Bioabsorbable implant of hyaluronic acid derivative for treatment of osteochondral and chondral defects
- Summary
- Abstract
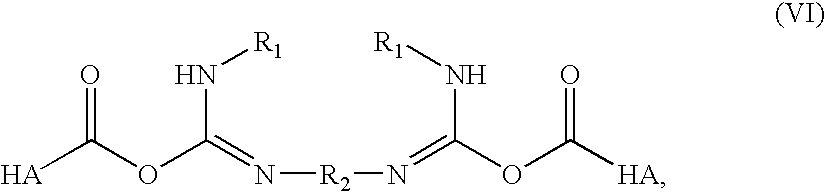
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
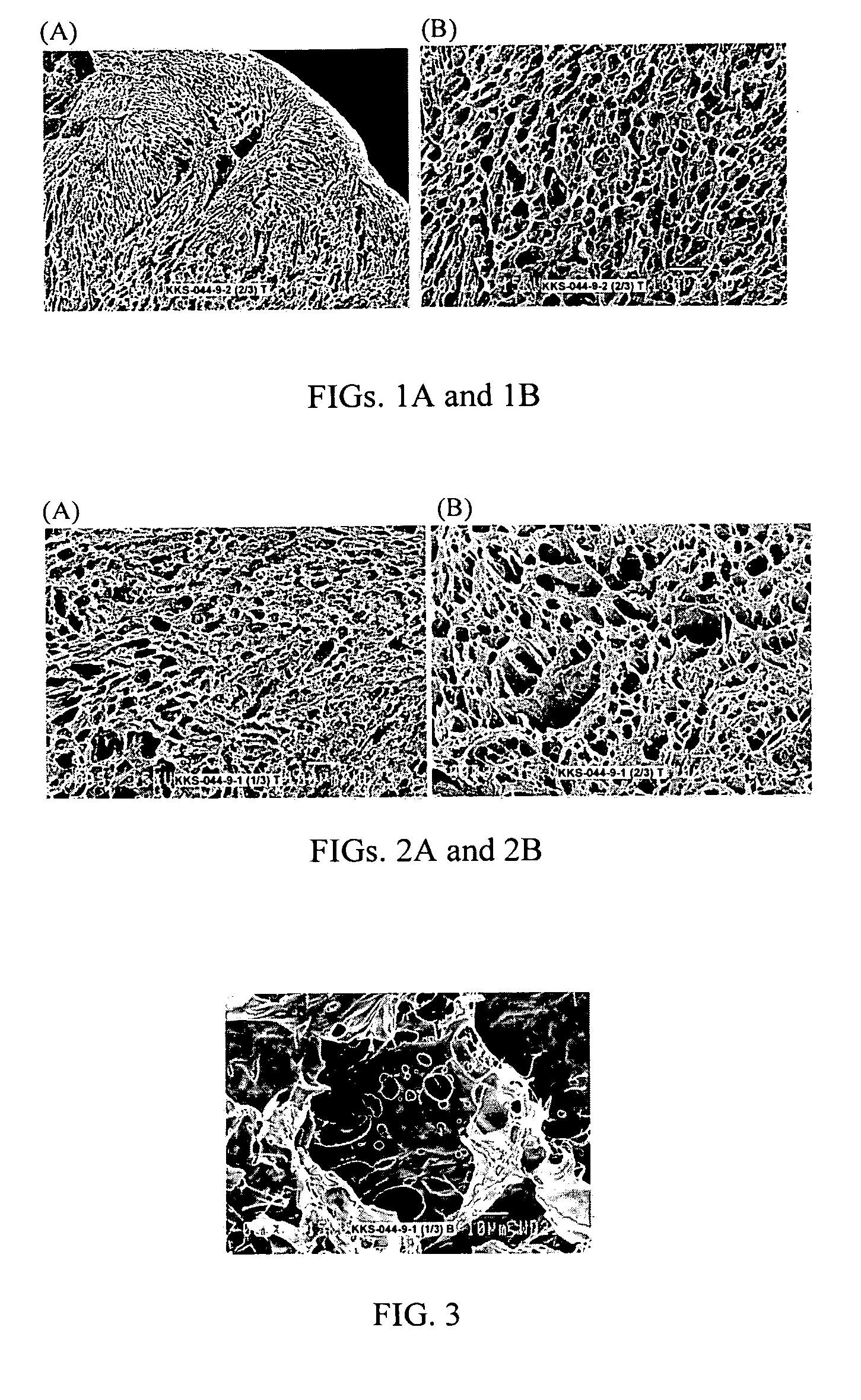
[0081] This example illustrates an embodiment of the invention in which a biscarbodiimide, p-phenylene-bis(ethylcarbodiimide), and HA are reacted at a molar equivalent ratio of 16.0%.
[0082] A solution of HA (5.4 mg / ml; 200-ml; 2.69 mequiv) was reacted with a solution of p-phenylene-bis(ethylcarbodiimide) (1 mg / ml in acetone; 46.1-ml; 0.215 mmol; 0.43 mequiv) according to a procedure described in U.S. Pat. Nos. 5,356,883, 5,502,081 and 6,013,679, the teachings of which are incorporated herein by reference in their entirety. The precipitate of the crosslinked HA was separated from the solution, washed, and resuspended in saline. The suspension was stirred for 2 days in a cold room to form a water-insoluble gel of about 4 mg / ml concentration. Chloroform equal to ½ of the volume of the aqueous solution was added to the solution and contents were vigorously stirred for seven days in the cold room. The reaction mixture was then centrifuged at 4° C. and 43 k rpm for one hour to remove chl...
example 2
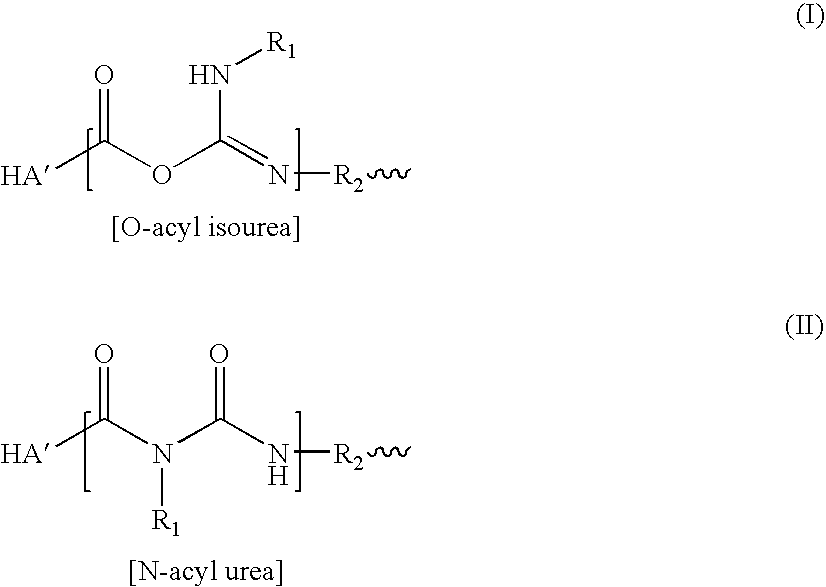
[0083] This example illustrates an embodiment of the invention in which a biscarbodiimide, p-phenylene-bis(ethylcarbodiimide), and HA are reacted at a molar equivalent ratio of 8.0%.
[0084] A solution of HA (5.4 mg / ml; 200-ml; 2.69 mequiv) was reacted with a solution of p-phenylene-bis(ethylcarbodiimide) (1 mg / ml in acetone; 23.0-ml; 0.108 mmol; 0.216 mequiv) according to a procedure described in U.S. Pat. Nos. 5,356,883, 5,502,081 and 6,013,679, the teachings of which are incorporated herein by reference in their entirety. The precipitate of the crosslinked HA was separated from the solution, washed, and resuspended in saline. The suspension was stirred for 2 days in a cold room to form a water-insoluble gel of about 4 mg / ml concentration. Chloroform equal to ½ of the volume of the aqueous solution was added to the solution and contents were vigorously stirred for seven days in the cold room. The reaction mixture was then centrifuged at 4° C. and 43 k rpm for one hour to remove chl...
example 3
[0085] This example illustrates an embodiment of the invention in which a biscarbodiimide, p-phenylene-bis(ethylcarbodiimide), and HA are reacted at a molar equivalent ratio of 8.0% in MES buffer.
[0086] A solution of HA (15.0 mg / ml; 133.3-ml; 4.99 mequiv) in MES buffer (pH 5.5) was reacted with a solution of p-phenylene-bis(ethylcarbodiimide) (15 mg / ml in acetone; 2.8-ml; 0.2 mmol; 0.4 mequiv) according to a procedure described in U.S. Patent Application 2005 / 0136122 A1. The reaction mixture was thoroughly mixed (mixing with either a glass rod or an overhead mechanical stirrer, e.g., for about 1 minute, results in a white paste from the clear reaction mixture), and the mixture was allowed to stand at room temperature for about 96 hours. Sodium chloride (6.5 g, to make the mixture 5% by weight of sodium chloride) was mixed into the resulting gel, which was allowed to stand for 1 hour. The crosslinked HA gel was precipitated by addition into about 1.2 L of vigorously stirred ethanol....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mole | aaaaa | aaaaa |
| Percent by mole | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com