Patents
Literature
1285 results about "Weight gain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Weight gain is an increase in body weight. This can involve an increase in muscle mass, fat deposits, excess fluids such as water or other factors. Weight gain can be a symptom of a serious medical condition.
Methods of using and compositions comprising (+) sibutramine optionally in combination with other pharmacologically active compounds
This invention encompasses methods for the treatment and prevention of disorders that include, but are not limited to, eating disorders; weight gain; obesity; irritable bowel syndrome; obsessive-compulsive disorders; platelet adhesion; apnea; affective disorders such as attention deficit disorders, depression, and anxiety; male and female sexual function disorders; restless leg syndrome; osteoarthritis; substance abuse including nicotine and cocaine addiction; narcolepsy; pain such as neuropathic pain, diabetic neuropathy, and chronic pain; migraines; cerebral function disorders; chronic disorders such as premenstrual syndrome; and incontinence. The invention further encompasses pharmaceutical compositions and dosage forms which comprise optically pure (+) sibutramine, optionally in combination with a phosphodiesterase inhibitor or a lipase inhibitor.
Owner:SEPACOR INC
Methods of treating and/or suppressing weight gain
Novel methods for the medical treatment and / or prevention of obesity, abdominal fat, and insulin resistance in susceptible warm-blooded animals including humans involves the administration of selective estrogen receptor modulators (SERMs). A combination of a SERM with an amount of estrogen or a sex steroid precursor selected from the group consisting of dehydroepiandrosterone, dehydroepiandrosterone sulfate, androst-5-ene-3b,17b-diol and compounds converted in vivo to one of the foregoing precursors or estrogen is also disclosed.
Owner:ENDORES & DEV
Smoking cessation treatments using naltrexone and related compounds
InactiveUS6541478B1Reduce weight gainPrevent relapseBiocideNervous disorderOpioid antagonistAntianxiety Agent
Nicotine dependency is treated by administration of an opioid antagonist. In some embodiments, rapid or ultra rapid detoxification techniques include using a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone or naltrexone or a mixture of any one of these, and either clonidine or related compounds either while awake, or while under sedation or anesthesia, followed by continued administration of an effective amount of an opioid antagonist with or without agents that enhance nicotine dependency treatment. Persons are also treated for nicotine dependency with more gradual detoxification methods using administration of a combination of an effective amount of an opioid antagonist such as nalmefene, naloxone, naltrexone, or a mixture of any of these, and an effective amount of agents used to treat nicotine withdrawal including nicotine, such as that delivered by a nicotine patch, nicotine chewing gum, nicotine inhaler or other methods for delivering nicotine, antidepressants and antianxiety agents, and / or clonidine and related compounds. Administration of an effective amount of an opioid antagonist to prevent relapse, attenuate craving, and reduce weight gain during and after treatment for nicotine dependency is continued in some embodiments.
Owner:YALE UNIV
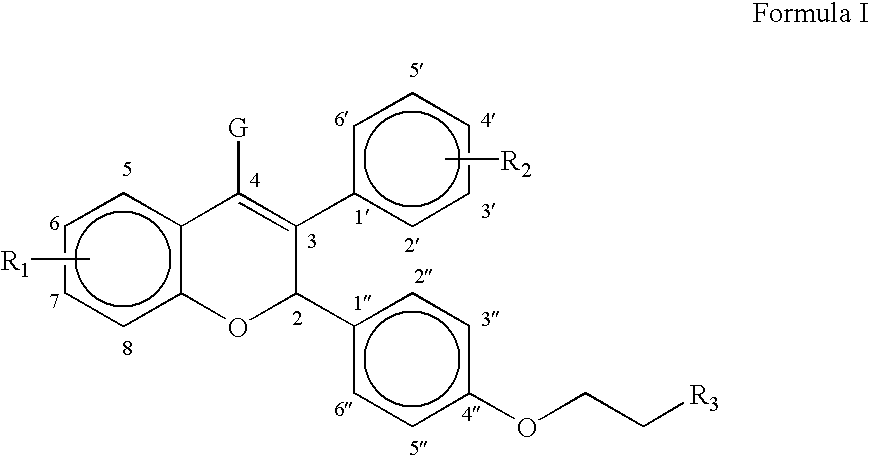
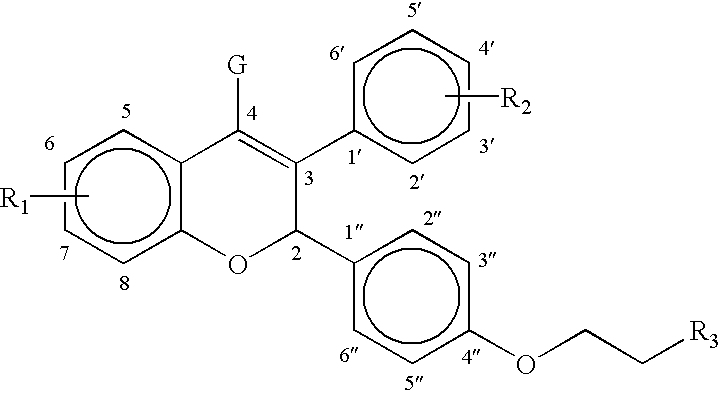
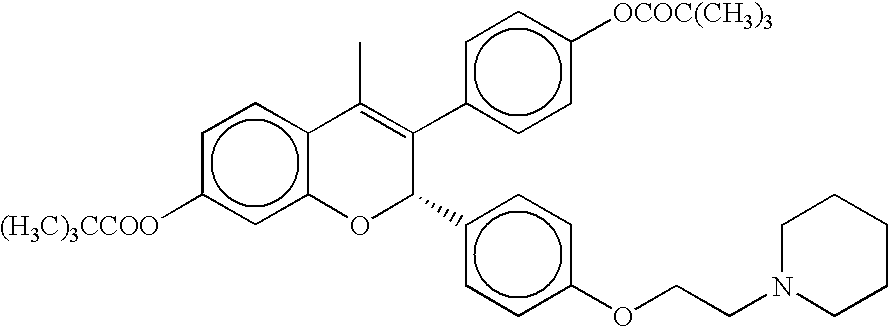
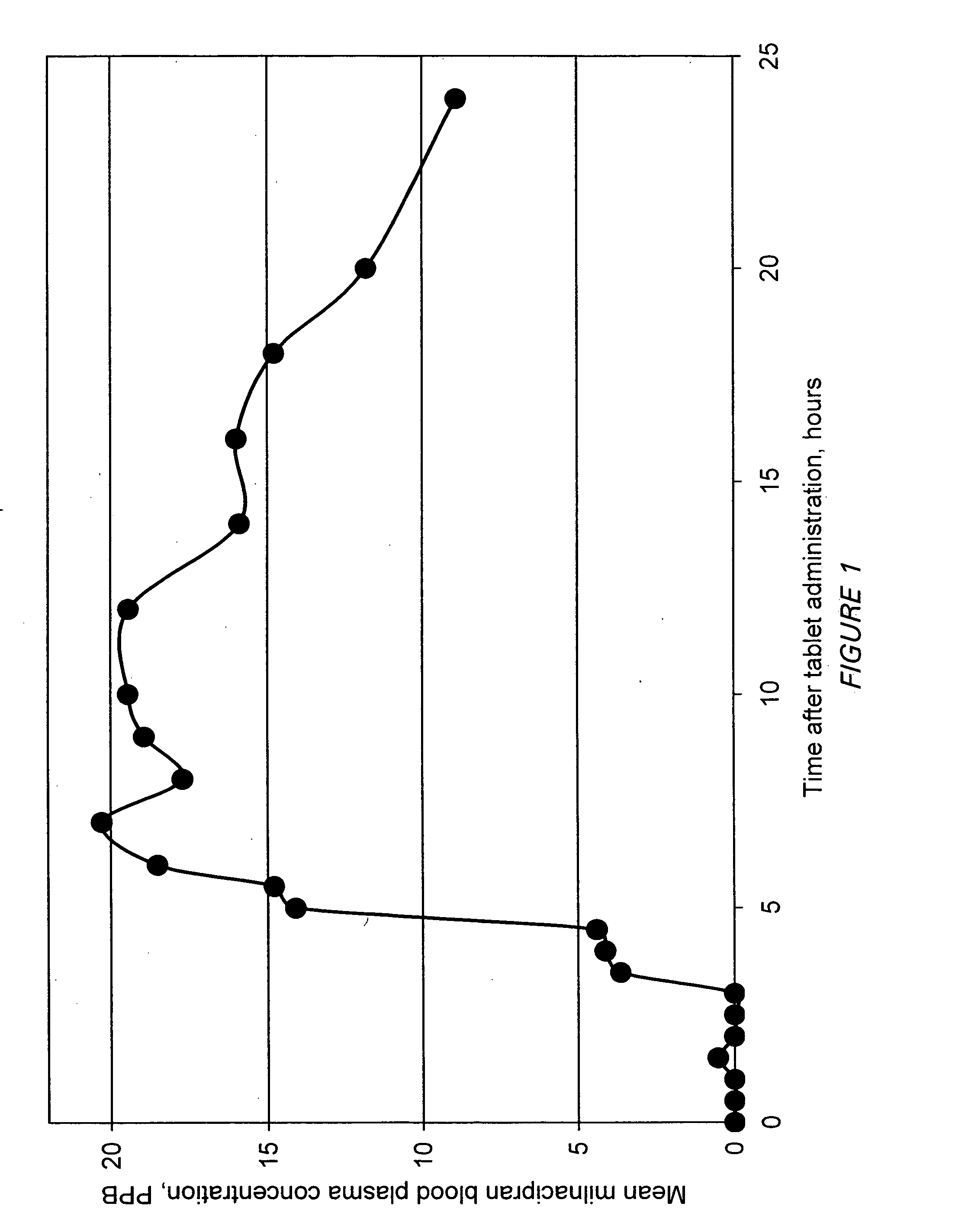
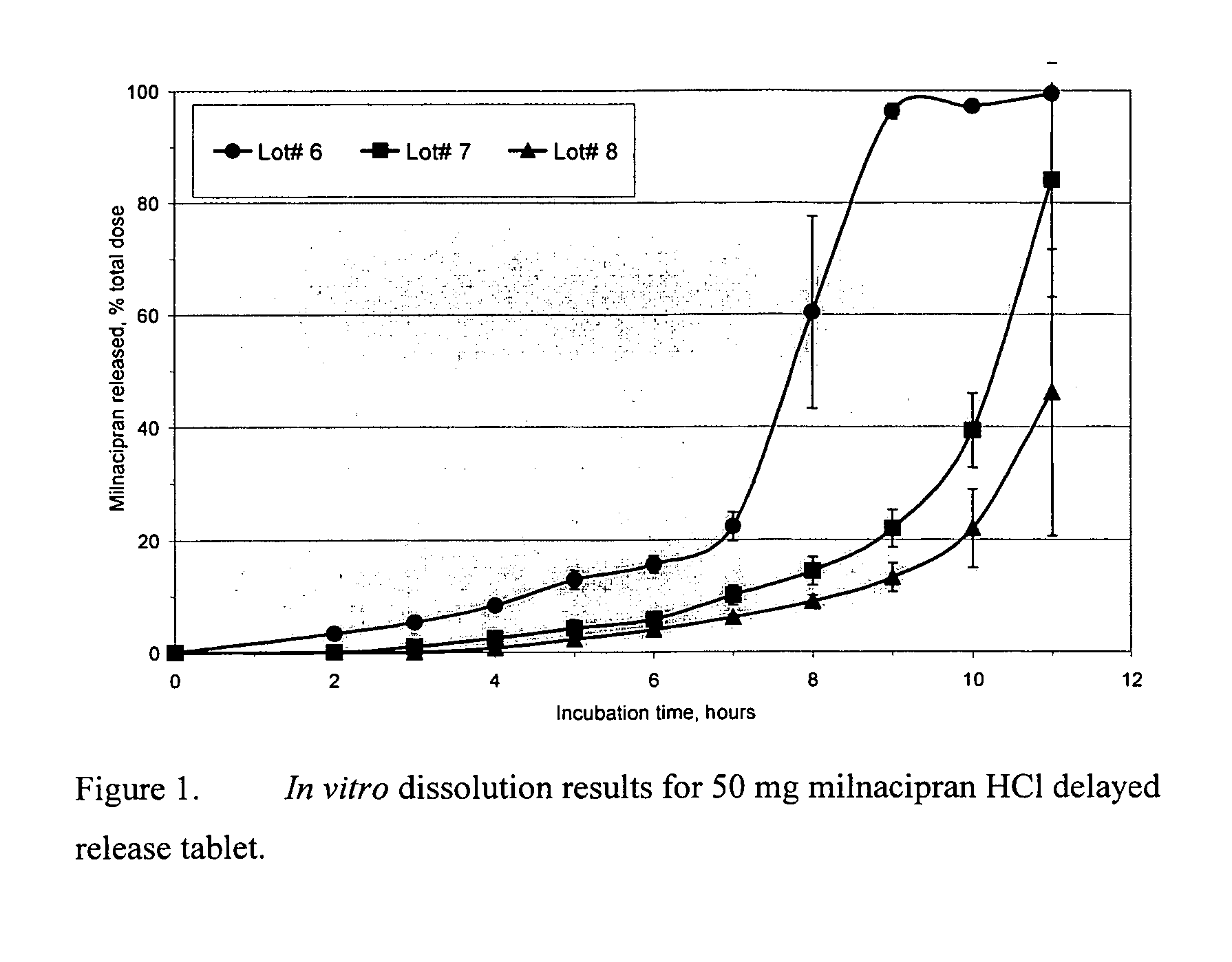
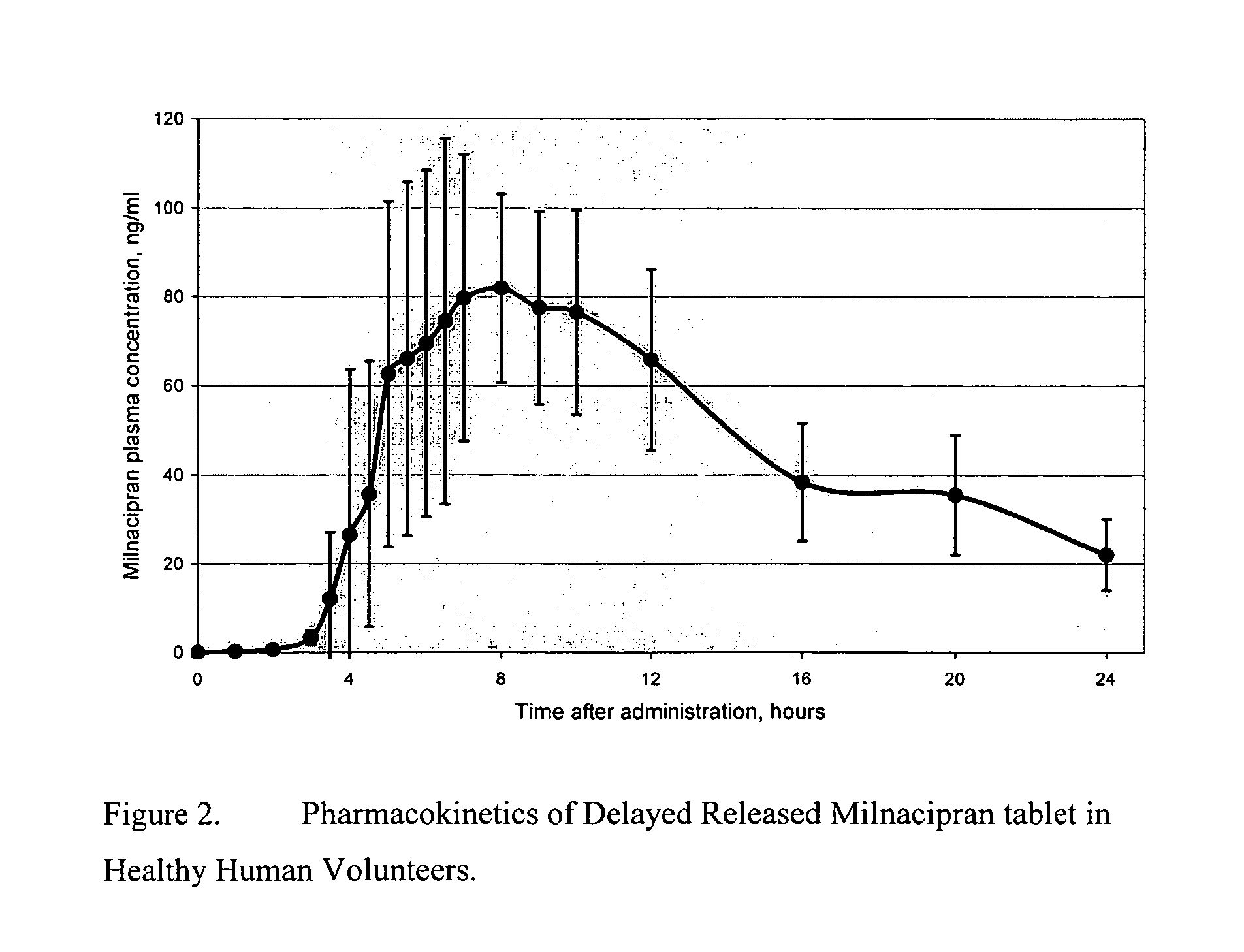
Modified release compositions of milnacipran
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Long-acting growth hormone and methods of producing same
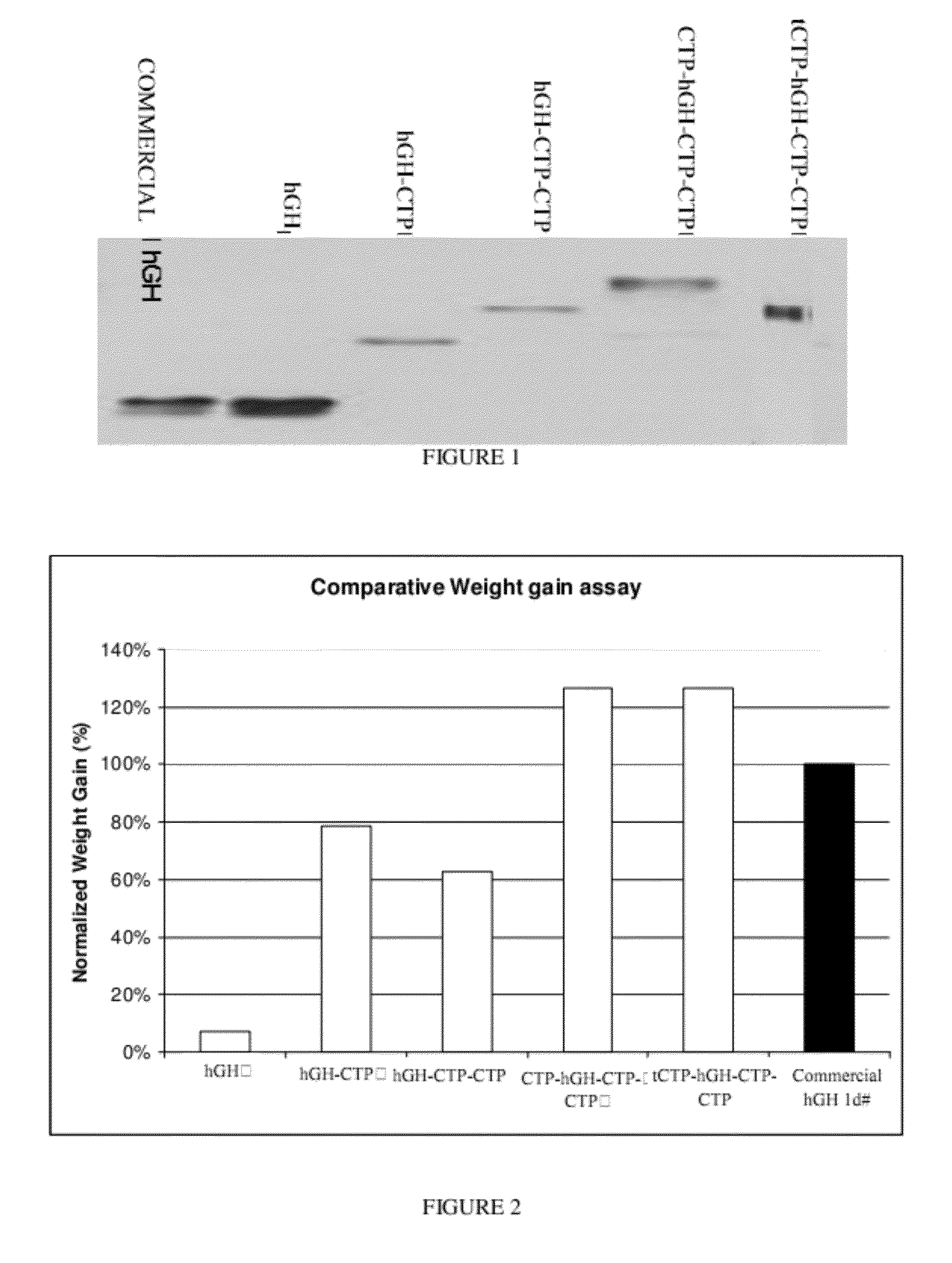
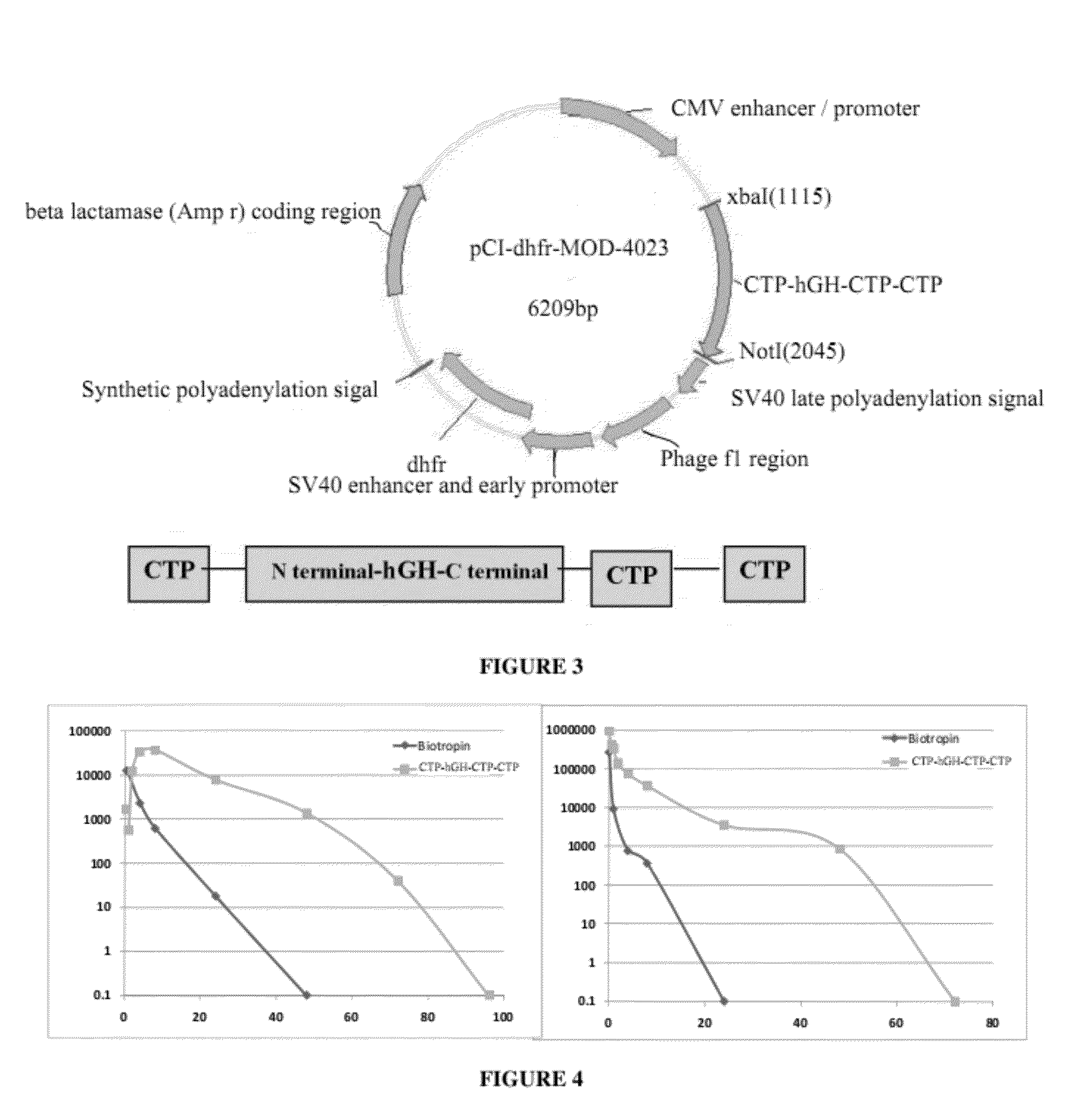
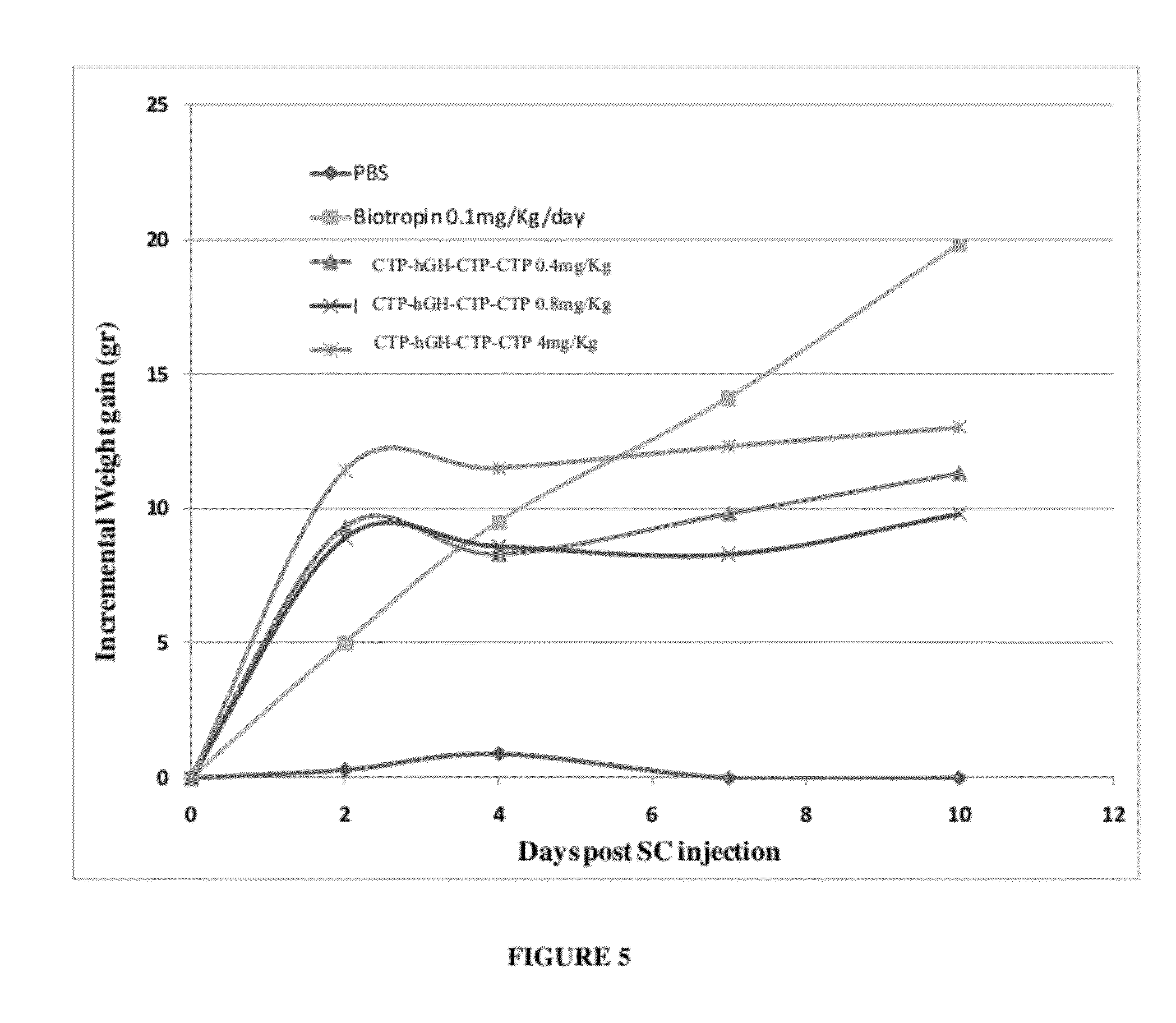
ActiveUS20120035101A1Decreasing body fatReduce weight lossPeptide/protein ingredientsMetabolism disorderSomatotropic hormoneNucleotide
Use of a growth hormone protein and polynucleotides encoding same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing growth or weight gain, method of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Combinations of 5-ht2a inverse agonists and antagonists with antipsychotics
InactiveUS20090053329A1Achieve effectQuick effectCompounds screening/testingBiocideSide effectAntipsychotic drug therapy
Combinations of 5-HT2A inverse agonists or antagonists such as pimavanserin with antipsychotics such as risperidone are shown to induce a rapid onset of antipsychotic action and increase the number of responders when compared to therapy with the antipsychotic alone. These effects can be achieved at a low dose of the antipsychotic, thereby reducing the incidence of side effects. The combinations are also effective at decreases the incidence of weight gain and increased glucose or prolactin levels caused by the antipsychotic.
Owner:ACADIA PHARMA INC
High gain, multi-beam antenna for 5g wireless communications
ActiveUS20170324171A1Improve directivityImprove performanceSimultaneous aerial operationsAntenna supports/mountingsFifth generationWeight gain
A high gain, multi-beam lens antenna system for future fifth generation (5G) wireless networks. The lens antenna includes a spherical dielectric lens fed with a plurality of radiating antenna elements. The elements are arranged around the exterior surface of the lens at a fixed offset with a predetermined angular displacement between each element. The number of beams and crossover levels between adjacent beams are determined by the dielectric properties and electrical size of the lens. The spherical nature of the dielectric lens provides a focal surface allowing the elements to be rotated around the lens with no degradation in performance. The antenna system supports wideband and multiband operation with multiple polarizations making it ideal for future 5G wireless networks.
Owner:AMPHENOL ANTENNA SOLUTIONS
Hydroxybutyrate ester and medical use thereof
A compound which is 3-hydroxybutyl 3-hydroxybutyrate enantiomerically enriched with respect to (3R)-hydroxybutyl (3R)-hydroxybutyrate of formula (I) is an effective and palatable precursor to the ketone body (3R)-hydroxybutyrate and may therefore be used to treat a condition which is caused by, exacerbated by or associated with elevated plasma levels of free fatty acids in a human or animal subject, for instance a condition where weight loss or weight gain is implicated, or to promote alertness or improve cognitive function, or to treat, prevent or reduce the effects of neurodegeneration, free radical toxicity, hypoxic conditions or hyperglycaemia.
Owner:UNITED STATES OF AMERICA +1
Compositions and methods for modulating metabolic pathways
ActiveUS20130017283A1Increasing sirtuin-pathway outputIncrease productionBiocideHydroxy compound active ingredientsIsocaproic acidFatty acid
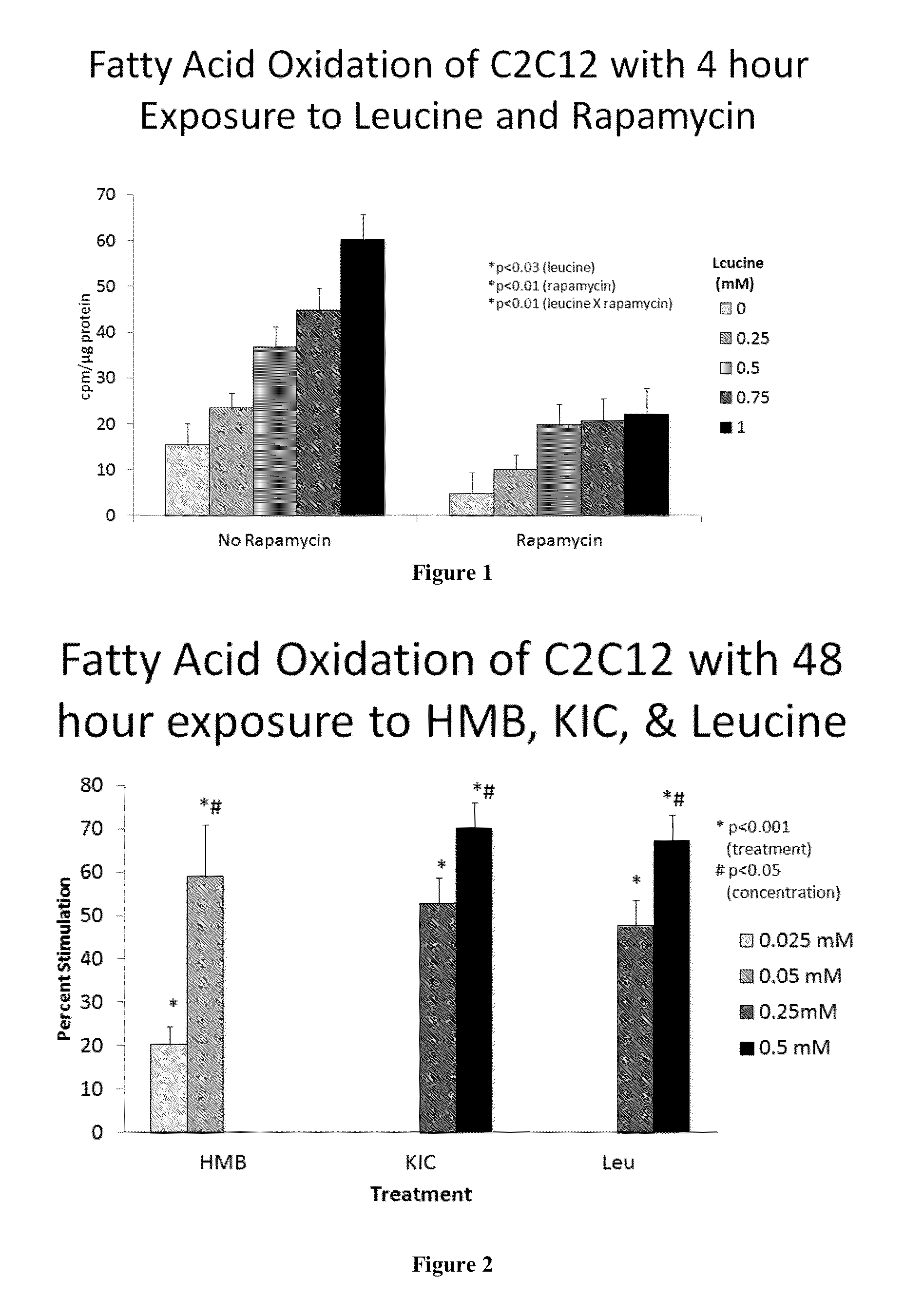
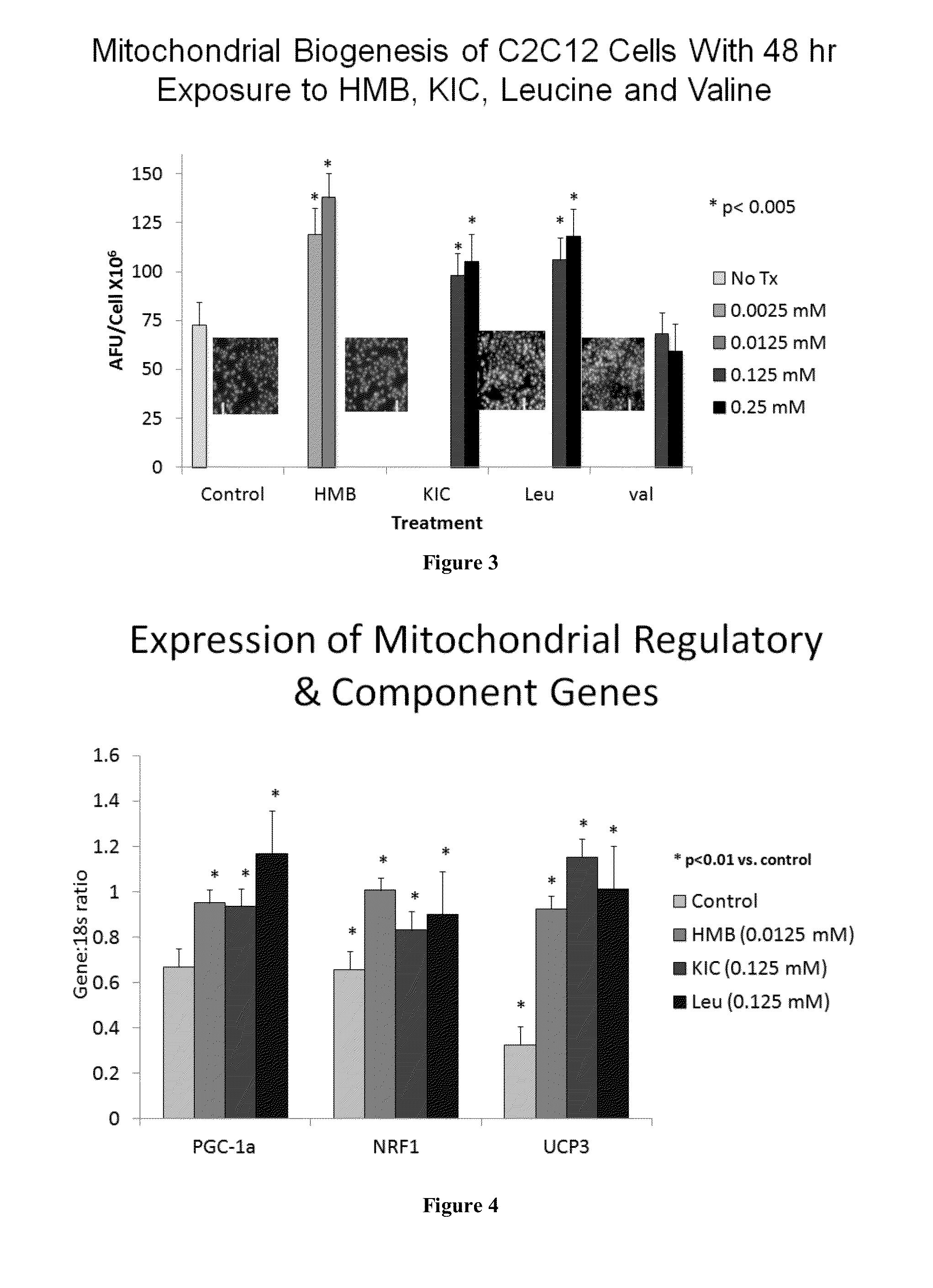
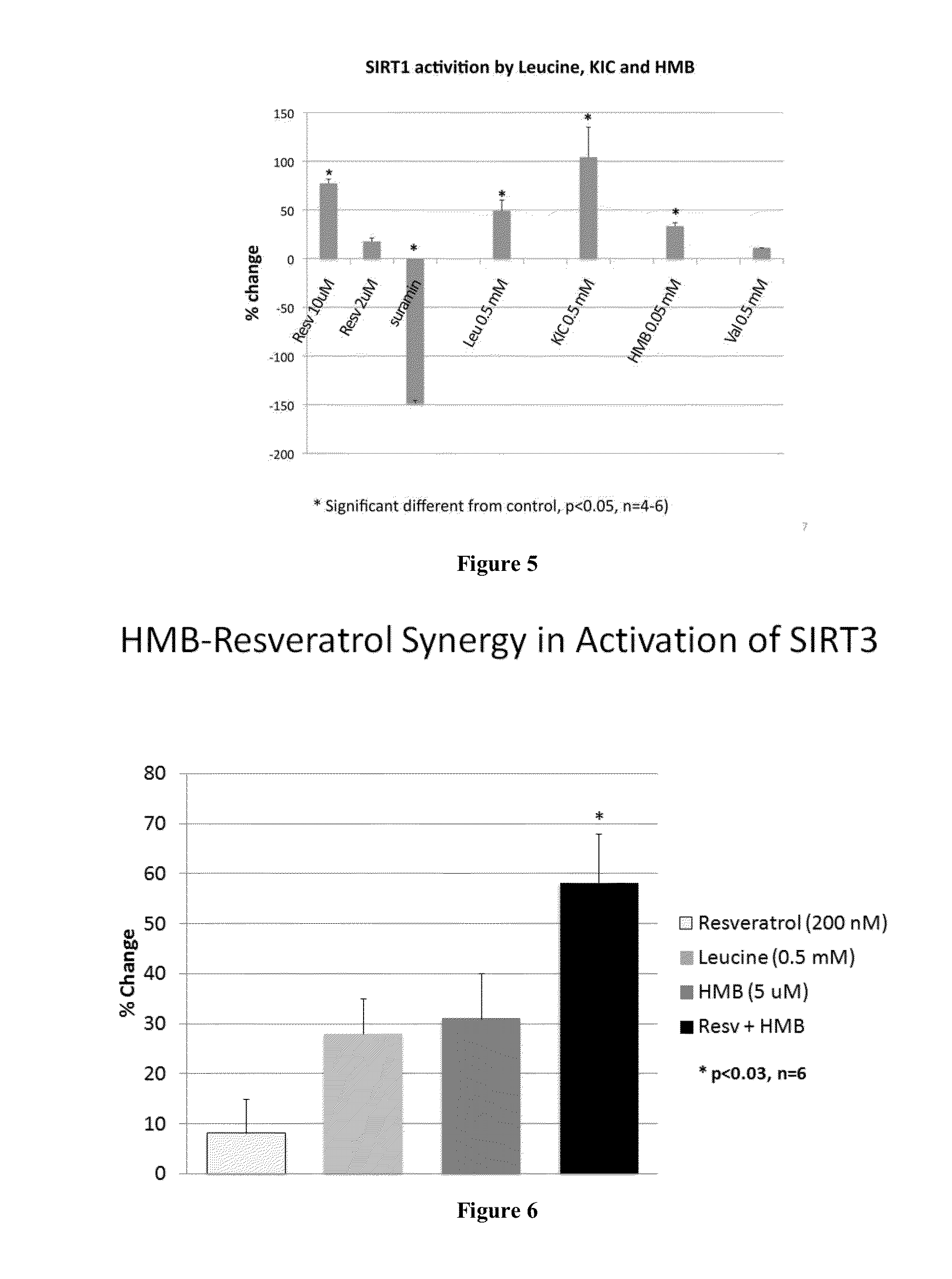
Compositions and methods useful for inducing an increase in fatty acid oxidation or mitochondrial biogenesis, reducing weight gain, inducing weight loss, or increasing Sirt1, Sirt3, or AMPK activity are provided herein. Such compositions can contain synergizing amounts of a sirtuin-pathway activators, including but not limited to resveratrol, in combination with beta-hydroxymethylbutyrate (HMB), keto isocaproic acid (KIC), leucine, or combinations of HMB, KIC and leucine.
Owner:NUSIRT SCI
Derivatives of (-)-venlafaxine and methods of preparing and using the same
InactiveUS6342533B1Potent activityReducing and avoiding adverse effectBiocideNervous disorderDementiaWeight gain
Methods of preparing, and compositions comprising, derivatives of (-)-venlafaxine are disclosed. Also disclosed are methods of treating and preventing diseases and disorders including, but not limited to, affective disorders such as depression, bipolar and manic disorders, attention deficit disorder, attention deficit disorder with hyperactivity, Parkinson's disease, epilepsy, cerebral function disorders, obesity and weight gain, incontinence, dementia and related disorders.
Owner:SUNOVION PHARMA INC
Acylated glucagon analogues
InactiveUS20120178670A1Avoid weight gainGood for weight lossPeptide/protein ingredientsAntipyreticDiabetes mellitusWeight gain prevention
The invention provides materials and methods for promoting weight loss or preventing weight gain, and in the treatment of diabetes and associated metabolic disorders. In particular, the invention provides novel acylated glucagon analogue peptides effective in such methods. The peptides may mediate their effect by having increased selectivity for the GLP-1 receptor as compared to human glucagon.
Owner:ZEALAND PHARM AS
Computerized repetitive-motion exercise logger and guide system
This system includes a self-contained, non-invasive system for collecting work and power performance data on nearly any kind of repetitive-motion exercise. See the glossary for definitions of terms used in this specification. It is non-invasive in that no permanent modifications need made to existing exercise equipment. It is a battery-powered system that remotely senses iterations of a moving part or body member, and records them with a time-stamp. It also records weight and distance of travel if they are input-but for some exercises this function may be deemed not necessary, or ignored. If time, weight, and distance are known then work and power metrics can be calculated on the data when it is analyzed on a host computer. This data can then be graphed to provide comparisons from workout session to workout session. The user can detect trends, and differences in performance as workout variables are manipulated. Workout variables include, but are not limited to: weight, distance of travel, time, order of exercise stations, number of sets, number of repetitions per set, pattern of weight increase / decrease for a given exercise station over the sets, pattern of extension and contraction per repetition, etc.
Owner:WARNER RICHARD D
Novel indoles compound having anti-tumor metastasis activity and anti-inflammatory activity, as well as synthesis and application of novel indoles compound
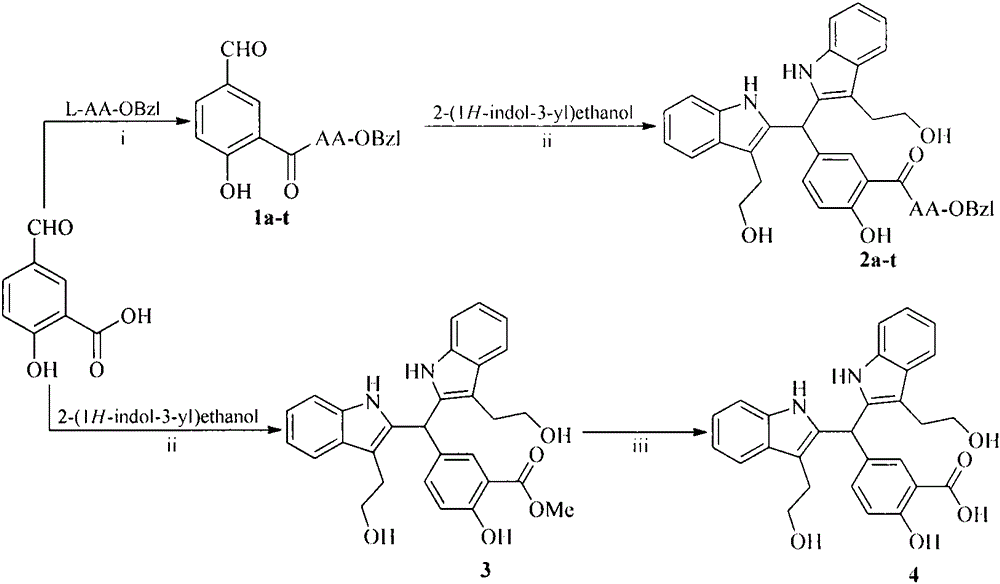
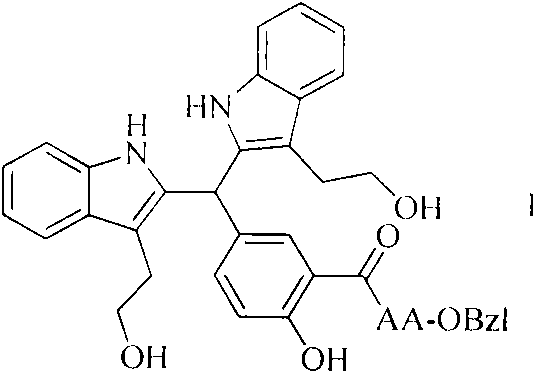
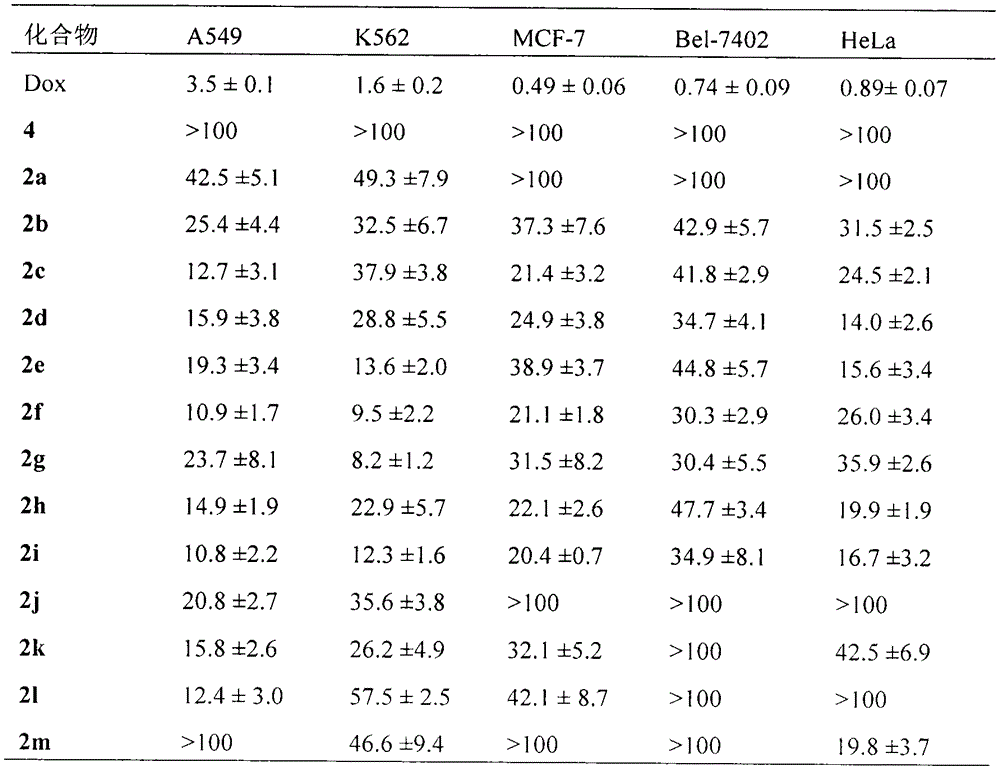
The invention discloses 20 kinds of 5-(bi(3-(2-ethoxy)-1H-indol-2)methyl)-2-hydroxy benzoyl-AA-OBzl, discloses a preparation method of the 5-(bi(3-(2-ethoxy)-1H-indol-2)methyl)-2-hydroxy benzoyl-AA-OBzl, discloses an effect of the 5-(bi(3-(2-ethoxy)-1H-indol-2)methyl)-2-hydroxy benzoyl-AA-OBzl in inhibiting invasion and metastasis of a tumor cell, discloses an activity of the 5-(bi(3-(2-ethoxy)-1H-indol-2)methyl)-2-hydroxy benzoyl-AA-OBzl in inhibiting weight gain of a tumor body of a S180-bearing mouse, and discloses an anti-tumor pulmonary metastasis activity and an anti-inflammatory activity. The compound has an excellent application prospect in preparing an antitumor drug, an anti-tumor metastasis drug and an anti-inflammatory drug.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Complex enzyme preparation for feeding piglets
InactiveCN102119768AImprove metabolic energyReduce chyme viscosityAnimal feeding stuffAccessory food factorsPectinaseDisease
The invention discloses a complex enzyme preparation for feeding piglets. The complex enzyme preparation for feeding piglets comprises the following seven enzymes: acid protease, amylase, xylanase, beta-glucanase, cellulase, pectinase and phytase; and the enzymatic activity ratio of the seven enzymes is 1: 1: (8-10): (3.3-4): 1: (0.5-0.55): (0-0.02). By applying the complex enzyme preparation disclosed by the invention, various anti-nutritional factors in feed can be degraded, the viscosity of chyme in an intestinal tract can be reduced, and the metabolic energy of the feed can be improved; the protein and starch digestibility of the piglets can be improved; excessive reproduction of harmful microorganisms in the intestinal tract can be reduced, damage to the intestinal wall can be reduced, and the microecological balance of the intestinal tract can be regulated; the survival rate and disease resistance of the piglets can be improved, the diarrhea rate can be reduced, and the overall uniformity is improved; and the weight gaining of the piglets can be promoted, and the cultivation cost is reduced.
Owner:BEIJING CHALLENGE AGRI SCI & TECH CO LTD
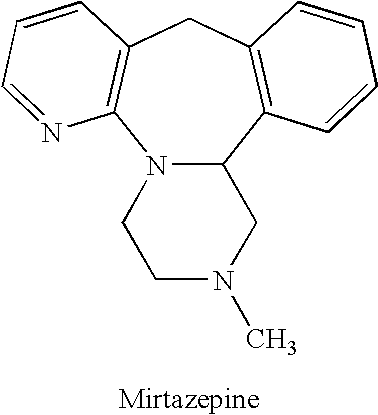
Methods for reducing the side effects associated with mirtzapine treatment
InactiveUS20060122127A1Eliminate side effectsBiocideCarbohydrate active ingredientsDiseaseNorepinephrine reuptake inhibitor
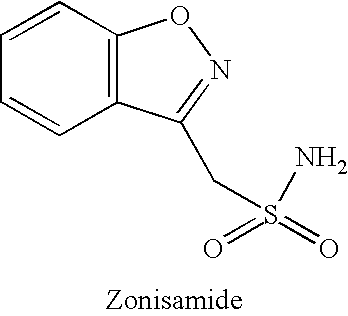
Compositions, and methods of use thereof, are provided for the prevention or treatment of side effects associated with the use of drugs that act as 5HT2 / 5HT3 serotonin receptor antagonists and alpha-2 adrenergic receptor antagonists (5HT2 / 5HT3 antagonist / alpha-2 antagonist). The method involves using dopamine-releasing compounds, such as amantadine, anticonvulsants, such as zonisamide, or dopamine / norepinephrine reuptake inhibitors, such as bupropion, in combination with 5HT2 / 5HT3 antagonist / alpha-2 antagonists, such as mirtazapine, to reduce the excessive daytime drowsiness and / or weight gain associated with 5HT2 / 5HT3 antagonist / alpha-2 antagonist use for the treatment of disorders, such as, depression, schizophrenia, anxiety disorders, sleep-related breathing disorders, insomnia, migraine headache, chronic tension-type headache, hot flashes, lower back pain, neuropathic pain and functional somatic syndromes. Formulations of dopamine-releasing compounds or anticonvulsants with 5HT2 / 5HT3 antagonist / alpha-2 antagonists are provided. In particular embodiments, combination therapy with mirtazapine and zonisamide provides relief from chronic low back pain, while reducing or avoiding side effects associated with monotherapy with mirtazapine or zonisamide.
Owner:CYPRESS BIOSCI
Dosage forms of O-desmethylvenlafaxine
InactiveUS20080132578A1Potent activityReducing and avoiding adverse effectOrganic active ingredientsBiocideClinical psychologyAttention deficits
Methods of preparing, and compositions comprising, derivatives of venlafaxine are disclosed. Also disclosed are methods of treating and preventing diseases and disorders including, but not limited to, affective disorders such as depression, bipolar and manic disorders, attention deficit disorder, attention deficit disorder with hyperactivity, Parkinson's disease, epilepsy, cerebral function disorders, obesity and weight gain, incontinence, dementia and related disorders.
Owner:WYETH LLC
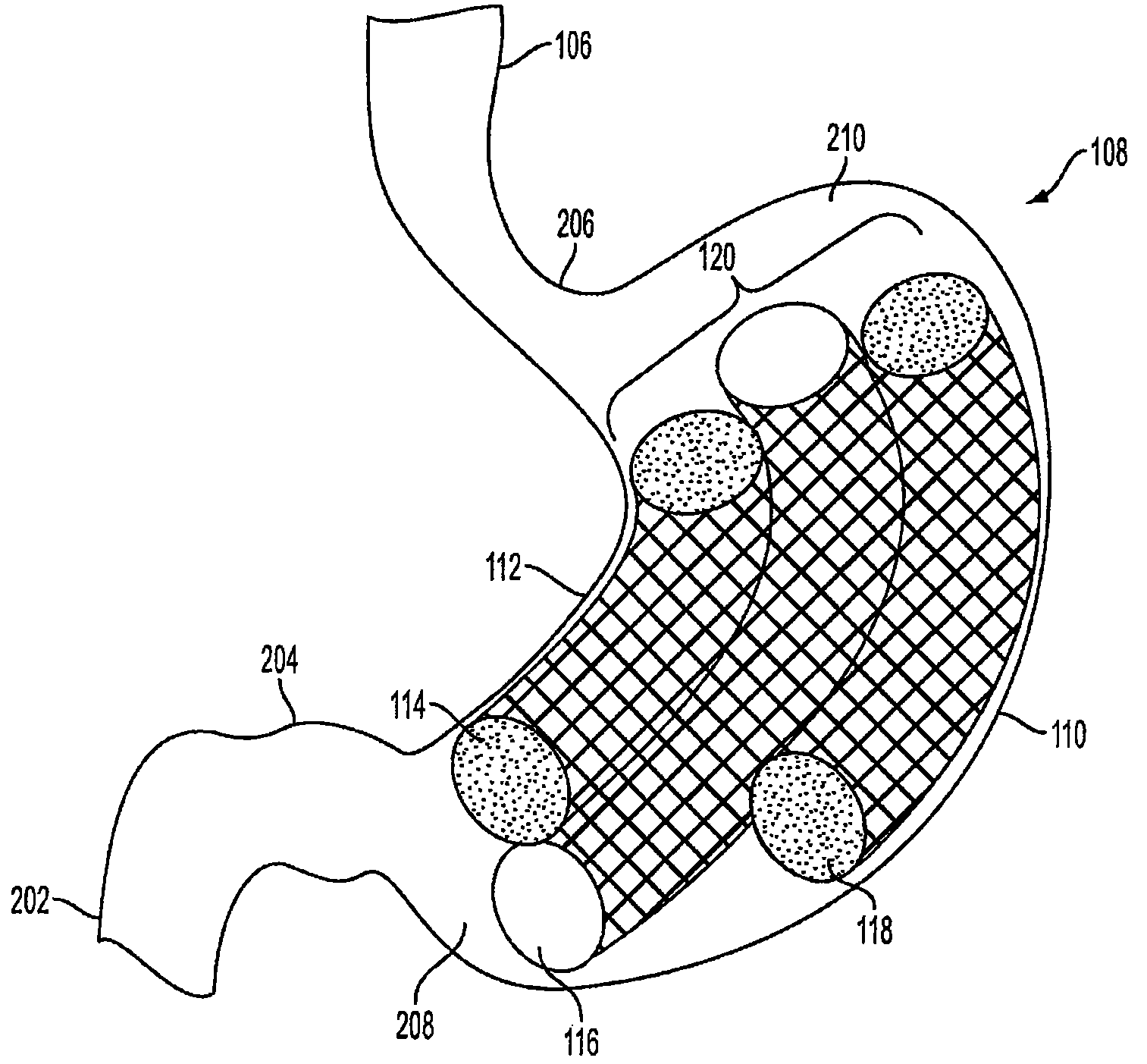
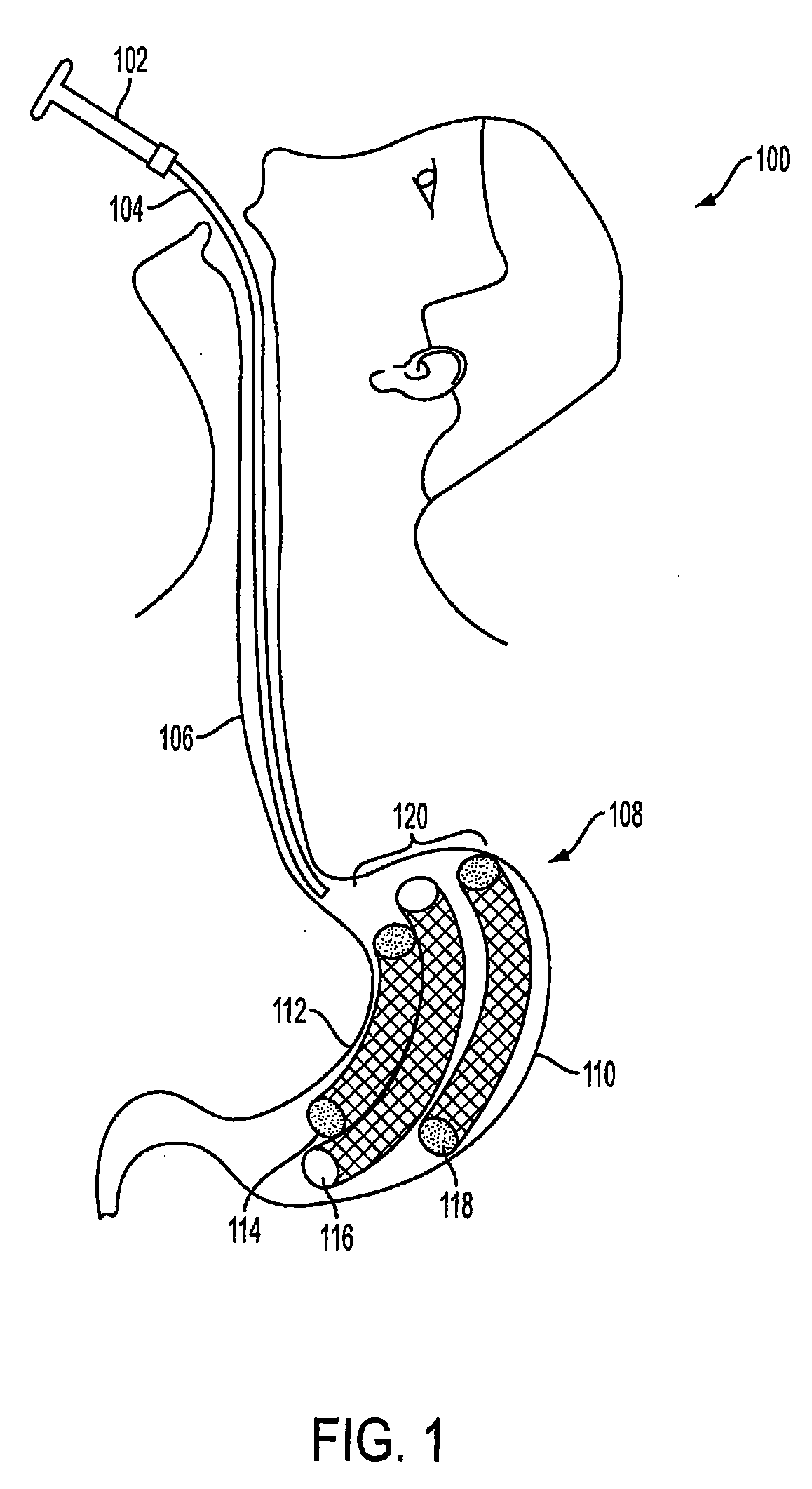
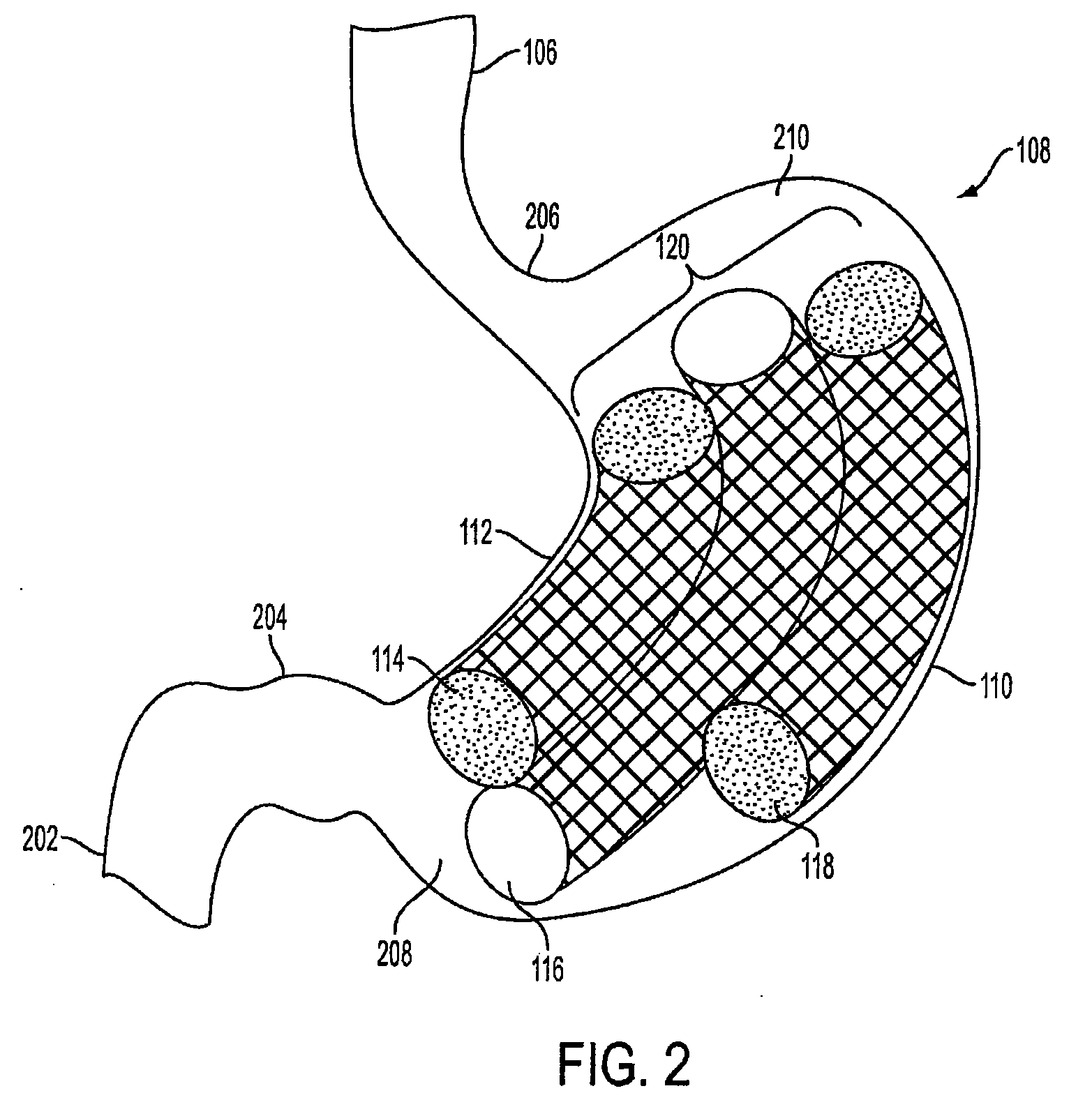
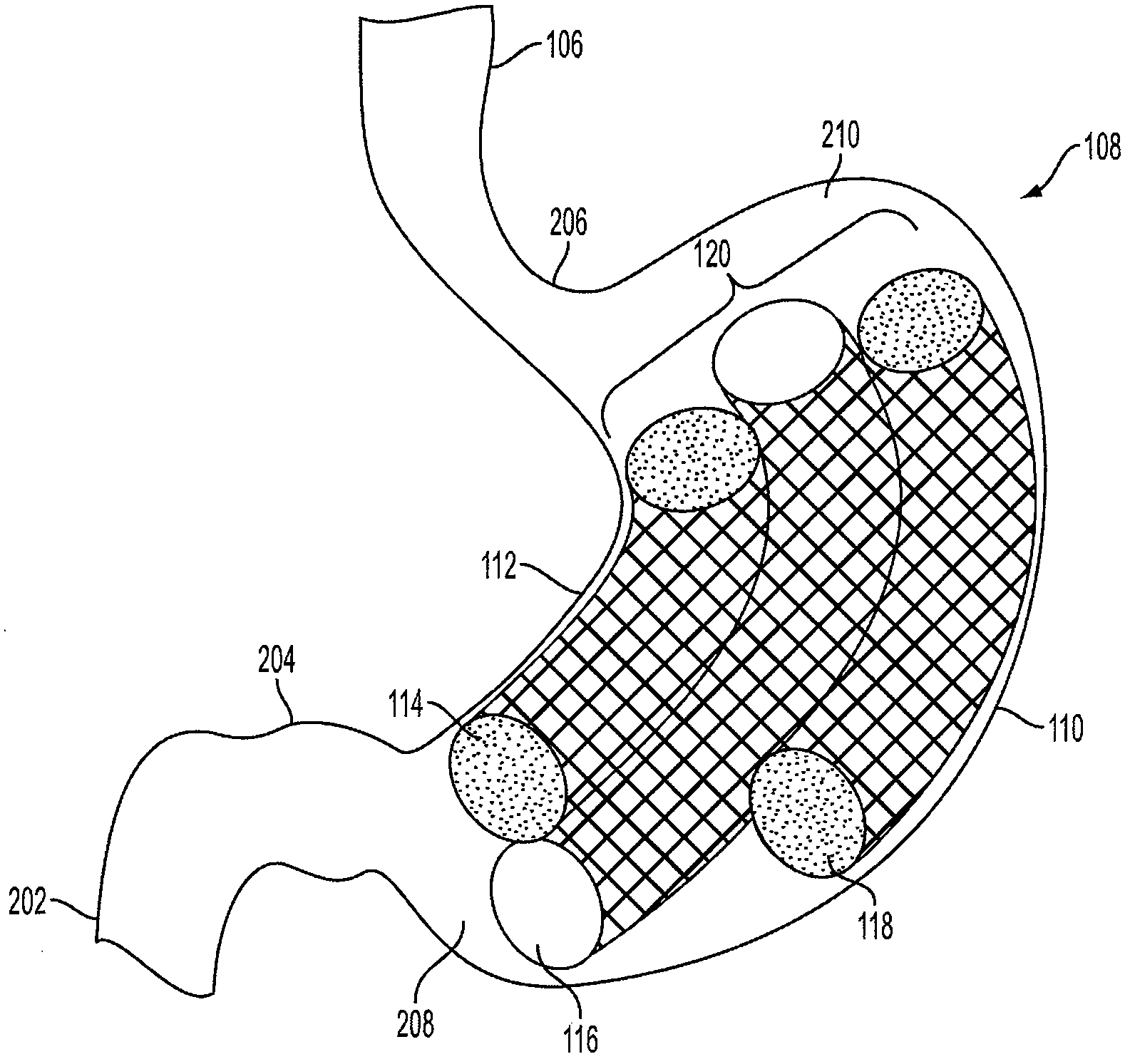
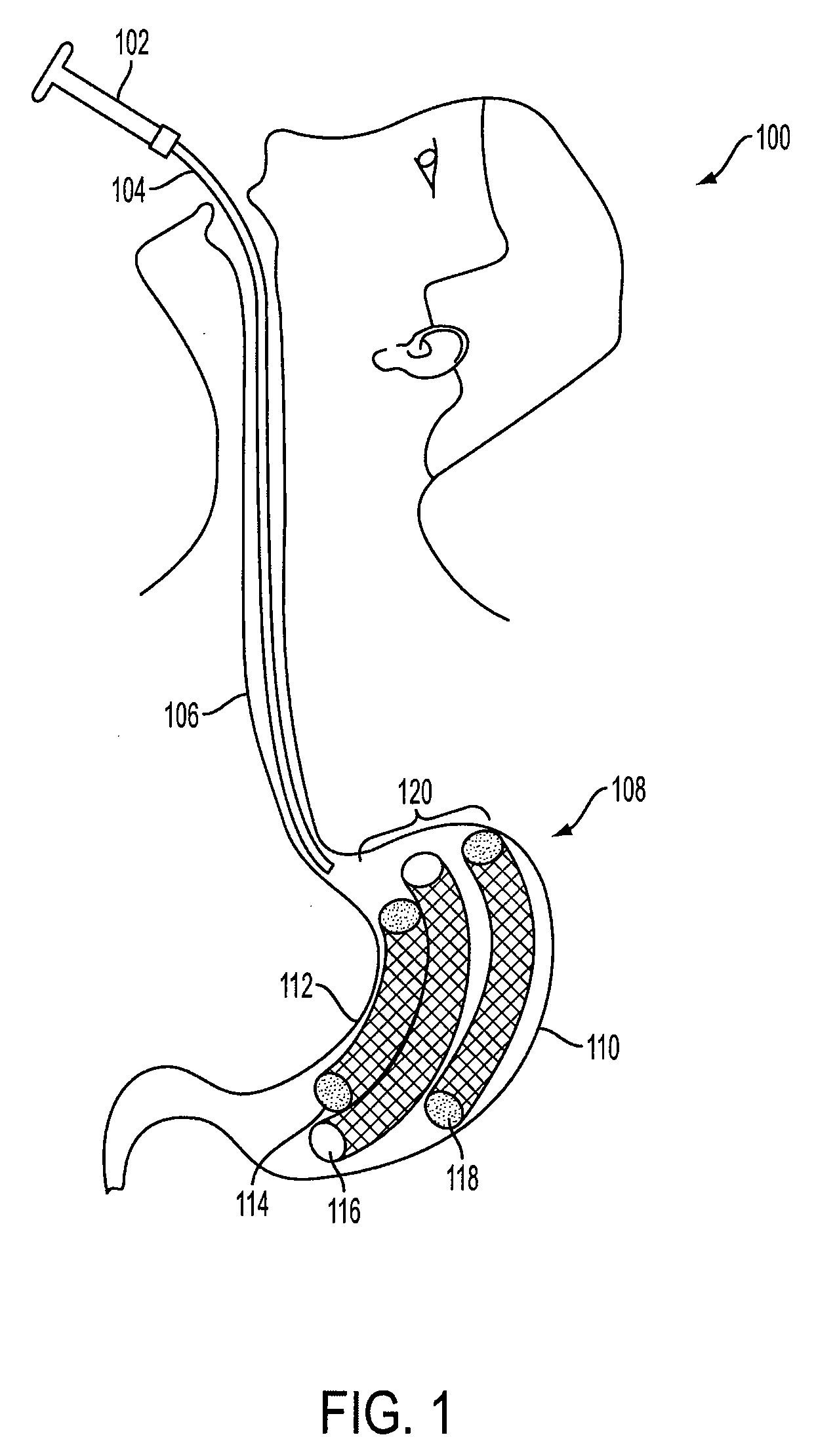
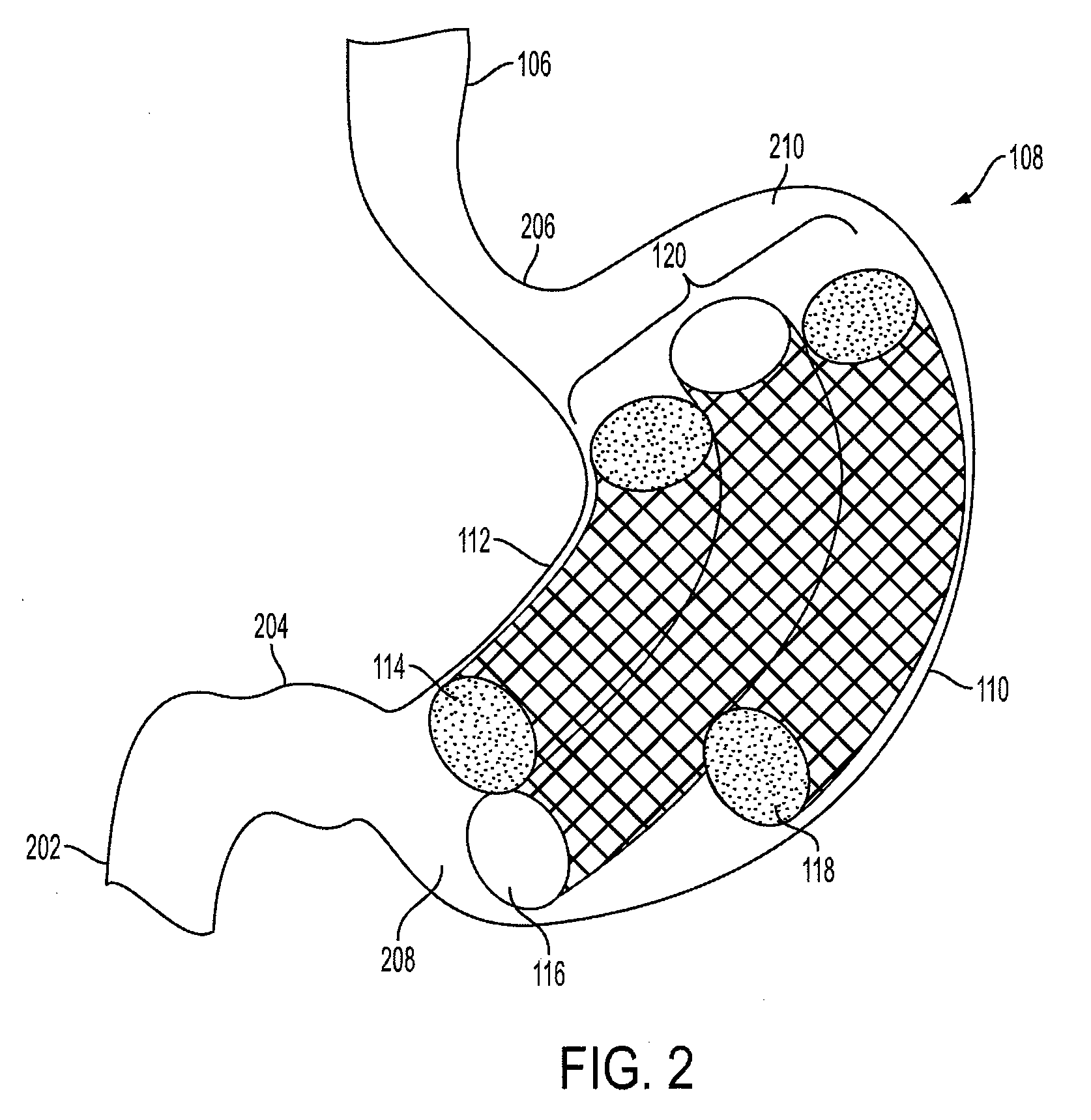
Method and apparatus for treating obesity and controlling weight gain using adjustable intragastric devices
The invention generally relates to an intragastric space-occupying device configured to be positioned within a stomach of a mammal for treating excessive weight or obesity. The intragastric space-occupying device may include an inner rod and an outer rod surrounding the inner rod, the outer rod moveable along the inner rod, the outer rod being movable between an outer first position and an inner second position. The intragastric space-occupying device may also include a plurality of support structures, each support structure having a first end connected to the inner rod and a second end connected to the outer rod, the plurality of support structures being in a collapsed position when the outer rod is in the outer first position and being in an extended position when the outer rod is in the inner second position and a covering positioned on the plurality of support structures.
Owner:ONCIOMED
Compositions of an anticonvulsant and mirtazapine to prevent weight gain
ActiveUS20060079501A1Avoid weight gainGood curative effectBiocidePowder deliveryWeight gainPharmacology
Disclosed are pharmaceutical compositions comprising mirtazapine and an anticonvulsant drug. Also disclosed are methods of preventing weight gain associated with the administration of mirtazapine comprising identifying a patient to whom mirtazapine is to be administered and administering to said patient a pharmaceutical composition comprising mirtazapine and an anticonvulsant drug. Further disclosed are methods of increasing the efficacy of mirtazapine comprising identifying a patient to whom mirtazapine is to be administered and administering to said patient a pharmaceutical composition comprising mirtazapine and an anticonvulsant drug.
Owner:DUKE UNIV
Use of organic acids and essential oils in animal feeding
The present invention relates to a novel feed composition for animals, for example poultry, comprising as active ingredient benzoic acid, derivatives or metabolites thereof, in combination with a mixture of at least two active compounds selected from the group consisting of thymol, eugenol and piperine. The inventors found that in addition to the well known function of benzoic acid, this compound can be used as a potential growth promoter when it is combined with a mixture of at least two active compounds selected from the group consisting of thymol, eugenol and piperine. In particular the inventors have been able to demonstrate that a mixture of these chemical compounds present in different parts of plants, used in synergy and in combination with an appropriate amount of benzoic acid, exhibits, in totally unexpected manner, the effects sought by the present invention of improving the digestibility of poultry feed, i.e. for improving feed conversion ratio and / or daily weight gain in animal.
Owner:DSM IP ASSETS BV
O-desmethylvenlafaxine and methods of preparing and using the same
InactiveUS20050197392A1Reduce weightPotent activityBiocideNervous disorderAttention deficitsEpileptic disorder
Methods of preparing, and compositions comprising, derivatives of venlafaxine are disclosed. Also disclosed are methods of treating and preventing diseases and disorders including, but not limited to, affective disorders such as depression, bipolar and manic disorders, attention deficit disorder, attention deficit disorder with hyperactivity, Parkinson's disease, epilepsy, cerebral function disorders, obesity and weight gain, incontinence, dementia and related disorders.
Owner:WYETH LLC
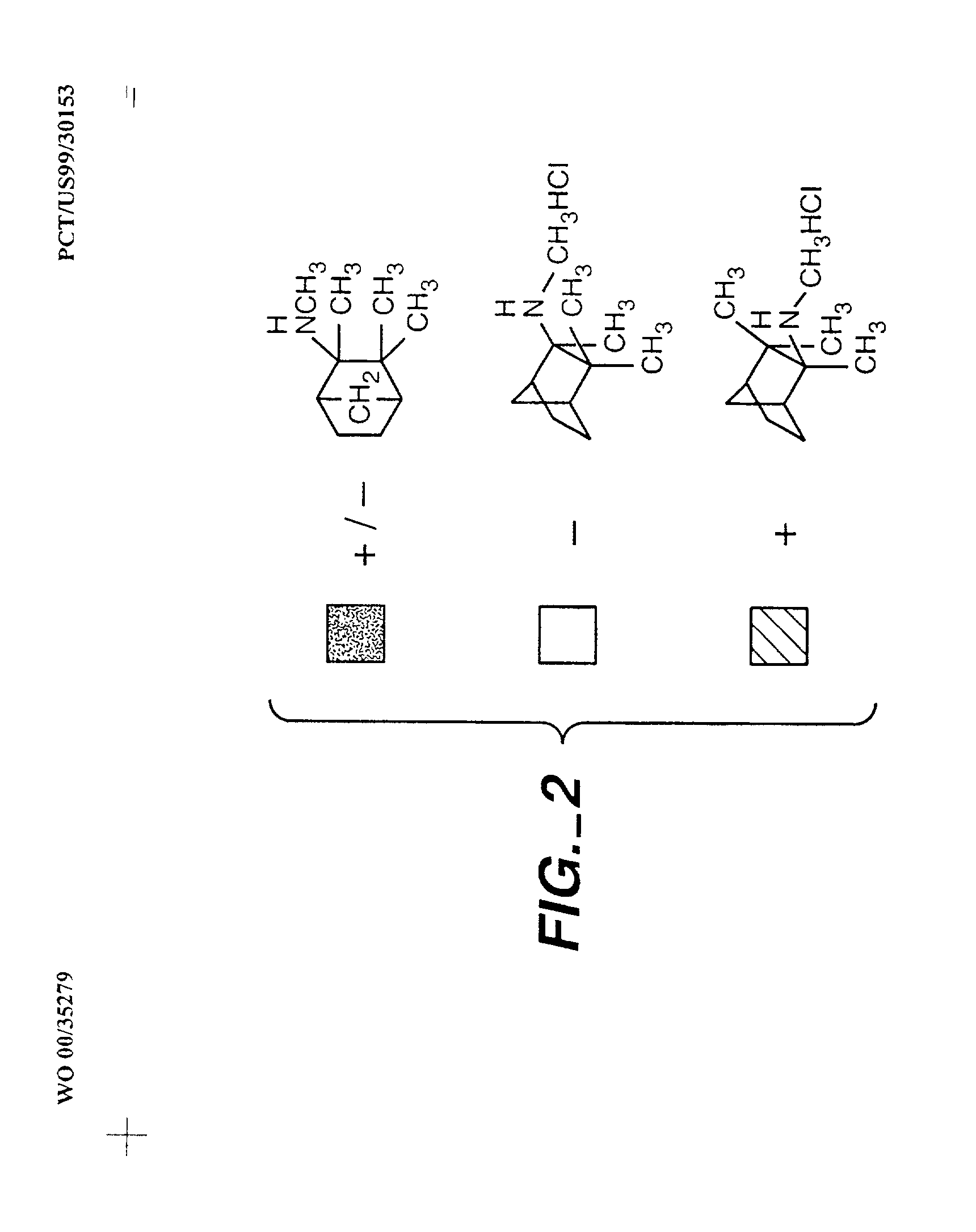
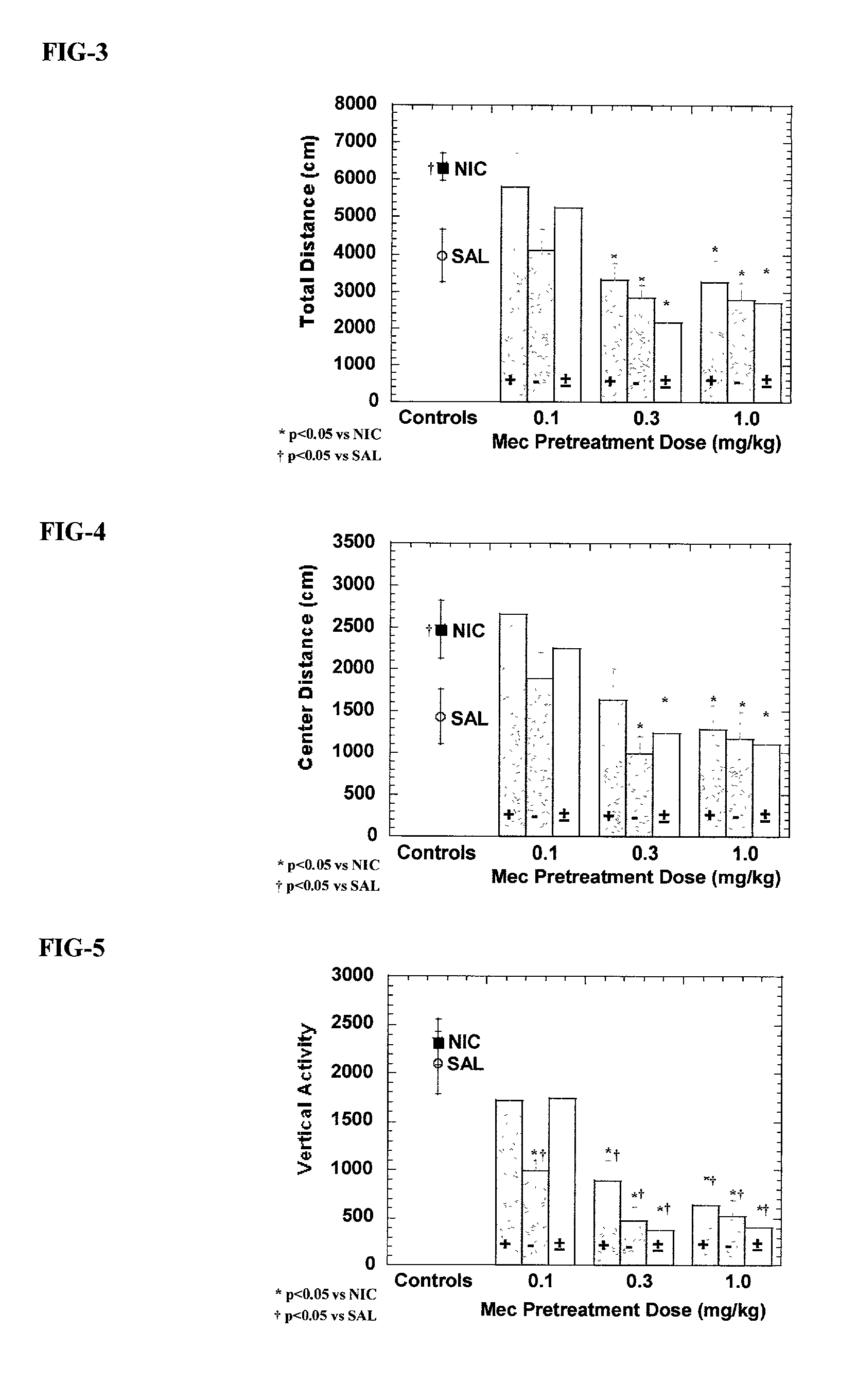
Exo-R-mecamylamine formulation and use in treatment
InactiveUS20020016370A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationStimulantS syndrome
A pharmaceutical composition includes a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-S-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-S-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
Composite conductive film, its preparation method and its application
ActiveCN102785437AImprove toughnessHigh impact damage toleranceSynthetic resin layered productsMetal layered productsFiberCarbon fibers
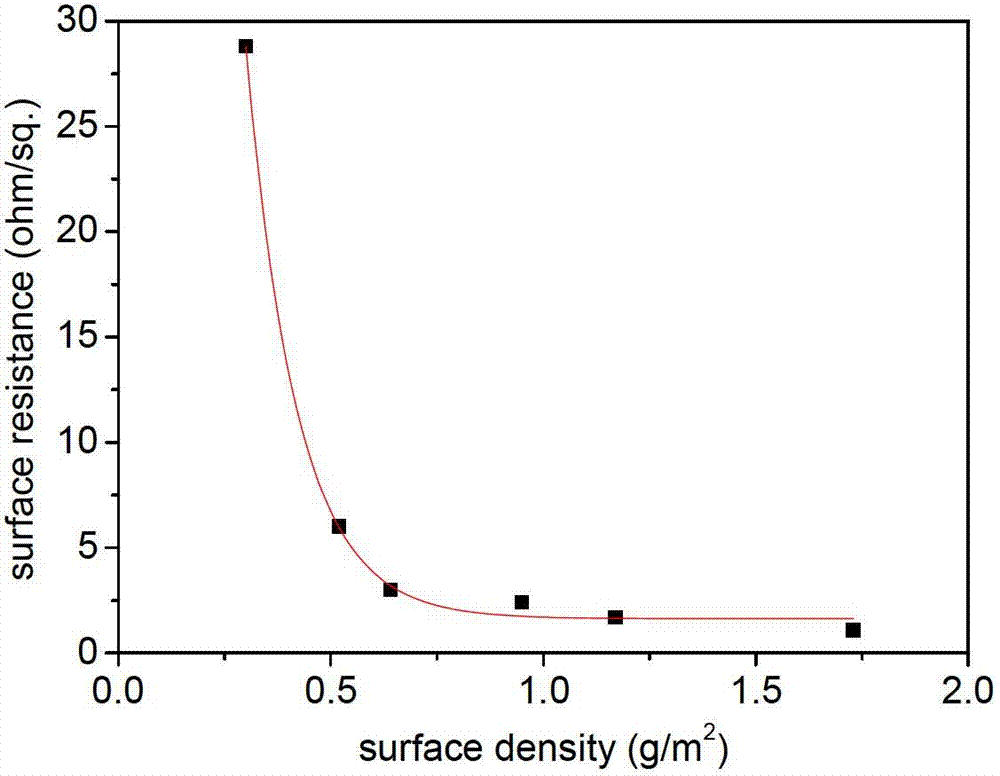
The invention relates to a design and a preparation method of a continuous carbon laminated fiber reinforced resin matrix structure composite material taking account of the conductivity and the high toughness, a corresponding intermediate composite conductive film and a final composite material product. The composite conductive film having a high conductivity and a toughness potential is prepared through utilizing a low-surface-density non-woven fabric having a network structure, a porous film or a fabric as a functional carrier, and loading highly-conductive nano-micro scale silver nanowires and other auxiliary conductive components, such as carbon nanotubes, graphene and the like; and the composite conductive film is disposed between layers of a routine carbon laminated fiber composite material through an intercalation technology, and is molded and cured to prepare the high-conductivity and high-toughness structure composite material. The method is simple to operate, the toughness of the obtained composite material is greatly improved, the resistivity in the layers and the resistivity between the layers are greatly decreased, the caused weight gain of the composite material is extremely small, and the high conductivity and the high toughness of the whole composite material are realized.
Owner:AVIC BEIJING INST OF AERONAUTICAL MATERIALS +1
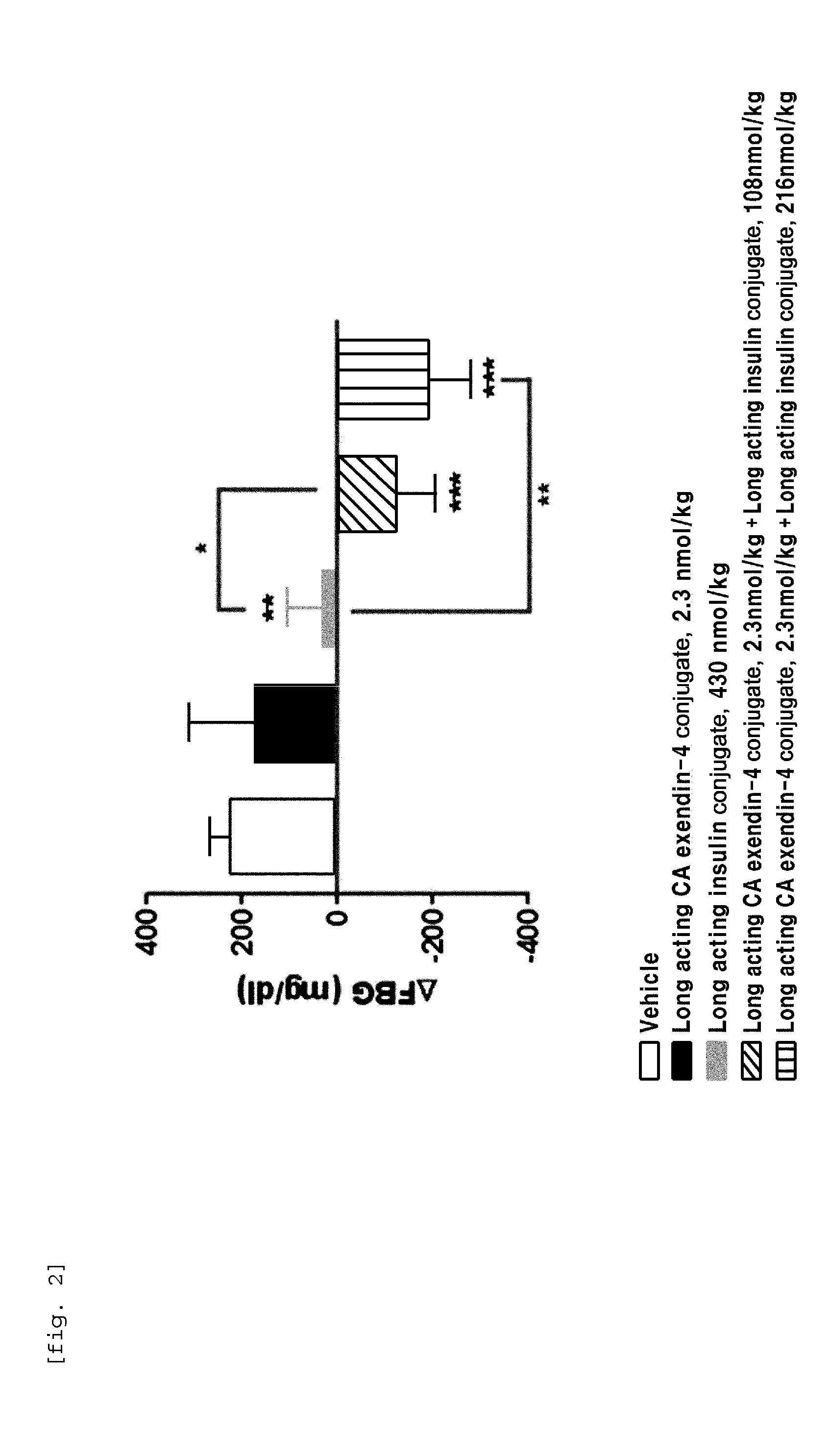
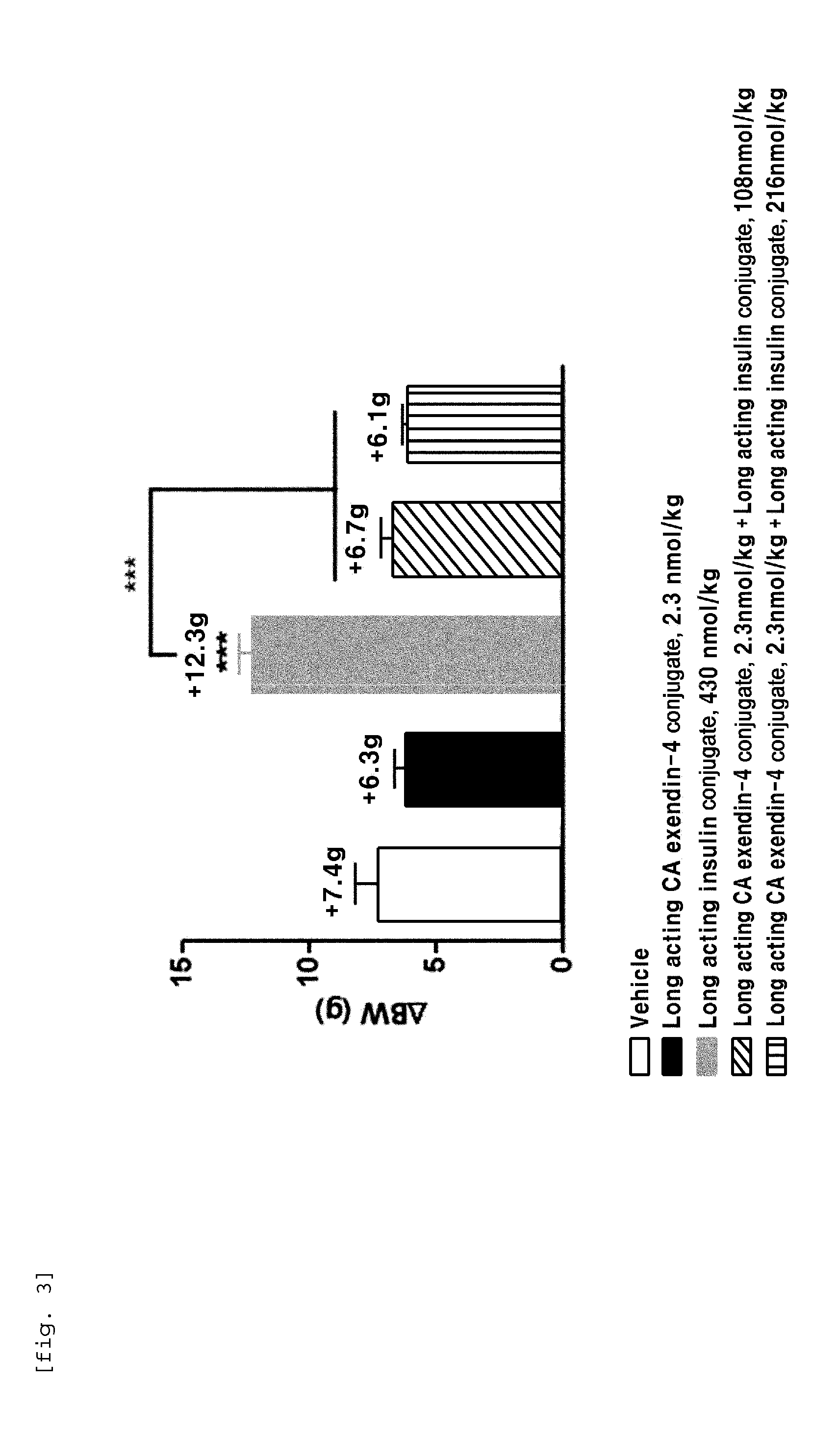
Composition for treating diabetes comprising long-acting insulin conjugate and long-acting insulinotropic peptide conjugate
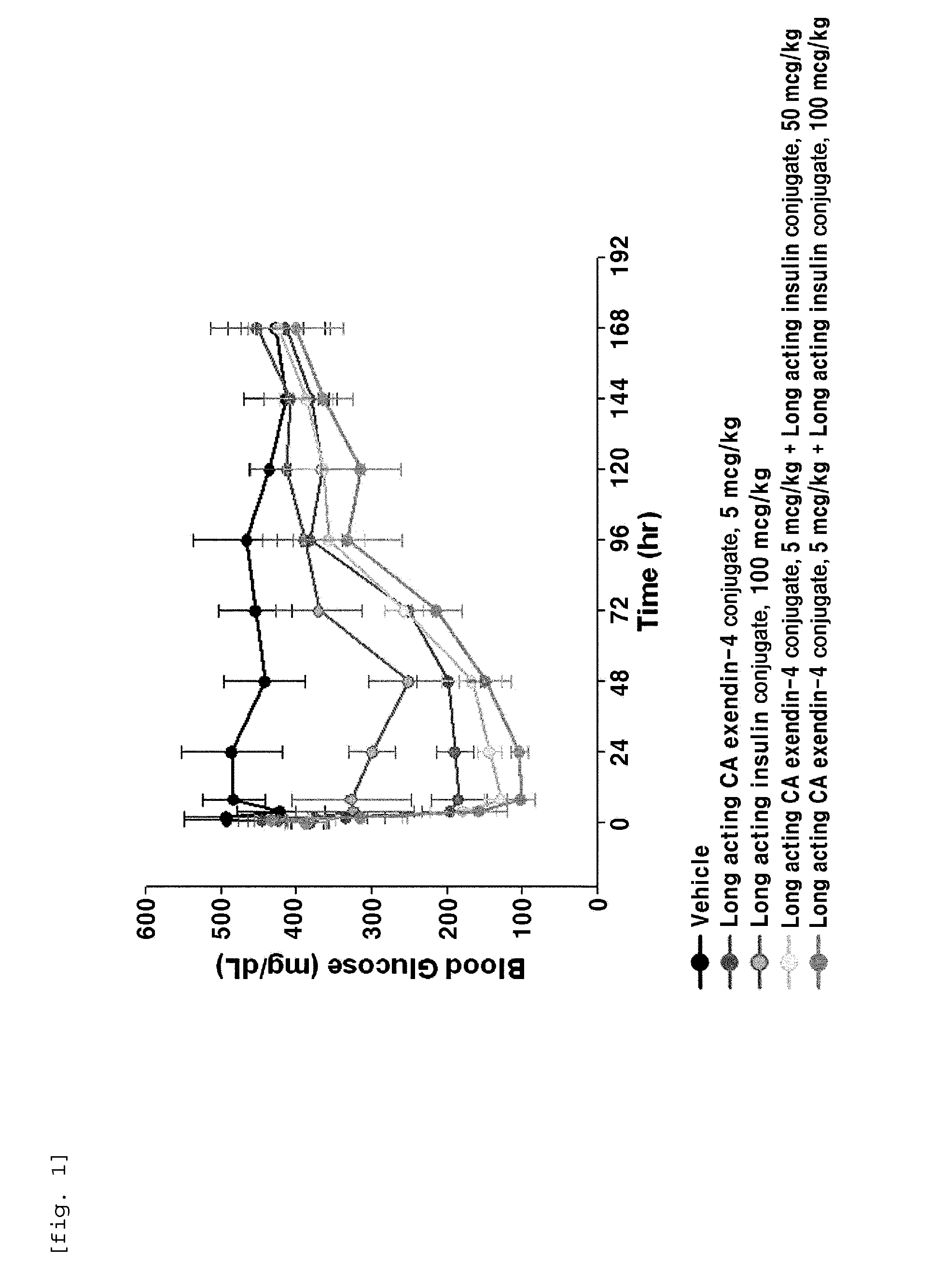
ActiveUS20140120120A1Good treatment effectImprove stabilityPeptide/protein ingredientsMetabolism disorderIn vivoWeight gain
The present invention relates to a composition for the prevention or treatment of diabetes comprising a long-acting insulin conjugate and a long-acting insulinotropic peptide conjugate, and a therapeutic method for the treatment of diabetes, and more particularly, concurrent administration of the long-acting insulin conjugate and the long-acting insulinotropic peptide conjugate inhibits weight gain caused by insulin treatment, and vomiting and nausea caused by insulinotropic peptide treatment, and reduces the required dose of insulin, thereby remarkably improving drug compliance. Moreover, each of the long-acting insulin conjugate and the long-acting insulinotropic peptide conjugate of the present invention is prepared by linking insulin or insulinotropic peptide with an immunoglobulin Fc region via a non-peptidyl linker, thereby showing improved in-vivo duration of efficacy and stability.
Owner:HANMI SCI CO LTD
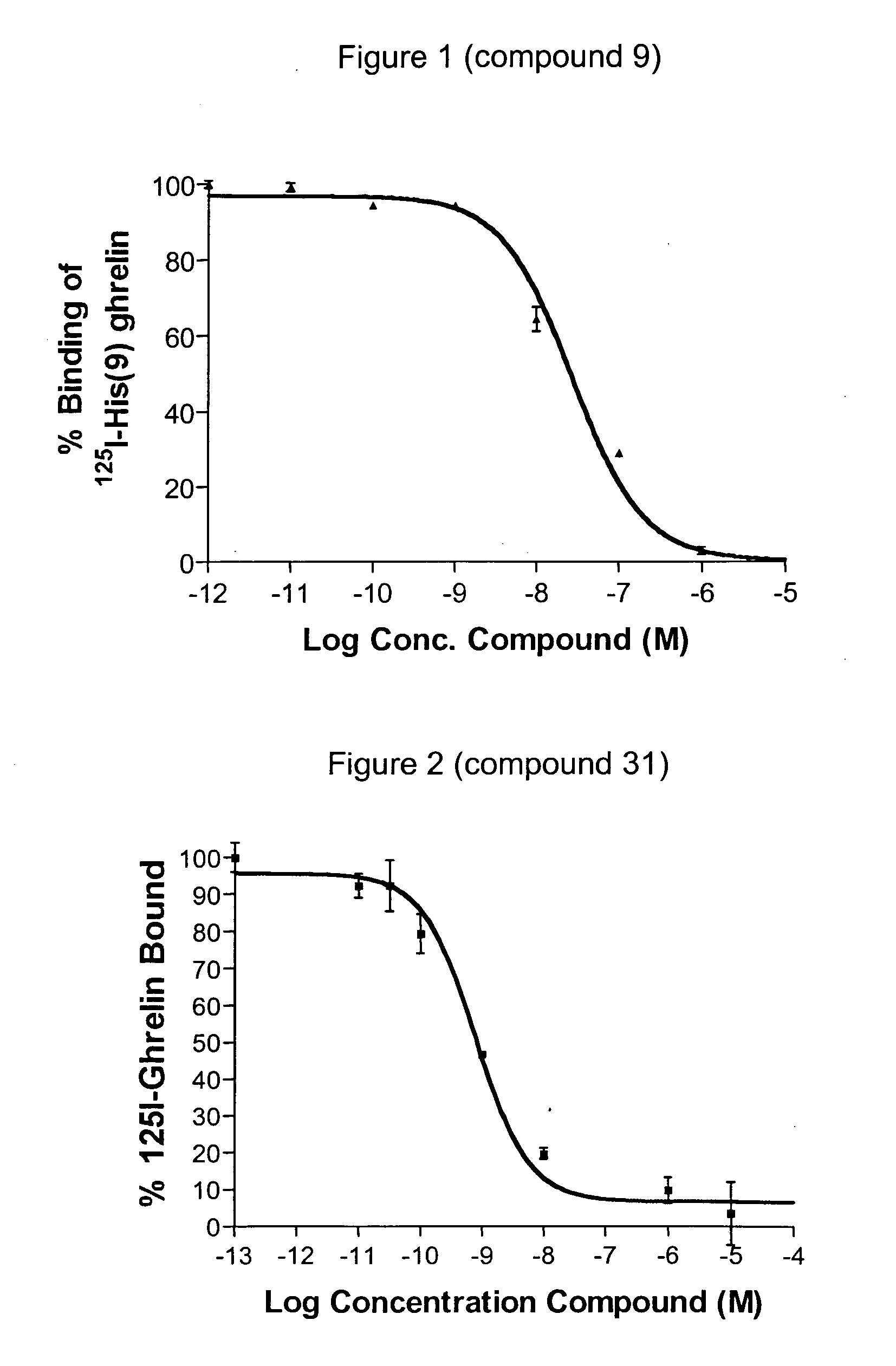
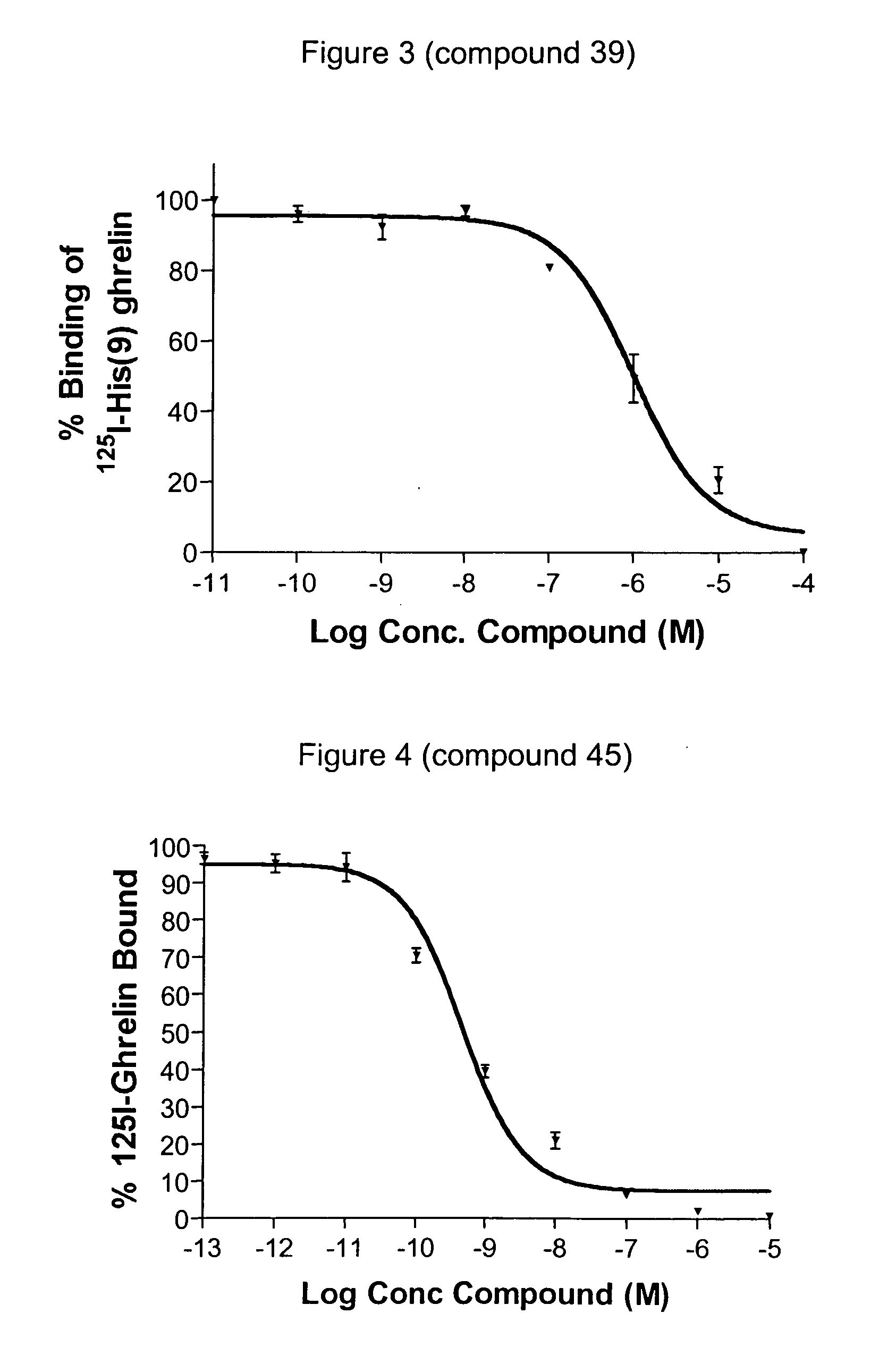
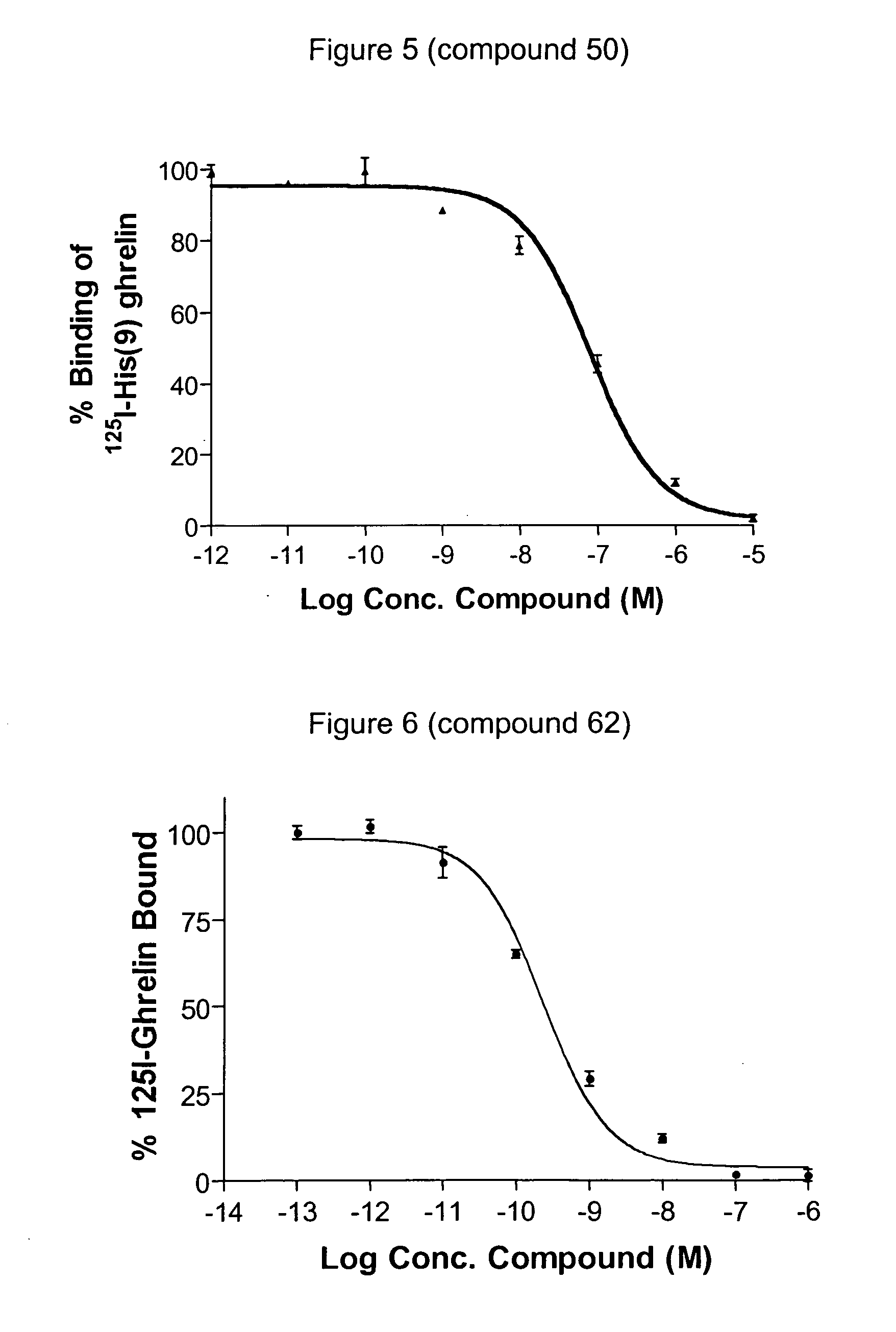
Novel triazole derivatives as ghrelin analogue ligands of growth hormone secretagogue receptors
ActiveUS20070037857A1Good metabolic stabilityImprove bioavailabilityBiocideSenses disorderAdipogenesisTriazole derivatives
The present invention provides novel triazole derivatives as ghrelin analogue ligands of growth hormone secretagogue receptors according to formula (I) that are useful in the treatment or prophylaxis of physiological and / or pathophysiological conditions in mammals, preferably humans, that are mediated by GHS receptors. The present invention further provides GHS receptor antagonists and agonists that can be used for modulation of these receptors and are useful for treating above conditions, in particular growth retardation, cachexia, short-, medium- and / or long term regulation of energy balance; short-, medium- and / or long term regulation (stimulation and / or inhibition) of food intake; adipogenesis, adiposity and / or obesity; body weight gain and / or reduction; diabetes, diabetes type I, diabetes type II, tumor cell proliferation; inflammation, inflammatory effects, gastric postoperative ileus, postoperative ileus and / or gastrectomy (ghrelin replacement therapy).
Owner:ZENTARIS GMBH +3
Nutritional supplement for the management of weight
InactiveUS20060159724A1Increase satietyDelay returnBiocidePharmaceutical non-active ingredientsNutrition supplementationFood grade
Described herein is a low-glycemic nutritional supplement to be incorporated into the diet of an overweight or obese patient comprising low glycemic index ingredients including carbohydrate source, a source of protein, and a source of fat, and further comprising a source of green tea extract, a source of 5-hydroxytryptophan (5-HTP), and a source of chromium. The supplement provides active food-grade ingredients to improve the management weight loss, prevention of weight gain, and a feeling of satiety.
Owner:ADVANCED FUNCTIONAL FOODS INT
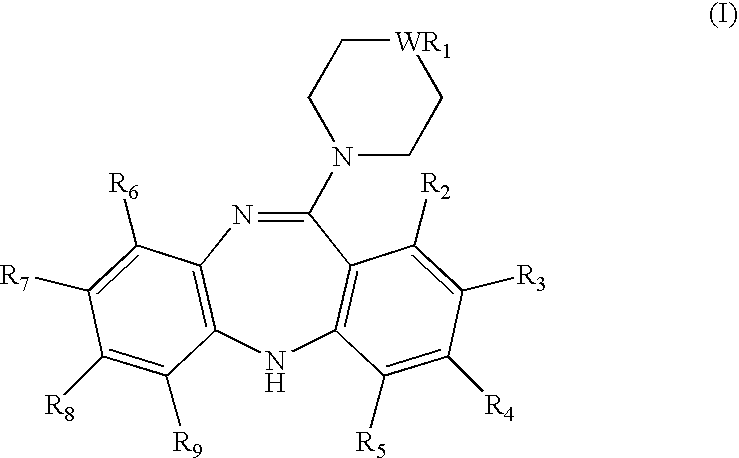
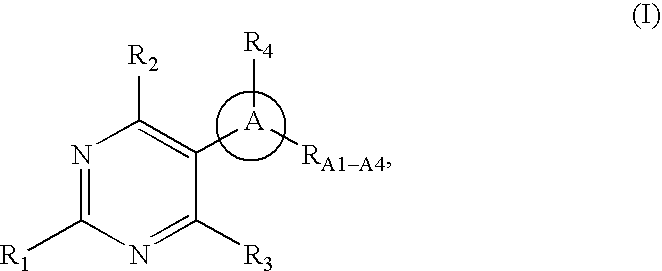
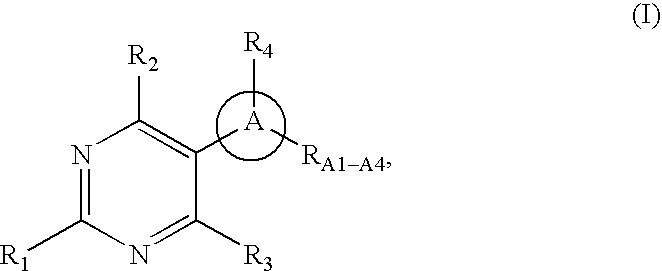
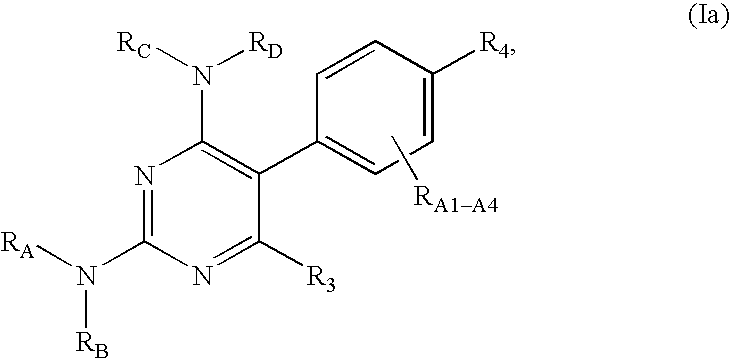
Pyrimidine derivatives as ghrelin receptor modulators
The present invention is related to compounds of formula (I), or a therapeutically suitable salt or prodrug thereof, the preparation of the compounds, compositions containing the compounds and the use of the compounds in the prevention or treatment of disorders regulated by ghrelin including anorexia, cancer cachexia, eating disorders, age-related decline in body composition, weight gain, obesity, and diabetes mellitus.
Owner:ABBOTT LAB INC
Compositions and methods of use for extracts of magnoliaceae plants
InactiveUS6582735B2Reduce and preventWithout reduction and loss of motor functionBiocideNervous disorderHonokiolMagnolol
The invention relates to compositions and methods for preventing, treating, or managing sleeplessness, restlessness, weight gain including weight gain due to stress or lack of sleep, or all three comprising the administration of a prophylactically and therapeutically effective amount of Magnoliaceae plant or extracts thereof to a mammal in need of such therapy. Preferably the mammal is human and the compositions have comprise at least two compounds selected from magnolol, honokiol, and magnoflorine. Alternatively, the compositions may also comprise about 2% honokiol by weight of the composition.
Owner:INTERHEALTH NUTRACEUTICALS
Nutrition-Pedometer
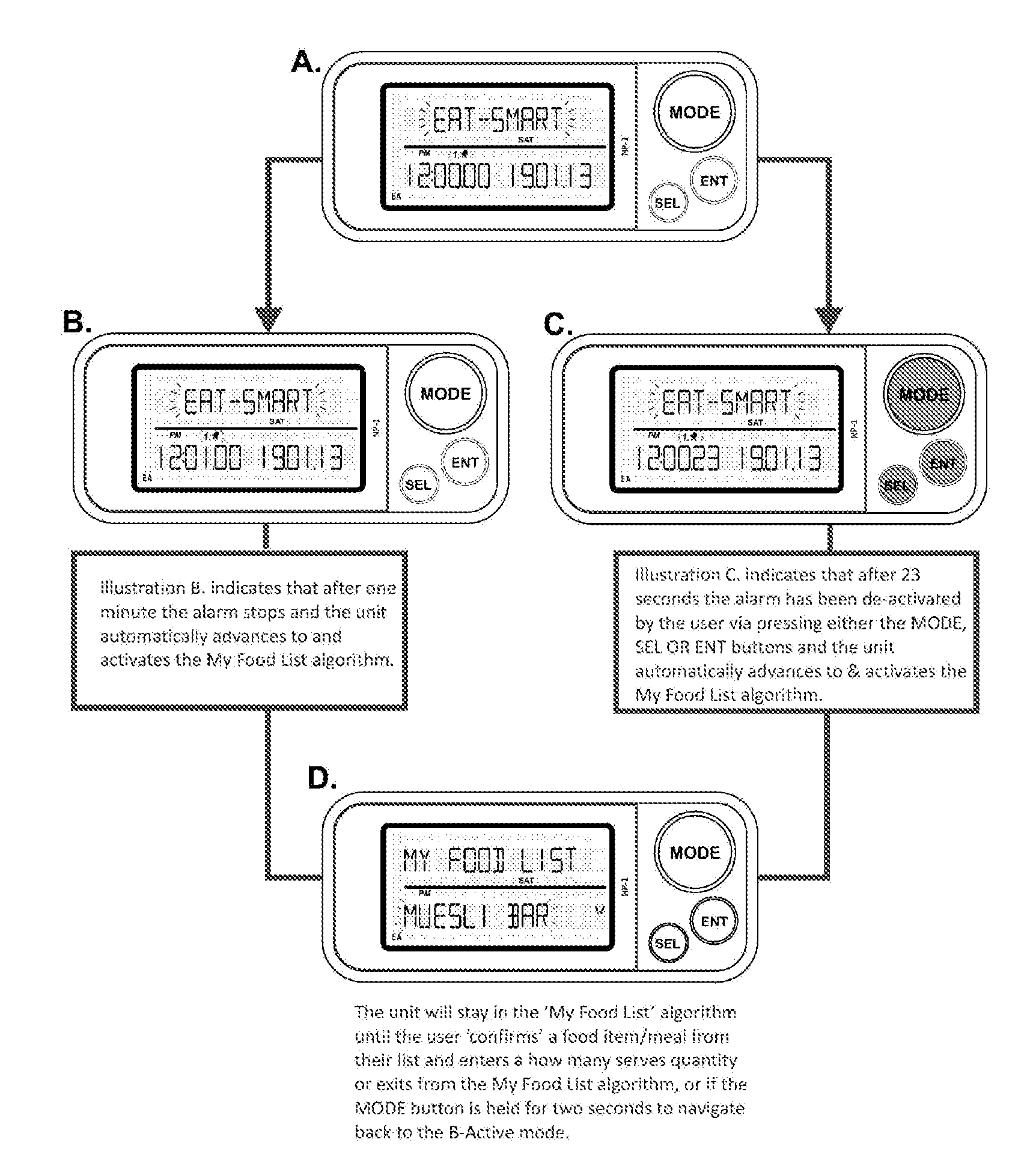
A system for determining the health and wellness of an individual user, the said system comprising a measuring device configured to measure and calculate (a) nutritional value of food items consumed, and (b) certain physical activities of the user, wherein the measuring device calculates and displays the weight gain or weight loss.
Owner:MACCALLUM DAVID +1
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Method and apparatus for treating obesity and controlling weight gain using self-expanding intragastric devices
The invention generally relates to a method and apparatus to treat obesity and controlling weight gain. In an exemplary embodiment, the invention relates to a covered cage device that is implanted within a human's stomach to occupy volume and cause a reduced desire for eating. The covered cage device is made from a wire-mesh, such a Nitinol, and can be adjustable and collapsible. In another embodiment, the covered cage device has edges that provide stimulation to the stomach to induce a feeling of fullness.
Owner:ACORN SUBSIDIARY HLDG LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com