Patents
Literature
71 results about "Chondral defect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
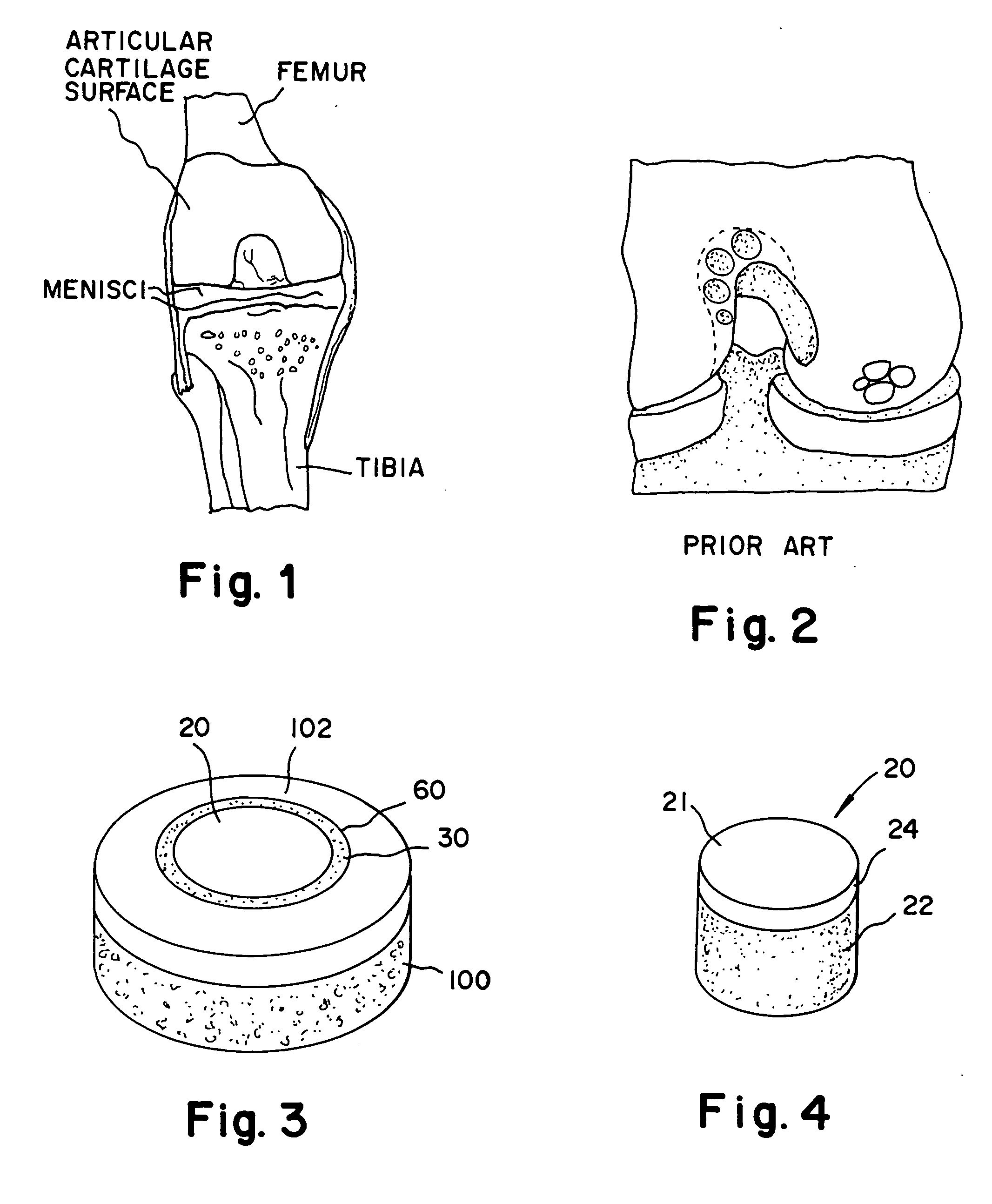
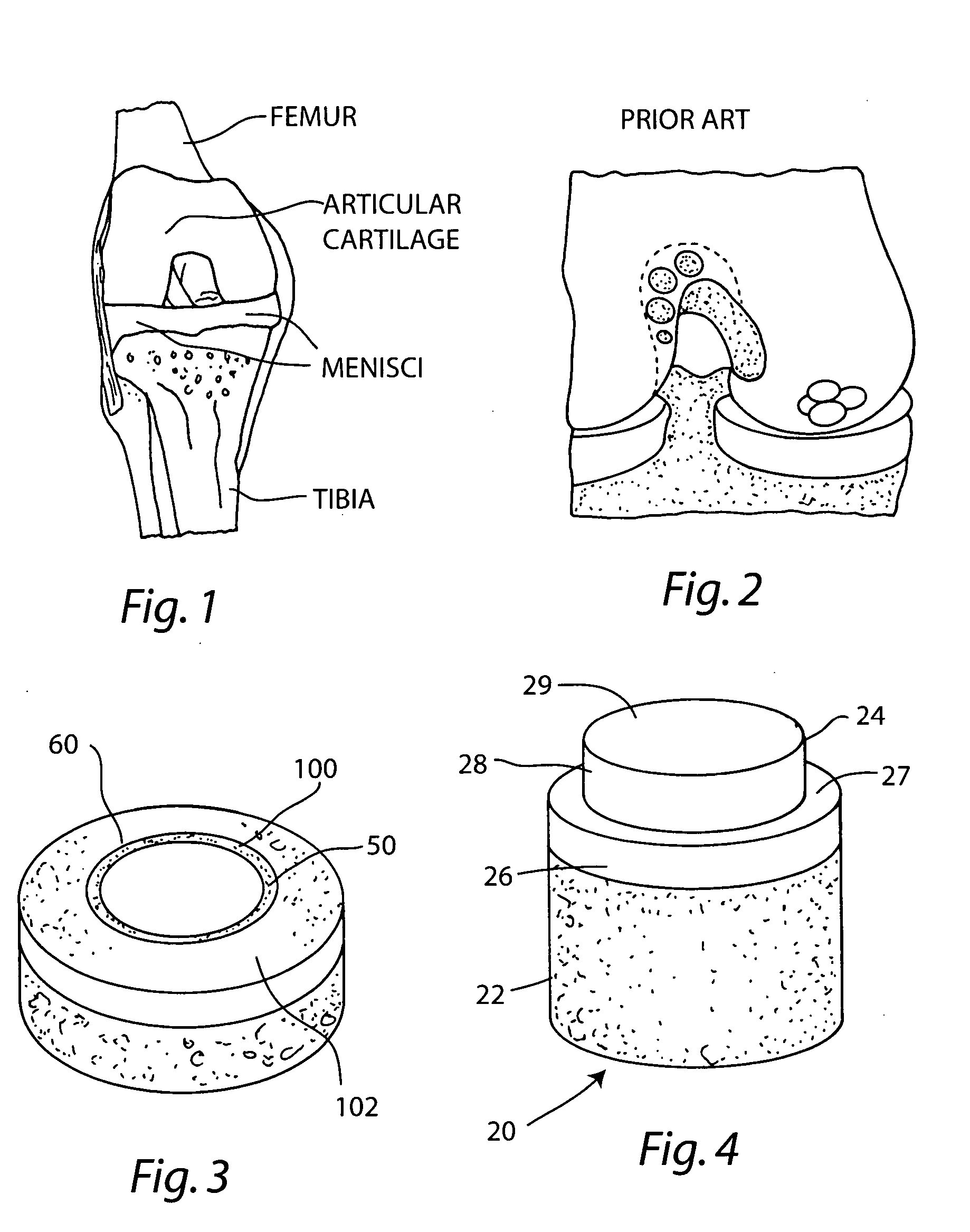
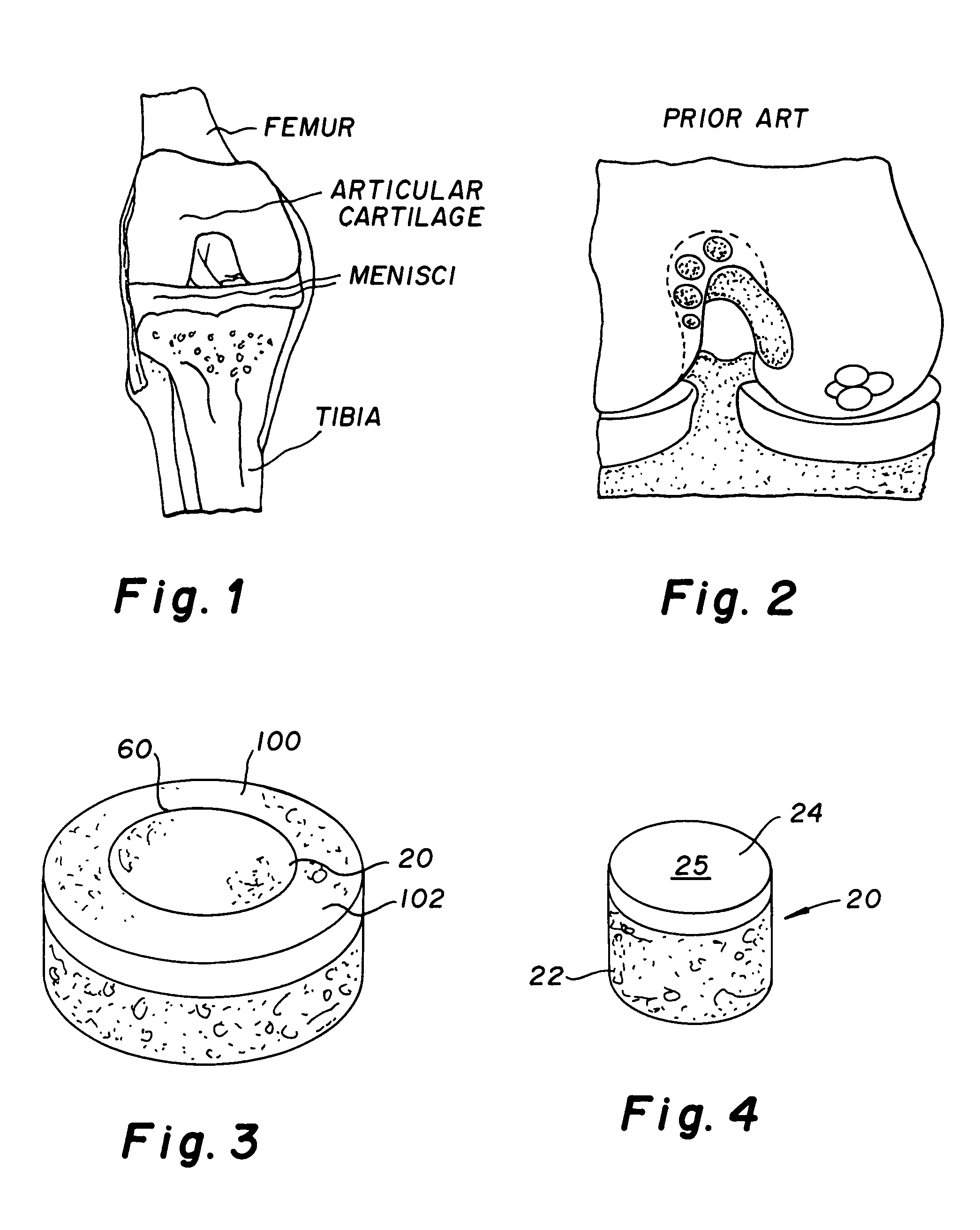
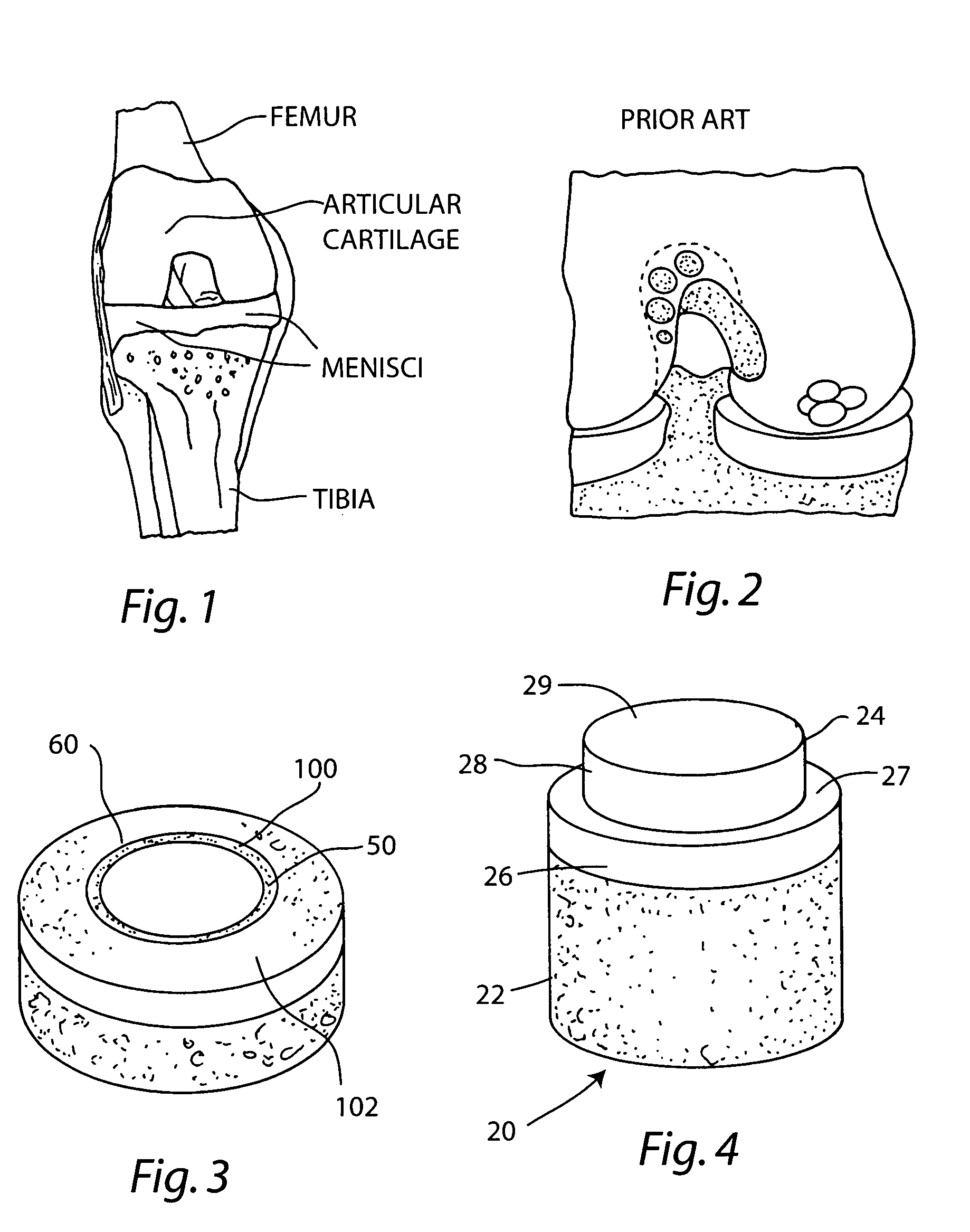
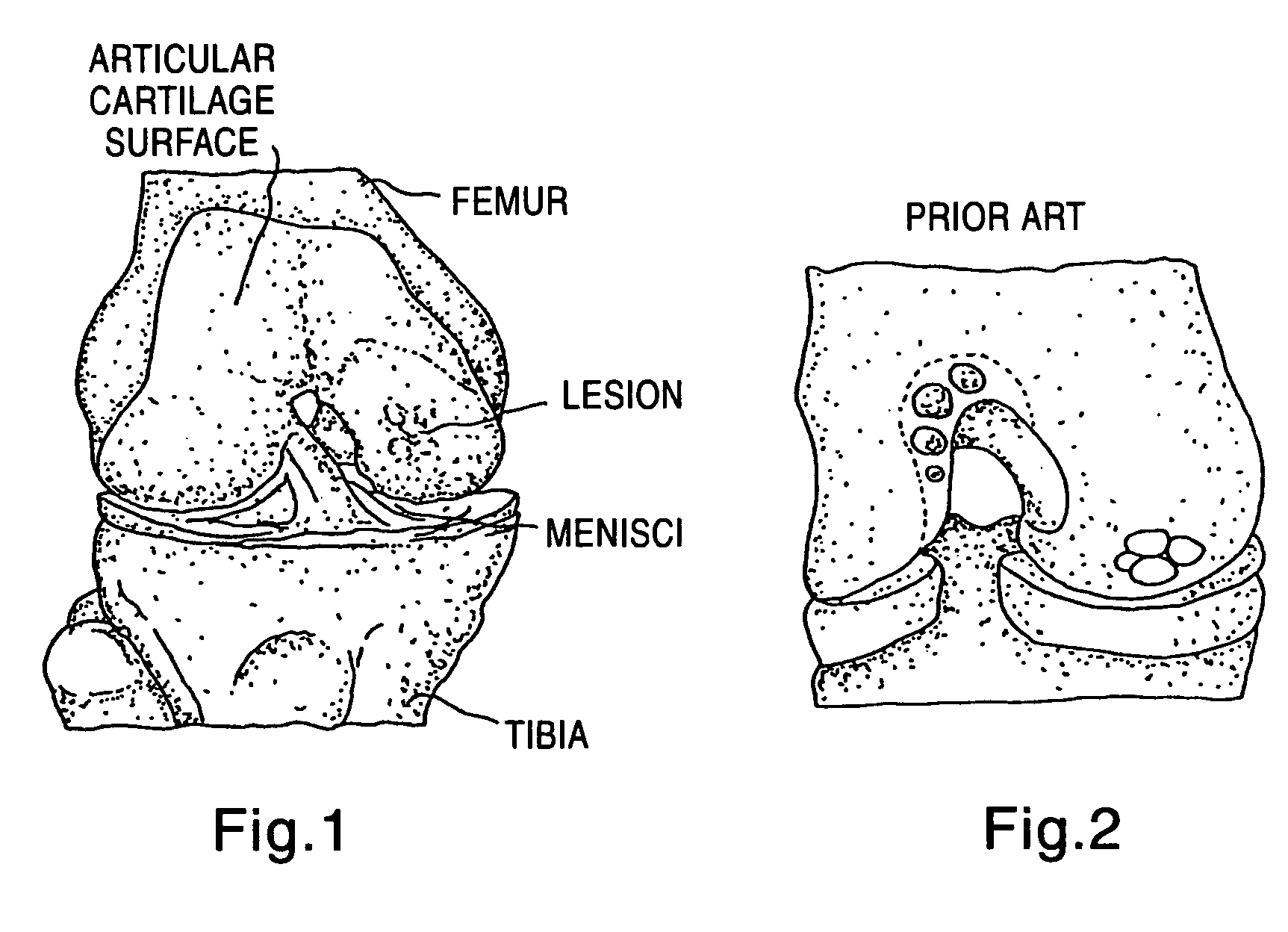
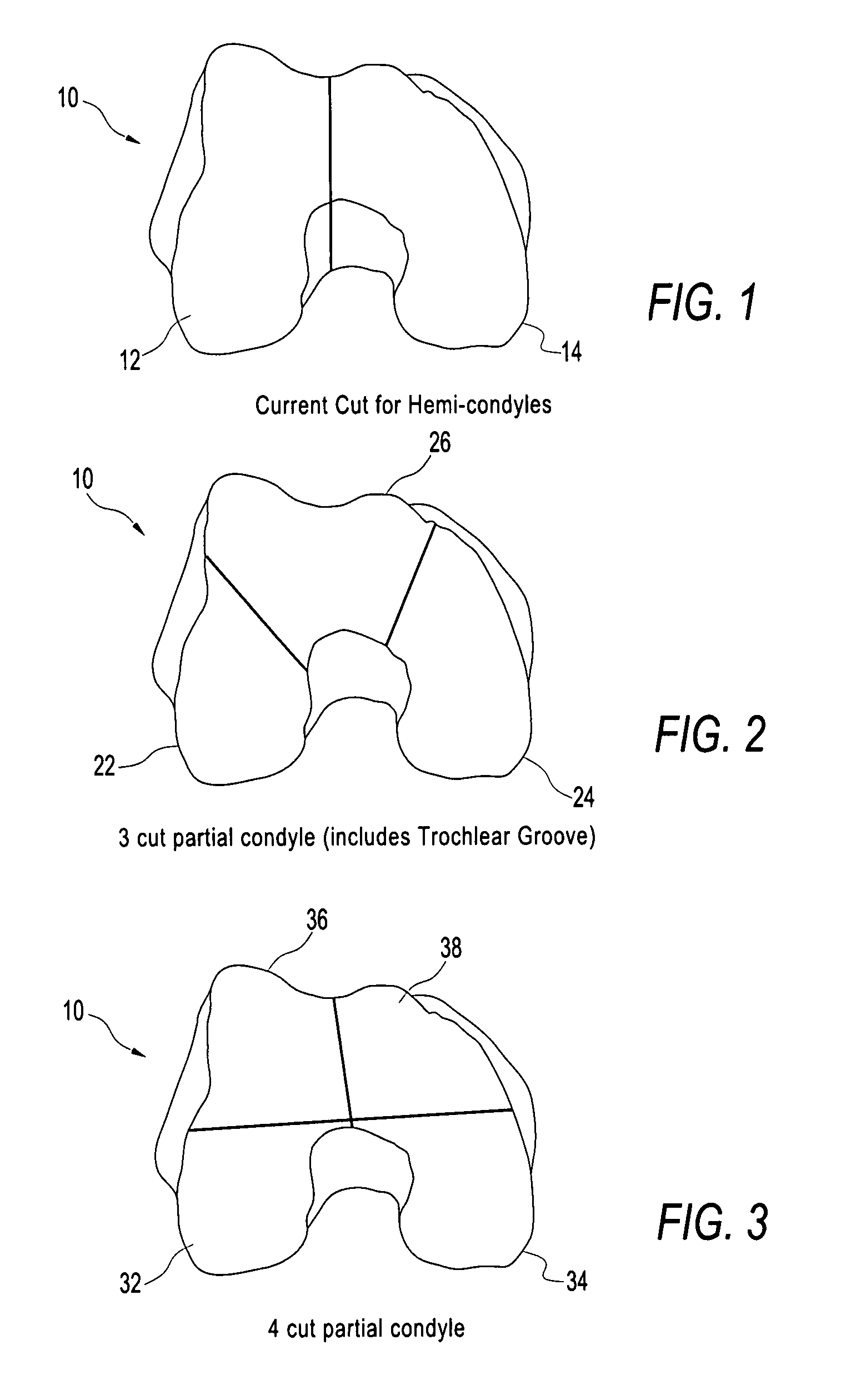
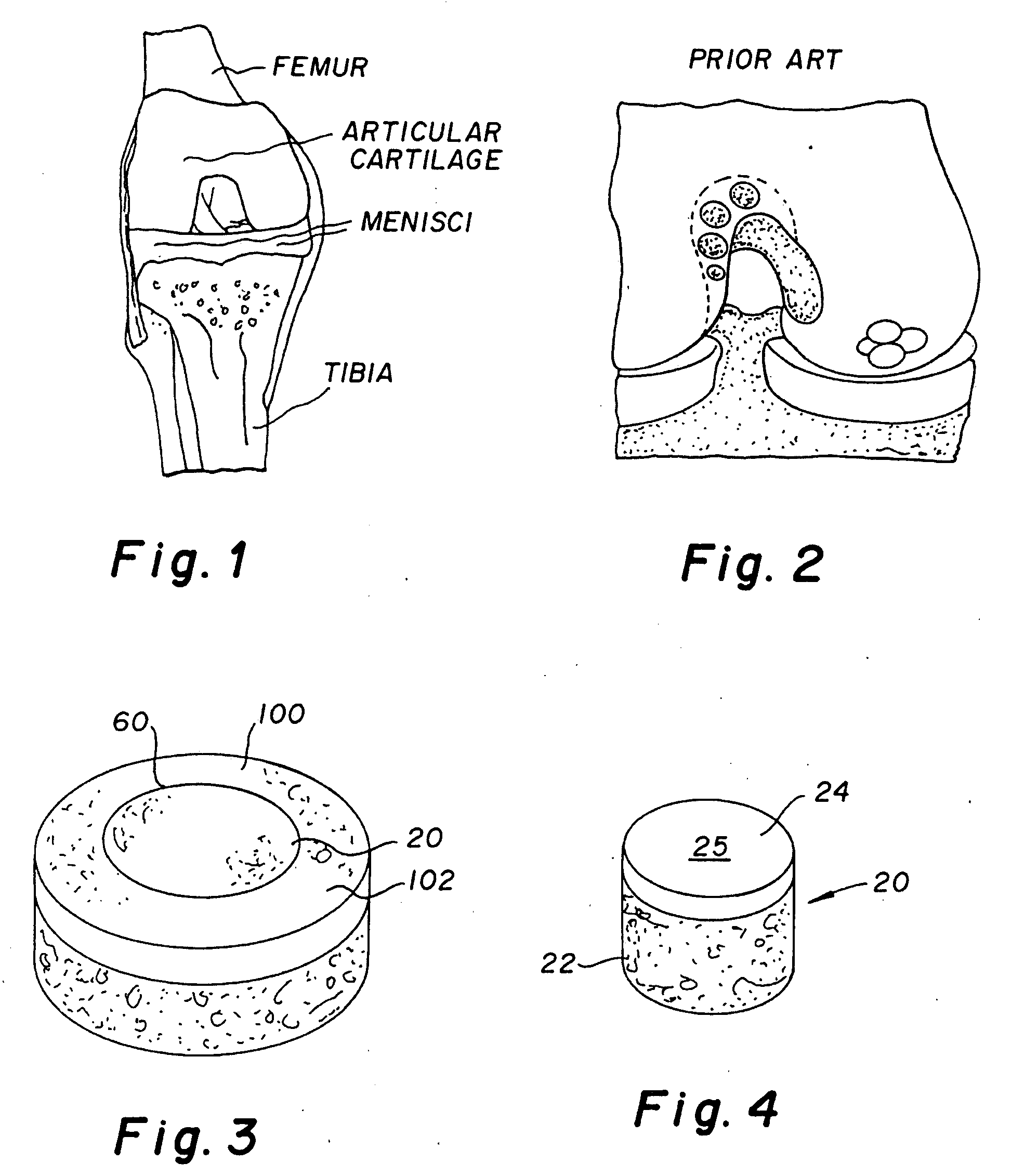
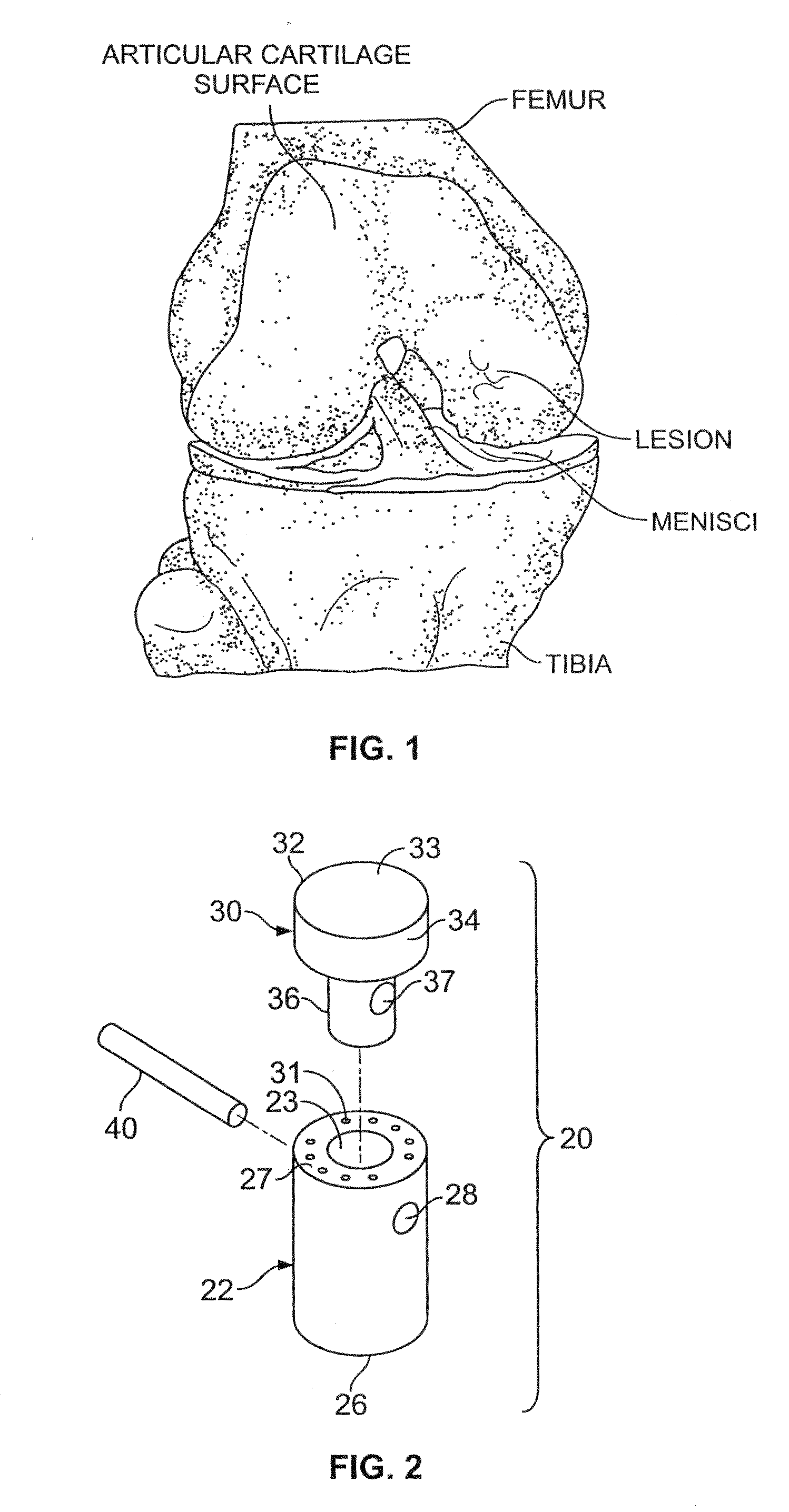
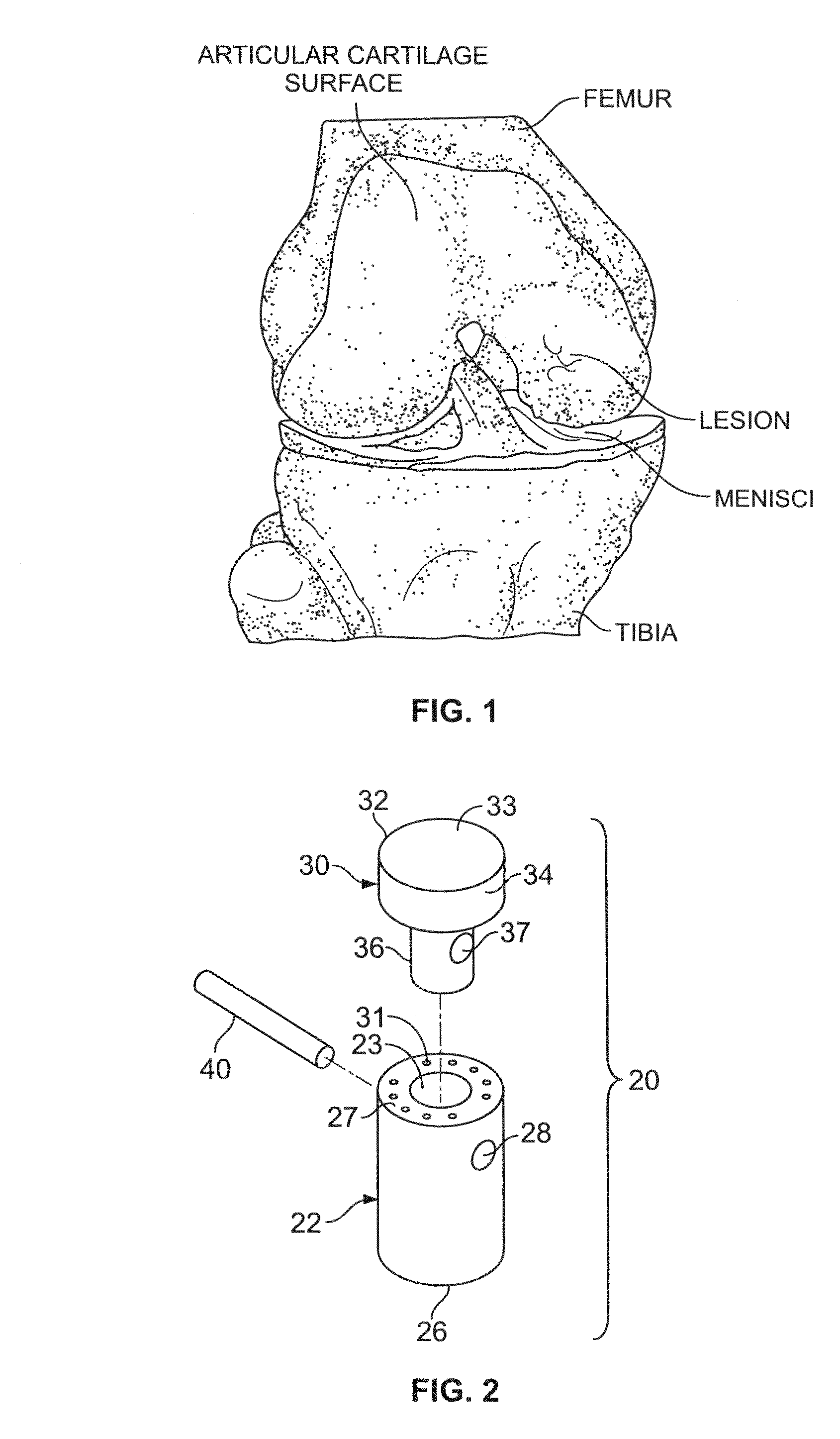
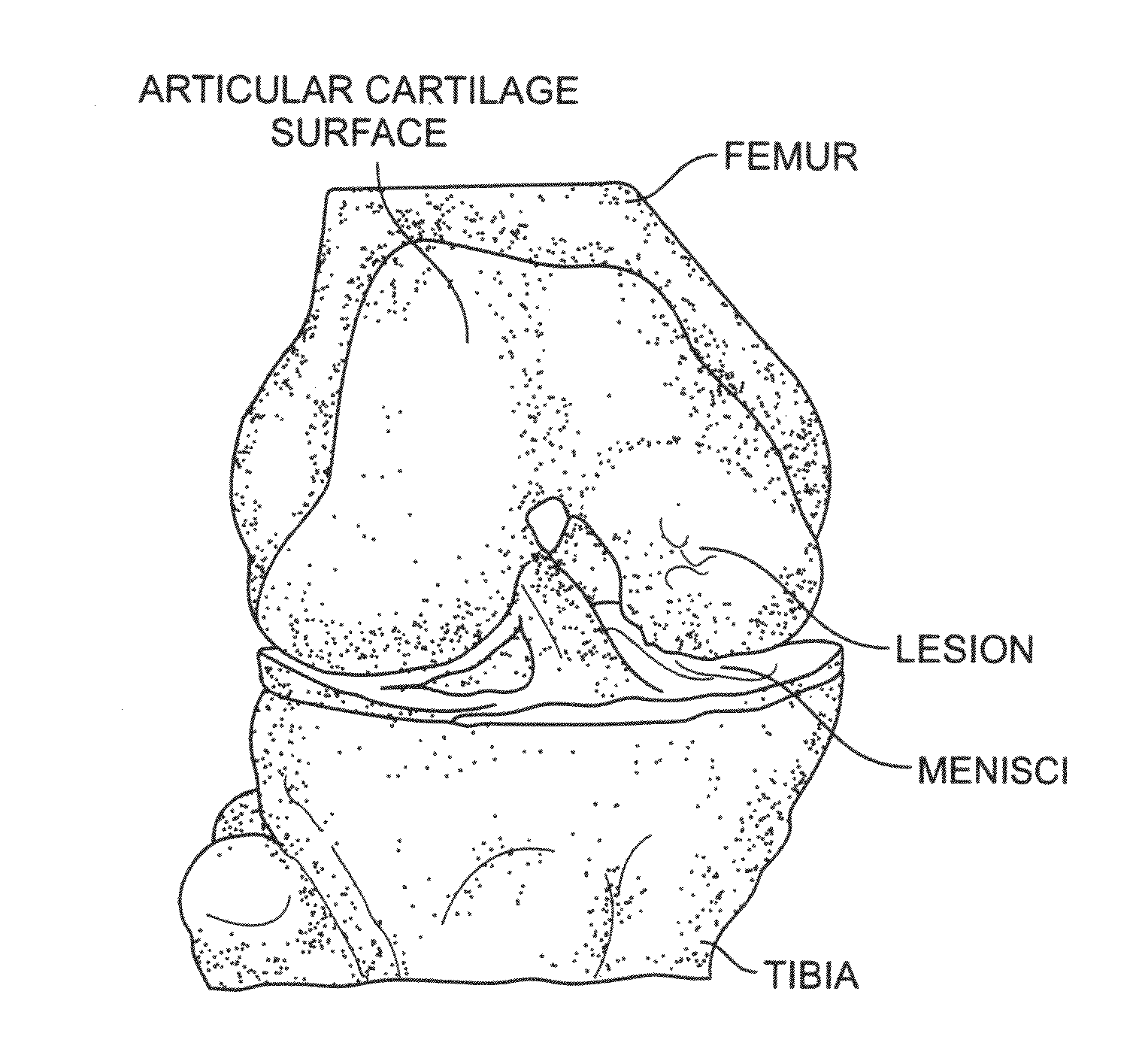
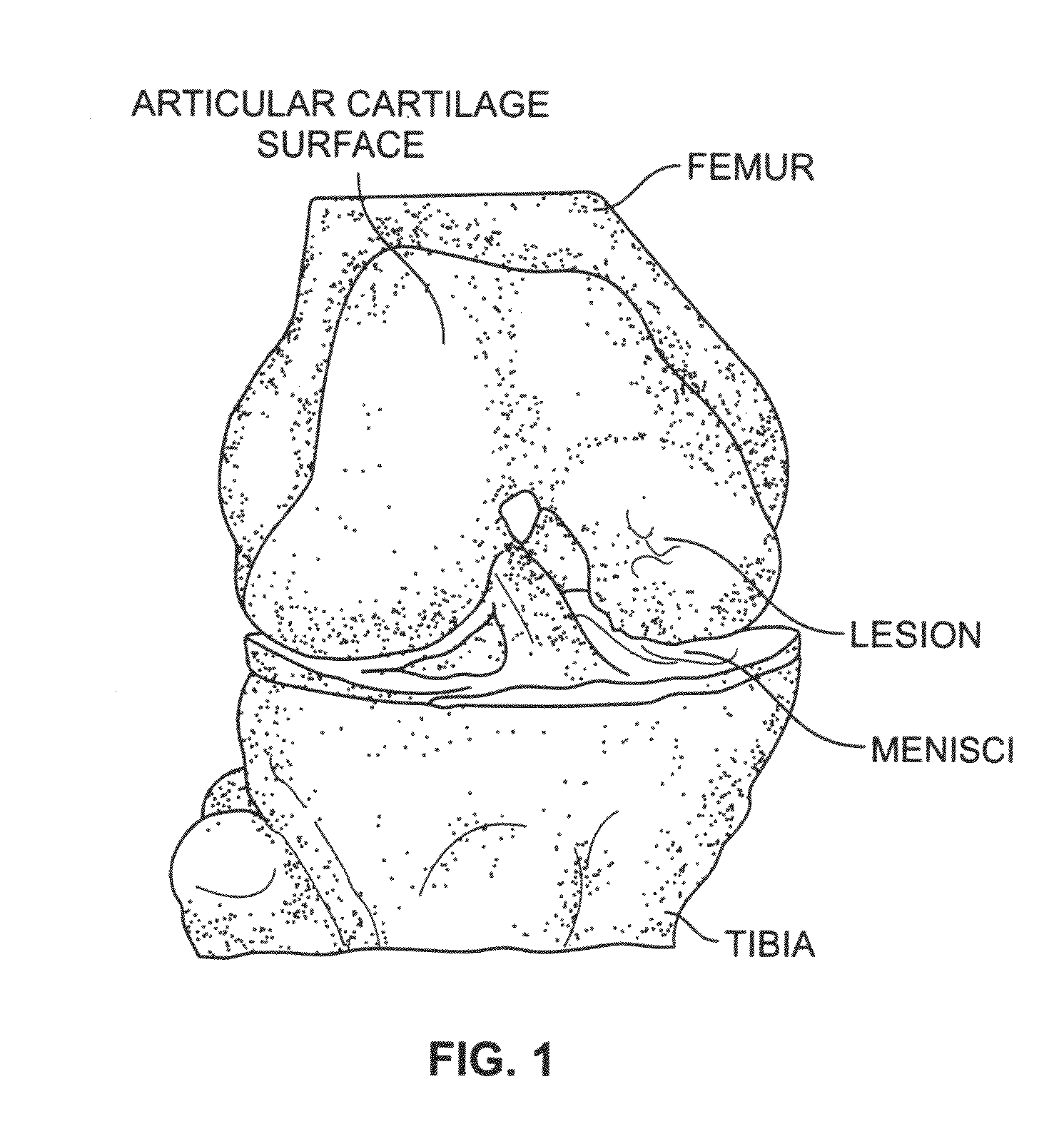
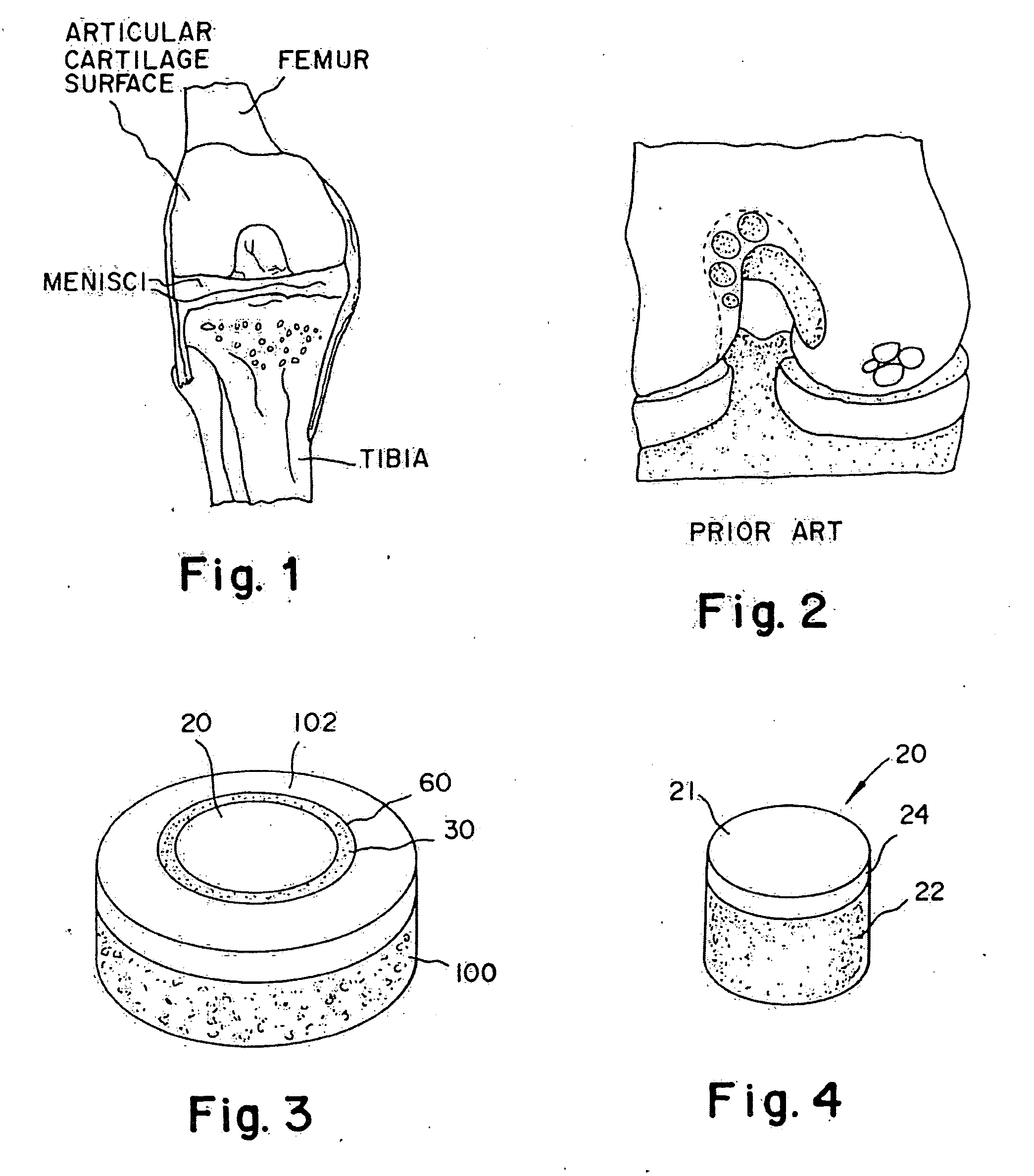
A chondral defect is a defect in the articular (hyaline) cartilage at the end of the bones. The defect is often on the femoral condyle (the rounded end of the thigh bone) and can result from an injury where there is a direct blow to the bent knee.
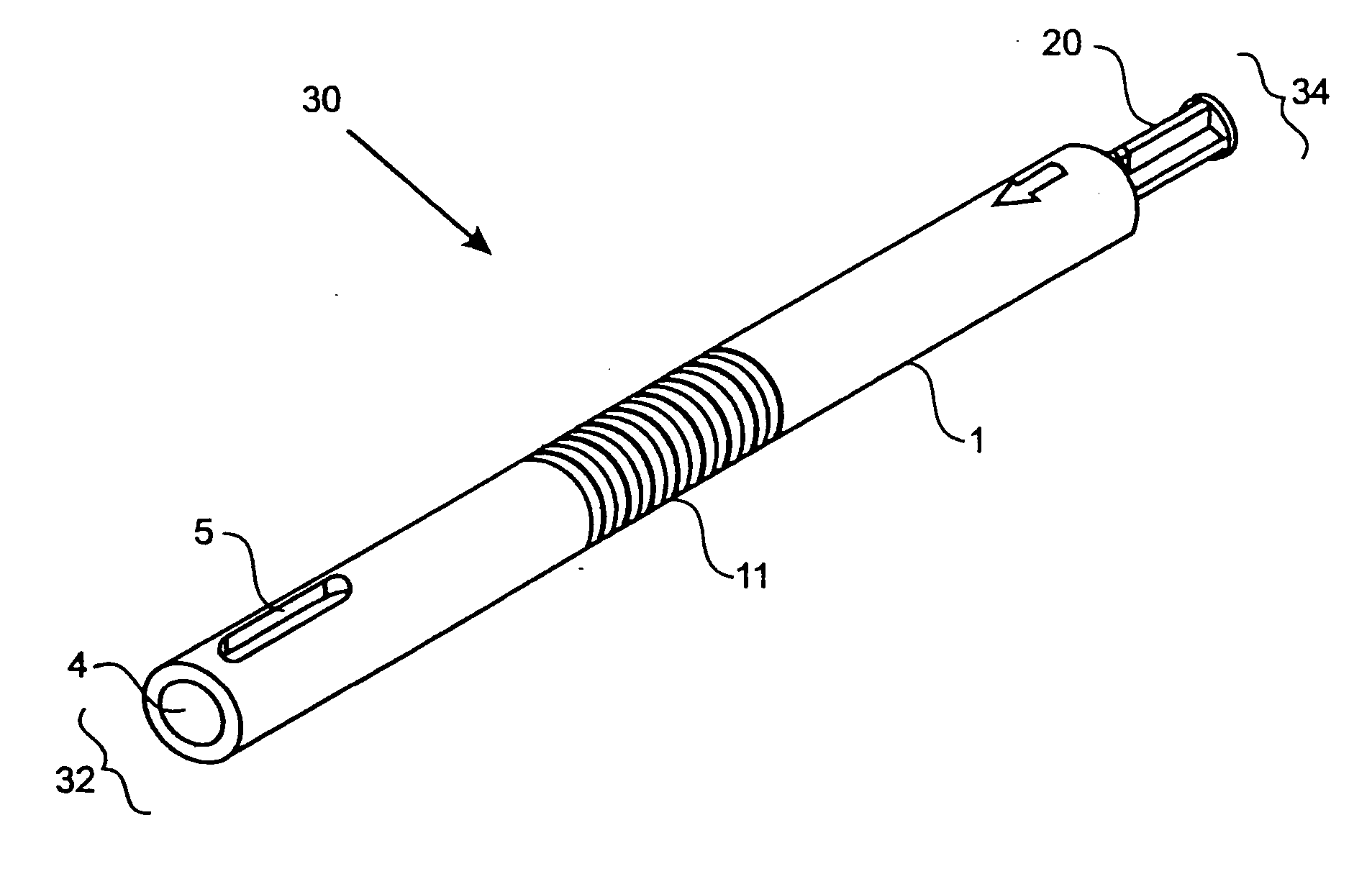
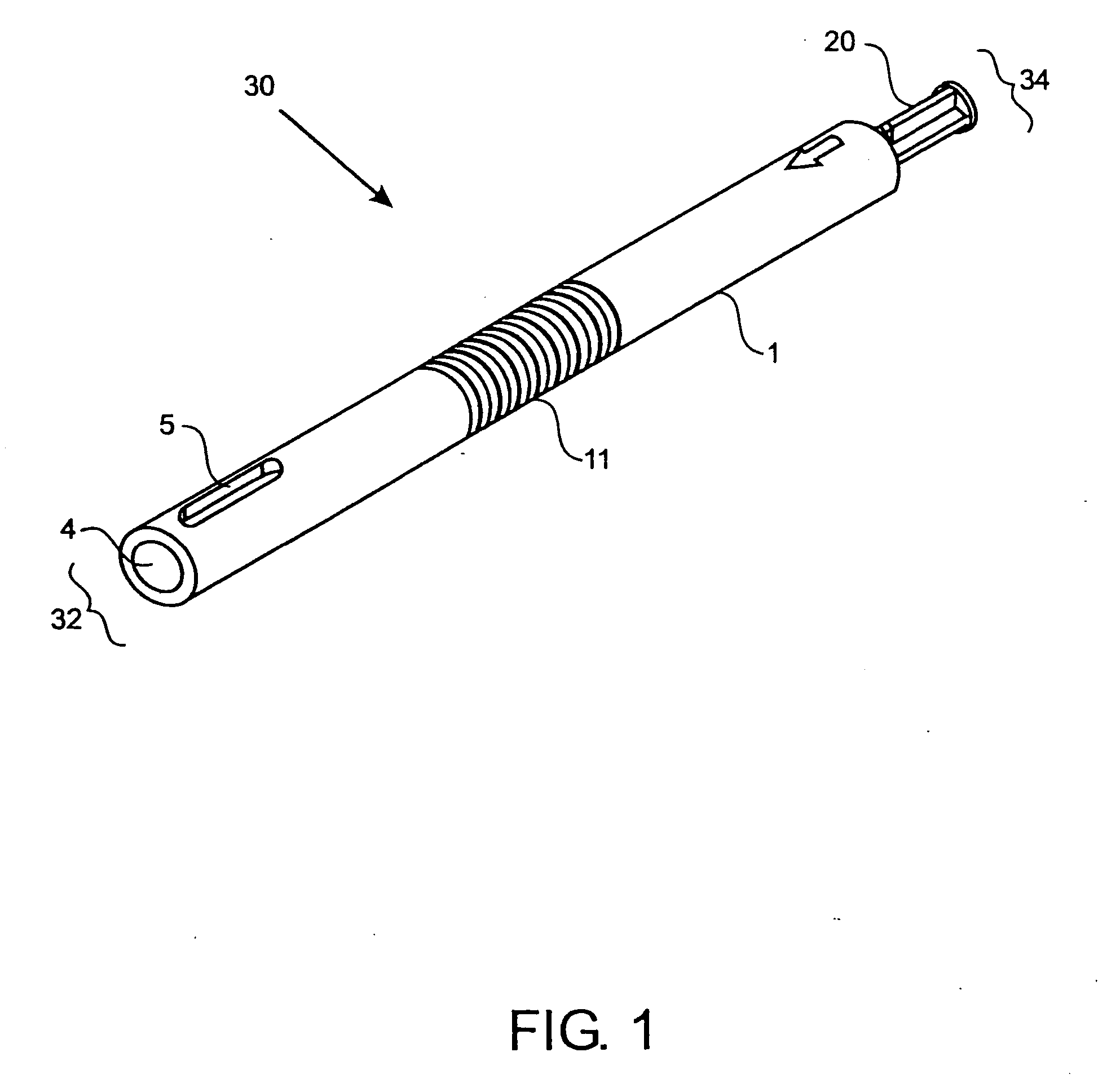
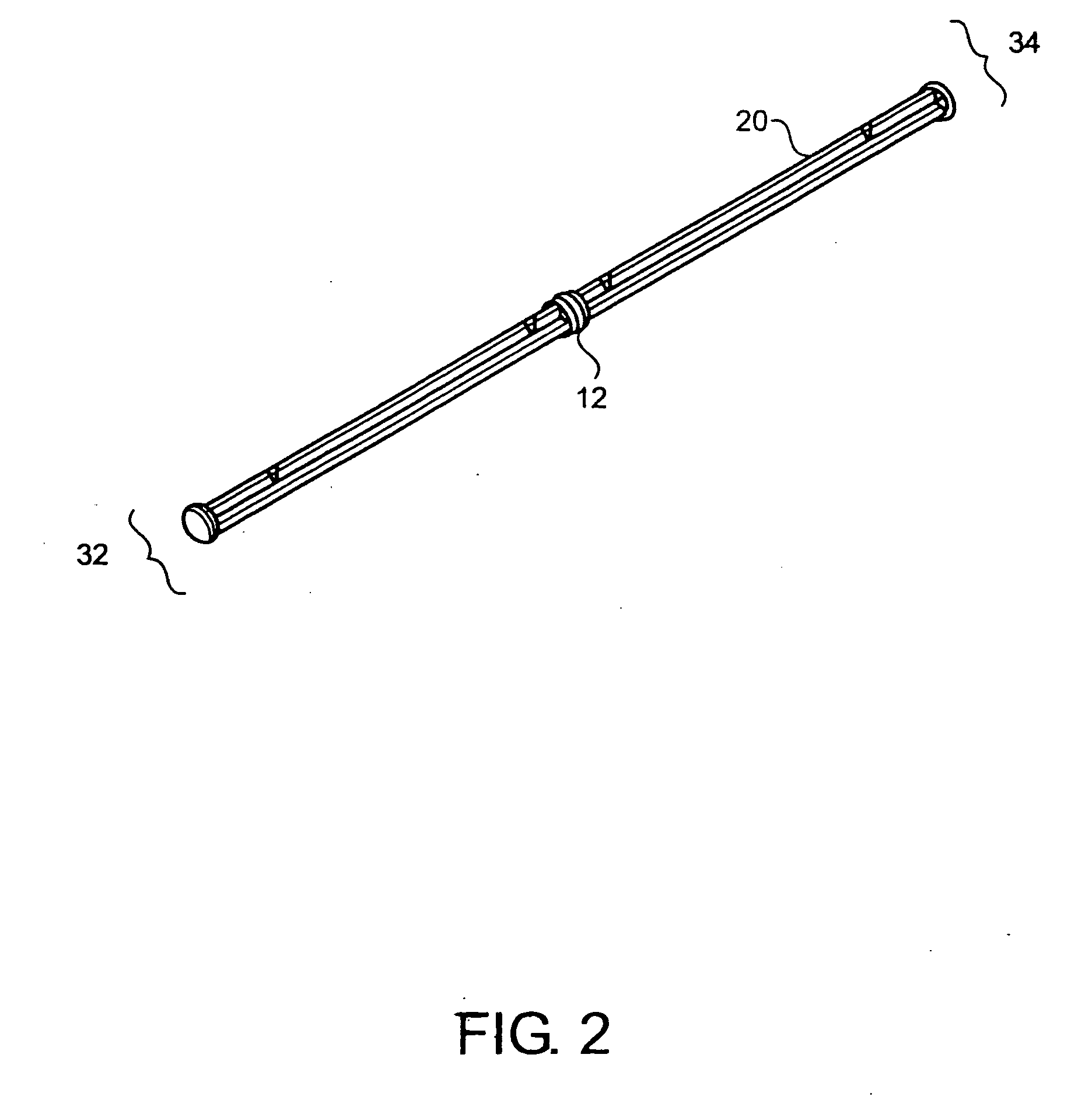
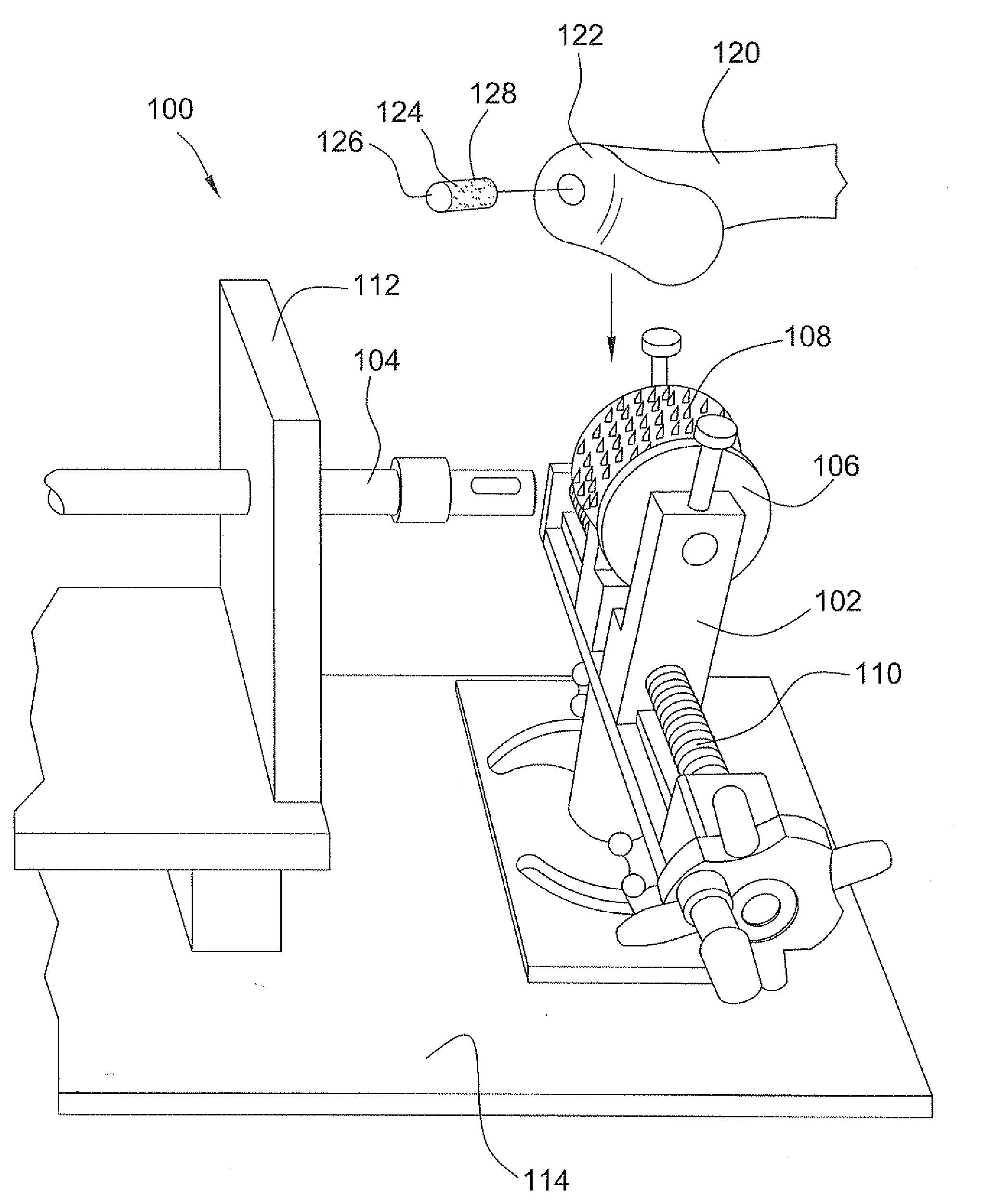
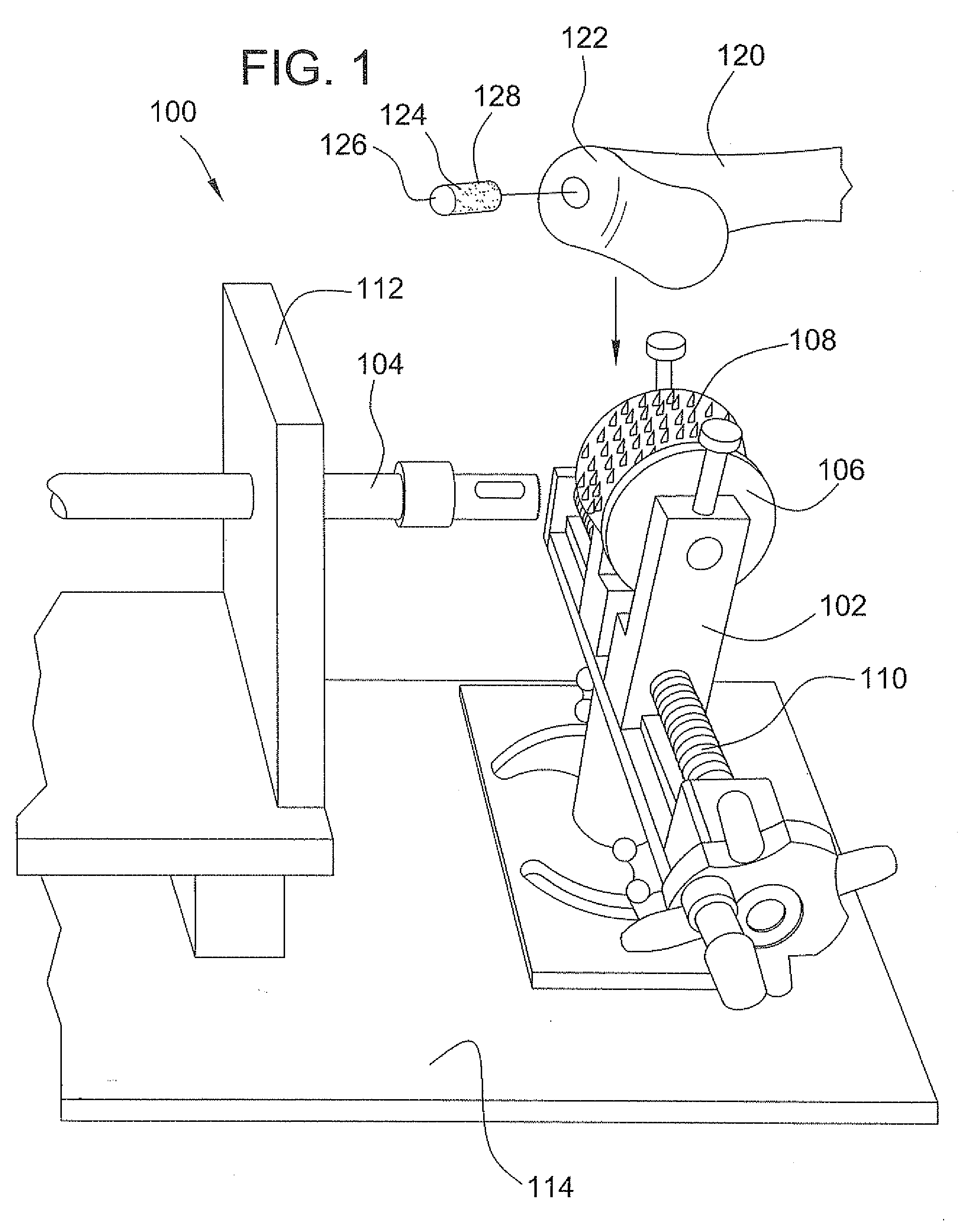
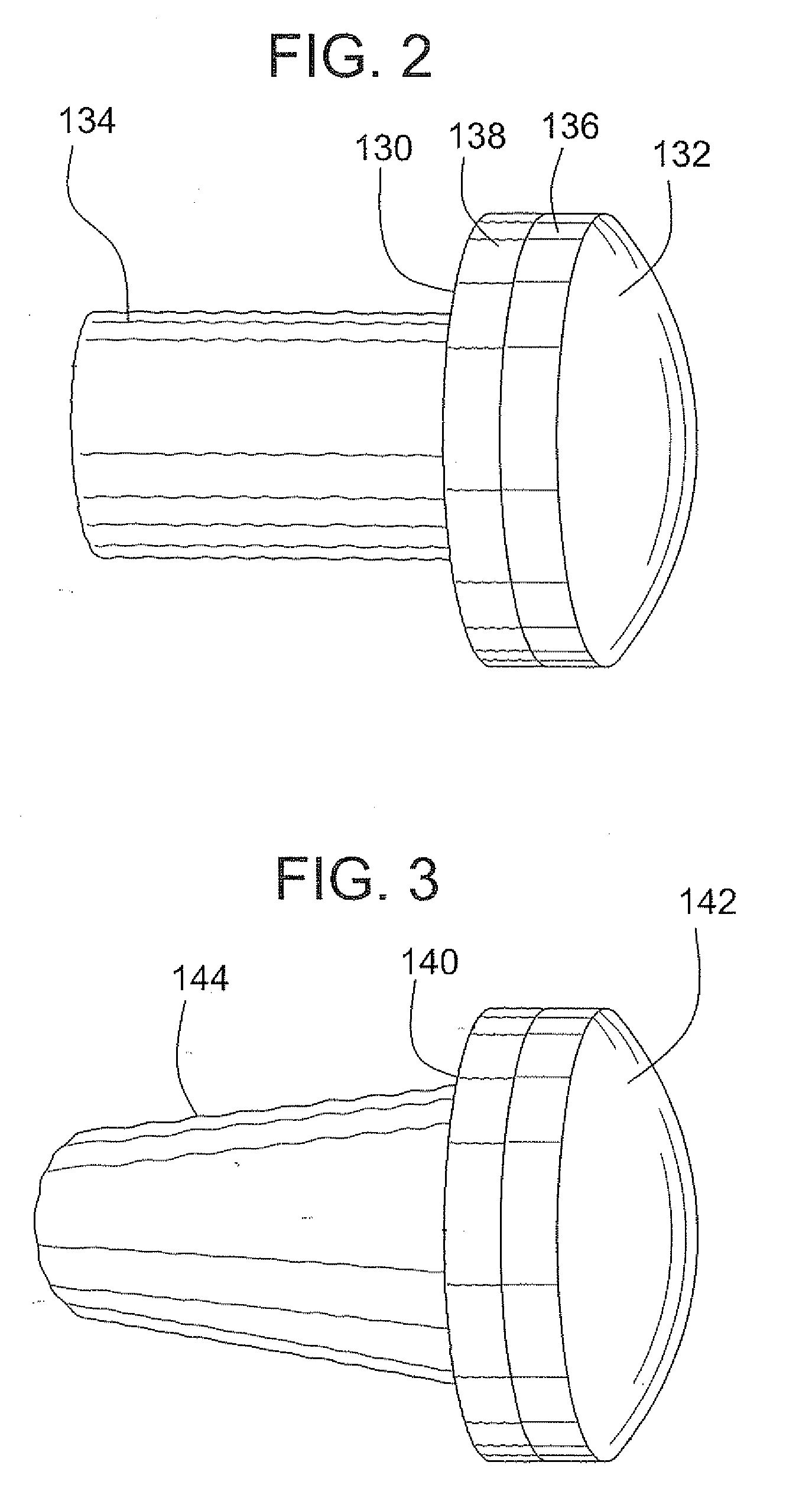
Bone and cartilage implant delivery device
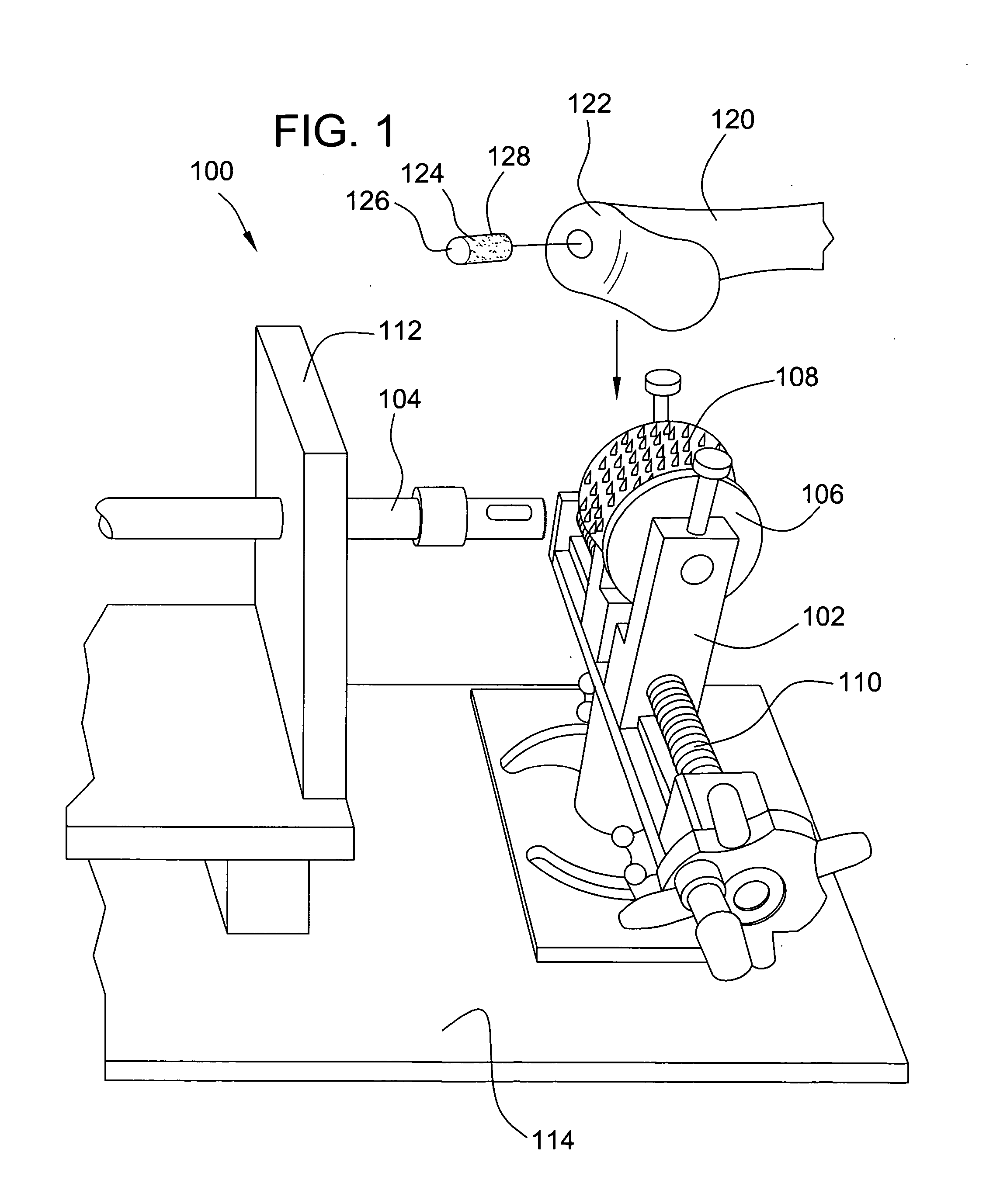
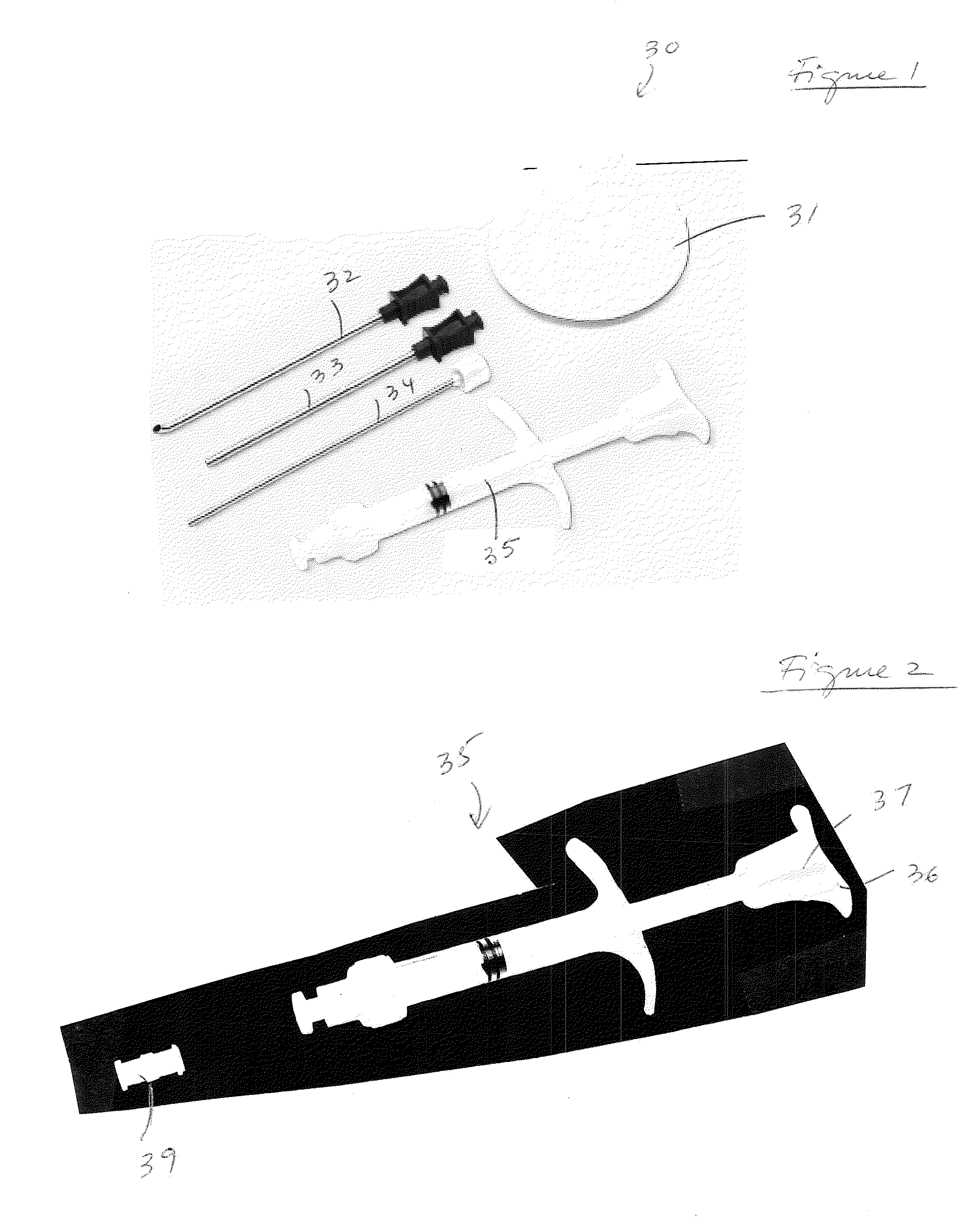
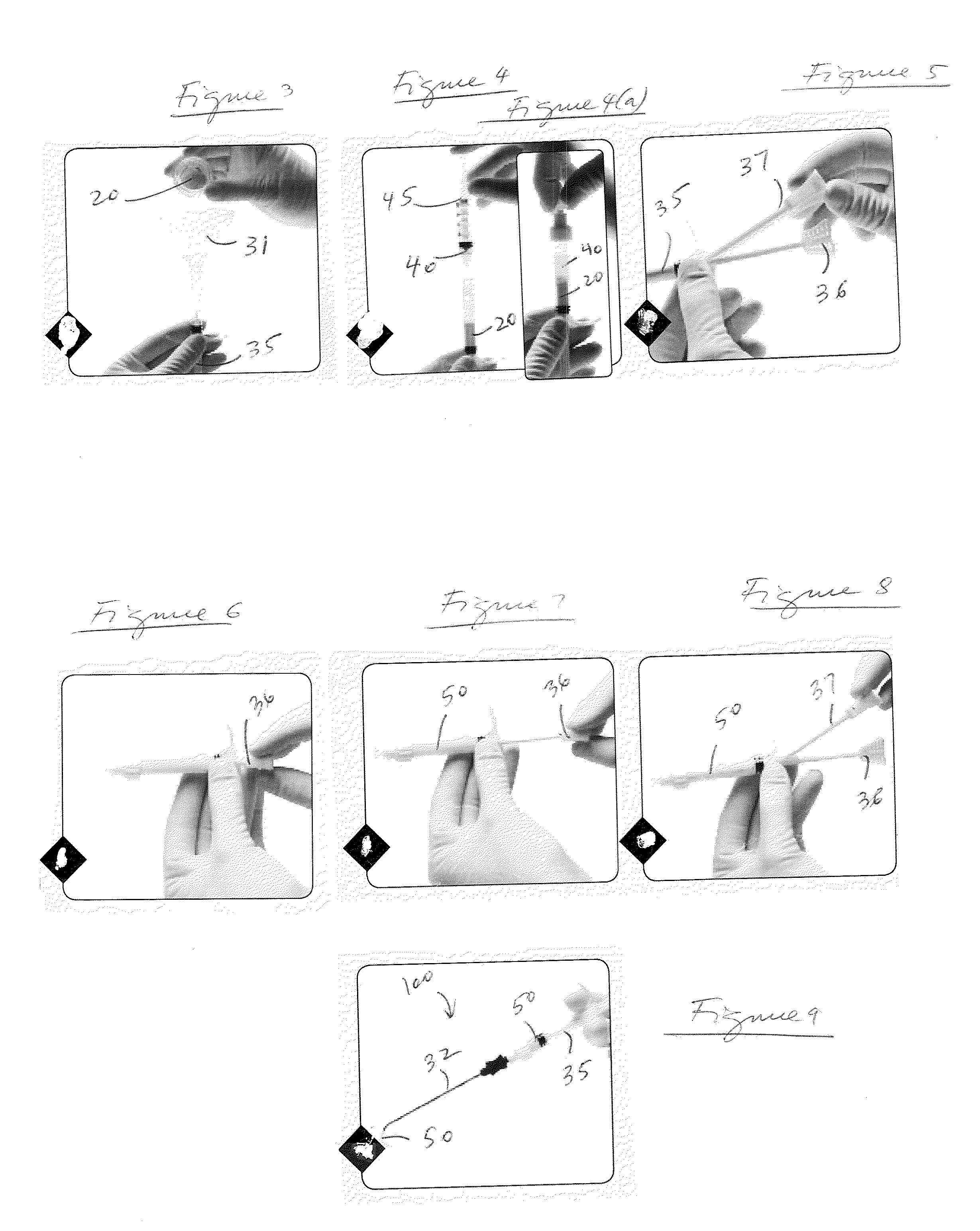
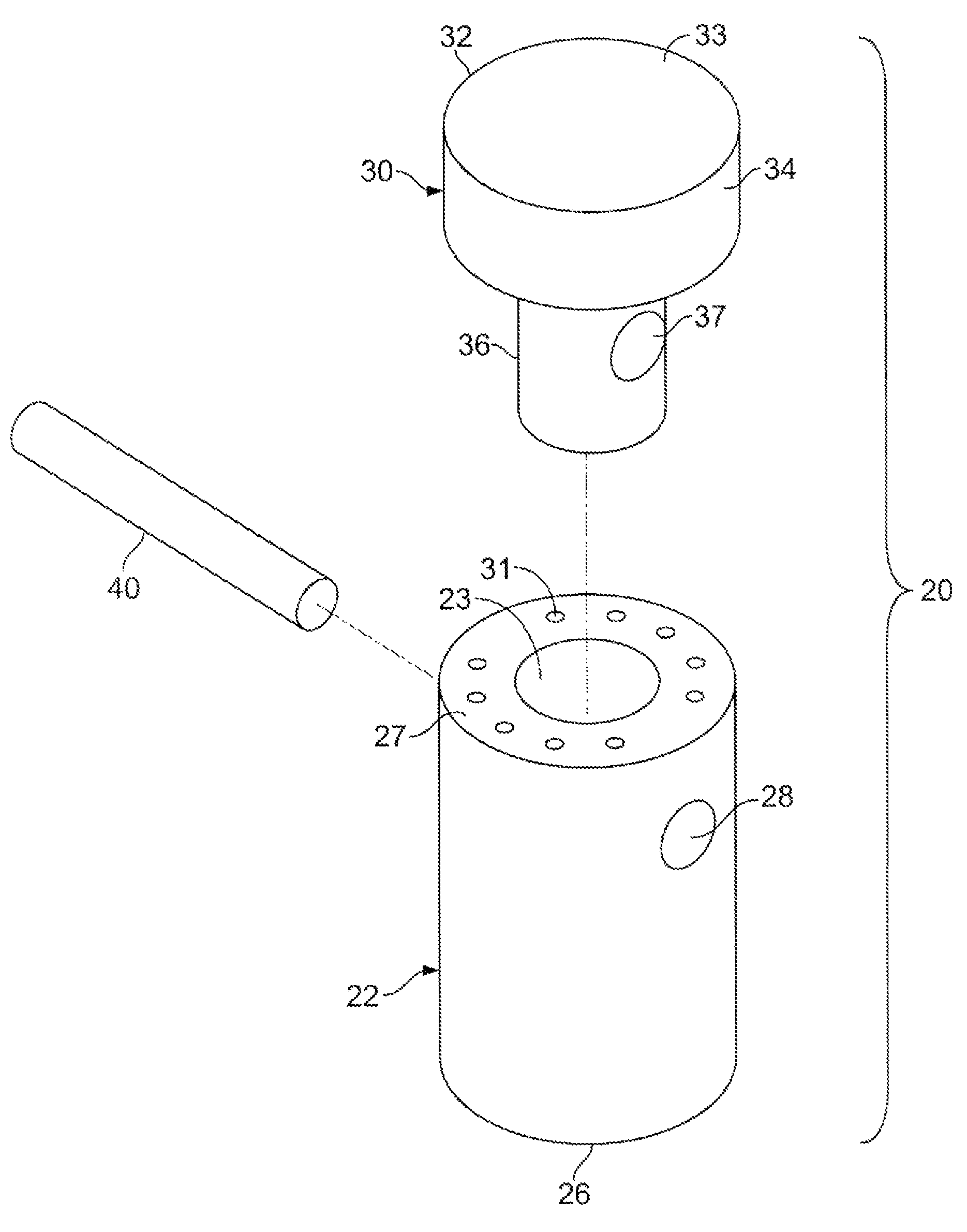
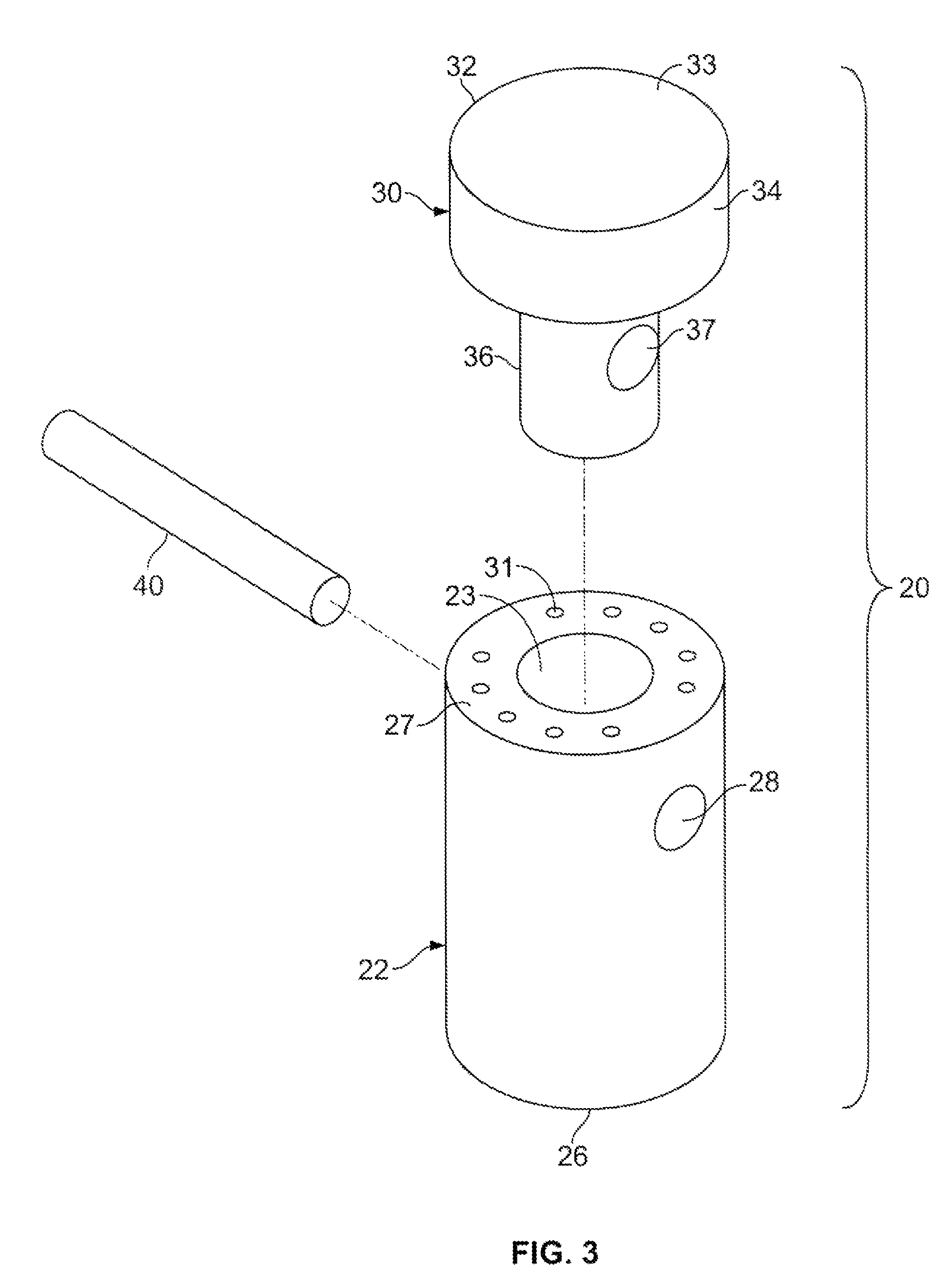
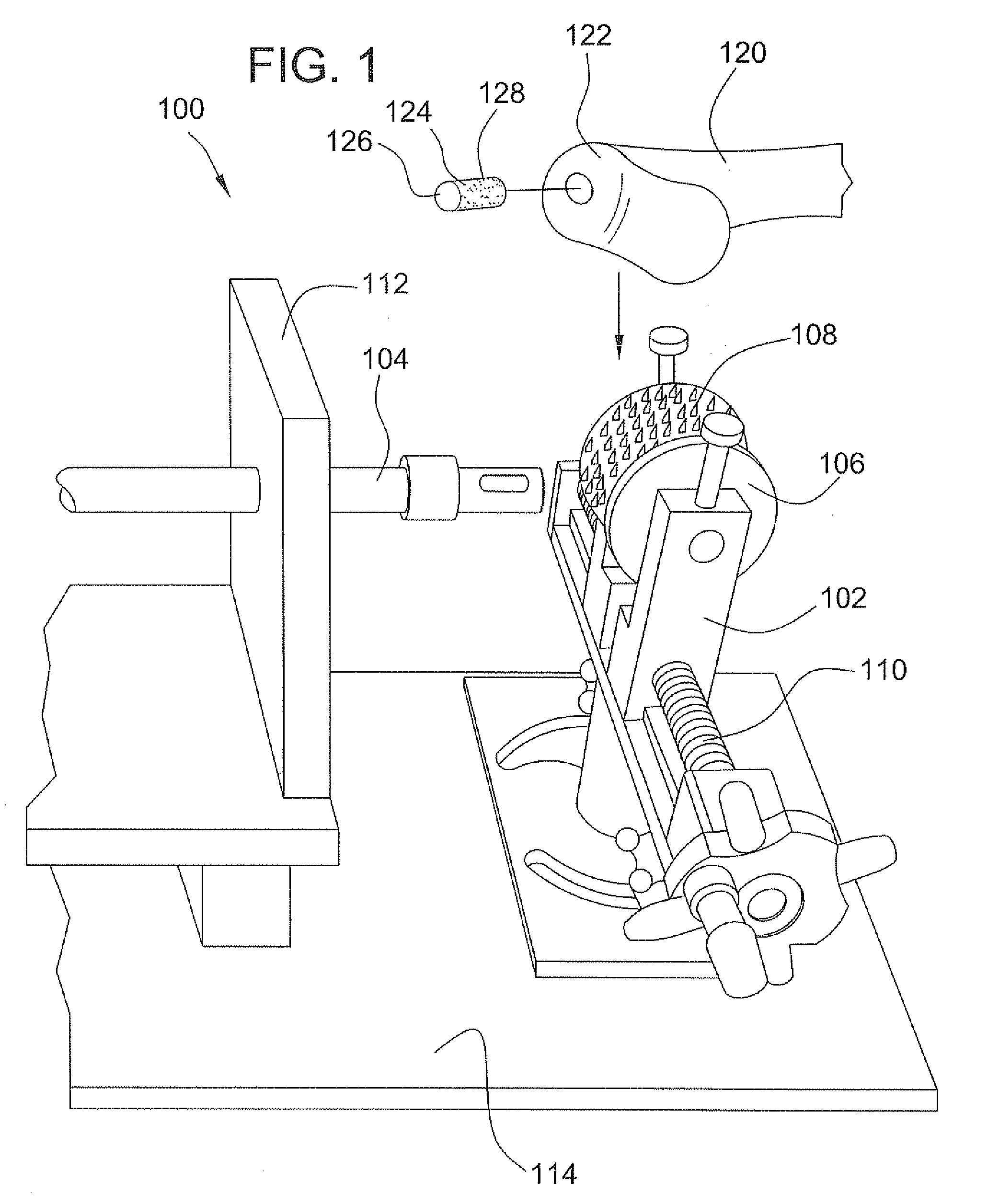
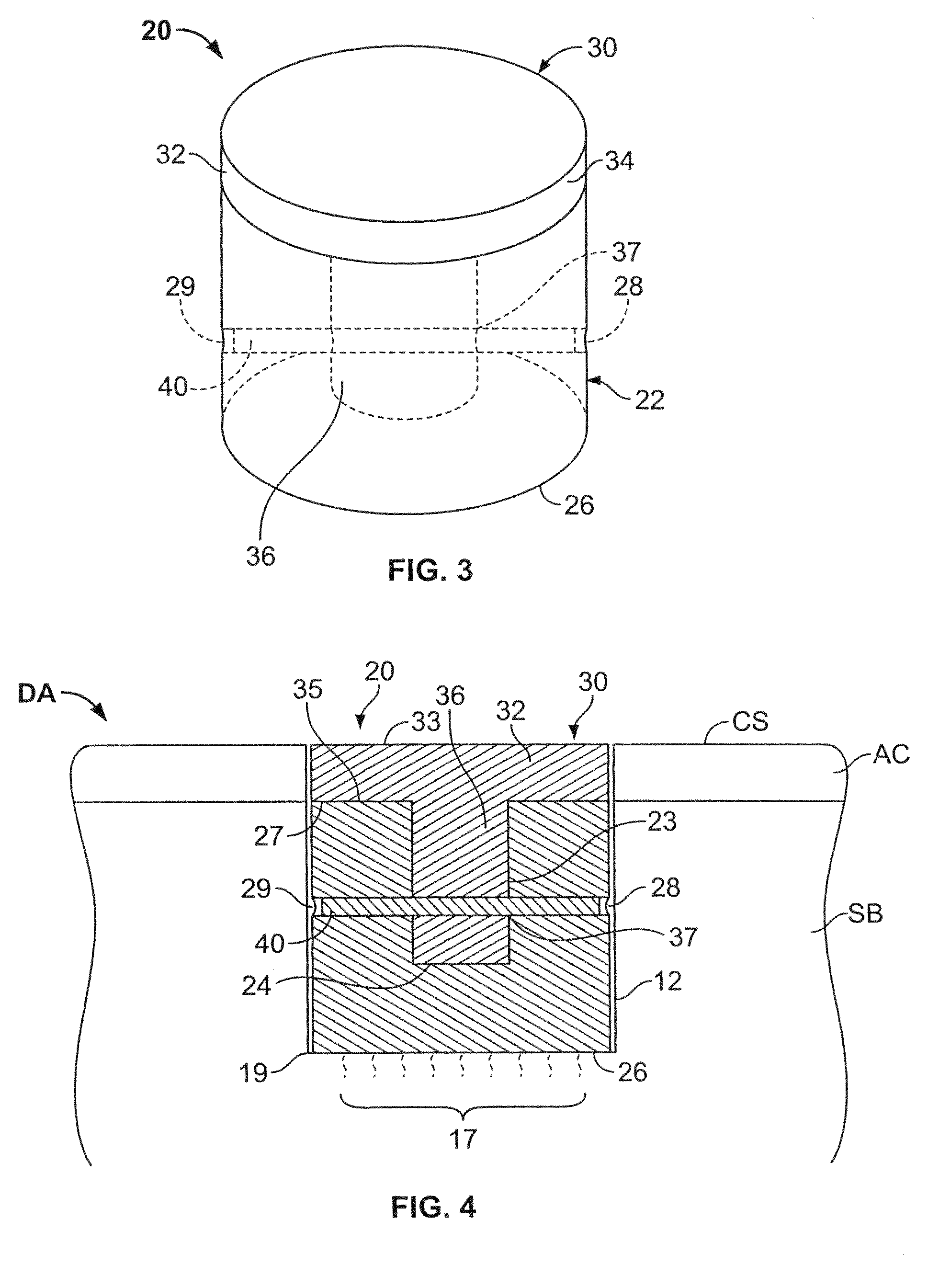
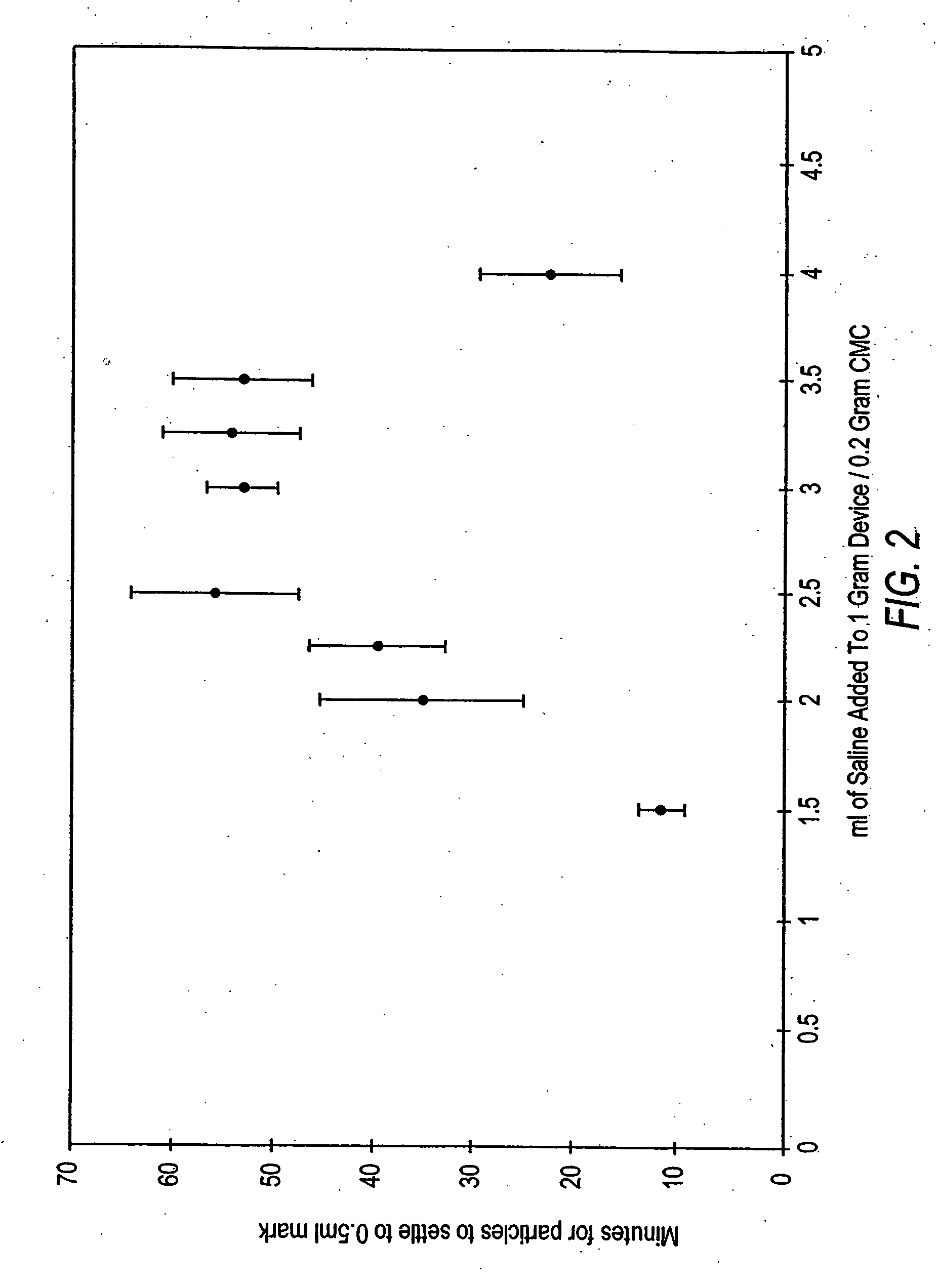
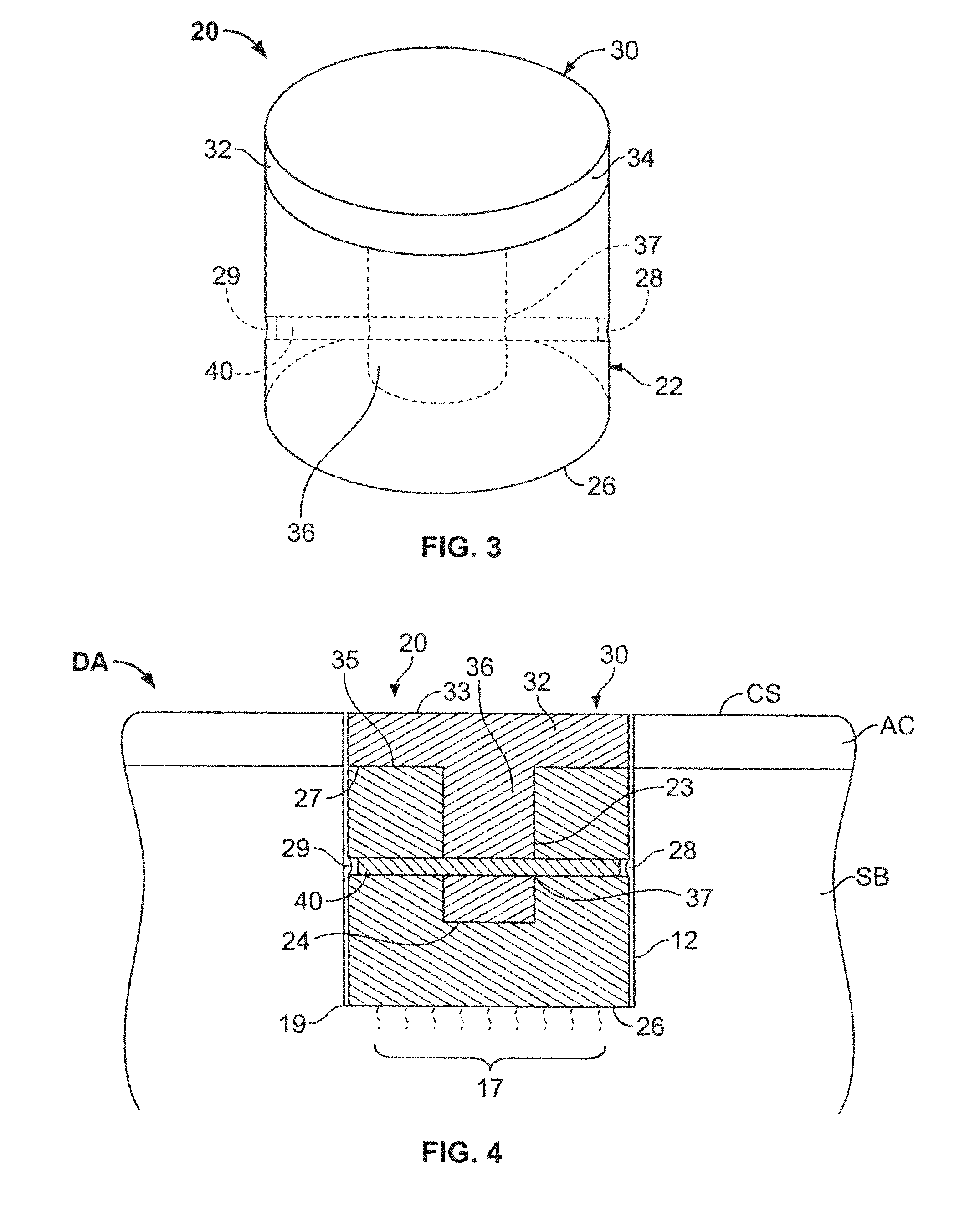
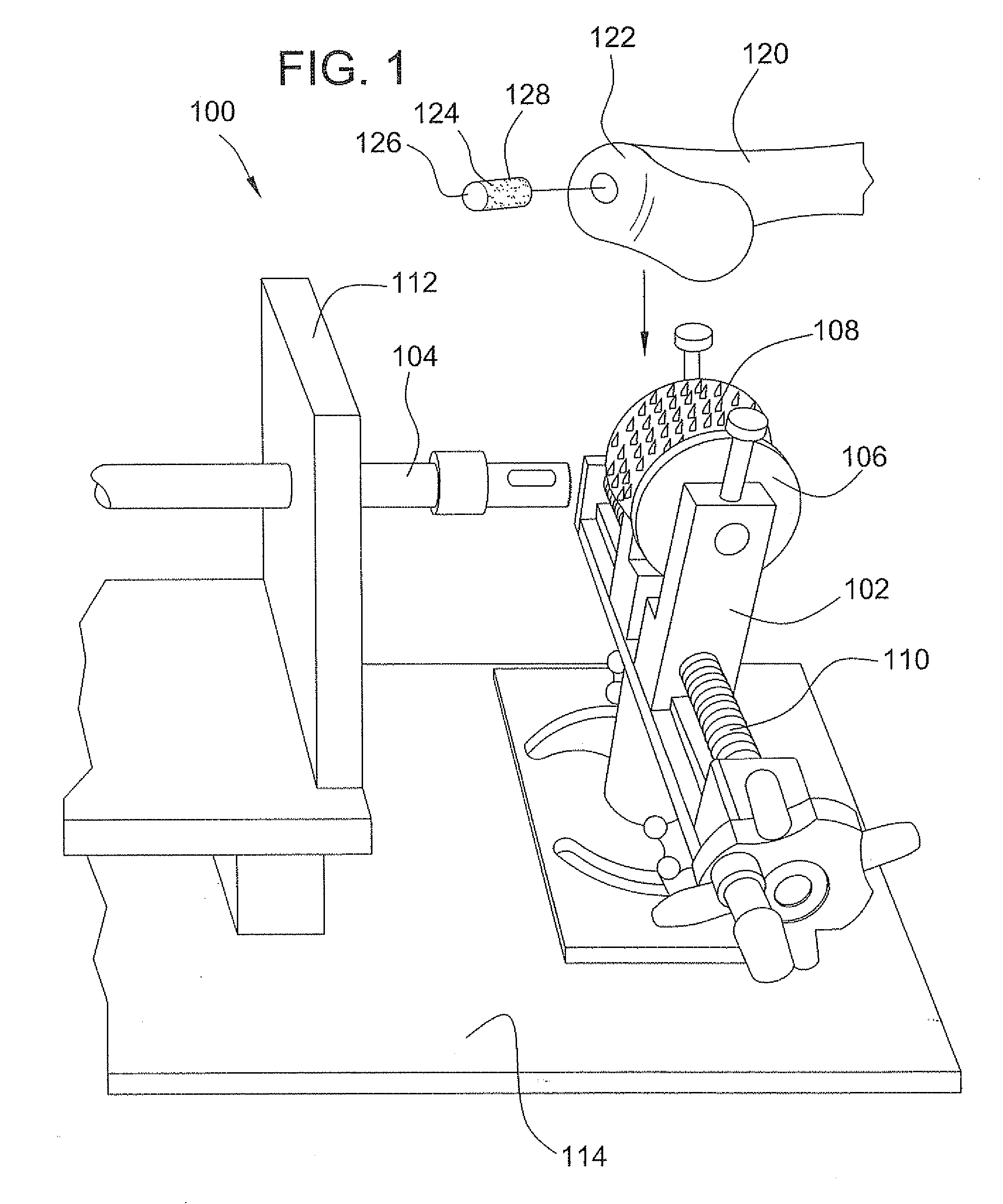
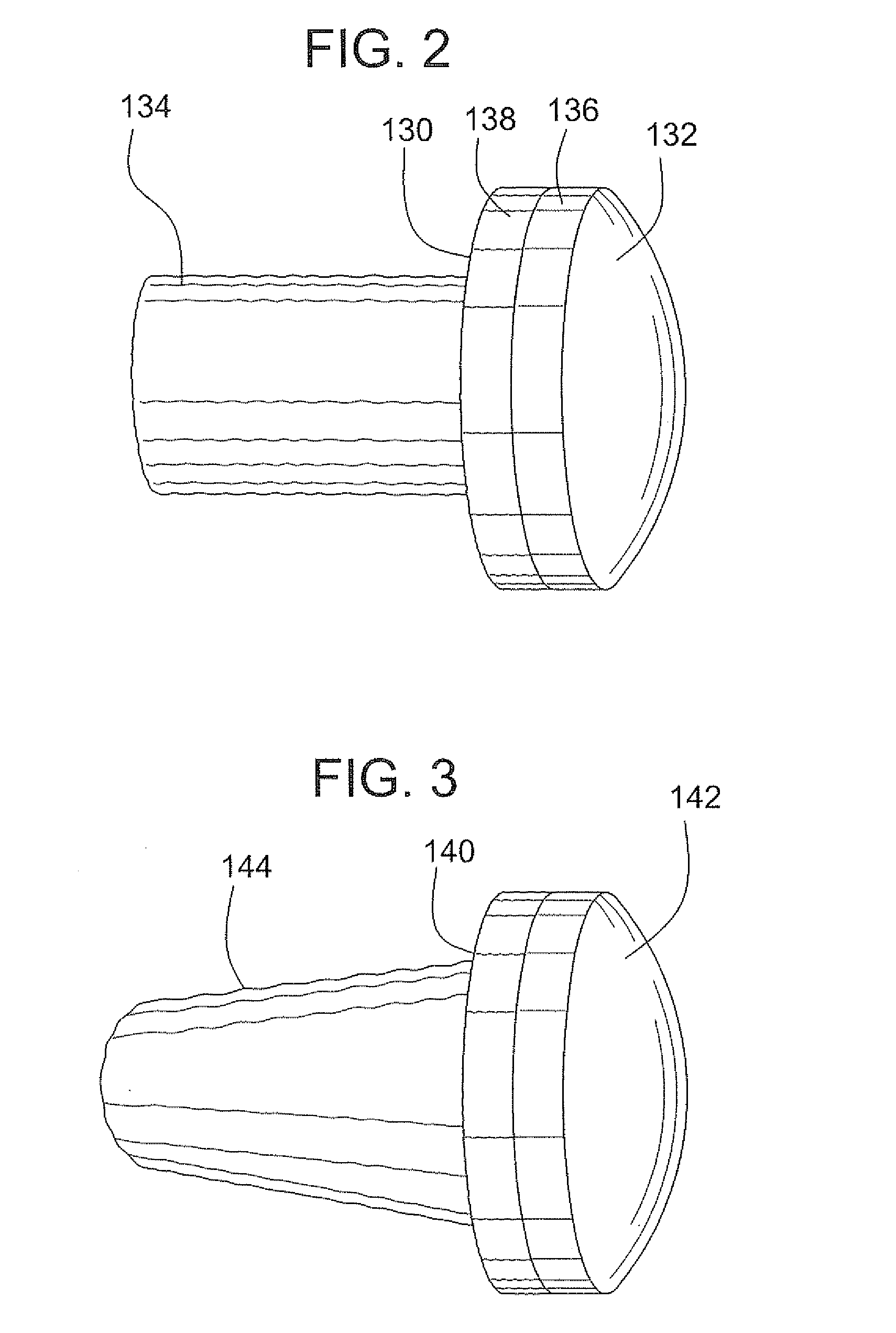
A method and device for inserting an implant of synthetic material or healthy bone or cartilage into a bone or cartilage defect of unknown depth. The device includes an inner shaft within a hollow outer shaft. One end of the inner shaft of the device is suitable for inserting into the bone or cartilage defect in order to determine the depth, while the other end of the outer shaft is suitable for holding an implant. The implant is cut to fit the defect. The device is partially transparent or translucent to allow visualizing of the implant and defect. The delivery device can be bent or curved to allow the device to be introduced to a defect at different angles and positions. The methods and devices are suitable for delivery of implants to defects having complex shapes.
Owner:OSTEOBIOLOGICS
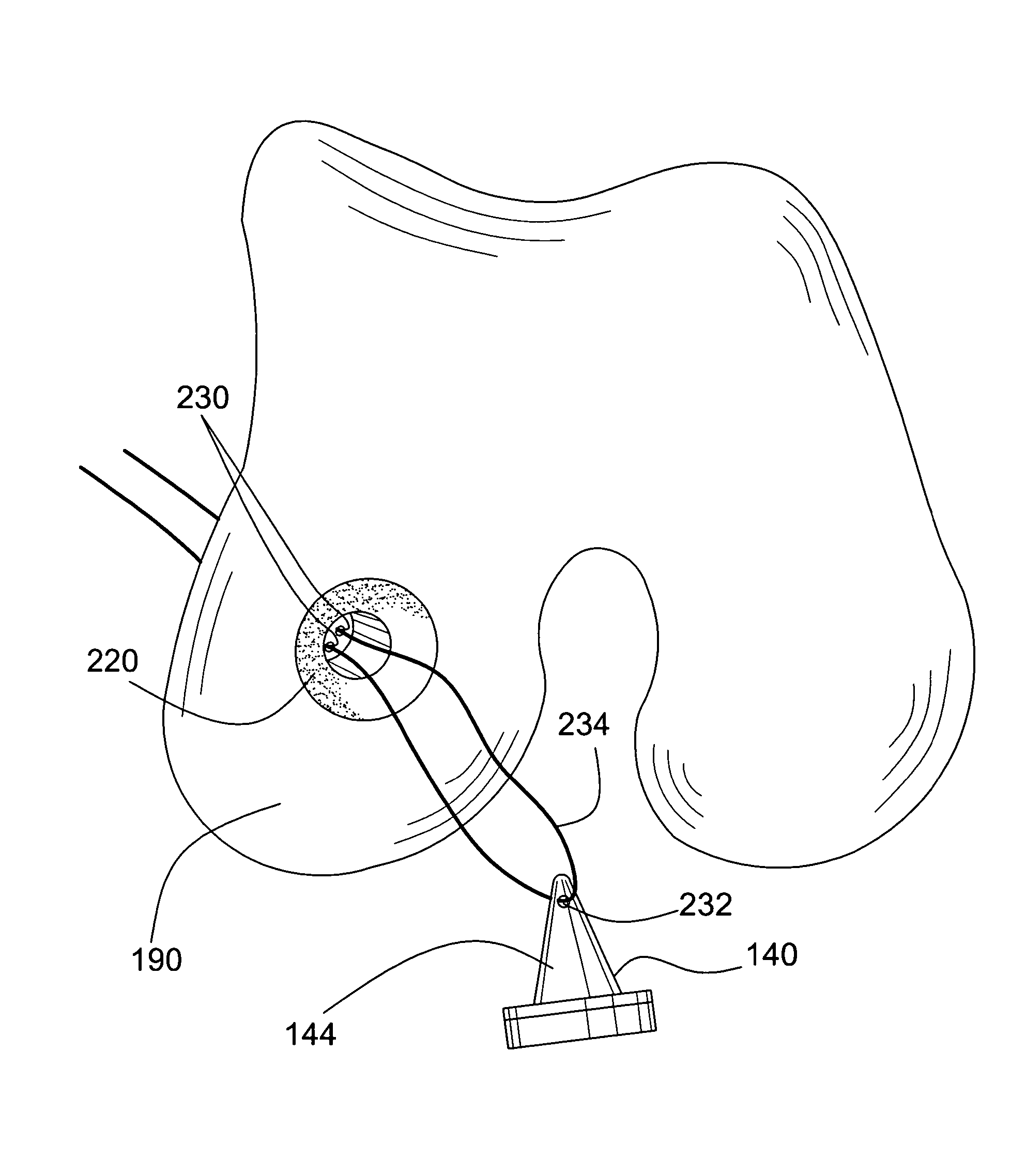
Method and instrumentation for the preparation and transplantation of osteochondral allografts
InactiveUS20070093896A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilnear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Cartilage implant assembly and method for implantation
InactiveUS20050222687A1Recovery functionRelief the painBone implantSurgerySubchondral boneInterference fit
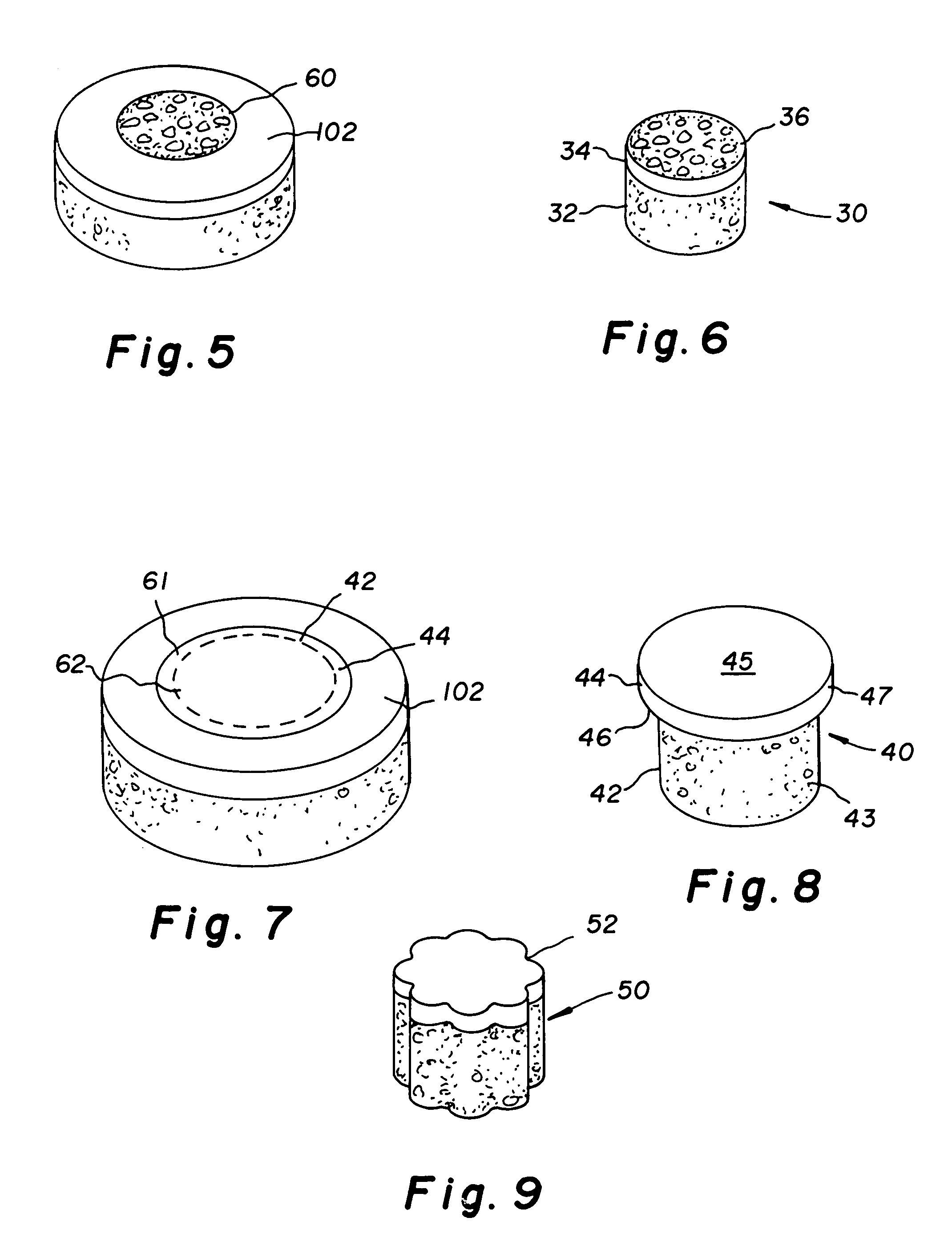
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore in an interference fit and is surrounded by milled cartilage and carrier. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
Cartilage allograft plug
InactiveUS20050251268A1Increase chondrocyte migrationIncreased proliferationBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Osteogenic devices and methods of use thereof for repair of endochondral bone and osteochondral defects
InactiveUS7041641B2Avoid undesirable formationOvercome problemsPowder deliveryImpression capsRepair tissueNon union
Disclosed herein are improved osteogenic devices and methods of use thereof for repair of bone and cartilage defects. The devices and methods promote accelerated formation of repair tissue with enhanced stability using less osteogenic protein than devices in the art. Defects susceptible to repair with the instant invention include, but are not limited to: critical size defects, non-critical size defects, non-union fractures, fractures, osteochondral defects, subchondral defects, and defects resulting from degenerative diseases such as osteochondritis dessicans.
Owner:MARIEL THERAPEUTICS
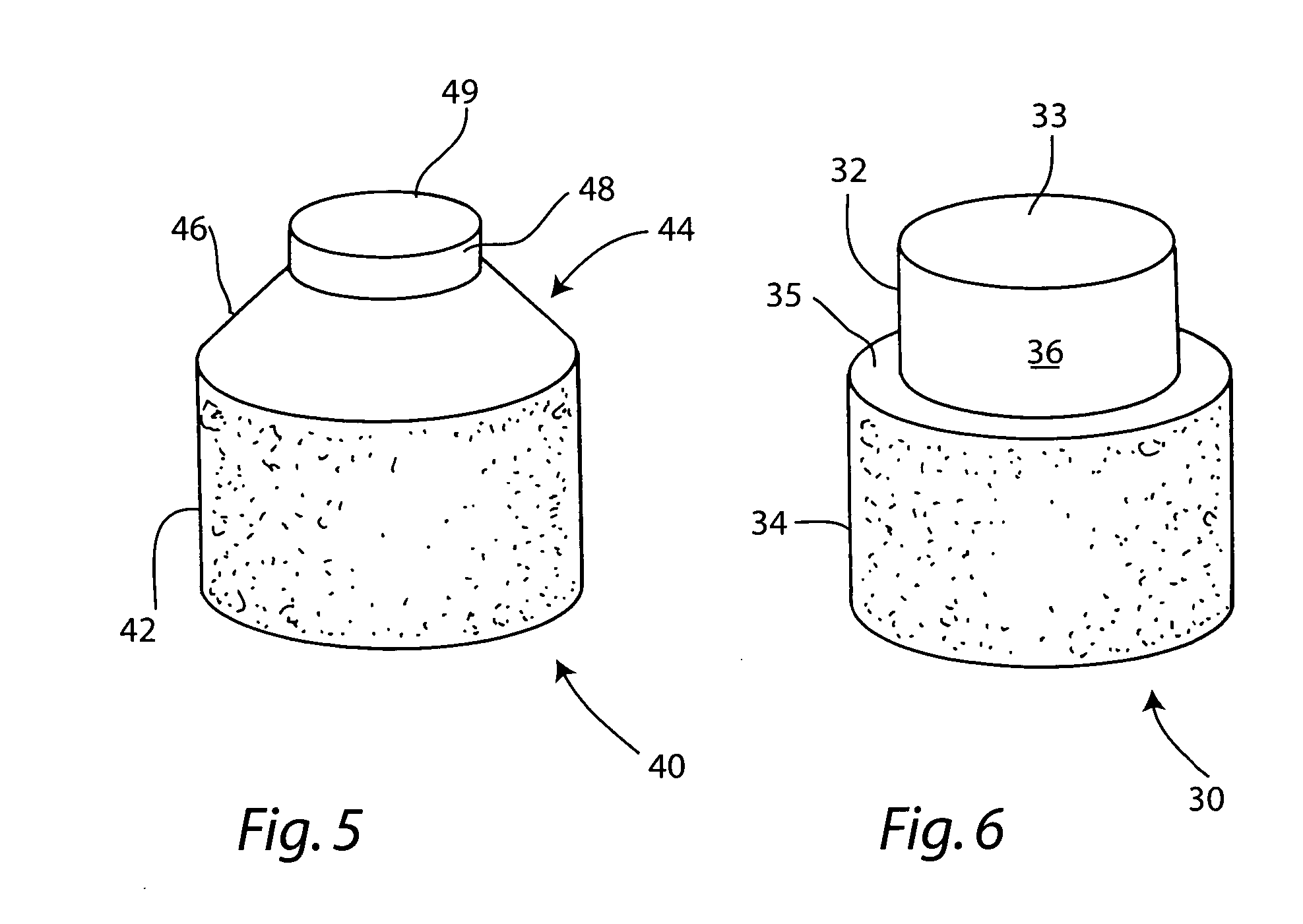
Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints
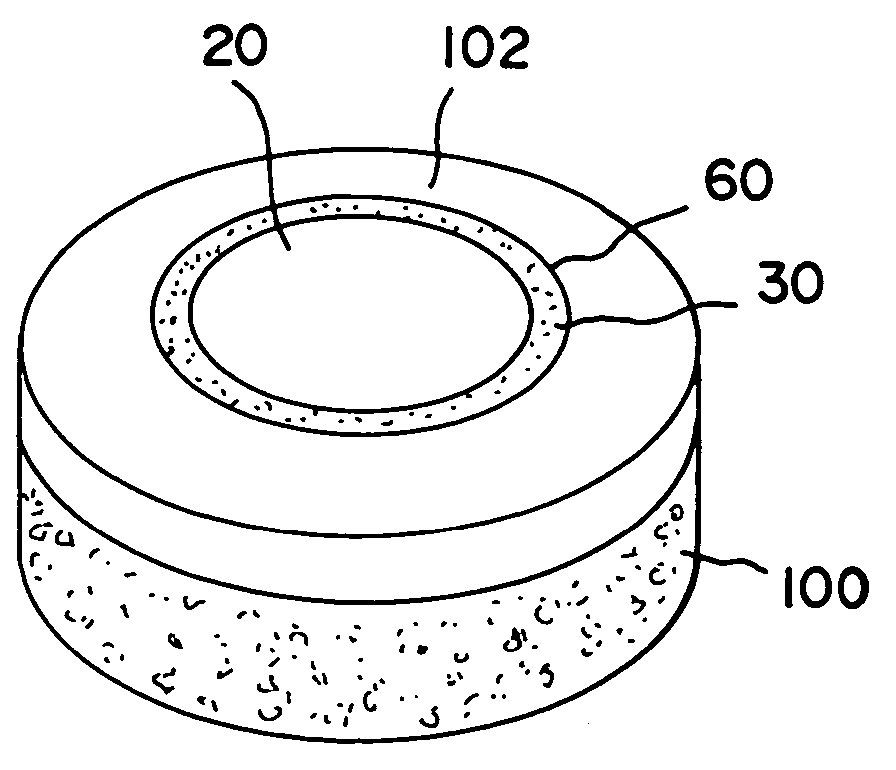
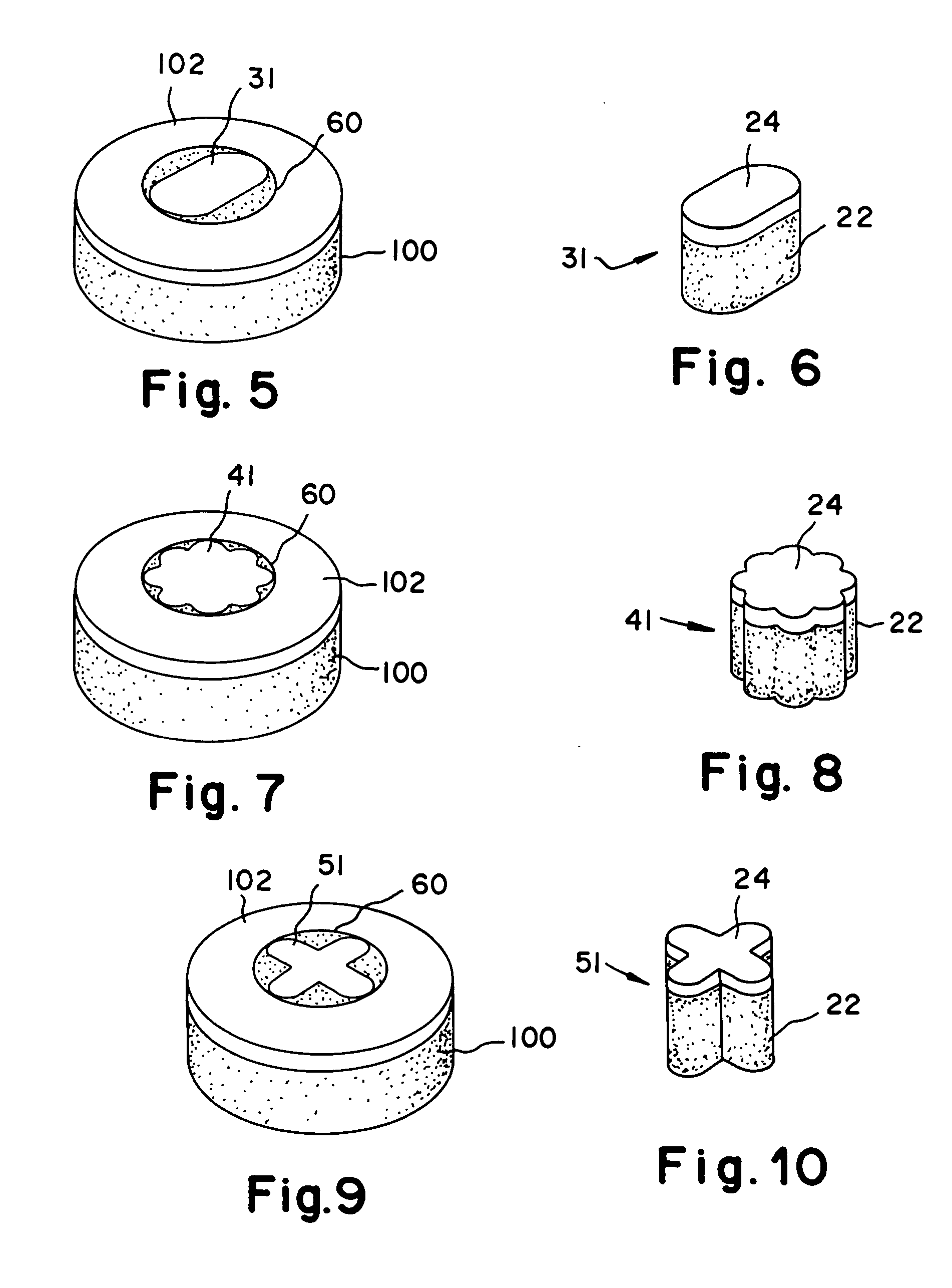
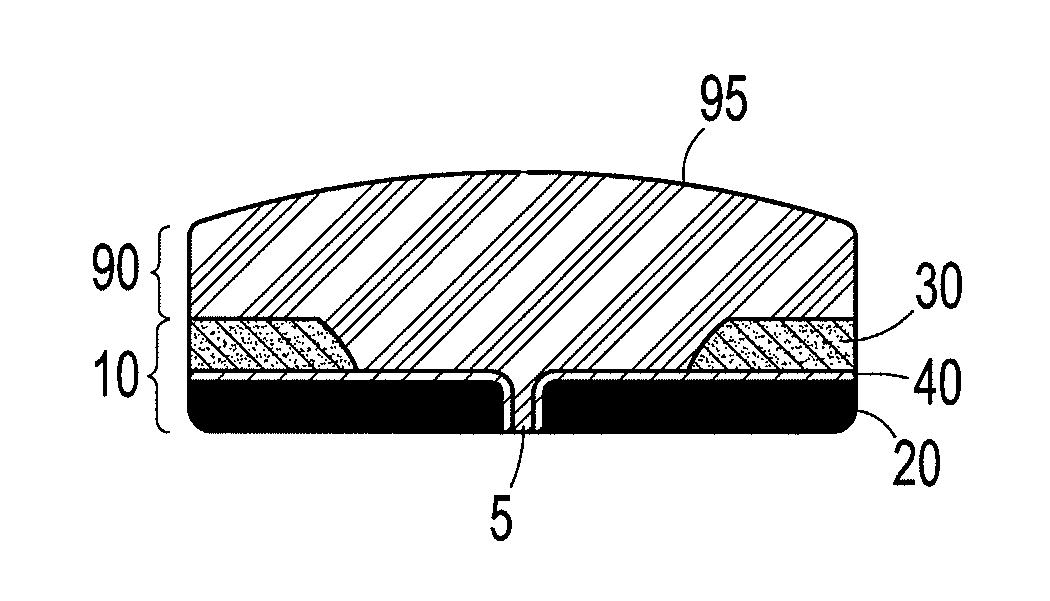
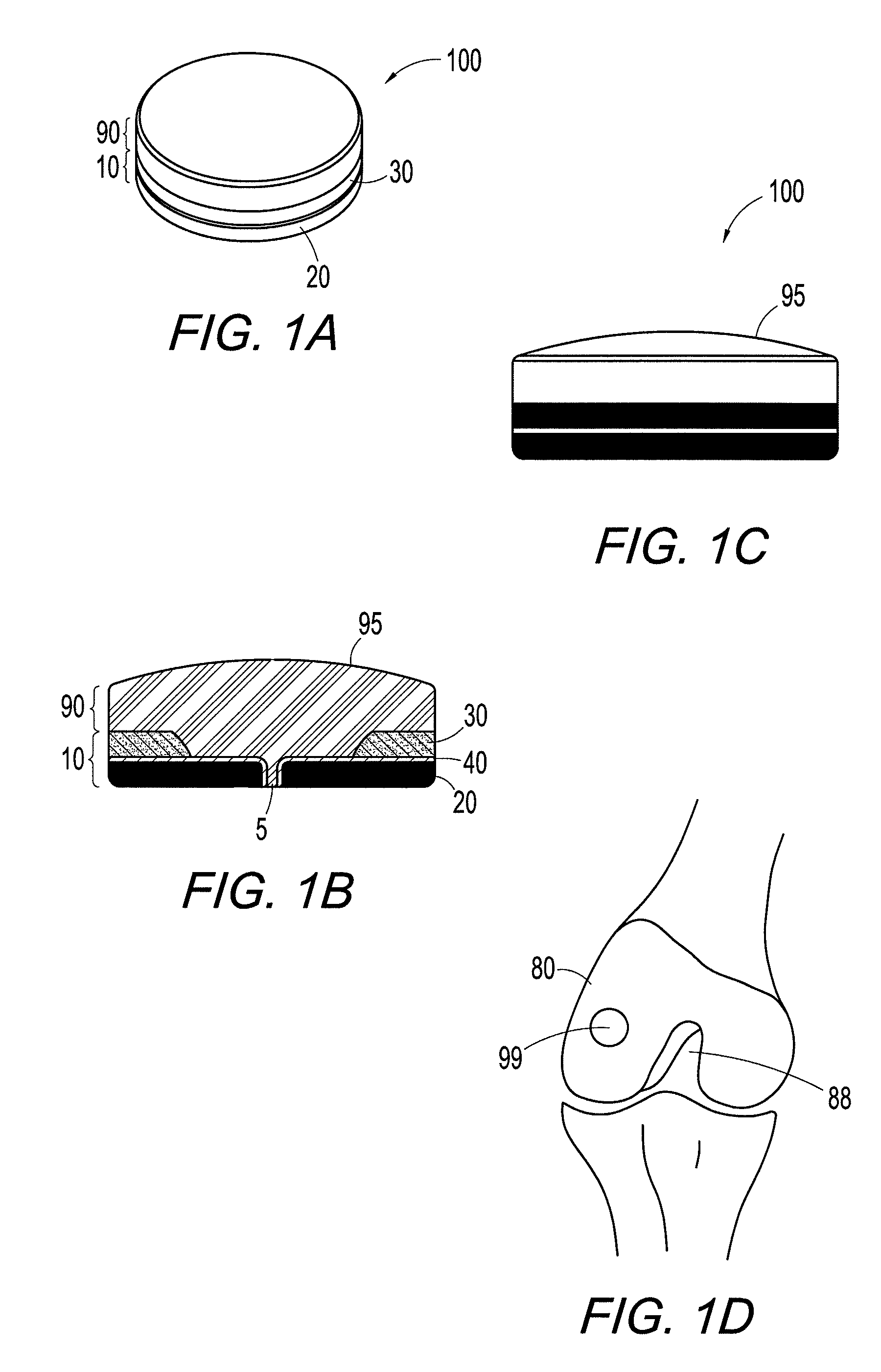
InactiveUS6858042B2Reduce absorptionResistant to resorptionBone implantJoint implantsRepair tissueCyst
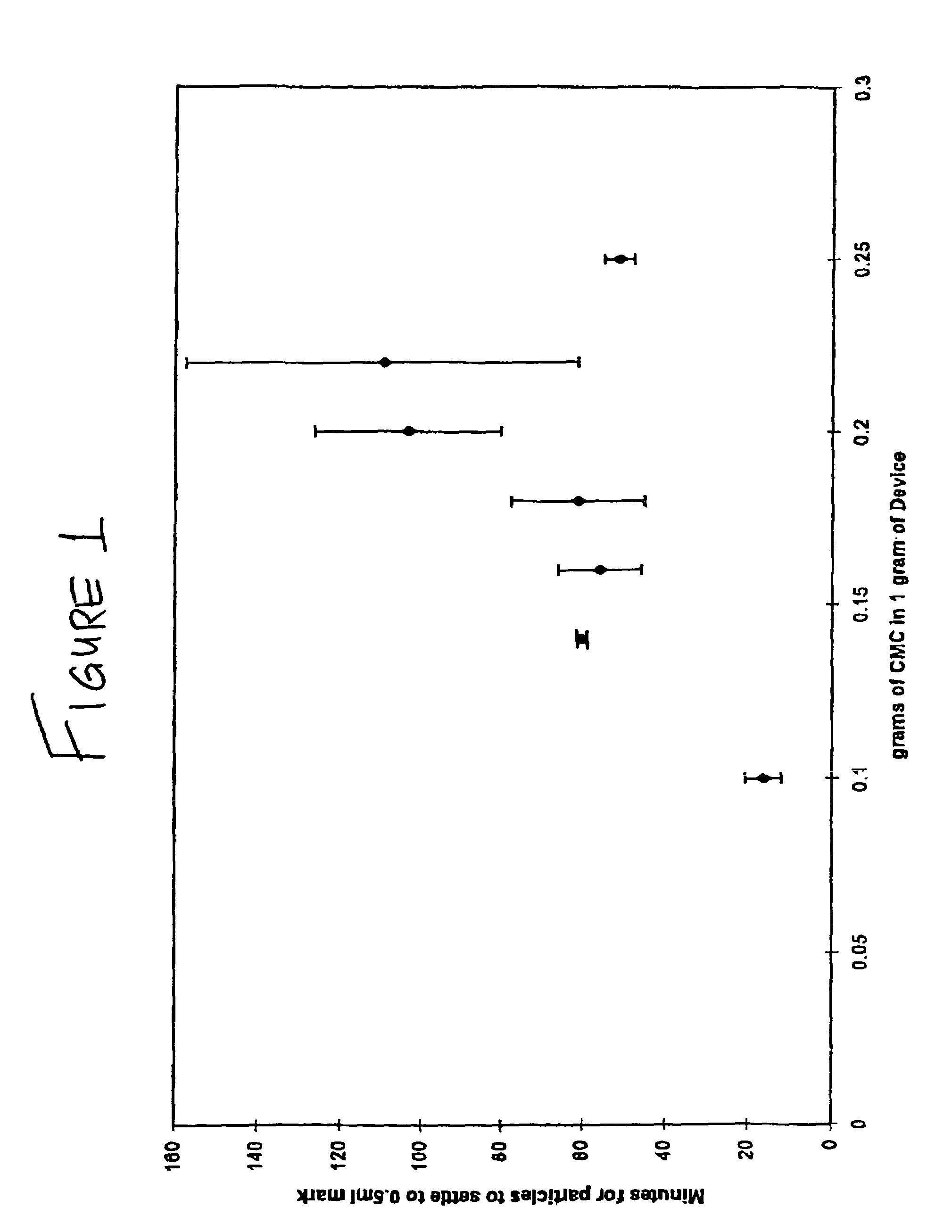
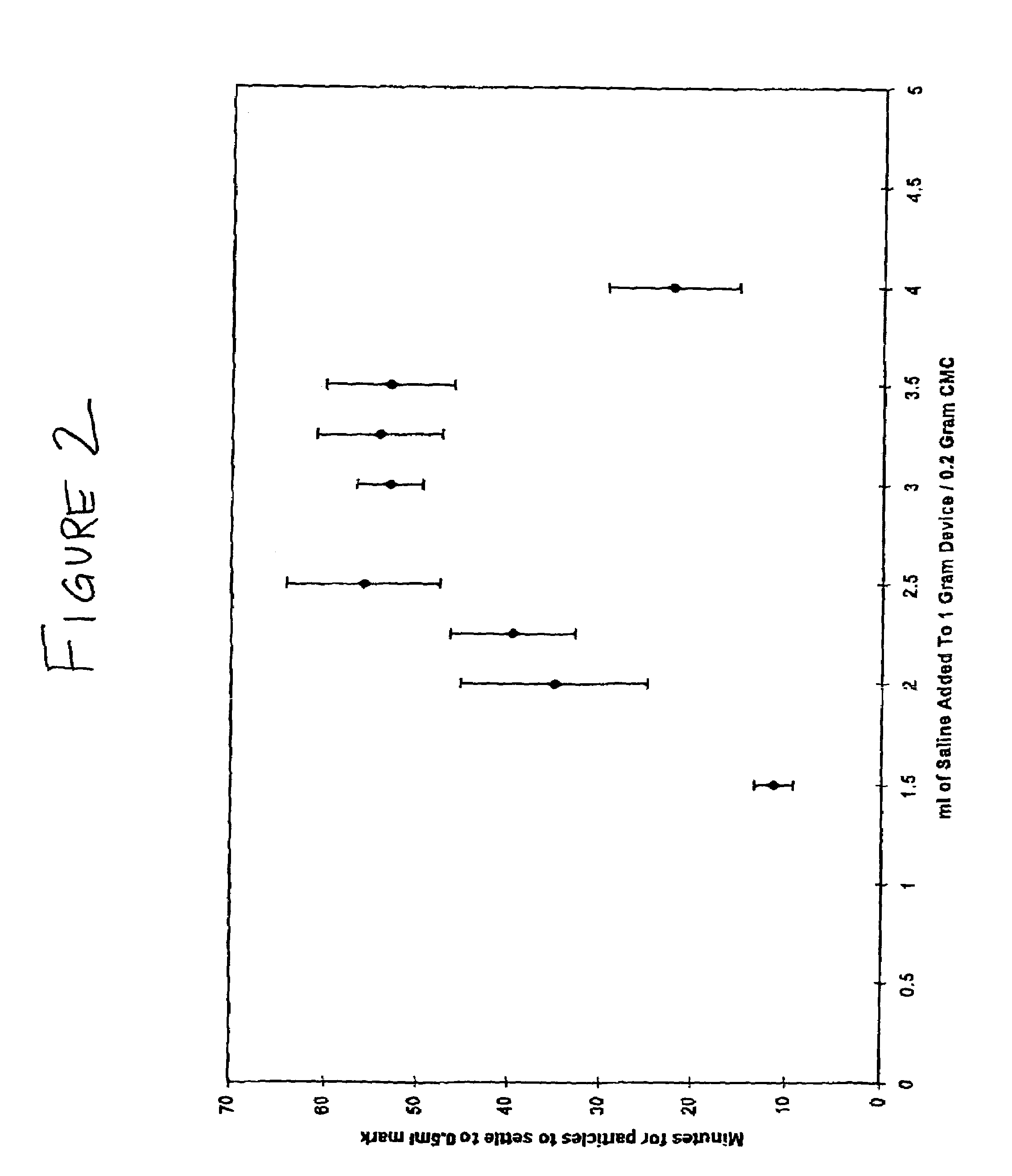
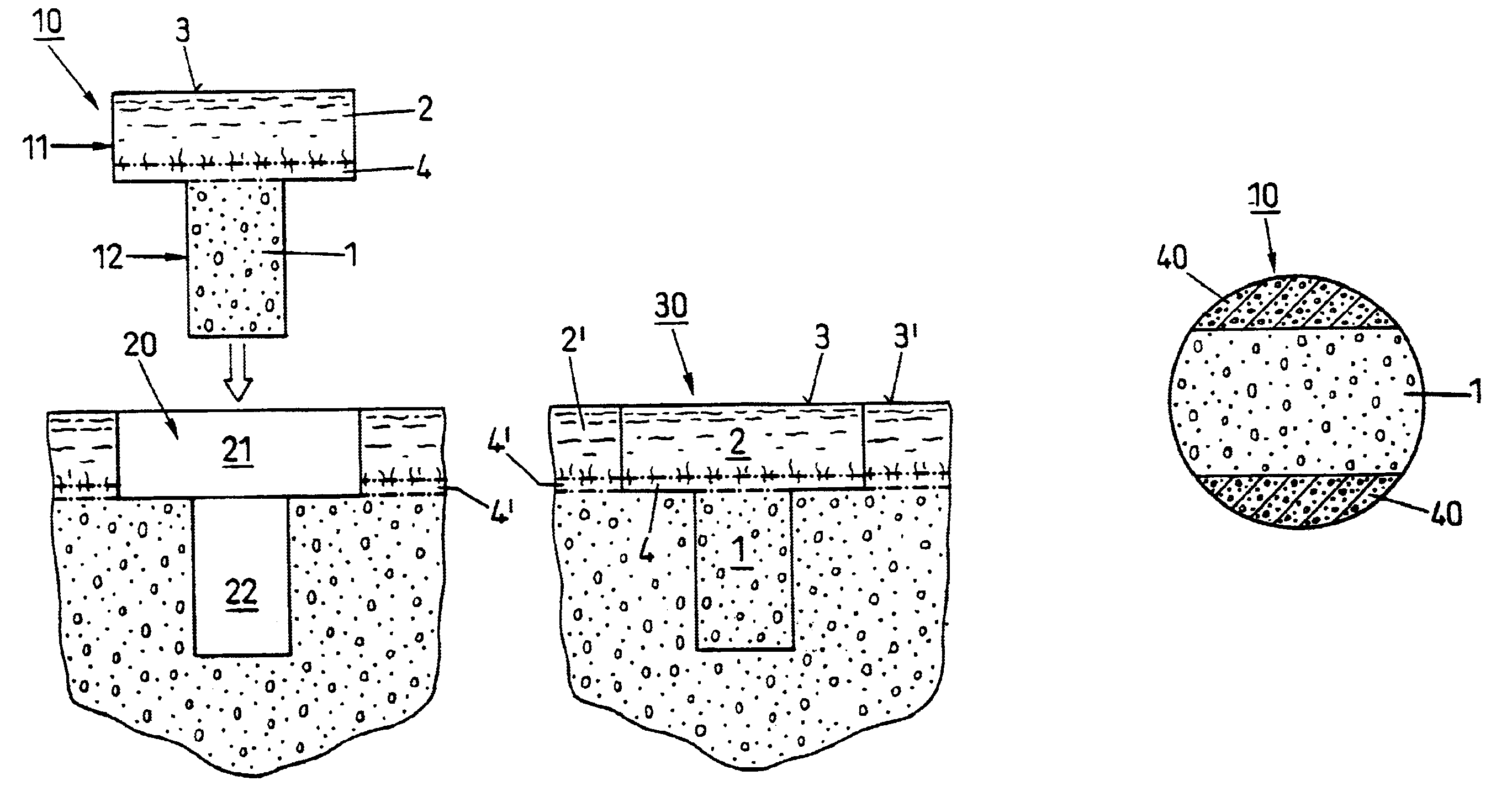
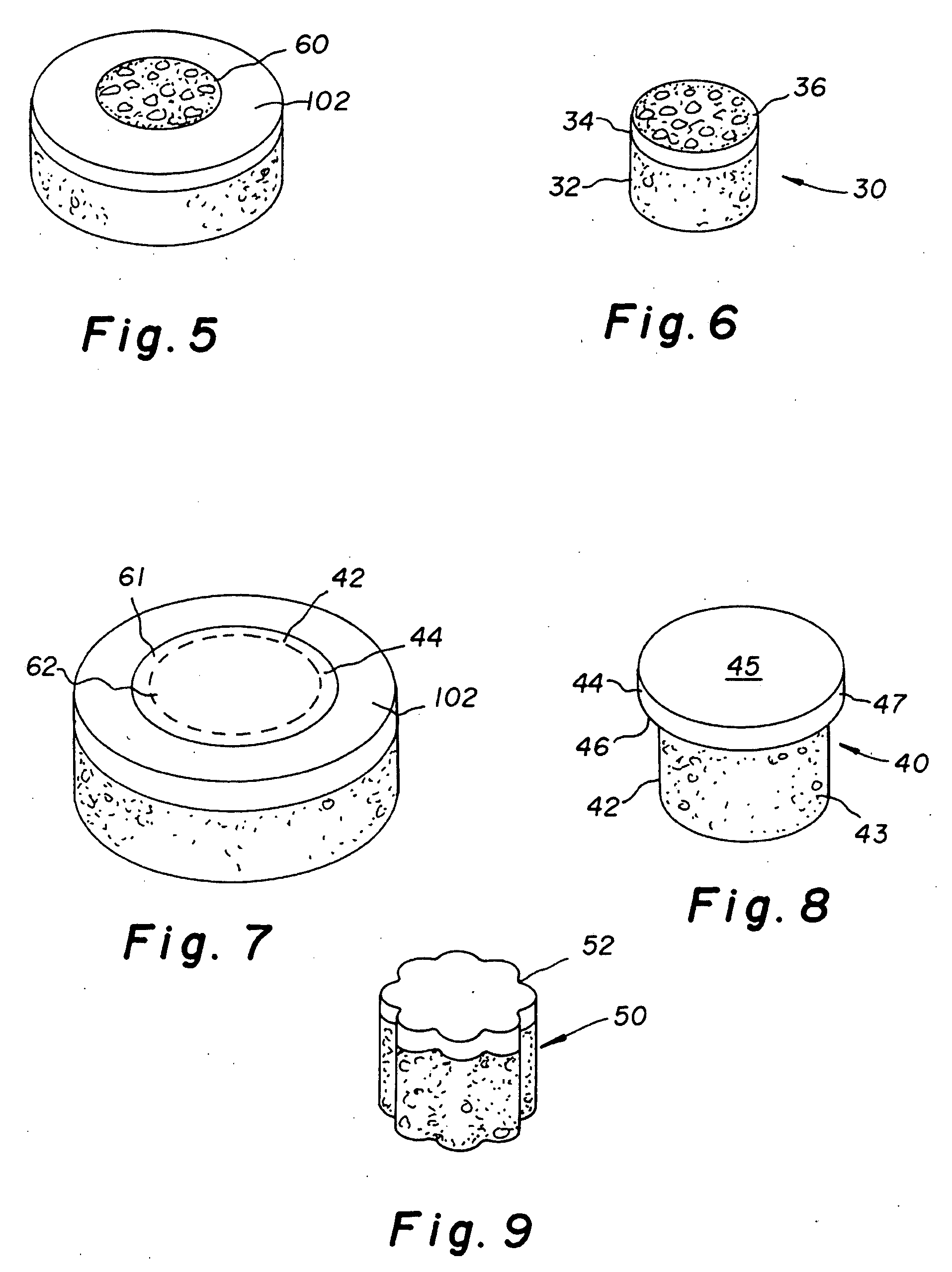
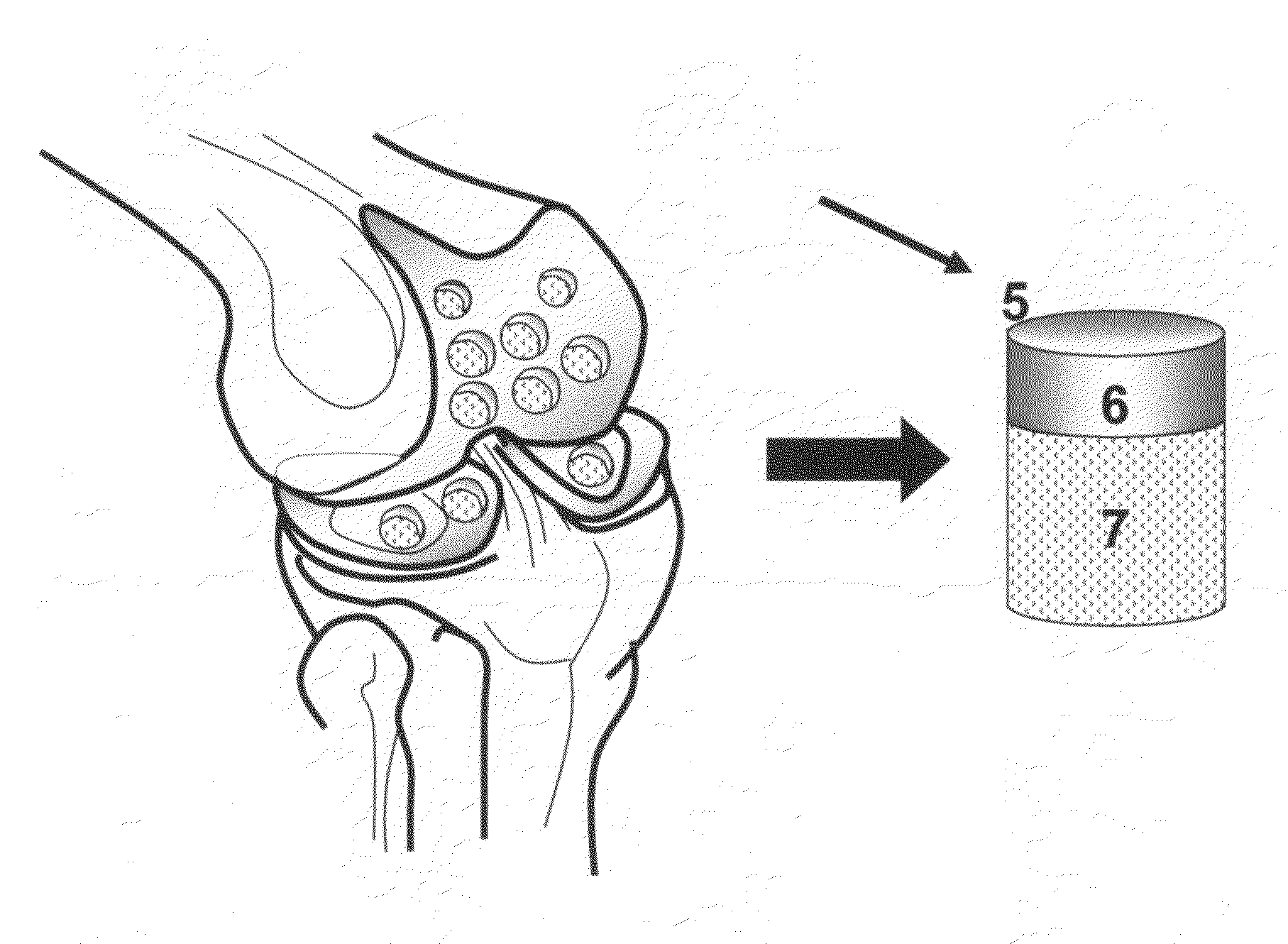
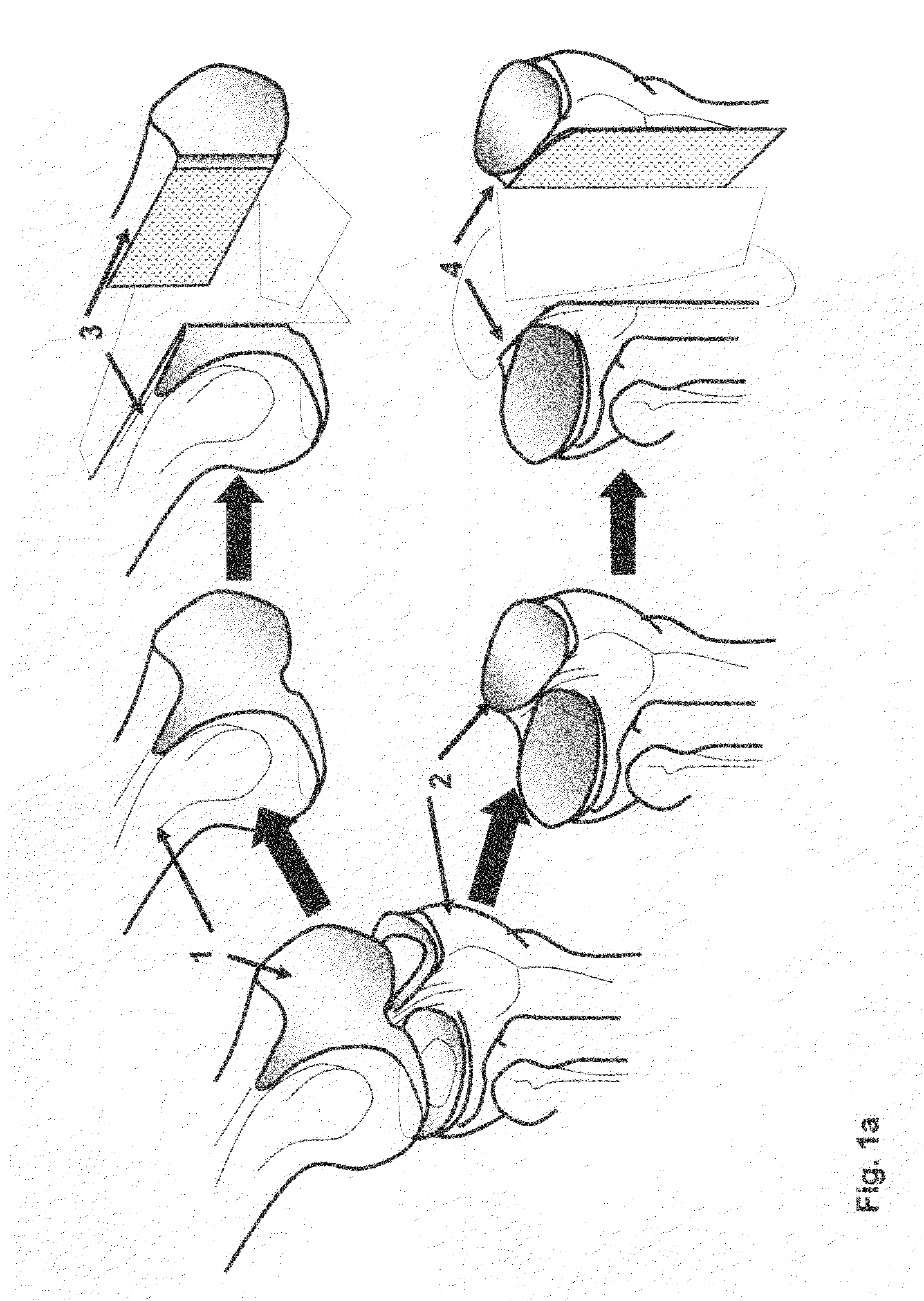
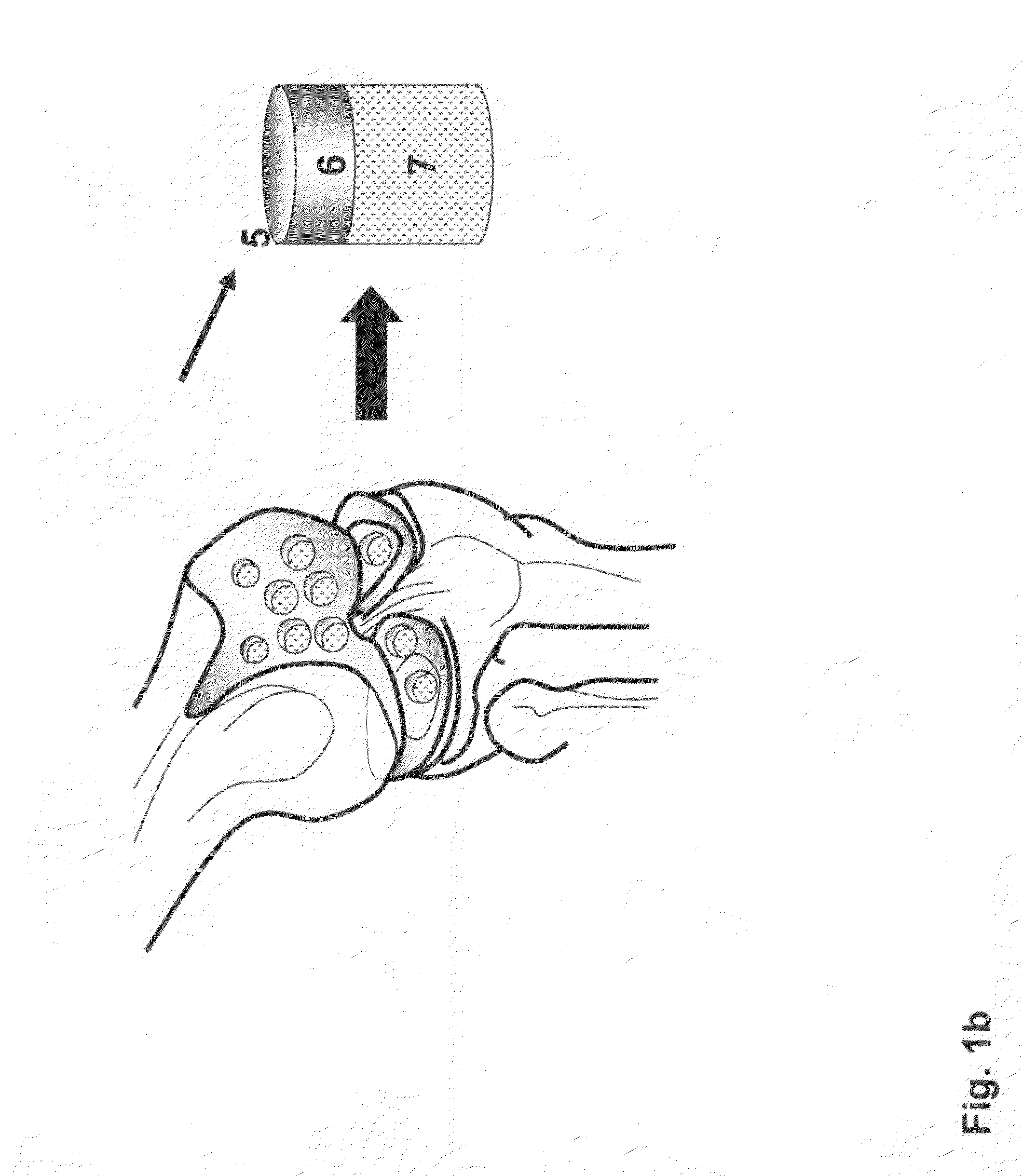
Repairs of cartilage defects or of cartilage / bone defects in human or animal joints with the help of devices including a bone part (1), a cartilage layer (2) and a subchondral bone plate (4) or an imitation of such a plate in the transition region between the cartilage layer (2) and the bone part (1). After implantation, the bone part (4) is resorbed and is replaced by reparative tissue only after being essentially totally resorbed. In a critical phase of the healing process, a mechanically inferior cyst is located in the place of the implanted bone part (1). In order to prevent the cartilage layer (2) from sinking into the cyst space during this critical phase of the healing process the device has a top part (11) and a bottom part (12), wherein the top part (11) consists essentially of the cartilage layer (2) and the subchondral bone plate (4) and the bottom part (12) corresponds essentially to the bone part (1) and wherein the top part (11) parallel to the subchondral bone plate (4) has a larger diameter than the bottom part (12). After implantation in a suitable opening or bore (20), the cartilage layer (2) and the subchondral bone plate (4) are supported not only on the bone part (1) but also on native bone tissue having a loading capacity not changing during the healing process. Therefore, the implanted cartilage layer cannot sink during the healing process.
Owner:ZIMMER GMBH
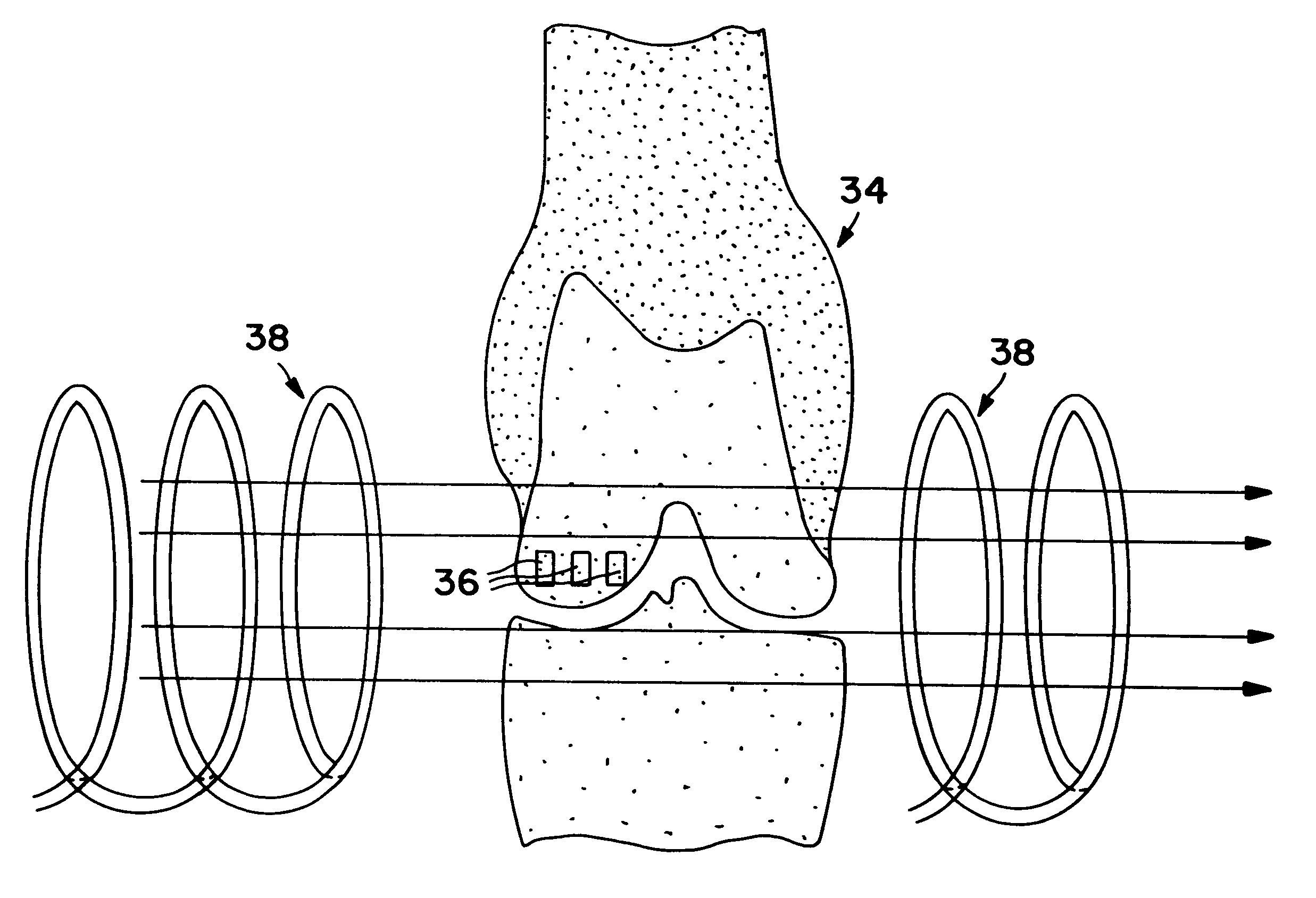
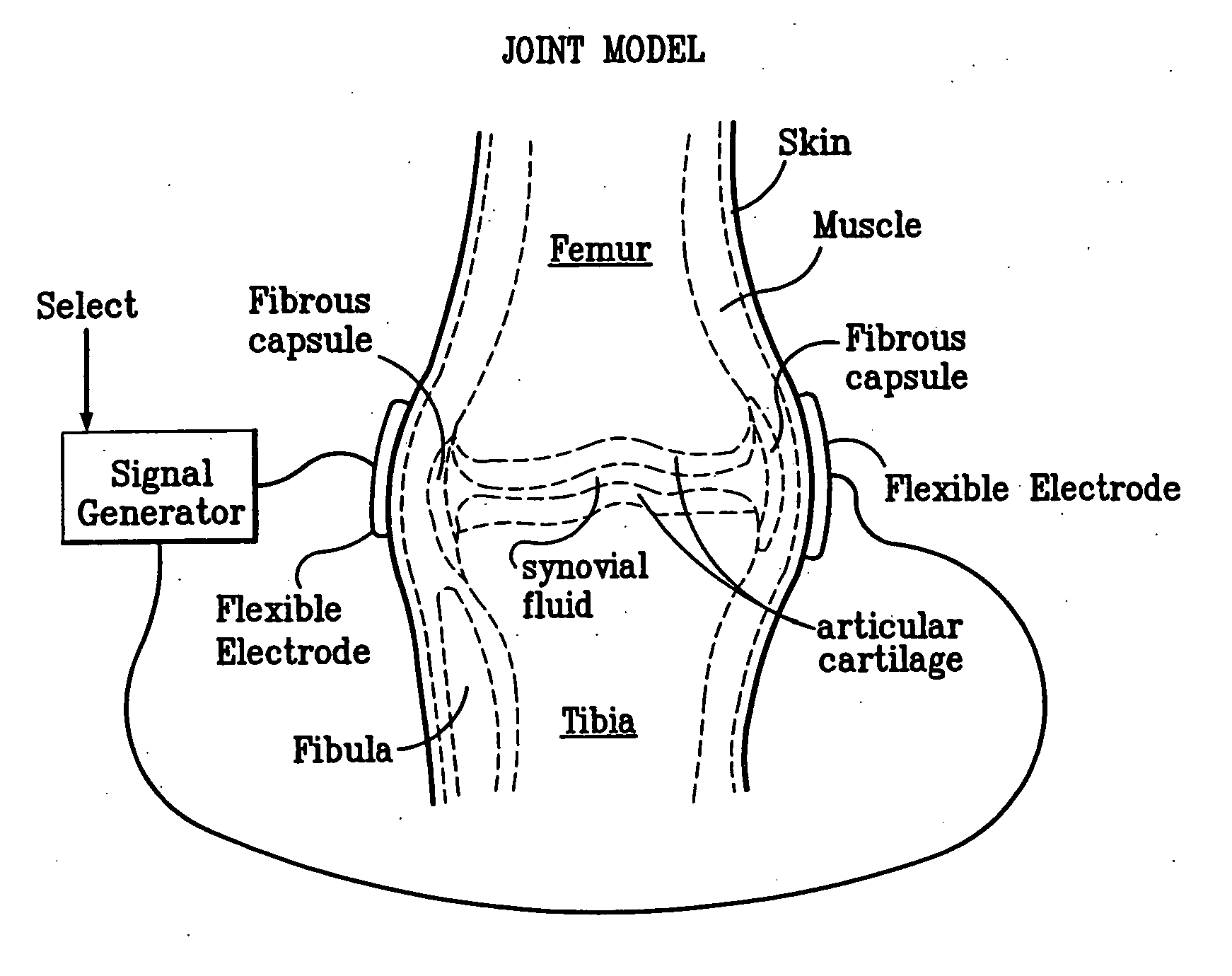
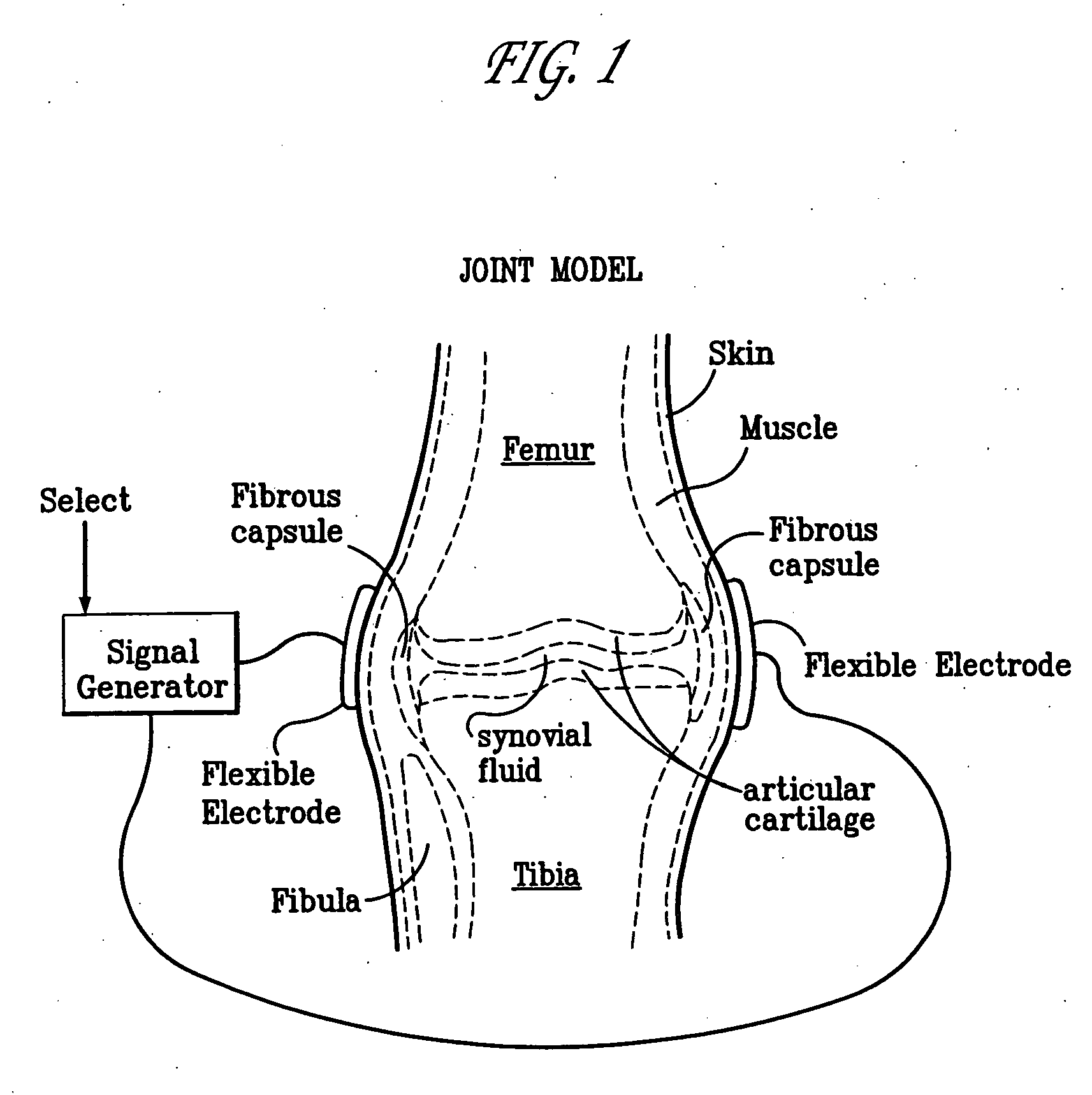
Method and device for treating osteoarthritis, cartilage disease, defects and injuries in the human knee
InactiveUS7022506B2Heart defibrillatorsMagnetotherapy using coils/electromagnetsHuman useCells transplantation
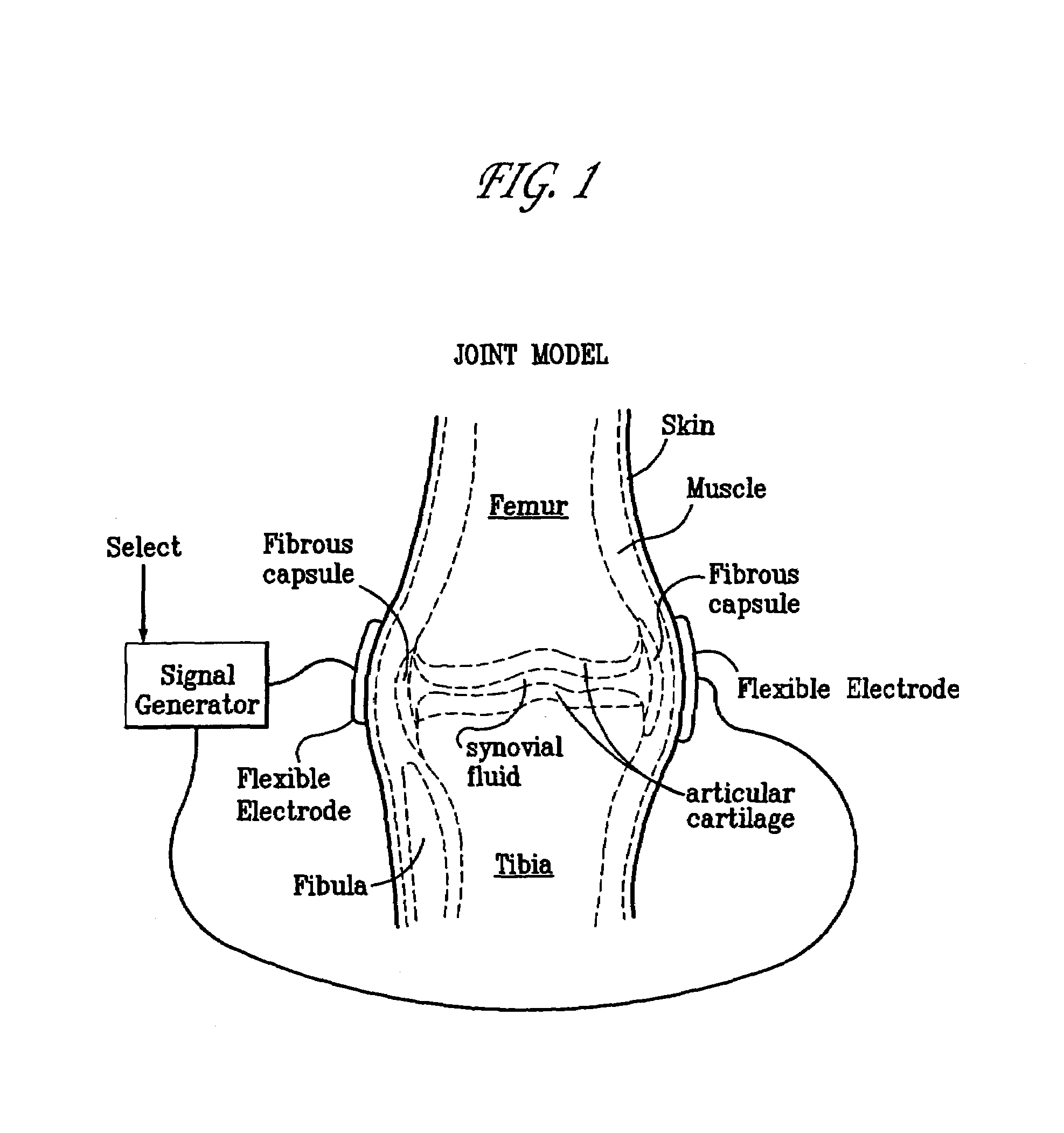
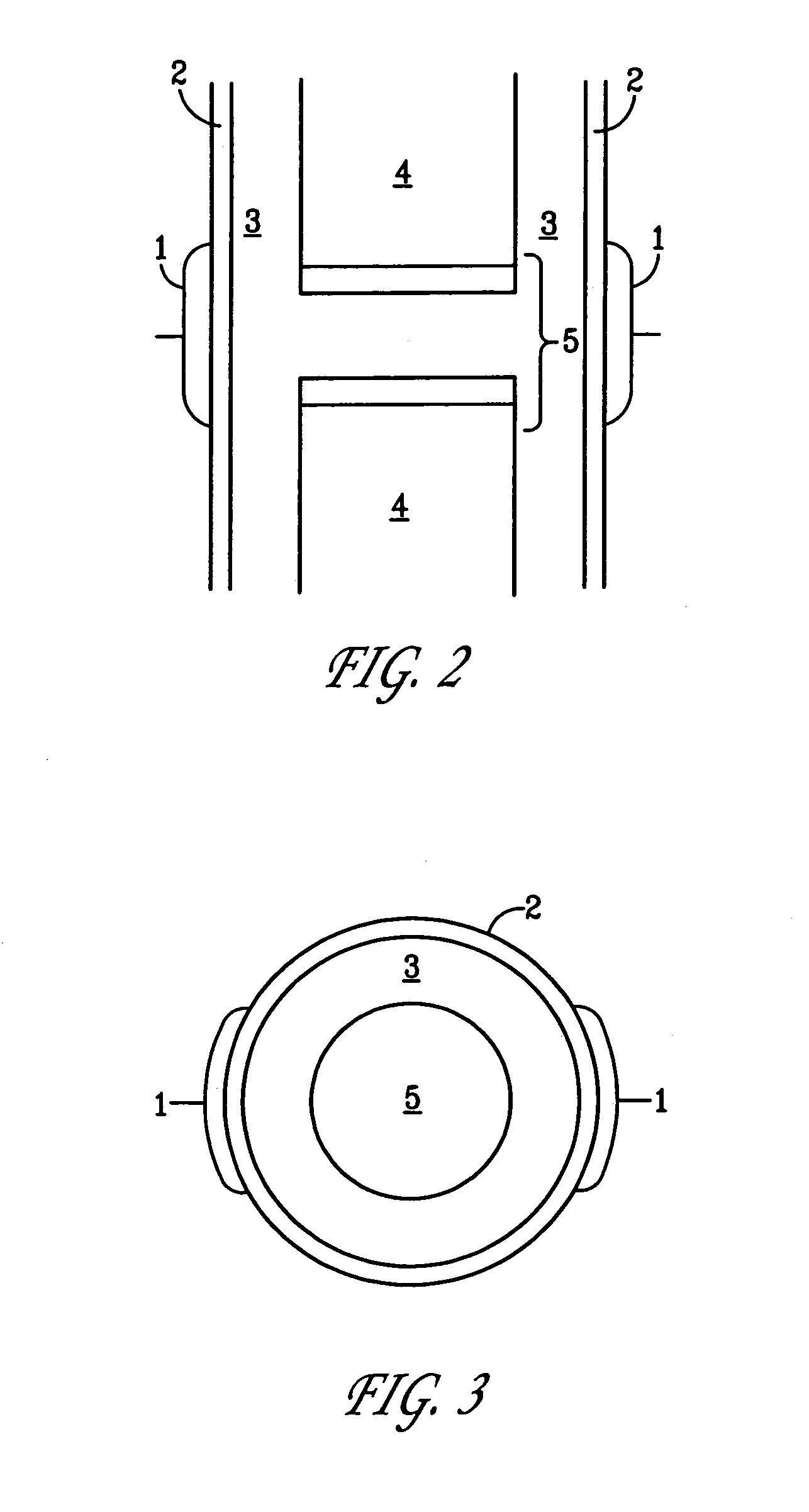
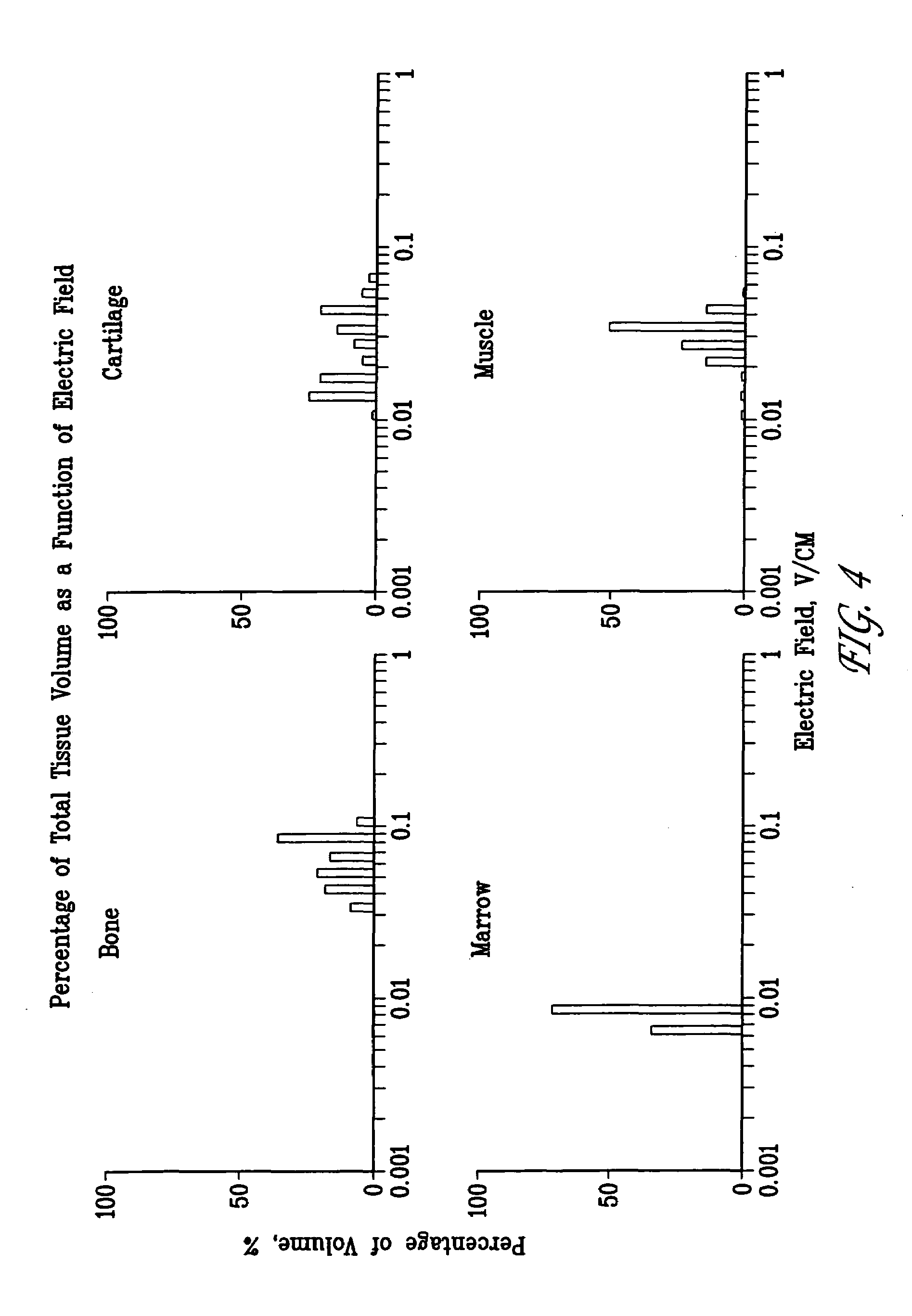
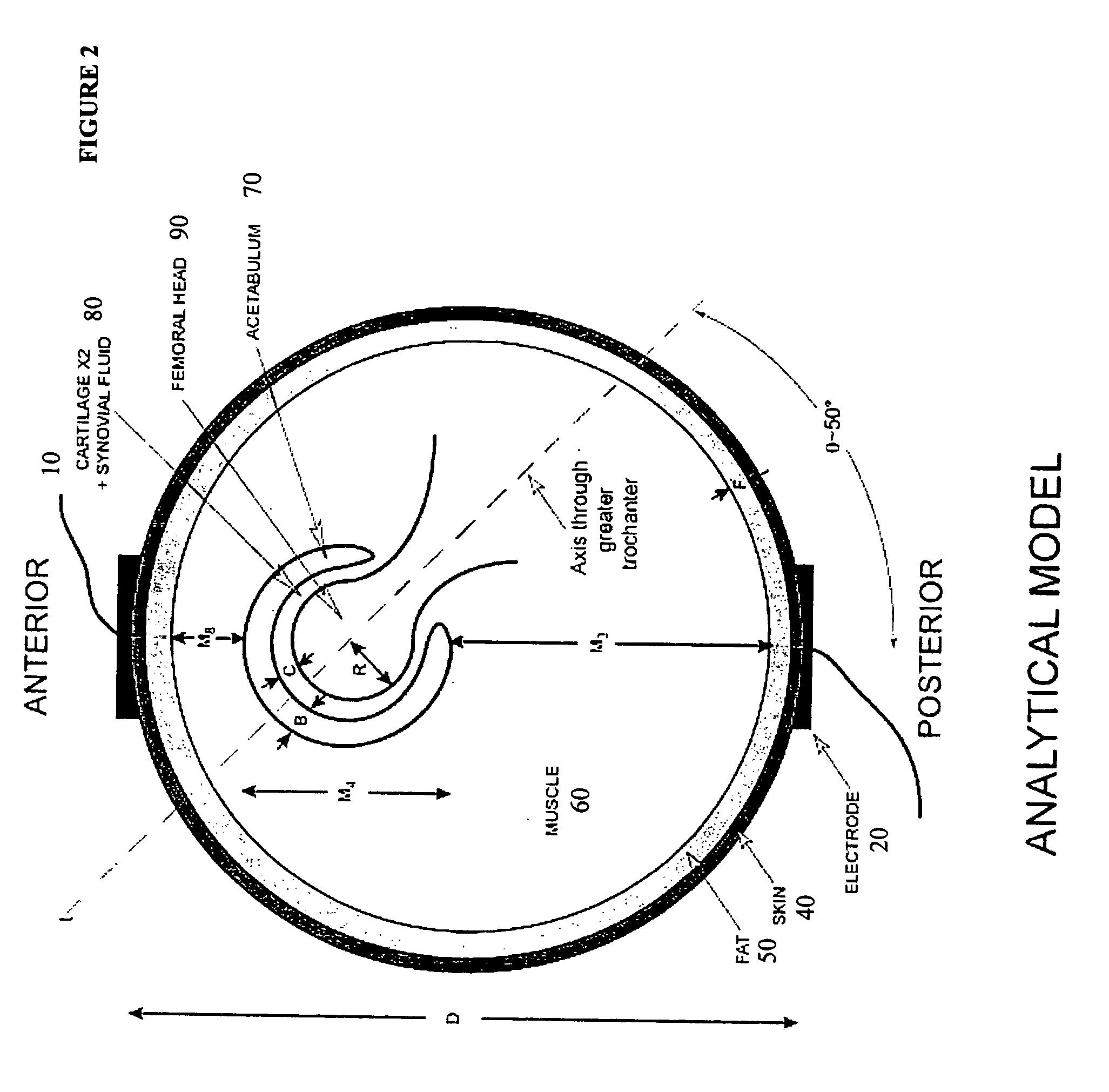
A method of determining the voltage and current output required for the application of specific and selective electric and electromagnetic signals to diseased articular cartilage in the treatment of osteoarthritis, cartilage defects due to trauma or sports injury, or used as an adjunct with other therapies (cell transplantation, tissue-engineered scaffolds, growth factors, etc.) for treating cartilage defects in the human knee joint and a device for delivering such signals to a patient's knee. An analytical model of the human knee is developed whereby the total tissue volume in the human knee may be determined for comparison to the total tissue volume of the diseased tissue in the animal model using electric field and current density histograms. The voltage and current output used in the animal model is scaled based on the ratio of the total tissue volume of the diseased tissue of the human to the total tissue volume of the diseased tissue in the animal model and the resulting field is applied to the diseased tissue of the human using at least two electrodes applied to the knee or a coil or solenoid placed around the knee. The voltage of the signal applied to the electrodes, coil or solenoid is varied based on the size of the knee joint; larger knee joints require larger voltages to generate the effective electric field.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Cartilage allograft plug
InactiveUS7901457B2Promote migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
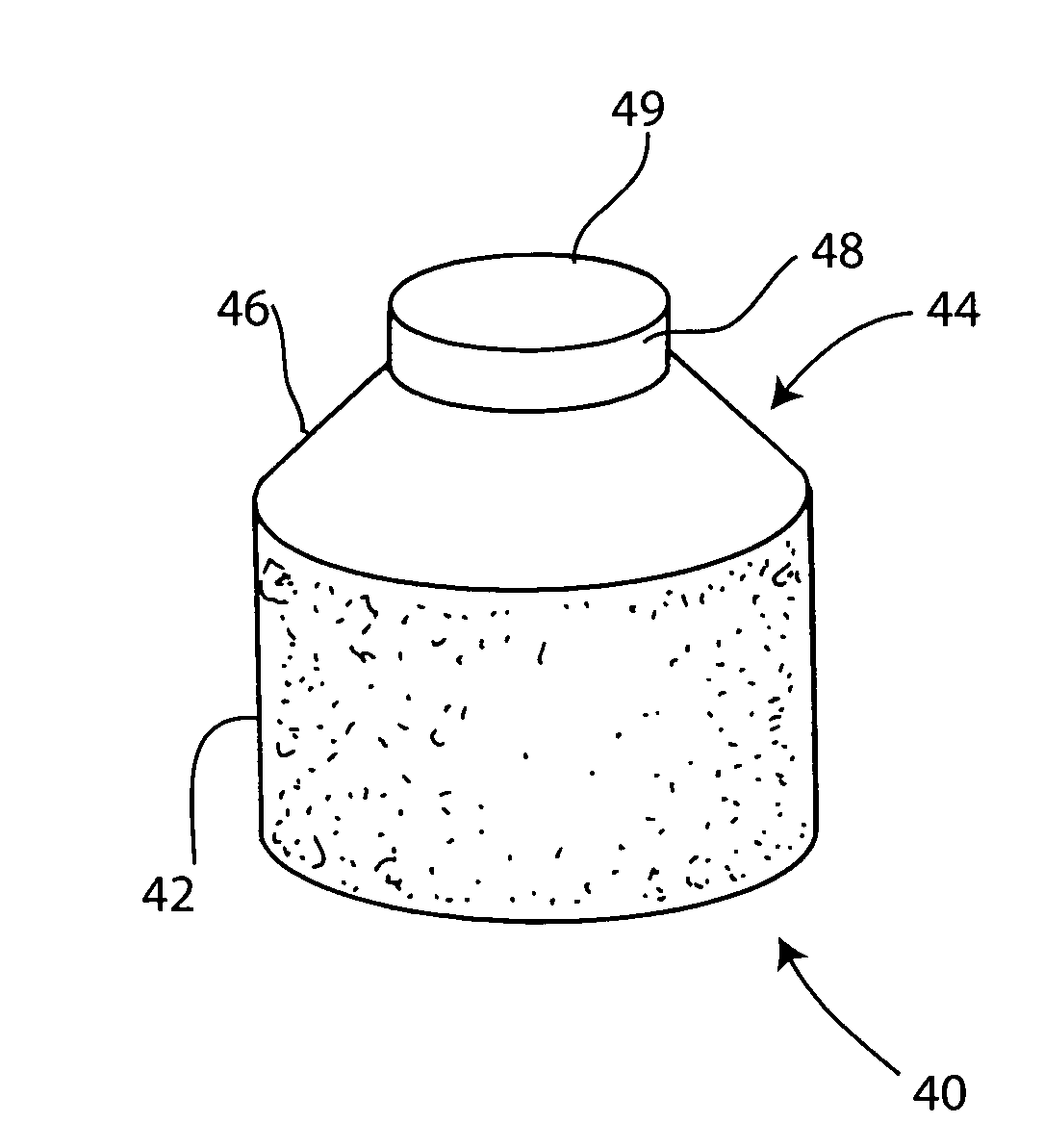
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Cartilage allograft plug
InactiveUS7488348B2Guaranteed functionEasily placed in defect areaBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Implantation of micronized allograft tissue over a microfractured defect
Techniques, mixtures, mixing and delivery kits, and improved delivery instruments for implantation of micronized allograft tissue over a microfractured defect. Allograft cartilage tissue is delivered over a cartilage defect that has been debrided and microfractured, without the need for a periosteal covering or separate type of patch sewn over the top. The allograft tissue may be any micronized cartilage particulates obtained by various methods, for example, cartilage delivered in its native form, dehydrated via lyophilization, “freeze-dried,” dehydrated via desiccation, or dehydrated by any other method.
Owner:ARTHREX
Means for cartilage repair
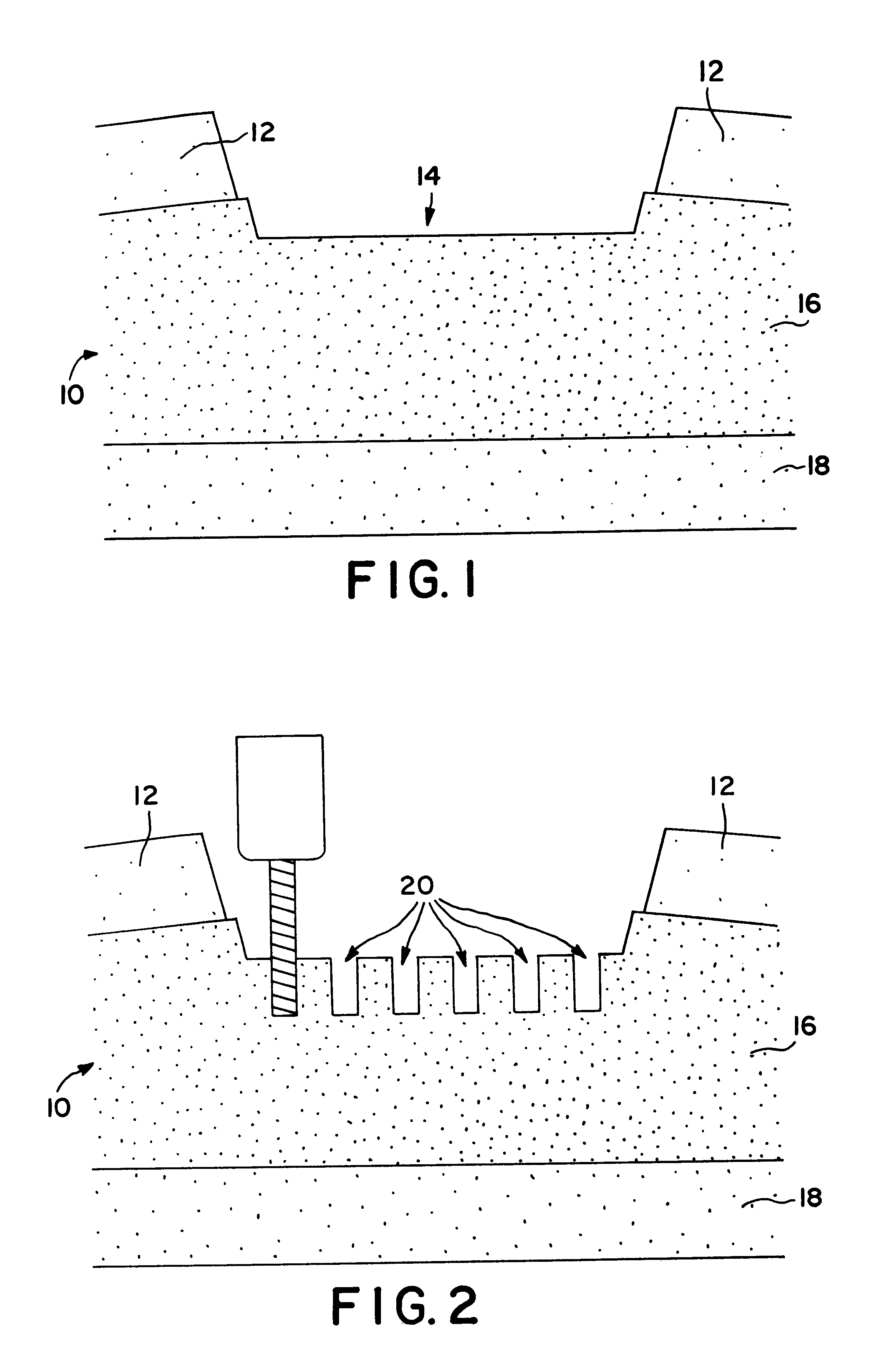
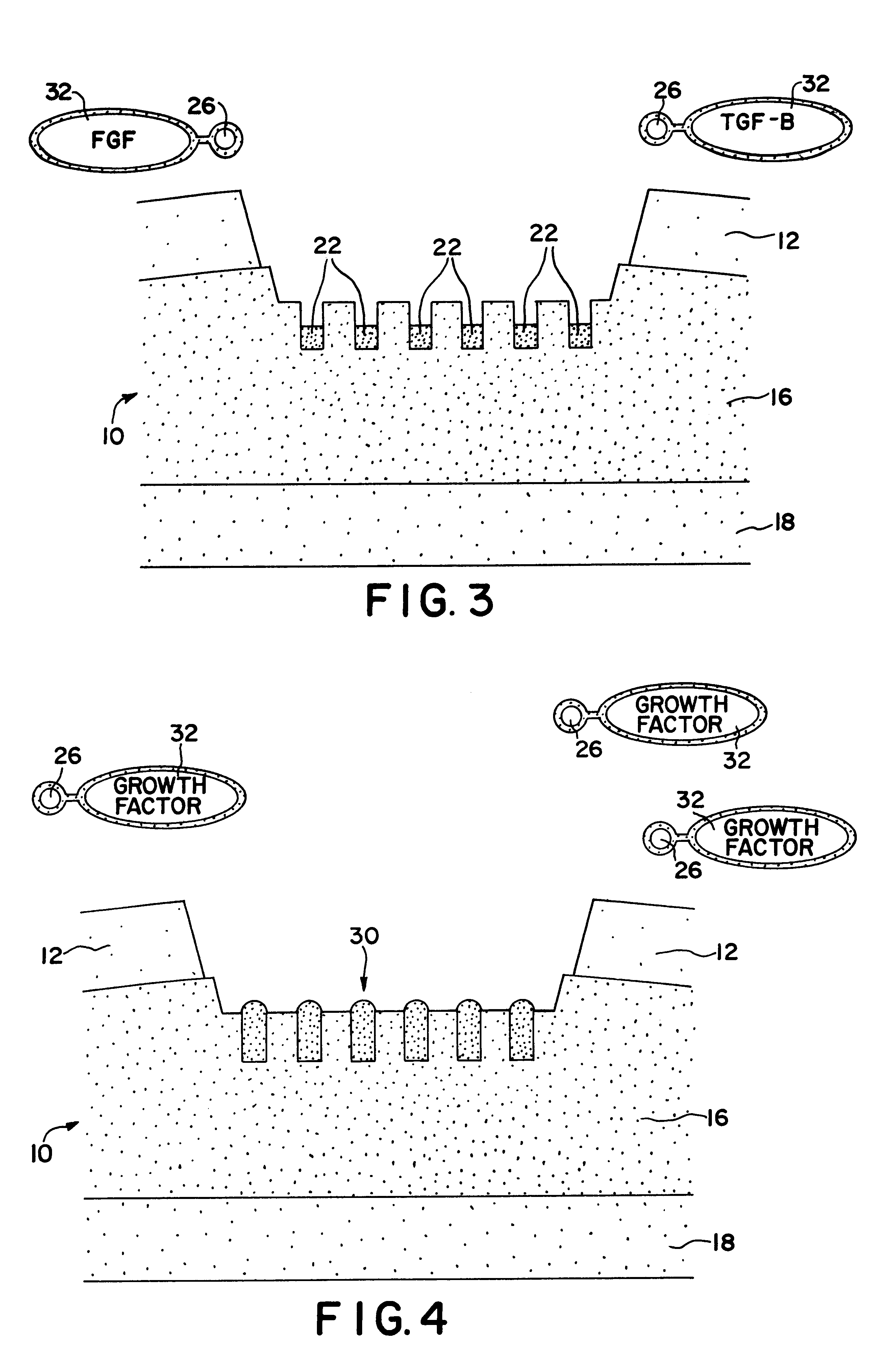
InactiveUS6179871B1Minimize extraneous effectEvenly spacedPowder deliveryElectrotherapyChondral defectGrowth promoting
A method for repairing a defect in cartilage, comprising the provision of apertures in the cartilage by drilling holes at the base of the cartilage defect, which holes may enter the mesenchymal depot, introducing a porous scaffold material containing a plurality of magnetic particles into the apertures, and subsequently and sequentially injecting magnetically-tagged cartilage growth promoting materials such as various growth factors or chondrocytes into the area of the defect. The magnetically tagged growth promoting material is then drawn into the apertures by magnetic attraction of the magnetic particles contained in the porous scaffold material, either by virtue of the particles being permanently magnetic, or by the imposition of an external magnetic field. The present application claims the biodegradable porous scaffold material containing the plurality of magnetic particles.
Owner:HALPERN ALAN A
Method and device for treating osteoarthritis, cartilage disease, defects and injuries in the human knee
InactiveUS20060190043A1ElectrotherapyMagnetotherapy using coils/electromagnetsHuman useArticular cartilage
A method of determining the voltage and current output required for the application of specific and selective electric and electromagnetic signals to diseased articular cartilage in the treatment of osteoarthritis, cartilage defects due to trauma or sports injury, or used as an adjunct with other therapies (cell transplantation, tissue-engineered scaffolds, growth factors, etc.) for treating cartilage defects in the human knee joint and a device for delivering such signals to a patient's knee. An analytical model of the human knee is developed whereby the total tissue volume in the human knee may be determined for comparison to the total tissue volume of the diseased tissue in the animal model using electric field and current density histograms. The voltage and current output used in the animal model is scaled based on the ratio of the total tissue volume of the diseased tissue of the human to the total tissue volume of the diseased tissue in the animal model and the resulting field is applied to the diseased tissue of the human using at least two electrodes applied to the knee or a coil or solenoid placed around the knee. The voltage of the signal applied to the electrodes, coil or solenoid is varied based on the size of the knee joint; larger knee joints require larger voltages to generate the effective electric field.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
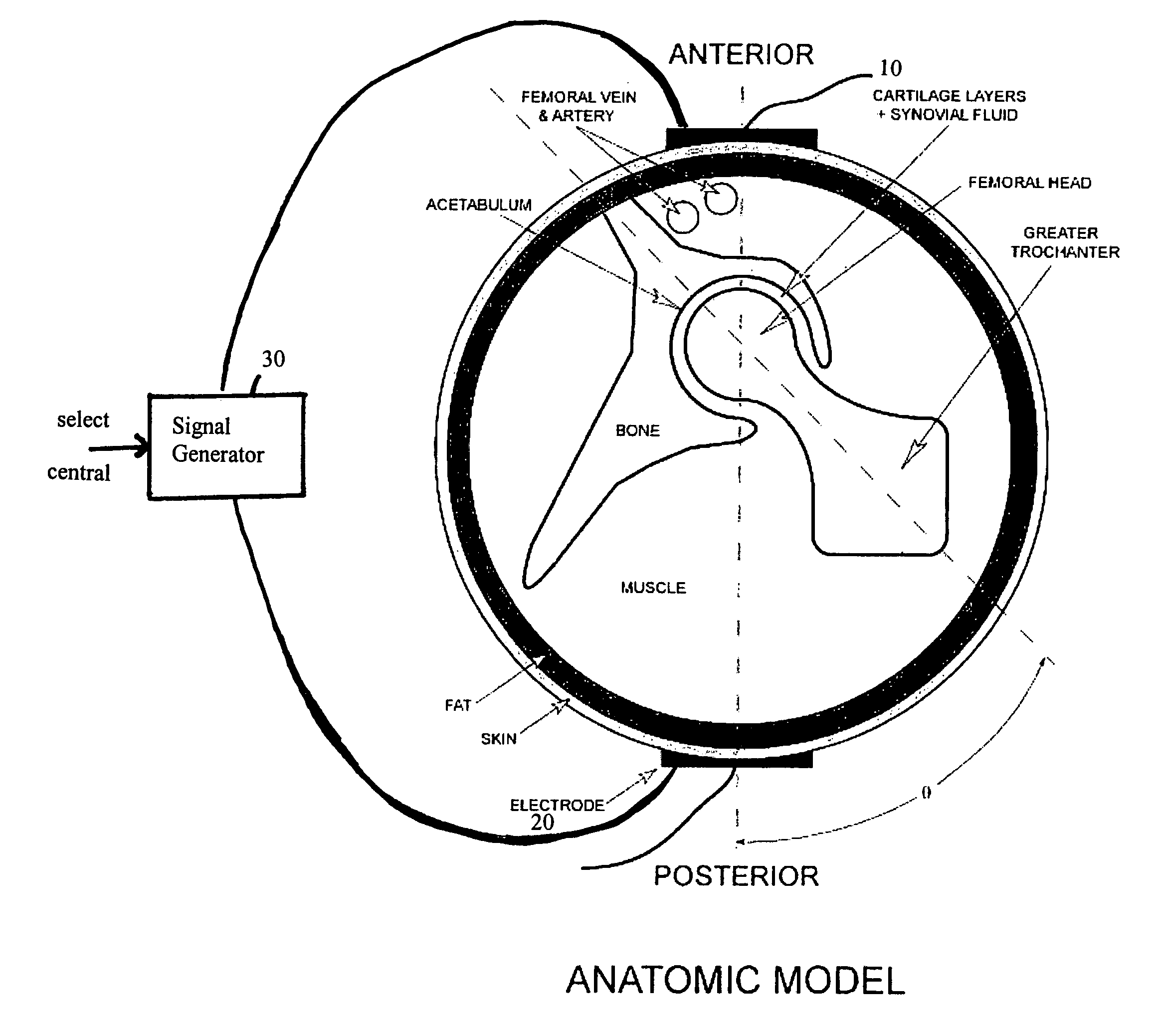
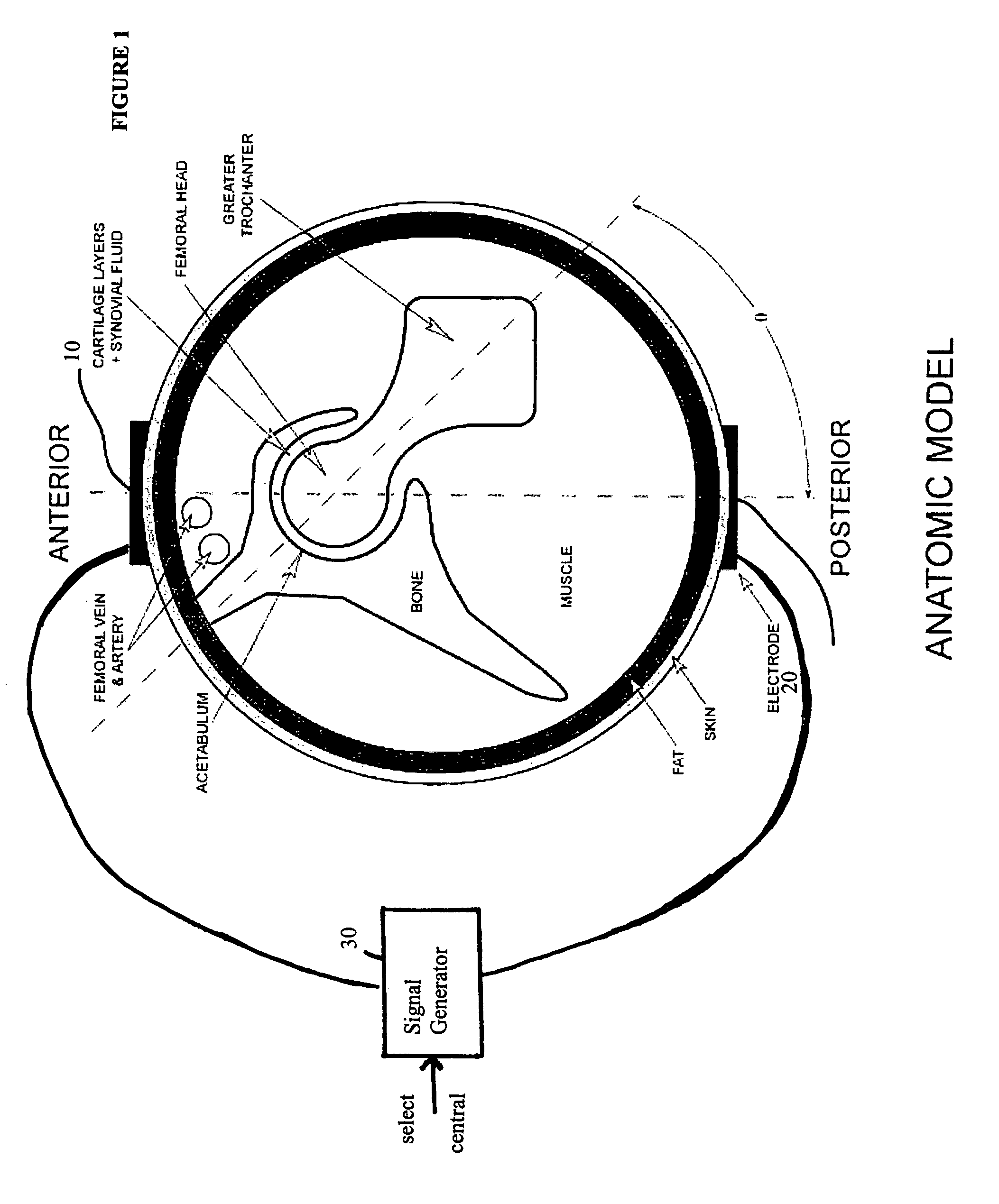
Method and device for treating osteoarthritis and cartilage disease, defects, and injuries in the human hip
A method of determining the voltage and current required for the application of specific and selective electric and electromagnetic signals to diseased articular cartilage in the treatment of osteoarthritis, cartilage defects due to trauma or sports injury, or used as an adjunct with other therapies (cell transplantation, tissue-engineered scaffold, growth factors, etc.) for treating cartilage defects in the human hip joint and a device for delivering such signals to a patient's hip. Anatomic, analytical, and planar circuit models are developed to determining the impedances, conductivities, and current flows in the human hip joint and its surrounding soft tissues and skin that are required to produce a 20 mV / cm electric field in the synovium and articular cartilage of the human hip. The voltage of the signal applied to the surface electrodes or to a coil(s) or solenoid is varied based on the size of the hip joint; larger hip joints require larger voltages to generate the effective electric field.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
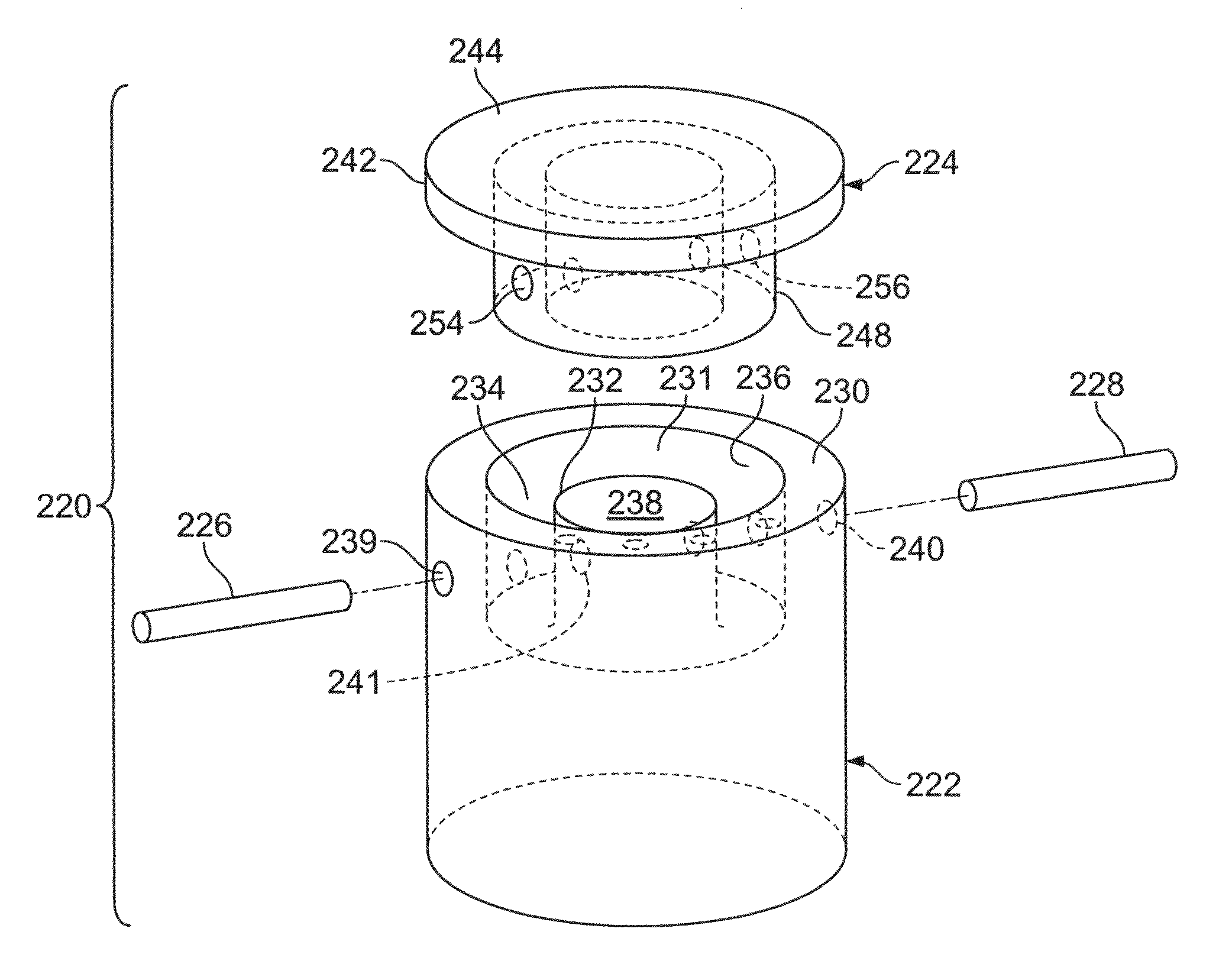
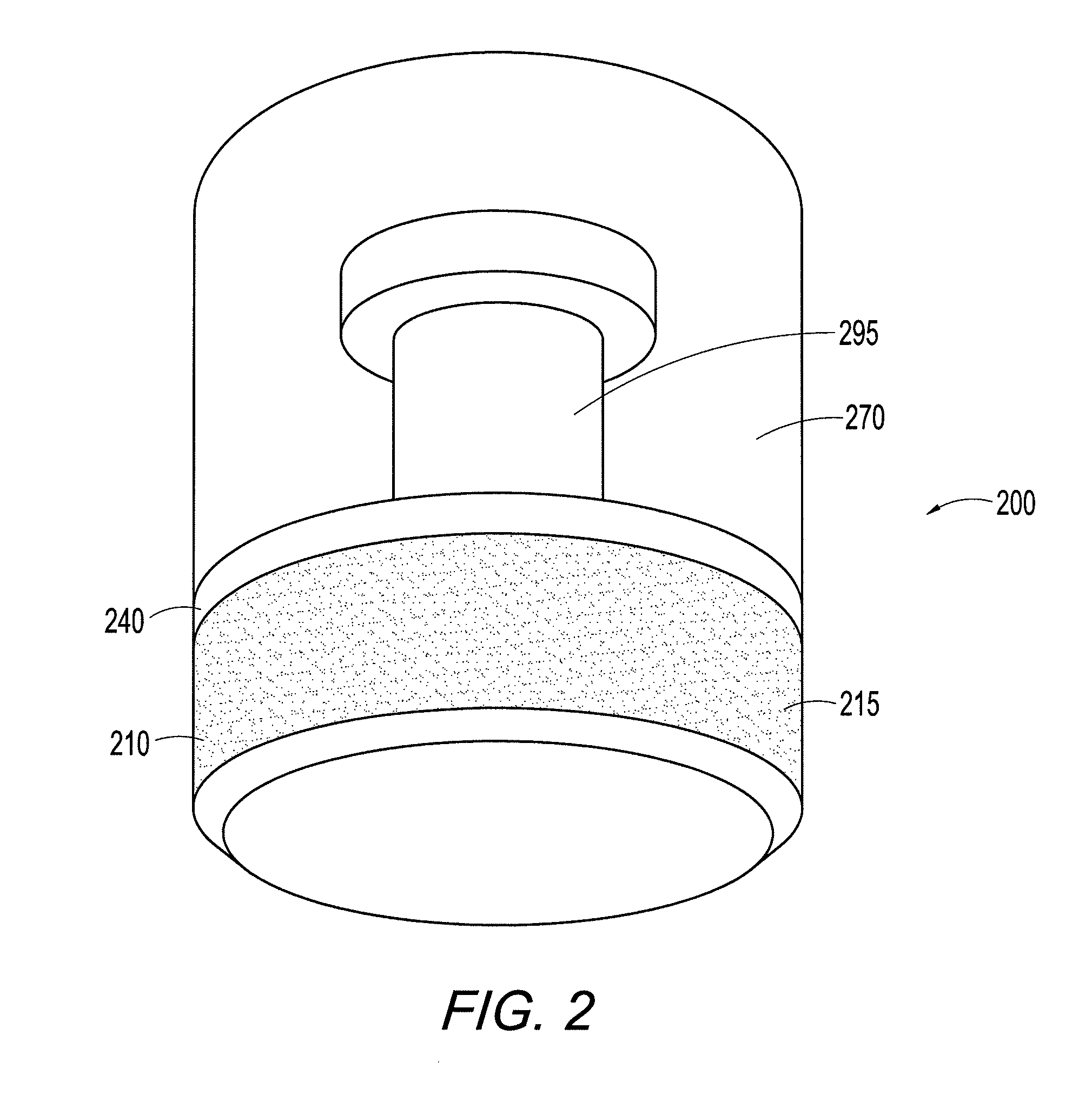
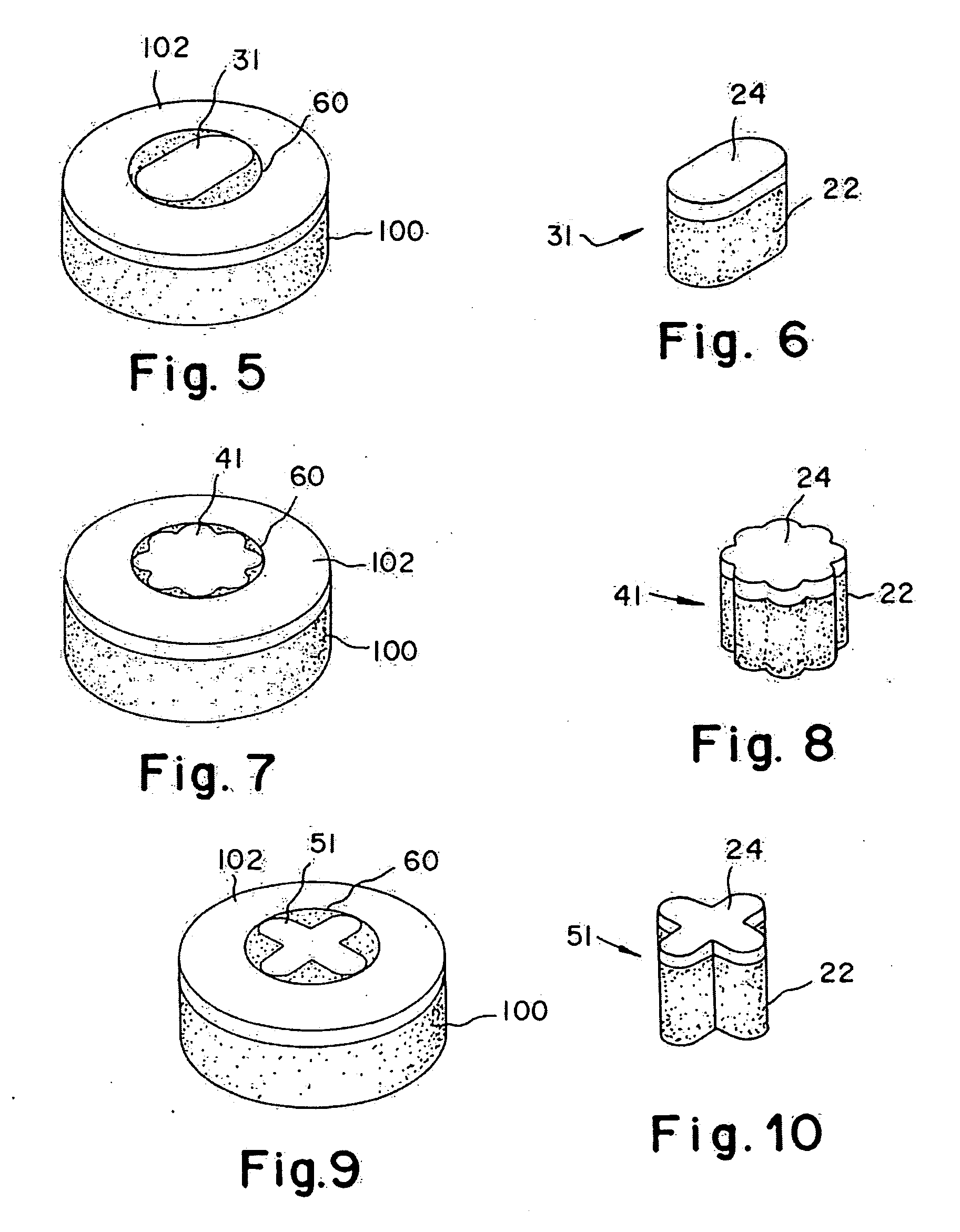
Two piece cancellous construct for cartilage repair
ActiveUS7837740B2Guaranteed functionEasily placed in defect areaBone implantTissue regenerationInterference fitChondral defect
The invention is directed toward a cartilage repair assembly comprising a shaped allograft two piece construct with a demineralized cancellous cap and a mineralized cylindrical base member defining a blind bore with a through going transverse bore intersecting the blind bore. The demineralized cancellous cap has a cylindrical top portion and a smaller diameter cylindrical stem extending away from the top portion which fits into the blind bore of the mineralized base member. The cap stem defines a transverse through going bore which is aligned with the through going bore of the base member to receive a cylindrical cortical pin holding the cap within the base member. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the assembly engages the side wall of the drilled bore in an interference fit.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
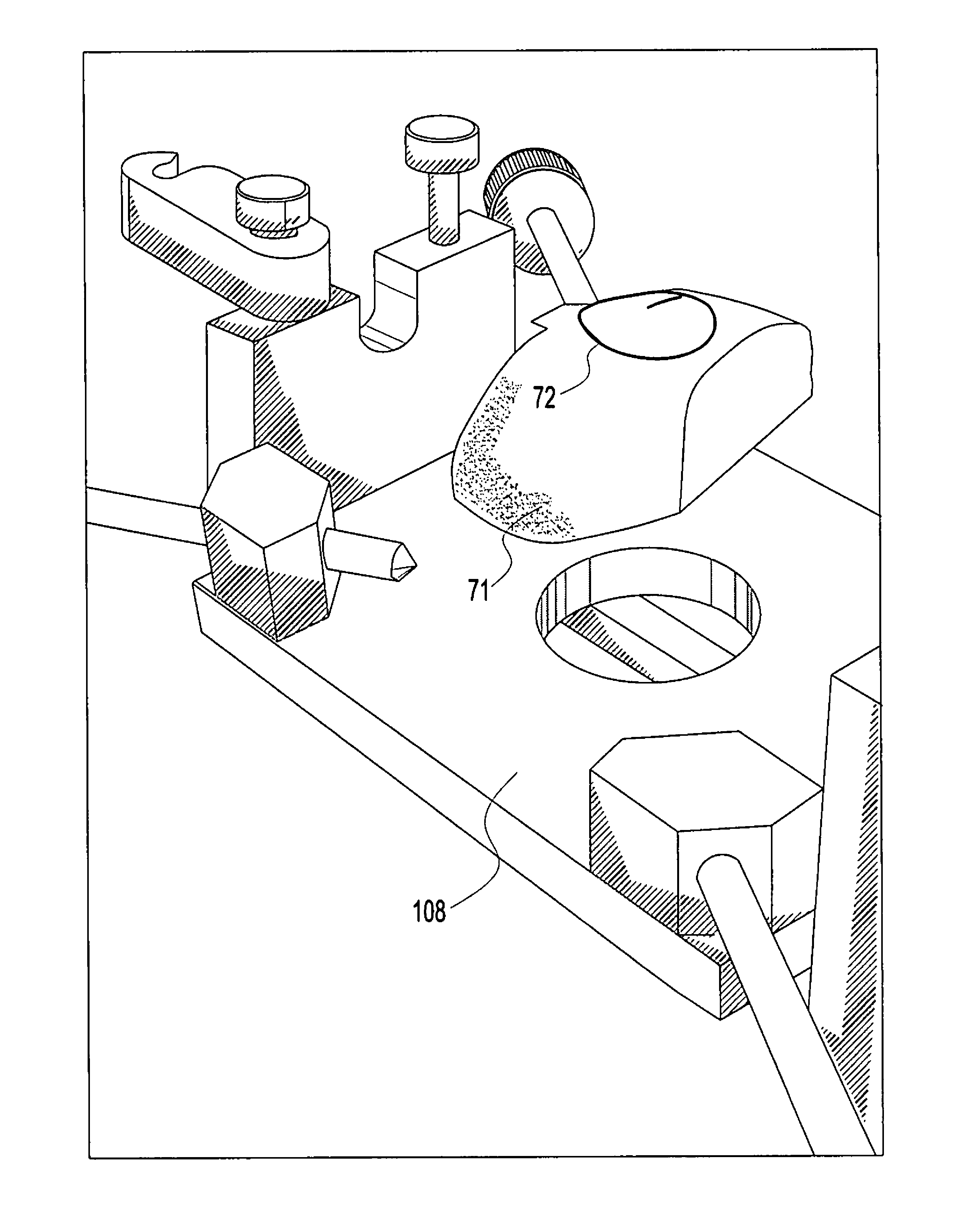
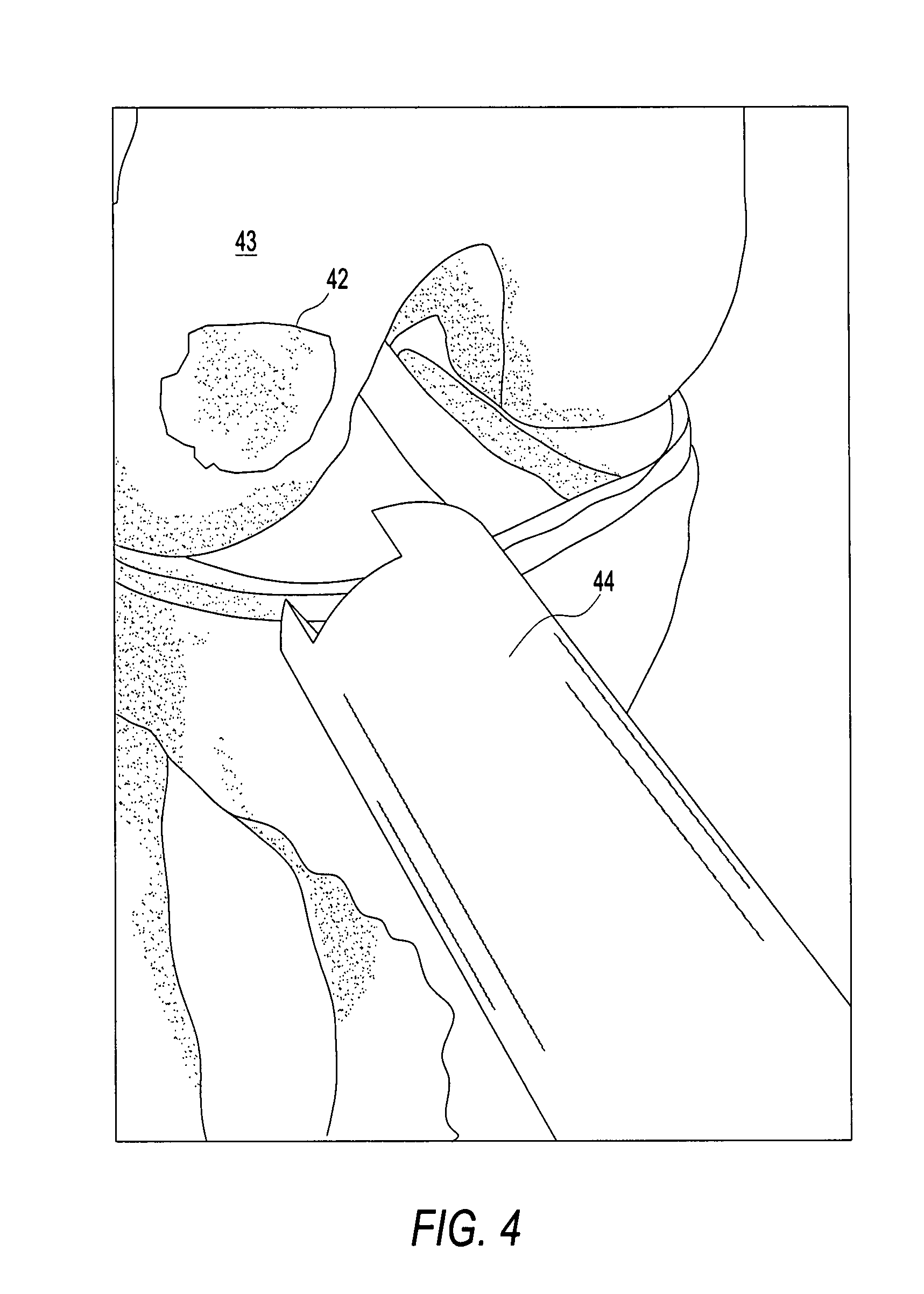
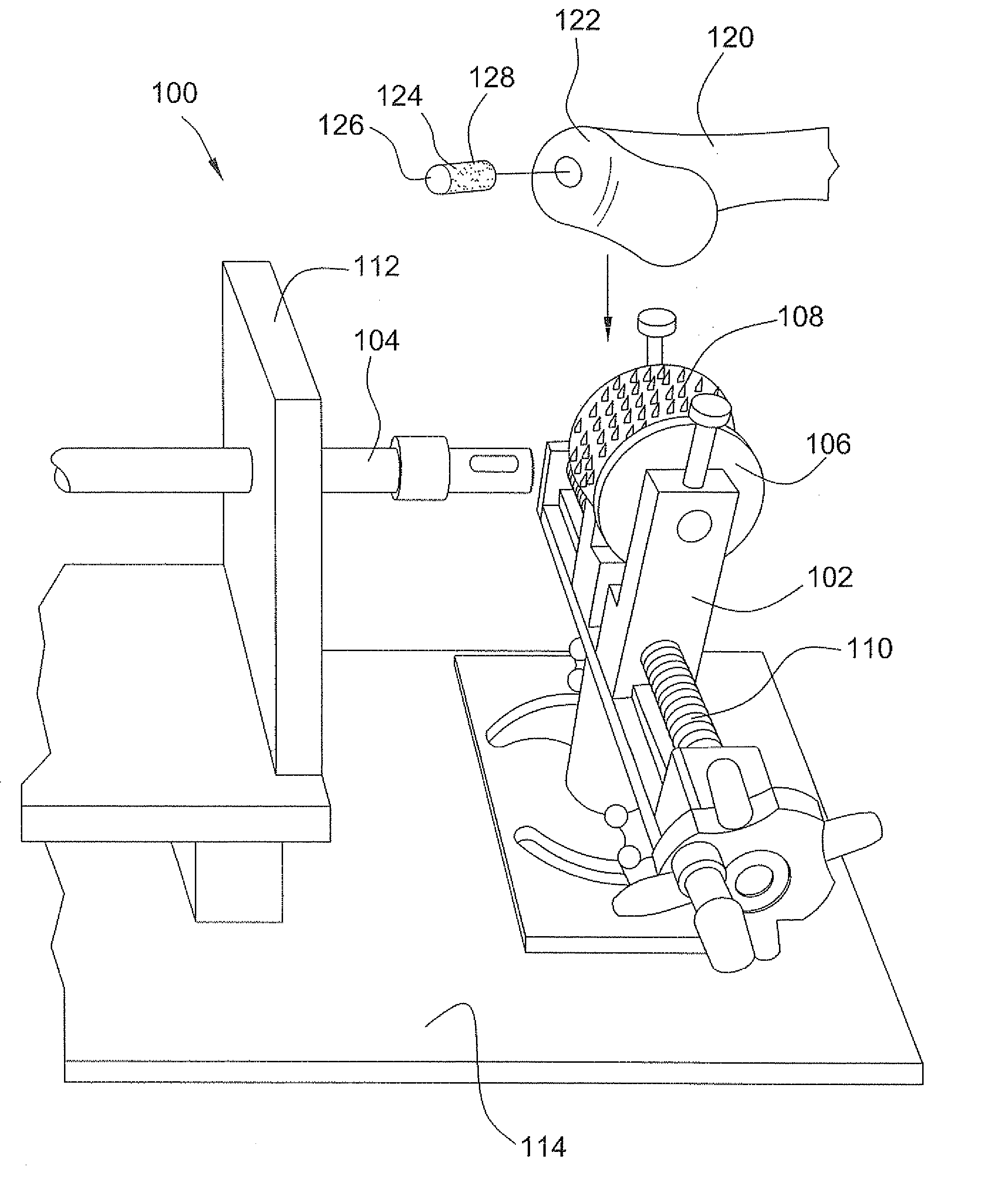
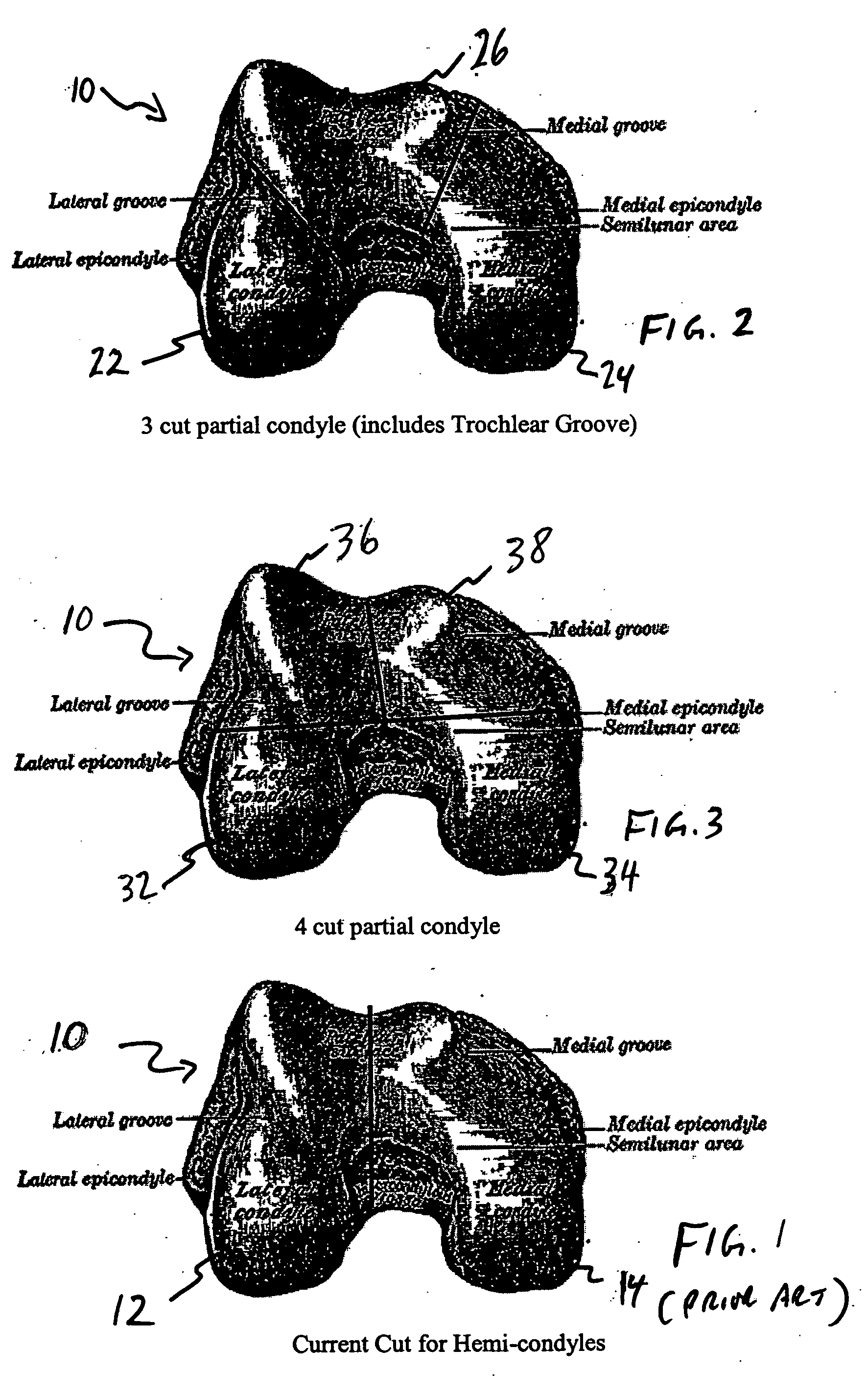
Osteochondral repair using plug fashioned from partial distal allograft femur or condyle
A method and apparatus for repairing isolated chondral defects using allograft implants. Lesions in articular tissue are corrected by forming a recipient socket in the tissue. A donor graft of a size corresponding to the recipient socket is harvested from a partial tissue specimen obtained from allograft material. The donor graft is implanted into the recipient socket.
Owner:ARTHREX
Instrumentation for the preparation and transplantation of osteochondral allografts
ActiveUS20070135918A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is forined corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Cartilage allograft plug
ActiveUS20090069901A1Increase chondrocyte migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles
Constructs that are at least partially constructed of allograft cancellous bone are disclosed, along with cartilage particles that may be used with the constructs for repairing articular cartilage defects. A multi-piece construct includes a base member, a cap member and at least one pin that secures the cap member to the base member. The base member may be constructed of mineralized cancellous bone, and is used to replace the subchondral bone removed when a surgeon cuts a bore in the area of an adjacent cartilage defect. The base member includes a blind bore and first and second through-going transverse bores in opposite sides of a wall of the base member. The cap member includes an upper section that has a thickness that is similar to that of a patient's surrounding articular cartilage layer and a stem depending from the upper section that is dimensioned to be received in and by the blind bore of the base member. The stem includes a transverse through-going bore, which may be aligned with the transverse through-going bores of the base member to receive the pin therein when the construct has been assembled. The cap member is at least partially formed of demineralized allograft cancellous bone, into which a mixture containing lyophilized, freeze-milled allograft cartilage particles may be infused for the repair of articular cartilage defects. The cartilage particles have a size within a range of from about 10 microns to about 210 microns.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Instrumentation for the preparation and transplantation of osteochondral allografts
ActiveUS20070135917A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Osteogenic devices and methods of use thereof for repair of endochondral bone and osteochondral defects
InactiveUS20060177475A1Restore an osteochondral or a chondral defectInhibition formationImpression capsPeptide/protein ingredientsRepair tissueOsteogenic proteins
Disclosed herein are improved osteogenic devices and methods of use thereof for repair of bone and cartilage defects. The devices and methods promote accelerated formation of repair tissue with enhanced stability using less osteogenic protein than devices in the art. Defects susceptible to repair with the instant invention include, but are not limited to: critical size defects, non-critical size defects, non-union fractures, fractures, osteochondral defects, subchondral defects, and defects resulting from degenerative diseases such as osteochondritis dessicans.
Owner:MARIEL THERAPEUTICS
Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles
InactiveUS20090319045A1Enhancing chondrogenesisMitigating fibrous tissue formationBone implantSurgerySubchondral boneChondral defect
Constructs that are at least partially constructed of allograft cancellous bone are disclosed, along with cartilage particles that may be used with the constructs for repairing articular cartilage defects. A multi-piece construct includes a base member, a cap member and at least one pin that secures the cap member to the base member. The base member may be constructed of mineralized cancellous bone, and is used to replace the subchondral bone removed when a surgeon cuts a bore in the area of an adjacent cartilage defect. The base member includes a blind bore and first and second through-going transverse bores in opposite sides of a wall of the base member. The cap member includes an upper section that has a thickness that is similar to that of a patient's surrounding articular cartilage layer and a stem depending from the upper section that is dimensioned to be received in and by the blind bore of the base member. The stem includes a transverse through-going bore, which may be aligned with the transverse through-going bores of the base member to receive the pin therein when the construct has been assembled. The cap member is at least partially formed of demineralized allograft cancellous bone, into which a mixture containing lyophilized, freeze-milled allograft cartilage particles may be infused for the repair of articular cartilage defects. The cartilage particles have a size within a range of from about 10 microns to about 210 microns.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Crafting of cartilage
The invention is directed to producing a shaped cartilage matrix isolated from a human or animal where the cartilage has been crafted to facilitate disinfection, cleaning, devitalization, recellularization, and / or integration after implantation. The invention relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
Cartilage particle tissue mixtures optionally combined with a cancellous construct
Mixtures, such as gels or pastes, comprising freeze-milled cartilage particles and exogenous growth factors are used for repairing chondral defects. Such mixtures may be applied to constructs comprising cancellous bone for implantation at the defect site. Suitable growth factors include variants of FGF-2, particularly variants that include a sole amino acid substitution for asparagine at amino acid 111 of the β8-β9 loop of the FGF-2 peptide. Such FGF-2 variants are released slowly and continuously at a constant rate from cartilage pastes. In other embodiments, the amino acid substituted for asparigine is glycine. Other variants that may be used include FGF-9 variants having truncated chains and a sole amino acid substitution in the β8-β9 loop of the FGF-9 peptide either for tryptophan at amino acid 144 or for asparagine at amino acid 143.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
Osteochondral repair using plug fashioned from partial distal allograft femur or condyle
A method and apparatus for repairing isolated chondral defects using allograft implants. Lesions in articular tissue are corrected by forming a recipient socket in the tissue. A donor graft of a size corresponding to the recipient socket is harvested from a partial tissue specimen obtained from allograft material. The donor graft is implanted into the recipient socket.
Owner:ARTHREX
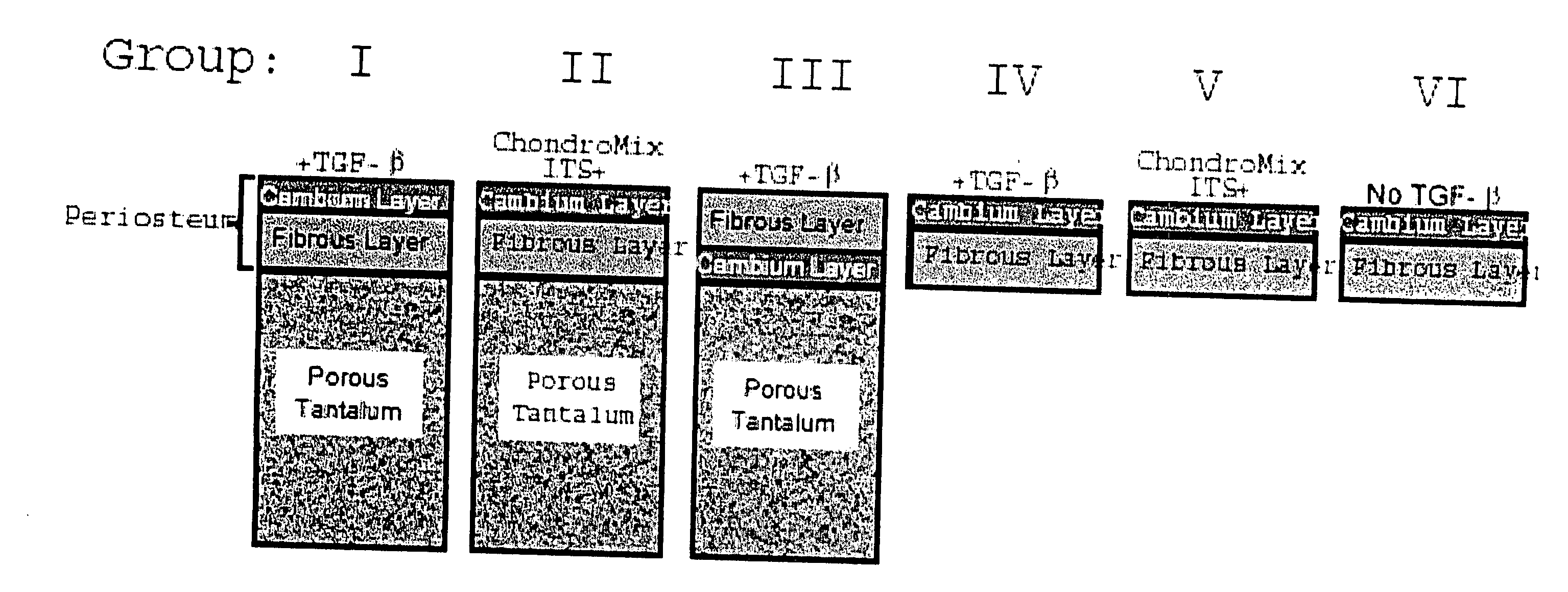
Biosynthetic composite for osteochondral defect repair
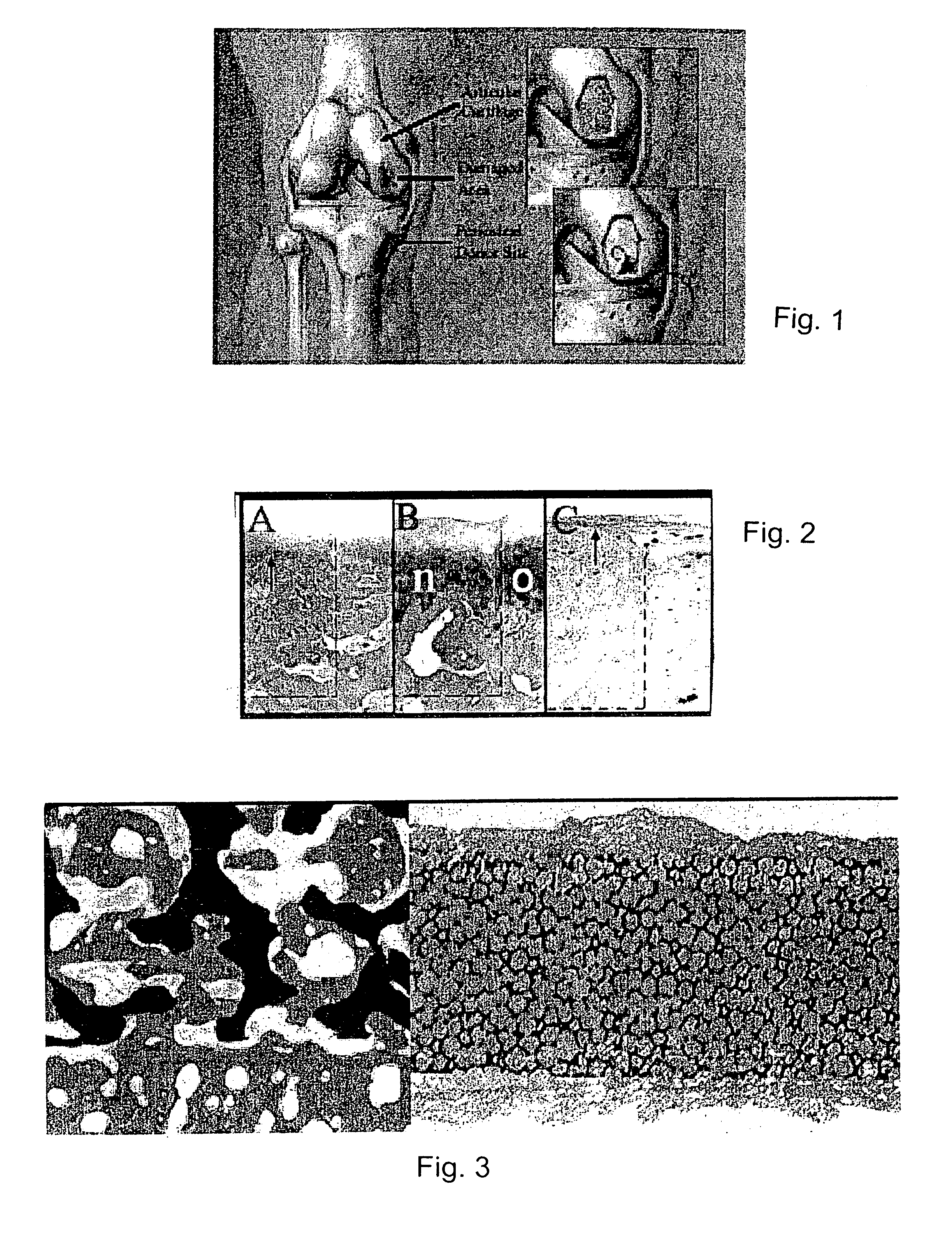
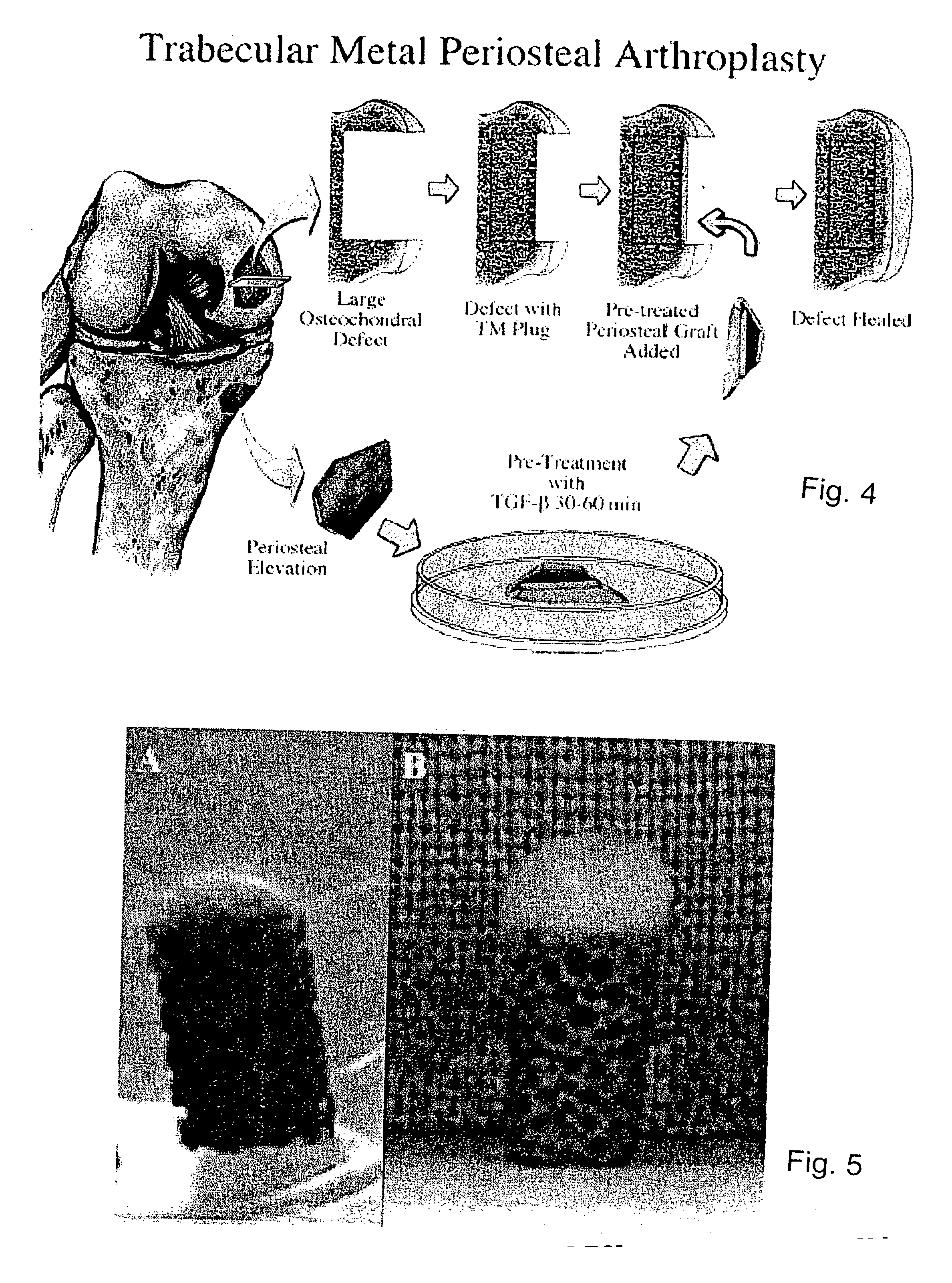
A composite for osteochondral defect repair includes a porous scaffold and a periosteal graft secured to a surface of the scaffold. The composite provides cartilage growth from autologous periosteum chondrogenesis. Biological resurfacing of large osteochondral defects, or a complete joint is feasible using the porous scaffold / autologous periosteal composite. The use of this composite eliminates the necessity of using normal cartilage surface as a donor site and its respective associated morbidity. In one form, the strong bone integration capacity of a porous metal (e.g., tantalum) scaffold and the high grade of integration observed from periosteal chondrogenesis into the normal cartilage eliminates the lack of chondral-chondral integration observed in the autologous osteochondral graft technique.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Osteochondral allografts
ActiveUS20070135928A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Hybrid polymer/metal plug for treating chondral defects
Osteochondral implants for repair of chondral defects and providing bone fixation through bone ongrowth and / or ingrowth. The implant is provided with a base allowing for bone ongrowth and / or ingrowth and a top attached to the base, the top being formed of a material having a compressive resistance similar to that of the cartilage. The material of the top is polycarbonate urethane, for example. The base may comprise a porous substrate for bony ingrowth formed of metal or PEEK and having a pattern porosity about similar to the porosity of cancellous bone. One side of the top attaches to the base for stability, and the other side of the top forms a surface for articulating with the opposing cartilage surface of the joint.
Owner:ARTHREX
Composition of cartilage therapeutic agents and its application
InactiveUS20070087032A1Large incisionLarge timePeptide/protein ingredientsSkeletal disorderSurgical operationKnee Joint
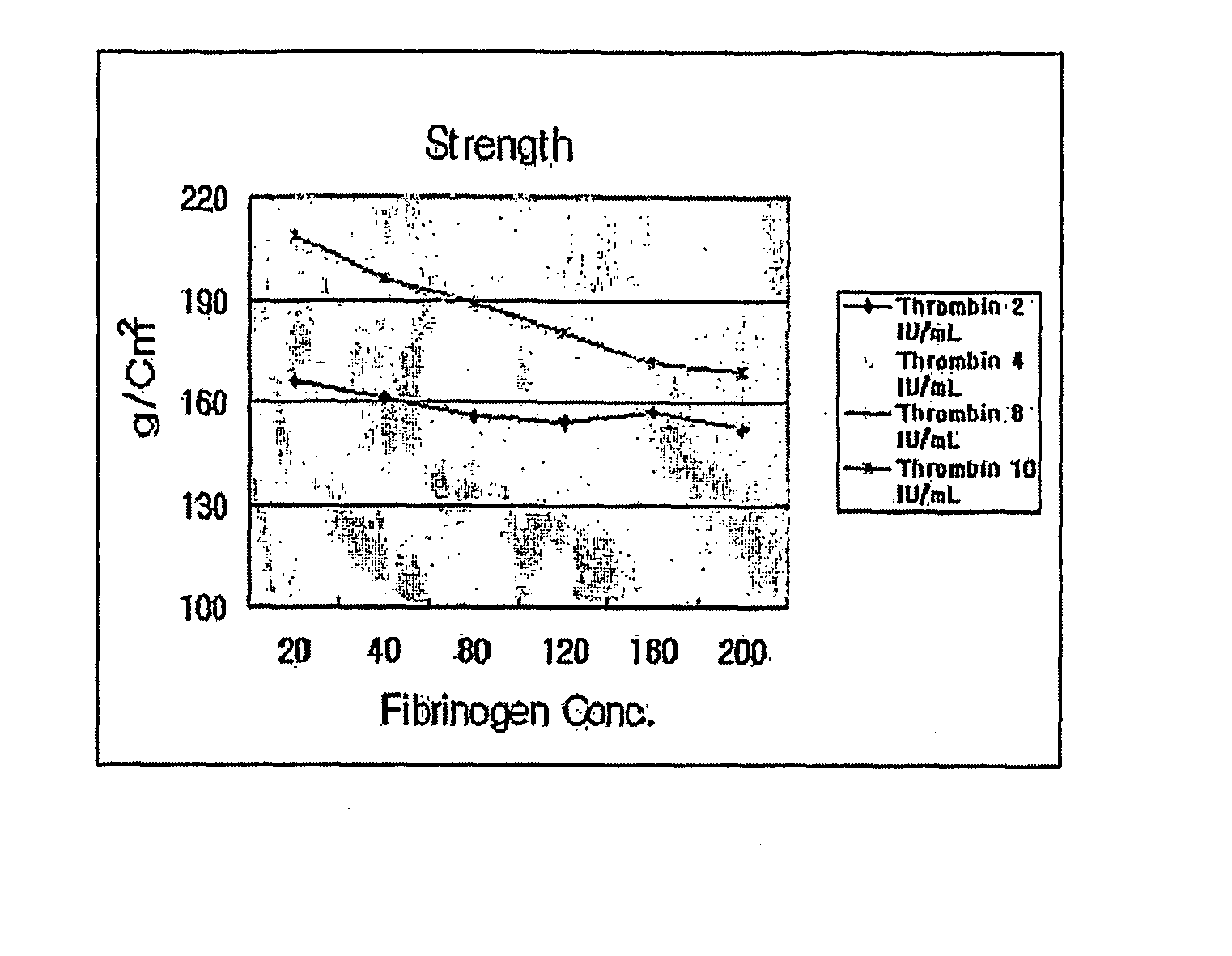
A cartilage therapeutic composition is developed for clinical transplantation into articulatio genu (knee joints) or ankle joints. It has clinical significance for symptomatic cartilage defects of the femoral condyle (medial, lateral, or trochlear) and bone cartilage defects of the talus (anklebone) in human or animal hosts, The cartilage therapeutic composition comprises a mixture of components of chondrocytes isolated and expanded or differentiated from a host such as a human or animal, and thrombin and a fibrinogen matrix containing fibrinogen. An application of the cartilage therapeutic composition is that a mixture of thrombin, chondrocyte components and a fibrinogen matrix is injected into a cartilage defect region followed by solidification therein. It provides rapid healing and effective regeneration of cartilage without surgical operation. It has the merits of safety and simplicity by allowing the use of an arthroscope for transplantation.
Owner:SEWON CELLONTECH CO LTD
Bioabsorbable implant of hyaluronic acid derivative for treatment of osteochondral and chondral defects
InactiveUS20070202084A1Speed up the repair processPromote cartilage regenerationBiocideOrganic active ingredientsChondral defectBiology
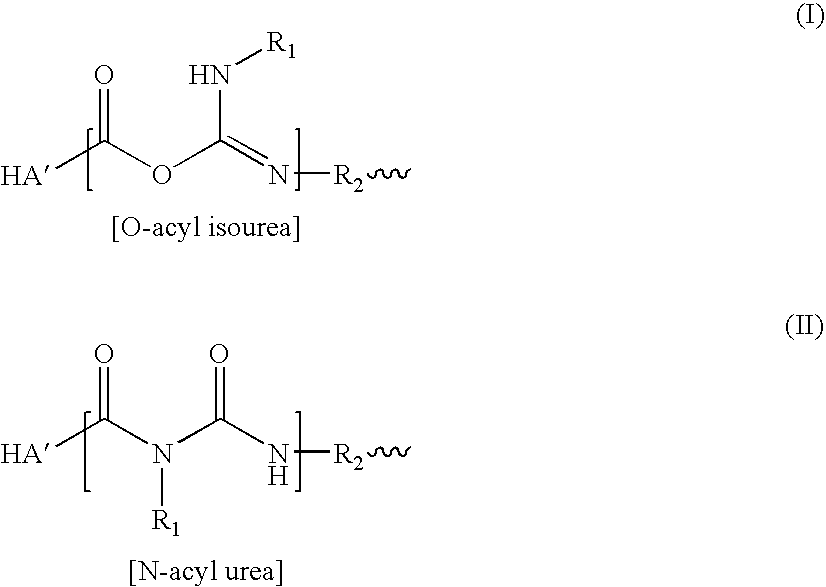
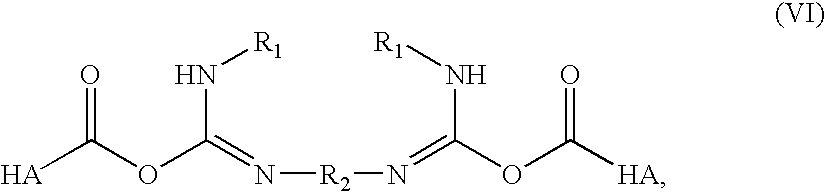
A method for treating an osteochondral defect or a chondral defect in a subject includes implanting a composite in a site of the osteochondral or chondral defect. The composite includes a hyaluronic acid derivative; and at least one member of the group consisting of a cell, a cellular growth factor and a cellular differentiation factor, which is impregnated in, or coupled to, the hyaluronic acid derivative. In one embodiment, carboxyl functionalities of the hyaluronic acid derivative are each independently derivatized to include an N-acylurea or O-acyl isourea, or both N-acylurea and O-acyl isourea. In another embodiment, the hyaluronic acid derivative is prepared by reacting an uncrosslinked hyaluronic acid with a biscarbodimide in the presence of a pH buffer in a range of between about 4 and about 8. The composite can be used for regenerating or stimulating regeneration of meniscal tissues in a subject in need thereof.
Owner:ANIKA THERAPEUTICS INC
Cartilage implant plug with fibrin glue and method for implantation
Owner:MASSACHUSETTS INST OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com