Extracellular Matrix Materials as Vaccine Adjuvants for Diseases Associated with Infectious Pathogens or Toxins
a technology of extracellular matrix and infectious pathogens, applied in the field of vaccines, can solve the problems of poor effect of alum for influenza vaccination, unfavorable th2 based immune response, and inability to offer optimal protection against several important diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods for ECM as an Adjuvant for a Vaccine against Diseases Associated with an Infectious Pathogen
[0062]The present example provides some examples of materials and methods that may be used in the practice of the present invention.
[0063]Small Intestinal Submucosa (SIS)
[0064]Small Intestinal Submucosa (SIS) was obtained from Cook Biotech, Inc. (West Lafayette, Ind.). Experimental grade material was provided for use in the present studies of an SIS preparation that was described as having been prepared by harvesting porcine jejunum and placing 10- to 20-cm lengths into saline solution (31-33). Following removal of all mesenteric tissues, the jejunal segment was everted and the tunica mucosa abraded using a longitudinal wiping motion with a scalpel handle and moistened gauze. The serosa and tunica muscularis were then gently removed using the same procedure. The remaining tissue was disinfected with peracetic acid, rinsed extensively in high purity water, and sterilized ...
example 2
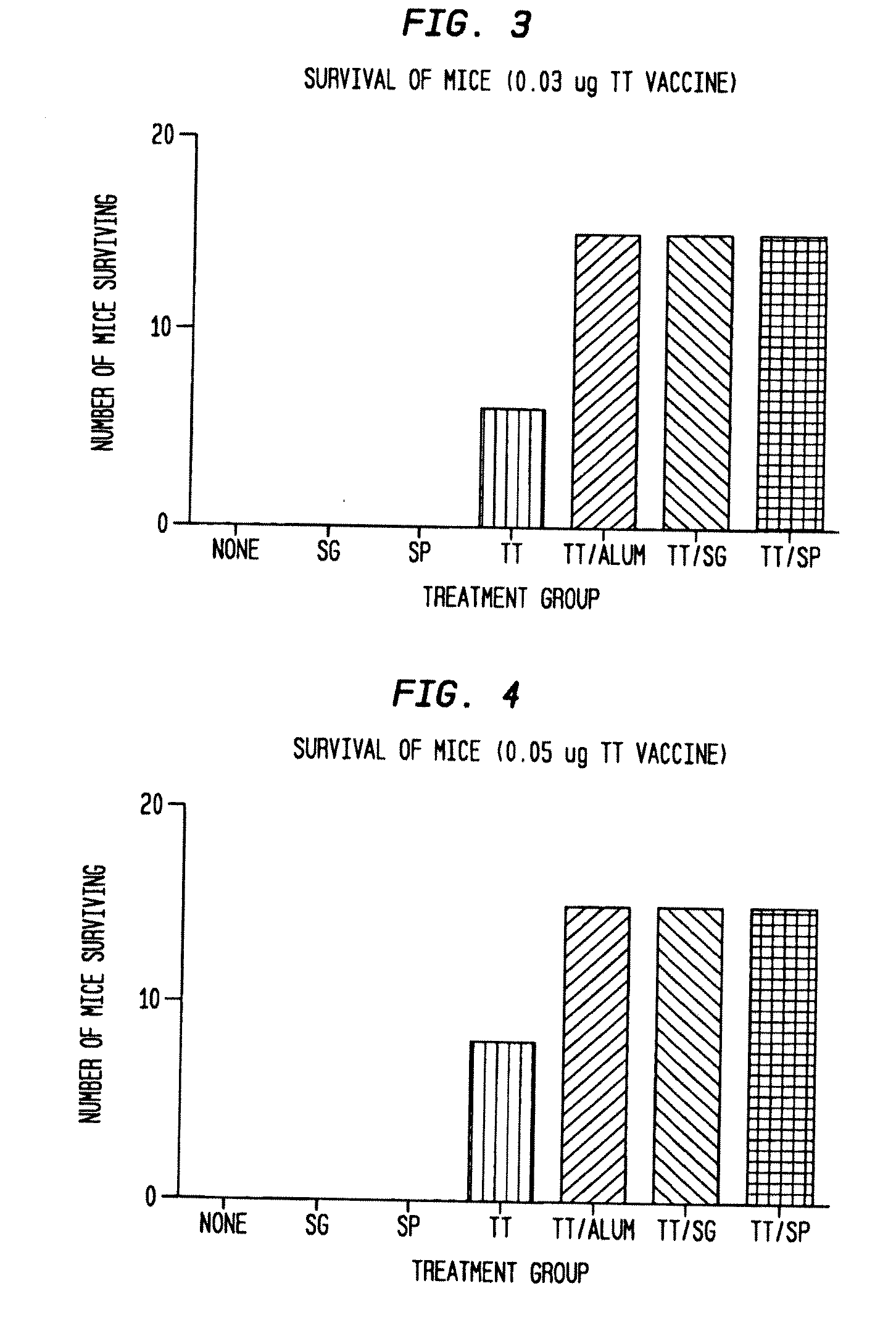
Use of Adjuvant in the Inhibition of Tetanus
[0071]To determine if SIS can act as an adjuvant for vaccines against diseases associated with infectious pathogens or biological toxins, preparations were made with tetanus toxoid, an inactivated form of tetanus toxin. Both gel SIS and SIS particles produced from a sheet of single layer SIS were evaluated as adjuvants. Briefly, groups of 15 Balb / C female mice (Harlan, Inc., Indianapolis, Ind.) were vaccinated initially (0.1 ml volume / dose) and again, five weeks later with one of the following:
[0072]Particulate SIS
[0073]Gel SIS
[0074]Tetanus toxoid (TT; 0.03 ug / dose)
[0075]TT (0.05 ug / dose)
[0076]TT (0.03 ug / dose)+alum (alhydrogel)
[0077]TT (0.05 ug / dose)+alum (alhydrogel)
[0078]TT (0.03 ug / dose)+particulate SIS
[0079]TT (0.05 ug / dose)+particulate SIS
[0080]TT (0.03 ug / dose)+gel SIS
[0081]TT (0.05 ug / dose)+gel SIS
[0082]Untreated control group
[0083]Five weeks after the second vaccination, mice were challenged with a lethal dose of tetanus toxin (1 ...
example 3
Exemplary Infectious Pathogens
[0087]The present example demonstrates the utility of the present invention with disease associated with a wide variety of infectious pathogens and biological toxins, including by way of example and not exclusion, tetanus, influenza, rabies, viral hepatitis, diphtheria, anthrax, Streptococcus pneumoniae infection, malaria, leishmaniasis, ricin toxicosis, and Staphylococcal enterotoxin B toxicosis.
TABLE 2Classification of Common Vaccines for HumansDisease or PathogenType of VaccineWhole Organisms:Bacterial cells:CholeraInactivatedPlagueInactivatedTuberculosisAttenuated BCG+Salmonella typhiAttenuatedViral Particles:InfluenzaInactivatedMeaslesAttenuatedMumpsAttenuatedRubellaAttenuatedPolio (Sabin / OPV)AttenuatedPolio (Salk / IPV)InactivatedV. zosterAttenuatedYellow feverAttenuatedType of Vaccine(Purified) MacromoleculesToxoids:DiphtheriaInactivated exotoxinTetanusInactivated exotoxinacellular PertussisInactivated exotoxinsCapsular polysaccharide:Haemophilus i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| biocompatibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com