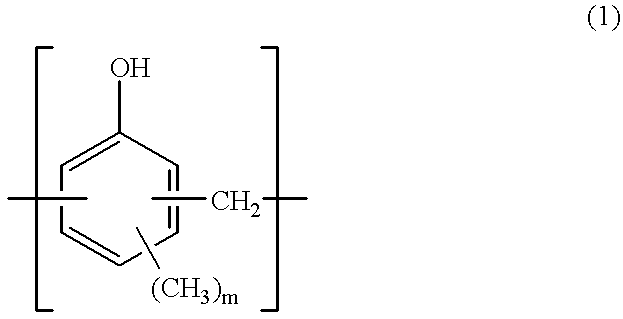
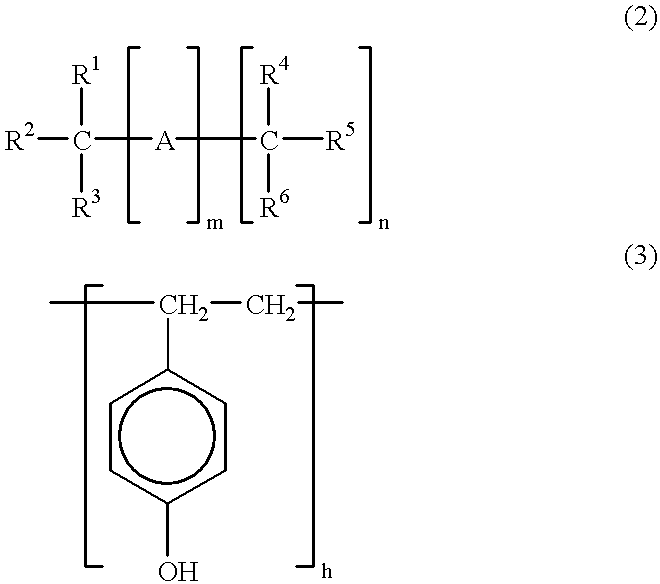
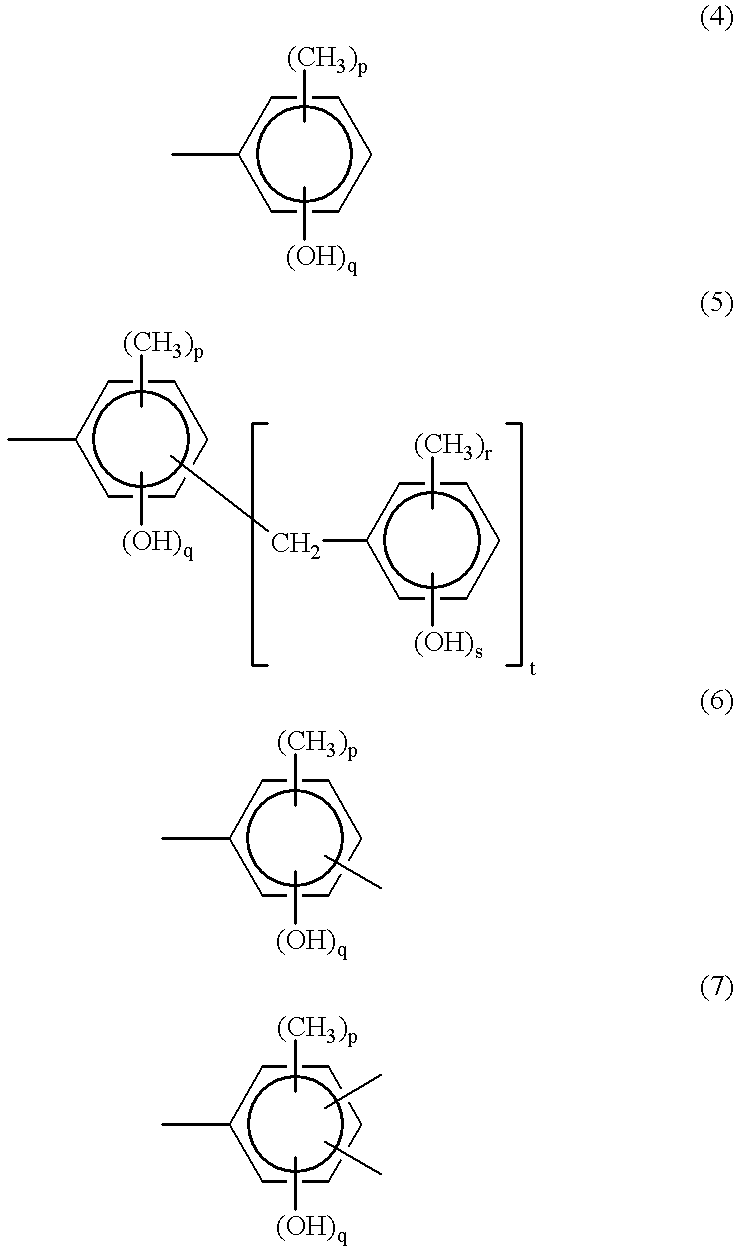
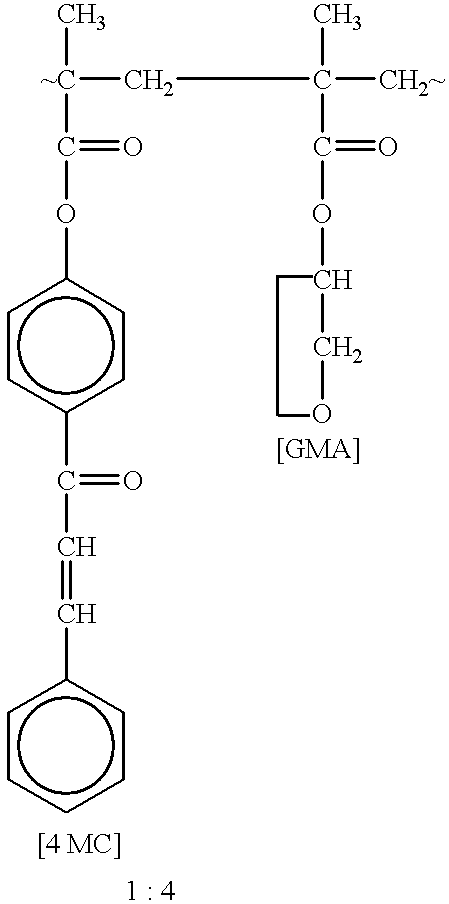
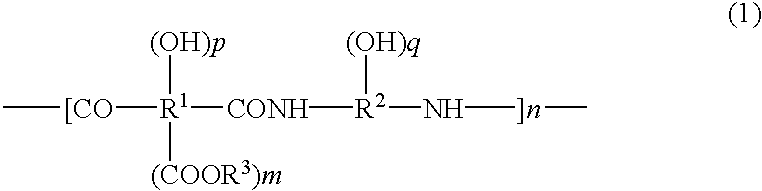Patents
Literature
723 results about "Naphthoquinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
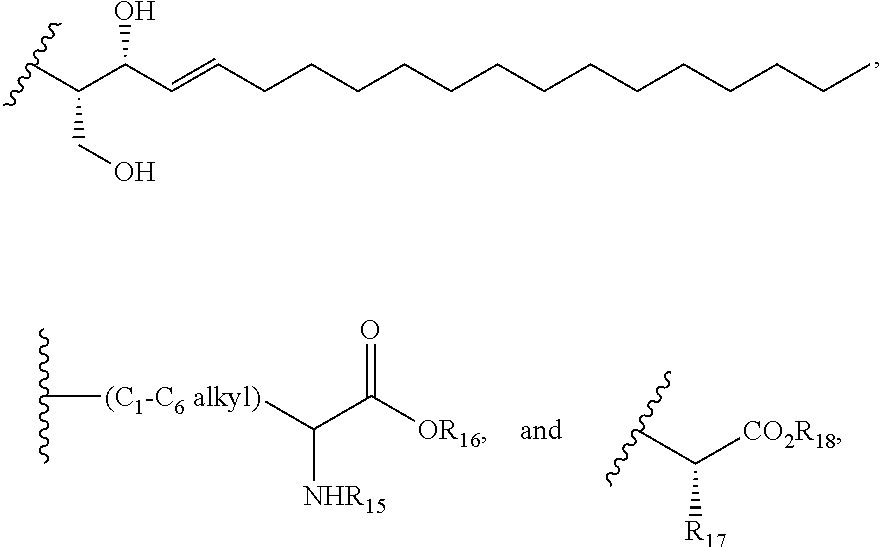
Naphthoquinone is a class of organic compounds structurally related to naphthalene.
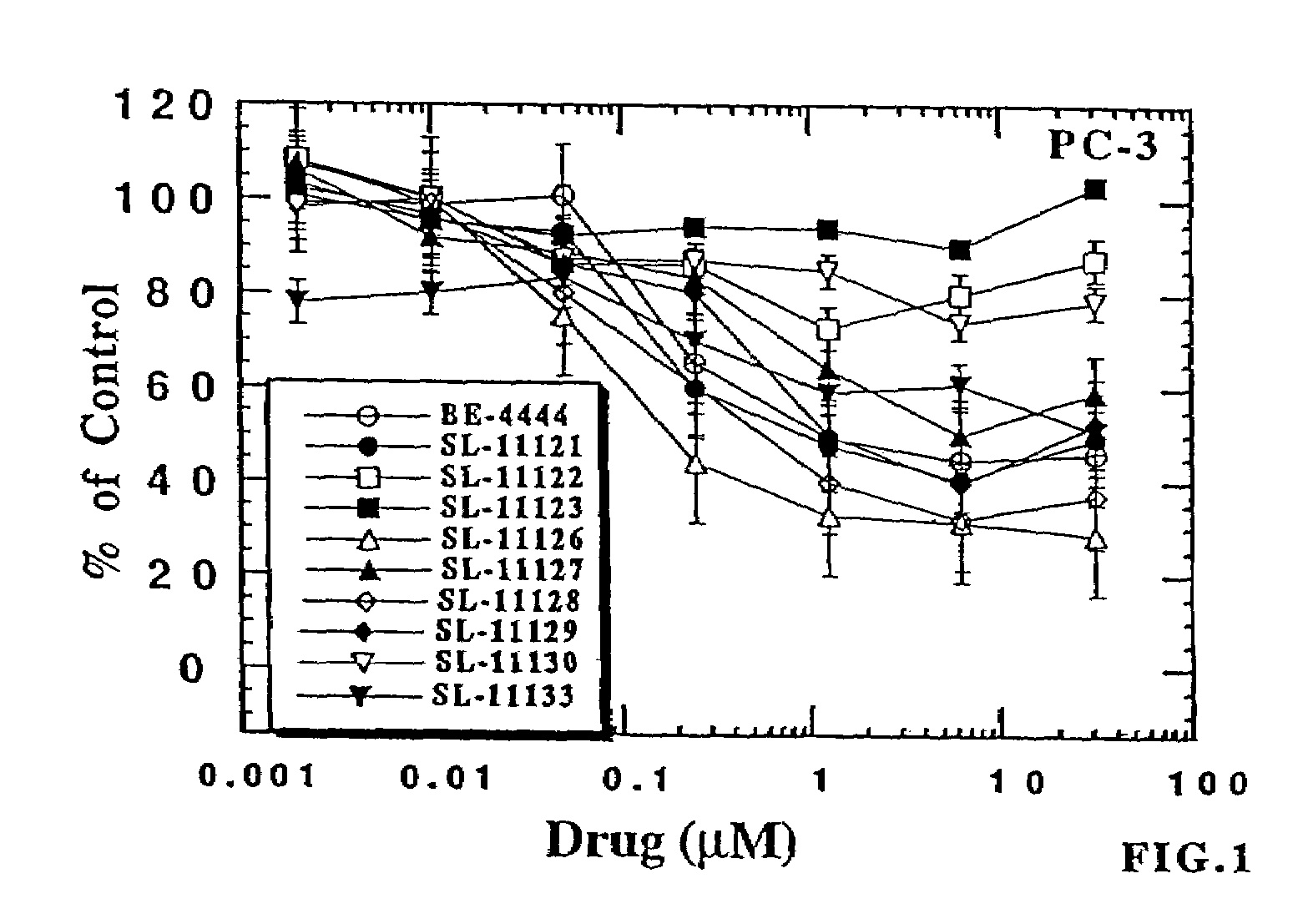
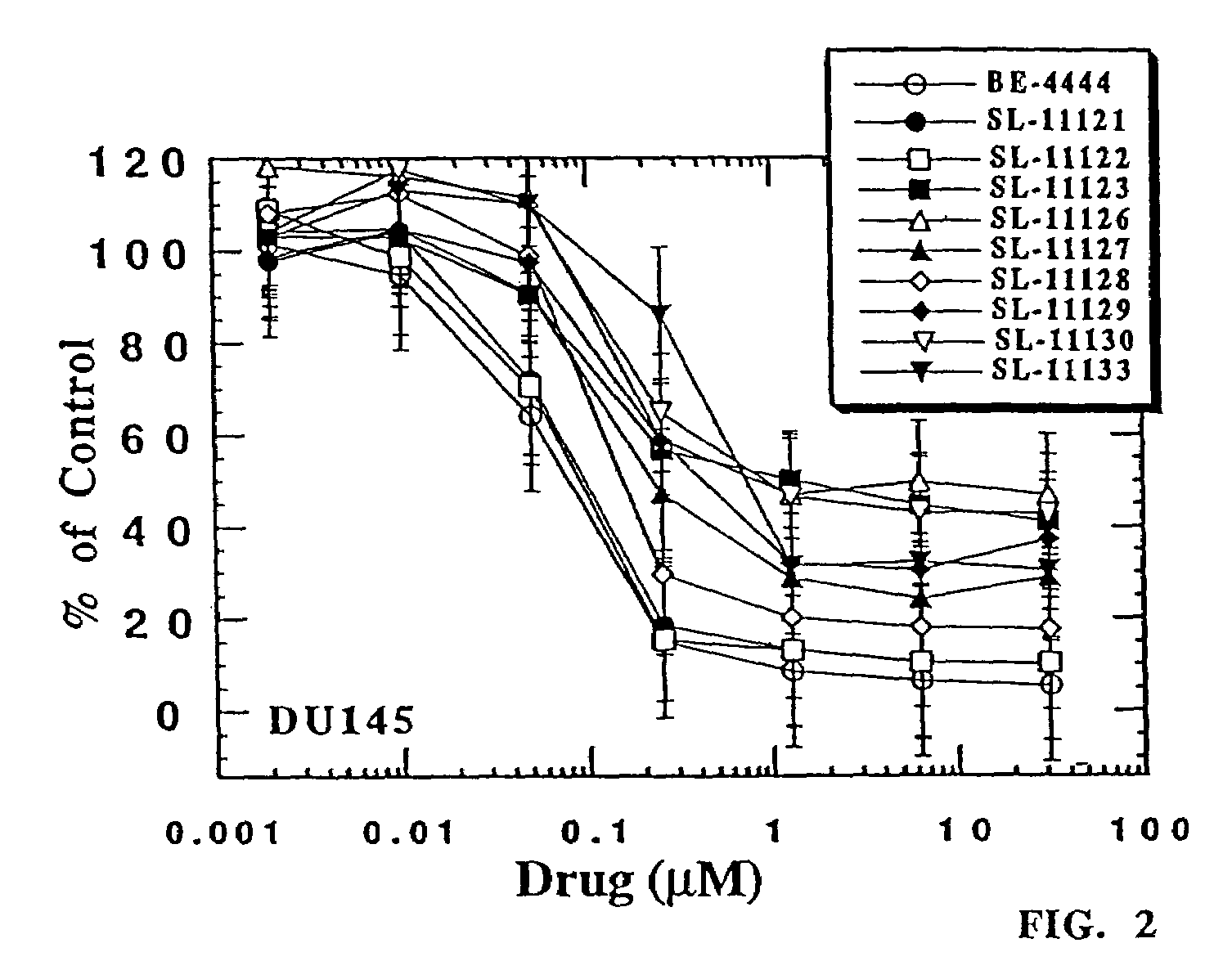
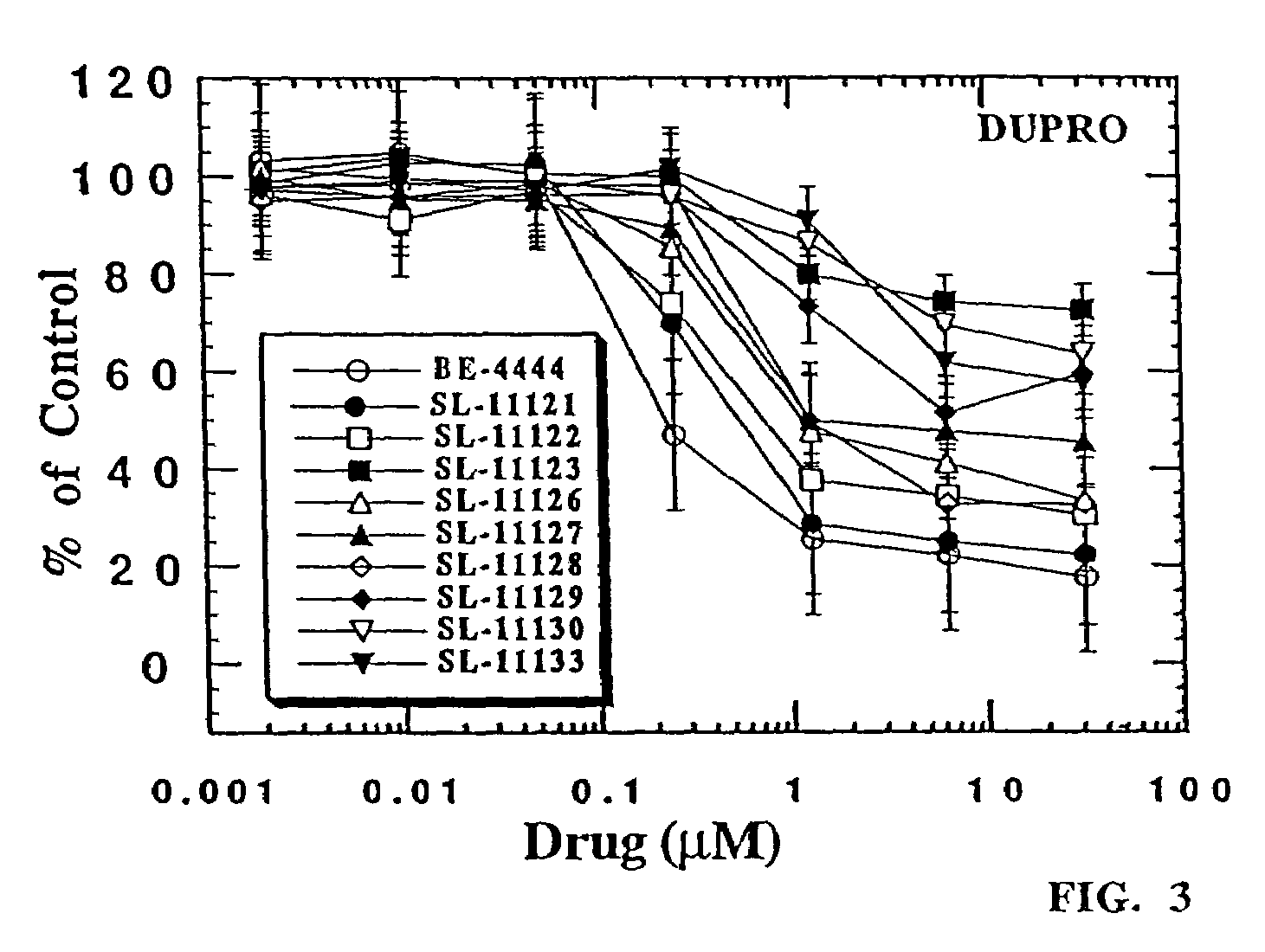
Polyamine analog conjugates and quinone conjugates as therapies for cancers and prostate diseases
Peptide conjugates in which cytocidal and cytostatic agents, such as polyamine analogs or naphthoquinones, are conjugated to a polypeptide recognized and cleaved by enzymes such as prostate-specific antigen (PSA) and cathepsin B are provided, as well as compositions comprising these conjugates. Methods of using these conjugates in the treatment of prostate diseases are also provided.
Owner:CELLGATE
Treatment of mitochondrial diseases with naphthoquinones
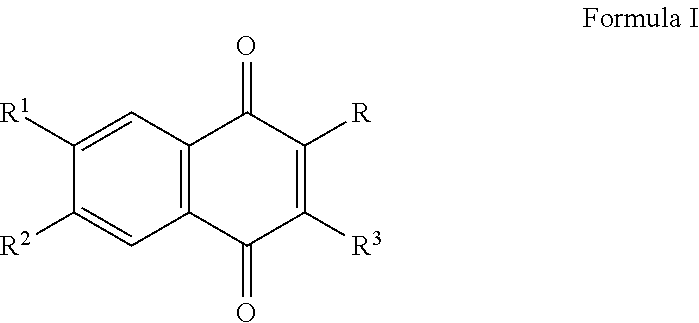
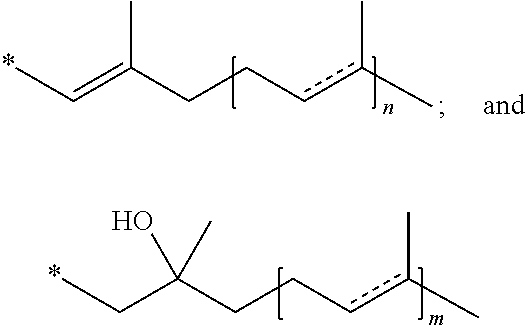
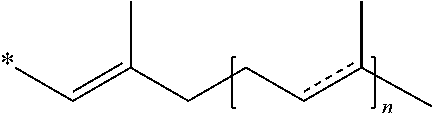
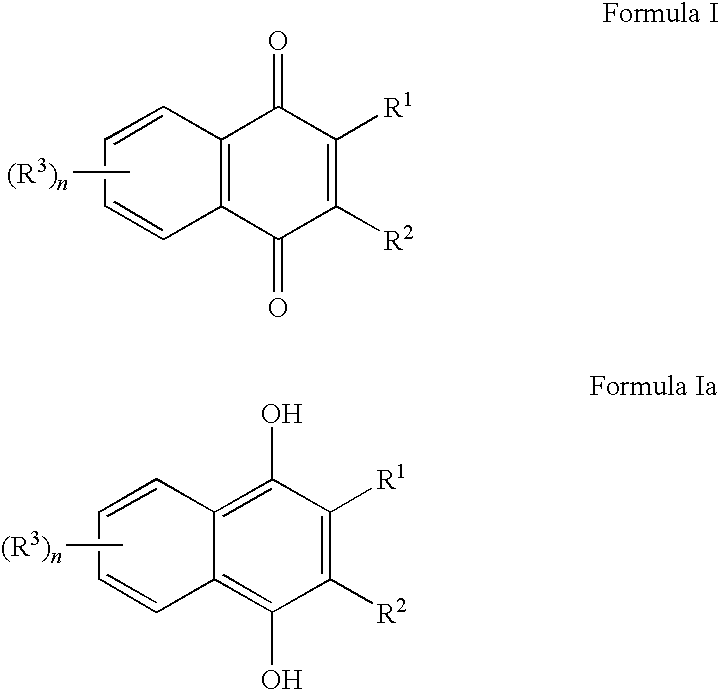
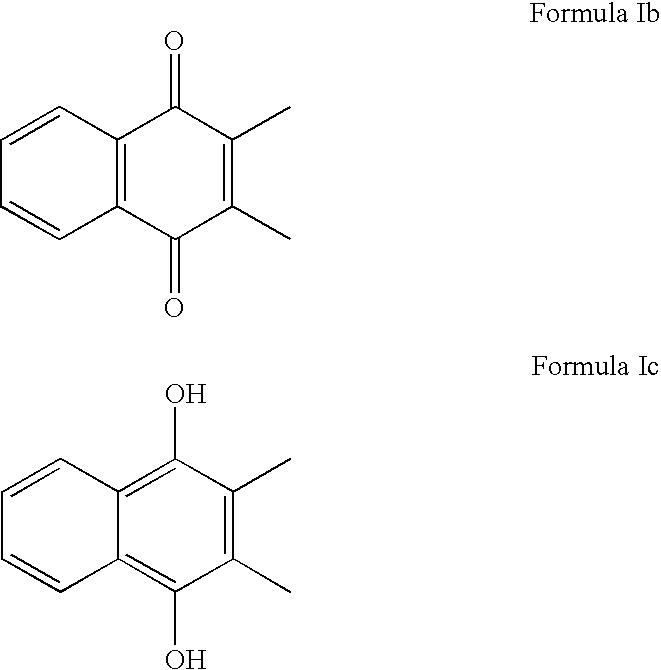
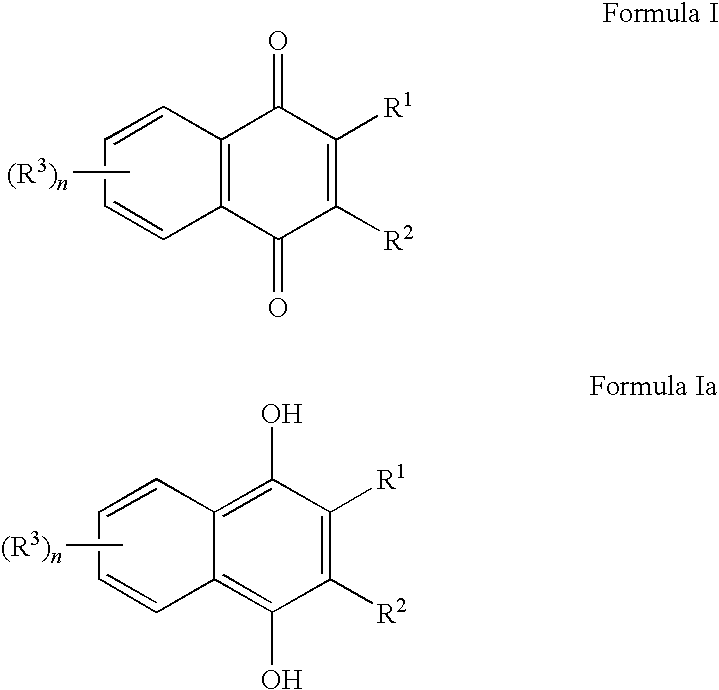
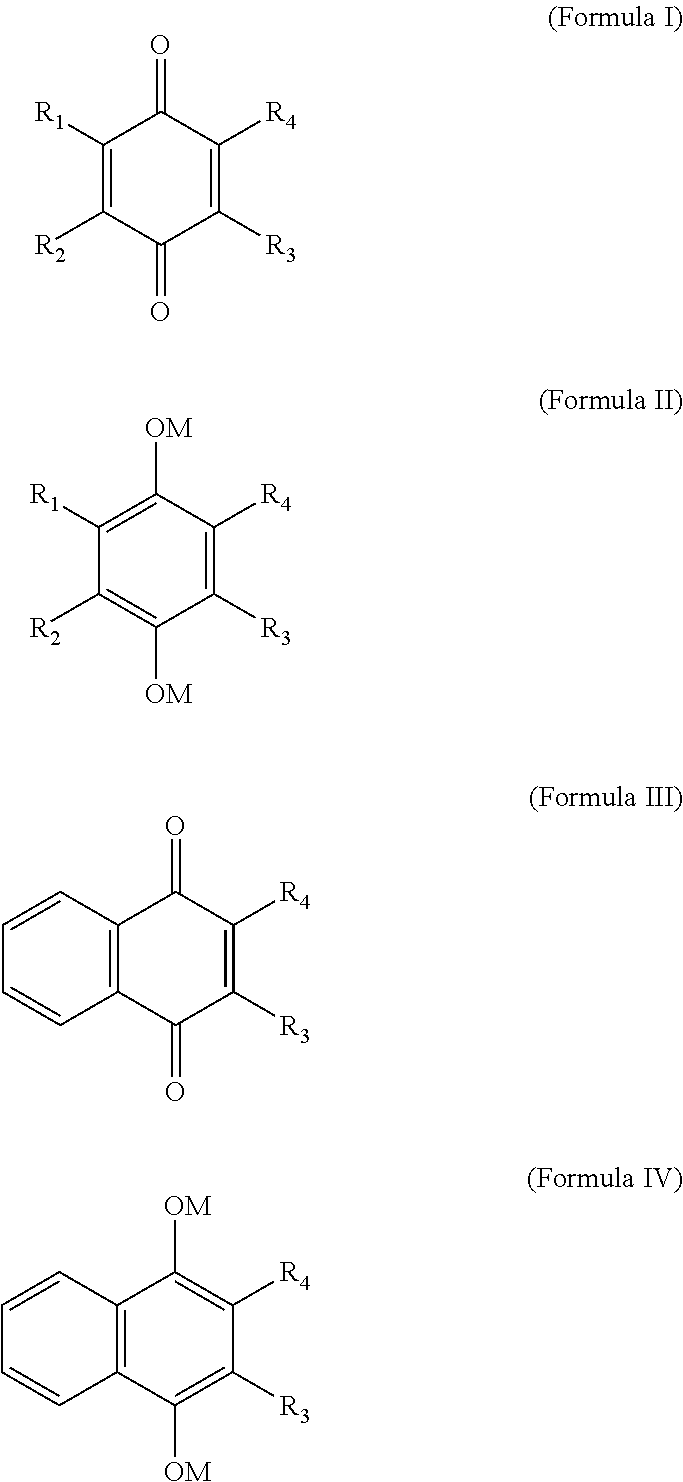
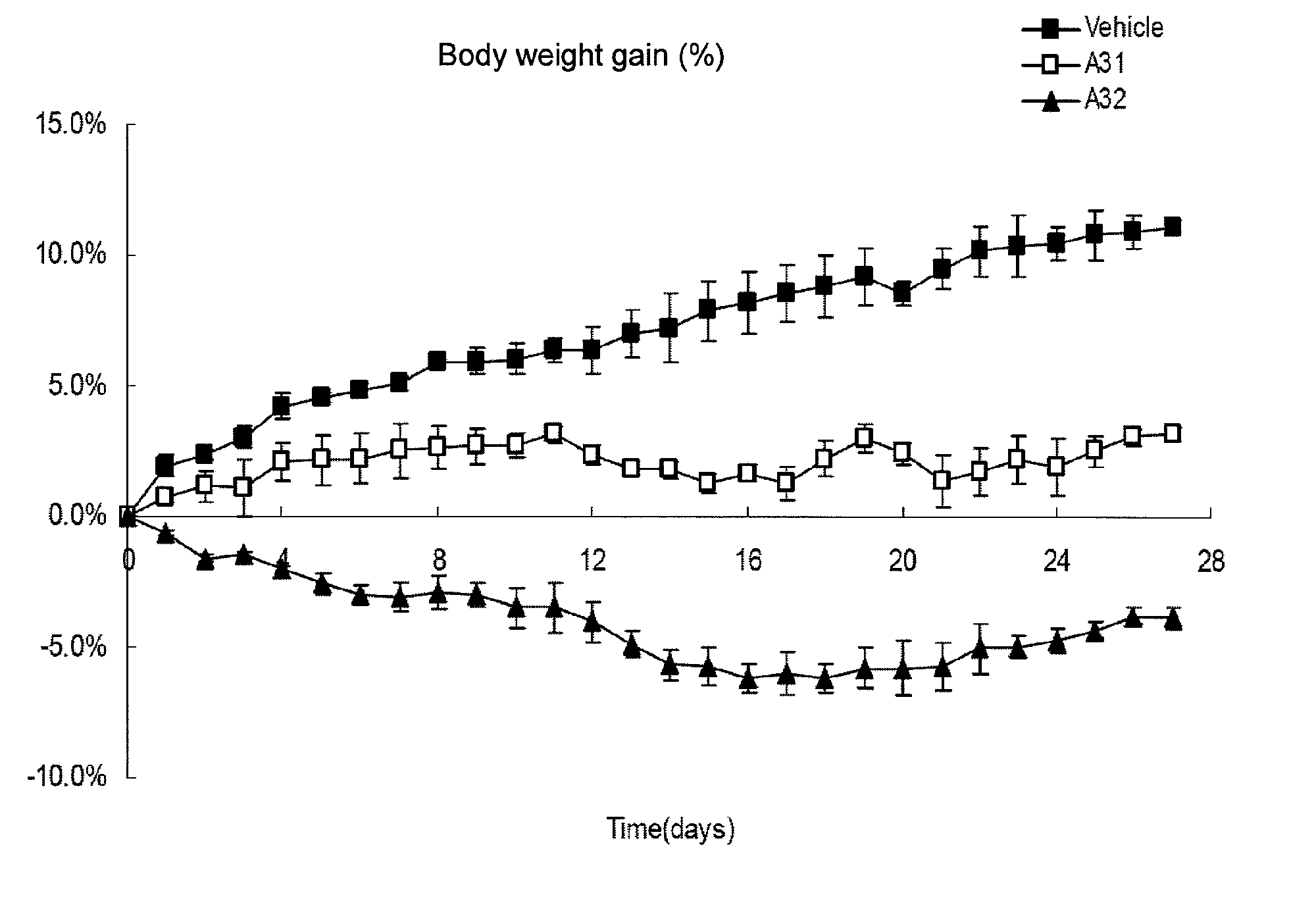
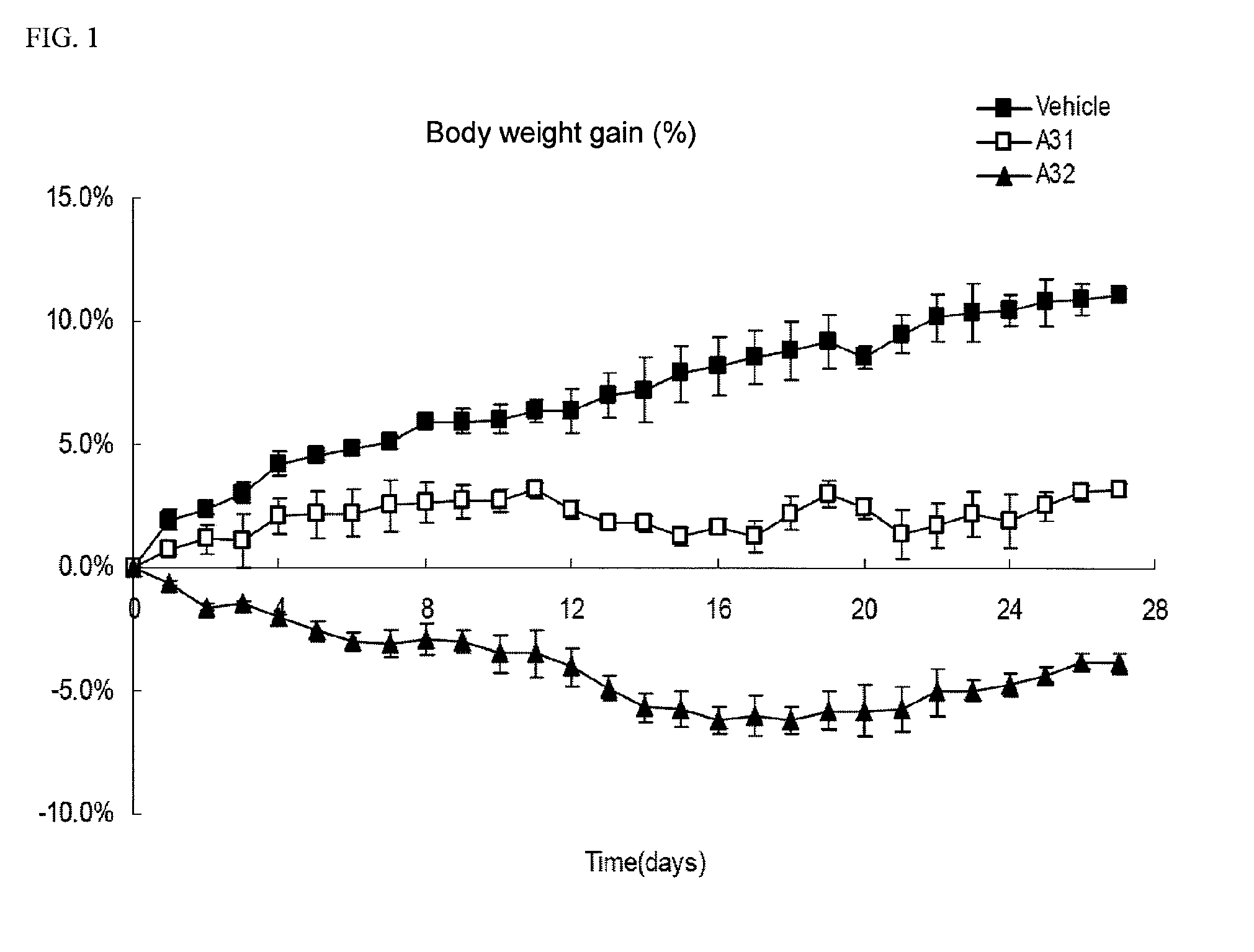
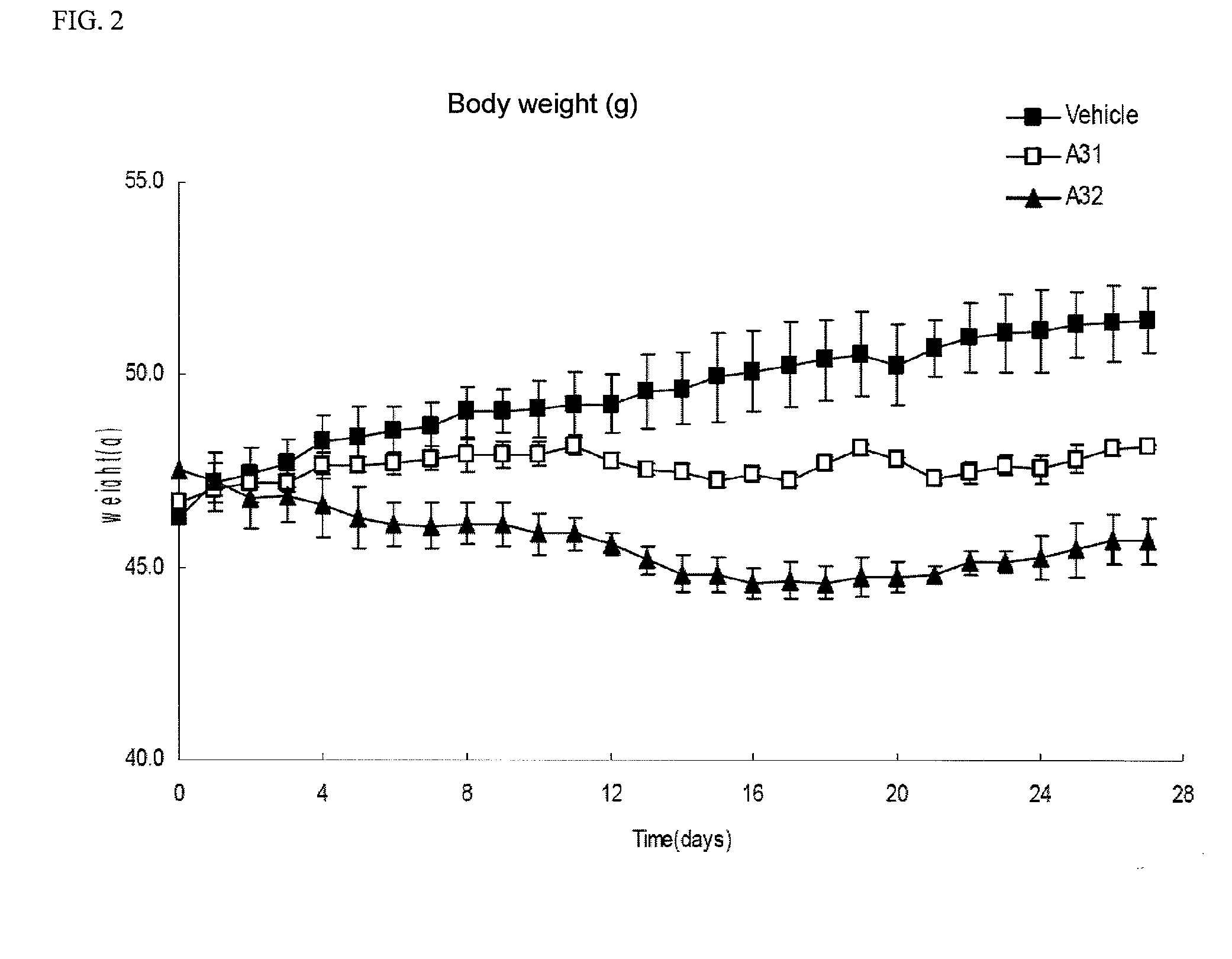
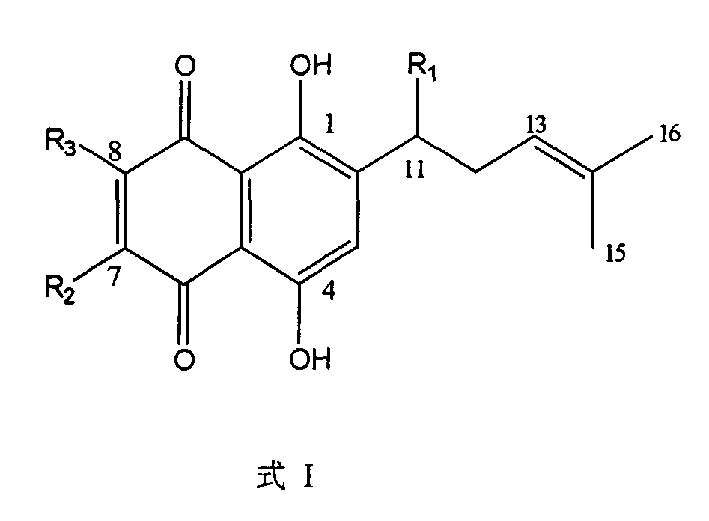
Methods of treating, preventing or suppressing symptoms associated with mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), dominant optic atrophy (DOA); mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Leigh syndrome or Kearns-Sayre Syndrome (KSS) with compounds of Formula (I) are disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods are also disclosed.
Owner:BIOELECTRON TECH CORP
Naphthoquinone compositions with Anti-aging, Anti-inflammatory and skin even-toning properties
InactiveUS20100029784A1Reduce and treat and prevent signEffective protectionCosmetic preparationsBiocideOxidative stressNaphthoquinone
The present invention relates to methods and compositions comprising naphthoquinones such as 2,3-dimethylnaphthalene-1,4-dione, for the use of treating, regulating or preventing a skin condition characterized by oxidative stress or a degenerative process. Methods of preventing, lightening or reducing the appearance of visible and / or tactile discontinuities of the skin resulting from skin pigmentation or skin aging are also disclosed.
Owner:AMPERE LIFE SCI
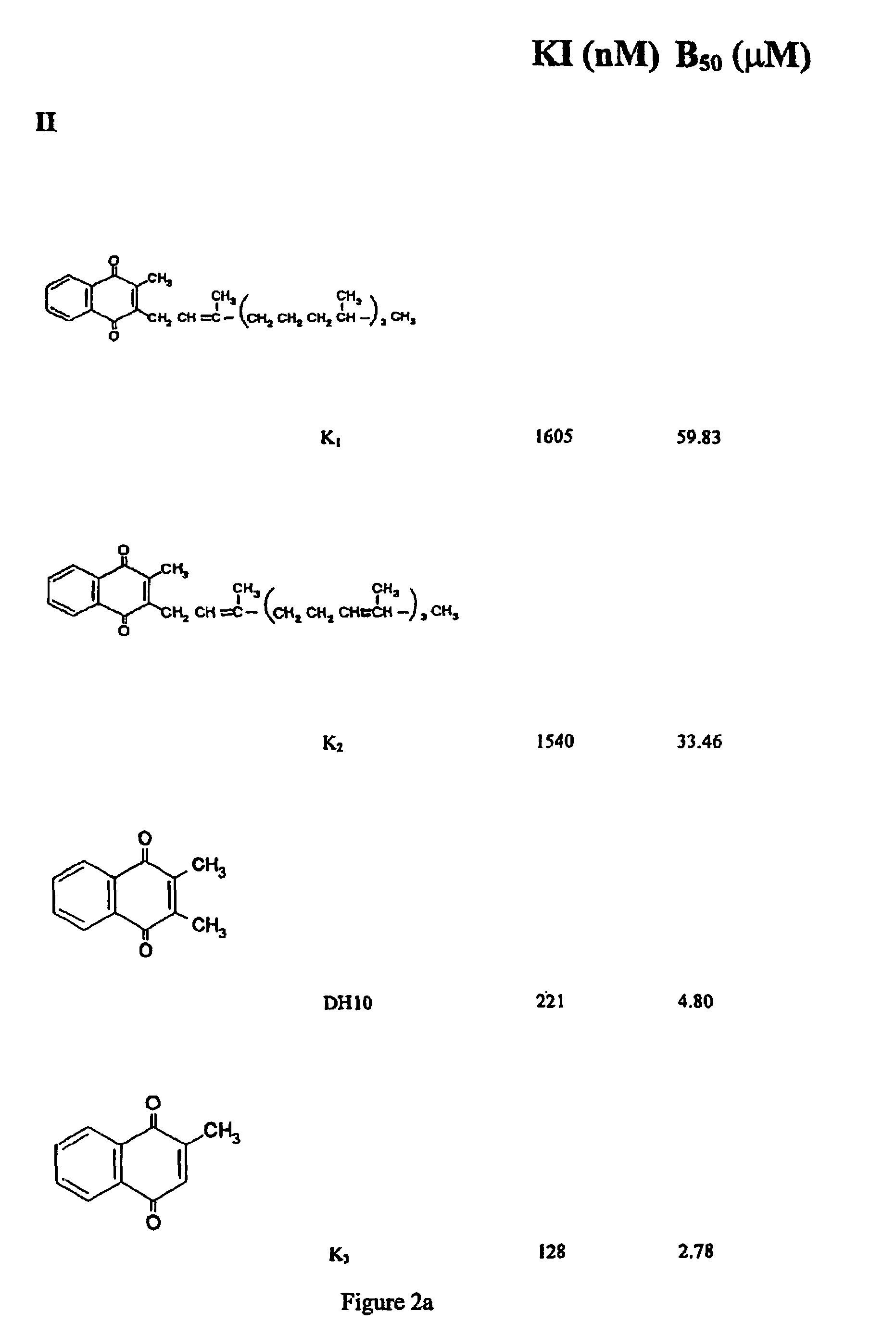
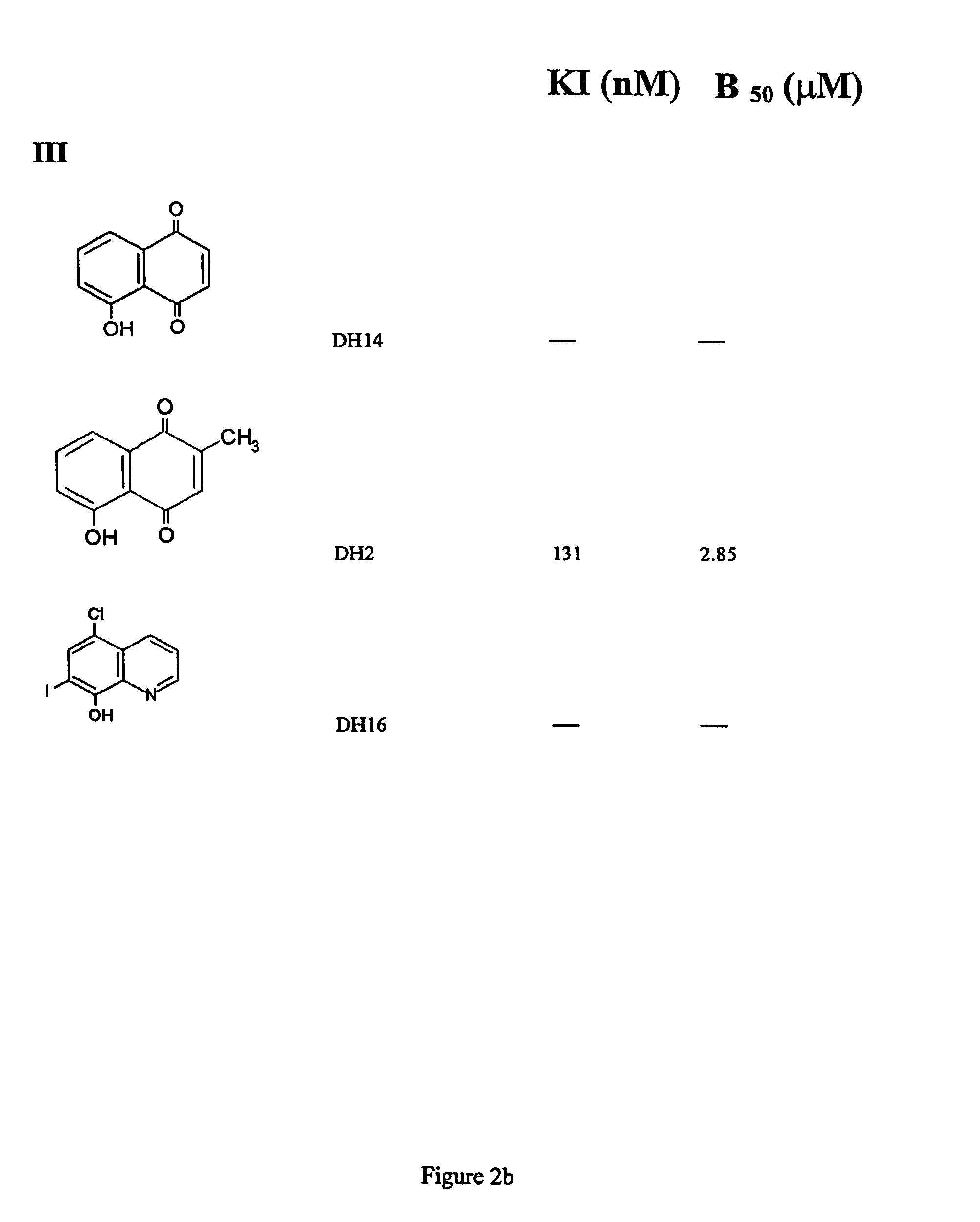
Napthoquinone derivatives as inhibitors of tau aggregation for the treatment of alzheimer's and related neurodegenerative disorders
Provided are napthoquinone-type compounds which can be used to modulate the aggregation of protein (e.g. tau) associated with neurodegenerative disease (e.g. Alzheimer's disease). Structure-function characteristics for oxidised and reduced napthoquinone-type compounds, such as menadione-related compounds, are disclosed. The invention further provides methods of treatment or prophylaxis of neurodegenerative diseases and / or clinical dementias based on the compounds.
Owner:WISTA LAB LTD
Positive resist composition suitable for lift-off technique and pattern forming method
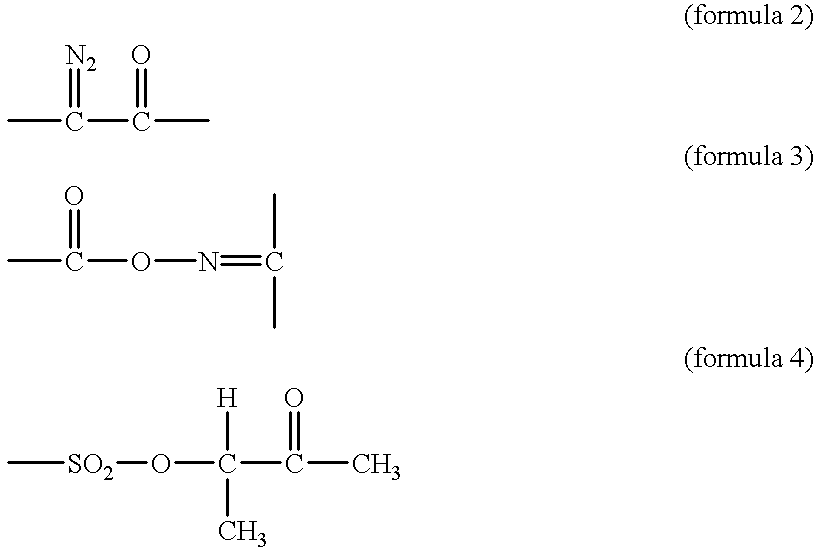
InactiveUS6210855B1High working precision and reliabilitySolve the lack of resistanceSemiconductor/solid-state device manufacturingDiazo compound compositionsBenzeneResist
A positive resist composition contains (A) a novolak resin having a weight average molecular weight calculated as polystyrene of 2,000-20,000 wherein 2.5-27 mol % of the hydrogen atom of a hydroxyl group is replaced by a 1,2-naphthoquinonediazidosulfonyl group and (B) a low molecular aromatic compound having phenolic hydroxyl groups and 2-20 benzene rings wherein the ratio of the number of phenolic hydroxyl groups to the number of benzene rings is between 0.5 and 2.5. By forming a resist layer on a substrate from the positive resist composition and baking the resist layer at 90-130° C., followed by exposure and development, there is formed a resist pattern having an undercut of desired configuration. Owing to high resolution and improved dimensional control, heat resistance and film retention, the resist pattern lends itself to a lift-off technique.
Owner:SHIN ETSU CHEM IND CO LTD
Liquid crystal alignment film, method of manufacturing the film, liquid crystal display using the film and method, and method of manufacturing the liquid crystal display
InactiveUS6368681B1Improve efficiencyImprove heat resistanceLiquid crystal compositionsMaterial nanotechnologyResistUltraviolet
A positive resist mainly composed of a novolak resin and comprising a naphthoquinone diazido-based photosensitizer as an energy beam sensitive resin (e.g., a photosensitive resin) is applied in a thickness of 0.1 to 0.2 mum to a surface of a glass substrate 1 provided with transparent electrodes and dried so as to form a photosensitive film. Next, using a mask, the film is exposed to ultraviolet rays (365 nm). Then, moisture in the air reacts with the resist in an exposed portion 2', thereby generating -COOH groups, with which CH3(CH2)18SiCl3 is allowed to react so as to cause a dehydrochlorination reaction, thereby forming a monomolecular chemisorption film 6 comprising carbon chains 8. This film is used as an alignment film. Thus, the present invention provides a method for producing a uniform and thin alignment film for use in a liquid crystal display panel with a high efficiency without performing a rubbing treatment, and a method for producing a display panel using the same.
Owner:PANASONIC CORP
Carboxylic acid derivatives for treatment of oxidative stress disorders
Disclosed herein are compounds and methods of using such compounds for treating or suppressing oxidative stress disorders, including mitochondrial disorders, impaired energy processing disorders, neurodegenerative diseases and diseases of aging, or for modulating one or more energy biomarkers, normalizing one or more energy biomarkers, or enhancing one or more energy biomarkers, wherein the compounds are quinone or naphthoquinone compounds with carboxylic acid or carboxylic acid derivative substituents.
Owner:BIOELECTRON TECH CORP
Positive photosensitive resin composition, cured film formed from the same, and device having cured film
ActiveCN102667625AImprove heat resistanceHigh transparencyCoatingsPhotosensitive materials for photomechanical apparatusHeat resistanceNaphthoquinone
Disclosed is a positive photosensitive resin composition which contains (a) a polisiloxane, (b) a naphthoquinone diazide compound, and (c) a solvent. The positive photosensitive resin composition is characterized in that the polysiloxane has: an organosilane-derived structure represented by the general formula (1): at a content ration of 20-80% inclusive of Si relative to the overall number of moles of Si atoms in the polysiloxane; and an organosilane-derived structure represented by general formula (2): The positive photosensitive resin composition exhibits high heat resistance, high transparency, and enables high sensitivity, high resolution patterning. The positive photosensitive resin composition can be used to form cured films such as planarization films used in TFT substrates, interlayer insulating films, core materials and cladding materials, and can be used in elements having cured films such as display elements, semiconductor elements, solid-state imaging elements, and optical waveguide elements.
Owner:TORAY IND INC
Use of prodrug composition containing naphthoquinone-based compound for manufacture of medicament for treatment or prevention of diseases involving metabolic syndrome
InactiveUS20090124680A1Prevent and alleviate and ameliorate symptomEffective amountOrganic active ingredientsBiocideNaphthoquinoneDisease cause
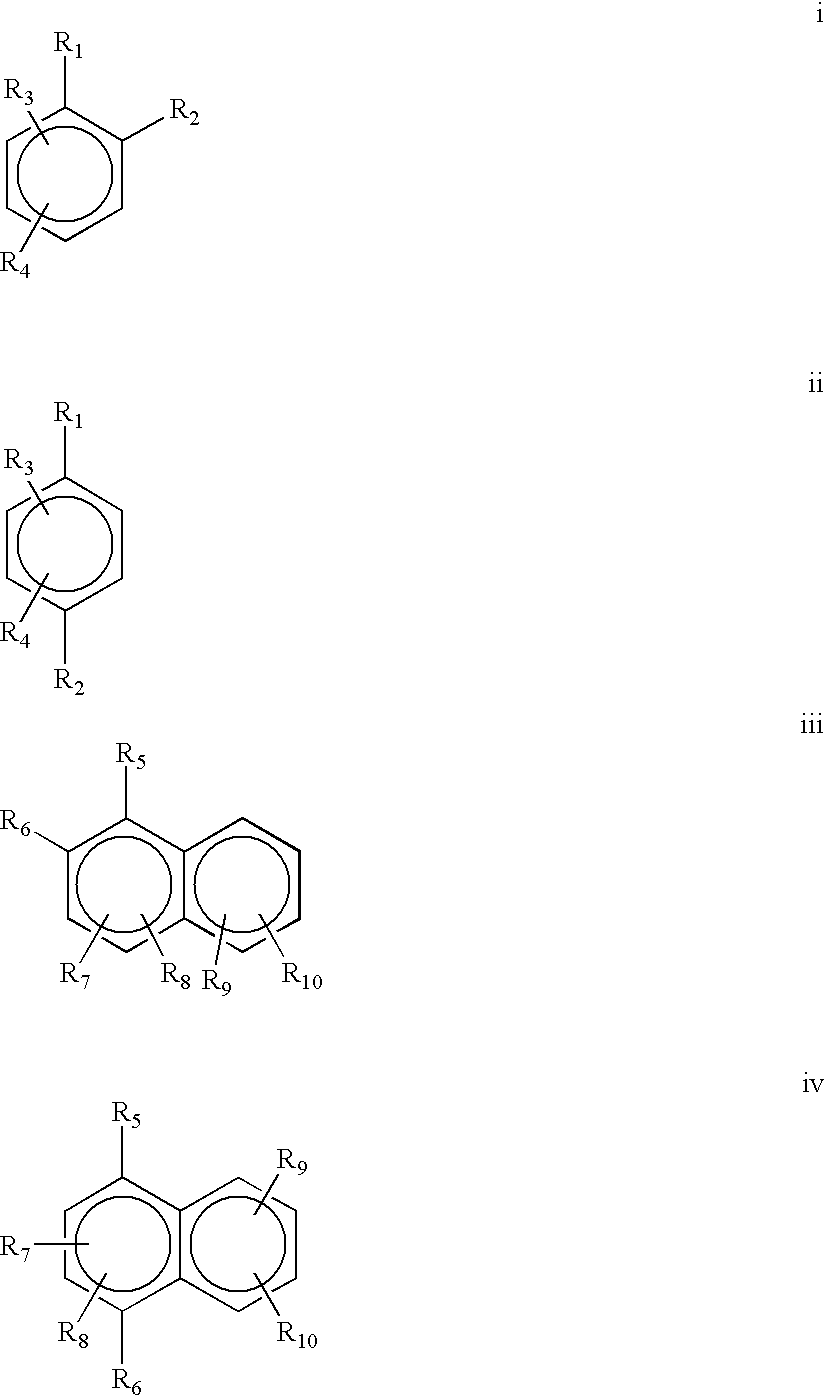
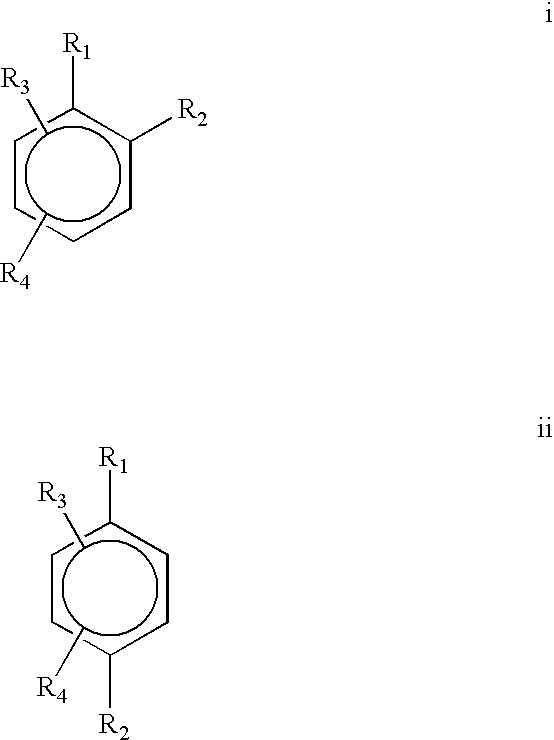
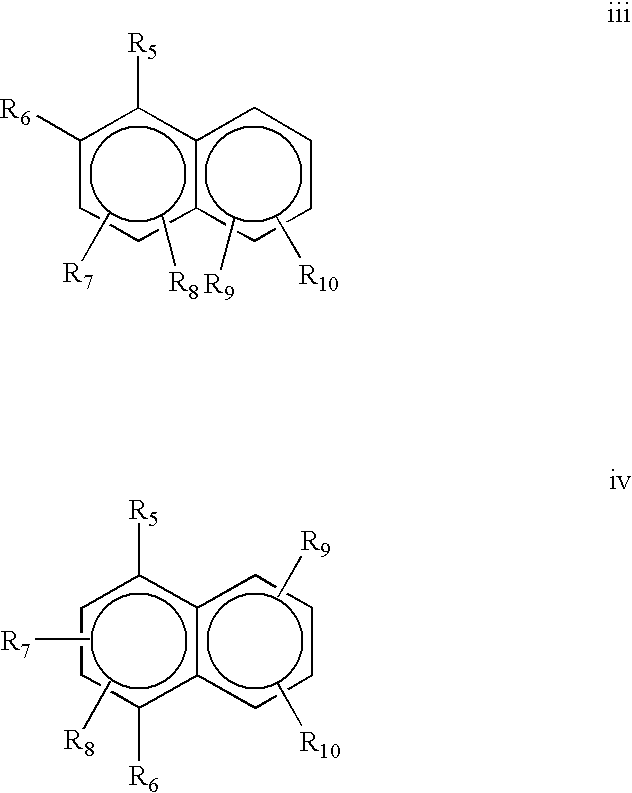
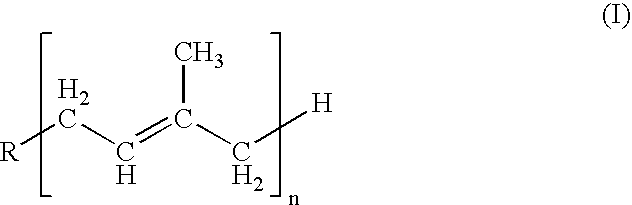
Provided is a use of a prodrug composition containing a naphthoquinone-based compound of Formula 1 for the manufacture of a medicament for treatment or prevention of metabolic syndrome diseases.wherein R1 to R10, X, m and n are as defined in the specification.
Owner:MAZENCE INC +1
Gronwell naphthaquinone derivative and its application in preparing anticancer medicine
The present invention relates to the gromwell naphthoquinone derivative and its application in preparing anticancer medicine. Experiment shows that the gromwell naphthoquinone derivative has obvious inhibition to tumor cell strain CNE2, Glc-82, S180A, EAC, HepA, etc, and the research on nude mouse transplantation tumor model of human cancer cell also proves the anticancer action of the gromwell naphthaquinone derivative.
Owner:SUN YAT SEN UNIV
Oxygen scavenger
InactiveUS20020100896A1Adequate effectExhibit excellent oxygen scavenging effectHydrogenOther chemical processesNaphthoquinonePhotochemistry
An oxygen scavenger, containing a heterocyclic compound having a N-substituted amino group as an effective component, can adequately exhibit the oxygen scavenging effect in not only feed water lines for high temperature water, a boiler, and steam and condensate return lines, but also feed water lines for low temperature water. The oxygen scavenger contains: a heterocyclic compound having a N-substituted amino group or a salt thereof; a hydroxybenzene derivative and / or a naphthoquinone derivative; and neutral amine.
Owner:KURITA WATER INDUSTRIES LTD
Isoprenyl derivatives and their use in the title treatment and prevention of osteoporosis and cardiovascular calcification
A non-toxic biologically active compound is provided having the following general formula (I): wherein n is an integer from 1 to 14, preferably from 2 to 4, and R is an organic moiety, preferably a group different from but structurally substantially similar to 2-methyl naphthoquinone, or a group P—C(R1)—P, where each P stands for a—PO(OH)2 group and R1 is a (poly)isoprenyl group, hydroxy, halogen (preferably chloro or bromo), or hydrogen, or a pharmaceutically acceptable derivative thereof. These compounds are useful for the treatment or prevention of certain disorders in a mammal, especially a human being, for example postmenopausal loss of bone in women, juvenile or senile osteoporosis in men and women, cardiovascular calcification and other ectopic calcifications.
Owner:NATTOPHARMA
Positive-type photosensitive polyimide precursor composition
InactiveUS6524764B1Diazo compound compositionsPhotosensitive material processingPolymer scienceStructural unit
The present invention relates to a positive type photosensitive resin precursor composition which is characterized in that it contains polymer having, as its chief component, structural units represented by the following general formula (1) and, furthermore, in that it satisfies the following conditions (a) and / or (b). The invention provides an alkali-developable photosensitive composition.(a) There is included an ester of a naphthoquinone diazide sulphonic acid and a phenol compound of dipole moment 0.1 to 1.6 debye(b) There is included a phenol compound represented by general formula (8) and a naphthoquinone diazide sulphonic acid and / or an ester of a phenol compound represented by general formula (8) and a naphthoquinone diazide sulphonic acid(In general formula (1), R1 represents a bivalent to octavalent organic group with at least two carbon atoms, R2 represents a bivalent to hexavalent organic group with at least two carbon atoms, and R3 represents hydrogen or an organic group with from one to ten carbons. n is an integer in the range 10 to 100,000, m is an integer in the range 0 to 2, and p and q are integers in the range 0 to 4, but p and q are not simultaneously 0.)(In the formula, R23, R24, R26 and R27 each represents a hydrogen atom or a C1-8 alkyl group, alkoxy group, carboxyl group or ester group. At least one R25 is hydroxyl group, while the rest represent hydrogen atoms and C1-8 alkyl groups. aa, bb, cc and dd represent integers in the range 0 to 3. However, aa+bb<=5, bb+dd<=5 and aa+bb>0. ee represents an integer in the range 1 to 3.)
Owner:TORAY IND INC
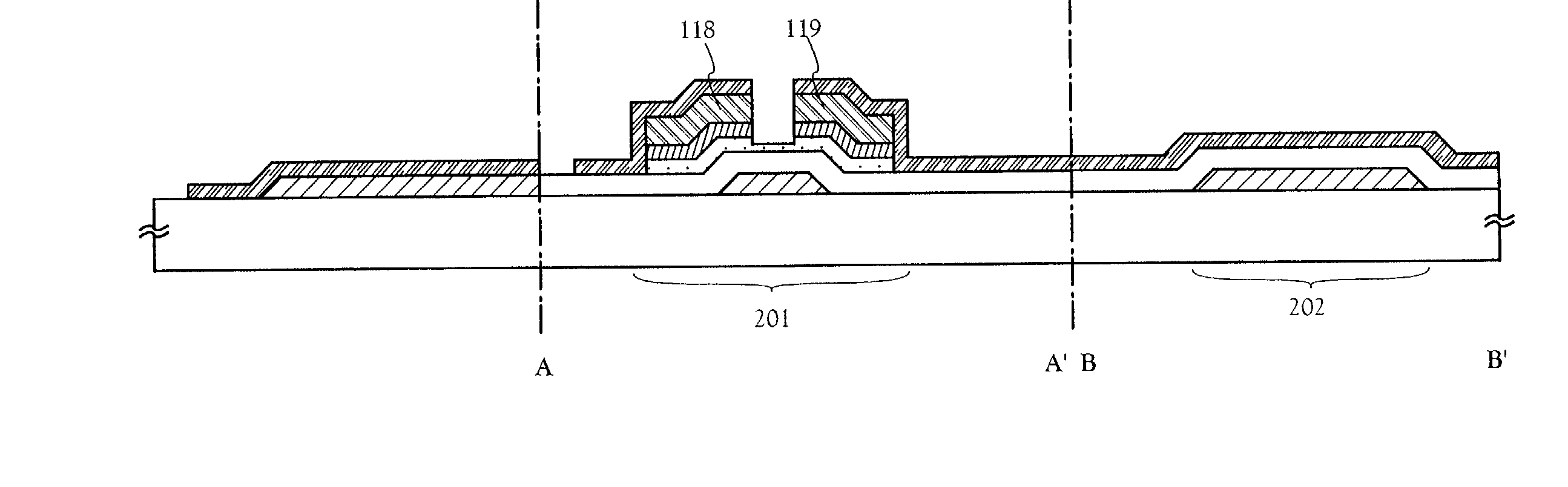
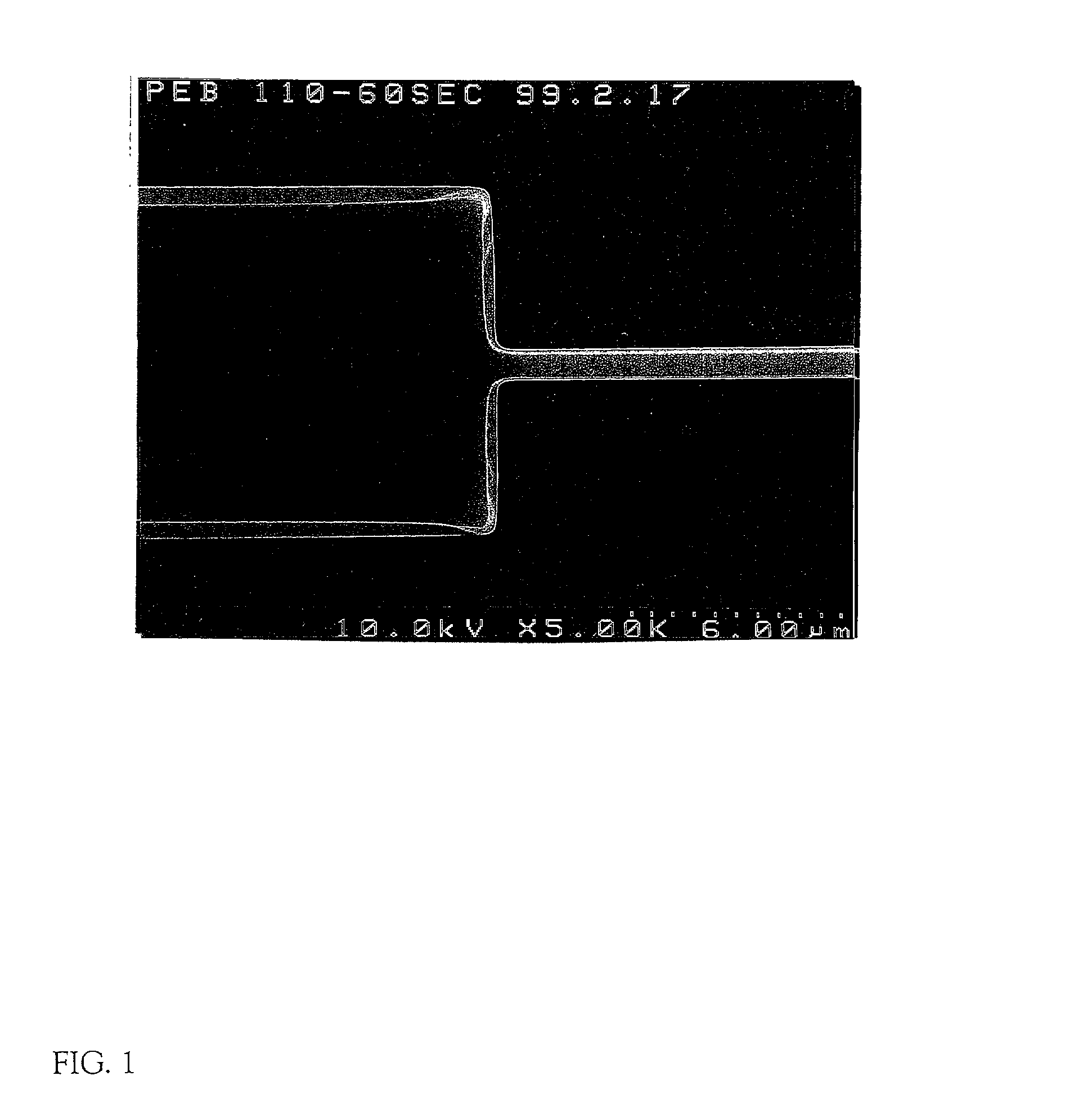
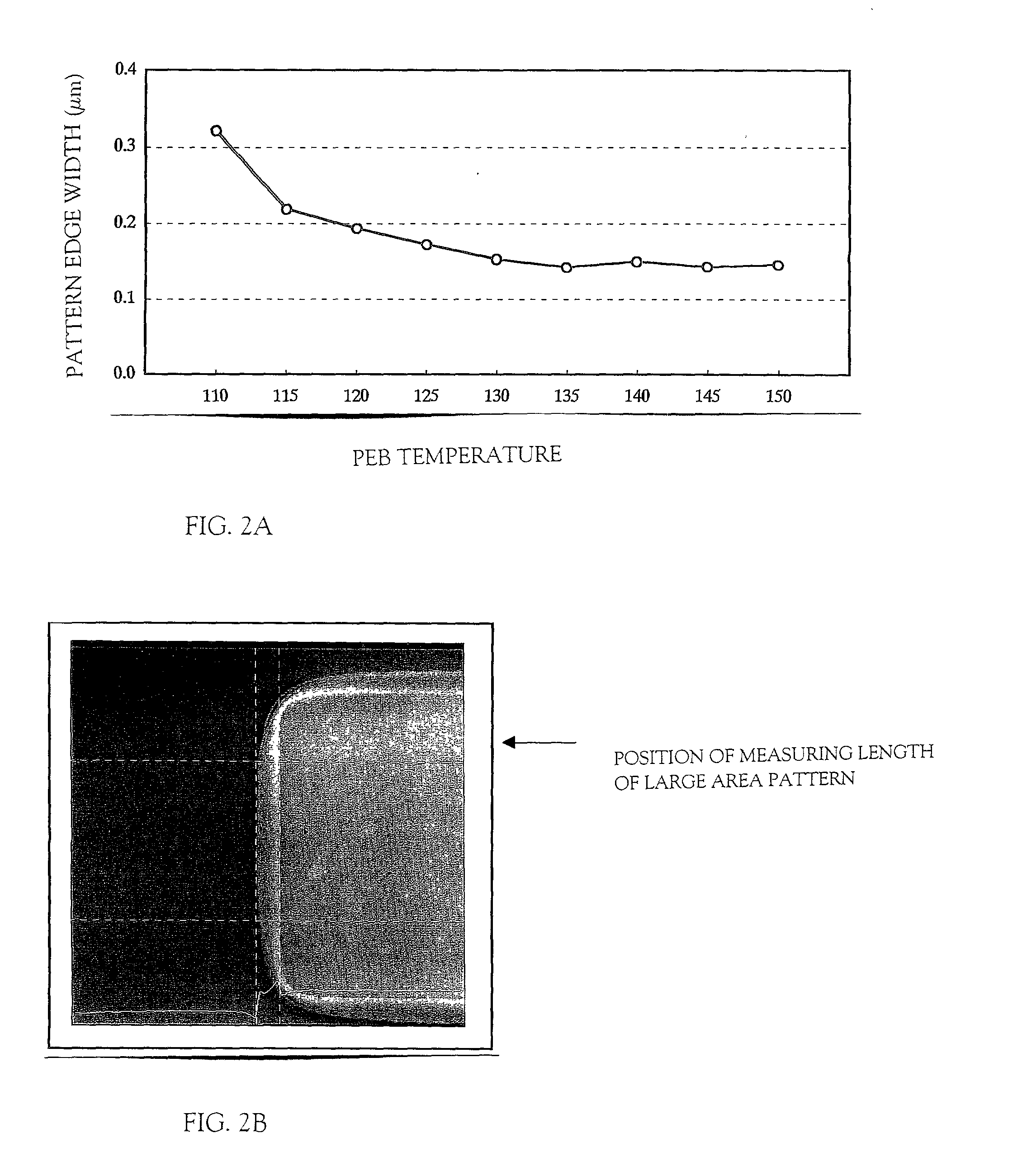
Method of manufacturing a semiconductor device
InactiveUS20020127887A1Evacuation of the solvent from the resist pattern at post-bake is relativelyEase of evacuationSemiconductor/solid-state device manufacturingNon-linear opticsResistNaphthoquinone
In a patterning process of a semiconductor device having inverted stagger type TFTs, a normal photolithography step using diazo naphthoquinone (DNQ)-Novolac resin based positive photo resist is applied, and a problem of the area dependency of the photo resist pattern side wall taper angle may occur. The problem is critical for the reason of influence on variation of an etching shape in a dry-etching step. The present invention has an object to solve the above problem. In a photolithography step, which is patterning step of a semiconductor device having inverted stagger type TFTs, by adjusting a pre-bake temperature or a PEB (post-exposure-bake) temperature, and positively performing evacuation of solvent in a state of a photo resist film, the volume contraction by evacuation of solvent at the post-bake is reduced, and the problem of the area dependency of the photo resist pattern side wall taper angle is solved, which is deformation due to the volume contraction.
Owner:SEMICON ENERGY LAB CO LTD
Positive photosensitive siloxane composition
ActiveUS20140335452A1High sensitivityImprove heat resistancePhotosensitive materialsPhotomechanical apparatusPolymer scienceSilanes
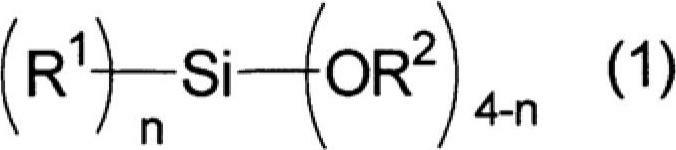
A positive photosensitive siloxane composition comprising at least three types of following polysiloxanes (A), (B) and (C) obtained by hydrolyzing and condensing a silane compound represented by general formula (1) R1nSi (OR2)4-n, a diazonaphthoquinone derivative, and a solvent: a polysiloxane (A) such that if pre-baked the film thereof will be soluble in a 5 weight % TMAH aqueous solution and the solution rate of said film will be 1,000 Å / sec or less; a polysiloxane (B) such that if pre-baked the solution rate of the film thereof will be 4,000 Å / sec or more relative to a 2.38 weight % TMAH aqueous solution; and a polysiloxane (C) such that if pre-baked the solution rate of the film thereof will be between 200 and 3,000 Å / sec relative to a 2.38 weight % TMAH aqueous solution. (In the formula, R1 represents a C1-20 linear or branched cyclic alkyl group, in which any methylene may be substituted by oxygen, or a C6-20 aryl group, in which any hydrogen may be substituted by fluorine; n represents a 0 or a 1; and R2 represents a C1-5 alkyl group.)
Owner:MERCK PATENT GMBH
An ultraviolet positive photoresist
ActiveCN109062008AHigh resolutionIncrease photosensitivityPhotomechanical apparatusSulfonyl chlorideSolvent
The invention provides an ultraviolet positive photoresist suitable for exposure under a UV light source. The photoresist is composed of, by mass percent, 1 to 10% of diazonaphthoquinone sulfonate photosensitizer with special structure, 10 to 50% of linear phenolic resin, 0.2 to 1% of sensitizer, 0.1 to 1% of toughening agent, 0.4 to 1% of adhesion promoter, 0.2 to 1% of leveling agent and the balance of solvent; wherein the diazonaphthoquinone sulfonate photosensitizer with special structure is obtained by subjecting 1, 1- P-hydroxyphenyl-[1-Biphenyl group-4-Isopropyl group-(1-o-methyl-4-phenol)] propane and 2-Diazo-1-Naphthoquinone-5-sulfonyl chloride to substitution reaction or esterification reaction according to the ratio of 1: 2; The linear phenol-formaldehyde resin is a mixture oflinear phenol-formaldehyde resin 1 and linear phenol-formaldehyde resin 2, and the weight ratio of linear phenol-formaldehyde resin 1 and linear phenol-formaldehyde resin 2 is 2-8: 8-2. The ultraviolet positive photoresist of the present invention has higher resolution, photosensitivity and size reducibility than the conventional diazonaphthoquinone sulfonate Phenolic resin system photoresist.
Owner:XILONG SCI CO LTD
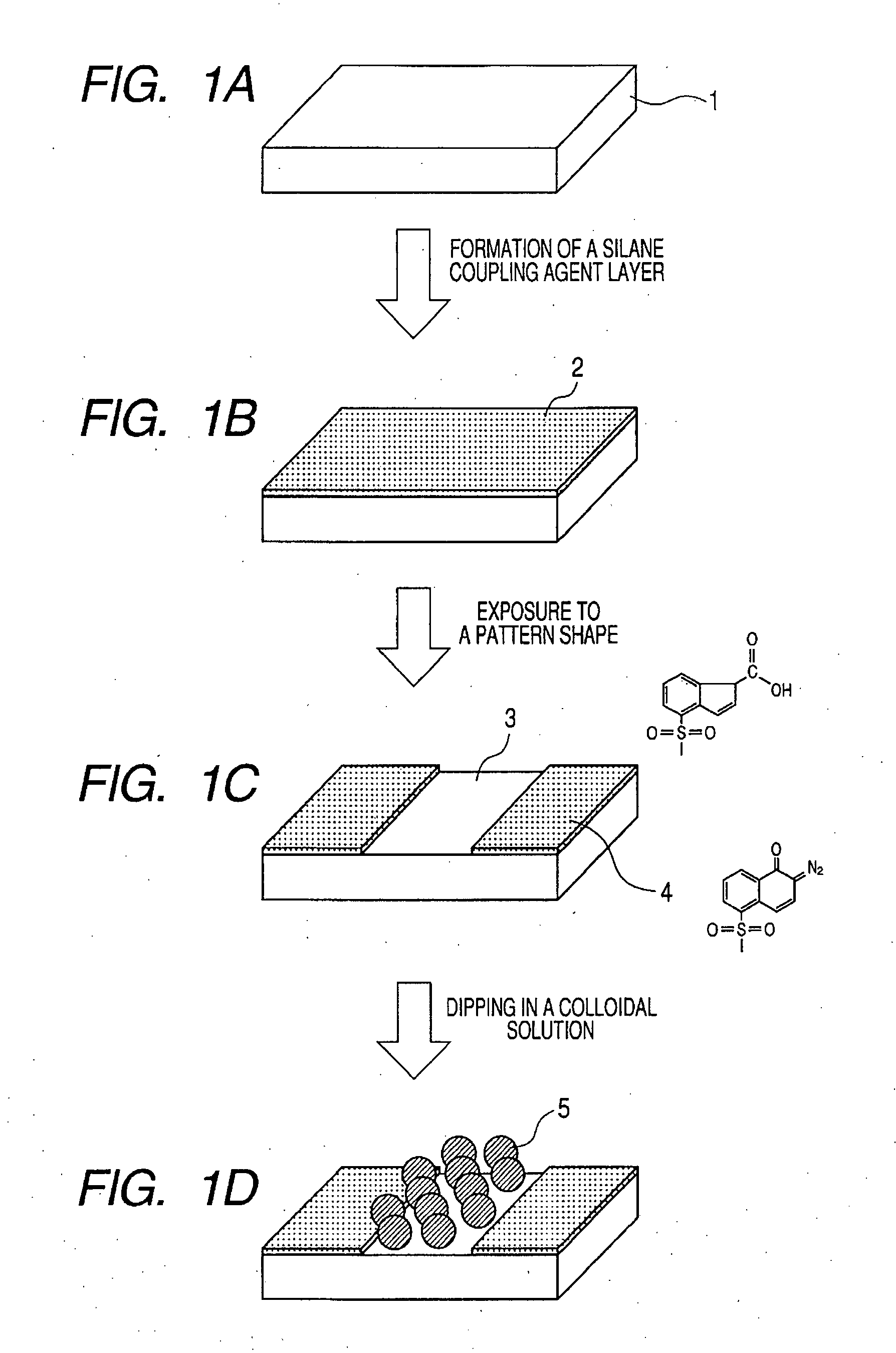
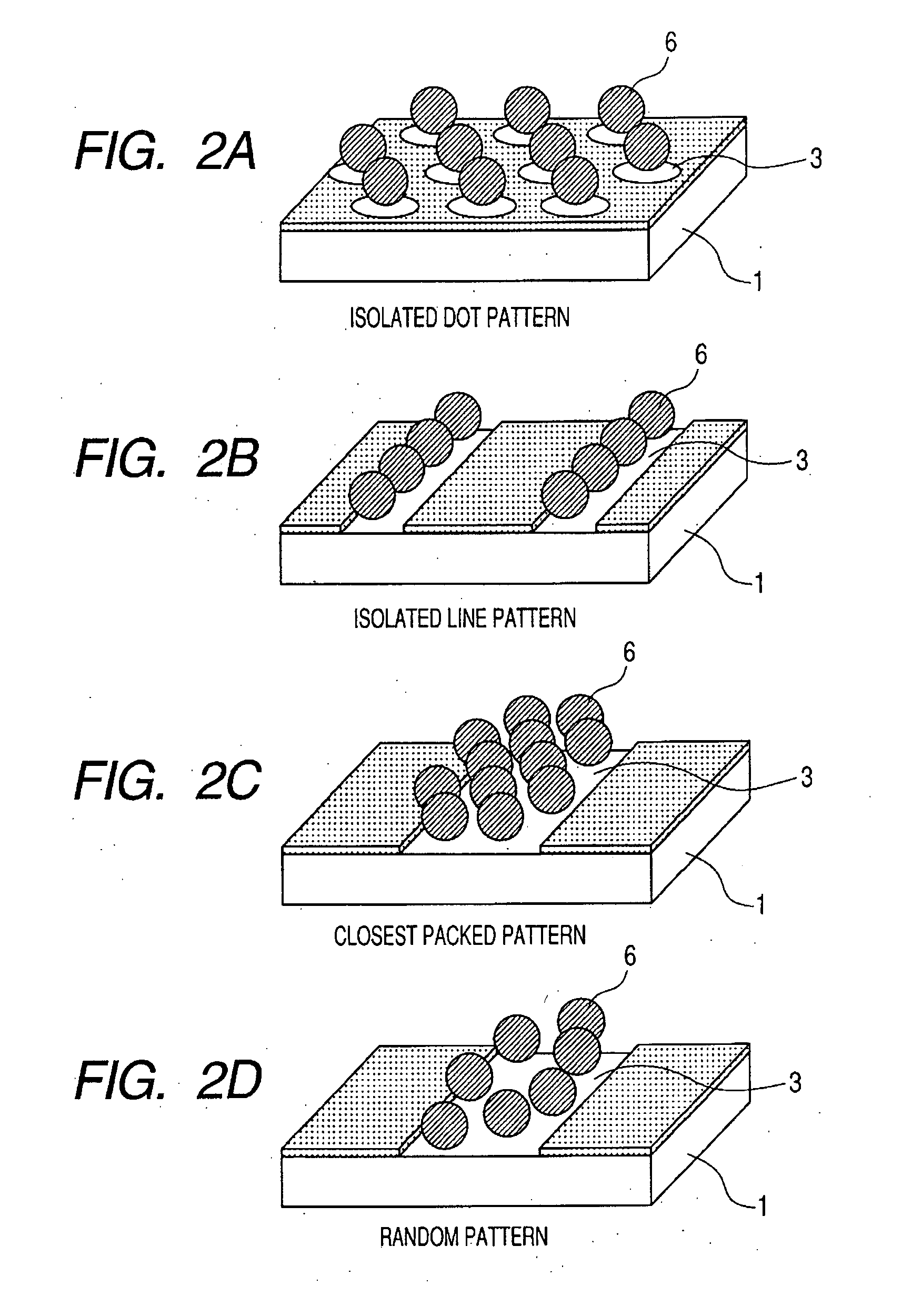
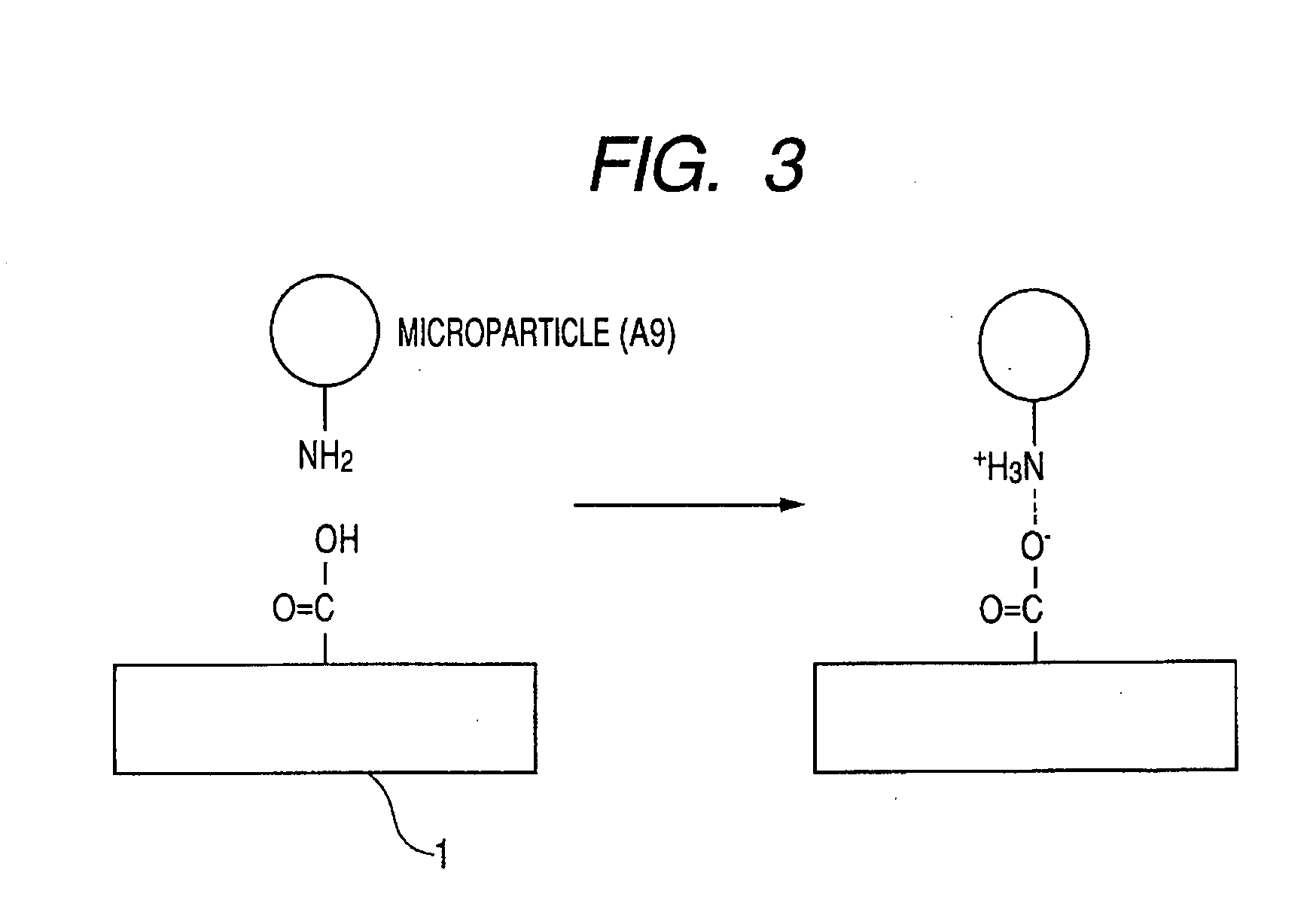
Photosensitive silane coupling agent, method of modifying surface, method of forming pattern, and method of fabricating device
InactiveUS20070218398A1Group 4/14 element organic compoundsPhotosensitive materials1,2-NaphthoquinoneNaphthoquinone
Provided are a photosensitive silane coupling agent for forming a low-defect microparticle pattern, dot array pattern, or hole array pattern through fewer steps, and a method of forming a pattern using such photosensitive silane coupling agent. Used is a photosensitive silane coupling agent comprising a 1,2-naphthoquinone-2-diazido-5-sulfonyl group or a 1,2-naphthoquinone-2-diazido-4-sulfonyl. group.
Owner:CANON KK
Preparation method and application of polypyrrole functional mediator doped with water-soluble anthraquinone or naphthoquinone compound
InactiveCN102277590ADoping ratio increaseHigh speedElectrolysis componentsTreatment with anaerobic digestion processesPolypyrrolePyrrole
The invention discloses a preparation method of a polypyrrole functional mediator doped with a water-soluble anthraquinone or naphthoquinone compound and application thereof. Specifically, the preparation method comprises: (1) preparing a polymerization solution: firstly preparing a saturated solution of the water-soluble anthraquinone or naphthoquinone compound, then adding 0.33-0.67mL of pyrrole to every 100mL of the saturated solution and mixing well; (2) pretreating active carbon felt and platinum plate electrodes; (3) embedding the pretreated platinum plate electrodes in step (2) into the active carbon felt, then conducting electrochemical polymerization in the polymerization solution, with a polymerization potential of 0.30-0.50V, a polymerization time of 1-3h and a potential changerate of 0.03-0.07V / s. The polypyrrole functional mediator provided in the invention plays an accelerating role in a microorganism denitrification process, and is recyclable and free of secondary pollution.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Process for preparing water base developing photosensitive polyimide material
InactiveCN1648154AHigh photosensitivityGood film formingPhotosensitive materials for photomechanical apparatusWater basedBenzoic acid
The present invention belongs to the preparation process of water base developing photosensitive polyimide material. Aromatic dialphanyl diacyl chloride, 3,5-diamino benzoic acid, 4, 4'-diamino-3, 3'-dihydroxy diphenyl methane and 4, 4'-diamino-3, 3'-dihydroxy diphenyl sulfone are polymerized to produce polyamate as one kind of imide prepolymer. The prepolymer has characteristic viscosity of 0.35-0.50, and when 2-diazo naphthoquinone derivative as photosensitizer is added, the prepolymer becomes excellent photosensitive material with high photosensitive performance, good filming performance and high heat resistance.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Immobilization support, process for producing the same, electrode, process for producing the same, electrode reaction utilizing apparatus and process for producing the same
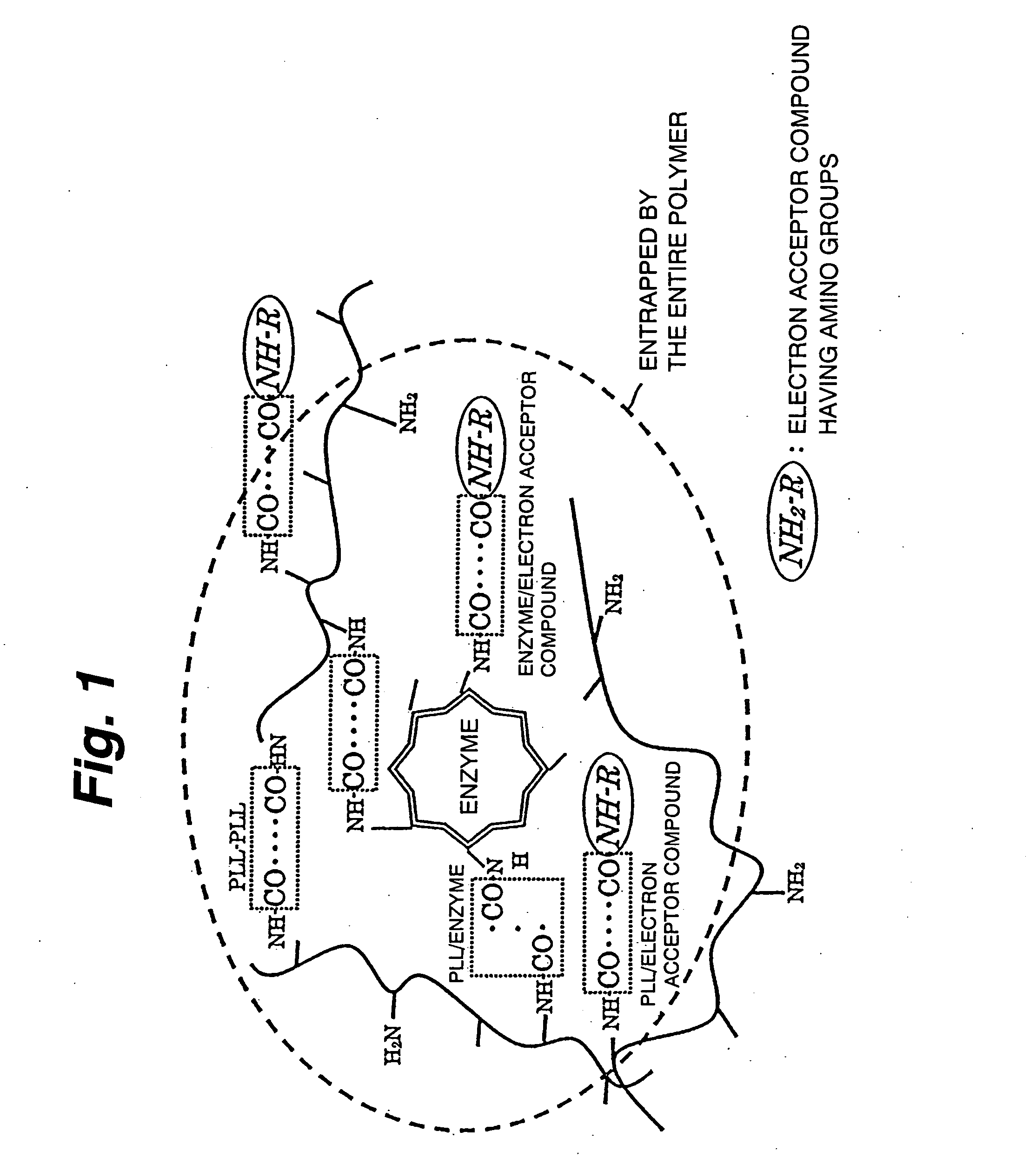
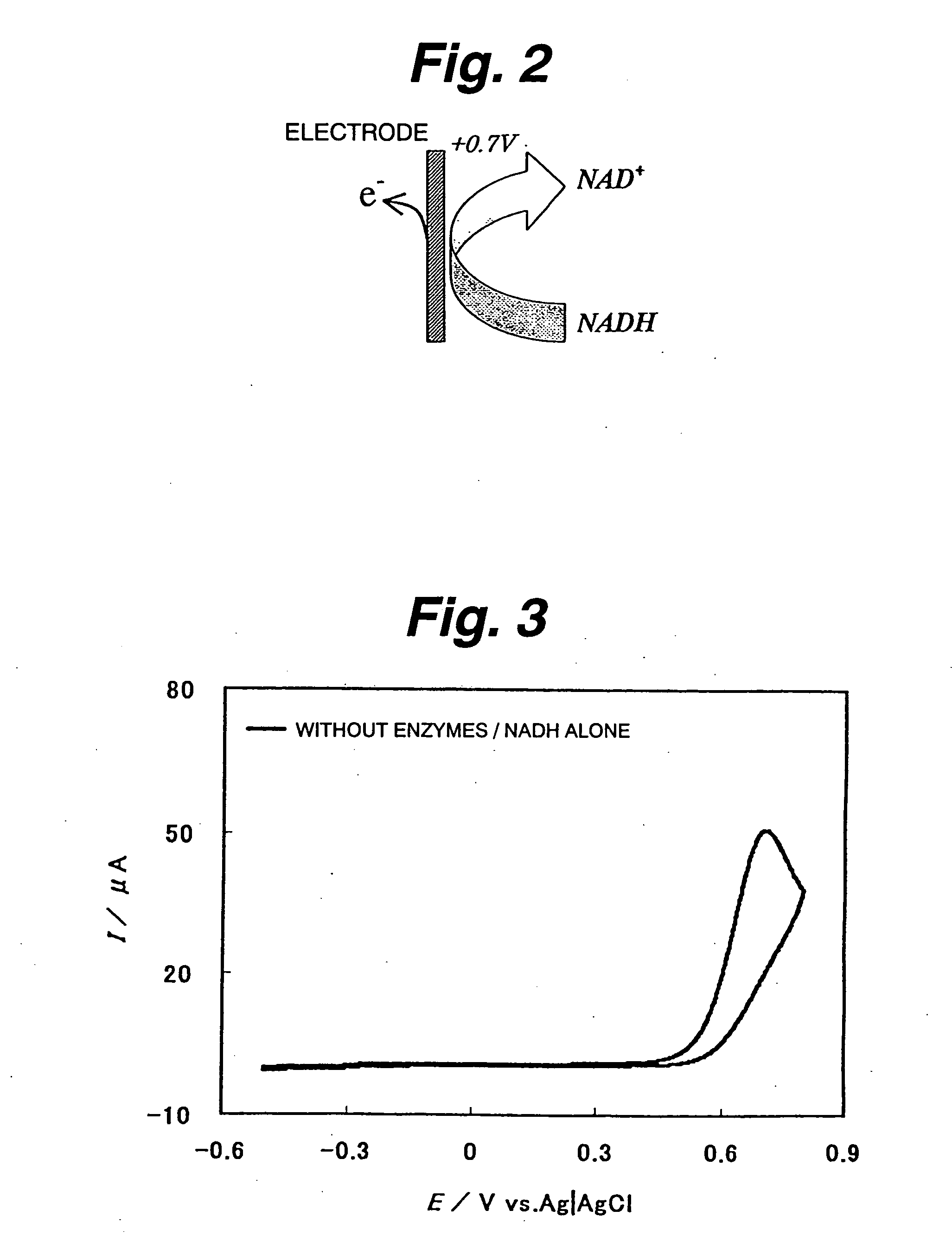
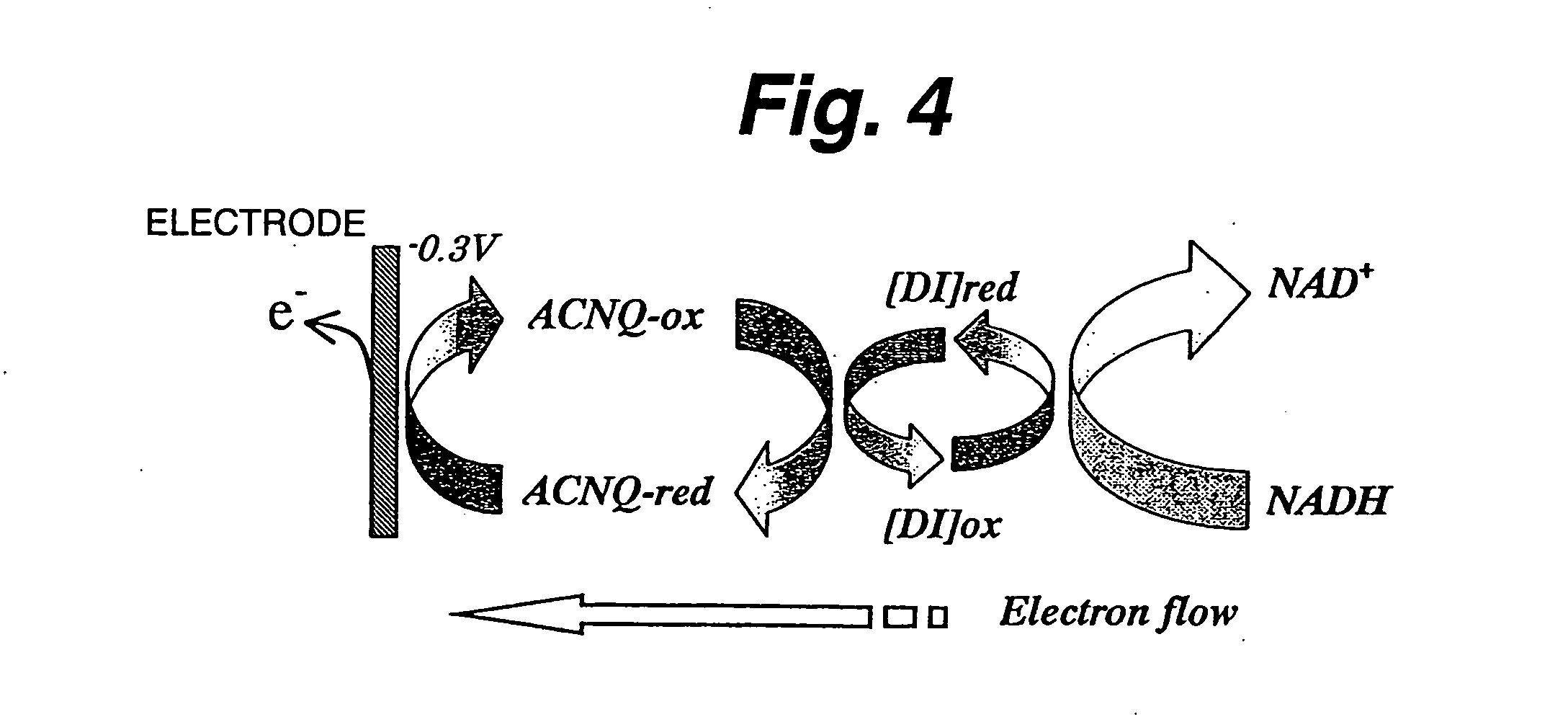
ActiveUS20060105418A1Electron transferFacilitates electron transferImmobilised enzymesBioreactor/fermenter combinationsNaphthoquinoneEnzyme
An immobilization carrier containing an electron acceptor compound is used in addition to glutaraldehyde and poly-L-lysine to immobilize an enzyme and an electron acceptor compound simultaneously to an electrode. For example, here are used diaphorase as the enzyme and 2-amino-3-carboxy-1,4-naphthoquinone (ACNQ) as the electron acceptor compound.
Owner:MURATA MFG CO LTD
Cyanide-free silver plating solution additive
The invention relates to a cyanide-free silver plating solution additive which comprises the following components by ratio: 0.1-10g / l of brightener, 5-10g / l of leveling agent, 100-600g / l of complexing agent and the balance of plasma water, wherein the brightener is one or mixture of more in nitrogen-containing compound, triazole, benzotriazole, 2-hydroxypyridine, pyridine, 22 dipyridyl, 1, 10-phenanthroline, triethylene tetramine and diethylene triamine according to any ratio; the leveling agent is one or mixture of more in aromatic hydrocarbon compounds, naphthalene, 1-methylnaphthalene, 1, 4-naphthoquinone and 1-naphthol according to any ratio; the complexing agent is one or mixture of more in disodium ethylenediamine tetraacetate, niacin, aminosulfonic acid and potassium pyrophosphate according to any ratio. The cyanide-free silver plating solution additive has the beneficial effects that the plating solution is stable, low in toxicity and good in dispersing ability; the obtained plating layer is bright and fine as well as good in binding force; the technology adopts the environment-friendly organic additive which does not contain heavy metal and sulfide; the plating layer is good in corrosion resistance. Furthermore, the cyanide-free silver plating solution additive can be directly used for parts such as brass, copper, chemical nickel and the like, preplating is not needed, and the binding force is also guaranteed.
Owner:HANGZHOU WIN WIN TECH CO LTD
Method for preparing perylenequinone pigment under catalysis of Shiraia bambusicola
InactiveCN101603062AImprove conversion rateLow costMicroorganism based processesFermentationNaphthoquinoneMicrobiology
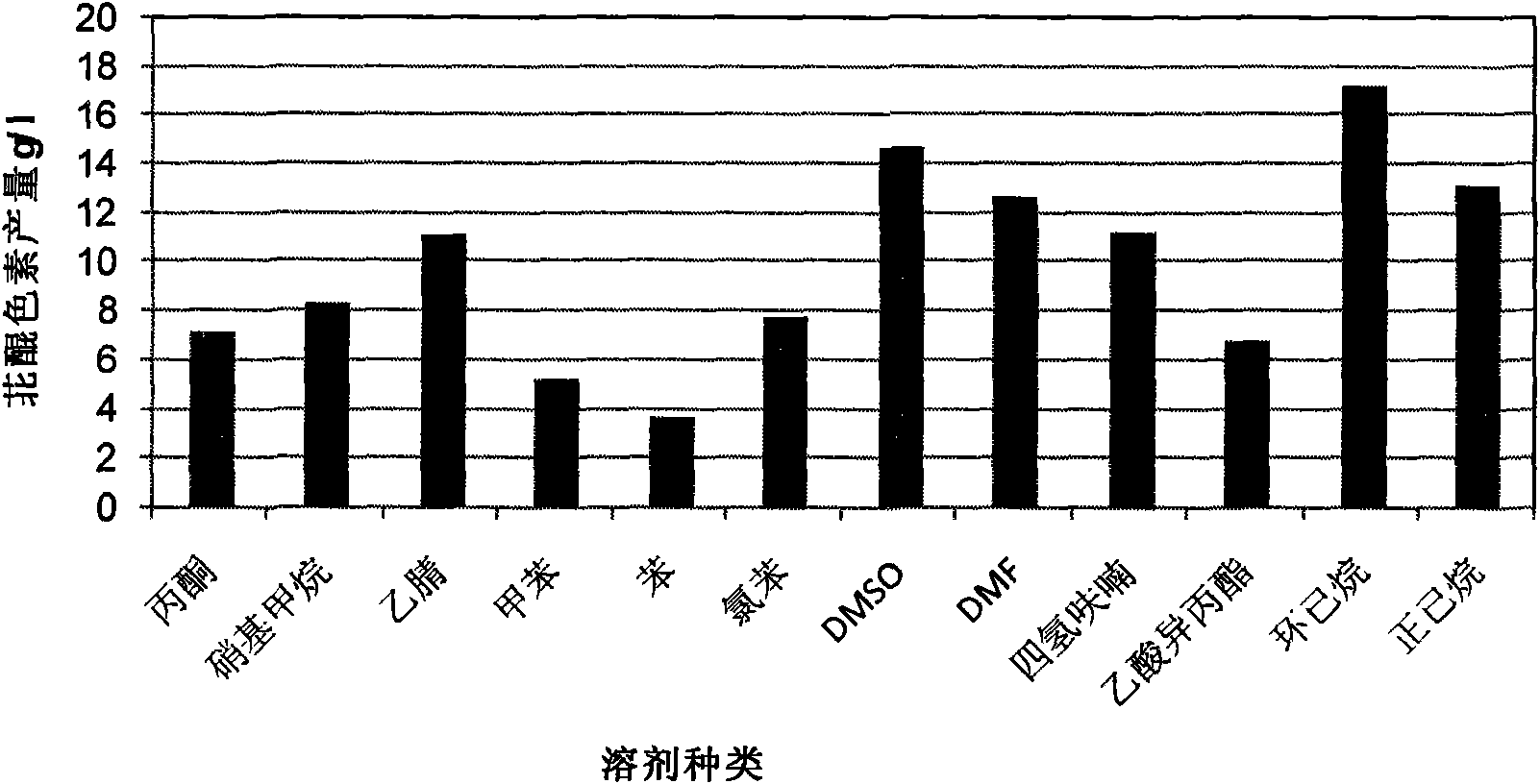
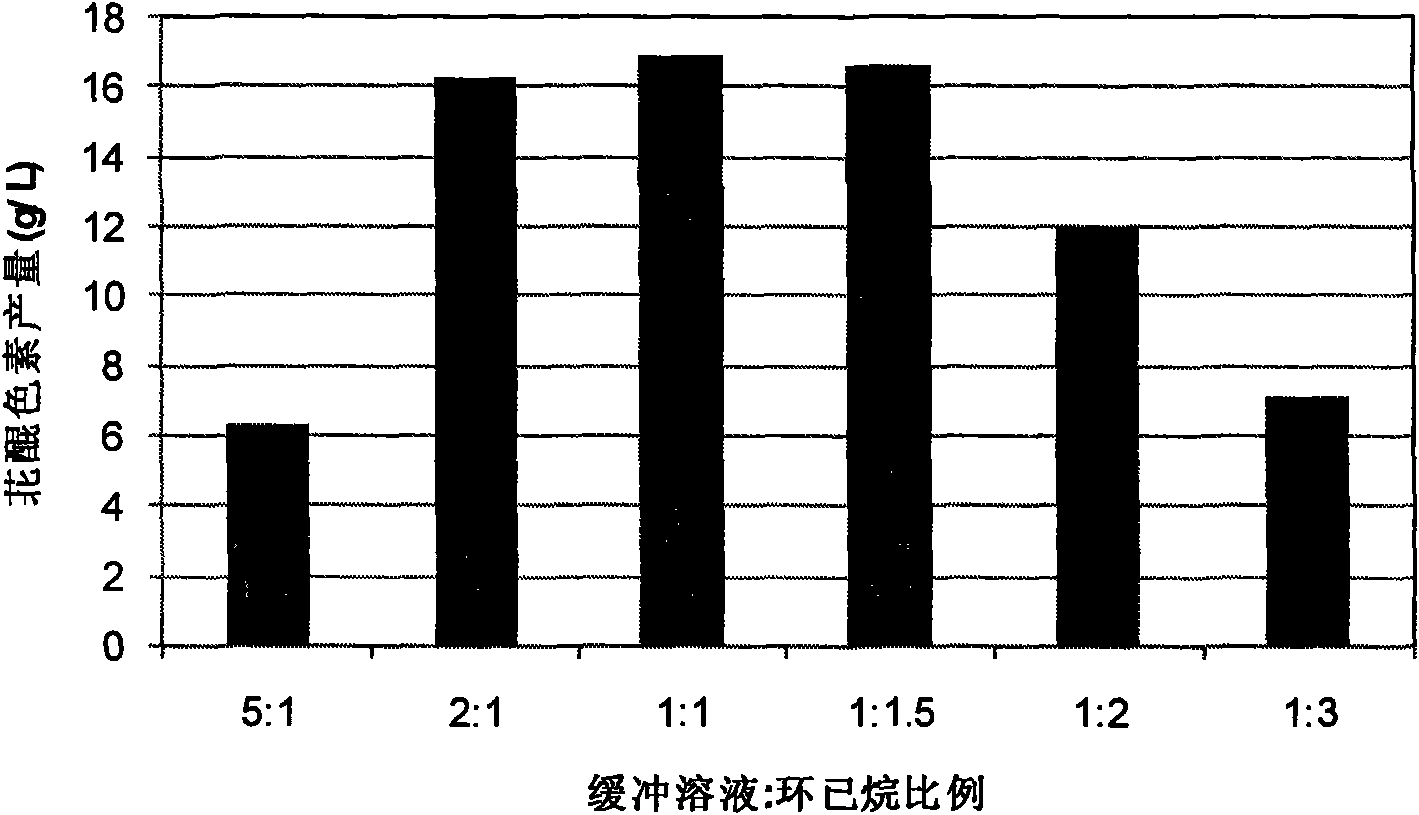
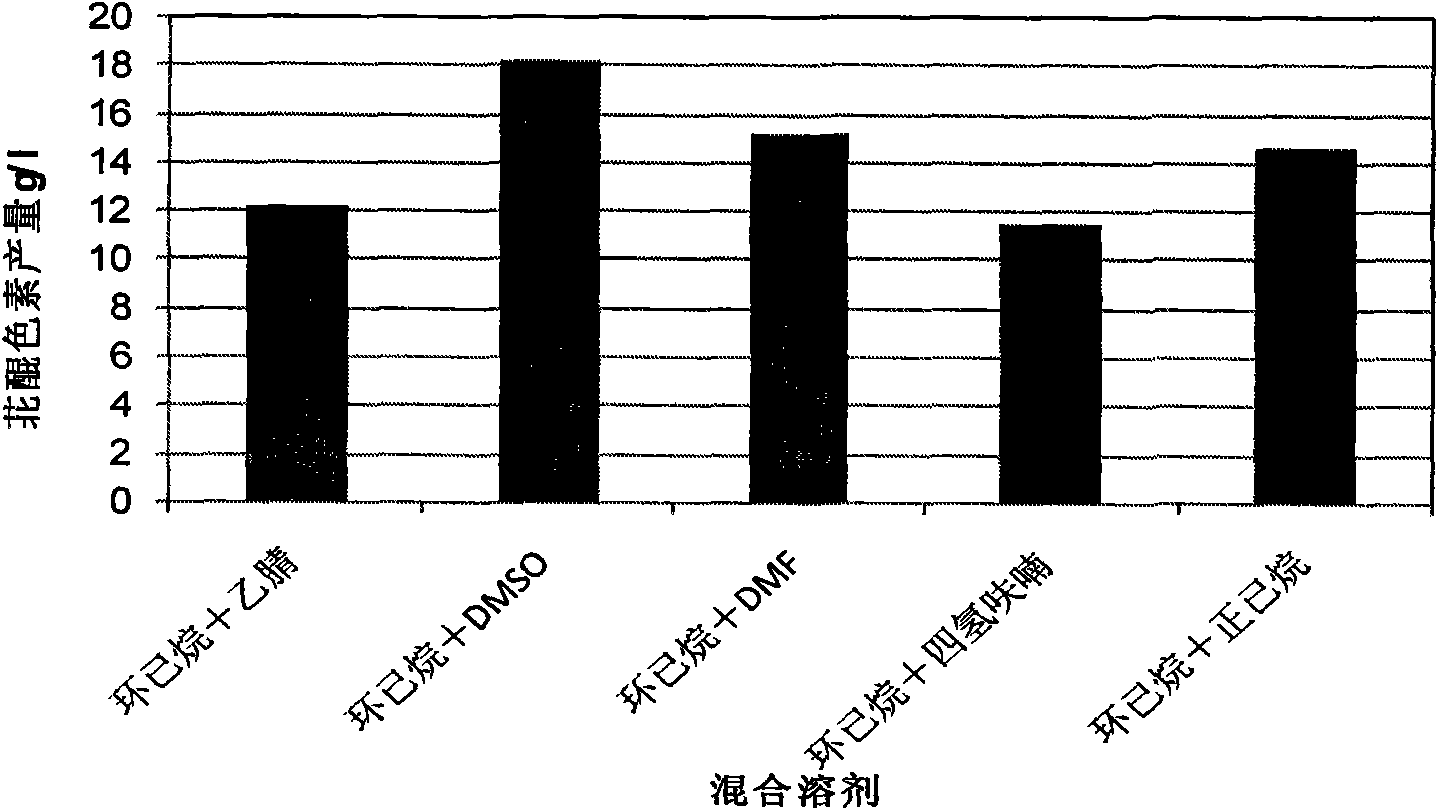
The invention discloses a method for preparing a perylenequinone pigment under the catalysis of Shiraia bambusicola and belongs to the technical field of bioengineering. In the method, naphthoquinone, cinnamic acid and the like which form a substrate are used to prepare the perylenequinone pigment under the catalysis of the Shiraia bambusicola. The catalytic process is implemented by: A), adding the substrate into culture system in a cell liquid culture process, wherein the perylenequinone pigment yield is up to 2.9g / L and the transformation rate is up to 9.7 percent; or B), placing cells obtained by liquid fermentation culture in a reaction system having the substrate, wherein the perylenequinone pigment yield is up to 35.3g / L and the transformation rate is up to 39.7 percent; or C), adding the substrate into a culture system in a cell solid culture process, wherein the perylenequinone pigment yield is up to 1.5g / 100g and the transformation rate is up to 46 percent. The method adopts a perylenequinone pigment-producing Shiraia bambusicola strain and specific substrate transformation to produce hypocrellin, is high in transformation rate, low in cost, less in step and applicable to industrial production and has excellent industrial application prospect.
Owner:江苏竹红生物科技有限公司
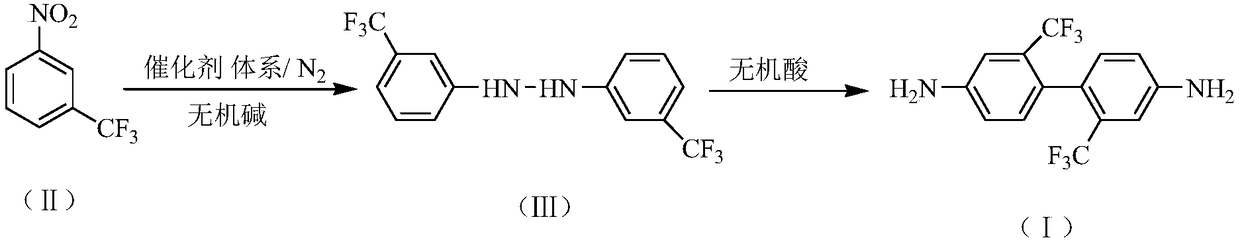
Preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl
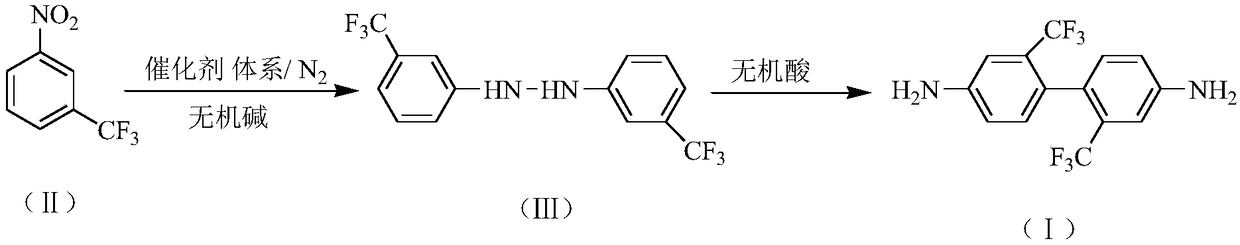
InactiveCN109232273AReduce generationHigh purityHydrazine preparationPreparation by rearrangement reactionsHydroxyanthraquinoneDodecylsulfonic acid
The invention relates to a preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl. The preparation method comprises the following steps: synthesizing 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic alkaline aqueous solution by adopting nitrobenzotrifluoride as a raw material, adopting a phase transfer catalyst, a co-catalyst and Pd / C as a catalytic system and adopting aromatic hydrocarbon as a solvent, and performing the re-arrangement reaction on the 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic acid aqueous solution, thus obtaining 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl, wherein the phase transfer catalyst is one or a mixture of more of sodium dodecyl benzene sulfonate, sodium dodecyl sulfate and cetyl trimethyl ammonium bromide, and the co-catalyst is one or a mixture of more of 2,3-dichloro-1,4-naphthoquinone, 2-hydroxyanthraquinone and 2,6-dioxyanthraquinone. The preparation method has the advantages of mild reaction condition, simple process, high product quality and yield, low production cost, environmental friendliness, suitability for continuous production and the like.
Owner:烟台海川化学制品有限公司
Process for preparation of 2-Methyl-1,4-naphthoquinone
InactiveUS6579994B2Low costReduce usageOrganic compound preparationQuinone preparation by oxidationAcetic acidNaphthoquinone
The present invention describes a process for the preparation of 2-Methyl-1,4-naphthoquinone by oxidizing 2-methylnaphthalene with hydrogen peroxide in the presence of acetic acid.
Owner:COUNCIL OF SCI & IND RES
Electroplating solution for copper plating and preparation method thereof
The invention discloses an electroplating solution for copper plating and a preparation method thereof. The method comprises the steps that (1) copper salt, a quinonyl compound, catechol, potassium sulfate, malic acid, 2,2'-dipyridyl, complexing agents, reducing agents, stabilizer and water are mixed, and a mixture M1 is obtained; (2) alkaline is added to the mixture M1, the pH value is adjusted to 11-13, and a mixture M2 is obtained; and (3) the mixture M2 is post-processed, and the electroplating solution for copper plating is obtained. The quinonyl compound is one or several of 1,2-naphthoquinone, 1,4-naphthoquinone and anthraquinone. The electroplating solution for copper plating prepared through the method is stable in performance, and a copper-plated layer obtained through electroplating with the electroplating solution for copper plating is high in adhesive force, large in hardness and not prone to expanding and blistering.
Owner:ANHUI JIANGWEI PRECISION IND
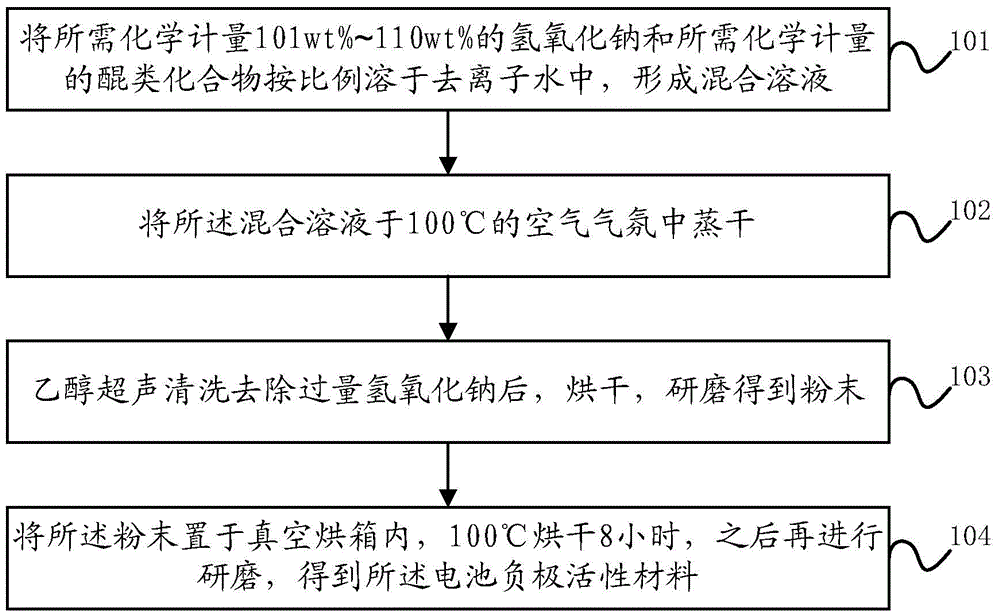
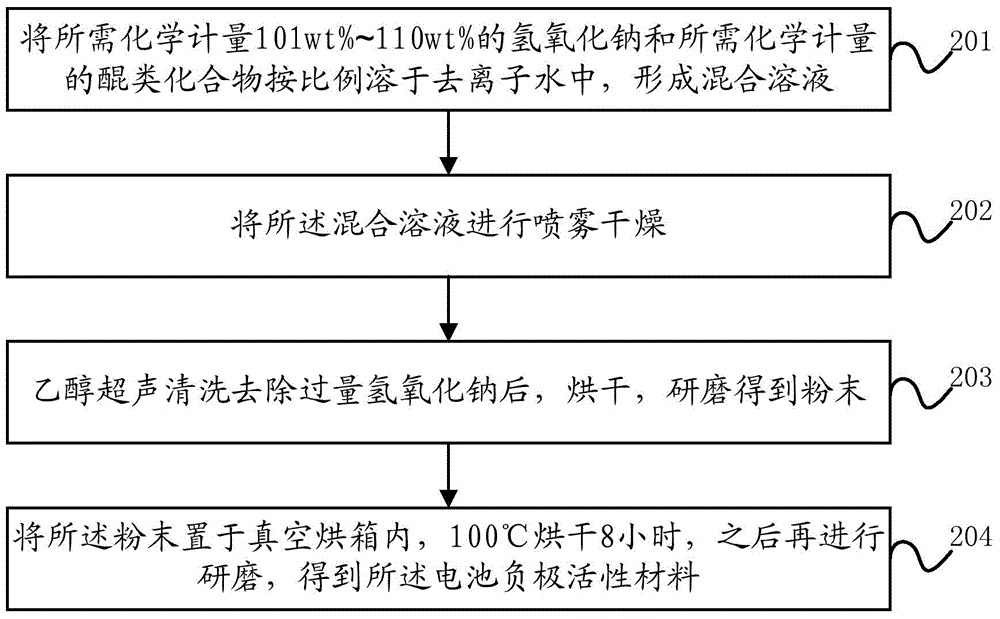
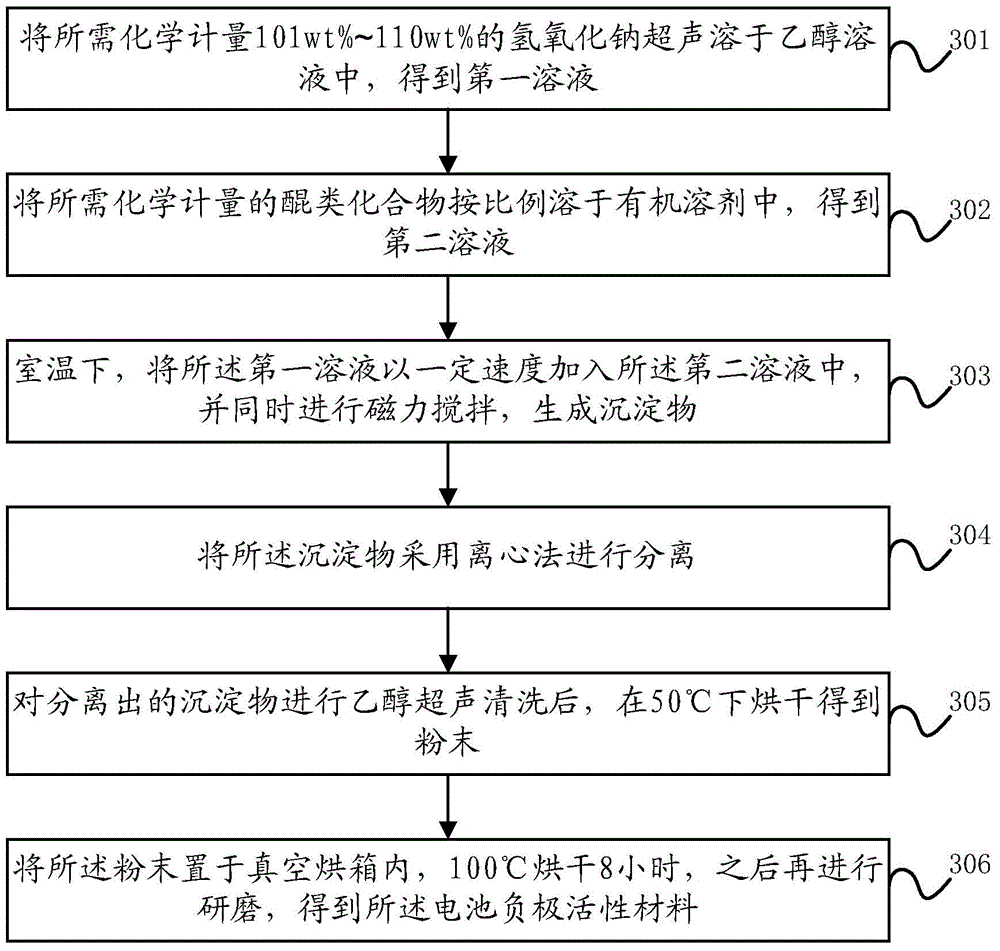
Battery negative electrode active material based on quinone structure and preparation method and application thereof
ActiveCN104795566AEasy to prepareIncrease working voltageNegative electrodesSecondary cellsNaphthoquinoneSodium salt
The invention discloses a battery negative electrode active material based on a quinone structure, and a preparation method and an application of the battery negative electrode active material. The battery negative electrode active material comprises a quinone compound taking the quinone structure as an electrochemical oxidation-reduction reaction site, wherein the quinone compound comprises any one of a benzoquinone sodium salt derivate, an anthraquinone sodium salt derivate, or a naphthoquinone sodium salt derivate. The benzoquinone sodium salt derivate, the anthraquinone sodium salt derivate, or the naphthoquinone sodium salt derivate comprises at least one of the following groups of -ONa, -SO3Na, or -COONa.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Novel aromatic prenyltransferases, nucleic acids encoding same and uses therefor
ActiveUS20060183211A1Sugar derivativesMicrobiological testing/measurementPrenyltransferase activityIsoprene
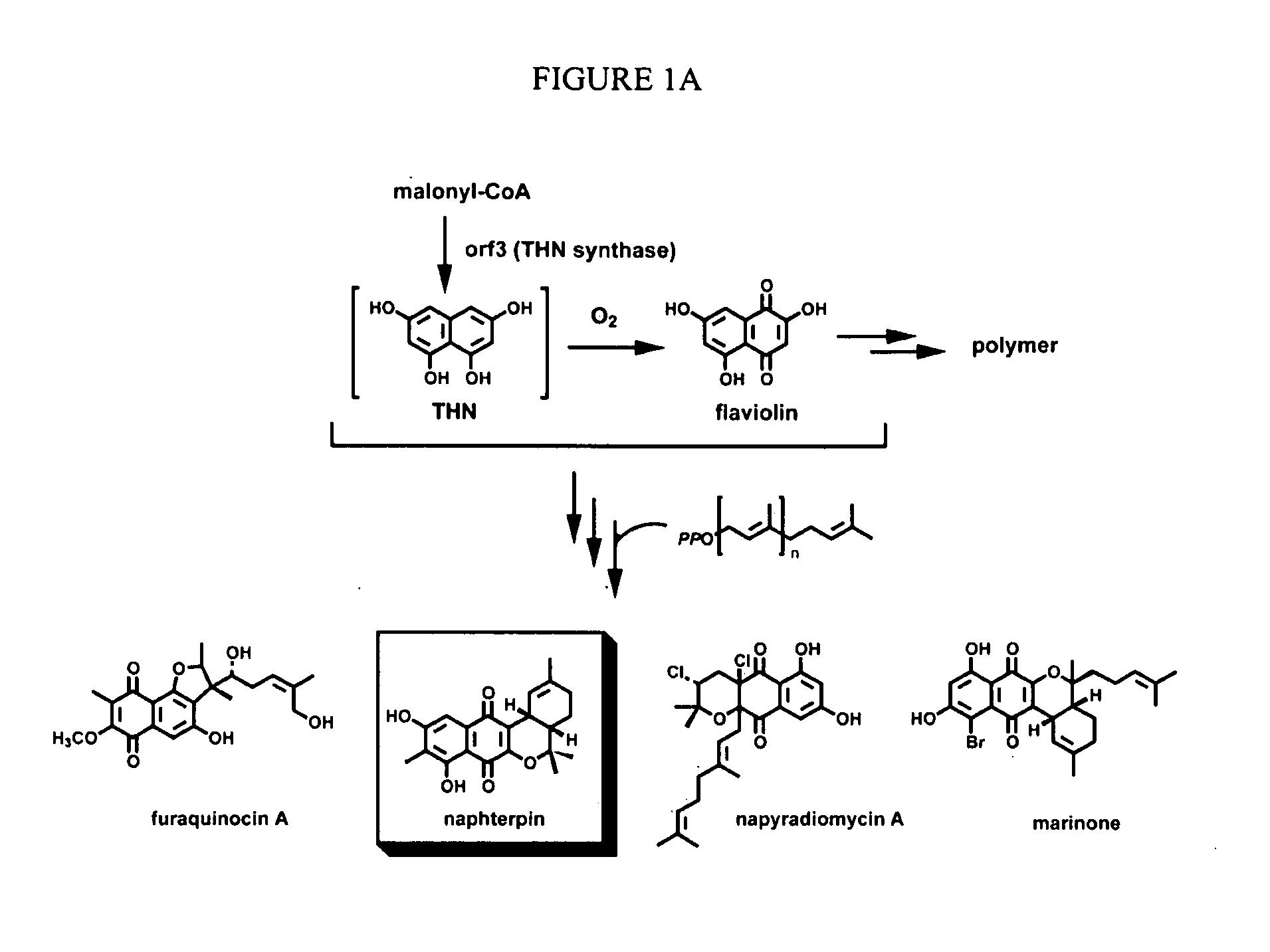
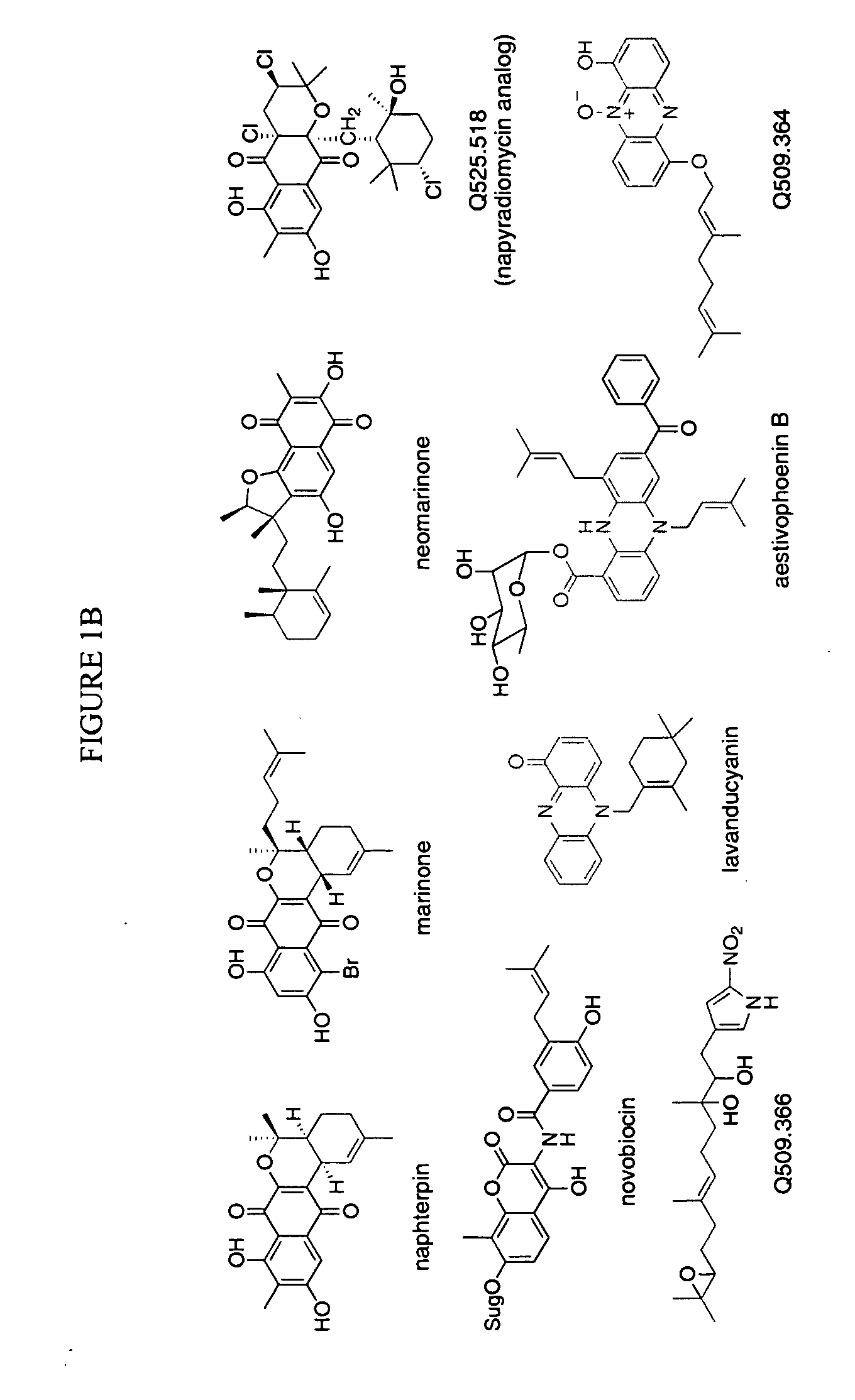
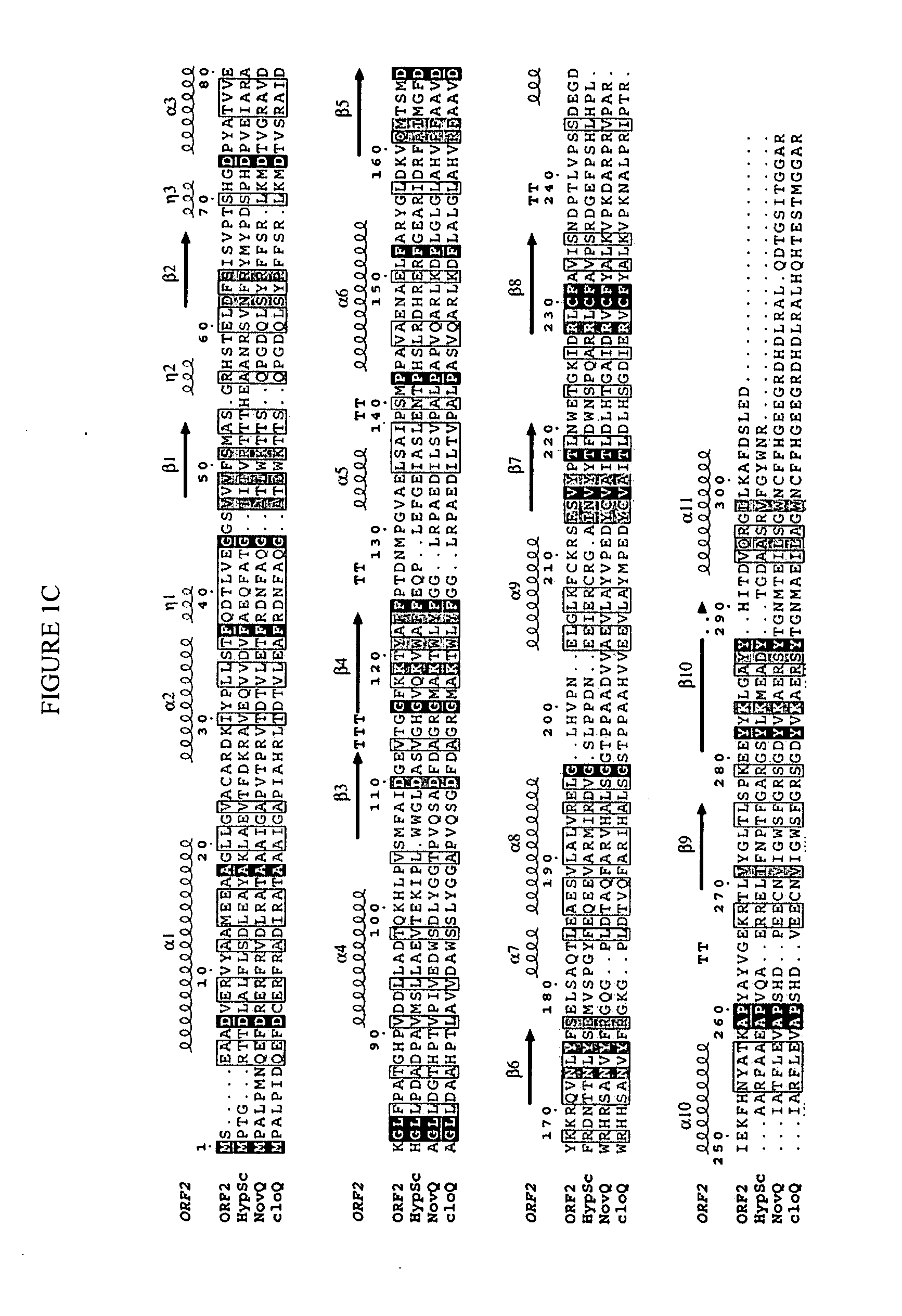
In accordance with the present invention, a novel aromatic prenyltransferase, Orf2 from Streptomyces sp. strain CL190, involved in naphterpin biosynthesis has been identified and the structure thereof elucidated. This prenyltransferase catalyzes the formation of a C—C bond between a prenyl group and a compound containing an aromatic nucleus, and also displays C—O bond formation activity. Numerous crystallographic structures of the prenyltransferase have been solved and refined, e.g., (1) prenyltransferase complexed with a buffer molecule (TAPS), (2) prenyltransferase as a binary complex with geranyl diphosphate (GPP) and Mg2+, and prenyltransferase as ternary complexes with a non-hydrolyzable substrate analogue, geranyl S-thiolodiphosphate (GSPP) and either (3) 1,6-dihydroxynaphthalene (1,6-DHN), or (4) flaviolin (i.e., 2,5,7-trihydroxy-1,4-naphthoquinone, which is the oxidized product of 1,3,6,8-tetrahydroxynaphthalene (THN)). These structures have been solved and refined to 1.5 Å, 2.25 Å, 1.95 Å and 2.02 Å, respectively. This first structure of an aromatic prenyltransferase displays an unexpected and non-canonical (β / α)-barrel architecture. The complexes with both aromatic substrates and prenyl containing substrates and analogs delineate the active site and are consistent with a proposed electrophilic mechanism of prenyl group transfer. These structures also provide a mechanistic basis for understanding prenyl chain length determination and aromatic co-substrate recognition in this structurally unique family of aromatic prenyltransferases. This structural information is useful for predicting the aromatic prenyltransferase activity of proteins.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Photochromic fiber as well as preparation method and application thereof
ActiveCN103556300AHas aesthetic valueGood Photochromic PropertiesFilament/thread formingConjugated synthetic polymer artificial filamentsFiberPolymer science
The invention provides a photochromic fiber as well as a preparation method and an application thereof. The photochromic fiber is a double-component composite fiber with a skin-core structure, wherein in a cross section of the double-component composite fiber with the skin-core structure, a skin layer and a core layer are in a concentric structure; the skin layer of the double-component composite fiber with the skin-core structure is made of low-smelting-point polyethylene terephthalate added with a phenoxy naphtho-naphthoquinone additive; the core layer is made of fiber-grade polyethylene terephthalate. The invention further provides the preparation method of the photochromic fiber. The photochromic fiber has good photochromism, heat stability, fatigue durability, short responding time, simple process and long service life, can serve as an anti-counterfeiting identification element for safe anti-counterfeiting and can also be used for garment materials and advertisements to increase the aesthetic feeling.
Owner:厦门翔鹭化纤股份有限公司
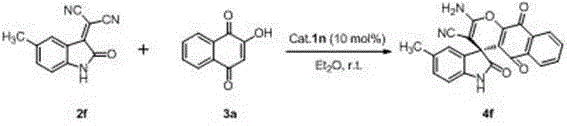
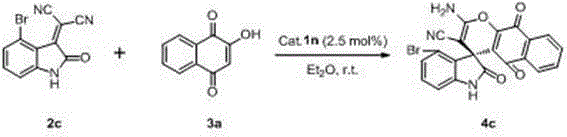
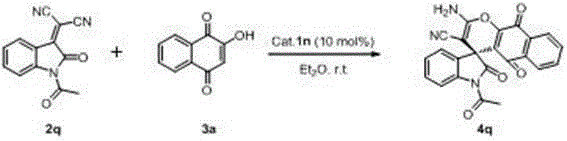
Preparation method of chiral spiro naphthoquinone benzopyran hydroxyindole compound
The invention discloses a preparation method of a chiral spiro naphthoquinone benzopyran hydroxyindole compound. The method comprises the following specific steps: reacting the condensation product of an isatin compound and propane dinitrile and a 2-hydroxyl-1,4-naphthoquinone compound in a solvent under the catalysis of quinine thiourea dihydride, thereby obtaining a product. According to the method, raw materials are simple and easily available, the reaction condition is mild, the post-treatment is simple and convenient, the applicative substrates are wide, the enantioselectivity is high, and the yield can reach 99%; therefore, the method is a novel method capable of efficiently synthesizing the chiral spiro naphthoquinone benzopyran hydroxyindole compound having important medicinal value and asymmetric synthesis value. The compound prepared by the method can be used for preparing intermediates of chiral drugs and has wide physiological and pharmacological activities.
Owner:SUZHOU UNIV
Method for treating naphthoquinone production liquid waste containing hexavalent chromium and vitamin K3 production wastewater and co-producing chromium oxide green
ActiveCN103613133AReduce COD valueEfficient disposalMultistage water/sewage treatmentChromium oxides/hydratesLiquid wasteNaphthoquinone
The invention relates to a method for treating naphthoquinone production liquid waste containing hexavalent chromium and vitamin K3 production wastewater and co-producing chromium oxide green. The method comprises the following steps: a) carrying out liquid phase reaction between the naphthoquinone production liquid waste and the vitamin K3 production wastewater at 80-260 DEG C, wherein the naphthoquinone production liquid waste is excessively used relative to a necessary dosage of complete reduction; b) adding a reducing agent into the obtained reaction mixture to reduce the residual hexavalent chromium; c) regulating the pH of the obtained reaction mixture to 5.5-8.5 to separate out chromium hydroxide, and separating to obtain the chromium hydroxide and filtrate; and d) transforming the chromium hydroxide obtained in the step c) into chromium oxide green. The method can be used for effectively treating the naphthoquinone production liquid waste and the vitamin K3 production wastewater generated in a vitamin K3 production chain and recycling effective components in the liquid waste to turn waste into wealth and improve the economic and environmental protection benefits at the same time.
Owner:绵阳市安剑皮革化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com