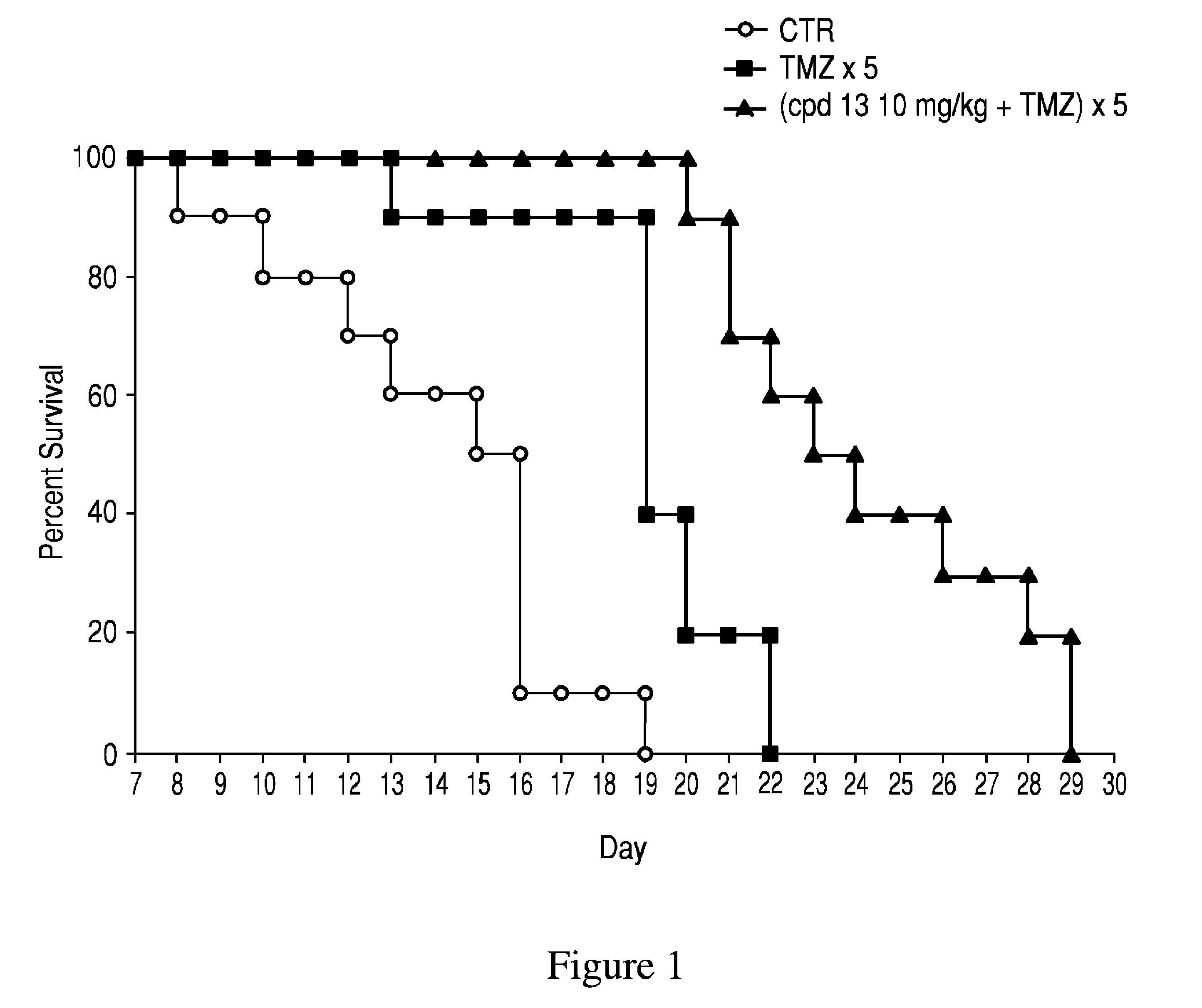
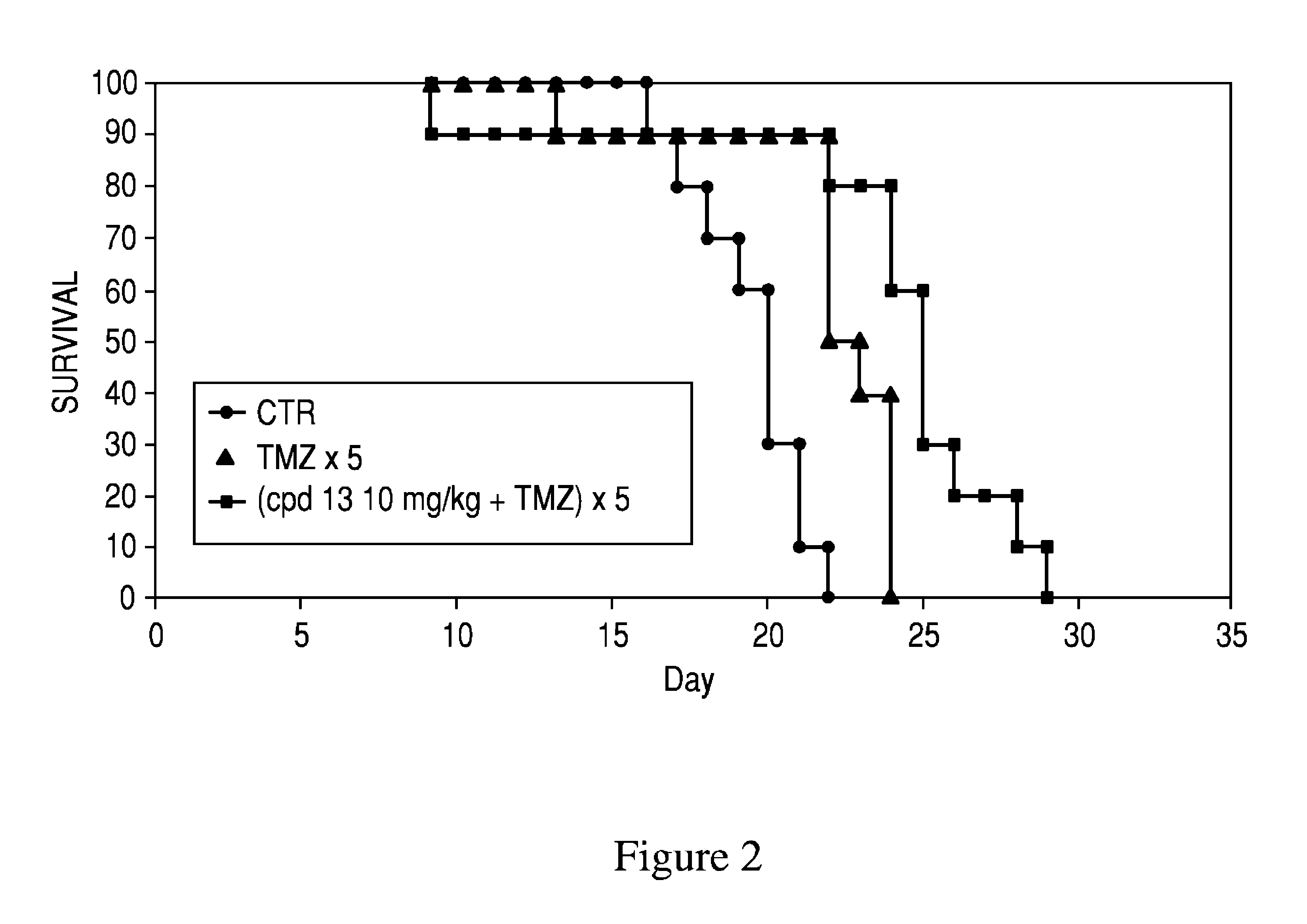
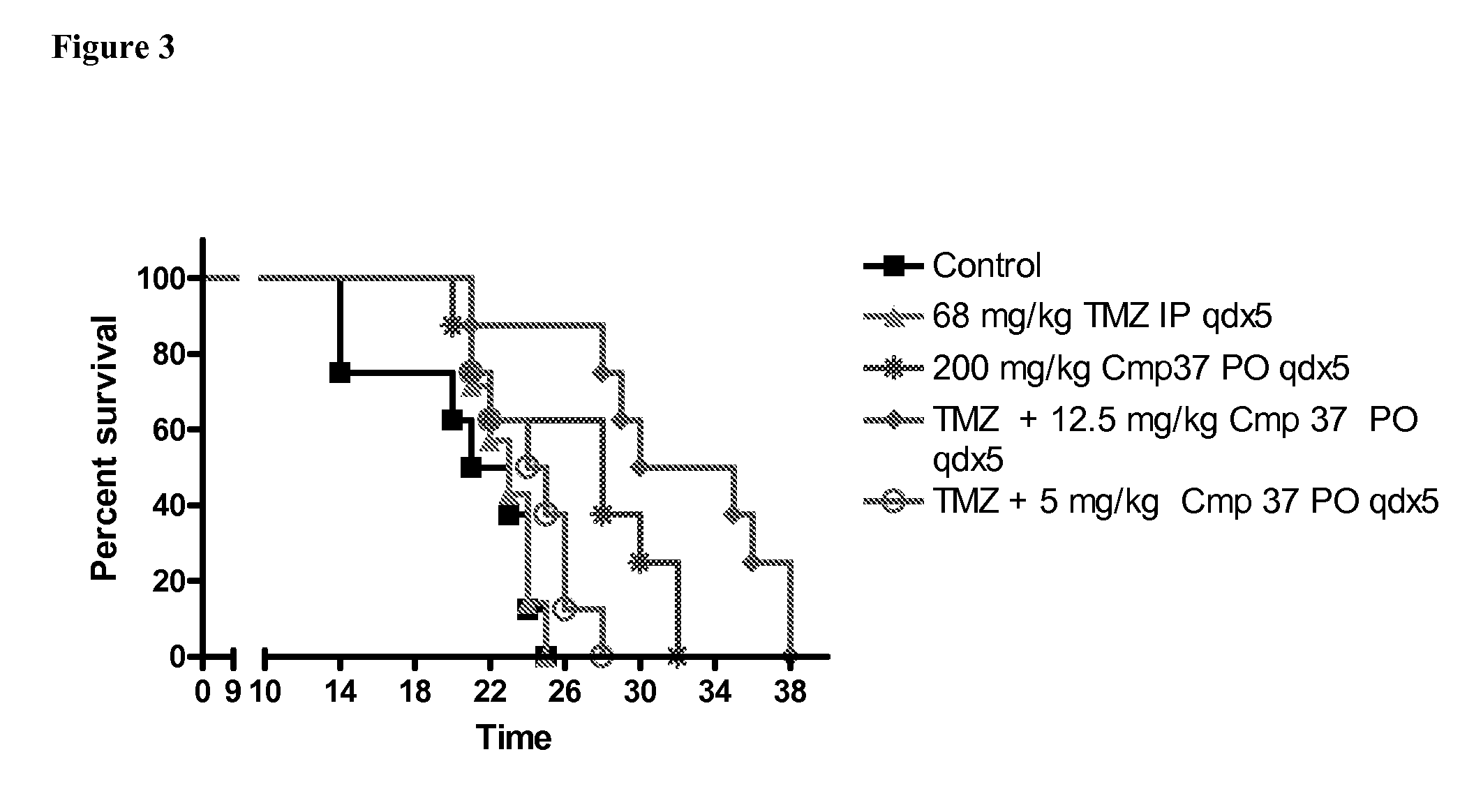
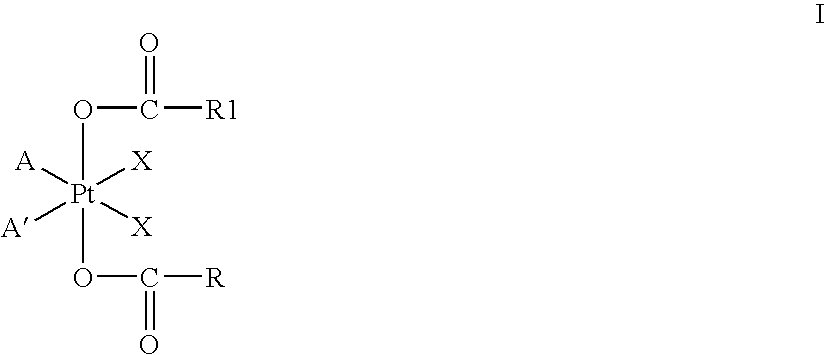Patents
Literature
180 results about "Temozolomide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
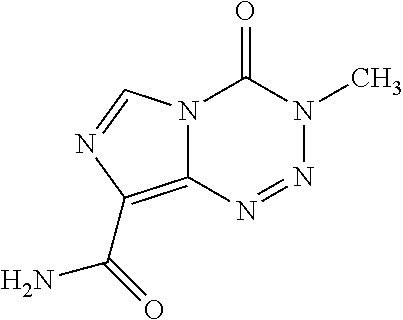
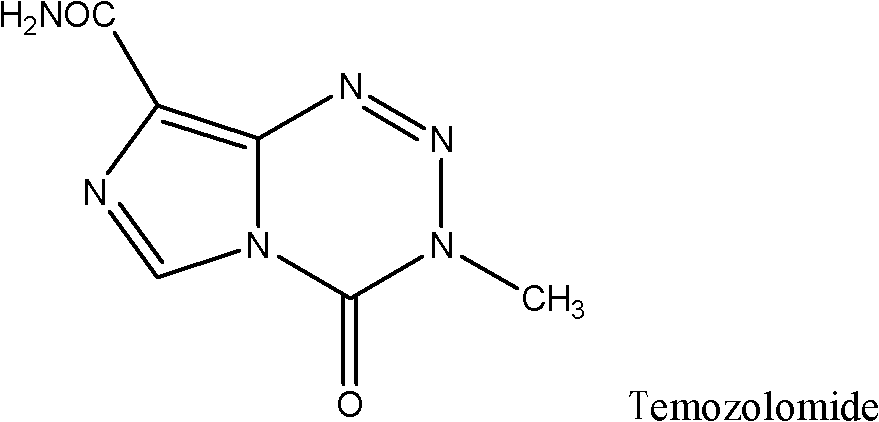
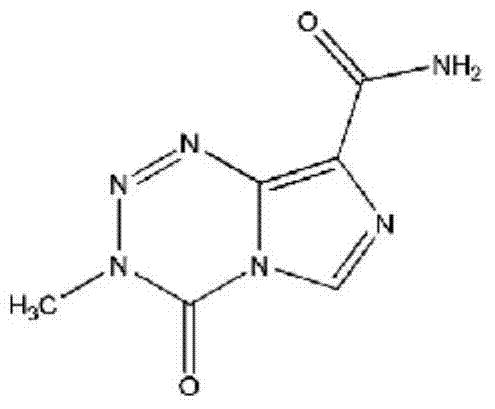
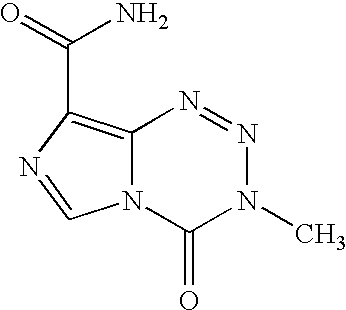
This medication is used to treat certain types of brain cancer.
Compositions and methods for the treatment of cancer
InactiveUS20020128228A1Reducing and avoiding adverse effectImprove toleranceBiocideAnimal repellantsIntestinal structureCancer prevention
This invention relates to compositions comprising temozolomide and thalidomide which can be used in the treatment or prevention of cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof. A particular composition comprises temozolomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof. The invention also relates to methods of treating or preventing cancer, in particular malignant melanoma, cancer of the skin, subcutaneous tissue, lymph nodes, brain, lung, liver, bone, intestine, colon, heart, pancreas, adrenals, kidney, prostate, breast, colorectal, or a combination thereof, which comprise the administration of temozolomide and thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of cancer chemotherapy or radiation therapy which comprise the administration of temozolomide and thalidomide to a patient in need of such reduction or avoidance.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
PARP inhibitor compounds, compositions and methods of use
ActiveUS8236802B2Improve survivalGrowth inhibitionHeavy metal active ingredientsBiocideSide effectPolymerase L
The present invention relates to tetraaza phenalen-3-one compounds which inhibit poly (ADP-ribose) polymerase (PARP) and are useful in the chemosensitization of cancer therapeutics. The induction of peripheral neuropathy is a common side-effect of many of the conventional and newer chemotherapies. The present invention further provides means to reliably prevent or cure chemotherapy-induced neuropathy. The invention also relates to the use of the disclosed PARP inhibitor compounds in enhancing the efficacy of chemotherapeutic agents such as temozolomide. The invention also relates to the use of the disclosed PARP inhibitor compounds to radiosensitize tumor cells to ionizing radiation. The invention also relates to the use of the disclosed PARP inhibitor compounds for treatment of cancers with DNA repair defects.
Owner:EISAI INC
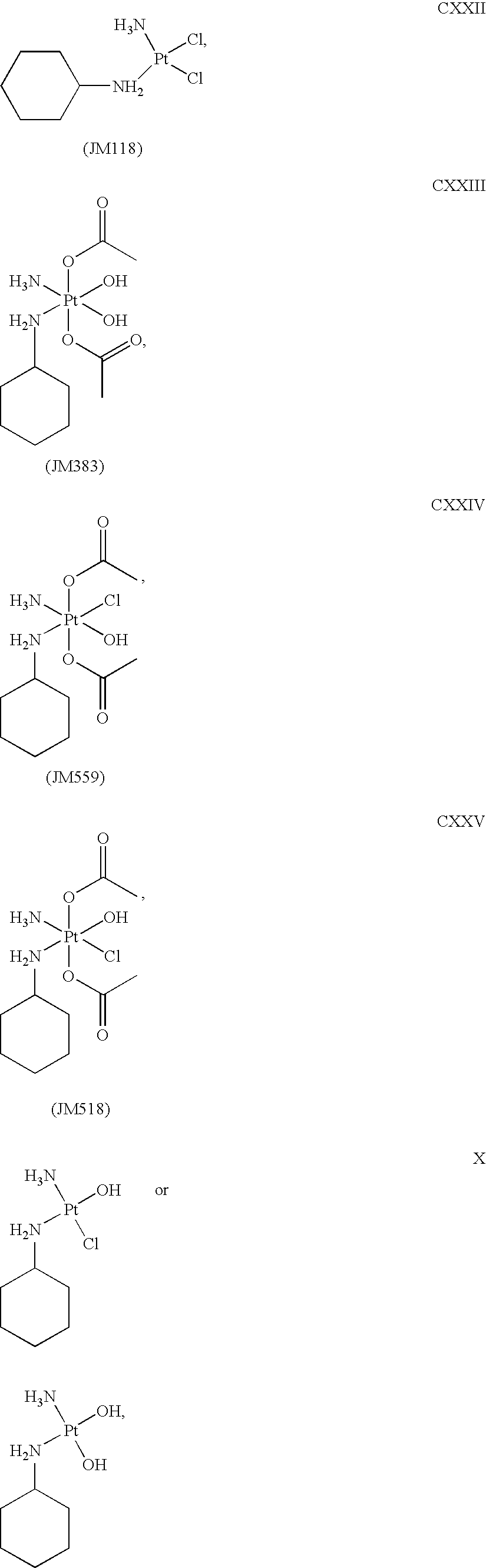
Platinum therapeutic combinations
The present invention provides combination compositions comprising Pt based compounds, including satraplatin, along with another chemotherapeutic agent such as temozolomide or lonafarnib. The combinations are useful for the prevention or treatment of cancer. Method of using the combinations to treat or prevent cancer are also provided
Owner:SCHERING CORP
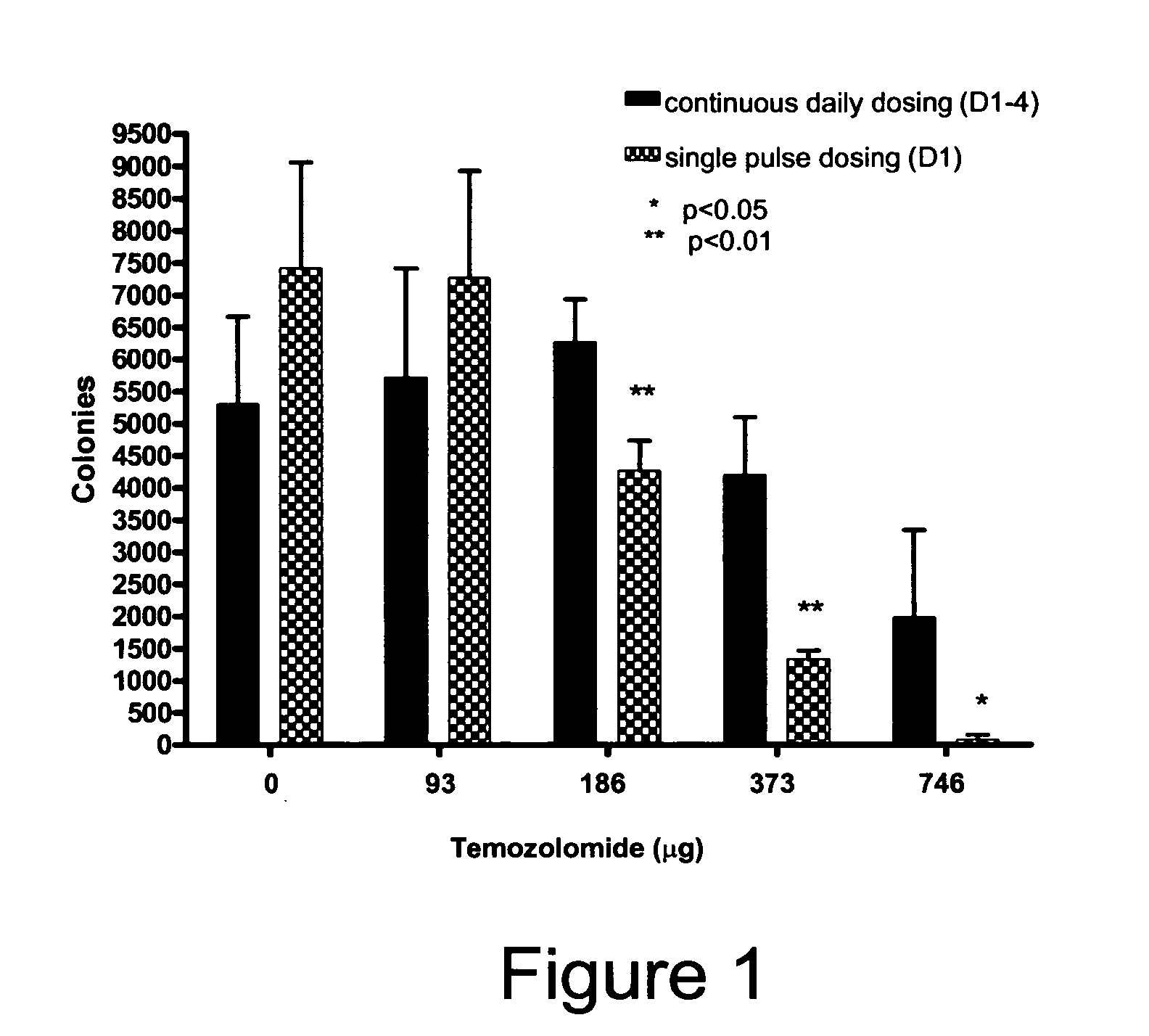
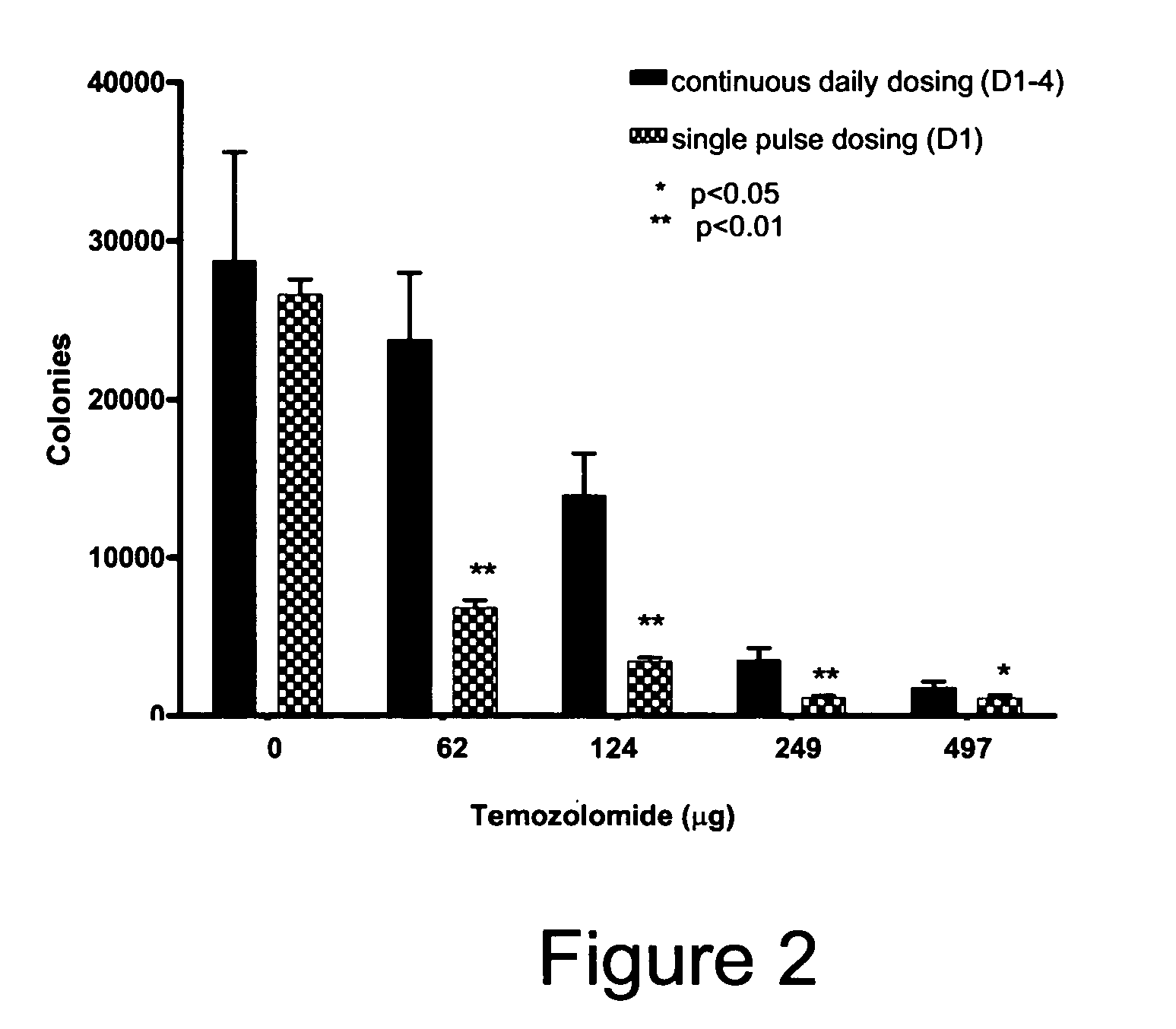
Treatment methods
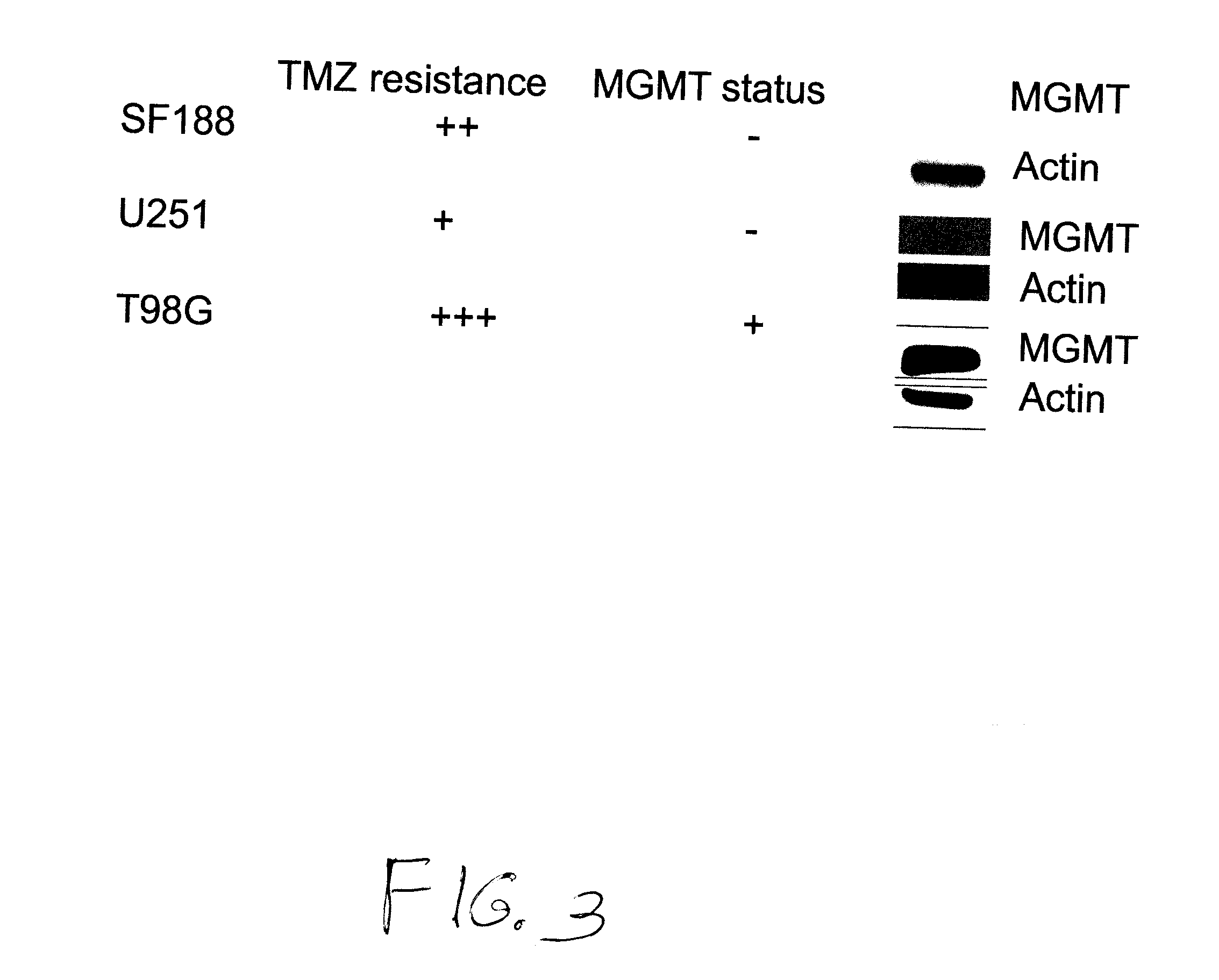
There are disclosed methods for treating cancer in a patient in need of such treating comprising administering temozolomide according to improved dosing regimen and / or schedules based on the patient's MGMT level. Additional improved methods for treating patients with temozolomide are also disclosed.
Owner:SCHERING CORP
Targeted liposomes
The present invention is in the field of drug delivery, and specifically, cationic liposome-based drug delivery. In embodiments, this invention provides methods of making ligand-targeted (e.g., antibody- or antibody fragment-targeted) liposomes useful for the delivery of liposomes to tumors, including brain tumors. In embodiments, the liposomes deliver temozolomide across the blood-brain barrier for treatment of primary or metastatic brain tumors. Additional cancers that can be treated with the liposomes include neuroendocrine tumors, melanoma, prostate, head and neck, ovarian, lung, liver, kidney, breast, urogenital, gastric, colorectal, cervical, vaginal, angiosarcoma, liposarcoma, rhabdomyosarcoma, choriocarcinoma, pancreatic, retinoblastoma and other types of cancer. In another embodiment the liposomes deliver melphalan for the treatment of multiple myeloma, other tumors of the blood or other solid tumors. In still other embodiments the liposomes can deliver other drugs such as pemetrexed or irinotecan for treatment of cancer or drugs including atropine for treatment of organophosphate poisoning.
Owner:GEORGETOWN UNIV
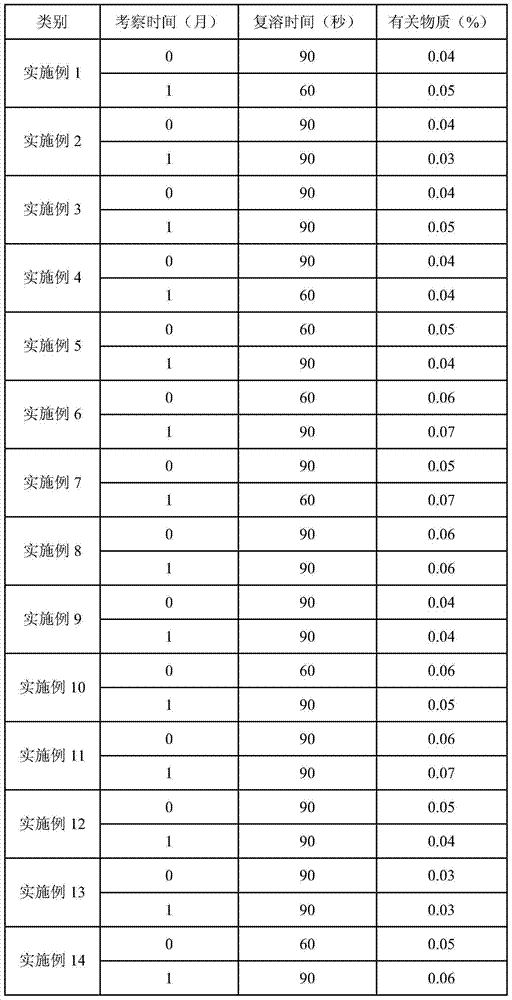
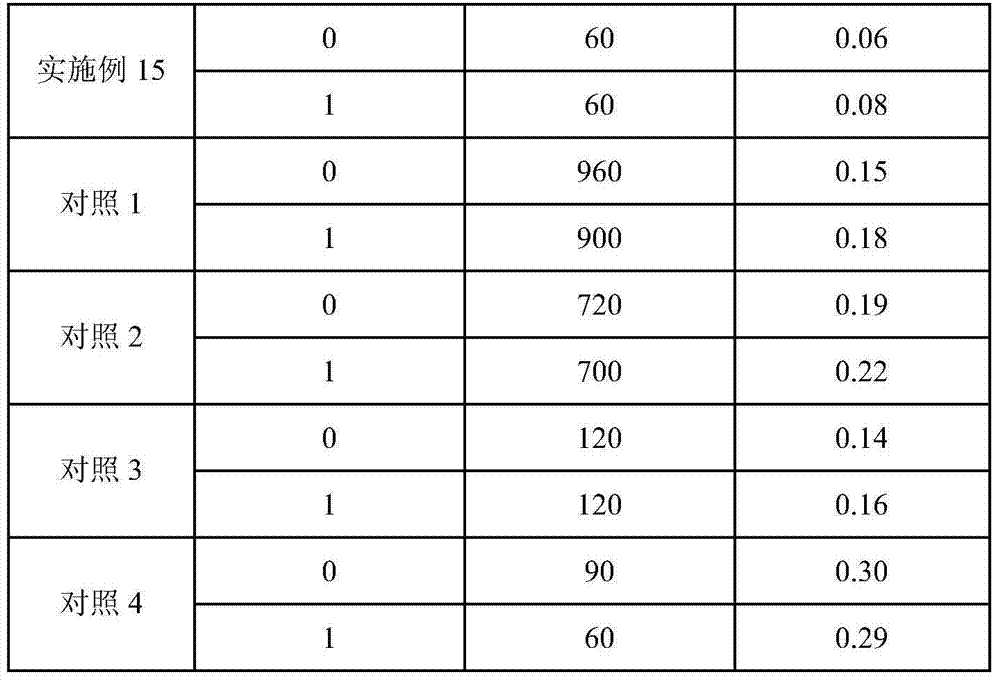
Temozolomide freeze-dried preparation
ActiveCN101869551ASolve the speed problemSolve poor resolubilityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
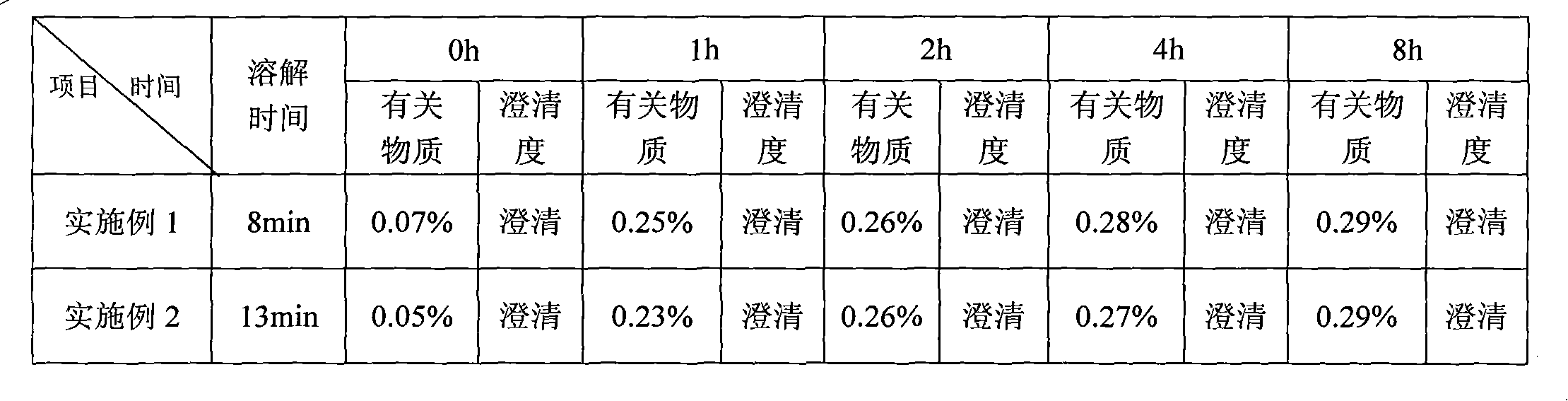
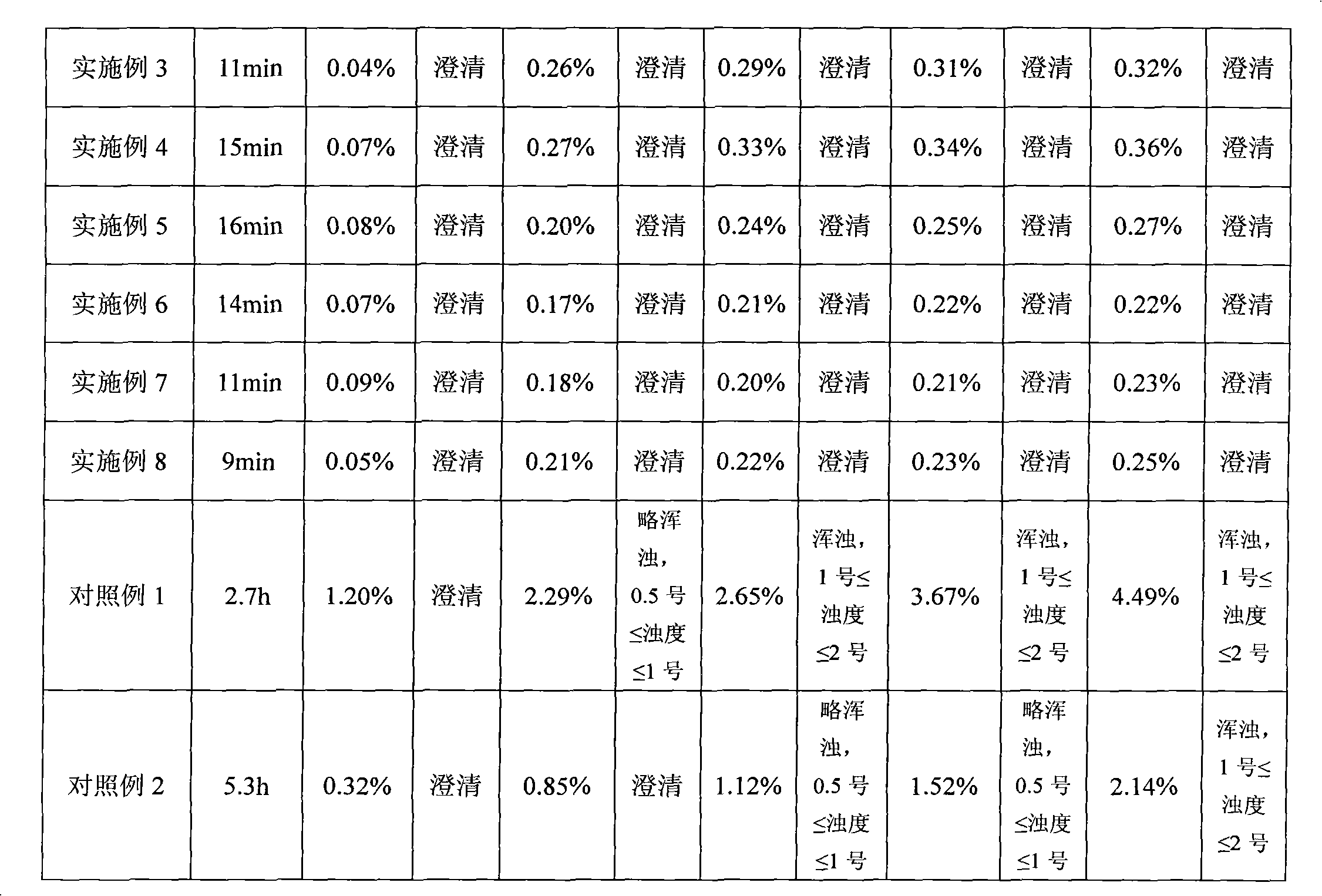
The invention discloses a temozolomide freeze-dried preparation. Every 100ml of the preparation comprises 1 to 2,000 mg of temozolomide, 1 to 2,000 mg of solubilizer, 801 to 2,000 mg of polysorbate, 5 to 5,000 mg of mannitol, 1 to 2,000 mg of buffering agent, 0.5 to 1,000 mg of hydrochloric acid and the balance of water. The preparation of the invention overcomes the shortcomings of slow dissolving speed, poor redissolution, fussy operation and unqualified solution clarity of the freeze-dried preparation, has the technical effects that: the preparation of the invention has high redissolution; solid is completely dissolved to be clear and colorless by adding water into the freeze-dried preparation and shaking slightly; purity is over 99.5 percent and single impurity content is below 0.3 percent by detecting the purity and the content by using HPLC; through stability experimental investigation, relative substances and content of the preparation are not changed remarkably; and quality is stable.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Methods of using temozolomide formulation intrathecally in the treatment of cancers
Methods are disclosed for treating cancer in a patient in need of such treating comprising intrathecally administering temozolomide in a pharmaceutical formulation in a therapeutically effective amount.
Owner:SCHERING CORP
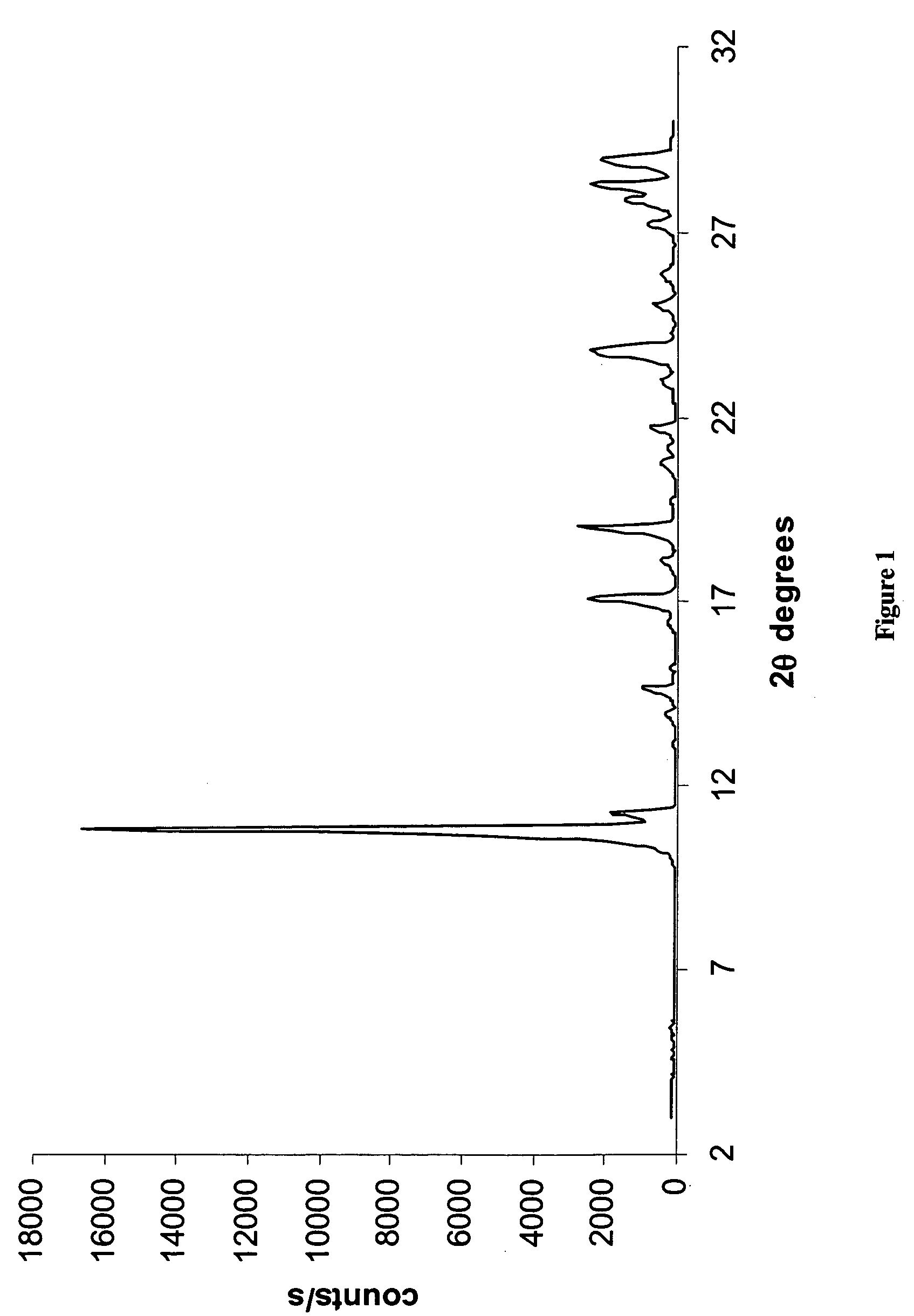
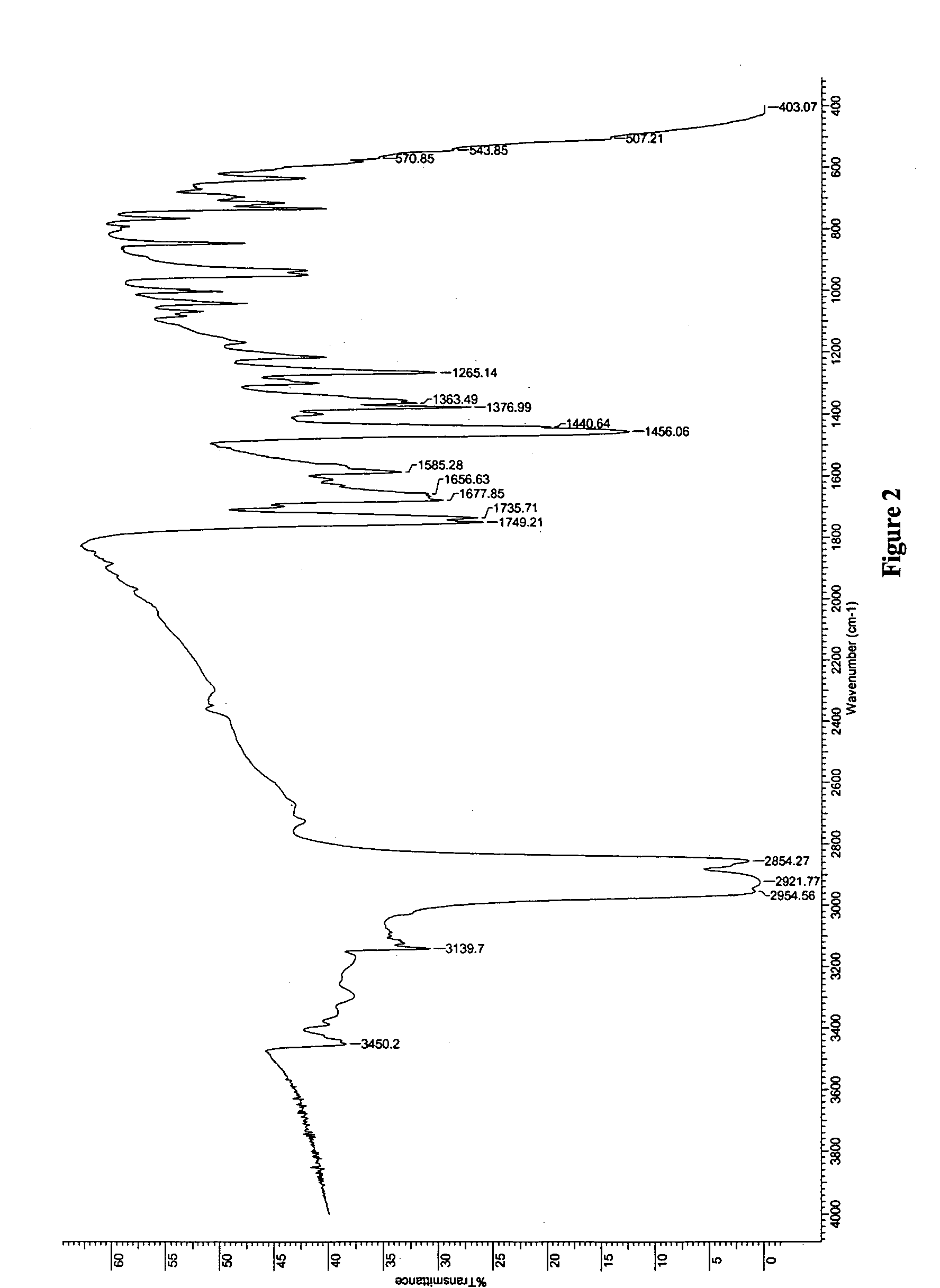
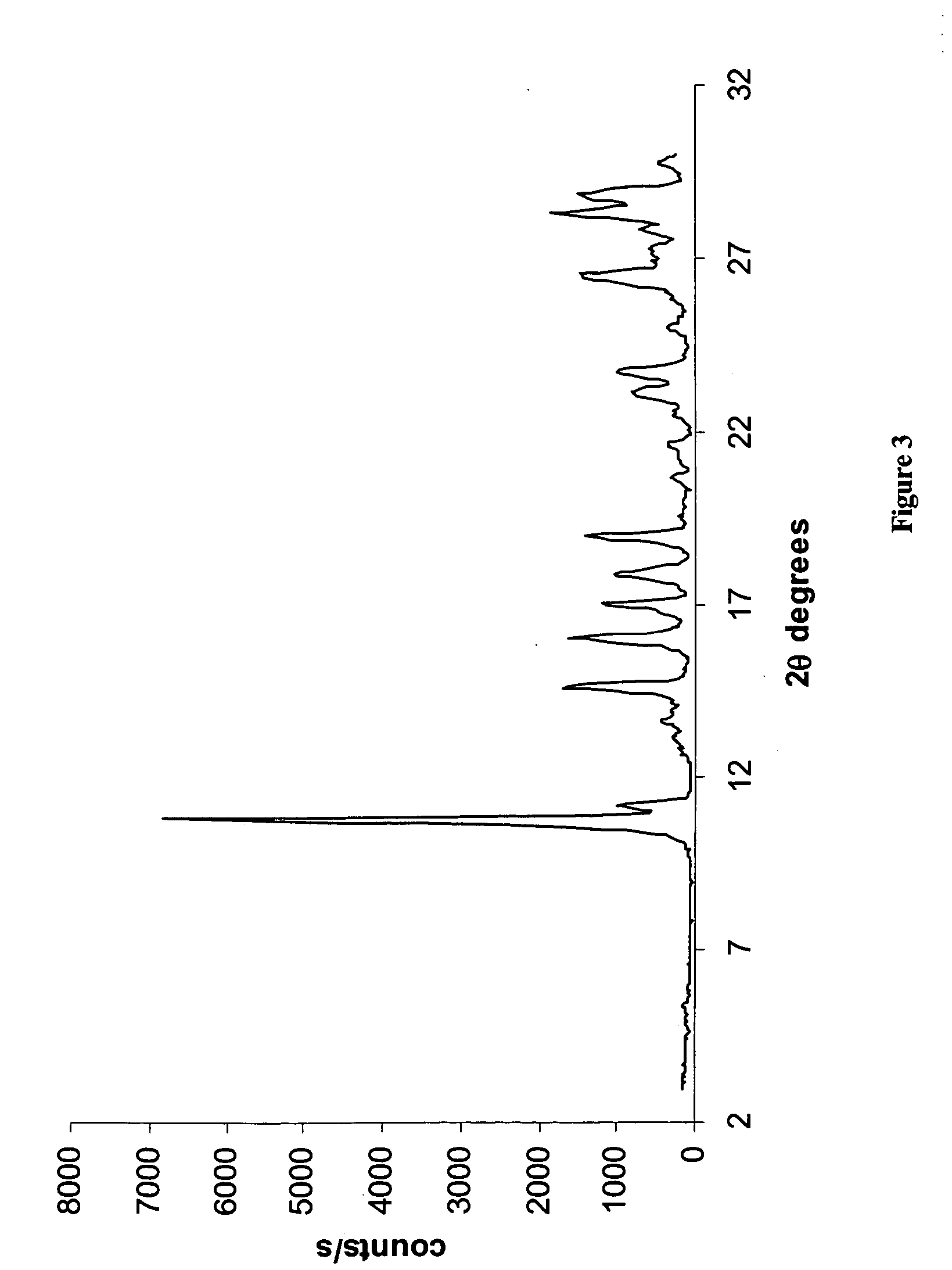
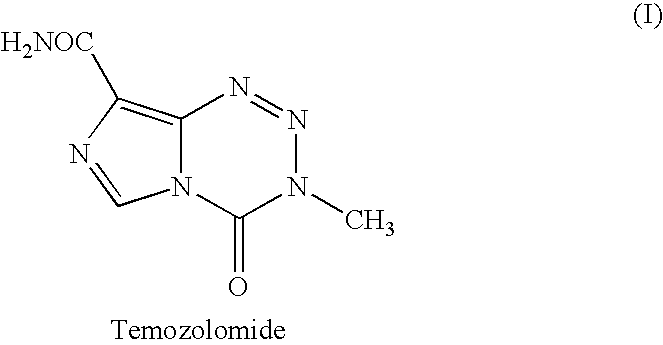
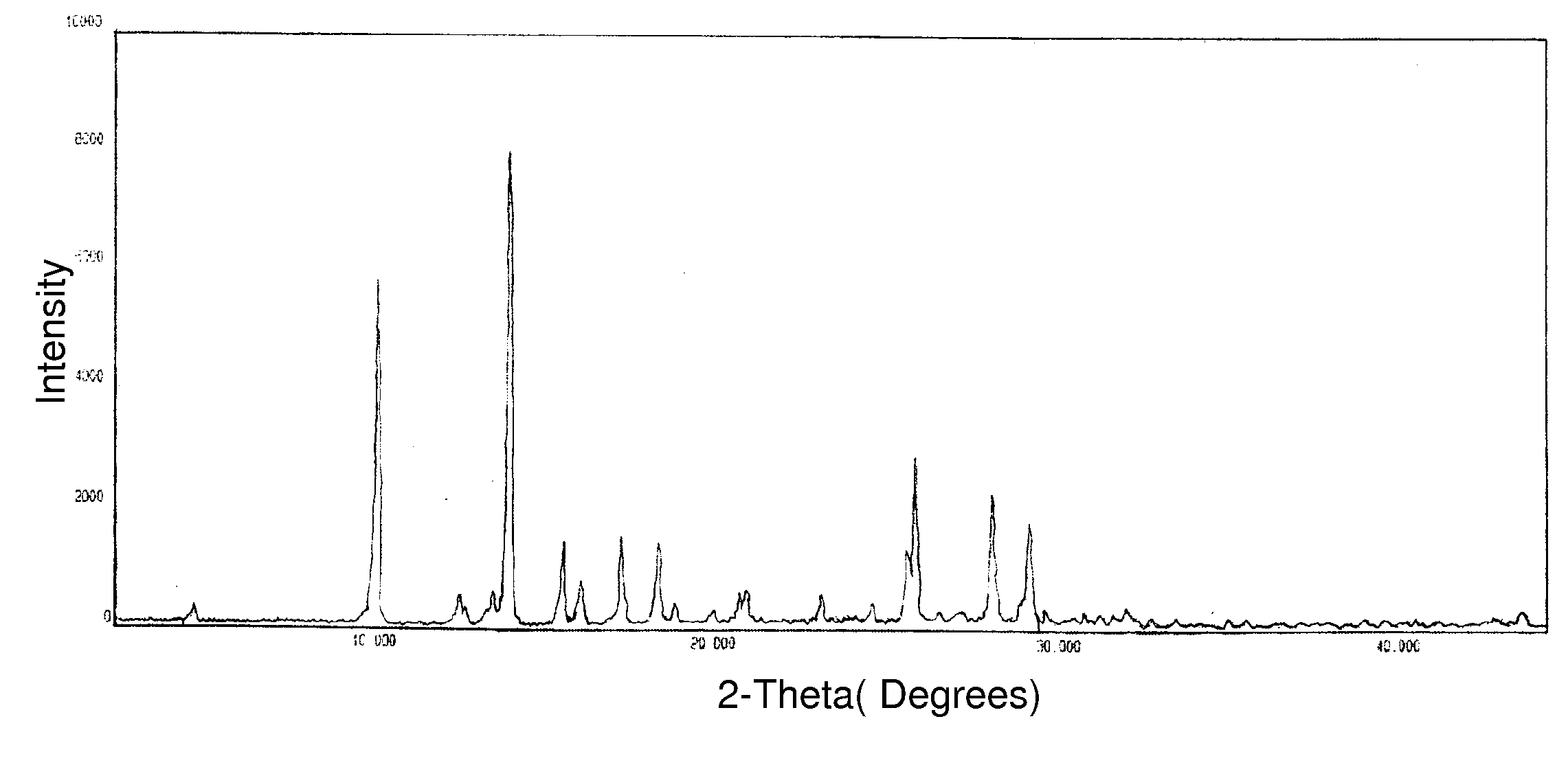
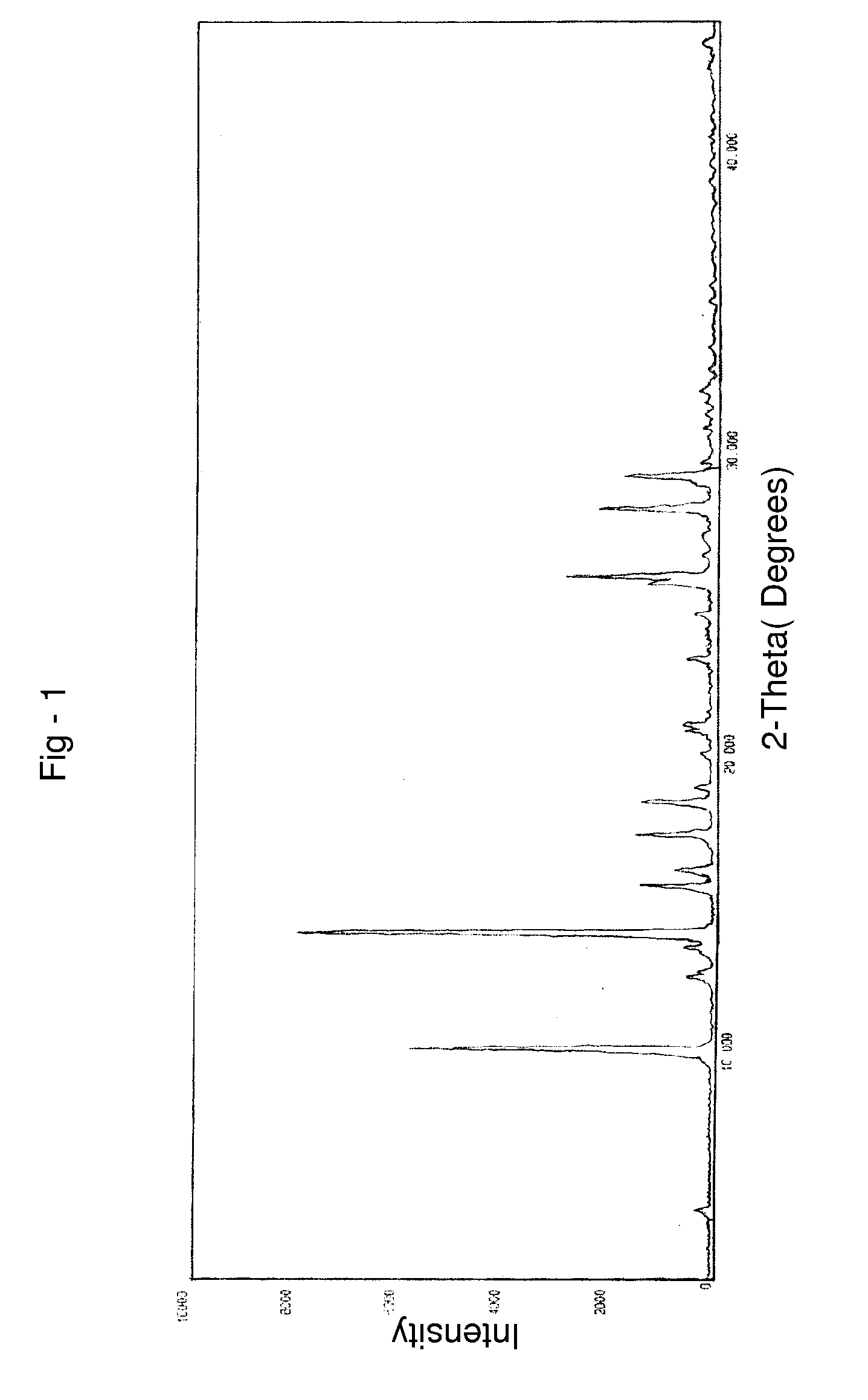
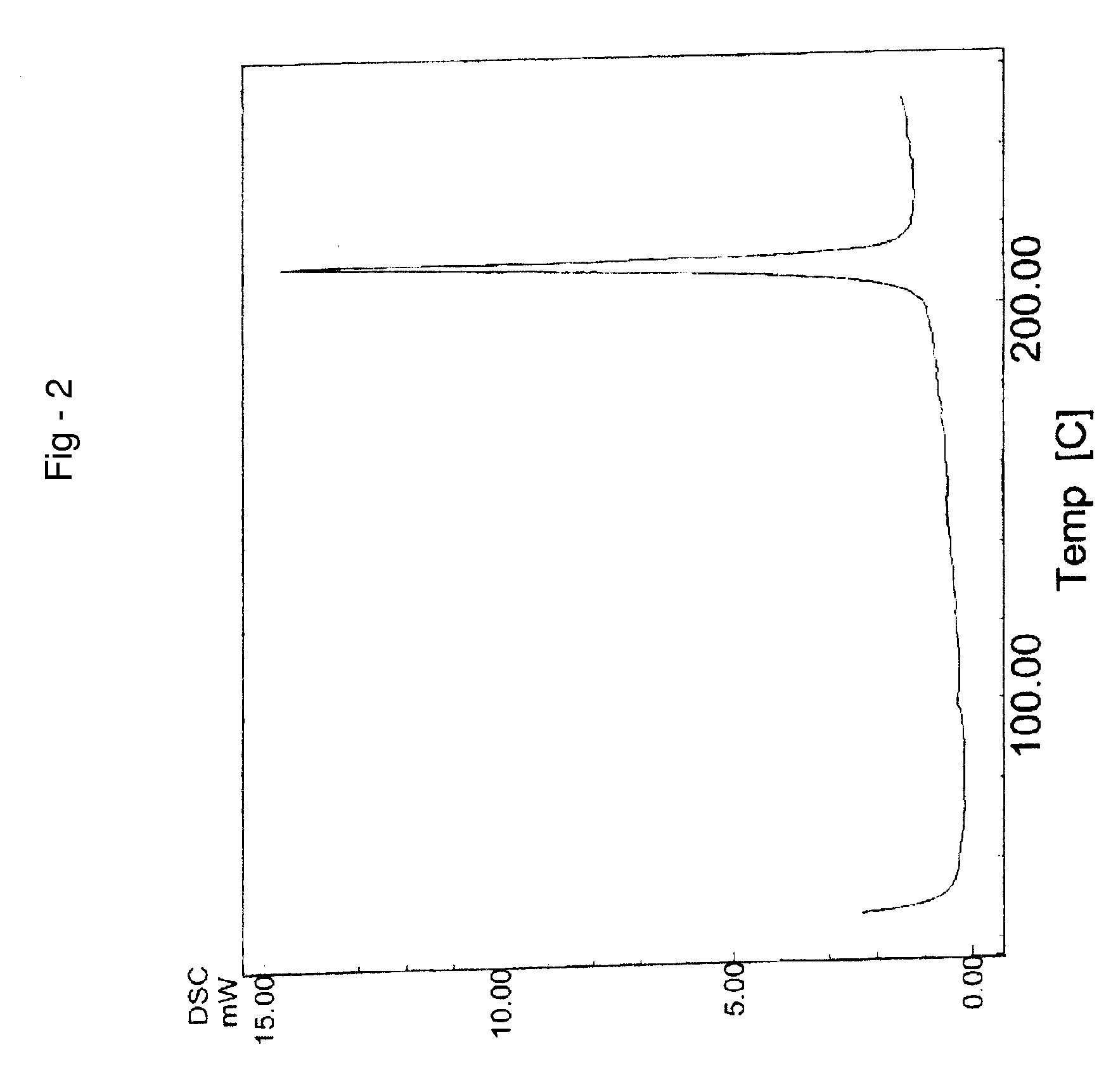
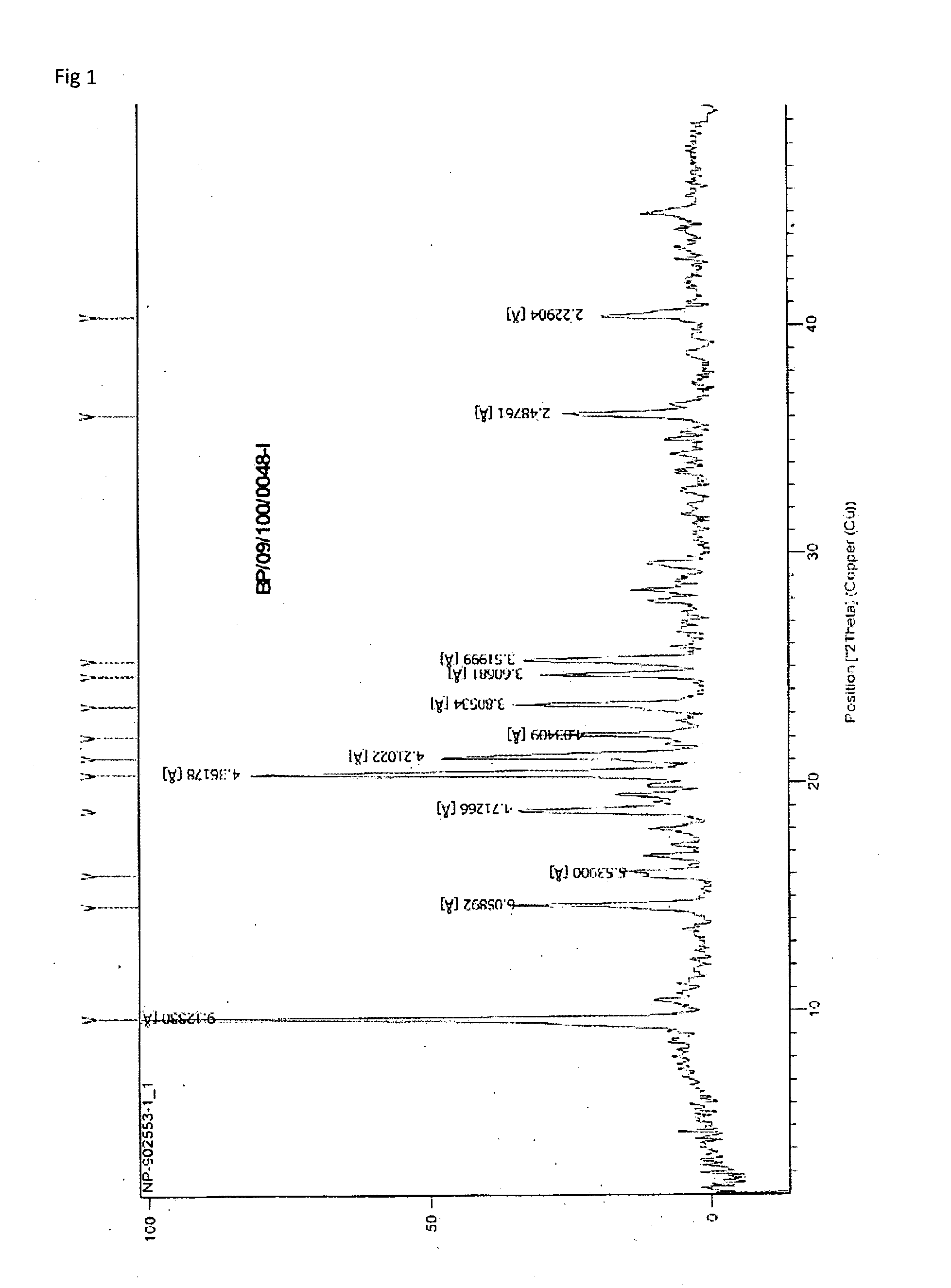
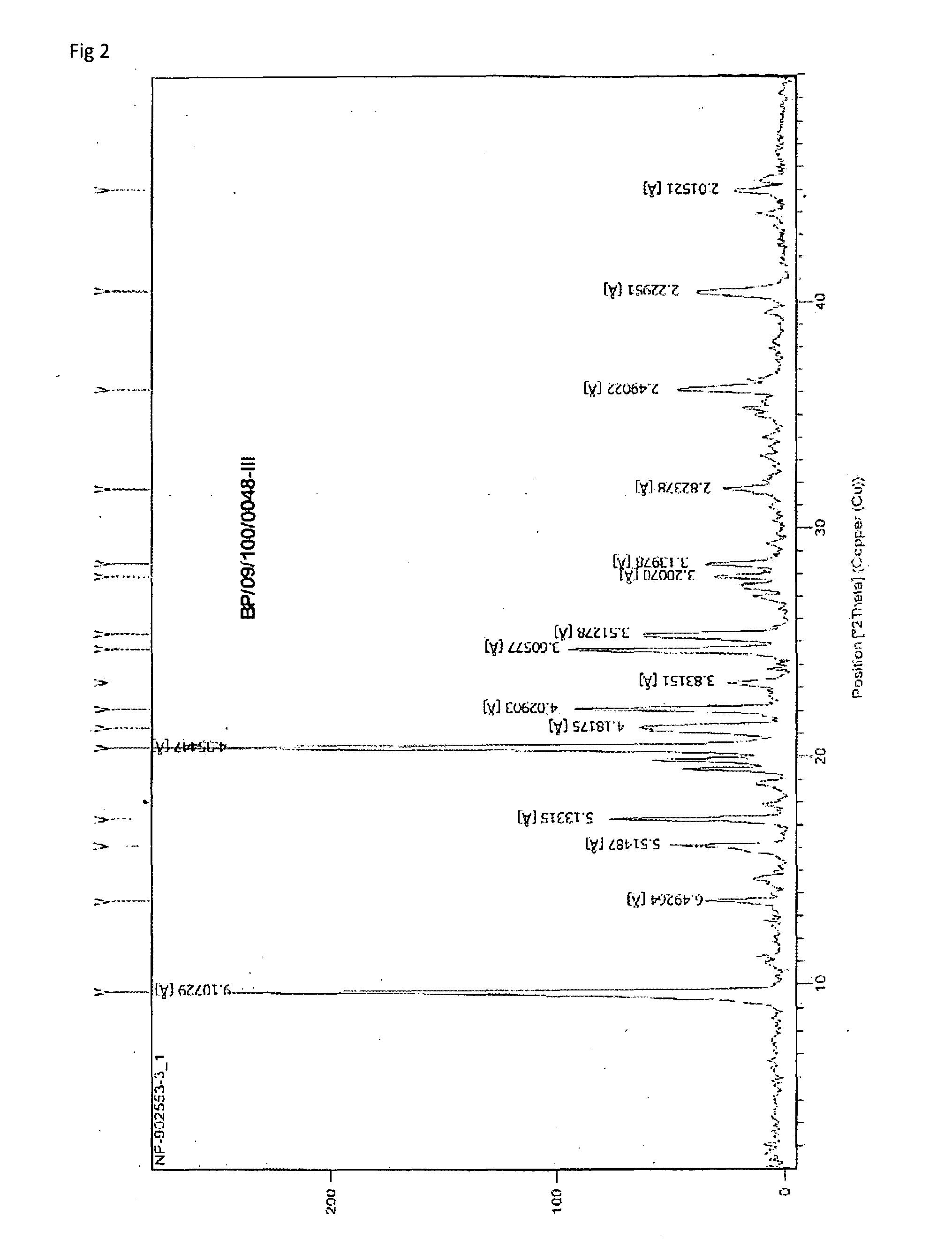
Novel crystalline forms of temozolomide
Disclosed are novel crystalline forms of Temozolomide, methods of preparation thereof, medicaments including the novel crystalline forms of Temozolomide, and uses thereof in the treatment of medical conditions.
Owner:WAVELENGTH ENTERPRISES LTD
Process for preparing temozolomide
InactiveUS20060183898A1High yieldStraightforward and cheap and easy to perform processOrganic chemistryIon-exchanger regenerationOrganic acidOrganic solvent
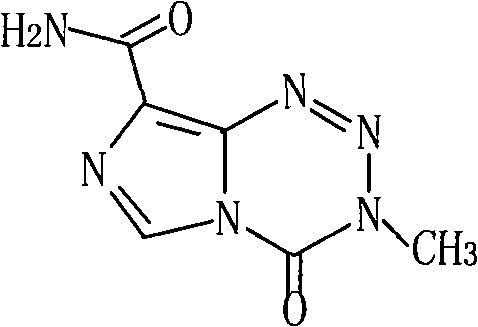
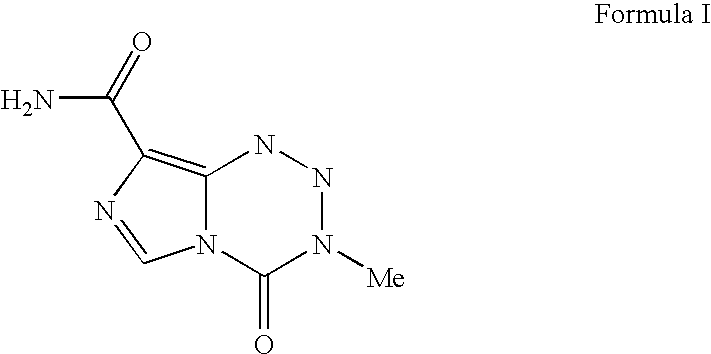
The present invention provides a process for preparing highly pure Temozolomide base which includes recovery from the purification mother liquors by using an anionic exchange resin. By treating Temozolomide hydrochloride with a mixture of an organic acid, a water miscible organic solvent, and water, Temozolomide free base is obtained in an acidic medium. Due to the high sensitivity of Temozolomide to basic pH values the recovery-including process is especially advantageous because it enables obtaining high yields of highly pure Temozolomide base in acidic conditions. The process for producing Temozolomide base includes hydrolysis of the starting material 8-cyano-3-methyl-[3H]-imidazo[5,1-d]-tetrazin-4-one in acidic medium to obtain highly pure Temozolomide hydrochloride in high yield.
Owner:WAVELENGTH ENTERPRISES LTD
Use of dianhydrogalactitol and analogs and derivatives thereof to treat glioblastoma multiforme
ActiveUS20140221442A1Improve survivalFree of side effectBiocideNervous disorderDianhydrogalactitolGlioblastoma
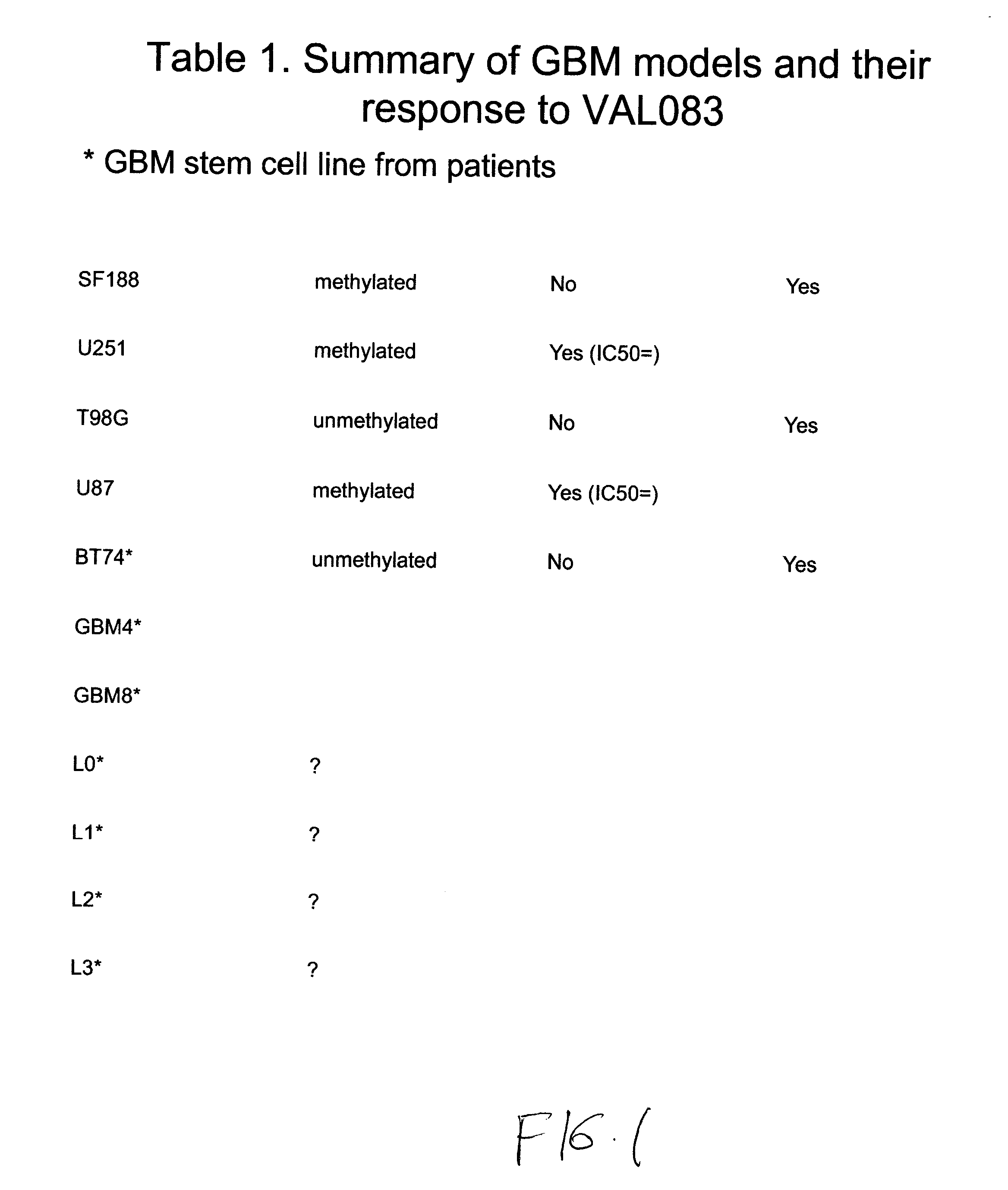
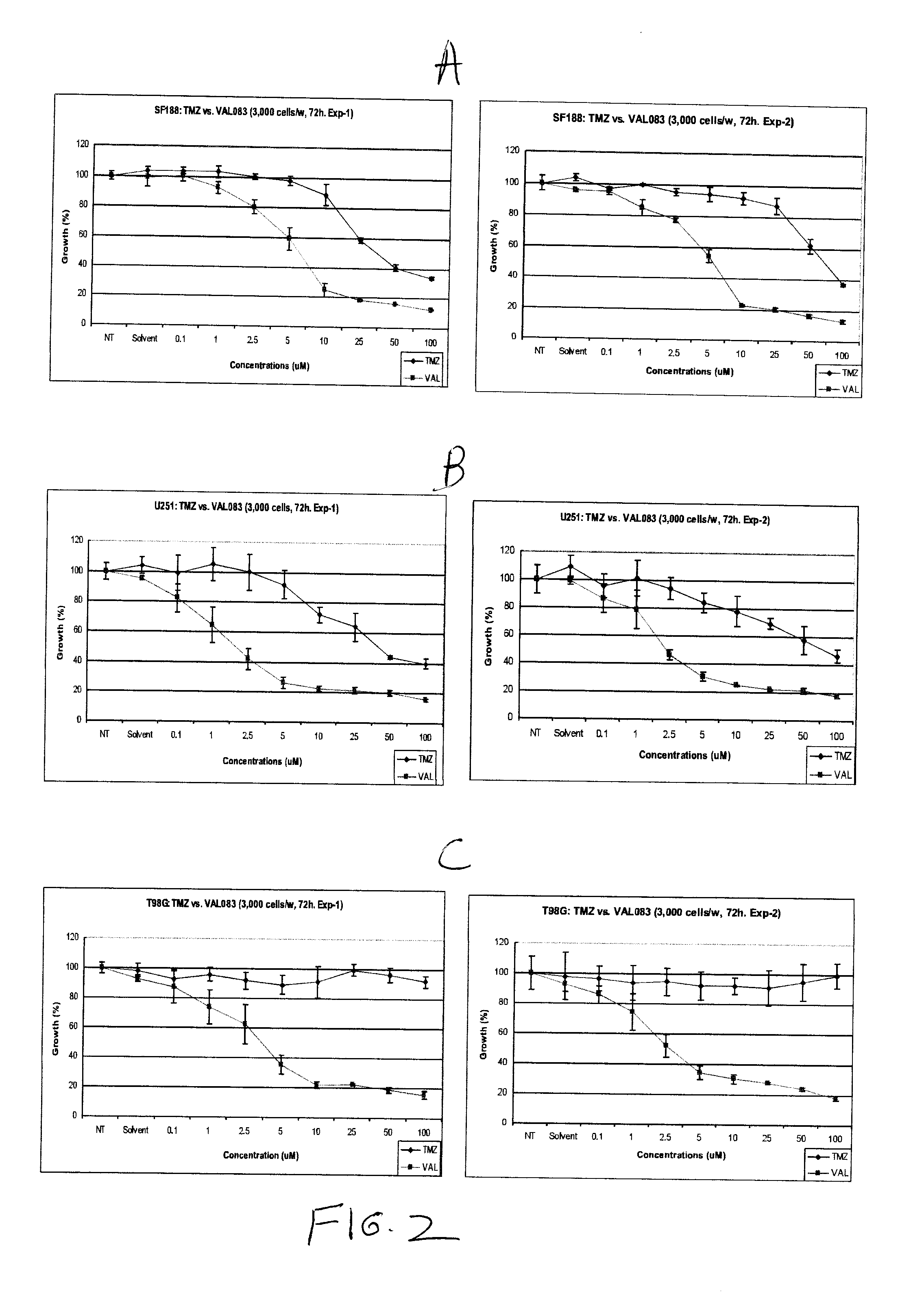
The use of dianhydrogalactitol provides a novel therapeutic modality for the treatment of glioblastoma multiforme. Dianhydrogalactitol acts as an alkylating agent on DNA that creates N7 methylation. Dianhydrogalactitol is effective in suppressing the growth of cancer stem cells and is active against tumors that are refractory to temozolomide; the drug acts independently of the MGMT repair mechanism.
Owner:DEL MAR PHARMA
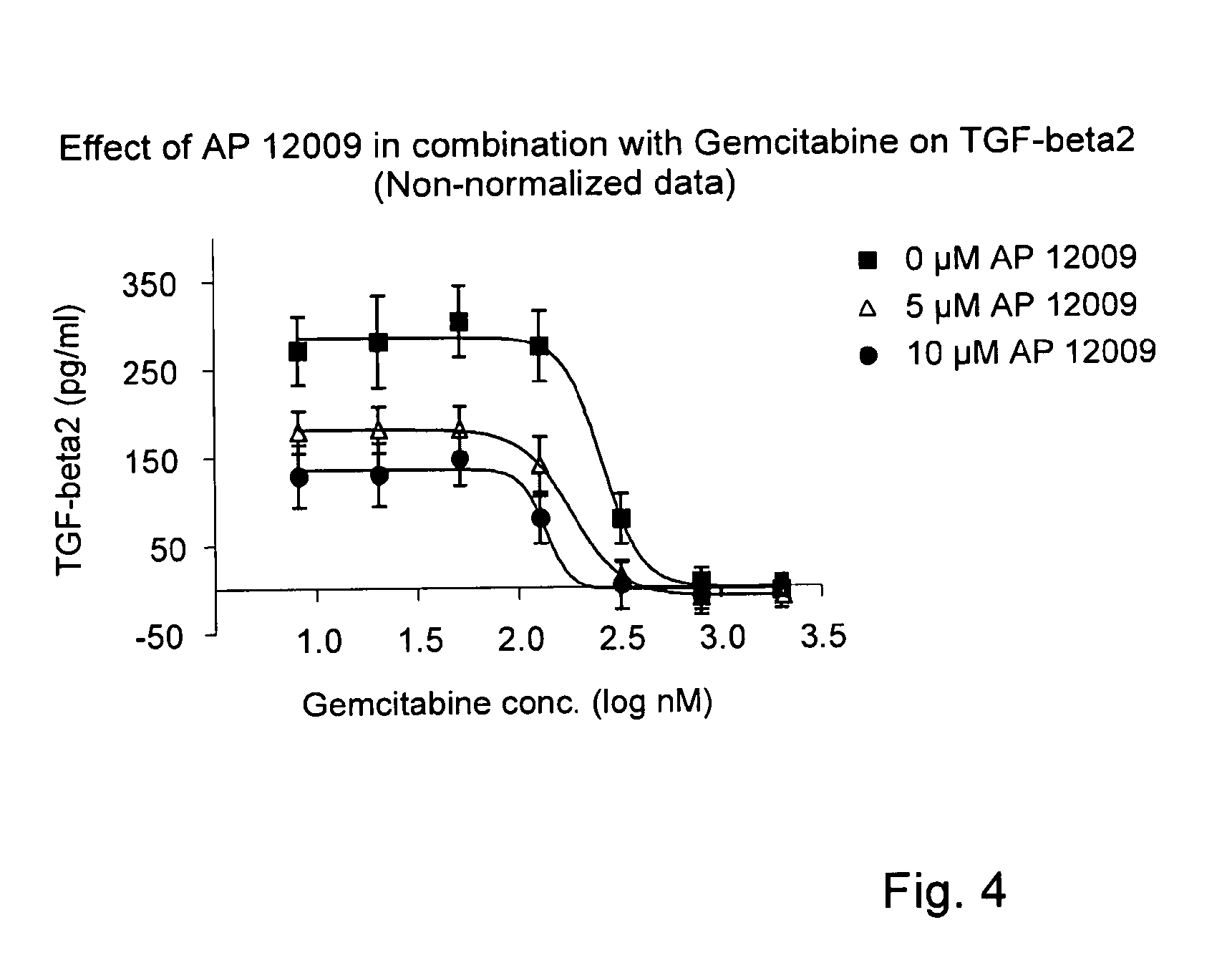
Combination of a chemotherapeutic agent and an inhibitor of the TGF-beta system
ActiveUS8476246B2Reduce IC50Improve efficiencyBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
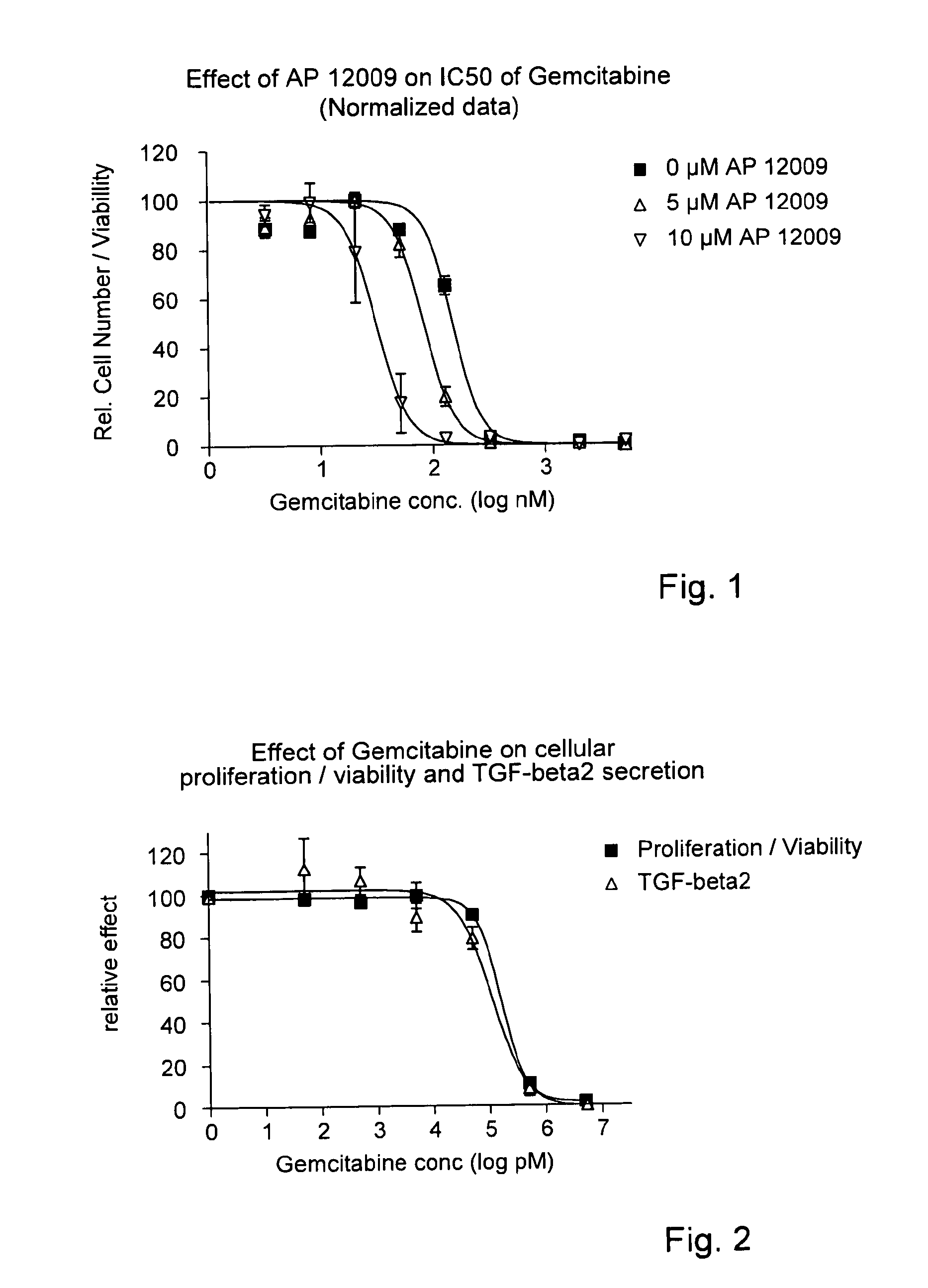
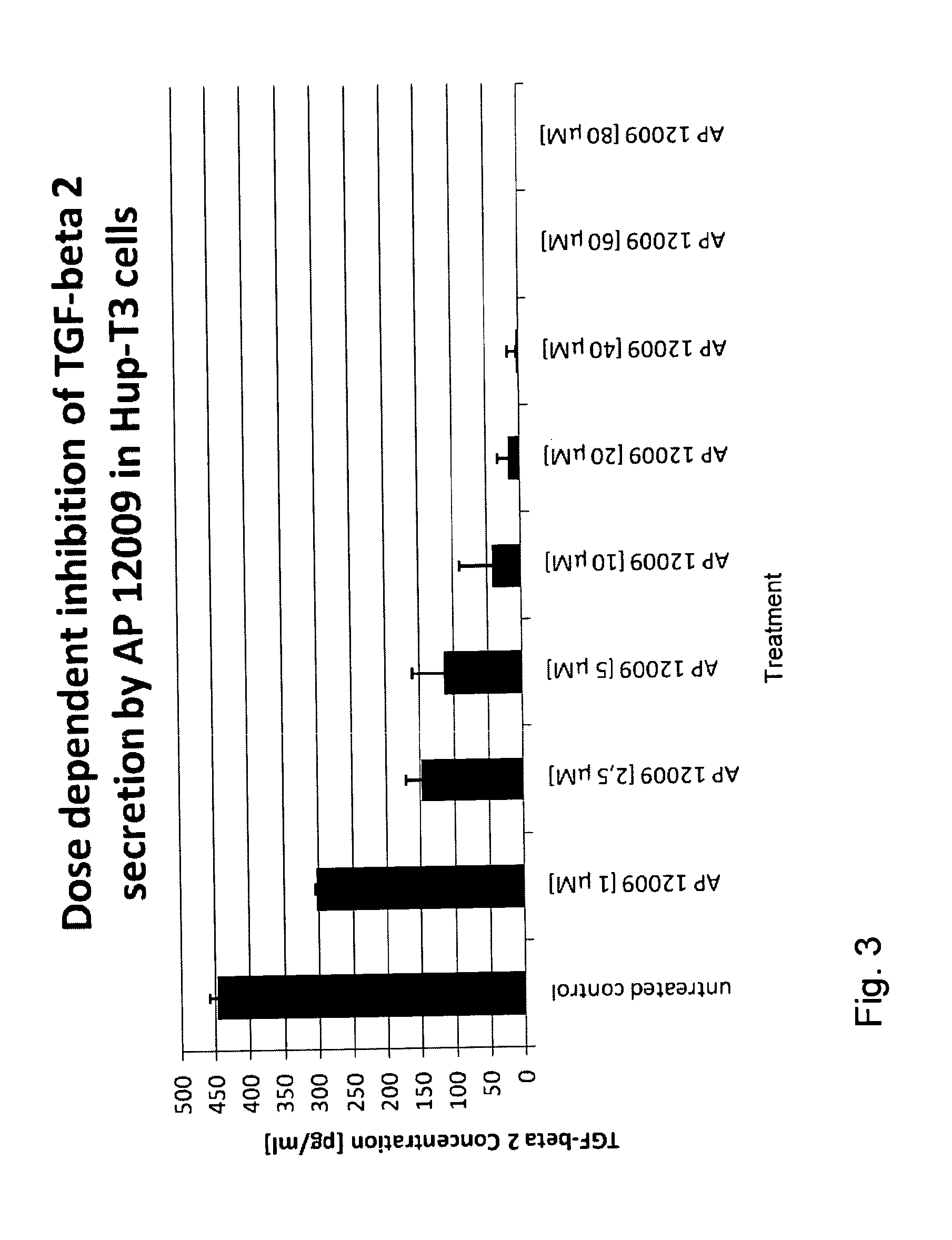
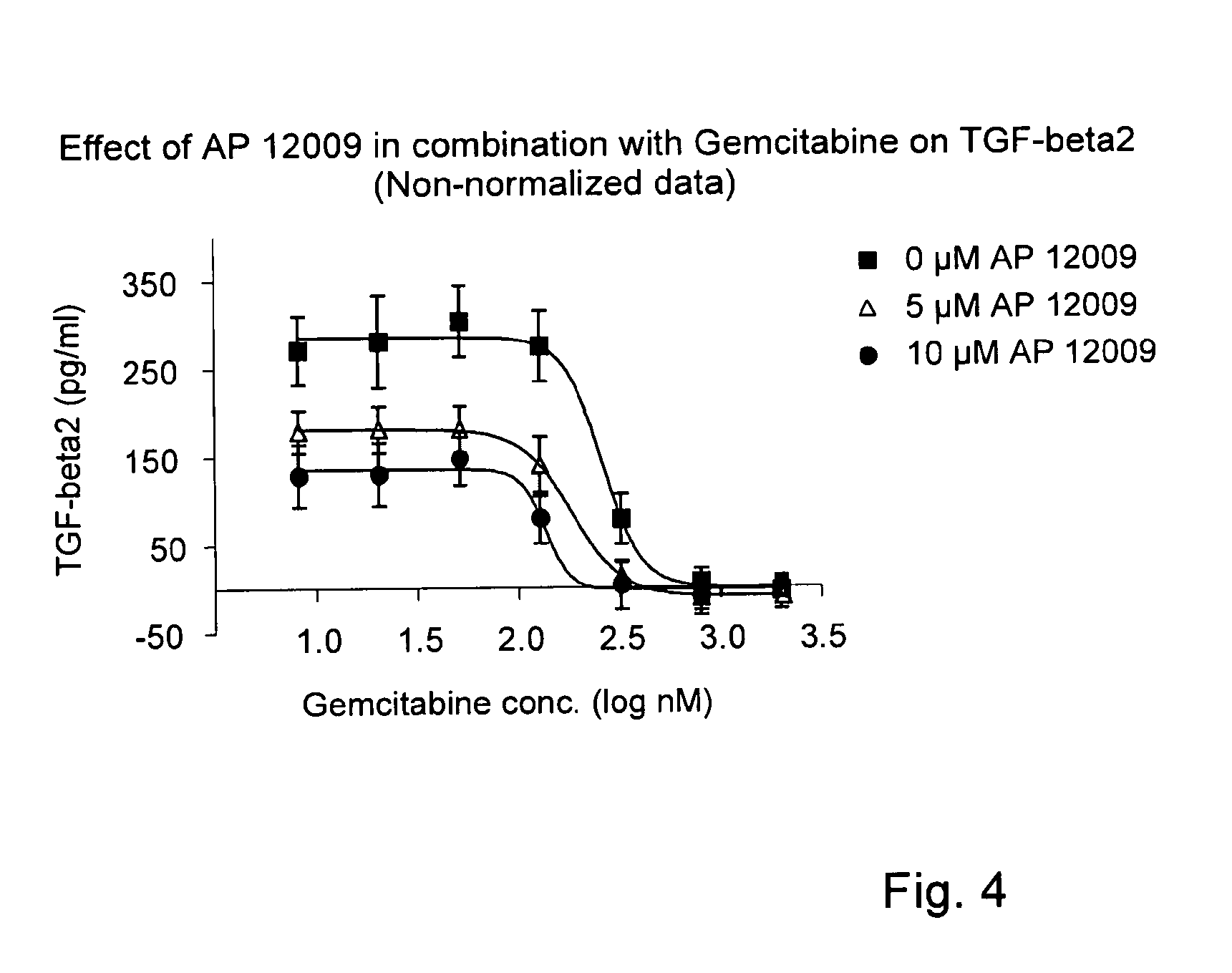
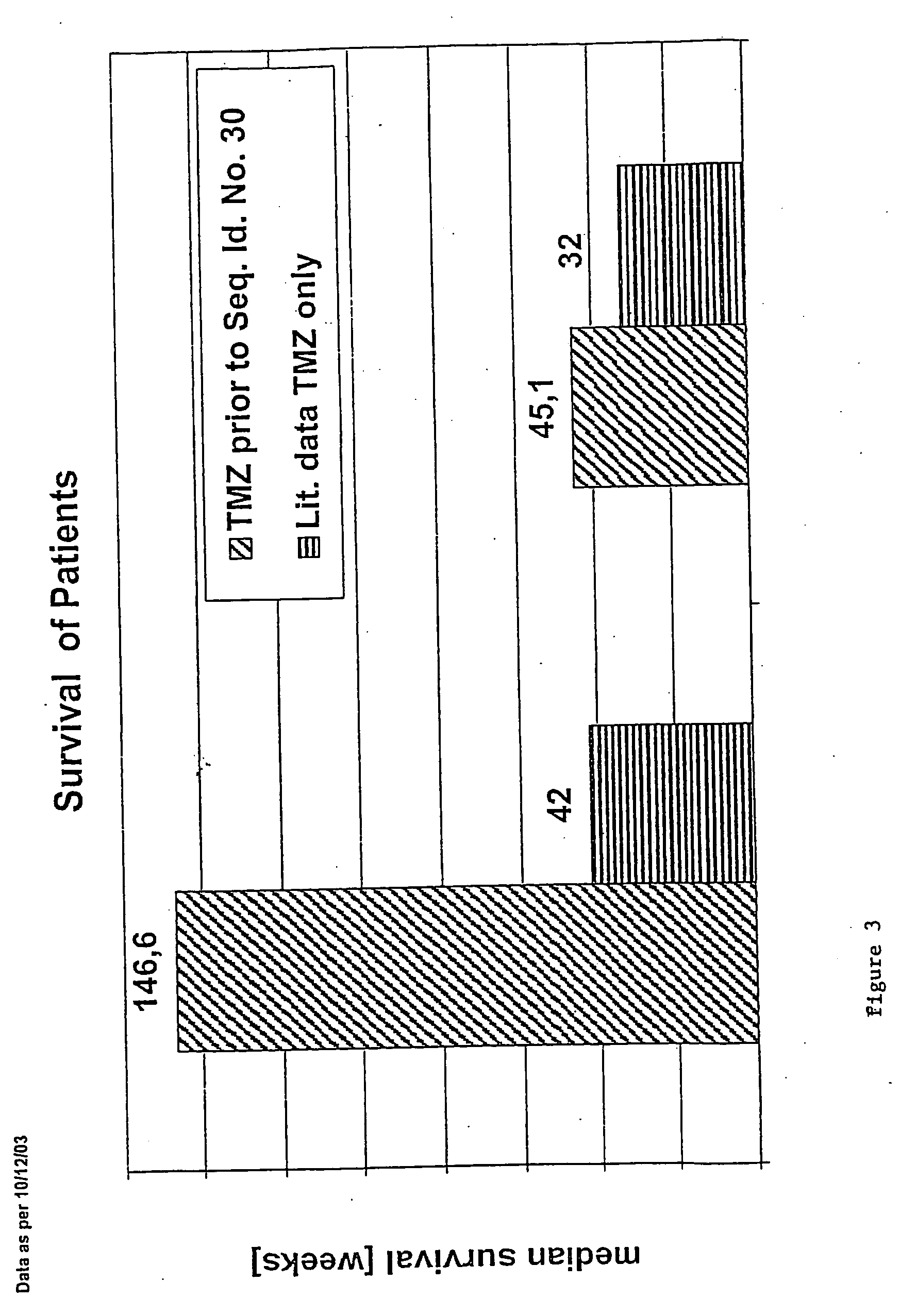
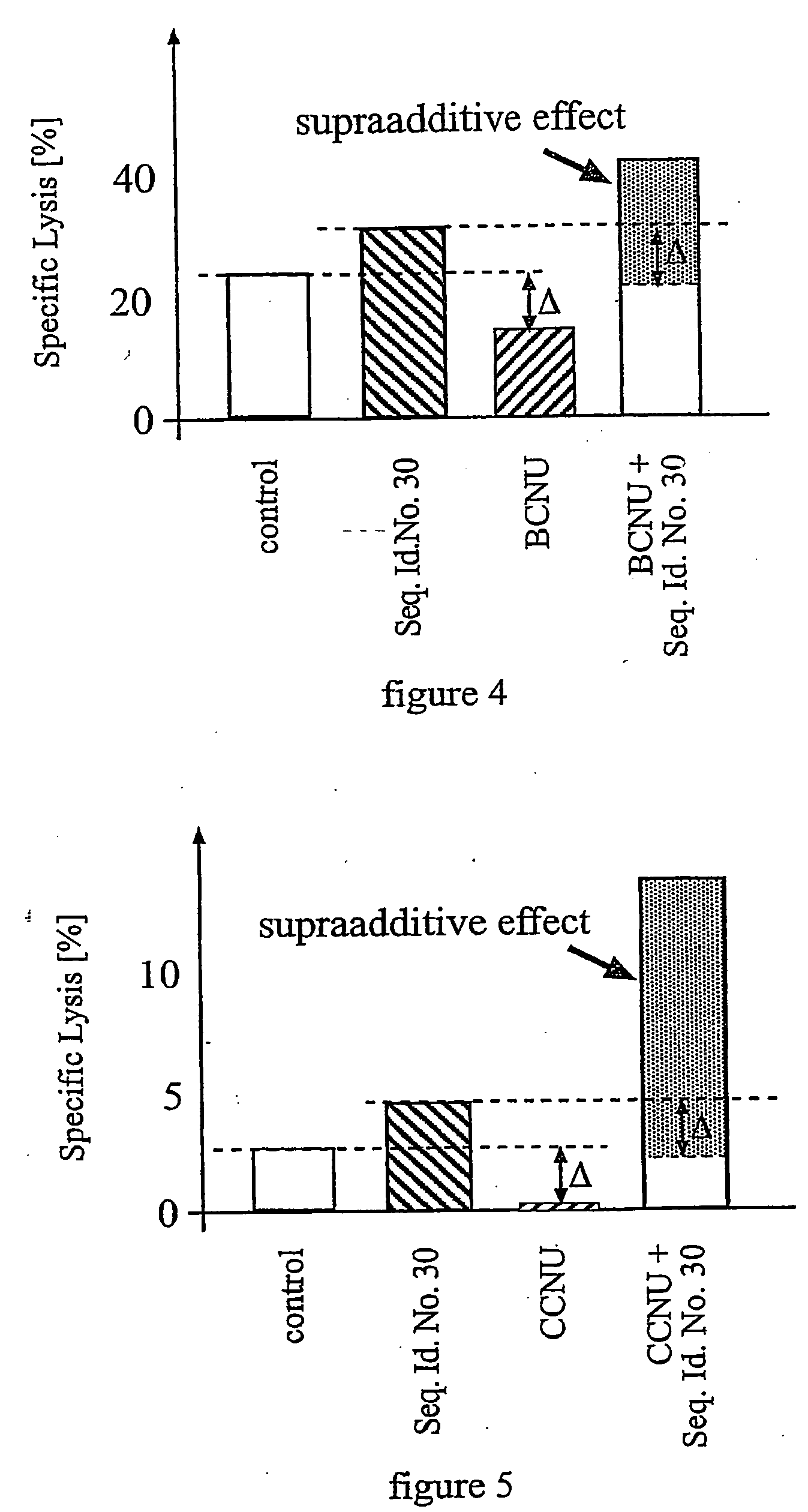
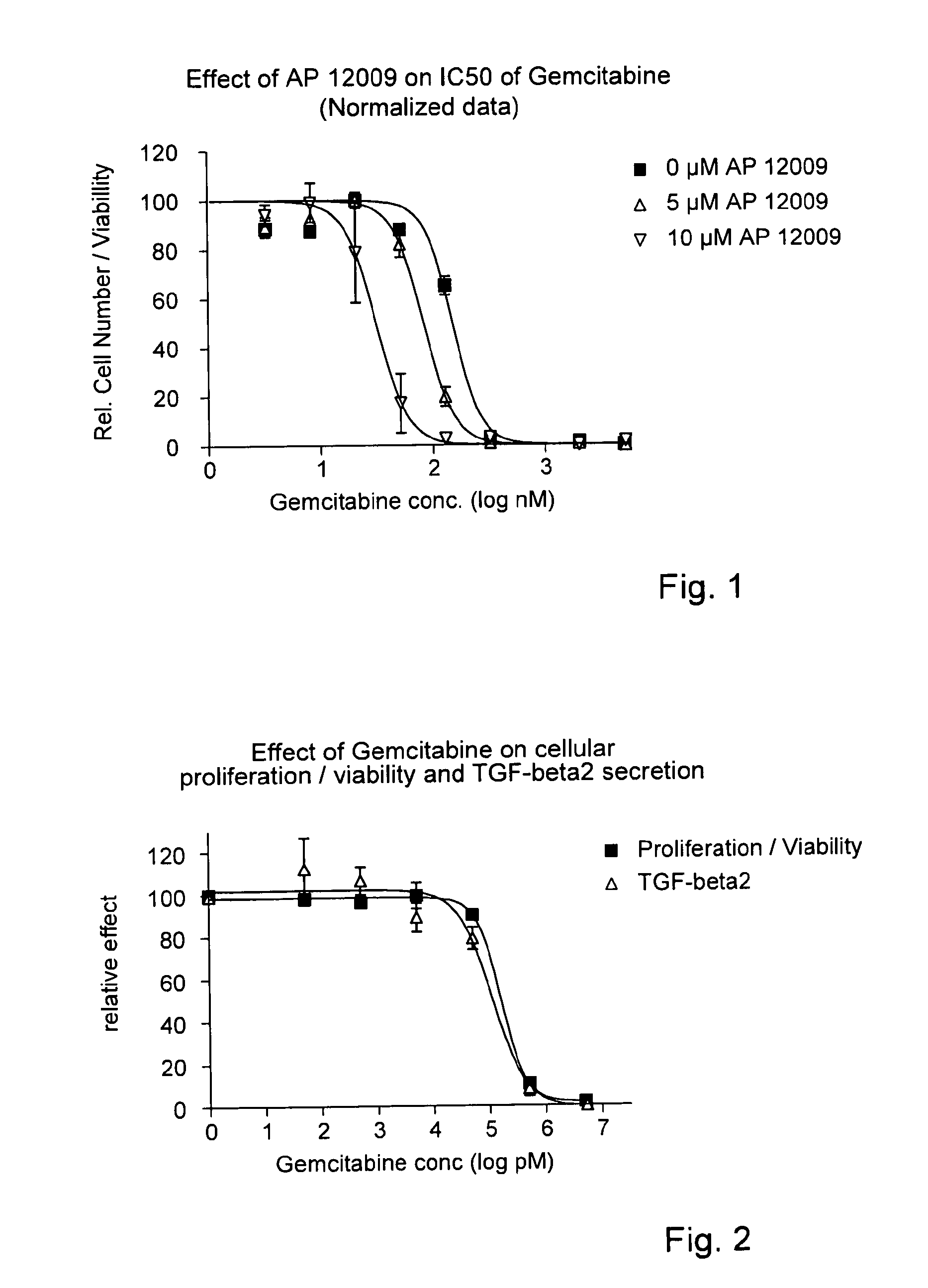
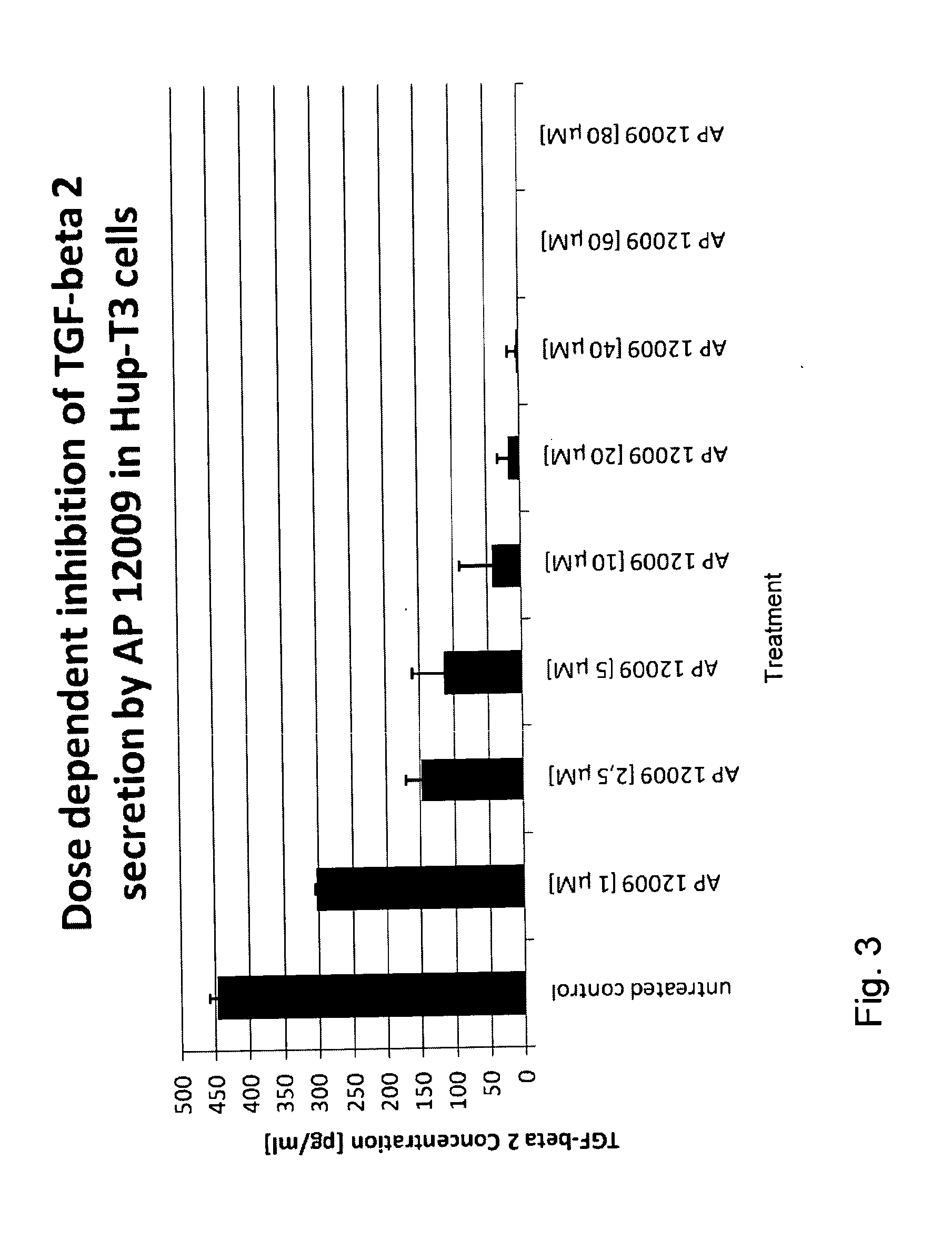
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
Methods and Means for the Treatment of Cancer
InactiveUS20090170880A1Increase volumeReduce transportationBiocideAnimal repellantsOncologyCombination therapy
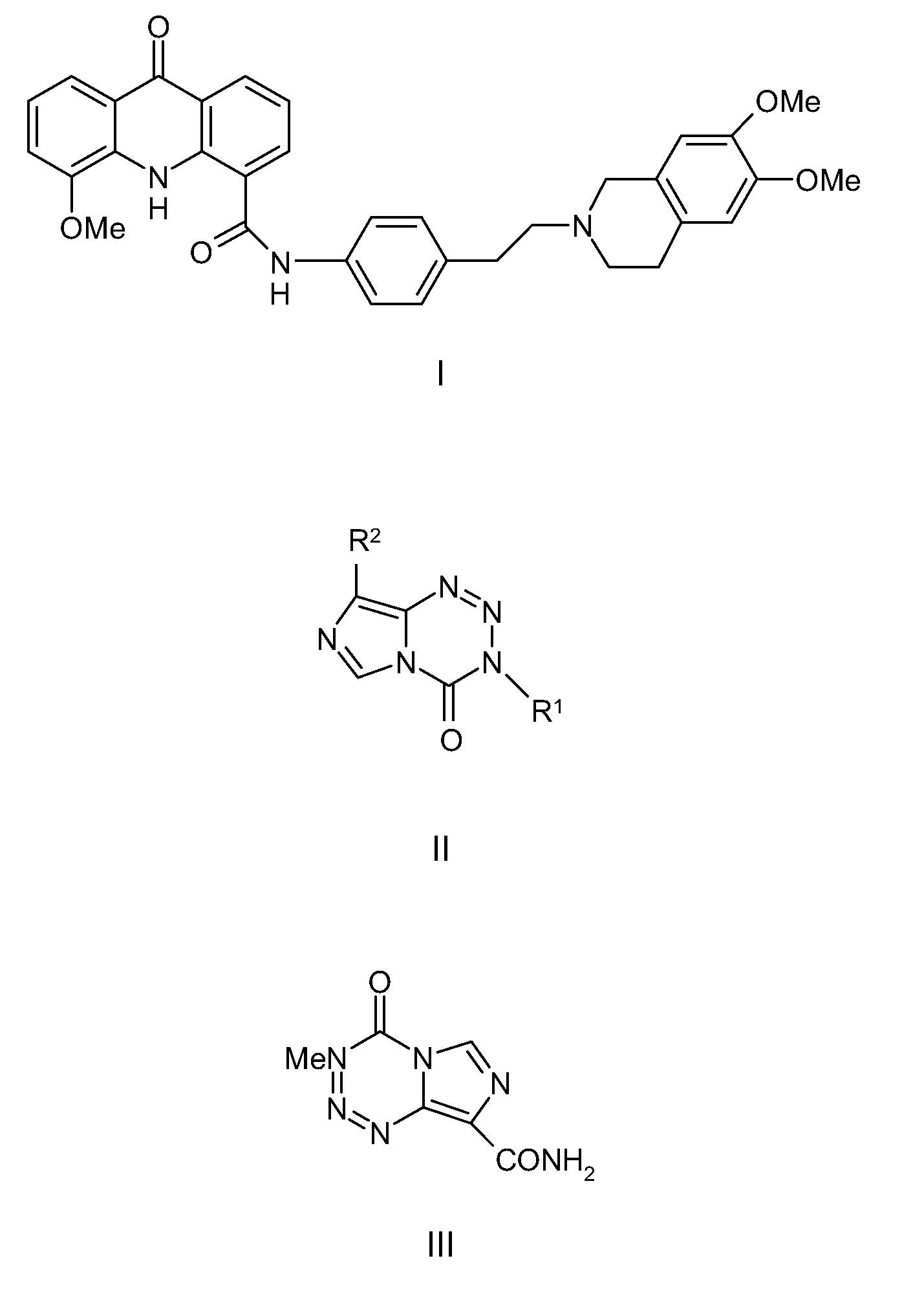
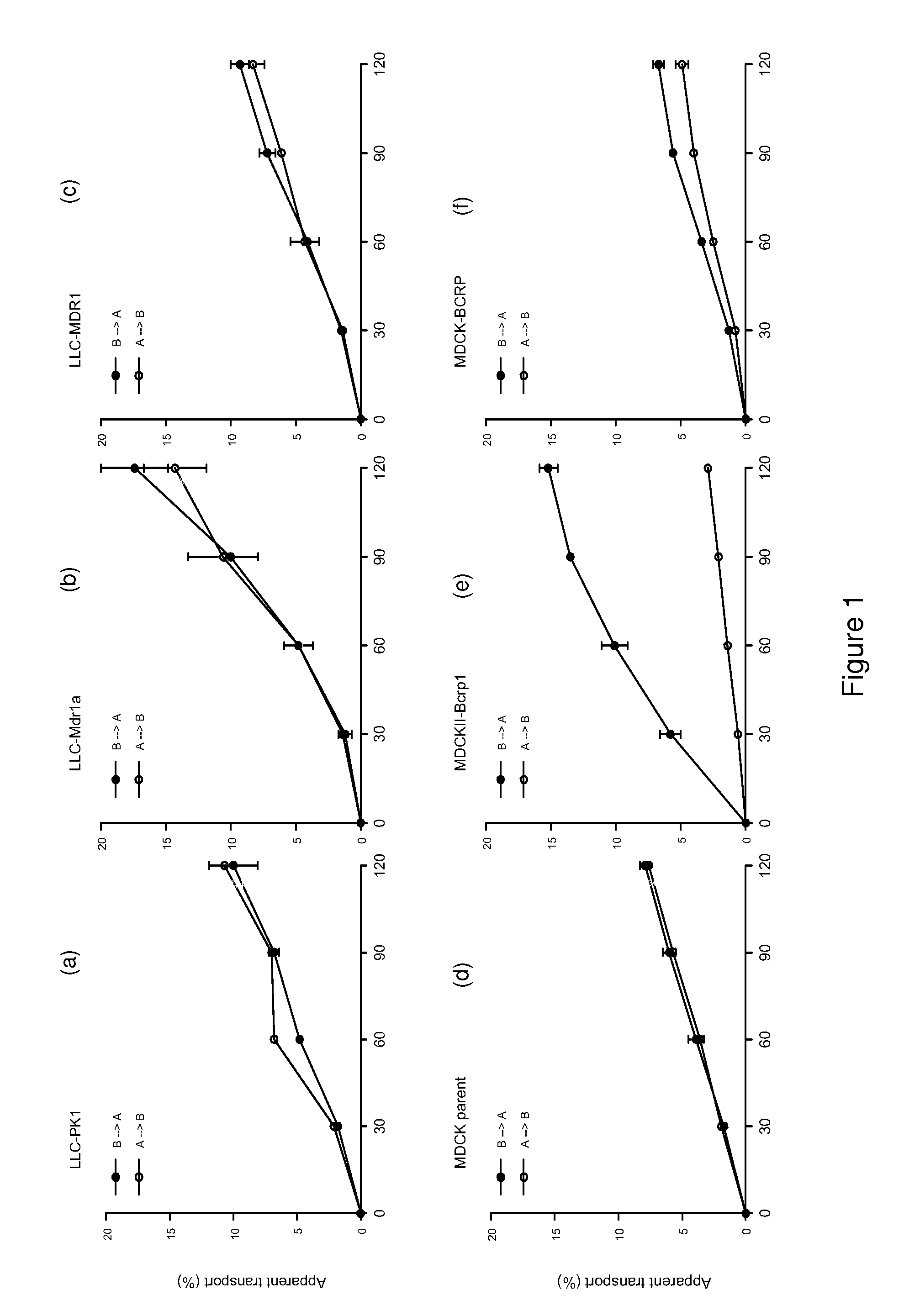
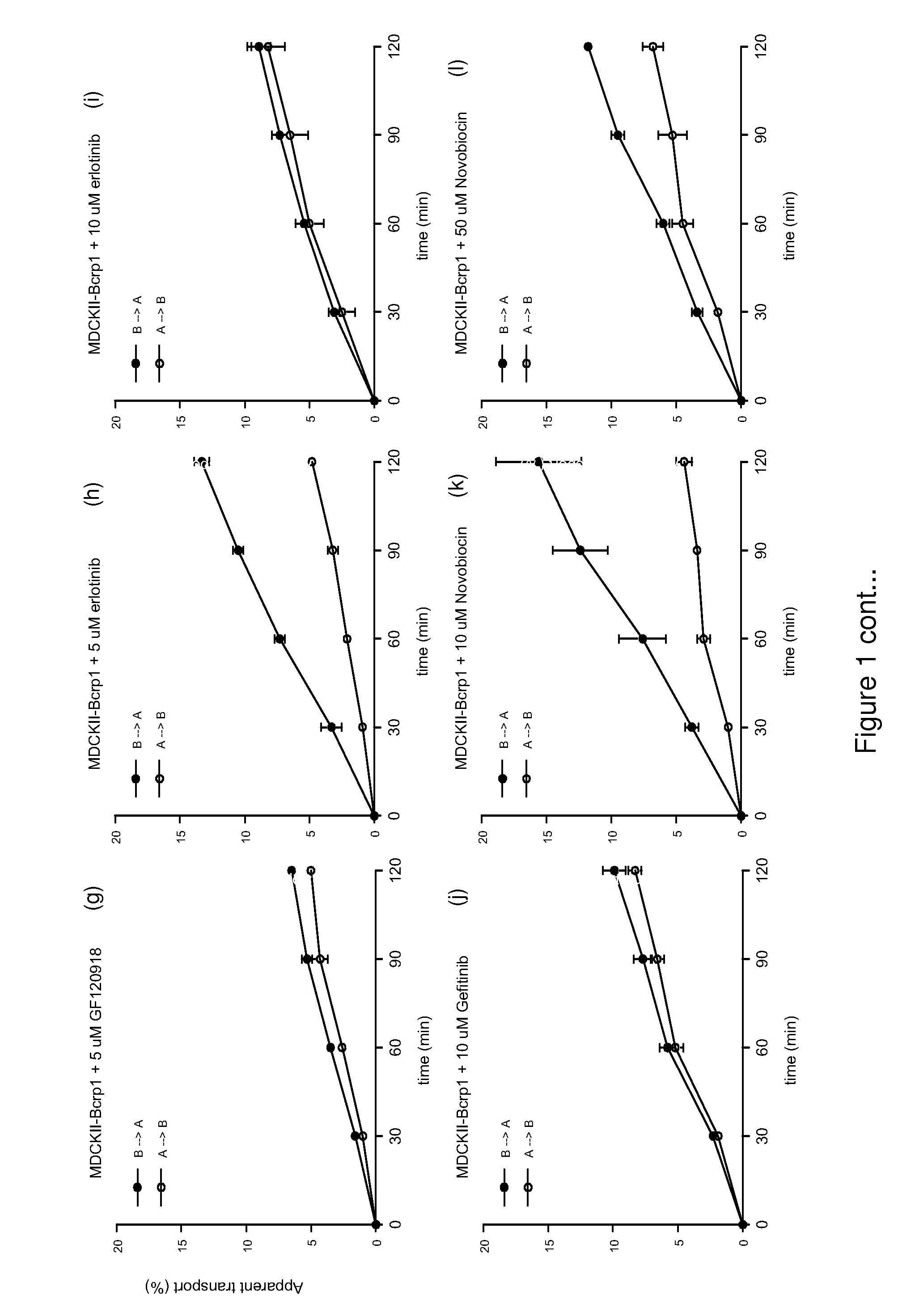
Provided are Breast Cancer Resistance Protein (BCRP) inhibitors, P-glycoprotein (P-gp) inhibitors and chemotherapeutic agents for use in the treatment of cancer by combination therapy of the BCRP inhibitor and / or P-gp inhibitor with the chemotherapeutic agent. The chemotherapeutic agent is an imidazotetrazine, e.g. temozolomide.
Owner:VAN TELLINGEN OLAF
Process for preparing temozolomide
Owner:DR REDDYS LAB LTD +1
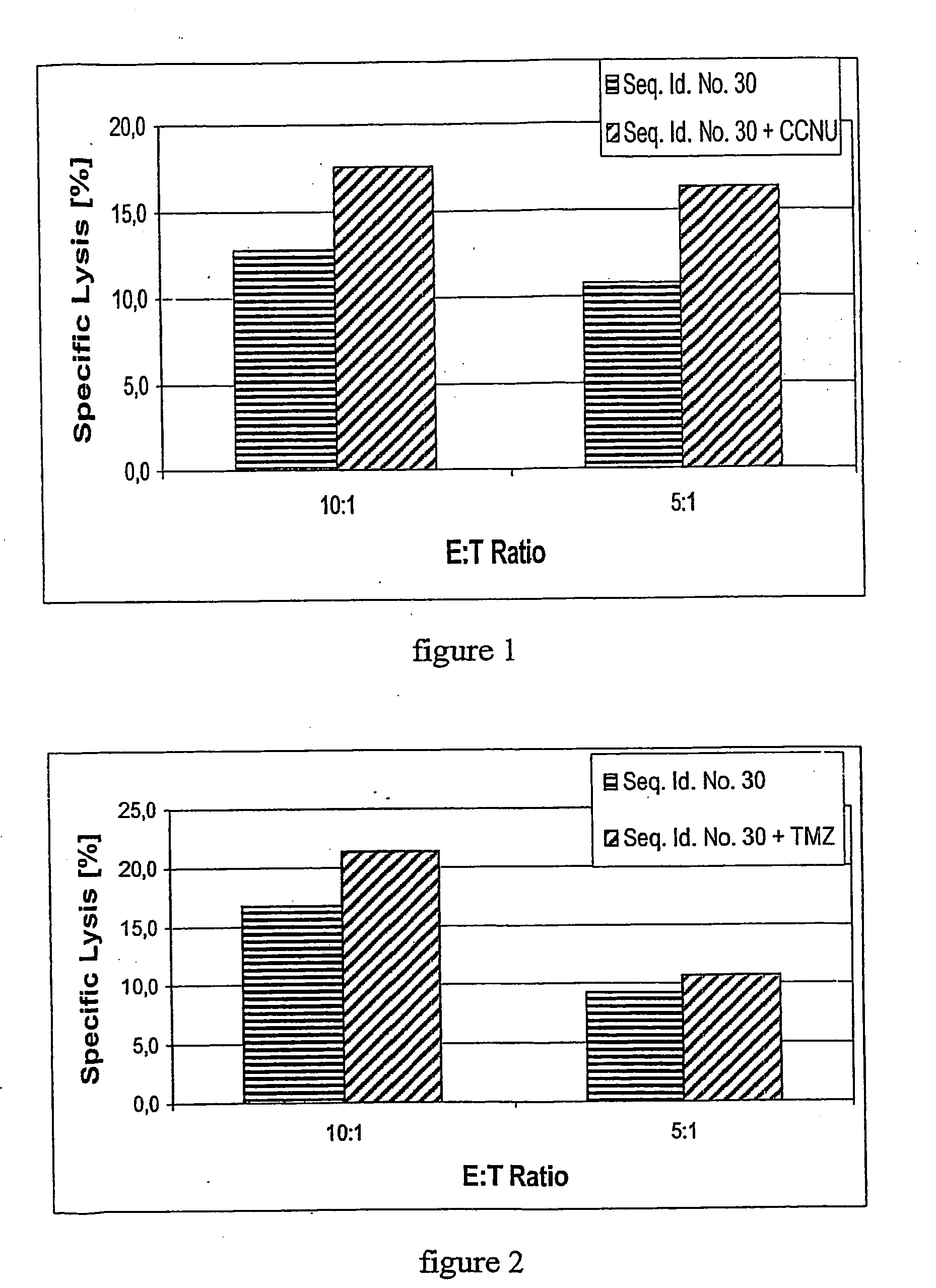
Concurrent chemotherapy and immunotherapy
InactiveUS20090220551A1Peptide/protein ingredientsAntibody mimetics/scaffoldsGlioblastomaConcurrent chemotherapy
The concurrent administration of chemotherapy and immunotherapy has been considered a contraindication because of the concern that the induced lymphopenia would ablate therapeutic efficacy of immunotherapy. Temozolomide has been shown to be an effective chemotherapeutic for patients with malignant gliomas and to deprive patients with glioblastoma (GBM) patients of this agent in order to treat with immunotherapy is controversial. Despite conventional dogma, we demonstrate that both chemotherapy and immunotherapy can be delivered concurrently without negating the effects of immunotherapy, in fact, the temozolomide induced lymphopenia may actually be synergistic with a peptide vaccine.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Formulations of Temozolomide for Parenteral Administration
The present invention relates to a parenteral formulation of Temozolomide comprising an excipient selected from the group consisting of bulking agents, buffers, pH adjusting agents, with the proviso that the formulation is free of dissolution enhancing agents.
Owner:SAHAJ LIFE SCI PVT
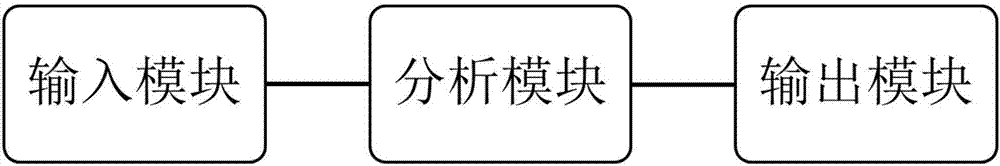
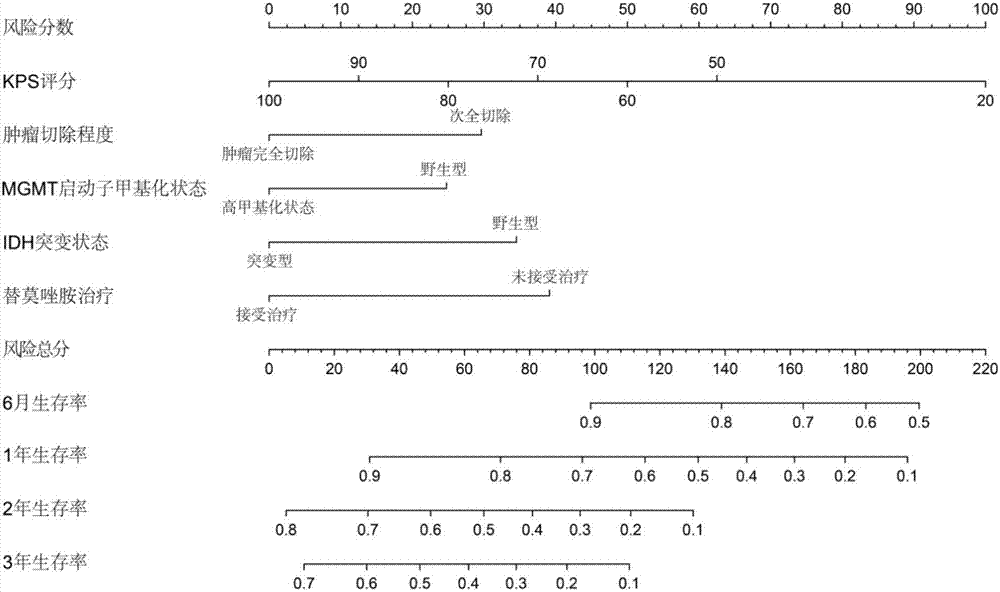
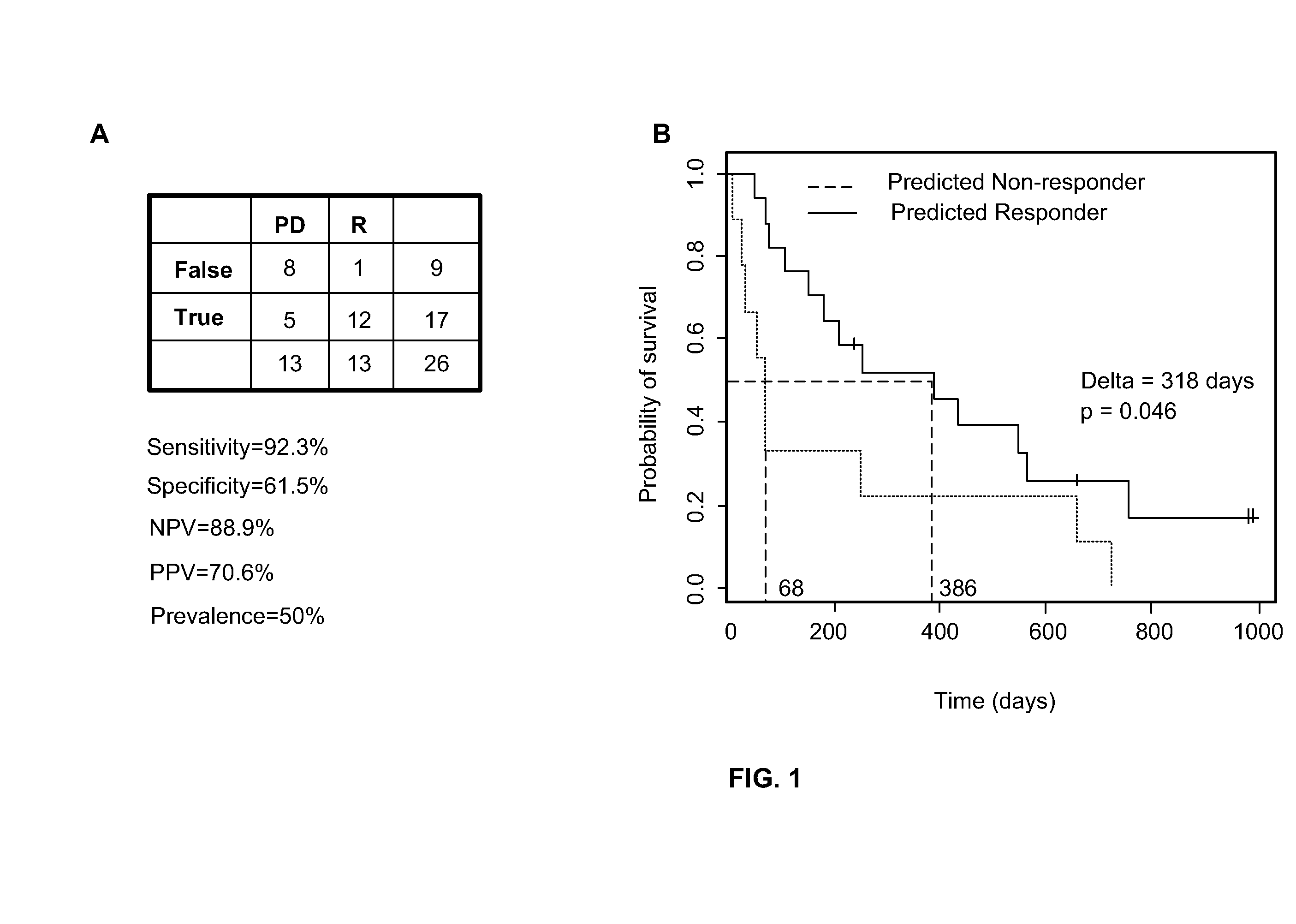
Lifetime predicting system for patient with glioblastoma multiforme
ActiveCN106874647APrediction is accurateShorten the timeMedical simulationHealth-index calculationGlioblastomaTotal risk
The invention discloses a lifetime predicting system for a patient with glioblastoma multiforme. The predicting system comprises an input module, an analyzing module and an output module; the analyzing module can establish a survival probability alignment chart based on variables input by a variable input module and calculate the total risk score, wherein the total risk score is a cumulative sum of Karnofsky performance score, tumour excision degree, MGMT gene promoter methylating state, IDH gene state and risk score of a temozolomide treatment policy; and the input module calculates a lifetime predicted value of the patient with glioblastoma multiforme according to the total risk score; the output module is used for outputting the lifetime predicted value of the patient with glioblastoma multiforme. The lifetime predicting system for the patient with glioblastoma multiforme, disclosed by the invention, integrates variables which are easily acquired clinically, and the five variables are input to quickly and accurately acquire a predicted result of the lifetime of the patient with glioblastoma multiforme, so that the time and vigor of a user are saved.
Owner:吴安华
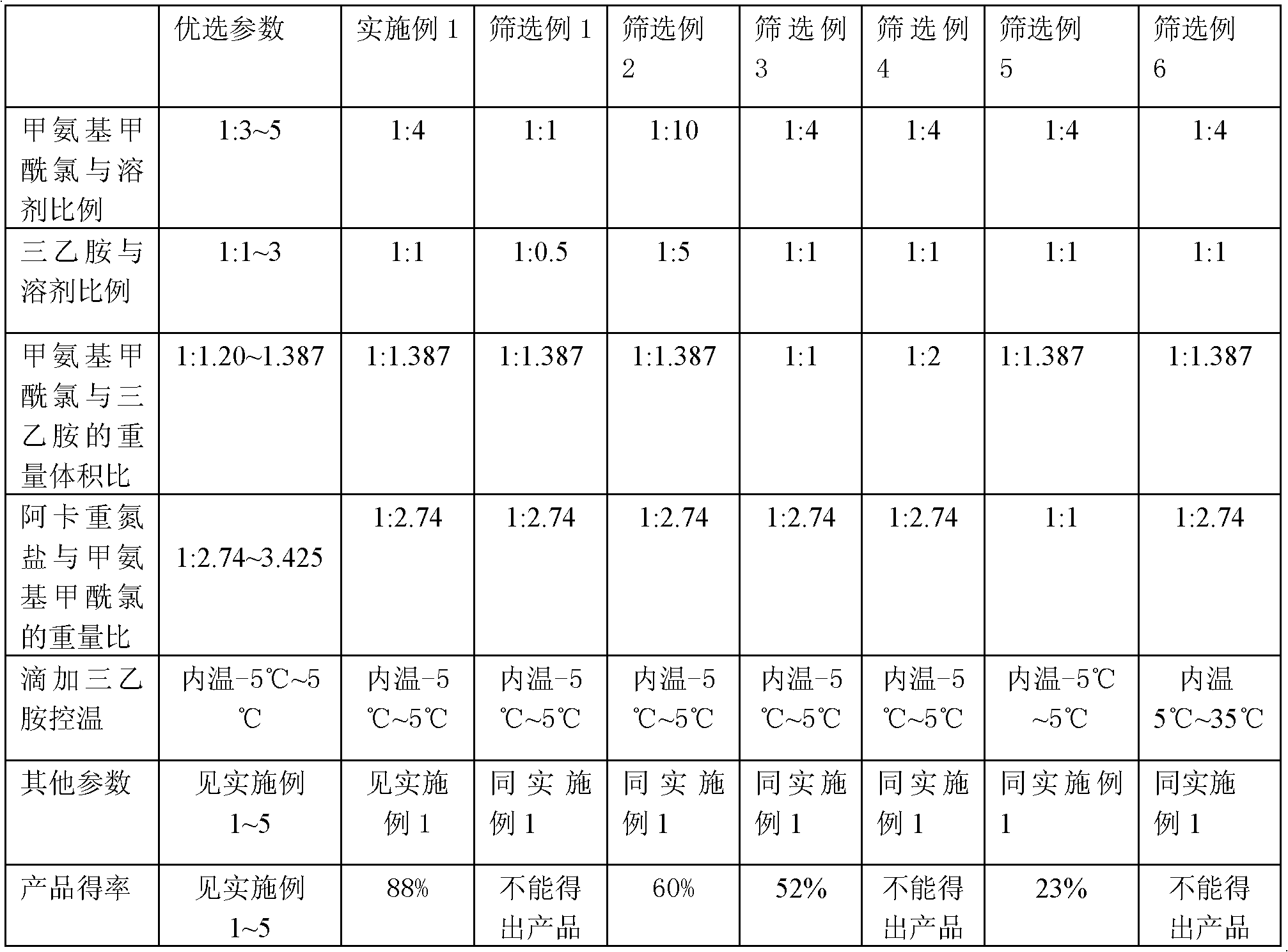
Method preparing temozolomide in one-pot mode and refining method of temozolomide
The invention discloses a method preparing temozolomide in a one-pot mode and further discloses a refining method of temozolomide. The refining method of temozolomide omits a rectification separation step of methyl isocyanate, and reduces the contacting times of temozolomide with the outside environment, thereby improving safety in the production process. By adjusting using quantities of a reaction substrate and a solvent, on the condition that the reaction steps are omitted, high yield of temozolomide can still be maintained. By screening various reaction conditions, the method achieves synthesis of temozolomide by one-pot boiling, and not only is production cost reduced, but also the industrialized mass production method is provided for production of temozolomide. Simultaneously, the improved refining method of temozolomide is adopted, recrystallization operation of temozolomide is effectively simplified, the recrystallization yield is remarkably improved, operation time for recrystallization is effectively shortened, and accordingly the whole production cycle is remarkably shortened.
Owner:凯默斯医药科技上海有限公司
Pharmaceutical composition
InactiveUS20100160208A1Solution to short lifeReduce dosageAntibacterial agentsSenses disorderMessenger RNAPurine
Owner:ANTISENSE PHARMA GMBH
Combination of a chemotherapeutic agent and an inhibitor of the tgf-beta system
ActiveUS20120027873A1Reduce IC50Improve efficiencyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
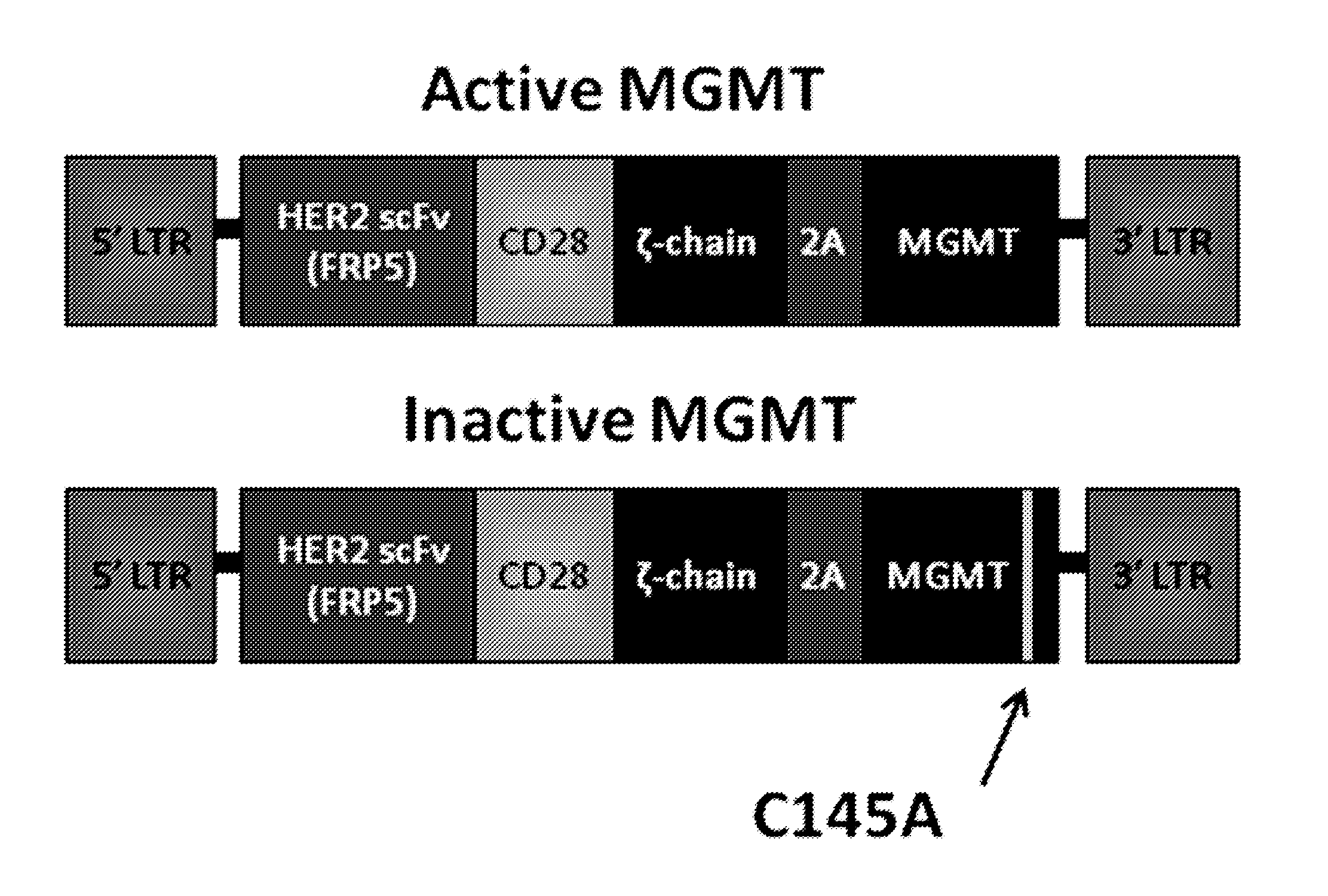
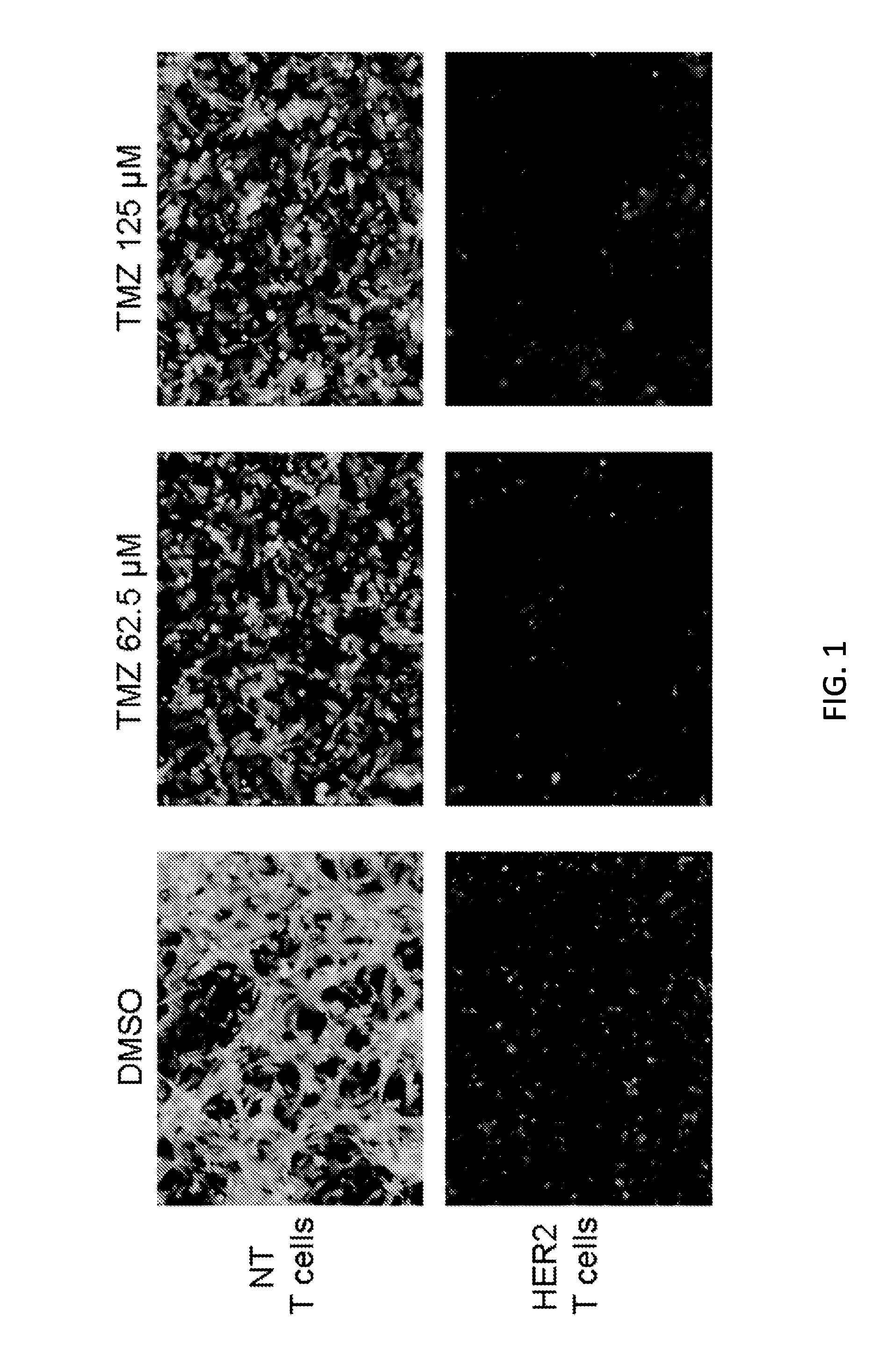
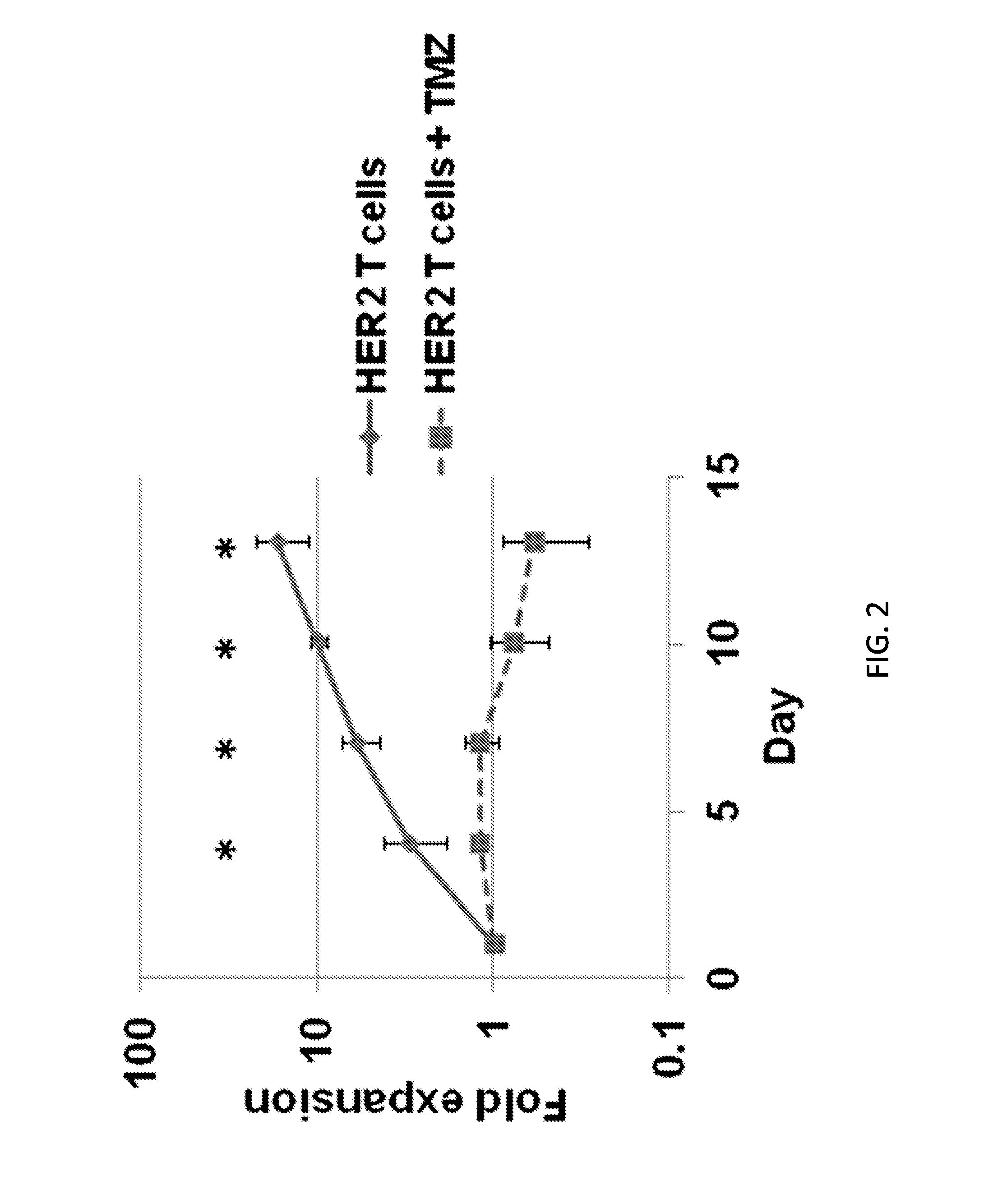
Chemotherapy-resistant immune cells
InactiveUS20160024175A1Effective immunotherapeutic responseOrganic active ingredientsVirusesTumor antigenT cell
Embodiments of the disclosure include compositions and methods useful for treating cancers that are sensitive to a chemotherapy, such as temozolomide. The methods allow effective cell immunotherapy to be used with chemotherapy when the cell immunotherapy is susceptible to being rendered ineffective by the chemotherapy. In specific aspects, the cancer is being treated by temozolomide (TMZ) and tumor antigen-specific T-cells that are resistant to TMZ.
Owner:BAYLOR COLLEGE OF MEDICINE
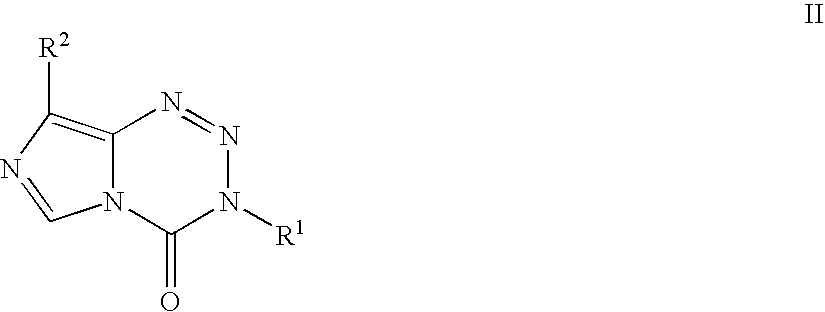
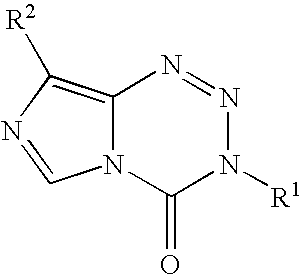
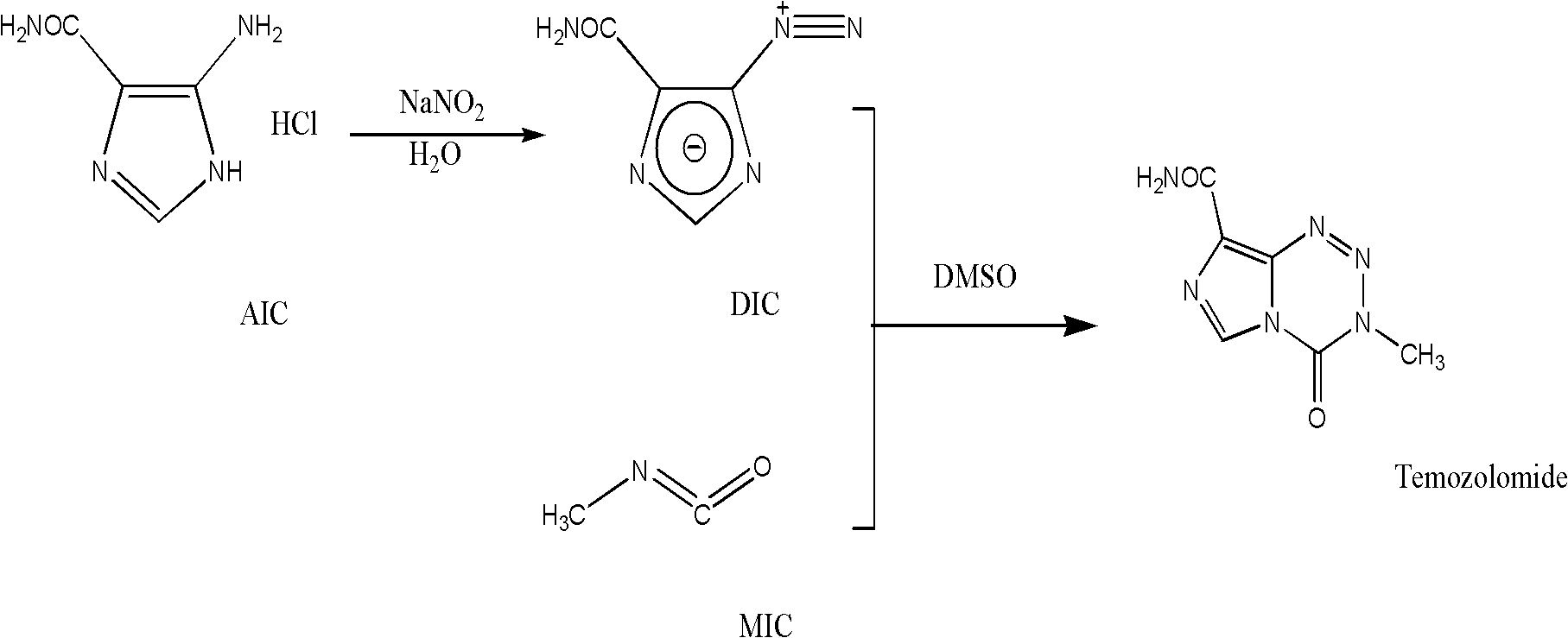
Synthesis of temozolomide and analogs
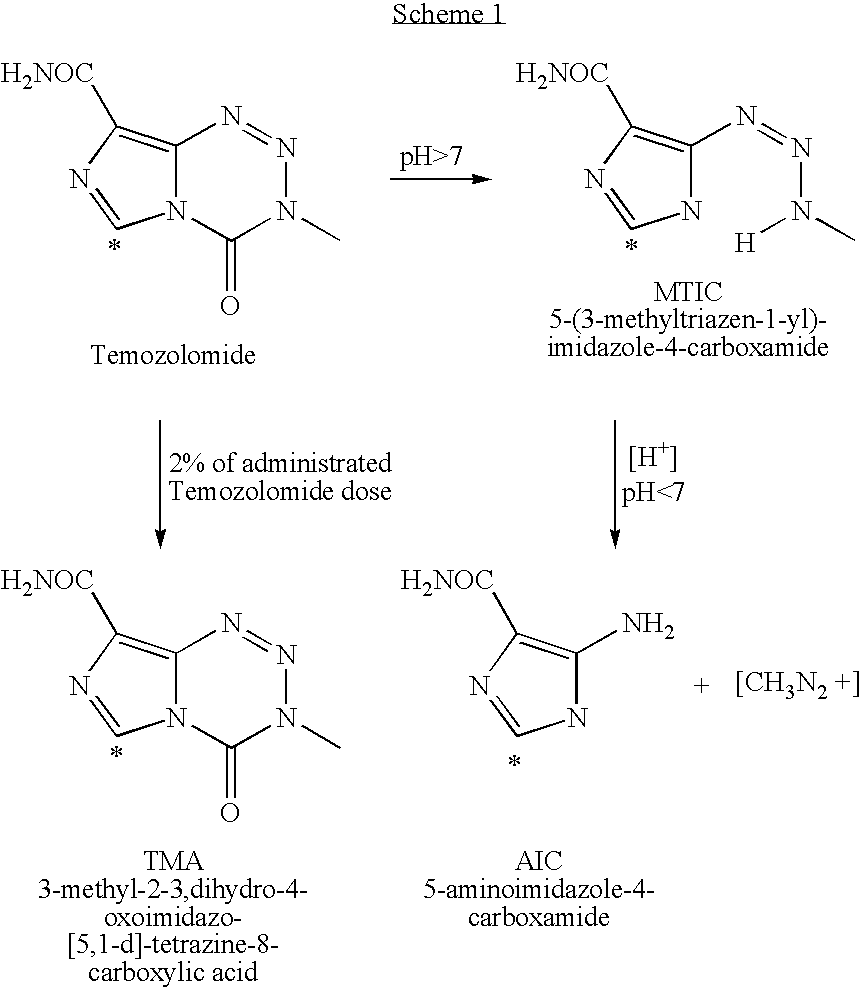
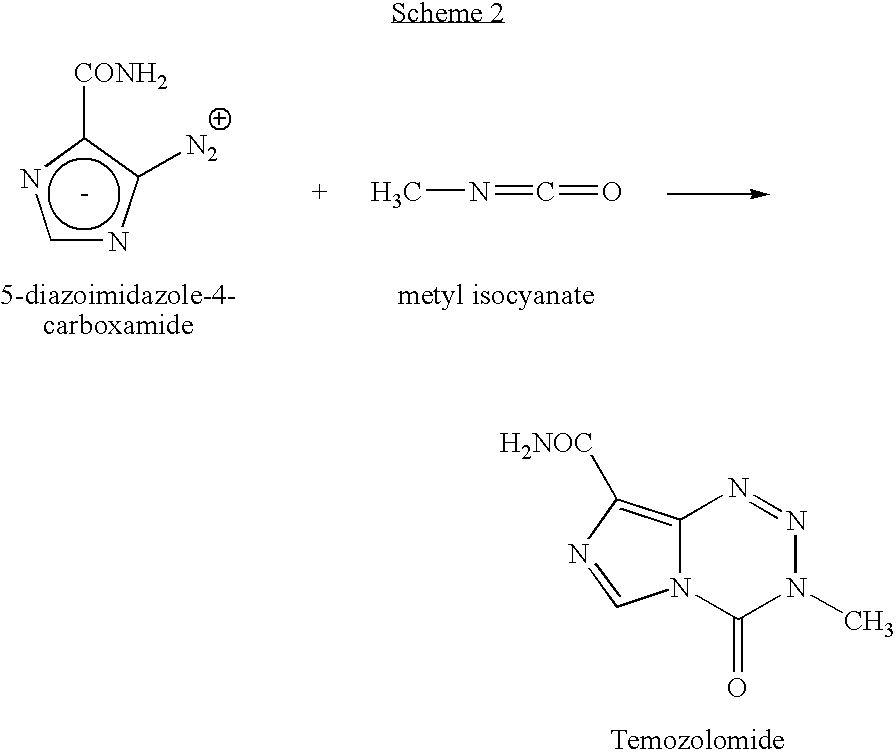
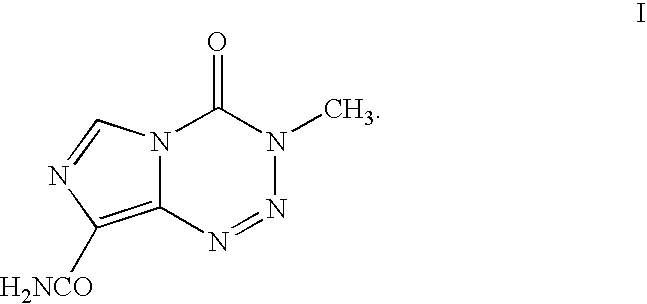
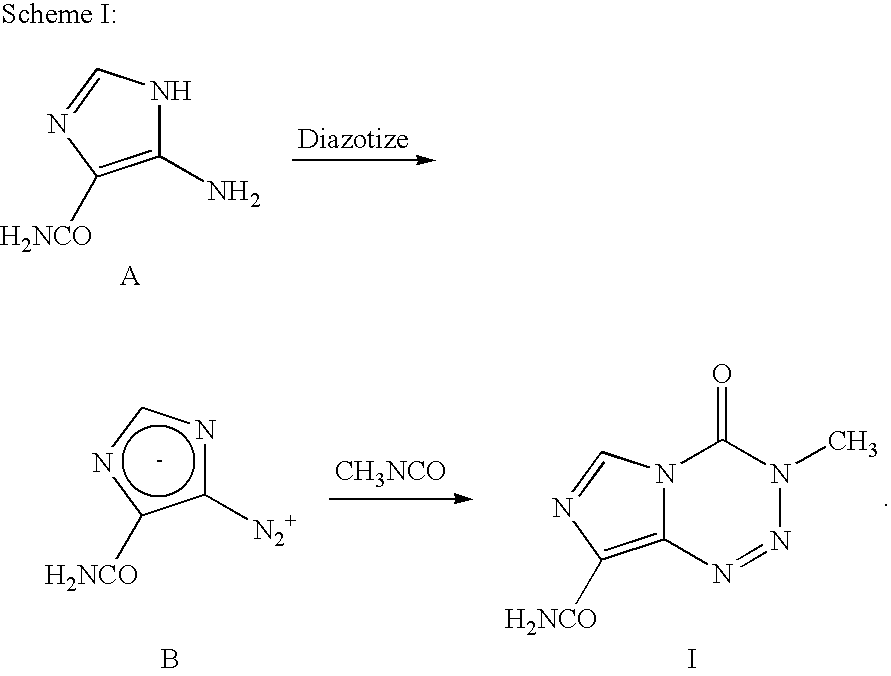
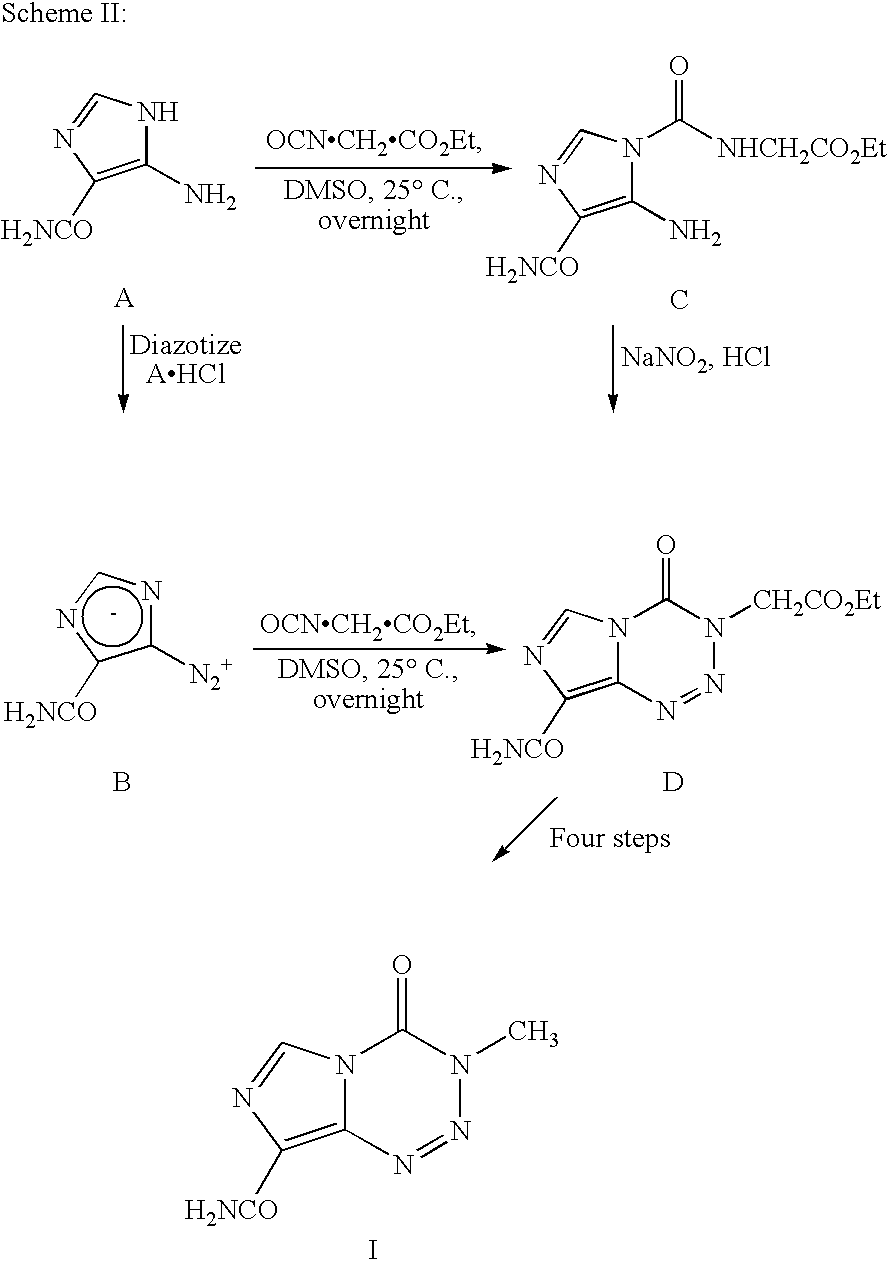
InactiveUS20050131227A1Promote correct directionOrganic active ingredientsOrganic chemistry methodsStereochemistryTemozolomide
This invention relates to a novel process for the synthesis of Temozolomide, an antitumor compound, and analogs, and to intermediates useful in this novel process.
Owner:SCHERING CORP
Method of determining acute myeloid leukemia response to treatment with farnesyltransferase inhibitors
ActiveUS20130130999A1Faster assayQuick forecastBiocideMicrobiological testing/measurementMyeloid leukemiaAptX
The disclosed method rapidly identifies with desired accuracy AML patients, including elderly AML patients, likely to respond to treatment with a combination of a farnesyltransferase inhibitor and one or more of etoposide, teniposide, tamoxifen, sorafenib, paclitaxel, temozolomide, topotecan, trastuzumab and cisplatinum. In an embodiment, the improvements include the use of whole blood rather than the customary bone marrow sample, thus making the assay more accurate, rapid, less intrusive, less expensive as well as less painful. The method includes evaluation of a two-gene expression ratio (RASGRP1:APTX), which with a corresponding threshold, provides sufficient accuracy for predicting the response to the combination treatment. In the preferred embodiment the combination treatment combines tipifarnib (R115777, ZARNESTRA®) with etoposide. Further, the elderly AML patients identified as being likely responsive to the combination treatment with tipinifarb and etoposide have a complete recovery rate comparable to the best therapy available for younger patients.
Owner:JANSSEN PHARMA NV
Process for preparing temozolomide
InactiveUS7612202B2High yieldStraightforward and cheap and easy to perform processOrganic active ingredientsOrganic chemistryOrganic acidOrganic solvent
The present invention provides a process for preparing highly pure Temozolomide base which includes recovery from the purification mother liquors by using an anionic exchange resin. By treating Temozolomide hydrochloride with a mixture of an organic acid, a water miscible organic solvent, and water, Temozolomide free base is obtained in an acidic medium. Due to the high sensitivity of Temozolomide to basic pH values the recovery-including process is especially advantageous because it enables obtaining high yields of highly pure Temozolomide base in acidic conditions.The process for producing Temozolomide base includes hydrolysis of the starting material 8-cyano-3-methyl-[3H]-imidazo[5,1-d]-tetrazin-4-one in acidic medium to obtain highly pure Temozolomide hydrochloride in high yield.
Owner:WAVELENGTH ENTERPRISES LTD
Pharmaceutical composition
InactiveUS20070196269A1Solution to short lifeReduce dosageAntibacterial agentsOrganic active ingredientsMessenger RNAPurine
The invention concerns a pharmaceutical composition comprising at least one stimulator of the immune cell functions and at least one substance inhibiting the cell proliferation and / or inducing cell death. In a preferred embodiment the stimulator of the function of the immune system and / or the immune cells are antagonists of TGF-beta selected from the group of oligonucleotides hybridizing with an area of the messenger RNA and or DNA encoding TGF-beta and the at least one substance inhibiting cell proliferation and / or inducing cell death is selected from the group of temozolomide, nitrosoureas, Vinca alkaloids, antagonists of the purine and pyrimidines bases, cytoststatic active antibiotics, caphthotecine derivatives, anti estrogens, anti-androgens and analogs of gonadotropin releasing hormon.
Owner:ANTISENSE PHARMA GMBH
Temozolomide lyophilized powder preparation and preparation method thereof
ActiveCN104721155AReduce adverse reactionsLow toxicityOrganic active ingredientsPowder deliveryDissolutionBULK ACTIVE INGREDIENT
The invention belongs to the technical field of pharmaceutical preparations and specifically relates to a temozolomide lyophilized powder preparation. The temozolomide lyophilized powder preparation comprises an active ingredient of temozolomide or a pharmaceutically acceptable salt thereof, and a solution before lyophilization further contains an excipient, a wetting agent, a buffer agent, an osmotic pressure regulating agent, a pH regulating agent, water for injection and an organic solvent, wherein the organic solvent is selected from one or any combination of ethanol, acetone, isopropanol, n-propanol, butanone, sec-butyl alcohol and methanol, and is preferably ethanol. The temozolomide lyophilized powder preparation provided by the invention has the advantages of stable quality, high re-dissolution speed and a small residual amount of the organic solvent. The invention further provides a method for preparing the preparation. The process provided by the invention is simple and convenient in preparation process and easy to control production links, the organic solvent accounts for a relatively small part of total volume of material liquid, the pollution to production equipment and environment caused by the organic solvent is reduced, and thus the method is suitable for large-scale production.
Owner:QILU PHARMA HAINAN
A temodar synthesis method
The invention discloses a process for synthesizing Temozolomide, which realizes the parameter optimization for the existing technique, and overcomes the deficits of the conventional processes.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Combination therapy (temozolomide and alpha-IFN) for advanced cancer
InactiveUS7294332B2High response rateEliminate side effectsBiocidePeptide/protein ingredientsInterferon alphaCombination therapy
There is disclosed a method for treating advanced cancer in patients in need of such treating. Temozolomide and alpha interferon are administered in combination in amounts sufficient to achieve a clinical response.
Owner:SCHERING CORP
Temozolomide intravenous injection fat emulsion and preparation method thereof
InactiveCN103405385ASolve the problem of low solubilityGood treatment effectOrganic active ingredientsEmulsion deliveryFat emulsionMedicine
The invention discloses a temozolomide intravenous injection fat emulsion which comprises the following components in percentage by weight: 0.01-5% of temozolomide, 5-30% of oil for injection, 0.2-5% of emulgator, 0.2-3% of stabilizer, 1-5% of isoosmotic adjusting agent and the balance of water for injection. The prepared temozolomide intravenous injection fat emulsion is uniform in appearance, narrower in distribution, high in drug loading capacity (1 mg / ml), stable in quality, used for intravenous injection, simple and practical in production process, high in controllability, and liable to large-scale industrial production; the average grain diameter is about 200 nm, the Zeta electric potential is about -40 mV, and the pH is 5.0-9.0; the temozolomide intravenous injection fat emulsion has excellent biocompatibility, can provide energy for an organism, and can realize therapeutic action to various tumours.
Owner:SHANDONG UNIV
Temozolomide-polymer local slow release chemotherapy medicine for treating malignant brain tumor
The present invention discloses a slow release system of Temozolomide as local slow release medicine and biodegraded polymer for treating malignant brain tumor, and the slow release system is implanted into operated area of excising malignant brain tumor for local slow release treatment of malignant brain tumor and prolonging the survival period of the patient. The present invention consists of Temozolomide and biodegraded polymer material in the weight ratio of 1.5 to 10. Temozolomide is fused into biodegraded polymer, and through the corrosion of the polymer and hydrolysis of the polymer, the medicine is released slowly, continuously and stably to act. The polymer is PLA, PGA, PLGA, PFAD-SA and / or the copolymer of p-carboxyl phenoxyl propane and sebasic acid.
Owner:赵世光 +1
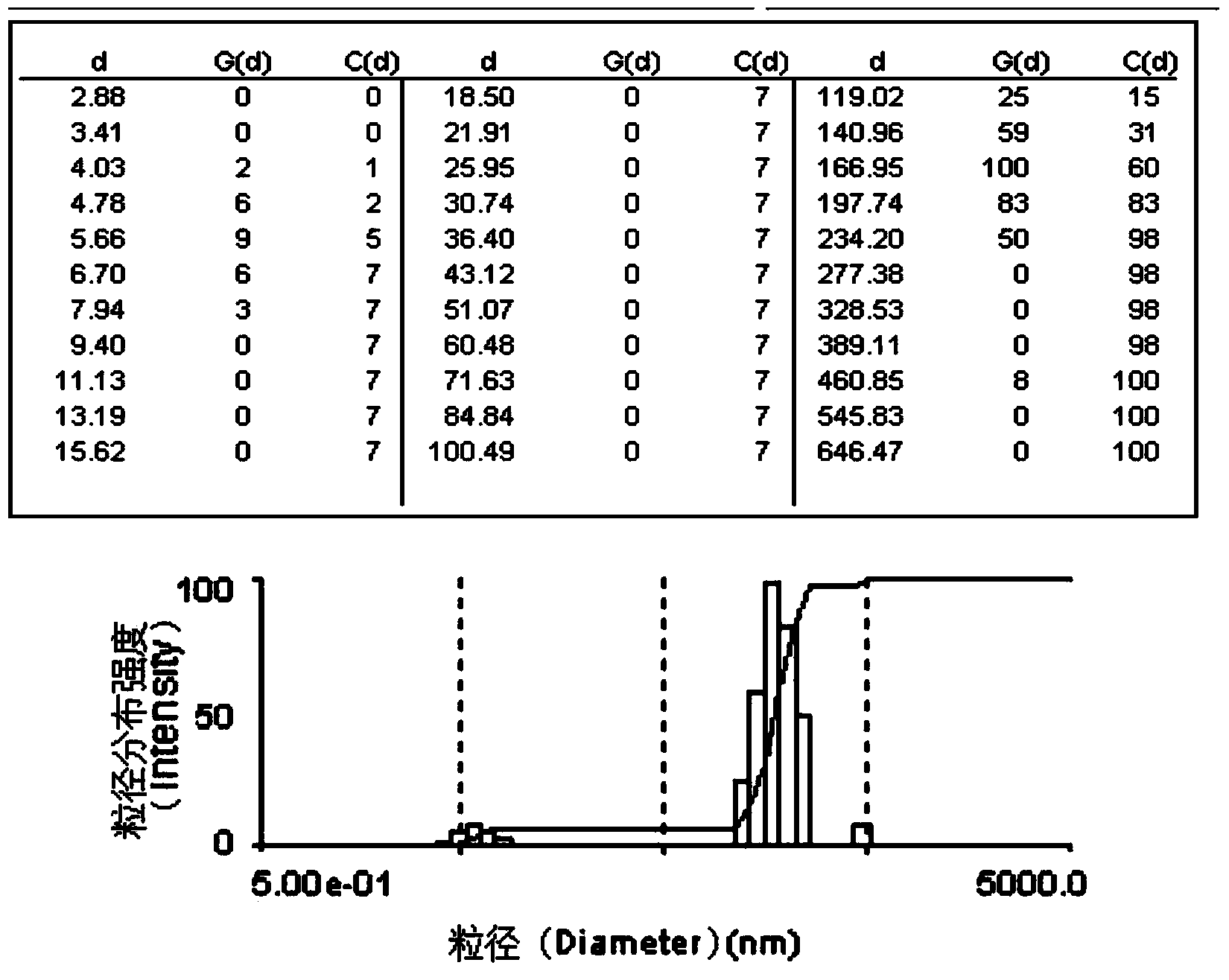
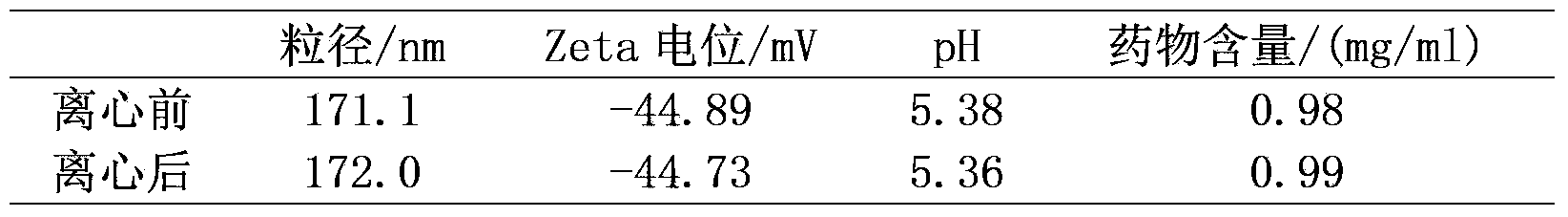
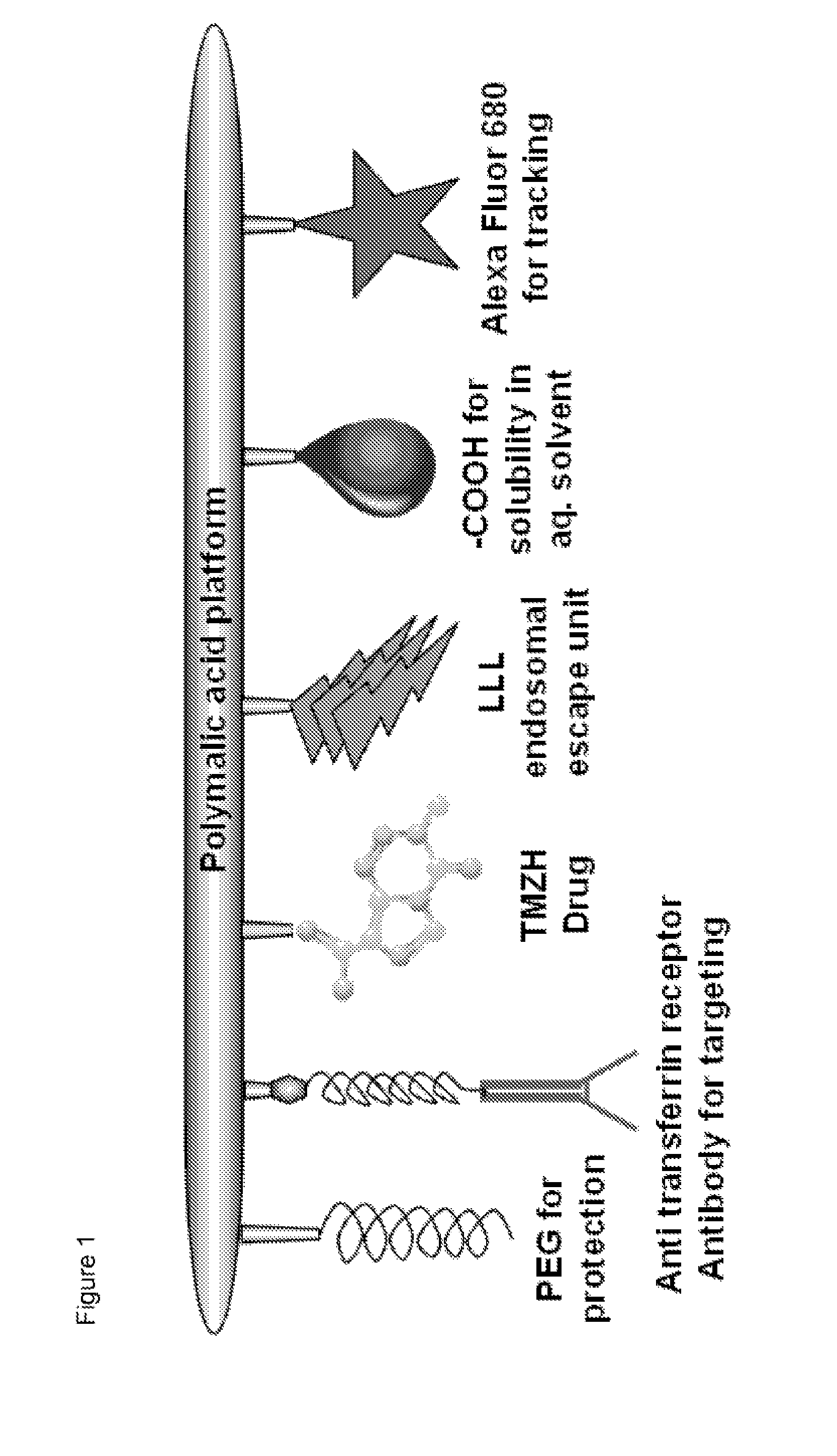
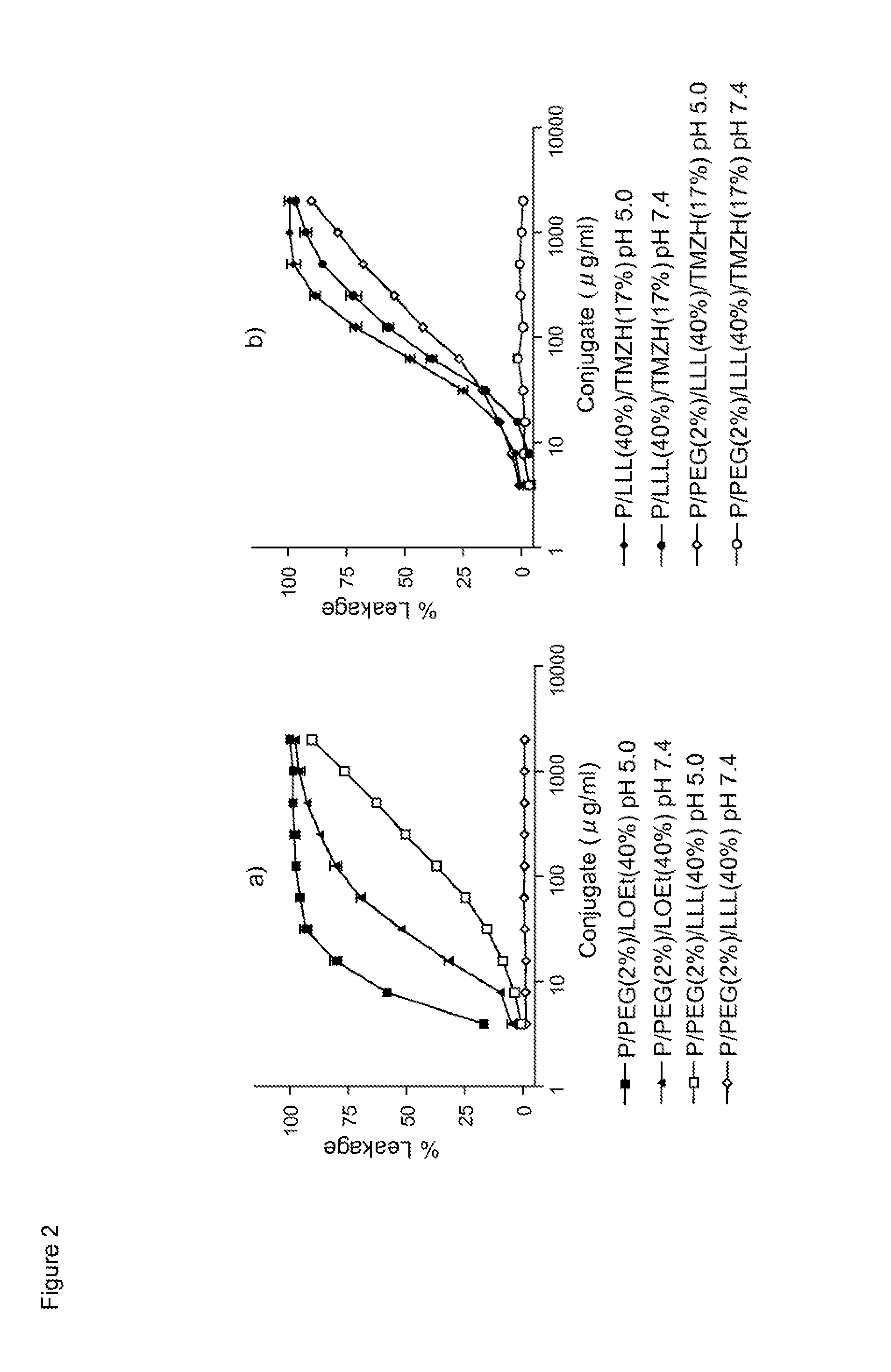
Drug delivery of temozolomide for systemic based treatment of cancer
The present invention relates to methods of drug delivery for the treatment of a condition or disease, such as cancer. In one embodiment, the invention provides a method of preparing a multifunctional nanoconjugate of temozolomide (TMZ) by conjugating TMZ in its hydrazide form to a polymalic acid platform. In another embodiment, the polymalic acid platform is conjugated to a monoclonal antibody to transferrin receptor, a trileucine (LLL) moiety, and / or a polyethylene glycol (PEG) moiety. The present invention relates to methods of drug delivery for the treatment of a condition or disease, such as cancer. In one embodiment, the invention provides a method of preparing a multifunctional nanoconjugate of temozolomide (TMZ) by conjugating TMZ in its hydrazide form to a polymalic acid platform. In another embodiment, the polymalic acid platform is conjugated to a monoclonal antibody to transferrin receptor, a trileucine (LLL) moiety, and / or a polyethylene glycol (PEG) moiety.
Owner:CEDARS SINAI MEDICAL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com