Patents
Literature
712results about How to "Increase dose" patented technology
Efficacy Topic
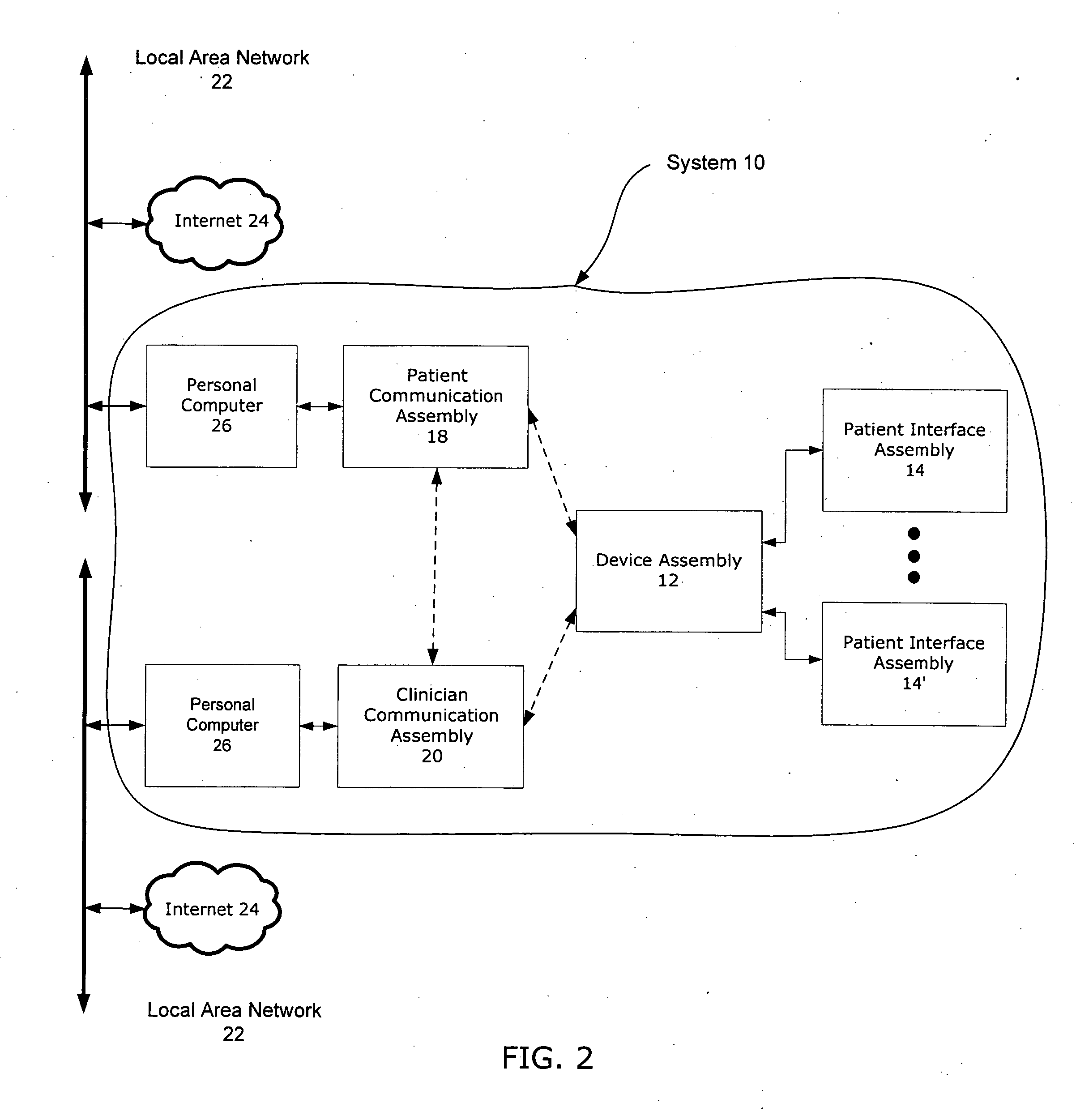
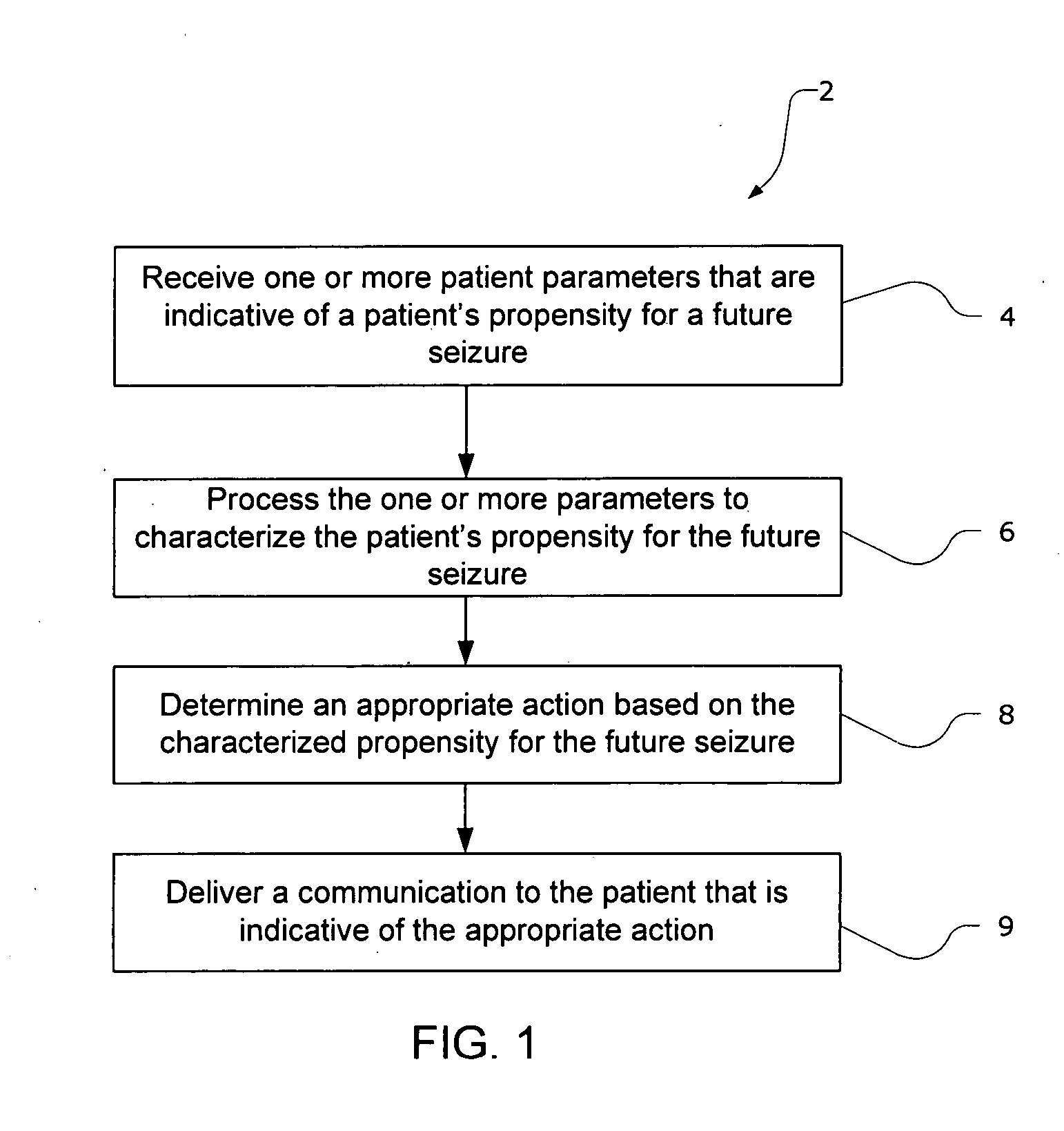
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
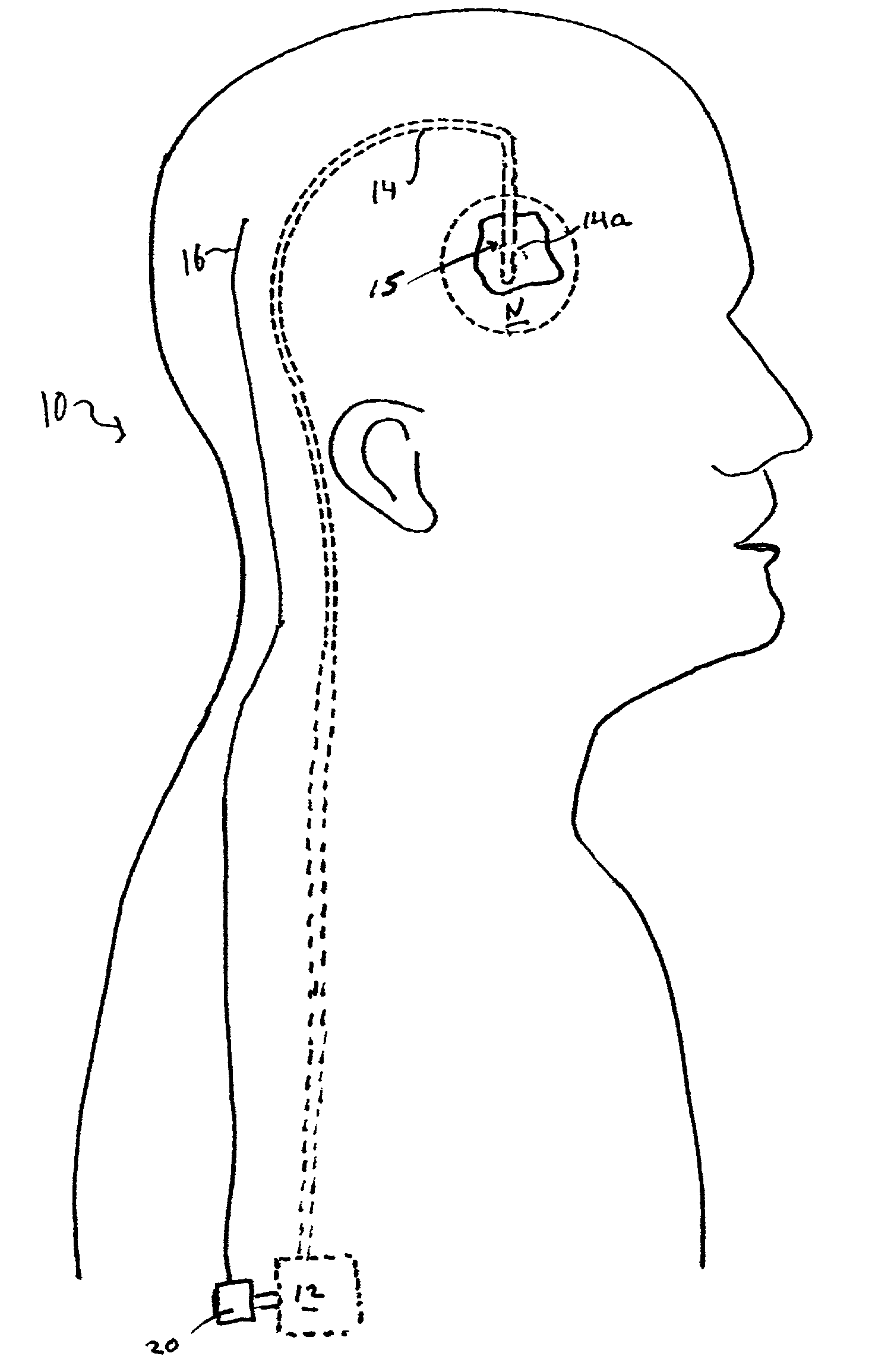
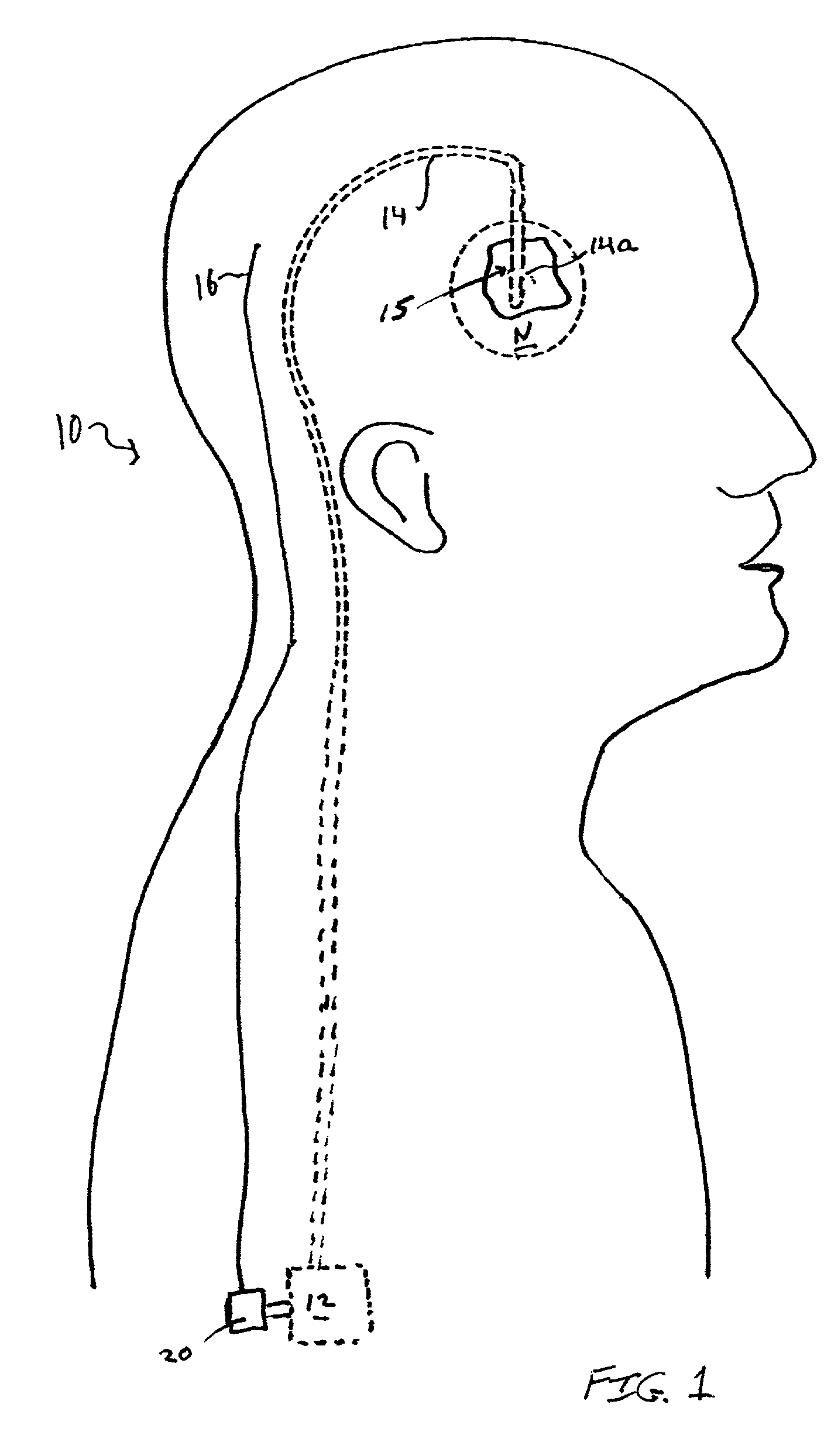
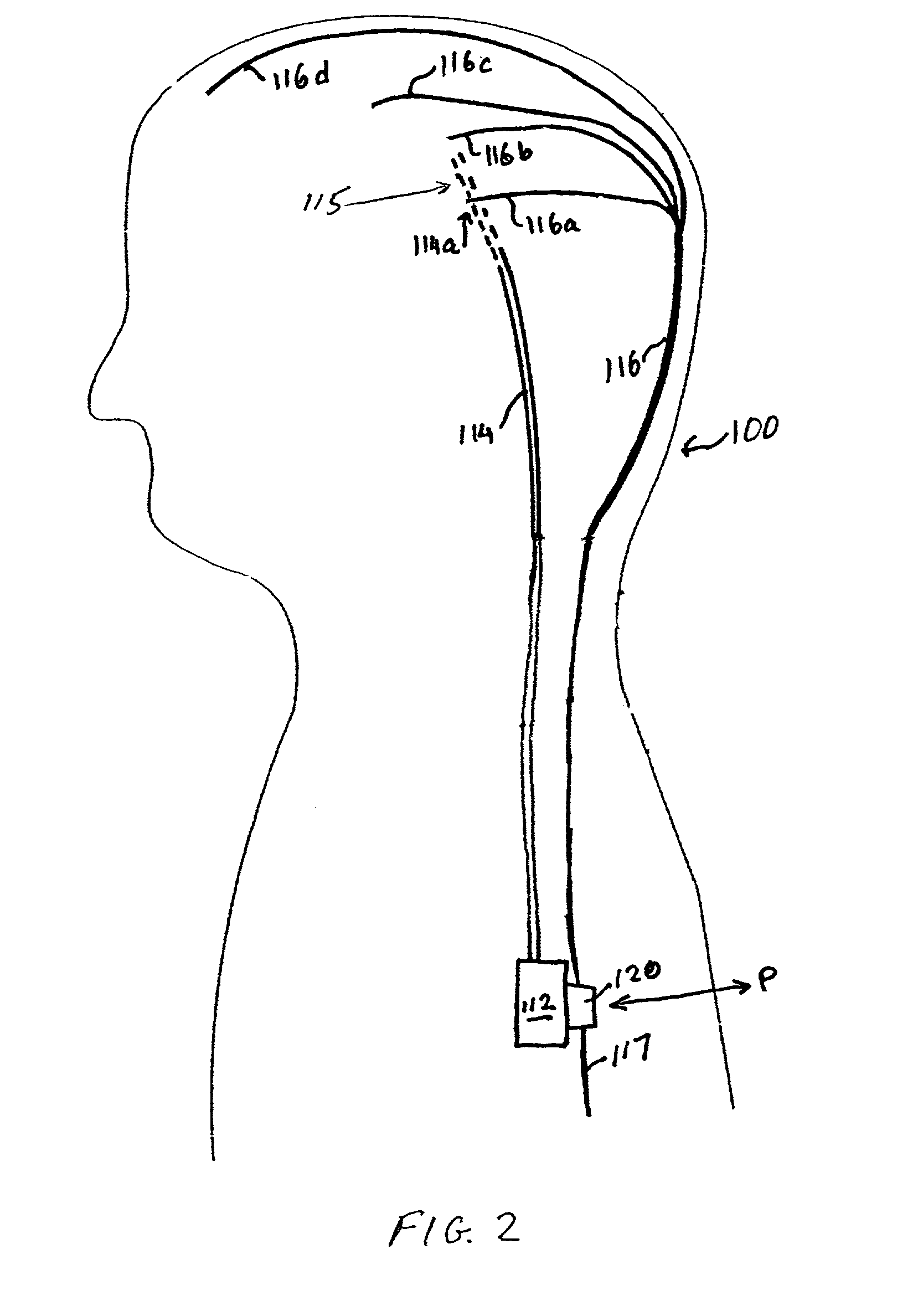
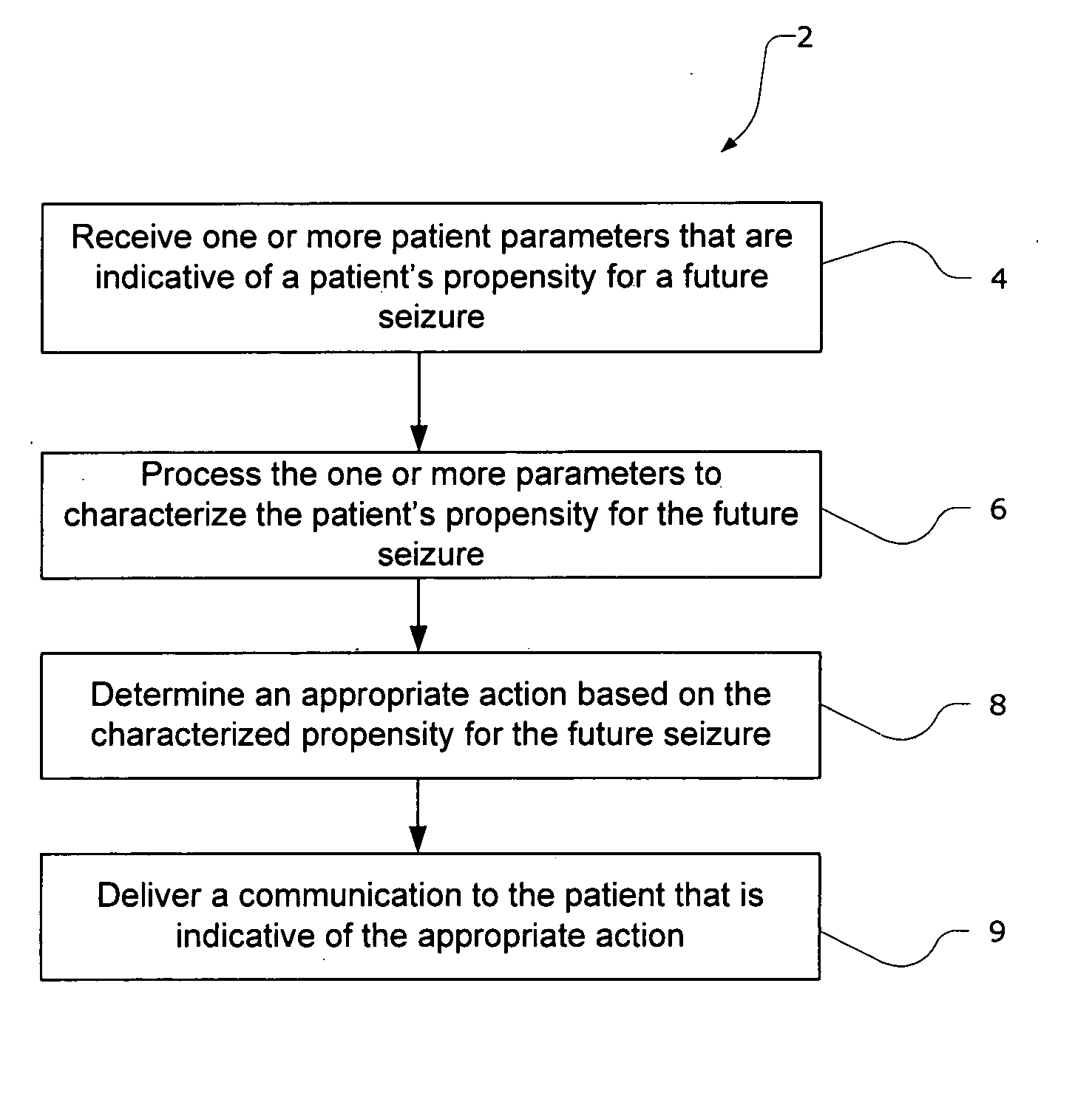
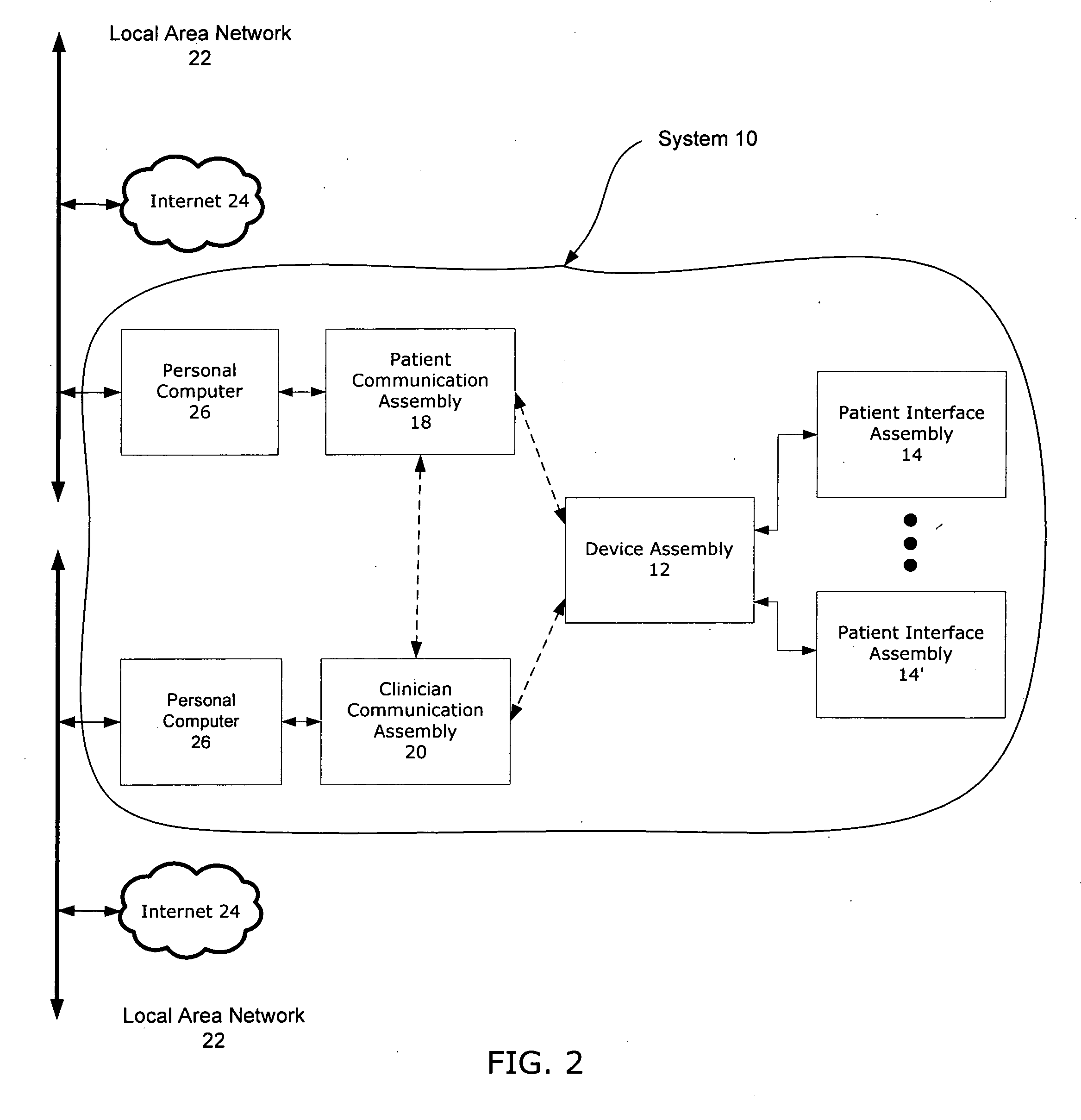
Closed-loop drug delivery system
InactiveUS7108680B2Safety and well be enhanceAdvantageousMedical devicesPressure infusionNervous systemImplantable Pump
A delivery system for a drug or bioactive agent includes an implantable pump and a delivery conduit that may be implanted in an organ or other tissue (e.g., the central or the peripheral nervous system) of a subject. A sensor is also implanted, and a controller unit receives the sensor output and directs drug delivery from within the patient pump accordingly. The sensor directly measures a primary biochemical material or state in the tissue or organ system, and the monitoring unit effects closed loop feedback control of the pump to achieve a desired end. The end may be the regulation of metabolism or maintenance of a stable metabolic or other state, or may be treatment regimen, e.g., by delivery of a dosage level or distribution of a drug in specific brain or nervous an system tissue. The sensed material may be the agent itself, a metabolite, or a related native material, tissue state or condition.
Owner:CODMAN & SHURTLEFF INC
Novel dosage form
ActiveUS20060024365A1Effectively control release rateReduce sizePill deliveryEster active ingredientsHigh dosesSolubility
A dosage form comprising of a high dose, high solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of modified release active ingredient per unit is from 500 mg to 1500 mg; a process for preparing the dosage form.
Owner:TORRENT PHARMA LTD
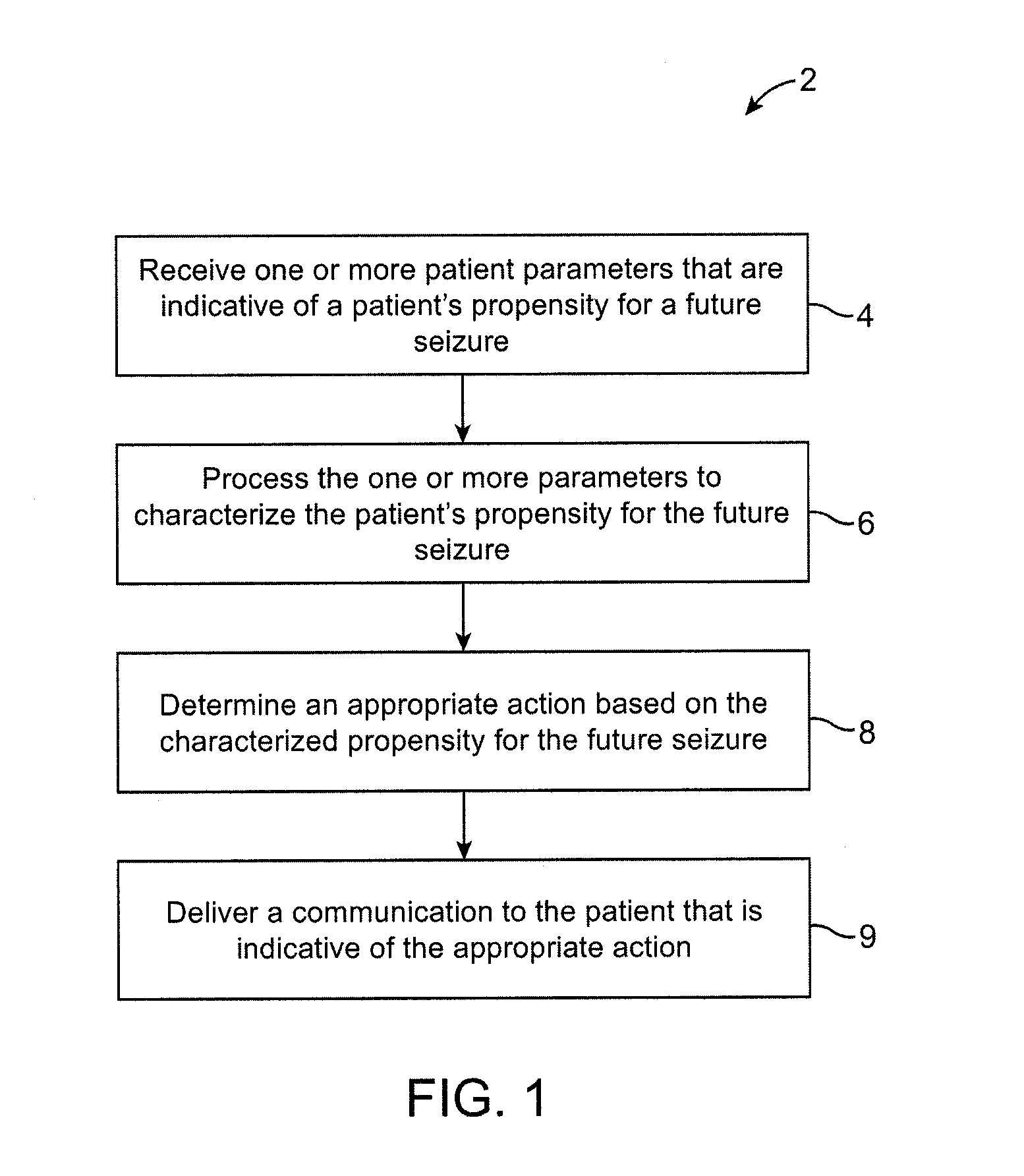
Methods and systems for recommending an appropriate action to a patient for managing epilepsy and other neurological disorders
ActiveUS20070150024A1Low profileIncrease probabilityElectroencephalographyPhysical therapies and activitiesNeurological disorderEpileptic seizure
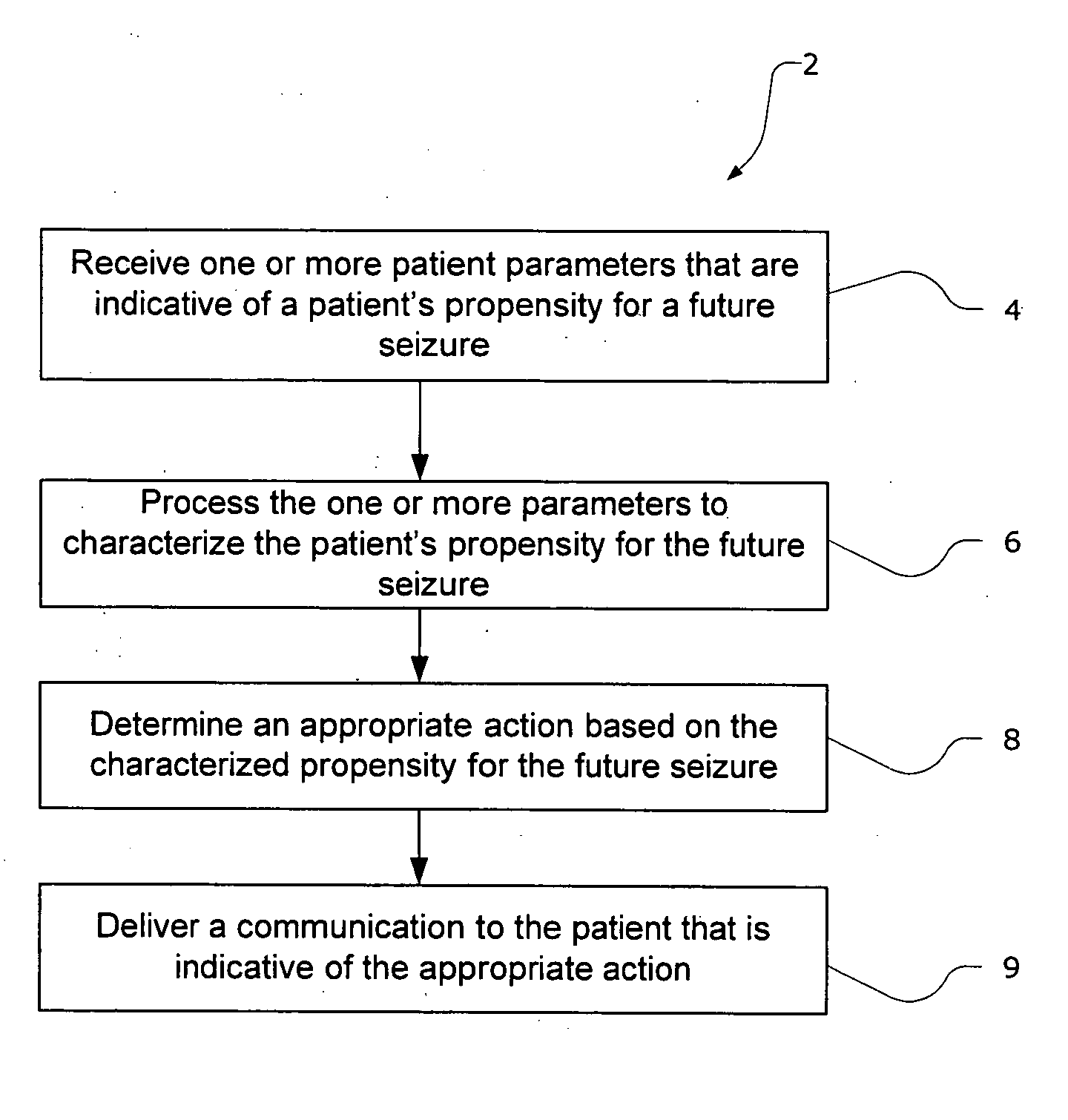
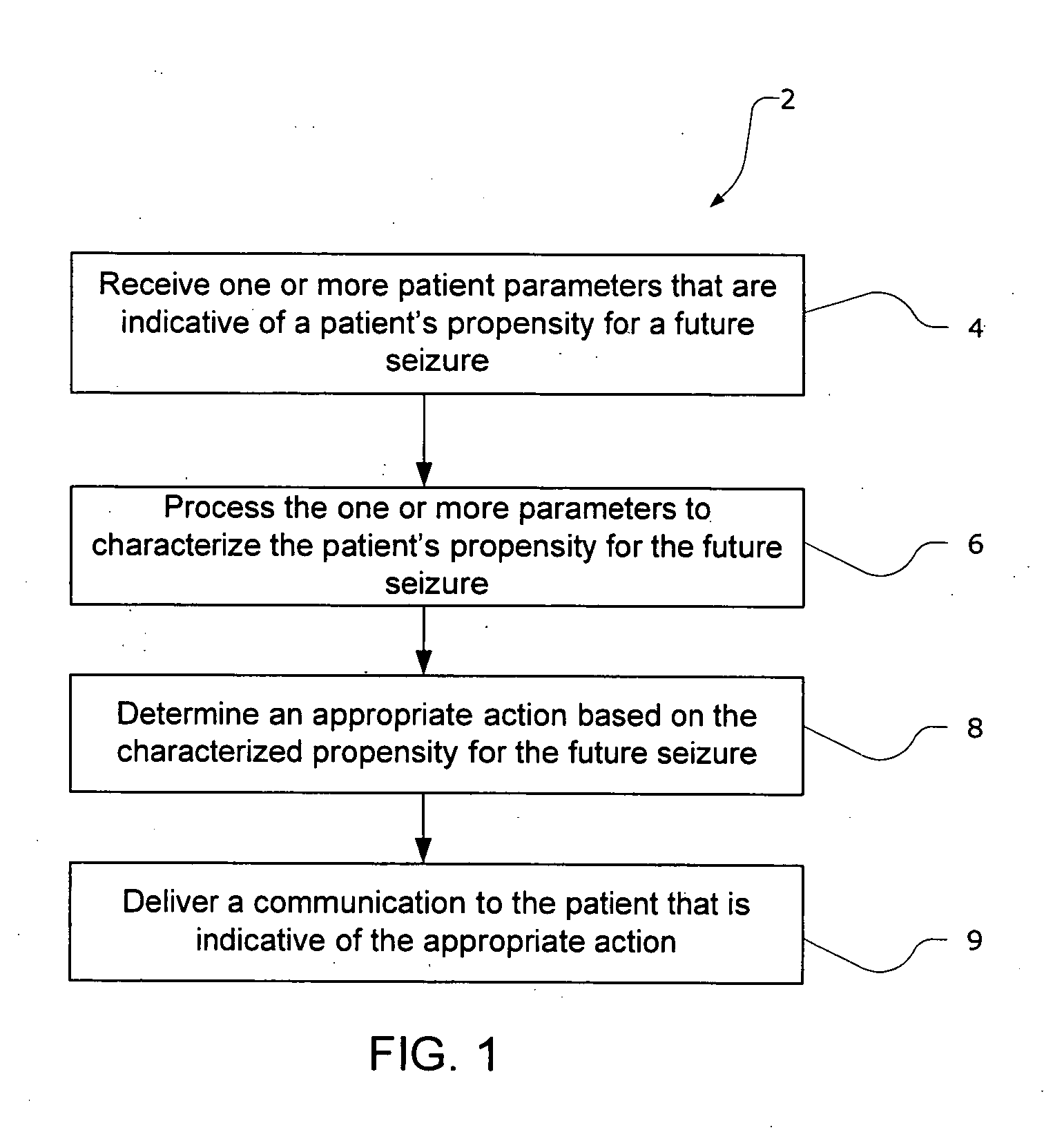
The present invention provides methods and system for managing neurological disorders such as epilepsy. In one embodiment, the method comprises measuring one or more signals from a patient and processing the one or more signals to characterize a patient's propensity for a future seizure. The characterized propensity for the seizure is thereafter used to determine an appropriate action for managing or treating the predicted seizure; and a recommendation is communicated to the patient that is indicative of the appropriate action.
Owner:CYBERONICS INC
Methods and systems for recommending an appropriate pharmacological treatment to a patient for managing epilepsy and other neurological disorders
ActiveUS20070150025A1Avoid it happening againReduced magnitudePhysical therapies and activitiesElectroencephalographyEpileptic disorderEpileptic seizure
The present invention provides systems and methods for managing epilepsy. In one embodiment, a method of the present invention characterize a patient's propensity for a future epileptic seizure and communicates to the patient and / or a health care provider a therapy recommendation. The therapy recommendation is typically a function of the patient's propensity for the future epileptic seizure.
Owner:CYBERONICS INC
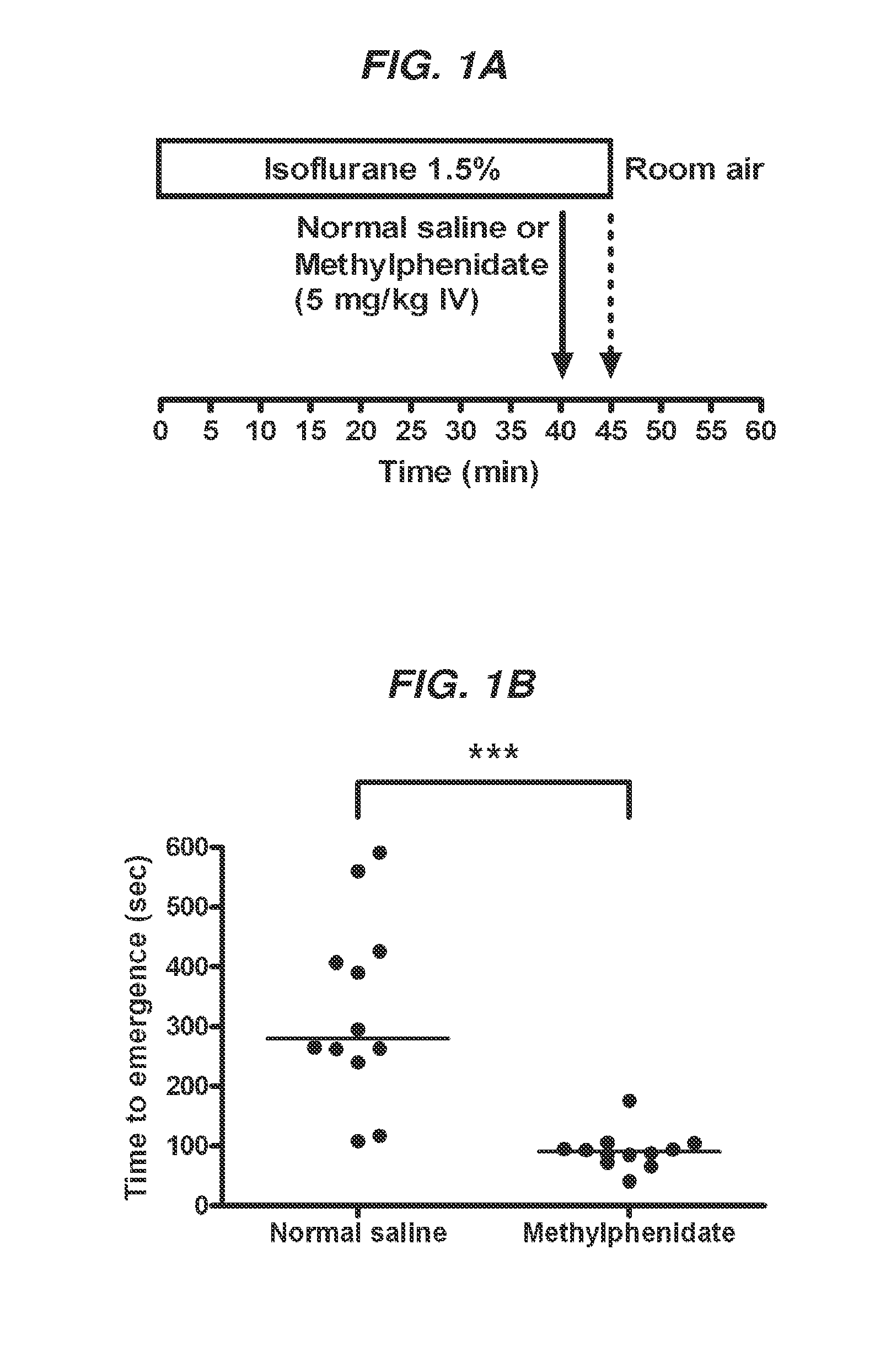
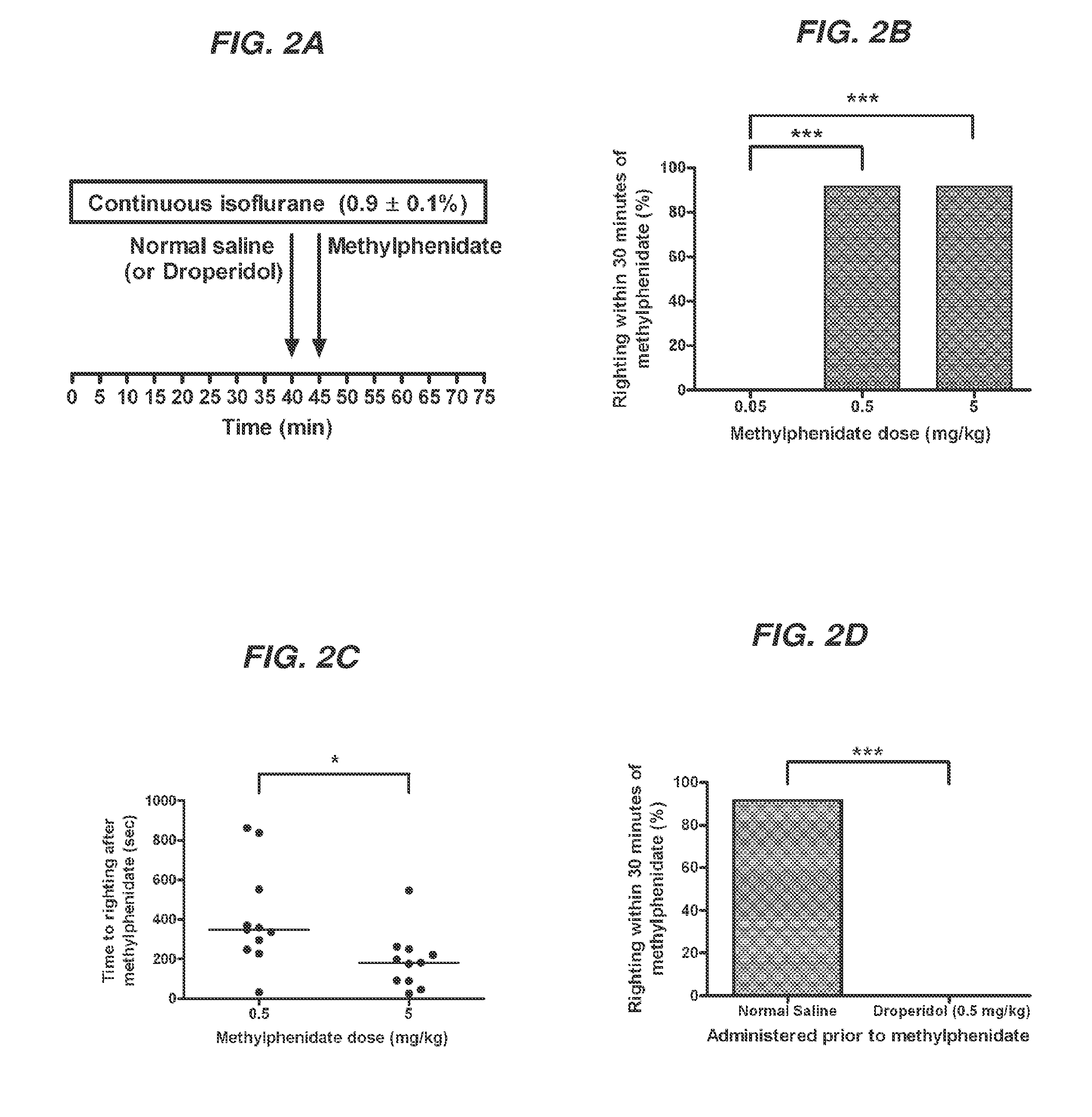
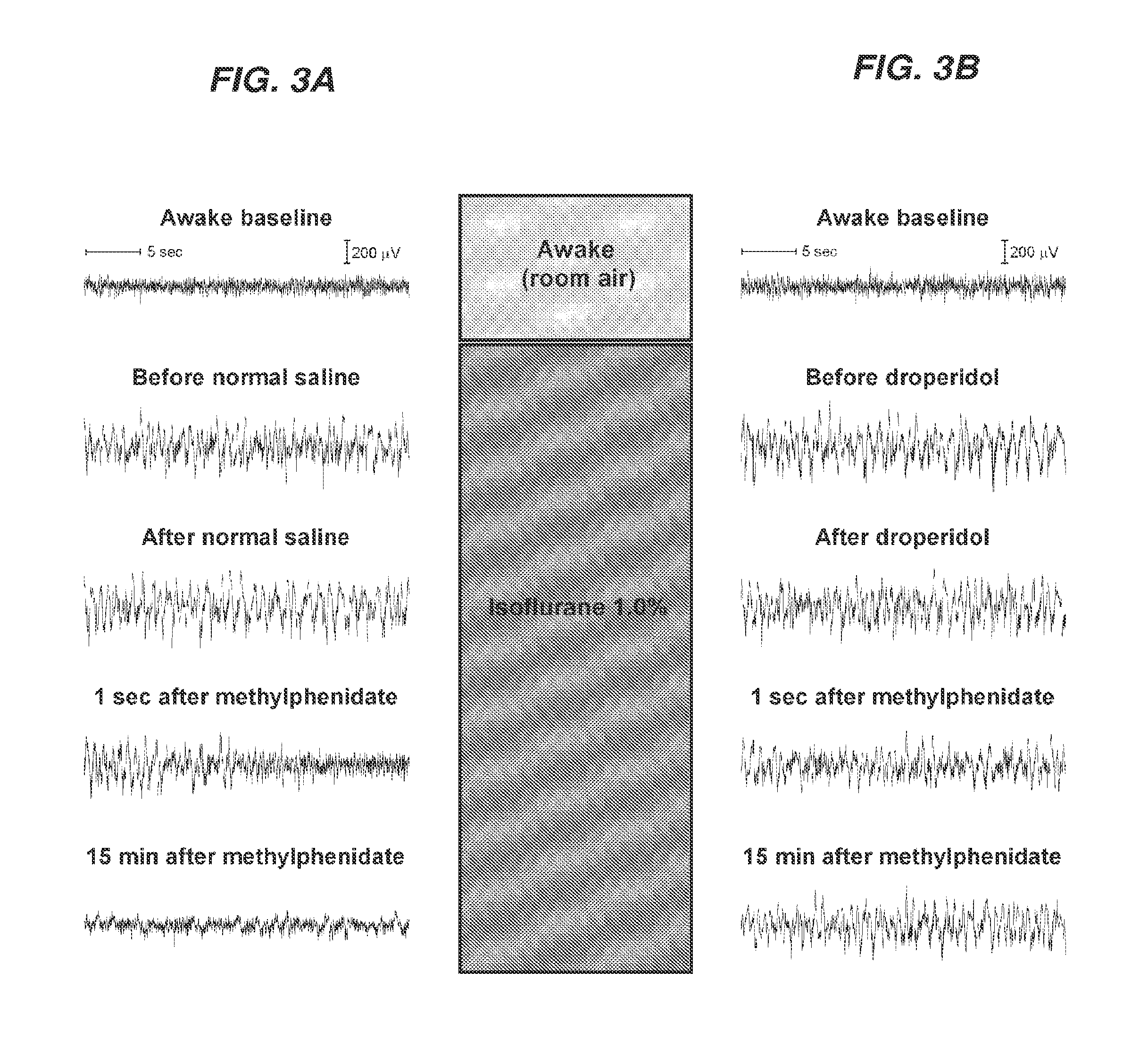
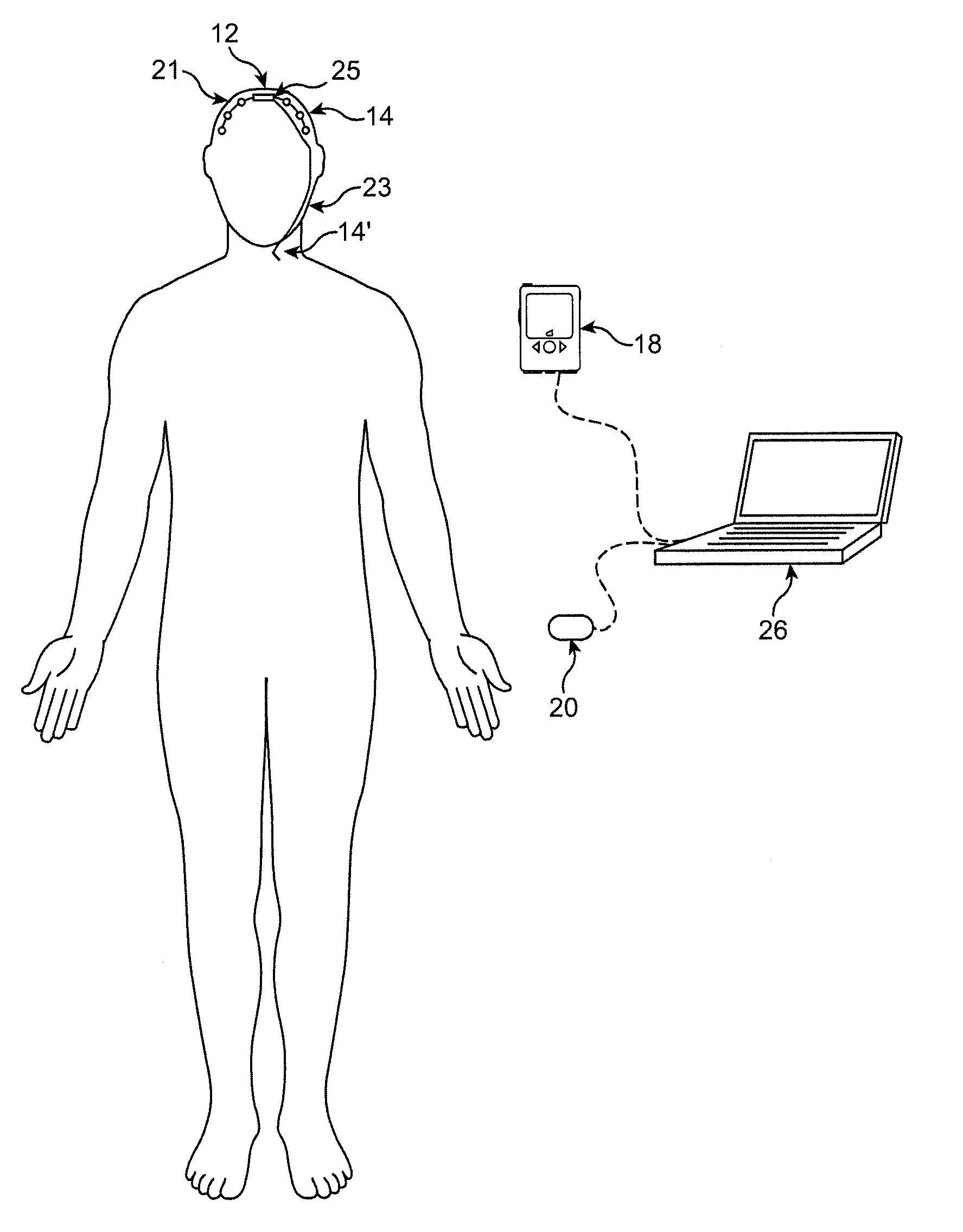
Reversal of General Anesthesia by Administration of Methylphenidate, Amphetamine, Modafinil, Amantadine, and/or Caffeine
ActiveUS20150196249A1High speedReduces and eliminates effectElectroencephalographyPharmaceutical delivery mechanismUnconsciousnessWhole body
Owner:THE GENERAL HOSPITAL CORP
Methods and systems for administering an appropriate pharmacological treatment to a patient for managing epilepsy and other neurological disorders
InactiveUS20070287931A1Reduced magnitudeShorten the durationElectroencephalographyBiocidePharmacometricsNeurological disorder
The present invention provides systems and methods for managing epilepsy. In one embodiment, a method of the present invention characterize a patient's propensity for a future epileptic seizure and facilitates administration of a pharmacological agent. The dosage of the pharmacological agent is typically a function of at least one of the patient's propensity for the future epileptic seizure and time period to seizure.
Owner:CYBERONICS INC
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
Inhaler
InactiveUS6880555B1Lifting efficiencyGood effectRespiratorsLiquid surface applicatorsInhalationMedicine
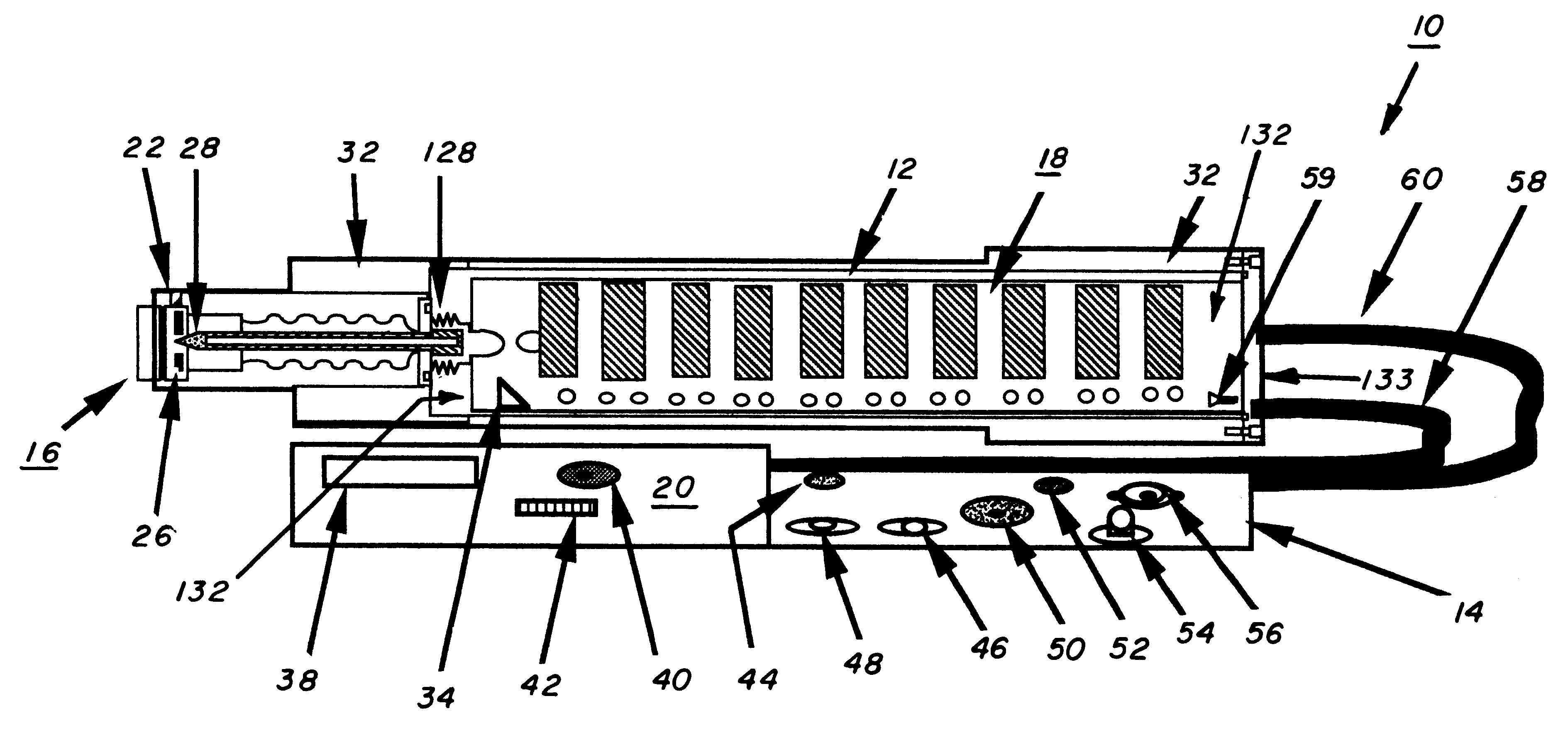
An inhaler for medicament in powder form with an opening intended for inhalation. The powder medicament is arranged in the inhaler in a number of enclosures, each enclosure including a specific dose of medicament. A member is provided for enabling access to the dose of medicament. The member is arranged and designed such that it is able to be inserted inside the enclosure and establish at least one outlet passage, between the interior of the enclosure and the inhalation opening, through which outlet passage the medicament is delivered to the patient upon inhalation.
Owner:SHL MEDICAL AG
Methods for implementing microbeam radiation therapy
InactiveUS7194063B2Reduce harmEasily damagedIrradiation devicesX-ray/gamma-ray/particle-irradiation therapyLight beamGadolinium
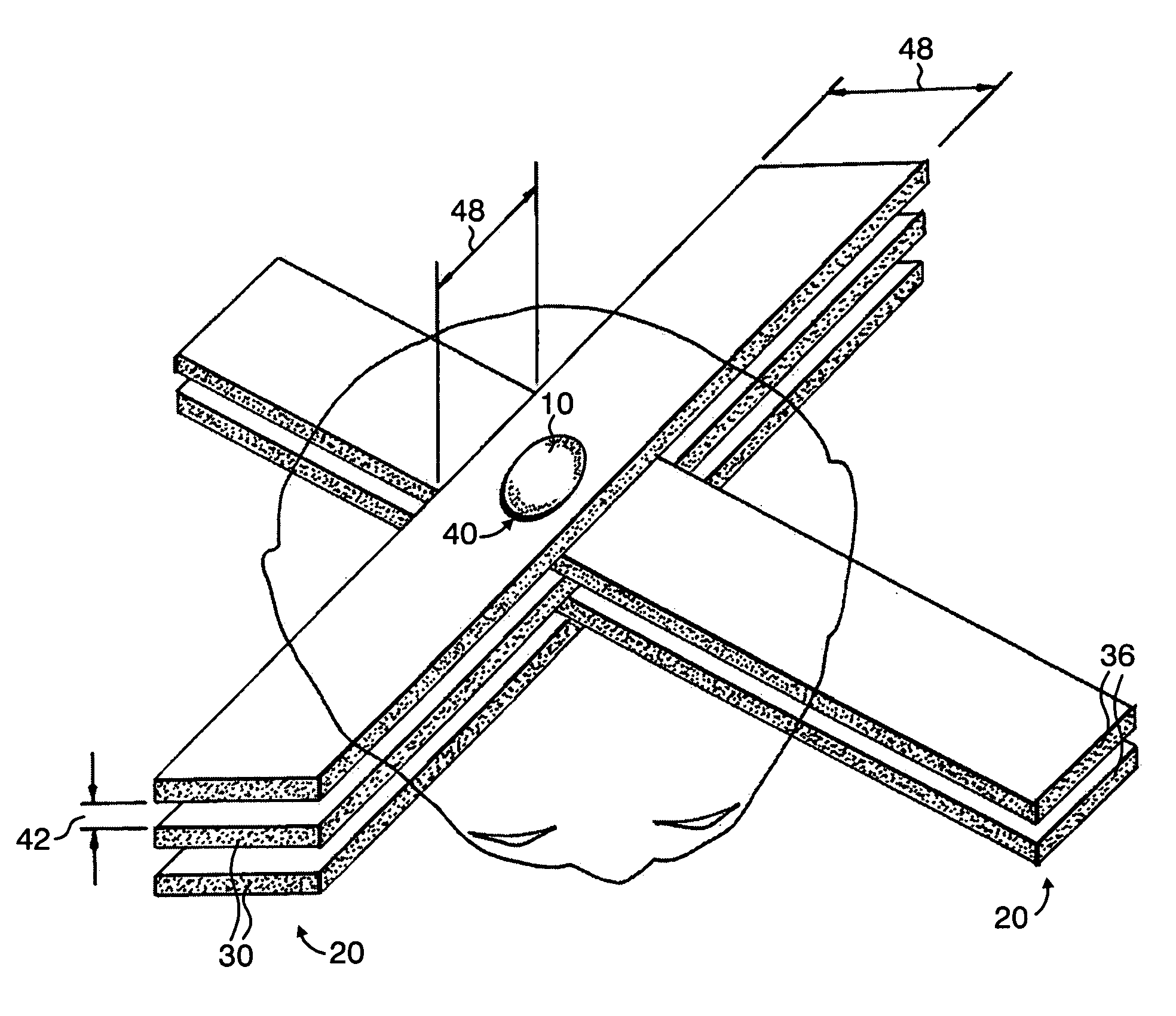
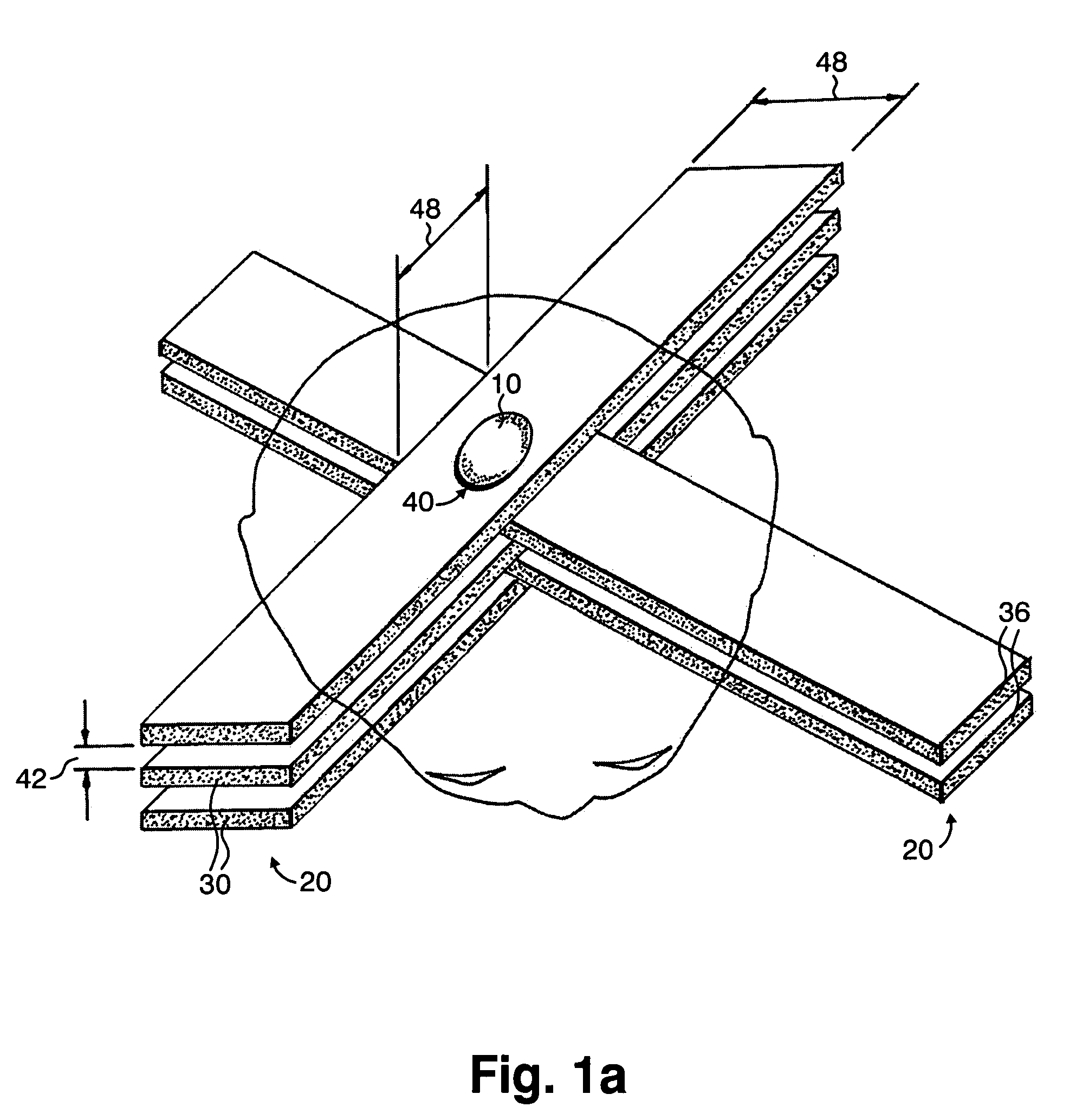
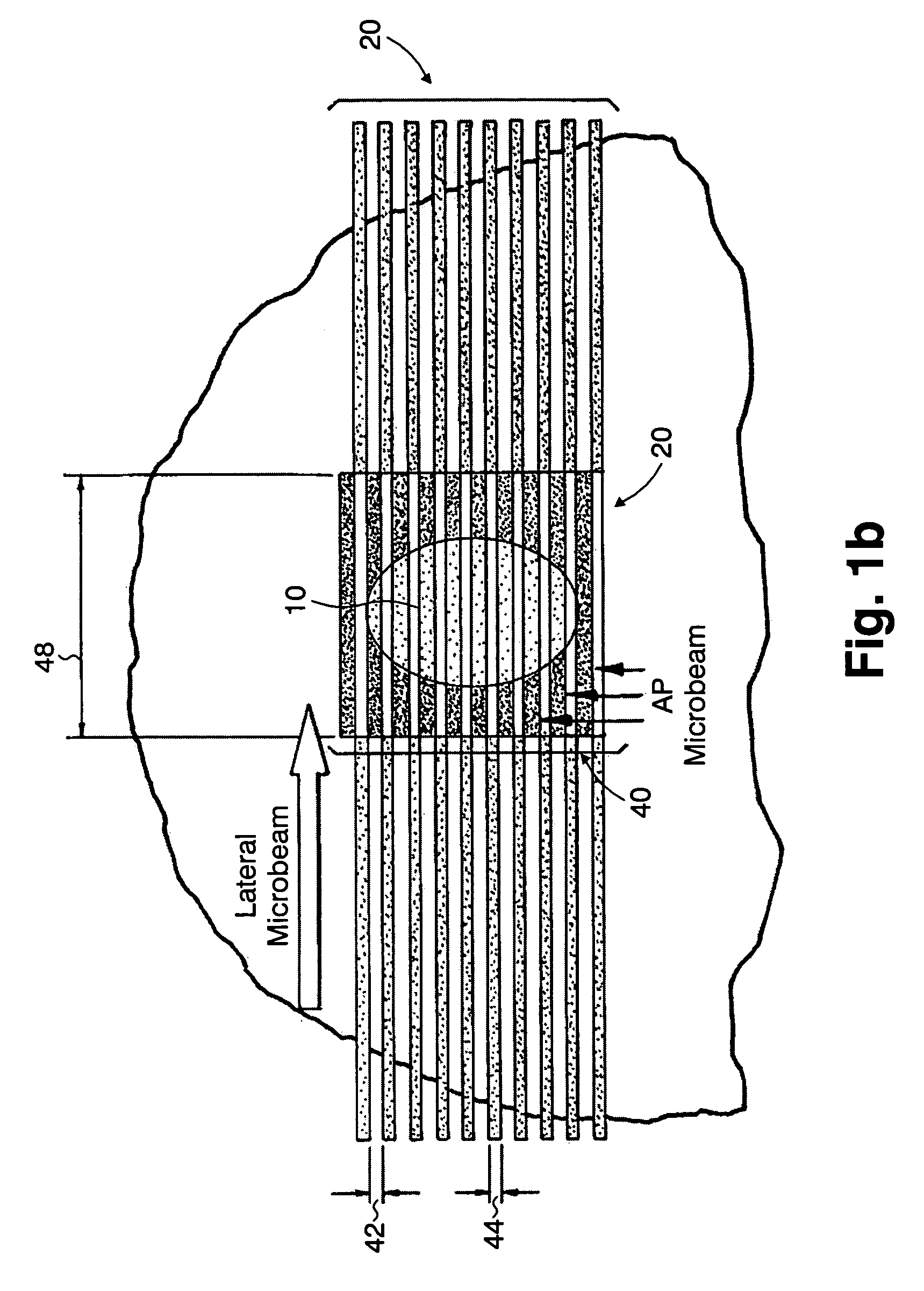
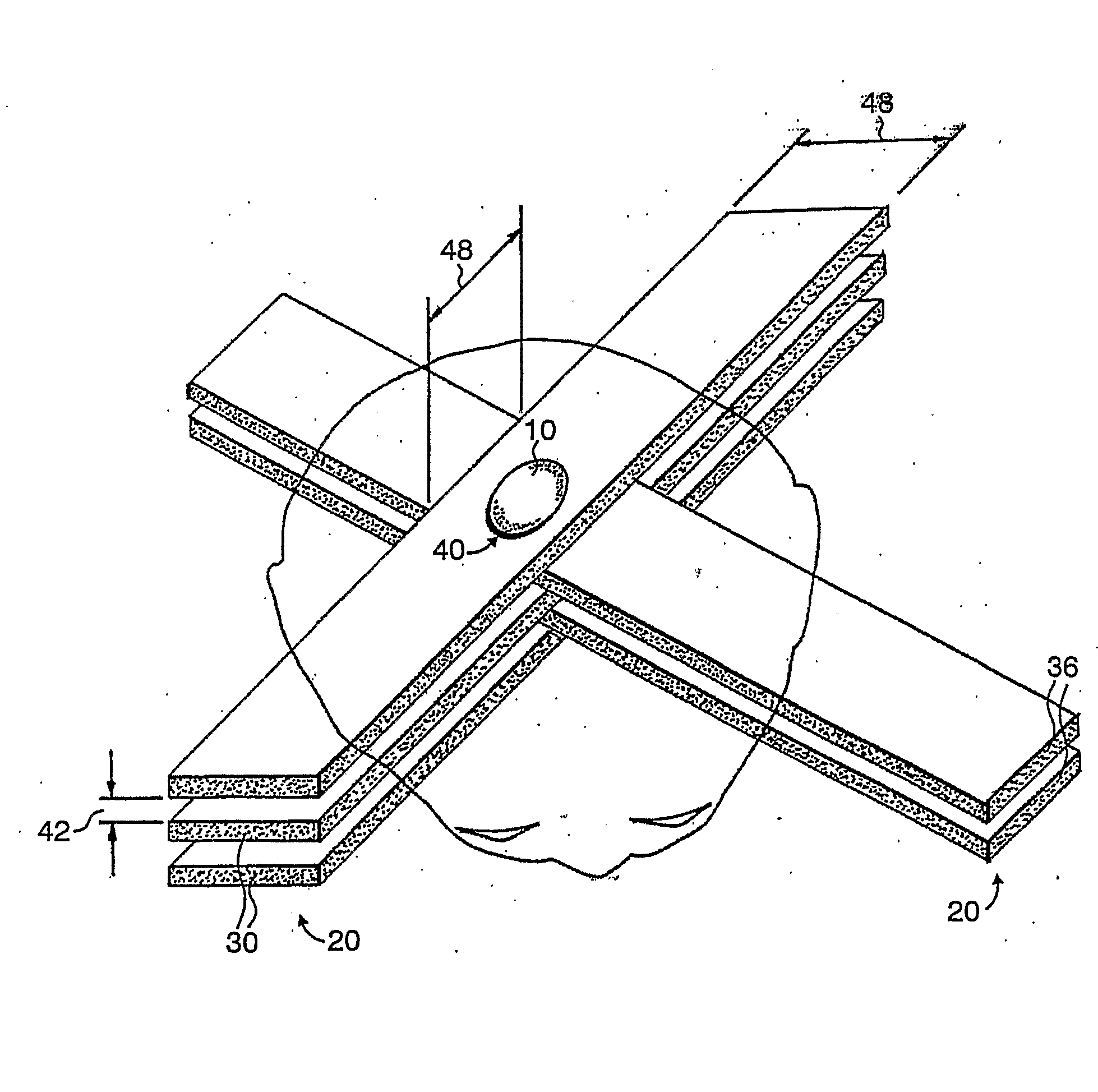
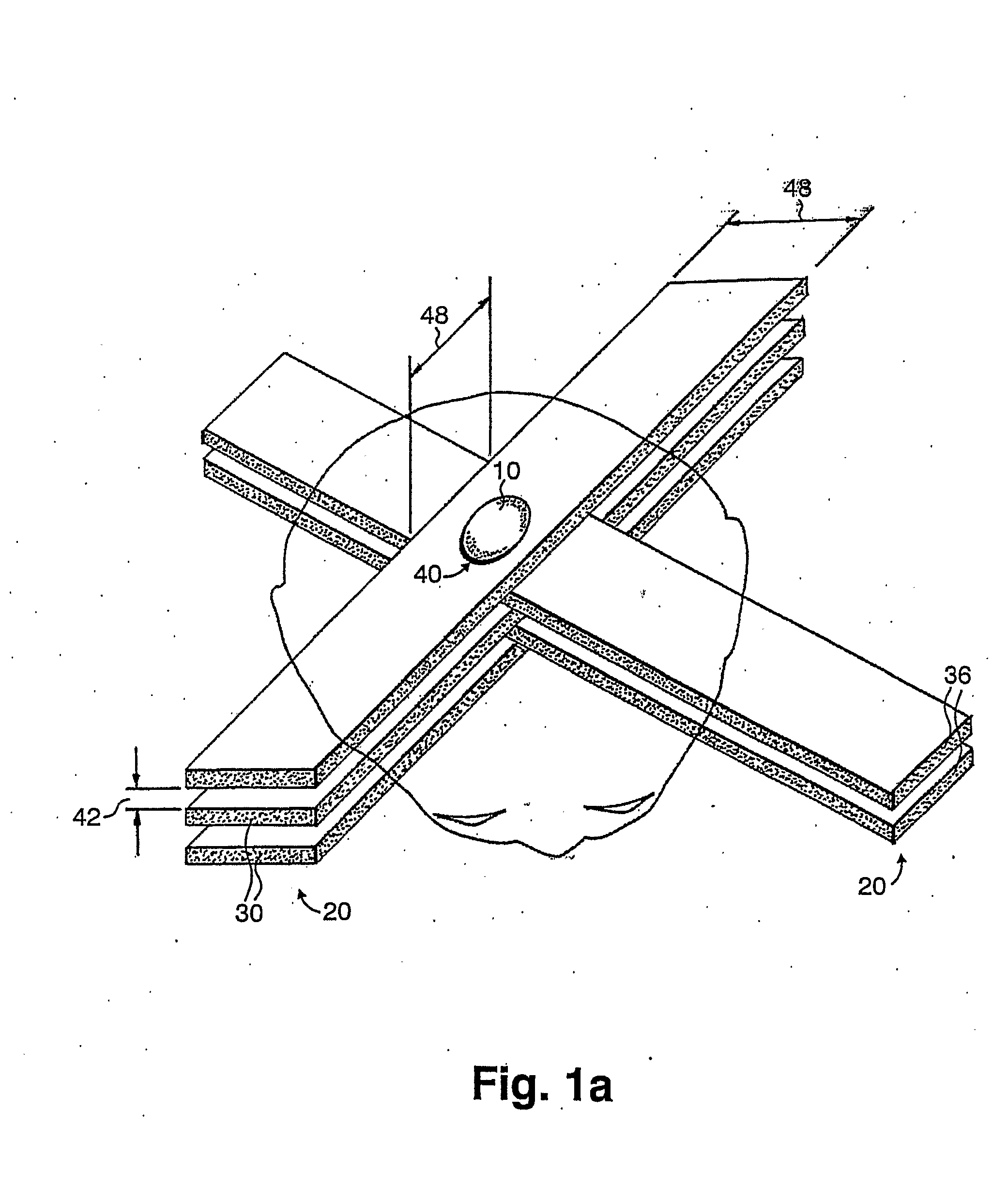
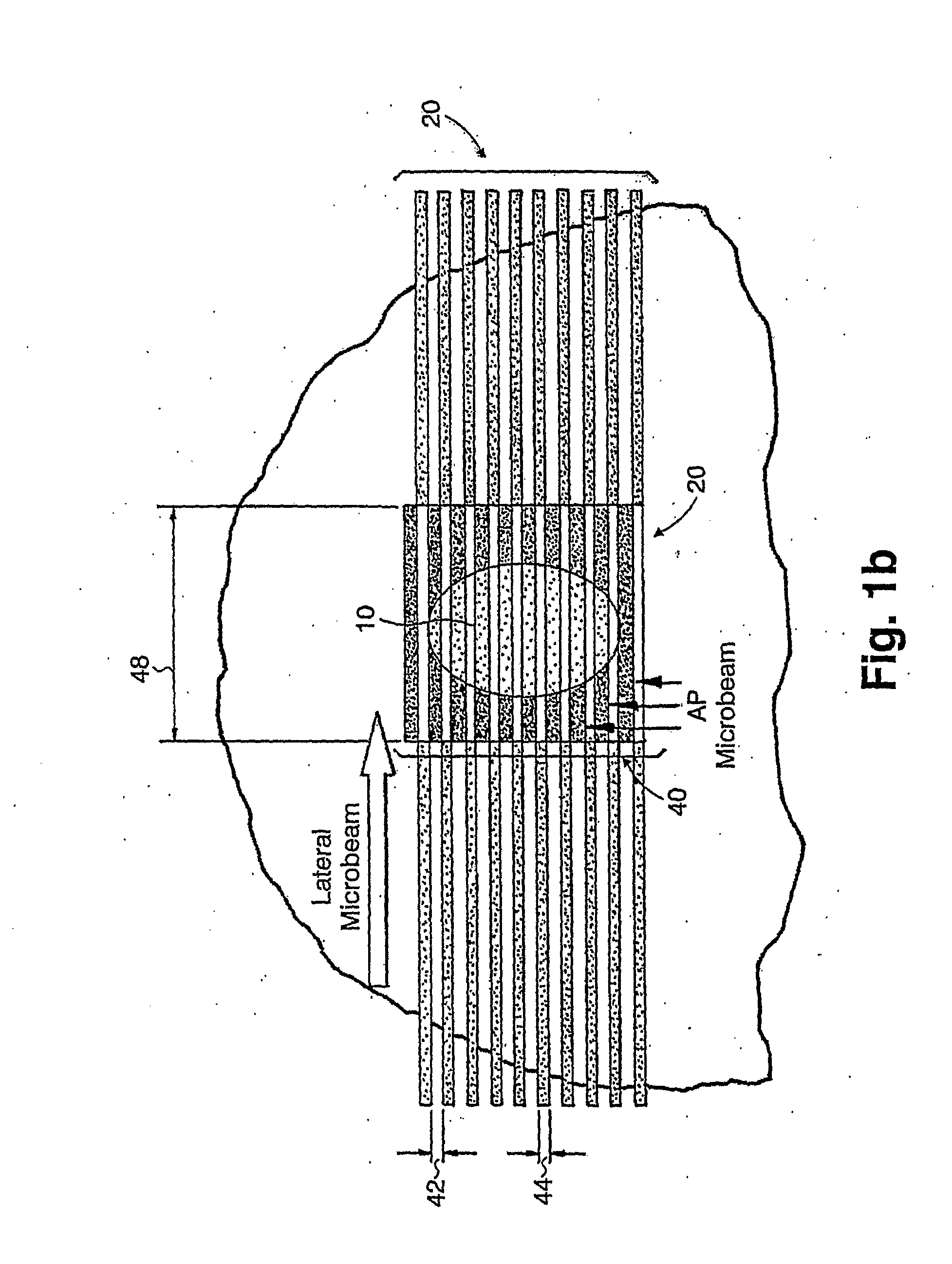
A method of performing radiation therapy includes delivering a therapeutic dose such as X-ray only to a target (e.g., tumor) with continuous broad beam (or in-effect continuous) using arrays of parallel planes of radiation (microbeams / microplanar beams). Microbeams spare normal tissues, and when interlaced at a tumor, form a broad-beam for tumor ablation. Bidirectional interlaced microbeam radiation therapy (BIMRT) uses two orthogonal arrays with inter-beam spacing equal to beam thickness. Multidirectional interlaced MRT (MIMRT) includes irradiations of arrays from several angles, which interleave at the target. Contrast agents, such as tungsten and gold, are administered to preferentially increase the target dose relative to the dose in normal tissue. Lighter elements, such as iodine and gadolinium, are used as scattering agents in conjunction with non-interleaving geometries of array(s) (e.g., unidirectional or cross-fired (intersecting) to generate a broad beam effect only within the target by preferentially increasing the valley dose within the tumor.
Owner:BROOKHAVEN SCI ASSOCS
Container inspection apparatus
InactiveUS6563903B2Easy to disassembleEasy to moveUsing wave/particle radiation meansMaterial analysis by transmitting radiationContinuous scanningModular unit
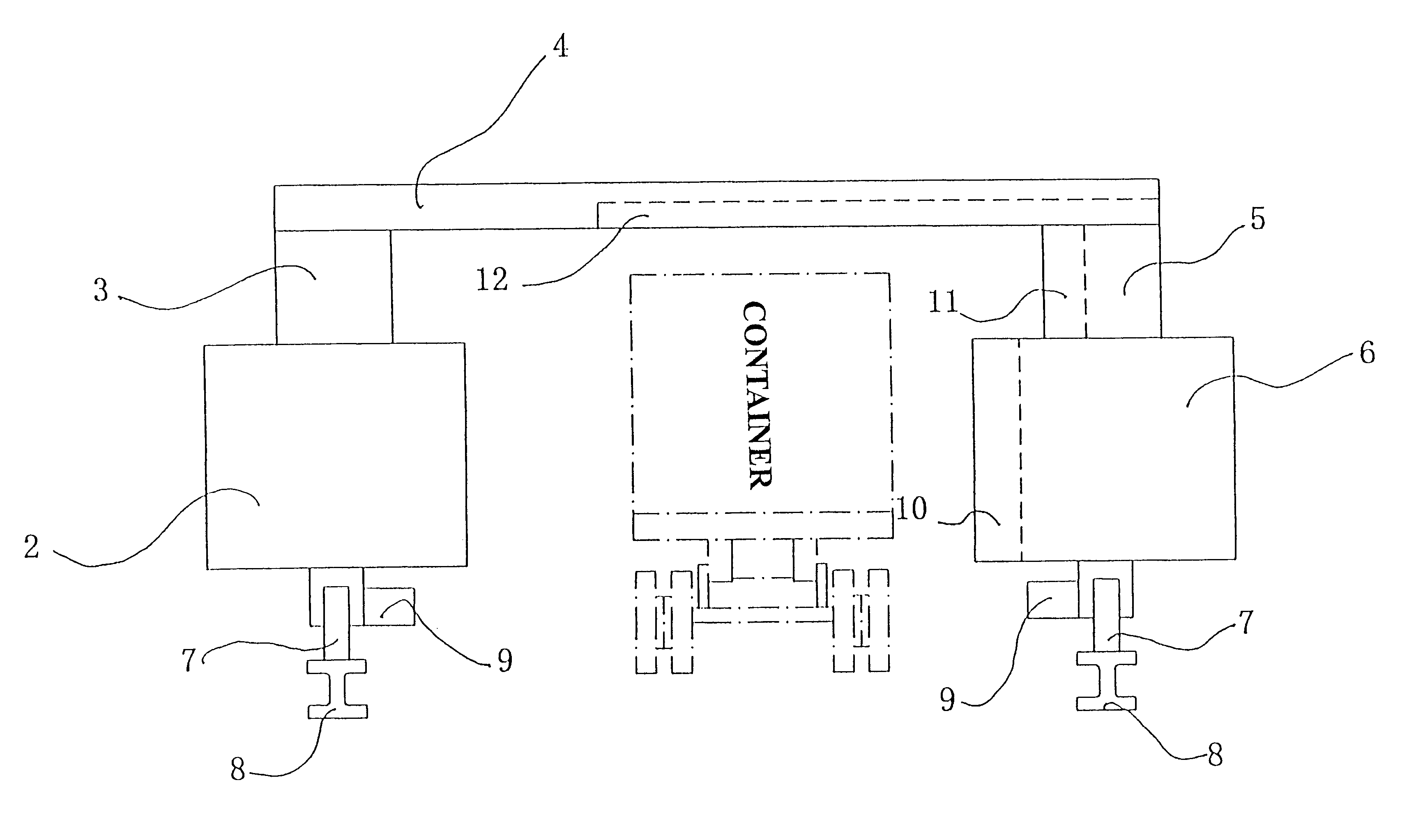
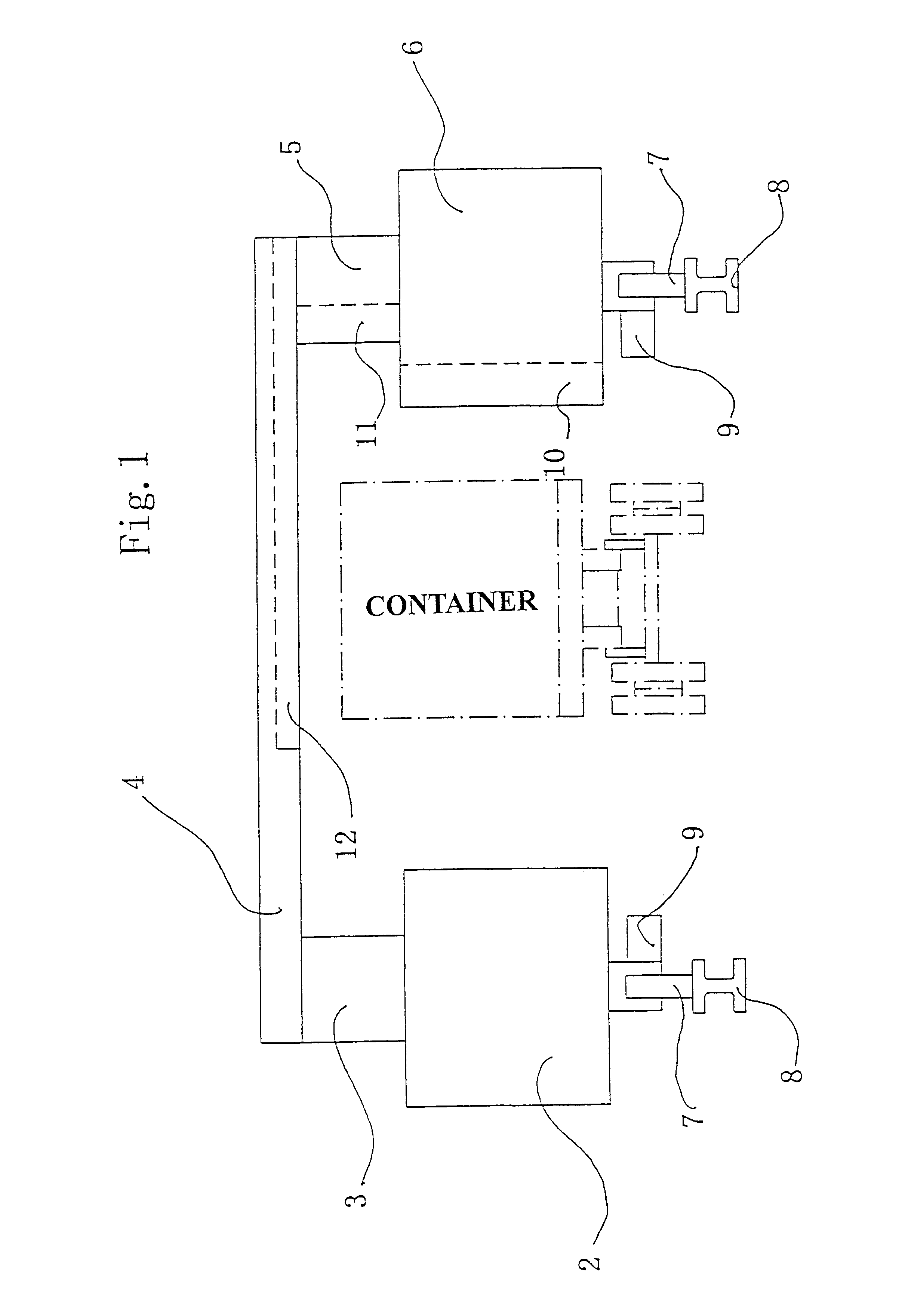
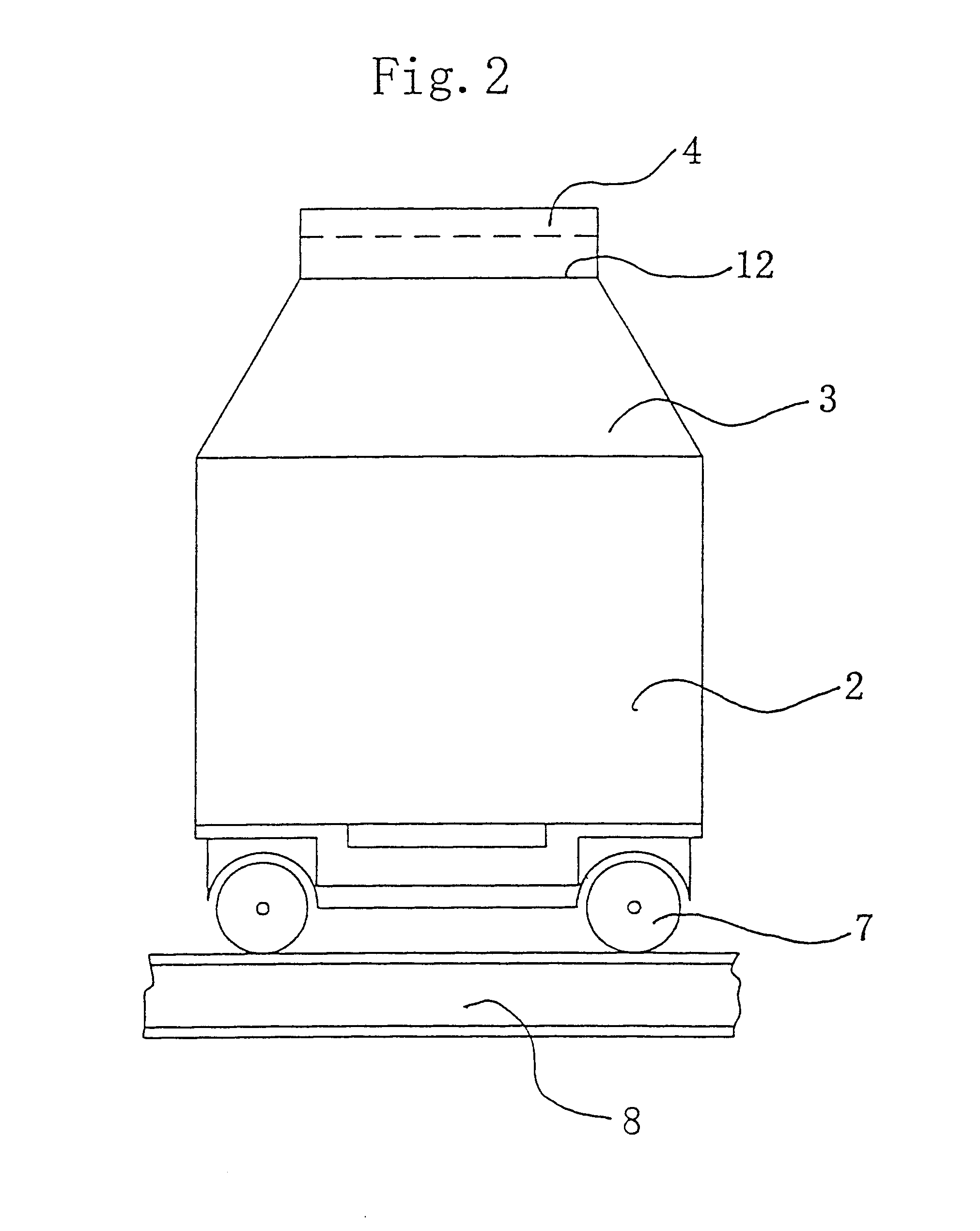
A relocatable container inspection device, which is constituted by modularized units, is used for inspecting a container. The device comprises: a first cabin unit including a radiation source for generating a beam of penetration radiation for penetrating the container to be inspected; a second cabin unit including detectors for detecting the beam of penetration radiation penetrating through the container being inspected and producing corresponding radiographic signals, the second cabin unit and the first cabin unit being arranged oppositely in both sides of an inspection passage; a gantry unit bridging the first cabin unit and the second cabin unit to form a portal-shaped frame adapted for straddling the container being inspected; means for moving the frame relative to the container being inspected along the inspection passage, so that the detectors receive the beam of the penetration radiation generated from the radiation resource and passing through the container being inspected, thereby continuously scanning the container and producing the corresponding radiographic signals, wherein the first cabin unit, the second cabin unit and the gantry unit are all individually made as modular units so as to be quickly and easily assembled and disassembled with each other on the inspection site. In the present invention, all of the modularized units of the container inspection device can be easily assembled and disassembled in the work field, and the dimensions of each modular unit are sized to be suitable for road transports and to be facilitate for the device's movement.
Owner:TSINGHUA UNIV +1
Screening methods to identify agents that selectively inhibit hepatitis C virus replication
InactiveUS6030785APrevent dimerizationBlock viral inhibitionFungiSsRNA viruses positive-senseCellular defenseViral infection
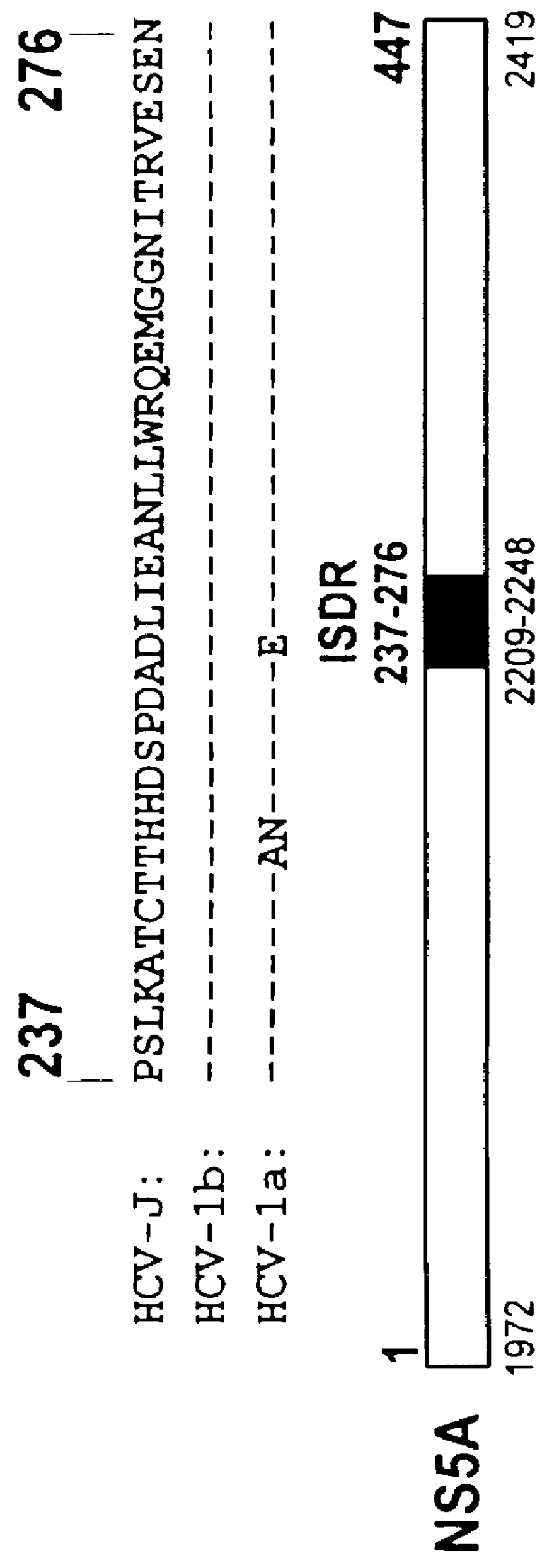
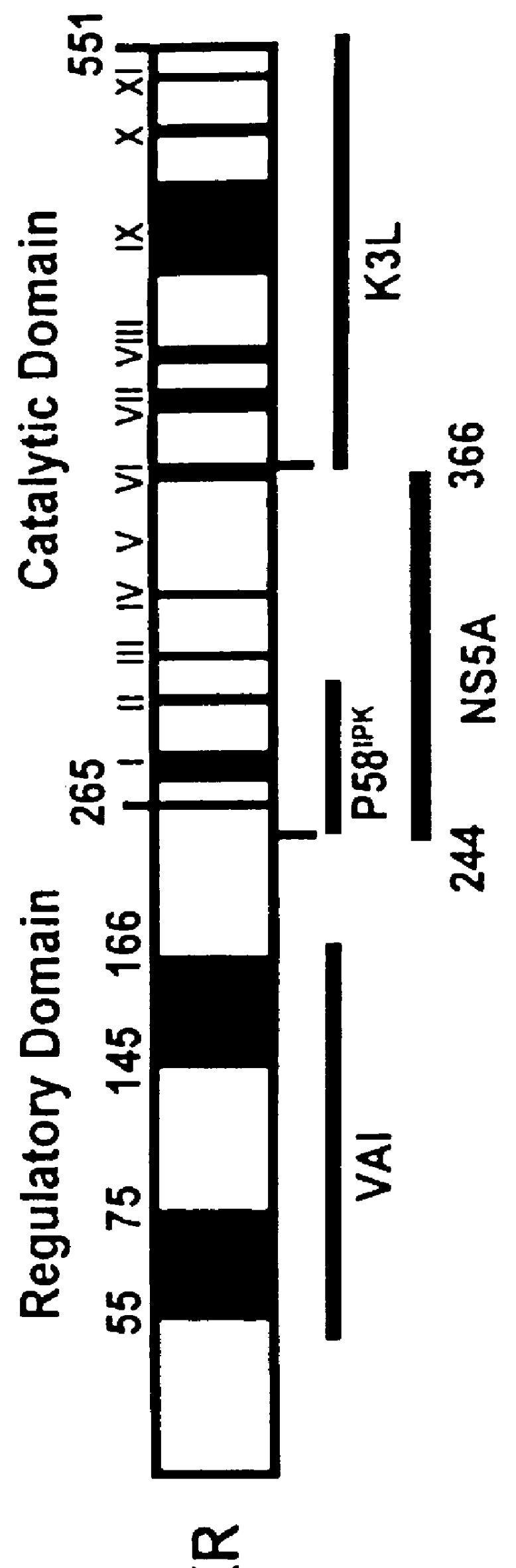
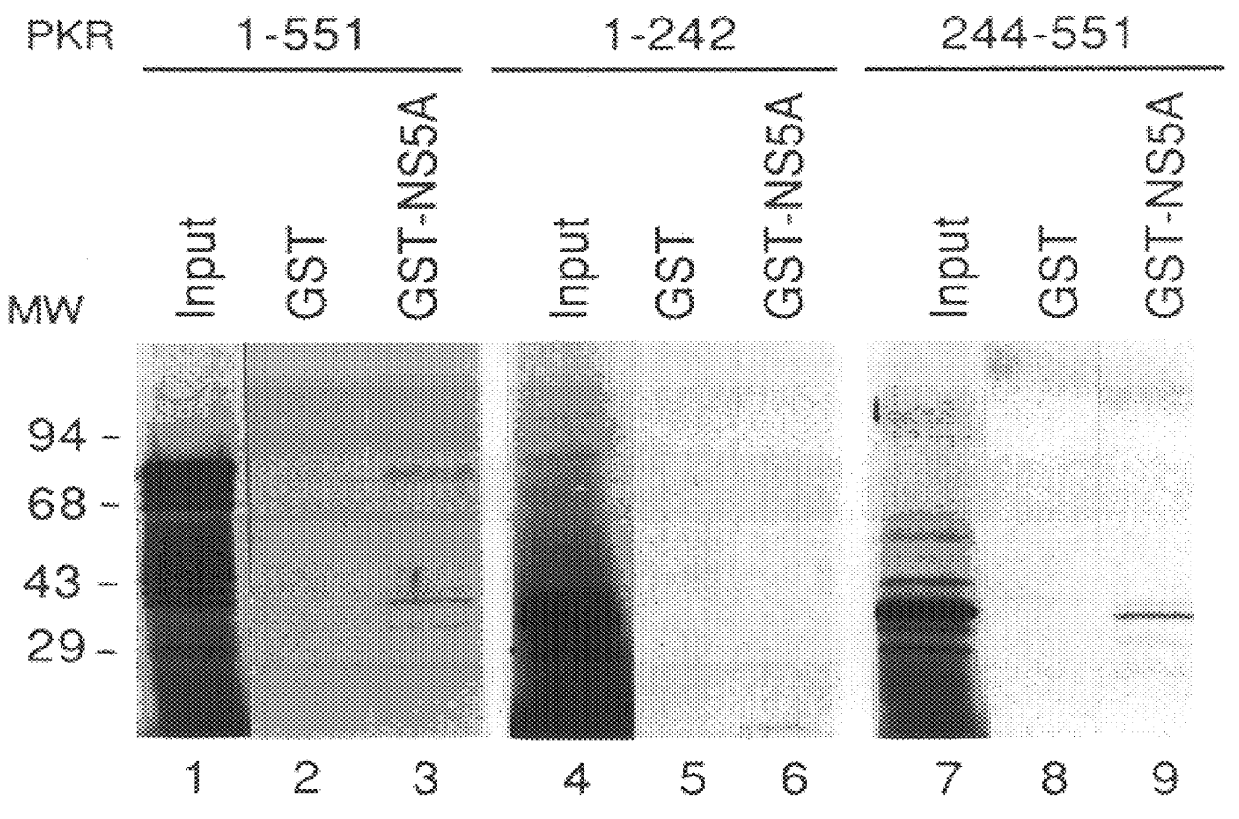
The present invention relates to novel methods for identifying antiviral agents which selectively interfere with viral proteins that override the interferon(IFN)-induced cellular defense mechanisms against viral infection. In particular, the present invention relates to screening assays that identify agents which selectively inhibit the interaction between viral proteins containing an interferon sensitivity determining region (ISDR) and IFN-induced PKR protein kinase. The present invention more particularly relates to screening assays that identify agents which selectively inhibit the interaction between hepatitis C virus (HCV) nonstructural 5A protein (NS5A), which contains an ISDR, and IFN-induced PKR protein kinase. The interaction between the viral ISDR and IFN-induced PKR protein kinase results in the override of IFN-induced cellular defense mechanisms to combat viral infection. Therefore the agents identified using the assays of the invention may have utility as antiviral agents.
Owner:UNIV OF WASHINGTON
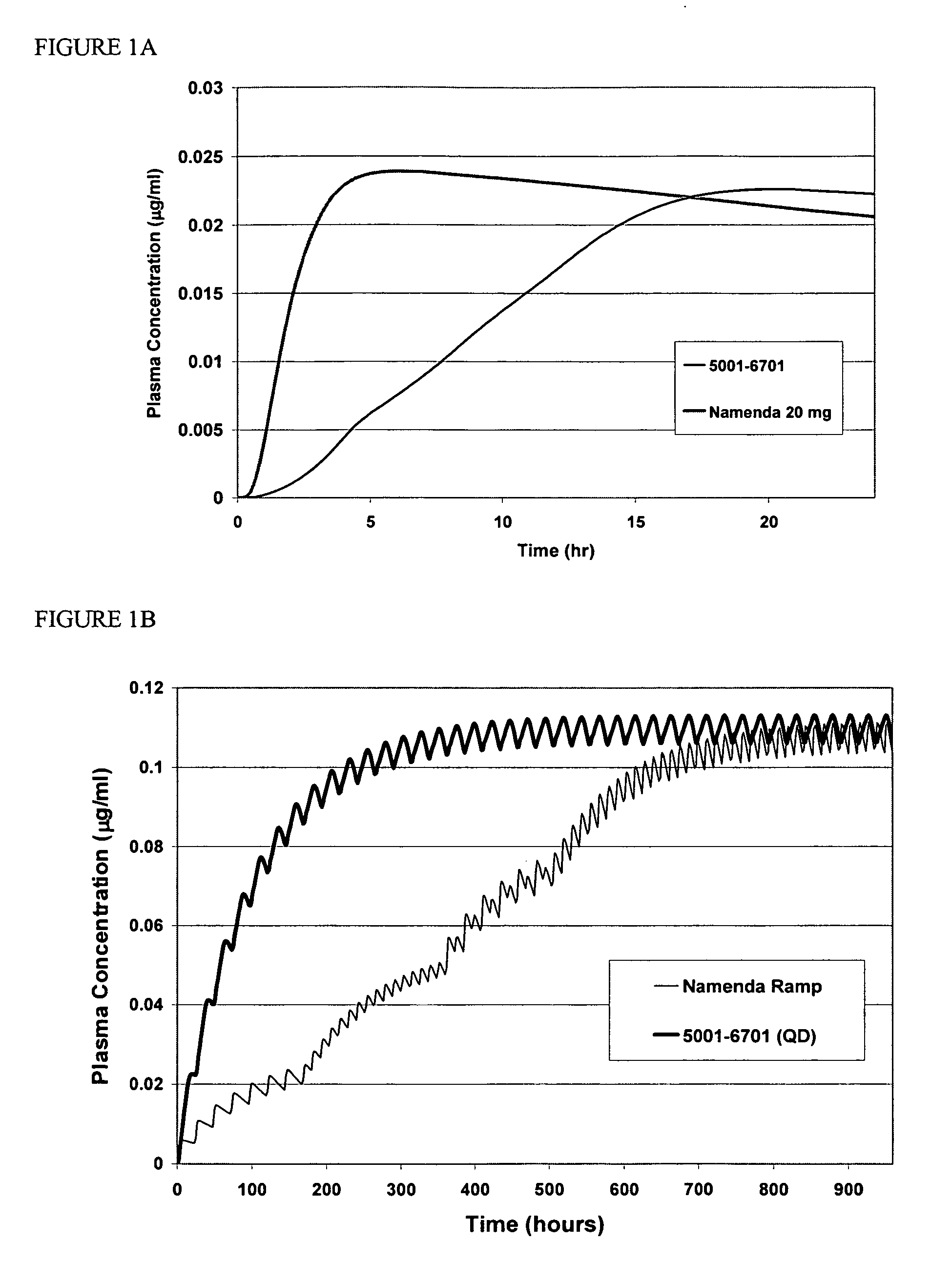
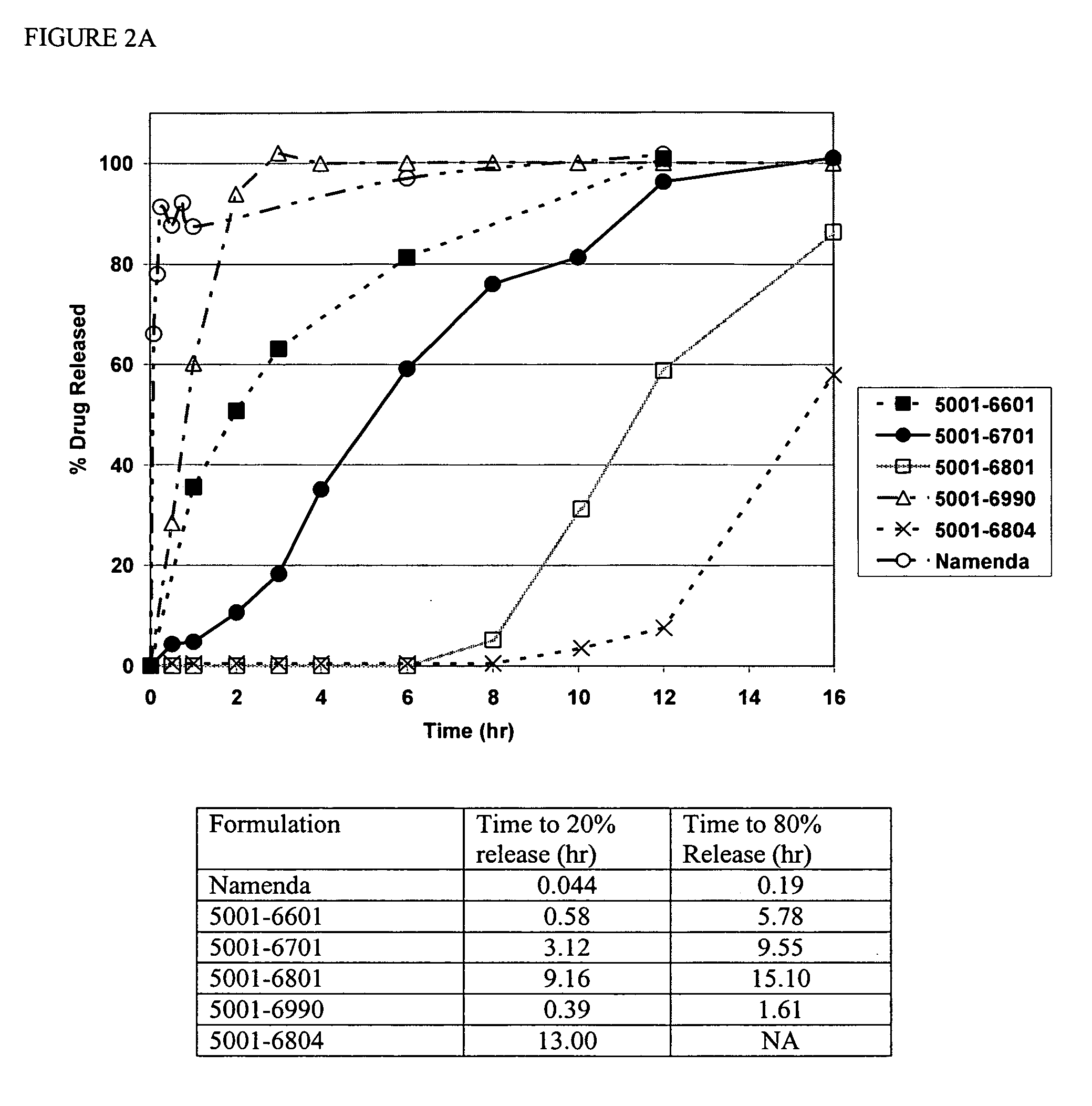
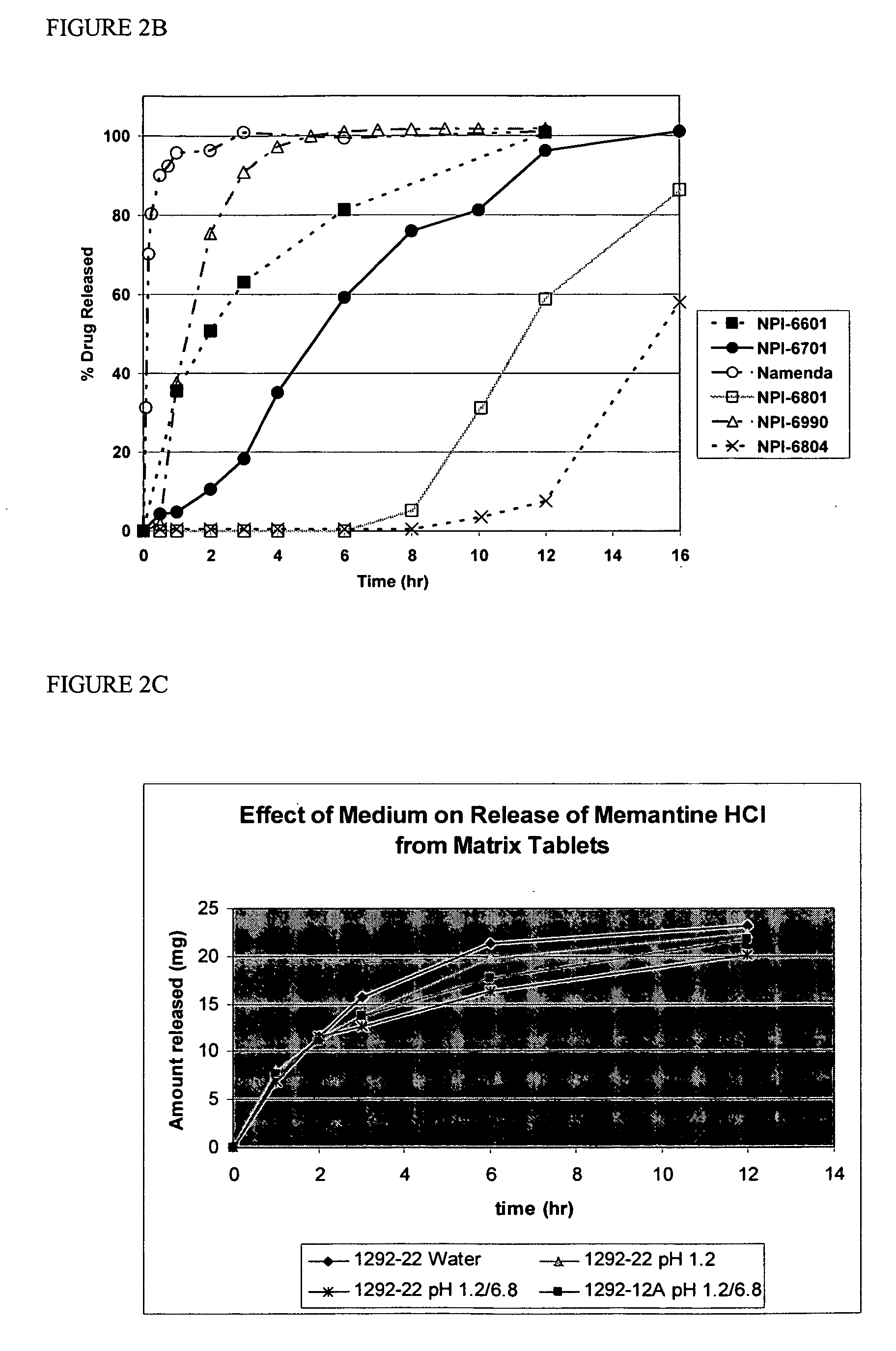
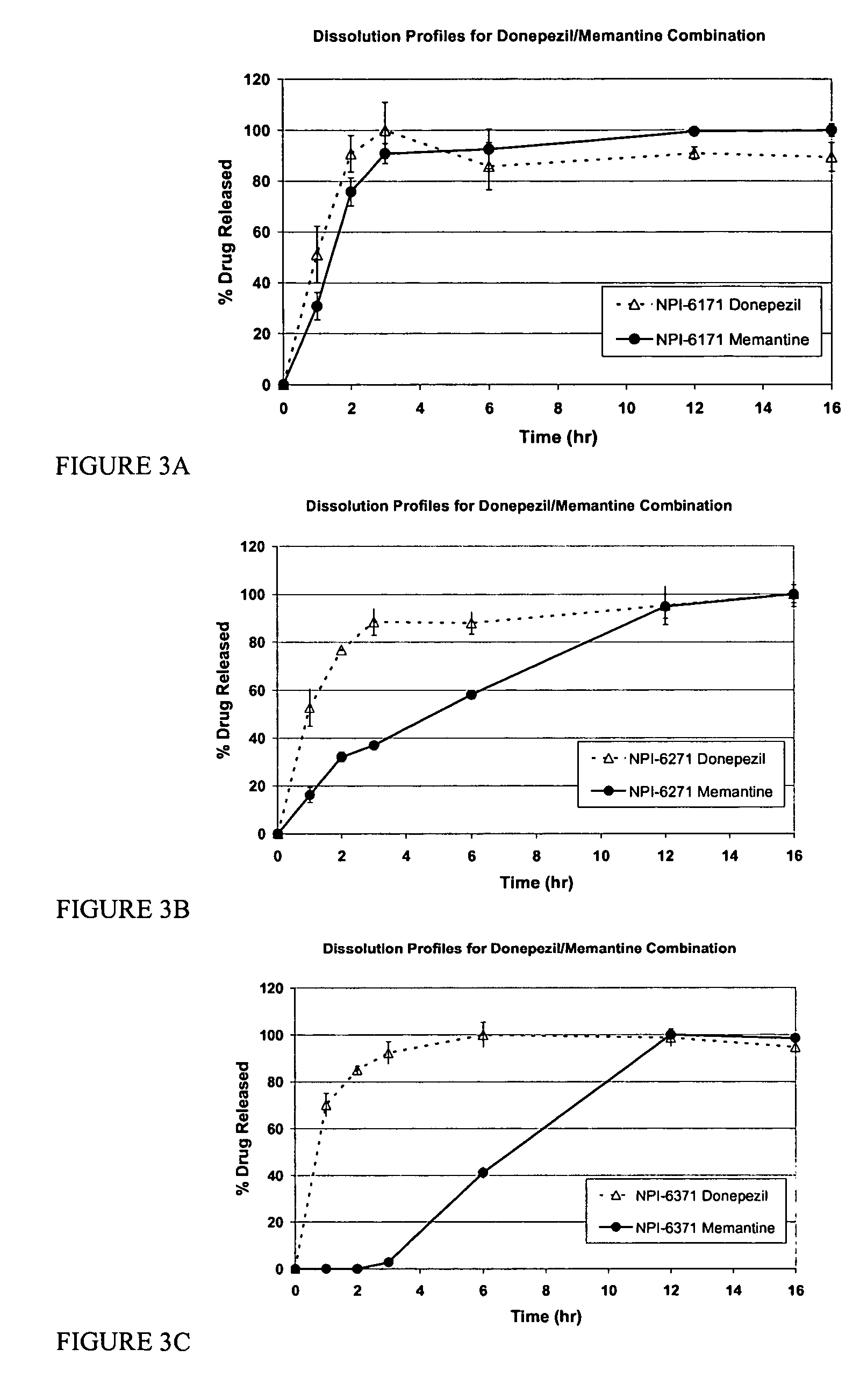
Method and composition for adminstering an NMDA receptor antagonist to a subject
ActiveUS20060142398A1Prevent adverse side effectsPatient compliance is goodBiocideNervous disorderNR1 NMDA receptorPharmacology
The invention provides methods and compositions for administering an NMDA receptor antagonist (e.g., memantine) to a subject.
Owner:ADAMAS PHARMA LLC
Method and apparatus for monitoring drug effects on cardiac electrical signals using an implantable cardiac stimulation device
InactiveUS7142911B2Increase aggressivenessIncrease dosePhysical therapies and activitiesDrug and medicationsEcg signalCardiac pacemaker electrode
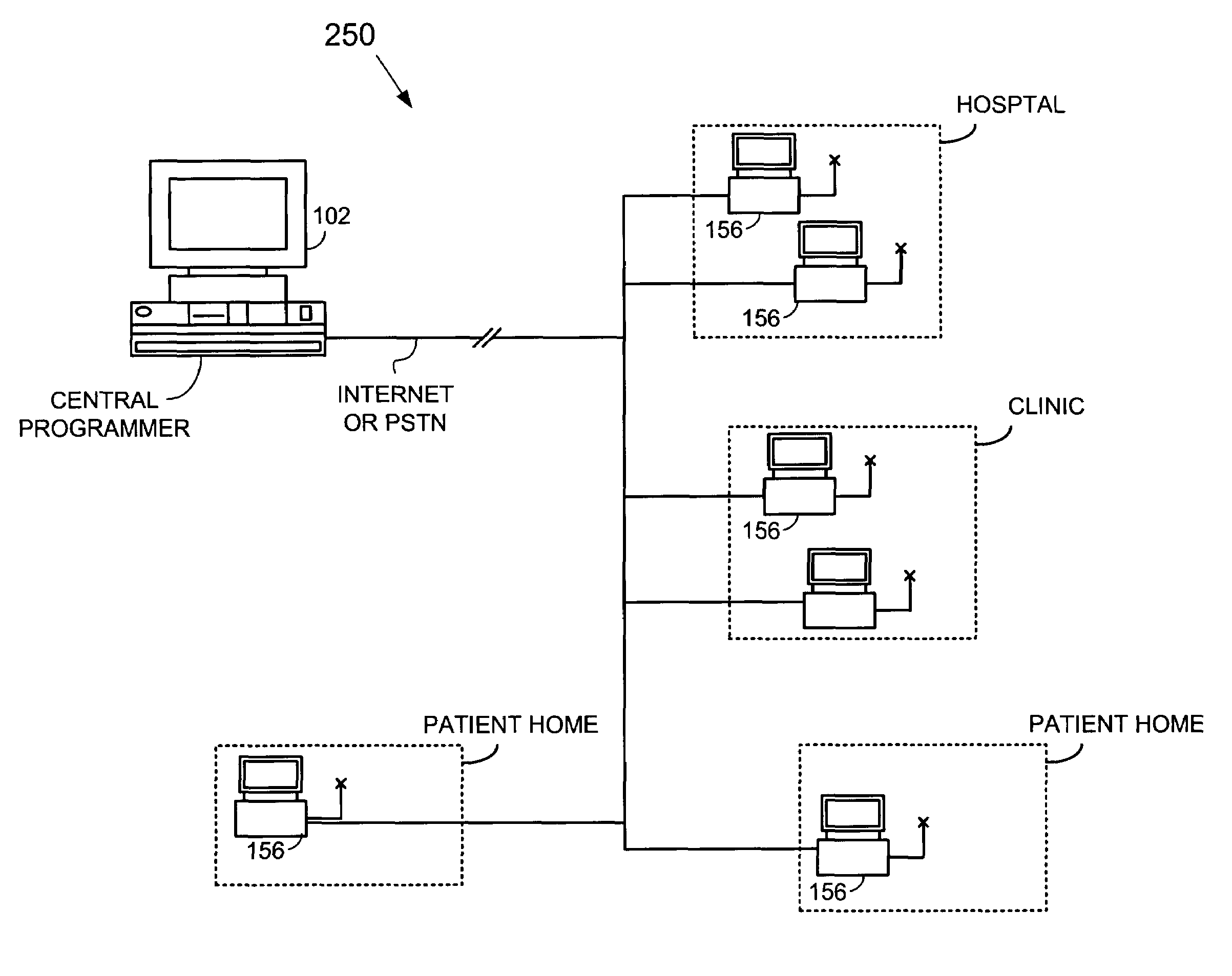
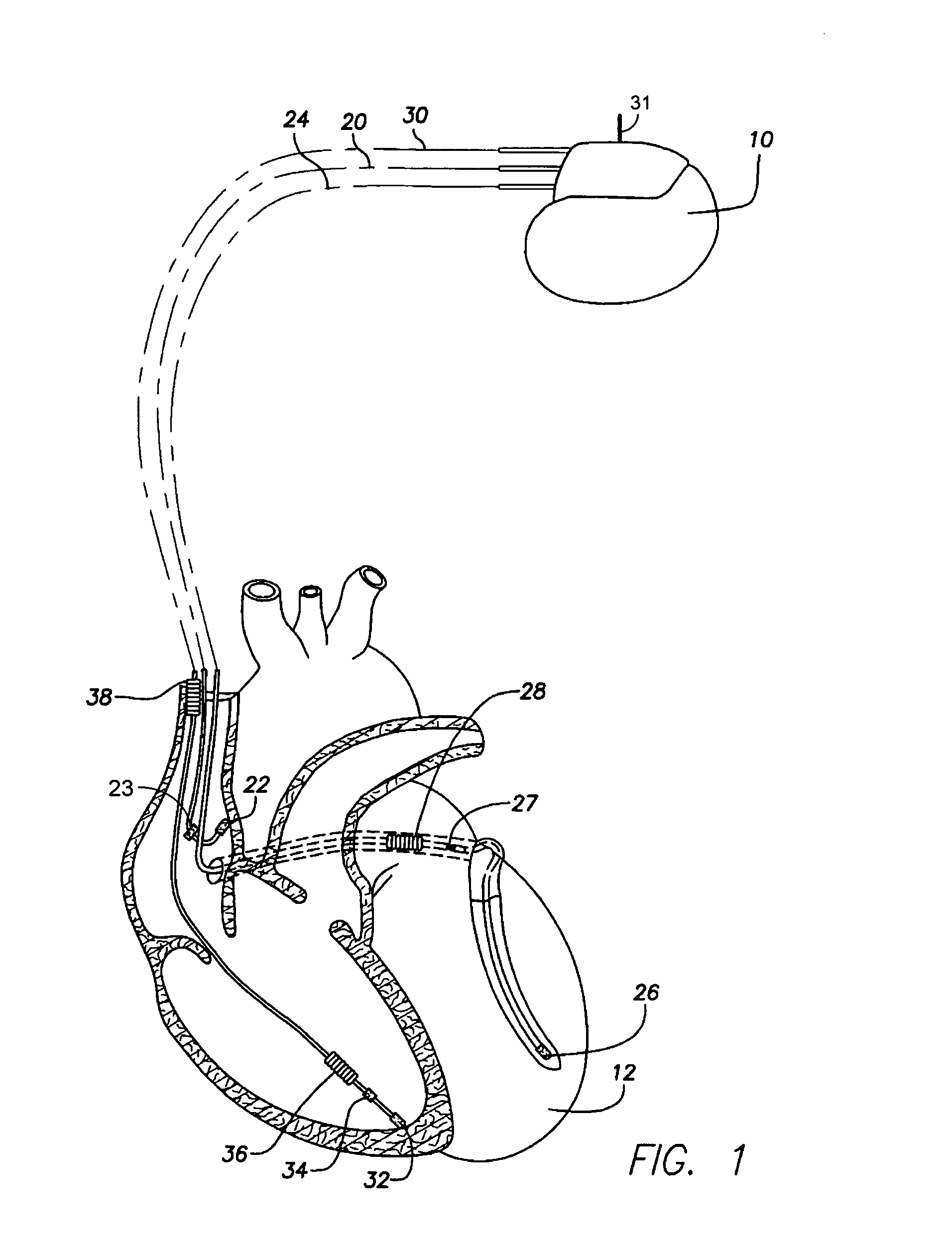
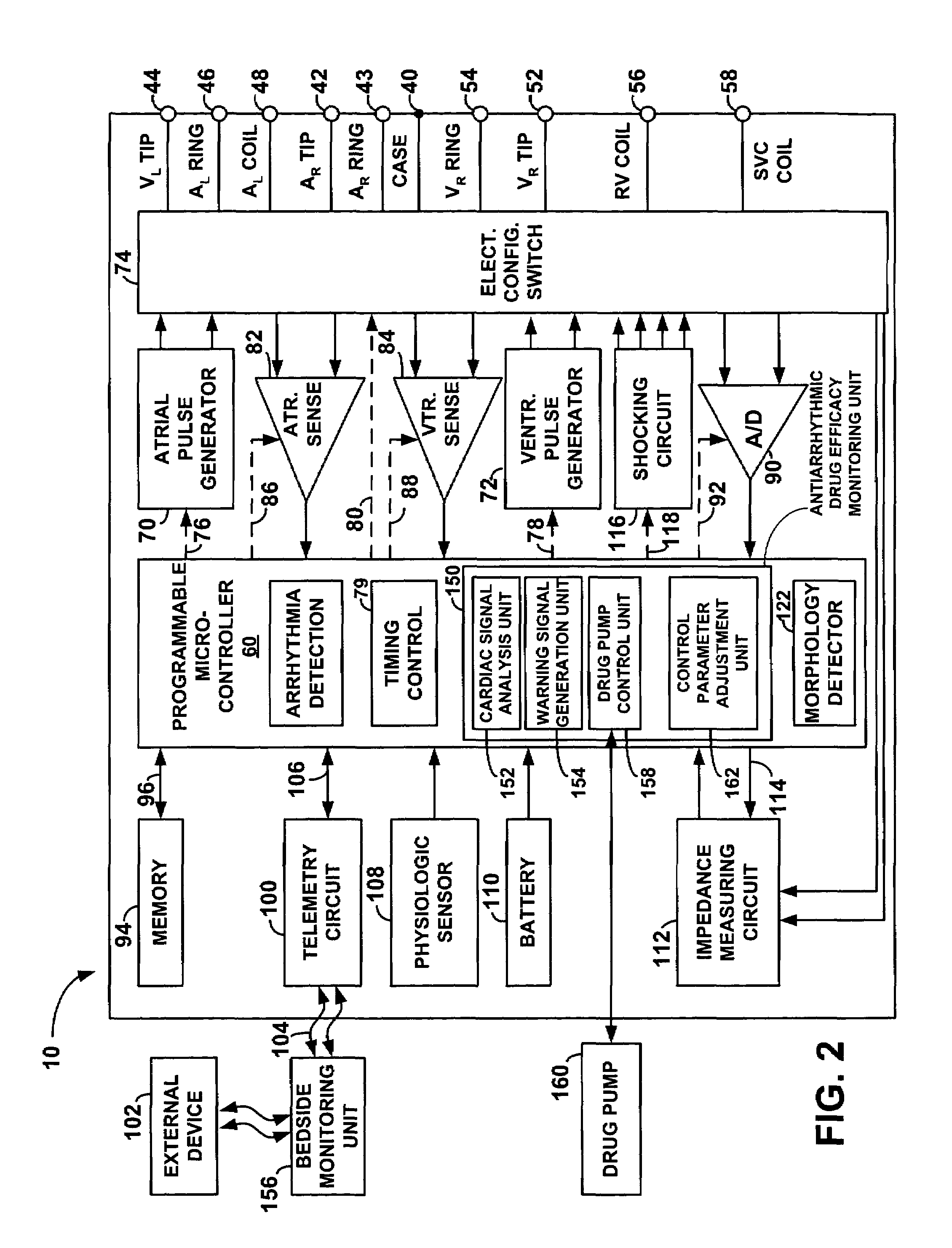
An implantable cardiac stimulation device, such as a pacemaker or Implantable Cardioverter Defibrillator, is configured to automatically monitor the effects of antiarrhythmic drugs on cardiac electrical signals within a patient to verify the efficacy of the drugs taken. In one example, an analysis of patient cardiac electrical signals is performed by comparing the cardiac electrical signals with values representative of the effects of different classes of antiarrhythmic drugs. If the implantable device determines that the prescribed antiarrhythmic drugs have not been effective, a warning signal is generated. The warning signal is conveyed directly to the patient via a bedside monitor and to the patient's physician via remote connection to an external programmer device so that both are notified of the drug efficacy problems. Additionally, the implantable device may be configured to automatically adjust pacing and defibrillation control parameters in an attempt to compensate for any lack of efficacy in the drugs. For example, the aggressiveness of overdrive pacing may be increased. Alternatively, a drug pump is controlled to adjust the dosage of antiarrhythmic drugs if an initial dosage is found to be ineffective.
Owner:PACESETTER INC
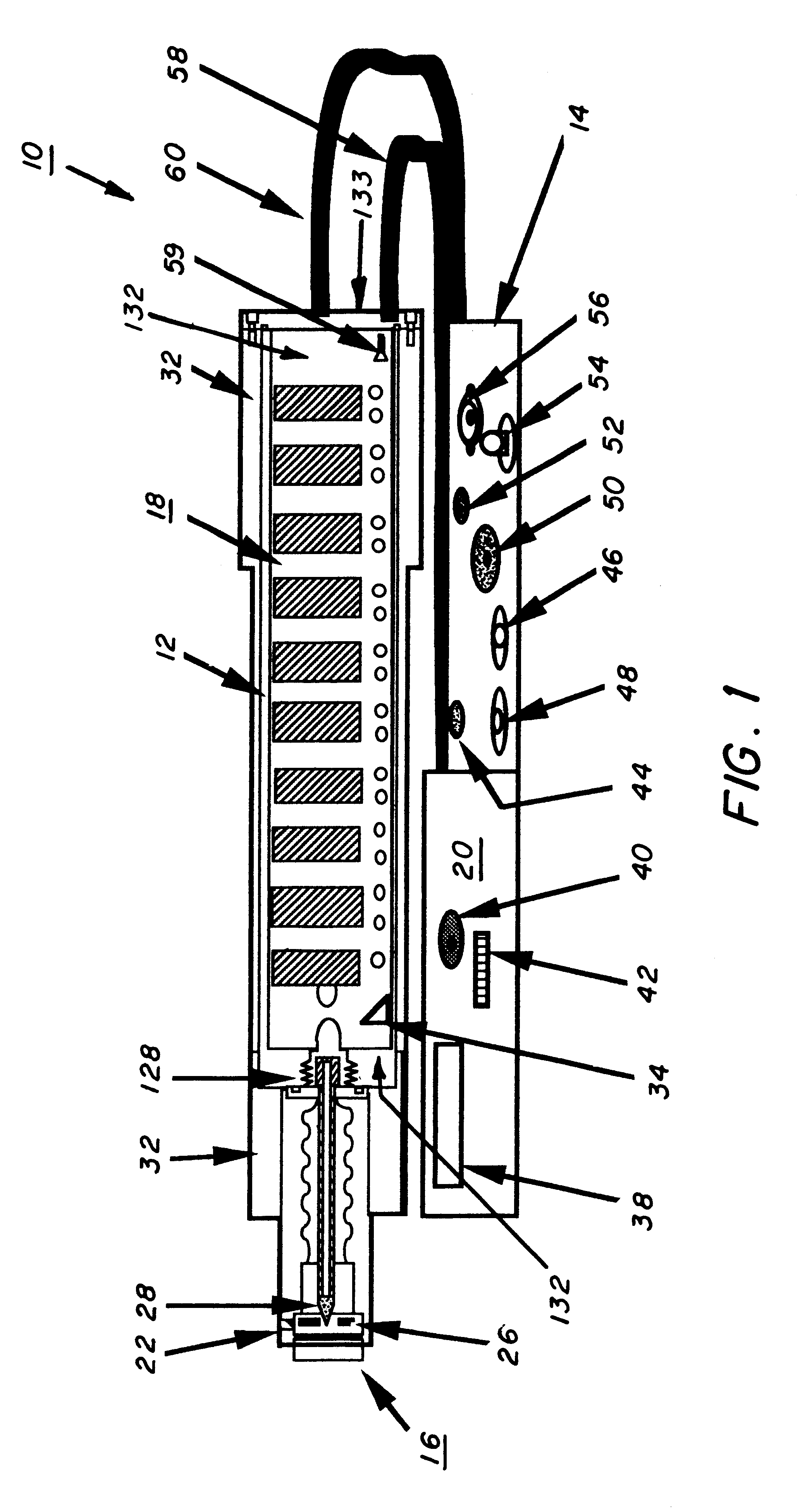
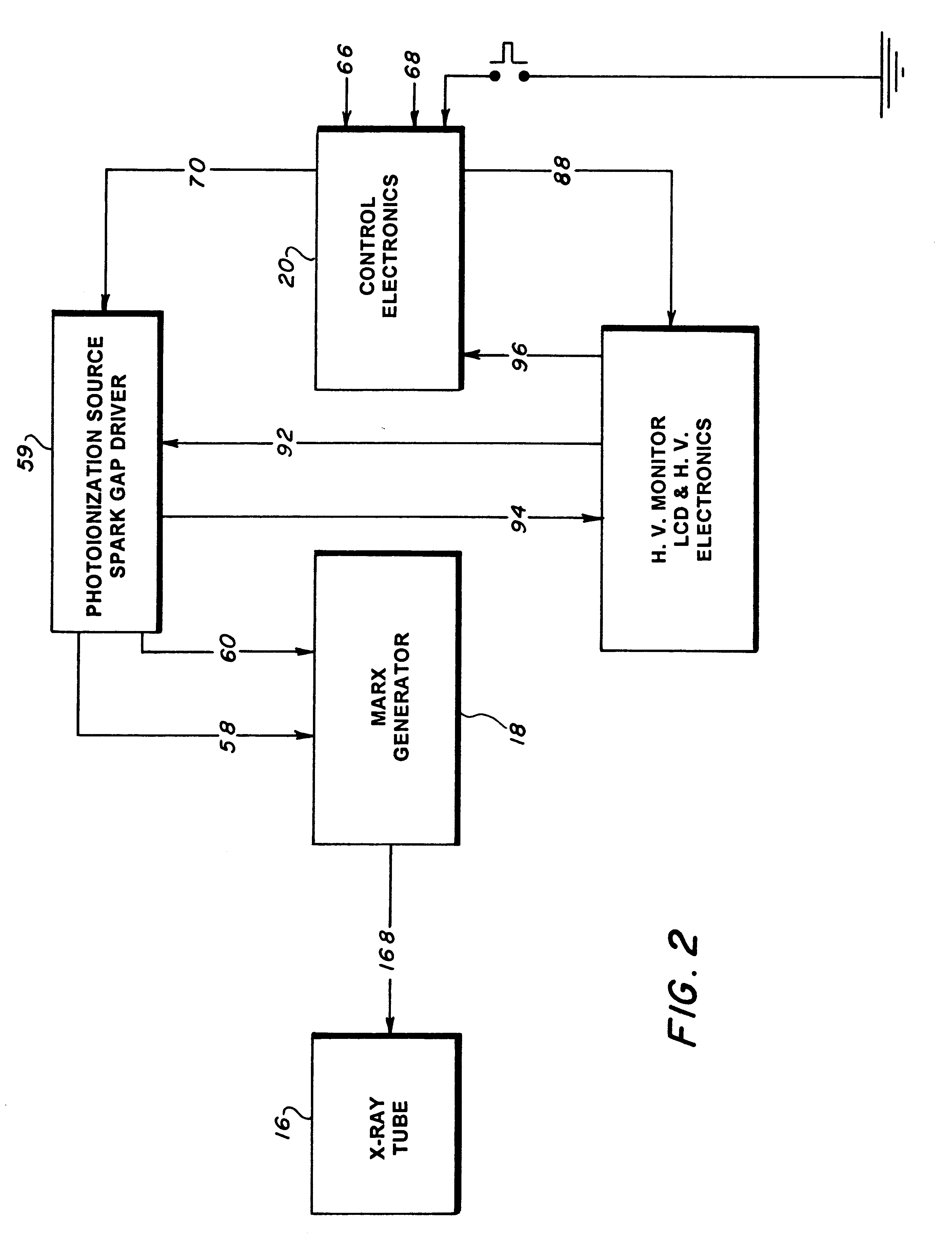
Mobile X-ray unit
A portable X-ray unit, of a relatively light-weight, occoping a volume of less then one-half a cubic foot containing an x-ray head assembly, a unique Marx generator, a plurality of spark-gap switches and control electronics is disclosed. The Marx generator allows for the development of a relatively high voltage in excess of 100 kV, yet allows for the discharge thereof within the nanosecond range. The Marx generator is enclosed by an acrylic insulator that cooperates with an aluminum enclosure, which functions as a return current path for the capacitors in the Marx generator and also as a shield against the escape of electromagnetic radiation from the pulsed x-ray unit. The Marx generator and spark-gap switches are confined within the pressurized chamber that may contain nitrogen gas to reduce the separation of the gap in the spark-gap switches.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Methods and compositions for the treatment of CNS-related conditions
ActiveUS20060252788A1Reduce and even minimize variationMinimizing CratioBiocideNervous disorderDiseasePsychiatry
The present invention provides novel methods and compositions for the treatment and prevention of CNS-related conditions. One of the CNS-related conditions treated by the methods and compositions of the invention is Alzheimer's disease.
Owner:ADAMAS PHARMA LLC
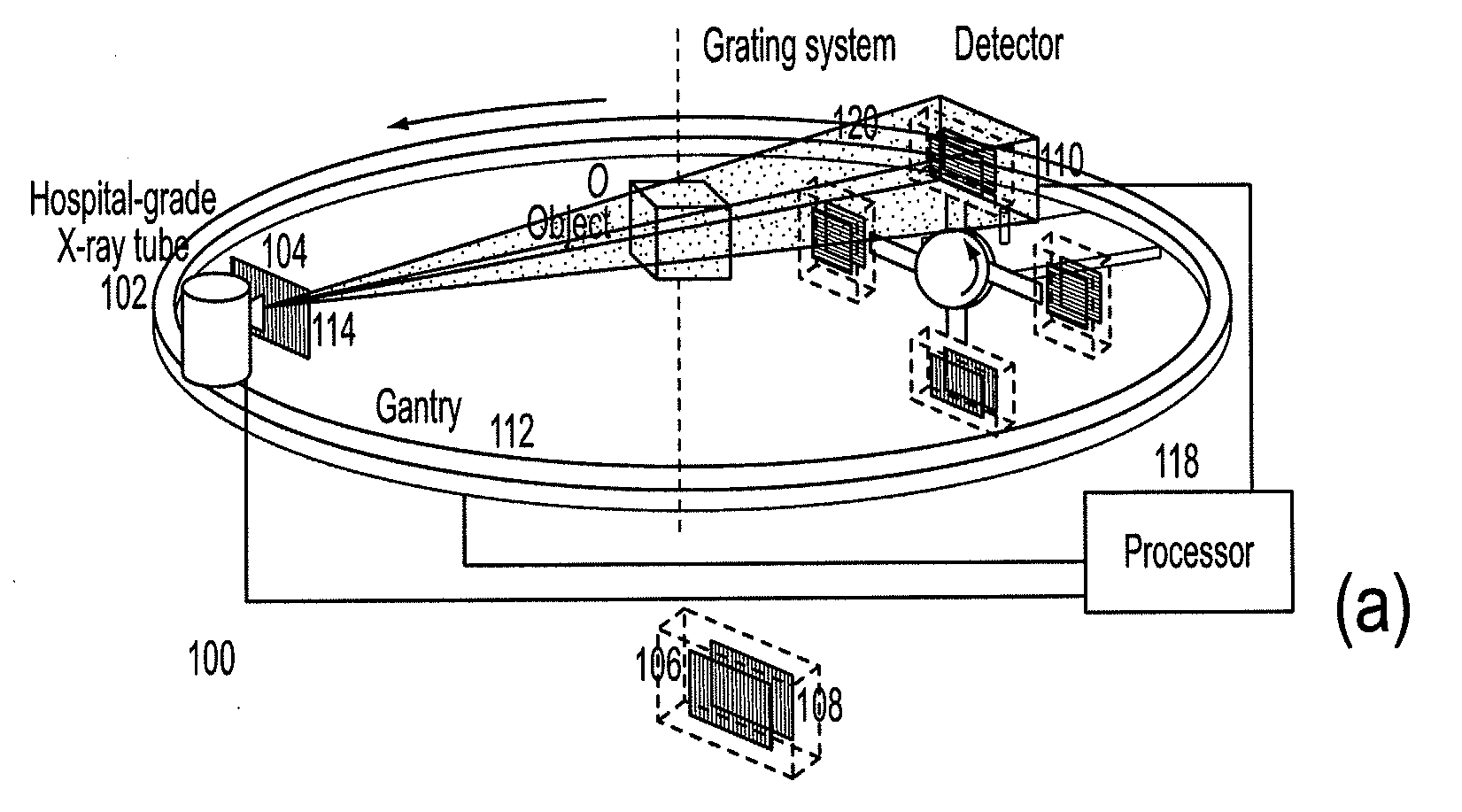
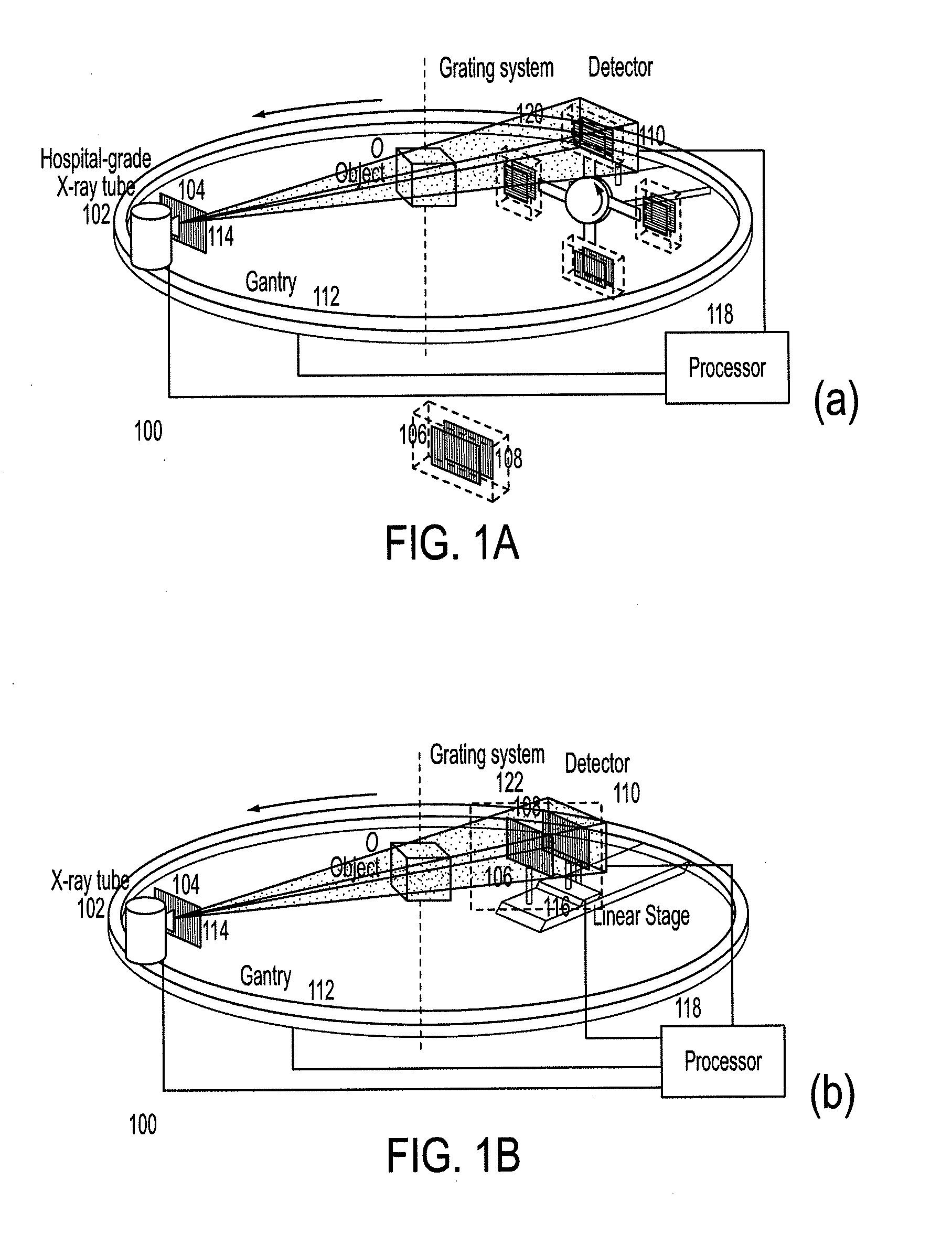
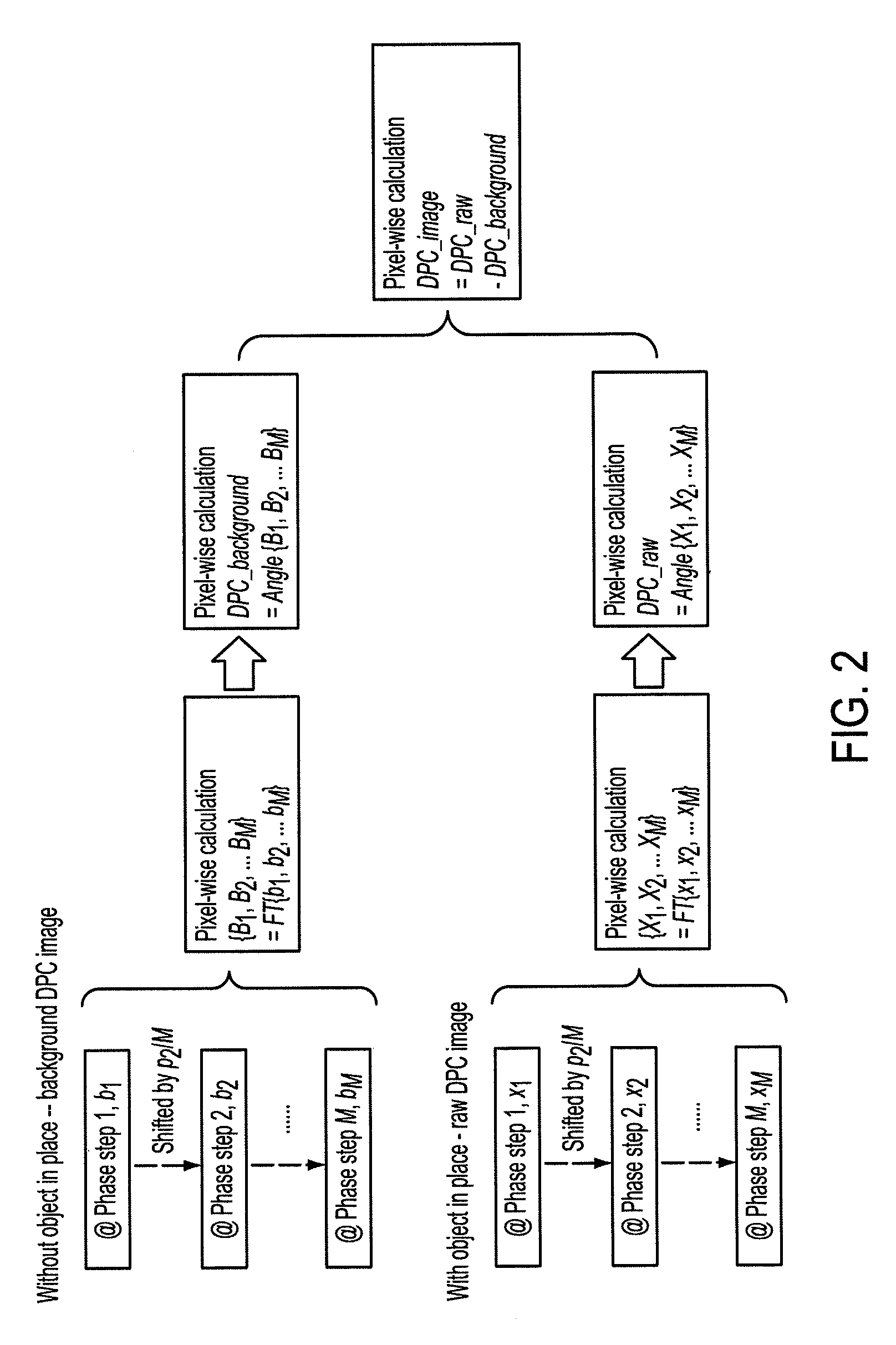
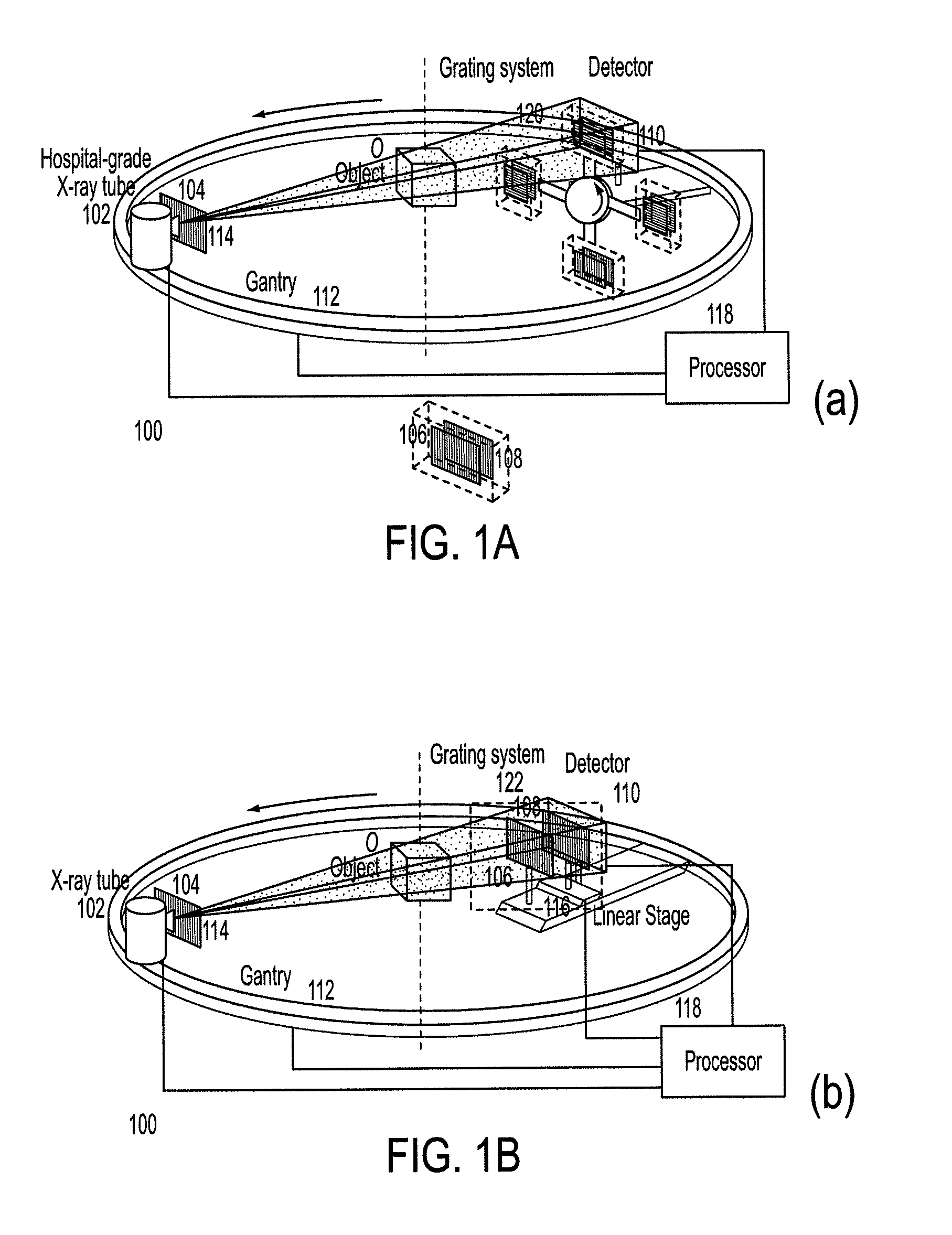
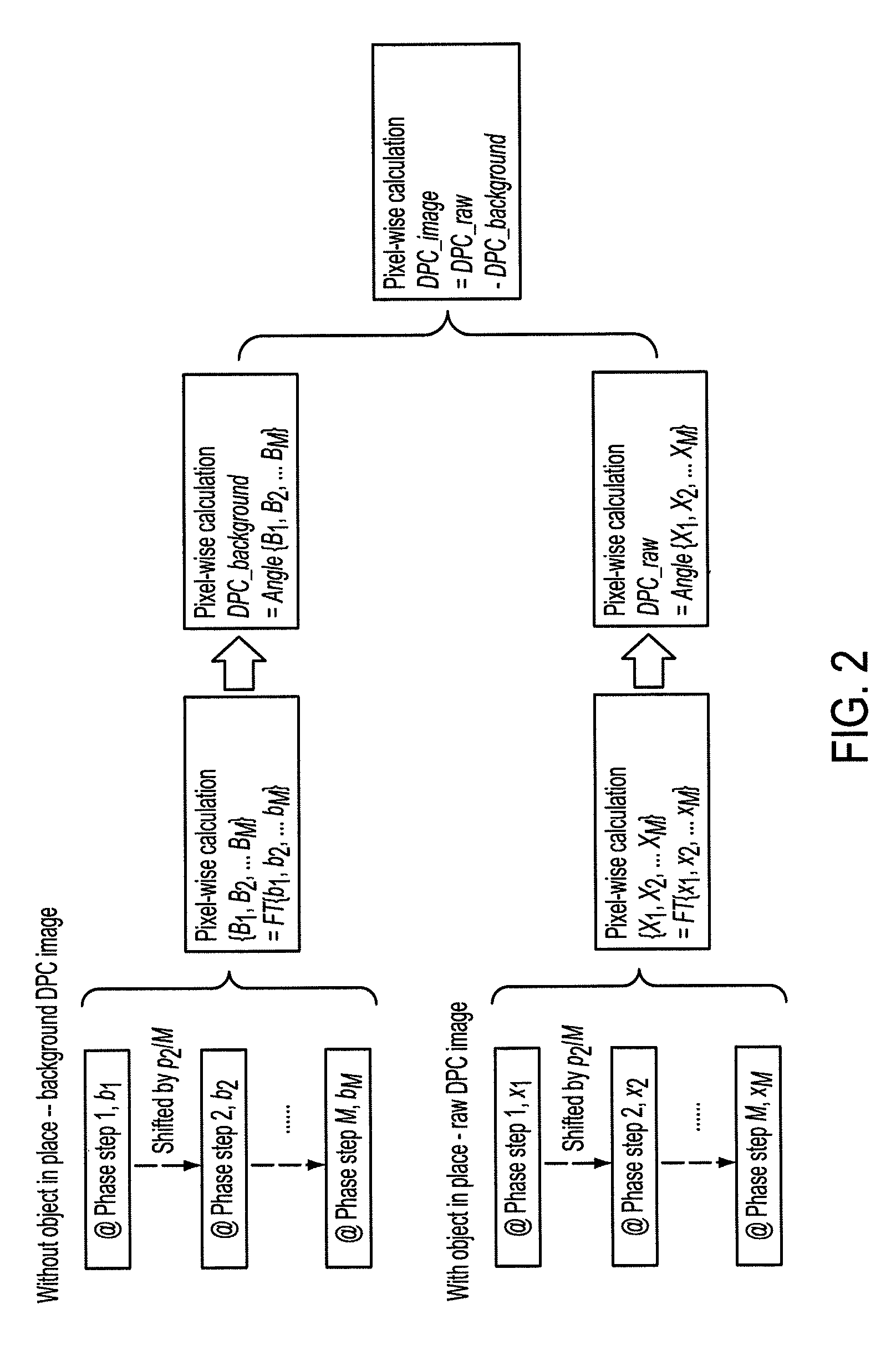
Methods and apparatus for differential phase-contrast fan beam ct, cone-beam ct and hybrid cone-beam ct
ActiveUS20100220832A1Improve spatial resolutionIncrease doseImaging devicesRadiation/particle handlingHybrid systemPhase grating
A device for imaging an object, such as for breast imaging, includes a gantry frame having mounted thereon an x-ray source, a source grating, a holder or other place for the object to be imaged, a phase grating, an analyzer grating, and an x-ray detector. The device images objects by differential-phase-contrast cone-beam computed tomography. A hybrid system includes sources and detectors for both conventional and differential-phase-contrast computed tomography.
Owner:UNIVERSITY OF ROCHESTER
Methods for Implementing Microbeam Radiation Therapy
InactiveUS20080192892A1Reduce damageReduce harmHandling using diaphragms/collimetersX-ray/gamma-ray/particle-irradiation therapyDrugEpilepsy
A method of performing microbeam radiation therapy (MRT) includes delivering a dose only to selected tissue in a target volume (10) with continuous broad beam, first, by interleaving arrays of microplanar beams (30,36) only at the target (10). Administered contrast agents can supplement the effect by preferentially increasing the target dose relative to dose in normal tissue. A broad beam effect is alternatively created using non-interleaving microbeam array(s) with scattering agents administered to selected tissue that preferentially increase valley dose (69) within target to approximate broad beam. The methods of interleaving microbeams are also applied to treat diseases and conditions by ablating at least a portion of selected tissue, or by damaging blood-brain barrier for efficient drug and / or cell administration. A system for performing interlaced microbeam radiosurgery preferably includes two orthogonal radiation source arms (102) for producing and interleaving microbeam arrays (30,36) at the target volume (10). The methods treat tumors, pain, epilepsy, and neurological diseases.
Owner:BROOKHAVEN SCI ASSOCS
Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes
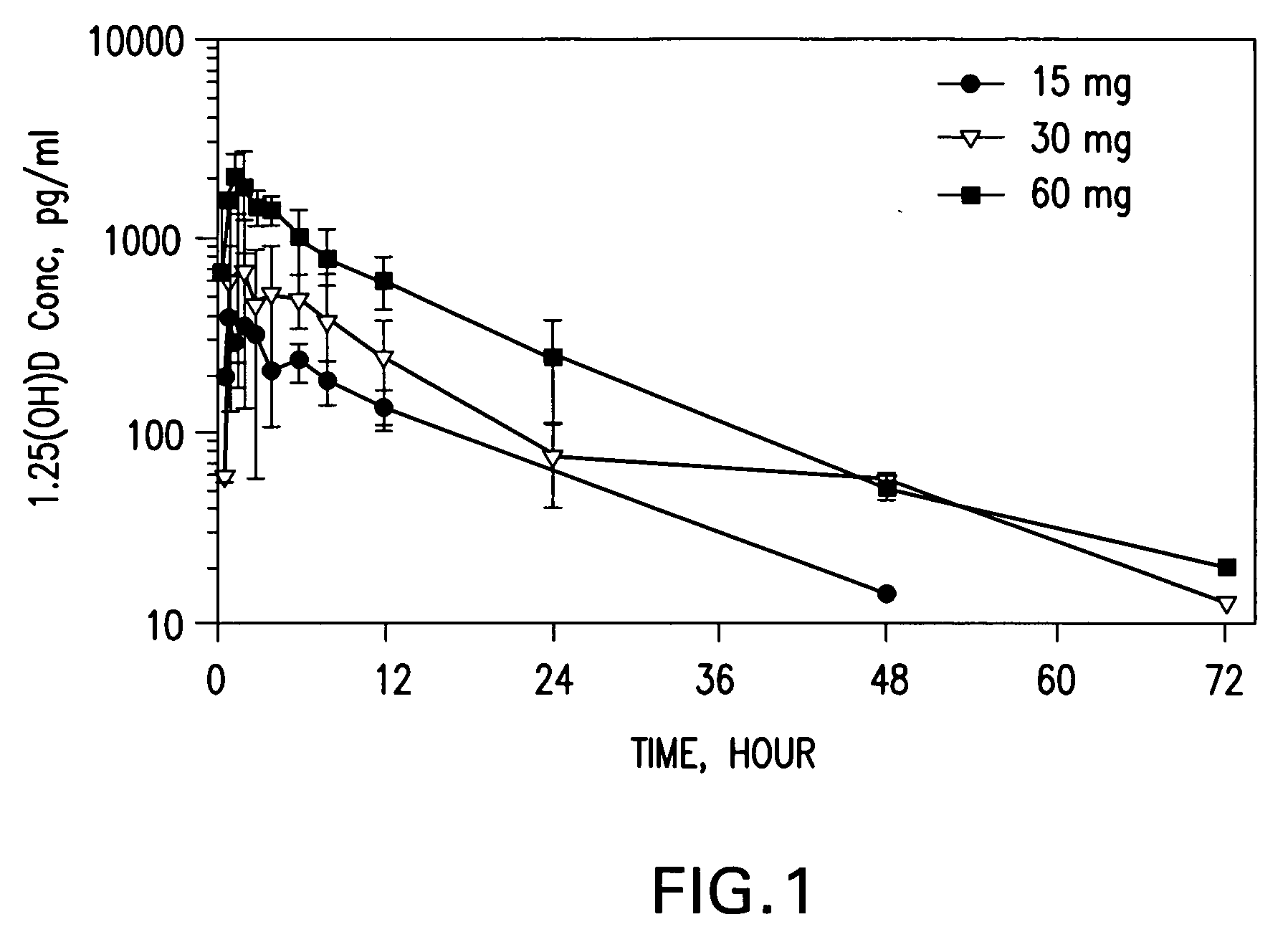
InactiveUS20050101576A1Minimizing and avoiding effectUseful in treatmentOrganic active ingredientsBiocideActive agentHigh doses
Methods of treating MDS, or ameliorating a symptom thereof, are disclosed. Specific methods encompass the administration of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Other methods include intermittent administration of a high dose of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Such intermittent administration allows high doses of the vitamin D compounds to be administered while minimizing or eliminating hypercalcemia.
Owner:NOVACEA INC
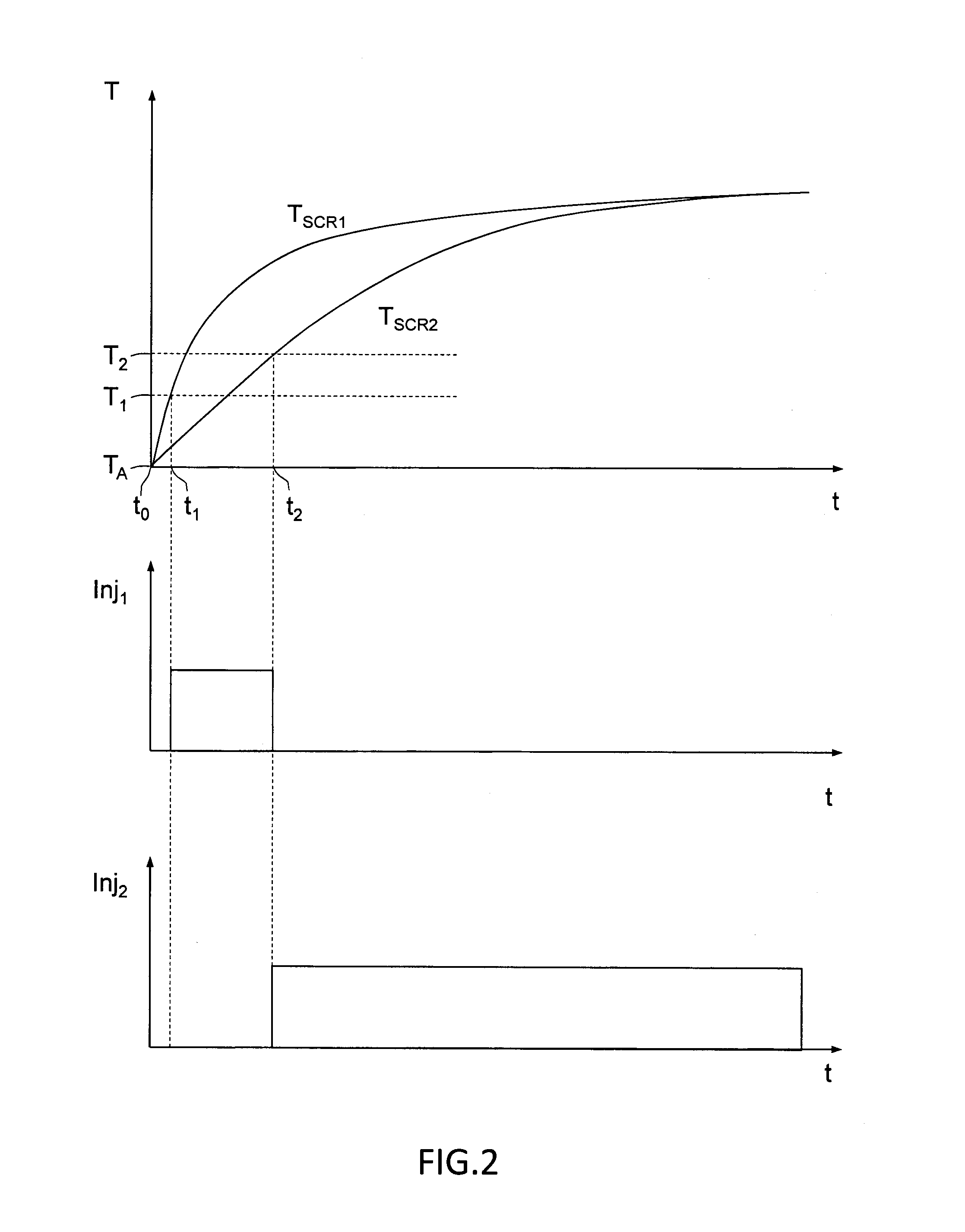
Exhaust aftertreatment system and method for operating the system
ActiveUS20140363358A1Fast warm-upFast and economical controlCombination devicesInternal combustion piston enginesEnvironmental engineeringGaseous ammonia
An exhaust system includes a first SCR catalyst and a second SCR catalyst positioned downstream of the first SCR catalyst. A first injector is provided upstream of the first SCR catalyst, and a second injector is provided upstream of the second SCR catalyst. The exhaust system further includes a gaseous ammonia supply device fluidly connected to the first injector for supplying gaseous ammonia to the exhaust gas by the first injector, and an ammonia-containing reductant reservoir fluidly connected to the second injector for supplying a fluid ammonia-containing reductant, such as urea, to the exhaust gas by the second injector. The first SCR catalyst has a smaller volume than the second SCR catalyst for a fast warm-up of the first SCR catalyst.
Owner:VOLVO LASTVAGNAR AB
Methods for administering weight loss medications
InactiveUS20080110792A1Good curative effectReduce adverse side effectsSmall article dispensingPill deliveryInternal medicineDrug
Methods and systems for administration of pharmaceuticals using a unit dosage package that includes a first unit dosage that has a first drug and a second drug, a second unit dosage that has the first drug and the second drug, where the second unit dosage includes a different amount of the second drug than the first unit dosage and a unit dosage package is configured to hold the first unit dosage and the second unit dosage. In preferred embodiments the methods and systems are used for administration of weight loss medications.
Owner:OREXIGEN THERAPEUTICS INC
Ultraviolet treatment for aqueous liquids
InactiveUS6773608B1Conveniently cleanedIncrease in amountWater/sewage treatment by irradiationLiquid separation by electricityProcess regionLight source
A process for treating an aqueous liquid. The process includes: passing the liquid by force of gravity through a treatment area, the liquid having an upper surface exposed to ambient pressure; disrupting the flow of the liquid as it passes through the treatment area, and exposing the upper surface of the liquid as the flow is disrupted to UV light. Disrupting the flow includes directing lower portions of the liquid toward the surface of the liquid to bring such portions into contact with UV light. A process for treating an aqueous liquid in which the treatment process is monitored. This process includes passing the liquid through a treatment area to bring the liquid into contact with reflective walls submerged below an upper surface of the liquid, and exposing the upper surface of the liquid to light emitted from a UV light source such that UV light penetrates the liquid to strike the submerged reflective surfaces and to be reflected therefrom to emerge through the upper surface of the liquid. The process also involves determining the intensity of the UV light emitted from the light source, determining the intensity of UV light received by a UV light sensor trained to receive emergent light and determining whether the treatment has a predetermined effectiveness based on the intensity of the UV light emitted from the light source and the intensity of the UV light received by the sensor. Apparatuses for carrying out processes of the invention are also described.
Owner:UV PURE TECH
Methods and apparatus for differential phase-contrast fan beam CT, cone-beam CT and hybrid cone-beam CT
ActiveUS7949095B2Increase doseImprove spatial resolutionImaging devicesRadiation/particle handlingPhase gratingHybrid system
Owner:UNIVERSITY OF ROCHESTER
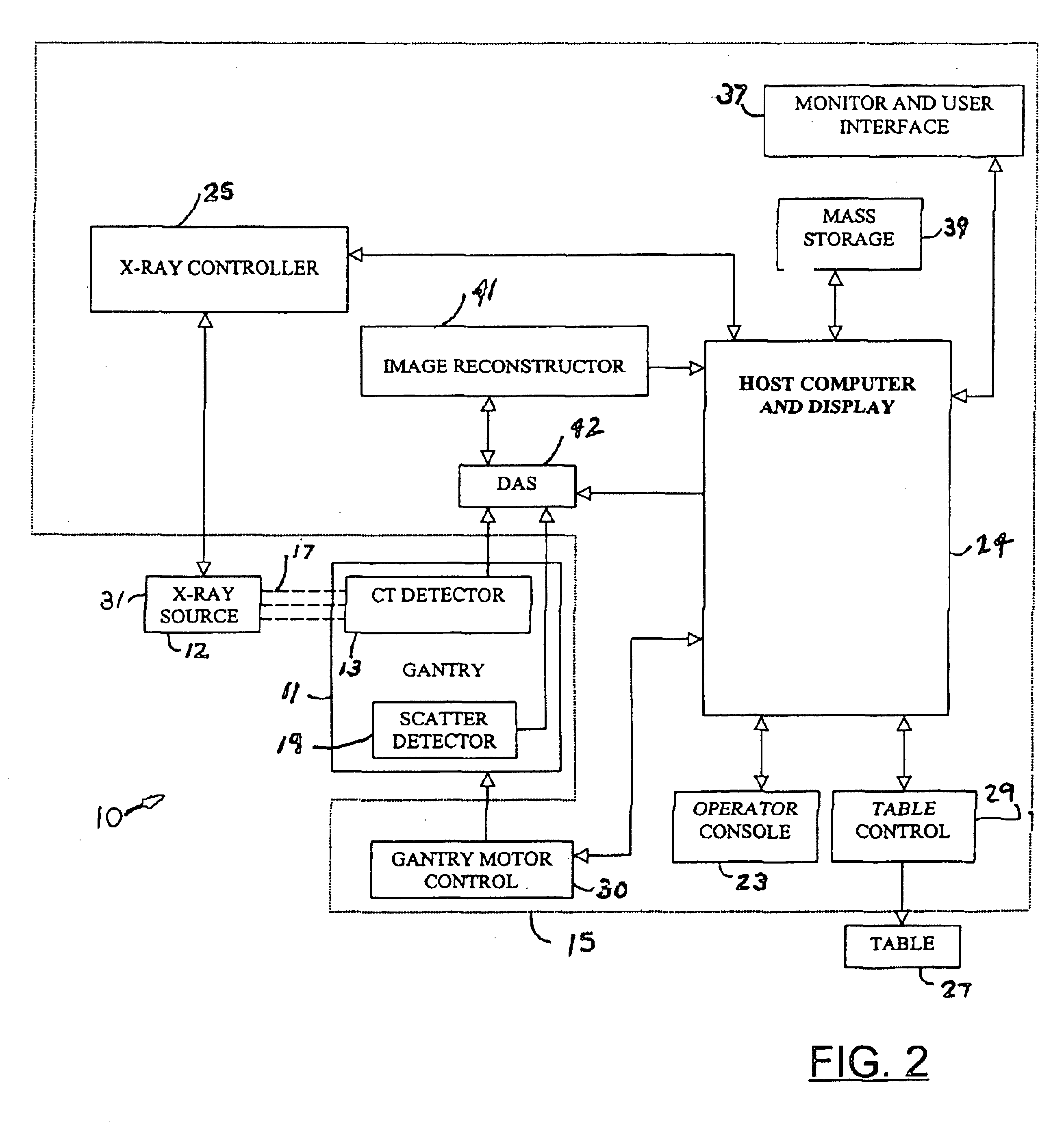
Computed tomography system with integrated scatter detectors
InactiveUS6879657B2Increase in numberIncrease dosageMaterial analysis using wave/particle radiationRadiation/particle handlingPhysicsComputed tomography
An imaging system includes an x-ray source coupled to a gantry. The x-ray source generates an x-ray flux, wherein a portion of the x-ray flux becomes scatter radiation. A scatter detector is also coupled to the gantry to receive the scatter radiation. The scatter detector generates a scatter signal in response to the scatter radiation, and a host computer receives the scatter signal.
Owner:GE MEDICAL SYST GLOBAL TECH CO LLC
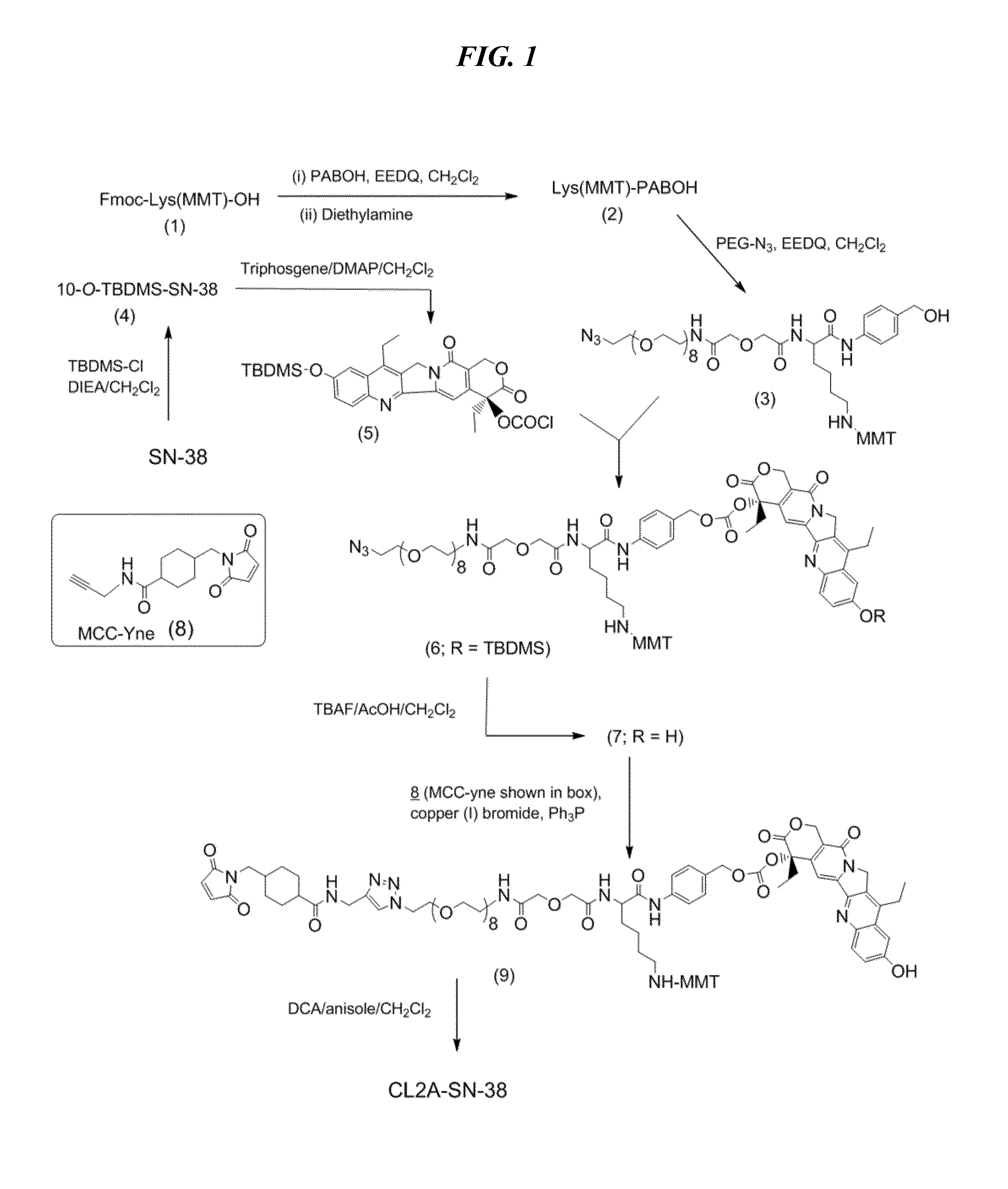
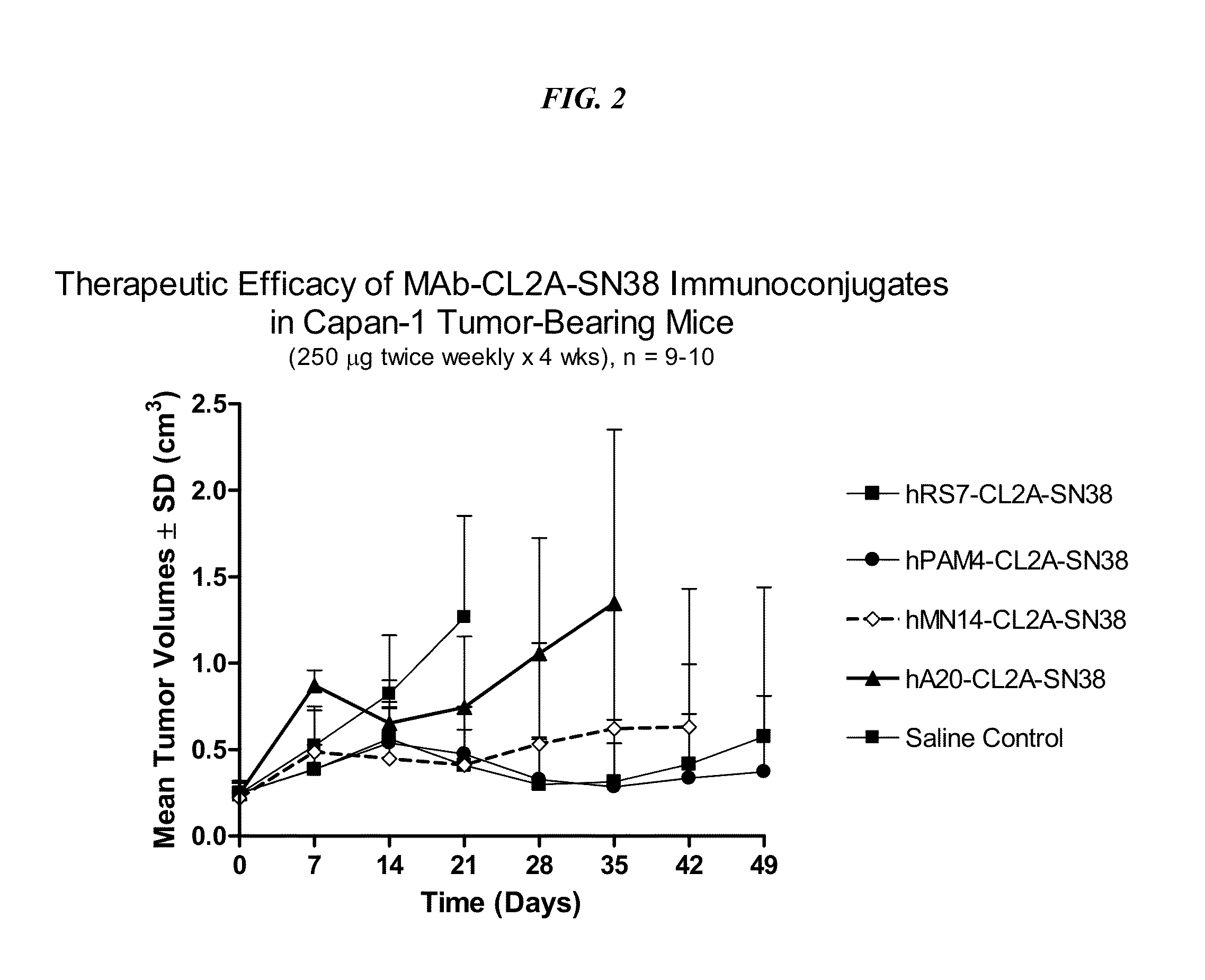
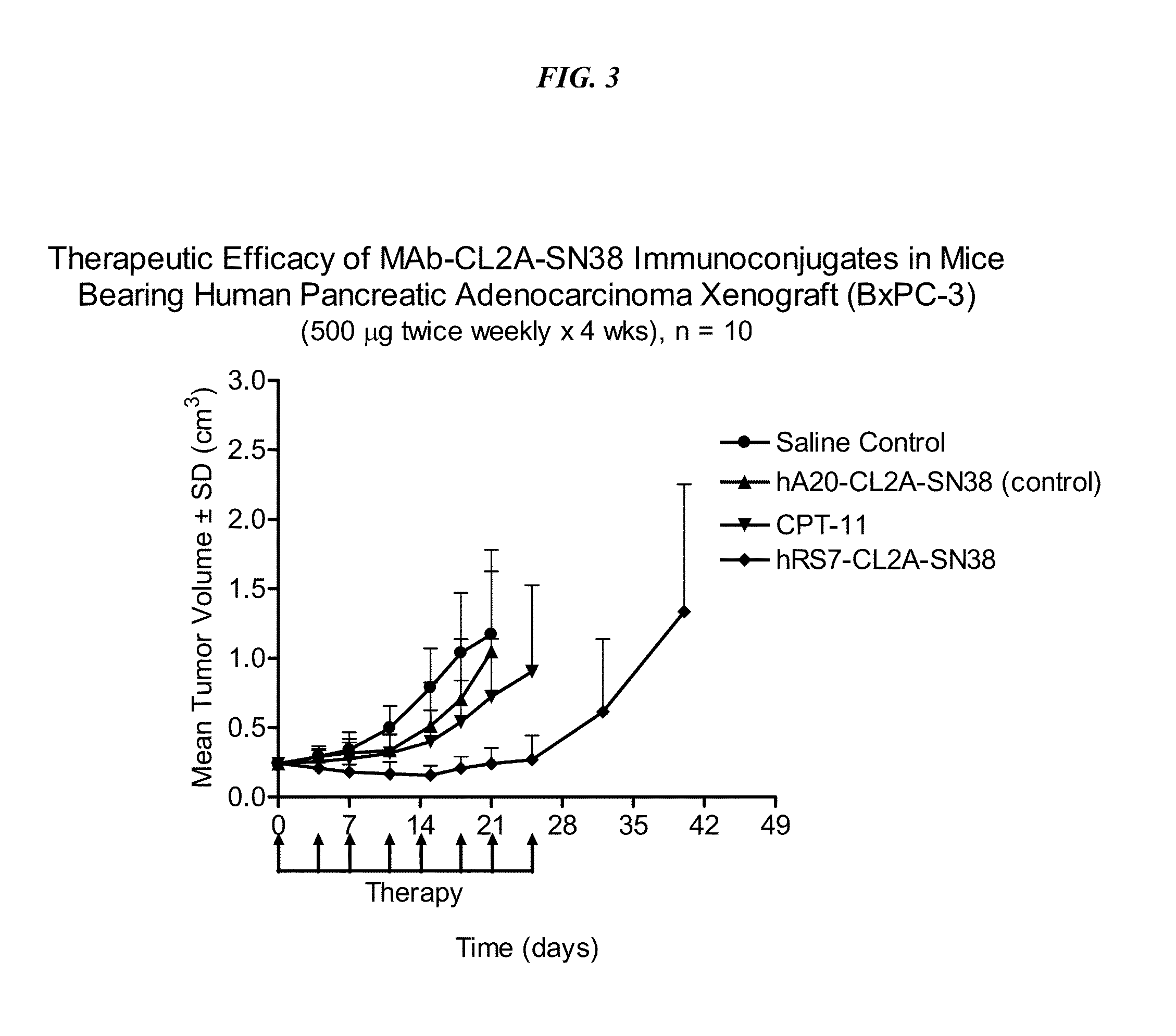
Antibody-sn-38 immunoconjugates with a cl2a linker
ActiveUS20140227180A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
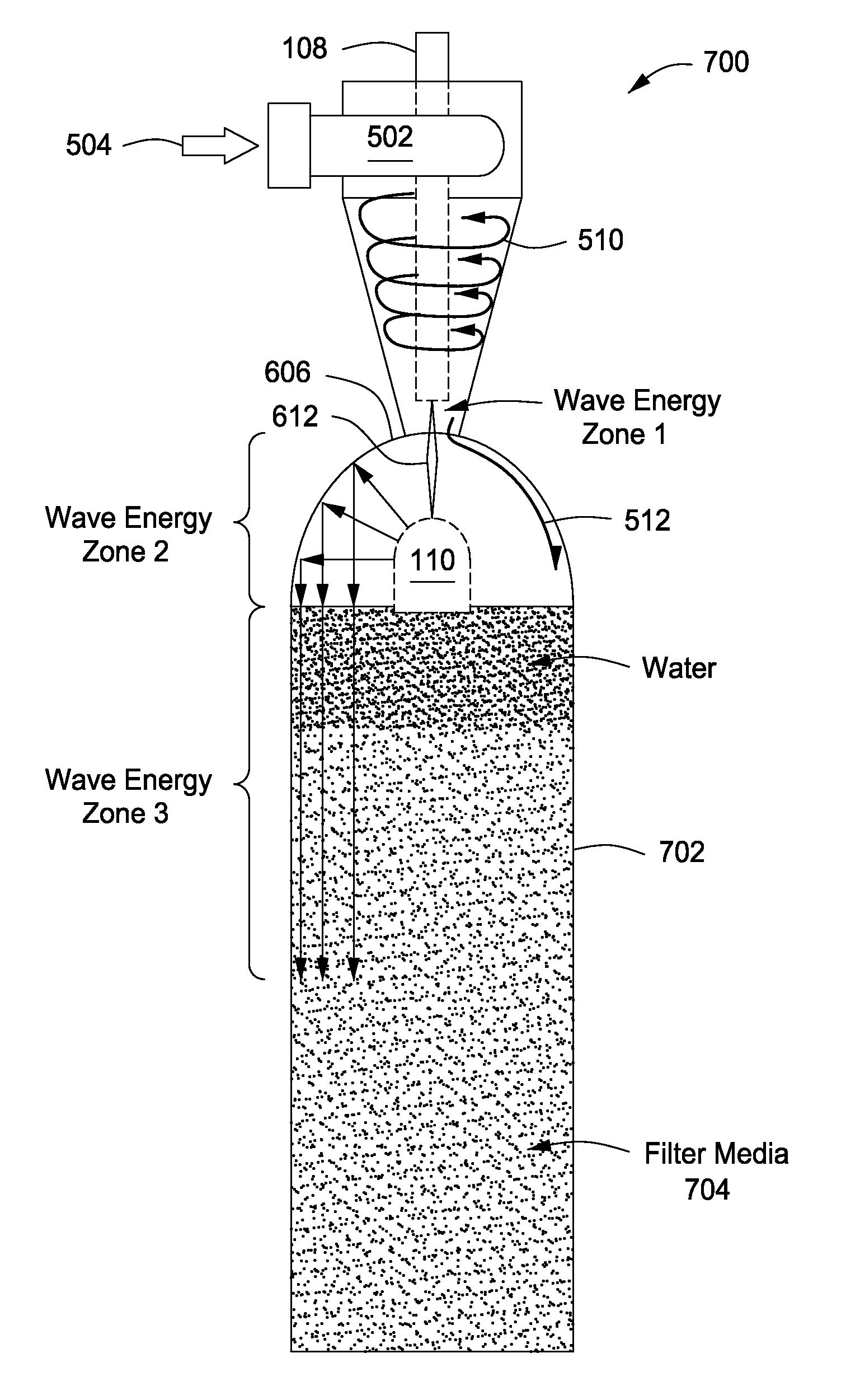
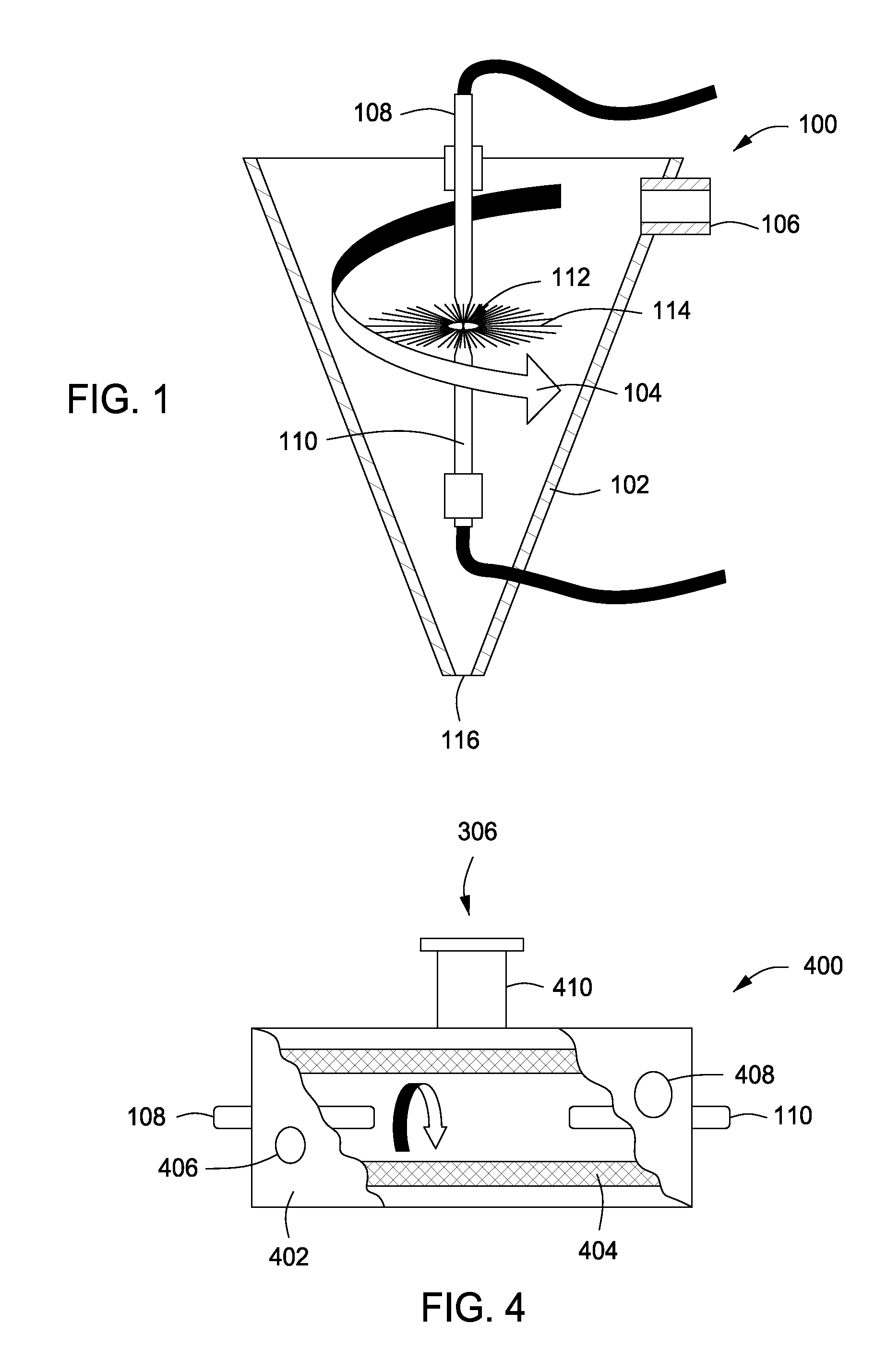
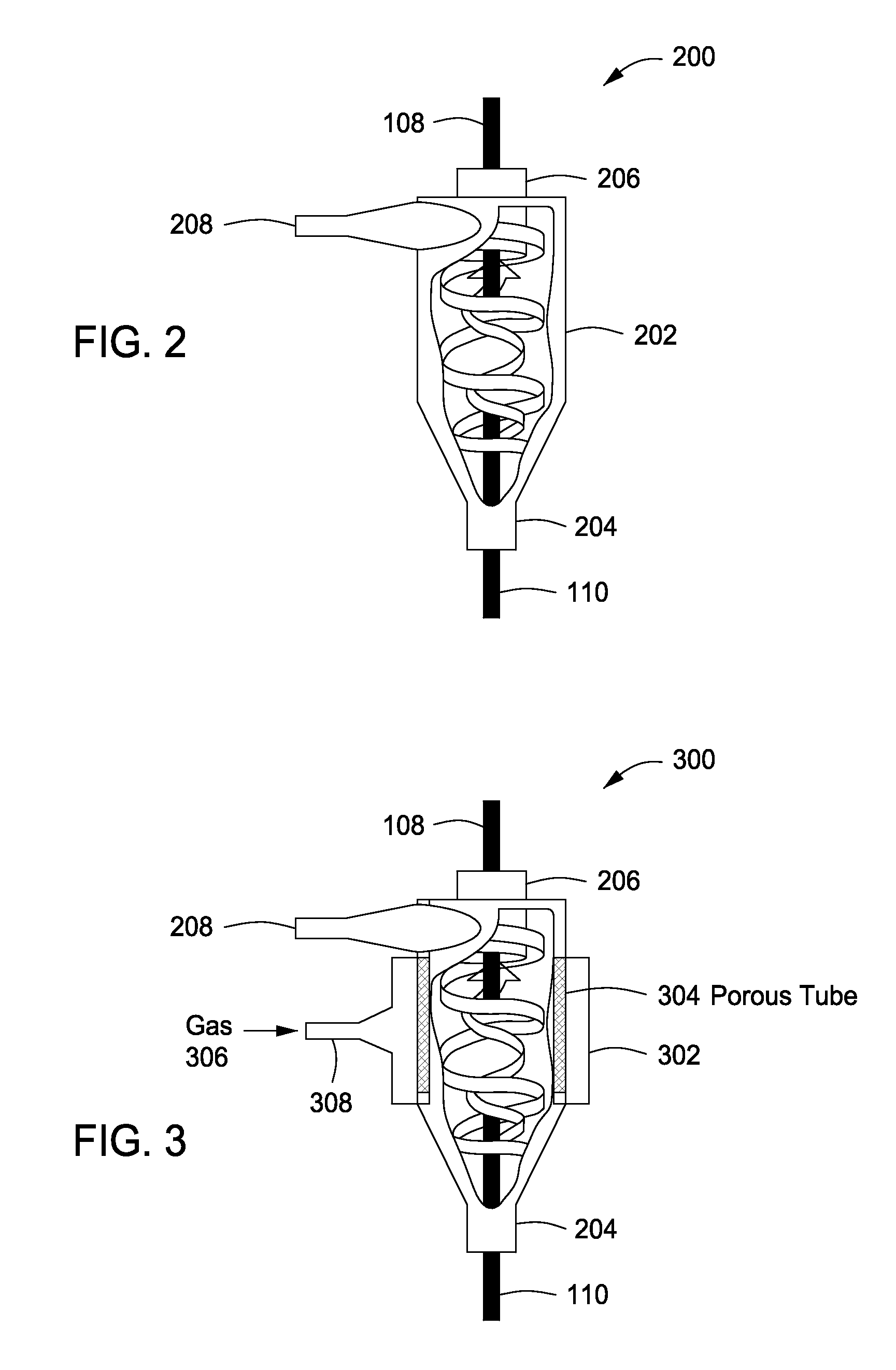
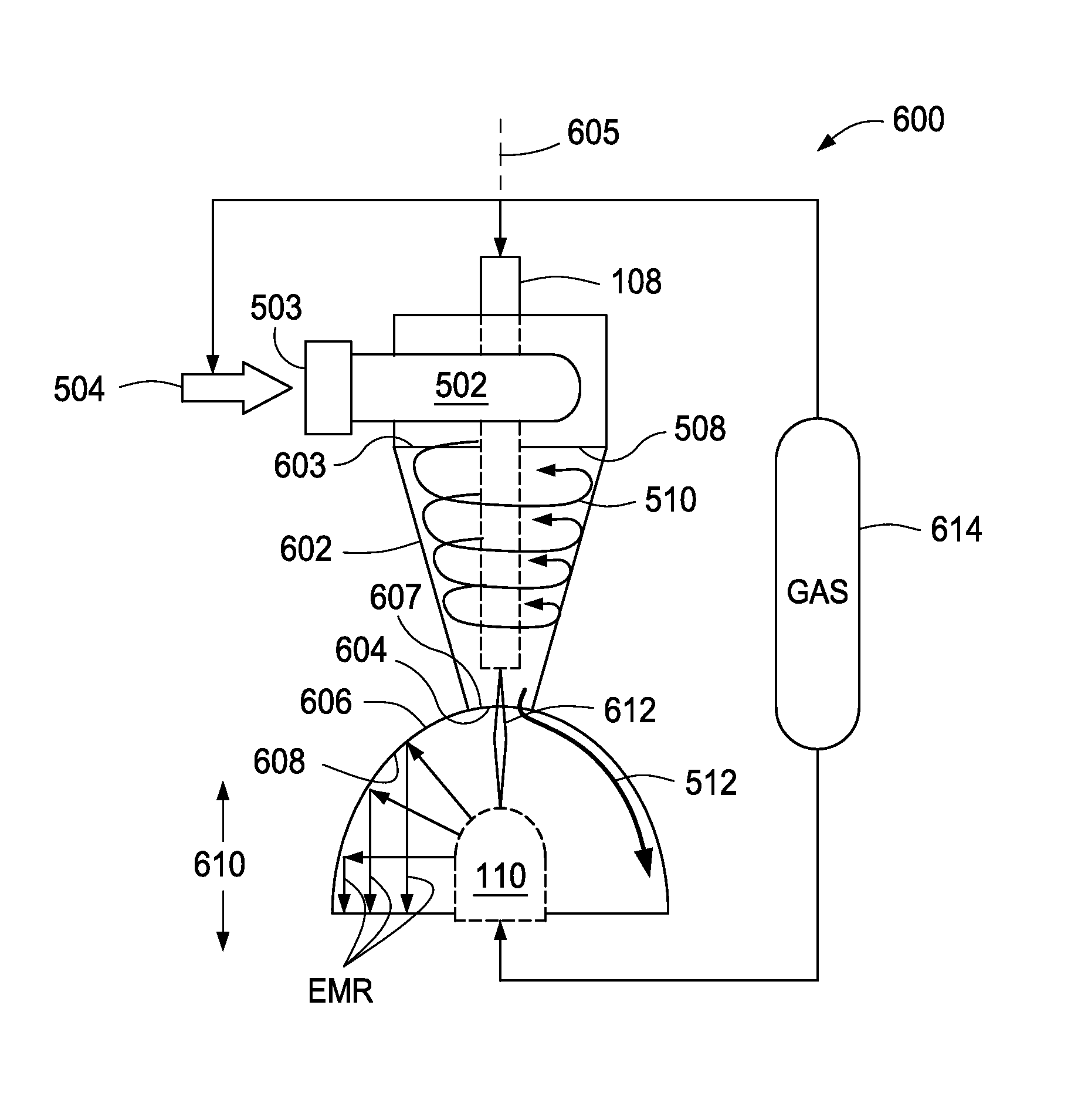
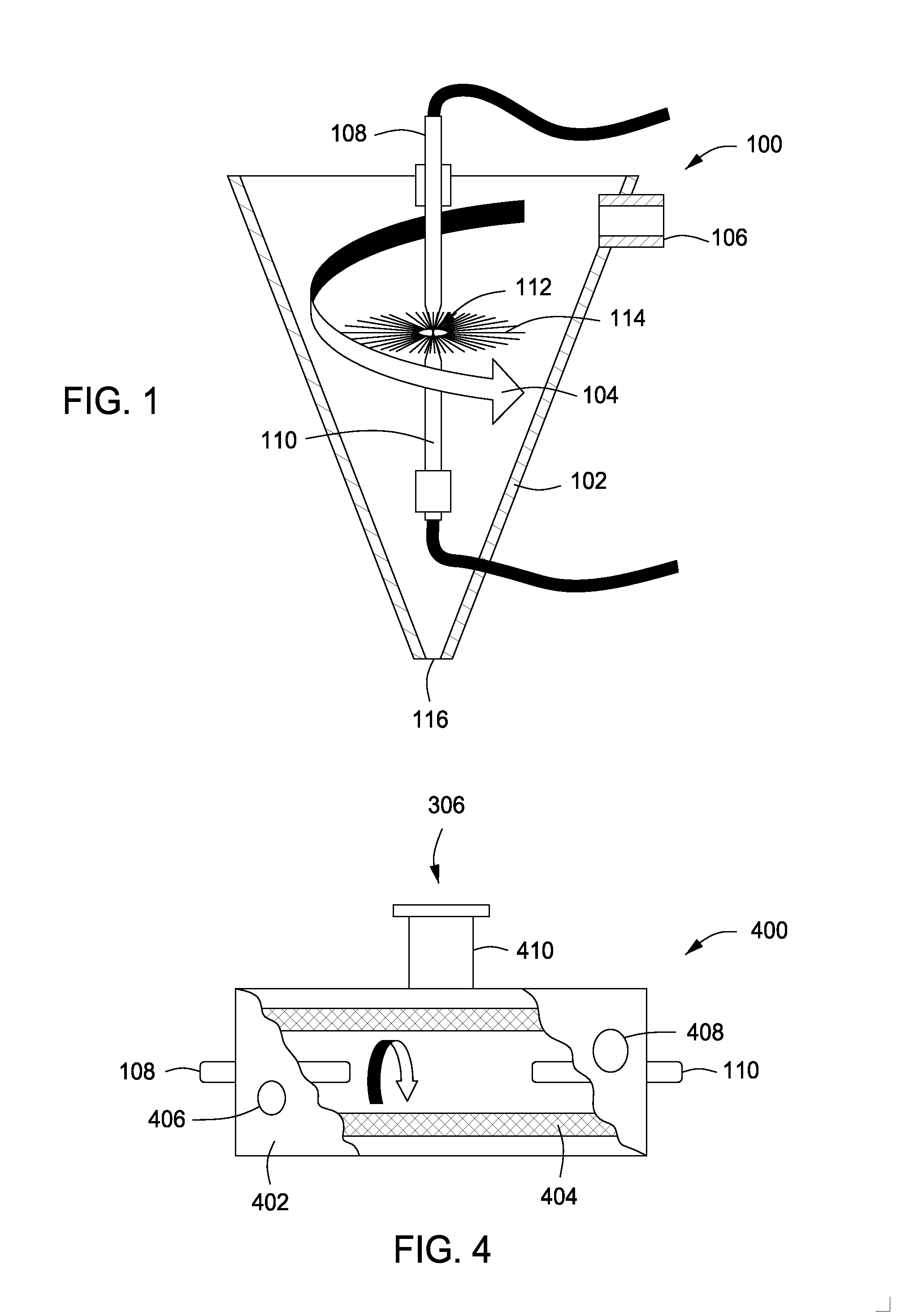
System, method and apparatus for treating liquids with wave energy from an electrical arc
ActiveUS20070240975A1Energy efficiencyReduce maintenanceWater distributersWaste water treatment from animal processingEngineeringEnergy source
The present invention provides a system, method and apparatus for treating a liquid by providing a wave energy source and creating a thin film of the liquid whirling around the wave energy source such that one or more wave energies irradiate the liquid. Likewise, the present invention provides a method of treating a liquid by providing three zones of wave energy and passing the liquid through the three zones of wave energy.
Owner:FORET PLASMA LABS
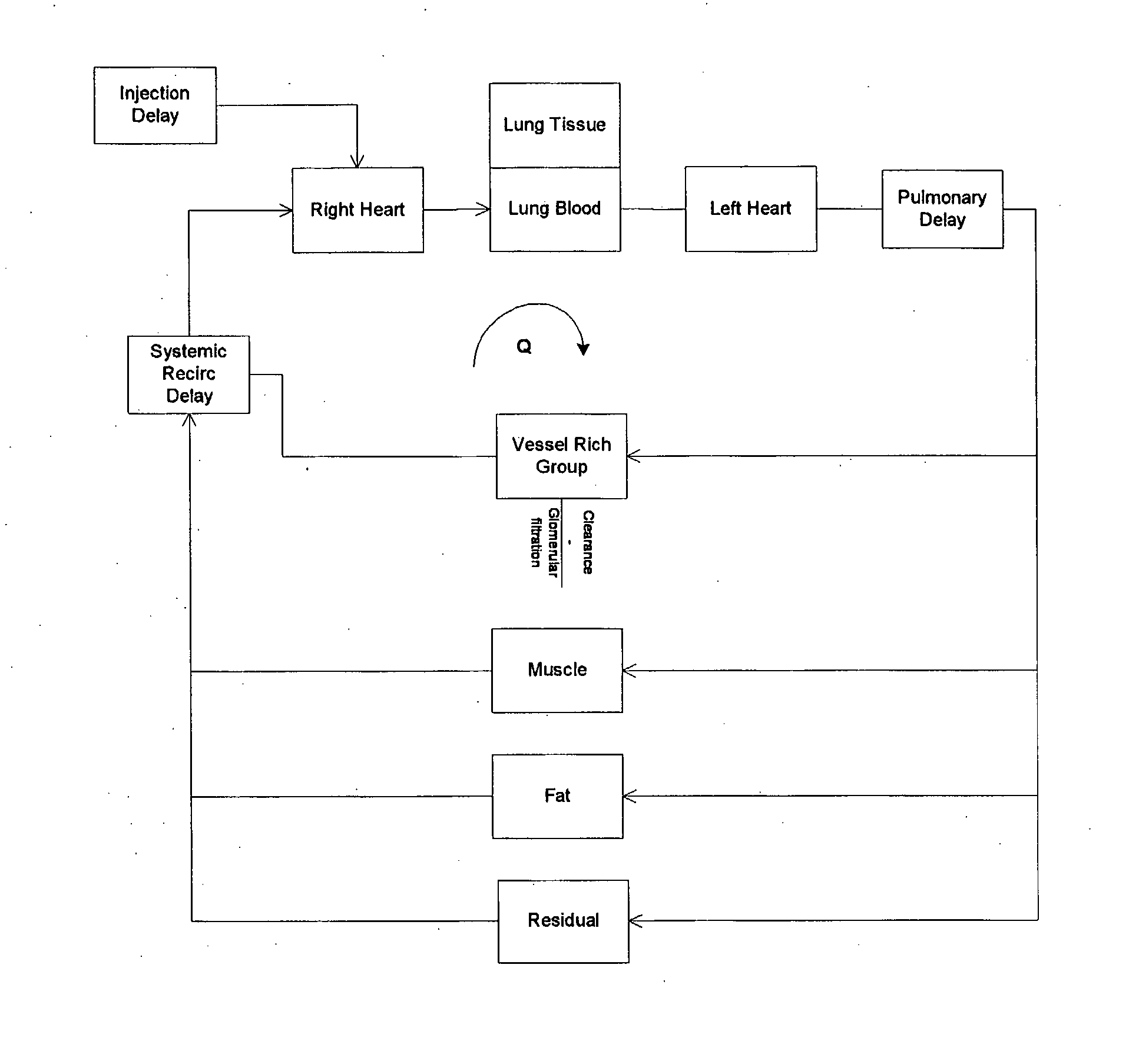
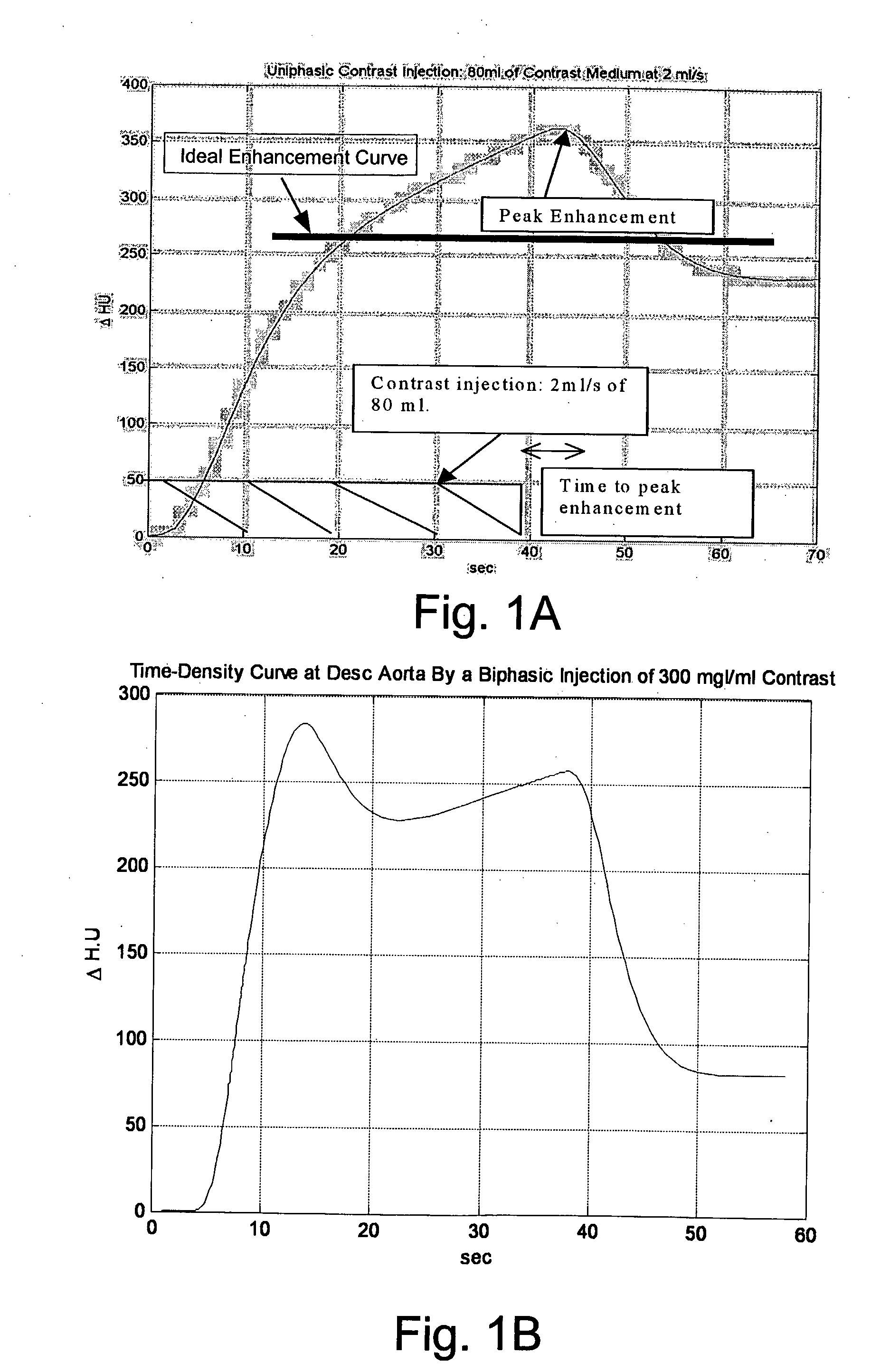
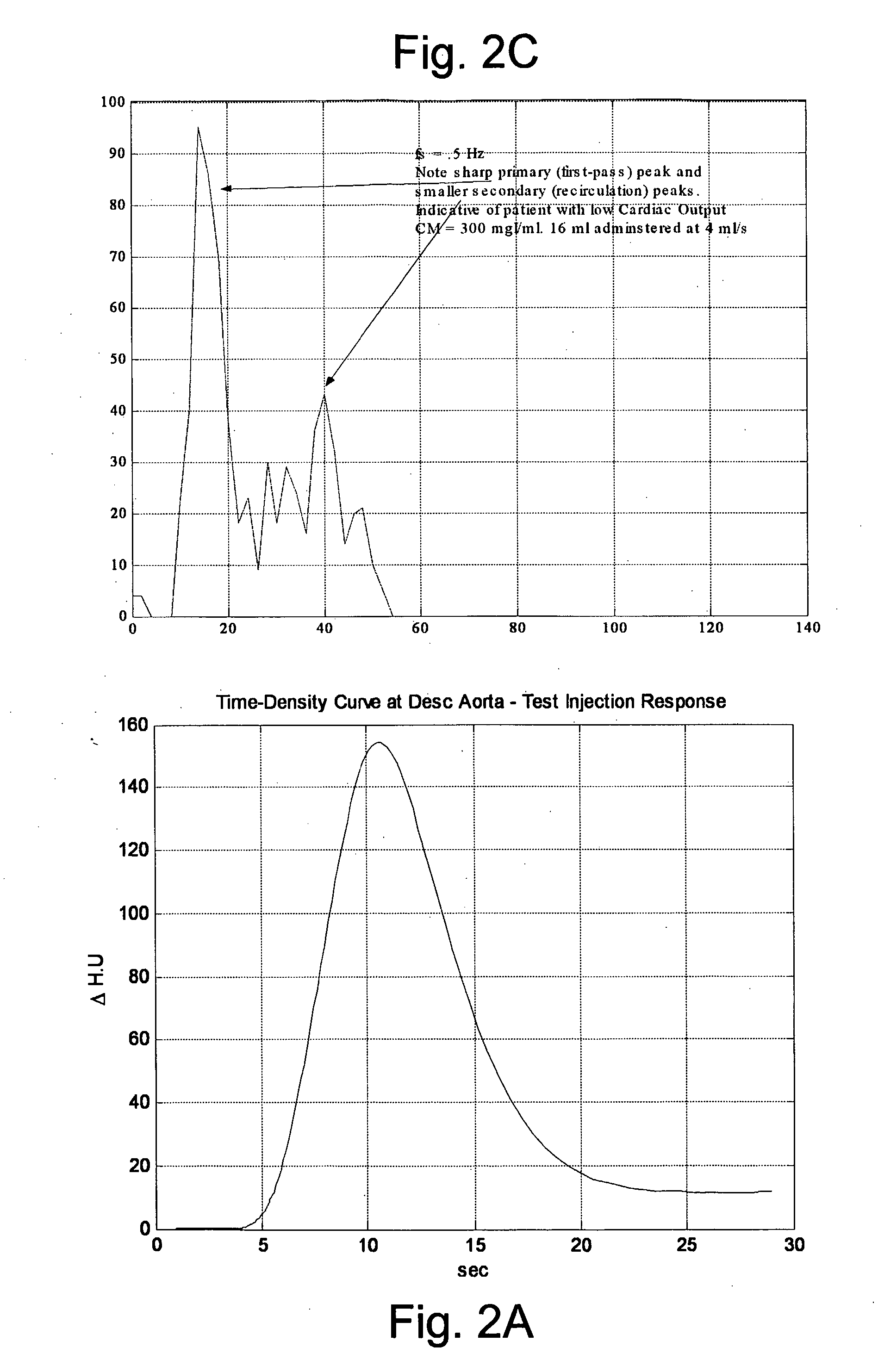
Modeling of Pharmaceutical Propagation
InactiveUS20080097197A1Overall light weightReduce contrastUltrasonic/sonic/infrasonic diagnosticsMagnetic measurementsMathematical modelContrast medium
A method of modeling propagation of a pharmaceutical fluid in a patient, includes: collecting data corresponding to a time response curve resulting from injection of the fluid; and determining at least one mathematical model describing the data. The mathematical model can, for example, be a model which is not determined by a continuous or a discrete-time Fourier transform of the data. A method of controlling injection of a pharmaceutical fluid into a patient using an injector in a medical procedure, includes: collecting data corresponding to a patient response curve resulting from injection of the fluid; determining at least one mathematical model describing the data; and controlling the injector during the medical procedure to control injection of the fluid into the patient to create patient response at least in part on the basis of the mathematical model. A method of controlling injection of a contrast medium into a patient using an injector in a medical imaging procedure using an imaging scanner, includes: determining at least one mathematical model to predict a time enhancement response resulting from injection of the contrast medium; determining an injection protocol to approximate a predetermined time enhancement response in the patient by determining a constrained input solution to the mathematical model; and using the injection protocol to control the injector during the medical imaging procedure to control injection of the contrast medium into the patient to create an image of a region of interest.
Owner:BAYER HEALTHCARE LLC
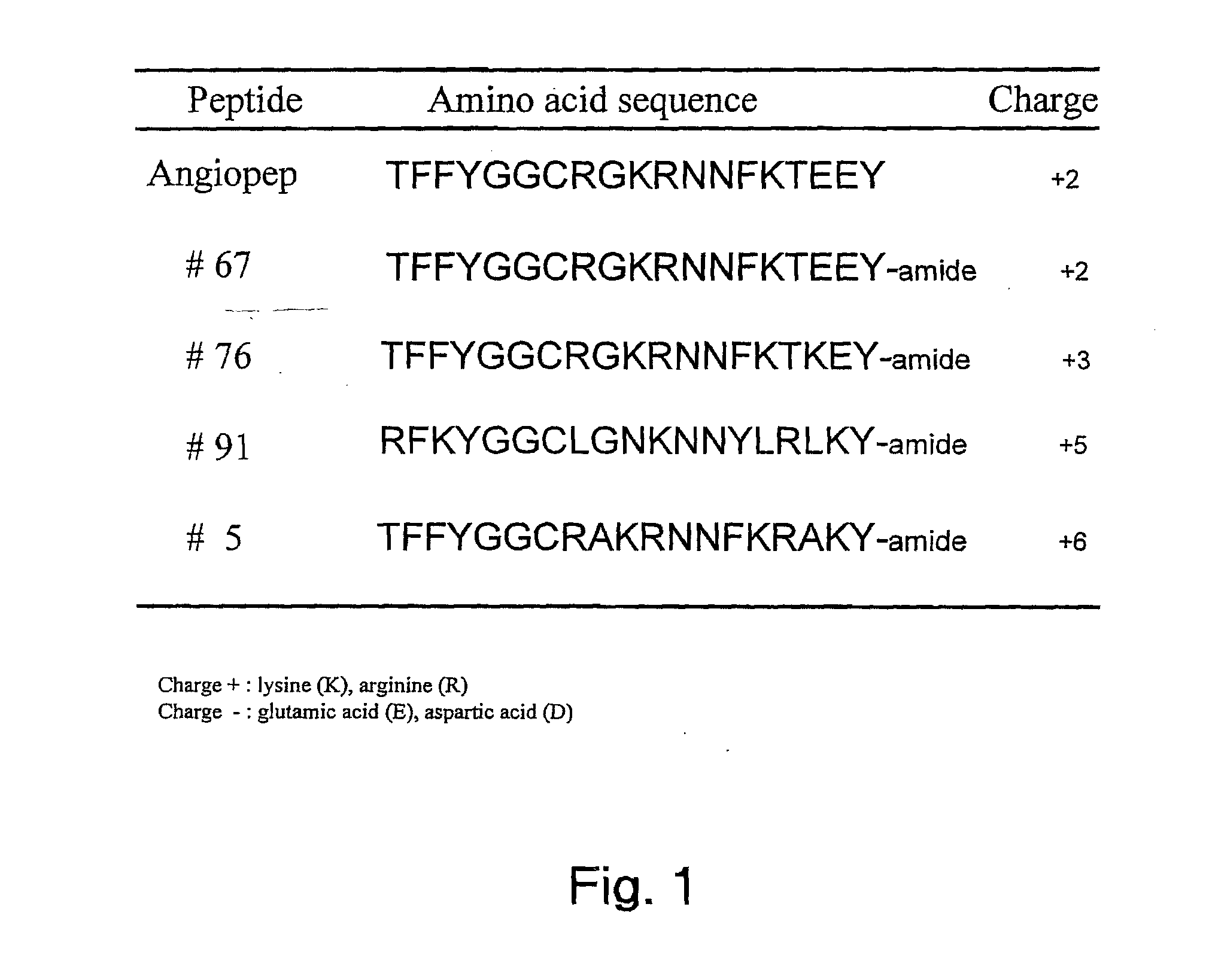
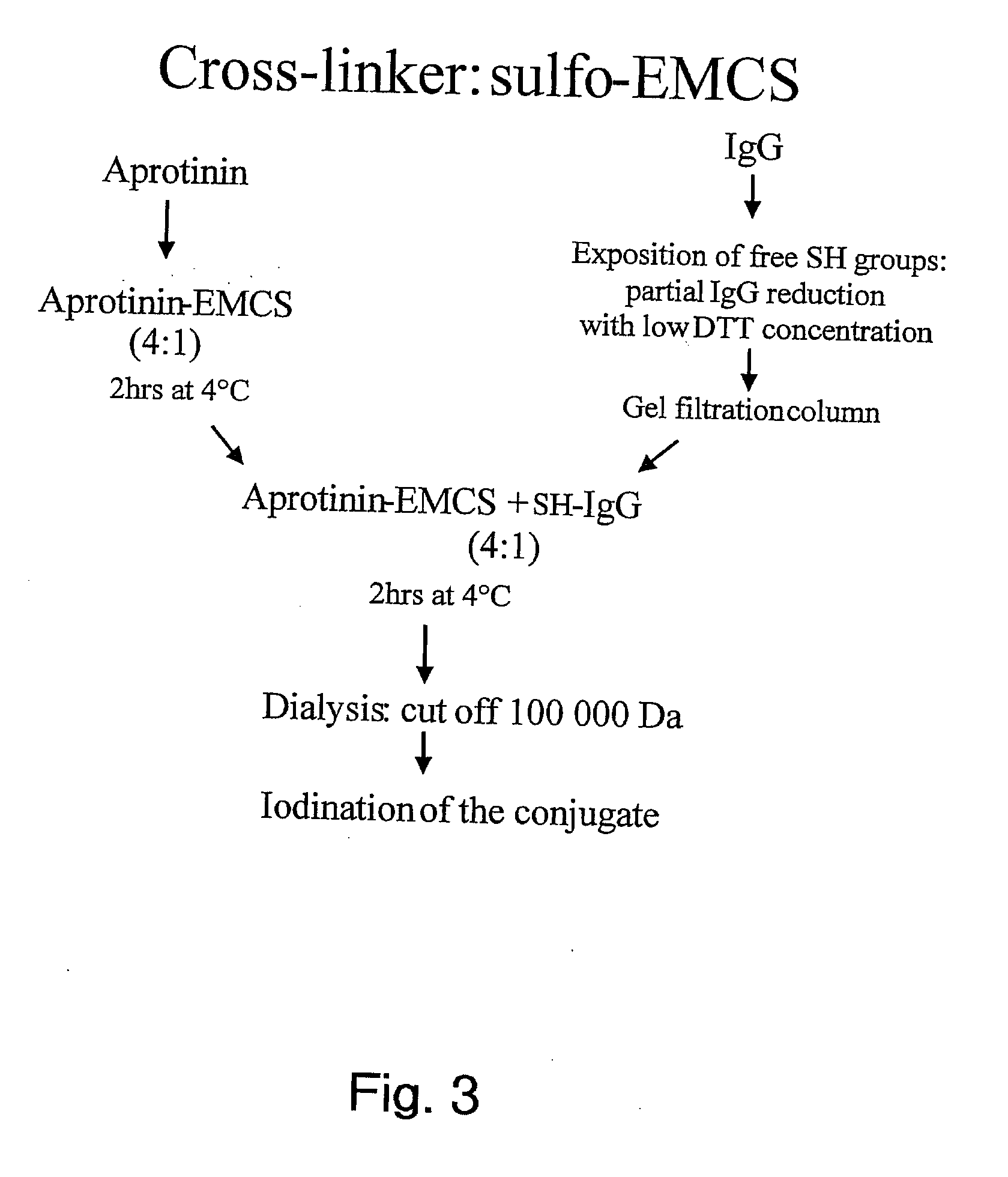
Aprotinin-like polypeptides for delivering agents conjugated thereto to tissues
ActiveUS20100297120A1Altered tissue distributionPromote accumulationPeptide/protein ingredientsPeptide preparation methodsDiseaseAprotinin
Based on our identification of a polypeptide (Angiopep-7) that is efficiently transported to cells such as liver, lung, kidney, spleen, and muscle, the invention provides polypeptides, conjugates including the polypeptides, and methods for treating diseases associated with these cell types. Unlike other aprotinin related polypeptides identified herein (including Angiopep-3, Angiopep-4a Angiopep-4b Angiopep-5, and Angiopep-6) which efficiently cross the blood-brain barrier (BBB), Angiopep-7 is not efficiently transported across the BBB.
Owner:ANGLACHEM INC
Microprojection array immunization patch and method
InactiveUS20050025778A1Adequate buffering capacityIncrease concentrationMicroneedlesSurgeryCurative effectVaccine antigen
Microprojection members (10) having a reservoir containing an antigenic agent and methods of using such members to vaccinate mammals (e.g., humans) are disclosed. The microprojection members are used to transdermally deliver an antigenic agent (e.g., a vaccine antigen) with substantially reduced skin reactions. This is achieved by delivering an induction amount and thereafter delivering one or more subsequent booster amounts. The induction amount is relatively larger than the booster amount. This technology has broad applicability for a wide variety of therapeutic vaccines to improve efficacy and convenience of use.
Owner:ALZA CORP
Therapeutic treatments using botulinum neurotoxin
ActiveUS20090232850A1Eliminates sensory inputImprove developmentBacterial antigen ingredientsNervous disorderDiseaseObstructive Pulmonary Diseases
Methods for treating a coronary risk factor (such as hypertension, diabetes, hyperlipidemia and obesity) and / or a respiratory disorder (such as asthma, chronic obstructive pulmonary disease and bronchitis) and / or arthritis by local administration of a botulinum neurotoxin to at least one of a head, neck or shoulder location (for example, by subdermal, subcutaneous or intramuscular administration of the botulinum neurotoxin) of a patient with a coronary risk factor, respiratory disorder or arthritis.
Owner:ALLERGAN INC
Apparatus for treating liquids with wave energy from an electrical arc
ActiveUS7857972B2Energy efficiencyReduce maintenanceWater distributersWaste water treatment from animal processingEngineeringEnergy source
The present invention provides a system, method and apparatus for treating a liquid by providing a wave energy source and creating a thin film of the liquid whirling around the wave energy source such that one or more wave energies irradiate the liquid. Likewise, the present invention provides a method of treating a liquid by providing three zones of wave energy and passing the liquid through the three zones of wave energy.
Owner:FORET PLASMA LABS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com