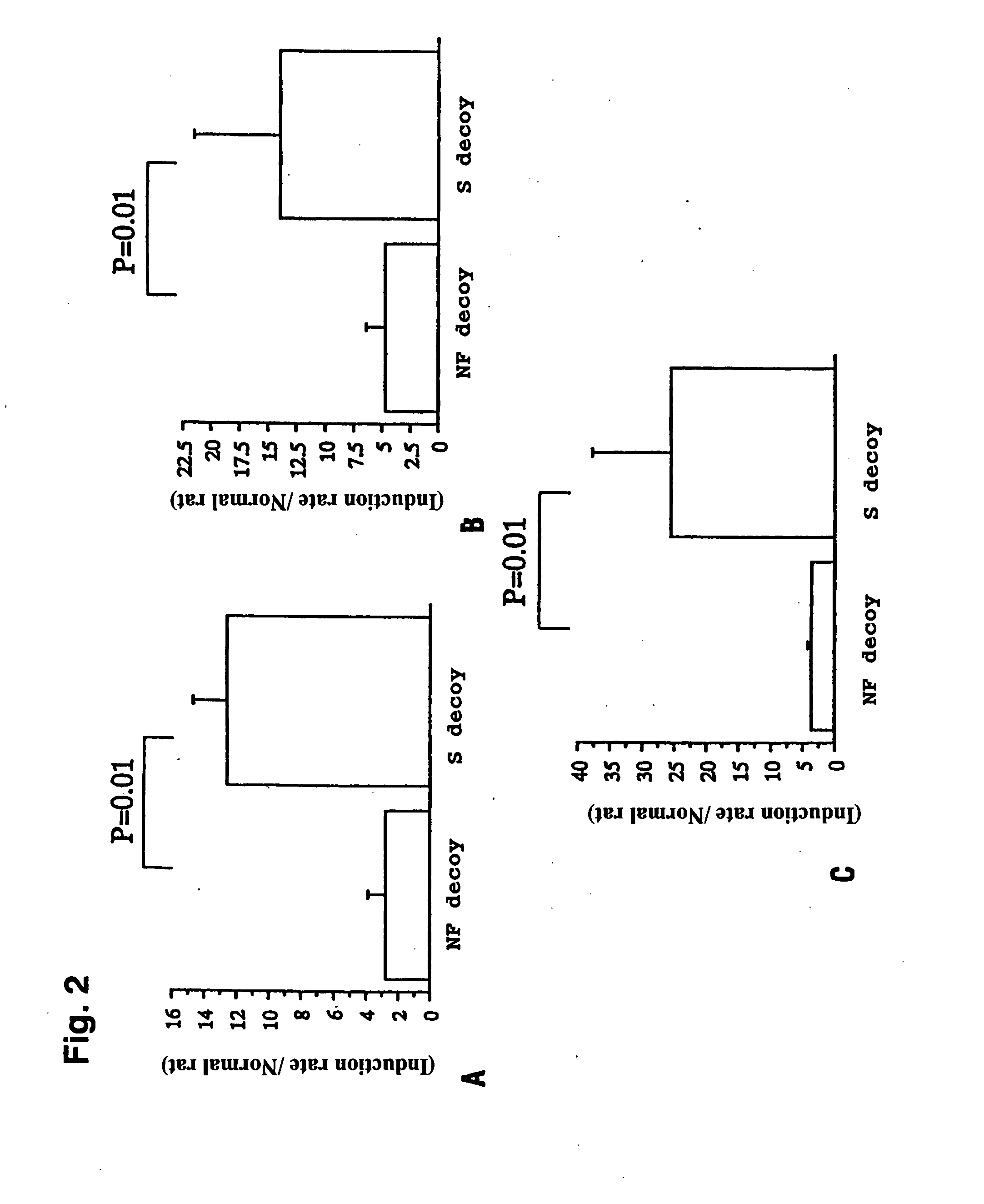
The present invention provides introduction of NF-κB decoy oligodeoxynucleotide into rat cranial nerve through a carotid artery during global brain ischemia. Polymerase chain reaction demonstrated that one hour after global brain ischemia, transfected NF-κB decoy oligodeoxynucleotide effectively suppressed expression of tumor necrosis factor α, interleukin 1β and intracellular adhesion molecule 1 messenger RNAs. Terminal deoxynucleotidyl transferase-mediated deoxyuridine nick-end labeling staining and immunohistochemistry using microtubule-associated protein 2 demonstrated that transfected NF-κB decoy oligodeoxynucleotide significantly attenuated neuronal damage seven days after global brain ischemia. Therapeutic transfection of NF-κB decoy oligodeoxynucleotide during brain ischemia may be effective for attenuation of neuronal damage, suggesting a strategy for protecting the cerebrum from global ischemia.
Owner:ANGES MG INC
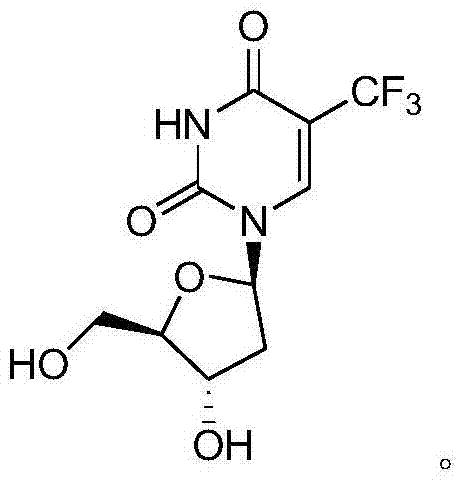
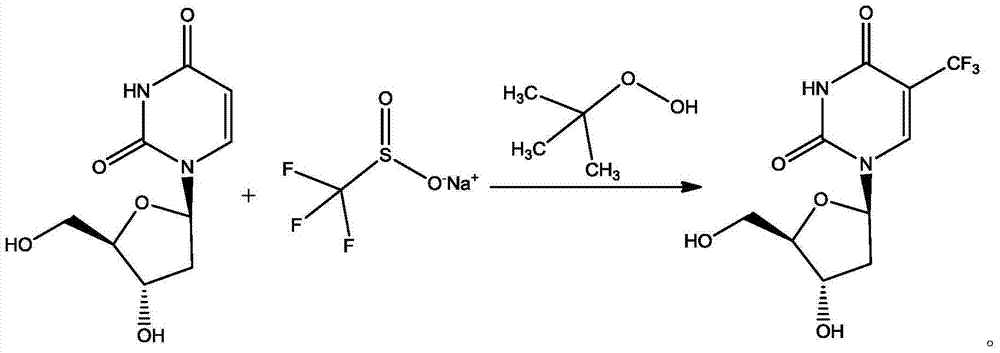
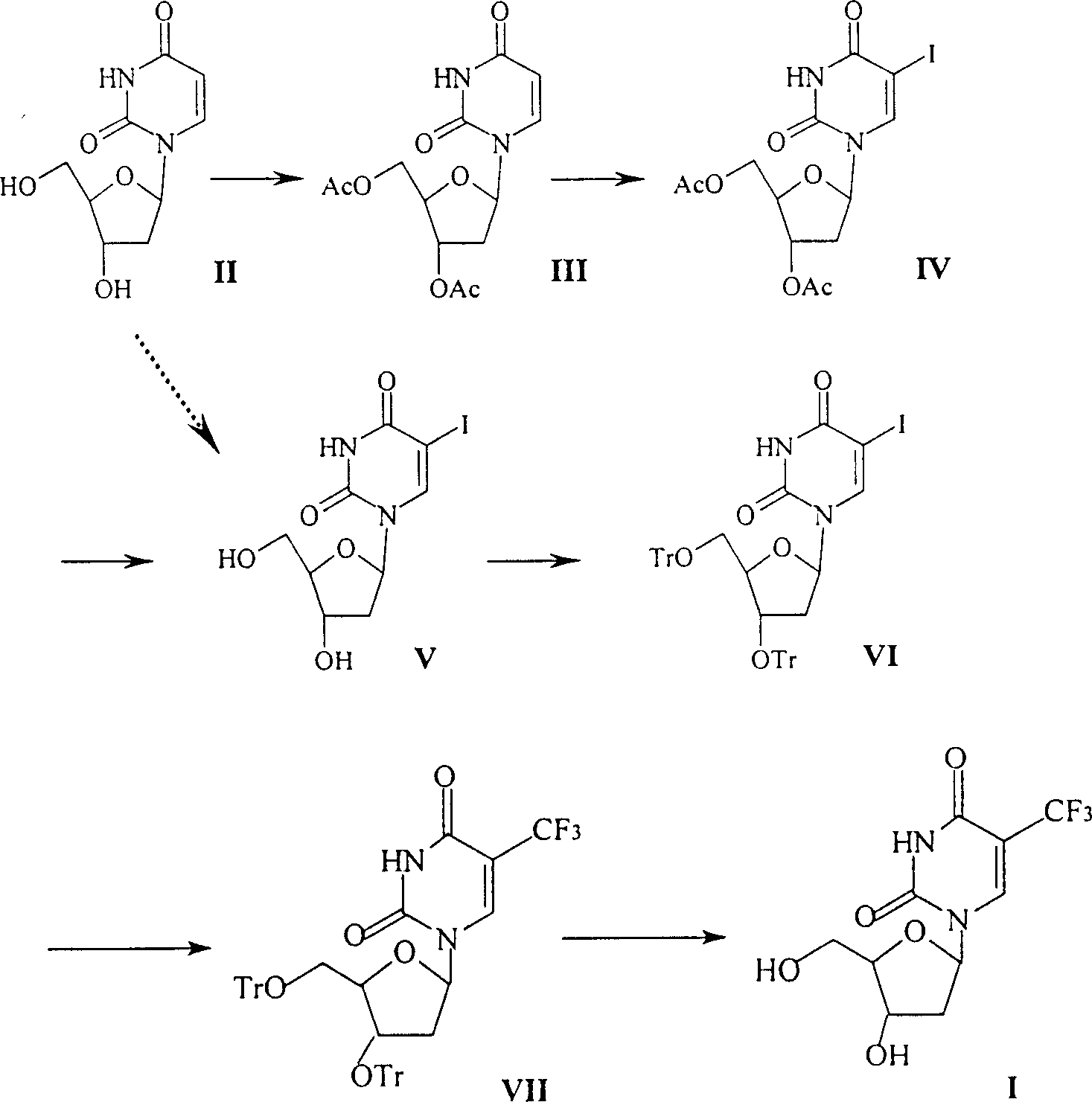
Preparation method of trifluridine
ActiveCN104761602AReduce the amount of feedMild reaction conditionsSugar derivativesSugar derivatives preparationClinical efficacyDeoxyuridine
The invention discloses a preparation method of trifluridine. The method comprises the specific steps of: adding 2'-deoxyuridine and trifluoromethyl sulfinate sodium to a reaction solvent, stirring and cooling to - 5 to - 3 DEG C, introducing of nitrogen for protection, stirring to dissolve, dropwise adding tert-butyl hydroperoxide, controlling the temperature at less than 5 DEG C, heating to 60-65 DEG C for reaction, and conducting posttreatment after the reaction to obtain a finished product. The method provided by the invention has mild reaction conditions, simple operation, little side reaction, short reaction time, great reduction of feeding amount of tert-butyl hydroperoxide, great saving of the production cost, and high yield and high purity of the product, and is especially applicable to industrial production, and has significance to the quality control of medicine and clinical curative effect.
Owner:SHANDONG CHENGCHUANG BLUE OCEAN PHARM TECH CO LTD
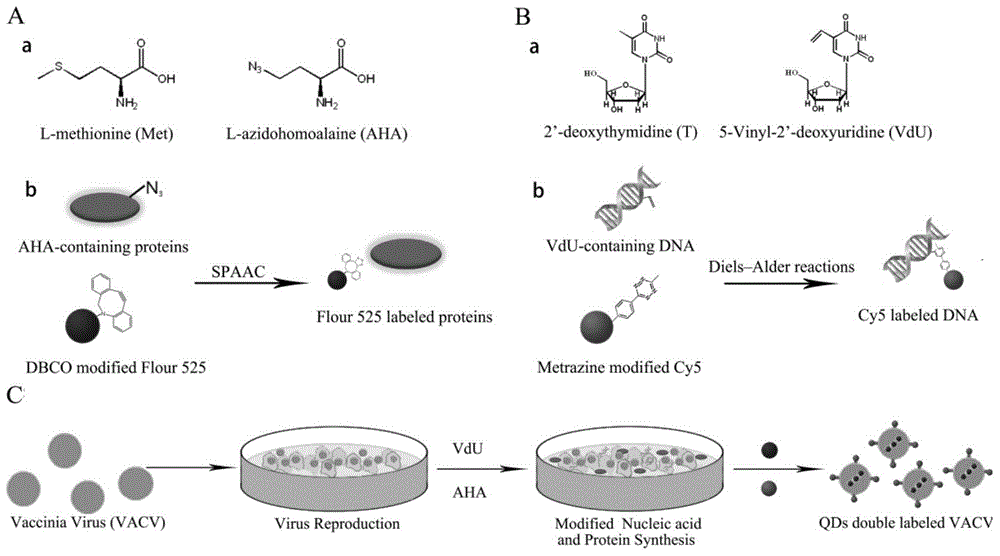
Novel virus double-fluorescence labeling method based on nucleic acid and protein biosynthesis
InactiveCN104894295ALittle effect on activitySimple and fast operationMicrobiological testing/measurementChemical reactionViral nucleic acid
The invention relates to a novel virus double-fluorescence labeling method based on nucleic acid and protein biosynthesis, and belongs to the field of chemistry and biomedicine. The method comprises specific steps as follows: a methionine analogue methionine azide and a deoxythymidine analogue ethylene deoxyuridine are synthesized; viruses are added to host cells for infection, methionine azide and ethylene deoxyuridine are added respectively, vinyl derivation of viral nucleic acid and azido derivation of protein are realized with biosynthesis processes of nucleic acid and protein; double-fluorescence labeling of viral nucleic acid and protein is naturally realized through two biological orthogonal reactions including a Diels-Alder reaction of tetrazine-oefin and a copper-free catalytic click chemical reaction of azido-cyclooctyne. The labeling method is simple, convenient and reliable, can be applied to all DNA (deoxyribonucleic acid) viruses including single-stranded DNA viruses, double-stranded DNA viruses, enveloped viruses and non-enveloped viruses, and is a universal virus fluorescence labeling method on an absolute basis.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Activity-based assay for ricin-like toxins
InactiveUS7172861B2Sensitive highEasy to adaptSugar derivativesMicrobiological testing/measurementPositive controlRicin
A method of detecting N-glycosylase activity in a sample involves incubating an oligodeoxyribonucleotide substrate containing a deoxyadenosine or deoxyuridine residue with the sample to be tested such that the N-glycosylase, if present, hydrolyzes the deoxyadenosine or deoxyuridine residue to result in an N-glycosylase product having an abasic site. A primer is annealed to the N-glycosylase product, and the primer is extended with a DNA polymerase, such as Taq DNA polymerase, that pauses at abasic sites. The resulting extension products are melted from the N-glycosylase product, allowed to form hairpins due to self-complementarity, and further extended in the presence of labeled precursors to result in labeled products. Extension products synthesized from undigested substrate as template do not result in labeled products. Thus, detection of labeled products results in detection of N-glycosylase activity. Oligodeoxyribonucleotide substrates, primer, and positive controls and a kit for N-glycosylase assay are also disclosed.
Owner:BATTELLE ENERGY ALLIANCE LLC
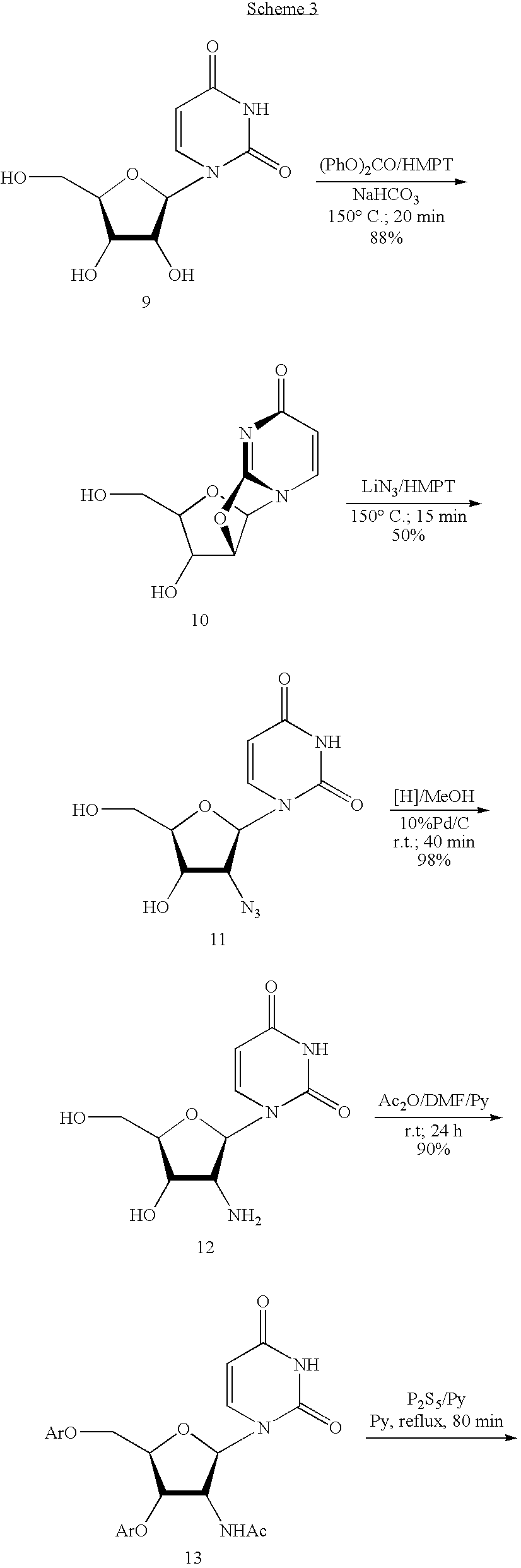
Preparation method of 3'-deoxyuridine
ActiveCN107033205AReduce usageAvoid generatingSugar derivativesSugar derivatives preparationAcetic anhydrideHigh pressure water
The invention relates to the field of pharmaceutical synthesis and particularly relates to a preparation method of 3'-deoxyuridine. The method comprises the steps: by adopting a compound 3 as a raw material, firstly protecting amino through acetic anhydride to obtain a compound 4, obtaining a compound 5 under the action of acetyl bromide, reducing through a hypophosphite system to obtain a compound 6; removing deacetylated amino under the action of high-pressure water vapor and an organic solvent to obtain a compound 8 or removing N-acetyl to obtain a compound 7; and finally removing all acetyl to obtain a mixture of 3'-deoxyuridine and 3'-deoxycytidine; separating and purifying to obtain 3'-deoxyuridine crystal and 3'-deoxycytidine crystal separately, or directly removing all acetyl through the compound 6 to obtain the 3'-deoxycytidine. Available natural products are taken as initial raw materials, so that the method is simple in operation and convenient to purify, and industrial large-scale production is extremely easy to implement.
Owner:SHANGHAI ZHAOWEI TECH DEV +1
Use of uridine and deoxyuridine to treat folate-responsive pathologies
ActiveUS20140080784A1Increased riskReduce the numberBiocideCarbohydrate active ingredientsMedicineDietary supplement
The present invention relates to a pharmaceutical or dietary composition comprising deoxyuridine and a pharmaceutically or dietetically suitable carrier. Another aspect of the present invention relates to a method of supplementing the dietary needs of a subject. This method includes administering to the subject a dietary supplementing effective amount of deoxyuridine. Yet another aspect of the present invention relates to a method of treating cancer in a subject. This method includes selecting a subject having cancer and administering to the selected subject a therapeutically effective amount of uridine, thereby treating the cancer in the selected subject.
Owner:CORNELL UNIVERSITY
Method for detecting biological activity of recombinant human keratinocyte growth factors
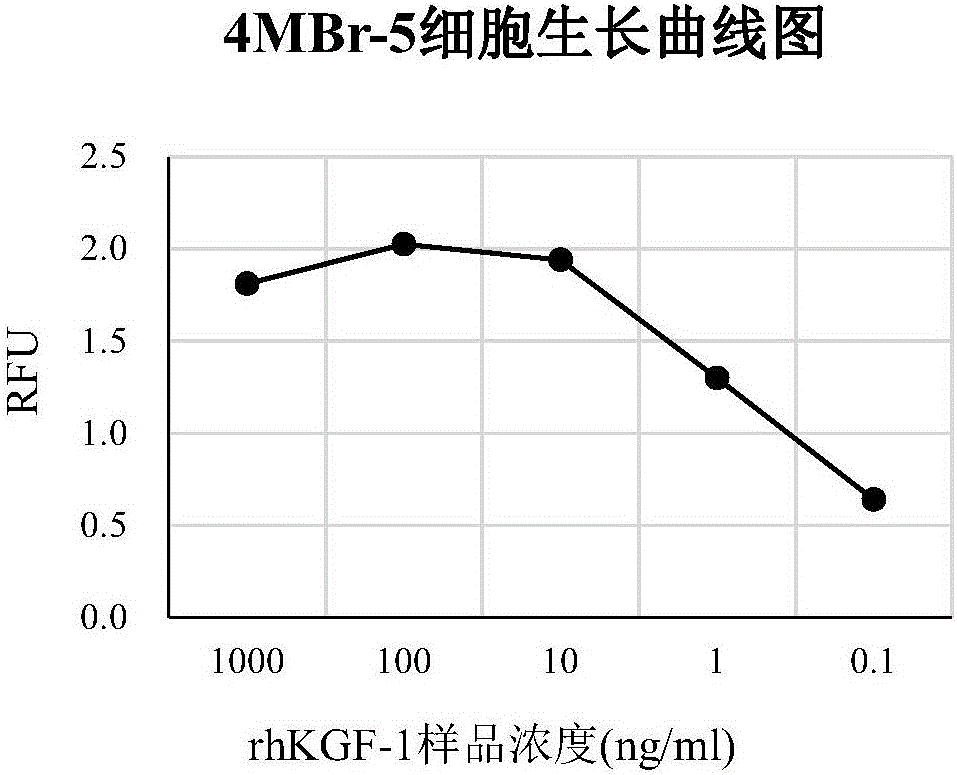
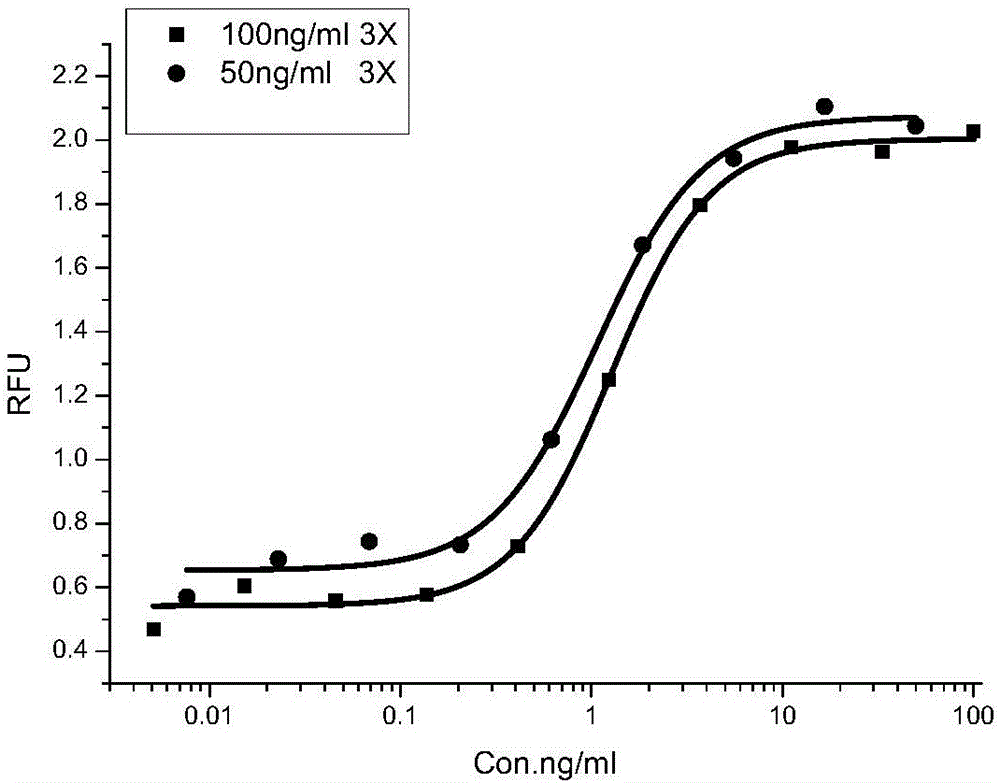
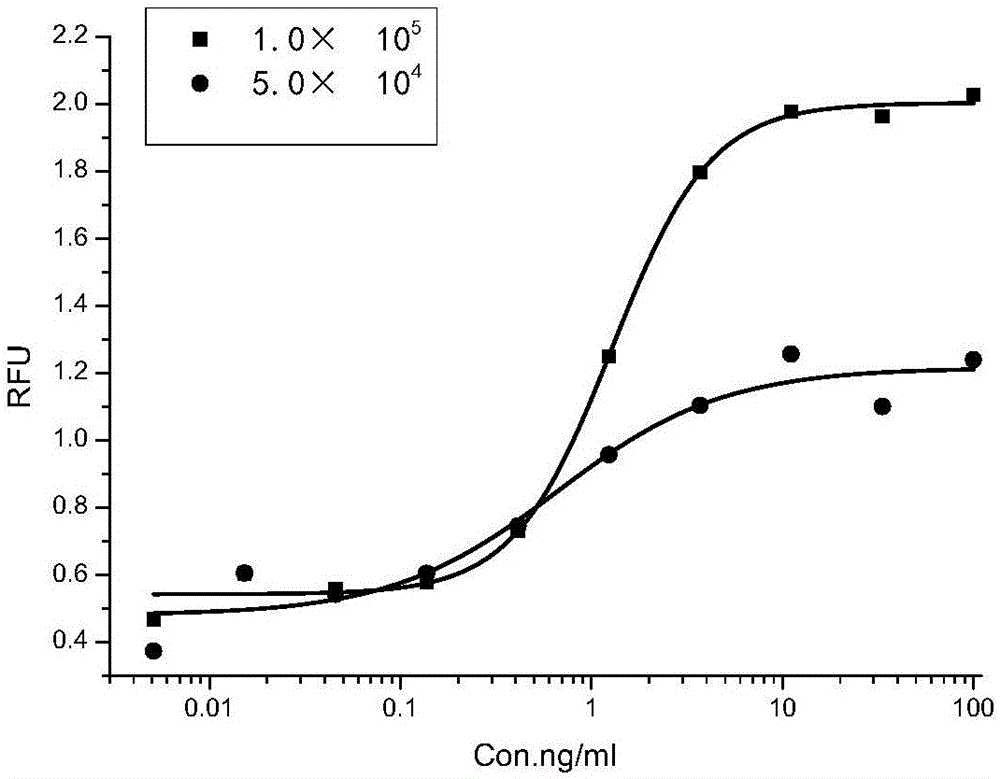
ActiveCN106226511APromote proliferationIntuitive and accurate measurementBiological testingRecombinant Human Keratinocyte Growth FactorVolumetric Mass Density
The invention provides a method for detecting recombinant human keratinocyte growth factors (rhKGF-1). The method includes steps of 1), cultivating keratinocytes; 2) inoculating the keratinocytes and controlling the density of the keratinocytes; 3), treating rhKGF-1 samples, carrying gradient dilution on the concentration of the rhKGF-1 samples and adding 5-acetenyl-2'-deoxyuridine (EdU) into the rhKGF-1 samples with various rhKGF-1 concentration dilution degrees to prepare loading samples with the series of rhKGF-1 concentration dilution degrees; 4), washing the keratinocytes in keratinocyte plates obtained at the step 2) and removing cultivation media; 5), loading the loading samples obtained at the step 3) into keratinocyte cultivation plates obtained at the step 4) and cultivating and incubating the keratinocytes; 6), solidifying and permeabilizing the keratinocytes, and then carrying out 'Click-iT' reaction on the EdU; 7), arranging the keratinocytes treated at the step 6) in a multifunctional microplate reader, reading the fluorescence intensity (RFU) and computing the biological activity of the rhKGF-1. The method has the advantages of low detection background, high sensitivity, specificity and accuracy, good repeatability and the like. Besides, the method is particularly applicable to detecting the biological activity of the rhKGF-1 and establishing quality standards.
Owner:信立泰(苏州)药业有限公司
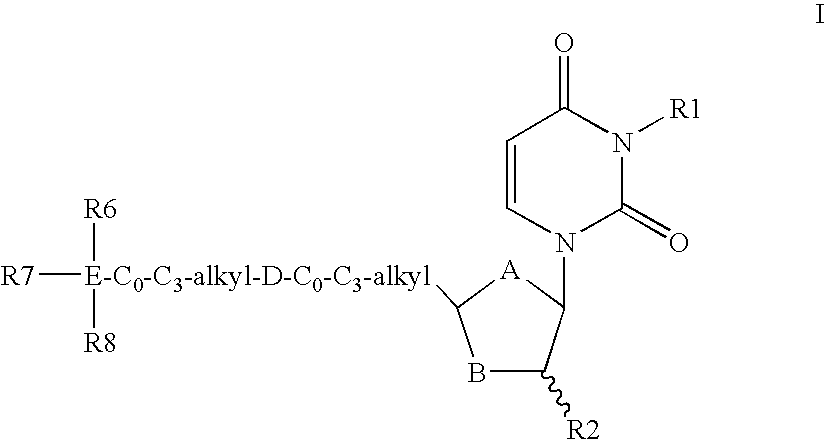
Dutpase Inhibitors
Deoxyuridine derivatives of Formula (I′); where A is O, S or CH2; B is O, S or CHR3; R1 is H, or various substituents; R2 is H, F; R3 is H, F, OH, NH2; or R2 and R3 together form a chemical bond; D is —NHCO—, —CONH—, —O—, —C(═O)—, —CH═CH, —C≡C—, —NR5—; R4 is hydrogen or various substituents; R5 is H, C1-C4 alkyl, C1-C4 alkanoyl; E is Si or C; R6, R7 and R8 are independently selected from C1-C8 alkyl, C2-C8 alkenyl, C2-C8 alkynyl, or a stable monocyclic, bicyclic or tricyclic ring system have utility in the prophylaxis of treatment of parasitic diseases such as malaria.
Owner:MEDIVIR AB
Fragrant acid ester derivative of 5-fluorine-2'-deoxidized uridine and synthesis thereof
InactiveCN101508714AGood choiceOvercoming selectivitySugar derivativesFermentationSynthesis methodsRegioselectivity
The invention discloses an aromatic acid ester derivative of 5-fluoro-2'-deoxyuridine and a synthesis method thereof. The synthesis method comprises the following steps: adding the 5-fluoro-2'-deoxyuridine with the concentration of 1.0-40mg / ml and an acyl donor to a non-aqueous medium, wherein, mol ratio of the 5-fluoro-2'-deoxyuridine to the acyl donor is 1:1.1-30; adding enzyme at a ratio of 0.1-10:1 based on the weight of the 5-fluoro-2'-deoxyuridine; performing an acylation reaction at the temperature of 20-60 DEG C and the normal pressure, and at an oscillation speed of 100-300rpm; and separating to obtain the aromatic acid ester derivative of 5-fluoro-2'-deoxyuridine. The method helps synthesize various aromatic acid ester derivatives of 5-fluoro-2'-deoxyuridine with novel structures and potential pharmaceutical value. The method has the advantages of mild condition, environmental protection, high regioselectivity of the reaction, simple and controllable reaction process, easy product separation and the like.
Owner:SOUTH CHINA UNIV OF TECH
Method for the production of cladribine
InactiveUS20100291632A1Improve isolationHigh yieldOrganic chemistryFermentationPurine nucleoside phosphorylaseSolvent
A method for producing cladribine (2-chloro-2′-deoxyadenosine) comprising the steps of:a) reaction of 2-deoxyuridine with 2-chloroadenine, in the presence of uridine phosphorylase (UPase) and purine nucleoside phosphorylase (PNPase) in an aqueous reaction medium possibly containing up to 40% v / v of an aprotic dipolar solvent, to obtain cladribine dissolved in said reaction medium;b) isolation of the cladribine by precipitation by means of concentration and alkalinisation of the reaction medium up to pH 11.5-12.5.
Owner:EXPLORA LAB
Method for synthesizing 5-trifluoro methyl-2'-desugarized uridine
InactiveCN100334100CHigh puritySimple and fast operationSugar derivativesTrifluoromethylationDeoxyuridine
The present invention relates to the synthesis process of 5-trifluoromethyl-2'-dexoy uridine. The compound is prepared with 2'-dexoy uridine as initial material and through iodizing, hydroxyl protection, trifluoromethylation and deprotection. The said process of the present invention has easy-to-obtain material, simple synthesis and no pollution.
Owner:FUBANG BIO PHARMA HANGZHOU
Deoxyuridine powder injection and its preparing method
ActiveCN1943559ARelieve nauseaAlleviate loss of appetitePowder deliveryOrganic active ingredientsCyclodextrinDeoxyuridine
A process for an injection from frozen powder of doxifluridine applying to mainline and its preparation method, said injection comprise 5 parts of doxifluridine, 0.1-100 parts of excipient or 0.1-100 parts of cyclodextrin.; said product can be dissolved quickly when meeting water and convenient for clinical use.
Owner:JIANGSU SHENLONG PHARMA
Cordyceps cytidine deaminase, coding gene and application thereof
ActiveCN103031295BIncrease productionEnhance expressive abilityBacteriaHydrolasesNucleotideDeoxyuridine
The invention relates to an enzyme from Bailing production bacterium Cordyceps Chinese Hirsutella for synthesizing metabolic (desoxy) uridine from (desoxy) cytidine, a gene for coding the enzyme and application thereof. The enzyme is cytidine deaminase which has more than 90% of homology with amino acid sequence disclosed as SEQ ID NO.1; and the coding gene has more than 90% of homology with nucleotide sequence disclosed as SEQ ID NO.2. The invention researches the metabolic pathway of (desoxy) cytidine synthesized (desoxy) uridine in details in principle. The cloned DNA (deoxyribonucleic acid) comprising the nucleotide sequence provided by the invention can be transformed into engineering bacterium by transduction, conversion and conjugal transfer. By adjusting the expression of the (desoxy) uridine biosynthesized gene, the host (desoxy) uridine is endowed with high expressivity, thereby providing an effective way for enhancing the yield of the (desoxy) uridine and having great application prospects.
Owner:ZHEJIANG UNIV OF TECH +1
Nucleoside disulfide dinitrogen derivative and preparation method thereof
The invention discloses a nucleoside disulfide dinitrogen derivative and a preparation method thereof and belongs to the technical field of the synthesis of radiopharmaceutical labelled precursor compounds. The invention provides 5-{2-sulfydryl ethyl-[2-(2-sulfydryl ethyl amino) acetyl] amino}-methyl-2'-deoxyuridine (ANMdU) and a preparation method thereof, and is used for preparing technetium marked radiopharmaceutical 99mTc-ANMdU for tumor imaging. Test results show that the labelled rate of the ANMdU is greater than 95 percent, and the ANMdU has good in-vitro stability, rapid metabolism in normal mice and high safety of animal tests and is a potential tumor imaging agent.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com