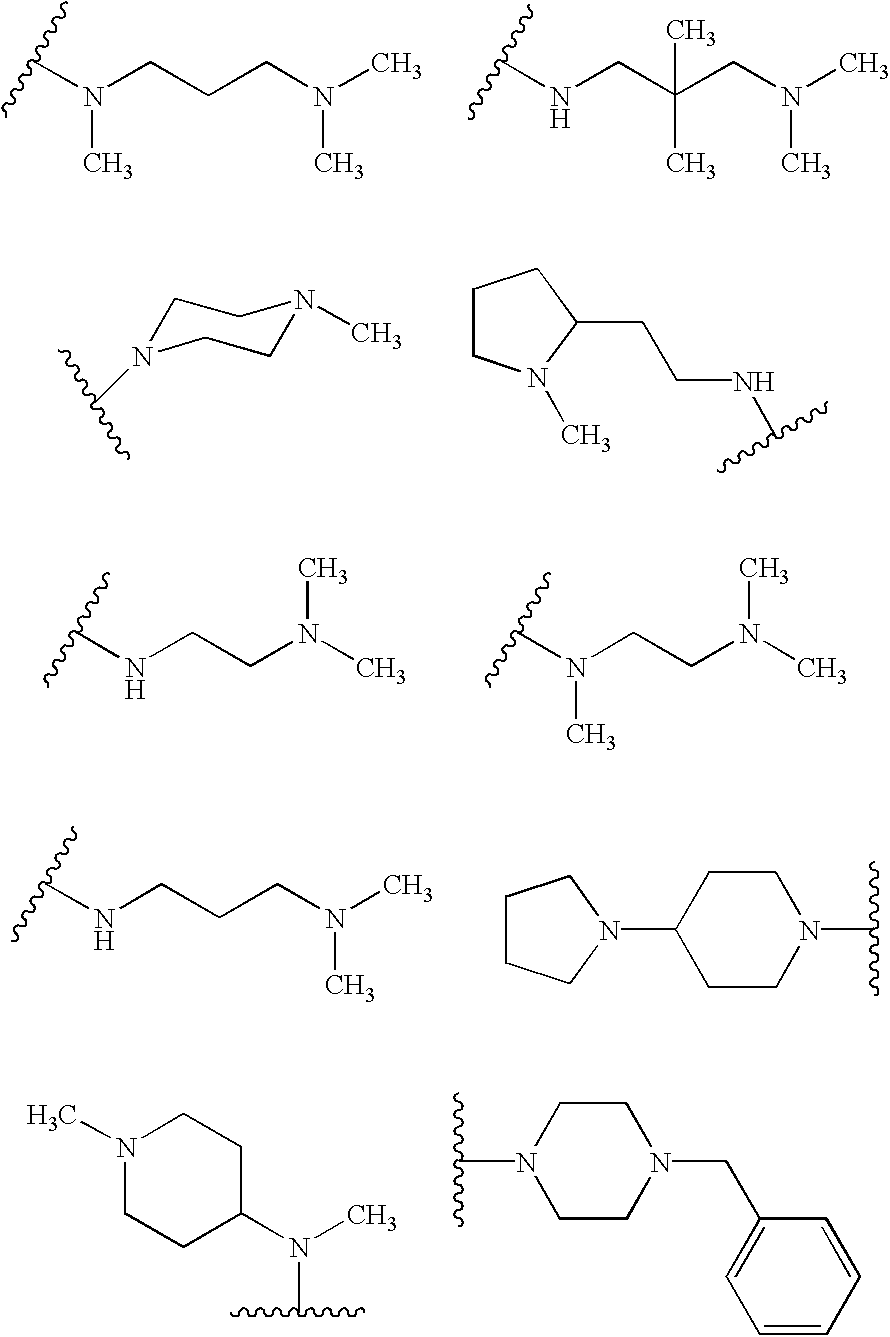
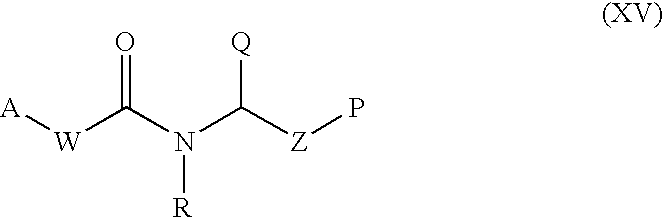
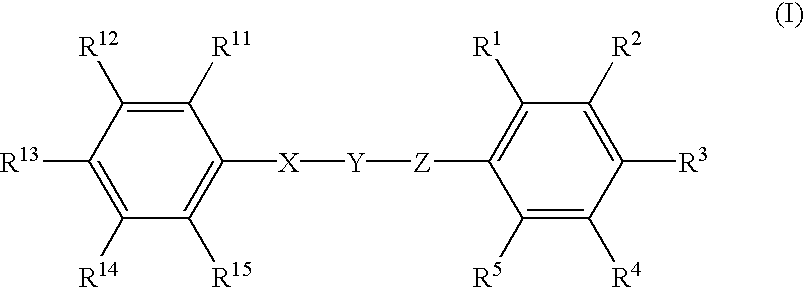
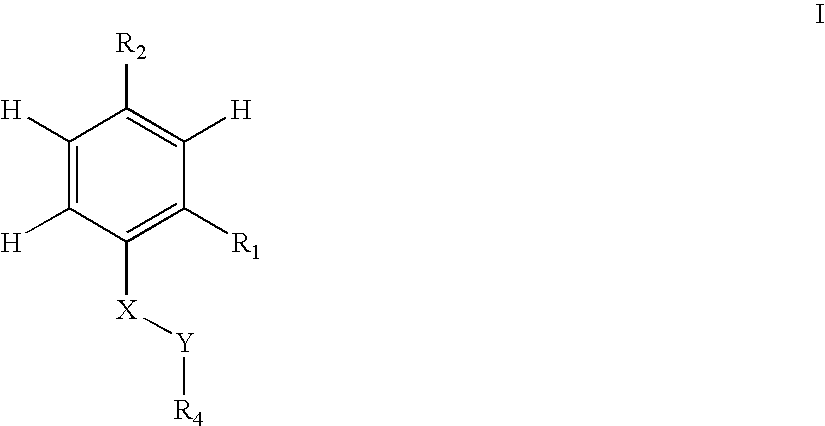
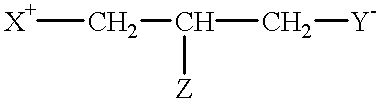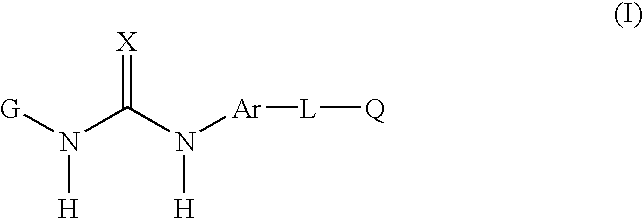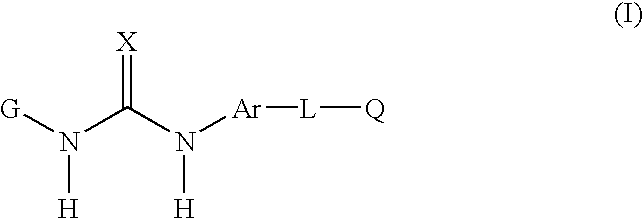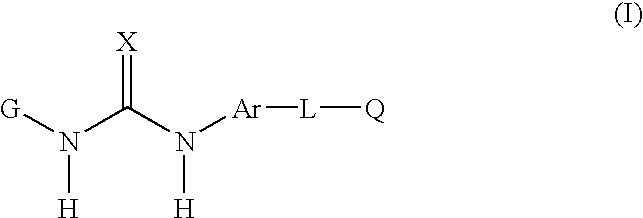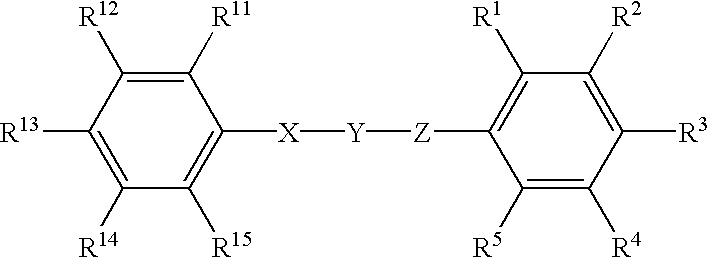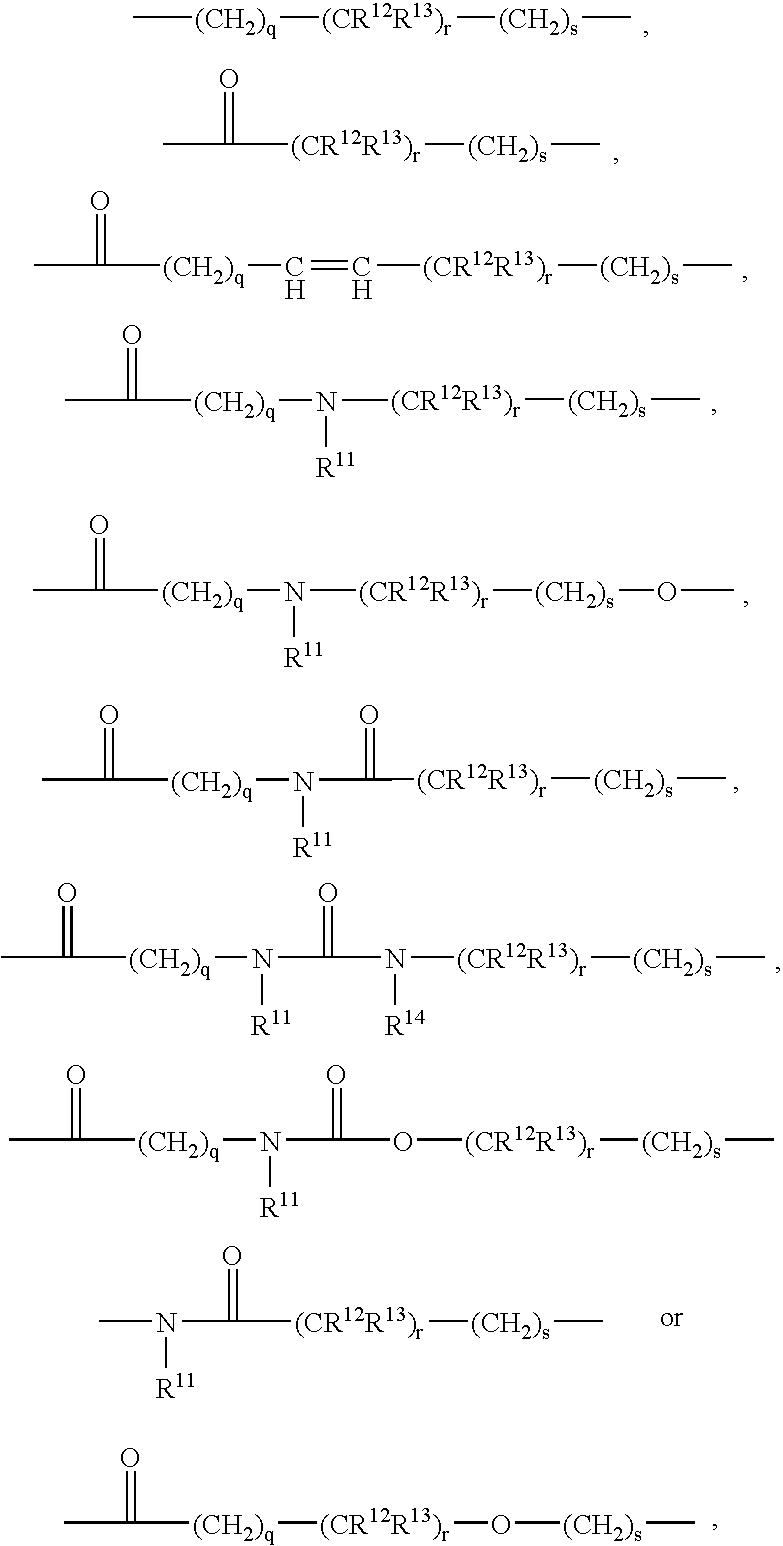Patents
Literature
2171results about "Urea derivatives preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Omega-carboxyaryl substituted diphenyl ureas as raf kinase inhibitors
This invention relates to the use of a group of aryl ureas in treating raf mediated diseases, and pharmaceutical compositions for use in such therapy.
Owner:BAYER HEALTHCARE LLC
Aryl and heteroaryl compounds, compositions, and methods of use
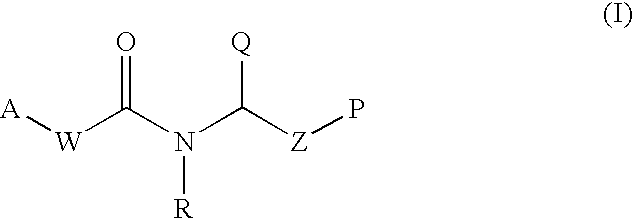
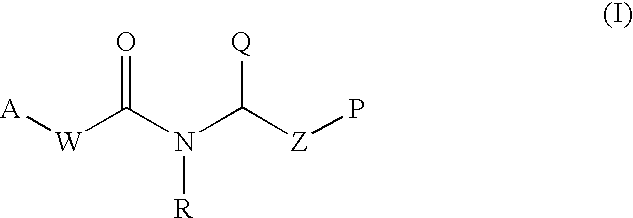
This invention provides aryl and heteroaryl compounds of Formula (I) as described herein, and methods of their preparation. Also provided are pharmaceutical compositions made with the compounds of Formula (I) and methods for making such compositions. Compounds of Formula (I) may be useful for treating viral infections including orthopox viruses, either alone or in combination with other therapeutic agents.
Owner:VTV THERAPEUTICS LLC
Amino acid-, peptide-and polypeptide-lipids, isomers, compositions, and uses thereof
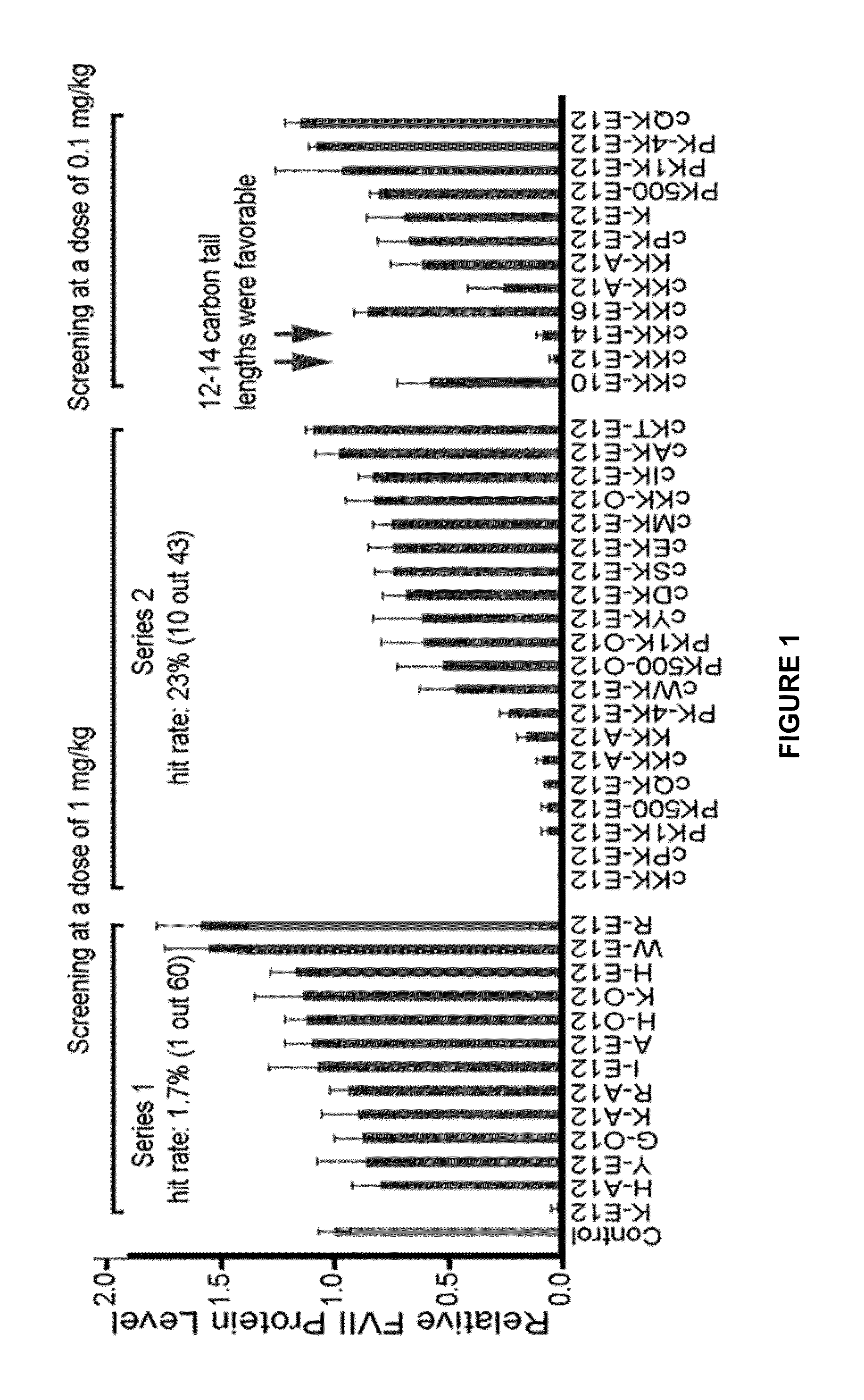
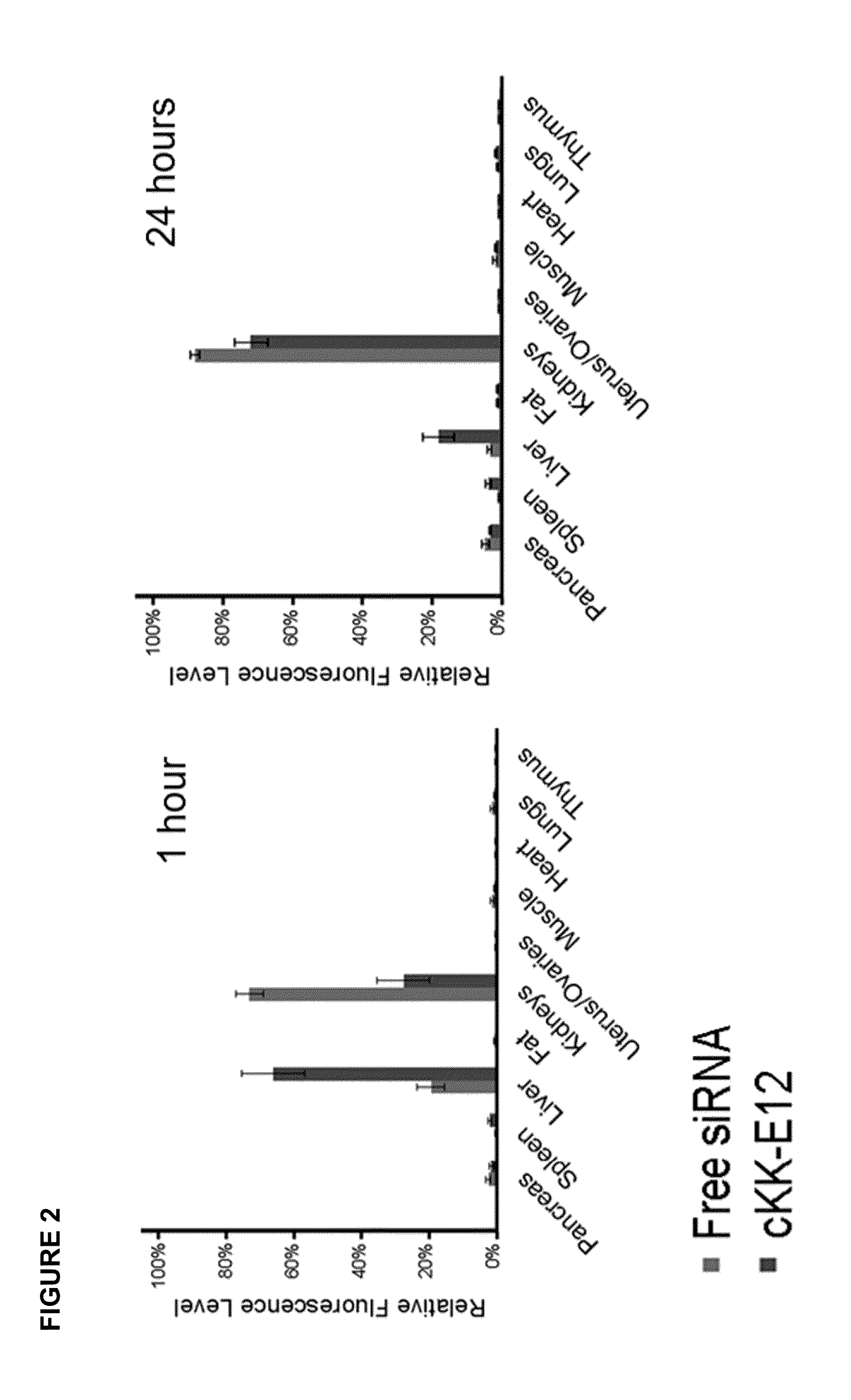
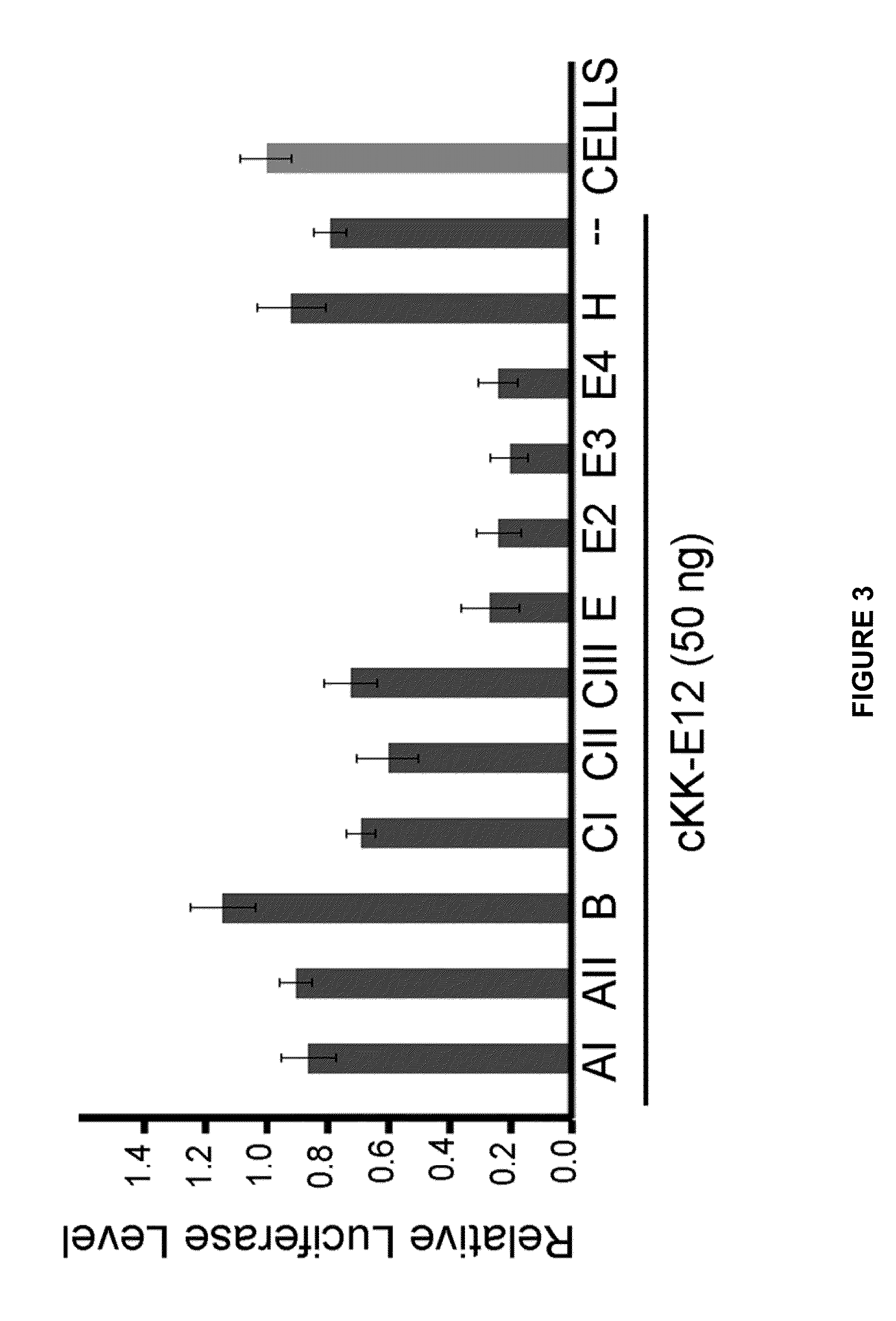
ActiveUS20130158021A1Improved nucleotide deliveryHigh transfection efficiencyUrea derivatives preparationOrganic active ingredientsCyclic peptideNucleotide
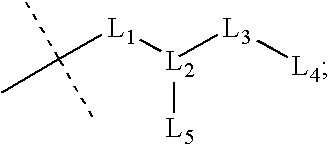
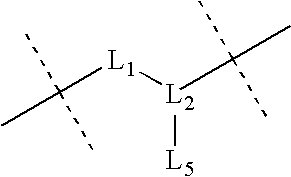
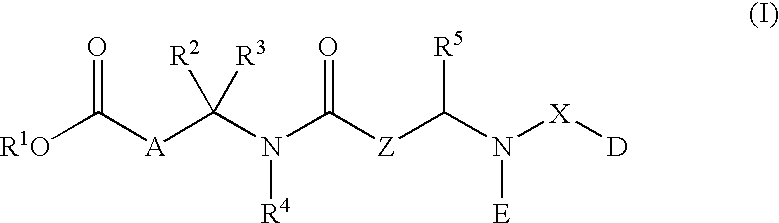
Described herein are compounds and compositions characterized, in certain embodiments, by conjugation of various groups, such as lipophilic groups, to an amino or amide group of an amino acid, a linear or cyclic peptide, a linear or cyclic polypeptide, or structural isomer thereof, to provide compounds of the present invention, collectively referred to herein as “APPLs”. Such APPLs are deemed useful for a variety of applications, such as, for example, improved nucleotide delivery. Exemplary APPLs include, but are not limited to, compounds of Formula (I), (II), (III), (IV), (V), and (VI), and salts thereof, as described herein:wherein m, n, p, R′, R1, R2, R3, R4, R5, R8, Z, W, Y, and Z are as defined herein.
Owner:MASSACHUSETTS INST OF TECH
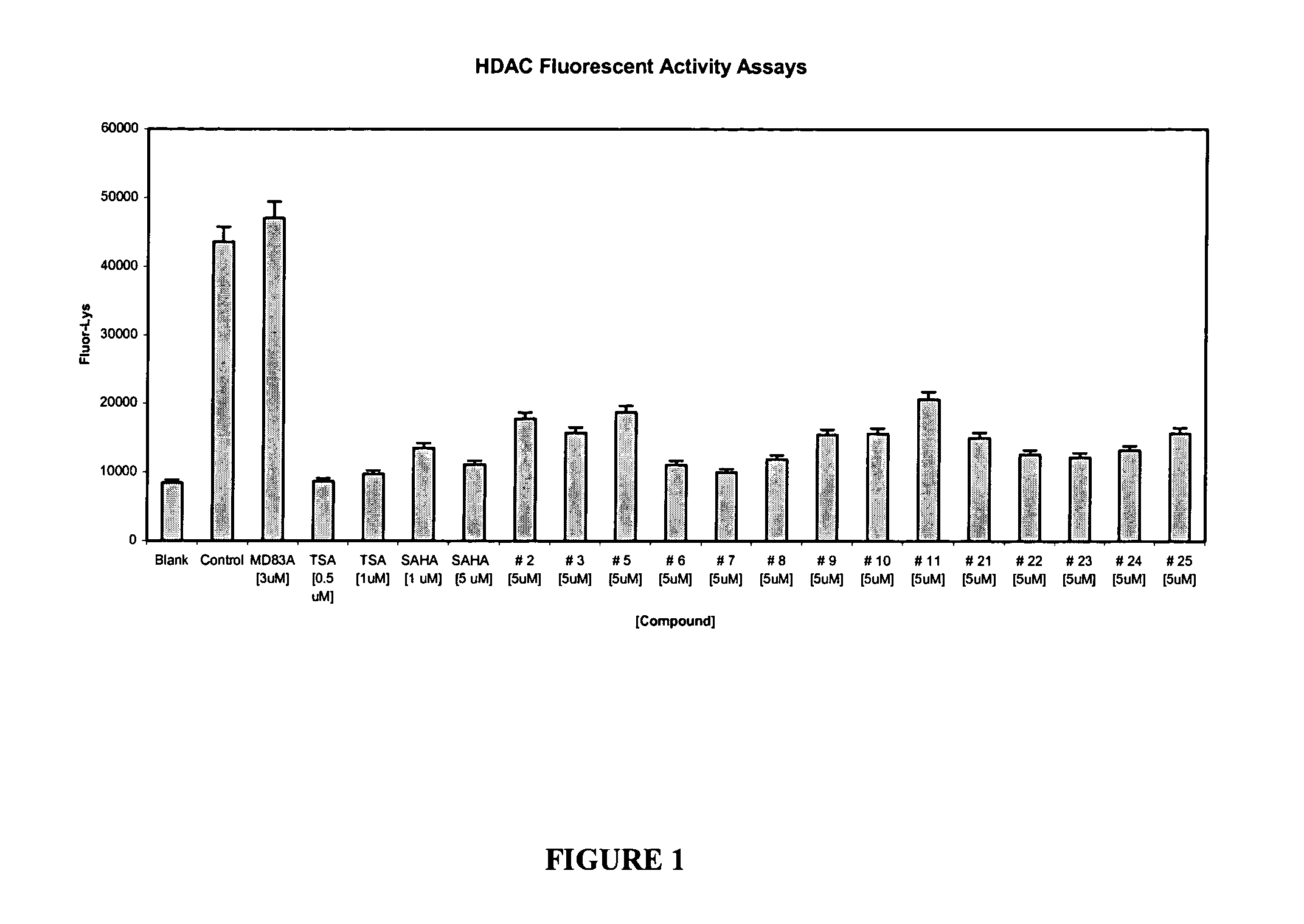
Histone deacetylase inhibitors and methods of use thereof
The invention provides novel classes of HDAC inhibitors. Methods of sensitizing a cancer cell to the cytotoxic effects of radiotherapy are also provided as well as methods for treating cancer and methods for treating neurological diseases. Additionally, the invention further provides pharmaceutical compositions comprising an HDAC inhibitor of the invention, and kits comprising a container containing an HDAC inhibitor of the invention.
Owner:GEORGETOWN UNIV
Halogenated selective androgen receptor modulators and methods of use thereof
InactiveUS7026500B2Unexpected anabolicUnexpected androgenicBiocideUrea derivatives preparationDiseaseAging male
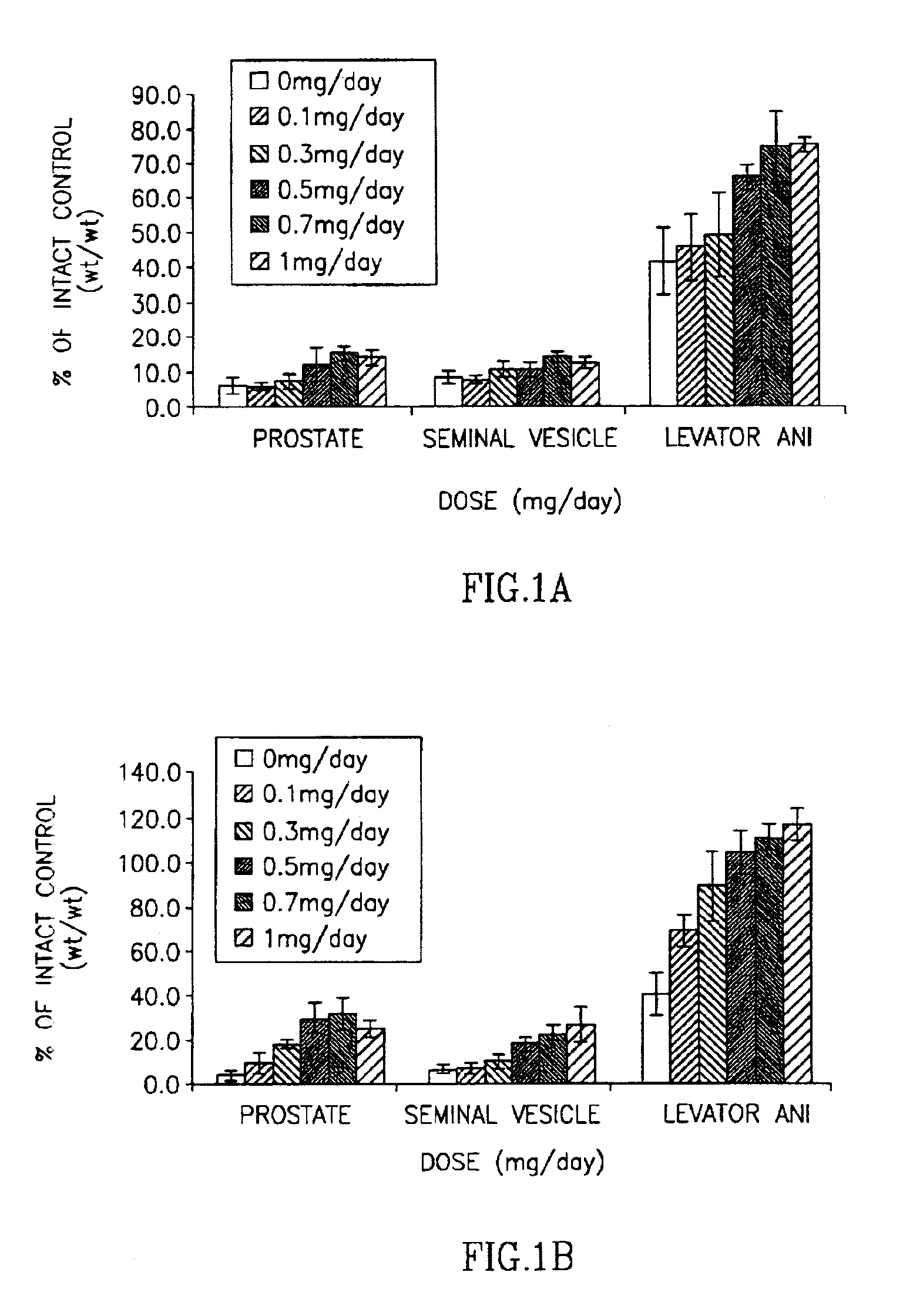
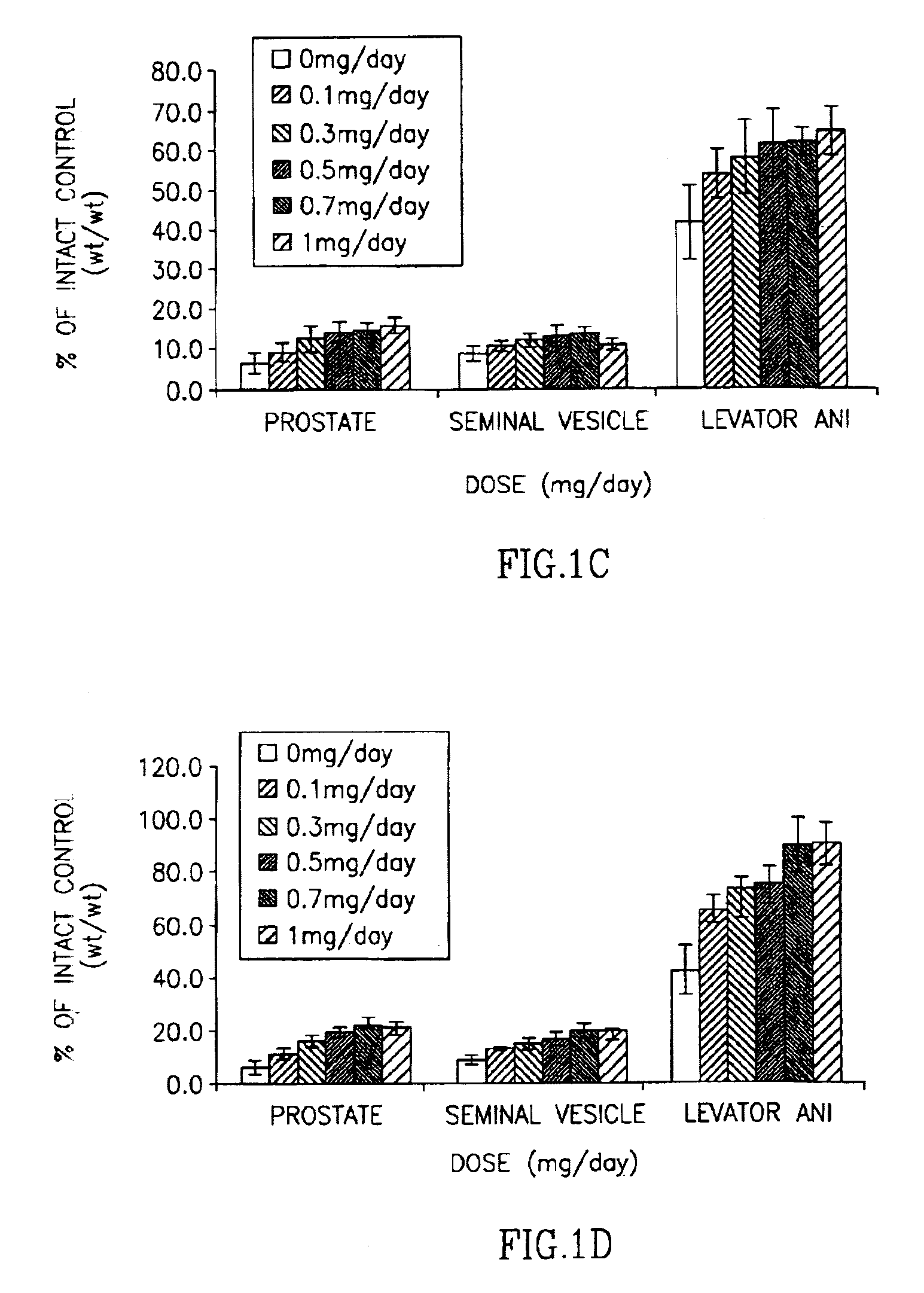
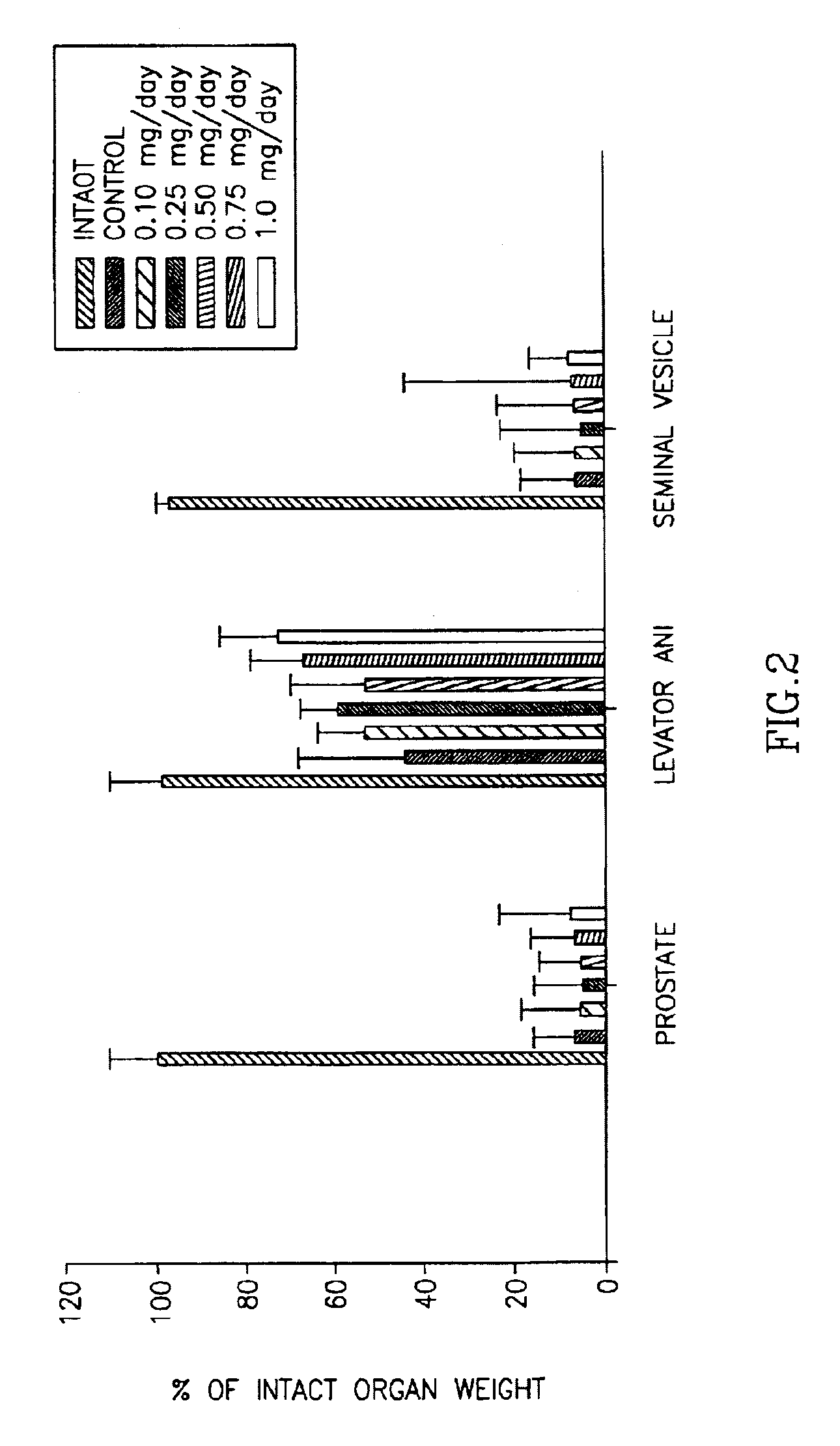
This invention provides a class of androgen receptor targeting agents (ARTA). The agents define a new subclass of compounds, which are selective androgen receptor modulators (SARM). Several of the SARM compounds have been found to have an unexpected androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. Other SARM compounds have been found to have an unexpected antiandrogenic activity of a nonsteroidal ligand for the androgen receptor. The SARM compounds, either alone or as a composition, are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia, osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of acute and / or chronic muscular wasting conditions; e) preventing and / or treating dry eye conditions; f) oral androgen replacement therapy; and / or g) decreasing the incidence of, halting or causing a regression of prostate cancer.
Owner:UNIV OF TENNESSEE RES FOUND
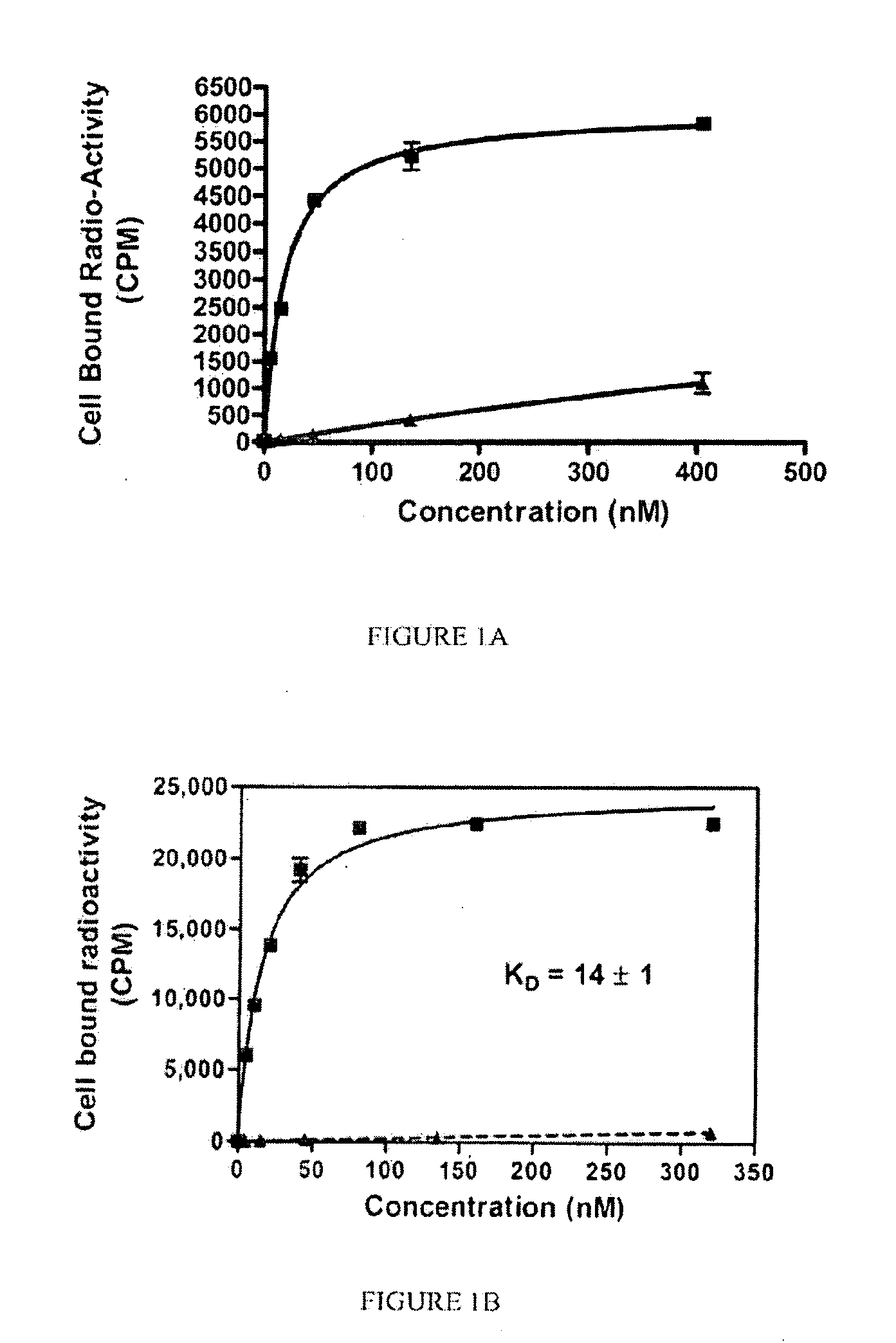
Psma binding ligand-linker conjugates and methods for using
Described herein are prostate specific membrane antigen (PSMA) binding conjugates that are useful for delivering therapeutic, diagnostic and imaging agents. Also described herein are pharmaceutical composition containing them and methods of using the conjugates and compositions. Also described are processes for manufacture of the conjugates and the compositions containing them.
Owner:PURDUE RES FOUND INC
Compounds having reversible inhibiting activity of carnitine palmitoyl-transferase
Compounds of formula (I)wherein the groups are as defined in the description are disclosed.The compounds of formula (I) are endowed with reversible inhibiting activity of carnitine palmitoyl-transferase and are useful in the preparation of medicaments useful in the pathologies related to a hyperactivity of carnitine palmitoyl-transferase, such as hyperglycemia, diabetes and pathologies related thereto, heart failure, ischemia.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Multi-substitued selective androgen receptor modulators and methods of use thereof
InactiveUS20050033074A1Decreased libidoAlteration in mood and cognitionUrea derivatives preparationSenses disorderDiseaseAging male
This invention provides a class of androgen receptor targeting agents (ARTA). The agents define a new subclass of compounds, which are selective androgen receptor modulators (SARM). Several of the SARM compounds have been found to have an unexpected androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. Other SARM compounds have been found to have an unexpected antiandrogenic activity of a nonsteroidal ligand for the androgen receptor. The SARM compounds, either alone or as a composition, are usefull for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia, osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of acute and / or chronic muscular wasting conditions; e) preventing and / or treating dry eye conditions; f) oral androgen replacement therapy; and / or g) decreasing the incidence of, halting or causing a regression of prostate cancer.
Owner:UNIV OF TENNESSEE RES FOUND
Compounds useful as anti-inflammatory agents
InactiveUS6492393B1Improve stabilityEasy to manageBiocideUrea derivatives preparationMedicinal chemistryDisease cause
Disclosed are novel aromatic compounds of the formula (I) wherein G, X, Ar, L and Q are defined herein. The compounds are useful in pharmaceutic compositions for treating diseases or pathological conditions involving inflammation such as chronic inflammatory diseases. Also disclosed are processes of making such compounds.
Owner:BOEHRINGER INGELHEIM PHARMA INC
Therapeutic agents and methods of use thereof for the modulation of angiogenesis
InactiveUS7084108B2Low toxicityInhibit methionine aminopeptidase 2Urea derivatives preparationOrganic active ingredientsDiseaseAngiogenesis growth factor
The present invention provides angiogenesis inhibitor compounds comprising a MetAP-2 inhibitory core coupled to a peptide, as well as pharmaceutical compositions comprising the angiogenesis inhibitor compounds and a pharmaceutically acceptable carrier. The present invention also provides methods of treating an angiogenic disease, e.g., cancer, in a subject by administering to the subject a therapeutically effective amount of one or more of the angiogenesis inhibitor compounds of the invention.
Owner:GLAXO SMITHKLINE LLC
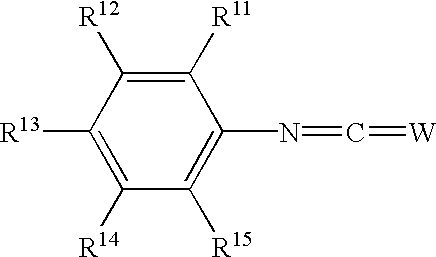
Phenyl derivatives containing an acidic group, their preparation and their use as chloride channel blockers
The present patent application relates to compounds having formula (I) or a pharmaceutically acceptable salt thereof wherein one of R1, R2 and R3 is a non-cyclic acidic group having a pKa value below 8 or a group which is in vivo convertible to such a group; R4, R5 and the other two of the substituents R1, R2 and R3 are each independently selected from hydrogen, alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, hydroxy, halogen, trifluoromethyl, trifluoromethoxy, cyano, nitro, amnino, and aryl, aralkyl, arylamino, aryloxy, aryl-CO-, or heteroaryl, wherein the aryl and heteroaryl groups may be substituted one or more times with substituents selected from alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, hydroxy, alkoxy, halogen, trifluoromethyl, trifluoromethoxy, cyano, nitro and amino; or R3 and R4 or R4 and R5 together form a fused 4- to 7-membered carbocyclic ring which may be unsaturated, or partially or fully saturated, while the other substituents R1, R2, R3, R4 and R5 are as defined above; Y is -CO-, -CS-, -SO2-, or -C(=N-R8)-, wherein R8 is hydrogen, alkyl, or cyano; X is -NH-, -CH2-NH-, or -SO2-NH-; Z is NR6, O, -CH=CH-, -C=C-, -N=CH-, or -CH=N-; wherein R6 is hydrogen, or alkyl; R11, R12, R13, R14 and R15 are each independently selected from hydrogen, alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, hydroxy, halogen, trifluoromethyl, trifluoromethoxy, cyano, nitro, amino, -NHSO2-R7, -COOR7, -SO2N(R7)2, -SO2OR7 and -CO-R7, wherein R7 is hydrogen alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, aryl or aralkyl; and aryl, aralkyl, arylamino, aryloxy, aryl-CO-, or heteroaryl, wherein the aryl and heteroaryl groups may be substituted one or more times with substituents selected from alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, hydroxy, alkoxy, halogen, trifluoromethyl, trifluoromethoxy, cyano, nitro and amino; or one of R11 and R12, R12 a
Owner:ANIONA APS
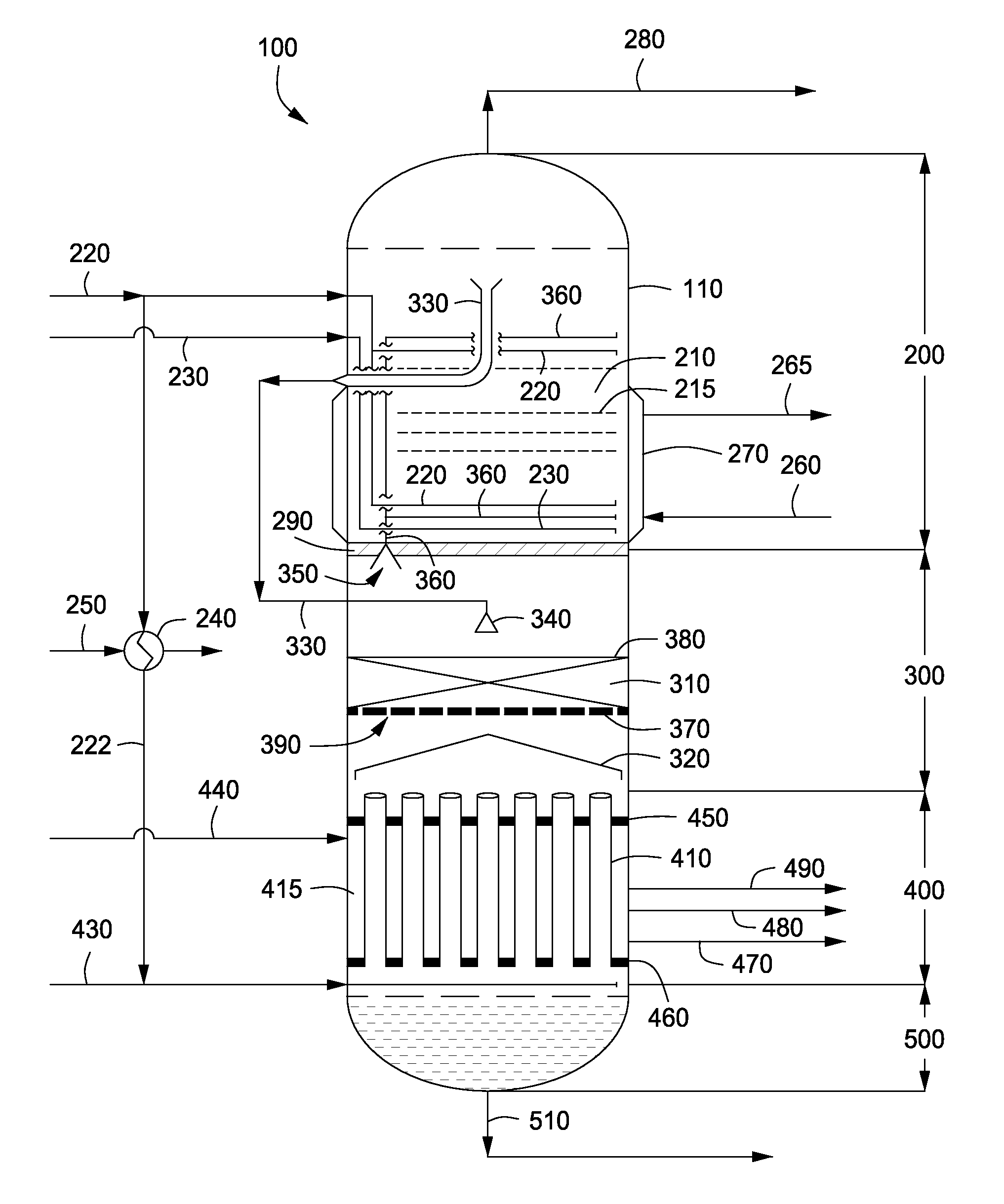
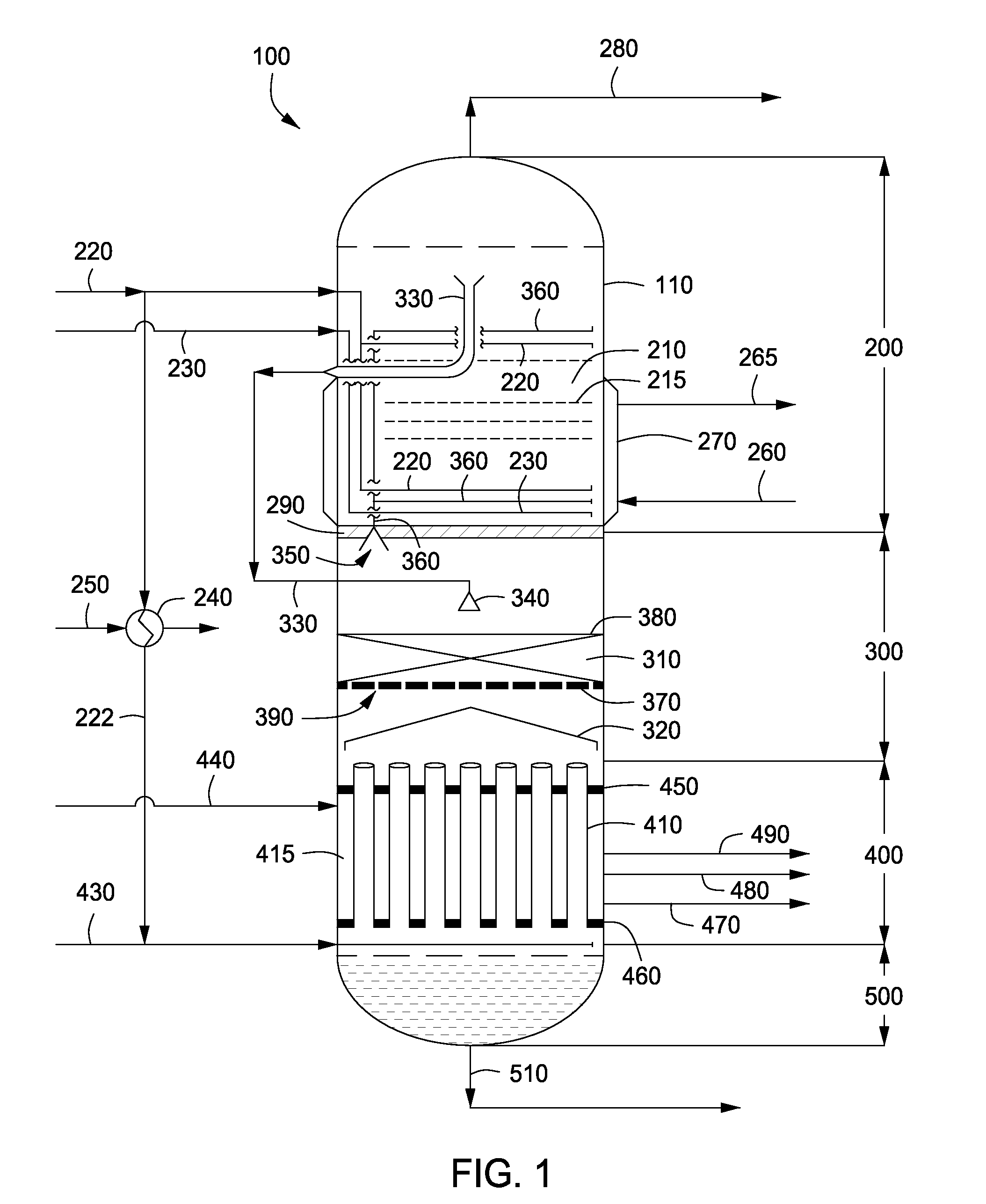
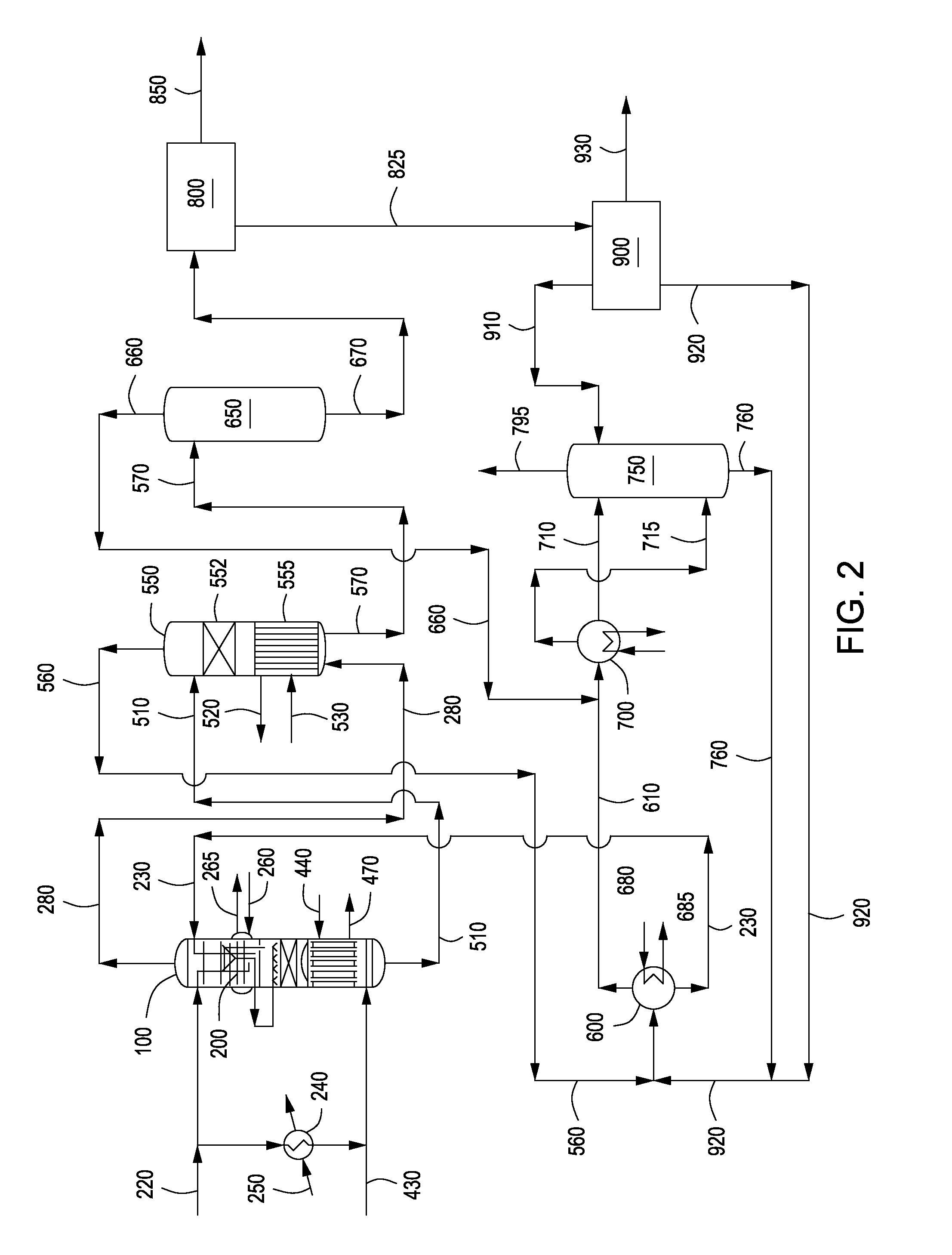
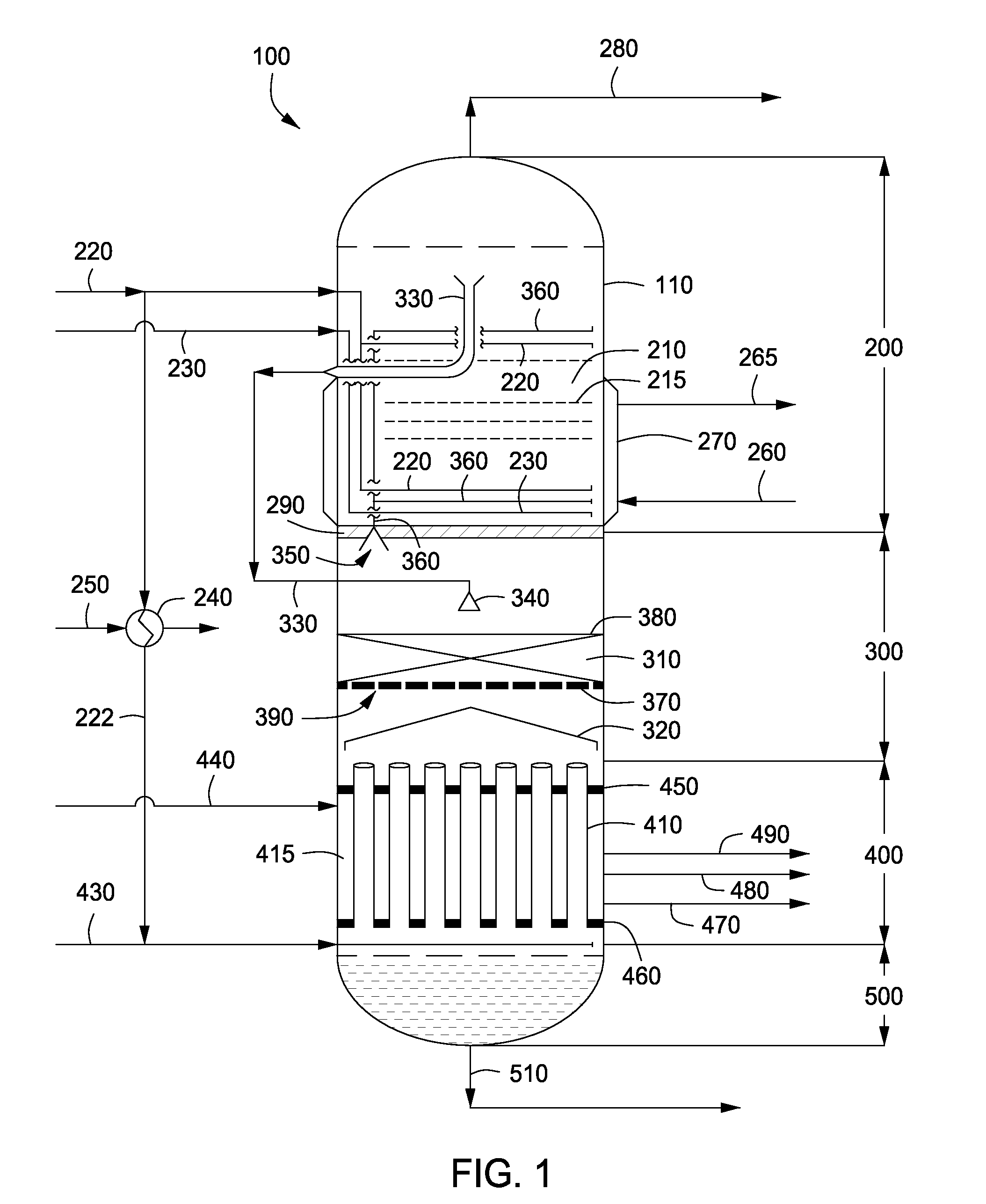
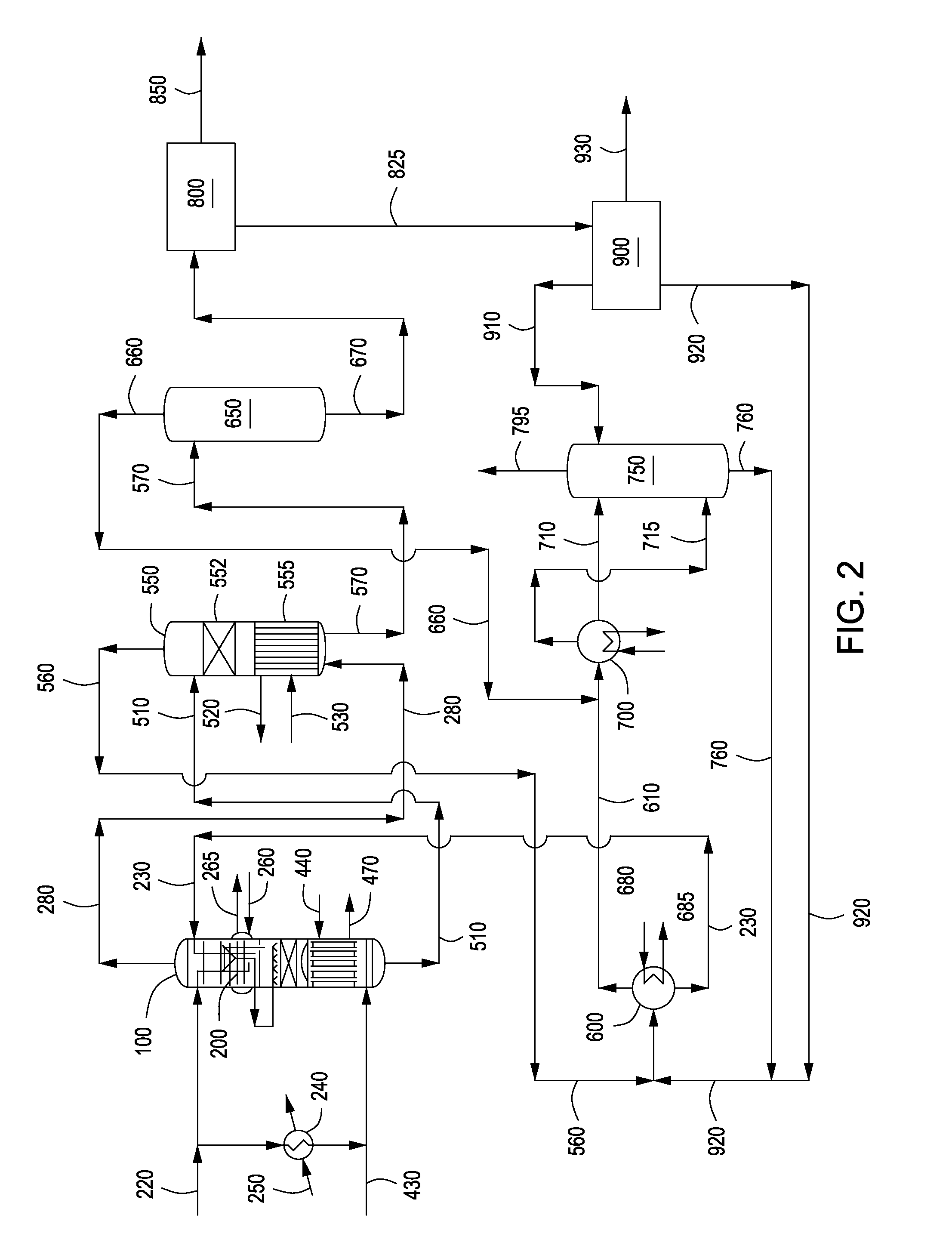
Apparatus and methods for urea production
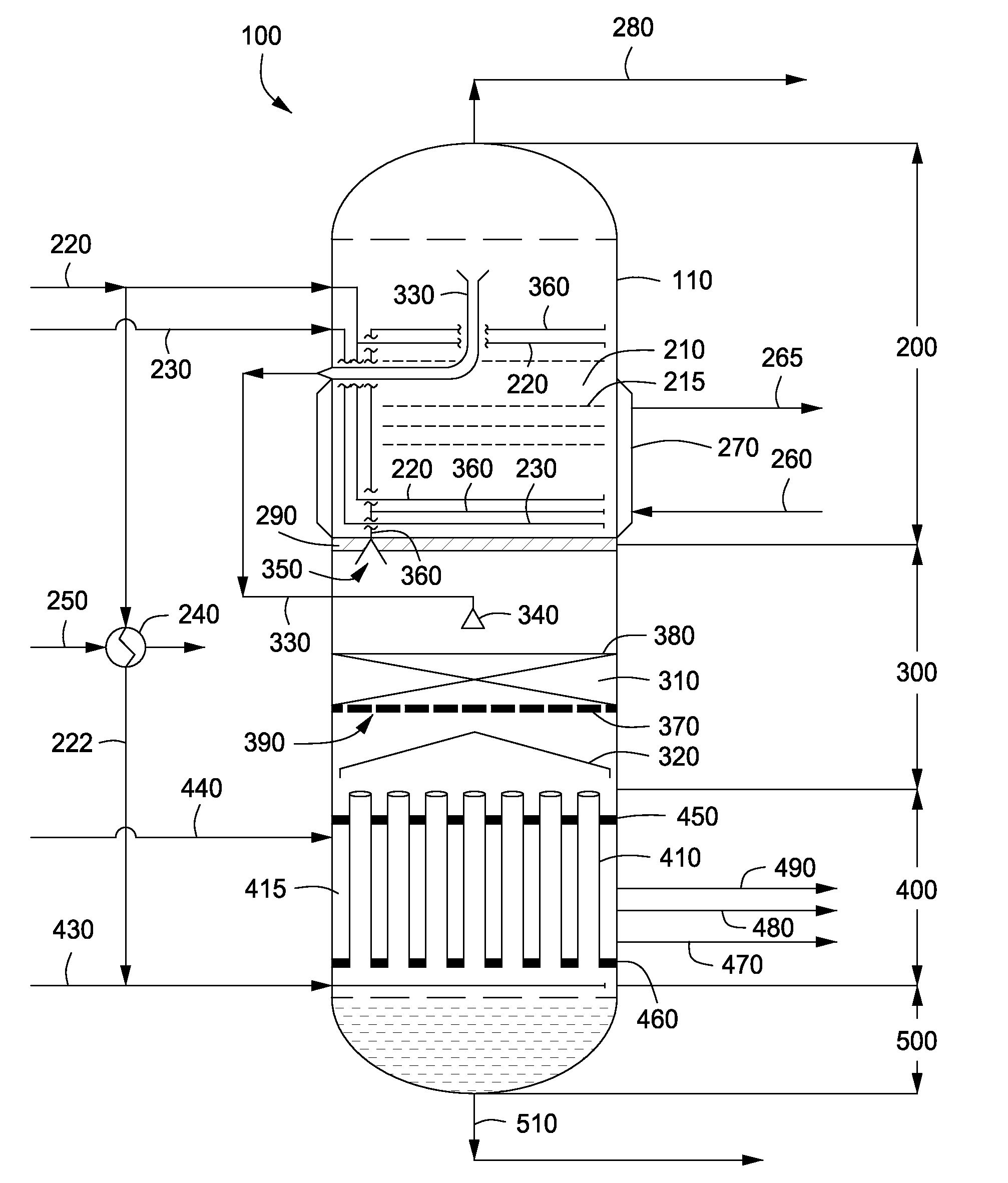
Apparatus and methods for producing urea are provided. In one or more embodiments, an apparatus for producing urea can include a first zone, which can include a first flow channel in fluid communication with a first tube disposed about a first end of a plurality of trays, a second flow channel in fluid communication with a second tube disposed about the first end of the trays and a second end of the trays, and a third flow channel in fluid communication with a third tube disposed about the first and second ends of the trays. The apparatus can include a second zone, which can include a fixed bed comprising one or more inert packing materials disposed therein to provide additional surface area. The apparatus can include a third zone, which can include a plurality of tubes disposed therein. The second zone can be disposed between the first and third zones.
Owner:KELLOGG BROWN & ROOT LLC
Method and apparatus for treating waste streams
InactiveUS6716360B2Overcome problemsUrea derivatives preparationSludge treatment by oxidationSufficient timeWaste stream
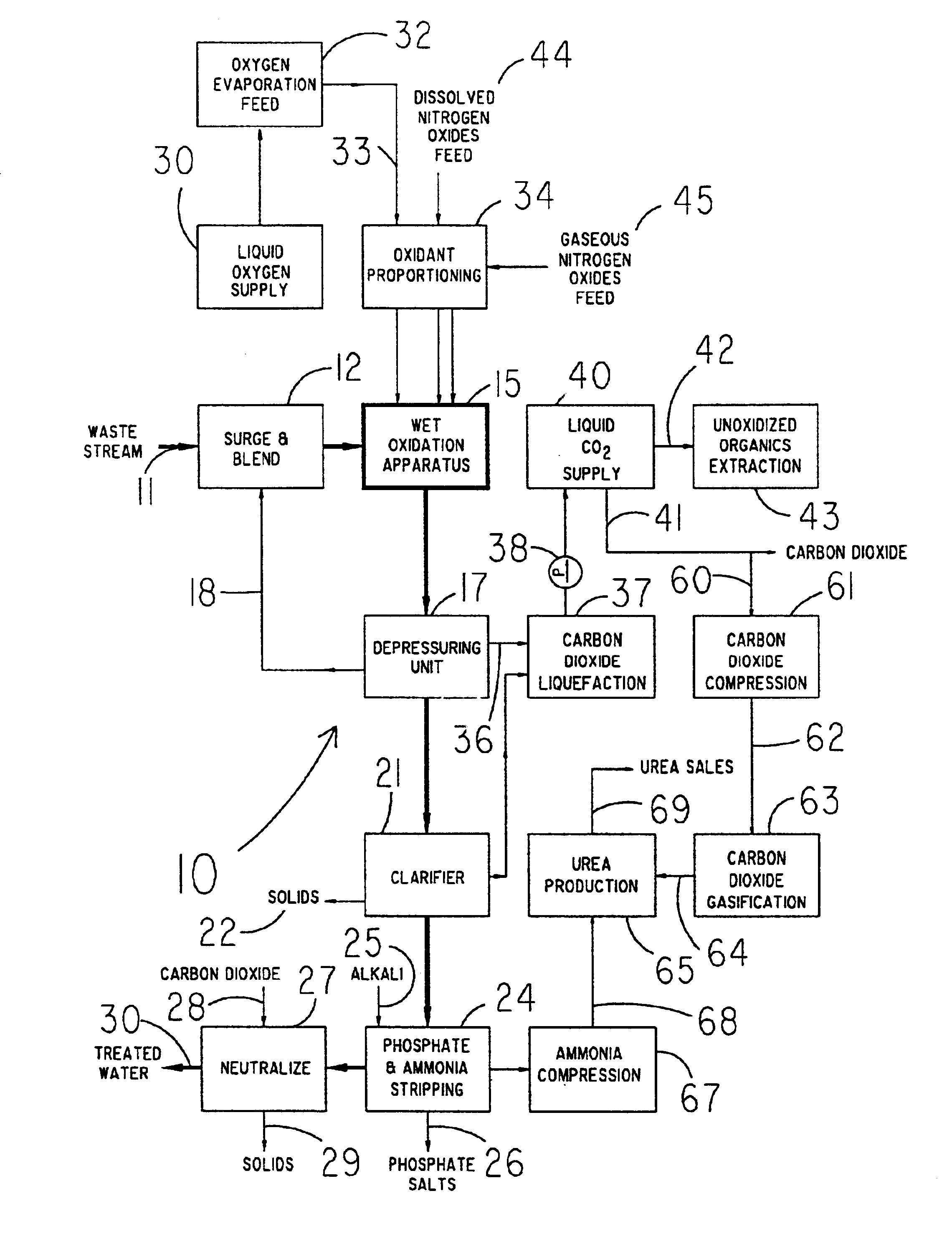
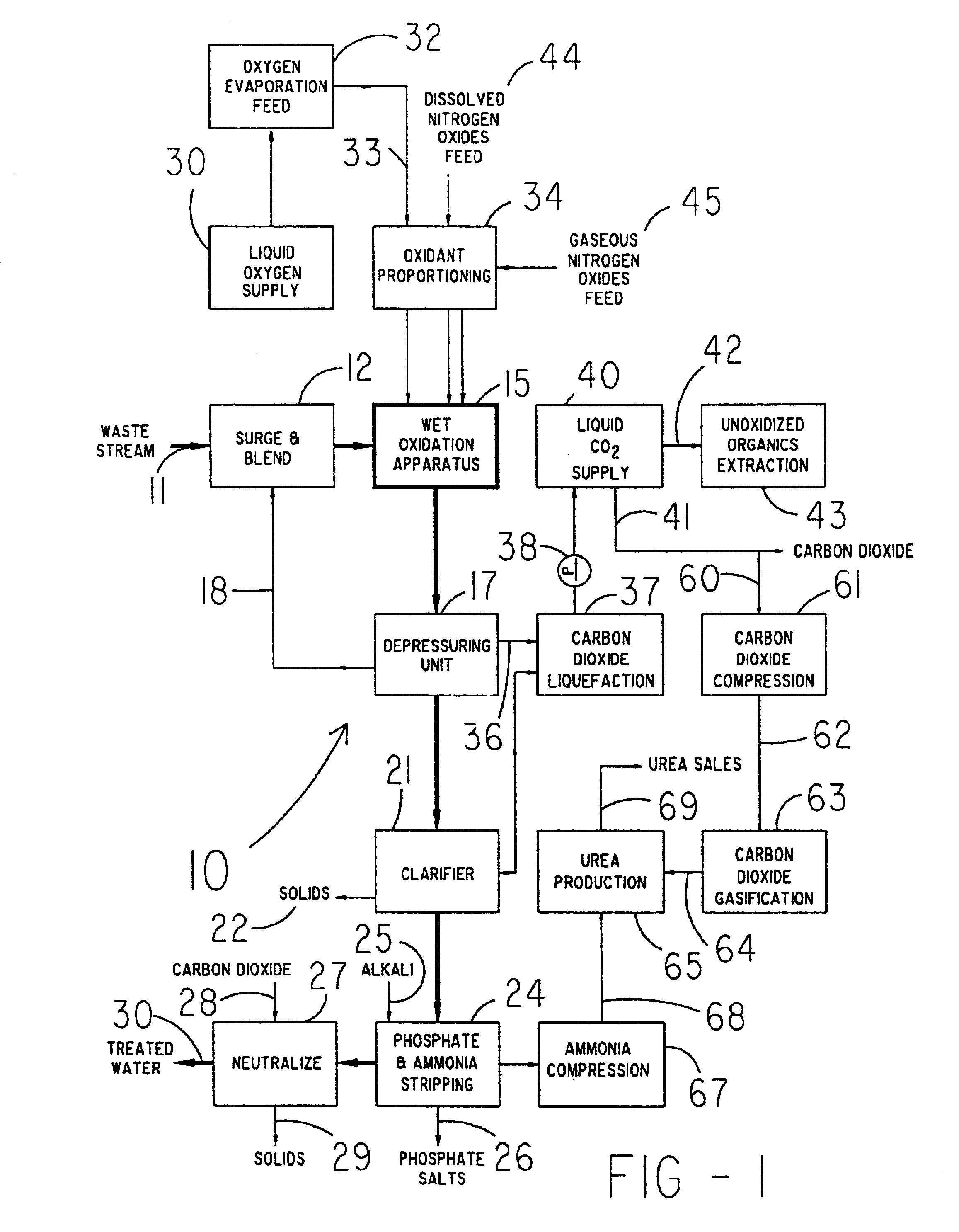
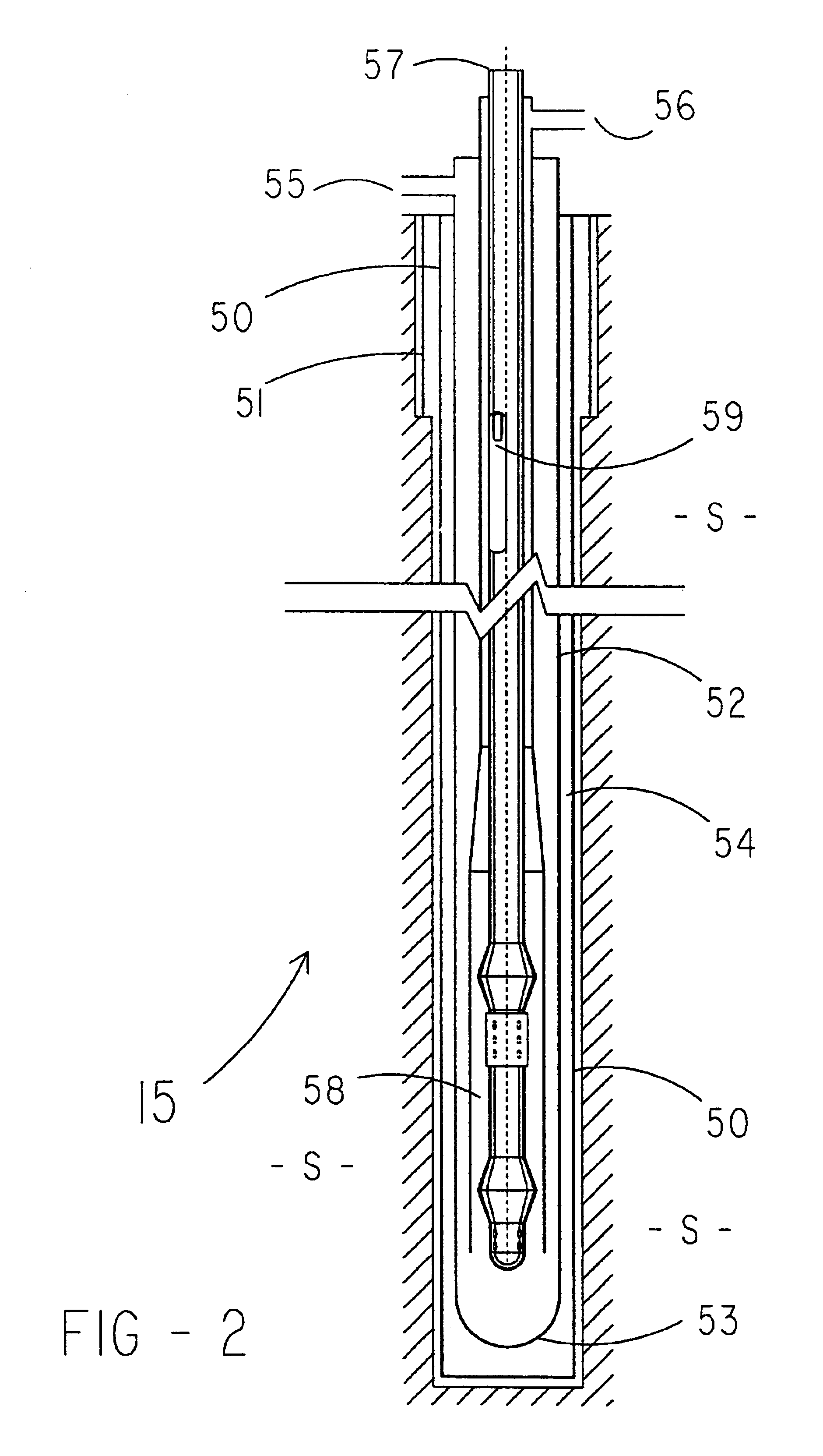
A method for treating a waste stream, the method including the steps of providing a waste stream that includes waste solids suspended in water, feeding the waste stream to the top of a hydraulic downdraft column, conducting the waste stream to the bottom of the hydraulic downdraft column, conducting the waste steam to a first reaction zone, introducing nitrogen-containing oxides into the first reaction zone so that the waste stream is contacted with the nitrogen-containing oxides, conducting the waste stream up a hydraulic updraft column into a second reaction zone, where the second reaction zone is configured to provide sufficient time so that a reaction between the nitrogen-containing oxides and waste solids can take place and substantially consume the nitrogen-containing oxides, introducing oxygen gas into the waste steam after the nitrogen-containing oxides are substantially consumed, thereby providing a second reactant that reacts with waste solids suspended within the stream, conducting the stream to the top of the hydraulic updraft column.
Owner:EAU VIRON
Apparatus and methods for urea production
ActiveUS20090216045A1Urea derivatives preparationOrganic compound preparationFilling materialsFixed bed
Apparatus and methods for producing urea are provided. In one or more embodiments, an apparatus for producing urea can include a first zone, which can include a first flow channel in fluid communication with a first tube disposed about a first end of a plurality of trays, a second flow channel in fluid communication with a second tube disposed about the first end of the trays and a second end of the trays, and a third flow channel in fluid communication with a third tube disposed about the first and second ends of the trays. The apparatus can include a second zone, which can include a fixed bed comprising one or more inert packing materials disposed therein to provide additional surface area. The apparatus can include a third zone, which can include a plurality of tubes disposed therein. The second zone can be disposed between the first and third zones.
Owner:KELLOGG BROWN & ROOT LLC
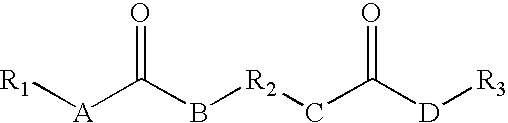
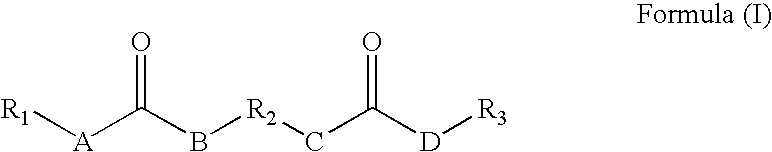
Rheology control agents
ActiveUS20060155146A1Urea derivatives preparationCarbamic acid derivatives preparationPolyesterEther
The present invention provides for a rheology control agent that includes a following compound represented by the following formula: wherein A, B, C and D equal CH2, CHR, NH, or O, and A, B, C and D may be the same or different and at least one of A and B equals NH and at least one of C and D equals NH; and wherein R1, R2, and R3 may be the same or different and represent a linear, branched, hyper-branched, or dendritic ether, polyether or hydrocarbon based chain, optionally forming at least one carbon-based ring, being saturated or unsaturated and R2 represents linear or branched alkylenes, ethers, polyethers, or polyester linkages and at least one of R1, R2, and R3 comprises an ester group or an amide group which is branched off from the main chain; excluded from Formula (1) is a compound wherein R2 is CH2—CH2—CH2—CH2—CH(C(O)OCH3), A, B, C, and D are equal to NH and R1 and R3 are both equal to a linear octyl hydrocarbon chain; the rheology control agent is suitable for solvent-borne and water-borne coating composition having improved rheology control useful for OEM refinishing or repainting the exterior of automobile and truck bodies and parts thereof.
Owner:AXALTA COATING SYST IP CO LLC
Glucagon antagonists/inverse agonists
A novel class of compounds, which act to antagonize the action of the glucagon hormone on the glucagon receptor. Owing to their antagonizing effect of the glucagon receptor the compounds may be suitable for the treatment and / or prevention of any glucagon-mediated conditions and diseases such as hyperglycemia, Type 1 diabetes, Type 2 diabetes and obesity.
Owner:PFIZER INC
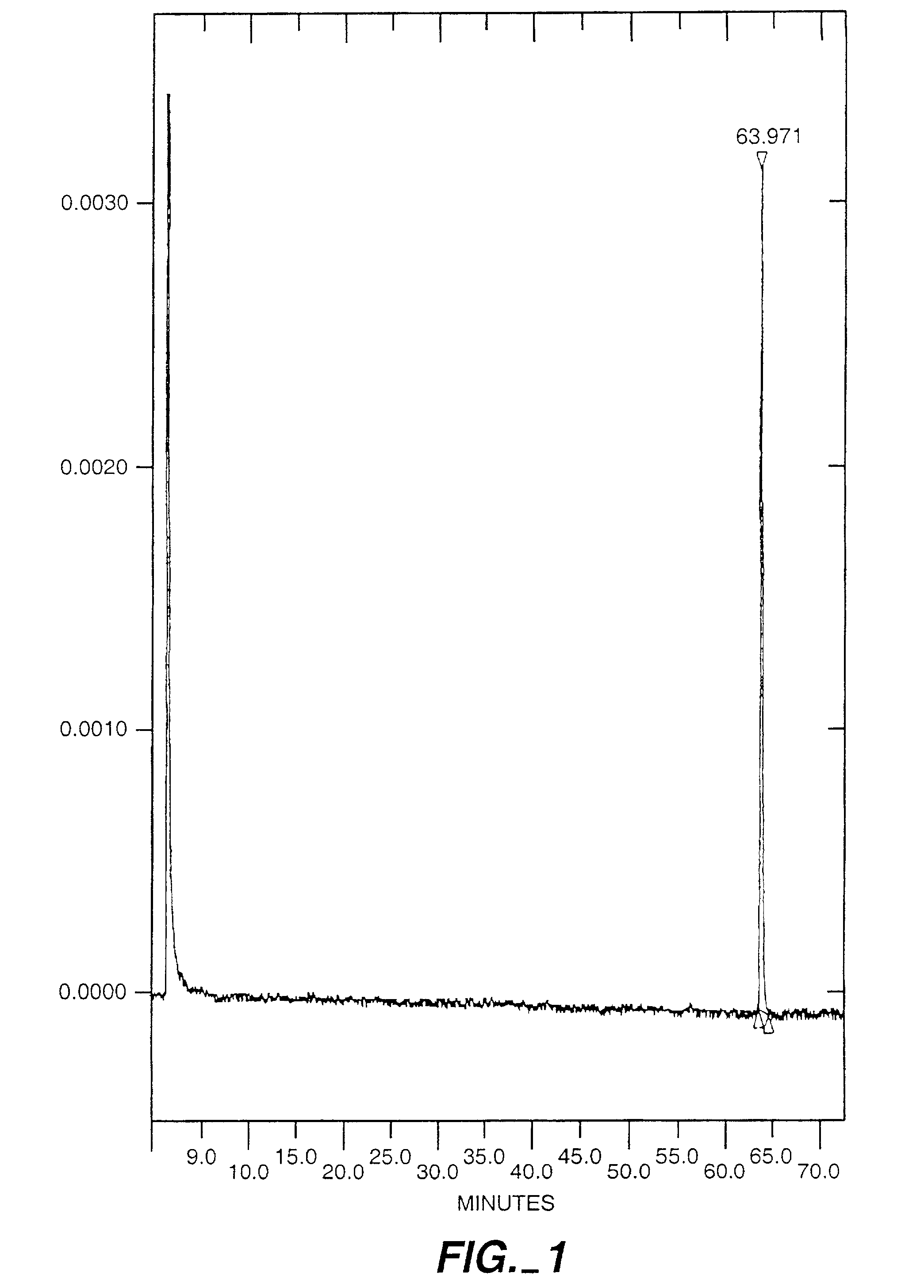
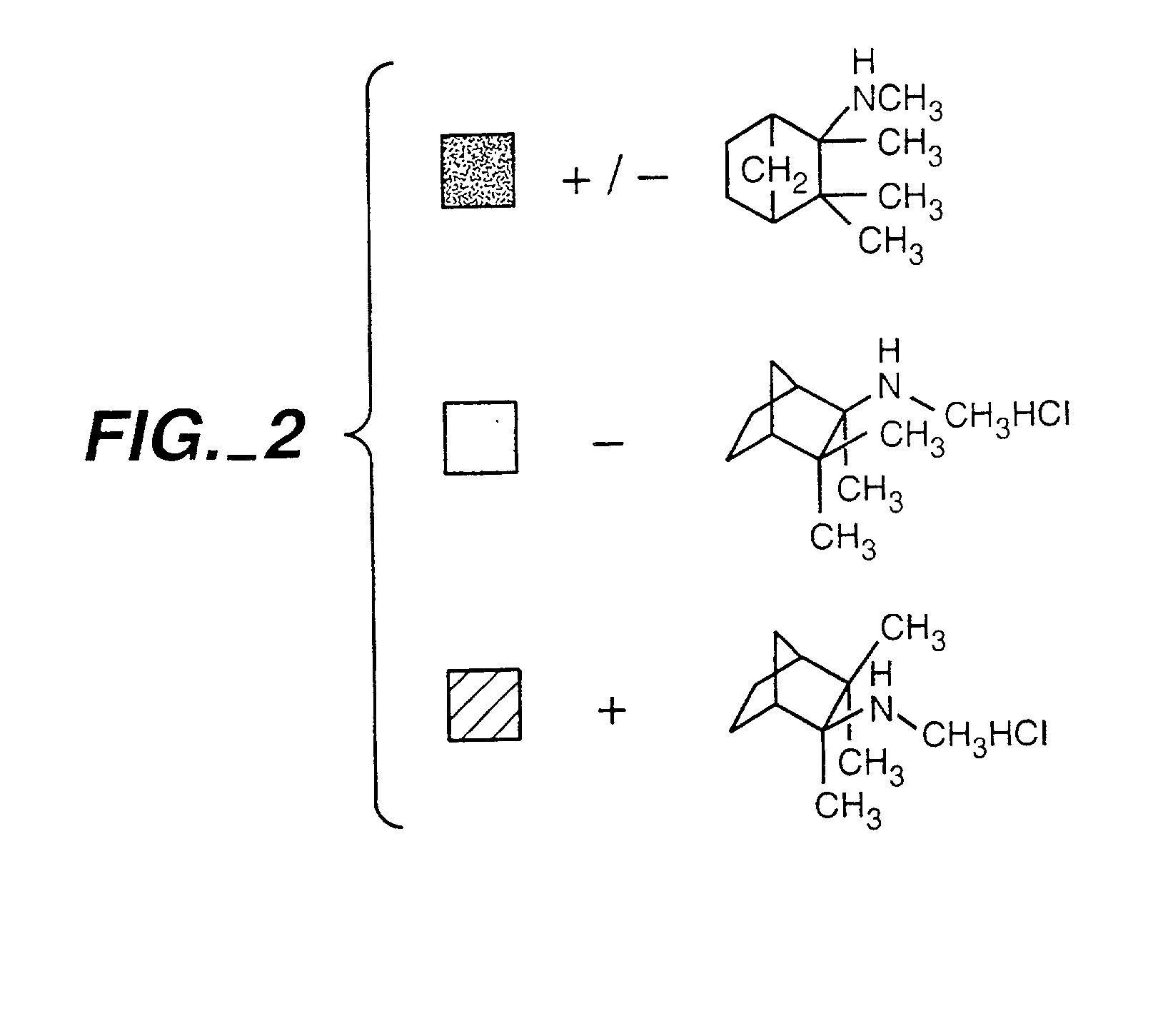
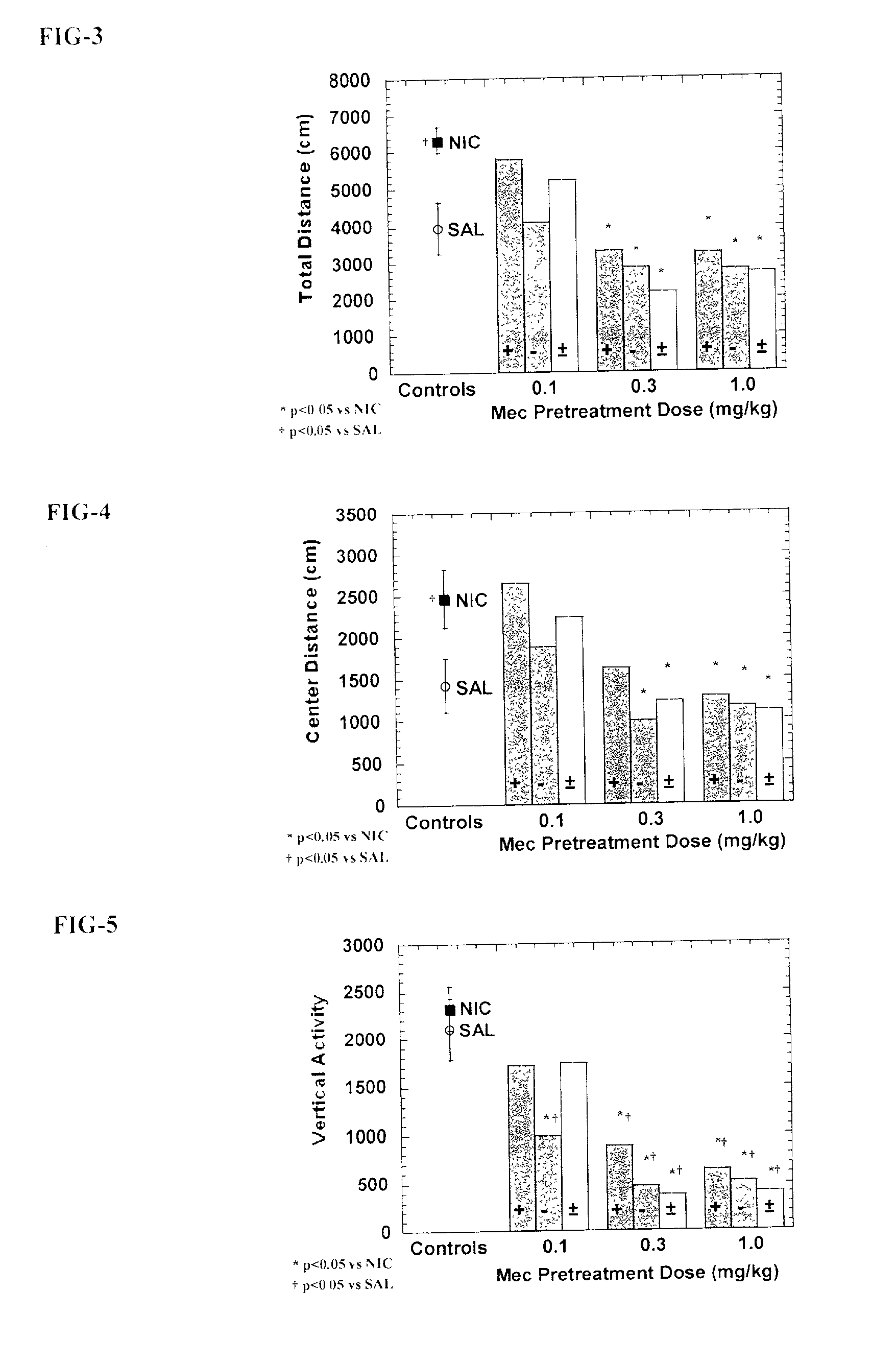
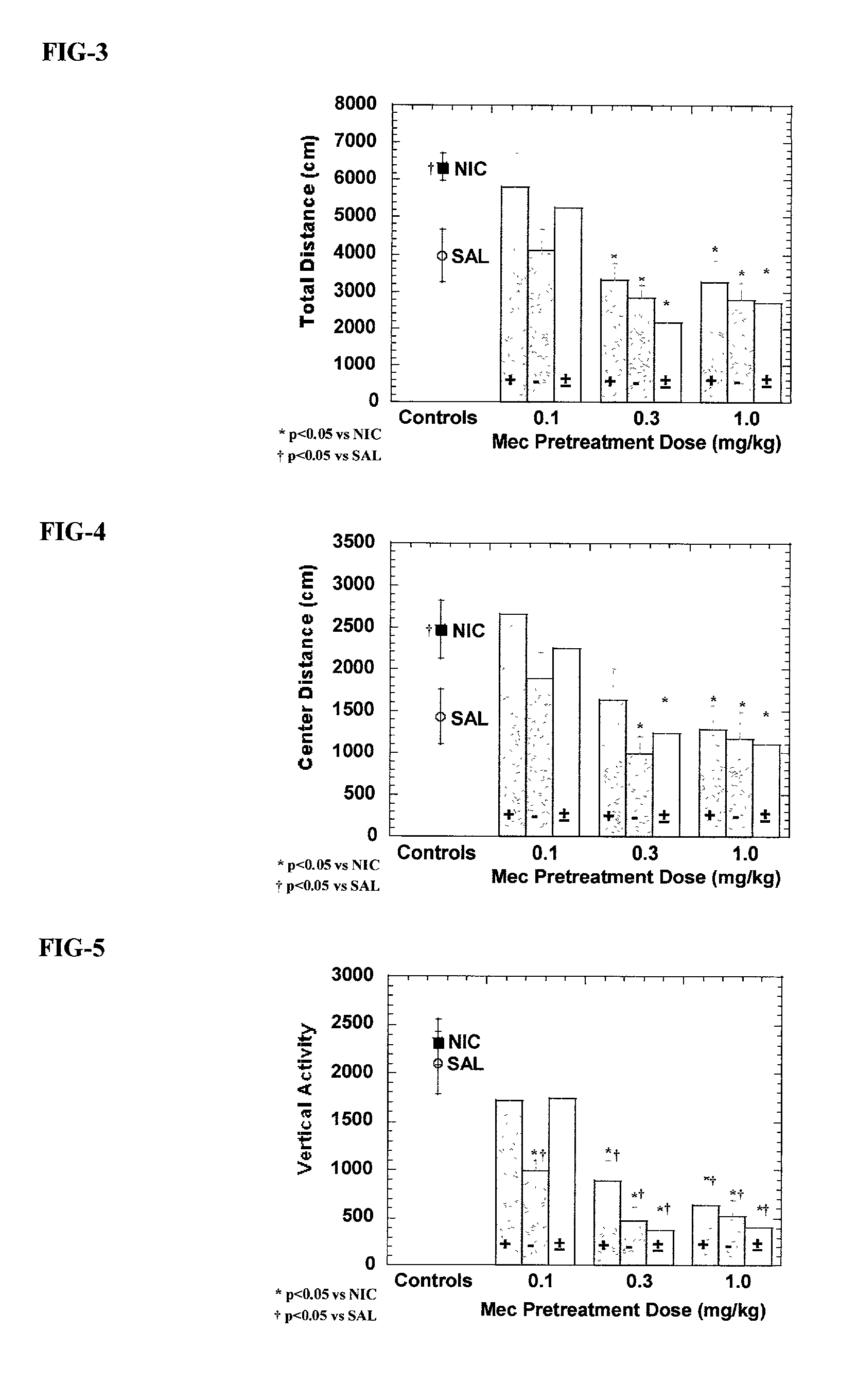
Exo-S-mecamylamine formulation and use in treatment
InactiveUS20020016371A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationWeight gainingSmoking cessation
A pharmaceutical composition includes a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-R-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-R-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
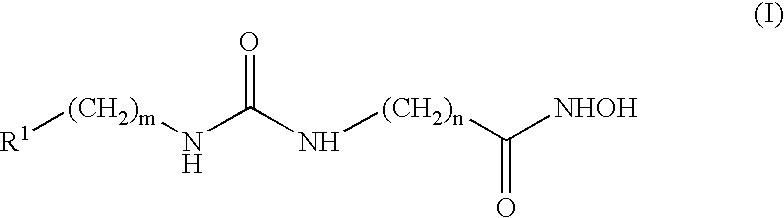
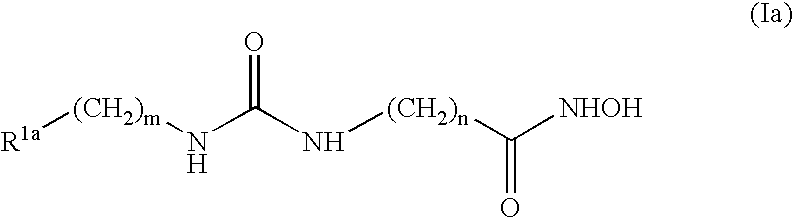
Urea derivatives having ACAT inhibitory activity, their preparation and their therapeutic and prophylactic use
Compounds of formula (I): wherein: R1 is alkyl; R2a, R2b, R2c and R2d are the same or different and each is hydrogen, optionally substituted alkyl or various other organic groups; R3 is alkyl; R4 is a group of formula (II), (III), (IV), (V), (VI), (VII), (VIII) or (IX): wherein: A1 is a single bond, alkylene or alkenylene; A2 is or alkenylene; A3 is a single bond, alkyleneor alkenylene; R5a and R5b are the same or different and each is hydrogen, alkyl or various other groups; R6 is alkyl or phenyl; R7 is hydrogen or alkyl; R8 is alkyl or various other groups; and n is 0 and pharmaceutically acceptable salts thereof have valuable inhibitory activity against acyl-CoA: cholesterol acyl transferase.
Owner:SANKYO CO LTD
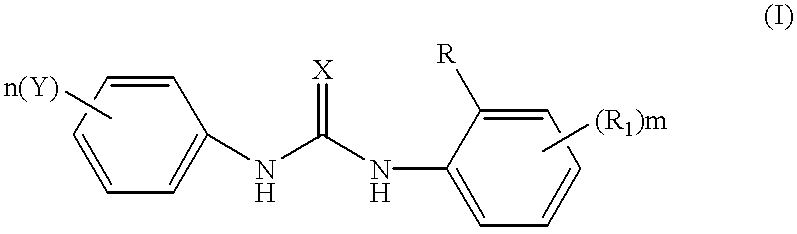
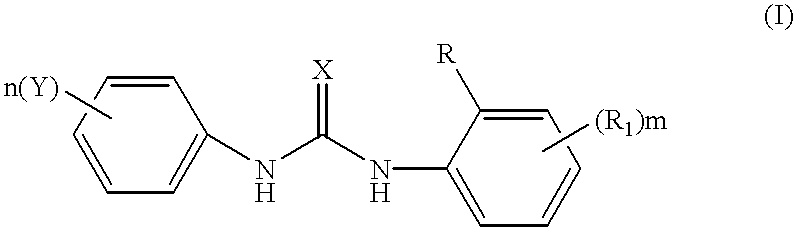
Phenyl urea antagonists of the IL-8 receptor
This invention relates to novel compounds of Formula (I), and a novel use of these compounds in treating chemokine mediated diseases, wherein the chemokine binds to an IL-8 a or b receptor. Compounds of Formula (1) are represented by the structure:wherein interalia, X is oxygen or sulfur;R is any functional moiety having an ionizable hydrogen and a pKa of 10 or less;R1 is independently selected from hydrogen; halogen; nitro; cyano; C1-C10 alkyl; halosubstituted C1-10 alkyl; C2-10 alkenyl; C1-10 alkoxy; halosubstituted C1-10 alkoxy; azide; (CR8R8)qS(O)tR4; hydroxy; hydroxy substituted C1-4 alkyl; aryl; aryl C1-4 alkyl; aryl C2-10 alkenyl; aryloxy; aryl C1-4 alkyloxy; heteroaryl; heteroarylalkyl; heteroaryl C2-10 alkenyl; heteroaryl C1-4 alkyloxy; heterocyclic, heterocyclic C1-4 alkyl; heterocyclic C1-4 alkyloxy; heterocyclic C2-10 alkenyl;q is 0 or an integer having a value of 1 to 10; n is an integer having a value of 1 to 3;m is an integer having a value of 1 to 3;n is an integer having a value of 1 to 3;Y is hydrogen; halogen; nitro; cyano; halosubstituted C1-10 alkyl; C1-10 alkyl; C2-10 alkenyl; C1-10 alkoxy; halosubstituted C1-1- alkoxy; azide; (CR8R8)qS(O)tR4, (CR8R8)qOR4; hydroxy; hydroxy substituted C1-4 alkyl; aryl; aryl C1-4 alkyl; aryloxy; aryC1-4 alkyloxy; aryl C2-10 alkenyl; heteroaryl; heteroarylalkyl; heteroaryl C1-4 alkyloxy; heteroaryl C2-10 alkenyl; heterocyclic, heterocyclic C1-4 alkyl; heterocyclic C2-10 alkenyl;or a pharmaceutically acceptable salt thereof.
Owner:GLAXO SMITHKLINE LLC
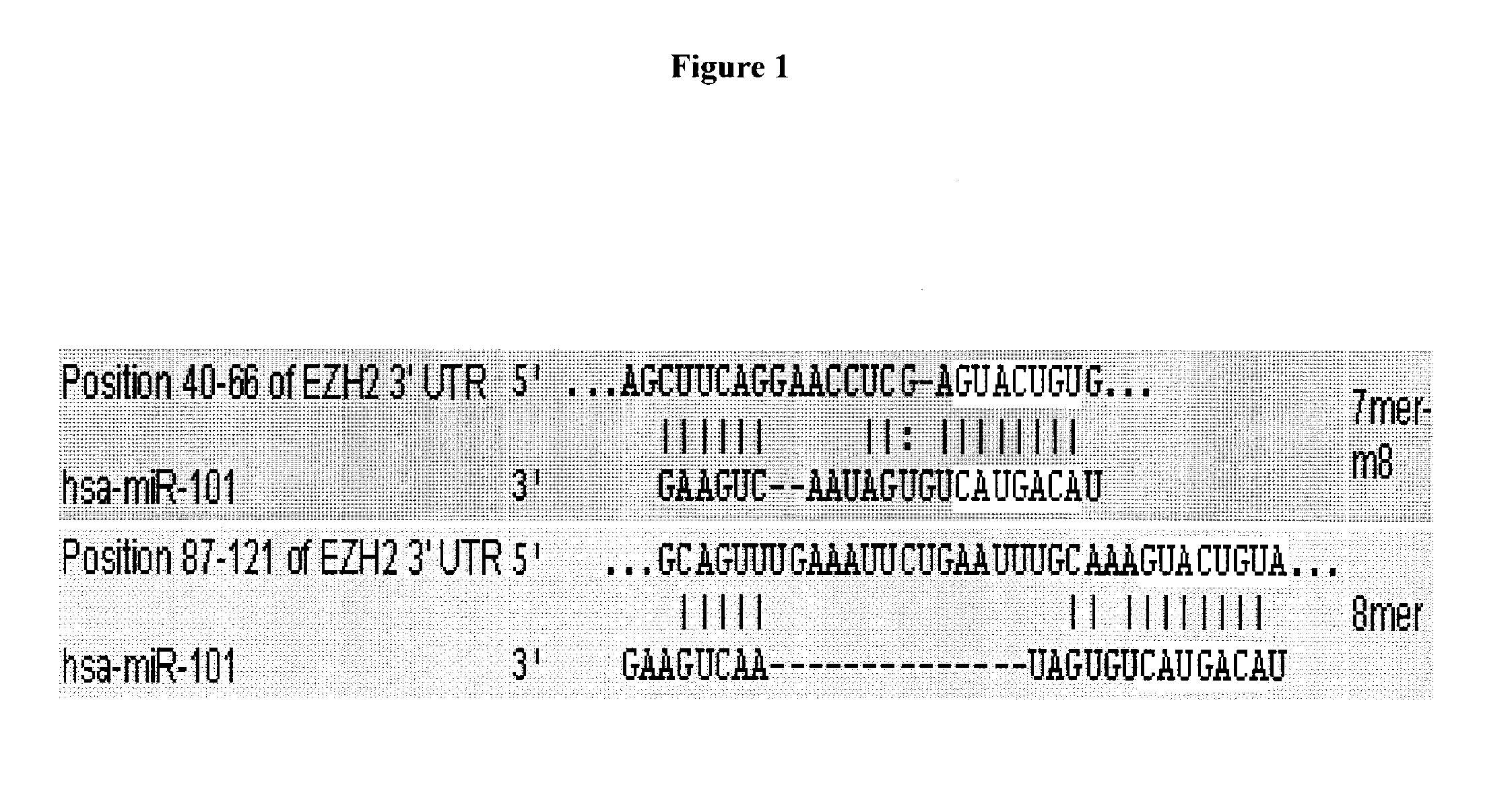
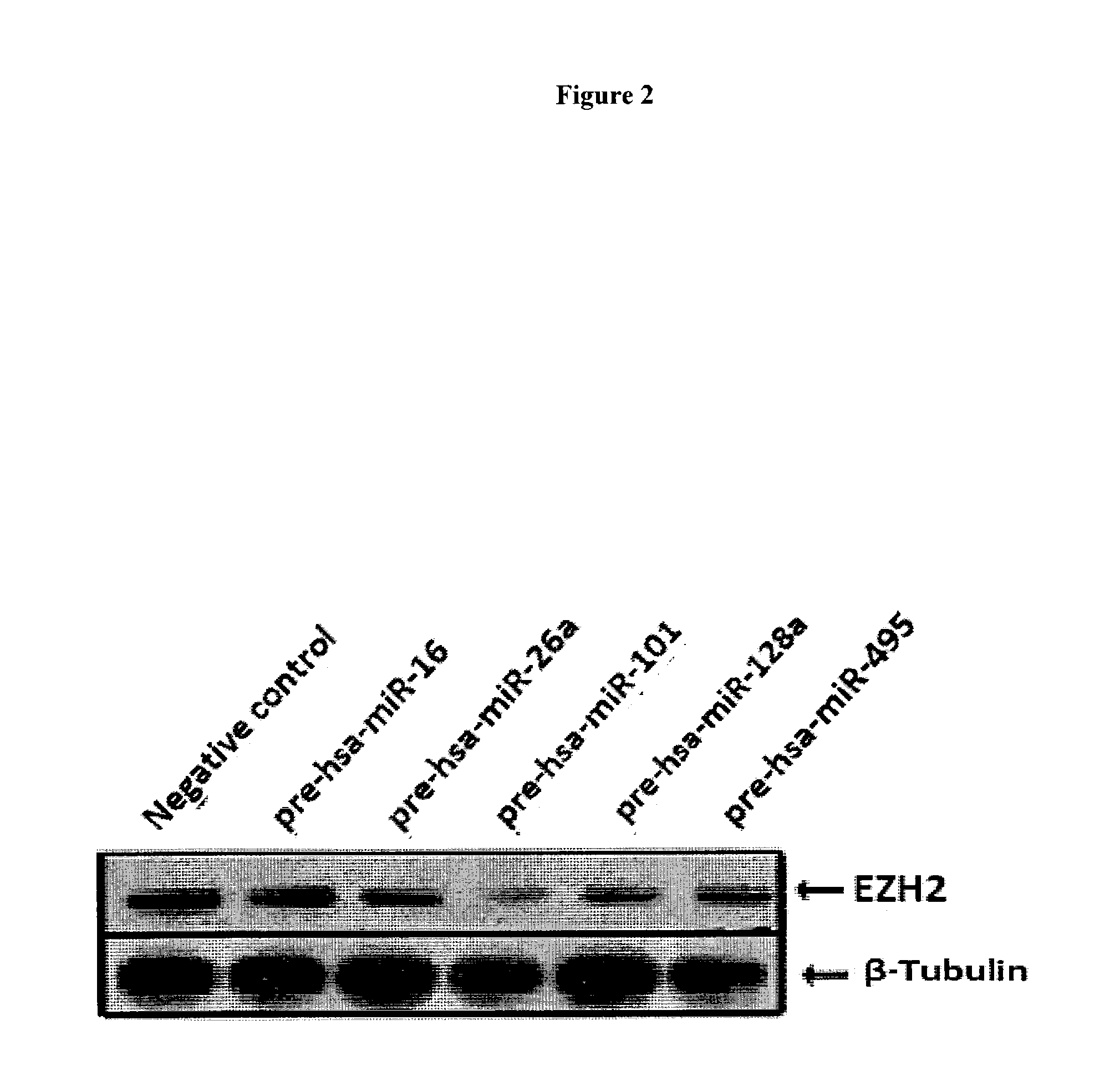
Compositions and methods for inhibiting ezh2
The present invention relates to therapeutic targets for cancer. In particular, the present invention relates to small molecules and nucleic acids that target EZH2 expression in cancer (e.g., prostate cancer, breast cancer, other solid tumors, multiple myeloma).
Owner:RGT UNIV OF MICHIGAN
Copper-catalyzed formation of carbon-heteroatom and carbon-carbon bonds
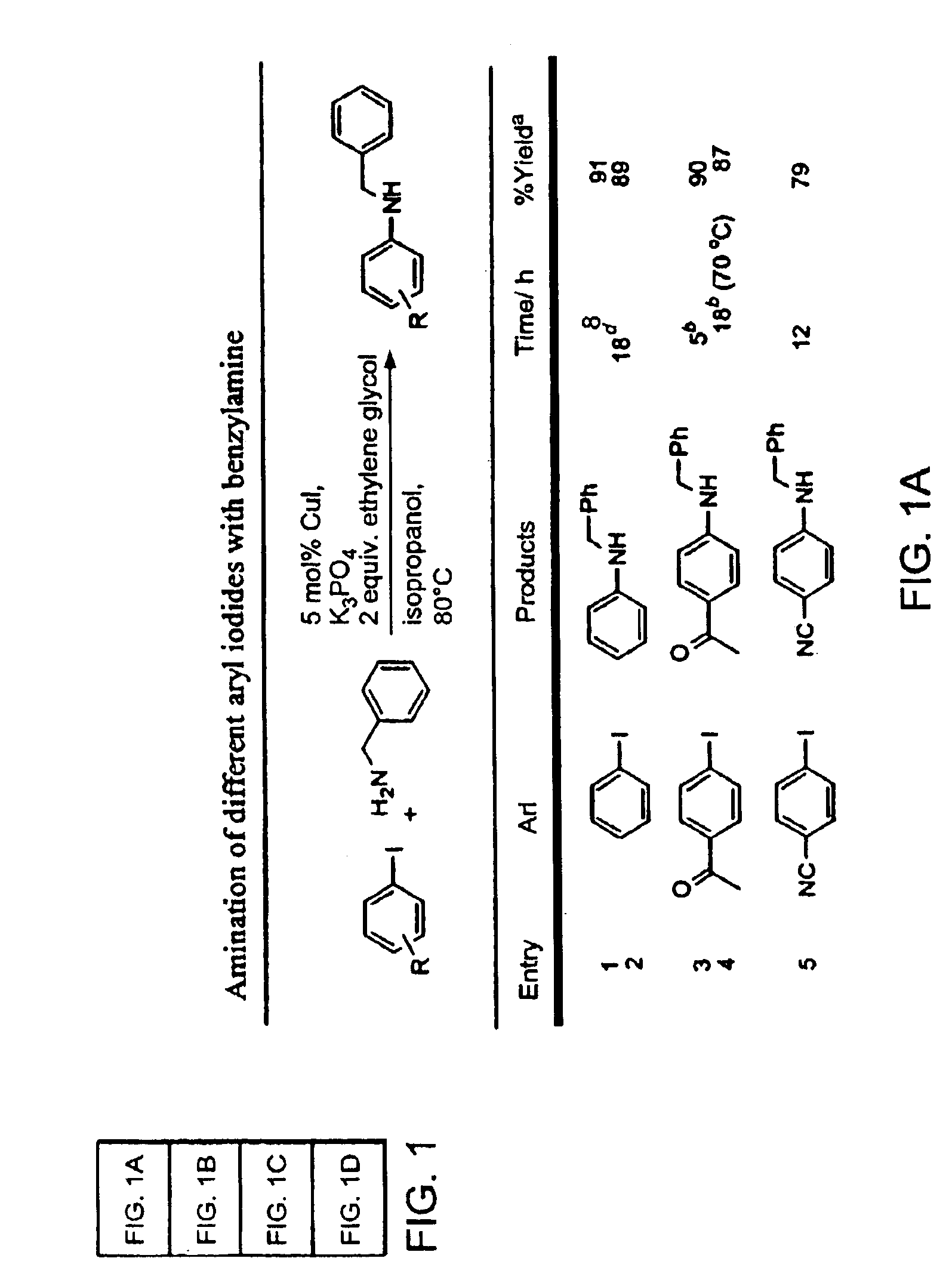
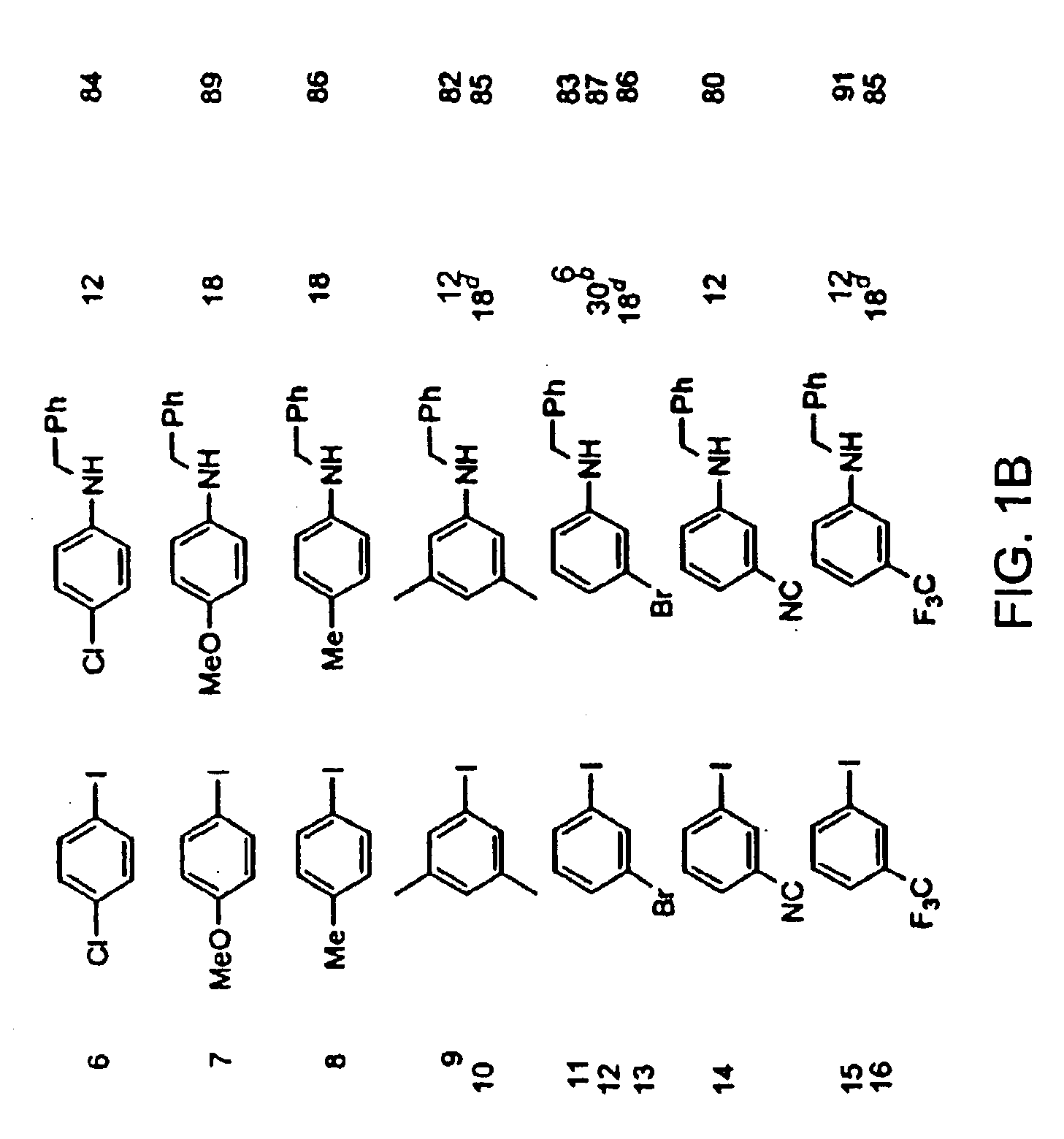
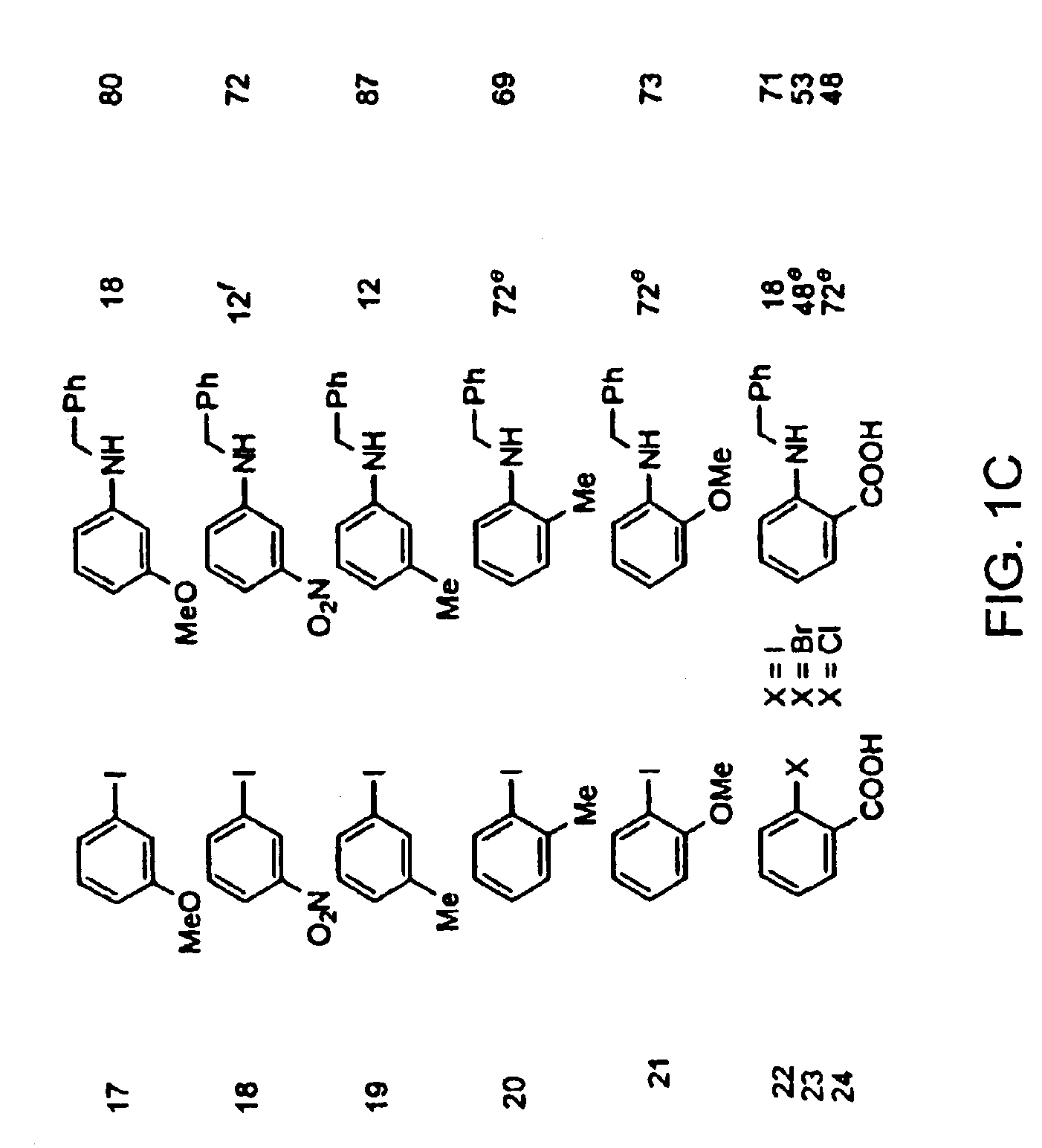
InactiveUS6867298B2Cheap and practicalLow costUrea derivatives preparationCarbamic acid derivatives preparationCarbon–oxygen bondHydrazine compound
The present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide or amine moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In additional embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between a nitrogen atom of an acyl hydrazine and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of a nitrogen-containing heteroaromatic, e.g., indole, pyrazole, and indazole, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-oxygen bond between the oxygen atom of an alcohol and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. The present invention also relates to copper-catalyzed methods of forming a carbon-carbon bond between a reactant comprising a nucleophilic carbon atom, e.g., an enolate or malonate anion, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. Importantly, all the methods of the present invention are relatively inexpensive to practice due to the low cost of the copper comprised by the catalysts.
Owner:MASSACHUSETTS INST OF TECH
Glucagon antagonists/inverse agonists
A novel class of compounds, which act to antagonize the action of the glucagon hormone on the glucagon receptor. Owing to their antagonizing effect of the glucagon receptor the compounds may be suitable for the treatment and / or prevention of any diseases and disorders, wherein a glucagon antagonistic action is beneficial, such as hyperglycemia, Type 1 diabetes, Type 2 diabetes, disorders of the lipid metabolism, such as dyslipidemia, and obesity.
Owner:PFIZER INC
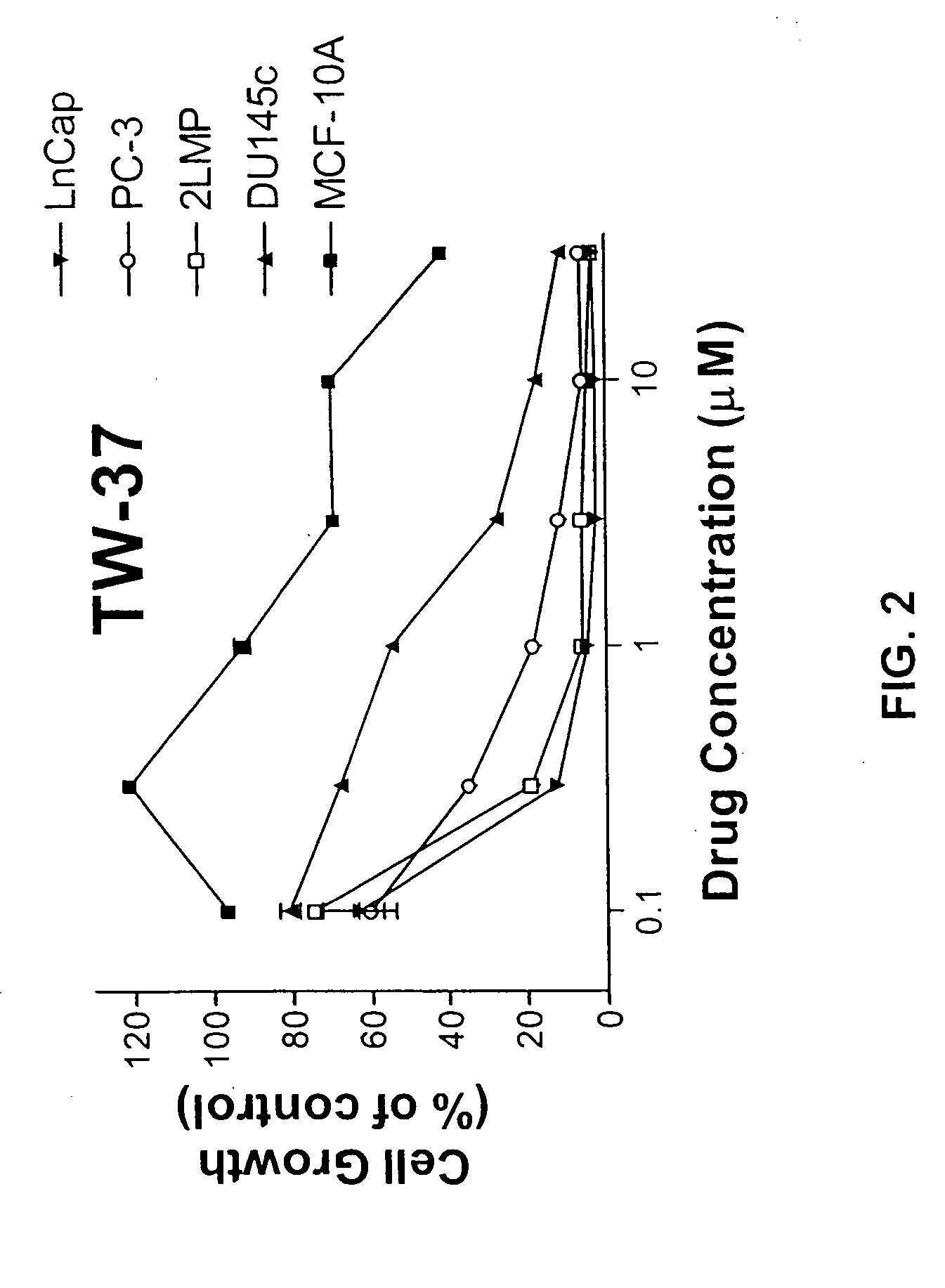
Small molecule inhibitors of anti-apoptotic BCL-2 family members and the uses thereof
ActiveUS20060084647A1Great clinical benefitGreat tumor responseUrea derivatives preparationBiocideSensitized cellBcl-2 Genes
The invention relates to small molecules which function as inhibitors of anti-apoptotic Bcl-2 family member proteins (e.g., Bcl-2 and Bcl-xL). The invention also relates to the use of these compounds for inducing apoptotic cell death and sensitizing cells to the induction of apoptotic cell death.
Owner:RGT UNIV OF MICHIGAN
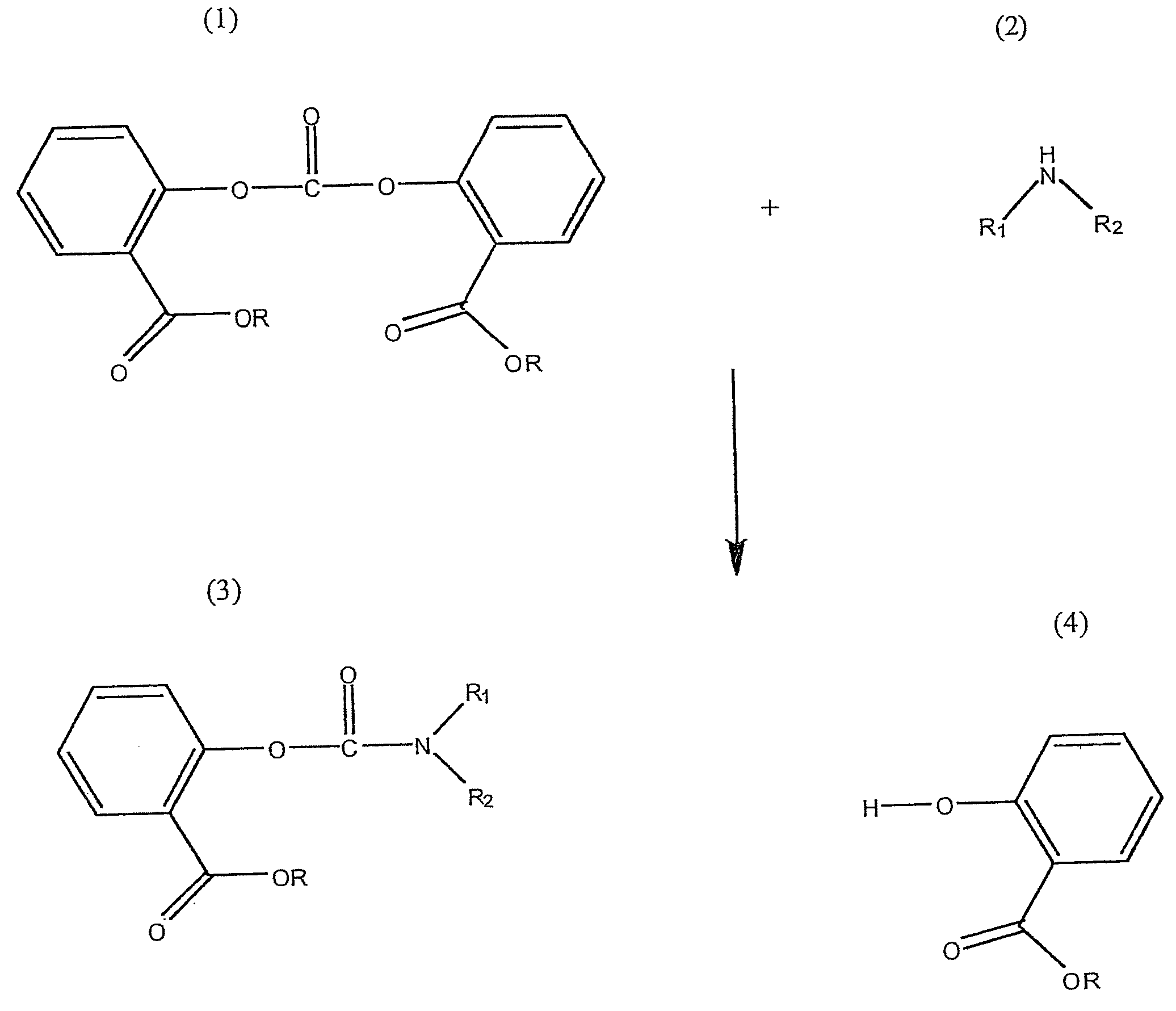
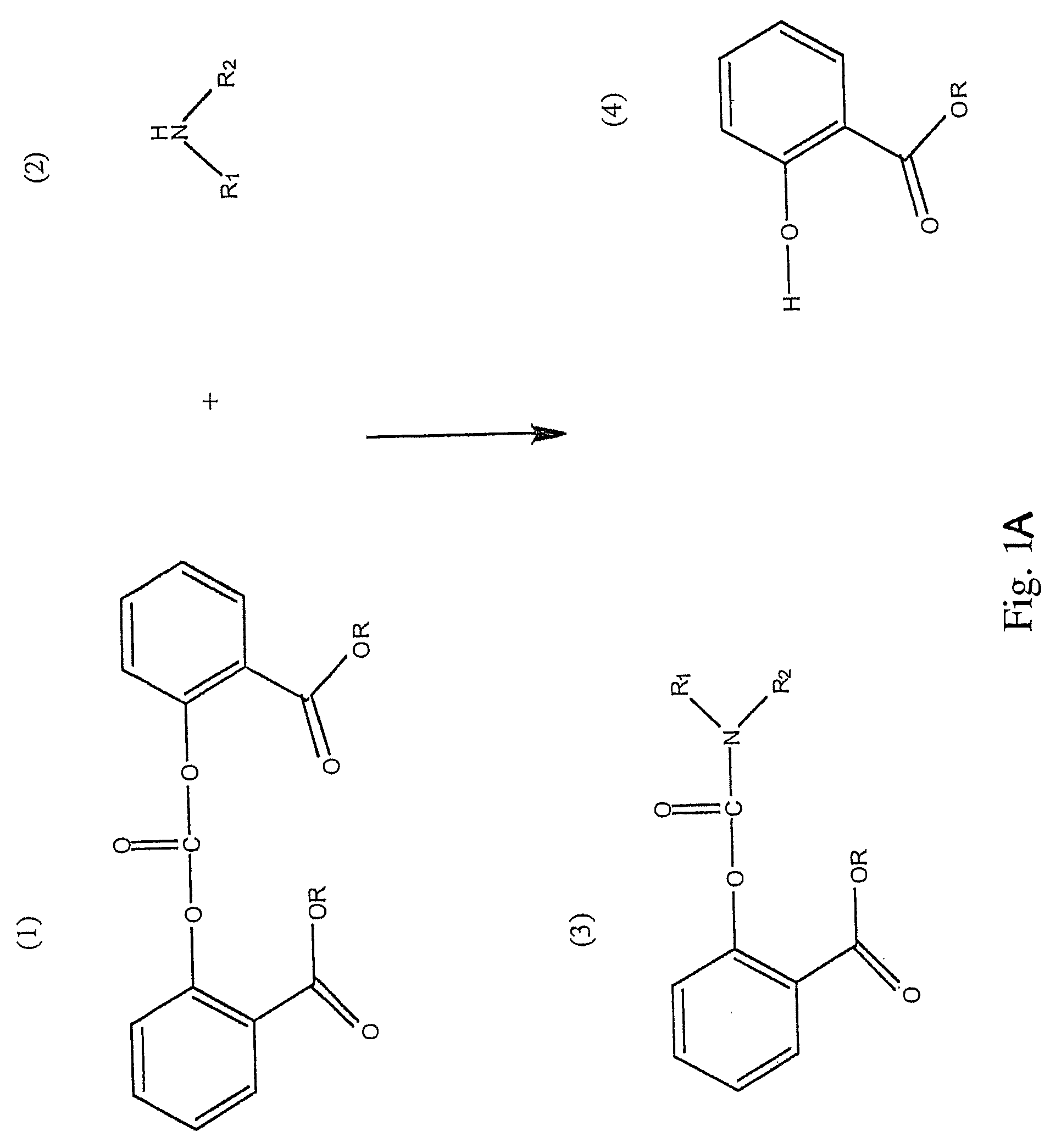
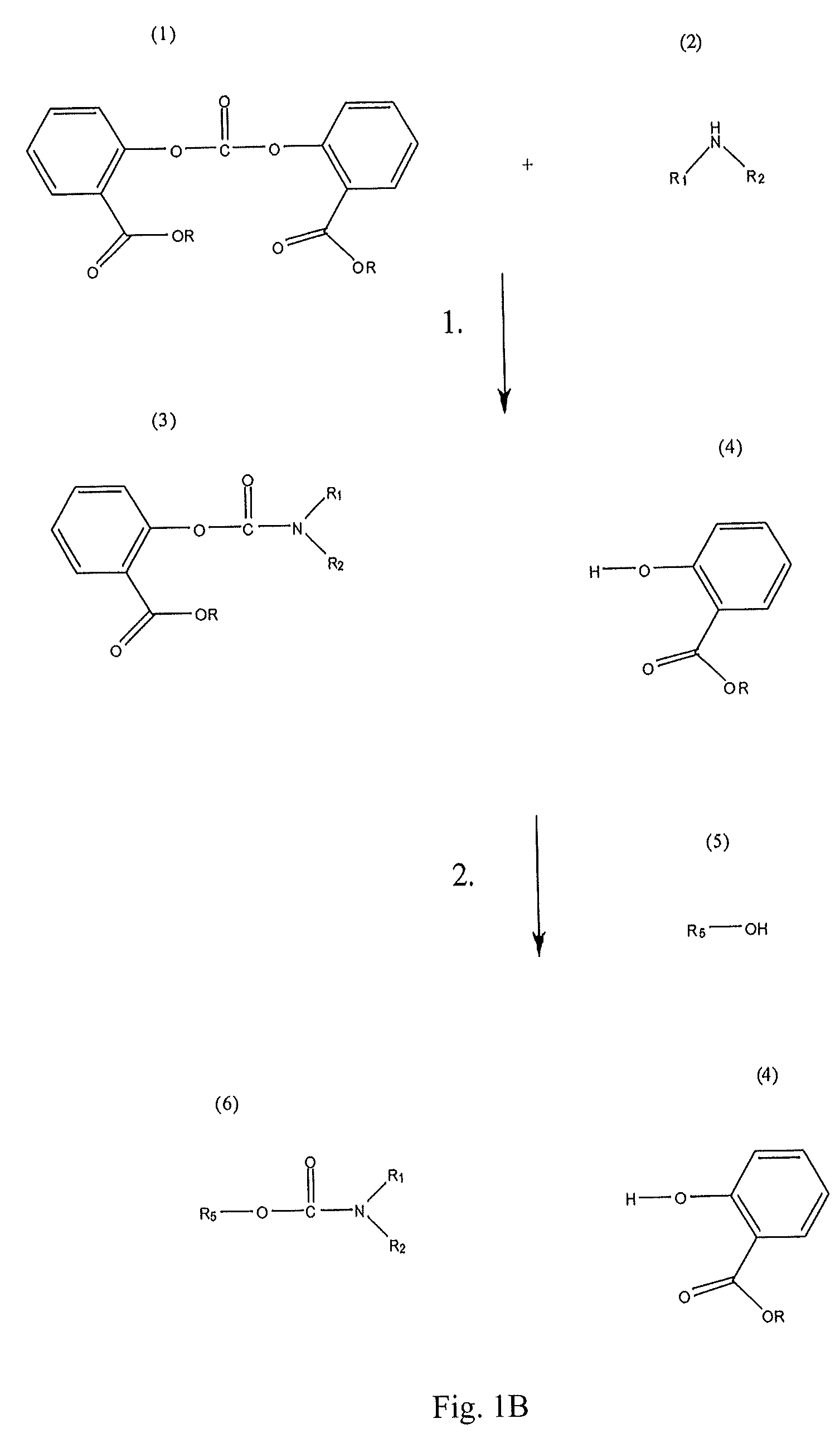
Method for Making Carbamates, Ureas and Isocyanates
InactiveUS20100113819A1Improve production yieldShort timeUrea derivatives preparationCarbamic acid derivatives preparationCarbamatePyrolysis
The present invention provides methods of forming carbamates, ureas, and isocyanates. In certain embodiments these methods include the step of reacting an amine with an ester-substituted diaryl carbonate to form an activated carbamate which can be further derivitized to form non-activated carbamate or a urea. The urea or carbamate can be subjected to a pyrolysis reaction to form isocyanate.
Owner:SABIC GLOBAL TECH BV
Antibacterial agents
InactiveUS20050113450A1Urea derivatives preparationOrganic active ingredientsCombinatorial chemistrySanitation
The present invention relates to antibacterial agents that are useful for sterilization, sanitation, antisepsis, and disinfection
Owner:PHARMACIA & UPJOHN CO
Guanylhydrazones and their use to treat inflammatory conditions
InactiveUS6180676B1Inhibit productionLimit and prevent damageUrea derivatives preparationBiocideDiseaseAbnormal tissue growth
This invention concerns new methods and compositions that are useful in preventing and ameliorating cachexia, the clinical syndrome of poor nutritional status and bodily wasting associated with cancer and other chronic diseases. More particularly, the invention relates to aromatic guanylhydrazone (more properly termed amidinohydrazone) compositions and their use to inhibit the uptake of arginine by macrophages and / or its conversion to urea. These compositions and methods are also useful in preventing the generation of nitric oxide (NO) by cells, and so to prevent NO-mediated inflammation and other responses in persons in need of same. In another embodiment, the compounds can be used to inhibit arginine uptake in arginine-dependent tumors and infections.
Owner:FERRING BV
Exo-R-mecamylamine formulation and use in treatment
InactiveUS20020016370A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationStimulantS syndrome
A pharmaceutical composition includes a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-S-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-S-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
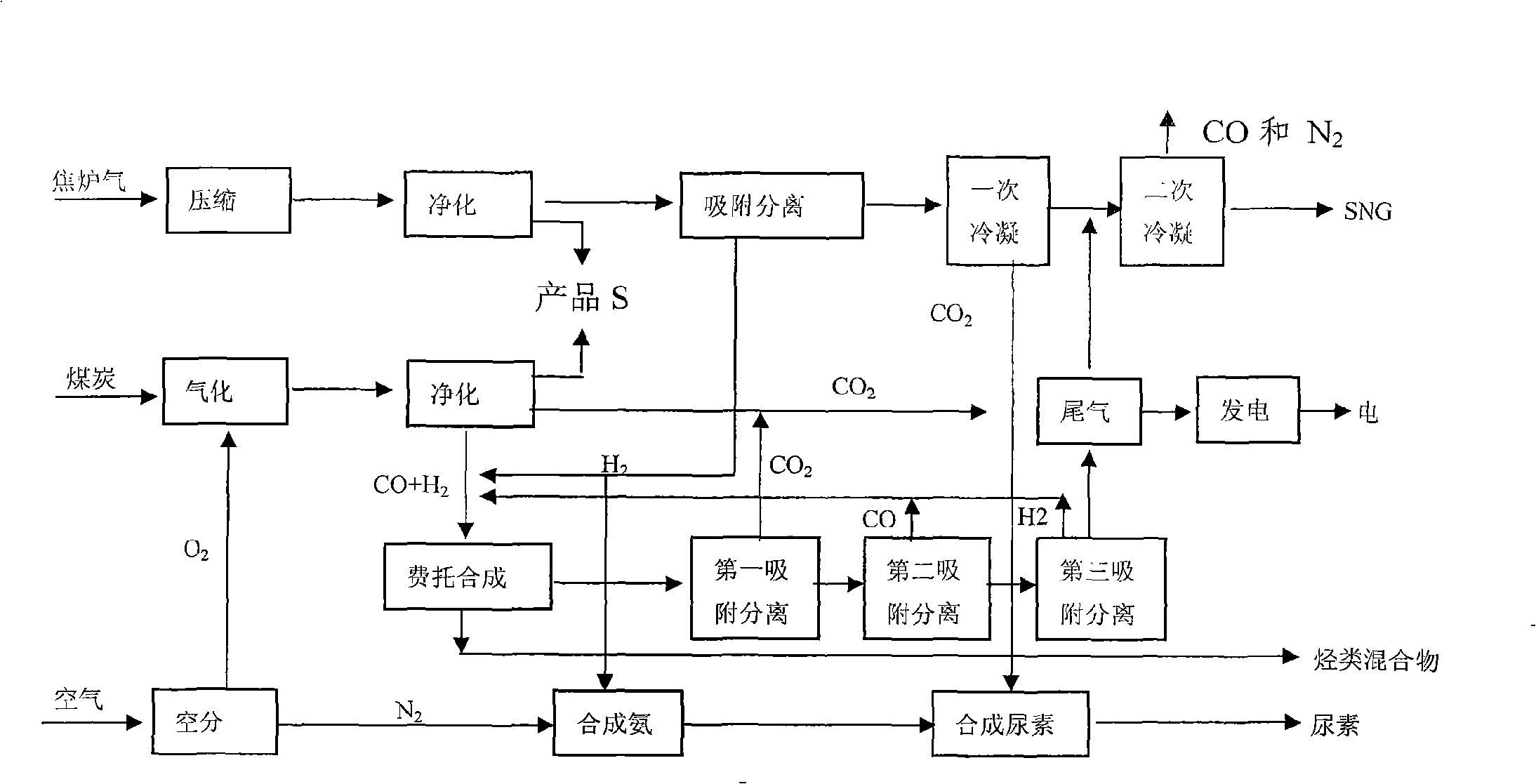
Poly-generation technique for using coal gas and coke oven gas as raw materials
ActiveCN101538483ANo conversionNo transform operationUrea derivatives preparationOrganic compound preparationHydrocarbon mixturesDesorption
The invention relates to a poly-generation technique for using coal gas and coke oven gas as raw materials. The poly-generation technique comprises the following steps of: carrying out mixing on part of H2 prepared by purified water gas and coke oven gas through pressure swing adsorption and tail gas obtained by Fischer-Tropsch synthesis, carrying out Fischer-Tropsch synthesis and obtaining hydrocarbon mixture and tail gas. The CO2 separated from tail gas separate by first pressure swing adsorption enters a urea synthesis unit for reaction, the CO and the hydrogen respectively obtained by separation of second pressure swing adsorption and third pressure swing adsorption of the residual tail gas are circulated back to a Fischer-Tropsch synthesis unit for reaction; and the residual gases can be used for generating power or obtaining SNG by secondary condensation. The coke oven gas enters pressure swing adsorption after being purified and desulphurized so as to separate H2; wherein one part of H2 is used as supplementation of H2 needed by Fischer-Tropsch synthesis and the other part of H2 is mixed with N2 for ammonia synthesis so as to obtain synthetic ammonia; and the synthetic ammonia is mixed with CO2 obtained by the first condensation and CO2 separated from the Fischer-Tropsch synthesis for urea synthesis so as to obtain urea. The CO2 separated from the first condensation of desorption gas by pressure swing adsorption of the coke oven gas is used for urea synthesis; and the residual gases is treated by the second condensation to obtain SNG and mixed gas of CO and N2. The invention has no emission of greenhouse gases, uses richness in carbon and deficiency in hydrogen of the coal gas and the richness in hydrogen and deficiency in carbon of the coke oven gas to carry out complementation, realizes modulation of product structure by Fischer-Tropsch synthesis and improves the economical efficiency of the process of Fischer-Tropsch synthesis.
Owner:中科潞安能源技术有限公司
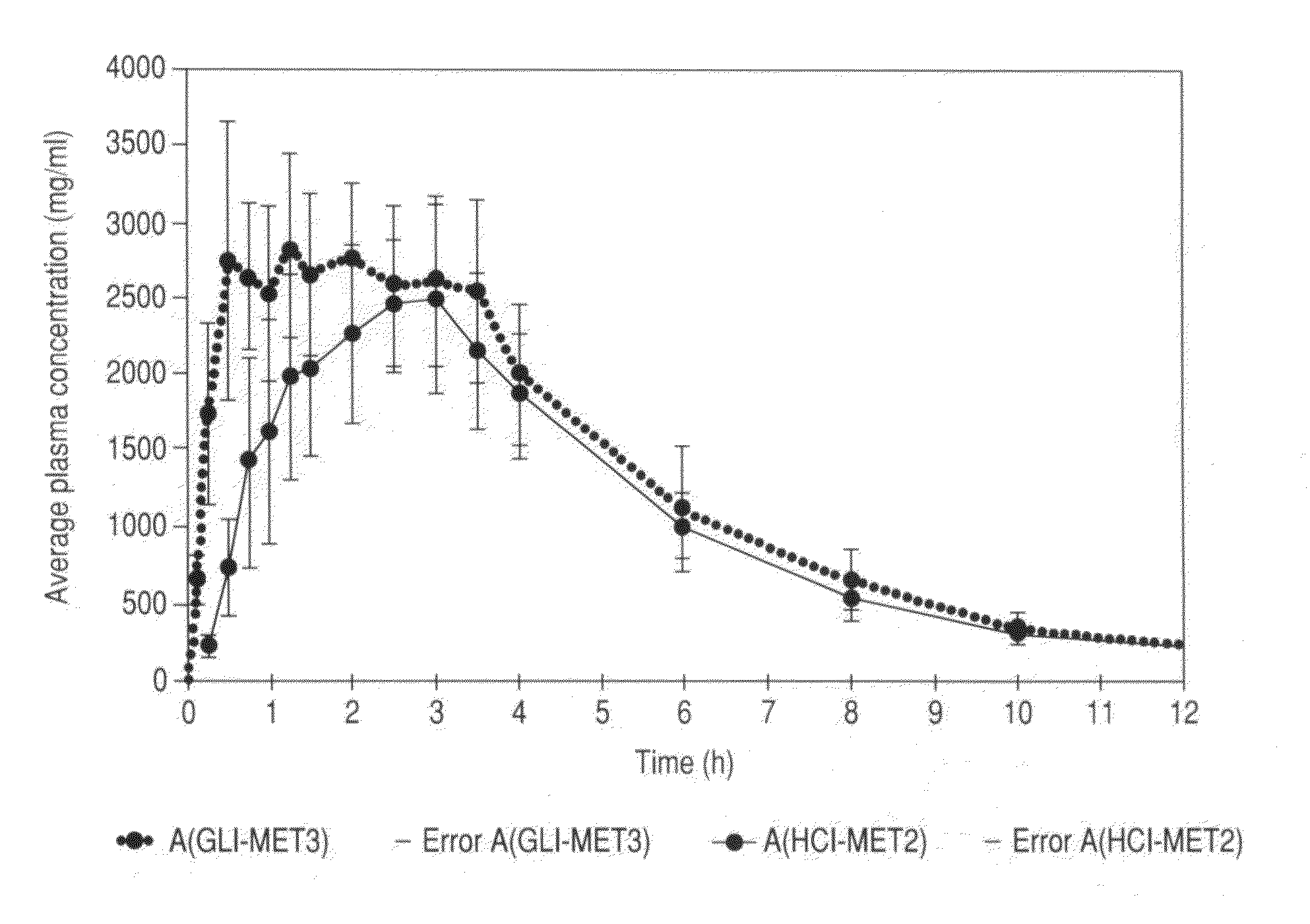
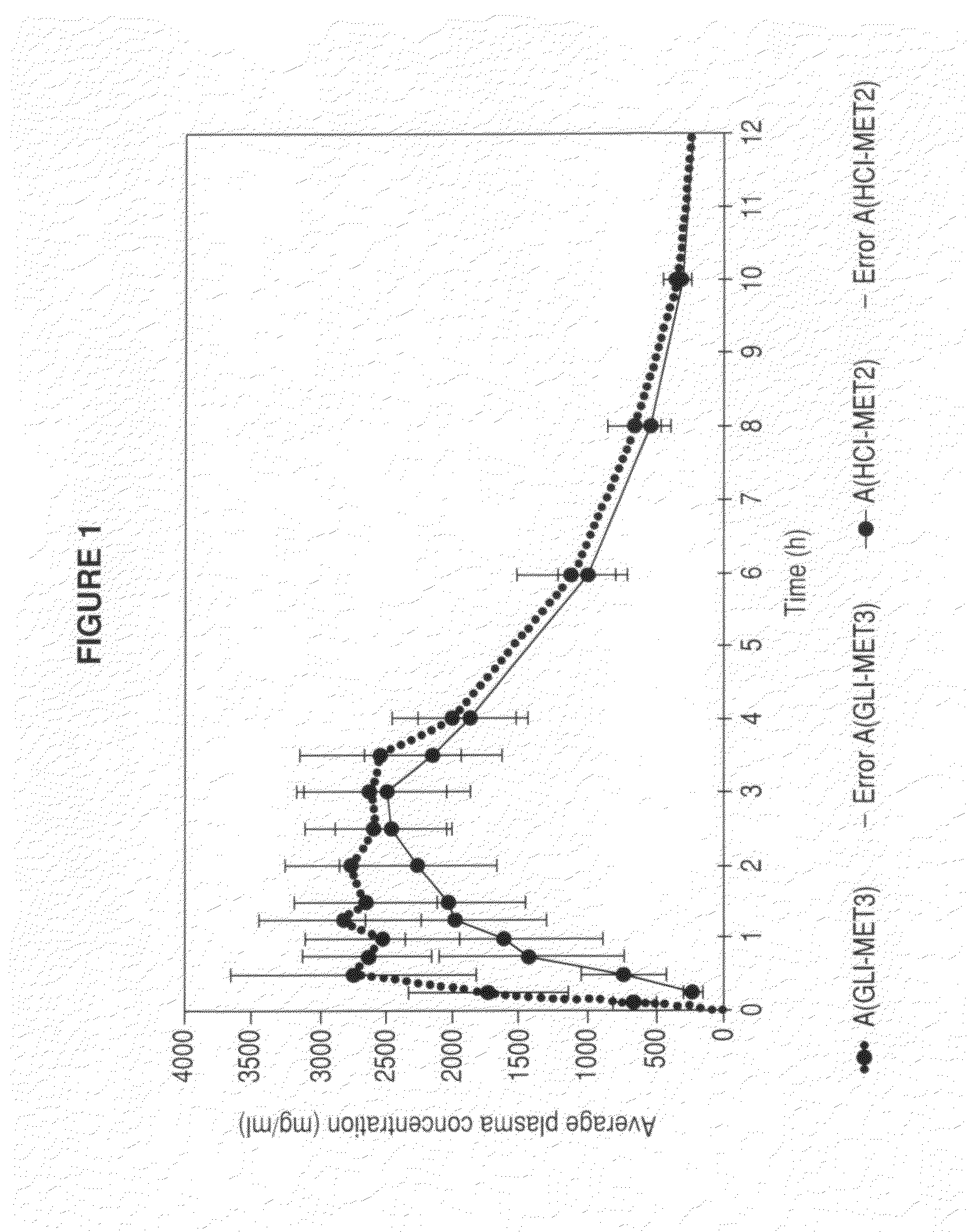
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
Therapeutic agents and methods of use thereof for the modulation of angiogenesis
InactiveUS7037890B2Low toxicityInhibit methionine aminopeptidase 2Urea derivatives preparationOrganic active ingredientsAngiogenesis growth factorDisease cause
The present invention provides angiogenesis inhibitor compounds comprising a MetAP-2 inhibitory core coupled to a peptide, as well as pharmaceutical compositions comprising the angiogenesis inhibitor compounds and a pharmaceutically acceptable carrier. The present invention also provides methods of treating an angiogenic disease, e.g., cancer, in a subject by administering to the subject a therapeutically effective amount of one or more of the angiogenesis inhibitor compounds of the invention.
Owner:GLAXO SMITHKLINE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com