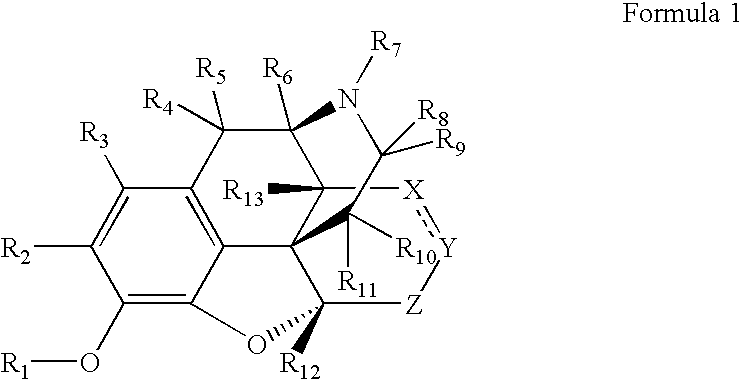
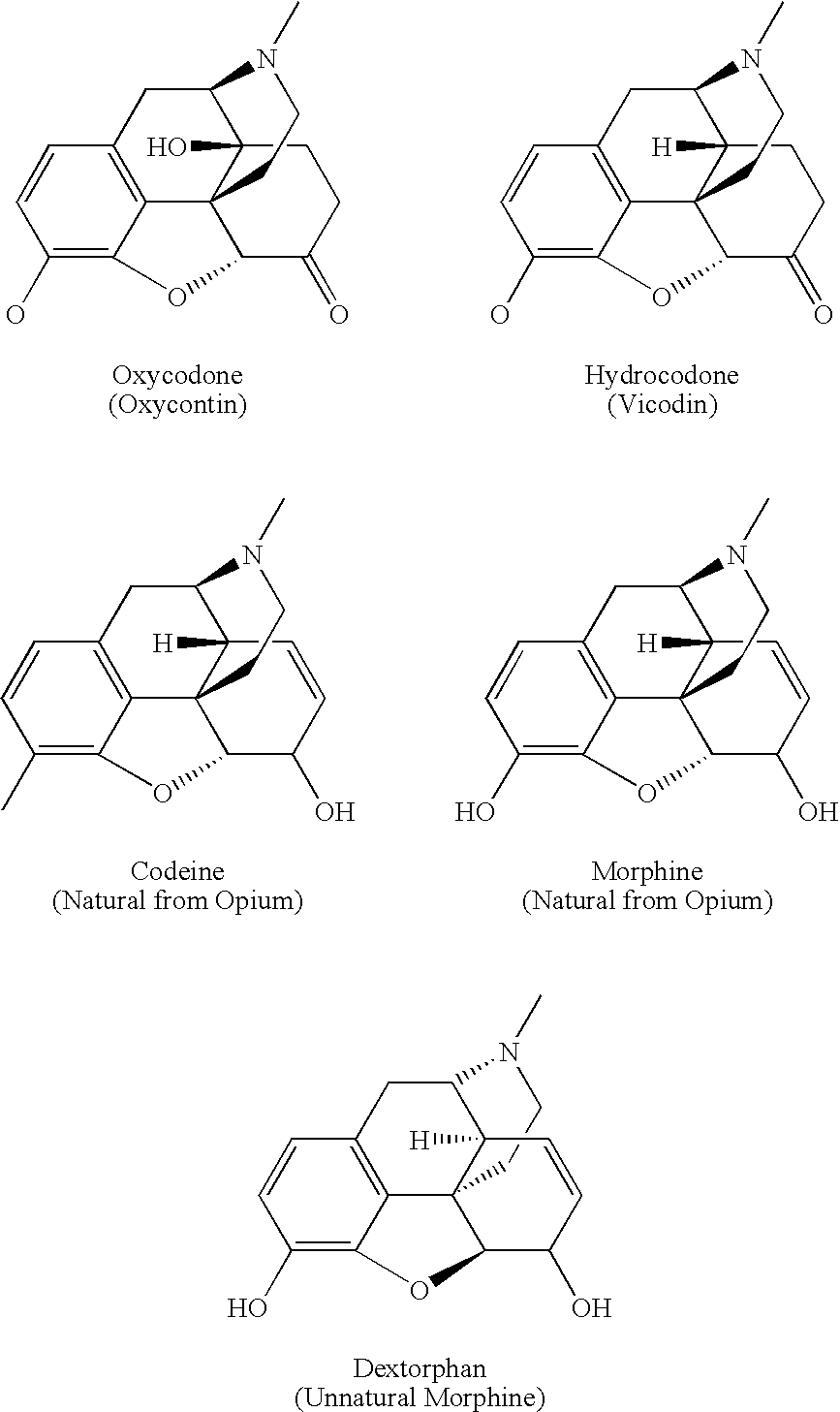
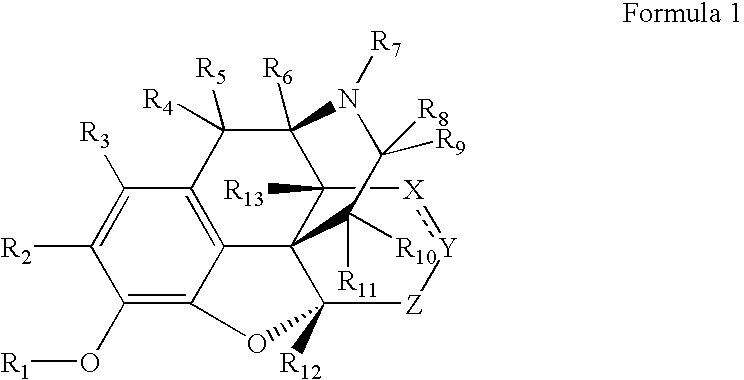
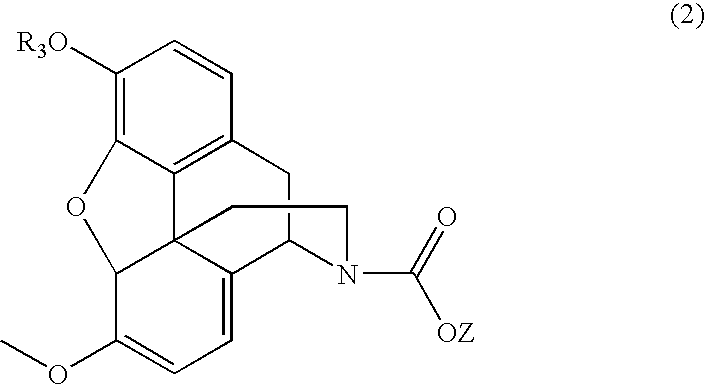
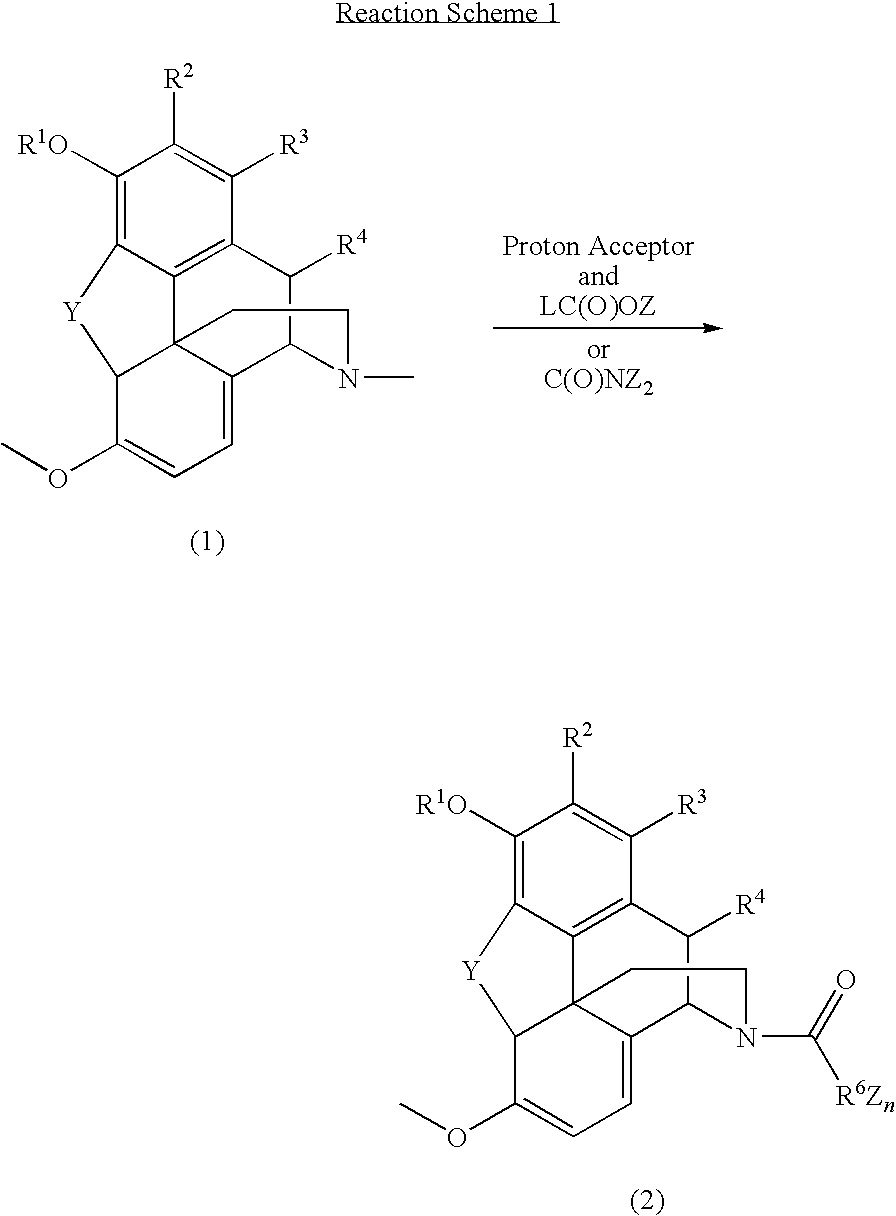
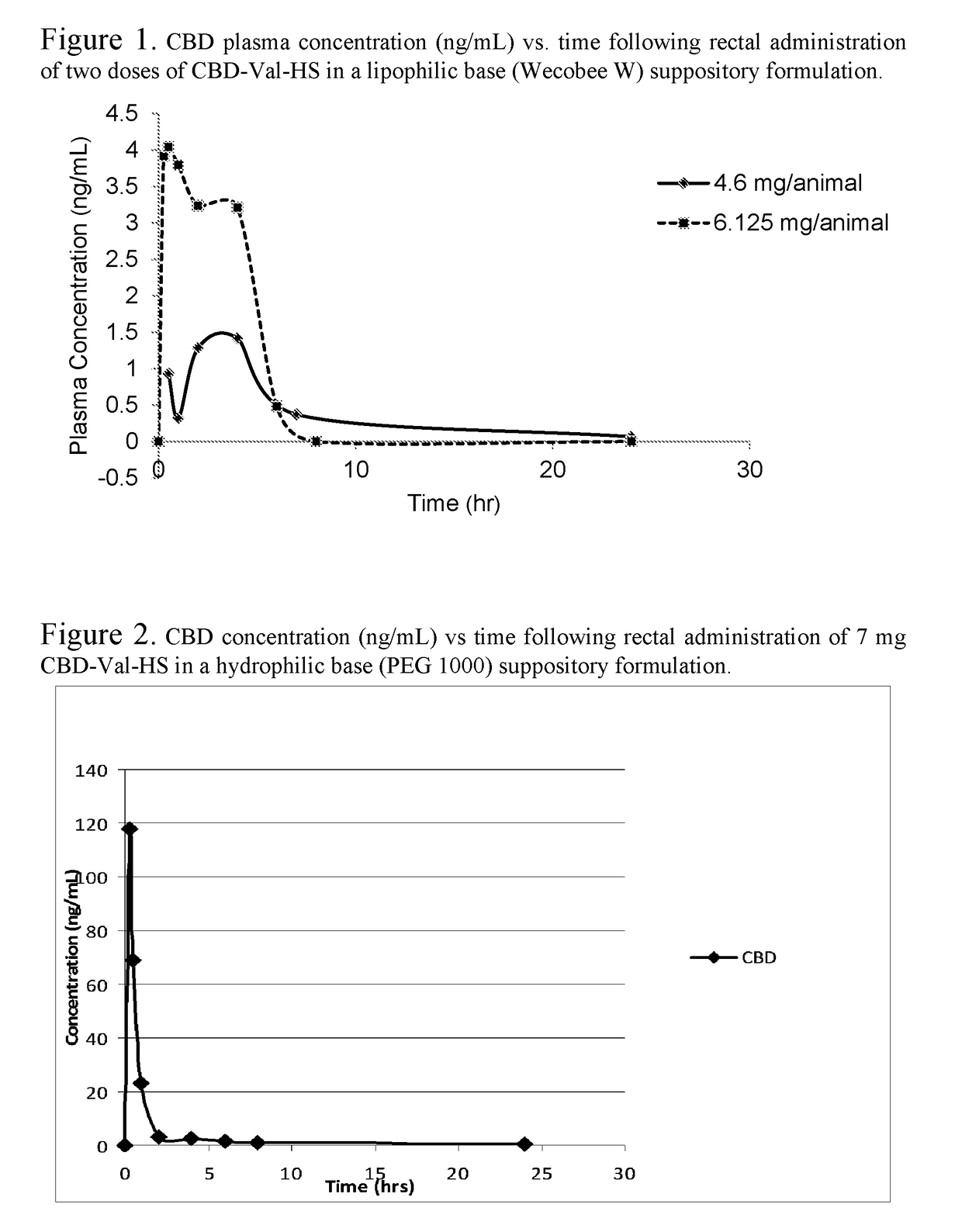
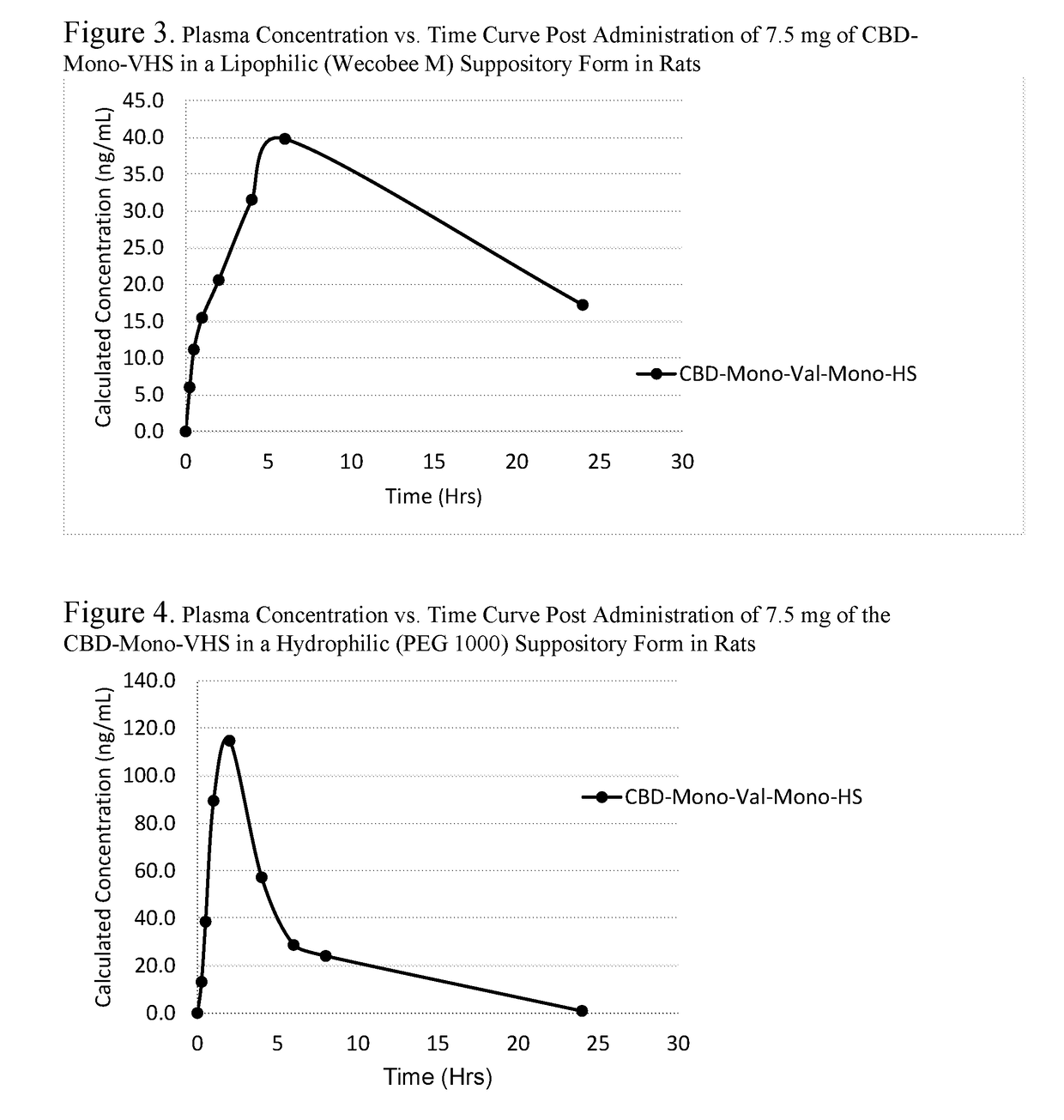
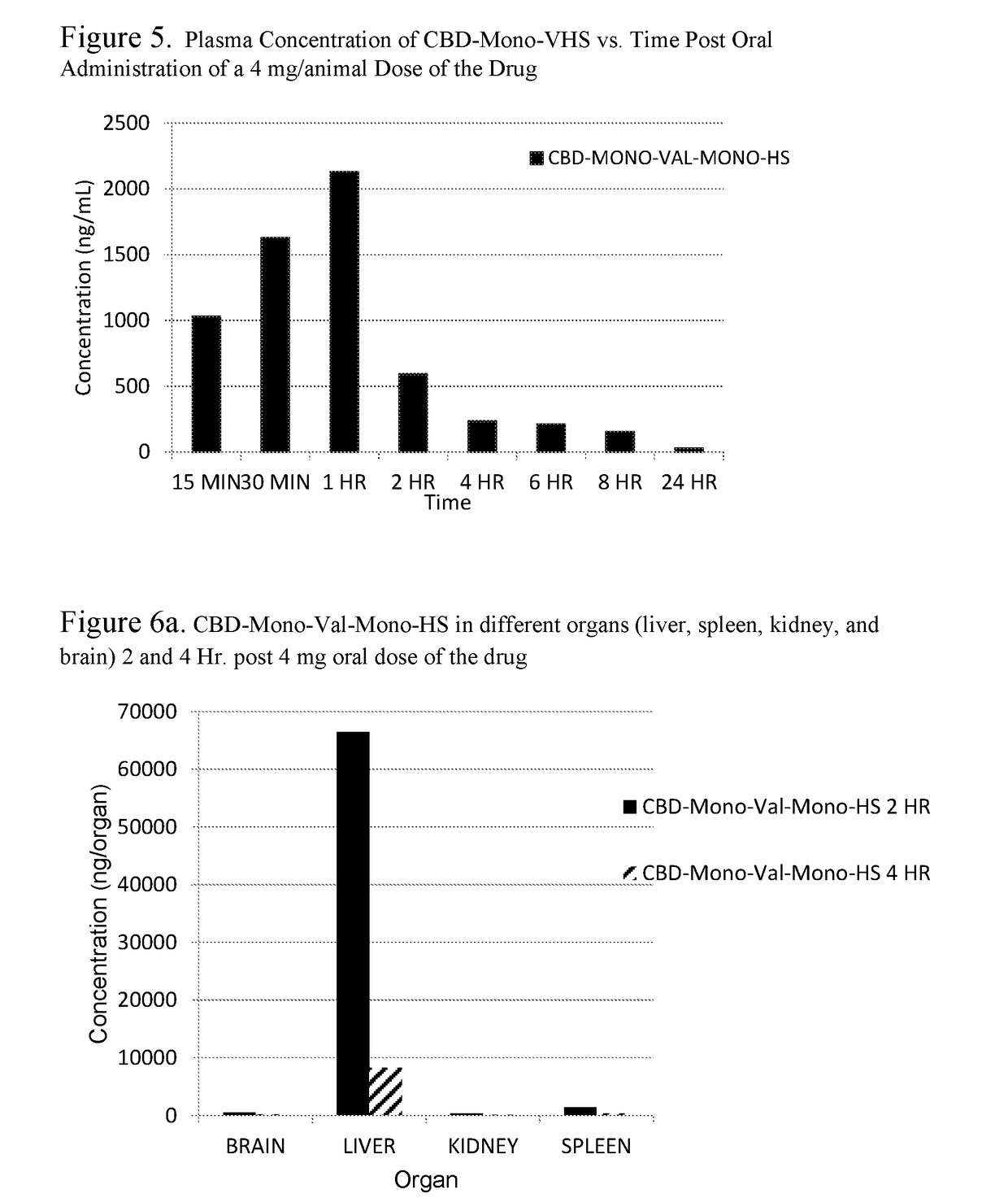
Patents
Literature
159 results about "Opiate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Opiate is a term classically used in pharmacology to mean a drug derived from opium. Opioid, a more modern term, is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain (including antagonists). Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS6893654B2Convenient and reliable and practicalReduce cravingsBiocidePowder deliveryOpiateInitial dose
A two-stage medicine delivery system provides an initial dose of medicine and a second dose of medicine. The initial and second doses are capable of achieving a rapid pharmacological effect and a prolonged pharmacological effect, respectively. The two-stage medicine delivery system preferably delivers a craving reduction substance, in which case, the rapid and prolonged pharmacological effects include a rapid and prolonged craving reduction. Preferably, the delivery system is a nicotine delivery system which is provided in chewing gum form or lozenge form and which provides the nicotine in a transmucosally absorbable form. The two-stage medicine delivery system preferably releases a buffering agent which increases a pH level in a user's mouth to facilitate absorption of the medicine when the delivery system is placed in the user's mouth. A method of making the medicine delivery system also is provided. The system and apparatus can be adapted to reduce cravings for alcohol, food, drugs (e.g., cocaine, opiates and the like) and tobacco products, especially tobacco products containing nicotine.
Owner:JSR NTI
Novel pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS20080058362A1Reduced plasma concentrationEffective pain managementBiocideAmide active ingredientsDextrorphanSustained release drug
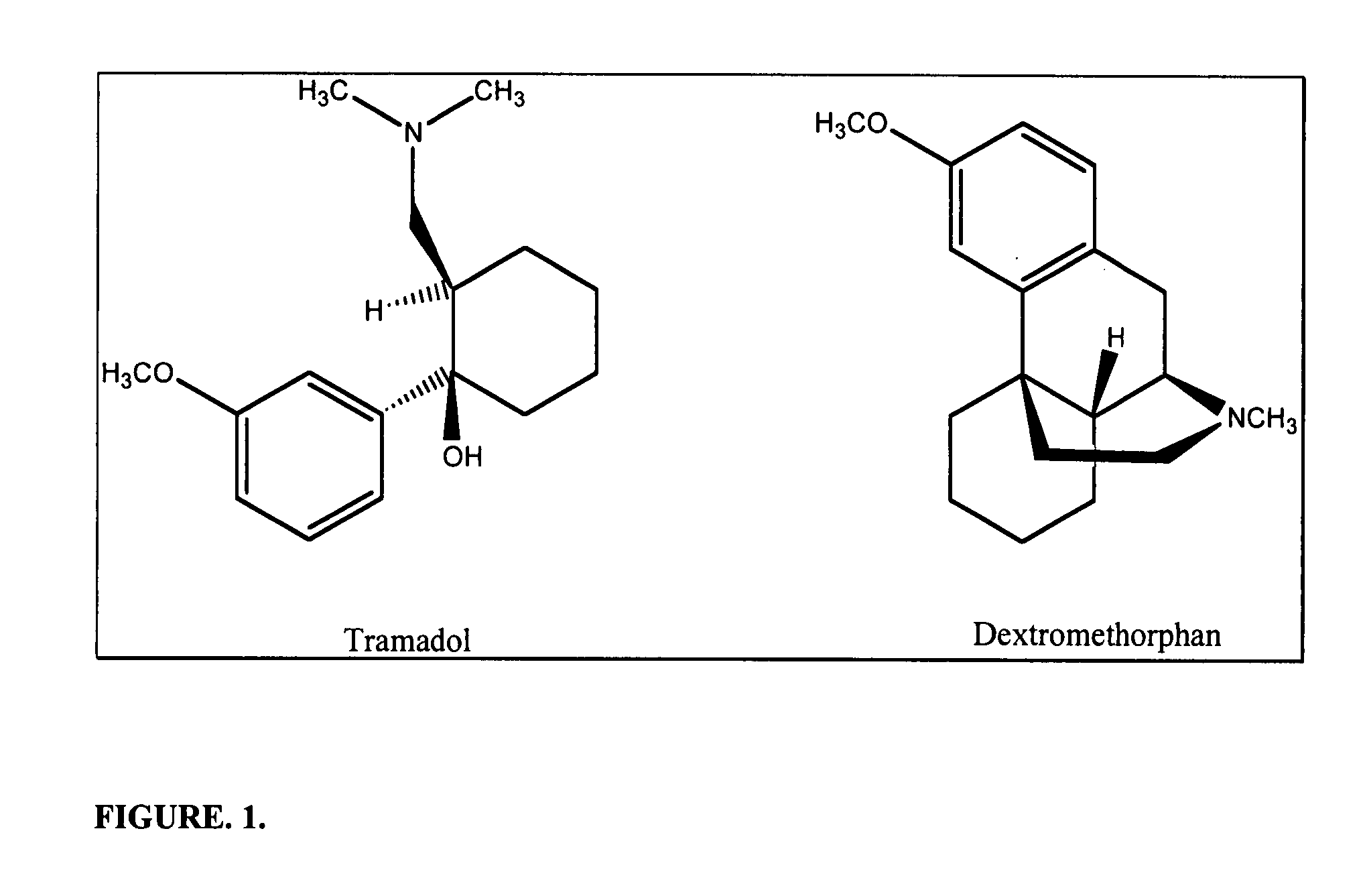
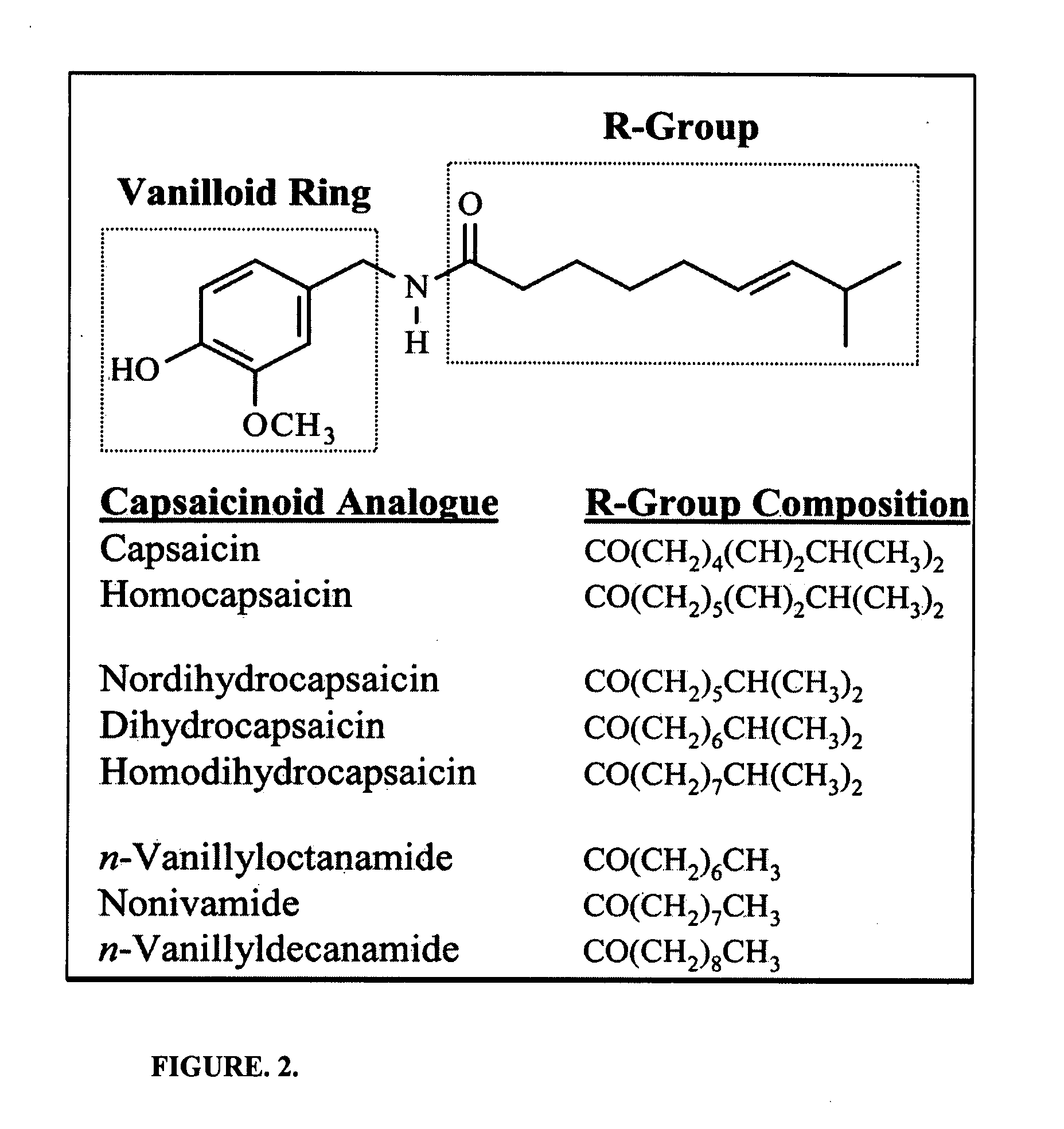
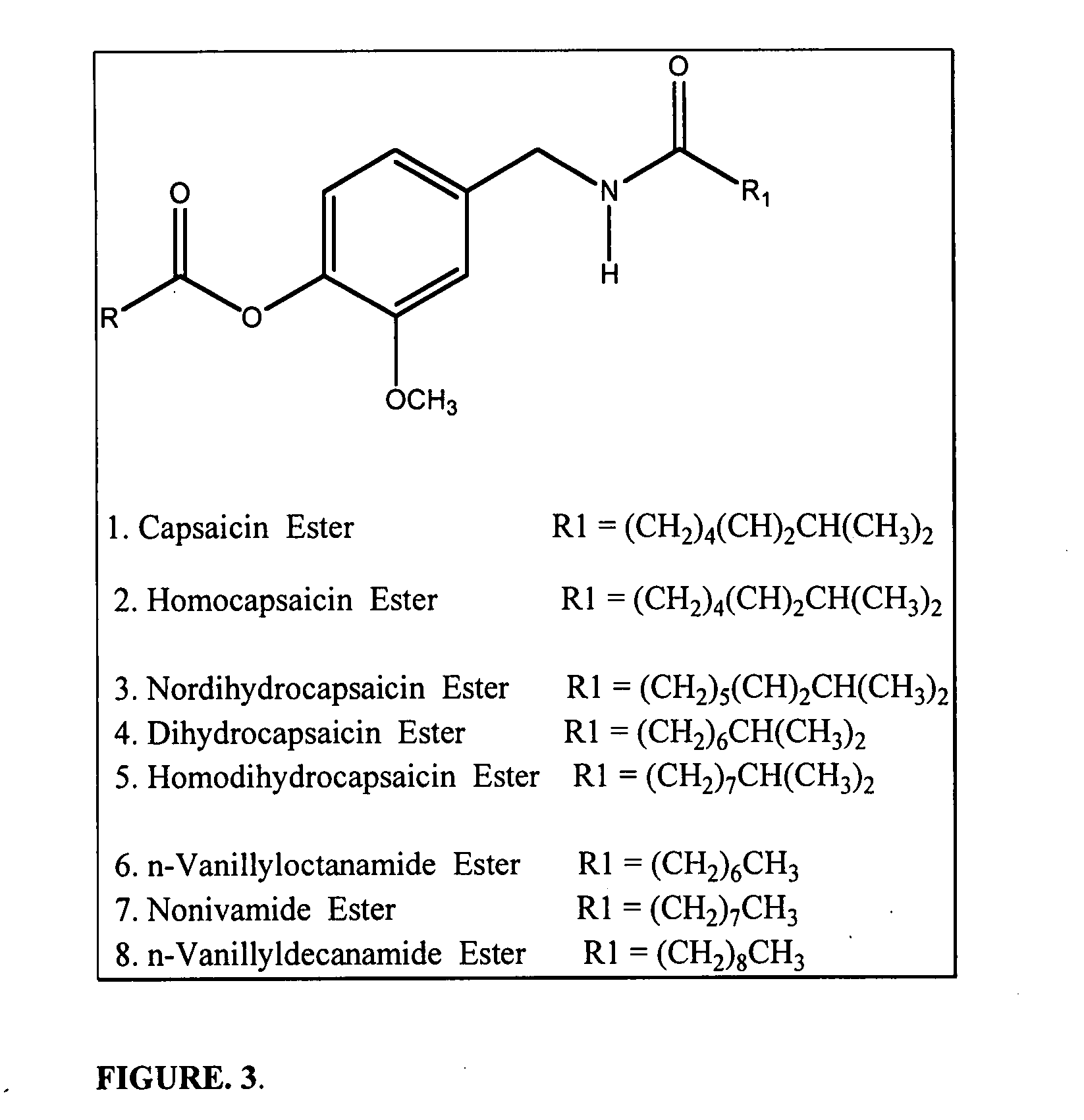
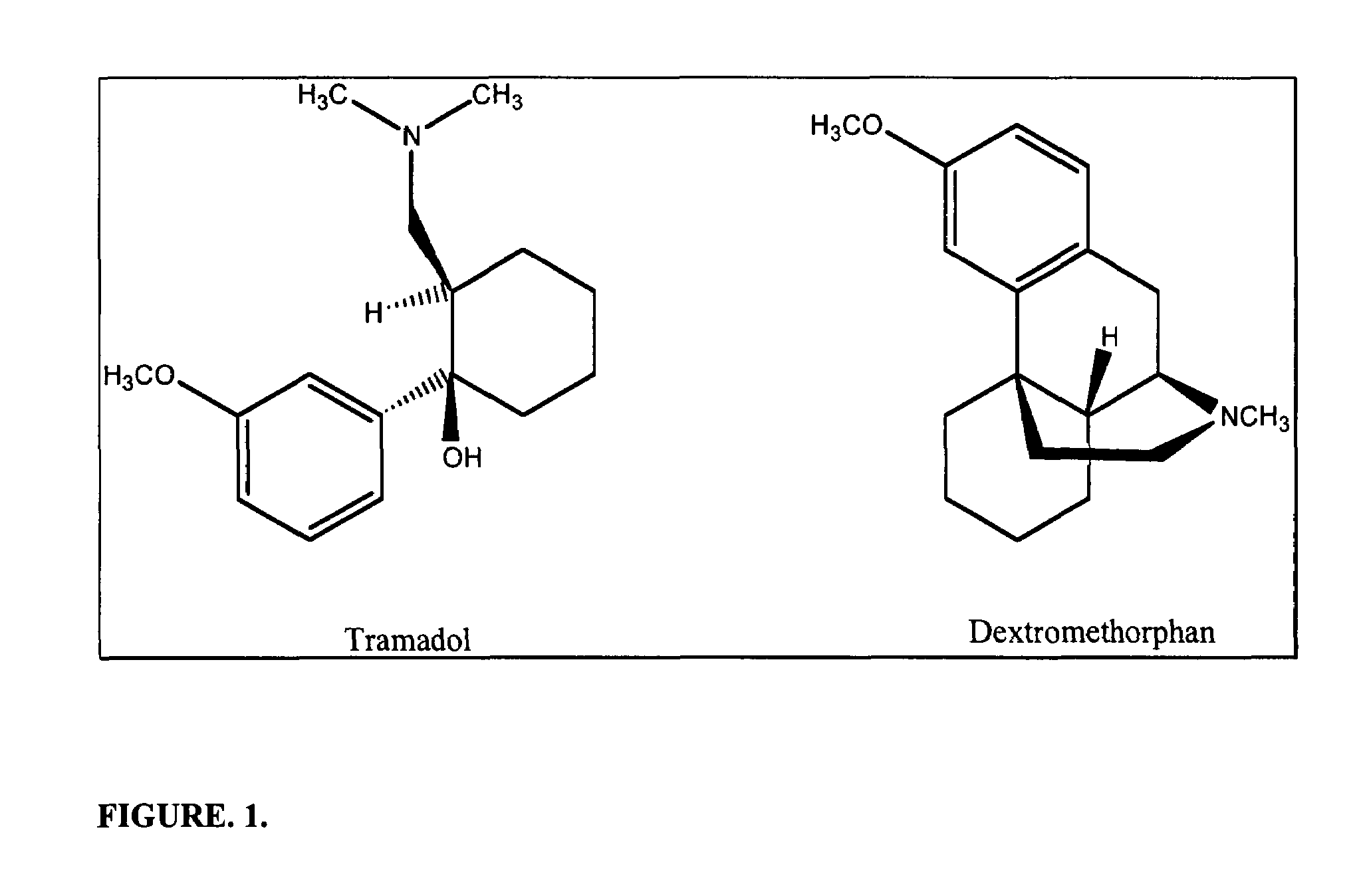
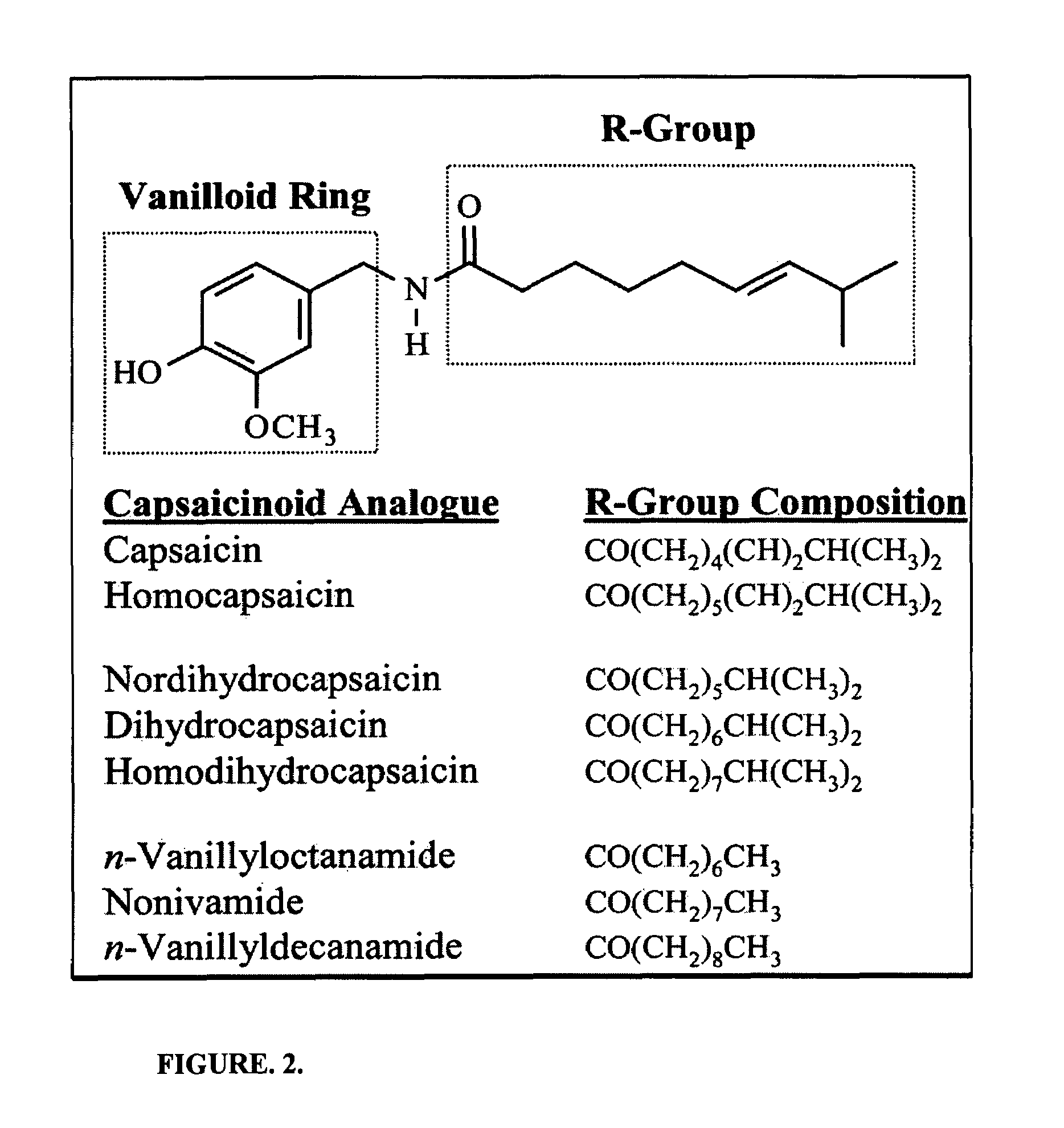
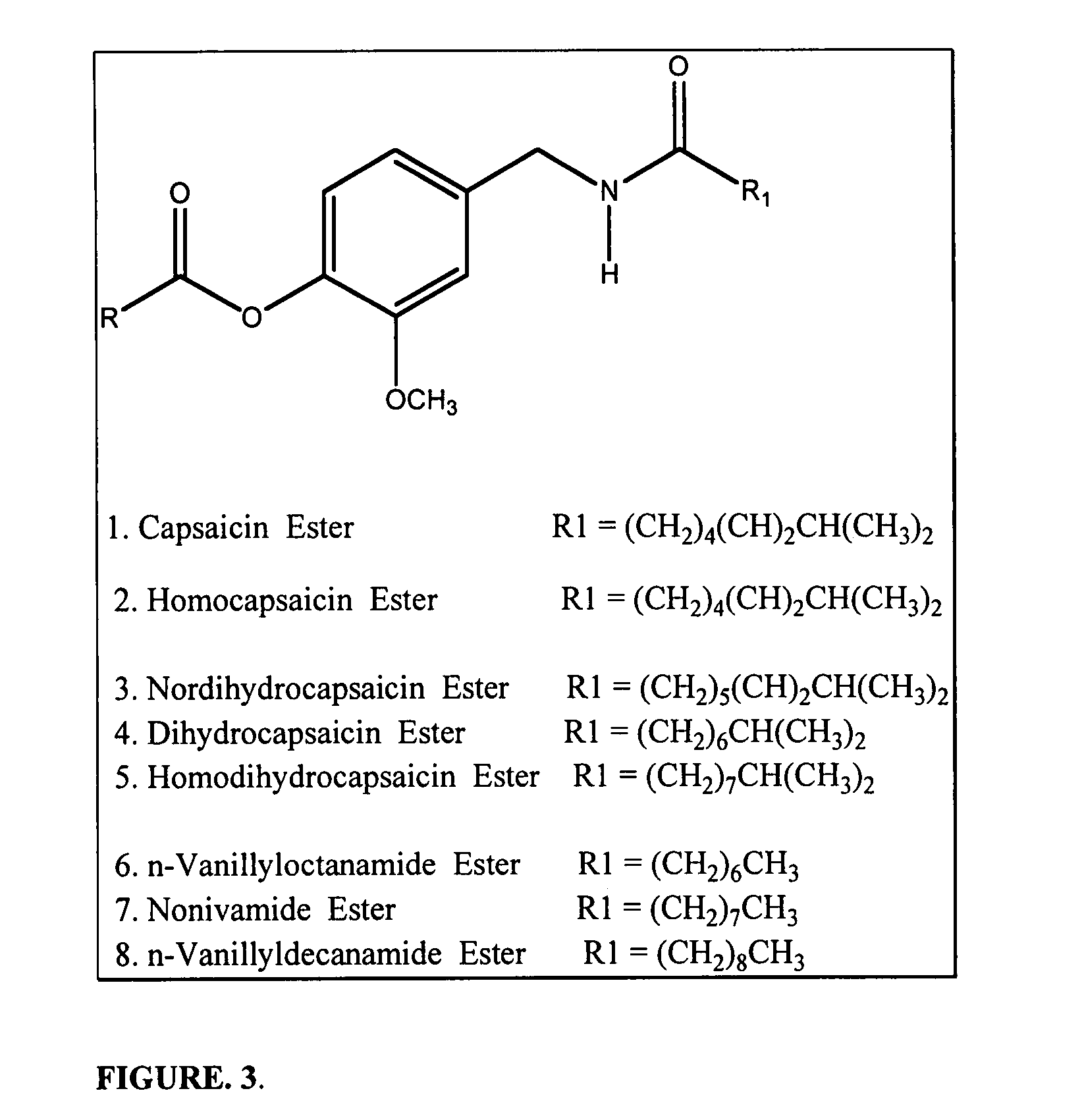
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS7645767B2Reduce concentrationEfficient managementBiocideAmide active ingredientsOpiatePharmaceutical medicine
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Exo-S-mecamylamine formulation and use in treatment
InactiveUS20020016371A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationWeight gainingSmoking cessation
A pharmaceutical composition includes a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-R-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-R-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
Effervescent oral opiate dosage forms and methods of administering opiates
Opiate containing dosage forms and methods using same are described. These dosage forms include substantially less opiates by weight than known oral formulations. These dosage forms are intended for oral administration across the oral mucosa.
Owner:CEPHALON INC
Exo-R-mecamylamine formulation and use in treatment
InactiveUS20020016370A1Convenient treatmentImprove Medication AdherenceBiocideUrea derivatives preparationStimulantS syndrome
A pharmaceutical composition includes a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of exo-S-mecamylamine in combination with a pharmaceutically acceptable carrier. Preferably the amount is about 0.5 mg to about 20 mg. Medical conditions are treated by administering a therapeutically effective amount of exo-R-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-S-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include but are not limited to substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), aiding smoking cessation, treating weight gain associated with smoking cessation, hypertension, hypertensive crisis, Tourette's Syndrome and other tremors, cancer (such as small cell lung cancer), atherogenic profile, neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder), chronic fatigue syndrome, Crohn's disease, autonomic dysreflexia, and spasmogenic intestinal disorders.
Owner:UNIV OF SOUTH FLORIDA
Oral dosage form safeguarded against abuse
The present invention relates to an abuse-proofed, oral dosage form with controlled opioid-release for once daily administration, characterised in that it comprises at least one opioid with potential for abuse (A), at least one synthetic or natural polymer (C), optionally delayed-release matrix auxiliary substances, physiologically acceptable auxiliary substances (B), optionally a wax (D) and optionally at least one delayed-release coating, component (C) or (D) in each case exhibiting a breaking strength of at least 500 N, preferably of at least 1000 N.
Owner:GRUNENTHAL GMBH
Quaternary opioid carboxamides
ActiveUS20090197905A1Good to excellent peripheral opioid antagonism activityLess susceptibleBiocideNervous disorderSide effectMedicine
Owner:RENESSELAER POLYTECHNIC INST
Methods and Compositions
InactiveUS20080039463A1Cause side effectNot induce sedationBiocideNervous disorderControl releaseActive agent
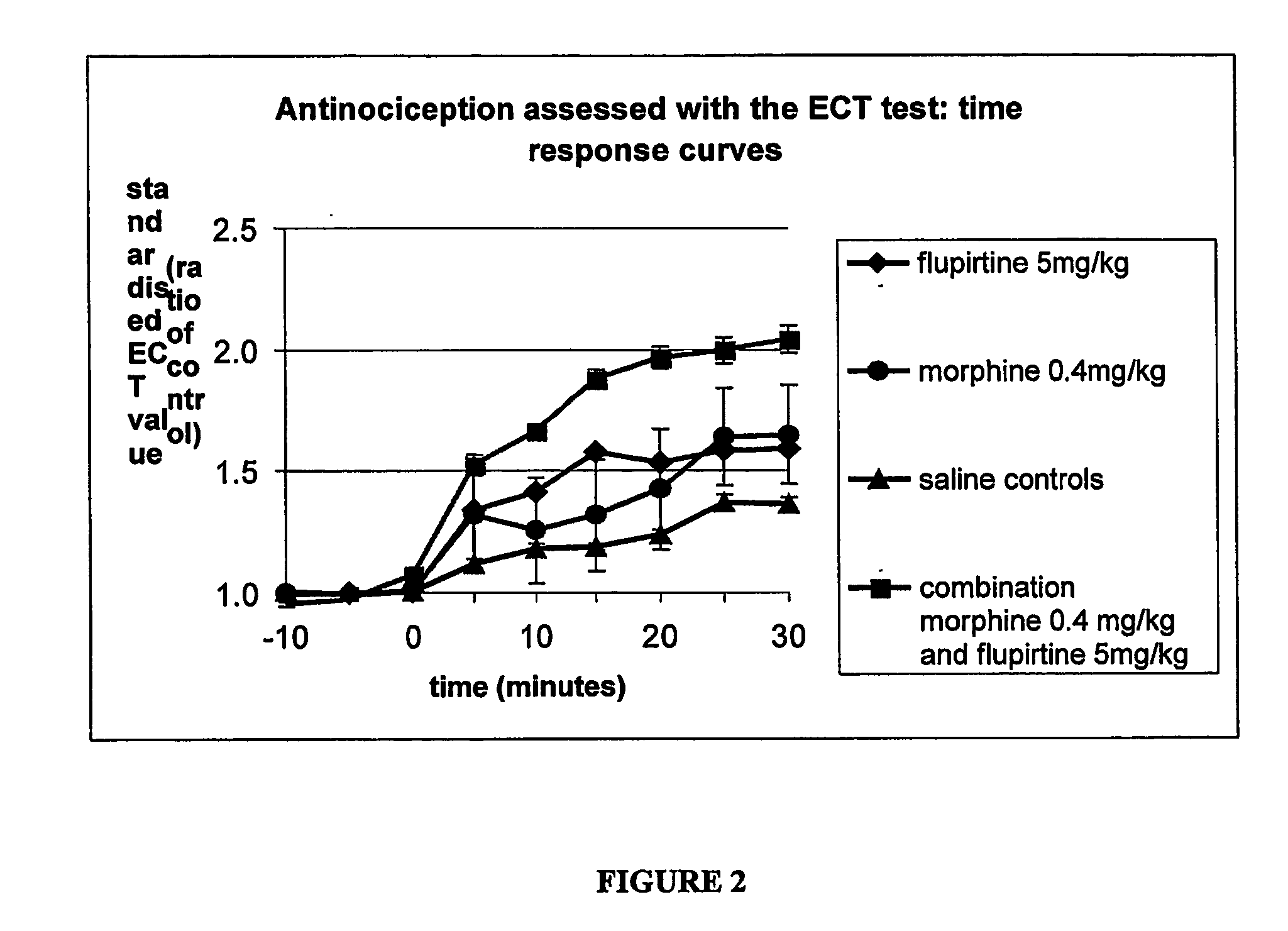
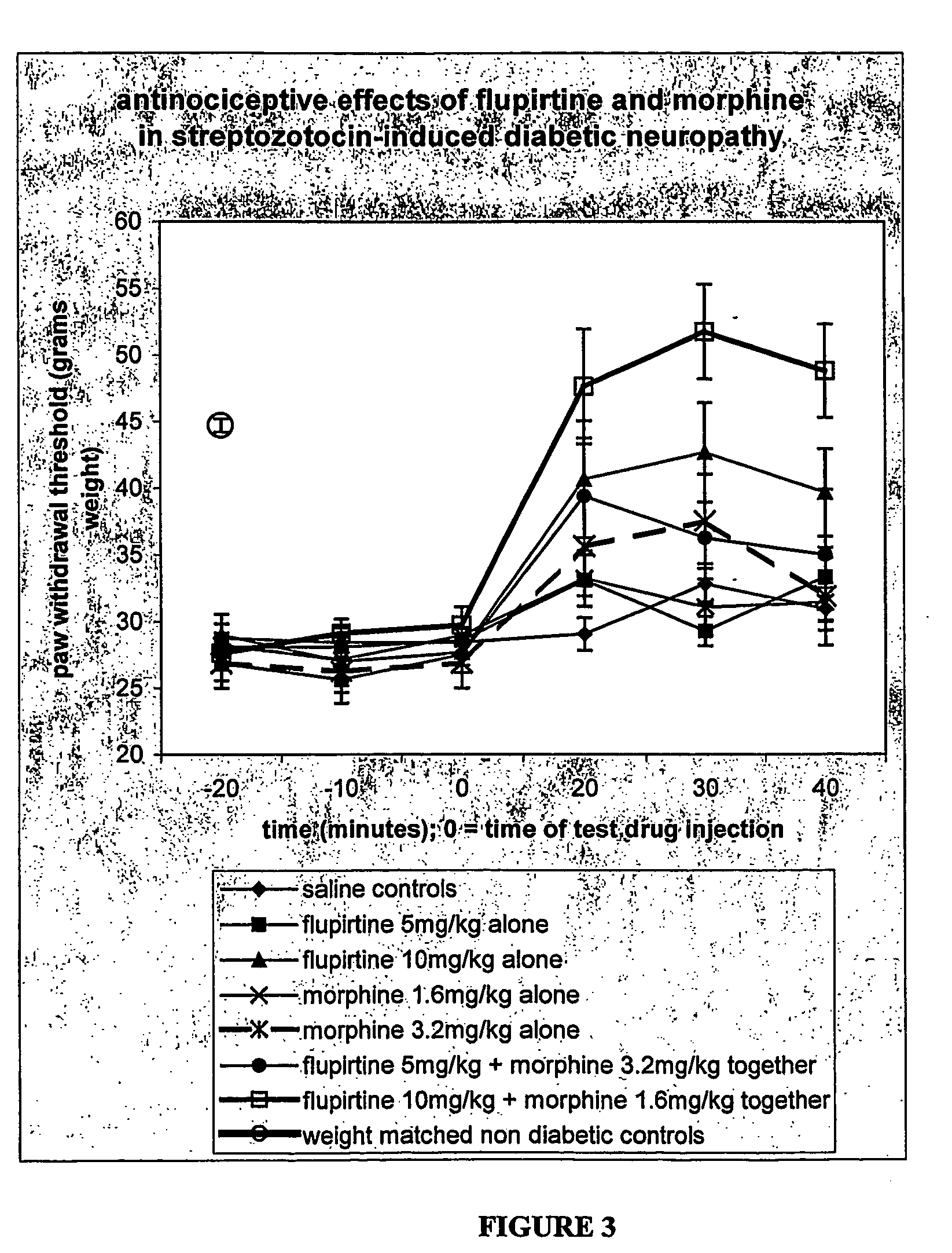
Compositions of flupirtine for management of neuropathic or inflammatory pain optionally including one or more other analgesics including opiates, NSAIDS and other active agents in immediate and controlled release forms. Methods and systems for administration of these compositions.
Owner:RELEVARE AUST
Exo-S-mecamylamine formulation and use in treatment
InactiveUS6734215B2Improve Medication AdherenceImprove the quality of lifeBiocideNervous disorderStimulantOpiate
Medical conditions are treated by administering a therapeutically effective amount of exo-S-mecamylamine or a pharmaceutically acceptable salt thereof, substantially free of its exo-R-mecamylamine, said amount being sufficient to ameliorate the medical condition. The medical conditions include substance addiction (involving nicotine, cocaine, alcohol, amphetamine, opiate, other psychostimulant and a combination thereof), Tourette's Syndrome, and neuropsychiatric disorders (such as bipolar disorder, depression, an anxiety disorder, schizophrenia, a seizure disorder, Parkinson's disease and attention deficit hyperactivity disorder).
Owner:UNIV OF SOUTH FLORIDA
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS20050214229A1Convenient and reliable and practicalReduce cravingsBiocideDrug compositionsOpiateInitial dose
Owner:JSR NTI
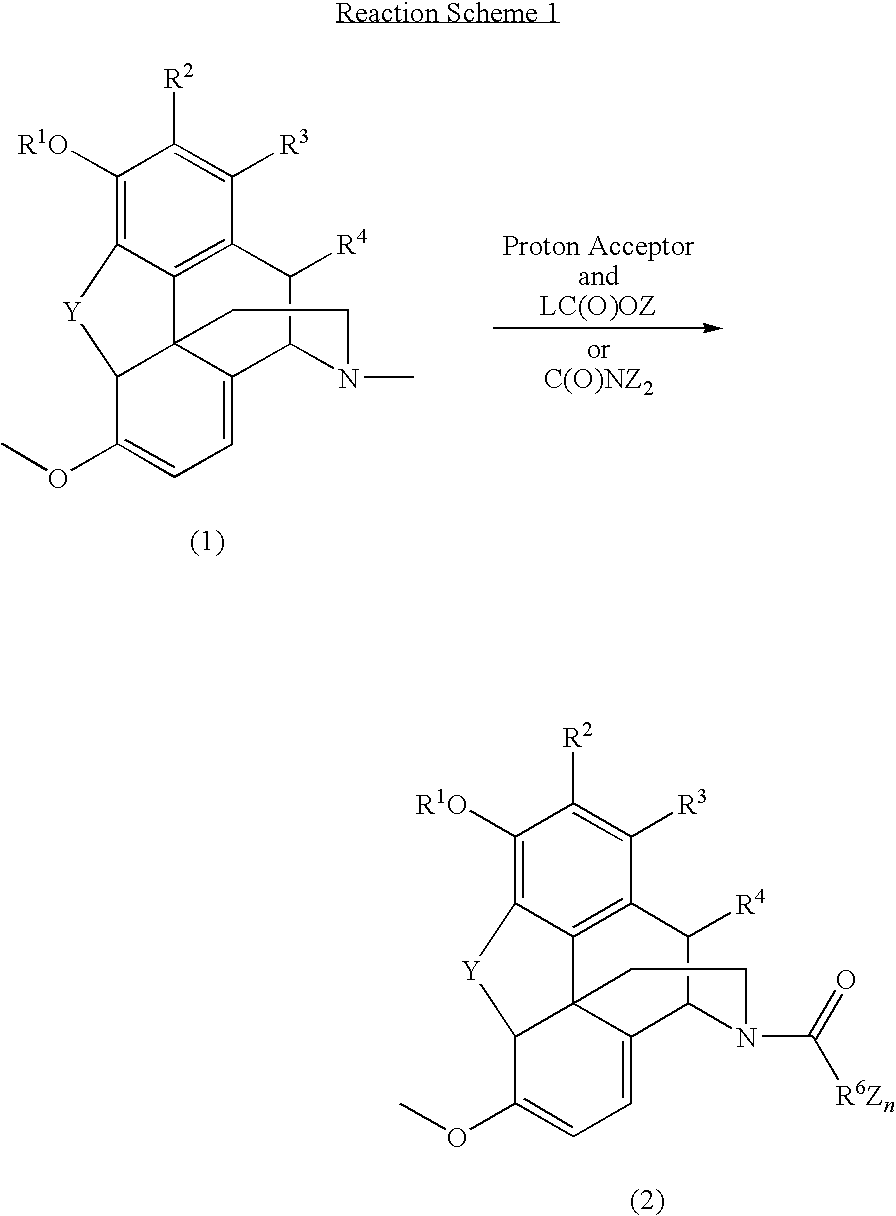
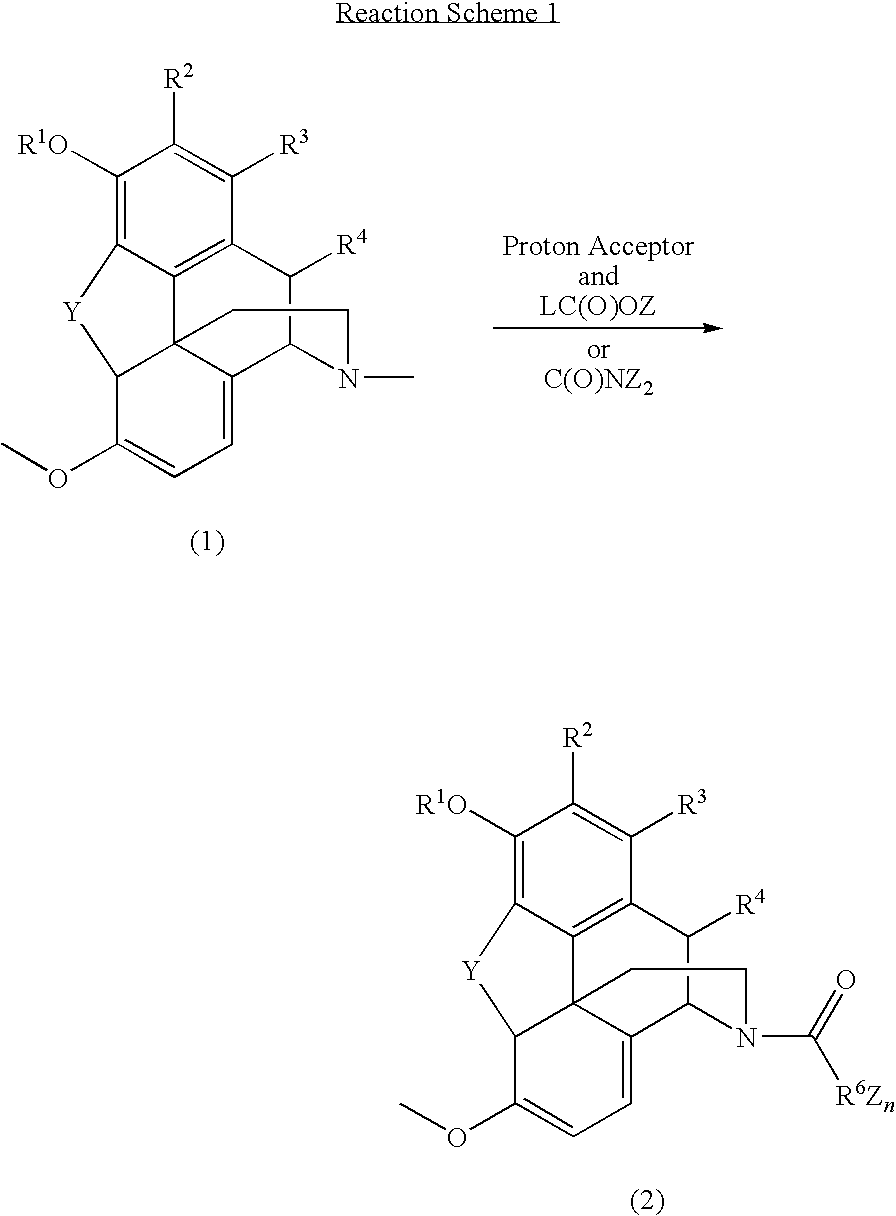
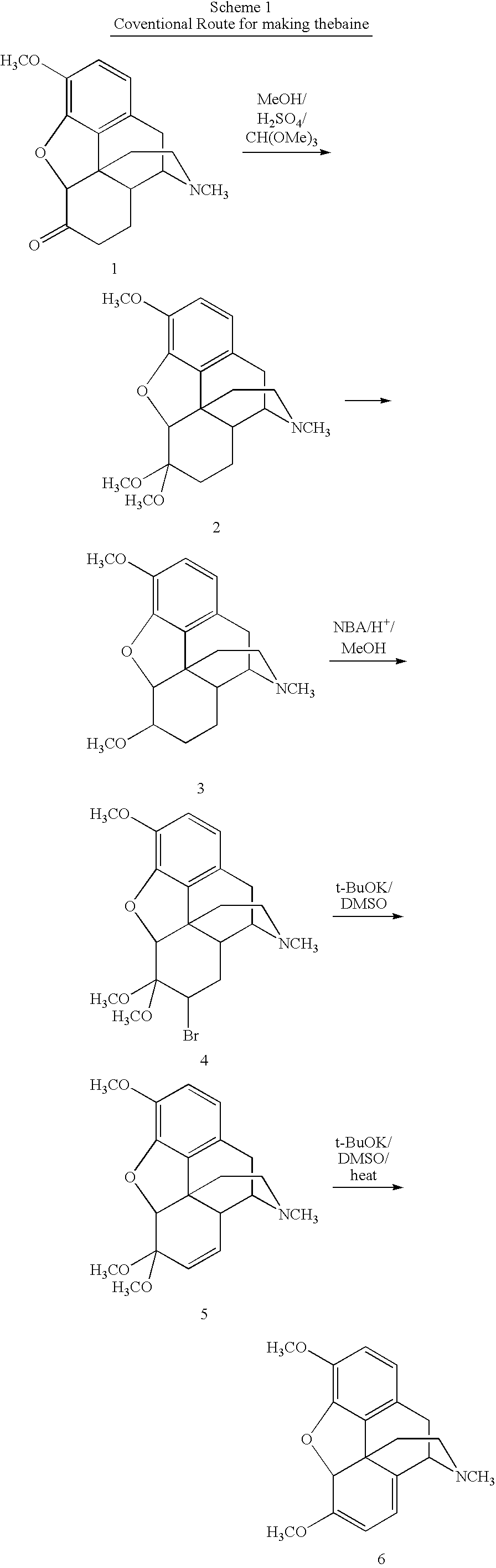
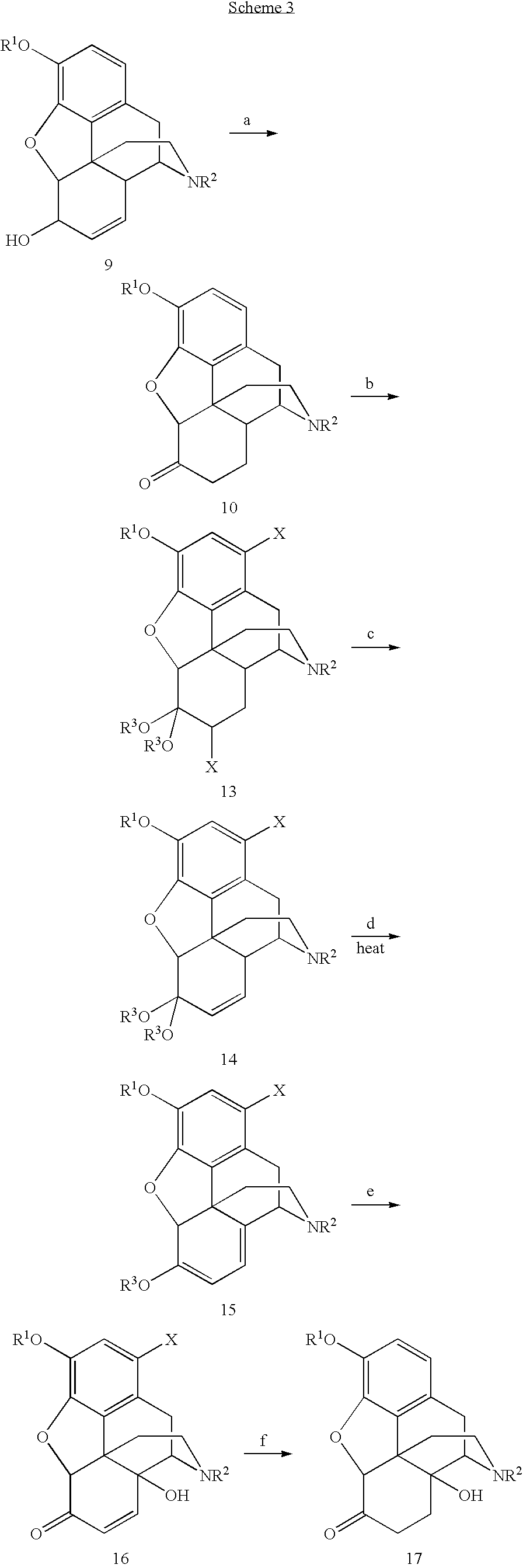
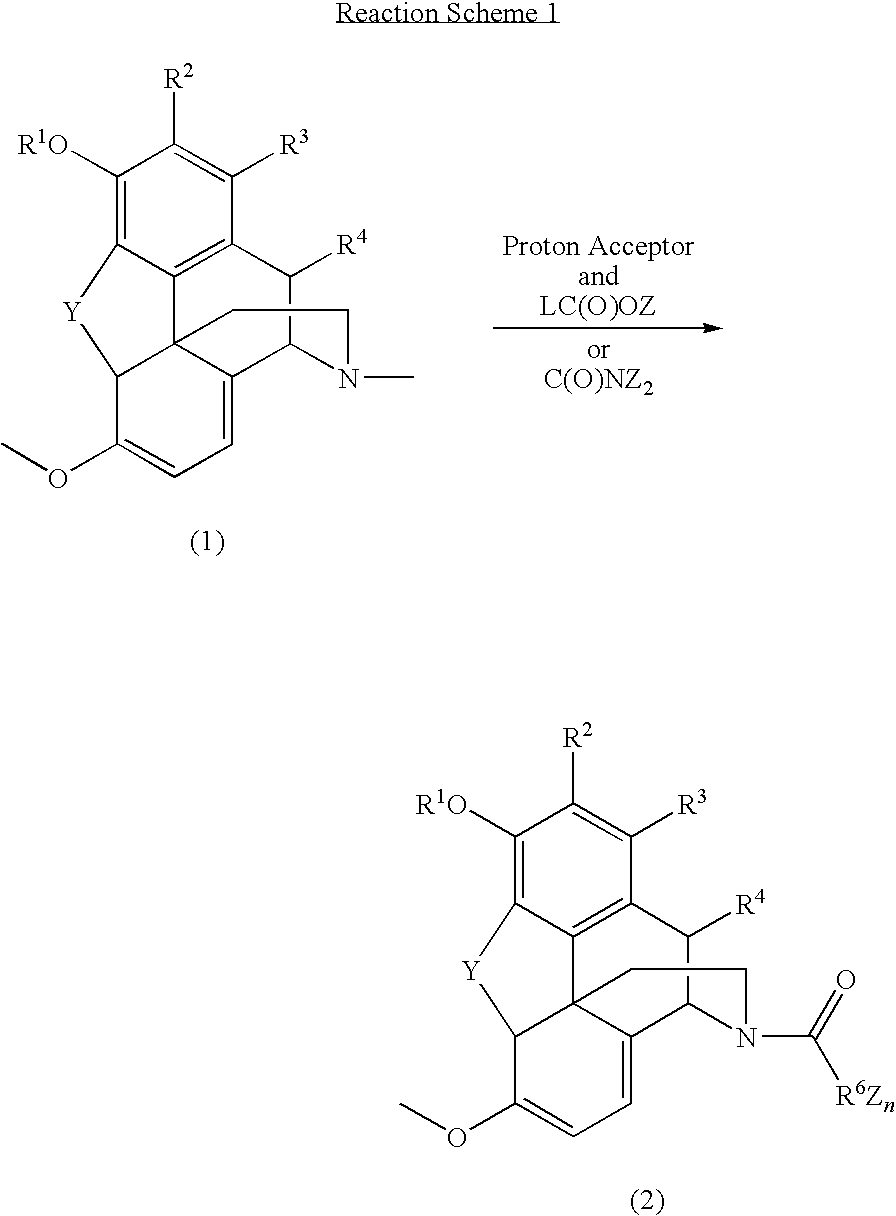
N-demethylation of N-methyl morphinans
The present invention provides a synthetic process for the N-demethylation of N-methyl morphinans. In particular, the invention provides improved synthetic methods for the preparation of N-demethylated morphinan compounds that may be employed as starting materials, for example, commonly available N-methyl opiates such as oripavine and thebaine, and C(3)-protected hydroxy derivatives of oripavine.
Owner:SPECGX LLC
Analgetic dosage forms that are resistant to parenteral and inhalation dosing and have reduced side effects
The invention provides a novel solid pharmaceutical dosage form which includes an opiate, an opiate antagonist admixed with the analgetic (opiate agonist) and an amount of a hydrocolloid containing excipient which is effective to form a non-injectable slurry when the dosage form is contacted with water. In addition the dosage form contains pure naloxone in enteric coated form which is designed to release in the colon to prevent or relieve constipation. Thus the formulation, because of the enteric coated naloxone and the hydrocolloid excipient(s), has reduced side effects as compared with formulations which do not contain these features.
Owner:GORDON MAXWELL
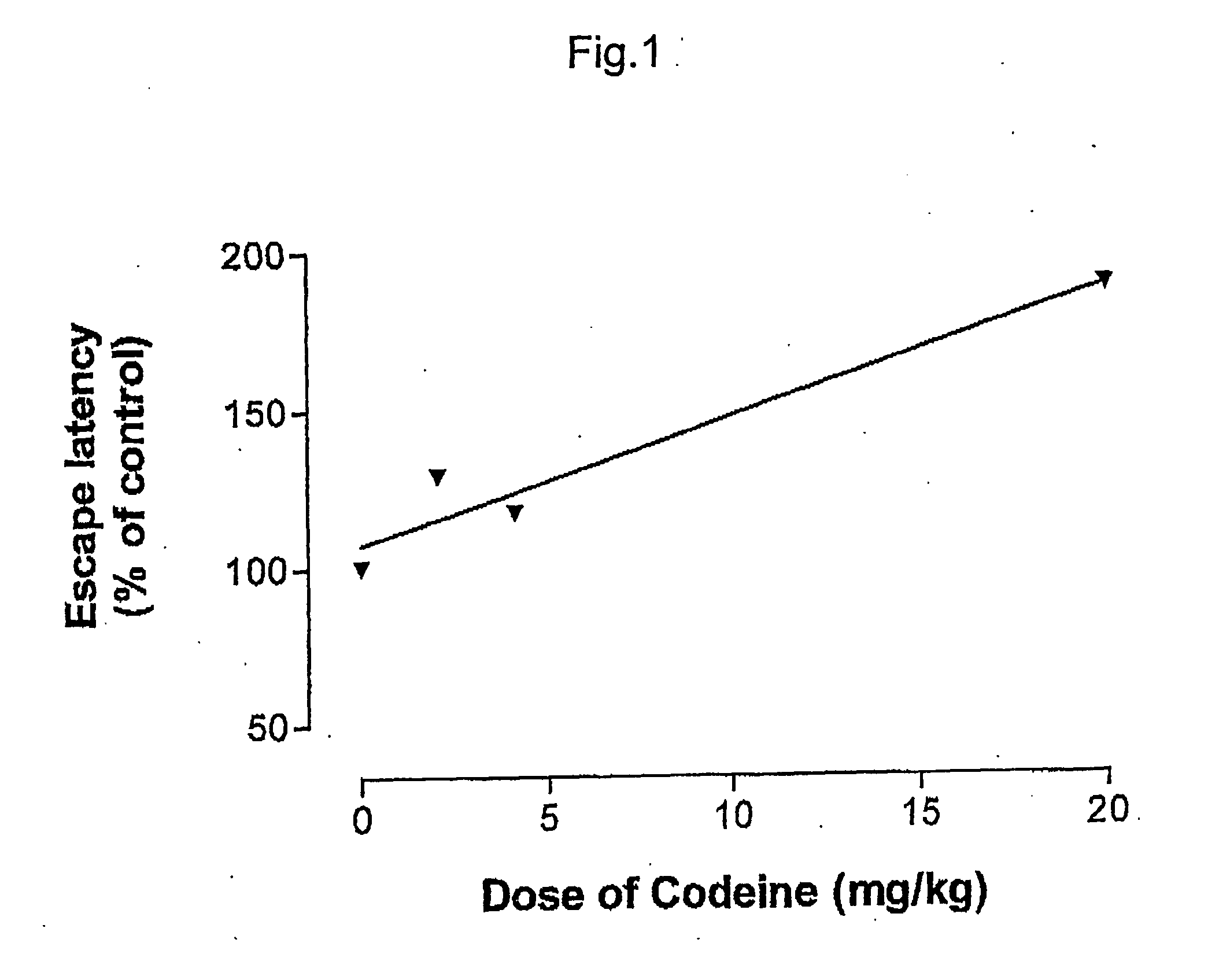
Pharmaceutical combinations of cox-2 inhibitors and opiates
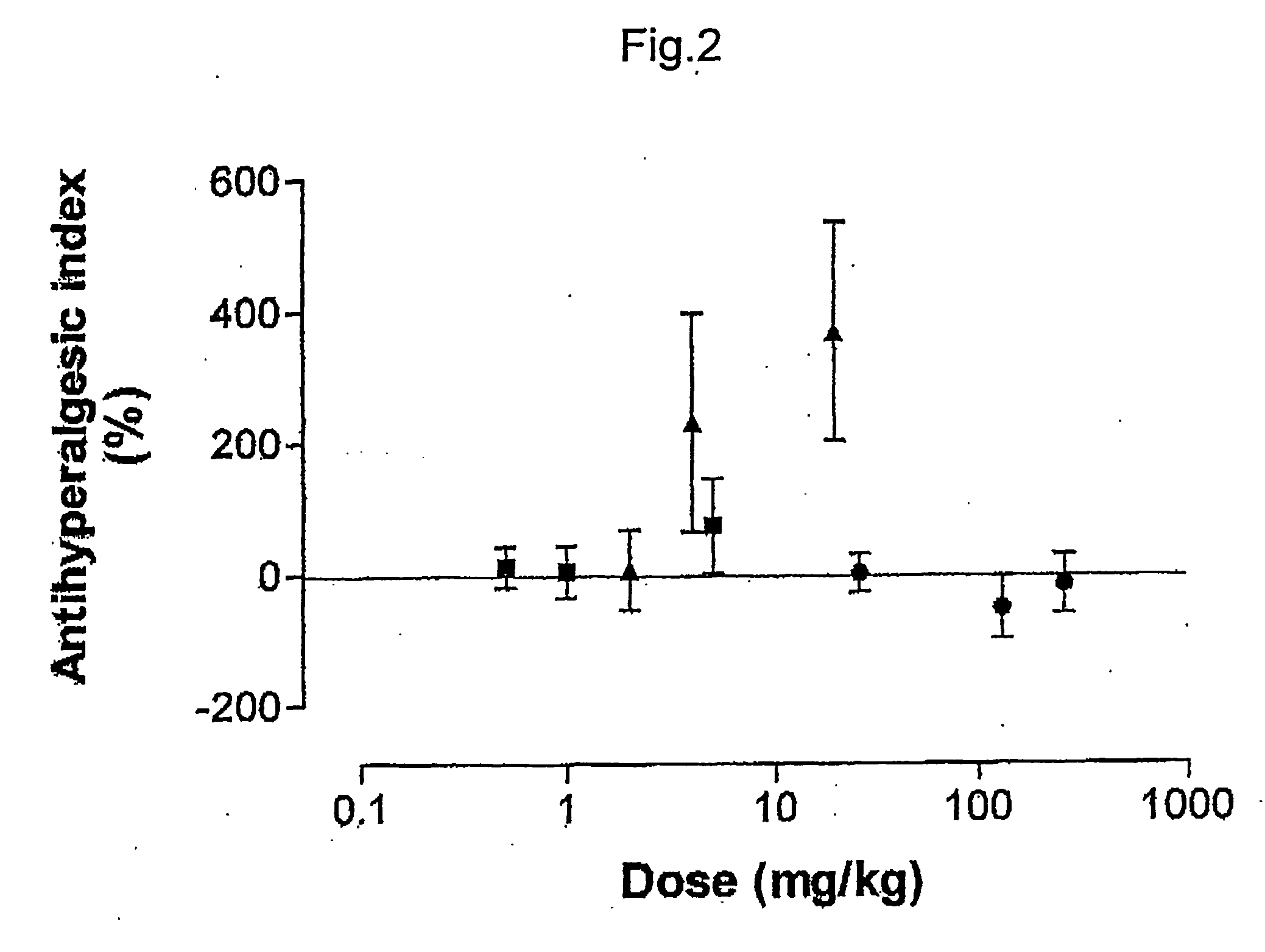
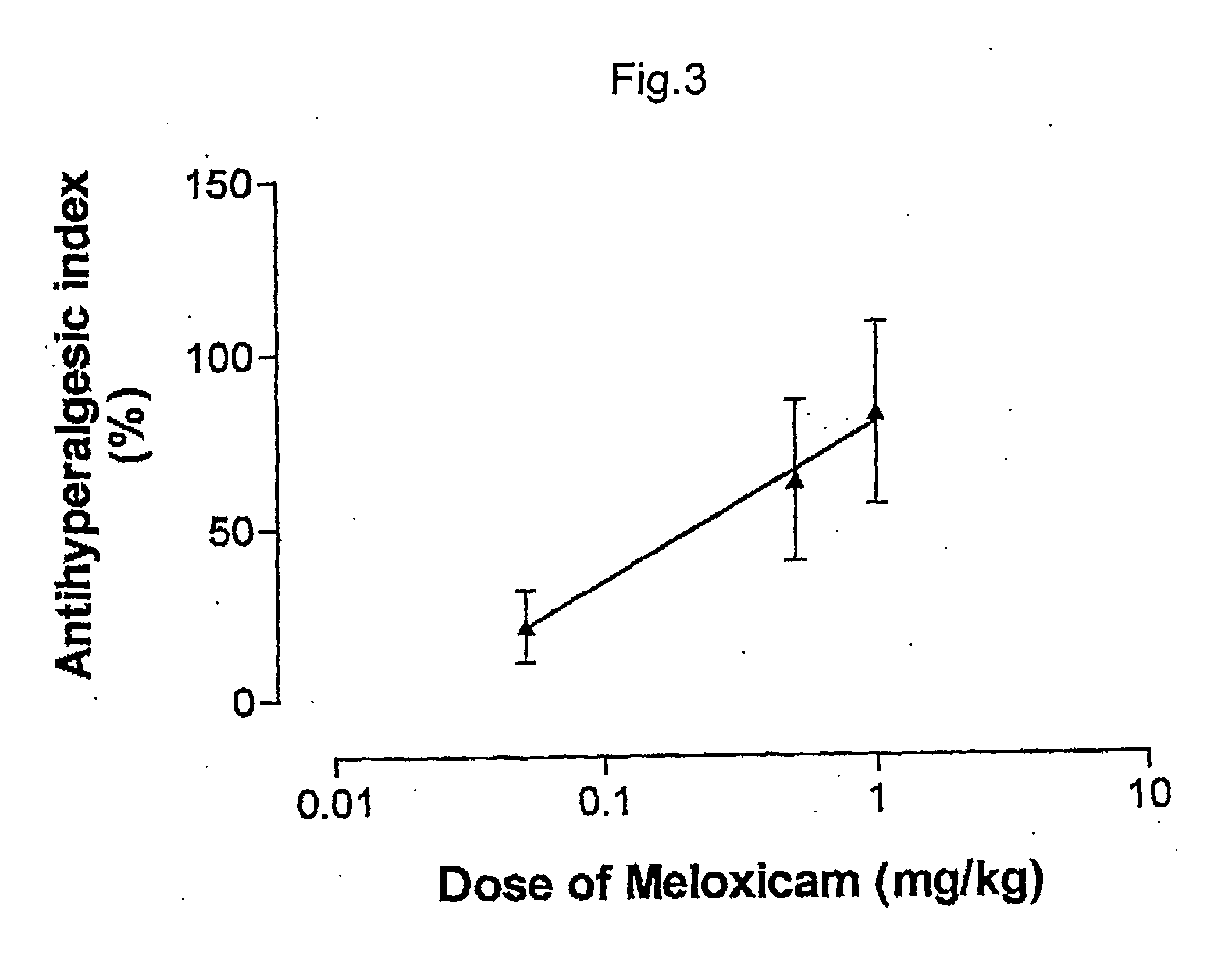
ABSTRACTA pharmaceutical composition comprises a combination of a selective or specific COX 2 inhibitor or a pharmaceutically acceptable salt or derivative thereof and an opiate or a pharmaceutically acceptable salt or derivative thereof, for example a combination of meloxicam and codeine, as active ingredients, and a pharmaceutically acceptable carrier. It may include a centrally-acting cyclo-oxygenase inhibitor such as paracetamol or its pharmaceutically acceptable salts or derivatives. The pharmaceutical compositions are used in methods of providing symptomatic relief or treatment of pain, in an algesic and / or hyperalgesic state, with or without fever, in particular that associated with inflammation such as that associated with trauma, osteoarthritis, rheumatoid arthritis, non-inflammatory myalgia or dysmenorrhoea
Owner:ADCOCK INGRAM LTD
Opiopathies
The present invention provides novel methods for classifying, diagnosing and / or treating a group of human and veterinary ailments involving endogenous opioid concentrations. Also provided is a novel use for an existing class of compounds, the opioids, to treat opiopathic ailments, particularly paresis / paralysis, pseudo-atrophy and / or opiopathic pain, and in the manufacture of pharmaceutical and veterinary formulations therefor. The invention also relates to neuropathic, polyneuropathic, neurologic and neurogenic ailments typically characterized by paresis / paralysis. These ailments can involve an abnormal concentration of one or more endogenous opioids, or the blockade, underexpression or overexpression of one or more opioid receptors. In that regard, the invention encompasses therapeutic uses, methods and compositions employing opiates and / or their receptors. In particular, the invention relates to certain laboratory testing methods, clinical testing methods, research and development methods, business methods, methods of treatment, novel therapeutic uses, and human and veterinary pharmaceutical dosage forms, dosing regimens and formulations, especially those pertaining to opiopathy (particularly hypo-opiopathy).
Owner:BROOKS KORN HOWARD
Acetaminophen compositions having minimized side effects including reduced hepatotoxicity
InactiveUS20080139654A1Promotes glutathione productionMitigate noxious smellBiocidePeptide/protein ingredientsSide effectOpiate
Solid tablets or gel capsules comprising acetaminophen and an agent that promotes glutathione production that mitigates adverse hepatic effects of acetaminophen. The glutathione production promoter is preferably n-acetylcysteine or other mercapto-2-amino alkyl carboxylic acid having glutathione production promoting properties. A preferred composition comprises acetaminophen (200 mg to 750 mg) and N-acetylcysteine (200 mg to 600 mg). Alternatively and / or in addition to N-acetylcysteine the composition can contain at least one of methionine and cysteine. Preferred compositions can contain at least one of an opiate (or synthetic equivalent), an antihistamine, an antiemetic, and a sedative. Physical encapsulation of the ingredients optionally with or in place of a fragrance is used to make the composition more acceptable to patients by mitigating noxious properties of the glutathione production promoter. Preferably, the compositions are prepared for patient self administration in tablet or gel capsule form wherein the patients can take the medication without the need for close oversight of a medical caregiver.
Owner:SODERLING ERIC MOTT
Opiopathies
The present invention provides novel methods for classifying, diagnosing and / or treating a group of human and veterinary ailments involving endogenous opioid concentrations. Also provided is a novel use for an existing class of compounds, the opioids, to treat opiopathic ailments, particularly paresis / paralysis, pseudo-atrophy and / or opiopathic pain, and in the manufacture of pharmaceutical and veterinary formulations therefor. The invention also relates to neuropathic, polyneuropathic, neurologic and neurogenic ailments typically characterized by paresis / paralysis. These ailments can involve an abnormal concentration of one or more endogenous opioids, or the blockade, underexpression or overexpression of one or more opioid receptors. In that regard, the invention encompasses therapeutic uses, methods and compositions employing opiates and / or their receptors. In particular, the invention relates to certain laboratory testing methods, clinical testing methods, research and development methods, business methods, methods of treatment, novel therapeutic uses, and human and veterinary pharmaceutical dosage forms, dosing regimens and formulations, especially those pertaining to opiopathy (particularly hypo-opiopathy).
Owner:BROOKS KORN HOWARD
Preparation and utility of opioid analgesics
The present disclosure is directed to modulators of opiate- and / or NMDA receptors and pharmaceutically acceptable salts and prodrugs thereof, the chemical synthesis thereof, and the use of such compounds for the treatment and / or management of pain, anxiety, neurodegeneration, drug dependence, coughing, muscular tension, and / or glaucoma and any other condition in which it is beneficial to modulate an opiate- and / or NMDA receptor.
Owner:AUSPEX PHARMA INC
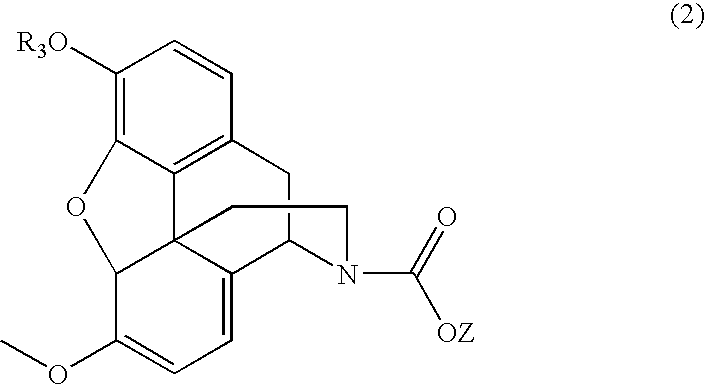
Processes for the production of buprenorphine with reduced impurity formation
The present invention provides process for the production of opiate alkaloids. In particular, the present invention provides processes for the production of buprenorphine or a derivative of buprenorphine that minimizes the formation of impurities.
Owner:SPECGX LLC
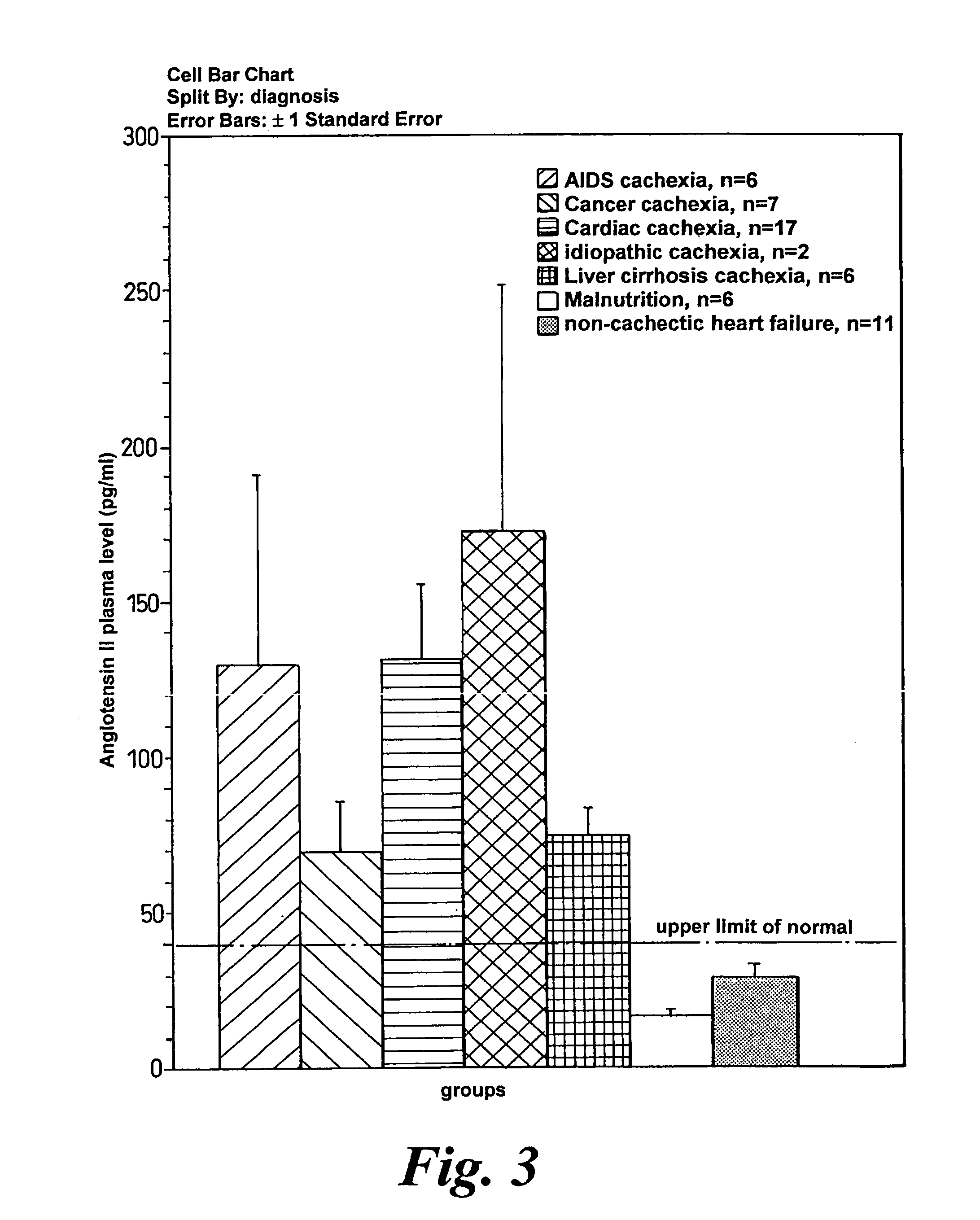
Methods of treating cachexia
InactiveUS7417038B1Reduced metabolic rateIncrease blood flowPeptide/protein ingredientsMetabolism disorderDiseaseImidazoline receptor
A method of treating weight loss due to underlying disease in a patient the method comprising administering to the patient an effective amount of an agent which reduces sympathetic nervous system activity. A method of treating weight loss due to underlying disease in a patient the 10 method comprising administering to the patient an effective amount of any one or more of the following: a compound which inhibits the effect of aldosterone such as an aldosterone antagonist; a chymase inhibitor; a cathepsin B inhibitor; a 13 receptor blocker; an imidazoline receptor antagonist; a centrally acting tx receptor antagonist; a peripherally acting ct receptor antagonist; a ganglion blocking agent; a drug that has an effect on cardiovascular reflexes and thereby reduce SNS activity such as an opiate; scopolamine; an endothelin receptor antagonist; and a xanthine oxidase inhibitor. The methods are particularly useful in treating cardiac cachexia.
Owner:IMPERIAL INNOVATIONS LTD
Di-substituted amides for enhancing glutamatergic synaptic responses
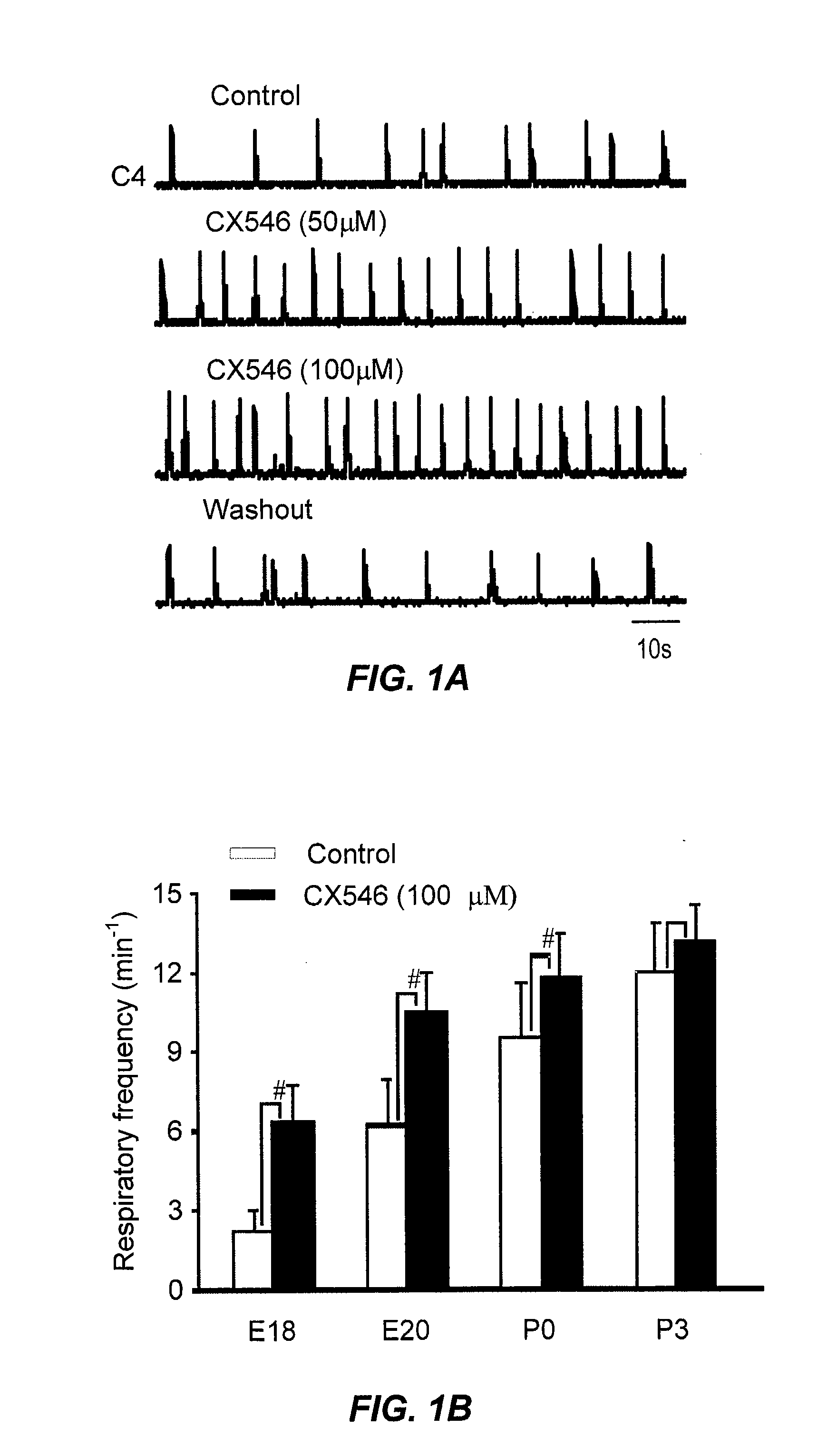
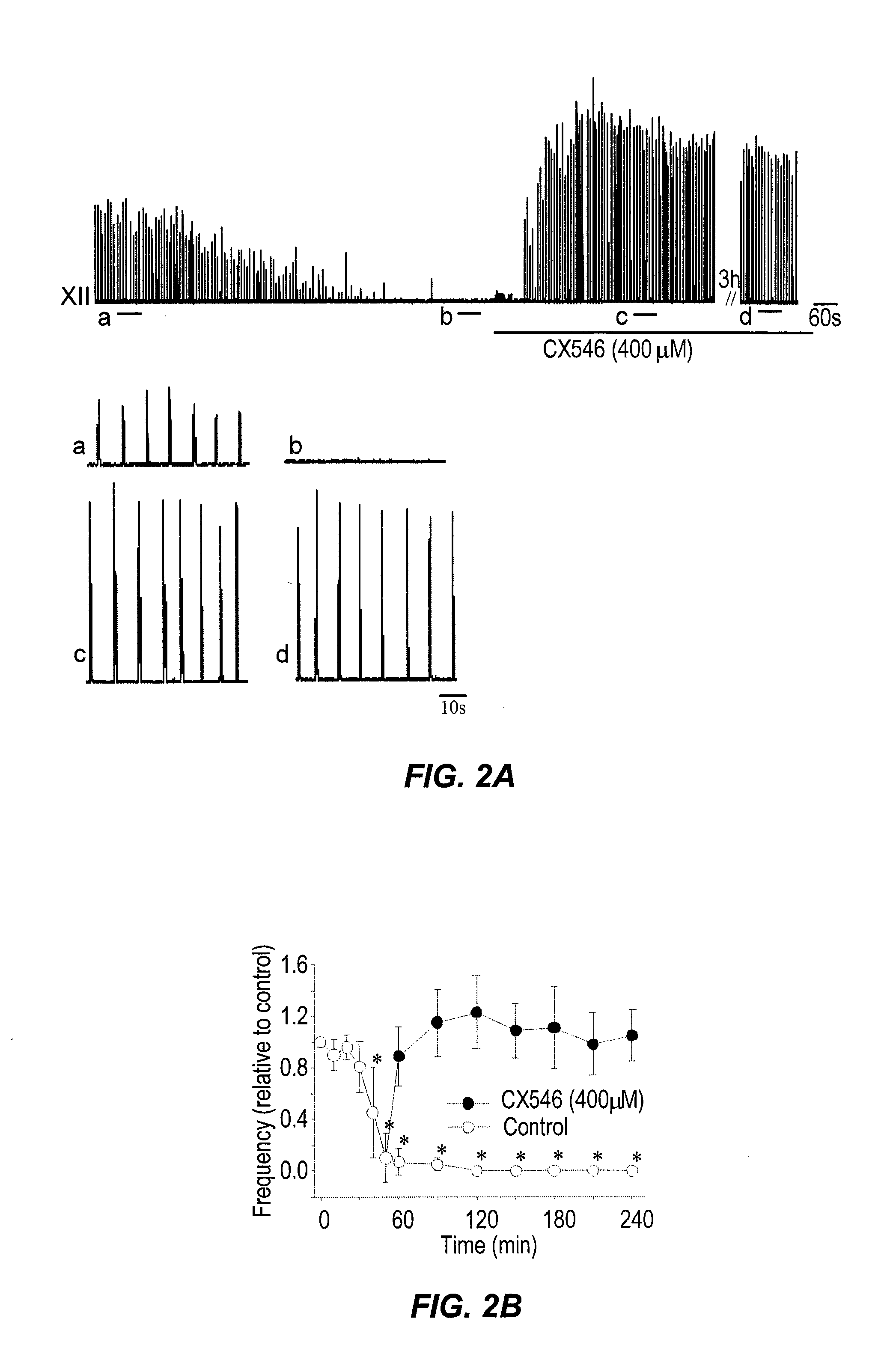
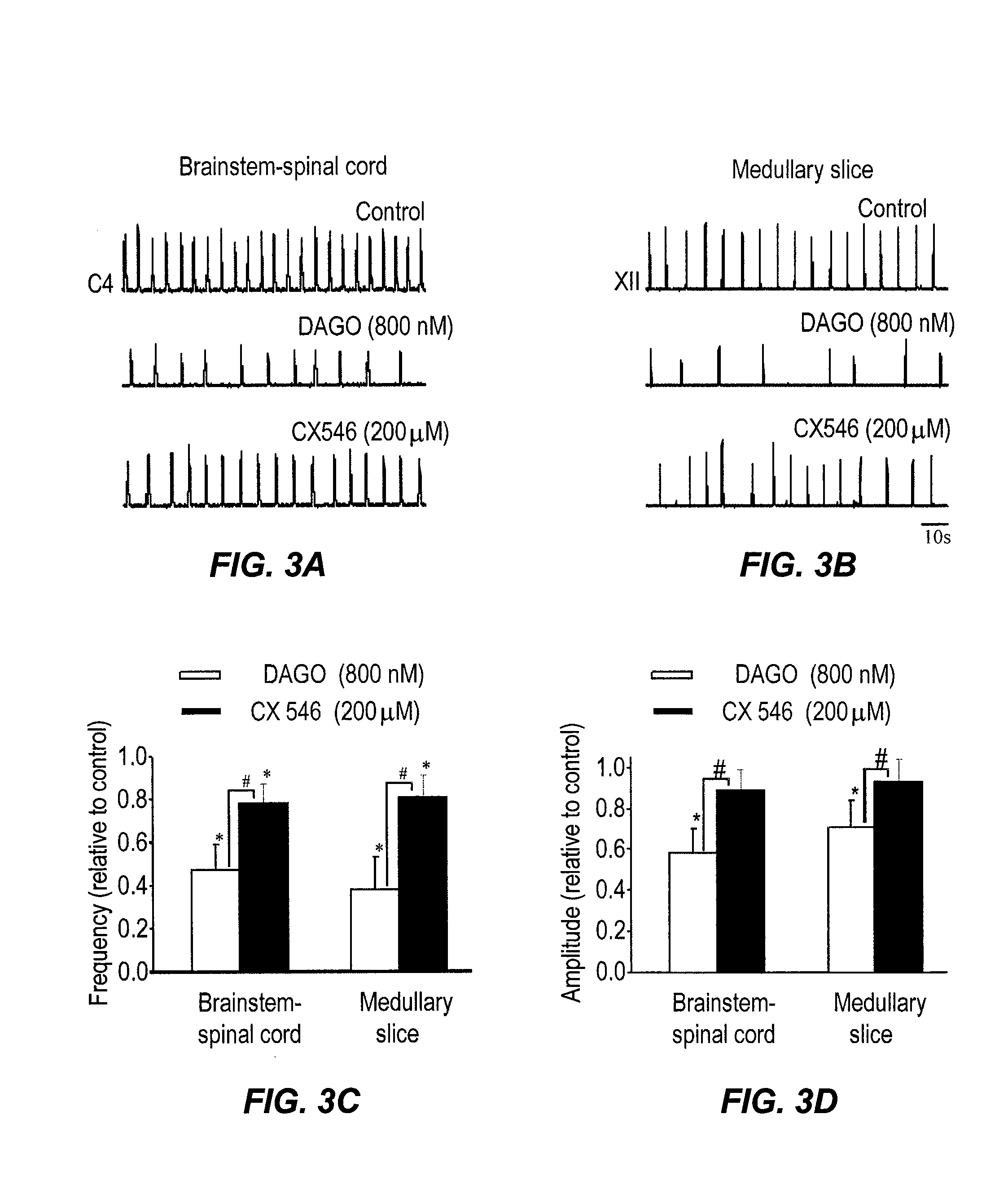
InactiveUS8013003B2Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stroke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning. In a particular aspect, the invention relates to compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA INC
Abuse resistant capsule
The present invention is directed to an immediate release and extended release capsule or capsule fill which mitigates the abuse of abuse-susceptible active pharmaceutical ingredients by direct intravenous injection. The fill comprises a parenteral abuse resistant liquid formulation which when mixed with water and heated, results in a turbid, viscous or bubbling mixture that is not injectable with a standard insulin syringe. The abuse-susceptible active pharmaceutical ingredient is selected from the group consisting of opiates, opioids, tranquillizers, stimulants and narcotics.
Owner:R P SCHERER TECH INC
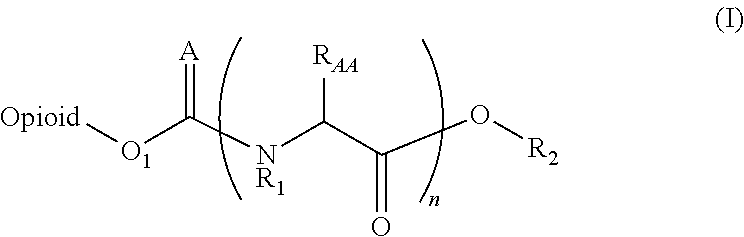
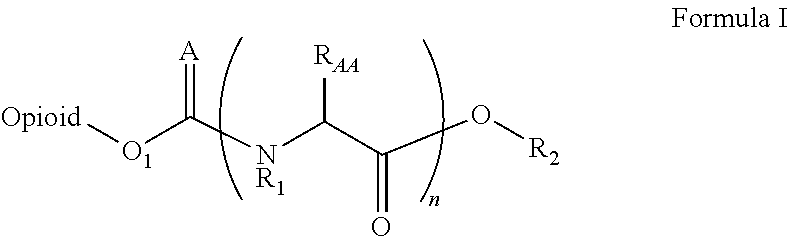
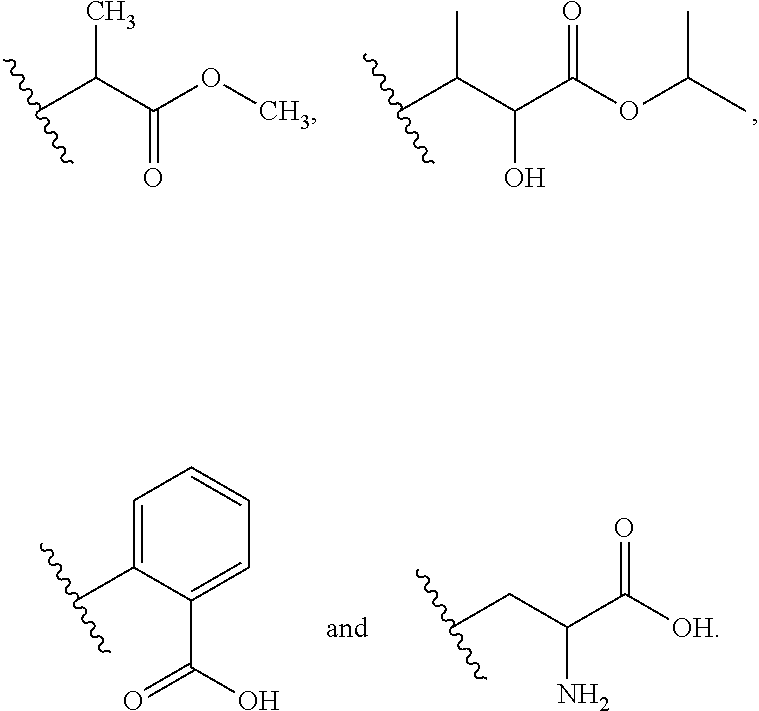
Novel carbamate amino acid and peptide prodrugs of opiates and uses thereof
Carbamate linked prodrugs of meptazinol and other opioid analgesics are provided. The prodrug moiety may comprise a single amino acid or short peptide. Additionally, the present invention relates to methods for reducing gastrointestinal side effects in a subject, the gastrointestinal side effects being associated with the administration of an opioid analgesic. The methods comprise orally administering an opioid prodrug or pharmaceutically acceptable salt thereof to a subject, wherein the opioid pro-drug is comprised of an opioid analgesic covalently bonded through a carbamate linkage to a prodrug moiety, and wherein upon oral administration, the prodrug or pharmaceutically acceptable salt minimizes at least one gastrointestinal side effect associated with oral administration of the opioid analgesic alone. Compositions for use with the method are also provided.
Owner:SHIRE PLC
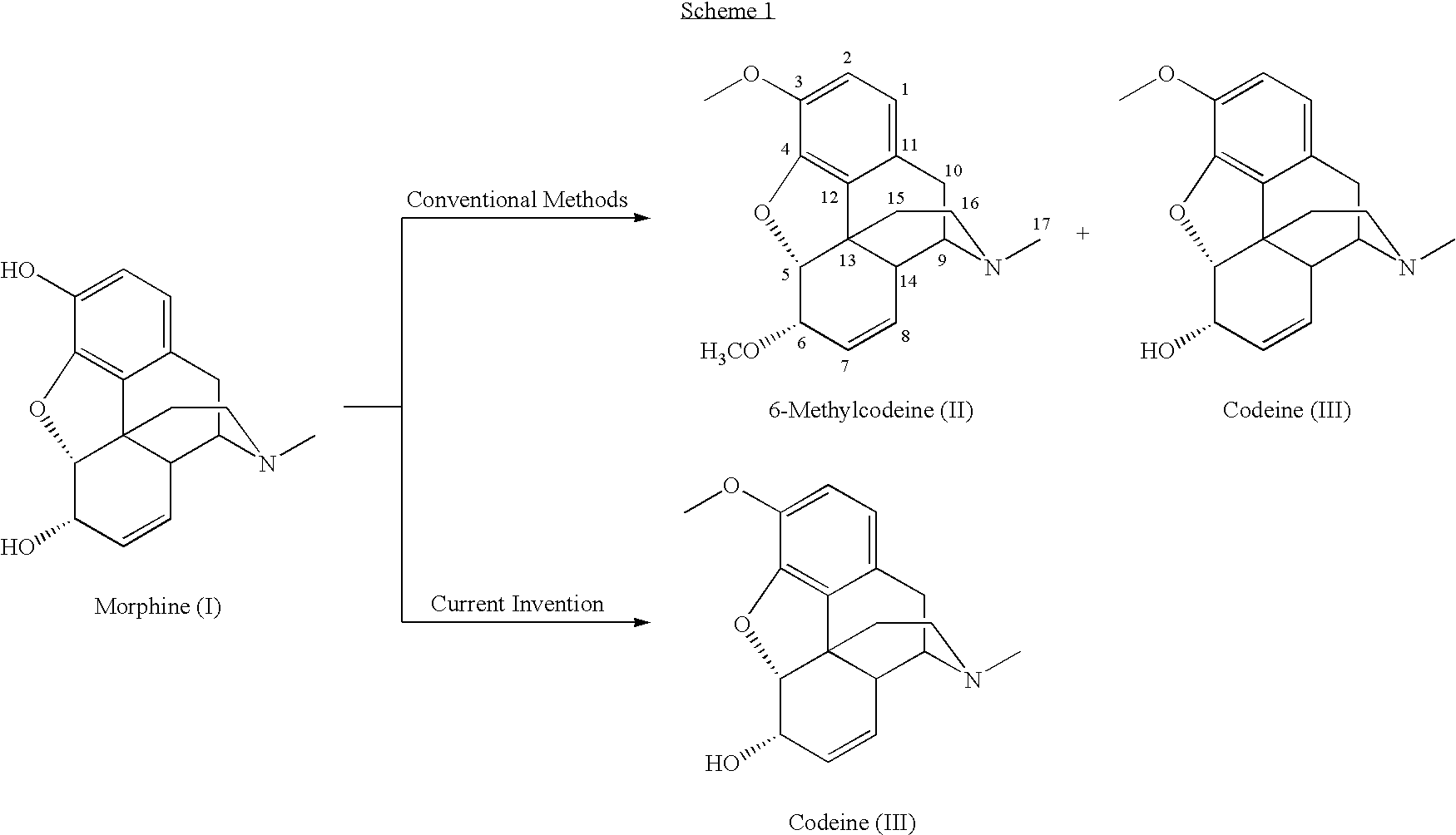
Process for the production of opiates
A morphine component, e.g., a concentrate of poppy straw, is converted into codeine in high yield and high purity and in a highly controlled manner. The conversion process involves the following steps: (a) providing a solution or suspension of a morphine component in an inert solvent or a mixture of solvents; (b) methylating the resultant solution or suspension with a methylating agent in the presence of an alkaline ingredient; and (c) recovering the resultant codeine as the free base or as a salt.
Owner:ACURA PHARMA
Method of inhibition of respiratory depression using positive allosteric ampa receptor modulators
ActiveUS20080261962A1Reducing and inhibiting respiratory depressionReduce and inhibit respiratory depressionBiocideNervous disorderDiseaseOpiate
The invention is directed to a method for alleviating respiratory depression in a subject as a result of disease of pharmacological agents such as opiates, opioids or barbiturates. The invention also discloses pharmaceutical compositions for use with the method, the composition containing in combination, an analgesic, anaesthetic, or a sedative and a positive allosteric AMPA receptor modulator in an amount sufficient to reduce or inhibit respiratory depression caused by the analgesic, anaesthetic, or sedative.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
N-demethylation of N-methyl morphinans
The present invention provides a synthetic process for the N-demethylation of N-methyl morphinans. In particular, the invention provides improved synthetic methods for the preparation of N-demethylated morphinan compounds that may be employed as starting materials, for example, commonly available N-methyl opiates such as oripavine and thebaine, and C(3)-protected hydroxy derivatives of oripavine.
Owner:SPECGX LLC
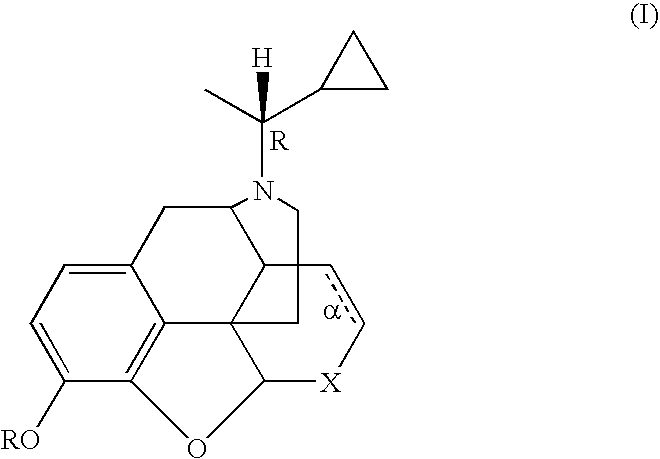
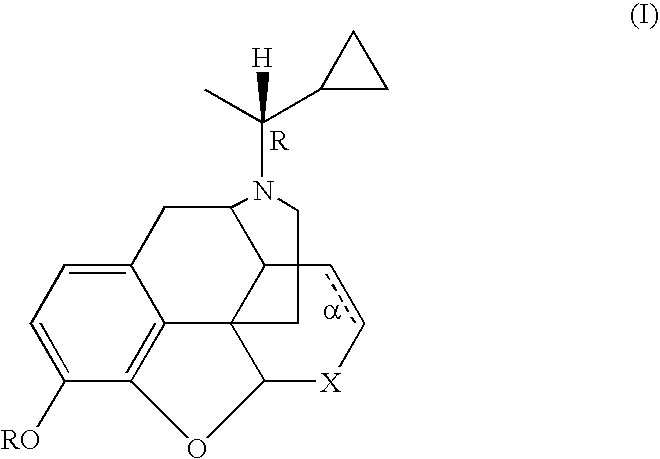
Treatment of chemical dependency with substantially nonaddicting normorphine and norcodeine derivatives
A method is provided for treating a drug-dependent individual so as to effect withdrawal from a drug of abuse, e.g., an opiate such as heroin or oxycodone, a stimulant such as cocaine, or alcohol. The method involves substitution therapy wherein a substantially nonaddicting normorphine or norcodeine derivative is substituted for the drug of abuse. The active agent has the structure of formula (I) wherein: R is H, alkyl, or acyl; X is CH(OR′) or C═O, wherein R′ is H or acyl; α is an optional double bond, with the proviso that when α is present, then X is necessarily CH(OH), or an acid addition salt thereof, wherein preferred such agents are in a stereoisomerically pure form that corresponds to that of N-[(1R)-1-cyclopropylethyl]-normorphine (1) which melts at approximately 188° C.-189° C.
Owner:LAWSON JOHN A
Di-substituted amides for enhancing glutamatergic synaptic responses
InactiveUS20100120764A1Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stroke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning. In a particular aspect, the invention relates to compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA
Biologically Active Cannabidiol Analogs
ActiveUS20190031601A1High analgesic activityPrevent addictionSenses disorderNervous disorderClinical settingsHydrogen
Biologically active cannabidiol analogs comprising a compound of the formulawherein one of R1 or R2 or both is / are the residue of a moiety formed by the reaction of an amino group of the amino acid ester of R1 or R2 or both with a dicarboxylic acid or a dicarboxylic acid derivative and the other R1 or R2 (in the case of the mono) is the residue of a dicarboxylic acid or dicarboxylic acid derivative or Hydrogen (H), (i.e. underivatized), and salts thereof. These CBD analogs are be useful in pain management in oncology and other clinical settings in which neuropathy is presented. Furthermore, these CBD-analogs are useful in blocking the addictive properties of opiates.
Owner:UNIVERSITY OF MISSISSIPPI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com