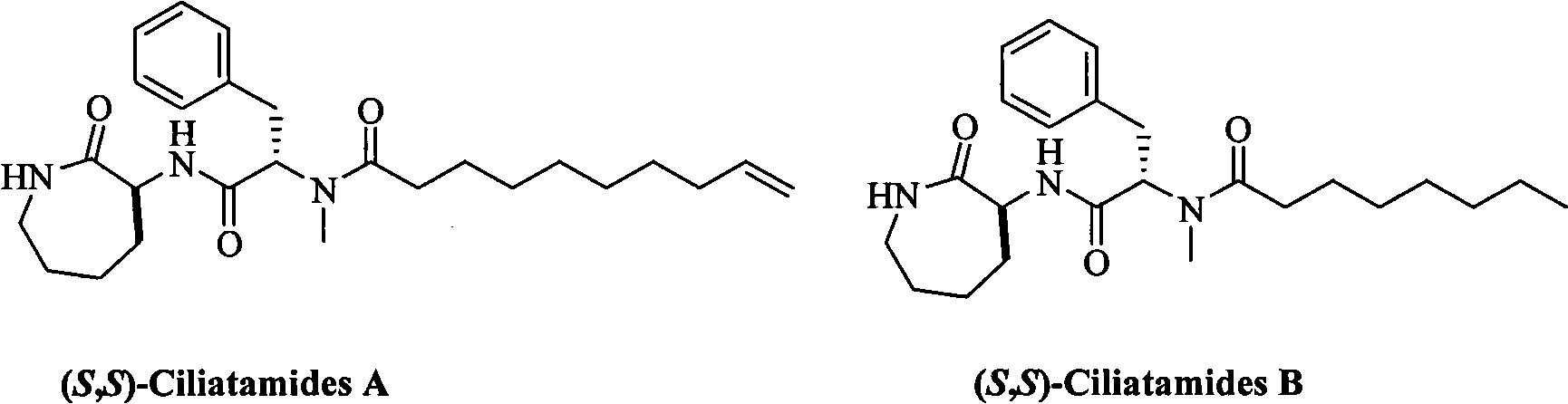
Process for preparing restraining agent for leishmaniasis
A leishmaniasis and inhibitor technology, applied in the chemical field, can solve problems such as lack of broad-spectrum, difficulty in mass production, difficult purification of cyclization products, etc., and achieve the effect of simple operation and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
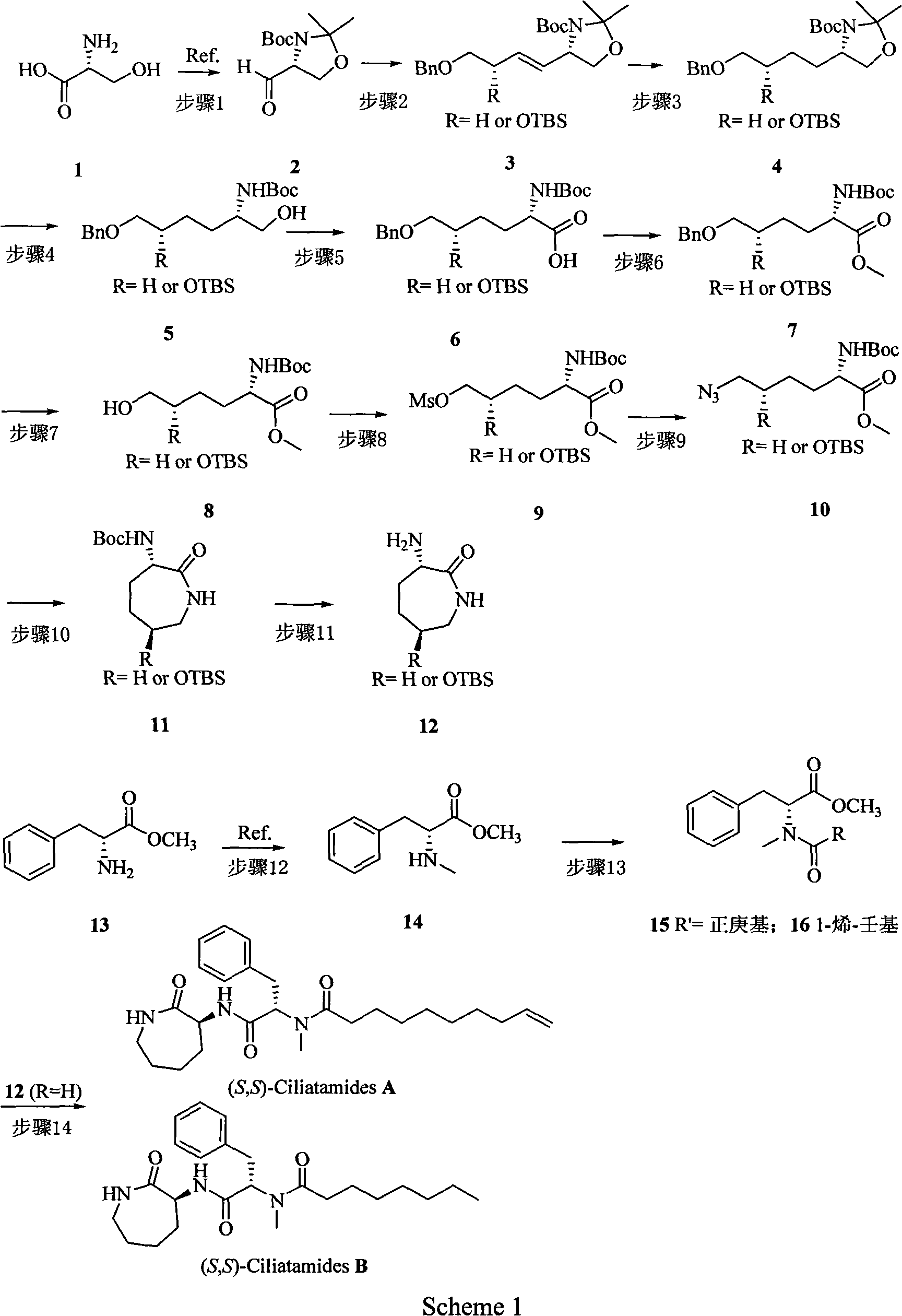
[0031]Step 1 Synthesis of (R)-tert-Butyl 4-formyl-2, 2-dimethyloxazolidine-3-carboxylate 2
[0032] This compound was prepared directly according to literature methods (Carner, P. et al., J. Org. Chem. 1987, 52, 2361-2364).
[0033] Step 2 Synthesis of (S, E)-tert-butyl 4-(4-(benzyloxy)but-1-enyl)-2,2-dimethyloxazolidine-3-carboxylate 3(R=H)
[0034] In a nitrogen atmosphere and at -78°C, a tetrahydrofuran solution in which compound 2 (2.42g, 10.55mmol) was dissolved was added dropwise to tetrahydrofuran in which 3-benzyloxypropane quaternary phosphine salt (9g, 18.3mmol) was dissolved, and after heating Stir overnight, the reaction system was diluted with water, extracted three times with ethyl acetate, the organic layer was washed three times with saturated brine, anhydrous Na 2 SO 4 After drying and concentration, the crude product was purified by silica gel column chromatography to obtain compound 3 (R=H) (85.0%).
[0035] Step 3 Synthesis of (S)-tert-butyl 4-(4-(benzyl...
Embodiment 2
[0059] Step 2 Synthesis of (S)-tert-butyl-4-((S,E)-4-(benzyloxy)-3-(tert-butyl dimethylsilyloxy)but-1-enyl)-2,2-dimethyloxazolidine-3-carboxylate 3 (R=OTBS)
[0060] In a nitrogen atmosphere and at -78°C, a solution of compound 2 (13 mmol) in tetrahydrofuran was slowly added dropwise to a solution of 1 benzyloxy-2-tert-butyldimethylsiloxypropane quaternary phosphine salt (25 mmol). tetrahydrofuran, warmed up and then stirred overnight. The reaction system was diluted with water, extracted three times with ethyl acetate, the organic layer was washed three times with saturated brine, anhydrous Na 2 SO 4After drying and concentration, the crude product was purified by silica gel column chromatography to obtain compound 3 (R=OTBS) (78.0%).
[0061] Step 3 Synthesis of (S)-tert-Butyl 4-((S)-4-(benzyloxy)-3-(tert-butyl dimethylsilyloxy)butyl)-2,2-dimethyloxazolidine-3-carb-oxylate 4(R=OTBS ).
[0062] Compound 3 (R=OTBS) (3.62 mmol) was dissolved in EtOH (15 mL), and 47 mg of 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com