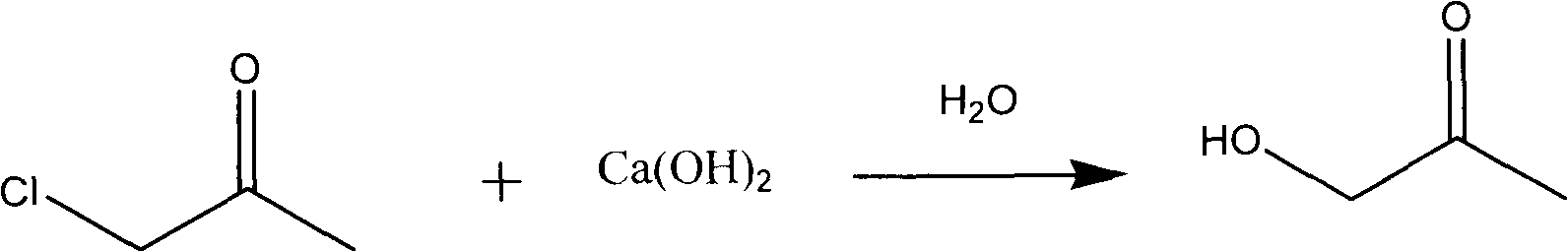Patents
Literature
329 results about "Isatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
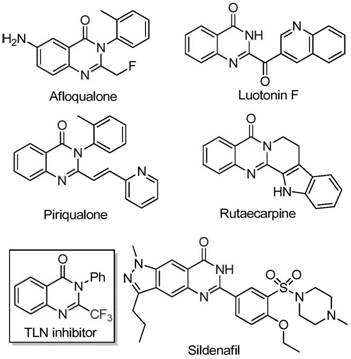
Isatin, also known as tribulin, is an organic compound derived from indole with formula C₈H₅NO₂. The compound was first obtained by Otto Linné Erdman and Auguste Laurent in 1840 as a product from the oxidation of indigo dye by nitric acid and chromic acids.
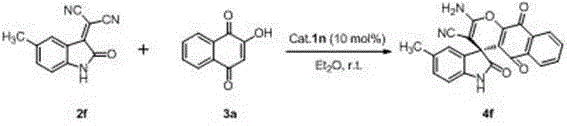
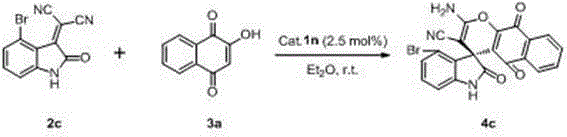
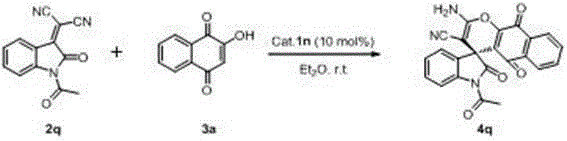
Preparation method of chiral spiro naphthoquinone benzopyran hydroxyindole compound
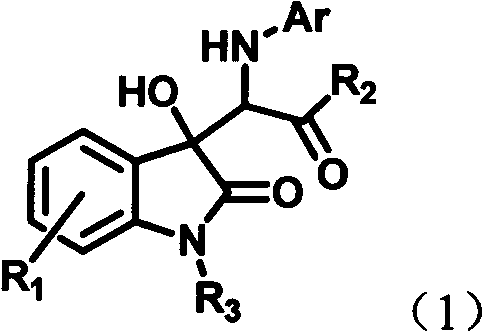
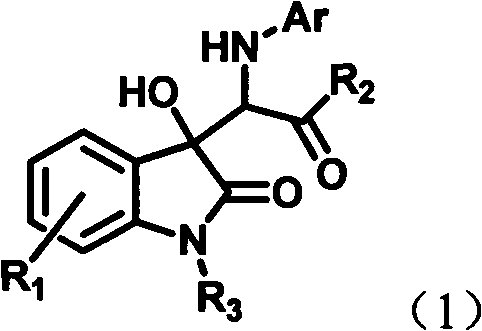
The invention discloses a preparation method of a chiral spiro naphthoquinone benzopyran hydroxyindole compound. The method comprises the following specific steps: reacting the condensation product of an isatin compound and propane dinitrile and a 2-hydroxyl-1,4-naphthoquinone compound in a solvent under the catalysis of quinine thiourea dihydride, thereby obtaining a product. According to the method, raw materials are simple and easily available, the reaction condition is mild, the post-treatment is simple and convenient, the applicative substrates are wide, the enantioselectivity is high, and the yield can reach 99%; therefore, the method is a novel method capable of efficiently synthesizing the chiral spiro naphthoquinone benzopyran hydroxyindole compound having important medicinal value and asymmetric synthesis value. The compound prepared by the method can be used for preparing intermediates of chiral drugs and has wide physiological and pharmacological activities.
Owner:SUZHOU UNIV
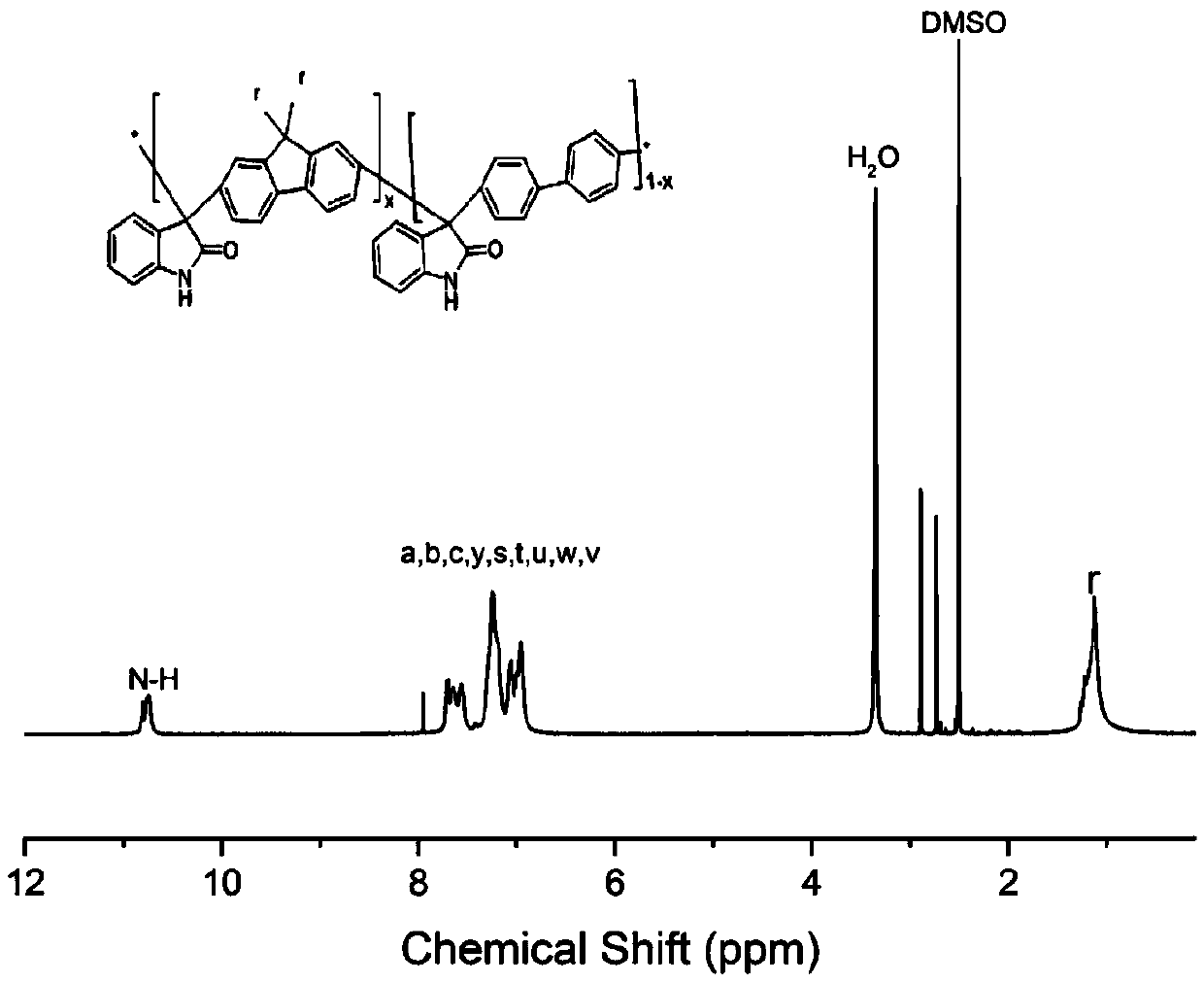
Cardo structure-containing isatin aromatic hydrocarbon copolymer, preparation method and application thereof
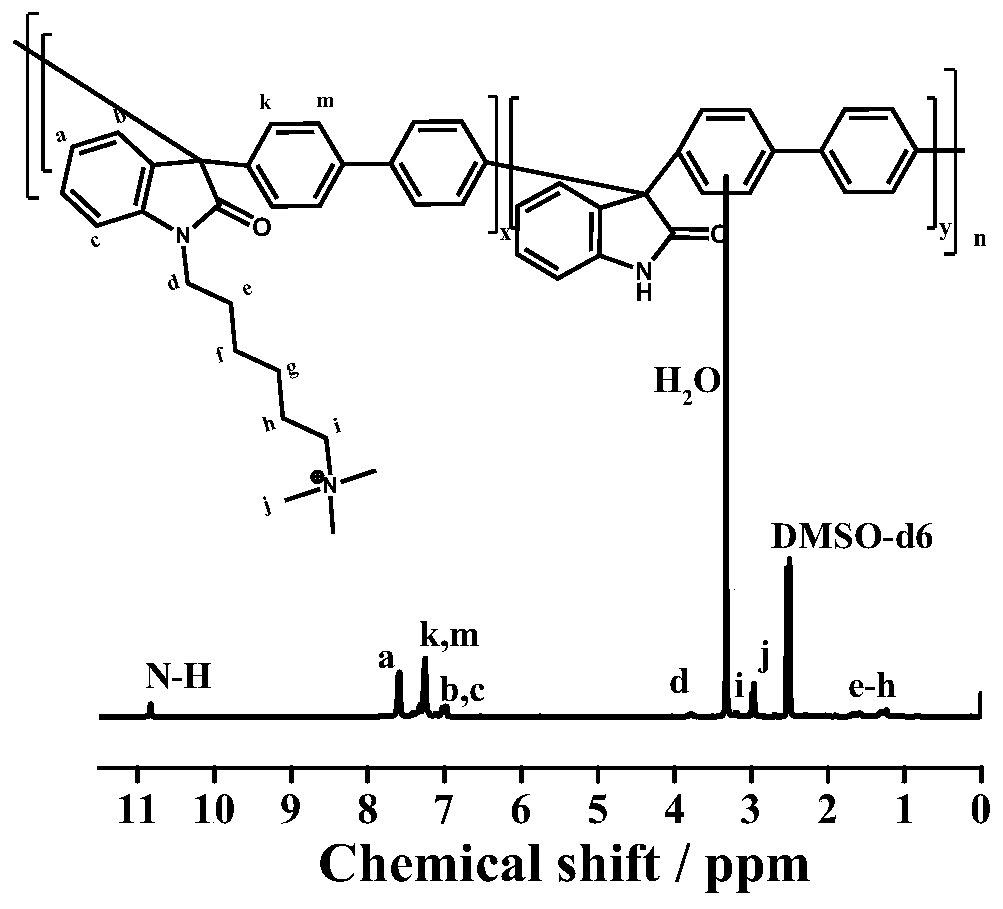
Belonging to the field of preparation of polymer materials and polymer ion exchange membranes thereof, the invention relates to a Cardo structure-containing isatin aromatic hydrocarbon copolymer, a preparation method and application thereof. Specifically, by means of superacid catalyzed hydroxyalkylation polycondensation reaction, isatin, 9, 9-disubstituted fluorene and aromatic hydrocarbon are subjected to a multi-component copolymerization reaction to synthesize an isatin-aromatic hydrocarbon copolymer containing Cardo structure and ammonium cations, so that an electrolyte solution and a tough anion exchange membrane can be conveniently prepared, and the polymerization reaction is simple and practicable. A large-volume Cardo structure is introduced into the polymer, thus reducing the adsorption on a catalyst surface and improving the fuel cell performance; an isatin structure is introduced for crosslinking or functionalization so as to further improve the chemical stability and mechanical properties. The copolymer electrolyte solution and the ion exchange membrane have high ionic conductivity, excellent alkali resistance / oxidation resistance and mechanical properties, and have wide application in fuel cells, energy storage batteries, electrolysis and other electrochemical devices or membrane separation related fields.
Owner:DALIAN UNIV OF TECH
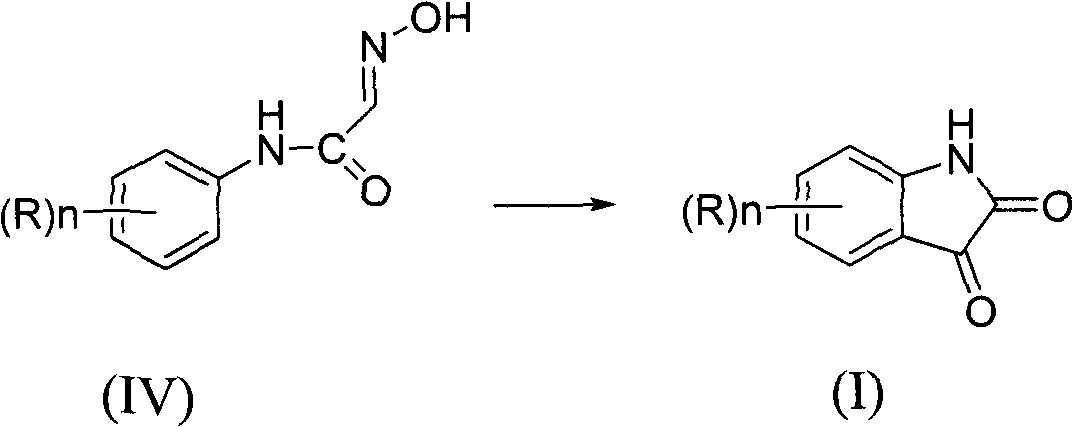
Synthesis method of isatin derivatives
The invention relates to a synthesis method of isatin derivatives, which comprises the following steps of: (1) under acidic conditions, reacting aniline derivatives with hydroxyl amine and trihalogen acetaldehyde at 30-90 DEG C to obtain N-(substituted phenyl)-2-(hydroxyl imino) acetamide; and (2) cyclizing the N-(substituted phenyl)-2-(hydroxyl imino) acetamide in the presence of strong acid orLewis acid as a catalyst to obtain the isatin derivatives. The invention has the advantages of high yield, less three wastes, simple technical processes, simple and convenient post-treatment, high product purity and low product cost.
Owner:滨海康杰化学有限公司
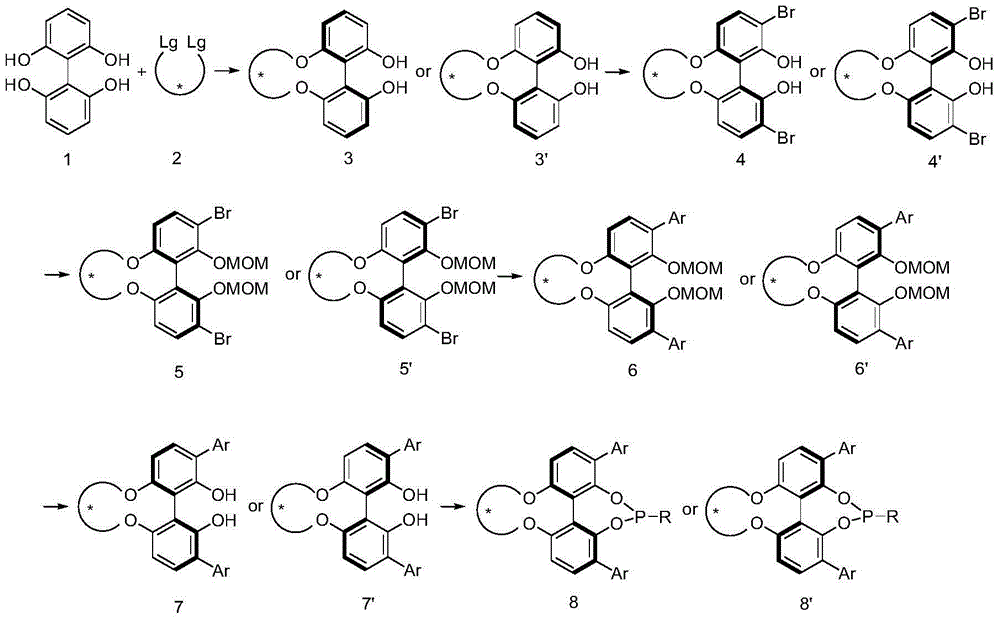
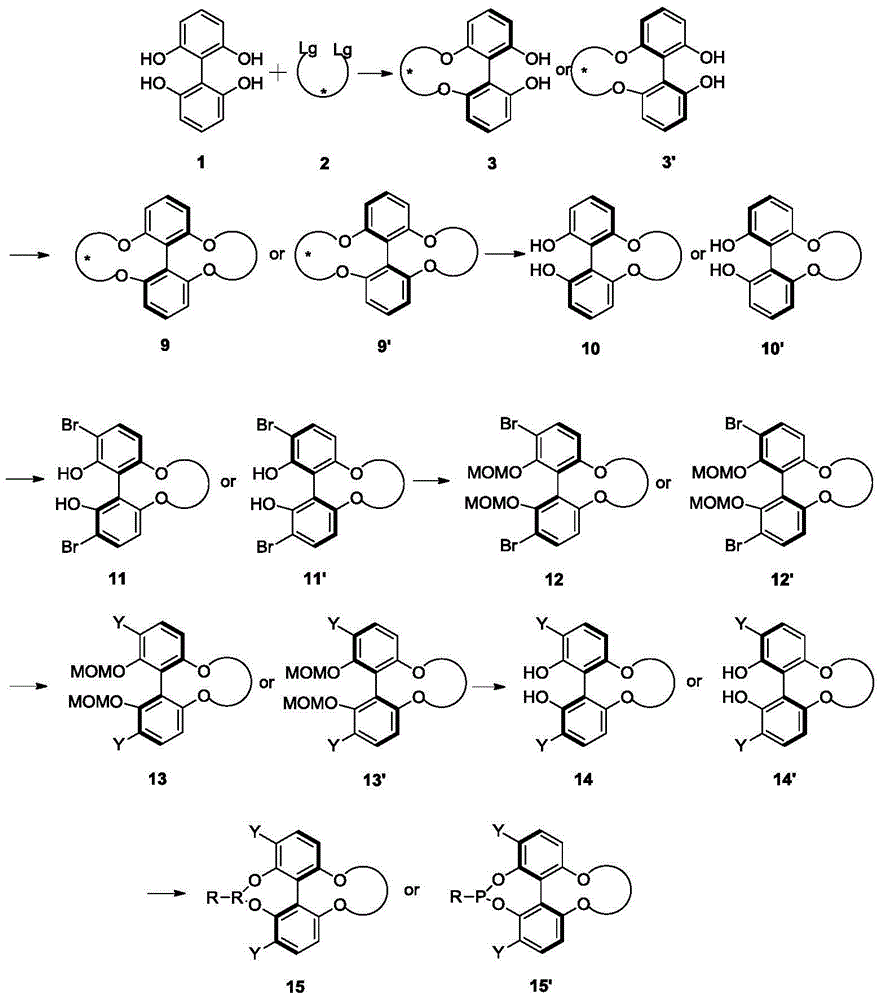
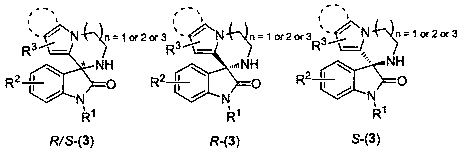
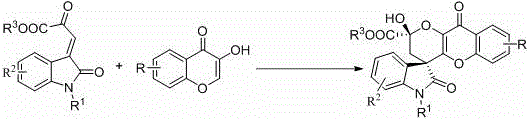
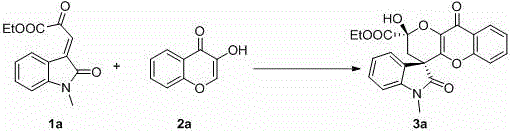
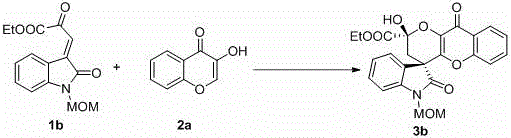
4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as synthetic method and application thereof
The invention discloses a 4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as a synthetic method and an application thereof. The synthetic method comprises the following steps: in the presence of a solvent, carrying out a reaction on substituted or unsubstituted unsaturated nitrile ethyl acetate and an isatin derivative under catalysis of an alkali compound; and treating the reaction product to obtain the 4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative. The method has the advantages that reaction time is agile, yield is high, operation is simple and convenient and the like, and is wide in application range and suitable for industrialized production. The 4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative disclosed by the invention can be used as an antibacterial agent.
Owner:海宁市袁花镇工业投资有限公司
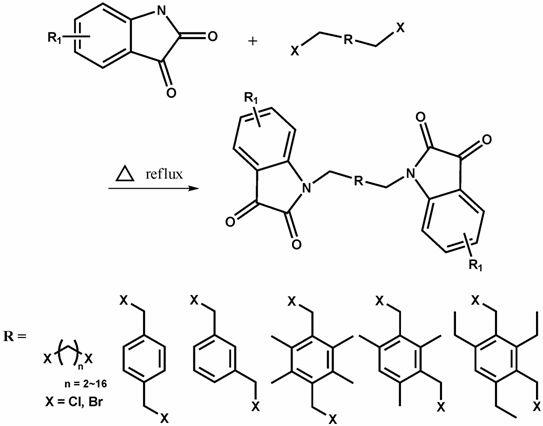
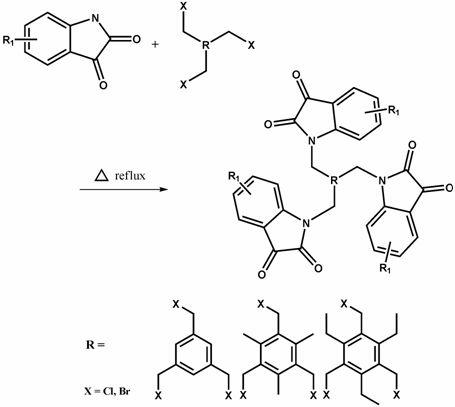
Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound
ActiveCN106432052AHigh yieldRaw materials are easy to obtainOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsRare earth
The invention discloses a method for catalytically preparing a spiral [cyclopropane-1,3'- indole] compound. According to the method, silicon amino rare earth compounds of [(Me3Si)2N]3Ln(mu-Cl)Li(THF)3 is used as a catalyst for catalytically replacing isatin, phosphite ester and olefin to prepare a product through one-pot reaction; in the catalyst, (Me3Si)2N represents trimethyl silicon amino; Ln represents positive trivalent rare earth metal irons and are one kind of metal irons selected from lanthanum, samarium, gadolinium, erbium or ytterbium; mu- represents a bridge key; THF represents tetrahydrofuran. In the method, the synthesis method of the catalyst is simple; the reaction raw materials are simple and can be easily obtained; the application range of a substrate is wide; the efficiency of the one-pot reaction method is high; the reaction conditions are mild; the yield of most target products can reach 85 percent or higher.
Owner:SUZHOU UNIV
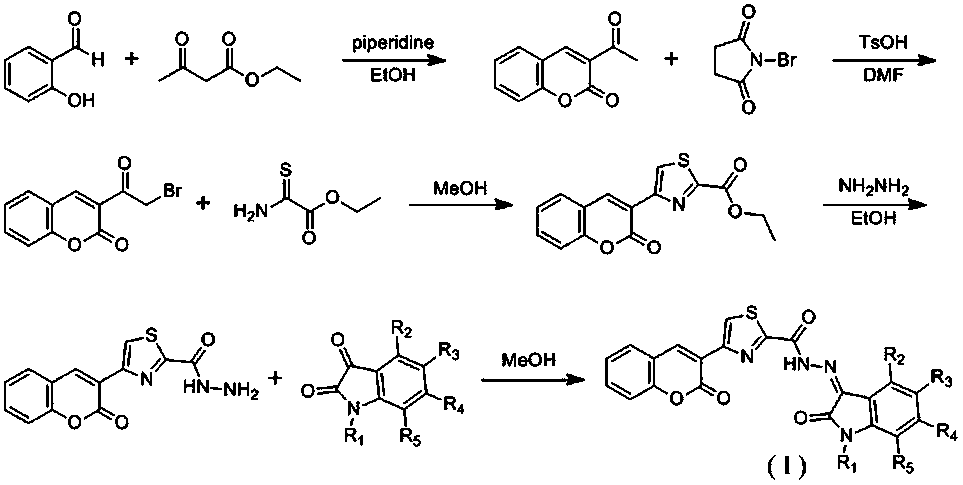
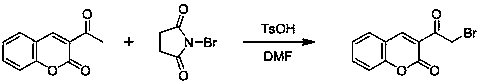
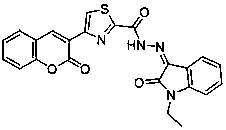
Coumarin-thiazole-indolone compounds, and preparation method and application thereof
InactiveCN104829608AEnhanced inhibitory effectHigh activityAntibacterial agentsOrganic active ingredientsAcetic acidSalicylaldehyde
The invention discloses coumarin-thiazole-indolone compounds, and a preparation method and application thereof. The compounds are disclosed as Formula (I). The preparation method comprises the following steps: reacting salicylaldehyde and ethyl acetoacetate to obtain 3-acetyl-2H-benzopyranyl-2-one, carrying out bromination, cyclization and hydrazinolysis reaction to obtain 4-(2-oxo-2H-benzopyranyl-3-yl)thiazolyl-2-formylhydrazine, and finally, reacting with various substituted isatin to obtain the target compounds. The compounds can be used as a raw material of antibacterial drugs. The preparation method has the advantage of simple and accessible raw materials, and is convenient to operate.
Owner:JISHOU UNIVERSITY
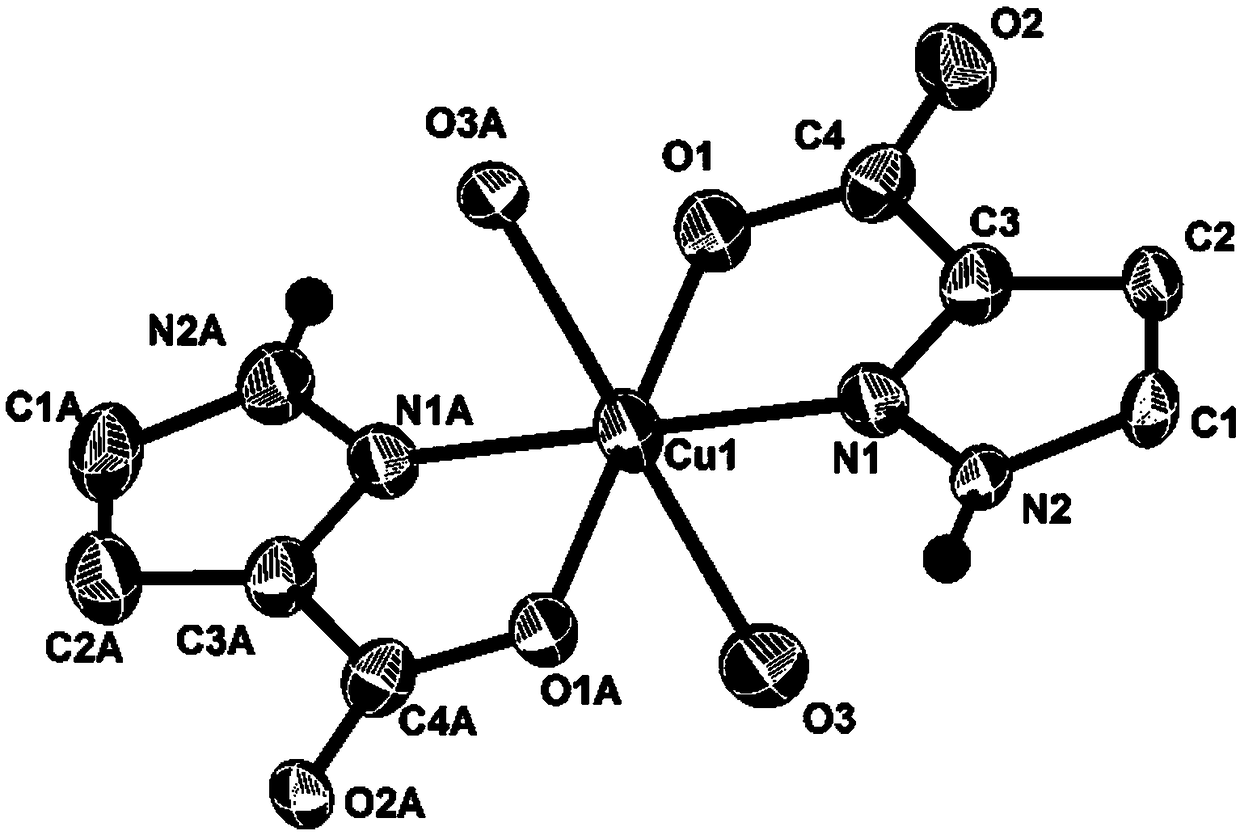
Pyrazolecarboxylic acid copper complex and preparation method and application thereof
PendingCN109020889AImprove stabilityHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsSpace groupSynthesis methods
The invention discloses a pyrazolecarboxylic acid copper complex, the pyrazolecarboxylic acid copper complex belongs to a triclinic system and a P-1 space group, and the unit cell parameters are shownin the specification: alpha is 97.058(4) DEG, beta is 92.589(4) DEG, and gamma is 107.30(4) DEG. The pyrazolecarboxylic acid copper complex disclosed by the invention can efficiently activate the terminal alkyne and catalyze an addition reaction thereof to an isatin compound, has good catalytic performance, and has advantages of good selectivity, mild reaction conditions, and high yield. Moreover, the synthesis method is simple, the product stability is high, and the method is easy to popularize and utilize.
Owner:南通沃兰化工有限公司 +1
Method for synthesizing 3-hydroxy-3-arylindole-2-one derivative
The invention discloses a method for synthesizing a 3-hydroxy-3-arylindole-2-one derivative shown as a structural formula (I), which comprises the following steps of: fully reacting raw materials such as an isatin compound shown as a structural formula (II) and arylboric acid shown as a structural formula (III) in an inert organic solvent in the presence of a copper catalyst, a nitrogen-containing bidentate ligand and an alkaline compound, and separating and purifying reaction liquid after the reaction is finished to obtain the 3-hydroxy-3-arylindole-2-one derivative, wherein the copper catalyst is one or any combination of copper trifluoromethanesulfonate, copper acetylacetonate, copper acetate, cuprous iodide, copper bromide, copper fluoride and copper chloride. The method is high in implementation value and good in social benefit and economic benefit.
Owner:WENZHOU UNIVERSITY
3-substituted-3-hydroxyindazolone derivatives, and preparation method and application thereof
InactiveCN102516151AHigh atomic economyHigh selectivityOrganic chemistryAntineoplastic agentsSolventDrug biological activity
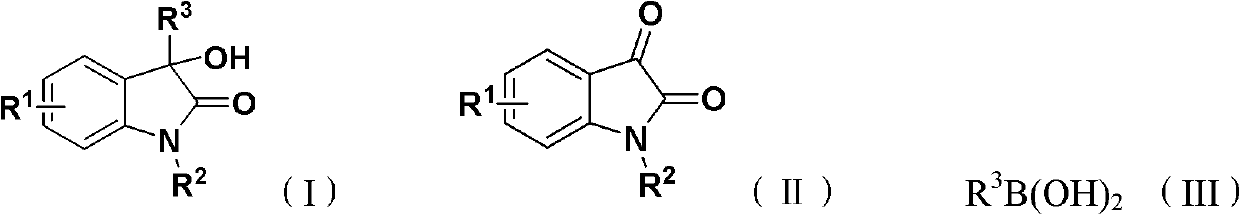
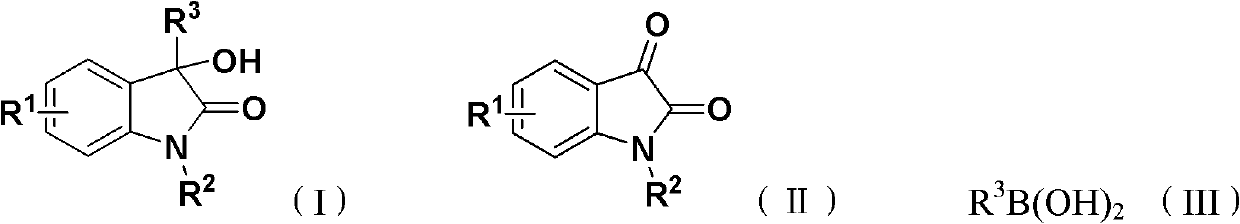
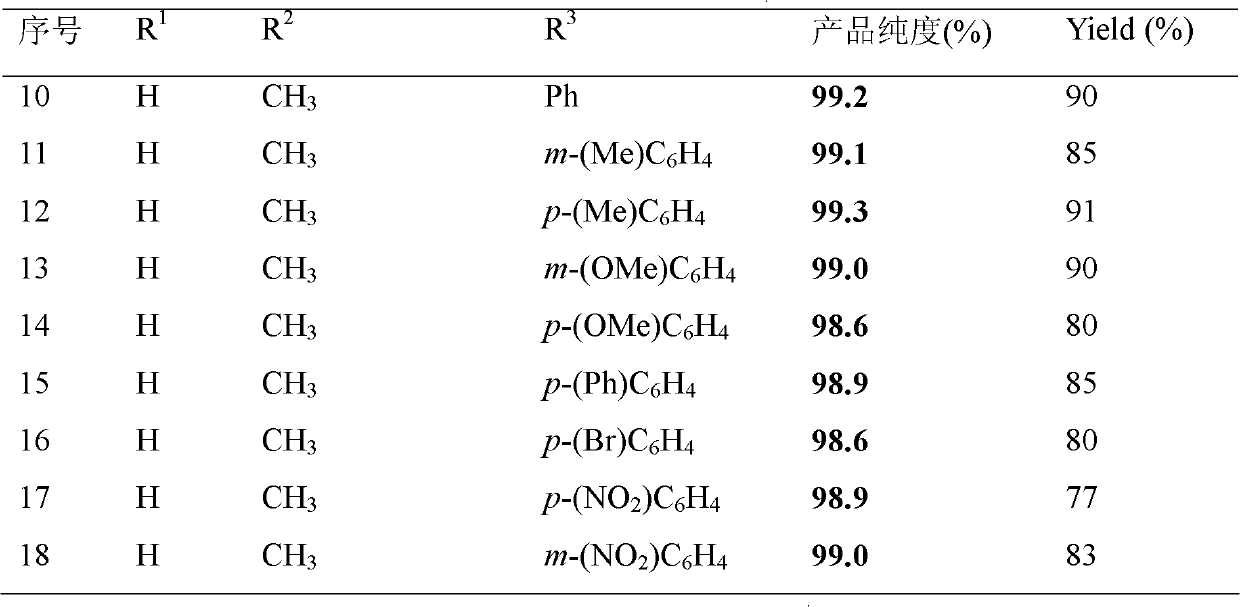
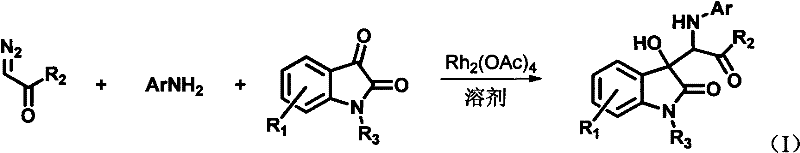
The invention discloses 3-substituted-3-hydroxyindazolone derivatives, and a preparation method and application thereof. Isatin, amine and diazo compound are used as raw materials, rhodium acetate is used as a catalyst, and an organic solvent is used as a solvent, a one-step reaction is carried out to obtain the product 3-substituted-3-hydroxyindazolone derivatives. The preparation method disclosed by the invention has the advantages of high atom economical efficiency, high selectivity, high yield and the like, and is simple and safe to operate. The 3-substituted-3-hydroxyindazolone derivatives have bioactivity, and are applicable to preparing antineoplastic drugs.
Owner:EAST CHINA NORMAL UNIVERSITY
Method for asymmetric catalytic synthesis of spirocyclic tetrahydrocarbazoline compound
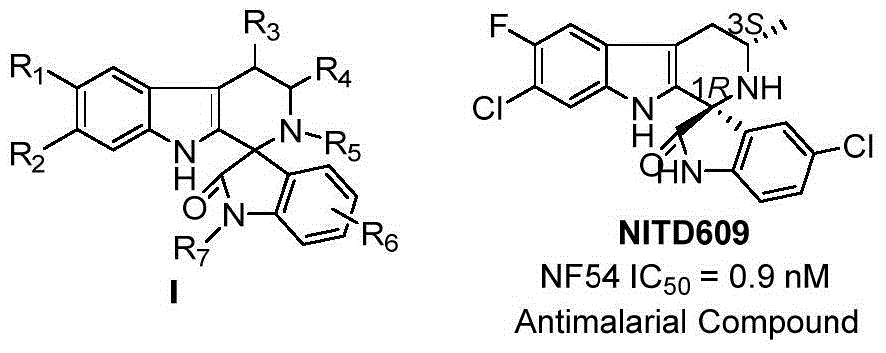
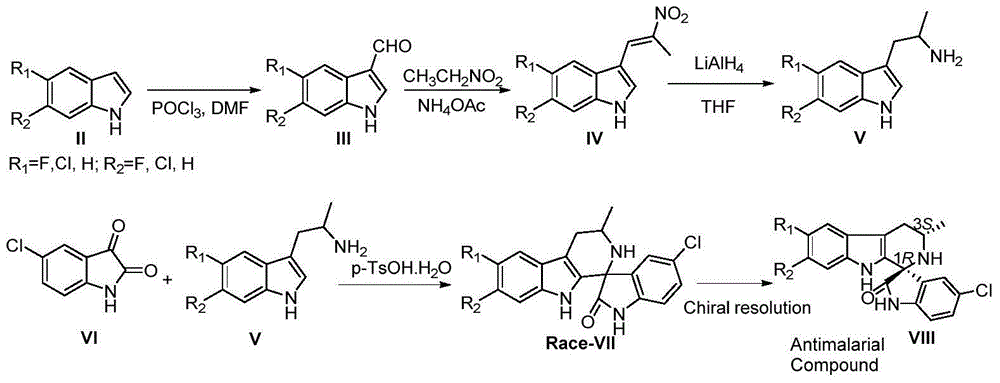
The invention discloses a method for asymmetric catalytic synthesis of a spirocyclic tetrahydrocarbazoline compound, comprising the steps: by taking 3-alkenyl indole and isatin derived ketimine as raw materials, a complex formed by chiral amine oxide and nickel trifluoromethane sulfonate as a catalyst and dichloromethane as a solvent, performing reaction on the raw materials at (-30) DEG C to (-10) DEG C at the normal pressure for 96-192h; then adding 6.0M HCl(solution) and performing reaction at 30 DEG C for 4-48h to obtain the chiral spirocyclic tetrahydro carbazoline compound, wherein the yield of the chiral spirocyclic tetrahydro carbazoline compound can reach up to 95%, and the enantioselectivity can reach up to 99%. According to the method provided by the invention, the catalytic reaction conforms with the green chemistry atom economy and has a good prospect in industrial application, thereby providing a new path for asymmetric synthesis of compounds with high anti-malarial activity (NITD609).
Owner:SICHUAN UNIV
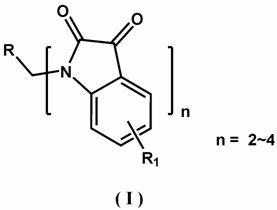
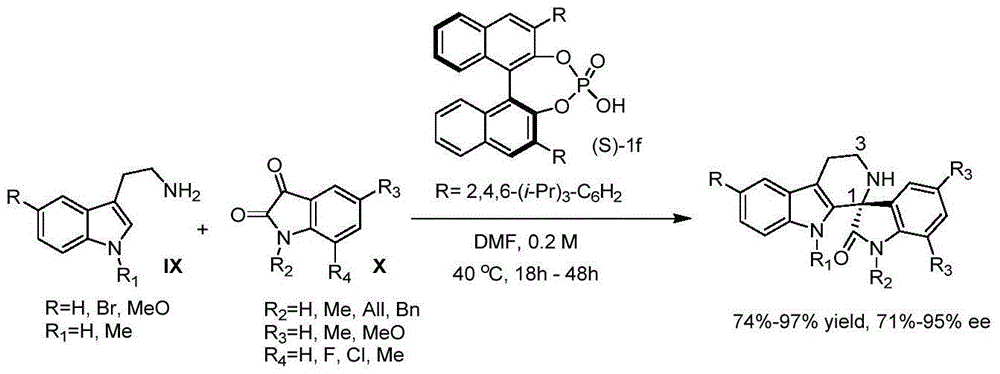
Application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of spiro[cyclopropane-1,3'-indole] compound
ActiveCN106423281AHigh yieldRaw materials are easy to obtainOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsRare earthLanthanum
The invention discloses application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of a spiro[cyclopropane-1,3'-indole] compound. The chemical formula of tris(bis(trimethylsilyl)amino)lanthanum is [(Me3Si)2N]3Ln(Mu-Cl)Li(THF)3, and can be taken as a catalyst for catalyzing substitution of isatin, phosphite ester and olefin to prepare the spiro[cyclopropane-1,3'-indole] compound through a one-pot reaction; in the catalyst, (Me3Si)2N represents trimethylsilylamino; Ln represents positive trivalent rare earth metal ions, and is selected from one of lanthanum, samarium, gadolinium, erbium or ytterbium; Mu- represents a bridged bond; THF represents tetrahydrofuran. In the method, a catalyst synthesizing method is simple, reaction raw materials are simple and readily available, a substrate has a wide application range, the one-pot reaction has high efficiency, reaction conditions are mild, and the yields of most target products are 85 percent or more.
Owner:SUZHOU UNIV
Preparation method of 2-trifluoromethyl substituted quinazolinone compound
ActiveCN111675662AImprove toleranceSimple and fast operationOrganic chemistryMolecular sievePtru catalyst
The invention discloses a preparation method of a 2-trifluoromethyl substituted quinazolinone compound. The preparation method comprises the following steps: adding ferric trichloride, sodium hydrogen, a molecular sieve, trifluoroethyleneimide chloride and isatin into an organic solvent, reacting at the temperature of 40 DEG C for 10 hours, heating to 120 DEG C, reacting for 20 hours, and after the reaction is completed, carrying out post-treatment to obtain the 2-trifluoromethyl substituted quinazolinone compound. The preparation method is simple and convenient to operate, cheap and easily available in initial raw materials and catalysts, high in reaction efficiency and wide in substrate compatibility range, diversified substituted quinazolinone compounds with trifluoromethyl can be synthesized through substrate design, and the practicability of the method is widened while operation is facilitated.
Owner:ZHEJIANG SCI-TECH UNIV
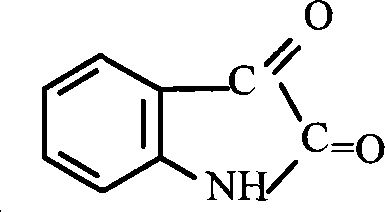
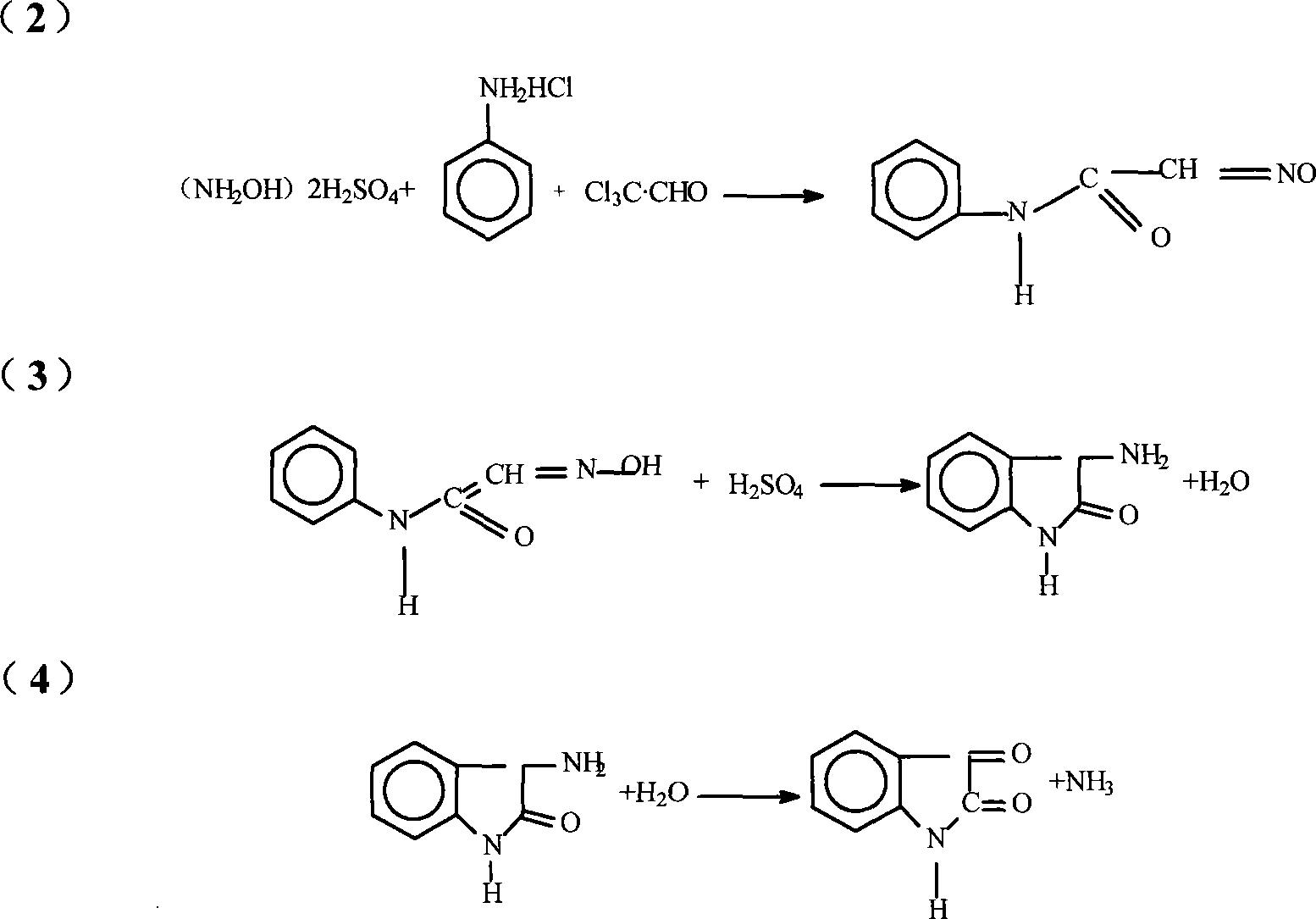
Method for preparing isatin
The invention relates to a preparative method for isatin, including the procedures as follows: compounding hydroxylamine sulphate; utilizing the hydroxylamine sulphate to synthesize oximido antifebrin; carrying out cyclization reaction and deamination reaction for the oximido antifebrin to product the coarse product of the isatin; and refining the coarse product of the isatin to obtain the isatin product, wherein, sodium nitrite, carbonate and supercarbonate of alkali metal and sulfur dioxide are adopted as the raw materials to synthesize the ydroxylamine sulphate. Te alkali metal is kalium or natrium. The isatin product produced by the method disclosed in the present invention has the advantages of high purity and good quality.
Owner:XIANGSHUI HENRYDA TECH CHEM
Preparation method of isatin derivatives
The invention discloses a novel method for synthesizing isatin derivatives. According to the method, a catalytic oxidation system of tert-butyl nitrite / O2 is adopted without using metal reagents, reaction is performed at room temperature under normal pressure, and isatin derivatives are obtained with high yield and high purity. The method has the advantages of simple technical operation process and low cost.
Owner:NINGBO UNIV
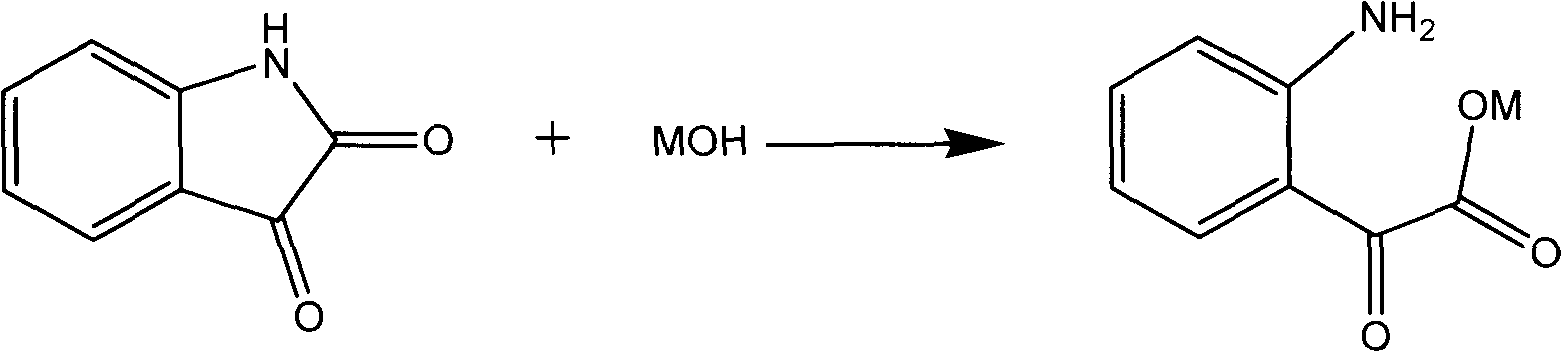
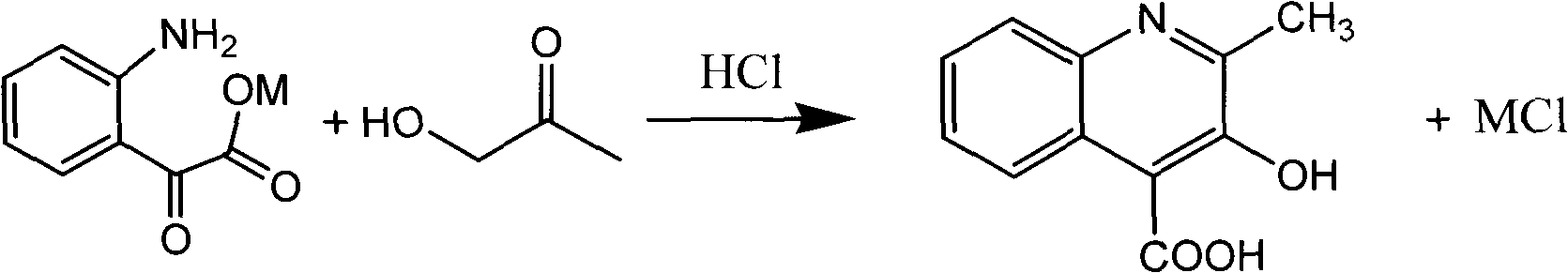
Novel method for preparing 3-hydroxylquinaldine-4-carboxylic acid
The invention relates to a novel method for preparing 3-hydroxylquinaldine-4-carboxylic acid. The 3-hydroxylquinaldine-4-carboxylic acid produced by the prior art has low purity. To solve the problem, the novel method for preparing the 3-hydroxylquinaldine-4-carboxylic acid comprises the following steps of: (1) adding 1.0 molar part of solid isatin into enough clear water, then adding 1.0 equivalent molar part of inorganic base, mixing and dissolving, filtering, and collecting filtrate; and (2) adding 1.0 equivalent molar part of inorganic base into the obtained filtrate, dropwise adding 1 to2.0 molar parts of monochloroacetone, then dropwise adding hydrochloric acid to adjust the pH value to be 7, then emptying and filtering, collecting a filter cake, and drying to obtain a finished product. BY the method, the content and purity of the 3-hydroxylquinaldine-4-carboxylic acid are improved on the premise of not increasing equipment, raw materials, processes and the like; and the purityof the 3-hydroxylquinaldine-4-carboxylic acid prepared by the method is over 97 percent, and the yield is more than or equal to 95 percent.
Owner:JIANGSU D I A N CHEM CO LTD
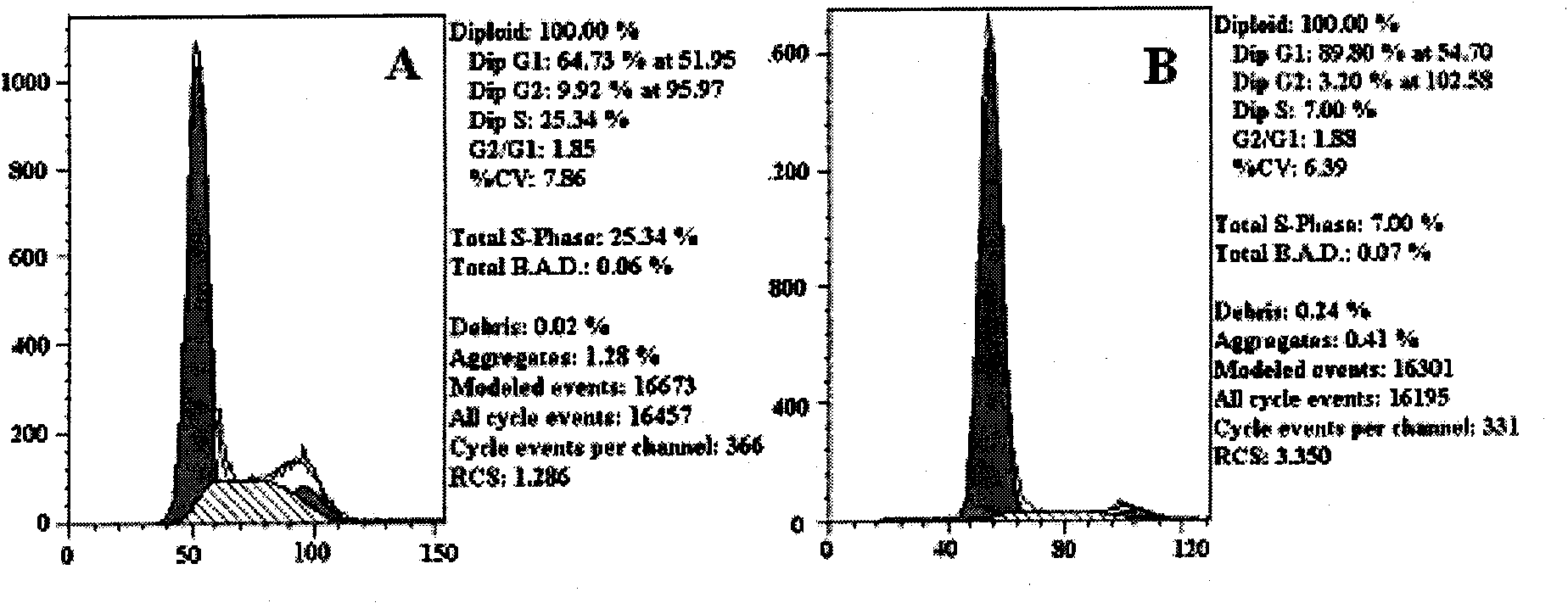
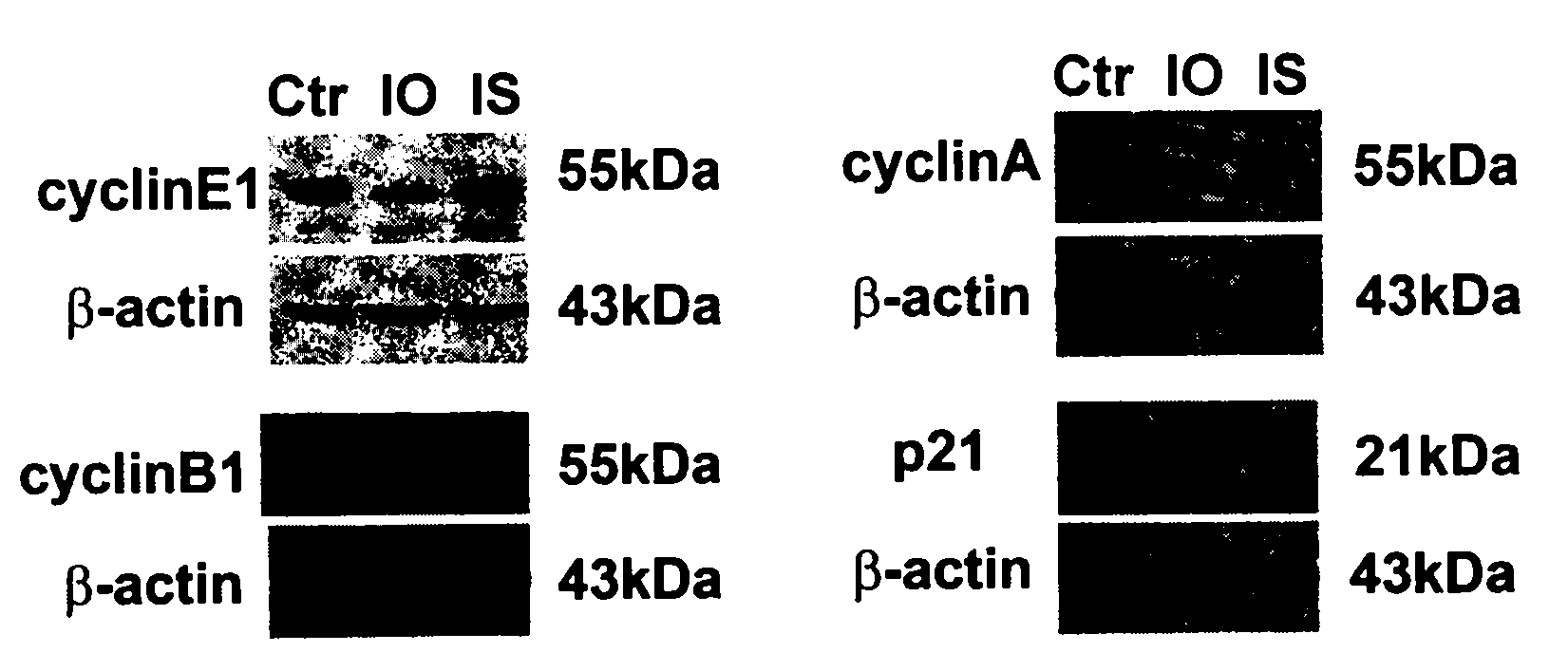
Preparation method of 2,3-indolediketone-3-oxime and application in prevention and control of cancer
InactiveCN101816649AGenotoxicIncreased drug resistanceOrganic active ingredientsOrganic chemistryCancer preventionCancer cell
The invention discloses a preparation method of 2,3-indolediketone-3-oxime or isatin-3-oxime and discloses application of the 2,3-indolediketone-3-oxime in preparing cancer prevention or control drugs. The application of the 2,3-indolediketone-3-oxime in prevention and control of cancer or other malignant tumours is characterized in that the substance has obvious growth inhibition action on various tumour cells, has specific G1-phase blocking action on cell generation cycle, i.e. the substance can stop the tumour cells carrying out DNA (deoxyribonucleic acid) replication, and has obvious down regulation action on the expression of cycle proteins cyclin E, cyclin B and cyclin A, obvious up regulation action on the expression of a cell cycle inhibiting factor p21, induction action on cancer cells or precancerosis cells and sensitization action on gene toxic chemotherapeutic agents.
Owner:PEKING UNIV
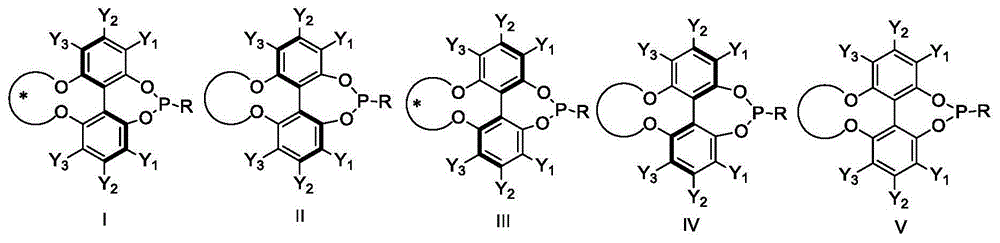
Phosphoramidite ligand as well as preparation method and application thereof
ActiveCN104610363AHigh reactivityHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsIridiumCatalytic effect
The invention relates to the field of chemical catalysis and discloses a phosphoramidite ligand as well as a preparation method and an application thereof. The synthesized phosphoramidite ligand and an enantiomer or a racemate of the phosphoramidite ligand take biphenyl as a framework and are subjected to an asymmetric induced reaction of axial chirality to realize complete transfer from central chirality to axial chirality. A synthetic method for the phosphoramidite ligand and the enantiomer or the racemate of the phosphoramidite ligand is simple and economic, and a common and complex chiral resolution process is avoided during chiral ligand preparation. The phosphoramidite ligand and the enantiomer or the racemate of the phosphoramidite ligand is employed to form the ligand together with active metal-iridium for catalyzing an asymmetric addition reaction of N-protected isatin and arylboronic acid, a very good catalytic effect is achieved, and a product can obtain the yield of more than 96% and the enantiomeric excess (ee) value of more than 92%.
Owner:SUN YAT SEN UNIV
Pyrrolo or indolo azacycloalkane structure containing chiral spiro oxyindole compound as well as racemate and preparation method thereof
ActiveCN108409746AImprove physiological activityOrganic chemistryAntineoplastic agentsEnantiomerPyrazine
The invention relates to the field of chemocatalysis and discloses a pyrrolo or indolo azacycloalkane structure containing chiral spiro oxyindole compound as well as a racemate and a preparation method thereof. The method comprises the following steps of by taking N-substituted isatin or benzene ring substituted isatin (1) and N-alkylamino group substituted pyrrole or indole (2) as reaction substrates and using chiral phosphoric acid or an enantiomer or a racemate thereof as a catalyst, directly preparing the pyrrolo or indolo azacycloalkane structure containing chiral spiro oxyindole compoundby one step by a way of catalyzing [m+1] cycloaddition in the environment of a solvent and an additive. Through a method of [5+1], [6+1] or [7+1] cycloaddition, by taking the chiral phosphoric acid as the catalyst, asymmetric synthesis is realized; and by using isatin which is not modified, a 3',4'-dihydro-2'H-spiro[indoline-3,1'-pyrrolo[1,2-a]pyrazine]-2-ketone compound is obtained for the firsttime through one-step reaction only, and the reaction can be extended to the cycloaddition process of a larger ring system. By adopting the method provided by the invention, an activity test is performed on the obtained compound, and a lead compound with very good physiological activity is obtained.
Owner:SUN YAT SEN UNIV
Multi-nitrogen substituted isatin derivative and synthetic method of multi-nitrogen substituted isatin derivative
InactiveCN102690226AHigh antibacterial activityGood acetylcholinesterase inhibitory activityOrganic chemistryAntibacterial activitySodium hydride
The invention discloses multi-nitrogen substituted isatin derivative and a synthetic method of the multi-nitrogen substituted isatin derivative. The multi-nitrogen substituted isatin derivative has a structure general formula of the formula (I); the synthetic method of the multi-nitrogen substituted isatin derivative comprises the steps of taking isatin derivative, dissolving into halogenated substance, refluxing reaction substrate in acetonitrile or N,N'-dimethyl formamide solvent for 2-8 hours under existence of inorganic bases such as potassium carbonate or sodium hydride, spin-drying, filtering, carrying out column chromatography and recrystallizing to obtain target compound. The synthetic method has the advantages of convenience, fast reaction speed, mild condition, simple treatment and high yield. Since the derivative has excellent antibacterial activity and acetylcholinesterase inhibitory activity, the multi-nitrogen substituted isatin derivative can be used as lead compound for resisting alzheimer's disease, and also can be applied to synthesis of pharmaceutical intermediates.
Owner:GUANGDONG UNIV OF TECH
Method for synthesizing chiral spirocyclo-oxindole-benzopyran-ketone-3,4-dihydro-pyran compound
ActiveCN106188078ASimple and fast operationHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsThioureaKetone
The invention discloses a method for synthesizing a chiral spirocyclo-oxindole-benzopyran-ketone-3,4-dihydro-pyran compound. The method comprises the following steps: using an isatin derivative beta, gama-unsaturated alpha-keto ester and 3-hydroxy-4-hydrogen-chromene-4-ketone as reactants, and synthesizing in a solvent under the catalysis of chiral multifunctional chiral quinine thiourea to obtain a product. The method provided by the invention has the advantages of simple and easy obtaining of raw materials, mild reaction conditions, simple and convenient post-treatment, wide range of suitable substrates, high yield and high enantioselectivity, and the synthesized product can be used for synthesizing intermediates of drugs, insecticides and photoelectric materials.
Owner:SUZHOU UNIV
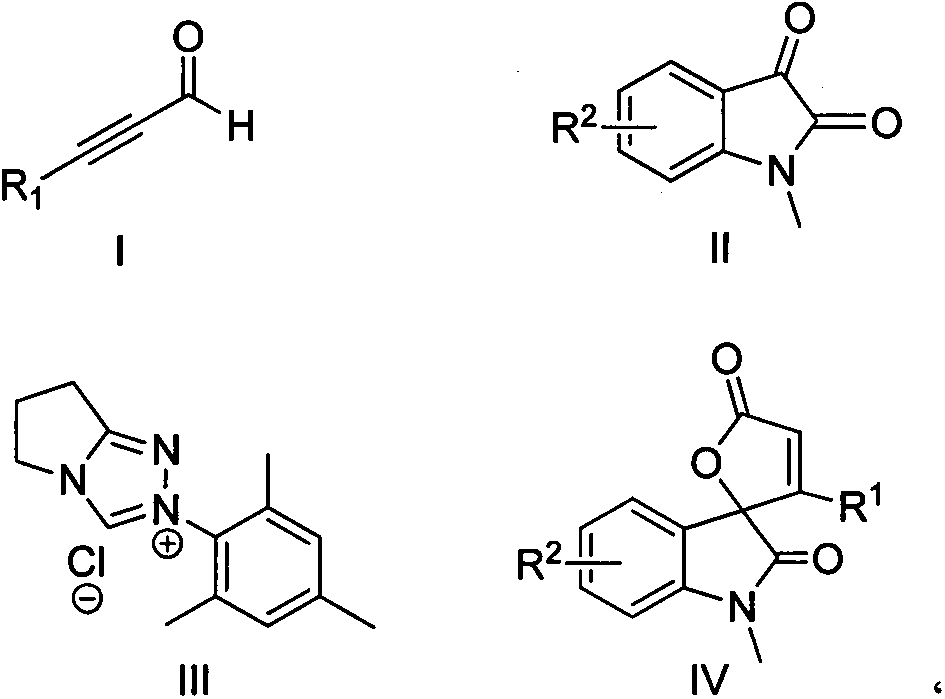
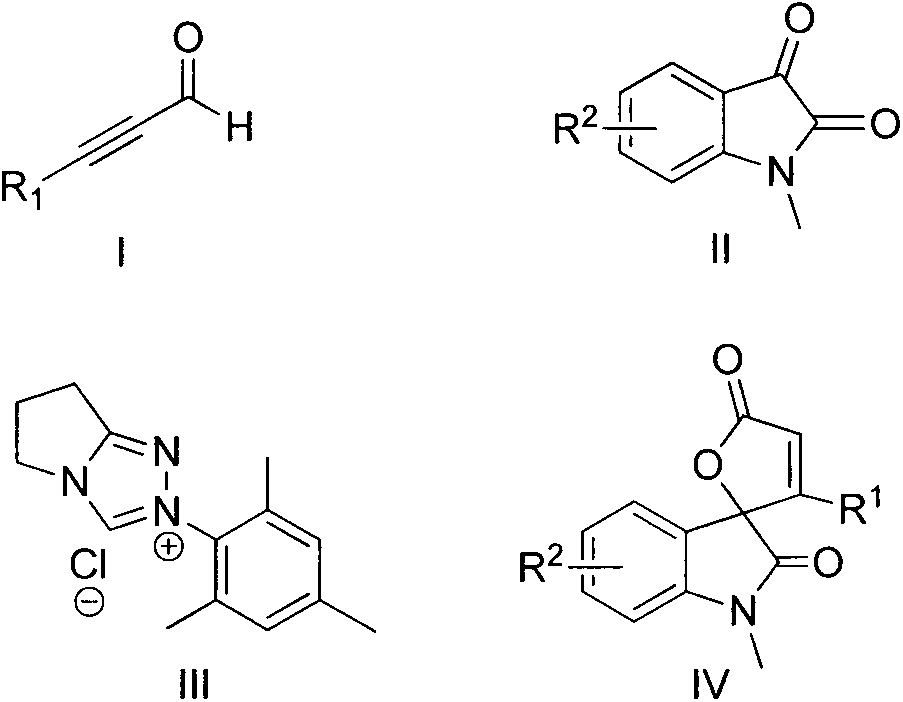
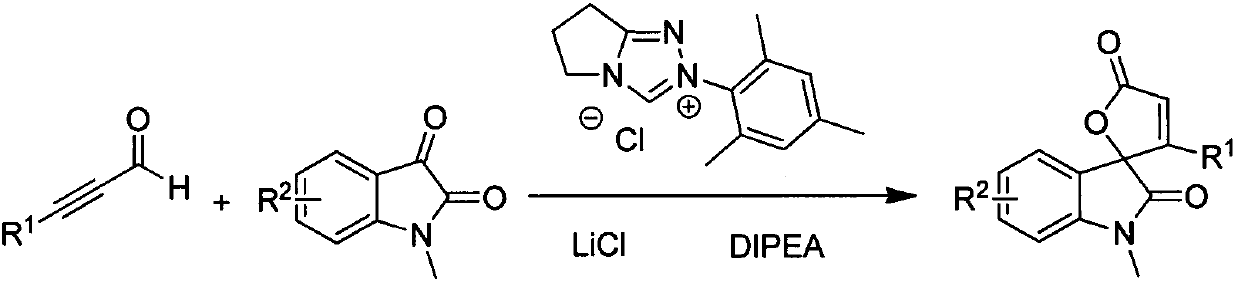
Synthesis method for spirally-epoxidized indole butenolide compound
InactiveCN103788102ASimple and fast operationMild reaction conditionsOrganic chemistryLithium chlorideSynthesis methods
The invention relates to the technical field of organic chemistry and particularly relates to a synthesis method for a spirally-epoxidized indole butenolide compound which is shown as the formula IV. The method comprises takes alkynal shown as the formula I and N-methyl isatin shown as the formula II as raw materials, and takes dioxane as a solvent in the presence of a triazole salt shown as the formula III, lithium chloride and diisopropyl ethyl amine to react for 3-72 hours under the condition of nitrogen protection and at 10-65 DEG C; a reaction solution is cooled and concentrated and a mixed solvent of a petroleum ether and acetone in the volume ratio of 25:1 is used as an eluting agent to carry out column chromatography elution; detected eluting solution parts of all products are collected and rotary evaporation is carried out so as to remove the solvent to obtain a spirally-epoxidized indole butenolide product. The synthesis method provided by the invention has good yield, and the application range of primers is wide; the synthesis method has the advantages of simplicity and convenience for operation, moderate reaction, convenience for post-treatment and the like.
Owner:CHINA PHARM UNIV
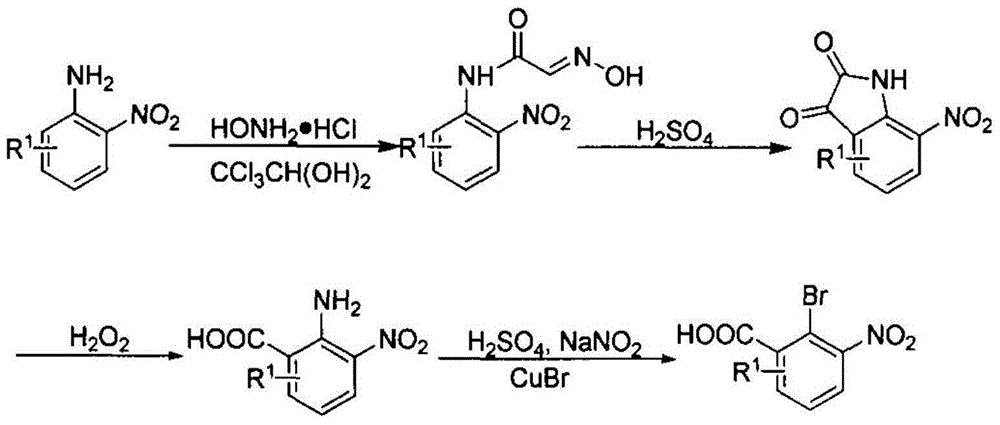
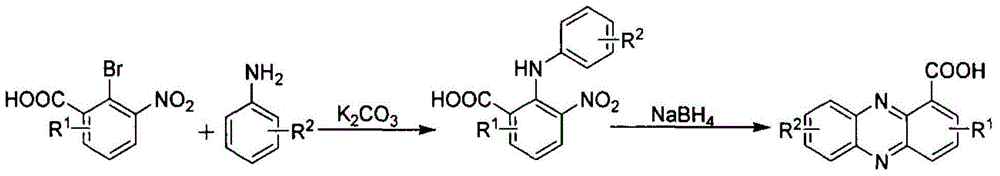
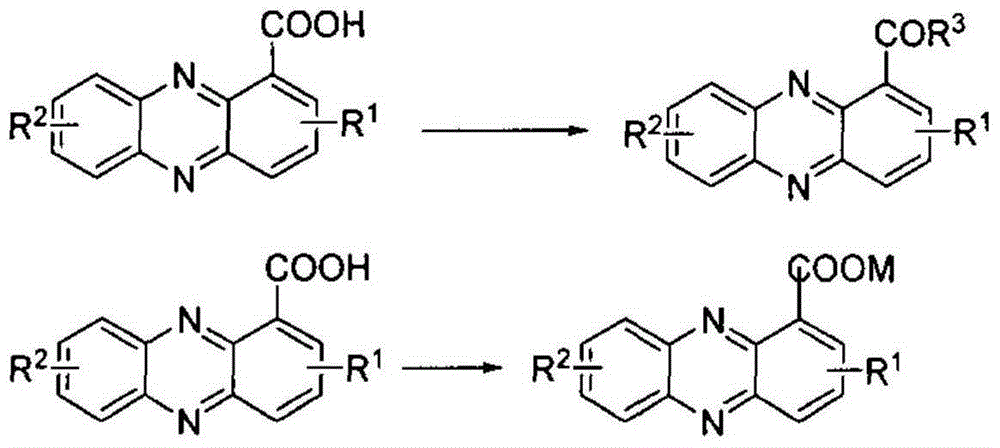
Preparation method for phenazine-1-carboxylic acid
ActiveCN104829544AReaction raw materials are readily availableHigh yieldOrganic chemistrySandmeyer reactionHydroxylamine
The invention relates to a preparation method for phenazine-1-carboxylic acid. The preparation method comprises the following steps: reacting aniline with chloral hydrate and hydroxylamine to produce alpha-oximidoacetanilide, treaing alpha-oximidoacetanilide with concentrated sulfuric acid to obtain isatin, reacting isatin with hydrogen peroxide so as to obtain 2-amino-3-nitrobenzoic acid and then carrying out Sandmeyer reaction to prepare 2-bromo-3-nitrobenzoic acid; and subjecting prepared 2-bromo-3-nitrobenzoic acid and aniline to Jourdan-Ullmann reaction so as to obtain substituted diphenylamine and carrying out ring closure to prepare phenazine-1-carboxylic acid. Compared with the prior art, the preparation method provided by the invention has the advantages of easy availability of raw materials, easy control of reaction, mild reaction conditions, easy post-treatment, high overall yield, as high as 32 to 47%, and suitability for industrial production; and compared with conventional method for production of shenqinmycin from ferment powder, the method provided by the invention enables cost to be greatly reduced.
Owner:SHANGHAI TAIHE INT TRADE CO LTD +1
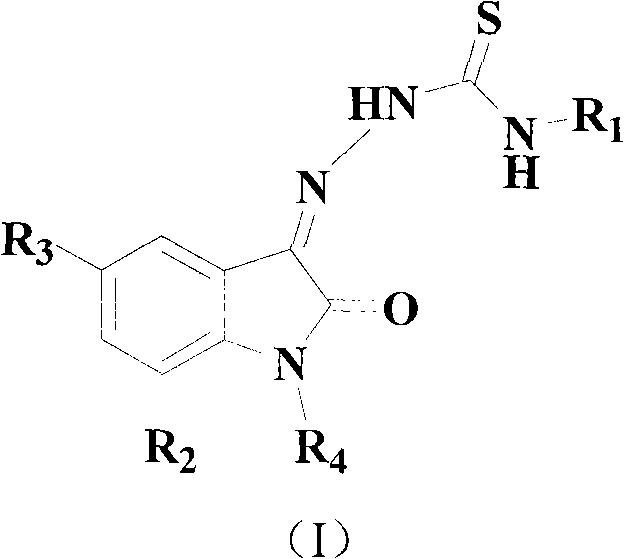
Isatin derivative and application thereof in preparation of medicines for resisting super-drug-resistance bacteria
InactiveCN102464603AAntibacterial agentsOrganic active ingredientsAntimicrobial drugSuspending Agents
The invention relates to an isatin derivative and application thereof in preparation of medicines for resisting super-drug-resistance bacteria. The isatin derivative has very strong inhibitory action on MRSA (Methicillin-resistant Staphylococcus Aureus) and can be used for preparing novel effective anti-microbial medicines. The medicines can be made into injections, tablets, pills, capsules, suspending agents or emulsions. The administration routes of the medicines can be of oral administration and percutaneous, venous or intramuscular injection. The isatin derivative is as shown in the chemical structural formula (I), wherein in the formula, R1 is H, phenyl or p-fluorophenyl; R2 is F, C1, Br, I or H; R3 is F, C1, Br, I or H; R4 is H, CH3 and o-fluorobenzyl, m-fluorobenzyl and p-fluorobenzyl.
Owner:NANKAI UNIV +1
Spiro-heterocyclic compound containing indole structures and preparation method of spiro-heterocyclic compound
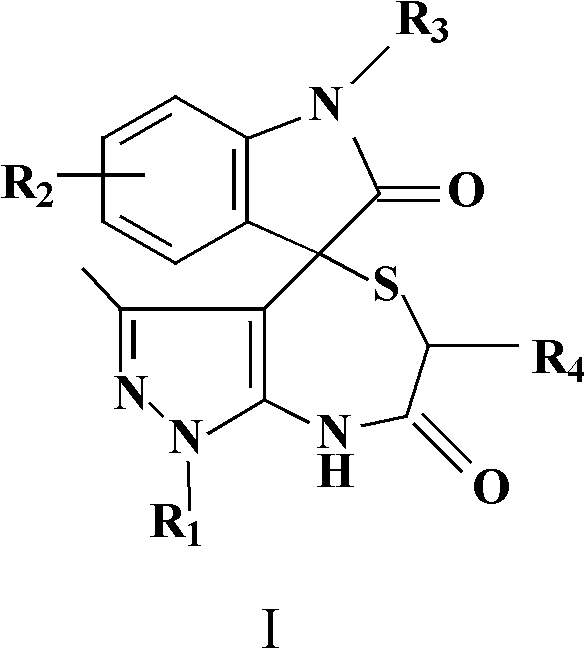
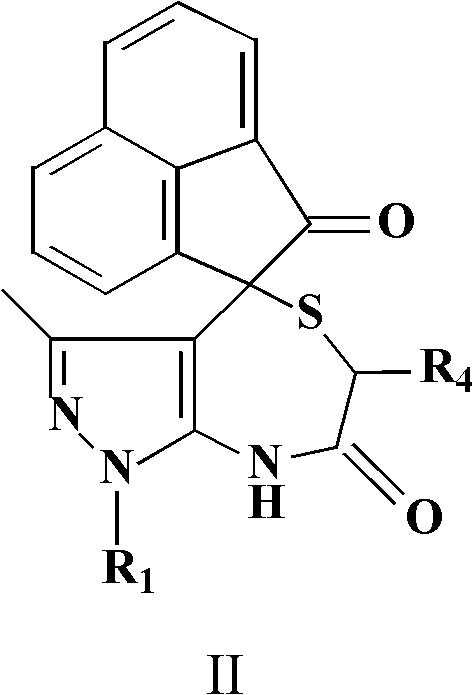
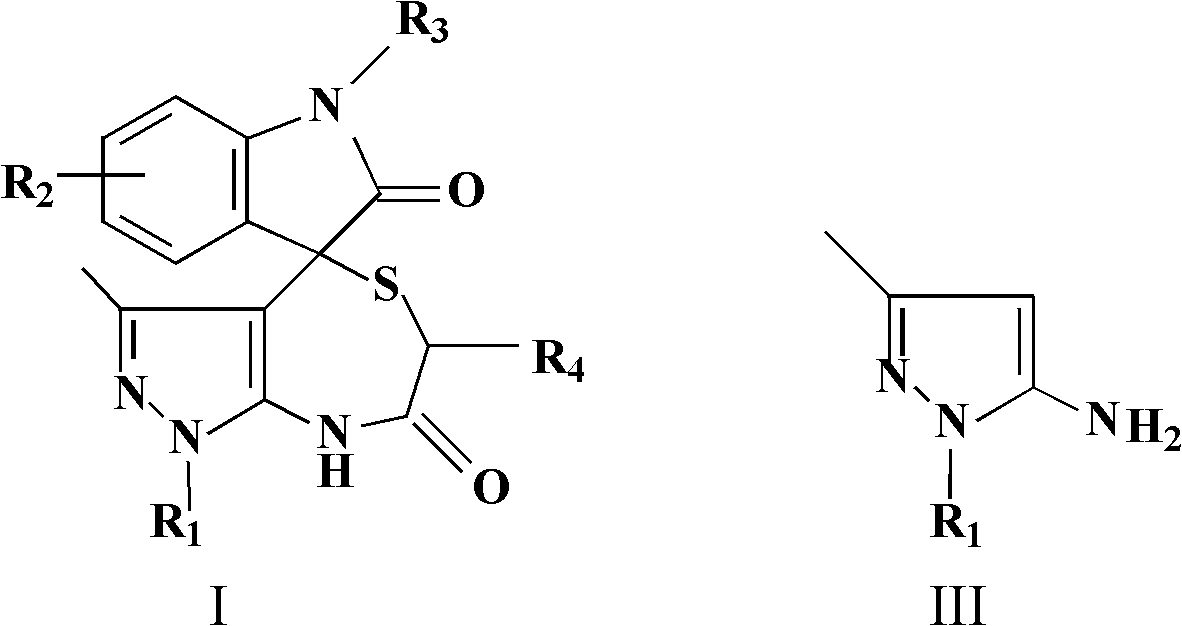
InactiveCN102584860ABiologically activePharmacologically activeOrganic chemistryAcenaphthyleneOrganic acid
The invention relates to the field of pharmaceutical chemistry, and discloses a spiro-heterocyclic compound containing indole structures and a preparation method of the spiro-heterocyclic compound. The spiro-heterocyclic compound containing the indole structures comprises a dihydro-spiro[indole-3,4'-pyrazolo[3,4-e][1,4]thiazepine] diketones compound and a dihydro-spiro[acenaphthylene-1,4'-pyrazolo[3,4-e][1,4]thiazepine] diketones compound, and adopts the structure which is shown in a formula I and a formula II, wherein R1 is -CH3 or -Ph, R2 is -H, -CH3, -F, -Cl or -Br, R3 is -H or -CH3, and R4 is -H or -CH3; in addition, the preparation method comprises the steps as follows: isatin or acenaphthylenedione, a 5-aminopyrazole compound and mercapto carboxylic acid are dissolved in a solvent, organic acid or mineral acid is taken as catalytic agents, and reaction is performed for 8 to 24 hours at the temperature ranging from 65 to 95 DEG C. The spiro-heterocyclic compound has stable properties, the preparation method is simple to operate, the productivity of reaction products is high, and the post-processing is simple.
Owner:SUZHOU UNIV
1, 3, 5-triazine-2-ketospirooxindole compound and preparation method
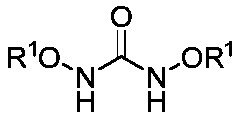
InactiveCN110256448AImprove chemical yieldEasy to operateOrganic chemistry methodsSynthesis methodsCompounded preparations
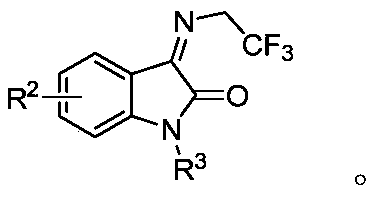
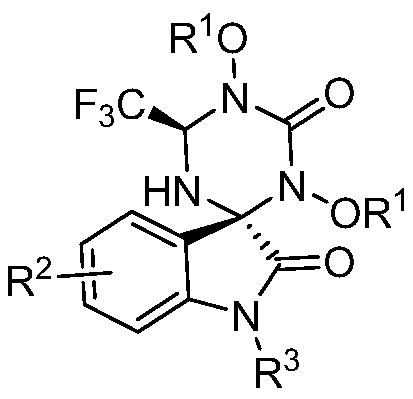
Belonging to the technical field of compound preparation, the invention relates to a 1, 3, 5-triazine-2-ketospirooxindole compound and a preparation method. The specific experimental method includes: weighing N, N'-dialkoxy substituted urea and trifluoroethyl substituted isatin-3-imide, dissolving the substances in DME, then adding the mixture into an NaH dissolved TFP solution at 0DEG C; then weighing an oxidant and performing dissolving in DME, then adding the obtained mixture dropwise into a reaction system, and fully stirring the reaction mixed solution (by TLC detection reaction) under a 0DEG C to room temperature condition till complete consumption of the trifluoroethyl substituted isatin-3-imide; and performing pressure reduced concentration, and subjecting a crude product to column chromatography, thus obtaining the 1, 3, 5-triazine-2-ketospirooxindole compound. The synthesis method can achieve excellent diastereomer selectivity and a chemical yield of medium level or above, and is a brand new method for efficient and concise synthesis of the 1, 3, 5-triazine-2-ketospirooxindole compound with potential bioactivity.
Owner:BEIJING UNIV OF TECH
Synthetic method of spiro-oxoindole ethylene oxide derivative
The invention discloses a spiro-indolone ethylene oxide derivative obtained with an o-trimethylsilylphenyl triflate, an N-methyl isatin derivative and aryl thioether as raw materials, CsF as an alkali and an organic solvent as a solvent and through a one-pot method, wherein an active intermediate benzyne is obtained by allowing o-trimethylsilylphenyl triflate to generate 1,2- elimination under fluorinion induction. In the synthetic method, the whole reaction adopts the one-pot method, reaction substrates are friendly to the environment, operations are simple, conditions are mild, product structures are diverse, and the product is widely applied in natural products and clinical medicines.
Owner:广东和博制药有限公司
Isatin imidazole compounds as well as preparation method and application thereof
InactiveCN107400121AAvoid drug resistanceAntibacterial agentsAntimycoticsAntifungal drugStructural formula
The invention relates to an isatin imidazole compound as well as a preparation method and application thereof. The isatin compounds are shown as general formulaa I-VI. The compounds have a certain inhibitory activities on Gram-positive bacteria, Gram-negative bacteria and fungi, can be used for preparing antibacterial and / or antifungal drugs, can serve as DNA intercalative agents, are simple in preparative raw materials, cheap, readily available and short in synthetic route and have significance in application of infection resistance. The structural formula is as shown in the specification.
Owner:SOUTHWEST UNIVERSITY
Metronidazole-isatin type compound as well as preparation method and application thereof
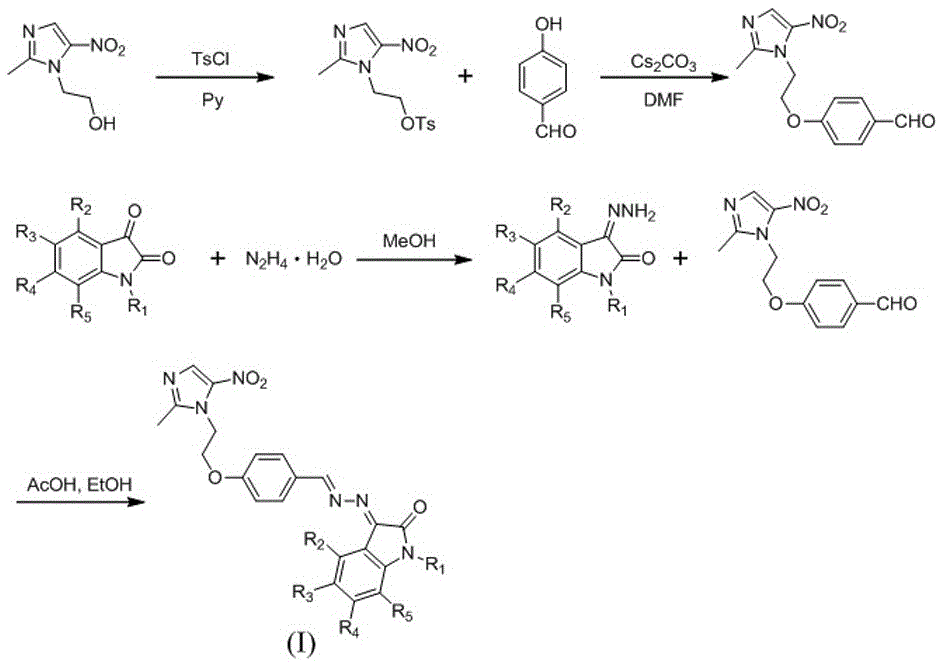
InactiveCN105541808AEnhanced inhibitory effectHigh activityAntibacterial agentsOrganic active ingredientsHydrazine compoundBenzaldehyde
The invention discloses a metronidazole-isatin type compound as well as a preparation method and an application thereof. The compound has a structure represented by a formula (I) (shown in the specification). The preparation method comprises the following steps: preparing 2-(2-methyl-5-nitro-1H-imidazole-1-yl)ethyl-4-methyl benzenesulfonate from metronidazole and paratoluensulfonyl chloride serving as raw materials, and reacting by virtue of cesium carbonate serving as alkali, N,N-dimethyl formamide serving as a solvent and p-hydroxy benzaldehyde, so as to obtain 4-(2-(2-methyl-5-nitro-1H-imidazole-1-yl)ethyoxyl)benzaldehyde; carrying out dehydration condensation on isatin, a derivative of isatin and hydrazine hydrate so as to obtain an intermediate, and carrying out dehydration condensation on the intermediate and 4-(2-(2-methyl-5-nitro-1H-imidazole-1-yl)ethyoxyl)benzaldehyde in the presence of glacial acetic acid serving as a catalyst, so as to obtain the target product. The compound can be used as a raw material of antibacterial drugs; and in the preparation method, the raw materials are simple and easily available, and the operation is convenient.
Owner:JISHOU UNIVERSITY
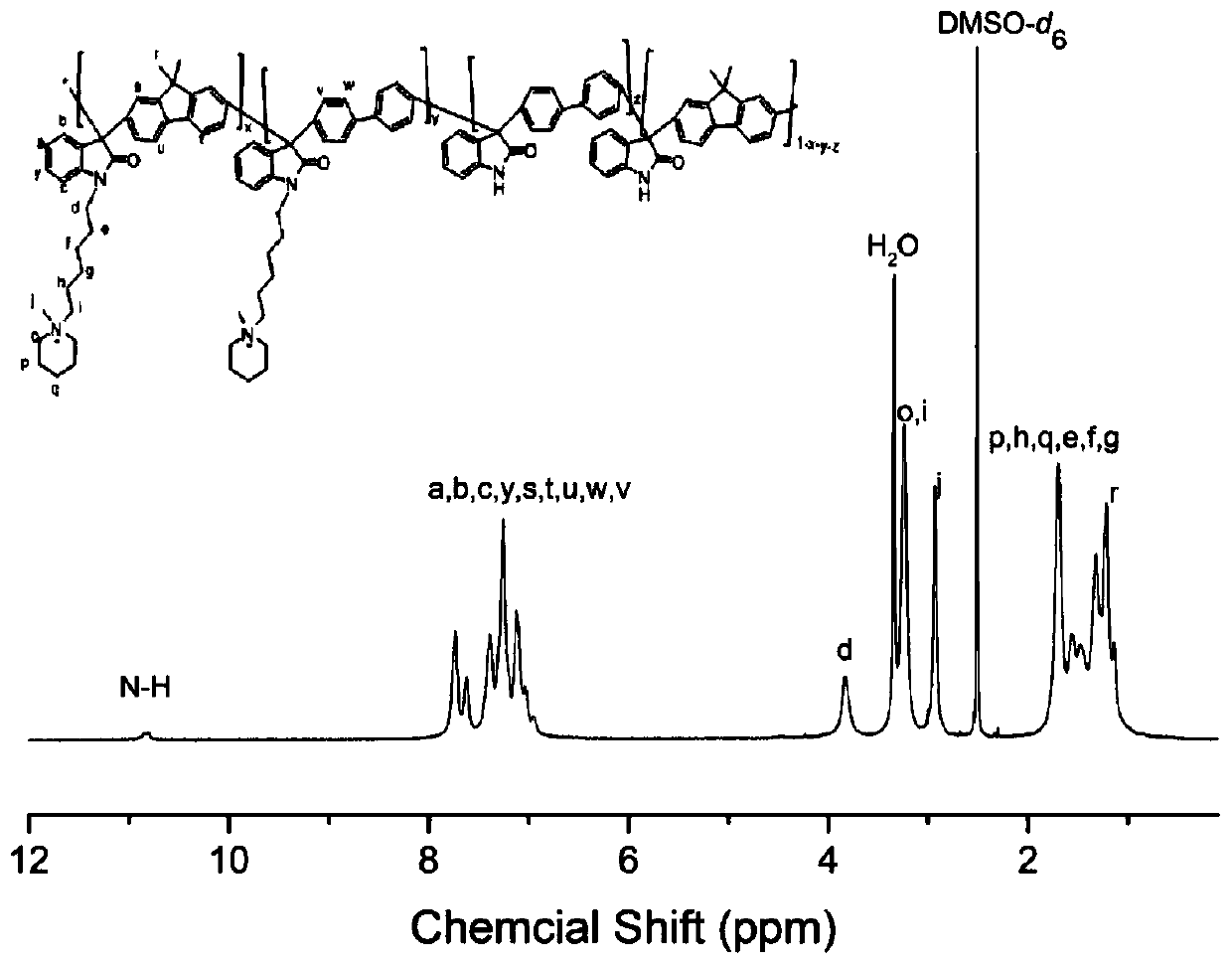
High-performance polyisatin aromatic hydrocarbon with long side chain ammonium salt, anion exchange membrane and preparation method thereof, and preparation method and application of anion exchange membrane
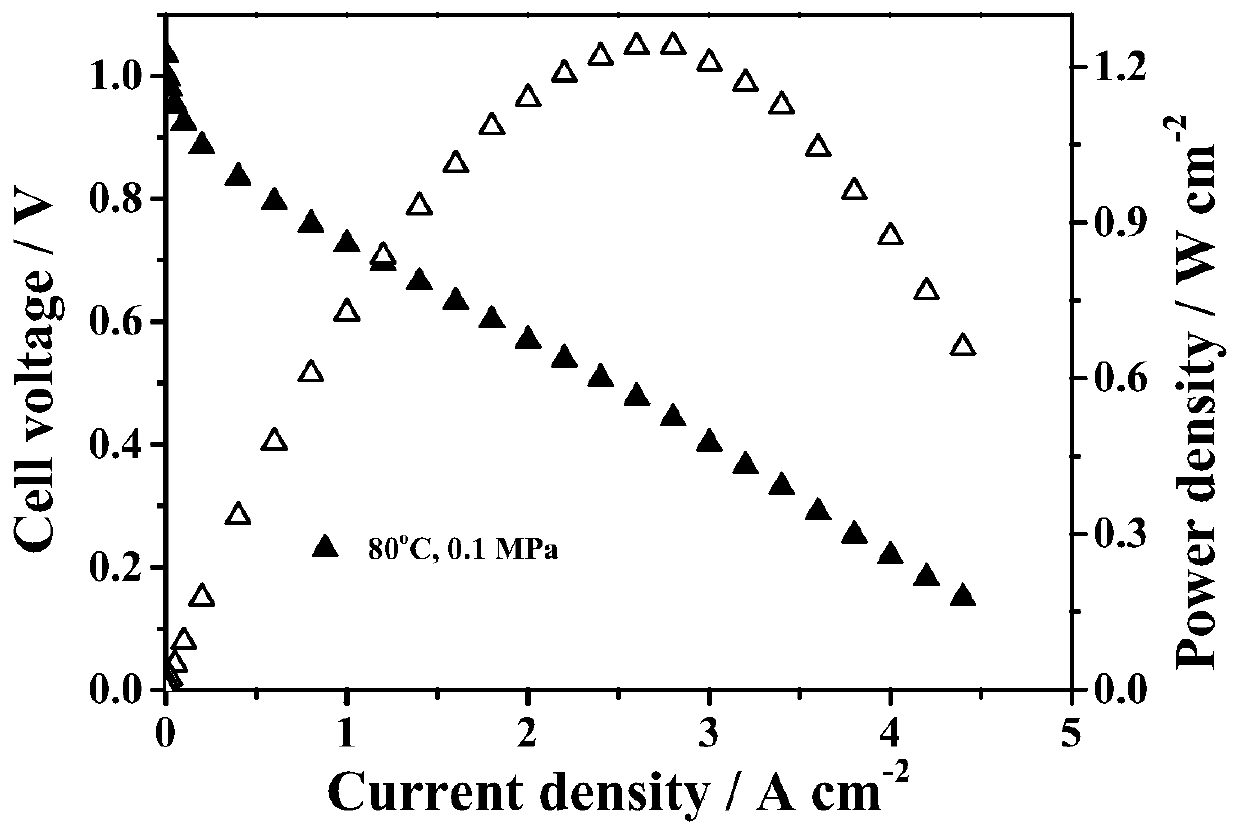
The invention discloses a polyisatin aromatic hydrocarbon with a long side chain ammonium salt, an anion exchange membrane and a preparation method thereof, and a preparation method and an applicationof the anion exchange membrane, and belongs to the technical field of polymer ion exchange membranes and batteries. Isatin and aromatic hydrocarbon undergo a polymerization reaction under the catalysis of a strong acid to obtain the polyisatin aromatic hydrocarbon. The polymer is grafted with the long-chain ammonium salt to form a membrane, and the membrane undergoes ion exchange to obtain the anion exchange membrane of the polyisatin aromatic hydrocarbon. The polyisatin aromatic hydrocarbon with the long side chain ammonium salt, and the anion exchange membrane thereof have the advantages ofhigh ionic conductivity, high alkali resistance stability and high oxidation resistance, allows the power density of cells to reach 1.24 W / cm<2> when applied to alkaline fuel cells, and can be applied to the fields of the fuel cells, energy storage cells, water electrolysis, resource recycling and other electrochemical devices involving anion exchange membranes.
Owner:DALIAN UNIV OF TECH
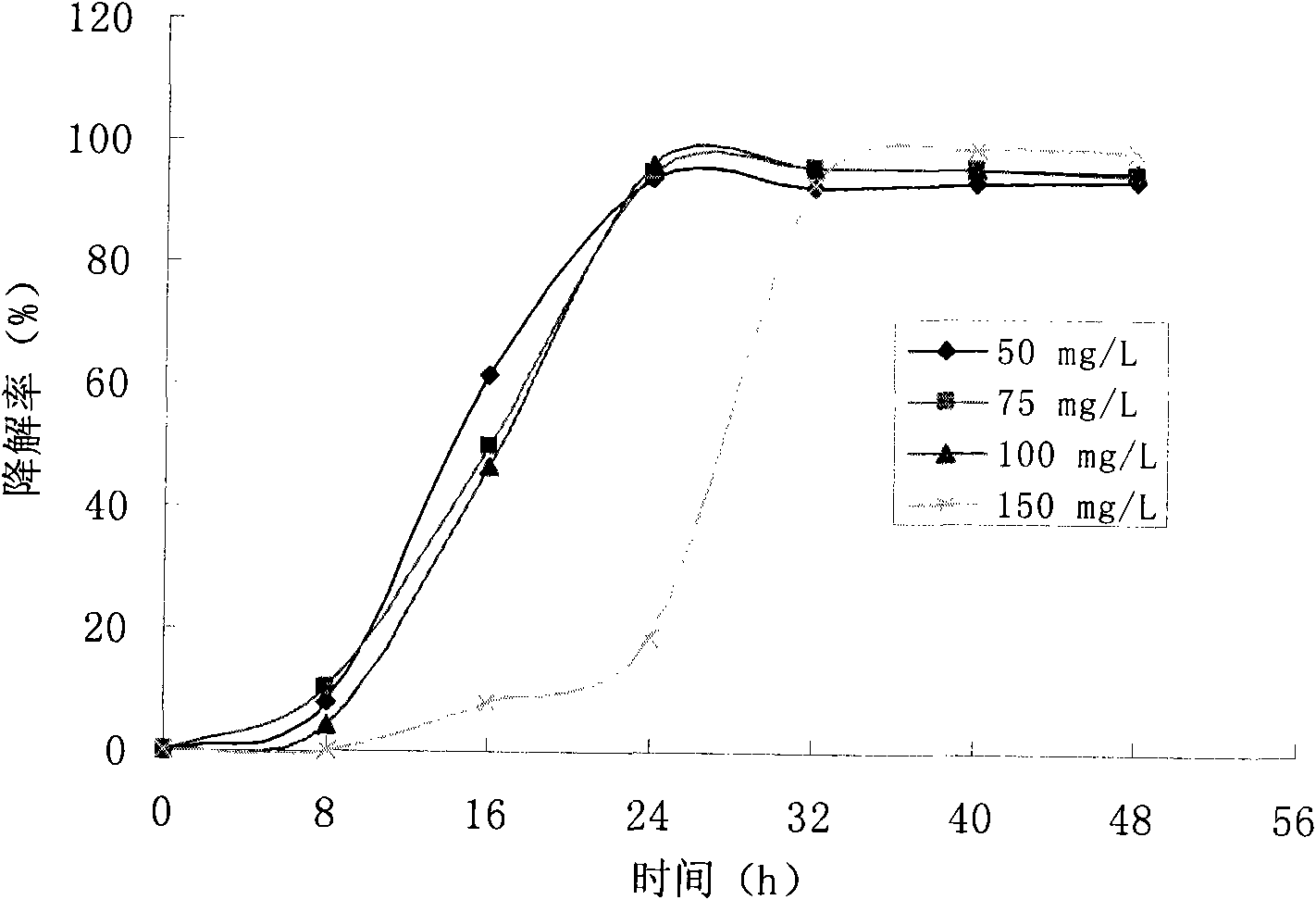
Achromobacter xylosoxidans strain and application thereof
InactiveCN101914467AEfficient degradationBacteriaWater contaminantsAchromobacter xylosoxidansWastewater
The invention discloses an Achromobacter xylosoxidans strain and application thereof. The strain is Achromobacter xylosoxidans N4 with the collection number of CGMCC No.3632. The Achromobacter xylosoxidans N4 GCMCC No.3632 can quickly and efficiently degrade isatin and o-aminobenzoic acid, and can be used for biologically treating calico printing wastewater.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
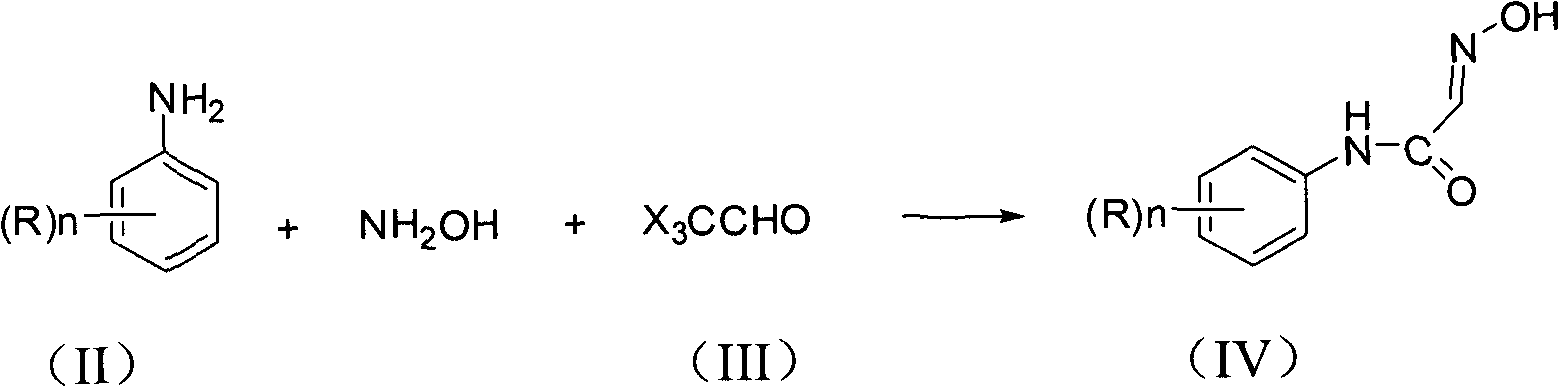
![4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as synthetic method and application thereof 4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as synthetic method and application thereof](https://images-eureka.patsnap.com/patent_img/0fedb1c2-f6c0-498f-901b-75431d3bb3c2/BDA0000459850840000011.PNG)
![4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as synthetic method and application thereof 4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as synthetic method and application thereof](https://images-eureka.patsnap.com/patent_img/0fedb1c2-f6c0-498f-901b-75431d3bb3c2/BDA0000459850840000012.PNG)
![4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as synthetic method and application thereof 4, 5-dyhydroxyl-3-H-spiro[furan-2, 3'-indole]-2'-ketone derivative as well as synthetic method and application thereof](https://images-eureka.patsnap.com/patent_img/0fedb1c2-f6c0-498f-901b-75431d3bb3c2/BDA0000459850840000021.PNG)
![Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound](https://images-eureka.patsnap.com/patent_img/3599ea27-31b2-43e9-b4e7-f03380afee07/100537DEST_PATH_IMAGE014.png)
![Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound](https://images-eureka.patsnap.com/patent_img/3599ea27-31b2-43e9-b4e7-f03380afee07/102811DEST_PATH_IMAGE021.png)
![Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound Method for catalytically preparing spiral [cyclopropane-1,3'- indole] compound](https://images-eureka.patsnap.com/patent_img/3599ea27-31b2-43e9-b4e7-f03380afee07/118281DEST_PATH_IMAGE005.png)
![Application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of spiro[cyclopropane-1,3'-indole] compound Application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of spiro[cyclopropane-1,3'-indole] compound](https://images-eureka.patsnap.com/patent_img/ecfedb37-fb27-41d4-a349-4f89c3968375/112593DEST_PATH_IMAGE006.png)
![Application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of spiro[cyclopropane-1,3'-indole] compound Application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of spiro[cyclopropane-1,3'-indole] compound](https://images-eureka.patsnap.com/patent_img/ecfedb37-fb27-41d4-a349-4f89c3968375/118311DEST_PATH_IMAGE024.png)
![Application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of spiro[cyclopropane-1,3'-indole] compound Application of tris(bis(trimethylsilyl)amino)lanthanum to catalyzed preparation of spiro[cyclopropane-1,3'-indole] compound](https://images-eureka.patsnap.com/patent_img/ecfedb37-fb27-41d4-a349-4f89c3968375/131081DEST_PATH_IMAGE018.png)