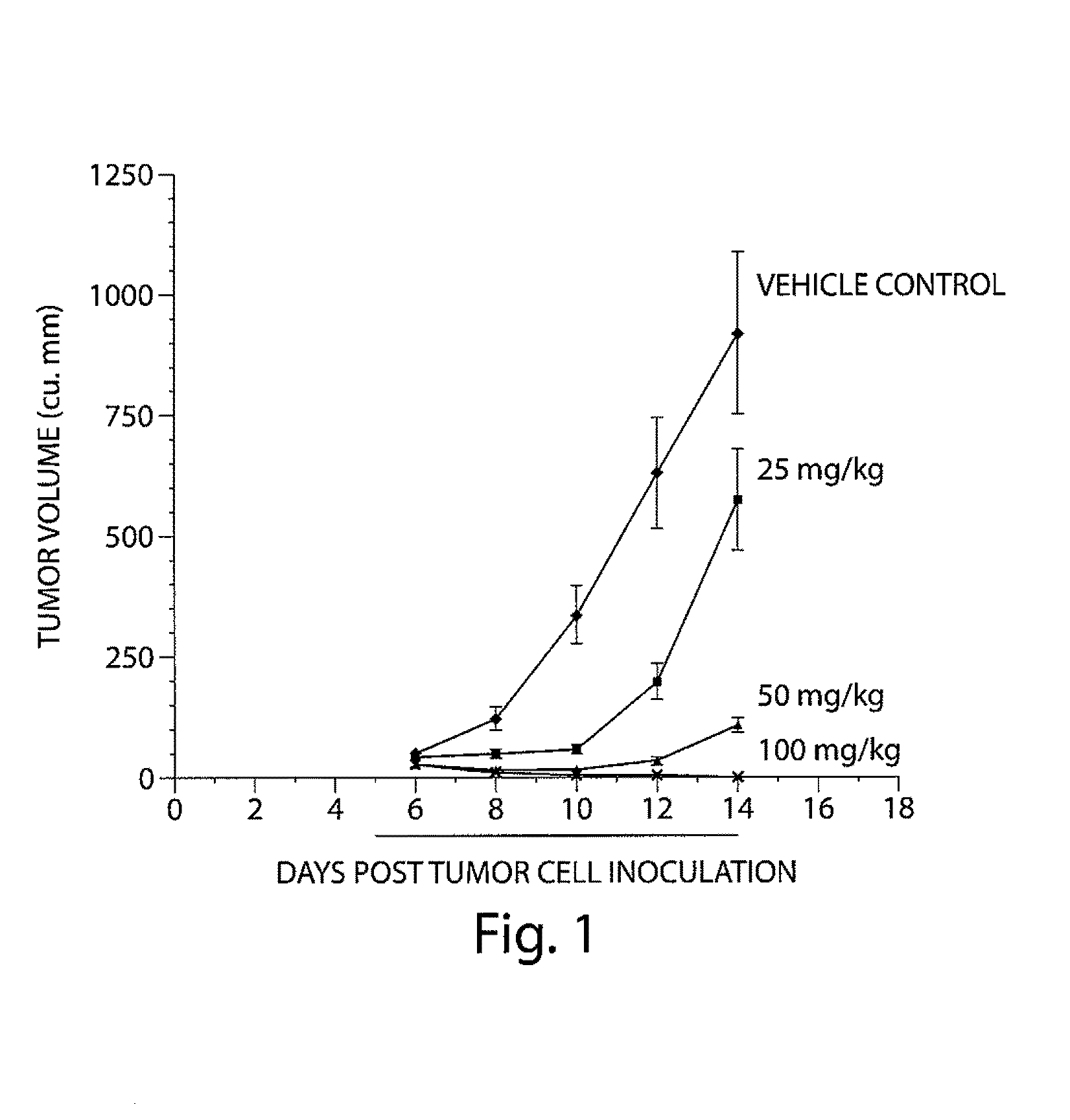
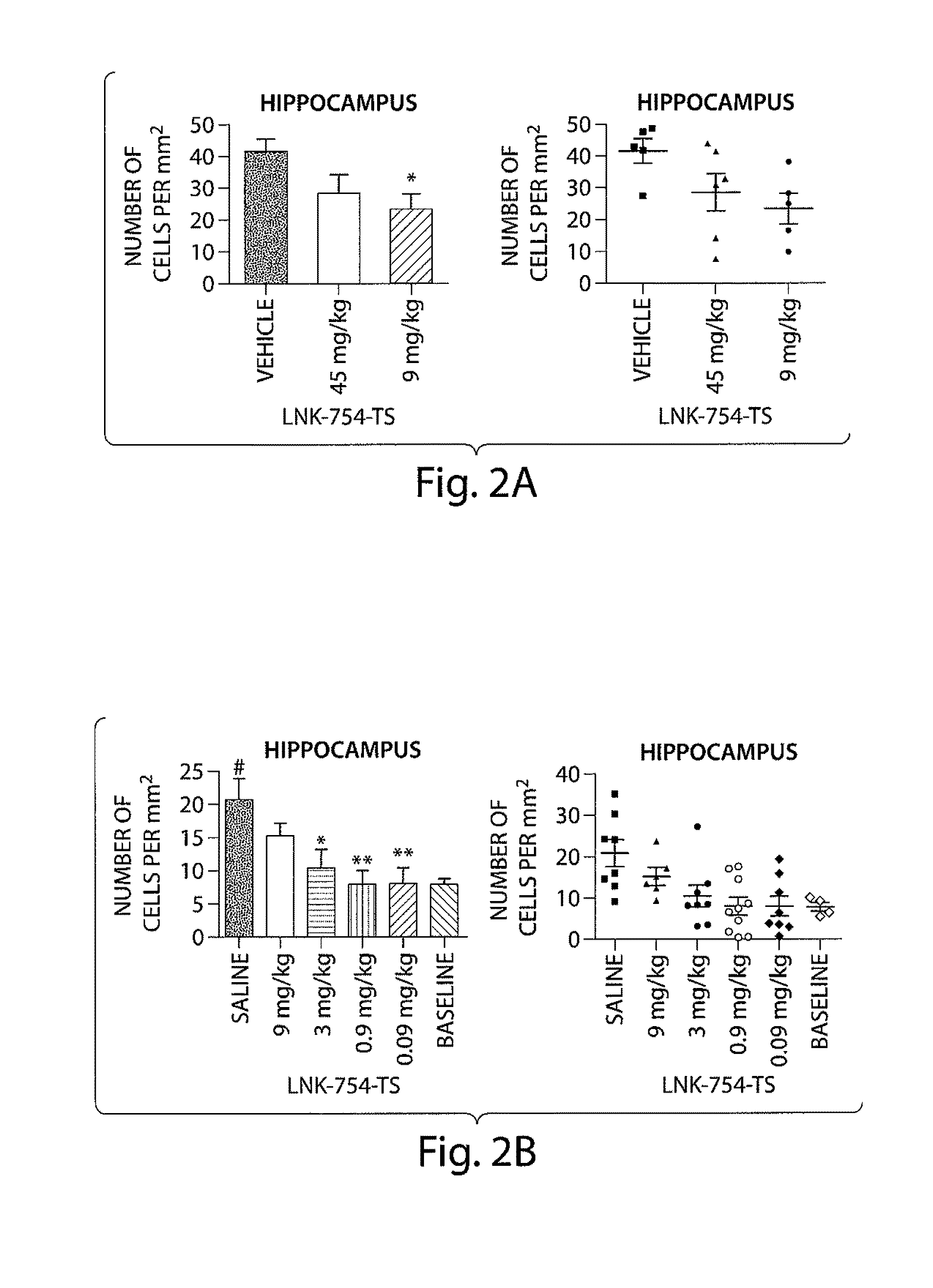
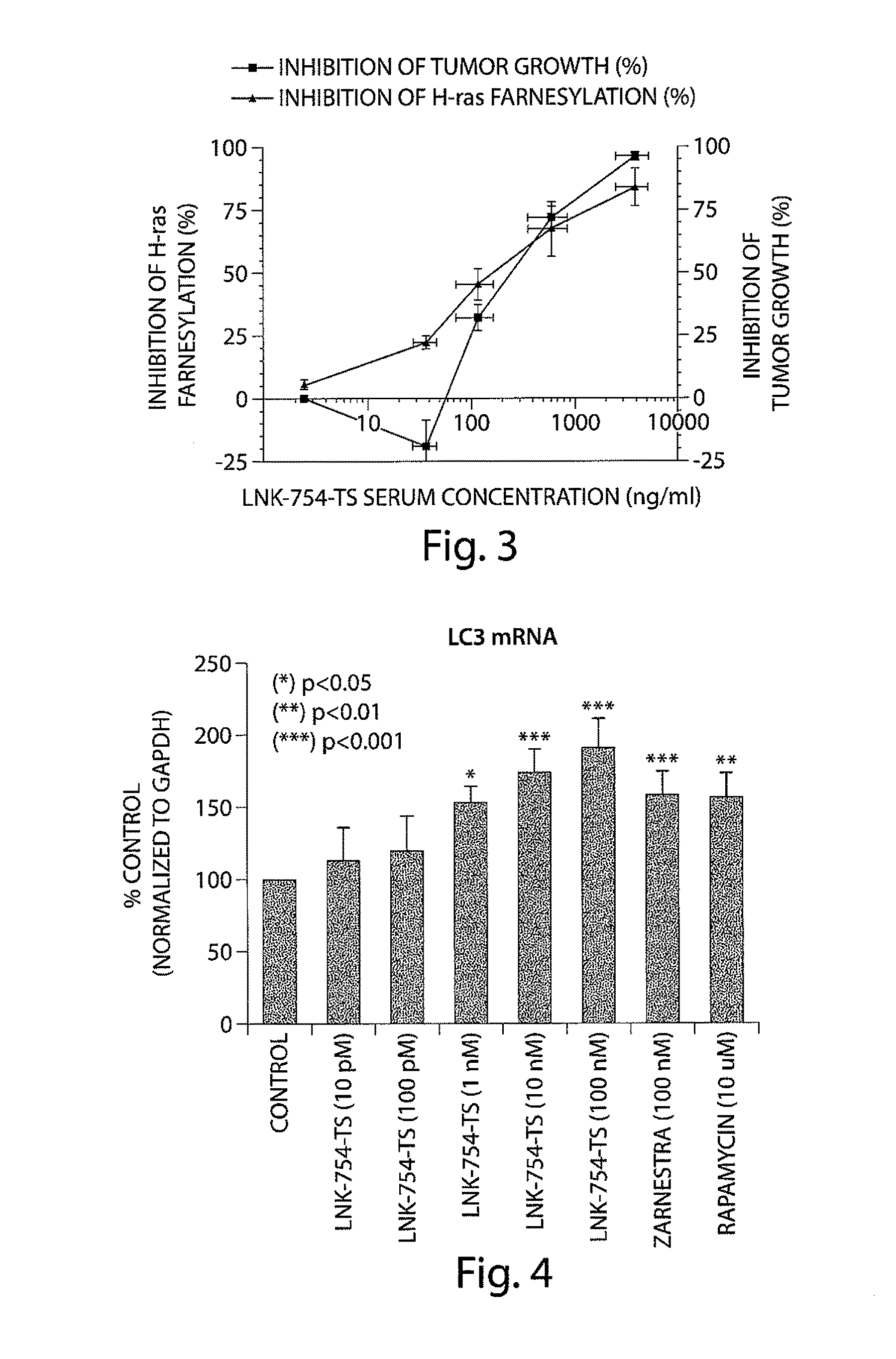
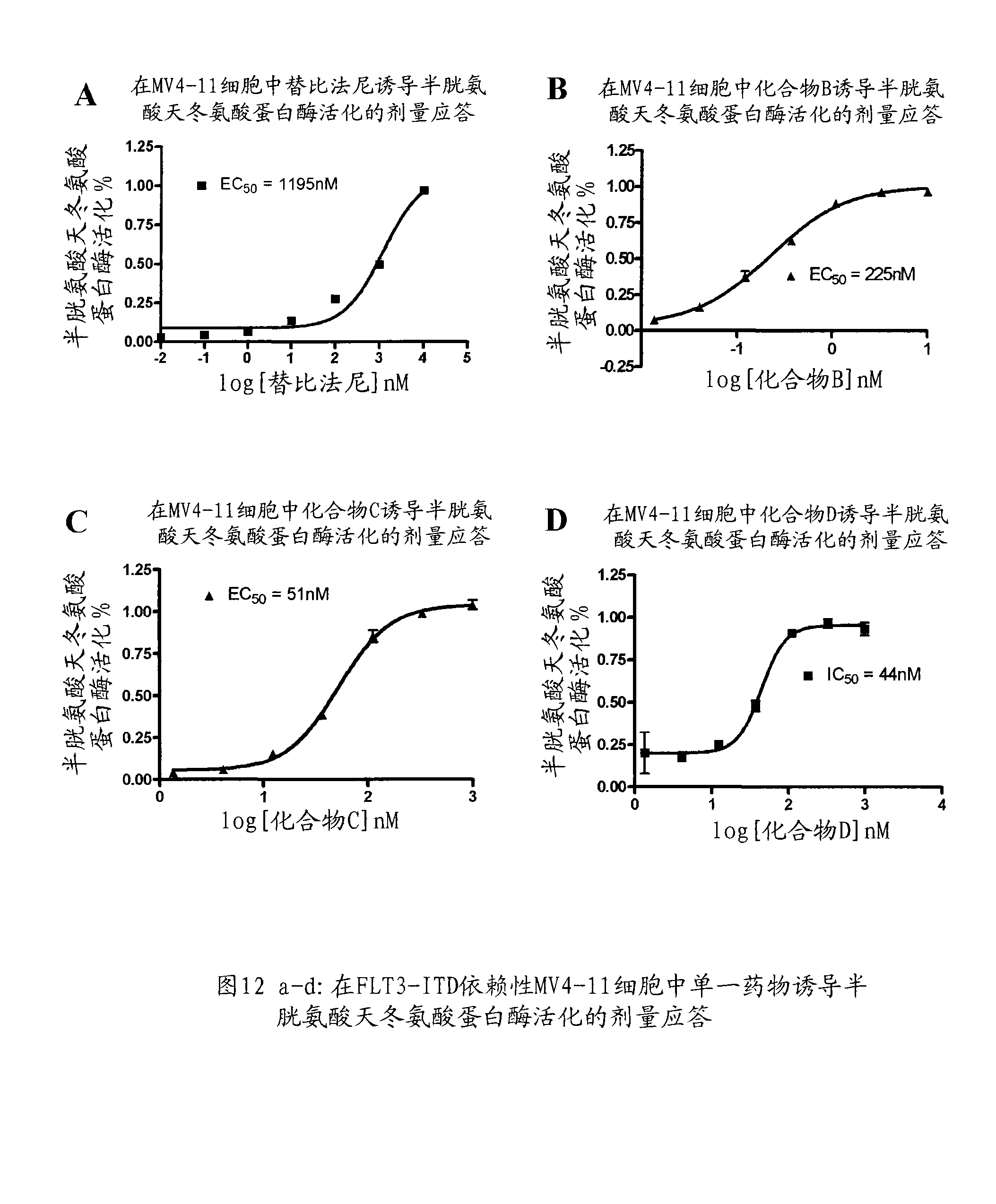
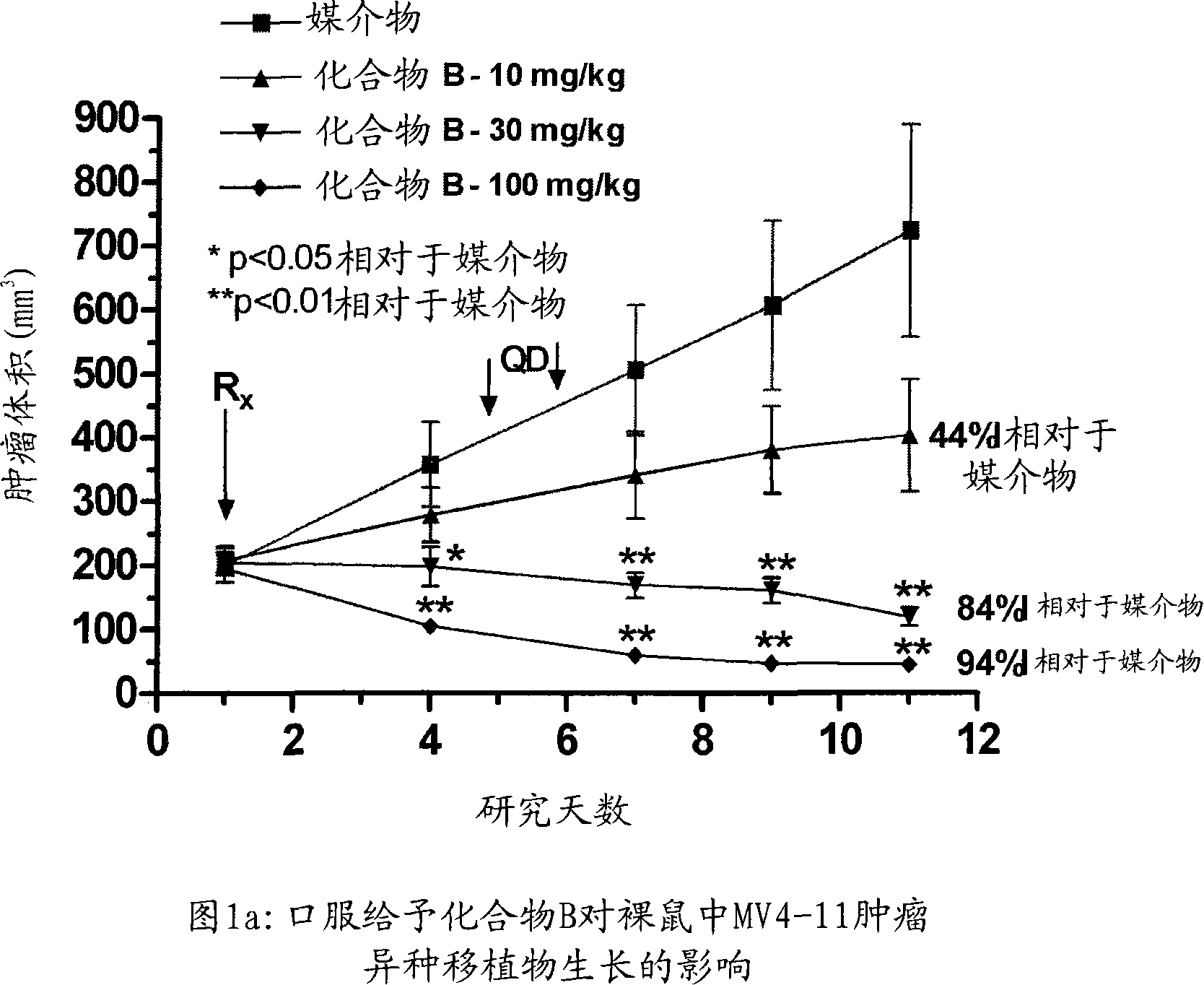
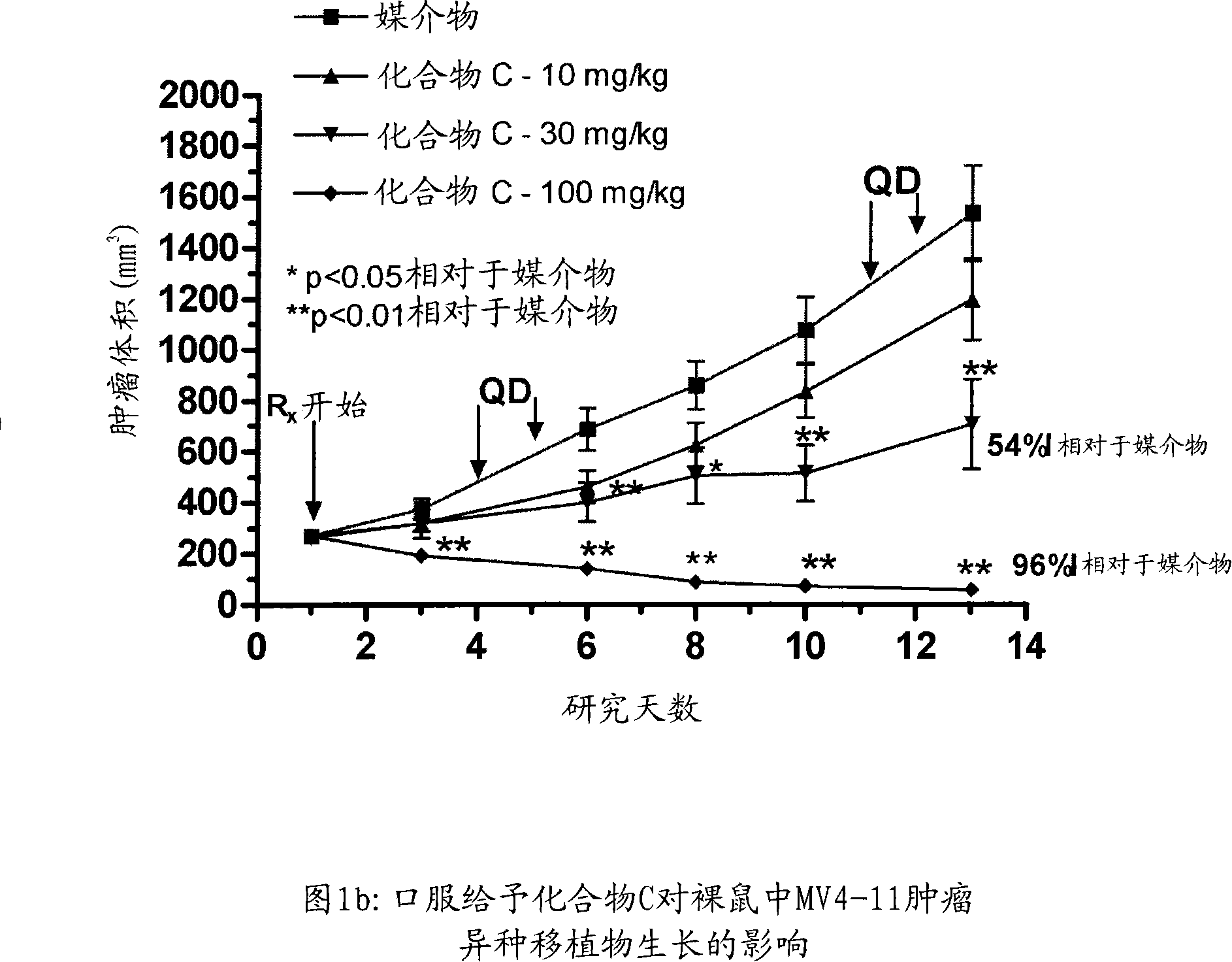
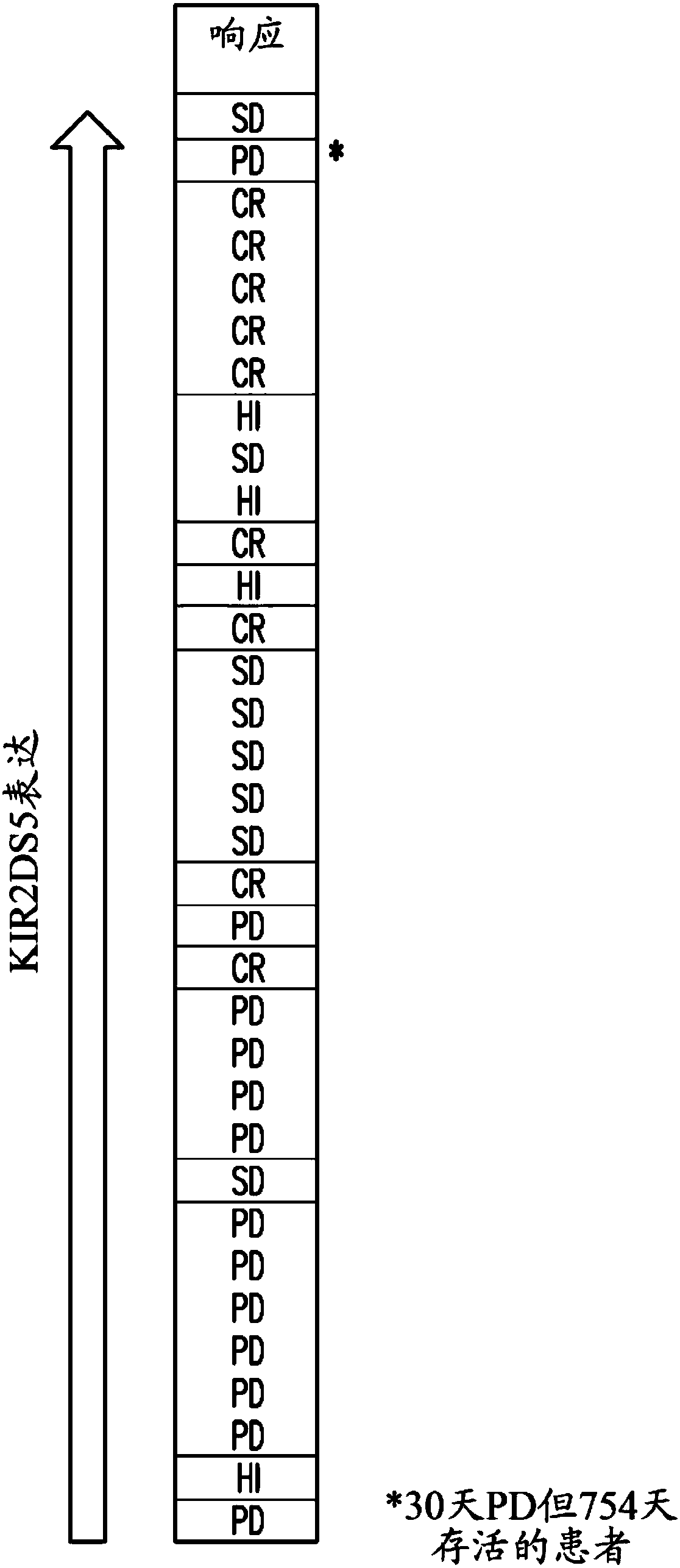
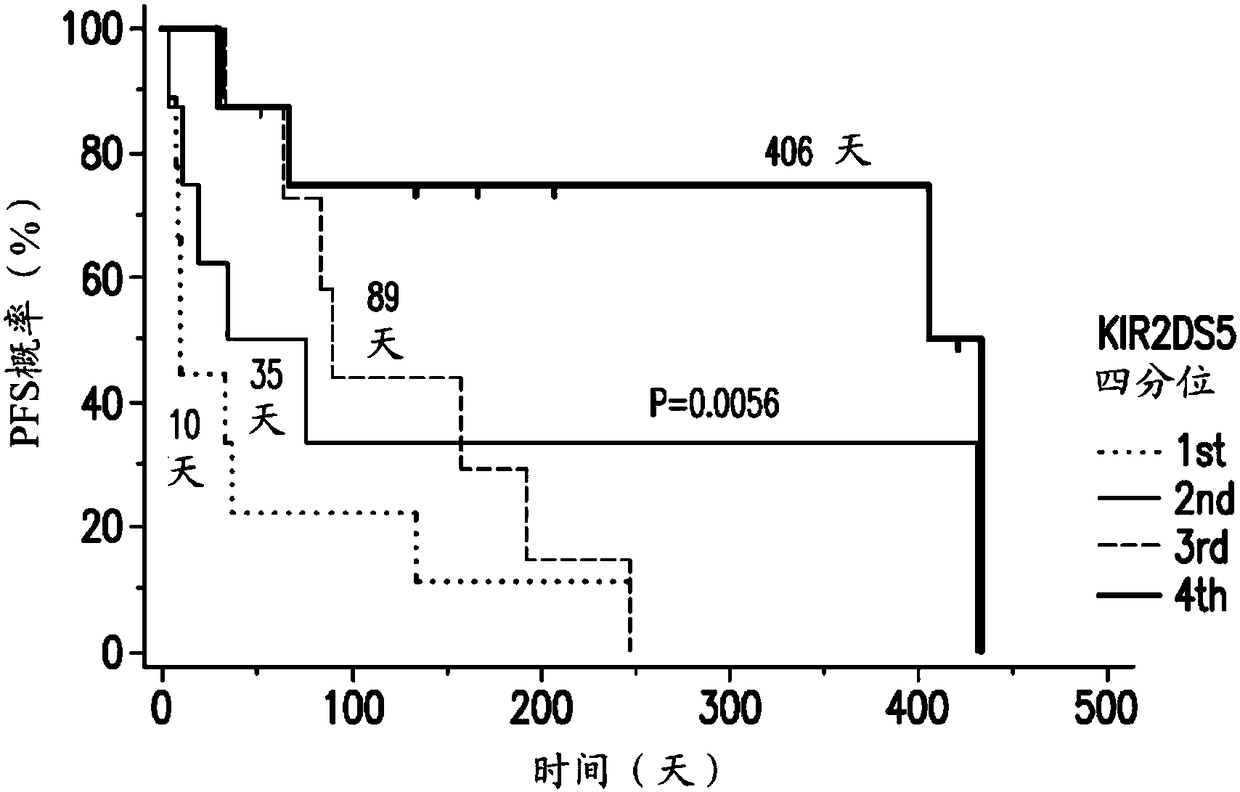
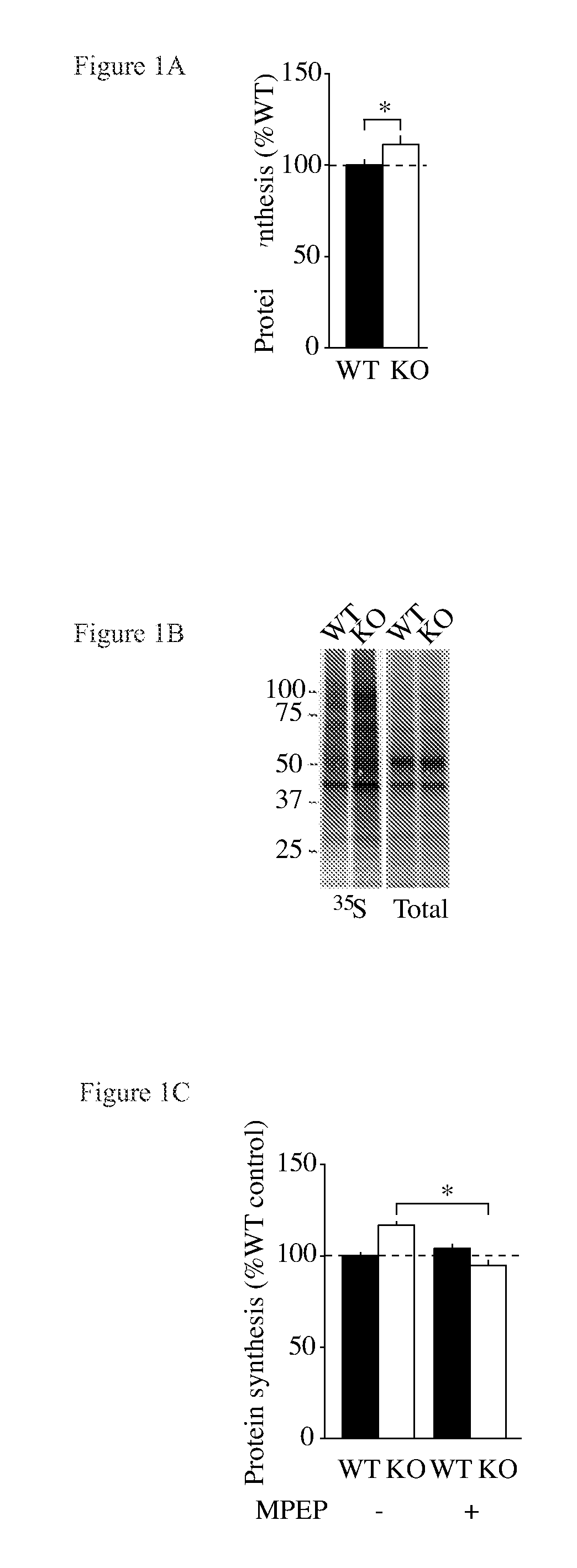
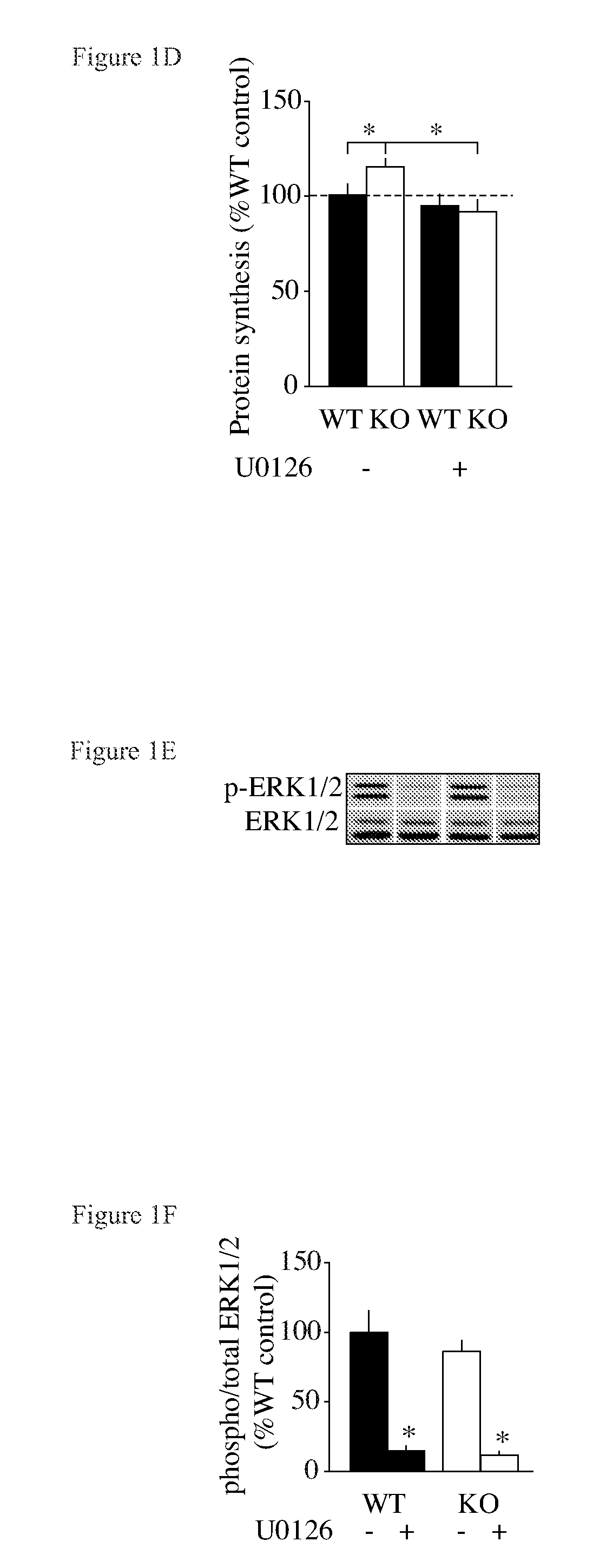
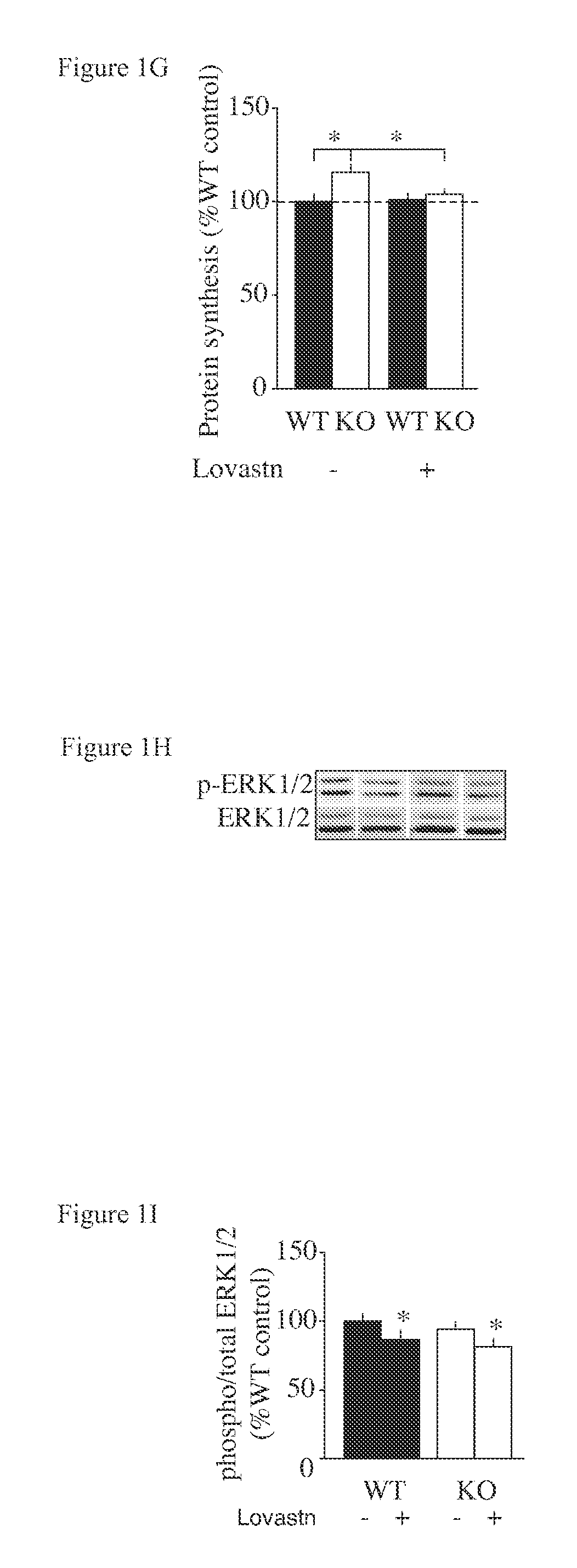
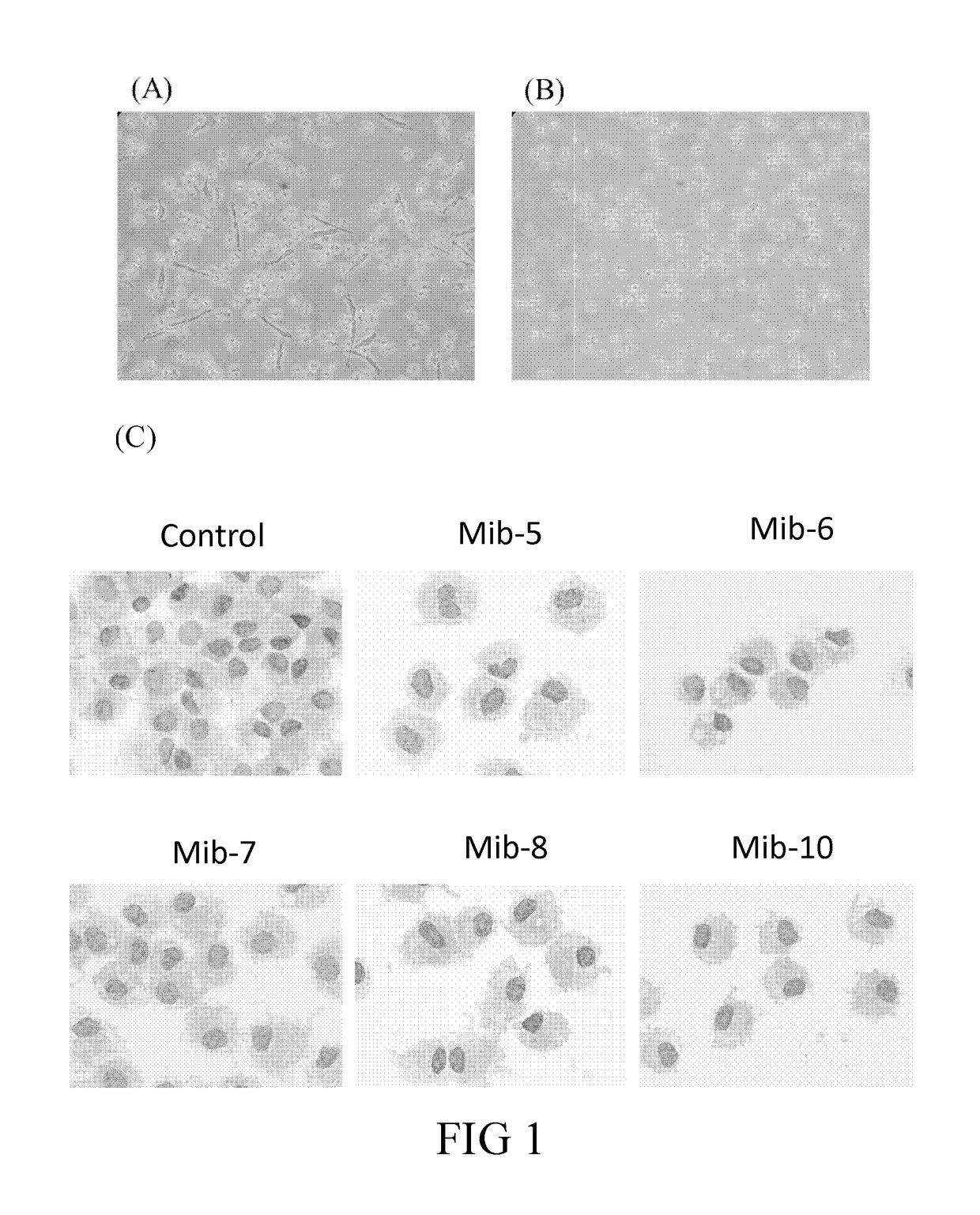
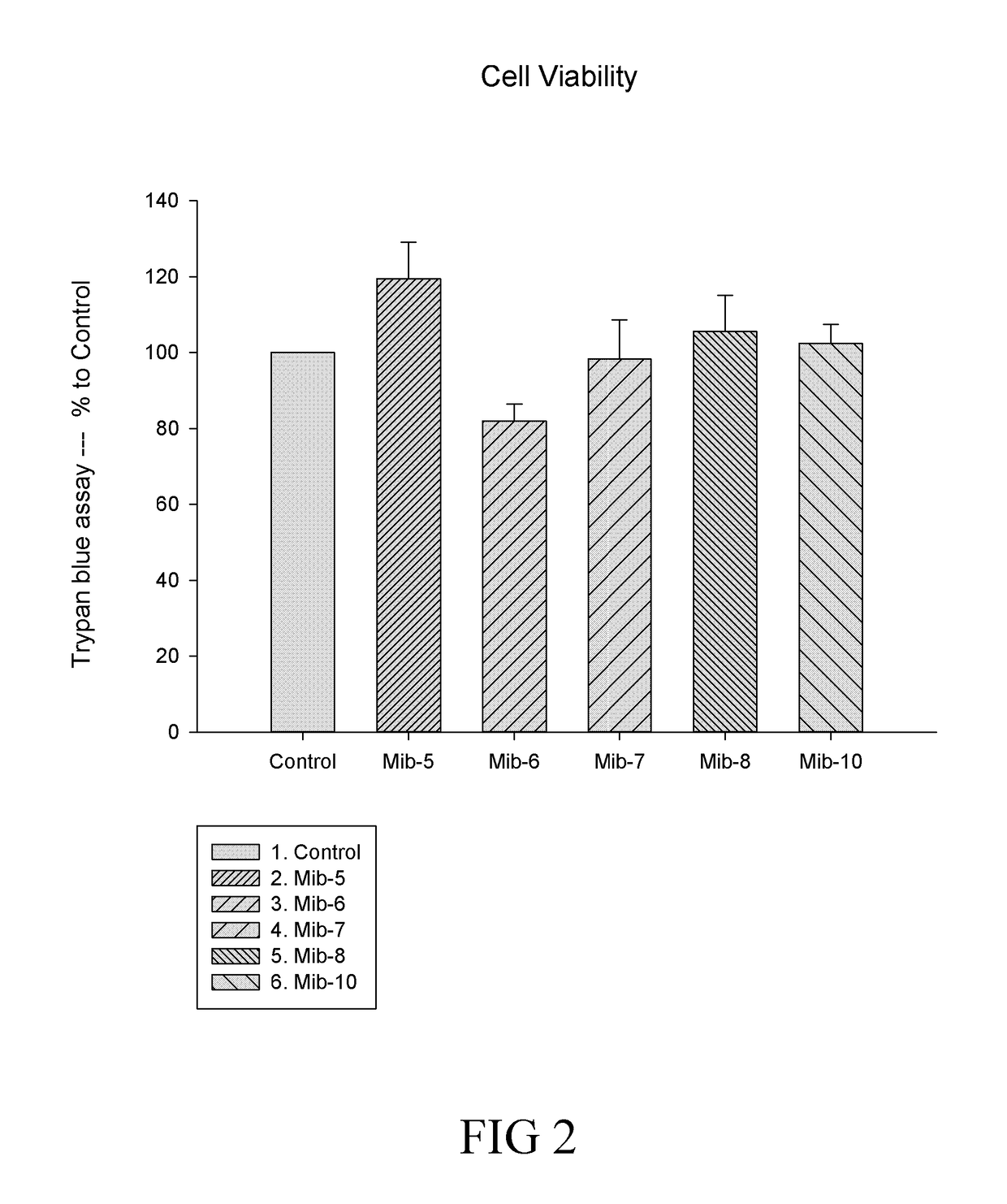
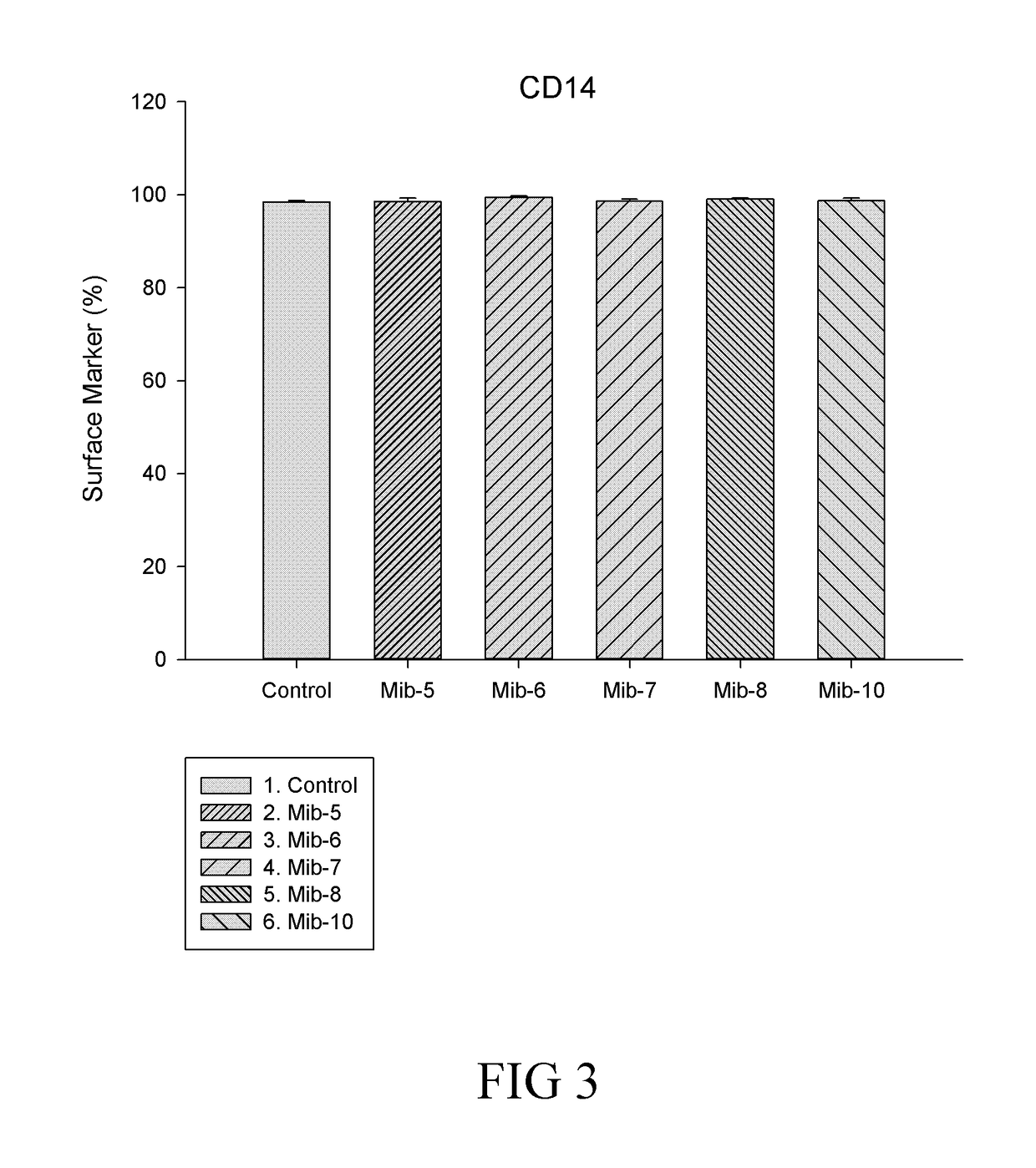
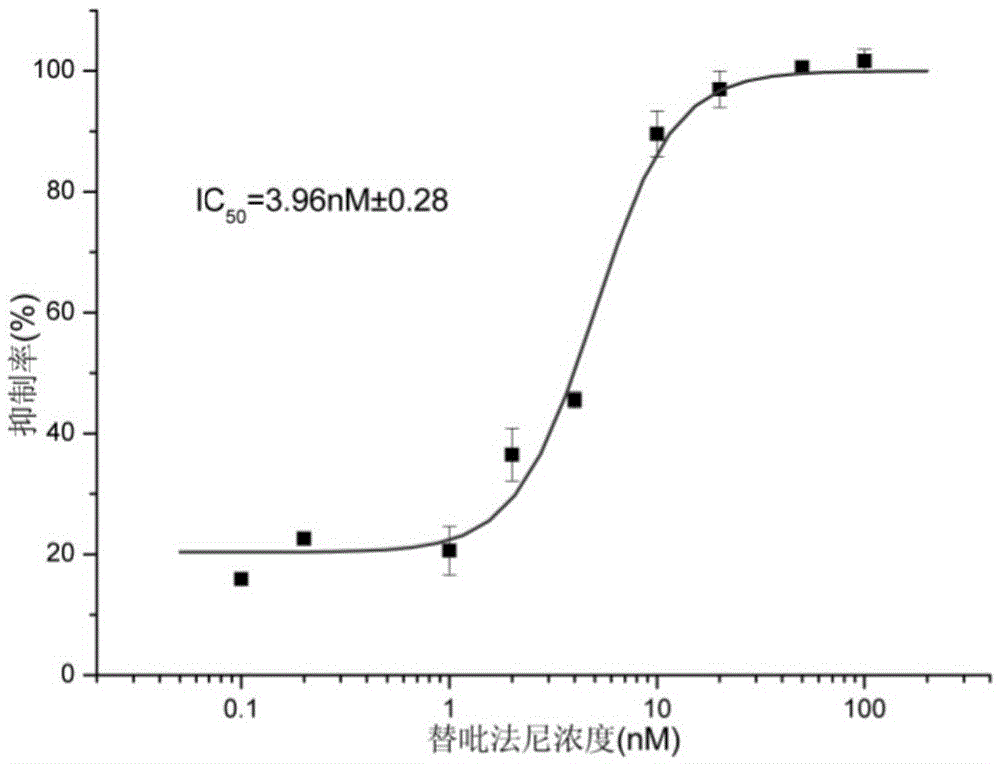
Patents
Literature
31 results about "Farnesyltransferase inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
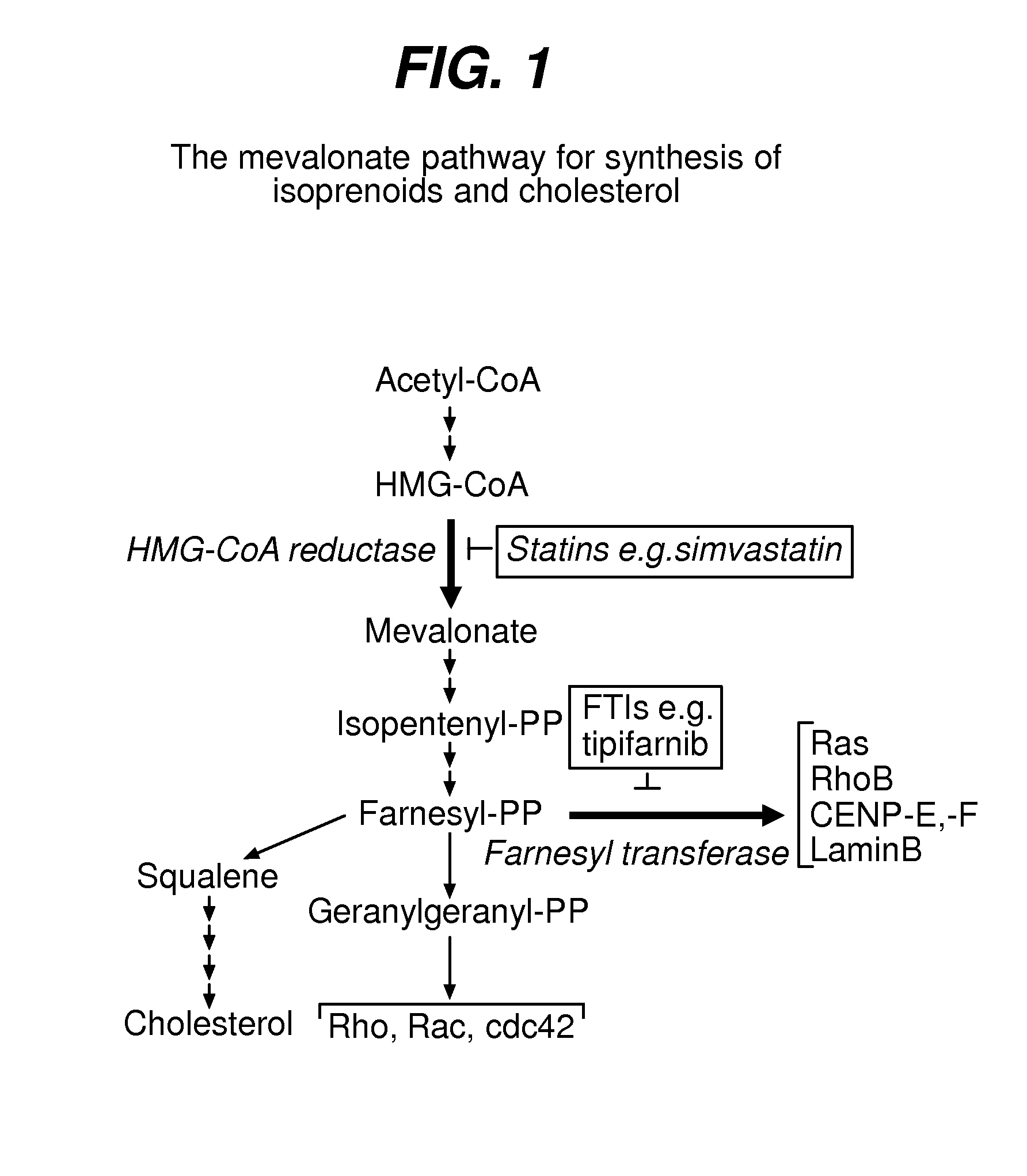
The farnesyltransferase inhibitors (FTIs) are a class of experimental cancer drugs that target protein farnesyltransferase with the downstream effect of preventing the proper functioning of the Ras (protein), which is commonly abnormally active in cancer.
Complex of ras-farnesyltransferase inhibitor and sulfobutylether-7-.beta.-cyclodextrin or 2-hydroxypropyl-.beta.-cyclodextrin and method
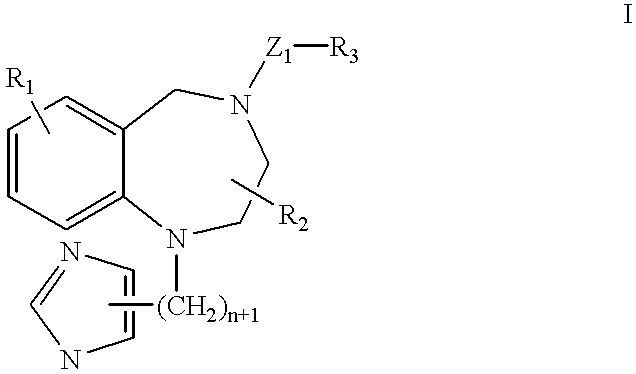
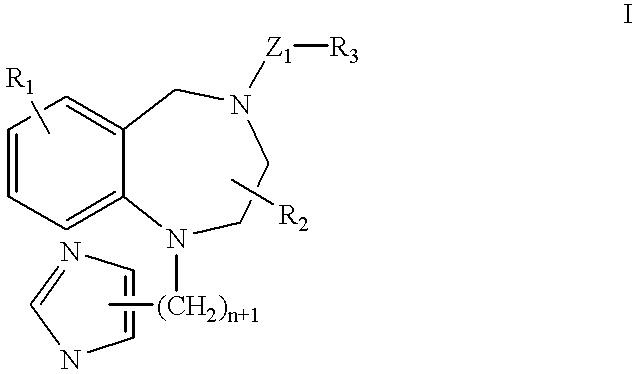
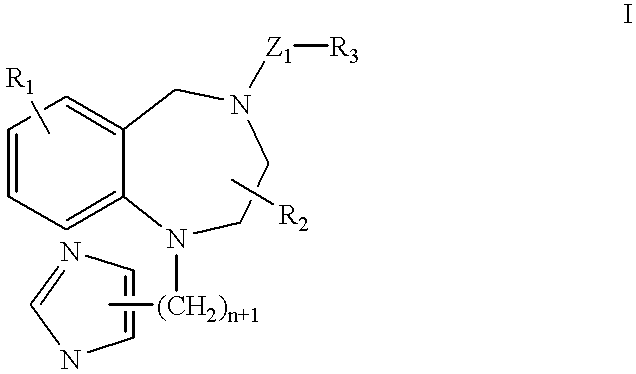
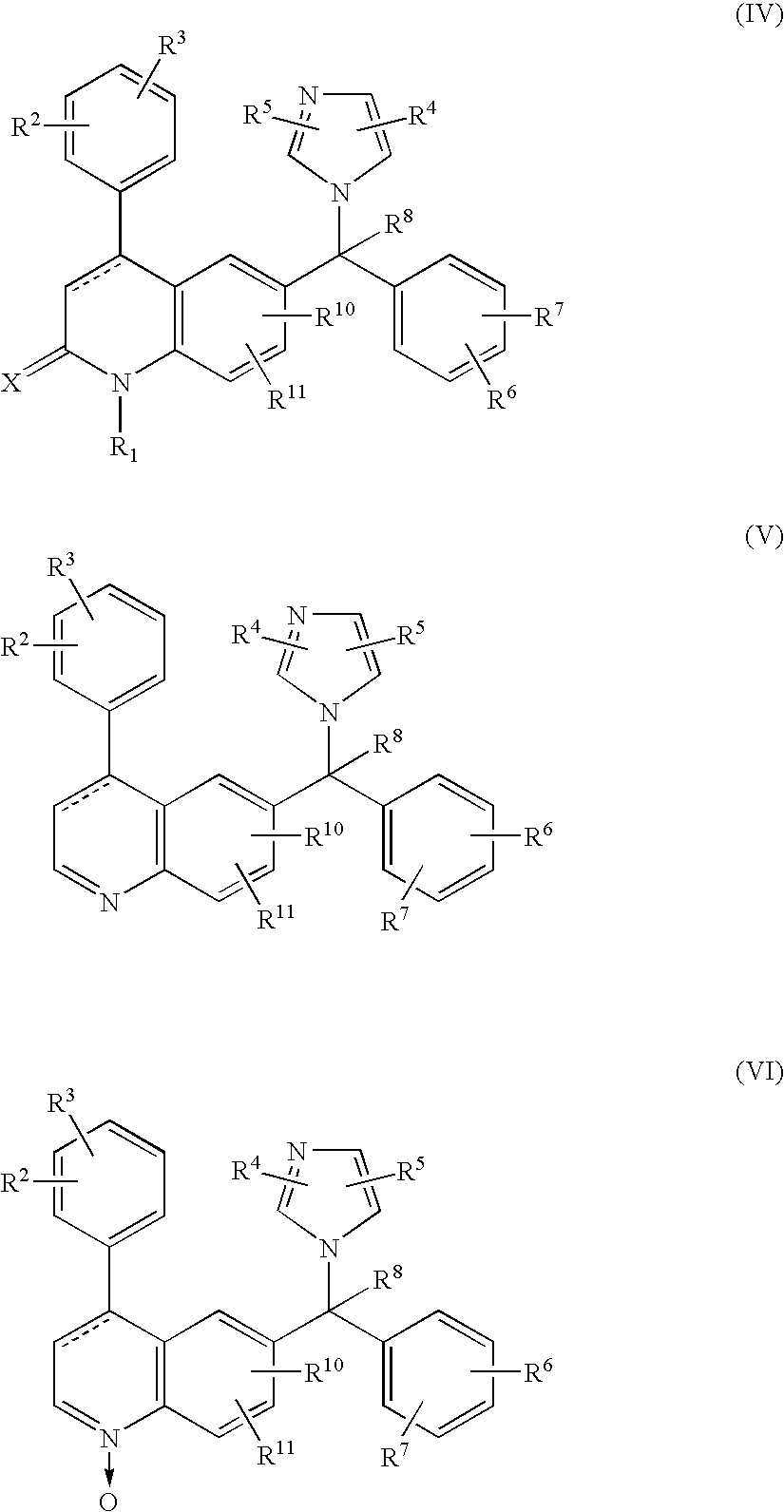
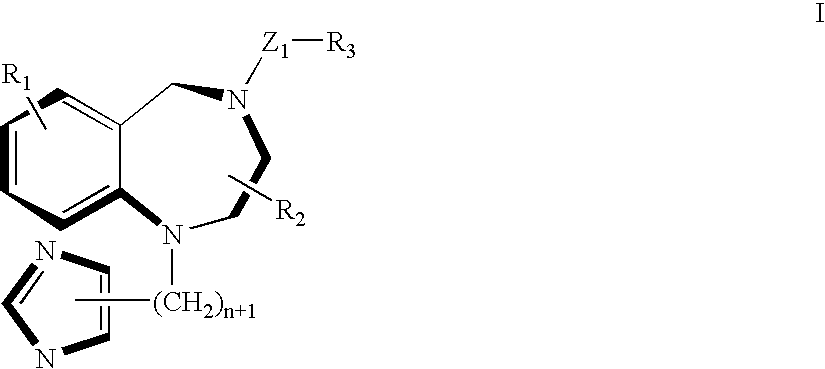
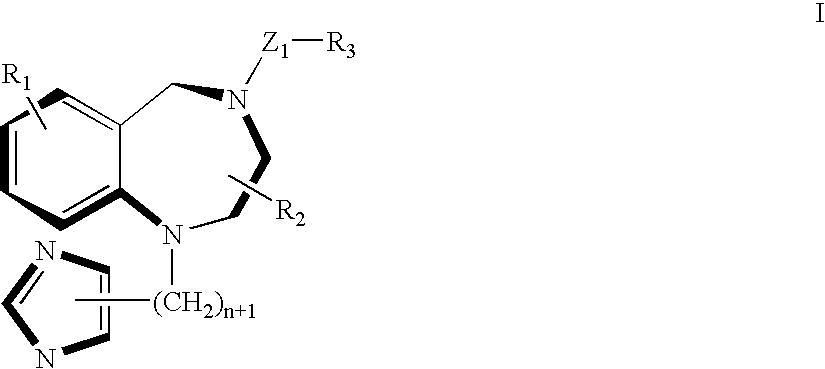
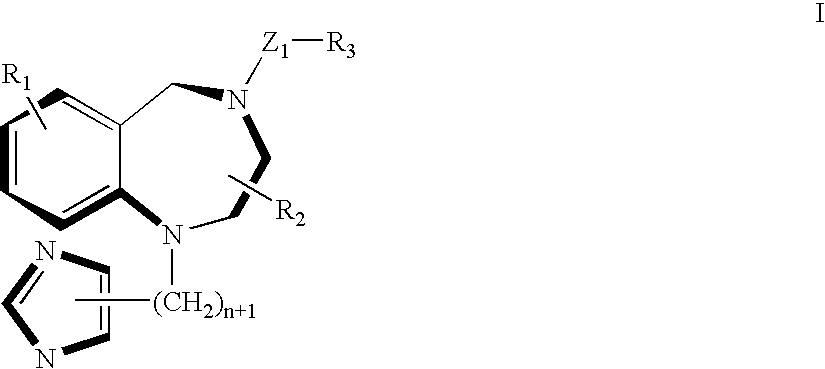
A ras-farnesyltransferase inhibitor complex formed of ras-farnesyltransferase inhibitor or a pharmaceutically acceptable salt thereof, of the formula I ##STR1## wherein n is 0 or 1; R.sub.1 is selected from Cl, Br, phenyl, pyridyl or cyano; R.sub.2 is aralkyl; R.sub.3 is selected from lower alkyl, aryl or substituted aryl or heterocyclo; Z.sub.1 is selected from CO, SO.sub.2, CO.sub.2, or SO.sub.2 NR.sub.5, R.sub.5 is selected from hydrogen, lower alkyl or substituted alkyl; and sulfobutylether-7-.beta.-cyclodextrin or 2-hydroxypropyl-.beta.-cyclodextrin is provided. The complex has unexpectedly high aqueous solubility of the ras-farnesyltransferase inhibitor and is useful for its intravenous delivery to humans with cancer. Also provided is a method for forming the complex. The ras-farnesyltransferase inhibitors are useful as anti-tumor agents.
Owner:BRISTOL MYERS SQUIBB CO
Therapeutic use of farnesyltransferase inhibitors and methods of monitoring the efficacy thereof
InactiveUS20060111398A1BiocideMicrobiological testing/measurementFarnesyltransferase inhibitorTipifarnib
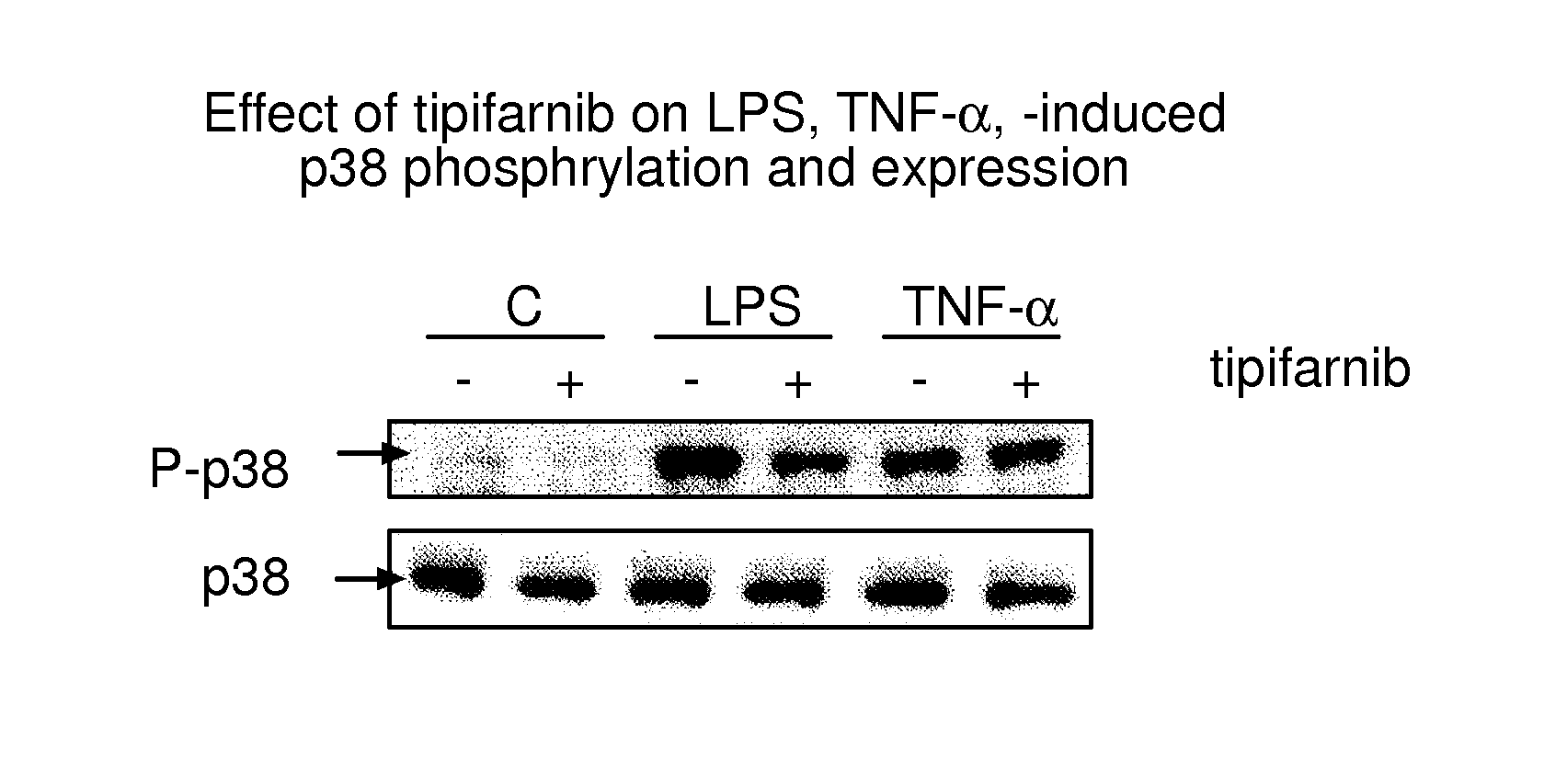
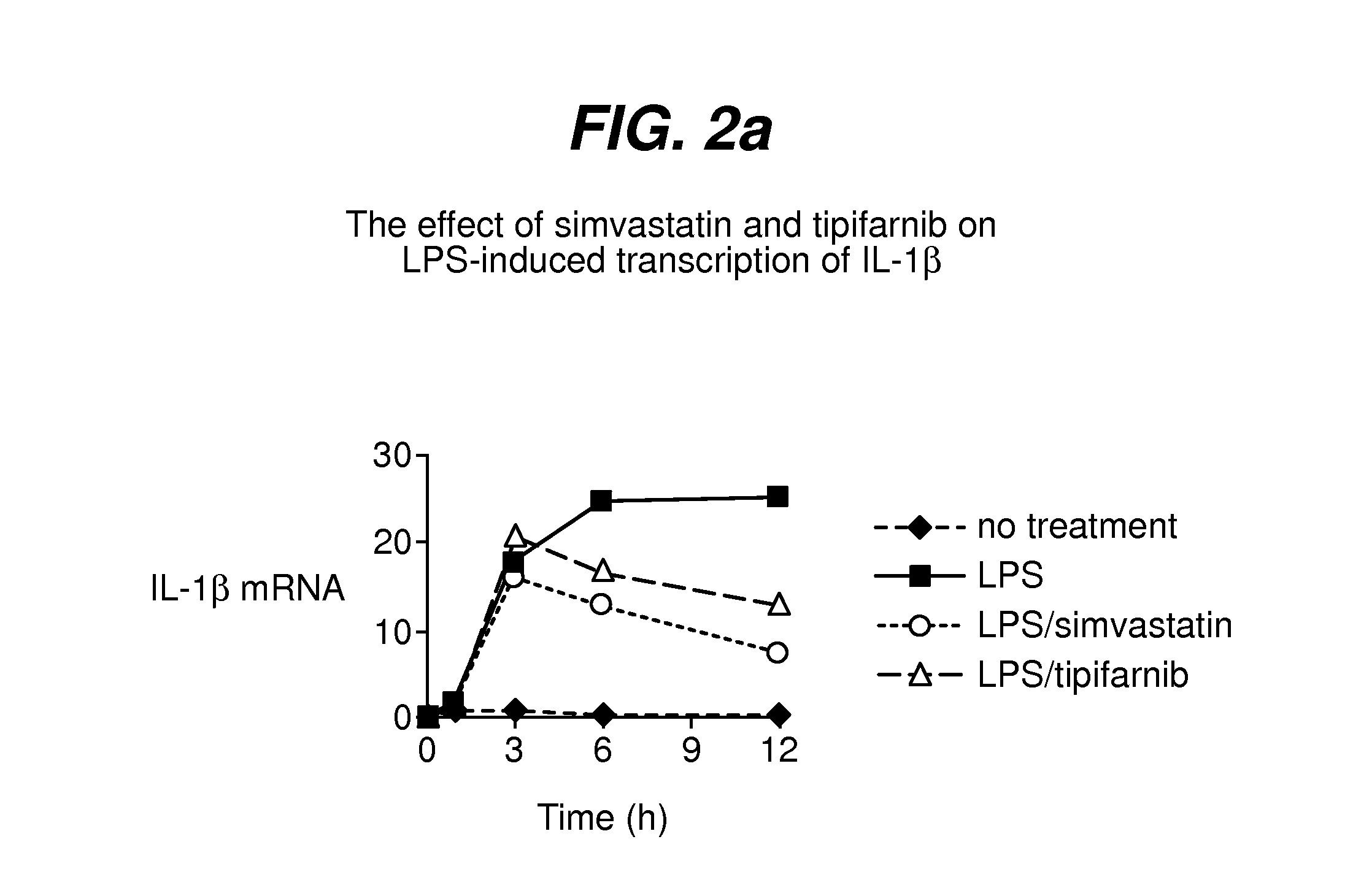
The therapeutic use of farnesyltransferase inhibitors (e.g., tipifarnib) for the treatment of sepsis is described. Methods useful to monitor the effectiveness of such treatment are also described.
Owner:JANSSEN PHARMA NV
Synergistic modulation of flt3 kinase using a flt3 inhibitor and a farnesyl transferase inhibitor
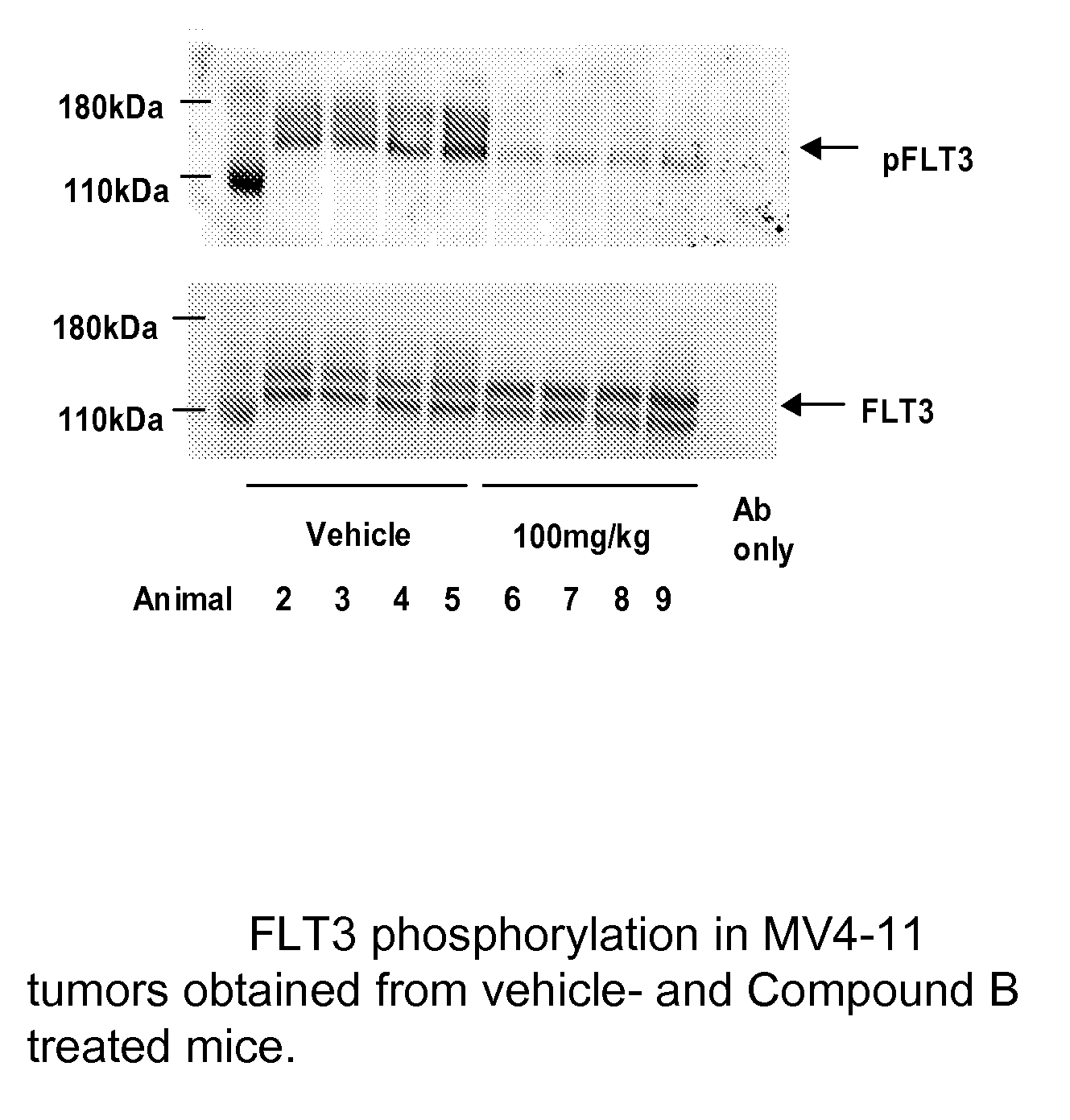
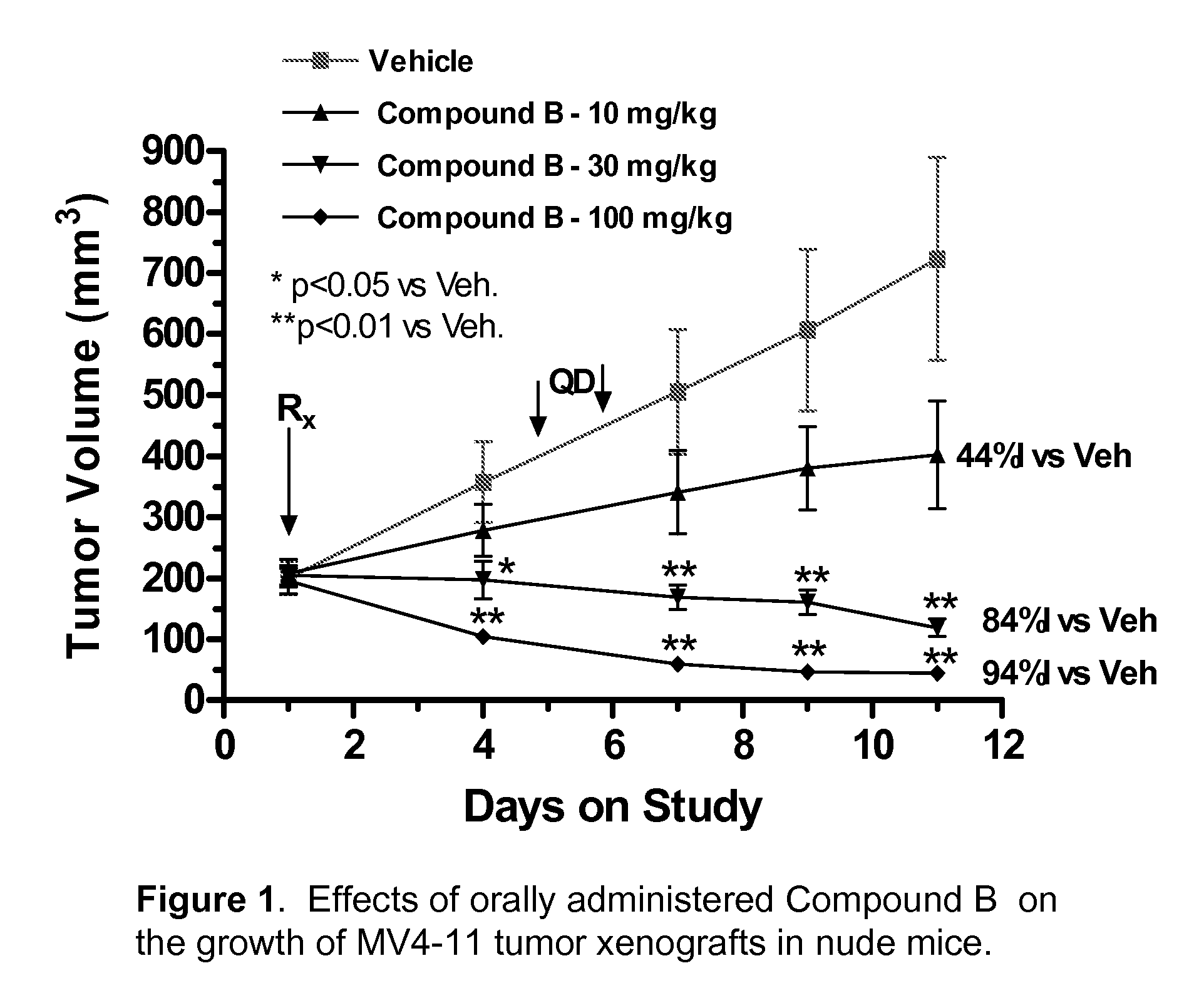
The invention is directed to a method of inhibiting FLT3 tyrosine kinase activity or expression or reducing FLT3 kinase activity or expression in a cell or a subject comprising the administration of a farnesyl transferase inhibitor and a FLT3 kinase inhibitor selected from compounds of Formula I′:Included within the present invention is both prophylactic and therapeutic methods for treating a subject at risk of (or susceptible to) developing a cell proliferative disorder or a disorder related to FLT3.
Owner:JANSSEN PHARMA NV
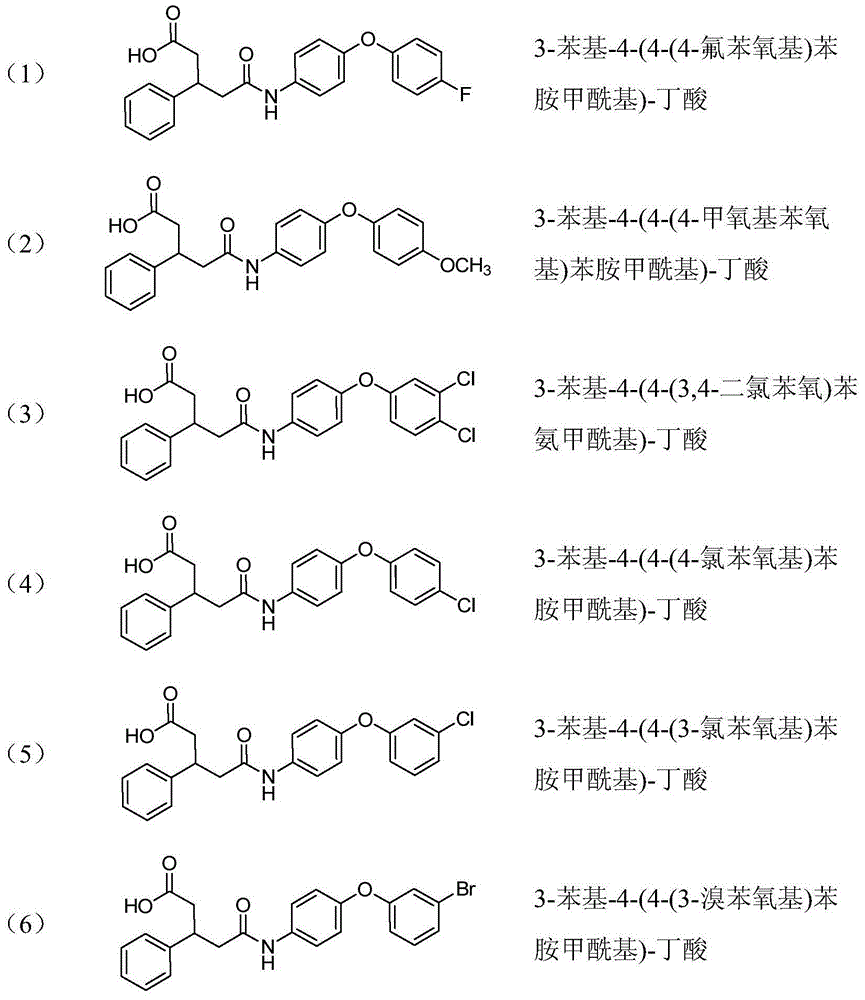
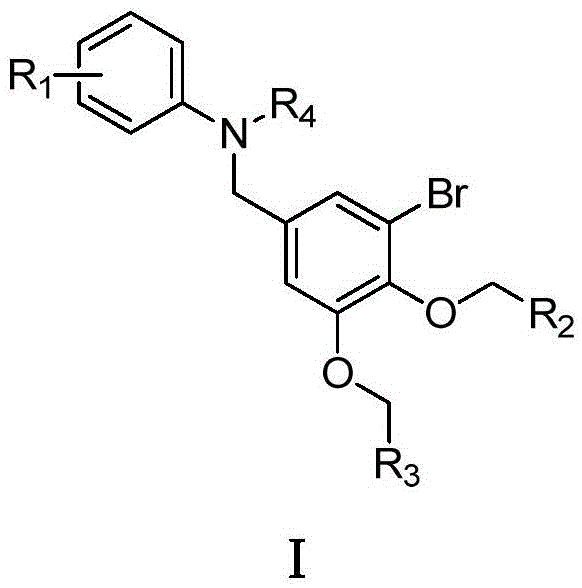
Benzyl-substituted aniline compound and application thereof
ActiveCN103787902ASignificant farnesyltransferase inhibitory activityGood drug prospectsCarboxylic acid nitrile preparationOrganic compound preparationDiseaseTransferase inhibitor
The invention provides a benzyl-substituted aniline compound represented by a formula I shown in a drawing or pharmaceutically acceptable salts thereof, wherein R1 is independently selected from -COOH, -CONH2, H, -COOR5 (R5 is methyl or ethyl), -CN, -OH and -NH2COCH3, R2 and R3 are independently selected from C6-C10 aryl, C3-C6 cyclane and C1-C3 saturated alkyl or unsaturated alkyl respectively, and R4 is independently selected from -H, ethyl and acetyl. The compound and the pharmaceutically acceptable salts thereof, provided by the invention, can be used as farnesyltransferase inhibitors or can be used for preparing drugs for preventing or treating diseases related to farnesyltransferase, thereby having good medicine preparation prospects.
Owner:EAST CHINA UNIV OF SCI & TECH
Method of determining acute myeloid leukemia response to treatment with farnesyltransferase inhibitors
ActiveUS20130130999A1Faster assayQuick forecastBiocideMicrobiological testing/measurementMyeloid leukemiaAptX
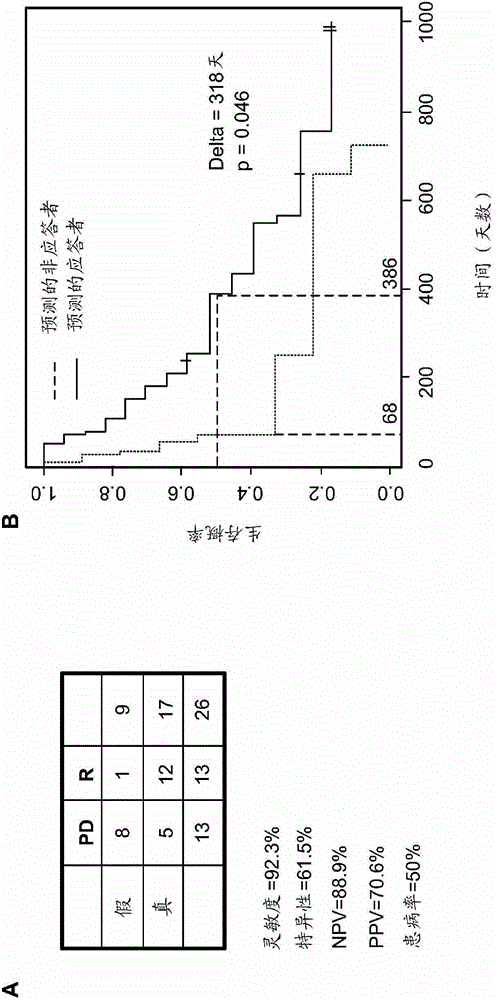
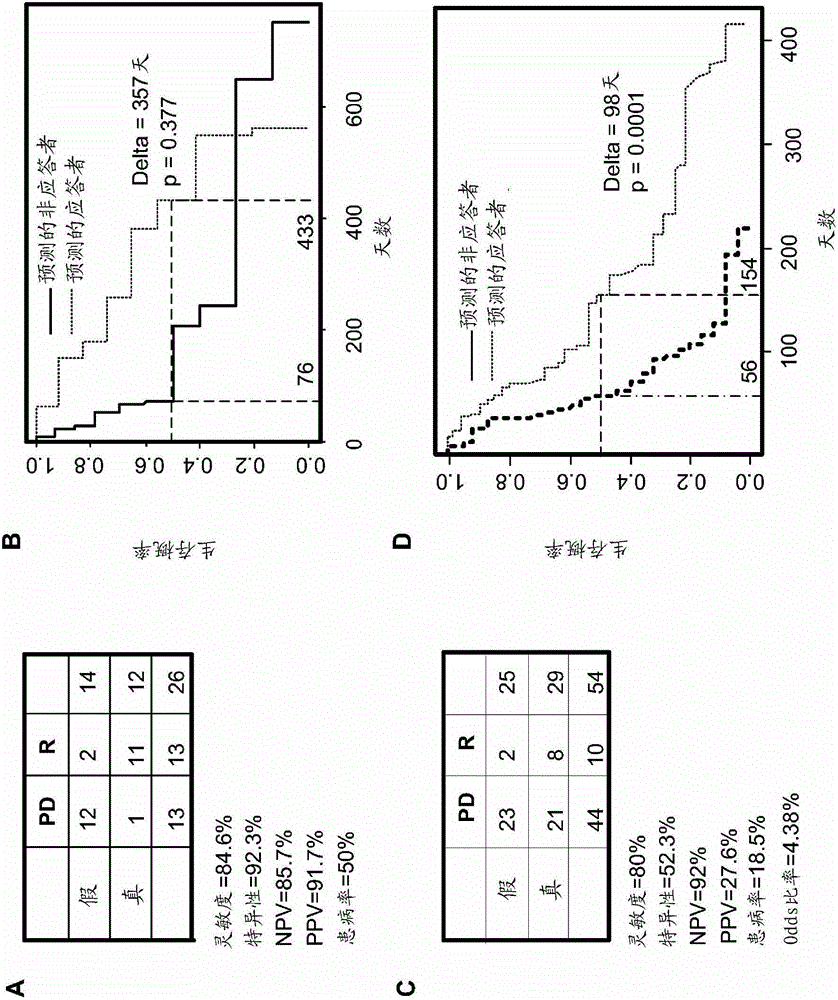
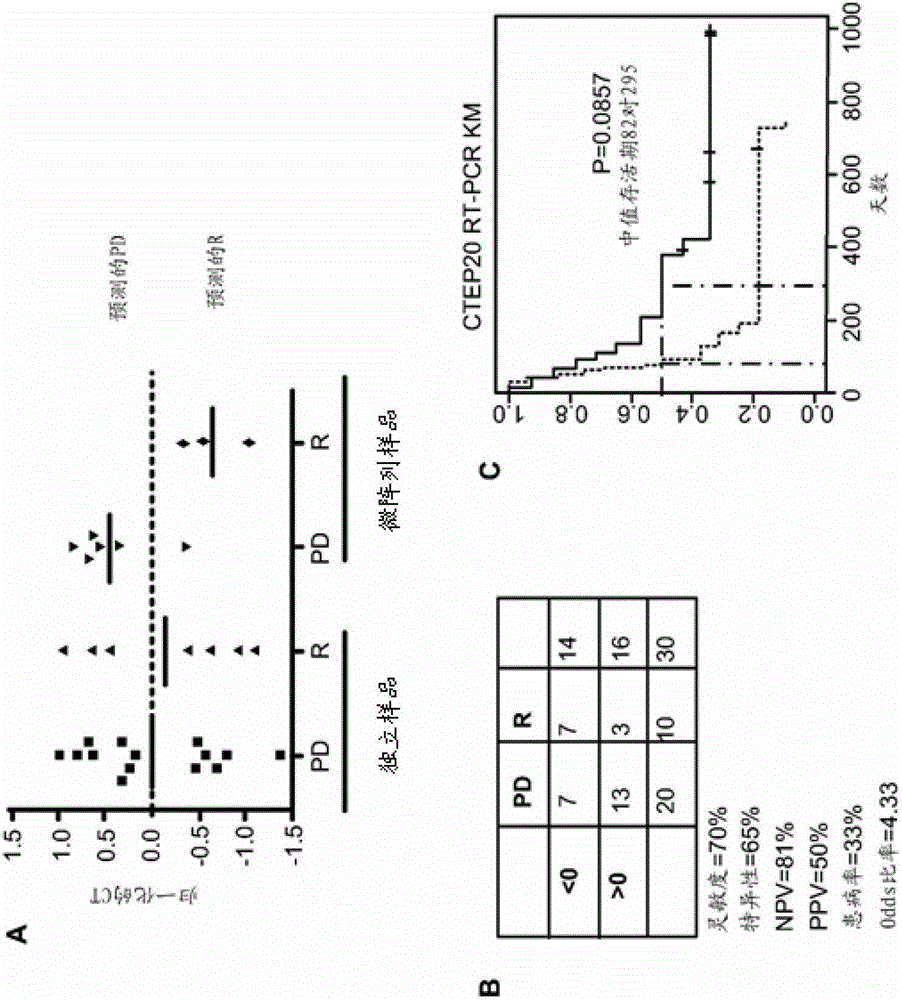
The disclosed method rapidly identifies with desired accuracy AML patients, including elderly AML patients, likely to respond to treatment with a combination of a farnesyltransferase inhibitor and one or more of etoposide, teniposide, tamoxifen, sorafenib, paclitaxel, temozolomide, topotecan, trastuzumab and cisplatinum. In an embodiment, the improvements include the use of whole blood rather than the customary bone marrow sample, thus making the assay more accurate, rapid, less intrusive, less expensive as well as less painful. The method includes evaluation of a two-gene expression ratio (RASGRP1:APTX), which with a corresponding threshold, provides sufficient accuracy for predicting the response to the combination treatment. In the preferred embodiment the combination treatment combines tipifarnib (R115777, ZARNESTRA®) with etoposide. Further, the elderly AML patients identified as being likely responsive to the combination treatment with tipinifarb and etoposide have a complete recovery rate comparable to the best therapy available for younger patients.
Owner:JANSSEN PHARMA NV
Therapeutic use of farnesyltransferase inhibitors and methods of monitoring the efficacy thereof
InactiveUS20110195419A1Organic active ingredientsMicrobiological testing/measurementTransferase inhibitorFarnesyltransferase inhibitor
The therapeutic use of farnesyltransferase inhibitors (e.g., tipifarnib) for the treatment of sepsis is described. Methods useful to monitor the effectiveness of such treatment are also described.
Owner:FOURIE ANNE MADELEINE
Treatment of mitochondrial disorders using a farnesyl transferase inhibitor
InactiveUS20100331363A1Avoid cell deathIncreased insulin secretionBiocideNervous disorderAntioxidantDepressant
Methods and pharmaceutical compositions comprising a low dose of a farnesyl transferase inhibitor useful in the treatment of proteinopathies are provided. These low doses are below the doses used in oncological treatments for which these compounds were initially designed. The treatment includes administering to a subject an amount of a farnesyl transferase inhibitor, wherein the amount administered is sufficient to cause an improvement in mitochondrial health in said subject. Treatments in accordance with the present invention may also include an acetylcholinesterase inhibitor, an activator of neurotrophic receptors, an NMDA anatagonist, an amyloid deposit inhibitor, an antipsychotic agent, an antidepressant, an anxiolytic, or an antioxidant.
Owner:ASTRAZENECA AB
Treatment of h-ras-driven tumors
ActiveUS20170042881A1Reduce tumor burdenReduce the burden onOrganic active ingredientsMicrobiological testing/measurementMEK inhibitorFarnesyltransferase inhibitor
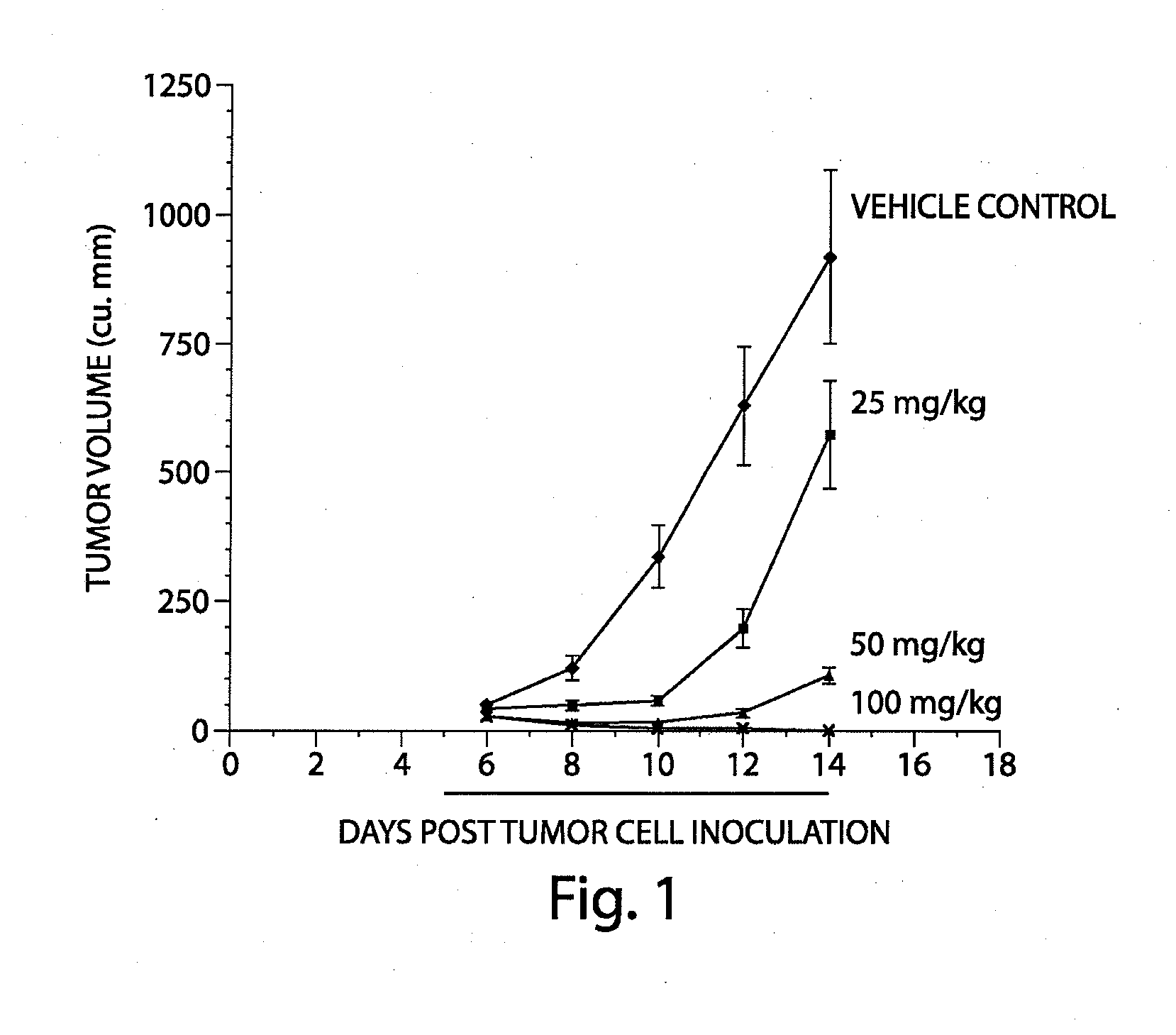
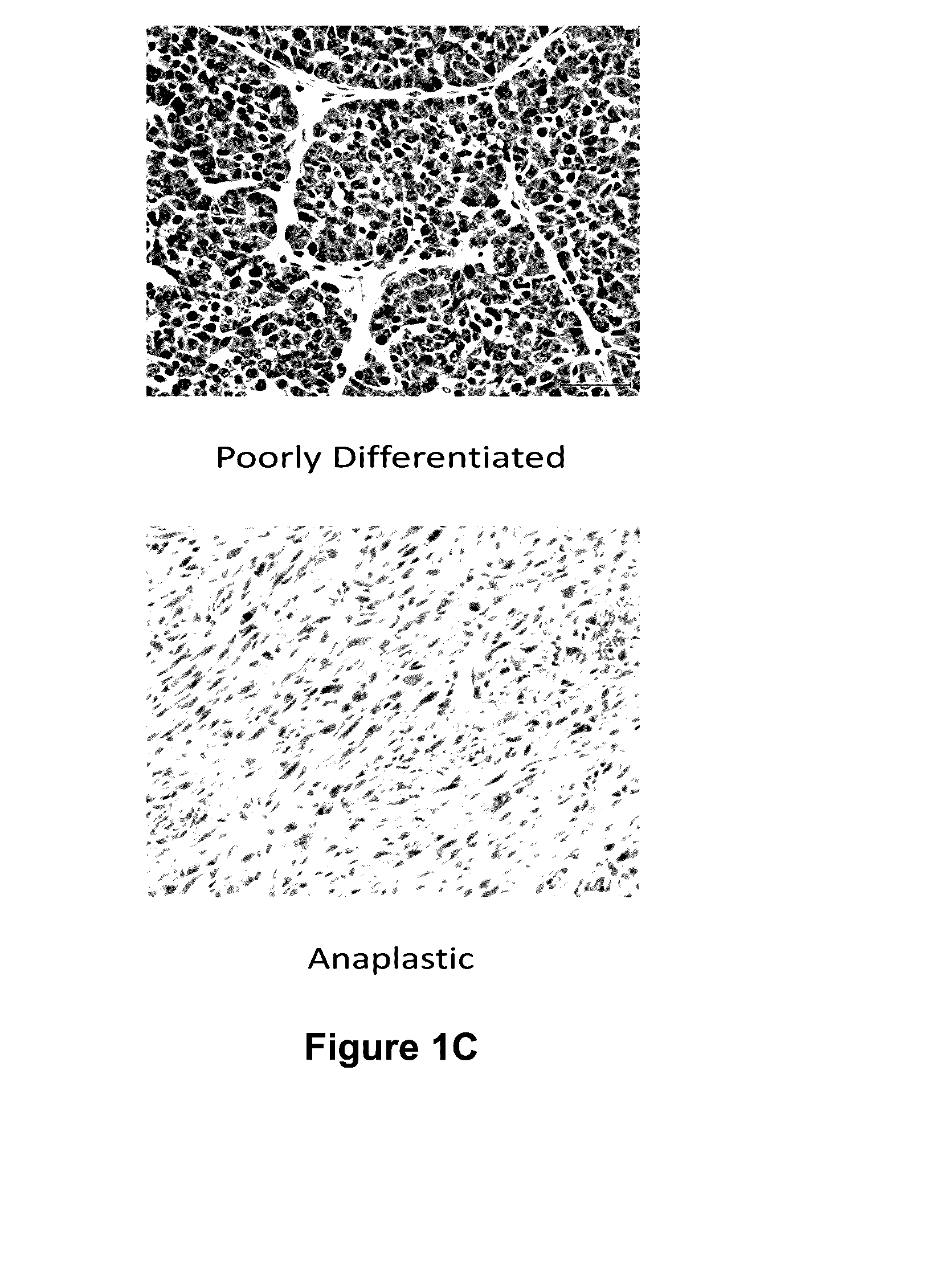
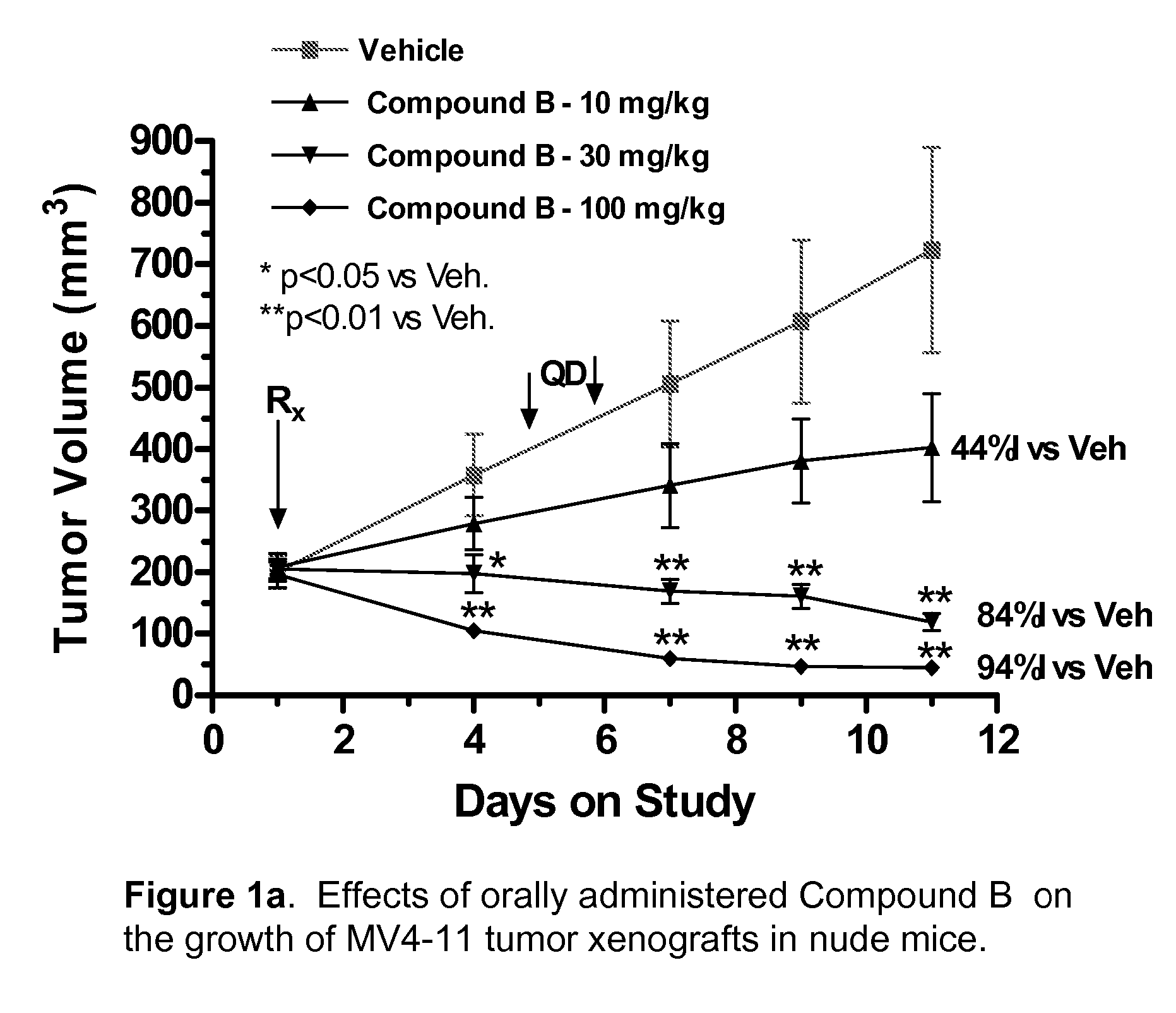
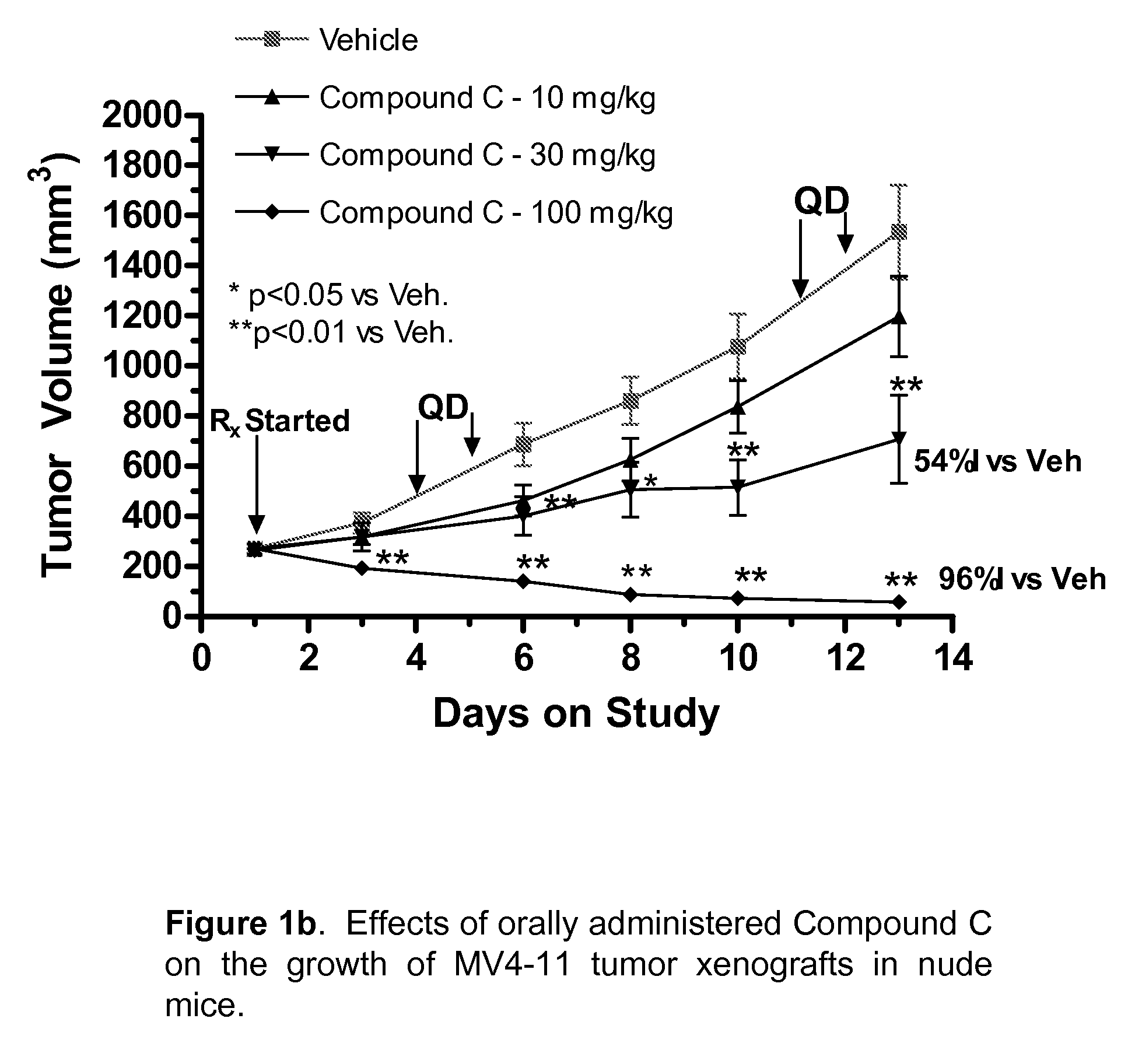
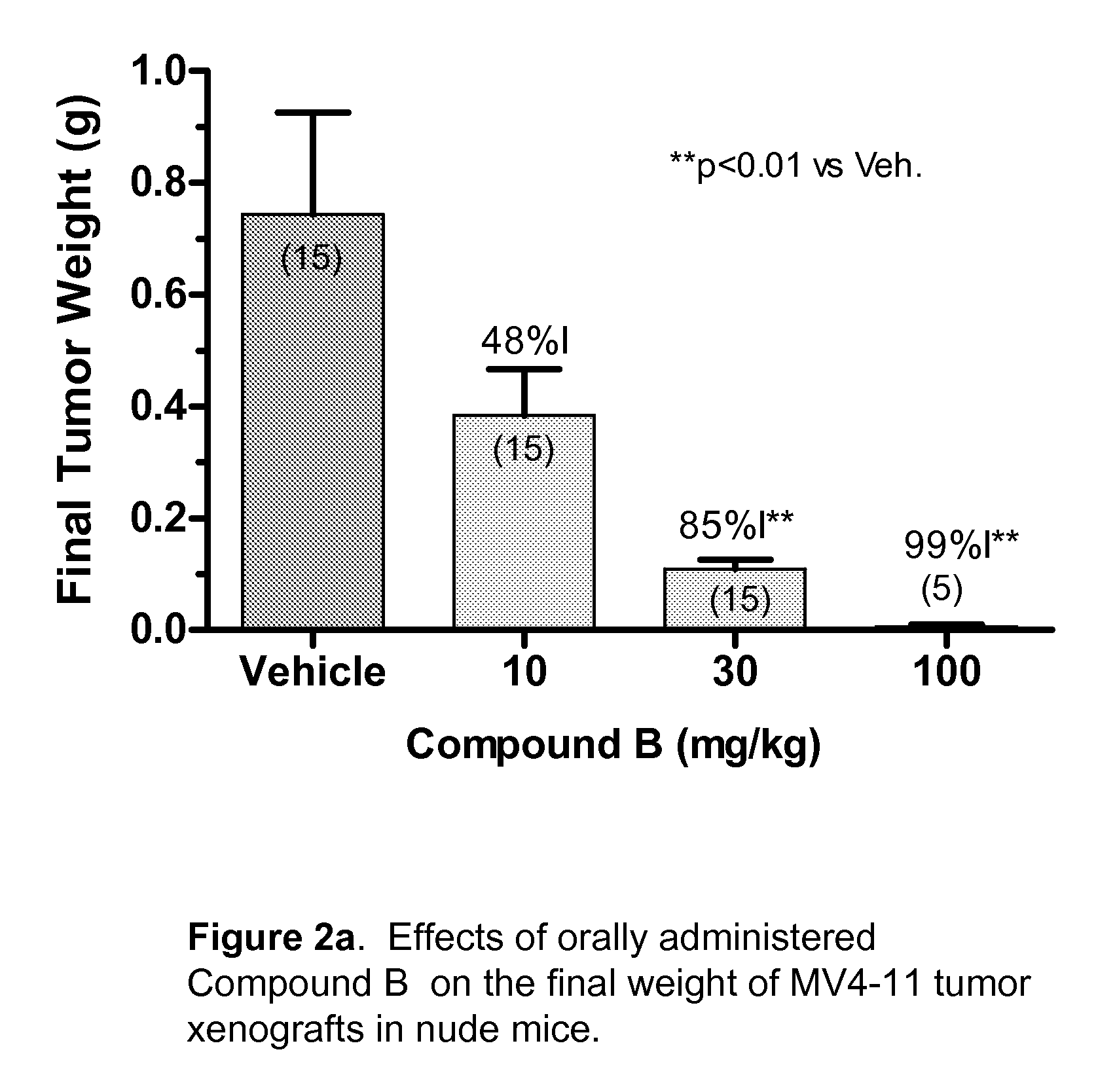
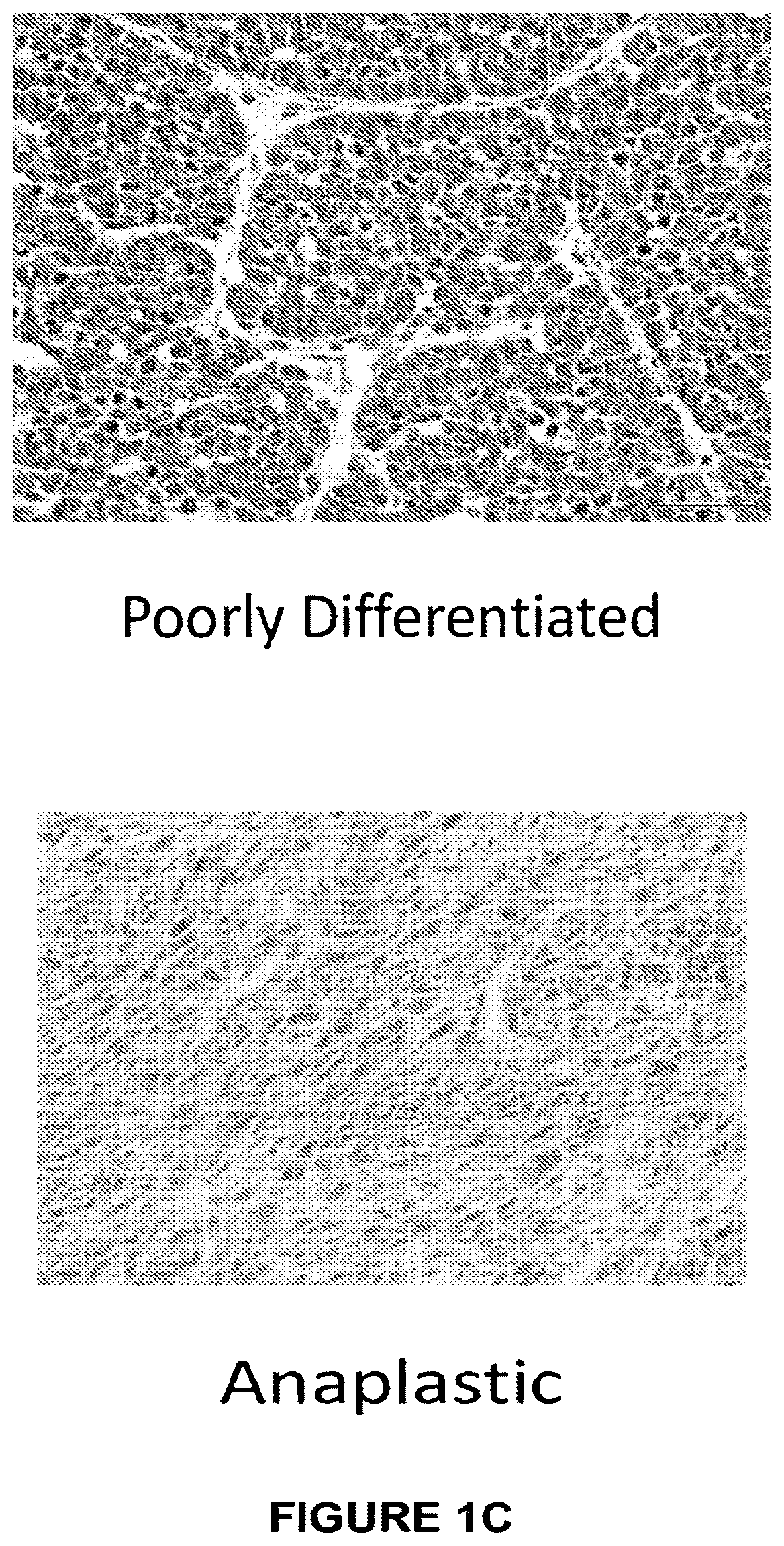
The present disclosure describes a compositions and methods for treatment of Hras-driven cancers. Administration of a farnesyltransferase inhibitor, for example, tipifarnib, alone or in combination with a MEK inhibitor can reduce tumor size and tumor growth in cancers such as poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC).
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Synergistic Modulation of Flt3 Kinase Using Alkylquinolines and Alkylquinazolines
InactiveUS20070004660A1Synergistic effectGrowth inhibitionBiocideGenetic material ingredientsKinase activityDisease
The invention is directed to a method of inhibiting FLT3 tyrosine kinase activity or expression or reducing FLT3 kinase activity or expression in a cell or a subject comprising the administration of a farnesyl transferase inhibitor and a FLT3 kinase inhibitor selected from alkylquinoline and alkylquinazoline compounds of Formula I′: where R1, R2, R3, Z, G, Q and X are as defined herein. Included within the present invention is both prophylactic and therapeutic methods for treating a subject at risk of (or susceptible to) developing a cell proliferative disorder or a disorder related to FLT3.
Owner:JANSSEN PHARMA NV
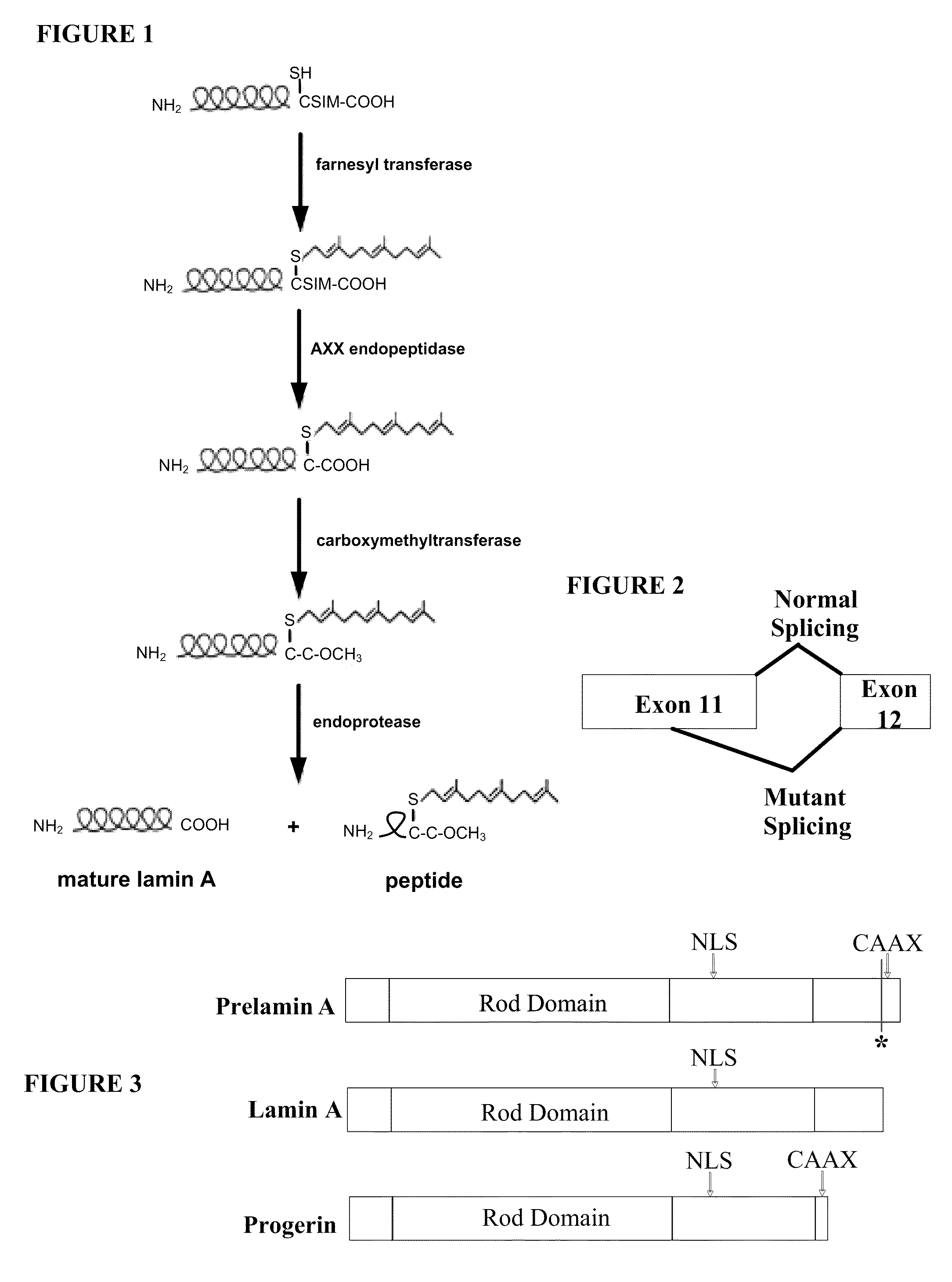
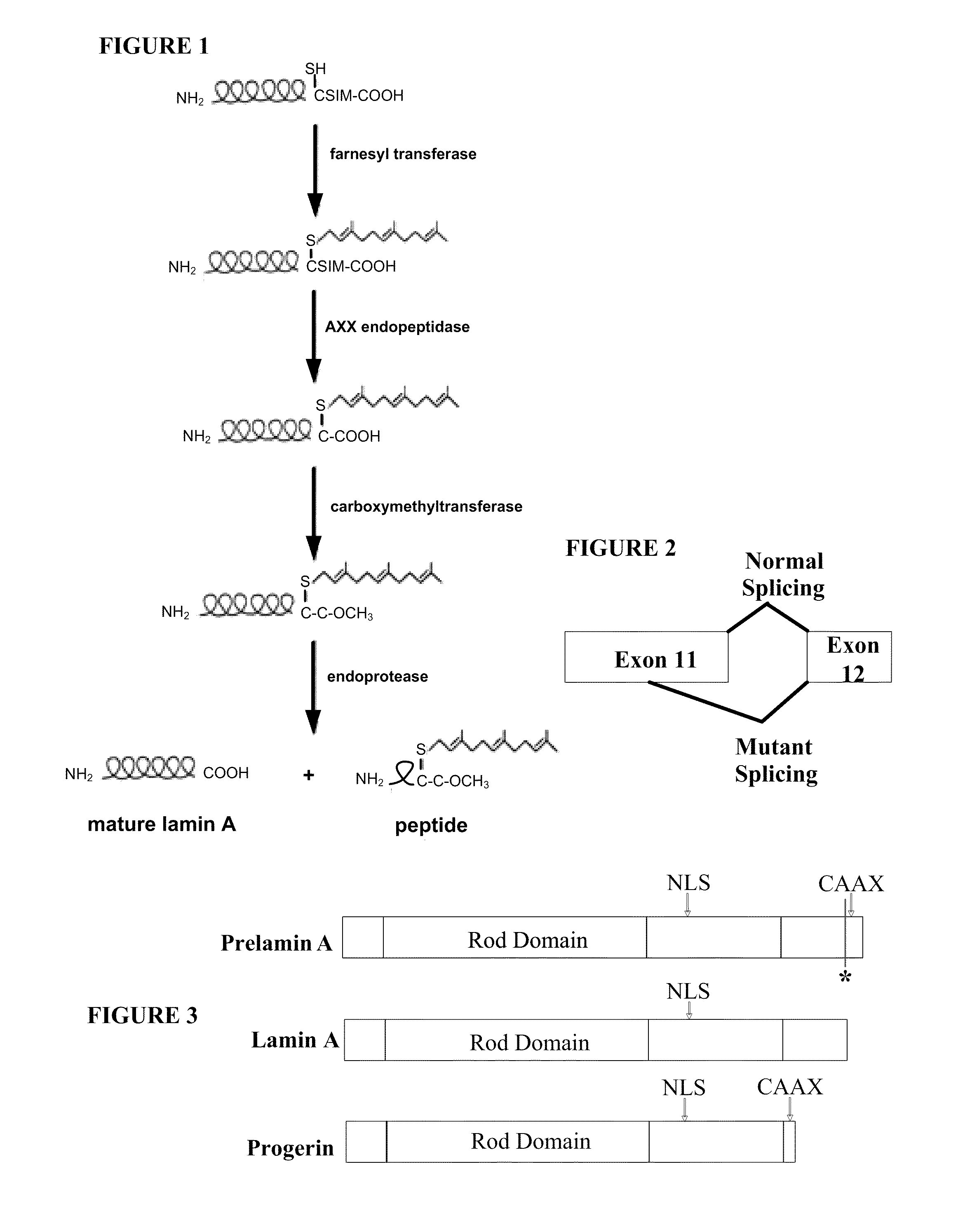
Farnesyltransferase inhibitors for treatment of laminopathies, cellular aging and atherosclerosis
ActiveUS7838531B2Additional elementDecrease in lamin A proteinCompound screeningBiocideNuclear membraneCellular Aging
Although it can be farnesylated, the mutant lamin A protein expressed in Hutchison Gilford Progeria Syndrome (HGPS) cannot be defarnesylated because the characteristic mutation causes deletion of a cleavage site necessary for binding the protease ZMPSTE24 and effecting defarnesylation. The result is an aberrant farnesylated protein (called “progerin”) that alters normal lamin A function as a dominant negative, as well as assuming its own aberrant function through its association with the nuclear membrane. The retention of farnesylation, and potentially other abnormal properties of progerin and other abnormal lamin gene protein products, produces disease. Farnesyltransferase inhibitors (FTIs) (both direct effectors and indirect inhibitors) will inhibit the formation of progerin, cause a decrease in lamin A protein, and / or an increase prelamin A protein. Decreasing the amount of aberrant protein improves cellular effects caused by and progerin expression. Similarly, treatment with FTIs should improve disease status in progeria and other laminopathies. In addition, elements of atherosclerosis and aging in non-laminopathy individuals will improve after treatment with farnesyltransferase inhibitors.
Owner:RGT UNIV OF MICHIGAN +3
Methods of treating cancer patients with farnesyl transferase inhibitors
ActiveCN107787373AOrganic active ingredientsMicrobiological testing/measurementTransferase inhibitorGenotyping
The present invention relates to the field of molecular biology and cancer biology. Specifically, the present invention relates to methods of treating a subject with a farnesyltransferase inhibitor (FTI) that include determining whether the subject is likely to be responsive to the FTI treatment based on genotyping and expression profiling of certain immunological genes and RAS mutation status inthe subject.
Owner:KURA ONCOLOGY
Farnesyltransferase inhibitors for treatment of laminopathies, cellular aging and atherosclerosis
InactiveUS20110027806A1Additional elementDecrease in lamin A proteinCompound screeningOrganic active ingredientsLaminopathyNuclear membrane
Although it can be farnesylated, the mutant lamin A protein expressed in Hutchinson Gilford Progeria Syndrome (HGPS) cannot be defarnesylated because the characteristic mutation causes deletion of a cleavage site necessary for binding the protease ZMPSTE24 and effecting defarnesylation. The result is an aberrant farnesylated protein (called “progerin”) that alters normal lamin A function as a dominant negative, as well as assuming its own aberrant function through its association with the nuclear membrane. The retention of farnesylation, and potentially other abnormal properties of progerin and other abnormal lamin gene protein products, produces disease. Farnesyltransferase inhibitors (FTIs) (both direct effectors and indirect inhibitors) will inhibit the formation of progerin, cause a decrease in lamin A protein, and / or an increase prelamin A protein. Decreasing the amount of aberrant protein improves cellular effects caused by and progerin expression. Similarly, treatment with FTIs should improve disease status in progeria and other laminopathies. In addition, elements of atherosclerosis and aging in non-laminopathy individuals will improve after treatment with farnesyltransferase inhibitors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +3
Treatment of mitochondrial disorders using a farnesyl transferase inhibitor
InactiveUS20110060005A1Avoid cell deathIncreased insulin secretionBiocideNervous disorderAntioxidantMITOCHONDRIAL PHERS
Methods and pharmaceutical compositions comprising a low dose of a farnesyl transferase inhibitor useful in the treatment of proteinopathies and mitochondrial disorders are provided. These low doses are below the doses used in oncological treatments for which these compounds were initially designed. The treatment includes administering to a subject an amount of a farnesyl transferase inhibitor, wherein the amount administered is sufficient to stimulate mitophagy in said subject. Treatments in accordance with the present invention may also include an acetylcholinesterase inhibitor, an activator of neurotrophic receptors, an NMDA anatagonist, an amyloid deposit inhibitor, an antipsychotic agent, an antidepressant, an anxiolytic, or an antioxidant.
Owner:ASTRAZENECA AB
Treatment of proteinopathies using a farnesyl transferase inhibitor
InactiveUS20100160372A1Reduces α-synuclein levelLess inclusionBiocideSenses disorderAntioxidantDepressant
Methods and pharmaceutical compositions comprising a low dose of a farnesyl transferase inhibitor useful in the treatment of proteinopathies are provided. These low doses are below the doses used in oncological treatments for which these compounds were initially designed. The treatment includes administering to a subject in need thereof a therapeutically effective amount of a farnesyl transferase inhibitor, wherein the amount is effective to inhibit the farnesylation of a non-Ras FTase substrate involved in the autophagy pathway without substantially affecting the farnesylation of Ras or other oncology related substrates. Treatments in accordance with the present invention may also include an acetylcholinesterase inhibitor, an activator of neurotrophic receptors, an NMDA antagonist, an amyloid deposit inhibitor, an antipsychotic agent, an antidepressant, an anxiolytic, or an antioxidant.
Owner:ASTRAZENECA AB
Synergistic modulation of FLT3 kinase using a farnesyl transferase inhibitor
The present invention relates to a method for inhibiting FLT3 tyrosine kinase activity or expression in a cell or a subject, or reducing FLT3 kinase activity or expression, comprising administering a farnesyl transferase inhibitor and an alkyl selected from formula (I') FLT3 kinase inhibitors of alkylquinolines and alkylquinazolines: where R 1 , R 2 , R 3 , Z, G, Q and X are as defined herein. The invention includes prophylactic and therapeutic methods for treating subjects at risk of (or susceptible to) developing a cell proliferative disorder or a FLT3-related disorder.
Owner:JANSSEN PHARMA NV
Methods of treating cancer patients with farnesyl transferase inhibitors
InactiveCN108371711AOrganic active ingredientsMicrobiological testing/measurementTransferase inhibitorGenotyping
The present invention relates to the field of molecular biology and cancer biology. Specifically, the present invention relates to methods of treating a subject with a farnesyltransferase inhibitor (FTI) that include determining whether the subject is likely to be responsive to the FTI treatment based on genotyping and expression profiling of certain immunological genes and RAS mutation status inthe subject.
Owner:KURA ONCOLOGY
Complex of ras-farnesyltransferase inhibitor, a cyclodextrin, and ethanol
A ras-farnesyltransferase inhibitor complex formed from a ras-farnesyltransferase inhibitor or a pharmaceutically acceptable salt thereof, a substituted cyclodextrin, and ethanol is provided. The complex has unexpectedly high aqueous solubility of the ras-farnesyltransferase inhibitor, improved dissolution, enhanced stability and is essentially free of particulate matter.
Owner:BRISTOL MYERS SQUIBB CO
Implant for treating or preventing an aneurysm
The invention relates to a medical product (1) which can be introduced into a blood vessel (2) and which has a medicament (3) for treating an aneurysm and arteriosclerotic diseases. The medicament (3) is a farnesyltransferase inhibitor.
Owner:雷扎·古特比
Aniline compound as farnesyltransferase inhibitor and application thereof
ActiveCN103787907BOrganic compound preparationCarboxylic acid amides preparationTransferase inhibitorFarnesyltransferase inhibitor
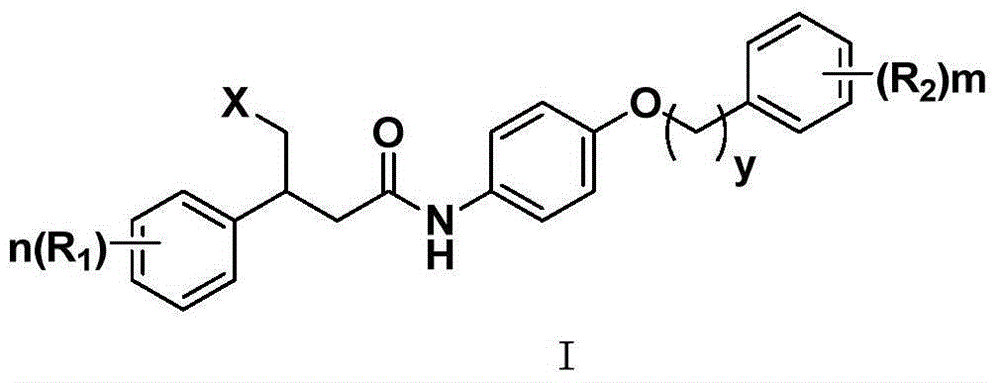
The invention provides an aniline compound represented by a formula I shown in a drawing or pharmaceutically acceptable salts thereof, wherein X is independently selected from COOH and SO2NH2, R1 and R2 are independently selected from H, C1-C3alkyl or alkoxy, CF3, F, Cl, Br, I, NH2 and NO2 respectively, m and n are respectively integers of 0-5, and y is 0 or 1. The compound and the pharmaceutically acceptable salts thereof, provided by the invention, can be used as farnesyltransferase inhibitors or can be used for preparing drugs for preventing or treating diseases related to farnesyltransferase, thereby having good medicine preparation prospects.
Owner:EAST CHINA UNIV OF SCI & TECH
Application of farnesyltransferase inhibitors in preparation of medicine for facilitated cholinergic nerve system
InactiveCN106667988AHigh activityHigh expressionOrganic active ingredientsNervous disorderIntraperitoneal routeCholinergic Nerves
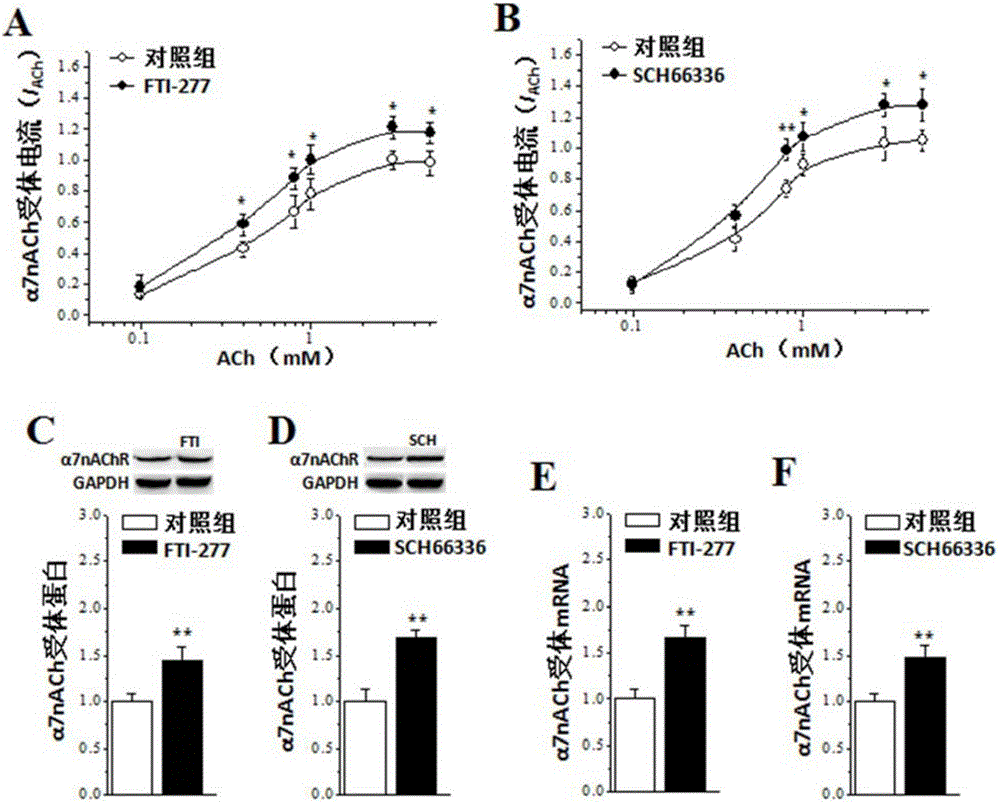
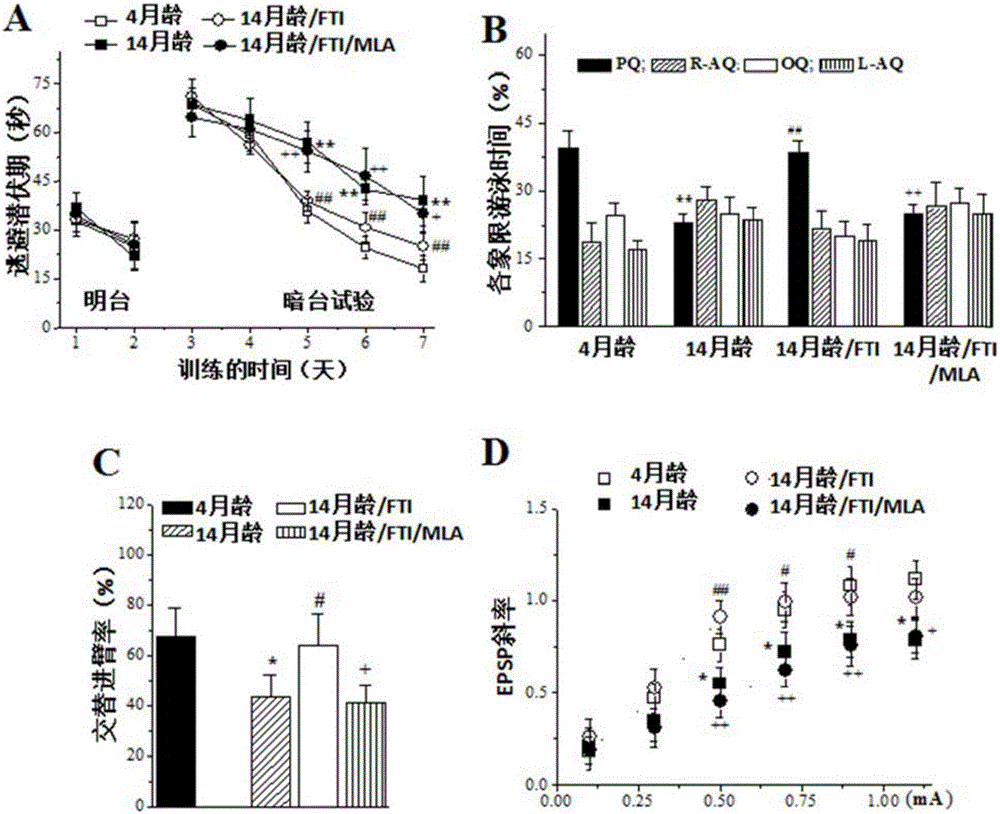
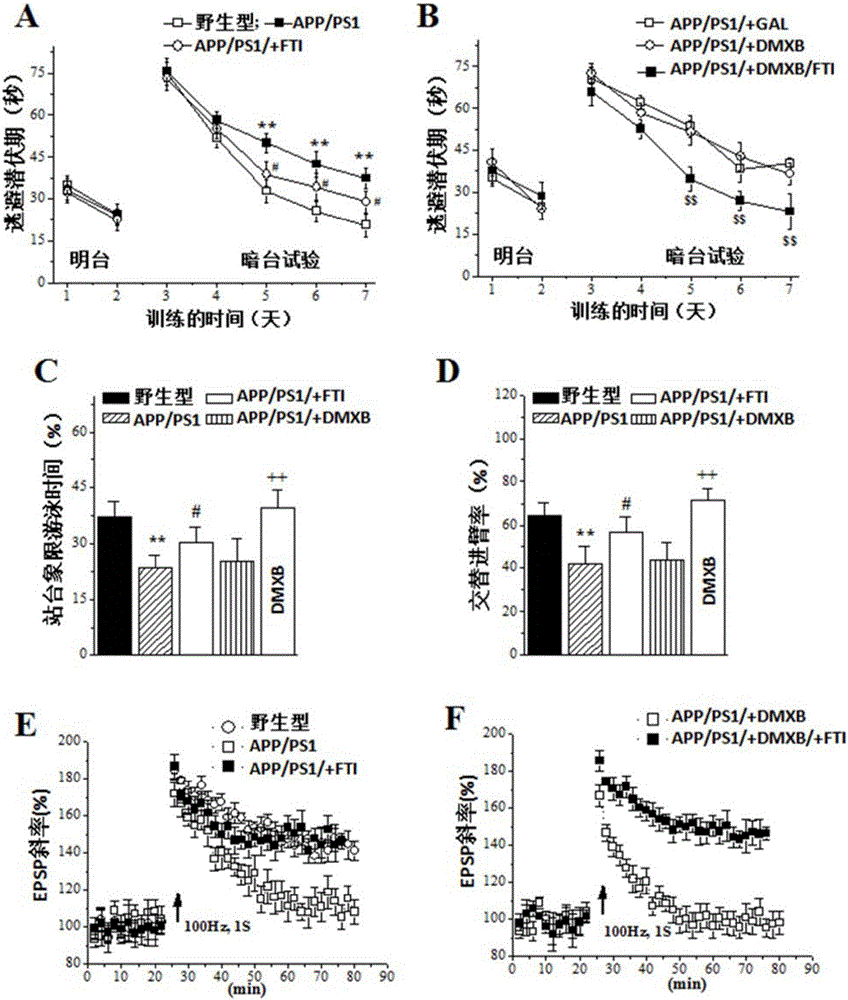
The invention relates to an application of farnesyltransferase inhibitors (FTI) in preparation of a medicine for a facilitated cholinergic nerve system. The farnesyltransferase inhibitors have an obvious improvement effect on senile dementia, Alzheimer's disease cognitive disorder, vascular dementia and Parkinson's disease cognitive function decline. Specifically speaking, in aging mice, Alzheimer's disease model mice, dementia model mice suffering cerebral ischemia and Parkinson's disease cognitive function decline model mice, the FTI, after subjected to treatment in the ways such as oral cavity injection, intraperitoneal injection, intracerebroventricular injection or cell incubation, can all dose-dependently (1) increase the activity and expression of a nerve cell acetylcholine receptor alpha7nACh, (2) improve dementia behavior, and (3) recover the synaptic transmission effect and long-term potentiation induction of the hippocampal brain.
Owner:NANJING MEDICAL UNIV
Methods of Treating Seizure Disorders
InactiveUS20130225671A1Reduces AGS severityReduce incidenceBiocideNervous disorderNeurofibromatosis type IFragile X chromosome
Seizure disorders are treated by Ras inhibitors. In an embodiment, the Ras inhibitor includes a farnesyl transferase inhibitor. In another embodiment, the farnesyl transferase inhibitor includes a 3-hydroxy-3-methylglutaryl-Coenzyme A reductase inhibitor. In another embodiment, the 3-hydroxy-3-methylglutaryl-Coenzyme A reductase inhibitor is not a Ras inhibitor. Subjects having fragile X syndrome, autism, Angelman syndrome, Costello syndrome, cardio facio cutaneous syndrome, neurofibromatosis type I, Noonan syndrome and Coffin-Lowry syndrome that have seizure disorders can be treated.
Owner:MASSACHUSETTS INST OF TECH
Method for treating cancer having resistance to kinase inhibitors
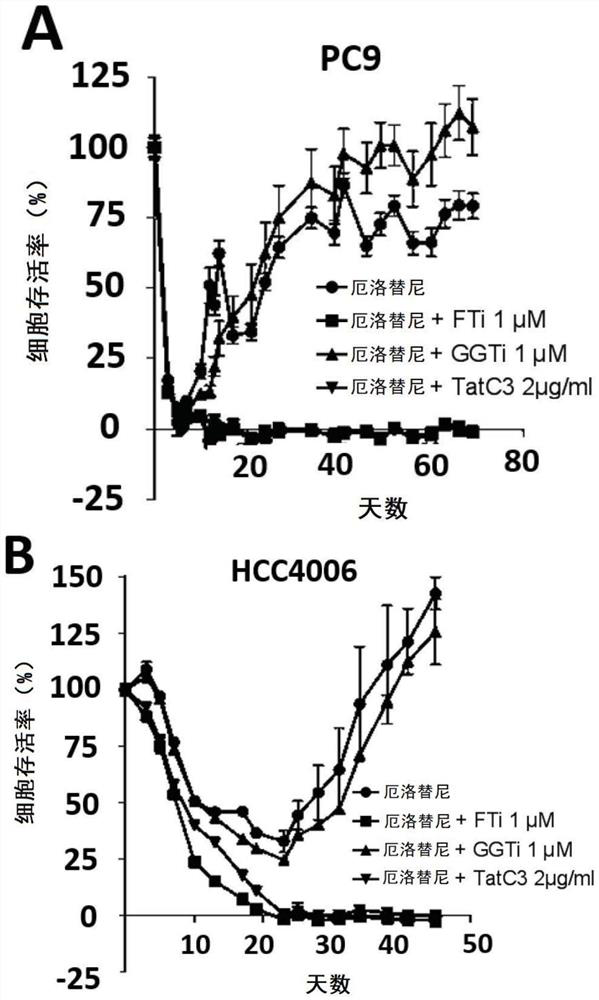
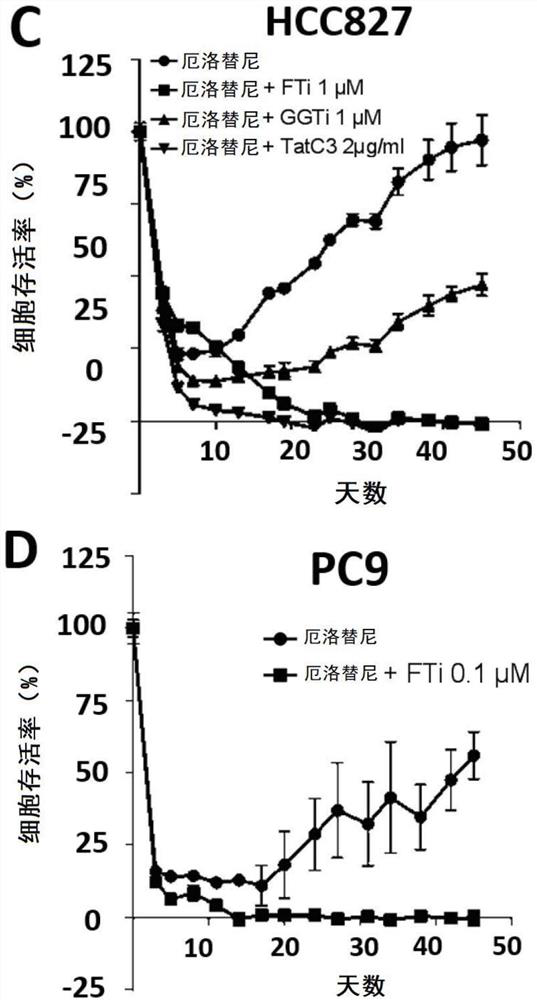
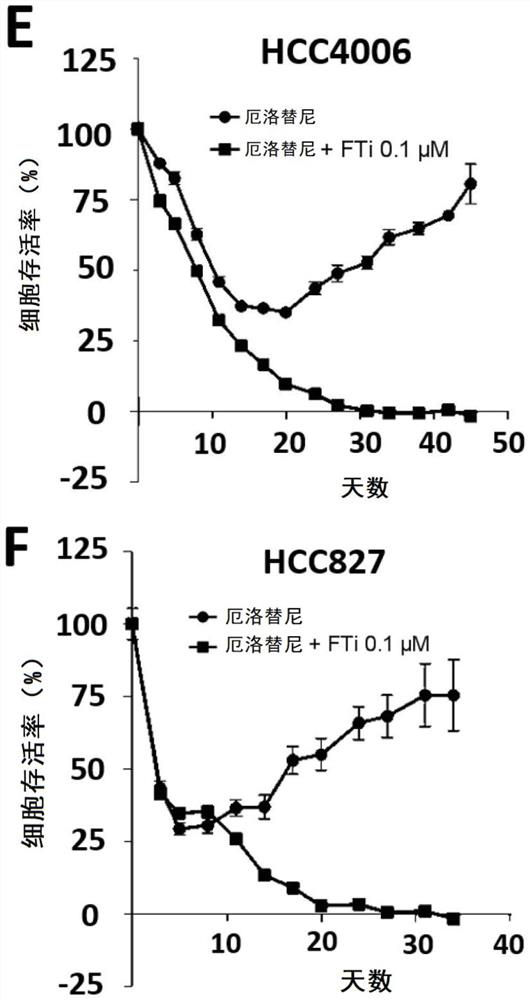
Resistance to kinase inhibitors is the biggest disorder of effective treatment for cancer patients. The recent research shows that the occurrence of the drug resistance may not only be generally considered by people to be explained by the existing drug-resistant subcloning drug selection, but also be caused by the generation of a small amount of drug-resistant cells (DTC) and entering a slow circulation state through the cells, so that the drug resistance has a resistance effect on treatment at the beginning. Therefore, a novel promising method should be adopted for the DTCs to prevent secondary drug resistance to kinase inhibitors. At present, the inventor has proved that inhibition of farnesyl transferase (but not geranyl geranyl transferase) can prevent the drug resistance in different carcinogenic environments. Particularly, the inventor determines the curative effect of a farnesyl transferase inhibitor (namely tipifarnib) and erlotinib which are jointly used in several EGFR (epidermal growth factor receptor) mutant cell lines in vitro. It is shown that the combination can effectively eliminate all drug-resistant cells and completely prevent the occurrence of drug-resistant cloning. It is interesting that similar results are also observed in other oncogenic models, such as ALK-translocated lung cancer cells or BRAF-mutated melanoma cells. Therefore, the present invention relates to the use of farnesyltransferase inhibitors in the treatment of kinase inhibitor resistant cancers.
Owner:INSERM法国国家健康医学研究院 +2
Methods of treating cancer with farnesyltransferase inhibitors
The present invention relates to the field of cancer therapy. Specifically, provided are methods of treating cancer in a subject having clinical signs of bone marrow homing of myeloid cells, includingneutropenia, isolated neutropenia, a low percentage of peripheral blood blasts with or without a high percentage of bone marrow blasts, and / or a low ratio of peripheral blood blasts to bone marrow blasts, with a farnesyltransferase inhibitor (FTI) that include determining whether the subject is likely to be responsive to the FTI treatment based on hematological characteristics indicating bone marrow homing of myeloid cells.
Owner:KURA ONCOLOGY
Methods of treating cancer with farnesyltransferase inhibitors
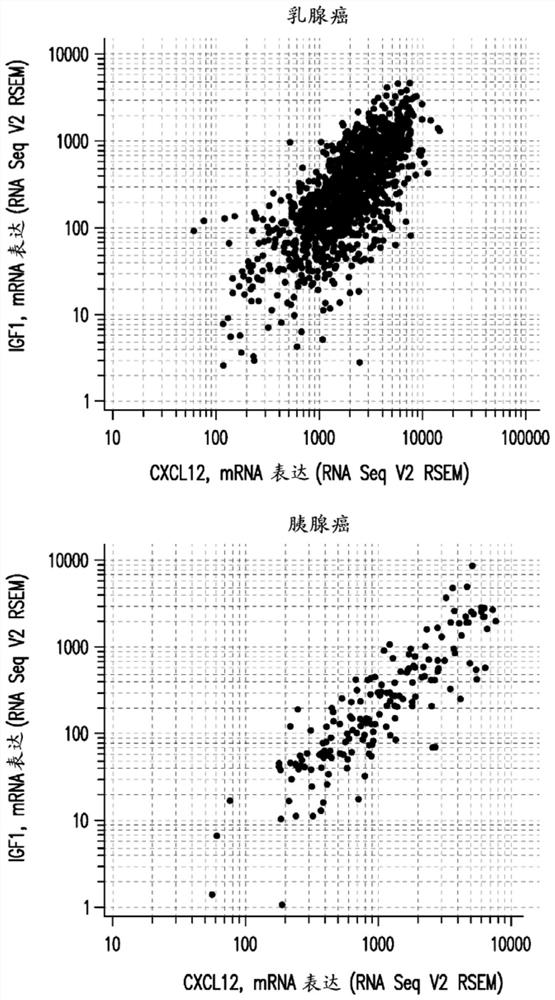
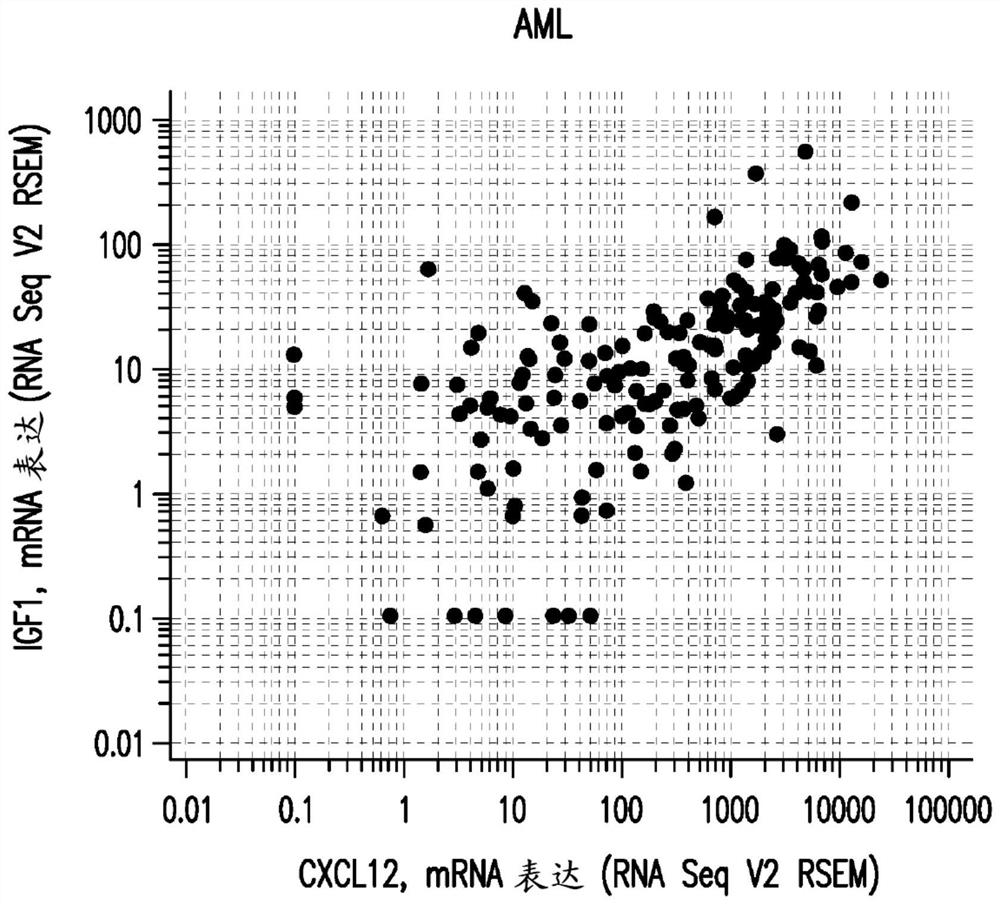
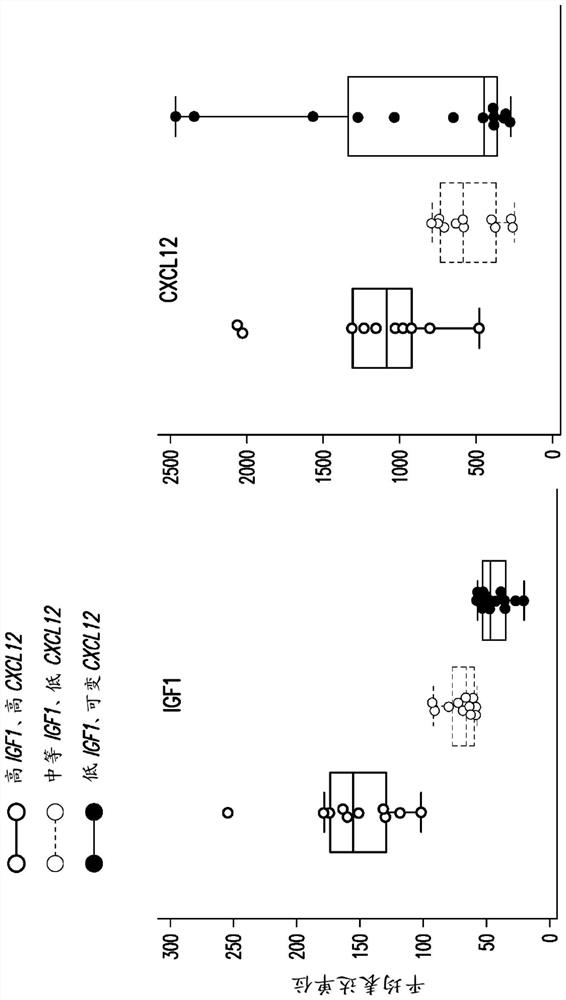
The present invention relates to the field of cancer therapy. Specifically, provided are methods of treating cancer in a subject with a farnesyltransferase inhibitor (FTI) that include determining whether the subject is likely to be responsive to the FTI treatment based on the activity of the CXCL12 / CXCR4 pathway, and / or the activity of the IGF1R pathway. Provided herein are also combination therapy of cancer treatment using FTI and either an IGF1R inhibitor or a CXCR4 antagonist.
Owner:KURA ONCOLOGY
Farnesyl transferase inhibitors and uses thereof
Disclosed herein are novel compounds and uses thereof. The present compounds may suppress the activity of farnesyl transferase and thus, may act as modulators of immune cells; therefor, they are useful for the development of a medicament for treating diseases that are associated with or caused by excessive levels of farnesyl transferase or immune response. Also disclosed herein are pharmaceutical compositions containing the present compounds.
Owner:MACKAY MEMORIAL HOSPITAL
Benzyl-substituted aniline compounds and their applications
ActiveCN103787902BCarboxylic acid nitrile preparationOrganic compound preparationArylTransferase inhibitor
The invention provides a benzyl-substituted aniline compound represented by a formula I shown in a drawing or pharmaceutically acceptable salts thereof, wherein R1 is independently selected from -COOH, -CONH2, H, -COOR5 (R5 is methyl or ethyl), -CN, -OH and -NH2COCH3, R2 and R3 are independently selected from C6-C10 aryl, C3-C6 cyclane and C1-C3 saturated alkyl or unsaturated alkyl respectively, and R4 is independently selected from -H, ethyl and acetyl. The compound and the pharmaceutically acceptable salts thereof, provided by the invention, can be used as farnesyltransferase inhibitors or can be used for preparing drugs for preventing or treating diseases related to farnesyltransferase, thereby having good medicine preparation prospects.
Owner:EAST CHINA UNIV OF SCI & TECH
Treatment of H-Ras-driven tumors
ActiveUS11331312B2Reduce the burden onOrganic active ingredientsMicrobiological testing/measurementTransferase inhibitorMEK inhibitor
The present disclosure describes a compositions and methods for treatment of Hras-driven cancers. Administration of a farnesyltransferase inhibitor, for example, tipifarnib, alone or in combination with a MEK inhibitor can reduce tumor size and tumor growth in cancers such as poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC).
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Method for Determining the Response of Acute Myeloid Leukemia to Farnesyltransferase Inhibitor Therapy
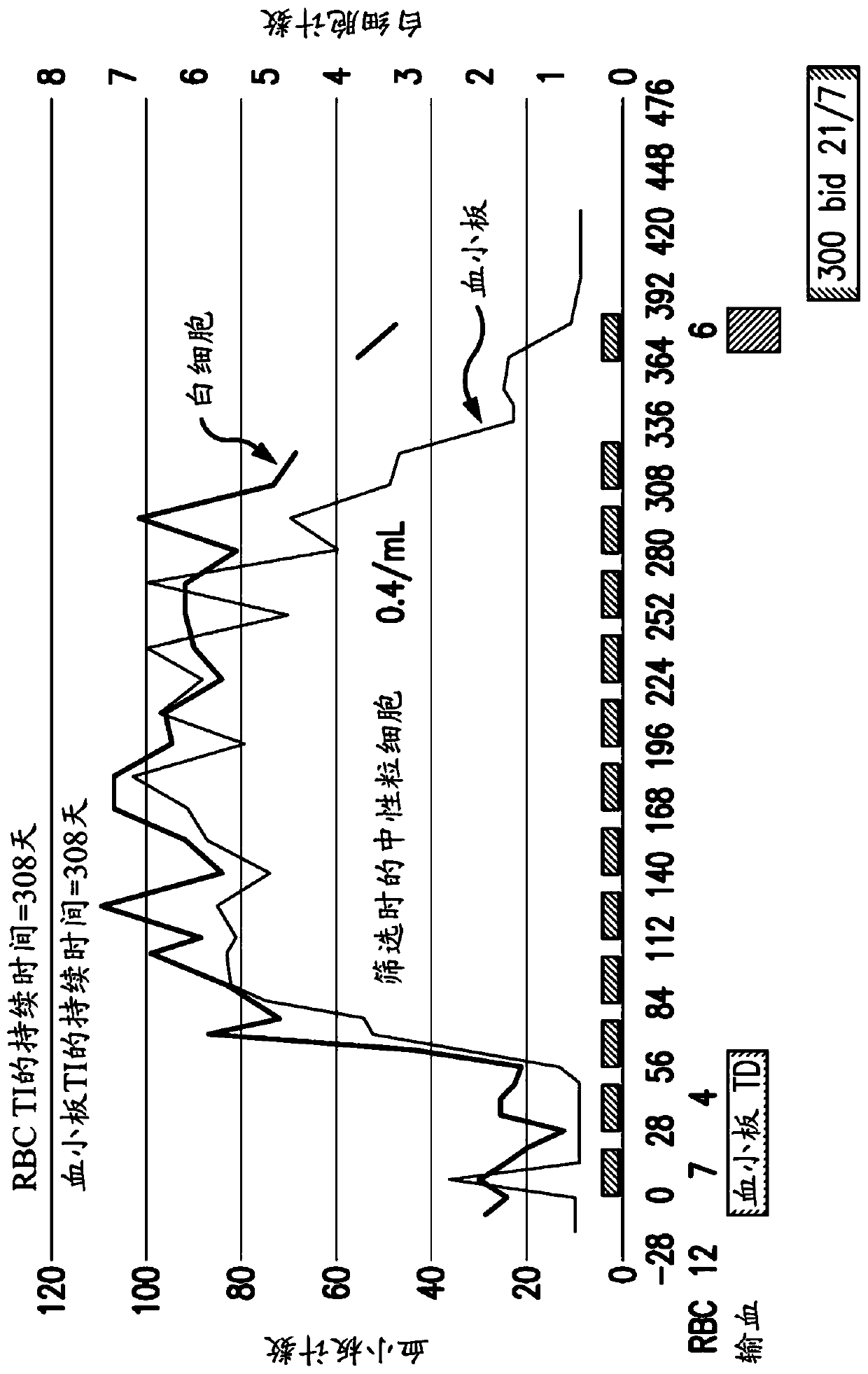
The method of the present invention can rapidly identify with expected accuracy those that are likely to respond to farnesyl transferase inhibitors and etoposide, teniposide, tamoxifen, sorafenib, paclitaxel, temozolomide, topotecan AML patients, including elderly AML patients, who respond to a combination of one or more of , trastuzumab, and cisplatin. In one embodiment, the improvement includes using whole blood instead of the usual bone marrow sample, making the test more accurate, faster, less intrusive, less expensive and less painful. The method includes evaluating the expression ratio of the two genes (RASGRP1:APTX) in combination with corresponding thresholds, which provides sufficient precision for the prediction of response to combination therapy. A preferred embodiment is the combination therapy of tipifarnib (R1 15777,) combined with etoposide. Further, elderly AML patients identified as likely to respond to the combination of tipifarnib and etoposide had full recovery rates comparable to younger patients under optimal treatment conditions.
Owner:JANSSEN PHARMA NV
Treatment of neurodegenerative conditions by disruption of Rhes
ActiveUS10864206B2Increase the gapNervous disorderKetone active ingredientsNeurophysinsNeuro-degenerative disease
The invention is directed to the treatment of tauopathic neurodegenerative conditions and the underlying processes thereof. Based on the discovery that Rhes acts as a negative regulator of normal tau clearance, the compositions and methods of the invention may be applied to inhibit Rhes and reduce pathogenic tau aggregation. Rhes may be disrupted by disrupting RAS2D gene expression or reducing the abundance of Rhes protein in neurons. Additionally, post-translational modifications of Rhes may be targeted, including the disruption of Rhes farnesylation by the administration of farnesyltransferase inhibitors.
Owner:RGT UNIV OF CALIFORNIA
Methods of treating squamous cell carcinomas with farnesyltransferase inhibitors
PendingCN113795253AGrowth inhibitionImprove responseHydroxy compound active ingredientsInorganic active ingredientsSquamous CarcinomasSquamous Cell Cancers
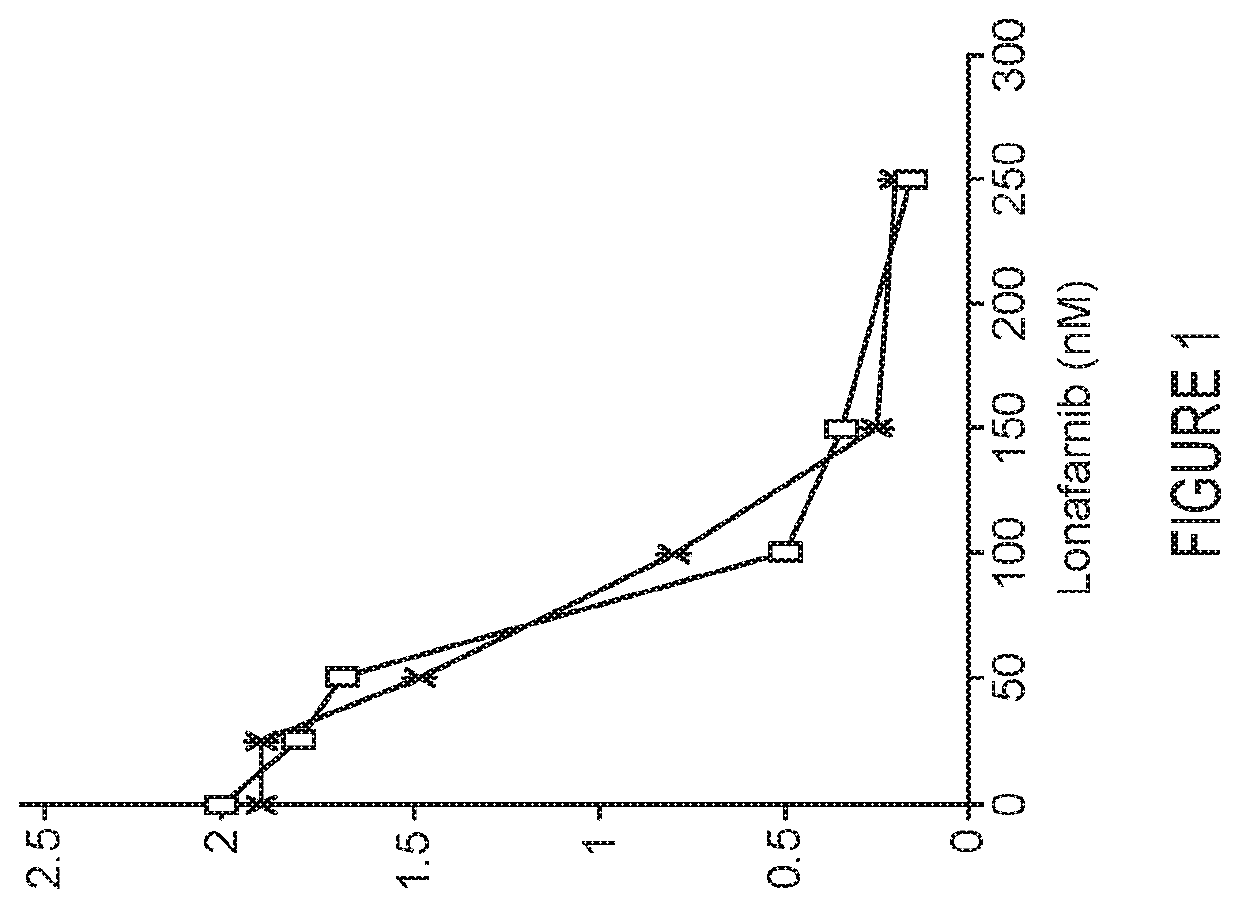
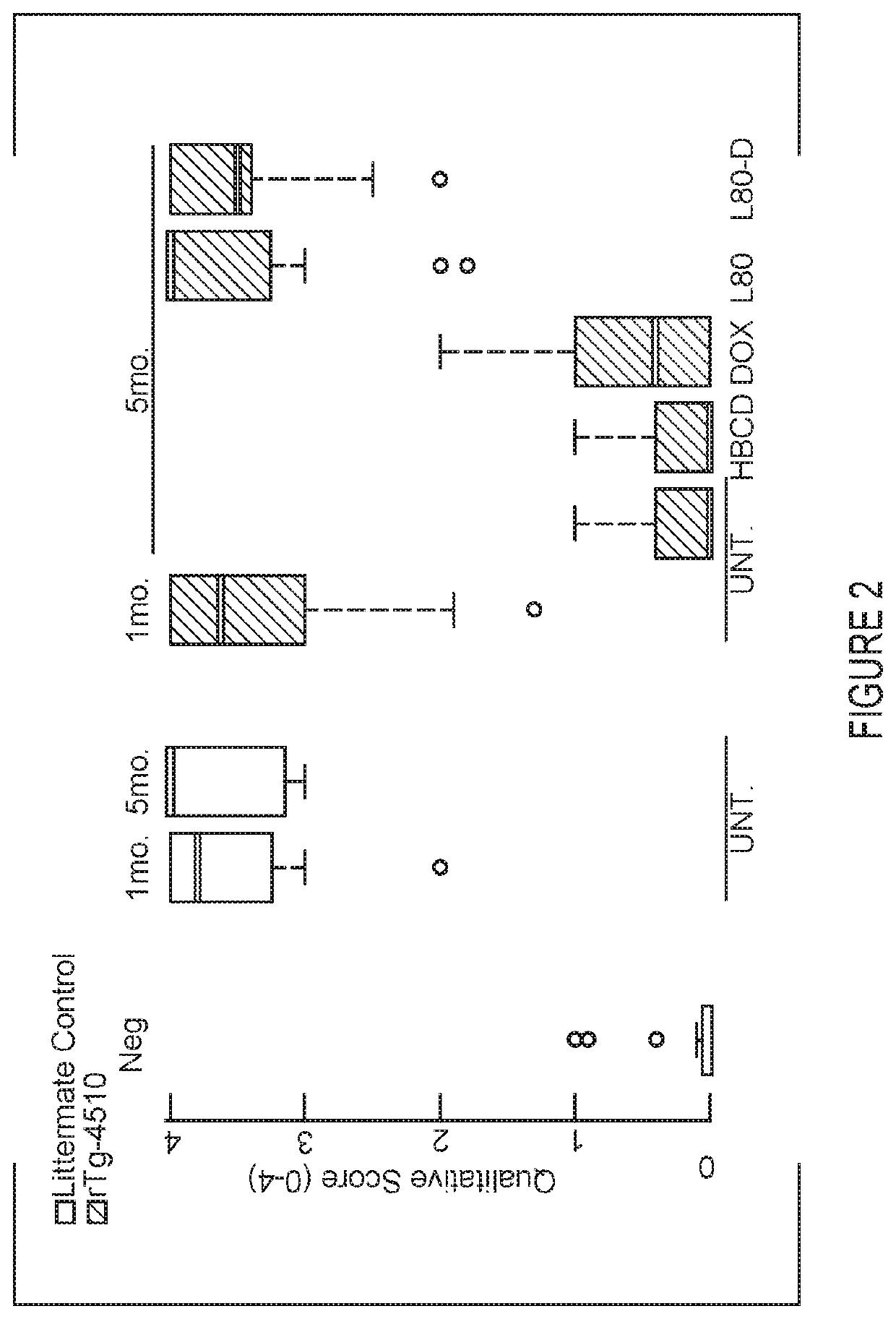
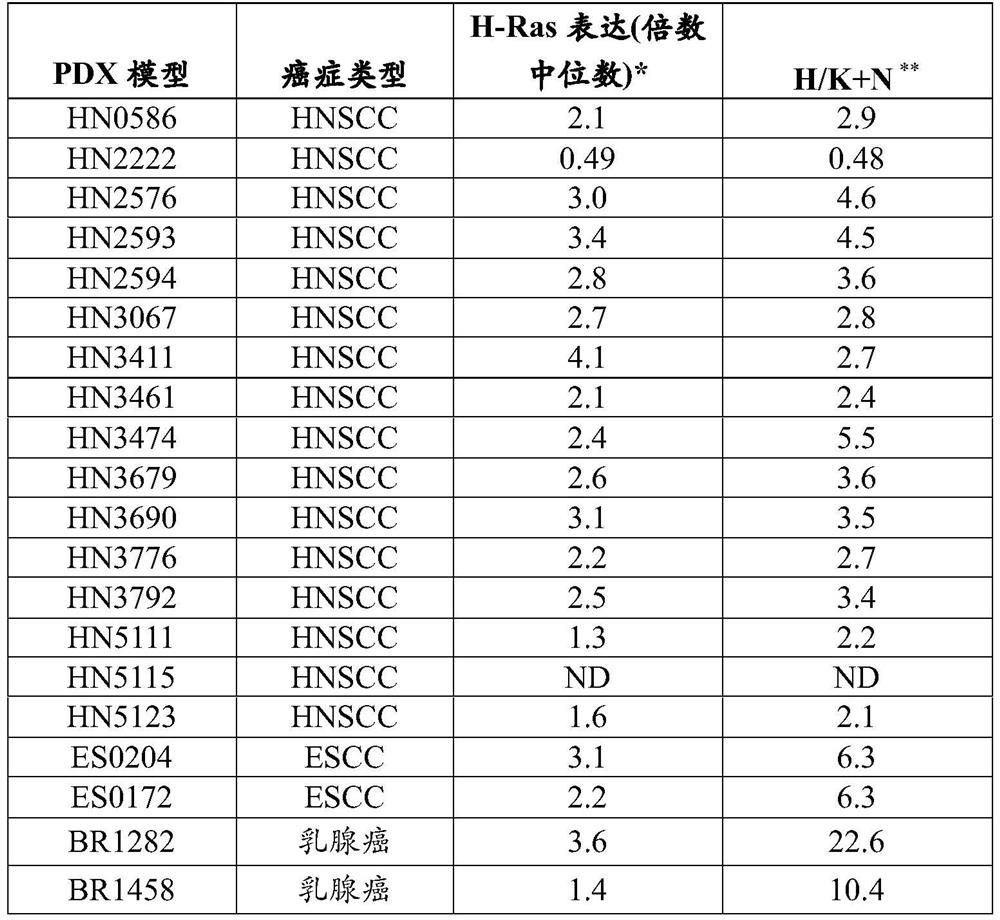
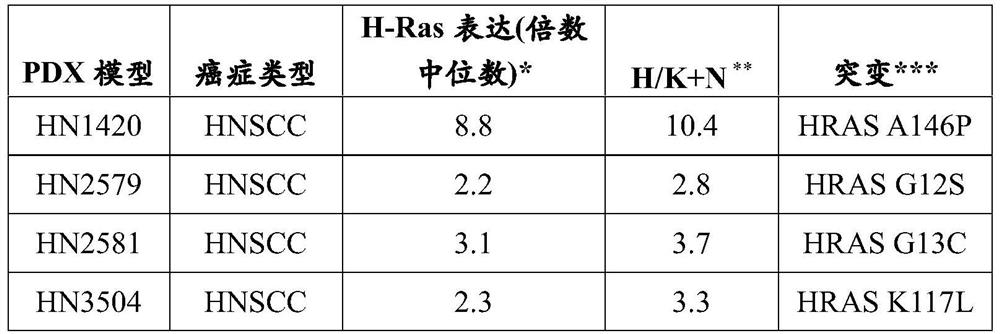
The present invention relates to the field of cancer therapy. Specifically, provided are methods of treating Squamous Cell Carcinoma in a subject with a farnesyltransferase inhibitor (FTI) that include determining whether the subject is likely to be responsive to the FTI treatment based on the expression level of H-Ras.
Owner:KURA ONCOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com