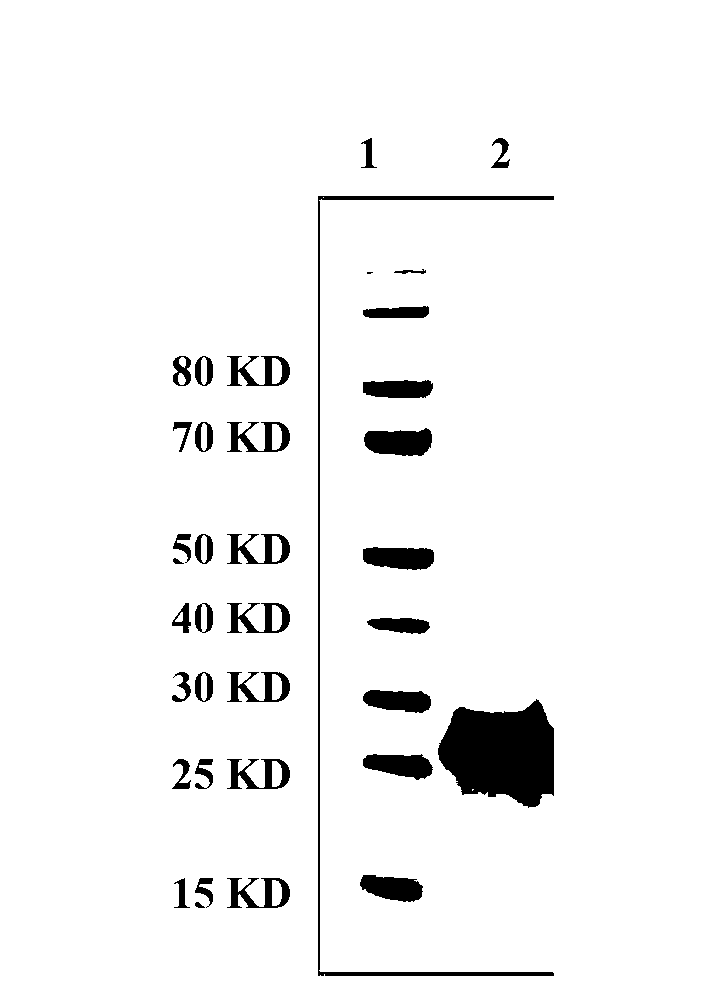
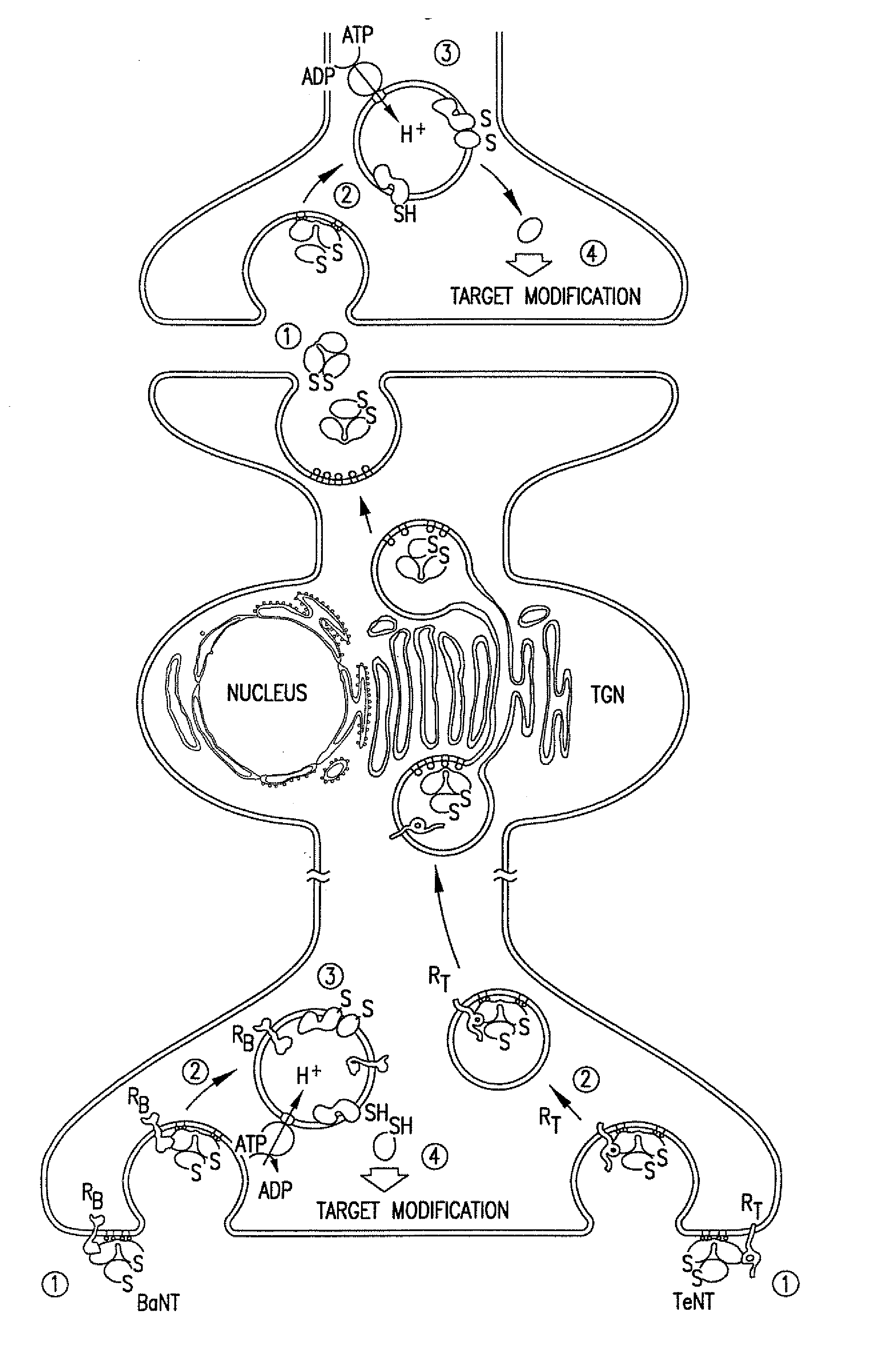
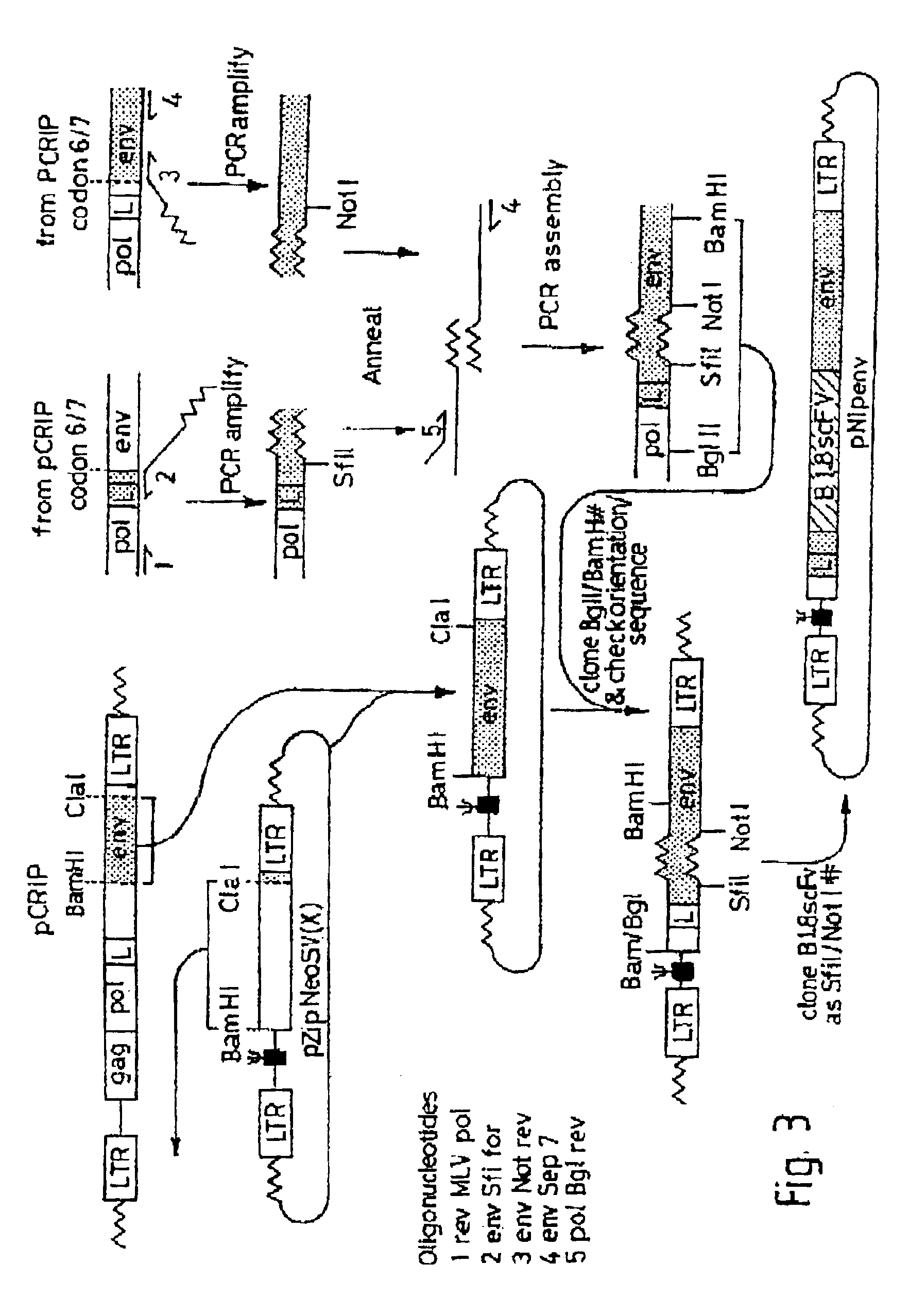
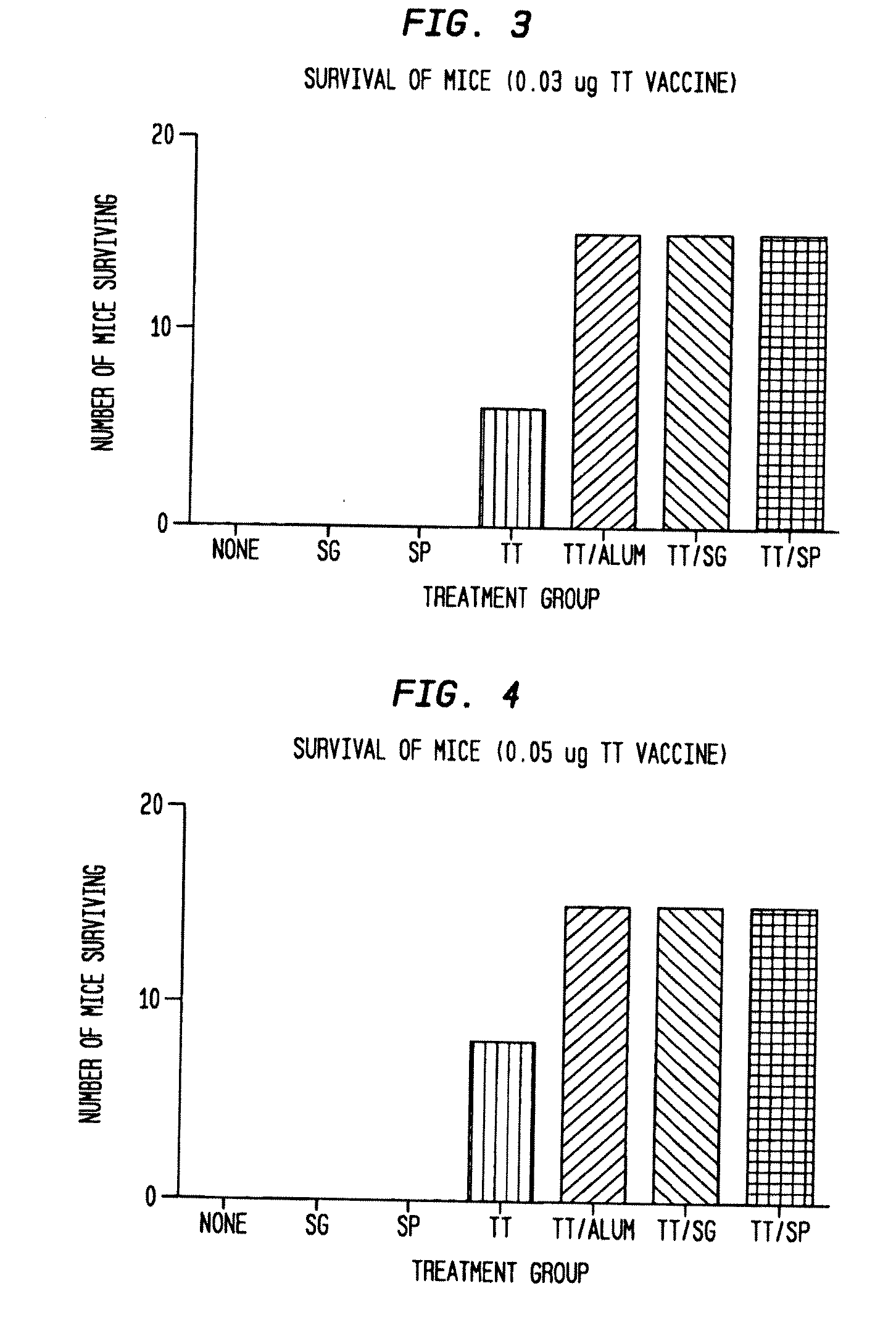
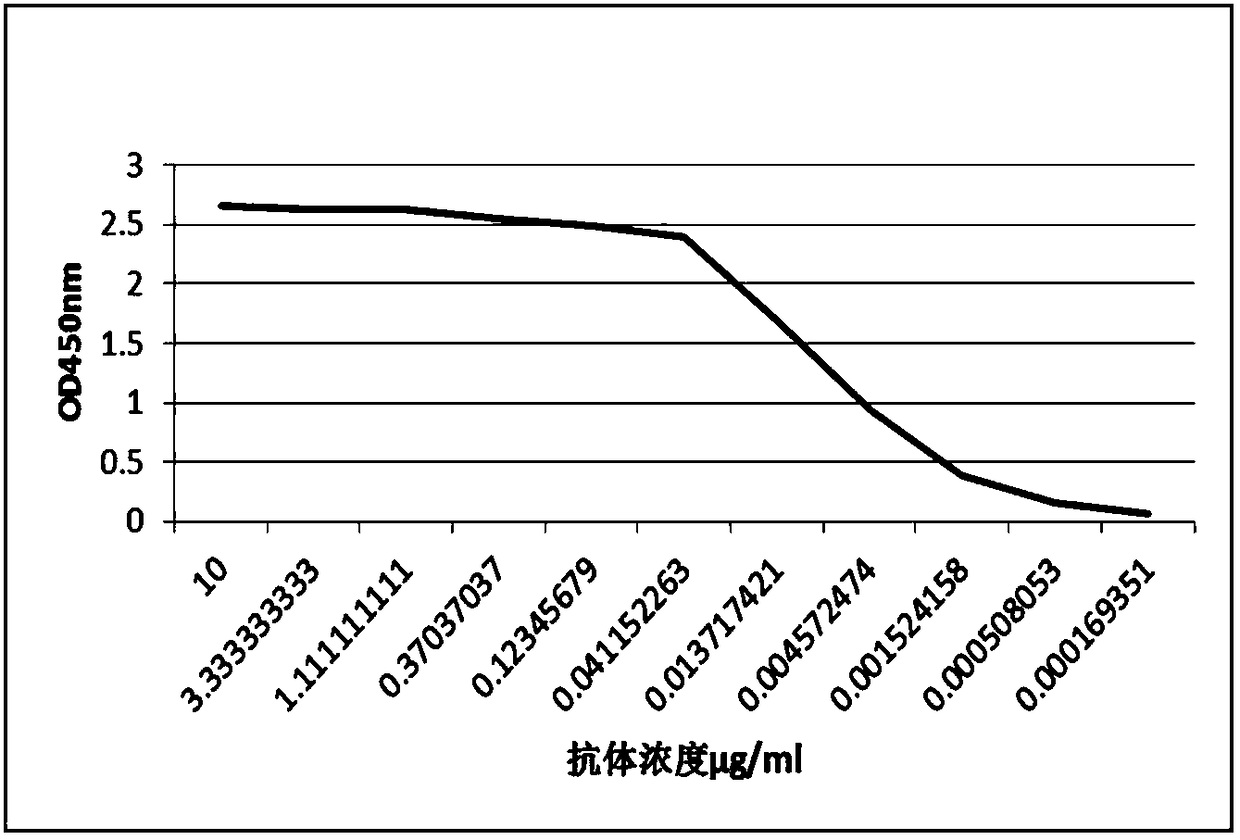
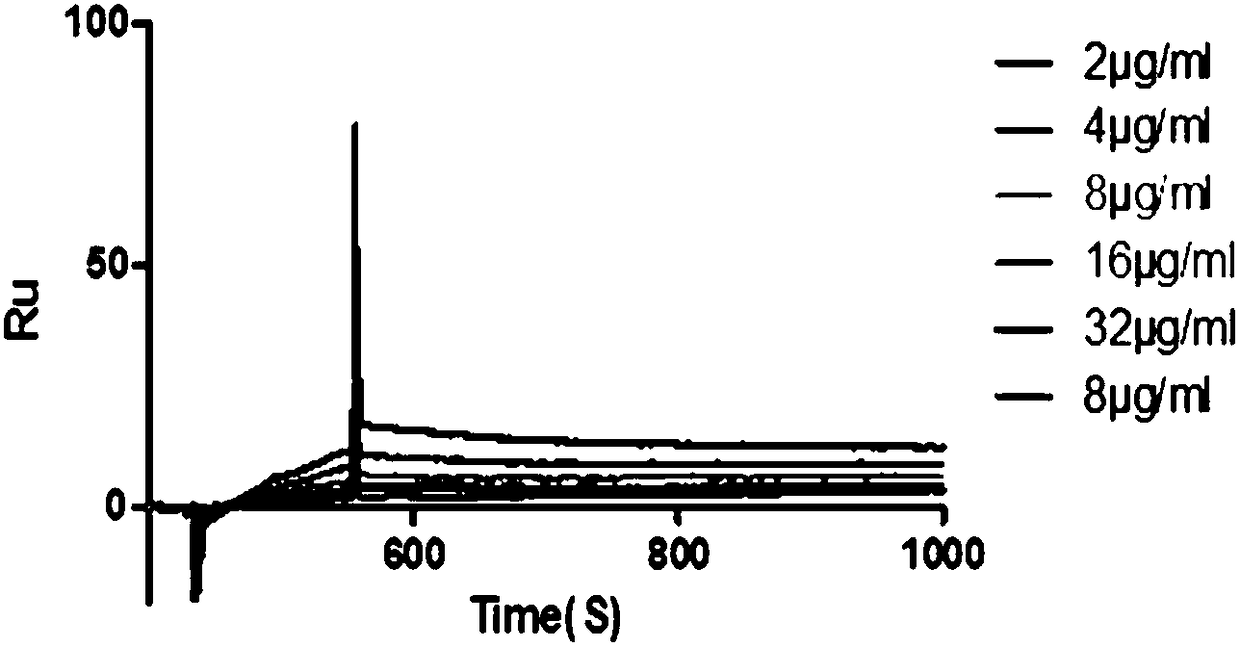
Patents
Literature
222 results about "Tetanus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A bacterial infection by Clostridium tetani that affects the nervous system.
Methods for using tetanus toxin for beneficial purposes in animals (mammals)
Methods of using tetanus toxin to modulate or control neural functions or nonneural cellular activities at selected sites in animals, particularly in mammals, and more particularly in humans, are provided. Pharmaceutical formulations to modulate neural functions or non-neural cellular activities of an animal at selected sites in animals, particularly in mammals, and more particularly in humans are also provided. Uses of tetanus toxin in preparation of medicaments for methods of treating clinical disorders or symptoms of animals, particularly mammals and more particularly humans are also provided.
Owner:SANDERS
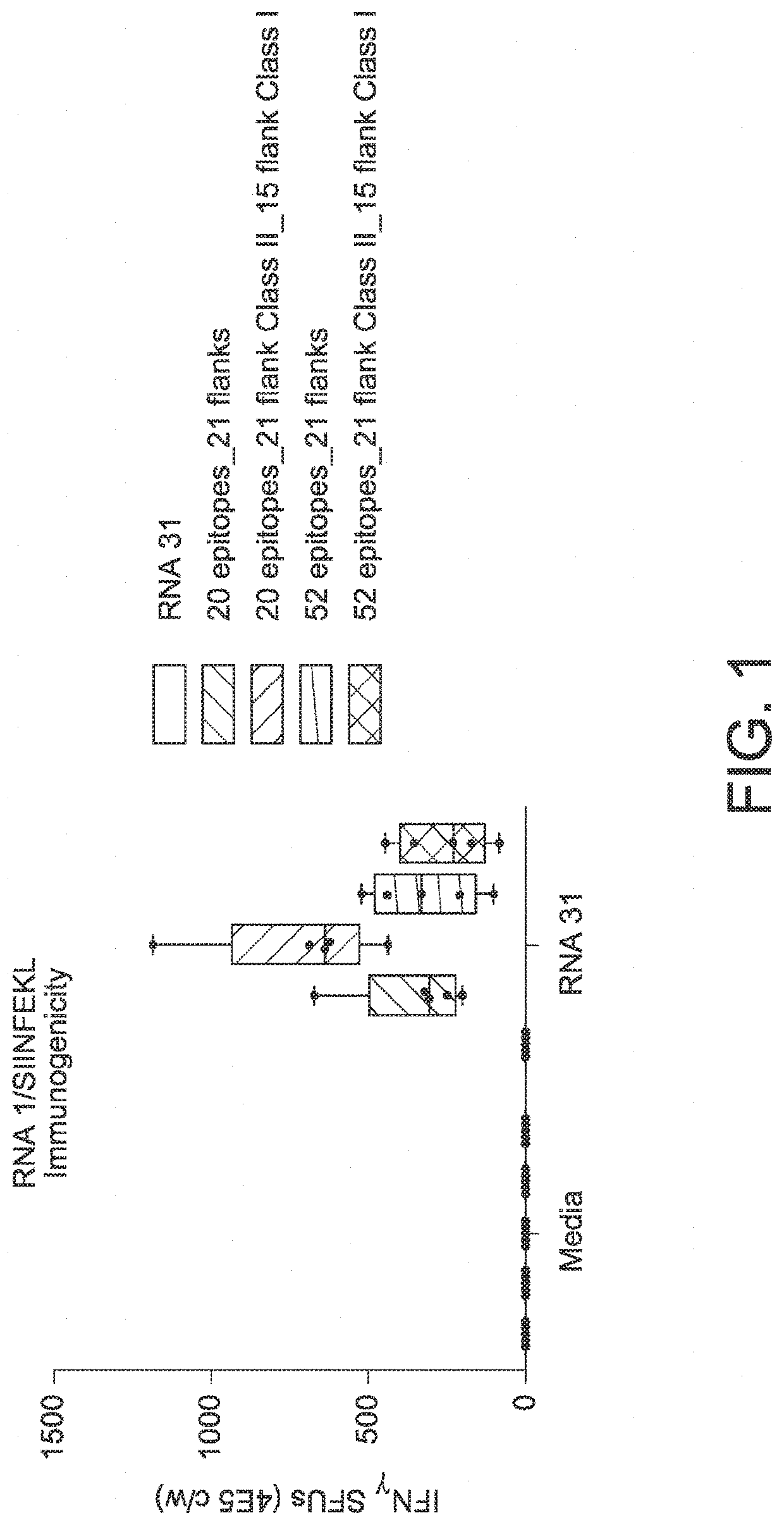
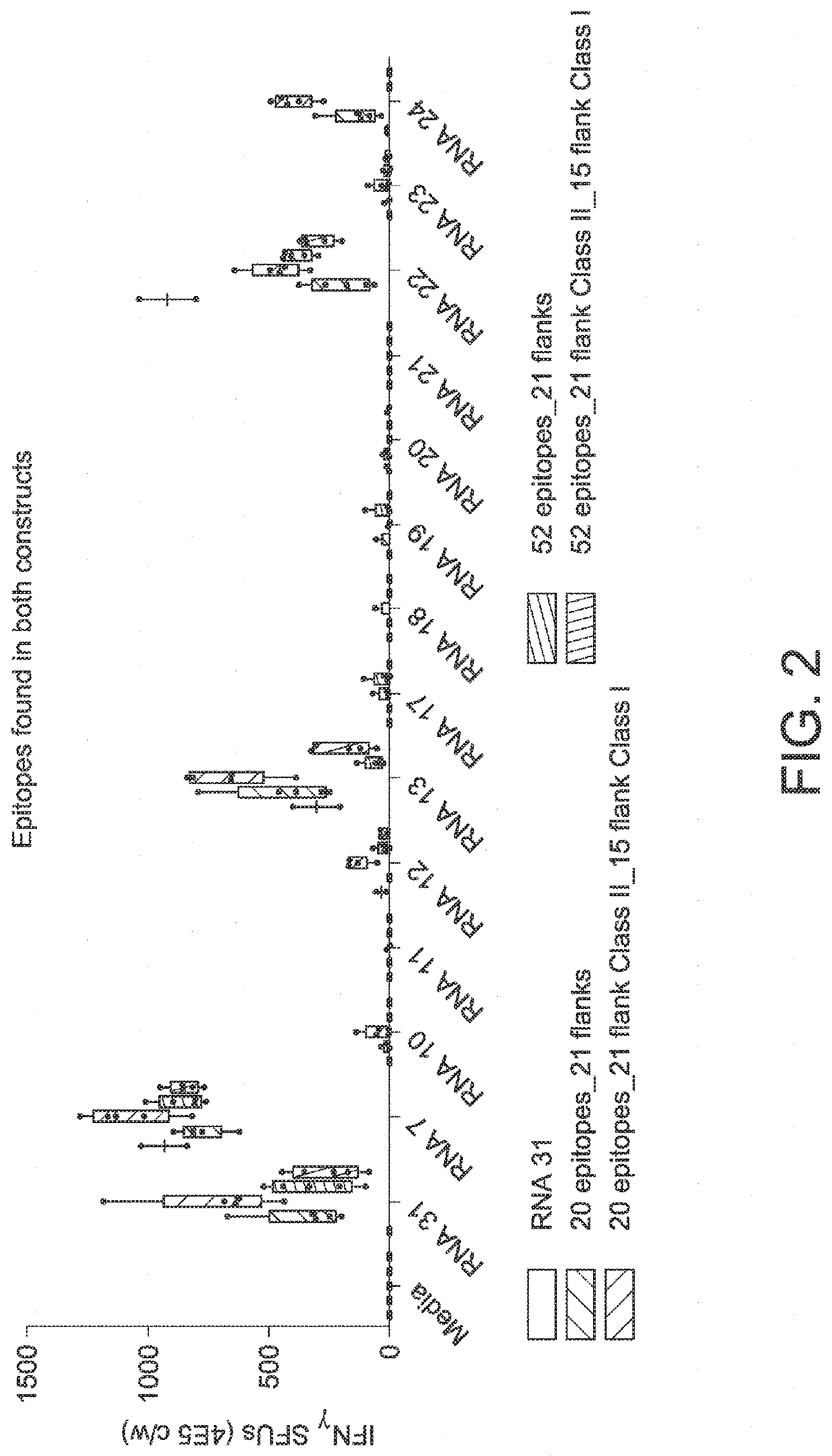
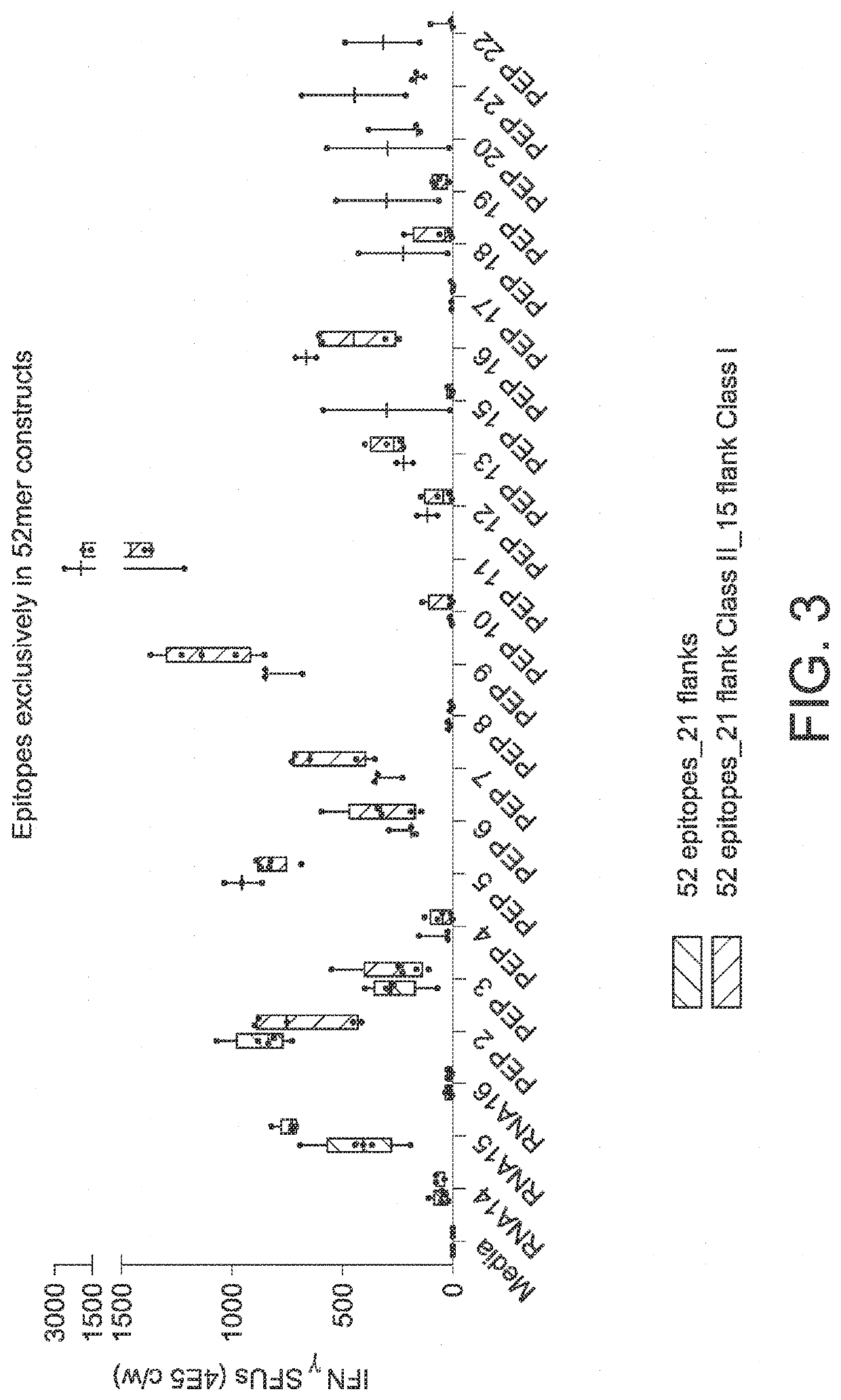
RNA cancer vaccines
PendingUS20190351040A1Balanced immune responseOrganic active ingredientsAntibody ingredientsTetanusAdjuvant
The disclosure relates to cancer ribonucleic acid (RNA) vaccines, as well as methods of using the vaccines and compositions comprising the vaccines. In particular, the disclosure relates to concatemeric mRNA cancer vaccines encoding several cancer epitopes on a single mRNA construct, i.e. poly-epitope mRNA constructs or poly-neo-epitope constructs. The disclosure further relates to p53 and KRAS mutations, as well as incorporation of immune enhancers such as STING, e.g. mRNA constructs further encoding an immune stimulator or adjuvant. The disclosure further relates to inclusion of universal T cell epitopes, such as tetanus or diphtheria toxins to elicit an enhanced immune response.
Owner:MODERNATX INC
Extracellular matrix materials as vaccine adjuvants for diseases associated with infectious pathogens or toxins
Disclosed are vaccines and vaccine adjuvants useful in the treatment and / or prevention of infection and diseases associated with infectious pathogens, such as tetanus, as well as diseases associated with biological toxins. Also provided are methods of preparing an adjuvant and the vaccine containing the adjuvant. Methods are also provided for vaccinating / immunizing an animal against infection and diseases associated with infectious pathogens, such as tetanus, and other diseases associated with biological toxins. Adjuvant materials are presented that are prepared from an extracellular matrix material. The adjuvants are demonstrated to enhance the immunogencity of an infectious pathogen antigen or biological toxin antigen of interest, as well as to enhance the survival of an immunized animal.
Owner:UNIV OF NOTRE DAME DU LAC +1
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS6822076B2Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsTetanusBasophilia
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-lIe-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Traditional Chinese medicine preparation for curing tetanus
InactiveCN101181534AEffectively preventEffective therapeuticAntibacterial agentsPowder deliveryCentipedeSide effect
The invention relates to a Chinese medicine preparation for curing tetanus. The formula includes earthworm, cicada ecdysis, rhizoma gastrodiae, notopterygium root, parsnip, herba schizonepetae, rhizoma arisaematis, uncaria, red peony, alum, ophiopogon japonicus, cortex moutan, Indian bread with hostwood, root poria, centipede, whole worm, atrina glass, honeysuckle, hemlock parsley, forsythia, safflower, peach seed and angelica. In the formula, the raw materials are pulverized, filtered and then made into pulvis. During the medicine taking, the medicine is mixed with cold boiled water, with two or three times per day and three days is one treatment period. The Chinese medicine preparation, compared with the prior art, is scientific and reasonable in the formula, simple in the regrouping ofthe raw materials and preparation process, effective in reducing the treatment cost and remarkable in treating tetanus. Having no toxic side effects to the human body, the invention is the medicine prepared commonly in homes.
Owner:张丽
Method for producing health care black bone chicken egg and employed pharmaceutical composition
InactiveCN101785828AEffective treatment of infertilityEffective treatment of irregular menstruationAnthropod material medical ingredientsMammal material medical ingredientsDiseaseAdjuvant
The invention relates to a method for producing health care black bone chicken egg and employed pharmaceutical composition. The production method of the health care black bone chicken egg comprises the following steps: (1) respectively grinding and then evenly mixing medicinal materials in the main drug; (2) respectively grinding and then evenly mixing medicinal materials in the adjuvant; (3) fully mixing the main drug and the adjuvant obtain in step (1) and step (2) to obtain the pharmaceutical composition, wherein the proportioning is weight of main drug: weight of adjuvant=80-150:100; (4) adding the pharmaceutical composition to the feed to be evenly mixed to form a mixed feed; wherein the additive amount is that weight of the pharmaceutical composition: weight of the feed=5-20:100; (5) feeding the mixed feed obtained in step (4) to egg-producing black bone chicken, then obtaining the health care black bone chicken egg which is produced after feeding the black bone chicken for 7 days.The pharmaceutical composition is rationally matched by selecting multiple medicines, through efficient biotransformation of the egg-producing black bone chicken, cream of Chinese herbal medicines are concentrated in the eggs, so that the health care black bone chicken eggs with multiple health care and treatment functions are obtained; the health care black bone chicken eggs enjoy excellent food therapy effect in preventing and treating diabetes, high blood pressure, hemiplegia, sequel of apoplexy, cardiovascular and cerebrovascular diseases, rheumatism and paralysis, tetanus, infertility and various cancers.
Owner:郭伟光
Fret protease assays for clostridial toxins
InactiveUS20080032318A1Used to determineDecreased acceptor fluorescence intensityPeptide-nucleic acidsMaterial analysis by observing effect on chemical indicatorSerotypeFluorophore
The present invention provides clostridial toxin substrates useful in assaying for the protease activity of any clostridial toxin, including botulinum toxins of all serotypes as well as tetanus toxins. A clostridial toxin substrate of the invention contains a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence that includes a cleavage site, where the cleavage site intervenes between the donor fluorophore and the acceptor and where, under the appropriate conditions, resonance energy transfer is exhibited between the donor fluorophore and the acceptor.
Owner:ALLERGAN INC
Anti-tetanotoxin antibody, and preparation method and application thereof
ActiveCN102875674AEffective neutralizationLess side effectsAntibacterial agentsBacterial antigen ingredientsBiotechnologyHeavy chain
The invention discloses an anti-tetanotoxin antibody. In the heavy chain variable region, the amino acid sequence of CDR1 is disclosed as SEQ ID NO.5, the amino acid sequence of CDR2 is disclosed as SEQ ID NO.6, and the amino acid sequence of CDR3 is disclosed as SEQ ID NO.7; and in the light chain variable region, the amino acid sequence of CDR1 is disclosed as SEQ ID NO.8, the amino acid sequence of CDR2 is disclosed as SEQ ID NO.9, and the amino acid sequence of CDR3 is disclosed as SEQ ID NO.10. The invention also discloses a nucleotide sequence for coding the anti-tetanotoxin antibody, and a corresponding recombinant plasmid and recombinant expression vector. The invention also discloses a preparation method and application of the anti-tetanotoxin antibody. The anti-tetanotoxin antibody disclosed by the invention is a full human antibody, has the advantages of low side reaction, high affinity and simple preparation method, and has wide industrial application prospects.
Owner:CHENGDU RONGSHENG PHARMA
Ultramicro wall-broken gastrodia elata composition and preparation method thereof
InactiveCN104189517AFully extractedQuality improvementNervous disorderDigestive systemConvulsionDisease
The invention discloses a preparation method of an ultramicro gastrodia elata decoction piece. The preparation method comprises selection of raw materials, cleaning of the raw materials, boiling, slicing, secondary boiling, drying, ultramicro wall breaking, obtaining of a preparation, and the like. Gastrodia elata processed by employing the processing and ultramicro fragmentation method has relatively high effective-composition dissolution rate, the bioavailability of a medicine is improved, the medicine effect is relatively good, and the toxicity is relatively low. The invention also discloses the decoction piece prepared by employing the method, and application of the decoction piece to prepare medicines for treating headache dizziness, numbness of limbs, infantile convulsion, epilepsy, twitch, tetanus and other diseases.
Owner:HUNAN BUTIAN PHARMA
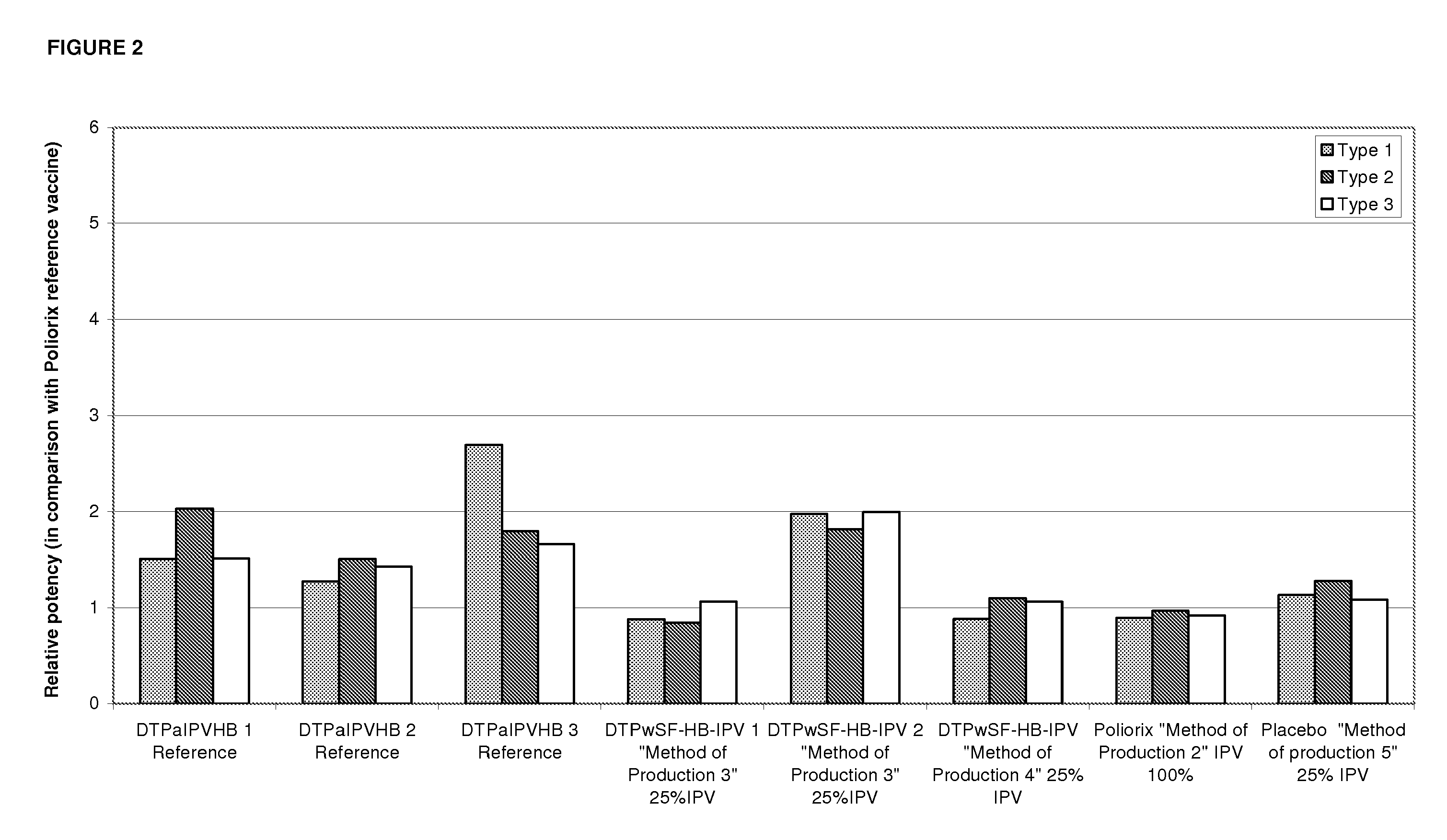
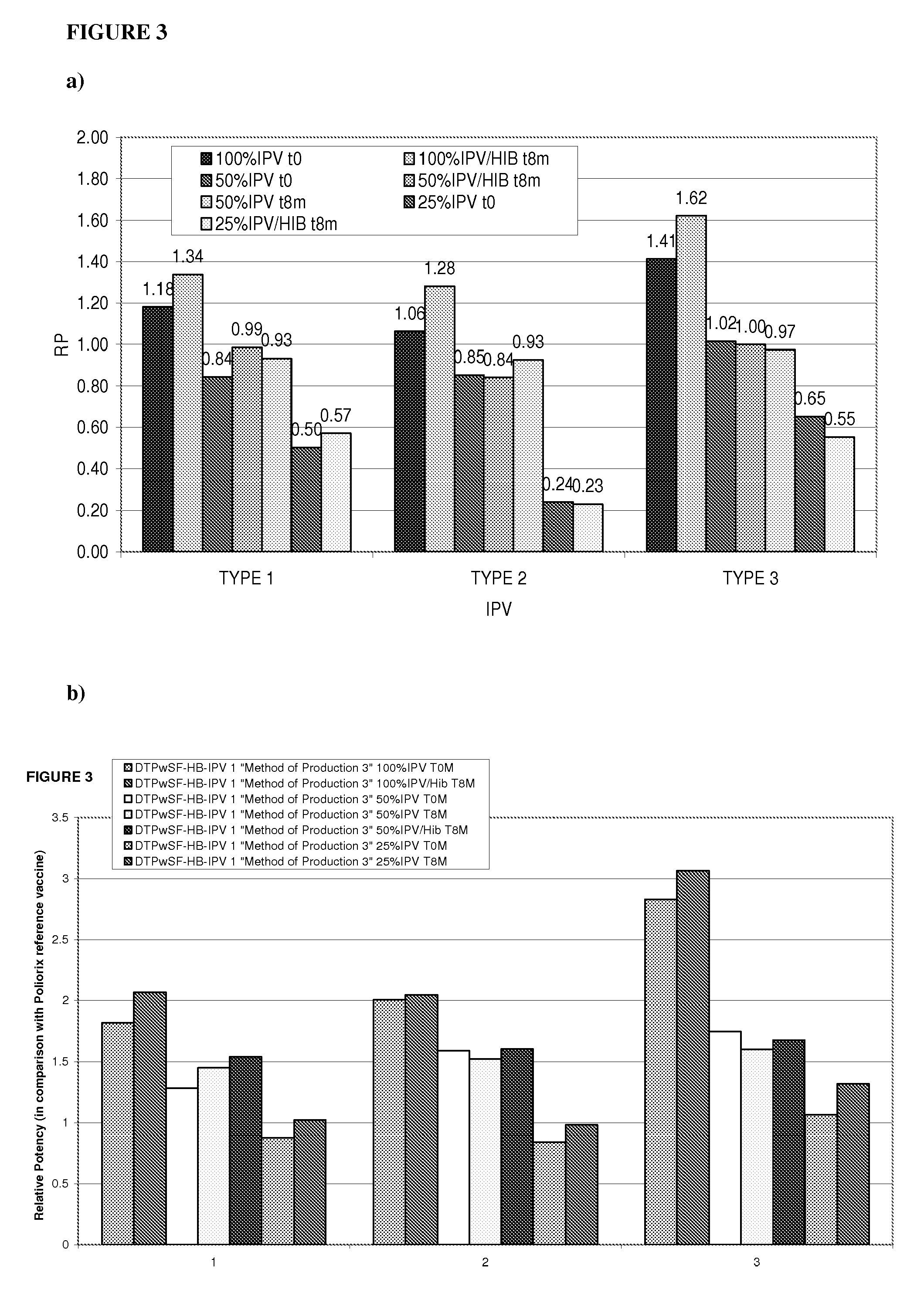
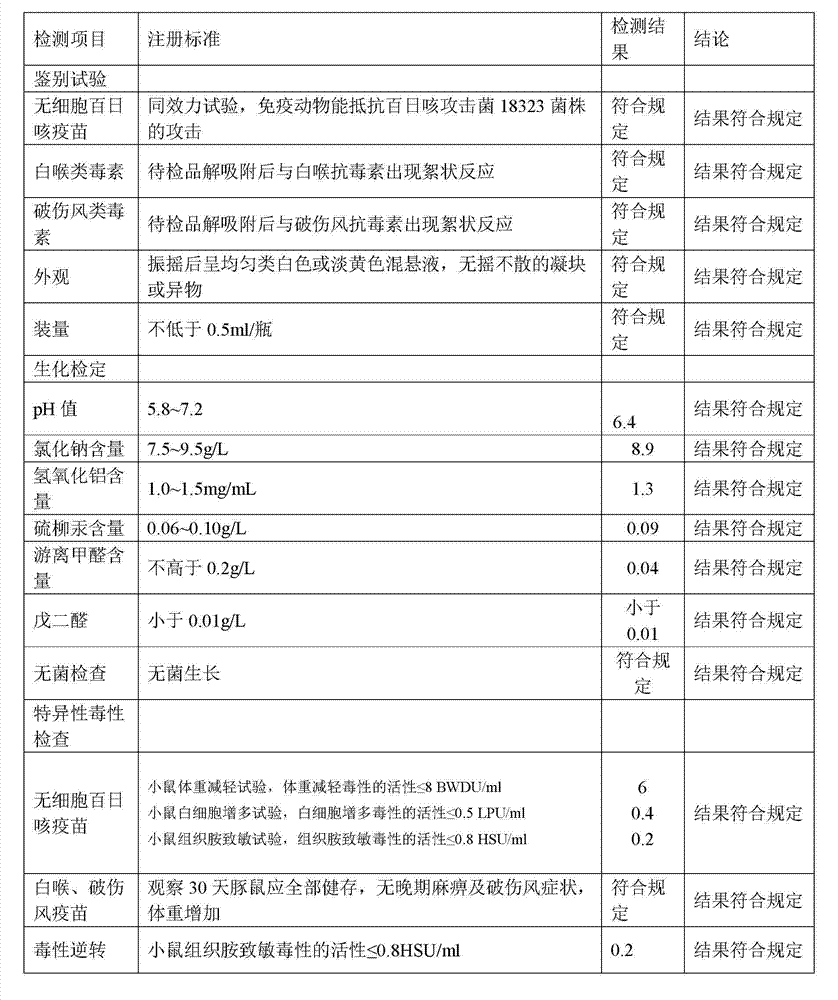
Combination vaccine with acellular pertussis
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, acellular pertussis, and infections caused by Haemophilus influenzae and polio viruses. The present invention also relates to inclusion of antigens for protection against infections caused Hepatitis virus and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
Immune responses to fusion proteins
InactiveUS6936464B1Improve aspectEnhance vaccine efficacyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsFusion Protein ExpressionIn vivo
The invention relates to a nucleic acid construct for delivery into living cells in vivo for inducing an immune response in a patient to an idiotypic determinant present on a malignant B cell in the patient; the construct directing the expression of a fusion protein, said fusion protein comprising the idiotypic determinant and at least one T helper cell epitope from tetanus toxin. The invention further relates to a method of making the nucleic acid construct, a method of treating a patient, and to a composition comprising the nucleic acid construct.
Owner:CANCER RES TECH LTD
Traditional Chinese medicine preparation for treating tetanus
InactiveCN101991781AImprove the body's immune functionImprove immune functionAntibacterial agentsAnthropod material medical ingredientsDiseaseTreatment effect
The invention discloses a traditional Chinese medicine preparation for treating tetanus, which comprises the following raw material medicaments in parts by weight: 15 parts of taraxacum, 15 parts of fish flabellate, 15 parts of exuviae cicada, 15 parts of scorpio, 8 parts of white silkworm, 8 parts of gastrodia, 8 parts of rhizoma arisaematis, 8 parts of hurricane lamp, 8 parts of angelica, 8 parts of herba schizonepetae, 8 parts of notopterygium root and 8 parts of annulus enclosure. The preparation comprises a pill, a powdery preparation, a tablet, an oral liquid preparation and a capsule prepared by a traditional preparation technology or a modern preparation technology. The adopted medical materials have the efficacy of clearing away heat and toxic material, removing carbuncle and eliminating stagnation, dispersing stagnated liver for regulating qi, promoting blood circulation for removing blood stasis, and relieving exterior syndrome for dispersing cold. The traditional Chinese medicine preparation enhances the immune function of an organism by oral administration, has good treating effect on the tetanus caused by various diseases, and is convenient to take. Clinical application proves that the traditional Chinese medicine preparation has the advantages of quick action, obvious treating effect, good durability, low cost and no any toxic or side effect.
Owner:栗素琴
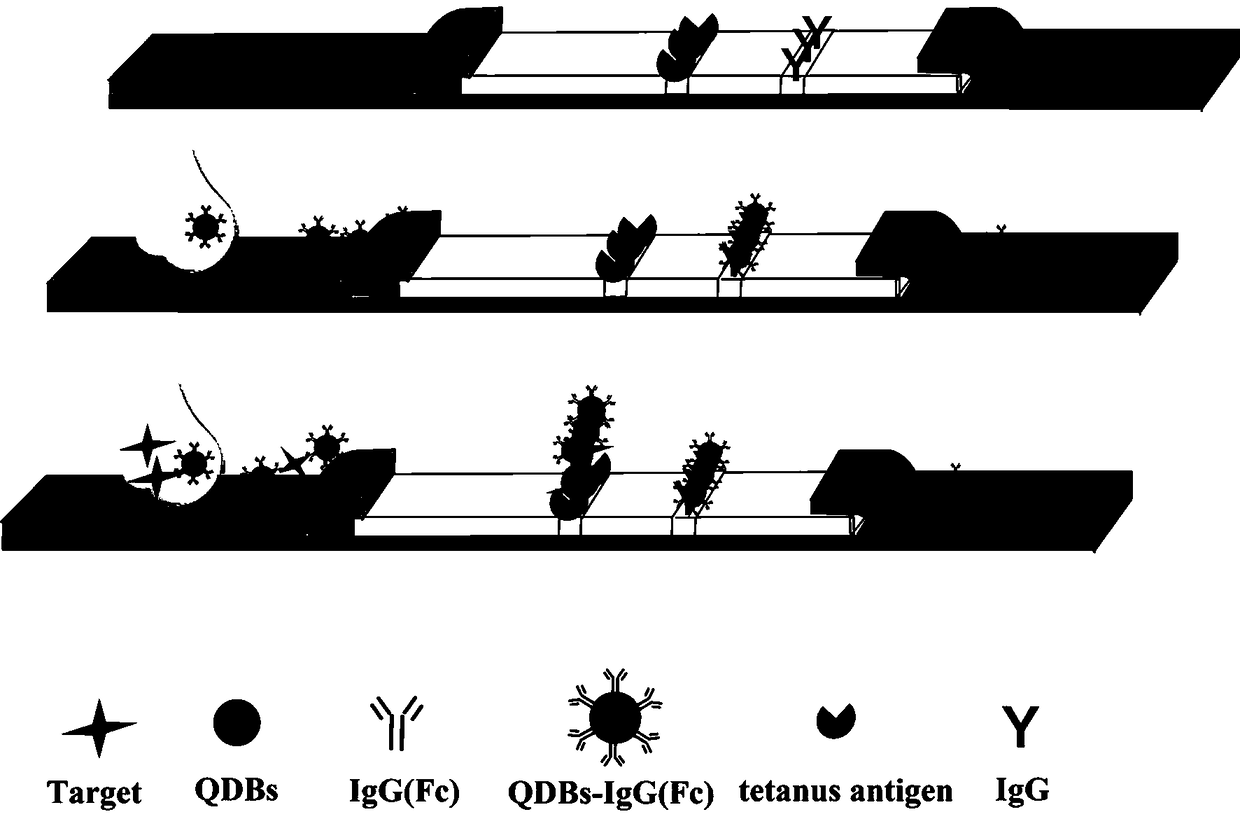
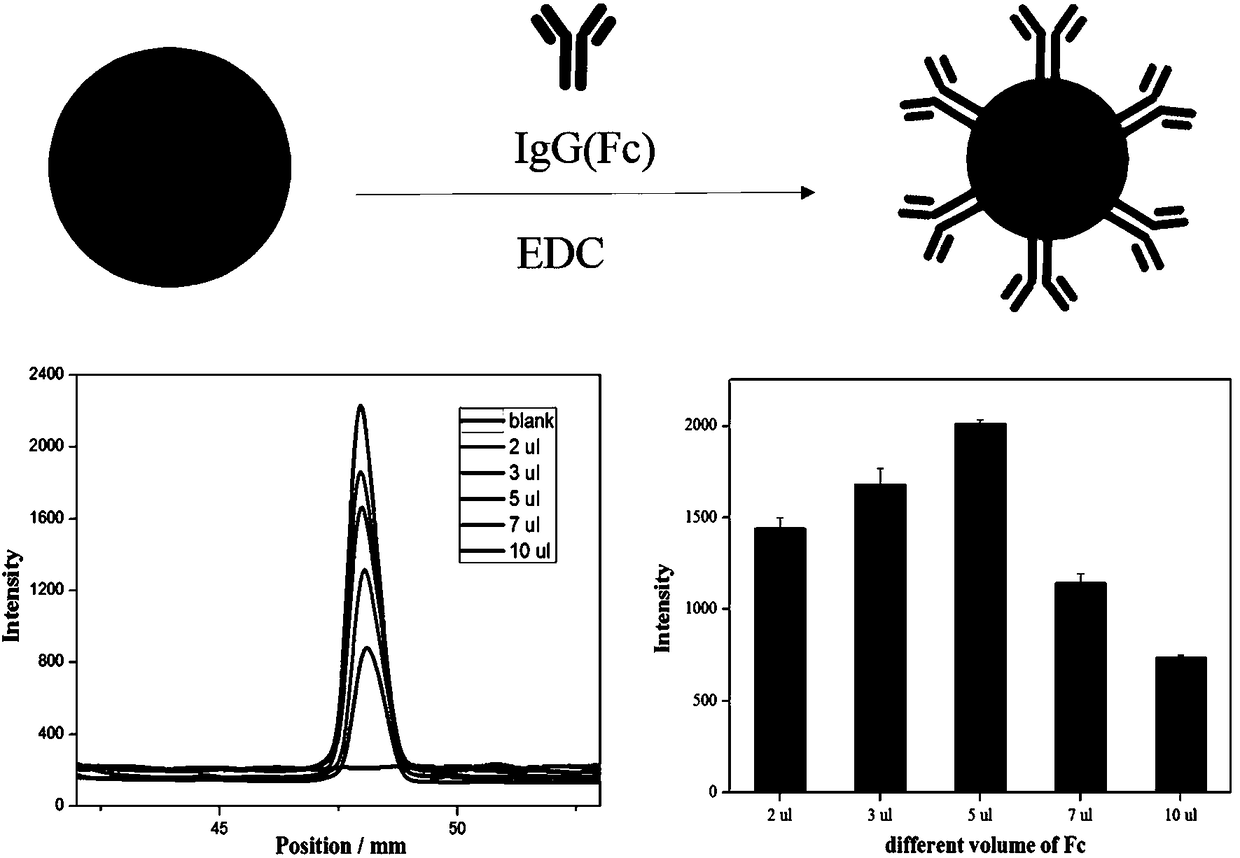
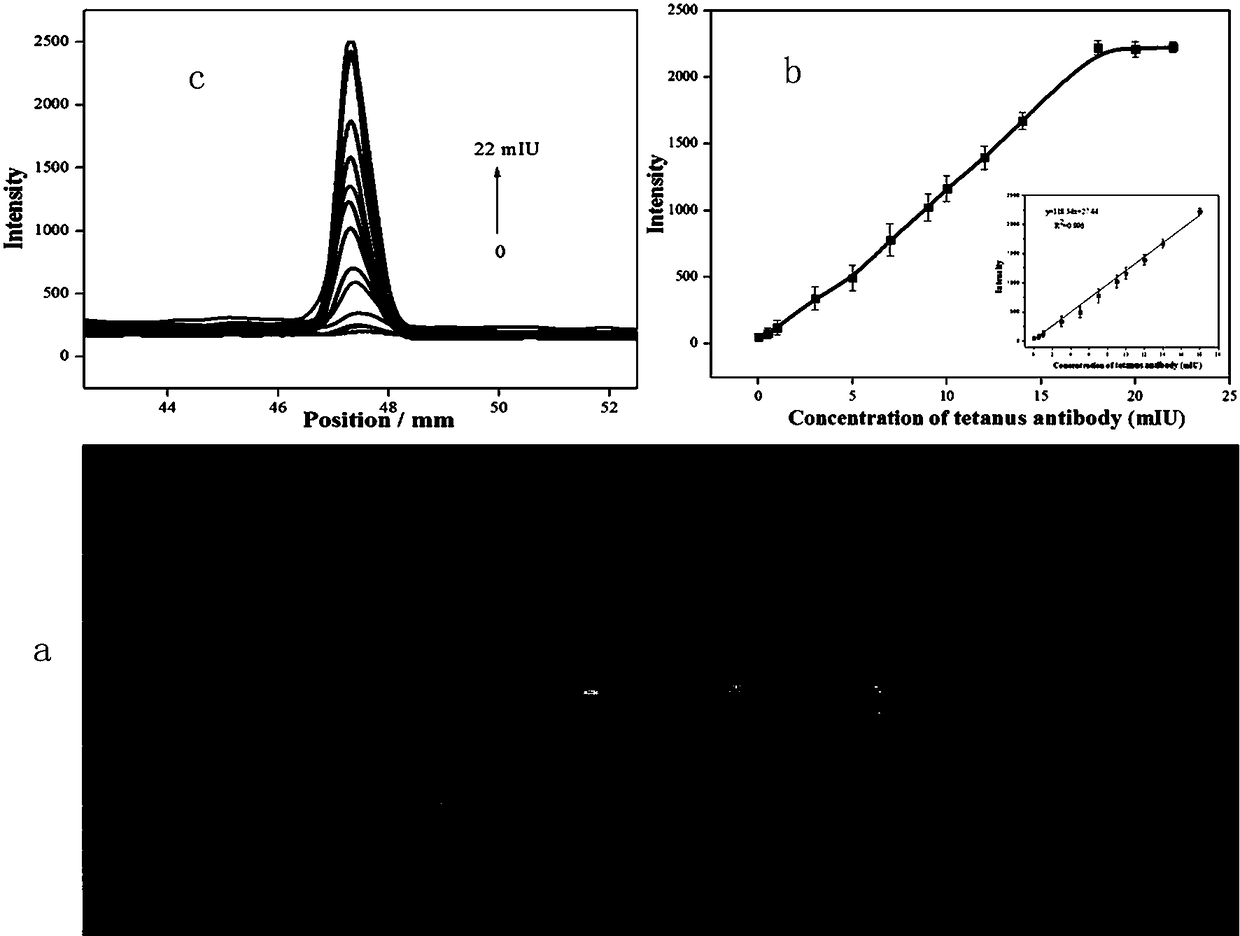
Test strip for detecting tetanus antibody on basis of quantum dot fluorescent microsphere chromatography and preparation method and using method thereof
The invention belongs to the technical field of lateral side stream chromatography and bioanalysis, particularly relates to a test strip for detecting tetanus antibody on basis of quantum dot fluorescent microsphere chromatography and a preparation method and using method thereof, and aims at solving the technical problems in the process of rapidly and portably detecting tetanus antibody content via low consumption. The preparation method of the test strip comprises the following steps of: firstly closing a sample pad by using solution with a TW20 mass concentration of 0.5-5%; marking a detection line and a quality control line on an NC membrane in sequence; drying the processed sample pad and the NC membrane at 37 DEG C for 1 hour; pasting the sample pad, the NC membrane and a water absorption pad on a PVC baseboard in sequence; carrying out overlapping in pairs to 2-3 mm; and finally cutting into 3.9 mm wide to obtain the finished test strip. Compared with traditional colloidal goldimmunochromatography test strips, the test strip is good in quantum dot microsphere marker stability and high in detection sensitivity, and is capable of realizing quantitative detection for low-concentration target objects.
Owner:ZHENGZHOU UNIV
Methods for direct visualization of active synapses
InactiveUS20050060761A1Easy to transportEasy to operateNervous disorderPeptide/protein ingredientsBiologic markerFragment C tetanus toxin
A method for visualizing an active synapse wherein said method comprises: (a) exposing cells forming the active synapse to a biomarker comprising at least fragment C of tetanus toxin and a reporter protein; and (b) visualizing the biomarker; wherein the accumulation of the biomarker into dendritic spines of the cells allows visualization of an active synapse. Also, a method for screening molecules capable of modulating synapse activity is provided. A kit useful for the early diagnosis of neurodegenerative disease comprises a biomarker comprising at least fragment C of tetanus toxin and a reporter protein.
Owner:INST PASTEUR +1
Vaccine
InactiveUS20100040647A1Adequate and improved level of protectionAntibacterial agentsSsRNA viruses positive-senseDiseaseTetanus
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus are provided
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Diphtheria, tetanus and acellular pertussis/Haemophilus influenzae type b - group A and C meningococcus combined vaccine
ActiveCN102813917AGood freeze-drying effectRelieve painAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus
The invention provides a diphtheria, tetanus and acellular pertussis / Haemophilus influenzae type b - group A and C meningococcus combined vaccine, which has the characteristics of high safety, high efficacy, high controllability and prevention of multiple diseases through one injection, satisfies relevant requirements on absorbed diphtheria, tetanus and acellular pertussis combined vaccines, Haemophilus influenzae type b combined vaccines and group A and C meningococcus combined vaccines in the Third Part of Chinese Pharmacopoeia (2010) and proposed Rules on Production and Inspection of Diphtheria, Tetanus and acellular Pertussis / Haemophilus influenzae Type b - Group A and C Meningococcus Combined Vaccines, and achieves an effect of putting into practical application.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Combination vaccines with low dose of hib conjugate
InactiveUS20090208526A1Facilitates correct measurementImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsTetanusDiphtheria vaccination
Provided herein are combination vaccines comprising antigens for protecting a subject against at least diphtheria, tetanus, pertussis and Hib, wherein: (a) the antigen for protecting against Hib is a conjugate of a Hib capsular saccharide; (b) the concentration of the Hib conjugate in the vaccine is <15 μg / ml; and (c) the Hib conjugate has never been lyophilised.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Single dose immunization against tetanus toxin cation dextran microspheres and preparation method thereof
InactiveCN101869704AReduce the number of injectionsImprove vaccination coverageAntibacterial agentsBacterial antigen ingredientsControlled releaseTetanus
The invention relates to the filed of pharmaceutical preparation, in particular to single dose immunization against tetanus toxin cation dextran microspheres and a preparation method thereof. The single dose immunization against tetanus toxin cation dextran microspheres are prepared by carrying tetanus toxin after electrostatic interaction on cation hydroxyethyl acrylate dextran microspheres. The tetanus toxin controlled release microspheres can reduce the injection frequency of tetanus vaccine, improve the vaccination coverage and reduce the drop-out rate, thereby effectively preventing tetanus and providing a single dose tetanus toxin controlled release vaccine preparation with long-term effect and realizing the whole course immunity by one injection.
Owner:CHINA PHARM UNIV
Substrate protein SNVP, and coding gene and application thereof
The invention discloses a clostridial neurotoxin substrate protein, and a coding gene and an application thereof. The clostridial neurotoxin substrate protein is a protein (a) composed of an amino acid sequence represented by sequence 2 in a sequence table, or is a protein (b) obtained through substituting and / or deleting and / or adding one or more amino acid residues to the amino acid sequence represented by the sequence 2, related to the clostridial neurotoxin detection and derived from the sequence 2. The substrate protein SNVP provided by the invention has seven toxin serotype botulinum and tetanus toxin enzyme hydrolysis sites, can specifically identify A, B, E, C, D, F and G type botulinum toxins and tetanus toxins, and can be used for the detection and the parting discrimination of the botulinum toxins and tetanus toxins as a core detection reagent.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Tetanus treatment traditional Chinese medicine preparation
InactiveCN103272121ASignificant effectQuick resultsAntibacterial agentsAnthropod material medical ingredientsTetanusSide effect
The invention relates to a tetanus treatment traditional Chinese medicine preparation, which is prepared from the following bulk drugs, by weight, 13-17 parts of Chinese bastardtoadflax herb, 13-17 parts of fragrant solomonseal rhizome, 13-17 parts of paniculate bolbostemma bulb, 13-17 parts of white peony root, 10-14 parts of milkvetch root, 10-14 parts of unprocessed rehmannia root, 10-14 parts of asiatic plantain herb, 9-11 parts of angelica, 9-11 parts of centipede, 9-11 parts of stiff silkworm, 6-8 parts of sharpleaf uncaria stem with hooks, 6-8 parts of giant typhonium tuber, 6-8 parts of divaricate saposhnikovia, 4-6 parts of scorpion, and 4-6 parts of reddish jackinthepulpit tuber. The tetanus treatment traditional Chinese medicine preparation has advantages of significant treatment effect, rapid effect and low toxic-side effect, wherein clinical trial results show that tetanus treatment efficiency is 96%.
Owner:郑立娜
Neutralizing monoclonal antibody resisting to tetanus toxin and application thereof
ActiveCN105542004AStrong specificityProlonged deathAntibacterial agentsImmunoglobulins against bacteriaLethal doseComplete antibody
The invention discloses a neutralizing monoclonal antibody resisting to tetanus toxin. Hybridoma cells are obtained from tetanus toxin heavy chain C fragment immune mice, light chain and heavy chain variable region genes of the antibody are taken, human-mouse chimeric complete antibody expression vectors are built, CHO cell transfection is carried out, purification is carried out, and then the antibody is obtained. The antibody has the activity of specific binding to tetanus toxin, the monoclonal antibody can partially protect mice against attack of tetanus toxin, and four antibodies can completely protect mice against a twofold lethal dose of tetanus toxin in combination.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Ointment for healing tranumatic injury fast
InactiveCN1383869ANo side effectsLow priceHydroxy compound active ingredientsAntipyreticInjury causeSide effect
The ointment for healing traumatic injury fast is prepared with 21 kinds of Chinese medicinal materials including ledebouriella root, dahurian angelica root, ground beetle, dragon's blood, cimicifugarhizome, etc. The preparation process includes soaking, drying, grinding into fine powder, sieving, mixing, bottling and other steps. When used, it is mixed with proper amount of turpentine, Vaselineor spirit to produce ointment. It has the integrated functions of stopping pain, stopping bleeding, relaxing muscles and joints, dispersing bloood clots, elimianting swelling, expelling wind and wetness, promoting healing of fracture and preventing tetanus and has no toxic side effect.
Owner:蓝华荣
Hydrophobia-tetanus double titer human immunoglobulin, method for preparing same and application thereof in pharmacy
ActiveCN101591393ARabies preventionTetanus preventionAntibacterial agentsSerum immunoglobulinsRabiesTetanus
The invention discloses a hydrophobia-tetanus double titer human immunoglobulin, wherein the purity of the immunoglobulin is more than or equal to 90 percent of total protein, the titer of the hydrophobia neutralizing antibody is more than or equal to 100IU / mL, the titer of the tetanus neutralizing antibody is more than or equal to 40IU / mL, and the content of protein is less than or equal to 180g / L. In addition, the invention also discloses a method for preparing the hydrophobia-tetanus double titer human immunoglobulin and application of the hydrophobia-tetanus double titer human immunoglobulin in preparing medicaments for preventing hydrophobia and tetanus.
Owner:TONROL BIOLOGICAL PHARM CO LTD
Extracellular Matrix Materials as Vaccine Adjuvants for Diseases Associated with Infectious Pathogens or Toxins
InactiveUS20100136050A1Antibacterial agentsBacterial antigen ingredientsPrevention infectionCell-Extracellular Matrix
Disclosed are vaccines and vaccine adjuvants useful in the treatment and / or prevention of infection and diseases associated with infectious pathogens, such as tetanus, as well as diseases associated with biological toxins. Also provided are methods of preparing an adjuvant and the vaccine containing the adjuvant. Methods are also provided for vaccinating / immunizing an animal against infection and diseases associated with infectious pathogens, such as tetanus, and other diseases associated with biological toxins. Adjuvant materials are presented that are prepared from an extracellular matrix material. The adjuvants are demonstrated to enhance the immunogenicity of an infectious pathogen antigen or biological toxin antigen of interest, as well as to enhance the survival of an immunized animal.
Owner:UNIV OF NOTRE DAME DU LAC +1
Combination vaccines with serogroup b meningococcus and d/t/p
InactiveUS20150190493A1Low amountEnhance immune responseAntibacterial agentsBacterial antigen ingredientsTetanusAdjuvant
Serogroup B meningococcus antigens can successfully be combined with diphtheria, tetanus and pertussis toxoids (“DTP”) to provide effective combination vaccines for protecting against multiple pathogens. These combinations are effective with a range of different adjuvants, and with both pediatric-type and booster-type DTP ratios. The adjuvant can improve the immune response which the composition elicits; alternatively, by including an adjuvant it is possible for the compositions to have a relatively lower amount of antigen while nevertheless having immunogenicity which is comparable to unadjuvanted combination vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Medical hemostasis first-aid belt and hemostyptic thereof
InactiveCN102783986ASimple structureEasy to useHeavy metal active ingredientsInorganic boron active ingredientsTetanusFirst aid
The invention discloses a medical hemostasis first-aid belt and a hemostyptic thereof. The first-aid belt comprises a body, wherein the periphery of the bottom surface of the body is provided with a gluing region at which an oily leather is arranged; the middle part of the body is provided with a hemostasis bag; and vacuum air bags are arranged at the periphery of the hemostasis bag. In addition, the invention further discloses the hemostyptic used for the medical hemostasis first-aid belt. The hemostyptic comprises medicaments with hemostasis, diminish inflammation, analgesia, infection prevent and tetanus prevention effects, such as common cephalanoplos herb, pseudo-ginseng, cicada slough, rhizoma arisaematis, corydalis tuber, bezoar, borneol, pearl powder, red paeonia, excrementum pteropi and dalbergia wood. Since the technical scheme is adopted, compared with the prior art, the medical hemostasis first-aid belt disclosed by the invention has the characteristics of simple structure, convenience in use, portability, good hemostasis effect, less possibility of infection, wide application range and the like.
Owner:李伟
Completely humanized monoclonal neutralizing antibody for tetanus toxin and application of neutralizing antibody
ActiveCN108314731AHigh affinityHigh activityAntibacterial agentsImmunoglobulins against bacteriaTetanusMonoclonal antibody
The invention discloses a completely humanized monoclonal neutralizing antibody for a tetanus toxin. The completely humanized anti-tetanus toxin neutralizing antibody is developed via a comprehensivehigh-throughput completely humanized monoclonal antibody research and development technical platform. The neutralizing antibody is high in affinity and specificity and has an important significance onprevention and treatment of tetanus infection.
Owner:CHANGCHUN BCHT BIOTECH
Chinese herba preparation for treating tetanus
InactiveCN104208552AQuick resultsShort course of treatmentAntibacterial agentsAnthropod material medical ingredientsTetanusSide effect
The invention discloses a Chinese herba preparation for treating tetanus. The selected crude drugs comprise the following components by weight: 20-30g of nam sing, 10-20g of gastrodia elata, 10-20g of periostracum cicada, 10-20g of bezoar, 10-20g of notopterygium incisium, 10-20g of pawpaw, 10-20g of cassia twig, 10-20g of tribulusterrestris, 10-20g of centipede, 8-15g of fructus evodiae, 8-12g of antelope horn, 8-12g of tabasheer, 8-12g of dandelion, 8-12g of coptis chinensis and 8-12g of astragalus membranaceus. The medicines are of good compatibility, plays the functions of supporting right and banking up the root, tranquilizing the mind, carrying out spasmolysis, activating blood, promoting the circulation of qi, perfusing meridian, relieving cough and eliminating phlegm. Clinical application finds that the Chinese herba preparation has the advantages of rapid effect taking, short treatment course, no side effect and high cure rate for treating tetanus, and is worthy of clinic promotion and application.
Owner:高建华
Chinese herbal medicine for treating tetanus
InactiveCN102940660AHeat-clearing and detoxifyingAnti-inflammatoryAntibacterial agentsPlant ingredientsMedicinal herbsTetanus
The invention discloses a Chinese herbal medicine for treating tetanus, which relates to a medicament preparation product and aims to increase a medicine variety for treating the tetanus. The Chinese herbal medicine comprises the following raw materials: 0.5-4g of climbing groundsel herb, 2-25g of Alangium platanifolium, 1-10g of serissa and 0.5-5g of wild chrysanthemum flower. The preparation method comprises the following steps of: cleaning the climbing groundsel herb, the Alangium platanifolium, the serissa and the wild chrysanthemum flower, mincing the raw materials and drying, crushing and milling, evenly mixing according to mass composition of each pharmaceutical formulation, and sealing and packaging and storing in a cool and dry place to obtain the medicine for later use. The usage method comprises the steps of boiling the medicine by adding with water, fumigating infected parts through steam, and washing the infected parts by warm medicinal water. The Chinese herbal medicine has the functions of clearing away heat and toxic materials, inflammation diminishing and swelling reducing, detoxifying, and being capable of restraining and killing clostridium tetani vitality. The raw materials are easily obtained, the cost is low, the preparation process is simple, and the medicine is suitable for patients of the tetanus.
Owner:张志荣
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com