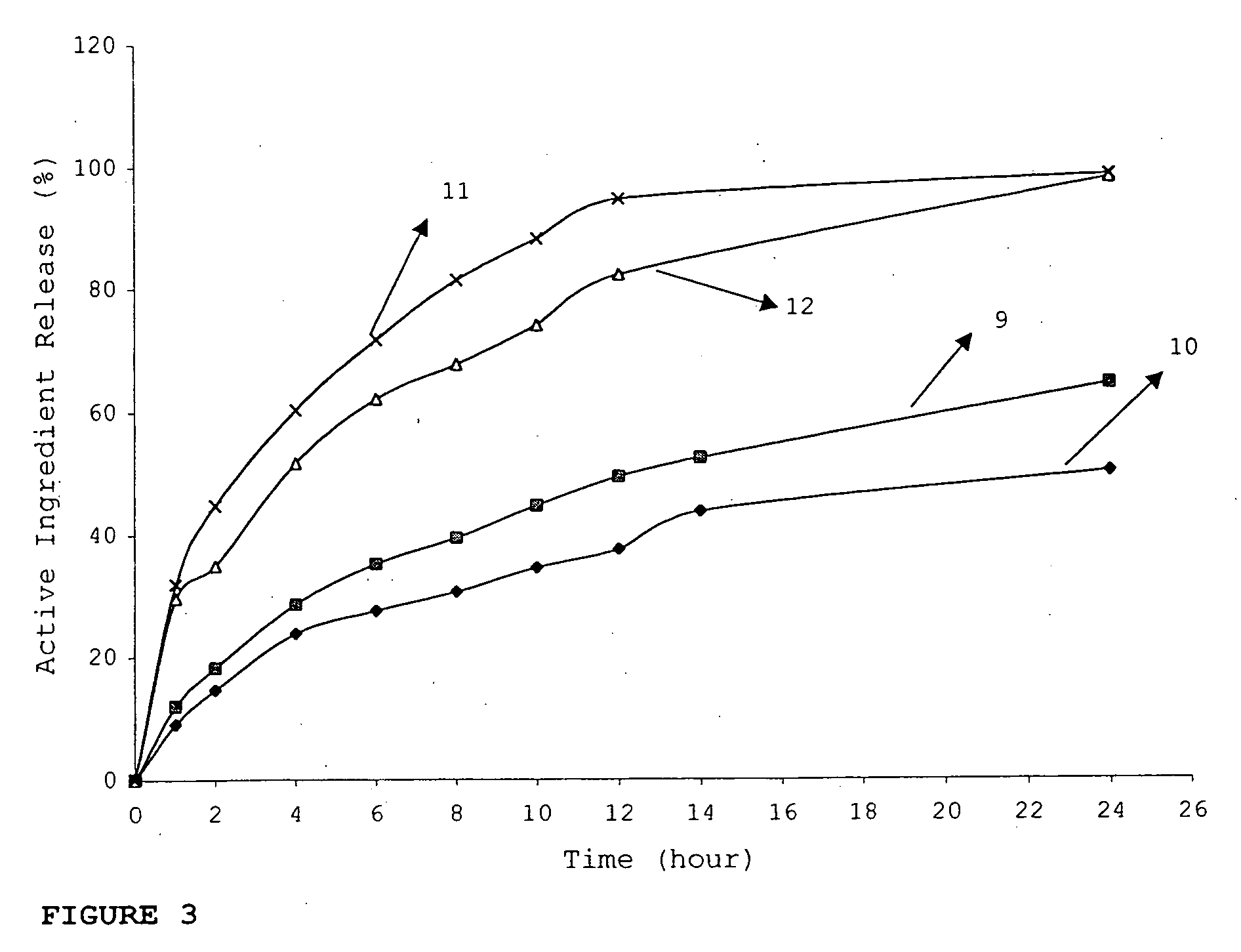
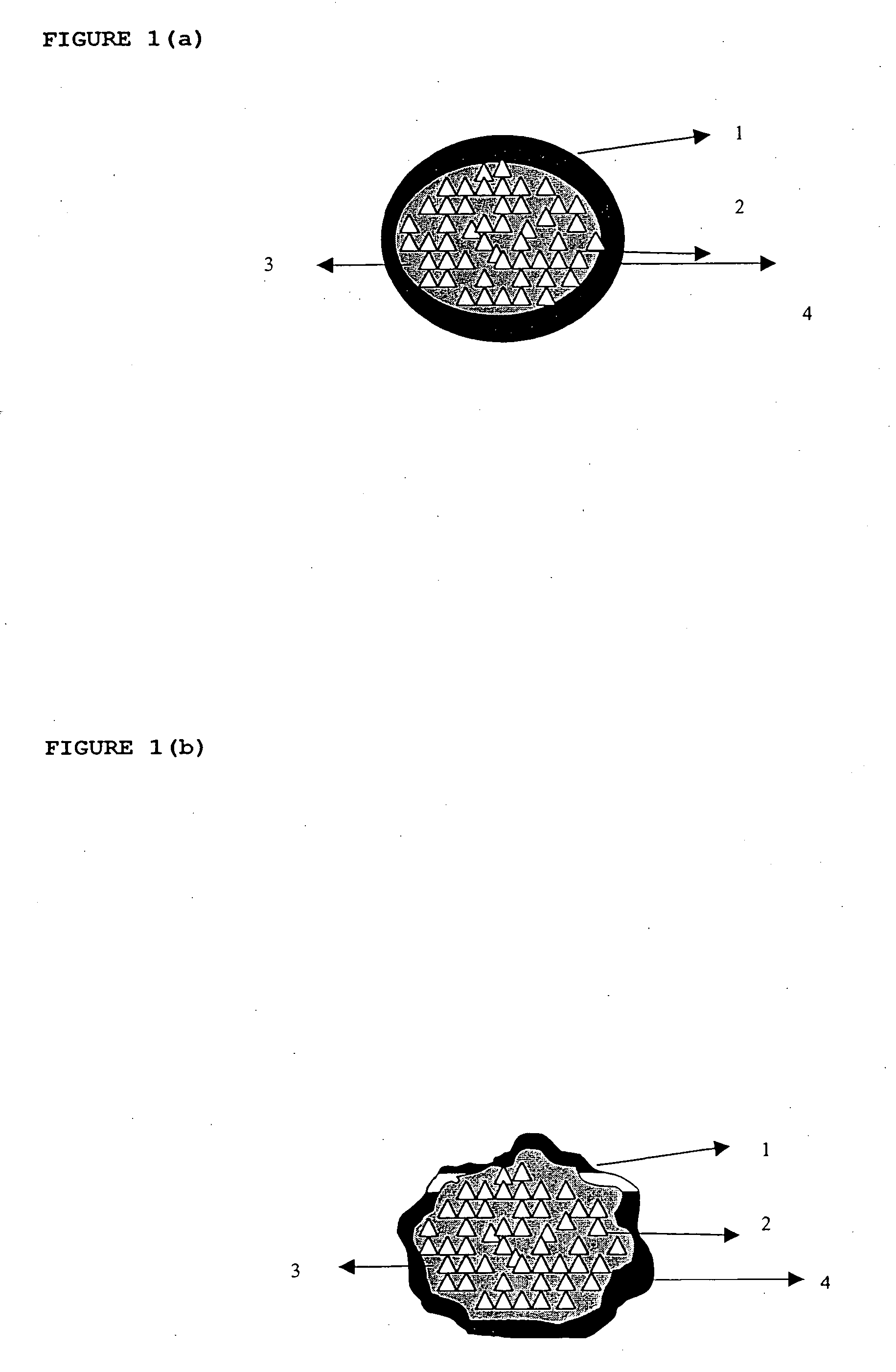
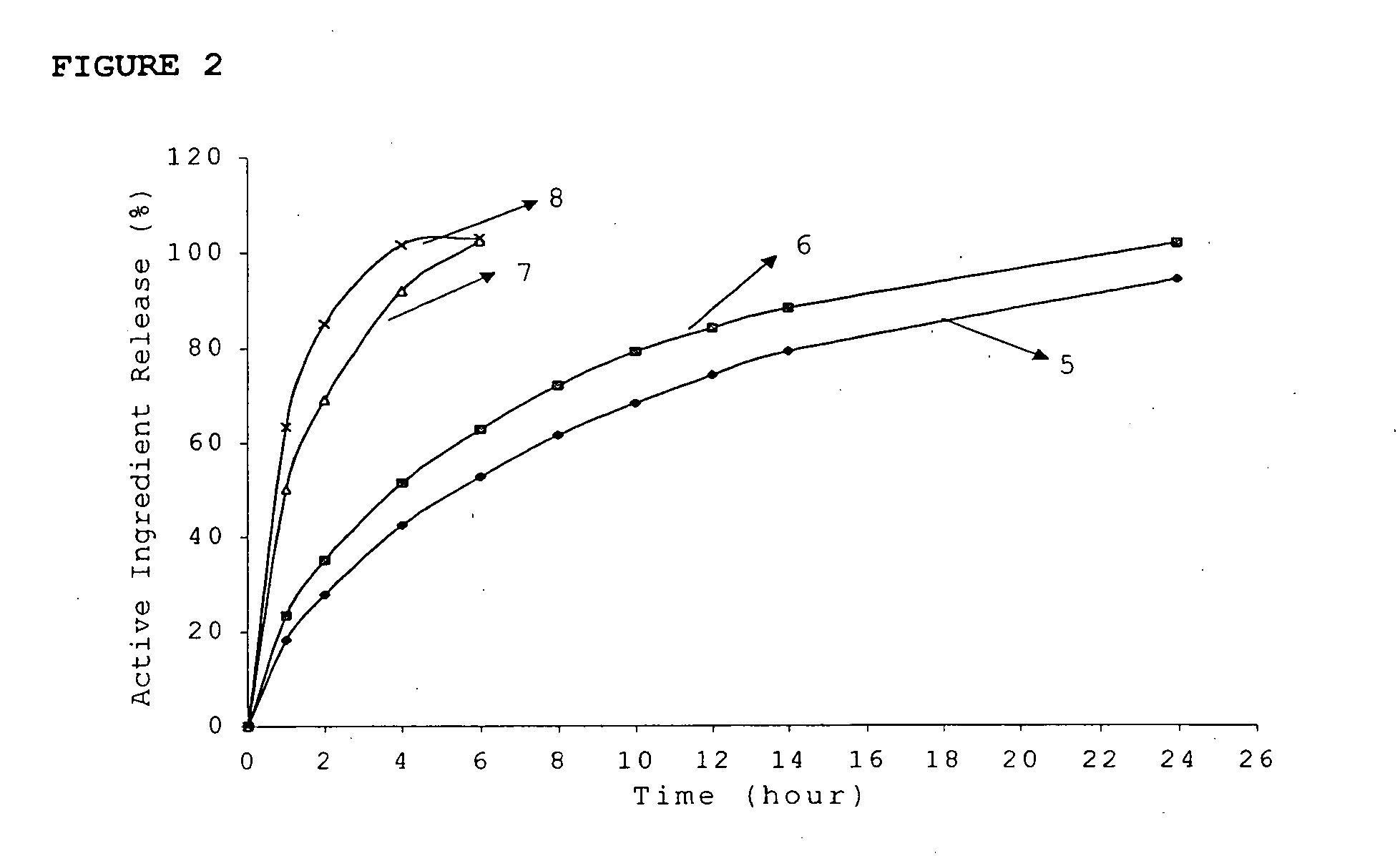
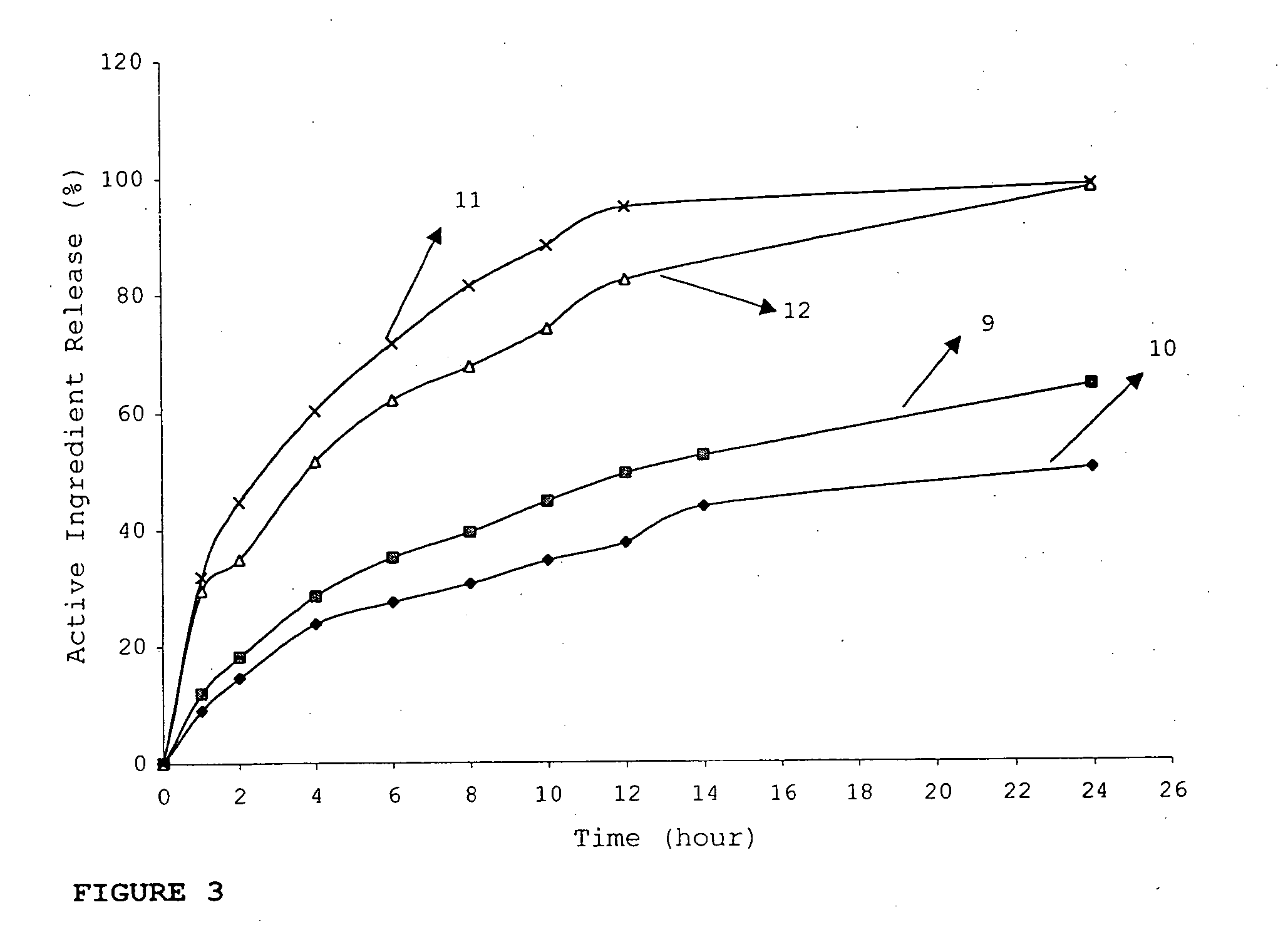
Patents
Literature
757results about How to "Easy to swallow" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel dosage form
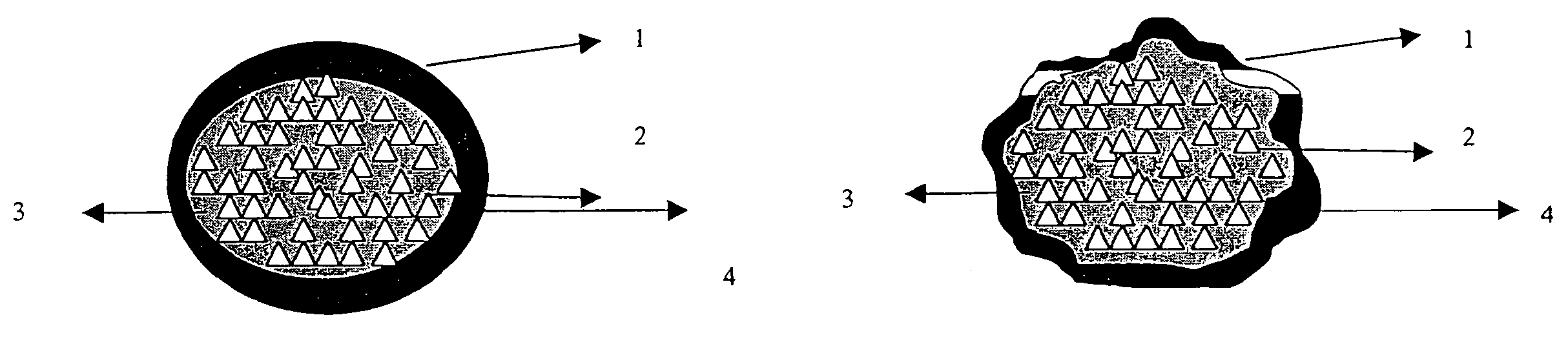
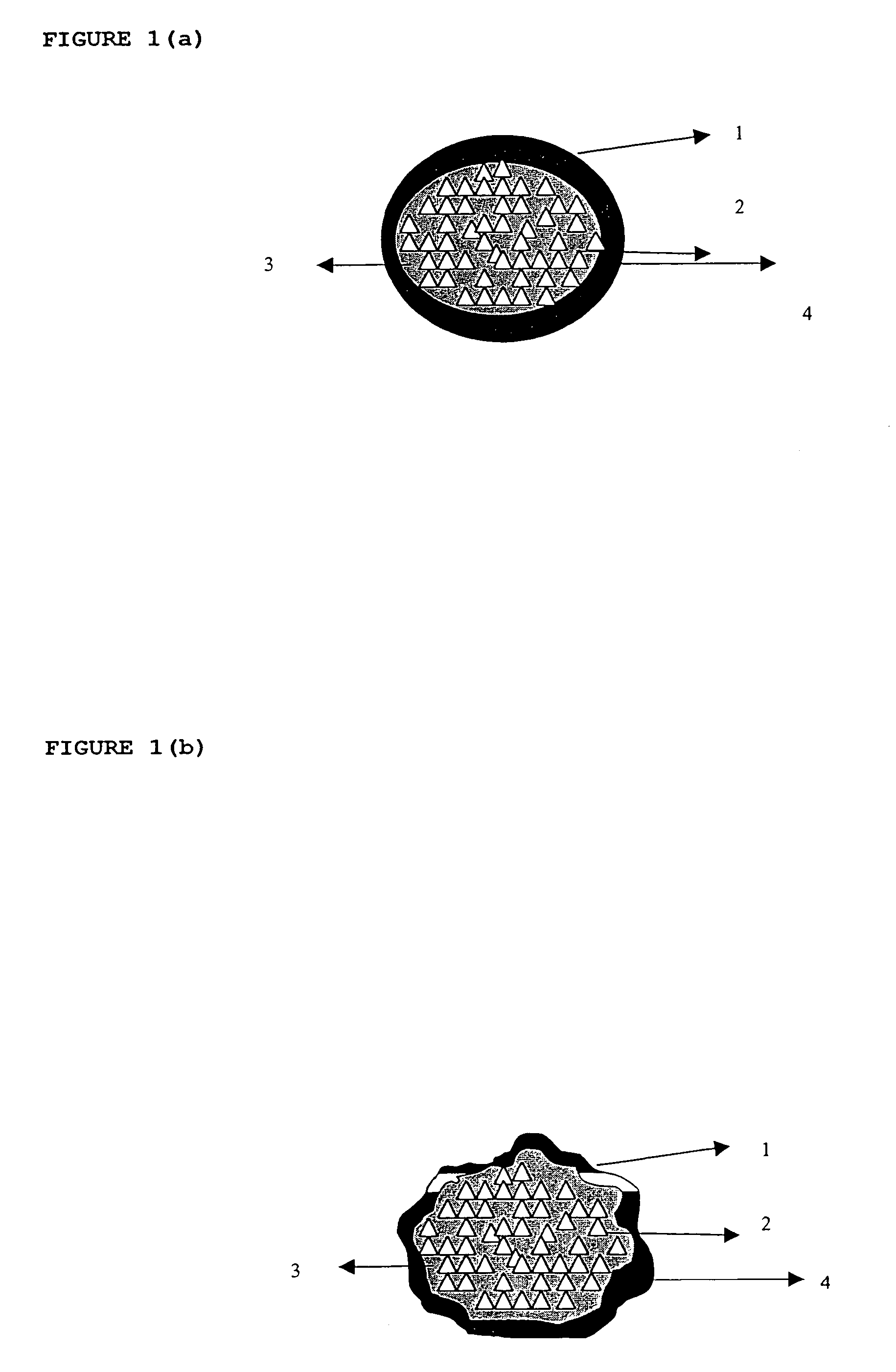
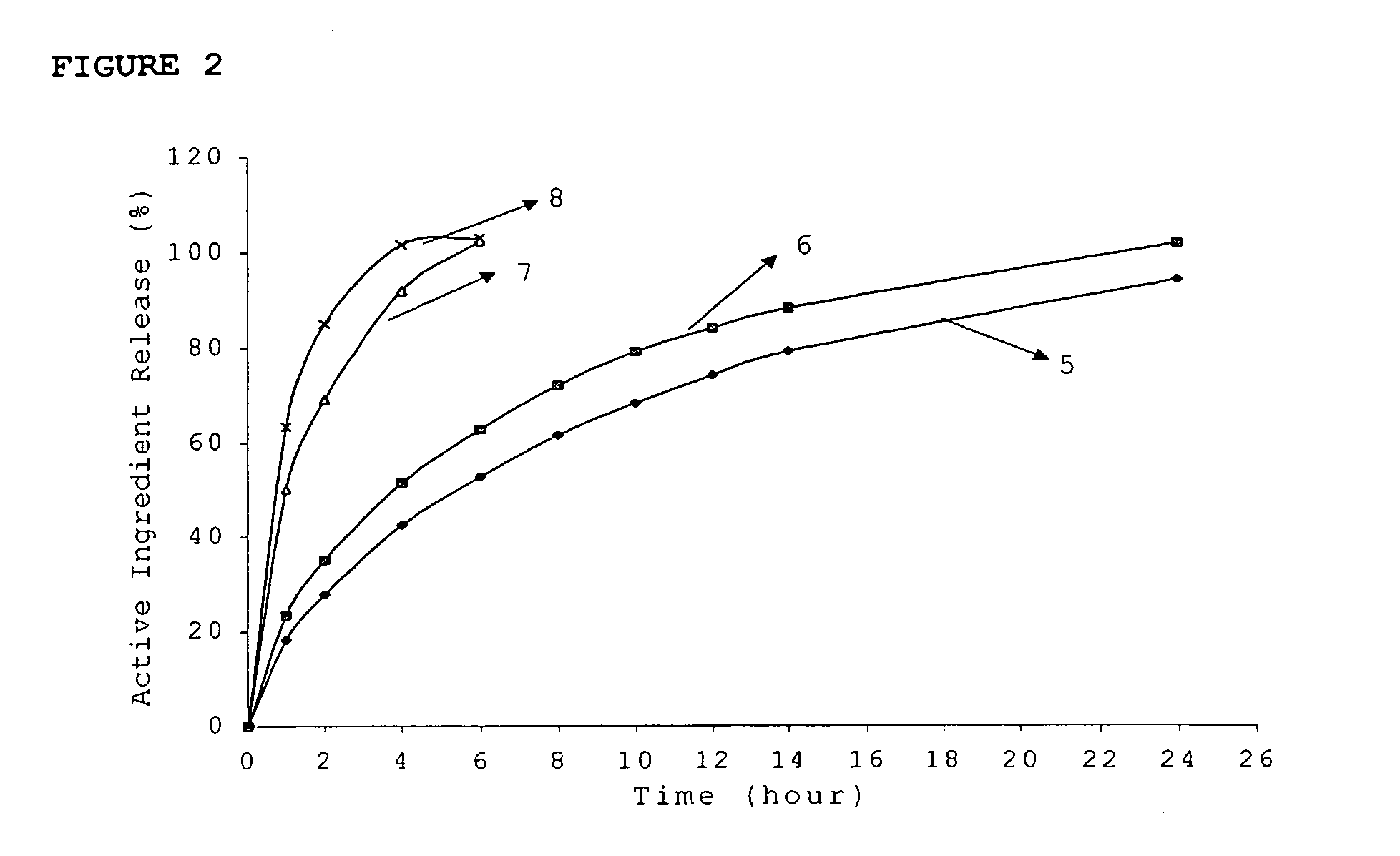
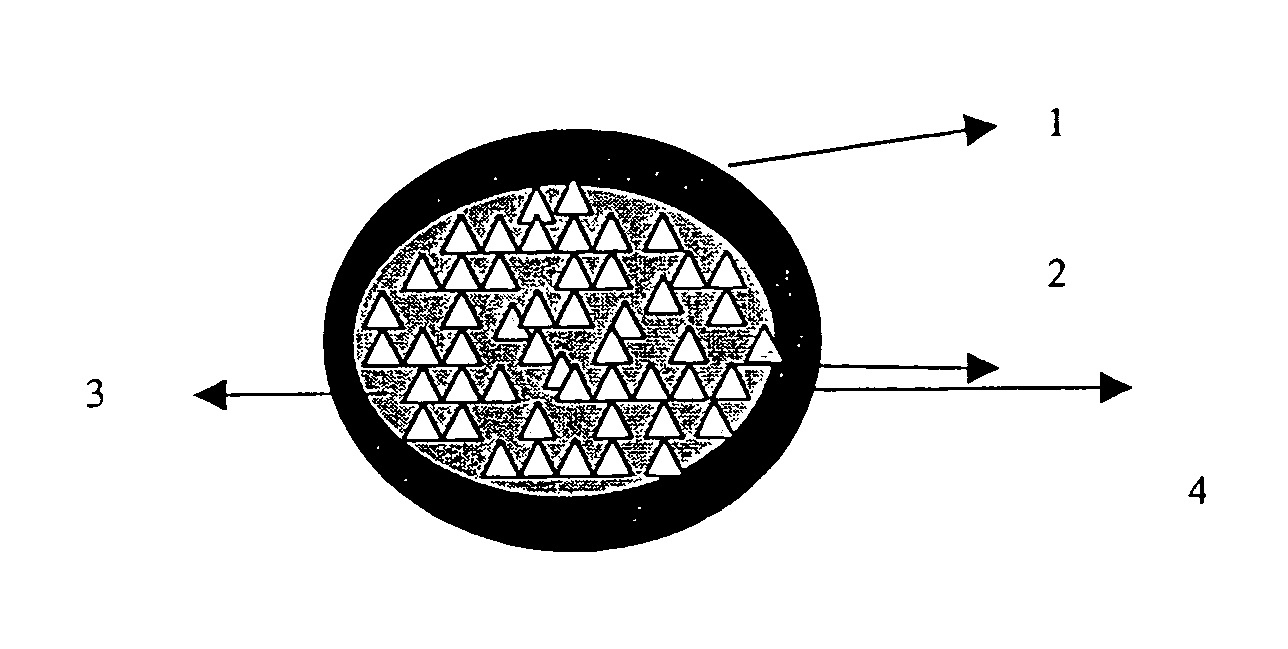
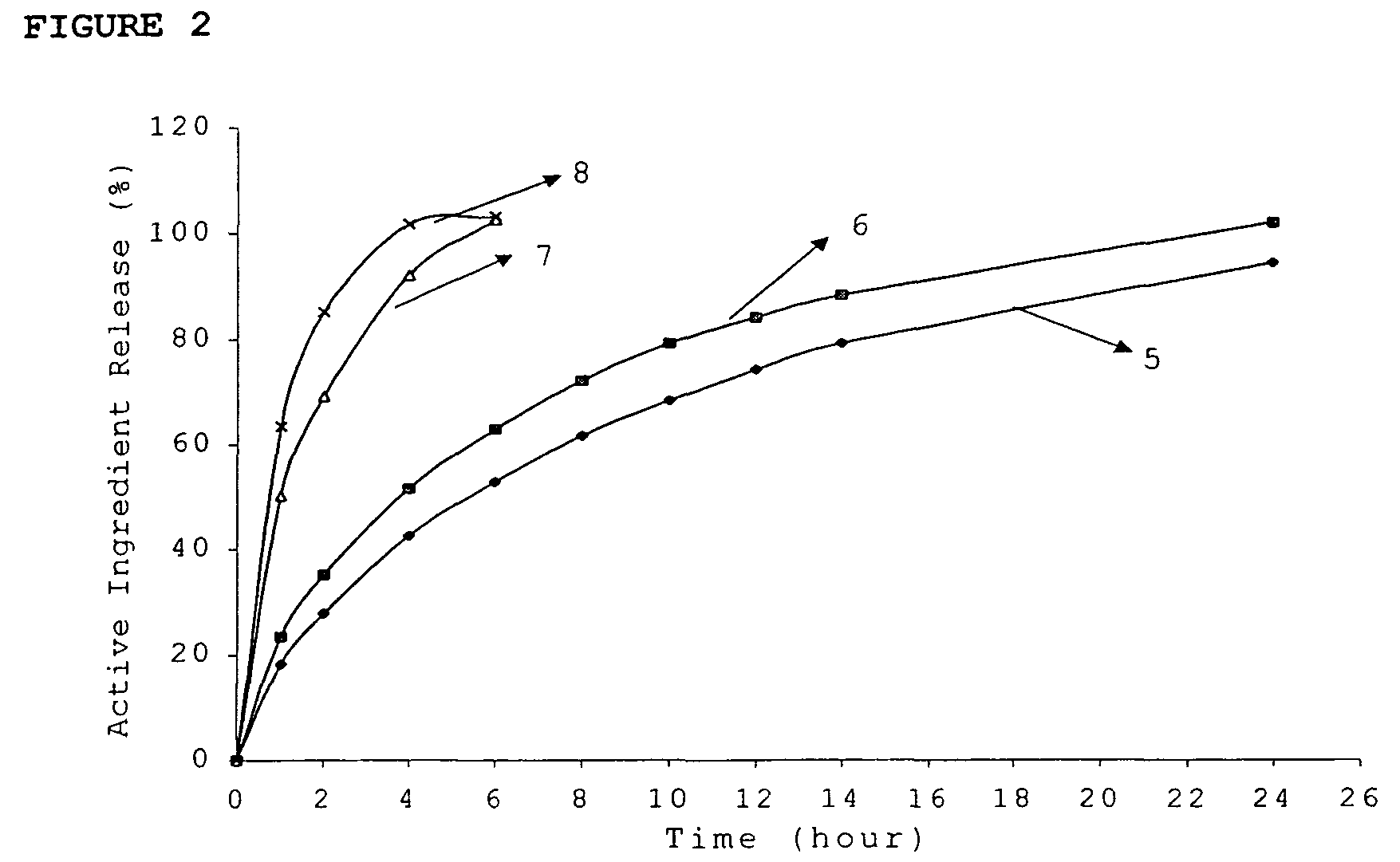
ActiveUS20060024365A1Effectively control release rateReduce sizePill deliveryEster active ingredientsHigh dosesSolubility
A dosage form comprising of a high dose, high solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of modified release active ingredient per unit is from 500 mg to 1500 mg; a process for preparing the dosage form.
Owner:TORRENT PHARMA LTD
Novel drug delivery system
InactiveUS20060018933A1Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Novel drug delivery system
InactiveUS20060018934A1Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Modified release composition for highly soluble drugs
InactiveUS8268352B2Effectively control release rateSmall sizePill deliveryMicrocapsulesSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
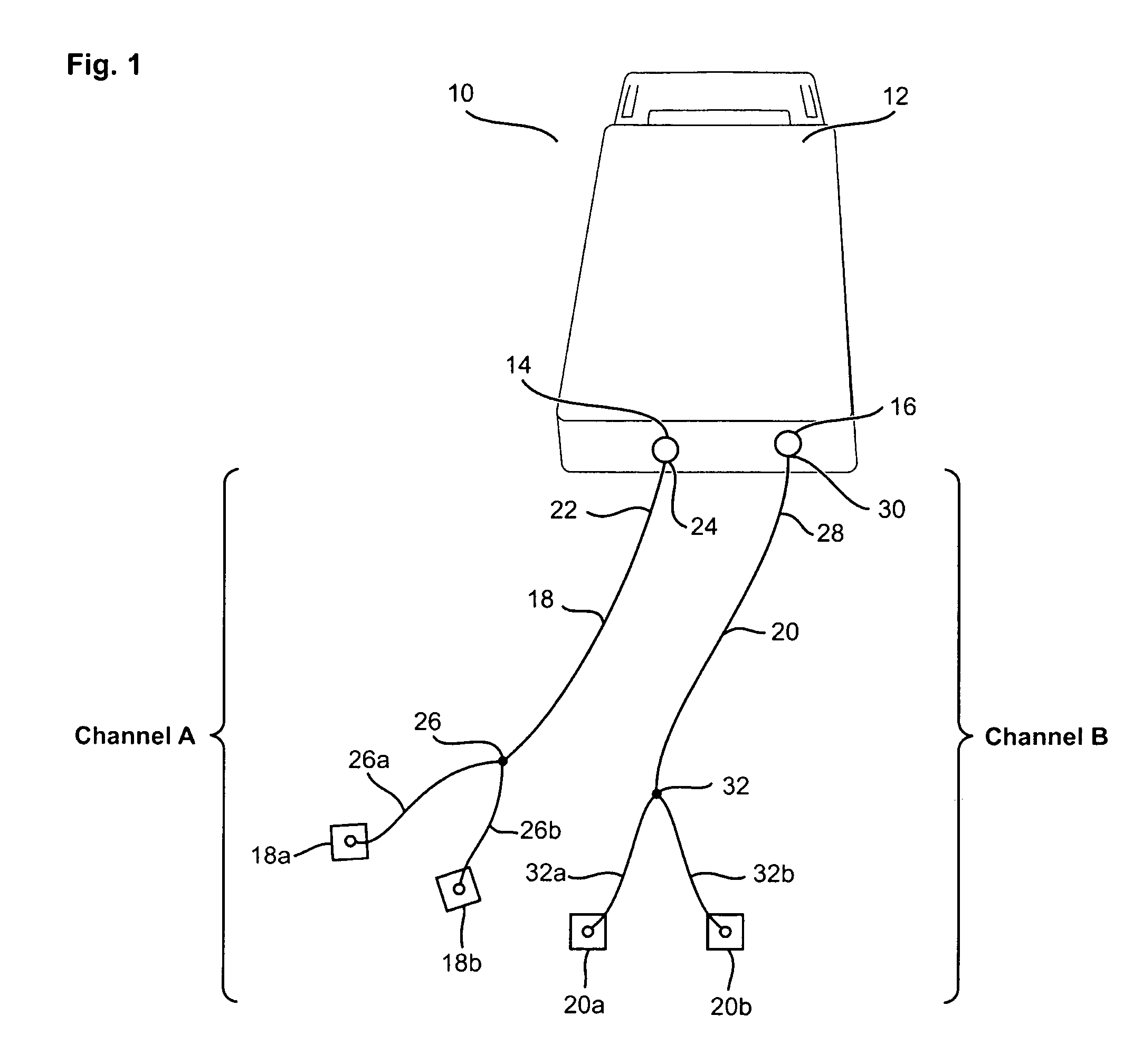
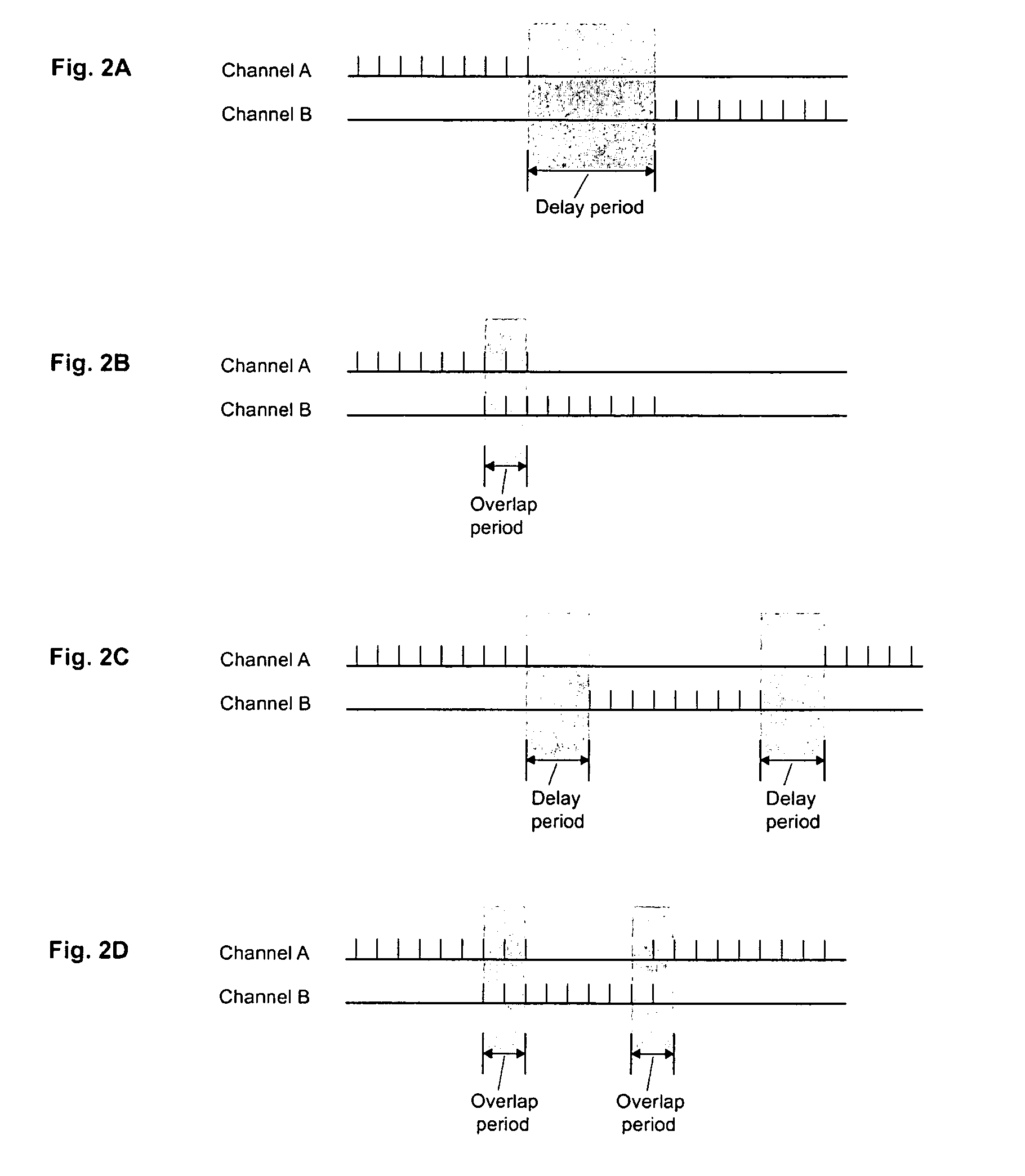
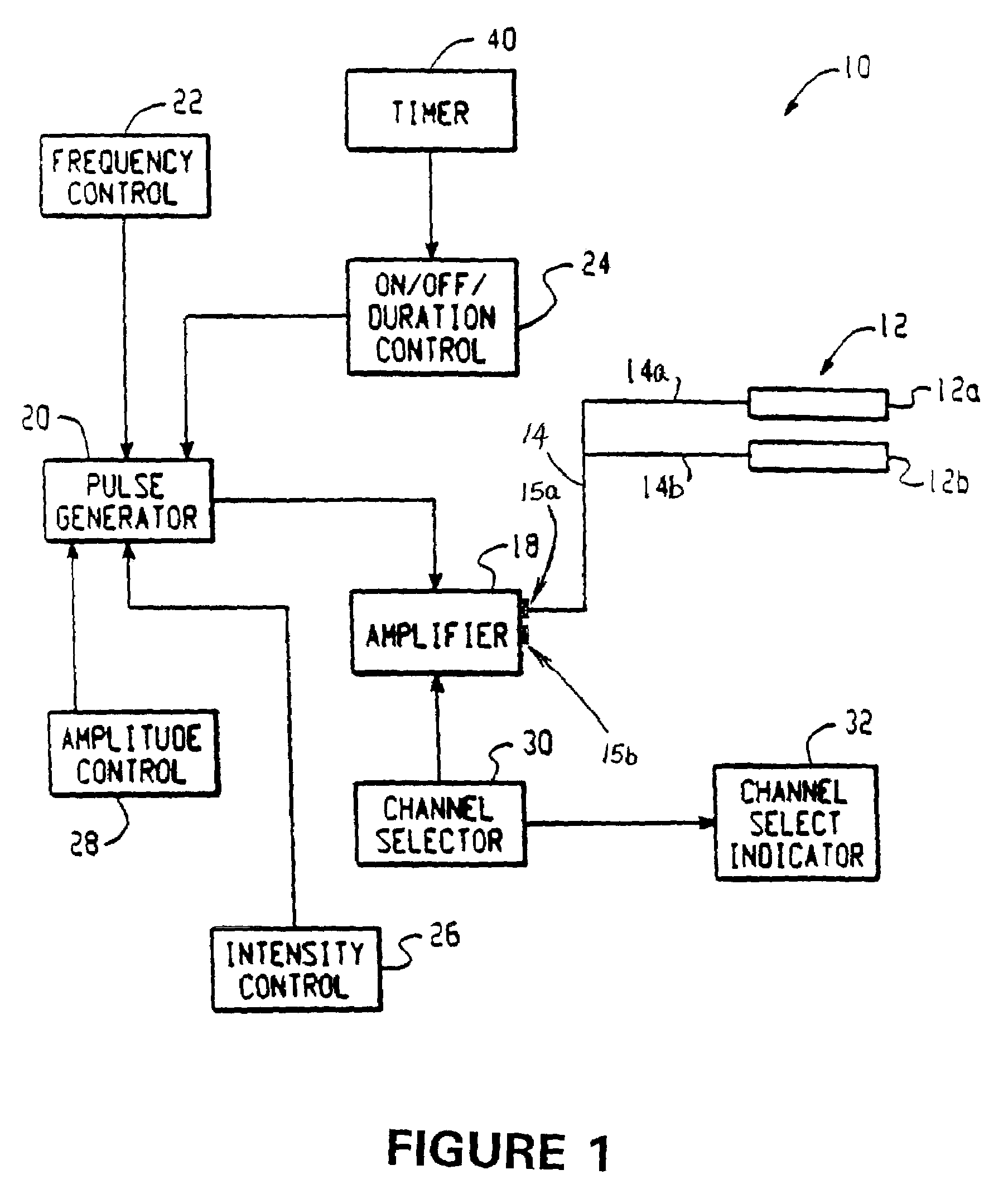
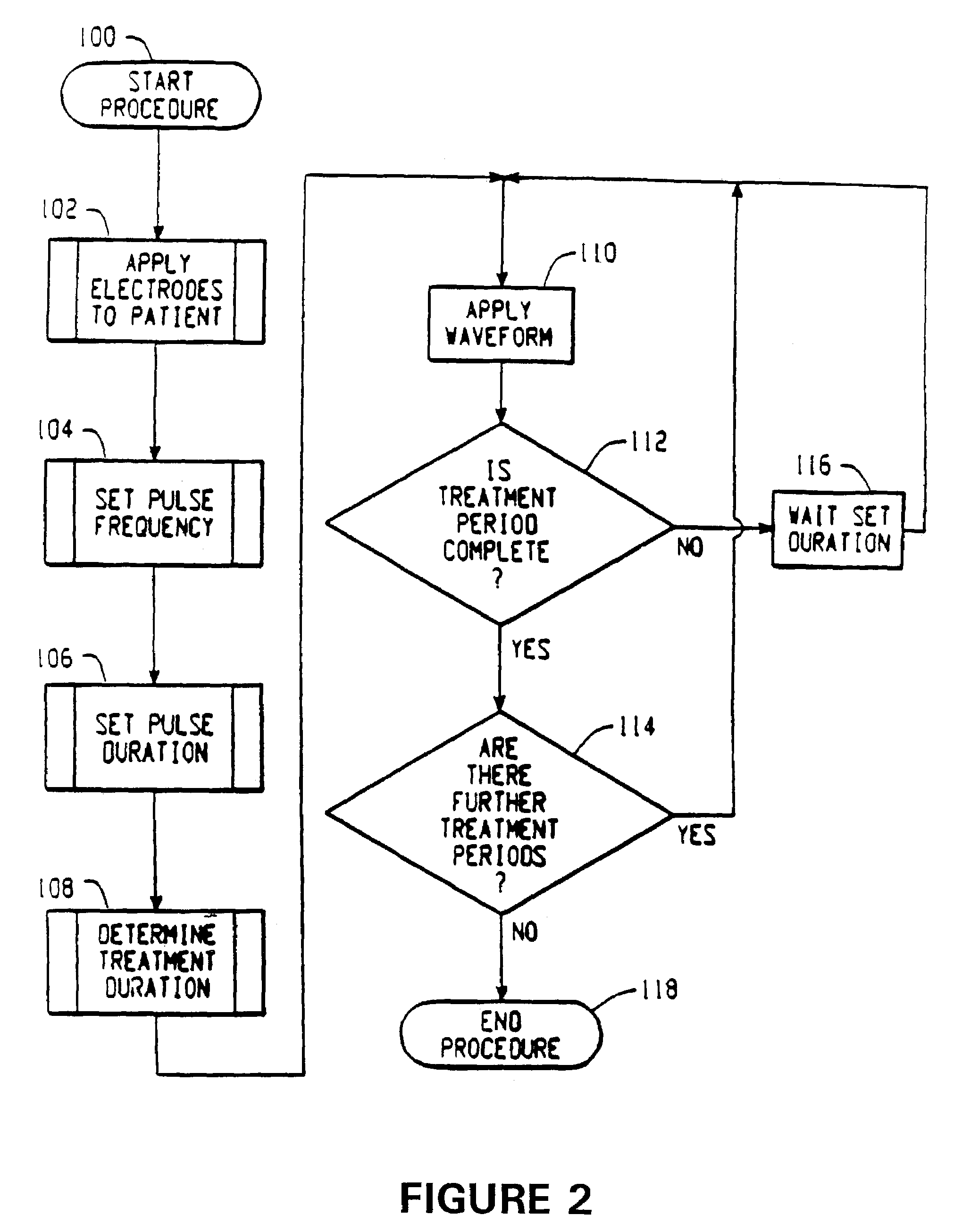
Electrical stimulation device and method for the treatment of dysphagia
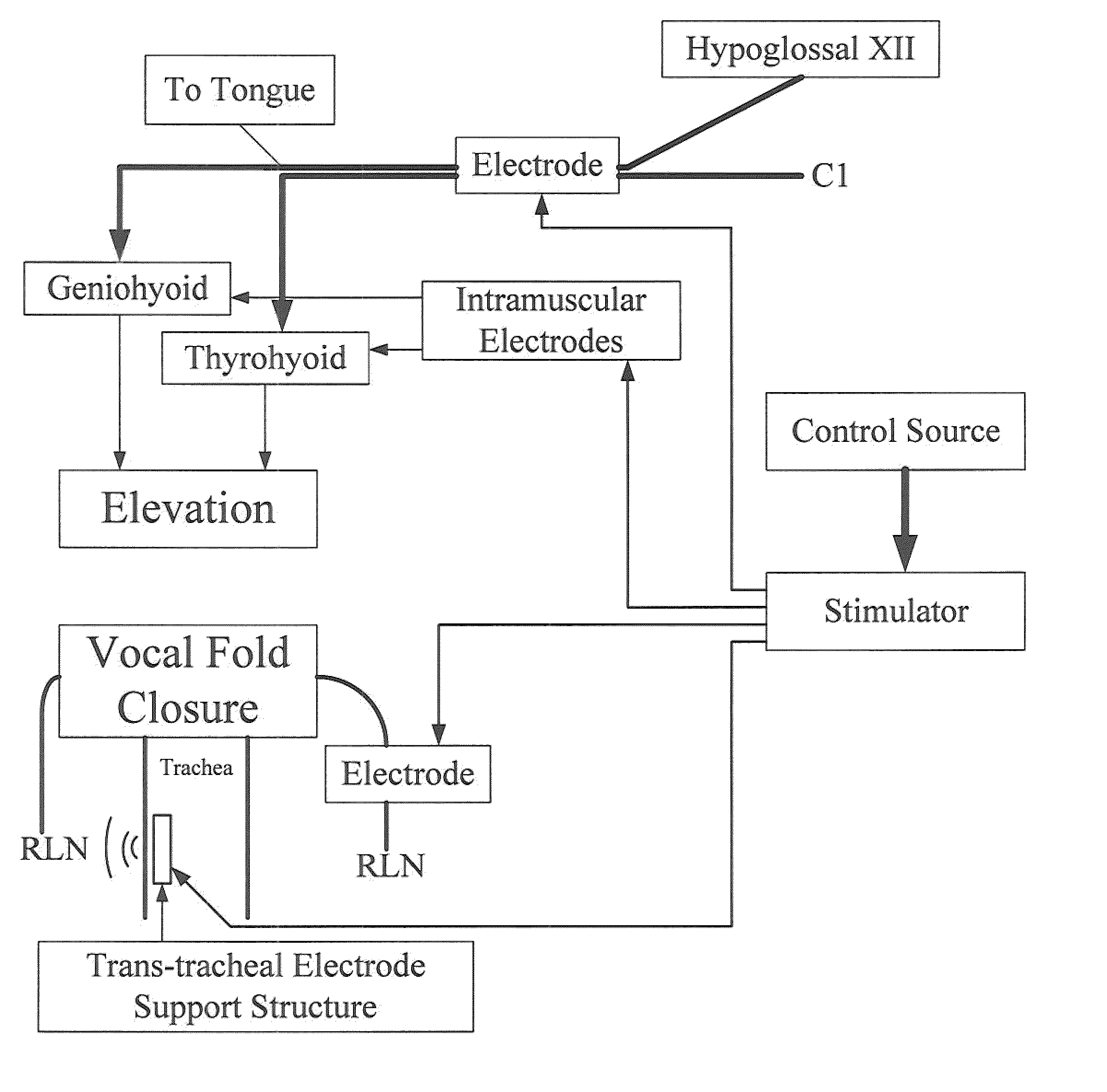
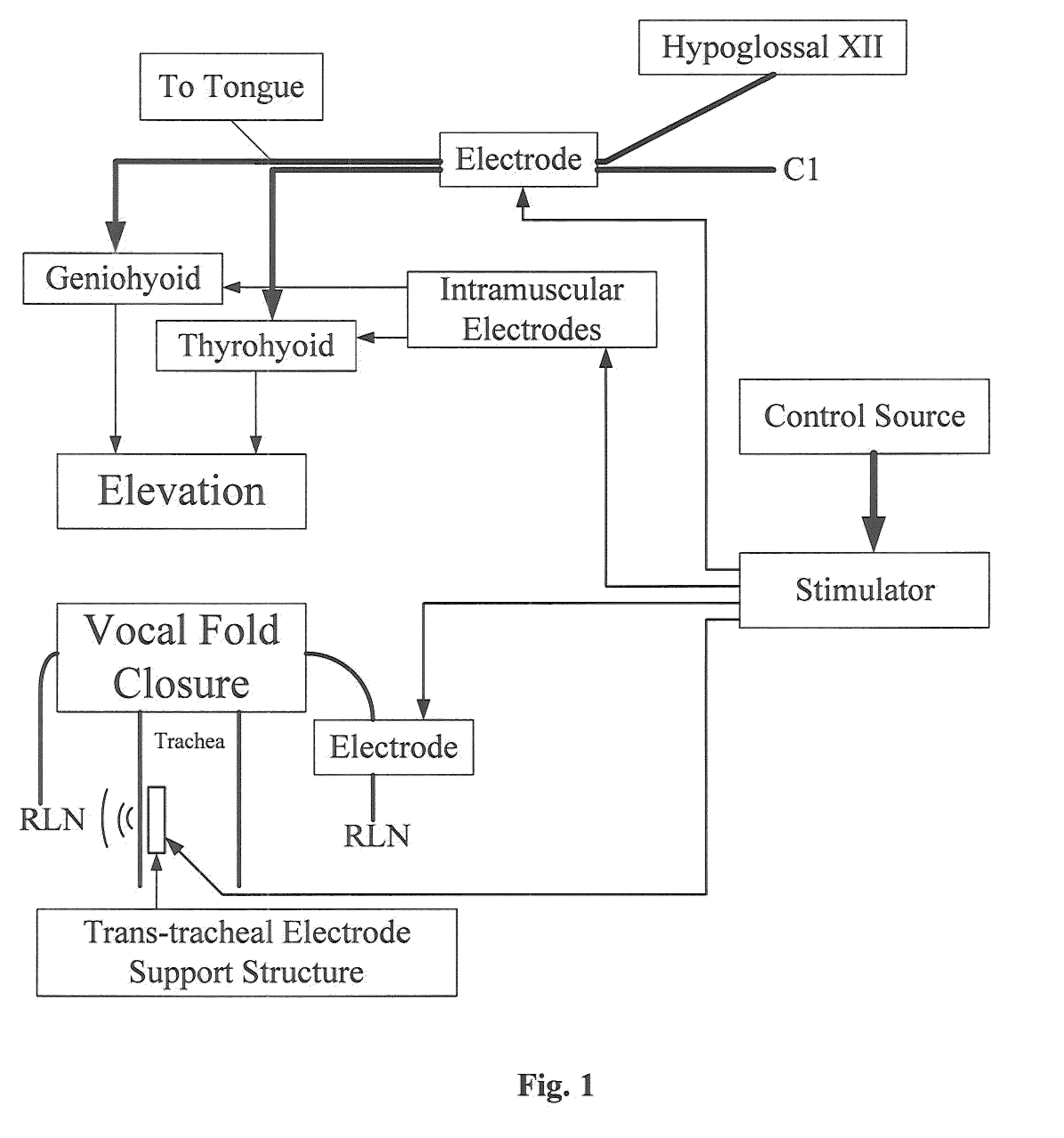
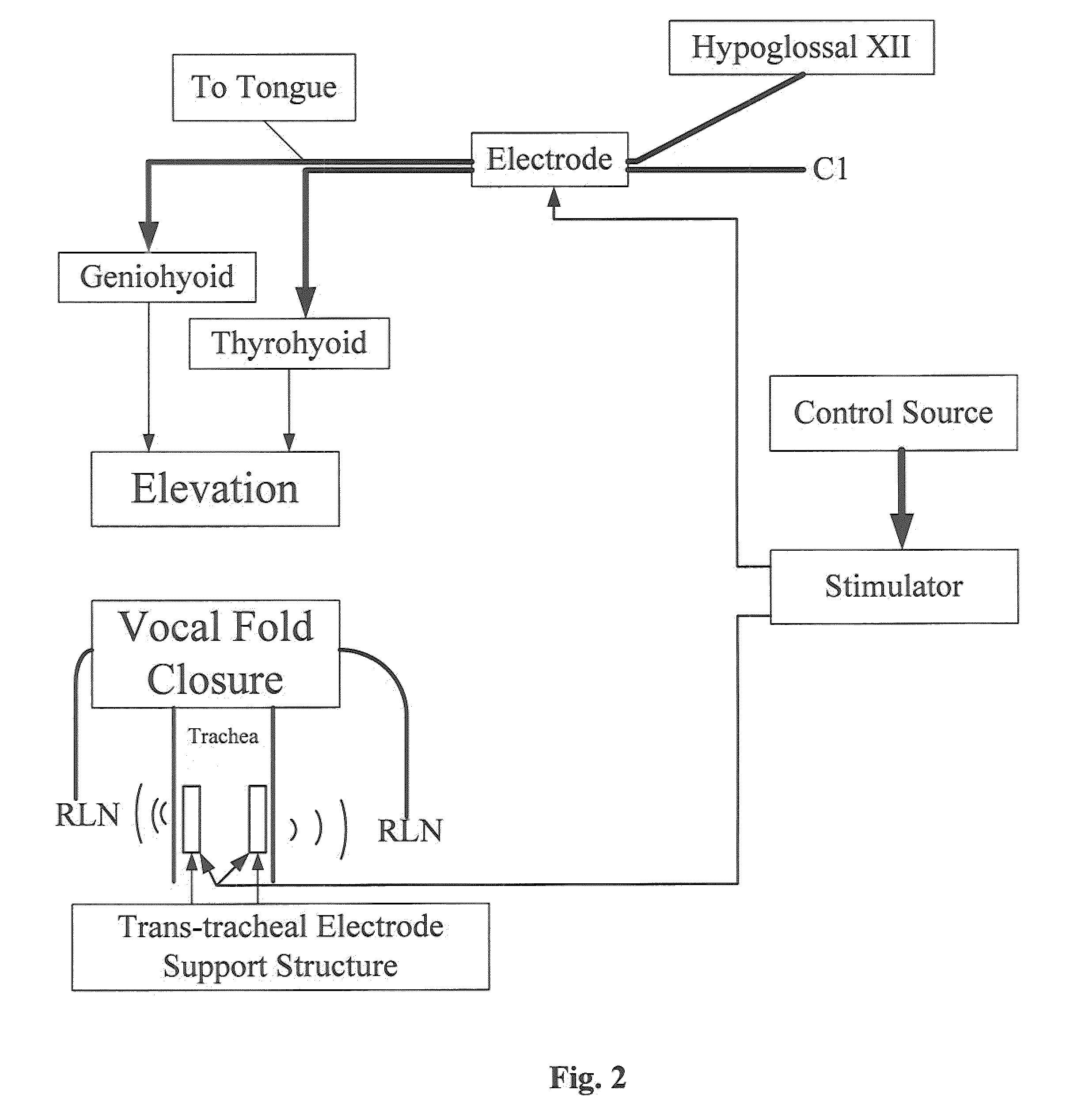
An electrical stimulation device and method for the treatment of dysphagia is disclosed. In a preferred embodiment, the electrical stimulation device includes one or more channels of electrodes each of which includes a first electrode positioned in electrical contact with tissue of a target region of a patient and a second electrode positioned in electrical contact with tissue of a posterior neck region or a posterior thoracic region of the patient. A series of electrical pulses are then applied to the patient through the one or more channels of electrodes in accordance with a procedure for treating dysphagia. The series of electrical pulses may comprise: a plurality of cycles of a biphasic sequential pulse train pattern; a plurality of cycles of a biphasic overlapping pulse train pattern; a plurality of cycles of a triphasic sequential pulse train pattern; a plurality of cycles of a triphasic overlapping pulse train pattern; a functional pulse train pattern; a low-frequency pulse train pattern; or a frequency-sequenced pulse burst train pattern. Various exemplary embodiments of the invention are disclosed.
Owner:ACCELERATED CARE PLUS CORP
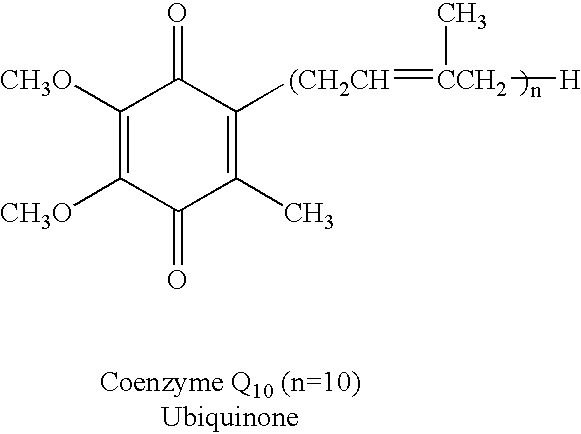
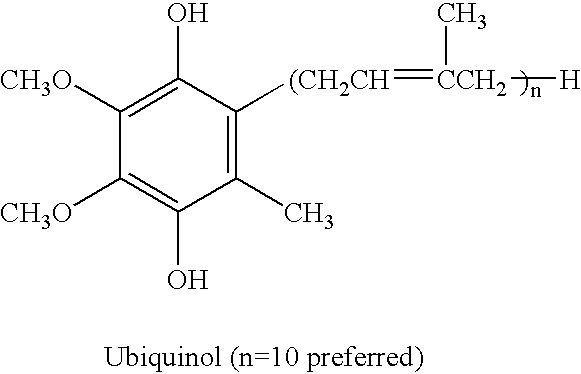
Reduced form of Coenzyme Q in high bioavailability stable oral dosage form
InactiveUS6740338B1Improve bioavailabilityReduced form requirementsBiocideEther/acetal active ingredientsOral medicationBioavailability
The present invention relates to a reduced form of Coenzyme Q also known as ubiquinol in oral dosage form such as a gelatin capsule, preferably a soft gelatin capsule. Compositions according to the present invention include storage stable compositions comprising effective amounts of ubiquinol in combination with an amount of a reducing agent effective to maintain ubiquinol in its reduced state when formulated in capsules, tablets and other orally administrable form. Methods of use are also disclosed.
Owner:QUTEN RES INST LLC
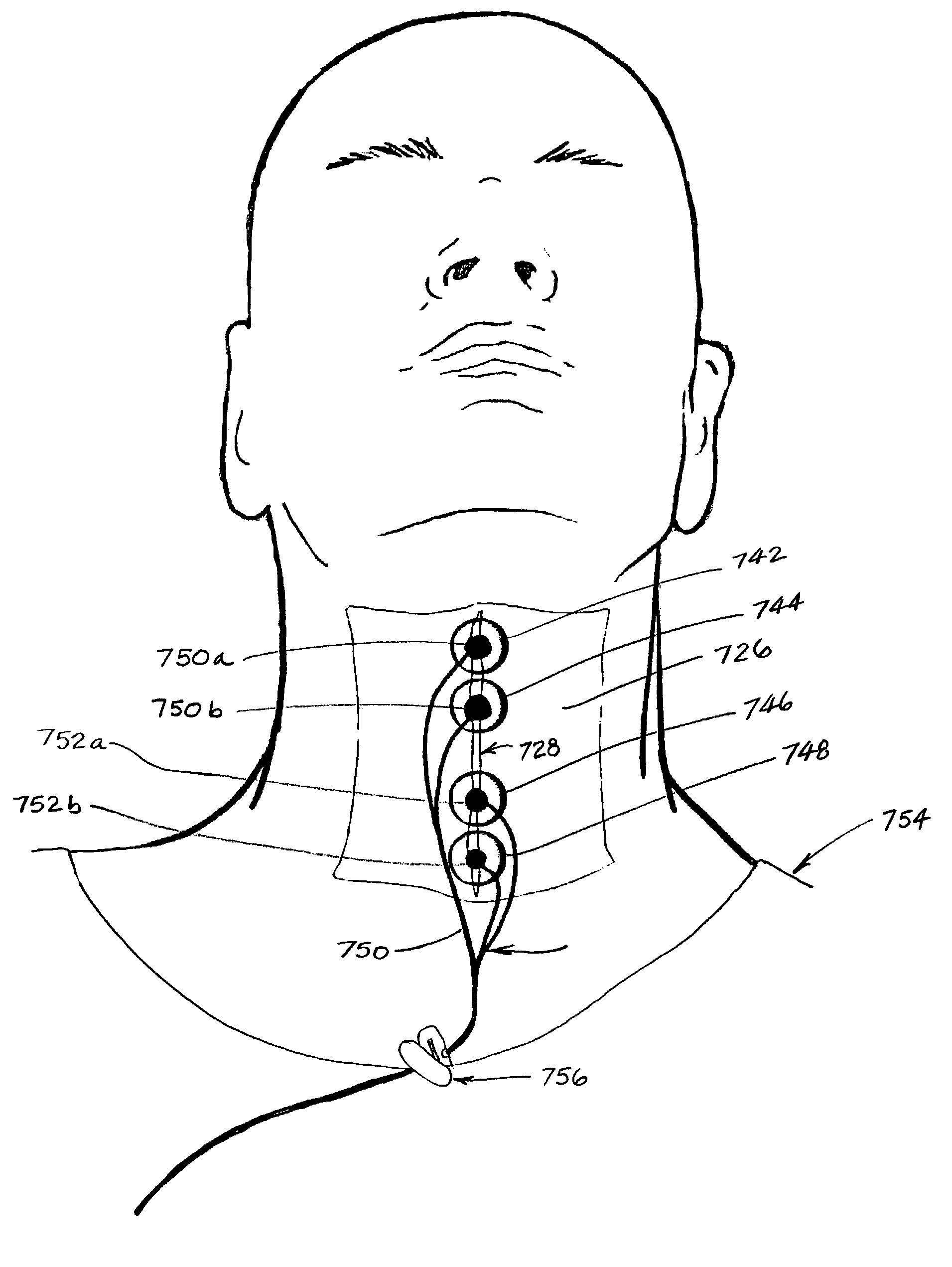
Method and apparatus for treating oropharyngeal disorders with electrical stimulation
InactiveUS7039468B2Simple, non-invasiveEasy to swallowAuscultation instrumentsDiagnostic recording/measuringElectricityThroat
A simple, non-invasive method and apparatus for treating oropharyngeal disorders provides electrical stimulation to the oropharyngeal region of a patient. The apparatus includes an electrical neuromuscular stimulator that includes a pulse generator for generating a series of electrical pulses and a processor coupled to the pulse generator for controlling its operation. The apparatus also includes a first electrode and a second electrode, each of which includes a snap eyelet having a connector to which a lead wire may be attached, a conductive film and an adhesive and conductive gel layer that is adapted to be attached to the skin of the patient. The apparatus also includes at least one lead wire for attachment of the electrodes to the pulse generator and at least one adhesively backed tape overlay for securing the first and second electrodes to the skin of the patient. According to the method, the electrodes are placed on the skin of the patient's throat, and the electrodes are secured to the skin of the patient's throat by applying at least one adhesively backed tape overlay to the patient's skin over at least a portion of each of the electrodes. The lead wires are attached to the connectors of the snap eyelets of the electrodes and to the output jack of the pulse generator, and a series of electrical pulses is generated using the pulse generator so as to apply the series of electrical pulses to the patient's throat using the electrodes.
Owner:ESD
Method and system for treatment of eating disorders
InactiveUS20070104754A1Simple processReduce the amount requiredBiocideOther chemical processesFood gradeFeeding disability
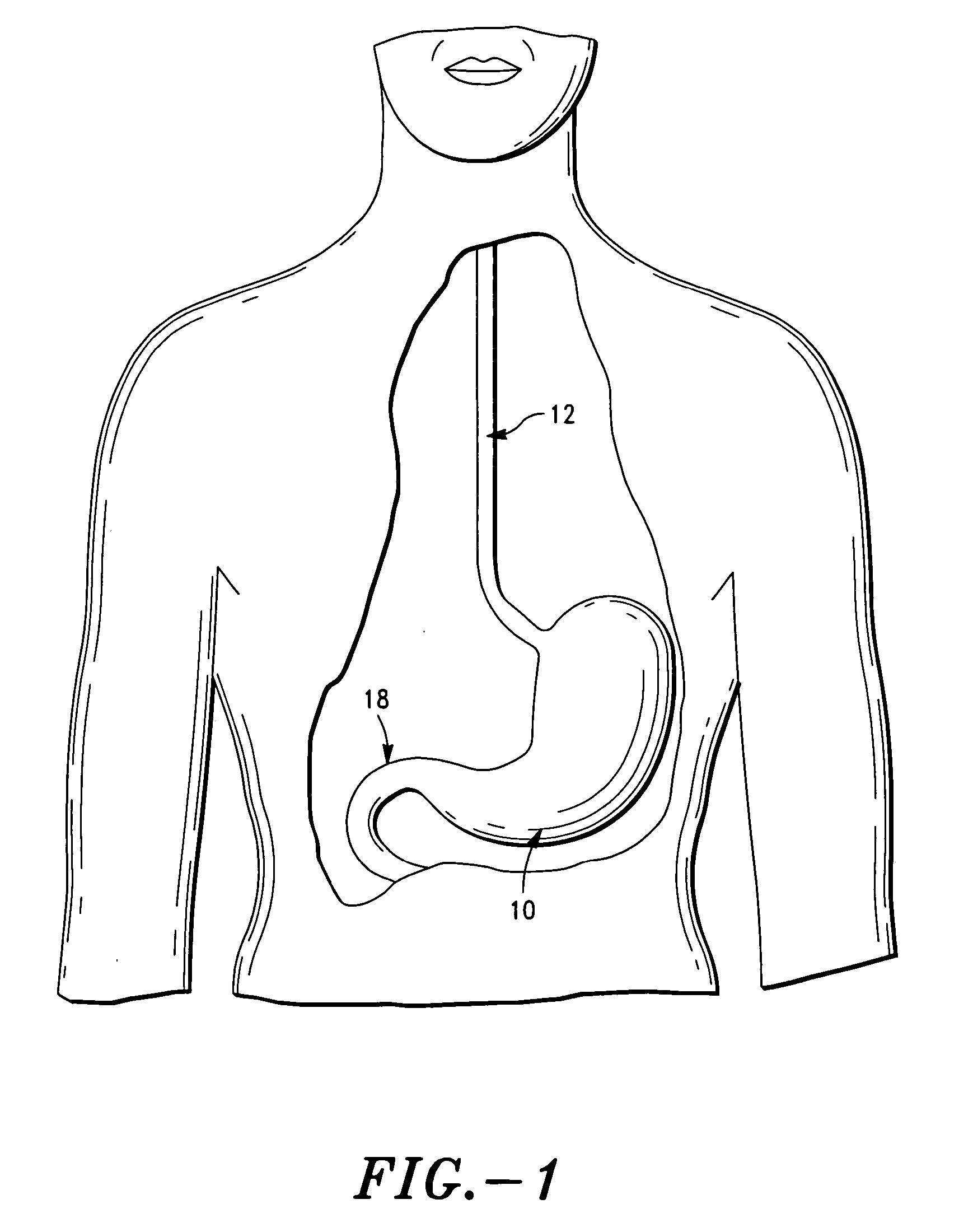
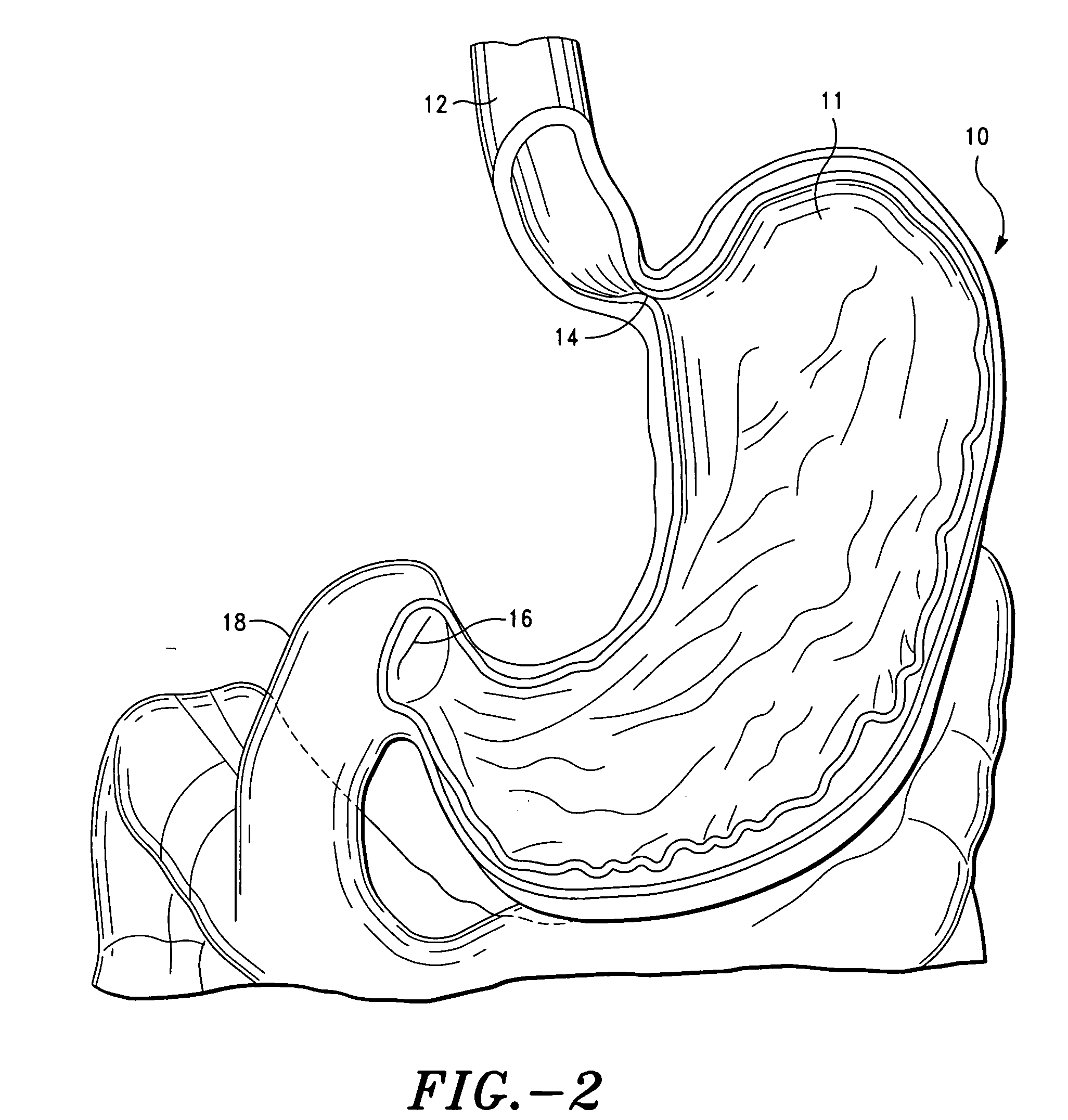
The invention comprises a gastric device having an outer region and a gas producing material. The gastric device hydrates within a patient's stomach and the gas producing material expands the device to a greater volume. Accordingly, the volume of the hydrated, expanded gastric devices occupy space in the stomach cavity and reduce the amount of food the patient will ingest before reaching the feeling of fullness. Preferably, the gastric device is made from food grade materials. The gastric device is configured to expand to a desired volume and then degrade after a residence time, allowing the device to be passed by the patient's normal digestive process.
Owner:PLENSAT
Novel dosage form
InactiveUS20060153916A1Effectively control release rateSmall sizePill deliveryEster active ingredientsMedicineImmediate release
A dosage form comprising of a high dose, high solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of modified release active ingredient per unit is from 500 mg to 1500 mg; a process for preparing the dosage form.
Owner:TORRENT PHARMA LTD
Powder compaction and enrobing
InactiveUS20050147710A1RobustQuicker release characteristicLayered productsConfectioneryMetallurgyMethyl cellulose
An apparatus and method is disclosed for forming a compacted powder slug coated with a film. The powder, e.g. of a medicament, is compacted and enrobed to produce compacted powder slugs by preferably mechanically compacting a powder and forming a film of a material, preferably hydroxy propyl methyl cellulose, by vacuum or pressure differential, about the surface of the powder thus compacted.
Owner:BIOPROGRESS TECH
Swallowing-assistive drink
InactiveUS6277395B1Easy to swallowNot disturbing efficacyPowder deliveryDispersion deliveryViscous liquidMedicine
To provide a swallowing-assistive drink for medicines that improves swallowing various medicines, is convenient and substitutable with ordinary drinking water, and does not disturb the efficacy of medicines and a swallowing method.A swallowing-assistive drink for helping swallowing medicines that contains water and an adhesive paste, forming a viscous liquid or a gelatinoid. If the drink is viscous liquid, the viscosity is 1,000-25,000 cP at 20° C., and if the drink is gelatinous, jelly strength is 10-100 g / cm2 at 20° C.
Owner:RYUKAKUSAN CO LTD
Hybrid method for modulating upper airway function in a subject
ActiveUS20110125212A1Easy to swallowSpinal electrodesHead electrodesPhysical therapyIntrinsic laryngeal muscle
A hybrid method is provided for modulating upper airway function in a subject. The method includes applying first and second therapy signals to the subject to modulate at least one extrinsic laryngeal muscle and at least one intrinsic laryngeal muscle to synergistically control laryngeal motion and vocal fold movement, respectively.
Owner:CASE WESTERN RESERVE UNIV
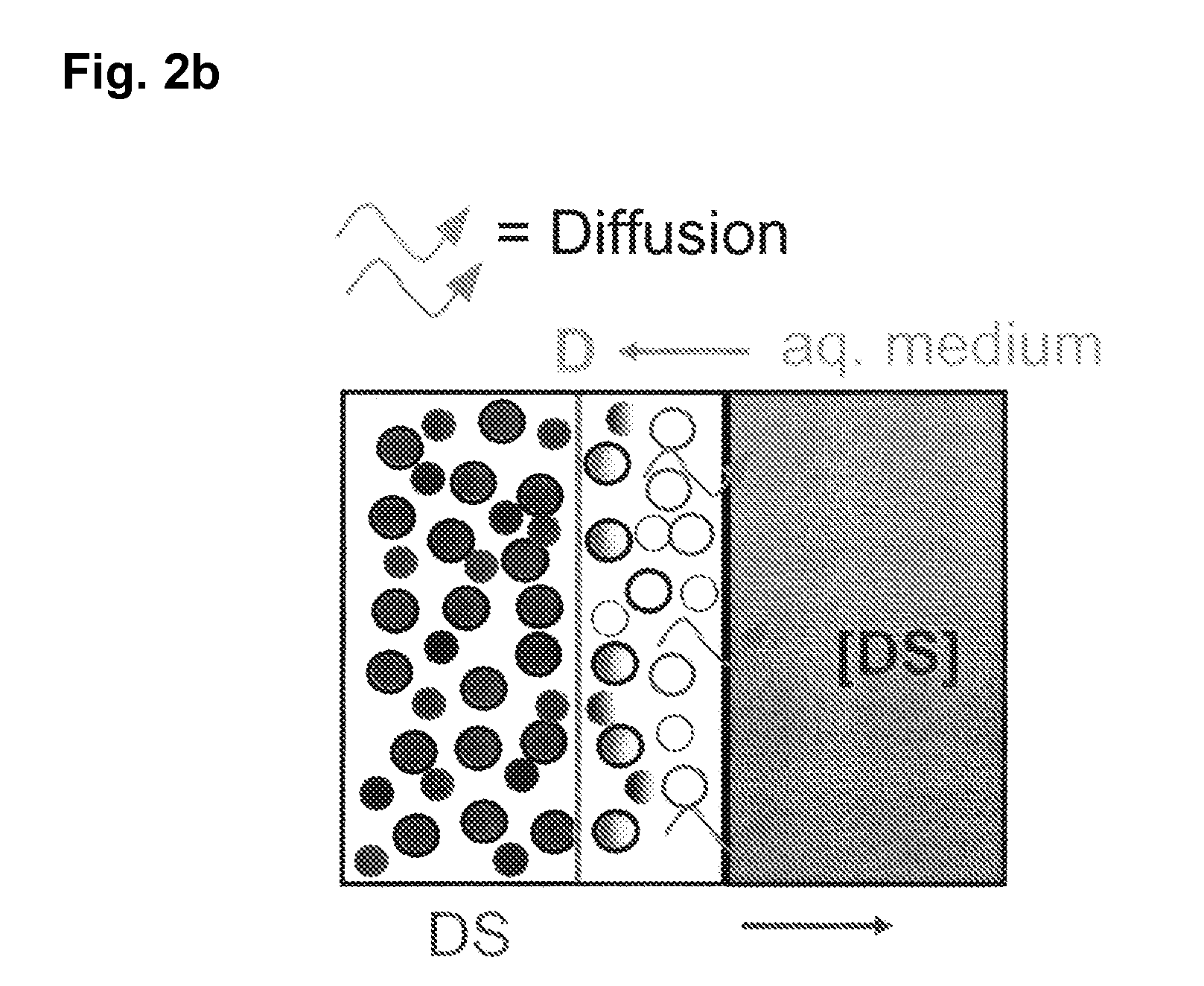
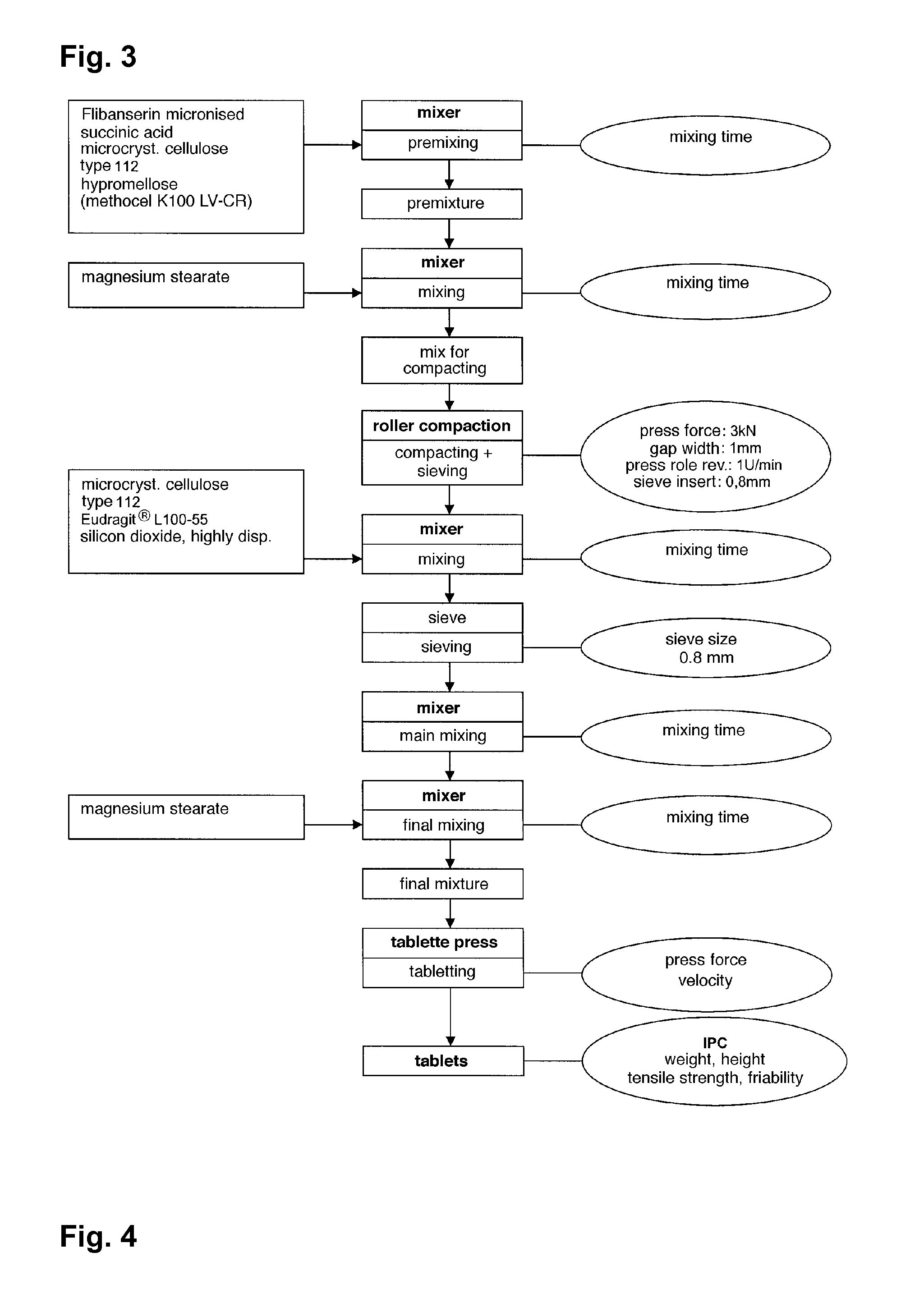
Extended release tablet formulations of flibanserin and method for manufacturing the same
ActiveUS20080038346A1Reduce solubilitySufficiently slow releasePowder deliveryOrganic active ingredientsOral medicationExtended release tablets
The invention is directed to a Pharmaceutical extended release system, particularly for oral administration, of a pH-dependent water-soluble active substance, comprising or essentially consisting of a) flibanserin or a pharmaceutically acceptable derivative thereof as active substance; b) one or more pharmaceutically acceptable pH-dependent polymers; c) one or more pharmaceutically acceptable pH-independent polymers; d) one or more pharmaceutically acceptable acids; and e) optionally one or more additives. The present invention provides a release profile of flibanserin which is independent on the pH in the gastrointestinal tract when administered orally resulting in a significantly improved bioavailability.
Owner:BOEHRINGER INGELHEIM INT GMBH
Cushioning wax beads for making solid shaped articles
InactiveUS6923984B1High tensile strengthSustained deliveryPowder deliveryPharmaceutical containersWaxCushioning
Biologically inactive cushioning beads comprise at least one compressible cushioning component consisting essentially of a microcrystalline hydrocarbon wax or a natural wax, the said wax being at least 30% by weight of the biologically inactive cushioning beads. Such beads are useful for making solid shaped articles containing biologically active ingredients by compression.
Owner:UNIV GENT
Oral medicament delivery system
InactiveUS20050136112A1Easily cut and severImprove compliancePowder deliveryDispersion deliveryDrugPharmaceutical formulation
Oral medicament delivery system comprising a pharmaceutical composition comprising a flexible matrix, said matrix formed of a plurality of fibers comprising a collagen-based carrier and a medicament, the composition orally dissolvable to deliver a unit dose of the medicament to a patient. The flexible composition can be dose titrated and co-administered with a second pharmaceutical formulation.
Owner:UNION SPRINGS PHARMA
Formulation
InactiveUS20100062057A1Increase volumeIncrease filling volumeOrganic active ingredientsSenses disorderBiomedical engineering
Owner:FMC BIOPOLYMER AS
Novel soft-gelatin capsule comprising S-adenosylmethionine and a method for producing the same
InactiveUS20020164369A1Easy to handleReadily availableBiocideSugar derivativesS-Adenosyl methionineGelatin film
The invention provides a novel soft gelatin capsule comprising a fill material consisting essentially of S-adenosylmethionine (SAMe) salt disposed within an enteric coated soft gelatin film.
Owner:ORCHID CHEM & PHARM LTD
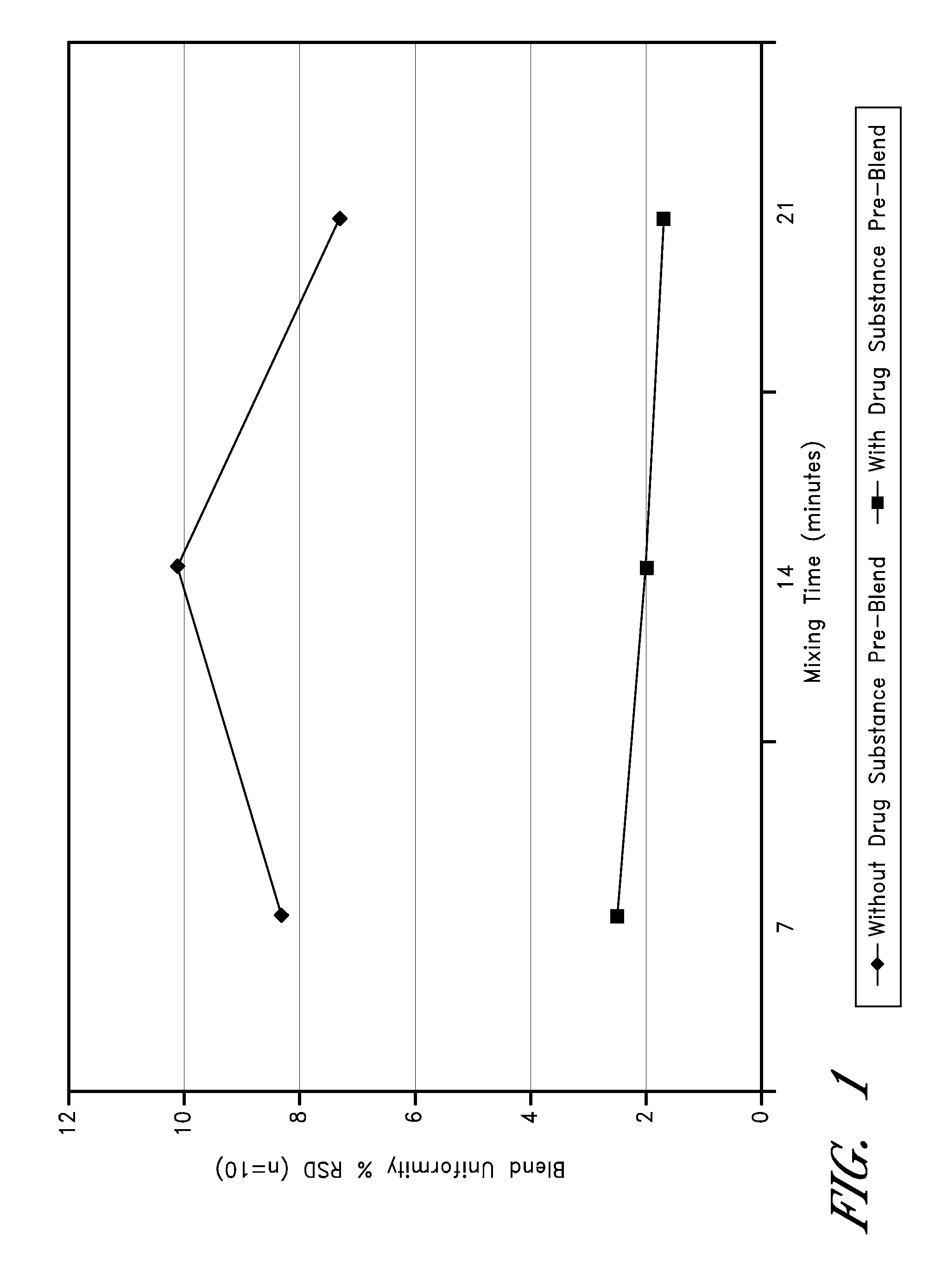
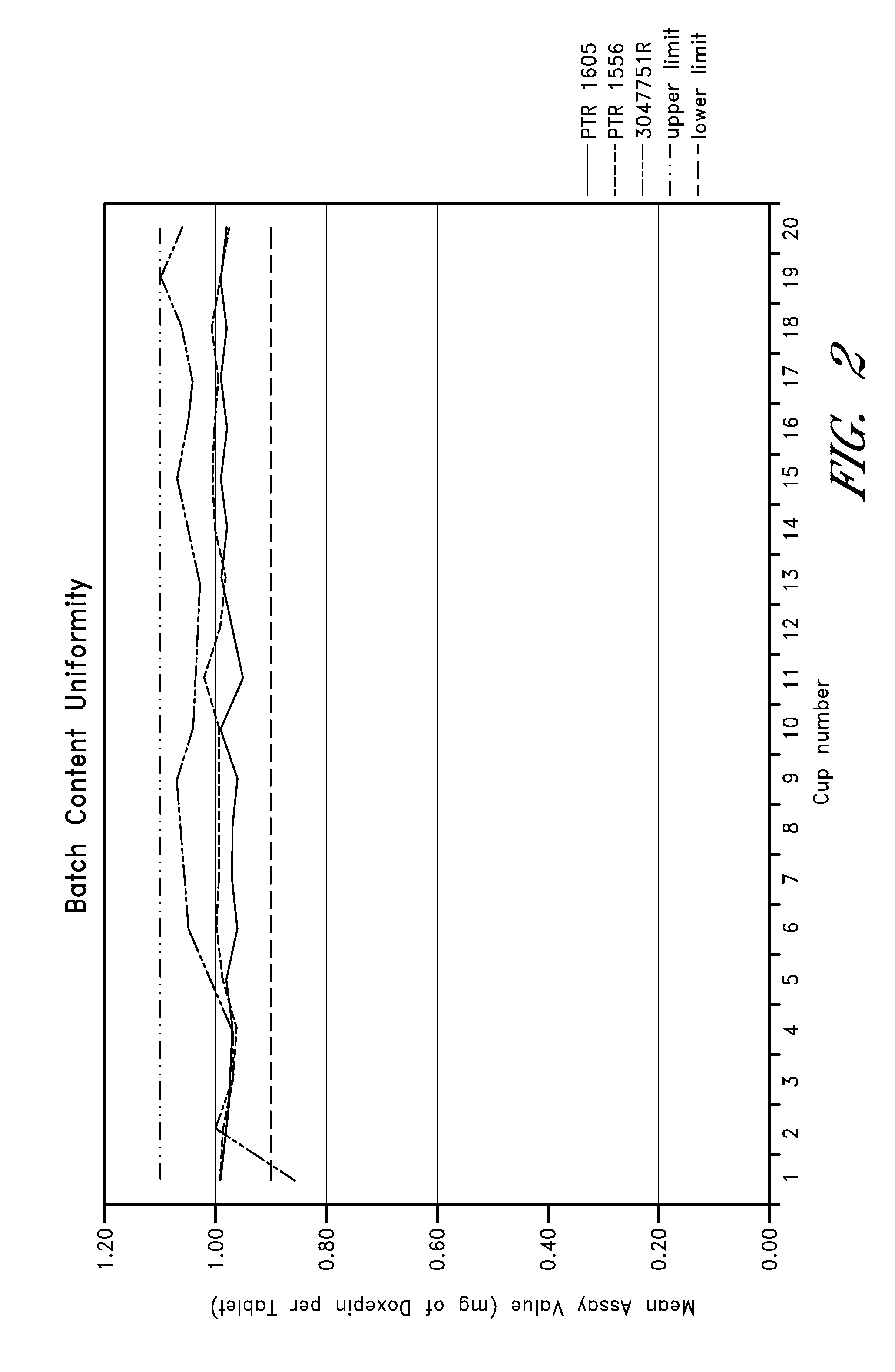
Low-dose doxepin formulations and methods of making and using the same
InactiveUS20090074862A1Dissolve fastEasy to swallowOrganic active ingredientsBiocideMedicinePharmaceutical formulation
The invention disclosed herein generally relates to low-dose oral doxepin pharmaceutical formulations and the use of these formulations to promote sleep.
Owner:SOMAXON PHARMA
Vitamin/mineral compositions with DHA
InactiveUS7704542B2Lower Level RequirementsReducing potential for clottingBiocideAntinoxious agentsDocosahexaenoic acidDisease
Compositions containing the fatty acid docosahexaenoic acid (DHA) in combination with at least one vitamin and mineral are provided to supplement nutrition in a mammalian diet. DHA is present in the composition in concentrated amounts, advantageously in a carrier such as marinol oil, to allow for quantities of DHA sufficient to supply expectant and new mothers and their children as recommended on a daily basis. This DHA may also be used to treat a variety of disorders in children and adults. The compositions advantageously include vitamins, minerals, and optionally other nutrients to provide a nutritional supplement which may be convenient to swallow and taken once a day.
Owner:SILICON VALLEY BANK
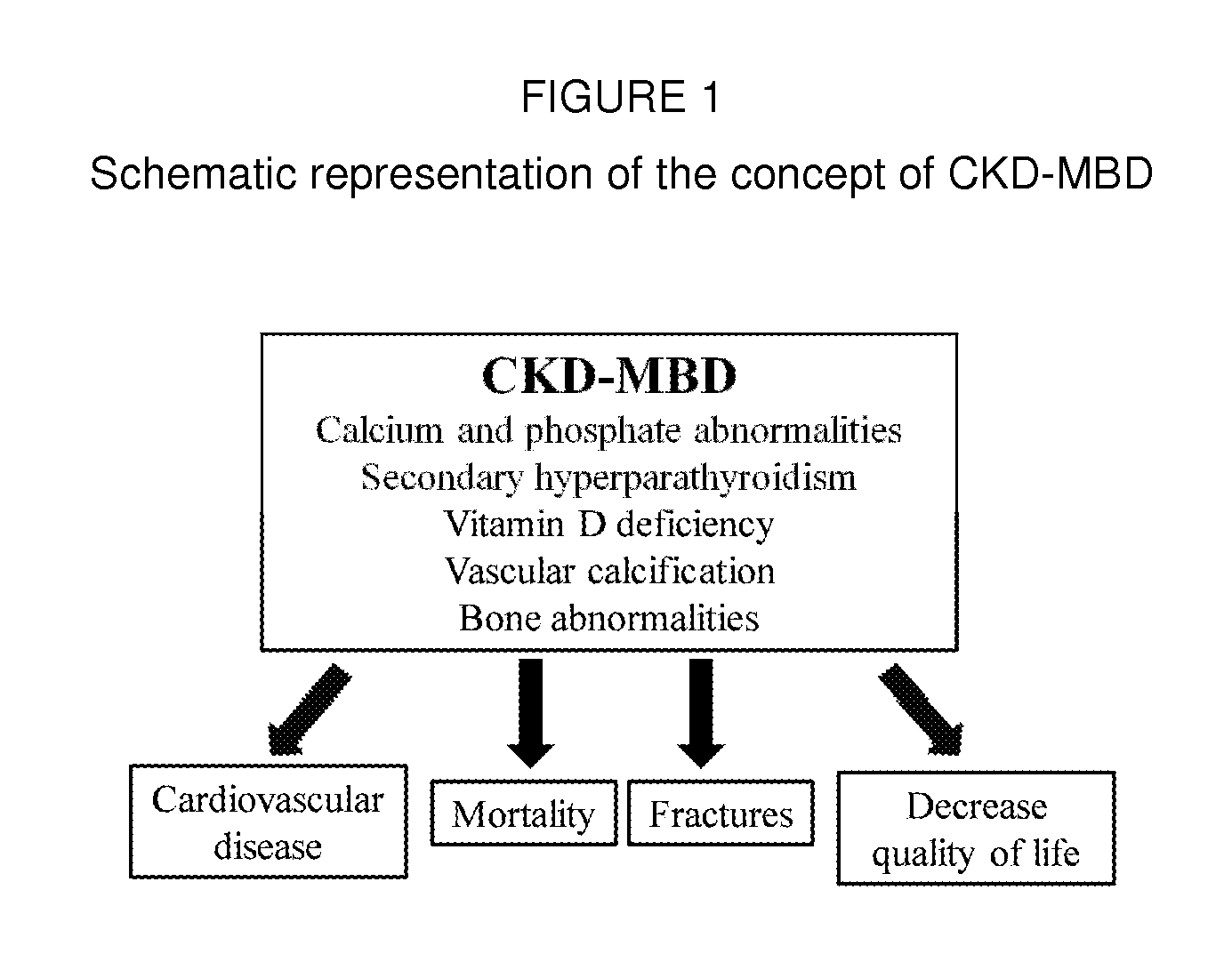
Pharmaceutical compositions comprising phosphate binder, calcium receptor-active compound and/or active vitamin d
InactiveUS20130085121A1Maintain good propertiesImprove bioavailabilityOrganic active ingredientsBiocideMineral bone diseaseCalcium Binder
The present invention is an oral solid pharmaceutical compositions for the treatment of kidney diseases and mineral bone disorder including a phosphate binder, a calcium receptor-active compound and at least one pharmaceutically acceptable excipient, the invention further including a method for preparing the pharmaceutical compositions including the steps of granulating cinacalcet and / or sevelamer and / or vitamin D by one of a wet and a dry granulation process, each with at least one pharmaceutically acceptable excipient to form cinacalcet granules and / or sevelamer granules and / or vitamin D granules, mixing at least two of the cinacalcet granules, sevelamer granules and vitamin D granules to form a granules mixture and compressing the granules mixture to tablets or encapsulating the granules mixture into capsules or pulverizing the granules mixture into a dispersion powder.
Owner:WEIFANG SYNERPHARM
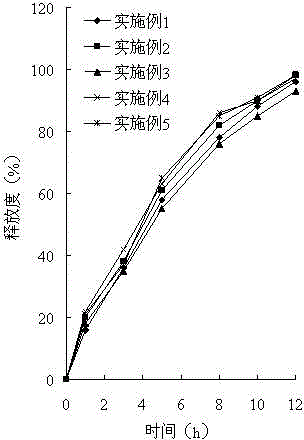
Palbociclib gastric-floating tablet and preparation method thereof
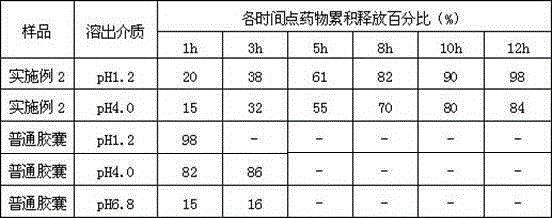
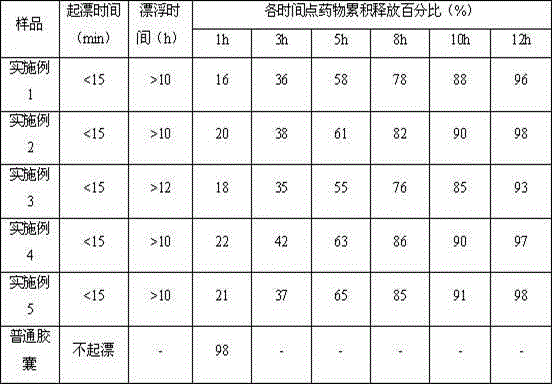
ActiveCN104887641ALow drift timeReduce dosageOrganic active ingredientsPill deliveryUse medicationPharmaceutical drug
The invention belongs to the technical field of medicine, and relates to a palbociclib gastric-floating tablet and a preparation method thereof. The palbociclib gastric-floating tablet comprises, by mass, 10%-30% of palbociclib, 20%-50% of hydroxypropyl methylcellulose, 20%-40% of bleaching auxiliaries, 2%-10% of foaming agents, 0%-25% of microcrystalline cellulose and 0.5%-3% of magnesium stearate. A dry granulating technology or a wet granulating technology can be used as the preparation technology. The palbociclib gastric-floating tablet is high in bioavailability, has a slow release tendency, and effectively lowers the total dosage. The palbociclib gastric-floating tablet and the preparation method thereof have the unique advantages that two different mechanisms are used for preparing the gastric-floating tablet, and accordingly the prepared tablet can keep floating in gastric juice by more than 10 hours and continuously release drugs in the hydrochloric acid solution with the pH being 1.2; the problem that the bioavailability is low due to the fact that drugs are extremely difficult to dissolve after the pH is higher than four is effectively solved; the medicine taking frequency is reduced; toxic and side effects are lightened; and the complaisance of a patient is effectively improved.
Owner:上海润泰医药科技有限公司
Lansoprazole orally disintegrating tablets
InactiveUS20070141151A1Dissolve fastPrevent degradationBiocidePill deliveryLansoprazoleOrally disintegrating tablet
The invention provides orally disintegrating tablets that readily disintegrates in the mouth, releasing enteric coated drug sub-tablets.
Owner:TEVA PHARM USA INC
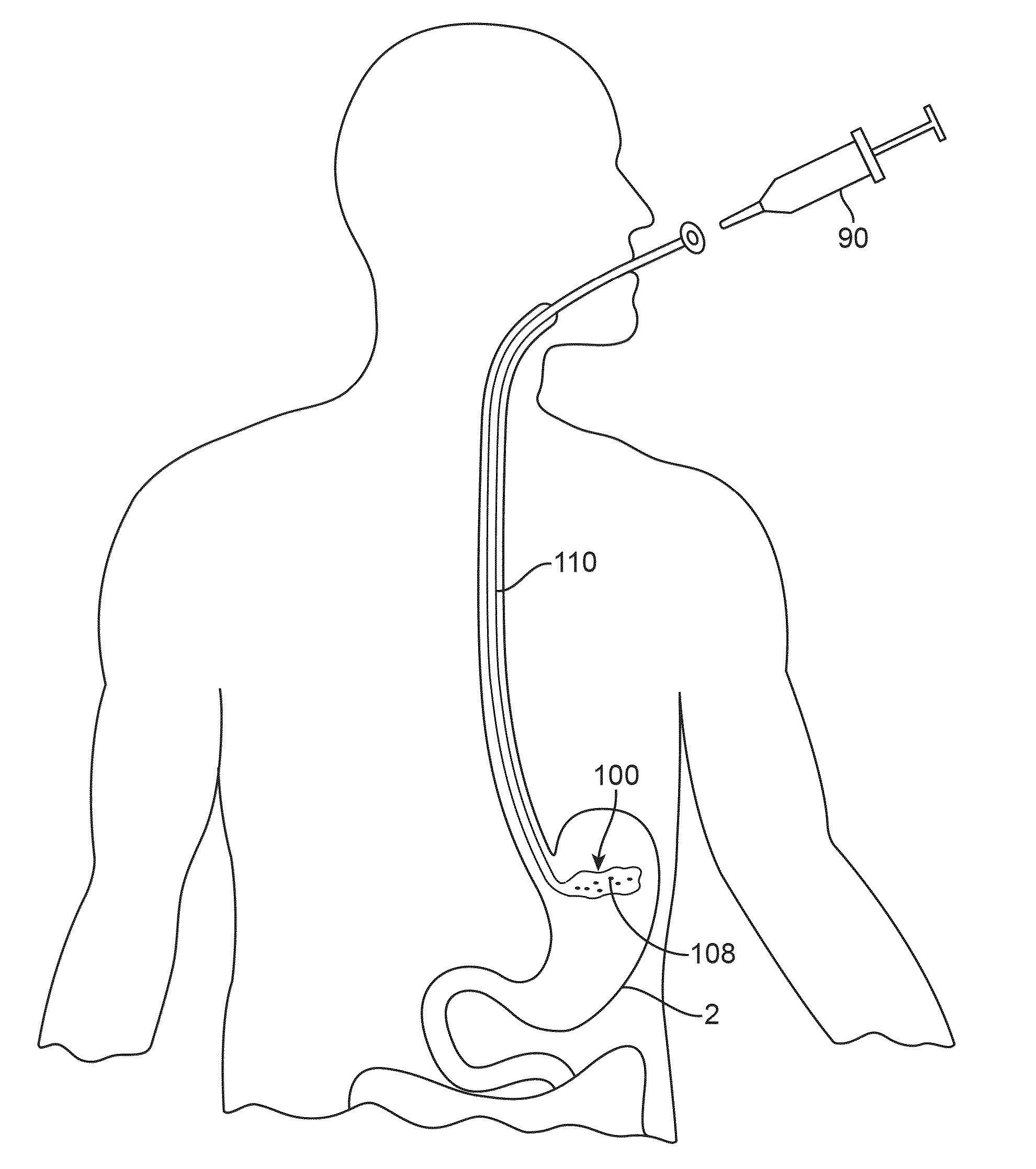
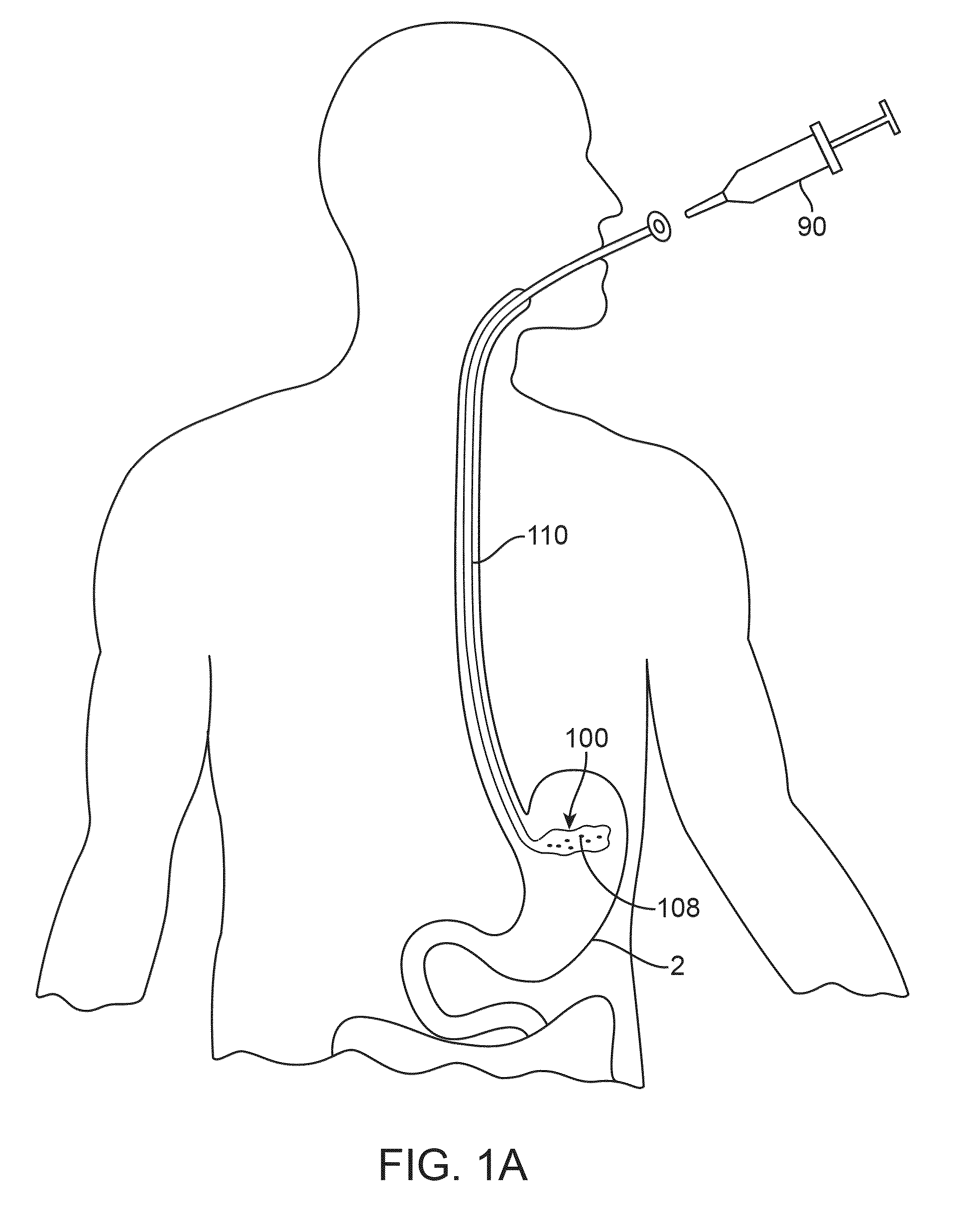
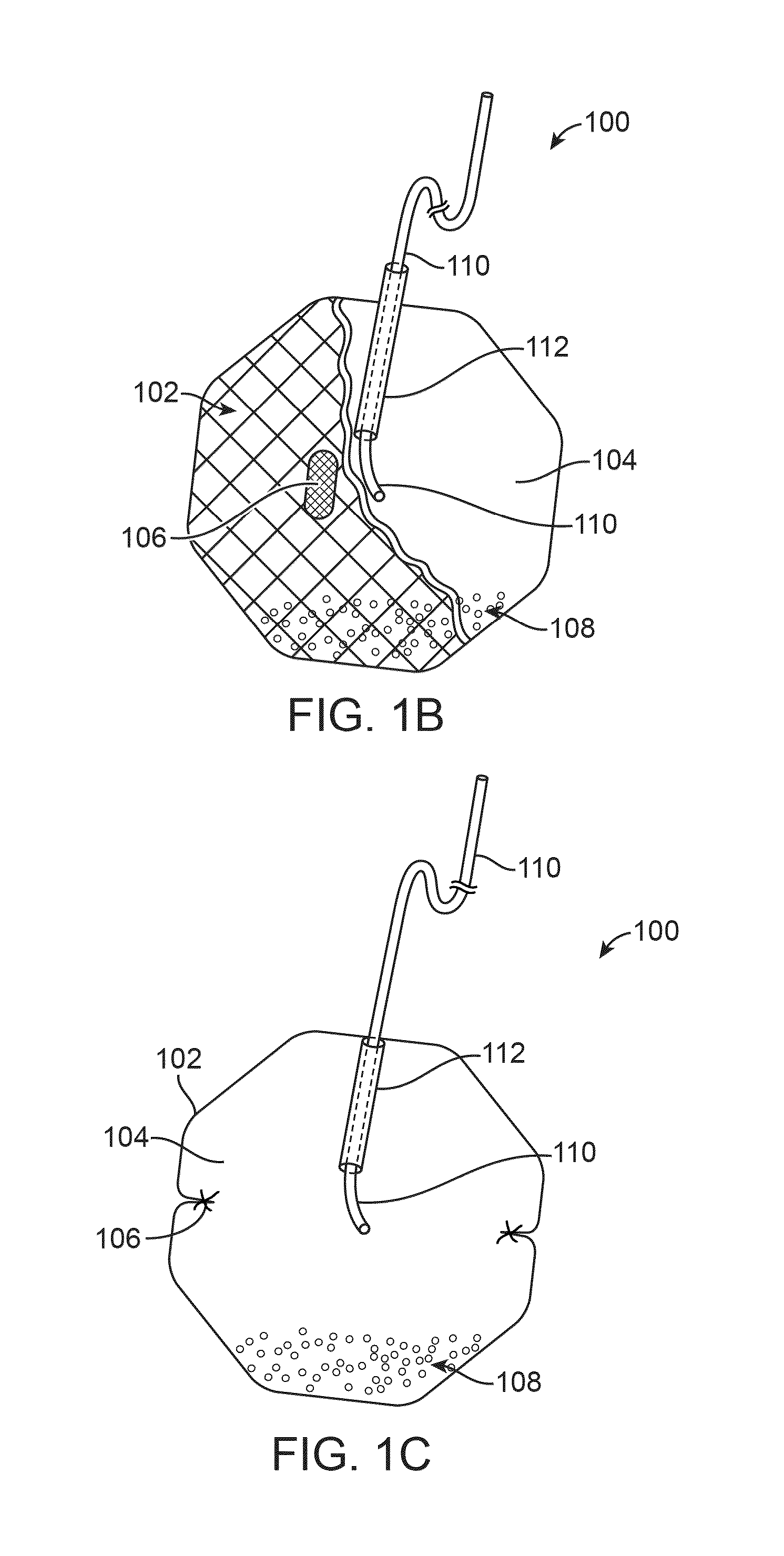
Anatomically adapted ingestible delivery systems and methods
ActiveUS20140066967A1Increase probabilityEasy to swallowBalloon catheterDilatorsDelivery systemGastroenterology
Owner:ALLURION TECH
Method and system for swallow control resulting in improved posture
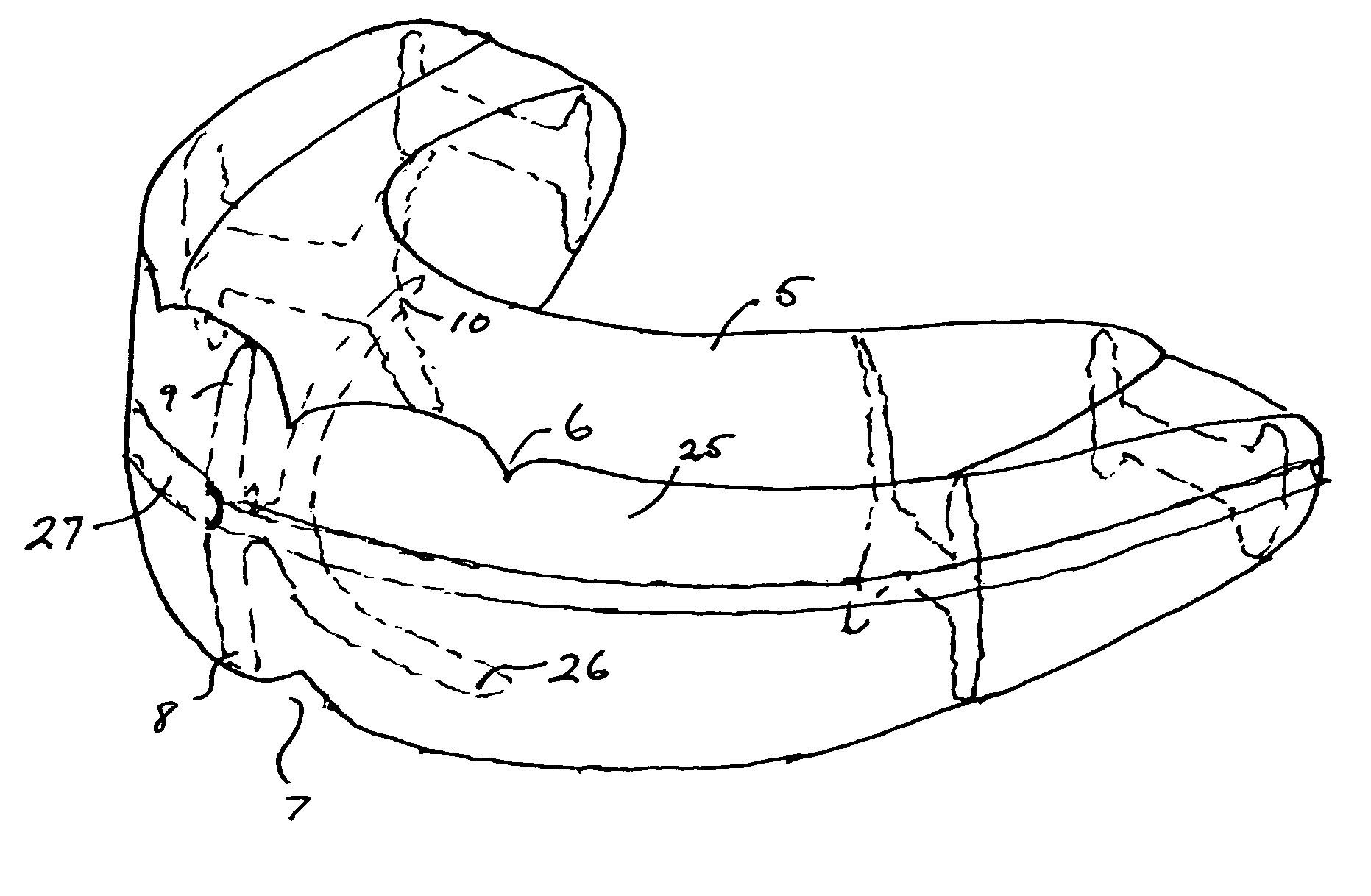
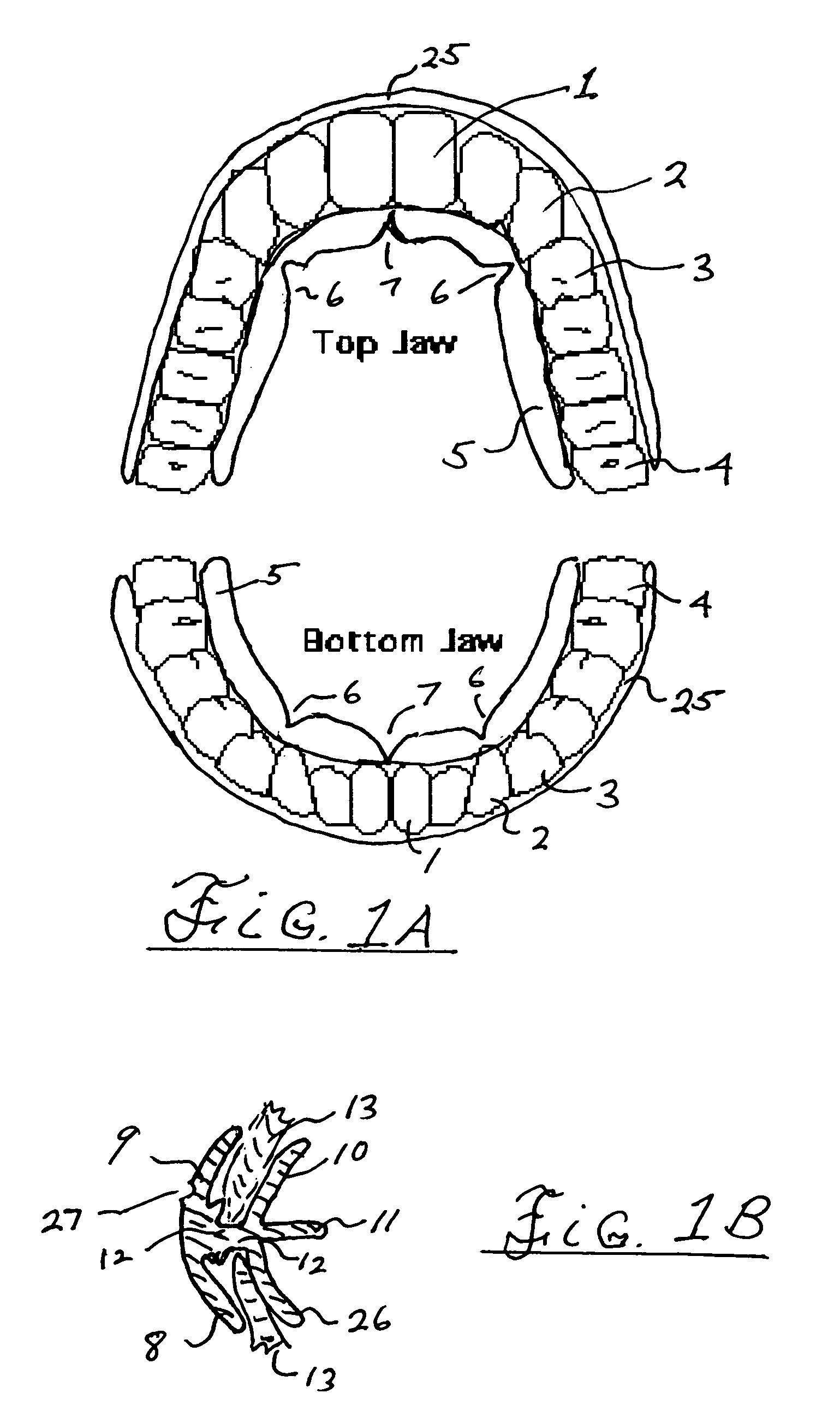
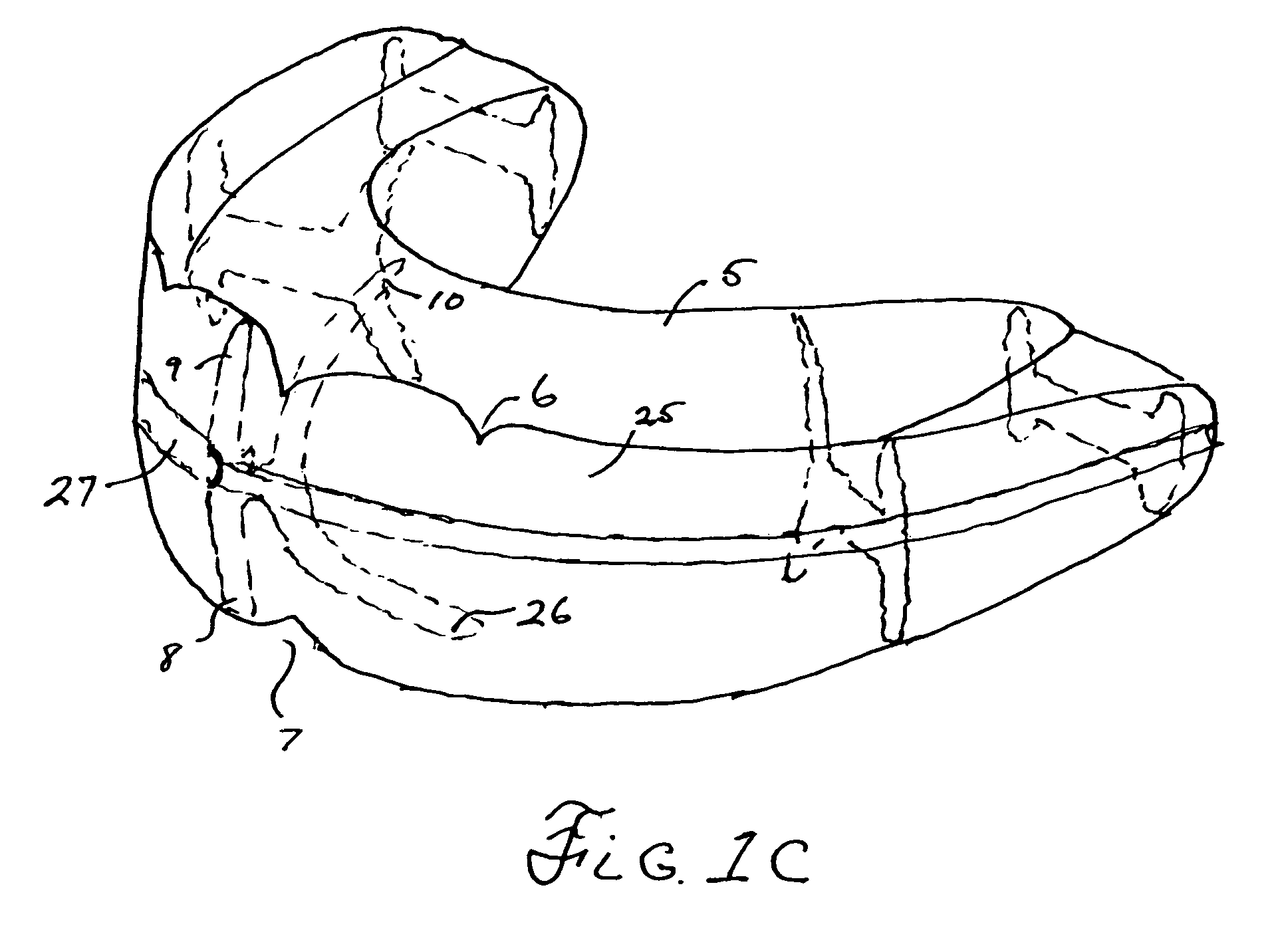
ActiveUS7798149B2Easy to swallowImprove postureOperating chairsTeeth fillingThermoplasticBiomechanics
A method and system for training a patient to improve swallowing, for retraining jaw muscles and for holding or keeping the jaw in a correct bite position. All of this in-turn causing an improvement in posture. The system includes a combination of exercise and oral apparatus pieces or mouth-guards to retrain and balance the facial muscles and to develop a correct swallow. The mouth-guards of the present invention can improve biomechanical imbalance, posture and in turn athletic performance. A particular embodiment of an oral apparatus of the present invention includes top and bottom troughs for receiving top and bottom teeth into the apparatus. The troughs can be adapted to hold the upper canine teeth lower than the central and lateral incisor teeth and hold the upper teeth outside the lower teeth from the incisors to the molars. The apparatus can be pre-fabricated or custom made for a particular patient. Using heat moldable thermoplastics, some embodiments of the device can be formed in the patient's mouth after heating in hot water.
Owner:HADUONG HAN
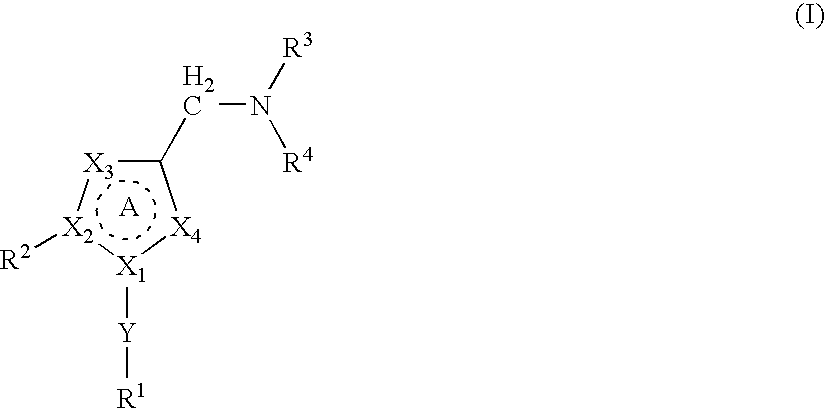
Aryl- or heteroaryl-sulfonyl compounds as acid secretion inhibitors
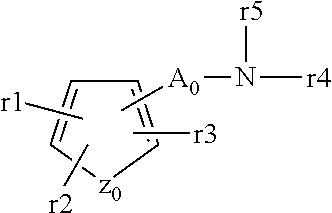
ActiveUS20110288040A1Low toxicityHigh expressionAntibacterial agentsBiocideAcyl groupProton-pump inhibitor
The present invention provides a compound having a superior acid secretion inhibitory action, an antiulcer activity and the like.A proton pump inhibitor containing a compound represented by the formula (I)wherein ring A is a saturated or unsaturated 5- or 6-membered ring group optionally having, as a ring-constituting atom besides carbon atom, 1 to 4 hetero atoms selected from a nitrogen atom, an oxygen atom and a sulfur atom, ring-constituting atoms X1 and X2 are each a carbon atom or a nitrogen atom, a ring-constituting atom X3 is a carbon atom, a nitrogen atom, an oxygen atom or a sulfur atom, R1 is an optionally substituted aryl group or an optionally substituted heteroaryl group, R2 is an optionally substituted alkyl group, an optionally substituted aryl group or an optionally substituted heteroaryl group, R3 is an aminomethyl group optionally substituted by 1 or 2 lower alkyl groups, which is a substituent on a ring-constituting atom other than X1, X2 and X3, and ring A optionally further has substituent(s) selected from a lower alkyl group, a halogen atom, a cyano group and an oxo group, or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Encapsulation of sensitive components using pre-emulsification
ActiveUS7431986B2Increase stabilityIncrease bioavailabilityPowder deliveryLiquid surface applicatorsWater contentOmega 3 fatty acid
A stabilized emulsion is employed to produce shelf stable, controlled release, discrete, solid particles or pellets which contain an encapsulated and / or embedded component, such as a readily oxidizable component, such as omega-3 fatty acids. An oil encapsulant component which contains an active, sensitive encapsulant, dissolved and / or dispersed in an oil is admixed with an aqueous component and a film-forming component to form an emulsion. An antioxidant for prevention of oxidation of the active, sensitive encapsulant, and a film-softening component or plasticizer for the film-forming component may be included in the emulsion. The emulsion is stabilized by subjecting it to homogenization. The pellets are produced by first reducing the water content of the stabilized emulsion so that the film-forming component forms a film around the oil droplets and encapsulates the encapsulant. In embodiments of the invention, the water content of the homogenized emulsion may be reduced by spray-drying to produce a powder. In other embodiments of the invention, after homogenization, the water content of the emulsion may be reduced by admixing the emulsion with at least one matrix material to thereby encapsulate the film-coated oil droplets within the matrix material. After the water content of the emulsion is reduced, a protective coating is applied on the film-coated oil droplets to obtain pellets.
Owner:GENERAL MILLS INC
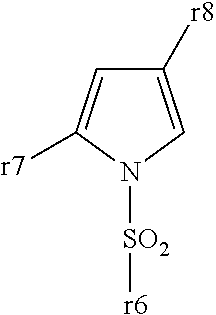
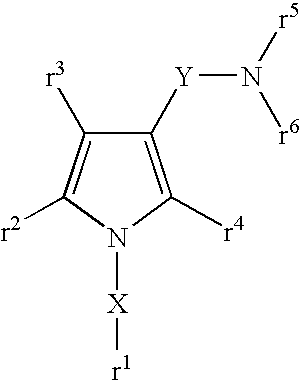
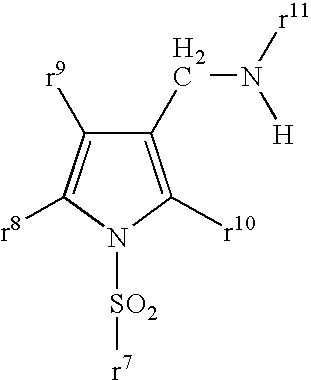
5-membered heterocyclic compound
Provided is a compound having a superior acid secretion suppressive action, which shows an antiulcer activity and the like.A compound represented by the formula (I) or a salt thereof:wherein ring A is a saturated or unsaturated 5-membered heterocycle containing, as a ring-constituting atom besides carbon atoms, at least one heteroatom selected from a nitrogen atom, an oxygen atom and a sulfur atom, the ring-constituting atoms X1 and X2 are the same or different and each is C or N, the ring-constituting atoms X3 and X4 are the same or different and each is C, N, 0 or S (provided that a pyrrole ring wherein X1 is N is excluded from ring A), and when the ring-constituting atom X3 or X4 is C or N, each ring-constituting atom optionally has substituent(s) selected from an optionally substituted alkyl, an acyl, an optionally substituted hydroxy, an optionally substituted mercapto, an optionally substituted amino, a halogen, a cyano and a nitro;R1 and R2 are each a cyclic group optionally having substituent(s); R3 and R4 are each H or alkyl, or R3 and R4 form, together with the adjacent N, an nitrogen-containing heterocycle; and Y is a spacer.
Owner:TAKEDA PHARMA CO LTD
Modified release composition of highly soluble drugs
InactiveUS7976871B2Effectively control release rateSmall sizePill deliveryAnhydride/acid/halide active ingredientsSolubilityModified Release Dosage Form
A novel modified release dosage form comprising of a high solubility active ingredient, which utilizes dual retard technique to effectively reduce the quantity of release controlling agents. Present invention can optionally comprise additionally another active ingredient as an immediate release form or modified release form. Present invention also relates to a process for preparing the said formulation.
Owner:TORRENT PHARMA LTD
Filter fish feed and farming method
InactiveCN1413489AHigh in nutrientsEasy to swallowClimate change adaptationAnimal feeding stuffFisheryAnimal protein
A feed for filter fish is a granular feed prepared from energy-type raw material (0-100 wt. portions), vegetable protein (0-100), animal protein (0-100) and minerals (0-20). The method for raising the said filter fish with the said feed features that the feed is loaded in bags and then suspended in the water. It can be dispersed in the water at moderate speed, so accelerating growth of fish.
Owner:任东升 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com