Patents
Literature
826 results about "Mutated protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
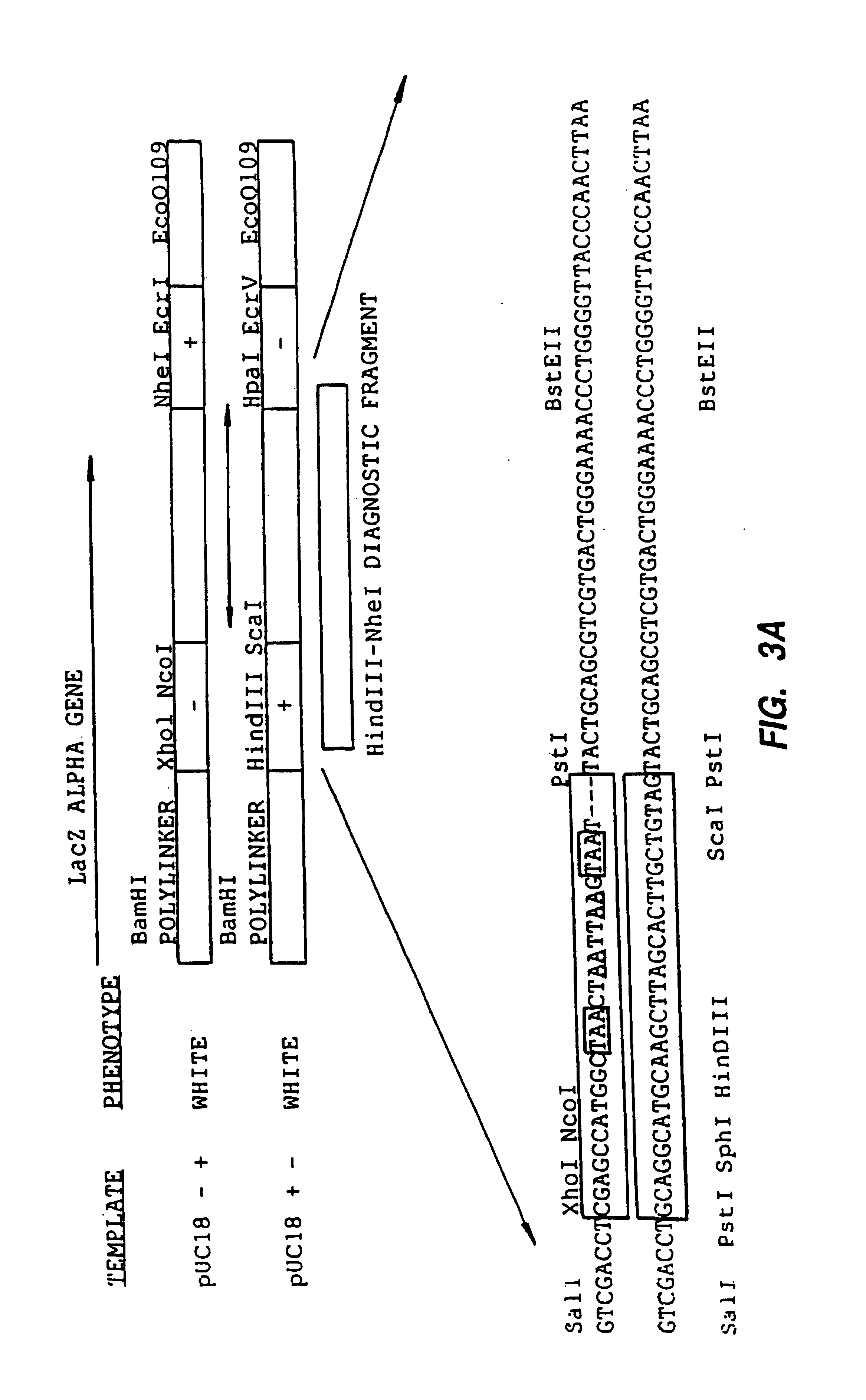
Mutant protein. A mutant protein is the protein product encoded by a gene with mutation. Mutated protein can have single amino acid change (minor, but still in many cases significant change leading to disease) or wide-range amino acid changes by e.g. truncation of C-terminus after introducing premature stop codon.
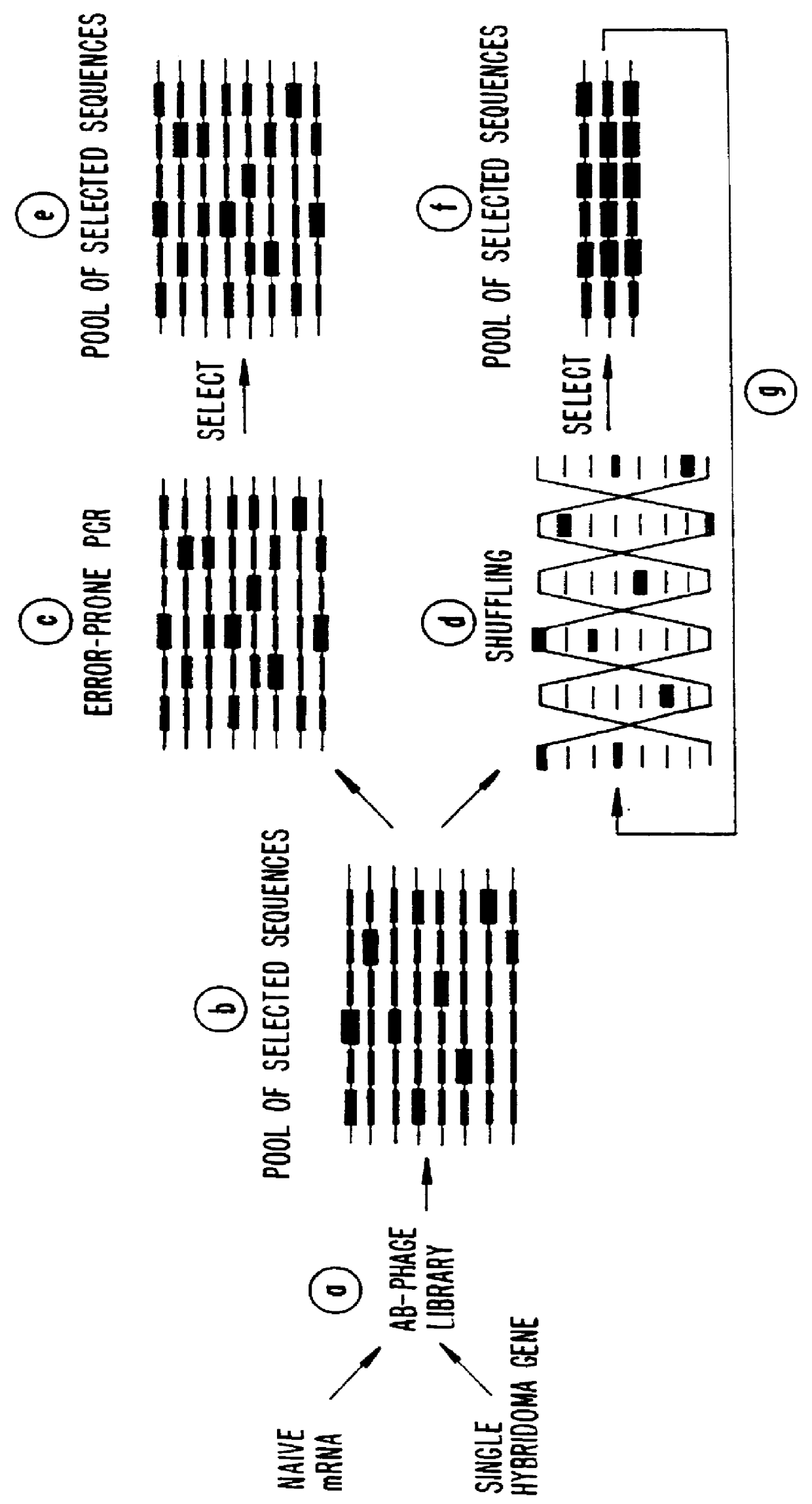
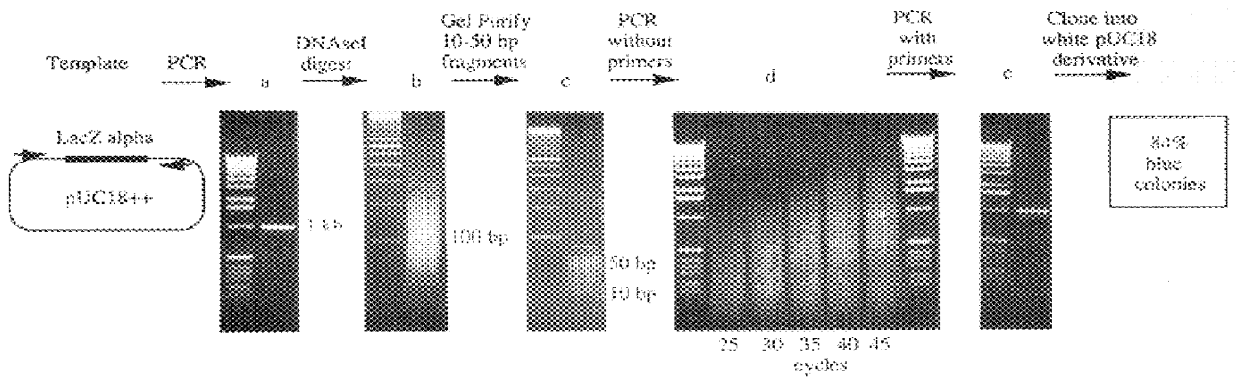
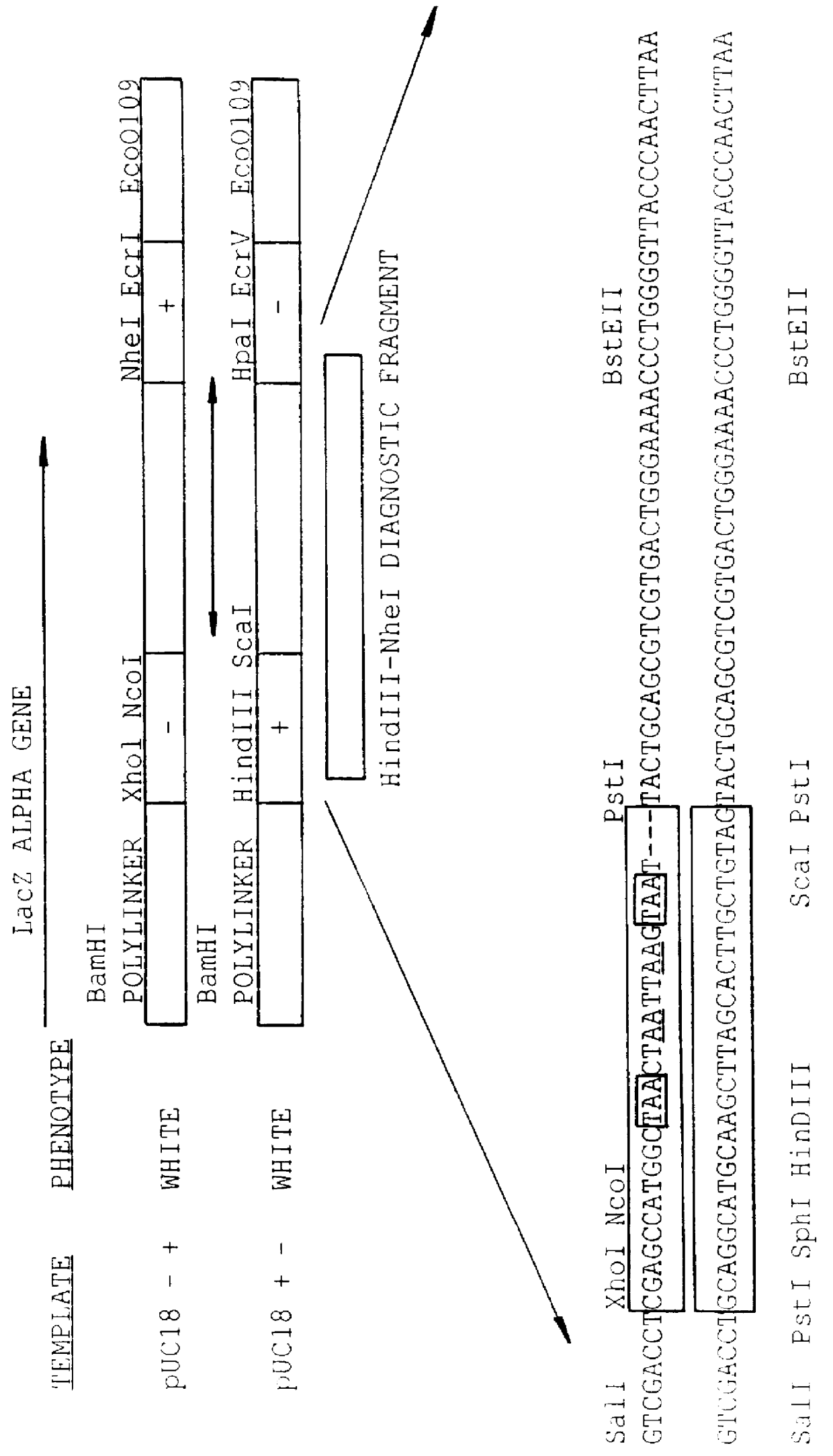
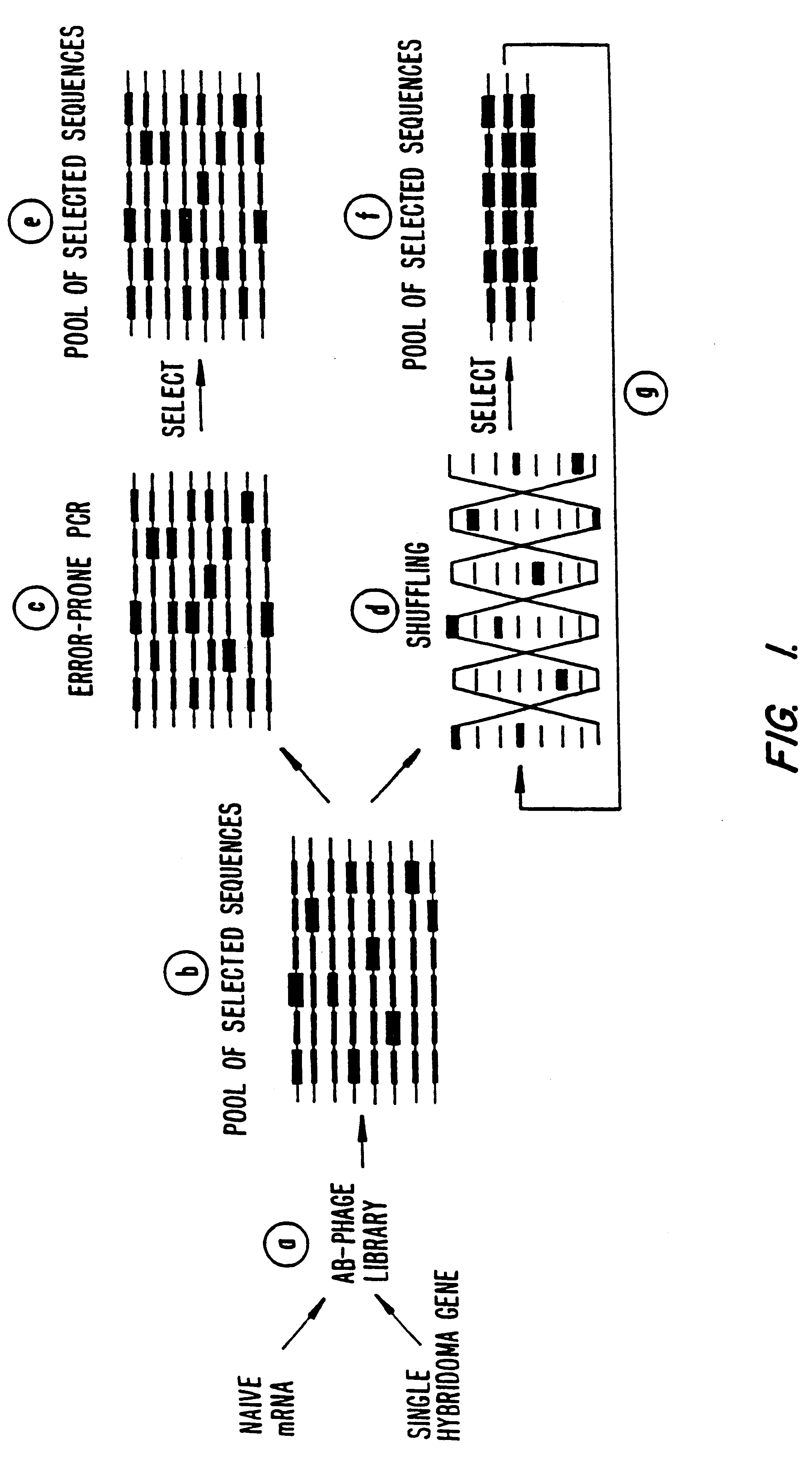
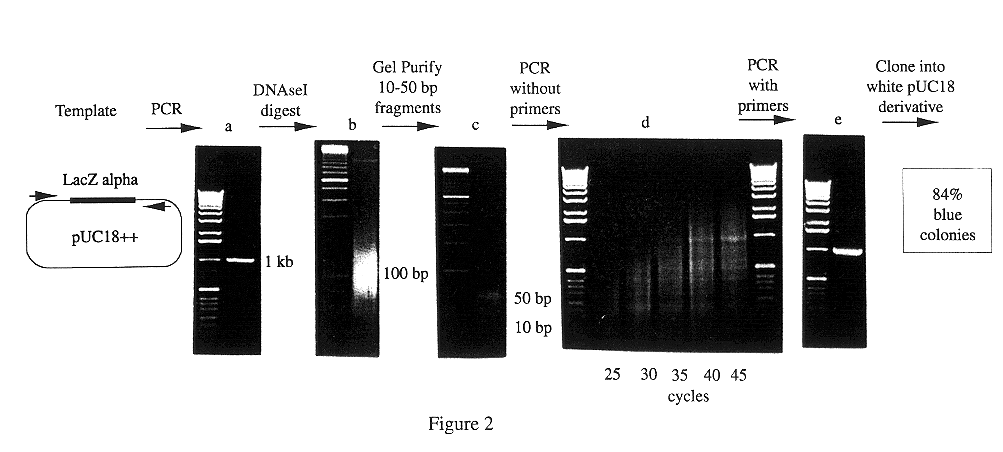
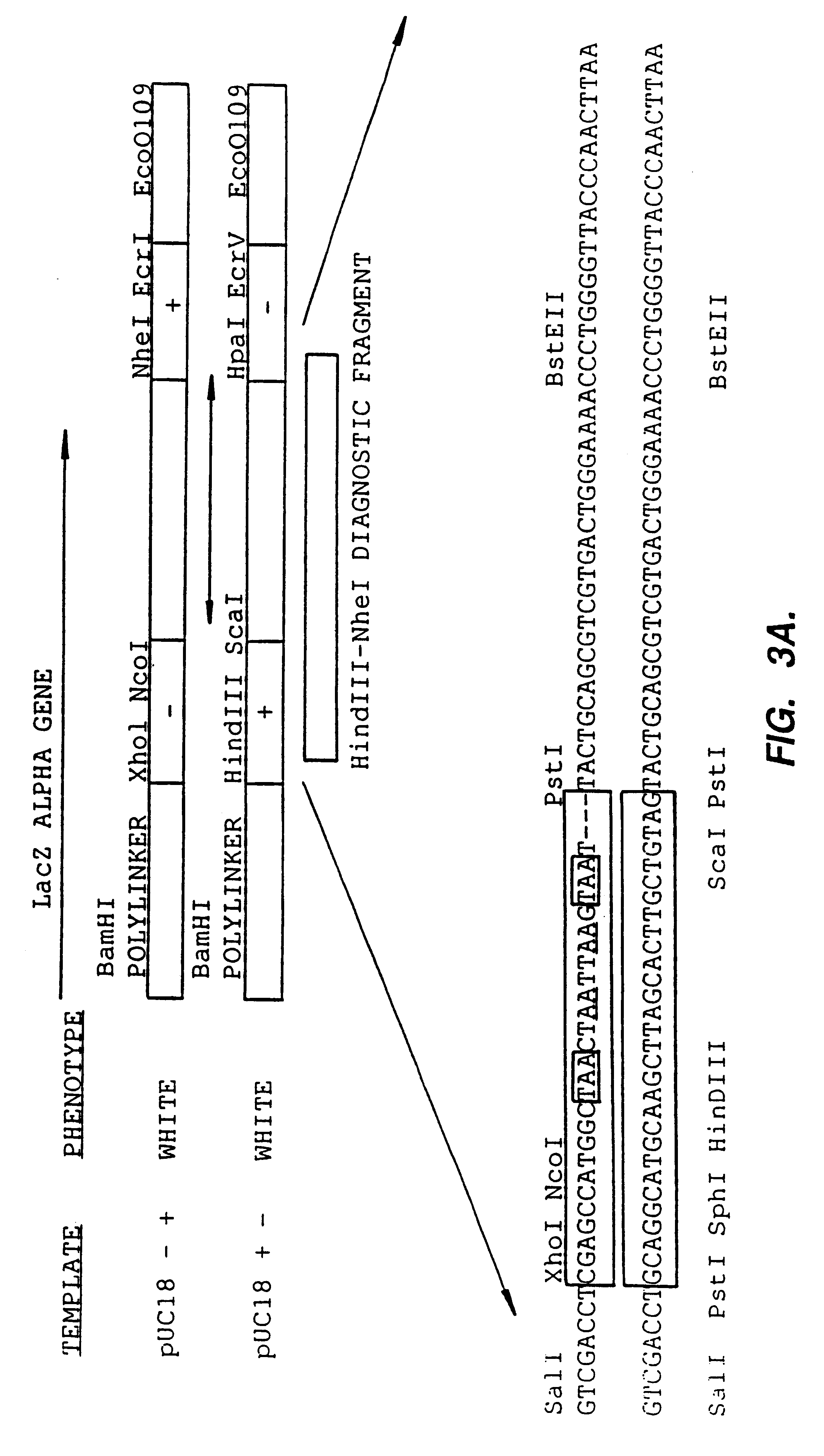
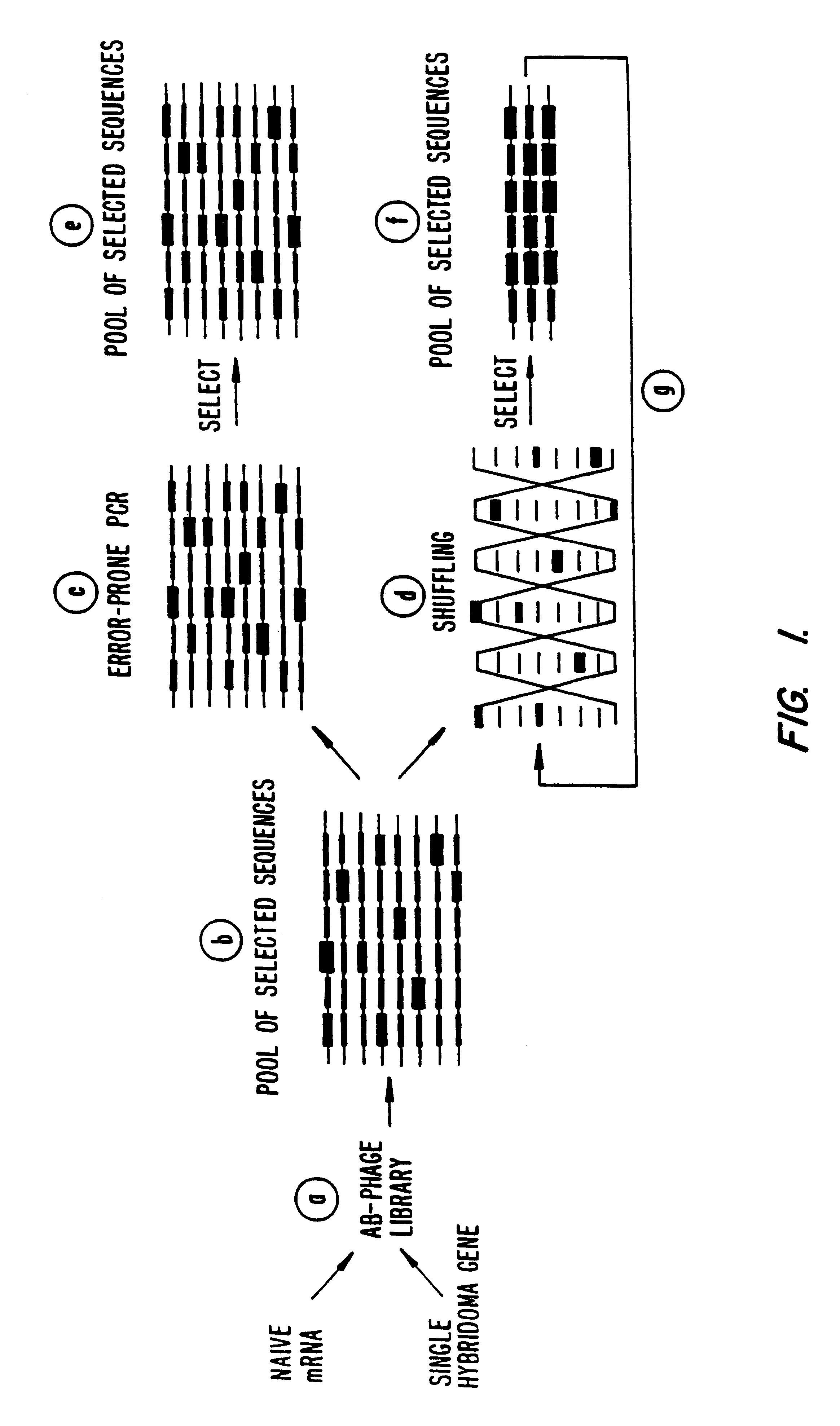
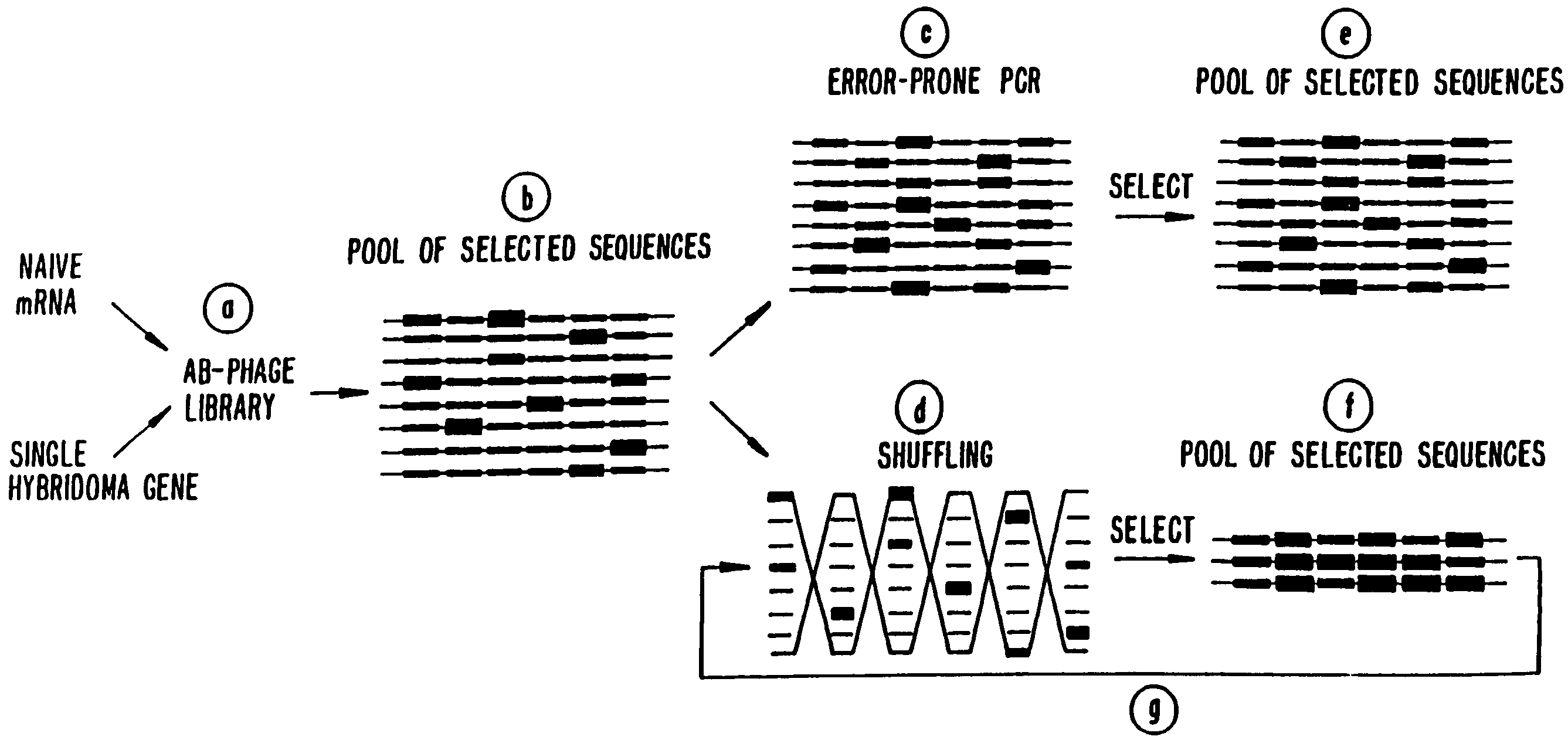
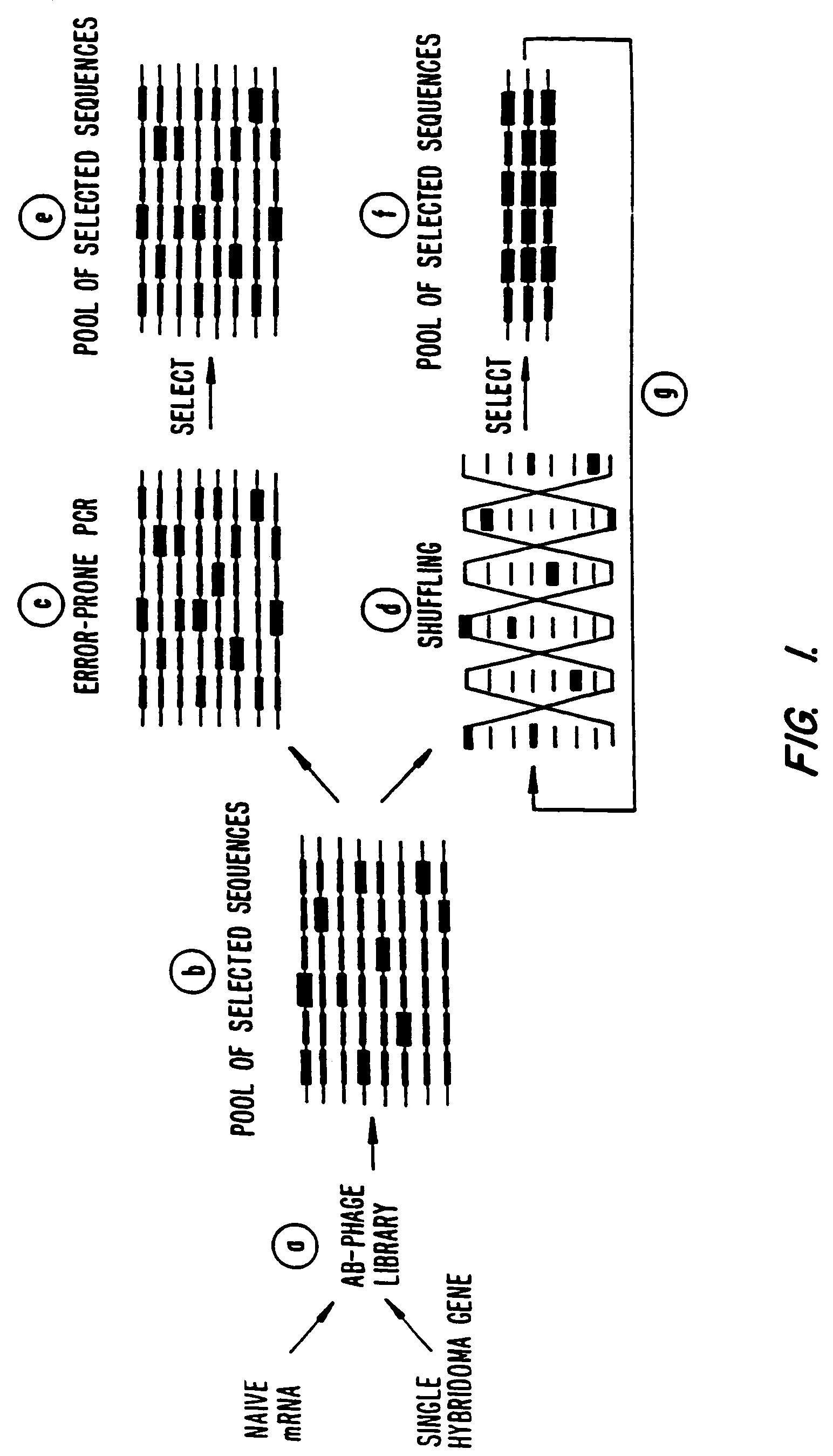
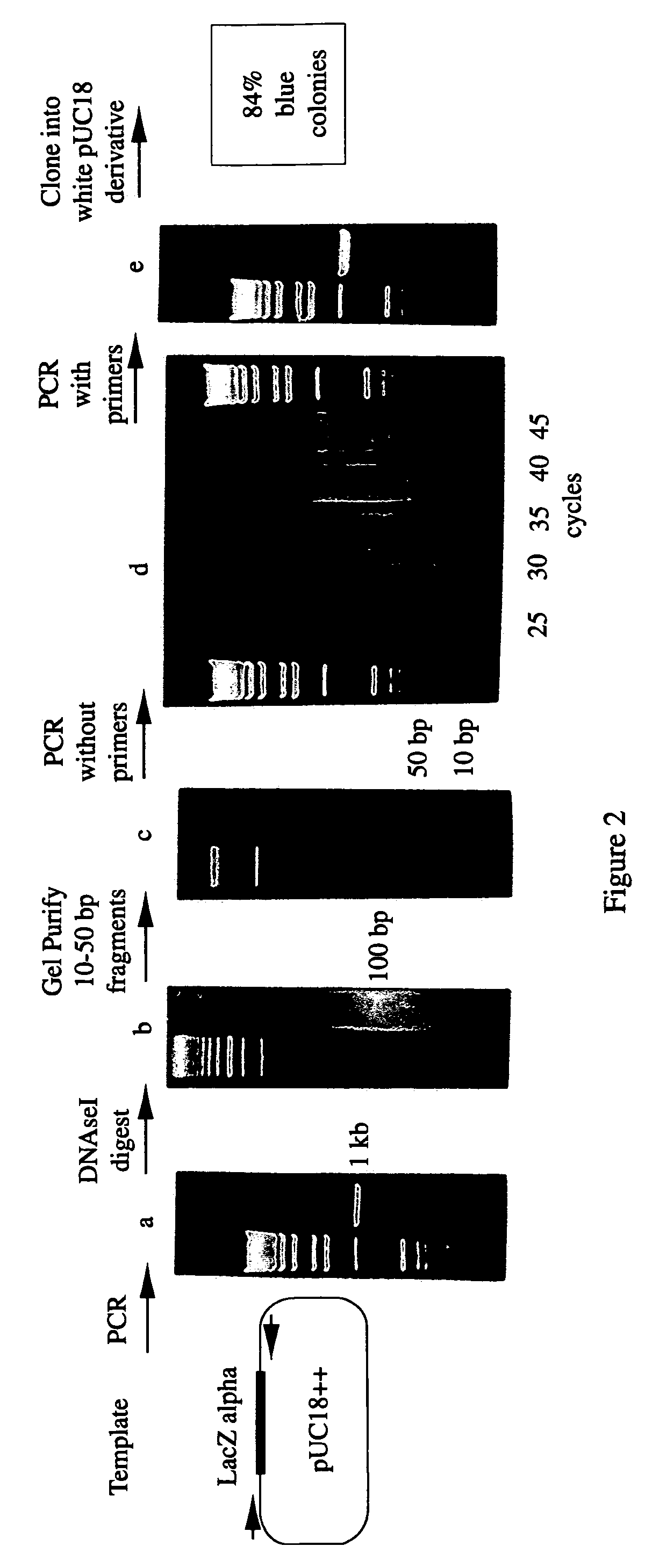
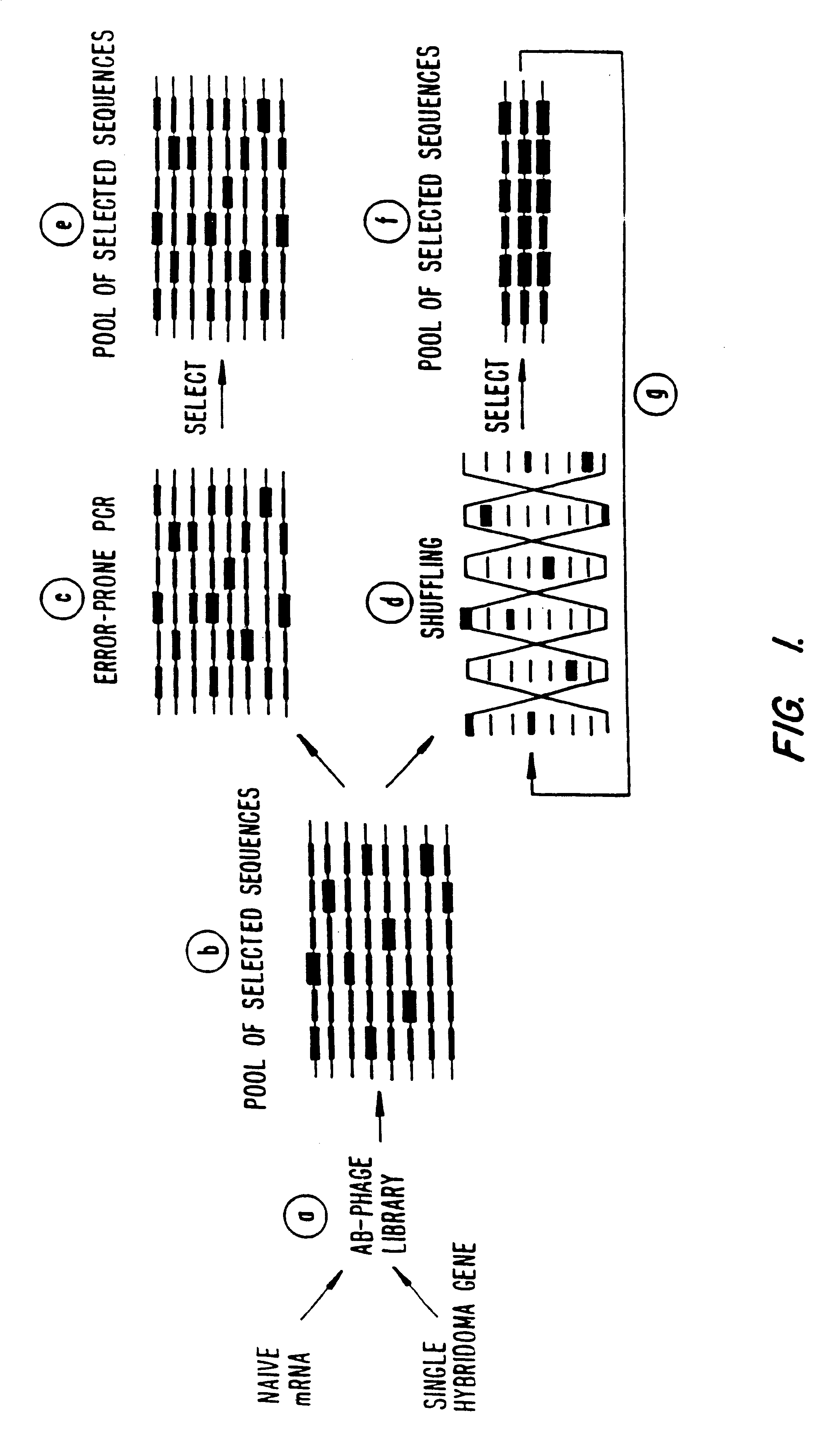
Methods for generating polynucleotides having desired characteristics by iterative selection and recombination
InactiveUS6117679ALess immunogenicLibrary screeningDirected macromolecular evolutionMutated proteinNucleic acid sequencing
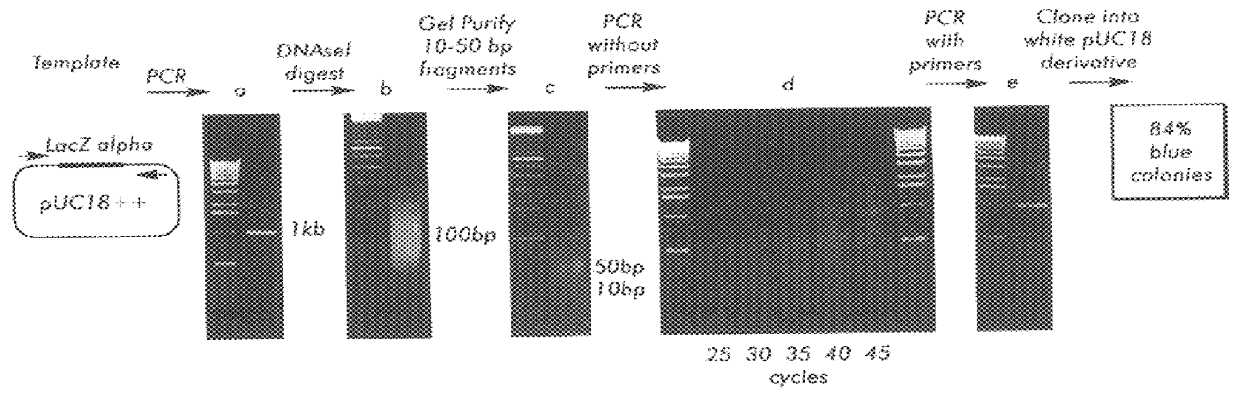
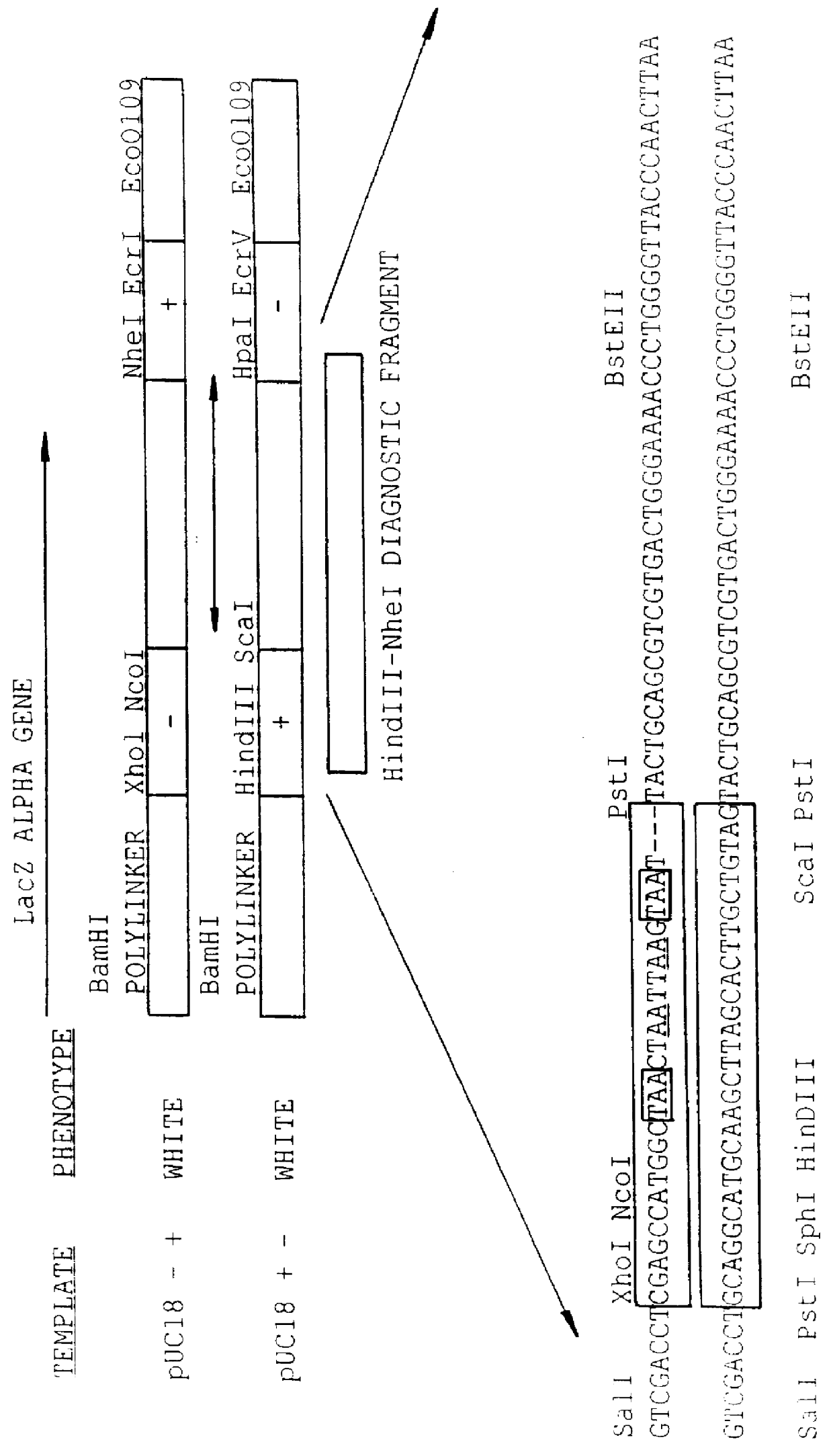
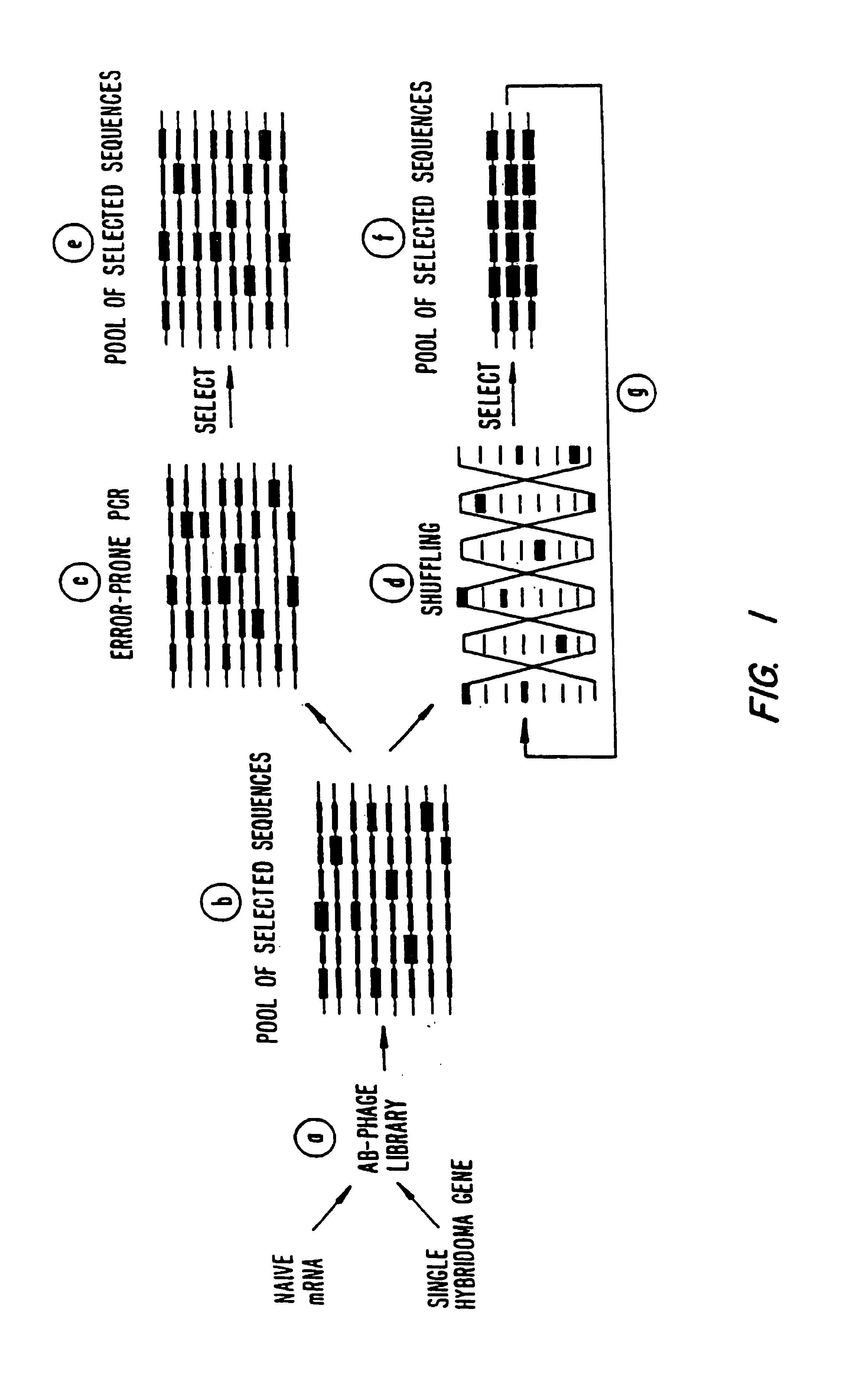
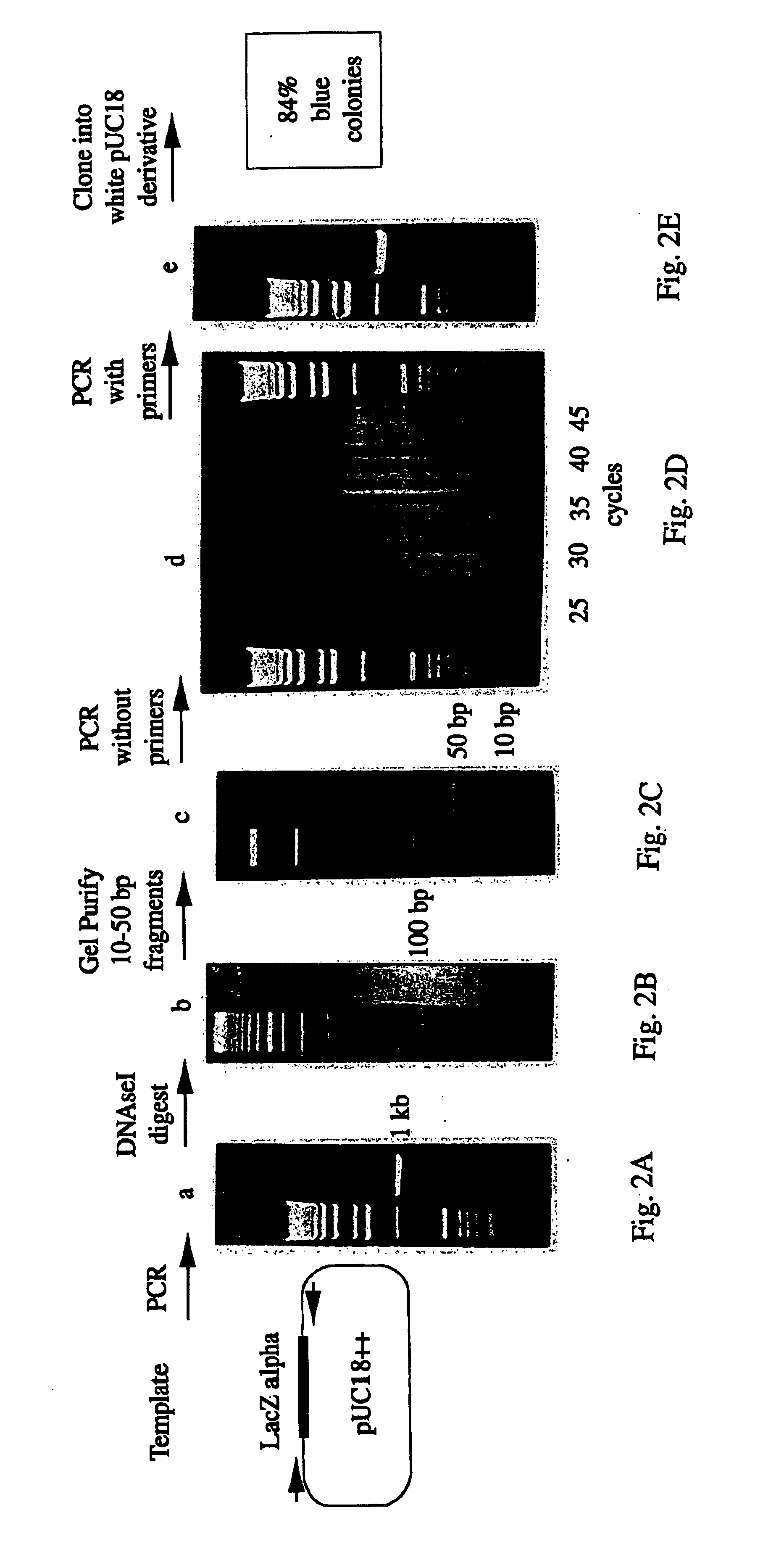
A method for DNA reassembly after random fragmentation, and its application to mutagenesis of nucleic acid sequences by in vitro or in vivo recombination is described. In particular, a method for the production of nucleic acid fragments or polynucleotides encoding mutant proteins is described. The present invention also relates to a method of repeated cycles of mutagenesis, shuffling and selection which allow for the directed molecular evolution in vitro or in vivo of proteins.
Owner:CODEXIS MAYFLOWER HLDG LLC
Methods for generating polynucleotides having desired characteristics by iterative selection and recombination
A method for DNA reassembly after random fragmentation, and its application to mutagenesis of nucleic acid sequences by in vitro or in vivo recombination is described. In particular, a method for the production of nucleic acid fragments or polynucleotides encoding mutant proteins is described. The present invention also relates to a method of repeated cycles of mutagenesis, shuffling and selection which allow for the directed molecular evolution in vitro or in vivo of proteins.
Owner:CODEXIS MAYFLOWER HLDG LLC
Methods for generating polynucleotides having desired characteristics by iterative selection and recombination
InactiveUS6180406B1Less immunogenicLibrary screeningDirected macromolecular evolutionMutated proteinNucleic acid sequencing
A method for DNA reassembly after random fragmentation, and its application to mutagenesis of nucleic acid sequences by in vitro or in vivo recombination is described. In particular, a method for the production of nucleic acid fragments or polynucleotides encoding mutant proteins is described. The present invention also relates to a method of repeated cycles of mutagenesis, shuffling and selection which allow for the directed molecular evolution in vitro or in vivo of proteins.
Owner:CODEXIS MAYFLOWER HLDG LLC
Methods for generating polynucleotides having desired characteristics by iterative selection and recombination
InactiveUS6165793ALess immunogenicDirected macromolecular evolutionImmunoglobulinsMutated proteinNucleotide
A method for DNA reassembly after random fragmentation, and its application to mutagenesis of nucleic acid sequences by in vitro or in vivo recombination is described. In particular, a method for the production of nucleic acid fragments or polynucleotides encoding mutant proteins is described. The present invention also relates to a method of repeated cycles of mutagenesis, shuffling and selection which allow for the directed molecular evolution in vitro or in vivo of proteins.
Owner:CODEXIS MAYFLOWER HLDG LLC
EGFR mutations
The present invention relates to mutations in Epidermal Growth Factor Receptor (EGFR) and methods of detecting such mutations as well as prognostic methods method for identifying a tumors that are susceptible to anticancer therapy such as chemotherapy and / or kinase inhibitor treatment. The methods involve determining the presence of a mutated EGFR gene or mutated EGFR protein in a tumor sample whereby the presence of a mutated EGFR gene or protein indicates the tumor is susceptible to treatment.
Owner:GENENTECH INC
Methods for generating polynucleotides having desired characteristics by iterative selection and recombination
InactiveUS6995017B1Enhance rate of recombinationEnhancing recombinationSugar derivativesMicrobiological testing/measurementMutated proteinNucleotide
A method for DNA reassembly after random fragmentation, and its application to mutagenesis of nucleic acid sequences by in vitro or in vivo recombination is described. In particular, a method for the production of nucleic acid fragments or polynucleotides encoding mutant proteins is described. The present invention also relates to a method of repeated cycles of mutagenesis, shuffling and selection which allow for the directed molecular evolution in vitro or in vivo of proteins.
Owner:CODEXIS MAYFLOWER HLDG LLC
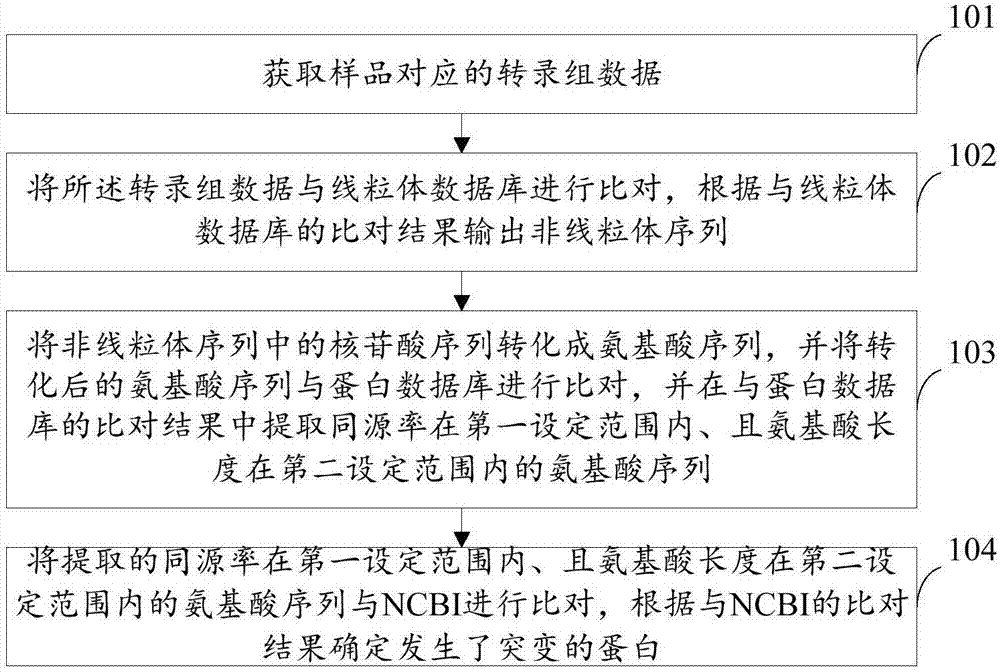
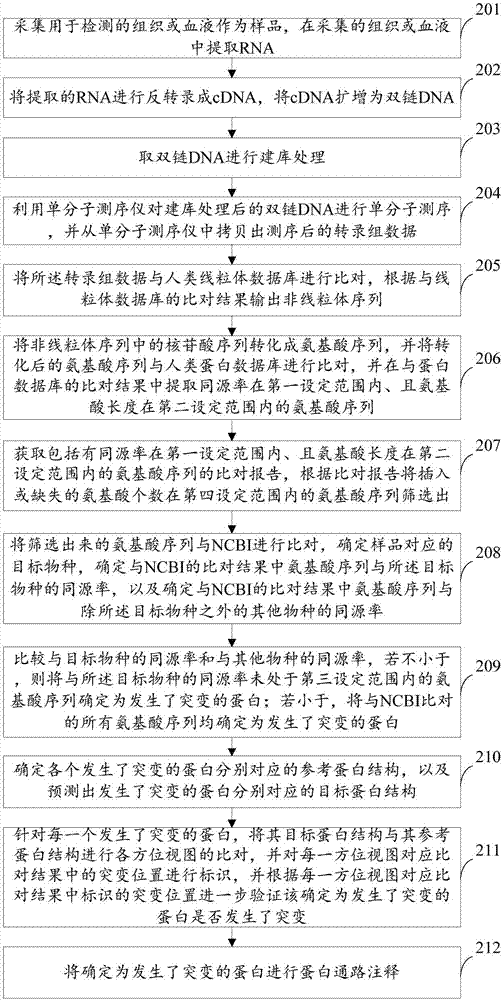
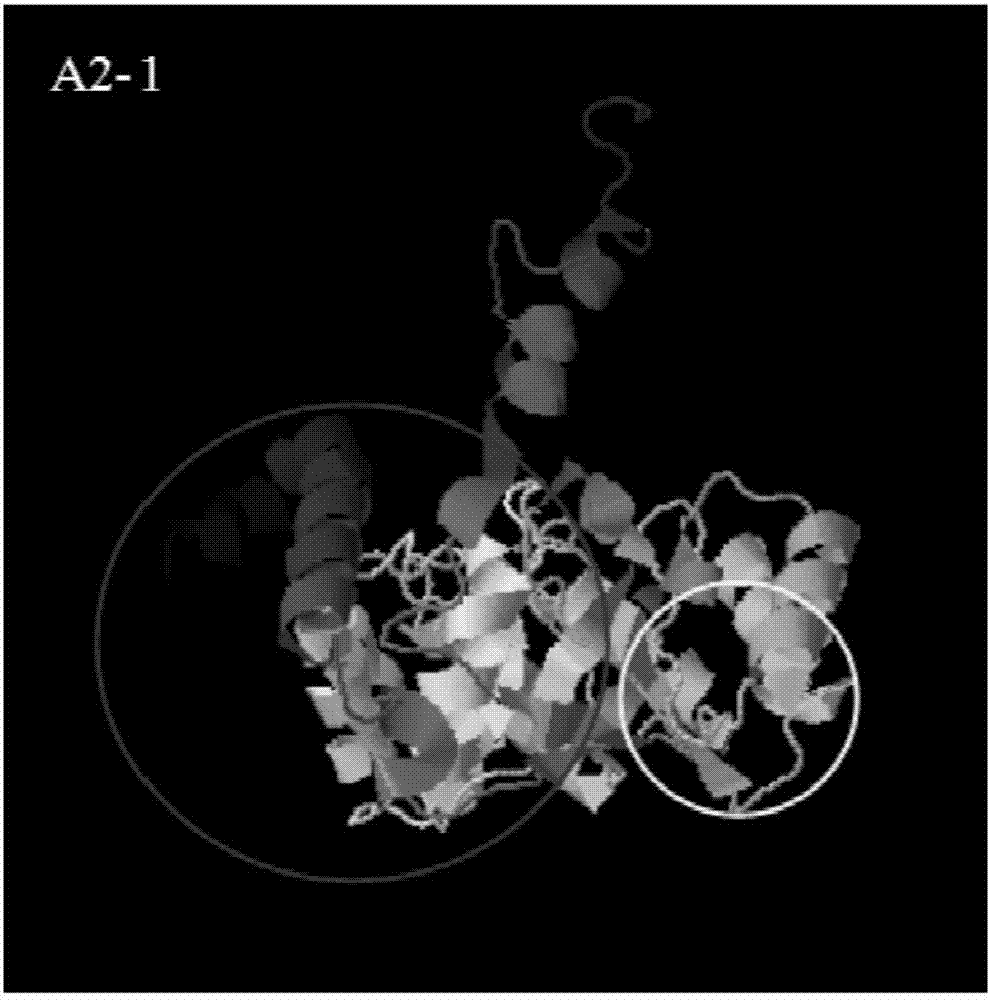
Method and device for detecting mutated proteins
The invention provides a method and a device for detecting mutated proteins. The method includes acquiring transcriptome data corresponding to samples; comparing the transcriptome data to mitochondrion databases and outputting non-mitochondrion sequences according to comparison results of the transcriptome data and the mitochondrion databases; transforming nucleotide sequences in the non-mitochondrion sequences into amino acid sequences, comparing the transformed amino acid sequences to protein databases and extracting amino acid sequences with homogenous rates in first set ranges and amino acid lengths in second set ranges from comparison results of the transformed amino acid sequences and the protein databases; comparing the extracted amino acid sequences with the homogenous rates in the first set ranges and the amino acid lengths in the second set ranges to NCBI (national center of biotechnology information) and determining the mutated proteins according to comparison results of the extracted amino acid sequences and the NCBI. According to the scheme, the method and the device have the advantage that the mutated proteins in the samples can be detected by the aid of the method and the device.
Owner:天津市湖滨盘古基因科学发展有限公司
Methods for generating polynucleotides having desired characteristics by iterative selection and recombination
InactiveUS7288375B2Less immunogenicPeptide/protein ingredientsImmunoglobulinsMutated proteinNucleotide
A method for DNA reassembly after random fragmentation, and its application to mutagenesis of nucleic acid sequences by in vitro or in vivo recombination is described. In particular, a method for the production of nucleic acid fragments or polynucleotides encoding mutant proteins is described. The present invention also relates to a method of repeated cycles of mutagenesis, shuffling and selection which allow for the directed molecular evolution in vitro or in vivo of proteins.
Owner:CODEXIS MAYFLOWER HLDG LLC
Methods for generating polynucleotides having desired characteristics by iterative selection and recombination
InactiveUS6518065B1Less immunogenicImmunoglobulinsLibrary member identificationMutated proteinNucleotide
Owner:CODEXIS MAYFLOWER HLDG LLC
Homogeneous preparations of IL-28 and IL-29
ActiveUS7157559B2Improve expression levelIncrease productionPeptide/protein ingredientsAntipyreticMutated proteinPolynucleotide
Homogeneous preparations of IL-28A, IL-28B, and IL-29 have been produced by mutating one or more of the cysteine residues in the polynucleotide sequences encoding the mature proteins. The cysteine mutant proteins can be shown to either bind to their cognate receptor or exhibit biological activity. One type of biological activity that is shown is an antiviral activity.
Owner:ZYMOGENETICS INC
Mutant protein
InactiveUS20060194950A1Improve stabilityIncreased pH-valuesBacteriaSerum immunoglobulinsMutated proteinComplementarity determining region
Owner:GE HEALTHCARE BIO SCI CORP
High-efficiency 14-valent pneumococcal conjugate vaccine
InactiveCN101590224AEffective protectionAntibacterial agentsBacterial antigen ingredientsCoccidiaHaemophilus
The invention relates to a high-efficiency 14-valent pneumococcal conjugate vaccine, which is formed by coupling and combining capsular polysaccharide extracted from fourteen types of serotype pneumococcuses and carrier protein; the fourteen types of the serotype pneumococcuses are 1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F; and the carrier protein is diphtherin non-toxic mutated protein CRM197, tetanus toxin protein and influenza haemophilus protein D. Compared with the prior pneumococcal conjugate vaccine, the high-efficiency 14-valent pneumococcal conjugate vaccine has the advantages that: (1) the cross-protection rate of the pneumococcuses of fourteen serotypes (1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F) reaches more than 90 percent; and (2) the high-efficiency 14-valent pneumococcal conjugate vaccine can generate effective protection on senior citizens which are susceptible to pneumonia and is suitable for children under two years old.
Owner:广州精达医学科技有限公司
Homogeneous preparations of IL-31
Homogeneous preparations of human and murine IL-31 have been produced by mutating one or more of the cysteine residues in the polynucleotide sequences encoding the mature proteins. The cysteine mutant proteins can be shown to either bind to their cognate receptor or exhibit biological activity.
Owner:ZYMOGENETICS INC
Muteins of fibroblast growth factor 21
ActiveUS7622445B2Reduce sensitivityReduced O-glycosylationPeptide/protein ingredientsMetabolism disorderMutated proteinNucleic acid sequence
The present invention relates to novel muteins of human fibroblast growth factor-21 with reduced susceptibility for proteolytic degradation when expressed in yeast. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, or metabolic syndrome.
Owner:ELI LILLY & CO
Methods of making non-transgenic herbicide resistant plants
The present invention relates to the production of a non-transgenic plant resistant or tolerant to a herbicide of the phosphonomethylglycine family, e.g., glyphosate. The present invention also relates to the use of a recombinagenic oligonucleobase to make a desired mutation in the chromosomal or episomal sequences of a plant in the gene encoding for 5-enol pyruvylshikimate-3-phosphate synthase (EPSPS). The mutated protein, which substantially maintains the catalytic activity of the wild-type protein, allows for increased resistance or tolerance of the plant to a herbicide of the phosphonomethylglycine family, and allows for the substantially normal growth or development of the plant, its organs, tissues or cells as compared to the wild-type plant irrespective of the presence or absence of the herbicide.
Owner:CIBUS
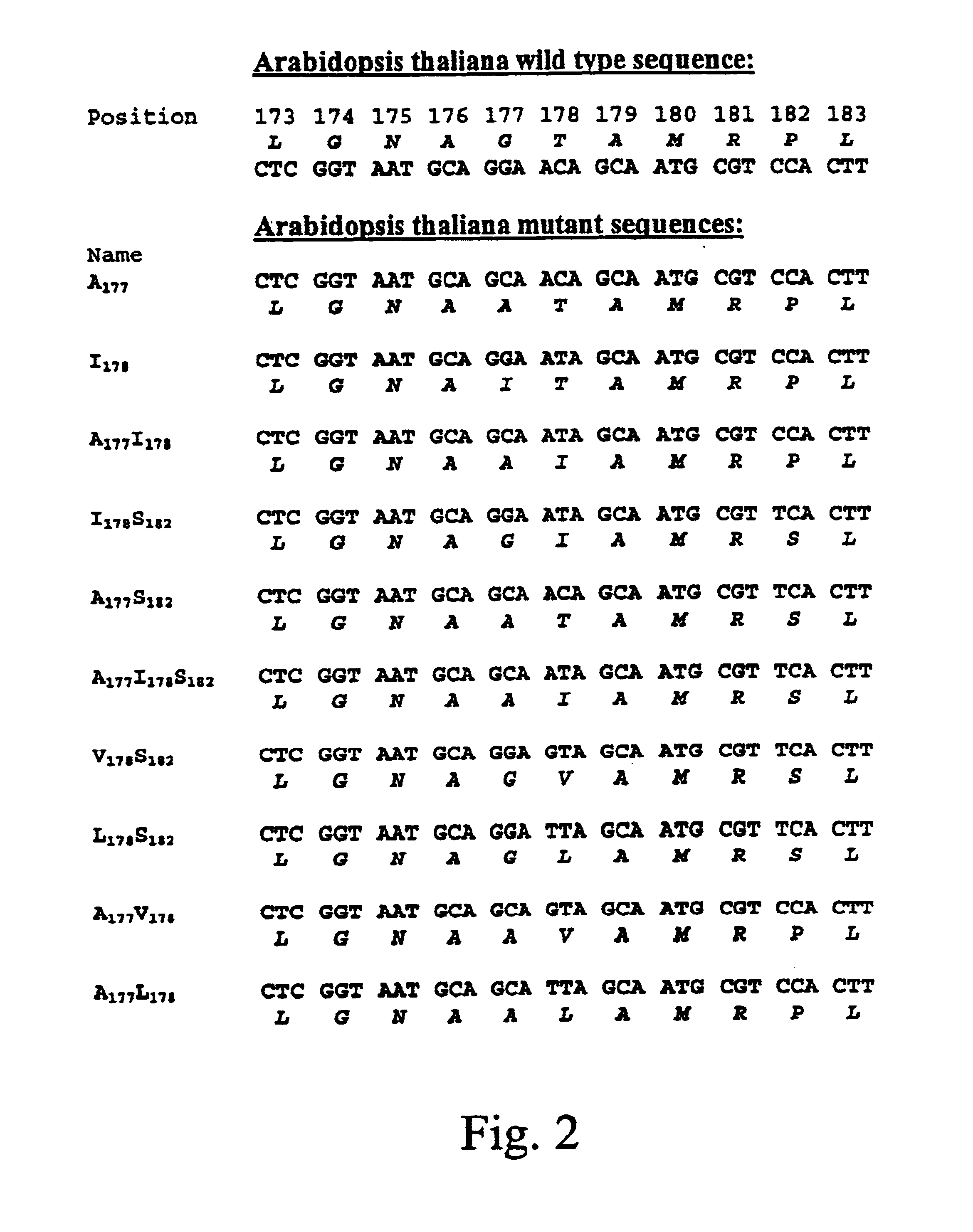
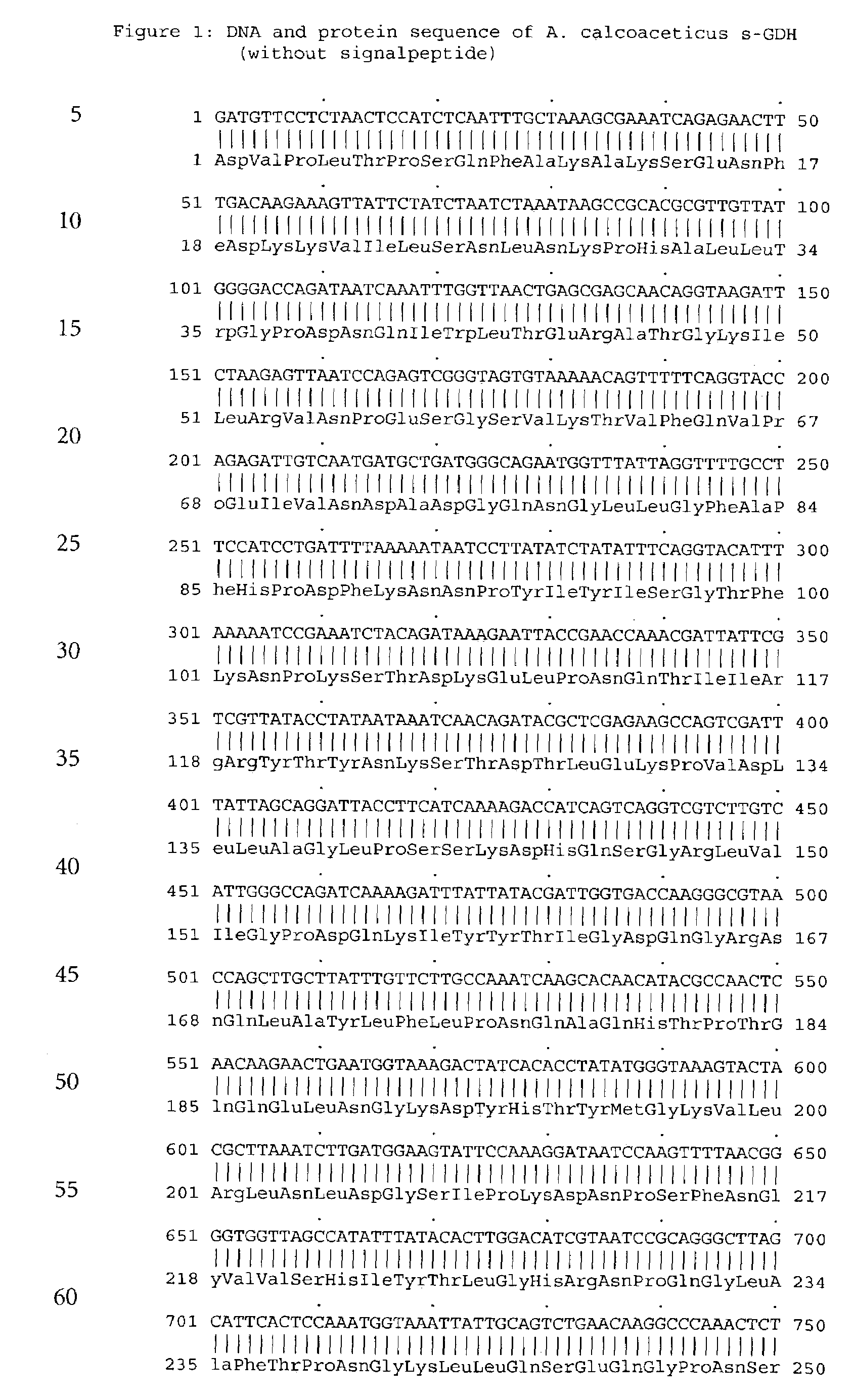
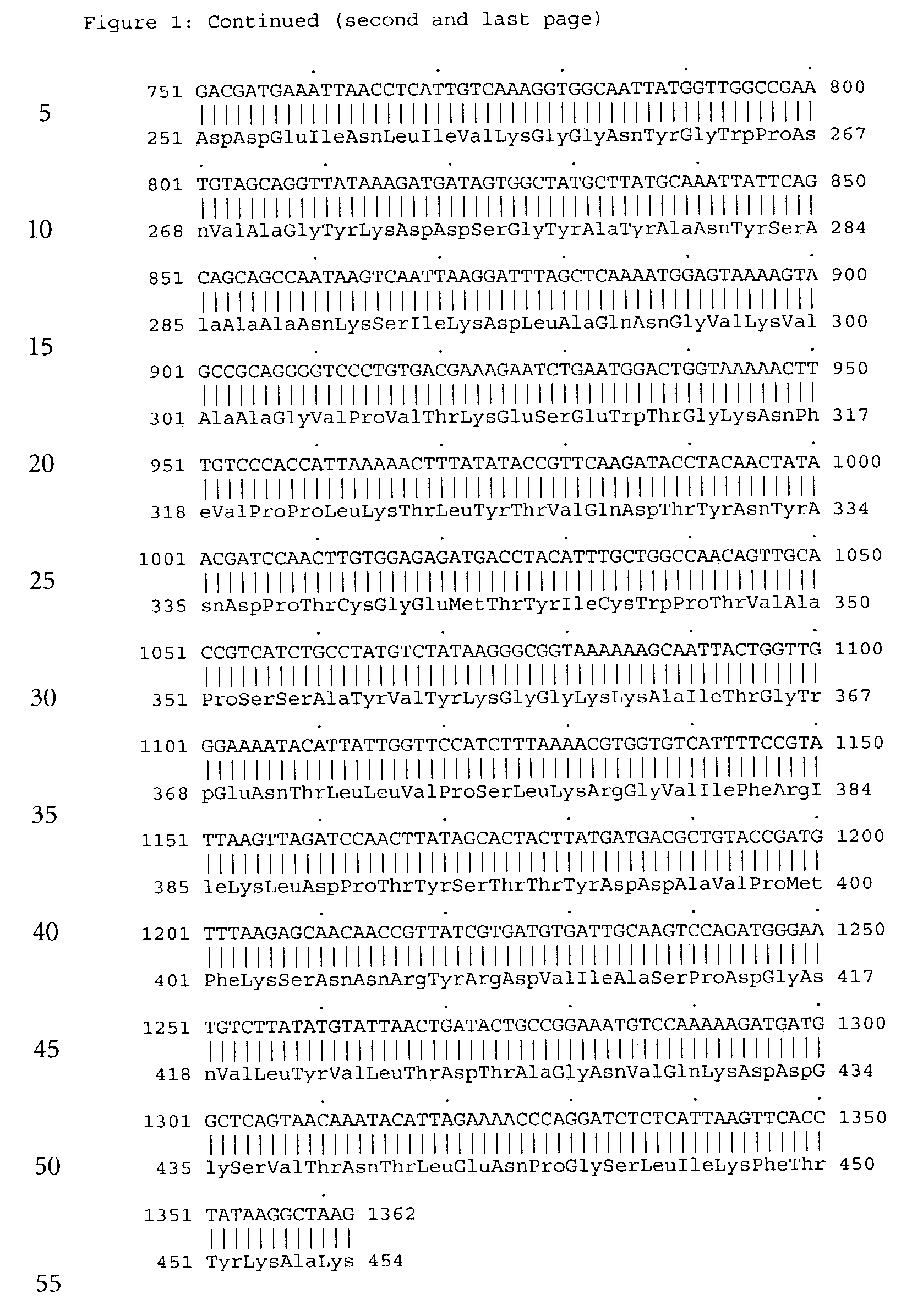
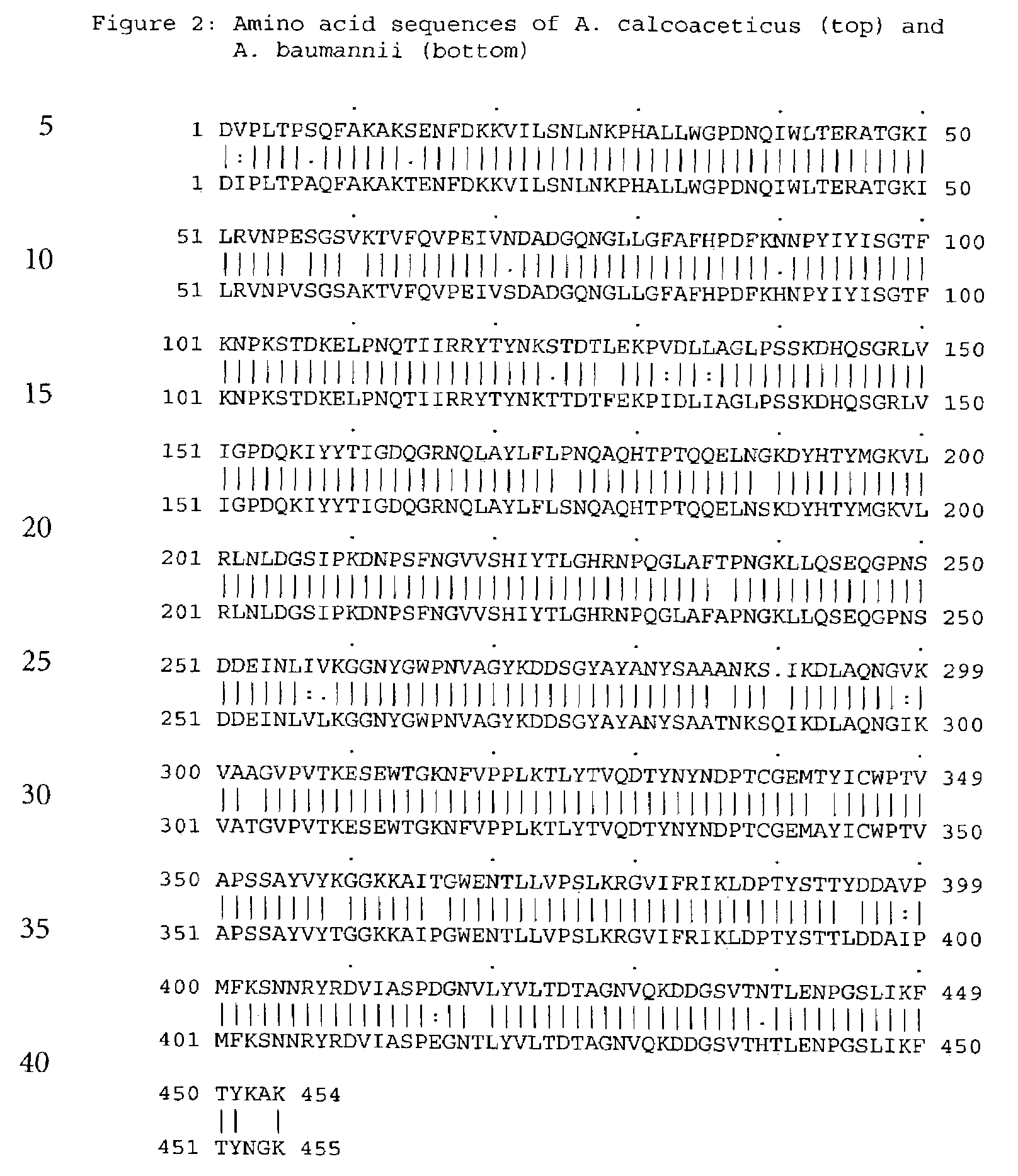
Forms of soluble pyrroloquinoline quinone-dependent glucose dehydrogenase
InactiveUS7132270B2Increased substrate specificityImprove propertiesFungiBacteriaMutated proteinGlucose polymers
The present invention relates to improved variants of soluble pyrroloquinoline quinone (PQQ)-dependent glucose dehydrogenases (s-GDH), to genes encoding mutated s-GDH, to mutant proteins of s-GDH with improved substrate specificity for glucose, and to different applications of these s-GDH variants, particularly for determining concentrations of sugar, especially of glucose in a sample.
Owner:ROCHE DIABETES CARE INC
Tumor necrosis factor-alpha mutants
The present invention has an object to provide a tumor necrosis factor mutant protein, particularly, a tumor necrosis factor mutant protein specific to TNF-R1 or TNF-R2; tumor necrosis factor inhibitor; or tumor necrosis factor preparation containing it as an effective ingredient, and the object is solved by providing a tumor necrosis factor mutant protein where one or more amino acid residues selected from the group consisting of 29th, 31st, 32nd, 145th, 146th and 147th, or the group consisting of 84th to 89th from the N-terminal of the amino acid sequence of SEQ ID NO:1 is / are replaced with other amino acid residue(s); a tumor necrosis factor inhibitor; and a tumor necrosis factor preparation containing it as an effective ingredient.
Owner:MAYUMI TADANORI +3
Treatment of CNS disorders associated with mutations in genes encoding lysosomal enzymes
Described is a method for treating an individual having a neurological disorder with an associated mutation or mutations in a gene encoding a lysosomal enzyme. Specifically, the individual is administered a specific pharmacological chaperone for the lysosomal enzyme which increases trafficking of the protein from the ER to the lysosome in neural cells, with or without concomitantly increasing enzyme activity in neural cells. Restoration of trafficking relieves cell stress and other toxicities associated with accumulation of mutant proteins. Restoration of enzyme activity relieves substrate accumulation and pathologies associated with lipid accumulation. In a specific embodiment, the neurological disorder is Parkinson's disease or parkinsonism which is associated with mutations in glucocerebrosidase.
Owner:AMICUS THERAPEUTICS INC
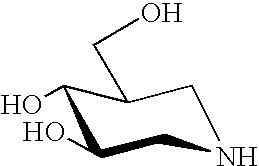
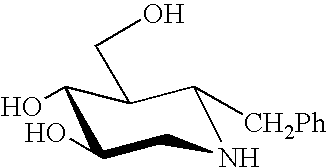
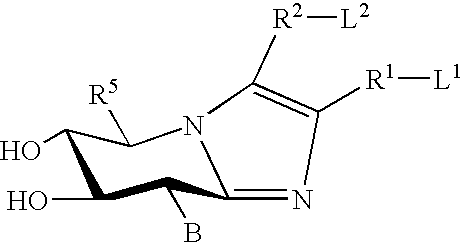
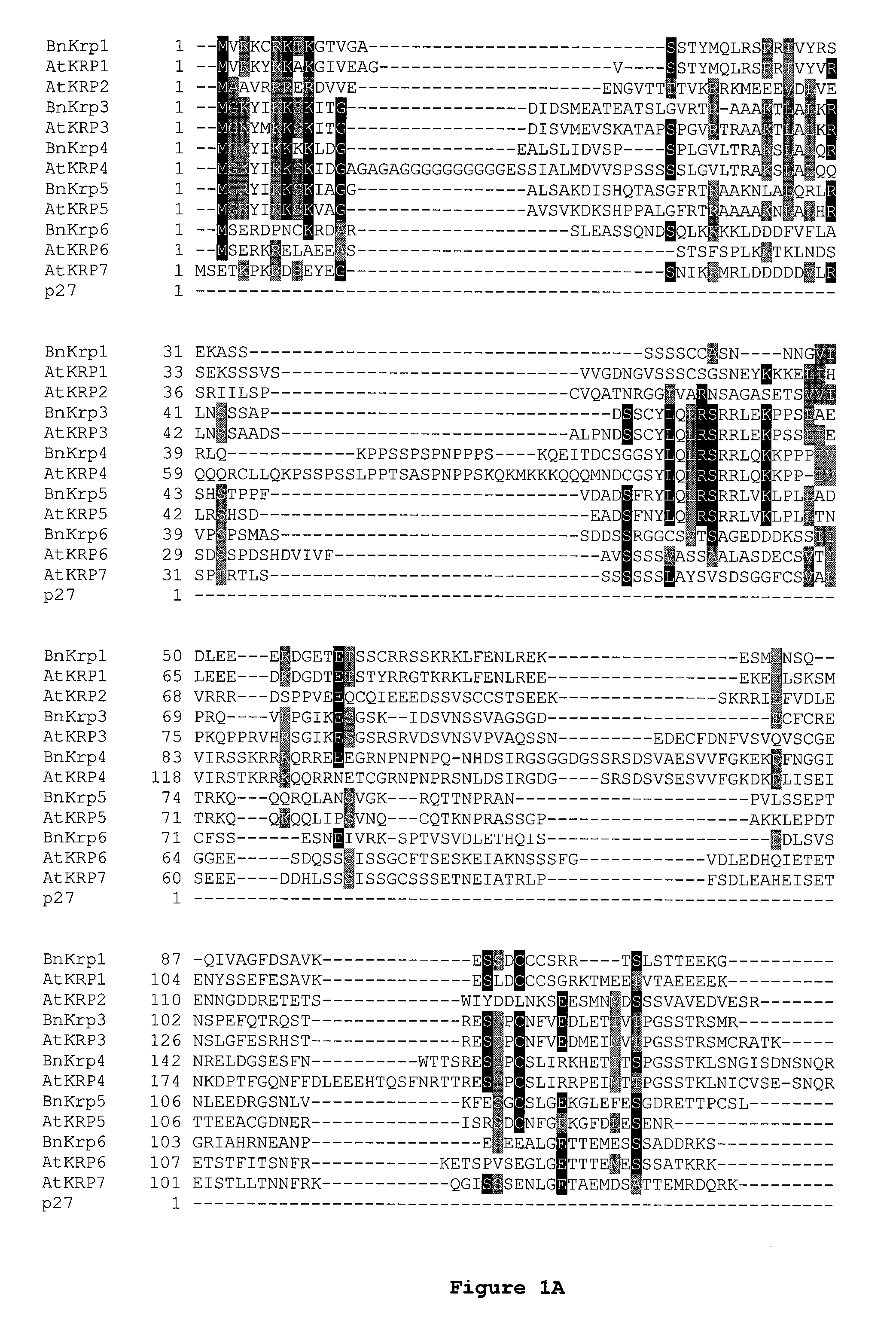
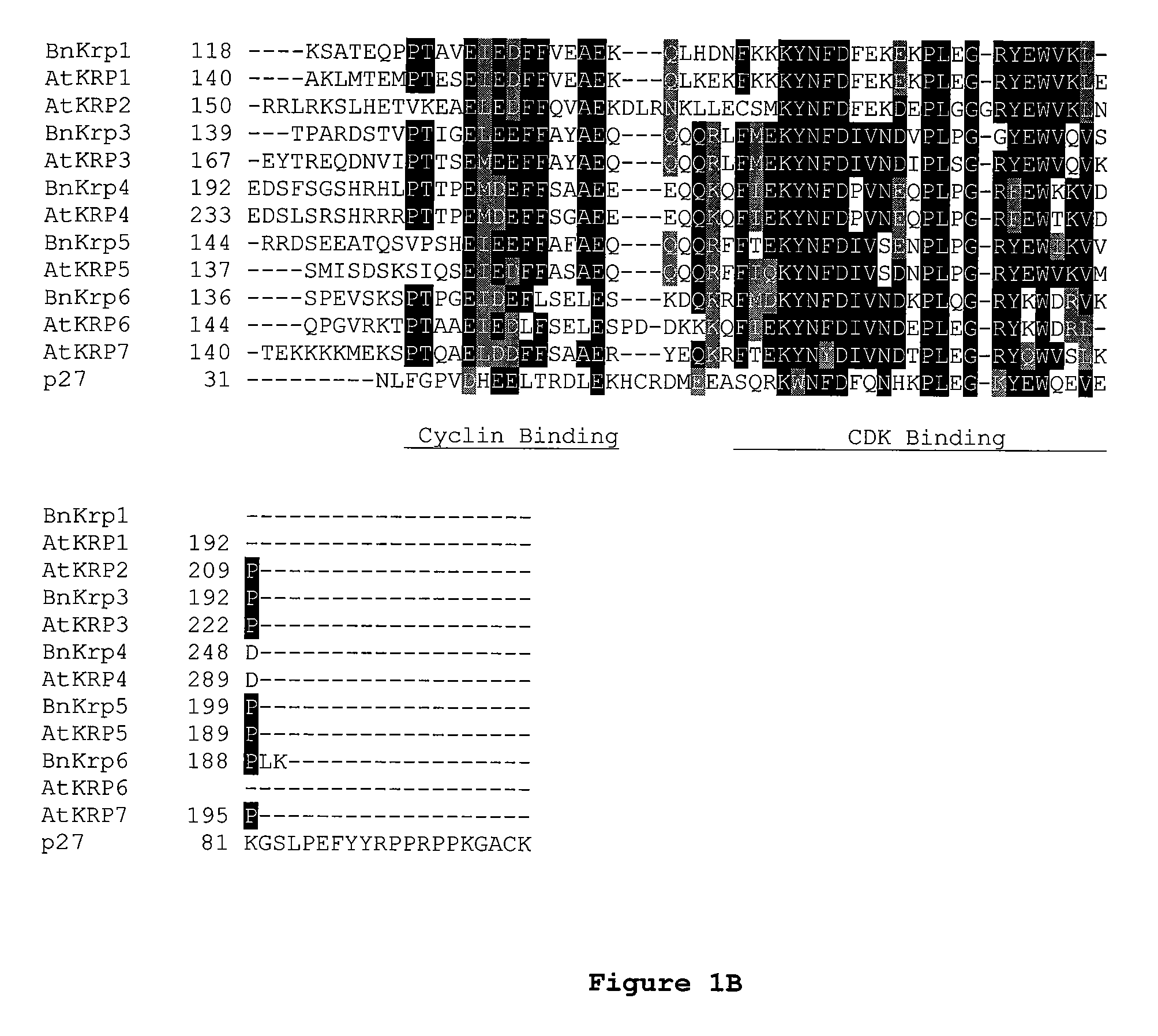
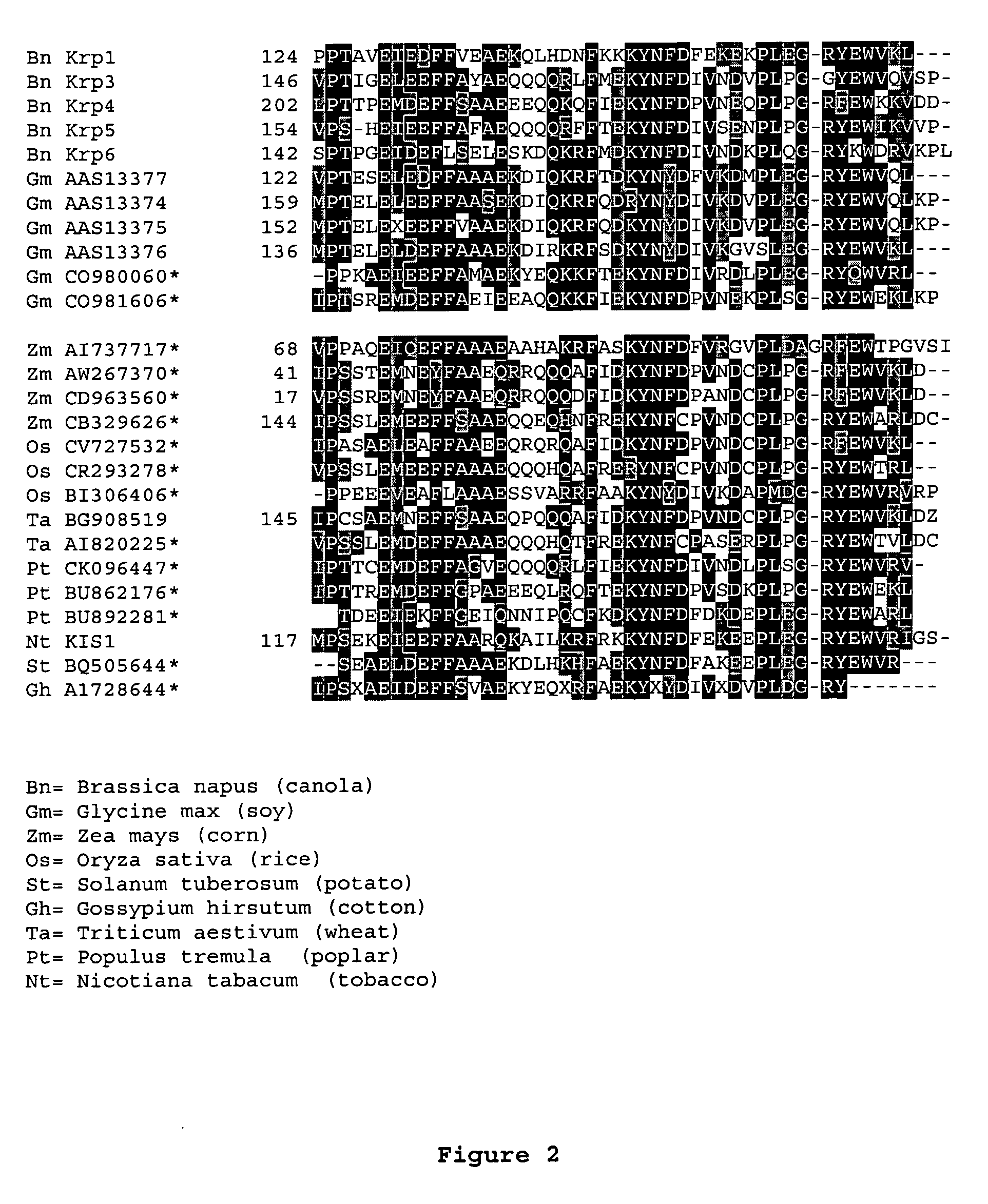
Dominant negative mutant krp protein protection of active cyclin-cdk complex inhibition by wild-type krp
InactiveUS20070056058A1Speed upIncreasing cell proliferationBacteriaSugar derivativesMutated proteinPlant cell
Disclosed are mutant CDK inhibitor (CKI) polypeptides having dominant negative antagonist activity against wild-type CKI proteins, as well as related compositions, including nucleic acids and vectors encoding the mutant CKI polypeptides and transformed host cells and transgenic plants comprising such nucleic acids and vectors. Also disclosed are related methods for using the mutant proteins to modulate cell division in cells, particularly plant cells.
Owner:TARGETED GROWTH
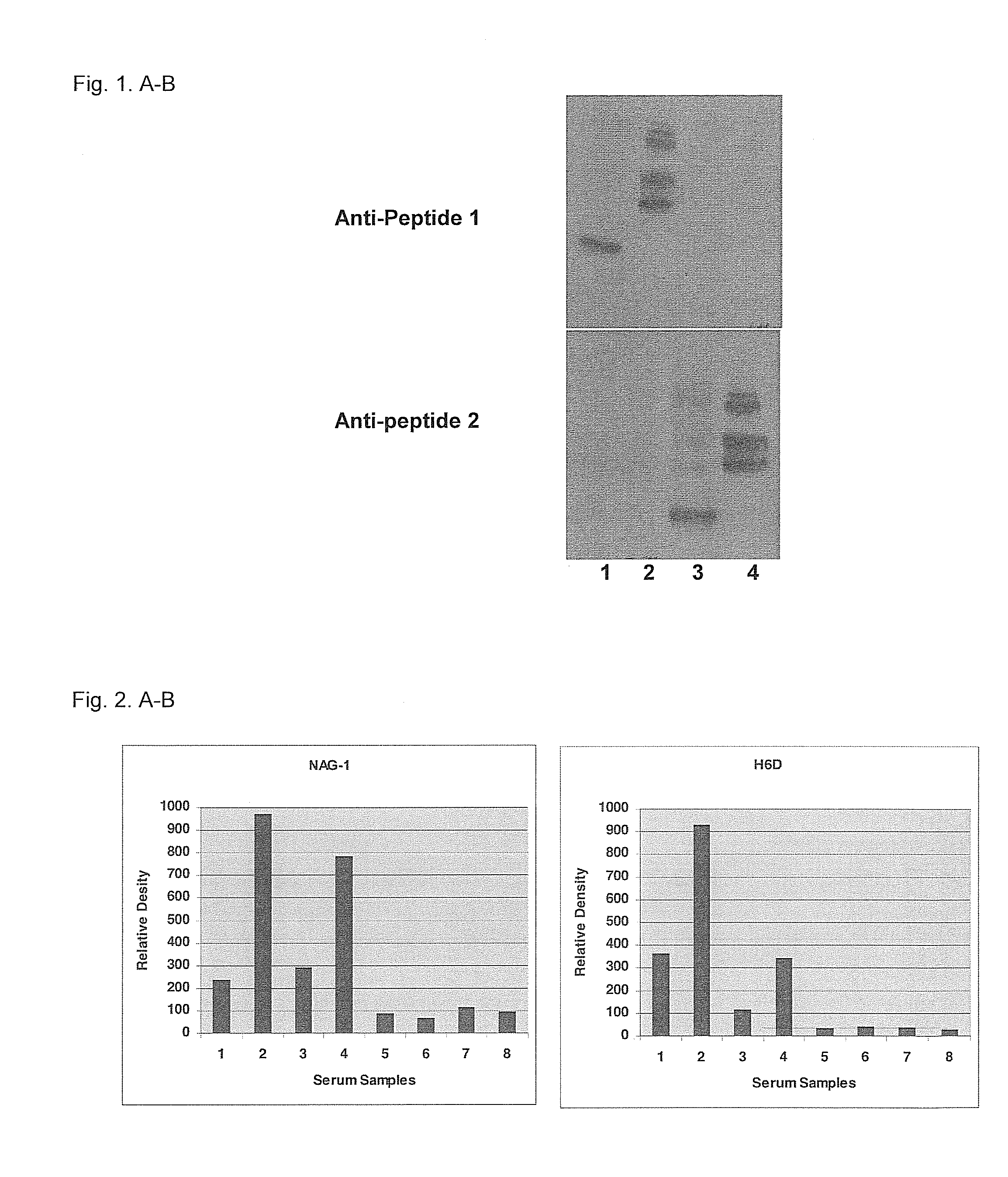
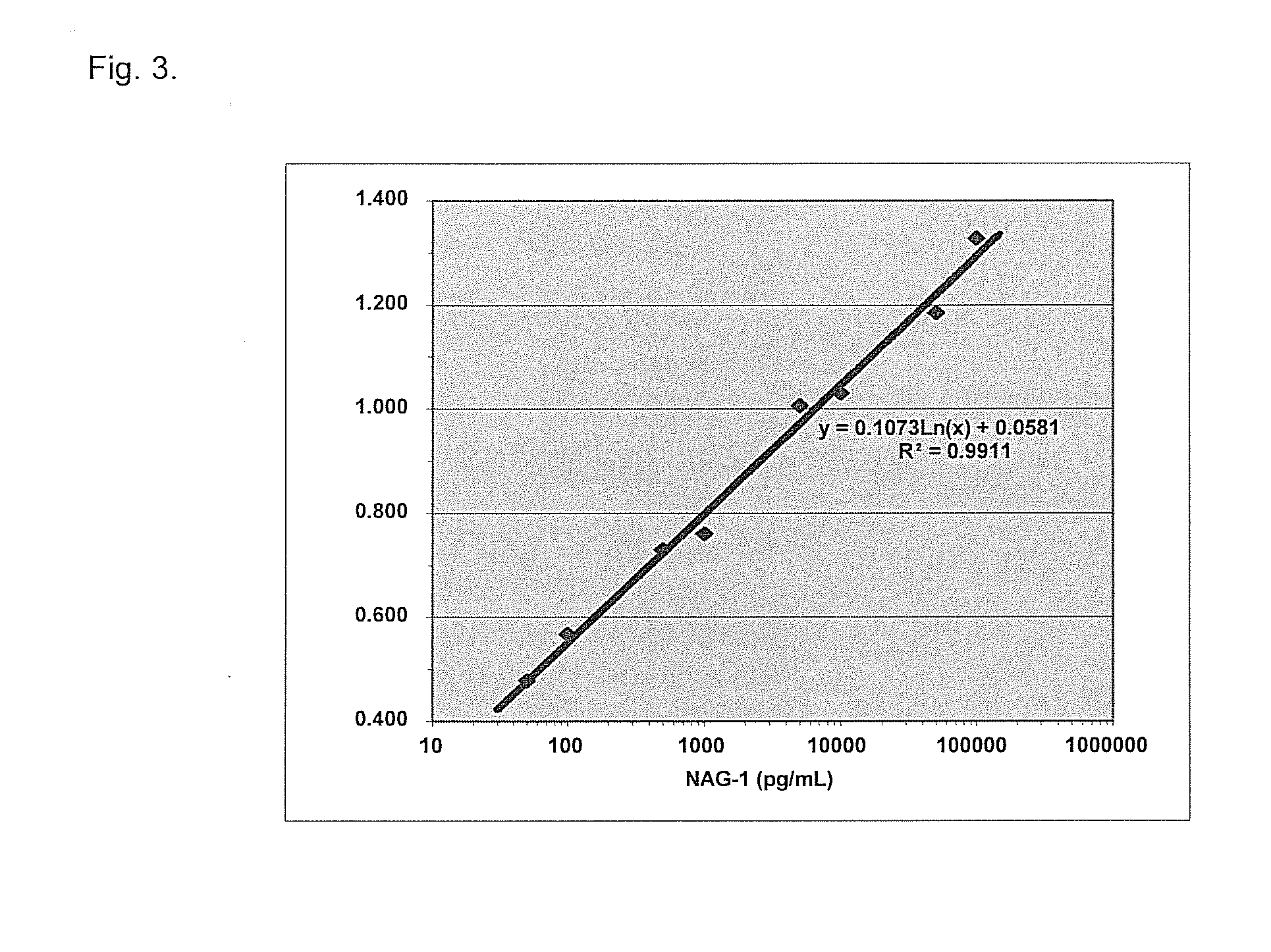
Form-specific antibodies for nag-1 (mic-1, gdf-15), h6d and other tgf-beta subfamily and heart disease and cancer diagnoses
A method of producing form-specific anti-peptide antibodies for a wild type protein and its one amino acid mutated protein using a peptide antigen, by obtaining a protein sequence of the wild type protein and its one amino acid mutated protein, selecting a continuous amino acid sequence without any internal cysteine residues that includes the one amino acid mutated sequence and wild type sequence corresponding to the mutated site at the end of the sequence to obtain a synthetic mutation peptide and a synthetic wild type peptide, conjugating the synthetic peptides to a carrier protein, and immunizing an animal to produce antibodies. Methods of detecting cancer and methods of treating cancer.
Owner:DETROIT R&D
FGFR Extracellular Domain Acidic Region Muteins
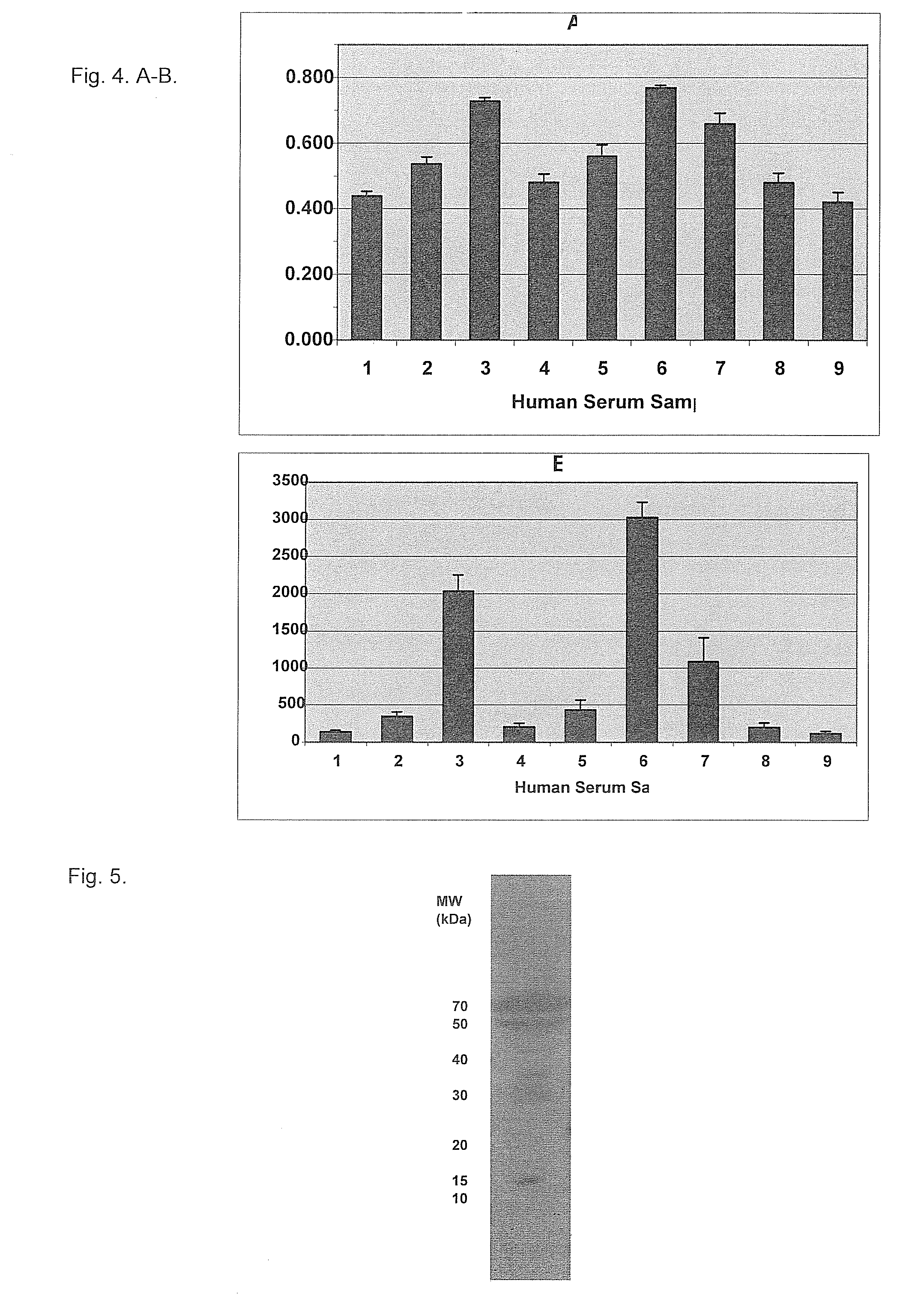
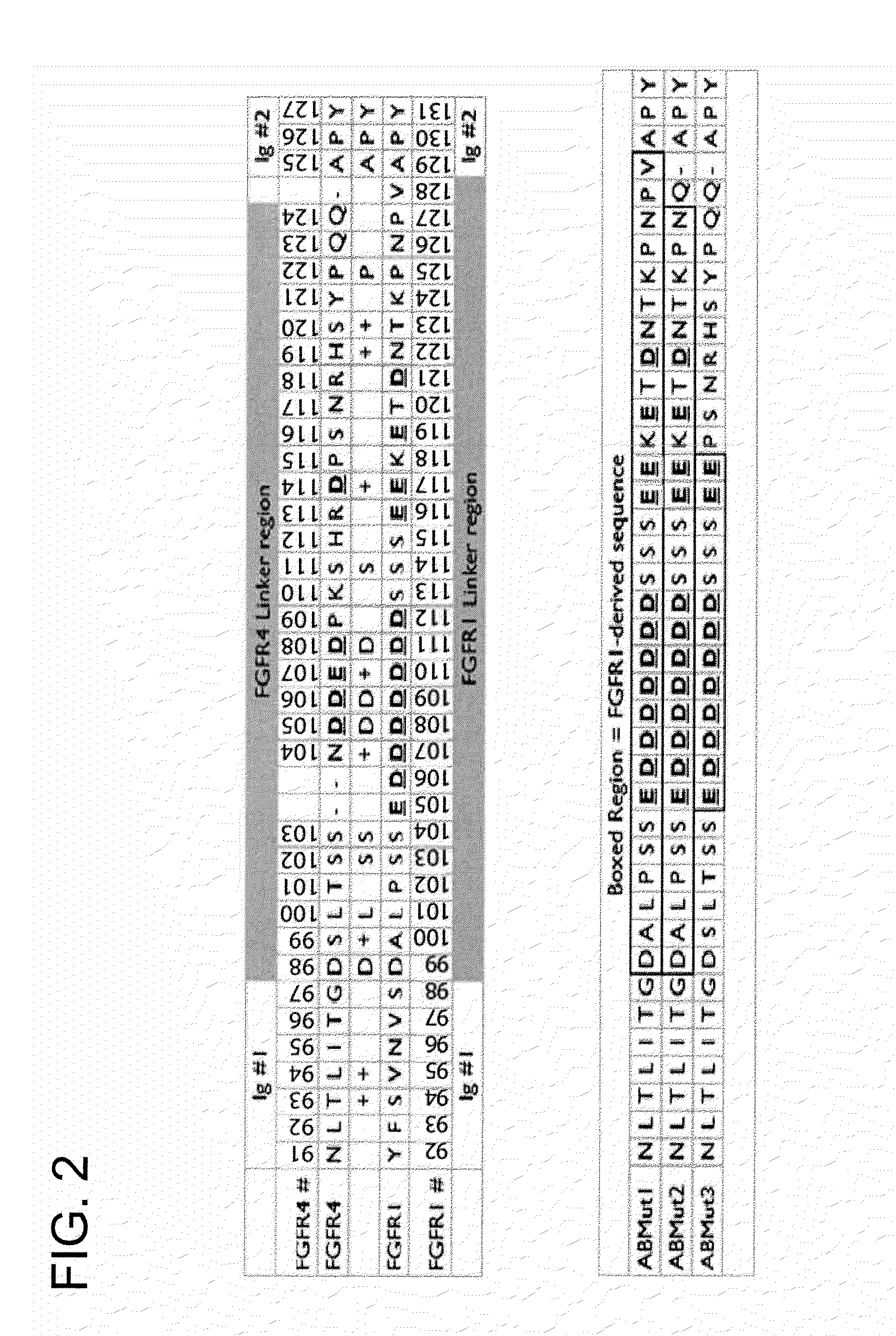
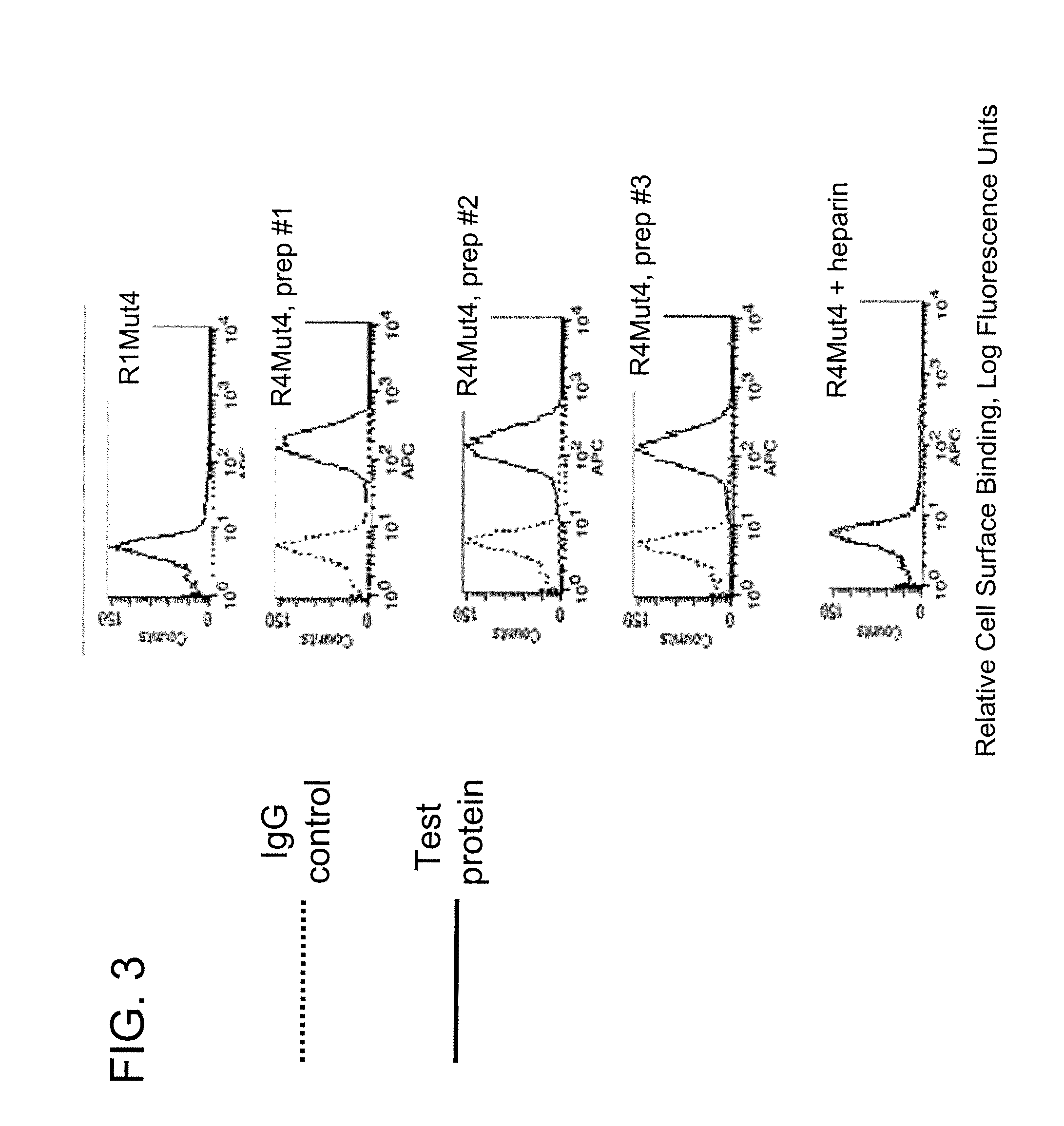
InactiveUS20100087627A1Decreased ECM bindingImprove bioavailabilitySenses disorderPeptide/protein ingredientsMutated proteinPolynucleotide
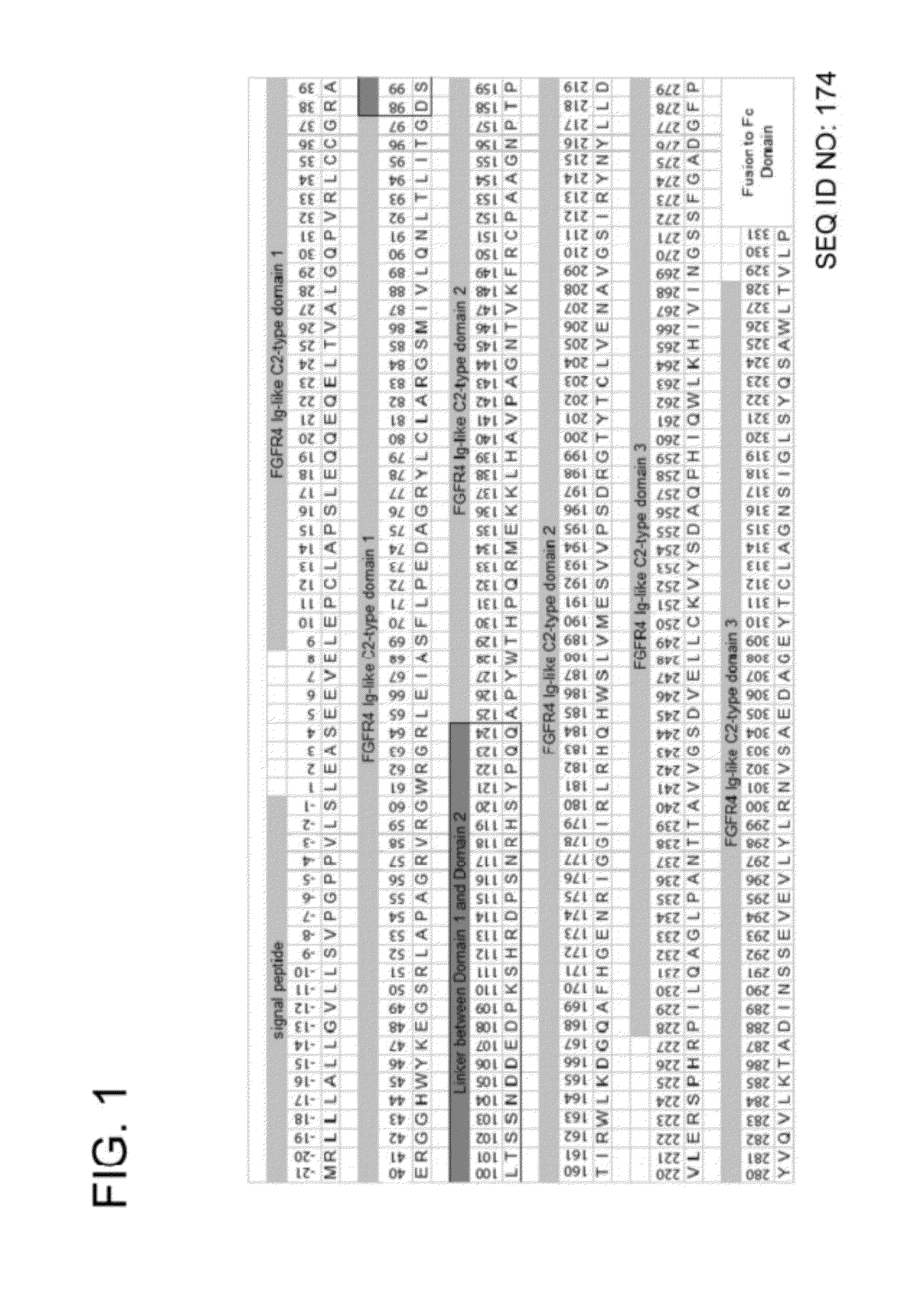
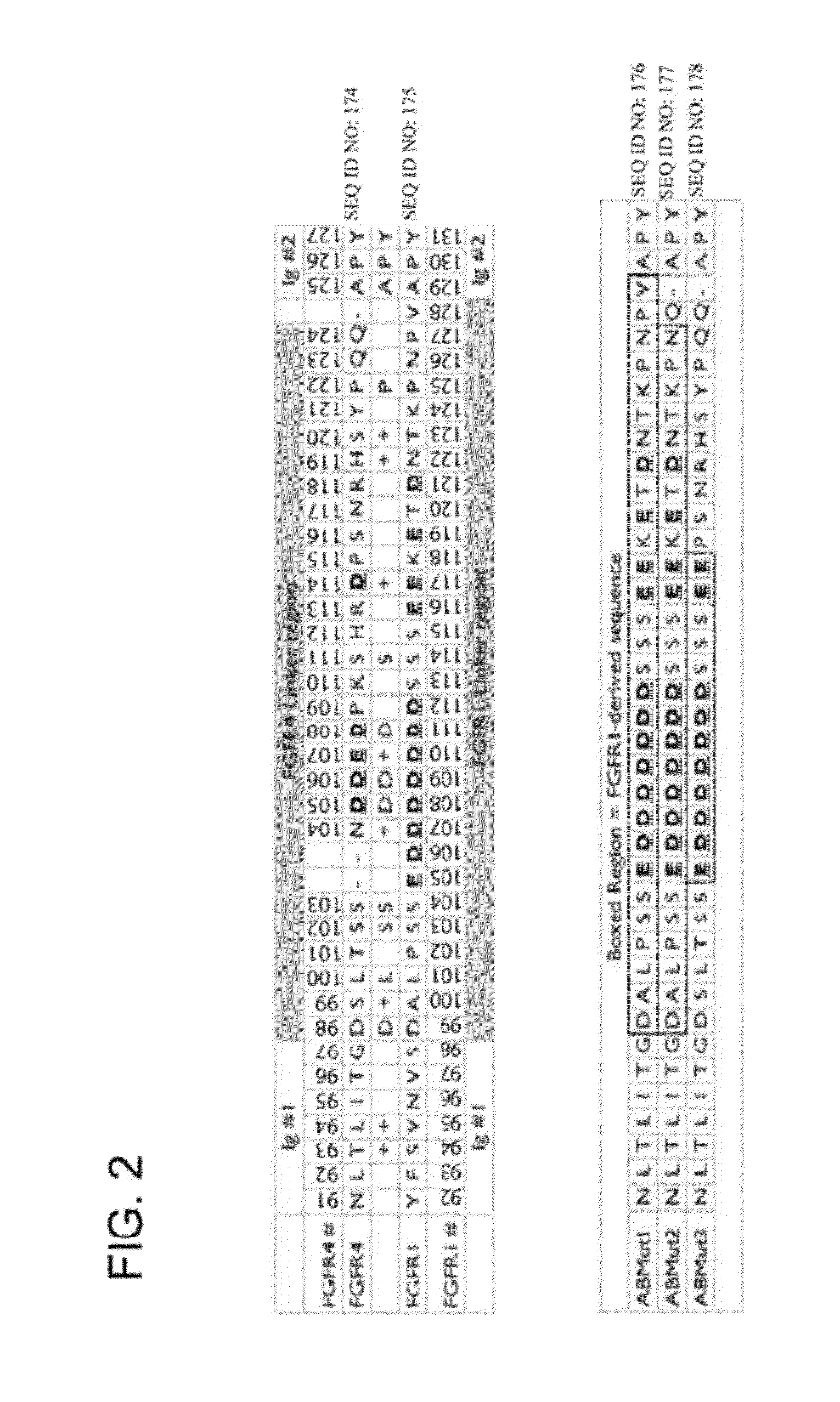
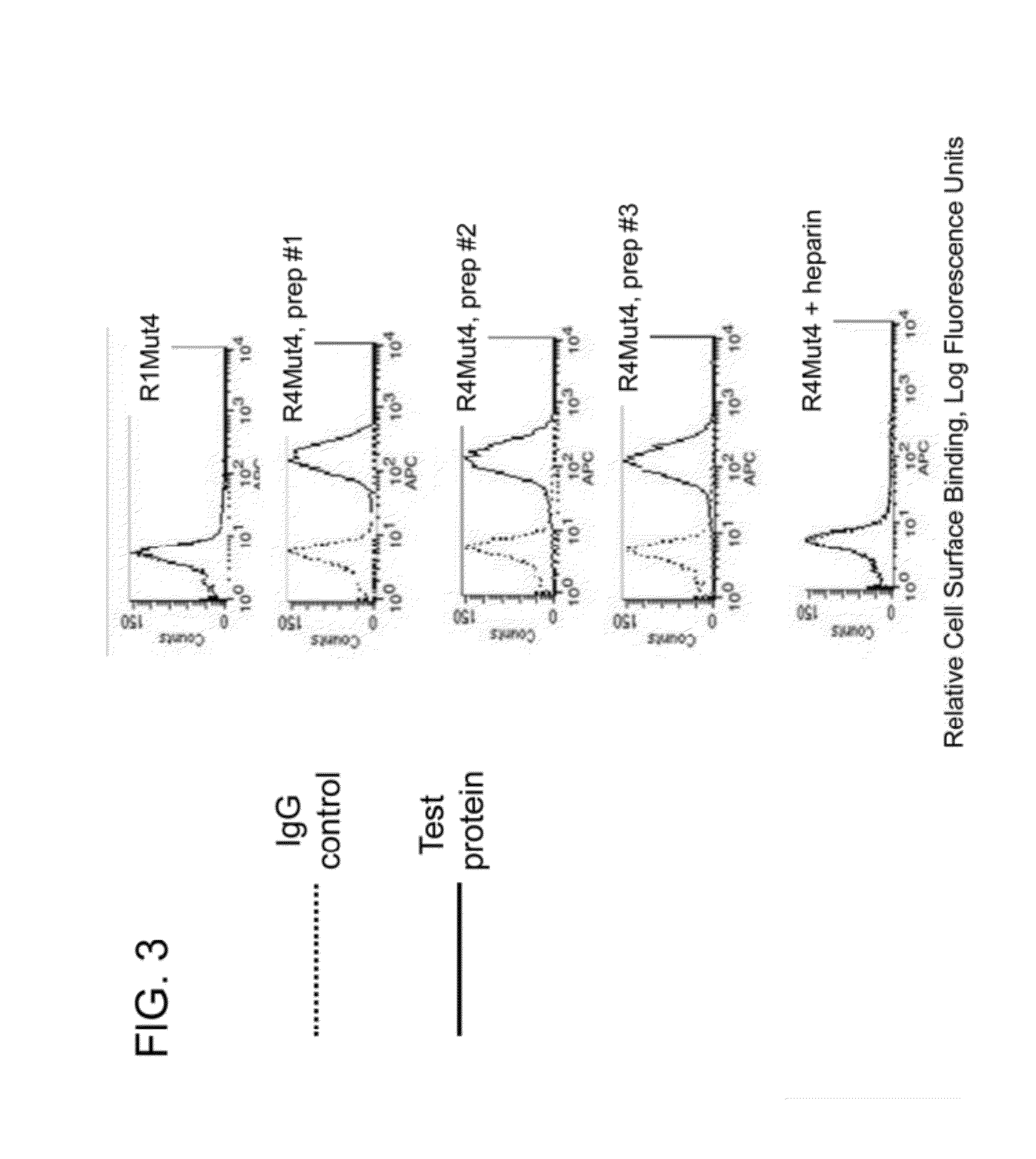
Fibroblast growth factor receptor (FGFR) extracellular domain (ECD) acidic region muteins that have been engineered to exhibit decreased tissue binding by increasing the number of acidic amino acid residues within the D1-D2 linker region are provided. Polynucleotides encoding FGFR ECD acidic region muteins are also provided. Methods of making FGFR ECD acidic region muteins, and methods of using such molecules to treat proliferative disorders, including cancers, disorders of angiogenesis, and macular degeneration, are also provided.
Owner:FIVE PRIME THERAPEUTICS
Nucleic acids encoding interleukin-1 inhibitors and processes for preparing interleukin-1 inhibitors
Compounds are disclosed having the general formula R1—X—R2, wherein R1 and R2 are biologically active groups, at least one of which is polypeptidic. X is a non-peptidic polymeric group. R1 and R2 may be the same or different. Preferred R1 and R2 groups are interleukin-1 receptor antagonist, 30 kDa TNF inhibitor, interleukin-2 receptors and CR1 and muteins thereof. Also included are site selectively modified interleukin-1 receptor antagonist and 30 kDa TNF inhibitor.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Homogeneous preparations of il-28 and il-29
ActiveUS20070042471A1Improve expression levelIncrease productionPeptide/protein ingredientsAntipyreticMutated proteinCysteine thiolate
Owner:ZYMOGENETICS INC
Mutant proteins, high potency inhibitory antibodies and fimch crystal structure
InactiveUS20030199071A1Great functional inhibitory activityStrong inhibitory activityHydrolasesImmunoglobulins against bacteriaPassive ImmunizationsMutated protein
The present invention provides bacterial immunogenic agents for administration to humans and non-human animals to stimulate an immune response. It particularly relates to the vaccination of mammalian species, especially human patients, with variants of the E. coli FimCH protein that elicit antibodies that have better functional inhibitory activity than antibodies raised against wild type protein. In particular, such variants include mutations that promote a more open confirmation of the FimH protein, particularly in regions involved in mannose binding, to expose regions previously poorly exposed and mutations that abolish a significantly reduce mannose binding. In another aspect, the invention provides antibodies against such proteins and protein complexes that may be used in passive immunization to protect or treat pathogenic bacterial infections. The present invention also provides machine readable media embedded with the three-dimensional atomic structure coordinates of FimCH bound to mannose, and subsets thereof, and methods of using the crystal structure to provide candidate amino acid residues for mutation.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Homogeneous preparations of il-28 and il-29
Homogeneous preparations of IL-28A, IL-28B, and IL-29 have been produced by mutating one or more of the cysteine residues in the polynucleotide sequences encoding the mature proteins. The cysteine mutant proteins can be shown to either bind to their cognate receptor or exhibit biological activity. One type of biological activity that is shown is an antiviral activity.
Owner:ZYMOGENETICS INC
Homogeneous preparations of il-28 and il-29
ActiveUS20070042470A1Improve expression levelIncrease productionPeptide/protein ingredientsAntipyreticMutated proteinCysteine thiolate
Homogeneous preparations of IL-28A, IL-28B, and IL-29 have been produced by mutating one or more of the cysteine residues in the polynucleotide sequences encoding the mature proteins. The cysteine mutant proteins can be shown to either bind to their cognate receptor or exhibit biological activity. One type of biological activity that is shown is an antiviral activity.
Owner:ZYMOGENETICS INC
Cytotoxic heteromeric protein combinatorial libraries
InactiveUS7713915B1Minimize ToxicityPeptide/protein ingredientsLibrary screeningMicroorganismMutated protein
A method is provided for constructing, identifying and using new therapeutic or diagnostic proteins capable of binding to a target cell. The new proteins are derived by mutating a binding subunit of a wild type heteromeric cytotoxic protein to create a library of microorganism clones producing mutant proteins which are then screened for their ability to specifically bind to and kill a target cell.
Owner:MOLECULAR TEMPLATES
RecA mutants
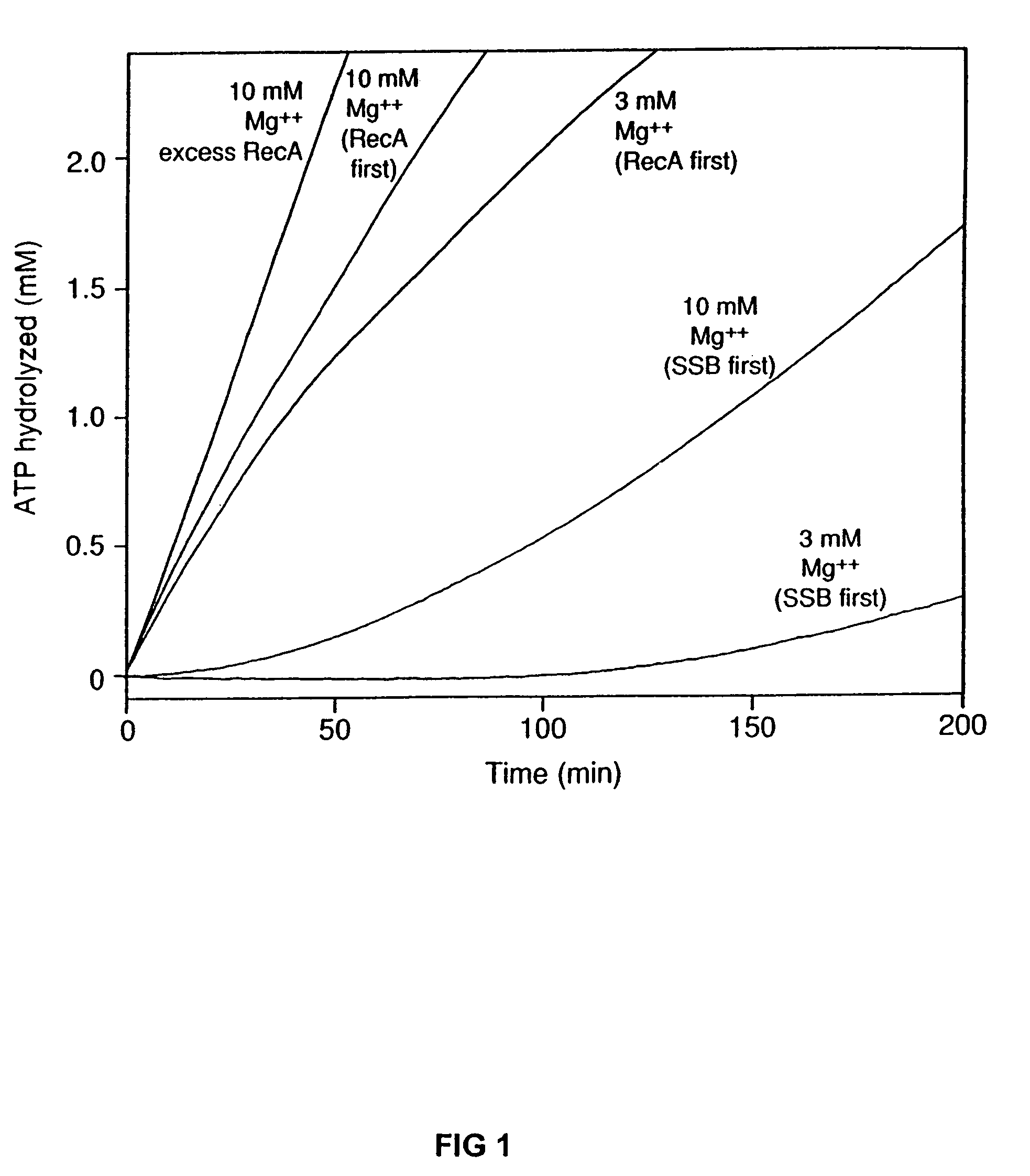
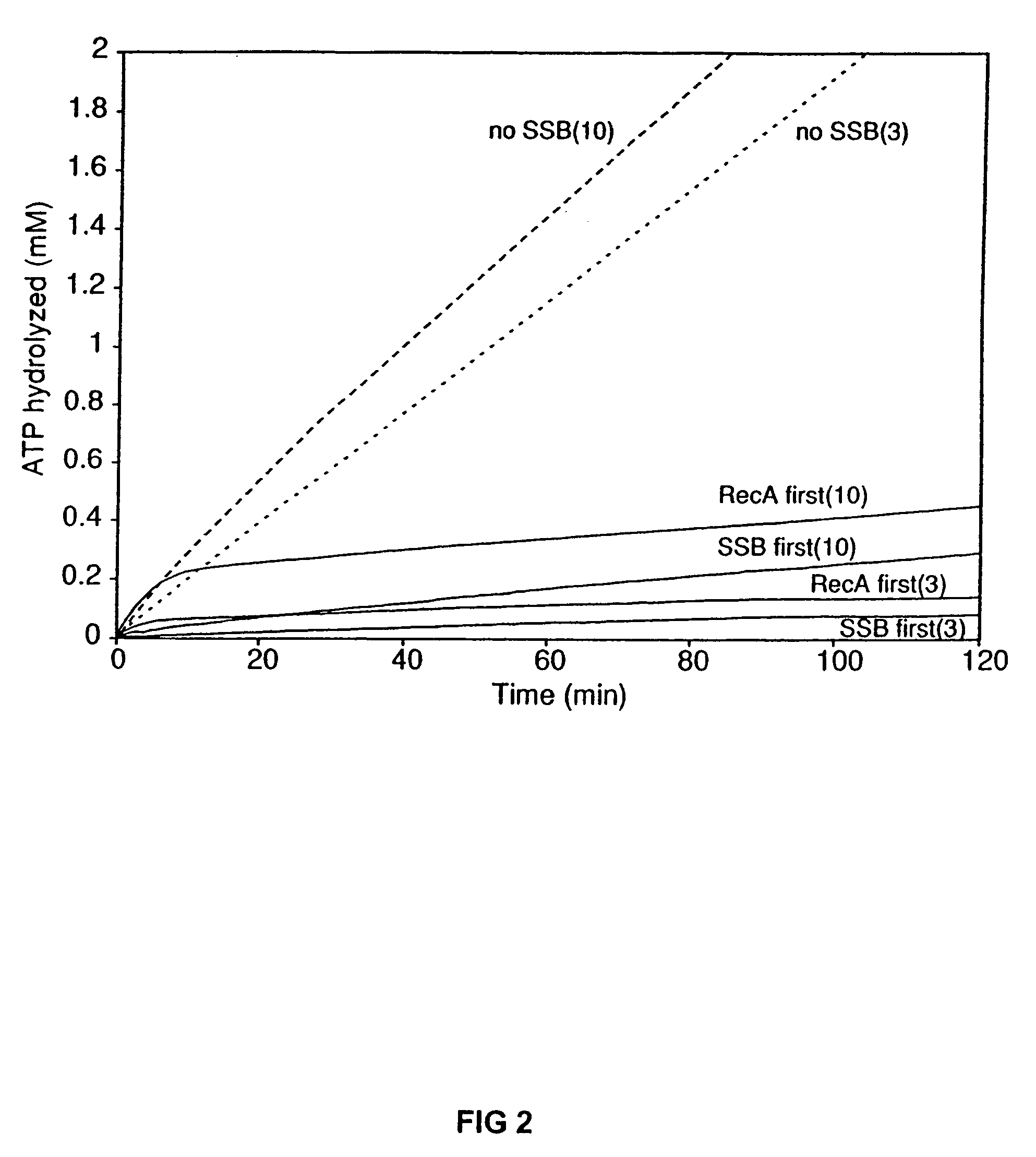
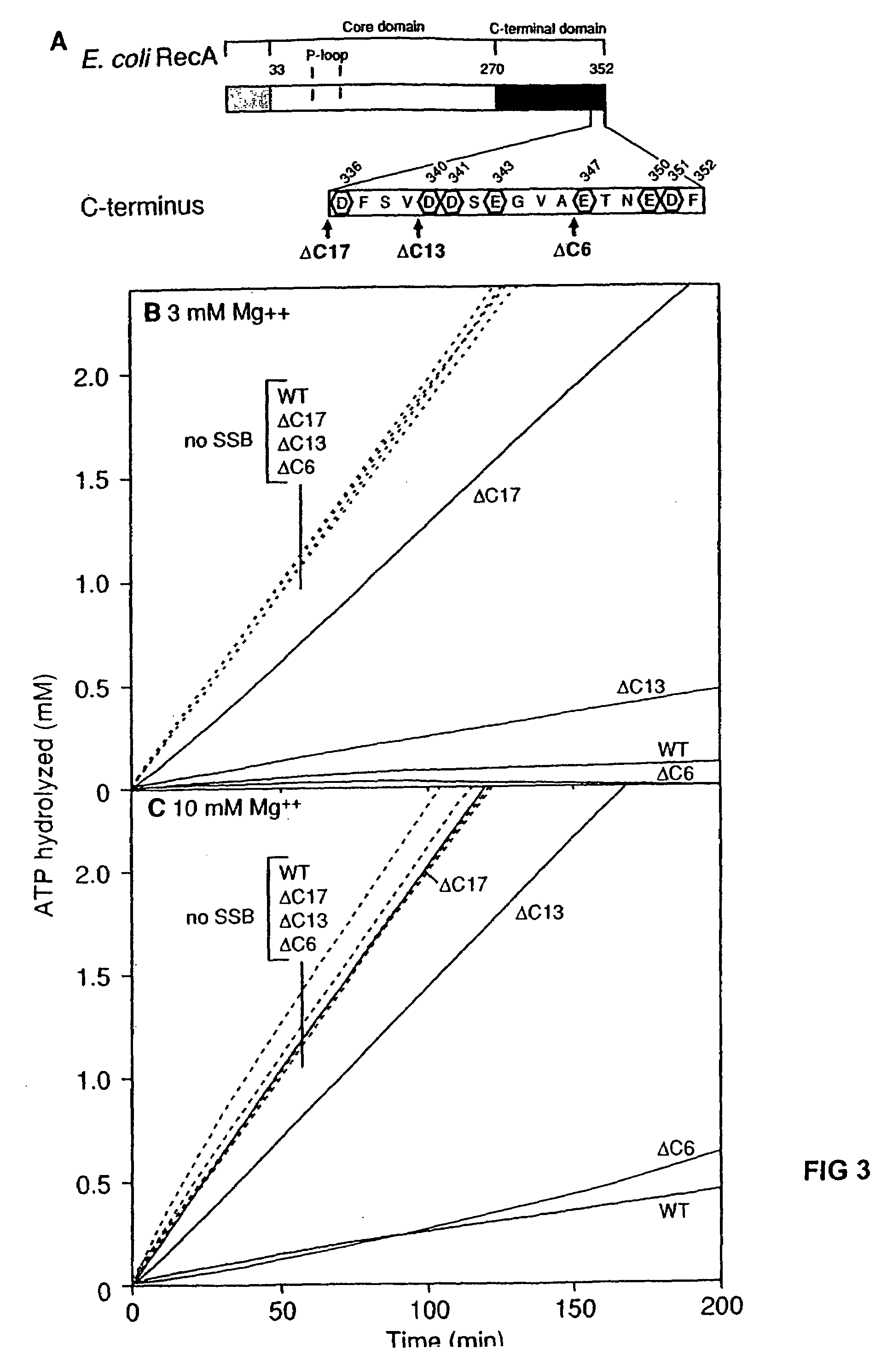
InactiveUS7176007B2Improve concentrationEliminate requirementsSugar derivativesMicrobiological testing/measurementMutated proteinDouble mutation
The present invention provides RecA mutant proteins, having either a single mutation or a double mutation. The RecA mutant proteins are highly proficient in both SSB displacement and steady state binding of DNA in the presence or absence of SSB as compared to the wild-type protein. The single RecA mutant, RecAΔC17, has 17 amino acid residues removed from the carboxyl terminus. The double mutant RecA, RecAΔC17 / E38K, combines the 17 amino acid residue C-terminal deletion of RecAΔC17, with a single amino acid change from Glutamate to Lysine at position 38. These RecA mutant proteins are pH sensitive allowing control over formation of products. Hence, methods of using the novel RecA mutants and kits having the RecA mutants as components thereof are also contemplated by the present invention.
Owner:WISCONSIN ALUMNI RES FOUND
Inhibitors of kras g12c mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I):or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein R1, R2, R3a, R3b, R4a, R4b, G1, G2, L, m1, m2 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
FGFR extracellular domain acidic region muteins
InactiveUS8338569B2Preventing tissue bindingGreater ECM bindingSenses disorderPeptide/protein ingredientsMutated proteinPolynucleotide
Fibroblast growth factor receptor (FGFR) extracellular domain (ECD) acidic region muteins that have been engineered to exhibit decreased tissue binding by increasing the number of acidic amino acid residues within the D1-D2 linker region are provided. Polynucleotides encoding FGFR ECD acidic region muteins are also provided. Methods of making FGFR ECD acidic region muteins, and methods of using such molecules to treat proliferative disorders, including cancers, disorders of angiogenesis, and macular degeneration, are also provided.
Owner:FIVE PRIME THERAPEUTICS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com