Patents
Literature
77 results about "Allograft bone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An allograft is a bone or tissue that is transplanted from one person to another. They typically come from a donor, or cadaver bone. The allograft is safe, ready to use and available in large amounts.
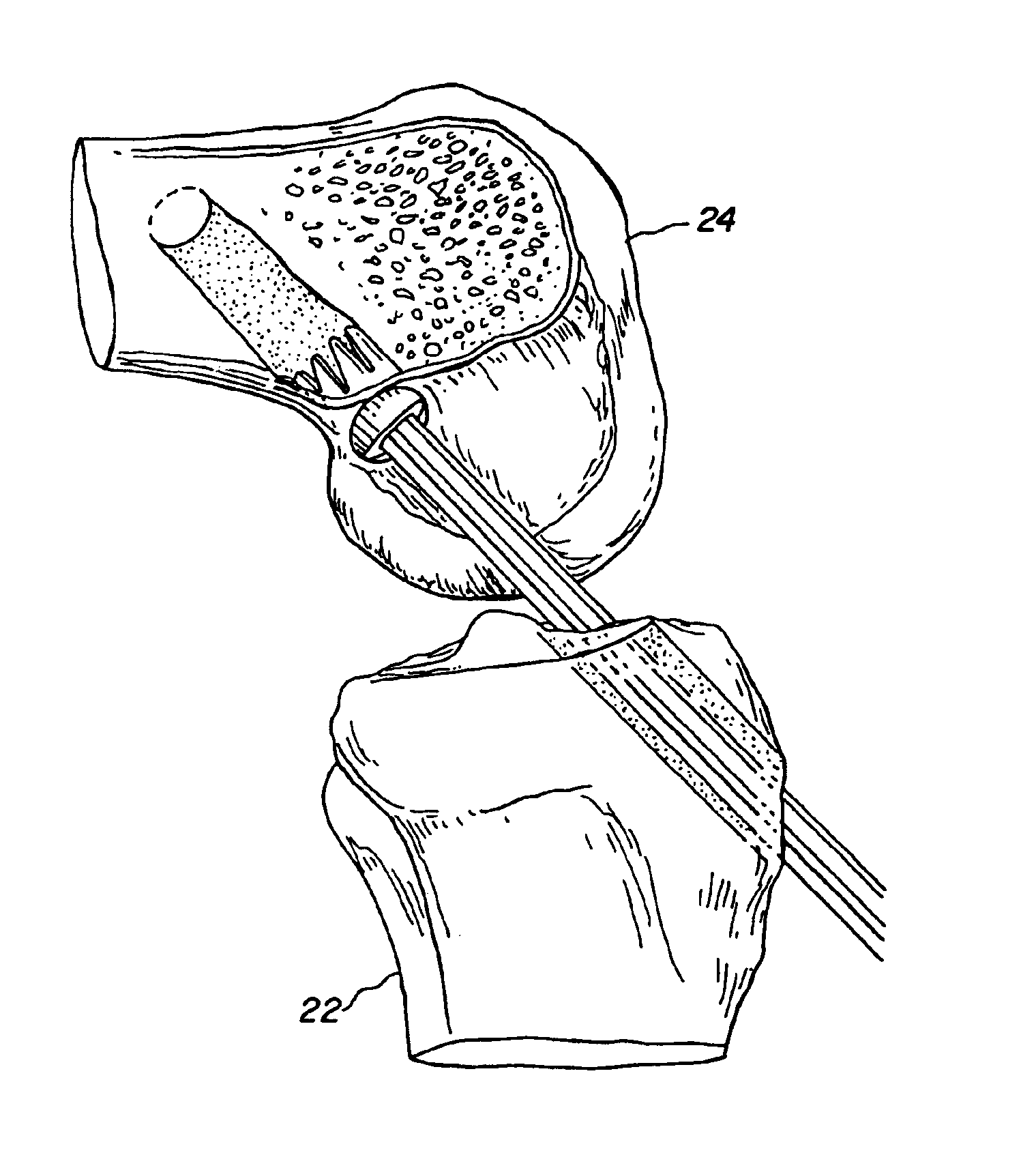
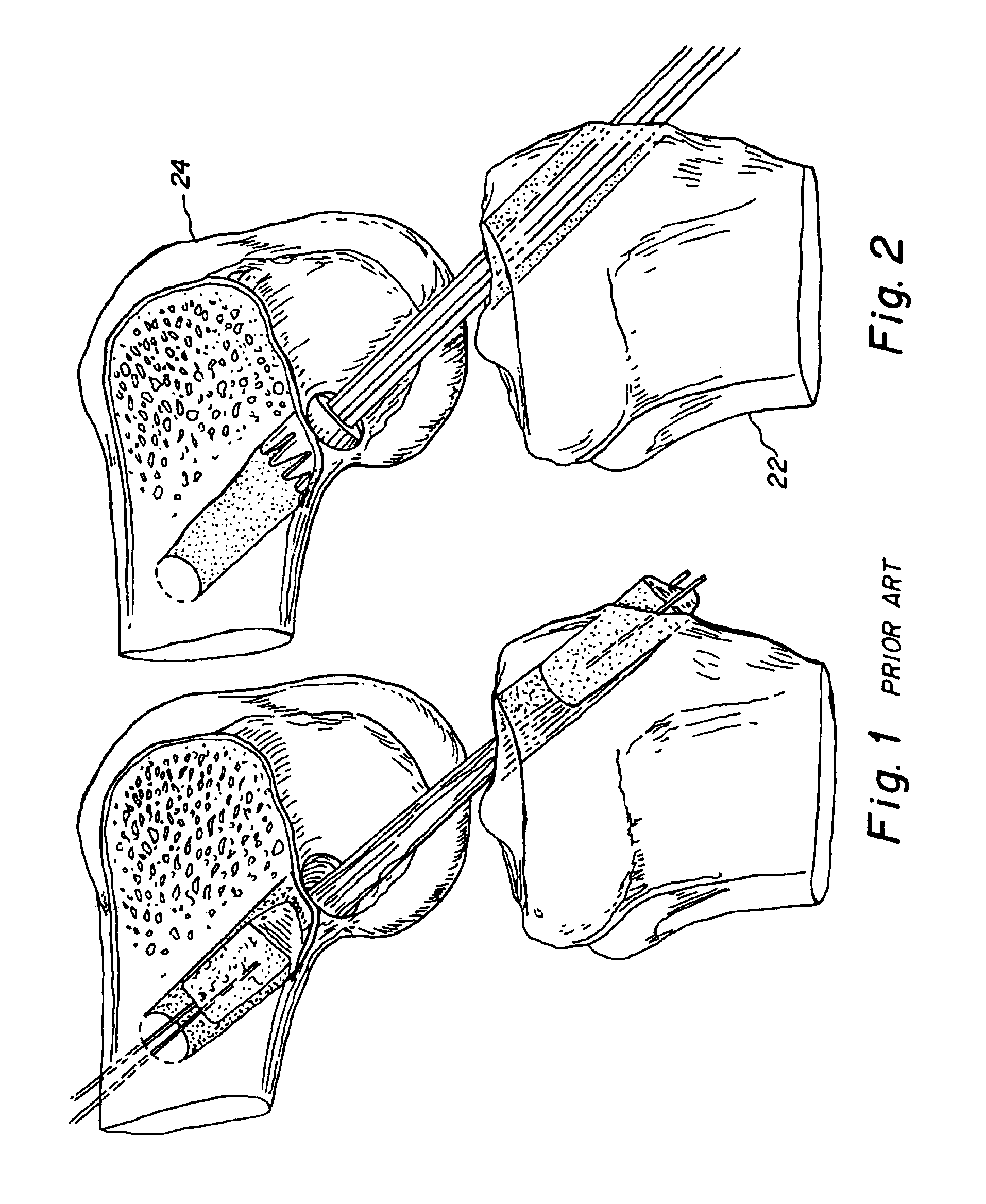
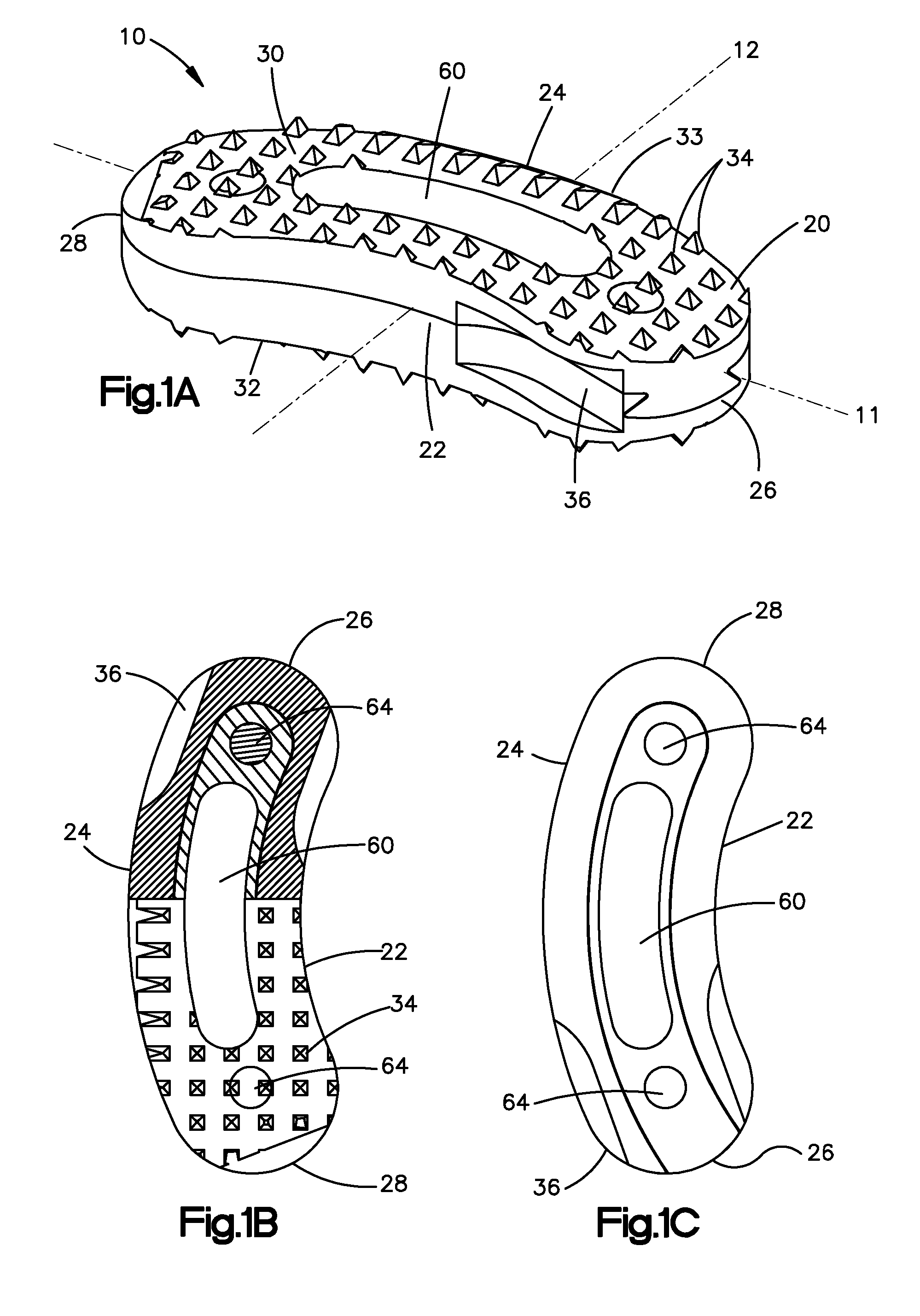
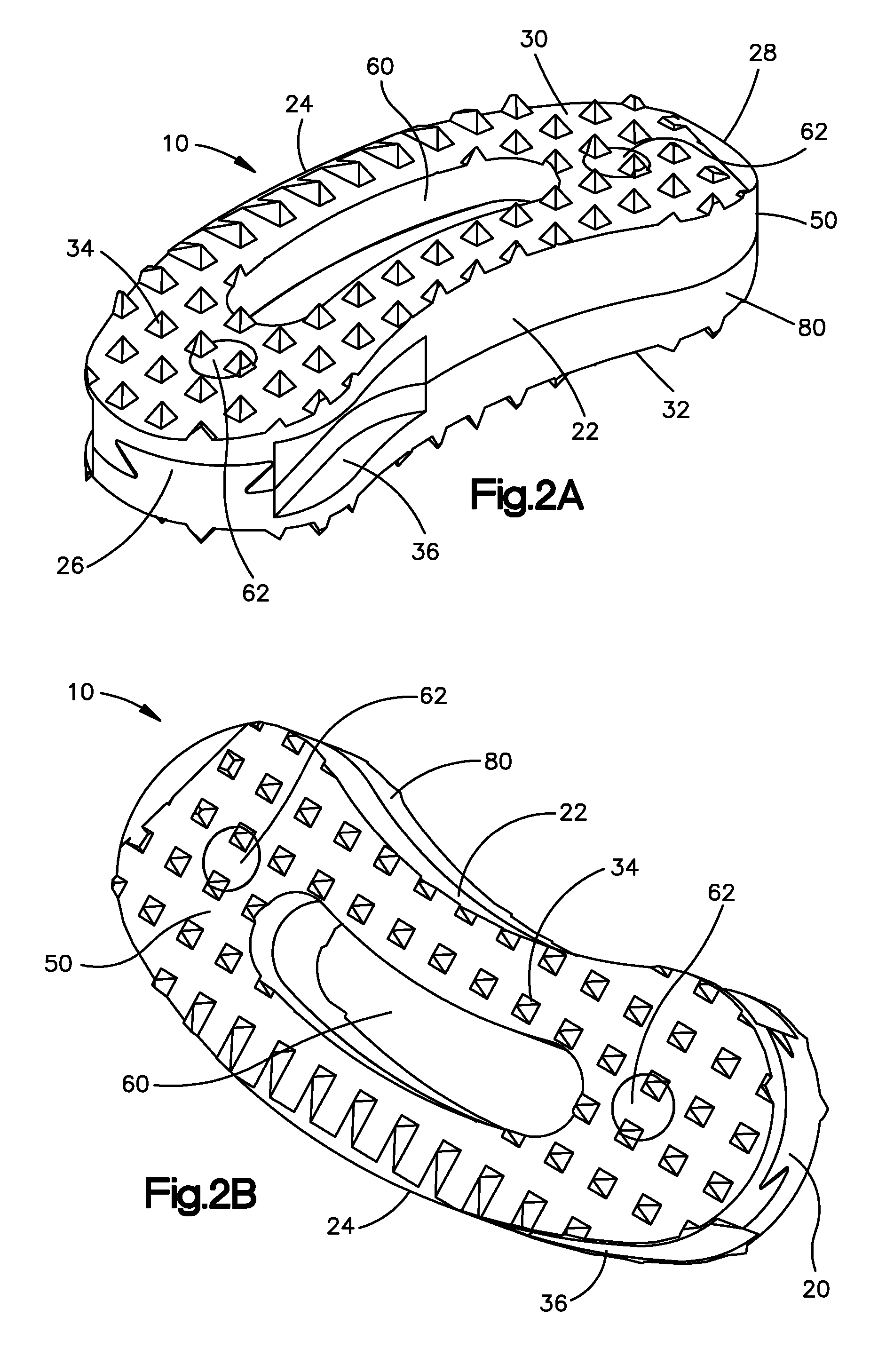
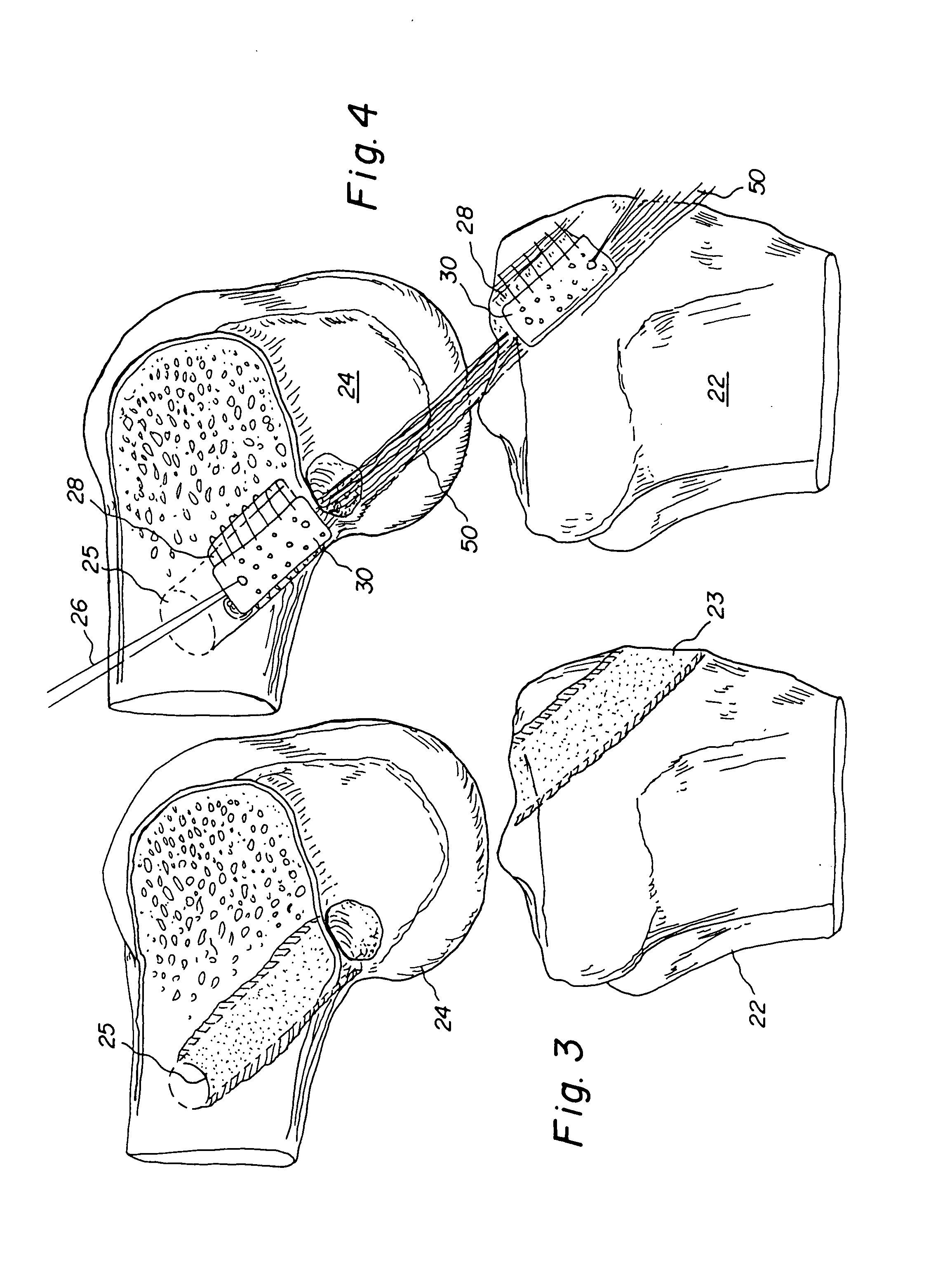
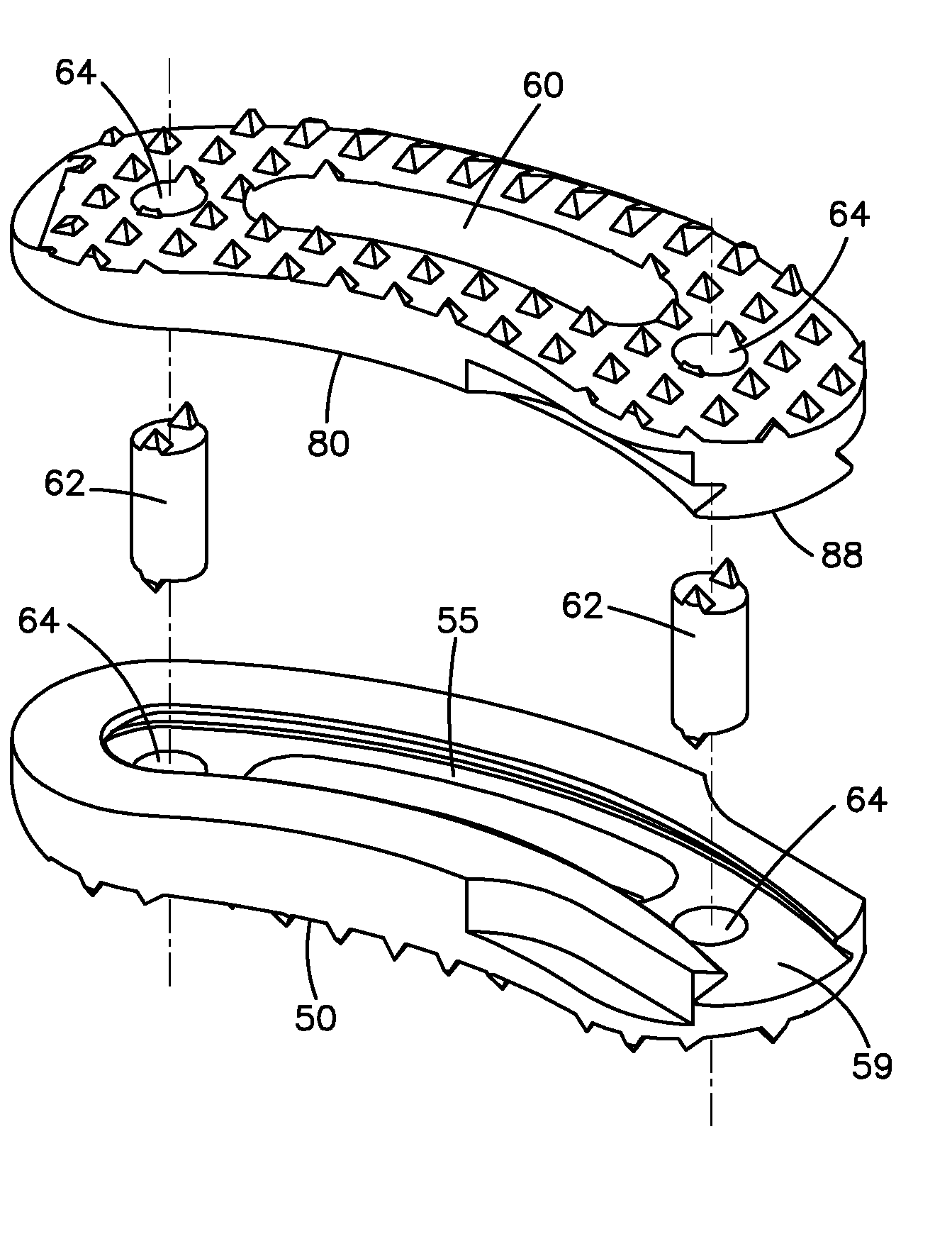
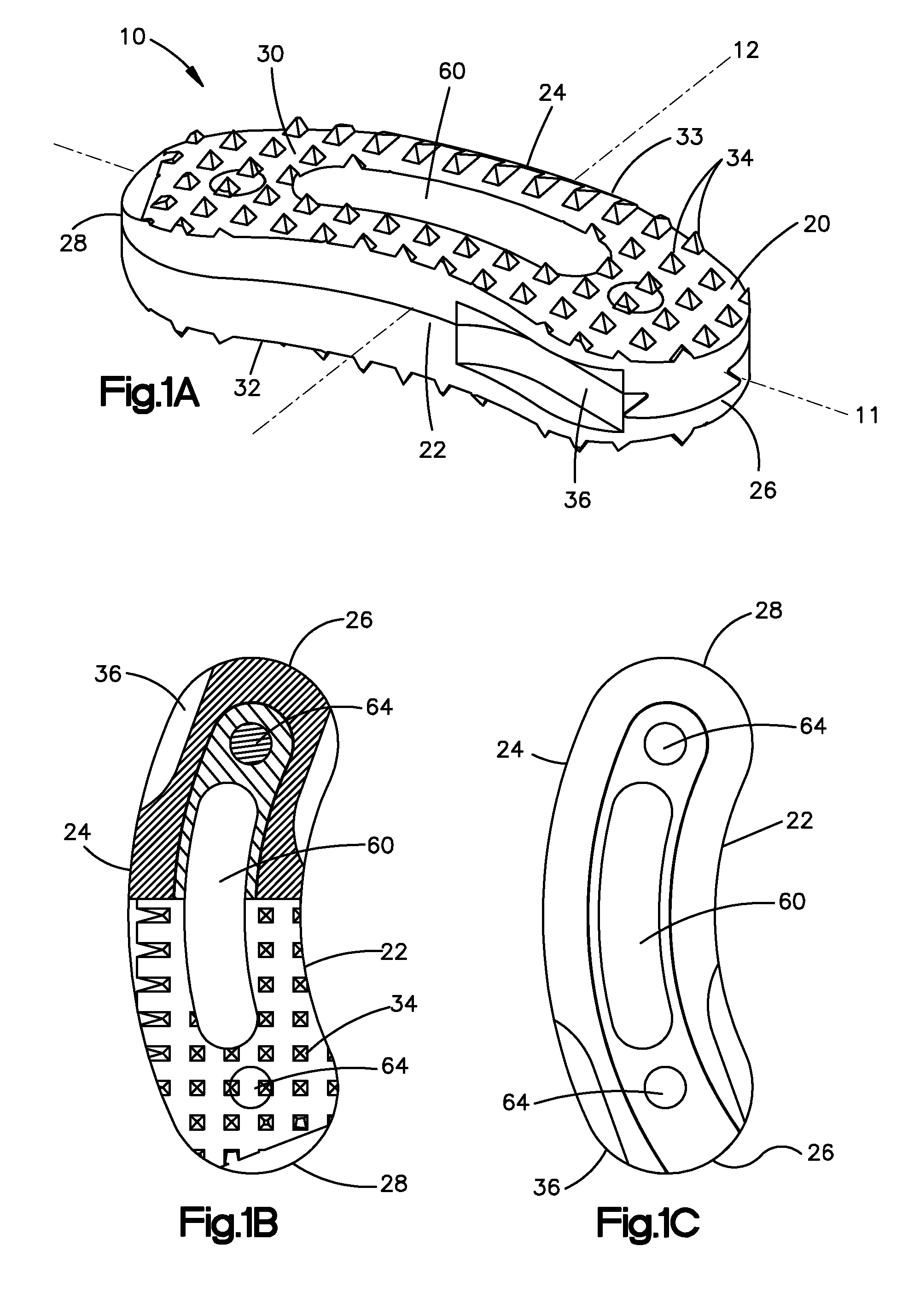
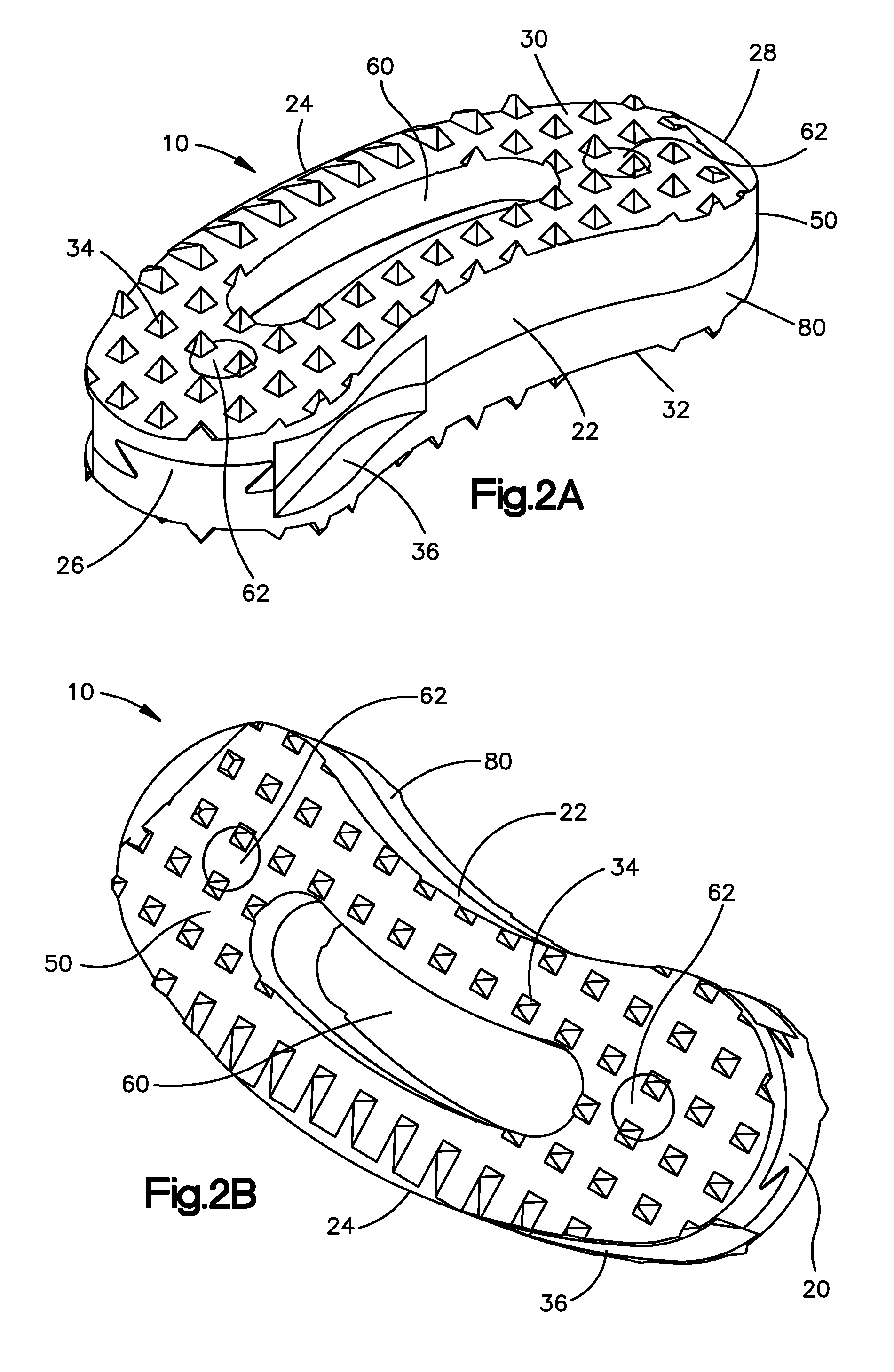
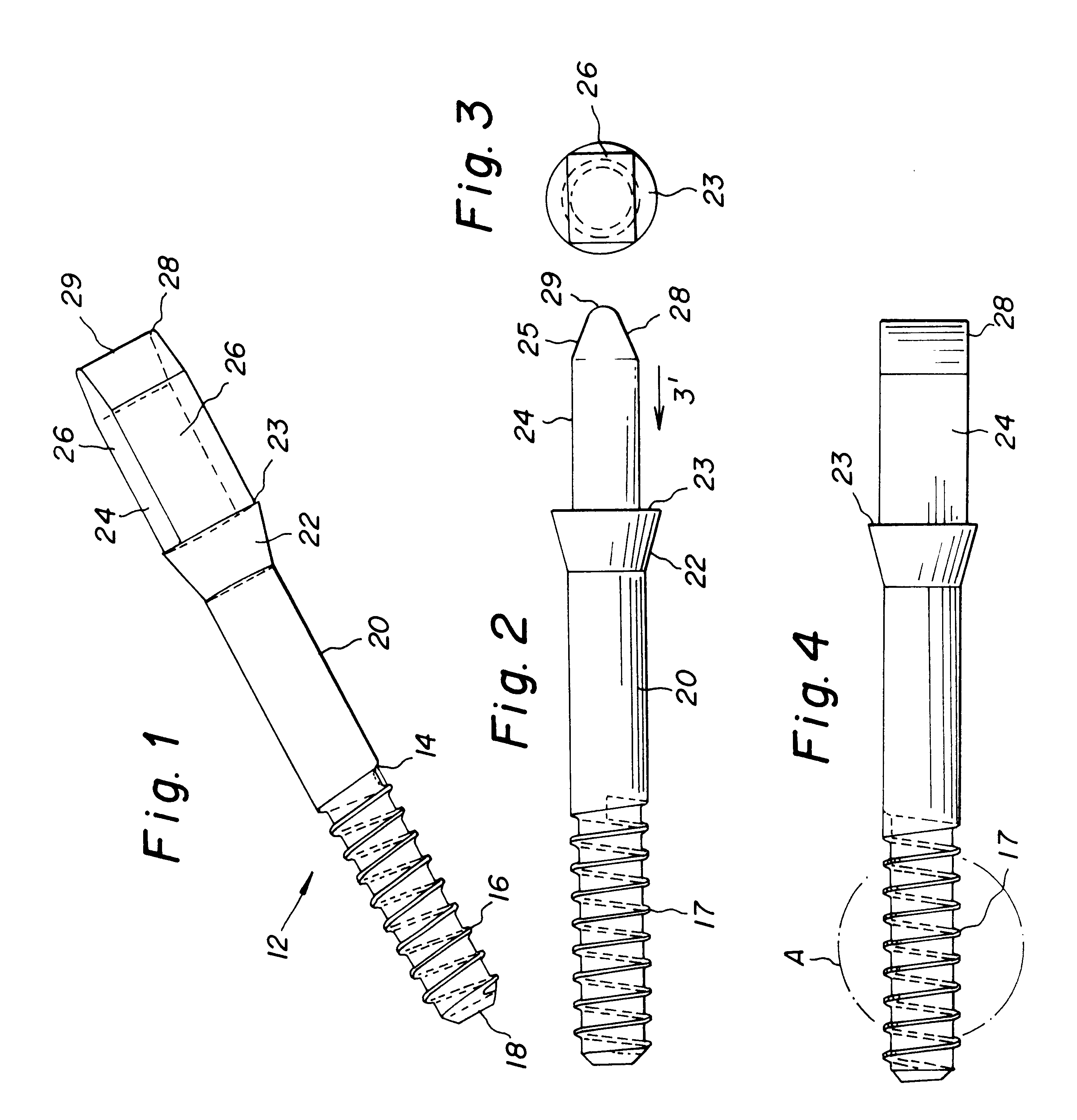
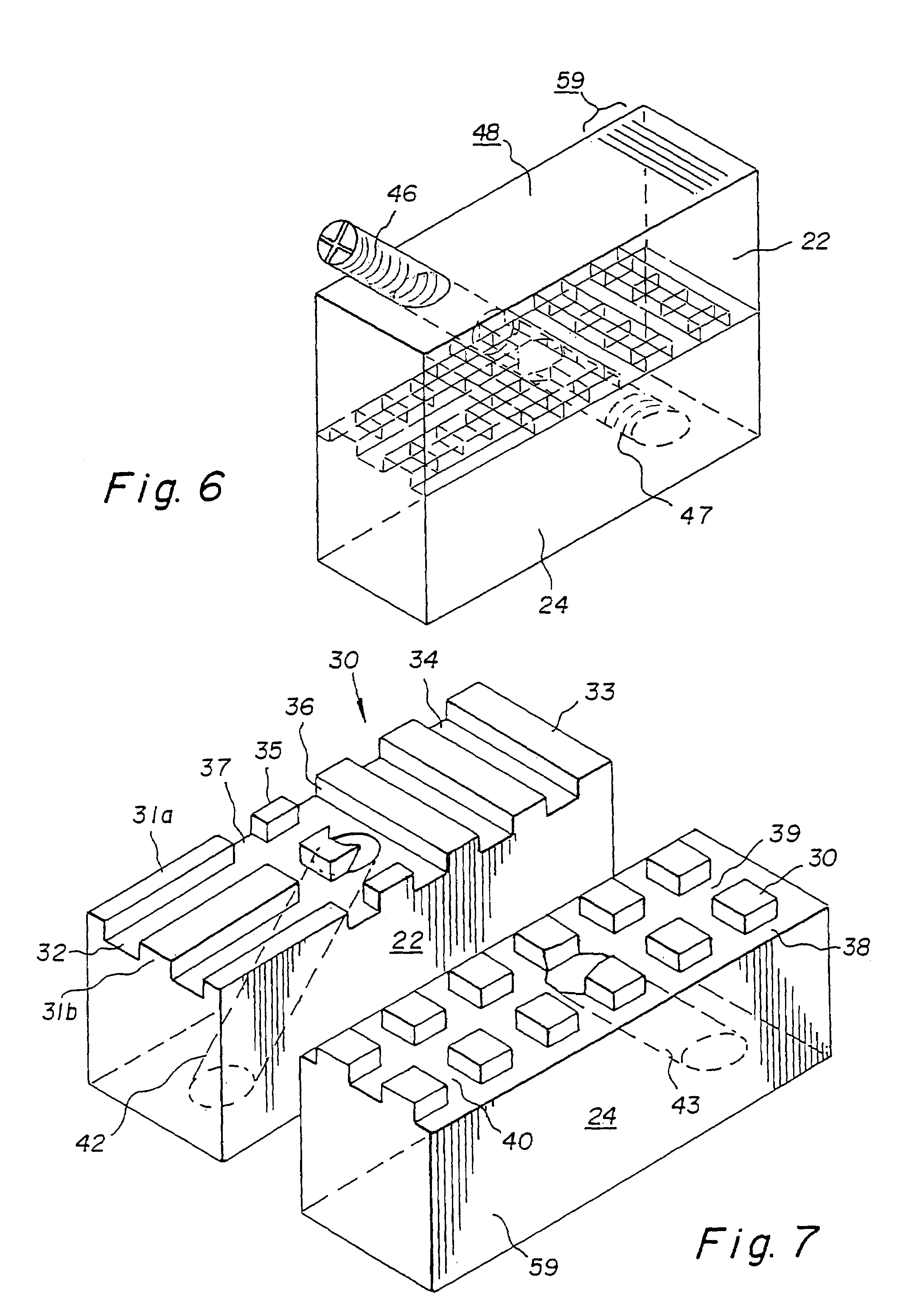
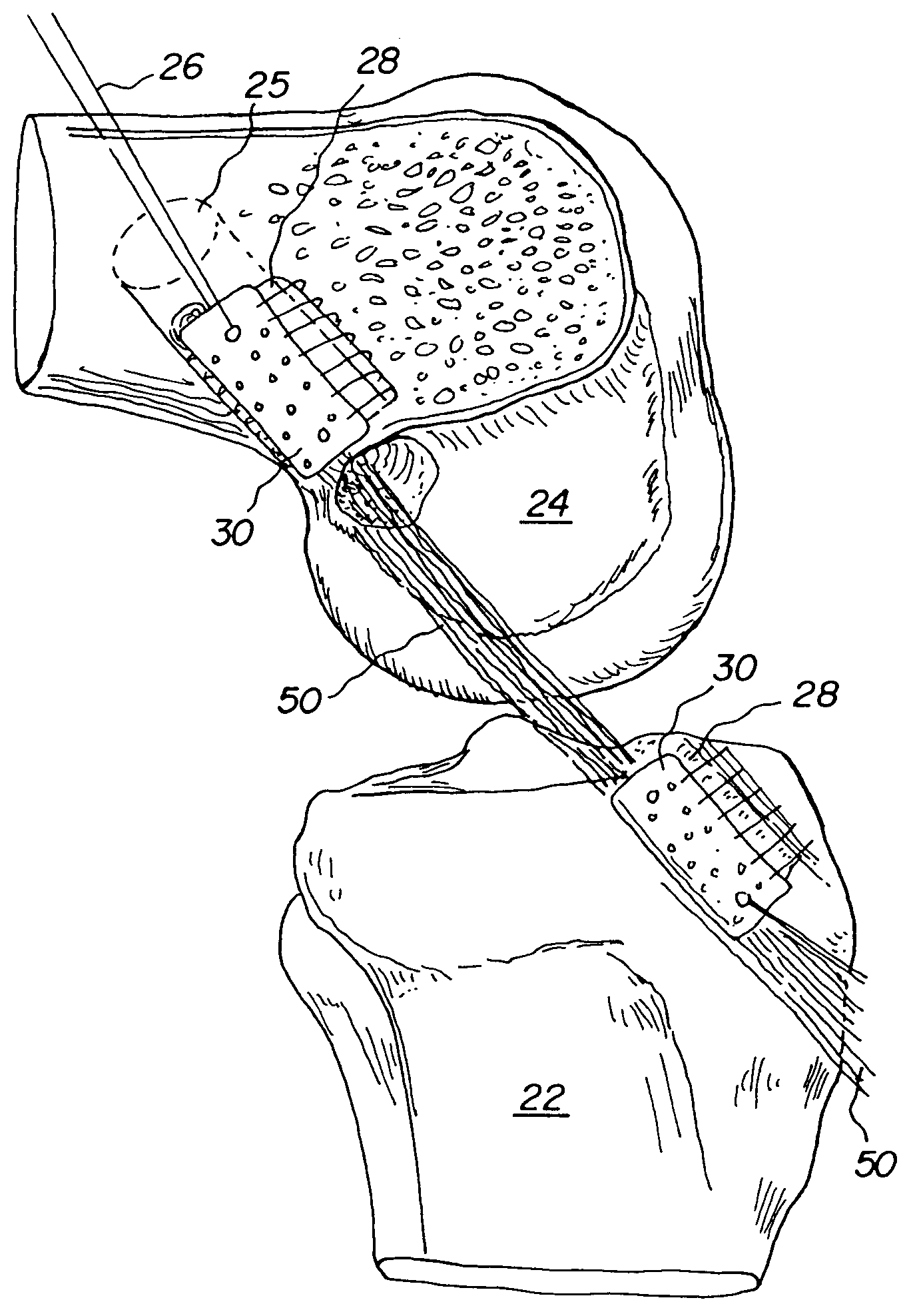
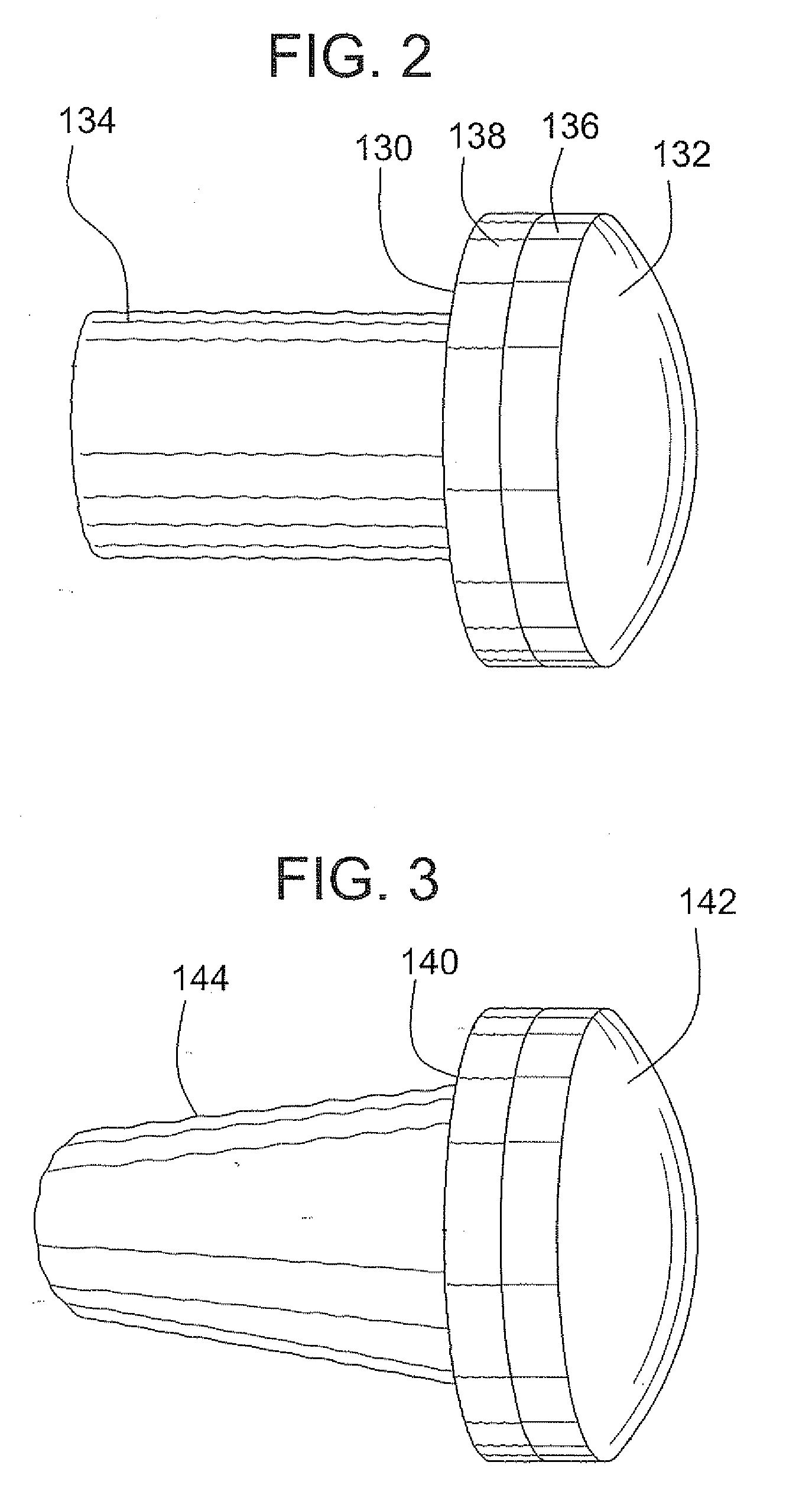
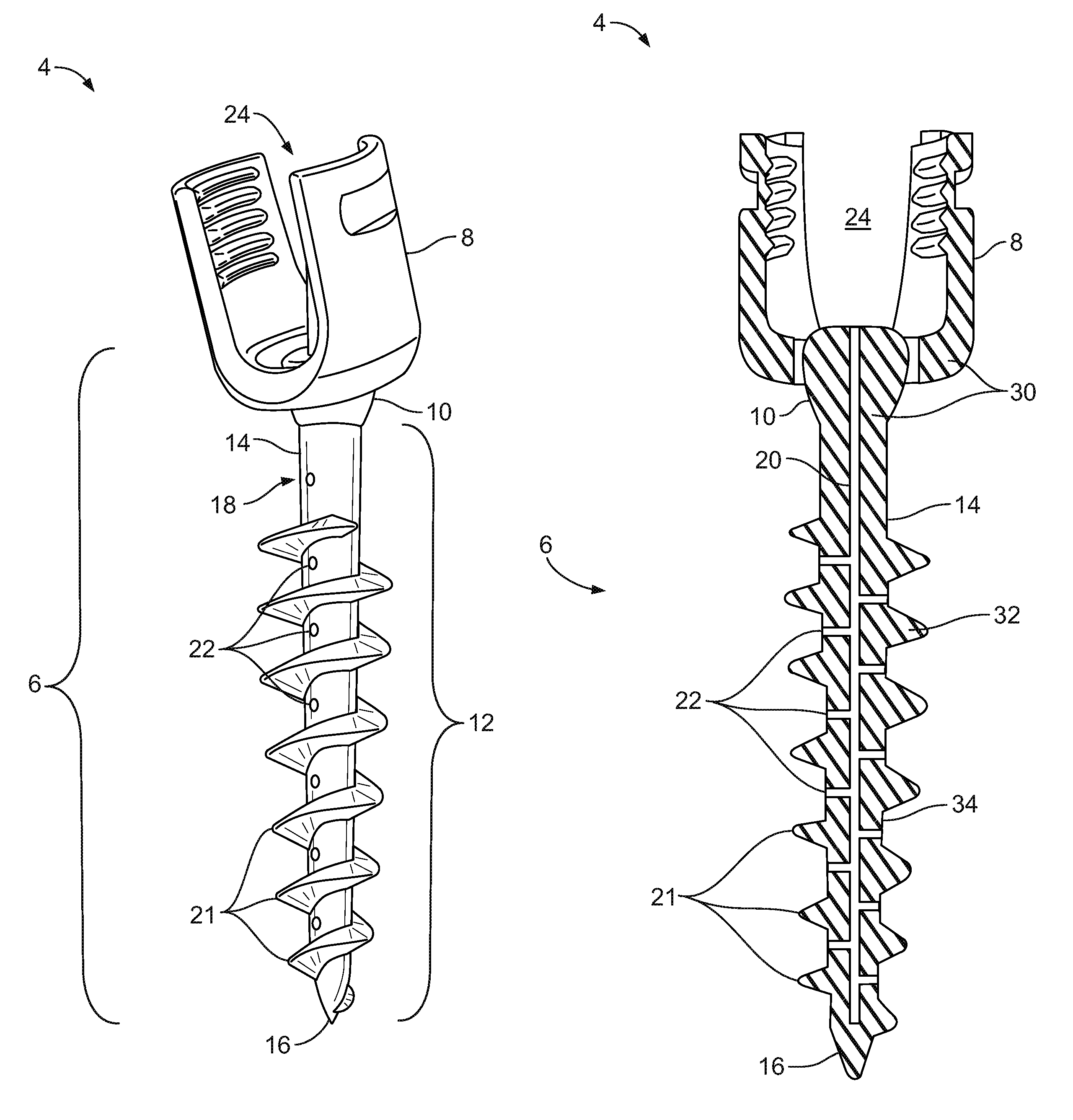
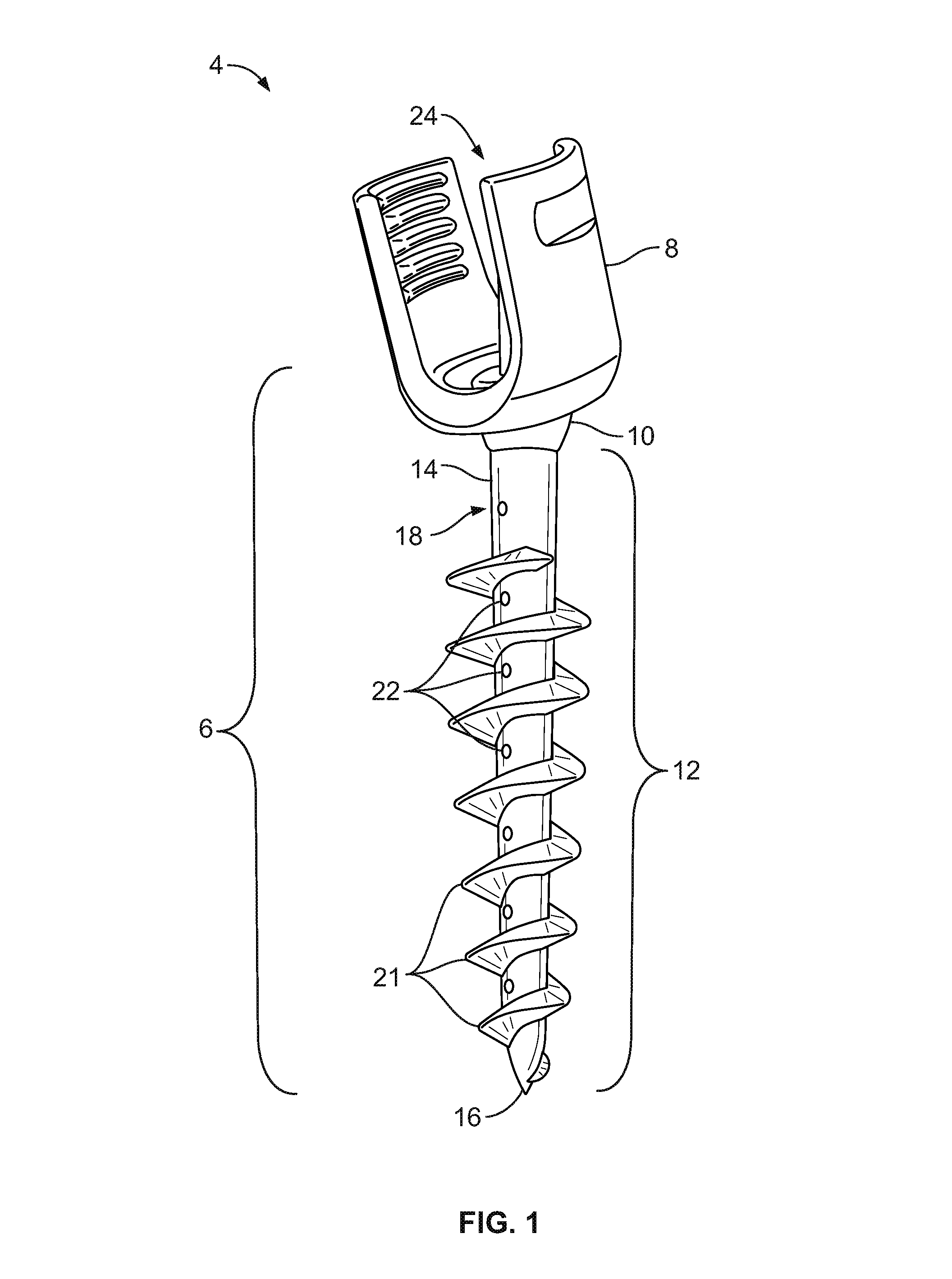
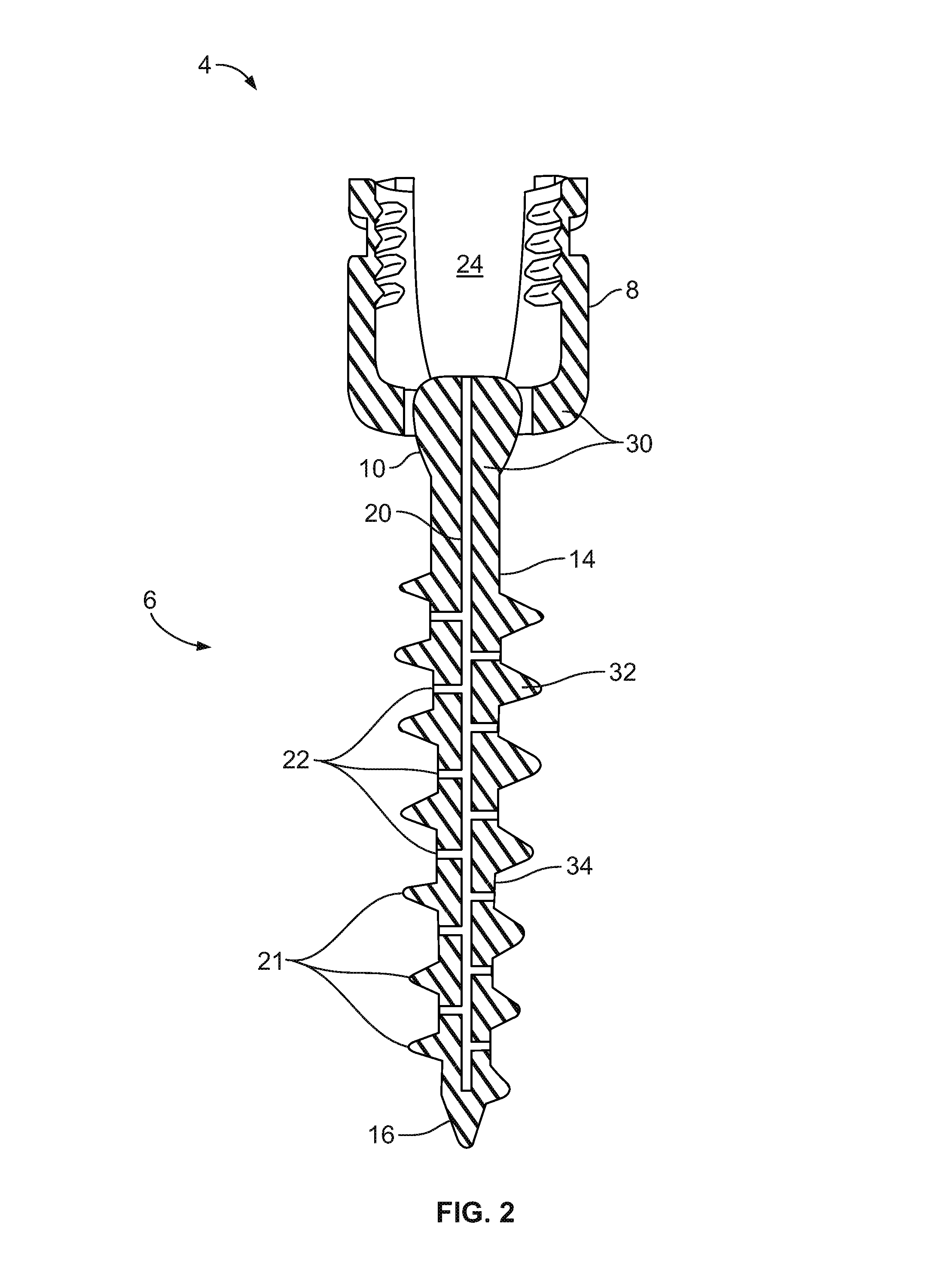
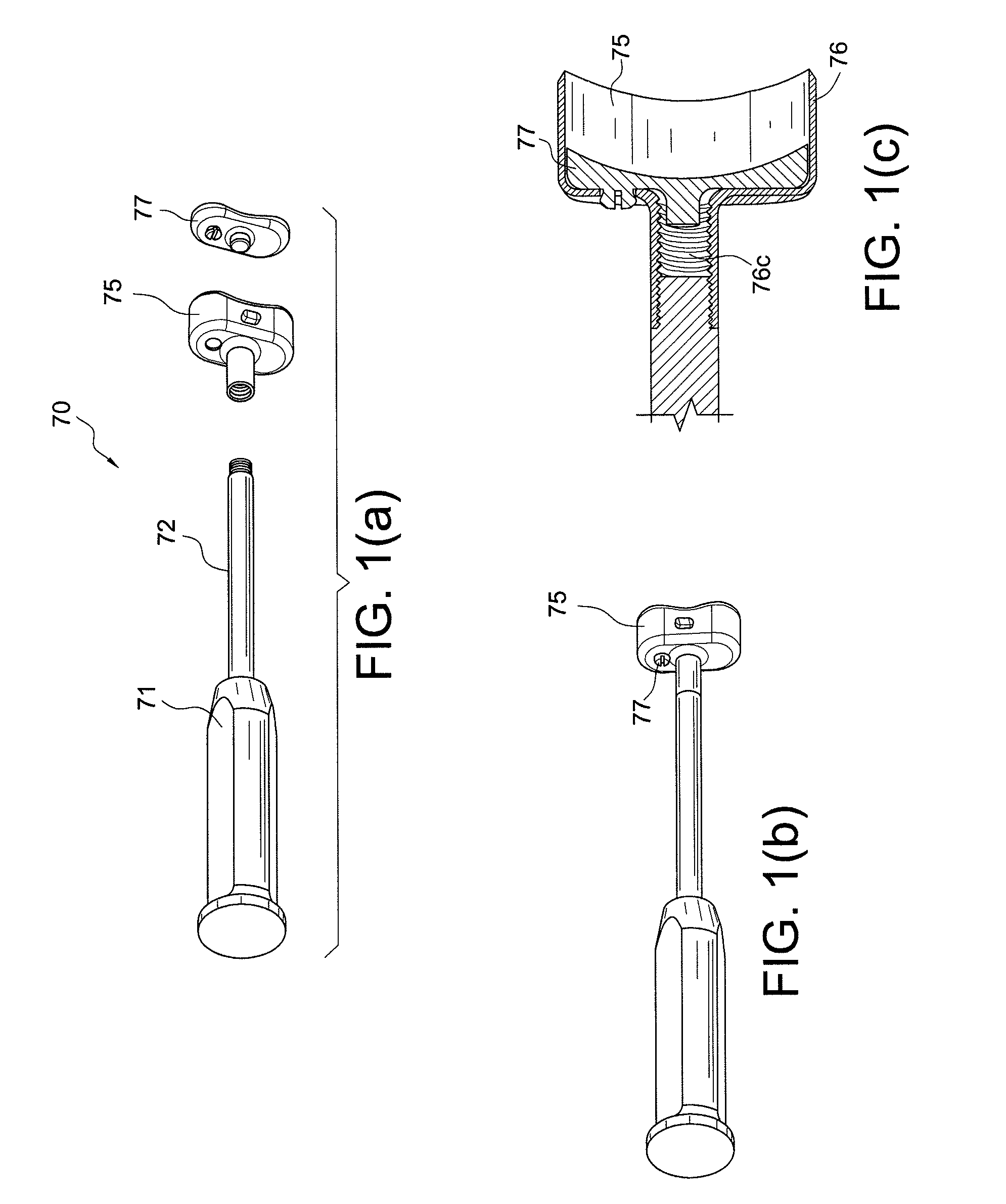
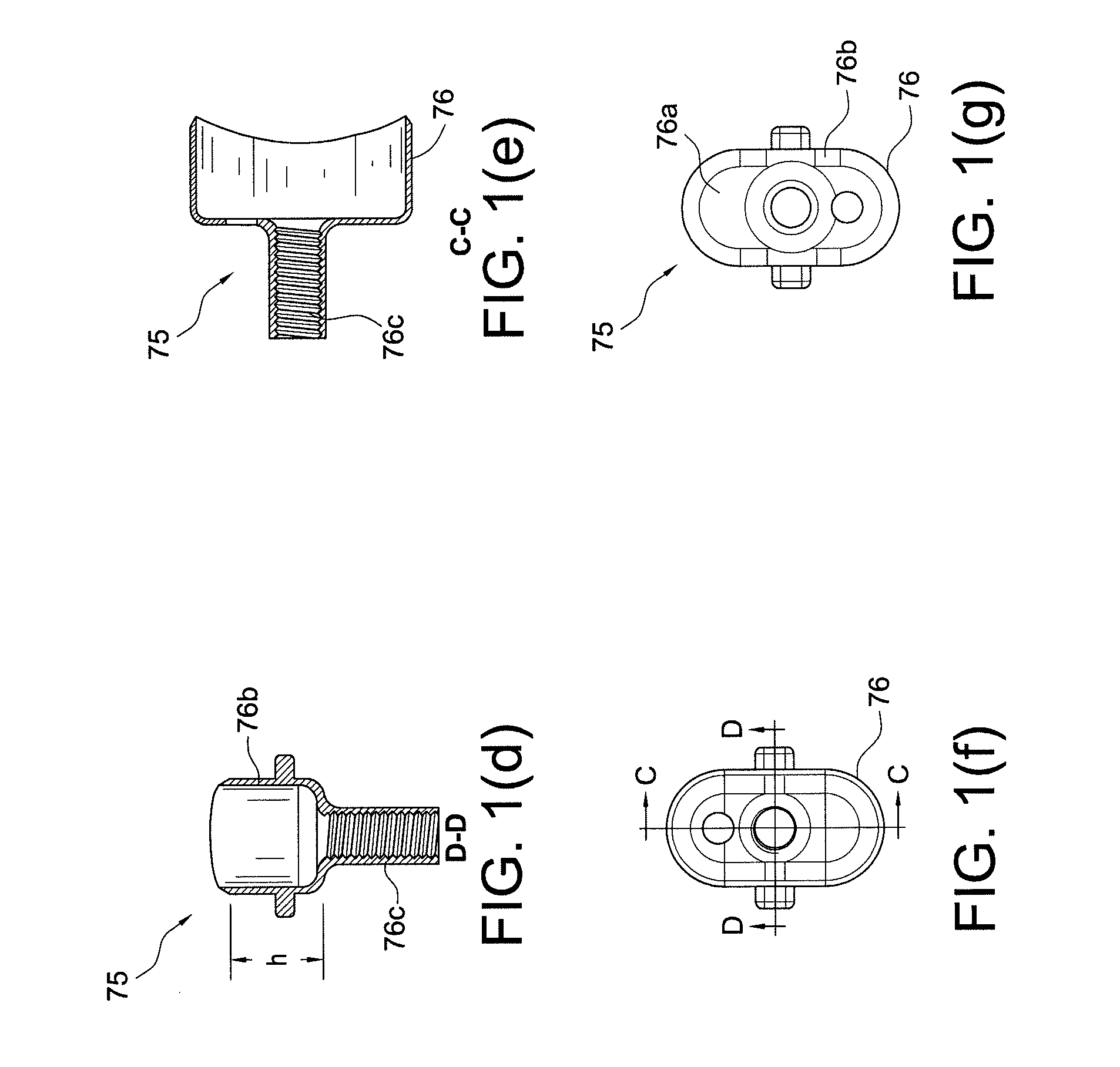
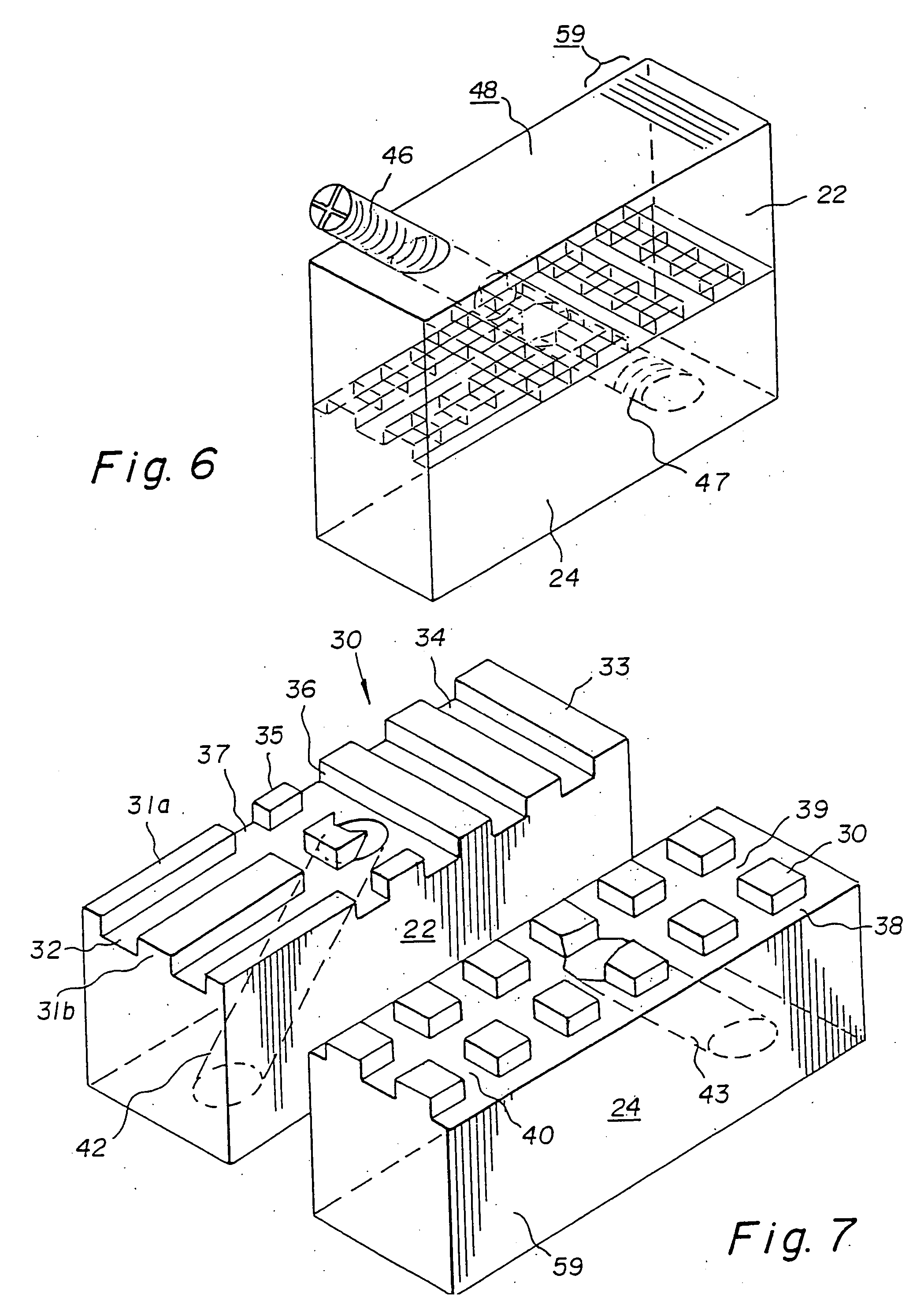
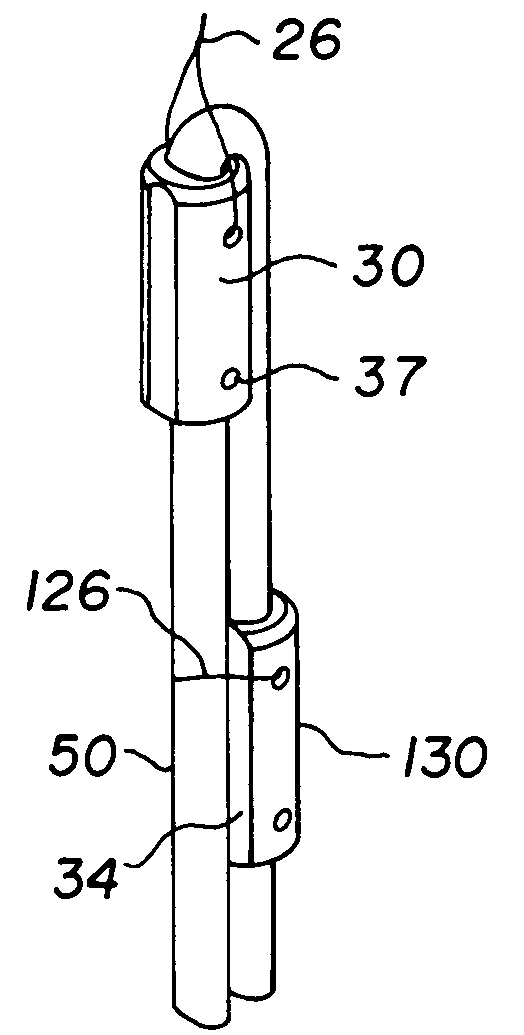
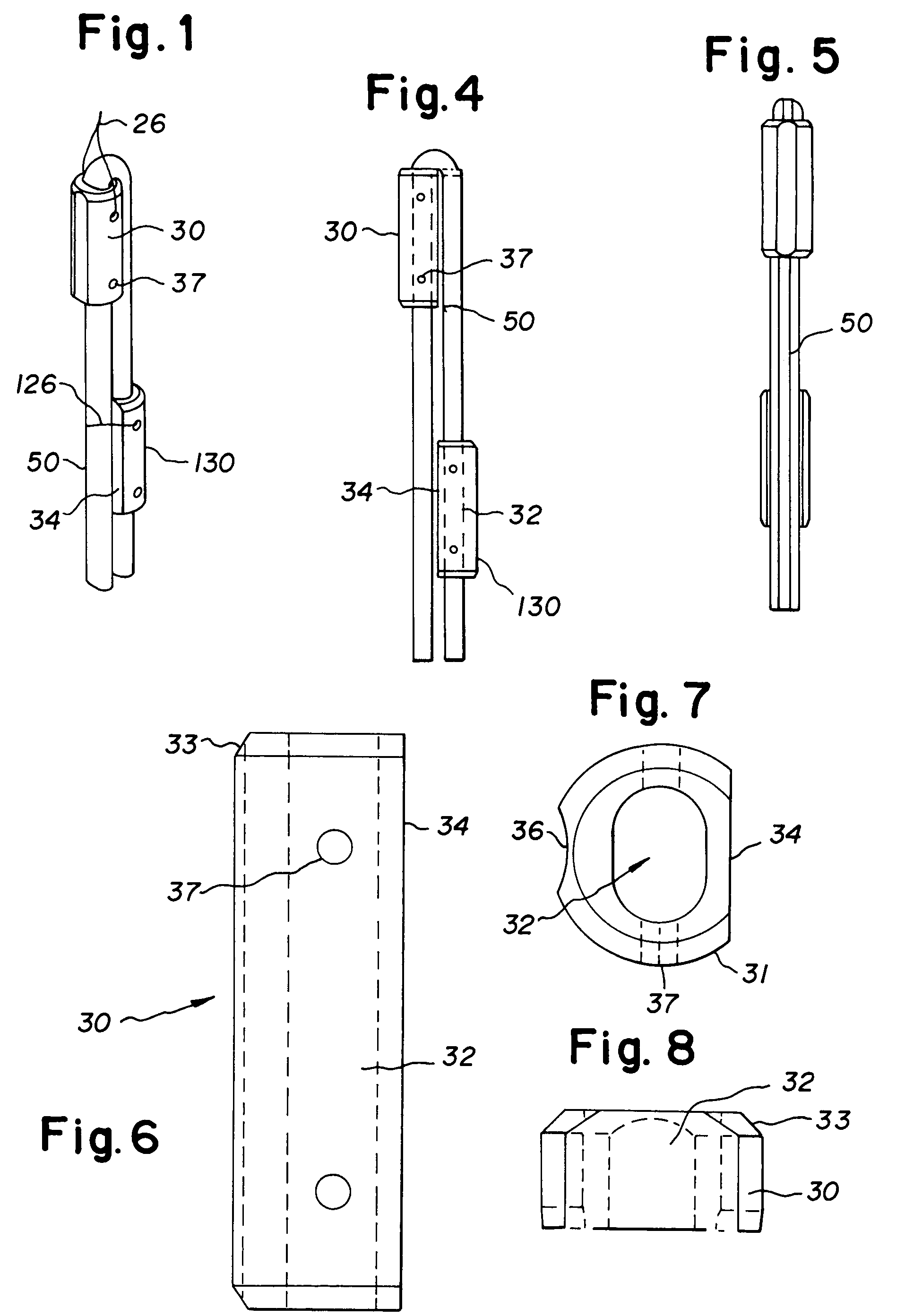
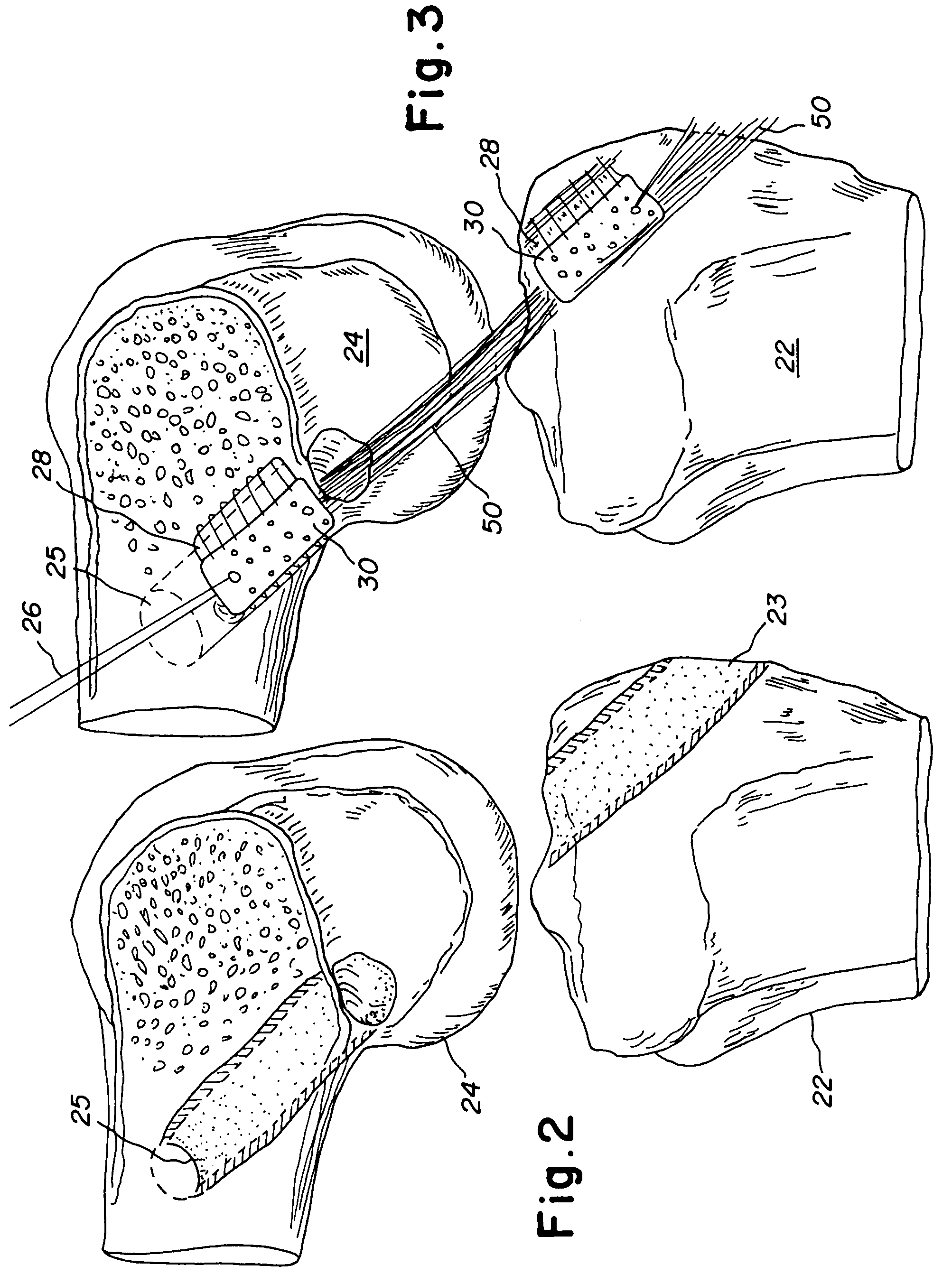
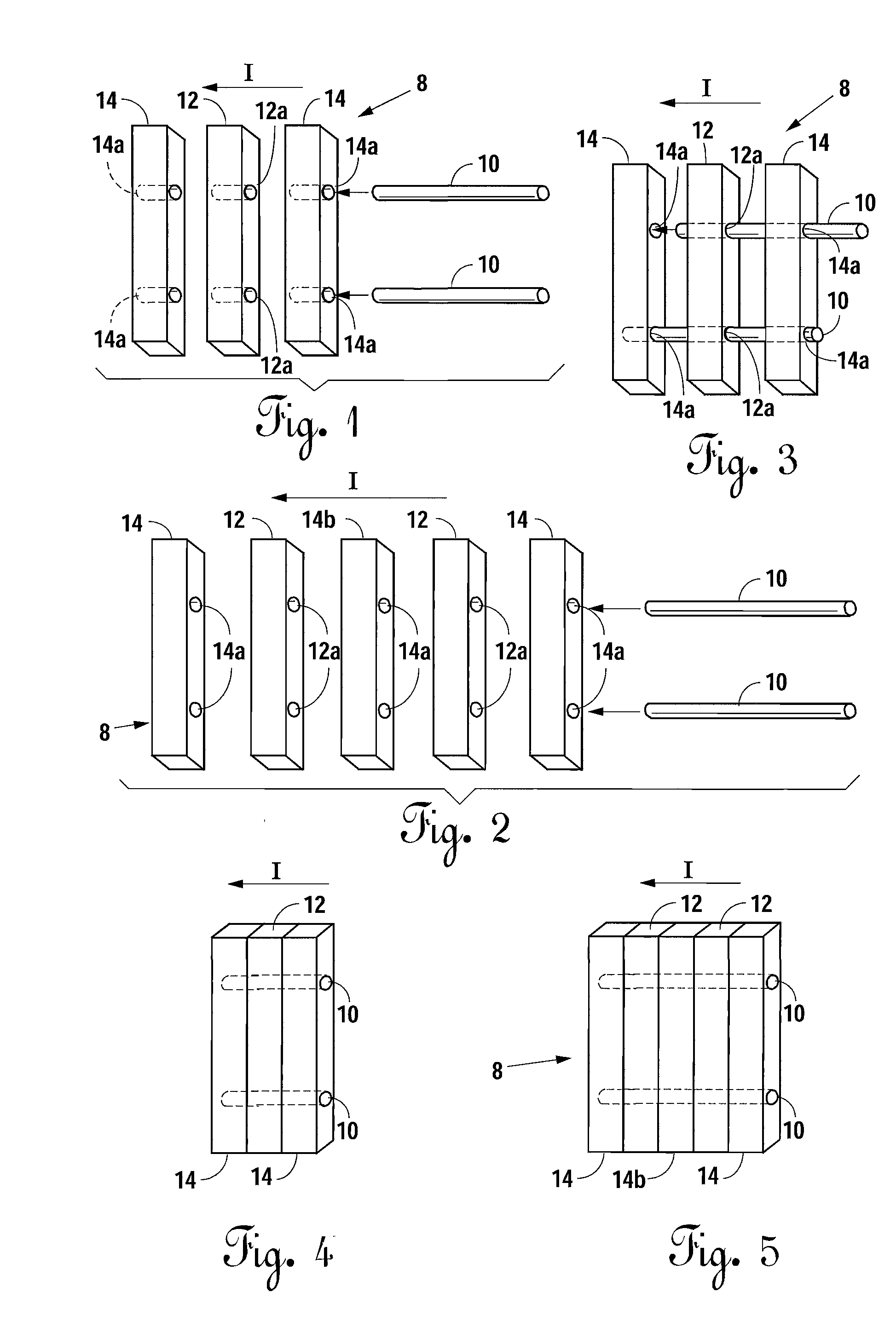
Bone-tendon-bone assembly with allograft bone block and method for inserting same
InactiveUS6890354B2Promote new bone growthPromote healingSuture equipmentsBone implantBone tunnelInterference screws
The invention is directed toward a bone block, a bone-tendon-bone assembly and method of tendon reconstruction in which at least one tendon replacement is extended between two bone blocks and fixed within each of two bone tunnels in the bones of a joint using interference screws. Each bone block has a central through going bore and at least one substantially parallel channel longitudinally cut in the exterior of the bone block body in which the ligament replacements are seated. One end of each bone block has a rounded recess leading from the central bore to the exterior parallel channel.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Malleable putty and flowable paste with allograft bone having residual calcium for filling bone defects
The invention is directed toward a malleable bone putty and a flowable pastel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of partially demineralized lyophilized allograft bone material having a residual calcium content ranging from 4 to 8% dry weight. The bone powder has a particle size ranging from about 100 to about 800 microns and is mixed in a high molecular weight hydrogel carrier containing a sodium phosphate saline buffer, the hydrogel component of the carrier ranging from about 1.00 to 50% of the composition and having a molecular weight of about at least 700,000 Daltons. The composition has a pH between 6.8-7.4 contains about 25% to about 35% bone powder and can be additionally provided with BMP's.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
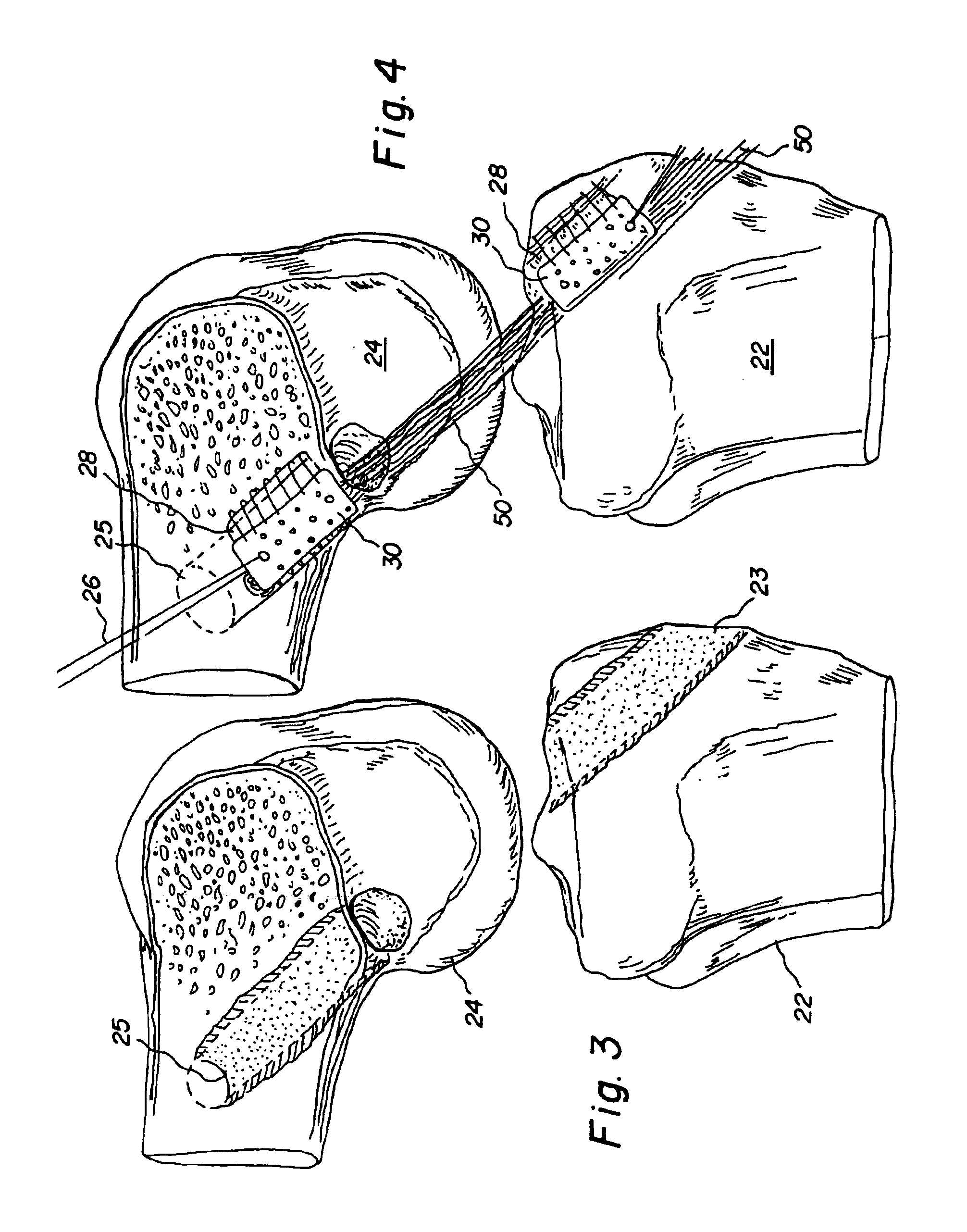
Allograft intervertebral implant and method of manufacturing the same
ActiveUS20080082173A1Facilitating spinal fusionBone implantSpinal implantsSacroiliac jointAllograft bone
The present invention is directed to an allograft intervertebral implant sized and configured for insertion between adjacent vertebral bodies in a spinal fusion surgery. The implant is preferably manufactured from two or more pieces of allograft bone joined together by a joint, more preferably a dovetail joint. The dovetail joint being sized and configured to substantially follow the exterior shape or surface (e.g. perimeter) of the intervertebral implant. The intervertebral implant may also include one or more bone pins for joining the allograft pieces, the pins being inserted into the implant at an angle substantially perpendicular with respect to the dovetail joint. The intervertebral implant may also include one or more through-bores for receiving ostegenic or bone graft material. The intervertebral implant is preferably sized and configured for insertion during a T-PLIF or PLIF procedure.
Owner:SYNTHES USA
Bone tendon bone assembly with allograft bone block
InactiveUS20050203620A1Provide strengthProvide to structureSuture equipmentsBone implantBone tunnelInterference screws
The invention is directed toward a bone block, a bone-tendon-bone assembly and method of tendon reconstruction in which at least one tendon replacement is extended between two bone blocks and fixed within each of two bone tunnels in the bones of a joint using interference screws. Each bone block has a central through going bore and at least one substantially parallel channel longitudinally cut in the exterior of the bone block body in which the ligament replacements are seated. One end of each bone block has a rounded recess leading from the central bore to the exterior parallel channel.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
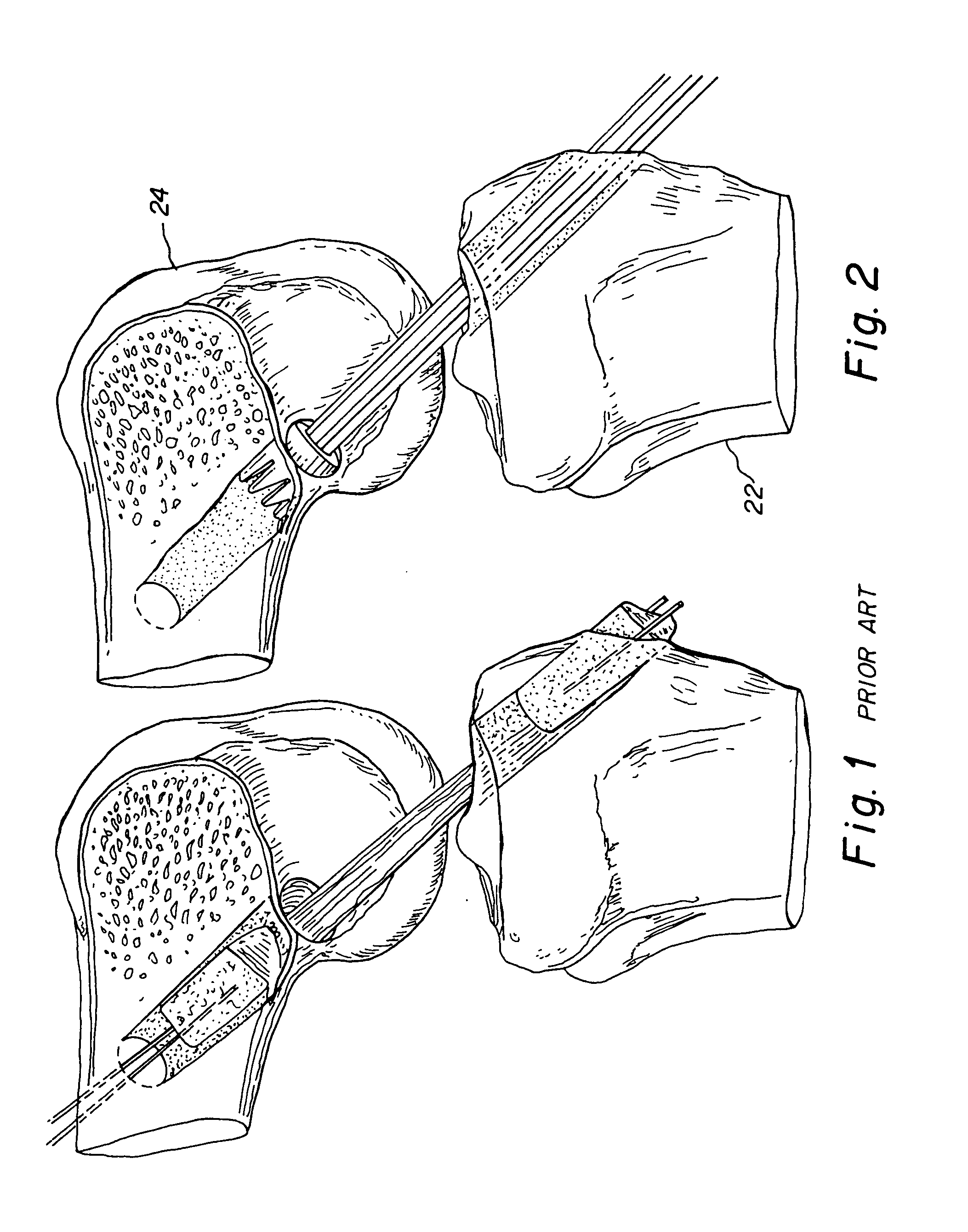
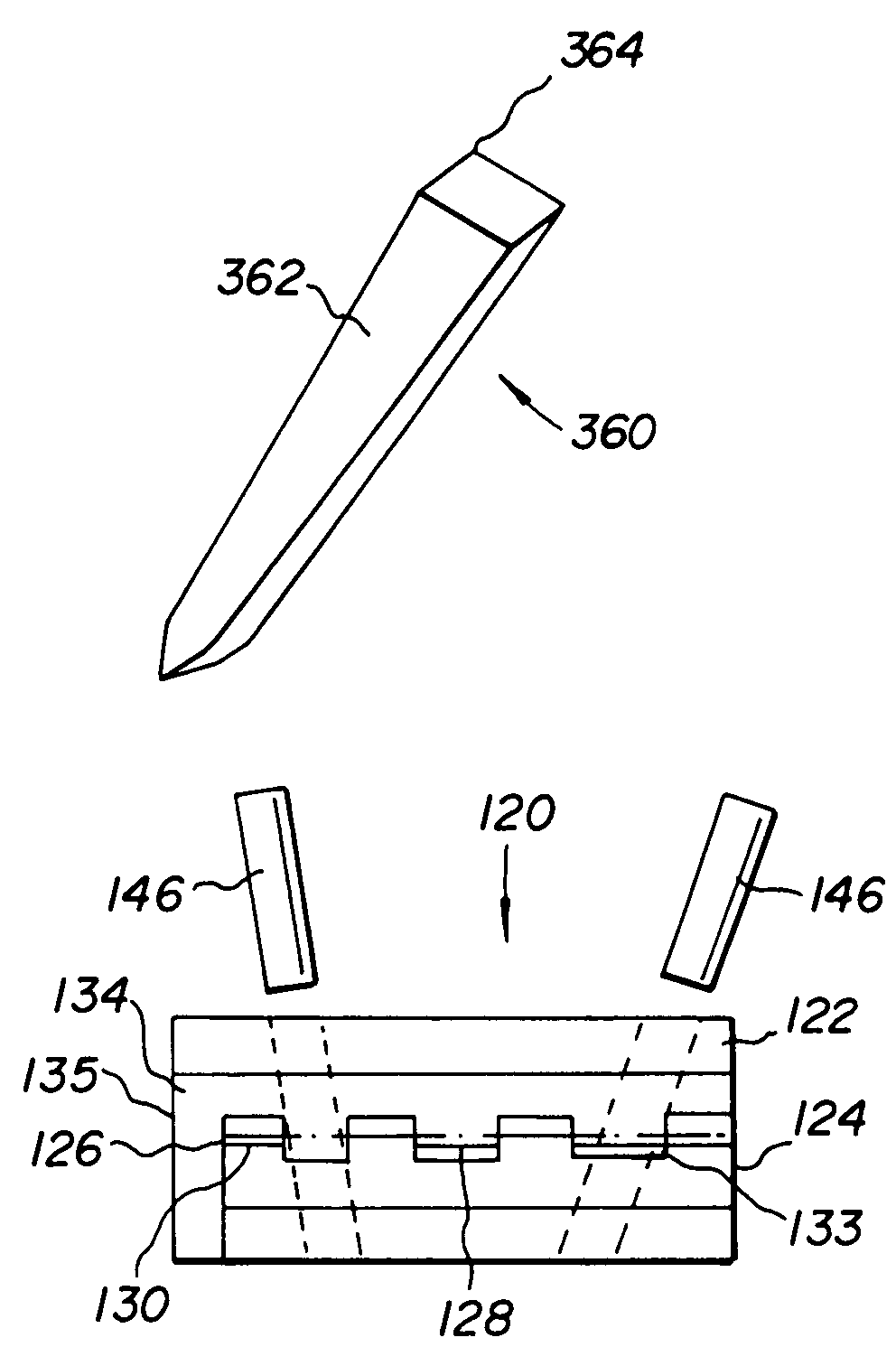
Method and instrumentation for the preparation and transplantation of osteochondral allografts
InactiveUS20070093896A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
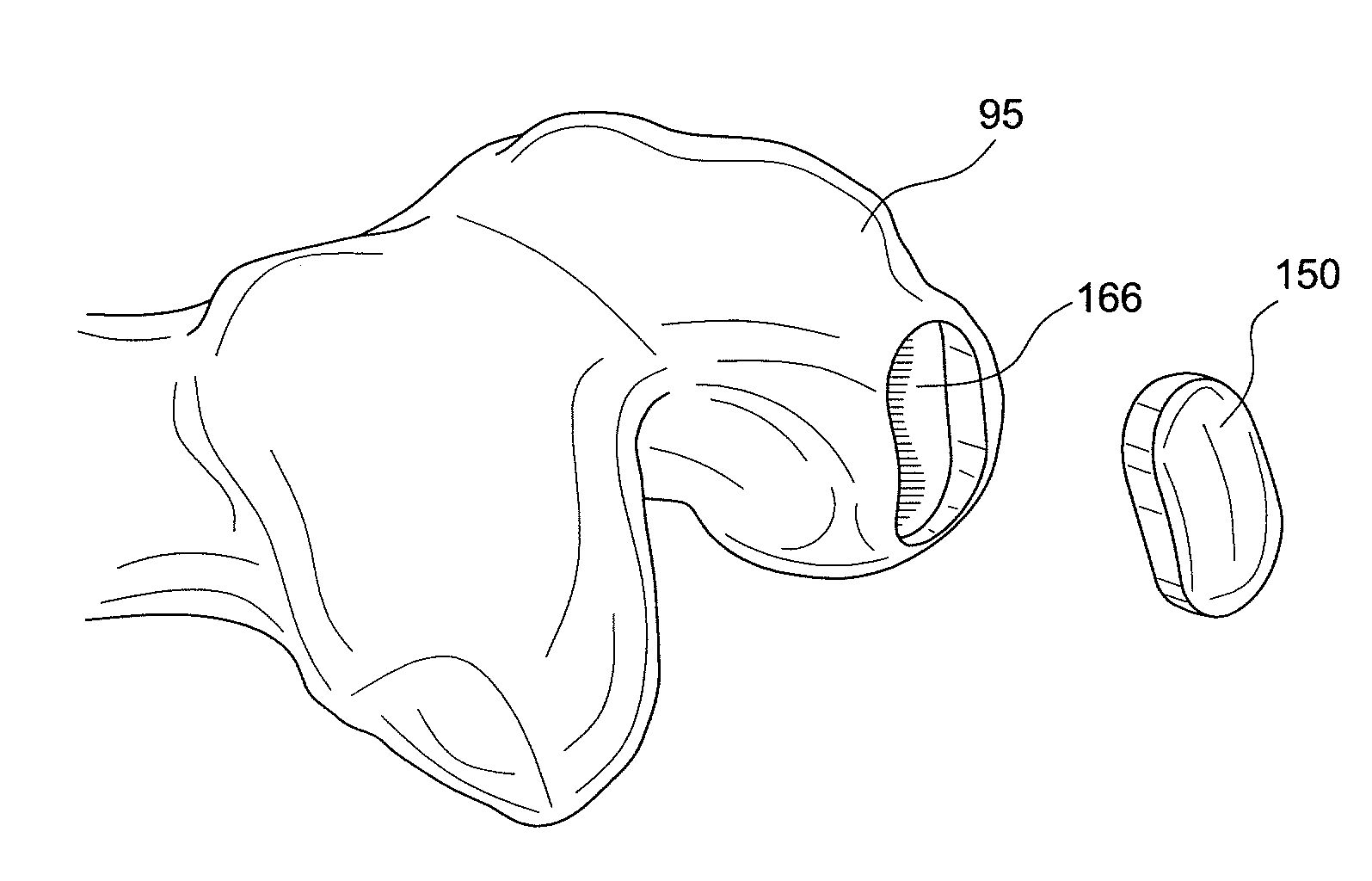
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilnear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Allograft bone composition having a gelatin binder
InactiveUS7045141B2Low tensile strengthIncrease delayImpression capsSurgical adhesivesCross-linkSolid structure
The invention is directed toward an osteoimplant for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing composition of demineralized allograft bone material mixed with an aqueous phosphate buffered gelatin which when lyophilized to remove water from the composition cross links the gelatin to form a solid structure.
Owner:MUSCULOSKELETAL TRANSPLANT
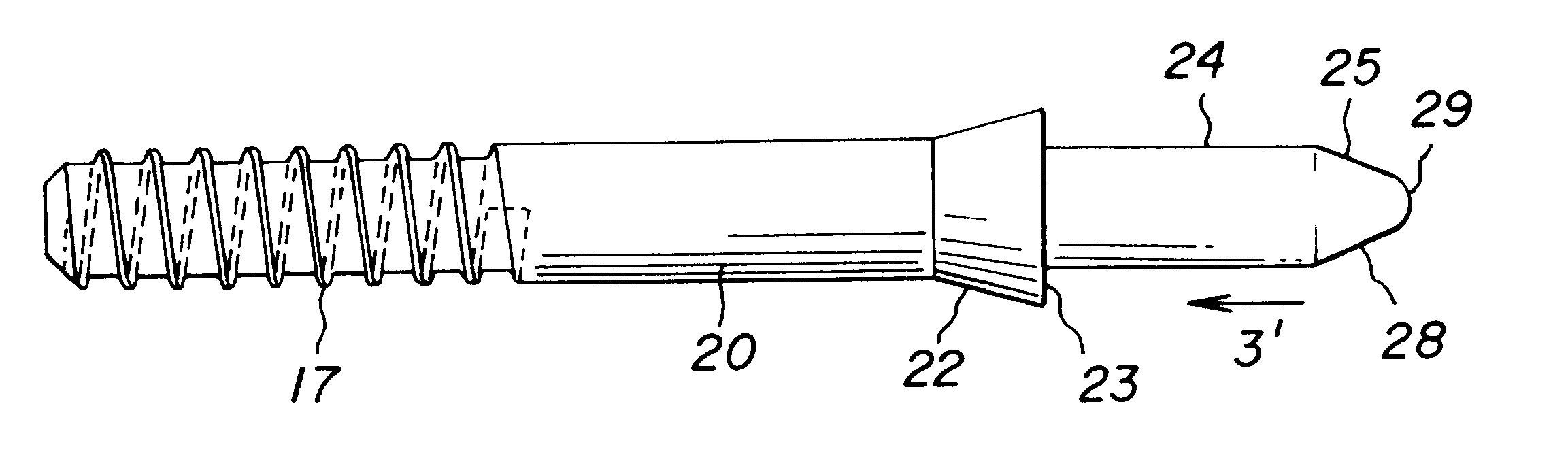
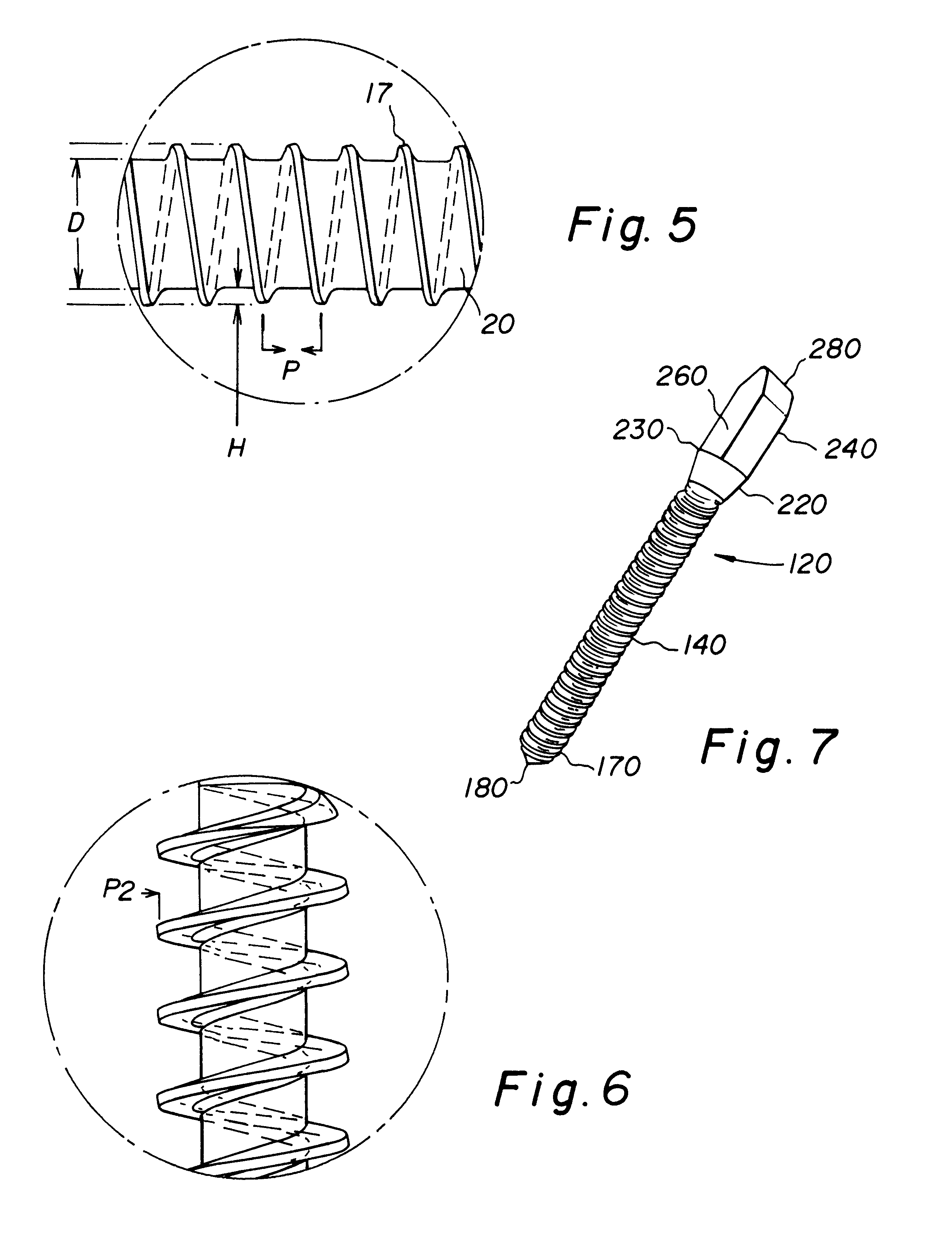
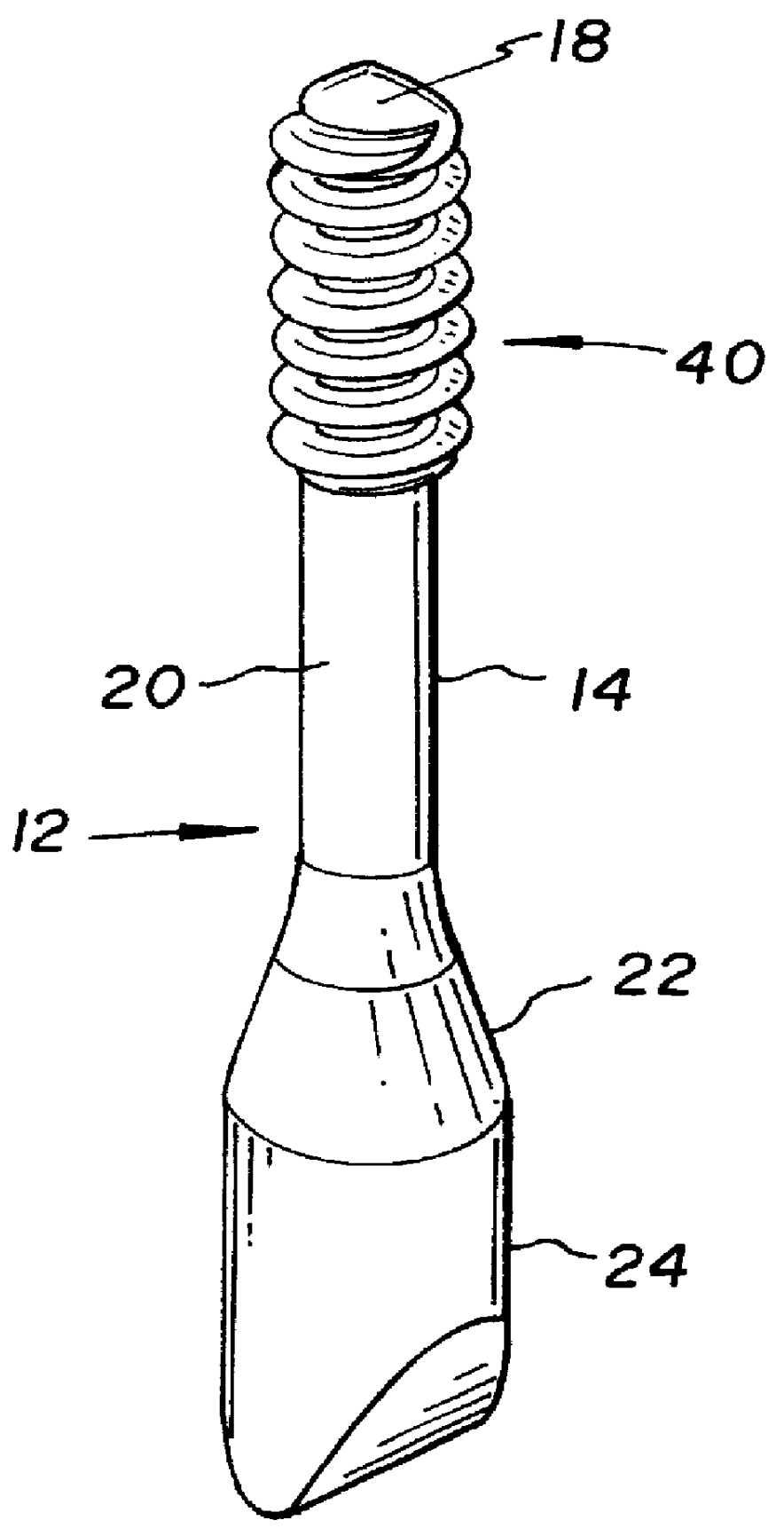
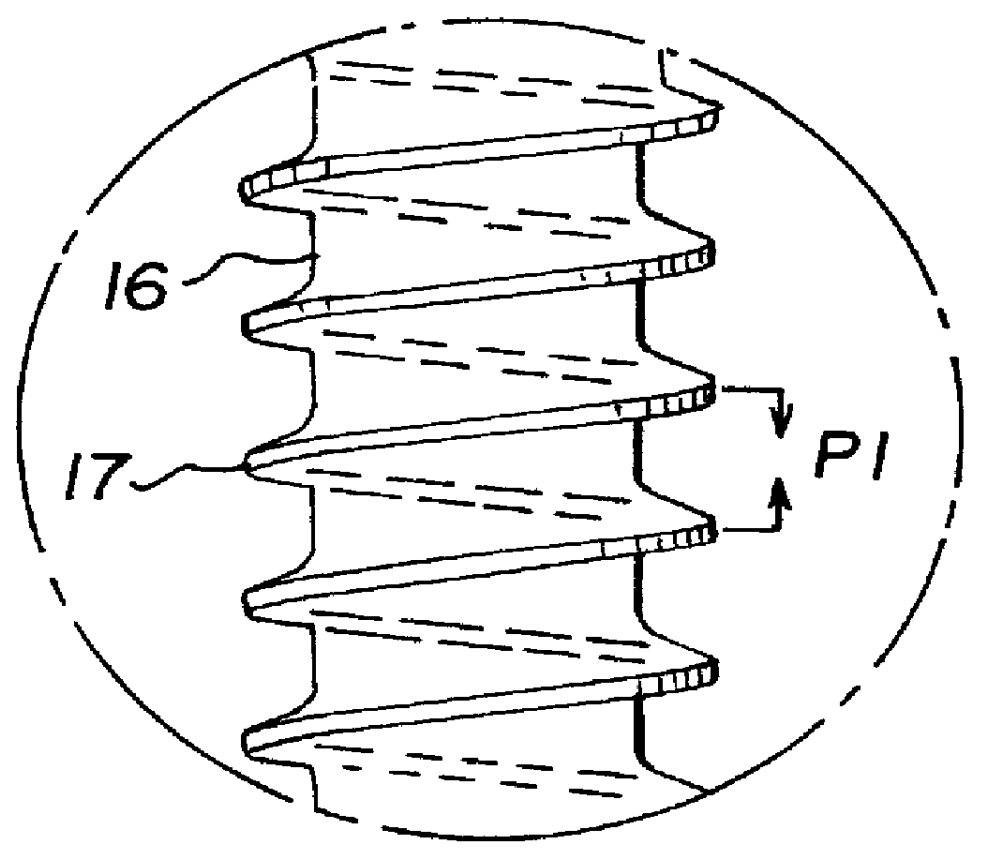
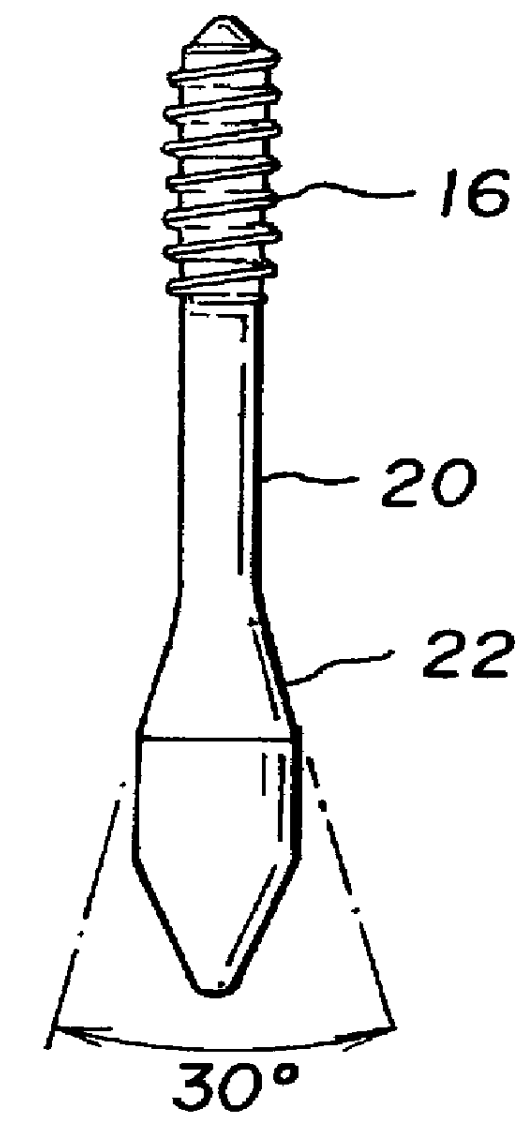
Allograft bone fixation screw
InactiveUS6506192B1Easy to useSuture equipmentsInternal osteosythesisAllograft boneBiomedical engineering
A sterile allograft bone screw comprising a screw shank with a uniform diameter threaded portion, an unthreaded portion with a outwardly tapered conical portion and a rectangularly shaped driving head portion with substantially wedge shaped end which can be engagably seated in a seat of a driver member.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Composition for filling bone defects
InactiveUS7019192B2Low tensile strengthIncrease delayBiocideSurgical adhesivesSodium phosphatesDemineralized bone
The invention is directed toward a formable bone composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone particles. The particle size ranges from about 0.1 mm to about 1.0 cm and is mixed in a hydrogel carrier containing a sodium phosphate saline buffer, the hydrogel component of the carrier ranging from about 1.0 to 5.0% of the composition and a pH between 6.8–7.4 with one or more additives of a cellular material, growth factor, demineralized bone chips or mineralized bone chips.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Malleable paste for filling bone defects
InactiveUSRE38522E1Easy to packFast absorptionSurgical adhesivesPeptide/protein ingredientsBone defectBiomedical engineering
The invention is directed toward a malleable bone putty and a flowable gel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone powder. The bone powder has a particle size ranging from about 100 to about 850 microns and is mixed in a high molecular weight hydrogel carrier, the hydrogel component of the carrier ranging from about 0.3 to 3.0% of the composition and having a molecular weight of about at least 10,000 Daltons. The composition contains about 25% to about 40% bone powder and can be additionally provided with BMP's and a sodium phosphate buffer.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
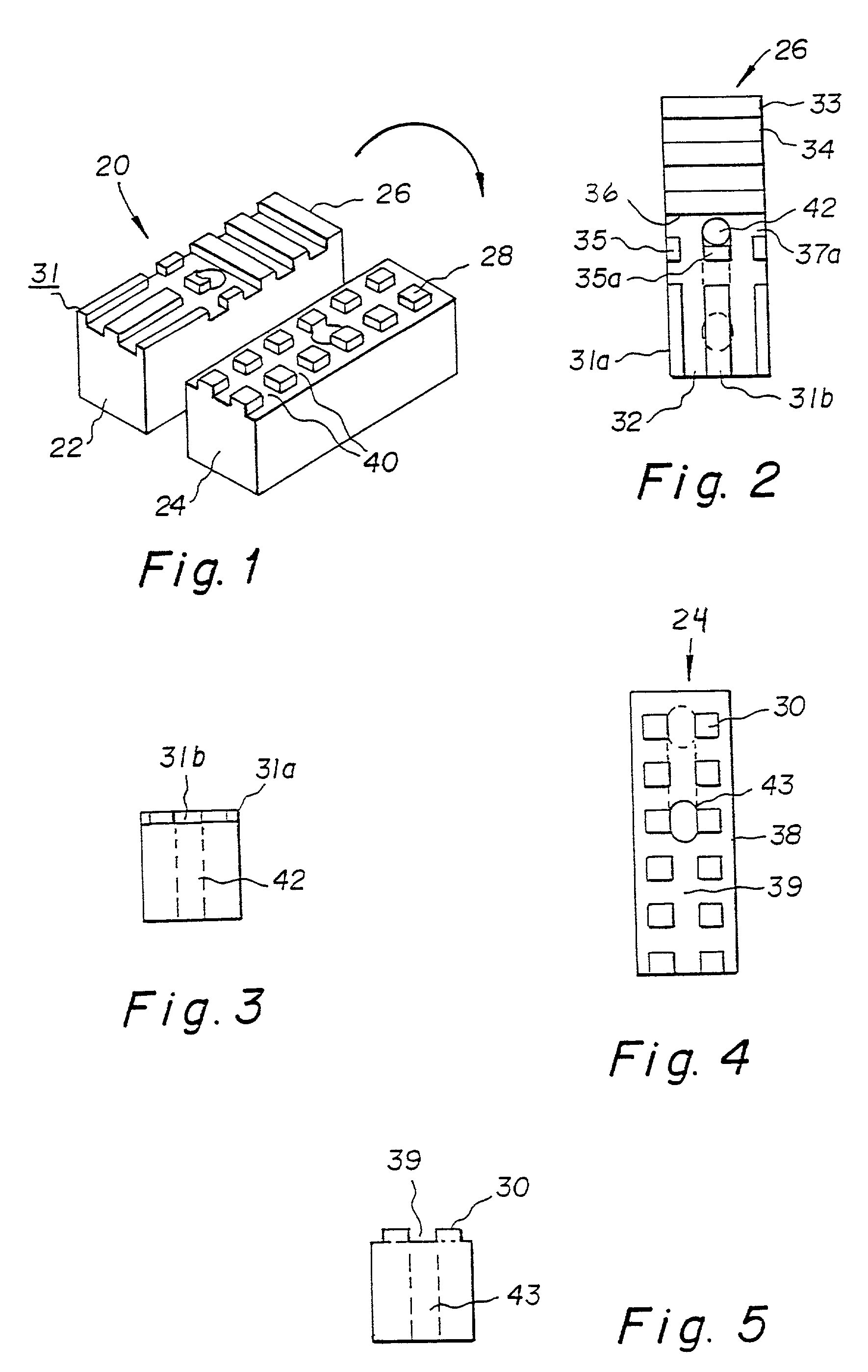
Compound bone structure of allograft tissue with threaded fasteners
A composite allograft bone device having a first bone member body with a face that defines a plurality of spaced projections forming a pattern and a second bone member body defining a face that forms a plurality of spaced projections forming a second pattern. The projections in the first face allow the two bodies to be mated together. The mated bodies form a composite bone device which is provided with a throughgoing bore and a threaded rod member mounted in the throughgoing bore extending into and engaging the bone member bodies holding the same together. Alternatively a rod member with a demineralized or knurled outer surface can be press fit into the throughgoing bore engaging the bone member bodies in an interference fit holding the same together. In another embodiment an inner central cancellous bone block is surrounded by plates or a U shaped base constructed of cortical bone material.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Allograft bone fixation screw method and apparatus
A combination driver and bone screw assembly comprising a screw shank with a uniform diameter threaded portion, an unthreaded portion with a outwardly tapered end and a driving end portion of substantially wedge shaped configuration which can be engagably seated in a wedge shaped notch seat of a driver member. The driver member comprises a body, a drive head defining a wedge shaped notch seat secured to the body forming a shoulder and a drive collar mounted around the drive head and seated on the shoulder. In operation a bore is drilled in the patients bone with the top portion of the bore having a tapered geometry which widens from the diameter of the bore. The bone screw driving end portion is placed in the wedge shaped notch seat of a driver member and a drive collar is mounted around the driver drive head and engages the shoulder. The driver member is rotated driving the bone screw into the previously cut bore in the patients bone until the tapered surface of the bore engages the tapered undercut surface of the screw.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Bone tendon bone assembly with cylindrical allograft bone block
InactiveUS20050203623A1Provide strengthProvide to structureSuture equipmentsBone implantInterference screwsBone tendon bone
The invention is directed toward a bone block, a bone-tendon-bone assembly and method of tendon reconstruction in which at least one tendon replacement is extended between two bone blocks and fixed within each of two bone tunnels in the bones of a joint using interference screws. Each bone block has a central through going bore and at least one substantially parallel channel longitudinally cut in the exterior of the bone block body in which the ligament replacements are seated. One end of each bone block has a rounded recess leading from the central bore to the exterior parallel channel.
Owner:MUSCULOSKELETAT TRANSPLANT FOUND
Allograft bone fixation screw method and apparatus
A bone screw assembly constructed of allograft bone comprising a screw shank with a uniform diameter threaded portion, an unthreaded portion with a outwardly tapered end terminating in a drive head defining a wedge shaped configuration to form an undercut for the drive head. In operation a bore is drilled in the two bone sections, with one bone section being over drilled with the top portion of the over drilled bore having a tapered geometry which widens from the diameter of the bore. The bone screw is driven into the previously cut bore in the bone sections until the tapered surface of the bore engages the tapered undercut surface of the screw.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Malleable paste for filling bone defects
InactiveUSRE39587E1Useful bulk viscosityAbsorb more quicklySurgical adhesivesPeptide/protein ingredientsBone defectBiomedical engineering
The invention is directed toward a malleable bone putty and a flowable gel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone powder. The bone powder has a particle size ranging from about 100 to about 850 microns and is mixed in a high molecular weight hydrogel carrier, the hydrogel component of the carrier ranging from about 0.3 to 3.0% of the composition and having a molecular weight of about at least 10,000 Daltons. The composition contains about 25% to about 40% bone powder and can be additionally provided with BMP's and a sodium phosphate buffer.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Instrumentation for the preparation and transplantation of osteochondral allografts
ActiveUS20070135918A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is forined corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Instrumentation for the preparation and transplantation of osteochondral allografts
ActiveUS20070135917A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Composite metal and bone orthopedic fixation devices
ActiveUS9173692B1Promote healingImprove bio-integrationInternal osteosythesisBone implantFiberOrthopedic devices
Composite orthopedic devices that facilitate spine stabilization, such as: bone screws, rods, plates, interbodies, and corpectomy cages are disclosed. They are designed to provide both strength and load carrying capabilities, while increasing bio-integration of the devices with the surrounding bone tissue. They are constructed of composite layers of allograft and / or autograft bone and a structural material, such as titanium alloy or carbon / graphite fiber composite. Cannulations within the device are loaded with a mixture of stem cells, particles of allograft and / or autograft bone, and bone growth factors, such as BMP-2. The cannulations are connected to the surface of the device via multiple fenestrations that provide pathways to supply the bone / stem cell mixture to the surface, allowing living bone tissue to grow and insure bio-integration. The devices can also have radiofrequency (RF) stimulation implantation within the structure of the implanted device, capable of responding to external RF stimulation of enhanced bone growth.
Owner:STC UNM
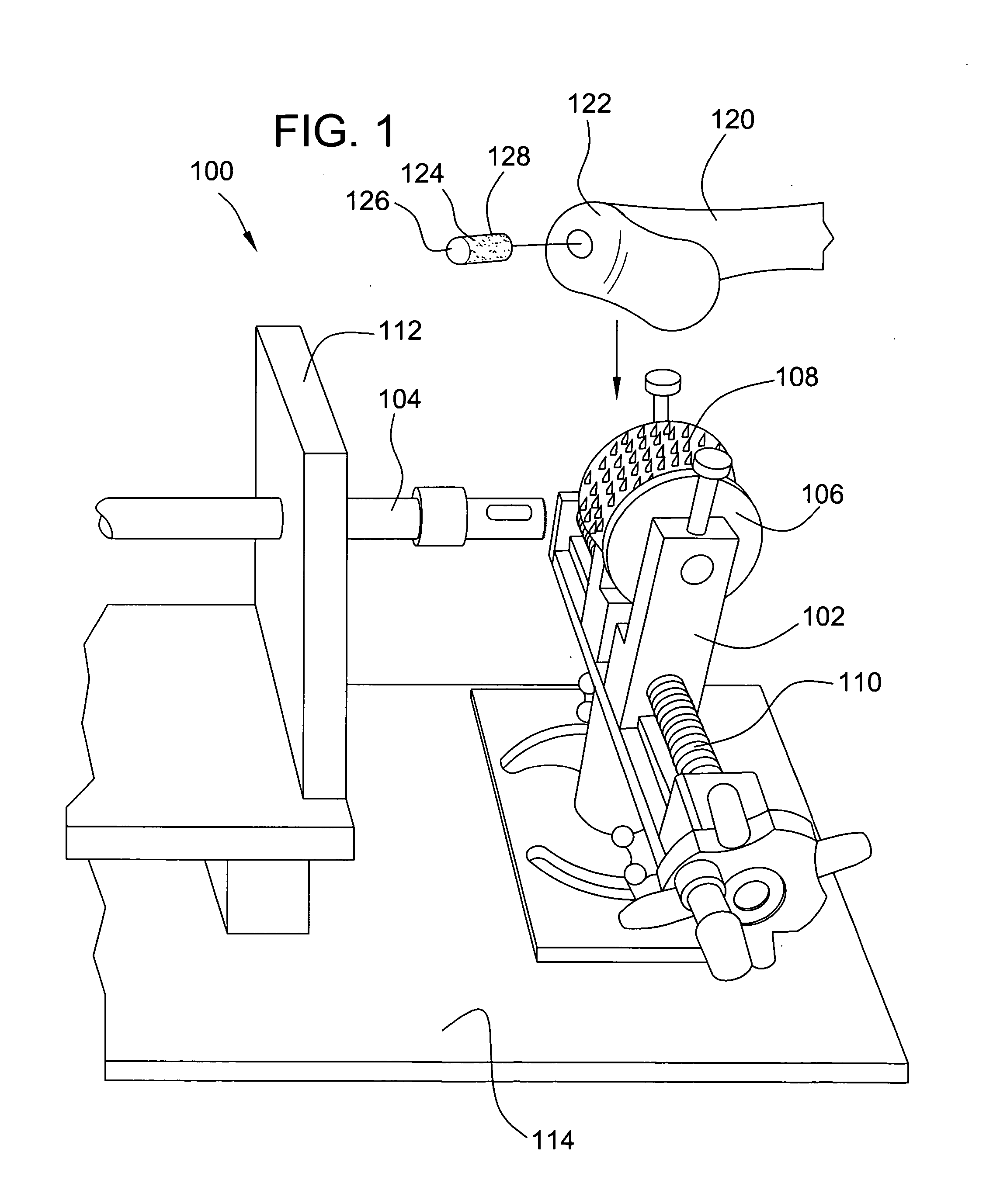
Methods and instruments for forming non-circular cartilage grafts
Techniques and instruments for knee replacement surgery that allow for the removal of an oval oblong-shaped allograft bone and cartilage plug from a donor distal femur. The instruments include (i) sizing guides to match the recipient's femoral size and curvature to that of a donor femur (the sizing guides also acting as a wide pin placement template for the donor distal femur); (ii) osteotomes that cut the curved and straight portions of the implant shape (these may be disposable or reusable); and (iii) templates that fit over the guide pins and have openings to allow the osteotomes to cut the donor femur plug to the correct size, shape and depth. The instruments allow for a non-circular shape to be extracted from a donor femur for use in a bone-saving osteoarthritis distal femur resurfacing procedure.
Owner:ARTHREX
Osteochondral allografts
ActiveUS20070135928A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Allograft bone composition having a gelatin binder
InactiveUS20060204544A1Easy to handleRapid bone formationImpression capsBone implantPhosphateSolid structure
The invention is directed toward an osteoimplant for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing composition of demineralized allograft bone material mixed with an aqueous phosphate buffered gelatin which when lyophilized to remove water from the composition crosslinks the gelatin to form a solid structure and when rehydrated is flexible
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Methods and instruments for forming non-circular cartilage grafts
Techniques and instruments for knee replacement surgery that allow for the removal of an oval oblong-shaped allograft bone and cartilage plug from a donor distal femur. The instruments include (i) sizing guides to match the recipient's femoral size and curvature to that of a donor femur (the sizing guides also acting as a wide pin placement template for the donor distal femur); (ii) osteotomes that cut the curved and straight portions of the implant shape (these may be disposable or reusable); and (iii) templates that fit over the guide pins and have openings to allow the osteotomes to cut the donor femur plug to the correct size, shape and depth. The instruments allow for a non-circular shape to be extracted from a donor femur for use in a bone-saving osteoarthritis distal femur resurfacing procedure.
Owner:ARTHREX
Compound bone structure of allograft tissue with threaded fasteners
A composite allograft bone device comprising a first bone member body with a face that defines a plurality of spaced projections forming a pattern and a second bone member body defining a face that forms a plurality of spaced projections forming a second pattern. The projections in the first face allow the two bodies to be mated together. The mated bodies form a composite bone device which is provided with a throughgoing bore and a threaded rod member mounted in the throughgoing bore extending into and engaging the bone member bodies holding the same together. Alternatively a rod member with a demineralized or knurled outer surface can be press fit into the throughgoing bore engaging the bone member bodies in an interference fit holding the same together. In another embodiment an inner central cancellous bone block is surrounded by plates or a U shaped base constructed of cortical bone material.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
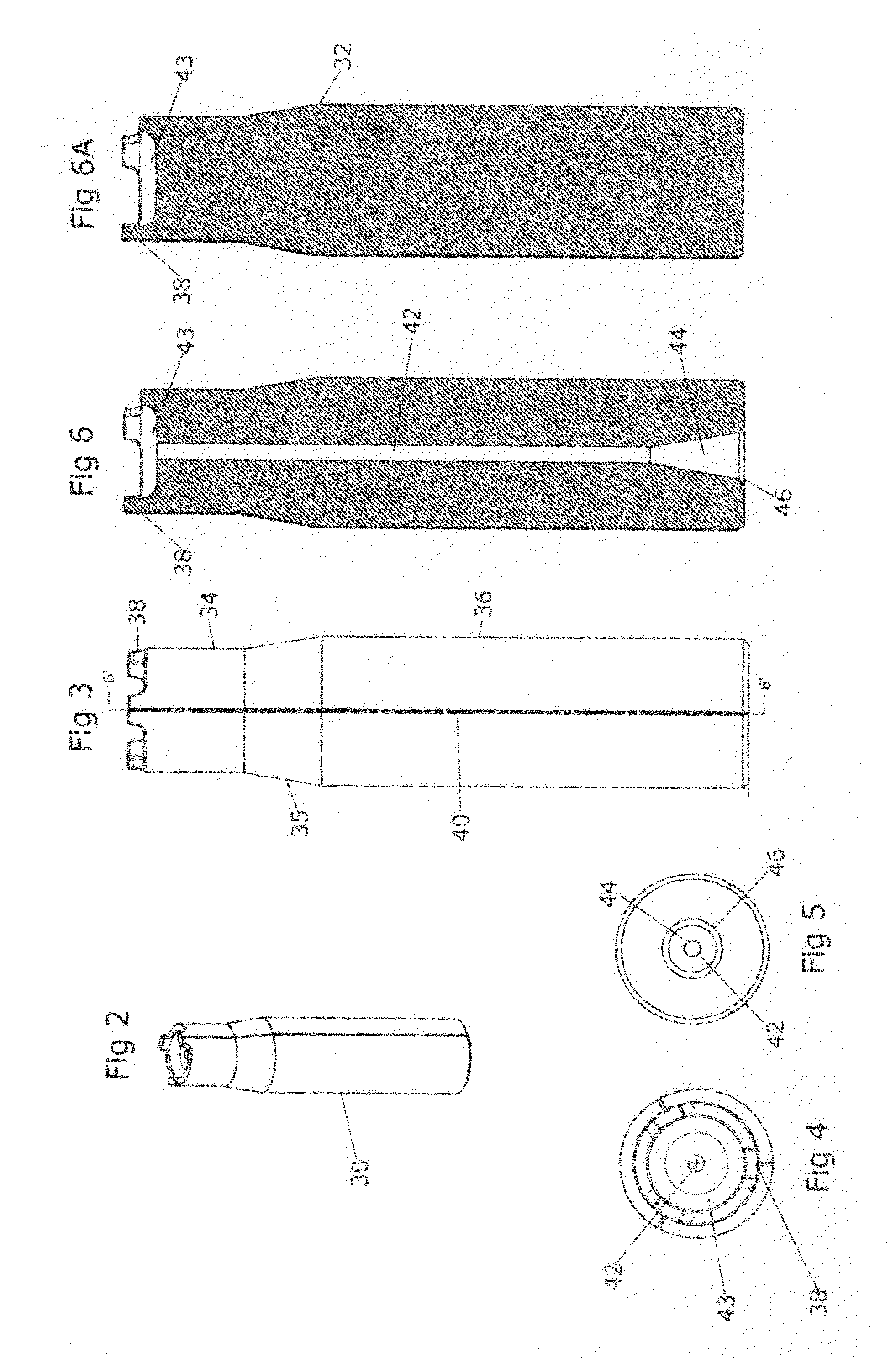
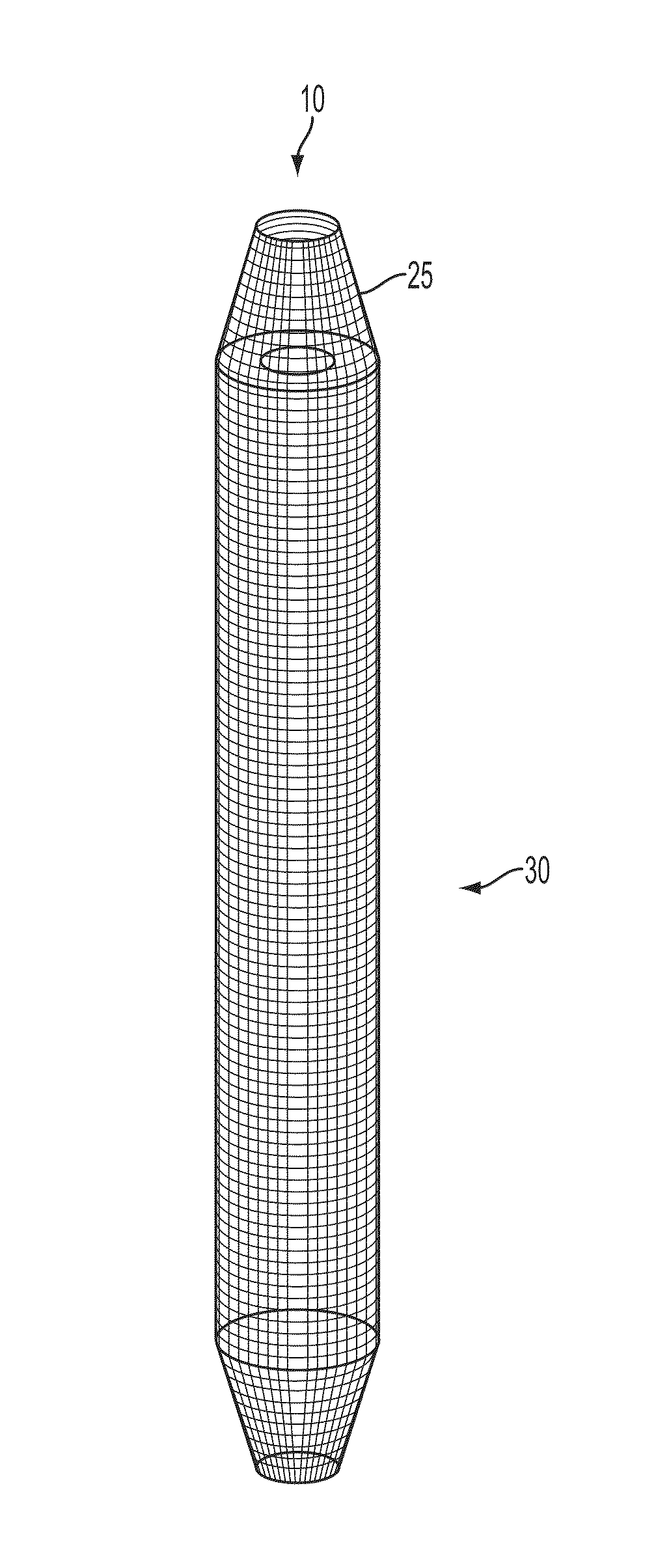
Bone-tendon- bone assembly with cancellous allograft bone block
InactiveUS7309356B2Provide strengthProvide to structureSuture equipmentsBone implantBone tunnelInterference screws
The invention is directed toward a bone-tendon-bone assembly extended between two shaped cancellous bone blocks and a method of inserting same in a joint. Each substantially cylindrically shaped cancellous bone block has a central through going bore, a flat exterior longitudinal surface and a channel longitudinally cut in the exterior of the bone block body opposite the flat longitudinal surface. A tendon replacement member is inserted through the central through going bore around the end of each block and looped back along the flat longitudinal side with each block being in a reversed orientation position as to the other block. A channel cut in the exterior surface of each block is adapted to receive an interference screw to keep the block anchored in a bone tunnel previously cut in the respective bones of a joint.
Owner:MUSCULOSKELETAL TRANSPLANT FOUNDATI
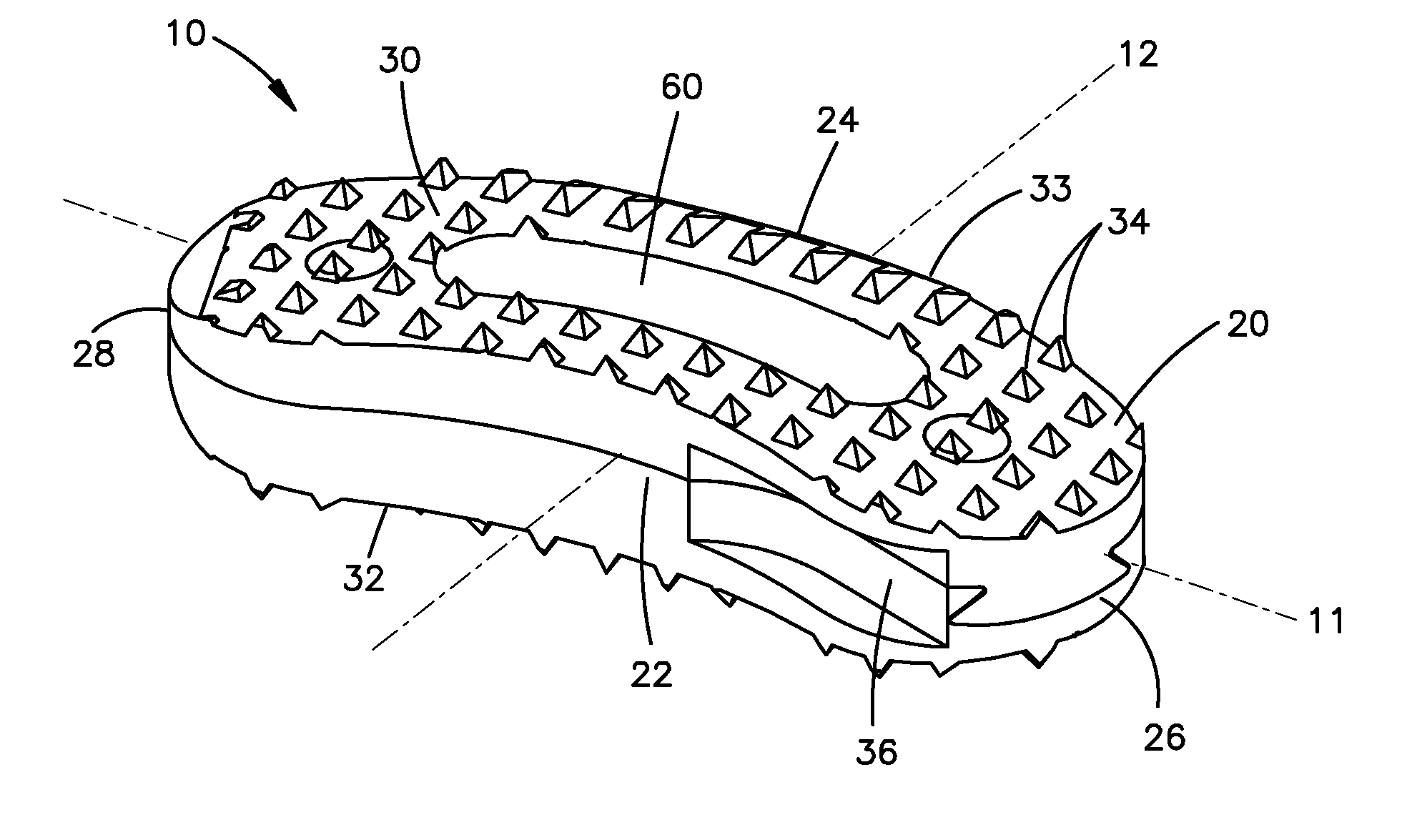
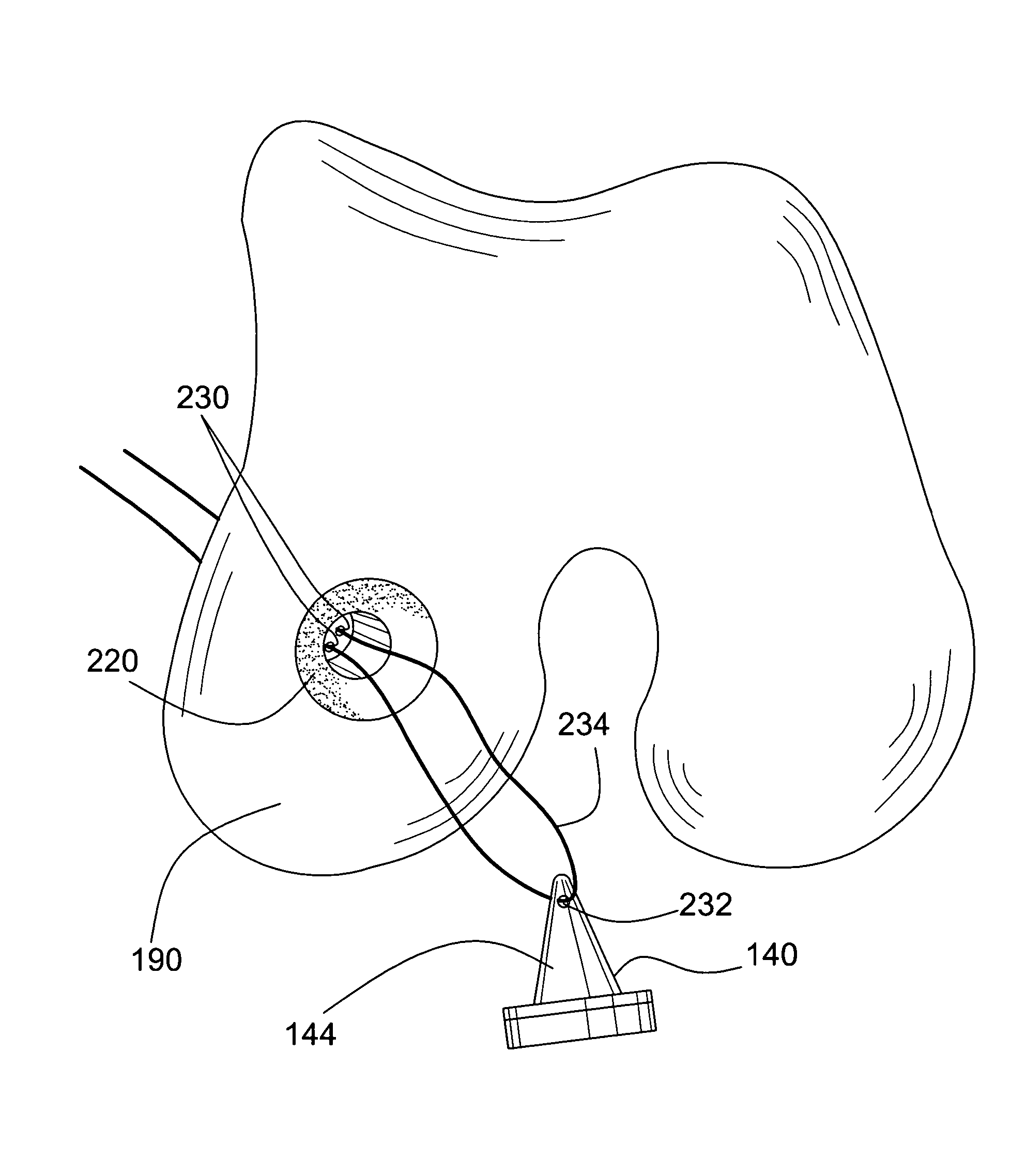
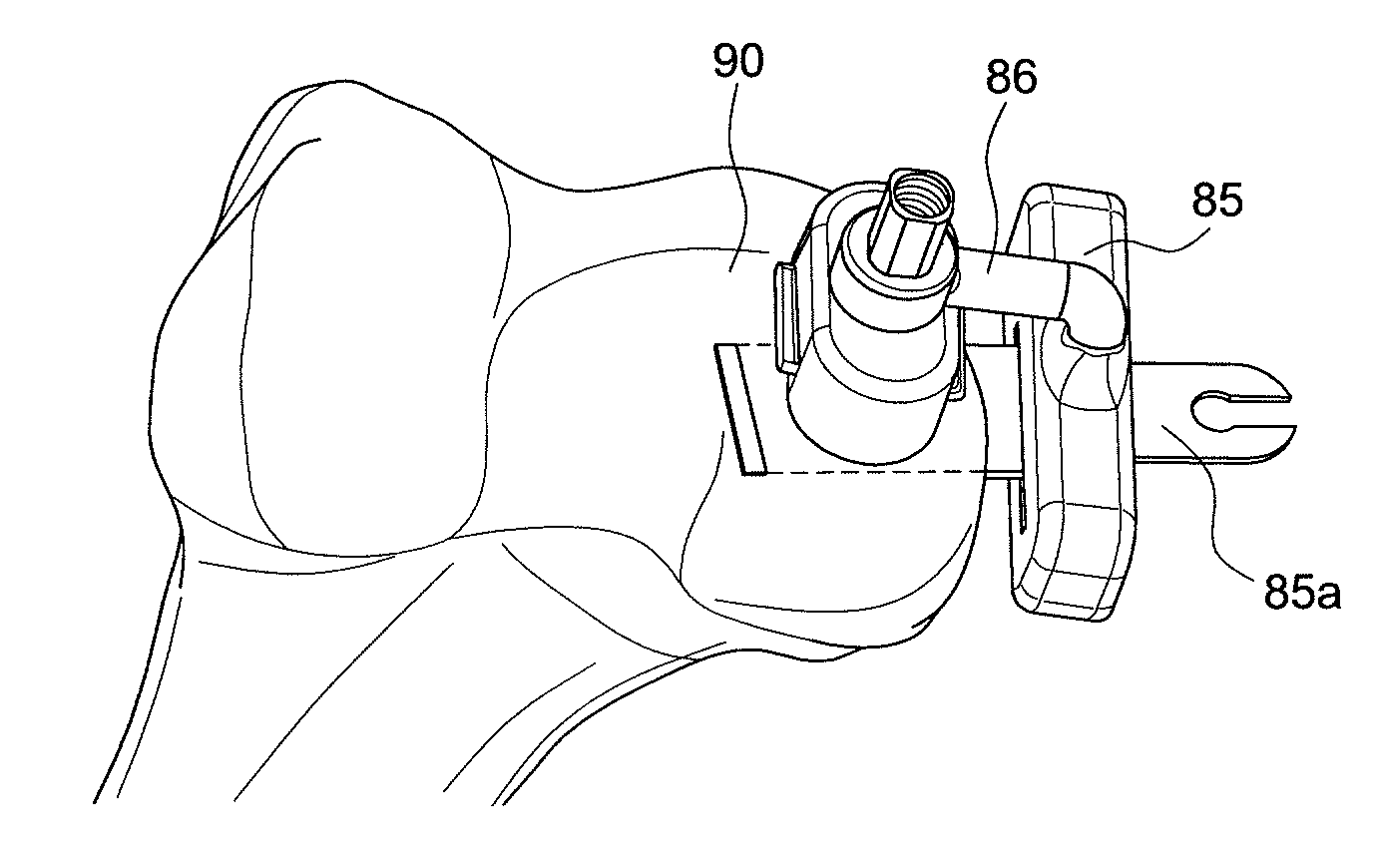
Facet joint implant device
A bone graft implant device for treating a tissue site is provided, the device including an implant body including a demineralized allograft bone material portion, and at least one engagement member protruding from the implant body including a mineralized allograft bone material portion. The engagement member may be integrally formed with the implant body, or alternatively, the implant body may include an aperture and the engagement member may be insertable therein. At least one of the amount of demineralization or area of the demineralized allograft bone material portion is adjustable to impart a desired flexibility to the implant body.
Owner:WARSAW ORTHOPEDIC INC
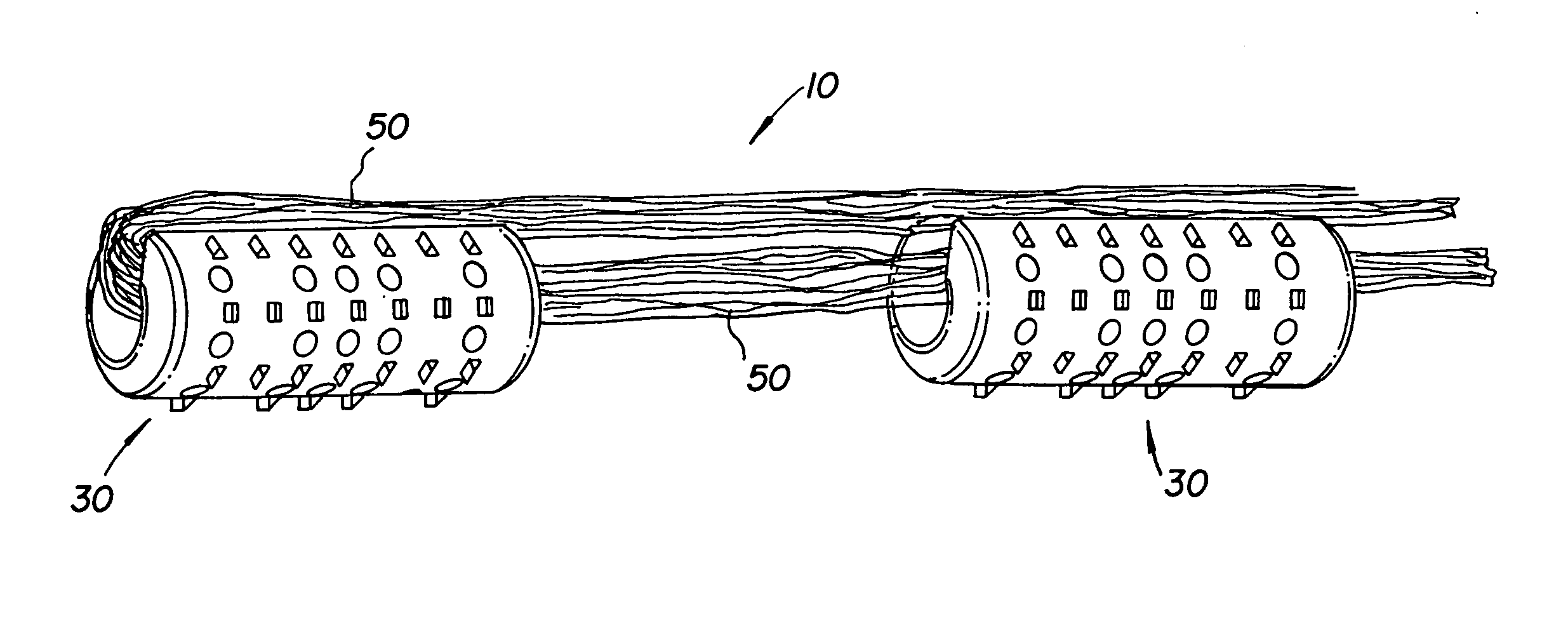
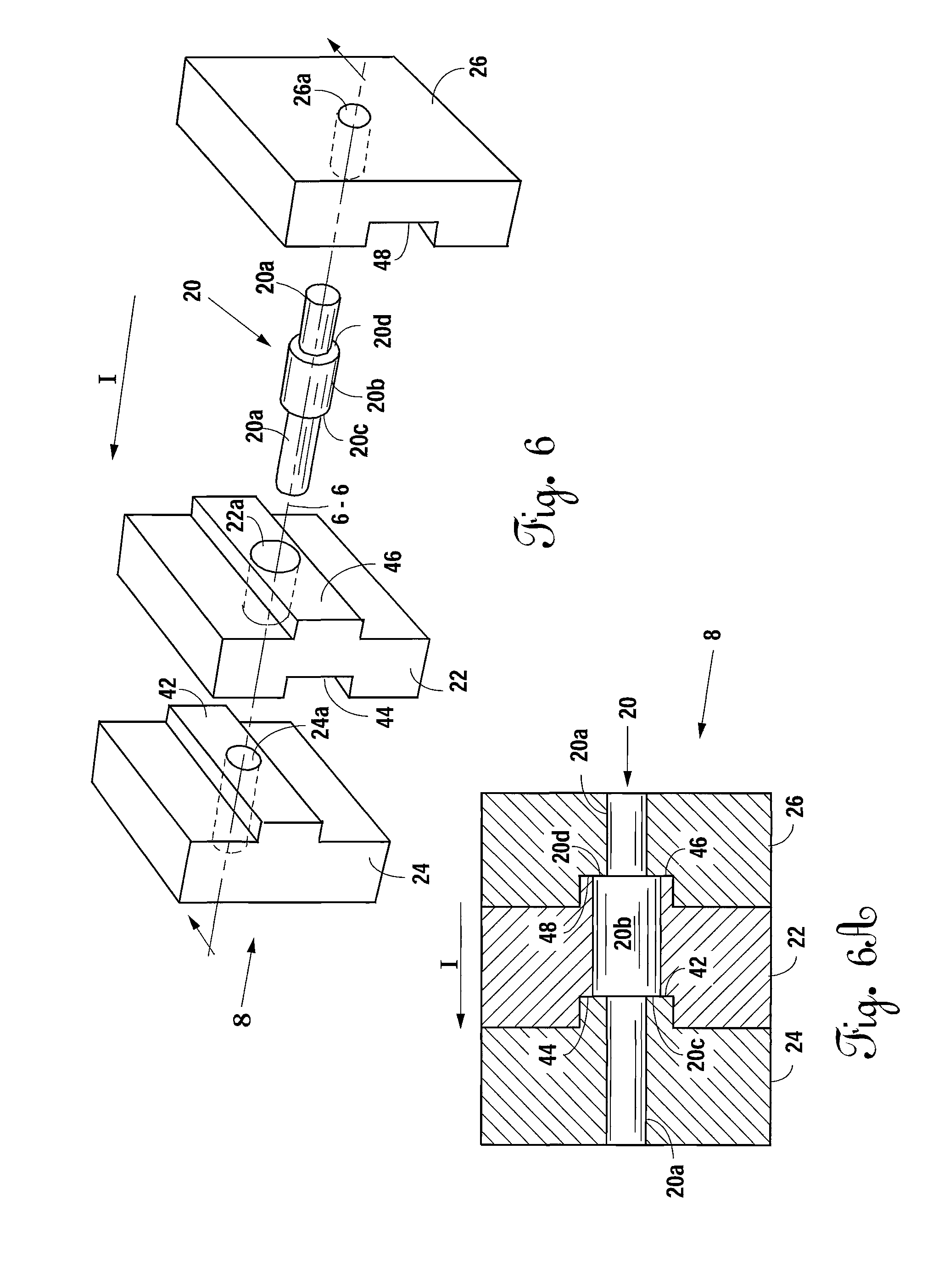
Multiple wafer cortical bone and cancellous bone allograft with cortical pins
InactiveUS20110137417A1Reduce the possibilityRelieve pressureBone implantSpinal implantsHuman bodyAllogeneic graft
A bone allograft and cortical pin for inserting into a surgically altered site of a human. The cortical pin is cylindrically shaped and has a thick middle diameter surrounded by two smaller end portions. The allograft has two end cortical wafers with small canals to receive the small ends of the cortical pin. At least one cancellous wafer is disposed adjacently between the end cortical bone wafers. A plurality of cancellous and / or cortical wafers may be inserted between the end cortical wafers to form the allograft. The wafers inserted between the end cortical wafers have larger canals to receive the thick diameter of the cortical pin. The size of the allograft may be adjusted as desired by either adding or removing inner wafers, or adjusting the size of the inner wafers of the allograft. The allograft and cortical pin are inserted parallel to the plane of insertion into the human.
Owner:TRANSPLANT TECH OF TEXAS
Allograft bone plugs, systems and techniques
ActiveUS8840677B2High strengthImprove fitSuture equipmentsInternal osteosythesisIliac screwUltimate tensile strength
The present invention provides a system, device, instruments and methods for inserting and / or improving the holding strength and purchase of a bone screw, bone pin, or bone dowel in bone. Embodiments include monolithic allograft tissue forms, multi-piece allograft tissue forms, distally expandable portions, partially and fully demineralized portions, and flexible connecting portions. Advantages of the allograft tissue forms of the present invention include improved pedicle screw blackout strength and improved filling of bone voids. Methods for making and instruments and techniques for inserting the augmentation device, system and screws or pins are also disclosed.
Owner:DEPUY SYNTHES PROD INC
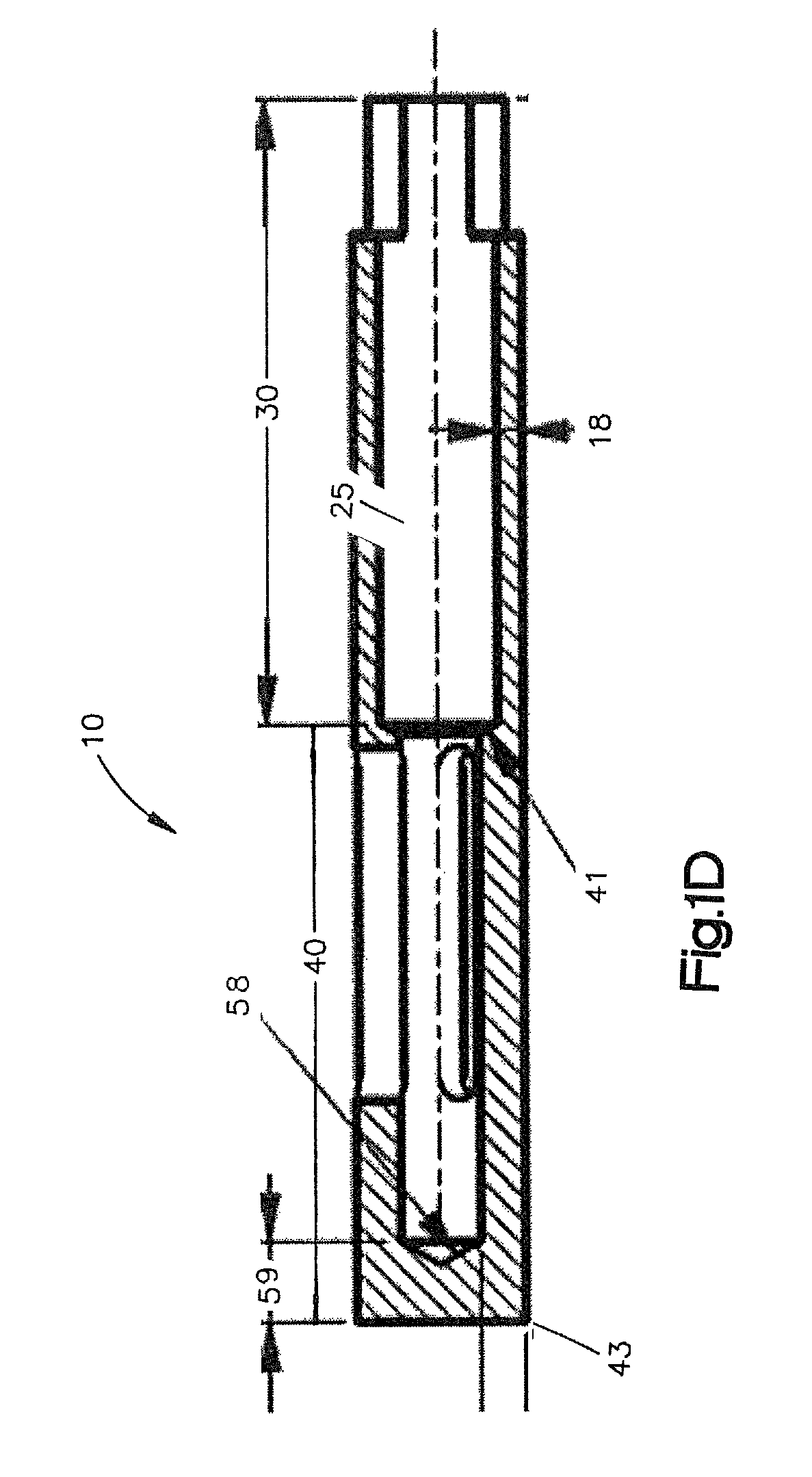
Modification of reactivity of bone constructs
A method of enhancing the binding of growth factors and cell cultures to a demineralized allograft bone material which includes applying ex vivo an effective quantity of an ionic force change agent to at least a portion of the surface of a demineralized allograft bone material to produce a binding-sensitized demineralized allograft bone material and implanting the binding-sensitized demineralized allograft bone material into a host bone. The ionic force change agent may include at least one of enzymes, pressure, chemicals, heat, sheer force, oxygen plasma, supercritical nitrogen, supercritical carbon, supercritical water or a combination thereof. A molecule, a cell culture, or a combination thereof is administered to the binding-sensitized demineralized allograft bone material.
Owner:WARSAW ORTHOPEDIC INC
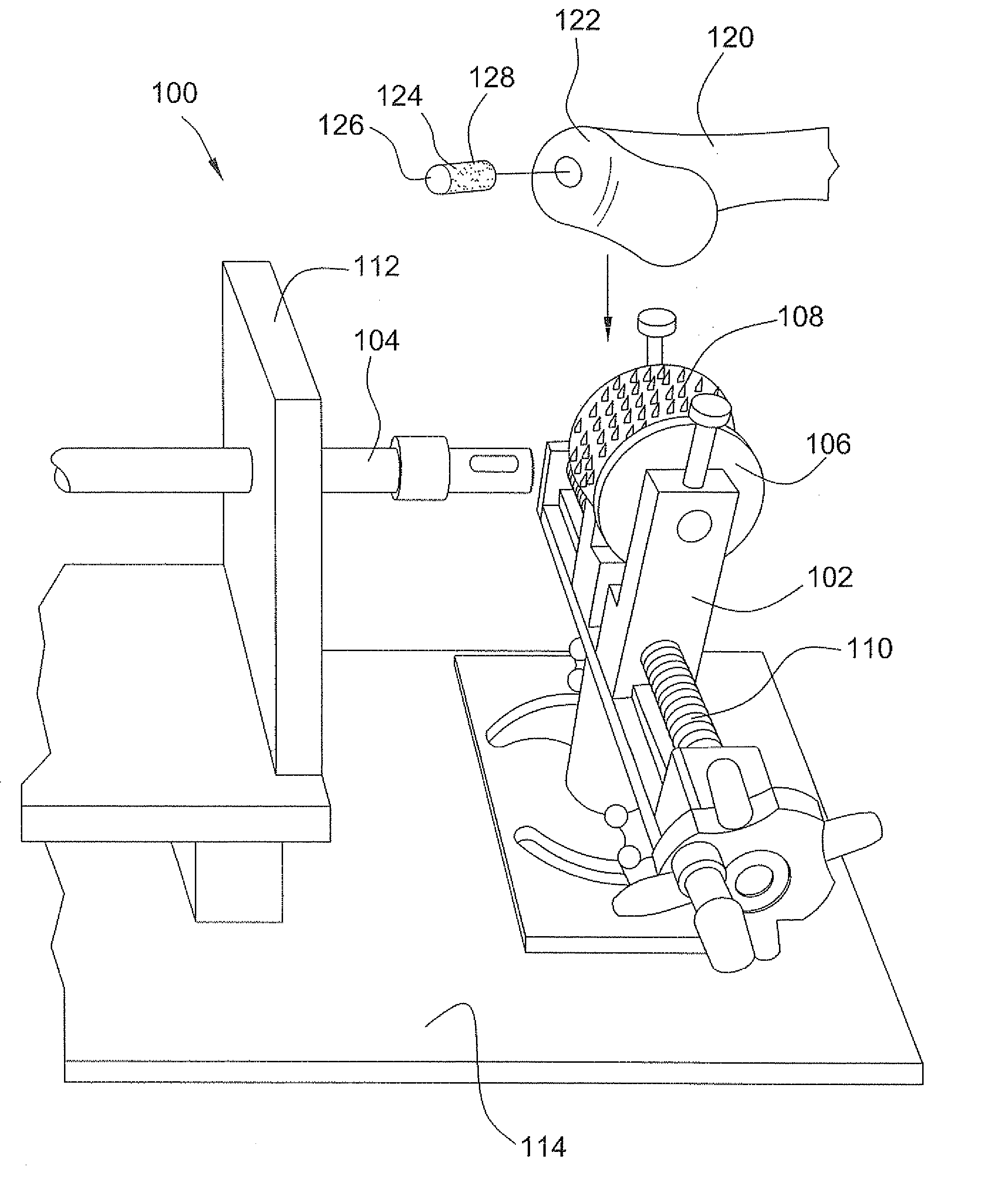
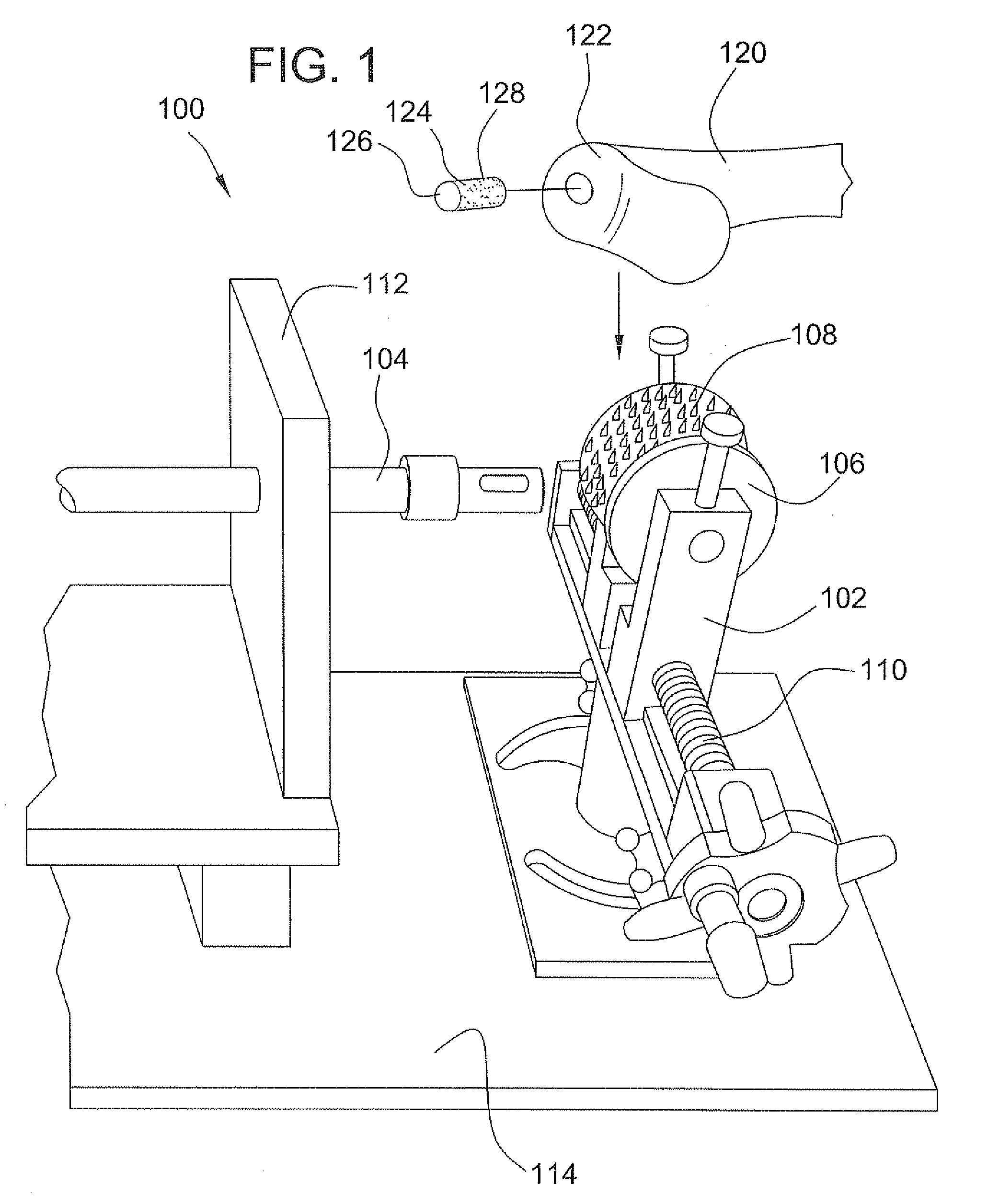
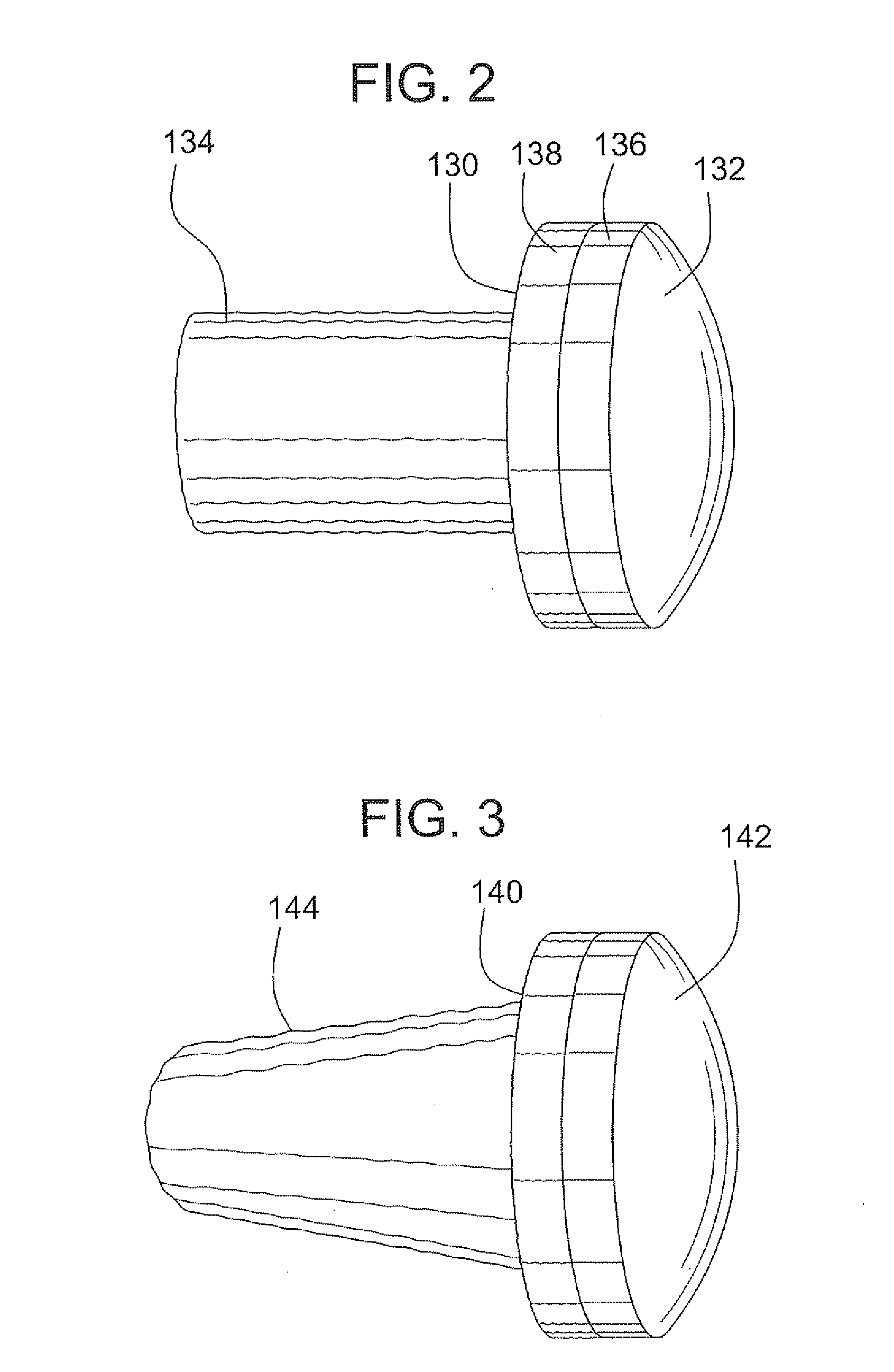
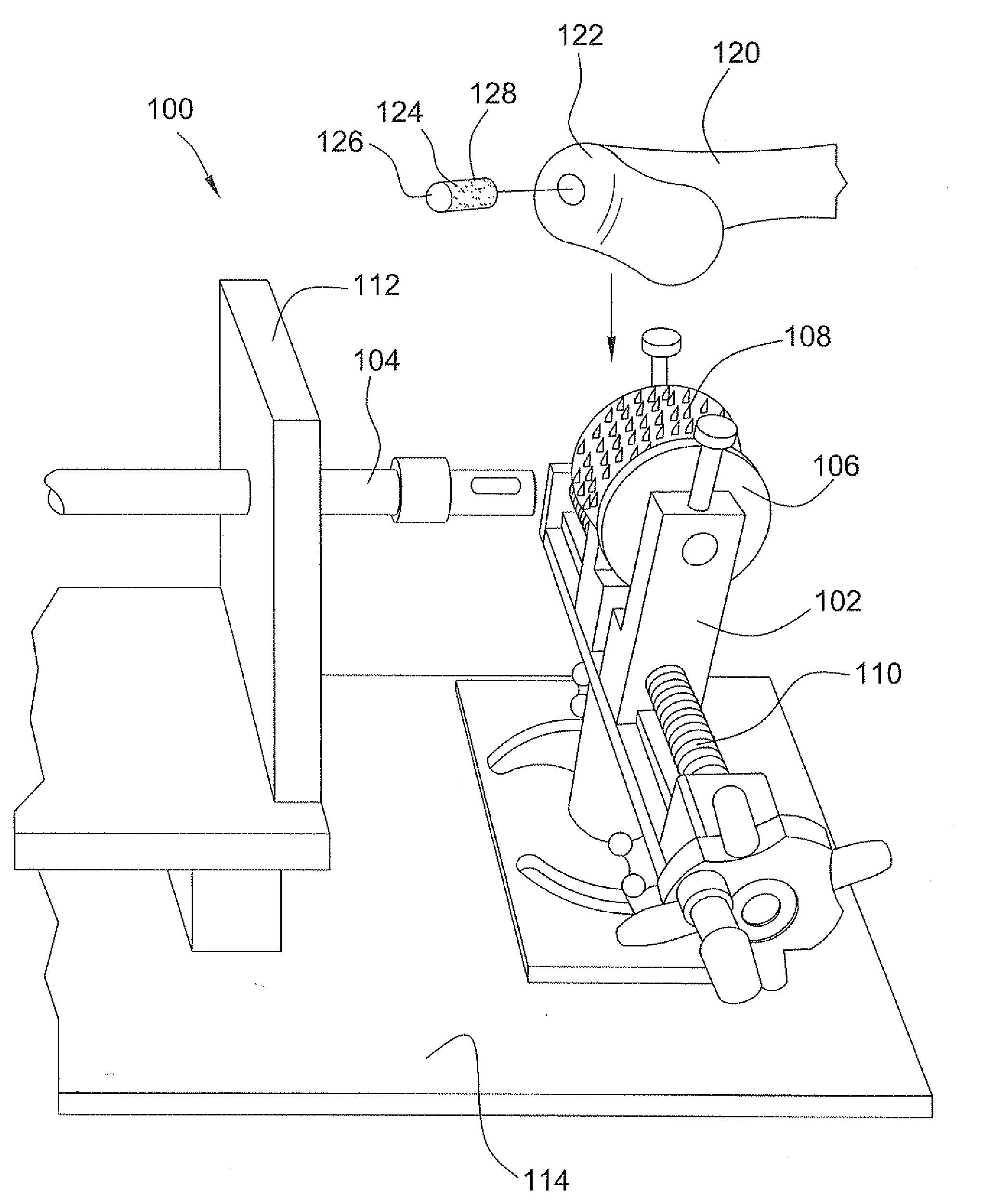
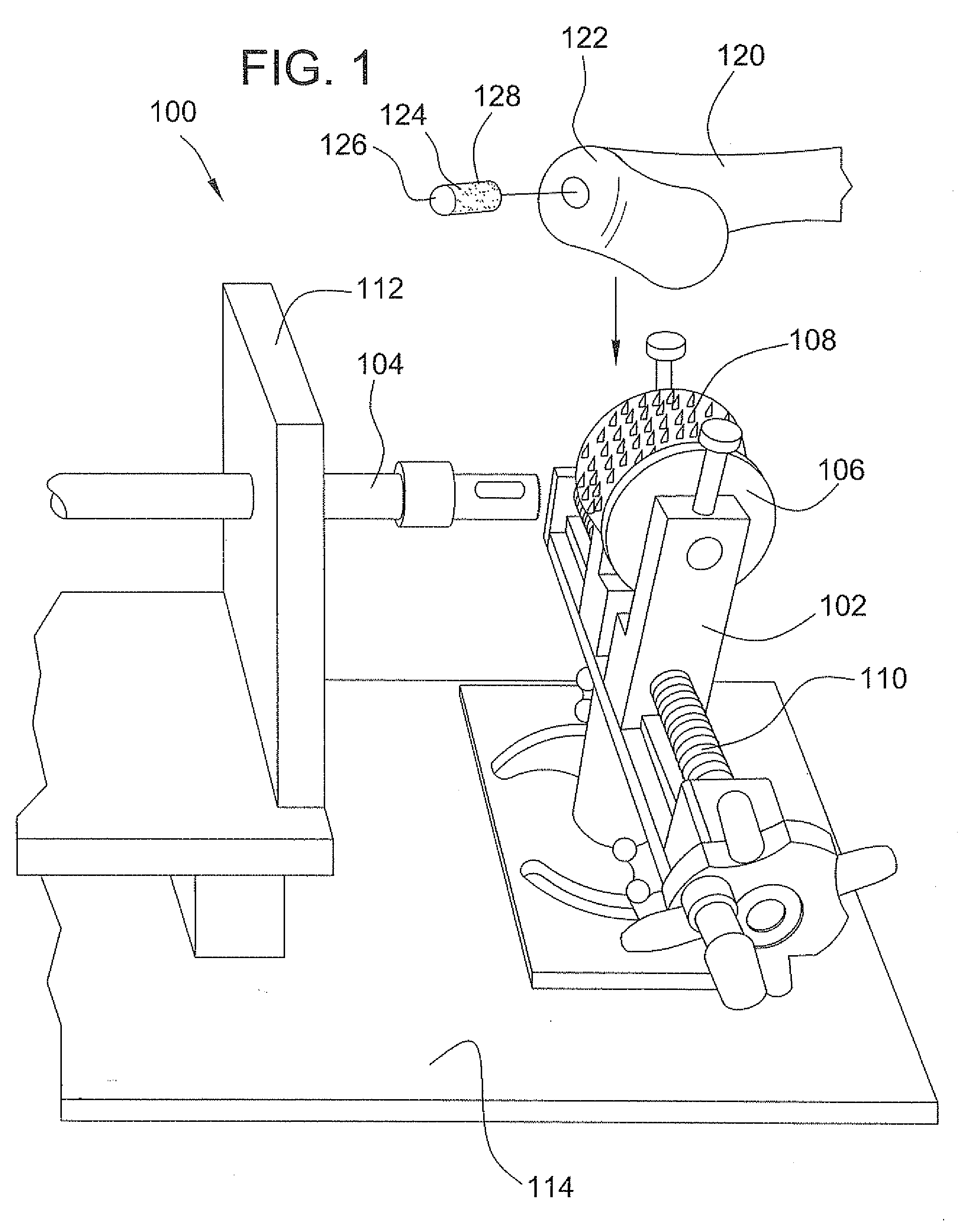
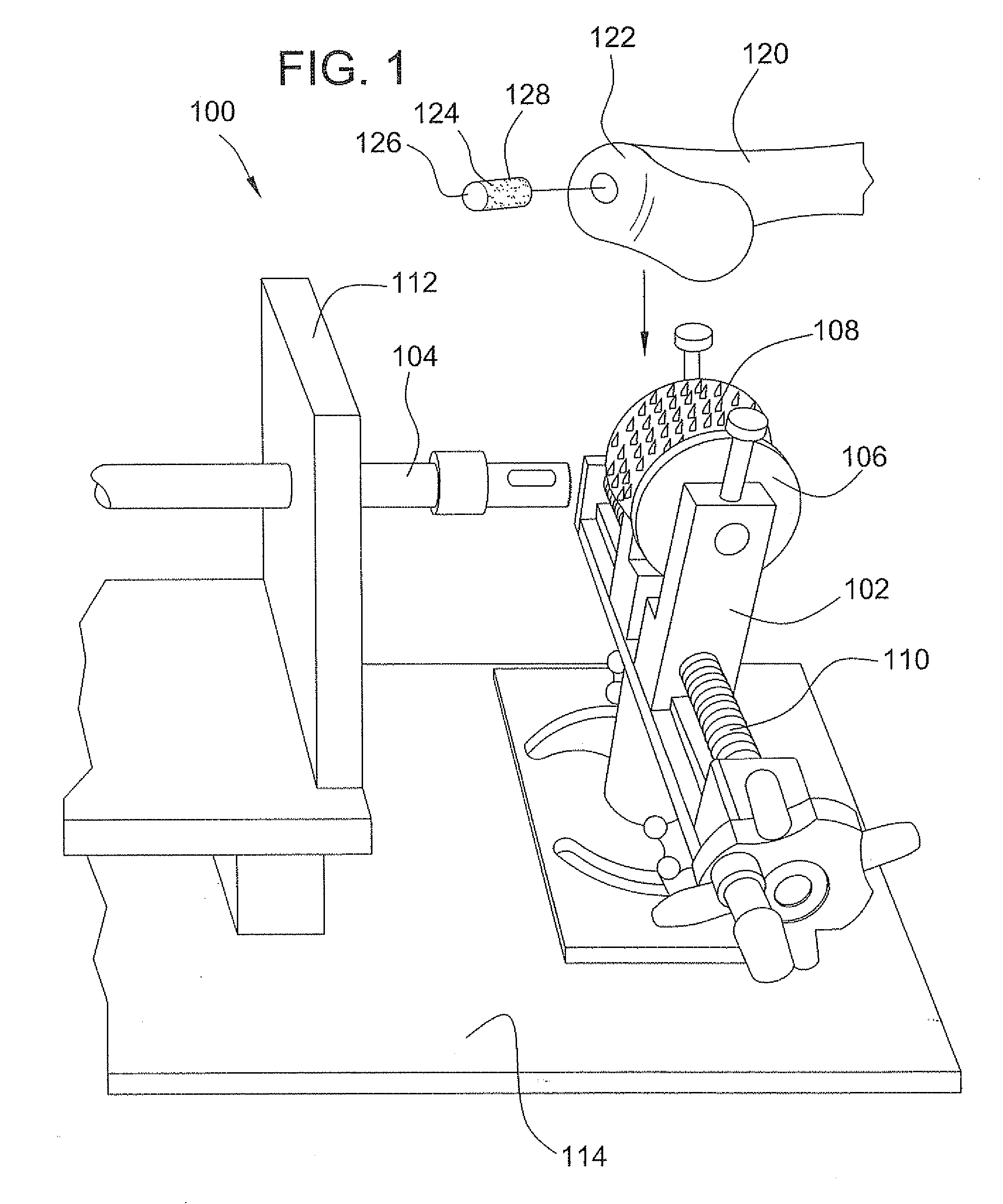
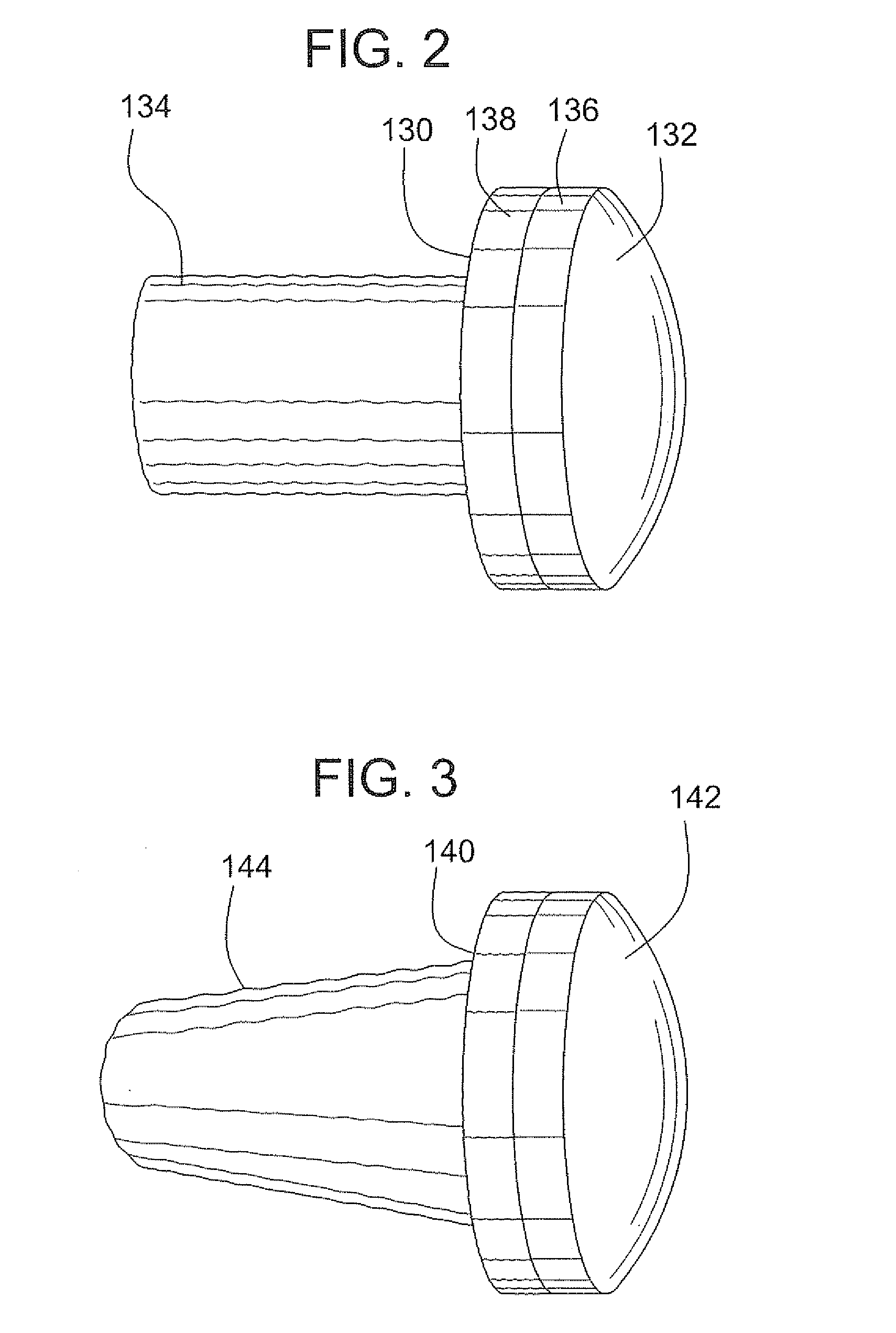
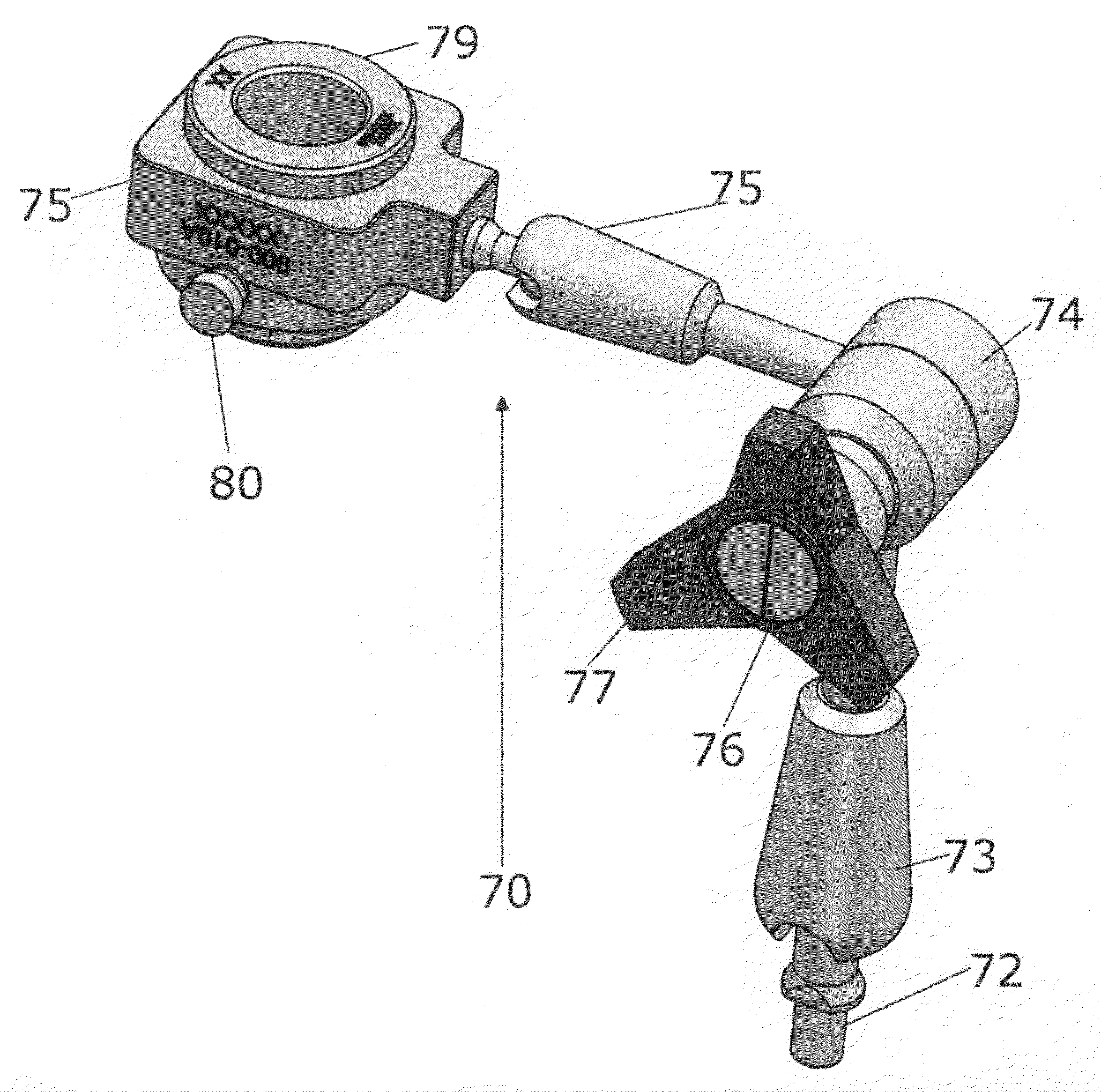
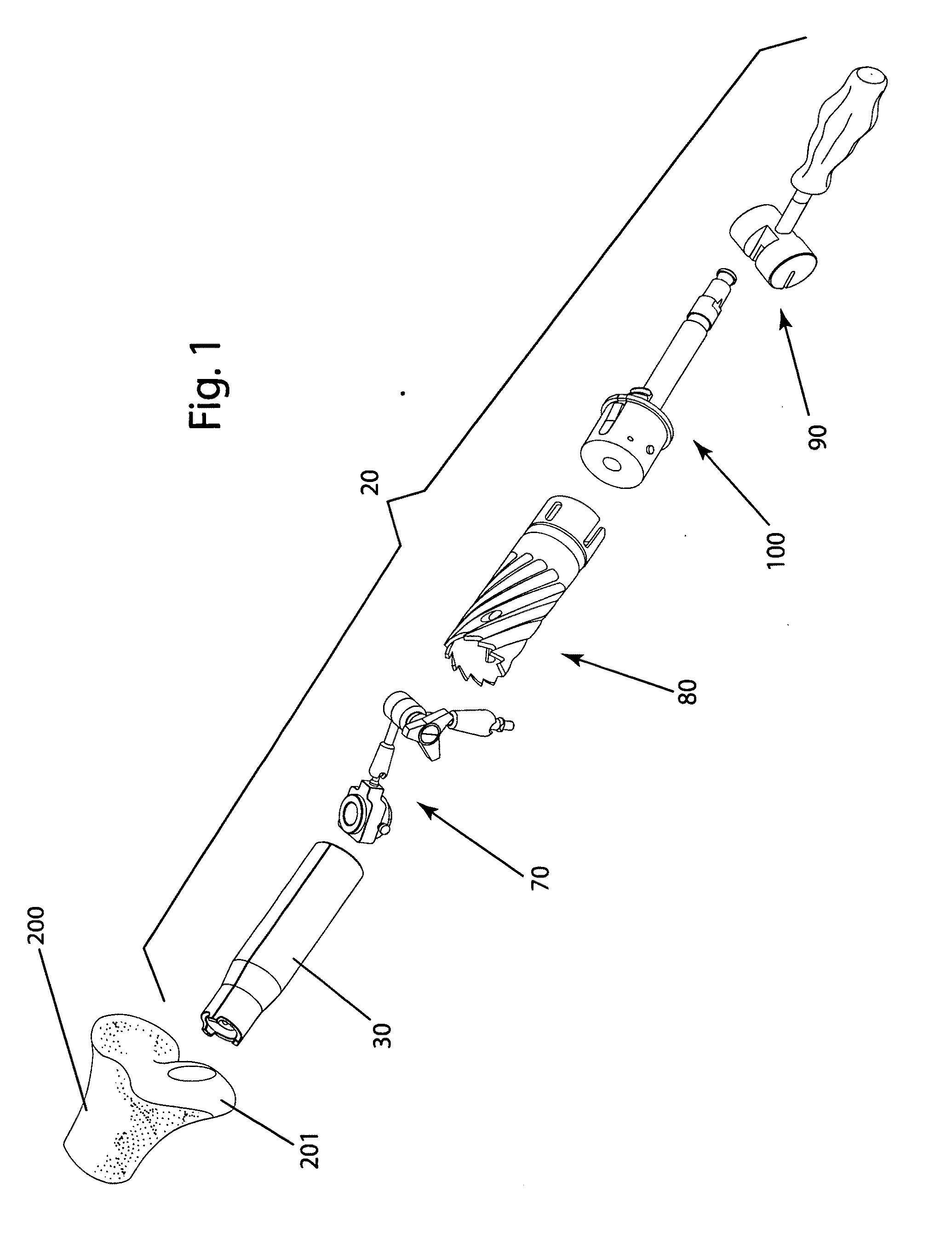
Surgical allograft bone plug cutting tool assembly and method of using same
ActiveUS20090299372A1Clear processEasy to useDiagnosticsNon-surgical orthopedic devicesAllograft boneBiomedical engineering
The invention is directed toward a surgical kit having component parts capable of use to prepare a cartilage implant plug from allograft condyle. The kit comprises a sizing gauge used to measure surface of the allograft condyle so that a cartilage implant plug can be removed that corresponds to the contours of the defect site excised on a patient. An adjustable guide member is mounted in a fixed orientation over the allograft condyle in which a cylindrical graft cutter for cutting a cylindrical allograft bone plug having a cartilage cap and a bone end portion. A chamfering tool is used for chamfering the bone end of the allograft bone plug.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com