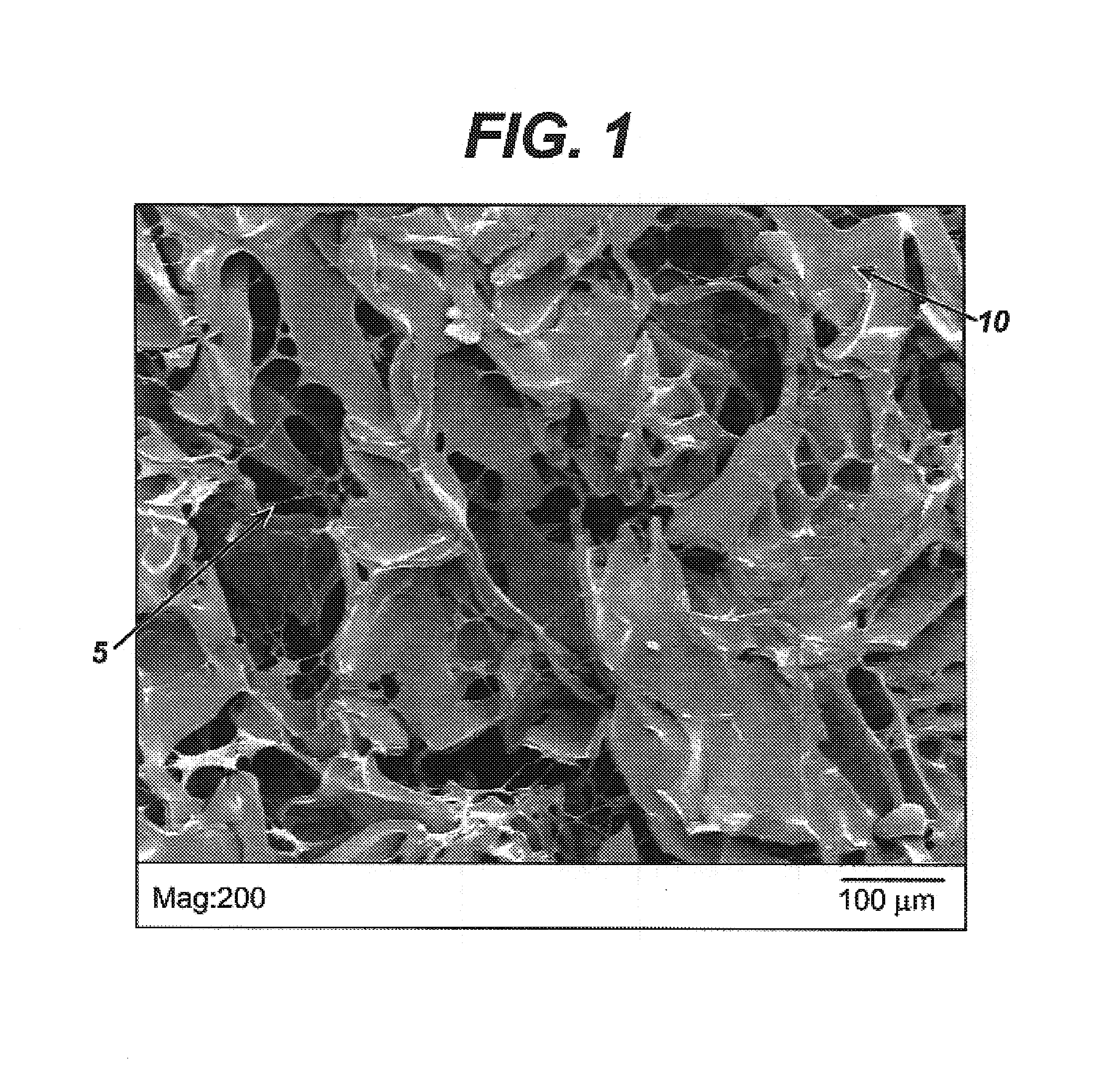
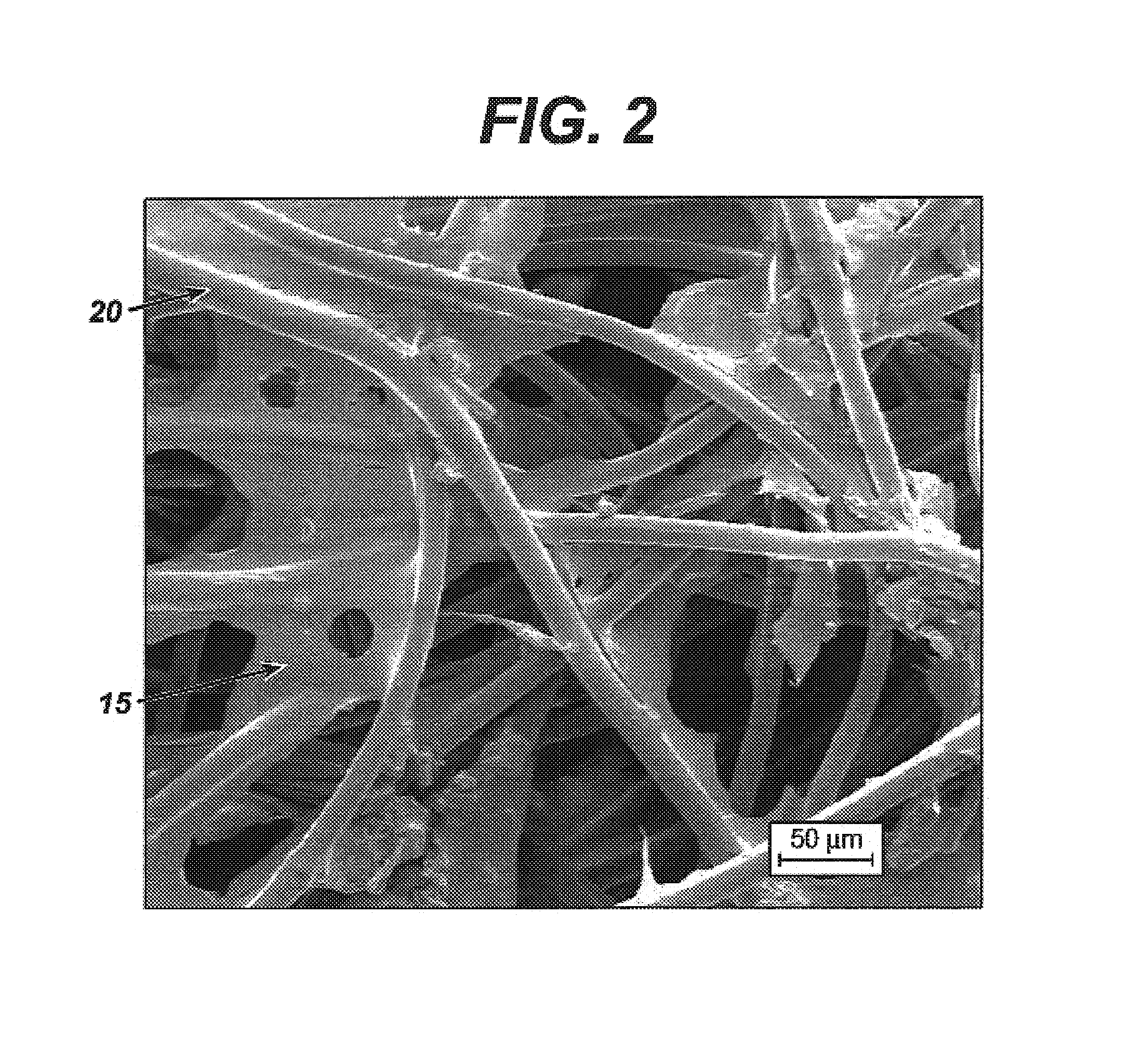
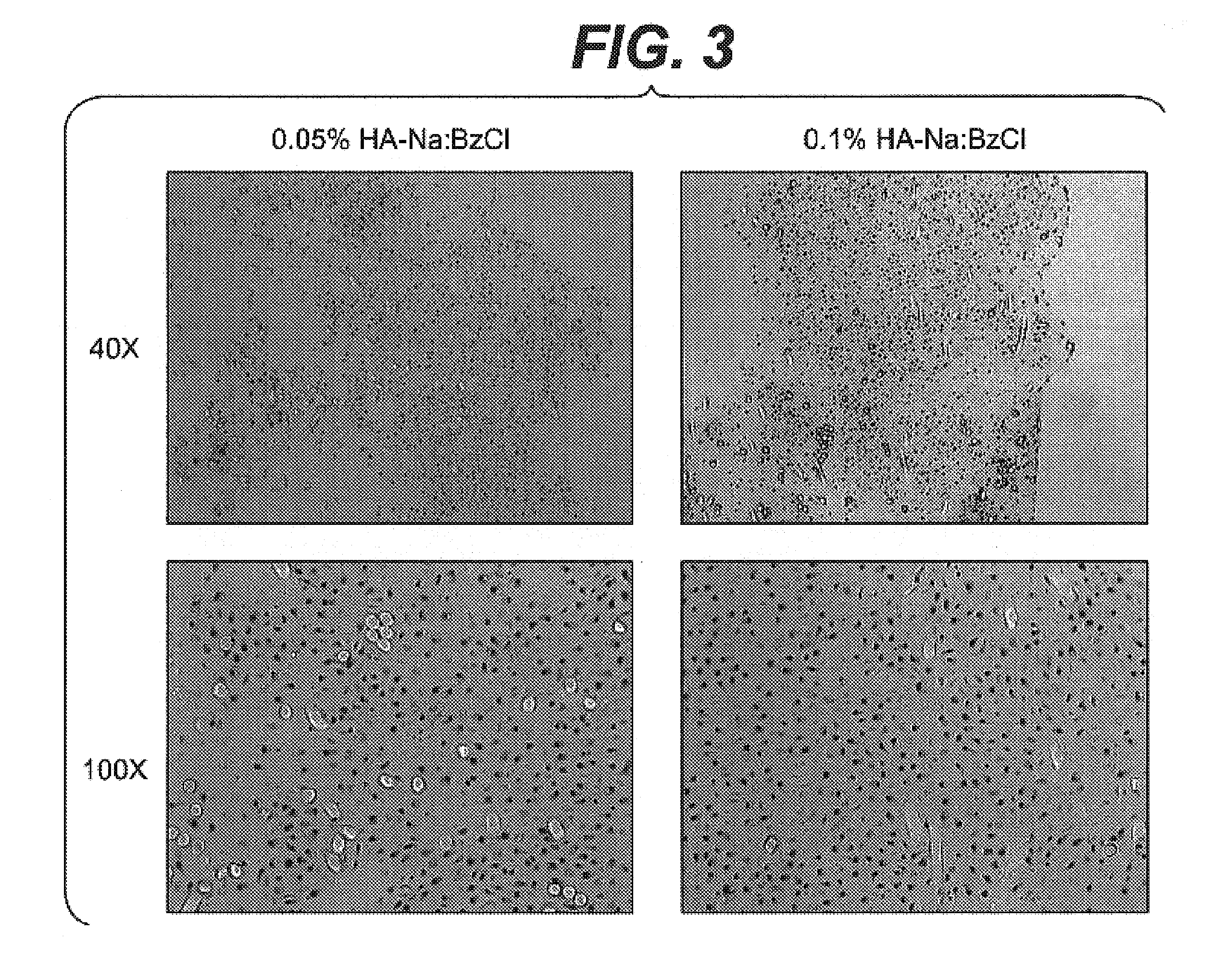
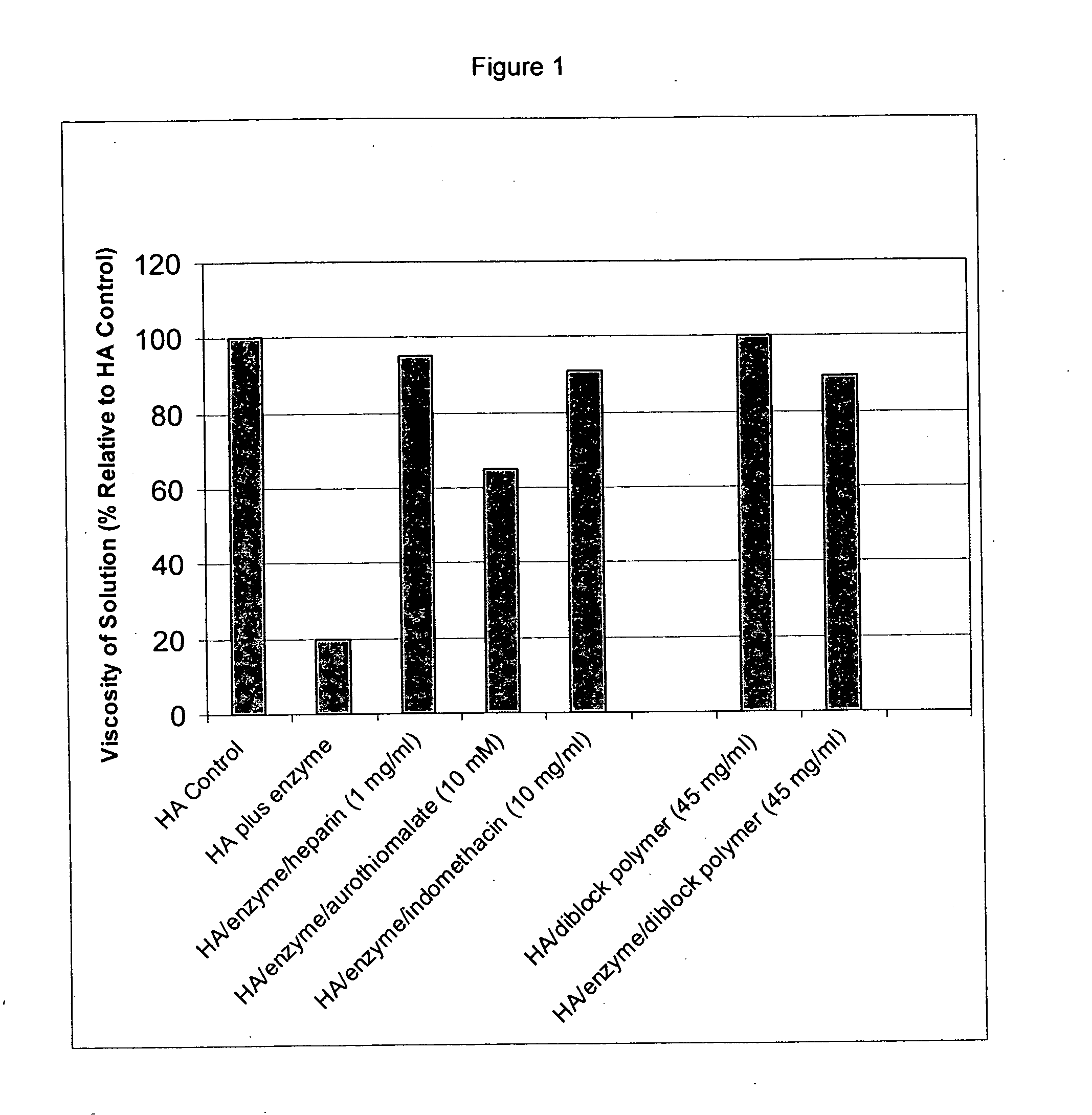
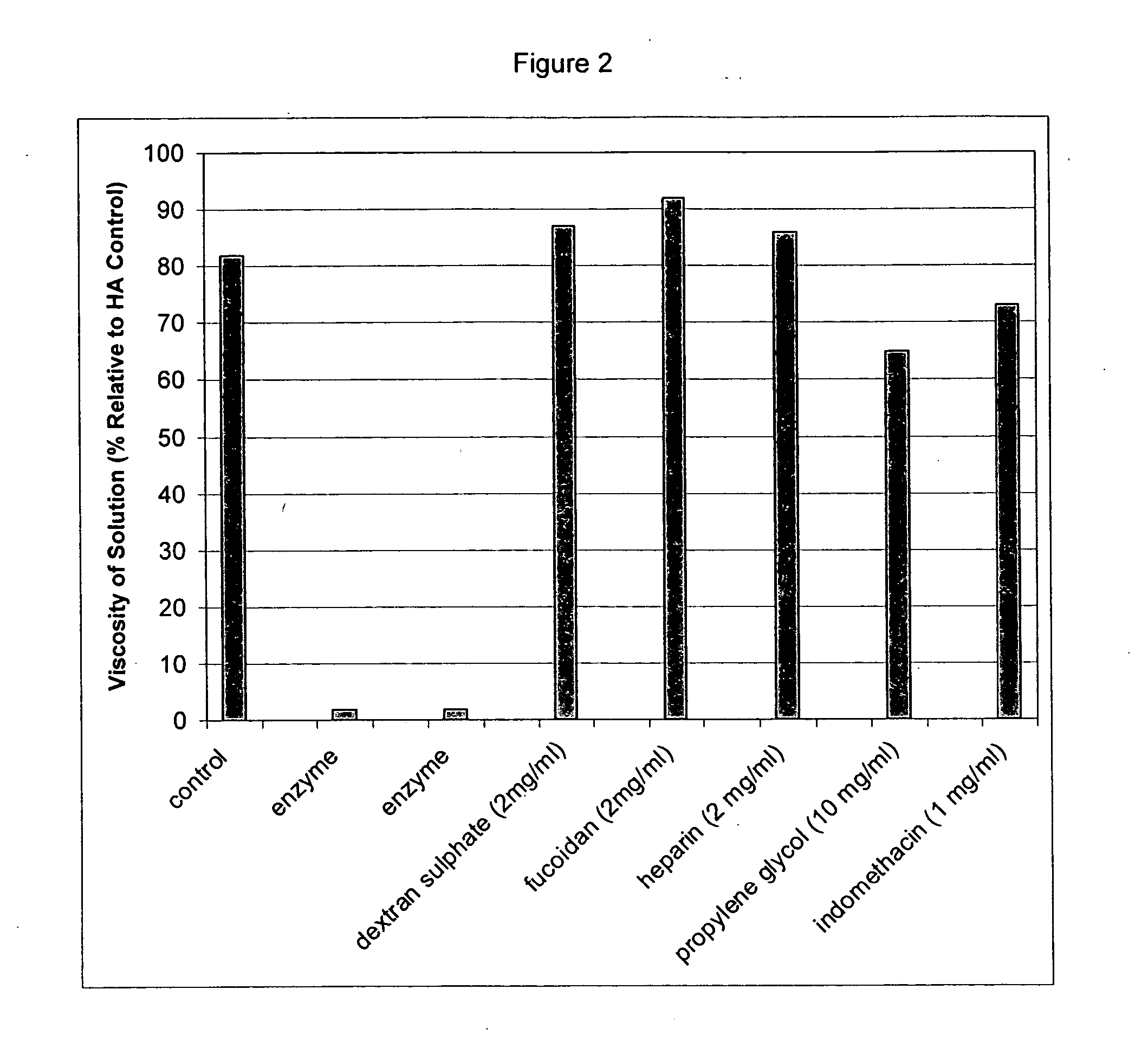
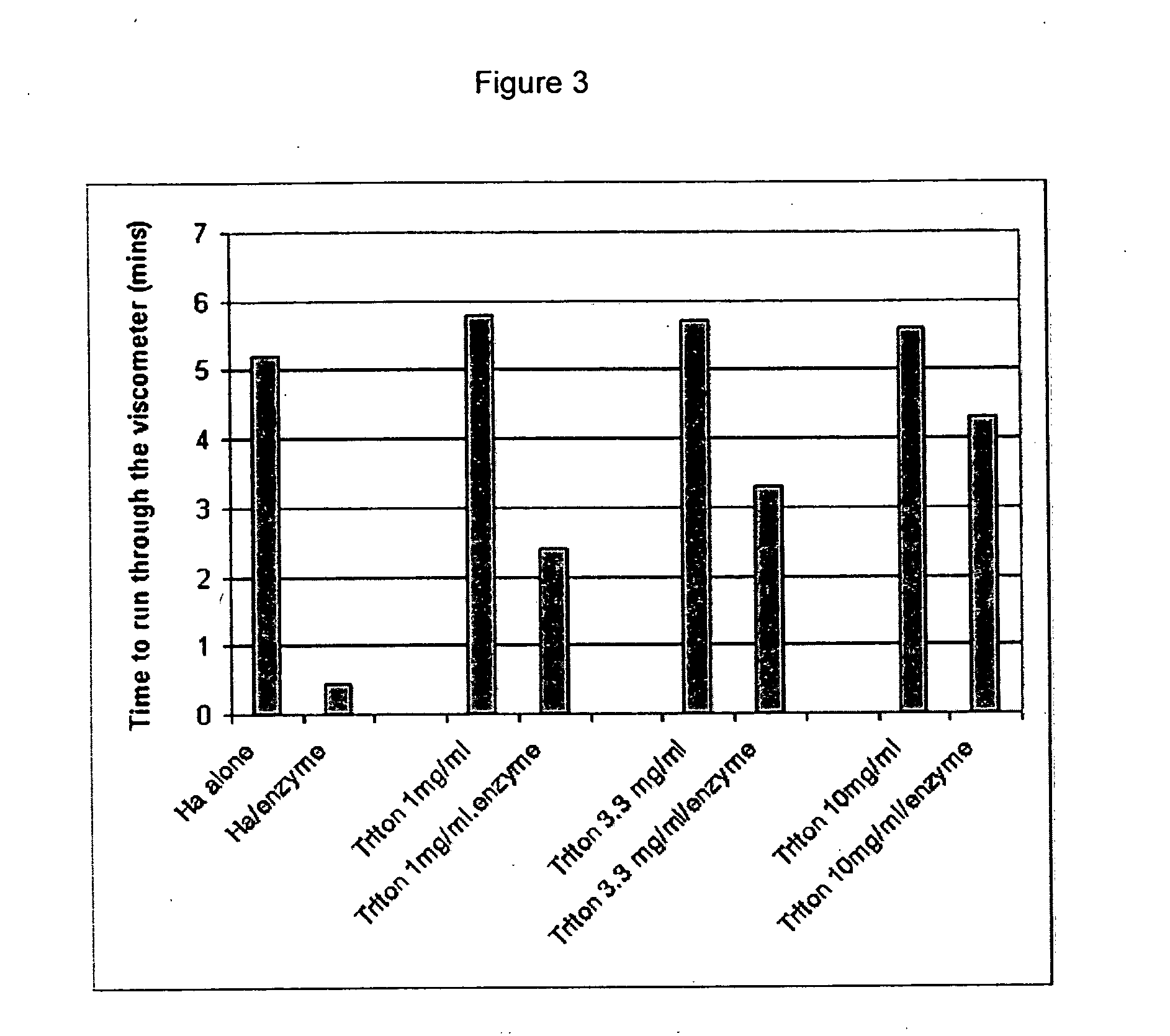
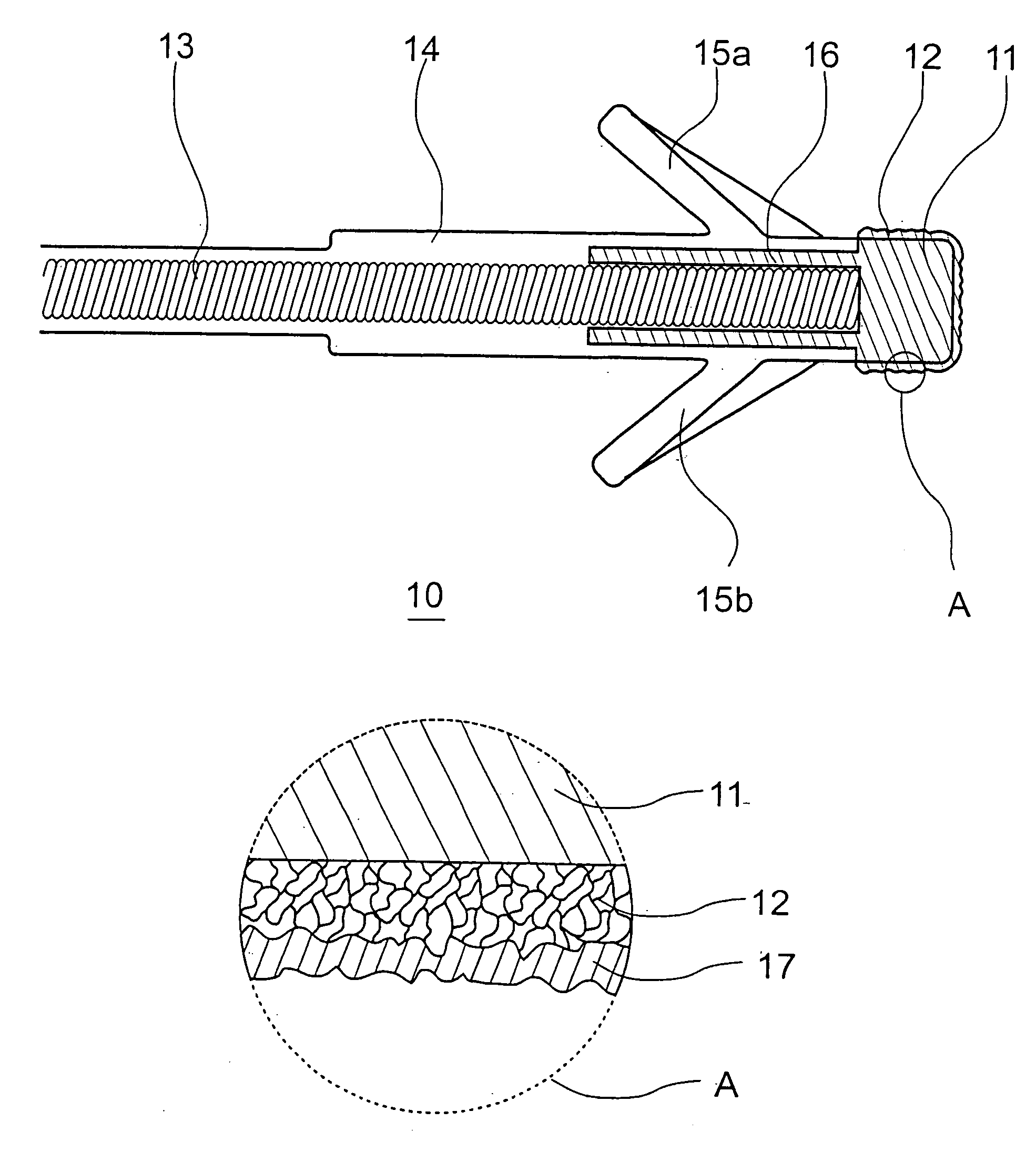
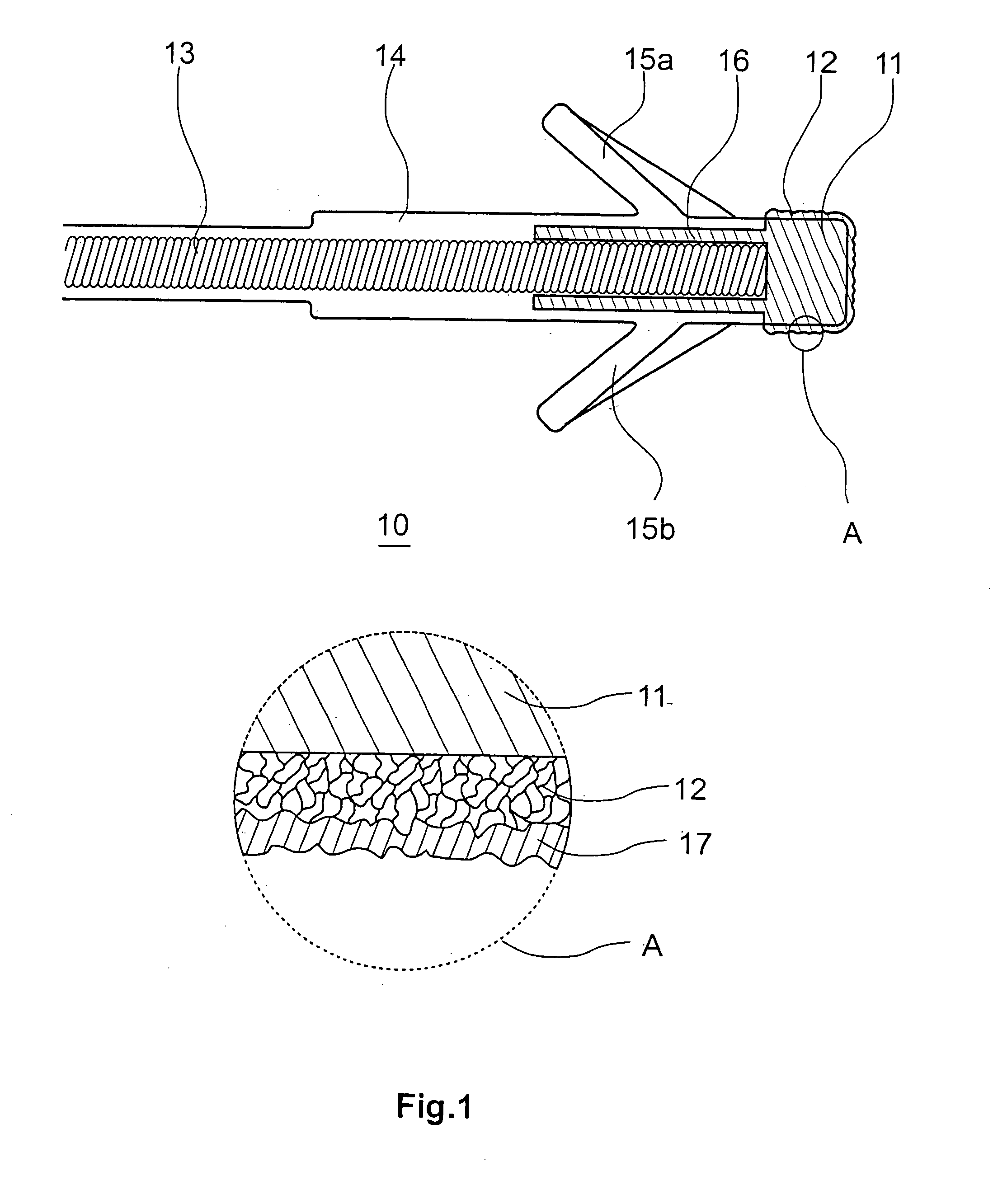
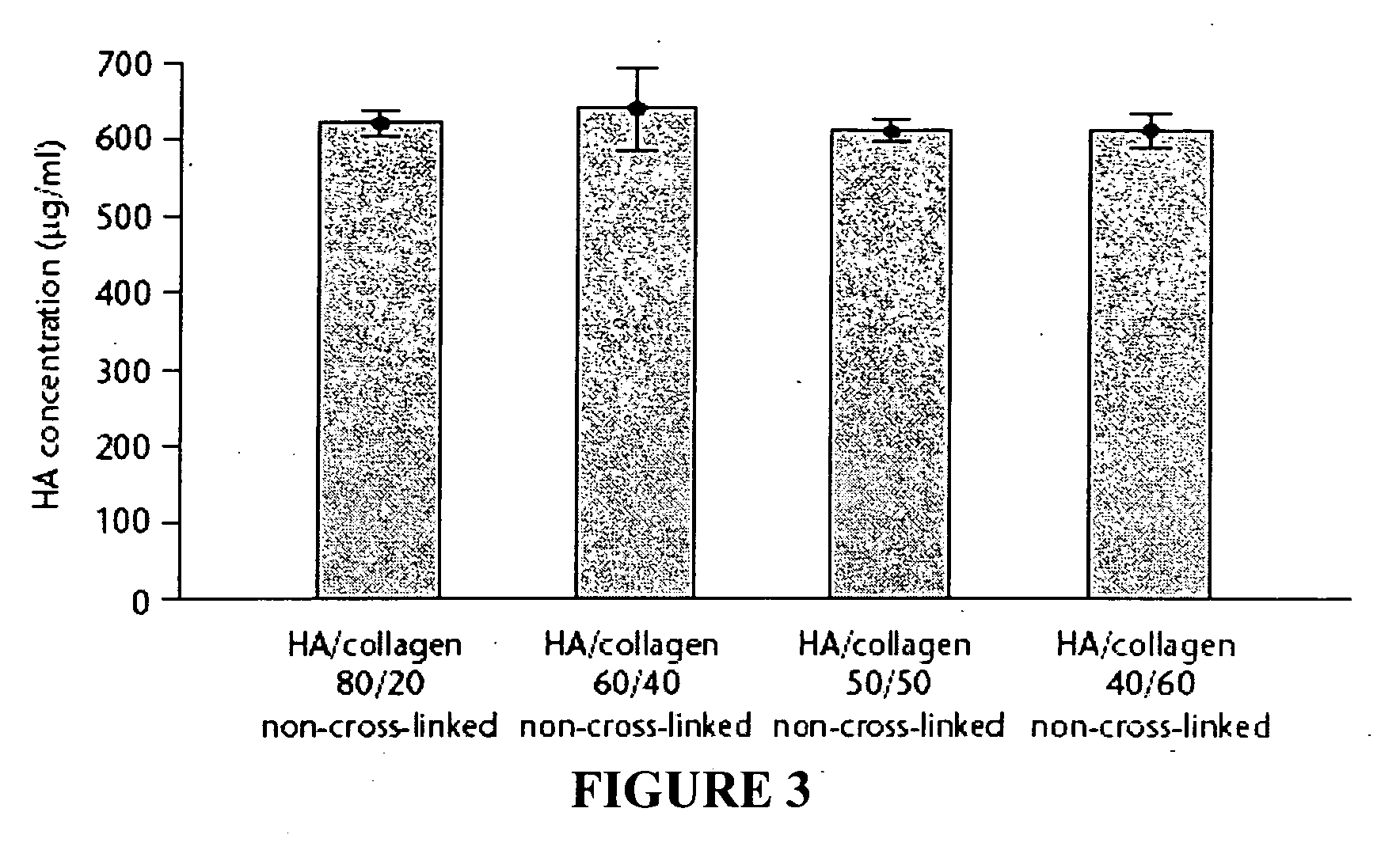
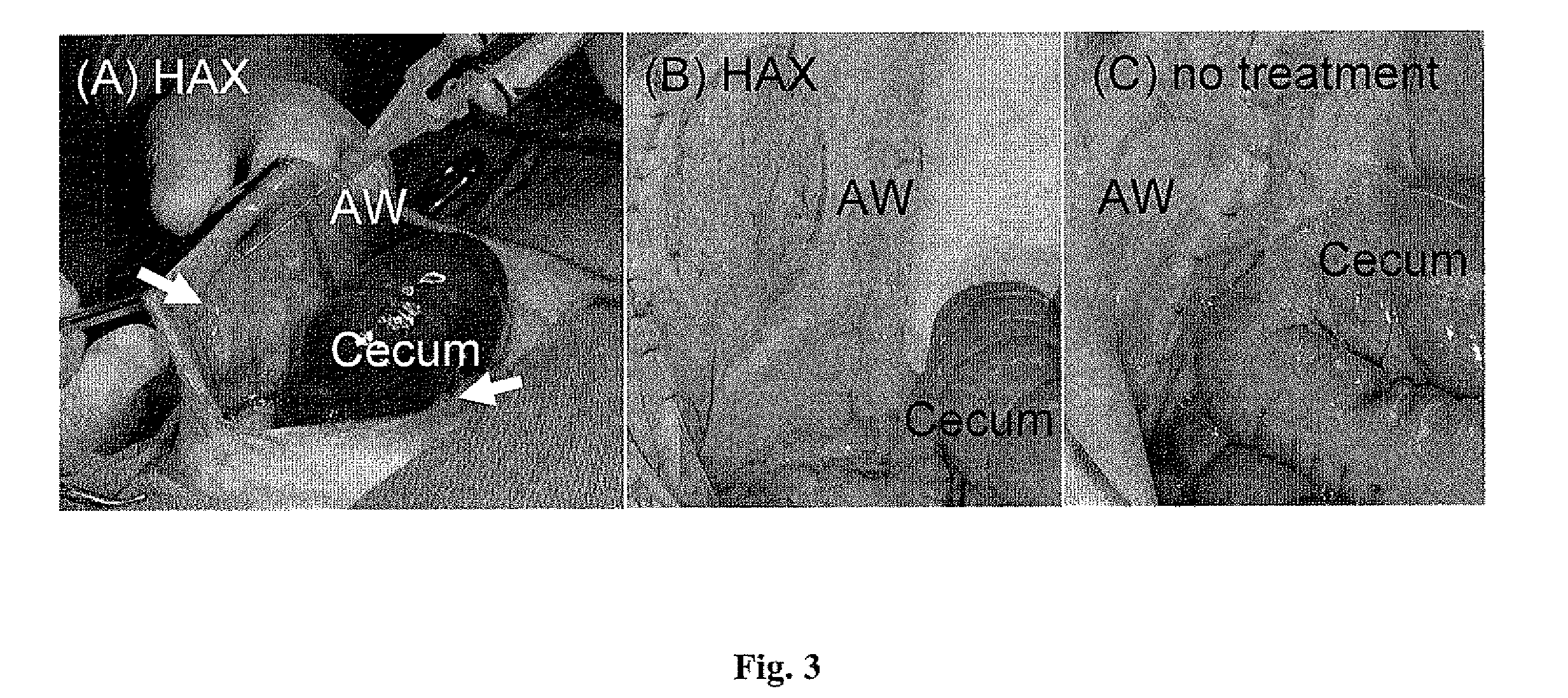
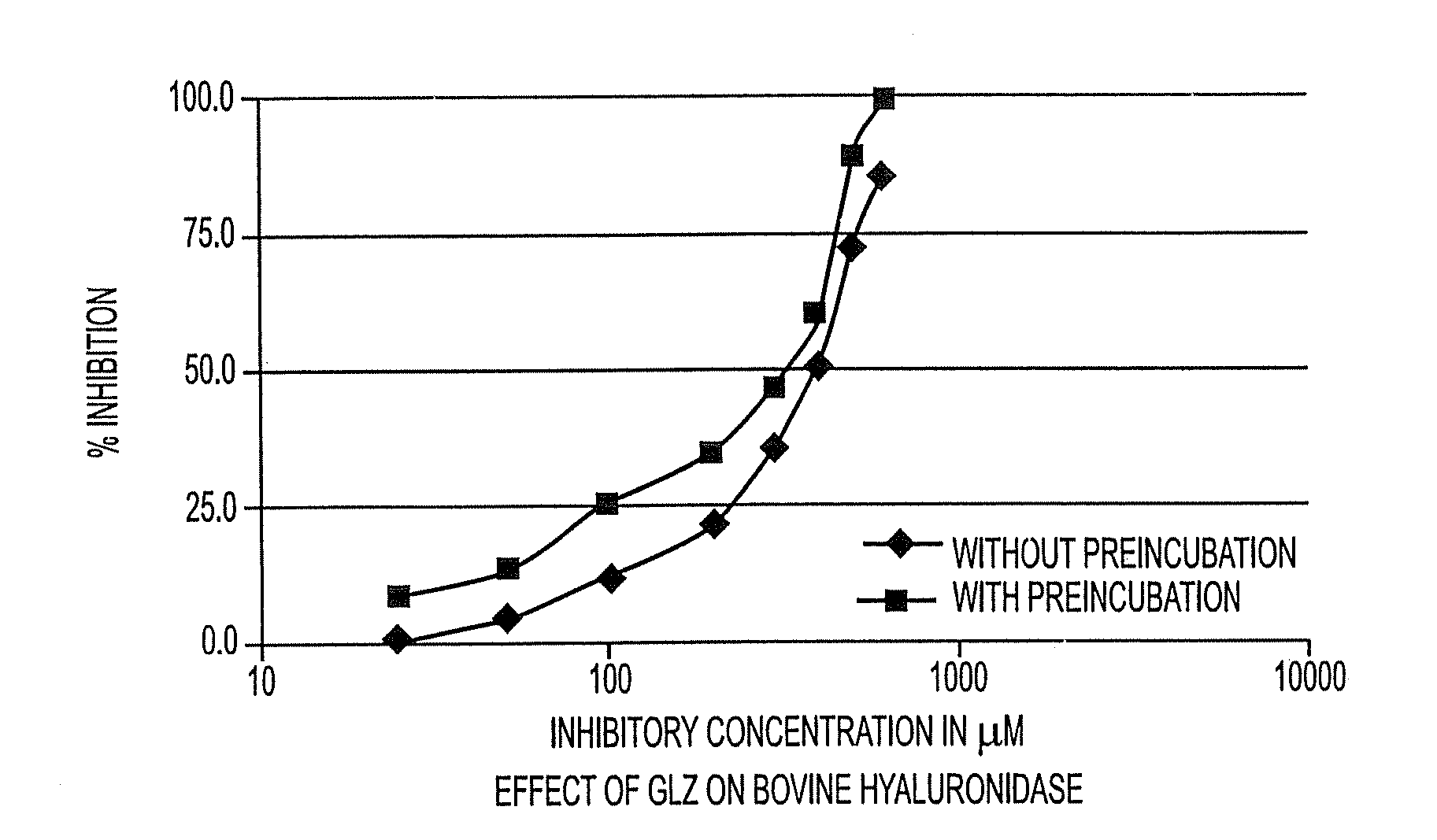
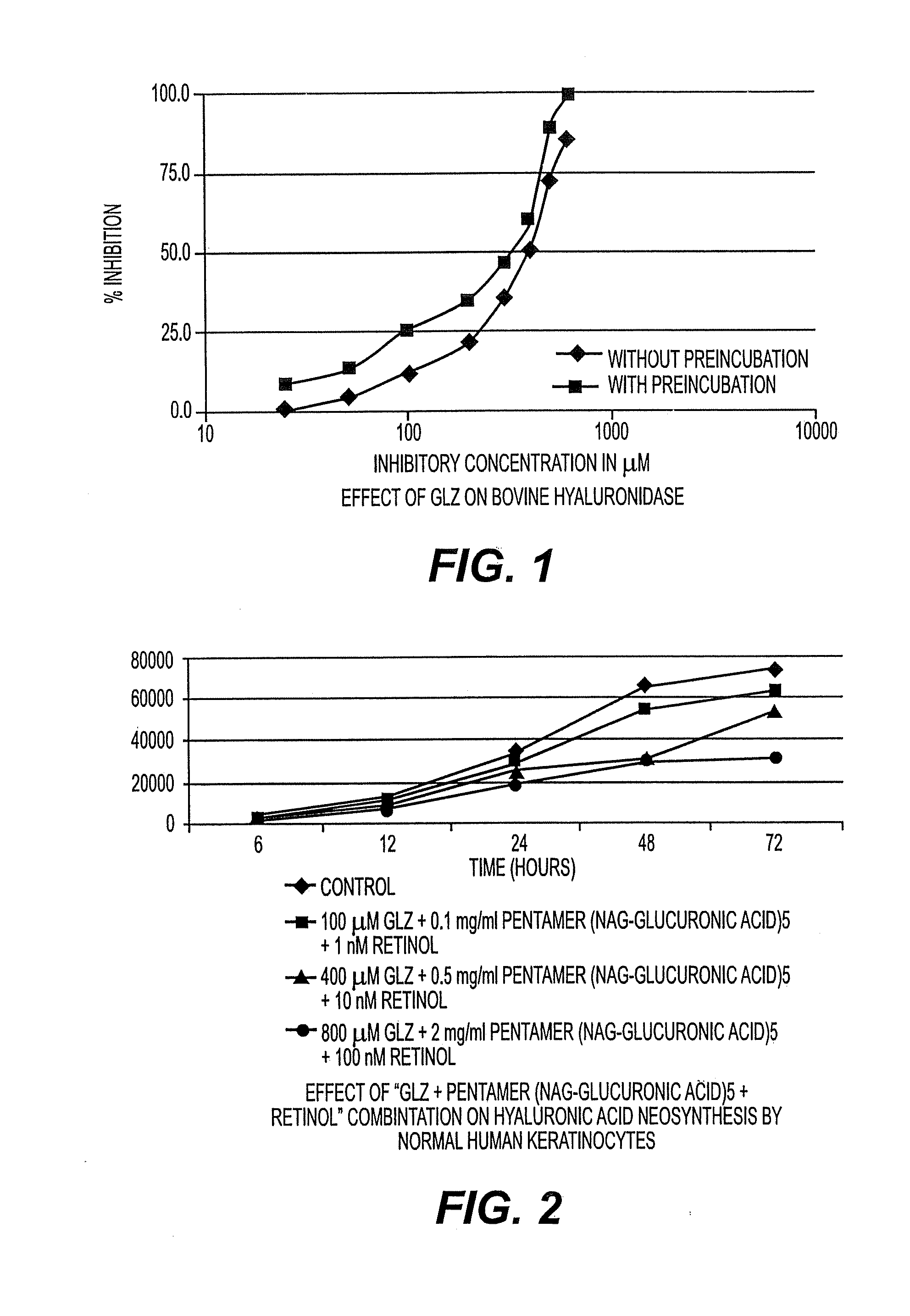
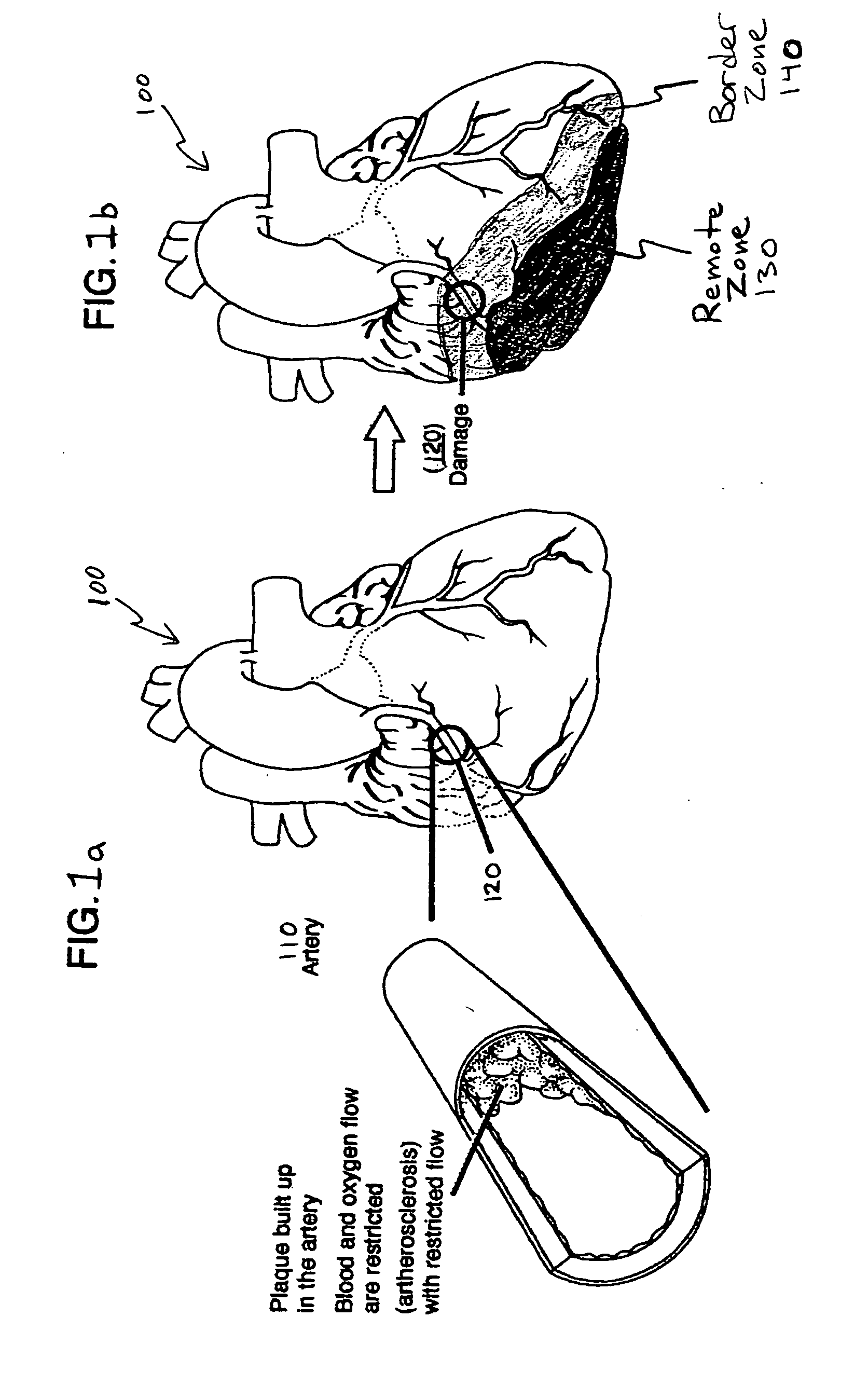
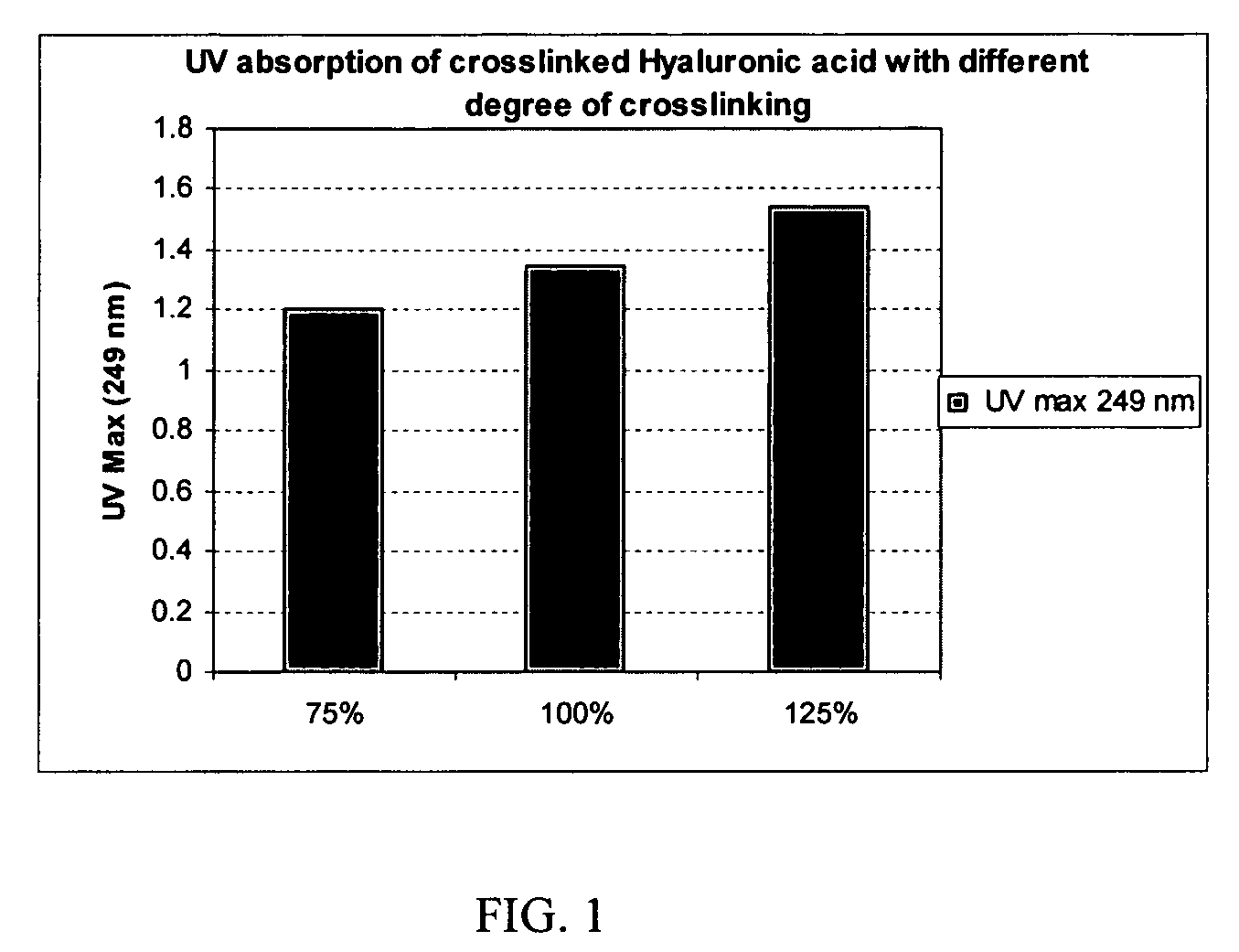
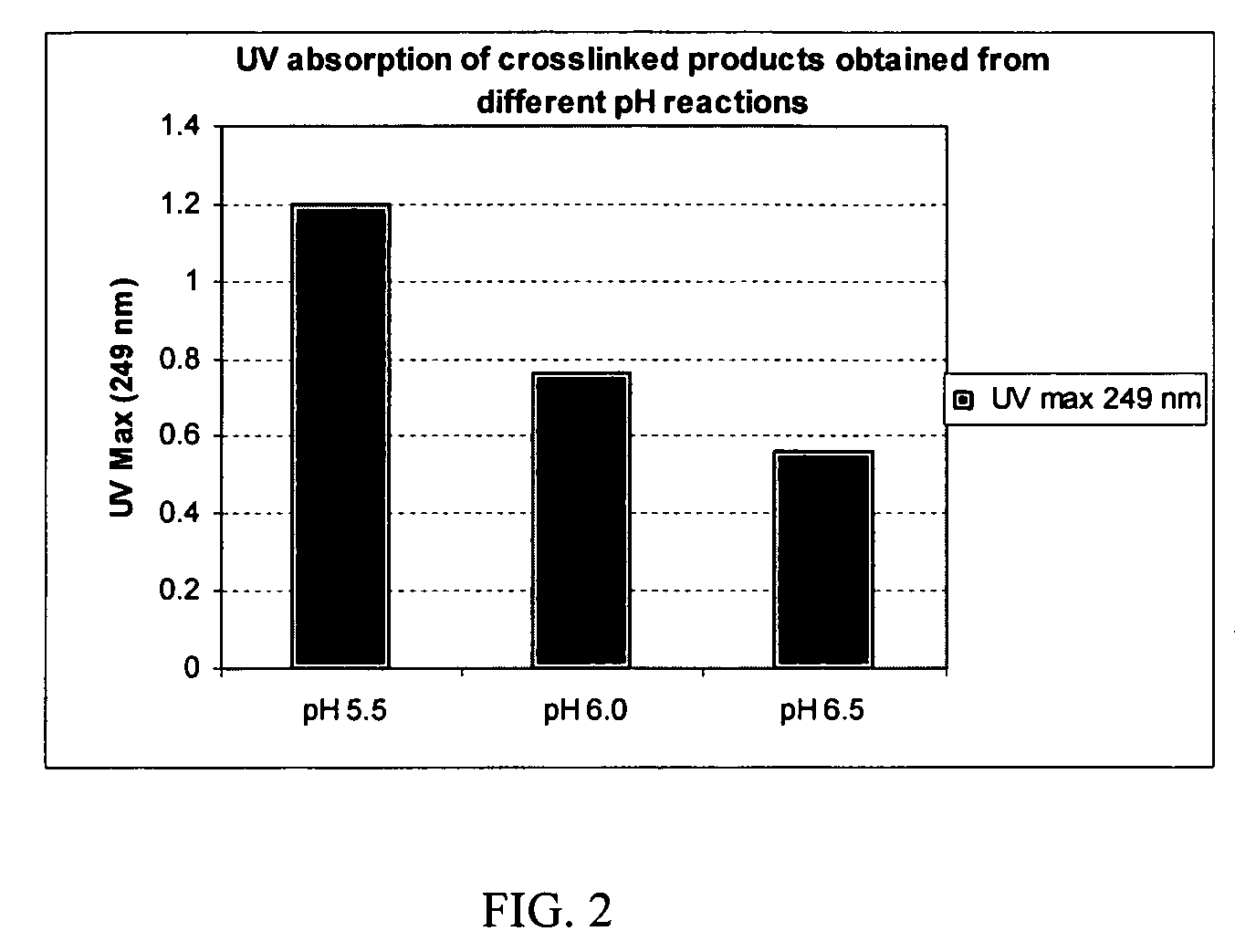
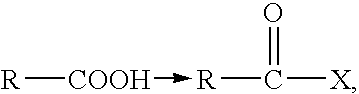Patents
Literature
8504 results about "Hyaluronic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
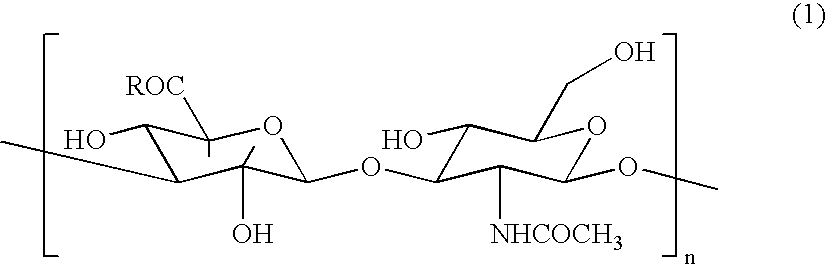
Hyaluronic acid (/ˌhaɪ.əljʊəˈrɒnɪk/; abbreviated HA; conjugate base hyaluronate), also called hyaluronan, is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. It is unique among glycosaminoglycans in that it is nonsulfated, forms in the plasma membrane instead of the Golgi apparatus, and can be very large: human synovial HA averages about 7 million Da per molecule, or about twenty thousand disaccharide monomers, while other sources mention 3–4 million Da. One of the chief components of the extracellular matrix, hyaluronan, contributes significantly to cell proliferation and migration, and may also be involved in the progression of some malignant tumors.
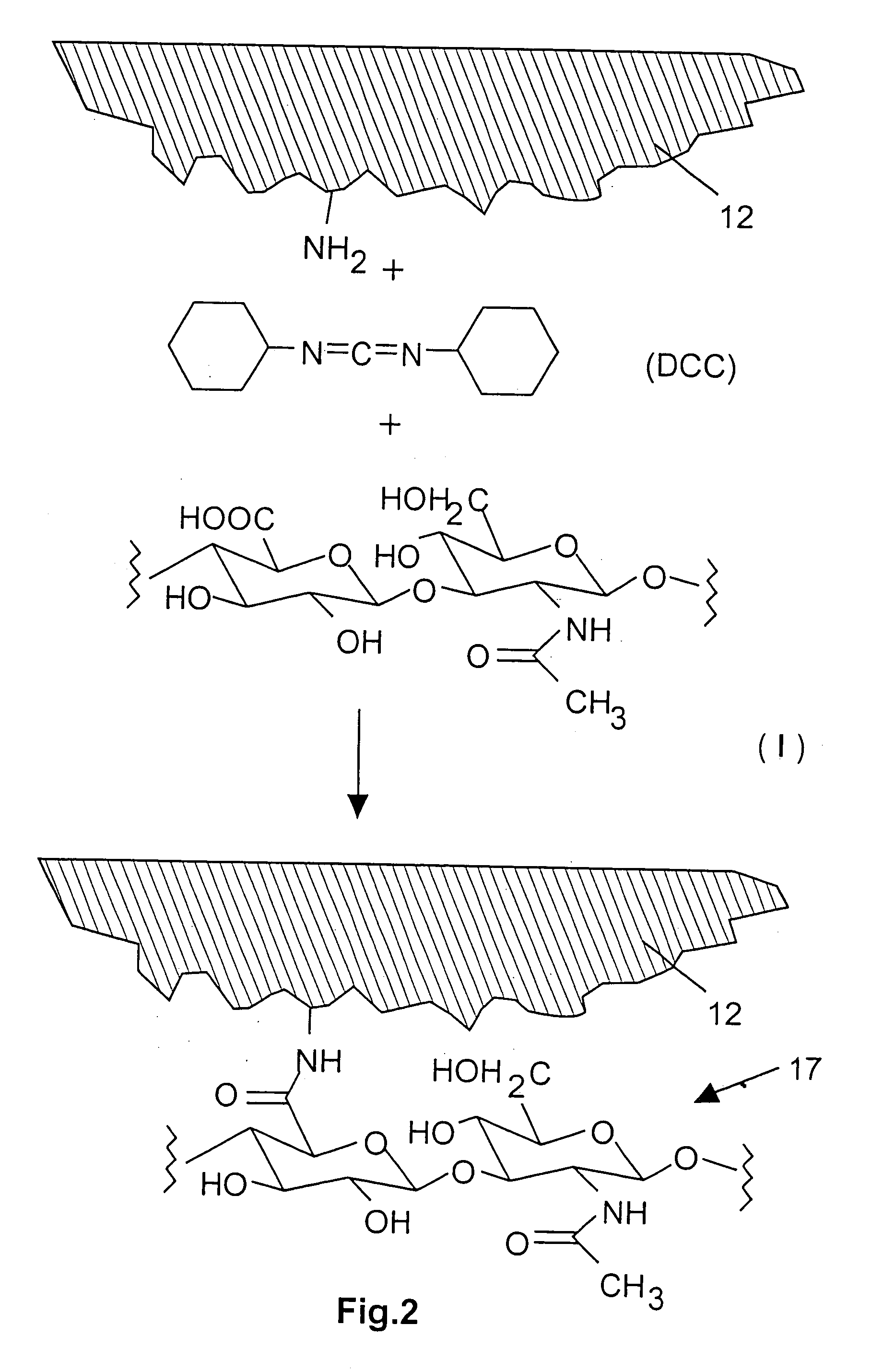
Modified hyaluronic acid for use in musculoskeletal tissue repair
The present invention includes hyaluronic acid complexes of a monovalent alkali metal salt of hyaluronic acid and a tetra alkyl ammonium halide that are suitable for incorporation with tissue scaffolds that are suitable for use in repair and / or regeneration of muscoloskeletal tissue and that include a biodegradable, porous substrate made from a biodegradable, hydrophobic polymer, where the hyaluronic acid complex is substantially insoluble in water at room temperature, yet soluble in mixtures of organic and aqueous solvents in which the selected hydrophobic polymer is soluble.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Compositions and methods using hyaluronic acid
InactiveUS20060040894A1Relieve painPreventing surgical adhesionBiocideHeavy metal active ingredientsCompound (substance)Hyaluronic acid
Compositions and devices including hyaluronic acid and a compound that inhibits degradation of hyaluronic acid, and methods of making and using same.
Owner:ANGIOTECH INT AG (CH) +1
Chondroprotective/restorative compositions and methods of use thereof
The instant invention provides a method of treating or preventing osteoarthritis, joint effusion, joint inflammation and pain, synovitis, lameness, post operative arthroscopic surgery, deterioration of proper joint function including joint mobility, the reduction or inhibition of metabolic activity of chondrocytes, the activity of enzymes that degrade cartilage, the reduction or inhibition of the production of Hyaluronic acid, said method comprising orally administering to a mammalian species a therapeutically effective amount of Hyaluronic Acid or pharmaceutically acceptable salts thereof. Additionally, compositions containing hyaluronic acid; chondroitin sulfate, and glucosamine sulfate in a paste formulation are also disclosed which can be administered on their own or can be used as a feed additive.
Owner:PIERCE SCOTT W
Implantable Stimulation Electrode with a Coating for Increasing Tissue Compatibility
InactiveUS20080234790A1Avoid tissue irritationGood biocompatibilitySurgeryInternal electrodesImplantable Stimulation ElectrodesIrritation
An implantable stimulation electrode for use with an implantable tissue stimulator, especially a pacemaker, a defibrillator, a bone stimulator or a neurostimulator includes a metal base body, optionally one or more intermediate layers disposed on the base body and a coating covering the base body and, optionally, intermediate layers in order to increase tissue compatibility. The coating should prevent tissue irritations after implantation and more particularly increase the stimulus threshold associated therewith, have very high biocompatibility and also has an anti-inflammatory effect. An increase in tissue compatibility is achieved by virtue of the fact that the coating has a polysaccharide layer made of hyaluronic acid and / or hyaluronic acid derivatives.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Chondroprotective/restorative compositions and methods of use thereof
The instant invention provides a method of treating or preventing osteoarthritis, joint effusion, joint inflammation and pain, synovitis, lameness, post operative arthroscopic surgery, deterioration of proper joint function including joint mobility, the reduction or inhibition of metabolic activity of chondrocytes, the activity of enzymes that degrade cartilage, the reduction or inhibition of the production of Hyaluronic acid, said method comprising orally administering to a mammalian species a therapeutically effective amount of Hyaluronic Acid or pharmaceutically acceptable salts thereof. Additionally, compositions containing hyaluronic acid; chondroitin sulfate, and glucosamine sulfate in a paste formulation are also disclosed which can be administered on their own or can be used as a feed additive.
Owner:PIERCE SCOTT W
High density fibrous polymers suitable for implant
InactiveUS6974862B2Easy to shapeEvenly dispersedFinger jointsInternal osteosythesisFiberHigh density
This invention includes malleable, biodegradable, fibrous compositions for application to a tissue site in order to promote or facilitate new tissue growth. One aspect of this invention is a fibrous component (e.g., collagen, chitosan, alginate, hyaluronic acid, poly-lactic acid, poly-capralactone, and polyurethane) that provides unique mechanical and physical properties. The invention may be created by providing a vessel containing a slurry, said slurry comprising a plurality of natural or synthetic polymer fibers and at least one suspension fluid, wherein the polymer fibers are substantially evenly dispersed and randomly oriented throughout the volume of the suspension fluid; applying a force, e.g., centrifugal, to said vessel containing said slurry, whereupon said force serves to cause said polymer fibers to migrate through the suspension fluid and amass at a furthest extent of the vessel, forming a polymer material, with said polymer material comprising polymer fibers of sufficient length and sufficiently viscous, interlaced, or interlocked to retard dissociation of said polymer fibers.
Owner:DSM IP ASSETS BV
Functionalized derivatives of hyaluronic acid, formation of hydrogels in situ using same, and methods for making and using same
InactiveUS7196180B2Good physical propertiesSimple structureOrganic active ingredientsSugar derivativesCross-linkIn situ polymerization
Methods for chemical modification of hyaluronic acid, formation of amine or aldehyde functionalized hyaluronic acid, and the cross-linking thereof to form hydrogels are provided. Functionalized hyaluronic acid hydrogels of this invention can be polymerized in situ, are biodegradable, and can serve as a tissue adhesive, a tissue separator, a drug delivery system, a matrix for cell cultures, and a temporary scaffold for tissue regeneration.
Owner:ORTHOGENE
Method for producing cross-linked hyaluronic acid-protein bio-composites
InactiveUS20060189516A1Uniform densityUniform porosityPeptide/protein ingredientsSkeletal disorderCross-linkFiber
This invention is concerned with a new method for producing cross-linked hyaluronic acid—protein bio-composites in various shapes. In the present process, a polysaccharide solution and a protein solution are mixed under moderate pH values in presence of salts to form a homogenous solution, which can be processed into various shapes, such as membrane, sponge, fiber, tube or micro-granular and so on. After then, the homogenous solution is subjected to a cross-linking reaction in organic solvent containing weak acid to produce an implantable bio composite material having excellent bio-compatibility, biodegradability, prolonged enzymatic degradation time, and good physical properties.
Owner:IND TECH RES INST
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Treatment of arthritis and other musculoskeletal disorders with crosslinked hyaluronic acid
InactiveUS20070203095A1Reduce frequencyFast pain reliefBiocideOrganic active ingredientsDiseaseCarboxyl radical
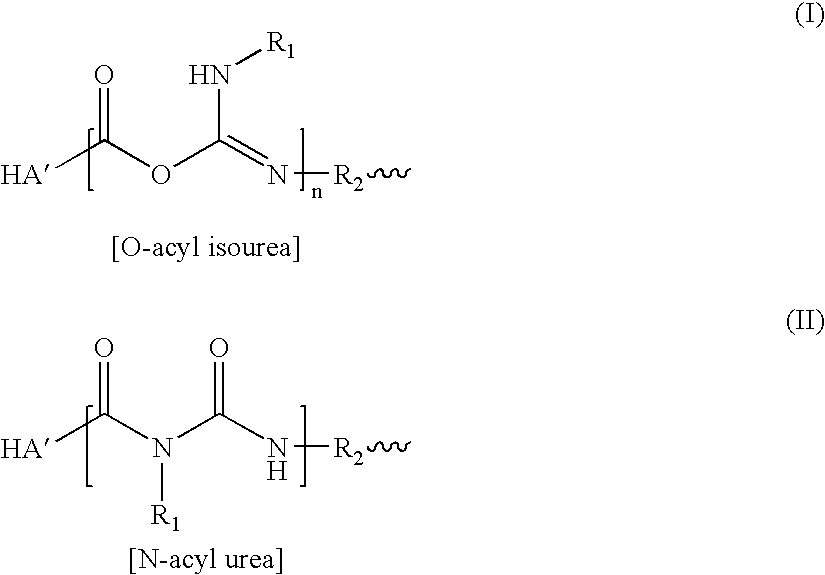
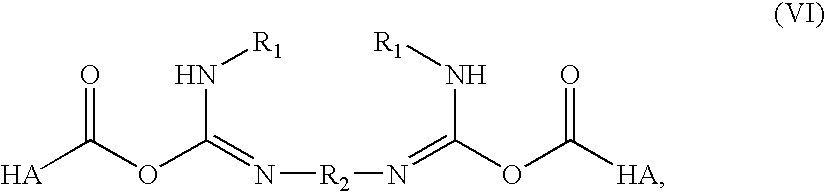
A method of treating a subject having a musculoskeletal disorder includes administering to a subject's articular site in need thereof an effective amount of a hyaluronic acid (HA) composition. In one embodiment, the HA composition includes an HA derivative, wherein carboxyl functionalities of the hyaluronic acid derivative are each independently derivatized to include an N-acylurea or 0-acyl isourea, or both N-acylurea and 0-acyl isourea. In another embodiment, the HA composition includes a crosslinked HA gel that is prepared by reacting an uncrosslinked HA with a biscarbodiimide in the presence of pH buffer in a range of between about 4 and about 8. The composite can optionally include at least one second bioactive agent other than the HA derivative, such as a steroid.
Owner:ANIKA THERAPEUTICS INC
Cross-linked polysaccharide composition
InactiveUS20070026070A1Good degradation propertiesPowder deliveryOrganic active ingredientsCross-linkHyaluronic acid
The present invention provides a process for making cross-linked polysaccharide gels under basic conditions. More particularly, the present invention provides a process for forming cross-linked hyaluronic acid gels under basic conditions. The resulting gels possess improved degradation characteristics, and are useful in a variety of medical and cosmetic applications.
Owner:ULTRACEUTICALS R&D
Suturable adhesion-preventing membrane
InactiveUS6977231B1High strengthGood biocompatibilitySuture equipmentsSynthetic resin layered productsCross-linkDecomposition
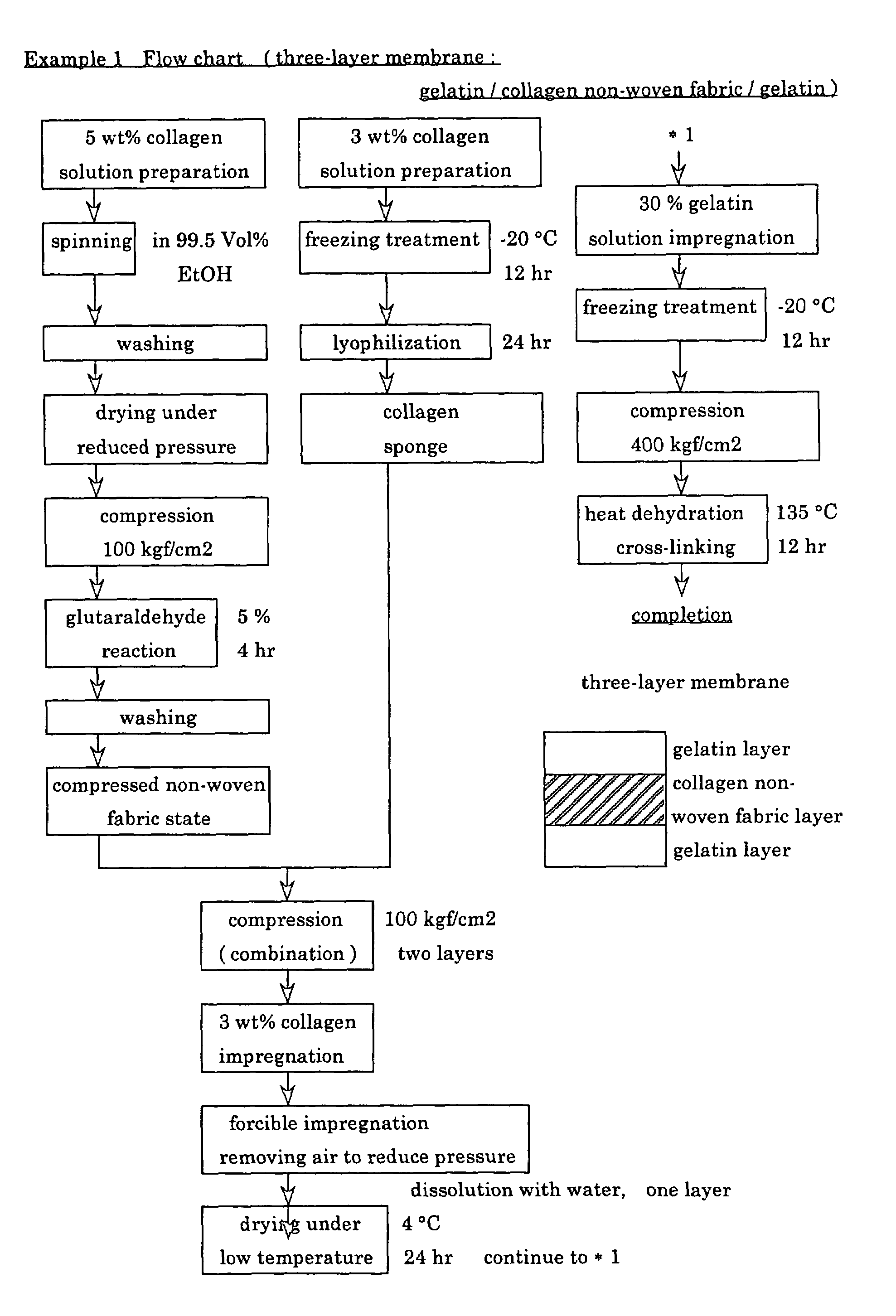
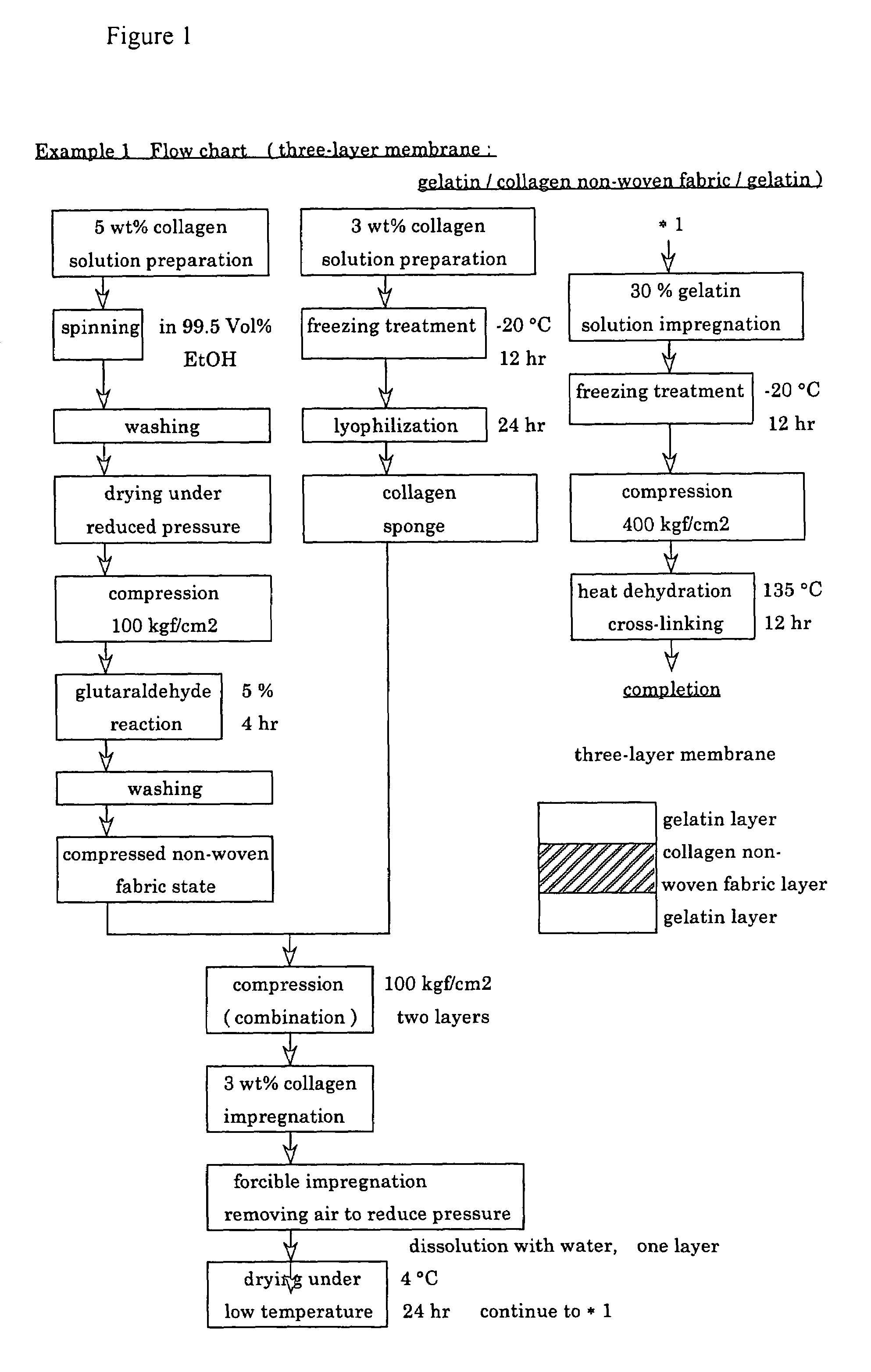
A suturable adhesion-preventing membrane has high suture strength, good biocompatibility, decomposition and absorption in a living body, sufficient adhesion-preventing effect, and desirable guided tissue regeneration. The membrane is composed of at least one non-woven fabric layer made of collagen fibers, or a laminated membranous substance consisting of at least one non-woven fabric layer made of collagen fibers and at least one sponge layer made of collagen, and a coating layer of gelatin or hyaluronic acid on the surface or surfaces of the above membrane. Preferably, the membrane comprises one to six compressed cross-linked collagen non-woven fabric layers wherein a layer has a fibers having a fiber diameter of 0.05 mm to 1.0 mm, a bulk density of 5.0×10−4 to 5 g / cm3 and a thickness of 0.1 mm to 50 mm, and a coating layer containing gelatin or hyaluronic acid and having a thickness of 0.05 mm to 20 mm, wherein the coating layer covers one or both sides or a part or whole of the surface of the membrane.
Owner:NIPRO CORP
Hyaluronic acid compositions for dermatological use
InactiveUS20110171286A1Increase of stability of gelBiocideCosmetic preparationsWrinkle skinHyaluronic acid
The disclosure provides hyaluronic acid (HA) gel formulations and methods for treating the appearance of the skin. The formulations hyaluronic acid and at least one additional constituent selected from the group consisting of vitamin B, C and vitamin E, wherein the formulation exhibits greater stability than an HA gel formulation without the additional constituent. Methods for treating lines, wrinkles, fibroblast depletions, and scars with the disclosed composition are provided as well.
Owner:ALLERGAN IND +1
Compositions And Methods For Inhibiting Adhesions
InactiveUS20080069857A1Fast cross-linkingLimitation on parameterPowder deliveryOrganic active ingredientsActive agentMicroparticle
The present invention provides compositions and methods for inhibiting adhesions. The methods involve administering solutions containing hydrogel precursors such as polysaccharide derivatives, e.g., derivatives of hyaluronic acid, cellulose, or dextran, to a subject at a site where adhesions may form, e.g., as a consequence of surgery, injury, or infection. The hydrogel precursors, e.g., polysaccharide derivatives, become crosslinked following their administration to form a hydrogel that maintains tissue separation. In certain embodiments of the invention one or both solutions contains particles, e.g., polymeric nanoparticles or microparticles, so that a composite hydrogel containing the particles is formed. The solution(s), particle(s), or both, may contain a biologically active agent such as an agent that contributes to inhibiting adhesions. The biologically active agent may be covalently attached to a hydrogel precursor.
Owner:EI DU PONT DE NEMOURS & CO +2
Phamaceutical/cosmetic compositions comprising hyaluronic acid and treatment of dermatological conditions therewith
InactiveUS20090018102A1Improve bioavailabilityReduce in quantityCosmetic preparationsBiocideFine lineWrinkle skin
Pharmaceutical / cosmetic compositions containing a dermatologically effective amount of hyaluronic acid, at least one retinoid and / or salt and / or derivative thereof, at least one oligosaccharide and at least one inhibitor of hyaluronic acid degradation, formulated into a physiologically acceptable medium therefor, are useful for the treatment of wrinkles, fine lines, fibroblast depletions and scars.
Owner:GALDERMA RES & DEV SNC
Hydrogel bioscaffoldings and biomedical device coatings
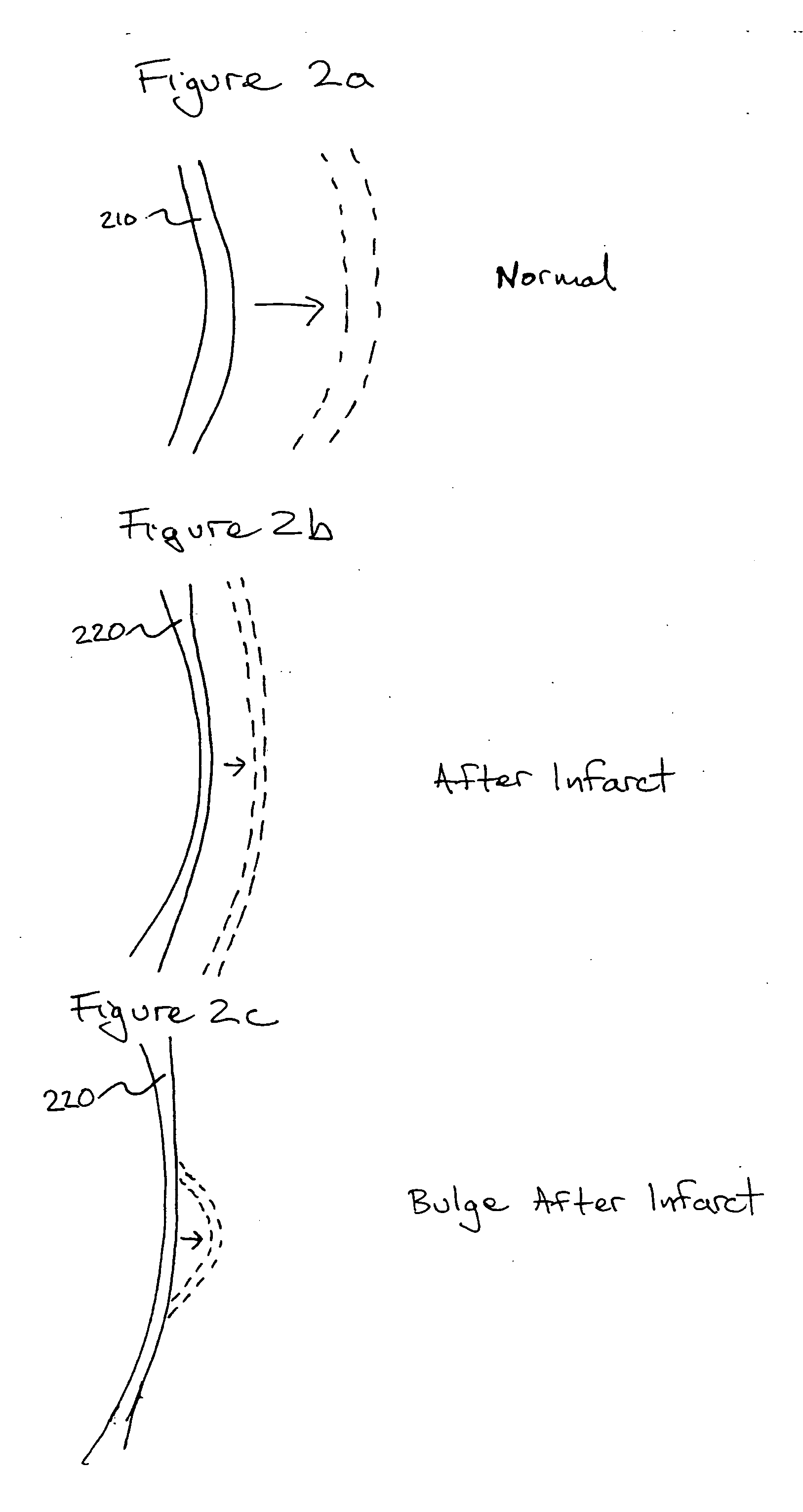
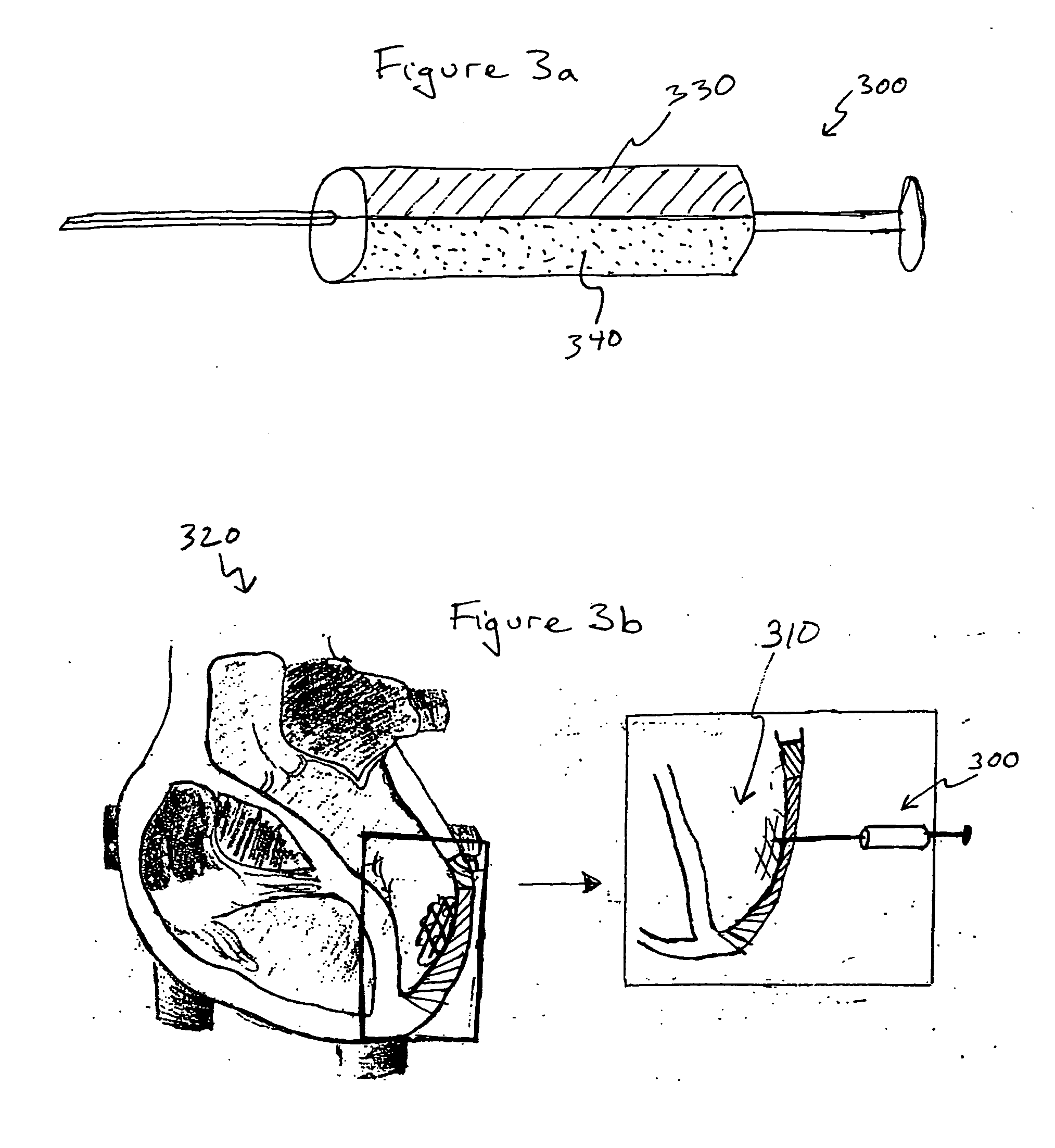
Bioscaffoldings formed of hydrogels that are crosslinked in situ in an infarcted region of the heart (myocardium) by a Michael's addition reaction or by a disulfide bond formed by an oxidative process are described. Each of the bioscaffoldings described includes hyaluronan as one of the hydrogel components and the other component is selected from collagen, collagen-laminin, poly-l-lysine, and fibrin. The bioscaffolding may further include an alginate component. The bioscaffoldings may have biofunctional groups such as angiogenic factors and stem cell homing factors bound to the collagen, collagen-laminin, poly-l-lysine, or fibrinogen hydrogel component. In particular, the biofunctional groups may be PR11, PR39, VEGF, bFGF, a polyarginine / DNA plasmid complex, or a DNA / polyethyleneimine (PEI) complex. Additionally, the hydrogel components may be injected into the infarct region along with stem cells and microspheres containing stem cell homing factors. The bioscaffolding may be formed on a stent or a cardiac medical device.
Owner:ABBOTT CARDIOVASCULAR
Crosslinked hyaluronic acid compositions for tissue augmentation
ActiveUS8124120B2Improve drug deliveryReduce frequencyAntibacterial agentsOrganic active ingredientsMedicineWater insoluble
Disclosed are hyaluronic acid (HA) compositions including crosslinked, water-insoluble, hydrated HA gel particles. Also disclosed are methods of making the HA compositions, and methods of using the HA composition to augment tissue in a subject.
Owner:ANIKA THERAPEUTICS INC
Topically applied Glucosamine Sulfate and all its related, precursor, and derivative compounds significantly increases the skin's natural produciton of hyaluronic acid for the rejuvenation of healthier younger-looking skin; while PhosphatidylCholine is required to replace its deficiency caused by topical Dimethylaminoethanol (DMAE)
InactiveUS20070092469A1Reducing eczemaReducing psoriasisBiocideCosmetic preparationsWrinkle skinPhysiology
A topical skin rejuvenation preparation to relieve wrinkles, increase the skin's natural production of hyaluronic acid, reverse the lack of suppleness, hydrate from within, erase spider veins, reduce varicose veins, lighten aging dark blotches (“liver spots” / Lentigos, Senile Lentigines), decrease acne, and reduce under eye puffiness includes Glucosamine (2-amino-2-deoxy-alpha-D-glucose), a hexosamine (6 carbon amino sugar), including its derivative and precursor compounds: Glucosamine Sulfate, Glucosamine Hydrochloride, Glucose-6-Phosphate, Acetyl Glucosamine, Fructose-6-phosphate, Glucosamine-6-Phosphate, to increase production of Hyaluronic acid and collagen from Glucosamine Sulfate, its precursors and derivatives and to increase skin muscle tone by Dimethylaminoethanol (DMAE) while over coming deficiency it creates in each cell's production of PhosphatidylCholine, whose deficiency damages cell membranes, as well as mitochondrial and lysosome membranes.
Owner:JACOBS ERIC
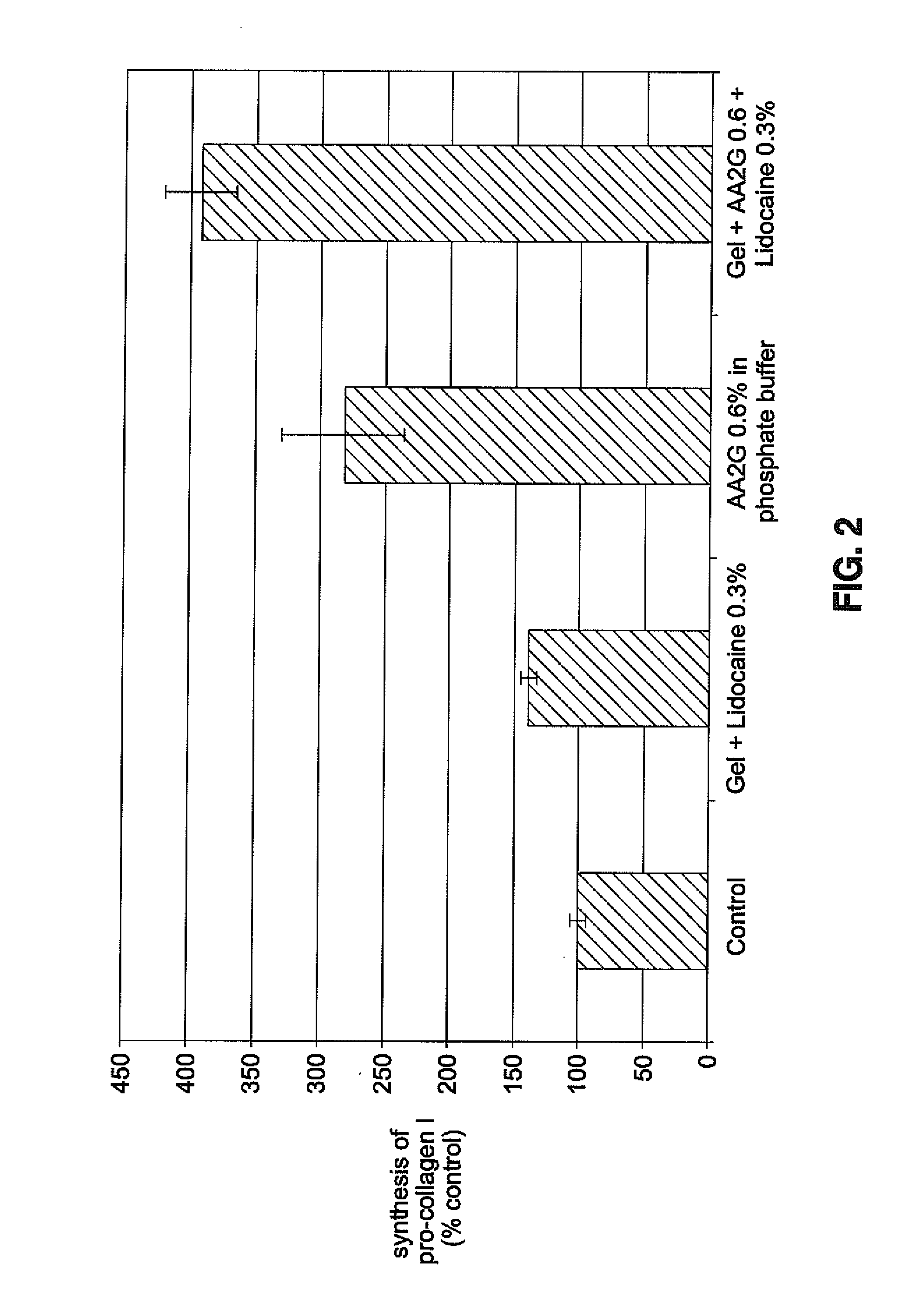
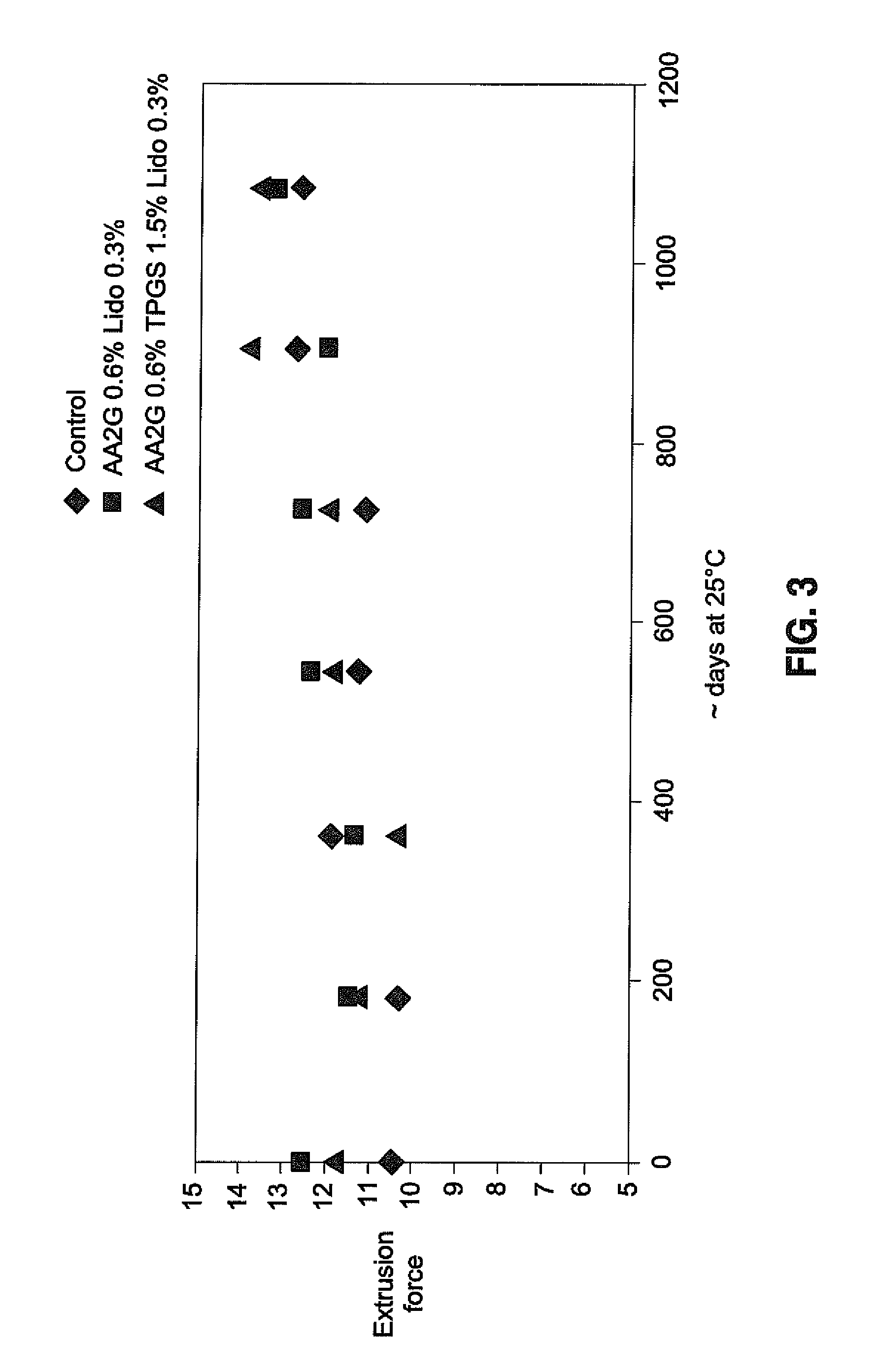
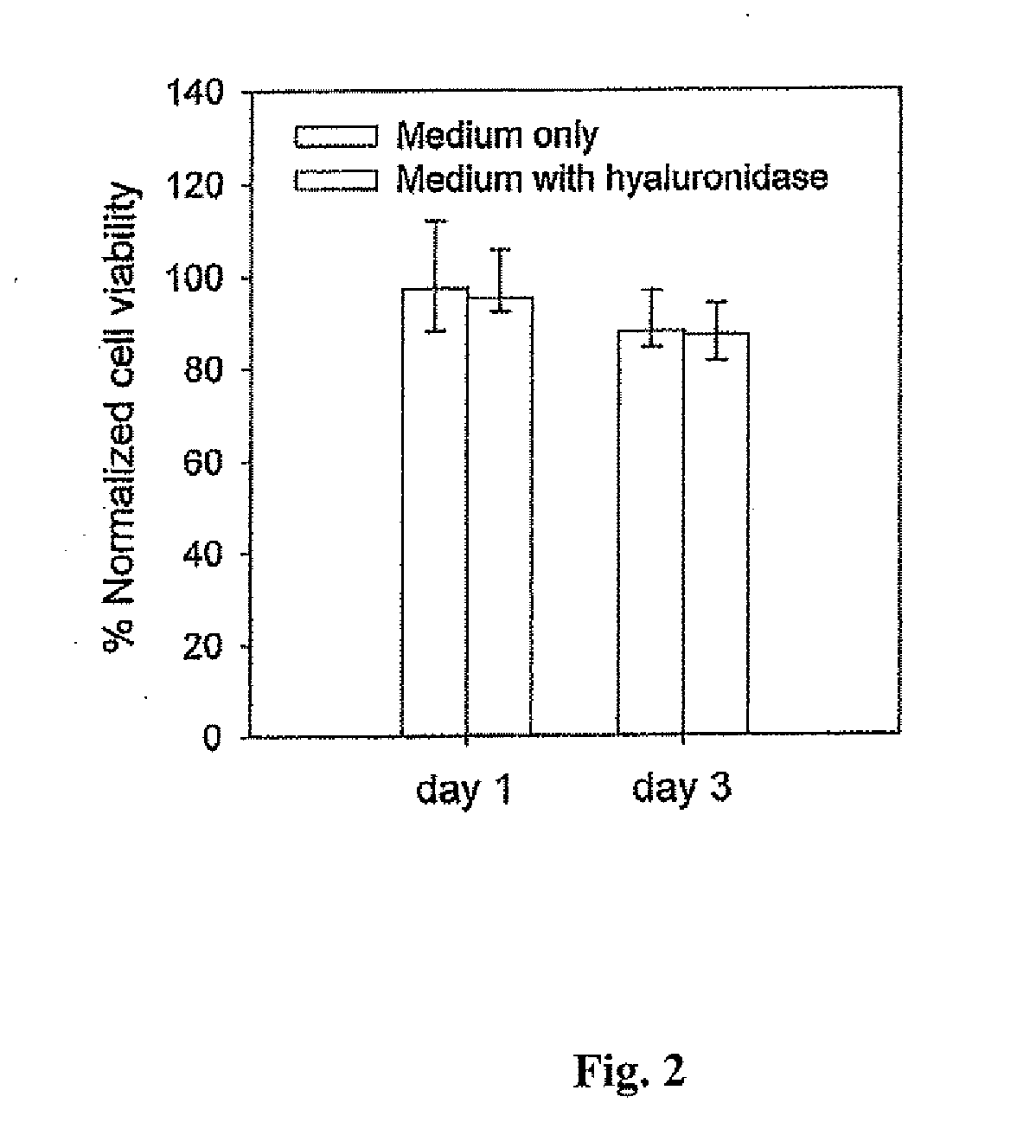
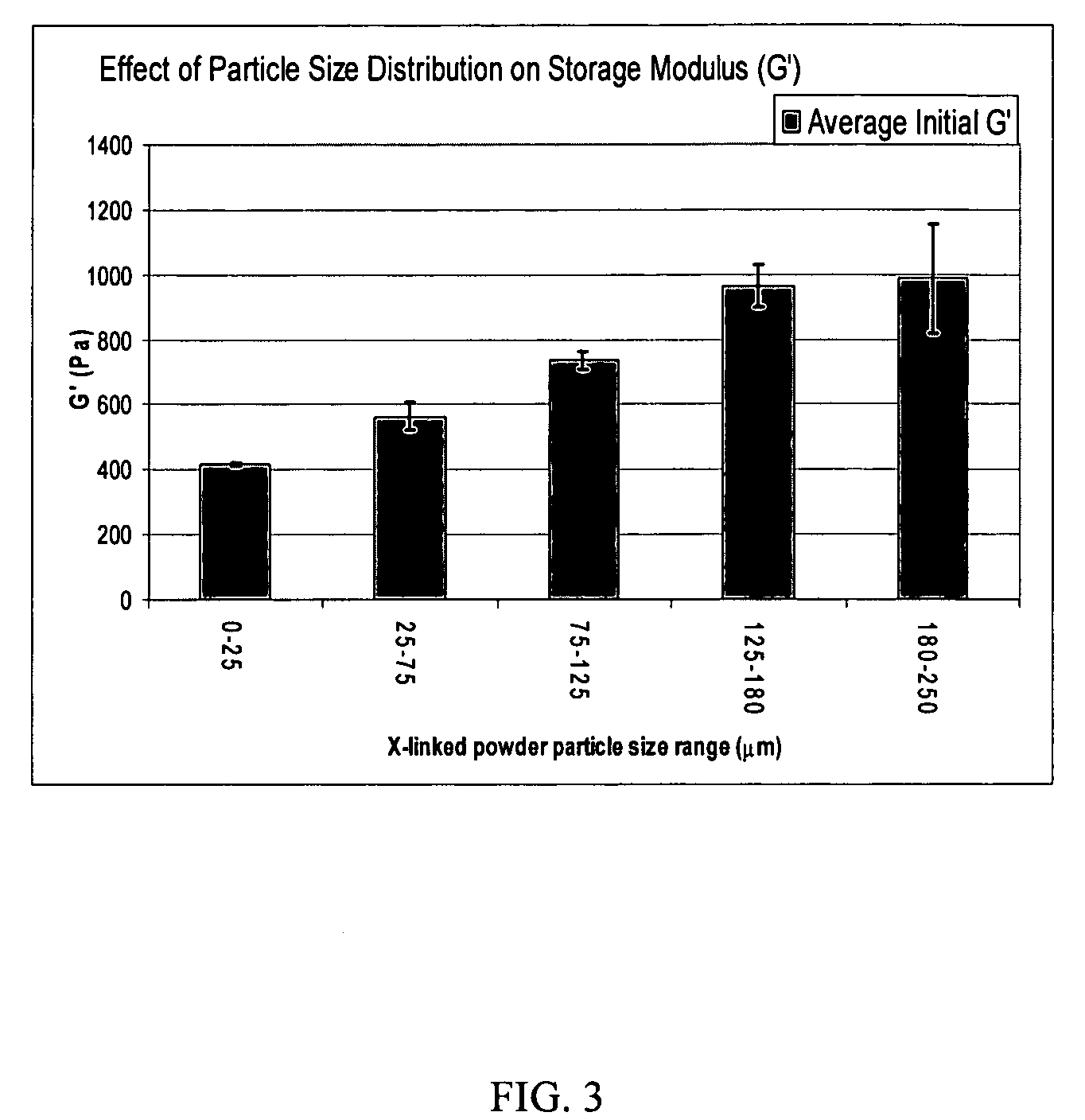
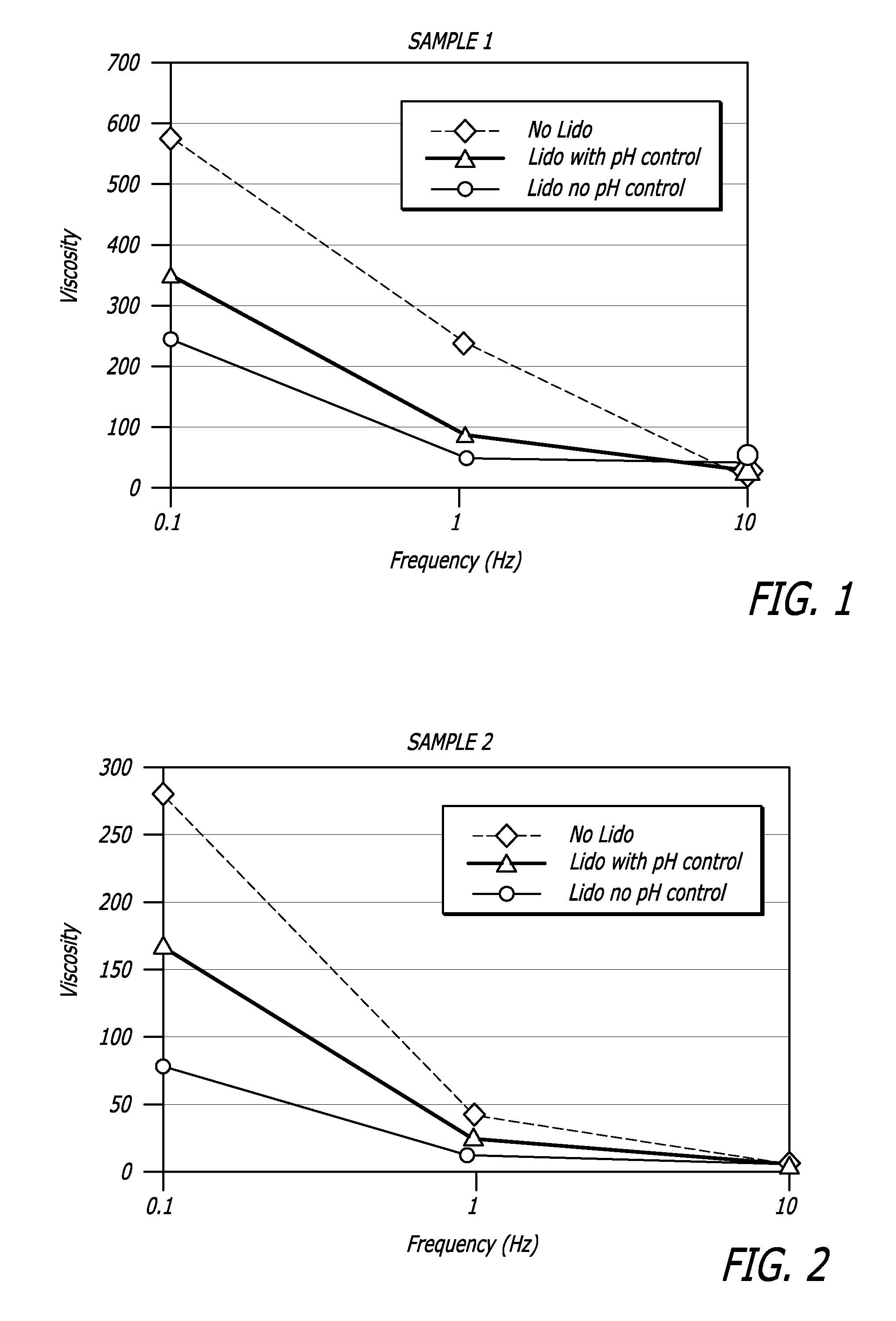
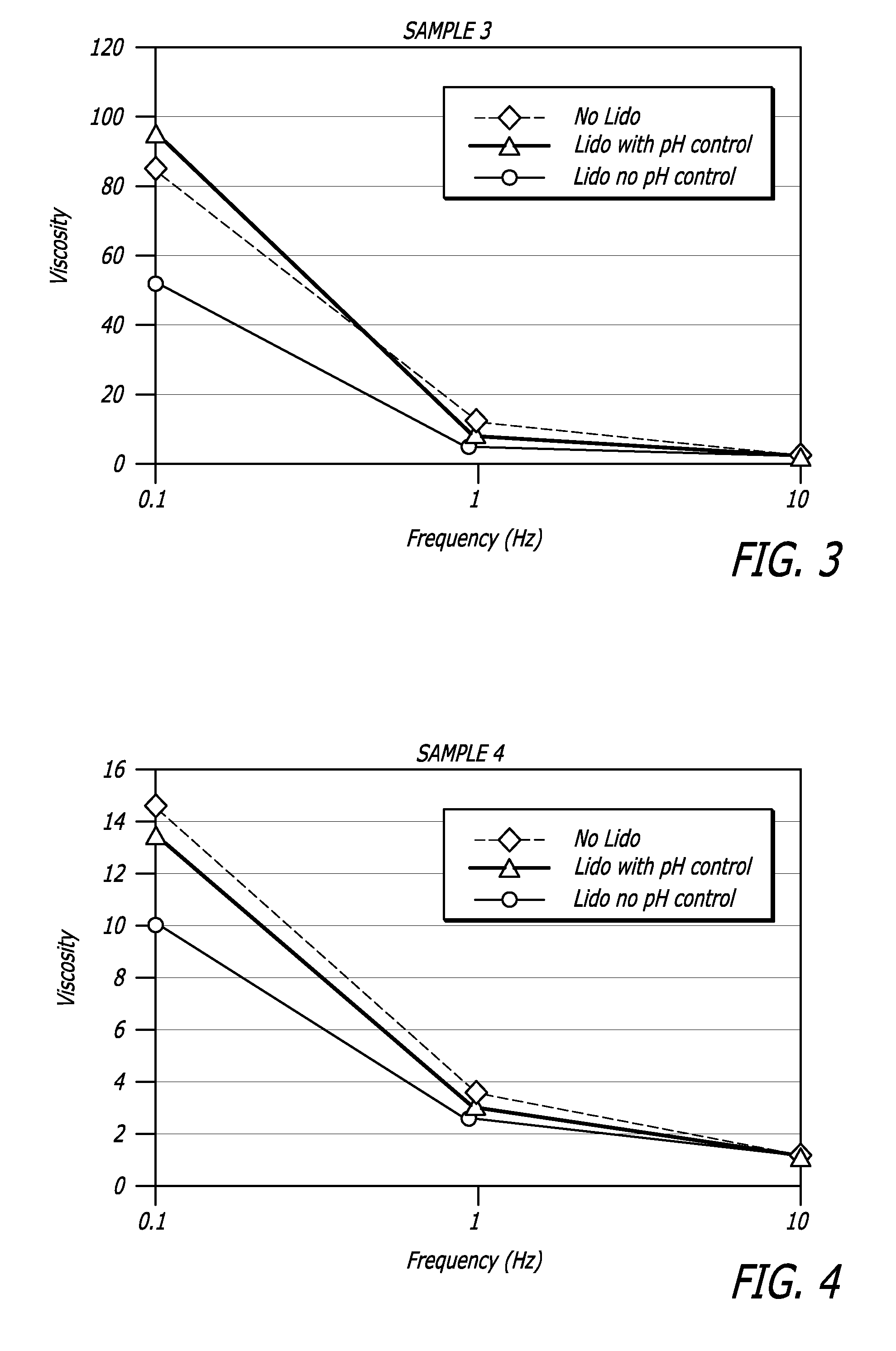
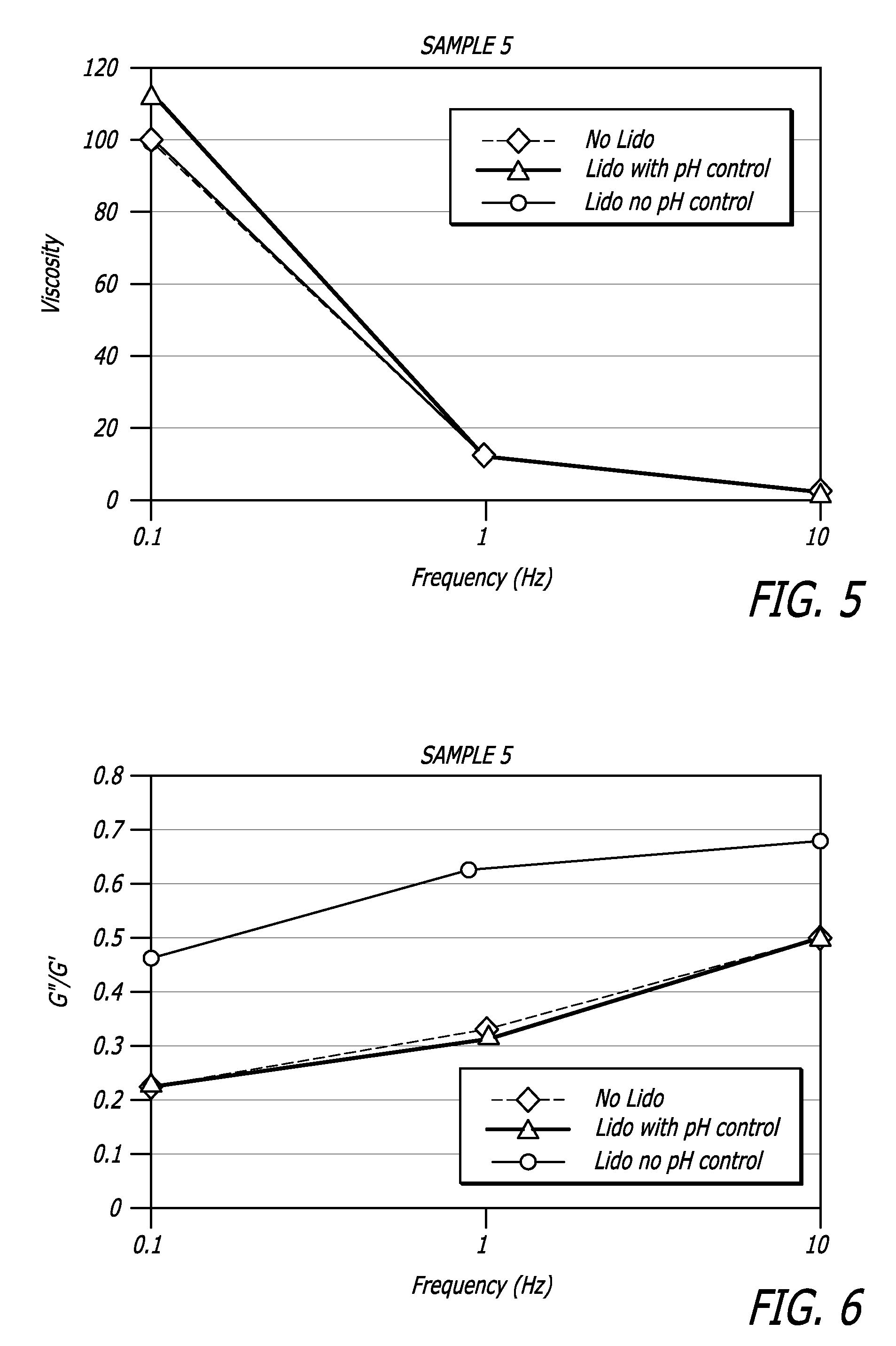
Hyaluronic acid-based gels including lidocaine
Disclosed herein are cohesive soft tissue fillers, for example, dermal and subdermal fillers, based on hyaluronic acids and pharmaceutically acceptable salts thereof. In one aspect, hyaluronic acid-based compositions described herein include a therapeutically effective amount of at least one anesthetic agent, for example, lidocaine. The present hyaluronic acid-based compositions including lidocaine have an enhanced stability and cohesivity, relative to conventional compositions including lidocaine, for example when subjected to sterilization techniques or when stored for long periods of time. Methods and processes of preparing such hyaluronic acid-based compositions are also provided.
Owner:ALLERGAN IND
Composition and method for healing tissues
InactiveUS20050147679A1Enhances chemotactic activityGood healing effectOrganic active ingredientsBiocideAdhesiveAdditive ingredient
The composition and method for healing tissues is a medicinal composition for facilitating the growth, protection and healing of tissues and cells in animals and humans. The composition is formulated as a either a powder, gel, paste, film, fluid injectable, rehydratable freeze-dried paste or sponge, sprayable solution, topically applied patch with adhesive and reservoir system, an intermediate for coatables such as films and bandages, a matrix for membranes, or as a matrix of flexible polymer(s), or delivered as either an orally ingestible liquid, tablet or capsule. The main ingredient of the formulated compositions is hydrolyzed collagen, which can be combined with polysulfated glycosaminoglycans, hyaluronic acid or salts thereof, or a glucosamine salt, and mixtures thereof. The composition may be formulated as an aqueous eye drop solution.
Owner:PETITO GEORGE D +1
Topical anti-wrinkle and anti-aging moisturizing cream
InactiveUS20100098794A1Restoring skinRestoring to stateOrganic active ingredientsBiocideWater basedMedicine
The present invention relates to a topical anti-wrinkle and anti-aging moisturizing cream designed (formulated) to repair and restore human adult skin to its original youthful state. This has successfully been achieved by using a water based molecular mixture of high molecular weight (0.5 to 2.0 million Daltons) hyaluronic acid (HA) and of low molecular weight hyaluronic acid oligosaccharides (small HA fragments less than 12000 Daltons molecular weight) to create this unique anti-aging moisturizer. An equal mixture of high molecular HA and HA-oligosaccharides at concentrations of 0.1-0.2% was found to be essential for providing our moisturizer its unique ability to repair and rejuvenate adult human skin.
Owner:ARMAND GERARD
Ester derivatives of hyaluronic acid for the preparation of hydrogel materials by photocuring
The present invention relates to hyaluronic acid ester derivatives, whose carboxylic groups are partially esterified with hydroxy groups of propiophenone derivatives, to the hydrogel materials consisting of the said hyaluronic acid ester derivatives, to their preparation process by photocuring of the hyaluronic acid ester derivatives, and their use in the biomedical, sanitary and surgical fields, and in the medical field as controlled release systems for drugs.
Owner:FIDIA FARM SPA
Method for texturing the surface of a synthetic implant
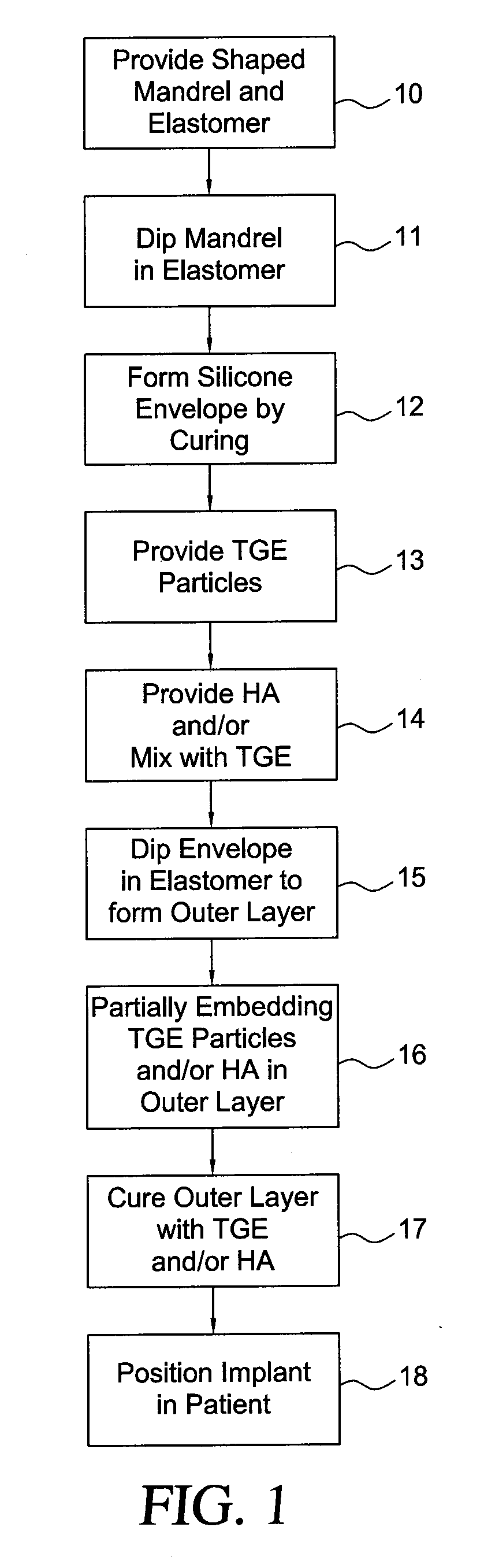
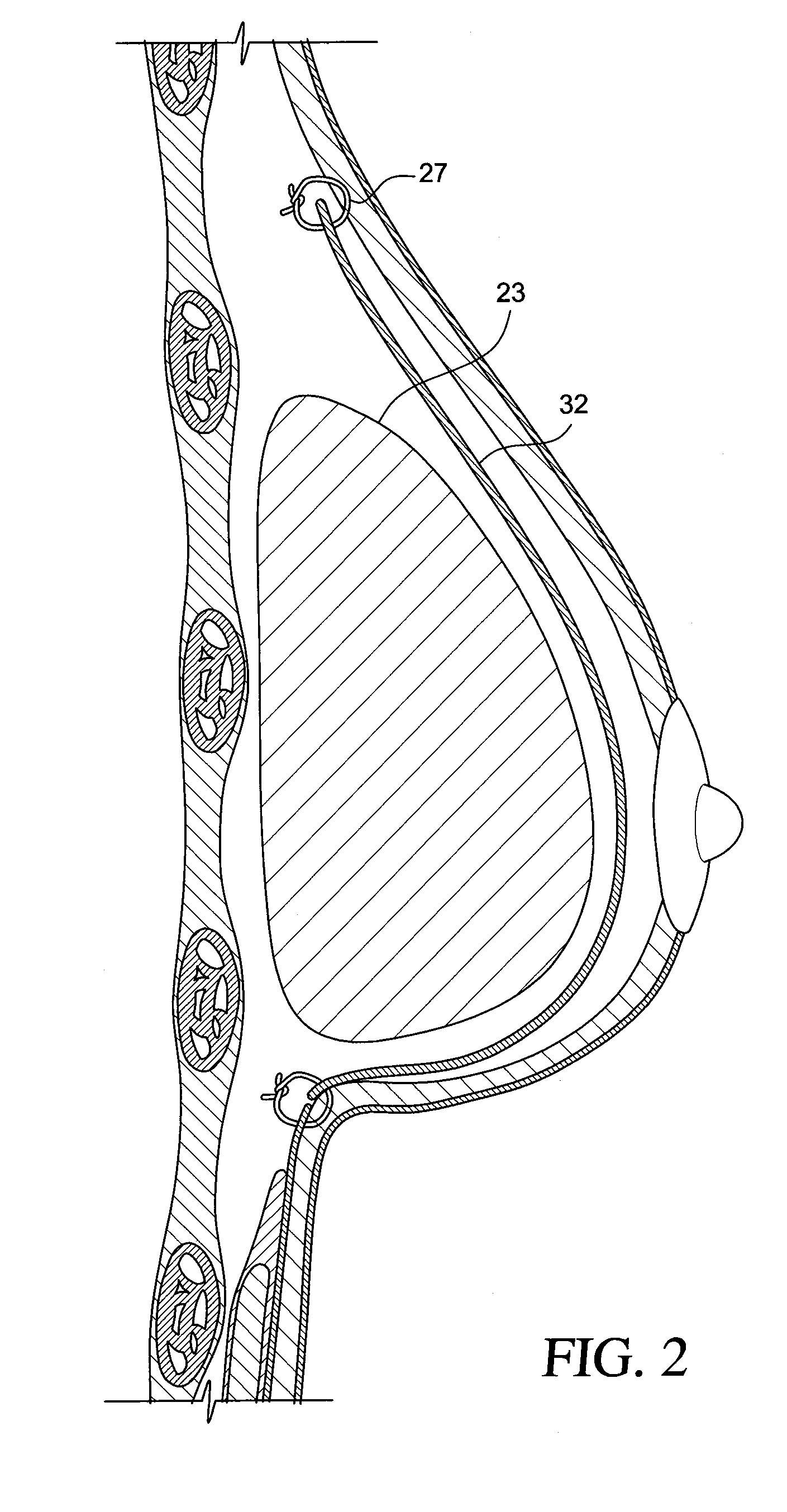
InactiveUS20090198333A1Increase heightReducing capsular contractionMammary implantsDiagnosticsBreast implantAcellular Dermis
A method for texturing the surface of a breast implant includes the step of partially impregnating a silicone outer surface of the implant with particles of a biologically active material such as acellular dermis of human or animal origin impregnated with hyaluronic acid. The biologically active material promotes tissue ingrowth into a plurality of cavities filled with a biologically active material.
Owner:BECKER HILTON
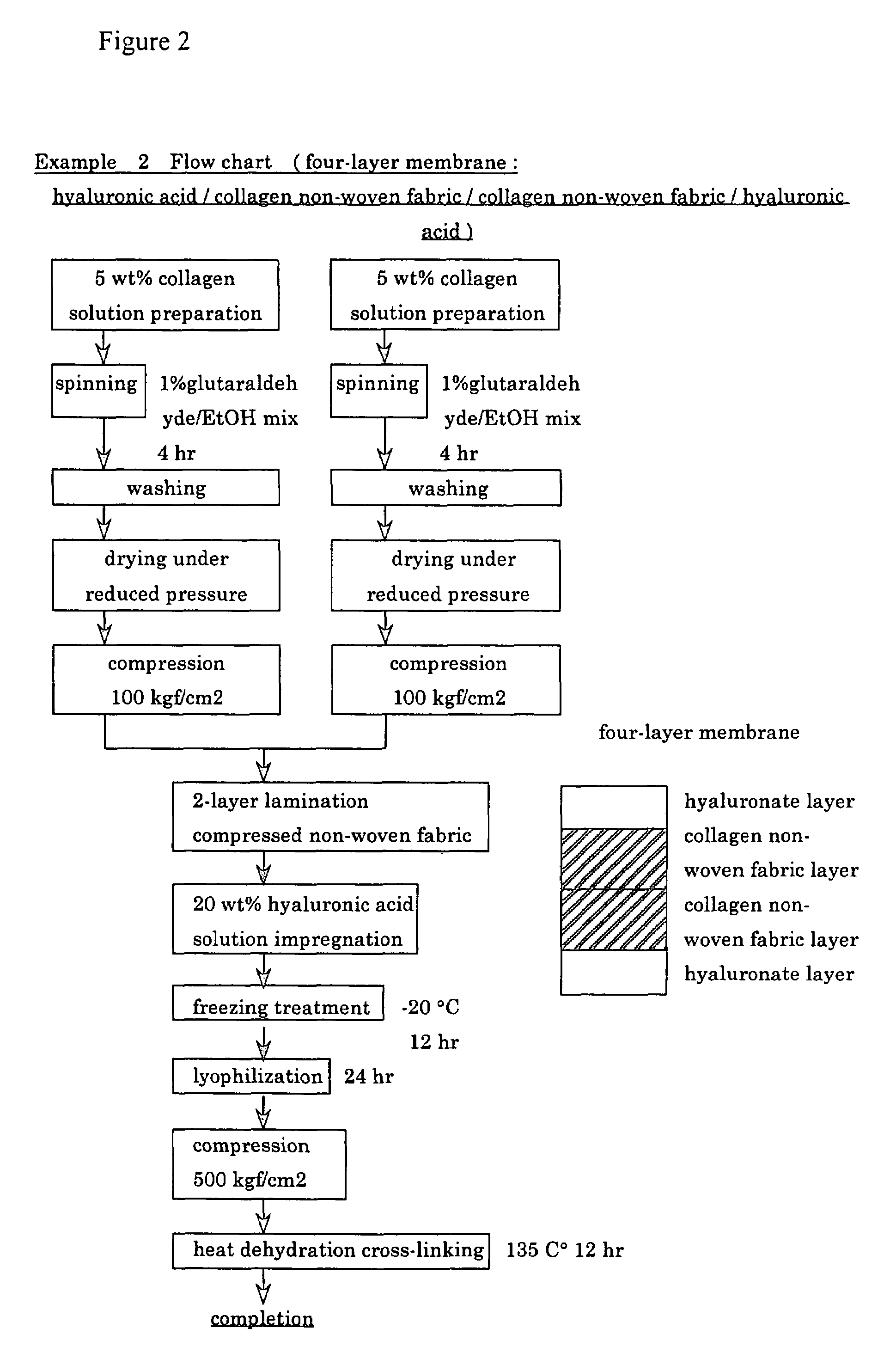
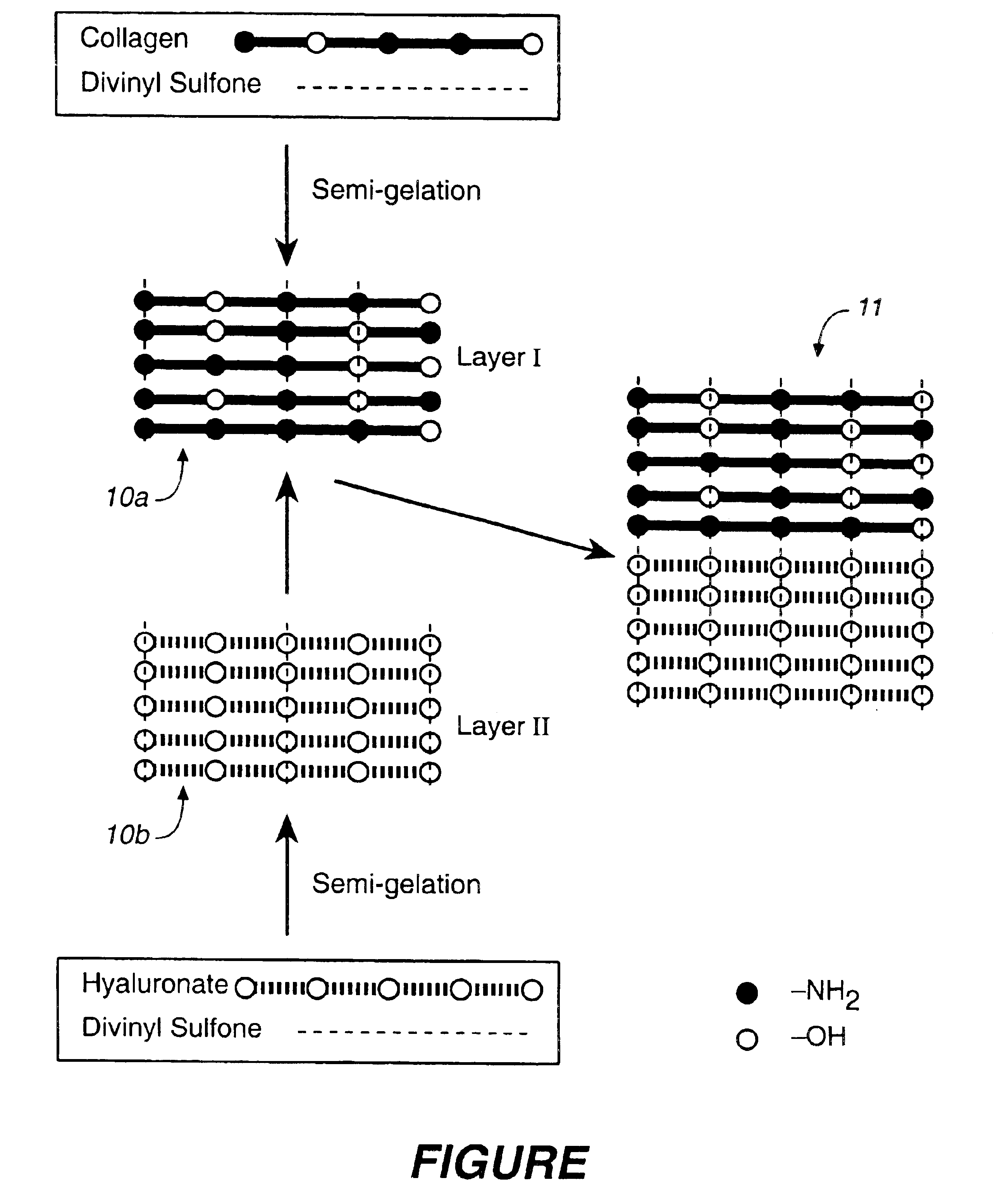
Collagen/polysaccharide bilayer matrix
Disclosed are bilayer matrices of a polysaccharide such as collagen (COL) and another polysaccharide such as hyaluronic acid (HA) with various COL / HA ratios. Each layer has a porous structure. These materials are useful for tissue regeneration, particularly when used with orthopedic implants and drug delivery.
Owner:DEPUY SPINE INC (US)
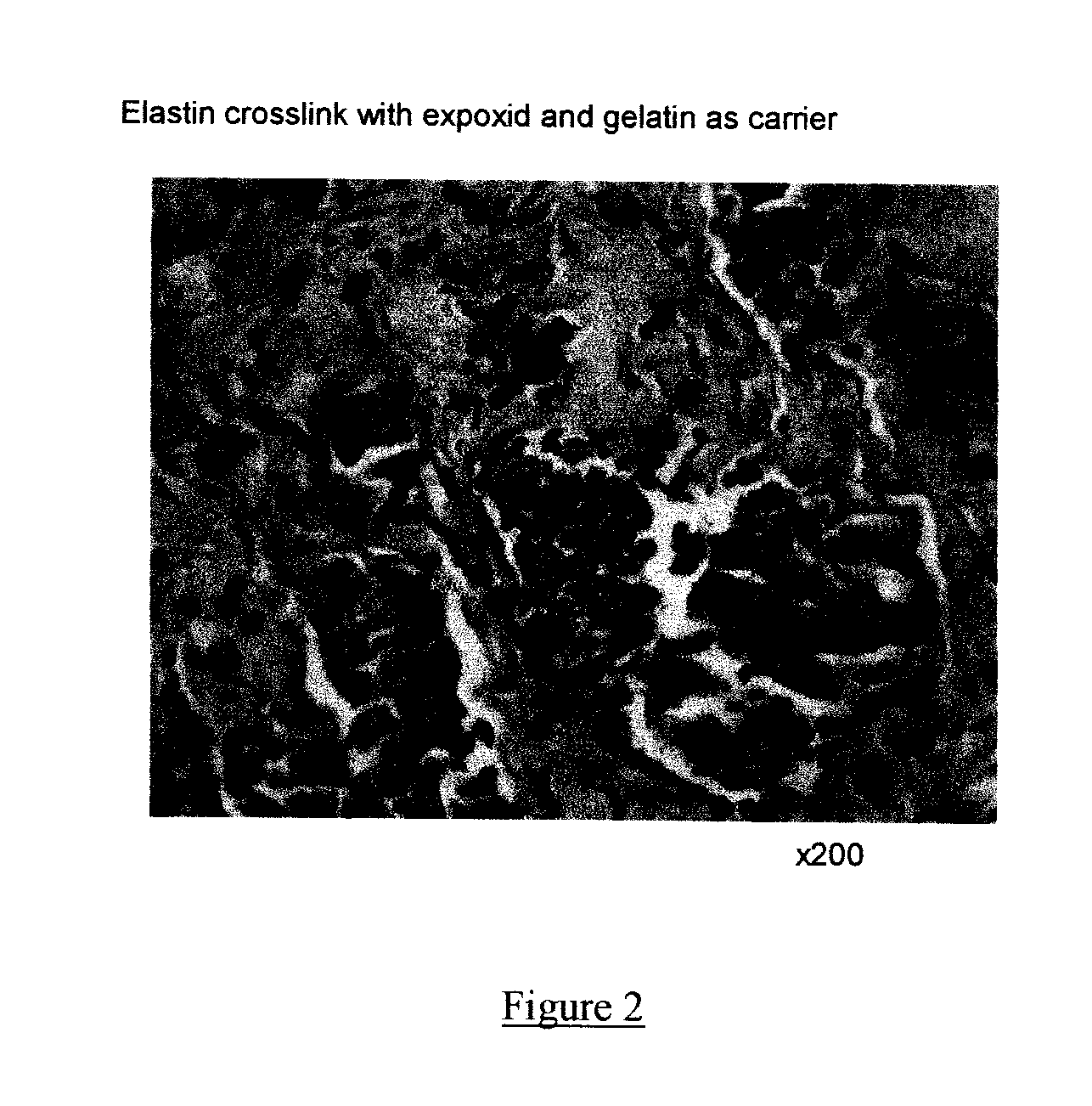
Composite containing collagen and elastin as a dermal expander and tissue filler
ActiveUS20120010146A1Easy injectionLesser tendency can be reactiveCosmetic preparationsPeptide/protein ingredientsTissue expansionCollagen VI
An injectable composition having dermal filling and tissue expanding activity comprises (1) a quantity of elastin sufficient to bring about dermal filling and tissue expansion when injected into a subject in need of dermal filling and tissue expansion, and (2) a pharmaceutically acceptable carrier. The composition can further comprise collagen, in other alternatives, the composition can further comprise hyaluronic acid, and one or more of the elastin, the collagen, and the hyaluronic acid, if present can be cross-linked, either intramolecularly or intermolecularly. The elastin, however, is the primary filler, even if collagen or hyaluronic acid are included in the composition
Owner:TRUELASTIN LAB INC
Ophthalmic formulations
InactiveUS6953776B2Improve bioavailabilityImprove toleranceOrganic active ingredientsBiocideCyclosporinsAqueous solution
A topical ophthalmic formulation in the form of an aqueous solution comprising a cyclosporin, hyaluronic acid or one of its salts, and polysorbate 80 is described.
Owner:LABE MEDIDOM
Biomaterials for preventing post-surgical adhesions comprised of hyaluronic acid derivatives
New biomaterials essentially constituted by esterified derivatives of hyaluronic acid or by cross-linked derivatives of hyaluronic acid for use in the surgical sector, particularly for use in the prevention of post-surgical adhesions.
Owner:ANIKA THERAPEUTICS SRL
Compositions and Methods for Improving the Appearance of Facial Texture
InactiveUS20120156146A1Good lookingAppearance of texture is enhancedBiocideCosmetic preparationsFacial skinMicro texture
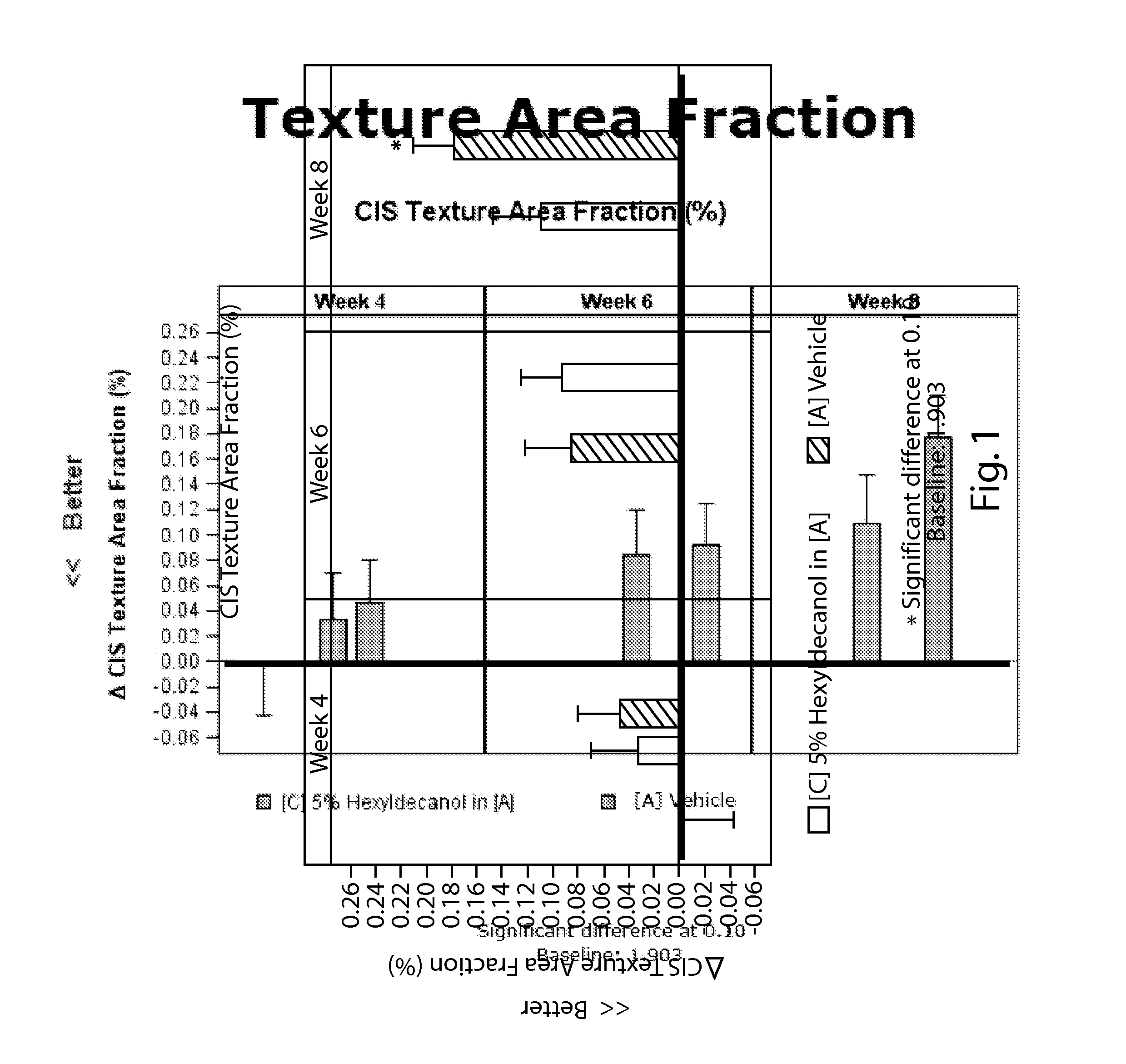
A method of improving the appearance of facial texture may comprise the step of applying a composition comprising an effective amount of a material that regulates hyaluronic acid synthesis to an area of textured facial skin, wherein the composition is applied for a period of time sufficient for the material to improve the appearance of the facial texture. In some embodiments, the material that regulates hyaluronic acid synthesis is hexyldecanol. The method may also include the step of identifying facial texture on a facial skin surface. In particular embodiments, the facial skin texture is selected from the group consisting of macro-texture, micro-texture, and combinations thereof.
Owner:THE PROCTER & GAMBLE COMPANY
Prion-free collagen and collagen-derived products and implants for multiple biomedical applications; methods of making thereof
InactiveUS6197935B1Preserve integrityPeptide/protein ingredientsImmunoglobulinsIn vivo biocompatibilityCollagen VI
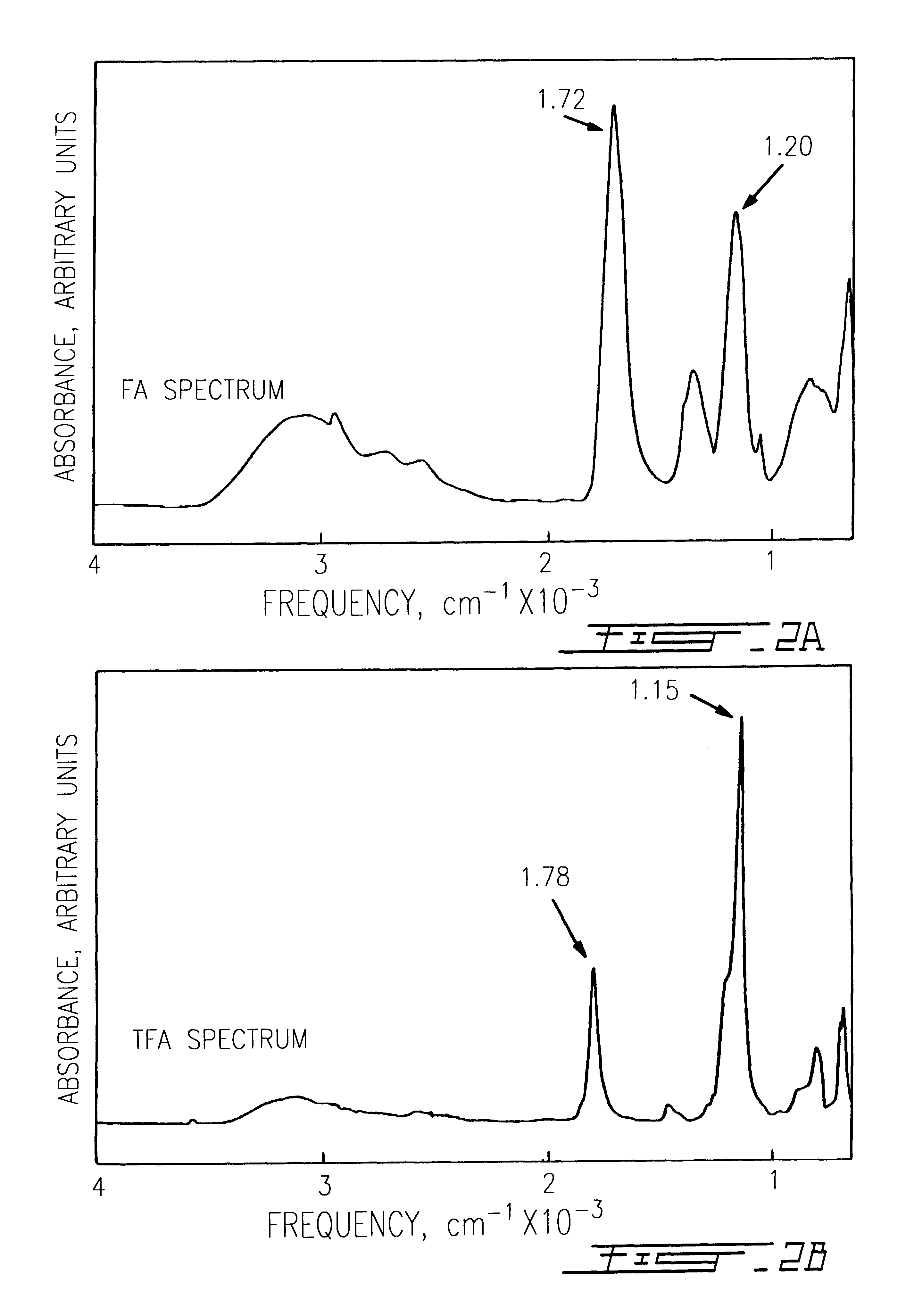
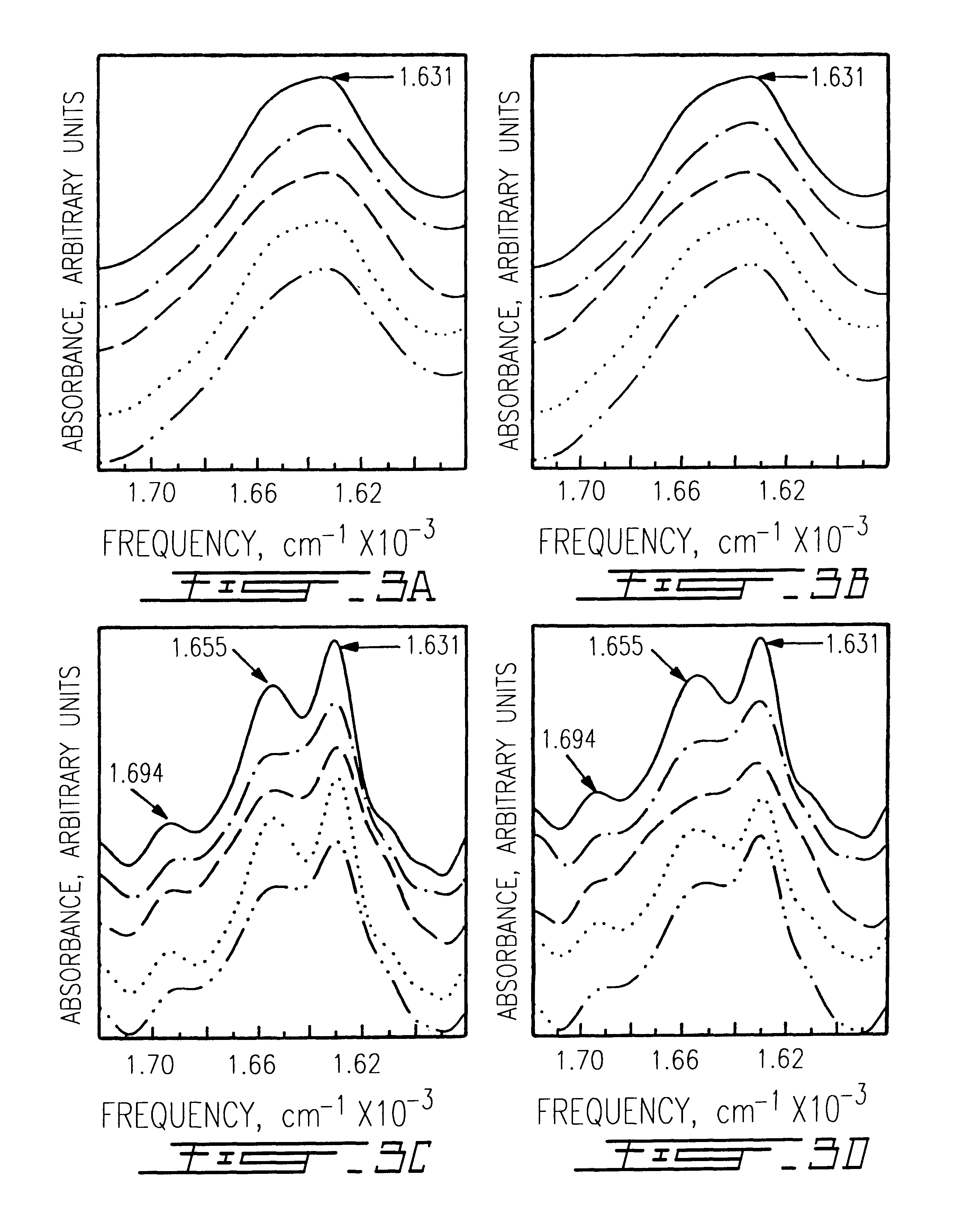
The use of collagen as a biomedical implant raises safety issues towards viruses and prions. The physicochemical changes and the in vitro and in vivo biocompatibility of collagen treated with heat, and by formic acid (FA), trifluoroacetic acid (TFA), tetrafluoroethanol (TFE) and hexafluoroiso-propanol (HFIP) were investigated. FA and TFA resulted in extensive depurination of nucleic acids while HFIP and TFE did so to a lesser degree. The molecules of FA, and most importantly of TFA, remained within collagen. Although these two acids induced modification in the secondary structure of collagen, resistance to collagenase was not affected and, in vitro, cell growth was not impaired. Severe dehydrothermal treatment, for example 110° C. for 1-3 days under high vacuum, also succeeded in removing completely nucleic acids. Since this treatment also leads to slight cross-linking, it could be advantageously used to eliminate prion and to stabilize gelatin products. Finally, prolonged treatment with TFA provides a transparent collagen, which transparency is further enhanced by adding glycosaminoglycans or proteoglycans, particularly hyaluronic acid. All the above treatments could offer a safe and biocompatible collagen-derived material for diverse biomedical uses, by providing a virus or prion-free product.
Owner:UNIV LAVAL
Hyaluronic Acid Compound, Hydrogel Thereof and Joint Treating Material
ActiveUS20080193538A1Good biological adaptabilityPowder deliveryBiocideBiocompatibility TestingHyaluronic acid
A hyaluronic acid compound which is a reaction product between hyaluronic acid and a phosphatidyl ethanolamine. This compound has biocompatibility and bio-safety, and can be formed into a hydrogel or molded form having a certain shape. Making use of these properties, it is used to treat a knee joint, prevent the accretion of a tissue after an operation and keep a skin wet.
Owner:TEIJIN LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com