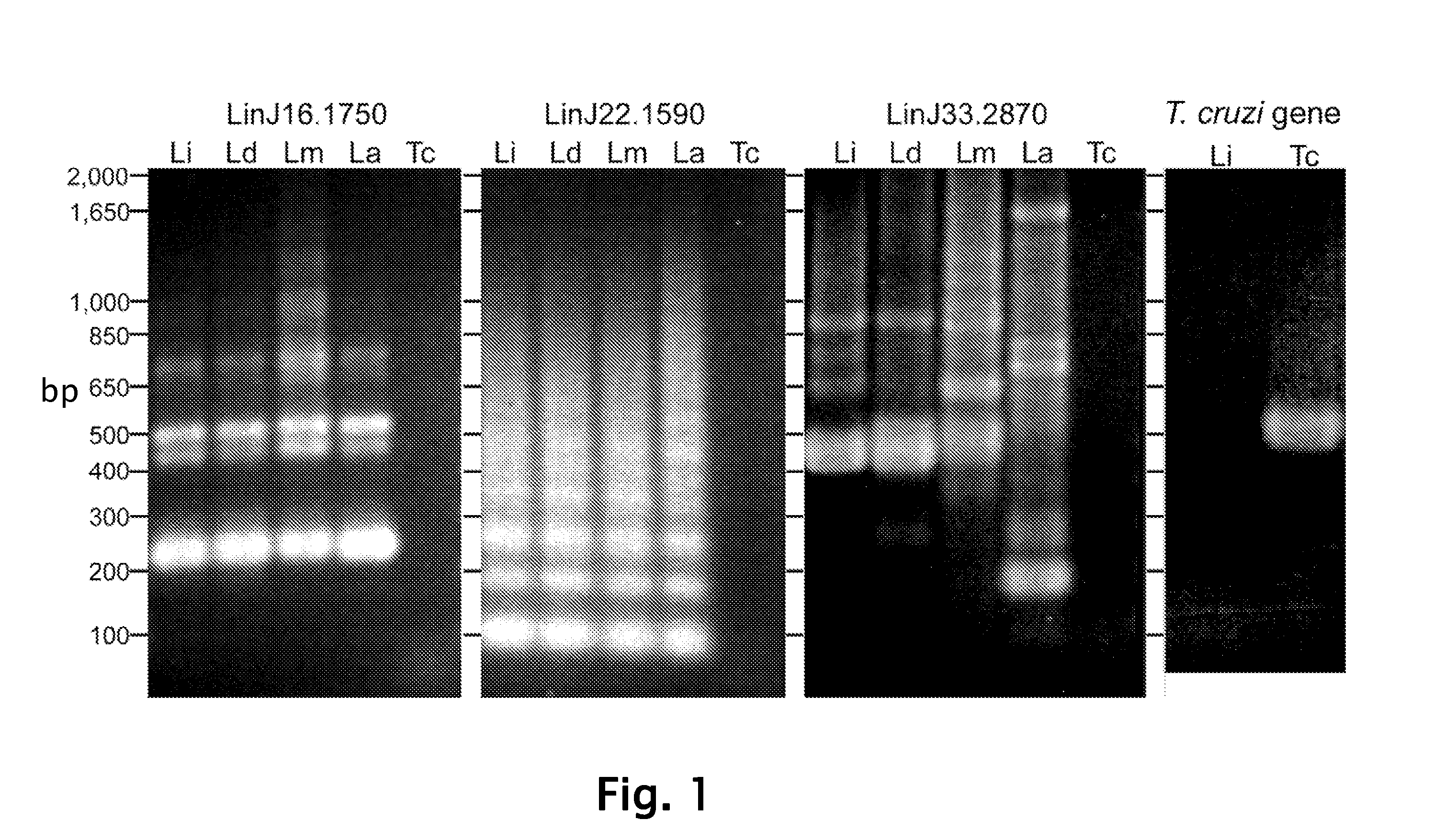
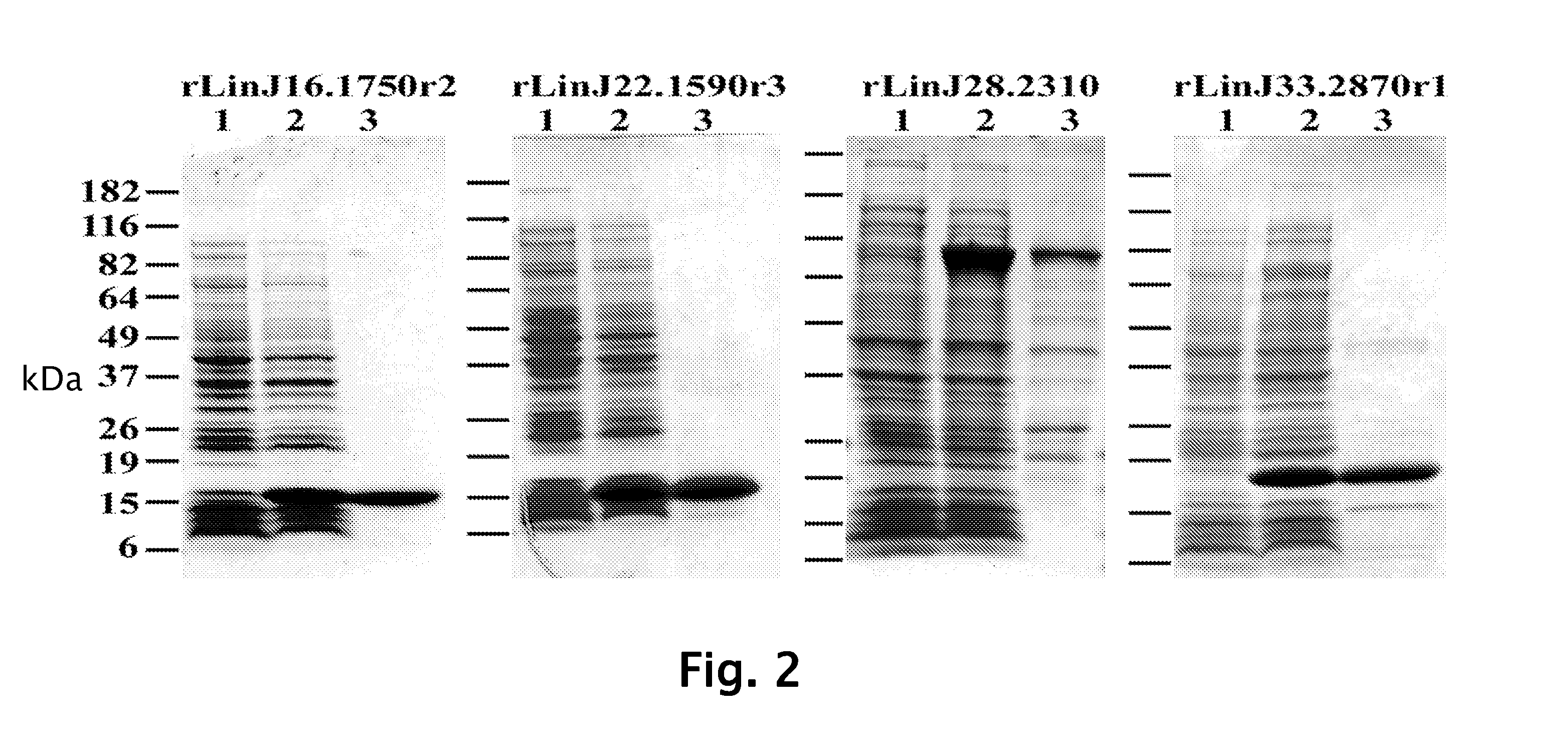
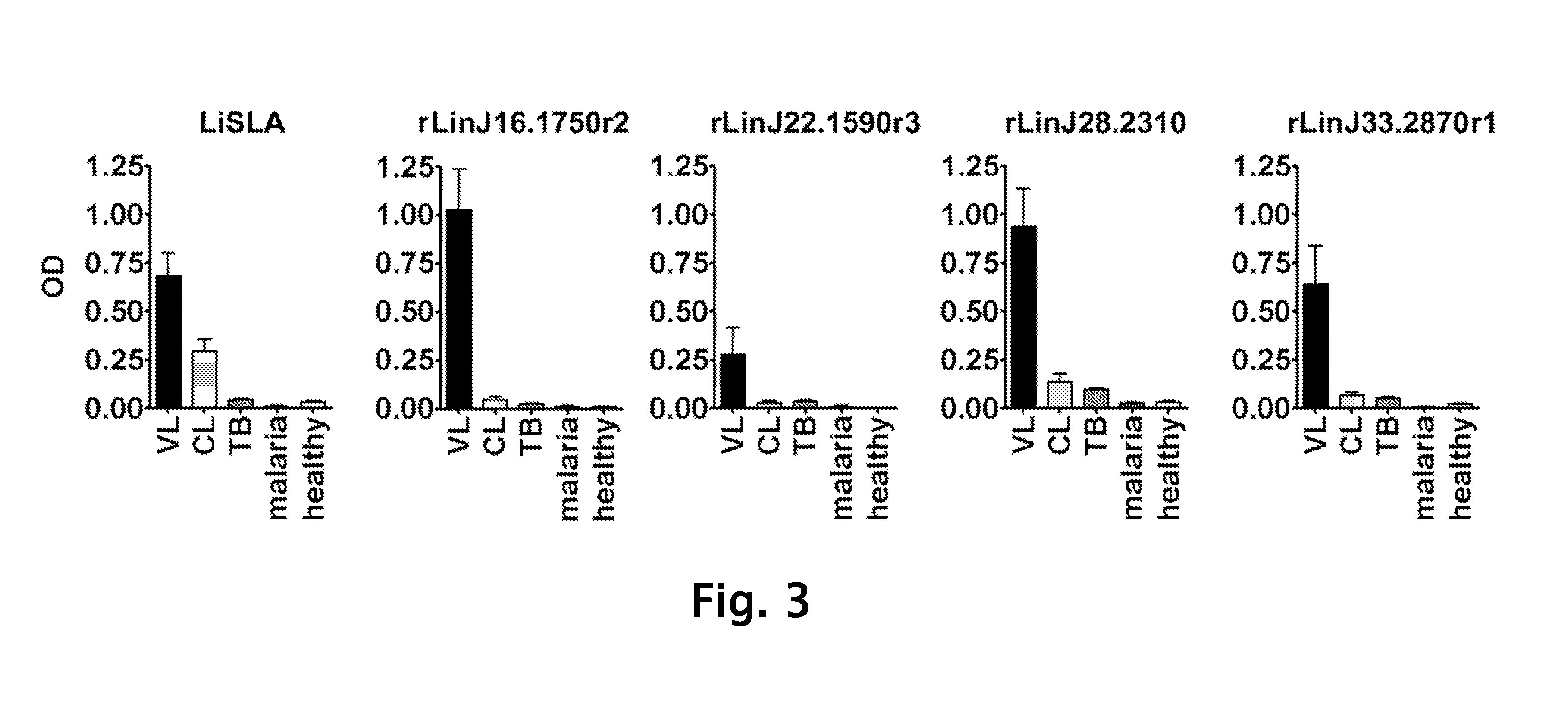
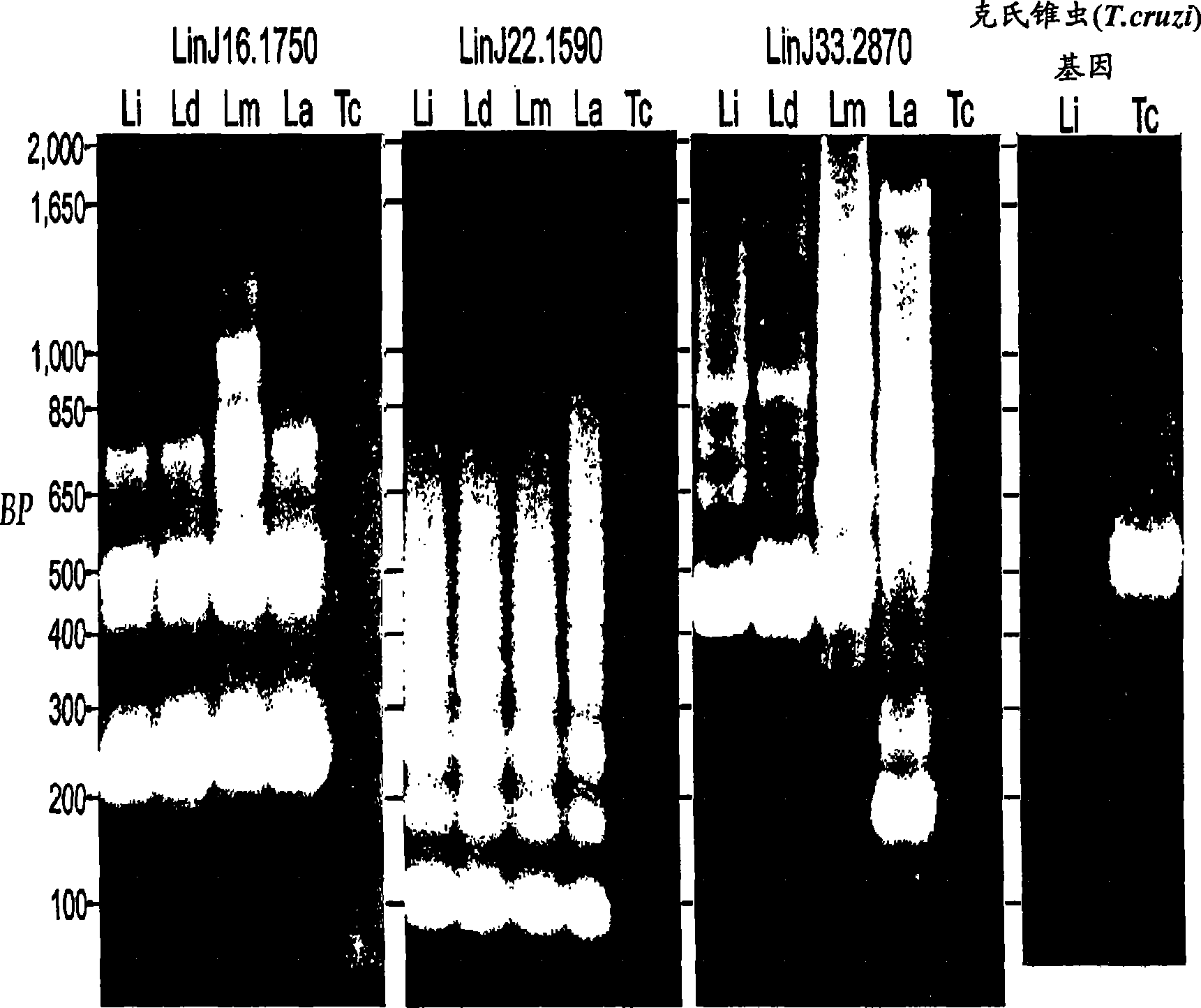
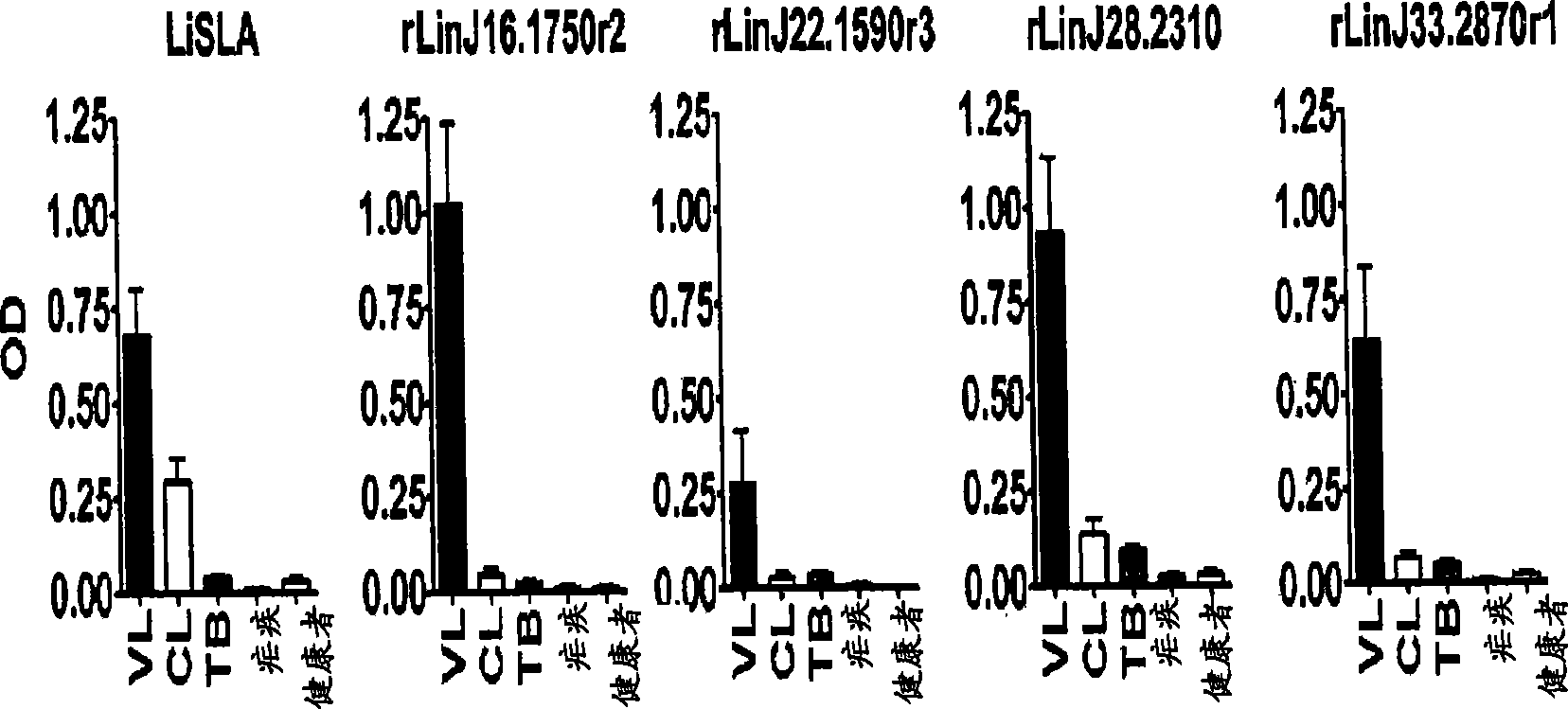
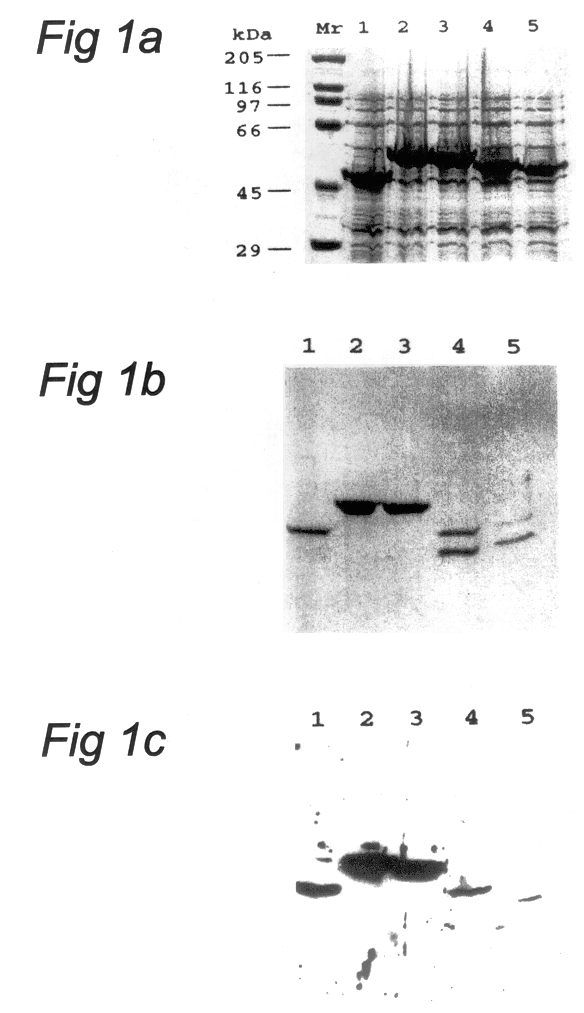
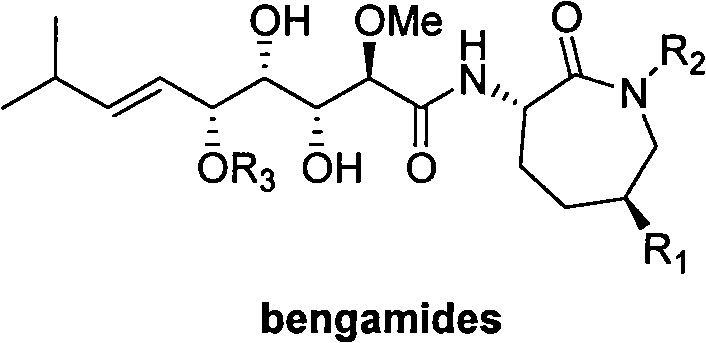
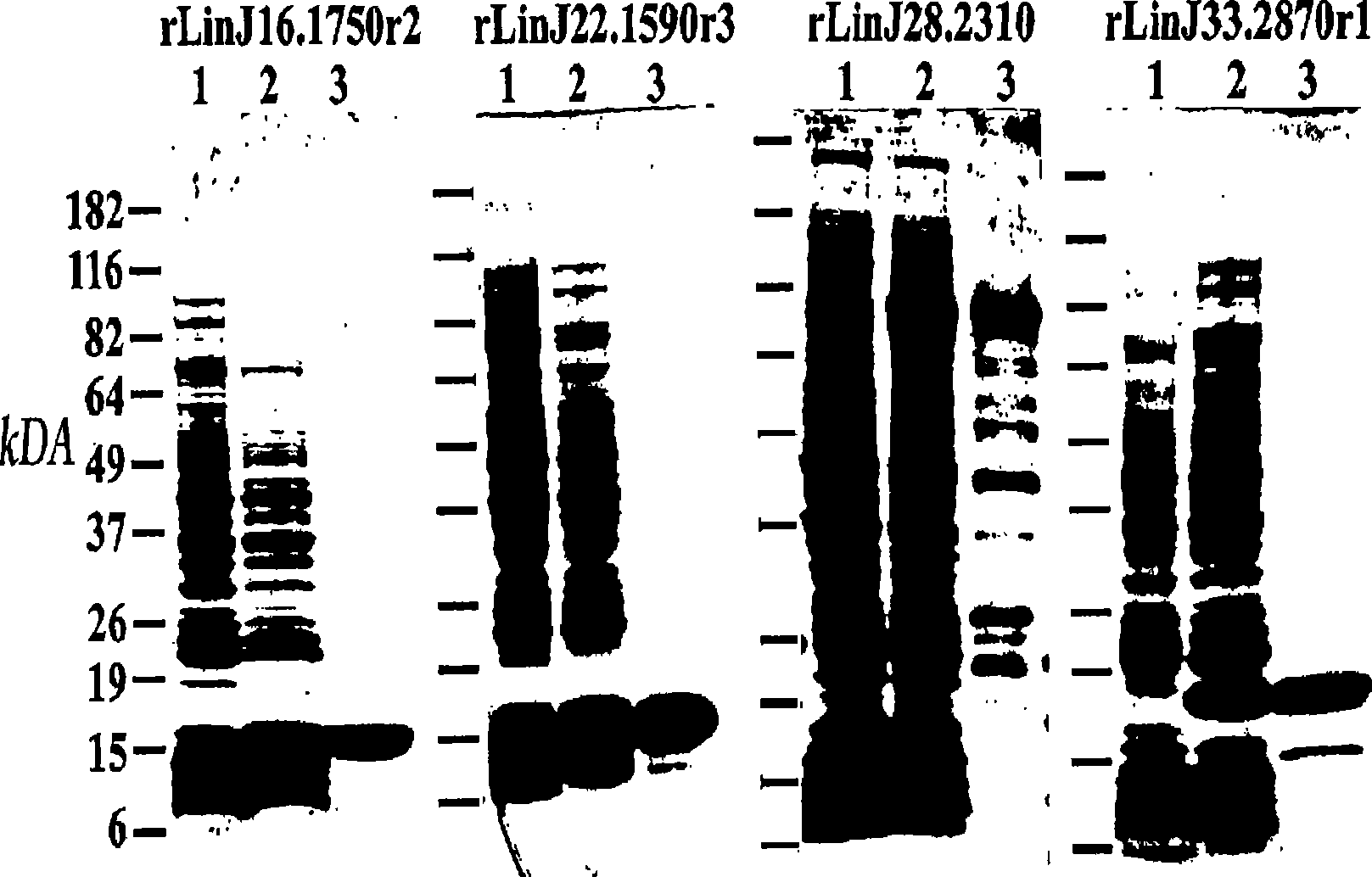Patents
Literature
107 results about "Leishmaniasis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An infection caused by the Leishmania parasite.
Short peptides useful for treatment of ischemia/reperfusion injury and other tissue damage conditions associated with nitric oxide and its reactive species
ActiveUS20080182797A1Avoid tissue damageLevel of protectionNervous disorderTetrapeptide ingredientsPregnancyAllograft rejection
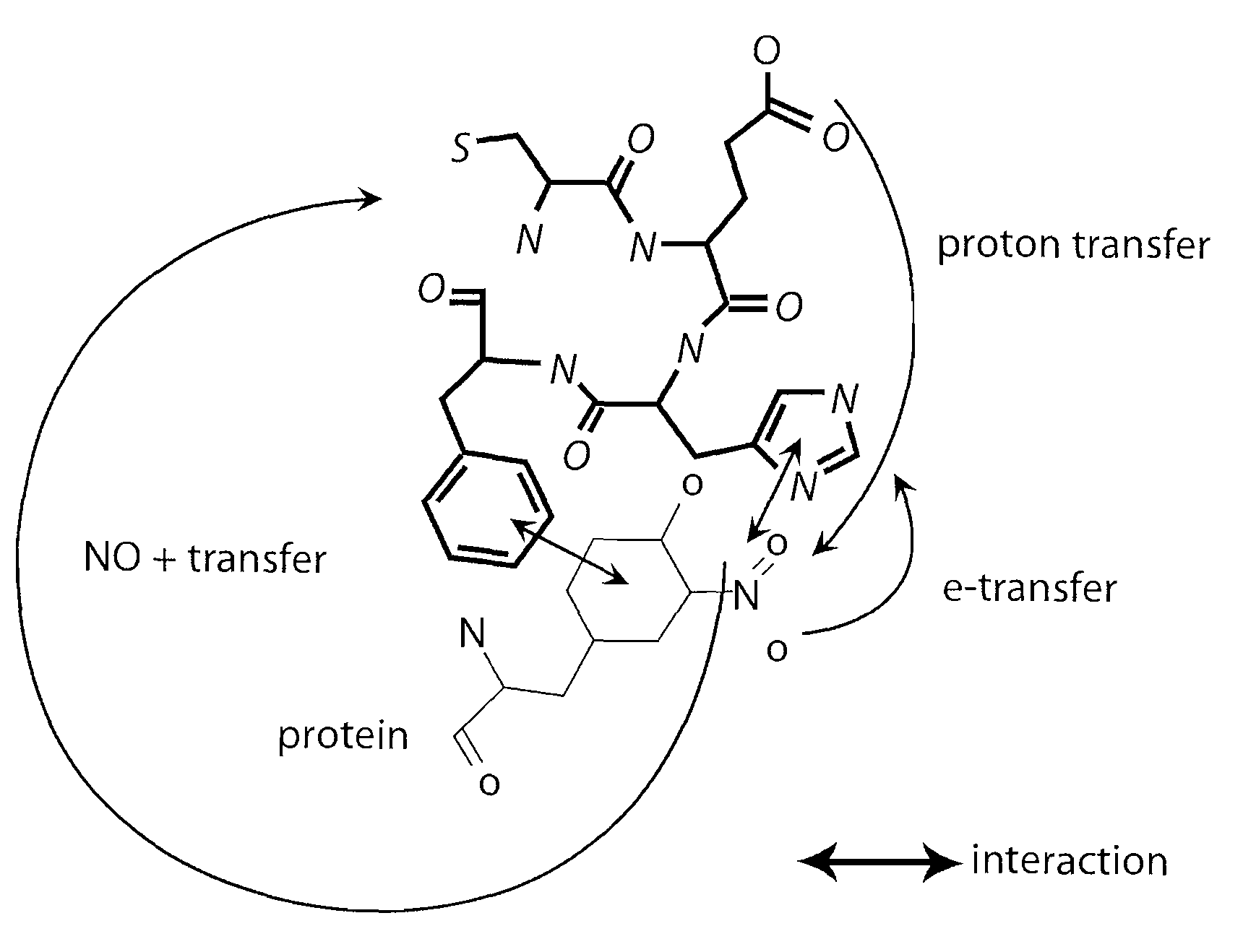
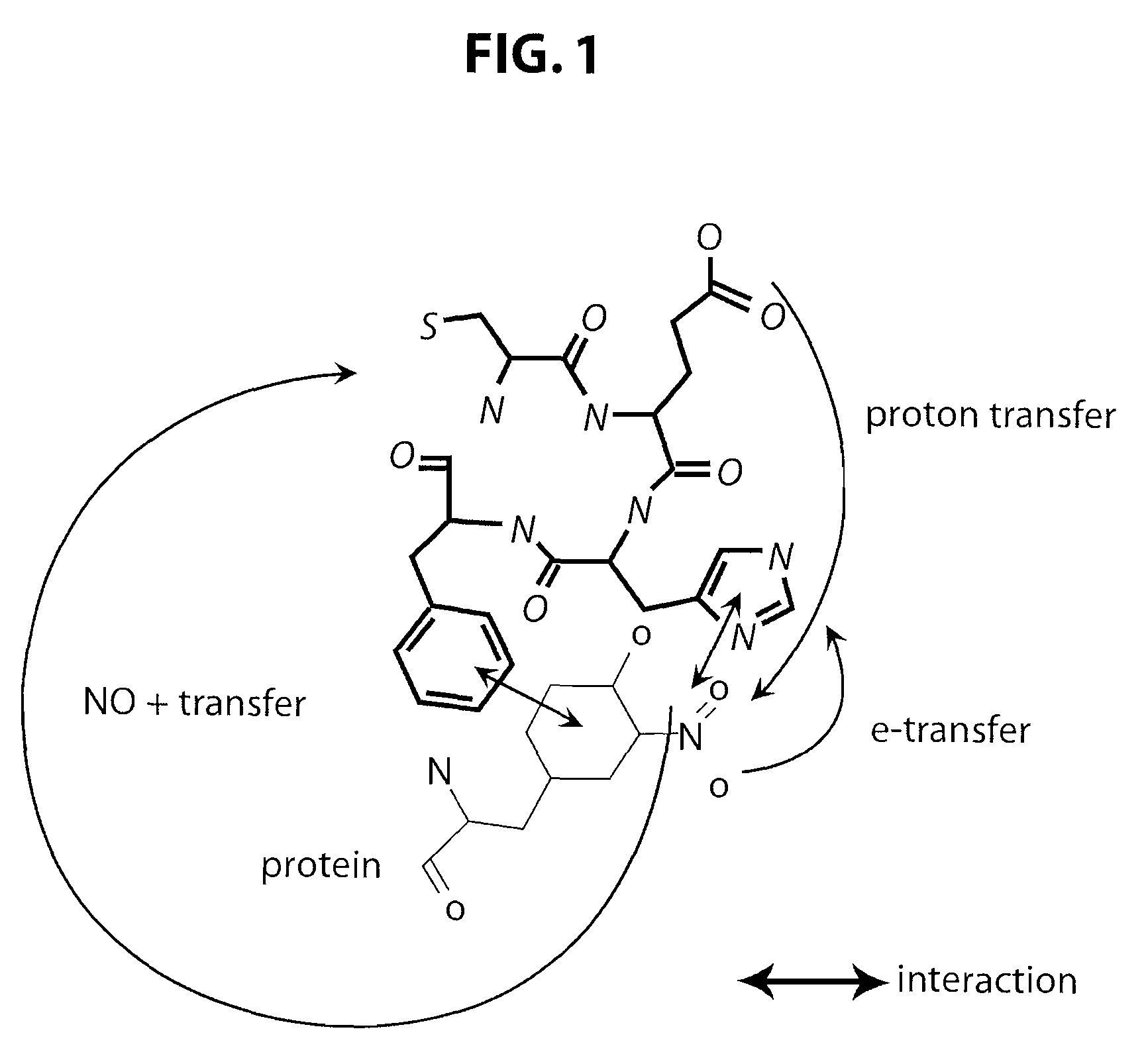
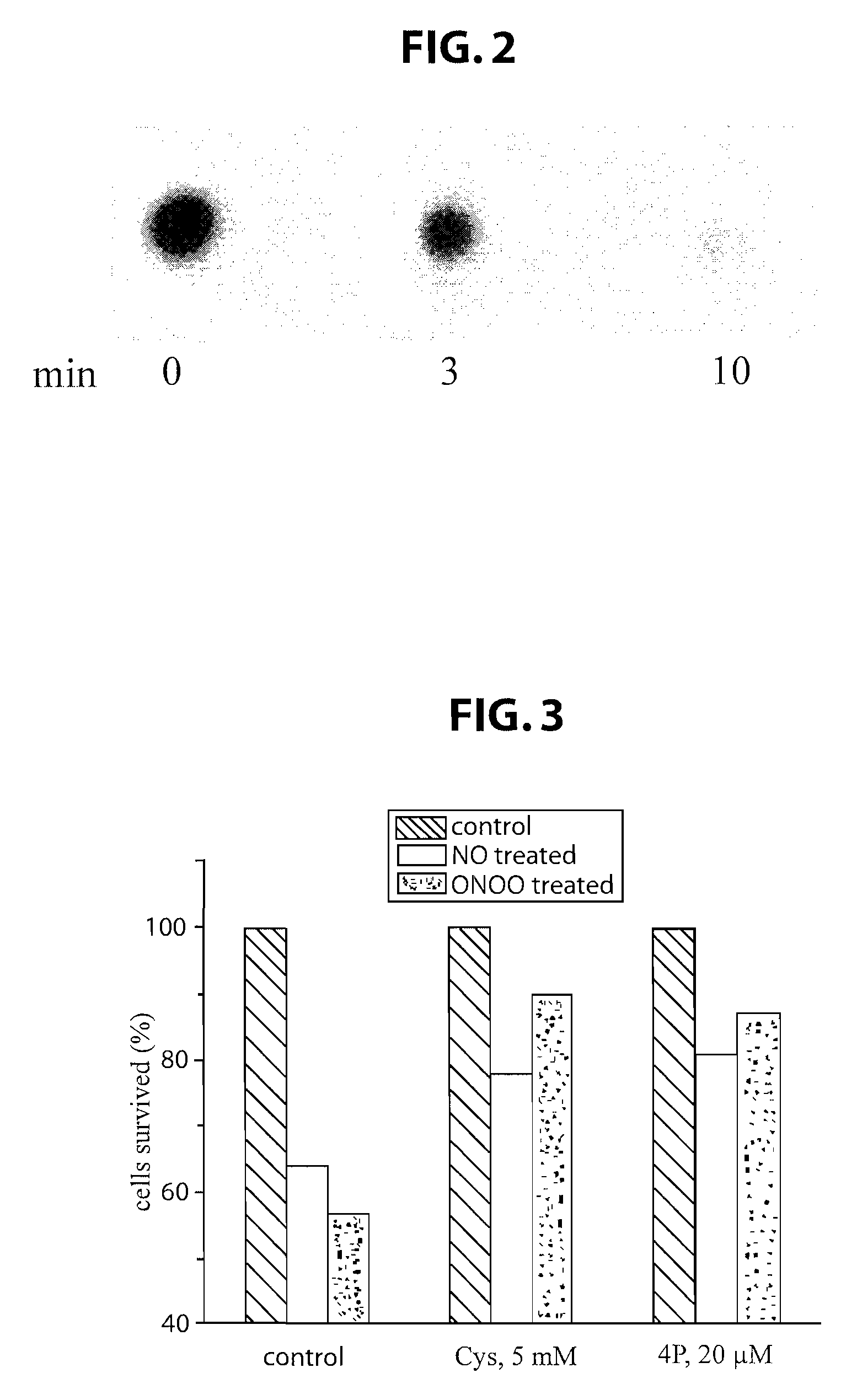
This invention discloses isolated short peptides comprising the amino acid sequence Cys-Glu-Phe-His (CEFH) and analogs thereof as well as compositions comprising CEFH peptides and analogs thereof. The CEFH peptides disclosed herein are effective in mediating the denitration of 3-nitrotyrosines (3-NT) in cellular proteins thereby preventing tissue damage associated with excess nitric oxide (NO) and its reactive species. The CEFH peptides disclosed herein are useful in the treatment of ischemia / reperfusion (I / R) injury of various tissues (e.g., I / R injury of heart muscle associated with heart attack or cardiac surgery, I / R injury of brain tissue associated with stroke, I / R injury of liver tissue, skeletal muscles, etc.), septic shock, anaphylactic shock, neurodegenerative diseases (e.g., Alzheimer's and Parkinson's diseases), neuronal injury, atherosclerosis, diabetes, multiple sclerosis, autoimmune uveitis, pulmonary fibrosis, oobliterative bronchiolitis, bronchopulmonary dysplasia (BPD), amyotrophic lateral sclerosis (ALS), sepsis, inflammatory bowel disease, arthritis, allograft rejection, autoimmune myocarditis, myocardial inflammation, pulmonary granulomatous inflammation, influenza- or HSV-induced pneumonia, chronic cerebral vasospasm, allergic encephalomyelitis, central nervous system (CNS) inflammation, Heliobacterium pylori gastritis, necrotizing entrerocolitis, celliac disease, peritonitis, early prosthesis failure, inclusion body myositis, preeclamptic pregnancies, skin lesions with anaphylactoid purpura, nephrosclerosis, ileitis, leishmaniasis, cancer, and related disorders.
Owner:NEW YORK UNIVERSITY
TH2-specific gene
InactiveUS6190909B1Increase the number of cellsEffective in number of cellOrganic active ingredientsFungiContact dermatitisTransgene
The present invention relates to the discovery, identification and characterization of nucleic acids that encode a novel protein differentially expressed within the TH2 cell subpopulation (hereinafter referred to as STIF). The invention encompasses STIF nucleotides, host cell expression systems, STIF proteins, fusion proteins, polypeptides and peptides, antibodies to the STIF protein, transgenic animals that express a STIF transgene, or recombinant knock-out animals that do not express the STIF protein, and compounds that modulate STIF gene expression or STIF activity that can be used for diagnosis, drug screening, clinical trial monitoring, and / or used to treat STIF based disorders, such as proliferative disorders and T-lymphocyte-related disorders including, but not limited to, chronic inflammatory diseases and disorders, such as Crohn's disease, reactive arthritis, including Lyme disease, insulin-dependent diabetes, organ-specific autoimmunity, including multiple sclerosis, Hashimoto's thyroiditis and Grave's disease, contact dermatitis, psoriasis, graft rejection, graft versus host disease, sarcoidosis, atopic conditions, such as asthma and allergy, including allergic rhinitis, gastrointestinal allergies, including food allergies, eosinophilia, conjunctivitis, glomerular nephritis, certain pathogen susceptibilities such as helminthic (e.g., leishmaniasis) and certain viral infections, including HIV, and bacterial infections, including tuberculosis and lepromatous leprosy.
Owner:MILLENNIUM PHARMA INC
Recombinant polyprotein vaccines for the treatment and diagnosis of leishmaniasis
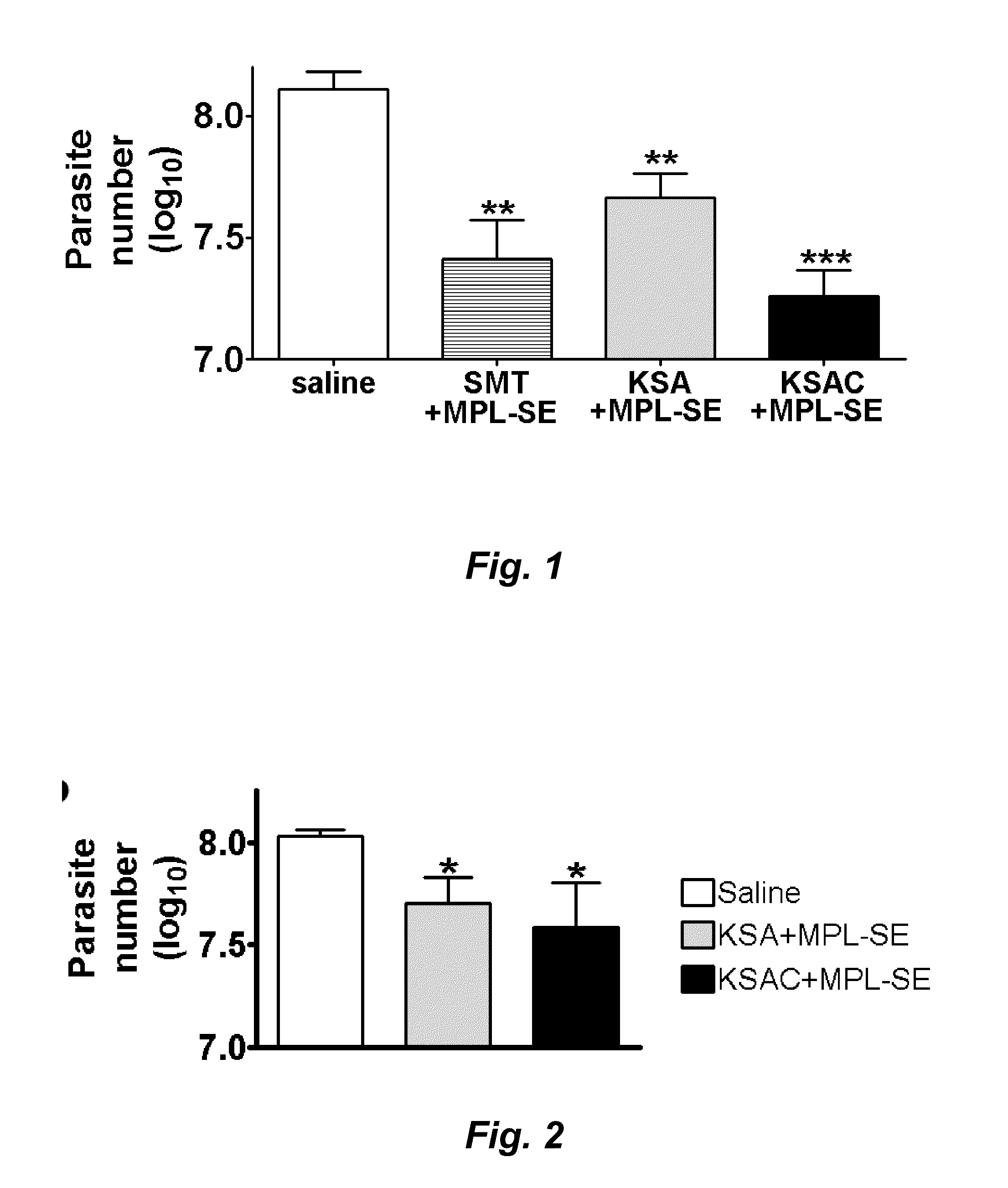
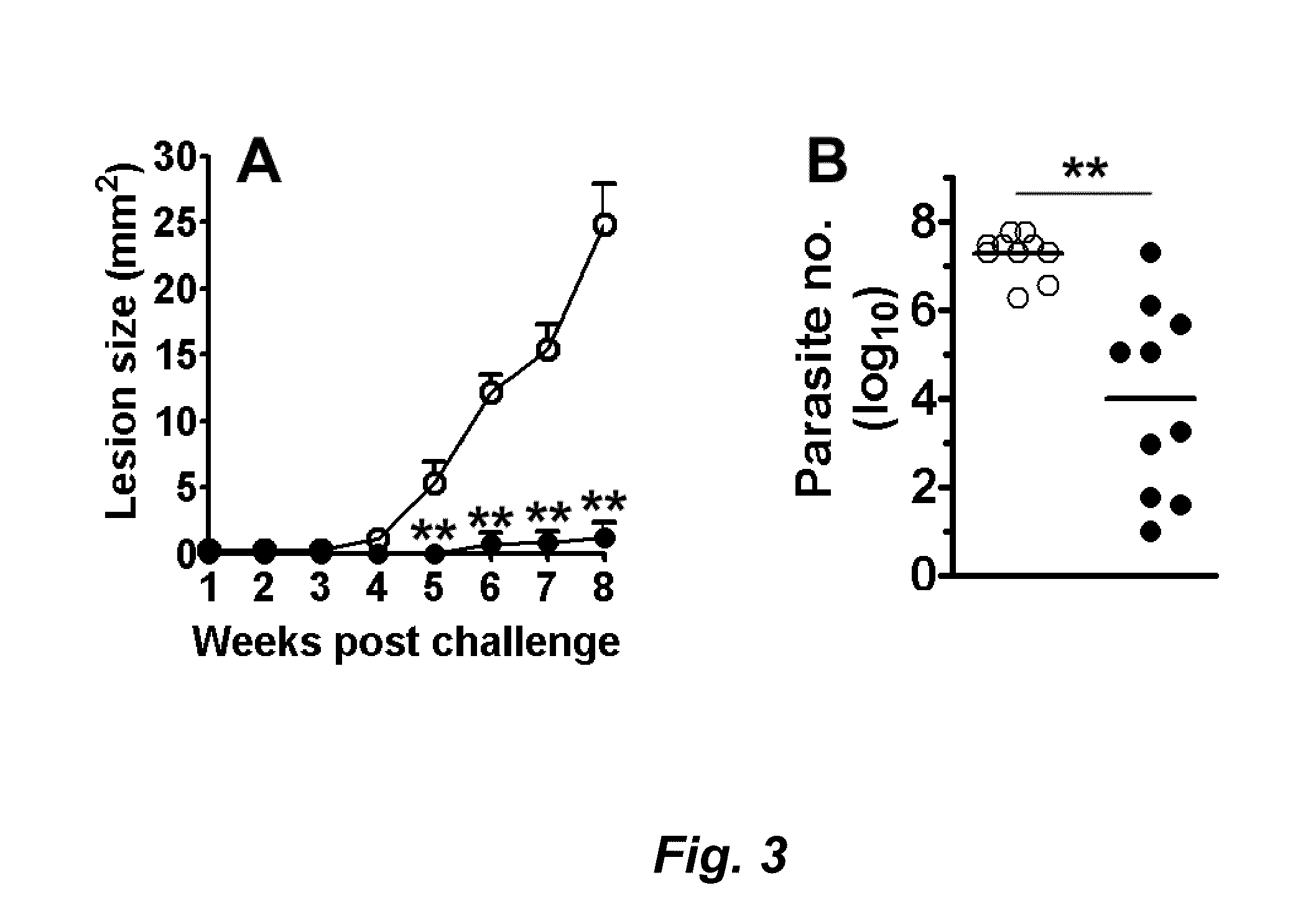
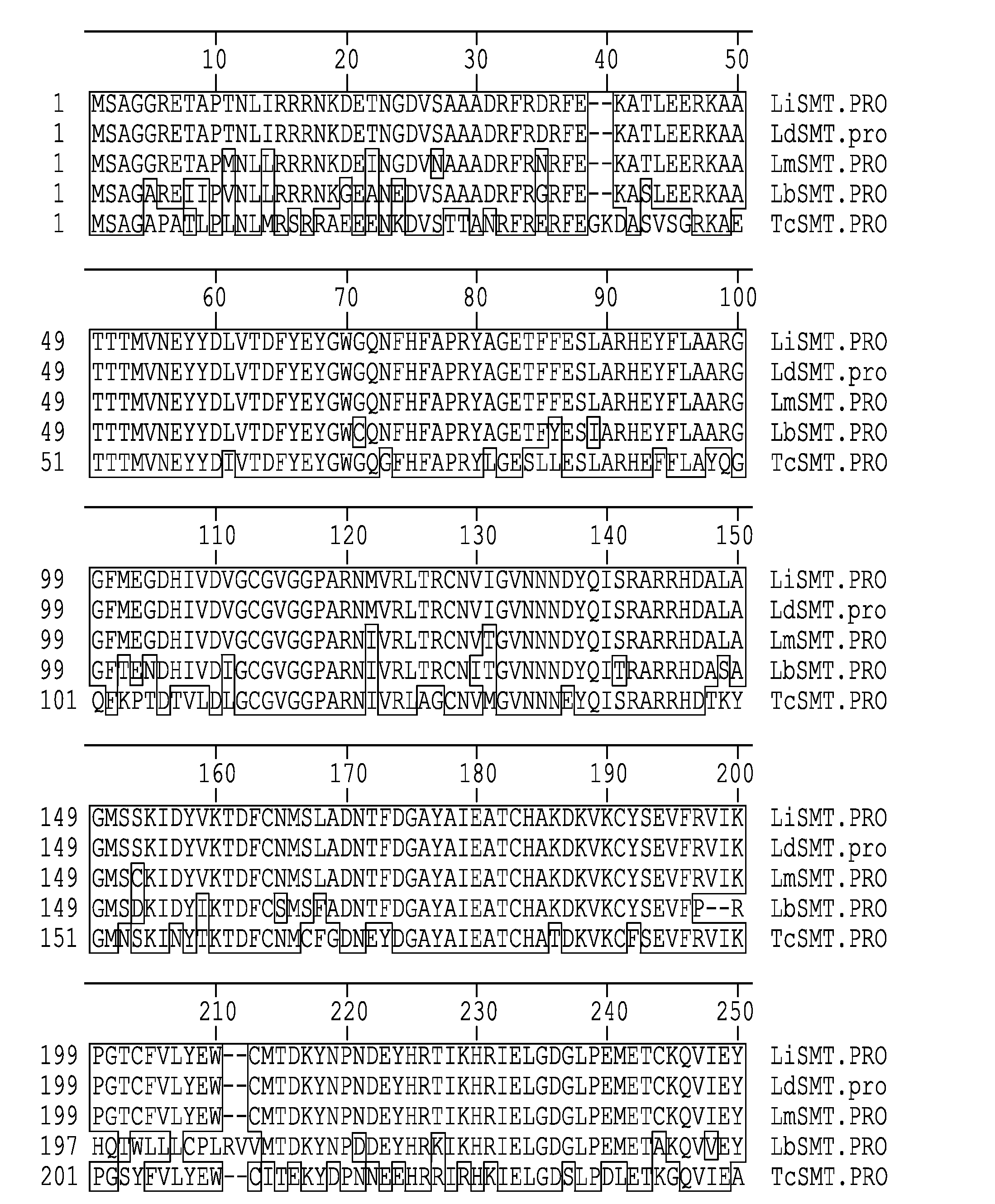
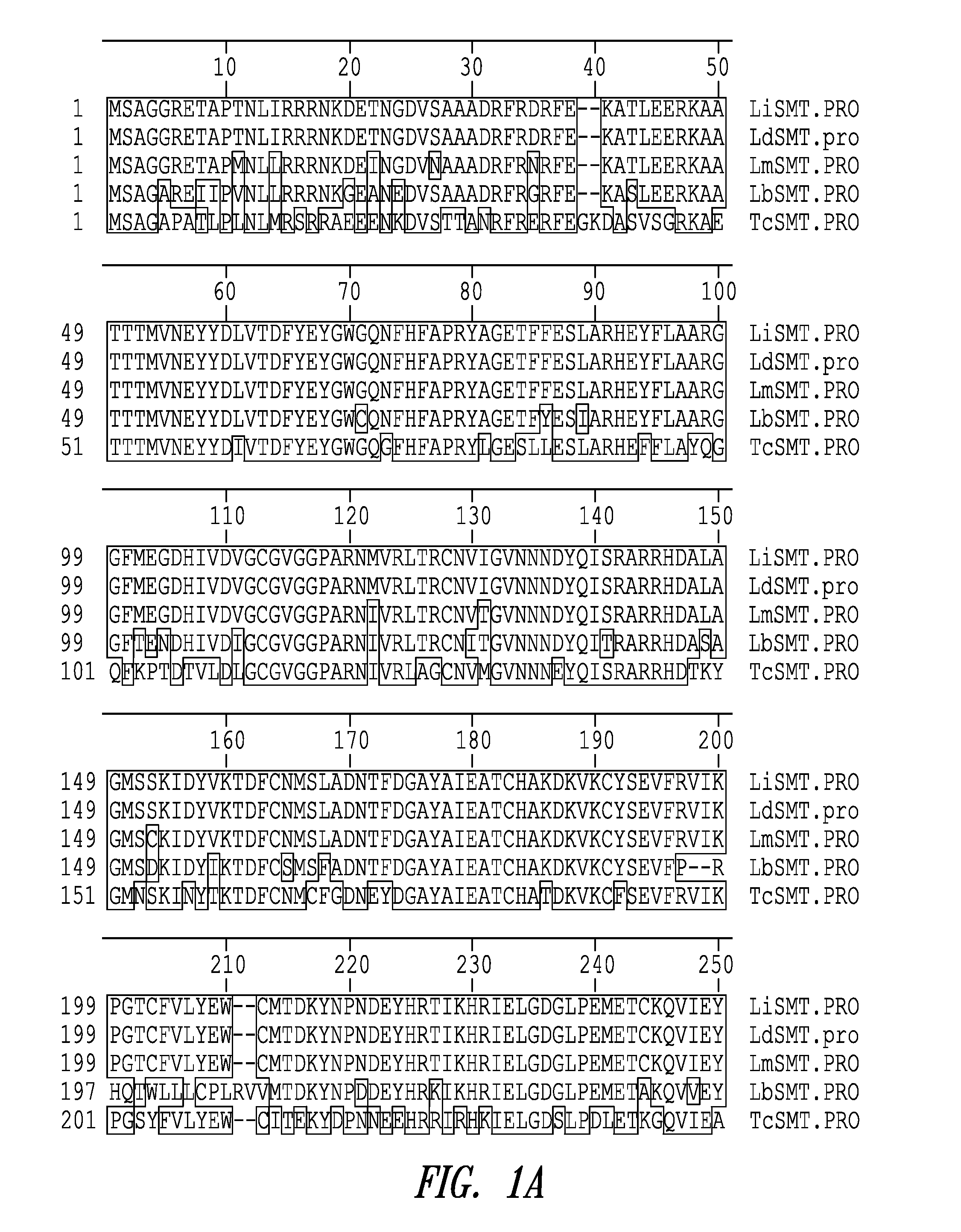
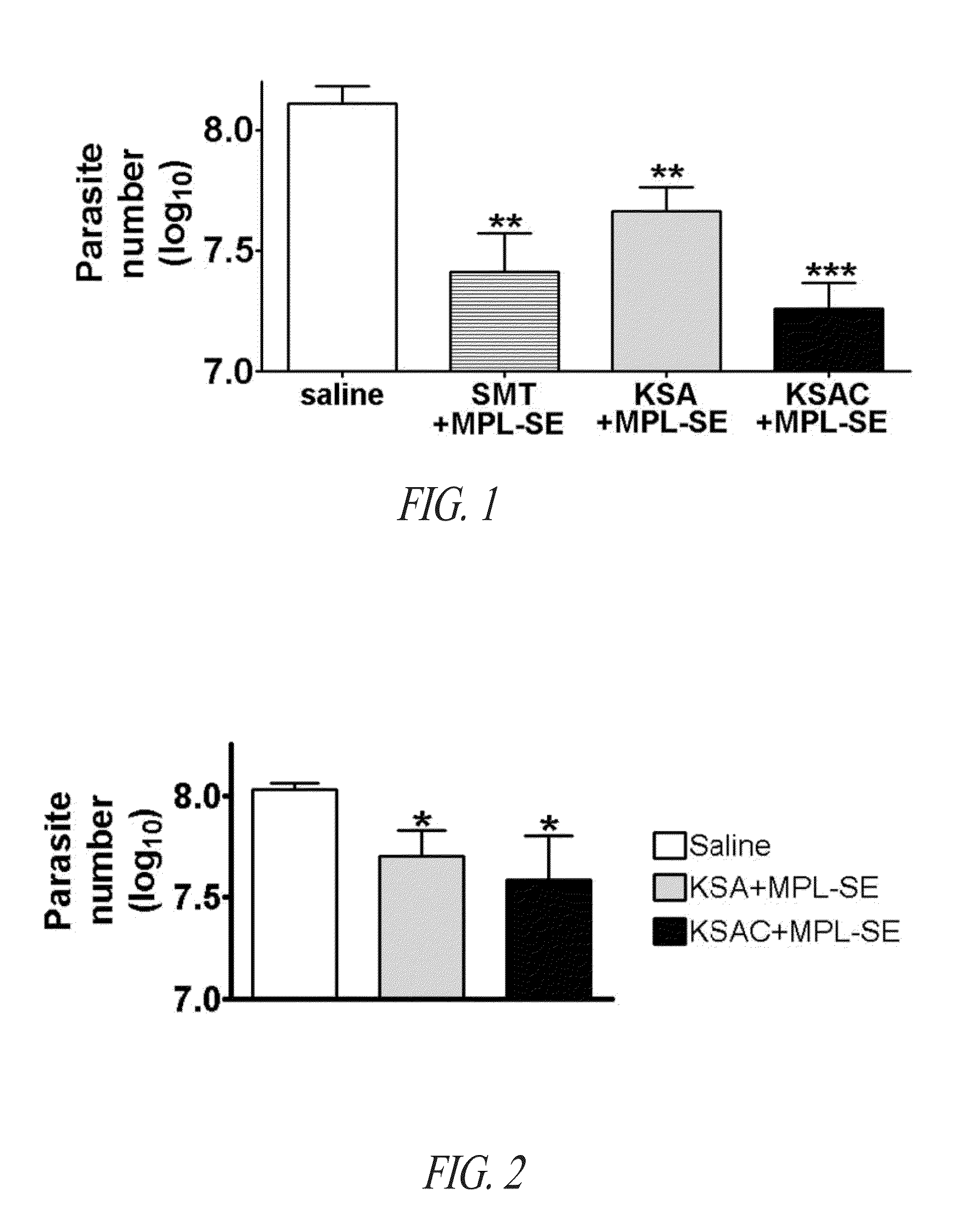
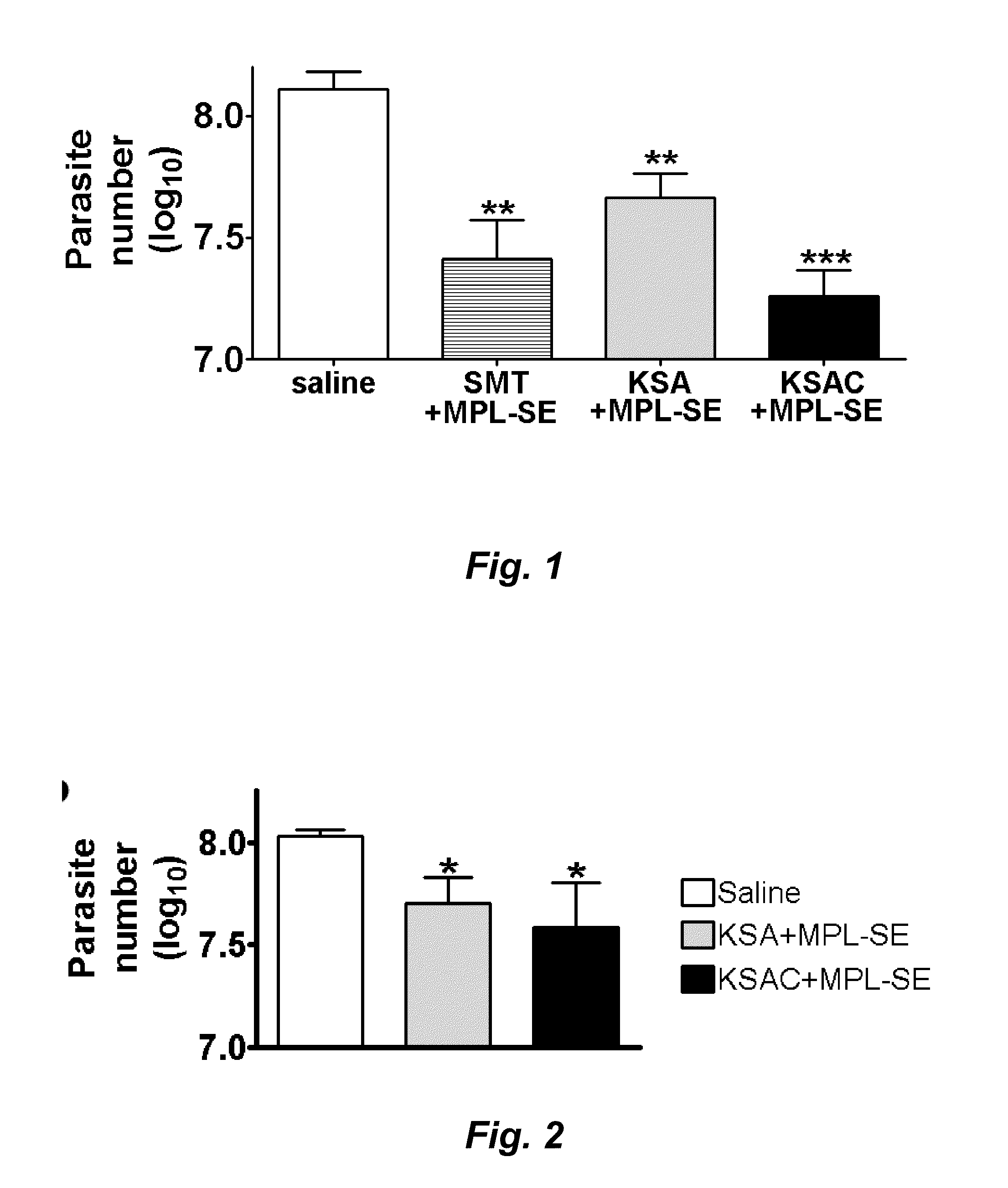
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising multiple Leishmania antigens, in particular, KMP11, SMT, A2 and / or CBP, or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:ACCESS TO ADVANCED HEALTH INST
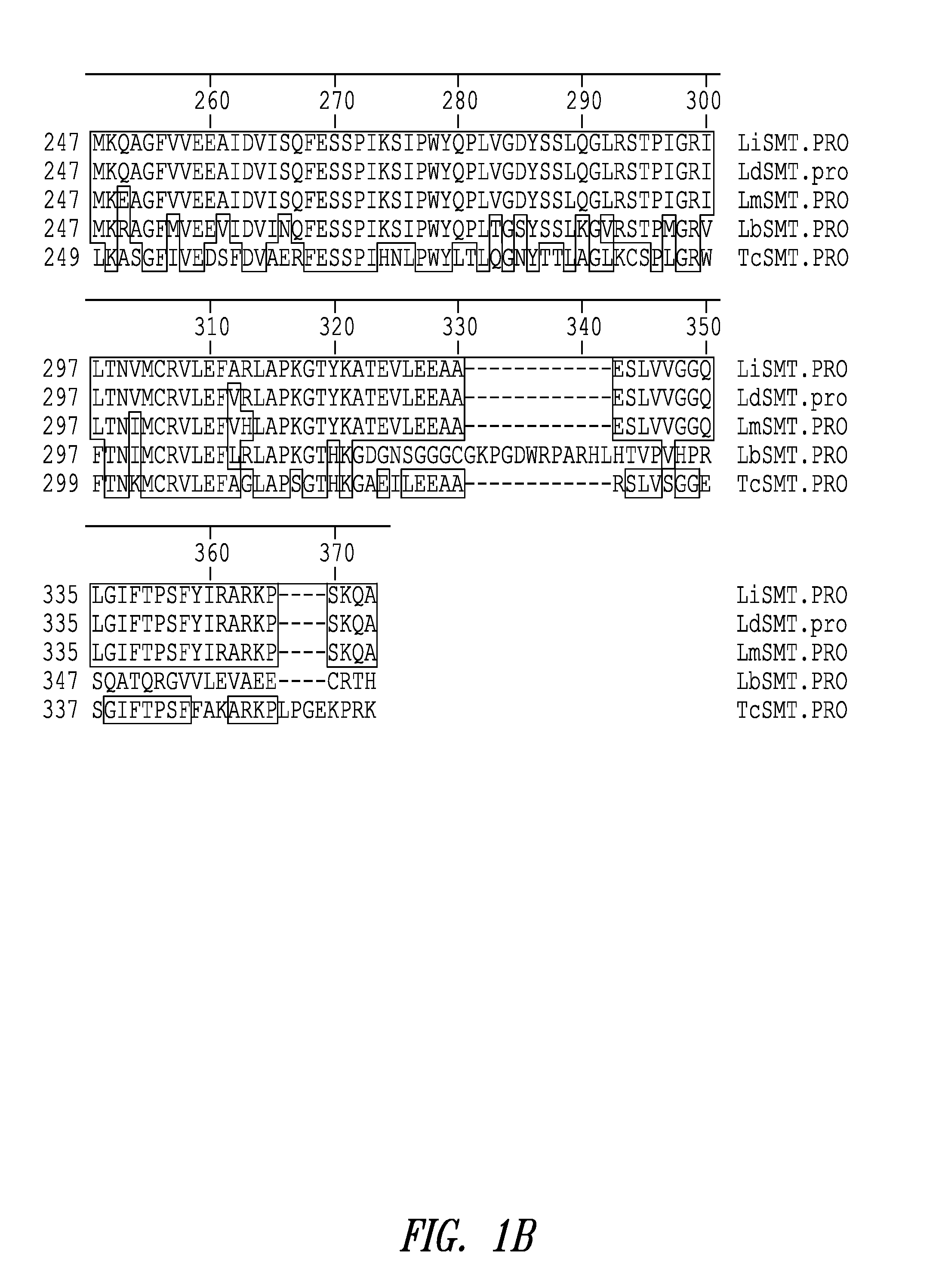
Leishmania sterol 24-c-methyltransferase compositions for the prevention, treatment and diagnosis of leishmaniasis
ActiveUS20090041798A1Preventing and treating and detecting leishmaniasisProtozoa antigen ingredientsPeptide/protein ingredientsLeishmaniasisSterol
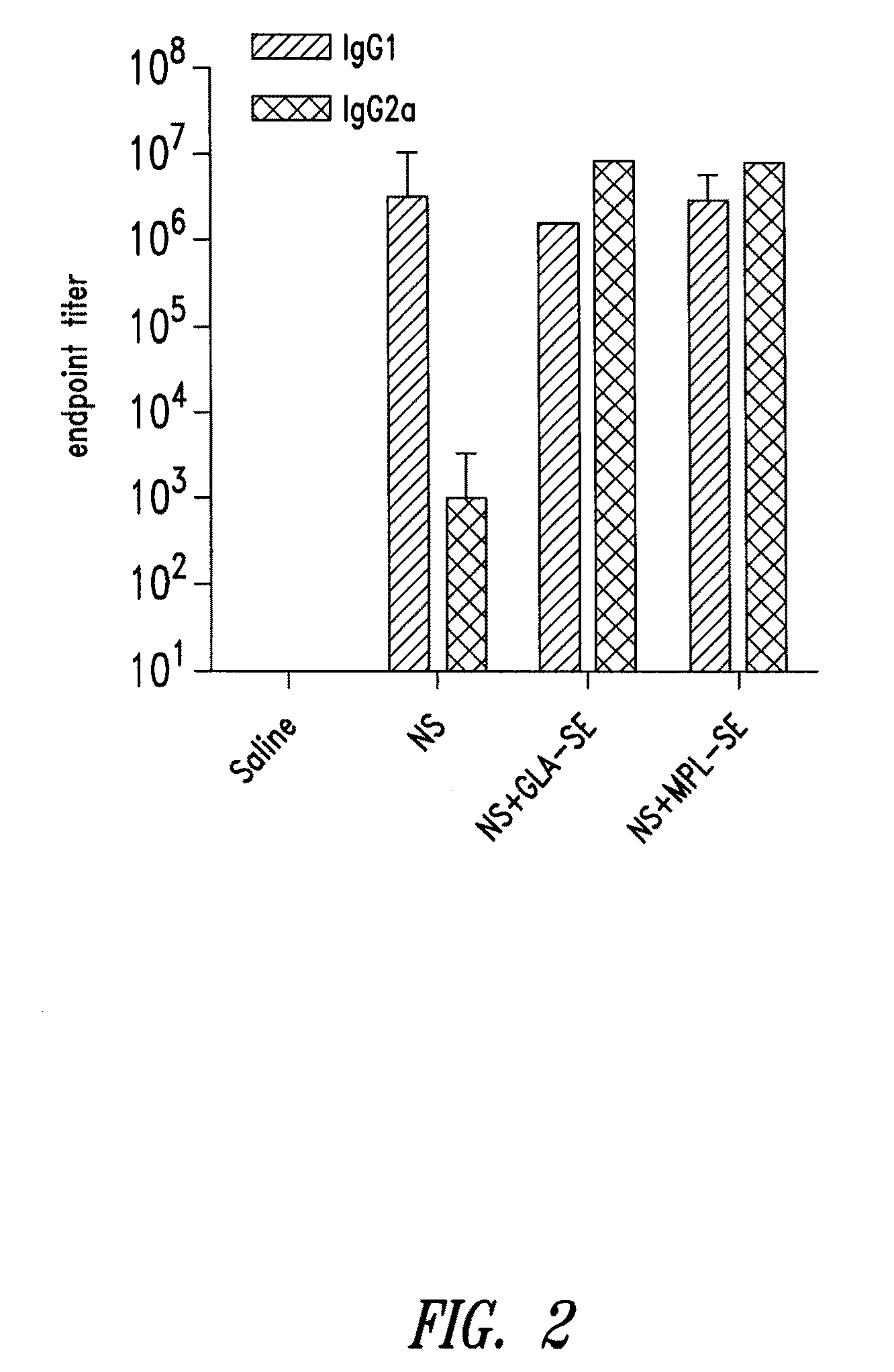
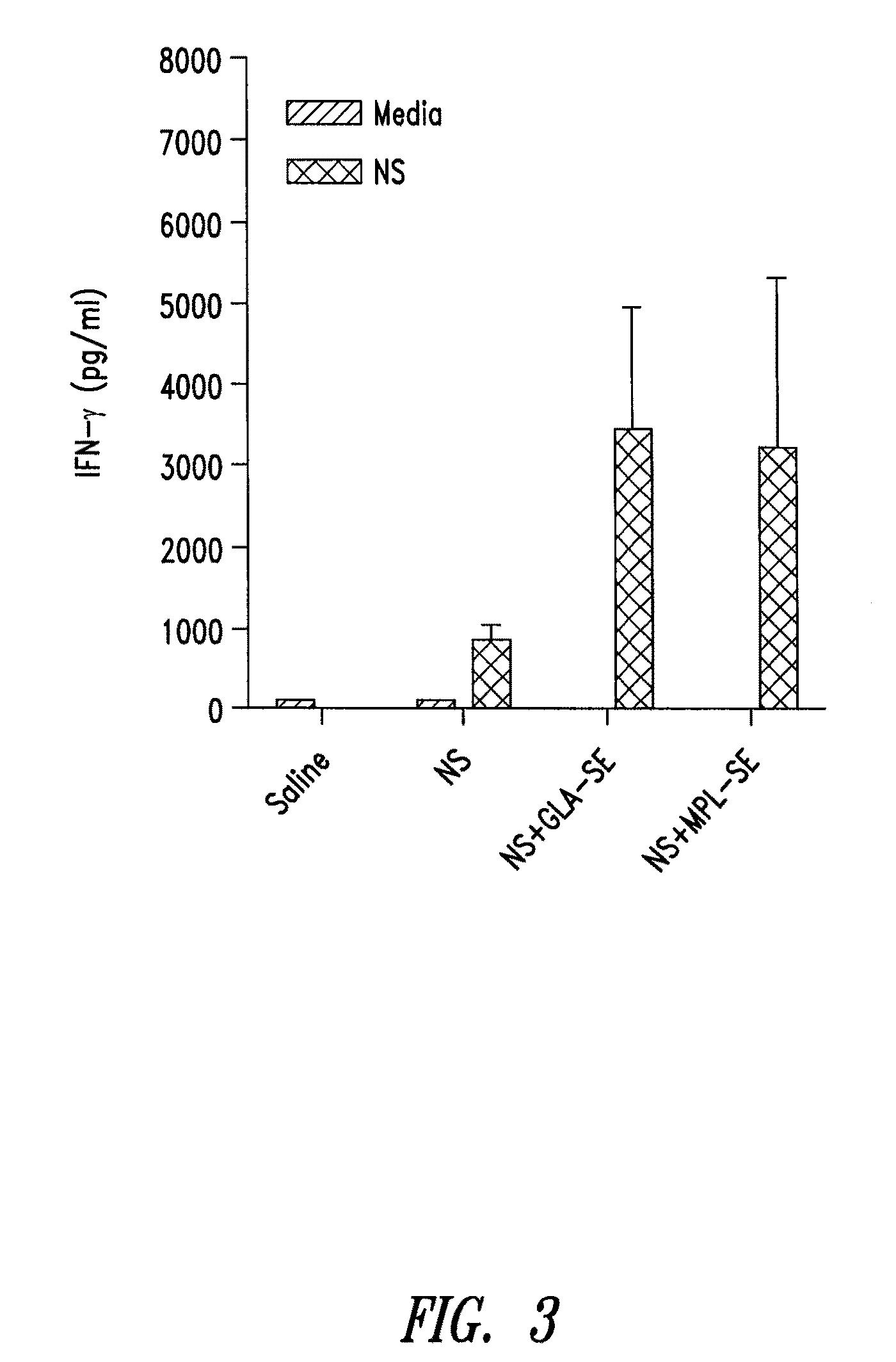
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise Leishmania sterol 24-c-methyltransferase (SMT) polypeptides, portions, variants and / or fusions, as well as polynucleotides encoding SMT polypeptides, portions, variants and / or fusions.
Owner:INFECTIOUS DISEASE RES INST
Anti-parasitic methods and compositions utilizing diindolylmethane-related indoles
ActiveUS20100055201A1Improve developmentShorten the counting processHeavy metal active ingredientsBiocideCoccidiosisTrypanosomiasis
The present invention includes methods and compositions for the treatment and prevention of protozoal parasitic infections utilizing Diindolylmethane-related indoles. Additive and synergistic interaction of Diindolylmethane-related indoles with other known anti-parasitic and pro-apoptotic agents is believed to permit more effective therapy and prevention of protozoal parasitic infections. The methods and compositions described provide new treatment of protozoal parasitic diseases of mammals and birds including malaria, leishmaniasis, trypanosomiasis, trichomoniasis, neosporosis and coccidiosis.
Owner:BIORESPONSE
Vaccines comprising non-specific nucleoside hydrolase and sterol 24-c-methyltransferase (SMT) polypeptides for the treatment and diagnosis of leishmaniasis
InactiveUS20120114688A1Preventing and treating and detecting leishmaniasisAntibody mimetics/scaffoldsTransferasesLeishmaniaLeishmaniasis
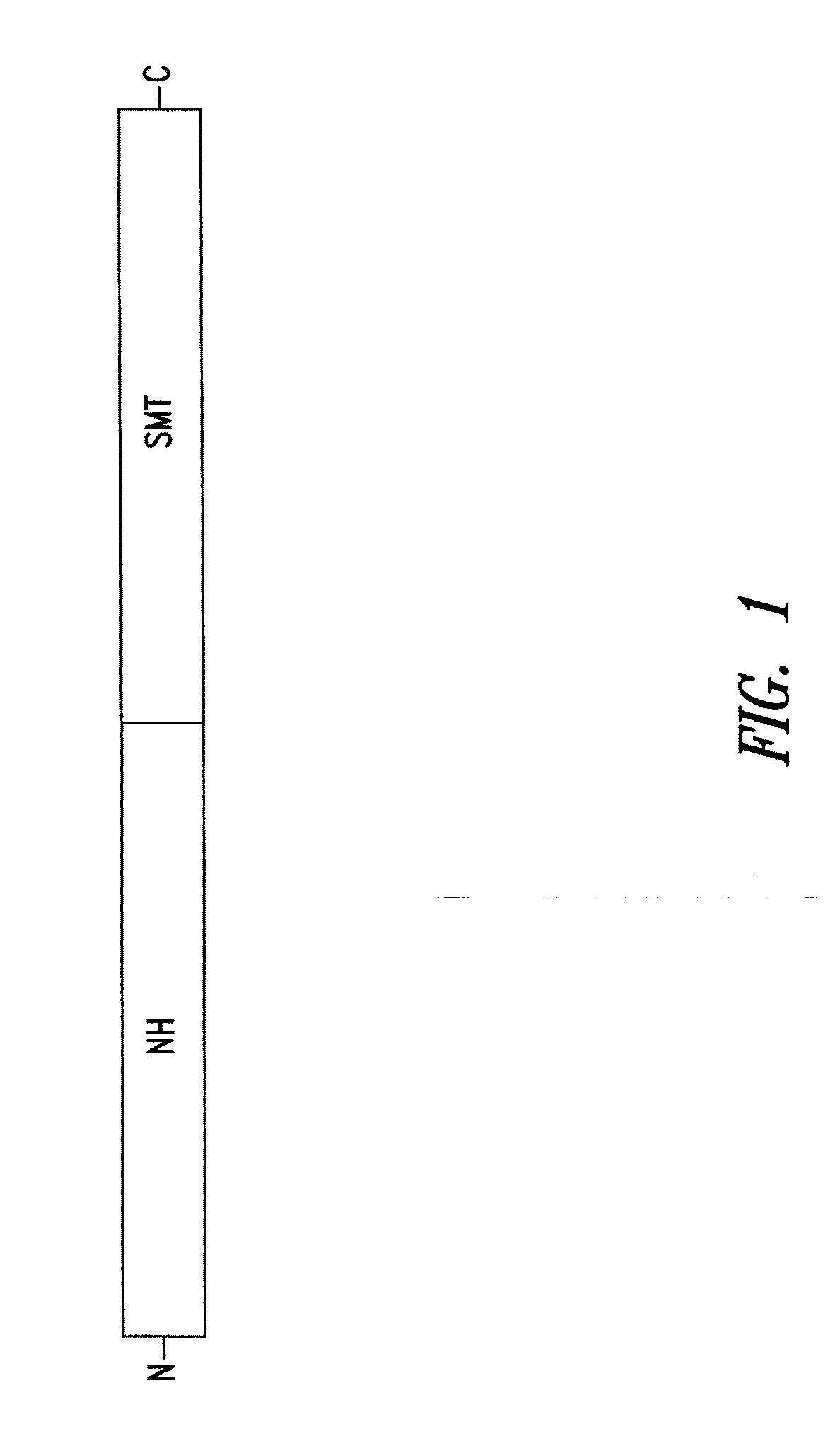
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising Leishmania antigens, in particular, SMT and NH antigens or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:INFECTIOUS DISEASE RES INST
Recombinant polyprotein vaccines for the treatment and diagnosis of leishmaniasis
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising multiple Leishmania antigens, in particular, KMP11, SMT, A2 and / or CBP, or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:ACCESS TO ADVANCED HEALTH INST
Betulin derived compounds useful as antiprotozoal agents
InactiveUS20100190795A1Improve solubilityLow toxicityAntibacterial agentsBiocideProtozoaLeishmaniasis
The invention relates to betulin derivatives, and to the use thereof as agents against protozoa of the genus Leishmania and against leishmaniasis in applications of pharmaceutical industry.
Owner:VALTION TEKNILLINEN TUTKIMUSKESKUS
Vaccine complex for preventing or treating leishmaniases
InactiveUS20060073170A1Facilitates emergenceInhibition of differentiationProtozoa antigen ingredientsAntiparasitic agentsSerum free mediaMammal
A therapeutic vaccine complex for preventing or treating leishmaniases and infections mediated by intracellular pathogenic micro-organism in mammals and in particular in humans, members of the dog, car and horse family. The invention is characterized in that it includes excretion secretion molecules derived from Leishmania sp. Promastigotes produced in a specific germ-free and serum-free medium.
Owner:BIO VETO TESTS BVT
Therapeutic film forming composition and treatment system therefor
By providing a film forming composition incorporating one or more therapeutic substances for application to nails and / or skin surfaces which can be employed independently or, if desired, in combination with an easily employed holding or support member for delivering heat directly to the application site an easily employed, convenient, consumer-oriented treatment system is achieved for treating nails and / or skin surfaces for a wide variety of medical problems. The treatment system of the present invention possesses broad applicability for a wide range of medical conditions, including the numerous diseases, disorders, and medical problems, all of which are capable of being treated using the present invention. In particular, diseases, disorders, and medical conditions which include, but are not limited to, psoriasis, skin cancers, warts, leishmaniasis, mycobacteria, and granuloma annulare can be specifically treated or improved due to the efficacy of the present invention and, when employed, the efficacy of heat penetration in treating these disorders.
Owner:DVORETZKY ISRAEL +1
Compounds and Methods for Diagnosis and Treatment of Leishmaniasis
Compounds and methods are provided for diagnosing, preventing, treating and detecting leishmaniasis infection and stimulating immune responses in patients are disclosed. The compounds disclosed are include polypeptides and fusion proteins that contain at least one immunogenic portion of one or more Leishmania antigens, or a variant thereof. Additionally, methods of screening a screening library for tandem repeat proteins that have immunogenic properties are disclosed. Vaccines and pharmaceutical compositions comprising polynucleotides, polypeptides, fusion proteins and variants thereof that may be used for the prevention and therapy of leishmaniasis, as well as for the detection of Leishmaniasis infection are described.
Owner:INFECTIOUS DISEASE RES INST
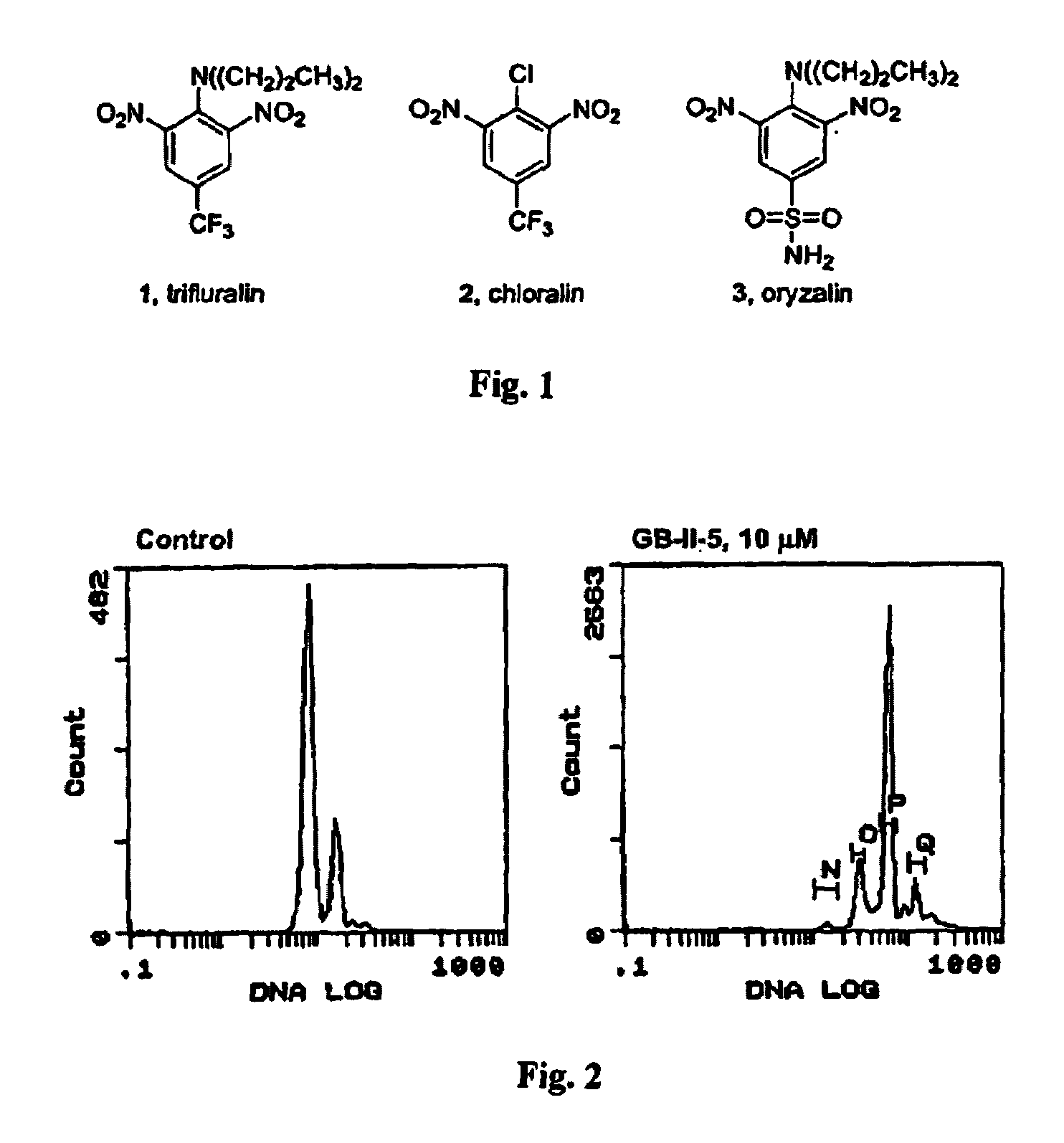
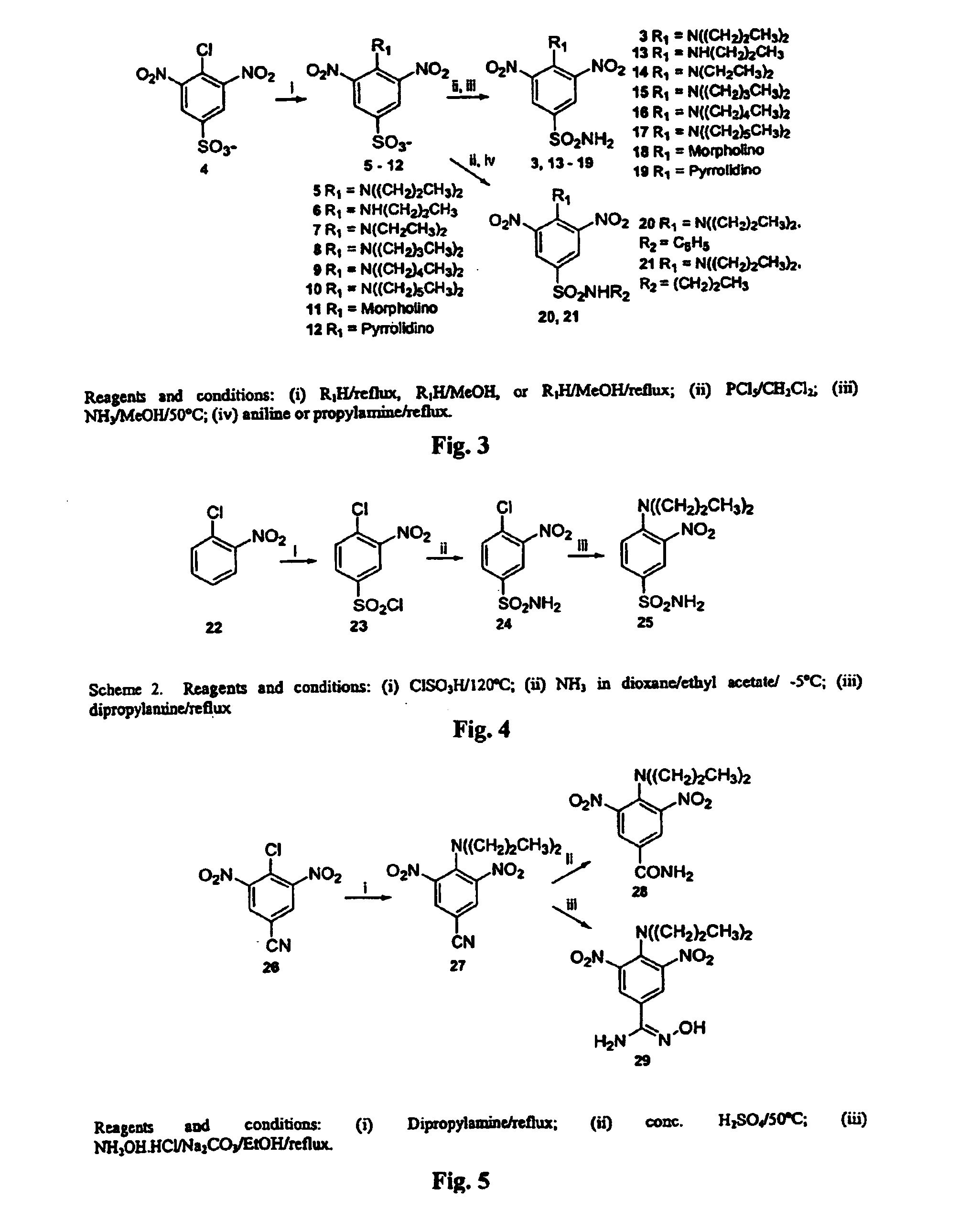
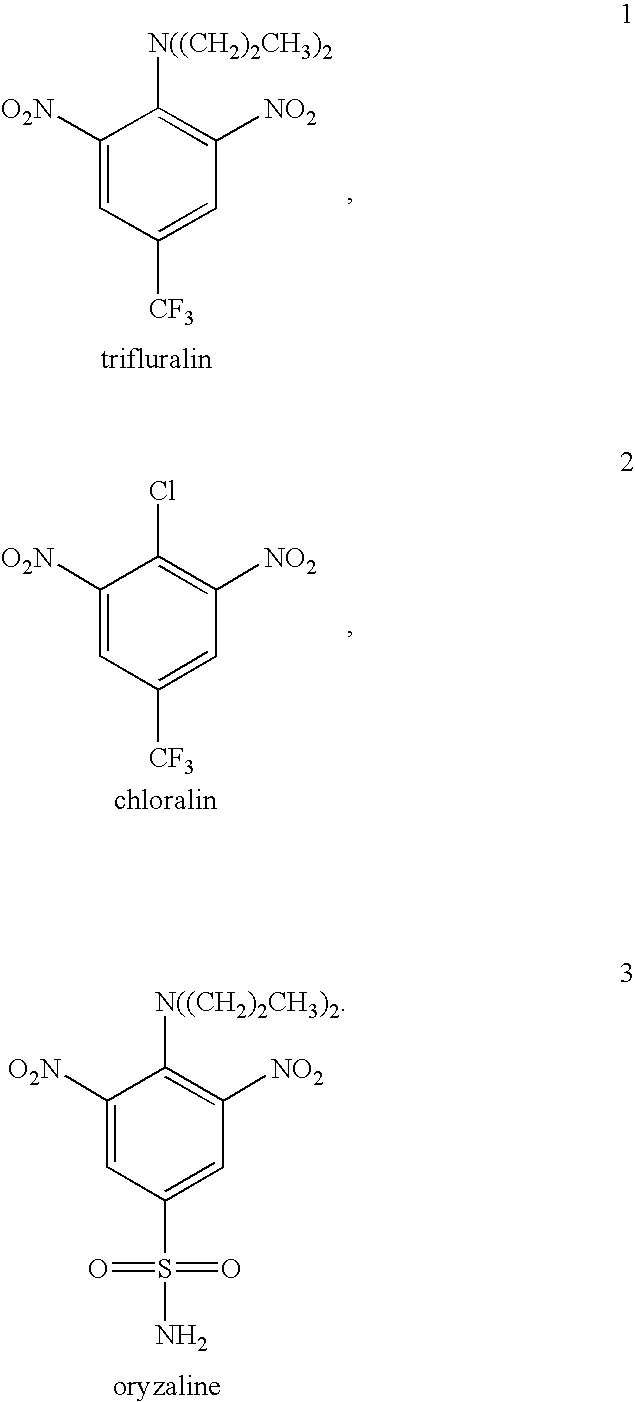
Antileishmanial dinitroaniline sulfanomides with activity against parasite tubulin
Dinitroaniline compounds useful for the treatment of diseases caused by parasitic protozoa in subjects in need of such treatment. The compounds are particularly useful in the treatment of leishmaniasis. The compounds are preferably less cytotoxic to normal cells than oryzalin. Also provided are methods of treating subjects having diseases caused by parasitic protozoa, preferably humans. The method comprising administering a therapeutically effective amount of a dinitroaniline compound of the present invention to a subject in need of such treatment
Owner:THE OHIO STATES UNIV +1
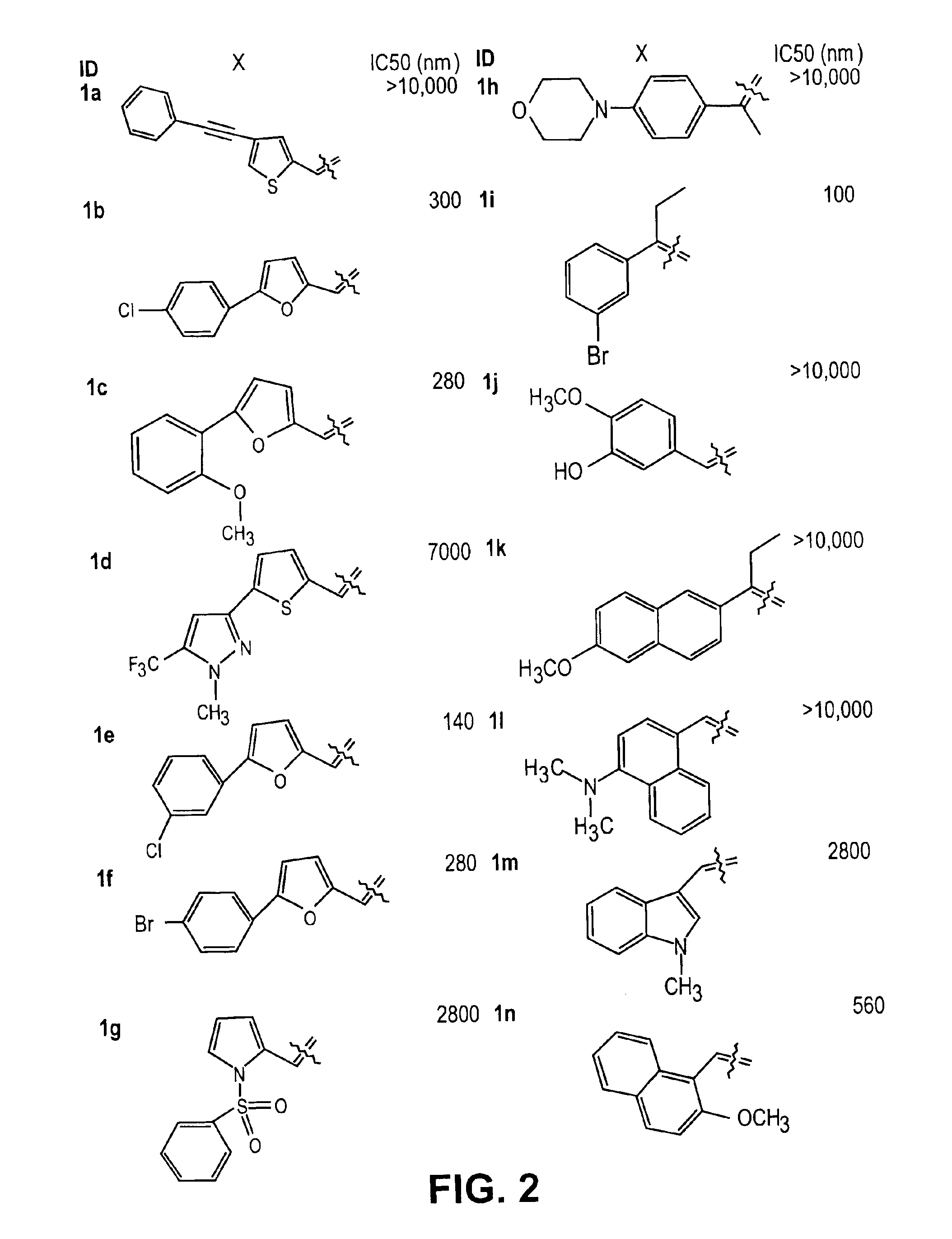
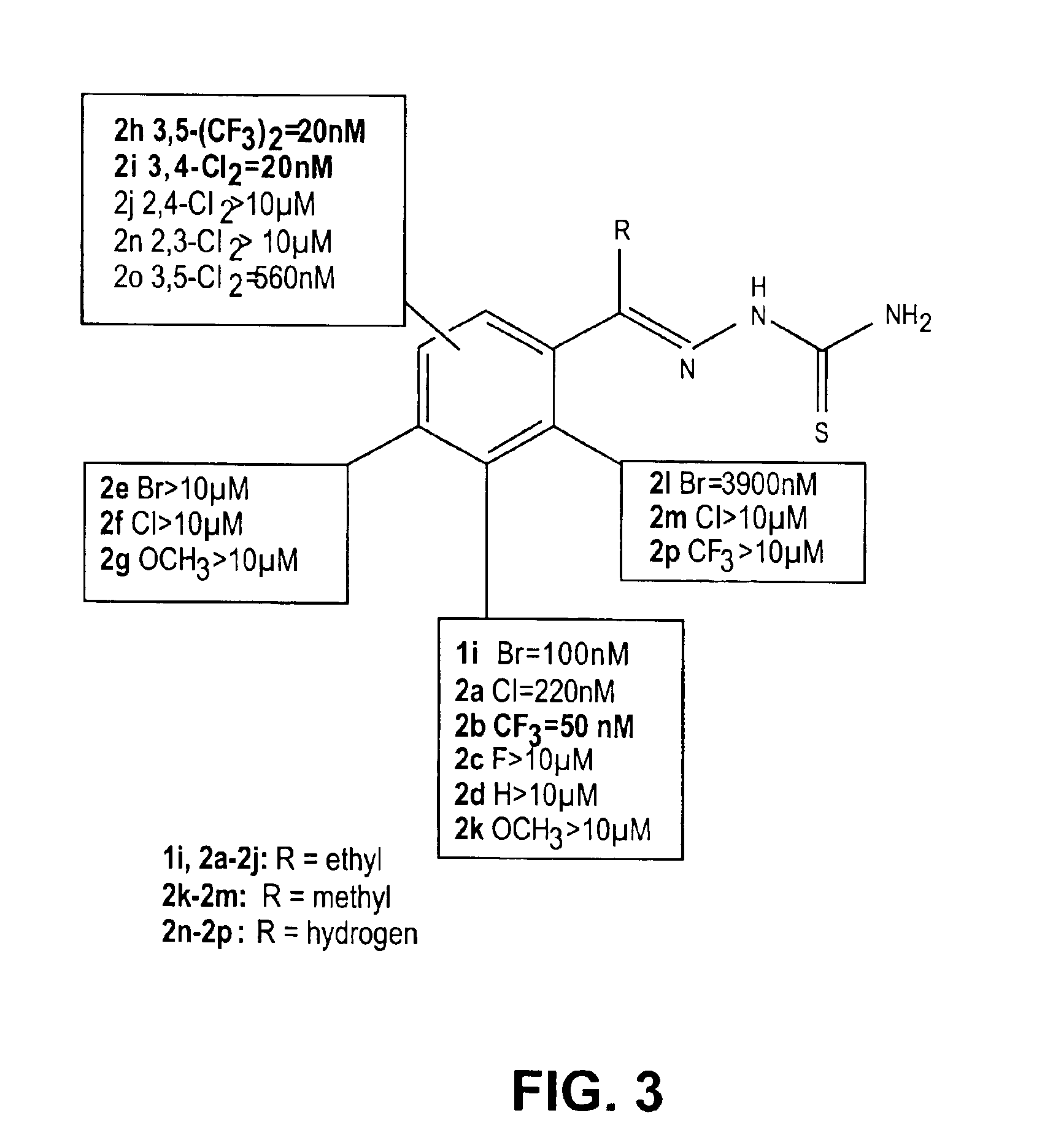
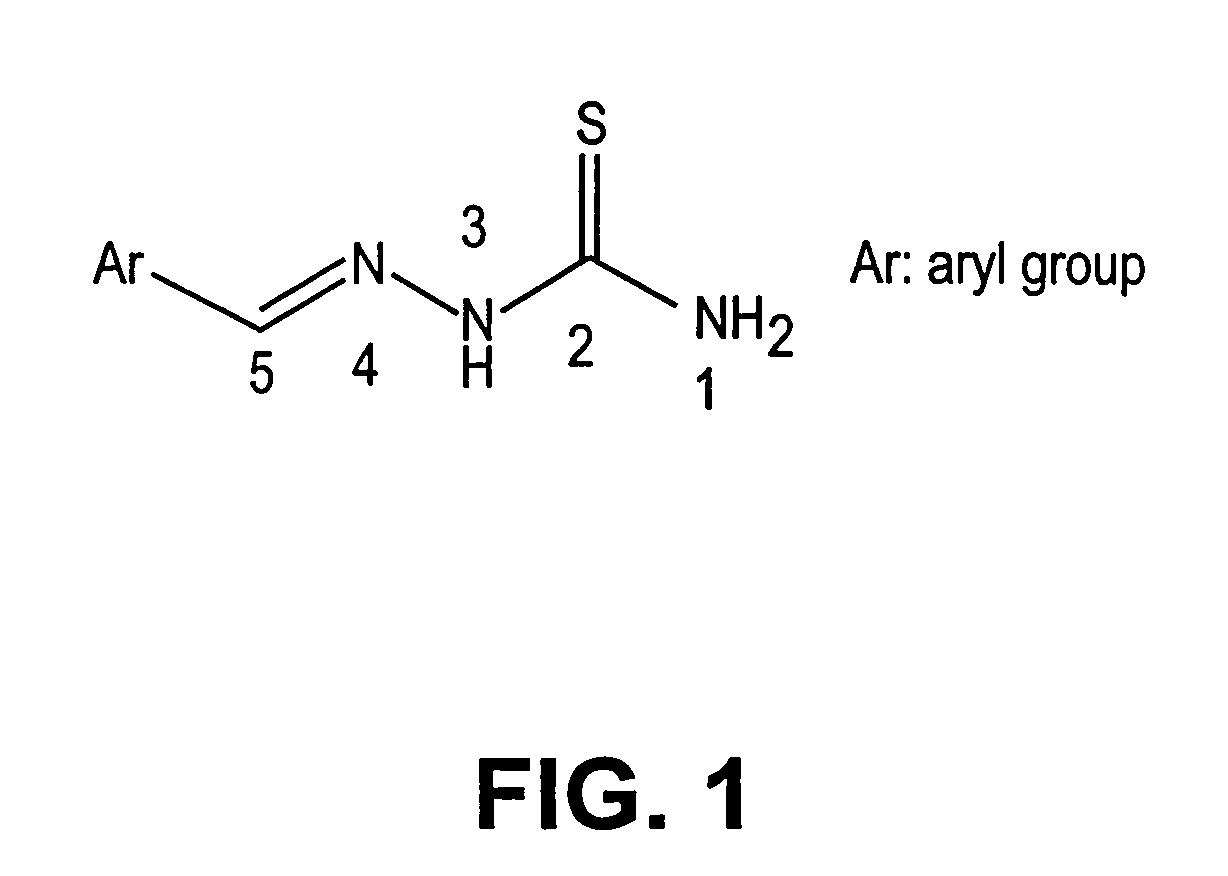
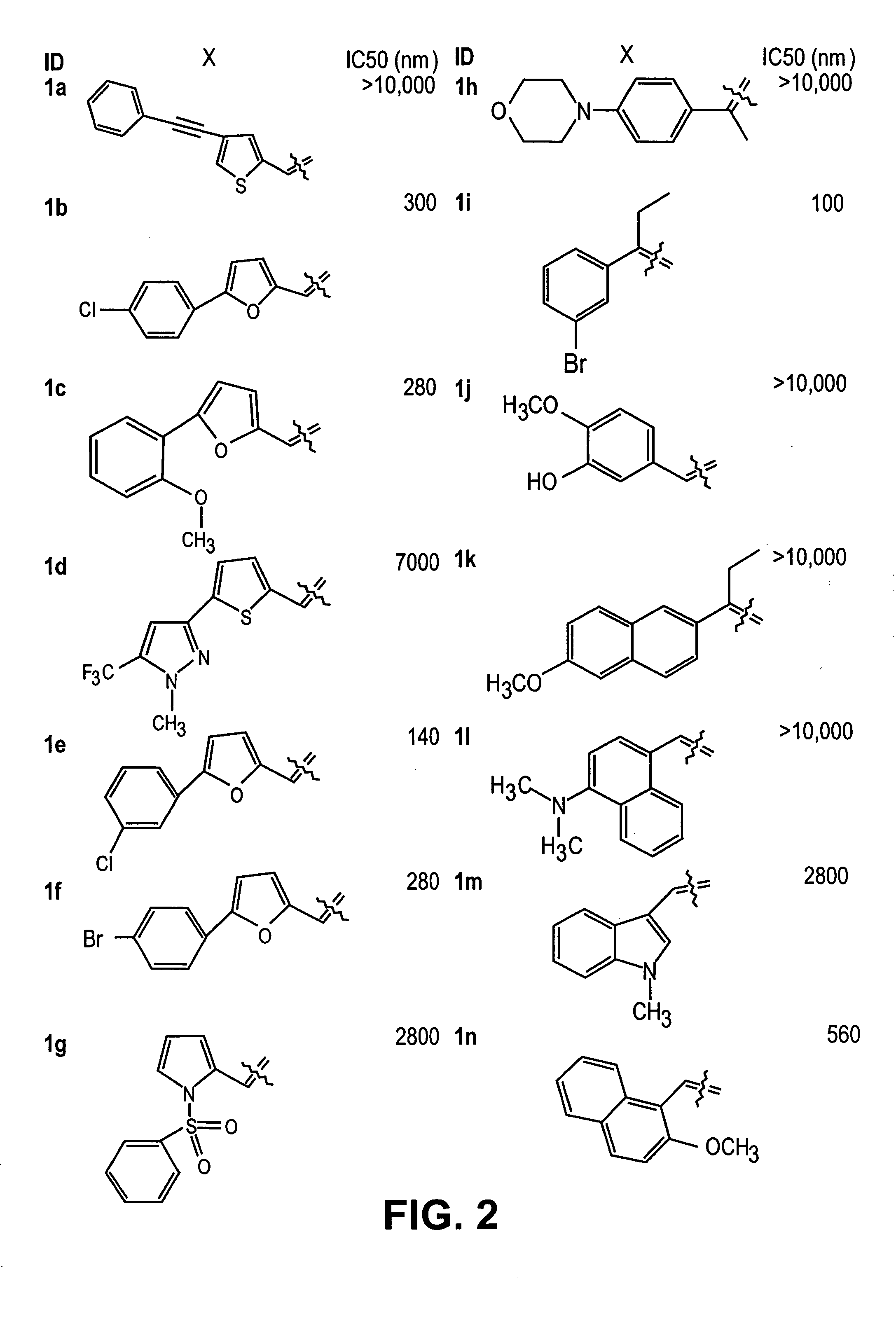
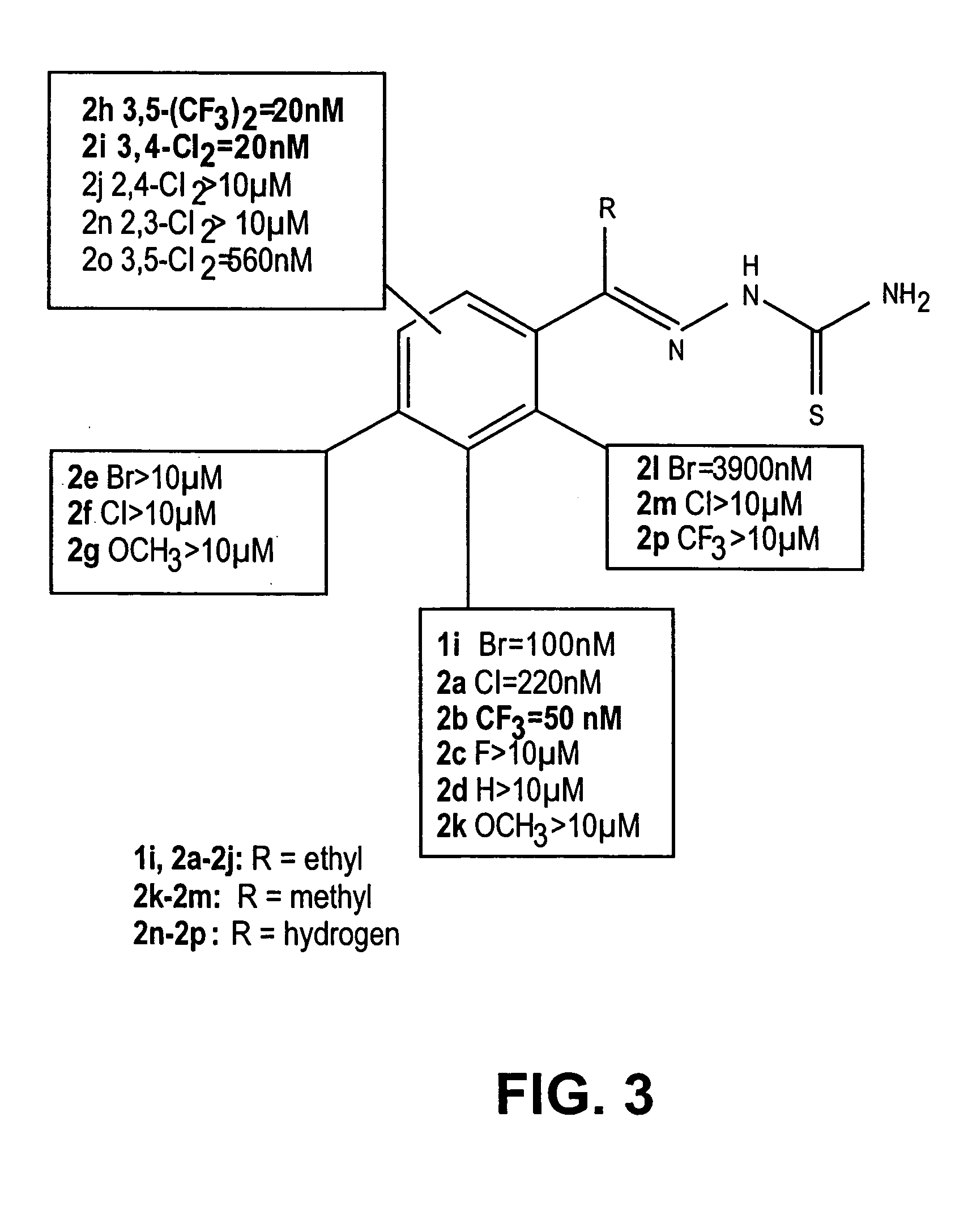
Thio semicarbazone and semicarbazone inhibitors of cysteine proteases and methods of their use
The present invention relates to thio semicarbazone and semicarbazone inhibitors of cysteine proteases and methods of using such compounds to prevent and treat protozoan infections such as trypanosomiasis, malaria and leishmaniasis. The compounds also find use in inhibiting cysteine proteases associated with carcinogenesis, including cathepsins B and L.
Owner:RGT UNIV OF CALIFORNIA
Agent for treating leishmania infections
InactiveUS20060194753A1Enhanced transfectionAvoid disadvantagesOrganic active ingredientsProtozoa antigen ingredientsLeishmaniasisTrademark
Use of a combination of DNA expression constructs for the production of a remedy for the immunization against infections with leishmaniasis, as well as a corresponding vaccine. The abstract of the disclosure is submitted herewith as required by 37 C.F.R. §1.72(b). As stated in 37 C.F.R. §1.72(b): A brief abstract of the technical disclosure in the specification must commence on a separate sheet, preferably following the claims, under the heading “Abstract of the Disclosure.” The purpose of the abstract is to enable the Patent and Trademark Office and the public generally to determine quickly from a cursory inspection the nature and gist of the technical disclosure. The abstract shall not be used for interpreting the scope of the claims. Therefore, any statements made relating to the abstract are not intended to limit the claims in any manner and should not be interpreted as limiting the claims in any manner.
Owner:MOLOGEN AG
Synthetic immunogen useful for generating long lasting immunity and protection against pathogens
InactiveUS20130183377A1Induced proliferationGenerating long lasting protective immunityAntibacterial agentsPowder deliverySynthetic ImmunogensTrypanosomiasis
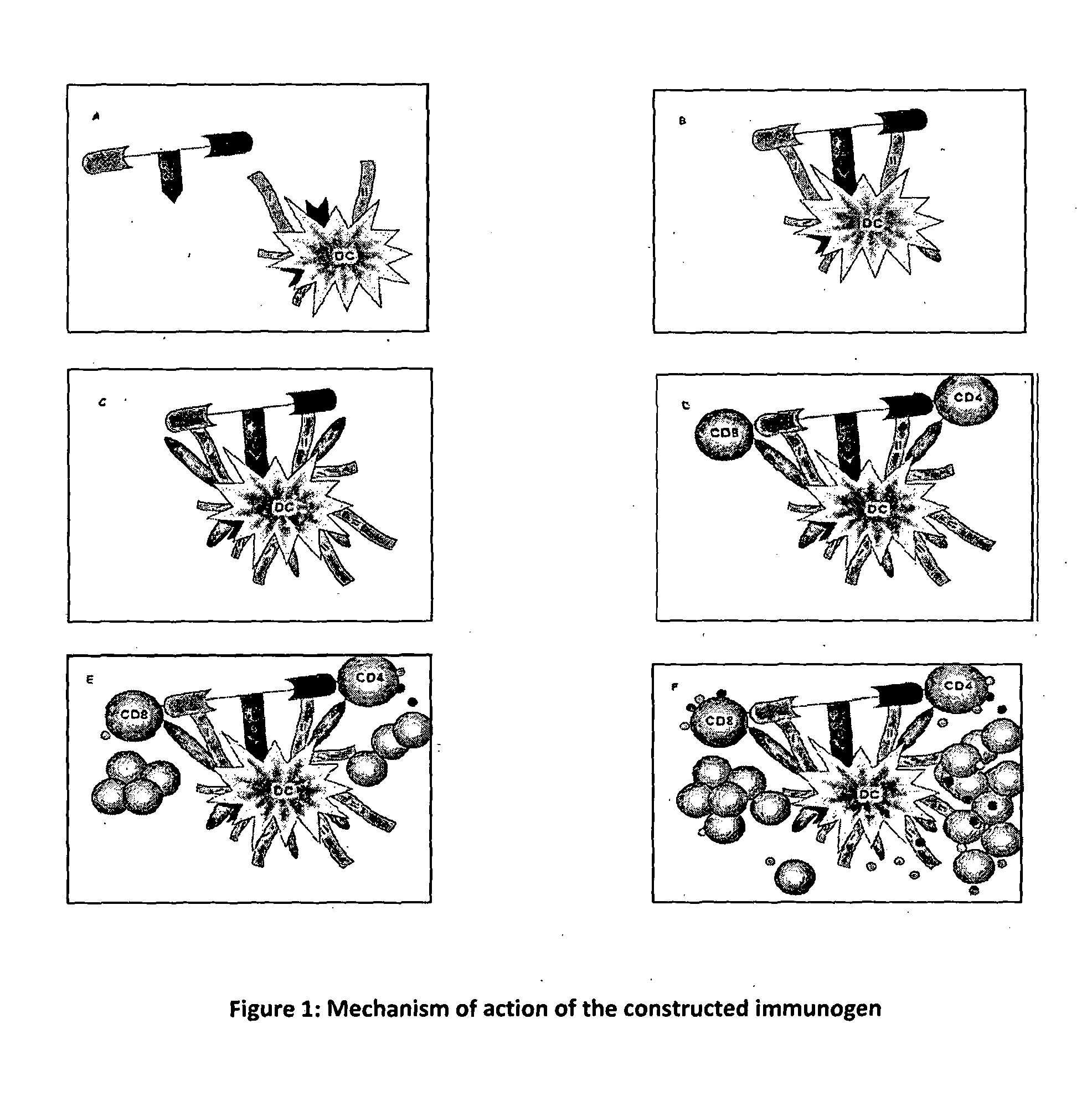
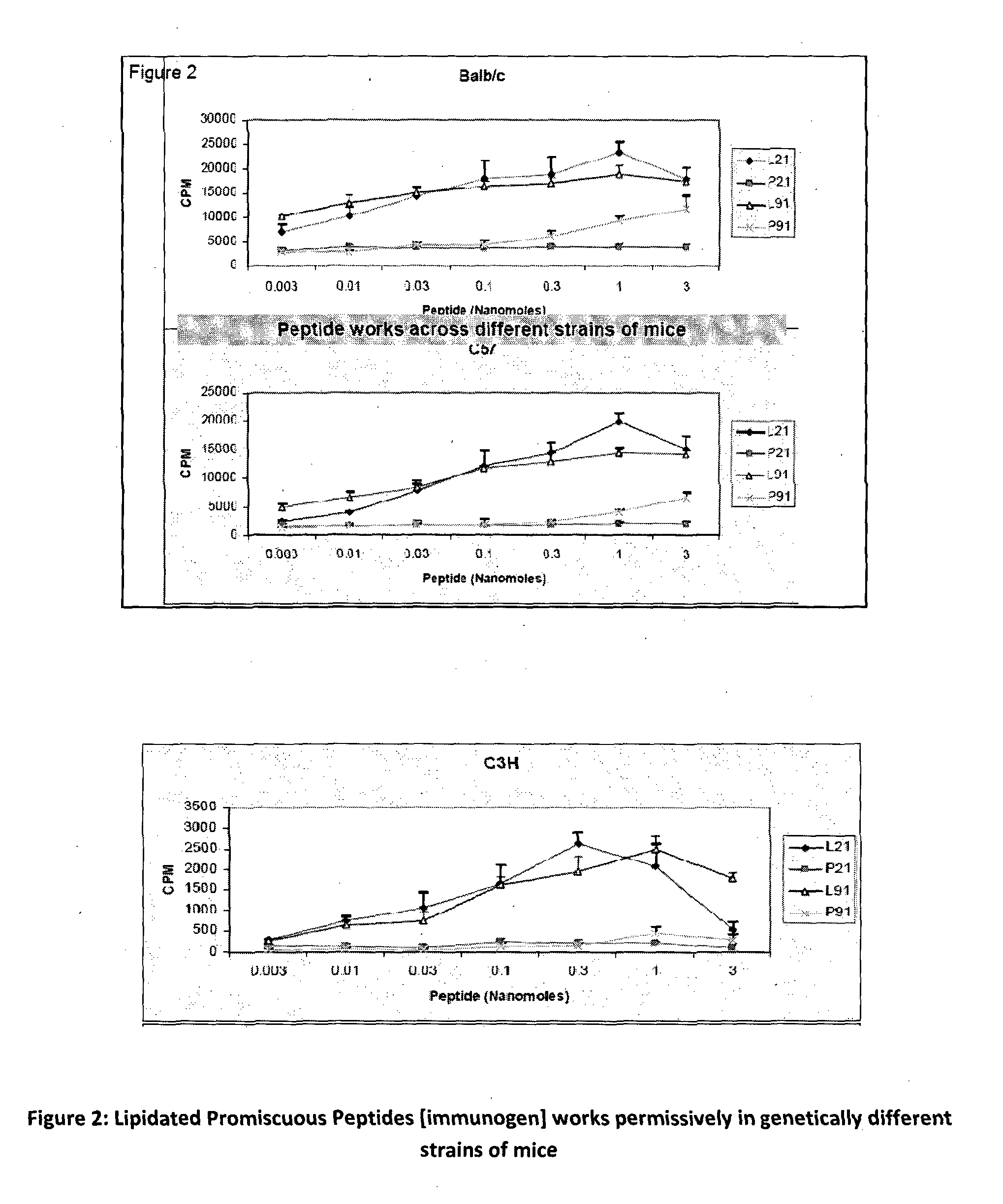
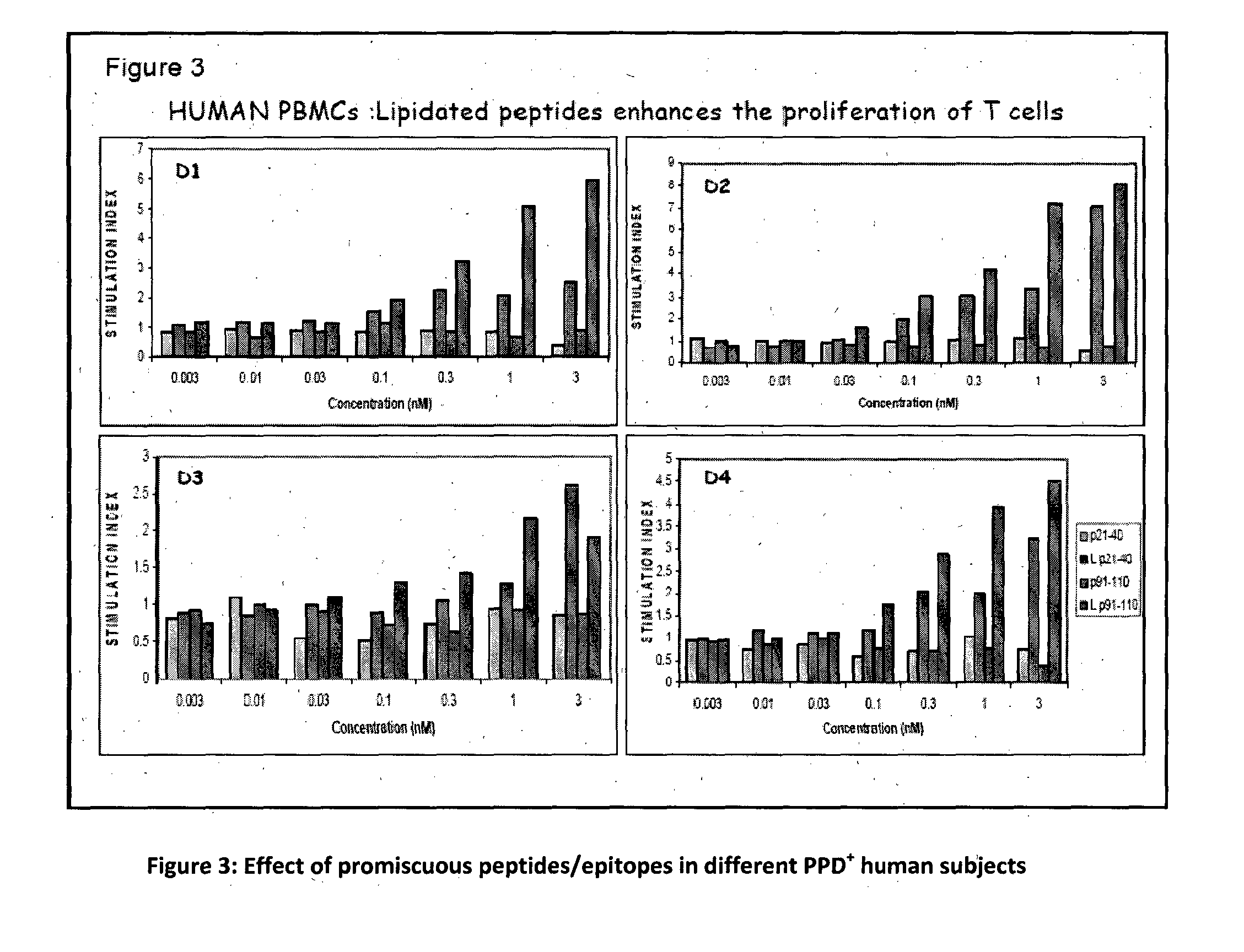
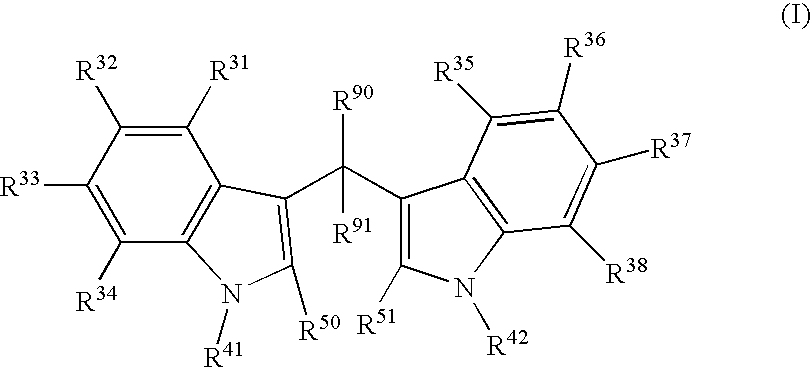
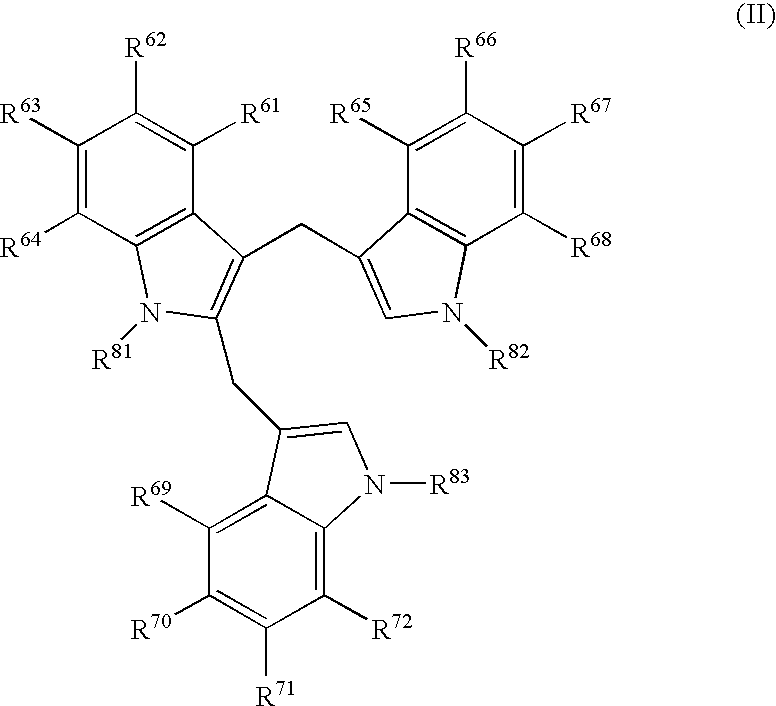
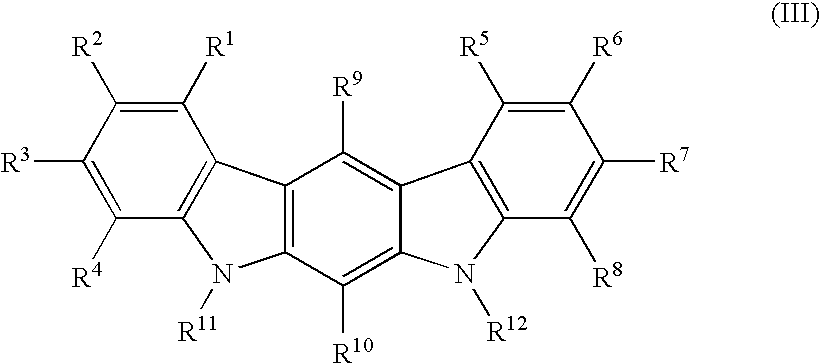
The present invention relates to a synthetic immunogen represented by the general formula 1, useful for generating long lasting protective immunity against various intracellular pathogens which are the causative agents of tuberculosis, leishmaniasis, AIDS, trypanosomiasis, malaria and also allergy, cancer and a process for the preparation thereof. The developed immunogen is able to circumvent HLA restriction in humans and livestock. The invention further relates to a vaccine comprising the said immunogen for generating enduring protective immunity against various diseases. The said vaccine is targeted against intracellular pathogens, more particularly the pathogen M. tuberculosis in this case. In the present invention, promiscuous peptides of M. tuberculosis are conjugated to TLR ligands especially; Pam2Cys to target them mainly to dendritic cells and therefore elicit long-lasting protective immunity. (The formula (I) should be inserted here) General formula (I) wherein, X1=a promiscuous CD4 T helper epitope selected from SEQ ID No. 1 to 98 OR nil; X2=a promiscuous CD8 T cytotoxic epitope selected from SEQ ID No. 99 to 103 OR nil; when X1=nil; X2=SEQ ID No. 99 to 103 and when X2=nil; X1=SEQ ID No. 1 to 98; Y=Lysine; and S=Serine.
Owner:COUNCIL OF SCI & IND RES +1
Anti-parasitic methods and compositions utilizing diindolylmethane-related indoles
ActiveUS8586621B2Shorten the counting processHeavy metal active ingredientsBiocideCoccidiosisTrypanosomiasis
The present invention includes methods and compositions for the treatment and prevention of protozoal parasitic infections utilizing Diindolylmethane-related indoles. Additive and synergistic interaction of Diindolylmethane-related indoles with other known anti-parasitic and pro-apoptotic agents is believed to permit more effective therapy and prevention of protozoal parasitic infections. The methods and compositions described provide new treatment of protozoal parasitic diseases of mammals and birds including malaria, leishmaniasis, trypanosomiasis, trichomoniasis, neosporosis and coccidiosis.
Owner:BIORESPONSE
Method for generating antigen-presenting cells
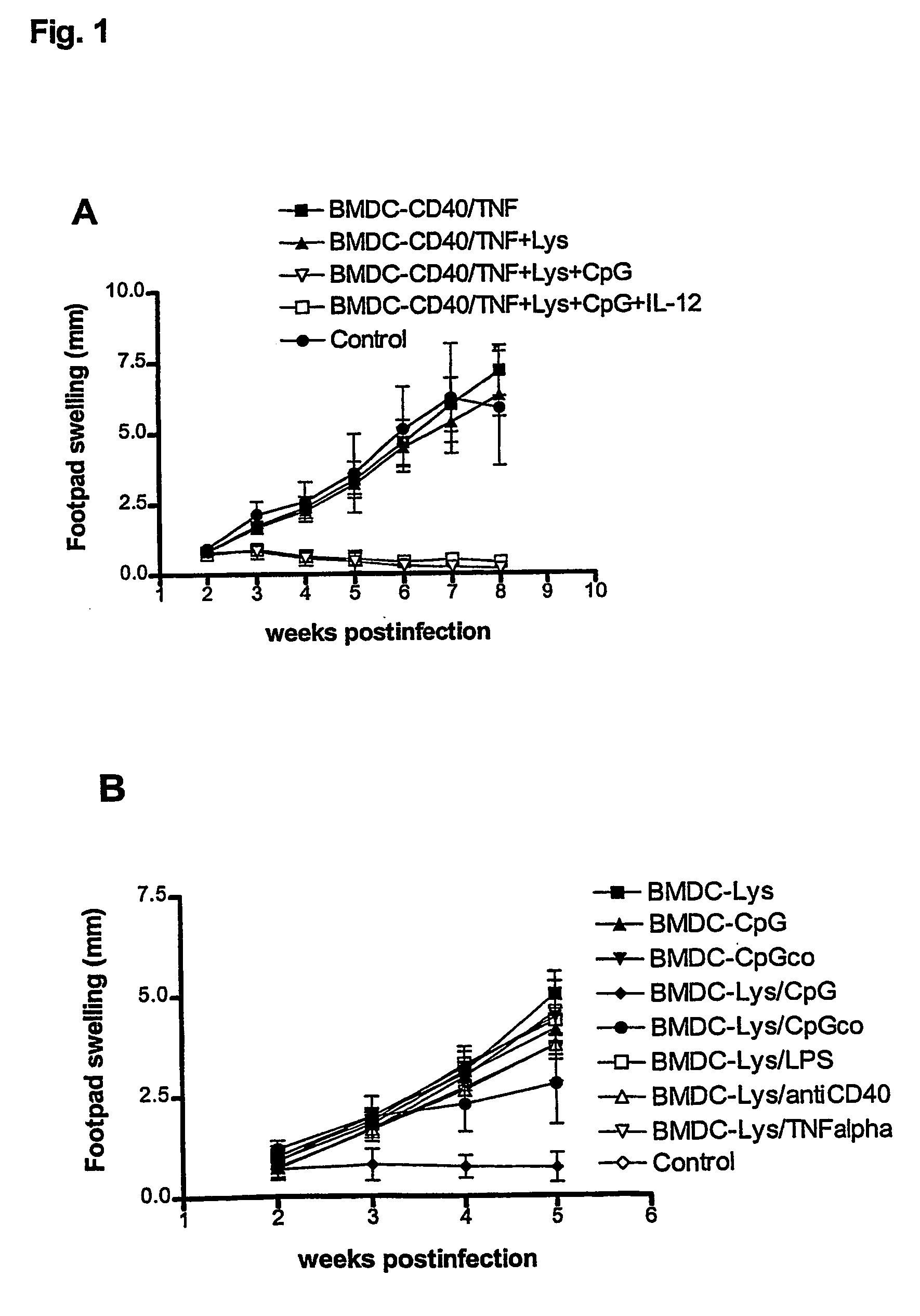
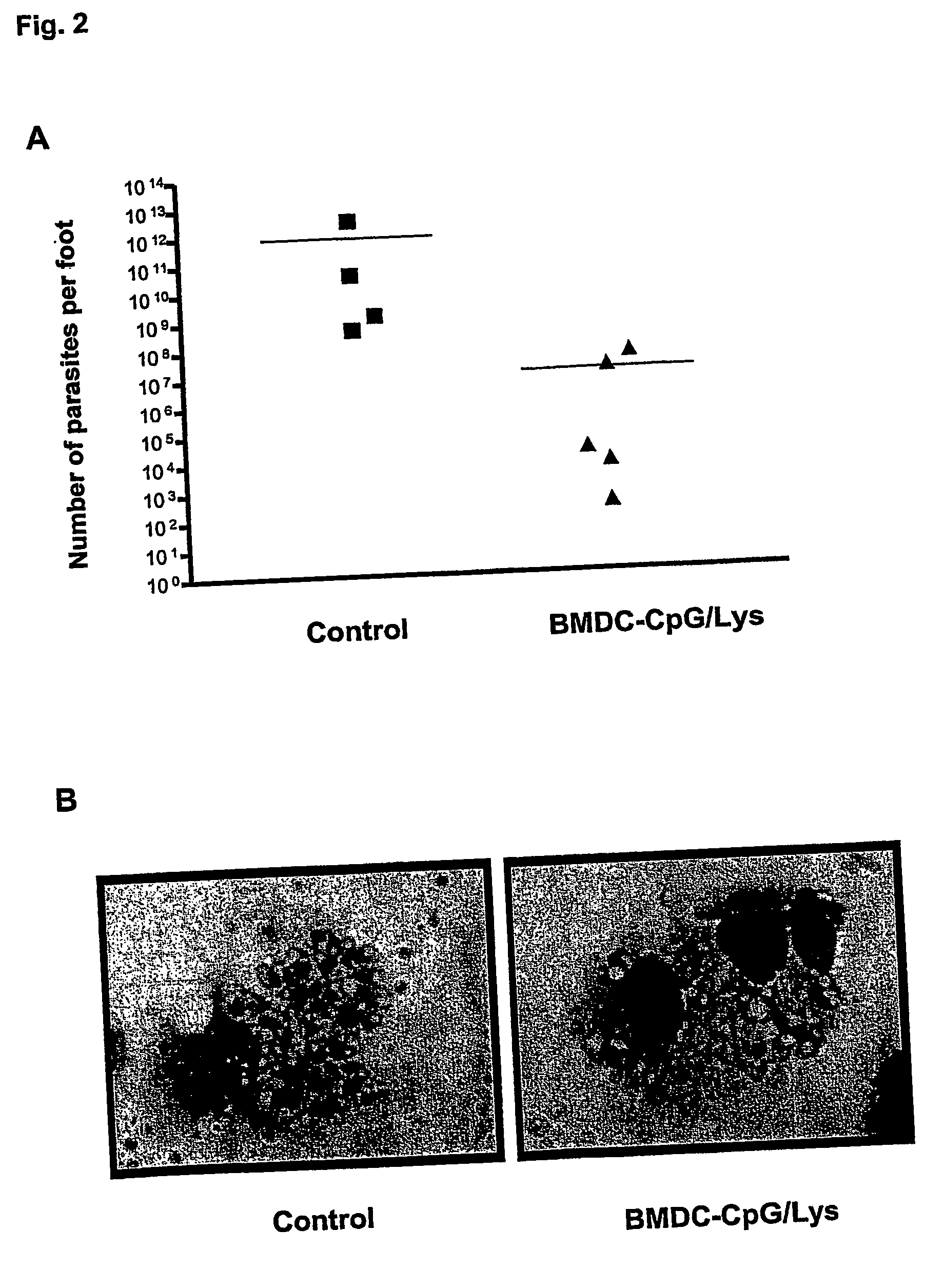
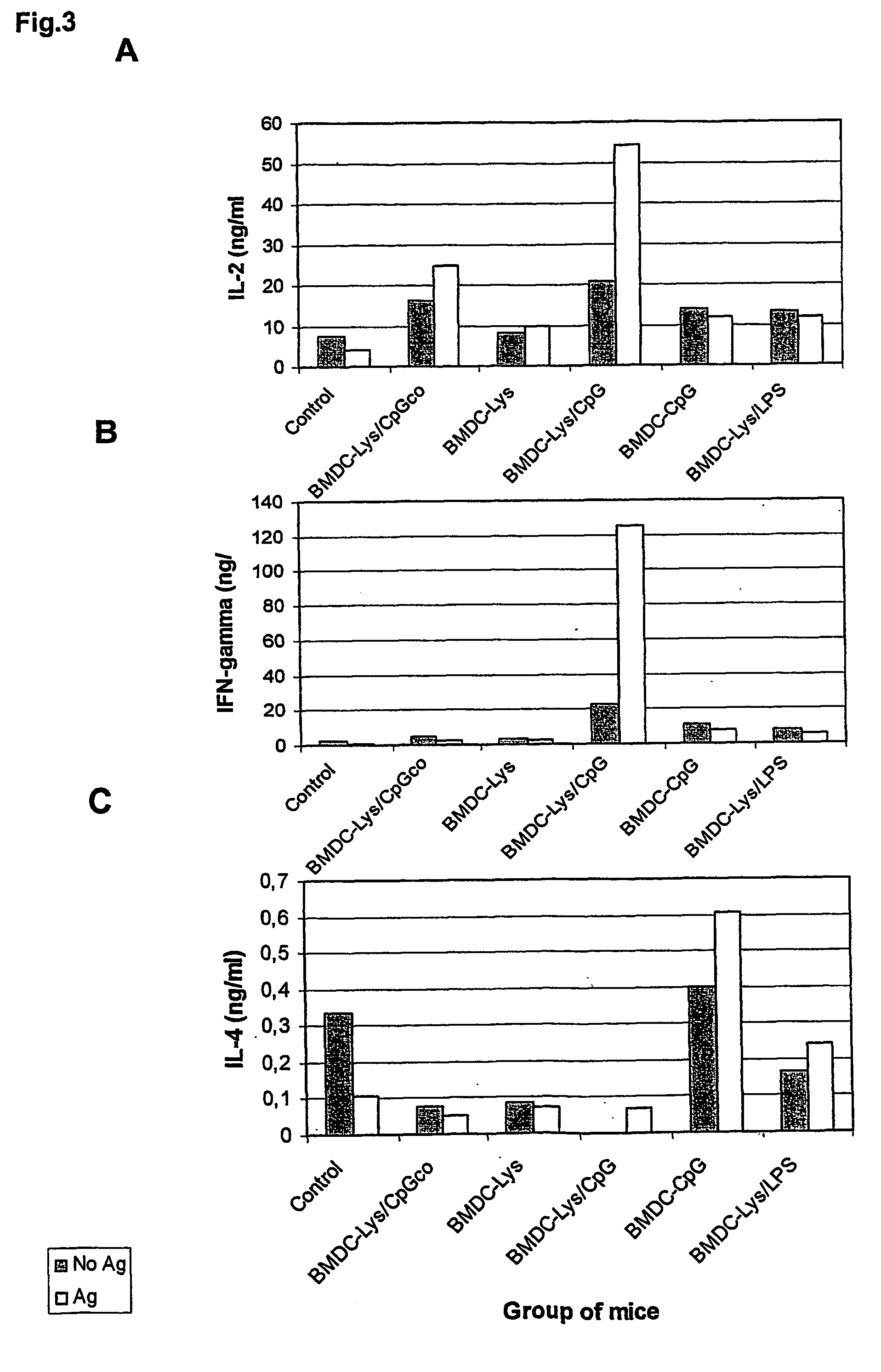
Described is a method for the generation of antigen-presenting cells (APC), preferably bone marrow-derived dendritic cells (BMDC) or peripheral blood-derived dendritic cells, as antigen carrier having immunostimulatory properties for anti-infective treatment comprising the steps of (a) pulsing the APC with antigen and (b) treating the APC with a CpG oligonucleotide. Said APC are useful as an immune prophylactic or immune therapeutic agent against diseases like AIDS, tuberculosis, malaria or leishmaniasis.
Owner:MERCK PATENT GMBH
Recombinant polyprotein vaccines for the treatment and diagnosis of leishmaniasis
Compositions and methods for preventing, treating and detecting leishmaniasis are disclosed. The compositions generally comprise fusion polypeptides comprising multiple Leishmania antigens, in particular, KMP11, SMT, A2 and / or CBP, or immunogenic portions or variants thereof, as well as polynucleotides encoding such fusion polypeptides.
Owner:ACCESS TO ADVANCED HEALTH INST
Anti-arthropod vector vaccines method of selecting and uses thereof
ActiveUS7388089B2Preventing LeishmaniasisPeptide/protein ingredientsImmunoglobulinsArthropod VectorLeishmaniasis
The present invention provides methods of selecting and uses of anti-arthropod vector vaccines to prevent Leishmaniasis. The present invention also provides compositions for vaccines to prevent Leishmaniasis.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Compounds and methods for diagnosis and treatment of leishmaniasis
Compounds and methods are provided for diagnosing, preventing, treating and detecting leishmaniasis infection and stimulating immune responses in patients are disclosed. The compounds disclosed are include polypeptides and fusion proteins that contain at least one immunogenic portion of one or more Leishmania antigens, or a variant thereof. Additionally, methods of screening a screening library for tandem repeat proteins that have immunogenic properties are disclosed. Vaccines and pharmaceutical compositions comprising polynucleotides, polypeptides, fusion proteins and variants thereof that may be used for the prevention and therapy of leishmaniasis, as well as for the detection of Leishmaniasis infection are described.
Owner:INFECTIOUS DISEASE RES INST
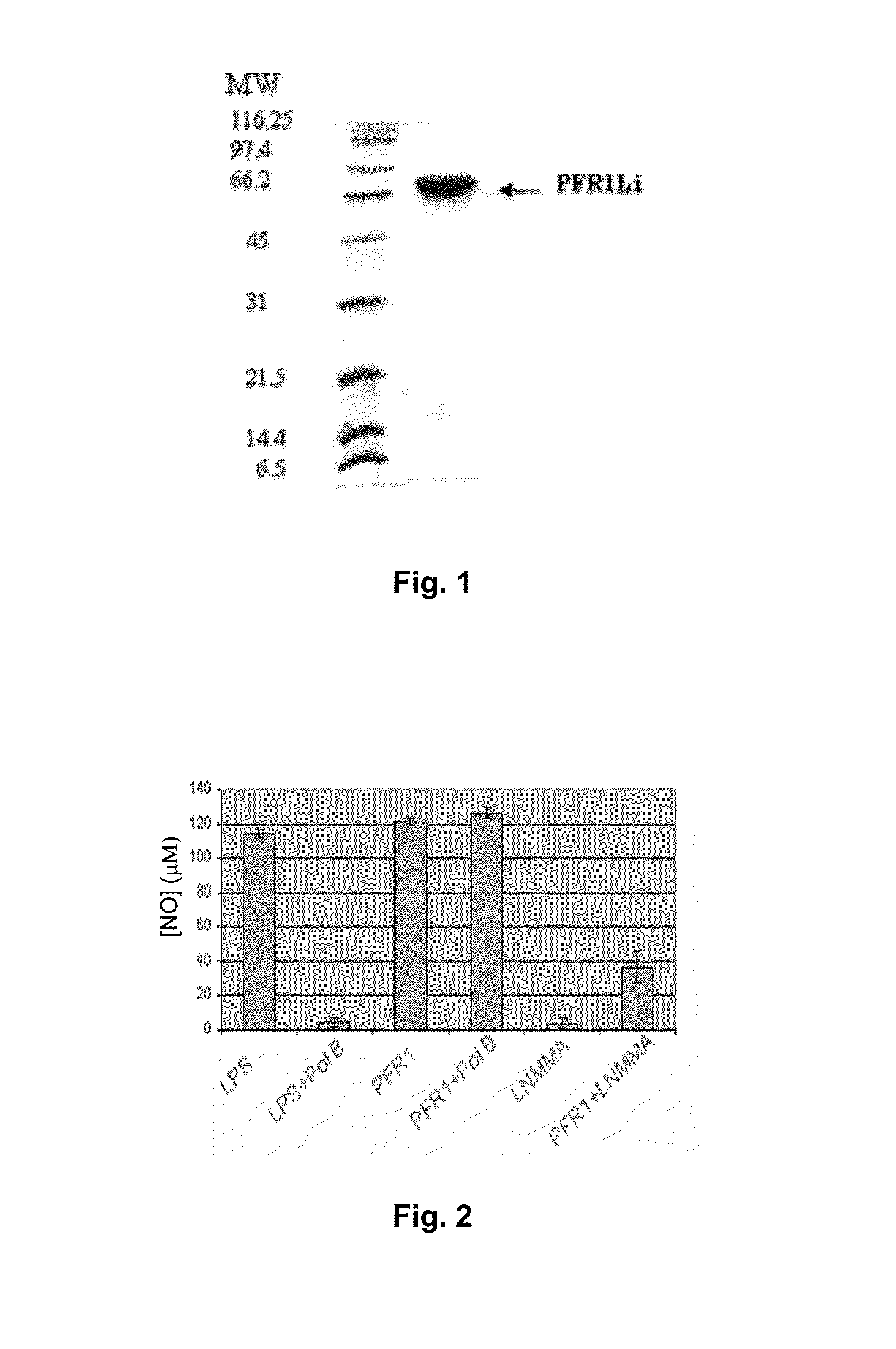
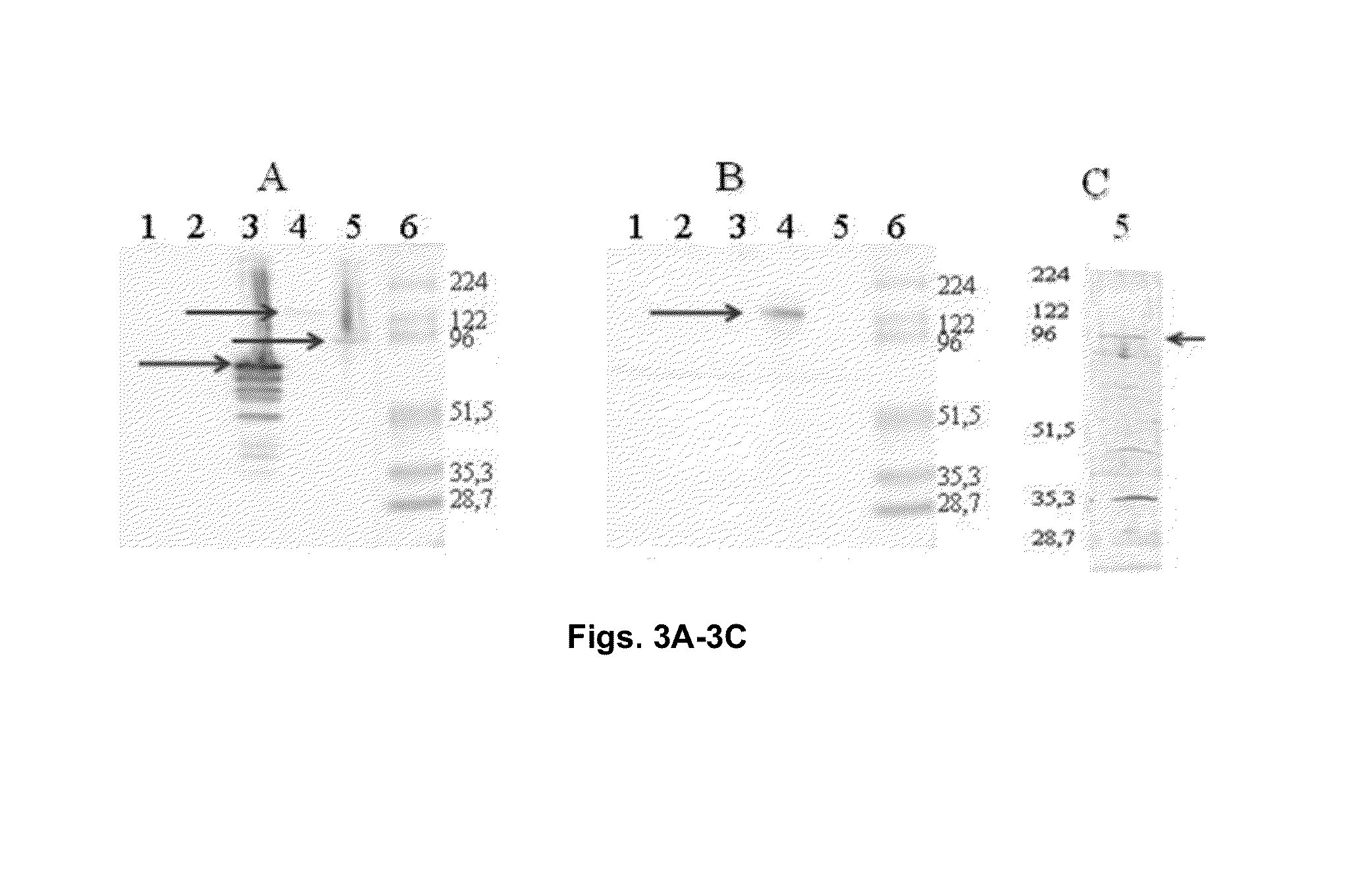
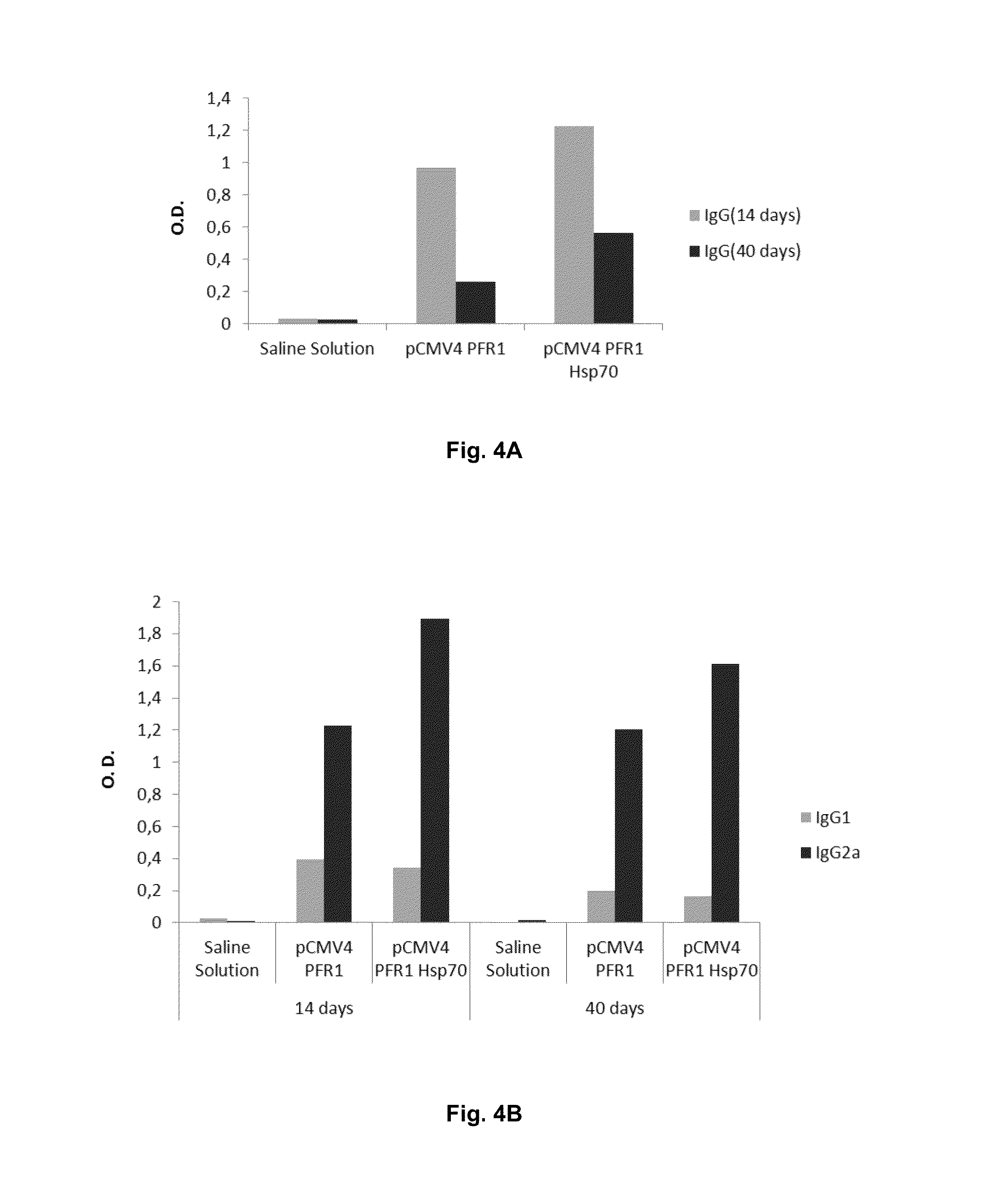
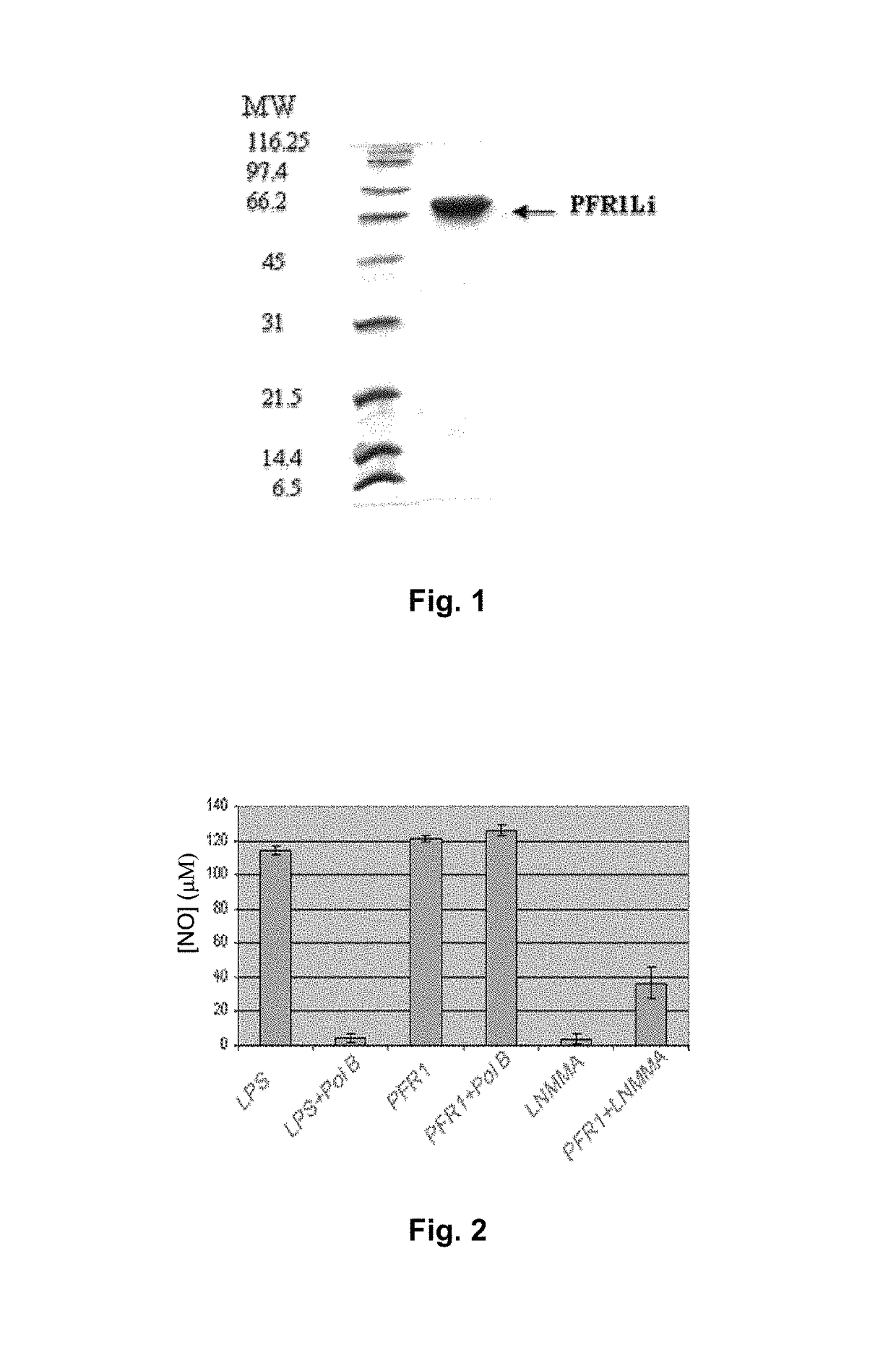
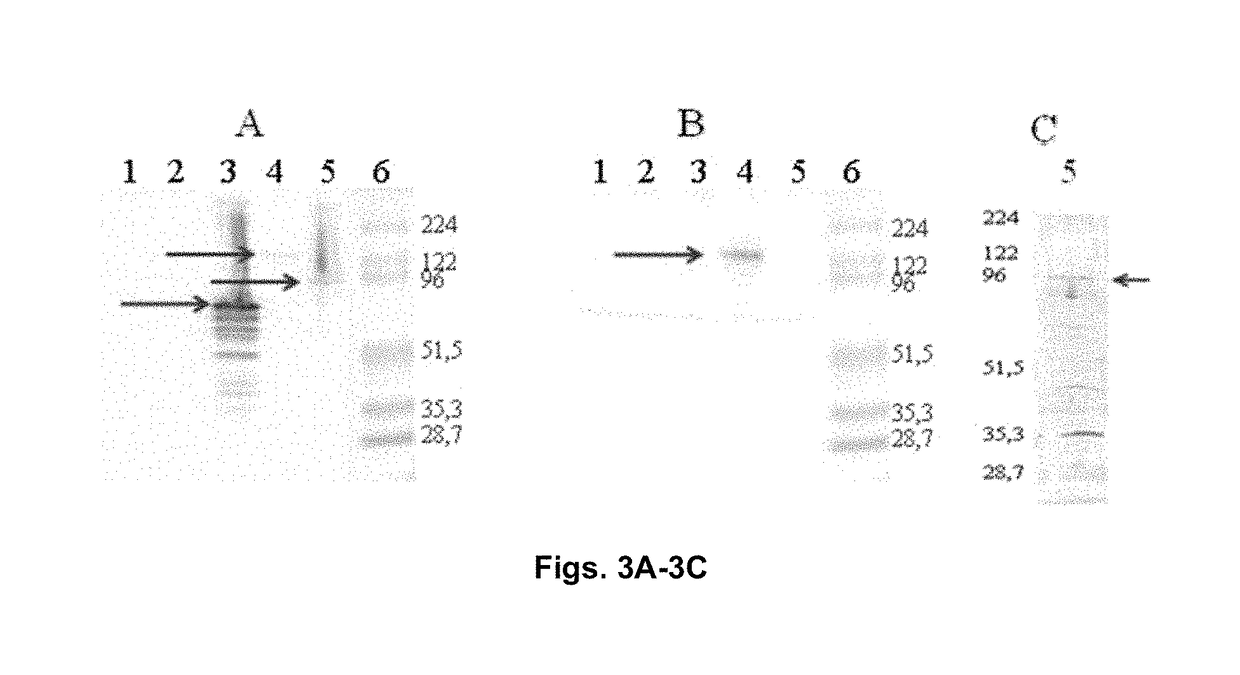
Chimeric molecule useful in immunotherapy for leishmaniasis, which includes a fragment of the pfr1 protein of leishmania infantum with specific immunodominant epitopes
The present invention claims an isolated nucleotide sequence characterized by encoding the PFR1 protein of Leishmania infantum or a fragment thereof. This PFR1 protein or a fragment thereof comprises at least a selected immunodominant epitope between the following group: SEQ ID No: 1, SEQ ID No: 2, SEQ ID No: 3, SEQ ID No: 4, SEQ ID No: 5, SEQ ID No: 6, SEQ ID No: 7 and SEQ ID No: 8, where the immunodominant epitope is able to induce an antigen-specific T cell cytotoxic immune response in an animal, against the kinetoplastids causing the leishmaniasis disease. The immunodominant epitopes are cytotoxic T-lymphocyte activators and they present a high binding affinity for A2 type MHC Class I molecule.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Compounds derived from artesunate, preparation process, pharmaceutical composition and use of the respective medicine
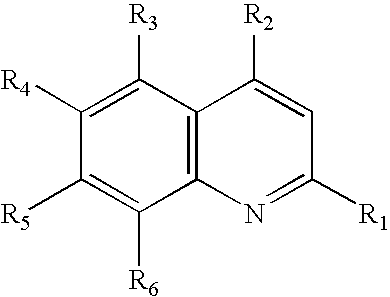
The present invention refers to new compounds derived from artesunate salts with quinolines represented by the general formula (I) where X is represented by the general formula (II) and Y is represented by the general formula (III) depending on the radicals substituted in X (formula II), the relation X to Y (formula III) may vary from 1:1 to 1:7, because the amount of Y depends on the amount of N available in X for the formation of the salt. The radicals R1, R2, R3, R4, R5, e R6 in the general formula (II) are represented by: R1═H, CF3, CH3, OCH3, NH2, halogen; R2═H, CH3, NH2, halogen, NH—CHCH3(CH2)3N(C2H5)(CH2CH2OH), CH(OH)-2(C5H11N), NH—R7—N—(C2H5)2; R3═H, m-OC6H4CF3, NH2; R4═H, CH3, OCH3, NH2, halogen; R5═H, CH3, CF3, NH2, halogen; R6═H, CF3, CH3, NH2, halogen, NH—R8—N—(C2H5)2, NHCH(CH3)(CH2)3NH2; R7═(CH2)2, (CH2)3, CHCH3CH2, (CH2)4, (CH2)5, CHCH3(CH2)3(CH2)6, (CH2)8, (CH2)10, (CH2)12; R8═CHCH3(CH2)3, CHCH3(CH2)CHCH3, (CH2)2, (CH2)3, (CH2)6, (CH2)3O(CH2)3. The present invention also refers to a process of preparation of these general formula (I) compounds, and the pharmaceutical compositions, in which these compounds are included especially their use as medicine for treatment or prevention or inhibition of malaria or other parasitic diseases such as: kaodzera, Chagas disease, leishmaniasis, amoebiasis, giardiasis, trichommoniasis, toxoplasmosis, schistosomiasis, as well as other helminthiases.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
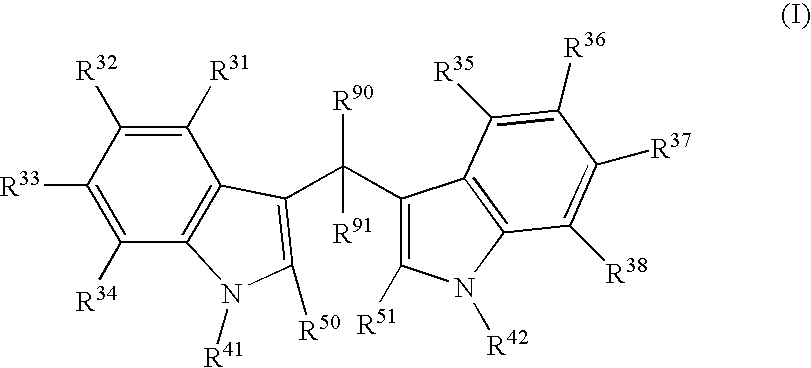
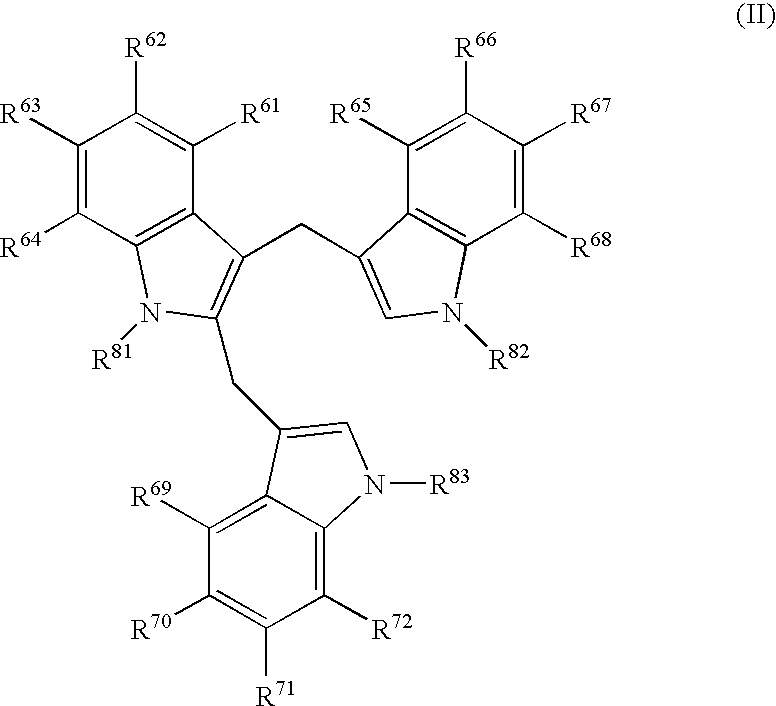
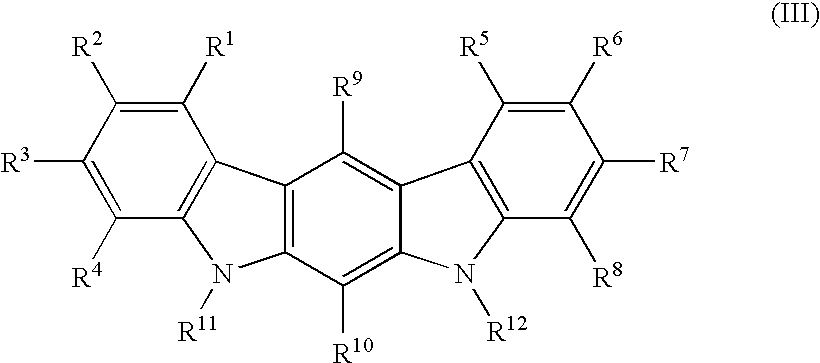
Compounds And Their Use As Inhibitors Of N-Myristoyl Transferase
This invention provides compounds of formula (I) and salts thereof, which have activity as inhibitors of N-myristoyl transferase (NMT). The invention also relates to uses of such compounds as medicaments, in particular in the treatment of a disease or disorder in which inhibition of N-myristoyl transferase provides a therapeutic or prophylactic effect, including protozoan infections (such as malaria and leishmaniasis), viral infections (such as human rhinovirus and HIV), and hyperproliferative disorders (such as B-cell lymphoma).
Owner:IMPERIAL INNOVATIONS LTD
Compounds and methods for diagnosis and treatment of leishmaniasis
Compounds and methods are provided for diagnosing, preventing, treating and detecting leishmaniasis infection and stimulating immune responses in patients are disclosed. The compounds disclosed are include polypeptides and fusion proteins that contain at least one immunogenic portion of one or more Leishmania antigens, or a variant thereof. Additionally, methods of screening a screening library for tandem repeat proteins that have immunogenic properties are disclosed. Vaccines and pharmaceutical compositions comprising polynucleotides, polypeptides, fusion proteins and variants thereof that may be used for the prevention and therapy of leishmaniasis, as well as for the detection of Leishmaniasis infection are described.
Owner:INFECTIOUS DISEASE RES INST IN
Chimeric gene formed of the DNA sequences that encode the antigenic determinants of four proteins of L. infantum, useful of serologic diagnosis of canine leishmaniosis and protein obtained
InactiveUS6525186B2Early diagnosisAccurate diagnosisPeptide/protein ingredientsAntibody mimetics/scaffoldsSAA proteinSerodiagnoses
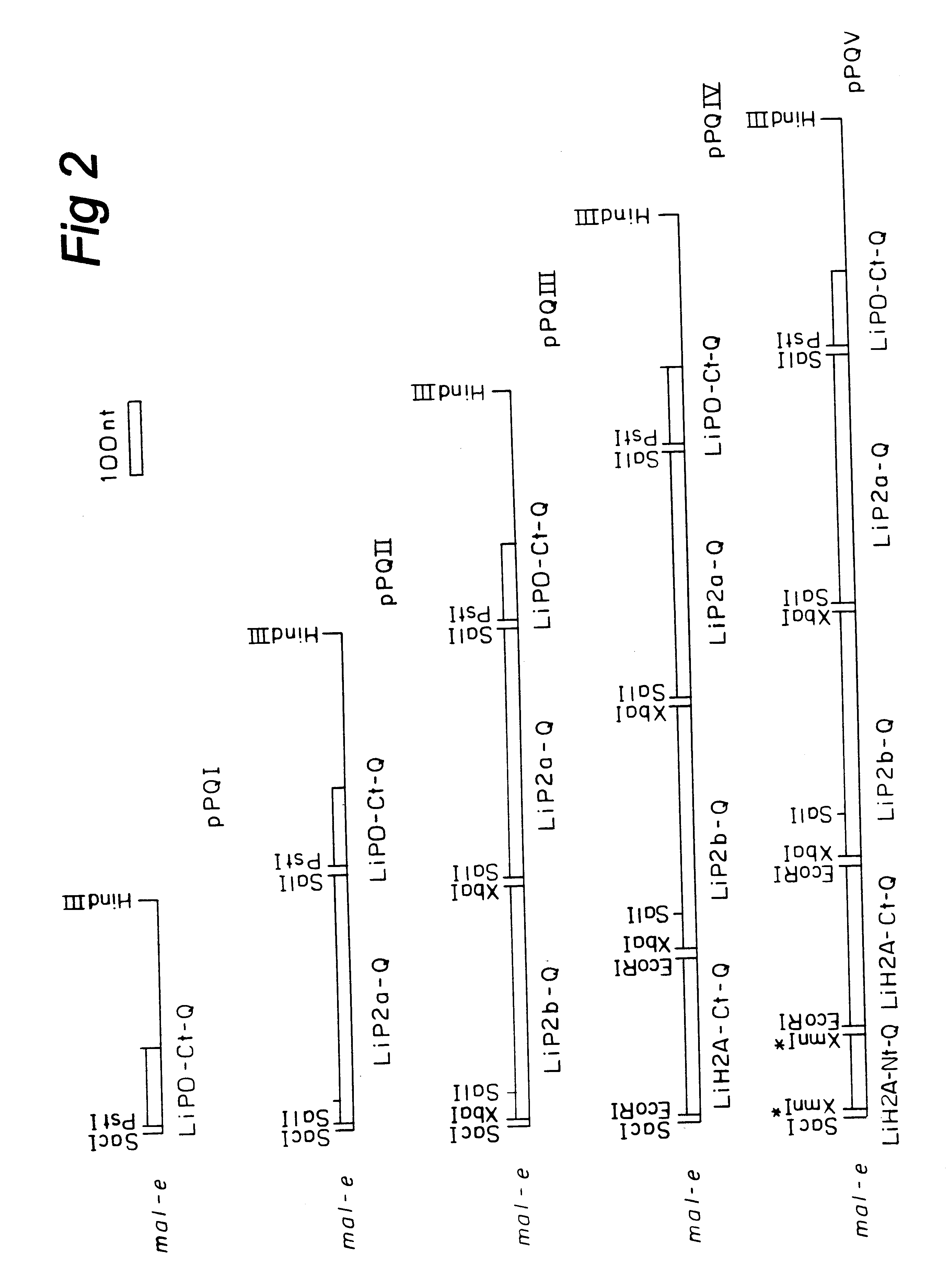
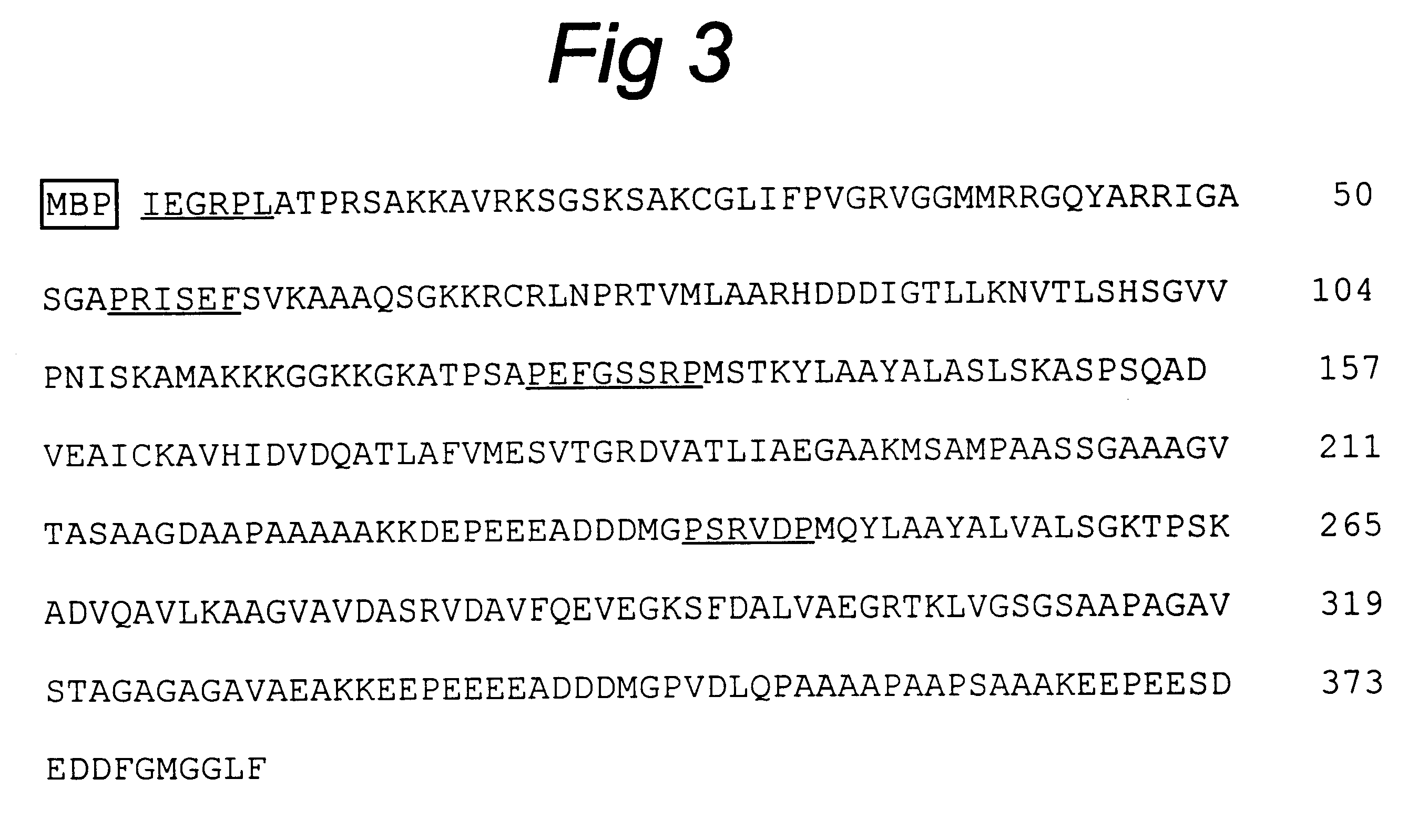
A chimeric polypeptide encoded by a chimeric gene formed by DNA sequences that encode four antigenic determinants of L. infantum is disclosed. These antigenic determinants are obtained from rLiP2a, rLiP2b, rLiH2A and rLiPO. The protein obtained, has a molecular mass of 38 KD with an isoelectric point of 7.37. This chimeric polypeptide is useful for diagnosing, preventing and / or treating leishmaniosis in animals or humans.
Owner:C B F LETI SL
Process for preparing restraining agent for leishmaniasis
InactiveCN101289426AHigh yieldHigh selectivityOrganic chemistryAntiparasitic agentsState of artLeishmaniasis
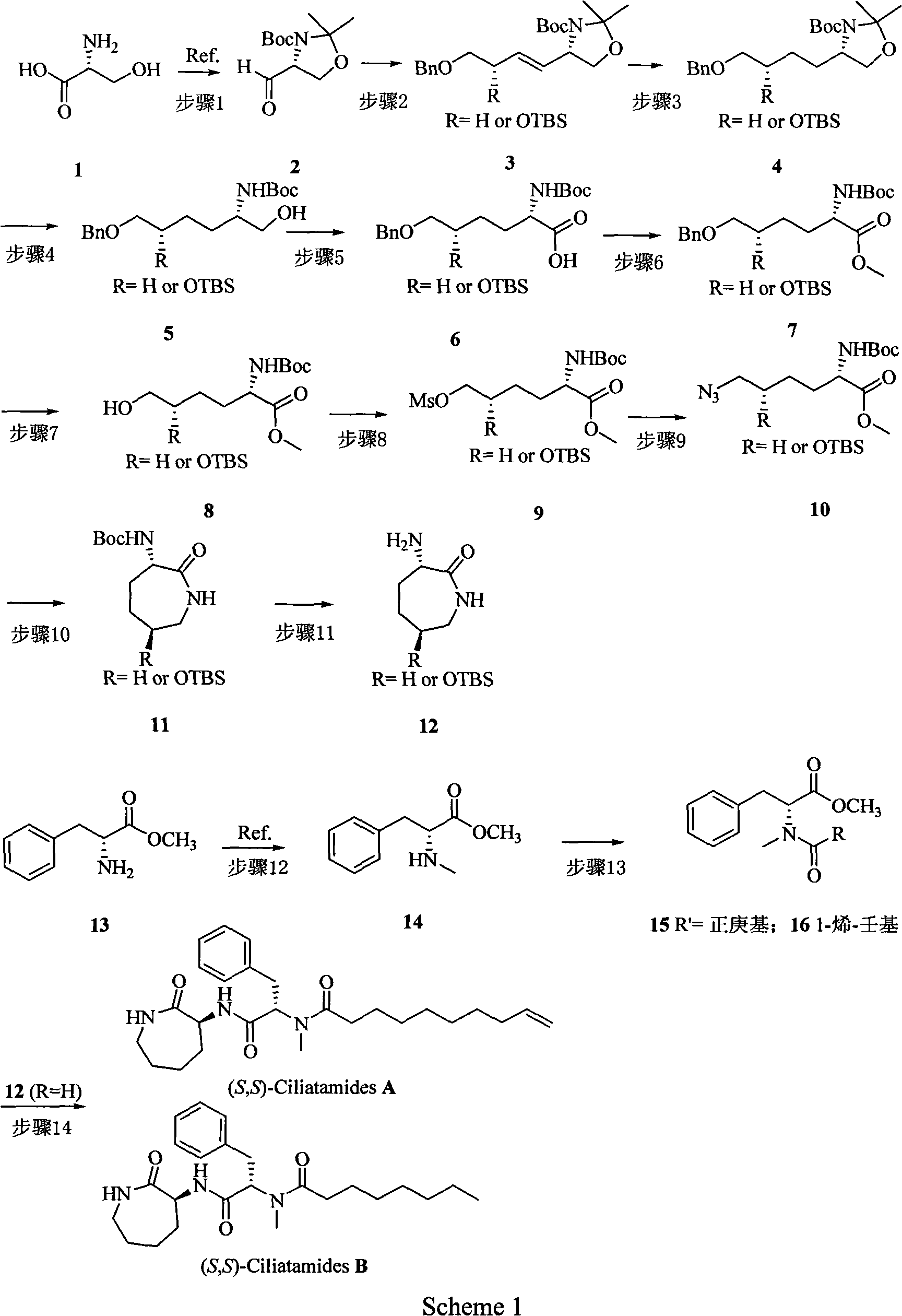
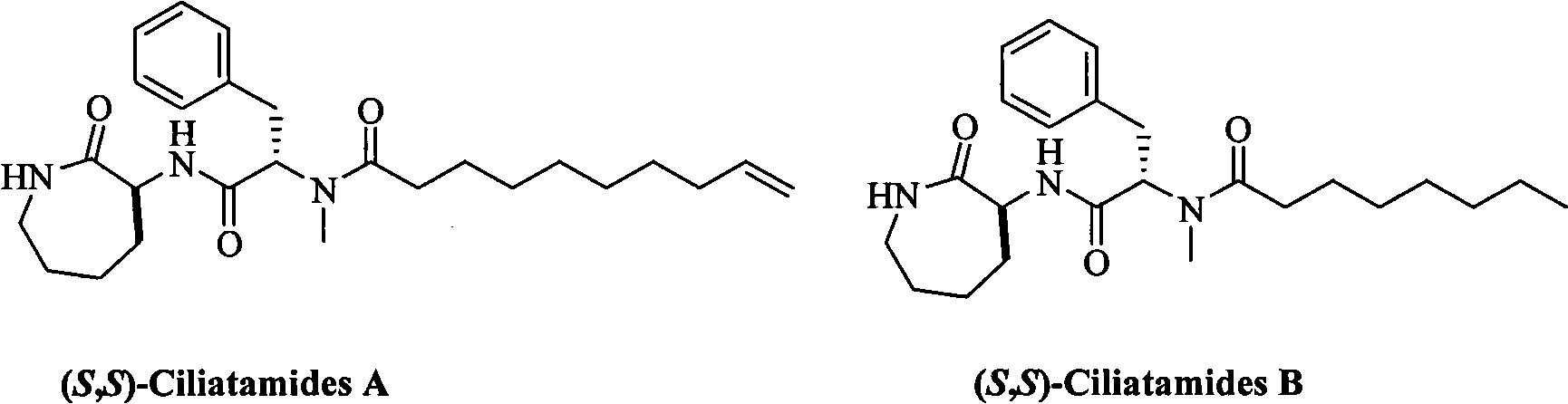
The invention belongs to the chemical field, which relates to a method for preparing leishmaniasis inhibitor. The invention overcomes the shortages of the prior art, and after a key intermediate compound 11 is synthesized with high yield and high electivity by using the cheap and available D-serine as raw material, asymmetric synthesis on marine natural product (S, S)-Ciliatamides A and B with inhibitive activity against the leishmaniasis and Hela cytotoxicity is carried out. Every step of the invention is simple in operation and high in yield; the used reagents are all common reagents which are cheap and easy to get; the preparation method of the used key intermediate is obviously different from synthesis methods of the prior art.
Owner:FUDAN UNIV
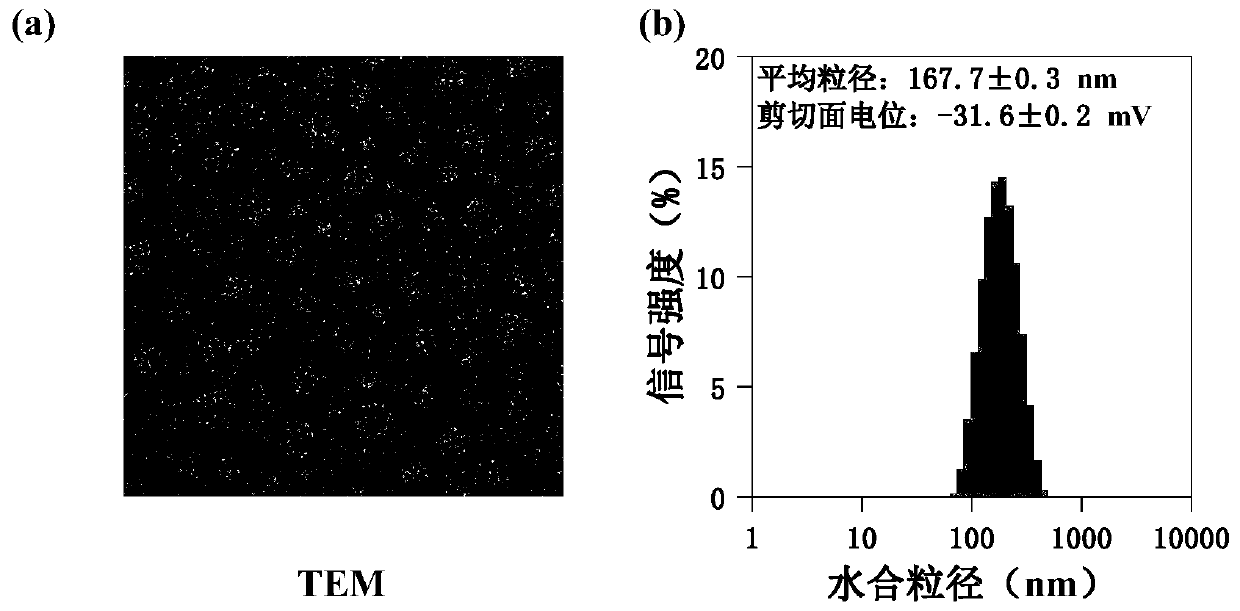
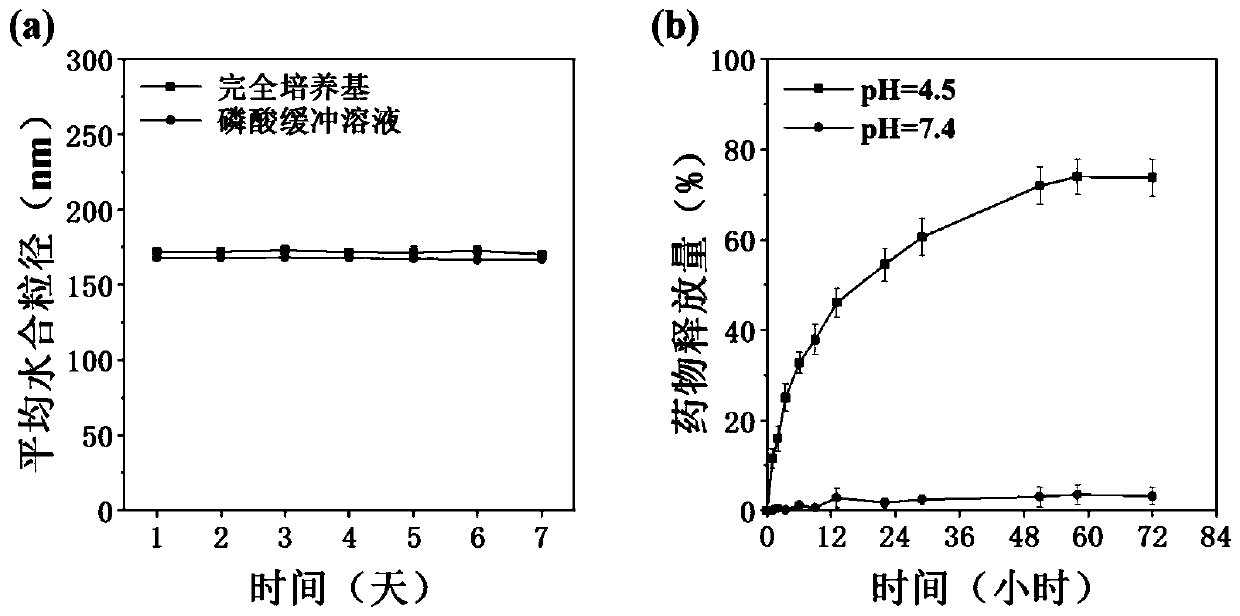
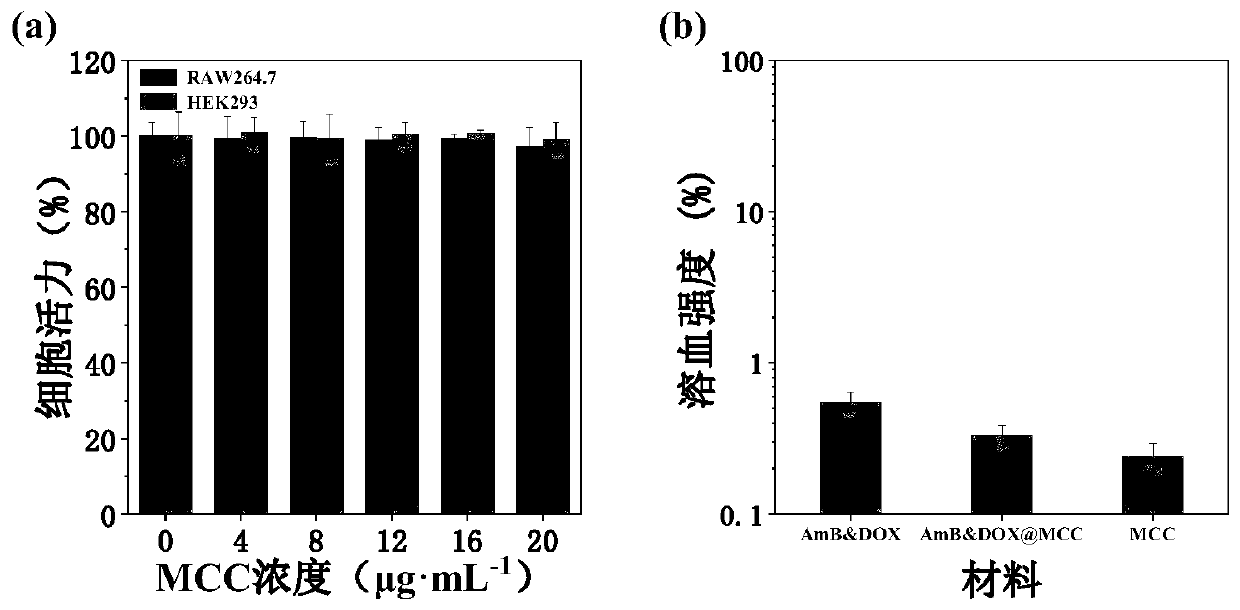
Targeted drug composition for co-loading amphotericin B and adriamycin and application of targeted drug composition
ActiveCN110801433AAchieving Synergistic TherapyImplement fixed-point releaseOrganic active ingredientsAntiparasitic agentsSide effectCyclodextrin
The invention discloses a targeted drug composition for co-loading amphotericin B and adriamycin and an application of the targeted drug composition, and belongs to the fields of medicines and pharmaceutics, and provides a novel medical composition for treating leishmaniasis. Two medicines of amphotericin B and adriamycin having fluorescent tracing properties are used and loaded in a pharmaceutical carrier material which is favorable in biological safety and has pH responsiveness to form the medical composition, wherein the carrier material consists of hydrophilicity mannose residues which actively target macrophages, beta-cyclodextrin and hydrophobic propionyl. The medical composition is good in biological safety (cytotoxicity and hemolytic activity), and the stability, pH response medicine release properties, target capacity, and treatment efficiency are verified in a cell model. Besides, the synergistic reaction of the amphotericin B and the adriamycin is realized, so that the consumption of the medicine is greatly reduced, the side effect is reduced, the economic burden of patients is alleviated, and the targeted drug composition is hopeful to exert great effects on clinical application.
Owner:JIANGNAN UNIV
Thio semicarbazone and semicarbozone inhibitors of cysteine proteases and methods of their use
Owner:RGT UNIV OF CALIFORNIA
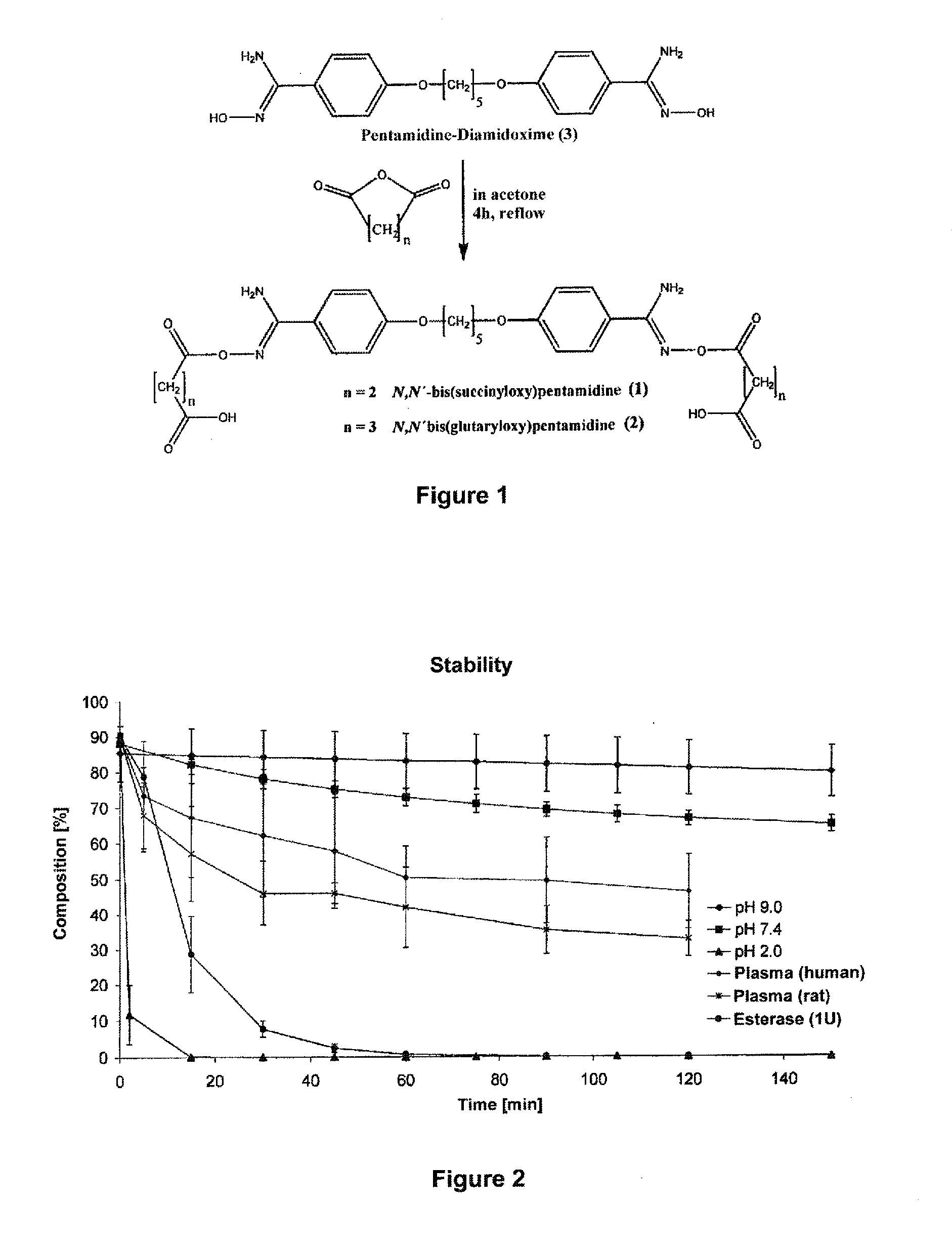
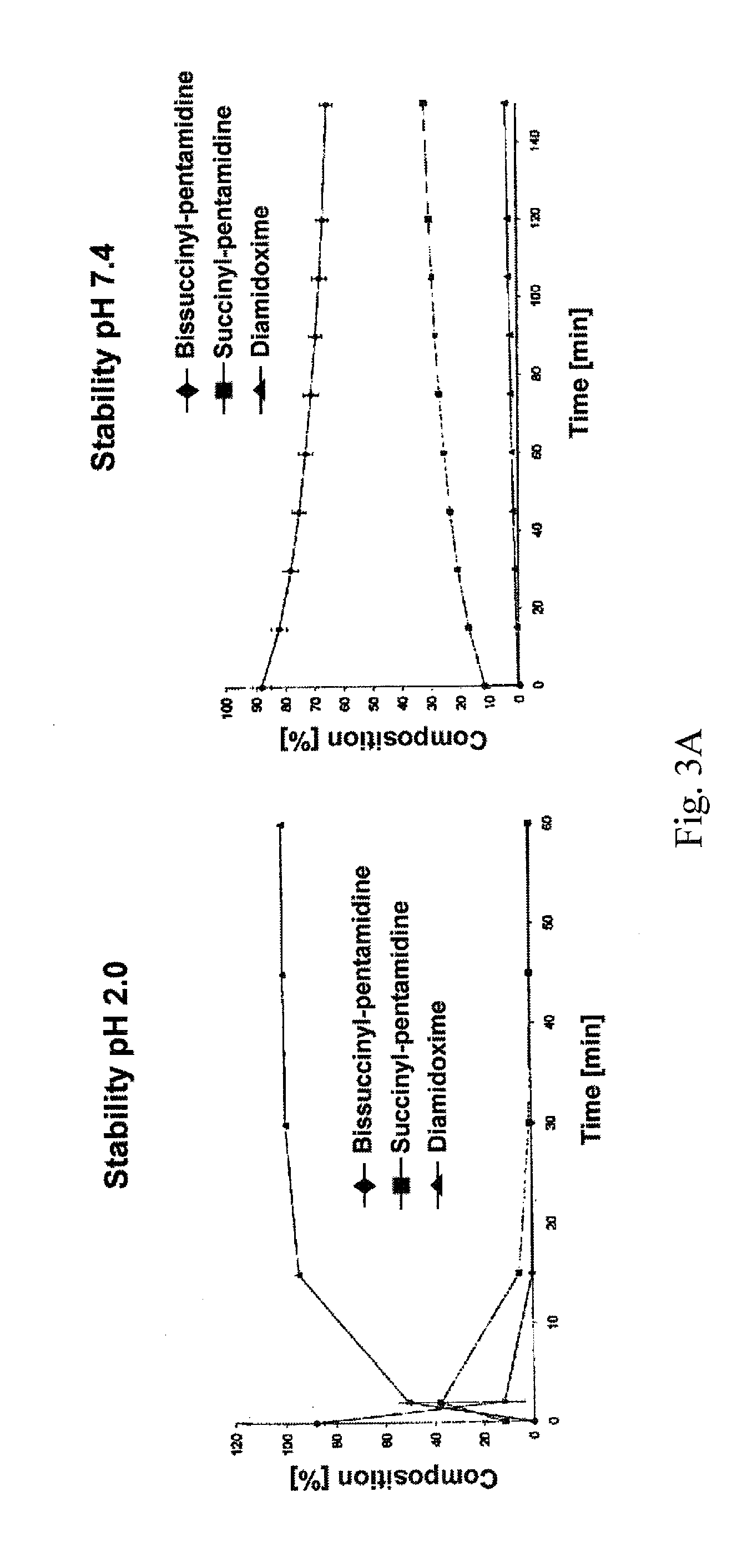
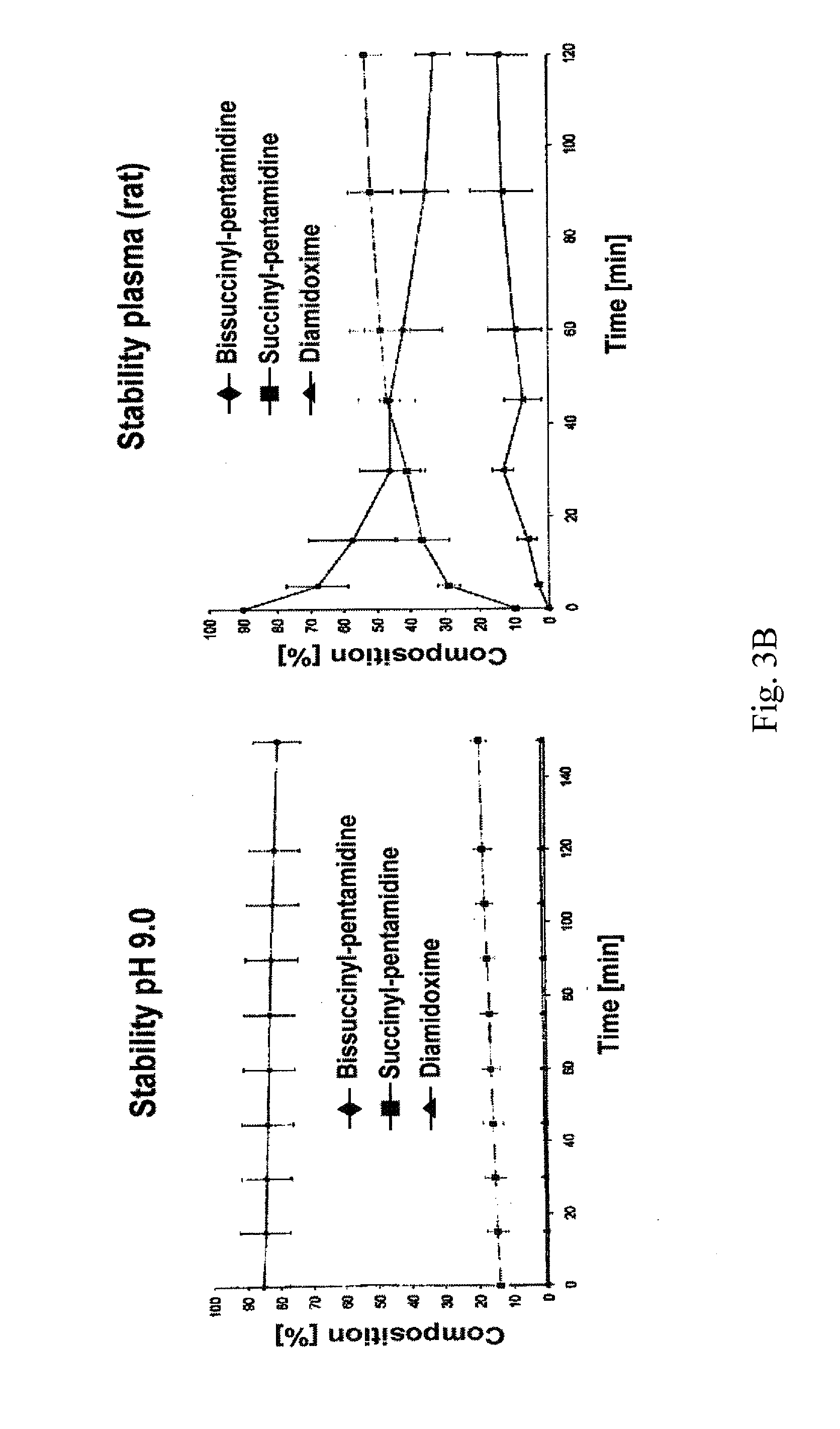
Oral bioavailable pentamidin prodrugs for treatment of diseases
InactiveUS20130085180A1Improve solubilityImprove oral bioavailabilityOrganic active ingredientsBiocideDiseaseLeishmaniasis
The present invention relates to prodrug derivatives of pentamidine, their use in the treatment and / or prophylaxis of diseases such as tumor diseases, as well as leishmaniasis, trypanosomiasis, pneumocystis carinii pneumonia (PcP), and malaria.
Owner:DRITTE PATENTPORTFOLIO BET GMBH & CO KG
Chimeric molecule useful in immunotherapy for leishmaniasis, which includes a fragment of the PFR1 protein of leishmania infantum with specific immunodominant epitopes
The present invention claims an isolated nucleotide sequence characterized by encoding the PFR1 protein of Leishmania infantum or a fragment thereof. This PFR1 protein or a fragment thereof comprises at least a selected immunodominant epitope between the following group: SEQ ID No: 1, SEQ ID No: 2, SEQ ID No: 3, SEQ ID No: 4, SEQ ID No: 5, SEQ ID No: 6, SEQ ID No: 7 and SEQ ID No: 8, where the immunodominant epitope is able to induce an antigen-specific T cell cytotoxic immune response in an animal, against the kinetoplastids causing the leishmaniasis disease. The immunodominant epitopes are cytotoxic T-lymphocyte activators and they present a high binding affinity for A2 type MHC Class I molecule.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com