Preparation method of chiral spiro naphthoquinone benzopyran hydroxyindole compound
A pyranoxindole compound and a compound technology are applied in the field of preparation of chiral spironaphthoquinopyranoxidole compounds, and can solve the problems of narrow substrate application range, harsh reaction conditions, narrow substrate range and the like, Achieve excellent enantioselectivity, mild reaction conditions, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
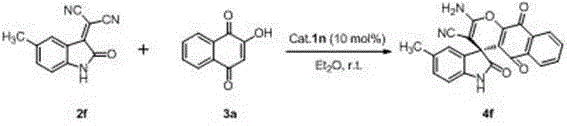
[0033] Xiang Shengyou 3a (34.8 mg, 0.2 mmol), dihydroquinine thiourea Cat. 1n (3.0 mg, 0.005 mmol) Add 2 mL of ether to the reaction bottle, and then add 2a (39.0 mg, 0.2 mmol), stirred at room temperature for 48 hours (reaction detected by TLC), after the reaction was complete, the crude product was subjected to column chromatography (eluent was ethyl acetate / petroleum ether=1 / 2, then two Chloromethane / methanol=40 / 1) to get the target product 4a (72.4 mg), the yield was 98%.
[0034] The target product was characterized and analyzed as follows: brown solid, 98% yield, 98% ee, [ α ] D 25 = -94.0 (c 0.50, CH 3 COCH 3 ); 1 H NMR (400 MHz, DMSO-d 6 ) δ 10.69 (s, 1H), 8.07 (d, J = 6.4 Hz, 1H), 7.91-7.77 (m, 3H), 7.59 (s, 2H), 7.28-7.15(m, 2H), 6.95-6.84(m, 2H); 13 C NMR (101 MHz, DMSO-d 6 ) δ 182.0, 177.8, 176.5, 158.8, 150.6, 141.8, 135.0, 134.6, 130.7, 130.4, 129.1, 126.4, 126.2, 124.4, 122.2, 119.7, 117.2, 109.8, (IR) ν max / cm -1 ): 3417, 33...
Embodiment 2
[0036]
[0037] Xiang Shengyou 3a (34.8 mg, 0.2 mmol), dihydroquinine thiourea Cat. 1n (3.0 mg, 0.005 mmol) Add 2 mL of ether to the reaction bottle, and then add 2b (45.9 mg, 0.2 mmol), stirred at room temperature for 6 hours (reaction detected by TLC), after the reaction was complete, the crude product was subjected to column chromatography (eluent was ethyl acetate / petroleum ether=1 / 2, then two Chloromethane / methanol=40 / 1) to get the target product 4b (79.9 mg), the yield was 99%.
[0038] The target product was characterized and analyzed as follows: brown solid, 99% yield, 91% ee, [ α ] D 25 = -39.0 (c 0.50, CH 3 COCH 3 ); 1 H NMR (400 MHz, DMSO-d 6 ) δ 11.04 (s, 1H), 8.11-8.02 (m, 1H), 7.93-7.82 (m, 3H), 7.79 (s, 2H), 7.33-7.21 (m, 1H), 6.99-6.85 (m, 2H); 13 C NMR (101 MHz, DMSO-d 6 ) δ 181.8, 176.7, 176.1, 159.7, 150.9, 143.8, 135.2, 134.8, 131.0, 130.2, 130.1, 129.7, 129.0, 126.3, 122.4, 116.7, 109.1, 48.5; IR (KBR) (KBR) (KBR) ν max / cm -1 ): ...
Embodiment 3
[0040]
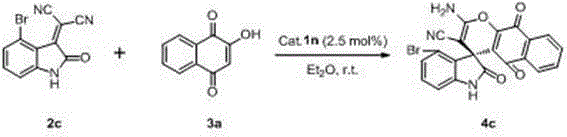
[0041] Xiang Shengyou 3a (34.8 mg, 0.2 mmol), dihydroquinine thiourea Cat. 1n (3.0 mg, 0.005 mmol) Add 2 mL of ether to the reaction bottle, and then add 2c (54.8 mg, 0.2 mmol), stirred at room temperature for 12 hours (reaction detected by TLC), after the reaction was complete, the crude product was subjected to column chromatography (eluent was ethyl acetate / petroleum ether=1 / 2, then two Chloromethane / methanol=40 / 1) to get the target product 4c (87.8 mg), the yield was 98%.
[0042] The target product was characterized and analyzed as follows: brown solid, 98% yield, 90% ee, [ α ] D 25 = -10.0 (c 0.50, CH 3 COCH 3 ); 1 H NMR (400 MHz, DMSO-d 6 ) δ 11.02 (s, 1H), 8.12-8.02 (m, 1H), 7.93-7.82 (m, 3H), 7.77 (s, 2H), 7.25-7.14 (m, 1H), 7.08 (d, J = 7.6 Hz, 1H), 6.95 (d, J = 7.2 Hz, 1H); 13 C NMR (75 MHz, DMSO-d 6 ) δ 181.7, 176.6, 176.2, 159.7, 151.1, 144.1, 135.3, 134.9, 131.2, 130.5, 130.2, 130.1, 126.6, 126.3, 118.6, 116.7, 109.5, 49.7; ν ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com