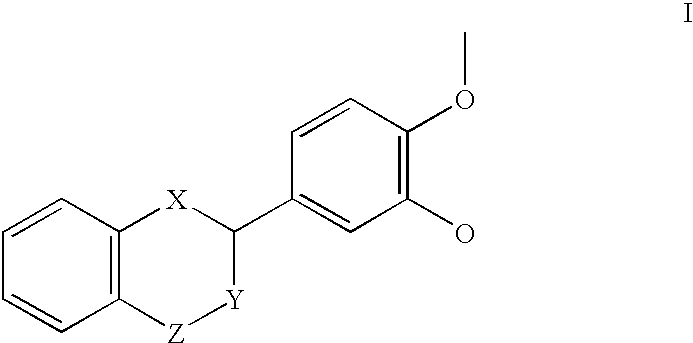
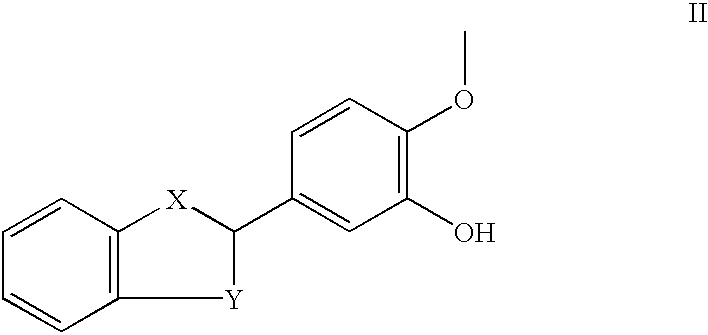
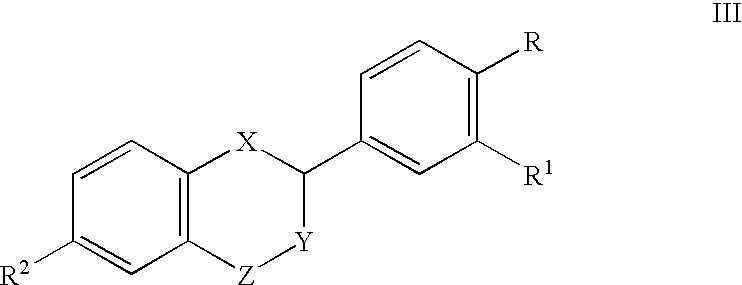
Patents
Literature
713 results about "Stimulant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and invigorating, or drugs that have sympathomimetic effects. Stimulants are widely used throughout the world as prescription medicines as well as without a prescription (either legally or illicitly) as performance-enhancing or recreational drugs. The most frequently prescribed stimulants as of 2013 were lisdexamfetamine, methylphenidate, and amphetamine. It is estimated that the percentage of the population that has abused amphetamine-type stimulants (e.g., amphetamine, methamphetamine, MDMA, etc.) and cocaine combined is between 0.8% and 2.1%.
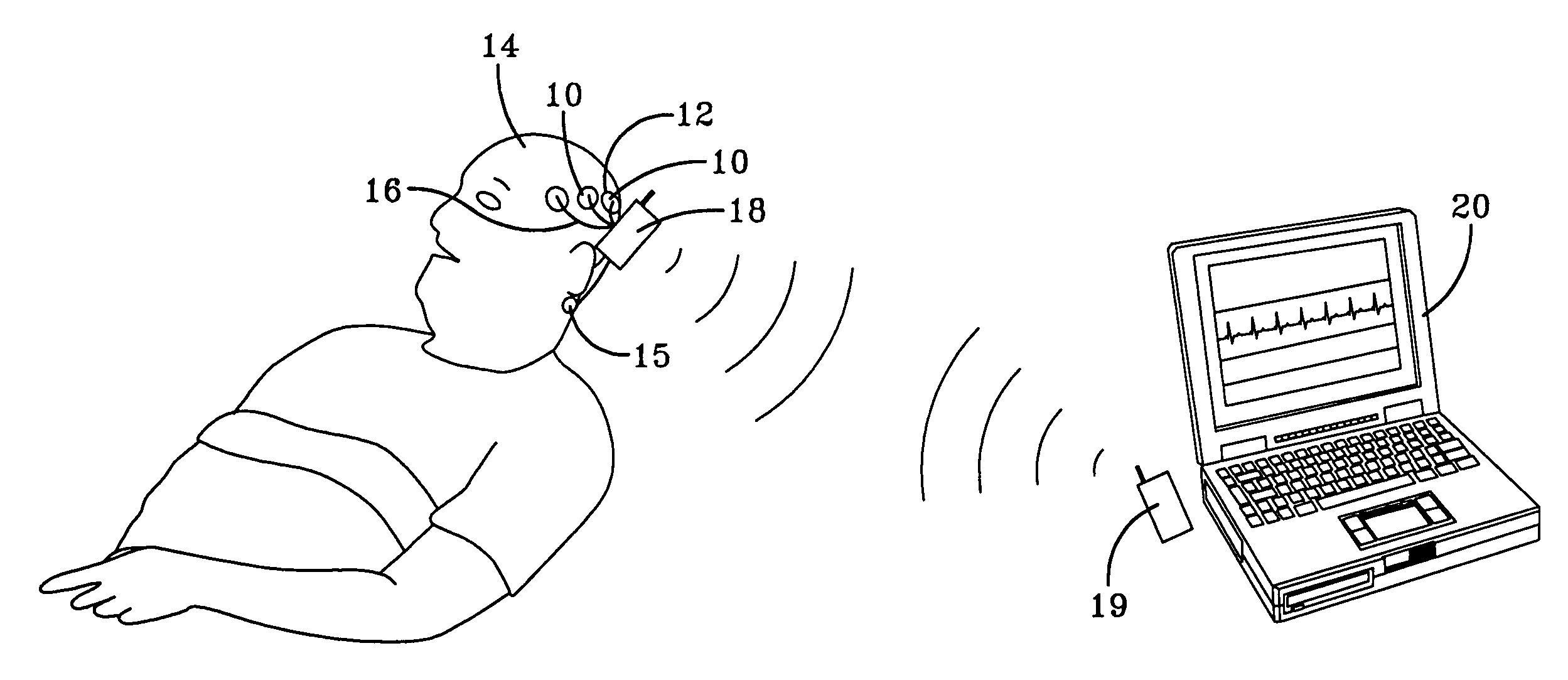
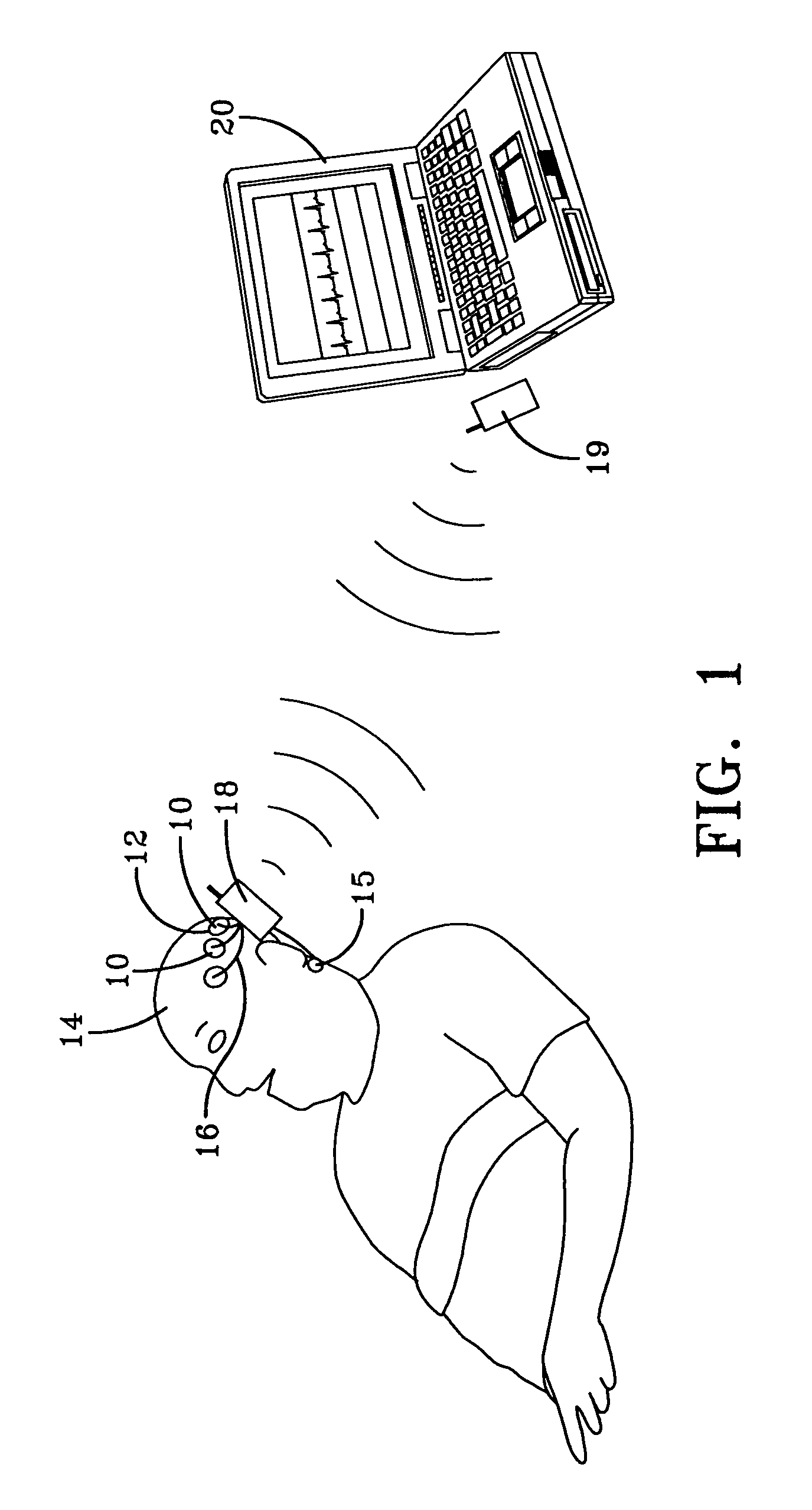
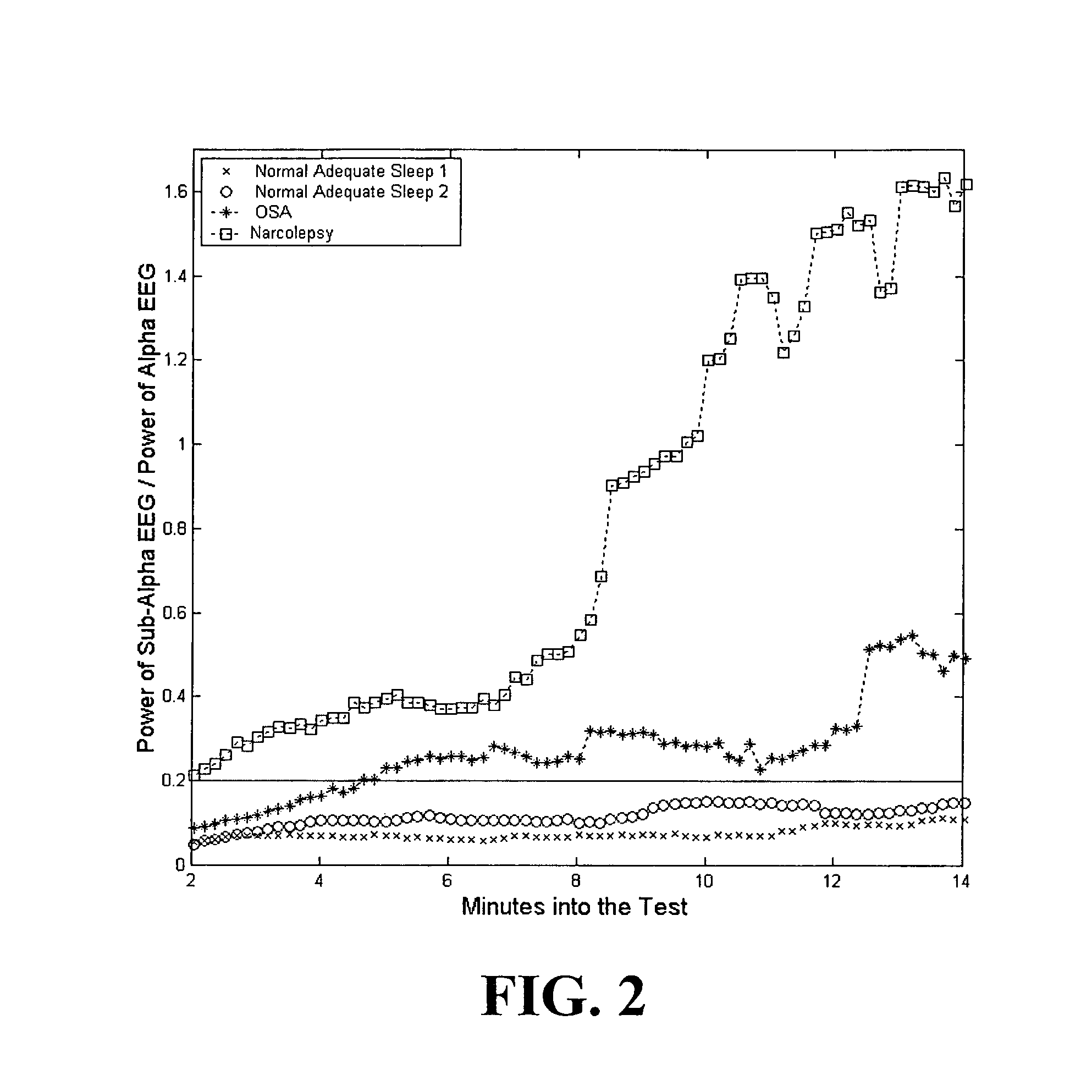
Quantitative sleep analysis method and system
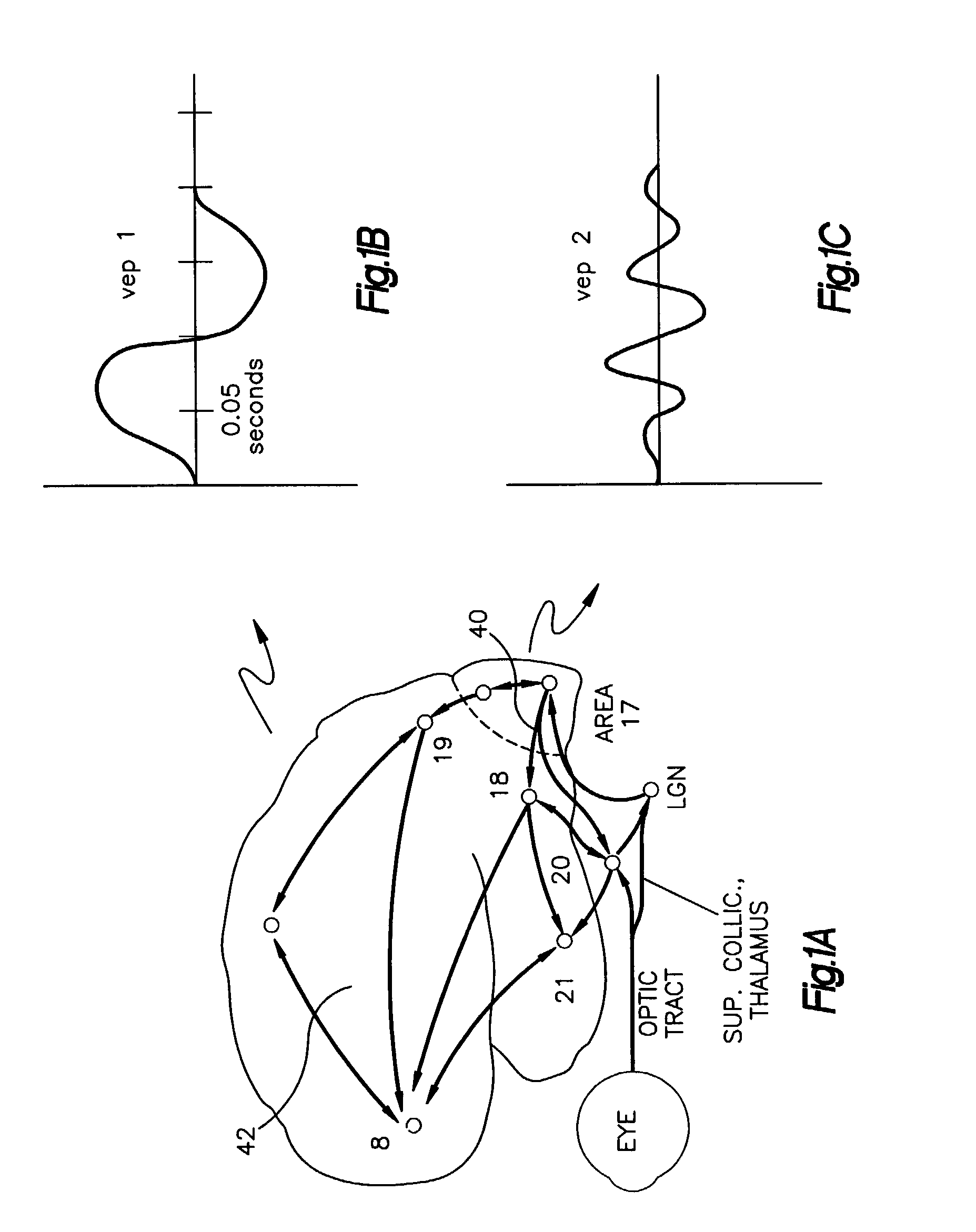
InactiveUS6993380B1Sure easyLow costElectroencephalographySensorsExcessive daytime sleepinessStimulant
The present invention relates to a method of analyzing a subject for excessive daytime sleepiness, and more particularly to a quick (short duration), quantitative method of sleep disorder analysis. The present invention additionally relates to a method, which can be used to quantitatively measure the treatment endpoints for the subject, i.e., appropriate levels of stimulants. Additionally, the present invention relates to a device for sleep disorder analysis.
Owner:CLEVELAND MEDICAL DEVICES
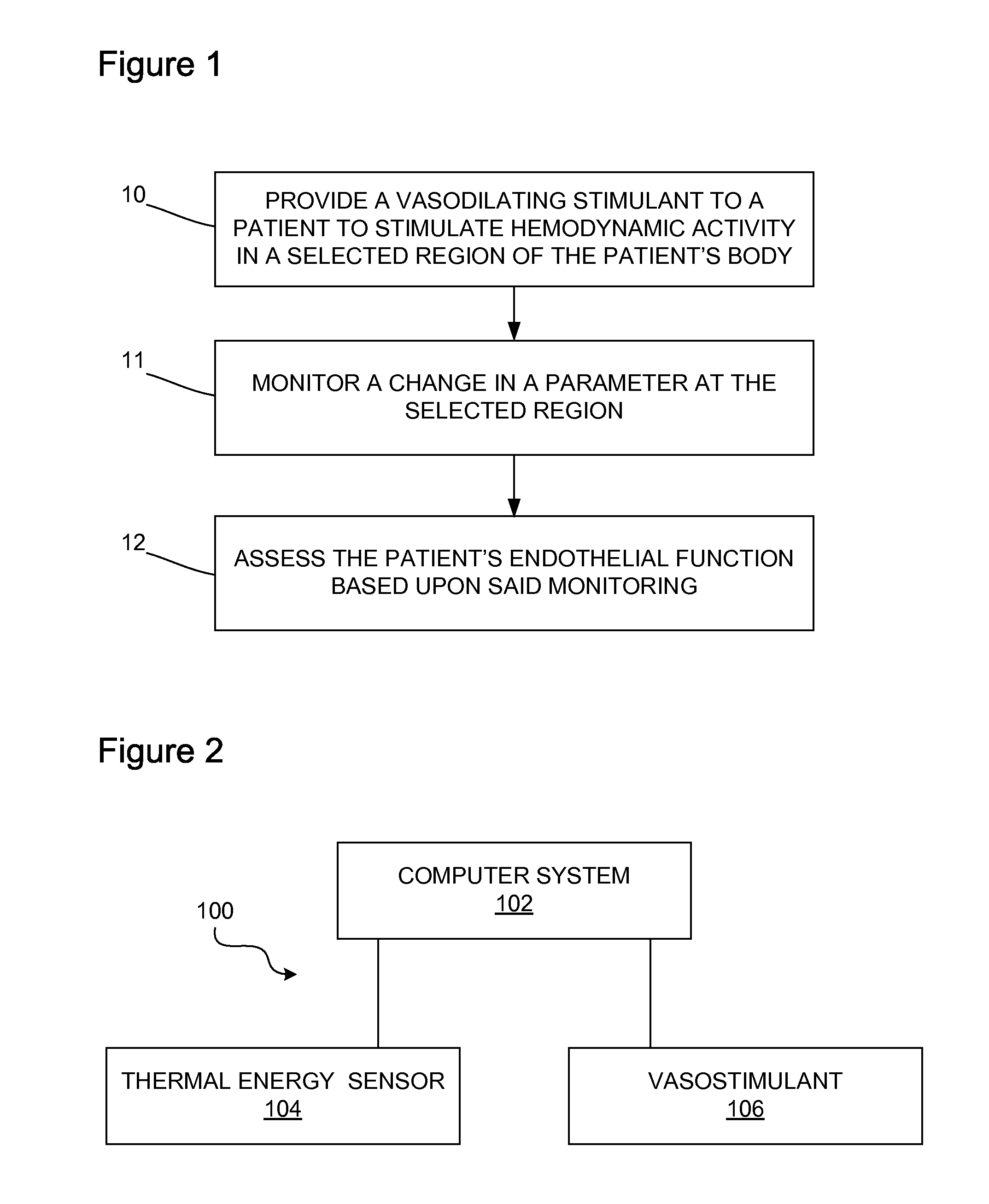
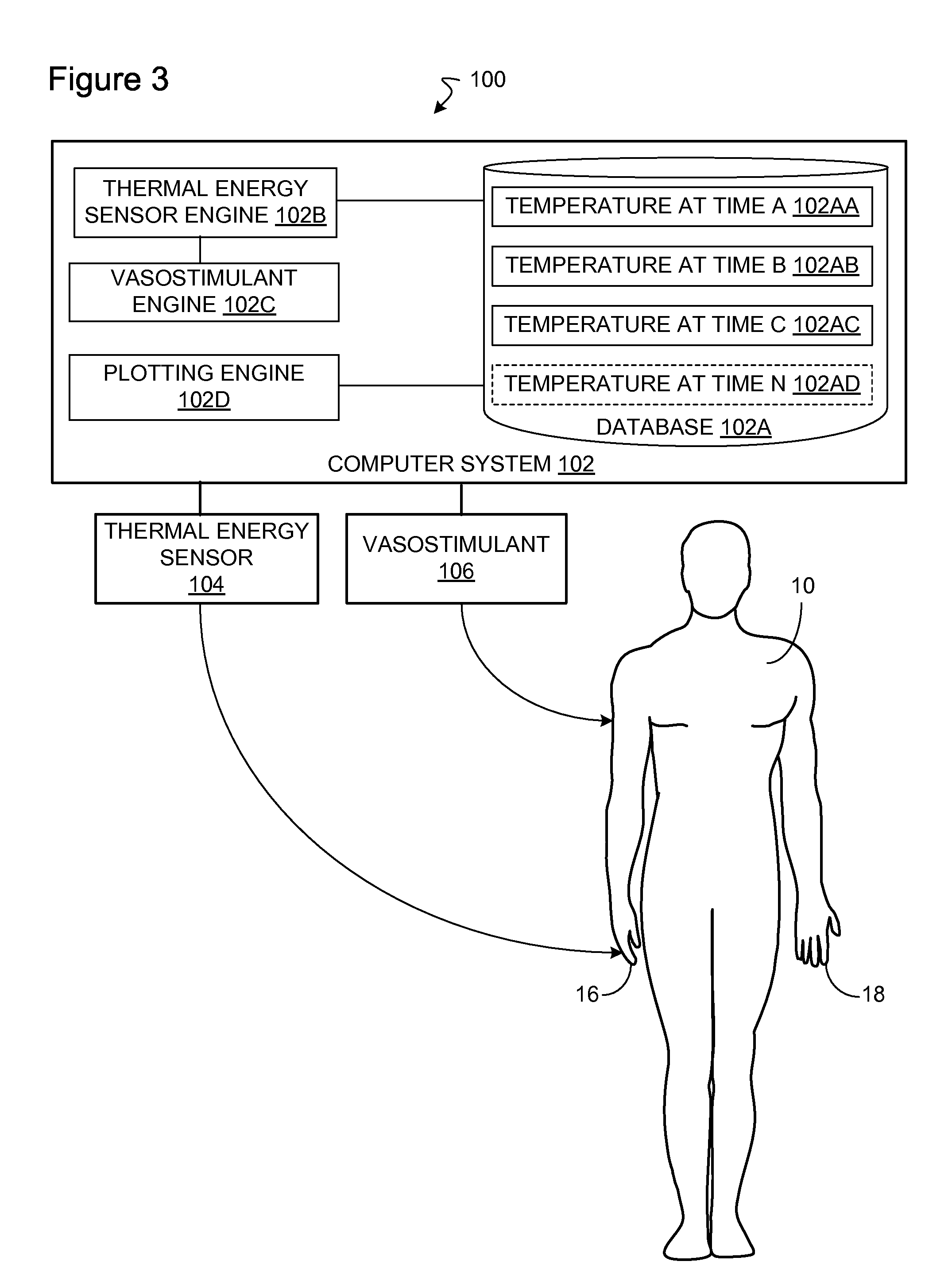
Method and apparatus for determining vascular health conditions
Owner:ENDOTHELIX
Methods and compositions for stimulating osteoblast proliferation or treating malignant cell proliferation and methods for selecting osteoblast proliferation stimulants
The present invention relates to methods for stimulating osteoblast proliferation and methods for selecting pharmacologically active compounds useful for stimulating osteoblast proliferation.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Composition for promoting healthy bone structure
InactiveUS6447809B1Increase bone densityPrevents radial bone lossBiocideHeavy metal active ingredientsVitamin CRegimen
A dietary supplement for benefitting human bone health includes a calcium source, a source of vitamin D activity, and an osteoblast stimulant. A preferred calcium source is microcrystalline hydroxyapatite, which also contains protein (mostly collagen), phosphorus, fat, and other minerals. A preferred source of vitamin D activity is cholecalciferol, and a preferred osteoblast stimulant is ipriflavone. In addition to these basic ingredients, the composition can further include various other minerals known to occur in bone, vitamin C, and glucosamine sulfate, all of which exert beneficial effects on growth and maintenance of healthy bone. A method for benefitting human bone health involves administering a daily regimen of the dietary supplement.
Owner:PHOENIX DICHTUNGSTECHN +1
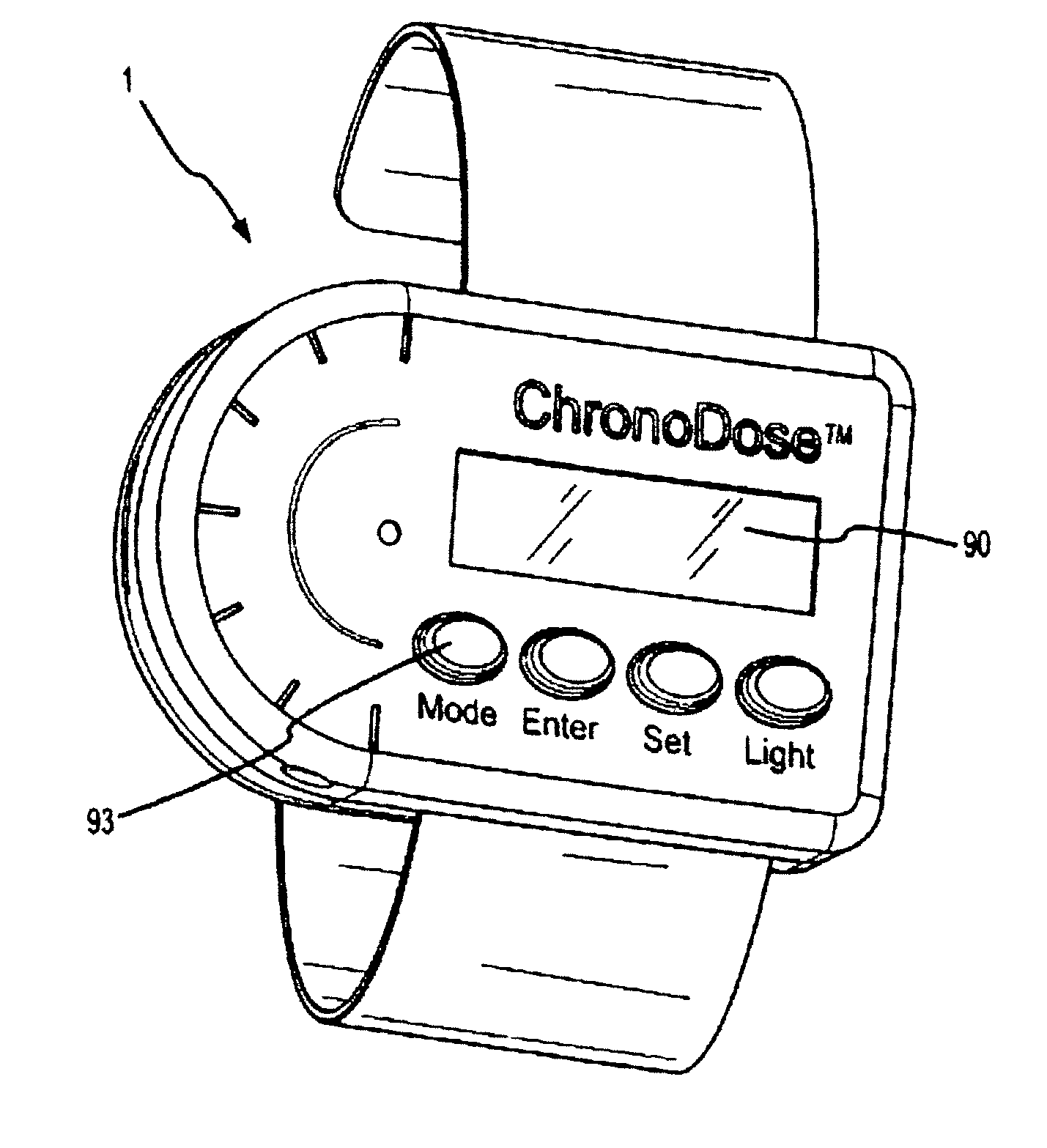
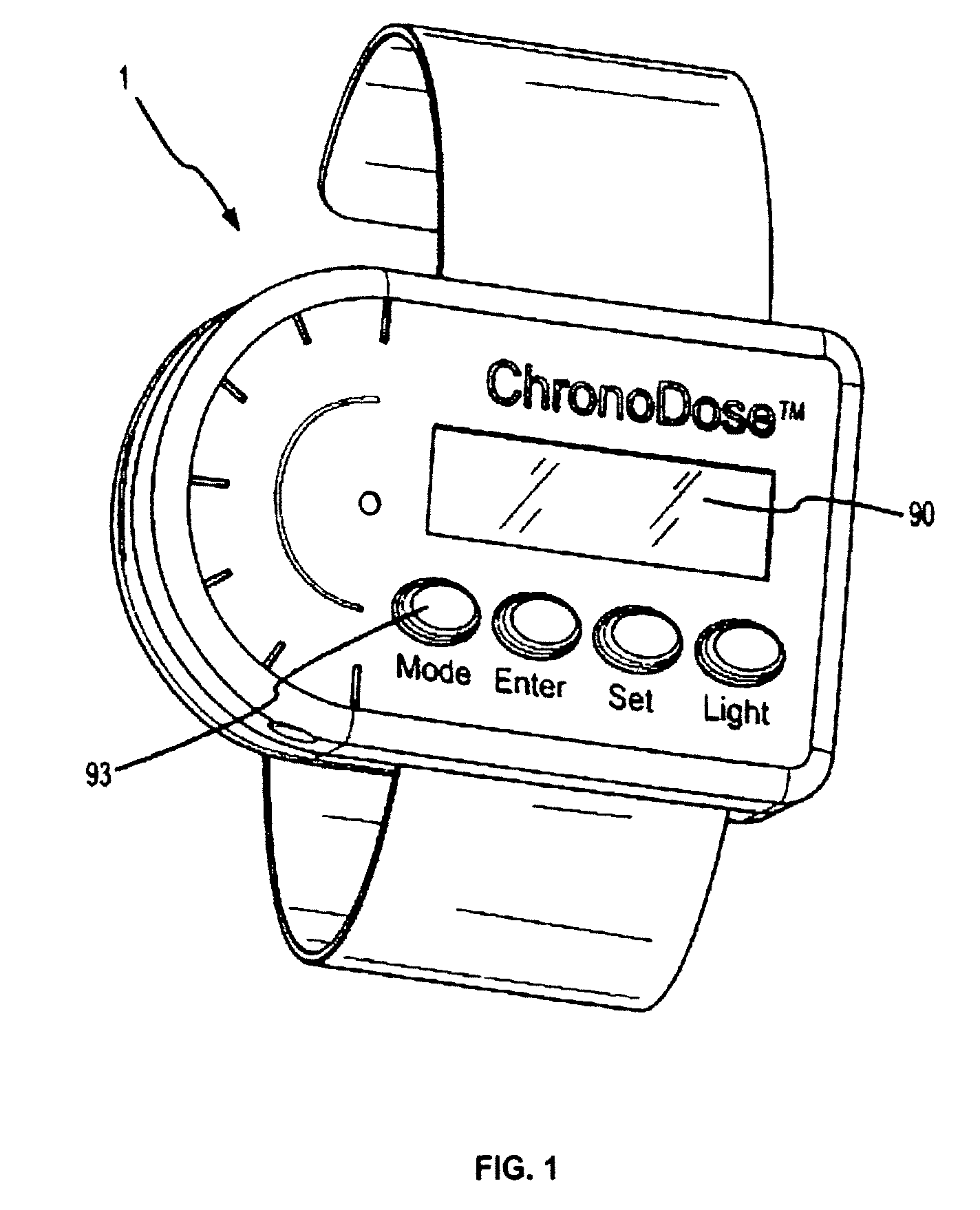
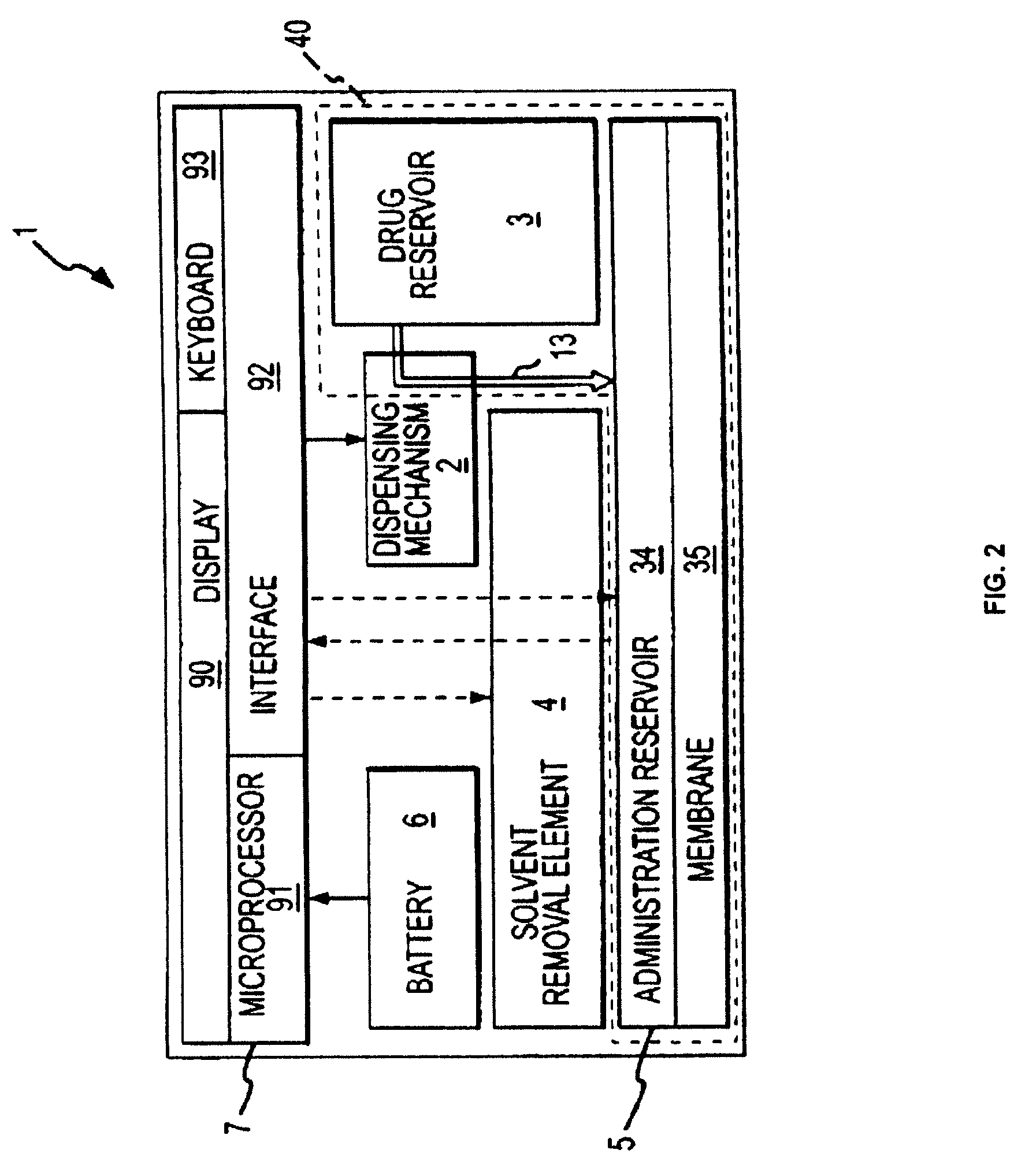
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
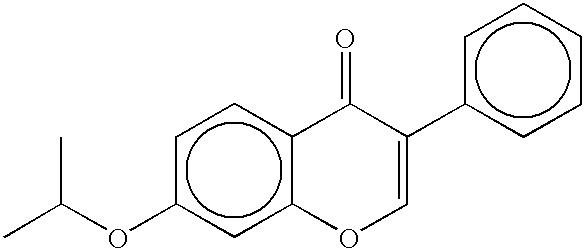
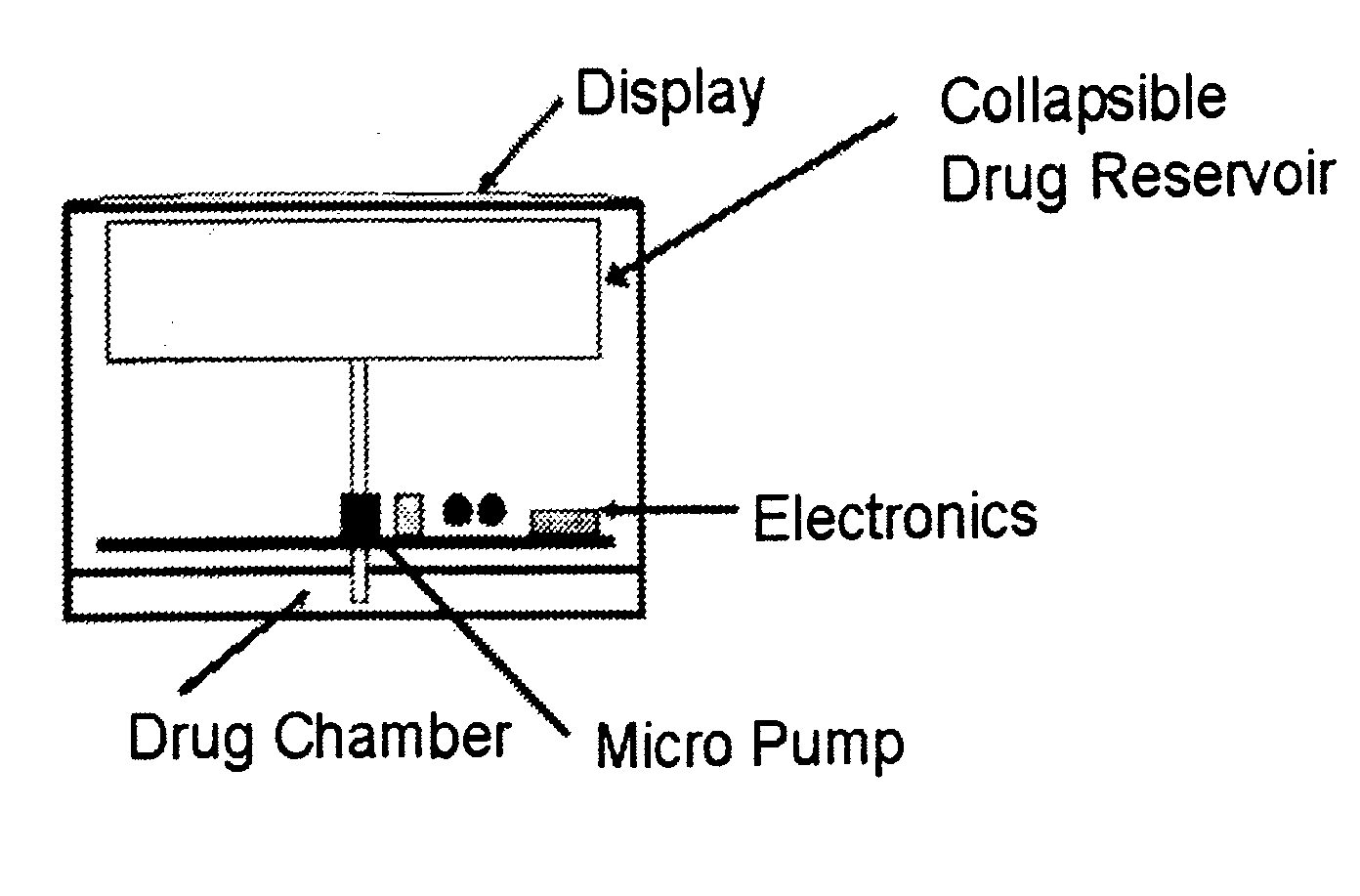
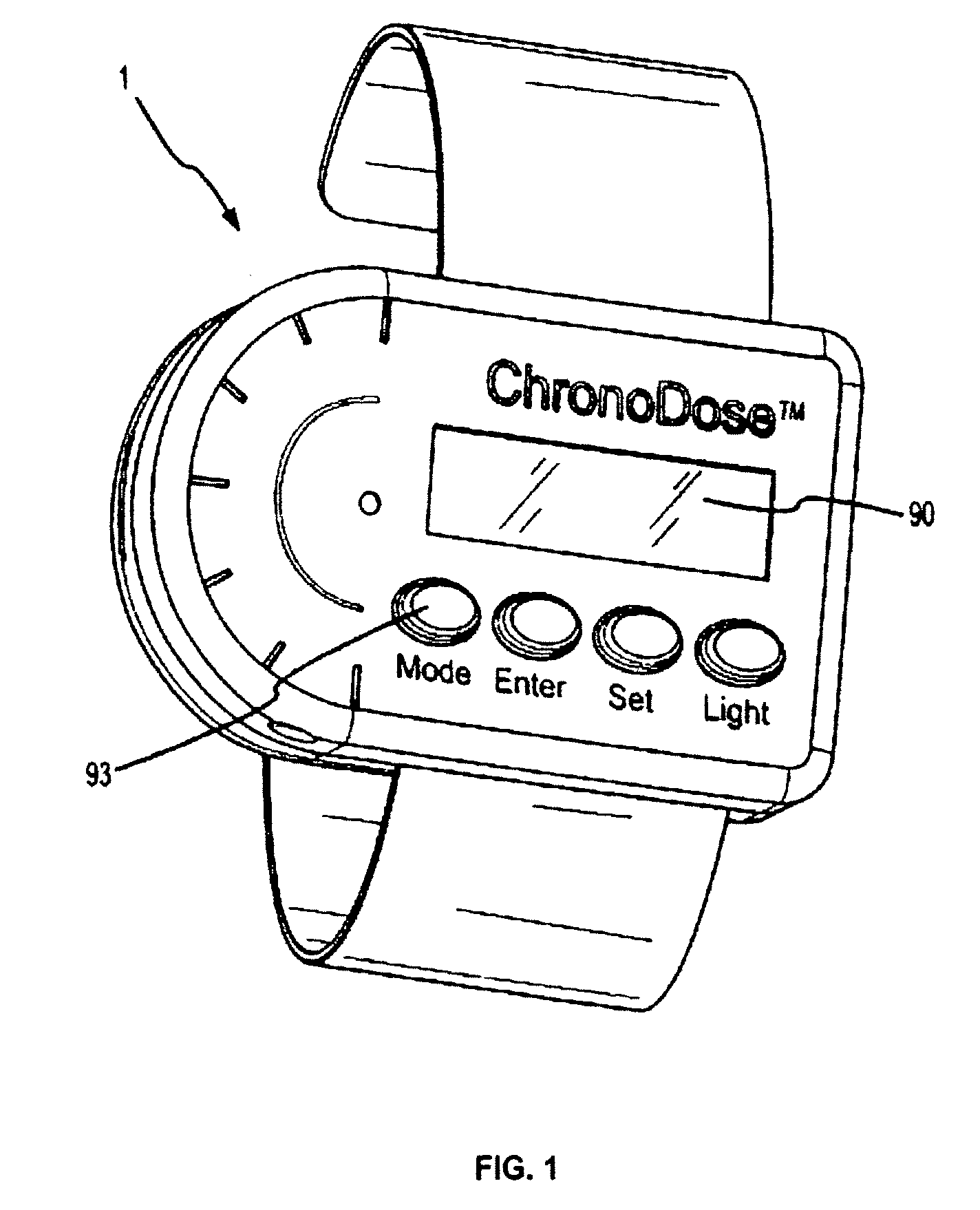
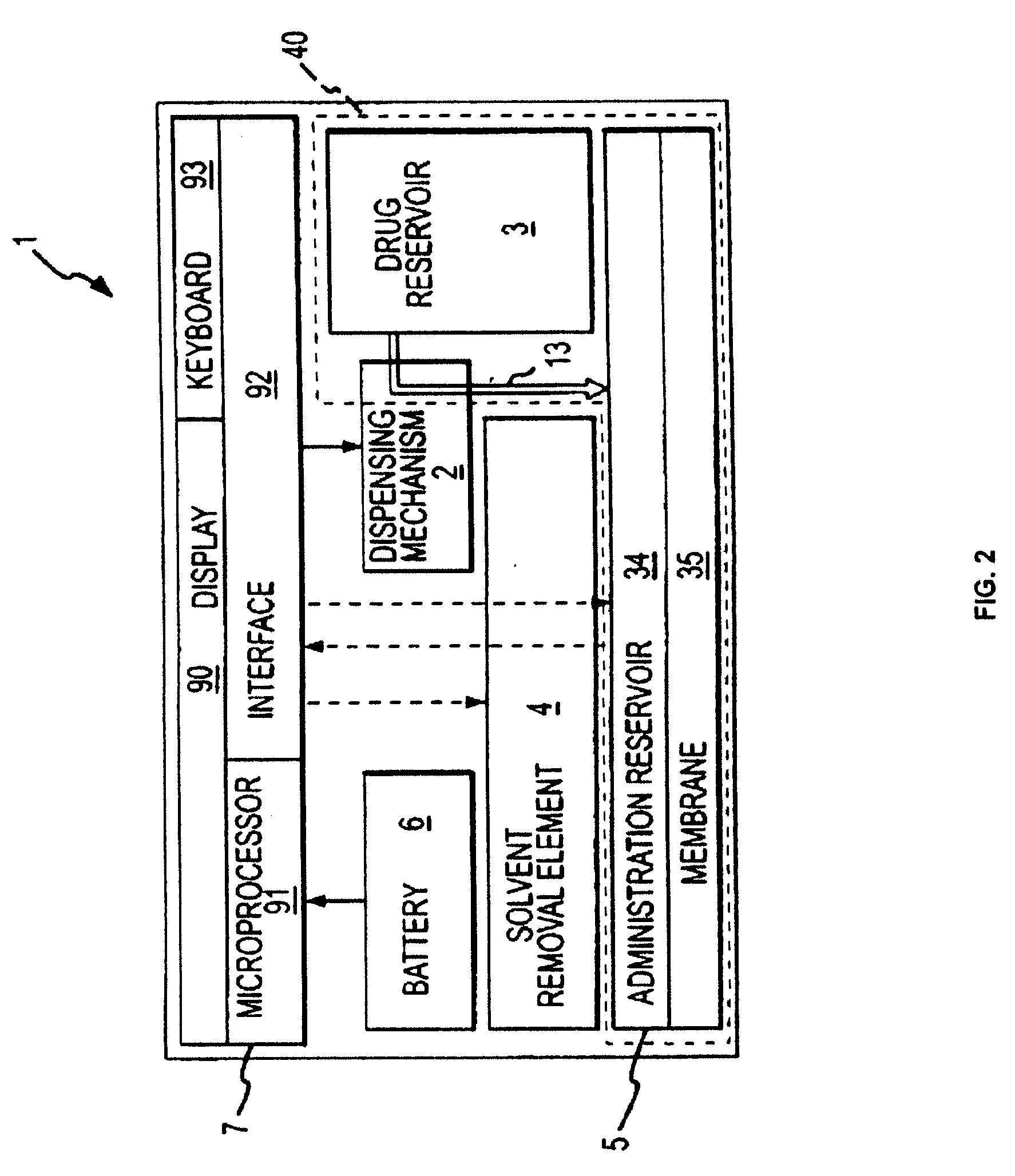
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
Nicotine containing stimulant unit
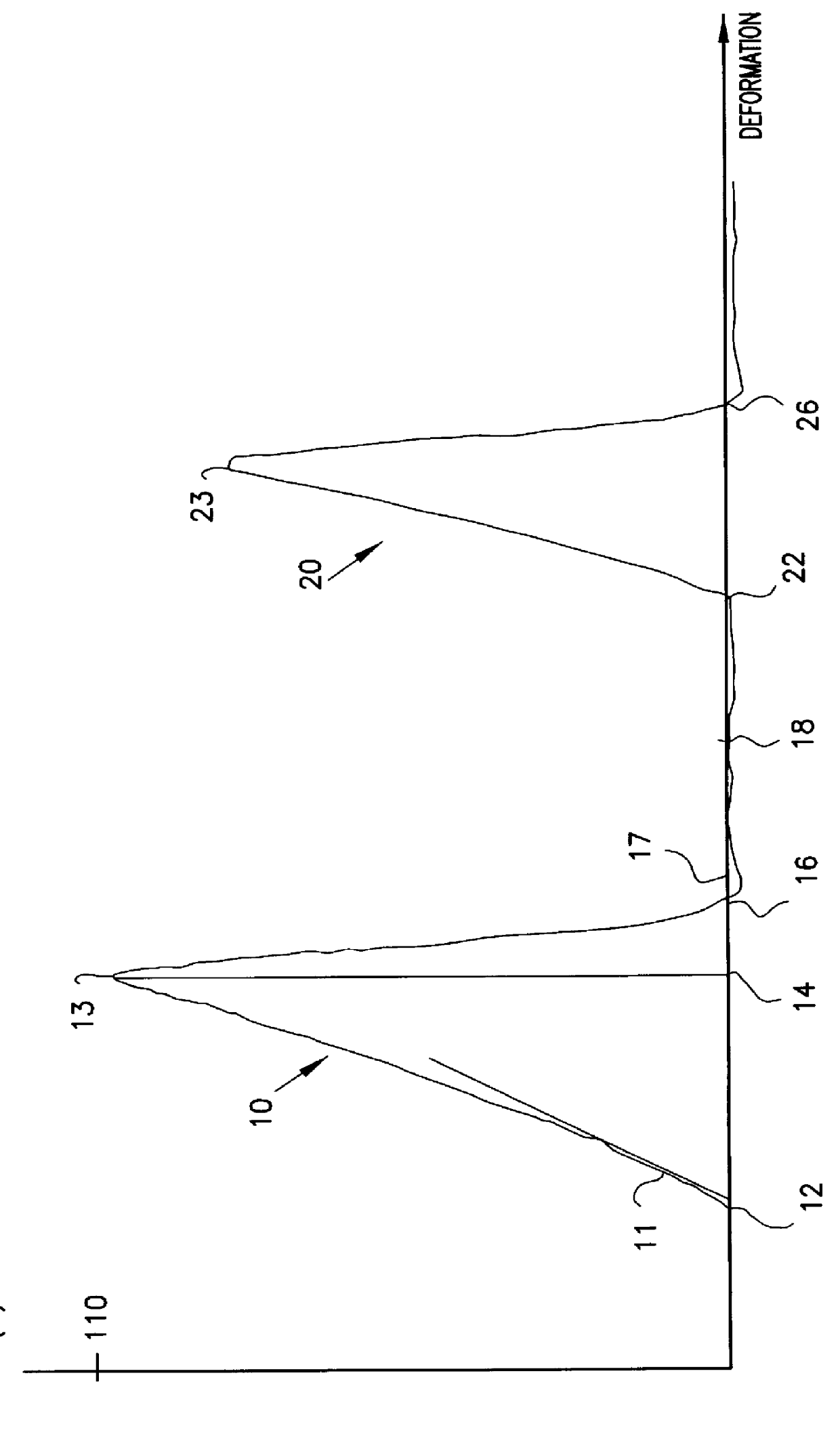
InactiveUS6110495AIncrease stimulationLarge deformationTobacco treatmentConfectioneryStimulantAdditive ingredient
A saliva-soluble stimulant unit comprising an active ingredient and optional ingredients comprising flavor and aroma additives incorporated in a gel prepared by gelling a water-binding gelling agent, in which the active ingredient comprises nicotine or other alkaloids with the same direction of activity, said unit having i) a texture profile, determined by texture profile analysis, with parameter values of firmness, hardness, brittleness, adhesiveness, elasticity, and cohesiveness within given ranges; (ii) a disintegration time within the range 5-60 minutes; and (iii) a nicotine content from 0.5 to 10 mg or a corresponding content of said alkaloids.
Owner:DAM ANDERS
Formulations and method for treating baldness
InactiveUS20060067905A1Lower Level RequirementsSlow, prevent, or even reverse hair lossCosmetic preparationsHair removalPersonal careApigenin
The present invention includes 1) a novel formulation for the treatment of hair loss comprising oleanolic acid (a 5α-reductase inhibitor), apigenin (a vasodilator), and biotinyl-GHK (a cell metabolism stimulant), 2) a novel additive for the treatment of hair loss comprising oleanolic acid, apigenin, biotinyl-GHK and a delivery agent, 3) a personal care, cosmetic, and / or dermopharmaceutical composition comprising oleanolic acid, apigenin, biotinyl-GHK, and at least one additional ingredient, and 4) a method for treating hair loss comprising the administration of oleanolic acid, apigenin, and biotinyl-GHK.
Owner:SEDERMA SA
Chewing gum products containing trigeminal stimulant and method of making the same
InactiveUS20050202118A1Prolonged flavor durationImproved sensory benefitConfectioneryChewing gumFlavorStimulant
Chewing gums and methods of making same that have improved flavor duration by stimulating a trigeminal nerve of a consumer of the chewing gum are provided. The chewing gums of the present invention includes a trigeminal stimulant. The trigeminal stimulant stimulates the trigeminal nerve of the consumer to provide longer lasting flavor duration.
Owner:WM WRIGLEY JR CO
Methods and compositions for stimulating osteoblast proliferation or treating malignant cell proliferation and methods for selecting osteoblast proliferation stimulants
InactiveUS20030119791A1Overcome limitationsPromote growthBiocideOrganic chemistryStimulantHormones regulation
The present invention relates to pharmacologically active compounds which are capable of binding to nuclear hormone receptors and are useful for the stimulation of osteoblast proliferation and ultimately bone growth. This invention also relates to the use of such compounds for the treatment or prevention of diseases and / or disorders associated with nuclear hormone receptor families.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Fibrous materials exhibiting thermal change during use
InactiveUS20050136765A1Exothermal chemical reaction heat productionSynthetic resin layered productsFiberThermal variation
Fibrous sheet materials, such as are useful as facial tissue, bath tissue and paper towels, for example, are provided with chemical agents, such as certain salts, which create a temperature change in the sheet when exposed to a particular stimulant, such as water, for example. These materials can provide a soothing feel to the user, either in the form of cooling or heating, depending upon the particular chemistry involved.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Method of treating depressive disorders
The invention provides methods of treating depressive disorders, in particular major depression but other depressive orders also, with prodrug stimulants or analogs including amphetamine prodrugs, methylphenidate prodrugs, and methylphenidate analogs, Such methods of treatment may utilize the prodrug stimulant or analog as monotherapy or, more commonly, as an adjunct to antidepressant medication treatment to augment their effect. The invention includes combination methods of treatment in which an amphetamine prodrug, methylphenidate prodrug, or methylphenidate analog is administered to an individual in need with one or more other active agents, either in separate forms or as a single pharmaceutical formulation. Packaged pharmaceutical compositions containing an amphetamine or methylphenidate prodrug, instructions for using the prodrug to treat certain disorders, and optionally one or more other active agents are provided by the invention.
Owner:LUCERNE BIOSCI
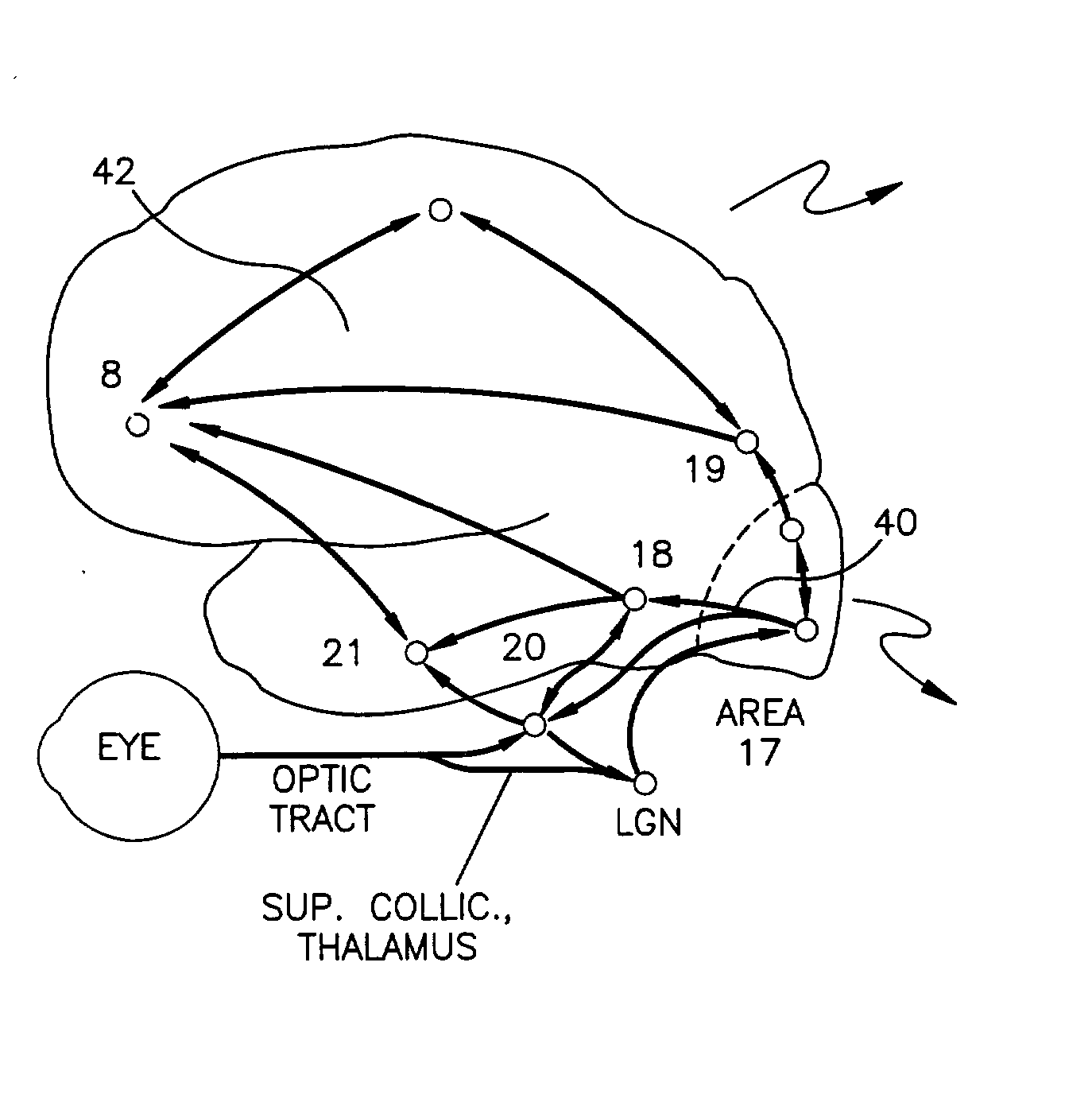
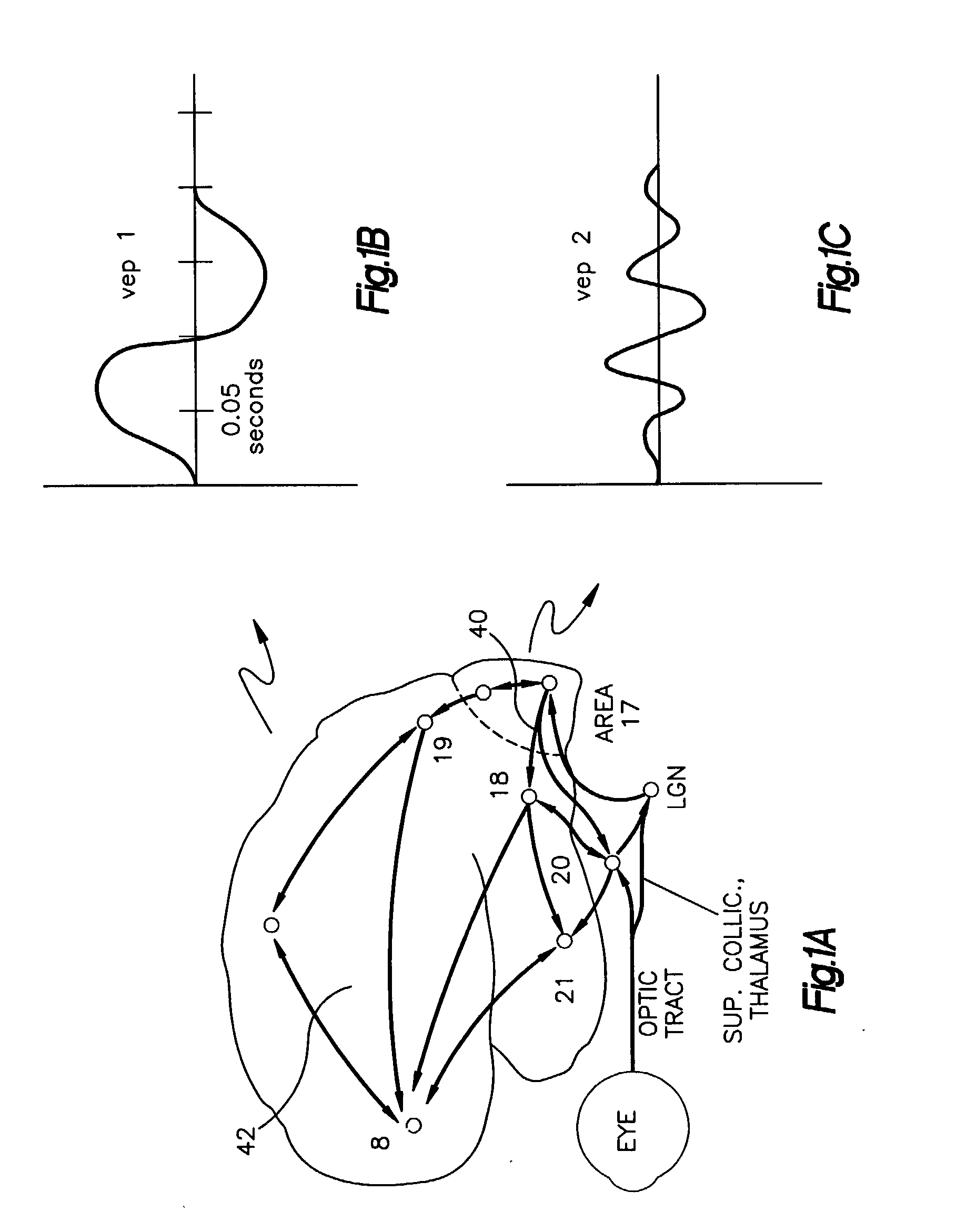
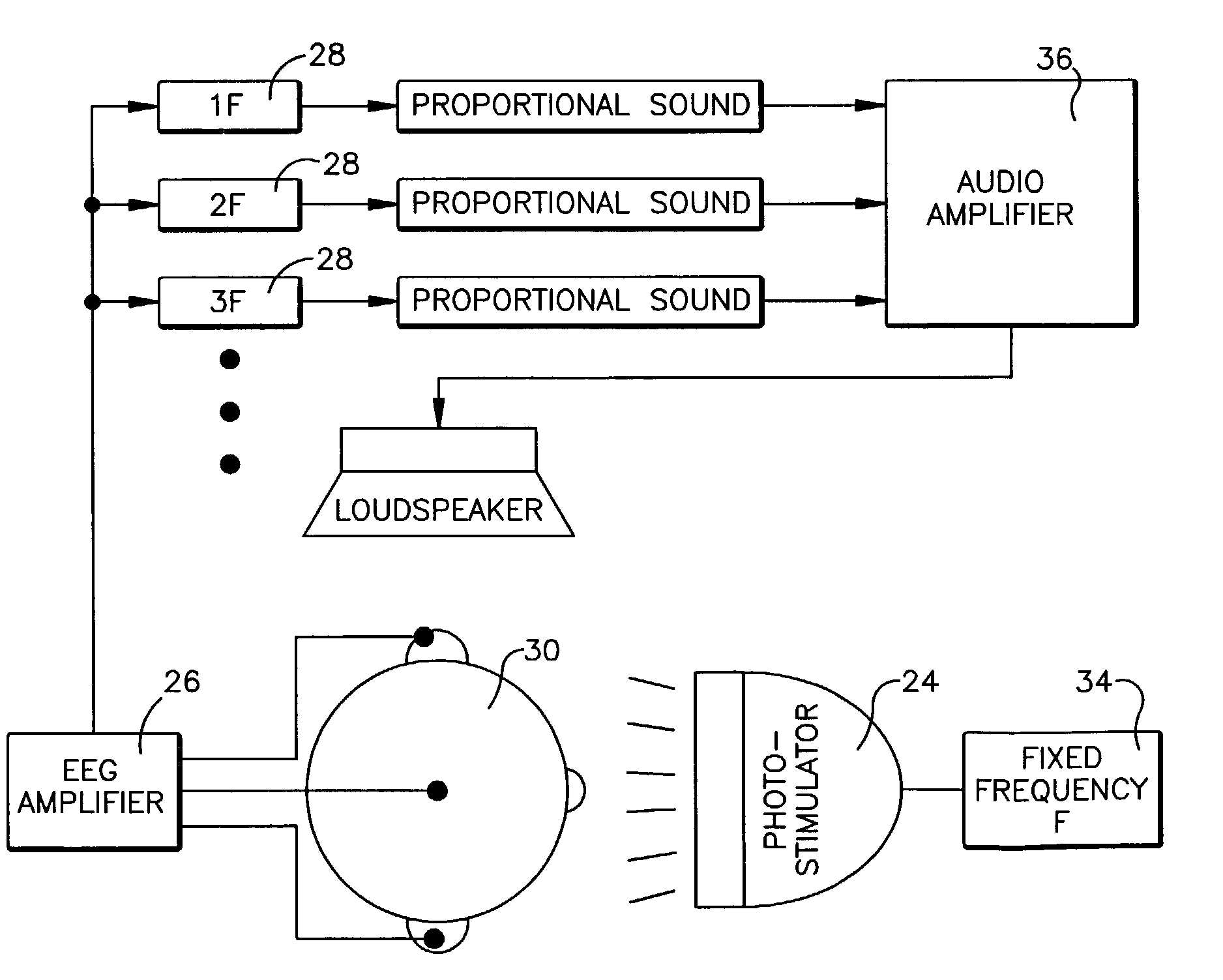
Repetitive visual stimulation to EEG neurofeedback protocols
An EEG neurofeedback and total evoked brain activity measurement methods utilize minimum ambient EEG activity as stimulant frequencies. A method of using repetitive stimulation in conjunction with EEG neurofeedback protocols is described. Electrodes, attached to a subject's scalp, transmit electroencephalographic (EEG) signals from the subject. These signals are in response to the visual and / or auditory stimuli being displayed to the subject. The resultant EEG signals are then filtered at pre-defined frequencies or frequency bands. The output from the filtered EEG signals is then analyzed and monitored for short-term state changes. The invention also uses flicker stimulation, real-time signal filtration and feedback, feedback during audio and visual stimulation derived from filtered outputs, and fundamental and integral harmonics in combination with total evoked response.
Owner:COLLURA THOMAS F
Self-molding permanent agent and method for proceeding free-rod and free-band type permanent
The present invention relates to a self-molding permanent agent and a method for proceeding free-rod and free-band type permanent, more particularly to a self-molding permanent agent comprising (a) a reducing composition containing a reducing agent reducing a disulfide bond of cystine on the hair and a molding stimulant spontaneously molding to fix a hair design; (b) a molding composition inducing to mold after reacting with the molding stimulant; and (c) a softening composition releasing the action of a molding stimulant, and a method for pressing a free-rod and free-band type permanent, which overcomes a disadvantage in the conventional method for pressing a permanent that needs to wear a curling device such as rods for a permanent (perm rod) or rubber band and improves to apply a wave set without a hair-curling device for a short time, since it has a self-molding feature.
Owner:KOREA RES INST OF CHEM TECH
Chewing gum having prolonged sensory benefits
InactiveUS7025999B2Prolong sensory benefitLong-lasting flavorContainers for annular articlesChewing gumSimple Organic CompoundsFlavor
Chewing gums and methods of making same that have prolonged and enhanced sensory benefits are provided. The chewing gums of the present invention include a hydrophobic sweetener, a sensorally active component or trigeminal stimulant, such as a flavor, in addition to other typical chewing gum ingredients. The hydrophobic sweeteners are composed of sweet organic compounds that have a low water solubility.
Owner:WM WRIGLEY JR CO
Residence structures and related methods
ActiveUS20170106099A1Facilitate dissociationTetracycline active ingredientsMedical devicesStimulantDissolution
Residence structures, systems, and related methods are generally provided. Certain embodiments comprise administering (e.g., orally) a residence structure to a subject (e.g., a patient) such that the residence structure is retained at a location internal to the subject for a particular amount of time (e.g., at least about 24 hours) before being released. The residence structure may be, in some cases, a gastric residence structure. In some embodiments, the structures and systems described herein comprise one or more materials configured for high levels of active substances (e.g., a therapeutic agent) loading, high active substance and / or structure stability in acidic environments, mechanical flexibility and strength in an internal orifice (e.g., gastric cavity), easy passage through the GI tract until delivery to at a desired internal orifice (e.g., gastric cavity), and / or rapid dissolution / degradation in a physiological environment (e.g., intestinal environment) and / or in response to a chemical stimulant (e.g., ingestion of a solution that induces rapid dissolution / degradation). In certain embodiments, the structure has a modular design, combining a material configured for controlled release of therapeutic, diagnostic, and / or enhancement agents with a structural material necessary for gastric residence but configured for controlled and / or tunable degradation / dissolution to determine the time at which retention shape integrity is lost and the structure passes out of the gastric cavity. For example, in certain embodiments, the residence structure comprises a first elastic component, a second component configured to release an active substance (e.g., a therapeutic agent), and, optionally, a linker. In some such embodiments, the linker may be configured to degrade such that the residence structure breaks apart and is released from the location internally of the subject after a predetermined amount of time.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
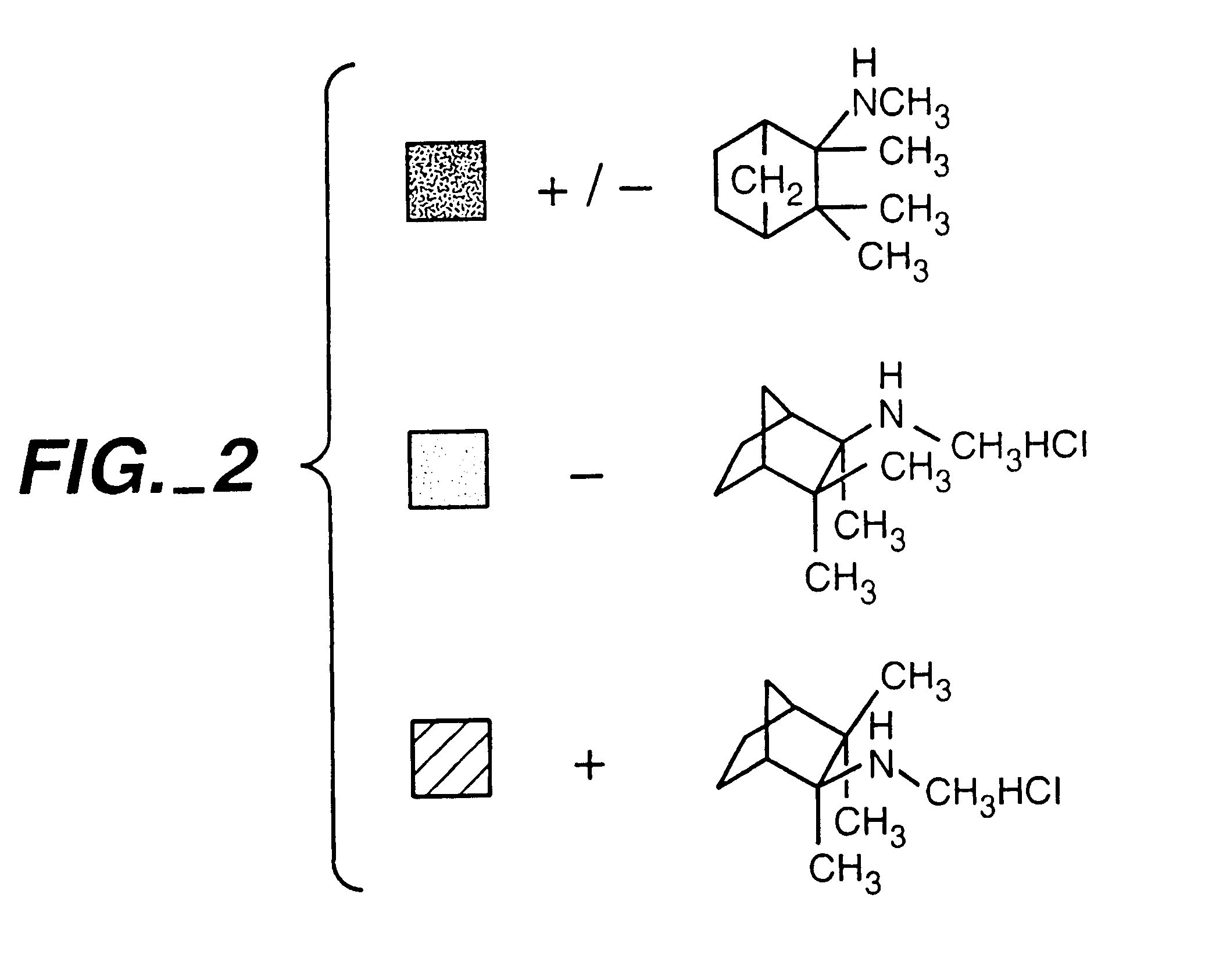
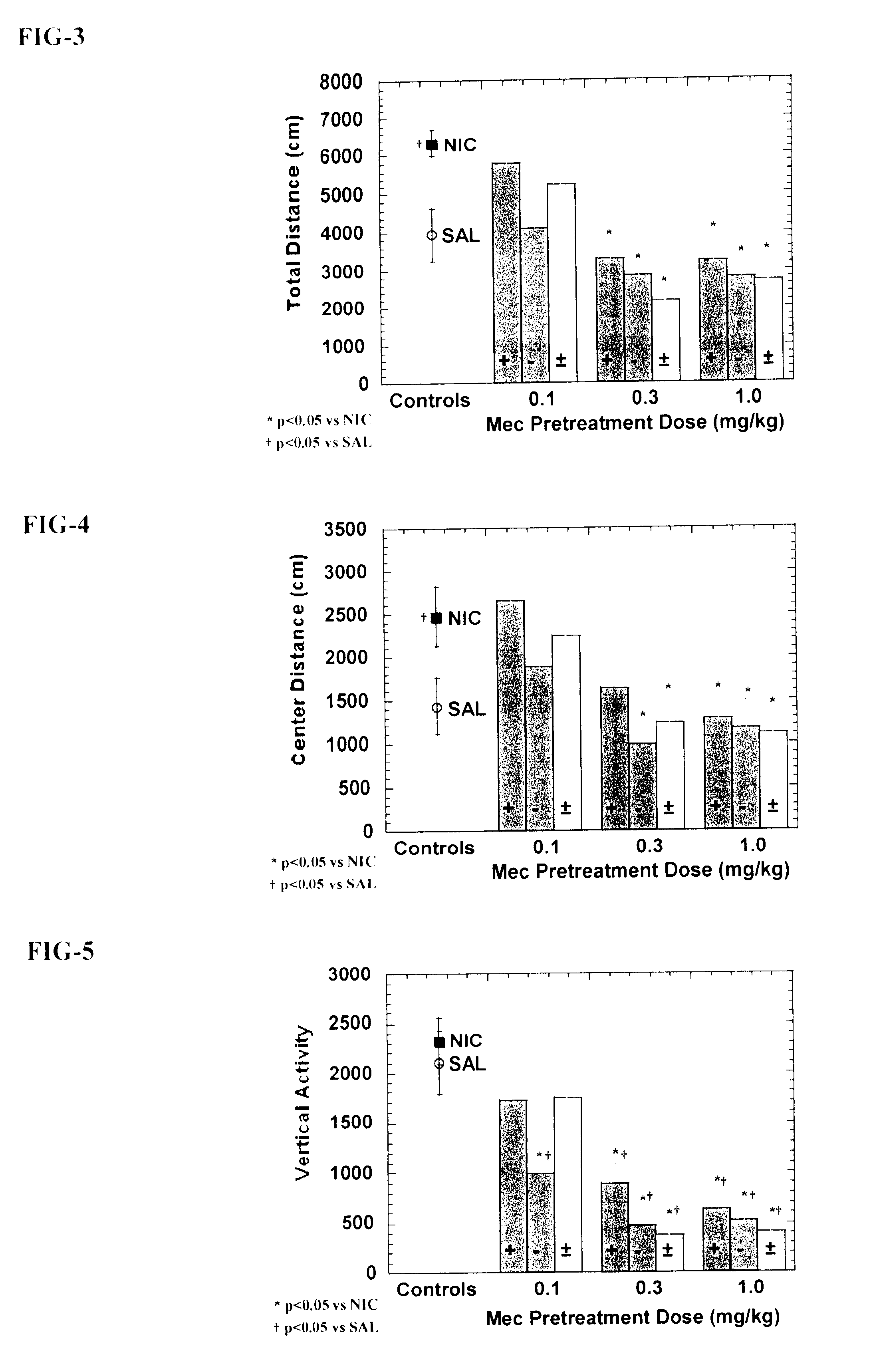
Exo-R-mecamylamine formulation and use in treatment
InactiveUS20020016370A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationStimulantS syndrome
A pharmaceutical composition includes a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-S-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-S-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
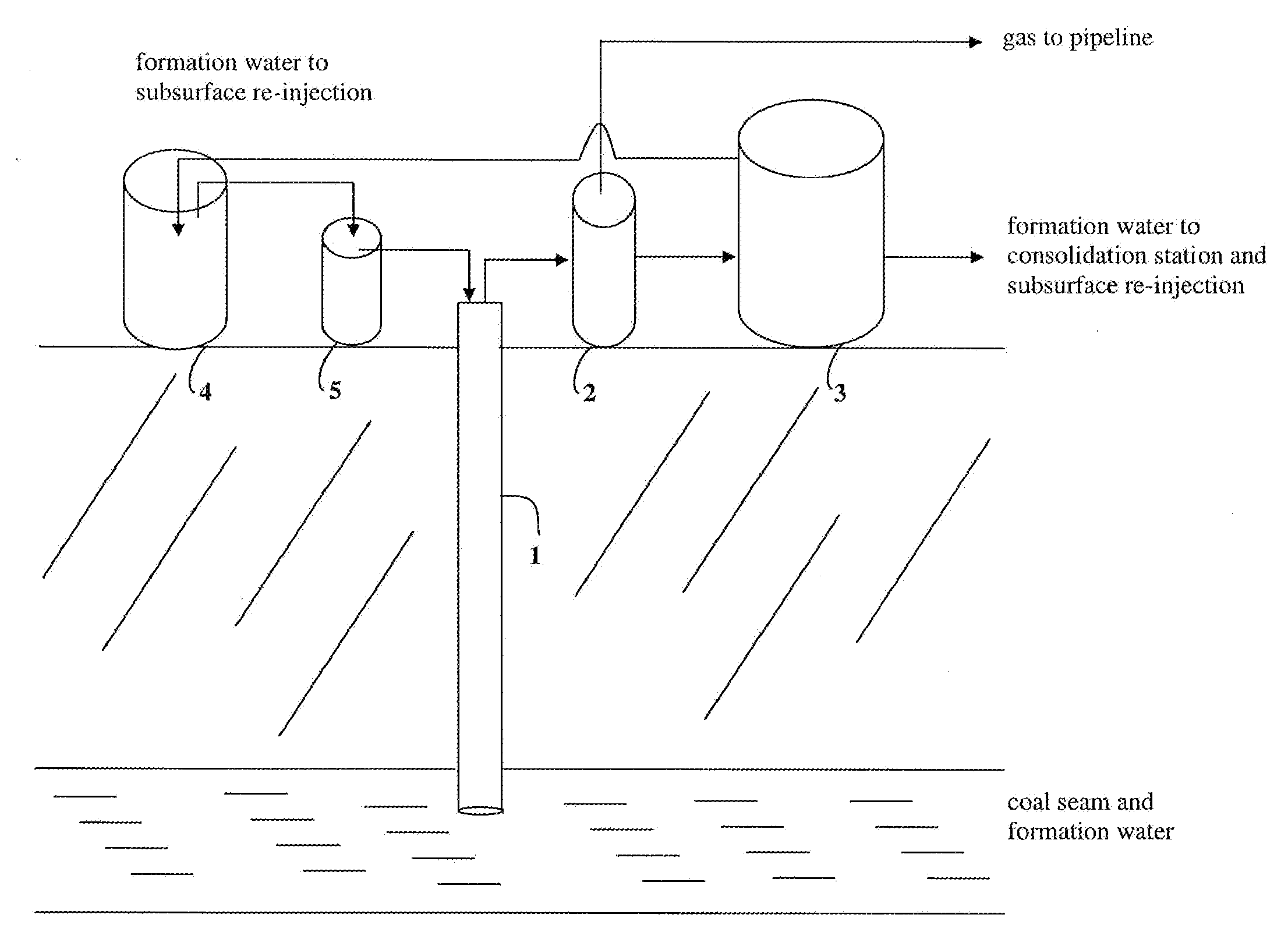
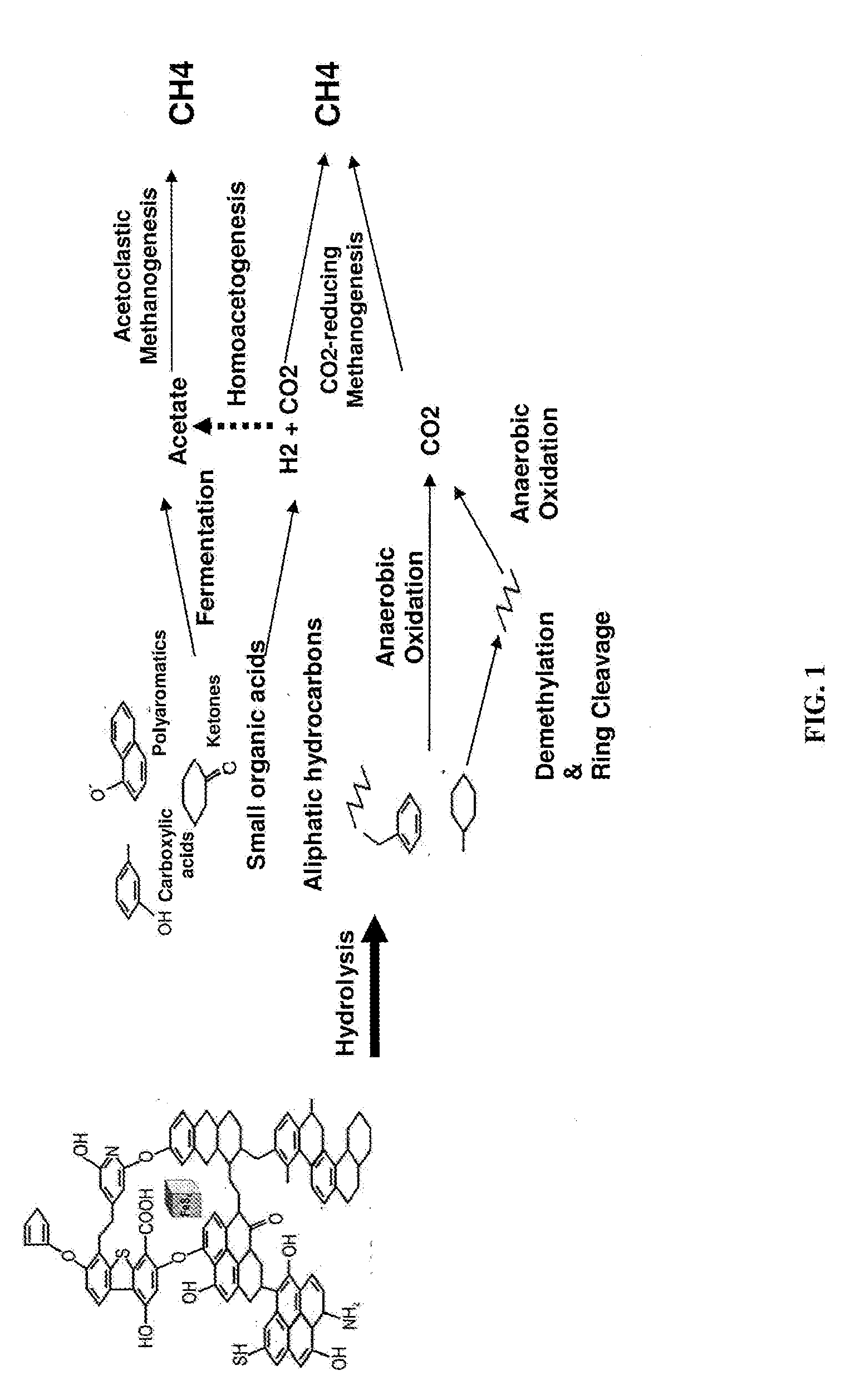
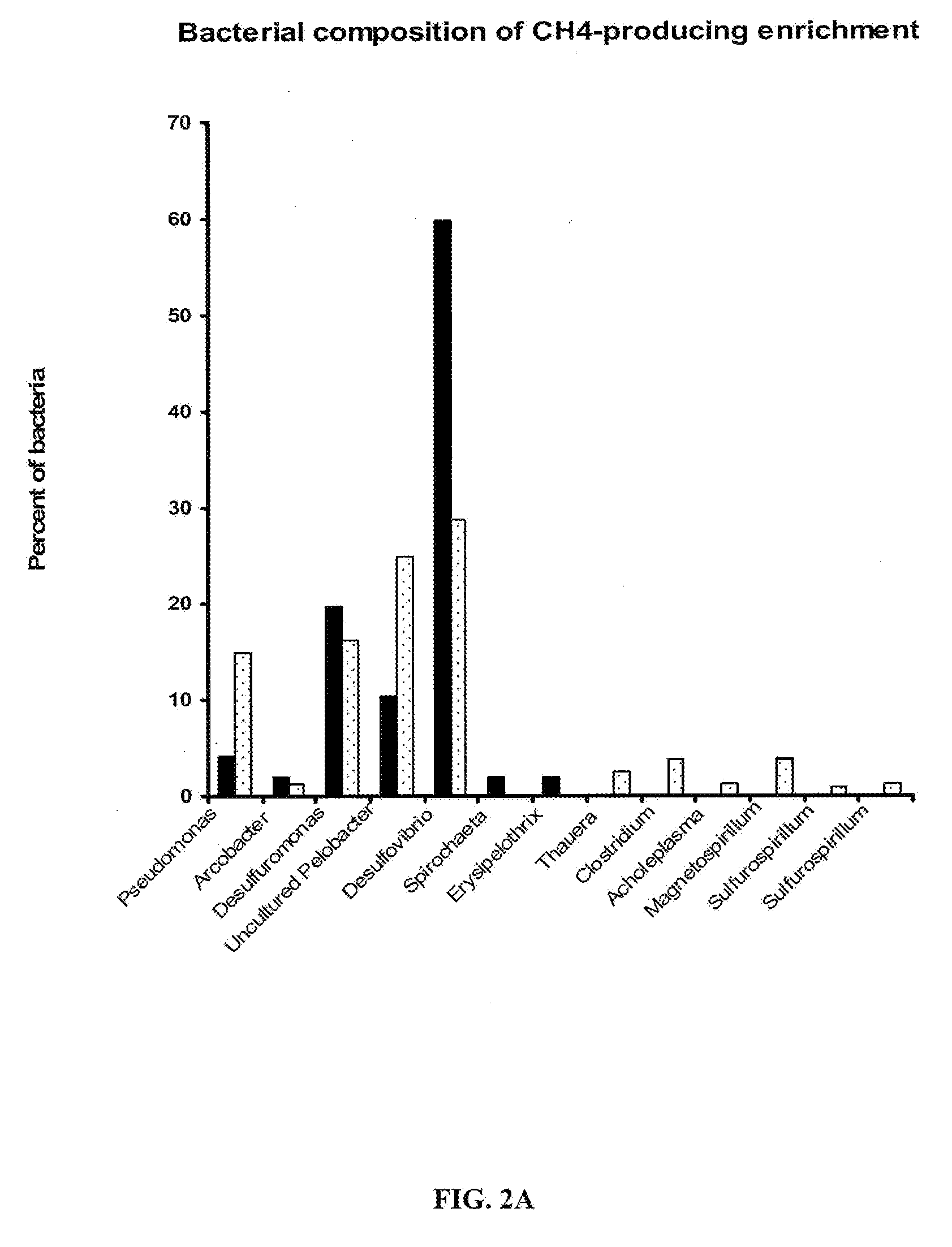
Methods to stimulate biogenic methane production from hydrocarbon-bearing formations
The present invention describes methods of identifying stimulants for the biogenic production of methane in hydrocarbon-bearing formations. Methods involve the use of microbial nucleic acid sequence information for the determination of gene products that are enzymes in a variety of pathways involved in the conversion of hydrocarbons to methane. Enzymes and stimulants identified by invention methods can be used in processes for enhancing biogenic methane production, for example, by addition to coal seams and coalbed methane wells.
Owner:SYNTHETIC GENOMICS INC
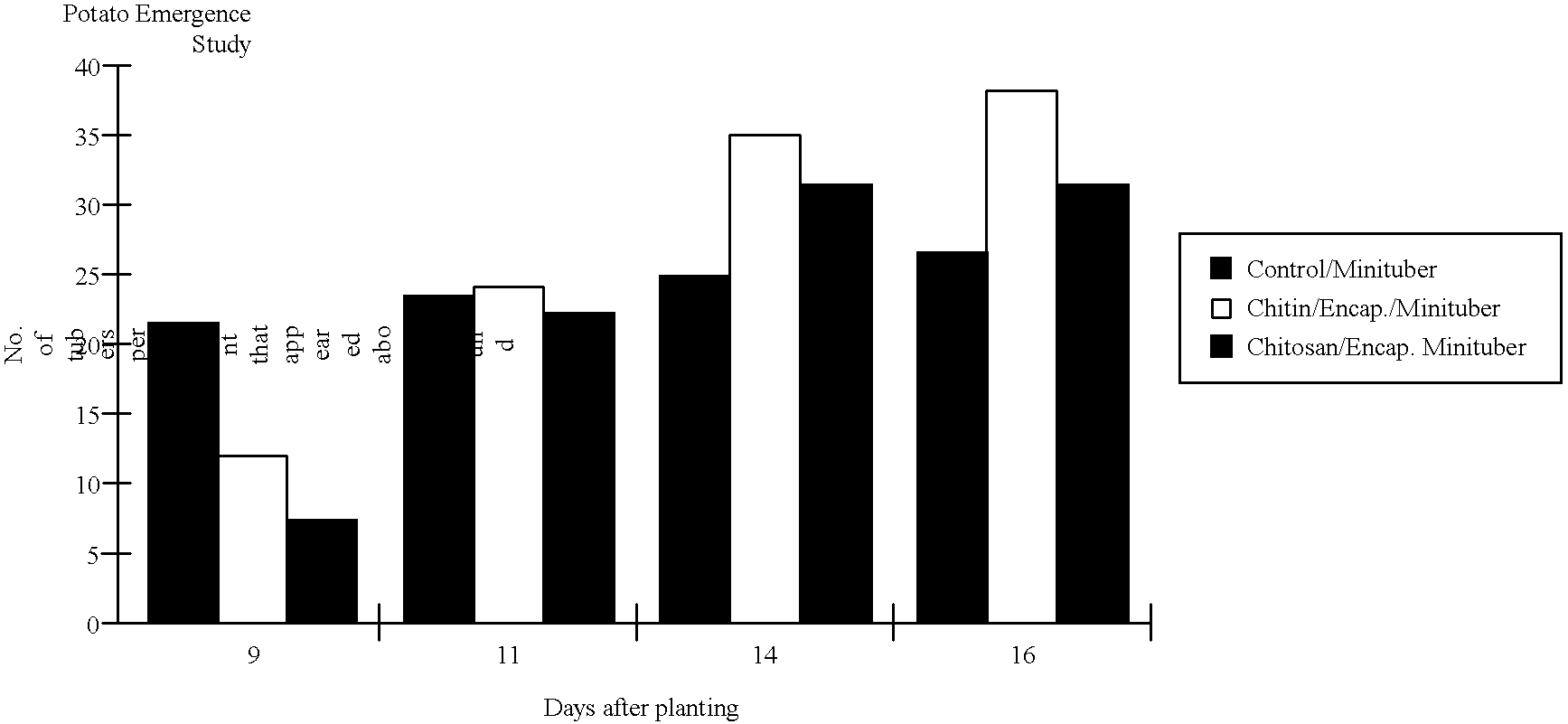
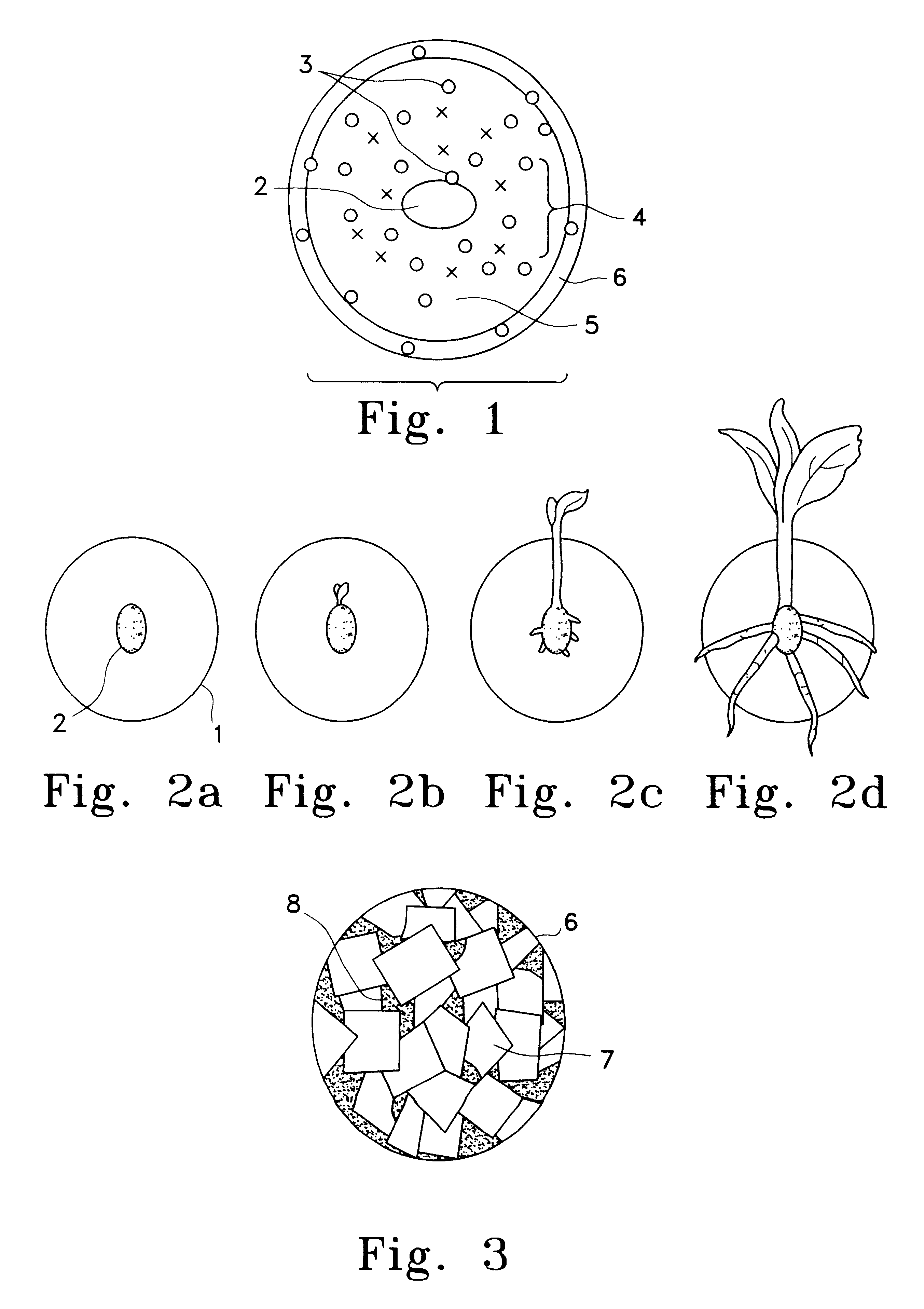
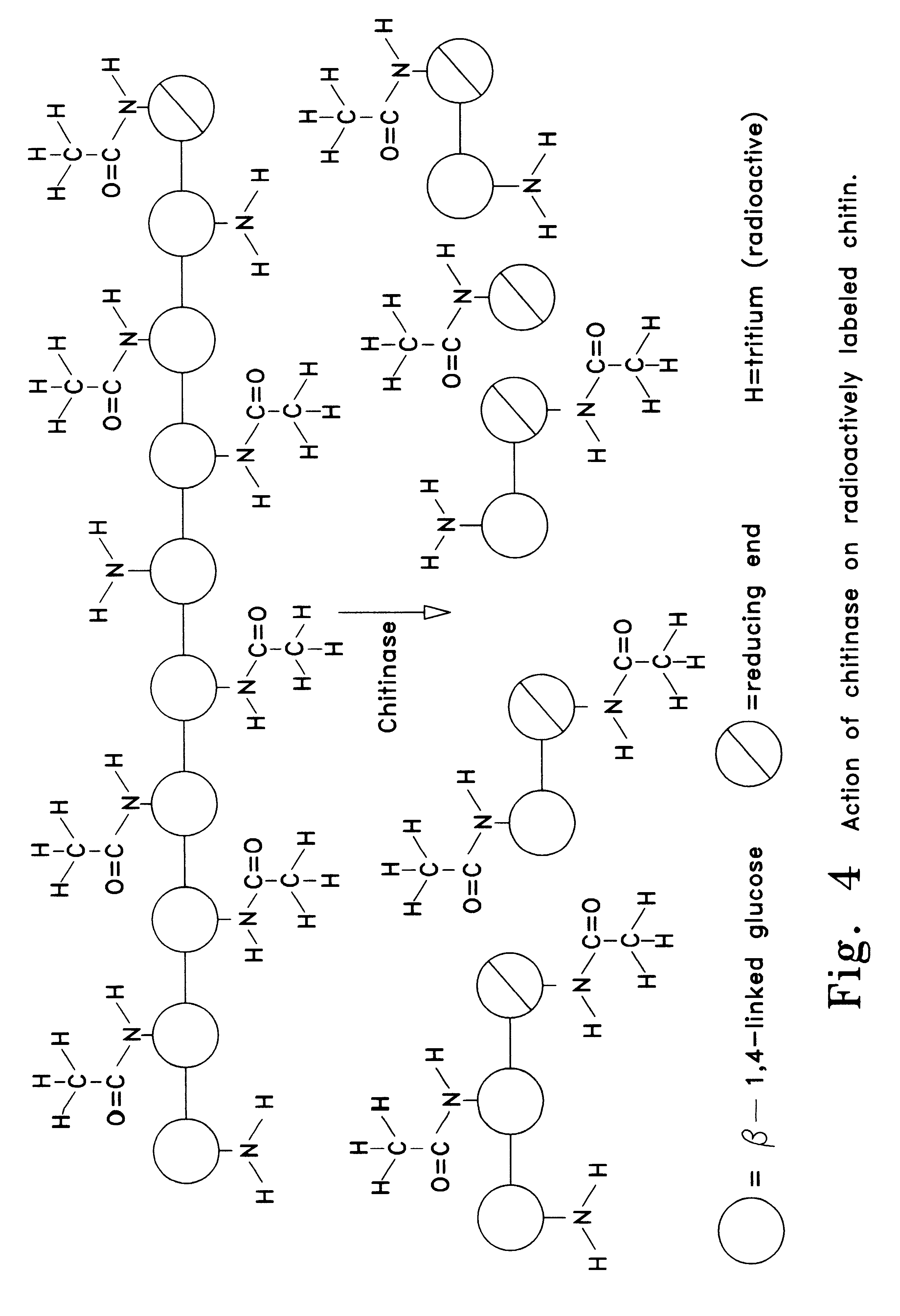
Tuber planting system comprising chitin or chitosan
InactiveUS6193988B1Increase emergenceHigh yieldBiocideDead animal preservationActivated carbonPropagule
A planting system involving a non-damaging stimulus (3) which is placed in the vicinity of a propagule (2) causes a naturally defensive substance (5) to be produced by the propagule. This substance may exist within an encapsulant (1) for a period of time so that the propagule may have enhanced disease control until it develops sufficiently to fend for itself. In one embodiment a large encapsulant surrounds a potato tuber. This encapsulant may include chitin to cause the release of chitinase through intermediate stimulants such as mRNA and activated carbon to absorb or release particular substances. The encapsulant is bound through the use of pentosan which acts with flaked chitin to achieve an outer casing and further protect the propagule both mechanically and chemically.
Owner:ENVIROGEN
Audible distance measurer-object discriminator cognition stimulant system device
InactiveUS20070085993A1Increase freedomEase the tormentOptical rangefindersHeight/levelling measurementDigital videoDiscriminator
An audible distance measurer object-face discriminator cognition stimulant system device housed in a handheld shaft, walking stick, belt attachment, or portable housing. The system device will capture, detect, merge, convert, process, compute and / or synthesize signals, measurements, images, objects-faces, colors, and shapes to output assistive phrases and measurements in electronic voice, to inform and make the user knowledgeable of objects and aware of surroundings and environment directly in path of movement-direction pointed, comprising in combination strategically positioned signal receivers, transmitters or satellite, embedded distance measurer sensor, digital video camera, cellular phone, radio, electronic travel aid (ETA), electronic orientation aid (EOA), position locator device (PLD), embedded restroom facility locator sensor, and embedded vehicle presence alert system (VPAS) sensor, which will generally aid sighted and visually impaired students, teachers and students of the No Child Left Behind (NCLB) Act, and more particularly, the elderly, and visually impaired military war veterans in a Right to Personal Privacy, while simultaneously providing sighted and blind-visually impaired children with an educational toy and interactive game.
Owner:BROWN ROBERT JR
Therapeutic Treatment
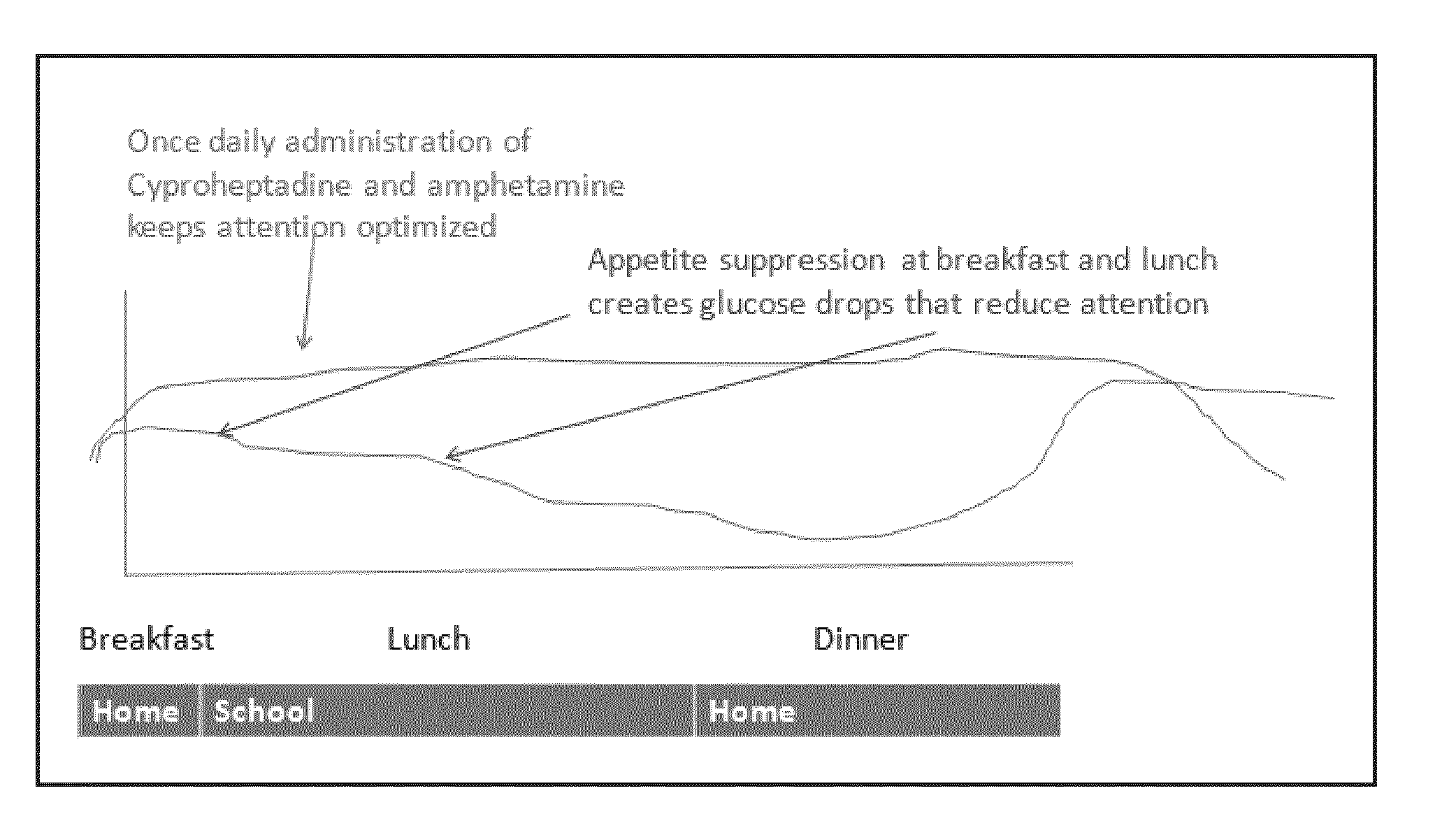
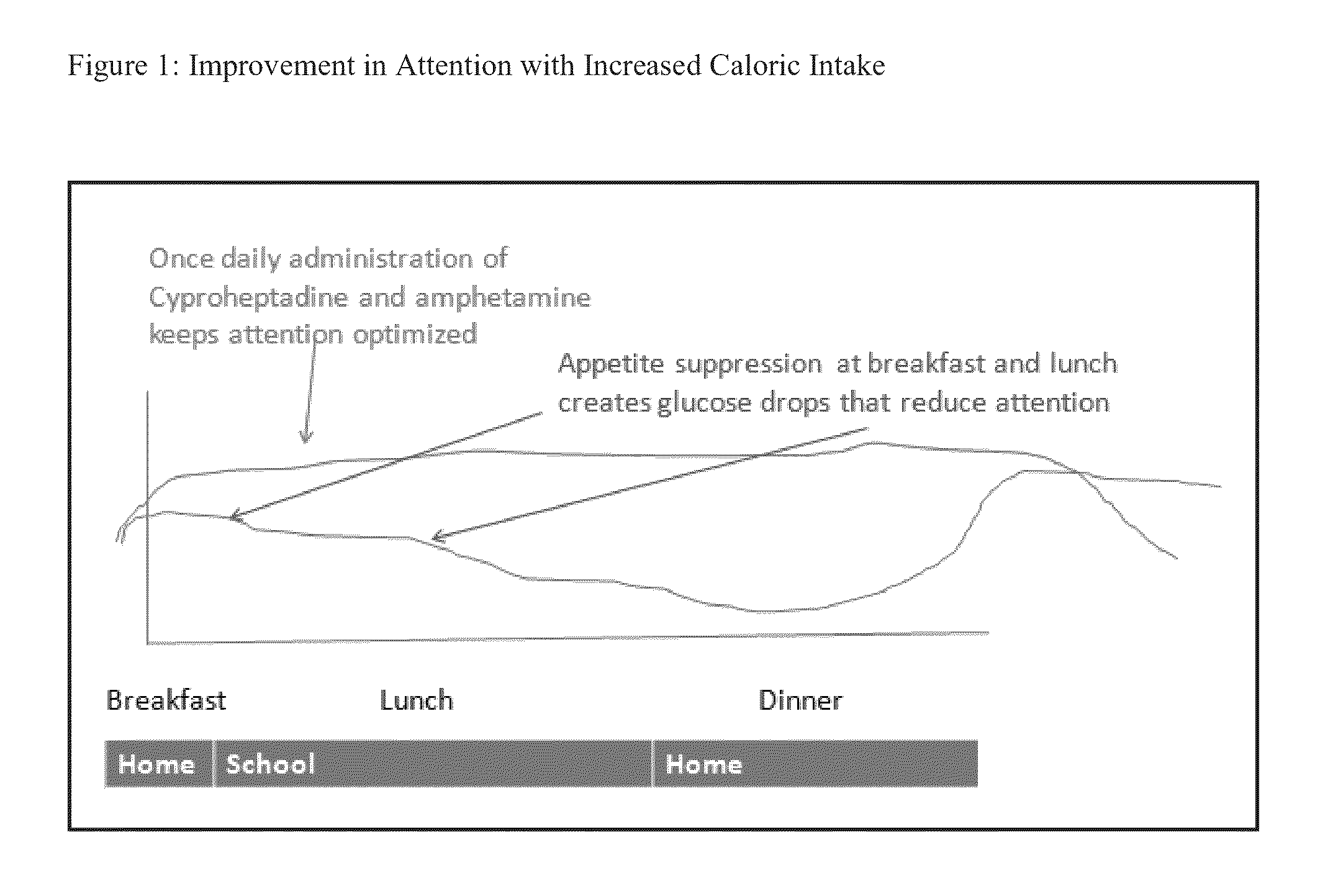
This invention discloses a treatment for a patient receiving medication to treat an attention deficit disorder such as ADHD wherein the treatment results in a loss of appetite and impairment of the patient's attentiveness. The treatment combines a treatment for an attention deficit disorder with an appetite stimulant, wherein the appetite stimulant increases the caloric intake of a patient, which can increase the patient's attentiveness. The combination treatment can be given for an indefinite, including, without limitation, life-long, to allow a patient to maintain normal caloric intake during treatment for an attention deficit disorder.
Owner:ATTENTIVE THERAPEUTICS INC
Root-specific, stimulant inducible promoter and its use
ActiveUS7196247B2Sugar derivativesOther foreign material introduction processesAntisense OrientationStimulant
This invention relates to an isolated isoflavone synthase 1 (IFS1) promoter nucleic acid fragment. The invention also relates to the construction of chimeric genes comprising all or a portion of the IFS1 promoter directing the expression of transgenes, in sense or antisense orientation.
Owner:CORTEVA AGRISCIENCE LLC +1
Reagent for quantitative detection of Beta-receptor stimulant through Europium chelate latex time-resolved immunochromatographic assay
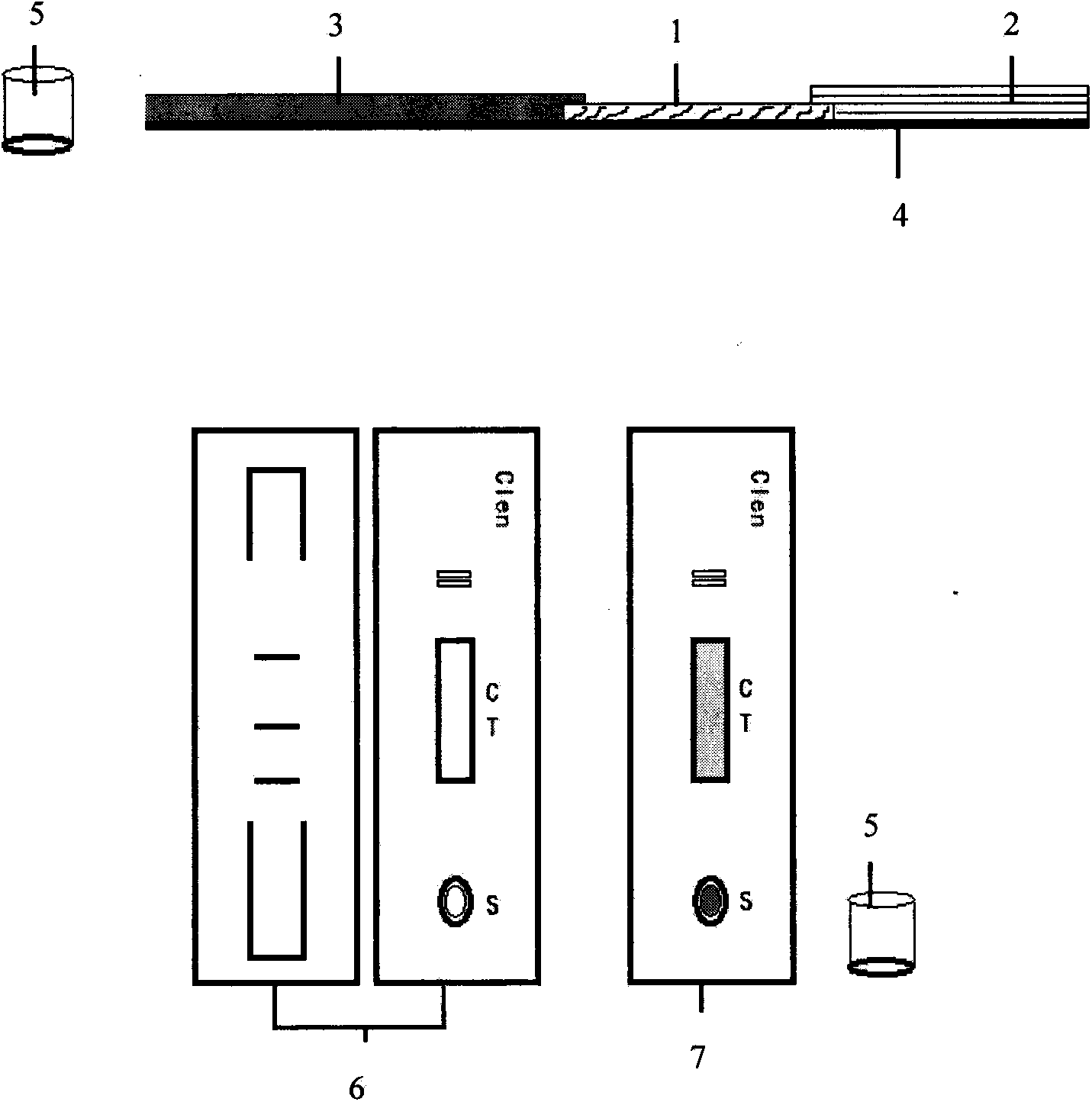
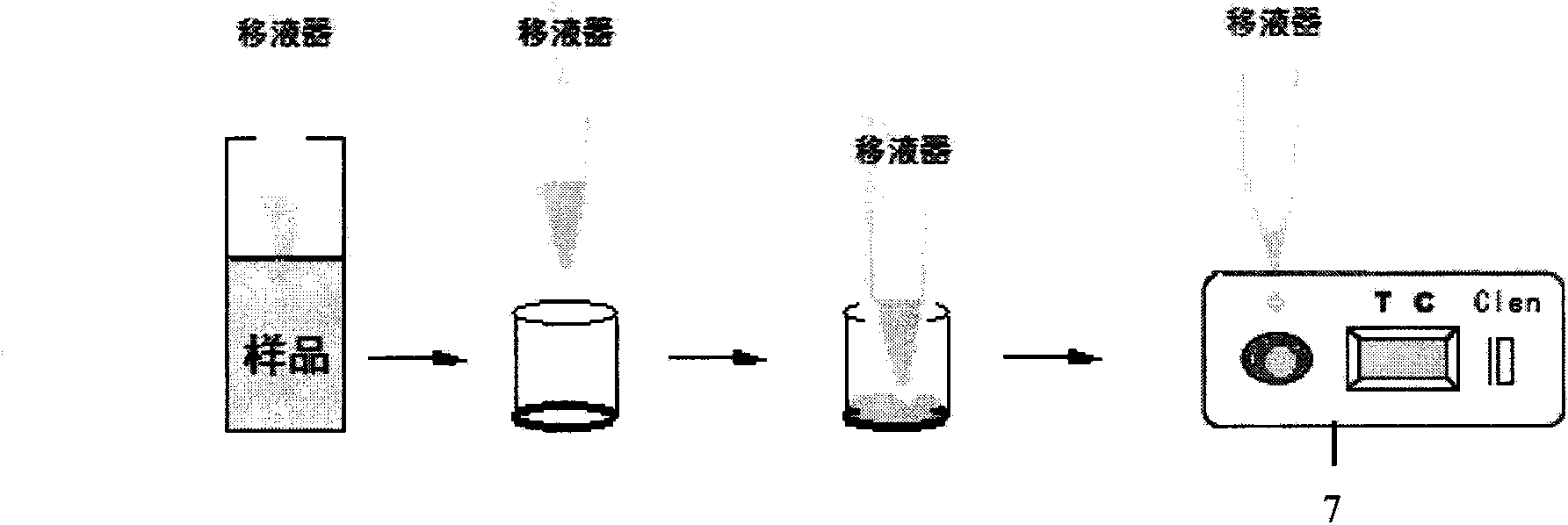
InactiveCN104090248AHigh sensitivityHigh quantitative sensitivityFluorescence/phosphorescenceMagnitude/direction of magnetic fieldsCelluloseReceptor
Disclosed is a reagent for quantitative detection of a Beta-receptor stimulant through Europium chelate latex time-resolved immunochromatographic assay. The reagent includes test card or test strip which includes a nitrocellulose membrane, absorbent paper, a sample pad and a PVC bottom plate, and a microporous container. A test sample firstly dissolves out Eu3+ fluorescent latex particles which are attached in the microporous container and marked with an anti-Beta-receptor-stimulant small-molecule antibody and after sufficient mixing, the test sample reacts completely with the marker and then a reaction liquid is dropped to the test card or test strip to carry out immunochromatography and at the same time, an immune competition reaction with a Beta-receptor-stimulant small molecule and BSA conjugate which envelopes the nitrocellulose membrane is carried out and five minutes later, the test card or the test strip is inserted into a fluorescent reading meter to measure a fluorescent value so as o obtain a test result. The method is high in sensitivity and quantitative and integrates the advantages of simple and convenient operation and rapidness, the method is applied to rapid detection of veterinary drug residuals such as the Beta-receptor stimulant and the like in food and raw materials on sites of production fields such as plantation, cultivation, animal husbandry and food processing and the like.
Owner:ROHI BIOTECH
Long acting sustained-release formulation containing dopamine-receptor stimulant medicine and its preparation process
ActiveCN1762495AReduce frequency of useImprove bioavailabilityNervous disorderPharmaceutical non-active ingredientsStimulantSustained Release Formulations
The invention relates to a long acting sustained-release formulation containing dopamine-receptor stimulant medicine, which comprises 5-5- wt% of effective dose of dopaminergic acceptor medicaments, and 50-95 wt% of medicinal macromolecular auxiliary materials.
Owner:SHANDONG LUYE PHARMA CO LTD
Composition and method for appetite and craving suppression and mood enhancement
A composition for suppressing appetite and cravings for substances such as nicotine, coffee, sweets or chocolate while improving energy and enhancing mood comprises theobromine or a salt thereof at an effective amount of from about 250 to 4000 mg. Using such relatively high proportions of theobromine, without added caffeine or ephedrine provides an effective method for promoting weight control or to halt substance cravings without the side effects associated with such stimulants. The composition also includes Rhodiola rosea extract to offset stress effects from reduced food or substance intake, and to further improve mood, and clarity of thought and ability to handle stress, and to also increase endurance while reducing muscle pain.
Owner:EURARK
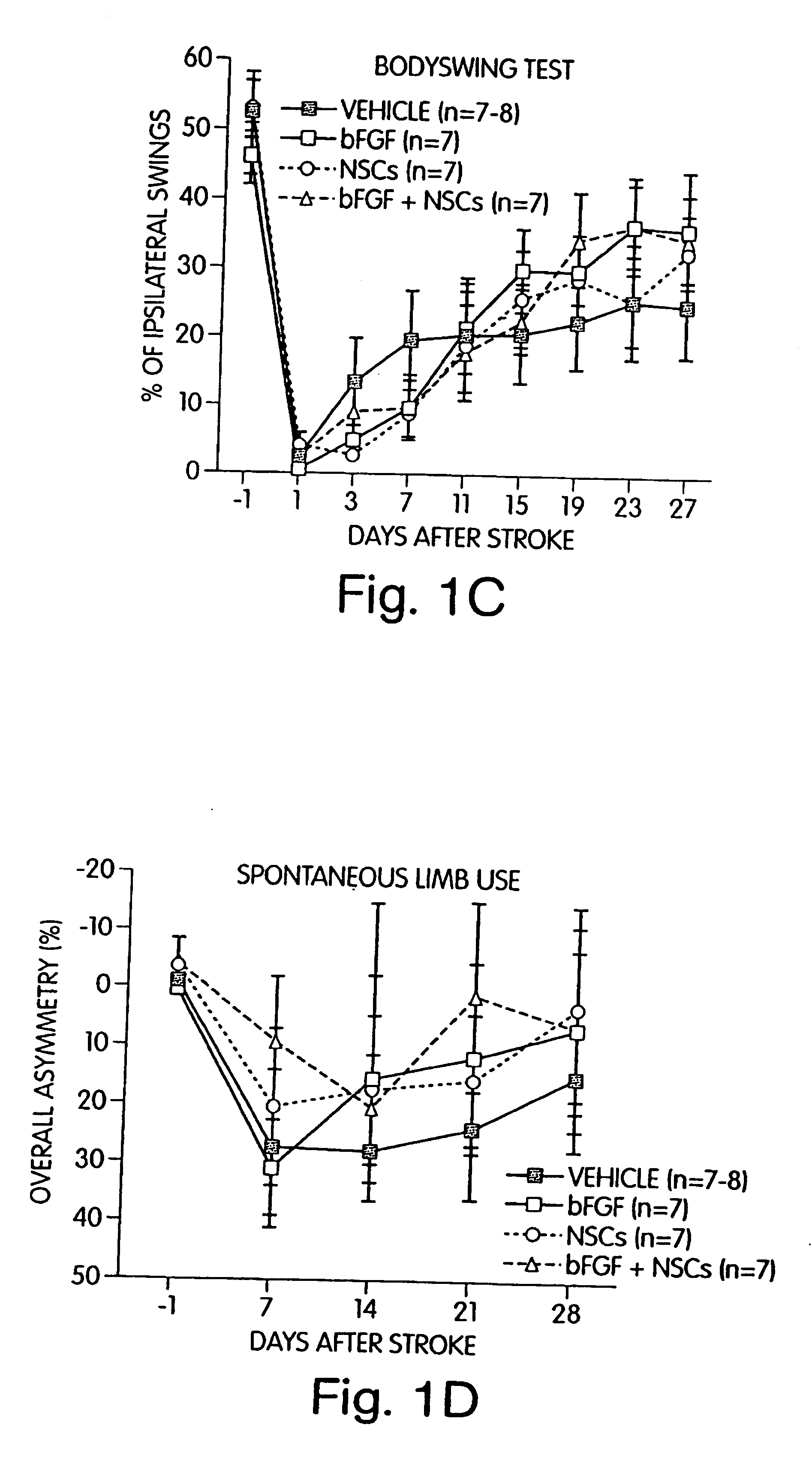
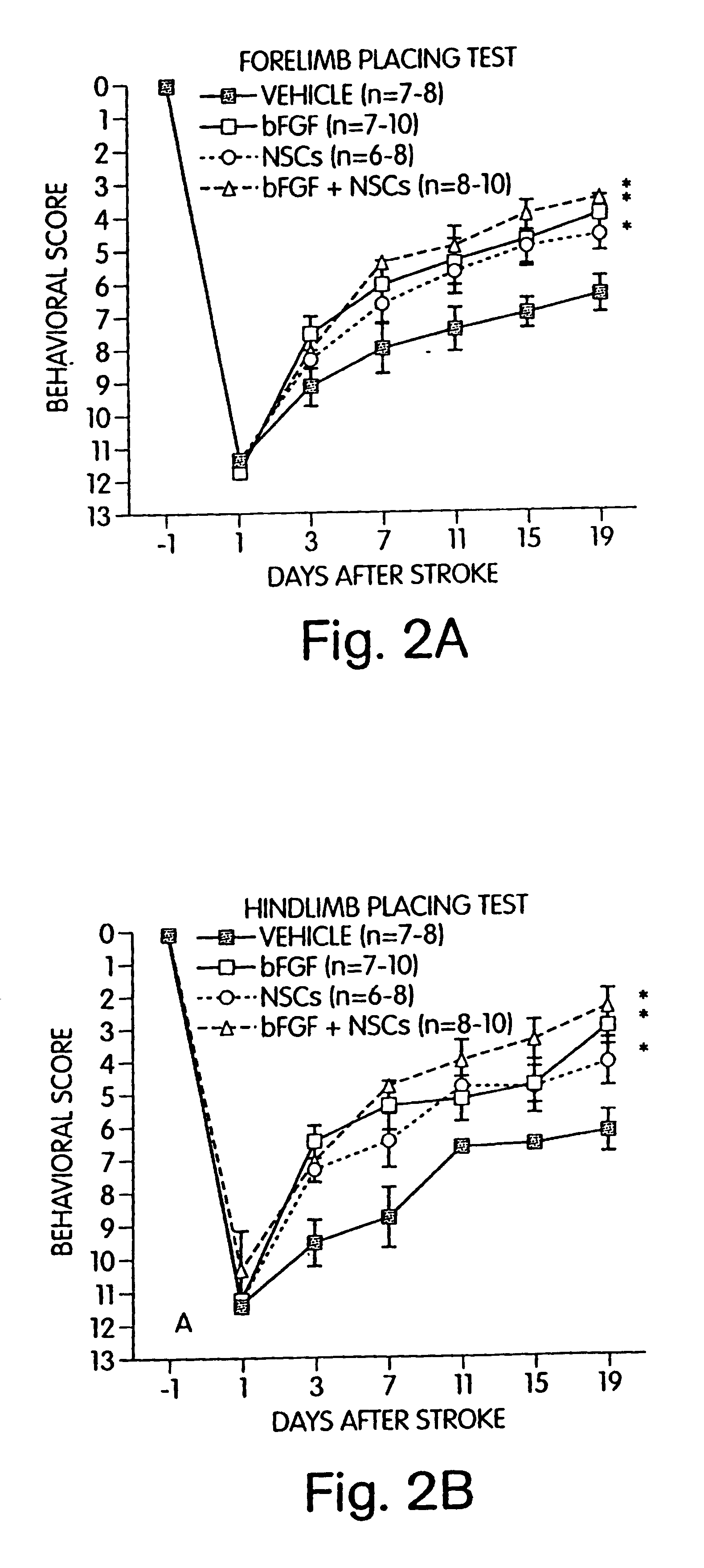
Methods, compositions and kits for promoting recovery from damage to the central nervous system
InactiveUS6749850B1Promote recoveryImprove abilitiesBiocideNervous disorderSensation movementStimulant
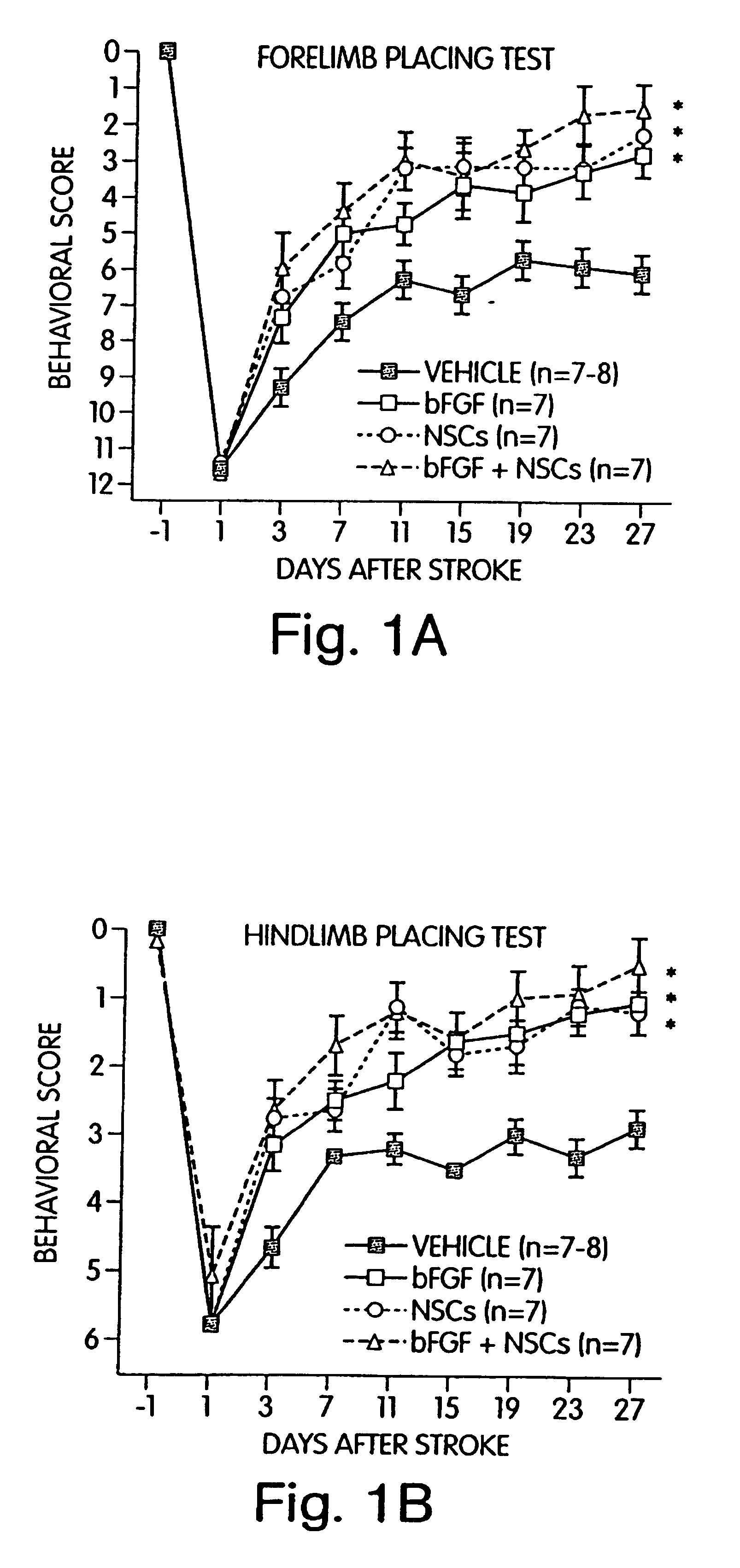
The present application relates to methods, kits and compositions for improving a subject's recovery from CNS injury. In certain aspects, methods of the invention comprise administering to a subject cells and a neural stimulant. Recovery may be manifest by improvements in sensorimotor or cognitive abilities, e.g., improved limb movement and control or improved speech capability. In certain embodiments, subject methods can be used as part of a treatment for damage resulting from ischemia, hypoxia or trauma.
Owner:CHILDRENS MEDICAL CENT CORP +1
Multifunctional Organic Agricultural Fertilizer Composition and Process for Preparation Thereof
ActiveUS20170166488A1Reduce volatilityInhibit complexation/tieOrganic phosphatic fertilisersAgriculture gas emission reductionAdjuvantStimulant
The invention disclosed herein is a multifunctional agricultural organic bio-complexed composition comprising essential and non-essential nutritional elements; useful as a fertilizer, nutrient, bio-stimulant, complexing agent, pH controller, pH indicator, pH corrector, hard water salts in-activator, surface tension reducer, Spreader, penetrator, adjuvant, alkaline hard water ill effects mitigator, water conditioner and drip system irrigation cleaner. The invention also disclosed herein a process for preparation thereof.
Owner:CHAUDHRY SUUNIL SUDHAKAR
Repetitive visual stimulation to EEG neurofeedback protocols
An EEG neurofeedback and total evoked brain activity measurement methods utilize minimum ambient EEG activity as stimulant frequencies. A method of using repetitive stimulation in conjunction with EEG neurofeedback protocols is described. Electrodes, attached to a subject's scalp, transmit electroencephalographic (EEG) signals from the subject. These signals are in response to the visual and / or auditory stimuli being displayed to the subject. The resultant EEG signals are then filtered at pre-defined frequencies or frequency bands. The output from the filtered EEG signals is then analyzed and monitored for short-term state changes. The invention also uses flicker stimulation, real-time signal filtration and feedback, feedback during audio and visual stimulation derived from filtered outputs, and fundamental and integral harmonics in combination with total evoked response.
Owner:COLLURA THOMAS F
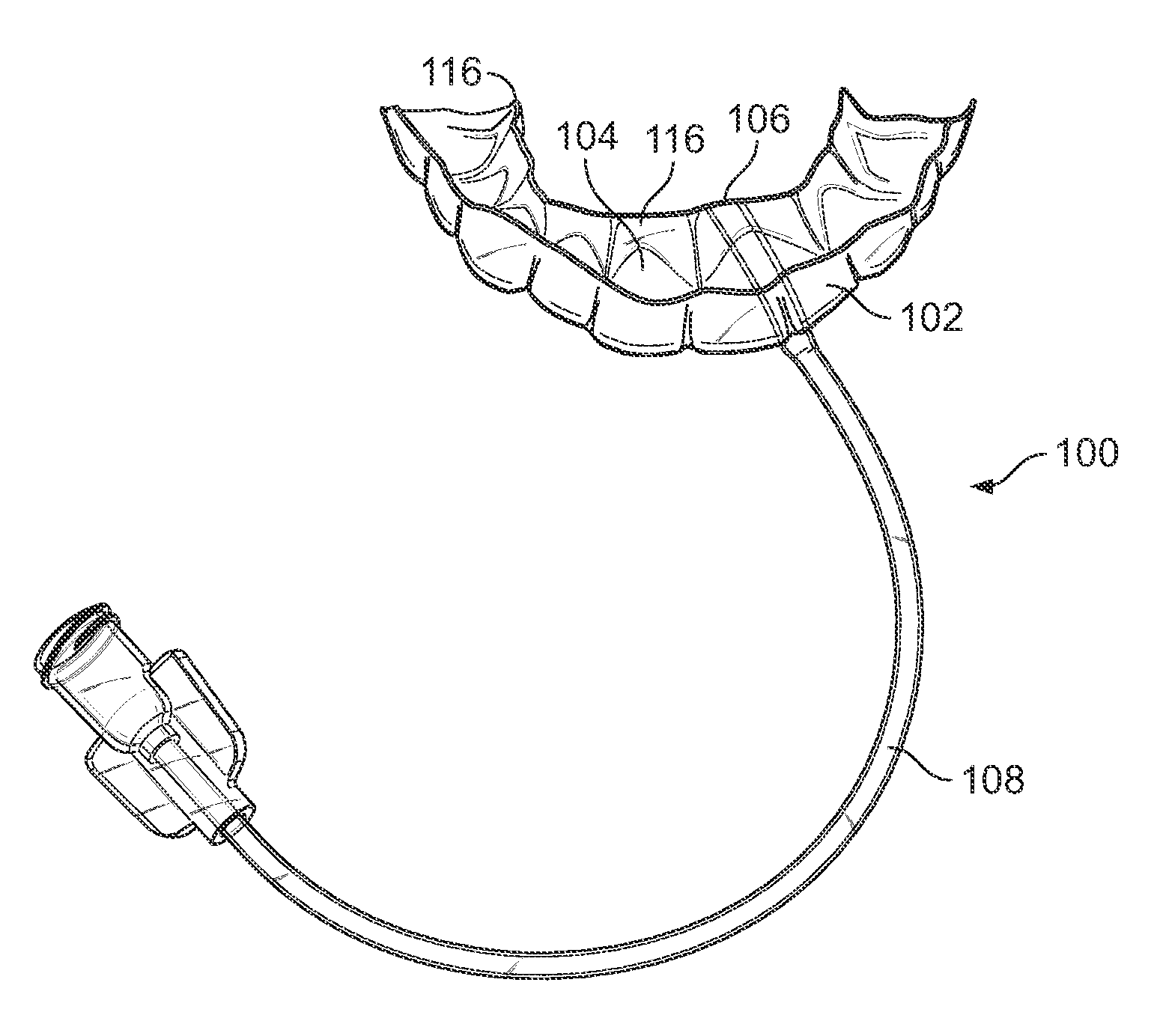
System and method for delivering a therapy and sensing a biological activity in the mouth
ActiveUS20130042876A1Triggering can be facilitatedReduce impactRespiratorsMedical devicesStimulantSleeping disorders
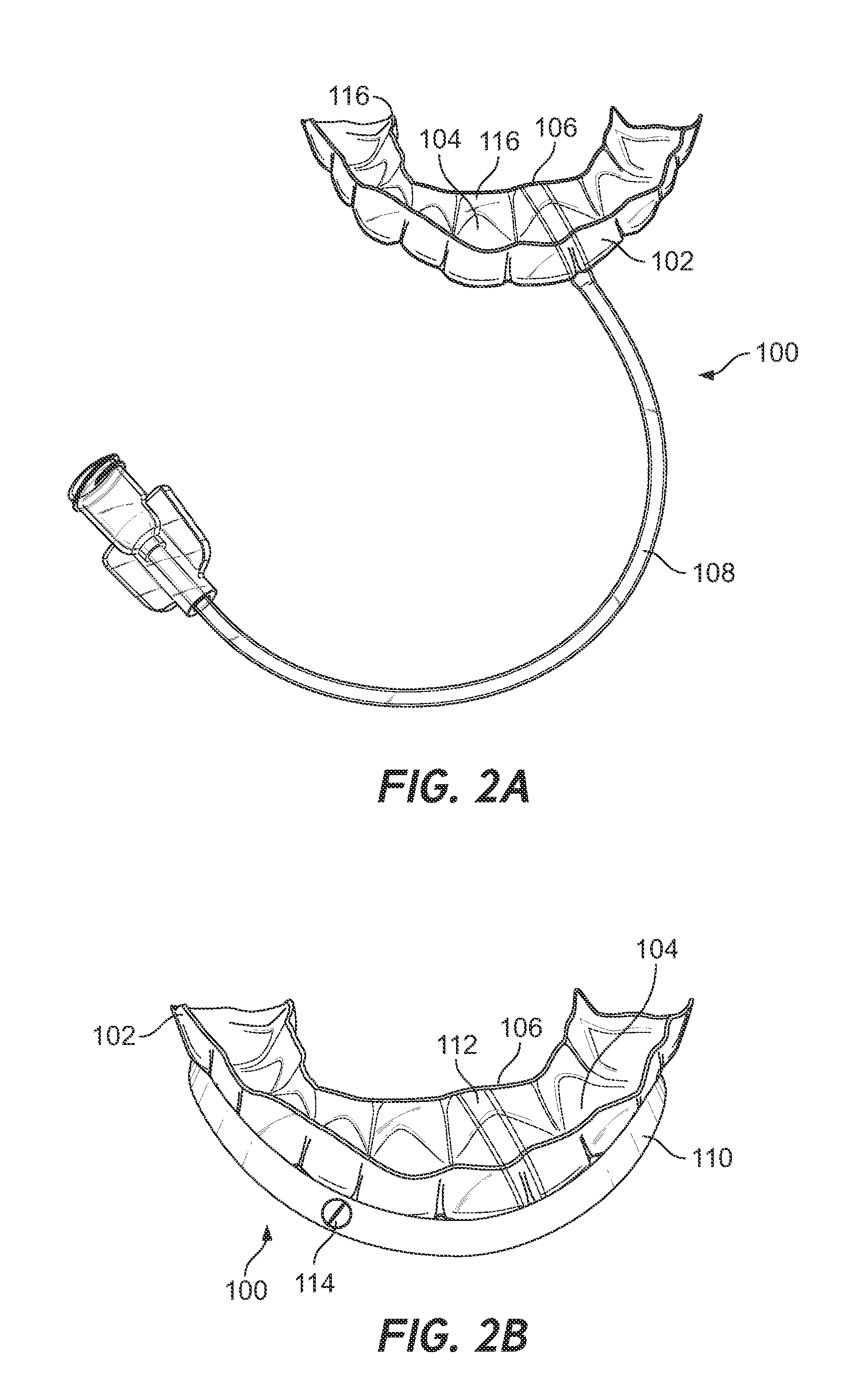
Methods, devices, and systems are disclosed for controlled delivery of a therapy, such as a stimulant, to a mouth of a subject via an oral device positioned in a secured configuration in the mouth. At least one of a tongue position stimulator (TST) and tongue position sensor (TSE) is provided, according to certain aspects. According to another aspect, a stimulus is delivered to the mouth and / or tongue via a mouthpiece secured to the subject's teeth. In another regard, a stimulus is delivered that generates a natural response to eliminate or reduce sleep disorders, such as for example at least one of snoring and obstructive sleep apnea.
Owner:SPLIT ROCK SCI INC
Exo-S-mecamylamine formulation and use in treatment
InactiveUS6734215B2Improve Medication AdherenceImprove the quality of lifeBiocideNervous disorderStimulantOpiate
Medical conditions are treated by administering a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-R-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), Tourette's Syndrome, and neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder).
Owner:UNIV OF SOUTH FLORIDA
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS8252321B2Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocideDiseasePhytochemical
Owner:MORNINGSIDE VENTURE INVESTMENTS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com