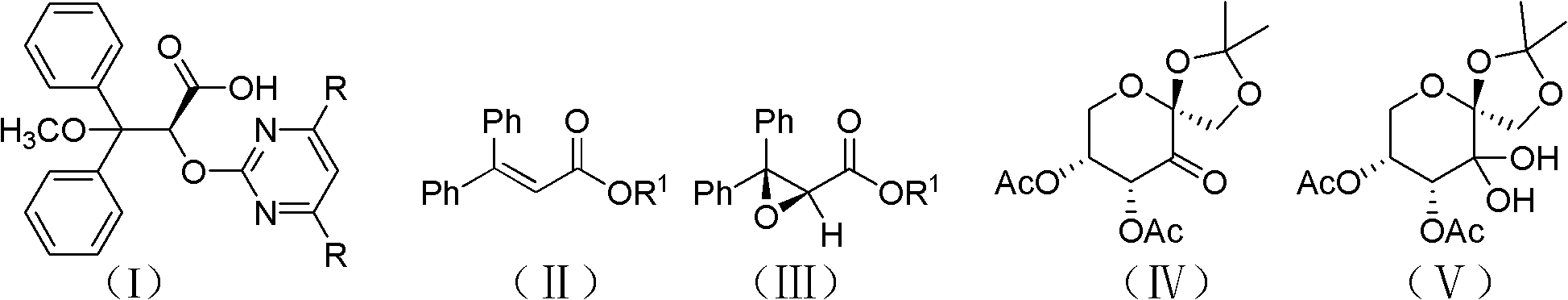
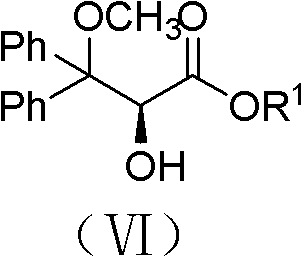
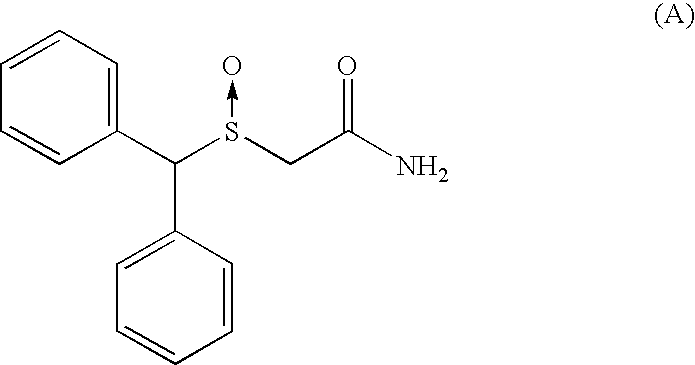
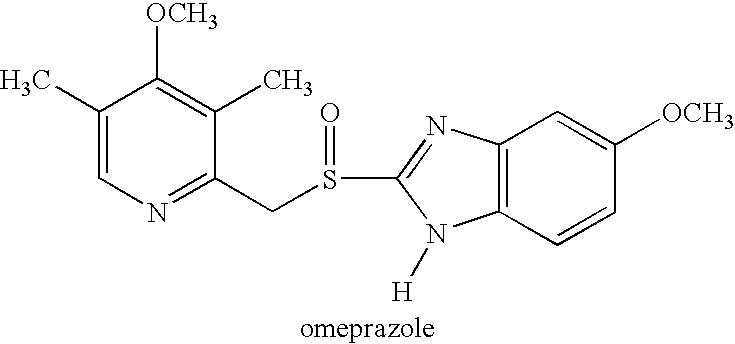
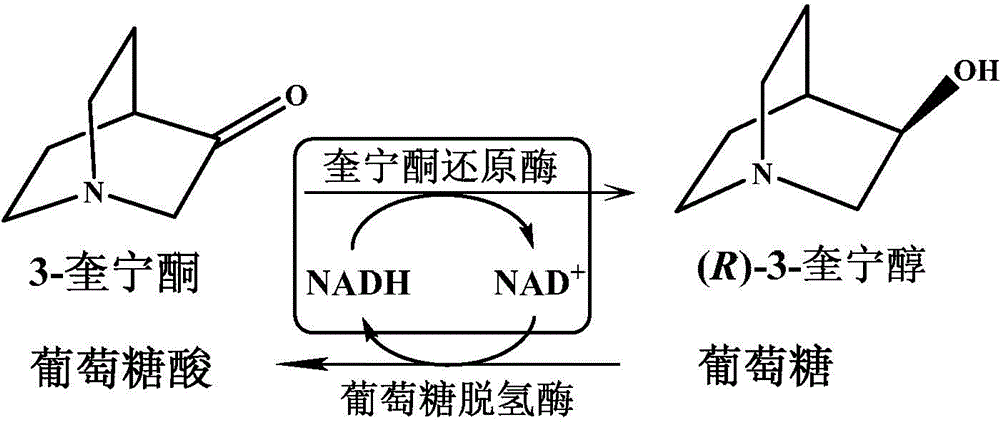
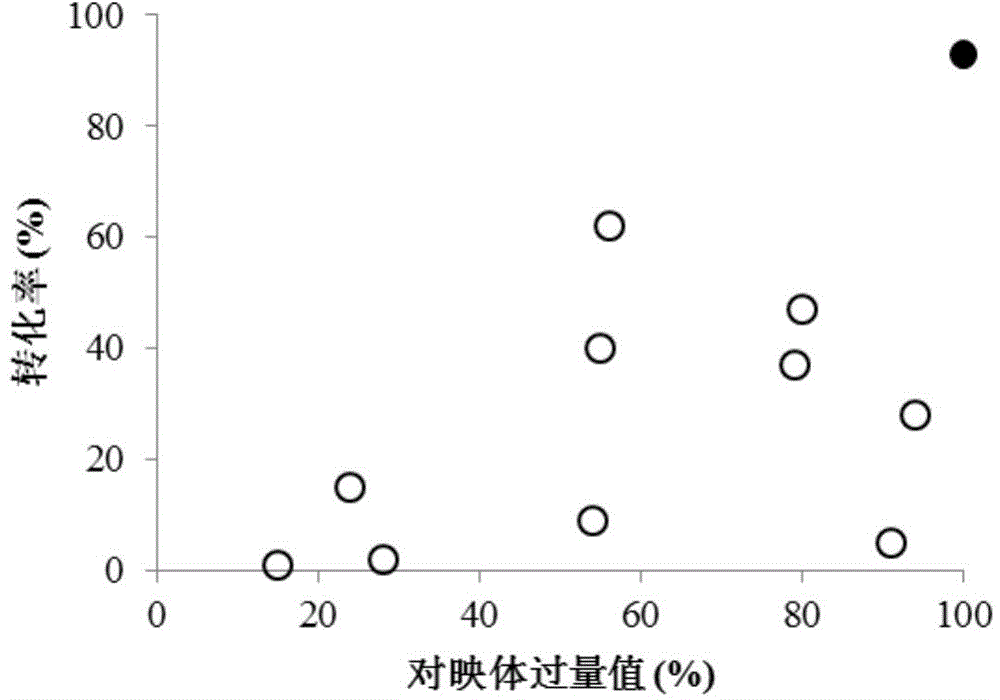
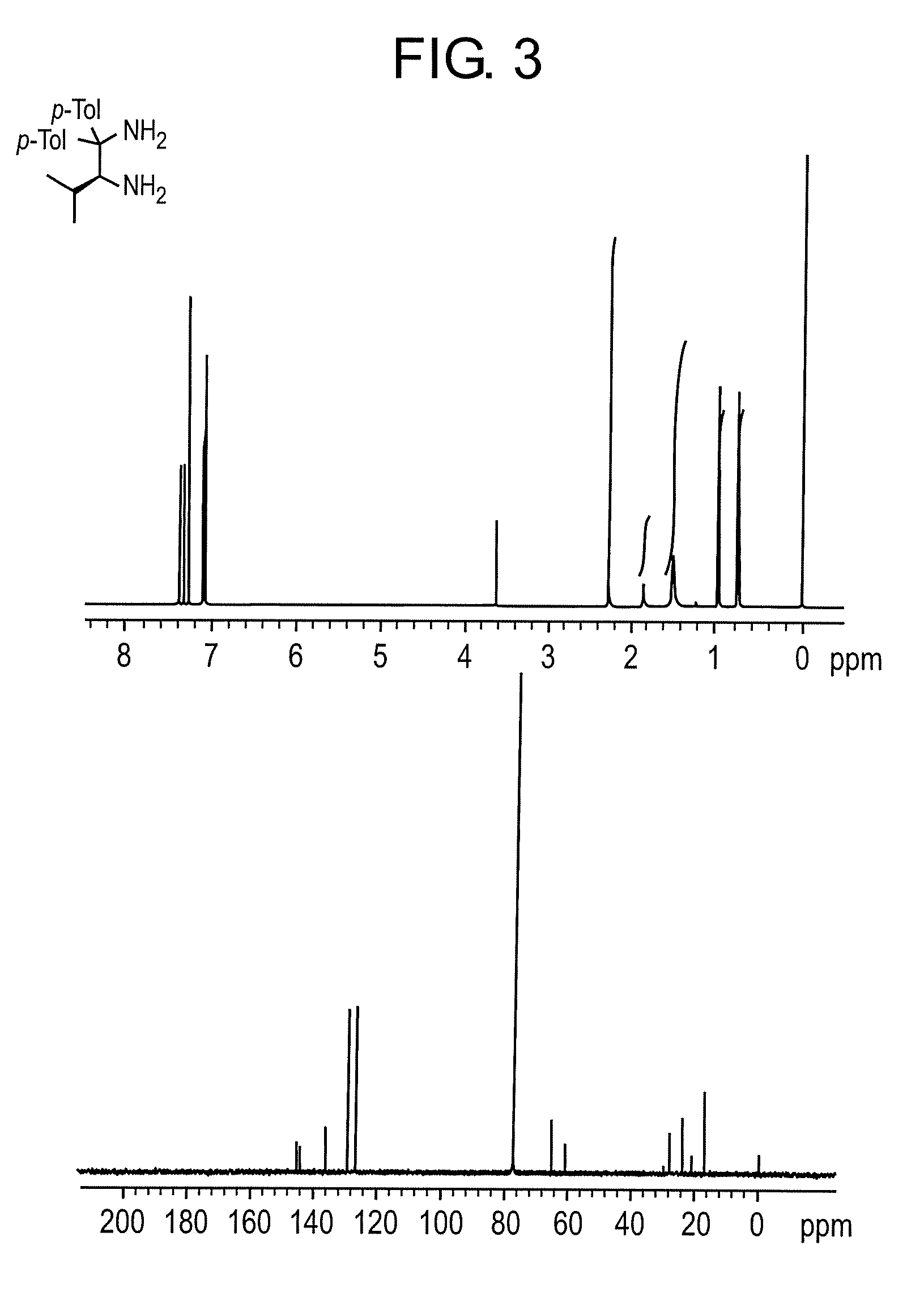
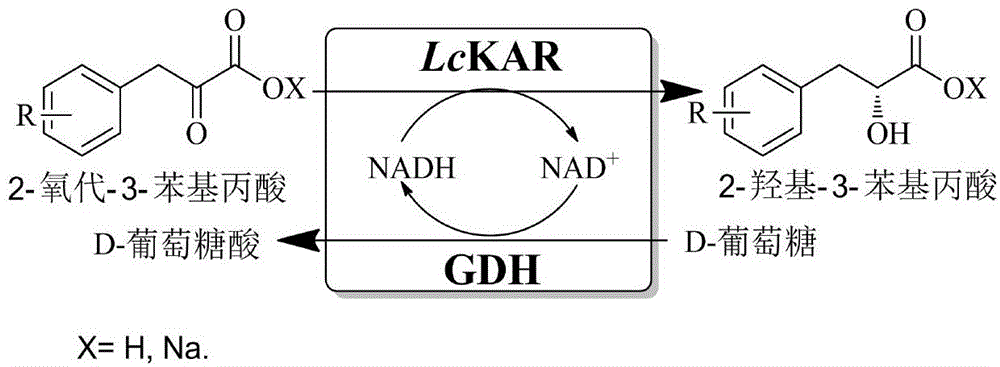
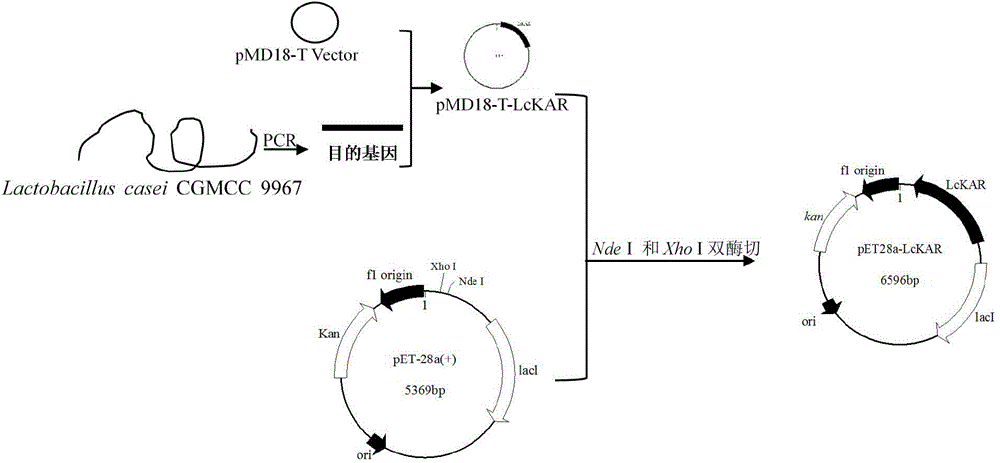
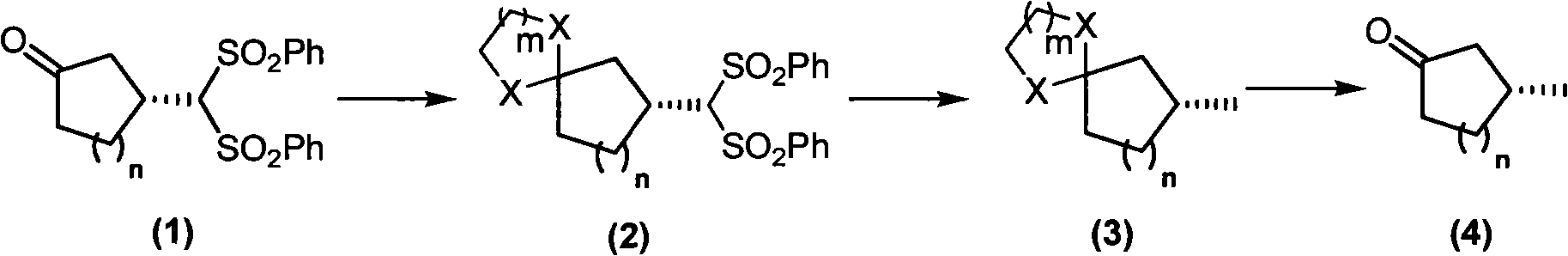
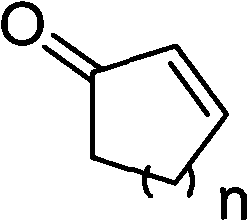
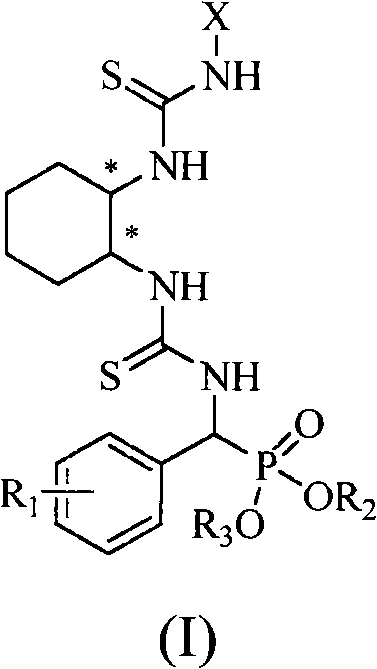
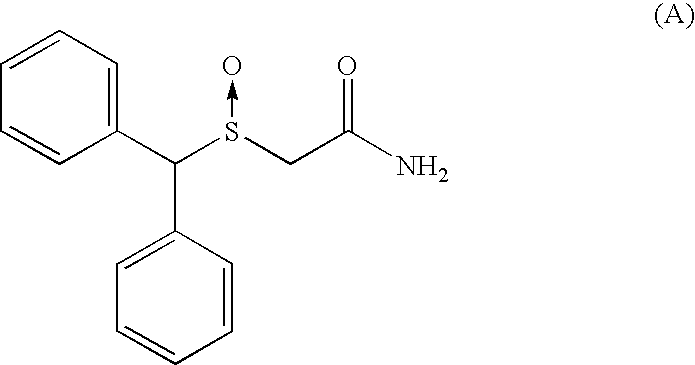
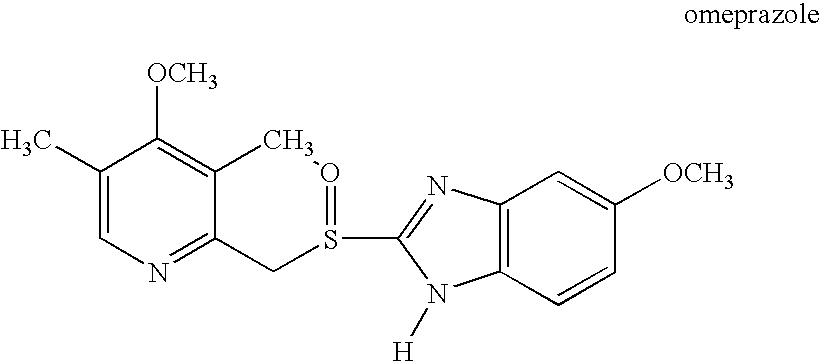
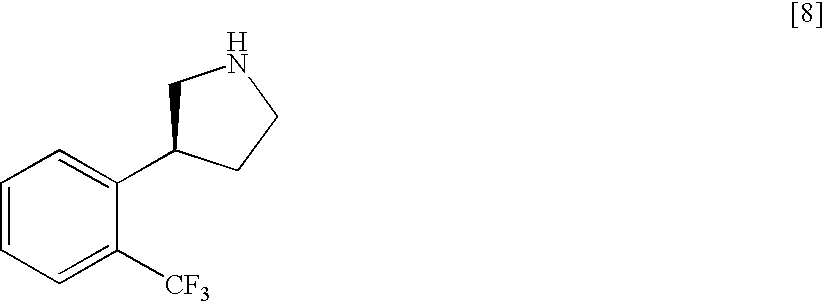
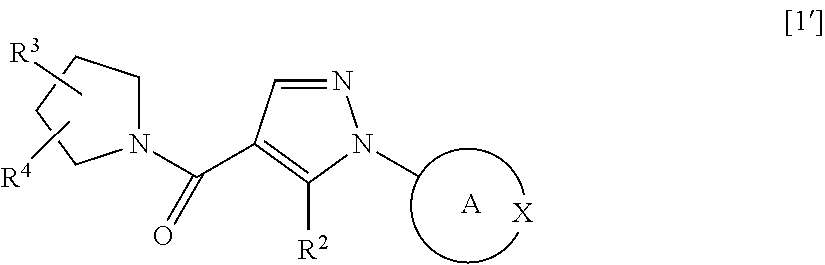
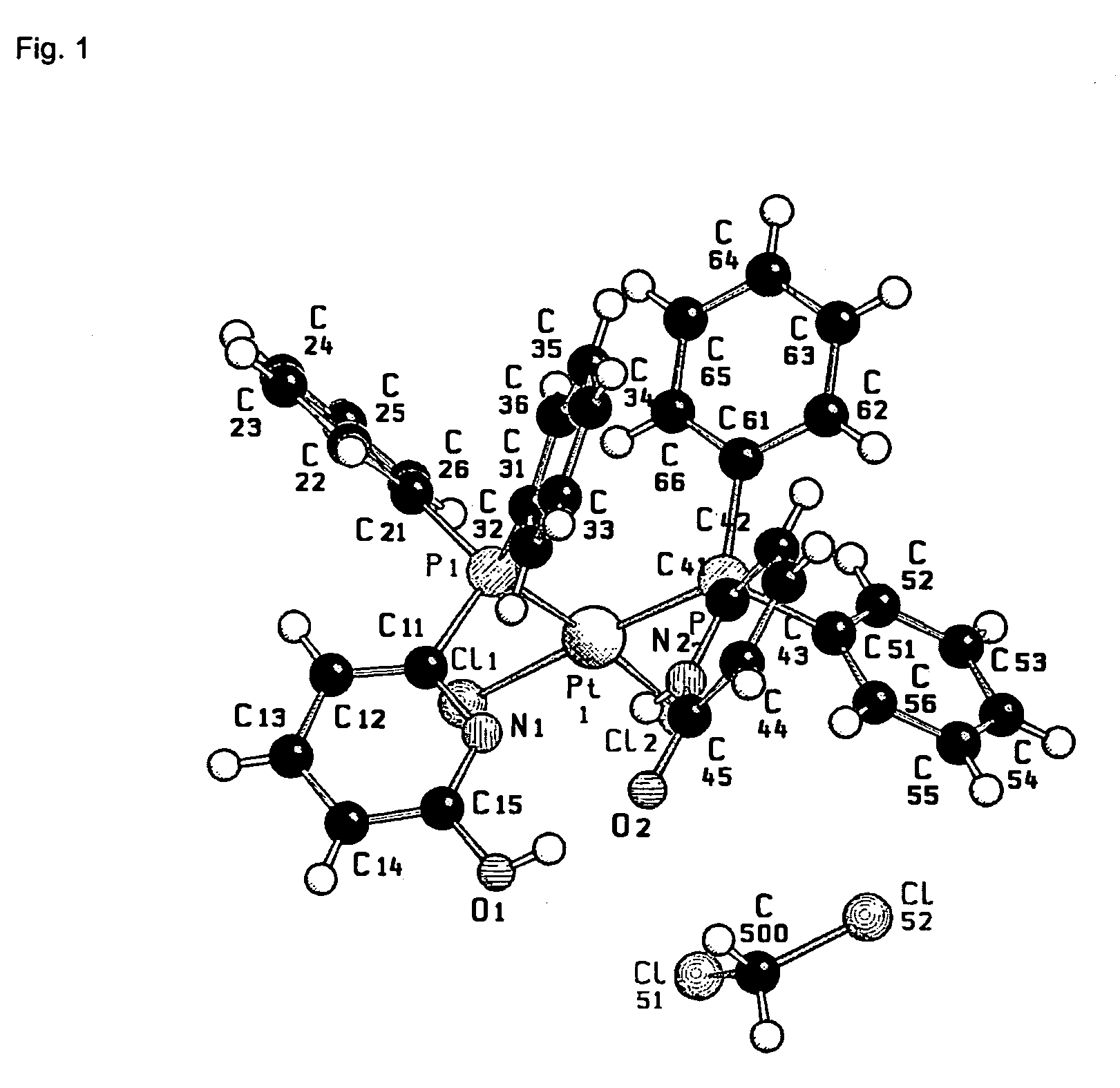
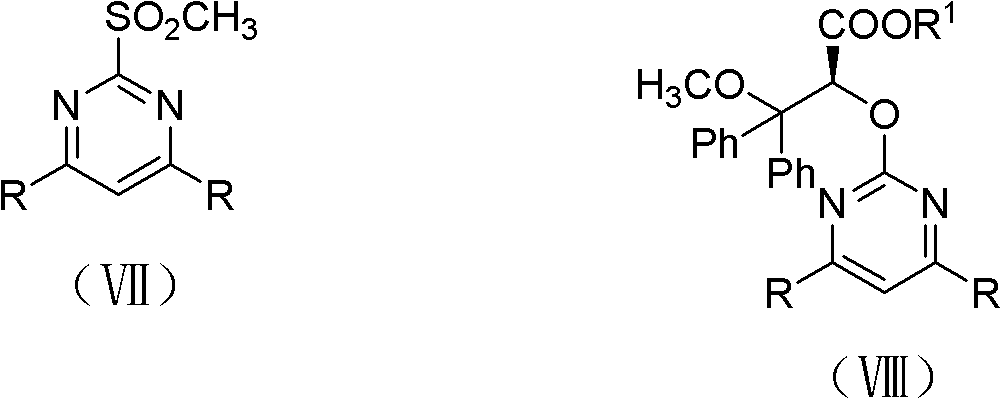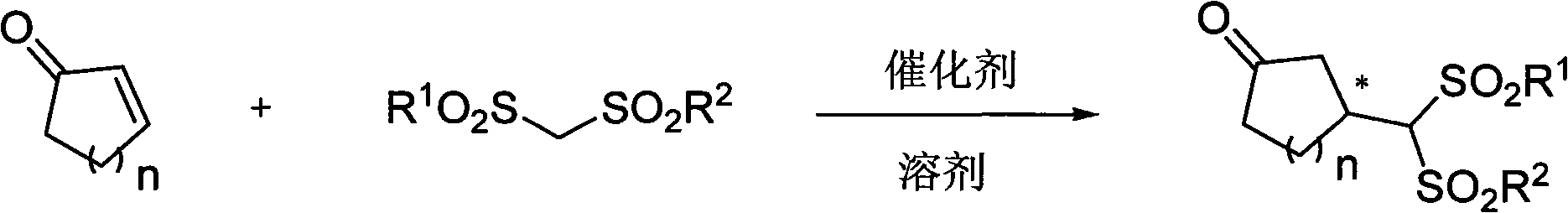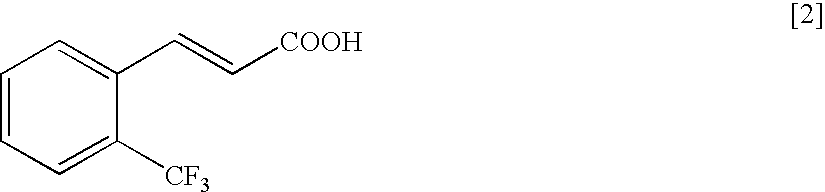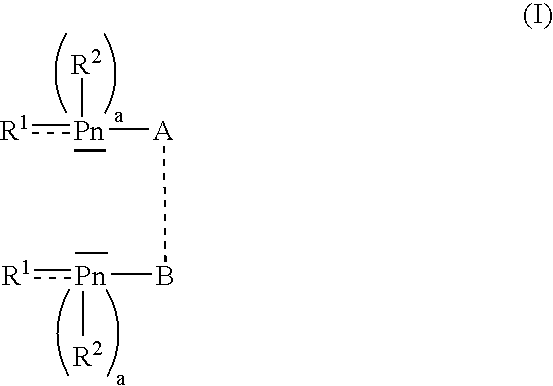Patents
Literature
269 results about "Enantioselective synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.
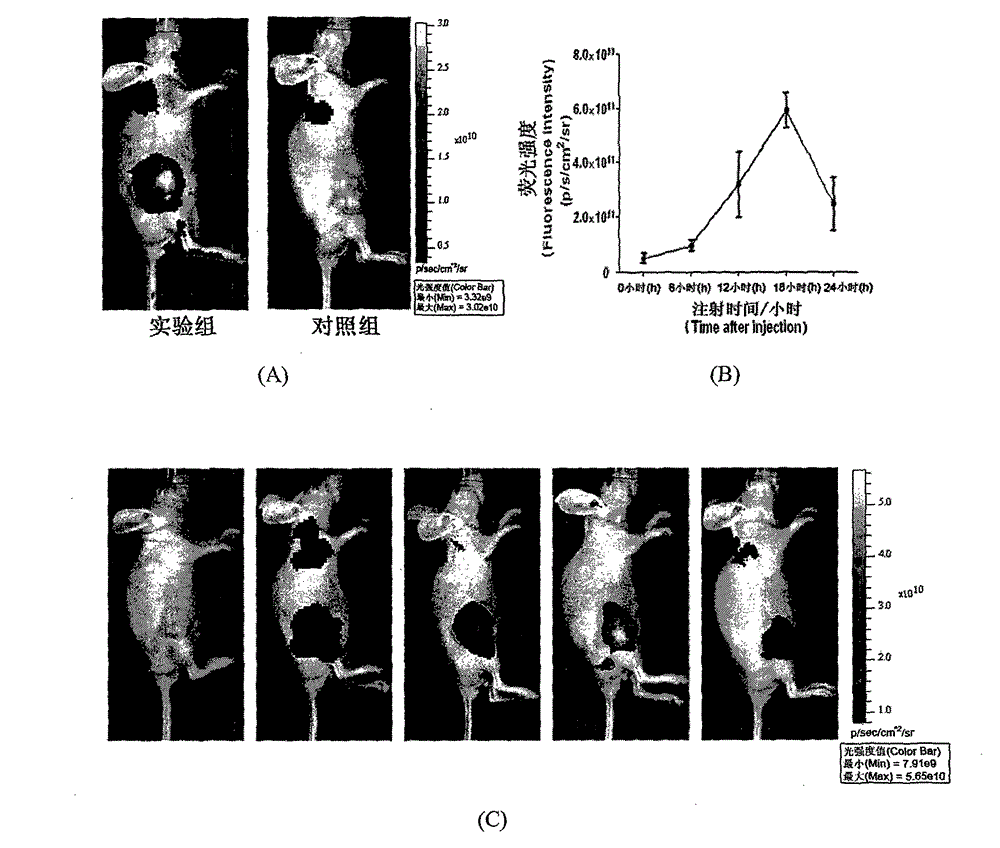
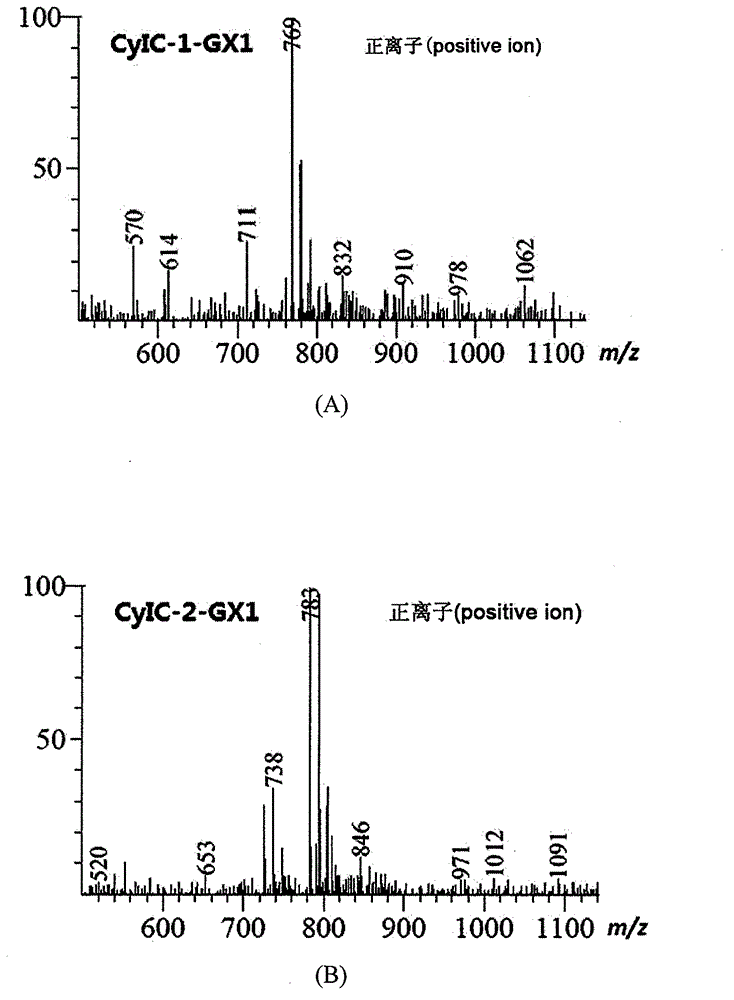
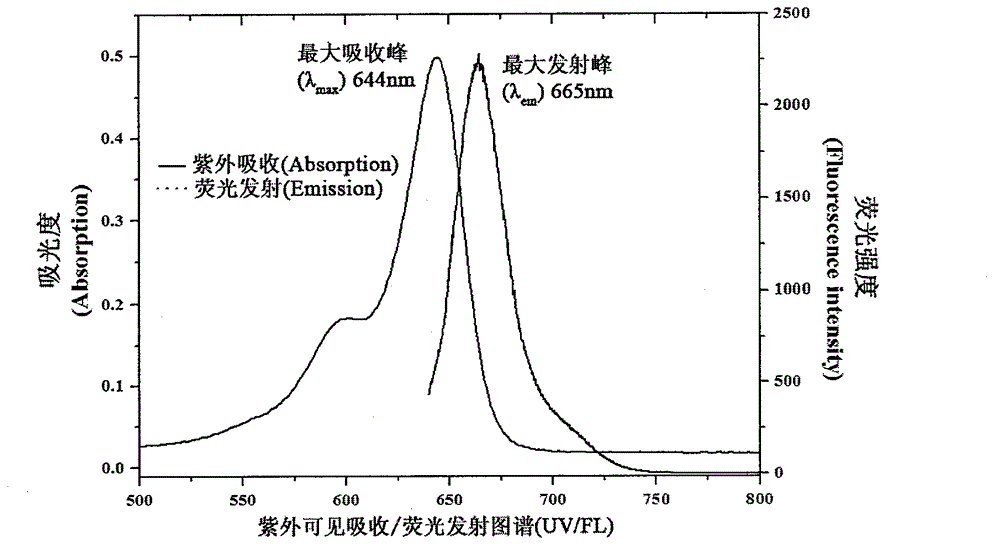
Symmetric pentamethyl cyanine dye and application thereof to molecular imaging
InactiveCN103146220AThe synthesis steps are simpleEasy to purifyMethine/polymethine dyesIn-vivo testing preparationsSynthesis methodsActive cell
A symmetric pentamethyl cyanine dye with a general formula I and application thereof to molecular imaging belong to the fields of organic compound and optical molecular imaging. In the general formula I, X represents Cl or S (CH2)nCOOH or II; R1 represents C1-6 alkyl, (CH2) pOR3 or (CH2) pC6H5; R2 represents H, methyl, hydroxy, halogen, nitro, benzyloxy, alkoxy or a water-soluble group SO3R4; and R4 represents H, sodium ion or potassium ion; and Y<-> represents halogen ion, PF6<-> or TsO<->. An asymmetric synthesis method is employed to prepare a pentamethyl cyanine dye fluorescence probe with the general formula I, in order to solve the problems of an asymmetric synthesis method, such as tedious steps, by-product and low yield. The dye has advantages of low toxicity, active cell membrane permeability and good light stability, and can be used for labeling live cells and in the field of molecular imaging.
Owner:XIDIAN UNIV
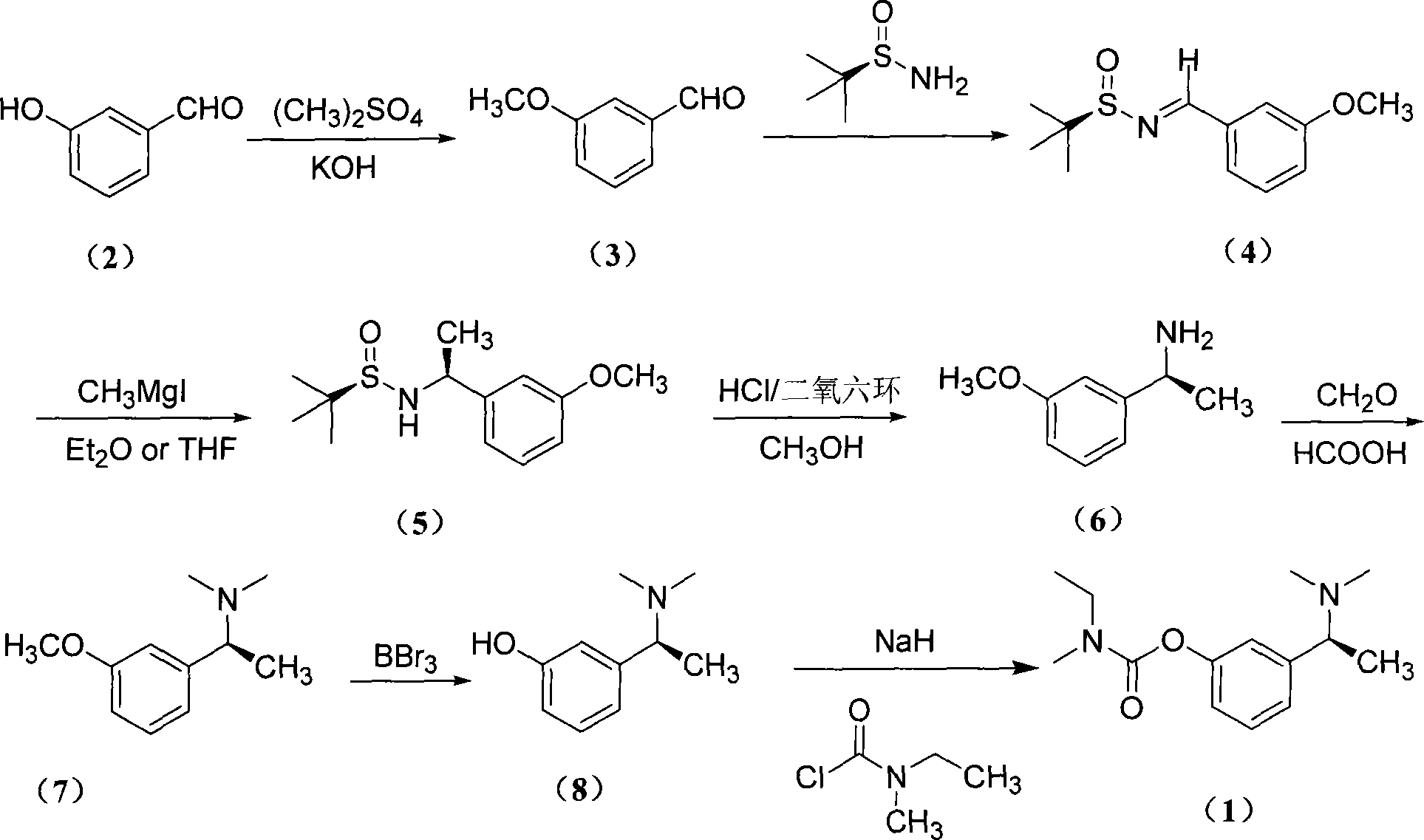
Asymmetric synthesis method of (S)-rivastigmine
InactiveCN101134738AFew reaction stepsHigh reaction yieldCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsGrignard reagent
The present invention is asymmetrical (S)-carbalatine synthesizing process including the following steps: protecting the phenolic hydroxyl group of m-hydroxy benzaldehyde, reacting with chiral tert-butyl sulfonamide to produce (R, E)-3-methoxyl-phenyl methylene tert-butyl sulfonamide, addition reacting with methyl Grignard reagent, hydrolyzing, Eschweiler-Clarke methylation reaction, demethylating BBr3 to synthesize important intermediate (S)-1-(3-hydroxy phenyl)-N, N-dimethyl ethyl amine, and esterification with methyl ethyl carbamyl chloride to obtain (S)-carbalatine. The present invention has less reaction steps, high yield, total yield up to 21.85 %, and low cost.
Owner:JINAN UNIVERSITY
A preparation method of optically pure (+)-ambisentan and optically pure (+)-dalusentan
ActiveCN102276536AEnvironmentally friendlyHigh yieldOrganic chemistryFructoseEnantioselective synthesis
Disclosed is a method for preparing optically pure (+)-ambrisentan and (+)-darusentan, comprising: firstly catalyzing the asymmetric epoxidation of a β-unsaturated alkene using a chiral ketone derived from fructose or a hydrate thereof as a catalyst, and then subjecting the product to an epoxy compound ring-opening reaction and substitution reaction successively to obtain optically pure (+)-ambrisentan and (+)-darusentan.
Owner:INST OF CHEM CHINESE ACAD OF SCI
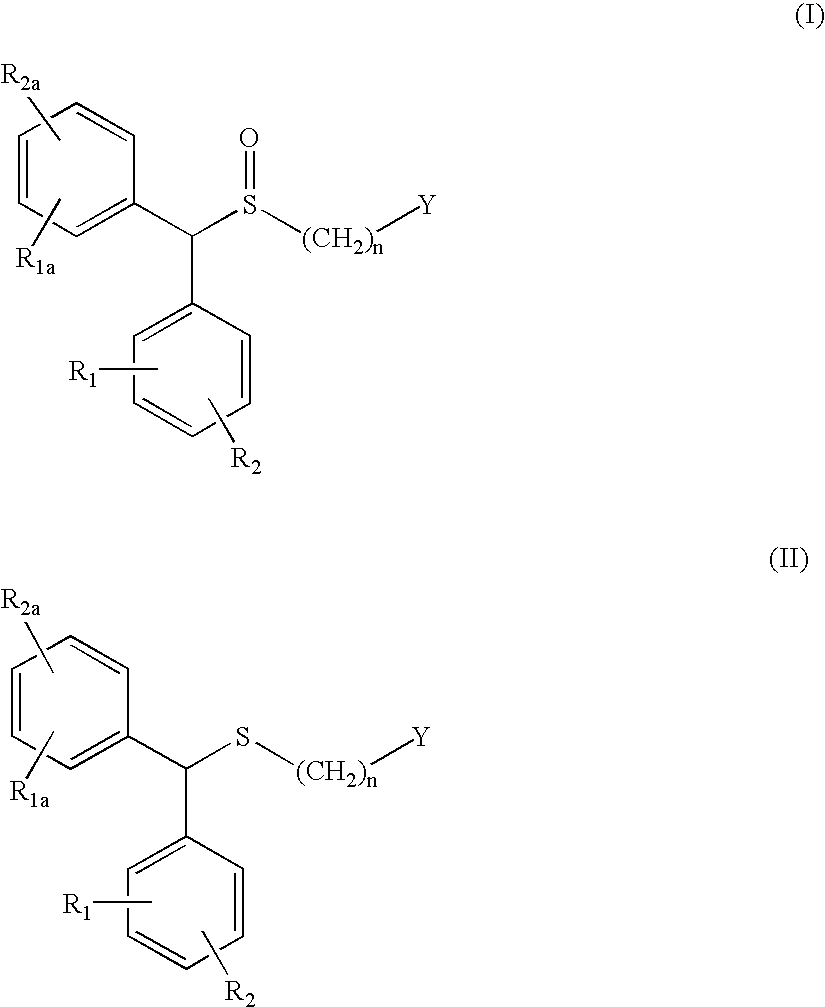
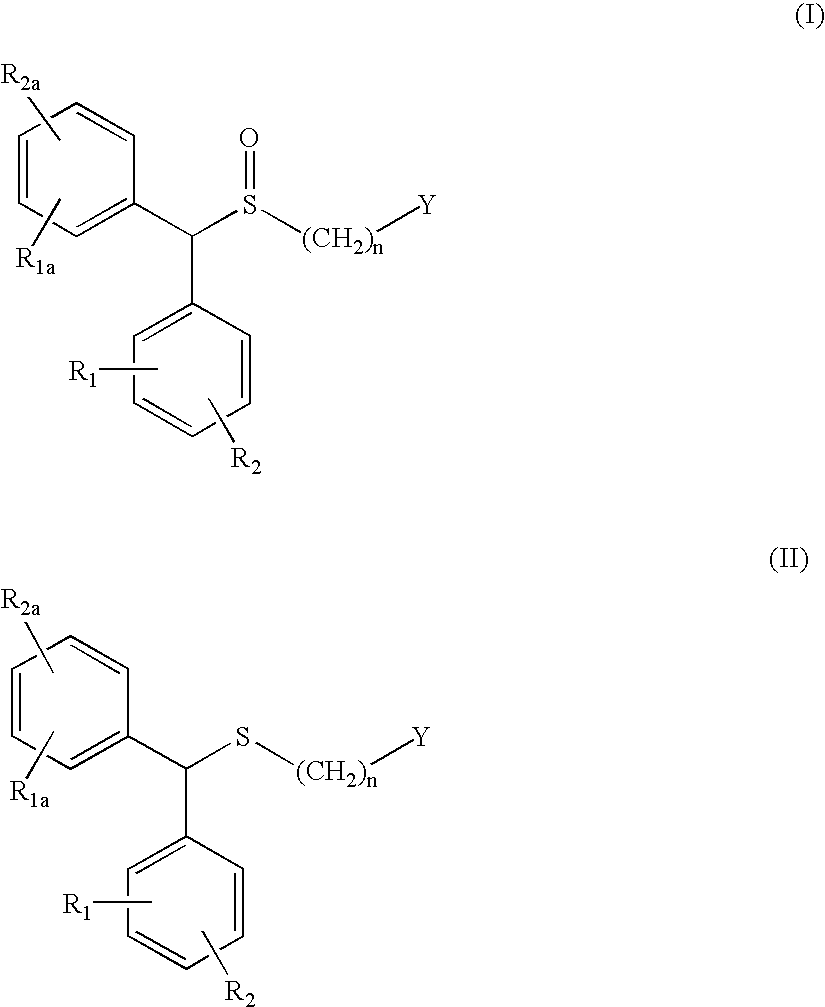
Process for enantioselective synthesis of single enantiomers of modafinil by asymmetric oxidation
InactiveUS7317126B2High yieldOrganic compound preparationOrganic chemistry methodsOrganic solventEnantiomer
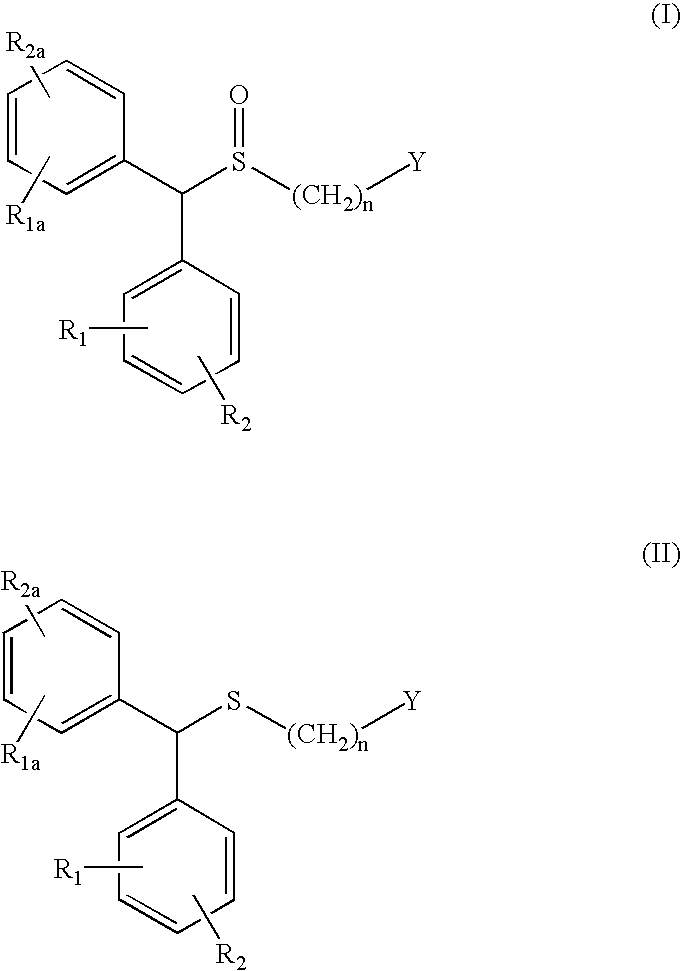
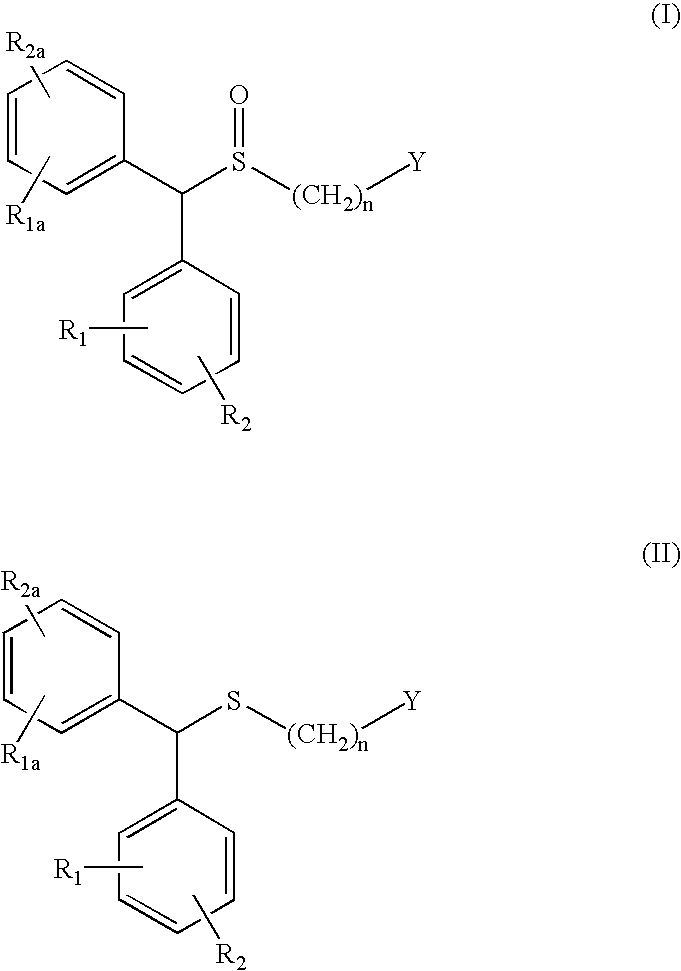
The invention relates to a method for preparing a sulphoxide compound of formula (I) either as a single enantiomer or in an enantiomerically enriched form, comprising the steps of:a) contacting a pro-chiral sulphide of formula (II) with a metal chiral complex, a base and an oxidizing agent in an organic solvent; and optionallyb) isolating the obtained sulphoxide of formula (I);wherein n, Y, R1, R1a, R2 and R2a are as defined herein.
Owner:TEVA SANTE
Quininone reductase and application thereof to asymmetric synthesis of (R)-3-quinuclidinol
ActiveCN103555608AIncrease concentrationMild reaction conditionsBacteriaMicroorganism based processesAnticholinergic DrugsEnantioselective synthesis
The invention discloses anagrobacterium radiobacter, a quininone reductase expressed thereby and a gene thereof, recombinant expression plasmid containing the gene, recombinant expression vector containing the gene, a recombinase of quininone, a preparation method of the recombinase , and application of the recombinase to asymmetric reduction of 3-quininone as a catalyst for preparation of (R)-3-quinuclidinol. Compared with other preparation methods for (R)-3-quinuclidinol, the (R)-3-quinuclidinol prepared by employing the quininone reductase provided by the invention is not only high in product concentration, but also good in optical purity; the reaction condition is mild, the operation is convenient, and the preparation is easy to amplify; and therefore the application of the quininone reductase to asymmetric synthesis of (R)-3-quinuclidinol has extremely good industrial application prospect in the production of intermediates of anticholinergic drugs.
Owner:EAST CHINA UNIV OF SCI & TECH
Inorganic mesoporous material having chiral twisted structure and process for producing the same
InactiveUS20090043003A1Easy to useAdjustable sizeMaterial nanotechnologyPolycrystalline material growthReaction fieldEnantioselective synthesis
A chiral inorganic mesoporous material characterized by having a chiral twisted structure and being mesoporous; a process for producing the material; and a method of using the material. The process for inorganic mesoporous material production is a method in which one or more polymerizable inorganic monomers selected from the group consisting of polymerizable inorganic monomers and polymerizable inorganic monomers having a functional group capable of having a charge are polymerized in the presence of a solvent using as a template a self-assembly of a chiral surfactant such as an N-(higher alkanoyl)amino acid salt. Examples of the use of the inorganic mesoporous material include the separation of racemates and reaction fields for asymmetric syntheses.
Owner:JAPAN SCI & TECH CORP
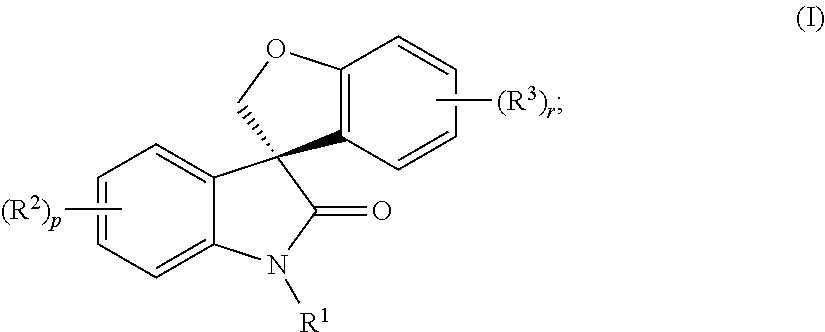
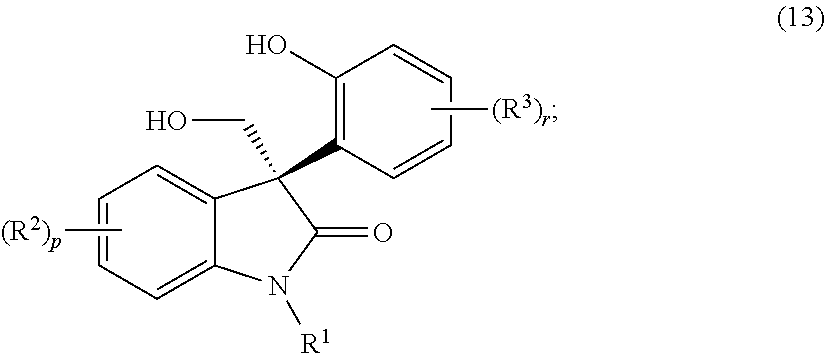
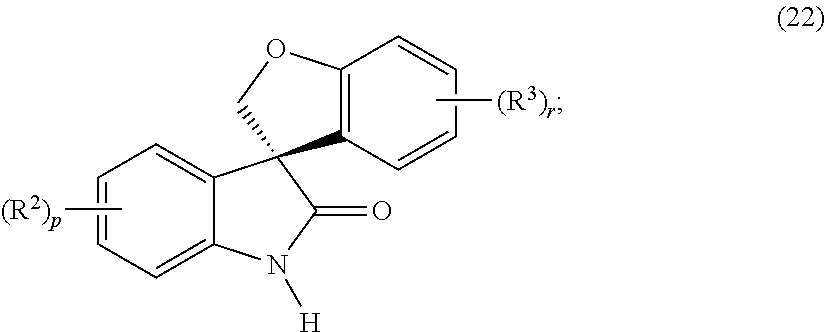
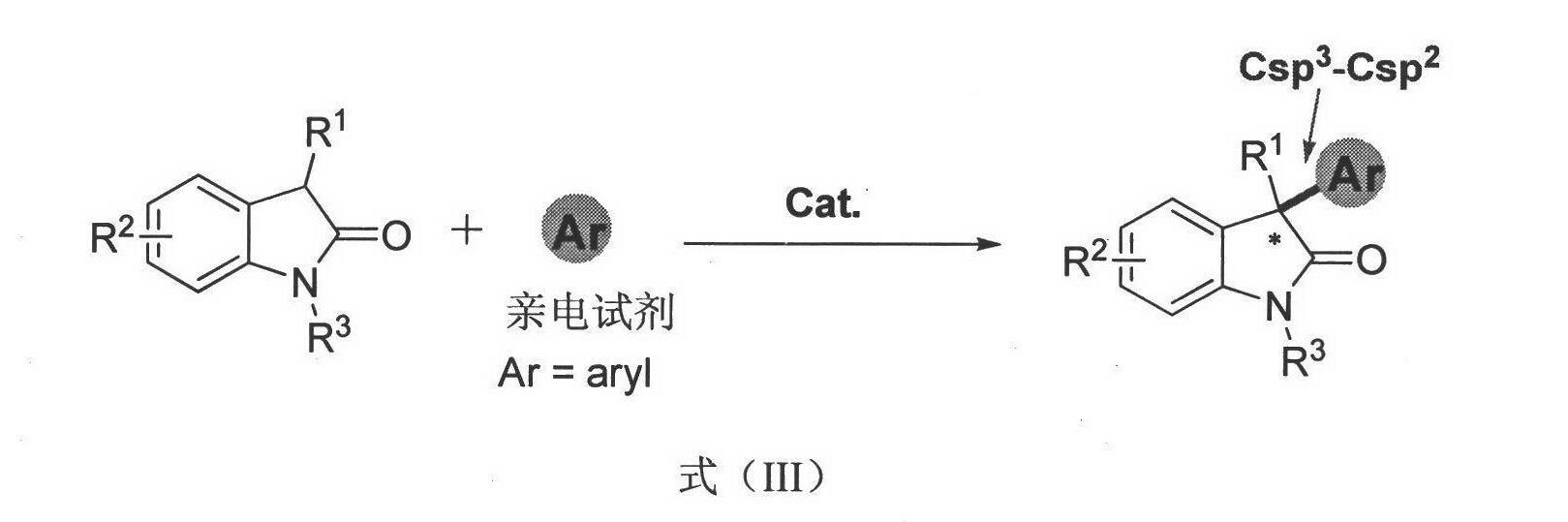
Asymmetric syntheses for spiro-oxindole compounds useful as therapeutic agents
ActiveUS20130274483A1Useful in treatmentNervous disorderOrganic chemistryDiseaseEnantioselective synthesis
Owner:PACIRA THERAPEUTICS INC
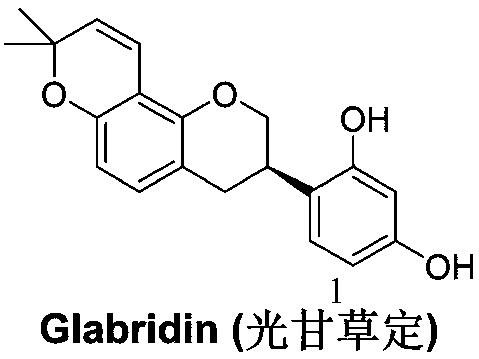
Method for asymmetrically synthesizing glabridin with optical purity under catalysis of ruthenium compound
ActiveCN108440553AHigh yieldHigh stereoselectivityOrganic chemistry methodsBulk chemical productionAbsolute configurationTrifluoroacetic acid
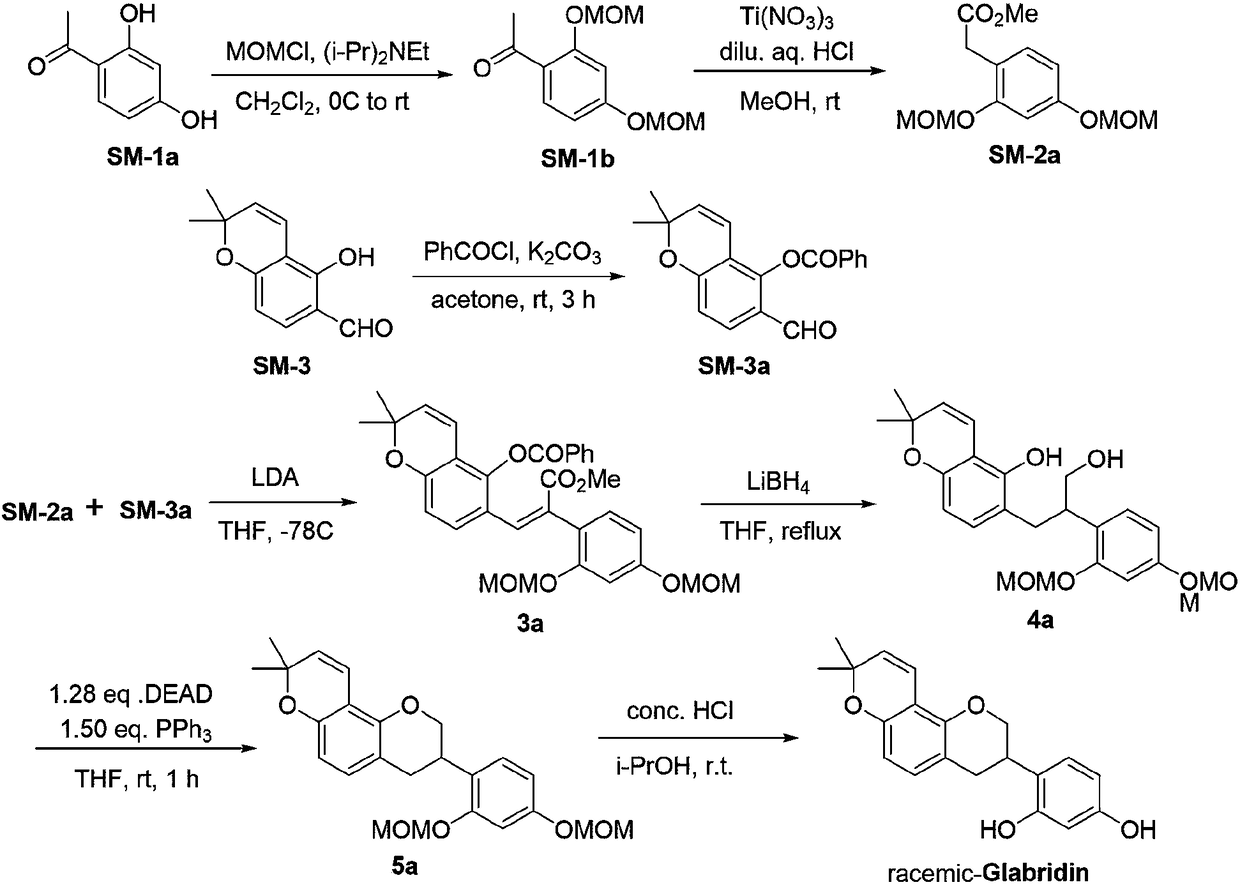
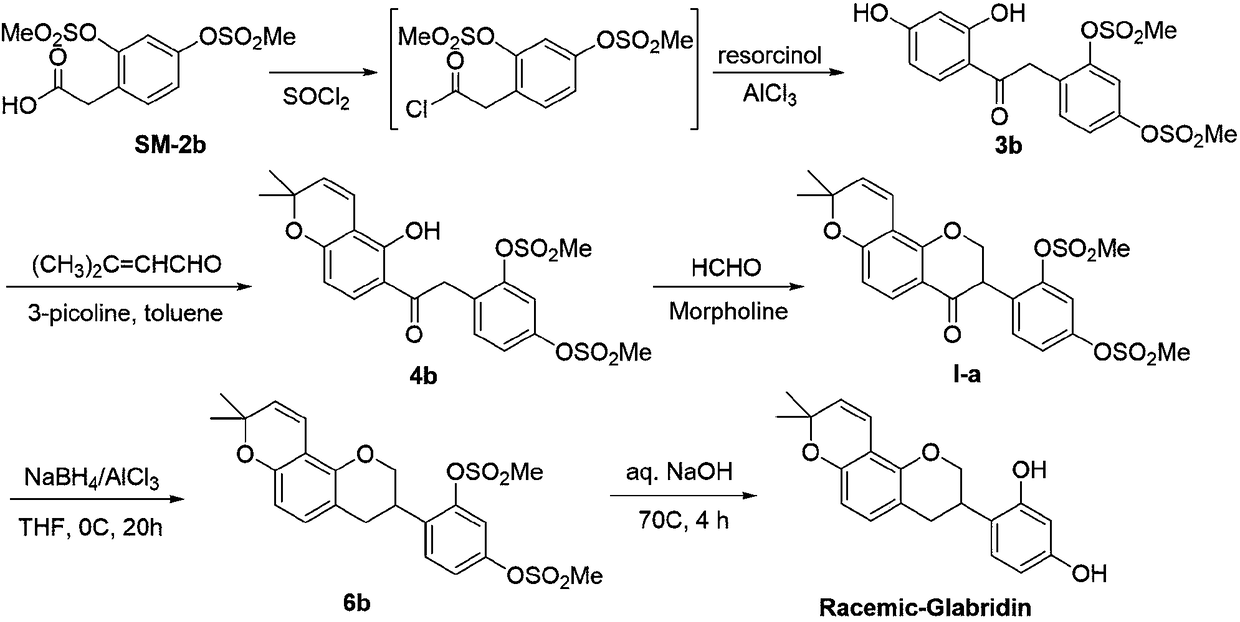
The invention relates to a method for asymmetrically synthesizing glabridin with optical purity under catalysis of a ruthenium compound. The method comprises the following steps: 1) taking isoflavoneprotected by a protection group as a raw material and carrying out dynamic kinetic asymmetric hydrogen transfer reaction under the catalysis effect of a ruthenium trichloride compound and the action of an acid-alkali buffering system to obtain chiral isoflavol with an absolute configuration being (3R, 4R); 2) removing hydroxyl of the chiral isoflavol under the action of triethylsilane and trifluoroacetic acid to obtain a product with an absolute configuration being (R); 3) removing a protection group of the product with the configuration being (R) in step 2) under an acidic or alkaline condition to obtain the glabridin with the configuration (R) and the optical purity. The method provided by the invention can be used for synthesizing the glabridin with the optical purity in a high-yield and high-stereoselectivity manner; the obtained product is completely the same as that of the glabridin extracted from glycyrrhiza glabra and can be used for replacing the glabridin derived from naturalplants to be industrially applied.
Owner:烟台六谛医药科技有限公司 +2
Silica gel bonded double-chirality active center chromatogram filler, preparation method and use thereof
The invention provides silica-bonded dual-chiral active central chromatography filler, a preparation method and applications thereof, belonging to the field of column filling. The preparation method comprises the following steps of: (1) activation of silica gel; (2) protection of microcrystalline cellulose 6-hydroxyl group; (3) carbonyl acyl chlorination; (4) derivative process of microcrystalline cellulose 2, 3-hydroxyl group and protection removal of 6-hydroxyl group; and (5) obtaining bonded chiral fixed phase by silica-bonded 2, 3-bonded 6-protection removal microcrystalline cellulose reaction and passivation of activated silica gel unreacted silanol group. The dual-chiral bonded chiral fixed phase is used for separating asymmetric synthesized chiral compounds, and n-hexane-isopropanol is used as flowing phase so as to separate the phenylethyl alcohol and ethanethioic acid, thus obtaining chromatograms. The filler, the preparation method and the application have high column efficiency, short separation time, and good separation effect on chiral compounds. The filling material generates no swelling even when in solvent, has good permeability performance and low column pressure; under the conditions of 100 percent n-hexane, flowing speed of 1ml / min and the chromatographic column of 150 multiplied by 4.6mm i.d, the column pressure is only 3.3MPa.
Owner:BEIJING UNIV OF CHEM TECH
Inorganic mesoporous materials with chiral nematic structures and preparation method thereof
The present invention describes a composition and a method for producing mesoporous silica materials with a chiral organization. In the method, a polymerizable inorganic monomer is reacted in the presence of nanocrystalline cellulose (NCC) to give a material of inorganic solid with cellulose nanocrystallites embedded in a chiral nematic organization. The NCC can be removed to give a stable porous structure that retains the chiral organization of the NCC template. The new materials may be obtained as iridescent free-standing films with high surface area. Through control of the reaction conditions, the colour of the films can be varied across the entire visible spectrum. These are the first materials to combine mesoporosity with long-range chiral ordering that leads to photonic properties. Examples of possible applications of the materials are: lightweight reinforcement materials, low k dielectric materials, tunable reflective filters, adsorbents, stationary phases for chromatography of chiral or achiral substances, supports for catalysts (e.g., for asymmetric synthetic transformations), and as a template to generate other new porous materials (e.g., porous carbon or porous metals), preferably with chiral nematic structures.
Owner:FPINNOVATIONS INC
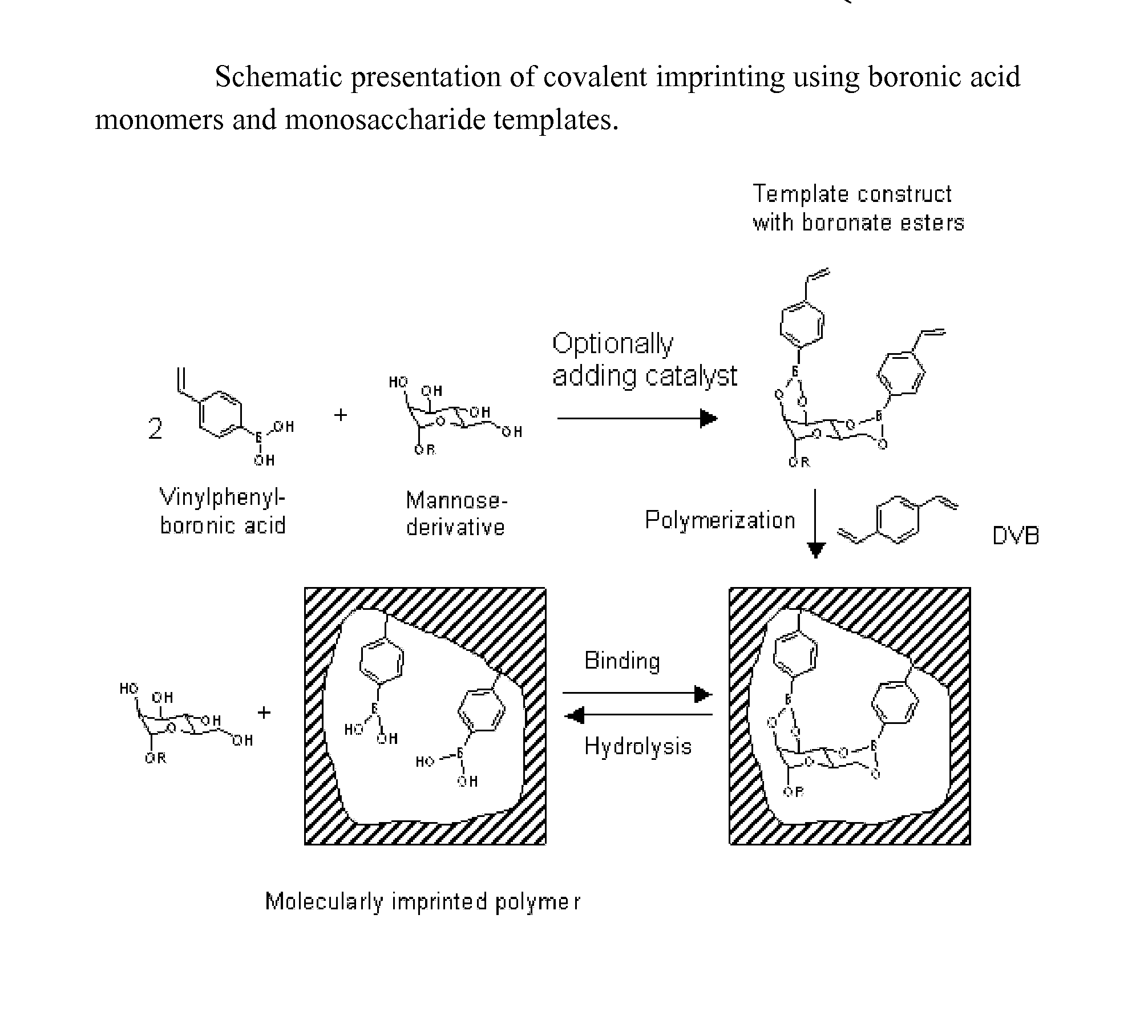
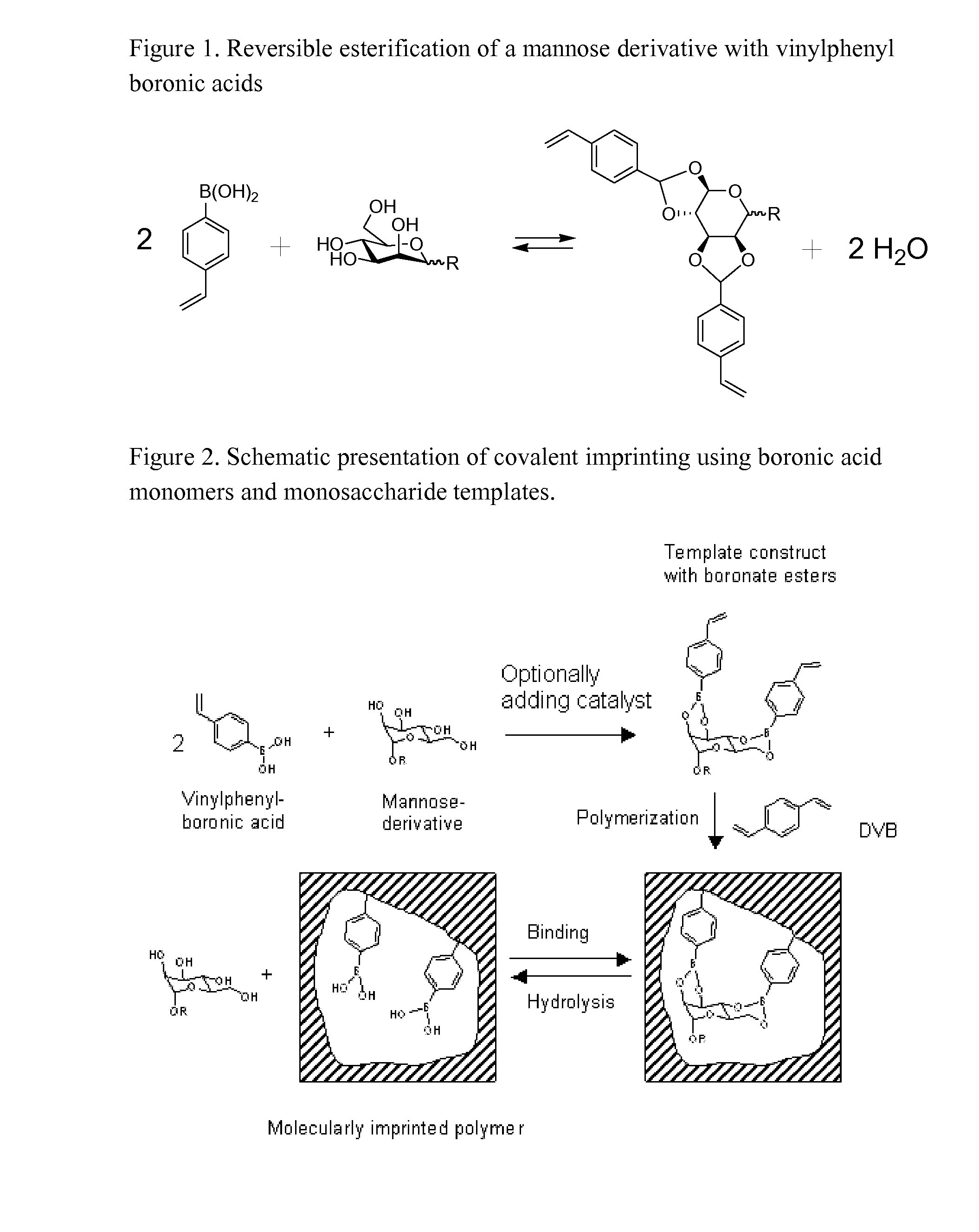
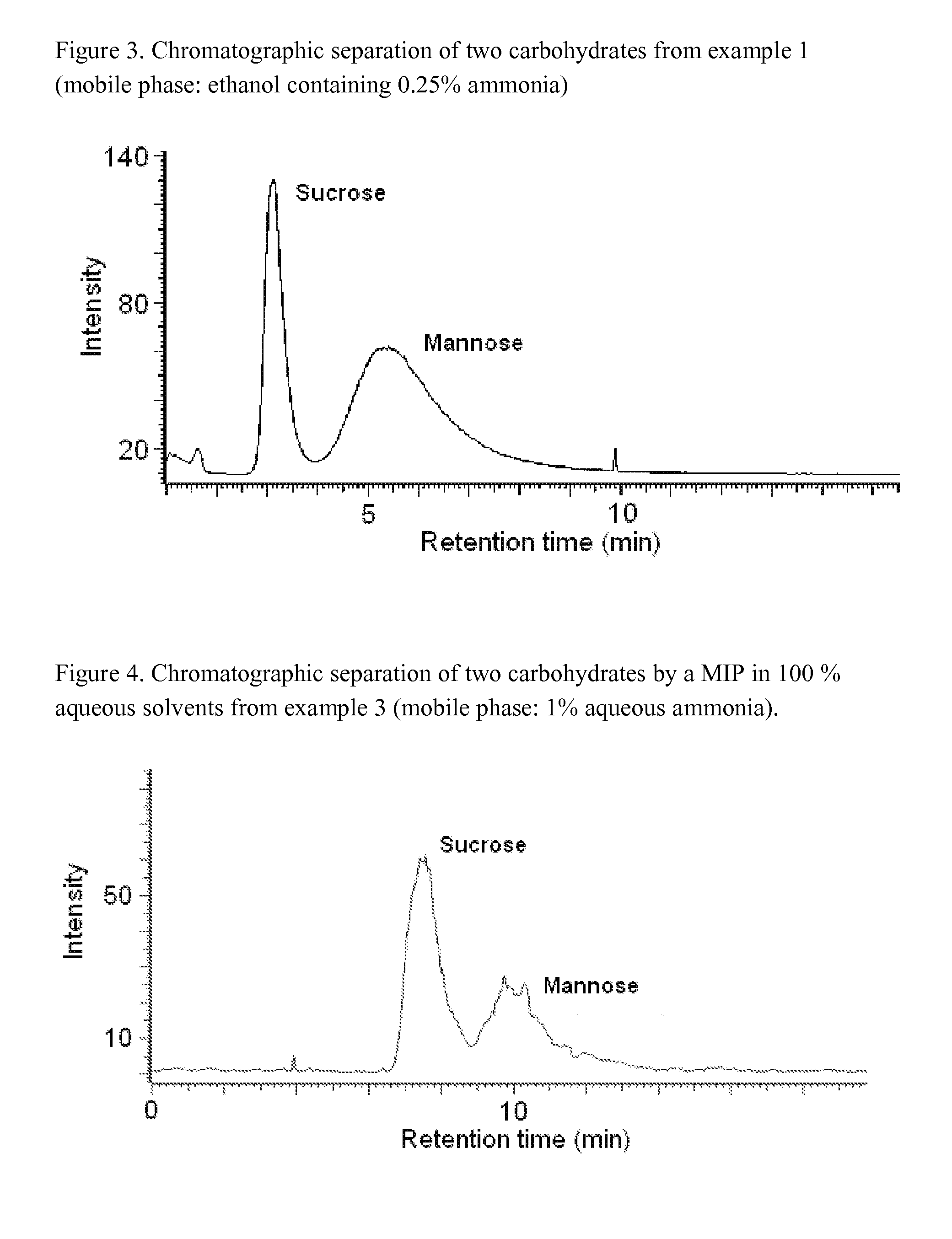
Imprinted polymers
ActiveUS20100113724A1Ensure solubilityPromote formationBioreactor/fermenter combinationsBiological substance pretreatmentsRegioselectivityEnantioselective synthesis
The present invention relates to molecularly imprinted polymers, methods for their preparation and use of said molecularly imprinted polymers in separation, chemical sensors, drug screening, catalysis and in regioselective and enantioselective synthesis.
Owner:BIOTAGE INC
CHIRAL TETRAAMINOPHOSPHONIUM SALTS, CATALYST FOR ASYMMETRIC SYNTHESIS AND METHOD FOR PRODUCING CHIRAL beta-NITROALCOHOL
ActiveUS20090131716A1High activityHigh steric controlOrganic compound preparationGroup 5/15 element organic compoundsHydrogen atomEnantioselective synthesis
A chiral tetraaminophosphonium salt represented by formula (1) and a method for producing chiral β-nitroalcohol comprising reacting an aldehyde or a ketone and a nitroalkane in the presence of the chiral tetraaminophosphonium salt represented by formula (1) and a base, or in the presence of a conjugated base of the chiral tetraaminophosphonium salt represented by formula (1):wherein R1 to R4 are independently a hydrogen atom or a monovalent hydrocarbon group; and, R1 and R2 are different groups or R3 and R4 are different groups.
Owner:MITSUI CHEM INC +1
Phenylpyruvate reductase and application thereof to asymmetric synthesis of (R)-phenyllactic acid
ActiveCN104560800AIncrease concentrationHigh optical purityBacteriaMicroorganism based processesPreservativeEnantioselective synthesis
The invention discloses phenylpyruvate reductase and application thereof to asymmetric synthesis of (R)-phenyllactic acid, and belongs to the technical field of bioengineering. The invention provides novel phenylpyruvate reductase and an encoding gene thereof. When the phenylpyruvate reductase, as a biocatalyst, is used for preparing the (R)-phenyllactic acid through asymmetric reduction of phenylpyruvate, the prepared (R)-phenyllactic acid is high in product concentration and high in optical purity; an expensive coenzyme is not required to be additionally added, the reaction condition is mild, the operation is simple, and the preparation is easily popularized, so that the phenylpyruvate reductase has a very good industrial application prospect in production of a biological preservative.
Owner:JIANGNAN UNIV +1
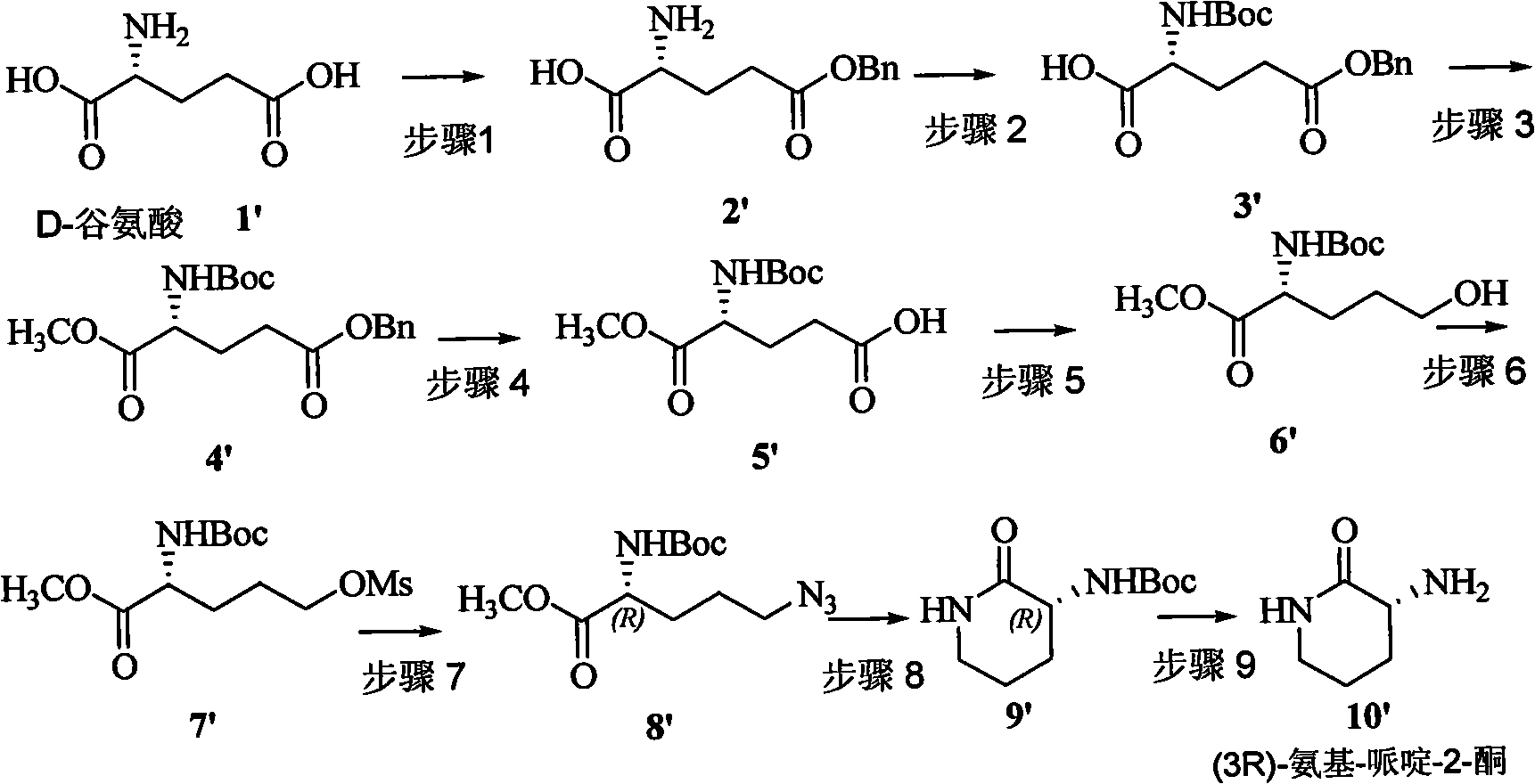
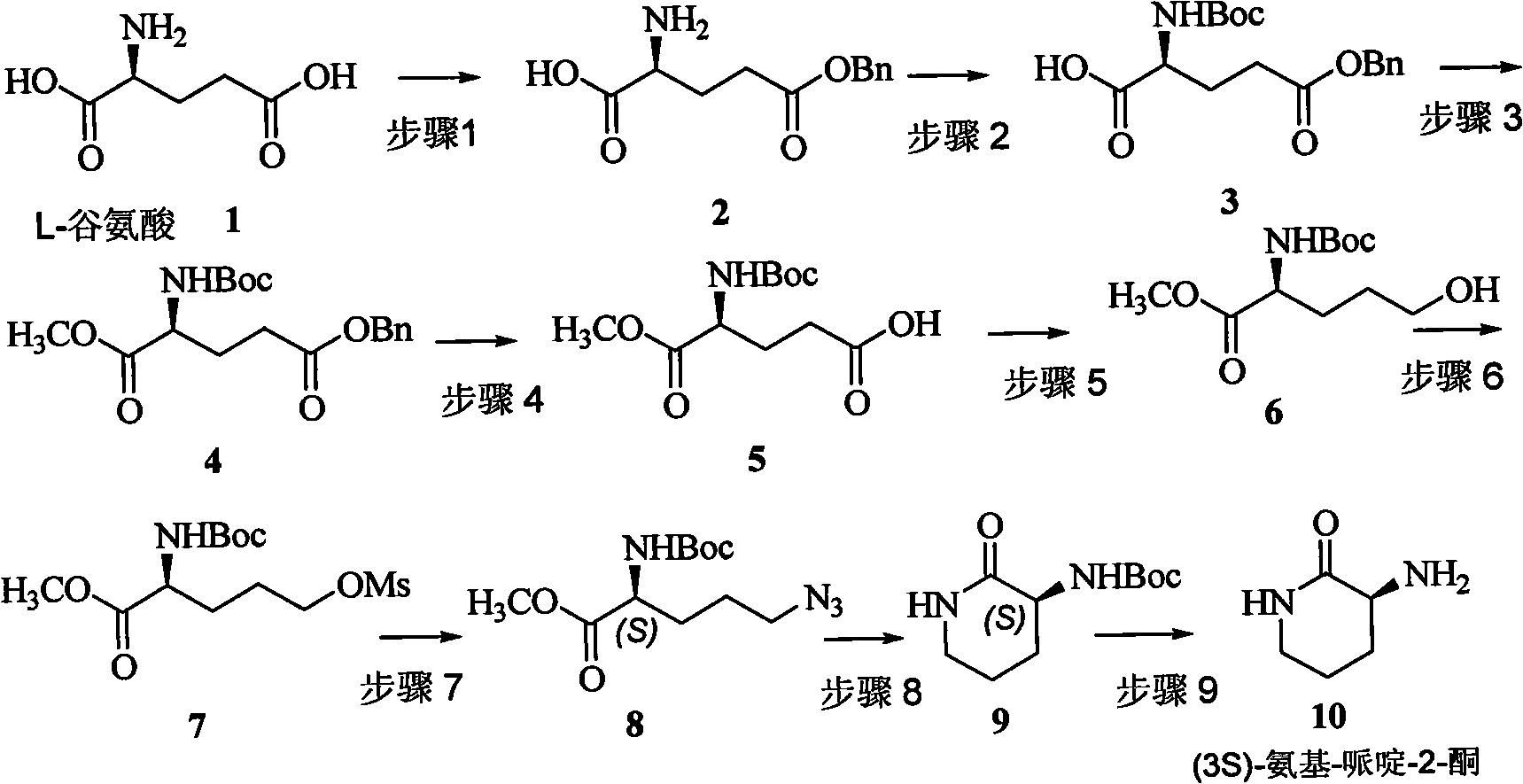
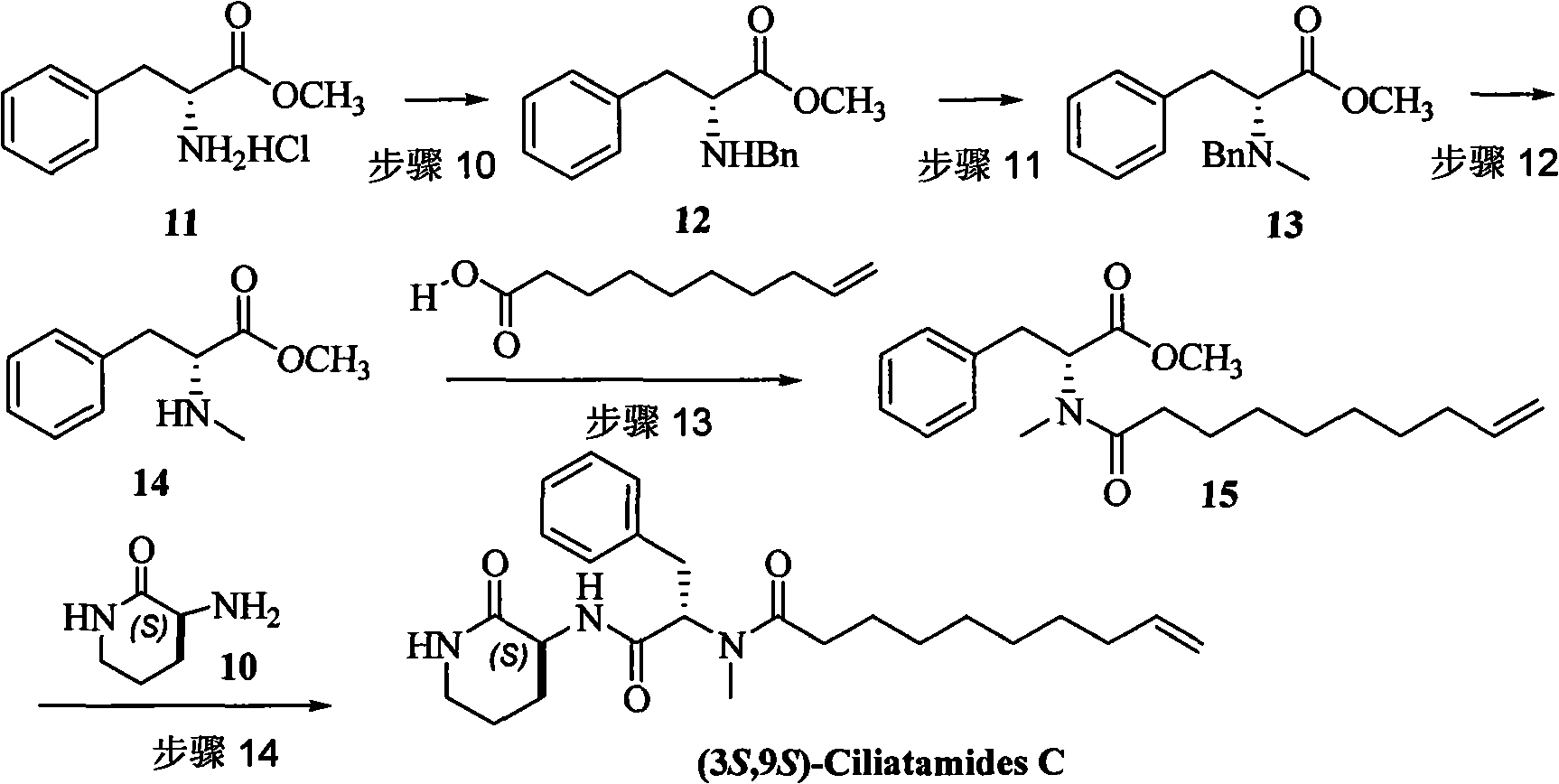
Method for preparing natural product (3S,9S)-Ciliatamides C
The present invention belongs to the field of chemical synthesis and relates to the asymmetric synthetic technology of chiral piperidine compounds, in particular to a preparation method of marine natural products, (3S, 9S)-Ciliatamides C and a synthetic fragment thereof, (3R) or (3S)-amido-piperidine-2-one. In the method, natural compound L or D-glutamic acid, which is cheap and can be easily prepared, is used as starting raw material to asymmetrically synthesize the compound (3R) or (3S)-amido-piperidine-2-one; simultaneously, the (3S)-amido-piperidine-2-one is used as the molecule of a key synthetic fragment to asymmetrically synthesize the marine natural products, (3S, 9S)-Ciliatamides C, which has inhibitory activity for the toxicity of HeLa cells. The method has the advantages of simple operation, easy separation, high yield and excellent stereoselectivity. And the agents that are used in the method are ordinary agents that are cheap and can be easily prepared.
Owner:FUDAN UNIV
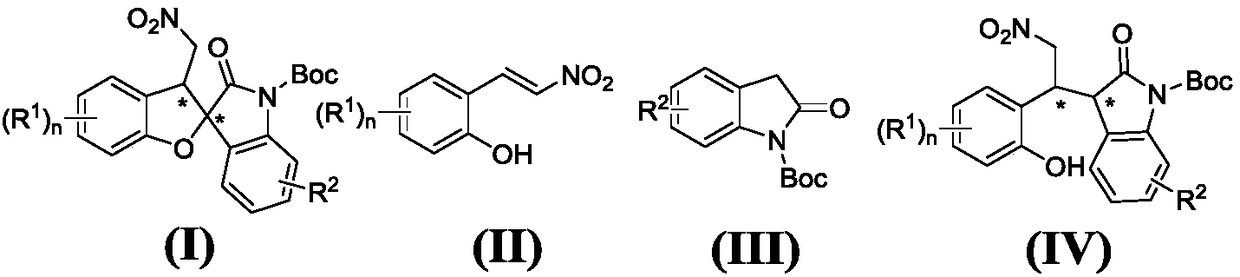
Asymmetric synthesis method of chiral benzofuran spirooxindole compound
The invention provides an asymmetric synthesis method of a chiral benzofuran spirooxindole compound as shown in a formula (I). The synthesis method is performed through the following steps of mixing ao-hydroxy nitroolefin compound as shown in the formula (II), with an oxoindole compound as shown in a formula (III), a chiral hydrogen key catalyst and an organic solvent, and performing a reaction at minus 40-60 DEG C for 1-240h so as to obtain a compound as shown in the formula (IV); and adding an iodine source additive and an oxidizer to the compound as shown in the formula (IV), performing areaction at minus 40-60 DEG C for 1-48h, and performing after-treatment on reaction liquid so as to obtain the chiral benzofuran spirooxindole compound as shown in the formula (I). According to the asymmetric synthesis method disclosed by the invention, the reaction condition is mild, the yield of products is high, and the selectivity is excellent. (As shown in the description).
Owner:ZHEJIANG UNIV OF TECH
Method for asymmetric synthesis of 3,3-disubstituted-2-oxindole compound
InactiveCN102659494AHigh ee valueSimple post-reaction handlingAsymmetric synthesesNatural productEnantioselective synthesis
The invention discloses a method for asymmetric synthesis of a 3,3-disubstituted-2-oxindole compound. The method is characterized in that a 3-monosubstituted-2-oxindole compound and a 1,4-naphthoquinone compound as reaction raw materials undergo a reaction in the presence of chiral organic catalysts in air to produce the 3,3-disubstituted-2-oxindole compound. The method has mild reaction conditions and adopts easily available raw materials. The 3,3-disubstituted-2-oxindole compound obtained by the method has a very high ee value, provides a key skeleton structure for the synthesis of many natural products and drugs, and can be widely used for large-scale industrial production.
Owner:EAST CHINA NORMAL UNIV +1
Process for enantioselective synthesis of single enantiomers of modafinil by asymmetric oxidation
InactiveUS7368591B2High enantioselectivityHigh yieldBiocideOrganic compound preparationOrganic solventEnantiomer
The invention relates to a method for preparing a sulphoxide compound of formula (I) either as a single enantiomer or in an enantiomerically enriched form, comprising the steps of:a) contacting a pro-chiral sulphide of formula (II) with a metal chiral complex, a base and an oxidizing agent in an organic solvent; and optionallyb) isolating the obtained sulphoxide of formula (I).wherein n, Y, R1, R1a, R2 and R2a are as defined in claim 1.
Owner:TEVA SANTE
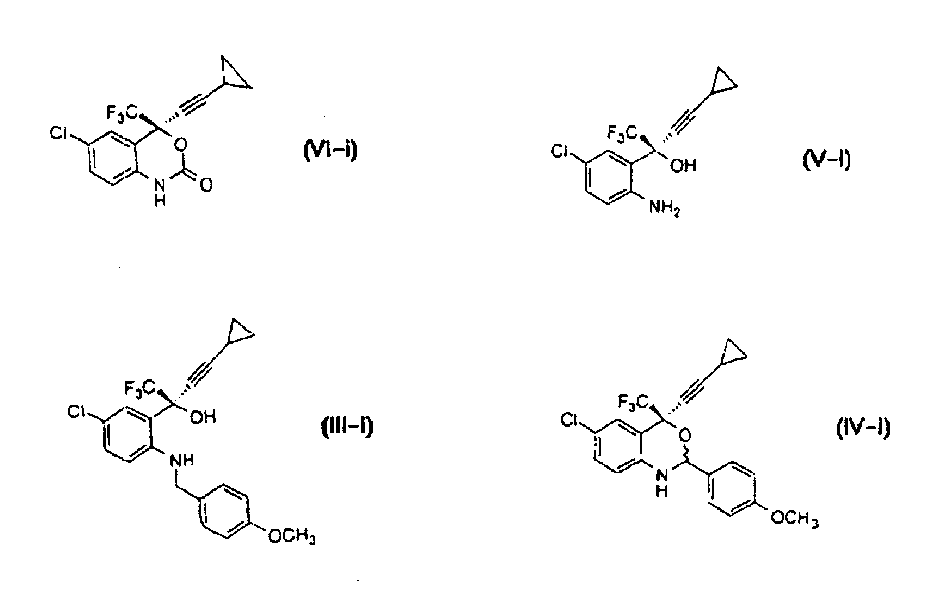
Asymmetric synthesis of benzoxazinones via new intermediates
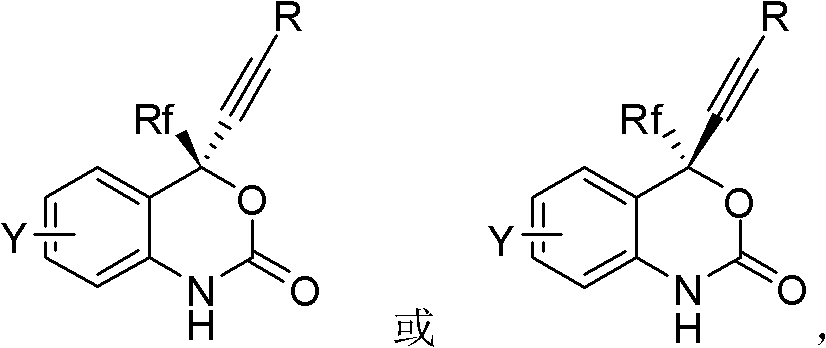
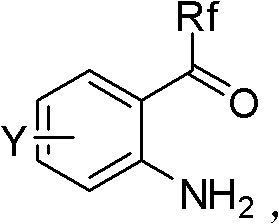
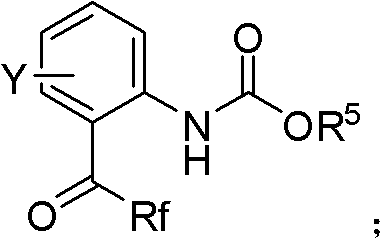
The present invention provides novel methods for the asymmetric synthesis of (S)-6-chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-2H-3,1-benzoxazin-2-one of formula (VI-i) which is useful as a human immunodeficiency virus (HIV) reverse transcriptase inhibitor. In an embodiment, the present invention provides a process for the preparation of an amino alcohol compound of formula (V-i) comprising adding a toluene solution of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone to a toluene solution of a compound of formula (III-i), via a compound of formula (IV-i).
Owner:DU PONT PHARMA CO +1
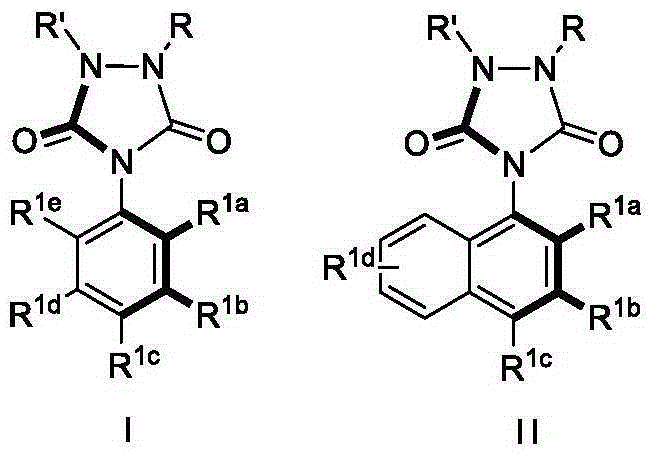
Urazole chiral axis compound and catalytic asymmetric synthesis method thereof
ActiveCN105330608AEasy to useConvenient sourceOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylHydrogen
The invention discloses an urazole chiral axis compound. The urazole chiral axis compound is represented by a formula I or II, wherein the R<1a> is selected from alkyl, halogen, phenyl, substituted phenyl and other groups shown in the descriptions, the R<5> is selected from halogen, alkyl and alkoxy, the R<6> and the R<7> are respectively and independently selected from an aliphatic substituent group and an aromatic substituent group, and the R<1b>, R<1c>, R<1d> and R<1e> are respectively and independently selected from hydrogen, halogen, phenyl, substituted phenyl, alkyl, alkoxy, hydroxyl, a ester group, an aldehyde group, a cyano group and amide. The R represents an aryl group free of a substituent group or provided with one or more of substituent groups, and the R' represents hydrogen or alkyl. The invention further discloses an asymmetric synthesis method of the compound. The high-yield high-ee-value urazole chiral axis compound is prepared through synthesis under the moderate reaction conditions. The synthesis method has very good substrate adaptability and can achieve synthesis of more than shown grams of the compound under the situation that the dosage of a catalyst is decreased. The compound can serve as a catalyst or a ligand for a certain asymmetric reactions.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Asymmetric synthesis of chiral muskone and other 3-methyl cyclic ketone
InactiveCN101982451AAchieve synthesisHigh optical purityOrganic-compounds/hydrides/coordination-complexes catalystsBulk chemical productionThioureaCyclic ketone
The invention relates to asymmetric synthesis of chiral muskone and other 3-methyl cyclic ketone. The asymmetric synthesis comprises the following steps: generating a Michael addition product by taking cyclic ketene and dual-sulfonyl methane as raw materials, the chiral catalyst containing one or more functional groups of primary amine, tertiary amine, urea or thiourea or salt thereof as a catalytic system and an organic solvent as a reaction carrier and conducting reaction at 0 to 100 DEG C for 2 and 10 days; and performing carbonyl protection, selective dual-sulfonyl removal and decarbonylation protection by taking the Michael addition product as a start raw material to synthesize the chiral muskone and other 3-methyl cyclic ketone. The invention realizes high-conversion and high-selectivity synthesis of the chiral muskone and other 3-methyl cyclic ketone for the first time, ensures that the synthesized muskone has the same structure as the natural muskone and overcomes the shortcomings of small content and high price of the natural muskone; and moreover, as the reaction conditions are mild, the operation is simple and the raw materials are cheap and readily available, the invention is suitable for industrial production and application.
Owner:EAST CHINA UNIV OF SCI & TECH
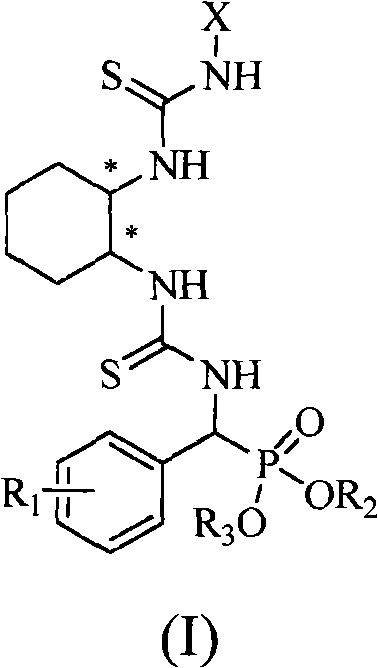
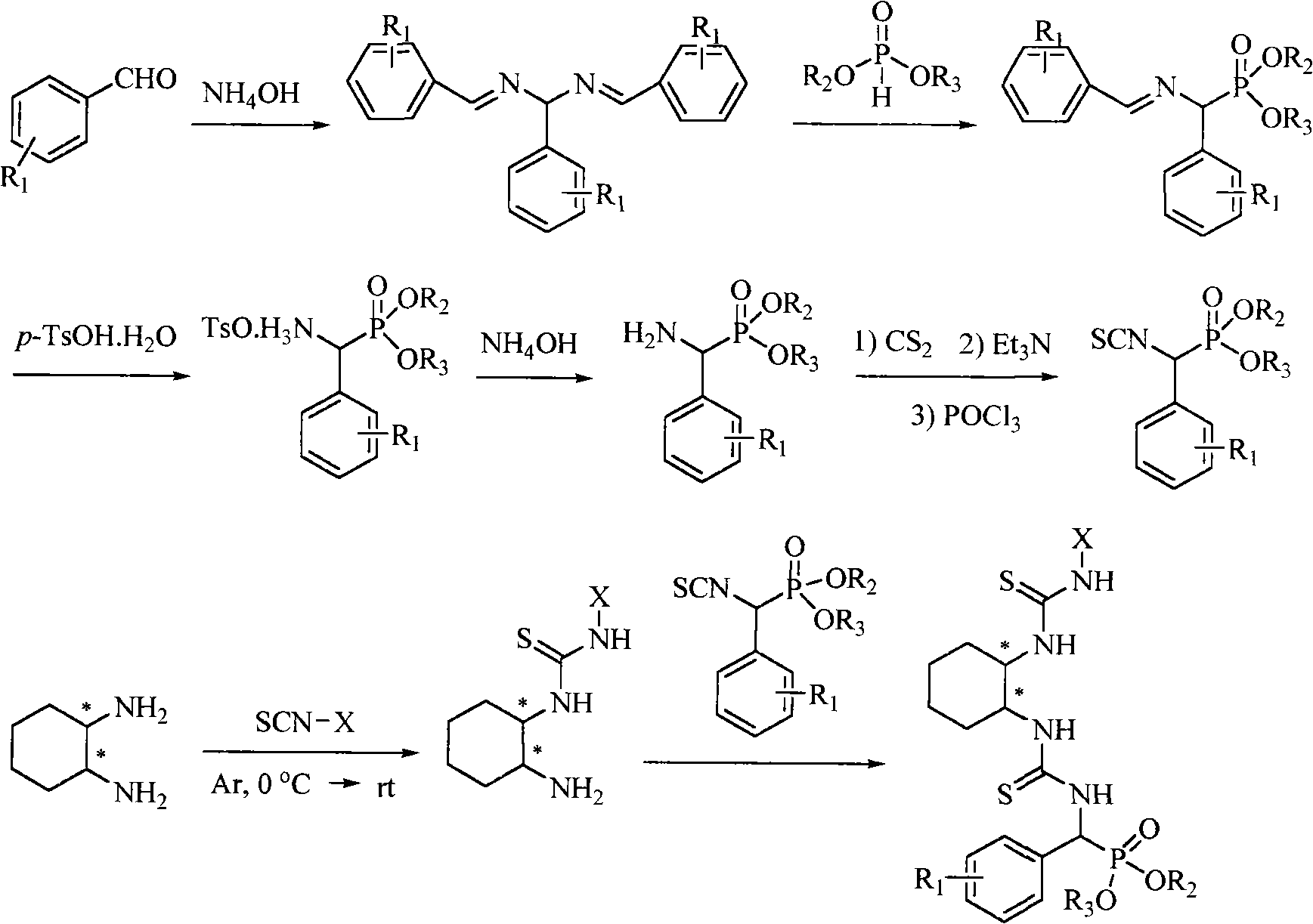
A chiral bithioureido derivate containing phosphonate as well as its preparing method and use
InactiveCN101550159AGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsDiseaseBenzaldehyde
The present invention discloses preparation method and biological activity of a compound for resisting plant virus, a chiral bithioureido derivate containing phosphonate, which has a compound structure represented by the following general formula. The invention describes a chiral bithioureido derivate containing phosphonate compounded through four-step reaction by taking chiral cyclohexanediamine, substituted isosulfocyanates, carbon disulfide, phosphorus oxychloride, triethylamine, substituted benzaldehyde, ammonia, dialkyl phosphite, p-toluenesulfonic acid and the like as raw materials. The inventive compounds a, e have higher treating inhibitory effect to tobacco mosaic virus (TMV) disease, and indicate better plant virus resisting activity; in addition, the compounds also have a certain anti-cancer activity, and can be taken as a chiral catalyst to perform asymmetric synthesis.
Owner:GUIZHOU UNIV
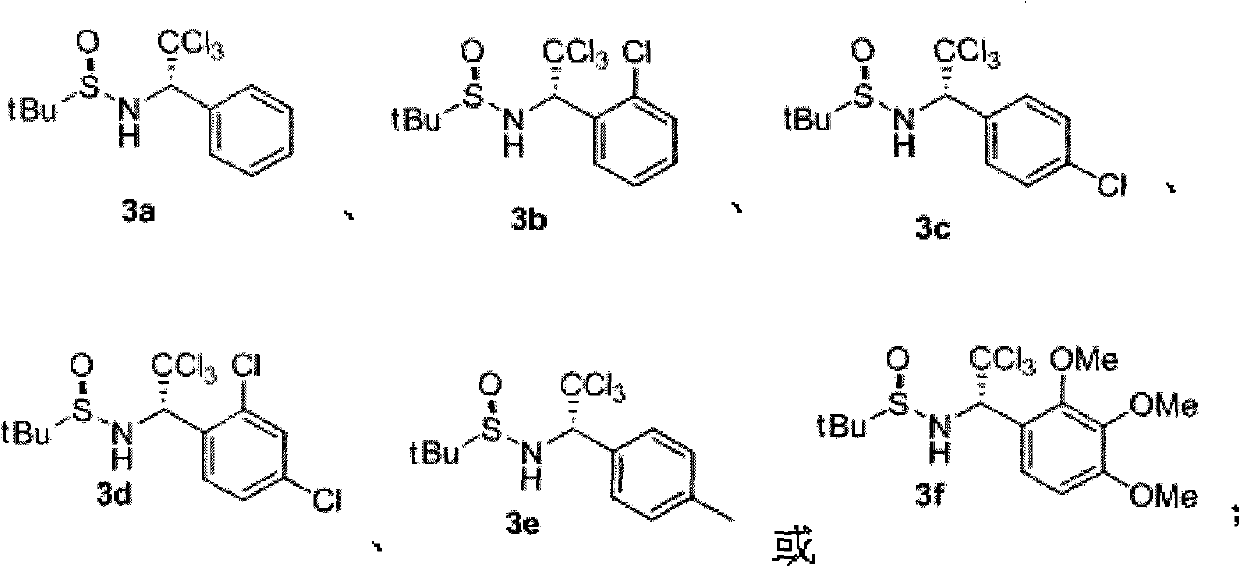
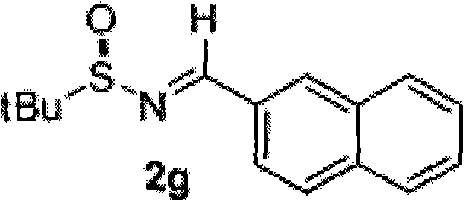
Chiral alpha-(trichloromethyl) amine compound and preparation method thereof
InactiveCN101857559AHigh optical purityThe preparation process conditions are mildAsymmetric synthesesEnantioselective synthesisAziridine
The invention discloses a chiral alpha-(trichloromethyl) amine compound and a preparation method thereof. The chiral alpha-(trichloromethyl) amine compound is a potential bioactive molecule synthesis building block and can serve as an important midbody for synthesizing chiral chloric amine compounds, such as 2,2-dichloro aziridine. The preparation method of the invention has moderate technological condition and abundant and cheap raw materials, and the prepared alpha-(trichloromethyl) amine has high optical purity and is convenient to industrially apply. The alpha-(trichloromethyl) amine prepared by the invention can be widely applied in the fields of asymmetric synthesis and medicine research and development. The structural general formula is disclosed in formula (3).
Owner:SHANGHAI UNIV OF ENG SCI
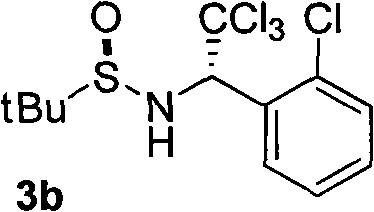
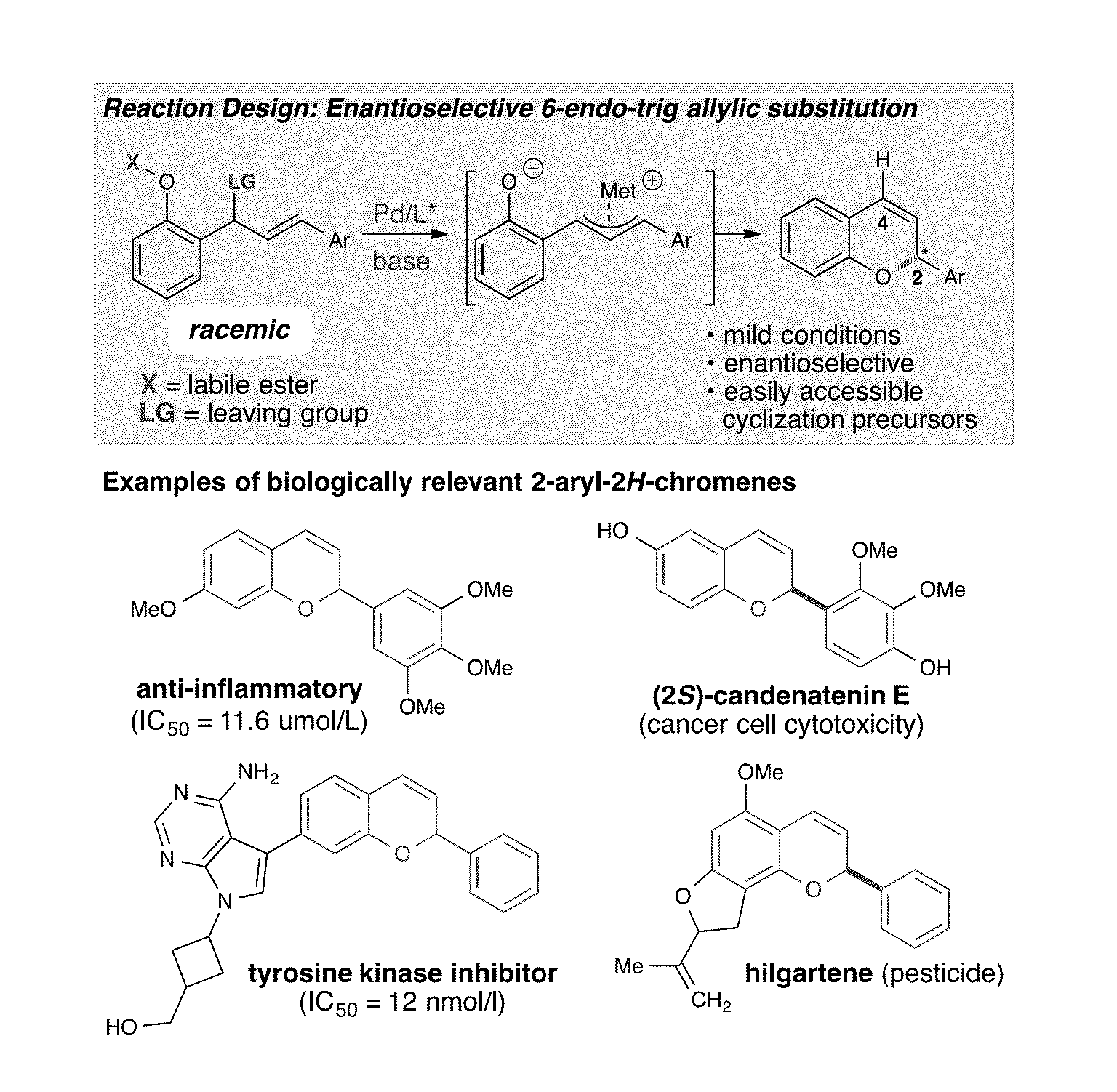
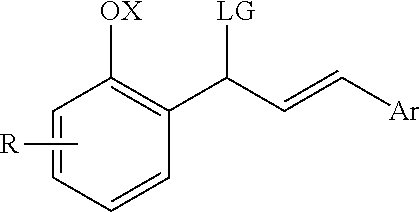
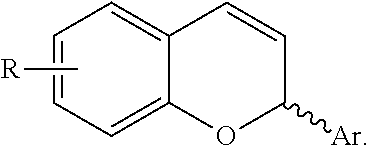
Catalytic enantioselective synthesis of 2-aryl chromenes and related phosphoramidite ligands and catalyst compounds
ActiveUS9309217B2Alleviating undue concernGroup 5/15 element organic compoundsAsymmetric synthesesArylEnantioselective synthesis
Owner:NORTHWESTERN UNIV
One pot method asymmetric synthesis process of HIV reverse transcriptase inhibitor Efavirenz compound
ActiveCN102584801AMild process conditionsSimple and fast operationGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsNucleoside Reverse Transcriptase InhibitorHydrogen
The invention relates to a novel one pot method asymmetric synthesis process of a (S)-6-chlorine-4-cylopropyl ethylnen-4-trifluoromethyl-1,4-dihydro-2H-1,3-benzoxazine-2-ketone (Efavirenz) compound, the compound can be used as an reverse transcriptase inhibitor for human immunodeficiency virus (HIV). The invention also relates to a novel aminoalcohol ligand used for the process.
Owner:然晟(上海)实业发展有限公司
Process for enantioselective synthesis of single enantiomers of modafinil by asymmetric oxidation
InactiveUS20050080256A1High enantioselectivityHigh yieldOrganic compound preparationOptically-active compound separationOrganic solventEnantioselective synthesis
The invention relates to a method for preparing a sulphoxide compound of formula (I) either as a single enantiomer or in an enantiomerically enriched form, comprising the steps of: a) contacting a pro-chiral sulphide of formula (II) with a metal chiral complex, a base and an oxidizing agent in an organic solvent; and optionally b) isolating the obtained sulphoxide of formula (I); wherein n, Y, R1, R1a, R2 and R2a are as defined herein.
Owner:TEVA SANTE
Method for producing pyrrolidine compound
InactiveUS20100010219A1Good achievabilityUse in synthesisOrganic chemistryMetabolism disorderEnantioselective synthesisMedicinal chemistry
Owner:JAPAN TOBACCO INC
Method for synthesizing (R)-salmeterol
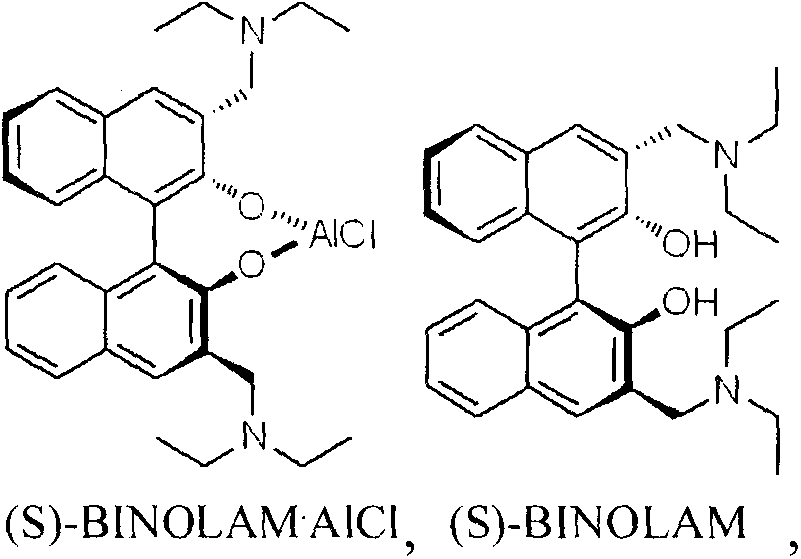
The invention discloses a method for synthesizing (R)-salmeterol. The method is implemented by taking p-hydroxy benzaldehyde as a raw material through the steps of carrying out a methylolation reaction on the p-hydroxy benzaldehyde so as to obtain prochiral aldehyde for protecting double hydroxide radicals in an acetal form; then, through taking (S)-BINOLAM.AlCl as a chiral catalyst, carrying out an asymmetric nucleophilic addition reaction so as to obtain a chiral cyanohydrin intermediate; and reducing the chiral cyanohydrin intermediate so as to obtain primary amine, reacting the primary amine with mesylate [6-(4 phenylbutoxy]hexane, and carrying out hydrolysis on the obtain product so as to remove protecting groups, thereby obtaining a final product (R)-salmeterol. In the invention, a chiral catalyst precursor (S)-BINOLAM can be reused; and the method has relatively high yield and relatively good enantioselectivity, and is short in synthetic route, simple and convenient in operation and low in cost.
Owner:NANJING UNIV OF TECH
Method for asymmetric synthesis
InactiveUS7442842B2High stereoselectivityHigh yieldPreparation by oxo-reaction and reductionOrganic compound preparationEnantioselective synthesisMetal
The present invention relates to a process for asymmetric synthesis in the presence of a chiral catalyst comprising at least one complex of a metal of transition group VIII with ligands capable of dimerization via noncovalent bonds, such catalysts and their use.
Owner:BASF AG
Method for asymmetric synthesis of (S)-2-chloro-1-phenylethanol derivative
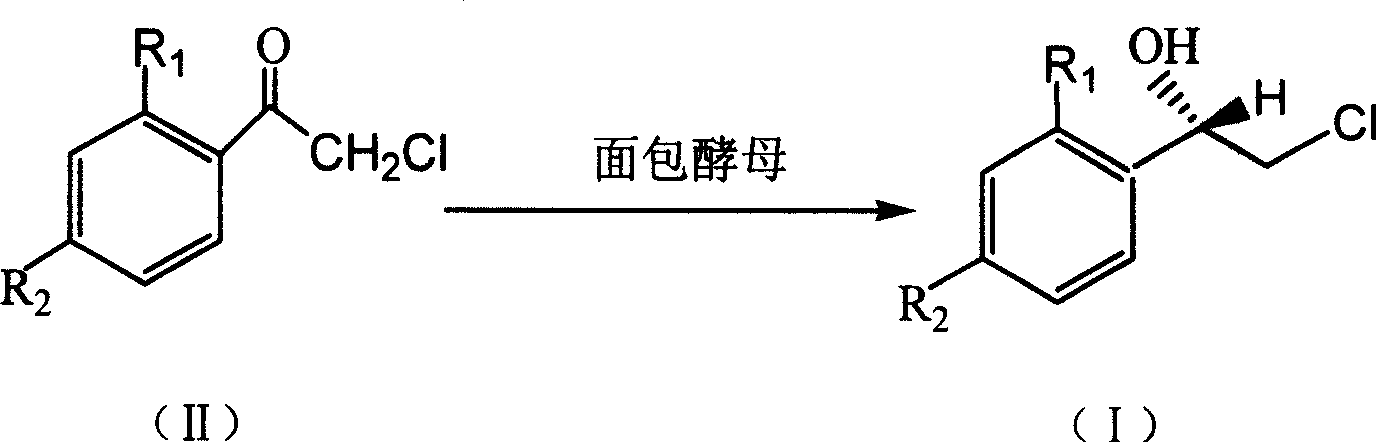
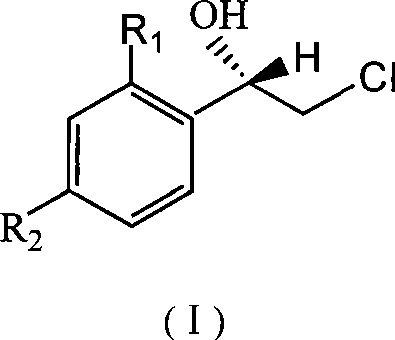
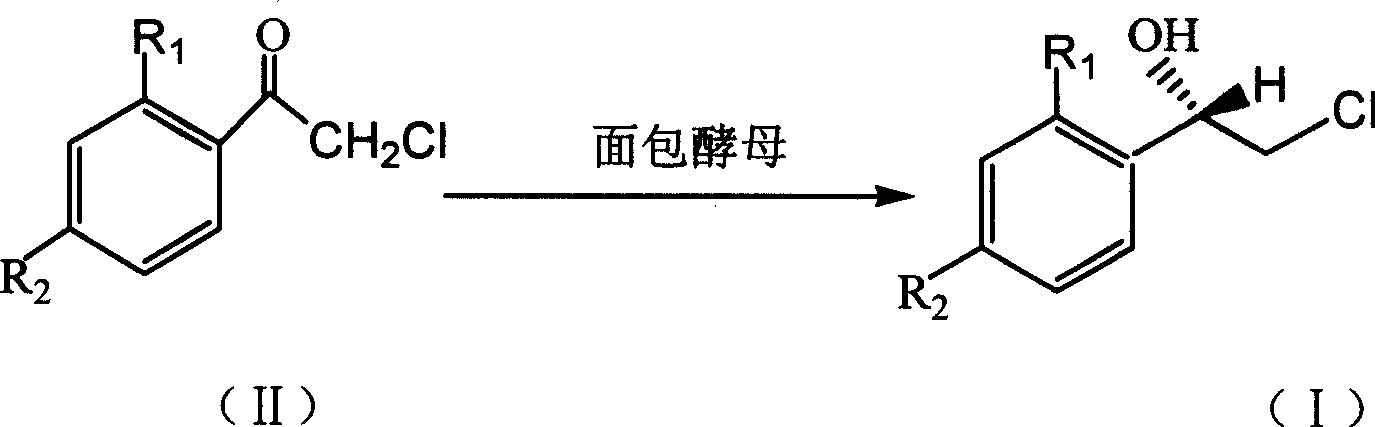
InactiveCN101503714ALow costHigh yieldMicroorganism based processesFermentationYeastEnantioselective synthesis
The invention discloses a method for asymmetrically synthesizing a derivative of (S)-2-clorine-1-phenyl ethanol. In the method, the bakery yeast is used for biologically catalyzing a derivative of 2-clorine-1-acetophenone shown in a formula (II) to asymmetrically synthesize the derivative of (S)-2-clorine-1-phenyl ethanol shown in a formula (I), wherein R1 and R2 are any two of H, F, Cl, Br, CH3 or C2H5. In the method, the bakery yeast is used as a catalyst to biologically asymmetrically catalyze the derivative of (S)-2-clorine-1-phenyl ethanol with single optical rotation property, the yield of the target product of the derivative of (S)-2-clorine-1-phenyl ethanol is over 74 percent, the enantioselectivity e.e is over 97 percent, thereby the method greatly simplifies the following split separation step; moreover, the method also has the advantages of simple step, high reaction stereoselectivity, mild reaction conditions, low cost and suitability for mass production.
Owner:GUANGDONG UNIV OF TECH
Process for enantioselective synthesis of single enantiomers of modafinil by asymmetric oxidation
InactiveUS20050222257A1High enantioselectivityHigh yieldBiocideOrganic compound preparationOrganic solventEnantioselective synthesis
The invention relates to a method for preparing a sulphoxide compound of formula (I) either as a single enantiomer or in an enantiomerically enriched form, comprising the steps of: a) contacting a pro-chiral sulphide of formula (II) with a metal chiral complex, a base and an oxidizing agent in an organic solvent; and optionally b) isolating the obtained sulphoxide of formula (I). wherein n, Y, R1, R1a, R2 and R2a are as defined in claim 1.
Owner:TEVA SANTE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com