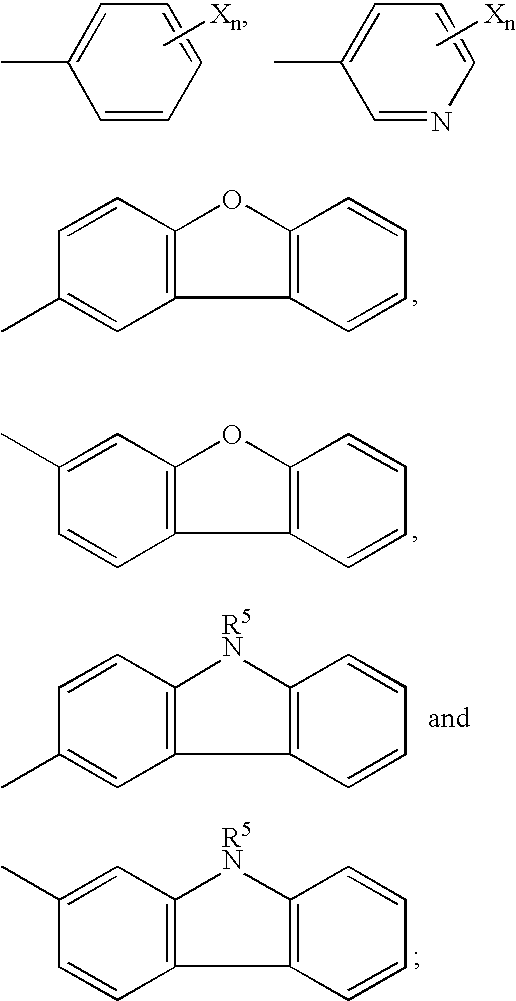
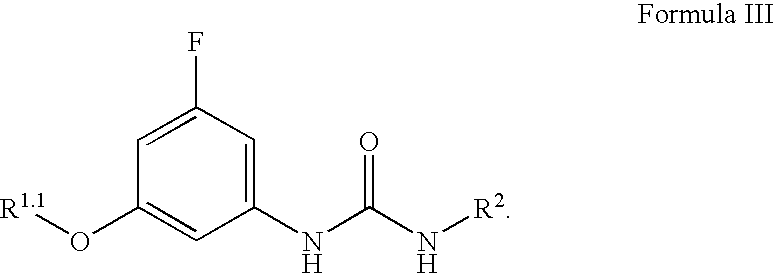
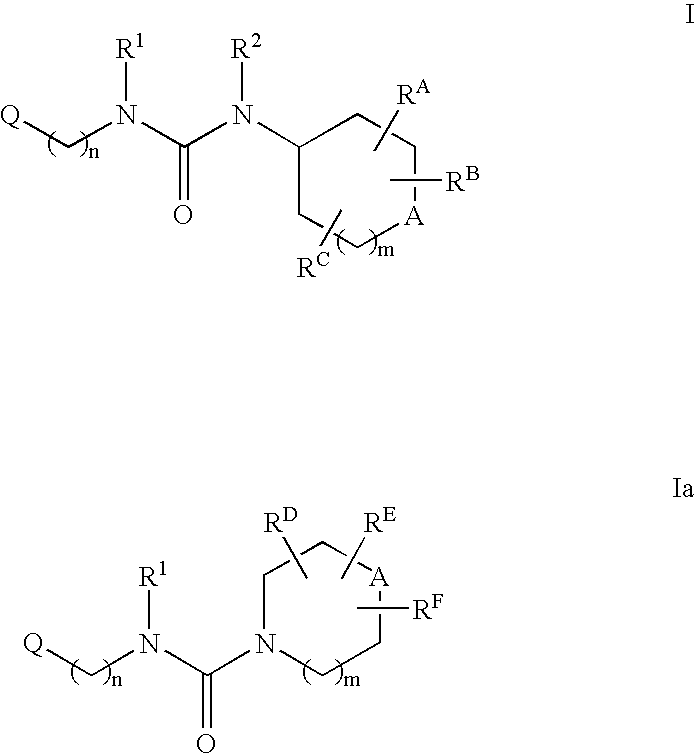
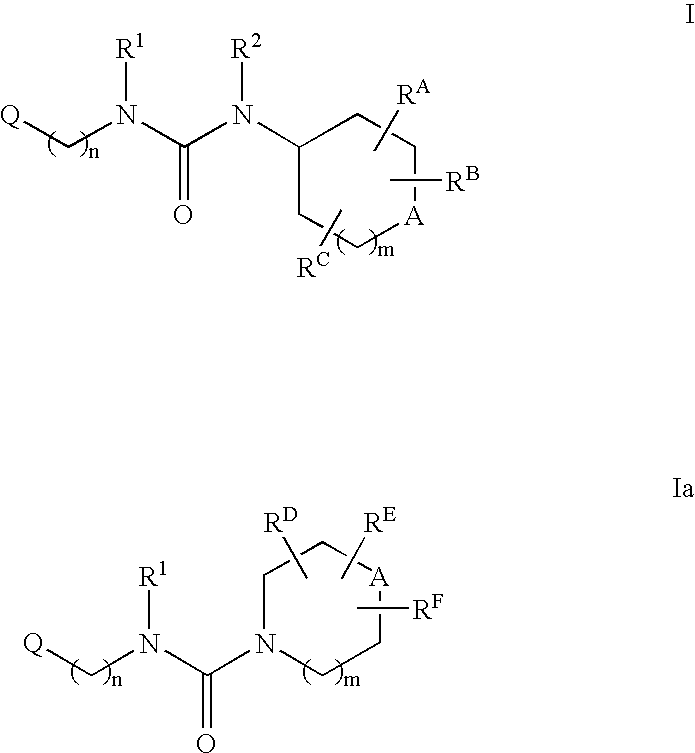
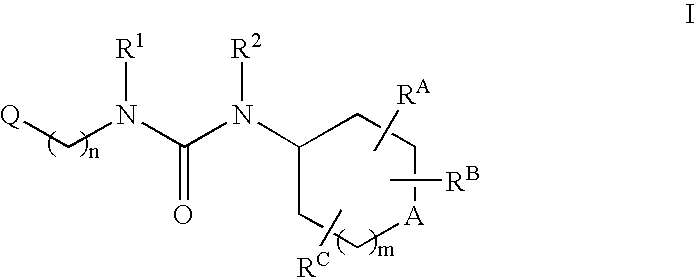
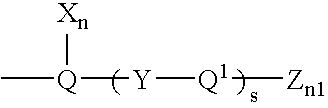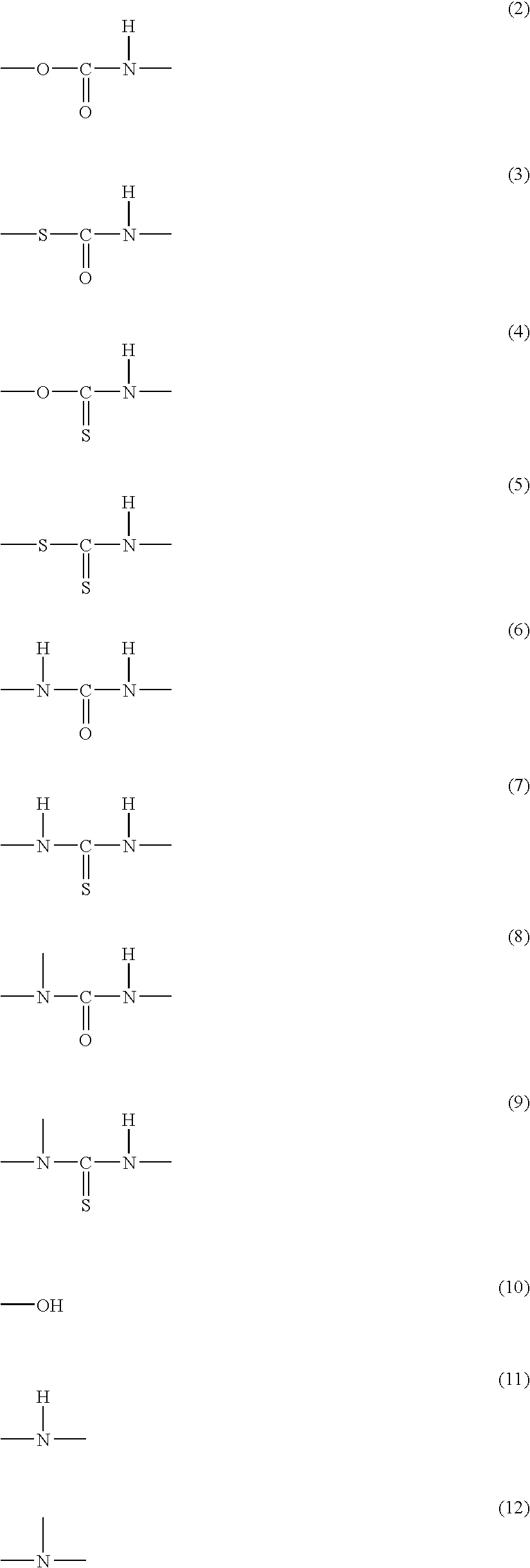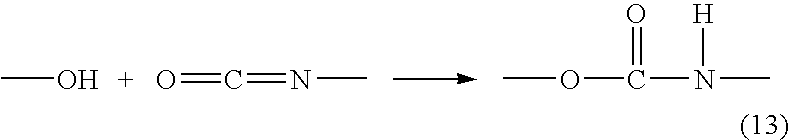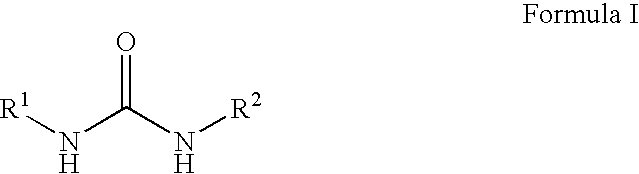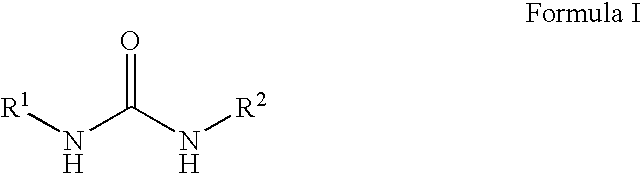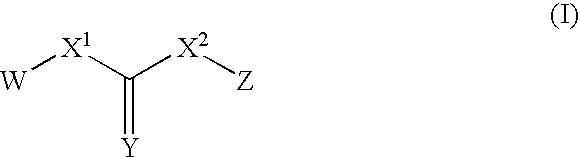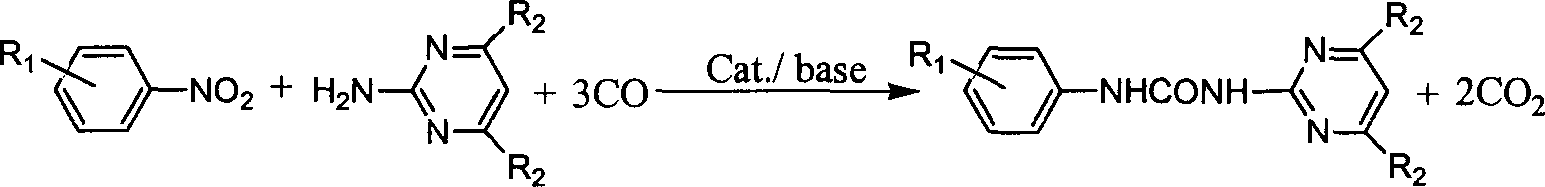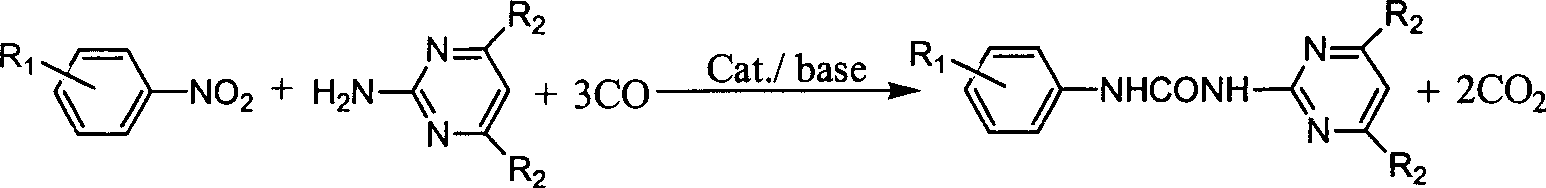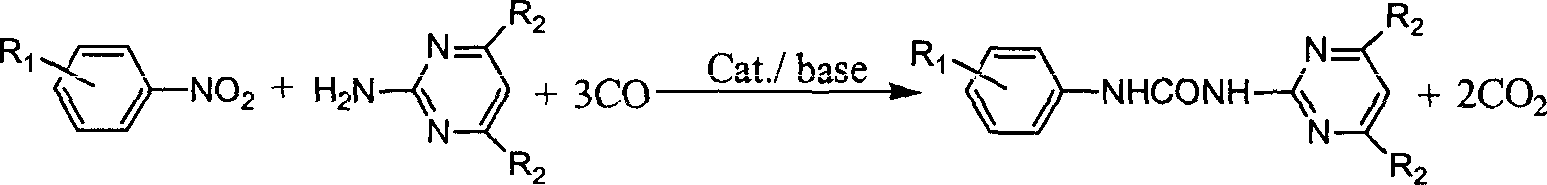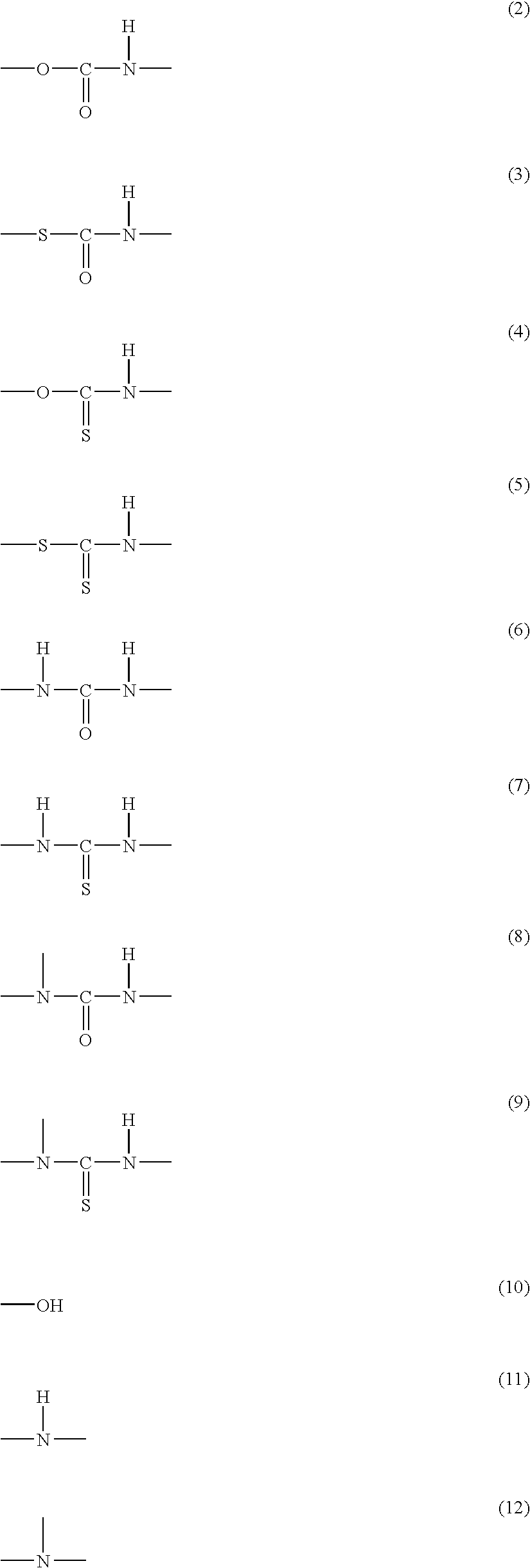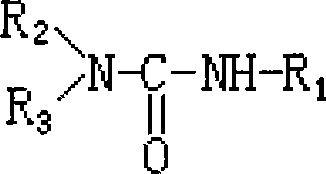Patents
Literature
153 results about "Substituted urea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
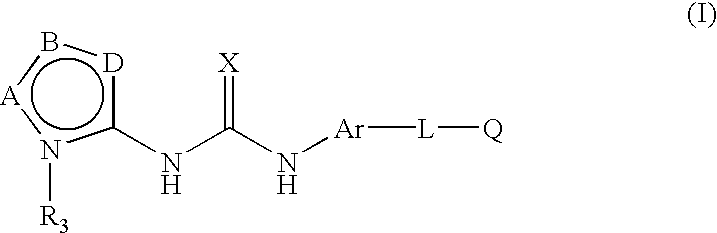
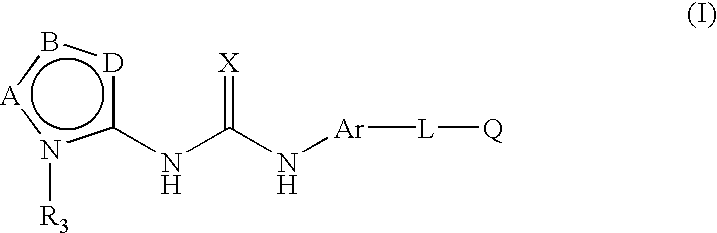
Inhibition of raf kinase using substituted heterocyclic ureas
Methods of treating tumors mediated by raf kinase, with substituted urea compounds, and such compounds per se.
Owner:BAYER HEALTHCARE LLC
Curable resin composition and cold-setting adhesive
Disclosed is a moisture-curing type curable resin composition containing: a curable resin intramolecularly having a silicon-containing functional group; and a Lewis acid or a complex of the Lewis acid as a curing catalyst, the Lewis acid being selected from the group consisting of metal halides and boron halides, which is rapidly cured at room temperature. The silicon-containing functional group is represented by general formula: —SiX1X2X3 or —SiR1X1X2 (wherein, X1, X2 and X3 respectively represent a hydrolytic group and may be the same as or different from each other, and R1 represents a substituted or unsubstituted organic group having 1 to 20 carbons). If the silicon-containing functional group is —SiR1X1X2, the curable resin further contains intramolecularly a polar component that is one of urethane, thiourethane, urea, thiourea, substituted urea, substituted thiourea, amide, and sulfide bonds, and hydroxyl, secondary amino and tertiary amino groups. Two-part type adhesive is constitutible with separating the curable resin from the curing catalyst.
Owner:KONISHI CO
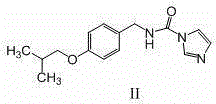
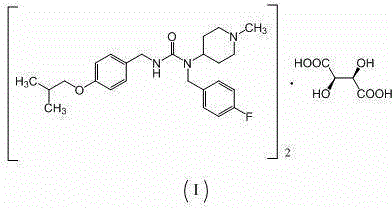
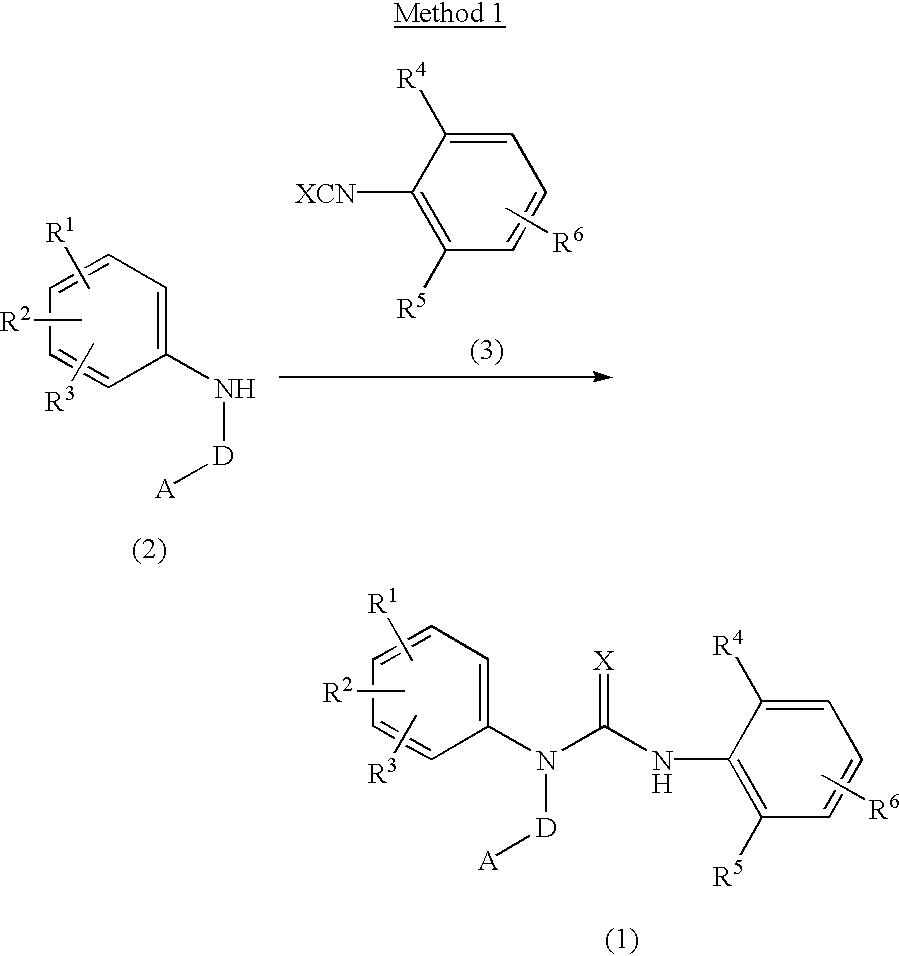
Preparation method of substituted urea derivative
InactiveCN105111135ANo pollution in the processSimple stepsOrganic chemistryUrea derivativesPimavanserin tartrate
The invention relates to a novel preparation method of 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidyl-4-yl)urea hemitartrate (pimavanserin tartrate), particularly a pharmaceutical compound for treating Parkinson's disease, of which the structural formula is disclosed as Formula (I). The invention also relates to a novel intermediate of the preparation method.
Owner:ANHUI YIXINMING PHARMA TECH
Compounds, compositions and methods
ActiveUS20050159416A1Improve therapeutic indexBiocideUrea derivatives preparationCardiac myosinUrea derivatives
Certain substituted urea derivatives selectively modulate the cardiac sarcomere, for example by potentiating cardiac myosin, and are useful in the treatment of systolic heart failure including congestive heart failure.
Owner:CYTOKINETICS INC
Inhibition of raf kinase using aryl and heteroaryl substituted heterocyclic ureas
Methods of treating tumors mediated by raf kinase, with substituted urea compounds, and such compounds per se,
Owner:BAYER HEALTHCARE LLC
Tetrasubstituted ureas as modulators of 11-beta hydroxyl steroid dehydrogenase type 1
InactiveUS20070293529A1Avoid conversionInhibit productionUrea derivatives preparationBiocideSubstituted ureaPharmaceutical Substances
The present invention relates to tetra-substituted urea compounds which are modulators of 11-β hydroxyl steroid dehydrogenase type 1 (11βHSD1), their pharmaceutical compositions, and methods of using the same.
Owner:INCYTE
Compounds, compositions, and methods
Certain substituted urea derivatives selectively modulate the cardiac sarcomere, for example by potentiating cardiac myosin, are useful in the treatment of systolic heart failure including congestive heart failure.
Owner:CYTOKINETICS INC
Compounds useful for inhibiting Chk1
Aryl- and heteroaryl-substituted urea compounds useful in the treatment of diseases and conditions related to DNA damage or lesions in DNA replication are disclosed. Methods of making the compounds, and their use as therapeutic agents, for example, in treating cancer and other diseases characterized by defects in DNA replication, chromosome segregation, or cell division also are disclosed.
Owner:ICOS CORP
Process for synthesis of heteroaryl-substituted urea compounds useful as antiinflammatory agents
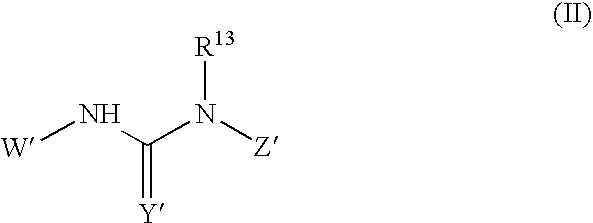
This invention relates to novel processes for preparing heteroaryl-substituted urea compounds of formula (I): which are useful for treating diseases and pathological conditions involving inflammation such as chronic inflammatory disease. X, Ar, L, Q and are described herein.
Owner:BOEHRINGER INGELHEIM PHARMA INC
A continuous process for synthesizing oxamide
ActiveCN102267921ALow costSufficient sourceOrganic compound preparationCarboxylic acid amides preparationDehydrogenationNitrogen gas
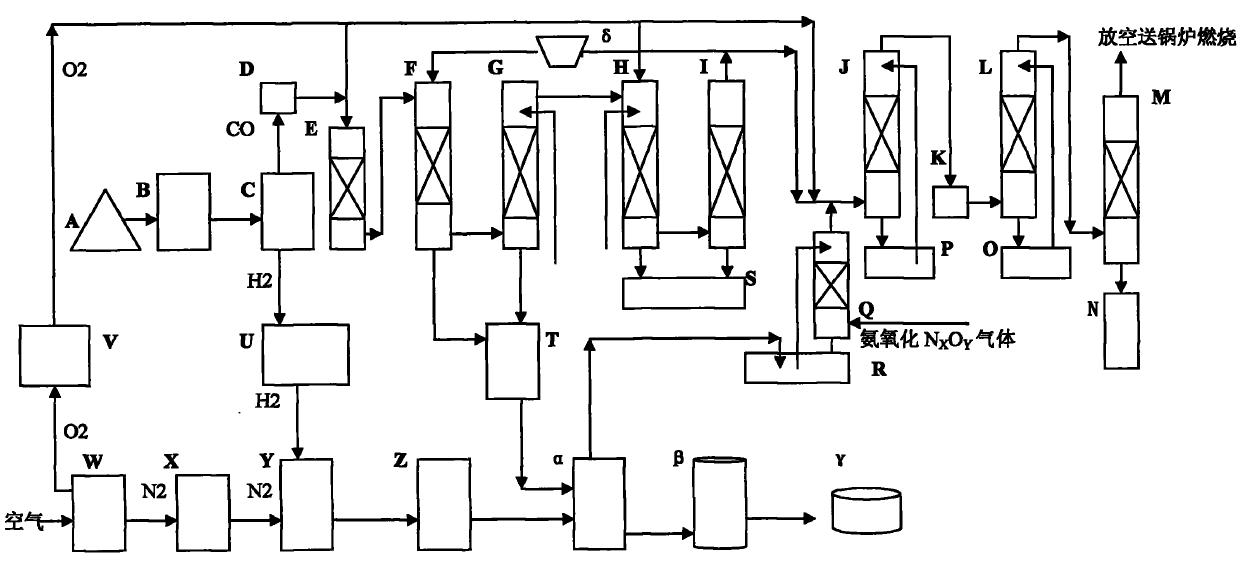
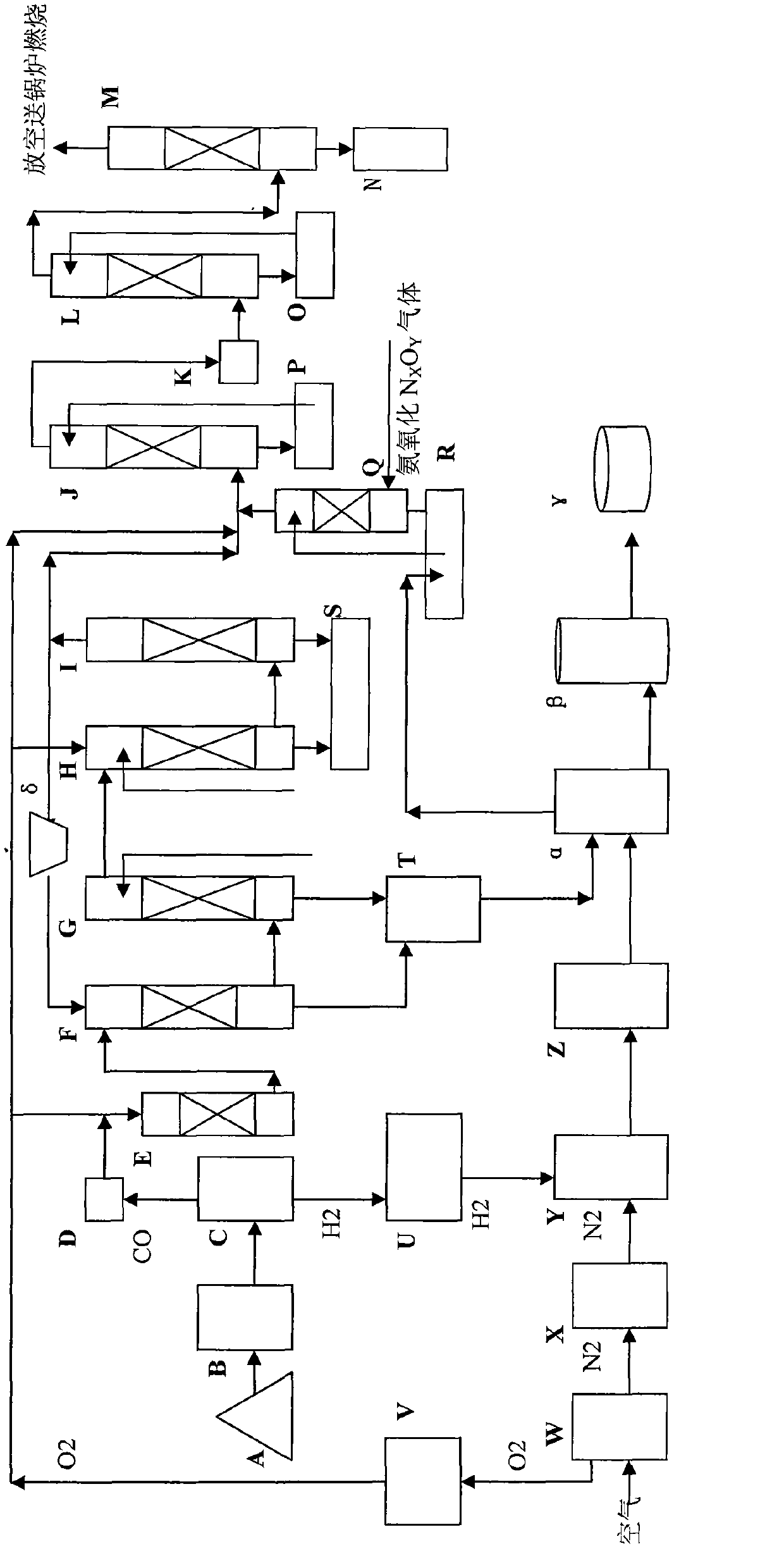
The invention discloses a continuous process for synthesizing oxamide, which belongs to the fields of catalysis, chemical industry, environmental protection and fertilizer. Coal-based synthesis gas is used to obtain H2≥99.0V% hydrogen and industrial CO through pressure swing adsorption separation technology; N2≥99.0V% nitrogen and industrial O2 are obtained through air through cryogenic separation technology. Use H2≥99.0v% hydrogen and N2≥99.0v% nitrogen to mix according to the ratio of N2:H2=1:3, and send them to the ammonia reaction tower to produce synthetic ammonia; industrial CO gas is sent to react with RONO gas after dehydrogenation, oxygen removal, and water removal The tower catalyzes the synthesis of oxalate, and the NO in the reaction tail gas is reacted with industrial O2 and an alcohol-water solution with an alcohol content of ≥10wt% to generate RONO for recycling; the synthesized oxalate and synthetic ammonia are directly sent to the reactor to synthesize oxamide, a slow-acting nitrogen fertilizer, Substituting urea or ammonium bicarbonate for agricultural and animal husbandry production has good economic and social benefits, and is an important development direction of C1 chemical and nitrogen fertilizer industries.
Owner:陈贻盾
Personal care compositions with enhanced fragrance delivery
A personal care product is provided which includes a fragrance, a substituted urea and a quaternary ammonium salt. The substituted urea and quaternary ammonium salt operate together as a scent boosting system to enhance volatilization of fragrance components upon the personal care composition being first applied to human skin or hair.
Owner:CONOPCO INC D B A UNILEVER
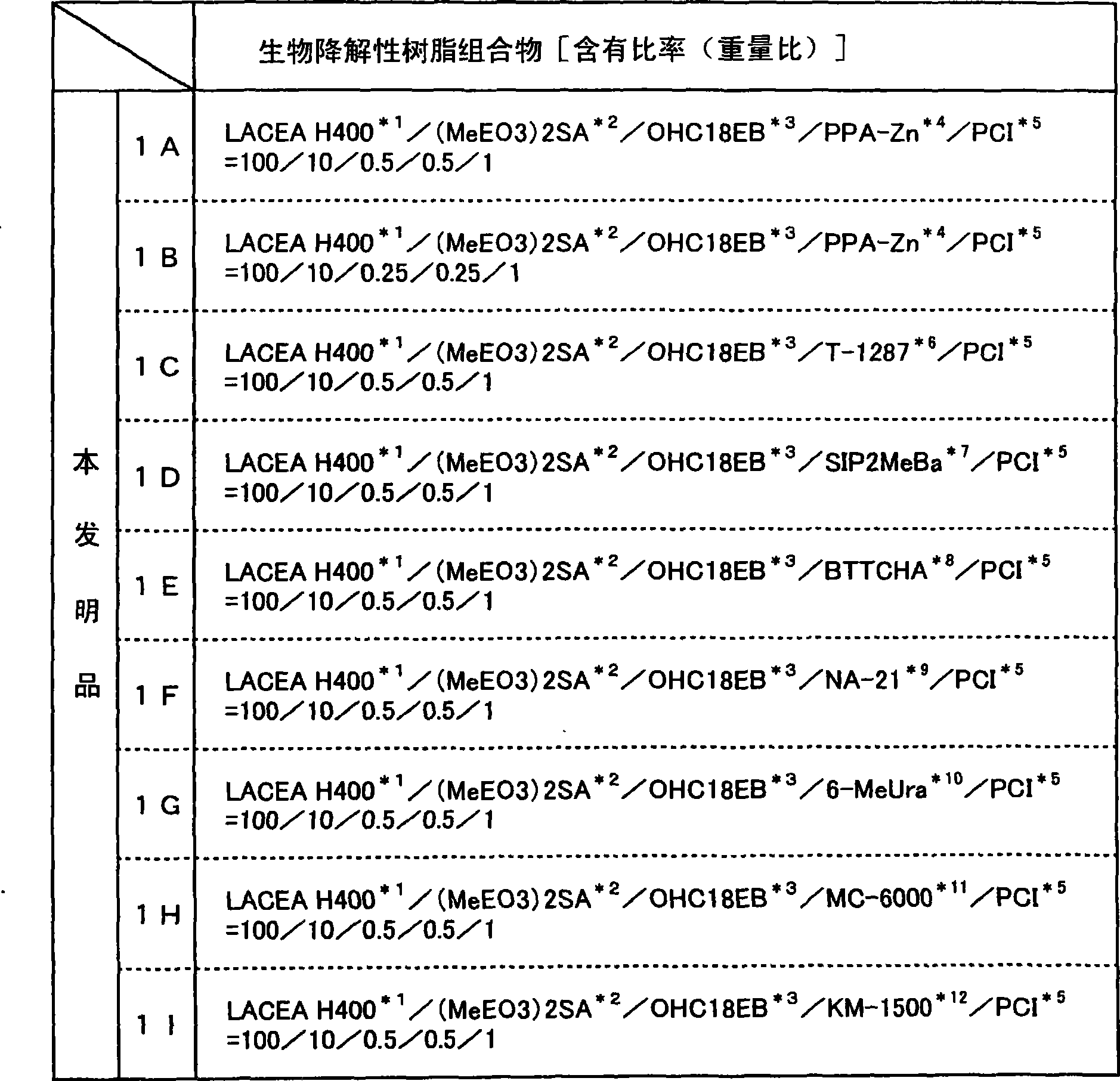
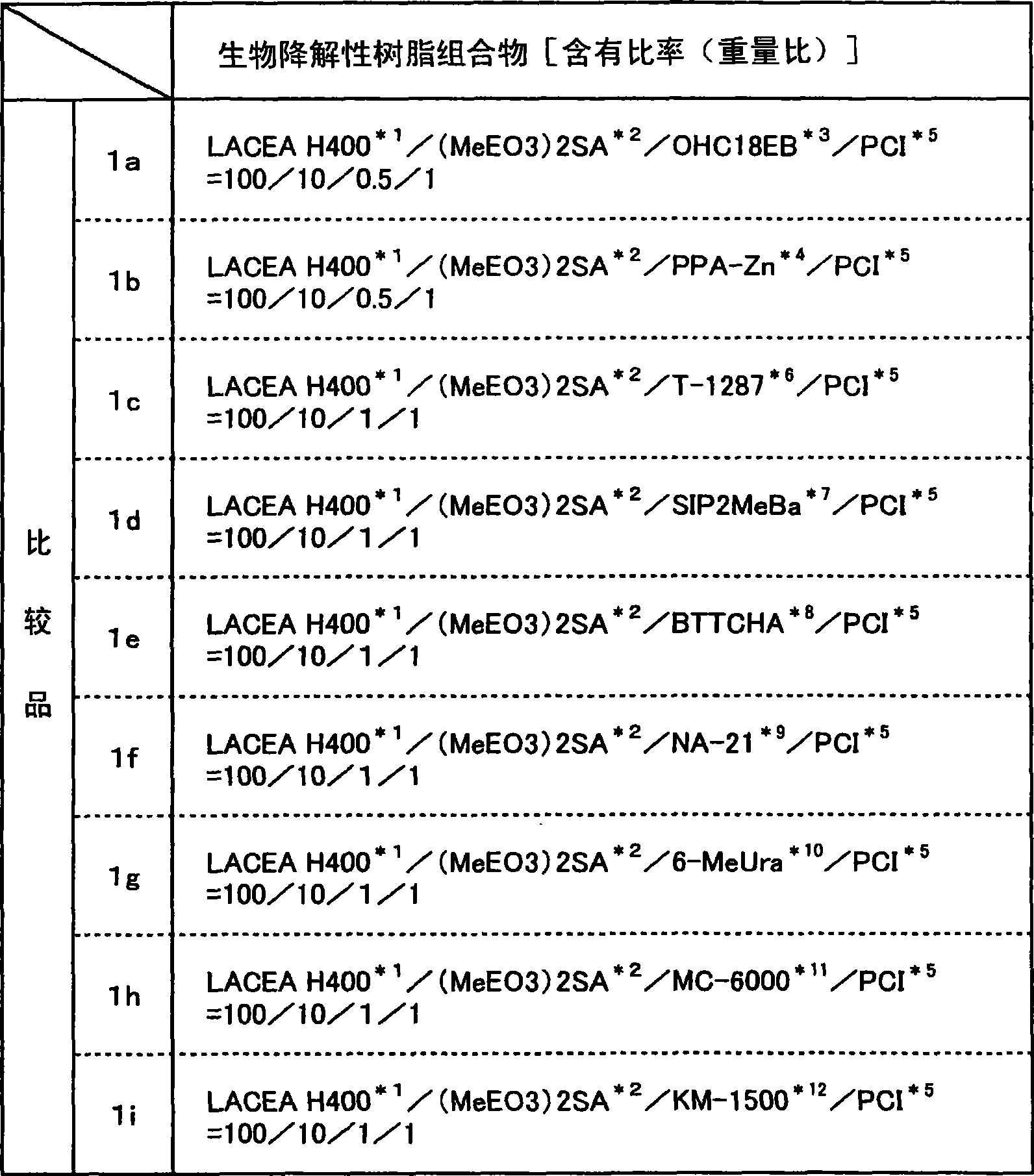
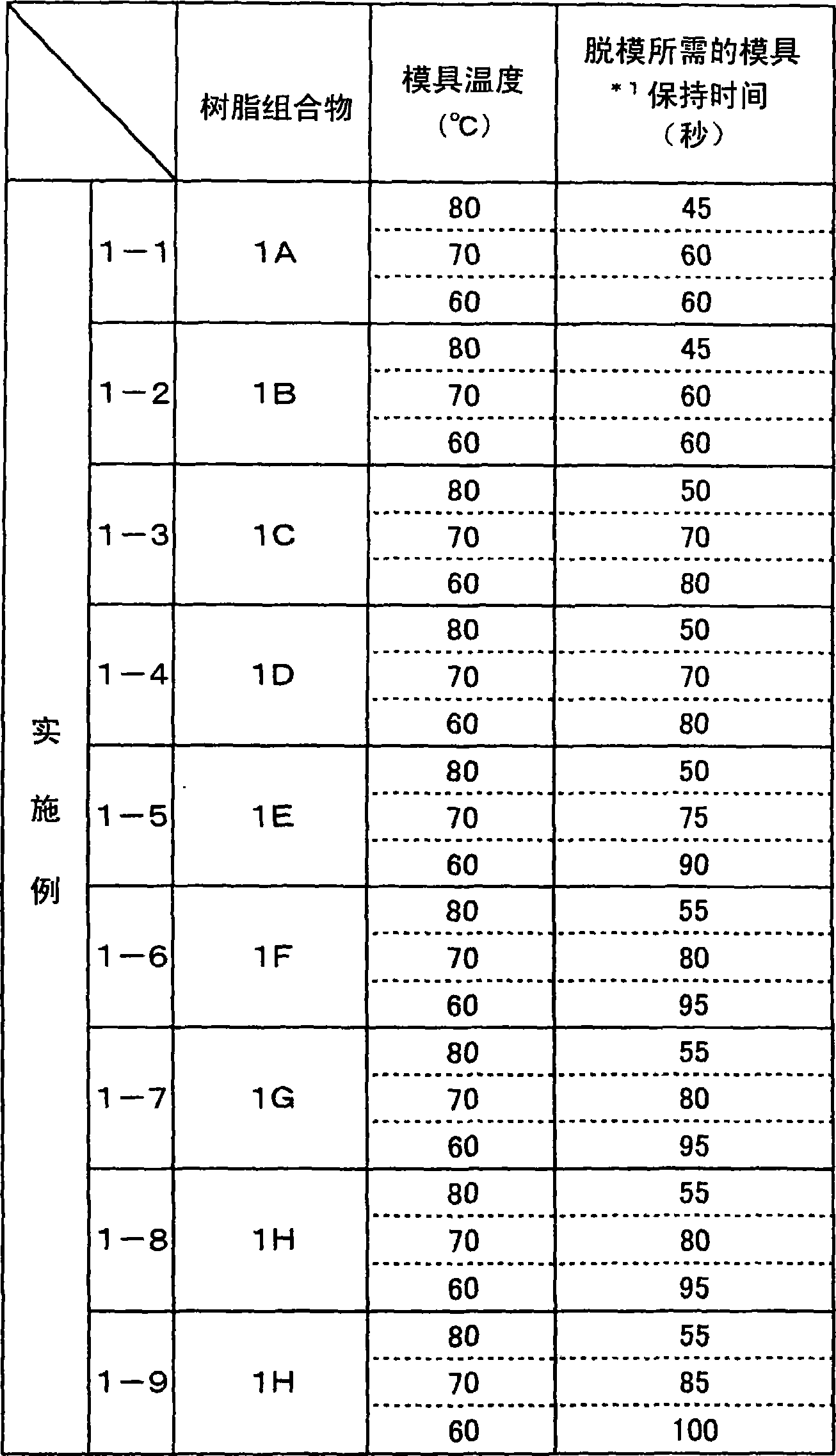
Biodegradable Resin Compositions and Molded Objects Thereof
InactiveUS20080033077A1Additional componentExcellent balance of physical propertiesOrganic detergent compounding agentsLayered productsPolyesterPolymer science
The present invention has its object to provide a resin composition excellent in impact resistance, tensile properties and transparency using a plant-derived biodegradable polymer obtained by positive fixation of carbon dioxide on the earth and, moldings thereof, and, further, provide a resin composition and moldings thereof improved in processability and thermal stability. The present invention relates to a resin composition which comprises (A) an aliphatic polyester type biodegradable polymer and (B) at least one copolymer selected from the group consisting of composite rubber graft copolymers (b1) and core-shell type graft copolymers (b2); or, a resin composition which comprises (A) an aliphatic polyester type biodegradable polymer and (C) at least one compound selected from the group consisting of sorbitol compounds (c1) having a particular chemical structure and urea bond-containing substituted urea compounds (c2).
Owner:MERIDIAN
Biodegradable resin compositions and molded objects thereof
The present invention has its object to provide a resin composition excellent in impact resistance, tensile properties and transparency using a plant-derived biodegradable polymer obtained by positive fixation of carbon dioxide on the earth and, moldings thereof, and, further, provide a resin composition and moldings thereof improved in processability and thermal stability. The present invention relates to a resin composition which comprises (A), an aliphatic polyester type biodegradable polymer and (B) at least one copolymer selected from the group consisting of composite rubber graft copolymers (b1) and core-shell type graft copolymers (b2); or, a resin composition which comprises (A) an aliphatic polyester type biodegradable polymer and (C) at least one compound selected from the group consisting of sorbitol compounds (c1) having a particular chemical structure and urea bond-containing substituted urea compounds (c2).
Owner:KANEKA CORP +1
Stabilization with substituted ureas against color degradation of personal care products
A personal care product is provided which includes an unsaturated organic material with at least one olefinic double bond susceptible to degradation into a color bearing substance, the unsaturated material being selected from C10-C50 terpenoids and C12-C48 unsaturated fatty compounds selected from the group consisting of fatty alcohols, fatty acids, fatty acid glycerides, fatty acid salts and combinations thereof, a substituted urea as a stabilization agent to prevent color body formation, and a cosmetically acceptable carrier. Particularly useful as a stabilizing agent is hydroxyethyl urea.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Single-component coating having alkoxysilane-terminated n-substittued urea resins
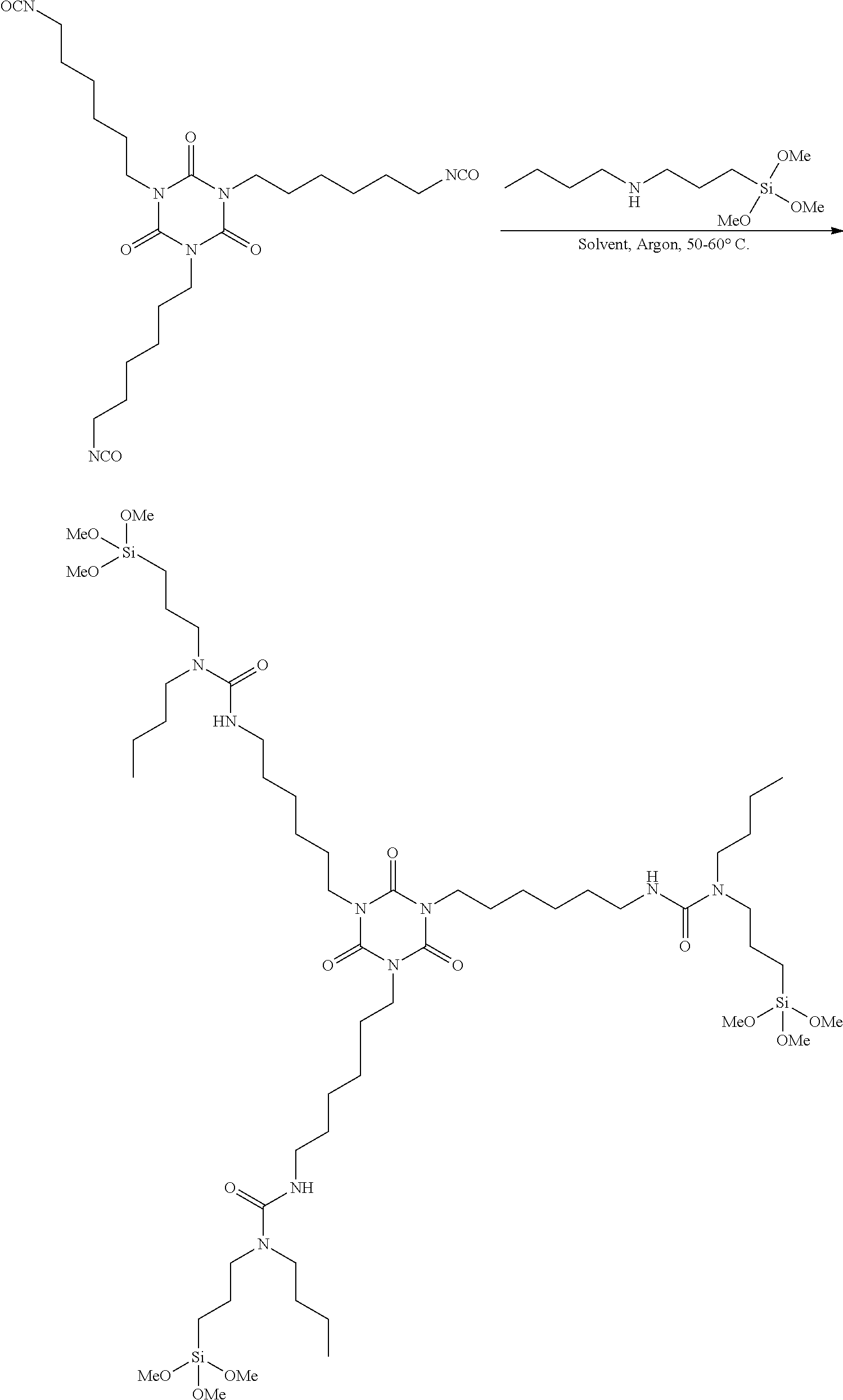
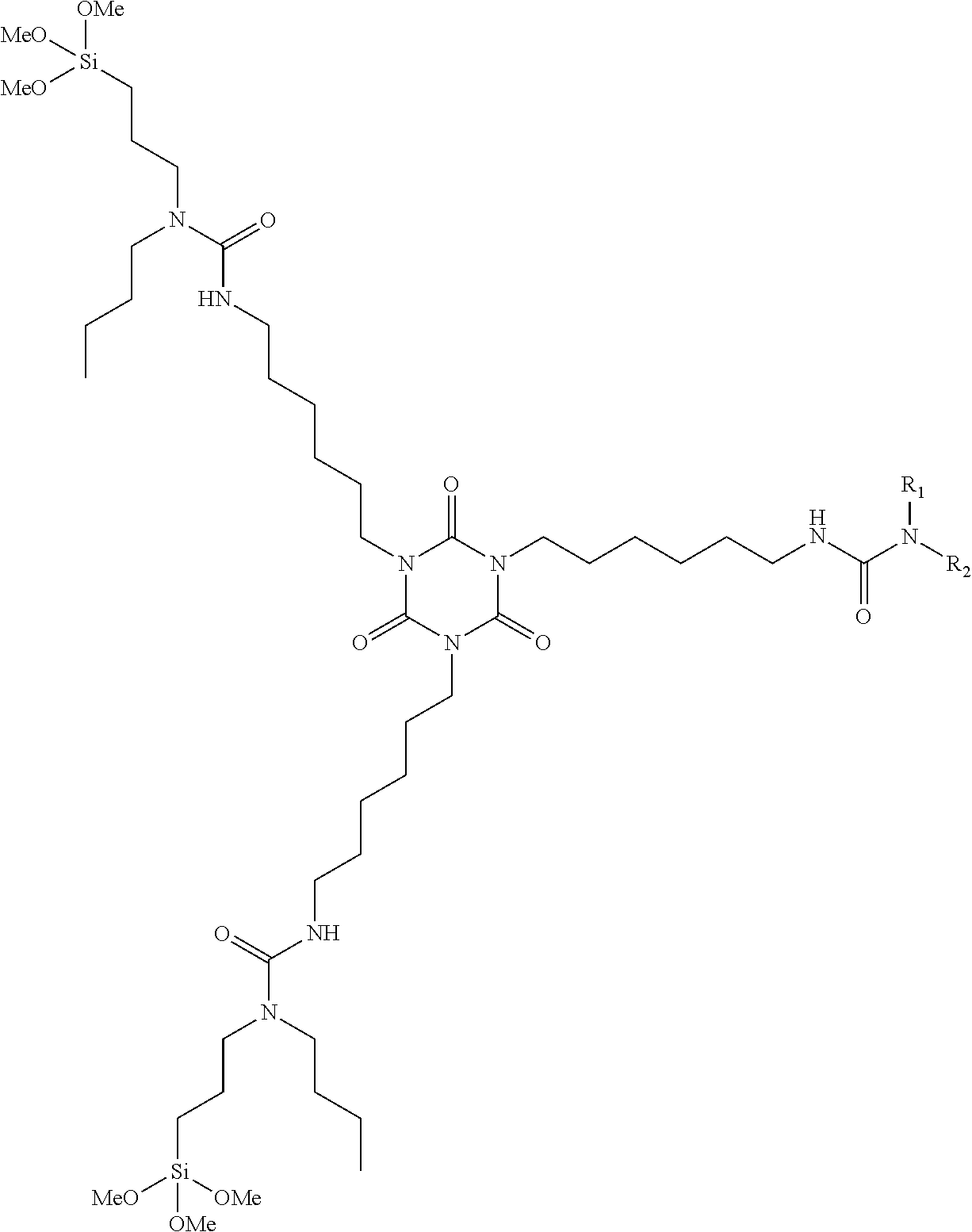
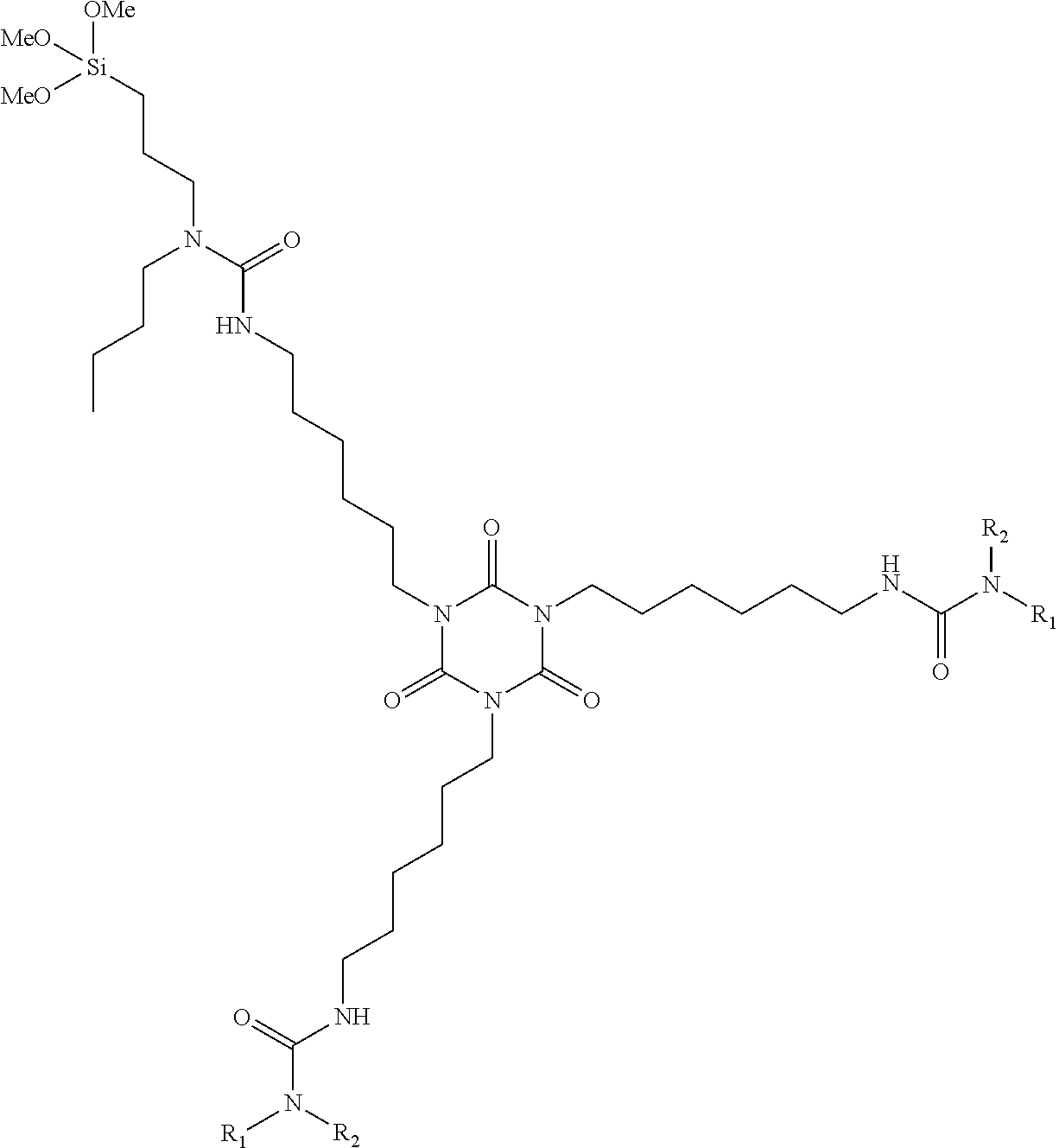
InactiveUS20110319555A1Improve external stabilityLow VOC contentPolyurea/polyurethane coatingsSilanesUltraviolet
The coating described herein attempts to provide this solution by synthesizing alkoxysilane-terminated N-substituted urea resins, then formulating them into moisture-curable single-component (1K) topside coatings. These coatings will provide greater external stability (to UV and visible radiation), cleanability, flexibility, cure times and lower VOC content than the currently qualified silicone alkyd topside coatings that are found on Navy ships. The single-component coating can include at least an alkoxysilane-terminated N-substituted urea resin, a reactive diluent, a pigment, a filler, and a catalyst. The resin can include an amino-functional silane substituted at the N-position and a non-aromatic isocyanate.
Owner:LEIDOS
N-phenyl-N'-pyrimidinyl-substituted urea derivative synthesizing method
The method for synthesizing N-phenyl-N'-pyrimidyl substituted urea derivative is characterized by that in the presence of CO it uses substituted aminopyrimidine derivative and substituted nitrobenzene compound as raw material, uses selenium dioxide as catalyst, and uses organic alkali of triethylamine as catalyst promotor and makes them implement reaction in organic solvent in a slosed high-pressure still. The substituent R1 on the nitrobenzene can be one or several kinds of electron-donating and / or electron-attacting groups or be hydrogen atoms, and the substitutent R2 on the aminopyrimidine derivative can be one or several kinds of inert groups or hydrogen atoms. The mole dose of selenium dioxide is 0.1-20% of substrate, its reaction time is 2-20 hr., reaction temp. is 50-200 deg.C, and CD reaction pressure is 1-10.0 MPa (gauge pressure).
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method and device for directly synthesizing oxamide granules
InactiveCN103242188ASimple processEasy to operateOrganic compound preparationCarboxylic acid amides preparationTemperature controlProcess engineering
The invention relates to a method and a device for directly synthesizing oxamide granules. Dimethyl oxalate and ammonia serving as reaction raw materials and methanol serving as a reaction solvent are subjected to an ammonolysis reaction under normal pressure, a stirring system and a cooling system are arranged in the reactor, a jacket temperature control device is arranged at the outer side of the reactor, and a condenser is connected to the upper part of the reactor for regulation. A front ammonia absorption tank is arranged at the front end of the reactor, the front end of the reactor is provided with a cooling system, the outlet of the condenser is respectively connected with the front ammonia absorption tank and the tail gas absorption tank. The reaction process and the granule forming process can be performed in the same reactor, the methanol recycling and tail absorption device are equipped, and the ammonia gas is not discharged into atmosphere. The reaction investment and consumption can be greatly saved, the environmental pollution is reduced, and the product can be produced in a large scale until the product is industrially applied, so that the urea and ammonium bicarbonate are replaced, and the occupancy of oxamide in the nitrogenous fertilizer market is increased.
Owner:TIANJIN UNIV
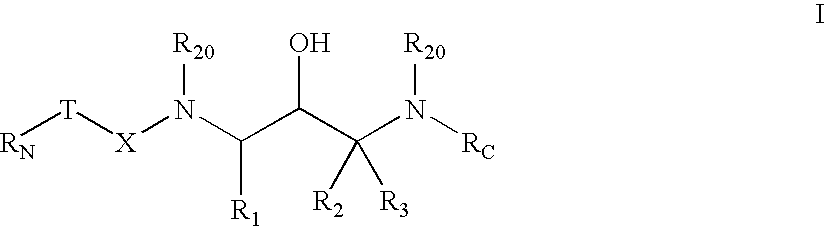
Substituted ureas and carbamates
InactiveUS7351738B2Preventing and delaying onsetSuppression problemBiocideUrea derivatives preparationCarbamateMammal
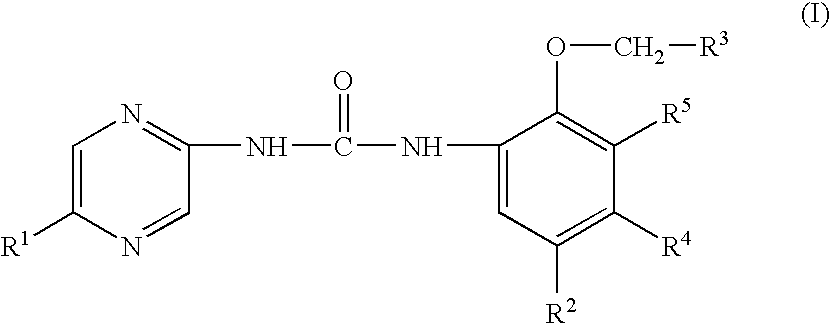
The invention provides compounds of formula I:useful in treating Alzheimer's disease and other similar diseases. These compounds include inhibitors of the beta-secretase enzyme that are useful in the treatment of Alzheimer's disease and other diseases characterized by deposition of A beta peptide in a mammal. The compounds of the invention are useful in pharmaceutical compositions and methods of treatment to reduce A beta peptide formation.
Owner:PHARMACIA & UPJOHN CO +1
Substituted urea retinoid agonists
The current invention provide novel compounds, methods of treating or preventing emphysema, cancer and dermatological disorders, pharmaceutical compositions suitable for the treatment or prevention of emphysema, cancer and dermatological disorders and methods for delivering formulations into the lung of a mammal suffering from emphysema, cancer and dermatological disorders.
Owner:SYNTEX (USA) INC
Curable resin composition
Disclosed is a moisture-curing type curable resin composition containing: a curable resin intramolecularly having a silicon-containing functional group; and a Lewis acid or a complex of the Lewis acid as a curing catalyst, the Lewis acid being selected from the group consisting of metal halides and boron halides, which is rapidly cured at room temperature. The silicon-containing functional group is represented by general formula: —SiX1X2X3 or —SiR1X1X2 (wherein, X1, X2 and X3 respectively represent a hydrolytic group and may be the same as or different from each other, and R1 represents a substituted or unsubstituted organic group having 1 to 20 carbons). If the silicon-containing functional group is —SiR1X1X2, the curable resin further contains intramolecularly a polar component that is one of urethane, thiourethane, urea, thiourea, substituted urea, substituted thiourea, amide, and sulfide bonds, and hydroxyl, secondary amino and tertiary amino groups. Two-part type adhesive is constitutible with separating the curable resin from the curing catalyst.
Owner:KONISHI CO
Biodegradable resin composition
ActiveUS20100063177A1Group 5/15 element organic compoundsConductive materialPhosphateCarboxylic acid
The invention relates to a biodegradable resin composition containing a biodegradable resin, a plasticizer and a crystal nucleus agent, the plasticizer being a compound containing two or more ester groups in the molecule thereof, wherein at least one alcohol component constituting the ester contains an alkylene oxide having 2 to 3 carbon atoms, added in the average amount of 0.5 to 5 moles per one hydroxyl group, and the crystal nucleus agent is a mixture of the following crystal nucleus agent (1) and crystal nucleus agent (2); the crystal nucleus agent (1) being the following crystal nucleus agent (1-1) or crystal nucleus agent (1-2): crystal nucleus agent (1-1): at least one selected from compounds having a hydroxyl group and an amide group in the molecule thereof; and crystal nucleus agent (1-2): at least one selected from hydroxy fatty acid esters; crystal nucleus agent (2): at least one selected from a metal salt of phenylphosphonic acid, a metal salt of a phosphate, a metal salt of an aromatic dialkyl sulfonate, a metal salt of rosinic acids, an aromatic carboxylic acid amide, rosinic acid amide, carbohydrazides, N-substituted ureas, salts of melamine compounds and uracils.
Owner:KAO CORP
Heteroaryl urea derivatives useful for inhibiting CHK1
Substituted urea compounds useful in the treatment of diseases and conditions related to DNA damage or lesions in DNA replication are disclosed. Methods of making the compounds, and their use as therapeutic agents, for example, in treating cancer and other diseases characterized by defects in DNA replication, chromosome segregation, or cell division, also are disclosed.
Owner:ICOS CORP
Compounds useful for inhibiting CHK1
InactiveUS20050245525A1Little or no activityUseful in treatmentBiocideOrganic chemistryArylCell division
Aryl- and heteroaryl-substituted urea compounds useful in the treatment of diseases and conditions related to DNA damage or lesions in DNA replication are disclosed. Methods of making the compounds, and their use as therapeutic agents, for example, in treating cancer and other diseases characterized by defects in DNA replication, chromosome segregation, or cell division also are disclosed.
Owner:ICOS CORP
Synergistic weeding composition containing fluroxypyr
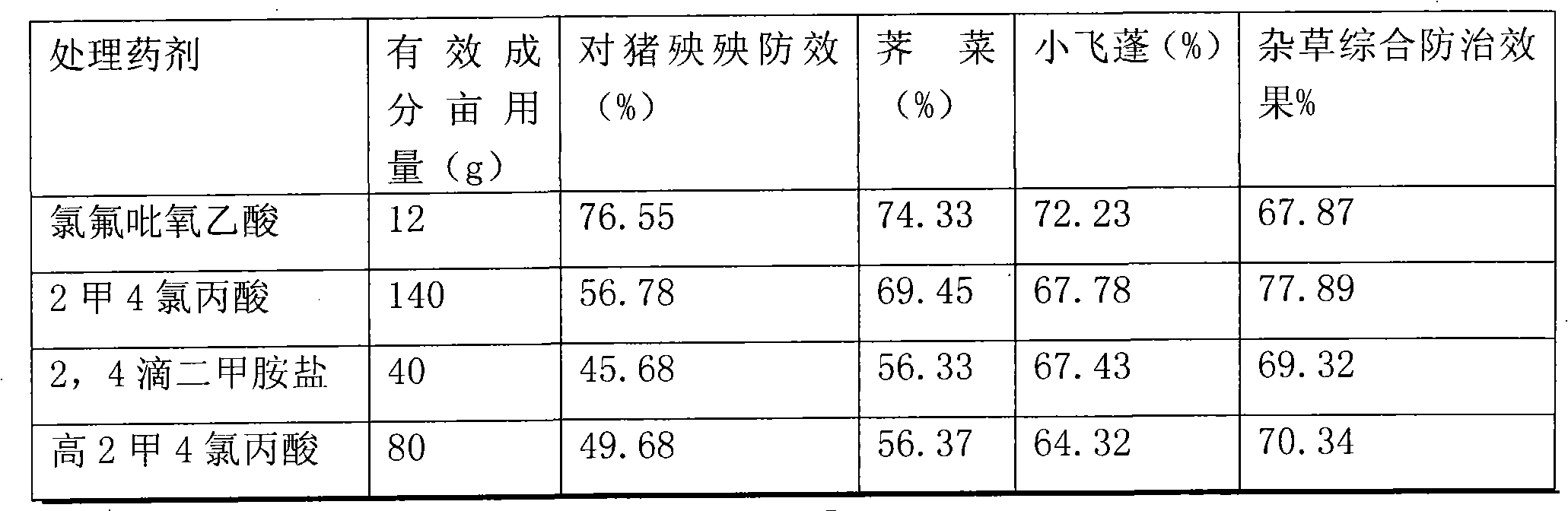
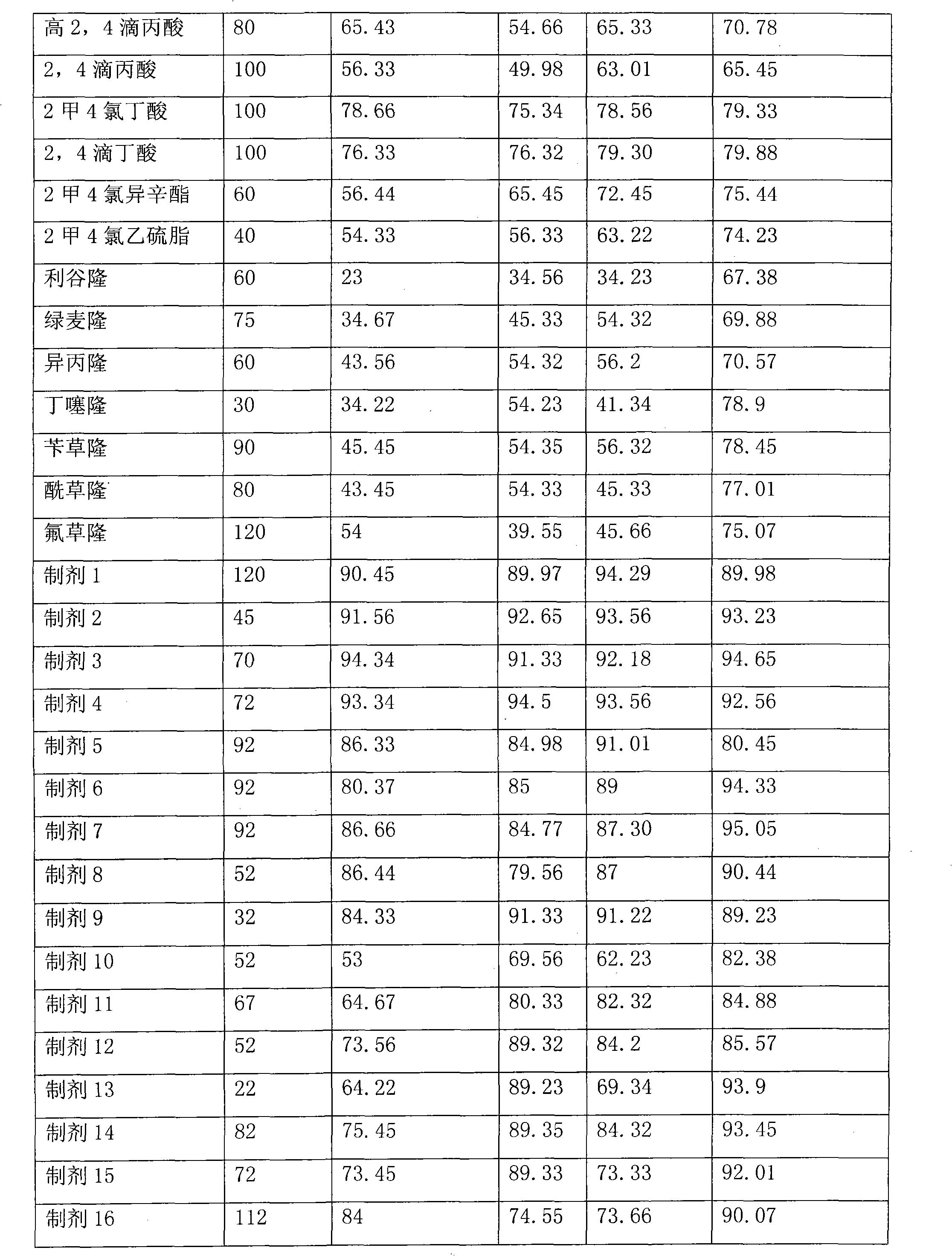
InactiveCN102318632APlay the effect of blocking double effectImprove efficiencyBiocideAnimal repellantsCompound aBenzoic acid
The invention relates to a synergistic weeding composition containing fluroxypyr, which is characterized by comprising compound A of fluroxypyr, compound B of phenoxy carboxylic acid and benzoic acid herbicides and substituted urea herbicide, wherein the weight percentage of the compound A and the compound B is 1 to 30 : 5 to 60. The composition can be widely used for preventing and curing broad-leaf weed in gramineous crop fields such as wheat, rice, corn and the like, can improve the prevention effect and can enlarge the weeding spectrum, is safe to the crops and has high efficiency.
Owner:GAUNGXI TIANYUAN BIOCHEM
Biodegradable resin composition
The invention relates to a biodegradable resin composition containing a biodegradable resin, a plasticizer and a crystal nucleus agent, the plasticizer being a compound containing two or more ester groups in the molecule thereof, wherein at least one alcohol component constituting the ester contains an alkylene oxide having 2 to 3 carbon atoms, added in the average amount of 0.5 to 5 moles per one hydroxyl group, and the crystal nucleus agent is a mixture of the following crystal nucleus agent (1) and crystal nucleus agent (2); the crystal nucleus agent (1) being the following crystal nucleus agent (1-1) or crystal nucleus agent (1-2): crystal nucleus agent (1-1): at least one selected from compounds having a hydroxyl group and an amide group in the molecule thereof; and crystal nucleus agent (1-2) : at least one selected from hydroxy fatty acid esters; crystal nucleus agent (2): at least one selected from a metal salt of phenylphosphonic acid, a metal salt of a phosphate, a metal salt of an aromatic dialkyl sulfonate, a metal salt of rosinic acids, an aromatic carboxylic acid amide, rosinic acid amide, carbohydrazides, N-substituted ureas, salts of melamine compounds and uracils.
Owner:KAO CORP
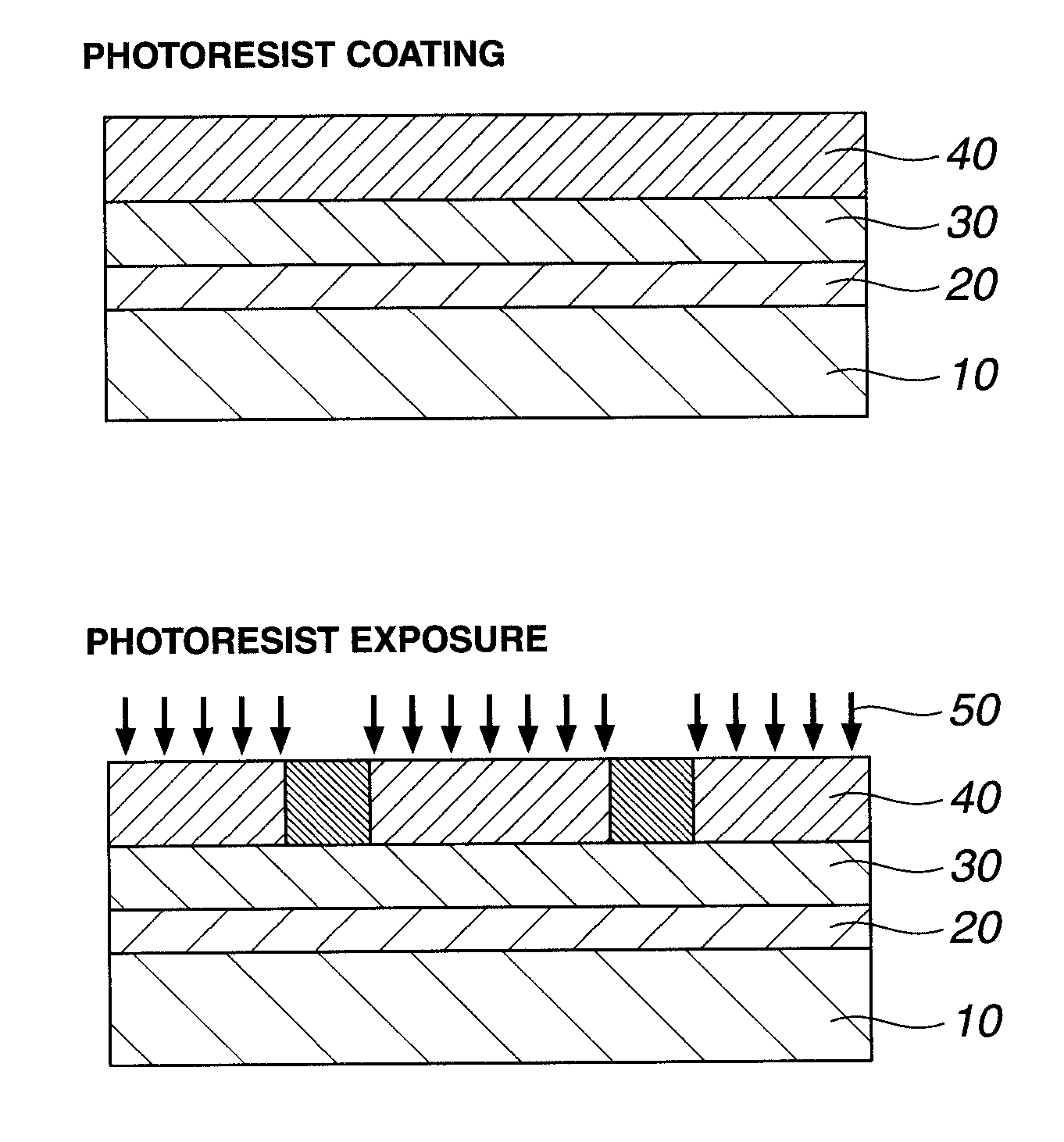
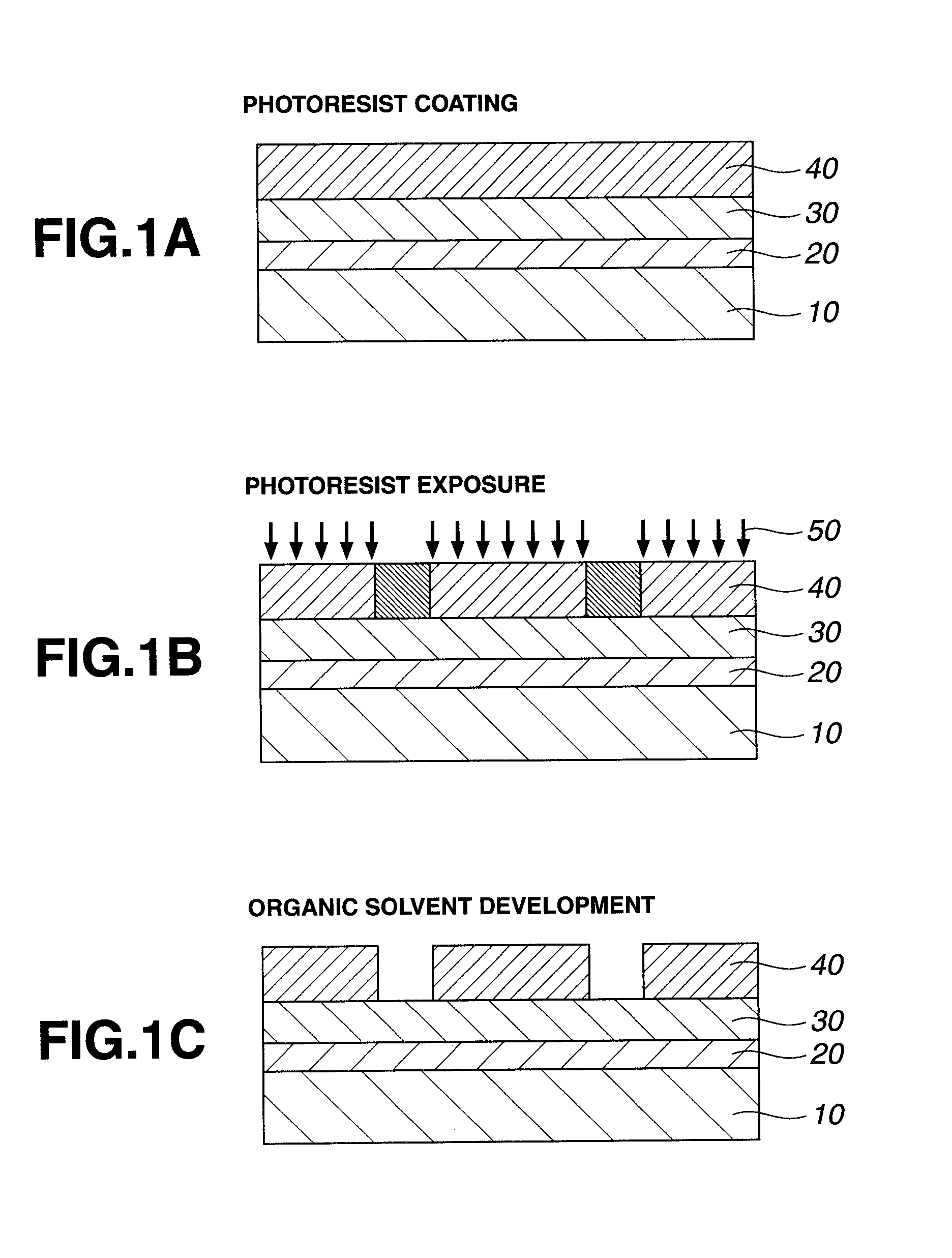
Patterning process and resist composition
ActiveUS20130052587A1High dissolution contrastHigh sensitivityElectric discharge tubesPhotosensitive materialsOrganic solventHigh energy
A negative pattern is formed by coating a resist composition comprising a methylol-substituted urea, amide or urethane compound, a polymer comprising recurring units having an acid labile group-substituted hydroxyl group, and an acid generator onto a substrate, prebaking, exposing to high-energy radiation, and developing in an organic solvent developer such that the unexposed region of resist film is dissolved away and the exposed region of resist film is not dissolved. In image formation via positive / negative reversal by organic solvent development, the resist film is characterized by a high dissolution contrast between the unexposed and exposed regions.
Owner:SHIN ETSU CHEM IND CO LTD
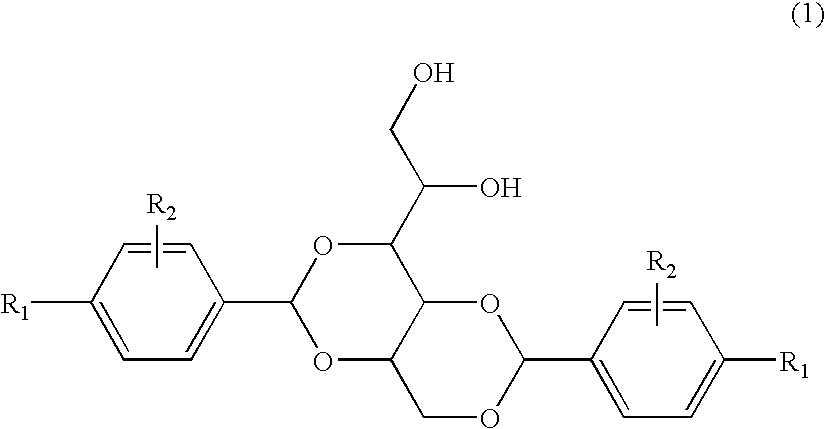
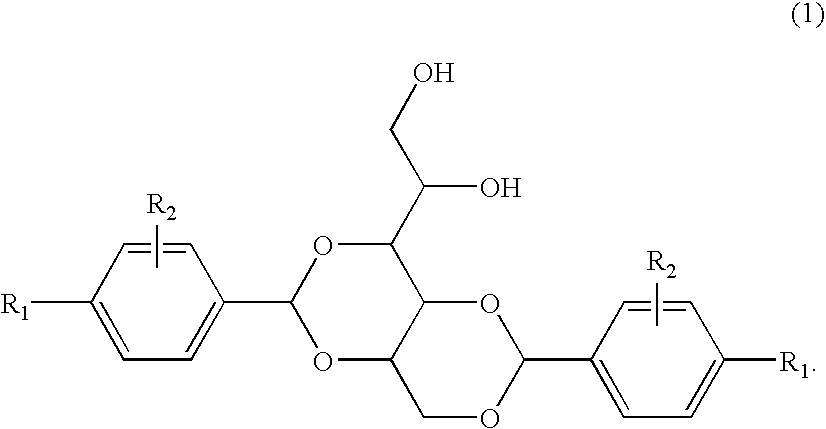
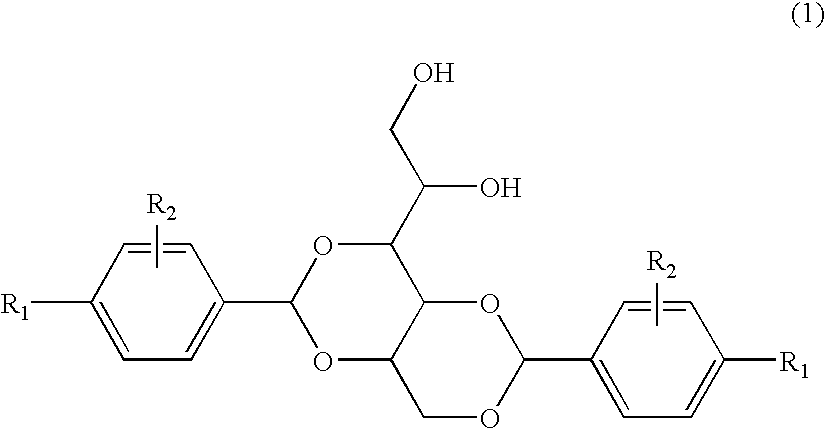
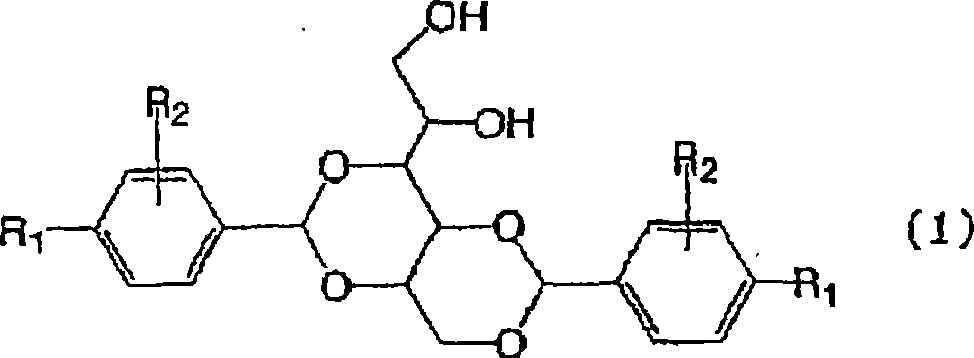
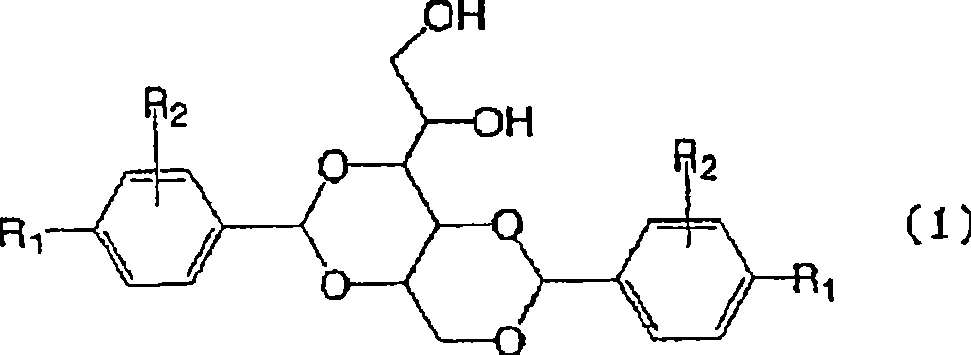
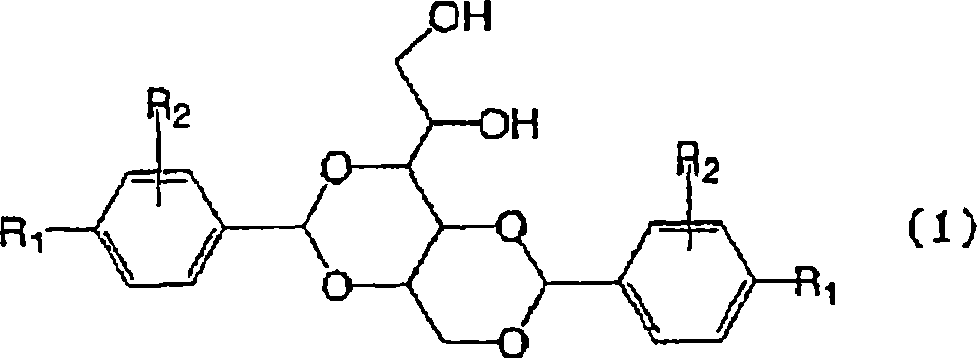
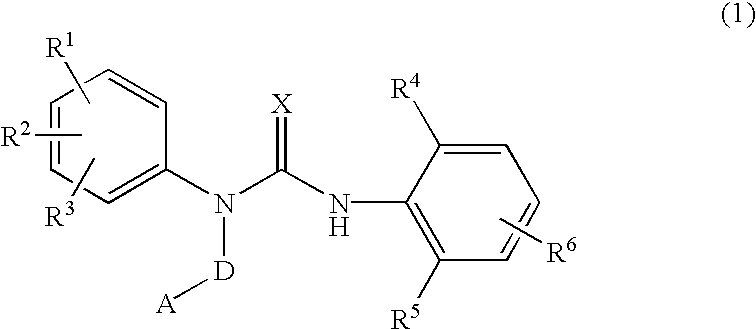
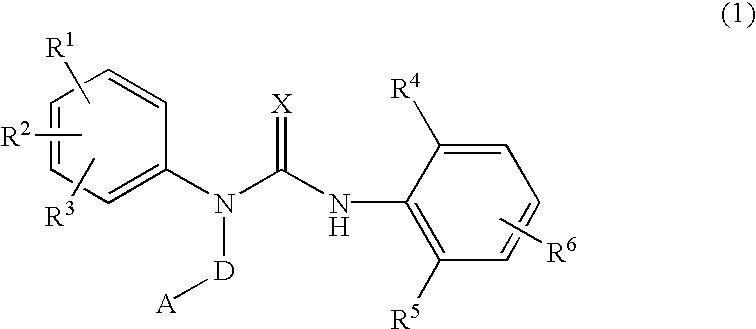
3-substituted urea derivatives and medicinal use thereof
InactiveUS7105567B2Antibacterial agentsUrea derivatives preparationRESPIRATORY DISTRESS SYNDROME ADULTWhite blood cell
The present invention relates to a urea derivative of the formula (1)wherein each symbol is as described in the specification, a pharmaceutically acceptable salt thereof and pharmaceutical use thereof. The compound of the present invention has a C5a receptor antagonistic action and is useful as an agent for the prophylaxis or treatment of diseases or syndromes due to inflammation caused by C5a [e.g., autoimmune diseases such as rheumatism, systemic lupus erythematosus and the like, sepsis, adult respiratory distress syndrome, chronic obstructive pulmonary disease, allergic diseases such as asthma and the like, atherosclerosis, cardiac infarction, brain infarction, psoriasis, Alzheimer's disease and serious organ injury (e.g., pneumonia, nephritis, hepatitis, pancreatitis and the like) due to activation of leukocytes caused by ischemia, trauma, burn, surgical invasion and the like]. In addition, it is useful as an agent for the prophylaxis or treatment of infectious diseases caused by bacteria or virus that invades via a C5a receptor.
Owner:MITSUBISHI TANABE PHARMA CORP
Preparation method of solid-phase extraction small column for molecular imprinting of substituted carbamide pesticide
InactiveCN101497035AWith dynamic "memory" functionImprove efficiencyOther chemical processesCross-linkFunctional monomer
The invention discloses a method for preparing a molecularly imprinted solid-phase extraction column of substituted urea pesticide, which belongs to the technical field of food safety. The method comprises the following steps: preparing a template molecule substituted urea pesticide, a functional monomer methacrylic acid and a cross-linking agent ethylene dimethacrylate into a molecularly imprinted polymer microsphere of the substituted area pesticide through suspension polymerization according to a mol ratio of the template molecule to the functional monomer to the cross-linking agent of 1:2-8:20; and weighing the molecularly imprinted polymer microsphere of the substituted urea pesticide to be filled in an empty pipe of polypropylene solid-phase extraction column to prepare the molecularly imprinted solid-phase extraction column of the substituted urea pesticide. The molecularly imprinted solid-phase extraction column of the substituted urea pesticide can be used for selective purification and selective enrichment of an extracted solution of a residual sample of the substituted urea pesticide in inspected food and environment. Compared with common liquid-liquid extraction method and C18 solid-phase extraction method, the method has the characteristics of easiness, rapidness, high purification efficiency and the like.
Owner:ANHUI AGRICULTURAL UNIVERSITY
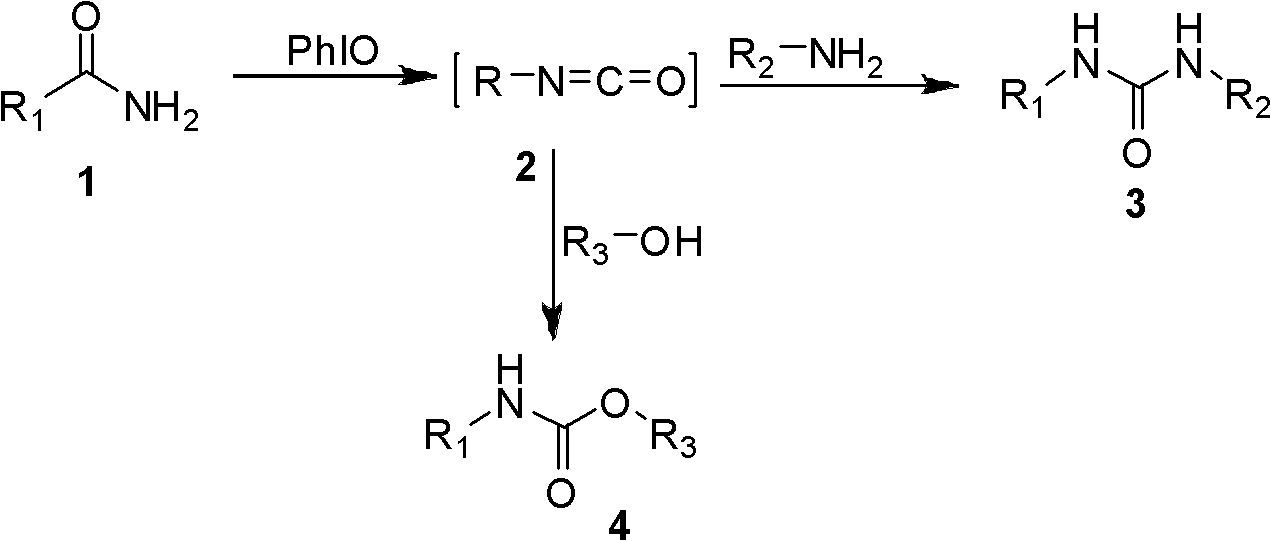
Synthetic method of 1, 3-two substituted ureas and carbamate
InactiveCN102503860AEasy to separateExperiment operation is simpleUrea derivatives preparationCarbamic acid derivatives preparationCarbamateAlcohol
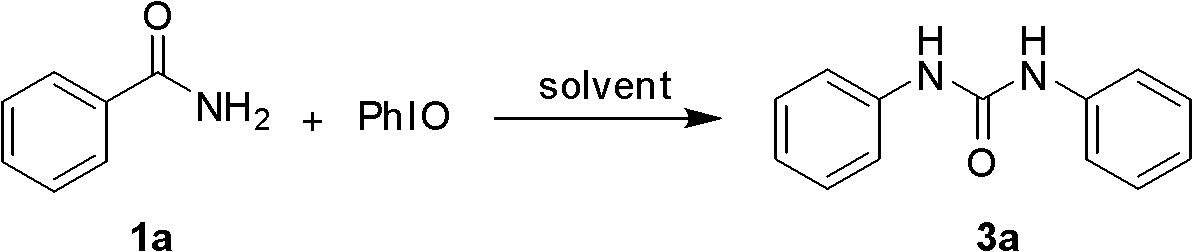
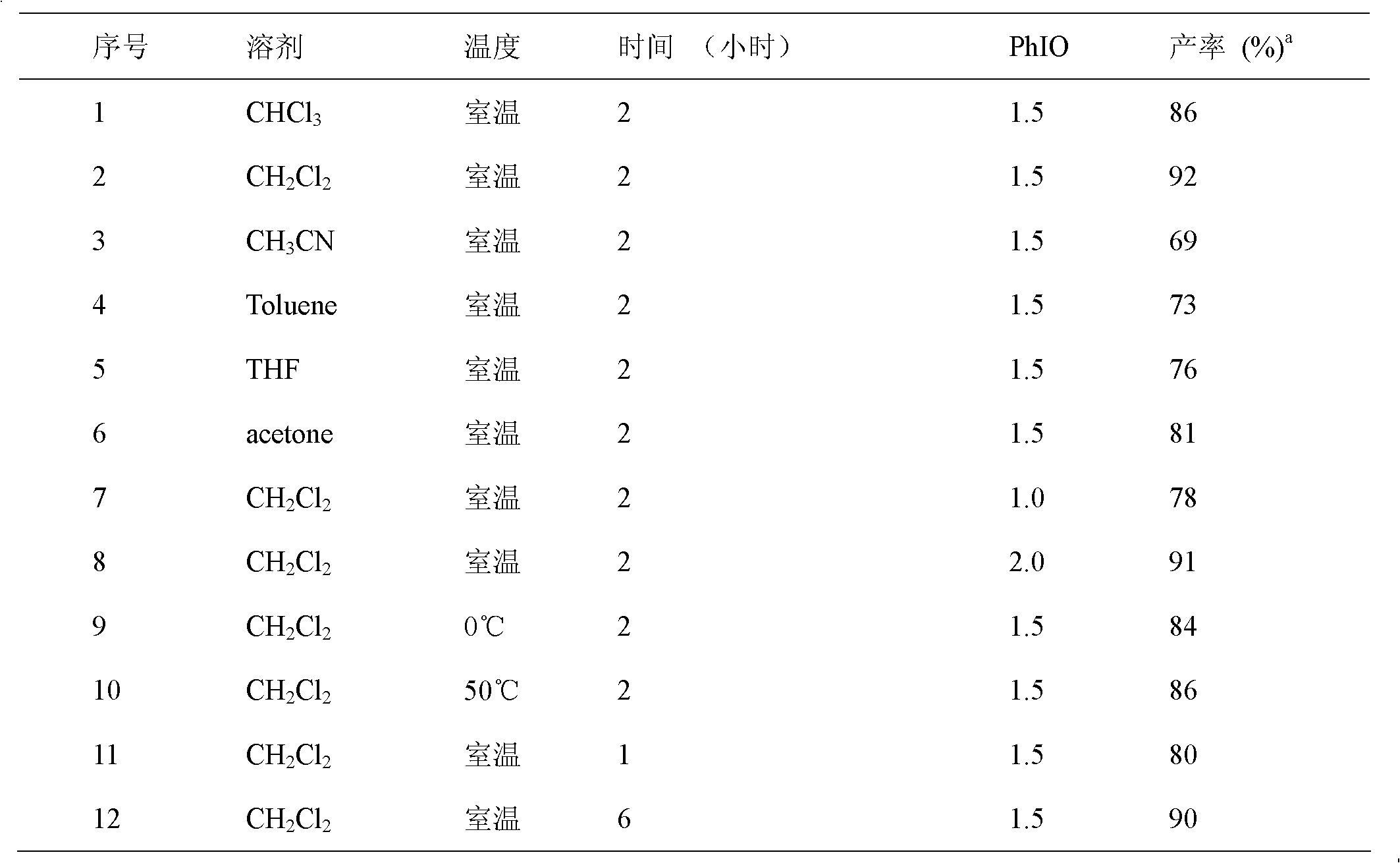
The invention relates to a simple and efficient preparation method of 1, 3-two substituted ureas and carbamate. The method comprises the steps as follows: amide is taken as substrate, and the 1, 3-two substituted ureas and carbamate are prepared by using Hofmann rearrangement reaction induced by hypervalent iodine. Only acid amide and oxidization iodobenzene are added into the reaction system, stirring is performed for 2 hours in methylene dichloride at a room temperature, and symmetrical 1, 3-two substituted ureas can be obtained with high yield. When acid amide, oxidization iodobenzene and nucleophilic agent amine are in reaction, and stirring is performed for 2 hours in methylene dichloride at a room temperature, unsymmetrical 1, 3- two substituted ureas can be obtained. When acid amide, oxidization iodobenzene and nucleophilic agent alcohol are in reaction, and stirring is performed for 2 hours in alcoholic solution at a room temperature, carbamate can be prepared. The method is simple to operate, the product selectivity is strong, and the application is wide.
Owner:WUHAN UNIV
Compounds useful for inhibiting CHK1
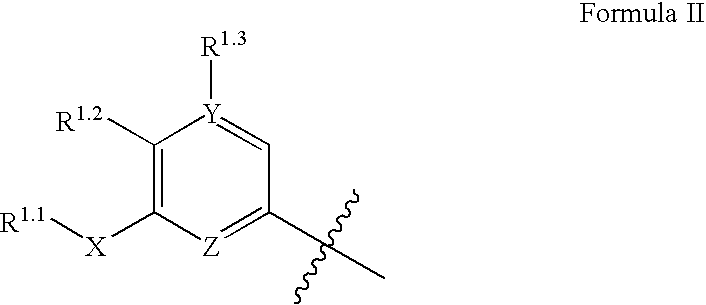
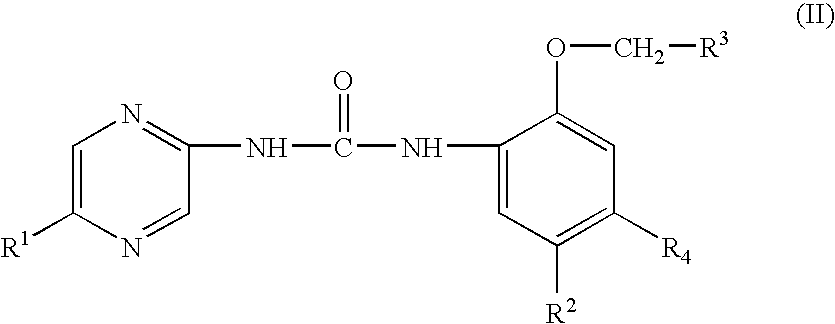
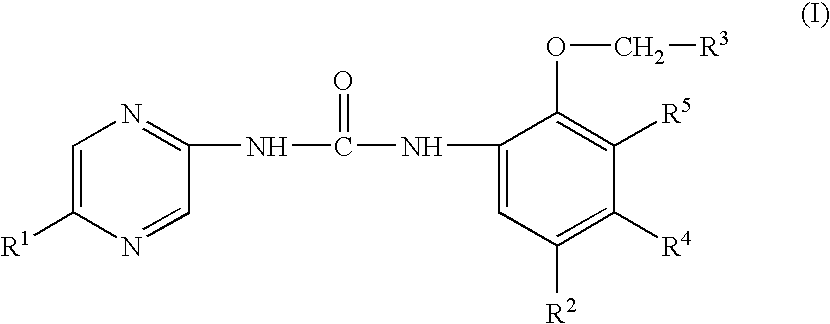
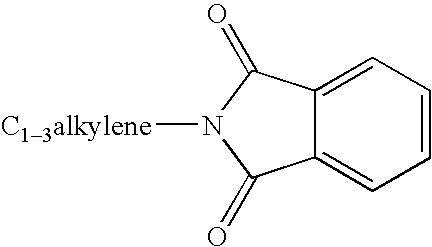
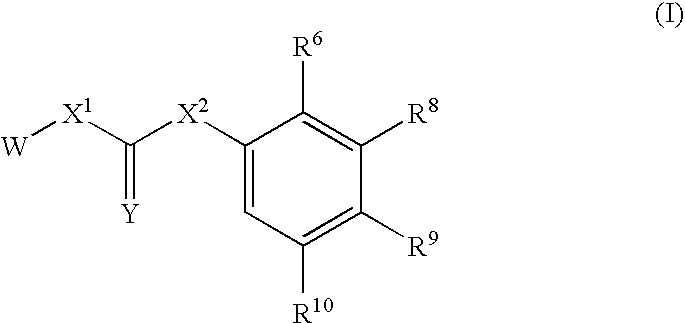
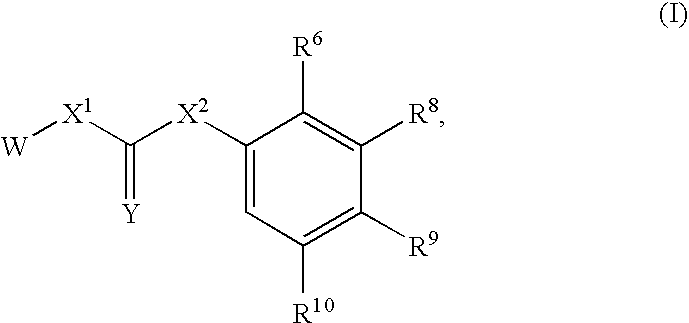
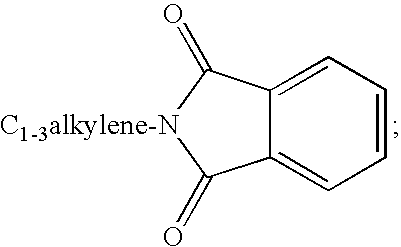
Substituted urea compounds useful in the treatment of diseases and C1-3alkyleneOR3 conditions related to DNA damage or lesions in DNA replication are disclosed formula (I), wherein X1 is null, —O—, —S—, —CH2—, or —N(R1)—; X2 is —O—, . -£>. -, or —N(R1)—,—. . Y xs 0 or S; or =y represents two hydrogen atoms attached to a common carbon atom, —W is selected from the group consisting of heteroaryl, aryl, heterocycloalkyl, cycloalkyl, and C1-6alkyl substituted with a heteroaryl. or aryl group; R6 is —C≡C—R7 or heteroaryl; R8, R9, and R10, independently, are selected from the group consisting of halo, optionally substituted C1-6alkyl, C2-6alkenyl, C2-6alkynyl, OCP3, CF3, NO2, CN, NC, N(R3)2, OR3, CO2R3, C(O)N (R3)2, C(O)R3, N(R1)COR3, N(R1)C(O)OR3, N(R8)C(O)OR3, N(R1)C(O)C1-3alkyleneC(O)R3, N(R1)C(O)C1 -3alkyleneC(O)OR3, N(R1)C(O)C1-3alkyleneOR3, N(R1)C(O)C1-3alkyleneNHC(O)OR3, N(R1)C(O)C1-3alkyleneSO2.NR3, C1-3alkyleneOR3, and SR3; Methods of making the compounds, and their use as therapeutic agents, for example, in treating cancer and other diseases characterized by defects in DNA replication, chromosome segregation, or cell division also are disclosed.
Owner:ICOS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com