Patents
Literature
1211results about How to "Reduce molecular weight" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composite proppant, composite filtration media and methods for making and using same
InactiveUS6632527B1Avoid condensationReduce molecular weightPretreated surfacesGlass/slag layered productsFiberFiltration
Composite particles made of a binder and filler material are provided for use in subterranean formations. The filler is finely divided mineral and optional fiber. The particles are proppants useful to prop open subterranean formation fractures. The particles are also useful for water filtration and artificial turf for sports fields. Methods of making the composite particles are also disclosed.< / PTEXT>
Owner:HEXION INC
Generating Acid Downhole in Acid Fracturing
An acid fracturing method is provided in which the acid is generated in the fracture by hydrolysis of a solid acid-precursor selected from one or more than one of lactide, glycolide, polylactic acid, polyglycolic acid, a copolymer of polylactic acid and polyglycolic acid, a copolymer of glycolic acid with other hydroxy-, carboxylic acid-, or hydroxycarboxylic acid-containing moieties, and a copolymer of lactic acid with other hydroxy-, carboxylic acid or hydroxycarboxylic acid-containing moieties. The solid acid-precursor may be mixed with a solid acid-reactive material to accelerate the hydrolysis and / or coated to slow the hydrolysis. Water-soluble liquid compounds are also given that accelerate the hydrolysis. The method ensures that the acid contacts fracture faces far from the wellbore.
Owner:SCHLUMBERGER TECH CORP
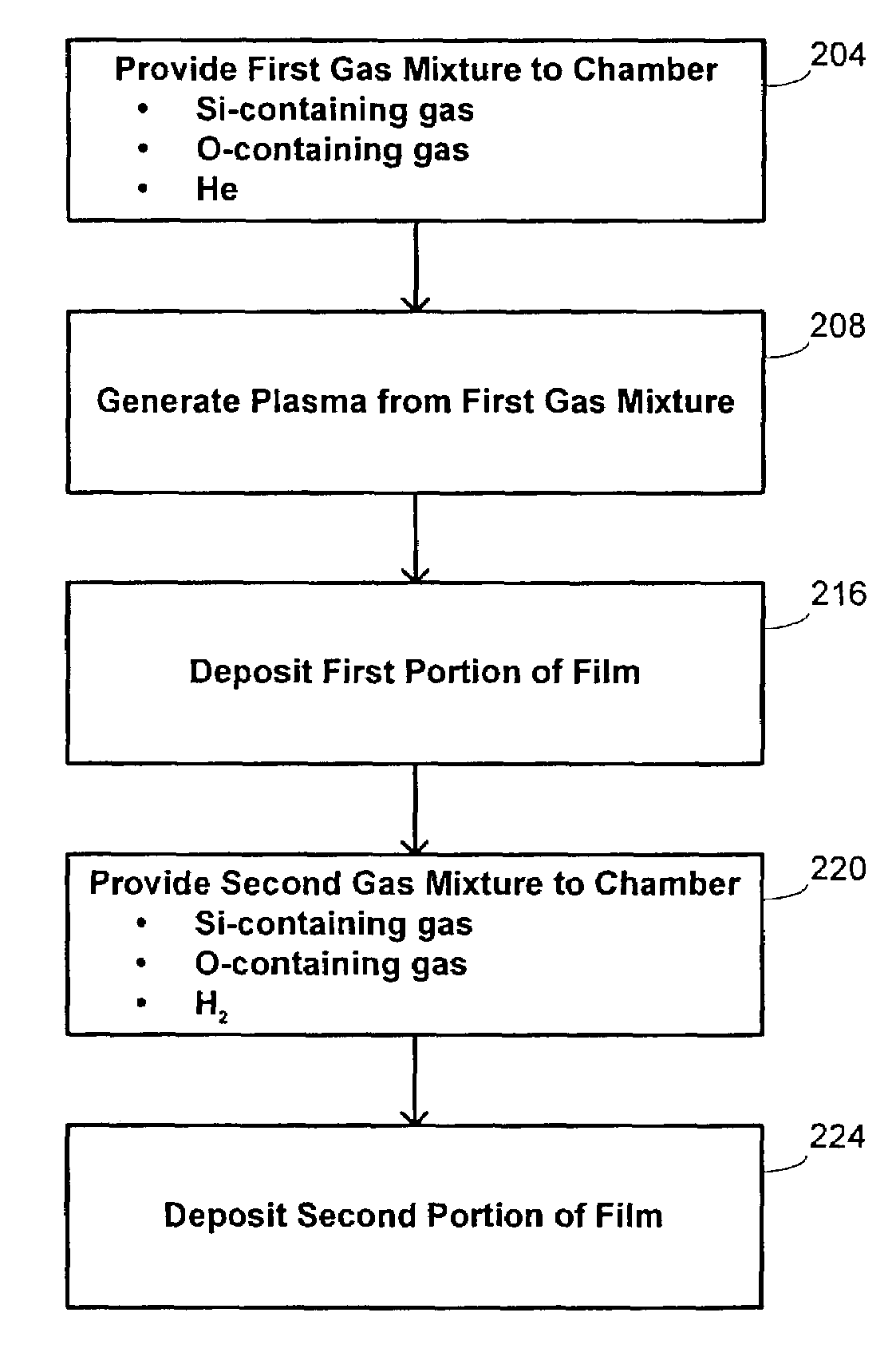
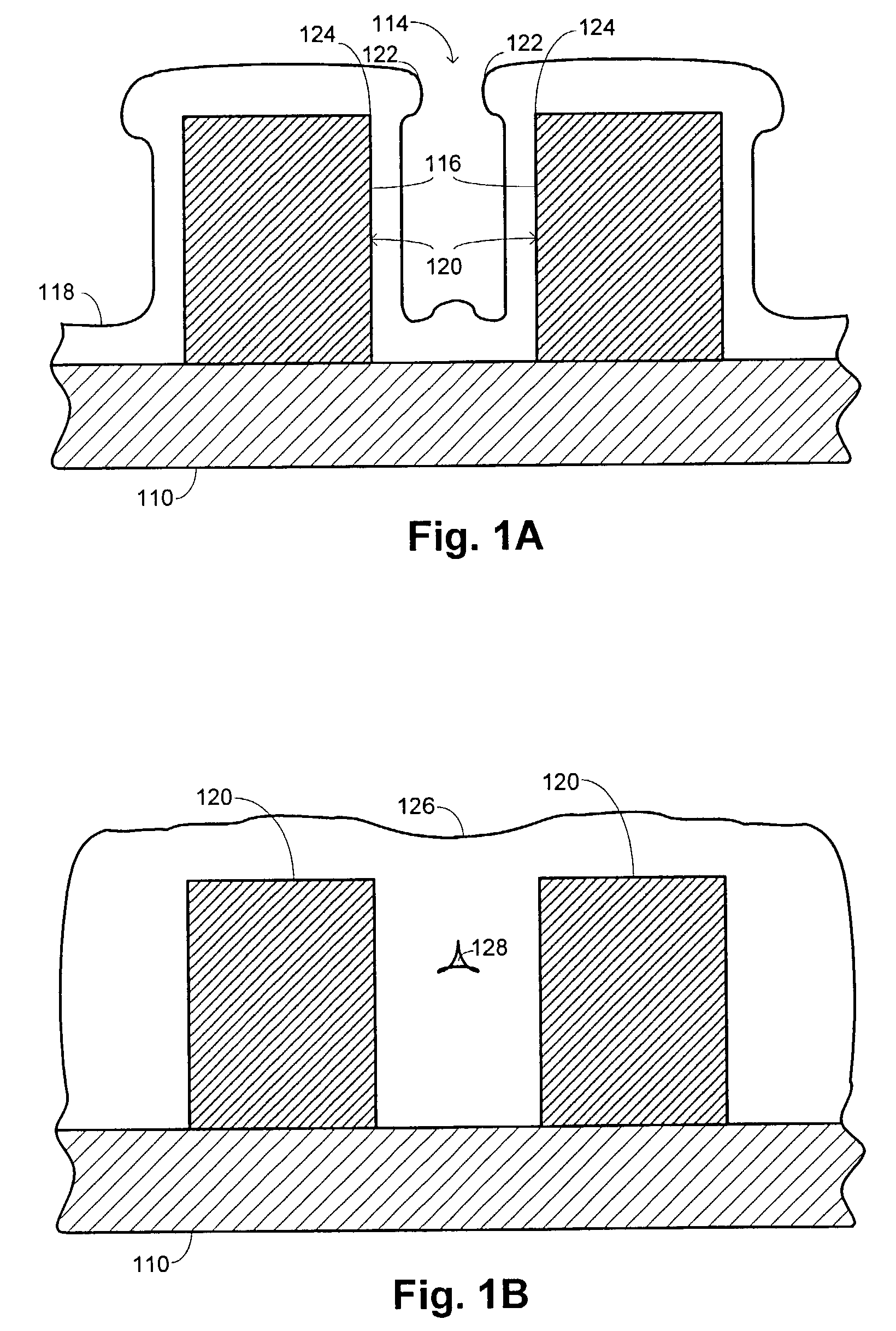
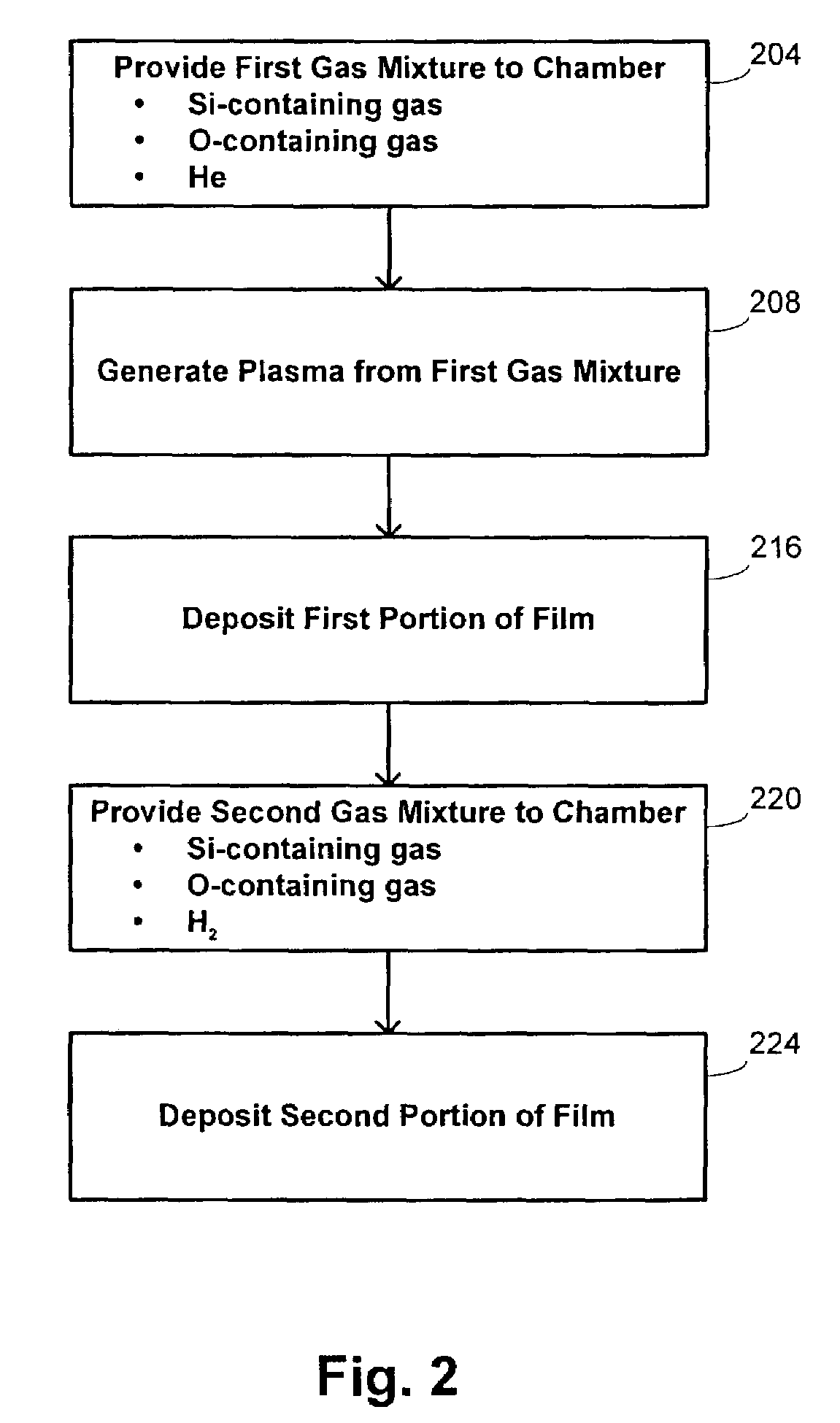
Hdp-cvd multistep gapfill process
InactiveUS20040245091A1Reduce molecular weightReduced sputtering characteristicVacuum evaporation coatingSputtering coatingProduct gasChemistry
Abstract of the Disclosure A gapfill process is provided using cycling of HDP-CVD deposition, etching, and deposition step. The fluent gas during the first deposition step includes an inert gas such as He, but includes H2 during the remainder deposition step. The higher average molecular weight of the fluent gas during the first deposition step provides some cusping over structures that define the gap to protect them during the etching step. The lower average molecular weight of the fluent gas during the remainder deposition step has reduced sputtering characteristics and is effective at filling the remainder of the gap.
Owner:APPLIED MATERIALS INC
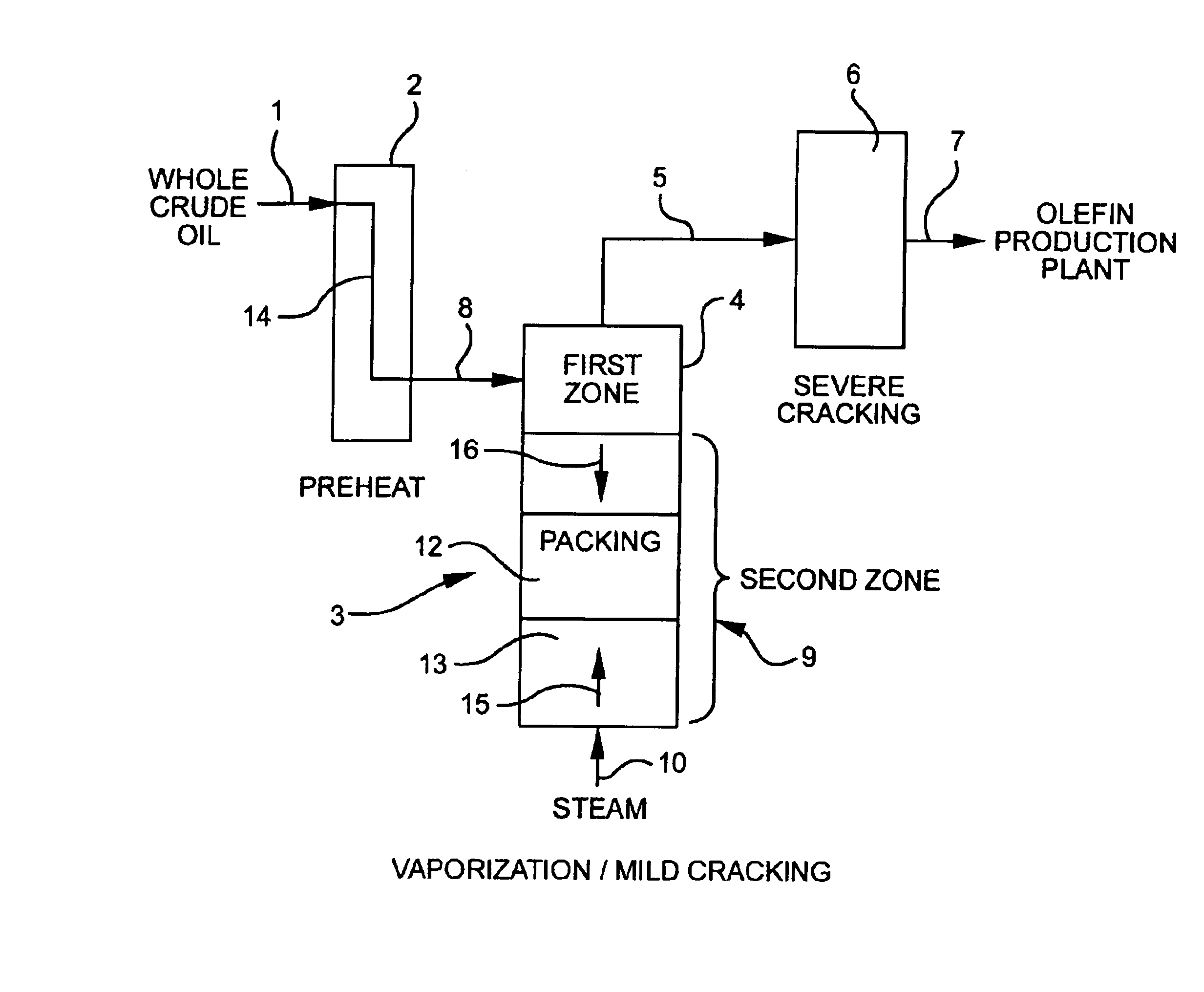
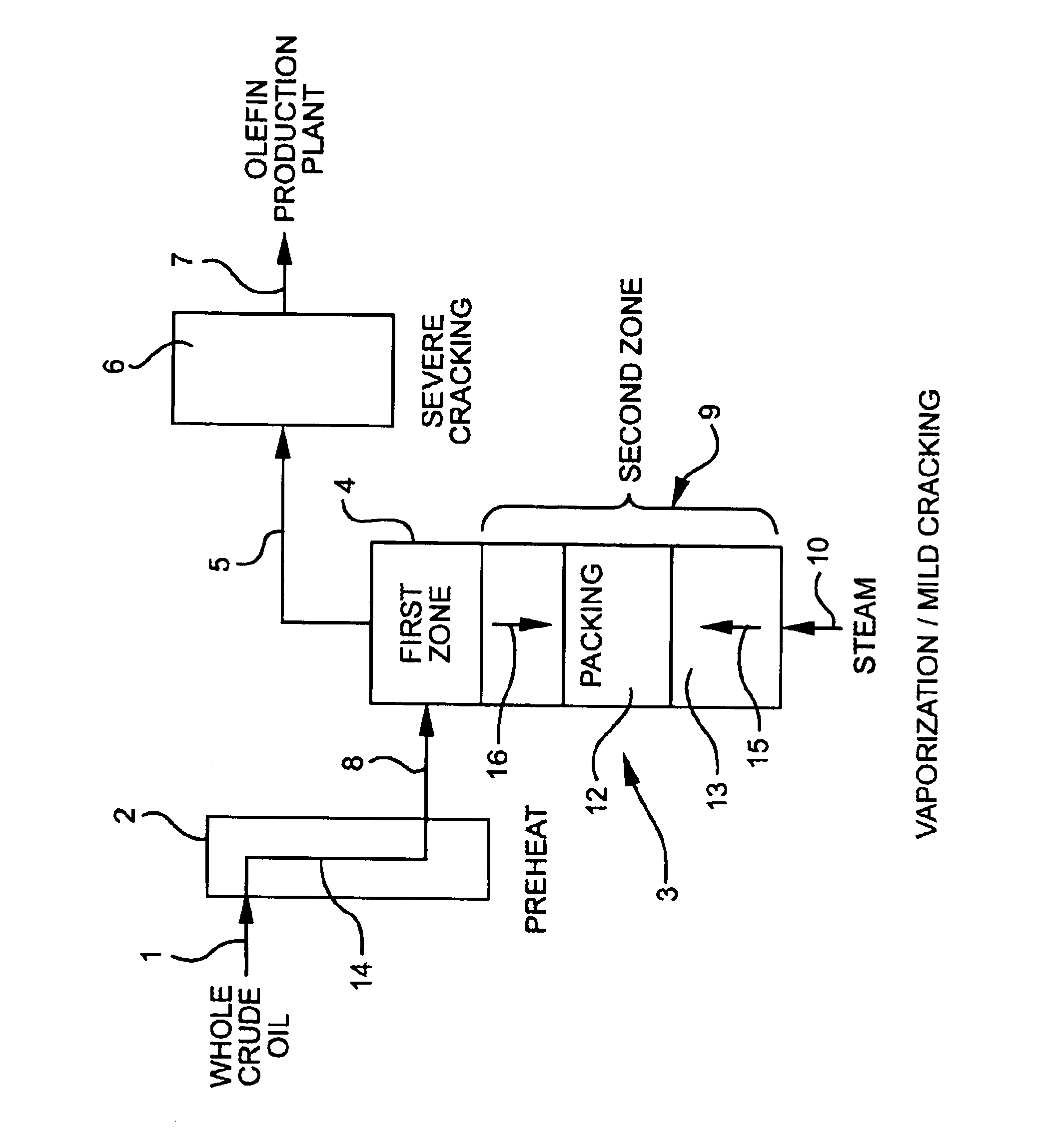
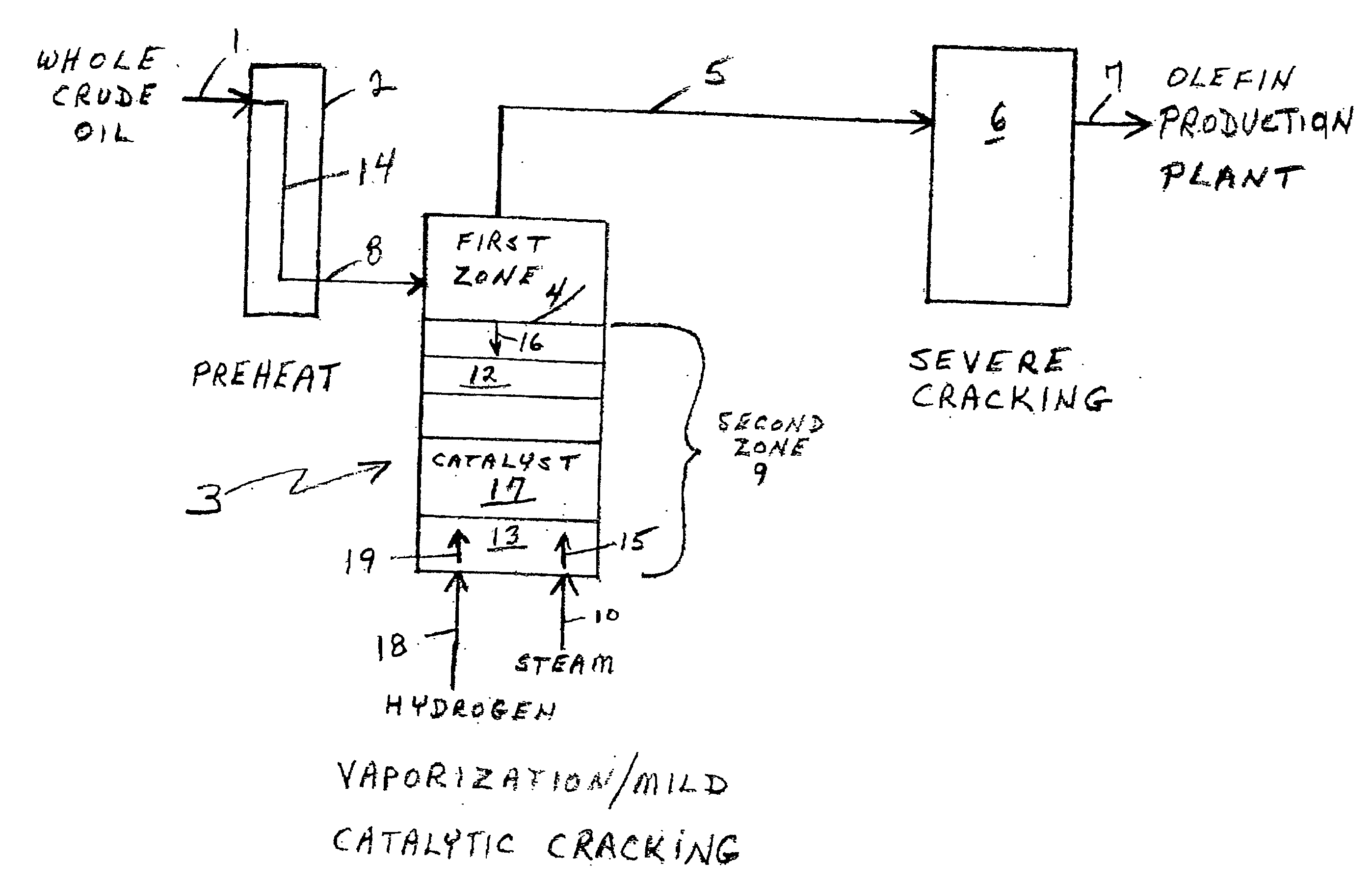
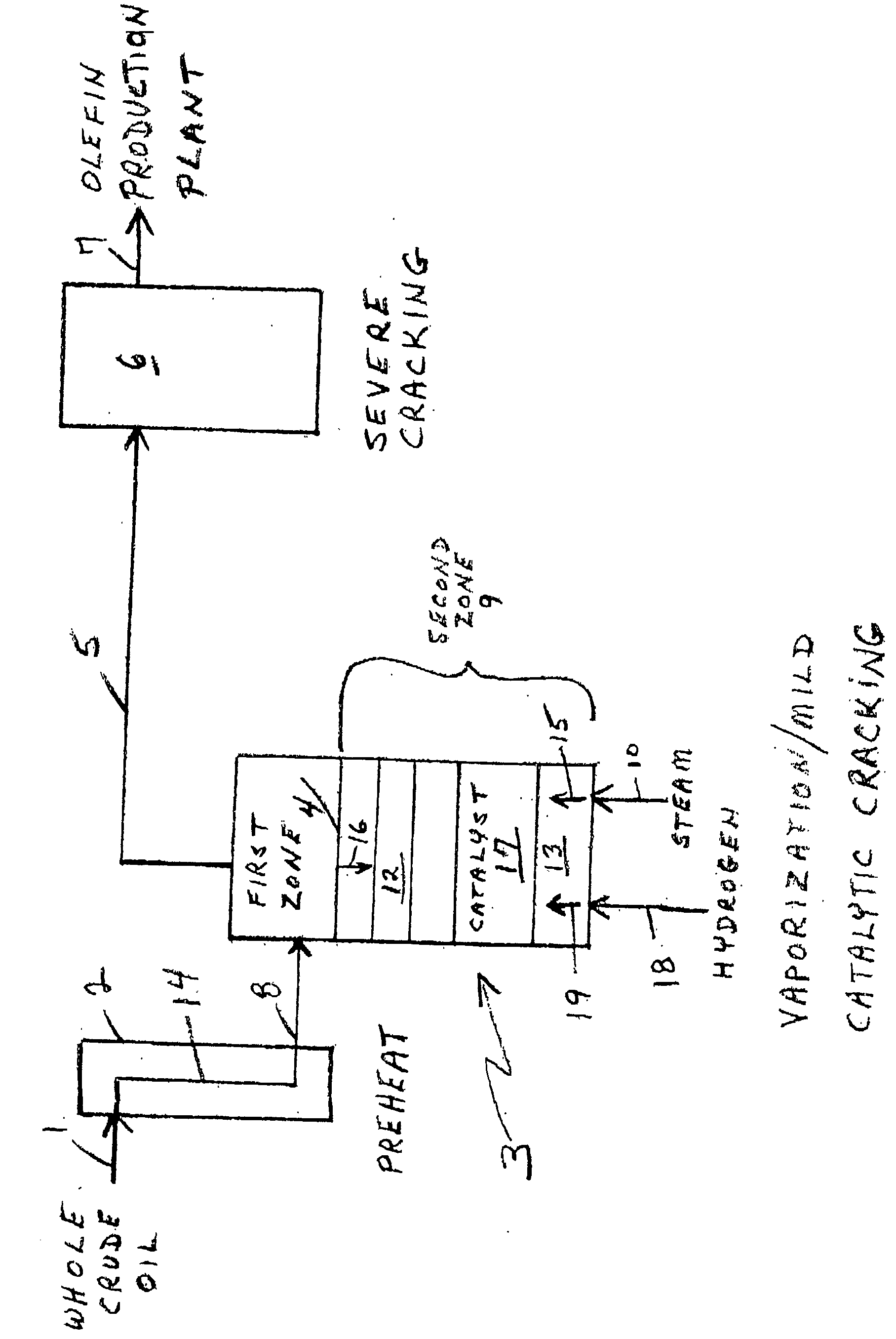
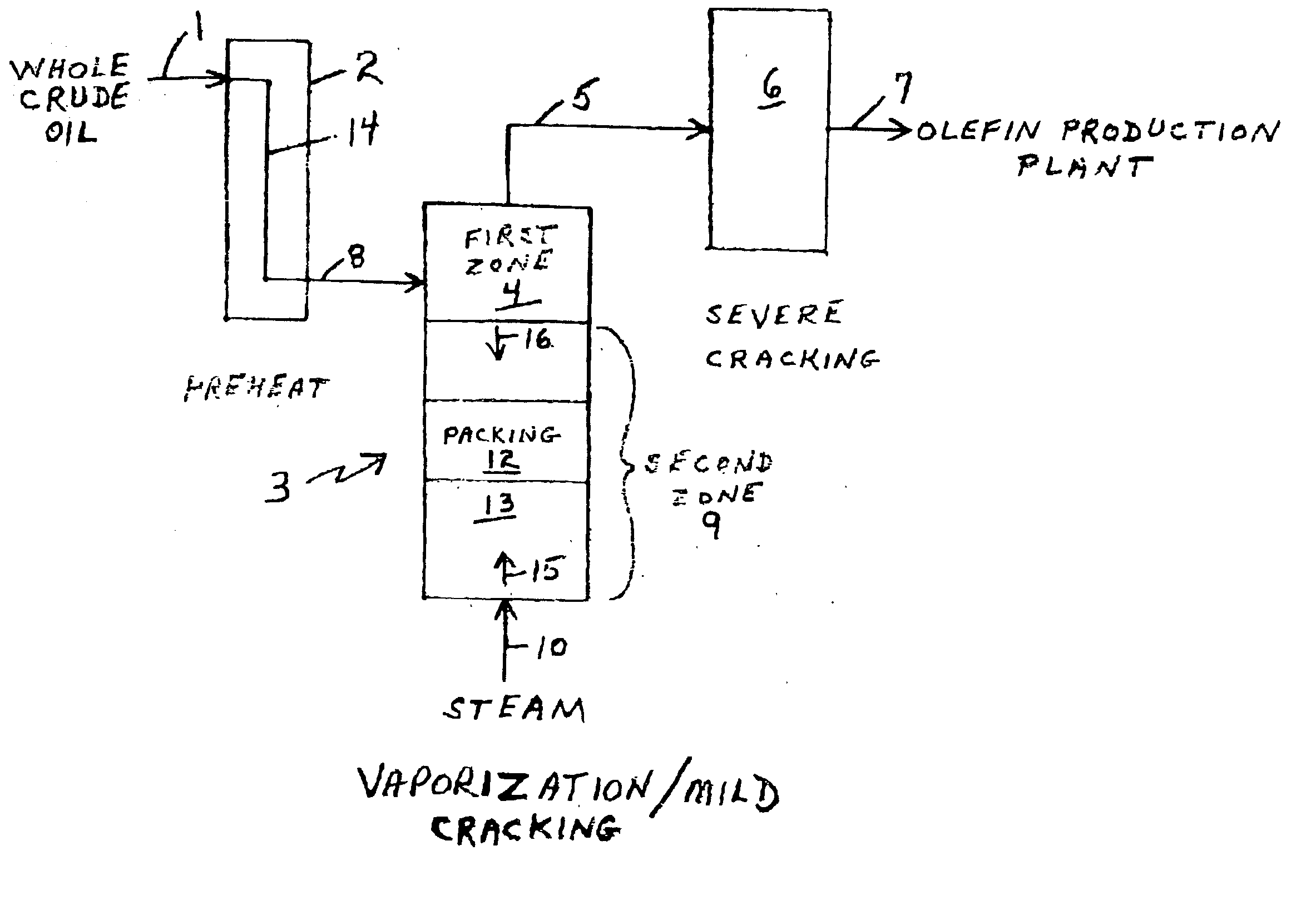
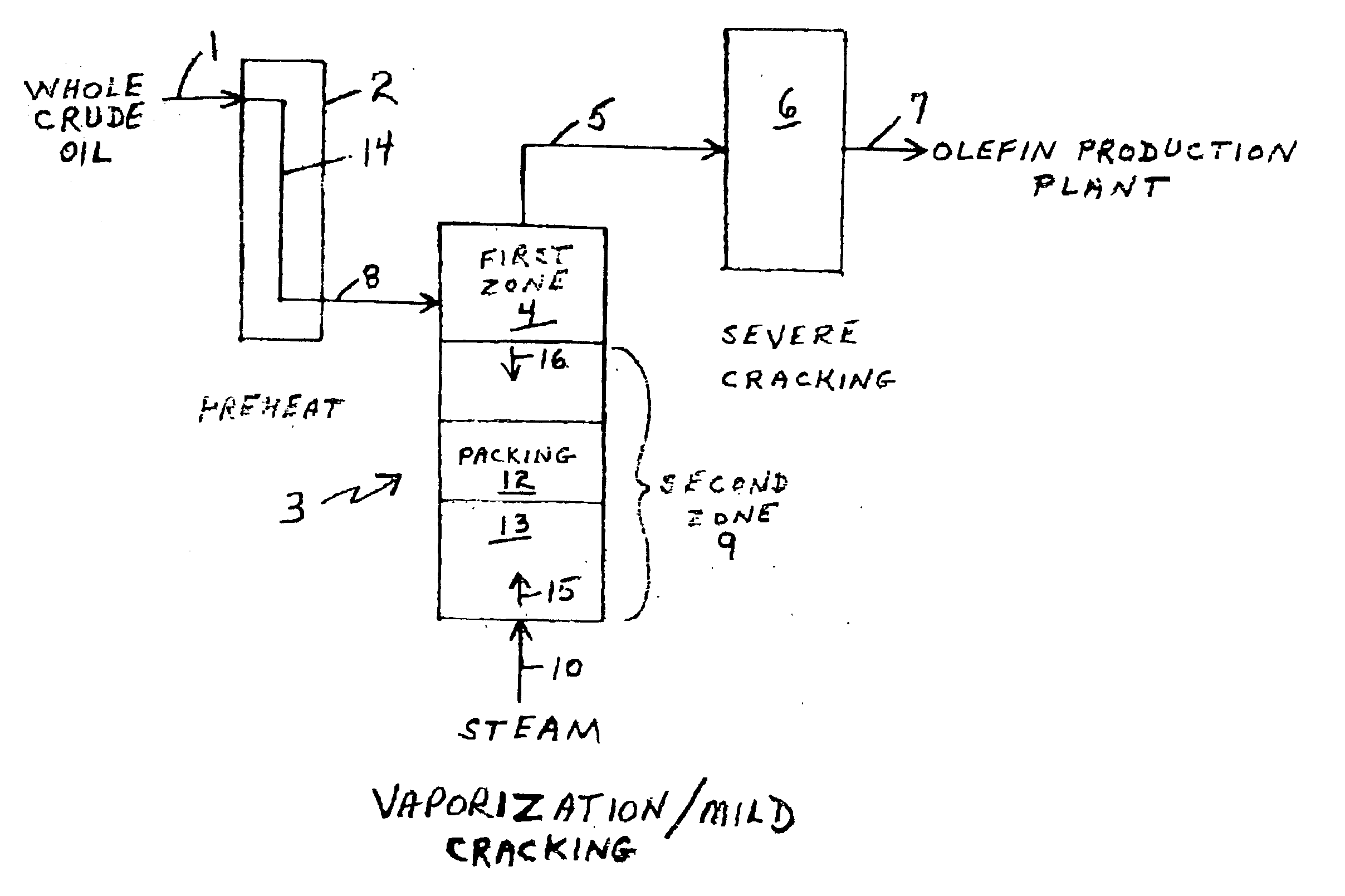
Olefin production utilizing whole crude oil
InactiveUS6743961B2Lower temperature rangeReduce molecular weightThermal non-catalytic crackingHydrocarbonsOil productionAlkene
A method for utilizing whole crude oil as a feedstock for the pyrolysis furnace of an olefin production plant wherein the feedstock after preheating is subjected to mild cracking conditions until substantially vaporized, the vapors from mild cracking being subjected to severe cracking in the radiant section of the furnace.
Owner:EQUSR CHEM LP
Methods for embolizing blood vessels
InactiveUS6335384B1Reduce molecular weightEasy to adjustHeavy metal active ingredientsOrganic active ingredientsParticulatesMedicine
Disclosed are methods useful for treating vascular lesions wherein a non-particulate agent such as a metal coil is introduced into a vascular site (e.g., an aneurysm cavity) in conjunction with an embolizing composition comprising a biocompatible polymer and a biocompatible solvent.The biocompatible solvent is miscible or soluble in blood and also solubilizes the polymer during delivery. The biocompatible polymer is selected to be soluble in the biocompatible solvent but insoluble in blood. Upon contact with the blood, the biocompatible solvent dissipates from the embolic composition whereupon the biocompatible polymer precipitates. Precipitation of the polymer in the presence of the non-particular agent permits the agent to act as a structural lattice for the growing polymer precipitate.In another embodiment, the biocompatible polymer composition can be replaced with a biocompatible prepolymer composition containing a biocompatible prepolymer.
Owner:MICRO THEREPEUTICS INC
Formable and settable polymer bone composite and method of production thereof
ActiveUS20050008672A1Reduce molecular weightEasy to insertBiocideSurgical adhesivesBone implantEngineering
A composite osteoimplant. The osteoimplant includes a polymer and bone-derived particles. The composite is adapted and constructed to be formable during or immediately prior to implantation and to be set after final surgical placement.
Owner:WARSAW ORTHOPEDIC INC
Sulfonium salt having polymerizable anion, polymer, resist composition, and patterning process
ActiveUS20080102407A1Feature size of to decreaseSmall feature sizeOrganic chemistryPhotosensitive materialsResistHigh energy
A sulfonium salt having a polymerizable anion generates a strong sulfonic acid upon exposure to high-energy radiation so that it facilitates effective scission of acid labile groups in chemically amplified resist compositions. It is useful as a monomer from which a base resin for use in radiation-sensitive resist compositions is derived.
Owner:SHIN ETSU CHEM IND CO LTD
Olefin production utilizing whole crude oil and mild catalytic cracking
InactiveUS20040054247A1Lower temperature rangeImproved vaporizationThermal non-catalytic crackingCatalytic crackingChemistryPyrolysis
A method for utilizing whole crude oil as a feedstock for the pyrolysis furnace of an olefin production plant wherein the feedstock after preheating is subjected to mild catalytic cracking conditions until substantially vaporized, the vapors from the mild catalytic cracking being subjected to severe cracking in the radiant section of the furnace.
Owner:EQUSR CHEM LP
Methods for embolizing blood vessels
InactiveUS6017977AReduce molecular weightEasy to adjustHeavy metal active ingredientsOrganic active ingredientsPrepolymerSolvent
Disclosed are methods useful for treating vascular lesions wherein a non-particulate agent such as a metal coil is introduced into a vascular site (e.g., an aneurysm cavity) in conjunction with an embolizing composition comprising a biocompatible polymer and a biocompatible solvent. The biocompatible solvent is miscible or soluble in blood and also solubilizes the polymer during delivery. The biocompatible polymer is selected to be soluble in the biocompatible solvent but insoluble in blood. Upon contact with the blood, the biocompatible solvent dissipates from the embolic composition whereupon the biocompatible polymer precipitates. Precipitation of the polymer in the presence of the non-particulate agent permits the agent to act as a structural lattice for the growing polymer precipitate. In another embodiment, the biocompatible polymer composition can be replaced with a biocompatible prepolymer composition containing a biocompatible prepolymer.
Owner:MICRO THEREPEUTICS INC
Method and apparatus for producing and treating novel elastomer composites
InactiveUS6929783B2Facilitate controlling and changing operating parameterImprove economyLiquid degasificationSpecial tyresParticulatesMasterbatch
Elastomer masterbatch is processed in a continuous compounder having multiple parallel elongate rotors axially oriented in an elongate processing chamber. Optionally, additional materials are compounded into the masterbatch, e.g., additives, other elastomeric compositions, etc. Preferably, the masterbatch then is further processed in an open mill. Excellent control of Mooney Viscosity is achieved.In certain preferred embodiments, elastomer composites are produced by novel continuous flow methods and apparatus in which fluid streams of particulate filler and elastomer latex are fed to the mixing zone of a coagulum reactor to form a coagulated mixture in semi-confined flow continuously from the mixing zone through a coagulum zone to a discharge end of the reactor. The particulate filler fluid is fed under high pressure to the mixing zone, such as to form a jet stream to entrain elastomer latex fluid sufficiently energetically to substantially completely coagulate the elastomer with the particulate filler prior to the discharge end without need of adding acid or salt solution or other coagulation step. The coagulated elastomer and particulate filler composite is fed into the aforesaid continuous compounder for processing and control of its moisture level and Mooney Viscosity. Novel elastomer composites are produced. Such novel elastomer composites combine material properties and characteristics, such as choice of filler, elastomer, level of filler loading, moisture level, Mooney Viscosity, balance between molecular weight and amount of bound rubber, and macro-dispersion not previously achieved.
Owner:CABOT CORP
Substituted pyridyl amine complexes, and catalysts
InactiveUS6900321B2Improve catalytic performanceIncrease temperatureSilicon organic compoundsMacromolecular libraries1-OcteneHafnium
New ligands, compositions, metal-ligand complexes and arrays with pyridylamine ligands are disclosed that catalyze the polymerization of monomers into polymers. Certain of these catalysts with hafnium metal centers have high performance characteristics, including higher comonomer incorporation into ethylene / olefin copolymers, where such olefins are for example, 1-octene, isobutylene or styrene. Certain of the catalysts are particularly effective at polymerizing propylene to high molecular weight isotactic polypropylene in a solution process at a variety of polymerization conditions.
Owner:FREESLATE
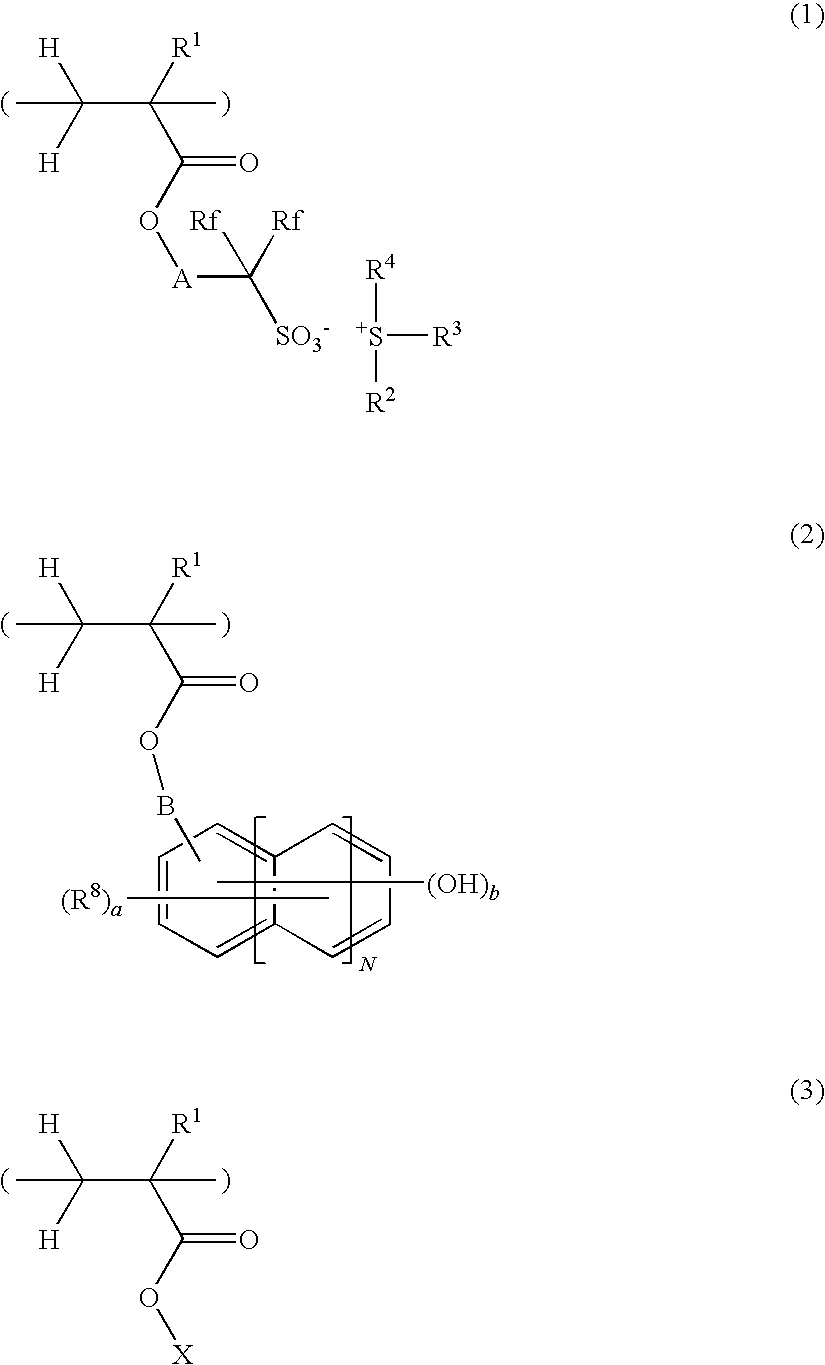
Sulfonium salt-containing polymer, resist composition, and patterning process
ActiveUS20090269696A1Promote divisionHigh sensitivity and resolutionPhotosensitive materialsRadiation applicationsResistAryl
A polymer comprising recurring units having formulae (1), (2) and (3) is provided as well as a chemically amplified resist composition comprising the same. R1 is H, F, CH3 or CF3, Rf is H, F, CF3 or C2F5, A is an optionally fluorine or oxygen-substituted divalent organic group, R2, R3 and R4 are alkyl, alkenyl, oxoalkyl, aryl, aralkyl or aryloxoalkyl, or may form a ring with the sulfur atom, N=0-2, R8 is H or alkyl, B is a single bond or optionally oxygen-substituted divalent organic group, a=0-3, b=1-3, and X is an acid labile group. The polymer generates a strong sulfonic acid which provides for effective cleavage of acid labile groups in a chemically amplified resist composition.
Owner:SHIN ETSU CHEM IND CO LTD
Water-based drilling fluids
InactiveUS20060019834A1Reduce molecular weightFlushingDrilling compositionWater basedInorganic salts
A water-based drilling fluid composition includes water and at least one rheology modifier and / or fluid loss control agent, and at least one other ingredient of polymeric additive, inorganic salts, dispersants, shale stabilizers, weighting agents, or finely divided clay particles, depending upon the desired attributes, wherein the rheology modifier and / or the fluid loss control agent comprises carboxymethylated raw cotton linters (CM-RCL) made from the baled raw cotton linters or comminuted raw cotton linters with increased bulk density.
Owner:HERCULES LLC
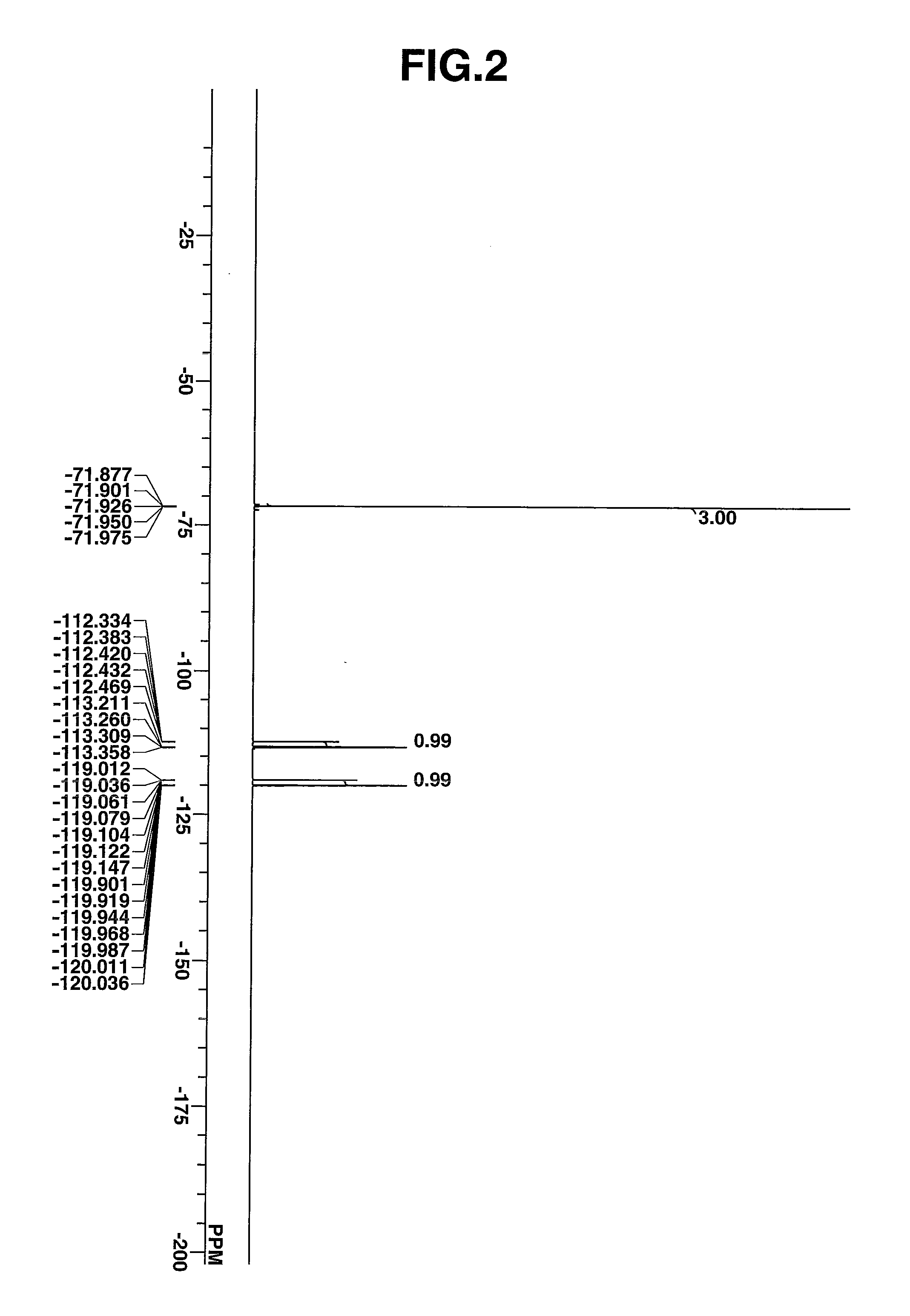
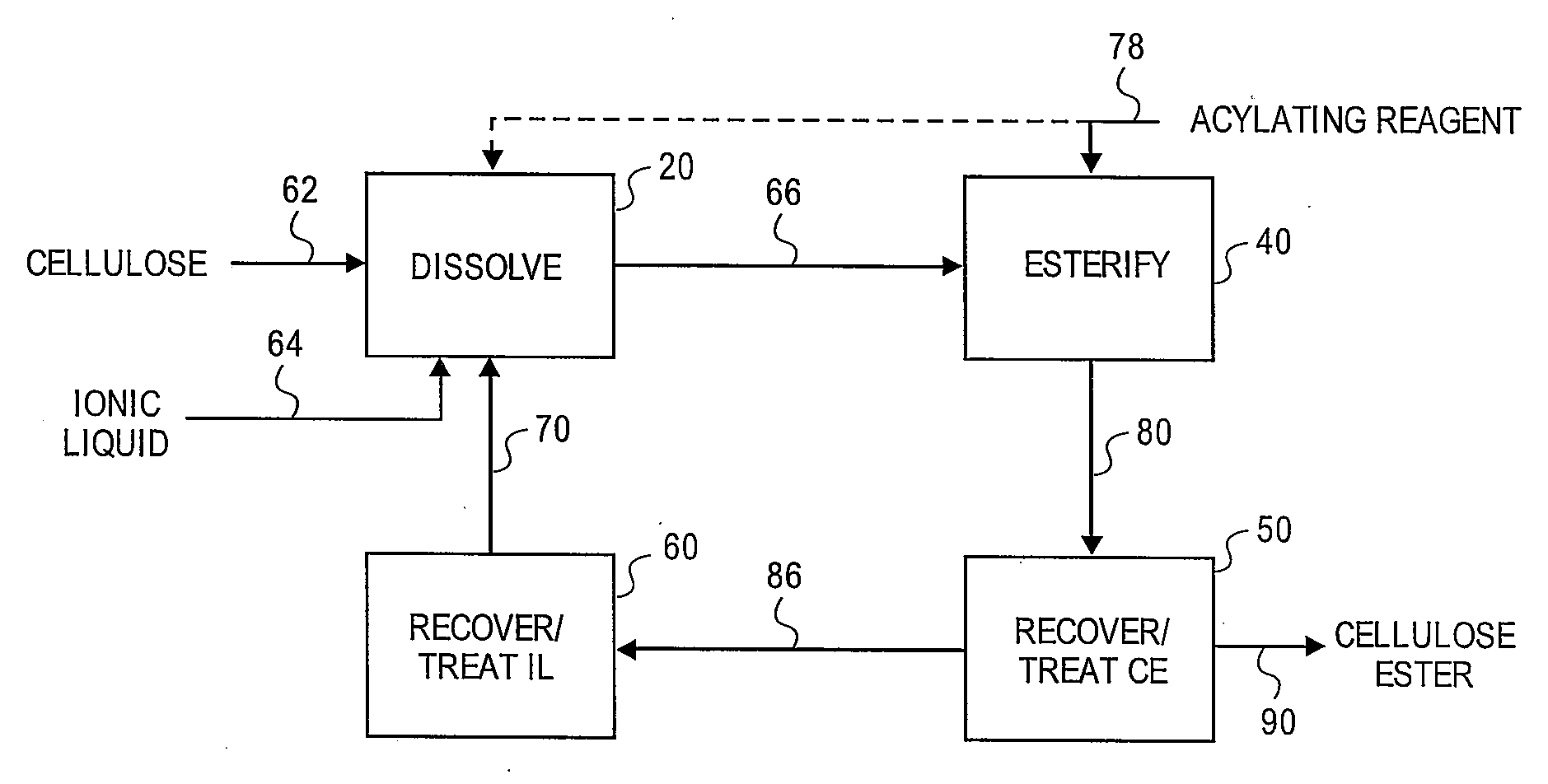
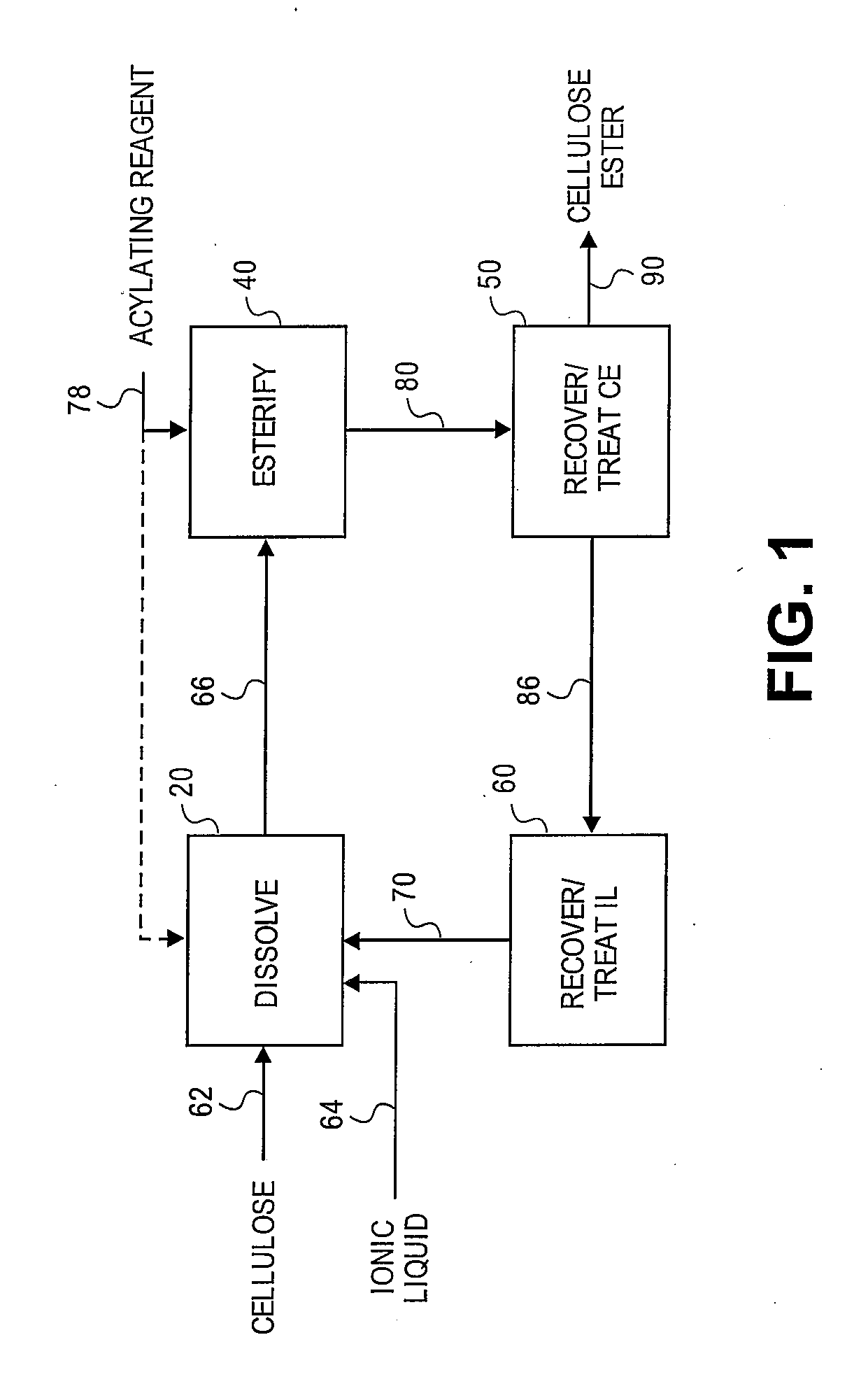
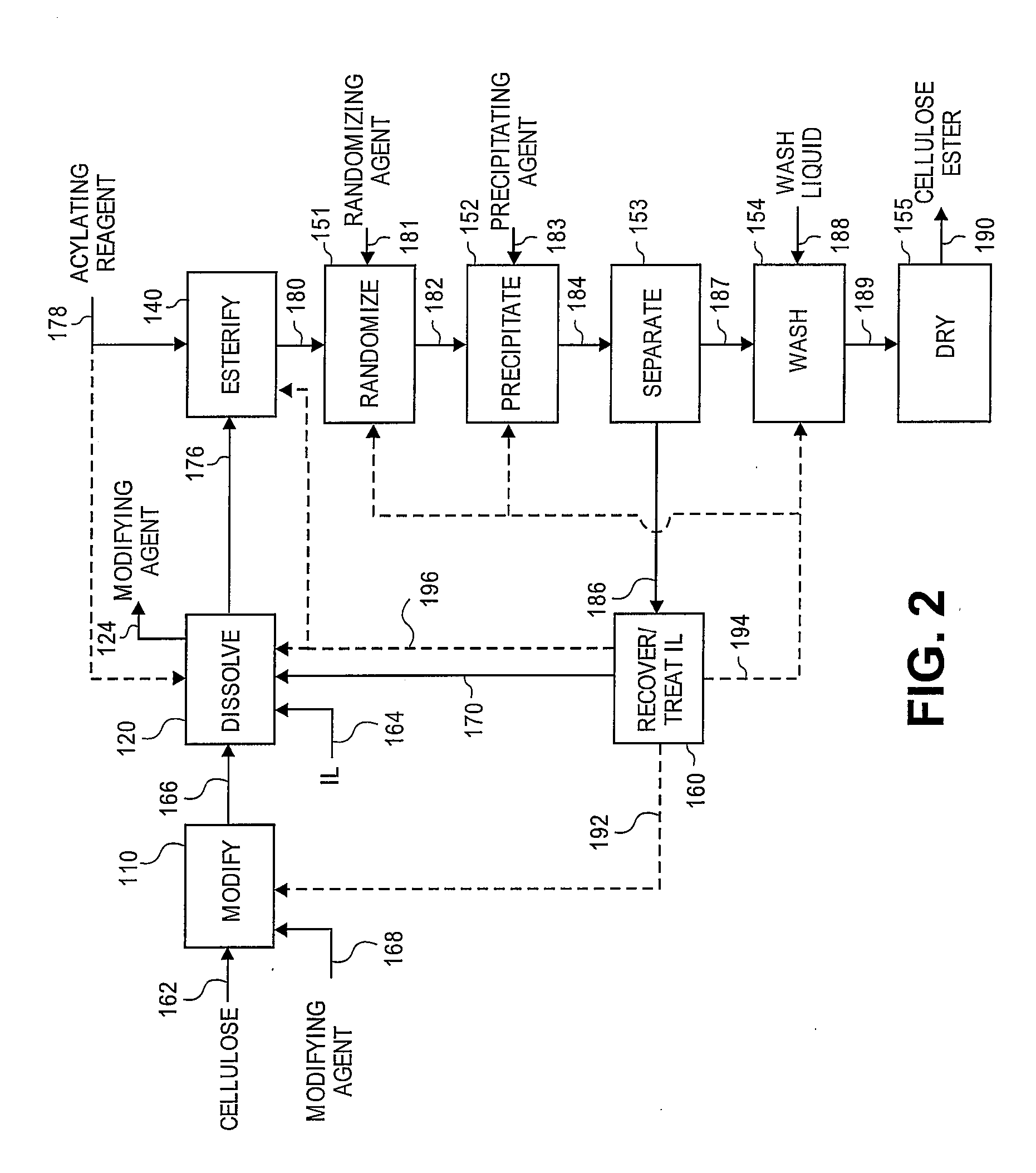
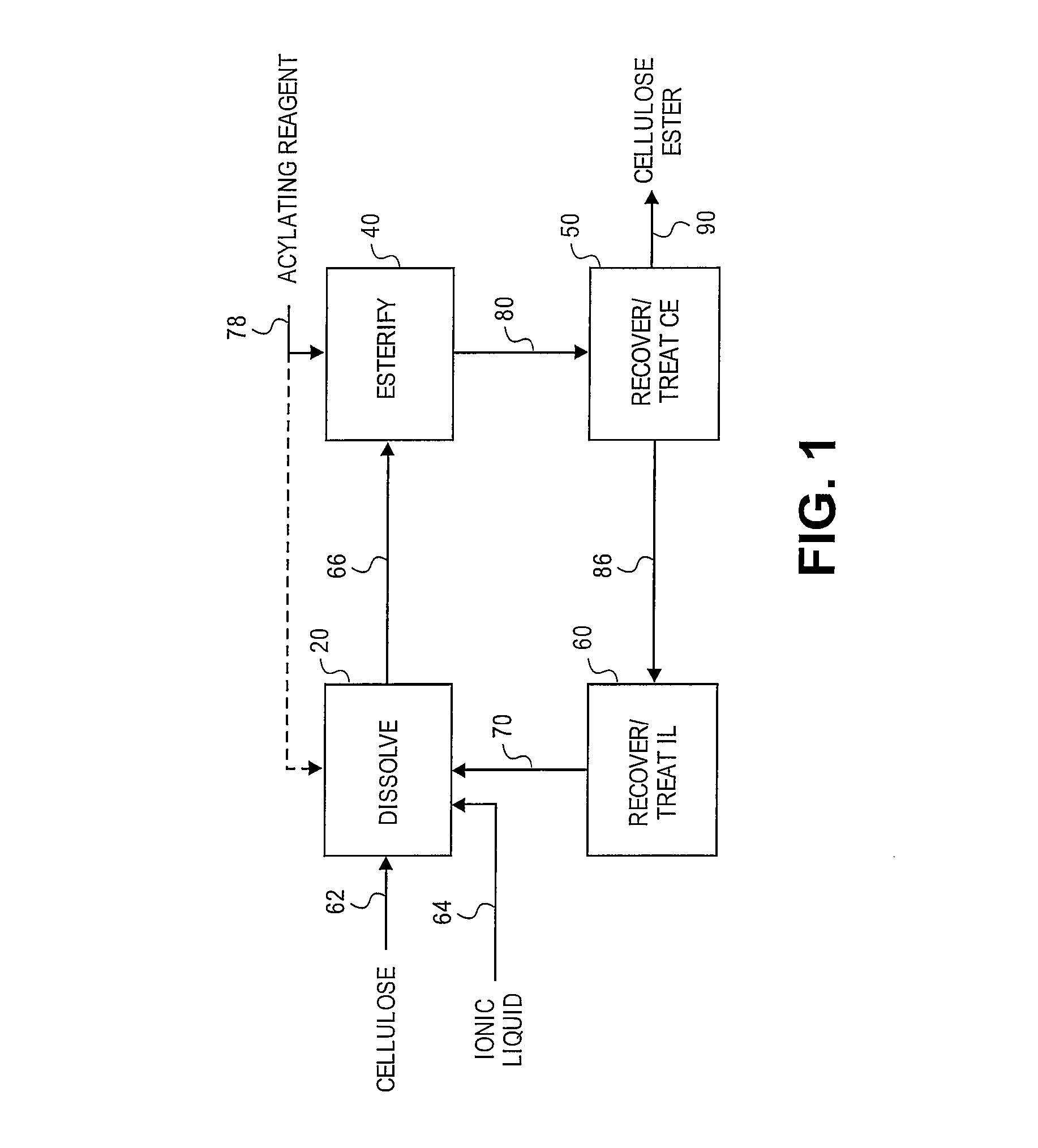
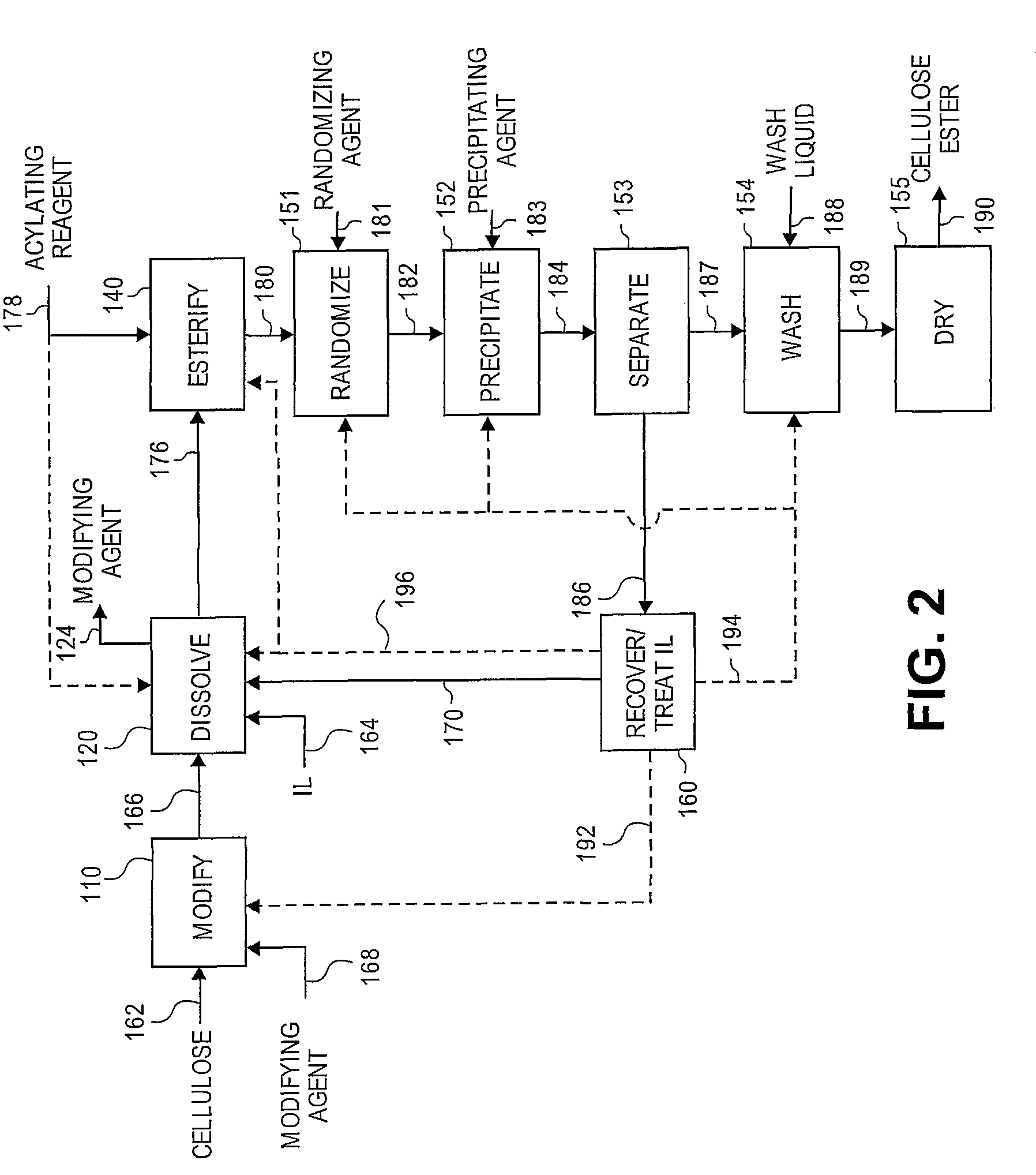
Regioselectively substituted cellulose esters produced in a carboxylated ionic liquid process and products produced therefrom
ActiveUS20100029927A1Increase ratingsPrevent gelOrganic chemistryOptical elementsCelluloseLiquid crystalline
This invention relates to novel compositions comprising regioselectively substituted cellulose esters. One aspect of the invention relates to processes for preparing regioselectively substituted cellulose esters from cellulose dissolved in ionic liquids. Another aspect of the invention relates to the utility of regioselectively substituted cellulose esters in applications such as protective and compensation films for liquid crystalline displays.
Owner:EASTMAN CHEM CO
Polycarboxy/polyol fiberglass binder
InactiveUS7067579B2Excellent recovery and rigidity propertyMany solutionsNon-fibrous pulp additionNon-woven fabricsGlass fiberPolyol
Provided is a novel fiberglass binder comprising a polycarboxy polymer and a polyol. The amount of polycarboxy polymer and polyol contained in the binder is such that the ratio of equivalents of hydroxyl groups to carboxy groups is preferably in the range from from about 0.6 / 1 to 0.8 / 1. It is further preferred that the molecular weight of the polycarboxy polymer is less than 10,000, and more preferably less than 5000.
Owner:JOHNS MANVILLE CORP
HDP-CVD multistep gapfill process
InactiveUS7205240B2Reduce molecular weightReduced characteristicsCellsLiquid surface applicatorsChemistryInert gas
Owner:APPLIED MATERIALS INC
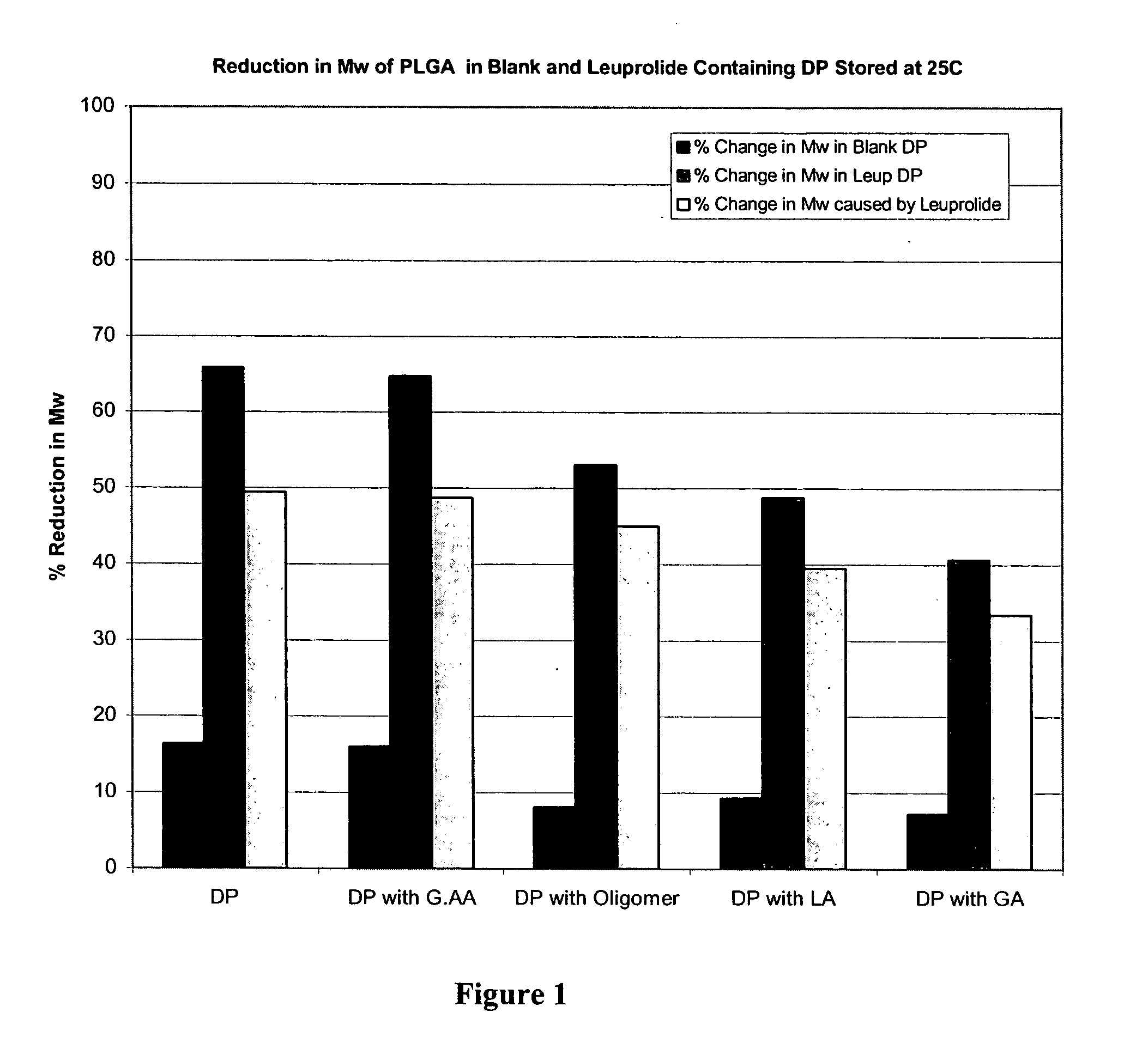
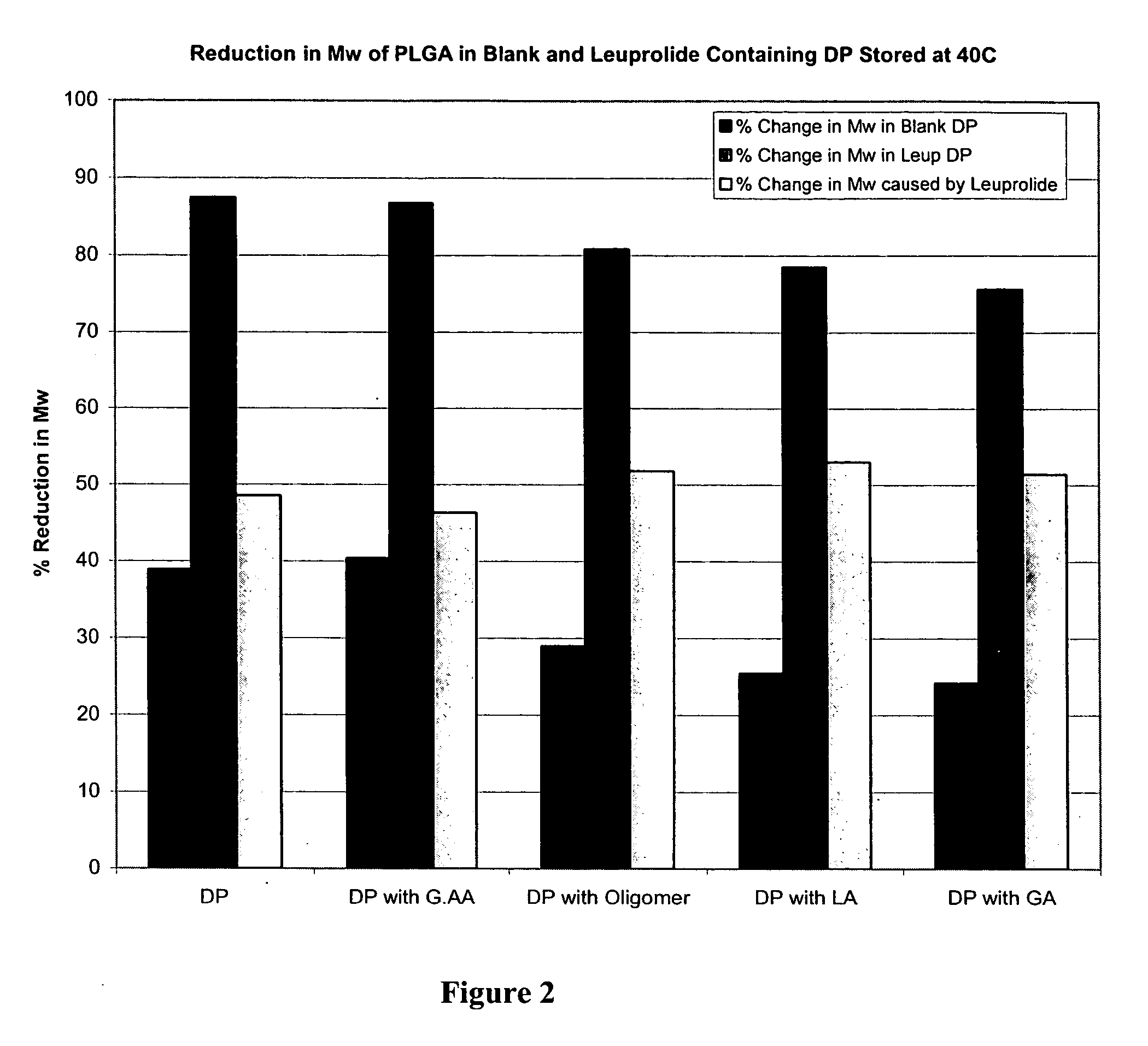
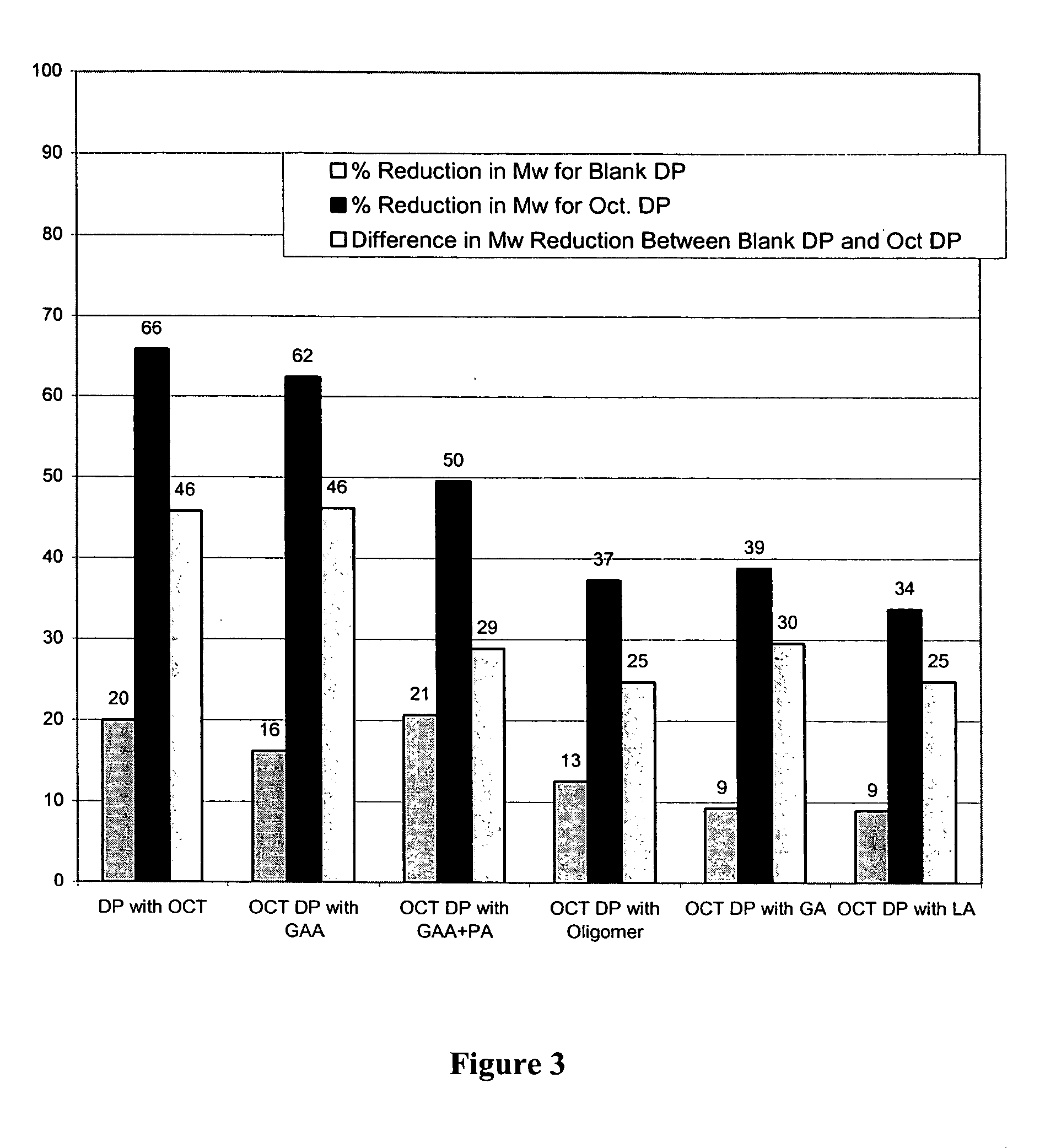
Prevention of molecular weight reduction of the polymer, impurity formation and gelling in polymer compositions
ActiveUS20050042294A1Reduce and eliminate molecular weight reductionReduce molecular weightPowder deliveryBiocideSolubilityActive agent
Polymer and drug containing compositions and method of preparing such compositions are disclosed. The dispersed phase formulation has a polymer, a pharmaceutically or biologically active agent and a small fraction of low pKa acid additive. Stable, filter sterilizable, non-gelling solutions containing GnRH analogues at least at levels typically used in sustained release formulations and a method of increasing solubility of a high level of a GnRH analogue or a freeze-dried antgonist of GnRH in a polymer containing solution are also disclosed. The amount of the acid additive in the polymer solution is such that it is sufficient to increase the solubility of the high level of the GnRH analogue in the polymer solution without affecting the release characteristics of the microspheres prepared therefrom.
Owner:OAKWOOD LAB LLC
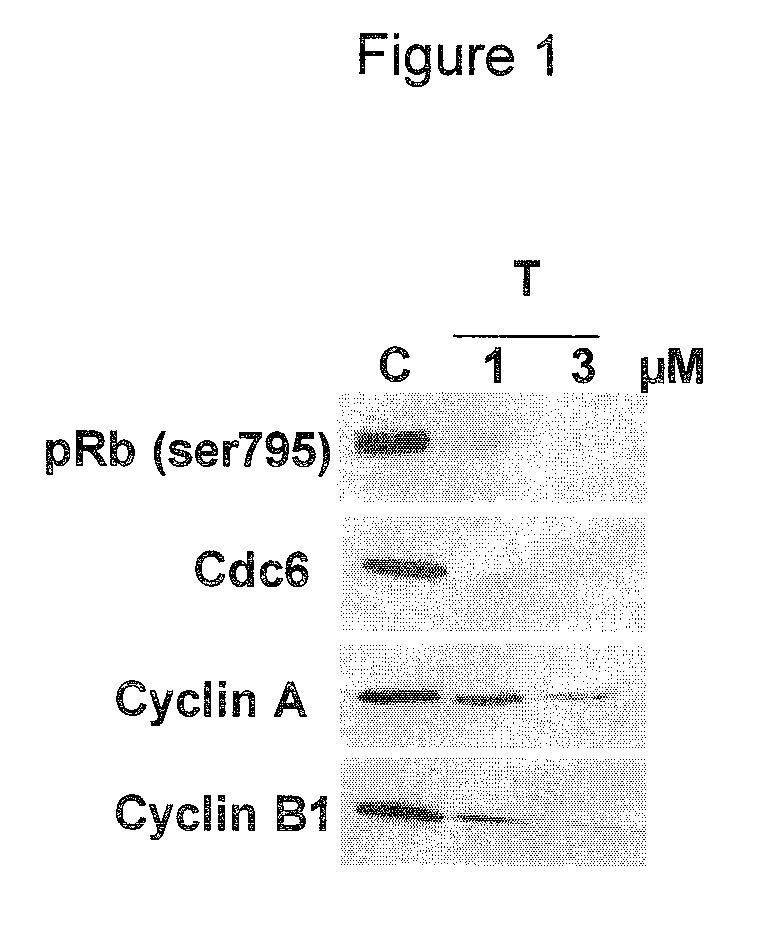
Use of CDK inhibitor for the treatment of glioma
ActiveUS8946226B2Prevent proliferationReduce molecular weightOrganic active ingredientsOrganic chemistryGlioblastomaCytotoxicity
Owner:NERVIANO MEDICAL SERVICES SRL
Olefin production utilizing whole crude oil
InactiveUS20040039240A1Lower temperature rangeReduce molecular weightThermal non-catalytic crackingHydrocarbonsOil productionAlkene
A method for utilizing whole crude oil as a feedstock for the pyrolysis furnace of an olefin production plant wherein the feedstock after preheating is subjected to mild cracking conditions until substantially vaporized, the vapors from mild cracking being subjected to severe cracking in the radiant section of the furnace.
Owner:EQUSR CHEM LP
Enhanced foam stability applications and methods
ActiveUS20120285694A1Improve foam stabilityBoost foam foam expansion ratio foamFluid removalDrilling compositionHydrogenMethyl group
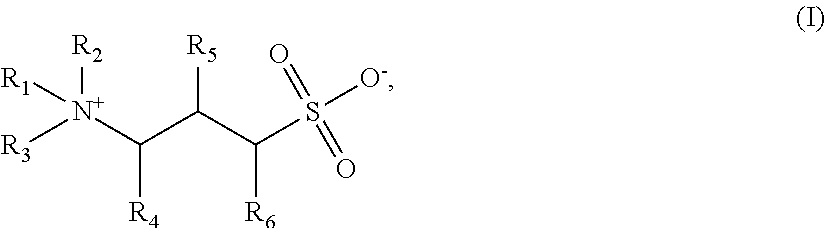
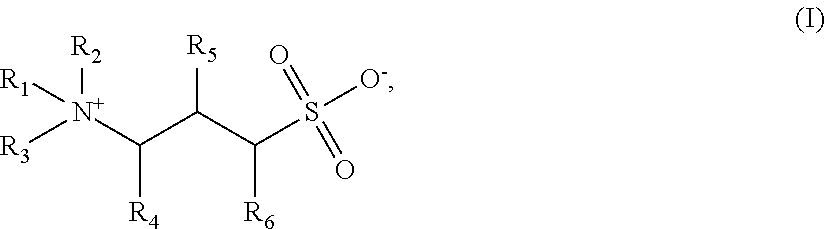
A method for enhancing foam stability in foaming compositions for use in oilfield treatment application, including but not limited to use in mobility control for gas flooding. The method comprises the step of adding to a foaming composition a foam stabilizer of formula (I):wherein R1 is an alkylamido group or a branched or linear alkyl group; R2 and R3 are individually hydrogen or a methyl group; R4, R5 and R6 are individually hydrogen or a hydroxy group, with the proviso that at least one of R4, R5 or R6 is a hydroxyl group; wherein the alkyl group has greater than about 10 carbon atoms. Also disclosed are methods to enhance foam stability in an aqueous foaming composition.
Owner:ENERGY SOLUTIONS (US) LLC
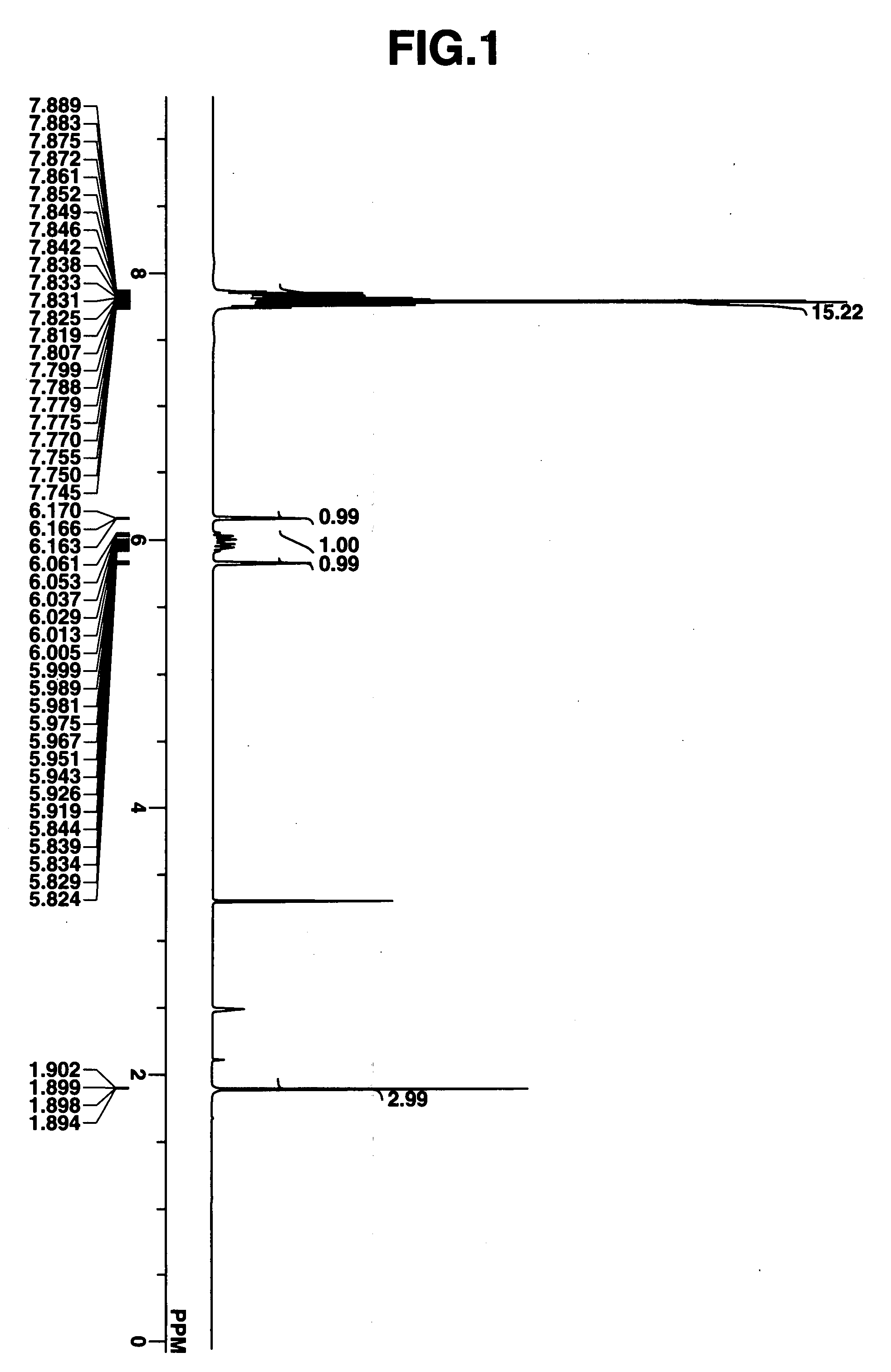
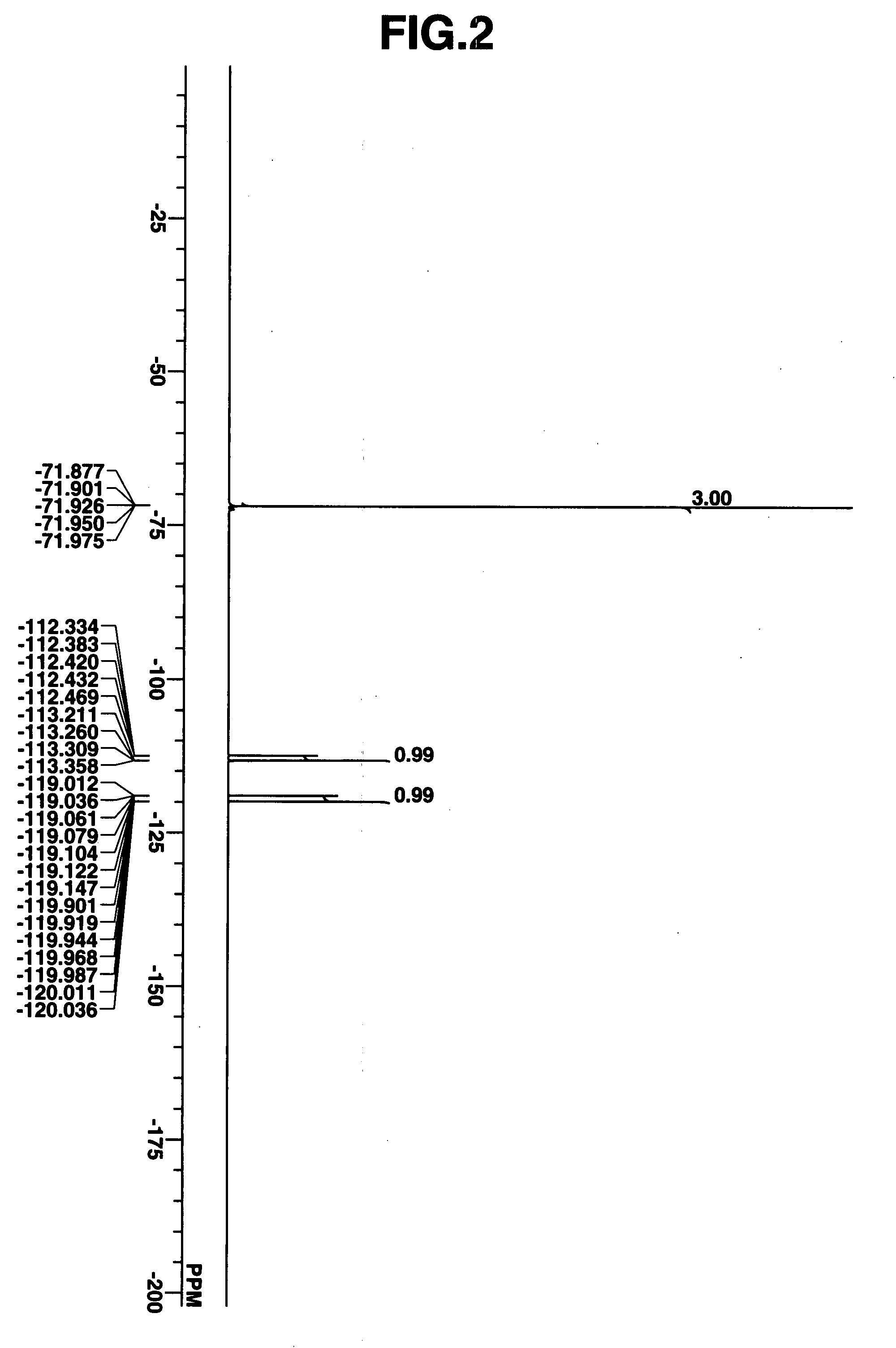
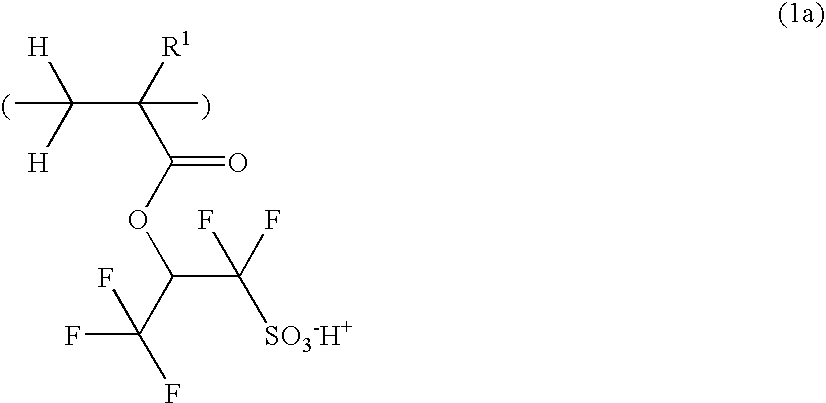
Novel sulfonate salts and derivatives, photoacid generators, resist compositions, and patterning process
ActiveUS20060228648A1Wide spectrum of molecular designReduce molecular weightOrganic chemistryPhotosensitive materialsResistSulfonate
Sulfonate salts have the formula: CF3—CH(OCOR)—CF2SO3−M+ wherein R is C1-C20 alkyl or C6-C14 aryl, and M+ is a lithium, sodium, potassium, ammonium or tetramethylammonium ion. Onium salts, oximesulfonates and sulfonyloxyimides and other compounds derived from these sulfonate salts are effective photoacid generators in chemically amplified resist compositions.
Owner:SHIN ETSU CHEM IND CO LTD
Coated Controlled Release Polymer Particles as Efficient Oral Delivery Vehicles for Biopharmaceuticals
ActiveUS20080268063A1Reduce molecular weightOrganic active ingredientsPowder deliveryActive agentNanoparticle
A composition for delivering an active agent to a patient. The composition includes a polymer core encapsulating the active agent and a mucoadhesive coating disposed about the core. The polymer may include covalently linked poly(ethylene glycol) chains, and the mucoadhesive coating may be selected to facilitate transfer of the particle through the intestinal mucosa. A molecular weight and cross-link density of the polymer may be selected such that the polymer core will decompose in a predetermined time interval. The fraction of the dose of the drug entering the system at circulation during the predetermined time interval may be between about 0.25% and about 25%. The composition may be formulated as a plurality of nanoparticles or microparticles that are combined with a pharmaceutically acceptable carrier to produce an edible or inhalable drug product.
Owner:THE BRIGHAM & WOMENS HOSPITAL INC +1
Thermal crystallization of a molten polyester polymer in a fluid
InactiveUS7192545B2Reduce molecular weightEnergy efficiencyDrying gas arrangementsCeramic shaping apparatusPolyesterLiquid medium
A process for crystallizing a polyester polymer by introducing a molten polyester polymer, such as a polyethylene terephthalate polymer, into a liquid medium at a liquid medium temperature greater than the Tg of the polyester polymer, such as at a temperature ranging from 100° C. to 190° C., and allowing the molten polyester polymer to reside in the liquid medium for a time sufficient to crystallize the polymer under a pressure equal to or greater than the vapor pressure of the liquid medium. A process flow, underwater cutting process, crystallization in a pipe, and a separator are also described.
Owner:ALPEK POLYESTER SA DE CV
Polymeric marker with high radiopacity for use in medical devices
InactiveUS20050255317A1Overcomes shortcomingImprove fill ratePowder deliverySurgeryPolymer resinRadiopaque agent
High radiopacity is achieved in a polymeric marker by combining a polymeric resin, a powdered radiopaque agent having uniformly shaped particles of a specific particle size distribution, and a wetting agent. The method to produce the marker calls for the blending and pelletization of these materials followed by extrusion onto support beading. The resulting supported tubing is subsequently cut to length with the beading still in place. After ejection of the beading remnant the marker is slipped into place on the device to be marked and attached by melt bonding. Marking of a guide wire allows lesions to be measured while the marking of balloon catheters allow the balloon to be properly positioned relative to a lesion.
Owner:ABBOTT CARDIOVASCULAR
Catalyst systems and their use for metathesis reactions
ActiveUS20090069516A1Narrow molecular weight distributionReduce molecular weightOrganic-compounds/hydrides/coordination-complexes catalystsAdhesive processes with surface pretreatmentNitrile rubberInorganic chemistry
Novel catalyst systems for metathesis reactions, in particular for the metathesis of nitrile rubber, which contain a specific salt additive in addition to the metathesis catalyst are provided.
Owner:ARLANXEO DEUT GMBH
Polymerizable anion-containing sulfonium salt and polymer, resist composition, and patterning process
A polymerizable anion-containing sulfonium salt having formula (1) is provided wherein R1 is H, F, methyl or trifluoromethyl, R2, R3 and R4 are C1-C10 alkyl, alkenyl or oxoalkyl or C6-C18 aryl, aralkyl or aryloxoalkyl, or two of R2, R3 and R4 may bond together to form a ring with S, A is a C2-C20 hydrocarbon group having cyclic structure, and n is 0 or 1. The sulfonium salt generates a very strong sulfonic acid upon exposure to high-energy radiation. A resist composition comprising a polymer derived from the sulfonium salt is also provided.
Owner:SHIN ETSU CHEM CO LTD
Supported catalysts having a controlled coordination structure and methods for preparing such catalysts
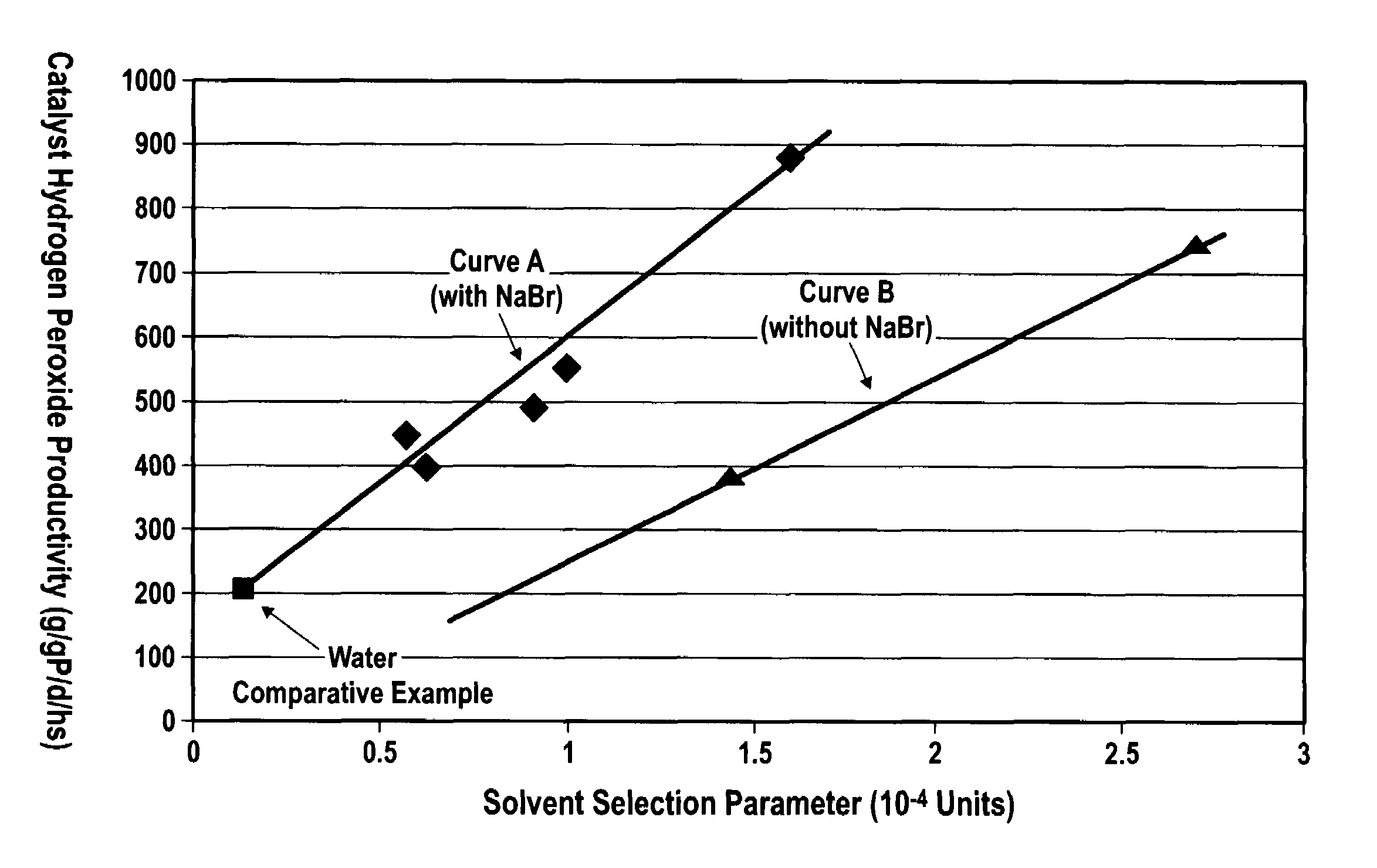
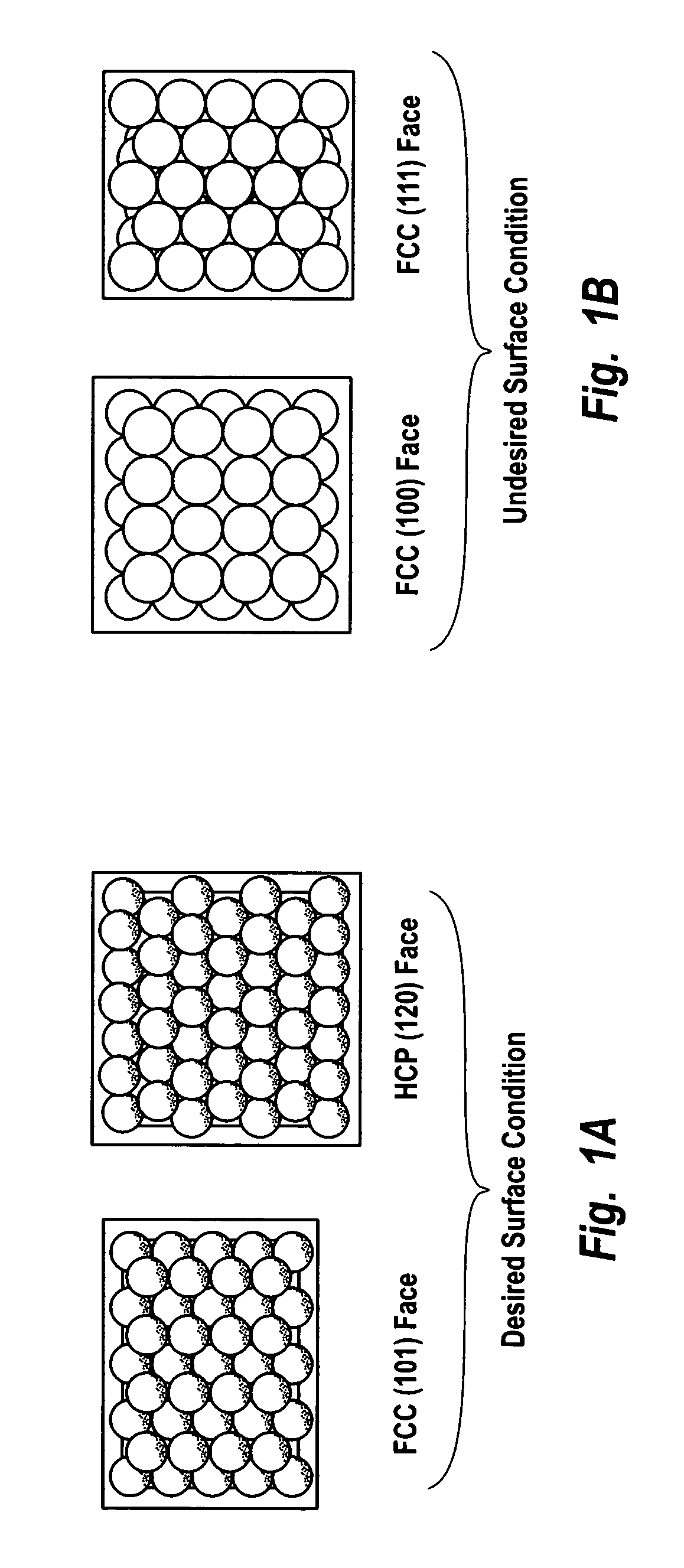
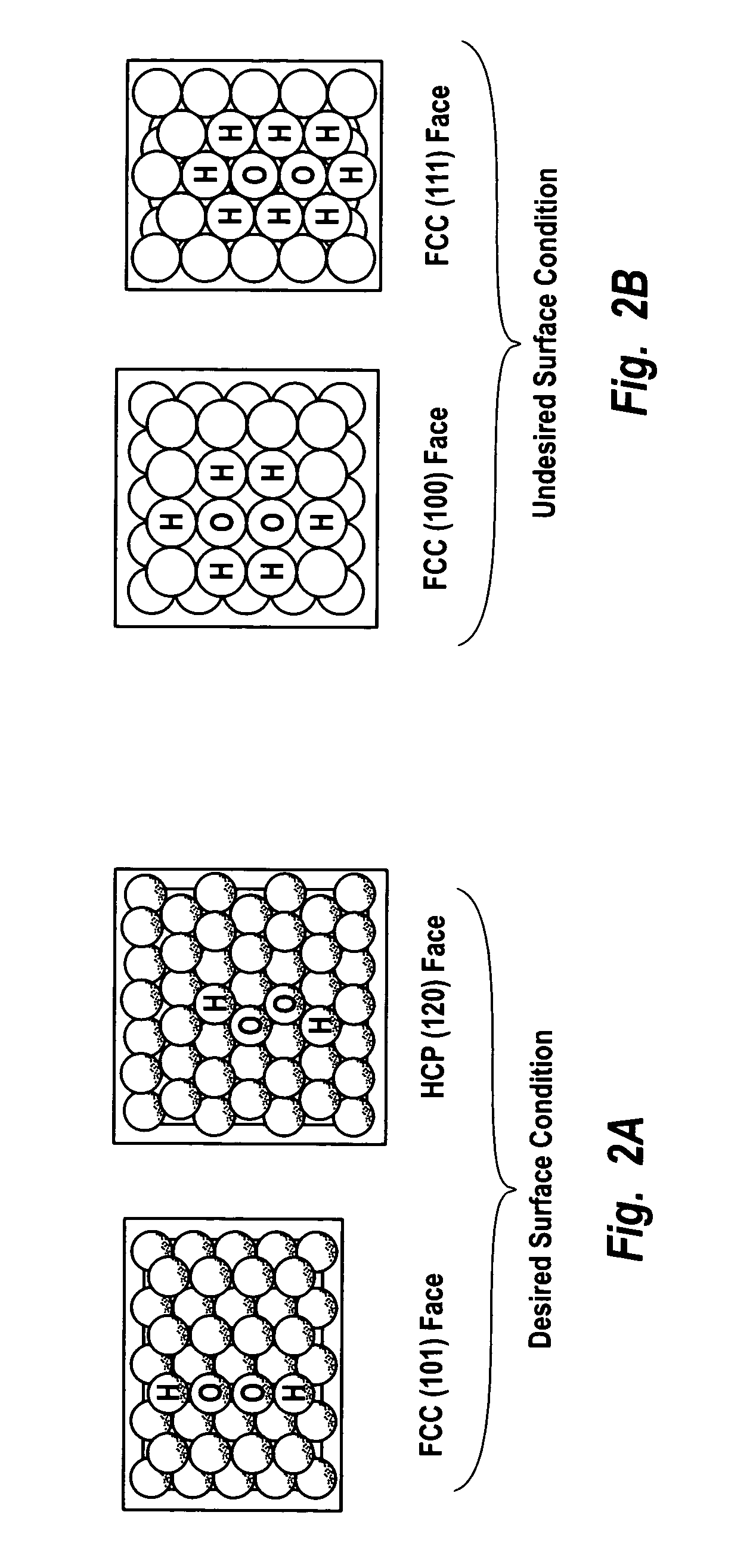
ActiveUS7011807B2Reduce molecular weightReduce the possibilityHydrogen peroxideHydrogenHydrogenOrganic fluid
Supported reactive catalysts having a controlled coordination structure and methods for their production are disclosed. The supported catalysts of the present invention are useful for the preparation of hydrogen peroxide with high selectivity in addition to other chemical conversion reactions. The supported catalyst comprises catalyst particles having top or outer layer of atoms in which at least a portion of the atoms exhibit a controlled coordination number of 2. The catalyst and methods may be used for the concurrent in situ and ex situ conversion of organic compounds. In addition, a process is provided for catalytically producing hydrogen peroxide from hydrogen and oxygen feeds by contacting them with the catalysts of the invention and a suitable organic liquid solvent having a Solvent Selection Parameter (SSP) between 0.14×10−4 and 5.0×10−4.
Owner:BORAL IP HLDG
Cellulose esters and their production in carboxylated ionic liquids
InactiveUS8148518B2Increase ratingsPrevent gelSugar derivativesSugar derivatives preparationCelluloseIonic liquid
Ionic liquids and cellulose ester compositions and processes and apparatus for producing ionic liquids and cellulose esters. Cellulose esters can be produced by dissolving cellulose in carboxylated ionic liquids and thereafter contacting the cellulose solution with at least one acylating reagent. Cellulose esters produced via the present invention can comprise ester groups that originate from the carboxylated ionic liquid and / or the acylating reagent.
Owner:EASTMAN CHEM CO
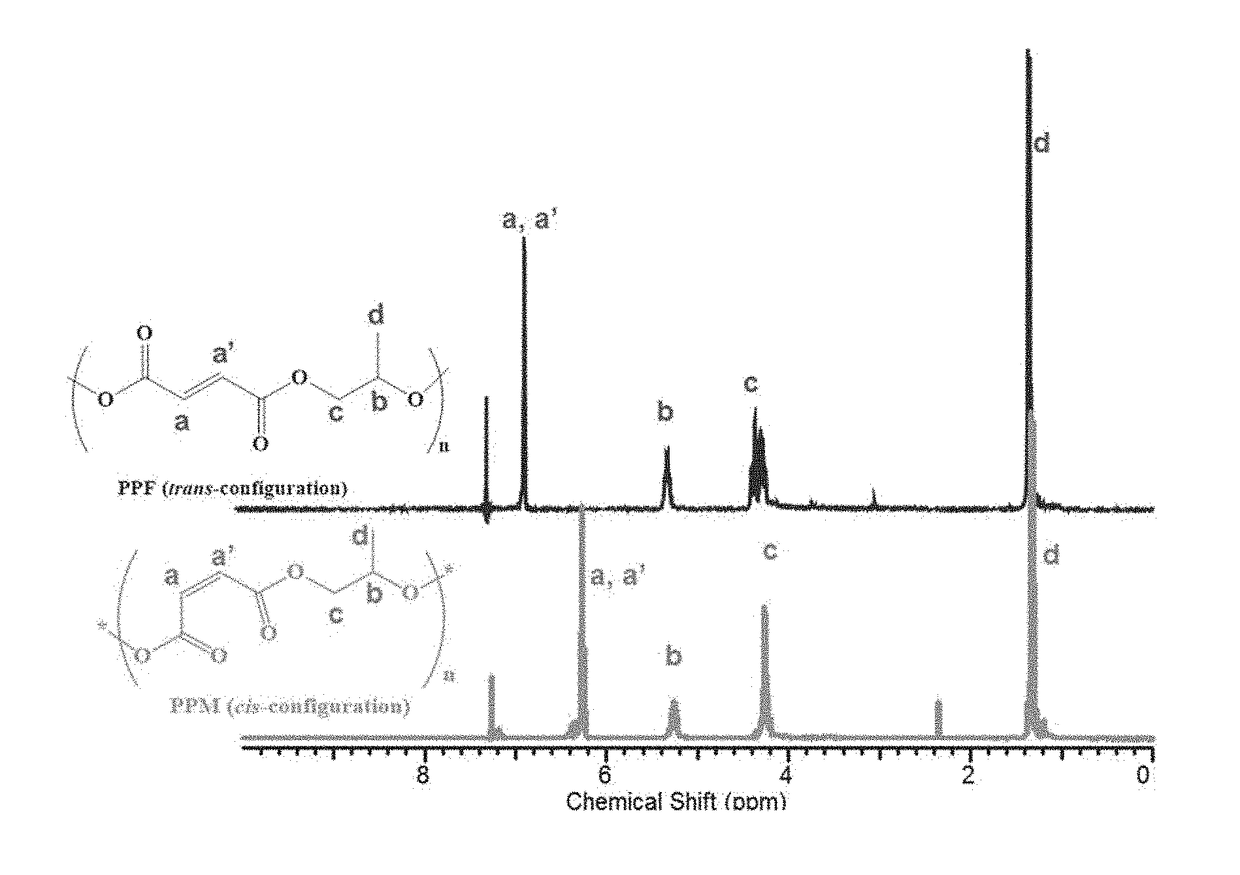
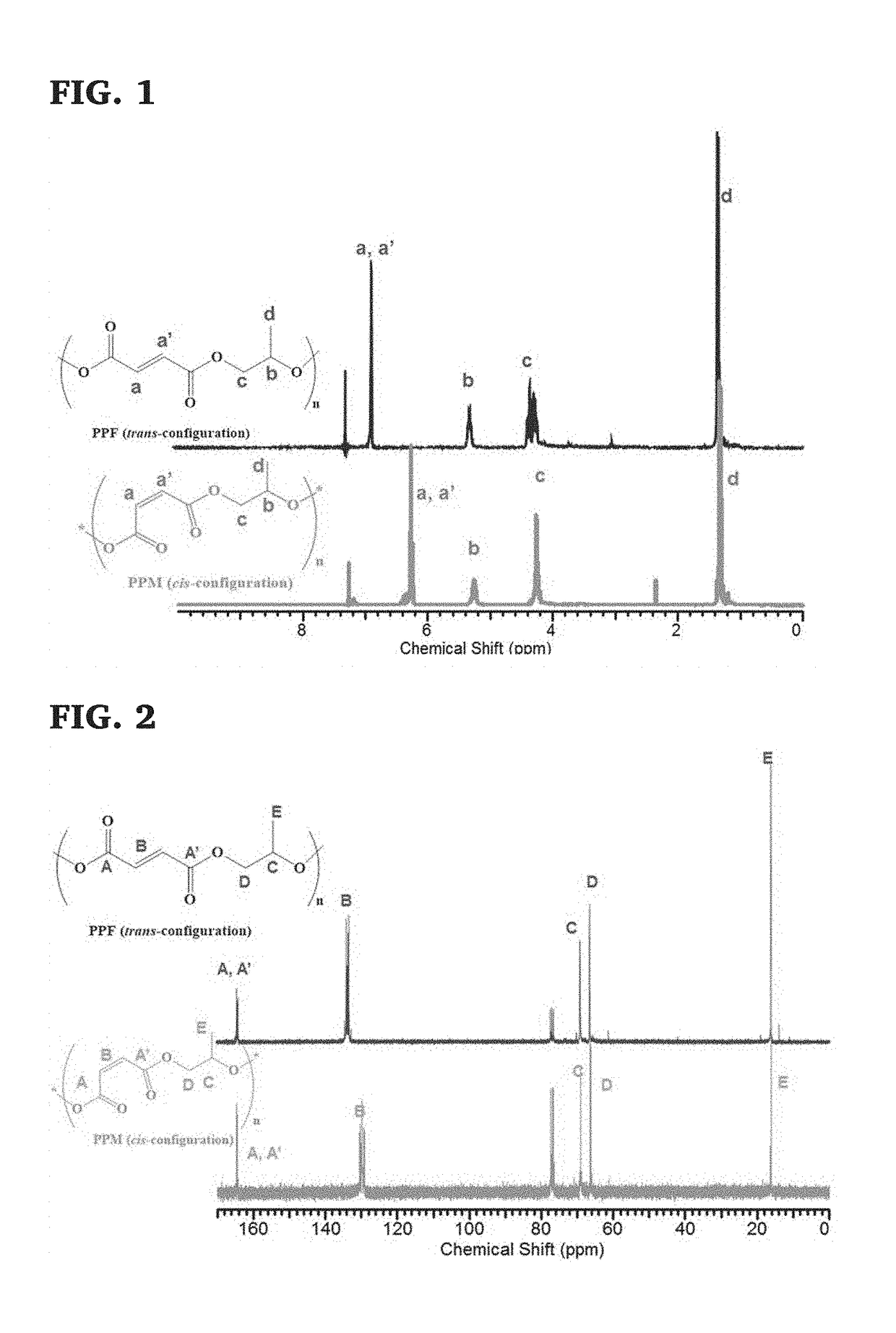
Well-defined degradable poly(propylene fumarate) polymers and scalable methods for the synthesis thereof
ActiveUS20170355815A1Constrained and predictable material propertyCheap to makeAdditive manufacturing apparatusProsthesisOrganic chemistryMedical device
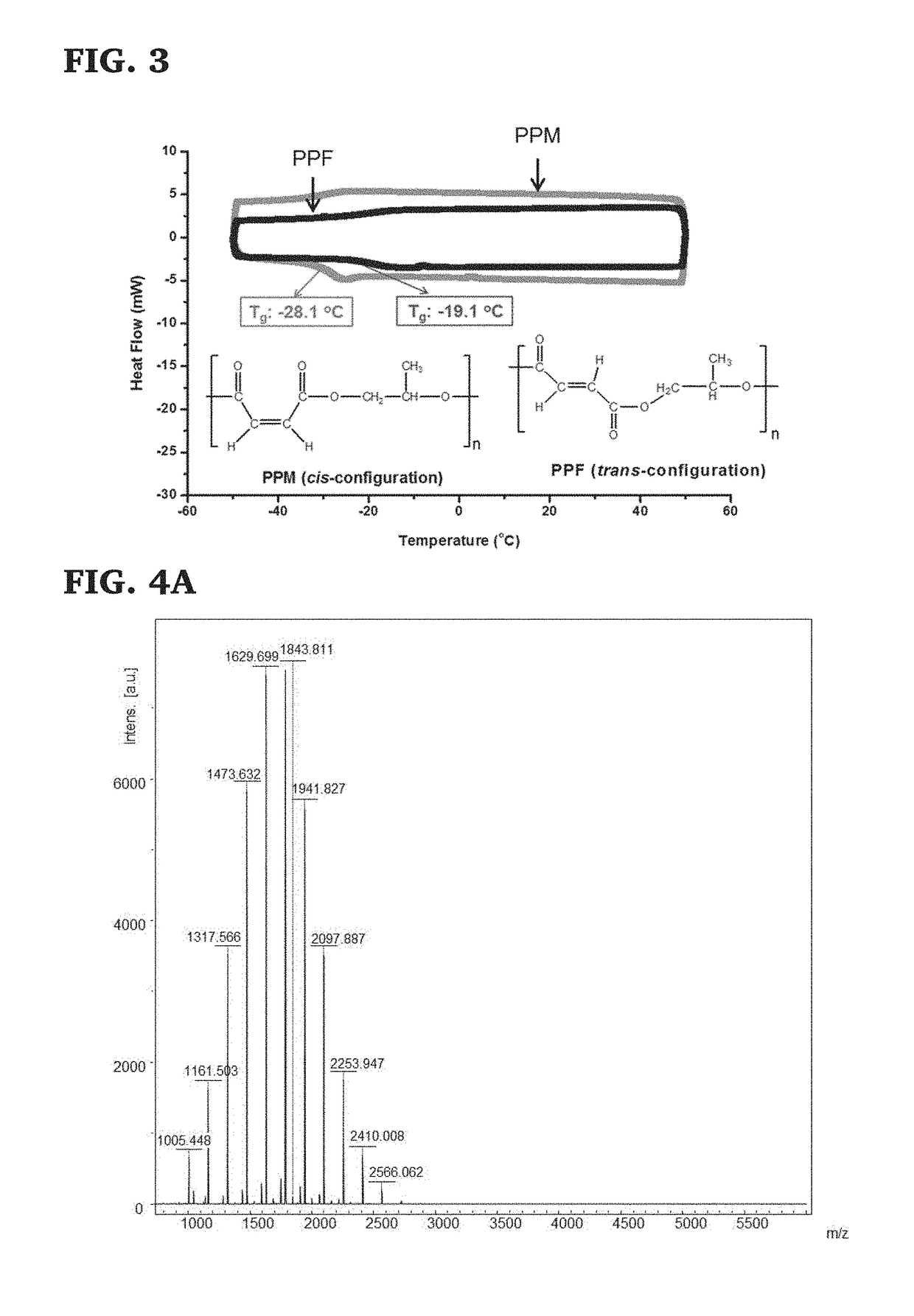
The present invention provides a low molecular mass PPF polymer (and related methods) that is suitable for 3D printing and other polymer device fabrication modalities and can be made inexpensively in commercially reasonable quantities. These novel low molecular mass PPF polymers have a low molecular mass distribution (m) and a wide variety of potential uses, particularly as a component in resins for 3D printing of medical devices. The ability to produce low m PPF creates a new opportunity for reliable GMP production of PPF. It provides low cost synthesis and scalability of synthesis, blending of well-defined mass and viscosity PPF, and reduced reliance on solvents or heat to (a) achieve mixing of 3D printable resins or (b) and flowability during 3D printing. These PPF polymers are non-toxic, degradable, and resorbable and can be used in tissue scaffolds and medical devices that are implanted within a living organism.
Owner:THE UNIVERSITY OF AKRON +1
Mutation analysis using mass spectrometry
InactiveUS6503710B2Rapid and economic sample preparationAccurate massSamplingSugar derivativesChemical treatmentFree form
The invention presents a method for examining genetic material (deoxyribonucleic acid, DNA) to detect the presence of pre-known mutations, especially single nucleotide polymorphisms (SNP), using mass spectrometry with ionization by matrix-assisted laser desorption (MALDI). The invention uses nucleoside triphosphates with modified sites for the method of primer extension in a duplicating, enzymatic reaction and at least partially removal of primers from the extension product, in combination with product neutralization by chemical treatment of the modified sites, so that the resulting DNA products can be, by using special matrix materials, preferredly ionized in an adduct-free form over other constituents in the reaction solution without any further cleaning. The method is particularly suitable for simultaneous identification of several mutations by multiplexing.
Owner:BRUKER DALTONIK GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com