Patents
Literature
5207results about "Surgical adhesives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
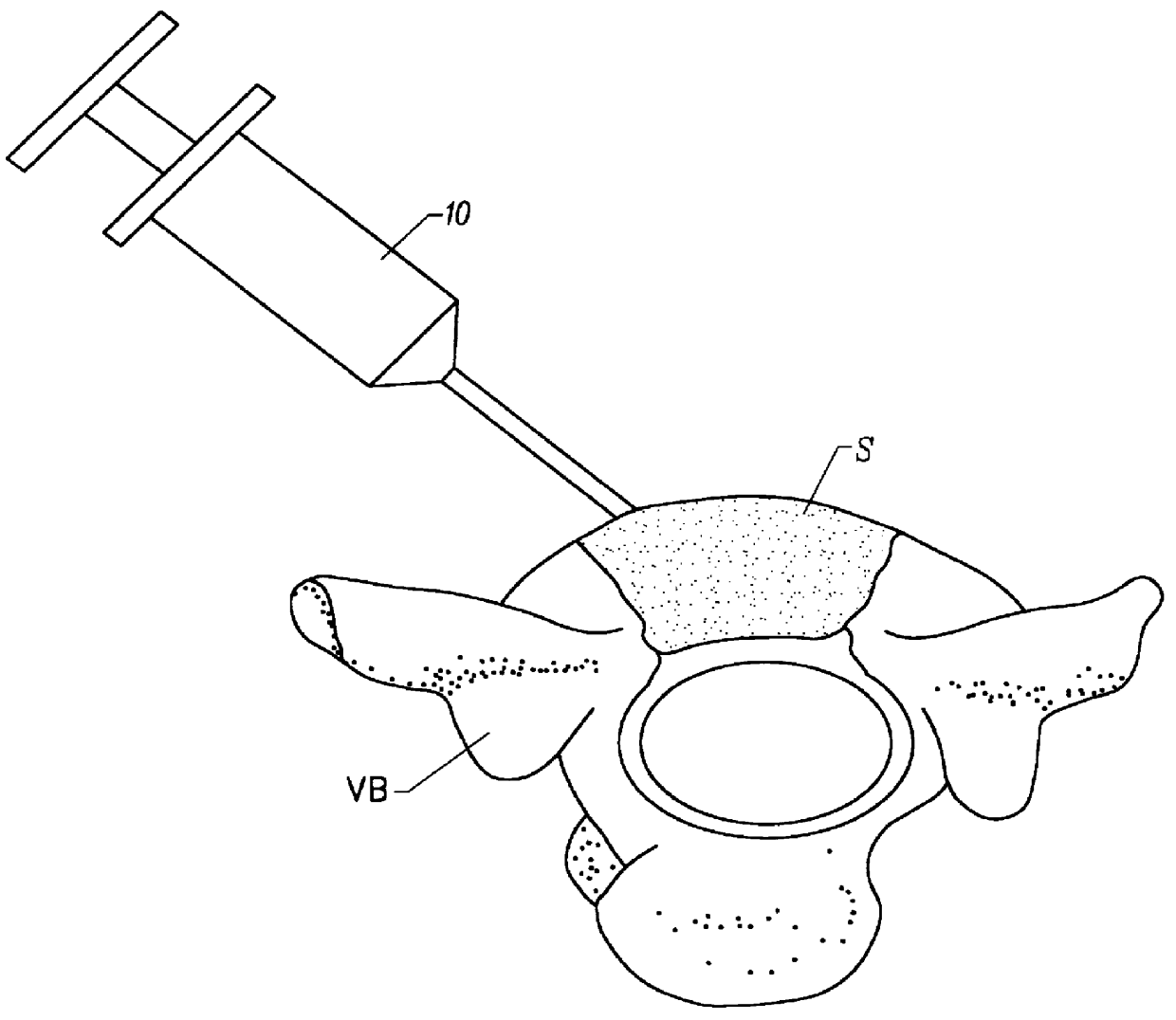
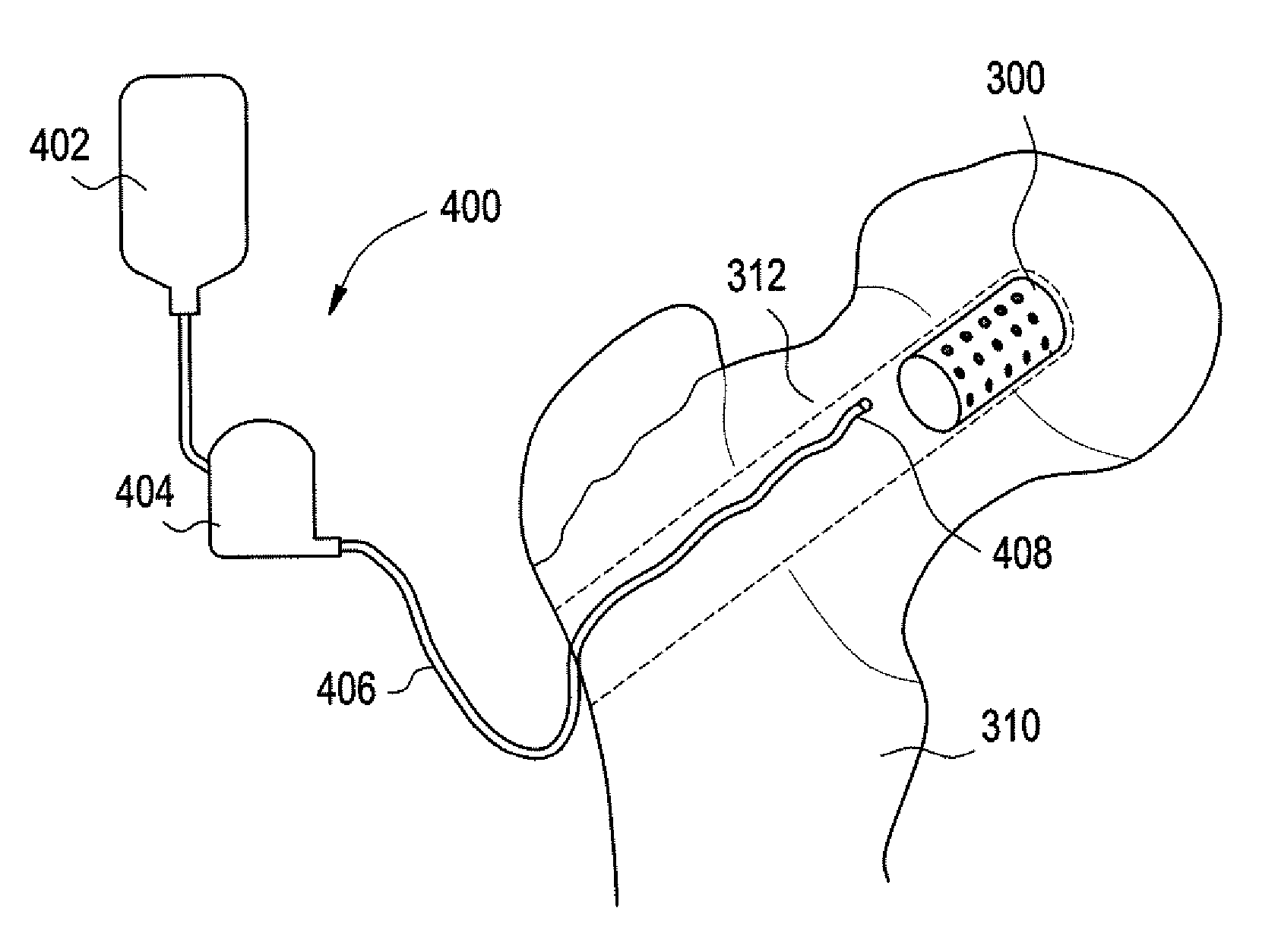
Vertebroplasty injection device
InactiveUS7008433B2Small sizeIncrease pressurePowder deliveryShaking/oscillating/vibrating mixersInjectable biomaterialBone cement
This invention relates to a mixing and delivery device suitable for delivering injectable biomaterials, and to preferred bone cement formulations.
Owner:DEPUY ACROMED INC
Water-swellable copolymers and articles and coatings made therefrom
Owner:TYCO HEALTHCARE GRP LP
Self-supporting, shaped, three-dimensional biopolymeric materials and methods
Self-supporting, shaped, three-dimensional cross-linked proteinaceous biopolymeric materials that may be implanted in vivo, and methods of making such materials are disclosed. The biopolymeric materials most preferably include reinforcing media, such as biocompatible fibrous or particulate materials. In use, the preformed, shaped biopolymeric materials may be applied to tissue in need of repair and then sealed around its edges with a liquid bioadhesive. In such a manner, repaired tissue which is capable of withstanding physiological pressures may be provided.
Owner:CRYOLIFE
Nanostructure-enhanced platelet binding and hemostatic structures
InactiveUS8319002B2Enhancing overall rate and strengthInduce platelet binding and efficient hemostasisBiocideSurgical adhesivesPlateletNanofiber
Methods, systems, and apparatuses for nanomaterial-enhanced platelet binding and hemostatic medical devices are provided. Hemostatic materials and structures are provided that induce platelet binding, including platelet binding and the coagulation of blood at a wound / opening caused by trauma, a surgical procedure, ulceration, or other cause. Example embodiments include platelet binding devices, hemostatic bandages, hemostatic plugs, and hemostatic formulations. The hemostatic materials and structures may incorporate nanostructures and / or further hemostatic elements such as polymers, silicon nanofibers, silicon dioxide nanofibers, and / or glass beads into a highly absorbent, gelling scaffold. The hemostatic materials and structures may be resorbable.
Owner:NANOSYS INC
Bioabsorbable Surgical Compositions
Owner:TYCO HEALTHCARE GRP LP
Hemostatic compositions for arresting blood flow from an open wound or surgical site
A hemostatic composition for stopping or decreasing blood flow from an open wound or medical or surgical procedure. Compositions of the invention comprise a mixture of a cationic polymer and a cation exchange material. In one embodiment, the composition comprises a mixture: (1) a high molecular weight copolymer of diallyl dimethyl ammonium chloride (DADMAC) and acrylamide [DADMAC copolymer], and (2) the hydrogen form of a crosslinked, sulfonated polystyrene (hydrogen resin). In an exemplified embodiment, a composition of the invention comprises the mixture of DADMAC copolymer and hydrogen resin provided in a dry powdered form. The compositions of the invention may be applied directly to a wound or treatment site, or they may be incorporated into a wound dressing, such as a bandage. The seal formed at a wound or treatment site treated with the present invention is adhesive and exhibits considerable toughness.
Owner:BIOLIFE
Non-metallic implant devices and intra-operative methods for assembly and fixation
This invention relates to orthopedic implants and to methods of treating bone defects. More specifically, but not exclusively, the present invention is directed to non-metallic implants and to methods for intra-operative assembly and fixation of orthopedic implants to facilitate medical treatment. The non-metallic implant assembly can be secured to underlying tissue by a fastener, such as a bone screw, that is capable of swelling on contact with fluid in the underlying tissue. Alternatively, the non-metallic implant assembly can be assembled intra-operatively using a fastener that is adhesively bonded to a bone plate or the bone plate can be deformed using heat, force, or solvents to inhibit withdrawal of the fastener. In preferred embodiments, both the fastener and the bone plate are formed of biodegradable material.
Owner:WARSAW ORTHOPEDIC INC
Fast curing compositions
Fast curing surgical adhesives and sealants contain an NCO-terminated hydrophilic urethane prepolymer derived from an aromatic diisocyanate and a polyol. Substantially all the aromatic diisocyanate used to prepare the NCO-terminated hydrophilic urethane prepolymer is in the para form. Optionally, the aromatic diisocyanate is substituted with at least one electron withdrawing group, such as, for example, a fluorine group.
Owner:TYCO HEALTHCARE GRP LP
Adhesive formulatiions
The disclosure relates to biocompatible components useful for forming compositions for use as medical / surgical synthetic adhesives and sealants. Biocompatible components of the present disclosure may include a multifunctional amine or multifunctional polyol core, with isocyanate and / or polyalkylene oxide arms, which may optionally be capped with electrophilic or nucleophilic groups. These biocompatible components may, in embodiments, be combined with optional cross linkers to form adhesive and / or sealant compositions.
Owner:TYCO HEALTHCARE GRP LP
Adhesive-containing wound closure device and method
InactiveUS20050182443A1Enlarging woundShorten operation timeSurgical adhesivesPharmaceutical delivery mechanismBiomedical engineeringWound closure
Owner:ETHICON INC
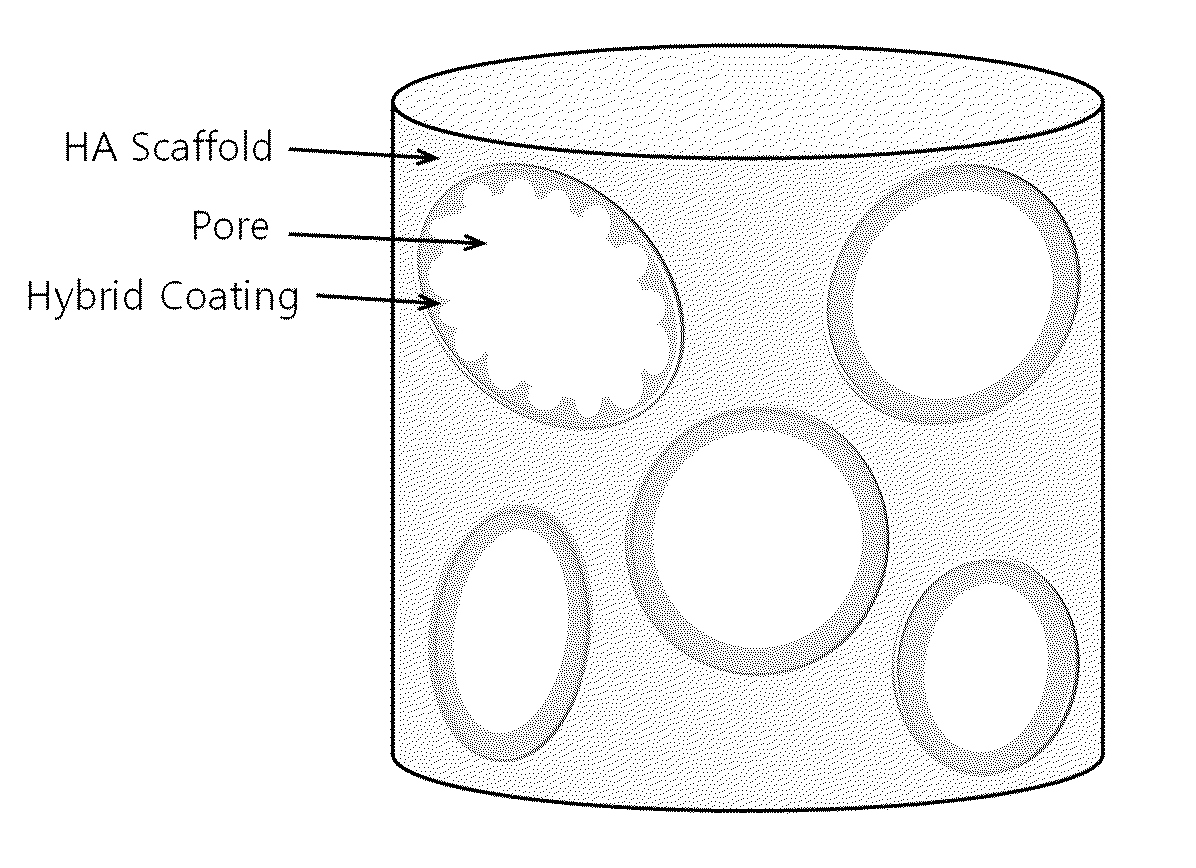
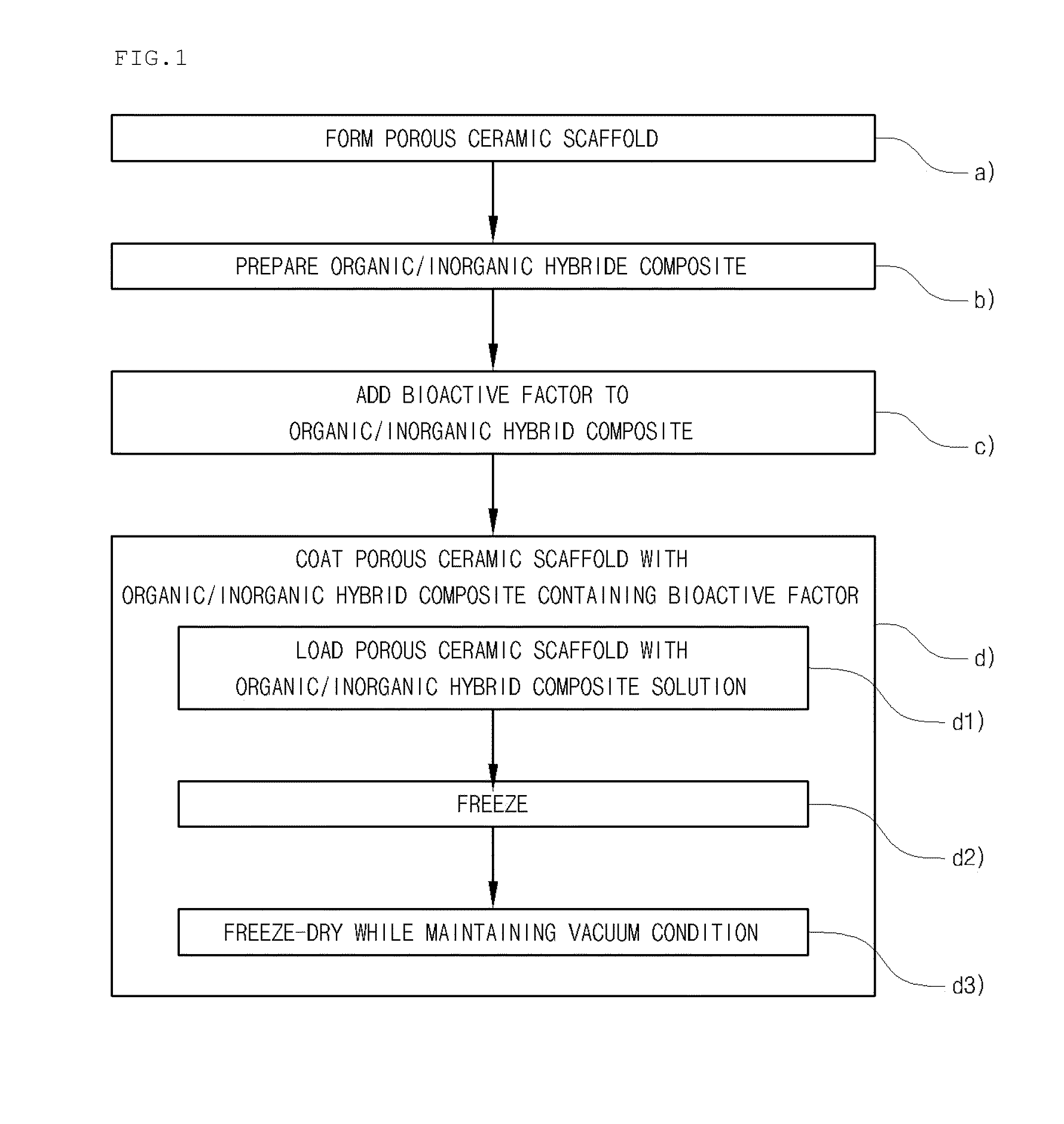
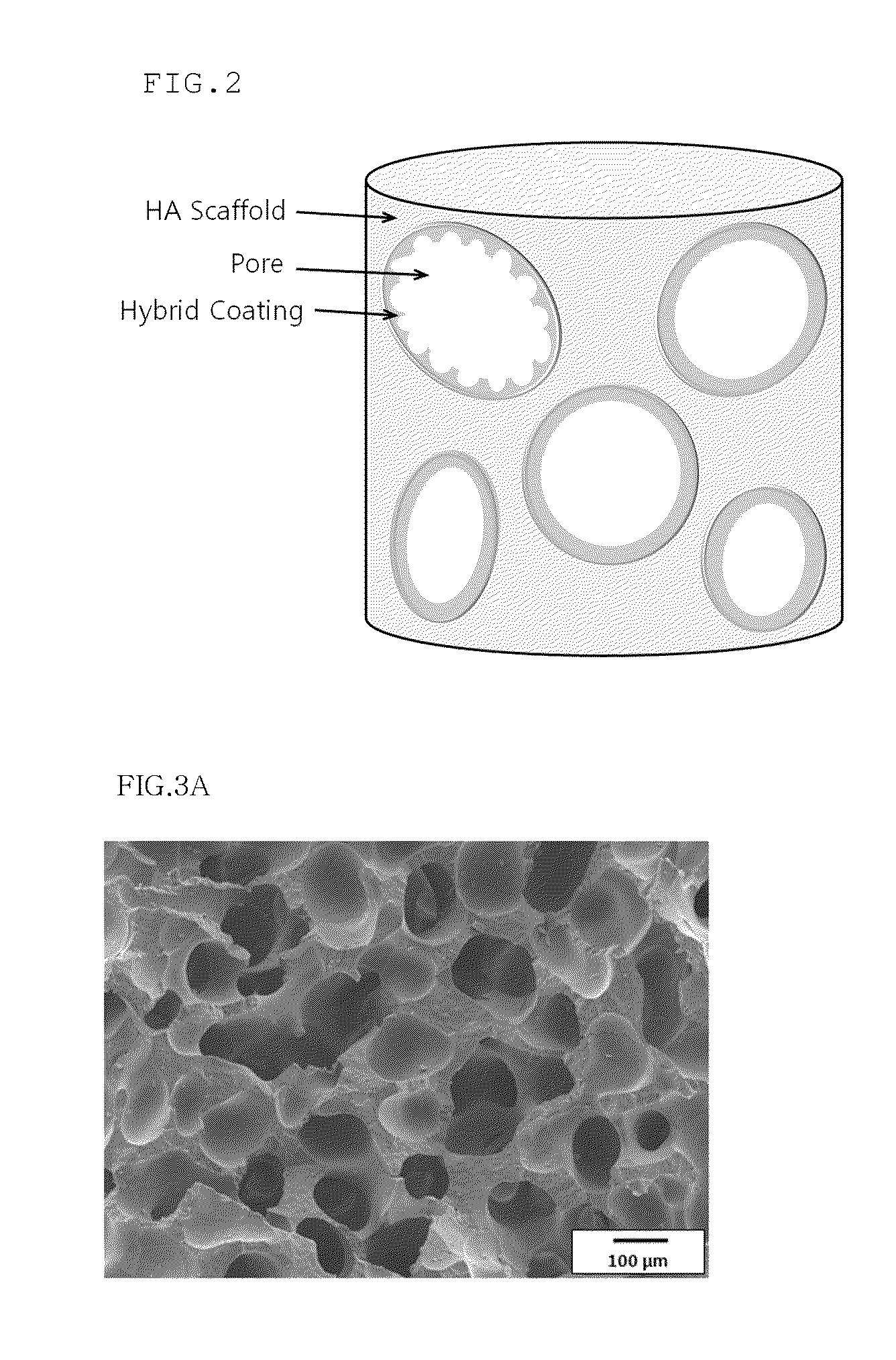
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
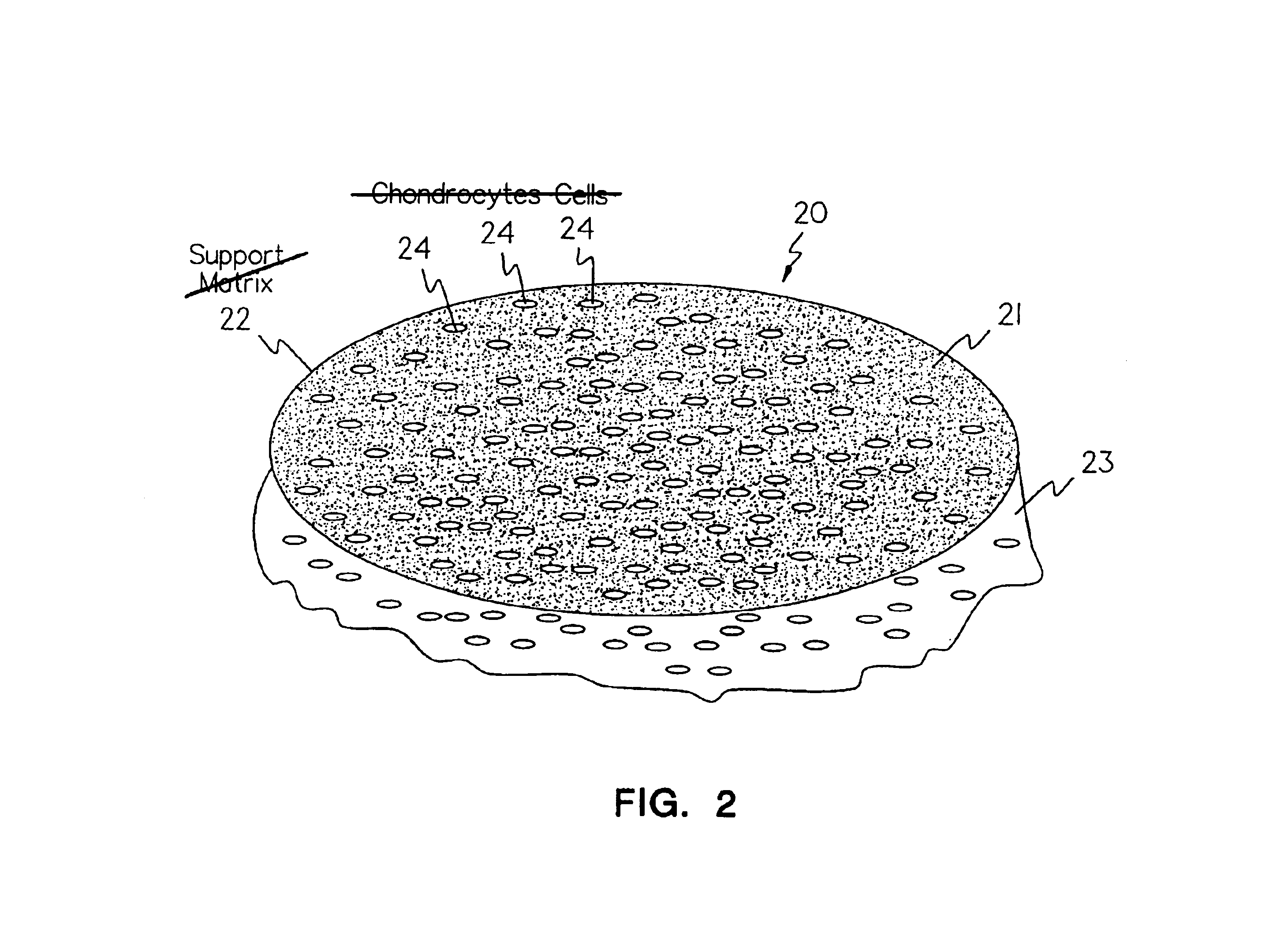
Methods, instruments and materials for chondrocyte cell transplantation
InactiveUS6866668B2Effective treatmentSuture equipmentsSurgical adhesivesSupport matrixTreated animal
A method for the effective treatment of articulating joint surface cartilage in an animal by the transplantation of an implantable article including chondrocyte cells retained to an absorbable support matrix. An instrument for placing and manipulating the implantable article at the site of implantation, and a retention device for securing the implantable article to the site of implantation. An implantable article for cartilage repair in an animal, the implantable article including chondrocyte cells retained on an absorbable support matrix, and a method of making same. An article comprising an absorbable flexible support matrix for living cells grown and adhered thereto.
Owner:VERIGEN TRANSPLANTATION SERVICE INT
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Fragmented polymeric compositions and methods for their use
InactiveUS6063061AImprove liquidityEasy to controlSurgical adhesivesSurgical drugsCross-linkBreast implant
Molecular cross-linked gels comprise a variety of biologic and non-biologic polymers, such as proteins, polysaccharides, and synthetic polymers. Such molecular gels may be applied to target sites in a patient's body by extruding the gel through an orifice at the target site. Alternatively, the gels may be mechanically disrupted and used in implantable articles, such as breast implants. When used in vivo, the compositions are useful for inhibiting post-surgical spinal and other tissue adhesions, for filling tissue divots, tissue tracts, body cavities, surgical defects, and the like.
Owner:BAXTER INT INC +1
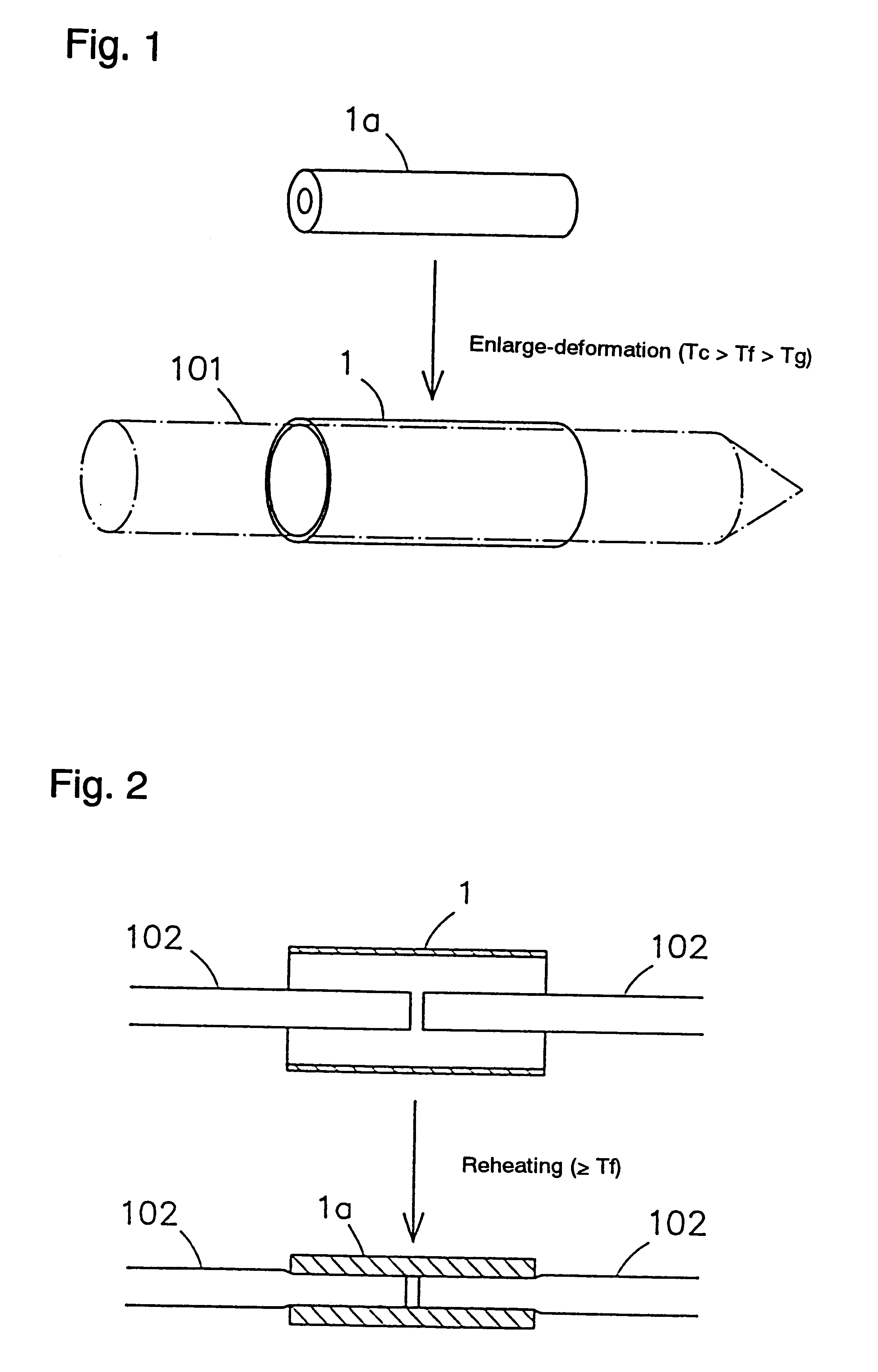
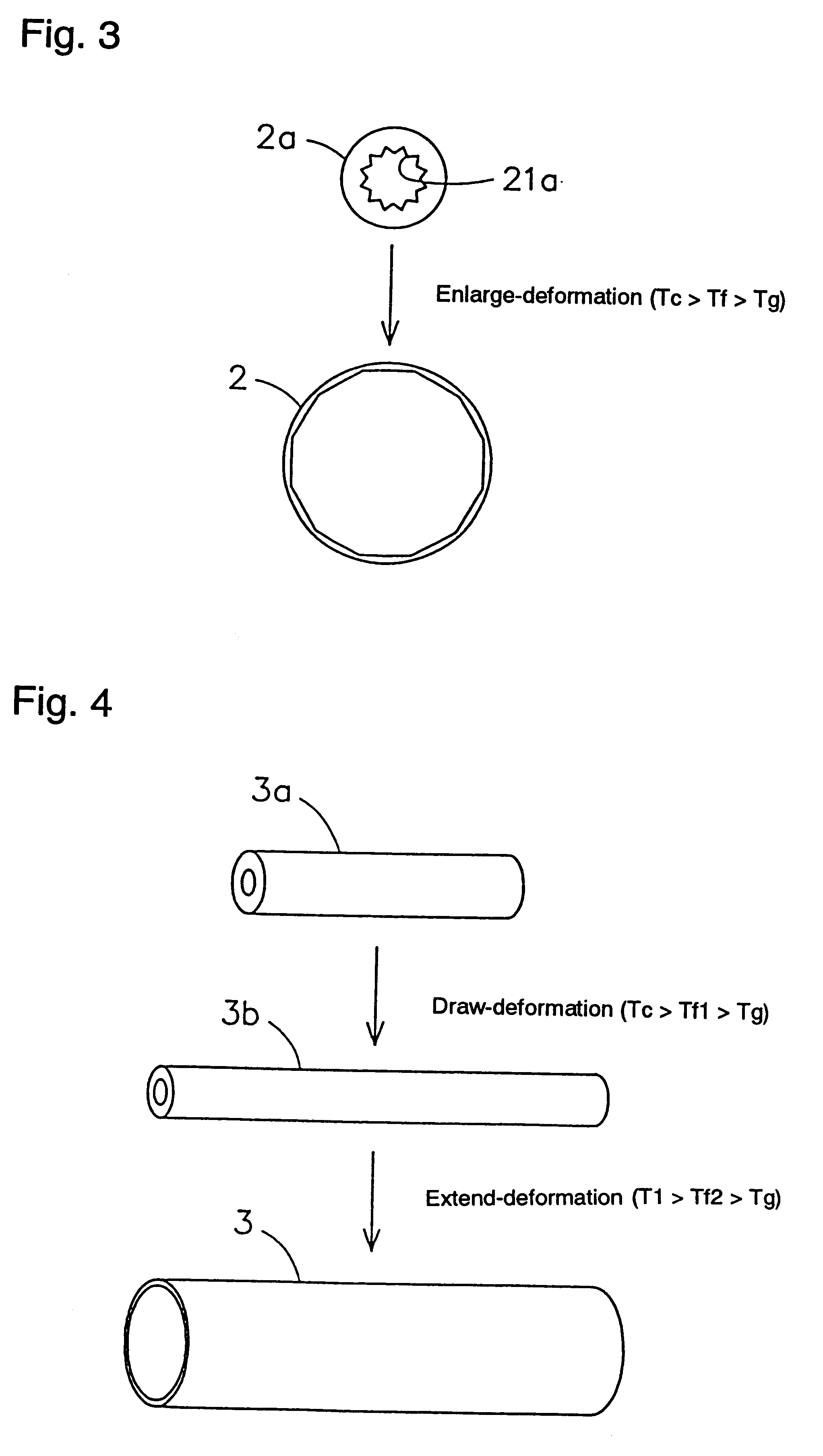
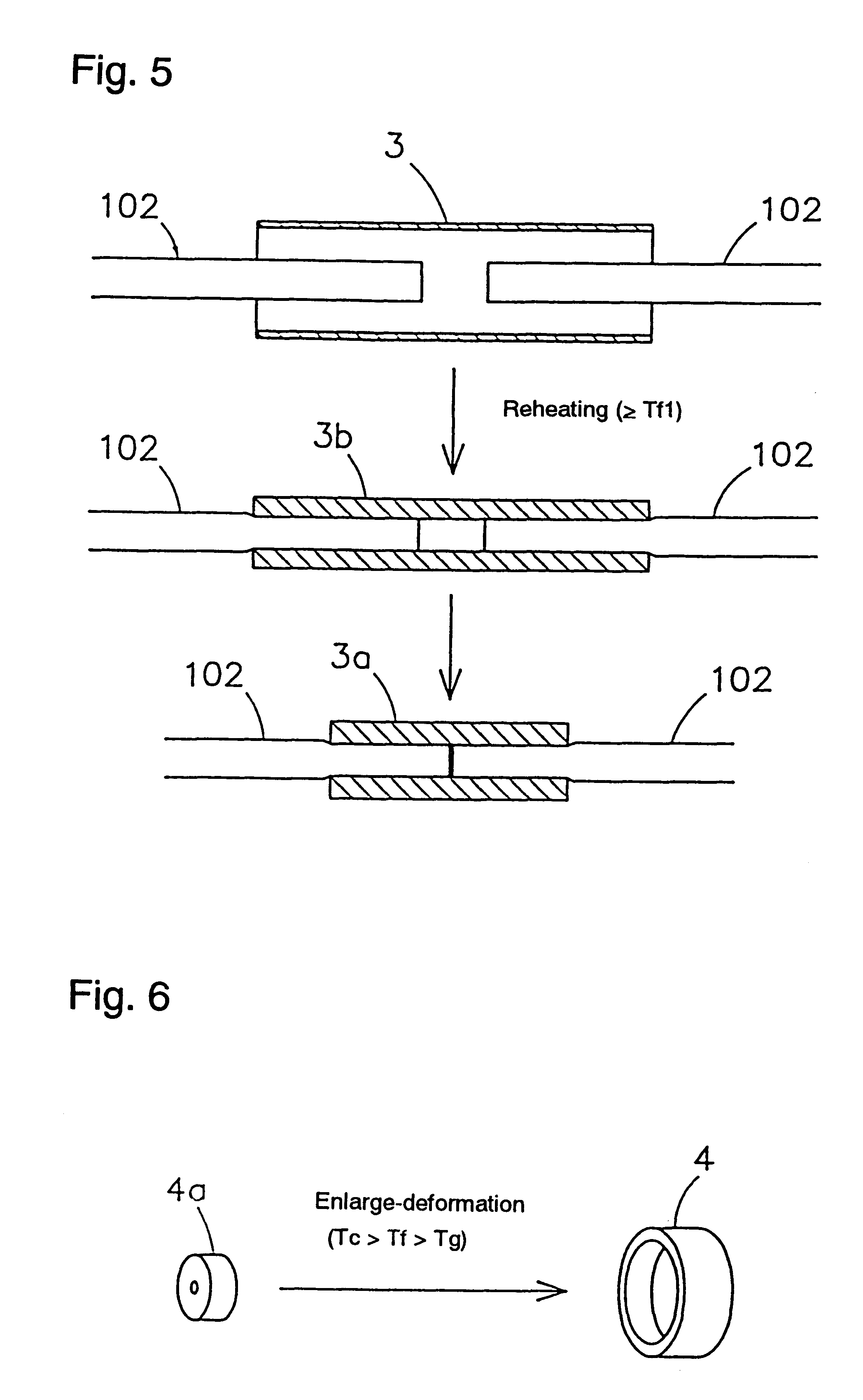
Shape-memory, biodegradable and absorbable material
InactiveUS6281262B1Easily and surely performSuture equipmentsCosmetic preparationsVitrificationPolymer science
Shape-memory biodegradable and absorbable materials which make it possible to easily treat vital tissues by suture, anastomosis, ligation, fixation, reconstitution, prosthesis, etc. without causing burn. These materials never induce halation in MRI or CT and never remain in vivo. Such a shape-memory biodegradable and absorbable material is a material made of a molded article of lactic acid-based polymer and can be recovered to the original shape without applying any external force thereto but heating to a definite temperature or above. It is obtained by deforming a molded article (a primary molded article) made of a lactic acid-based polymer and having a definite shape into another molded article (a secondary molded article) having another shape at a temperature higher than the glass transition temperature thereof but lower than the crystallization temperature thereof (or 100.degree. C. when the molded article has no crystallization temperature) and then fixing said molded article to the thus deformed shape by cooling it as such to a temperature lower than the glass transition temperature. When this material is heated to the above-mentioned deformation temperature or above, it is immediately recovered to the original shape. The lactic acid-based polymer is hydrolyzed and absorbed in vivo.
Owner:TAKIRON CO LTD
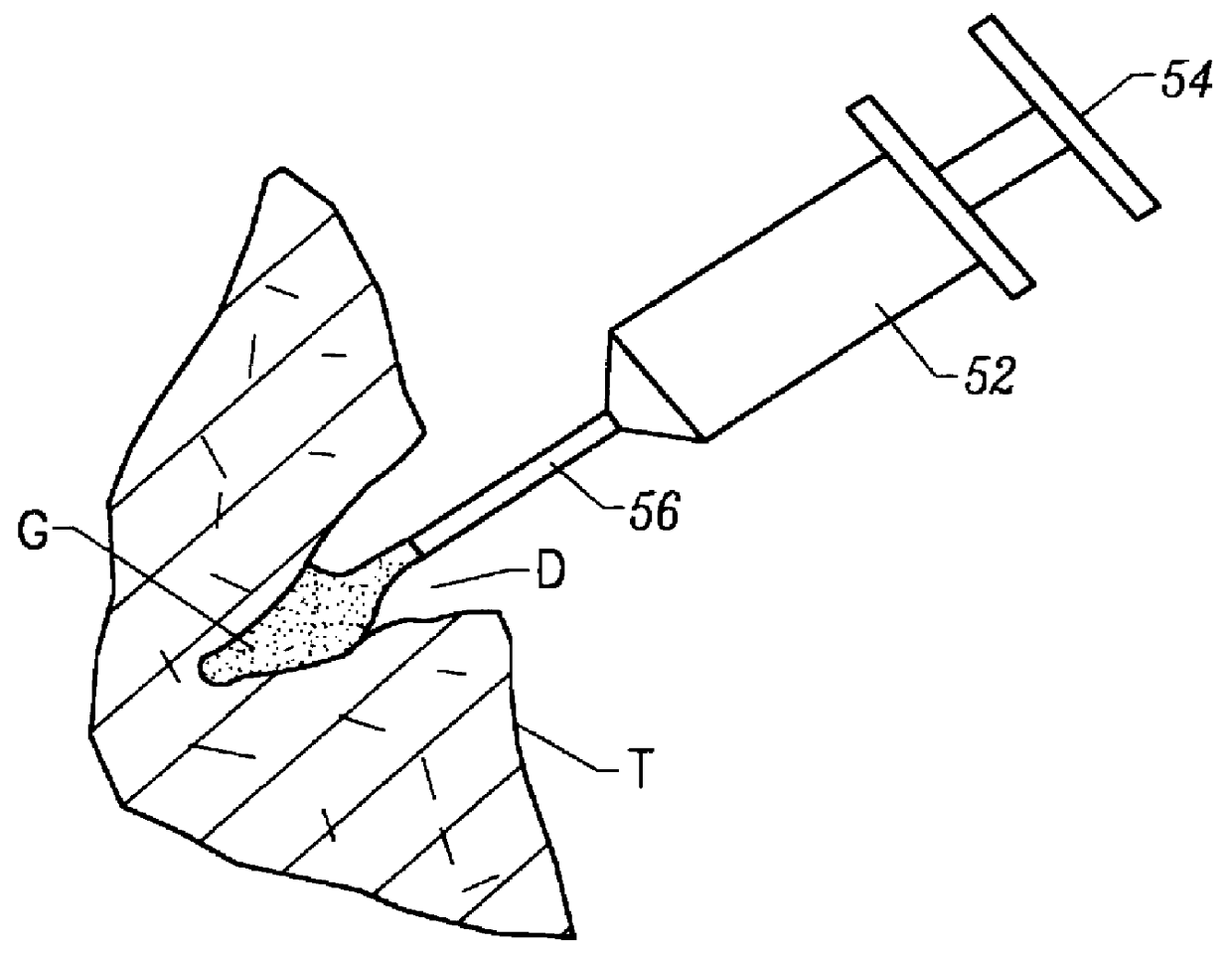
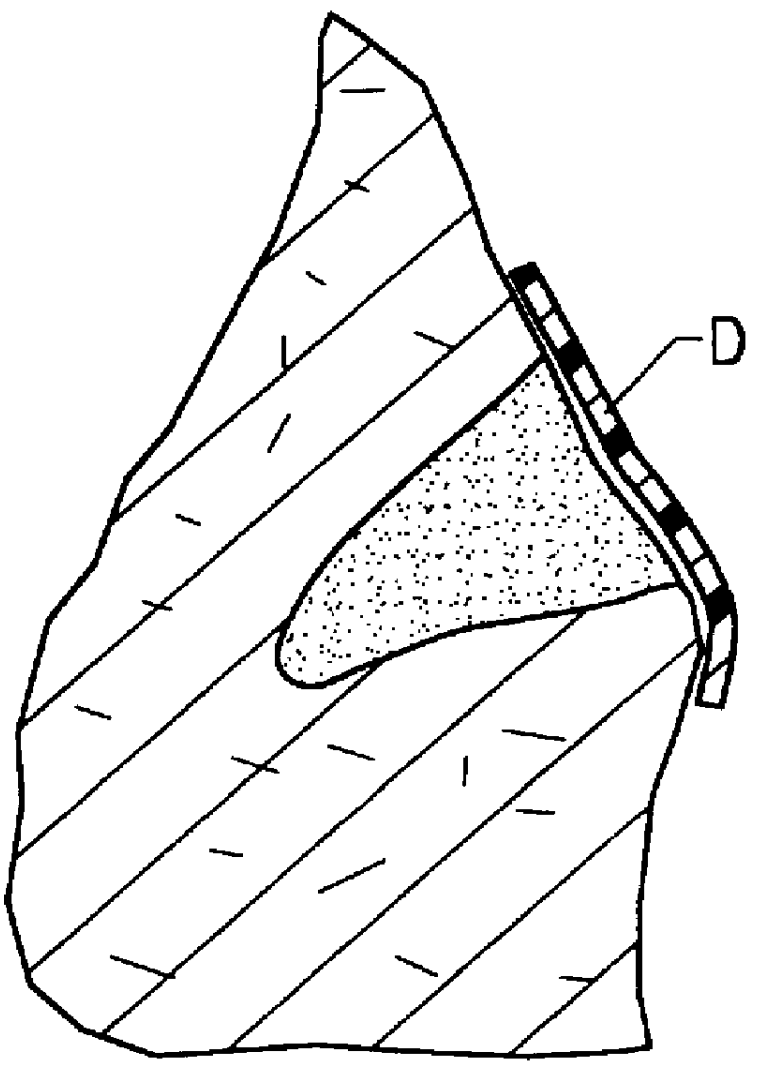
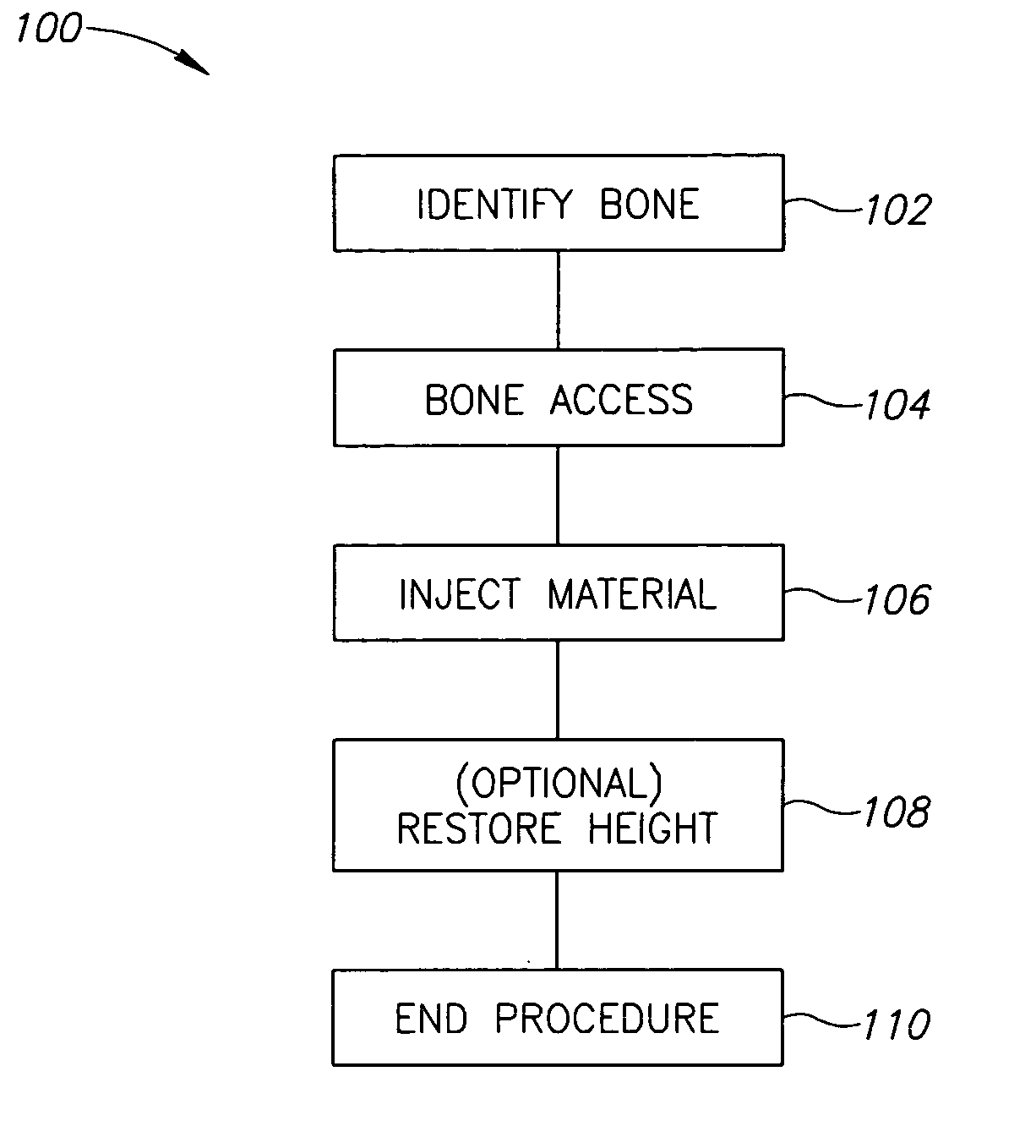
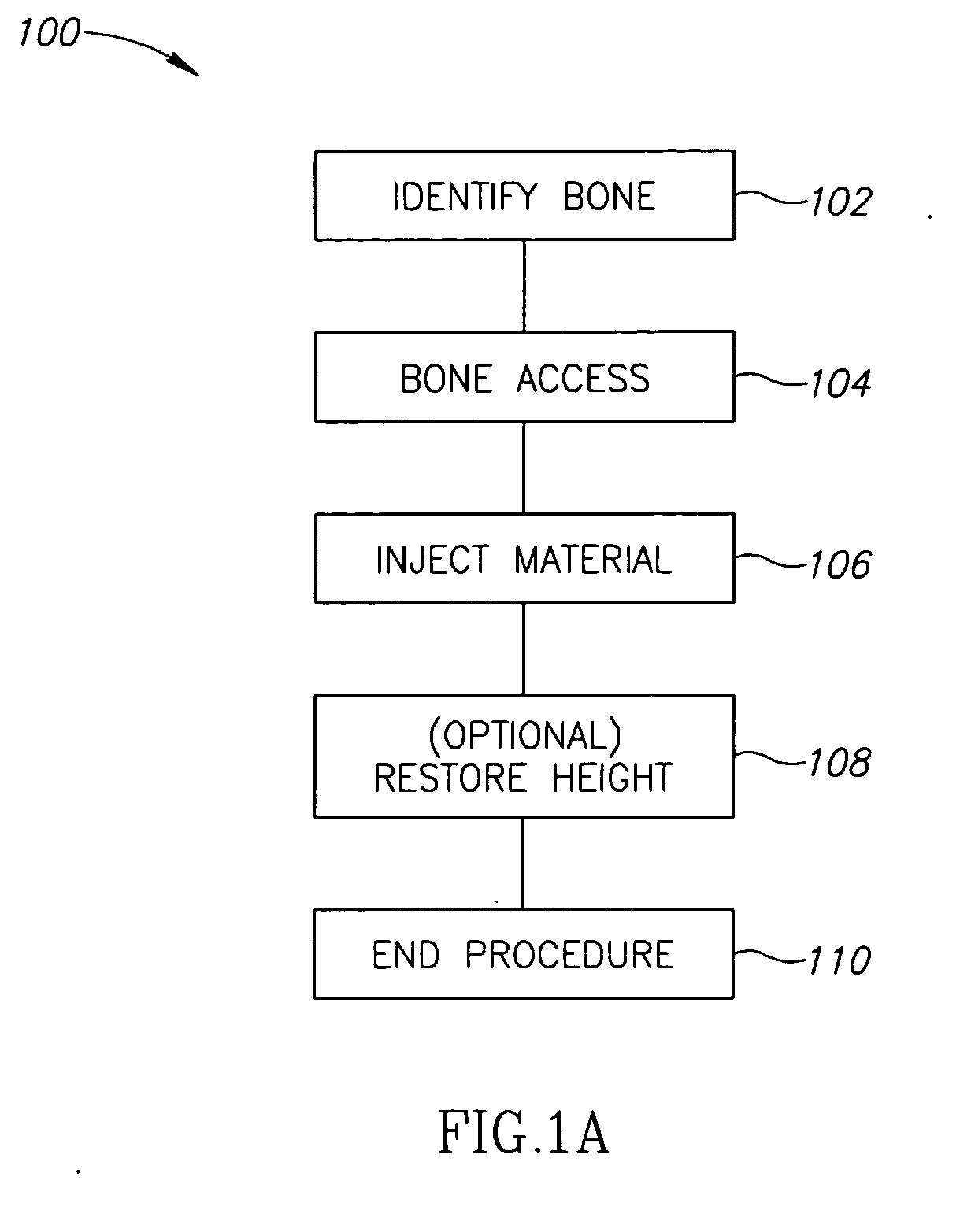
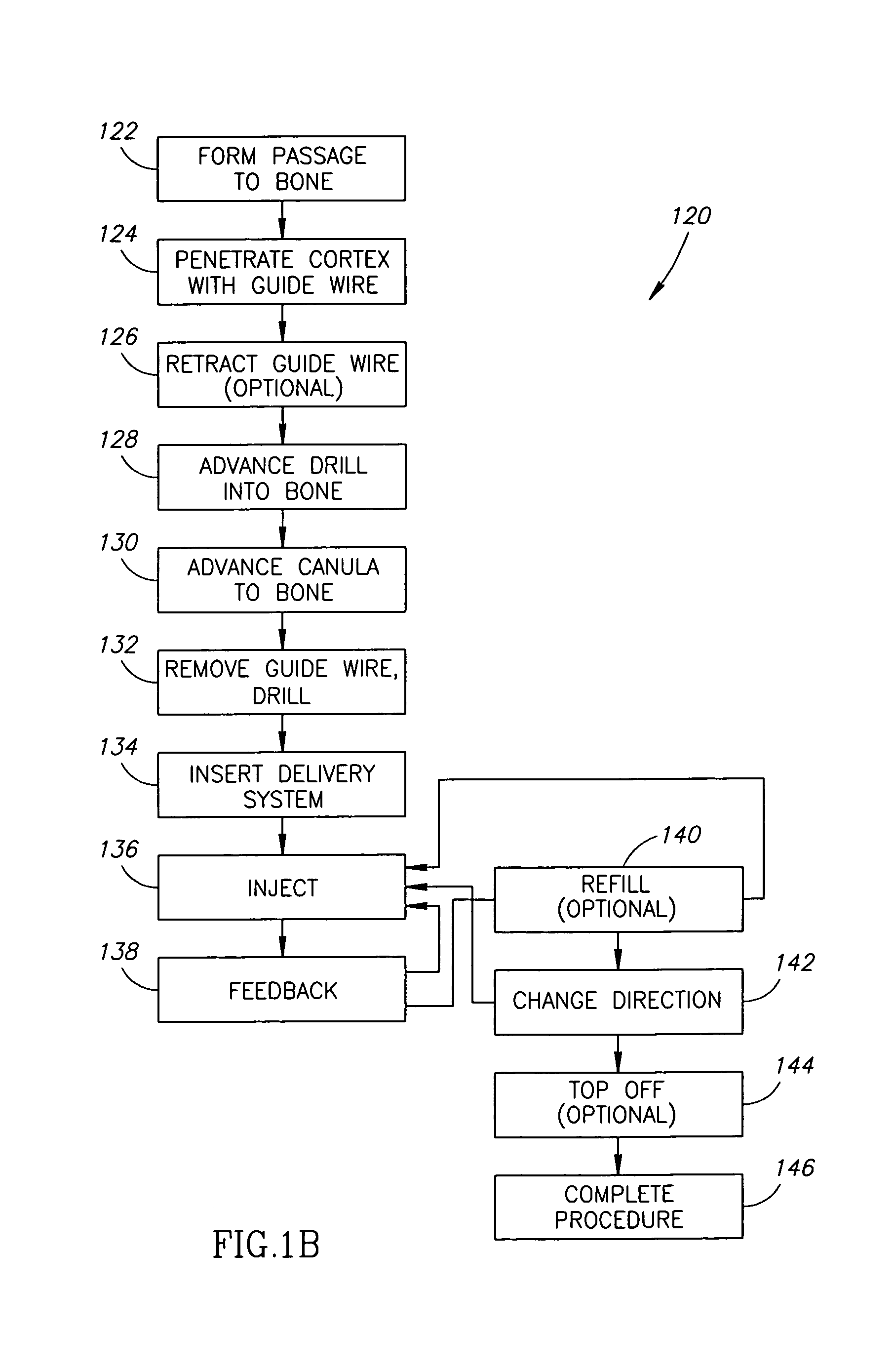
Methods, materials and apparatus for treating bone and other tissue
ActiveUS20060079905A1Avoid less flexibilityEasy to useImpression capsSurgical adhesivesBiomedical engineeringVertebra
A method of treating a vertebra, comprising: (a) accessing an interior of a vertebra; and (b) introducing a sufficient amount of artificial biocompatible material which does not set to a hardened condition in storage, into said bone, with sufficient force to move apart fractured portions of said bone.
Owner:DEPUY SYNTHES PROD INC
Means for Controlled Sealing of Endovascular Devices
InactiveUS20130331929A1Improve sealingEliminate prosthetic-annular incongruenceSurgical adhesivesHeart valvesProsthesisFunctional integrity
Expandable sealing means for endoluminal devices have been developed for controlled activation. The devices have the benefits of a low profile mechanism (for both self-expanding and balloon-expanding prostheses), contained, not open, release of the material, active conformation to the “leak sites” such that leakage areas are filled without disrupting the physical and functional integrity of the prosthesis, and on-demand, controlled activation, that may not be pressure activated.
Owner:ENDOLUMINAL SCI
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Implantable medical devices and methods for making same
An implantable medical device is disclosed. The device is fabricated at least in part from a biocompatible, biodegradable composition. The composition is composed of a biocompatible, biodegradable polymer and a biocompatible, biodegradable wax. The concentration of the wax at the surface of the device is greater than the concentration of the wax in the body of the implantable device. The increased concentration of wax at the device surface may be attained when the device is heated to a temperature greater than the melting point of the wax and the glass transition temperature of the polymer, but lower than melting point of the polymer.
Owner:ETHICON INC
Methods, materials, and apparatus for treating bone and other tissue
ActiveUS20070027230A1Avoid less flexibilityEasy to useCosmetic preparationsImpression capsMedicineIn vivo
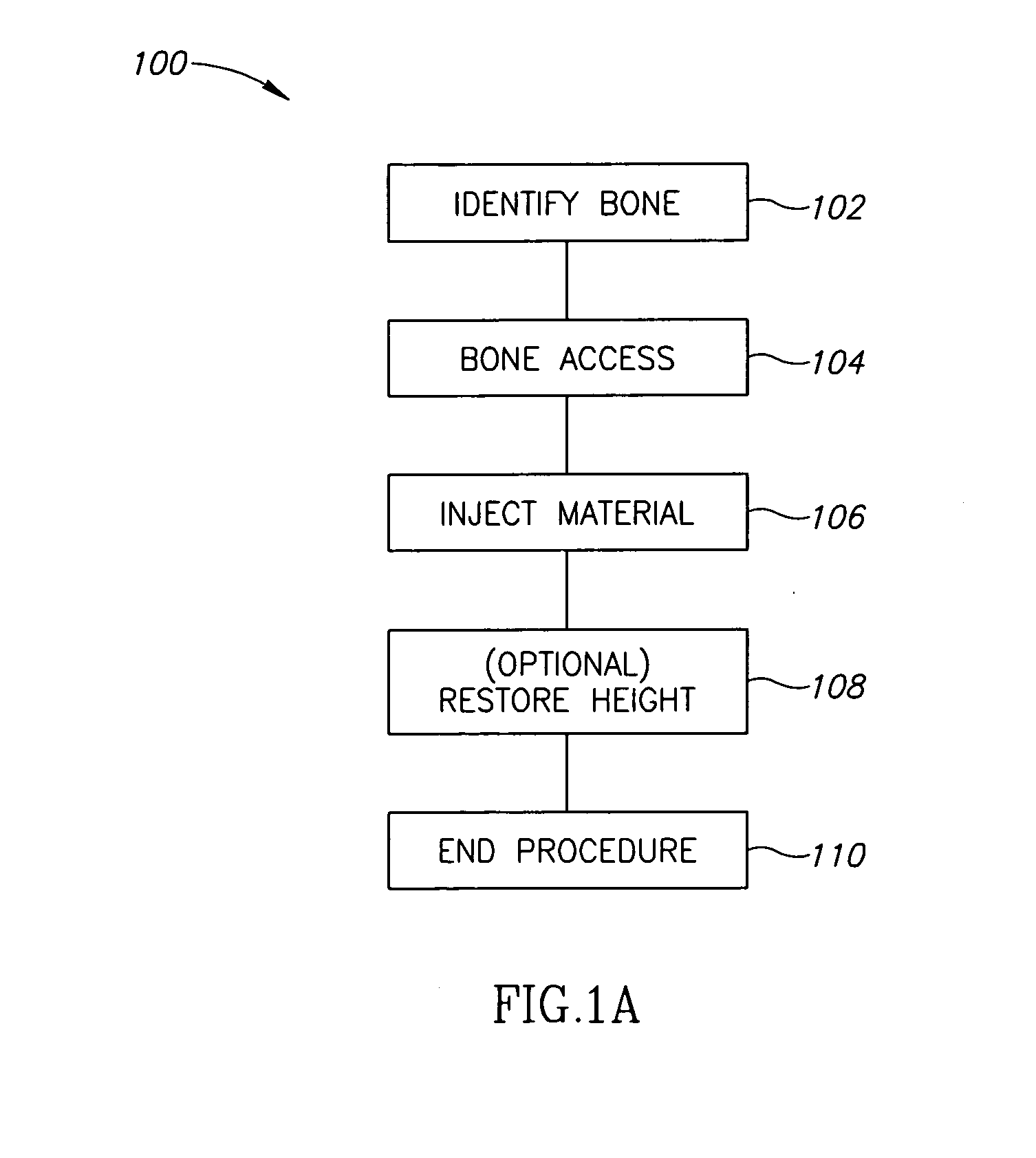
A bone cement comprising a first component and a second component, wherein contacting the first component and the second component produces a mixture which attains a high viscosity an initial period and the viscosity of the mixture remains relatively stable for a working time of at least 5 minutes after the initial setting period, and the mixture is suitable for in-vivo use.
Owner:DEPUY SYNTHES PROD INC
Medical and dental implant devices for controlled drug delivery
Implantable devices and methods for use in the treatment of osteonecrosisare provided. The device includes at least one implant device body adapted for insertion into one or more channels or voids in bone tissue; a plurality of discrete reservoirs, which may preferably be microreservoirs, located in the surface of the at least one implant device body; and at least one release system disposed in one or more of the plurality of reservoirs, wherein the release system includes at least one drug selected from the group consisting of bone growth promoters, angiogenesis promoters, analgesics, anesthetics, antibiotics, and combinations thereof. The device body may be formed of a bone graft material, a polymer, a metal, a ceramic, or a combination thereof. The device body may be a monolithic structure, such as one having a cylindrical shape, or it may be in the form of multiple units, such as a plurality of beads.
Owner:MICROCHIPS INC
Porous β-tricalcium phosphate granules for regeneration of bone tissue
InactiveUS6949251B2Improve regenerative abilitySurgical adhesivesSkeletal disorderActive agentBone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
Topically applied clotting material
A composition, system, articles and method for the enhancement of clotting in wounds with extravascular blood flow, especially where the surface of the tissue has been broken is described. The system consists of biotolerable, porous particulates applied to the surface of a wound with liquid blood thereon. The porous nature of the particulate material, either free-flowing or packaged or restrained on or in a surface, enhances clotting. Chemical or biochemical agents, such as additional clotting agents, therapeutic agents, antibiotics, clot strengthening agents (such as fibrous structural materials), and the like may optionally be included on, with or within the porous particles. The particles may comprise such diverse materials as organics, metallics, inorganics, ceramics, and the like, both natural and artificial. It is generally preferred that the pore size distribution lies within a general range, and this range may vary from animal to animal and condition to condition, but generally falls within about 0.5 to 1000 nanometers or 3,000 to 200,000 Daltons.
Owner:MEDAFOR
Hydrogels and methods of making and using same
The invention is directed to methods of making novel porous and solid polyvinyl alcohol hydrogels. These hydrogels are particularly suited for use in the replacement and augmentation of soft tissue or non-load bearing bone of the face, head and cranium.
Owner:POREX CORP
Malleable putty and flowable paste with allograft bone having residual calcium for filling bone defects
The invention is directed toward a malleable bone putty and a flowable pastel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of partially demineralized lyophilized allograft bone material having a residual calcium content ranging from 4 to 8% dry weight. The bone powder has a particle size ranging from about 100 to about 800 microns and is mixed in a high molecular weight hydrogel carrier containing a sodium phosphate saline buffer, the hydrogel component of the carrier ranging from about 1.00 to 50% of the composition and having a molecular weight of about at least 700,000 Daltons. The composition has a pH between 6.8-7.4 contains about 25% to about 35% bone powder and can be additionally provided with BMP's.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Process for medical implant of cross-linked ultrahigh molecular weight polyethylene having improved balance of wear properties and oxidation resistance
A medical implant of ultrahigh molecular weight polyethylene having an improved balance of wear properties and oxidation resistance is prepared by irradiating a preform of ultrahigh molecular weight polyethylene, annealing the irradiated preform in the absence of oxygen to a temperature at or above the onset of melting temperature, and forming an implant from the stabilized cross-linked polymer. Implants prepared according to the process of the present invention have comparable oxidation resistance and superior wear performance compared to unirradiated ultrahigh molecular weight polyethylene.
Owner:DEPUY ORTHOPAEDICS INC
Bioresorbable hydrogel compositions for implantable prostheses
Crosslinked compositions formed from water-insoluble copolymers are disclosed. These compositions are copolymers having a bioresorbable region, a hydrophilic region and at least two cross-linkable functional groups per polymer chain. Crosslinking of these polymers can be effected in solution in organic solvents or in solvent-free systems. If crosslinking occurs in a humid environment, a hydrogel will form. If crosslinking occurs in a non-humid environment, a xerogel will form which will form a hydrogel when exposed to a humid environment and the resulting crosslinked materials form hydrogels when exposed to humid environments. These hydrogels are useful as components in medical devices such as implantable prostheses. In addition, such hydrogels are useful as delivery vehicles for therapeutic agents and as scaffolding for tissue engineering applications.
Owner:LIFESHIELD SCI
Biologic replacement for fibrin clot
InactiveUS20040059416A1Promote regenerationWound closure is subsequentlySuture equipmentsVirusesLigament structureBiomedical engineering
Owner:CHILDRENS MEDICAL CENT CORP +1
Calcium based neutral and bioresorbable bone graft
InactiveUS20030055512A1Improve mechanical propertiesReduce decreaseSurgical adhesivesBone implantSulfateBone implant
An injectable and moldable putty comprising biodegradable calcium-based compounds including calcium sulfate, hydroxyapatite, and tricalcium phosphate is invented. The putty hardens into a solid body when mixed with water, saline, serum, or other neutral aqueous solutions. The hardening time of the putty can be tailored in order to meet the specific requirements of various dental or orthopedic applications. The pH of the putty is neutral during and after mixing. The invented putty may be used as bone graft, bone implant, or implantable drug delivery device.
Owner:BERKELEY ADVANCED BIOMATERIALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com