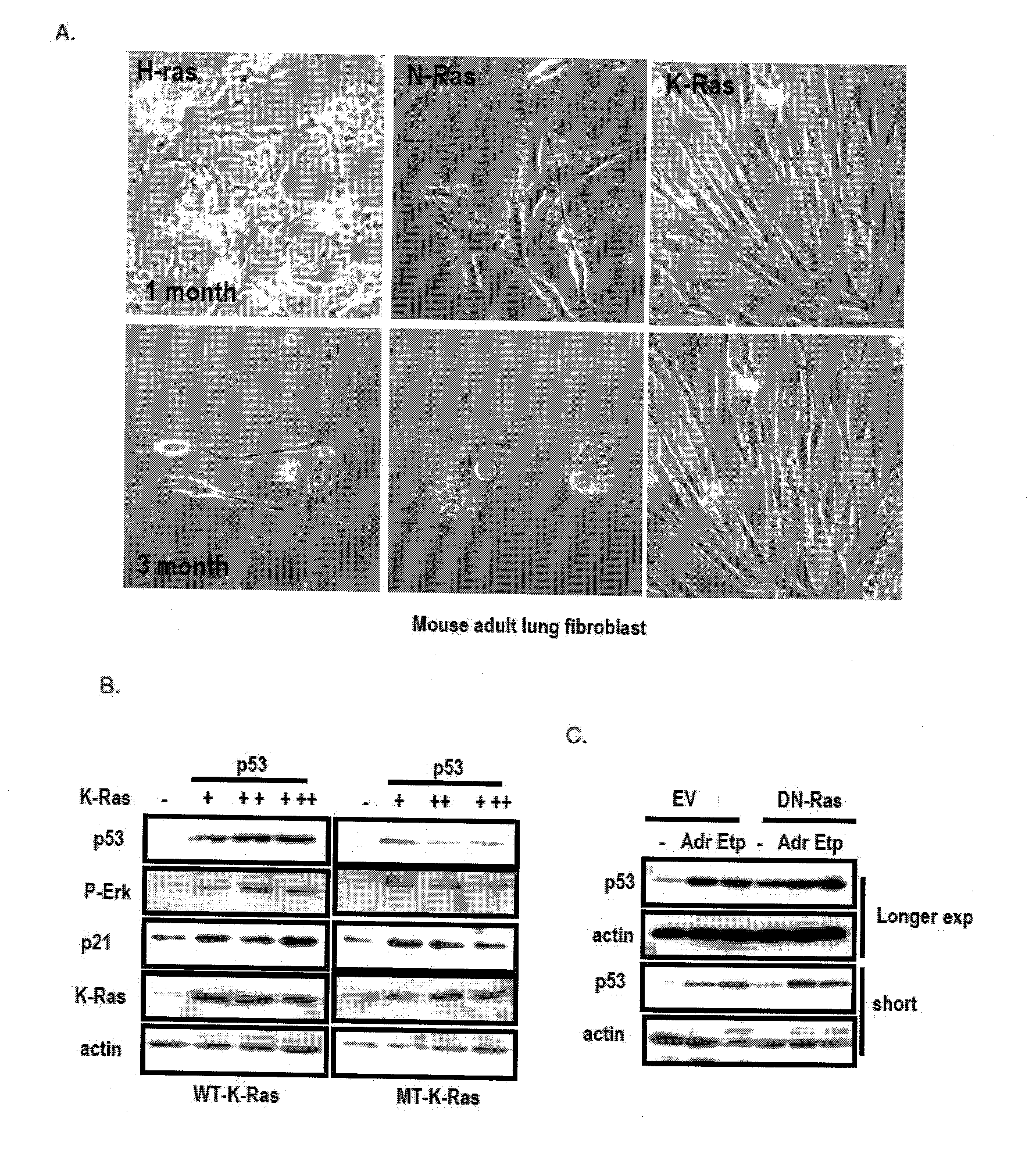
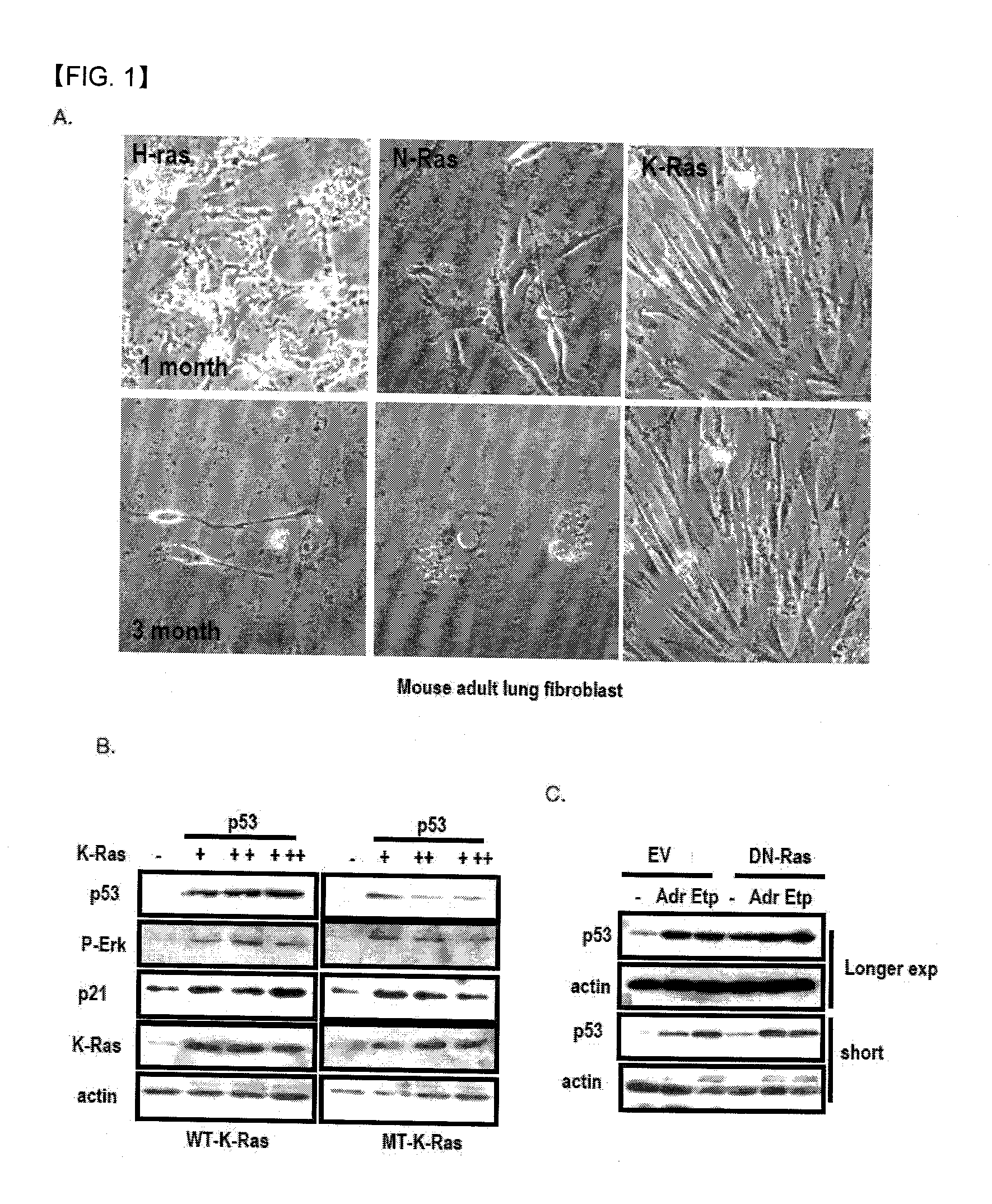
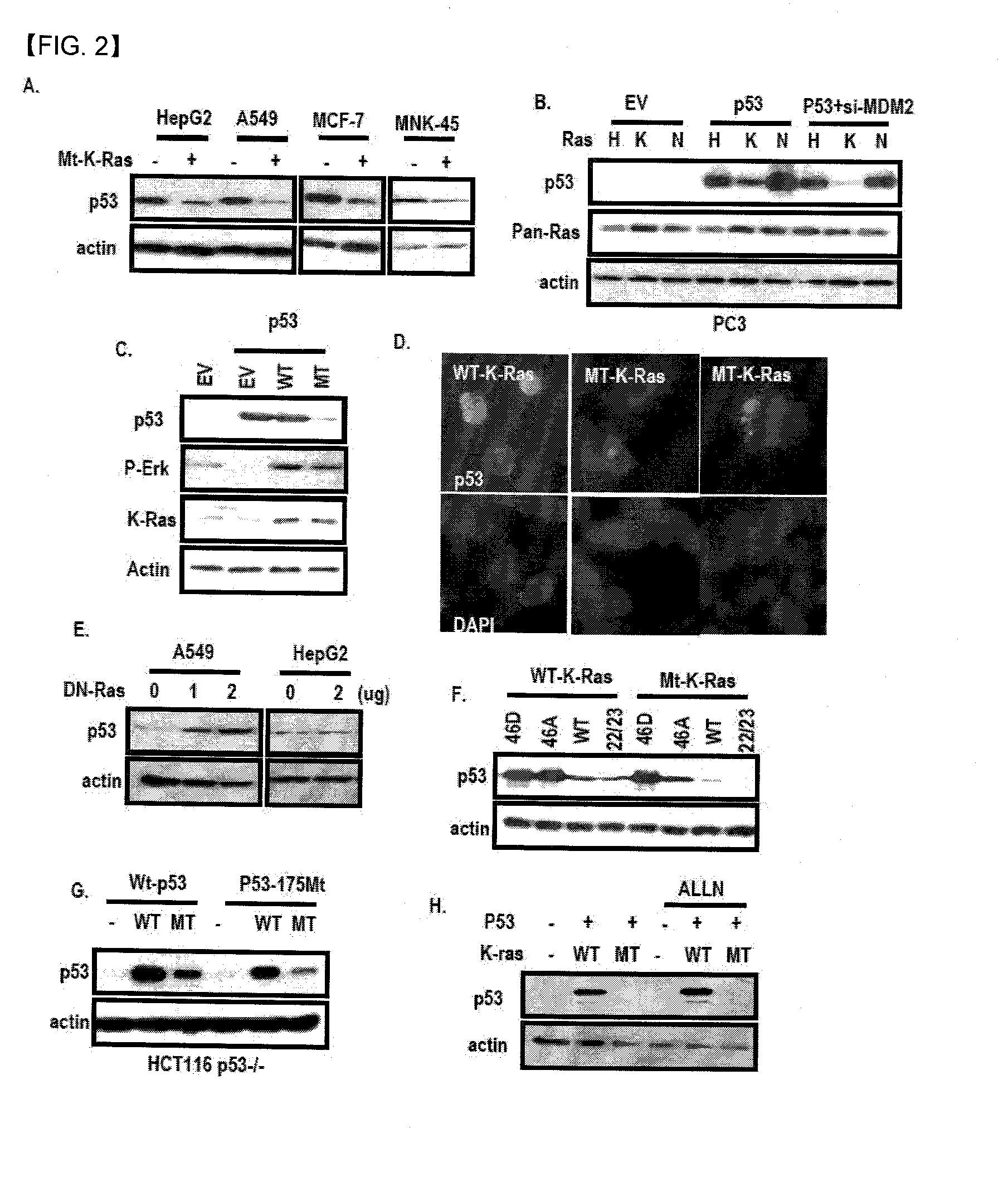
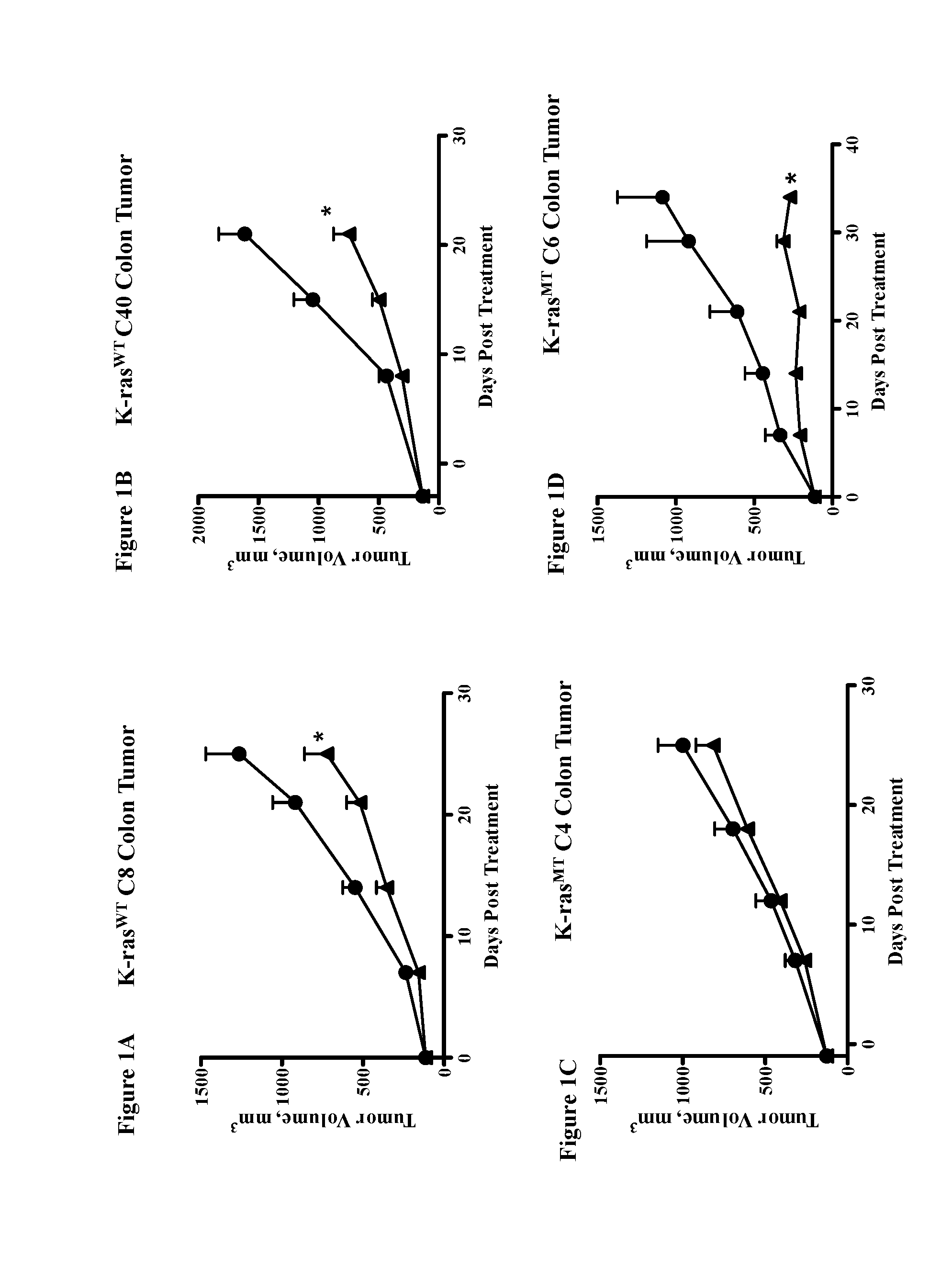
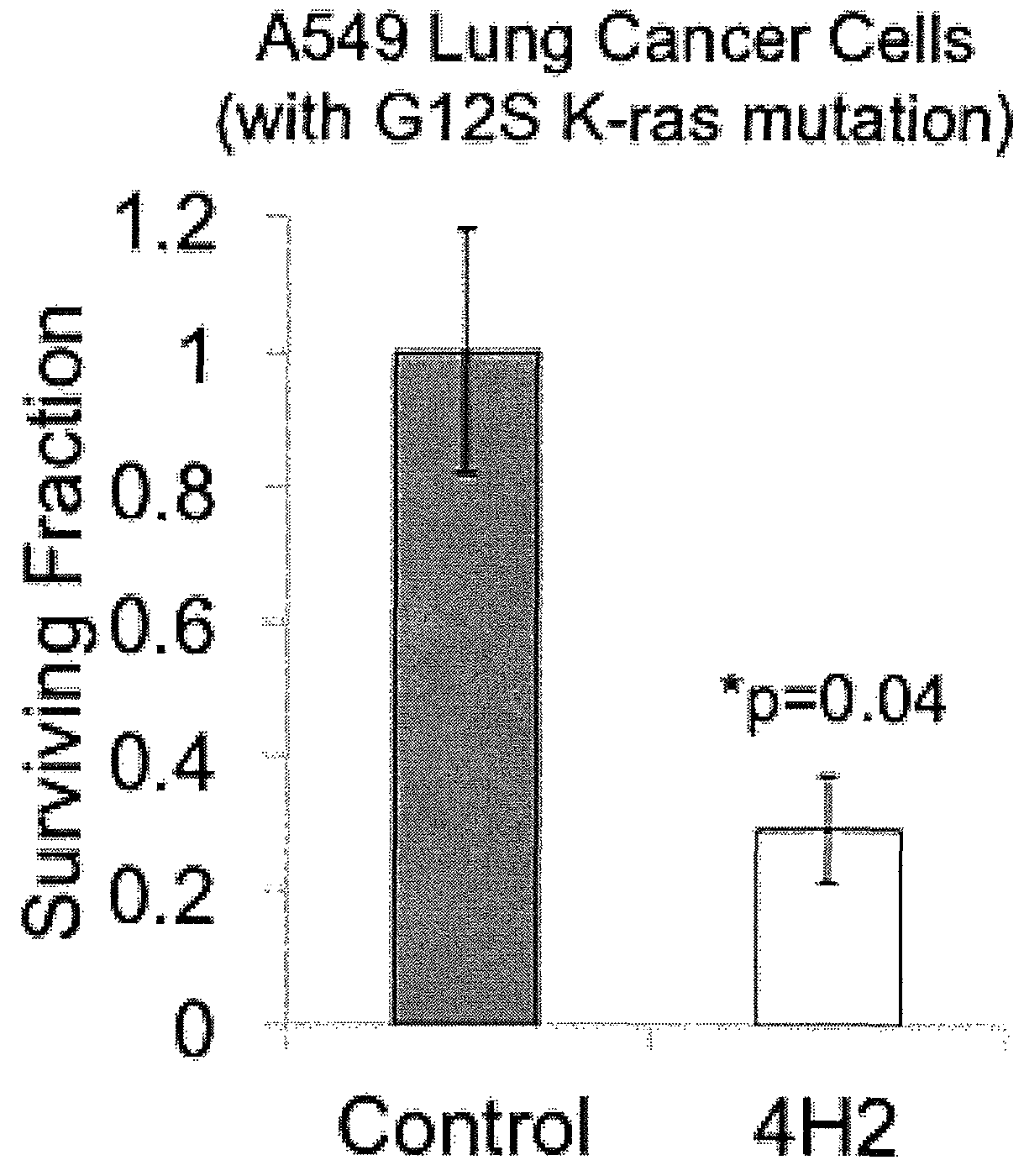
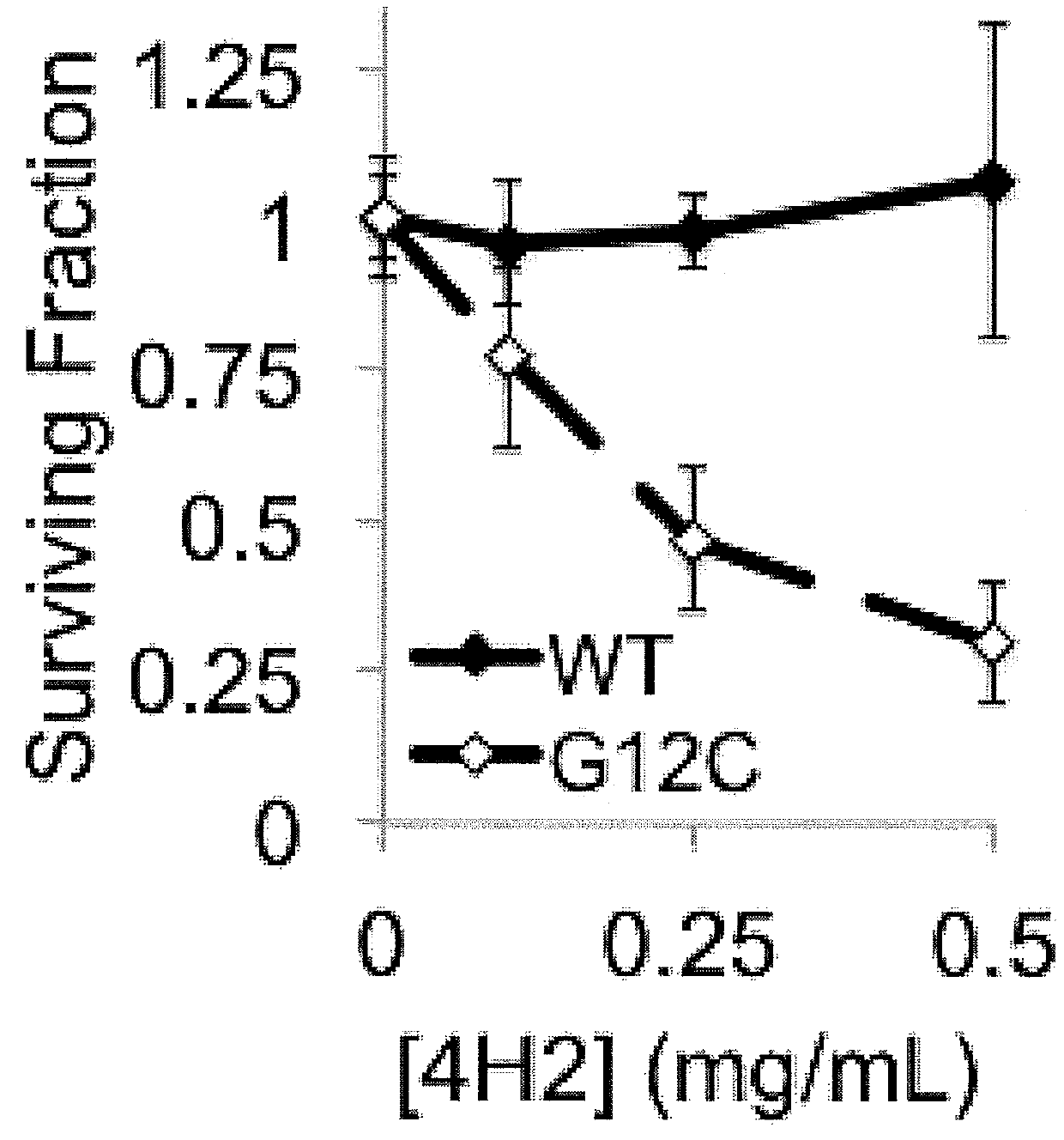
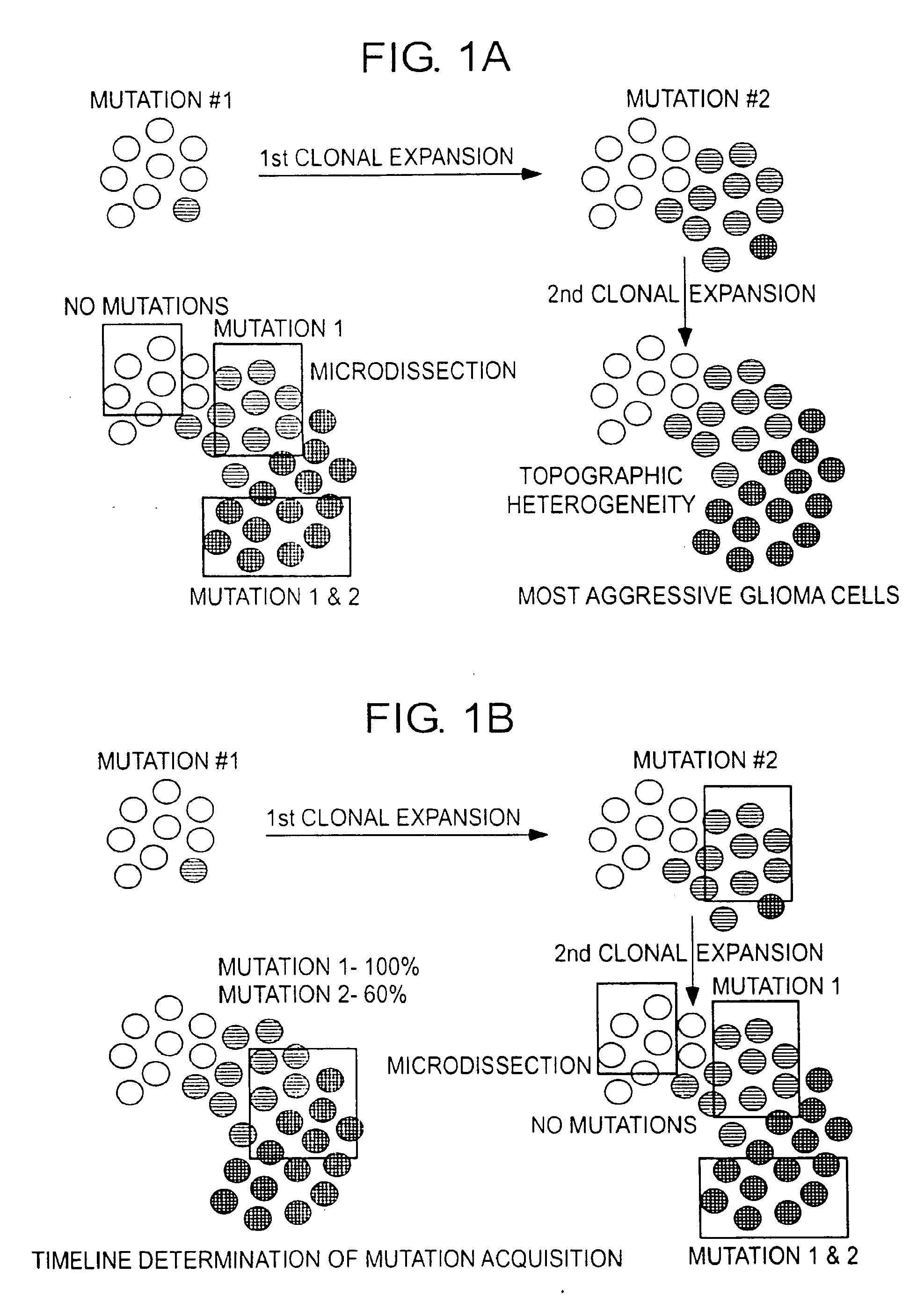
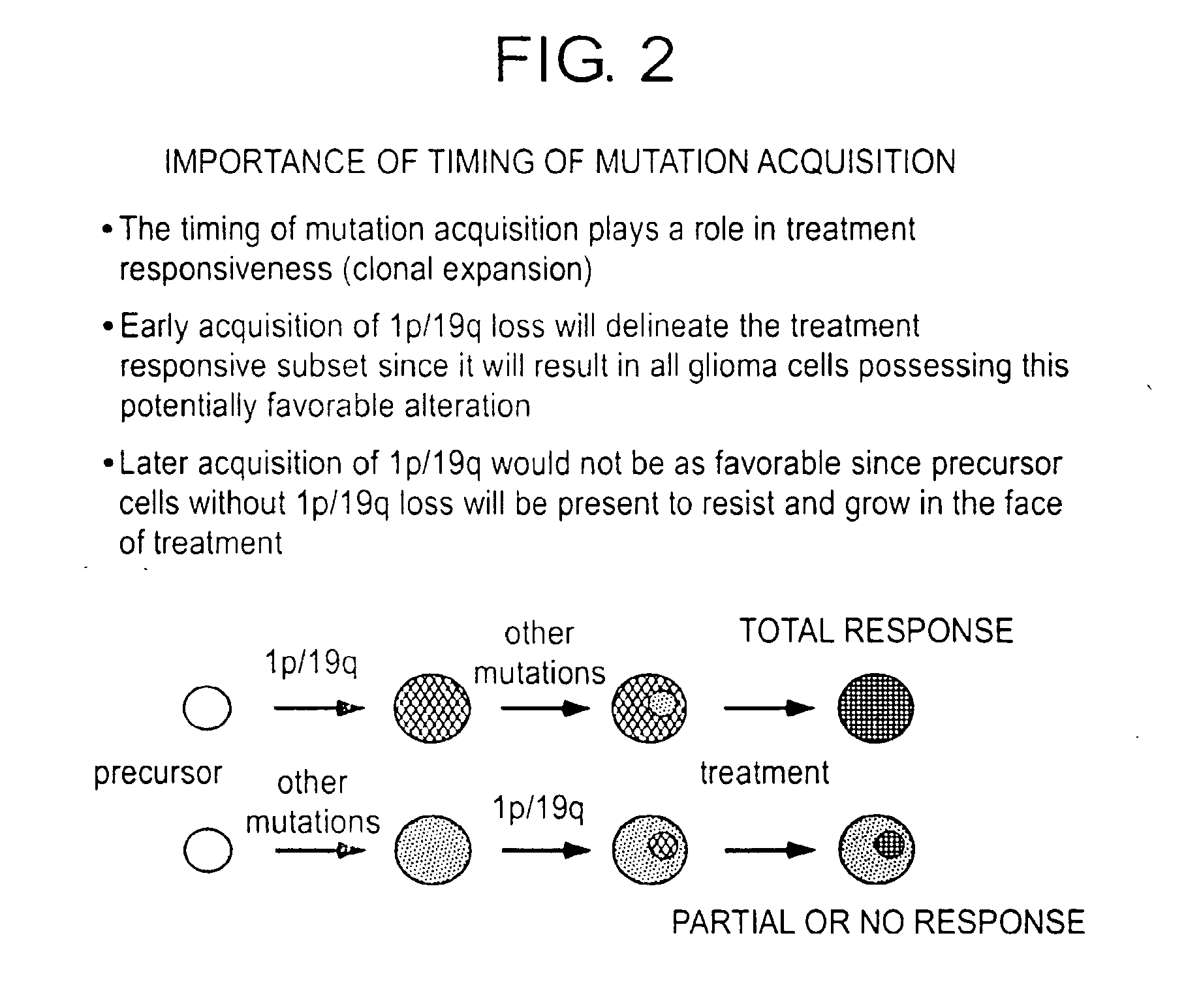
Patents
Literature
49 results about "RAS Mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ras mutation and compositions and methods related thereto
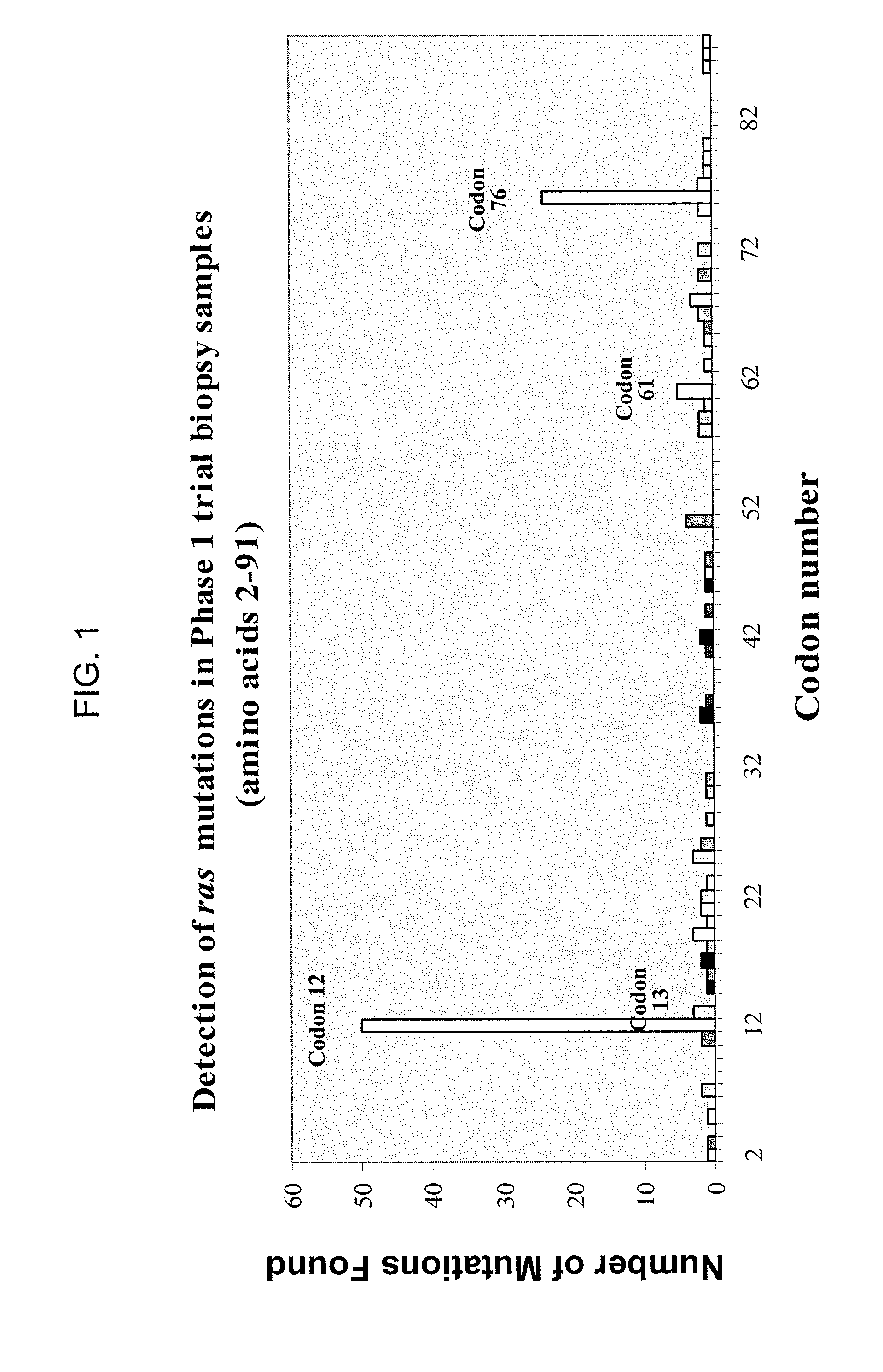
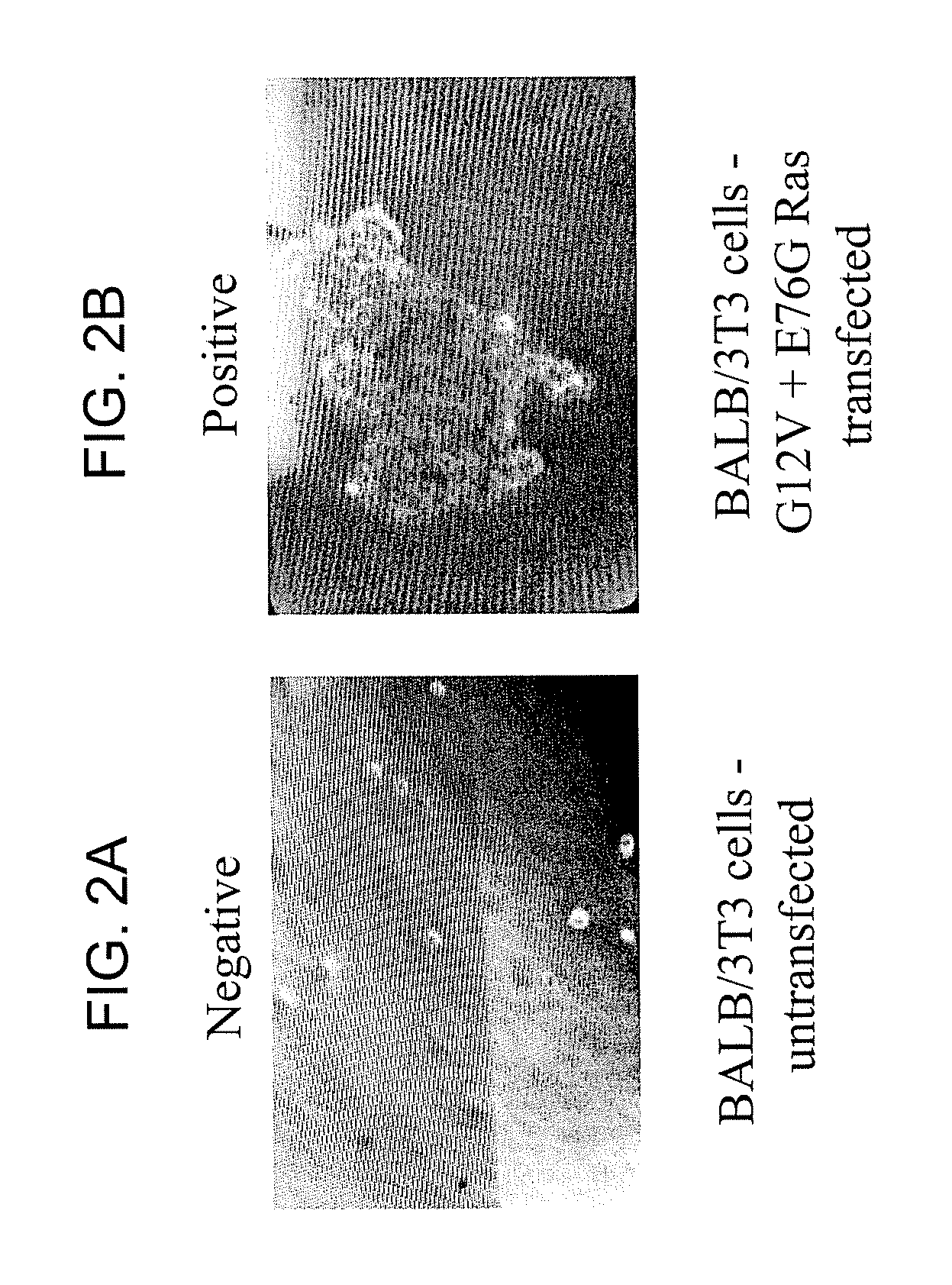
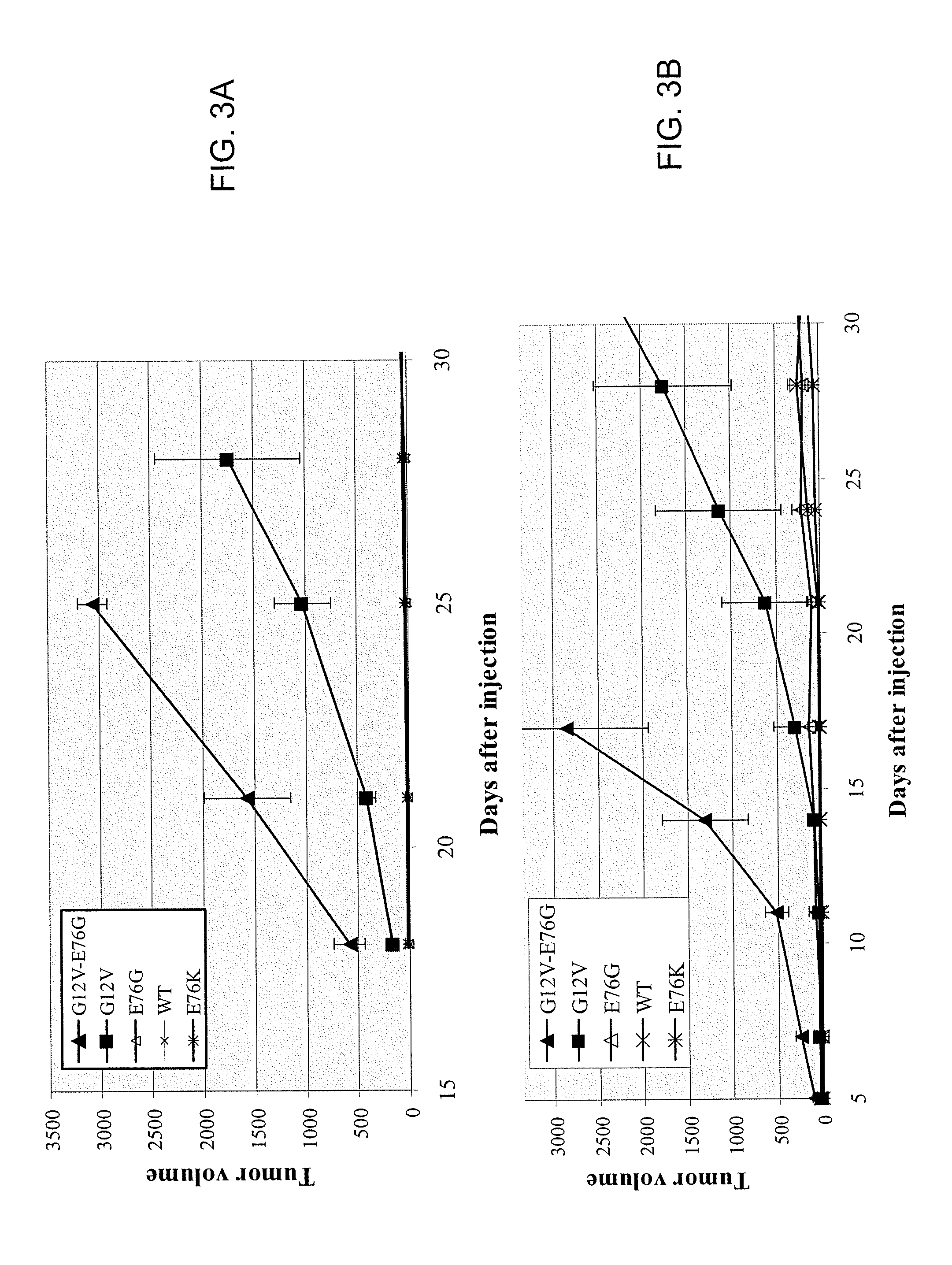
Disclosed are newly discovered Ras mutations and combinations of mutations, proteins and peptides and fusion proteins containing these mutations, nucleic acid molecules encoding such proteins, peptides, and fusion proteins, and a variety of tools and diagnostic, therapeutic, and screening methods associated with the use of such mutations.
Owner:GLOBE IMMUNE INC
Methods of determining acute myeloid leukemia response to treatment with farnesyltransferase
InactiveUS7932036B1Improve accuracyHigh response rateSugar derivativesMicrobiological testing/measurementNewly diagnosedClinical study
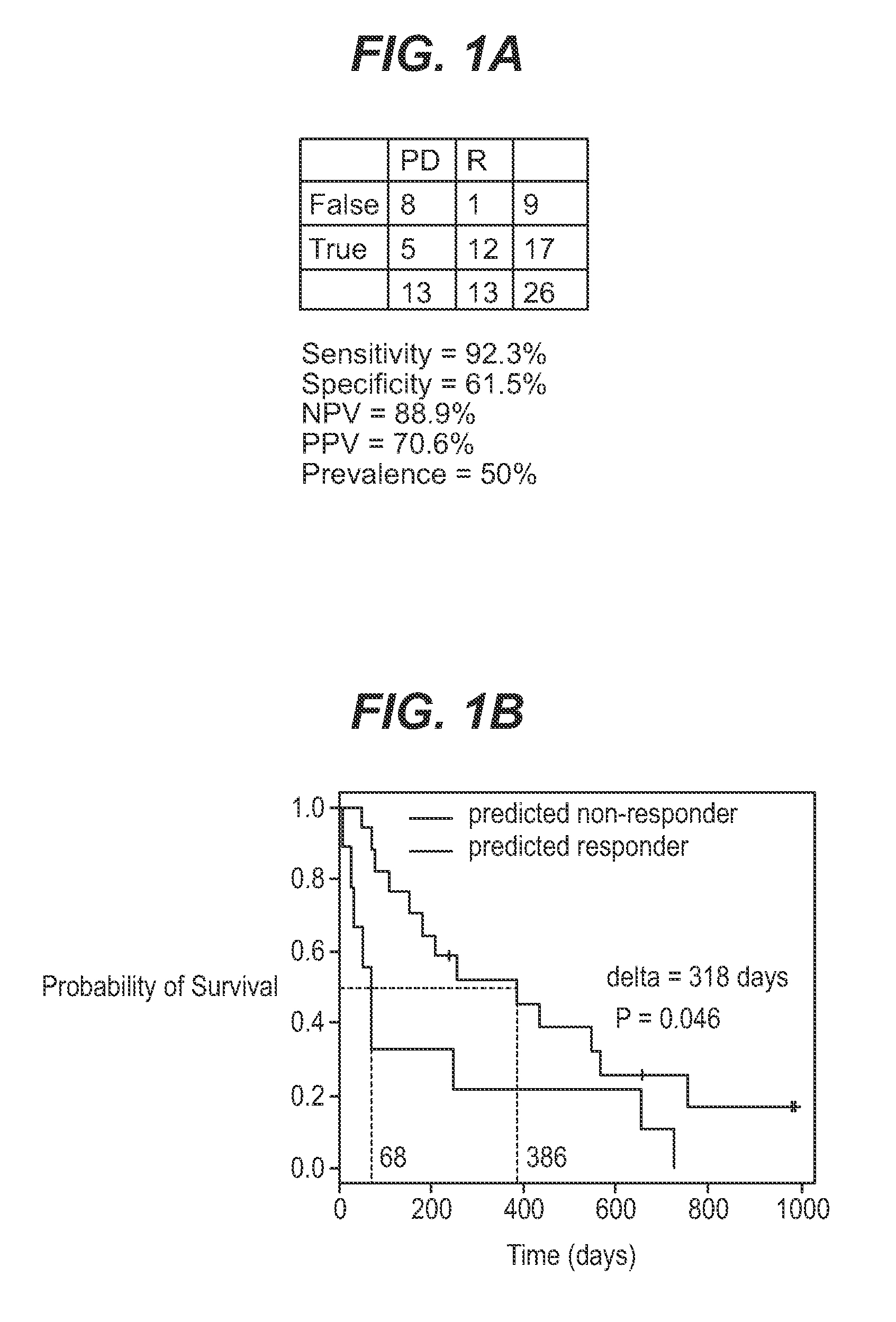
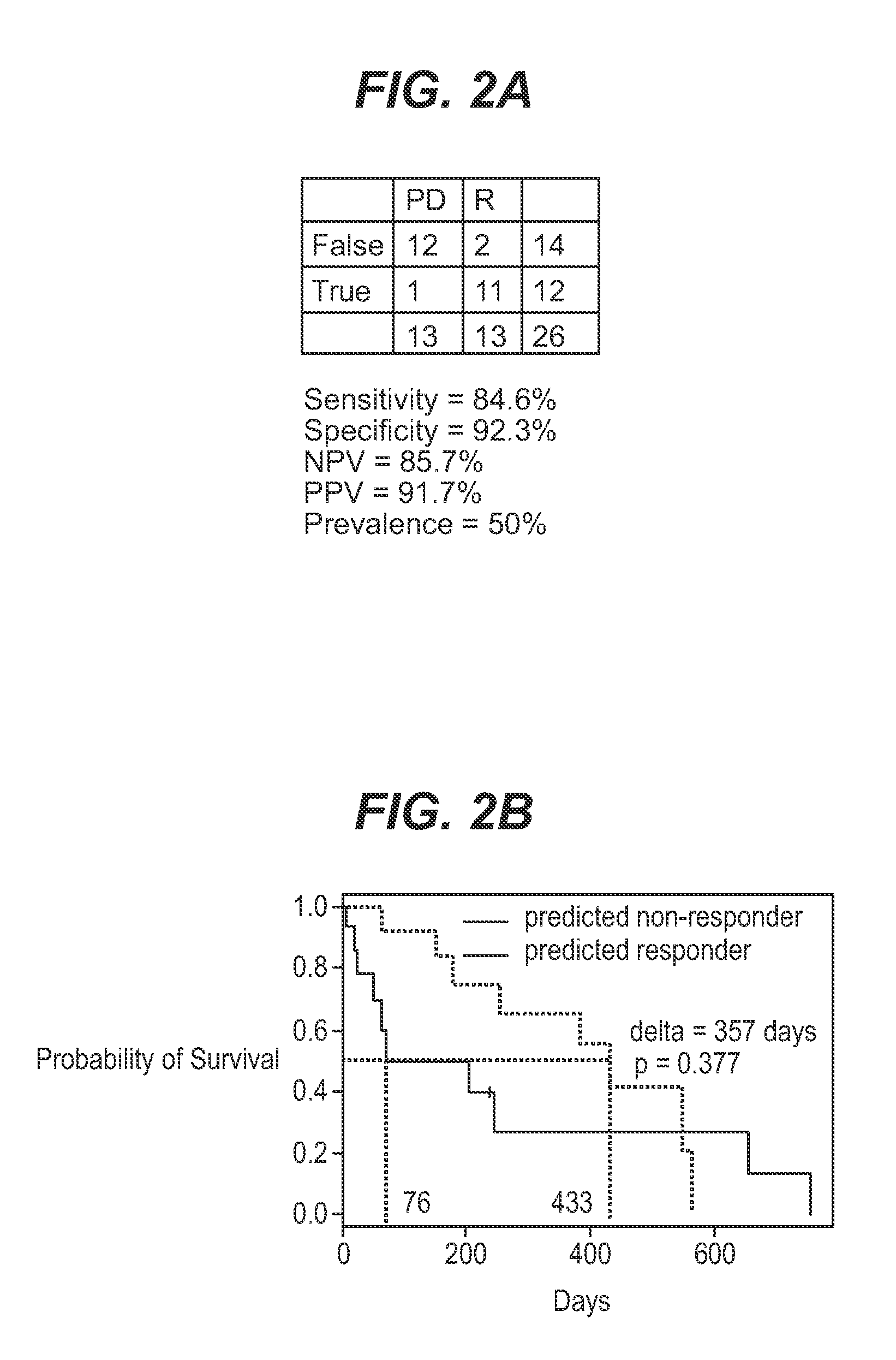
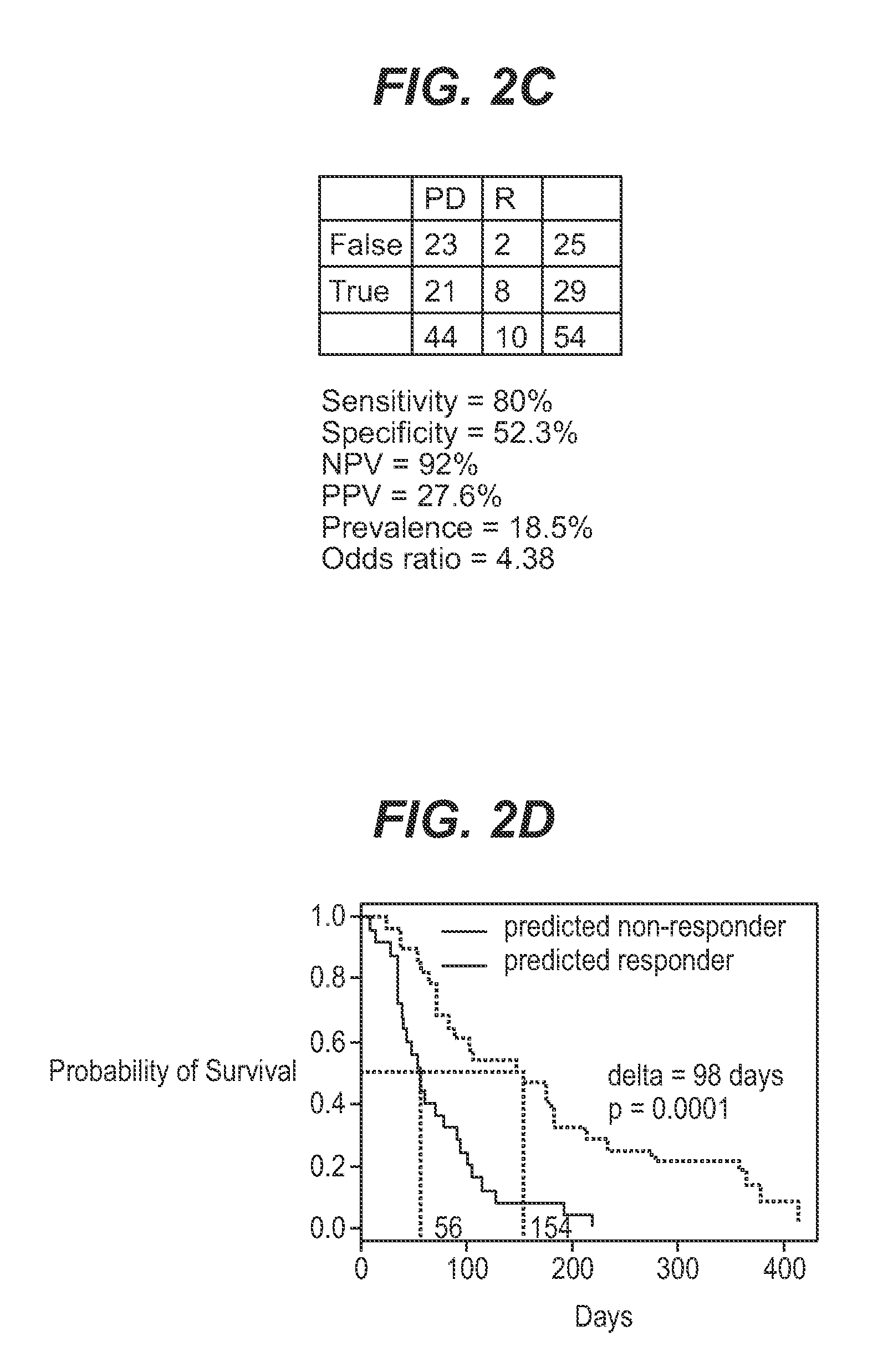
We analyzed bone marrow from 67 patients from a phase 2 study of farnesyltransferase inhibition with tipifarnib (R115777, ZARNESTRA®), in older adults with previously untreated, poor-risk acute myeloid leukemia (AML) for N-Ras mutations, global gene expression, and / or quantitative PCR (qPCR) of specific genes. Microarray profiling identified a two-gene expression ratio (RASGRP1:APTX) which provided the greatest accuracy for predicting response to tipifarnib. We demonstrated that this classifier could predict response to tipifarnib in an independent set of 54 samples from relapsed or refractory AML, with a NPV and PPV of 92% and 28%, respectively (odds ratio of 4.4). Therefore, in both newly diagnosed and relapsed or refractory AML, this classifier improves the overall response rate by approximately 50% while maintaining a high NPV, and significantly improves patient overall survival. The two-gene classifier was also validated by qPCR in thirty AML samples from the same clinical study demonstrating a negative predictive value (NPV) and positive predictive value (PPV) of 81% and 50%, respectively (odds ratio of 4.3). These data indicate that a simple two-gene expression assay may have utility in diagnosing a population of AML patients who are more likely to respond to tipifarnib.
Owner:JANSSEN DIAGNOSTICS LLC
Method for treating cancer based on mutation status of k-ras
The present invention provides methods and compositions for treating cancer by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and / or b) a therapeutic agent (e.g., gemcitabine) based upon K-ras mutation status.
Owner:ABRAXIS BIOSCI LLC
Methods and compositions for inhibition of ras
Inhibitors of Ras protein, methods to modulate the activity of Ras protein, and methods of treatment of disorders mediated by Ras protein are provided. A method for regulating activity of a K-Ras, H-Ras or N-Ras mutant protein with a compound is described. Disorders that can be treated include cancer, such as hematological cancer, pancreatic cancer, MYH associated polyposis, colorectal cancer, or lung cancer.
Owner:ARAXES PHARMA LLC
Ras mutation and compositions and methods related thereto
Disclosed are newly discovered Ras mutations and combinations of mutations, proteins and peptides and fusion proteins containing these mutations, nucleic acid molecules encoding such proteins, peptides, and fusion proteins, and a variety of tools and diagnostic, therapeutic, and screening methods associated with the use of such mutations.
Owner:GLOBE IMMUNE INC
Topographic genotyping for determining the diagnosis, malignant potential, and biologic behavior of pancreatic cysts and related conditions
InactiveUS20060088870A1Easy to analyzeEnhancing definitive diagnosisHealth-index calculationMicrobiological testing/measurementMolecular analysisMicrosatellite
The application relates to a method of a predicting the presence of invasive pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions comprising detailed molecular analysis incorporating DNA quality and quantity, K-ras mutational analysis and a broad spectrum of tumor suppressor gene linked microsatellite LOH. Methods of diagnosing, determining prognosis of and determining a course of treatment for pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions are also provided.
Owner:REDPATH INTEGRATED PATHOLOGY INC
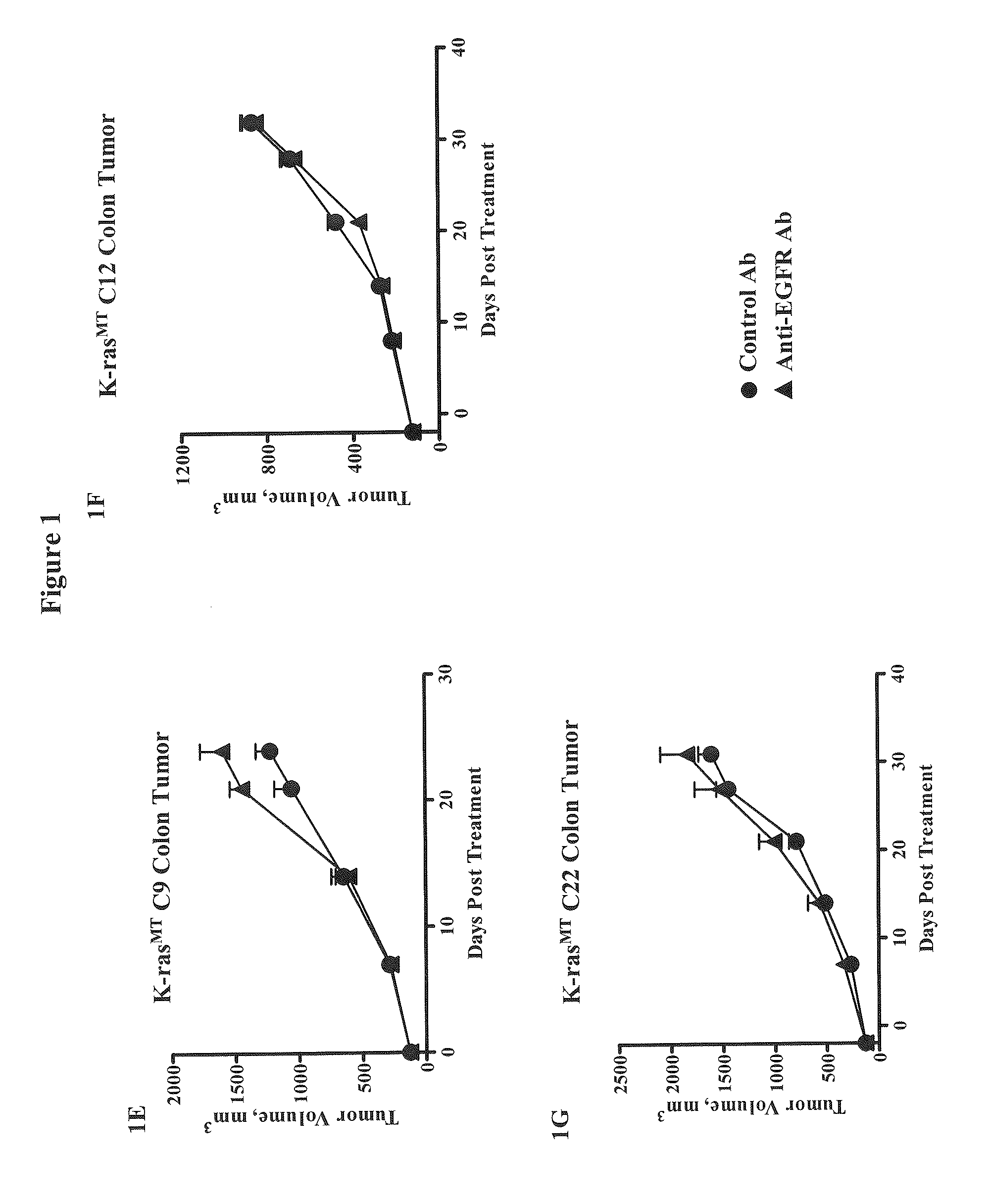
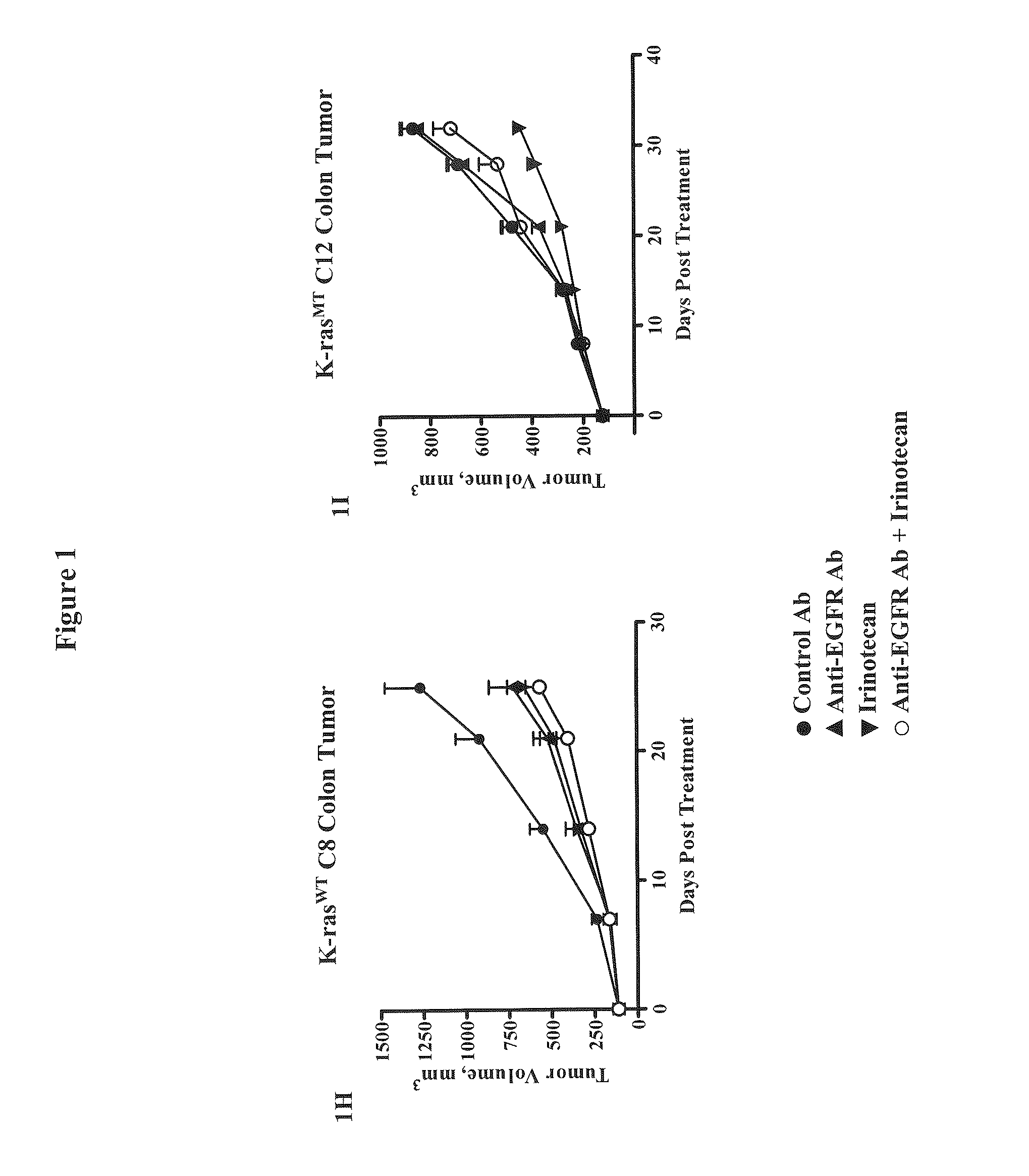
Methods for Treating Cancers Comprising K-ras Mutations
InactiveUS20110165162A1Inhibit tumor growthTreat cancerImmunoglobulins against growth factorsAntibody ingredientsExtracellular StructurePolynucleotide
Methods of inhibiting tumor growth, methods of treating cancer, and methods of reducing the frequency of cancer stem cells in a tumor are described. Particularly, the methods are directed to tumors or cancers that comprise a K-ras mutation. The methods described comprise administering a DLL4 antagonist (e.g., an antibody that specifically binds the extracellular domain of human DLL4) to a subject. Related polypeptides and polynucleotides, compositions comprising the DLL4 antagonists, and methods of making the DLL4 antagonists are also described.
Owner:ONCOMED PHARMA
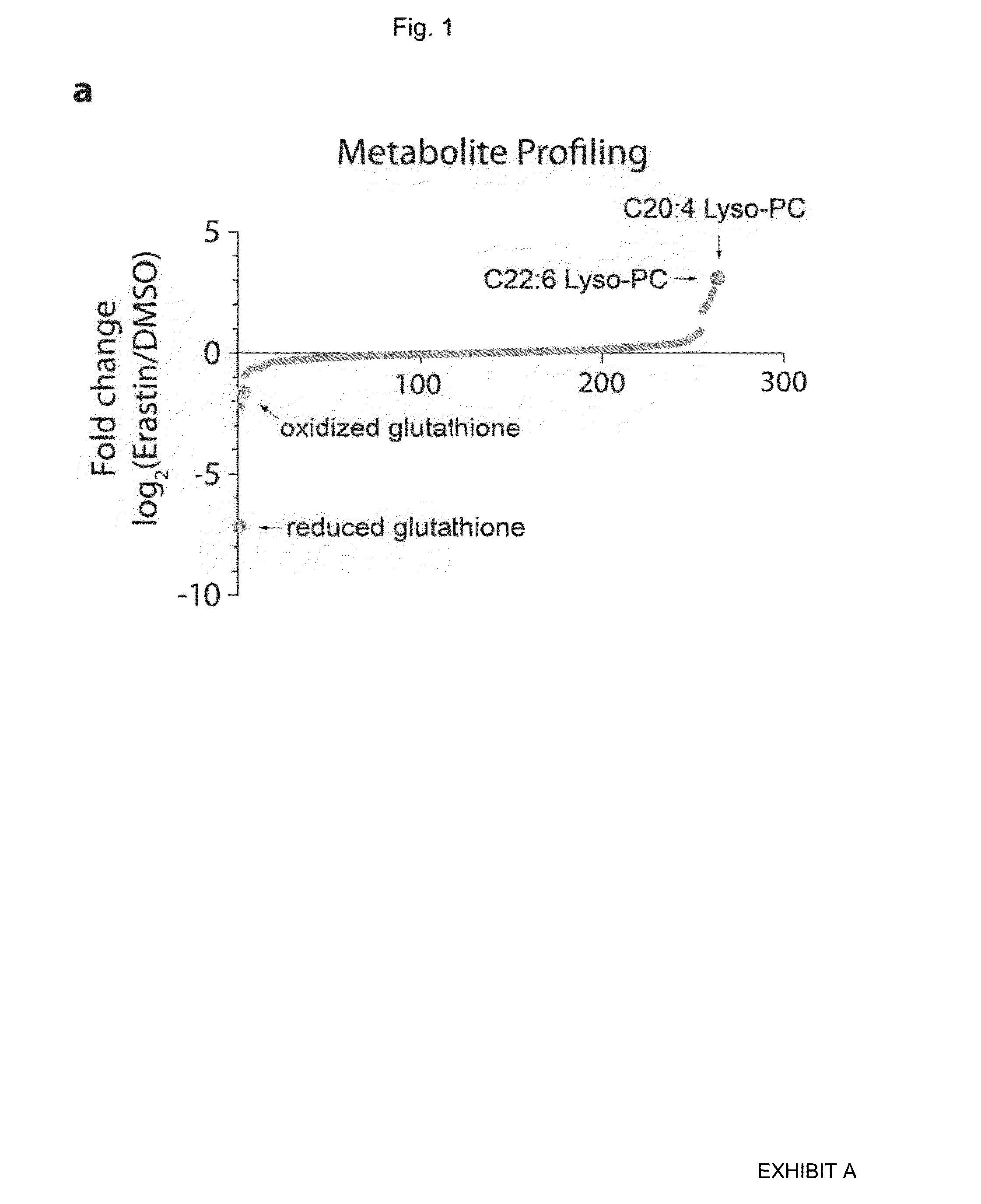
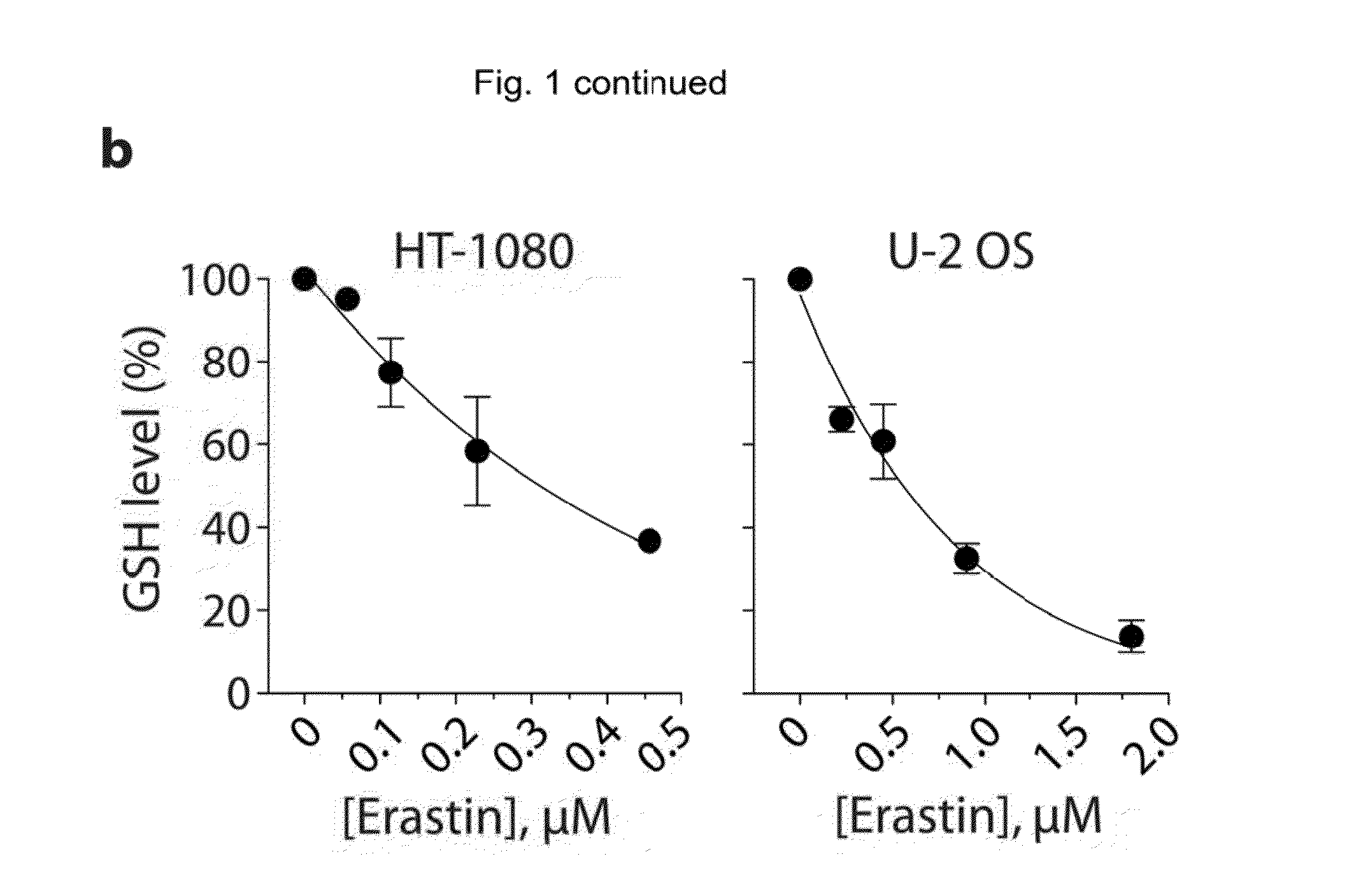
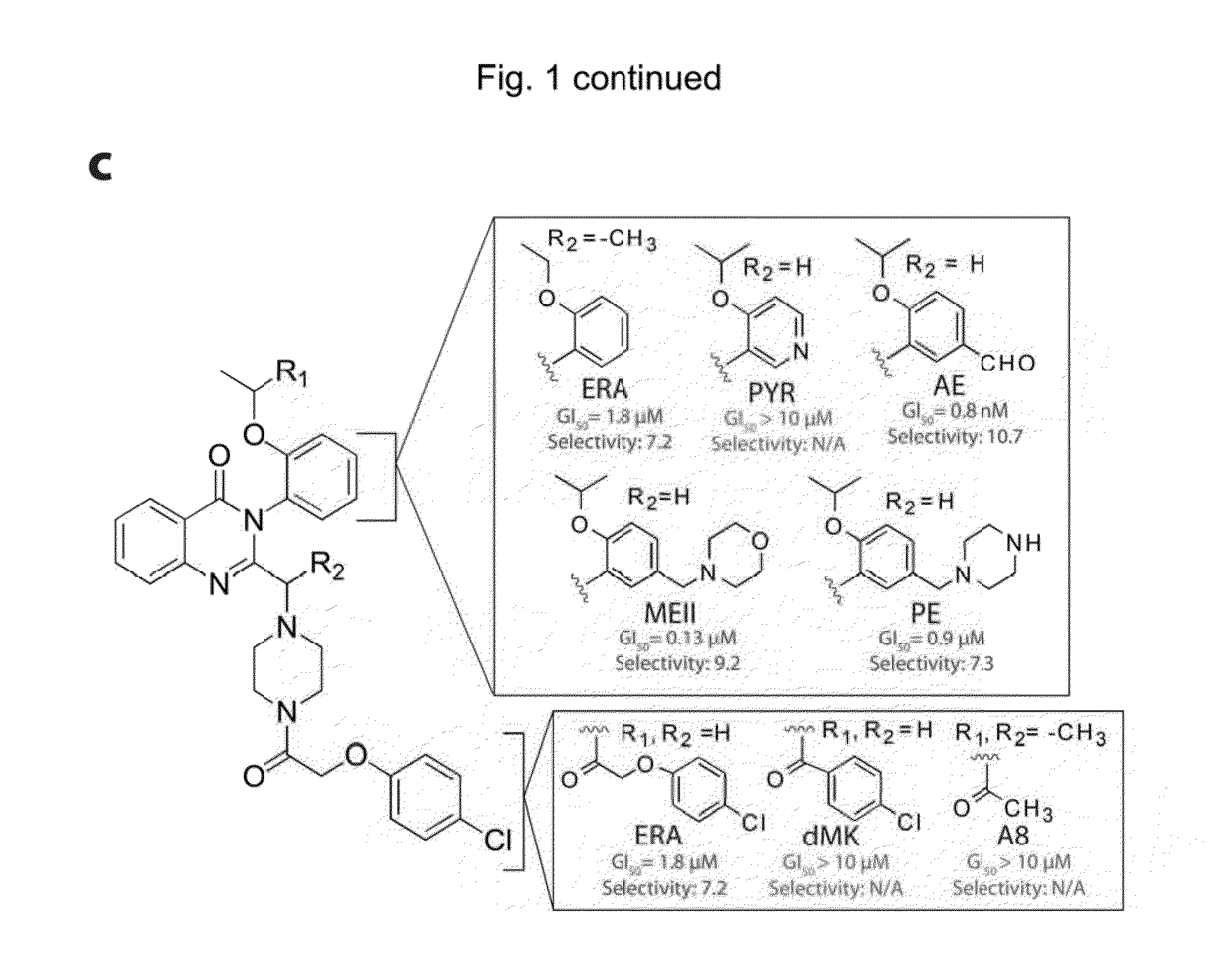
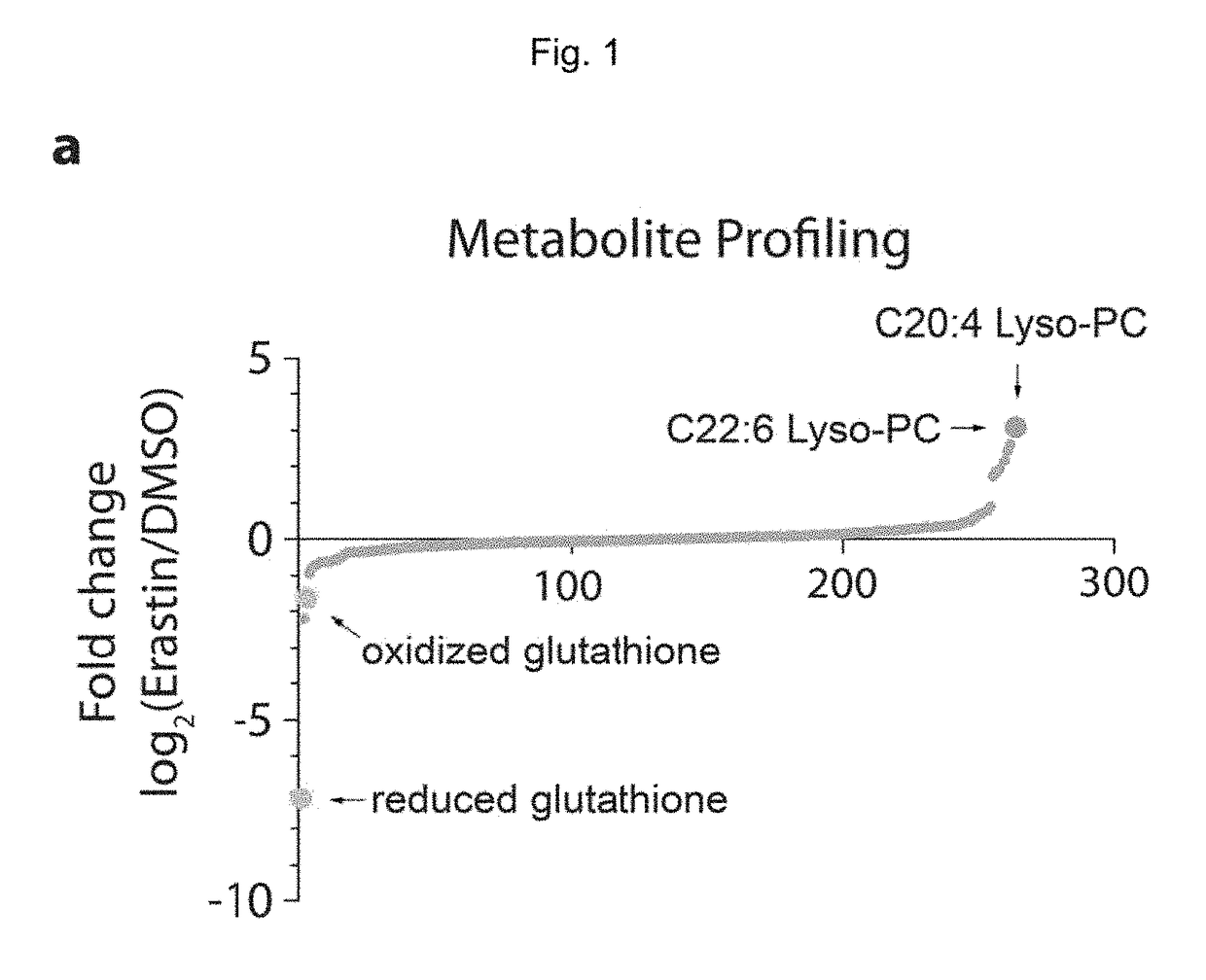
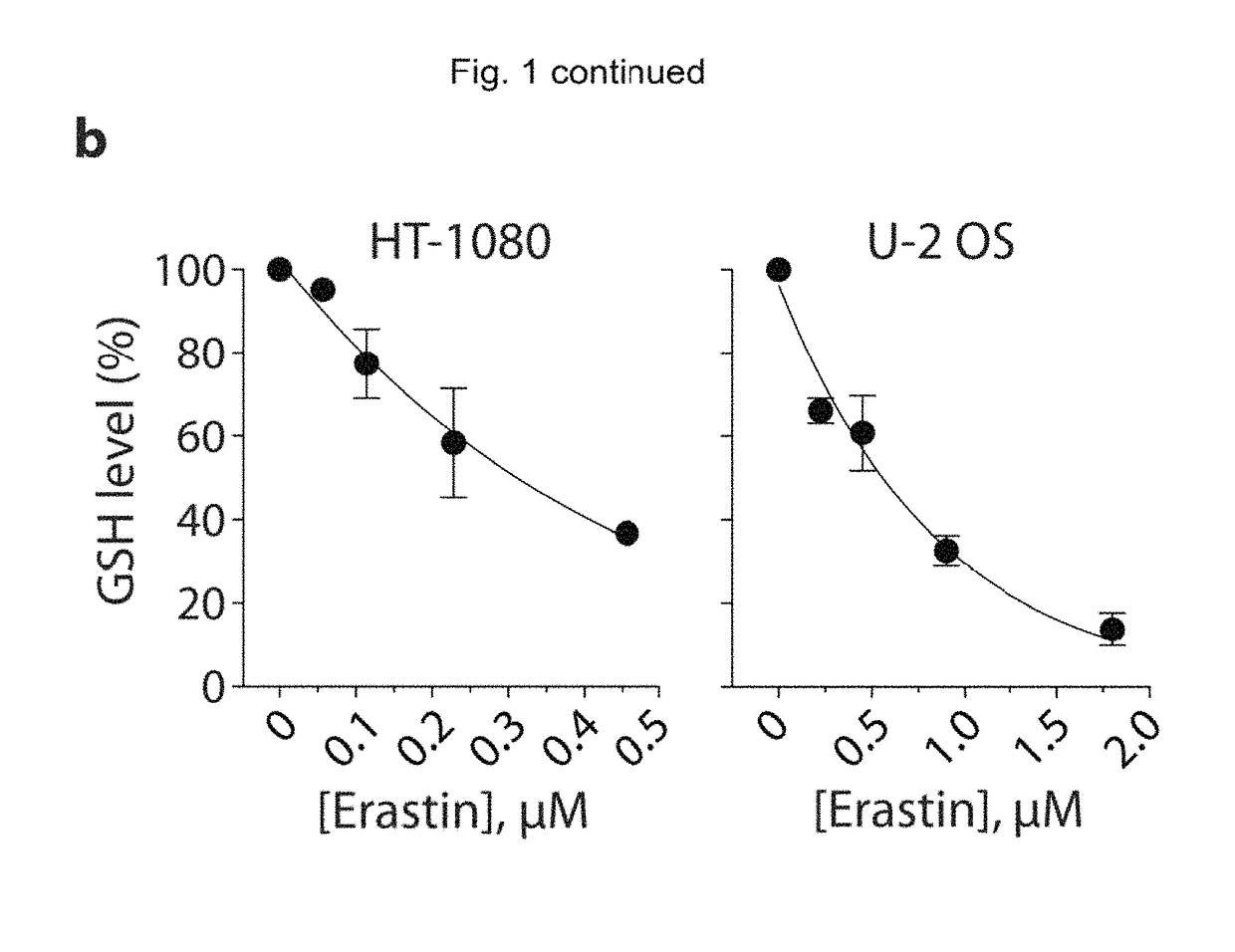
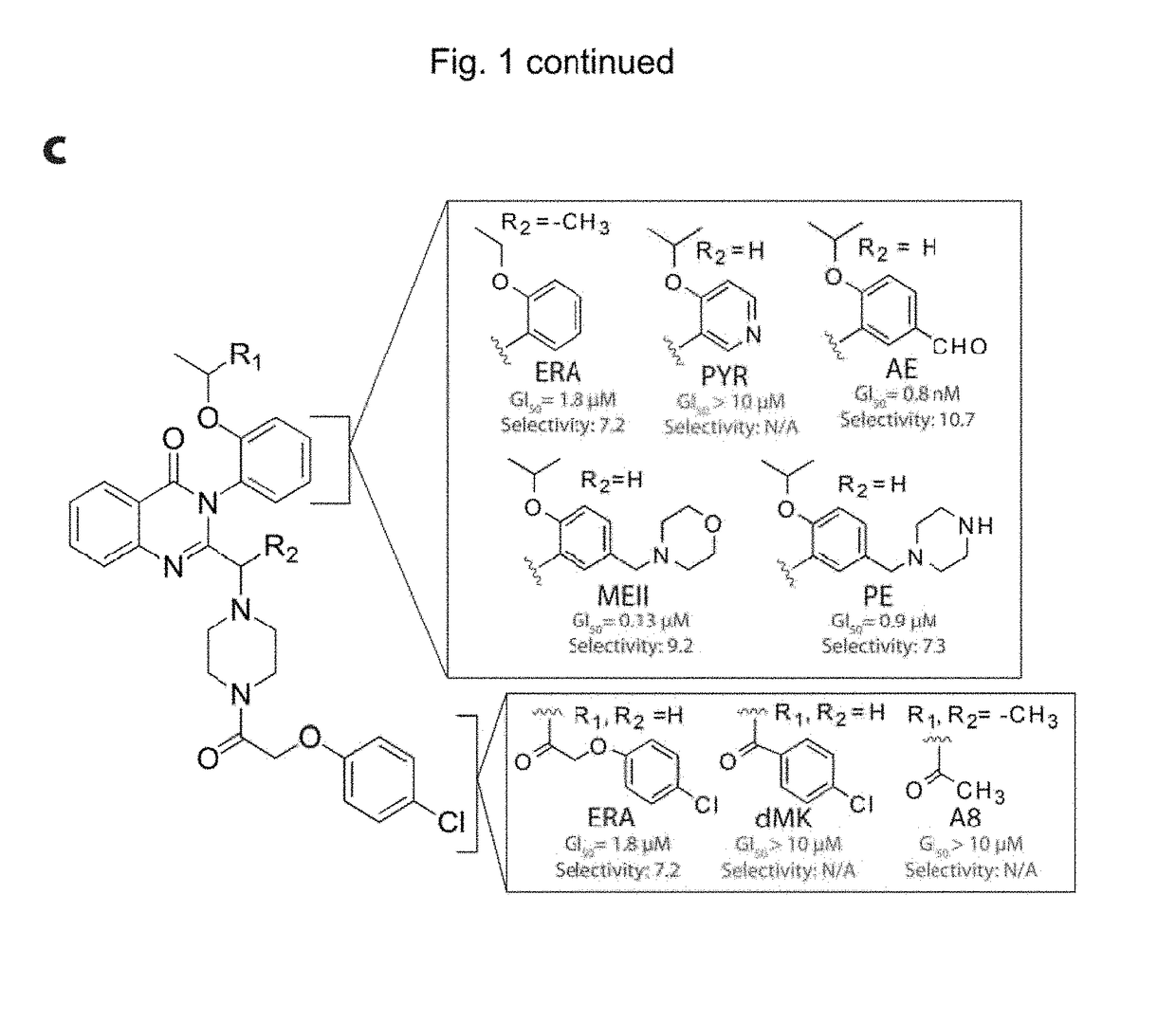
Quinazolinone-based oncogenic-ras-selective lethal compounds and their use
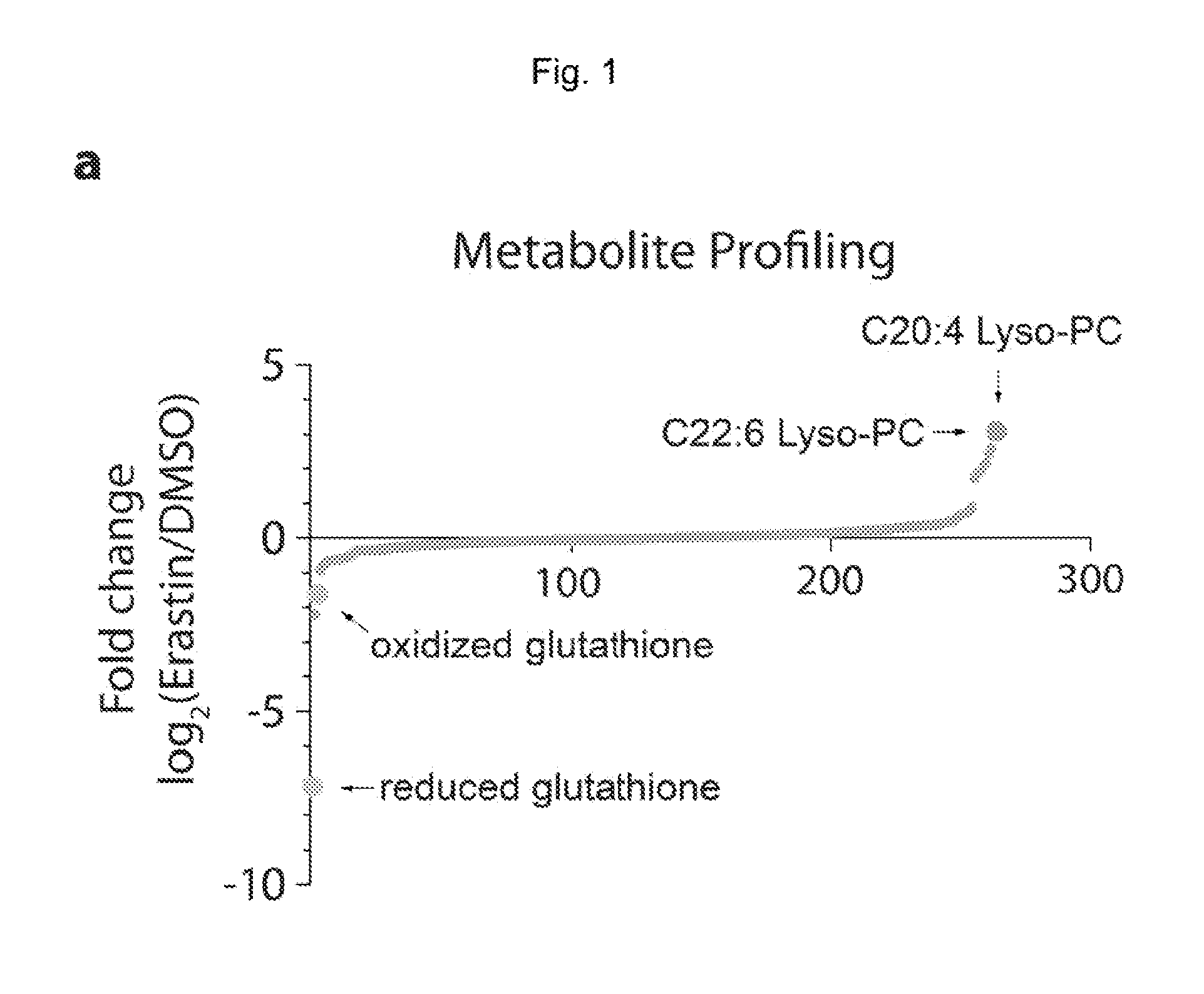
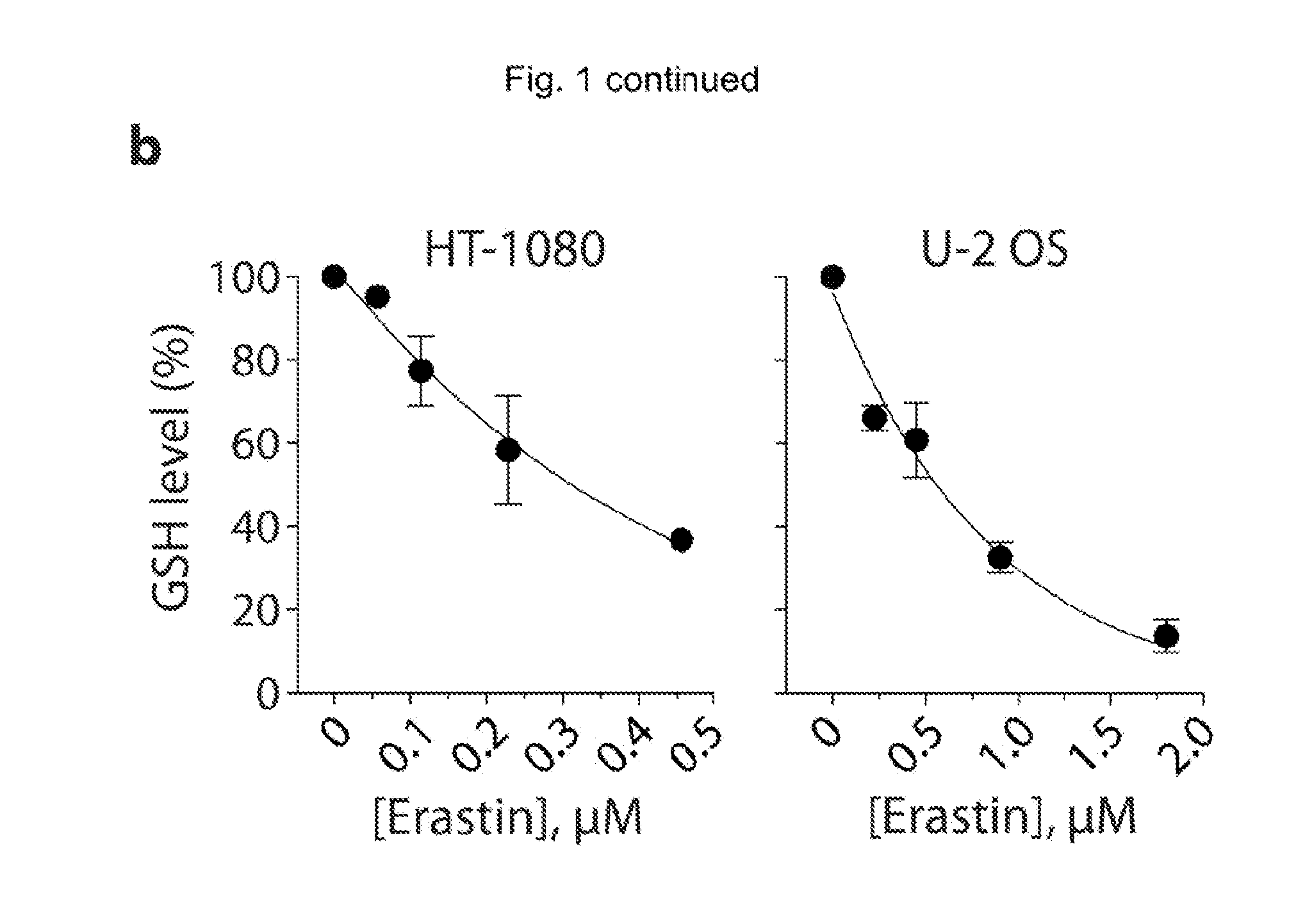
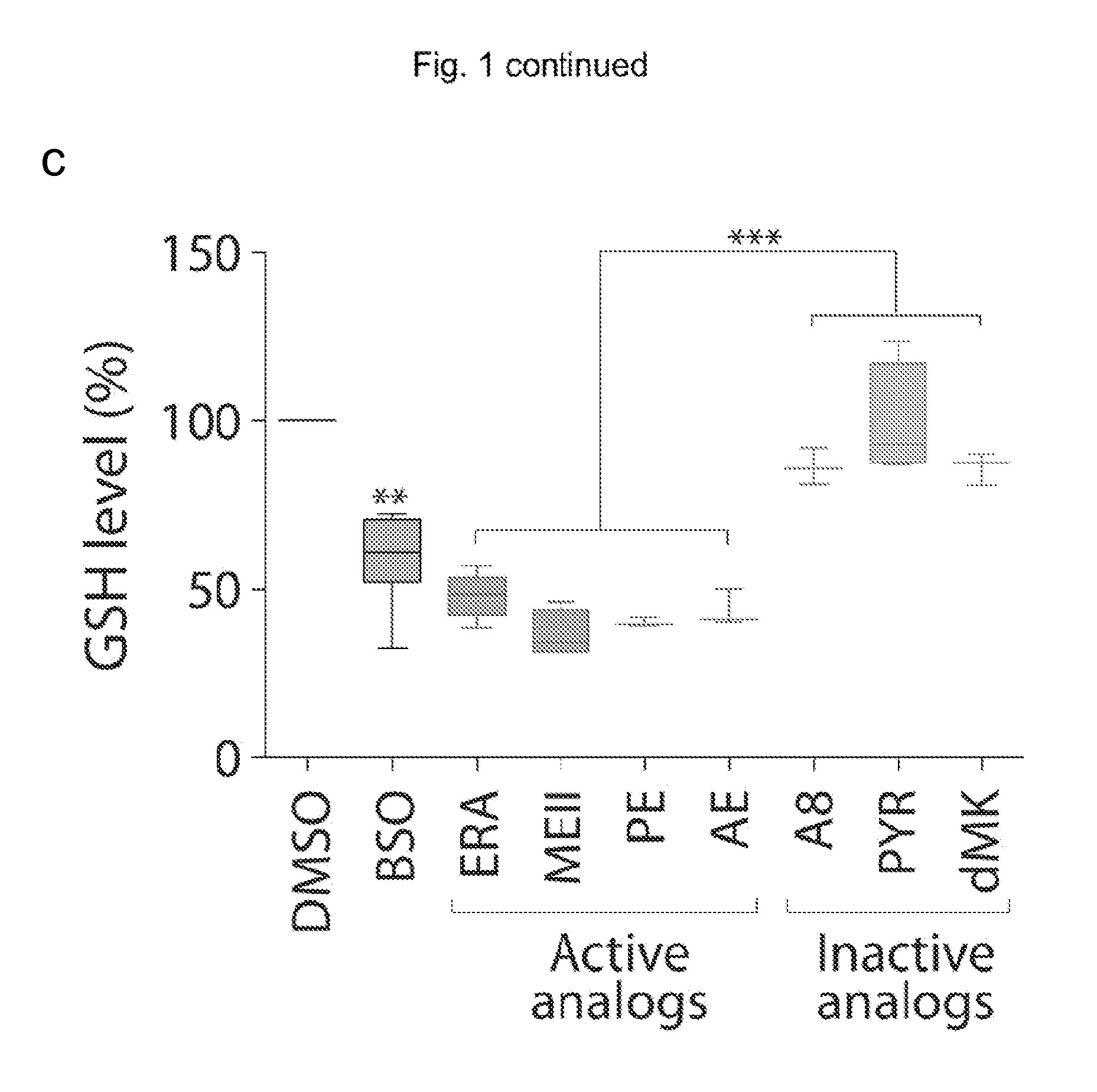
The present invention provides, inter alia, compounds having the structure (1) compositions containing such compounds are also provided. Methods for using such compounds or compositions for treating or ameliorating the effects of a cancer having a cell that harbors an oncogenic RAS mutation, for modulating a lipoxygenase in a ferroptosis cell death pathway, and for depleting reduced glutathione (GSH) in a cell harboring an oncogenic RAS mutation are further provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Primer, probes and detection kit used for full RAS mutation detection
InactiveCN104762410AHigh sensitivityEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationKRASA-DNA
The invention discloses a primer and probes used for full RAS mutation detection. The primer and probes comprise a full RAS specific primer and the probes, and the full RAS specific primer and the probes comprise twenty seven mutation types of KRAS 2,3,4 exons and twenty three mutation types of NRAS 2,3,4 exons. A detection kit used for full RAS mutation detection extracts a DNA sample and performs a PCR reaction. The specific MGB Blocker probe, the NRAS specific probe and the KRAS specific probe and the primer are adopted and can detect RAS mutation in DNA in tumor tissue; MGB Blocker is used for retarding nonspecific augmentation of NRAS and KRAS, and sensitivity is improved and higher than 1% of ARMS; RAS mutation can be detected in circular tumor DNA. The primer and the probes are easy to operate, low in detection cost, capable of being popularized in a large-scale mode and high in detection speed, and the detection process can be finished after about 2 hours.
Owner:张道允 +1
Cancer treatment with retroviral vectors comprising wild-type p53
InactiveUS6998117B1Maintain stabilityImproved ability to inhibit and provideBiocidePeptide/protein ingredientsAntisense OrientationGenomic Segment
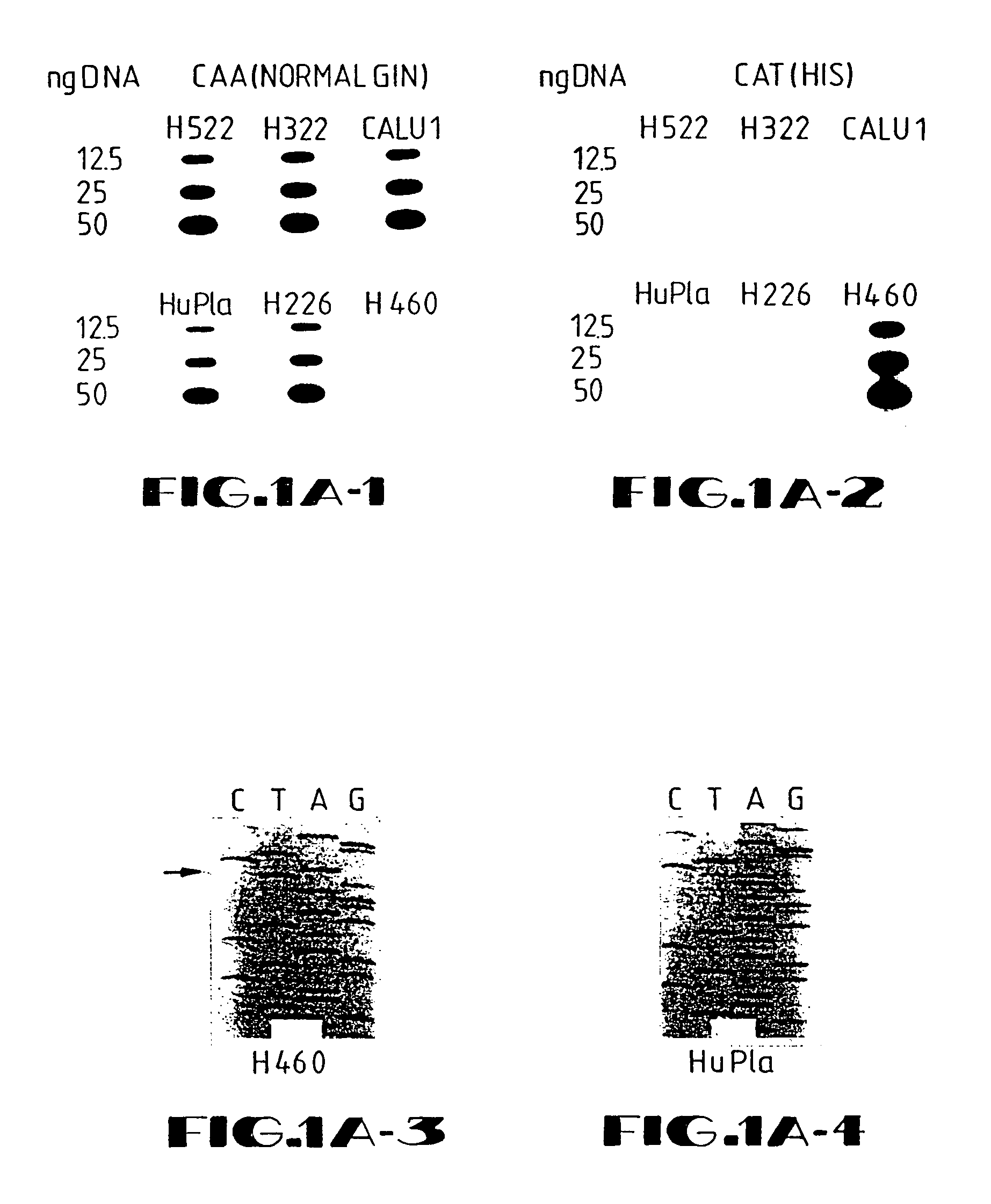
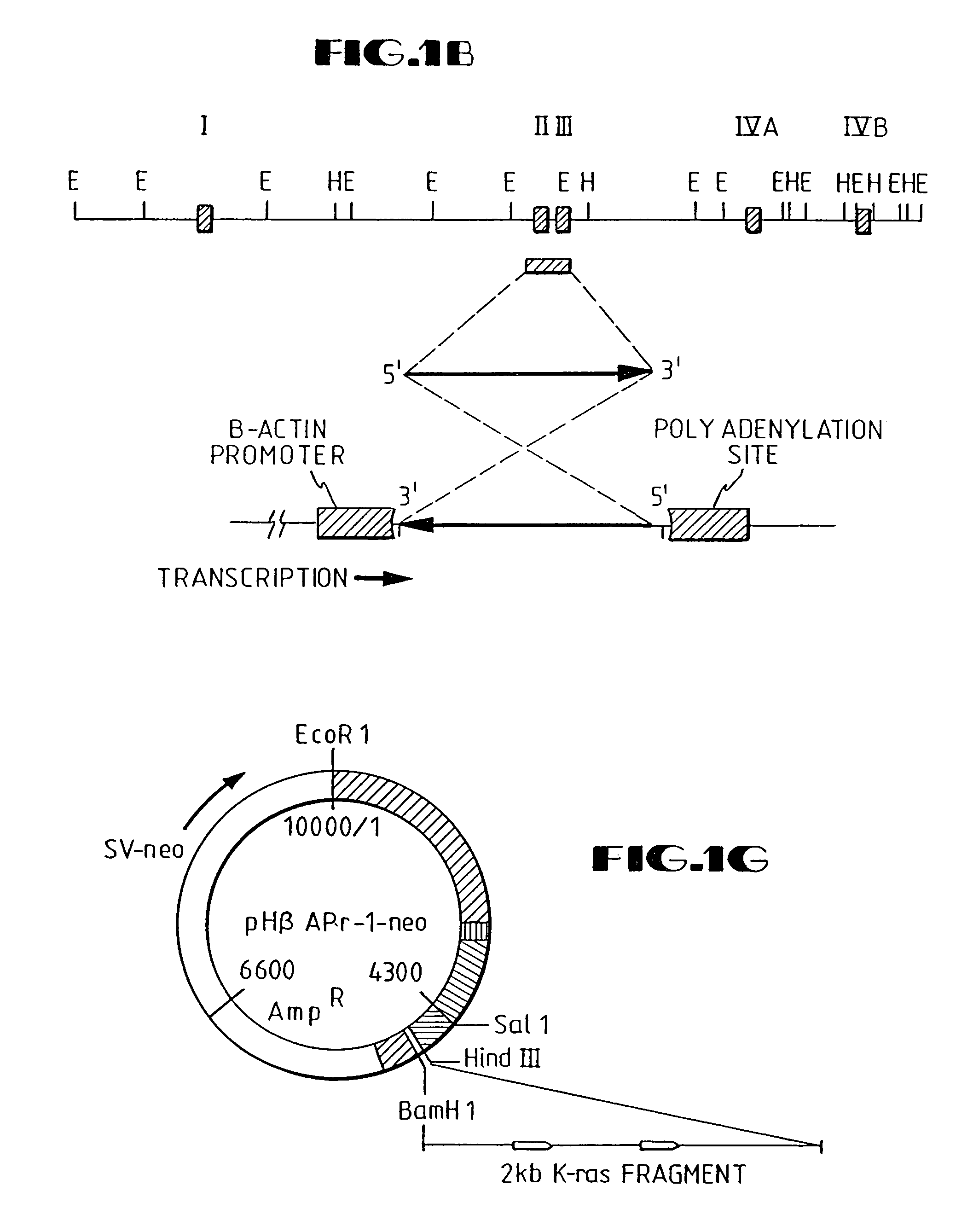
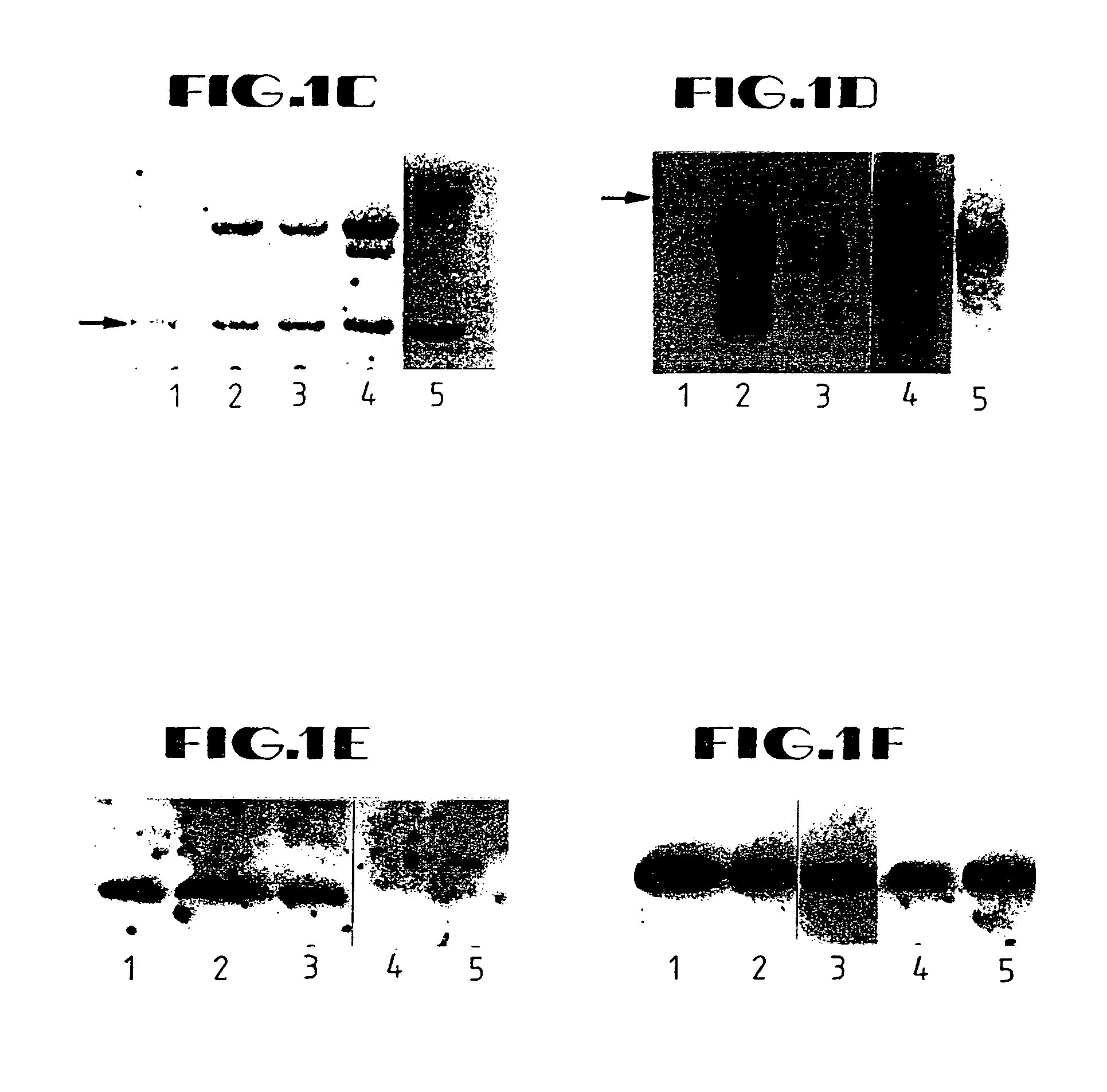
Disclosed are methods and compositions for the selective manipulation of gene expression through the preparation of retroviral expression vectors for expressing antisense sequences, such as K-ras oncogene antisense sequences, or sequences encoding a desired product, such as wild type p53 sequences. Preferred retroviral vectors of the present invention incorporate the β-actin promoter in a reverse orientation with respect to retroviral transcription. Preferred antisense RNA constructs of the present invention employ the use of antisense intron DNA corresponding to distinct intron regions of the gene whose expression is targeted for down-regulation. In an exemplary embodiment, a human lung cancer cell line (NCI-H460a) with a homozygous spontaneous K-ras mutation was transfected with a recombinant plasmid that synthesizes a genomic segment of K-ras in antisense orientation. Translation of the mutated K-ras mRNA was specifically inhibited, whereas expression of H-ras and N-ras was unchanged. A three-fold growth inhibition occurred in H460a cells when expression of the mutated ras p21 protein was down-regulated by antisense RNA and cells remained viable. The growth of H460a tumors in nu / nu mice was substantially reduced by expressed K-ras antisense RNA.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
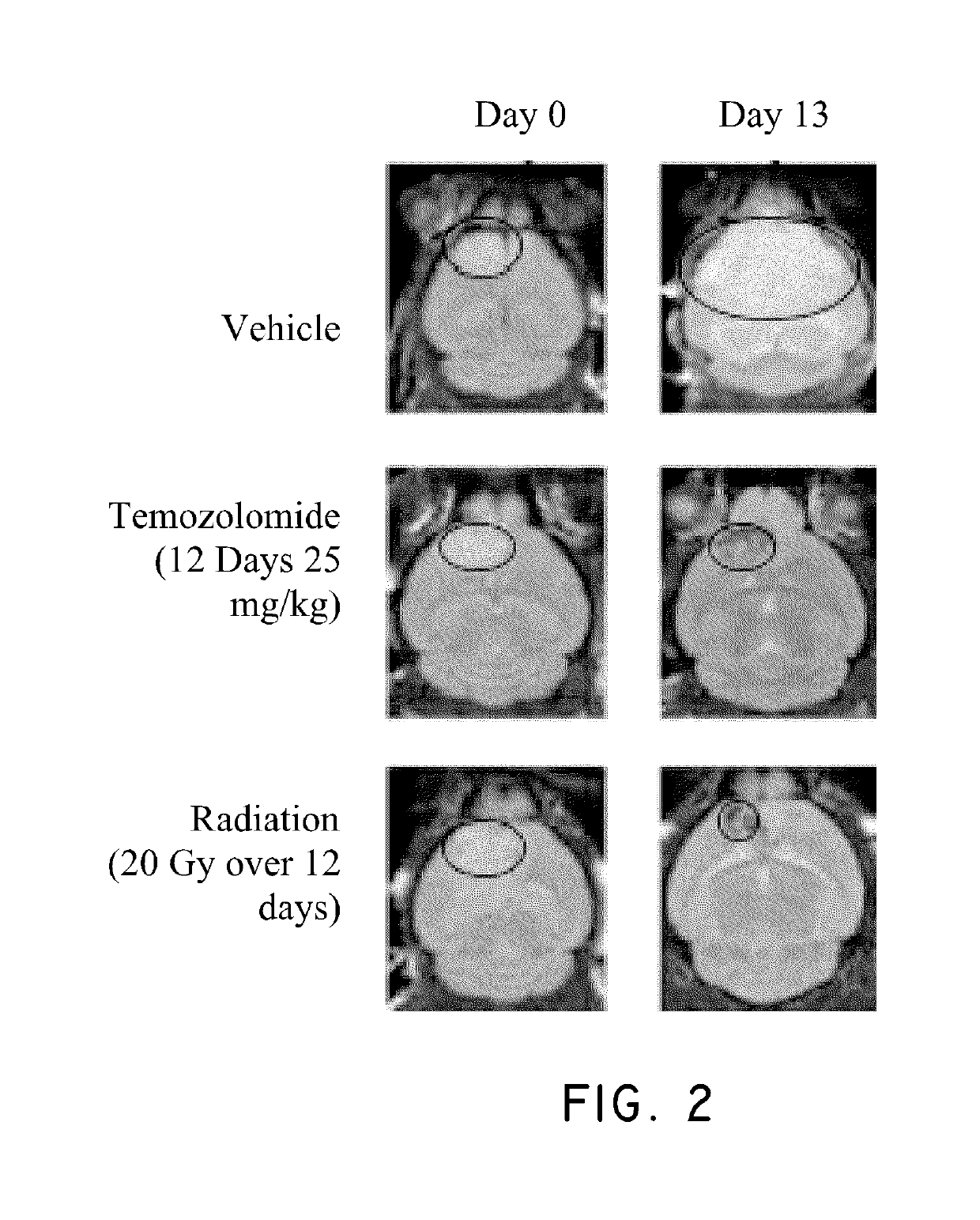
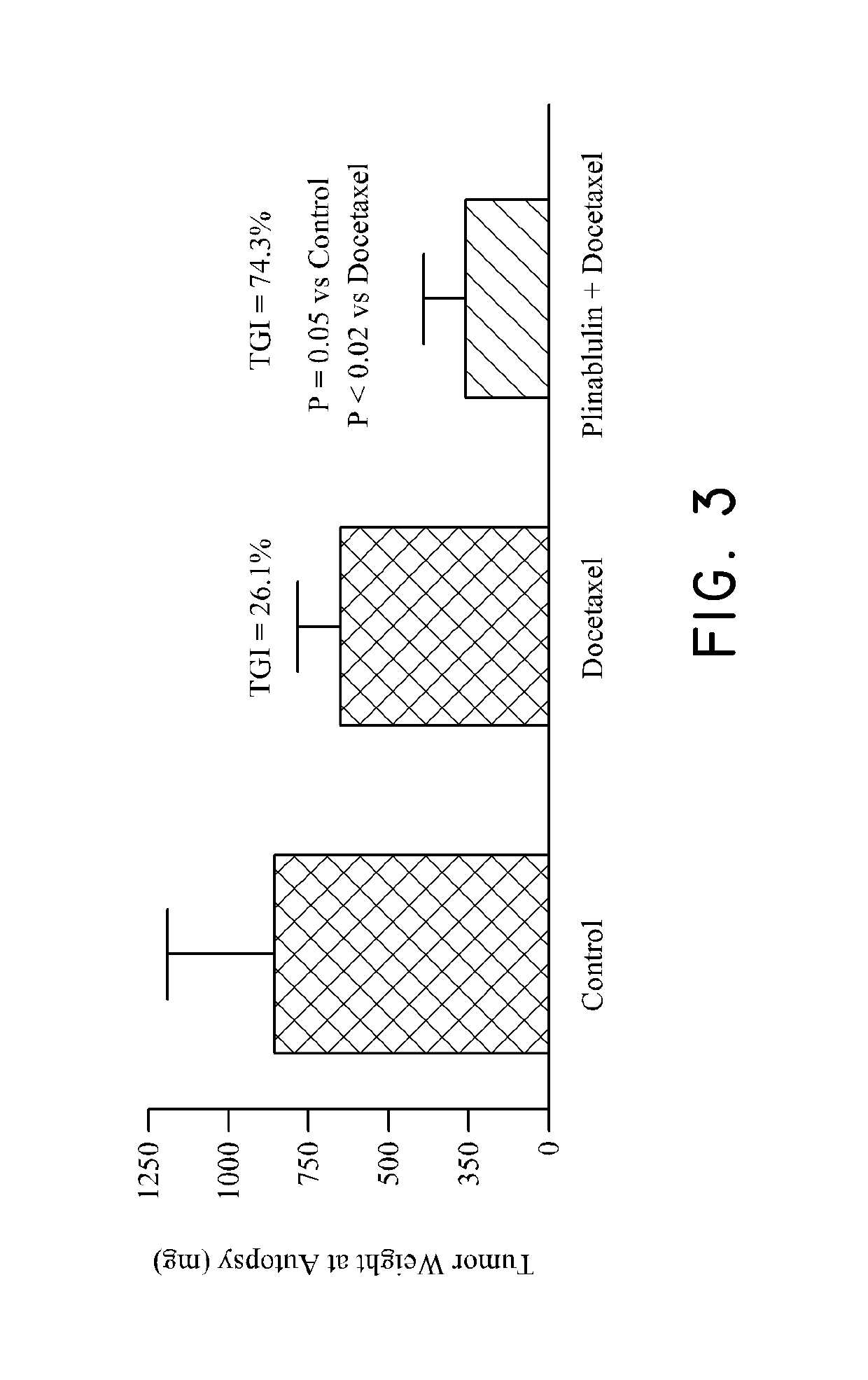
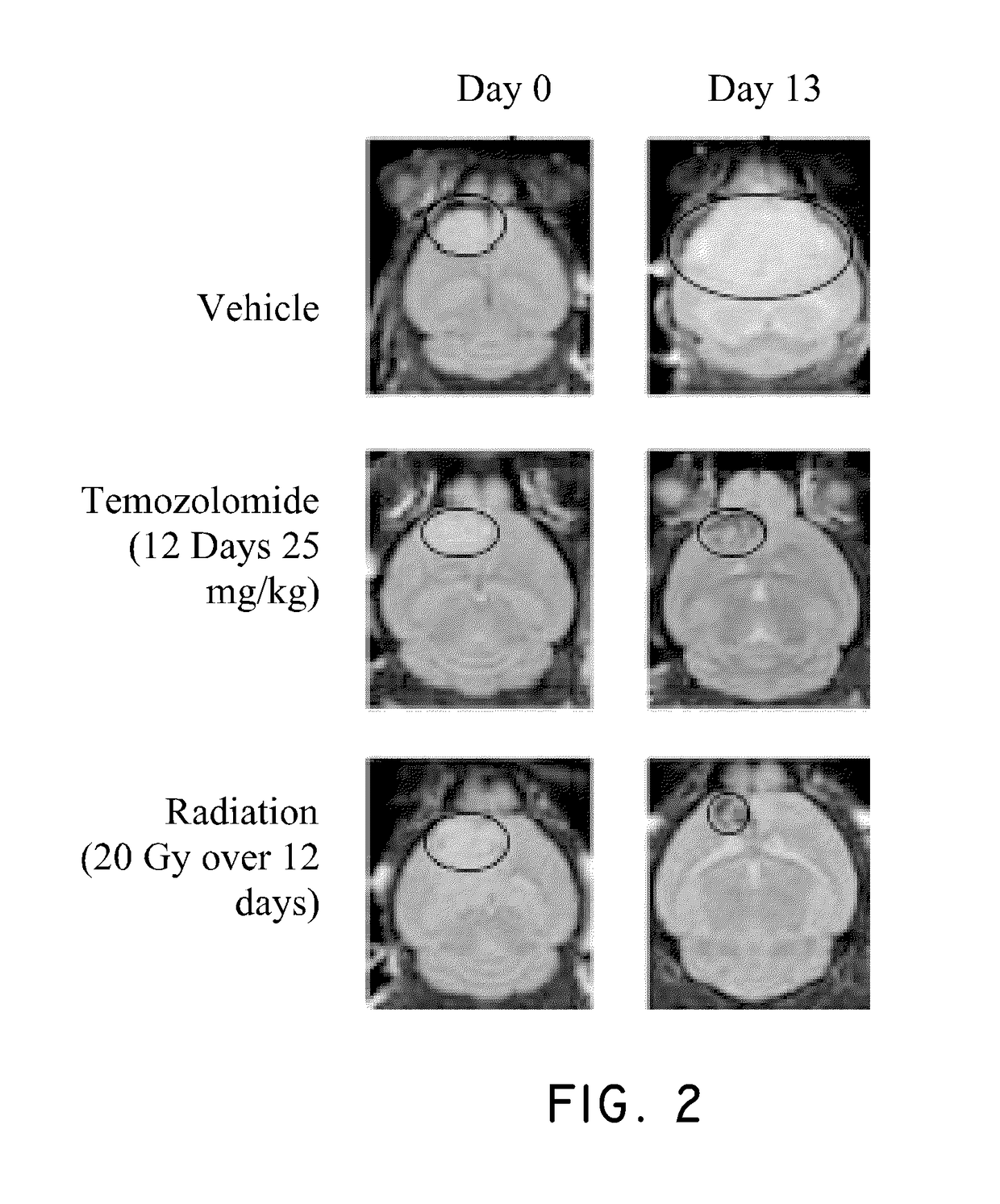
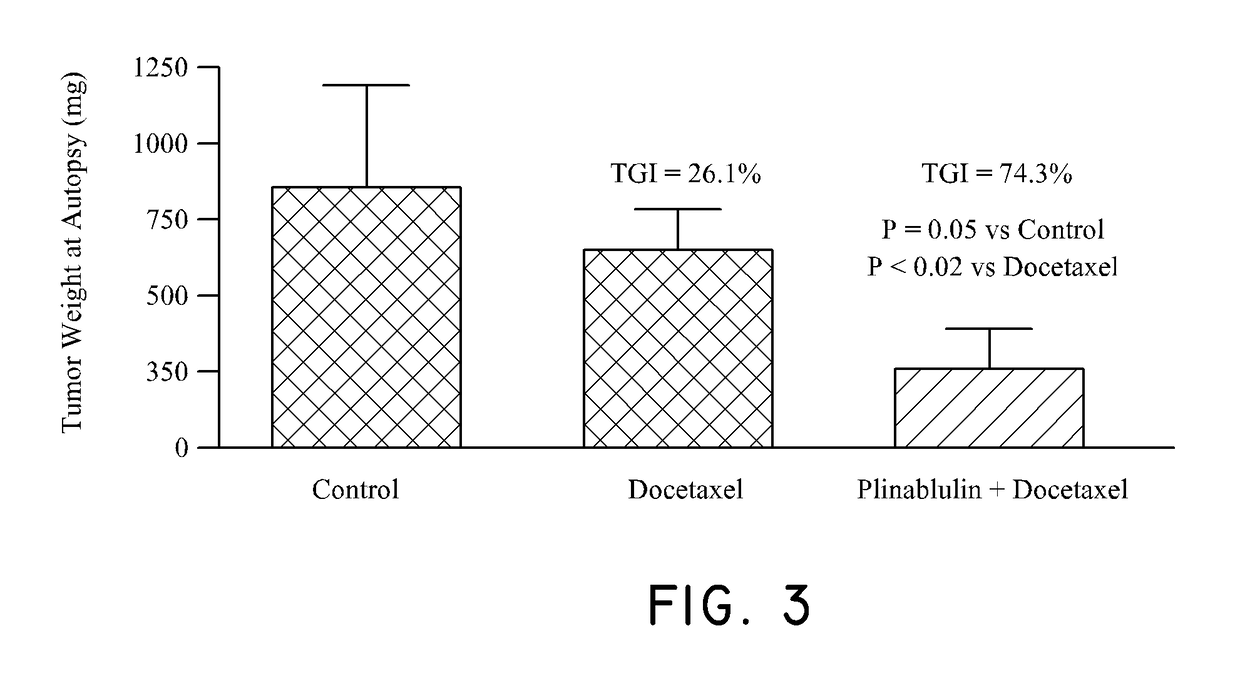
Method of treating cancer associated with a RAS mutation
ActiveUS10238650B2Inhibit progressOrganic active ingredientsAntineoplastic agentsPlinabulinRAS Mutation
Owner:BEYONDSPRING PHARMA INC
Quinazolinone-based oncogenic-RAS-selective lethal compounds and their use
The present invention provides, inter alia, compounds having the structure (1) compositions containing such compounds are also provided. Methods for using such compounds or compositions for treating or ameliorating the effects of a cancer having a cell that harbors an oncogenic RAS mutation, for modulating a lipoxygenase in a ferroptosis cell death pathway, and for depleting reduced glutathione (GSH) in a cell harboring an oncogenic RAS mutation are further provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Leukemia mouse model based on gene co-transfection technology and preparation method thereof
ActiveCN102864172AIncrease success rateImprove featuresFermentationGenetic engineeringBone marrow cellViral vector
The invention relates to the technical field of biology, in particular to a leukemia mouse model based on a gene co-transfection technology and a preparation method thereof. The preparation method of the leukemia mouse model comprises the following steps: performing construction and packaging of K-ras mutants and AML1-ETO fusion gene lentivirus vectors; performing bone marrow cell separation and virus infection condition monitoring; implanting infection cells in a mouse to build a leukemia animal model; and performing model identification. The method of building the leukemia mouse model in a mode of utilizing caudal vein injection to lead in the manual site-directed mutagenesis K-ras mutants and AML1-ETO fusion gene lentivirus vectors is adopted initiatively. The leukemia mouse model is high in success rate, pathological characteristics are high in similarity to morbidity conditions of clinical leukemia, and the novel animal model can be provided for leukemia extramedullary infiltration mechanism research, leukemia medicine screening and gene and molecule target treatment.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Carbonyl erastin analogs and their use
The present invention provides, inter alia, compounds having the structure of formula (I): Compositions containing such compounds are also provided. Methods for using such compounds or compositions for treating or ameliorating the effects of a cancer having a cell that harbors an oncogenic RAS mutation, for modulating a lipoxygenase in a ferroptosis cell death pathway, and for depleting reduced glutathione (GSH) in a cell harboring an oncogenic RAS mutation are further provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Composition and method for treating cancer
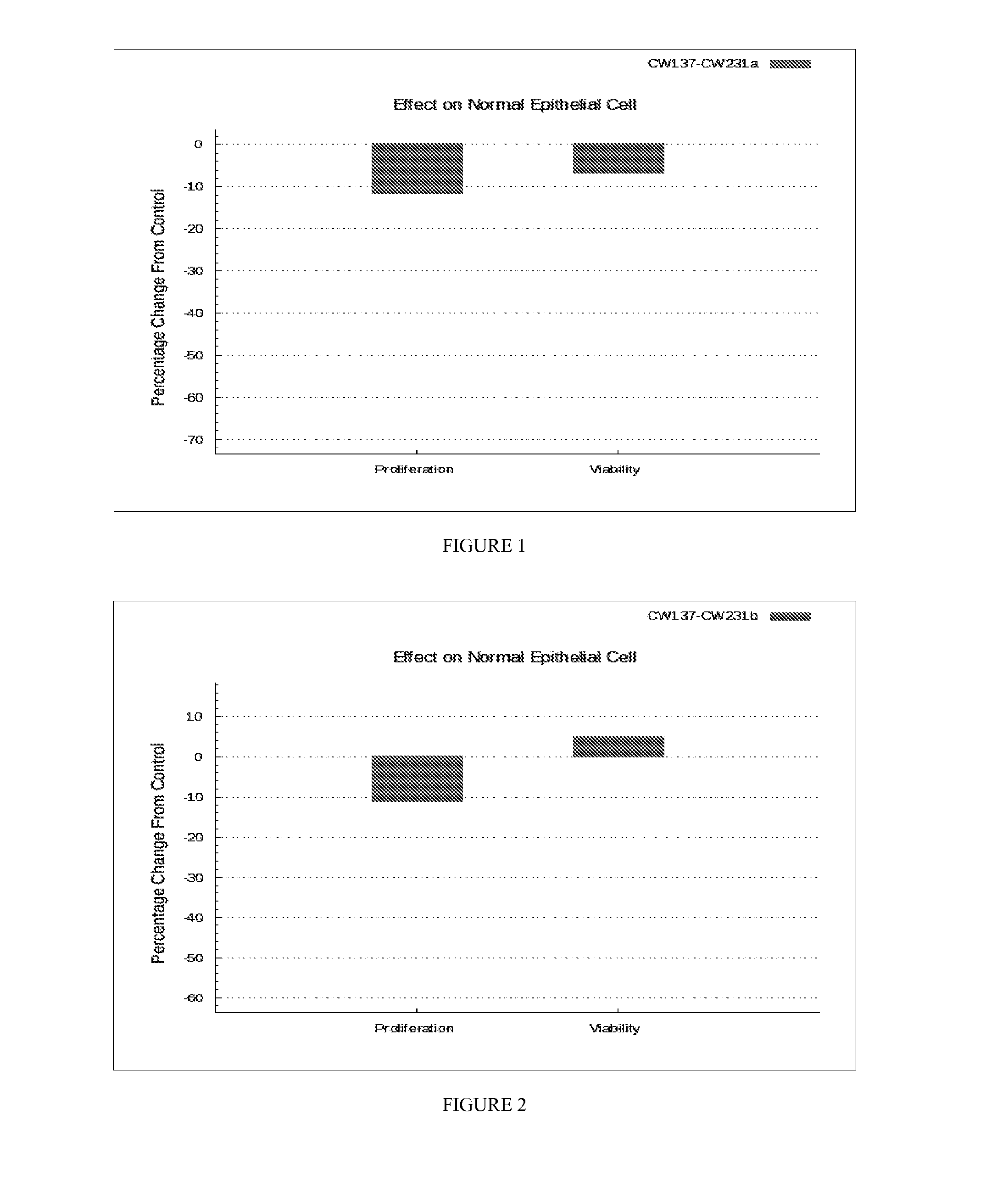
The present disclosure relates to a pharmaceutical composition and a kit to treat cancer. The disclosure provides a combination of compounds for use in the treatment of cancer. The disclosure further provides a process of preparing the composition and a method of treating a cancer associated with a RAS mutation or a RAS mutation along with any other mutation.
Owner:CELLWORKS GROUP
K-ras mutations and antagonists
The present application relates to K-Ras mutations, to polynucleotides encoding mutant K-Ras polypeptides, and to methods of identifying small molecule antagonists using K-Ras mutations. The present application also relates to K-Ras small molecule antagonists and use thereof in the treatment of tumors.
Owner:STEVENS INSTITUTE OF TECHNOLOGY
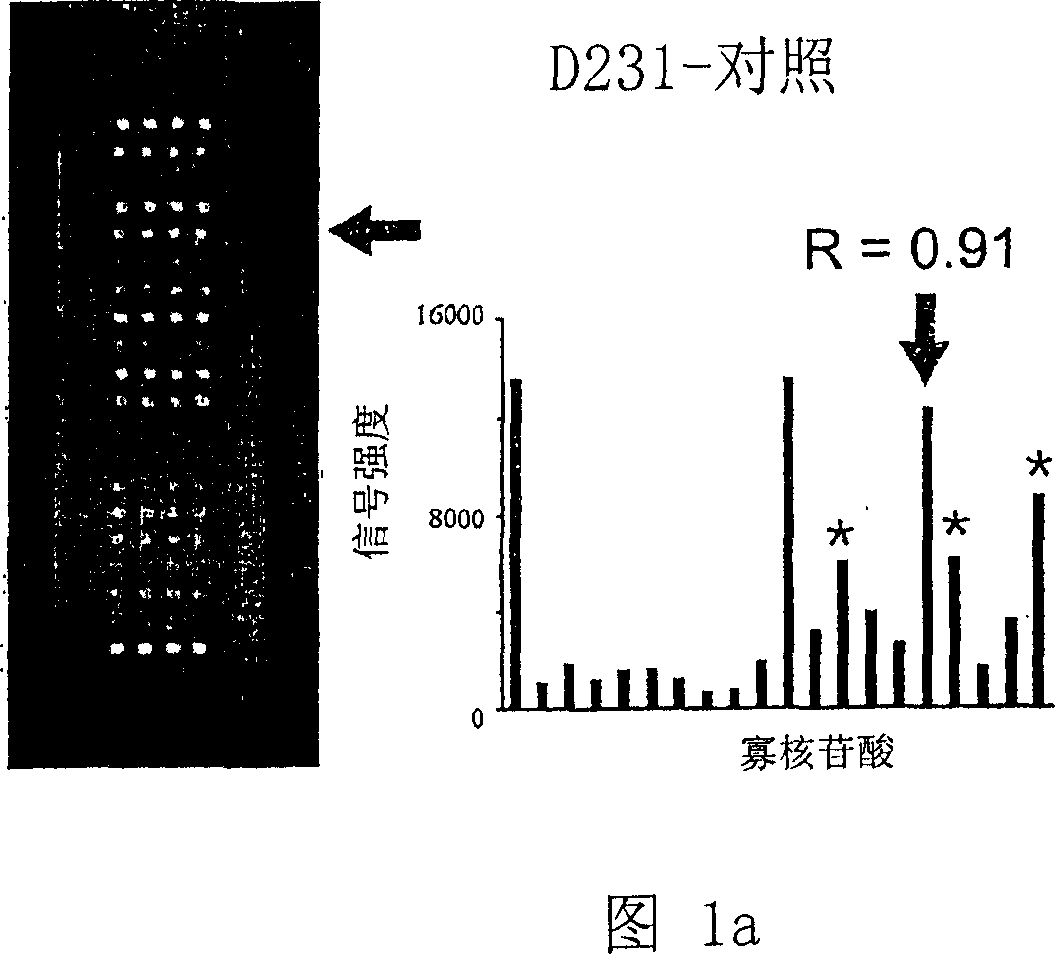
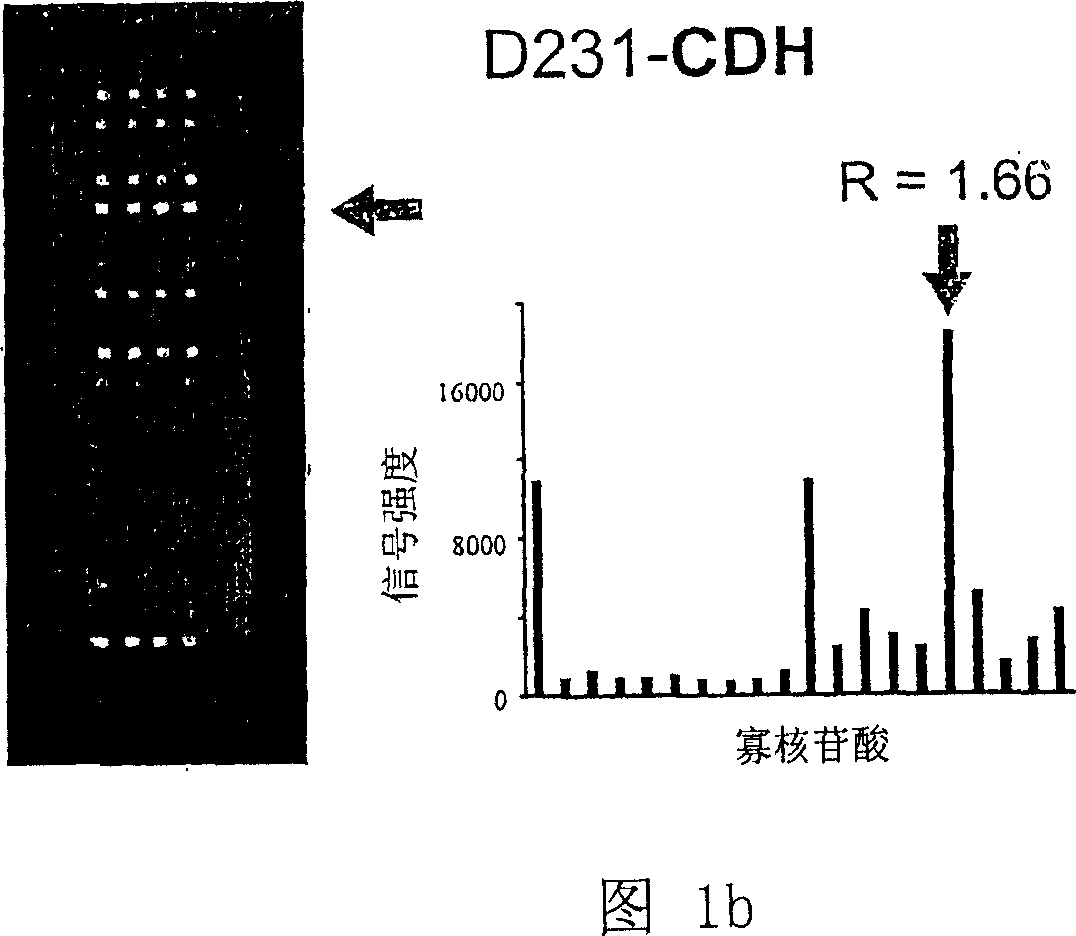
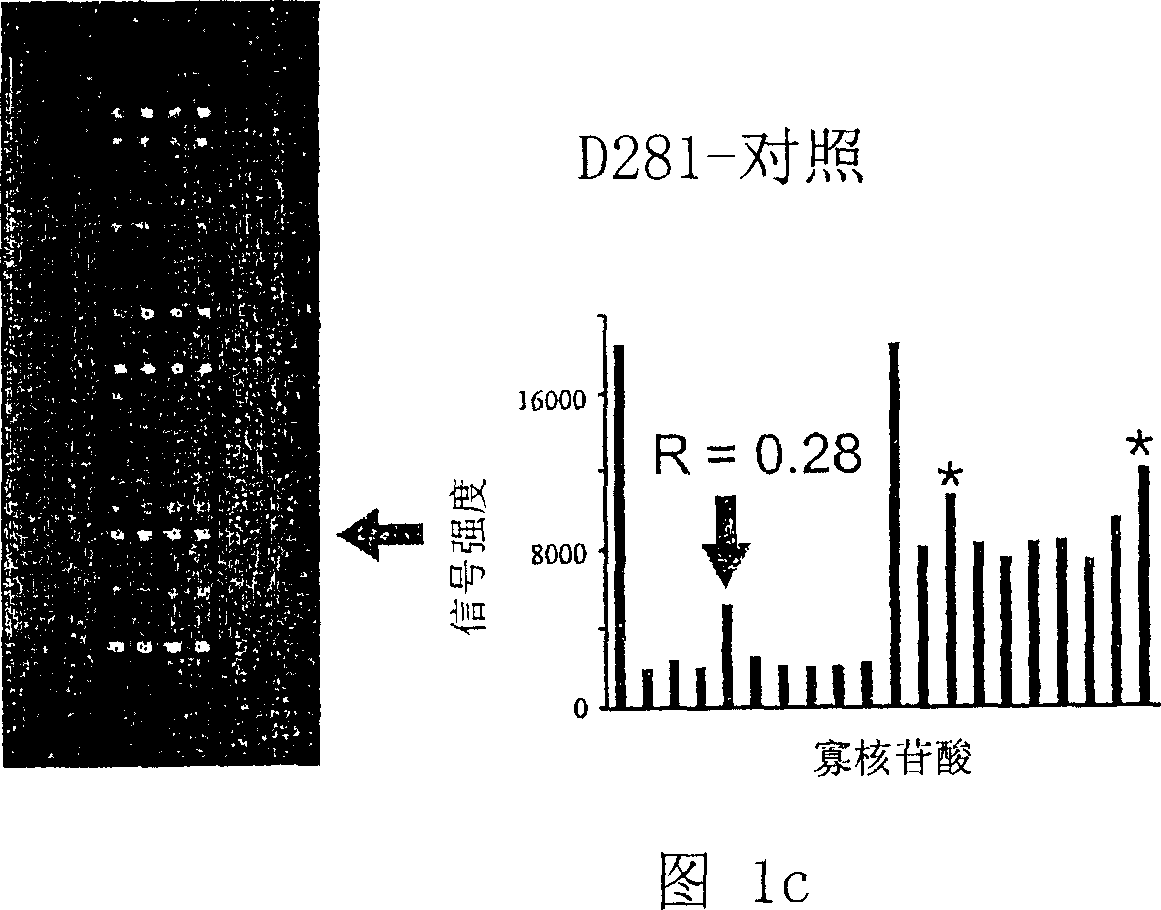
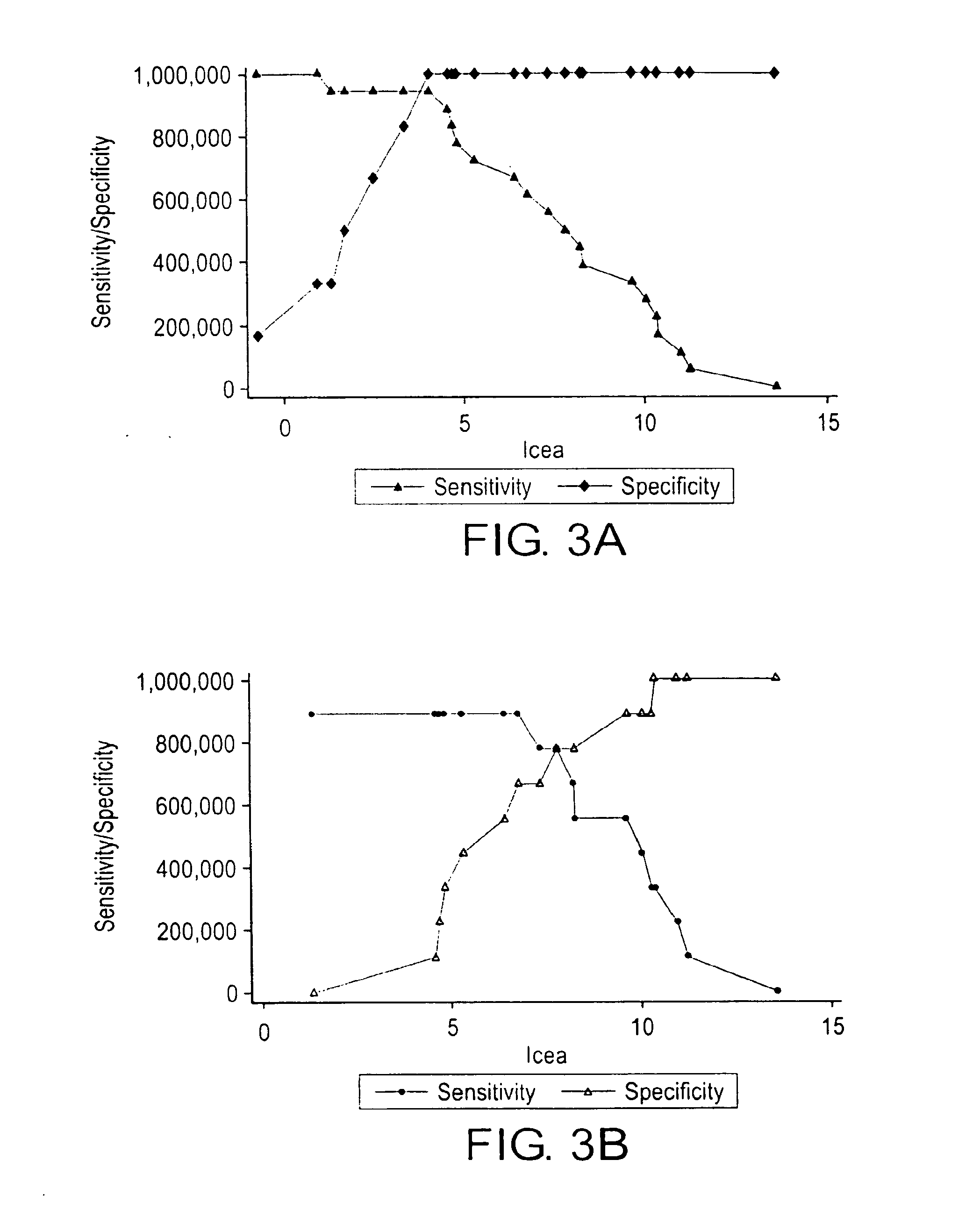
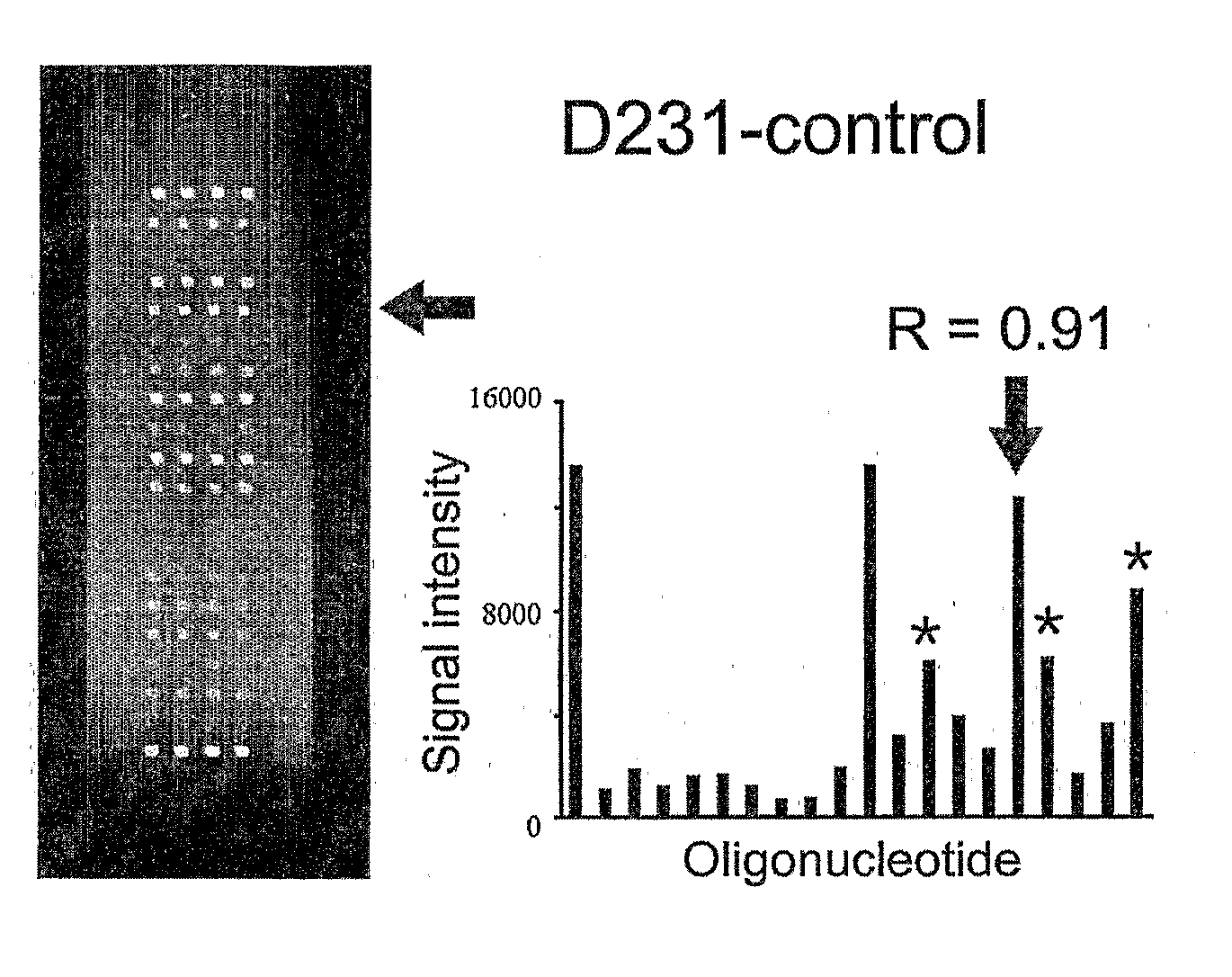
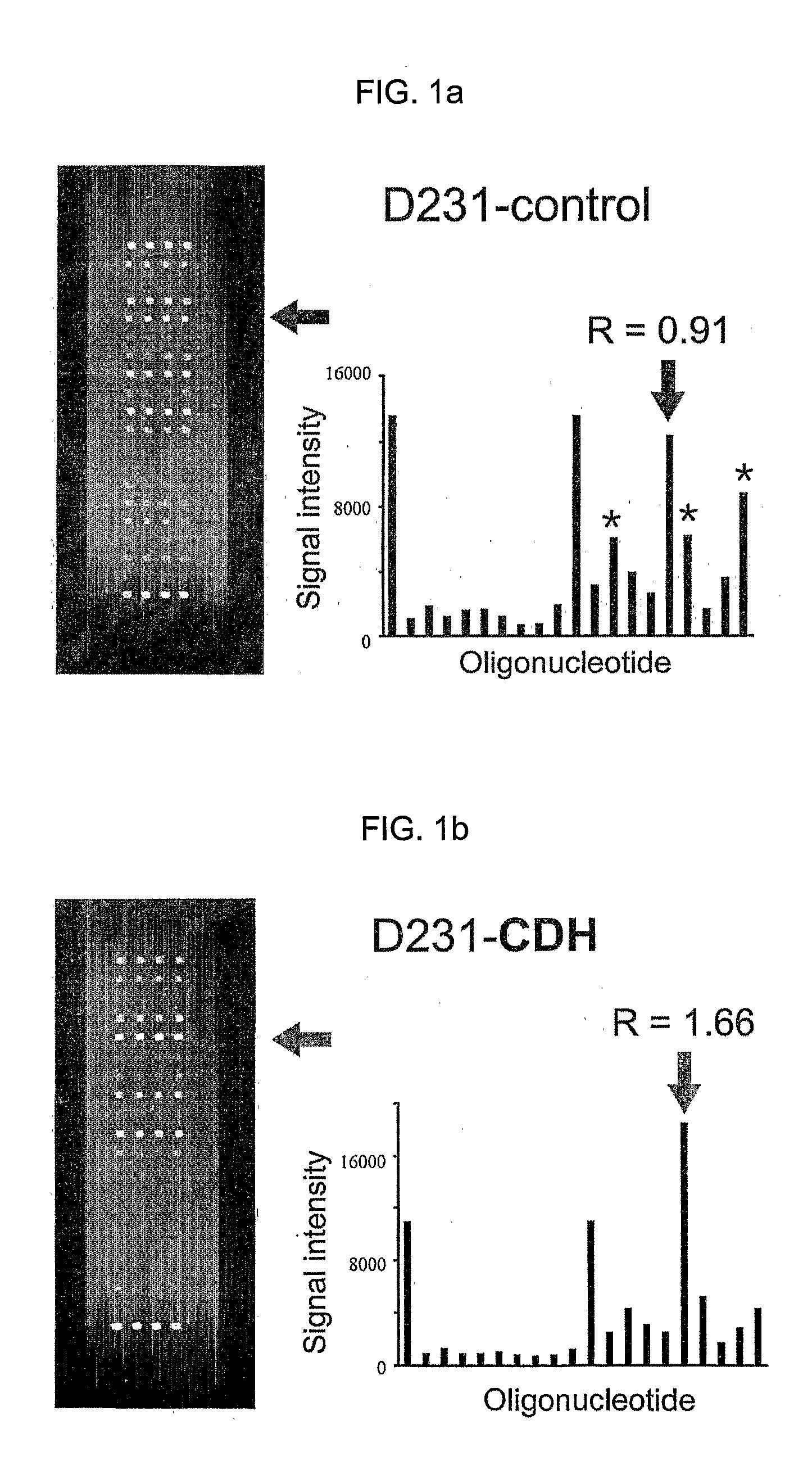
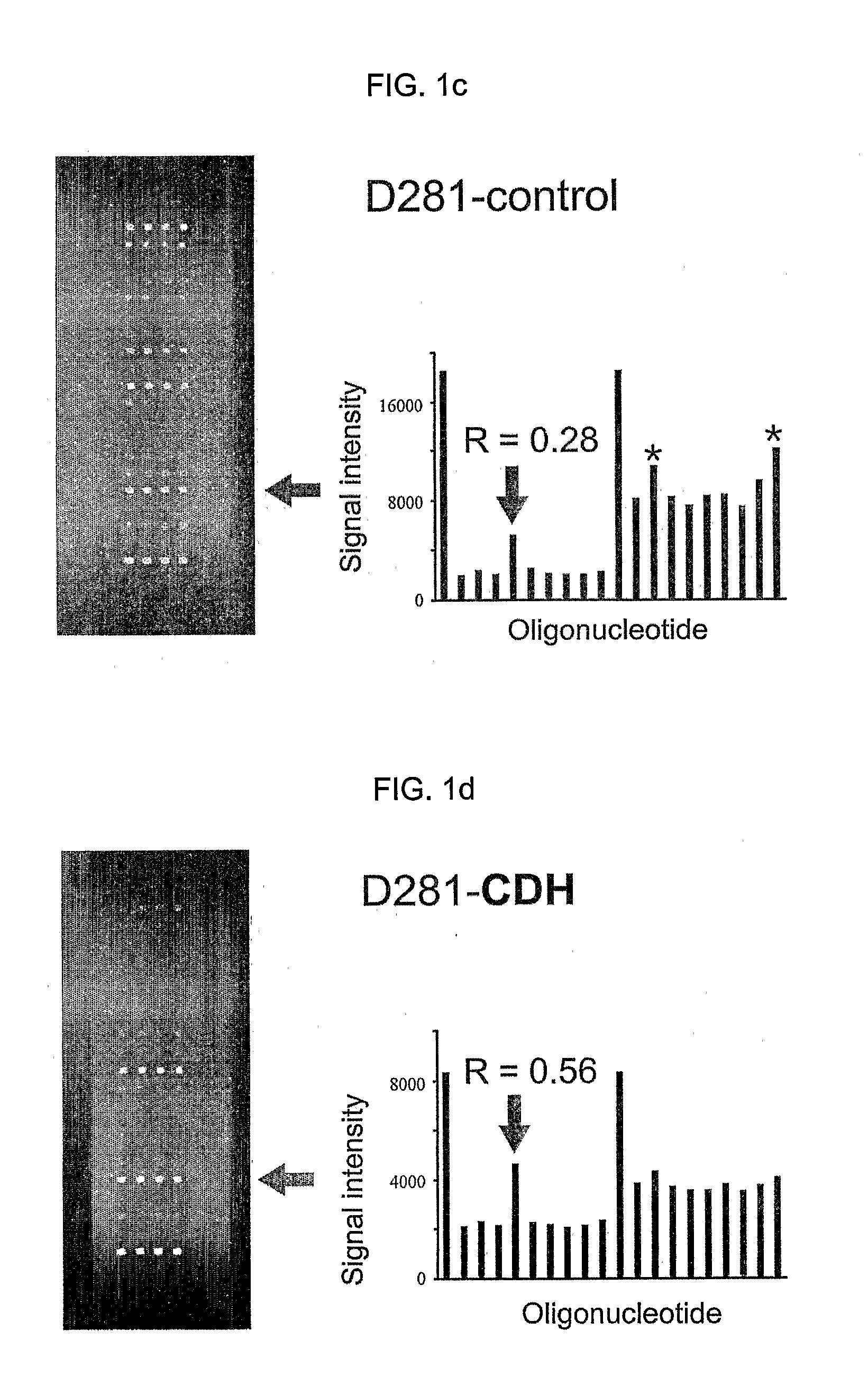
K-ras oligonucleotide microarray and method for detecting K-ras mutations employing the same
Owner:NAT CANCER CENT
Method of treating cancer associated with a ras mutation
ActiveUS20180036304A1Inhibit progressOrganic active ingredientsAntineoplastic agentsPlinabulinCancer research
Owner:BEYONDSPRING PHARMA INC
Chinese lung adenocarcinoma cell system and application thereof
ActiveCN107083365APromote proliferationIn vitro tumorigenic abilityCell dissociation methodsMicrobiological testing/measurementKras mutationCell system
The invention discloses a Chinese lung adenocarcinoma cell system CAFQ1 and application thereof. The preservation number of the cell system is CCTCC NO: C201774. The cell system has similar protein expression as that of primary tumor tissue, also has chromosome abnormal karyotype and has tumor properties of in-vitro tumor formation properties. By adopting the cell system, properties of lung adenocarcinoma tumor cells can be maintained after continuous in-vitro subculture, and the cell system is applicable to cell materials for researching and developing immunity treatment, diagnosis and medicines for lung adenocarcinoma. The cell system disclosed by the invention comprises ALK: Kras mutations, the two mutations can result normal pulmonary epithelial cell mutations and tumor formation and can promote tumor cell proliferation, therefore, the cell system can be used as powerful tool cells for studying target medicines with ALK; Kras as targets.
Owner:SHANGHAI CHEST HOSPITAL
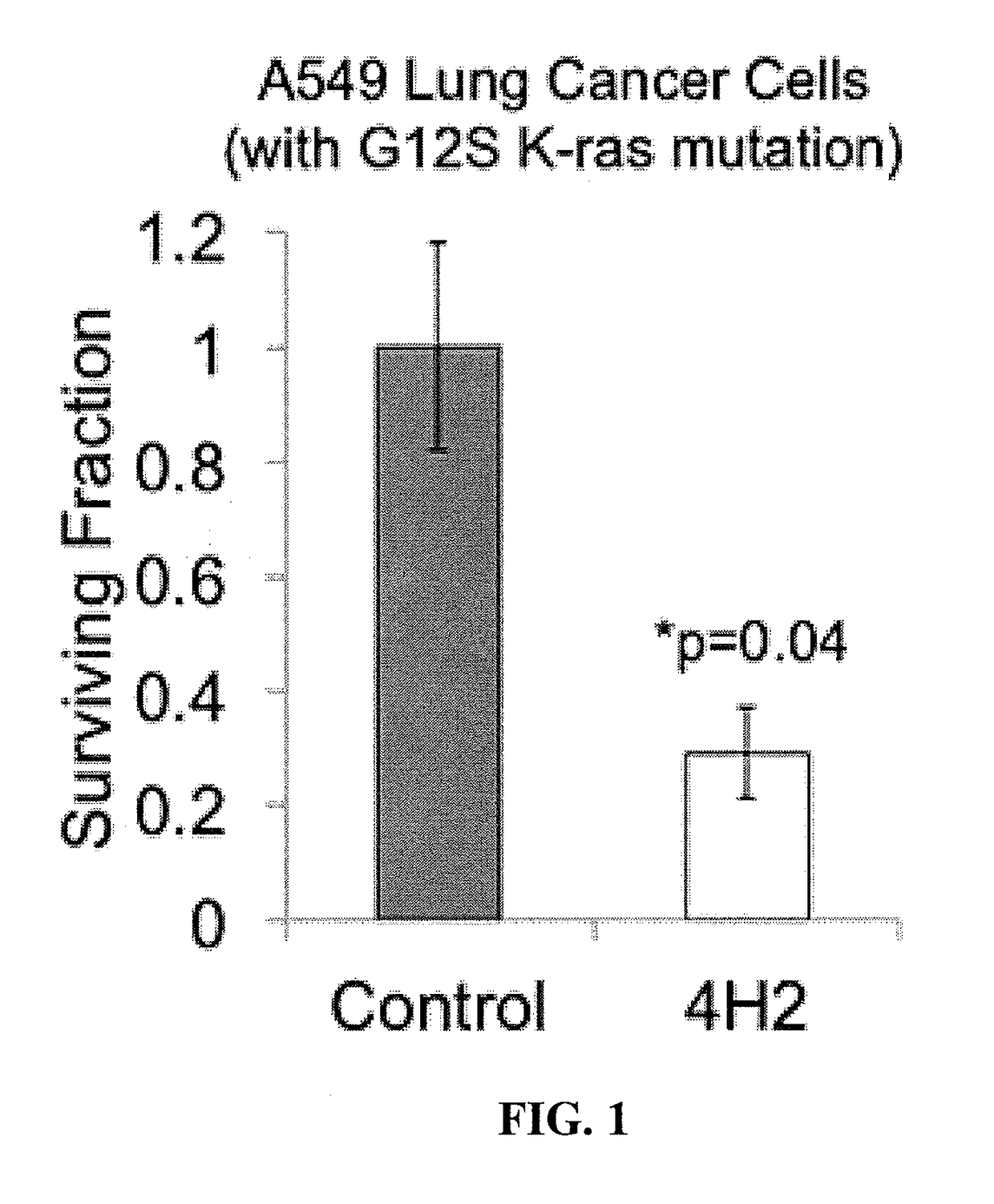
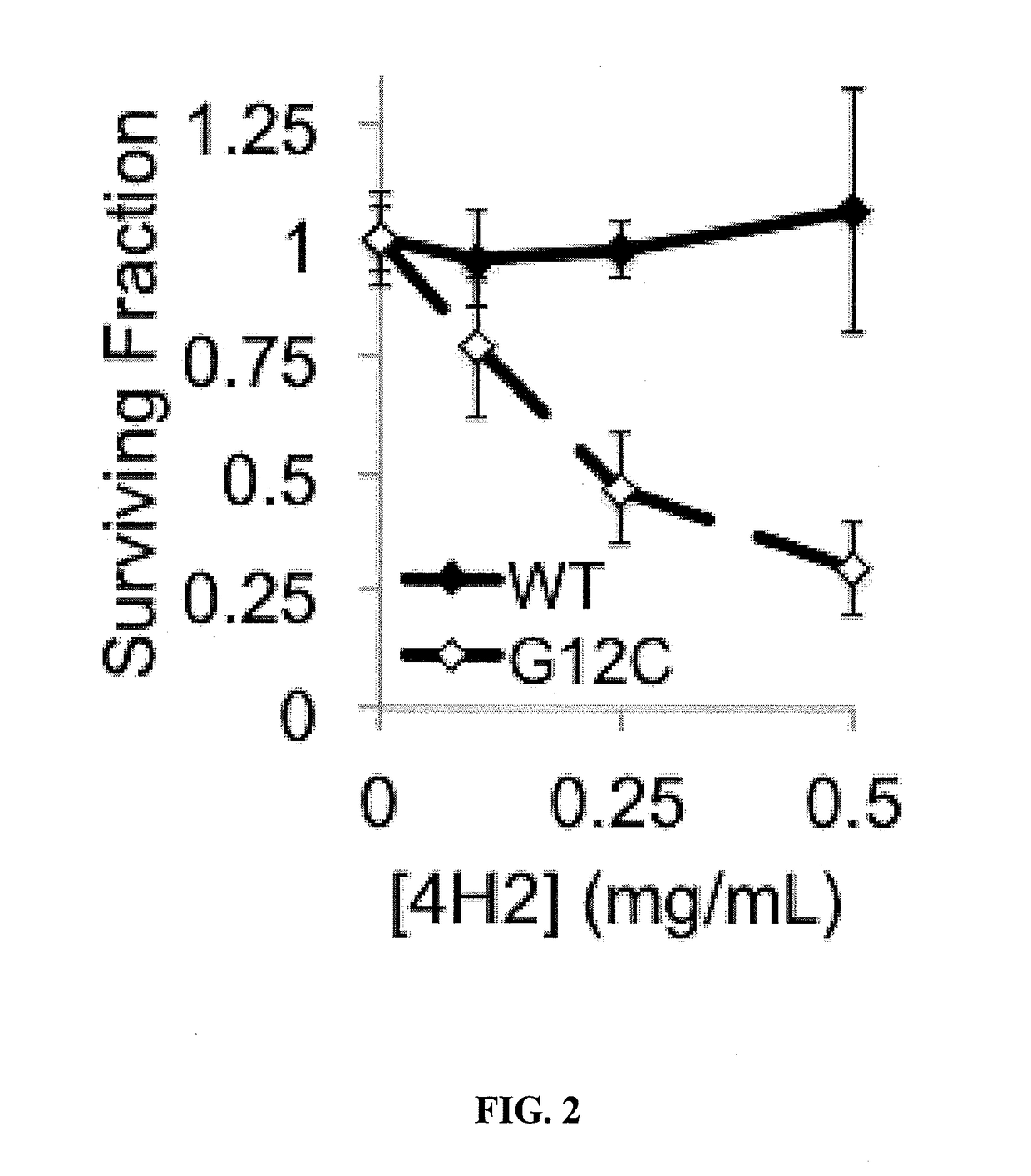
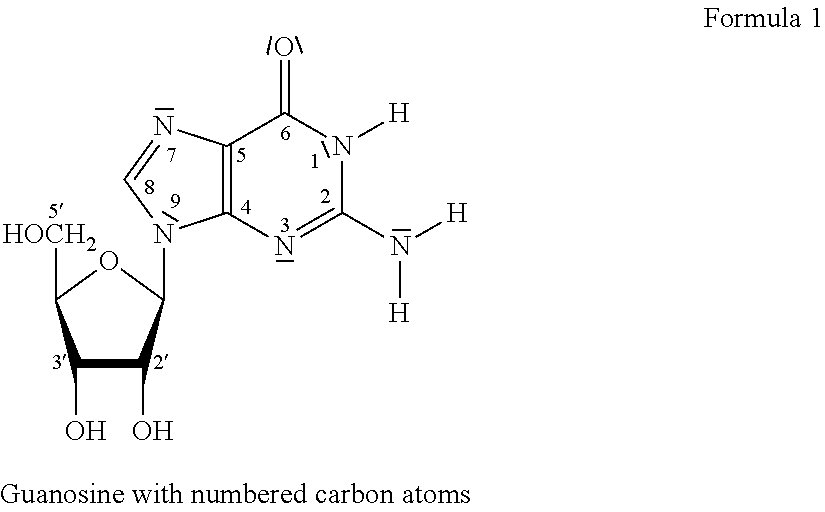
Cell penetrating Anti-guanosine antibody based therapy for cancers with ras mutations
ActiveUS20170073429A1Reduce in quantityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSmall GTPaseCancer cell
It has been established that cancer cells with oncogenic mutants in the small GTPase K-Ras are susceptible to antibodies that bind intracellular guanosine, but delivery of antibodies into cells can be challenging. A subset of lupus autoantibodies is associated with anti-guanosine activity, and is capable of cellular penetration. These antibodies have potential as therapeutic agents targeted towards K-Ras associated malignancies.
Owner:THE UNITED STATES GOVERNMENT AS REPRESENTED BY THE DPARTMENT OF VETERANS AFFAIRS +1
Chemical inhibitor of p53-snail binding and pharmaceutical composition for treating cancer disease containing same as its active ingredient
InactiveUS20120015960A1Effectively treatingEffectively preventingBiocideOrganic chemistryBULK ACTIVE INGREDIENTLung cancer
Provided are compounds for inhibiting Snail-p53 binding and therapeutic agents for cancer including the compounds as an effective component. The Snail-p53 binding inhibitors induce expression of p53 in K-Ras mutant cell lines, thereby enabling effective treatment or prevention of K-Ras mutant cancer, such as, pancreatic cancer, lung cancer, cholangioma, and colon cancer, of which diagnosis or treatment is not easy.
Owner:PUSAN NAT UNIV IND UNIV COOPERATION FOUND +1
Methods for treating cancers comprising k-ras mutations
InactiveUS20160243223A1Immunoglobulins against growth factorsAntibody ingredientsExtracellular StructureWilms' tumor
Methods of inhibiting tumor growth, methods of treating cancer, and methods of reducing the frequency of cancer stem cells in a tumor are described. Particularly, the methods are directed to tumors or cancers that comprise a K-ras mutation. The methods described comprise administering a DLL4 antagonist (e.g., an antibody that specifically binds the extracellular domain of human DLL4) to a subject. Related polypeptides and polynucleotides, compositions comprising the DLL4 antagonists, and methods of making the DLL4 antagonists are also described.
Owner:ONCOMED PHARMA INC
Methods of treating cancer patients with farnesyl transferase inhibitors
ActiveCN107787373AOrganic active ingredientsMicrobiological testing/measurementTransferase inhibitorGenotyping
The present invention relates to the field of molecular biology and cancer biology. Specifically, the present invention relates to methods of treating a subject with a farnesyltransferase inhibitor (FTI) that include determining whether the subject is likely to be responsive to the FTI treatment based on genotyping and expression profiling of certain immunological genes and RAS mutation status inthe subject.
Owner:KURA ONCOLOGY
Kit for quantitative detection of K-ras mutation
ActiveCN102115782AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesEGFR Tyrosine Kinase Inhibitors
The invention relates to a kit for quantitative detection of K-ras mutation. The invention specifically relates to a detecting method and a detecting kit for K-ras gene mutation relevant with the curative effect of molecular targeted anti-cancer drugs. The invention especially relates to fluorescence quantification PCR detecting method for K-ras gene mutational hotspot region detecting mutational, kit and the application of the fluorescence quantification PCR detecting method. The method of the invention detects the mutation of K-ras gene at a particular locus, predicts the curative effect of molecule targeting anticancer medicament EGFR tyrosine kinases inhibitor, furthermore provides clinic individualized medication scheme for a tumour patient with guidance.
Owner:BEIJING ACCB BIOTECH
Cell penetrating anti-guanosine antibody based therapy for cancers with Ras mutations
ActiveUS10040867B2Increases cell 's sensitivityIncrease cell 's sensitivityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSmall GTPaseCancer cell
Owner:THE UNITED STATES GOVERNMENT AS REPRESENTED BY THE DPARTMENT OF VETERANS AFFAIRS +1
Topographic genotyping for determining the diagnosis, malignant potential, and biologic behavior of pancreatic cysts and related conditions
InactiveUS20140296103A1Easy to analyzeEasy diagnosisHealth-index calculationMicrobiological testing/measurementMolecular analysisMicrosatellite
The application relates to a method of a predicting the presence of invasive pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions comprising detailed molecular analysis incorporating DNA quality and quantity, K-ras mutational analysis and a broad spectrum of tumor suppressor gene linked microsatellite LOH. Methods of diagnosing, determining prognosis of and determining a course of treatment for pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions are also provided.
Owner:REDPATH INTEGRATED PATHOLOGY INC
K-Ras Oligonucleotide Microarray and Method for Detecting K-Ras Mutations Employing the Same
InactiveUS20070298419A1Fast and reliableBioreactor/fermenter combinationsBiological substance pretreatmentsK-ras GenesBiology
Since the K-ras oligonucleotide microarray of the present invention can detect K-ras mutations by applying a competitive DNA hybridization method to the oligonucleotides spotted on a solid matrix different from the previously reported method for detecting a mutation, it makes the more precise analysis and can reduce experimental cost and time. Accordingly, the K-ras oligonucleotide microarray of the present invention can be used in studies to detect K-ras mutations and unravel the signal transduction mechanism and tumorigenesis related to K-ras gene. Further, since the microarray of the present invention can be applied to other genes having mutational hot spot regions such as K-ras, it has wide applicable range.
Owner:NAT CANCER CENT
Establishment and application of MMHRL1 transgenic mouse liver tumor cell line
InactiveCN106635999AStable traitsStable for multiple passagesCompounds screening/testingGenetically modified cellsRenin–angiotensin systemMitogen-activated protein
The invention provides establishment and application of an H-ras 12V transgenic male mouse liver tumor cell line MMHRL1. Specifically, the H-ras 12V transgenic male mouse liver tumor cell line MMHRL1 provided by the invention has stable properties, can be stably passed for a plurality of times, is applicable to establishment of a liver tumor animal model and is an ideal cell line for foundation and clinical prophase application researches of liver tumor. Furthermore, the H-ras 12V transgenic male mouse liver tumor cell line MMHRL1 can be used for expressing H-ras mutated cancer genes and has highly-activated Ras / MAPK (Renin-angiotensin system / Mitogen-activated Protein Kinase) and PI3K / AKT / mTOR (Phosphoinositide 3-kinase / protein kinase B / mammalian Target of Rapamycin) signal paths. The invention further provides the application of the cell line to the aspects of establishment of model animals, drug screening and the like.
Owner:DALIAN MEDICAL UNIVERSITY
Kits for quantitative detection of k-ras mutations
InactiveUS20120288862A1Accurately and quantitatively ratioEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesEGFR Tyrosine Kinase Inhibitors
The present invention relates to an assay kit for quantitatively detecting k-ras gene mutations. Particularly, the present invention relates to detection method and a detection kit for K-ras gene mutations, which relates to the therapeutic efficacy of targeted molecular anti-cancer drugs. More particularly, the present invention relates to a fluorescent quantitative PCR method and kit for detecting mutations at hotspots of K-ras gene, together with the use thereof. The present invention detects the mutations at specific sites of K-ras gene, and can predict the therapeutic efficacy of anti-EGFR tyrosine kinase inhibitors. Therefore, it can provide a guidance to individualized treatments for cancer patients.
Owner:BEIJING ACCB BIOTECH
Rapid detection kit for K-ras gene mutation
InactiveCN110004224AThe detection process is fastMicrobiological testing/measurementK-ras GenesFluorescence
The invention relates to a rapid detection kit for K-ras gene mutation, and belongs to the technical field of molecular biology. The kit consists of an ARMS upstream primer, a downstream primer, a trigger primer and a fluorescent probe for detection of 10 sensitive mutation sites. The K-ras gene detection kit can detect 10 major mutations simultaneously under a single-tube condition. With design of the trigger primer, a reaction system generates fluorescence when any kind of mutation exists, and the effect of rapid detection and determination of K-Ras mutation can be achieved. The kit has theadvantages of fast detection speed and the sensitivity degree reaching 0.001%.
Owner:邓定平
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com