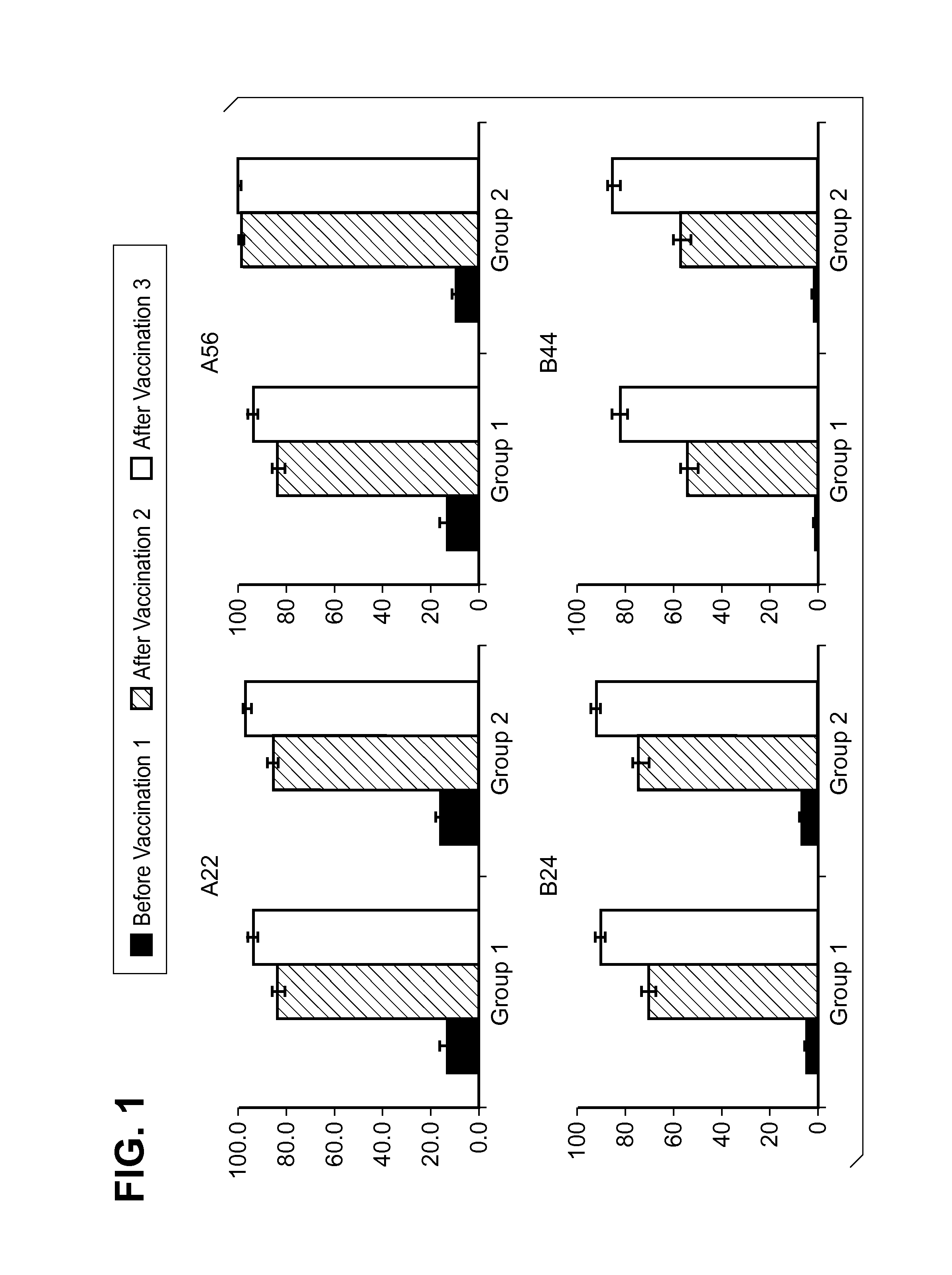
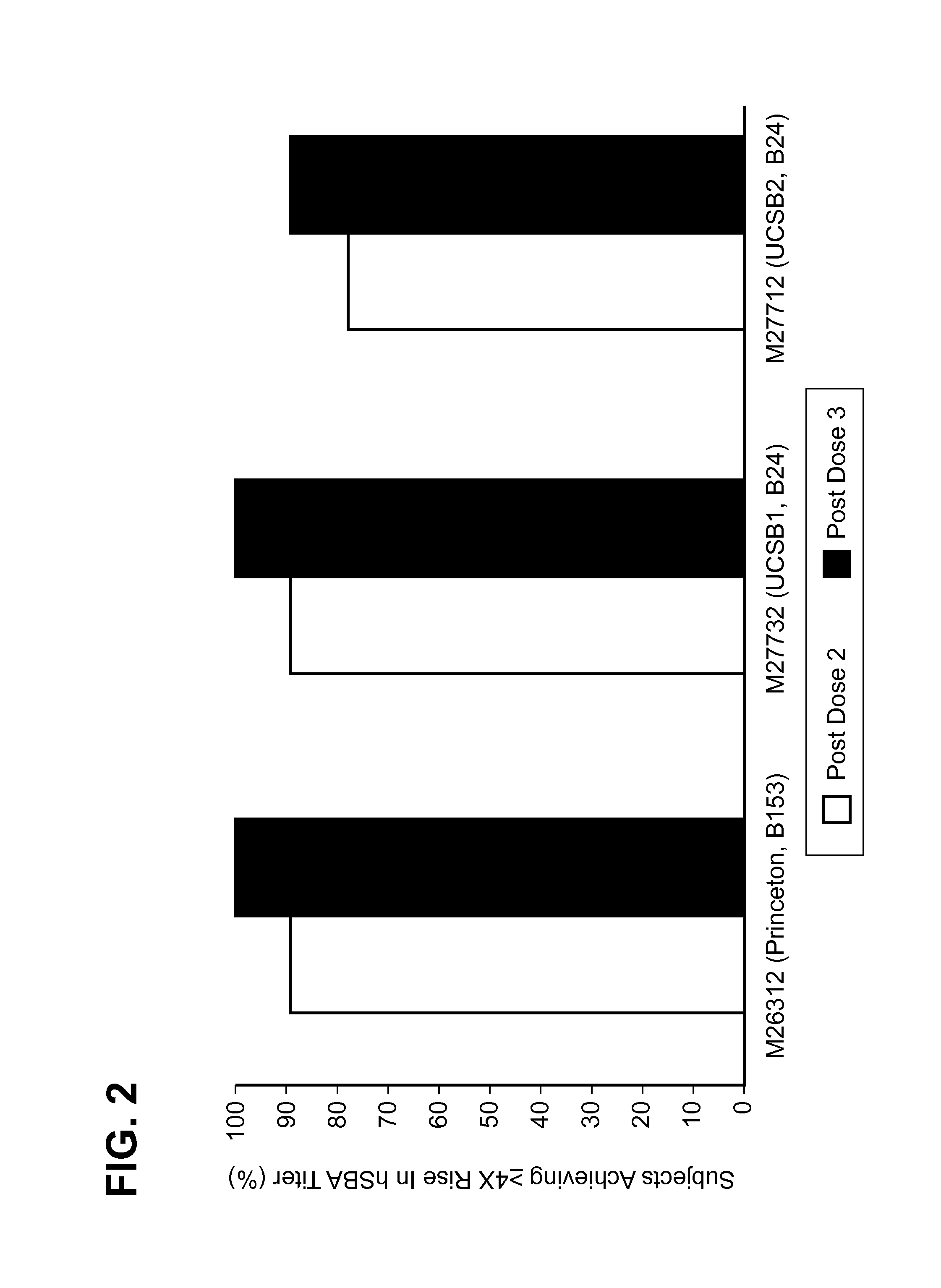
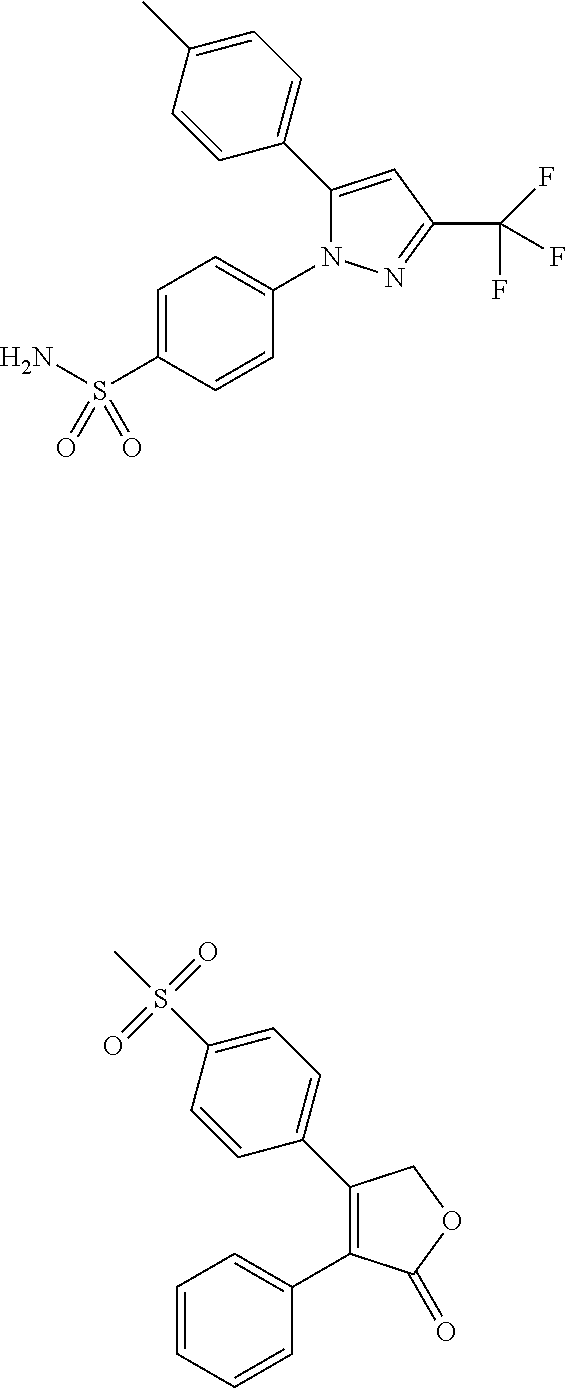
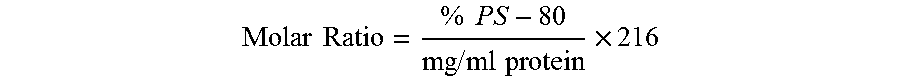Patents
Literature
1018 results about "Polysorbate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
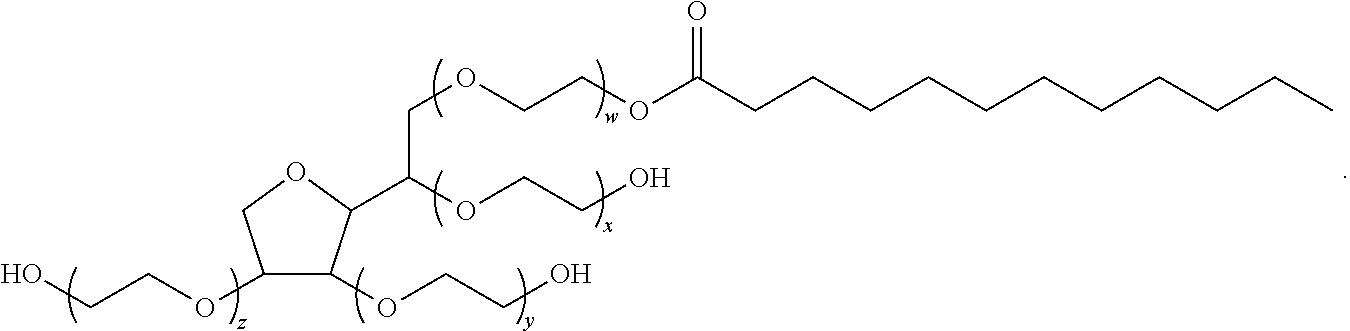
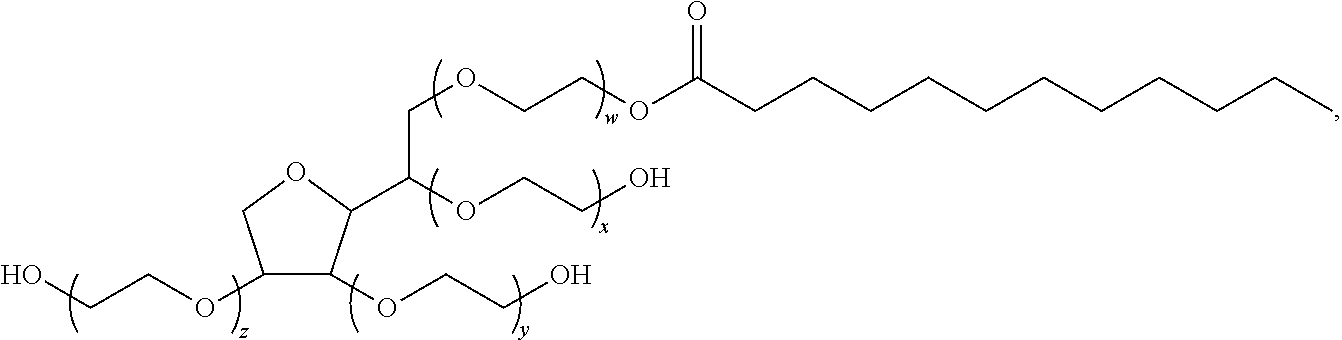
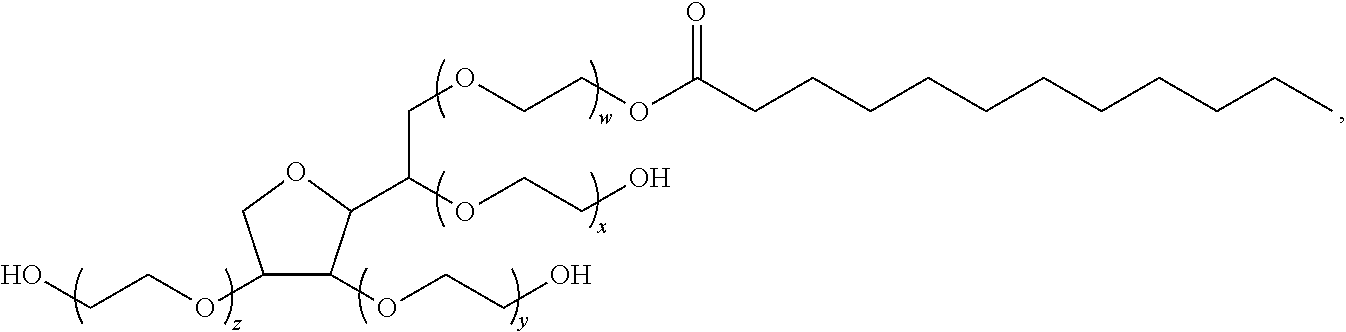
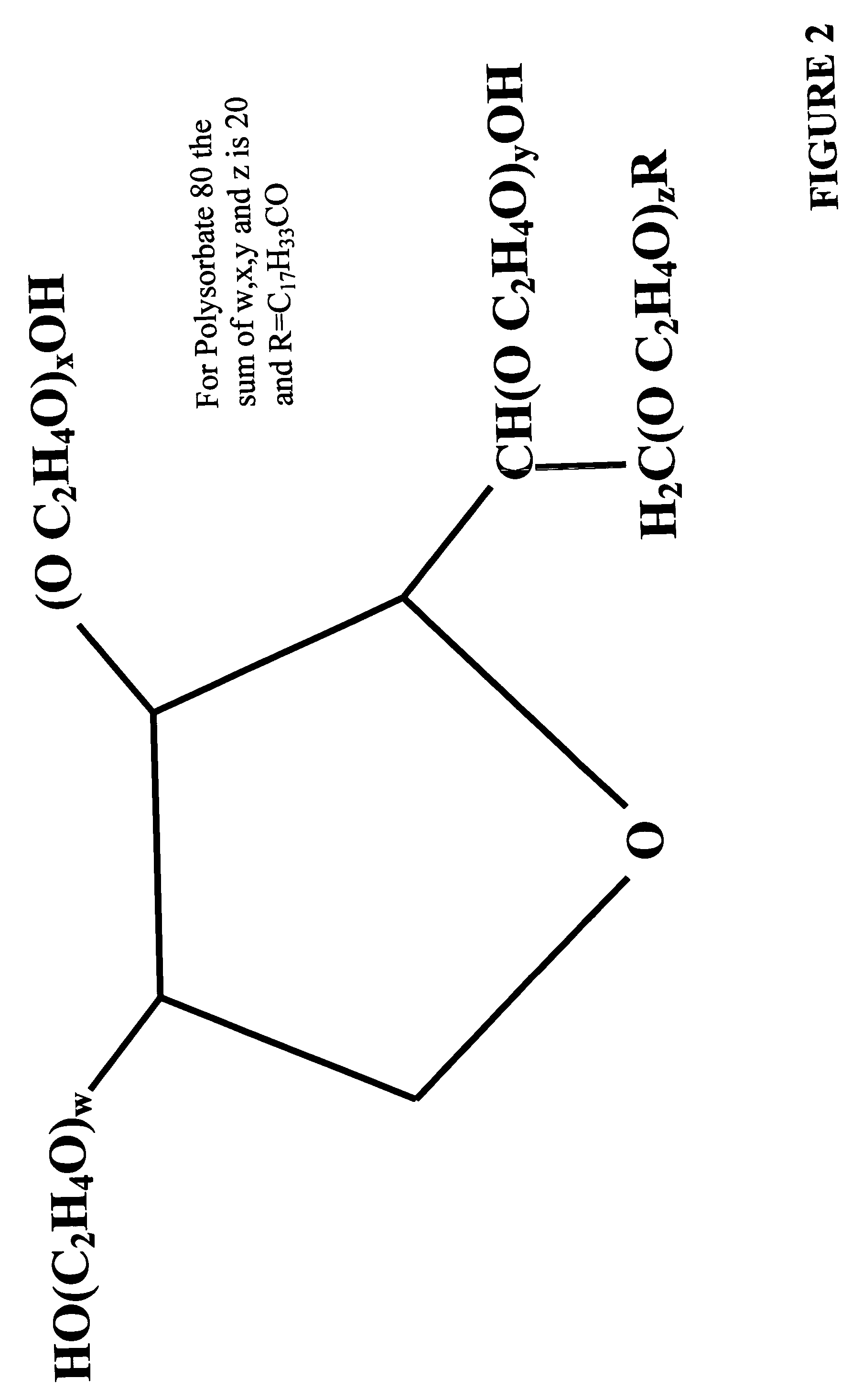
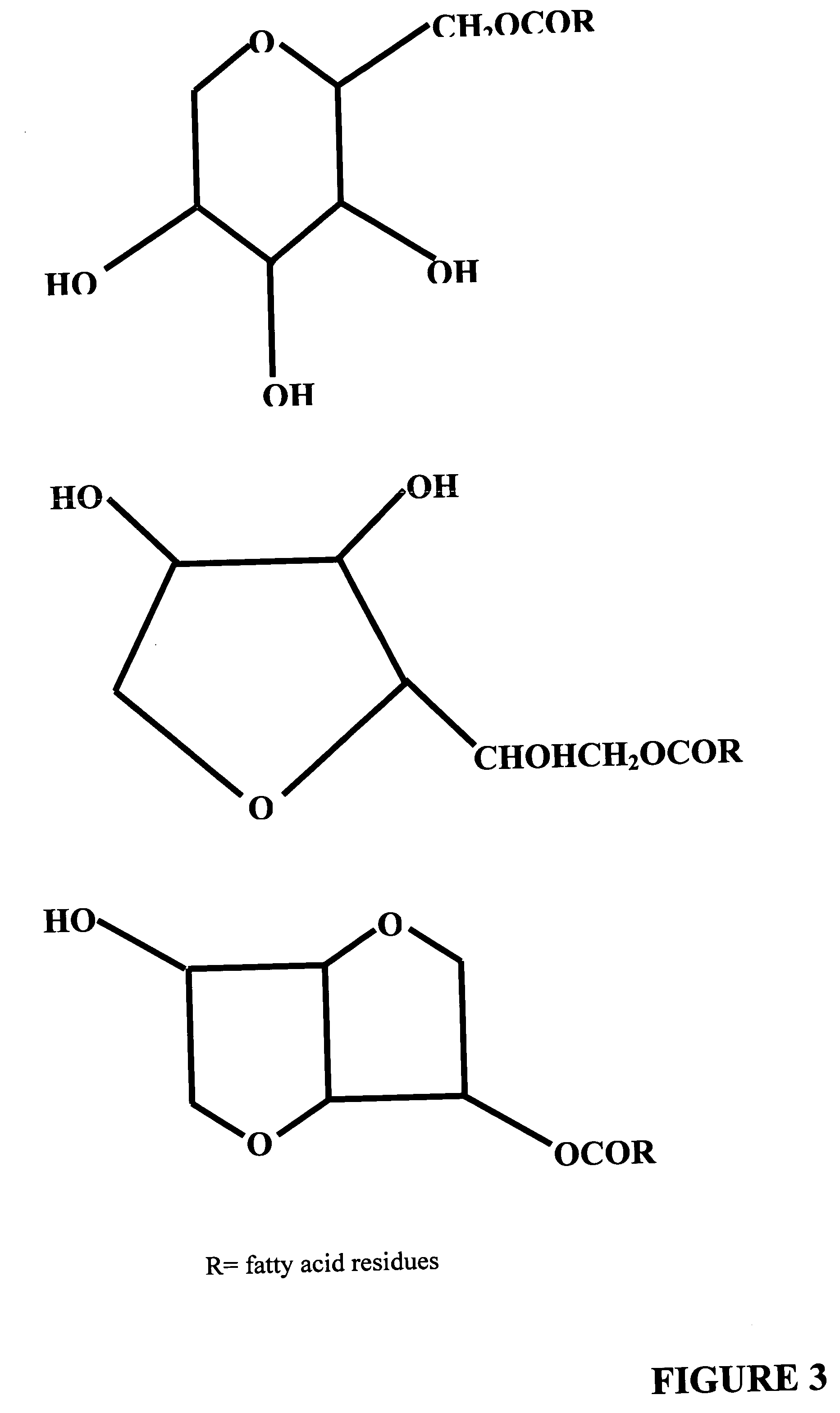
Polysorbates are a class of emulsifiers used in some pharmaceuticals and food preparation. They are often used in cosmetics to solubilize essential oils into water-based products. Polysorbates are oily liquids derived from ethoxylated sorbitan (a derivative of sorbitol) esterified with fatty acids. Common brand names for polysorbates include Scattics, Alkest, Canarcel, and Tween.
Injectable compositions of nanoparticulate immunosuppressive compounds
InactiveUS20060210638A1Improve complianceImprove efficacyPowder deliveryBiocideDepressantCompound (substance)
The invention is directed to an injectable nanoparticulate immunosuppressant composition for the formation of a subcutaneous or intramuscular depot. The invention is also directed to an injectable composition of nanoparticulate tacrolimus and / or sirolimus which eliminates the need to use polyoxyl 60 hydrogenated castor oil (HCO-60) and / or polysorbate 80 as a solubilizer. This invention further discloses a method of making an injectable nanoparticulate tacrolimus and / or sirolimus composition and is also directed to methods of treatment using the injectable nanoparticulate formulations comprising tacrolimus, sirolimus, or combination thereof for a subcutaneous or intramuscular depot for the prophylaxis of organ rejection and for the treatment of psoriasis or other immune diseases
Owner:ELAN PHRMA INT LTD
Stable lyophilized pharmaceutical formulation of IgG antibodies
InactiveUS7592004B2Process stabilityAvoid formingPowder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-IL2 ReceptorHigh concentration
Owner:ABBVIE BIOTHERAPEUTICS
Method for preparing high-efficient organic-inorganic compound fertilizer from biogas slurry
ActiveCN103626575AConvenient supplementImprove overall utilizationWaste based fuelFertilizer mixturesFermentationOrganic inorganic
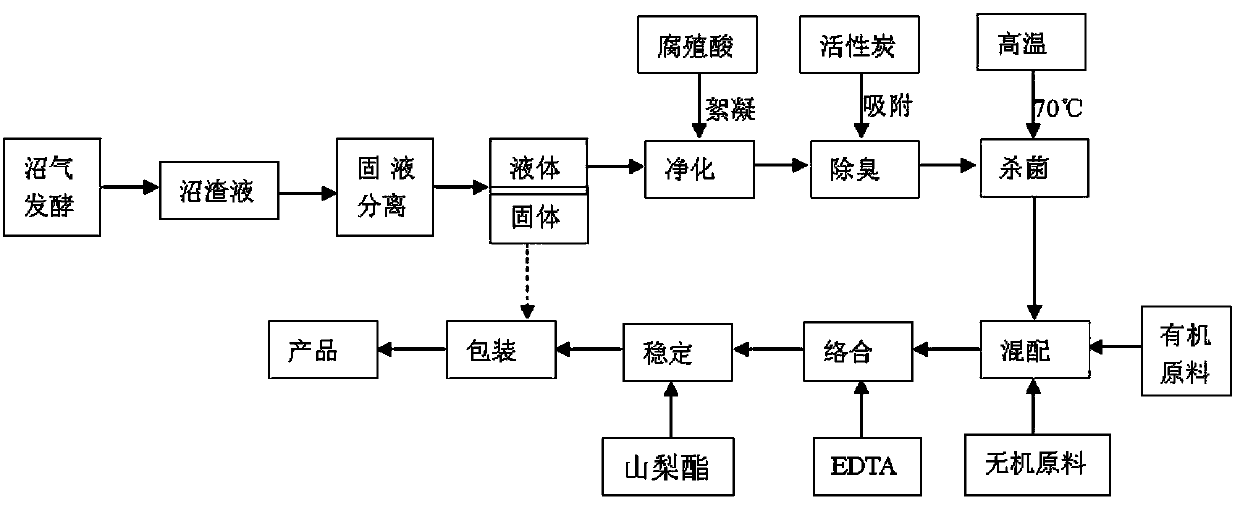
The invention discloses a method for preparing a high-efficient organic-inorganic compound fertilizer from biogas slurry. The method comprises the following steps: a) performing solid-liquid separation on a biogas residue liquid obtained from biogas fermentation to respectively obtain a solid and a liquid; b) purifying the liquid by utilizing humic acid as a flocculant; c) removing smelly substances in the liquid by utilizing activated carbon; d) performing high-temperature sterilization on the liquid; e) reducing the temperature of the biogas slurry to room temperature and then adding organic raw materials and inorganic raw materials for mixing; f) adding EDTA (ethylene diamine tetraacetie acid) for performing complexing, and adding polysorbate-80 for stabilizing; and g) uniformly mixing to obtain the organic-inorganic liquid fertilizer. A product base liquid prepared by using the method disclosed by the invention is a fermented product which can be dissolved in water at higher speed and be absorbed by crops easier in comparison with ordinary products, further has the advantages of easiness in storage, easiness in transportation, easiness in application and good effects, and can reduce the labor intensity in the liquid fertilizer application process, reduce the agricultural ecological environmental pollution and reduce the environmental pollution caused by disordered emission of the biogas slurry in a period without fertilizer application.
Owner:中化农业生态科技(湖北)有限公司
Liquid formulation of a VEGF antagonist
ActiveUS10576128B2Short timeLow costSenses disorderPeptide/protein ingredientsInorganic saltsPharmaceutical Substances
The present invention relates to liquid pharmaceutical compositions of a VEGF antagonist for intravitreal administration comprising a histidine buffer, an inorganic salt, a carbohydrate and a polysorbate.
Owner:FORMYCON AG
Docetaxel formulations with lipoic acid
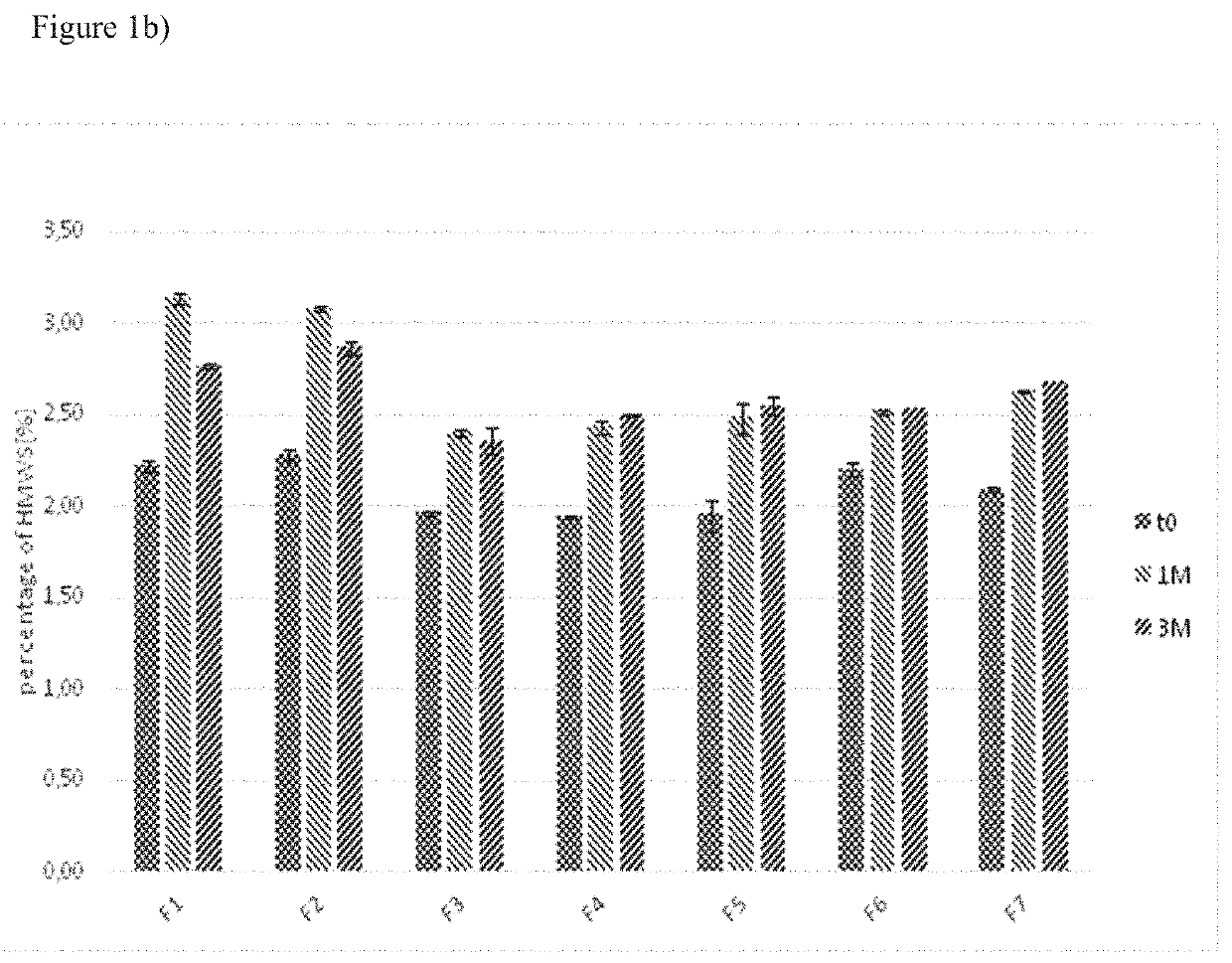
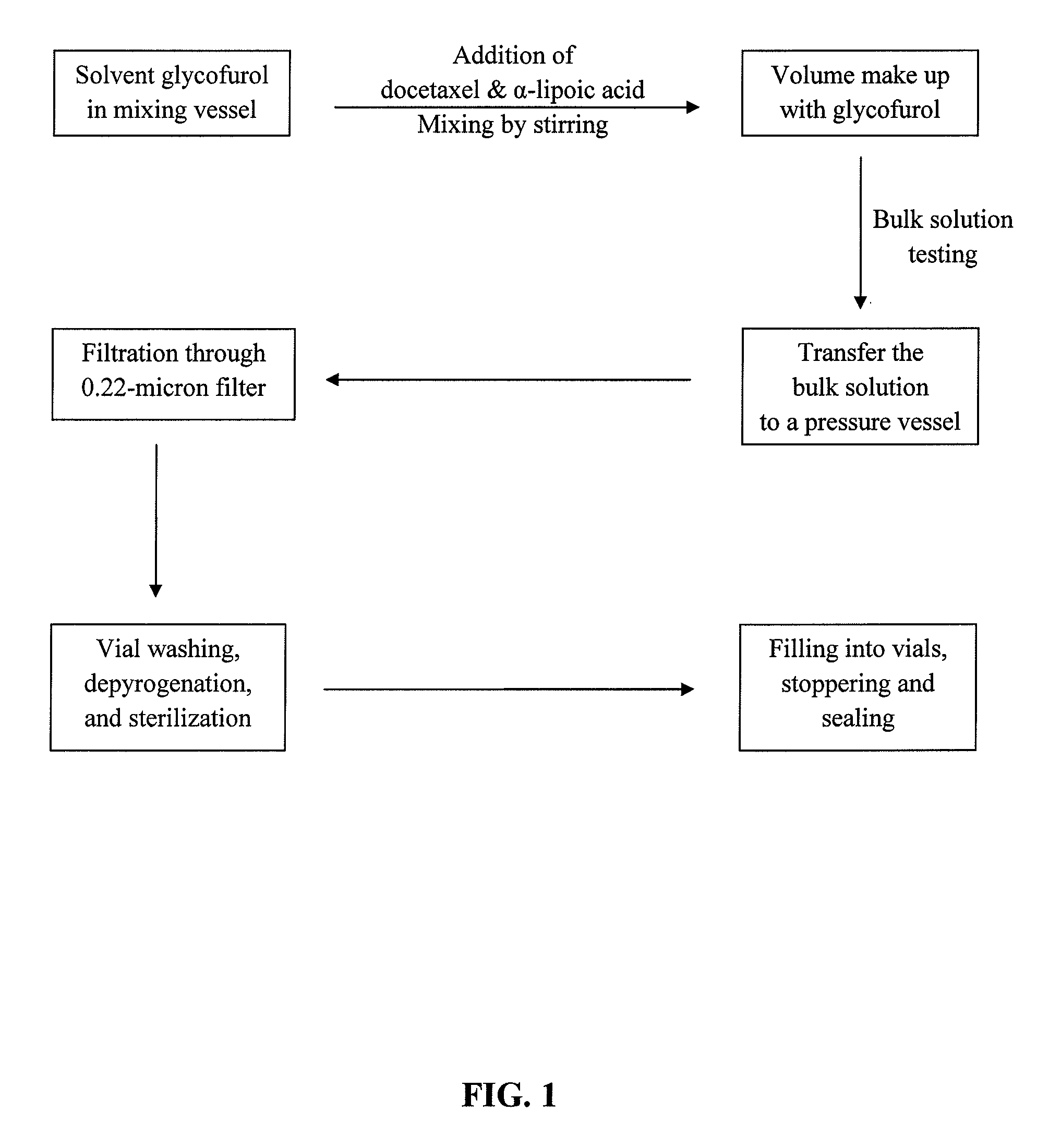
Pharmaceutical formulations comprising docetaxel, solubilizer, and α-lipoic acid, wherein the formulation is substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol, acetic acid, benzyl alcohol, or ethanol. The α-lipoic acid, at certain concentrations, may impart stability and prevent degradation of docetaxel while the formulations are in storage. The formulations may be combined with a diluent, which comprises one or more hydrotropes such as tocopherol polyethylene glycol succinate and polyethylene glycol. The formulations combined with the diluent also exhibit stability after storage. Methods of administering docetaxel comprise preparing the formulation comprising docetaxel, solubilizer, and α-lipoic acid; mixing the formulation with a diluent; diluting the resulting formulation in saline, water for injection, or the like; and then injecting the formulations into patients in need thereof.
Owner:SCIDOSE
Cabazitaxel formulations and methods of preparing thereof
InactiveUS20120065255A1Organic active ingredientsBiocideHydrotropeTocopherol polyethylene glycol succinate
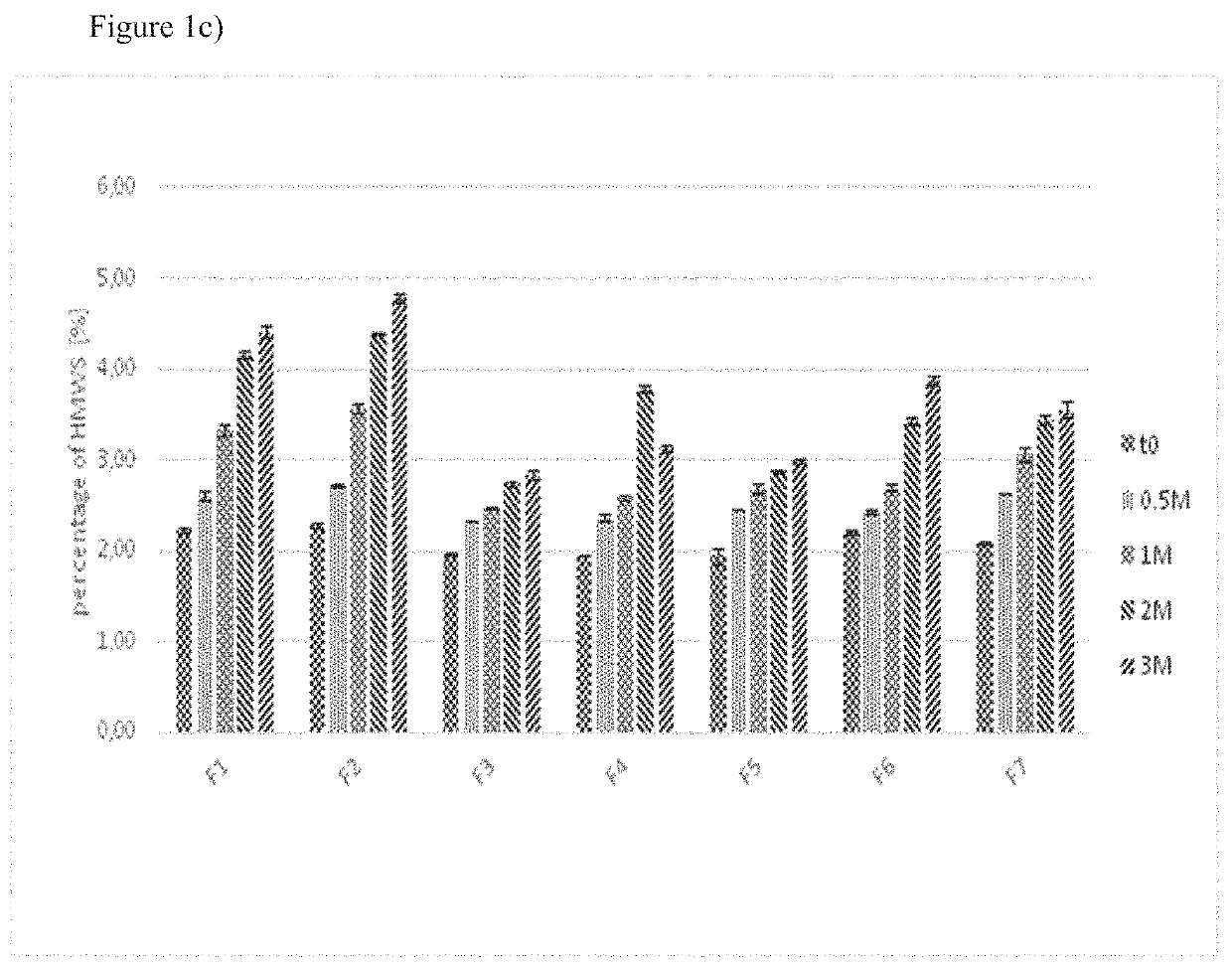
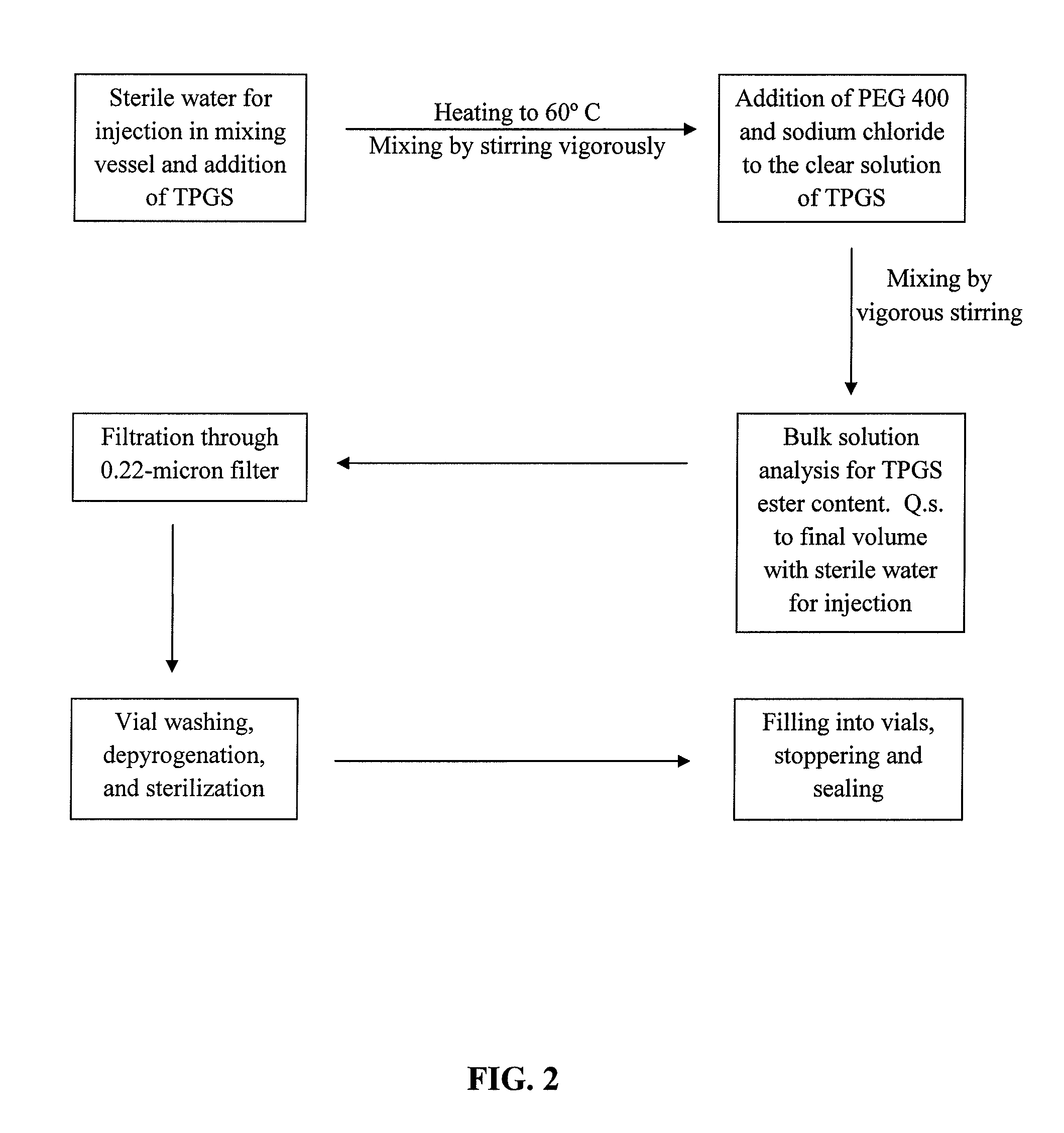
Pharmaceutical formulations comprising cabazitaxel, solubilizer, tocopherol polyethylene glycol succinate (TPGS), one or more hydrotropes, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol or ethanol. Pharmaceutical formulations may alternatively comprise cabazitaxel, solubilizer, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. These formulations may be combined with a diluent, which comprises TPGS and one or more hydrotropes. Methods of administering the cabazitaxel formulations include combining the formulations with an infusion solution.
Owner:SCIDOSE
Topical composition containing ibuprofen
The present invention generally relates to the transdermal delivery of various compositions. In some aspects, the transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising ibuprofen and / or an ibuprofen salt, a nitric oxide donor, and optionally, a hostile biophysical environment. In some cases, the composition may be stabilized using a stabilization polymer such as xanthan gum, KELTROL® BT and / or KELTROL® RD; propylene glycol; and a polysorbate surfactant such as Polysorbate 20, which unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Acne treatment composition and application thereof and acne treatment cream containing acne treatment composition
ActiveCN105012479ASoothe rednessRelieve discomfort such as inflammationCosmetic preparationsToilet preparationsCentella asiatica extractEthylhexyl palmitate
The invention discloses an acne treatment composition and application thereof and an acne treatment cream containing the acne treatment composition. The acne treatment composition comprises shea butter, Chinese corktree bark extract, salix nigra bark extract, herba portulacae extract, purple perilla extract, asiatic pennywort herb extract and mung bean extract, effectively conditions acne skins and inhibits reproduction of acne, smoothens acne and removes acne marks, and can be applied to skin directly or added into acne treatment products. The acne treatment cream comprises the acne treatment composition, cetearyl alcohol, ethylhexyl palmitate, polydimethylsiloxane, glyceryl stearate, PEG-100 stearate, caprylic / capric triglyceride, propylene glycol, polyacrylamide, polysorbate-60, sorbitan stearate, laurocapram, PEG-20 methl glucse sesquistearate, menthol, borneol, edta disodium, methylparaben, propyl hydroxybenzoate and water. The acne treatment cream prepared by a hypo-allergenic formula is mild and non-irritating, easy to absorb, non-greasy, safe and effective.
Owner:广州科玛生物科技股份有限公司
Instant lyophilized fibrinogen and fibrin ferment formulation composition, preparation method and use thereof
ActiveCN101371921ASolve the shortcoming of short storage timeImprove solubilityPeptide/protein ingredientsBlood disorderArginineIrritation
The invention provides a composition of instant freeze-dried fibrinogen and thrombin preparation, a preparation method and a use thereof, and the composition comprises composition 1 composed of 35-70mg / ml of fibrinogen, 9-15mg / ml of sodium citrate, 7-12mg / ml of sodium chloride, 0.3-0.6mg / ml of polysorbate-80, 10-15mg / ml of mannitol, 4 -8mg / ml of arginine and 3.5-5.5mg / ml of glutamic acid by mg / ml and composition 2 composed of 700-1,200 mg / ml of thrombin, 3-6mg / ml of dextran 20, 15-25mg / ml of glycine, 5-7mg / ml of sodium chloride and 4-7mg / ml of calcium chloride by mg / ml. A sealant avoids the risk of spreading of AIDS virus, and the like, increases the drug stability, reduces the degeneration during the virus inactivation process by dry-heat method, protects biological activity, avoids local liquid storage of the using part caused by high permeability and the irritation of a large amount of inorganic salts to tissues, is conductive to the healing of trauma sites and ensures product safety and independent package to the maximum extent, thereby increasing storage time, facilitating use and meeting the clinical and field first-aid needs.
Owner:HANBANG MEDICAL SCI & TECH HARBIN CITY
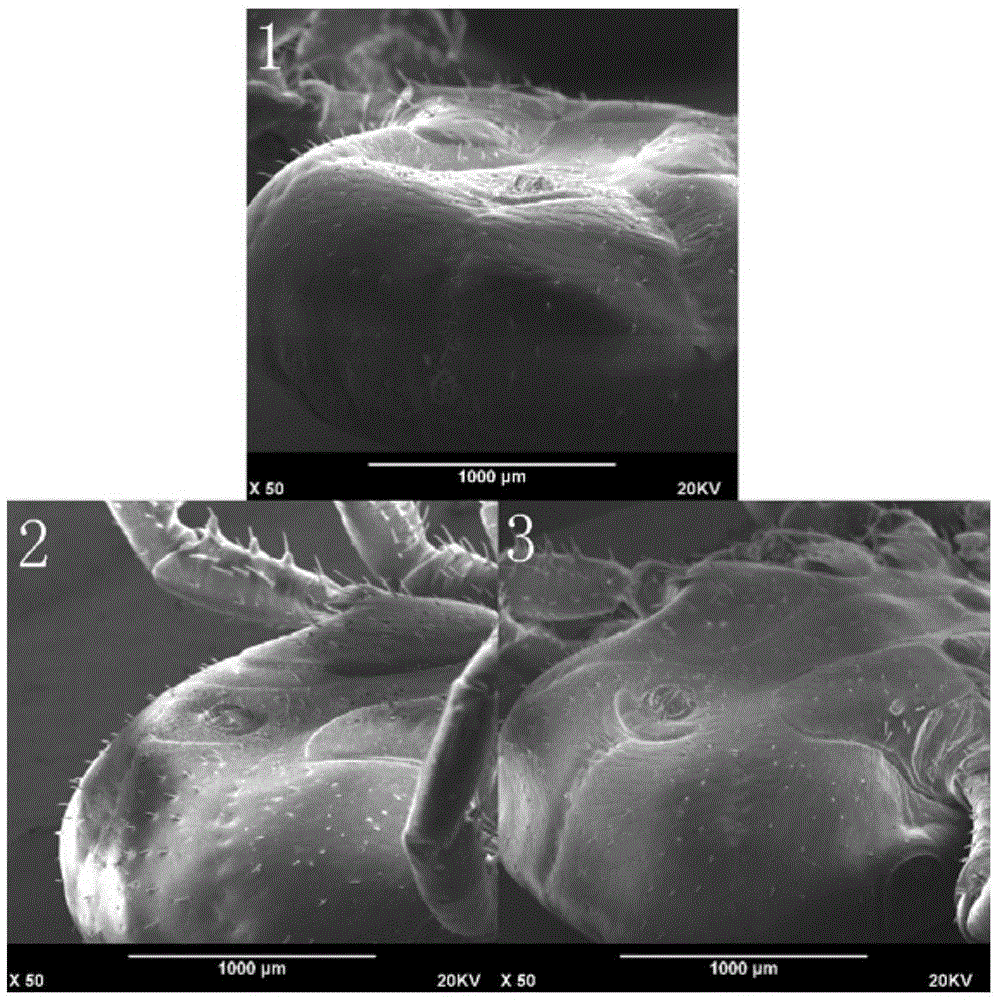
Formula and method for immobilizing tick sample for scanning electron microscopy
InactiveCN105486554ADry and completeShort timePreparing sample for investigationFreeze-dryingHigh energy
The invention relates to a formula and method for immobilizing a tick sample for scanning electron microscopy, which belongs to the technical field of methods for immobilizing samples for scanning electron microscopy. The immobilization formula comprises a PBS buffer solution, polysorbate and glutaraldehyde, and a glutaraldehyde immobilization liquid is prepared from the above-mentioned components. The method comprises the following steps: (1) observing whether host tissue is left in basis capituli of the tick sample and if so, removing the host tissue; (2) cleaning the surface of the tick sample; (3) immobilizing the tick sample at room temperature by using the glutaraldehyde immobilization liquid; (4) carrying out gradient dehydration with ethanol on the immobilized tick sample; (5) putting the tick sample into mixed liquor of absolute ethyl alcohol and acetone for displacement, and then putting the tick sample into acetone for displacement; and (6) drying the tick sample in a vacuum freeze drying instrument for drying and spraying gold on the tick sample. During scanning electron microscopic observation, the phenomenon of fuzzy and foggy images or incapable imaging due to ionization discharging of the sample as water vapor produced after electron beam bombardment of the sample encounters high-energy electron streams is prevented.
Owner:XINJIANG AGRI UNIV
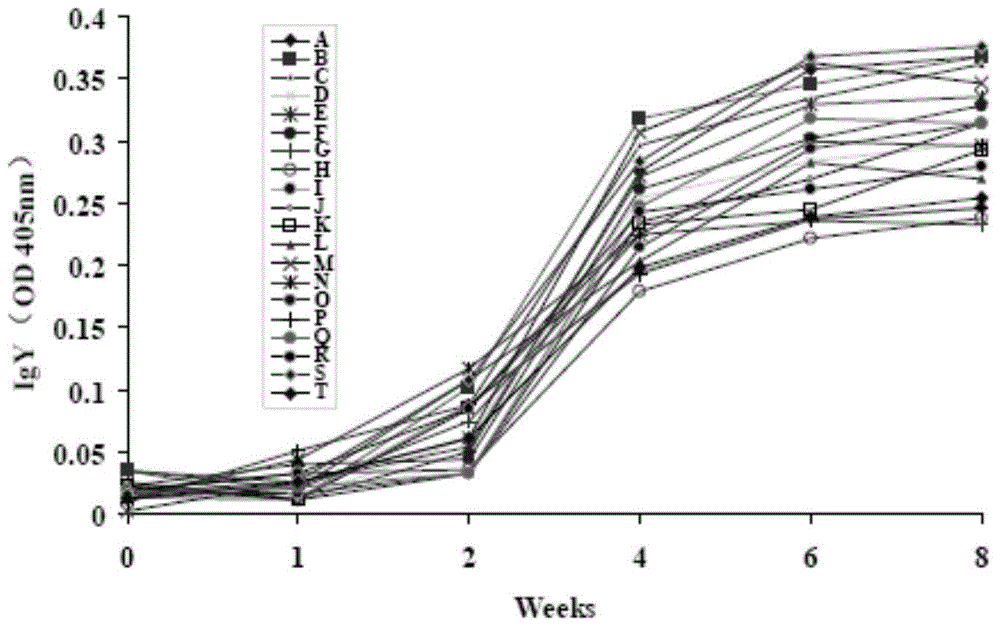
Specificity yelk immune globulin composition for preventing pathogenic bacteria and preparation thereof
ActiveCN104013958AGood killing effectImprove securityAntibacterial agentsAntimycoticsIrritationGlycerol
The invention relates to a specificity yelk immune globulin composition for preventing pathogenic bacteria. The composition is prepared by preparing compound antigen, performing supplementary immunization on chicken, preparing crude extract and purifying. The invention further relates to a preparation prepared from the composition. The preparation comprises the following components in percentage by mass: 0.15-0.3% of specificity yelk immune globulin composition for preventing pathogenic bacteria or crude extract of the composition, 0.3-0.6% of povidone K30, 1.0-2.0% of glycerol, 0.3-0.5% of polysorbate, 0.10-0.30% of fresher essence, 0.05-0.1% of edible essence and the balance of distilled water of 100%. The specificity yelk immune globulin composition for preventing pathogenic bacteria and the preparation of the composition are high in antibody activity, high in specificity, good in specificity sterilization effect, free of microecological balance interference, free of adverse irritation on human bodies and high in safety performance.
Owner:GUANGZHOU PHGO BIOTECH
Topical composition containing naproxen
The present invention generally relates to the transdermal delivery of various compositions. In some aspects, the transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising ibuprofen and / or an ibuprofen salt, a nitric oxide donor, and optionally, a hostile biophysical environment. In some cases, the composition may be stabilized using a stabilization polymer such as xanthan gum, KELTROL® BT and / or KELTROL® RD; propylene glycol; and a polysorbate surfactant such as Polysorbate 20, which unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Composition with azelaic acid
The invention relates to a pharmaceutical composition having the following constituents: azelaic acid, polyacrylic acid, triacylglyceride, propylene glycol, polysorbate, soya lecithin, water and salts. The composition is a hydrogel which is suited for the treatment of rosacea, presbyderma, melasma or skin irritations.
Owner:LEO PHARMA AS
Organic and inorganic composite flushing liquid as well as preparation method and applications thereof
ActiveCN102732239AImprove the interface bonding strengthImprove flushing efficiencyDrilling compositionWater basedPolyethylene glycol
The invention discloses a preparation method of an organic and inorganic composite flushing liquid, and belongs to the technique of oil well cement well cementation. The flushing liquid comprises the following components: polysorbate-80, alkyl phenol polyethylene glycol ether, dodecyl benzene sulfonic acid sodium, sodium silicate, sodium pyrophosphate and water, wherein the mass ratio of each component is as follows: 20%-30% of polysorbate-80, 10%-15% of alkyl phenol polyethylene glycol ether, 5%-10% of dodecyl benzene sulfonic acid sodium, 10%-20% of sodium silicate, 5%-10% of sodium pyrophosphate and the balance of water. The flushing liquid is suitable for both the oil-based mud and water-based mud, has high flushing efficiency, improves the replacement efficiency of a well drilling liquid and cement sheath interfacial cementation quality, and can be used under the condition of 50 DEG C-200 DEG C.
Owner:天津科力奥尔工程材料技术有限公司
Neisseria meningitidis compositions and methods thereof
In one aspect, the invention relates to a composition including a first polypeptide having the sequence set forth in SEQ ID NO: 1 and a second polypeptide having the sequence set forth in SEQ ID NO: 2. In one embodiment, the composition includes about 120 μg / ml of a first polypeptide including the amino acid sequence set forth in SEQ ID NO: 1, 120 μg / ml of a second polypeptide including the amino acid sequence set forth in SEQ ID NO: 2, about 2.8 molar ratio polysorbate-80 to the first polypeptide, about 2.8 molar ratio polysorbate-80 to the second polypeptide, about 0.5 mg / ml aluminum, about 10 mM histidine, and about 150 mM sodium chloride. In one embodiment, a dose of the composition is about 0.5 ml in total volume. In one embodiment, two-doses of the composition induce a bactericidal titer against diverse heterologous subfamily A and subfamily B strains in a human.
Owner:PFIZER INC
Cox-2 inhibitors and related compounds, and systems and methods for delivery thereof
InactiveUS20140004177A1Improve efficiencyReduce riskBiocideNervous disorderMedicineCompound (substance)
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising a COX-2 inhibitor and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Face mask containing plant material residues
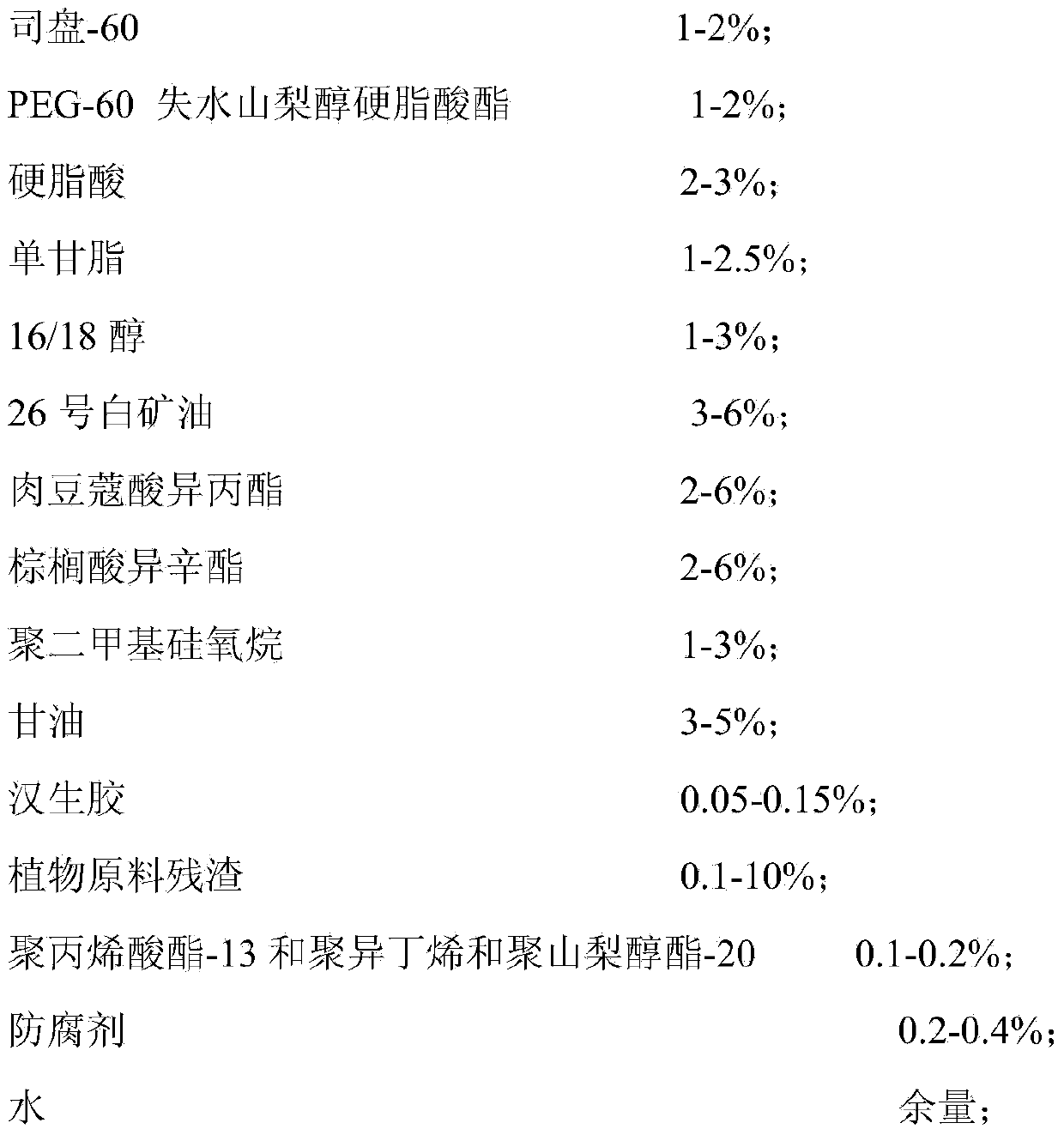
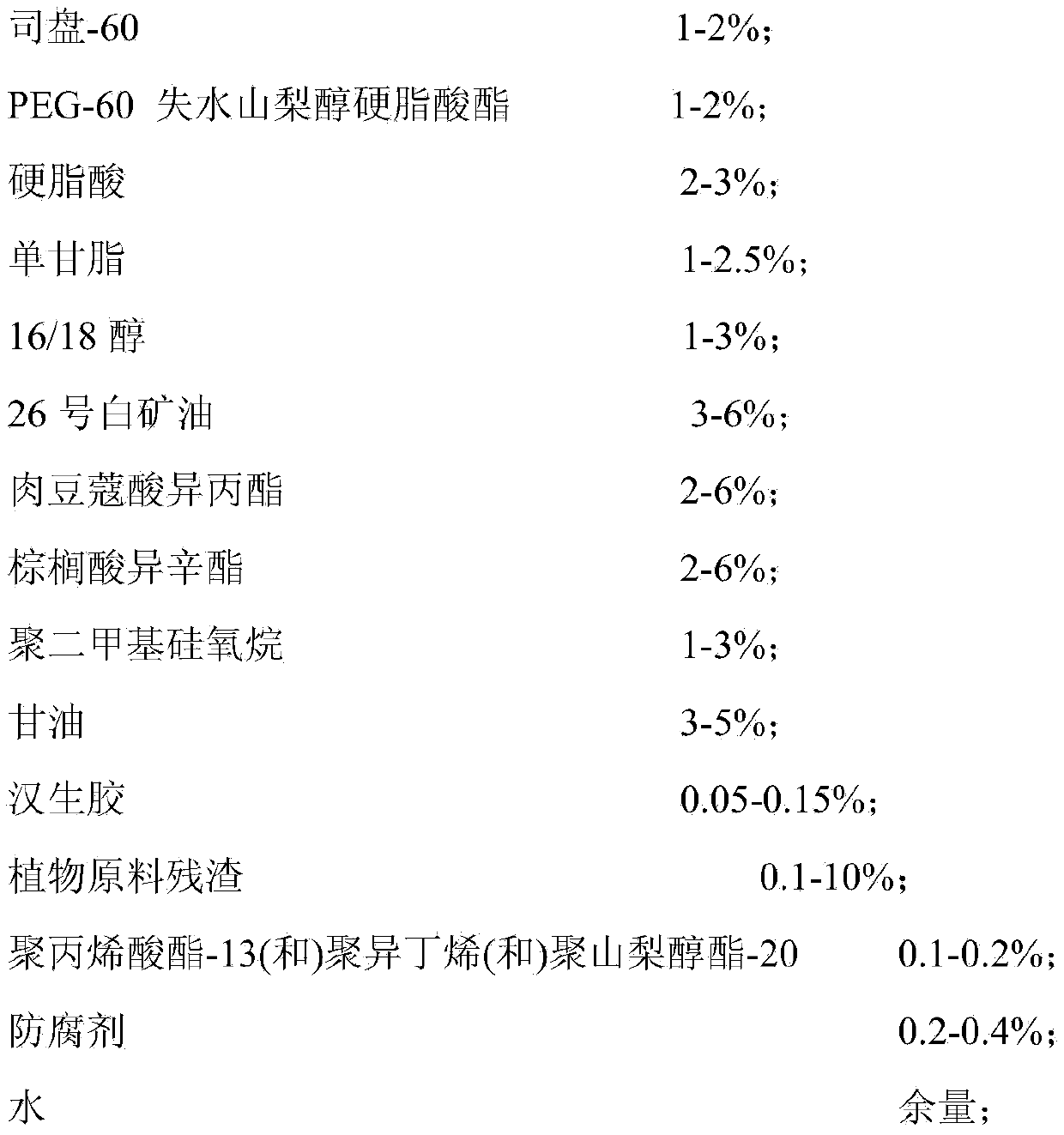
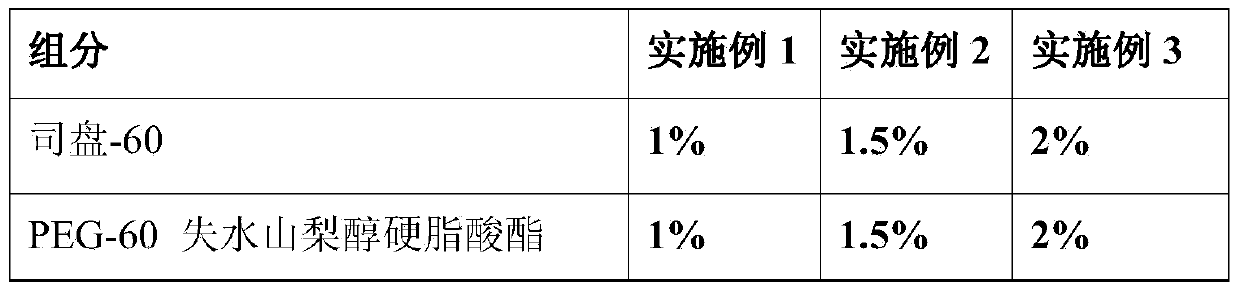
ActiveCN103432043ATake advantage ofIncrease profitCosmetic preparationsToilet preparationsMonoglyceridePolyethylene glycol
The invention provides a face mask containing plant material residues. A formula of the face mask comprises the following components in percentage by weight: 1%-2% of span 60, 1%-2% of PEG-60 (polyethylene glycol-60) sorbitan stearate, 2%-3% of stearic acid, 1%-2.5% of monoglyceride, 1%-3% of 16 / 18 alcohol, 3%-6% of 26# white mineral oil, 2%-6% of isopropyl myristate, 2%-6% of isooctyl palmitate, 1%-3% of polydimethylsiloxane, 3%-5% of glycerinum, 0.05-0.15% of xanthan gum, 0.1-10% of the plant material residues, 0.1%-0.2% of polyacrylate-13 (and) polyisobutene (and) polysorbate-20, 0.2%-0.4% of preservative and the balance of water. According to the face mask provided by the invention, the material residues after plant materials are extracted and utilized are turned into wealth, the production cost is reduced, and the utilization rate of the plant materials is increased.
Owner:广州市千纤草化妆品有限公司
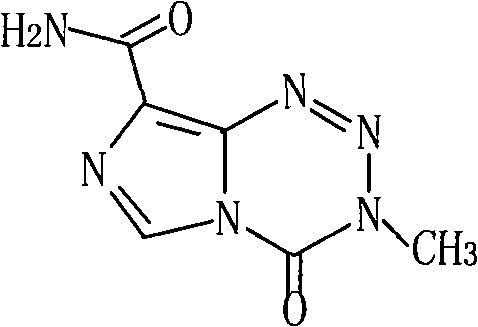
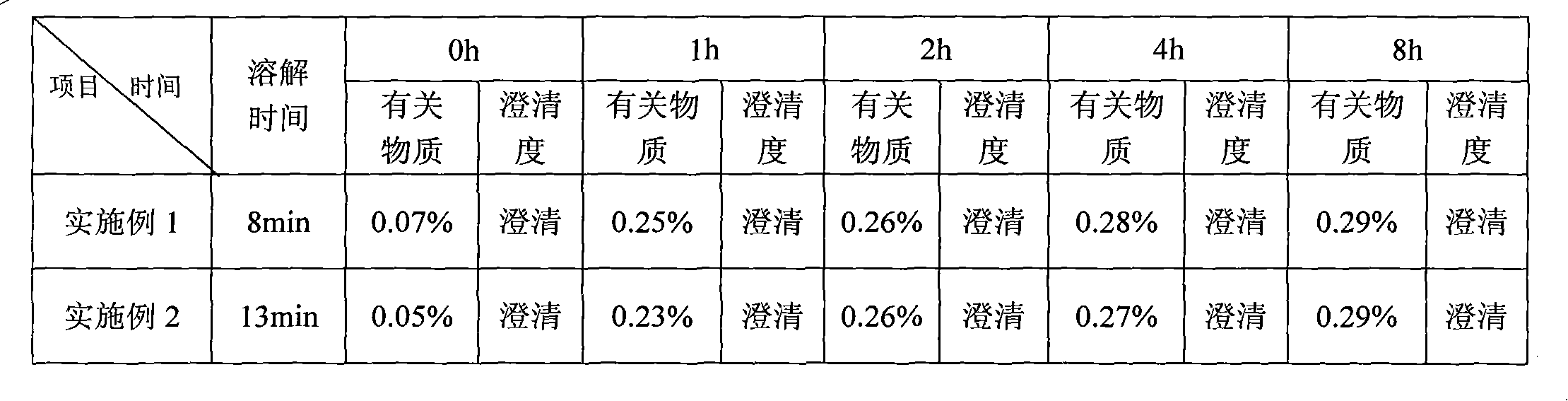
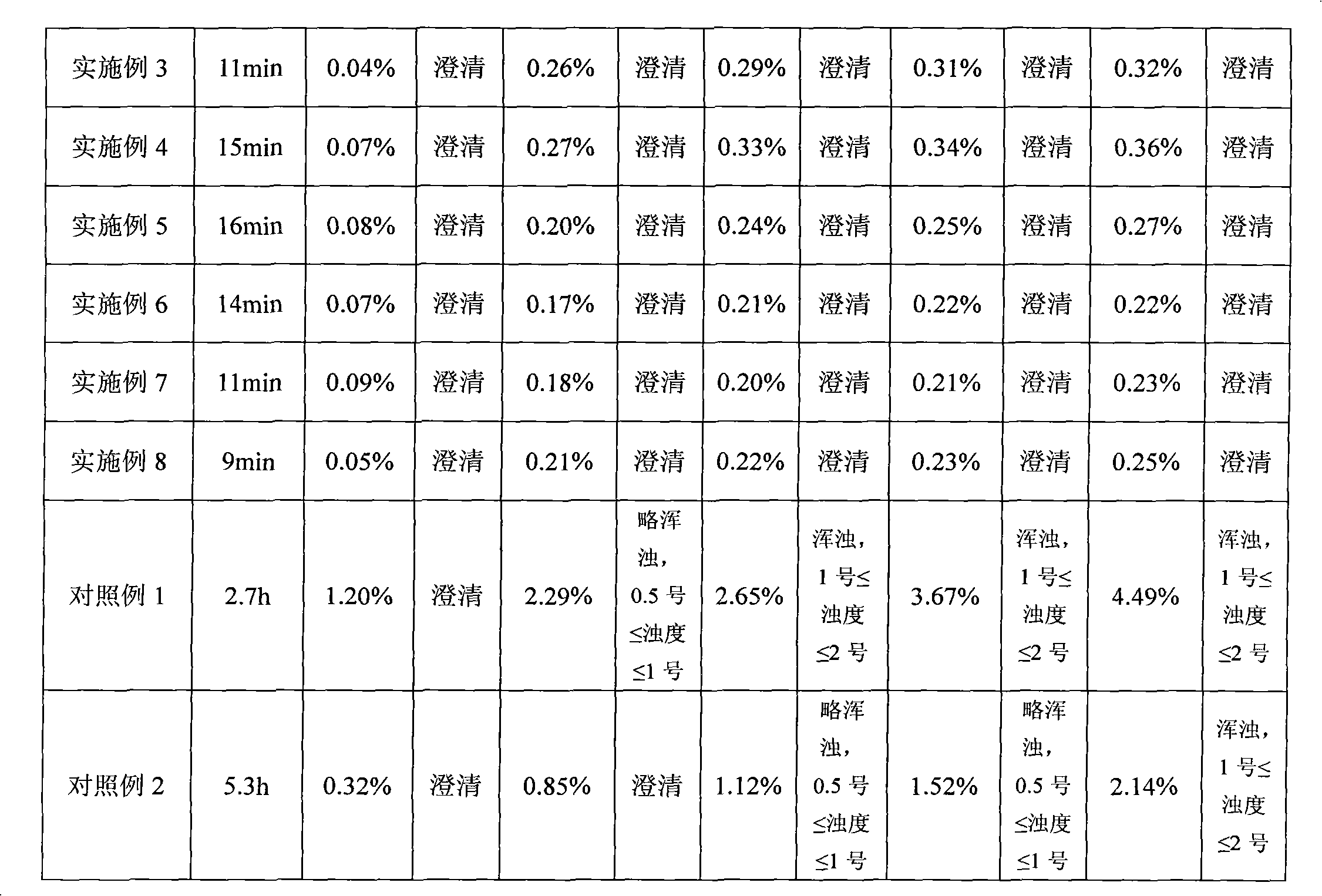
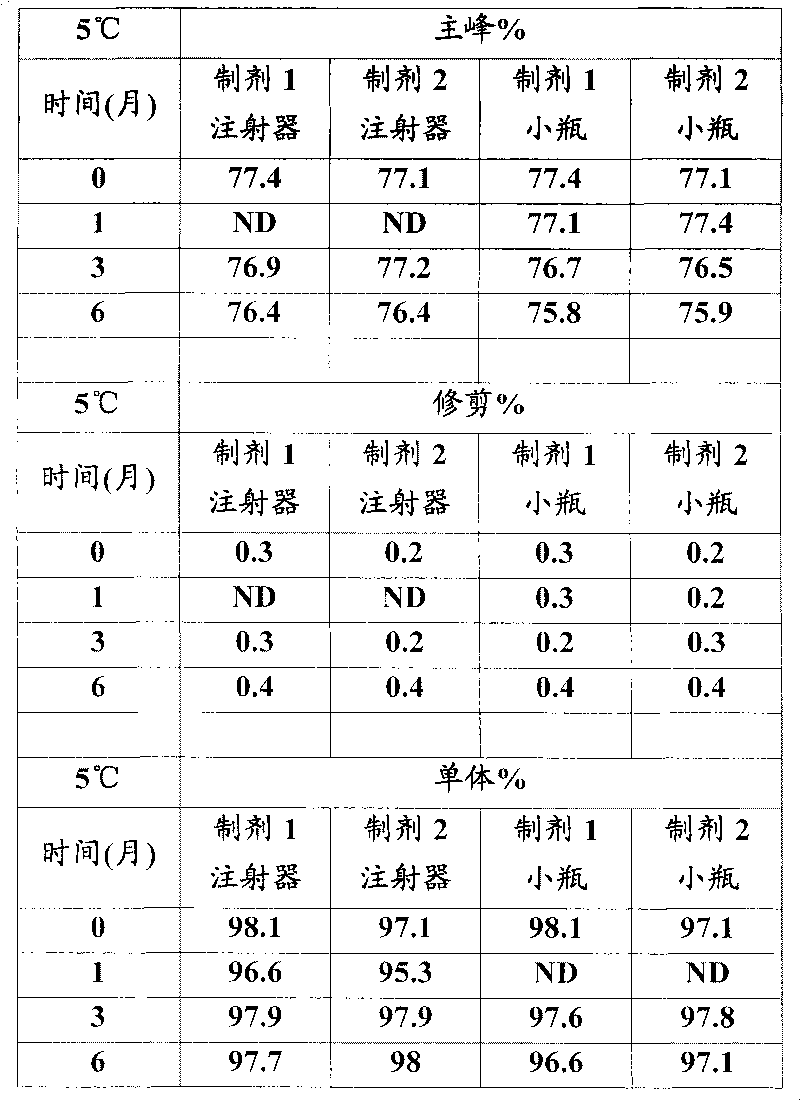
Temozolomide freeze-dried preparation
ActiveCN101869551ASolve the speed problemSolve poor resolubilityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
The invention discloses a temozolomide freeze-dried preparation. Every 100ml of the preparation comprises 1 to 2,000 mg of temozolomide, 1 to 2,000 mg of solubilizer, 801 to 2,000 mg of polysorbate, 5 to 5,000 mg of mannitol, 1 to 2,000 mg of buffering agent, 0.5 to 1,000 mg of hydrochloric acid and the balance of water. The preparation of the invention overcomes the shortcomings of slow dissolving speed, poor redissolution, fussy operation and unqualified solution clarity of the freeze-dried preparation, has the technical effects that: the preparation of the invention has high redissolution; solid is completely dissolved to be clear and colorless by adding water into the freeze-dried preparation and shaking slightly; purity is over 99.5 percent and single impurity content is below 0.3 percent by detecting the purity and the content by using HPLC; through stability experimental investigation, relative substances and content of the preparation are not changed remarkably; and quality is stable.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Glp-1-fc fusion protein formulation
InactiveCN101730523APeptide/protein ingredientsMetabolism disorderDiabetes mellitusMANNITOL/SORBITOL
The invention provides a stable solution formulation comprising a therapeutically effective amount of a GLP-1-Fc fusion protein at about pH 6.5 in citrate buffer with polysorbate-80 and mannitol. The formulation is useful in treating diabetes and obesity as well as a variety of other conditions or disorders.
Owner:ELI LILLY & CO
Stable lyophilized pharmaceutical formulation of IgG antibodies
InactiveUS20060029599A1Process stabilityAvoid formingPowder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-IL2 ReceptorHigh concentration
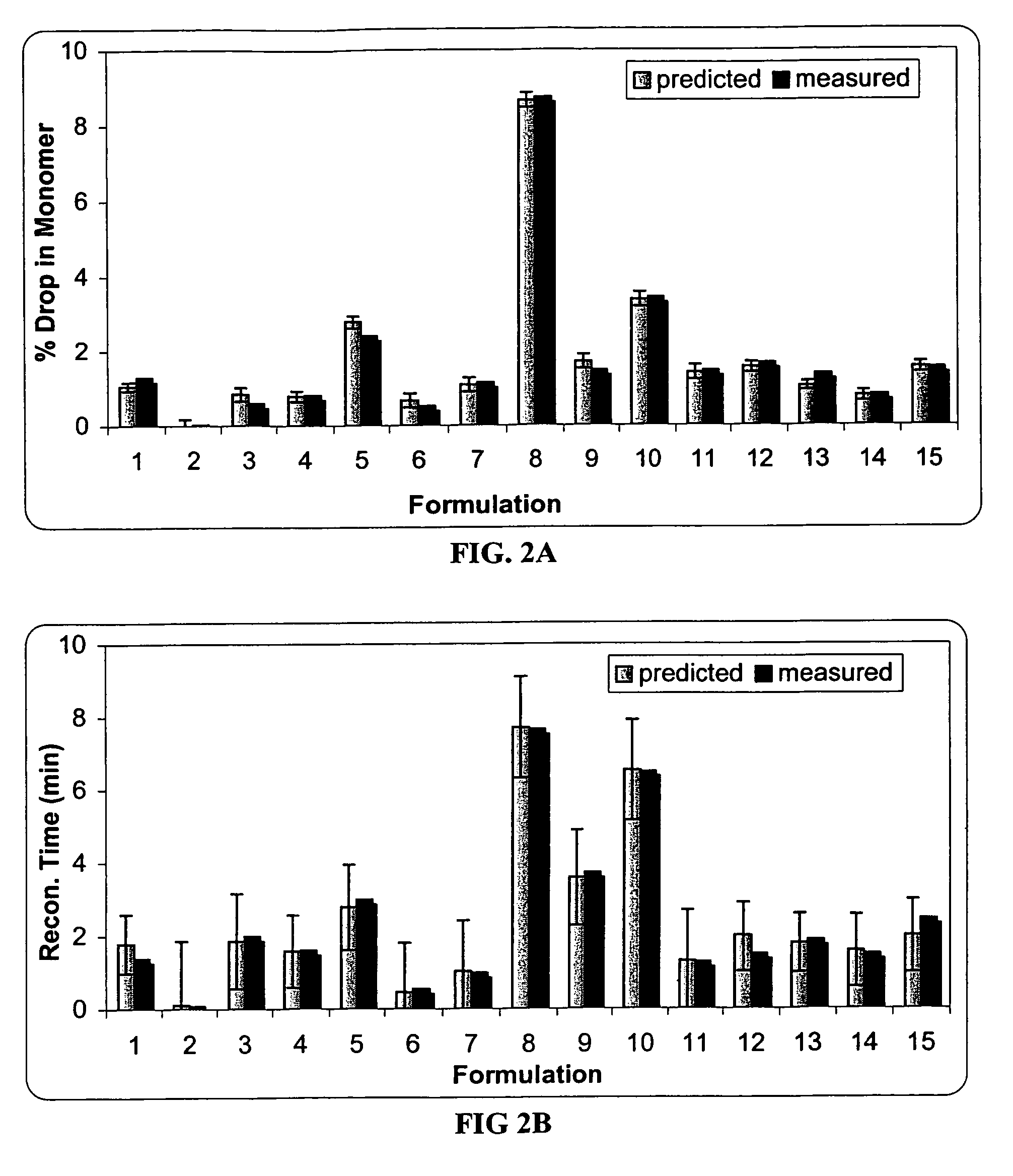
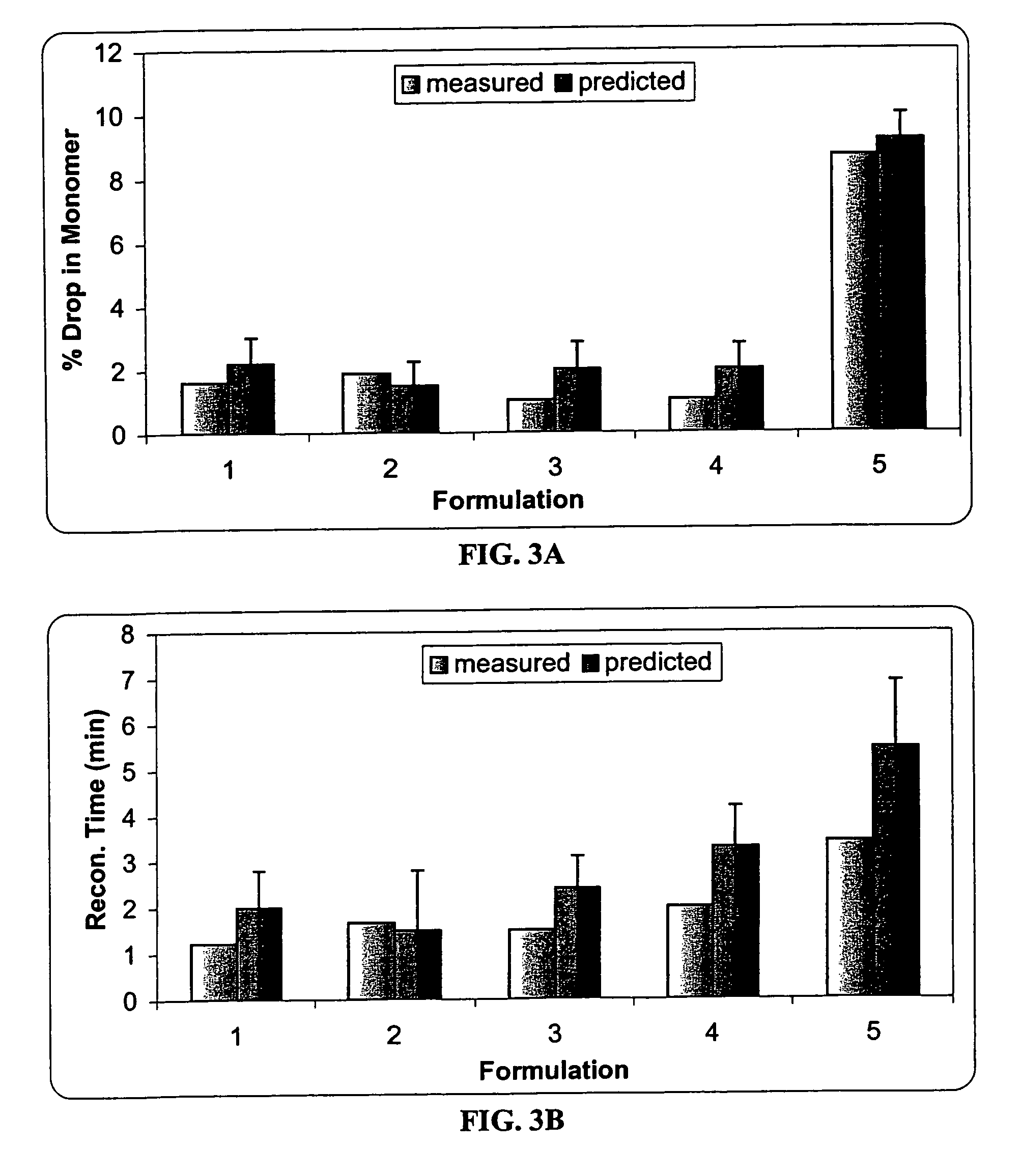
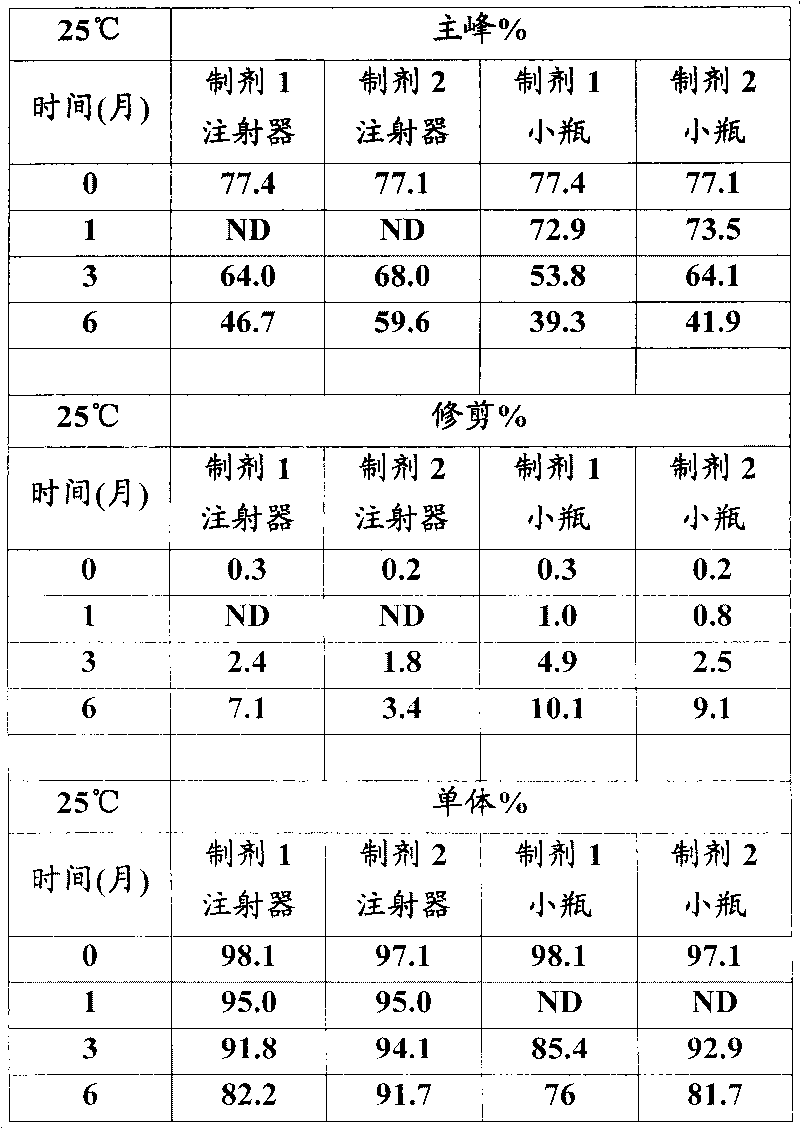
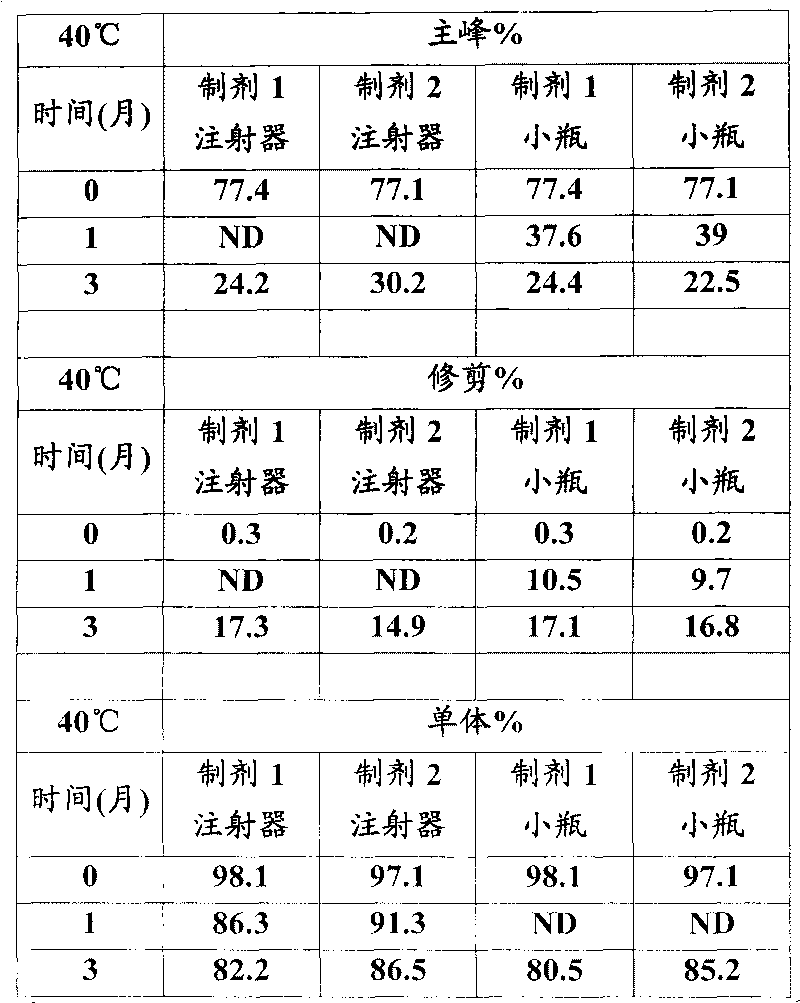
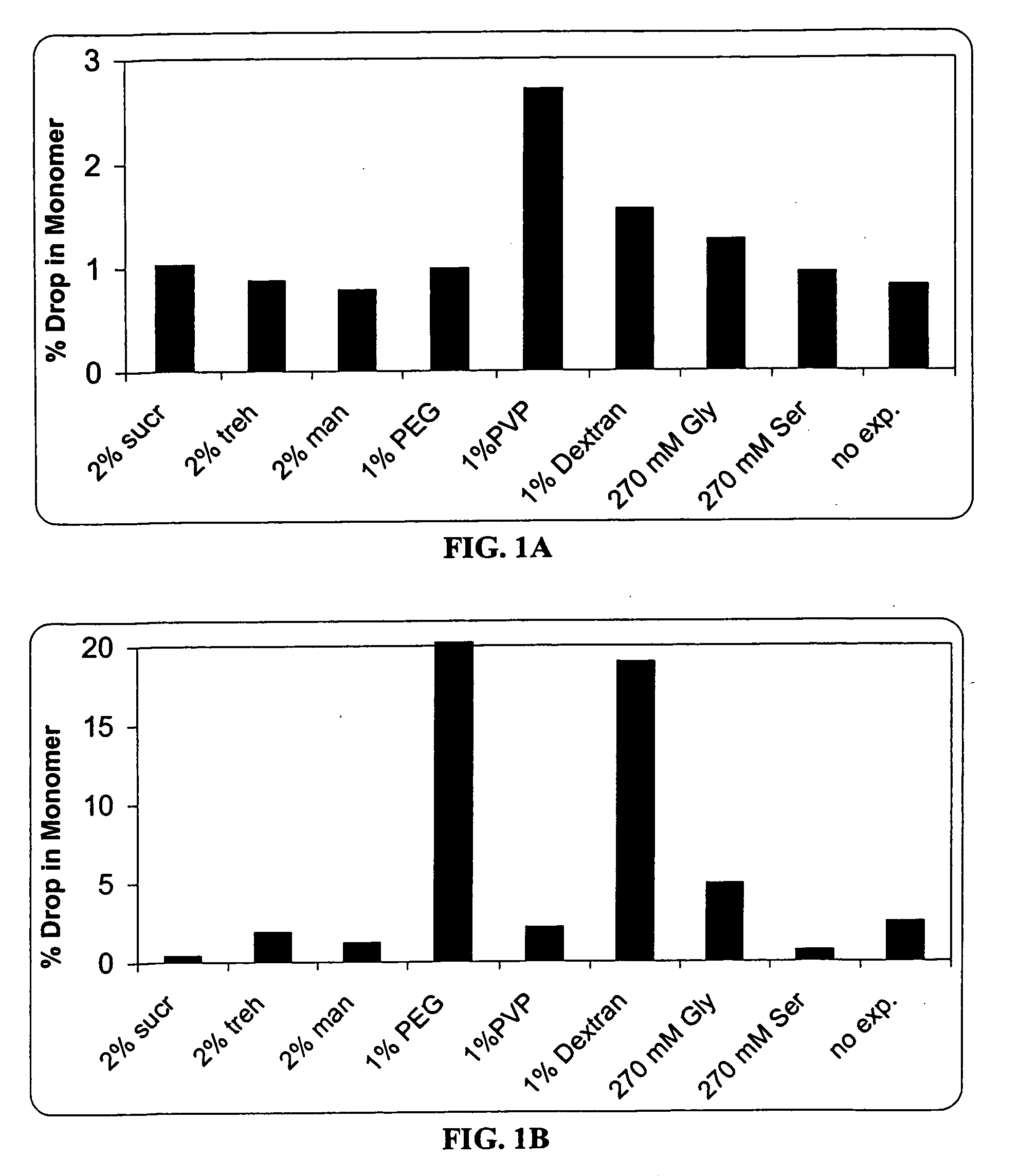
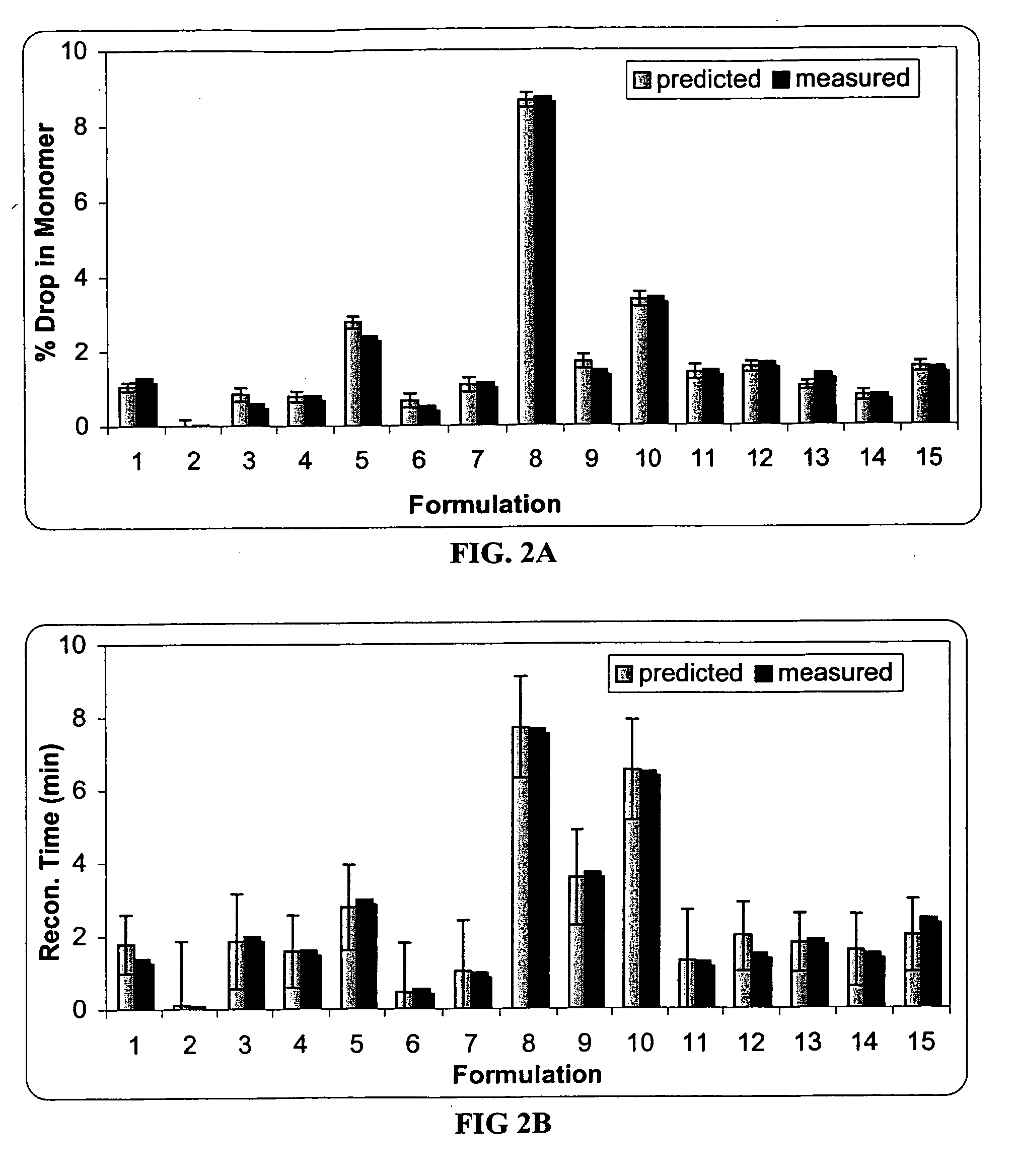
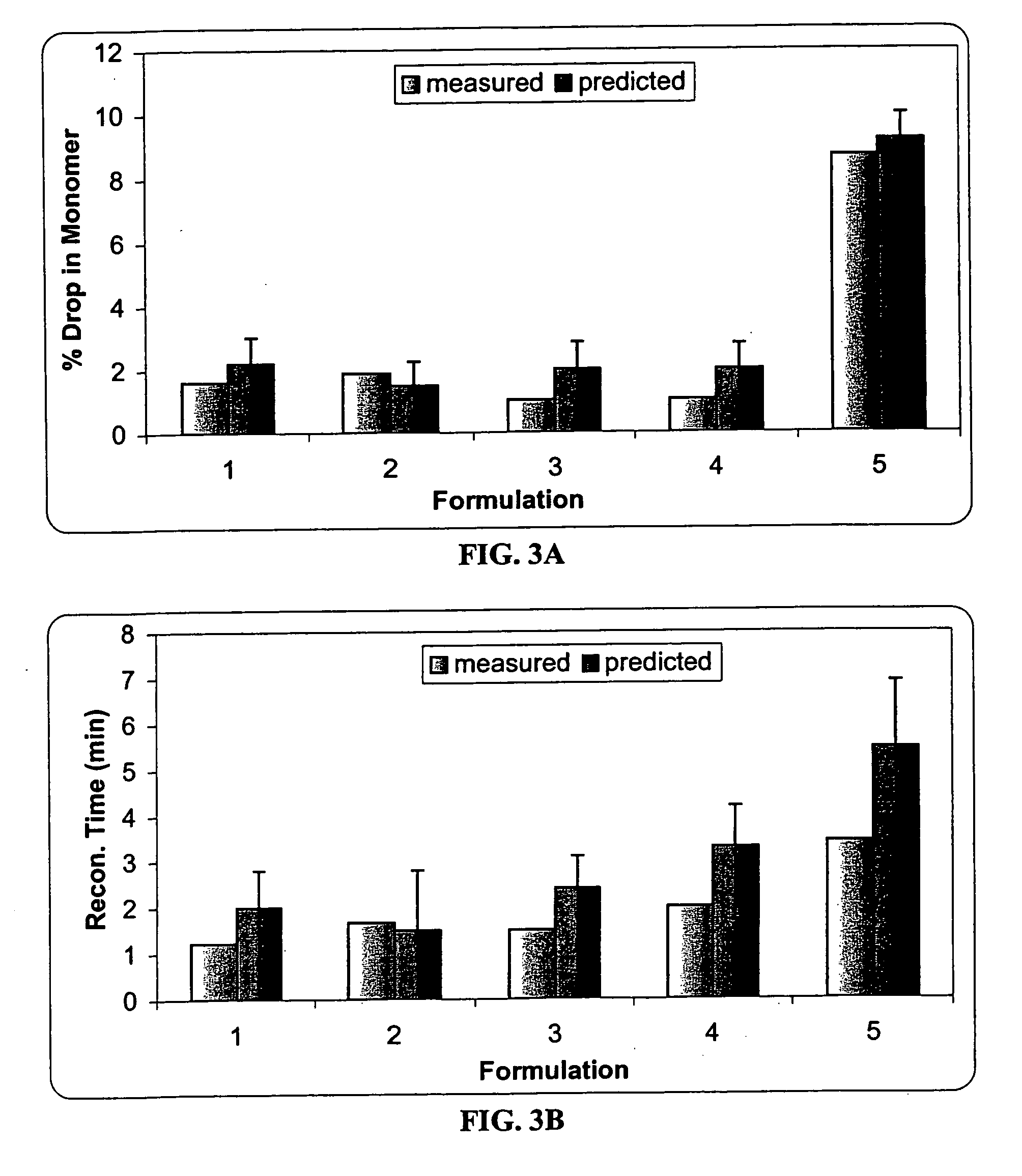
This invention is directed to a stable lyophilized pharmaceutical formulation prepared by lyophilizing an aqueous formulation comprising a high concentration, e.g. 50 mg / ml or more, of an IgG antibody in about 5-25 mM histidine buffer having pH from about 5.5 to about 6.5, about 0.005%-0.03% polysorbate, sucrose, and optionally serine, and / or mannitol. This lyophilized formulation is stable at room temperature for at least 6 months, and preferably 1 year. This lyophilized formulation has a short reconstitution time of less than 2 minutes, and is suitable for parenteral administration such as intravenous, intramuscular, intraperitoneal, or subcutaneous injection. This invention is exemplified by the anti-IL2 receptor antibody.
Owner:ABBVIE BIOTHERAPEUTICS
Topical composition containing ibuprofen
The present invention generally relates to the transdermal delivery of various compositions. In some aspects, the transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising ibuprofen and / or an ibuprofen salt, a nitric oxide donor, and optionally, a hostile biophysical environment. In some cases, the composition may be stabilized using a stabilization polymer such as xanthan gum, KELTROL® BT and / or KELTROL® RD; propylene glycol; and a polysorbate surfactant such as Polysorbate 20, which unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Treatment of erectile dysfunction and other indications
ActiveUS20140086980A1Improve efficiencyFast actionBiocideInorganic non-active ingredientsNitric oxidePhosphodiesterase Type 5 Inhibitors
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising a phosphodiesterase type 5 inhibitor and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
Treatment of erectile dysfunction and other indications
InactiveUS20140051707A1Enhancing local deliveryReduce systemic amount of compoundBiocideInorganic non-active ingredientsNitric oxidePhosphodiesterase Type 5 Inhibitors
The present invention generally relates to the transdermal delivery of various compounds. In some aspects, transdermal delivery may be facilitated by the use of a hostile biophysical environment. One set of embodiments provides a composition for topical delivery comprising a phosphodiesterase type 5 inhibitor and / or a salt thereof, and optionally, a hostile biophysical environment and / or a nitric oxide donor. In some cases, the composition may be stabilized using a combination of a stabilization polymer (such as xanthan gum, KELTROL® BT and / or KELTROL® RD), propylene glycol, and a polysorbate surfactant such as Polysorbate 20, which combination unexpectedly provides temperature stability to the composition, e.g., at elevated temperatures such as at least 40° C. (at least about 104° F.), as compared to compositions lacking one or more of these.
Owner:STRATEGIC SCI & TECH
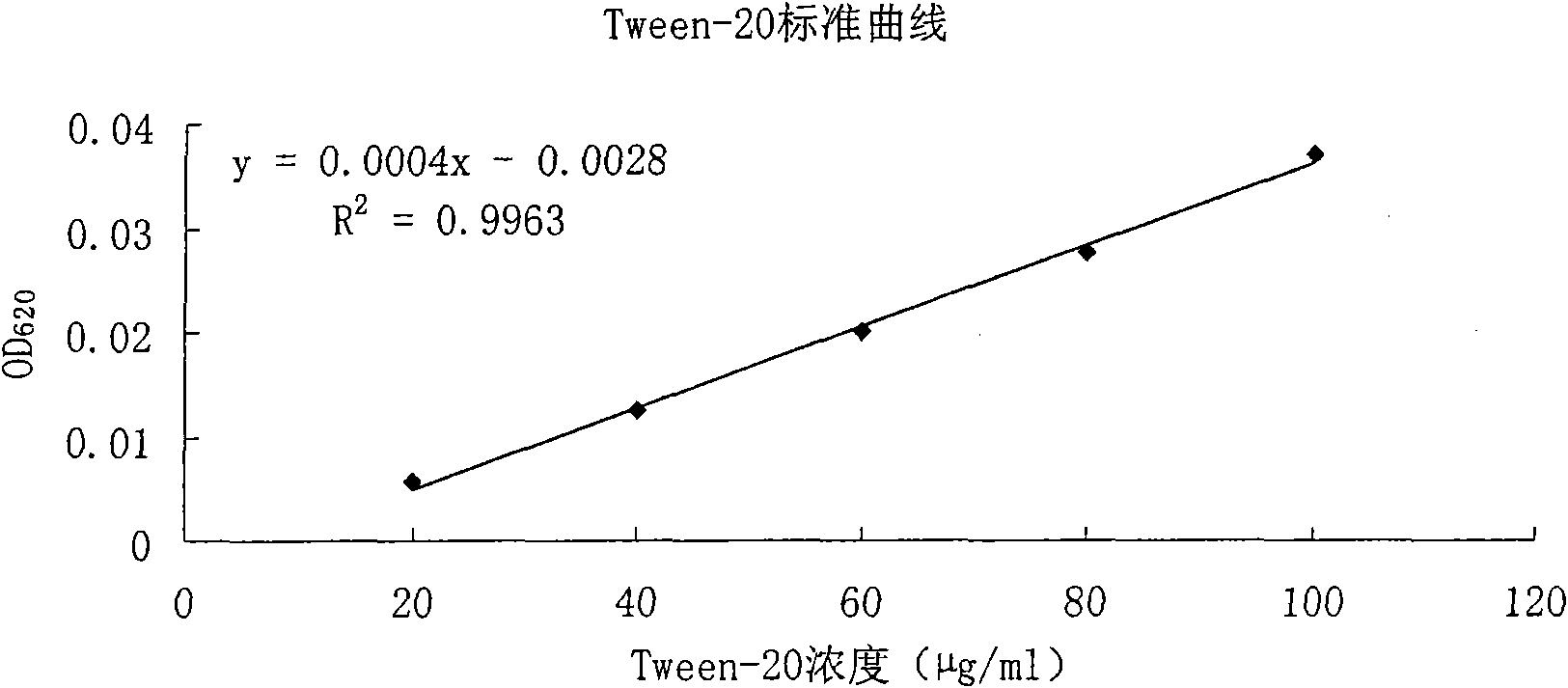
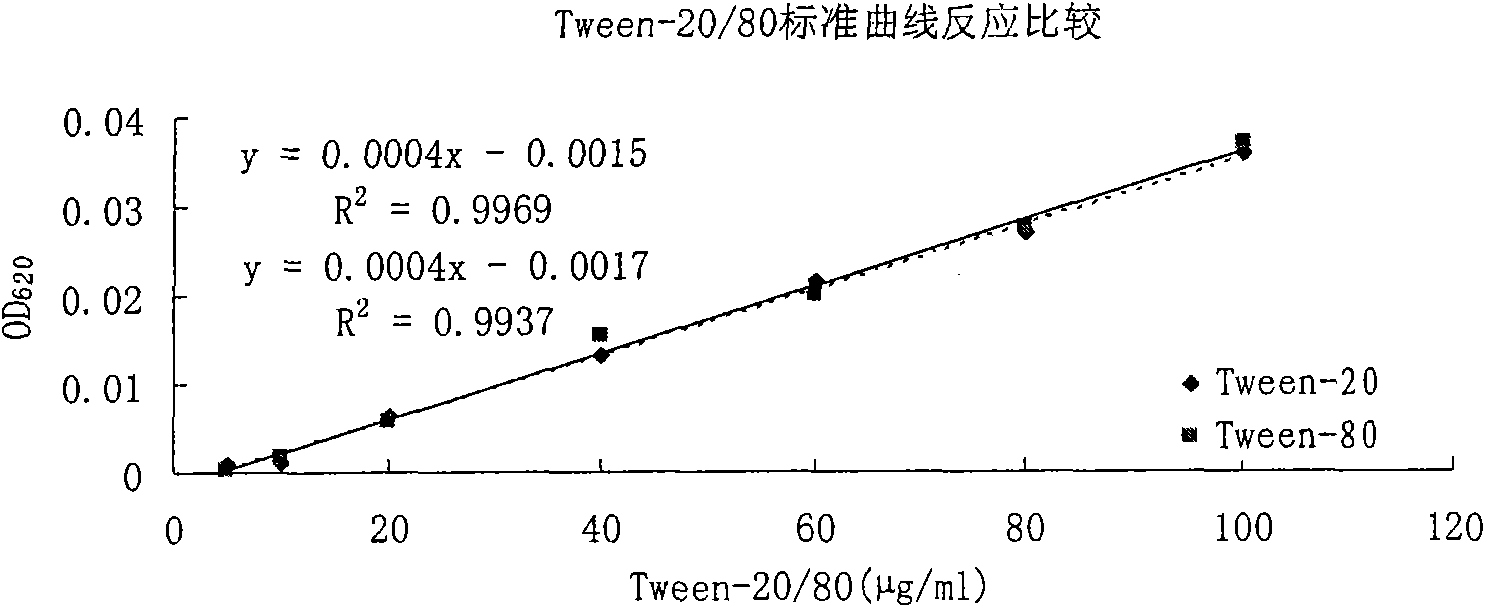
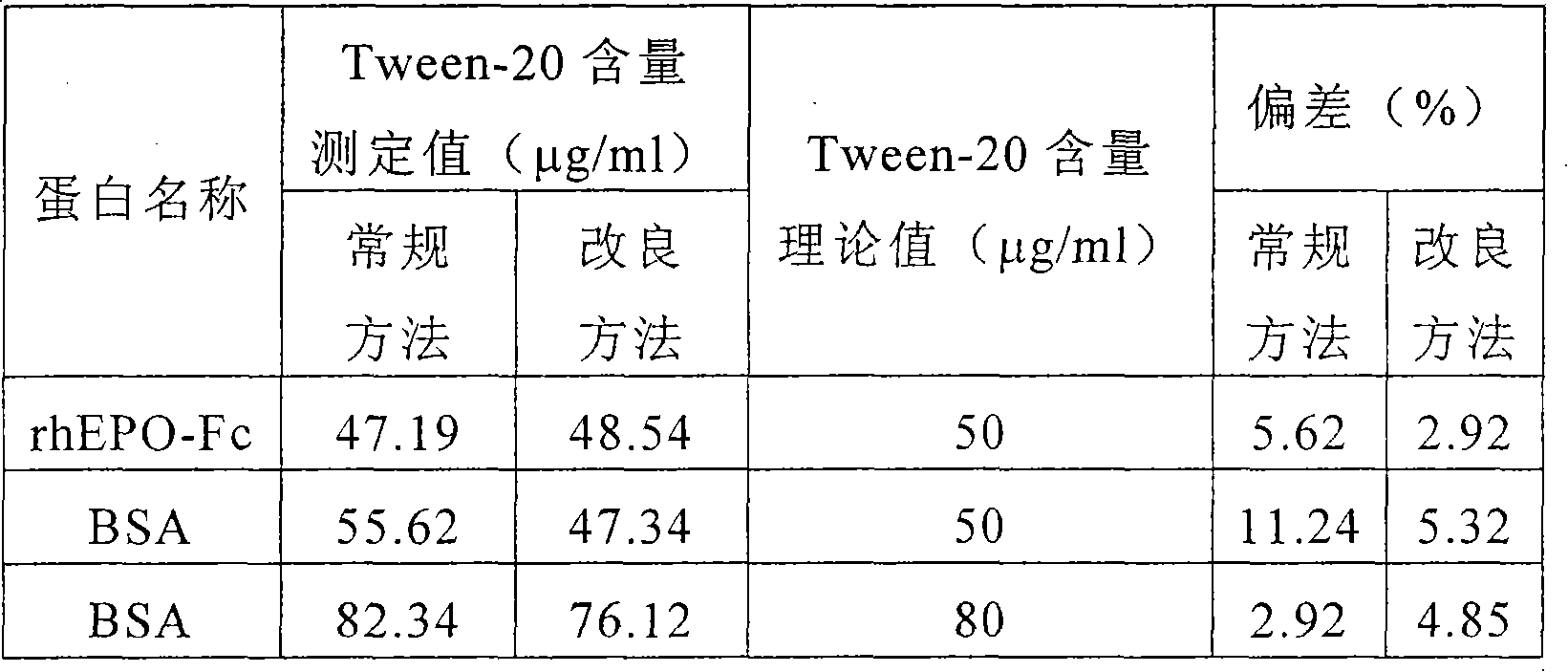
Method for measuring polysorbate content in protein solution
InactiveCN101639439AOvercoming discrepancies caused by long detection intervalsSimple and fast operationColor/spectral properties measurementsProtein solutionChloride
The invention relates to a method for measuring polysorbate content in a protein solution. The method comprises the following steps: (1) mixing a measured sample with methenyl chloride and an ammoniumcobalt thiocyanate aqueous solution to obtain a mixed solution; (2) demixing the mixed solution; (3) measuring the absorbance of the layered methenyl chloride layer under a ultraviolet-visible wavelength; and (4) calculating the concentration of the polysorbate in the measured sample according to the measured absorbance. The method performs the measurement without sedimentation of the protein inthe preparation or an overnight reaction of the sample, and overcomes the differences caused by the overlong measuring time interval because methylene chloride is volatile easily. The method can be used for controlling the polysorbate content in the protein solution.
Owner:DONGGUAN TAILI BIOTECH
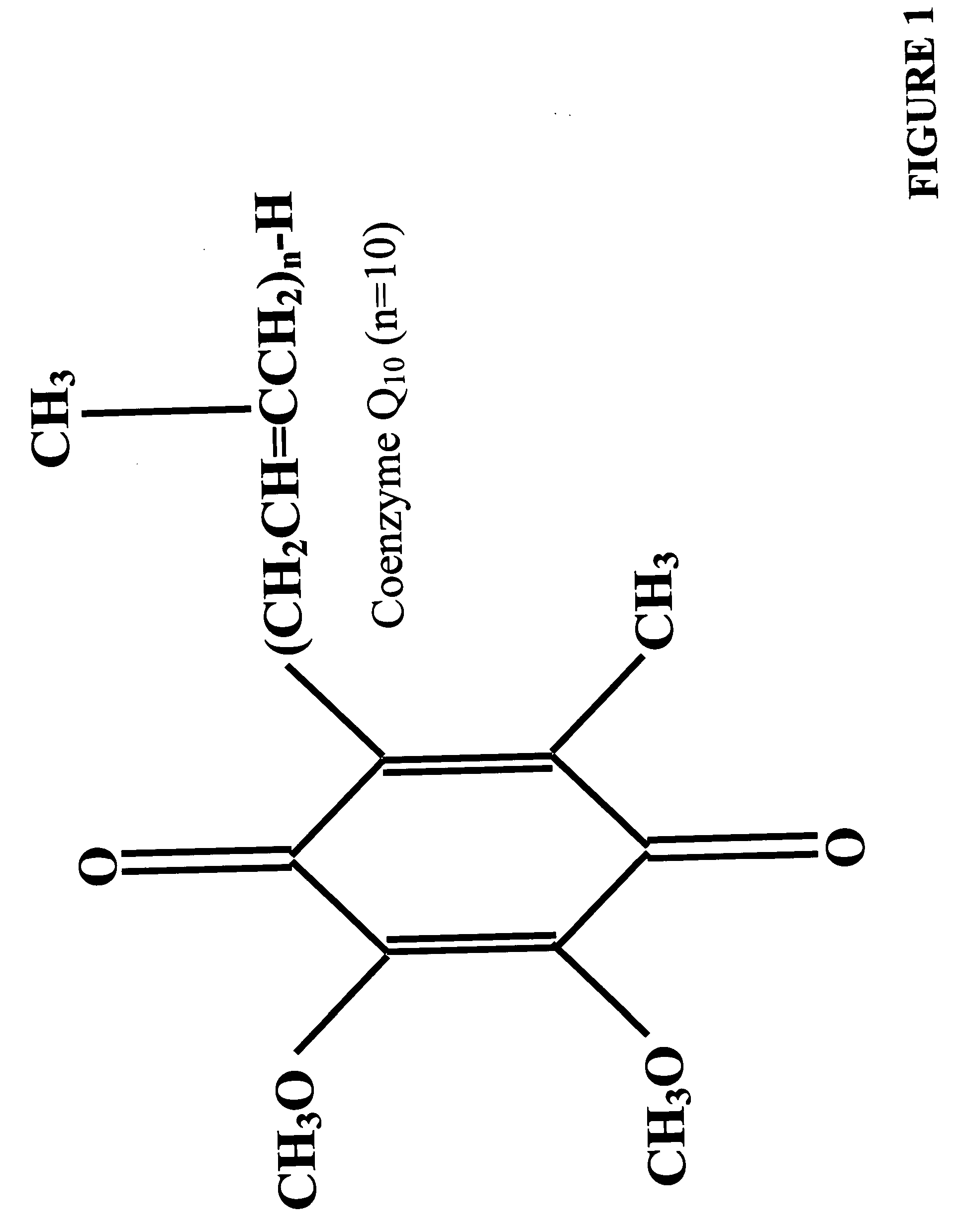
Culture medium containing enhancers of oxidative phosphorylation
The present invention relates to a culture medium suitable for mammalian cell, tissue or embryo growth in vitro in which enhancers of oxidative phosphorylation are included. These enhancers include coenzyme Q, alpha.lipoic acid, acetyl-L-carnitine, alpha.tocopherol, These products are introduced into the medium in a soluble form through the use of either a polysorbate surfactant such as Tween ™ or a preparation containing lecithin and surfactant.
Owner:WILDING MARTIN GRAHAM +2
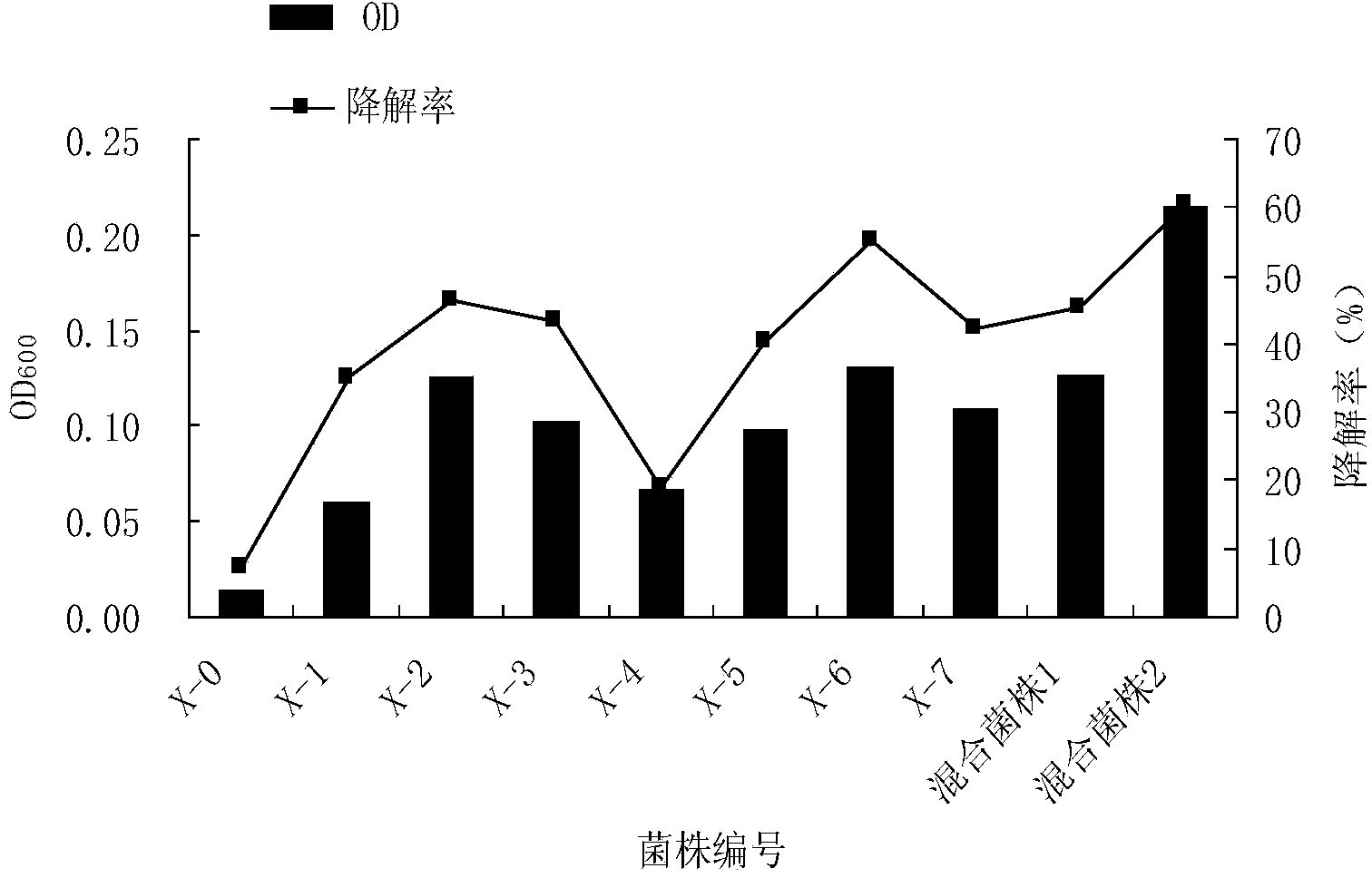
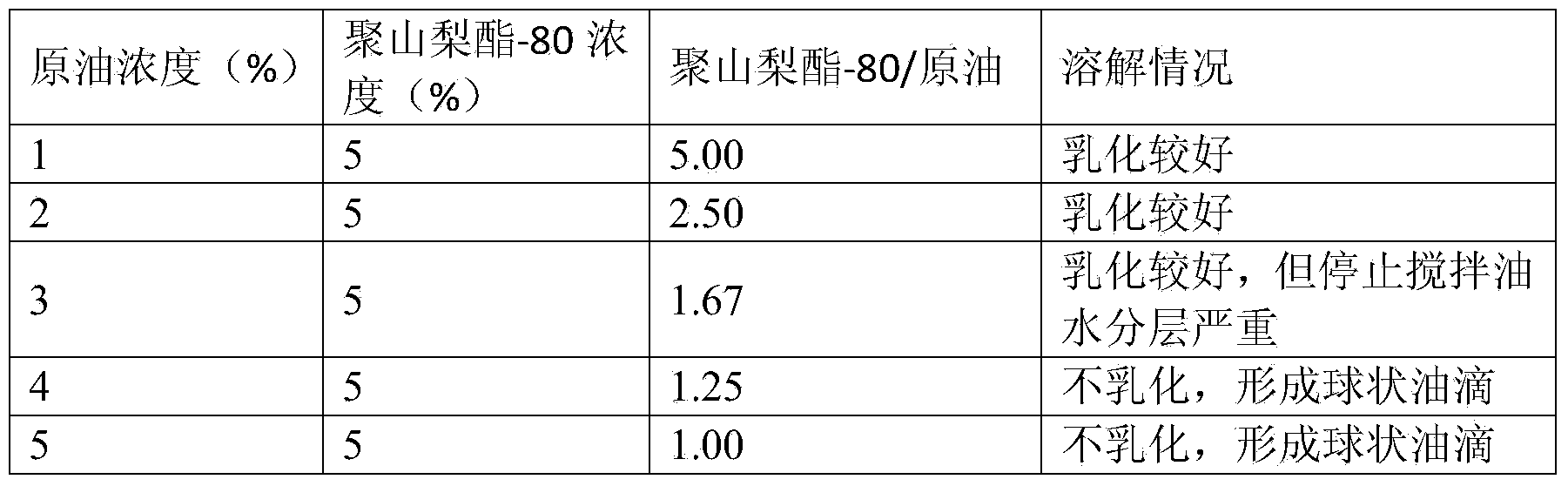
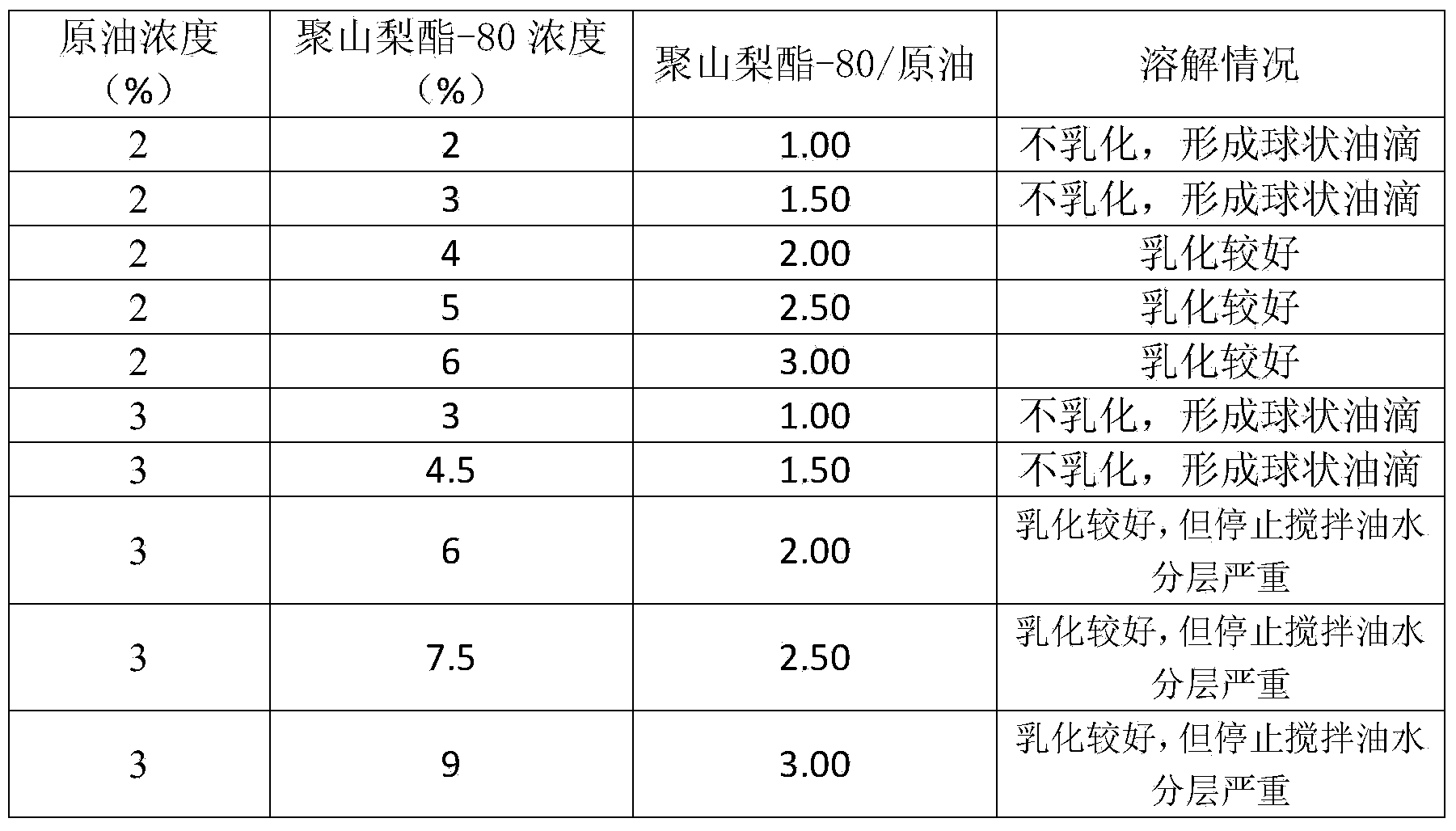
Screening method for petroleum degrading bacteria, method for preparing petroleum degrading bacteria inoculant from screened bacteria, and application of inoculant
ActiveCN104388312AImprove solubilitySolve the difficulty of dissolutionContaminated soil reclamationMicrobiology processesPrimary screeningScreening method
The invention relates to a screening method for petroleum degrading bacteria. An enrichment medium, a screening medium and a domestication medium, which are used in the method, are media employing petroleum as a unique carbon source, and specifically, petroleum polysorbate-80 solubilizing fluid is specifically added into the media. The method has the beneficial effects that a nonionic surfactant polysorbate-80 is used as a solubilizing agent, so that the dissolution effects of petroleum in water can be improved, and the problem of difficulty in the dissolution of petroleum in the media is solved; petroleum is actually the unique carbon source, and the petroleum polysorbate-80 solubilizing fluid is used as the carbon source of the media in processes of primary screening, domestication, purification and the like of the petroleum degrading bacteria; the screening of the petroleum degrading bacteria comprises the processes of primary screening, domestication, purification and the like, a strain is gradually domesticated to be adapted to growth and reproduction under the condition of employing petroleum as the unique carbon source in the domestication medium of which the petroleum concentration gradient gradually rises, and tolerance to petroleum and the degradation efficiency are improved.
Owner:QINGDAO AGRI UNIV
Pharmaceutical compositions comprising docetaxel and methods for preparation thereof
InactiveUS20080306137A1High stability and solubilityEasy to makeBiocideOrganic active ingredientsSolubilityDocetaxel-PNP
A pharmaceutical composition of docetaxel comprising an effective amount of docetaxel, a polysorbate (TWEEN® compound) and a co-solvent, wherein the co-solvent is at least one member selected from the group consisting of glycerol and polyethylene glycol. The composition is an injectable solution or a freeze-dried powder for injection. The solubility of decetaxel is improved by adding a polysorbate and a co-solvent. Methods of preparation of the pharmaceutical composition are also disclosed.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
Method for synthesizing high-purity polysorbate-80
ActiveCN101701065AHigh purityLight colorTransportation and packagingMixingSorbitanOleic Acid Triglyceride
The invention relates to a method for synthesizing high-purity polysorbate-80. The polysorbate-80 (I) is a partial esterified product of a sorbitan ethyoxyl compound and oleic acid. The method comprises the following steps of: (1) partially dehydrating sorbitol as a raw material under the action of an acid catalyst in a state of vacuum to obtain sorbitan (II); (2) carrying out addition polymerization on the sorbitan (II) and oxirane under the action of a base catalyst to obtain sorbitan polyethenoxy ether (III), wherein the addition number of the oxirane is 20; (3) reacting the sorbitan polyethenoxy ether (III) with the high-purity oleic acid under the action of an esterifying catalyst and refining to obtain the high-purity polysorbate-80. The invention leads the emulsifying and solubilizing performance of products to be more perfect and the quality of the products to easily meet the requirement of an injection class and has easily controlled quality and good stability; and the high-purity polysorbate-80 has lower blood dissolving rate in same concentration, and predictable and safer clinical use by being used as an auxiliary material for injection.
Owner:NANJING WELL BIOCHEM
Cream for chap removal
InactiveCN104546562APrevent chappingCracked isolationCosmetic preparationsToilet preparationsGlycerolOil phase
The invention discloses a cream for chap removal, which is composed of a water phase, an oil phase and a functional phase, wherein in parts by weigh, the water phase is composed of 45-85 parts of water, 0.1-0.5 part of allantoin and 0.05-0.2 part of diazolidinyl urea, the oil phase is composed of 0.5-1.5 parts of cetostearyl alcohol, 1-3 parts of glyceryl stearate, 0.5-1.5 parts of PEG-100 stearate, 2.5-5 parts of glycerin, 1-5 parts of polydimethylsiloxane, 2.5-5 parts of propylene glycol, 1-3 parts of isopropyl palmitate, 1-3 parts of petrolatum, 0.5-1.5 parts of polysorbate-60, 5-15 parts of mineral oil, 0.1-0.3 part of methylparaben, and 0.05-0.15 part of propyl hydroxybenzoate; and the functional phase is composed of 1-4 parts of one or more of ammonium polyacrylate, C13-16 isoparaffin, and laureths-25, 0.15-0.45 part of essence and 0.25-0.75 part of vitamin E. Experiments show that the cream both has the functions of water locking and moisturizing and has the effects of chap prevention and chap removal, therefore, the cream has a good market prospect.
Owner:无锡樱花梦美容制品有限公司
Pharmaceutical composition for improving safety of Shenmai injection and method for preparing same
ActiveCN101518617ADelays the problem of significant drops in pHImprove stabilityPowder deliveryPharmaceutical non-active ingredientsMedicineHydroxystearic Acid
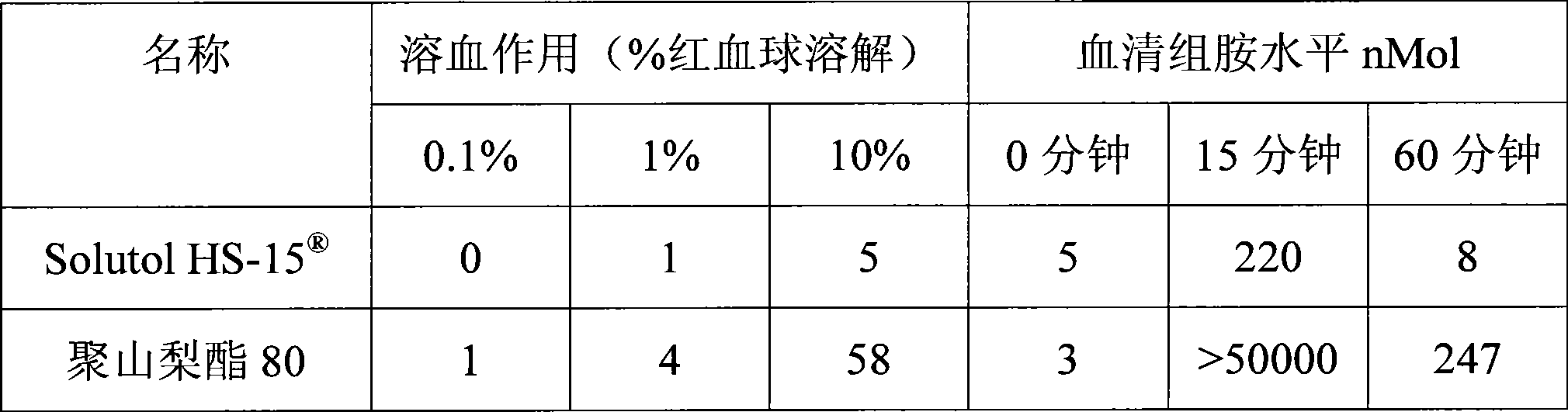
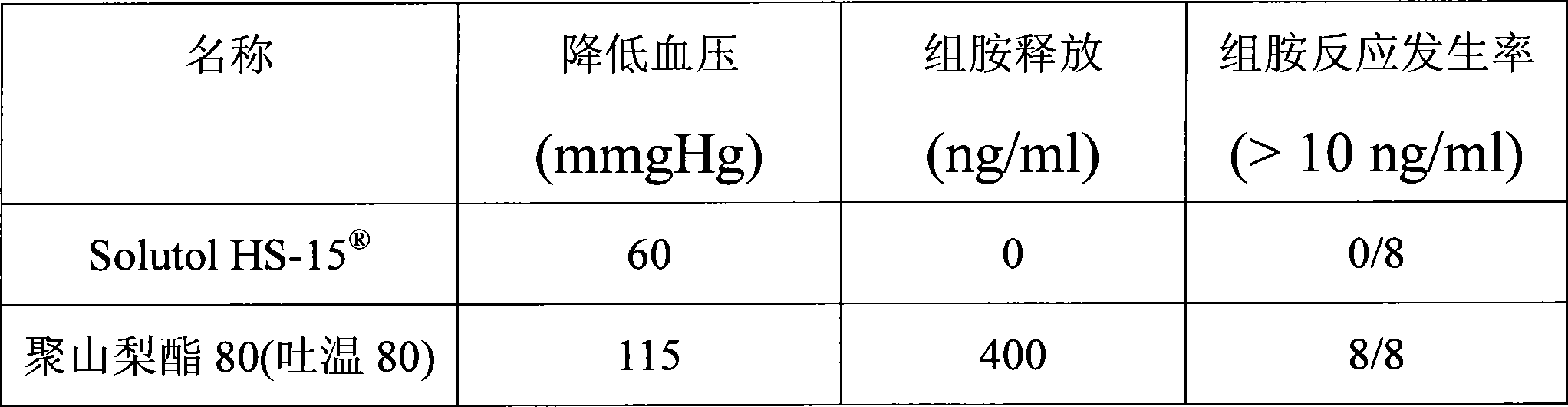
The invention discloses a pharmaceutical composition for improving the safety of Shenmai injection and a method for preparing the same. The pharmaceutical composition is a pharmaceutical composition for injection and is mainly prepared from red ginseng extract, dwarf lilyturf root extract and polyethylene glycol 12-hydroxy stearic acid ester. The pharmaceutical composition adopts a latent solvent with better safety and more obvious hydrotropy to replace the latent solvent polysorbate-80 which has potential safety hazard and influences the product quality, thus the reduction of the pH value of the Shenmai injection in the processes of storing and high temperature sterilization is obviously delayed, and the stability of the pharmaceutical composition is increased; besides, the safety of the polyethylene glycol 12-hydroxy stearic acid ester is higher than that of the polysorbate-80 and the dosage thereof is lower, thus the probability and the risk of untoward reactions of the pharmaceutical composition is reduced, and the safety for clinical application is improved.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com