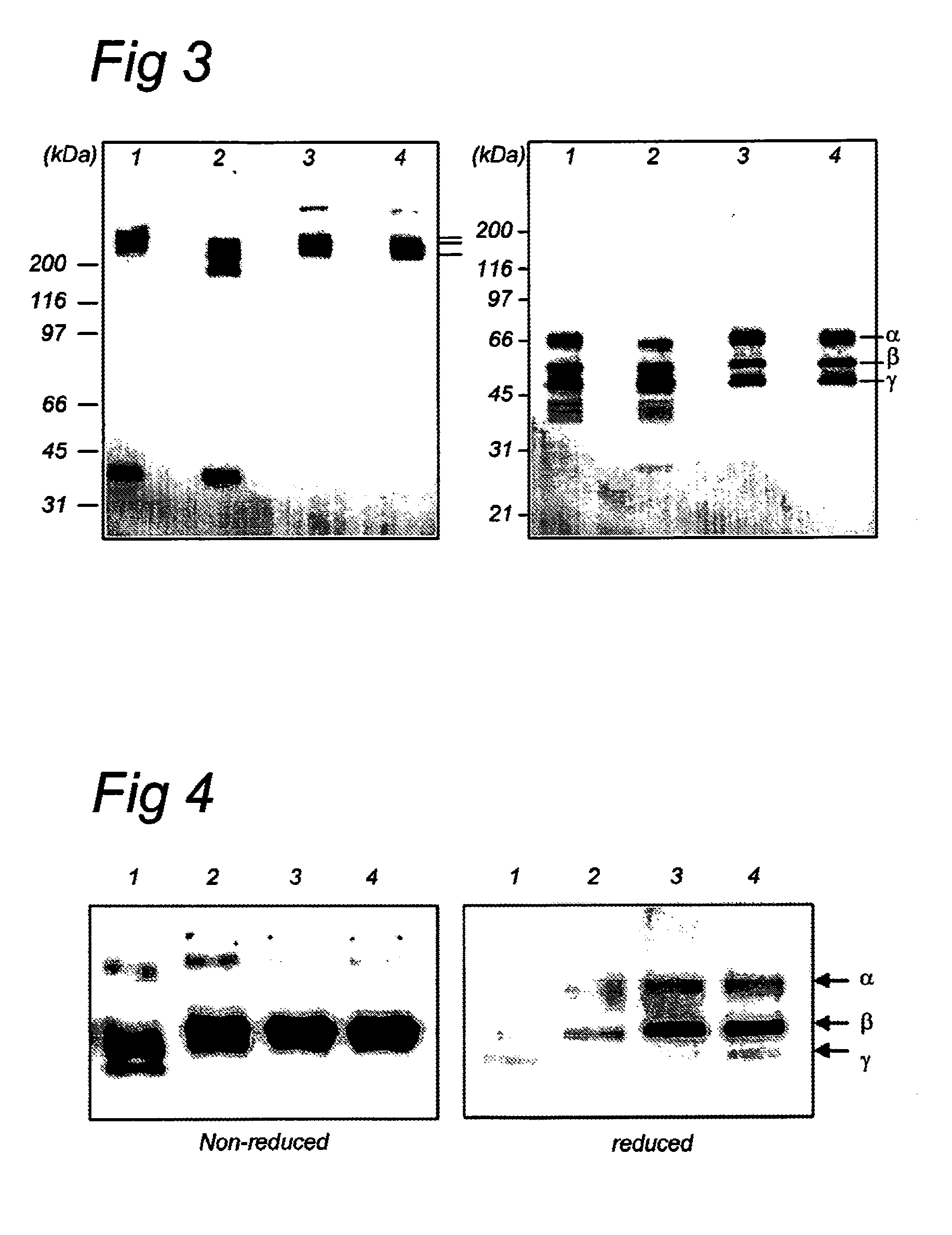
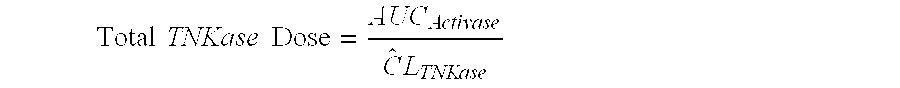Patents
Literature
62 results about "Initial dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Initial dose. a comparatively large dose given at the beginning of treatment to get the patient under the influence of the drug. Synonym: loading dose.
Method and apparatus for monitoring drug effects on cardiac electrical signals using an implantable cardiac stimulation device
InactiveUS7142911B2Increase aggressivenessIncrease dosePhysical therapies and activitiesDrug and medicationsEcg signalCardiac pacemaker electrode
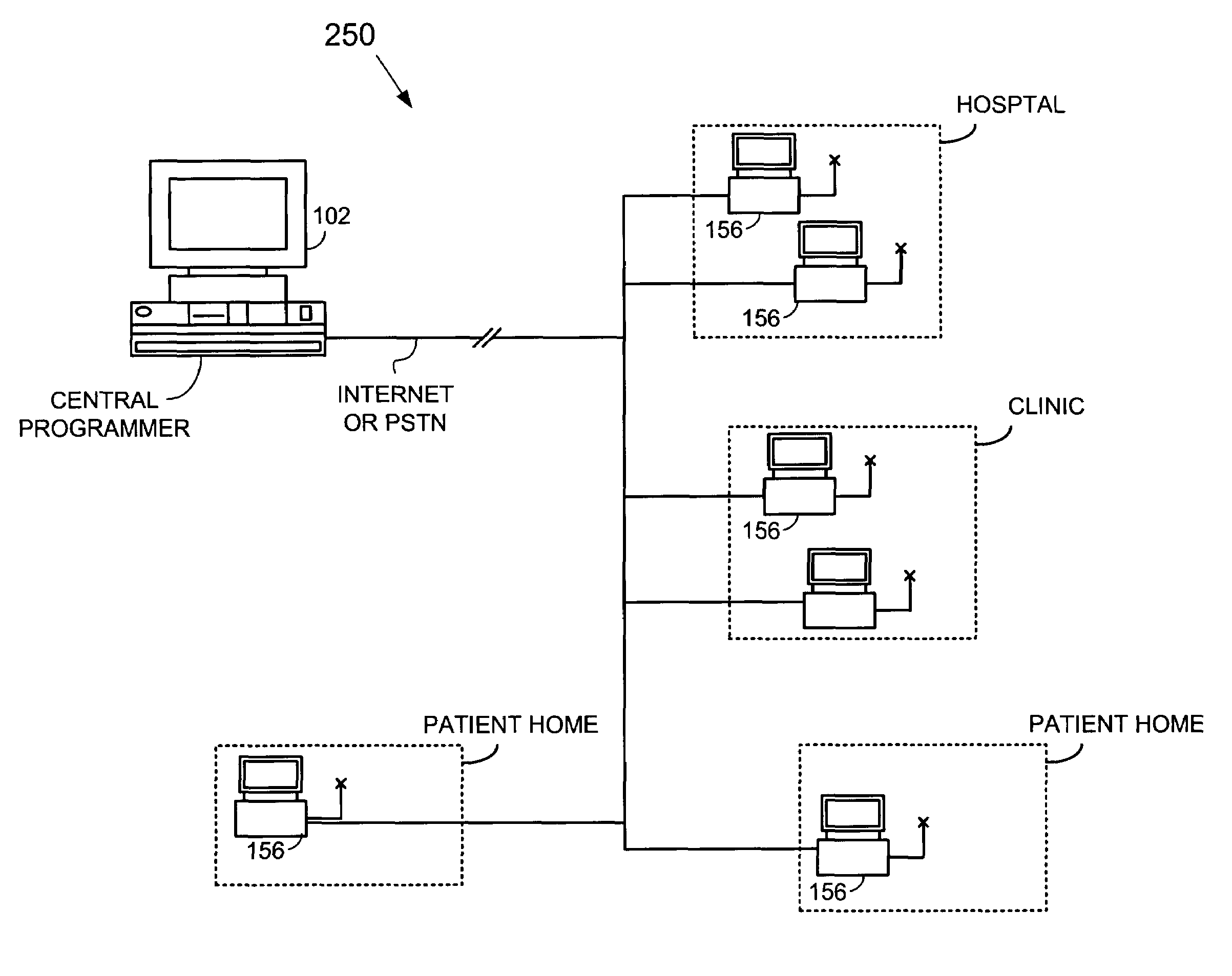
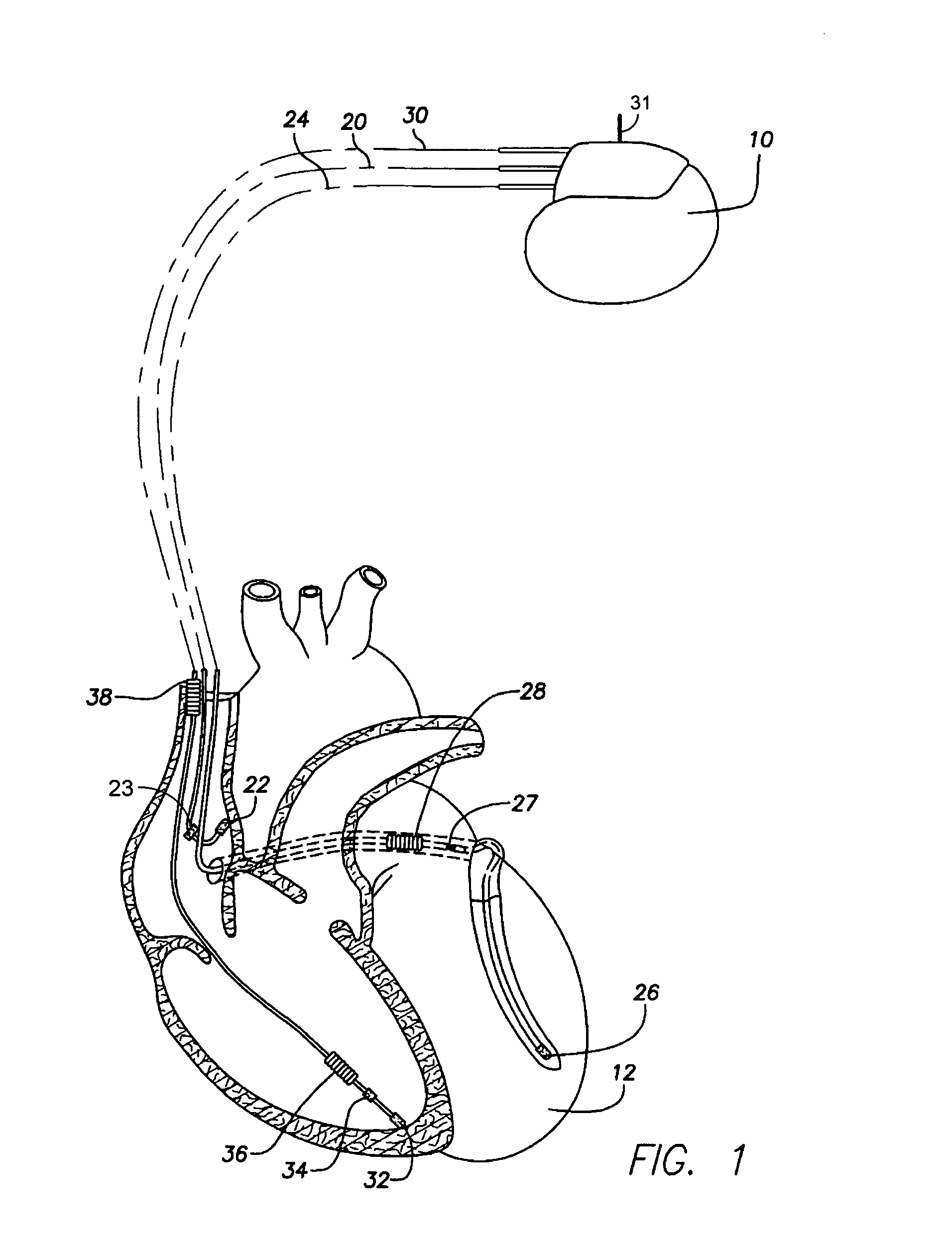
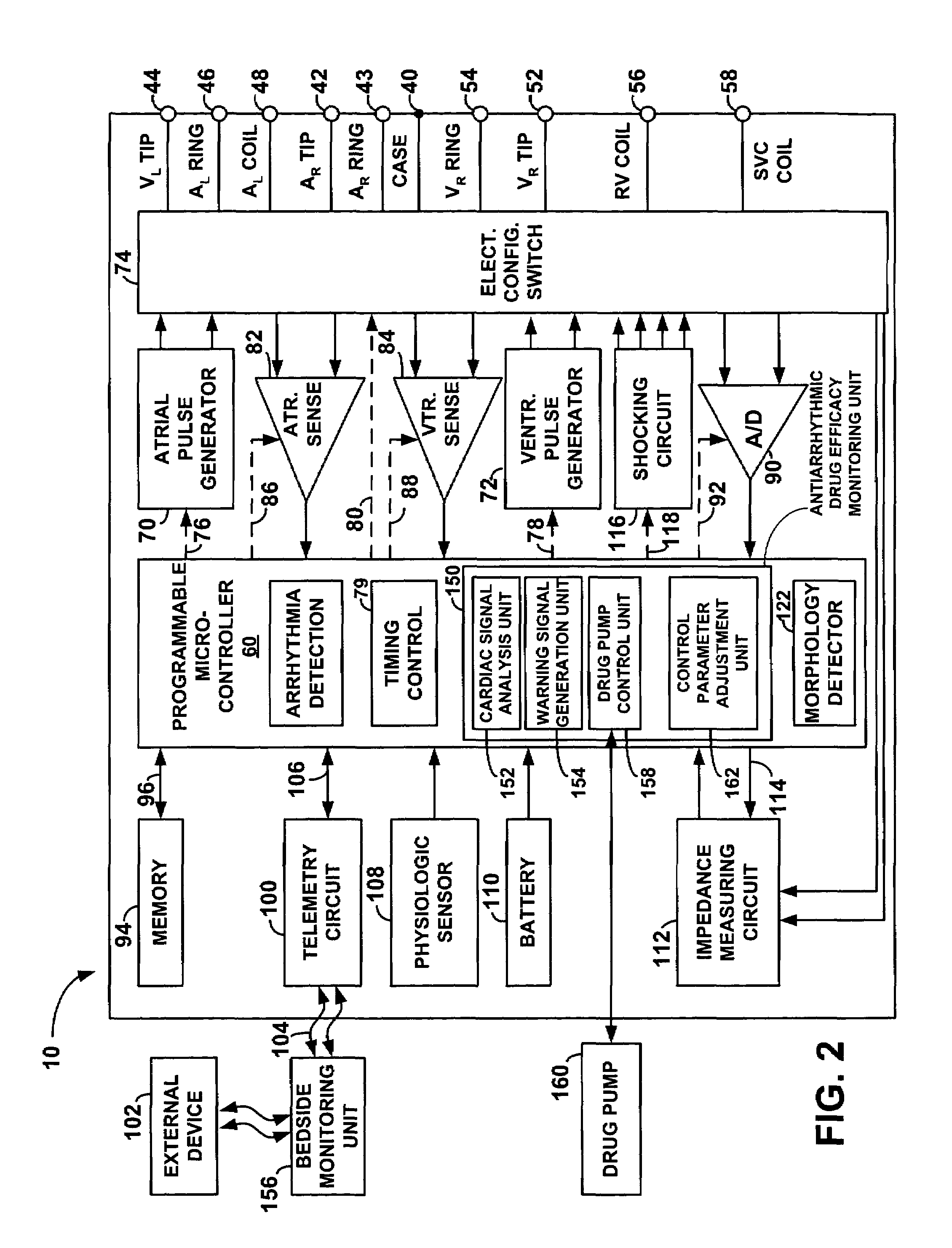
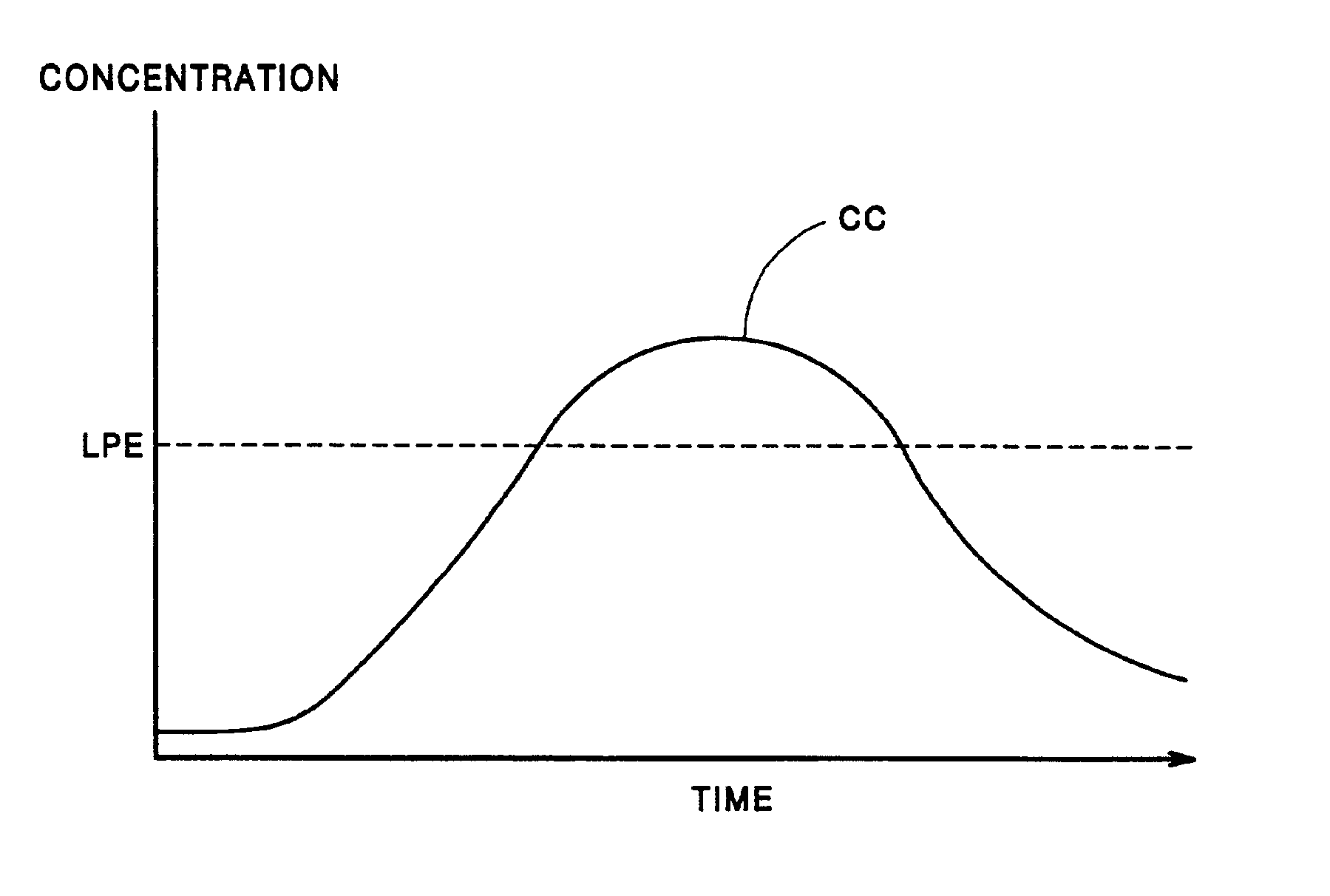
An implantable cardiac stimulation device, such as a pacemaker or Implantable Cardioverter Defibrillator, is configured to automatically monitor the effects of antiarrhythmic drugs on cardiac electrical signals within a patient to verify the efficacy of the drugs taken. In one example, an analysis of patient cardiac electrical signals is performed by comparing the cardiac electrical signals with values representative of the effects of different classes of antiarrhythmic drugs. If the implantable device determines that the prescribed antiarrhythmic drugs have not been effective, a warning signal is generated. The warning signal is conveyed directly to the patient via a bedside monitor and to the patient's physician via remote connection to an external programmer device so that both are notified of the drug efficacy problems. Additionally, the implantable device may be configured to automatically adjust pacing and defibrillation control parameters in an attempt to compensate for any lack of efficacy in the drugs. For example, the aggressiveness of overdrive pacing may be increased. Alternatively, a drug pump is controlled to adjust the dosage of antiarrhythmic drugs if an initial dosage is found to be ineffective.
Owner:PACESETTER INC
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS6893654B2Convenient and reliable and practicalReduce cravingsBiocidePowder deliveryOpiateInitial dose
A two-stage medicine delivery system provides an initial dose of medicine and a second dose of medicine. The initial and second doses are capable of achieving a rapid pharmacological effect and a prolonged pharmacological effect, respectively. The two-stage medicine delivery system preferably delivers a craving reduction substance, in which case, the rapid and prolonged pharmacological effects include a rapid and prolonged craving reduction. Preferably, the delivery system is a nicotine delivery system which is provided in chewing gum form or lozenge form and which provides the nicotine in a transmucosally absorbable form. The two-stage medicine delivery system preferably releases a buffering agent which increases a pH level in a user's mouth to facilitate absorption of the medicine when the delivery system is placed in the user's mouth. A method of making the medicine delivery system also is provided. The system and apparatus can be adapted to reduce cravings for alcohol, food, drugs (e.g., cocaine, opiates and the like) and tobacco products, especially tobacco products containing nicotine.
Owner:JSR NTI
Drug delivery apparatus and method for automatically reducing drug dosage
InactiveUS20060047270A1Reduce probabilityMedical devicesPressure infusionMicrocontrollerDrug reservoir
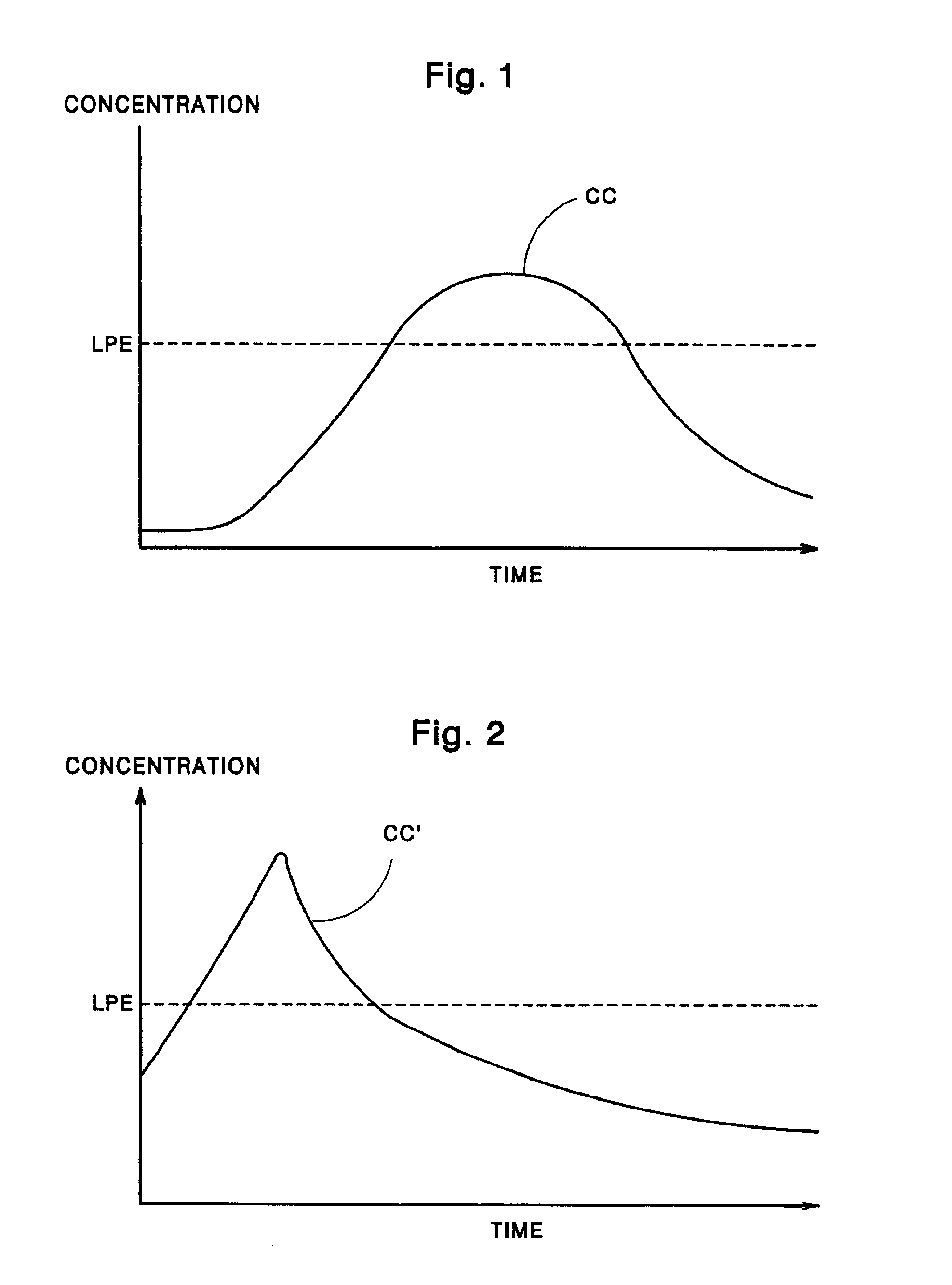
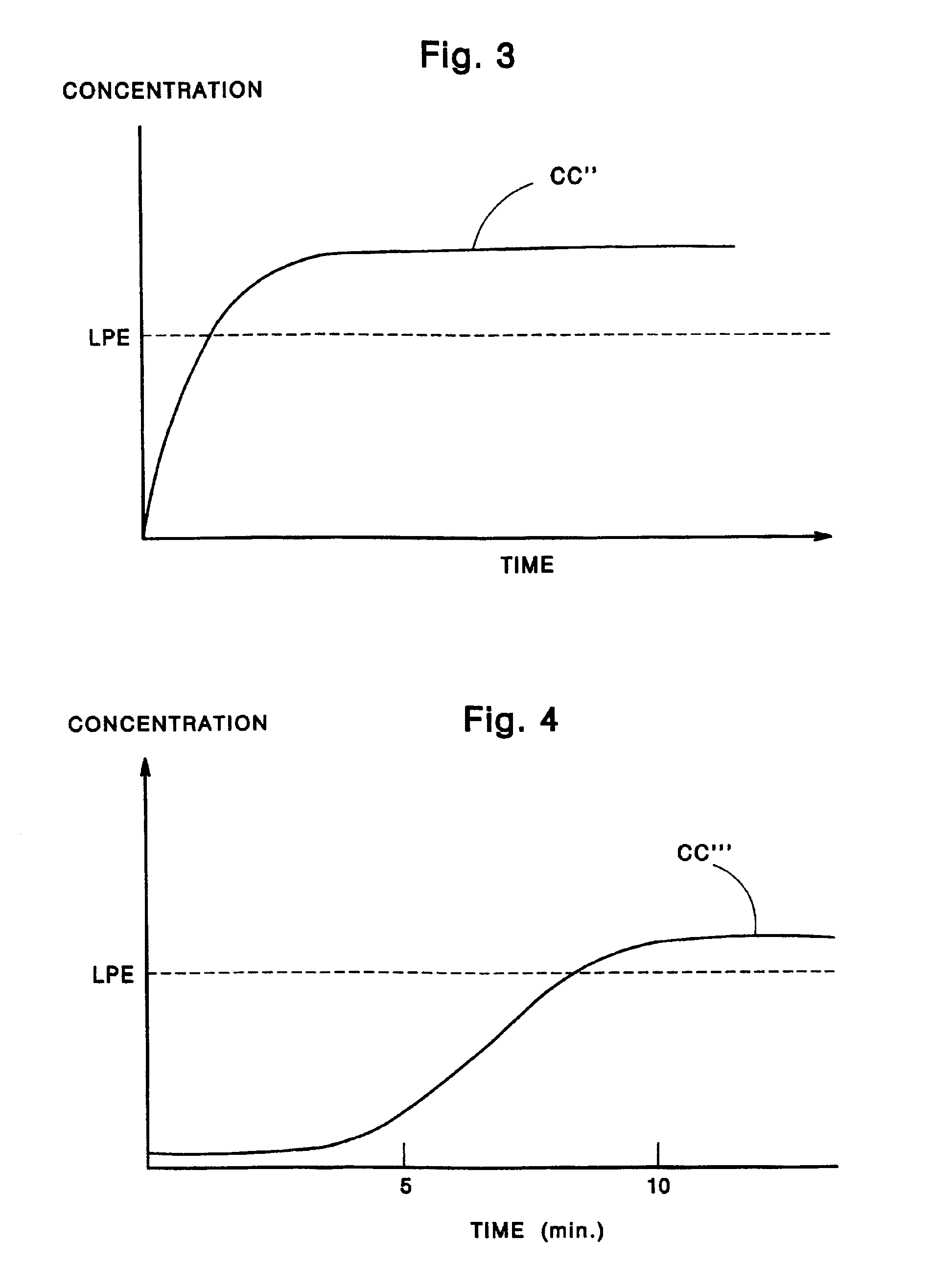
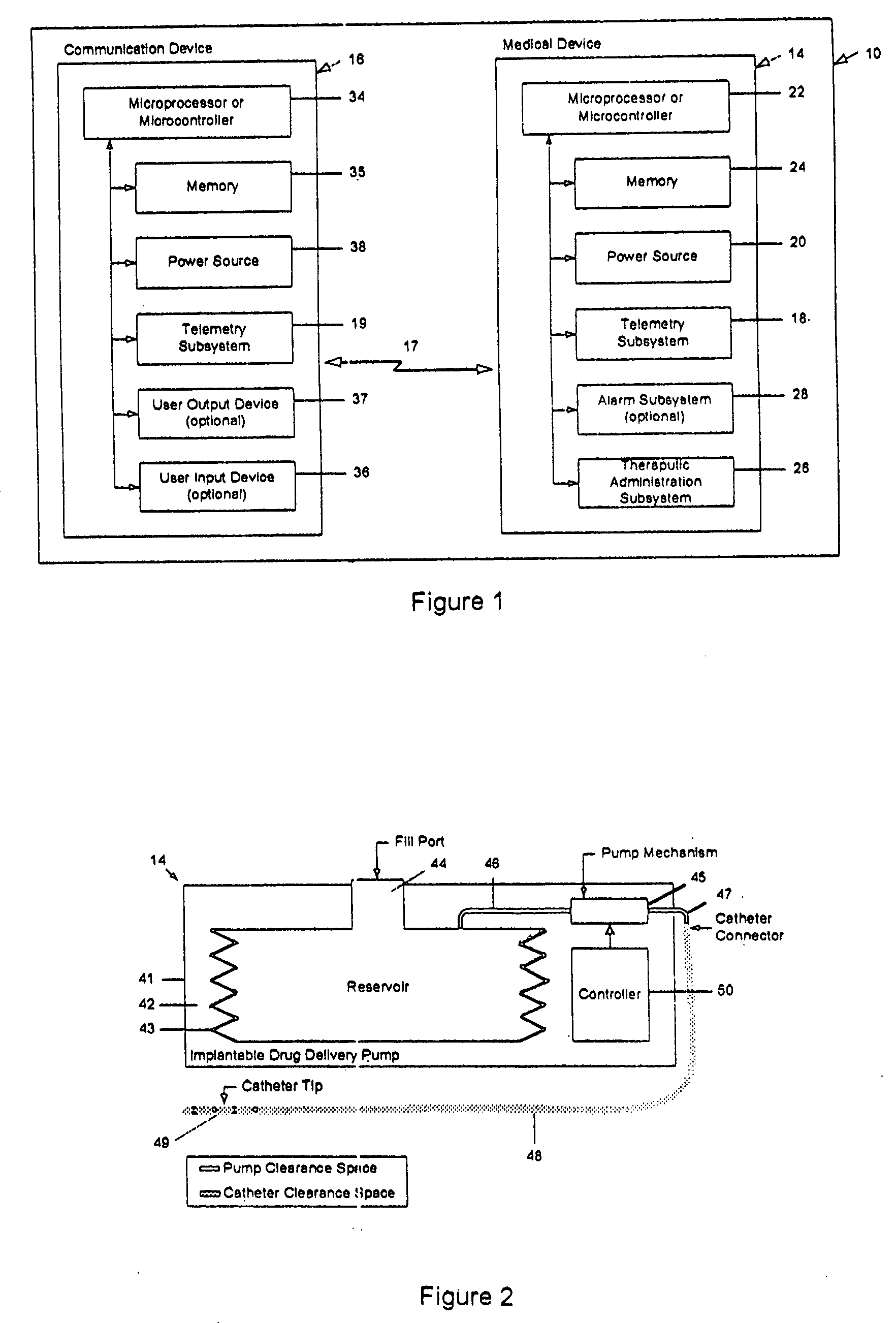
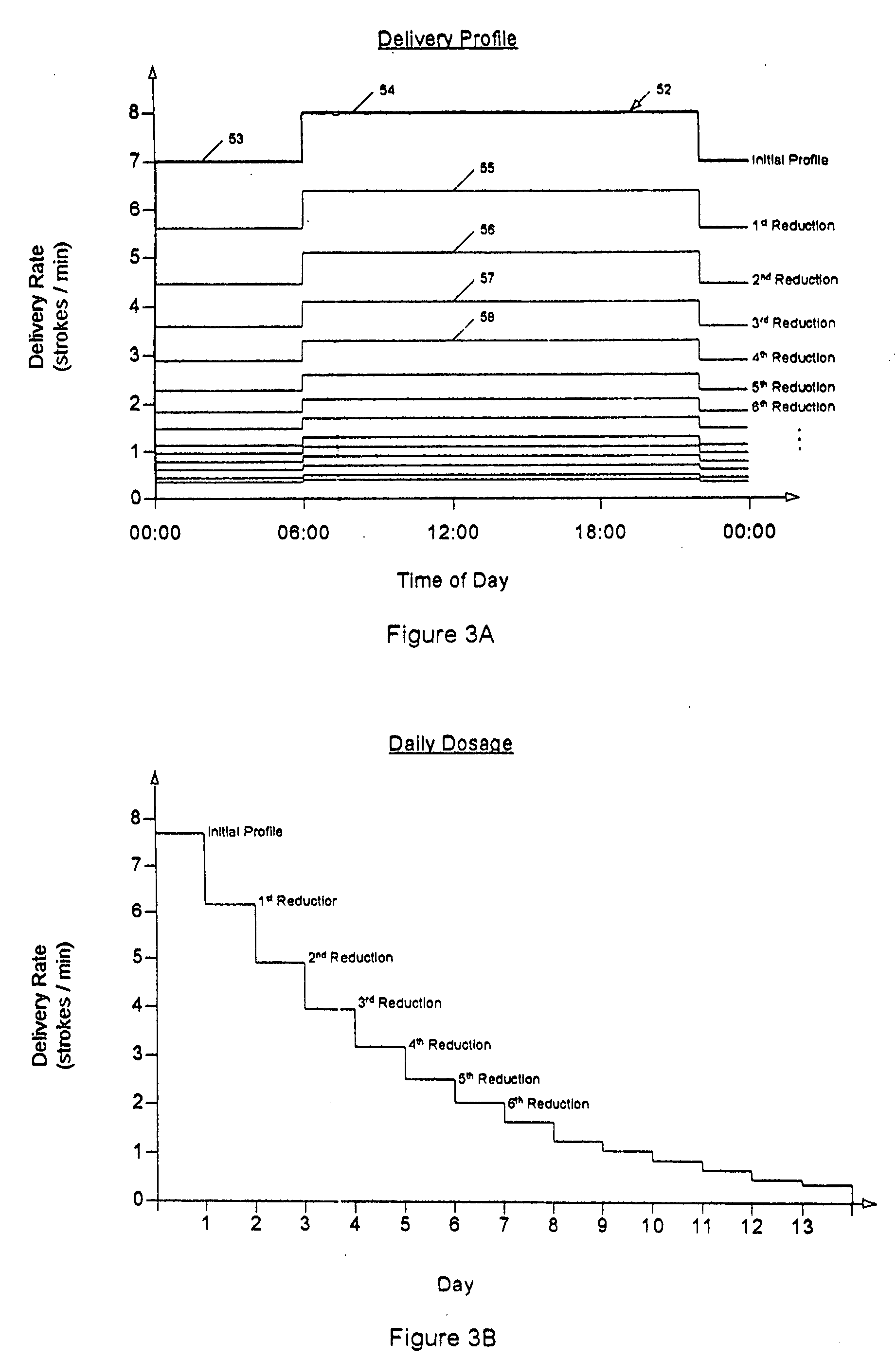
A drug delivery device which includes a fluid drug reservoir, a catheter, a controllable fluid transfer device, e.g., a pump mechanism or valve, and a drug delivery control means. The drug delivery control means comprises a controller, e.g., a microprocessor or microcontroller which is operable to automatically reduce the rate of drug delivery over a certain reduction interval (e.g., multiple days) from an initial dosage value to a final dosage value.
Owner:INFUSION SYST
Method of treating stroke with thrombolytic agent
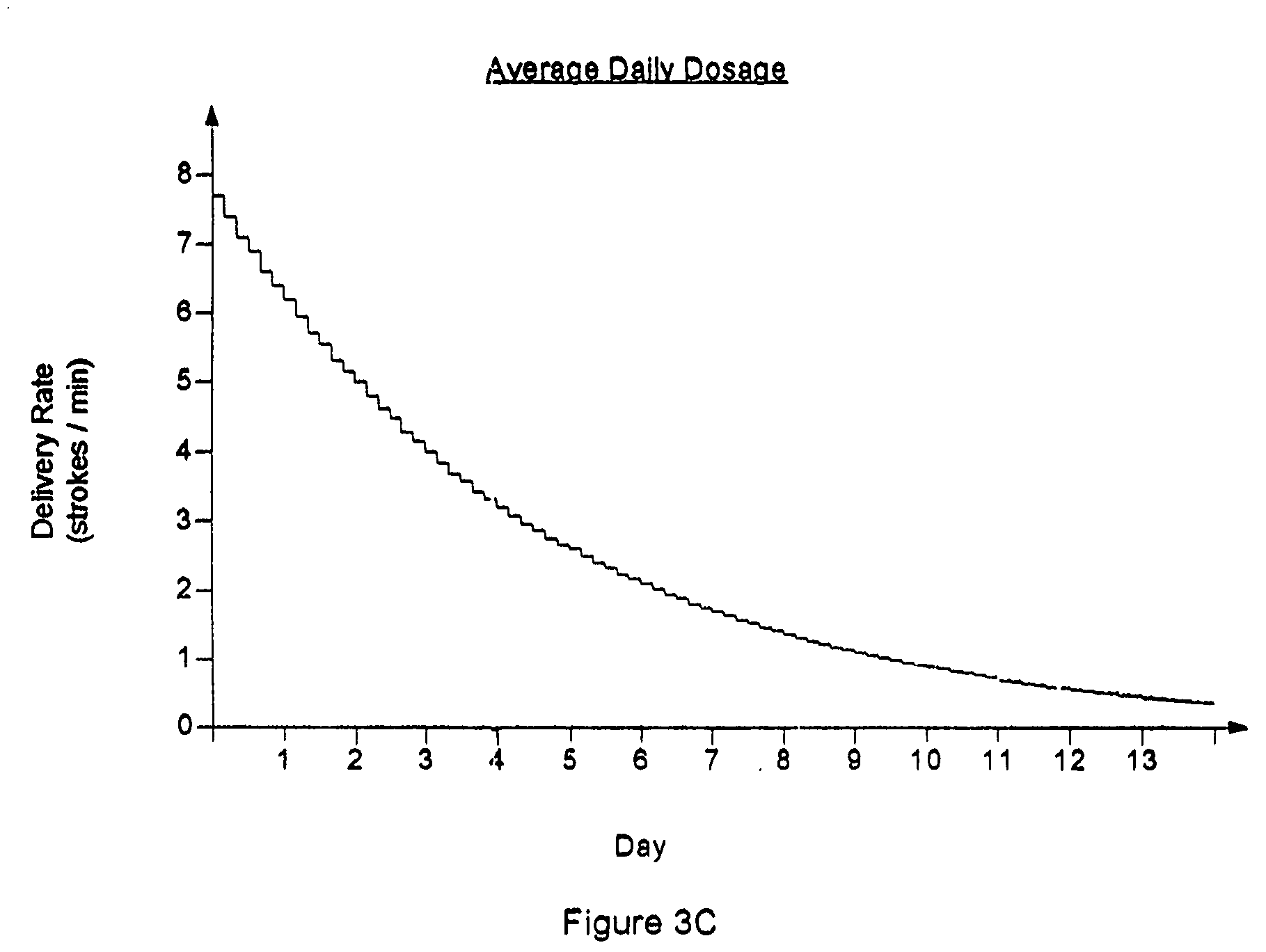
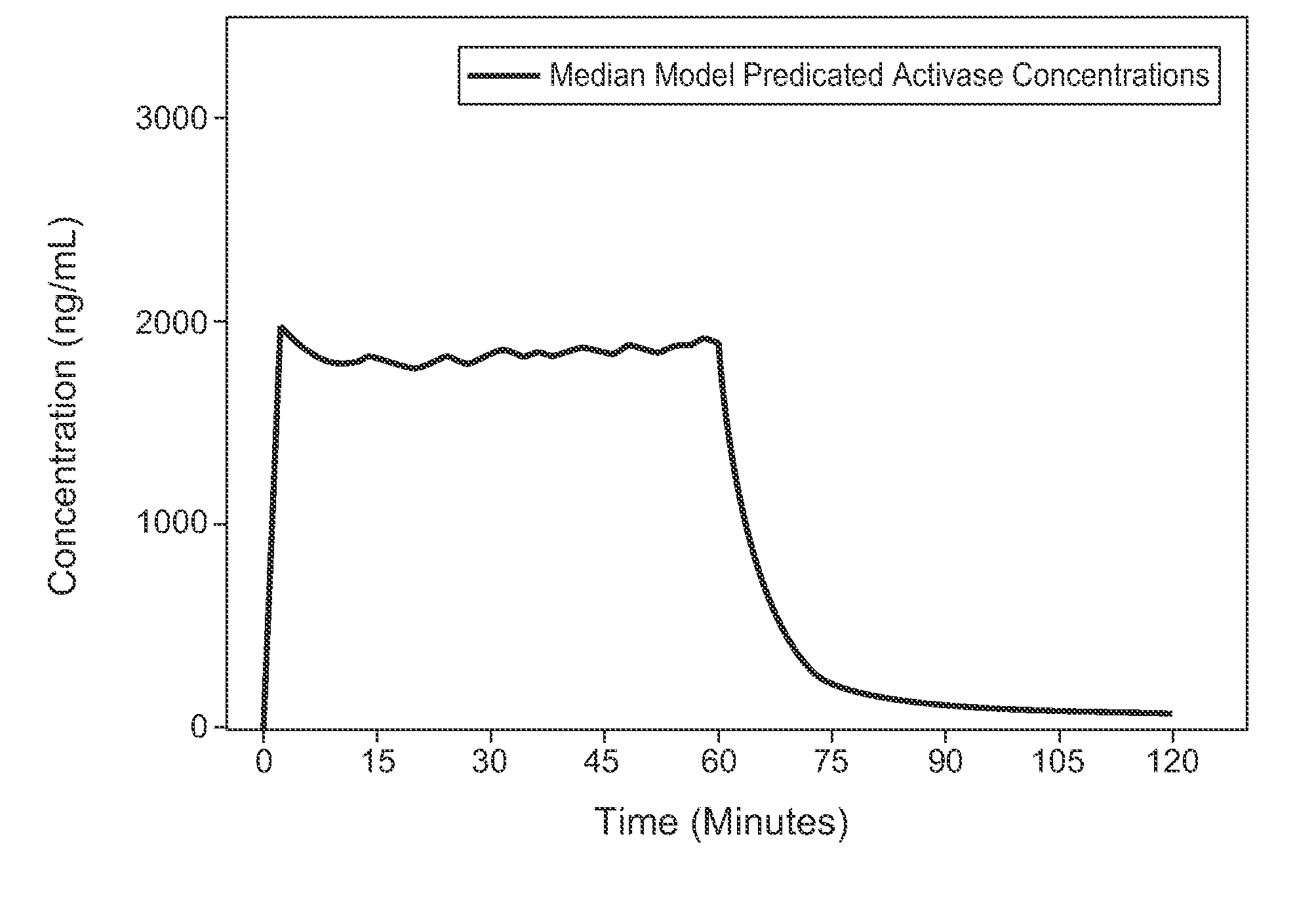
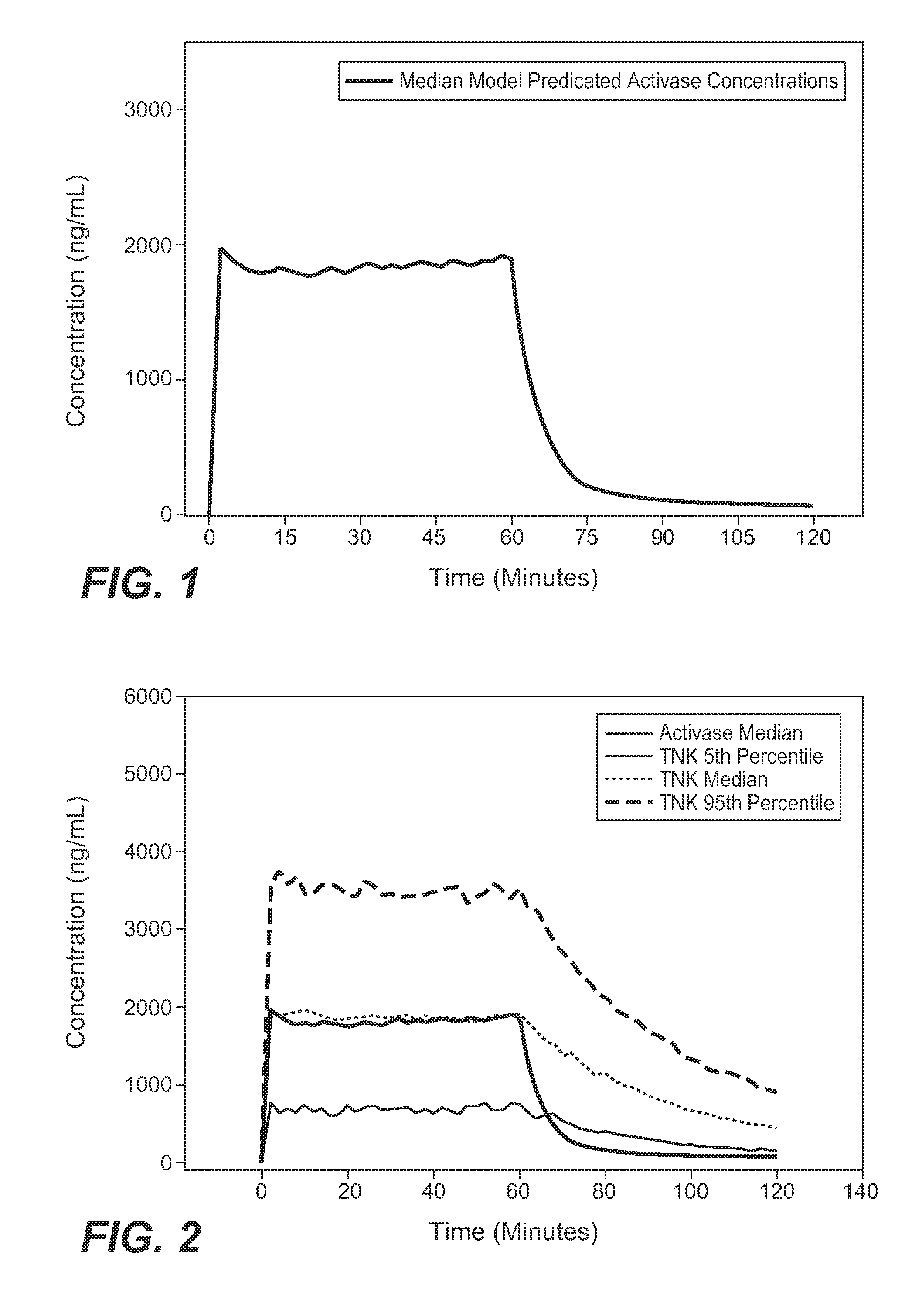
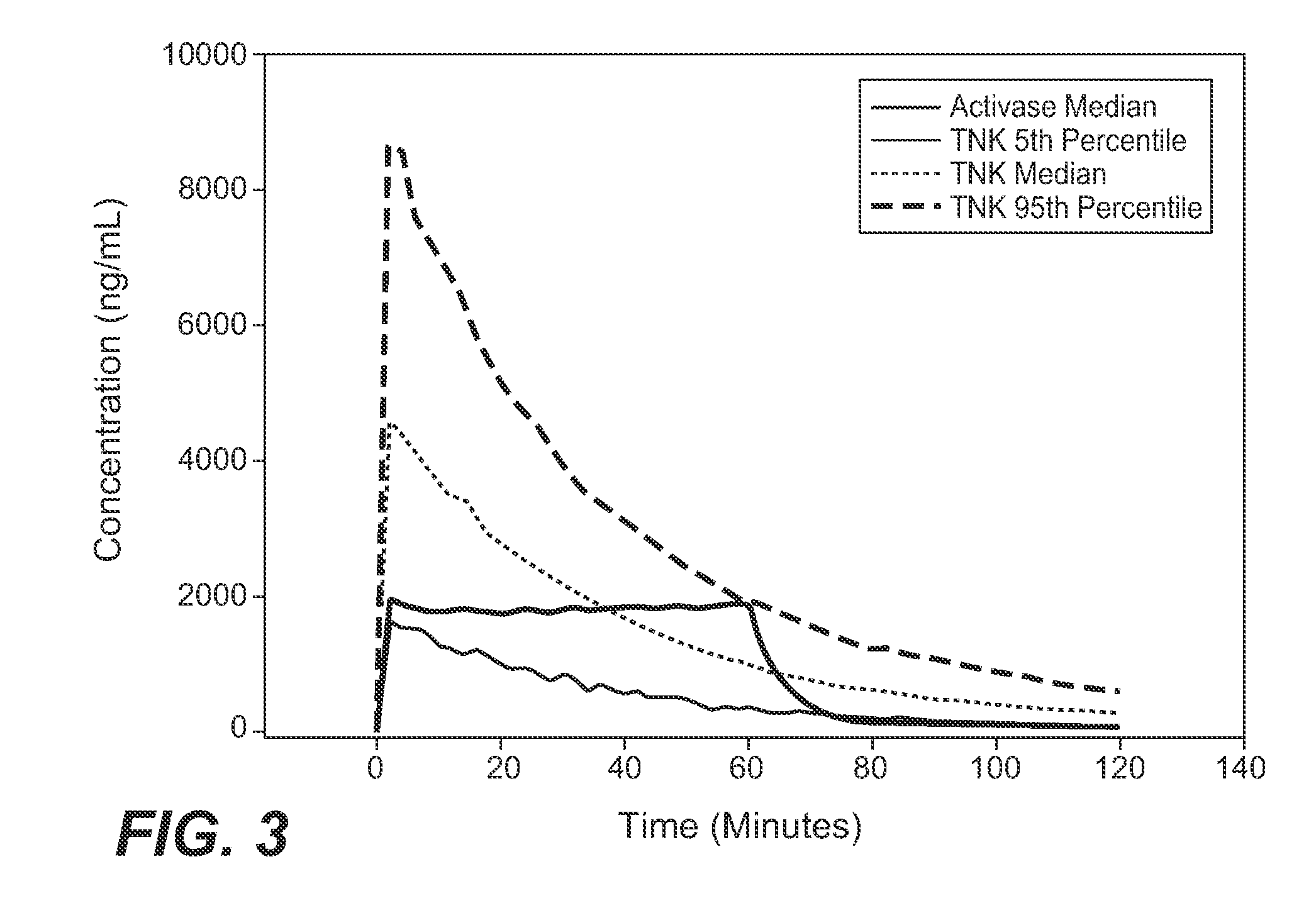
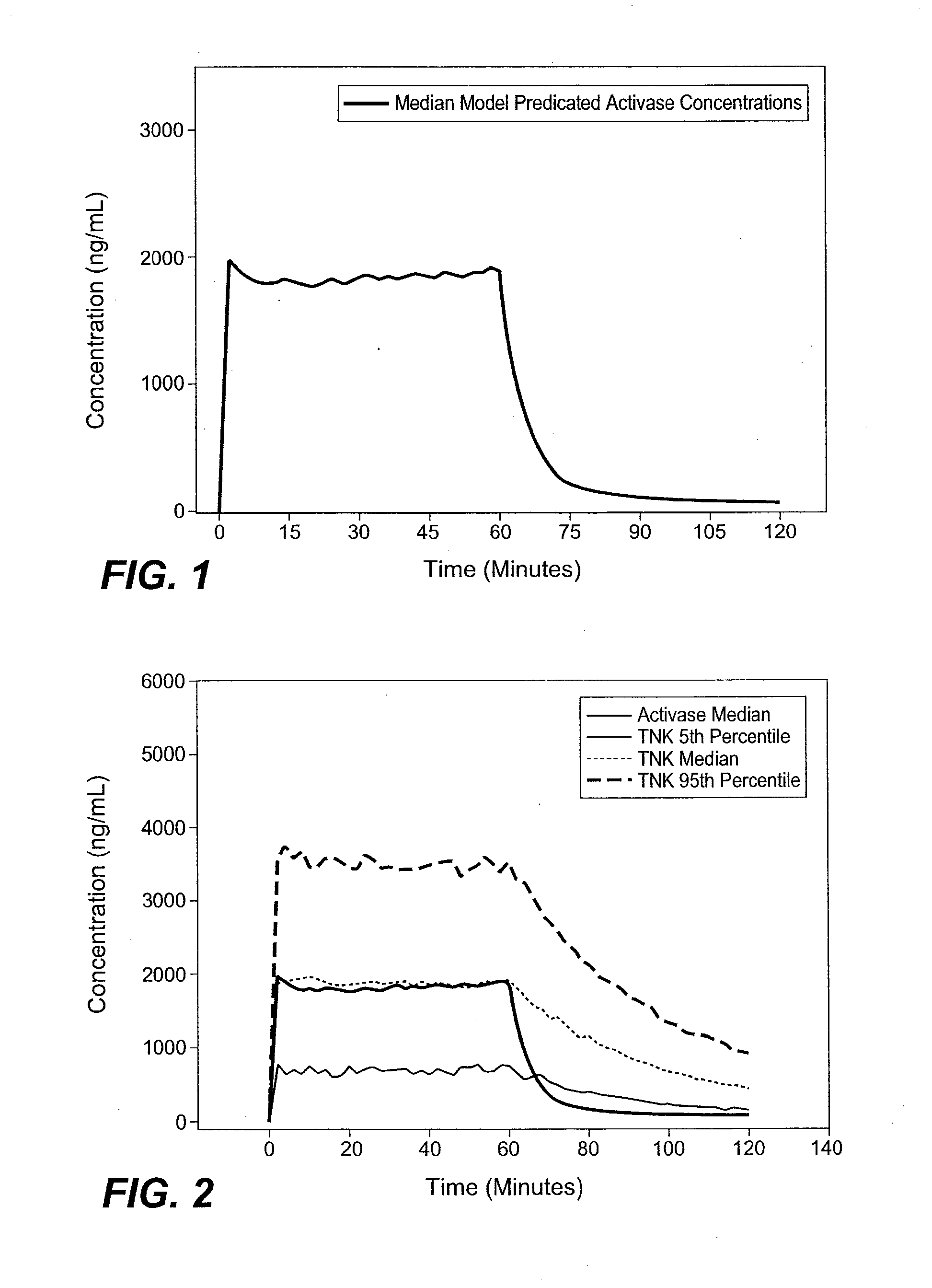
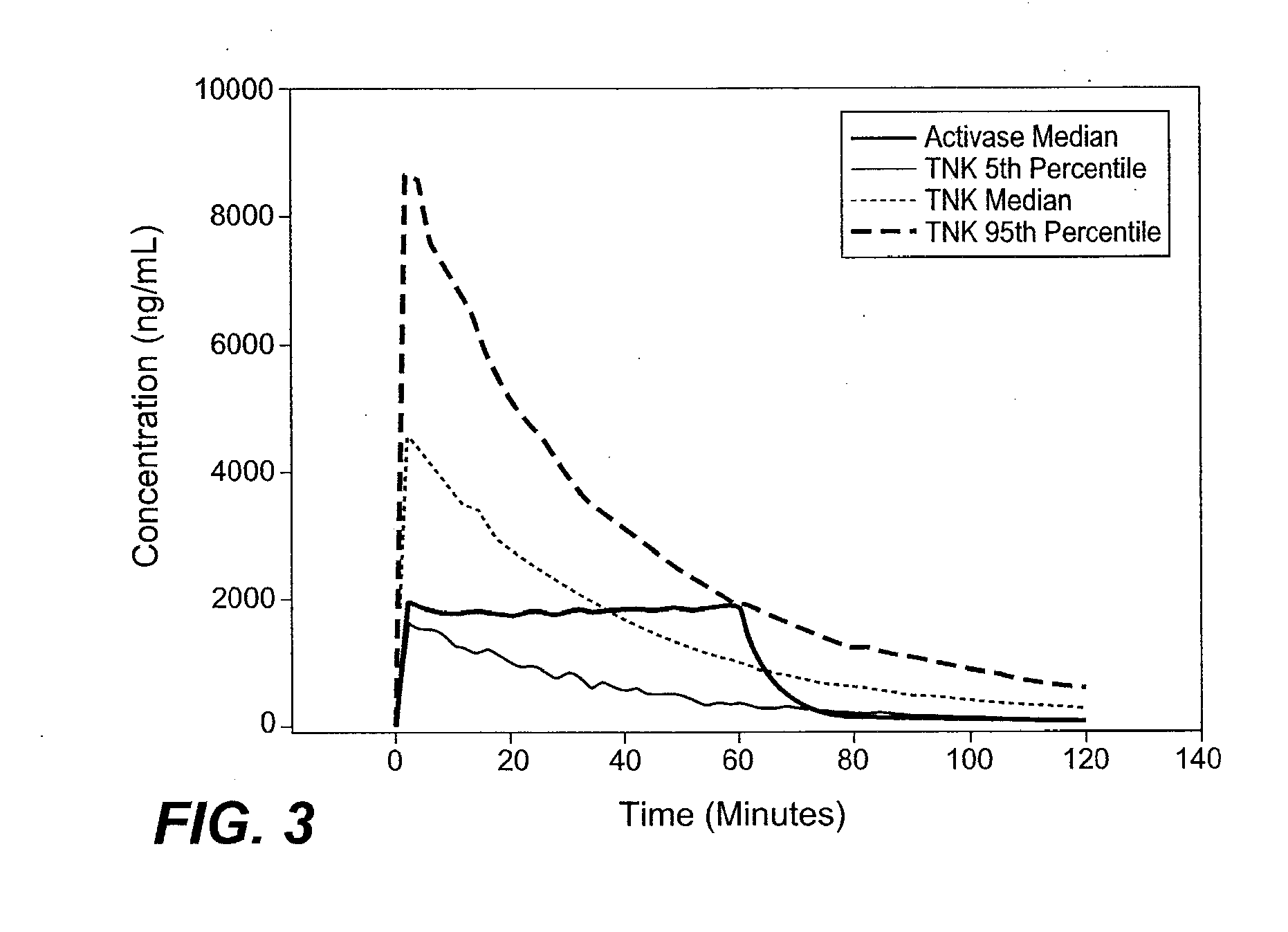
A method for treating acute ischemic stroke in a human comprises administering tenecteplase to the human in a total dose of about 0.05 to 0.5 mg / kg, given as (a) an initial bolus dose of about 0.015 to 0.15 mg / kg, followed by infusion of an amount equaling the total dose minus the initial dose over a period of about 50-90 minutes, or (b) a bolus. Also described are kits for carrying out this method.
Owner:GENENTECH INC
Method of treating stroke with thrombolytic agent
A method for treating acute ischemic stroke in a human comprises administering tenecteplase to the human in a total dose of about 0.05 to 0.5 mg / kg, given as (a) an initial bolus dose of about 0.015 to 0.15 mg / kg, followed by infusion of an amount equaling the total dose minus the initial dose over a period of about 50-90 minutes, or (b) a bolus. Also described are kits for carrying out this method.
Owner:GENENTECH INC
Extremely low duty-cycle activation of the cholinergic anti-inflammatory pathway to treat chronic inflammation
ActiveUS9211410B2Reduce power consumptionMaintain curative effectImplantable neurostimulatorsArtificial respirationInitial doseCholinergic anti-inflammatory pathway
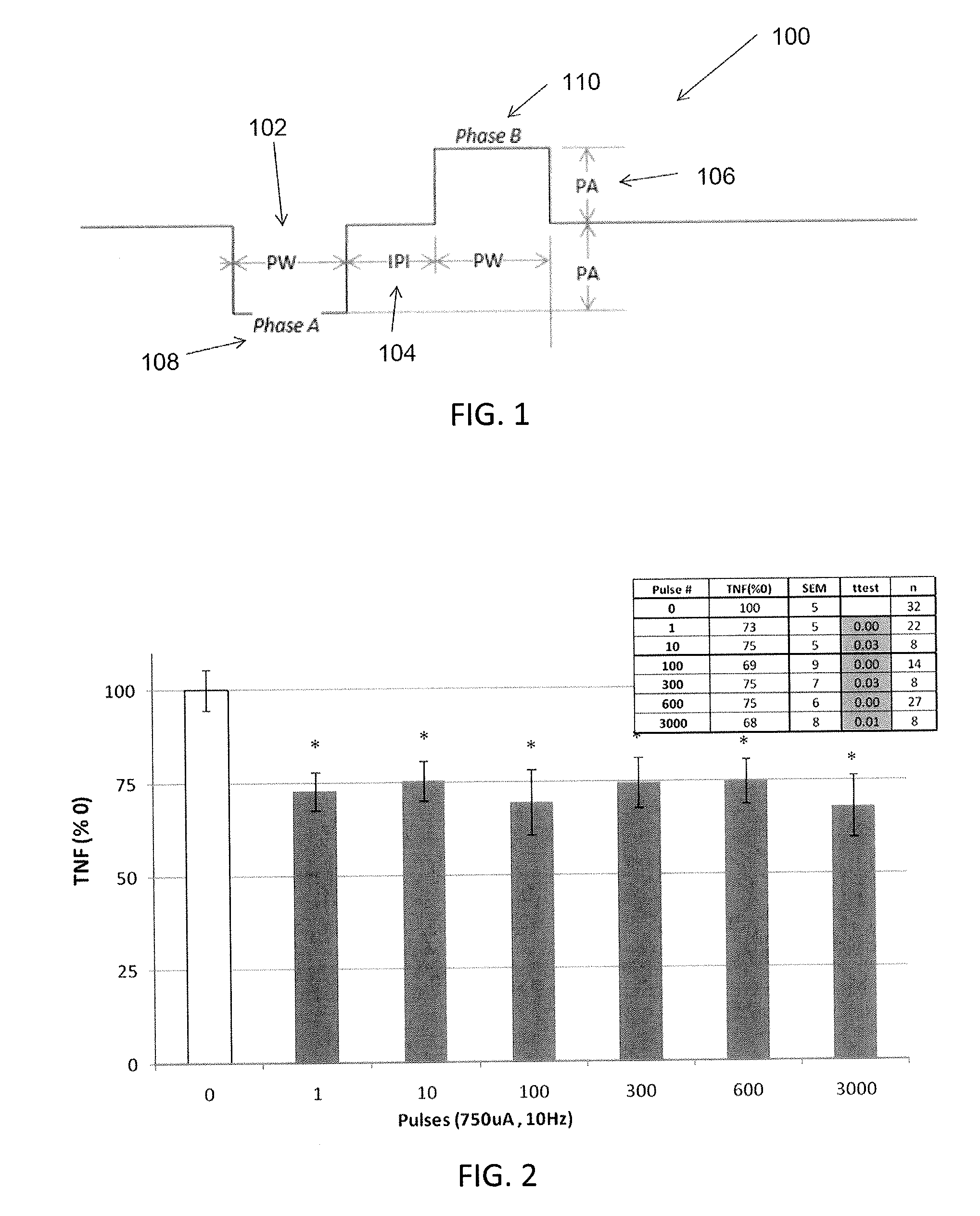
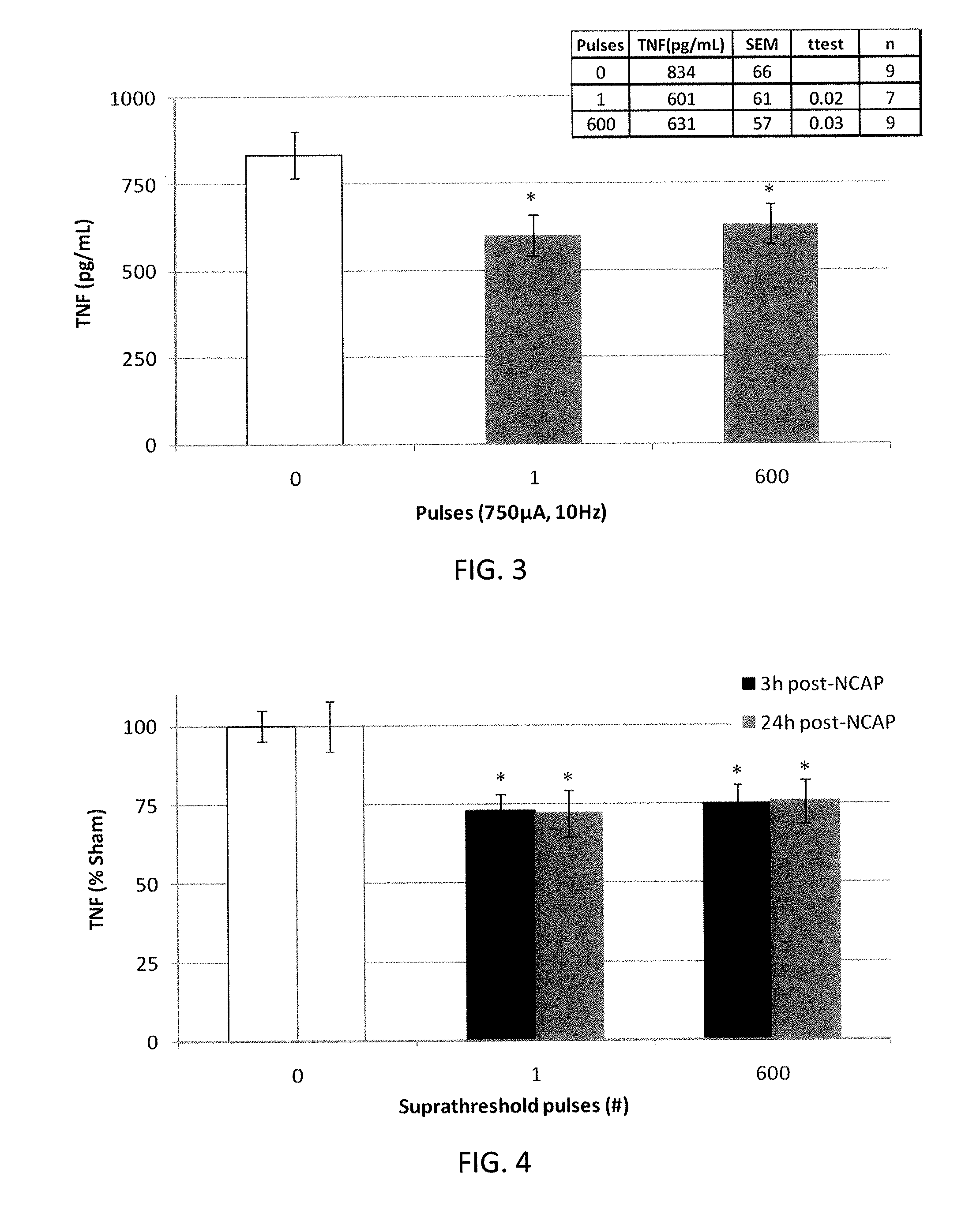
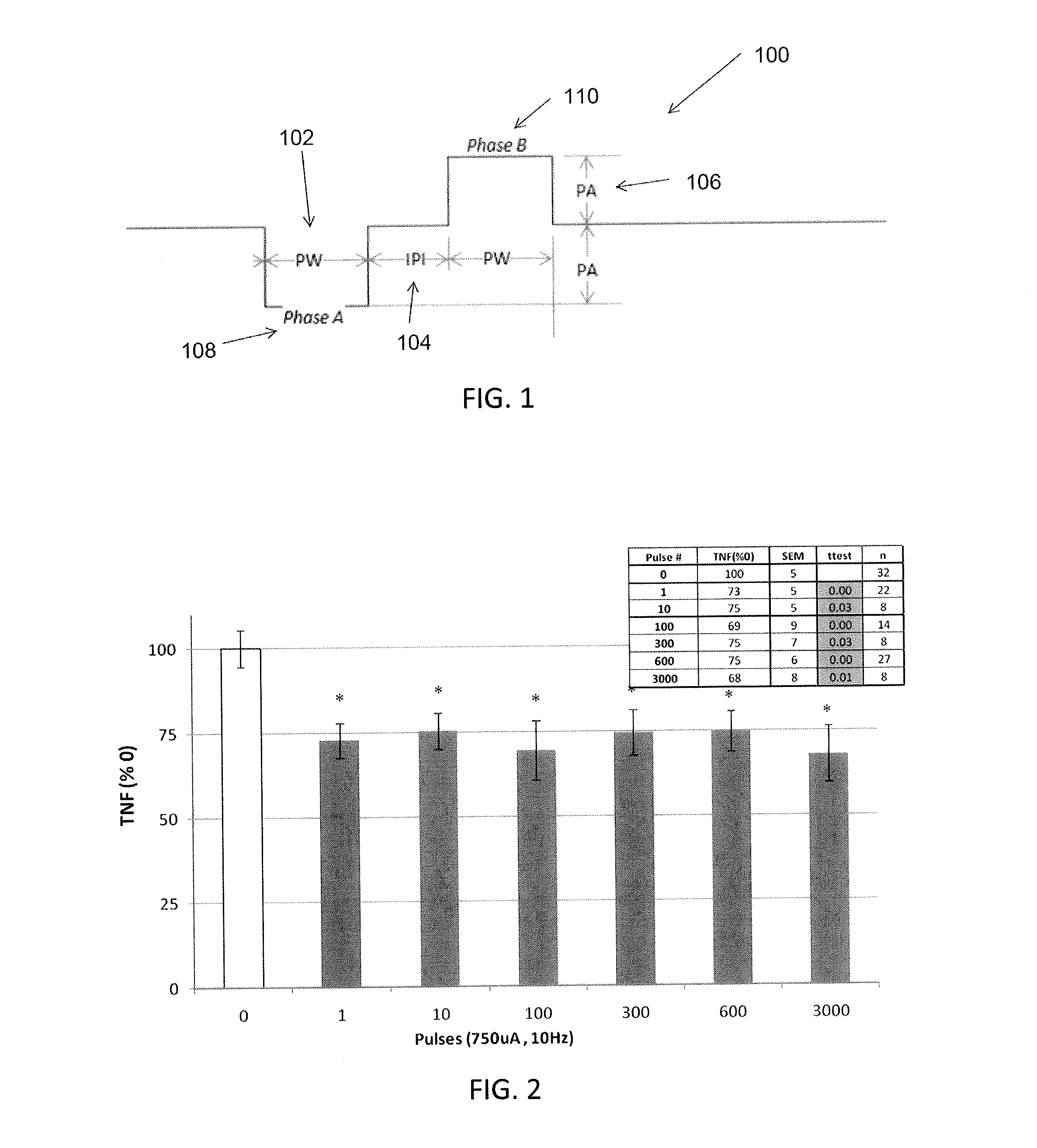
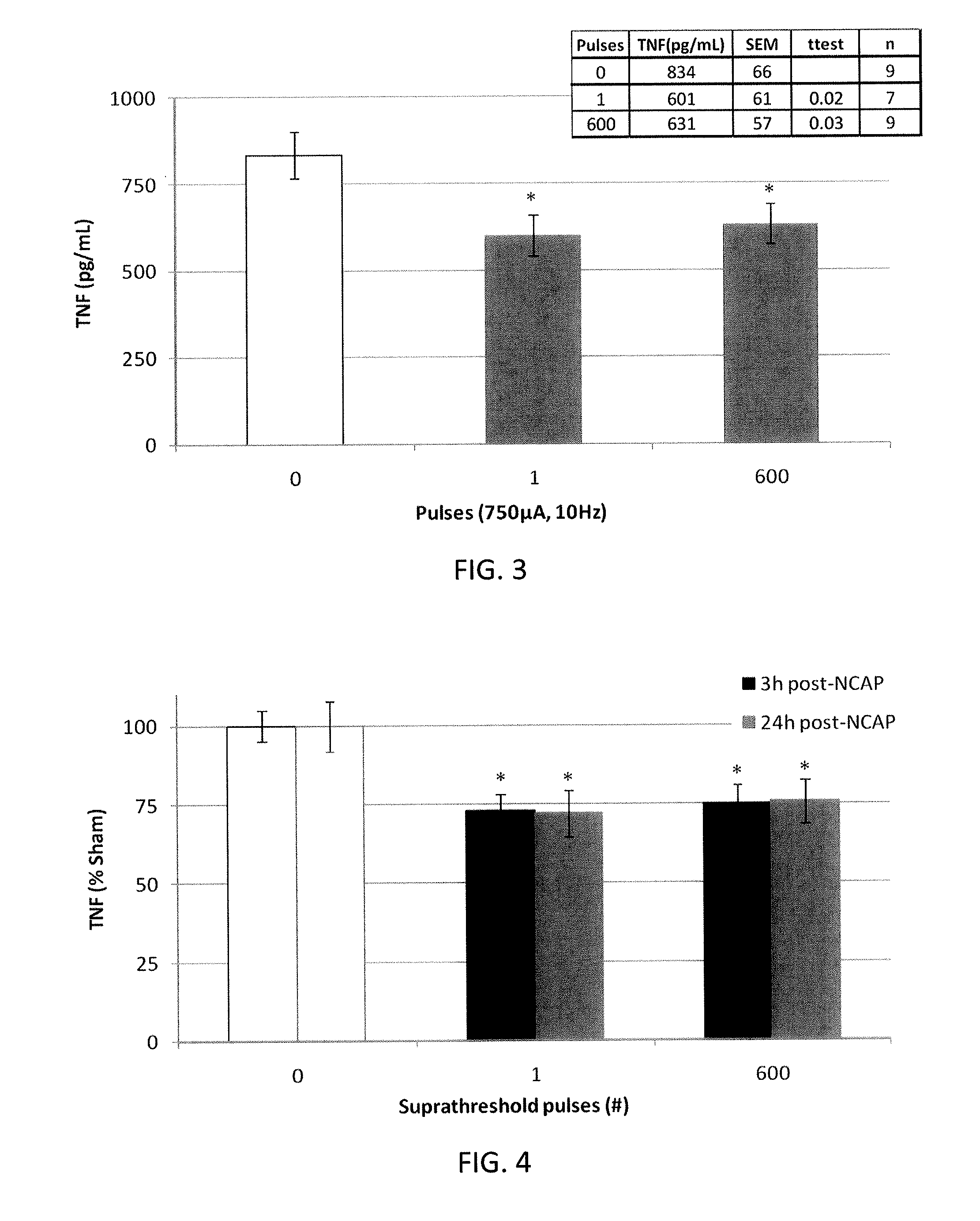
Described herein are systems and methods for applying extremely low duty-cycle stimulation sufficient to treat chronic inflammation with progressively longer delays (off periods) from an initial stimulation. In particular, described herein are supra-threshold pulses of electrical stimulation sufficient to result in a long-lasting (e.g., >48 hours) inhibition of pro-inflammatory cytokines and / or effects of chronic inflammation; the delay between initial doses (which may be single-pulse doses) may be extended for subsequent doses, potentially dramatically enhancing battery and device longevity.
Owner:SETPOINT MEDICAL CORP
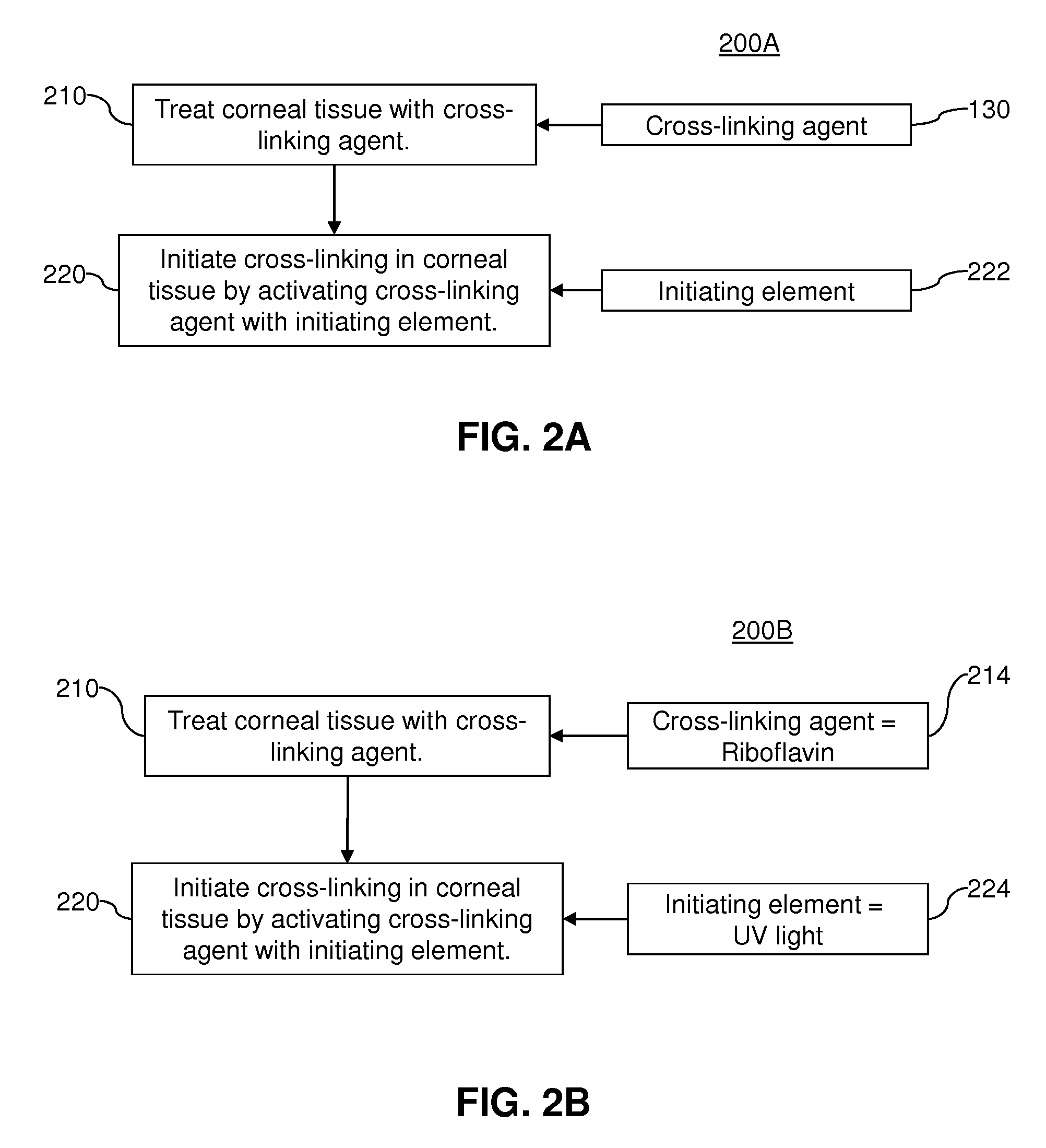
Sterilizing application of cross-linking agent
InactiveUS20120283621A1Reduce the risk of infectionPromote absorptionLaser surgeryElectrotherapyPhotosensitizerInitial dose
A method for treating an eye includes applying, to an outer protective layer of an area of an eye, one or more initial doses of a cross-linking agent that acts as a photosensitizer. The method also includes delivering, from a light source, one or more initial doses of ultraviolet light to the area of the eye. The cross-linking agent increases absorption of the ultraviolet light by the area of the eye, and the absorption of the ultraviolet light sterilizes the area of the eye before the outer protective layer is penetrated. Additionally, the method includes penetrating the outer protective layer of the eye to provide access to an area below the outer protective layer. Moreover, the method includes applying a treatment to the eye via the access provided by cutting the outer protective layer. In some embodiments, the cross-linking agent is Riboflavin, and the treatment is LASIK surgery.
Owner:AVEDRO
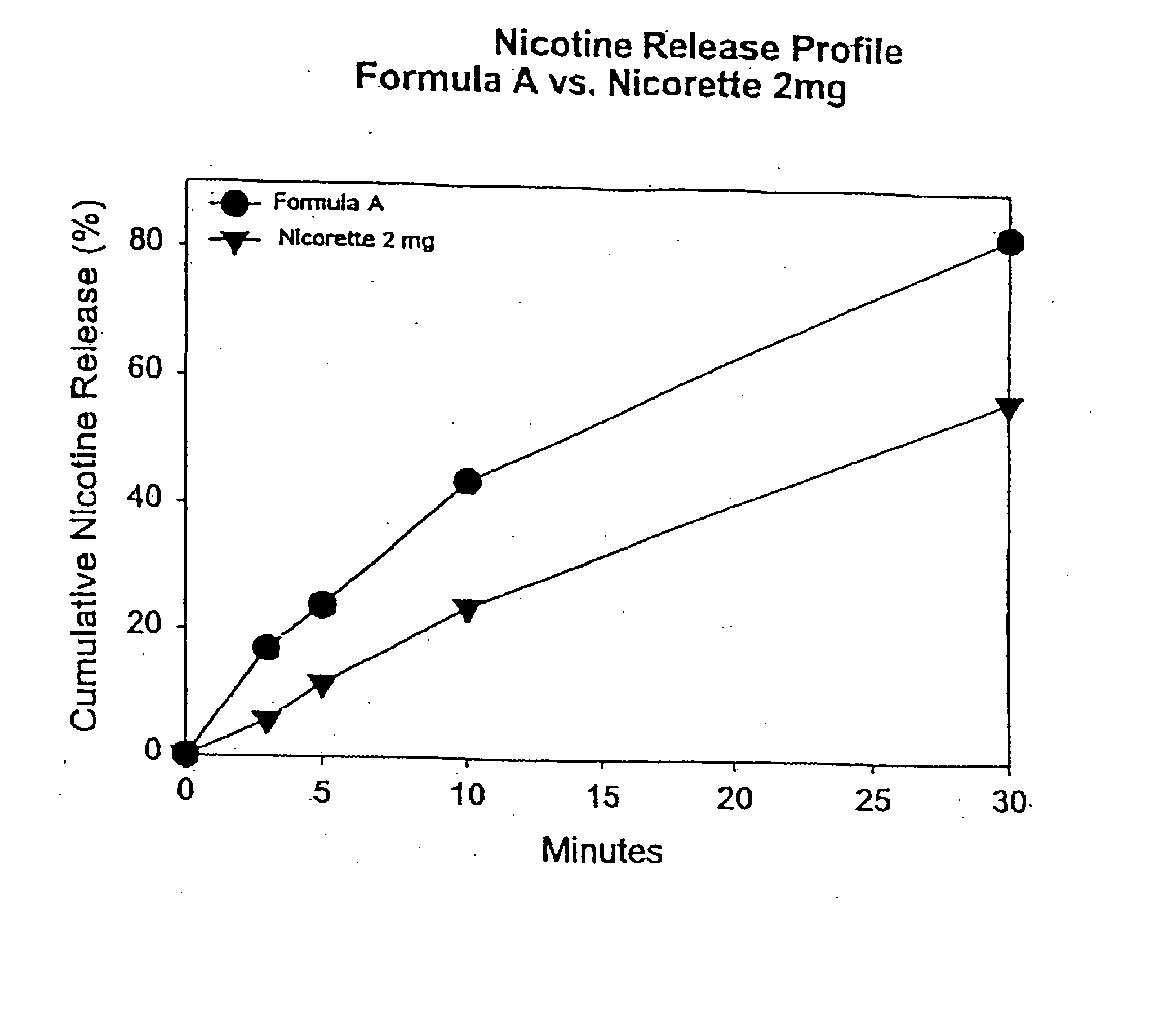
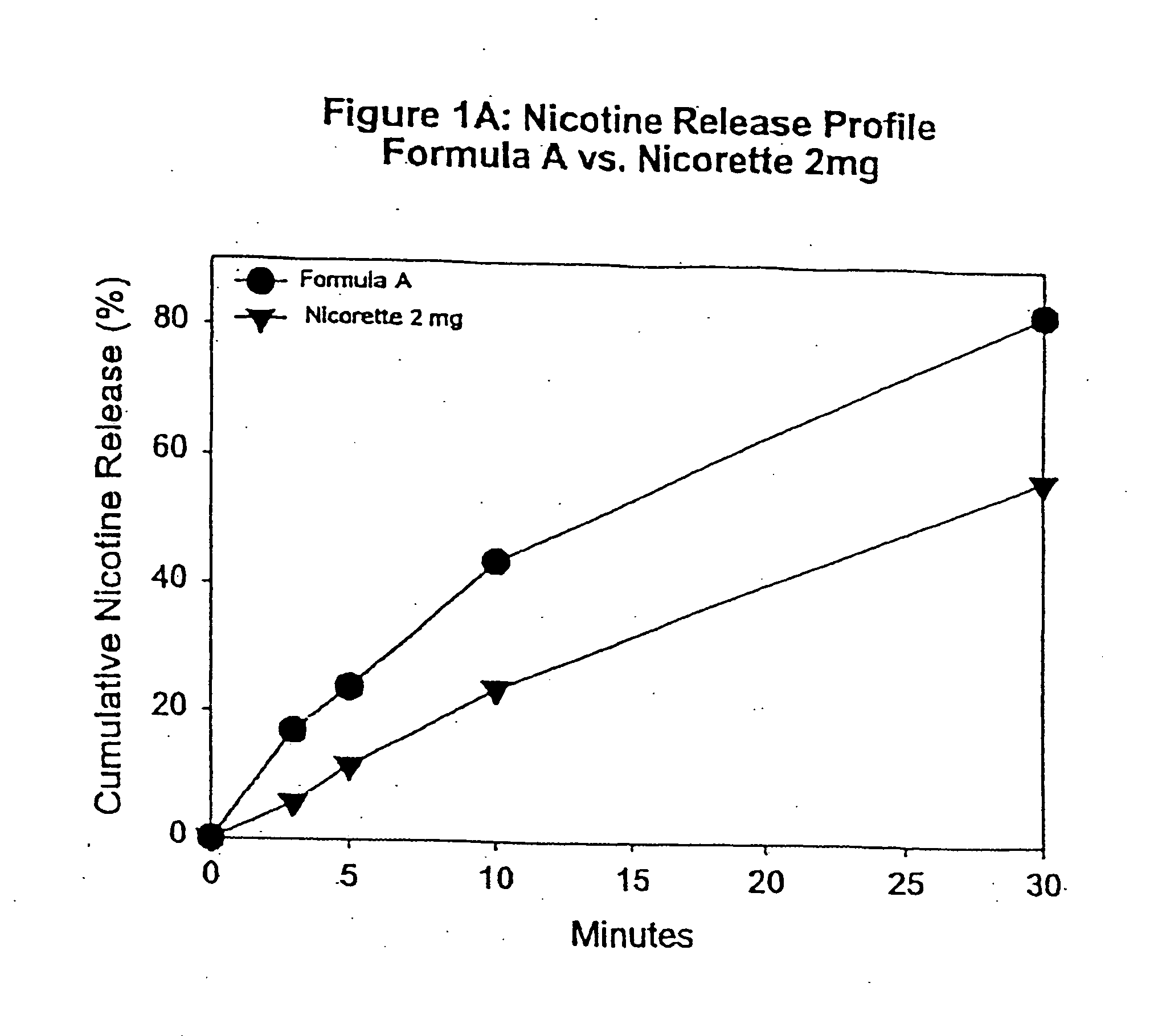
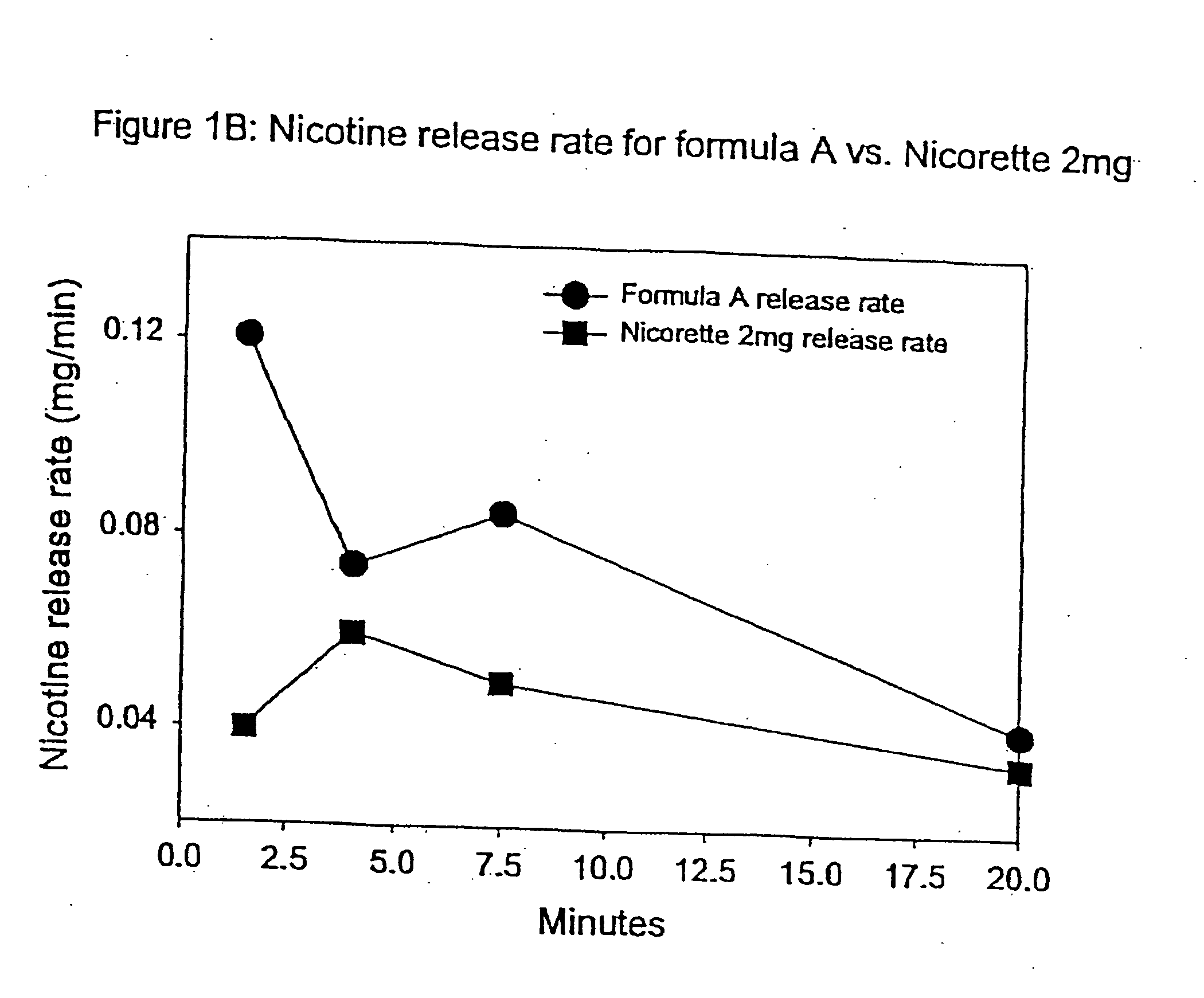
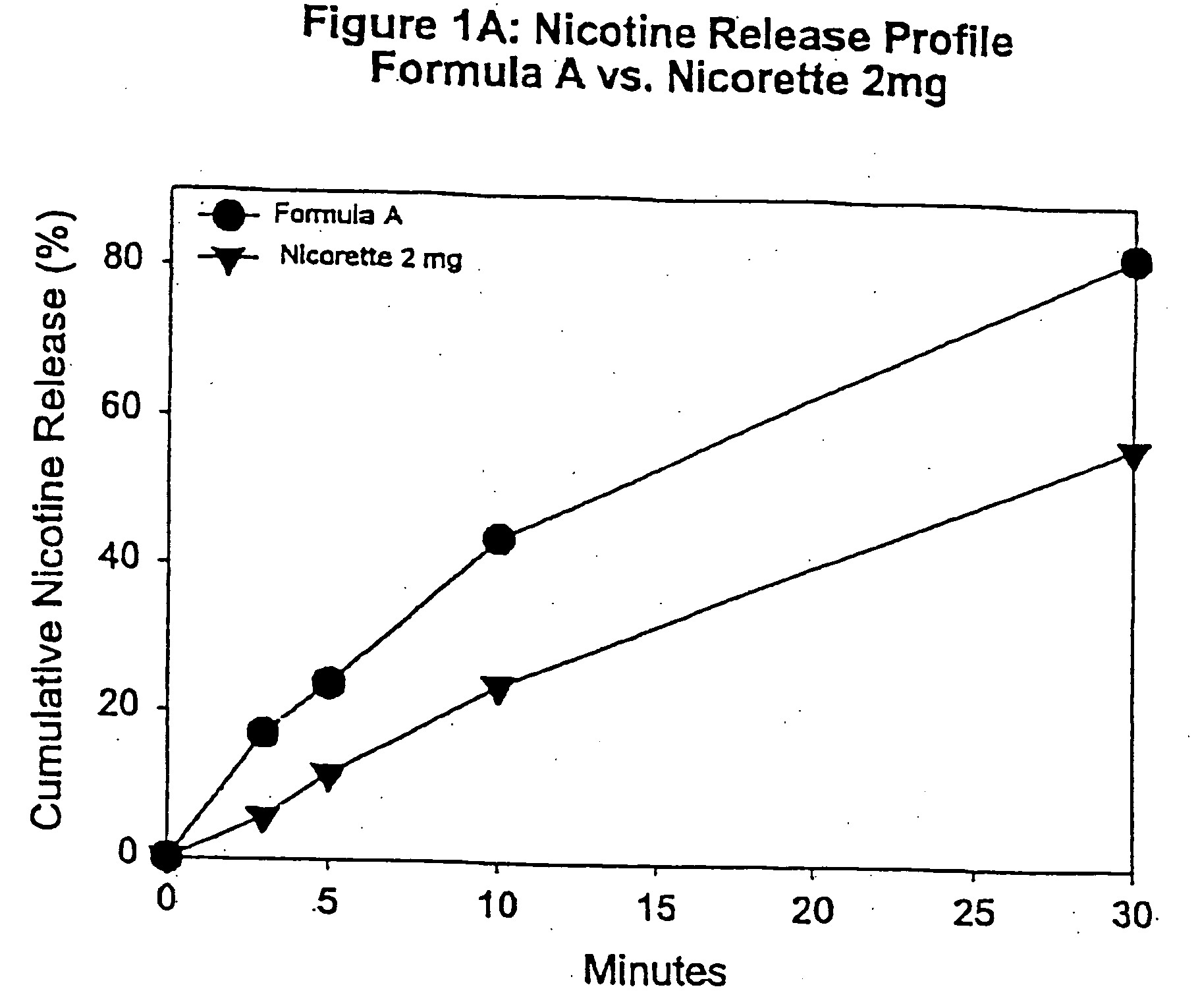
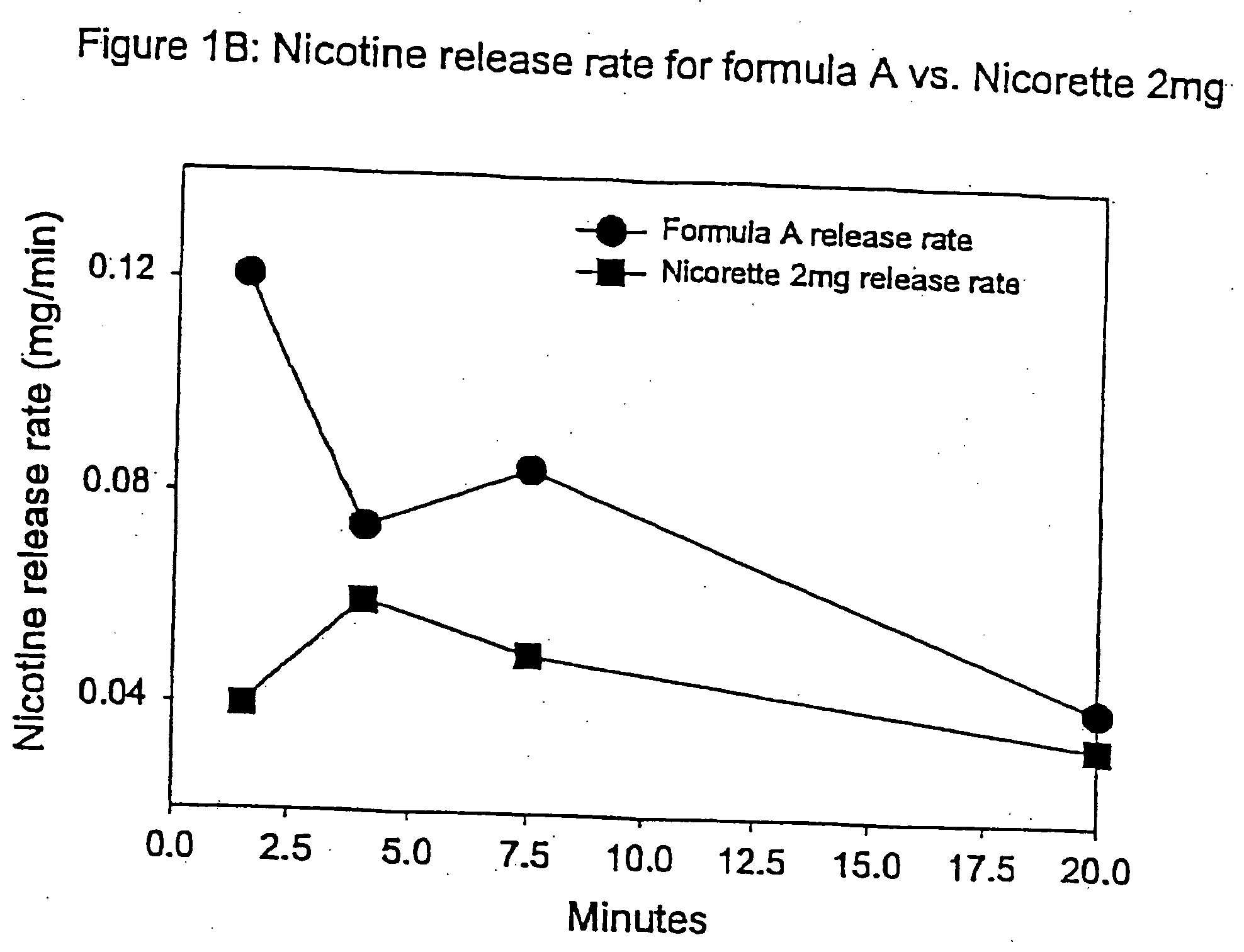
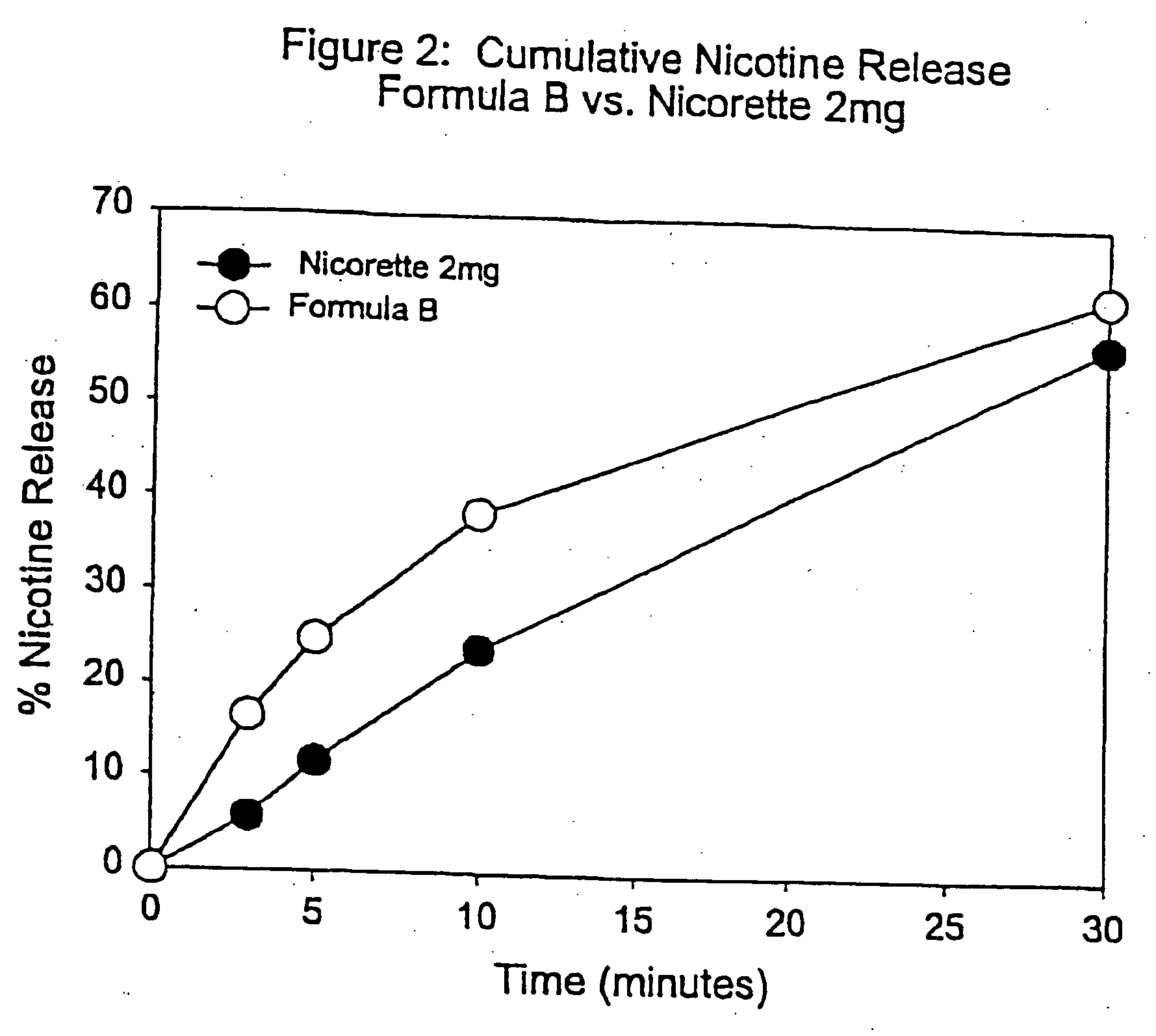
Medicated chewing gum delivery system for nicotine
InactiveUS20070014887A1Quick reliefQuick releaseInorganic non-active ingredientsChewing gumMedicated chewing-gumInitial dose
A chewing gum delivery system has nicotine, gum base and a buffer system with an improved release rate for the nicotine. The resulting delivery system advantageously provides a convenient, reliable, practical, and relatively painless system for delivering an active. The delivery system is capable of delivering initial and second doses of a craving reduction active or other actives (e.g., nicotine), the combination of which rapidly reduces cravings, or provides some other pharmacological effect, and provides the pharmacological effect or protection from such cravings over a prolonged period of time beyond the initial dose. Notably, the delivery system is capable of rapidly achieving a pharmacologically effective concentration of the active (e.g., nicotine) in the bloodstream (e.g., within 5 minutes, or more desirably within 3 minutes, or in some cases, within 1-2 minutes), and is also capable of keeping the concentration of the active in the bloodstream at or near the pharmacologically effective concentration for at least 20 minutes after chewing of the delivery system begins, or more desirably about 30 minutes to about 50 minutes after chewing begins.
Owner:JSR NTI
Application of initial doses of LHRH analogues and maintenance doses of LHRH antagonists for the treatment of hormone-dependent cancers and corresponding pharmaceutical kits
ActiveUS20080032935A1Negative hormone withdrawal symptomsPeptide/protein ingredientsLuteinising hormone-releasing hormoneGynecologyInitial dose
LHRH analogues and LHRH antagonists for use in the treatment or prophylaxis of hormone-dependent cancers, in particular prostate cancer, prostate carcinoma and / or advanced prostate carcinoma, by administering an initial dose of an LHRH analogue over a first period sufficient to effect hormonal castration, then administering a maintenance dose of an LHRH antagonist over a second period, the dose being insufficient to achieve and / or maintain hormonal castration.
Owner:AETERNA ZENTARIS GMBH
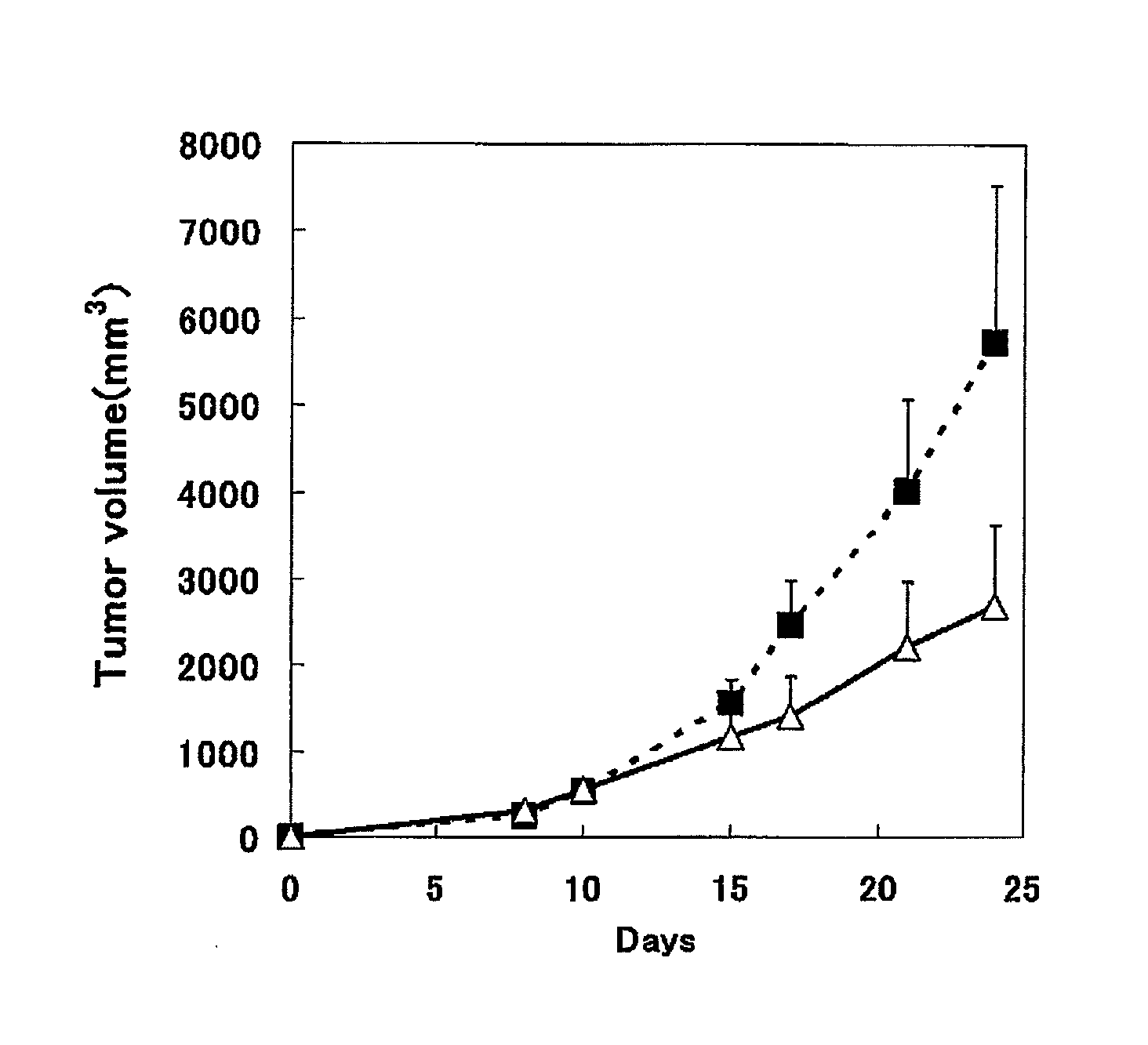
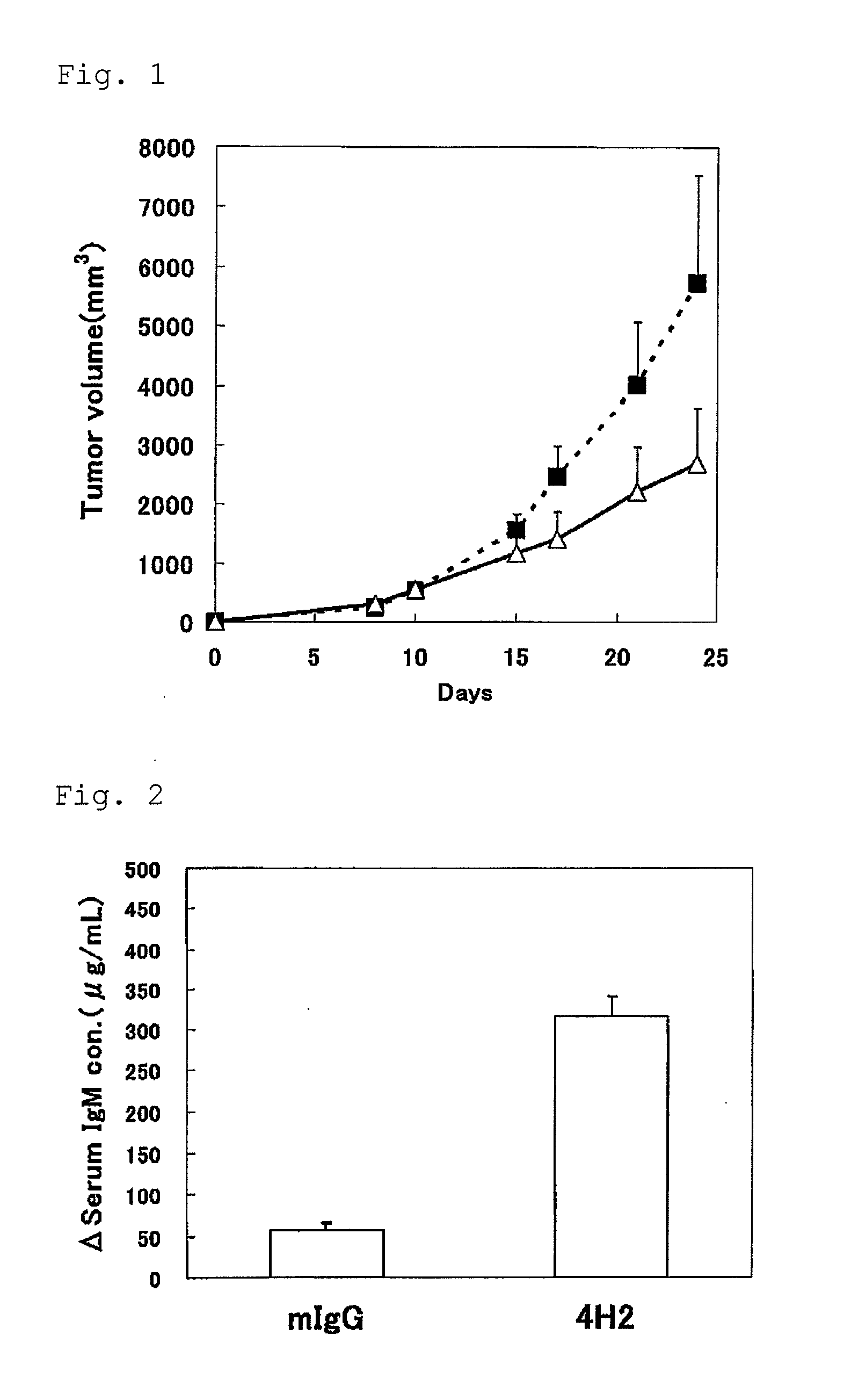
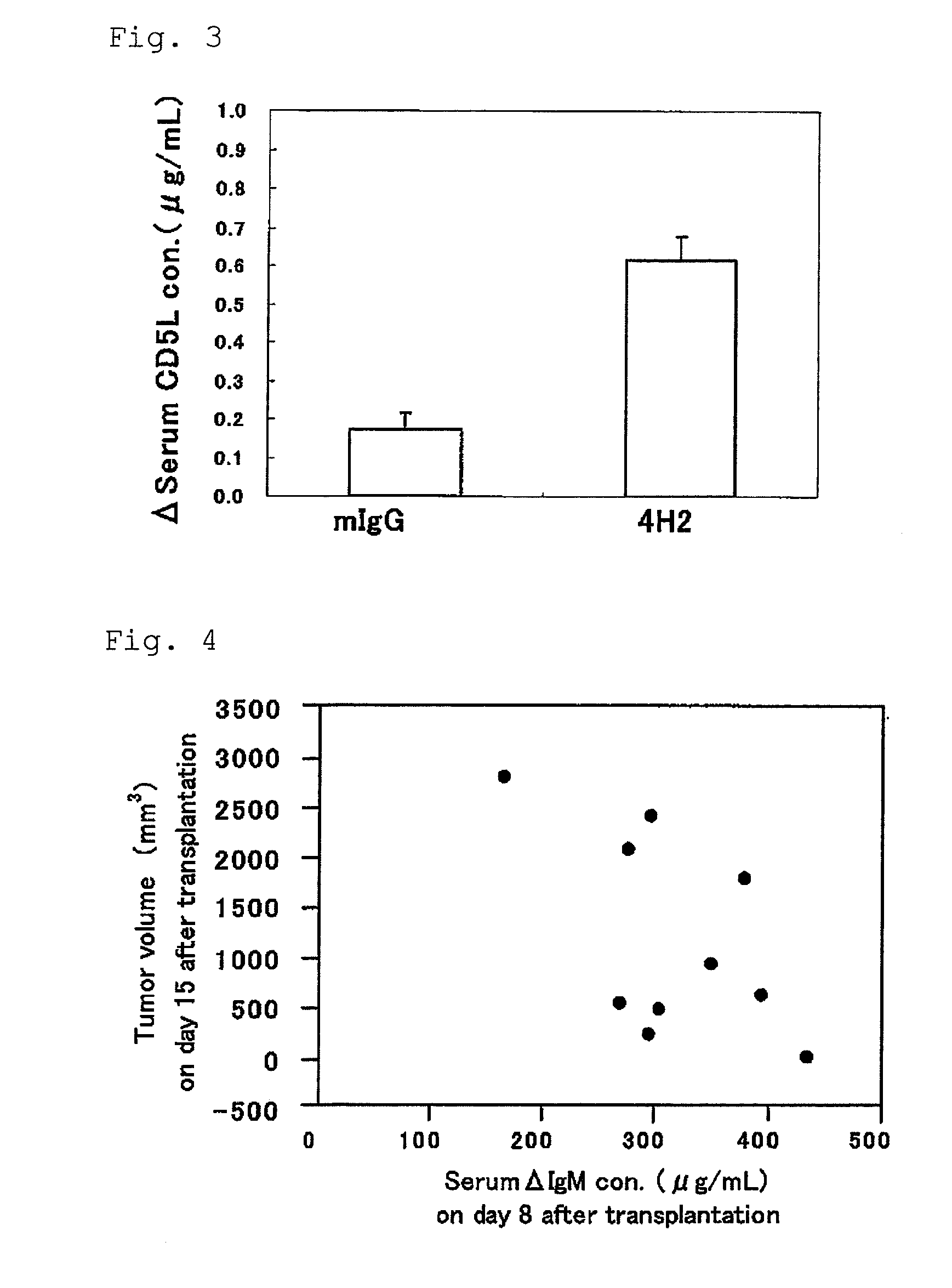
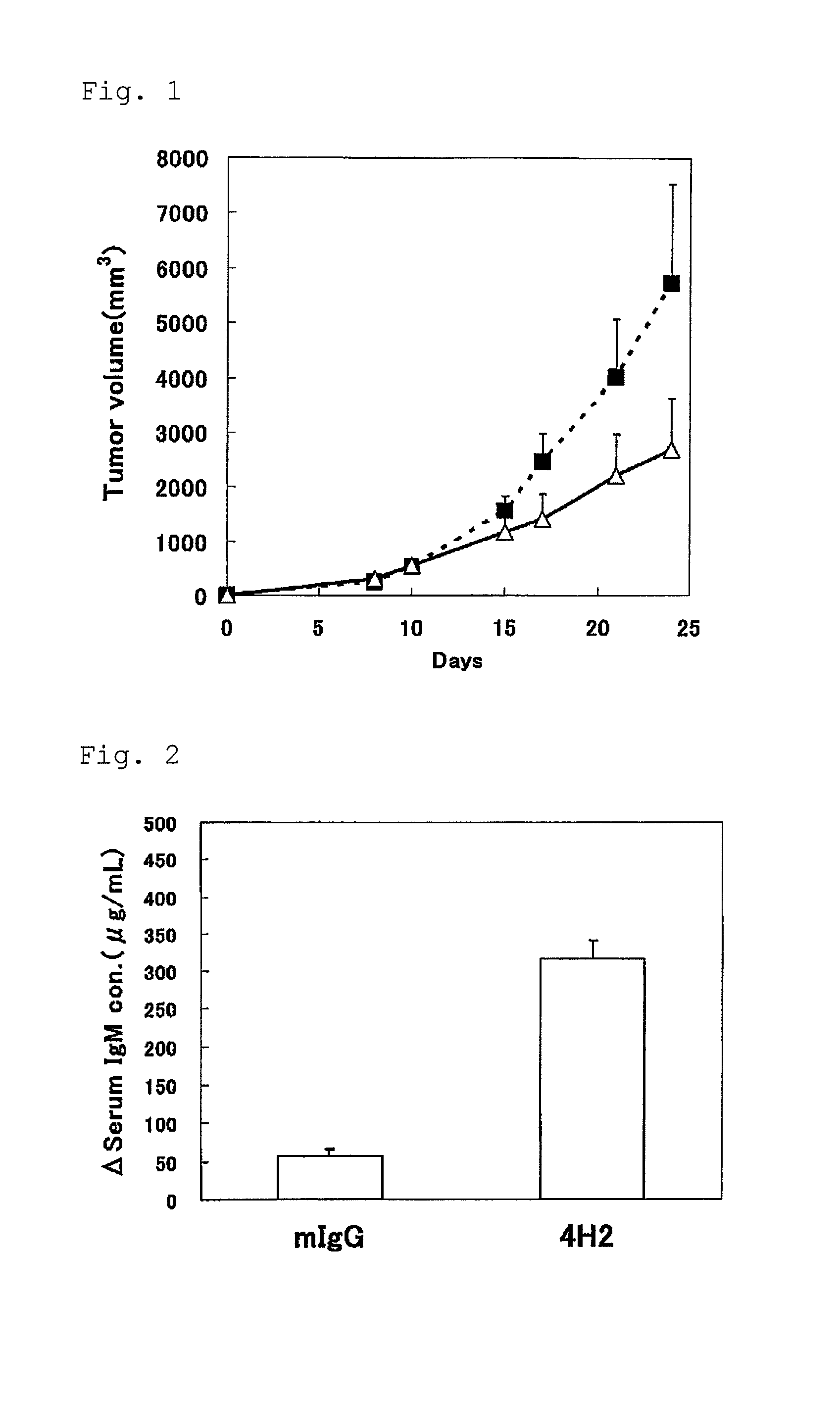
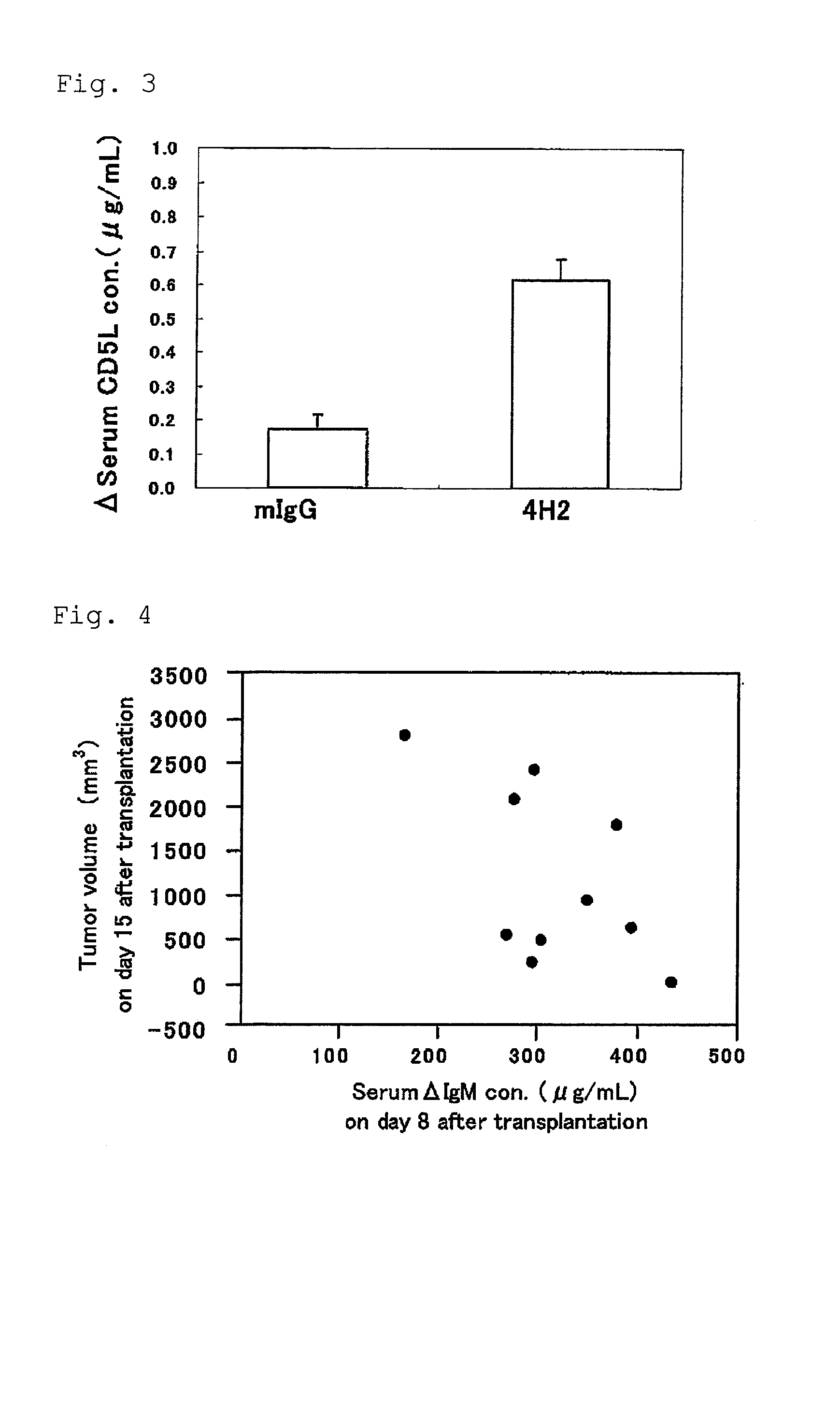
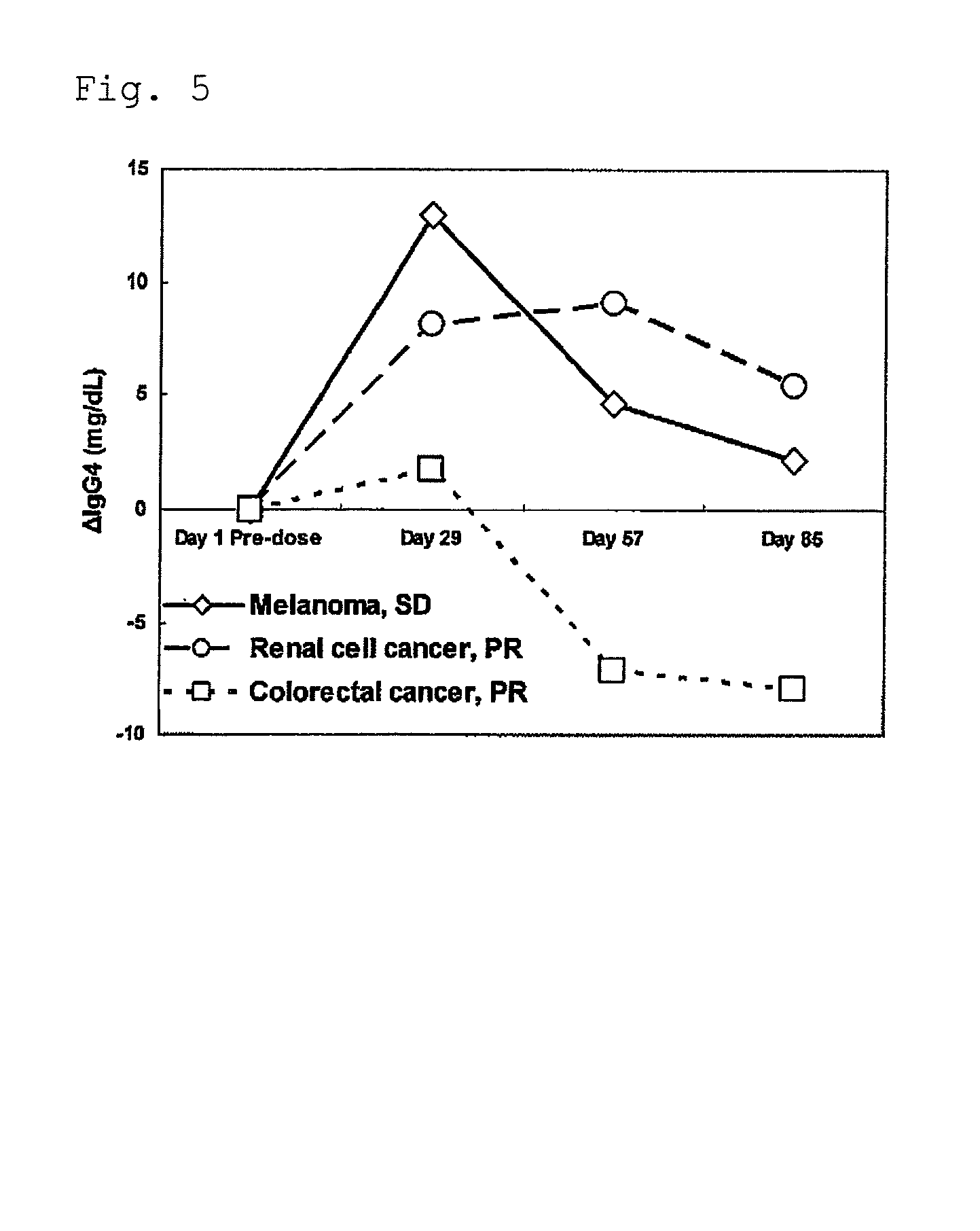
Use of an efficacy marker for optimizing therapeutic efficacy of an Anti-human PD-1 antibody on cancers
InactiveUS20110123550A1Effectively prescribeAntibody ingredientsDisease diagnosisInitial doseTherapeutic effect
A purpose of the present invention is to provide a method capable of more effectively prescribing an anti-human PD-1 antibody for anti-cancer therapy, a method for estimating or optimizing therapeutic efficacy thereof, and further an efficacy marker that can be used in methods thereof. The present invention enables selection of the cancer patient in whom the therapeutic efficacy of the anti-human PD-1 antibody can be expected in future, by measuring the change which is more than a certain level of several kinds of efficacy markers in blood after administering the initial dose or doses of the anti-human PD-1 antibody compared to that prior to administering the initial dose, and provides a new prescription of the anti-human PD-1 antibody for anti-cancer therapy.
Owner:ONO PHARMA CO LTD +1
Use of an efficacy marker for optimizing therapeutic efficacy of an anti-human PD-1 antibody on cancers
A purpose of the present invention is to provide a method capable of more effectively prescribing an anti-human PD-1 antibody for anti-cancer therapy, a method for estimating or optimizing therapeutic efficacy thereof, and further an efficacy marker that can be used in methods thereof. The present invention enables selection of the cancer patient in whom the therapeutic efficacy of the anti-human PD-1 antibody can be expected in future, by measuring the change which is more than a certain level of several kinds of efficacy markers in blood, after administering the initial dose or doses of the anti-human PD-1 antibody compared to that prior to administering the initial dose, and provides a new prescription of the anti-human PD-1 antibody for anti-cancer therapy.
Owner:ONO PHARMA CO LTD +1
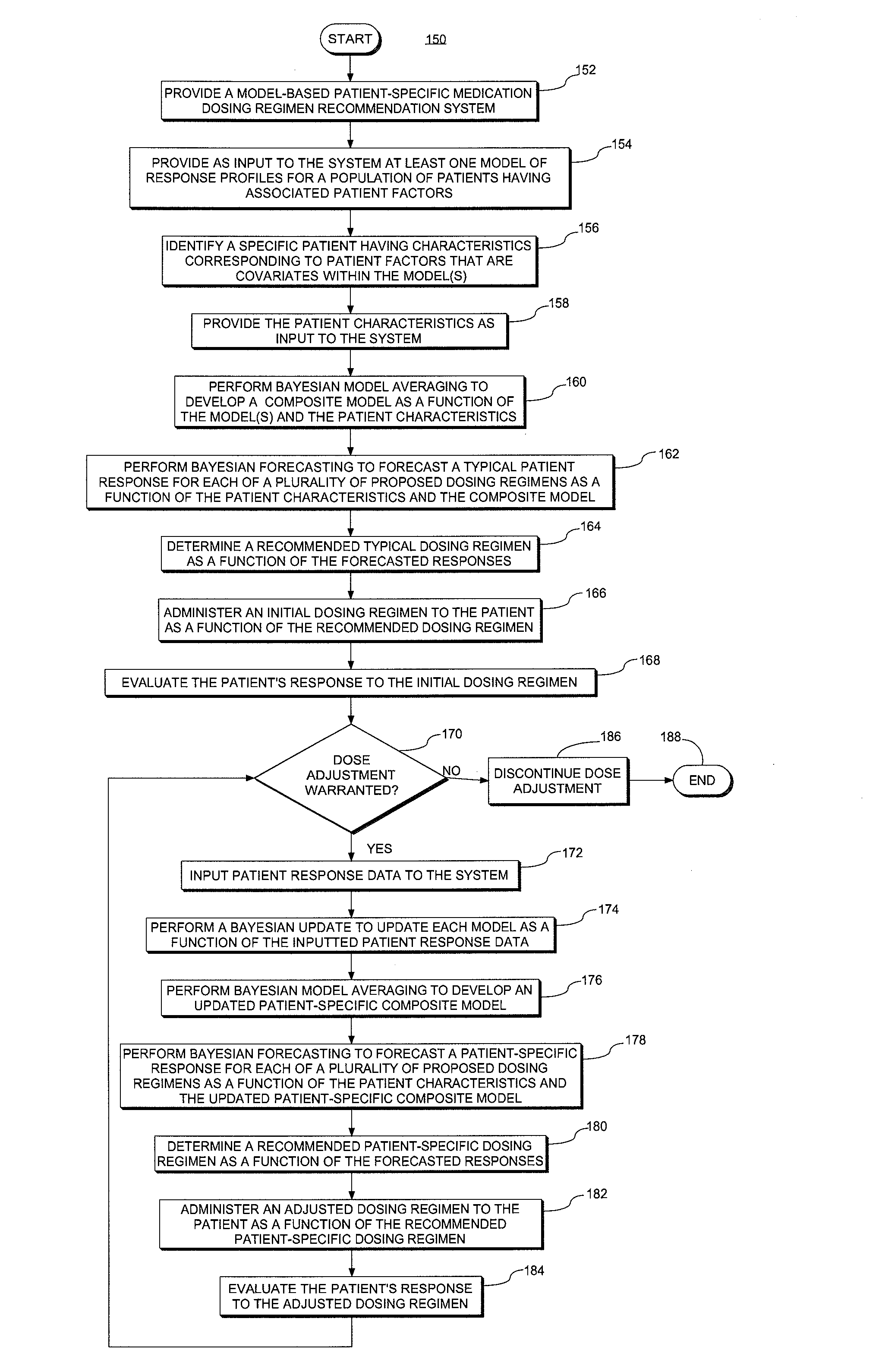
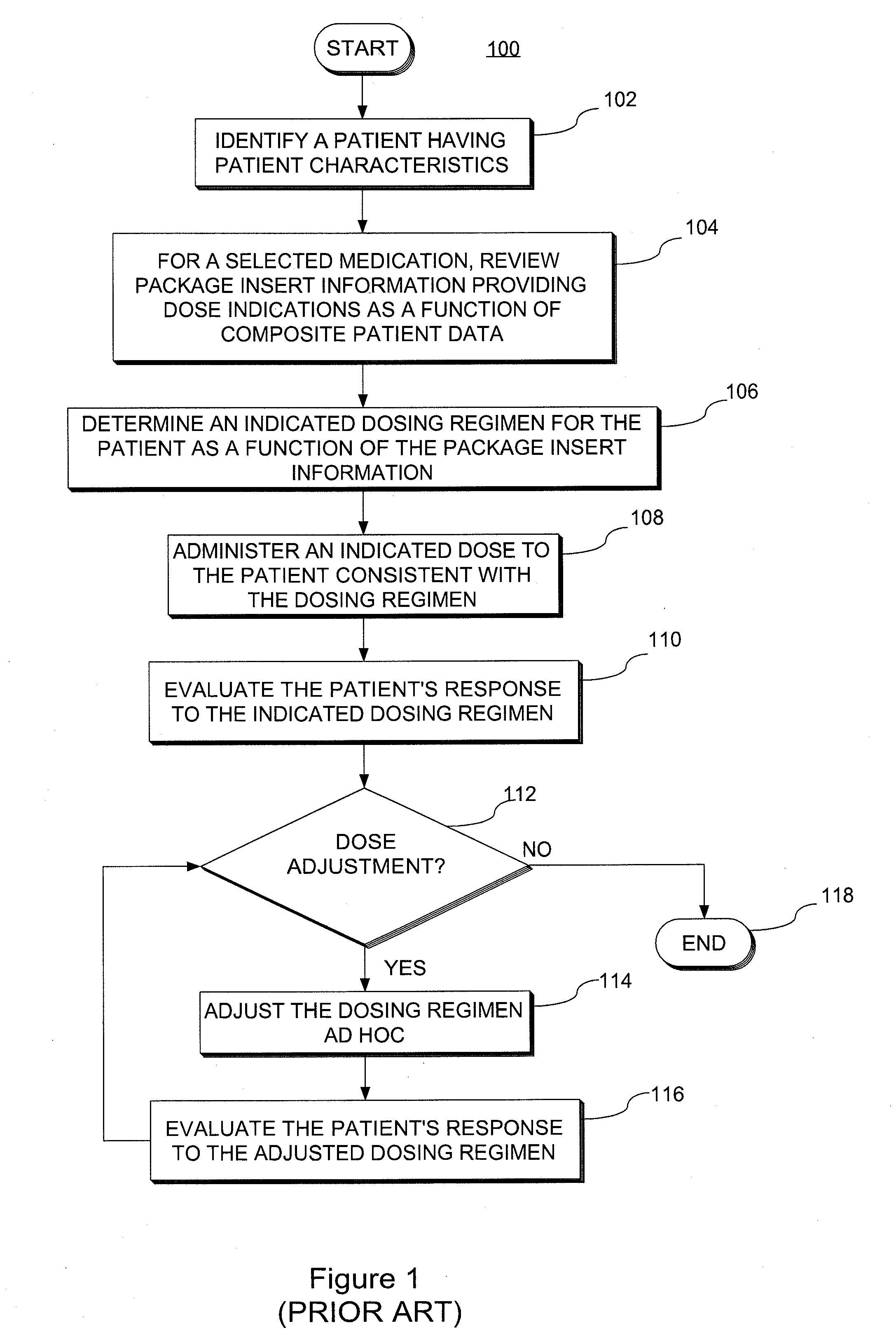
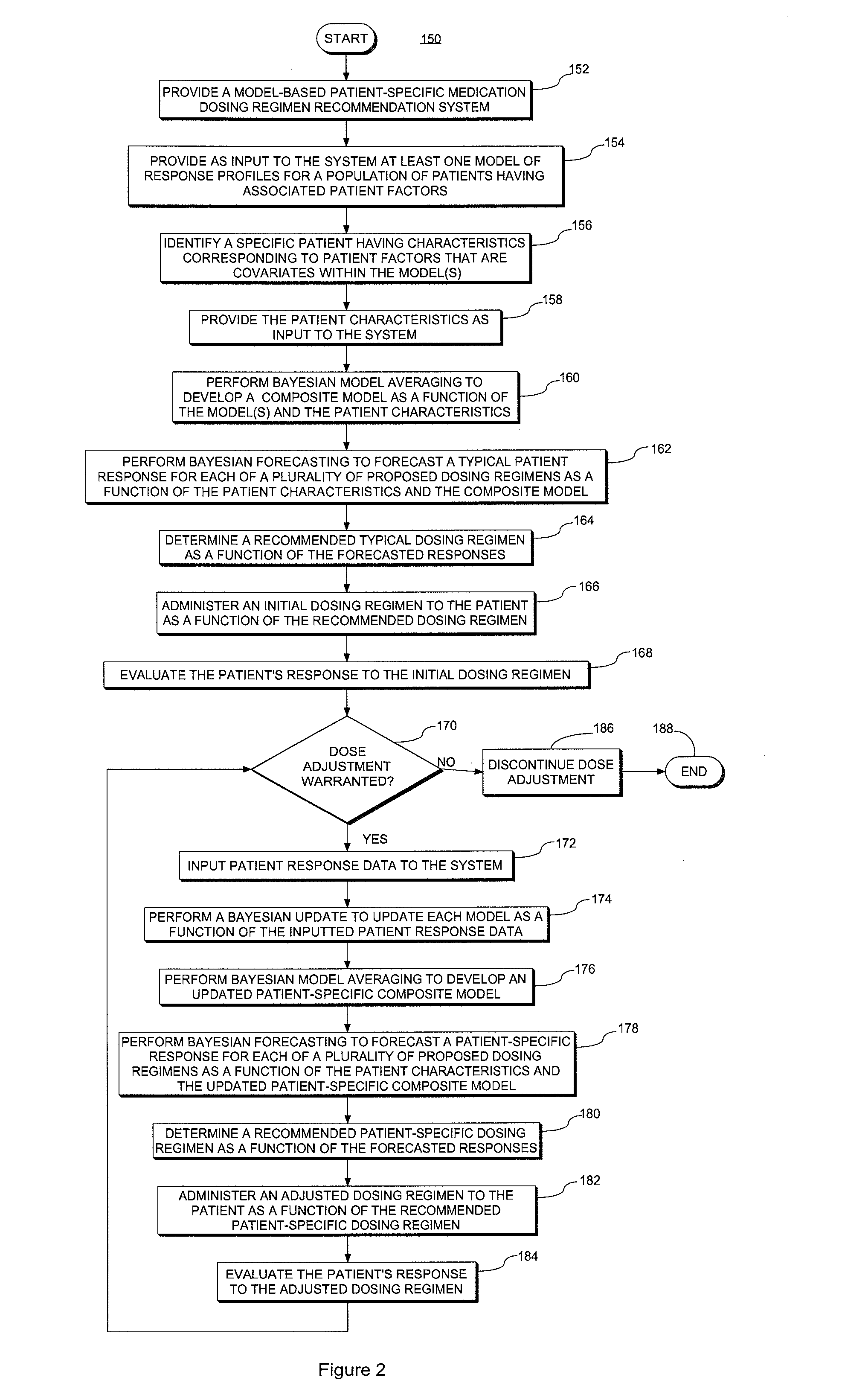
System and method for providing patient-specific dosing as a function of mathematical models updated to account fro an observed patient response
ActiveUS20140100829A1Good precisionAccurate predictionMedical simulationMathematical modelsDosing regimenRegimen
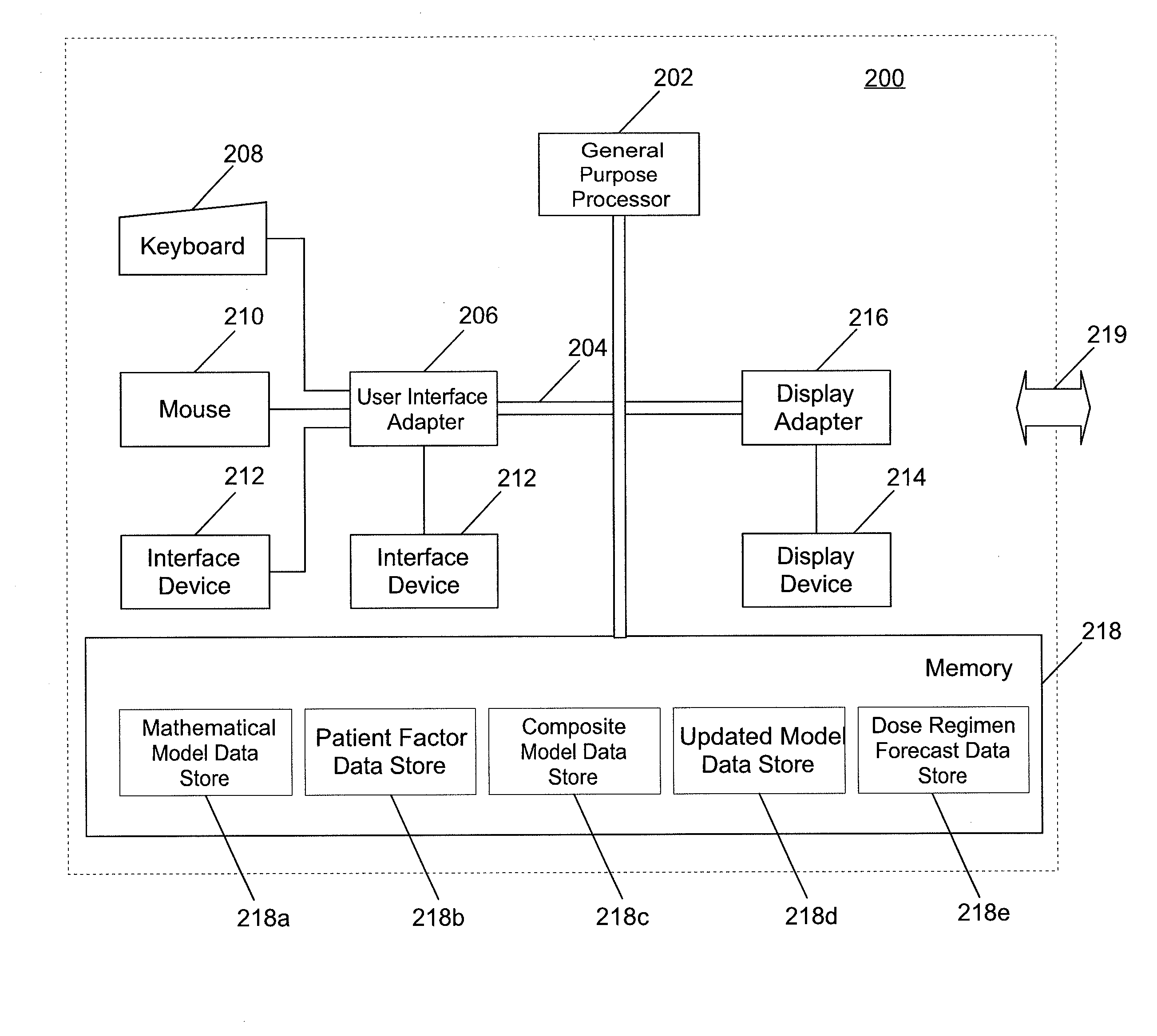
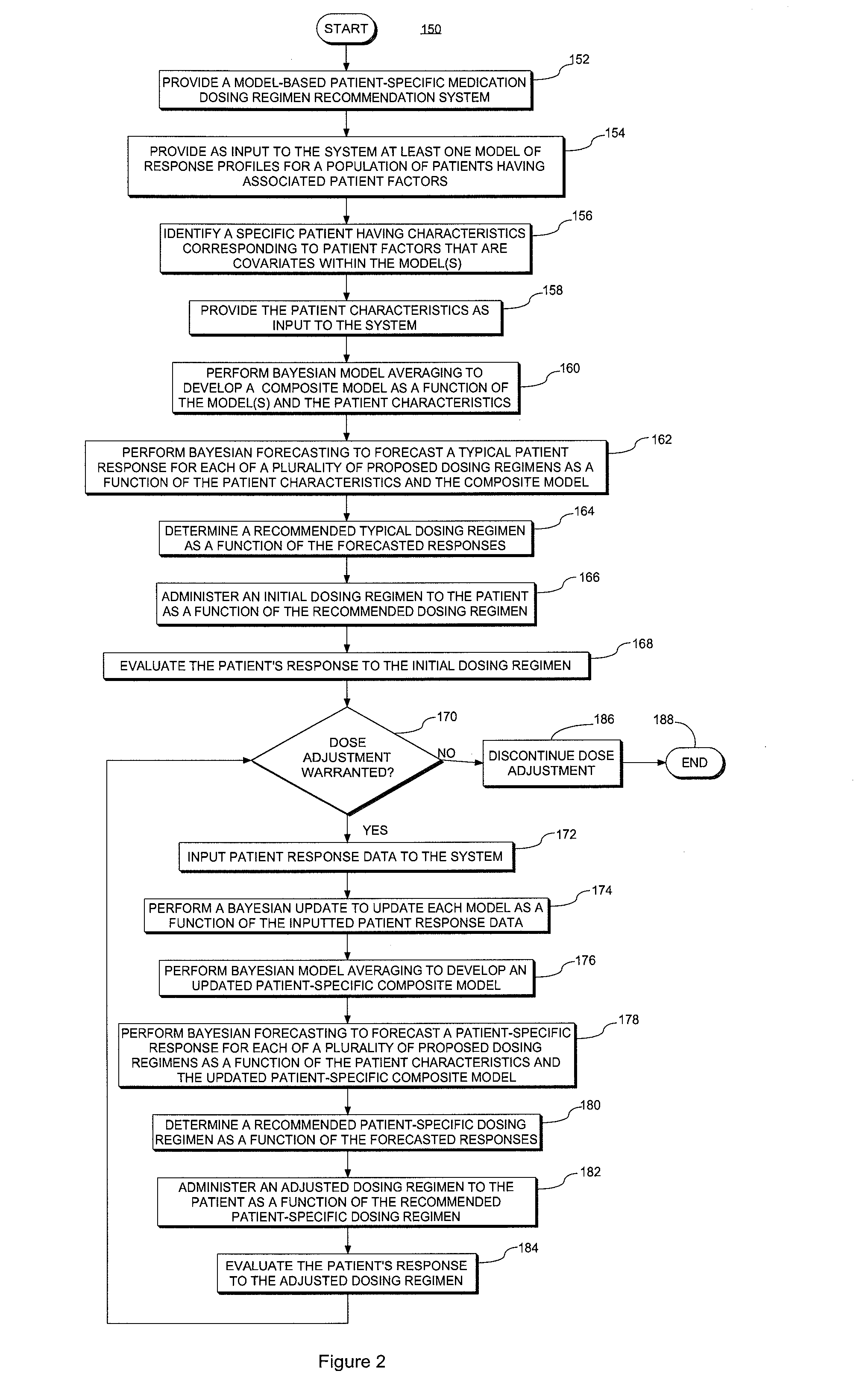
A system and method for predicting, proposing and / or evaluating suitable medication dosing regimens for a specific individual as a function of individual-specific characteristics and observed responses of the specific individual. Mathematical models of observed patient responses are used in determining an initial dose. The system and method use the patient's observed response to the initial dose to refine the model for use to forecast expected responses to proposed dosing regimens more accurately for a specific patient. More specifically, the system and method uses Bayesian averaging, Bayesian updating and Bayesian forecasting techniques to develop patient-specific dosing regimens as a function of not only generic mathematical models and patient-specific characteristics accounted for in the models as covariate patient factors, but also observed patient-specific responses that are not accounted for within the models themselves, and that reflect variability that distinguishes the specific patient from the typical patient reflected by the model.
Owner:MOLD DIANE R
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS20050214229A1Convenient and reliable and practicalReduce cravingsBiocideDrug compositionsOpiateInitial dose
Owner:JSR NTI
Medical system and method for providing glycemic control based on glycemic response information
ActiveUS20120065894A1Improved carbohydrate calculationOptimizationDrug and medicationsNutrition controlInitial doseBlood sugar
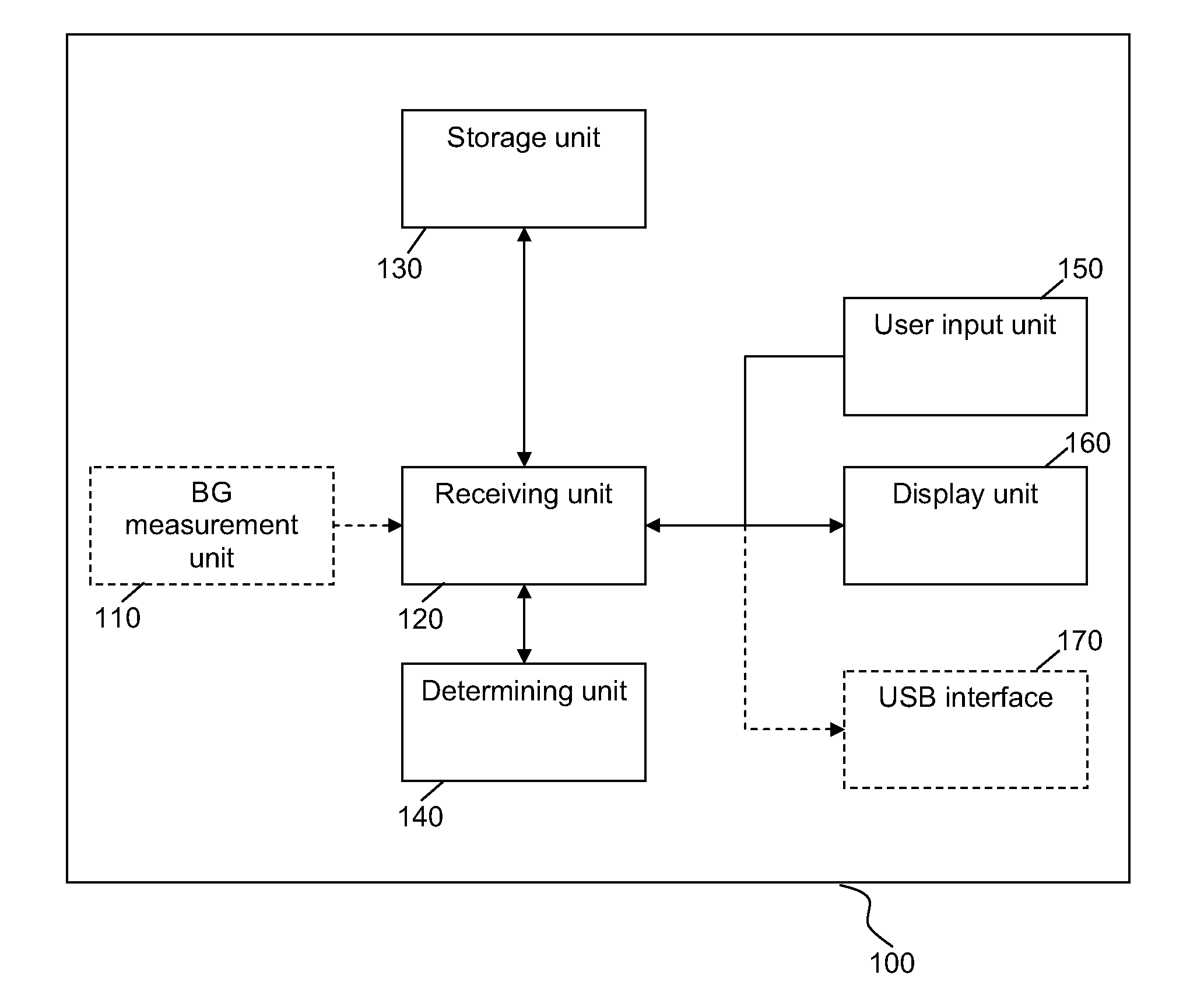
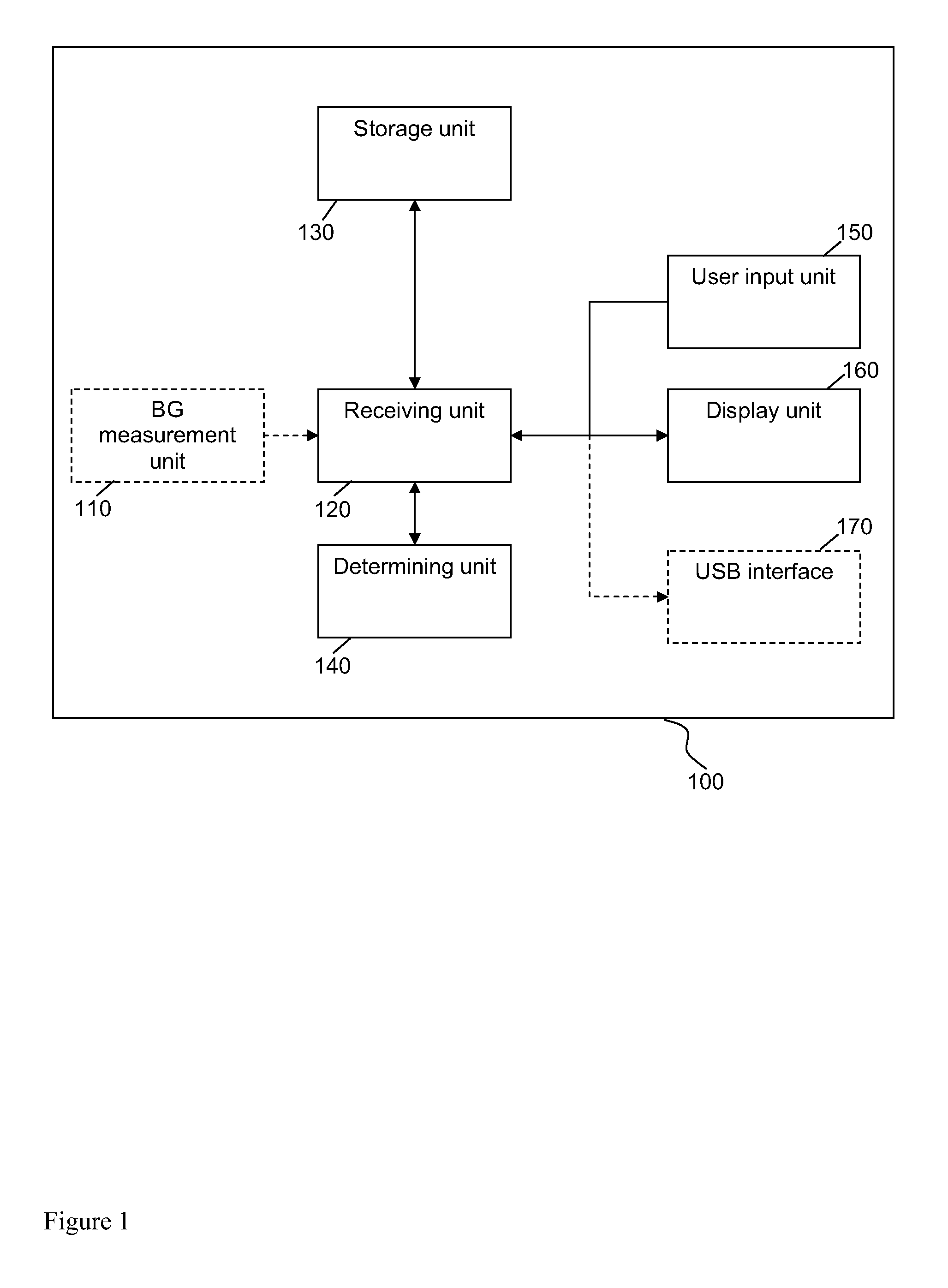
A medical device for providing information for glycemic control is provided, wherein the device comprises a storage unit arranged to store information on an initial dose of insulin and to store information on a blood glucose level measured after the initial dose of insulin was administered and after specific food was consumed, and a determining unit arranged to determine a subsequent dose of insulin to be administered before the specific food is consumed based at least on said information on the initial dose of insulin and said information on the blood glucose level.
Owner:SANOFI AVENTIS DEUT GMBH
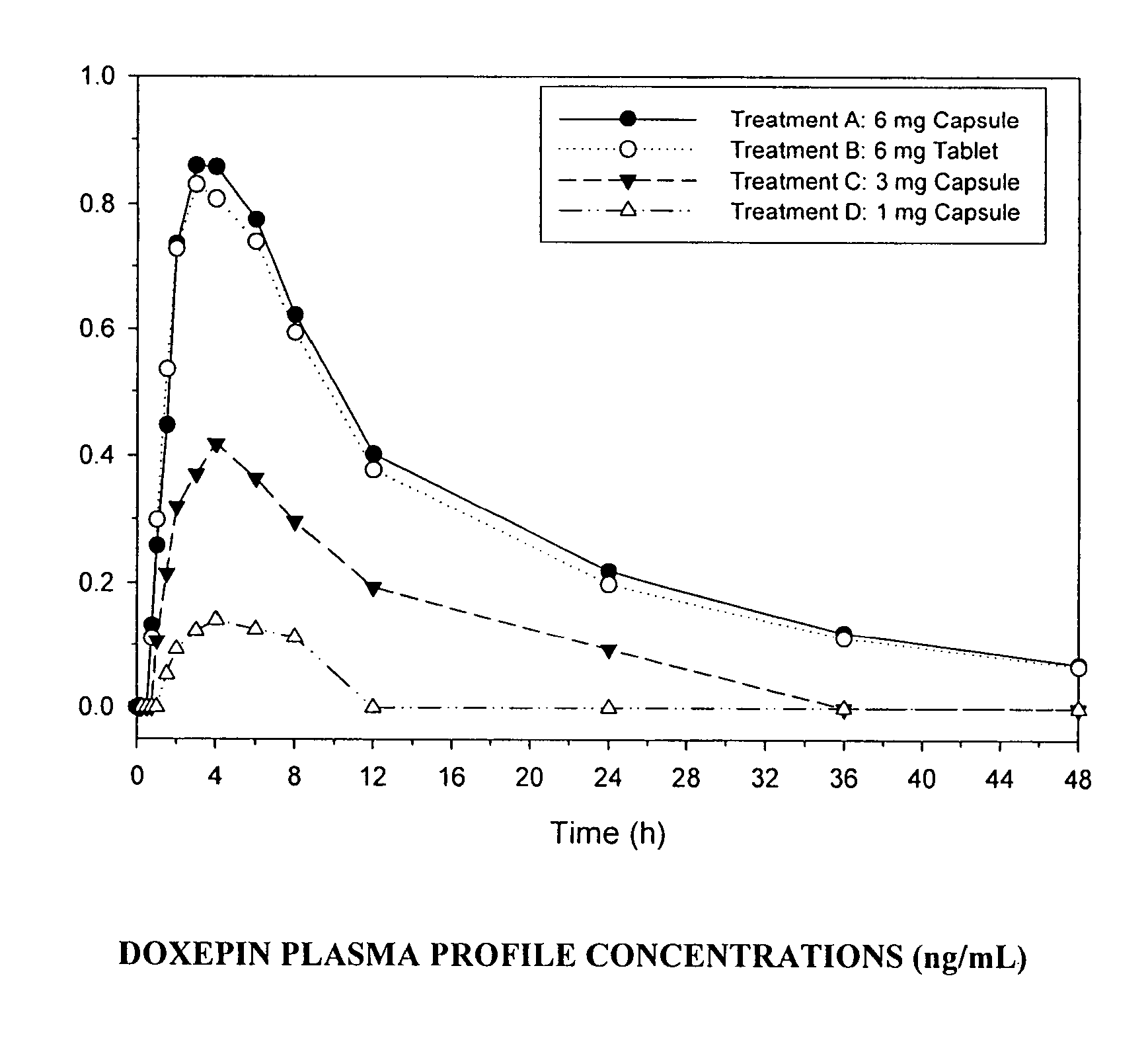
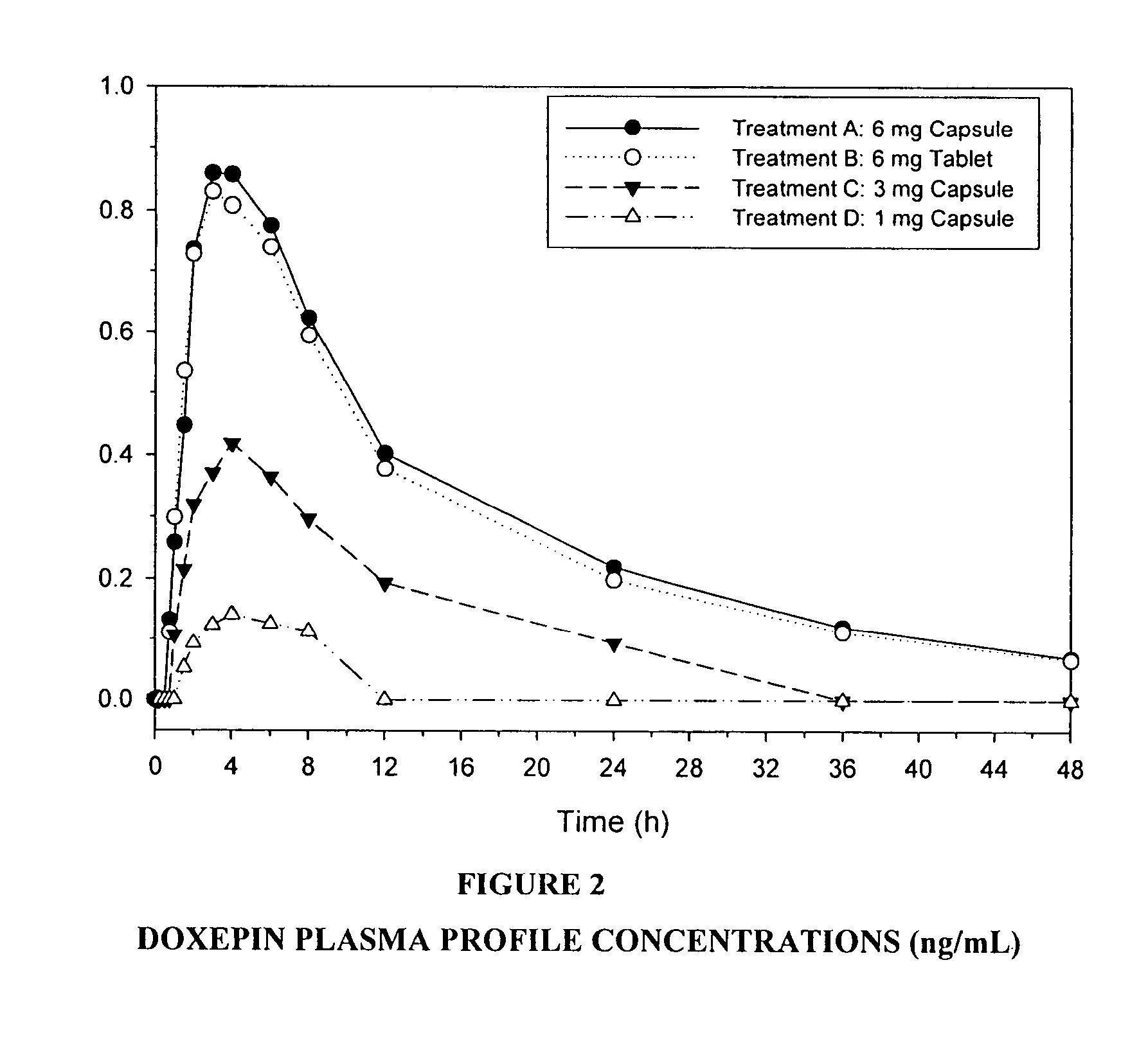
Low-dose doxepin for treatment of sleep disorders in elderly patients
Owner:SOMAXON PHARMA
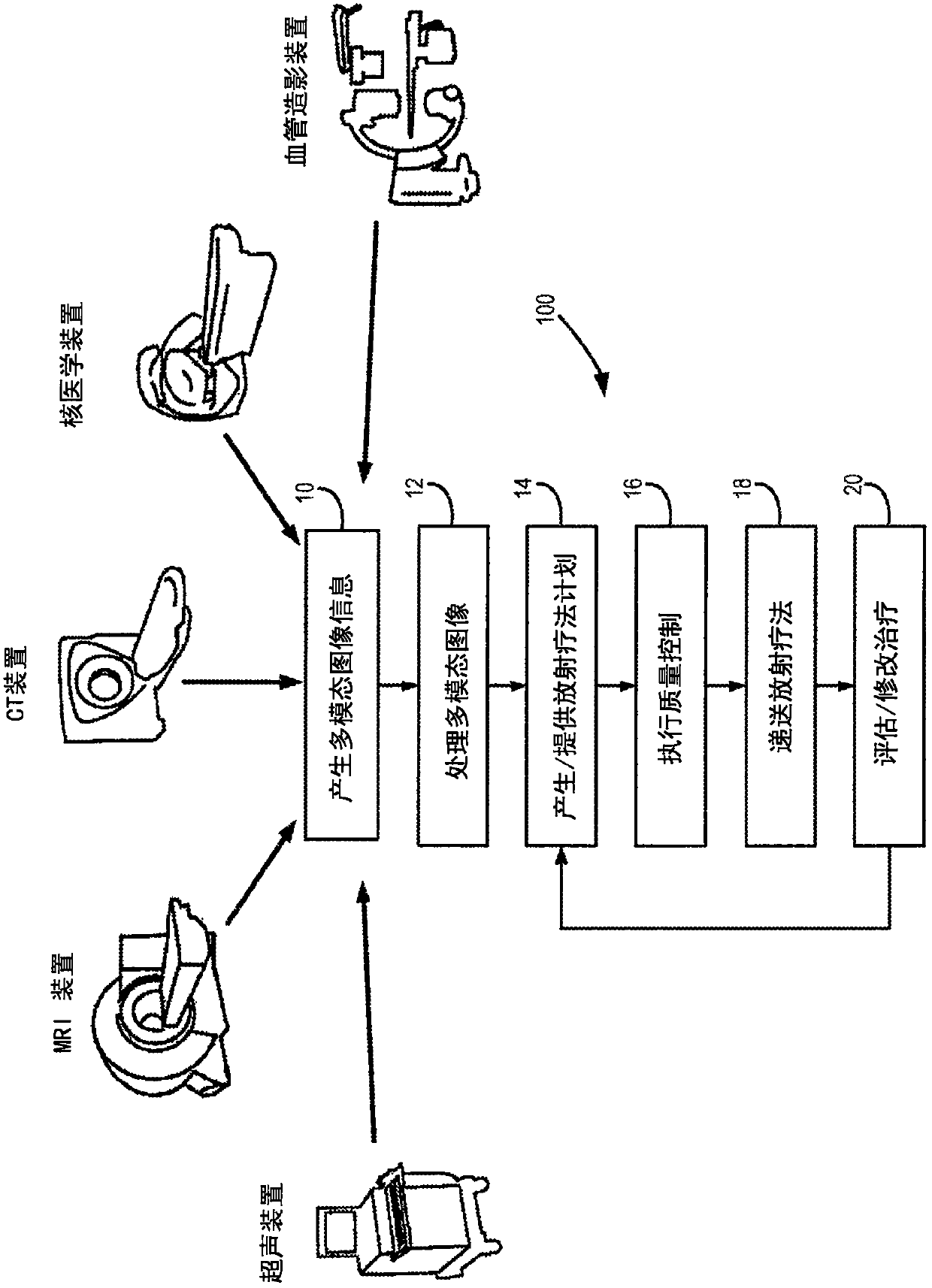
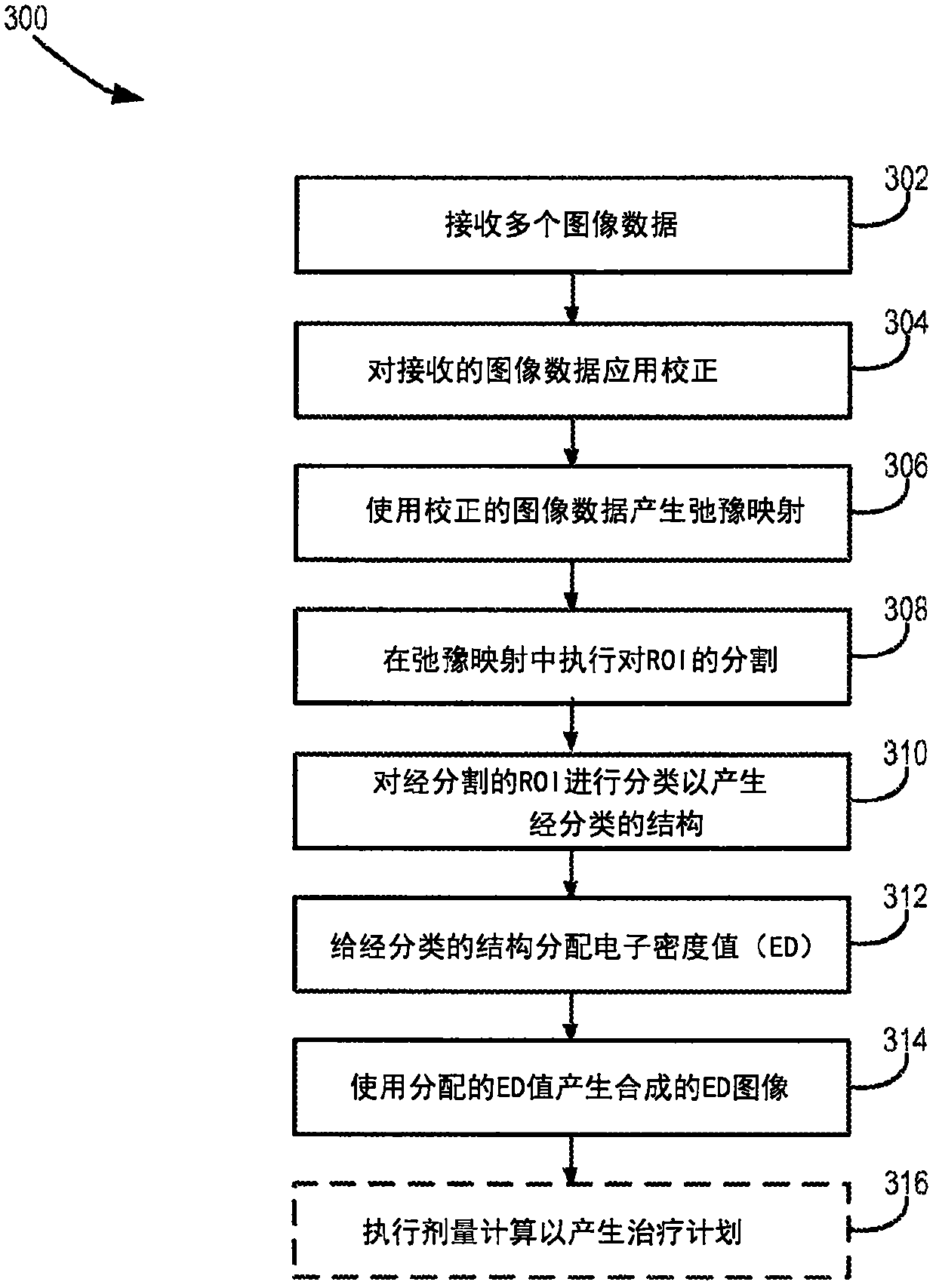
Adaptive replanning based on multimodality imaging
ActiveCN107072595AUltrasonic/sonic/infrasonic diagnosticsImage enhancementDose gradientAdaptive learning
Systems and methods directed to adaptive radiotherapy planning are provided. In some aspects, provided system and method include producing synthetic images from magnetic resonance data using relaxometry maps. The method includes applying corrections to the data and generating relaxometry maps therefrom. In other aspects, a method for adapting a radiotherapy plan is provided. The method includes determining an objective function based on dose gradients from an initial dose distribution, and generating an optimized plan based on updated images, using aperture morphing and gradient maintenance algorithms without need for organ-at-risk contouring. In yet other aspects, a method for obtaining 4D MR imaging using a temporal reshuffling of data acquired during normal breathing, a method for deformable image registration using a sequentially applied semi-physical model regularization method for multimodality images, and a method to generate 4D plans using an aperture morphing algorithm based on 4D CT or 4D MR imaging are provided.
Owner:THE MEDICAL COLLEGE OF WISCONSIN INC
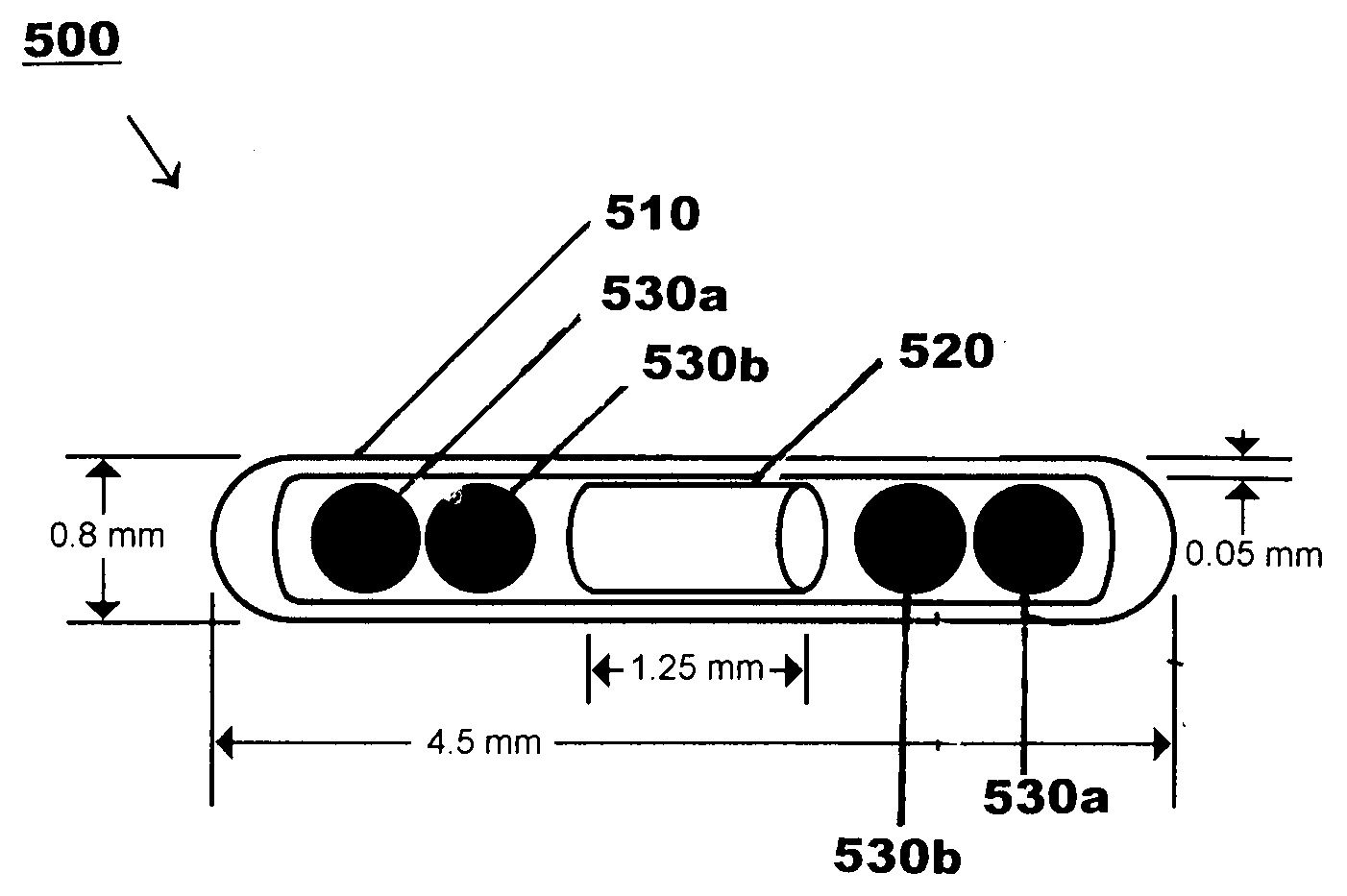
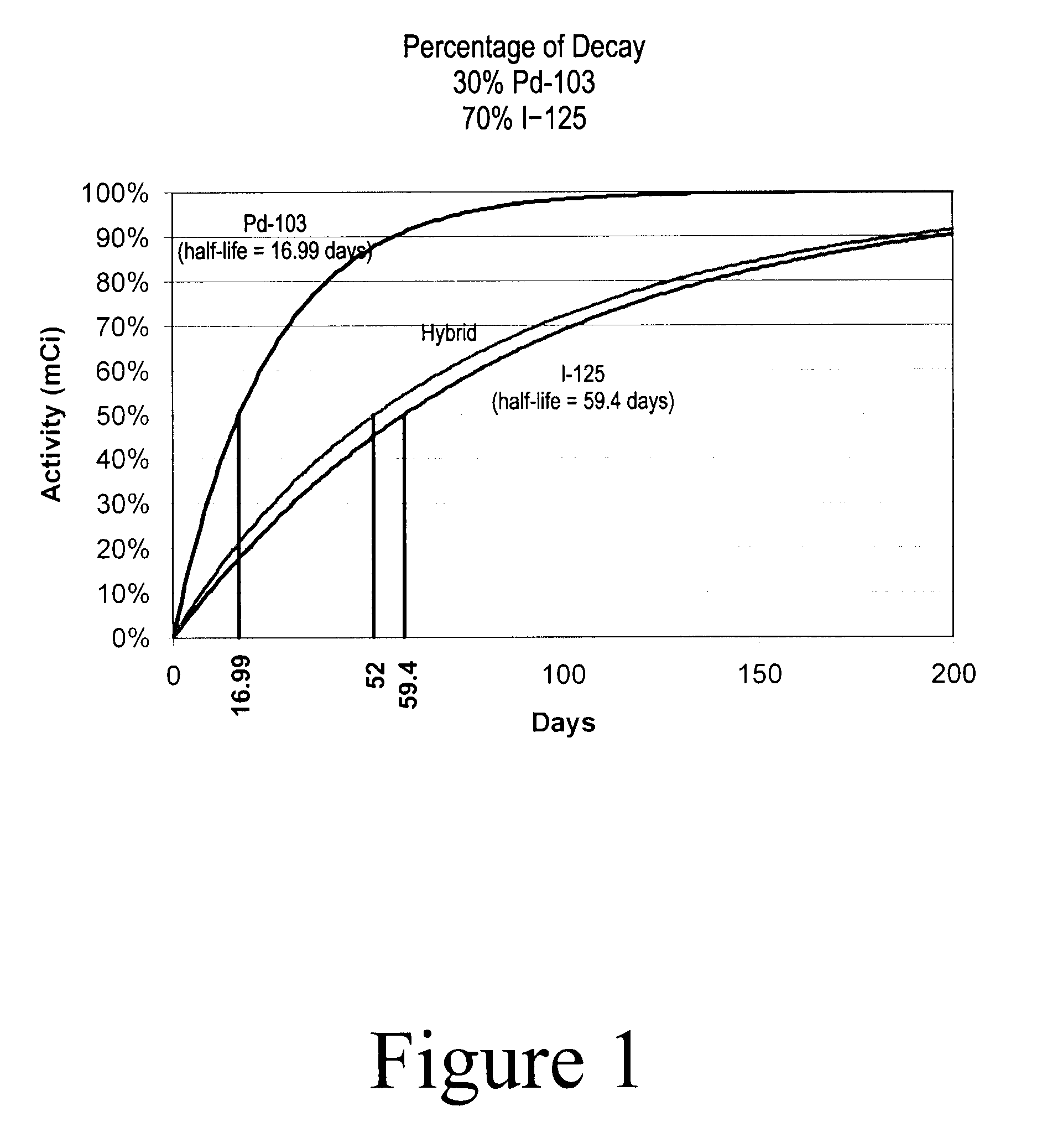
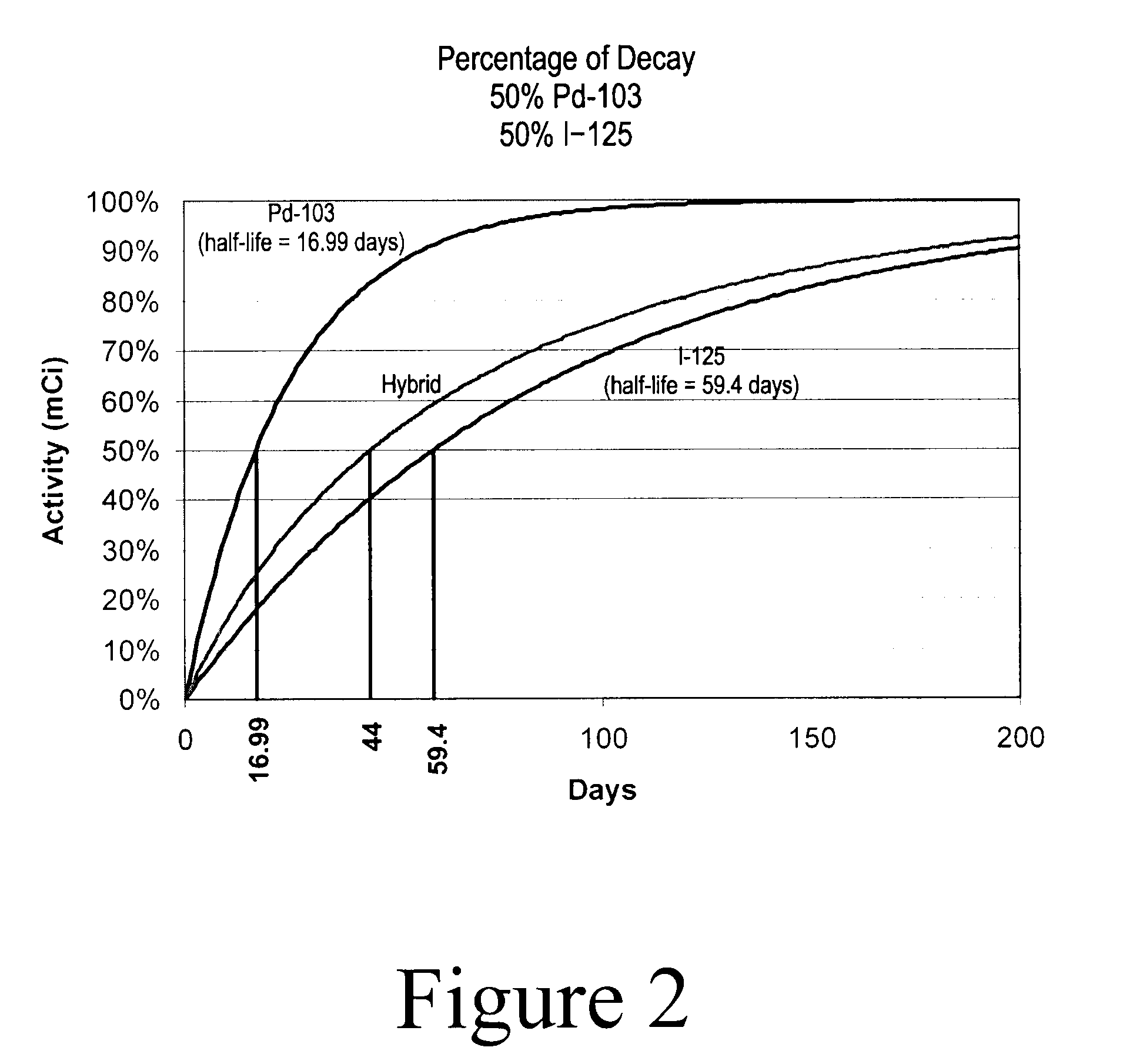
Hybrid Source Containing Multi-Radionuclides for Use in Radiation Therapy
InactiveUS20080249398A1Avoid damageGood curative effectDiagnostic recording/measuringSensorsInitial doseBrachytherapy
A hybrid multi-radionuclide sealed source for use in brachytherapy comprising a plurality of radionuclides is disclosed. The differing decay rates of the radionuclides in the hybrid multi-radionuclide sealed source combine a large initial dose of radiation followed by an extended dose of radiation contained within the single source. The sealed source may comprise a seed, a flexible strand, a rigid strand, a wire, a coil or a catheter.
Owner:HARDER GEORGE FRED +1
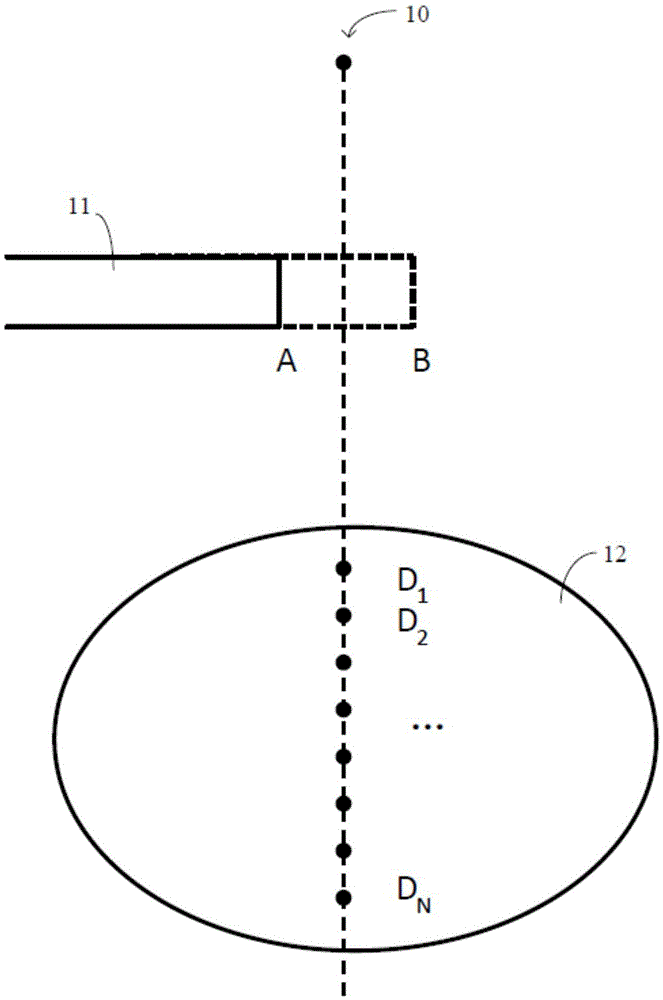
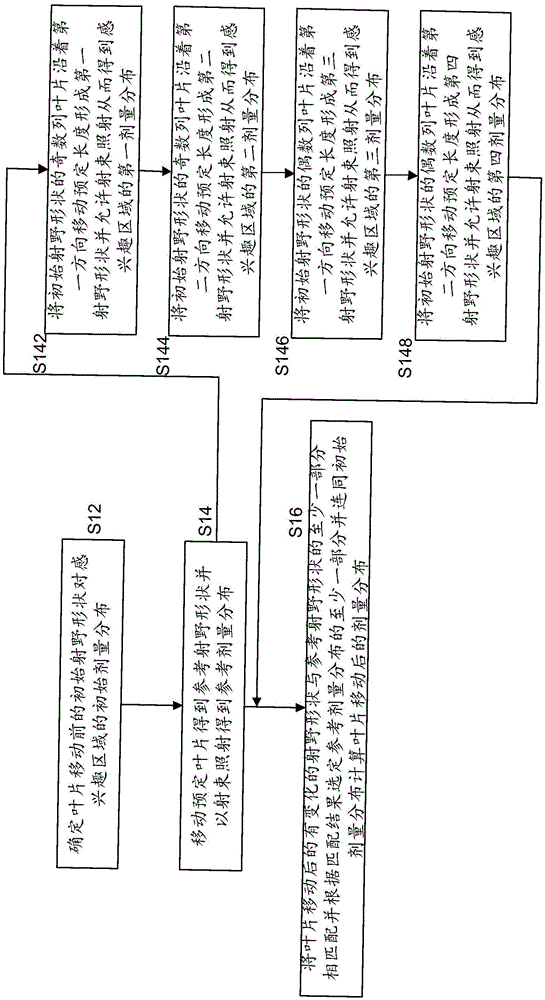
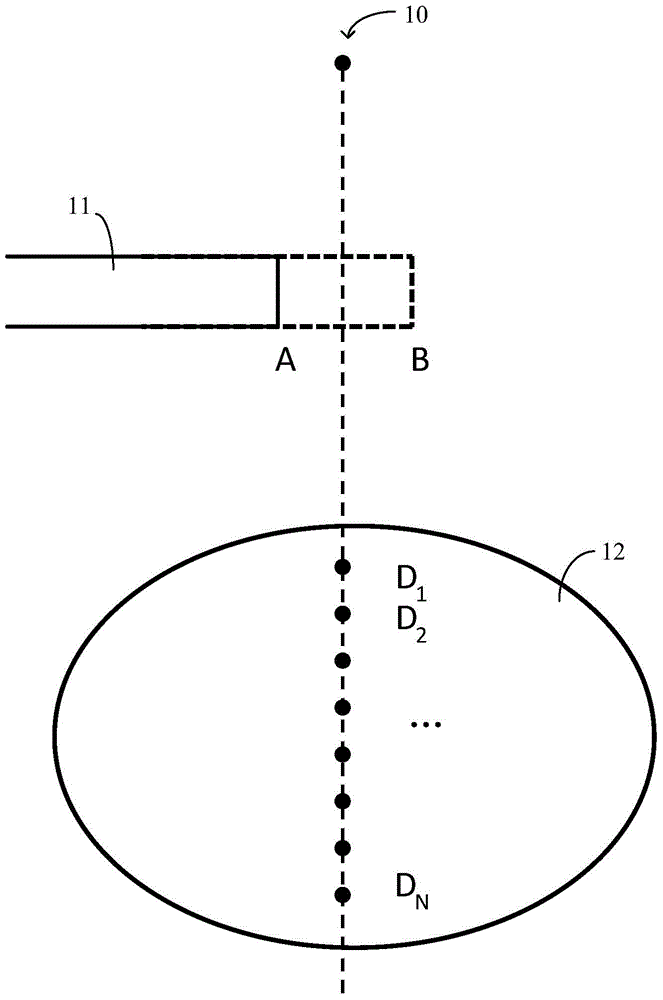
Dose distribution estimation method and sub-field optimization method
InactiveCN105617535AGuaranteed calculation accuracyImprove efficiencyX-ray/gamma-ray/particle-irradiation therapyInitial doseEstimation methods
The present invention provides a dose distribution estimation method which comprises a step of determining the initial dose distribution of an interested area by an initial radiation field shape before leaf movement, a step of moving a predetermined blade which forms the initial radiation field shape to a predetermined length to obtain a reference radiation field shape, and carrying out radiation with the beam from a target to obtain the predetermined dose distribution of the interested area, wherein the change of the reference radiation field shape is regular compared with the initial radiation field shape, and a step of matching a changed part of the radiation field shape after leaf movement compared with the initial radiation field shape and at least a part of the reference radiation field shape, and correspondingly selecting at least a part of the predetermined dose distribution to calcualte the dose distribution after leaf movement with the combination with the initial dose distribution according to a matching result.
Owner:SHANGHAI UNITED IMAGING HEALTHCARE
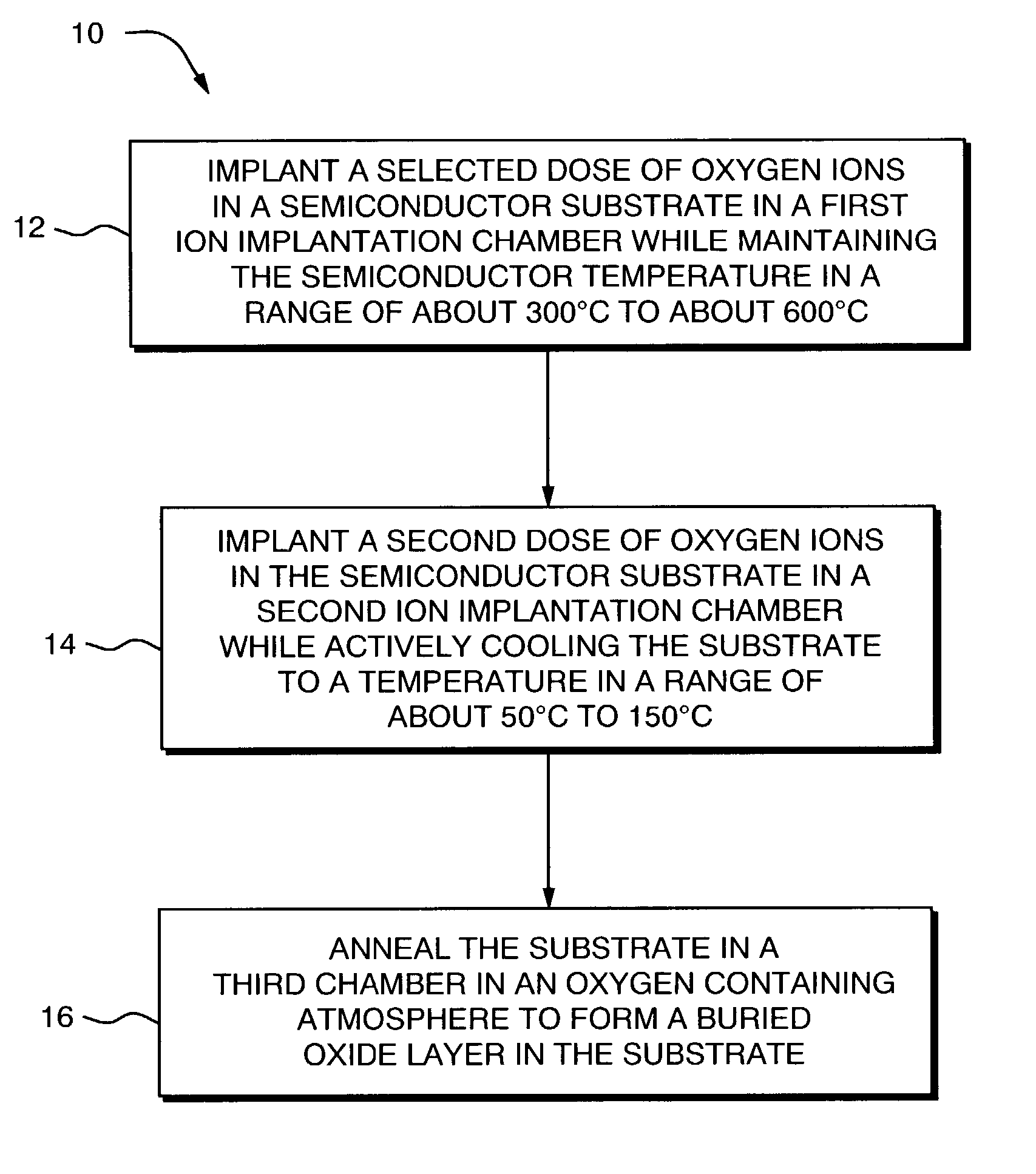
Active wafer cooling during damage engineering implant to enhance buried oxide formation in SIMOX wafers
InactiveUS6998353B2Solid-state devicesSemiconductor/solid-state device manufacturingInitial doseActive cooling
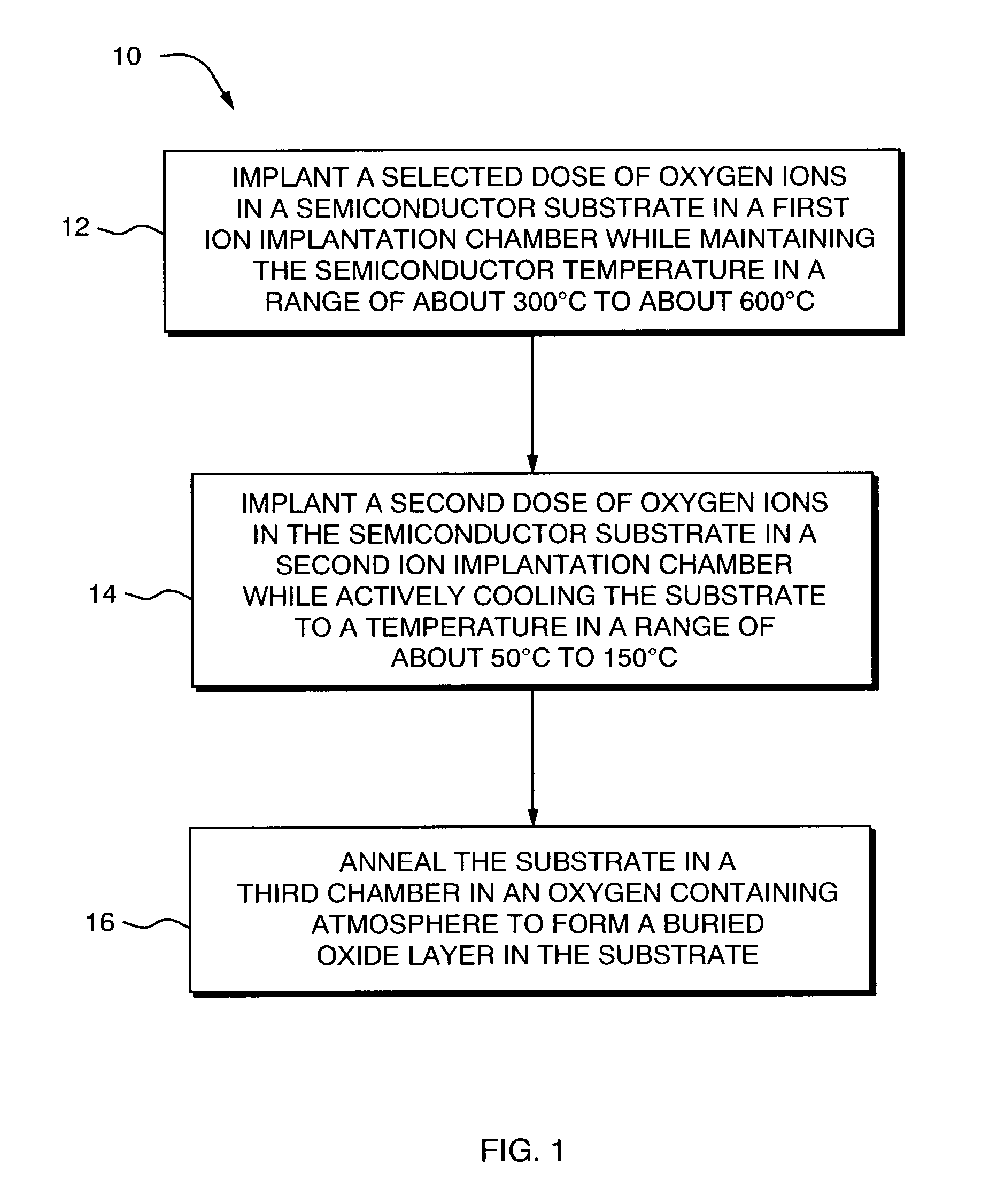
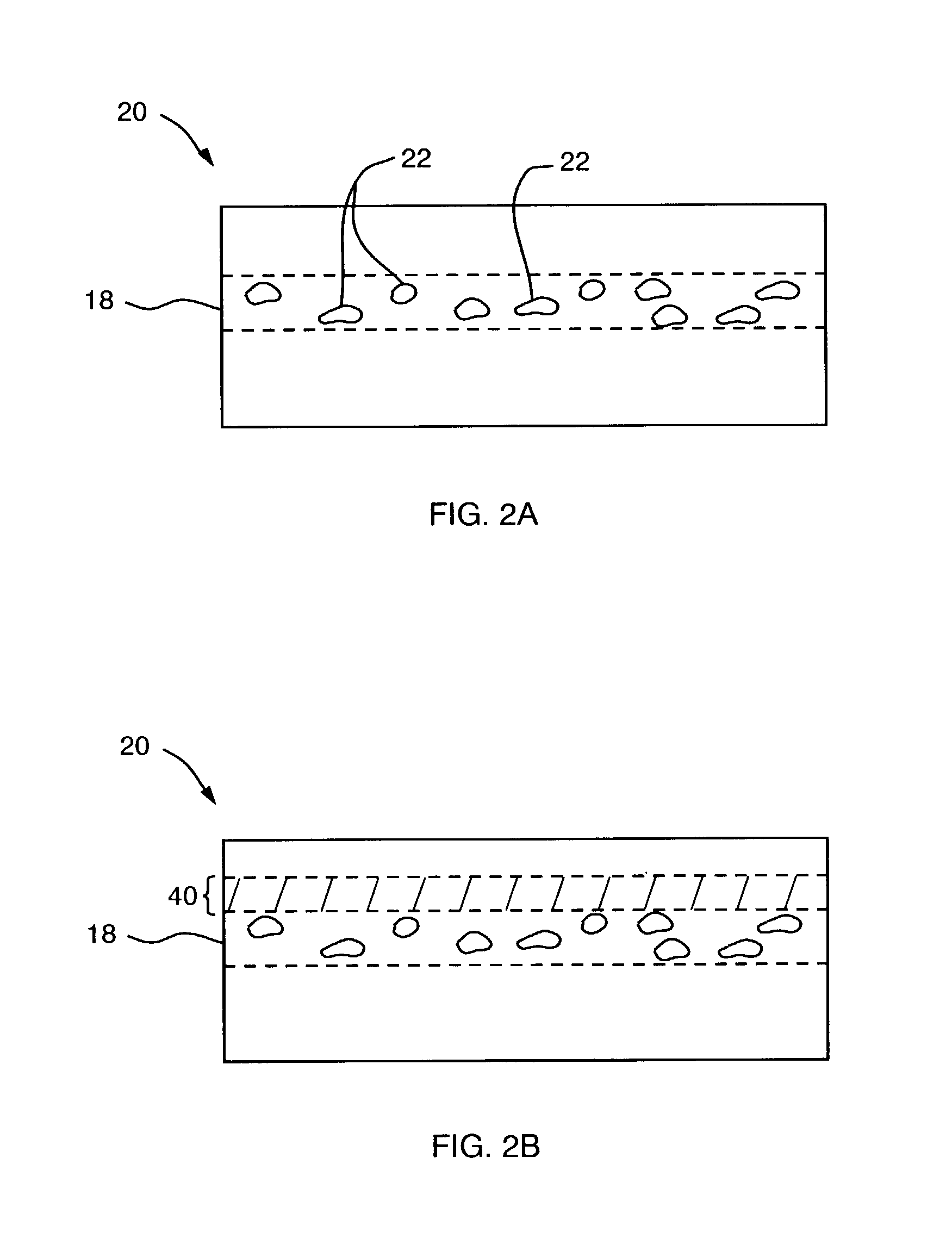
The present invention provides methods and system for forming a buried oxide layer (BOX) region in a semiconductor substrate, such as, a silicon wafer. In one aspect, in a method of the invention, an initial dose of oxygen ions is implanted in the substrate while maintaining the substrate temperature in a range of about 300° C. to 600° C. Subsequently, a second dose of oxygen ions is implanted in the substrate while actively cooling the substrate to maintain the substrate temperature in range of about 50° C. to 150° C. These ion implantation steps are followed by an annealing step in an oxygen containing atmosphere to form a continuous BOX region in the substrate. In one preferred embodiment, the initial ion implantation step is performed in a chamber that includes a device for heating the substrate while the second ion implantation step is performed in a separate chamber that is equipped with a device for actively cooling the substrate. The annealing step can be performed in a third chamber or in either of the first or second chambers.
Owner:IBIS TECH
Extremely low duty-cycle activation of the cholinergic Anti-inflammatory pathway to treat chronic inflammation
ActiveUS20140330349A1Maintain curative effectReduce power consumptionImplantable neurostimulatorsArtificial respirationInitial doseCholinergic anti-inflammatory pathway
Described herein are systems and methods for applying extremely low duty-cycle stimulation sufficient to treat chronic inflammation with progressively longer delays (off periods) from an initial stimulation. In particular, described herein are supra-threshold pulses of electrical stimulation sufficient to result in a long-lasting (e.g., >48 hours) inhibition of pro-inflammatory cytokines and / or effects of chronic inflammation; the delay between initial doses (which may be single-pulse doses) may be extended for subsequent doses, potentially dramatically enhancing battery and device longevity.
Owner:SETPOINT MEDICAL CORP
System and method for providing patient-specific dosing as a function of mathematical models updated to account for an observed patient response
ActiveUS20140351197A1Good precisionEffectively personalize the modelsMedical simulationDrug and medicationsMedication doseRegimen
A system and method for predicting, proposing and / or evaluating suitable medication dosing regimens for a specific individual as a function of individual-specific characteristics and observed responses of the specific individual. Mathematical models of observed patient responses are used in determining an initial dose. The system and method use the patient's observed response to the initial dose to refine the model for use to forecast expected responses to proposed dosing regimens more accurately for a specific patient. More specifically, the system and method uses Bayesian averaging, Bayesian updating and Bayesian forecasting techniques to develop patient-specific dosing regimens as a function of not only generic mathematical models and patient-specific characteristics accounted for in the models as covariate patient factors, but also observed patient-specific responses that are not accounted for within the models themselves, and that reflect variability that distinguishes the specific patient from the typical patient reflected by the model.
Owner:MOLD DIANE R
Child tuberous sclerosis treatment drug
The present invention relates to the field of medicines, and in particular discloses a child tuberous sclerosis treatment drug including rapamycin, the child tuberous sclerosis includes child tuberous sclerosis combined intractable epilepsy, child tuberous sclerosis combined cardiac rhabdomyoma, child tuberous sclerosis combined depigmentation, facial steatadenoma, and child tuberous sclerosis combined renal angioleiomyolipoma, and the drug use method is as follows: the initial dose of the rapamycin is 1mg / (m2.d), and the blood drug concentration is maintained at 5-10ng / mL.
Owner:邹丽萍 +1
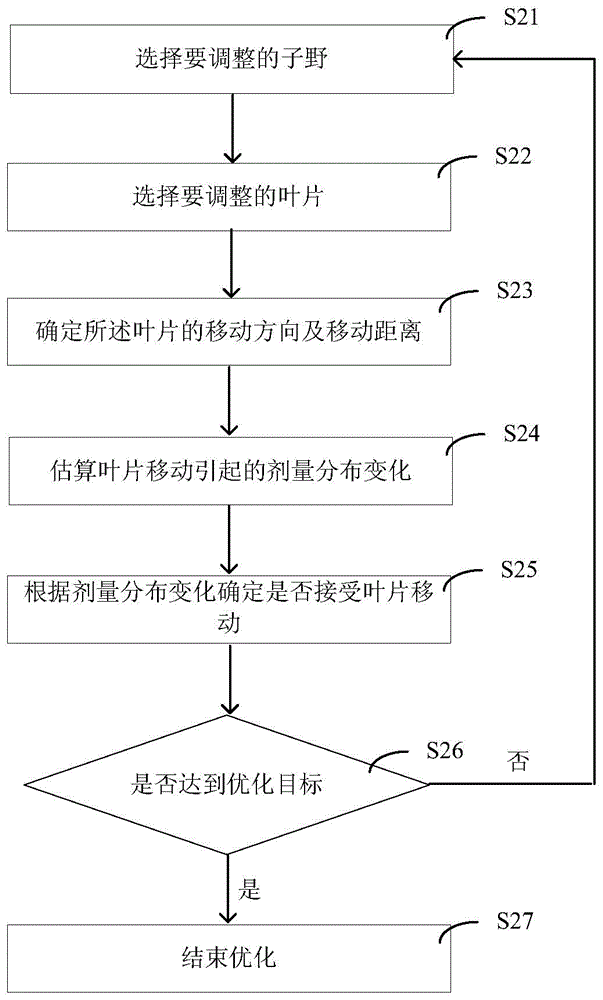
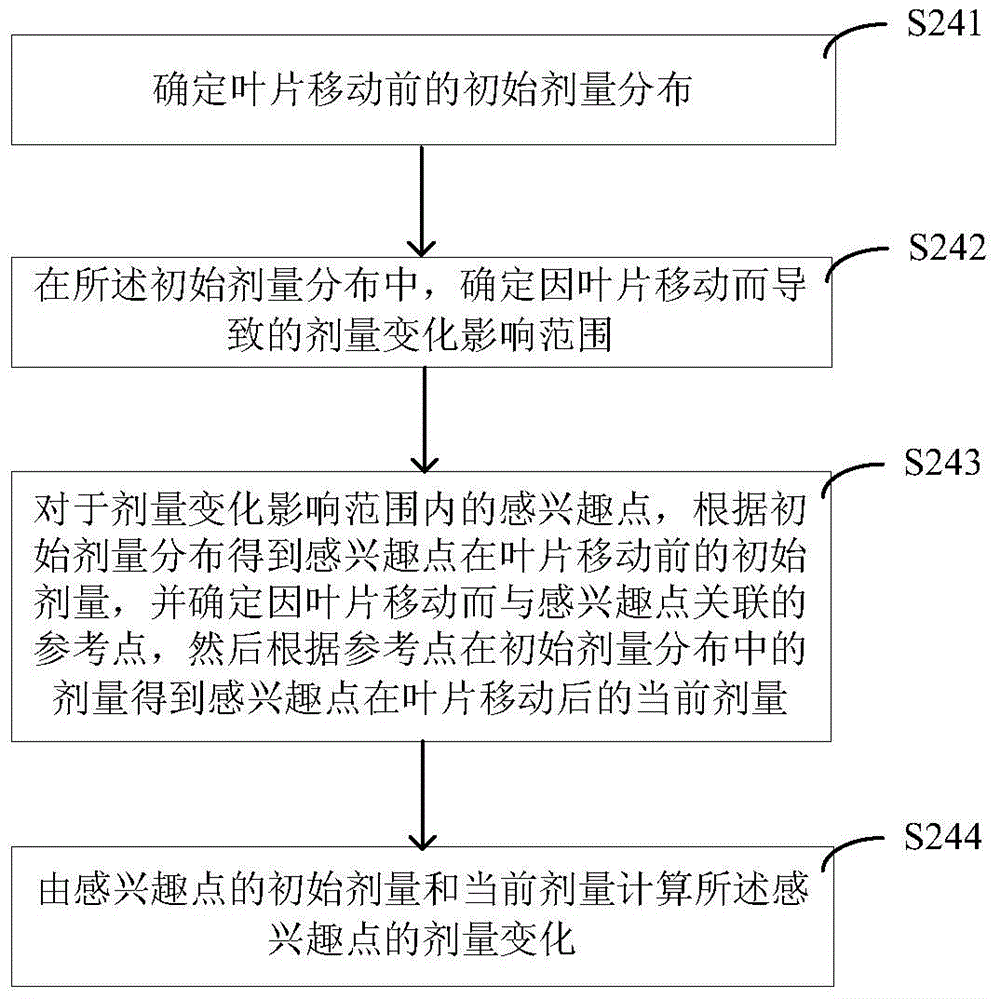
Method and device for estimating dose distribution change, and method and system for direct machine parameter optimizing
ActiveCN104636832ACalculation speedSmall amount of calculationForecastingComputer-assisted treatment prescription/deliveryInitial doseEstimation methods
The invention provides a method and a device for estimating dose distribution change, and a method and a system for direct machine parameter optimizing. The estimation method comprises the following steps of determining the initial dose distribution before a blade is moved; in the initial dose distribution, determining the dose change influence range caused by the blade moving; for an interest point in the dose change influence range, according to the initial dose distribution, obtaining the initial dose of the interest point before the blade moving, determining a reference point correlated with the interest point because of the blade moving, and according to the dose of the reference point in the initial dose distribution, obtaining the current dose of the interest point after the blade moving; according to the initial dose and the current dose of the interest point, calculating the dose change of the interest point. The estimation method has the advantages that the estimation accuracy on the dose distribution change is higher, the calculation speed is high, the calculation amount is less, the memory occupation is less, and the like.
Owner:SHANGHAI UNITED IMAGING HEALTHCARE
Medicated chewing gum delivery system for nicotine
InactiveUS20050123489A1Quick reliefQuick releaseBiocideInorganic non-active ingredientsMedicated chewing-gumInitial dose
A chewing gum delivery system has nicotine, gum base and a buffer system with an improved release rate for the nicotine. The resulting delivery system advantageously provides a convenient, reliable, practical, and relatively painless system for delivering an active. The delivery system is capable of delivering initial and second doses of a craving reduction active or other actives (e.g., nicotine), the combination of which rapidly reduces cravings, or provides some other pharmacological effect, and provides the pharmacological effect or protection from such cravings over a prolonged period of time beyond the initial dose. Notably, the delivery system is capable of rapidly achieving a pharmacologically effective concentration of the active (e.g., nicotine) in the bloodstream (e.g., within 5 minutes, or more desirably within 3 minutes, or in some cases, within 1-2 minutes), and is also capable of keeping the concentration of the active in the bloodstream at or near the pharmacologically effective concentration for at least 20 minutes after chewing of the delivery system begins, or more desirably about 30 minutes to about 50 minutes after chewing begins.
Owner:JSR NTI
Treatment of bleeding with low half-life fibrinogen
InactiveUS20110263503A1Improved efficacy side effect profileReduced half-lifeFibrinogenPeptide/protein ingredientsHalf-lifeInitial dose
The invention provides low-half life fibrinogen as a result of recombinant expression or enzymatic and chemical removal. The low-half life fibrinogen is useful in treating or effecting prophylaxis of bleeding particularly in situations of an acute nature in which a high initial dose and rapid decline to normal or below normal levels is desirable.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
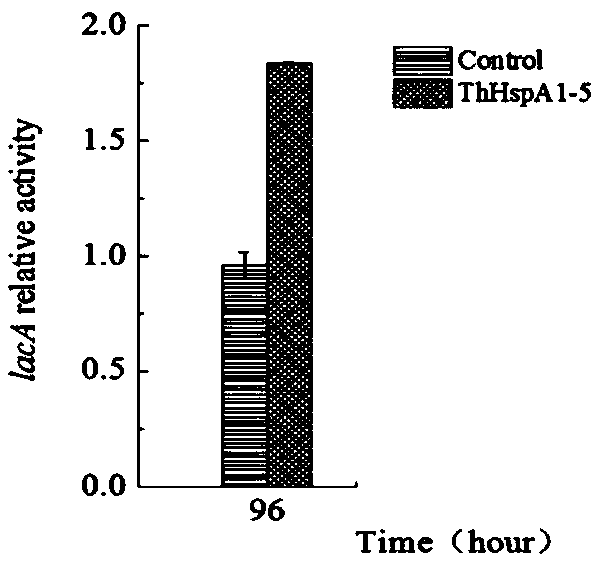
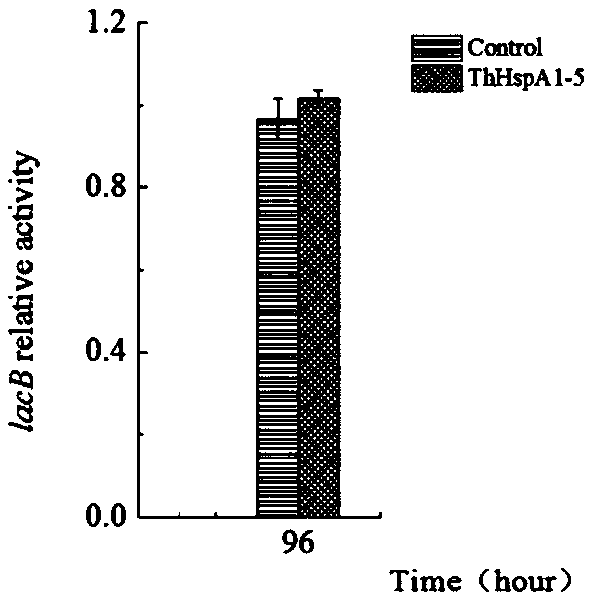
Fungal-laccase expression strain and establishing method and application thereof
ActiveCN109666592AIncrease productionFor industrial applicationsFungiMicroorganism based processesInitial doseTrametes hirsuta
The invention discloses a fungal-laccase expression strain and an establishing method and application thereof. A laccase high-producing strain-trametes AH28-2 is modified with a genetic mean, namely,heat shock protein ThHspA1 is subjected to overexpression in the strain, and through the modified strain Trametes hirsuta AH28-2 (ThHspA1-5), the laccase yield can be increased by 2 times under the condition that 1 / 10 initial dose of an inductive agent is added, and therefore laccase produced through the strain has the advantages of being efficient, environmentally friendly and the like.
Owner:ANHUI UNIVERSITY
Regimen for suppressing organ rejection
ActiveUS9168246B2Low fluctuation and swingSuppression of rejectionAntibody ingredientsPill deliveryOral treatmentRegimen
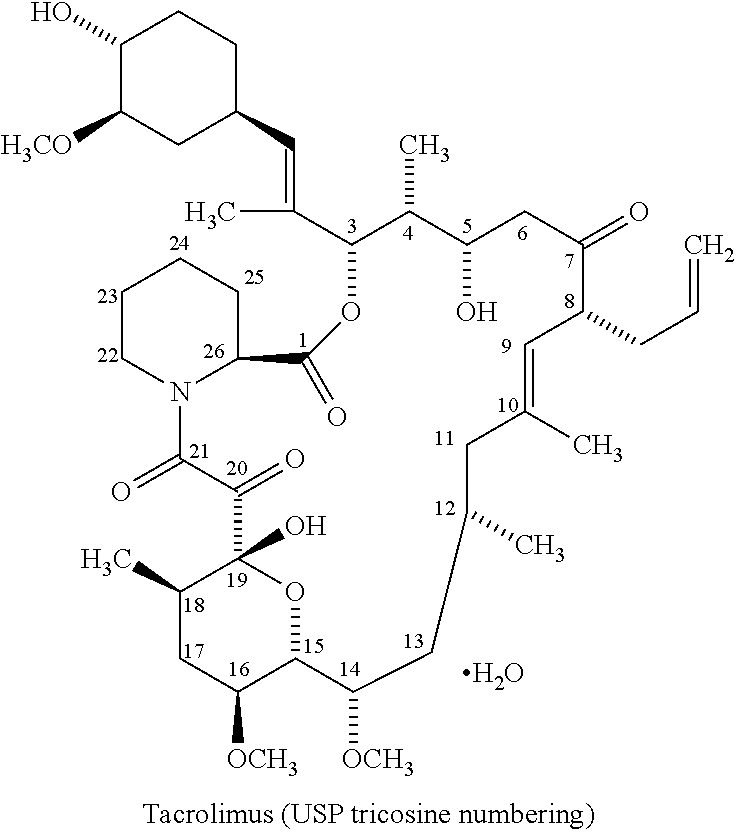
The present invention relates to a method of suppressing organ rejection in a patient receiving an organ transplant by initiating oral treatment with a once-daily extended release tacrolimus dosage form, for example, at an initial dose of from about 0.15 to about 0.20 mg / kg / day within 24 or 48 hours following transplantation. The once-daily extended release tacrolimus dosage form (i) provides low fluctuation and / or swing of tacrolimus, (ii) provides a significantly lower Cmax than an immediate release formulation of tacrolimus while providing the same or greater area under the curve (AUC), (iii) releases the tacrolimus substantially in the colon and / or the lower ileum, (iv) releases at most 63.5% of the tacrolimus in the dosage form at the 12 hour time point, or (v) any combination of any of the foregoing.
Owner:VELOXIS PHARM INC
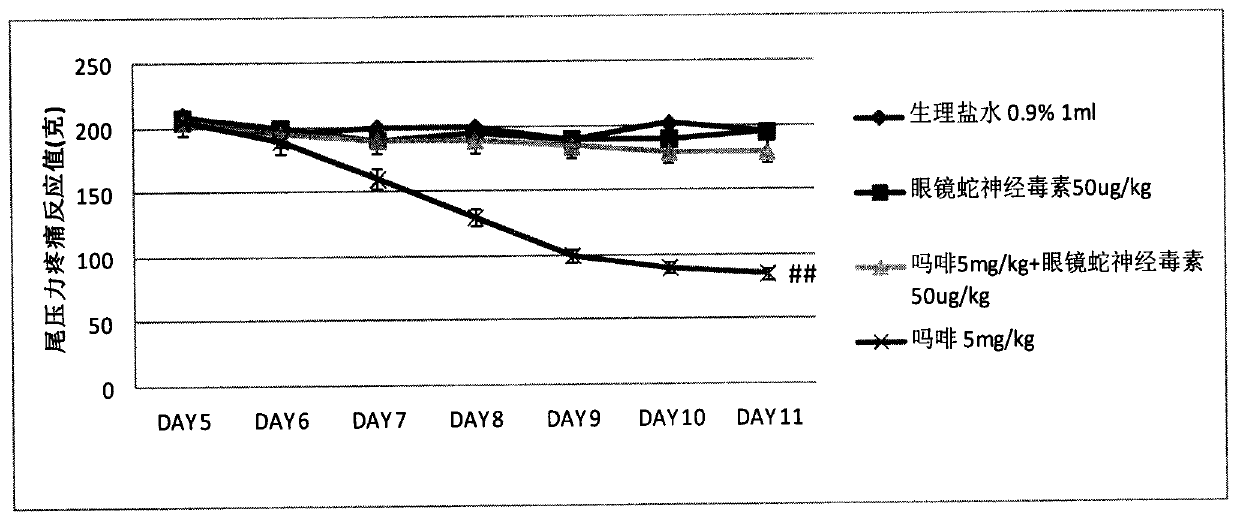
Synergy effect of cobra neurotoxin polypeptide on hyperalgesia and tolerance and alleviating pain of opioids
An opioid receptor agonist is a powerful effect analgesic medicine which is clinically and widely used, morphine is representative, and the medicine can effectively relieve pain of patients, particularly for pain of people after an operation and in the terminal cancer. However, opioids are easy to cause tolerance and hyperalgesia of patients, and the tolerance and the hyperalgesia can be easily formed within one week. Once the tolerance and the hyperalgesia are formed, dosage is continuously increased to continue to maintain the effect of alleviating pain, namely that organisms need a massivedose of medicines to achieve the effect of alleviating pain as the initial dose, but the higher dose can cause serious hyperalgesia, tolerance, constipation, addiction and respiratory depression. Cobra neurotoxin polypeptide can restrain the hyperalgesia and the tolerance of the opioids, and can also improve the treatment effects of opioids, and when the cobra neurotoxin polypeptide and the opioids are combined together for use, the opioids can alleviate pain at the lower dose and does not generate tolerance and hyperalgesia, so that the clinical technical problem that the opiods cannot be used for a long term can be solved.
Owner:江苏毫末医药生物科技有限公司
Prediction model for initial dose of tacrolimus after liver transplantation and individualized application of prediction model
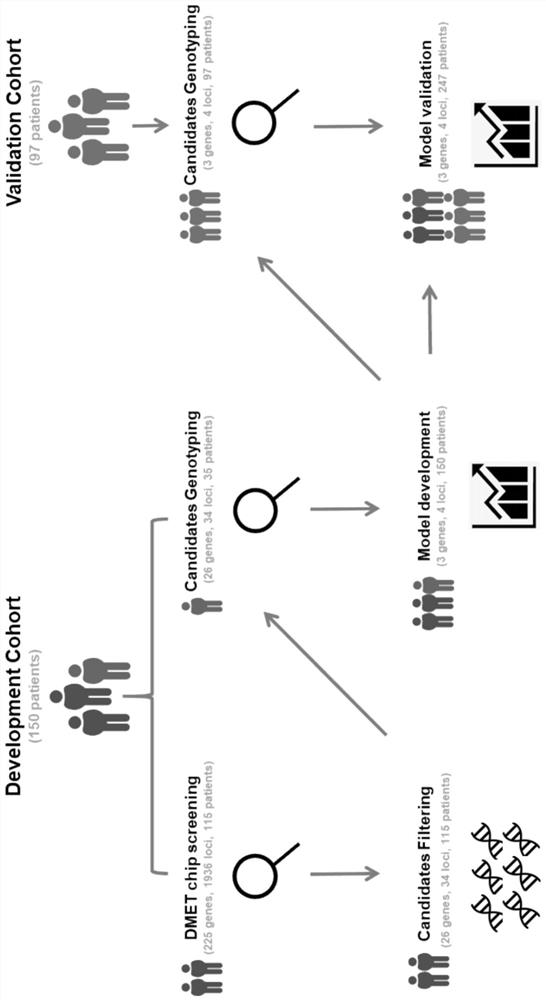
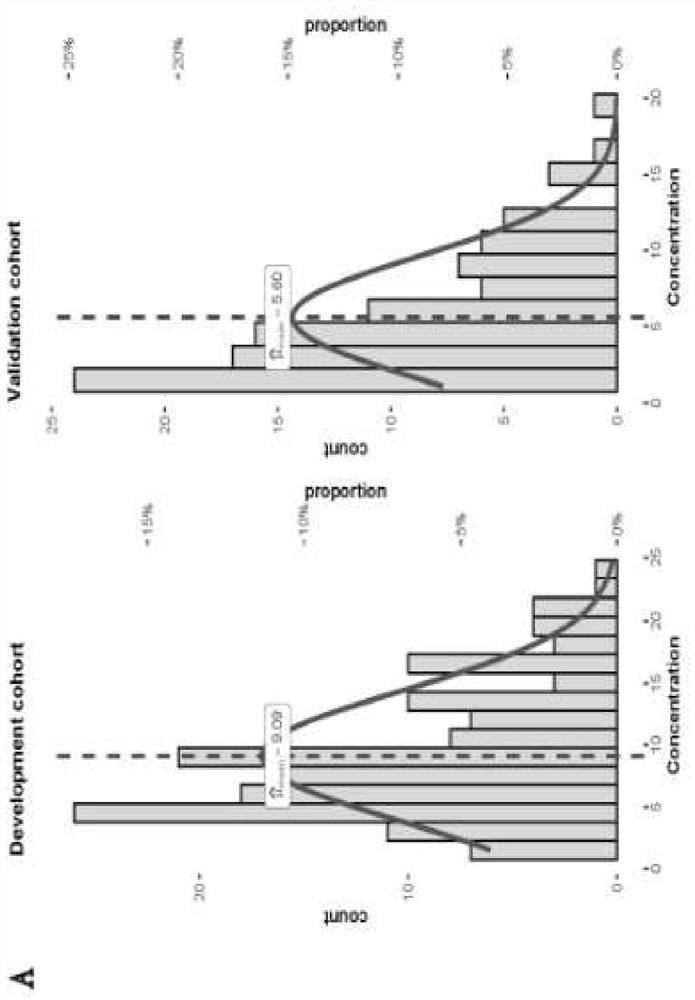
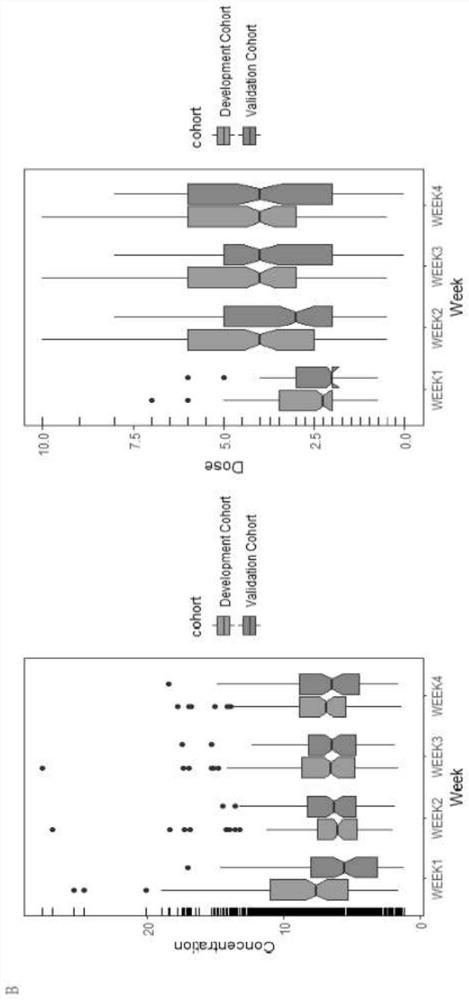
ActiveCN113930495AConvenient supplementReduce morbidityMicrobiological testing/measurementDNA/RNA fragmentationReceptorInitial dose
The invention relates to the technical field of biology, in particular to a prediction model for the initial dose of tacrolimus after liver transplantation and an individualized application of the prediction model. According to the invention, 247 cases of orthotopic liver transplantation patients from 2 transplantation centers are studied, donor and receptor DMET Plus chips are used for screening important sites, and liver genotyping is performed on 150 cases of recipients from the center 1 and livers of corresponding donors of the recipients. Besides, clinical variables are further contained in the model, a tacrolimus pharmacokinetic prediction model is constructed through LASSO regression, results show that AUC in a development queue and AUC in a verification queue are 0.878 and 0.790 respectively, after tacrolimus is given within the recommended concentration range (RCR, 4-10 ng / mL), the incidence rate of new metabolic syndromes is low, and a basis is provided for personalized drug delivery of clinical liver transplantation patients.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Application of baicalin in treatment of acute pancreatitis
InactiveCN1736387ASignificant effectAcute onsetOrganic active ingredientsDigestive systemVeinCase fatality rate
The invention relates to the use of baicalin in treating acute pancreatitis, which mainly comprises applying an initial dose of 10mg / 100g through jugular vein channel, and sustaining intra-vascular administration 1ml / h / 100g through trace quantity transfusion pumps. The invention also discloses the computation method for the 5% baicalin dosage.
Owner:张喜平
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com