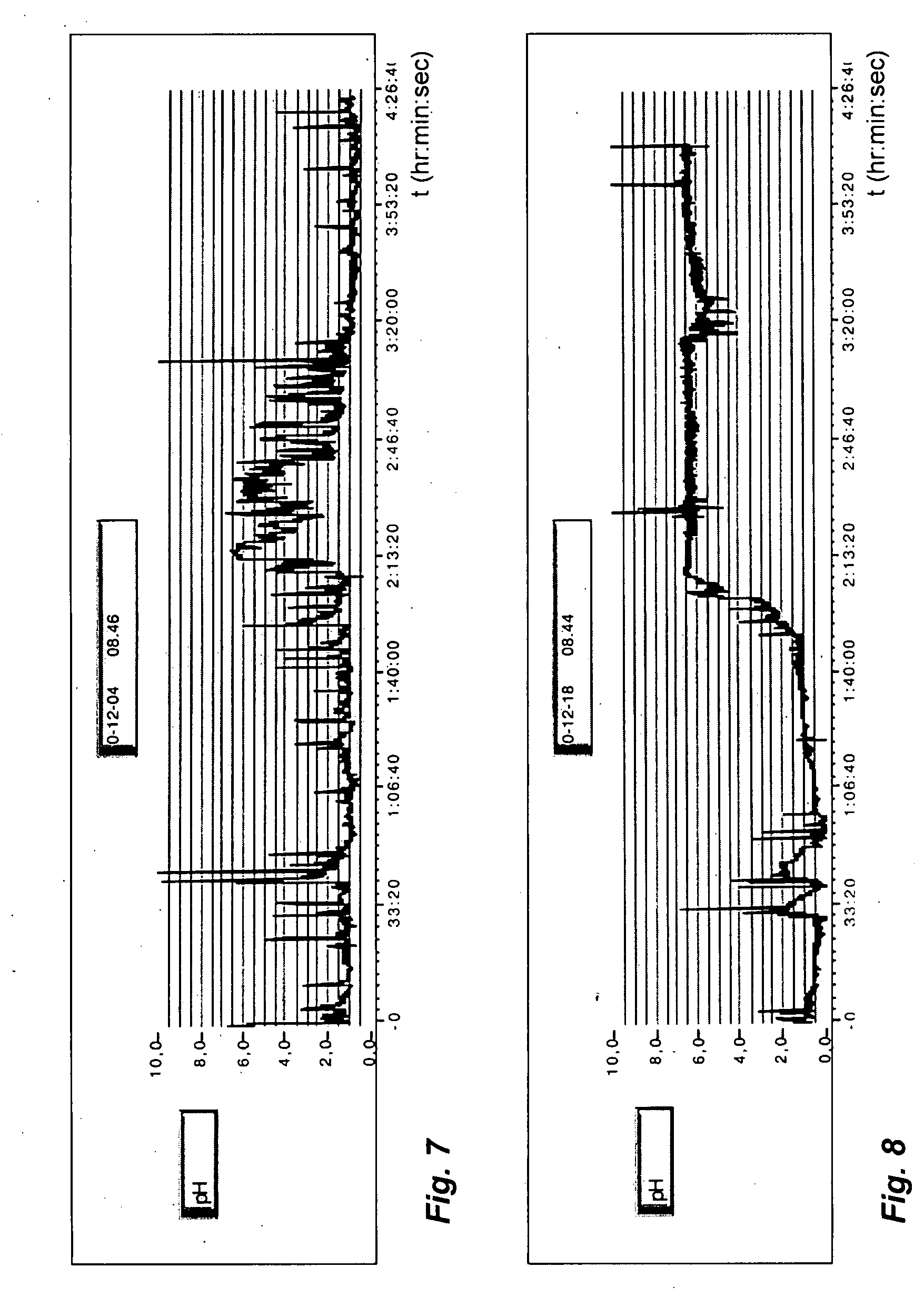
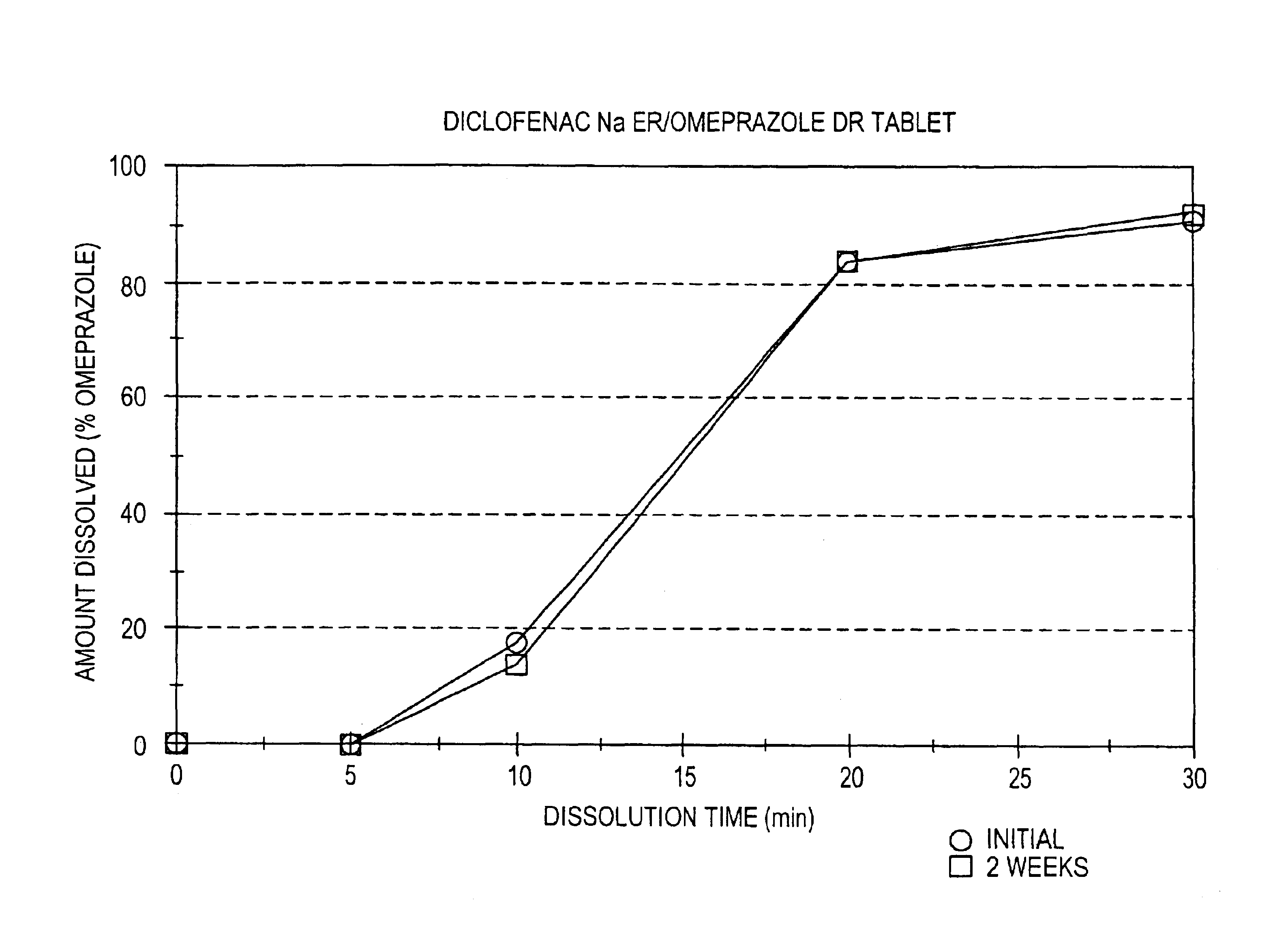
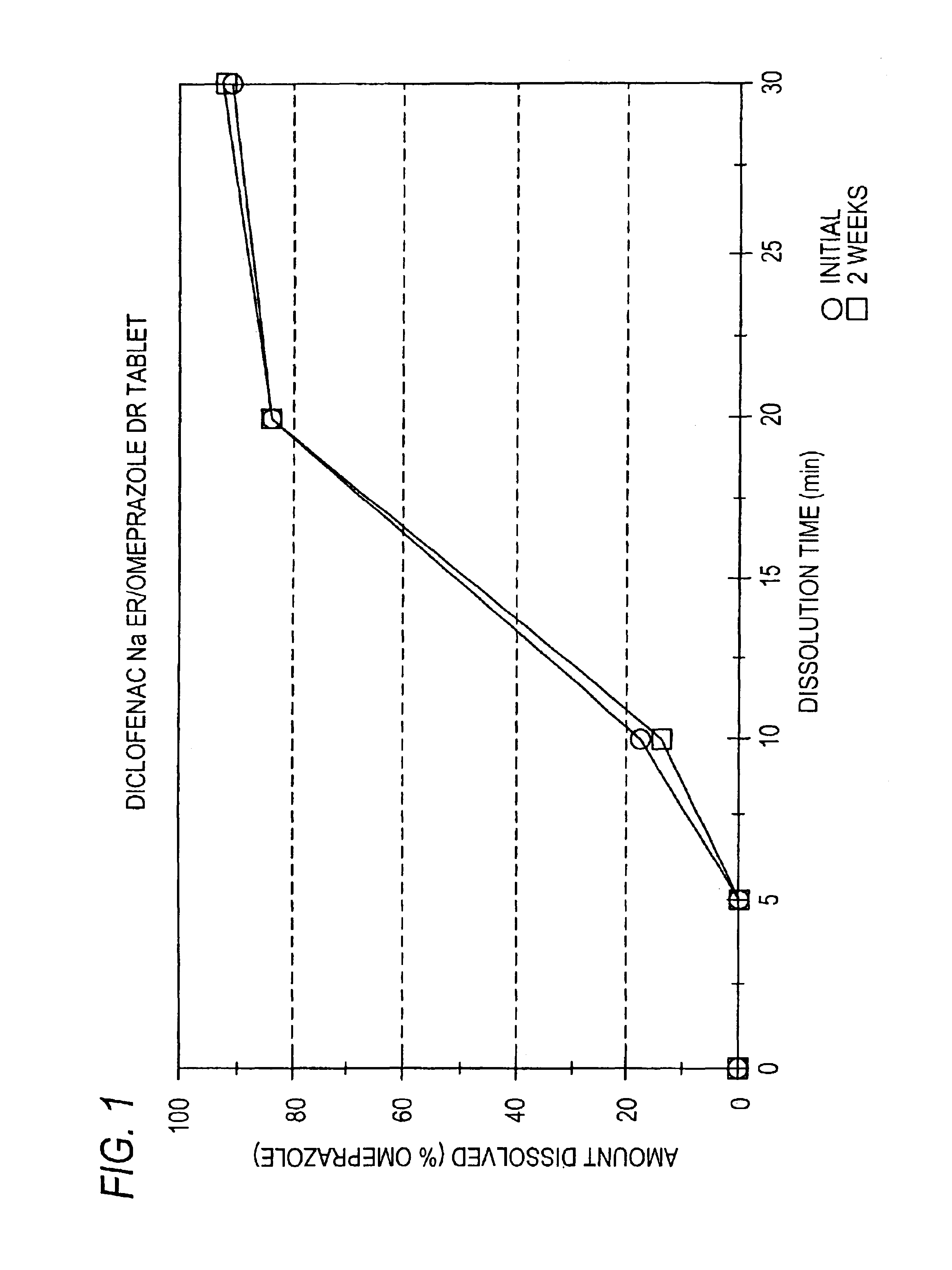
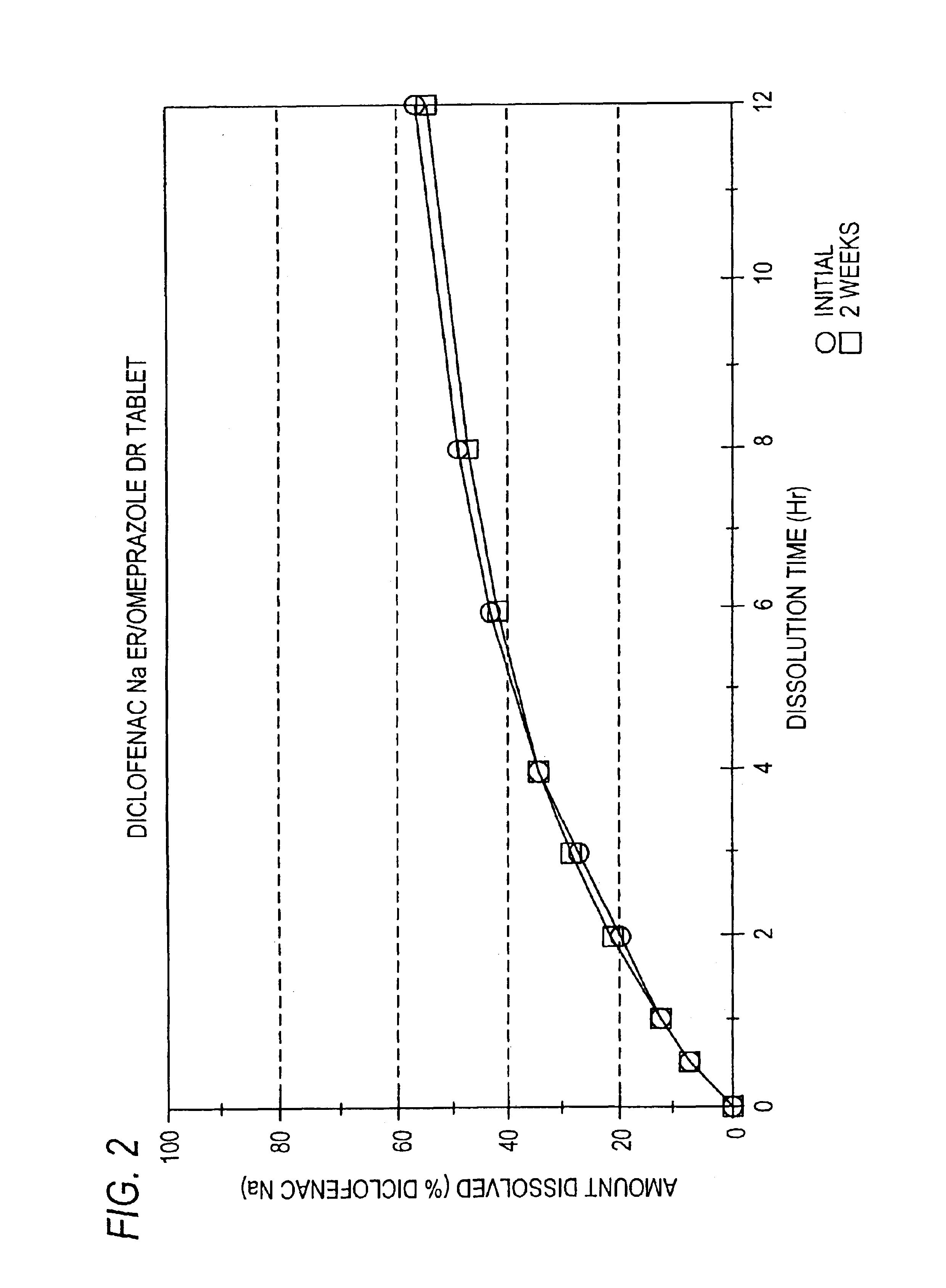
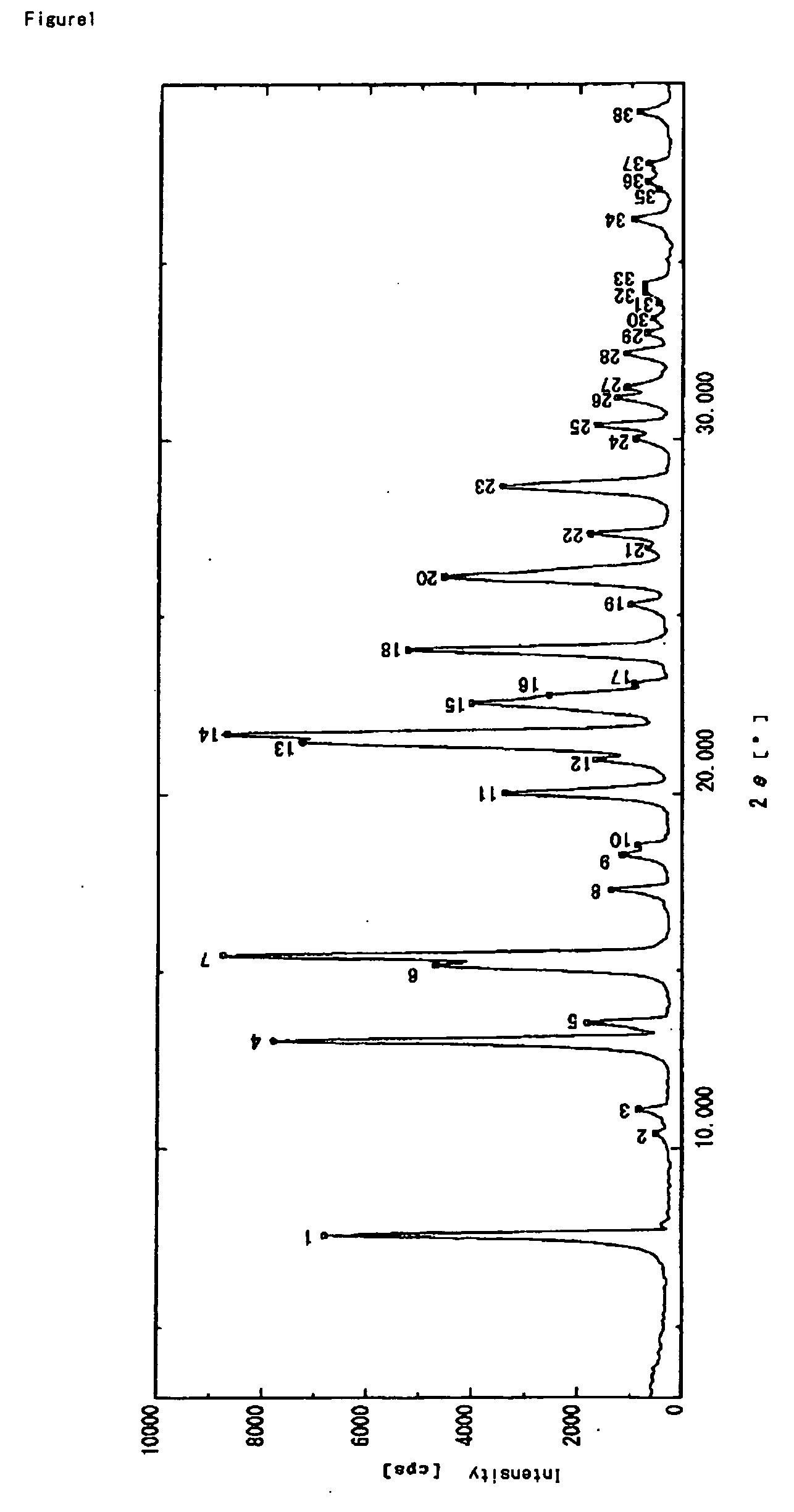
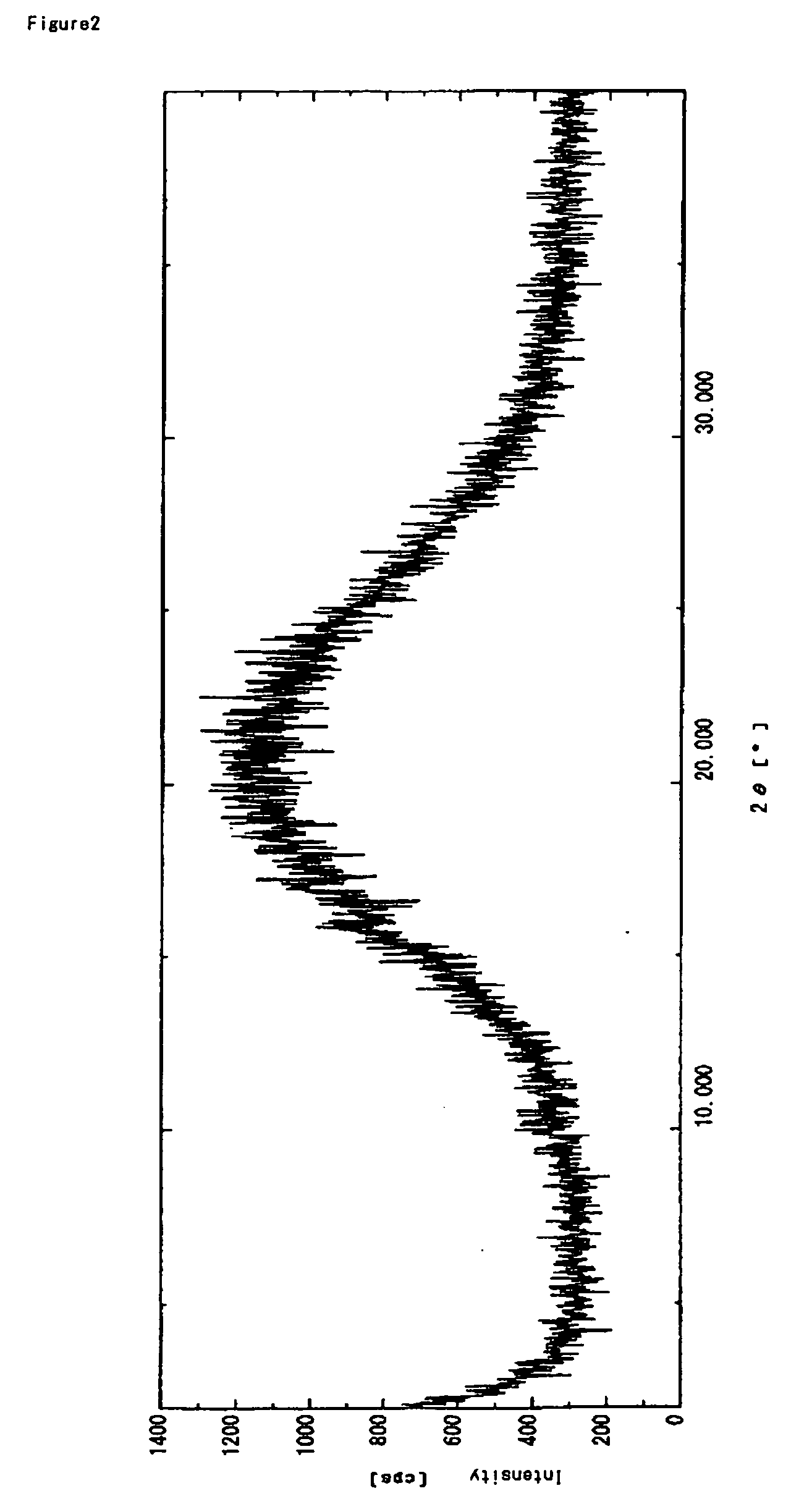
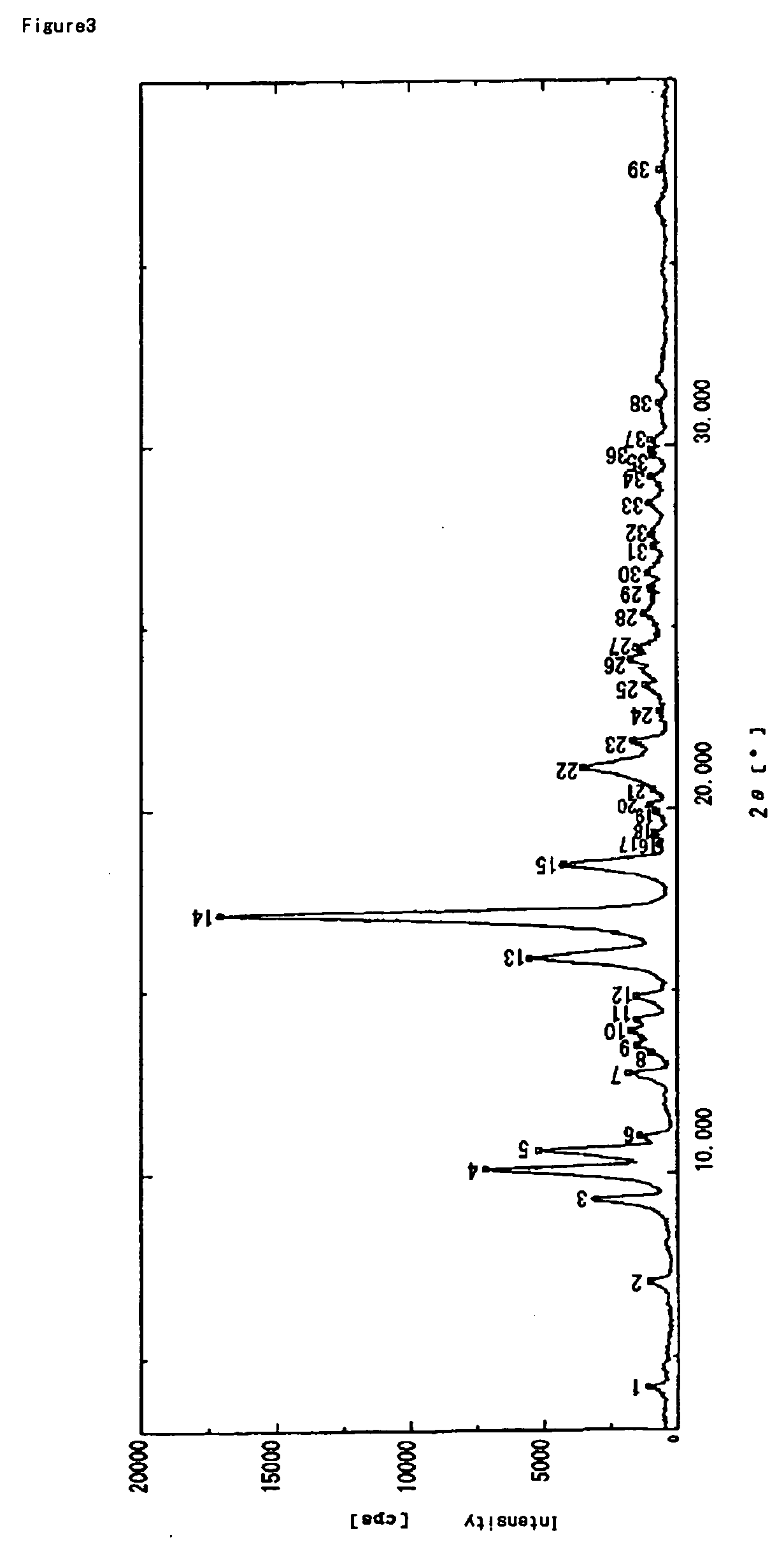
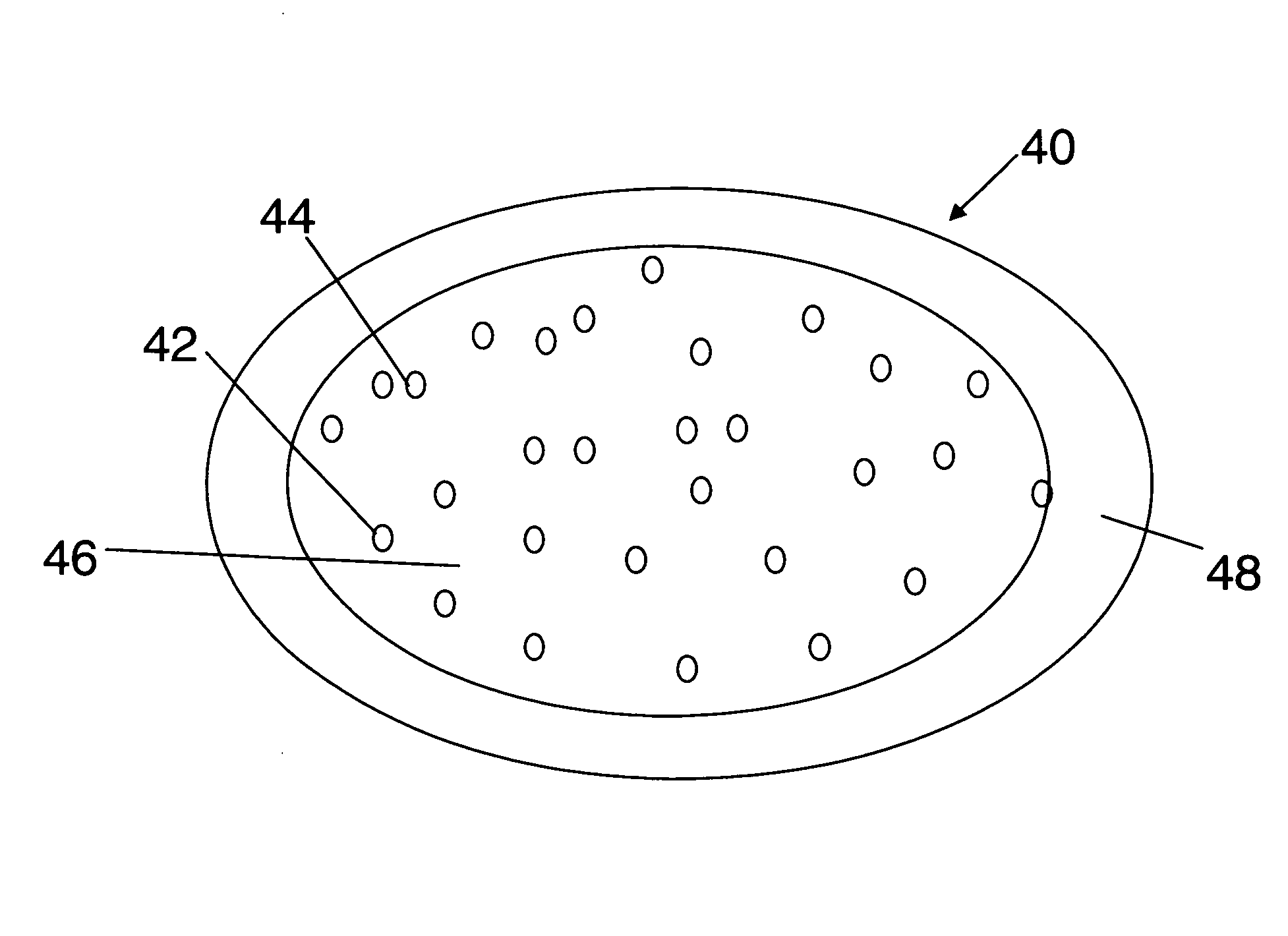
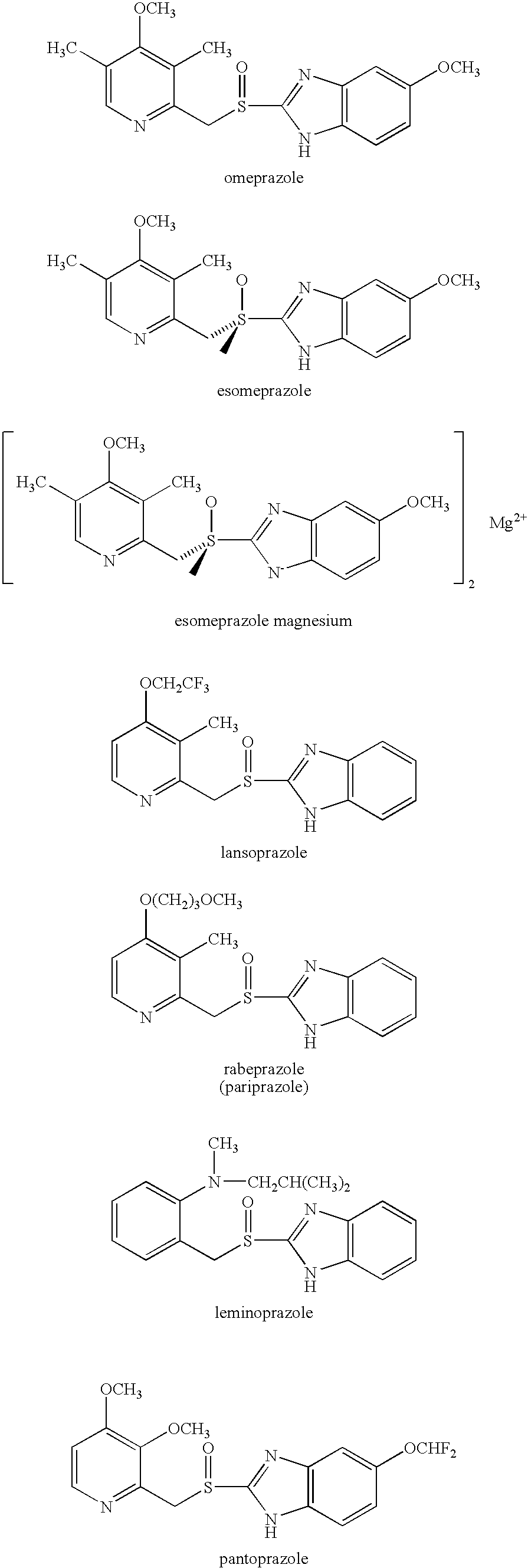
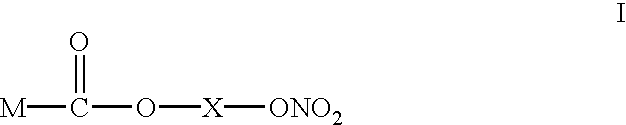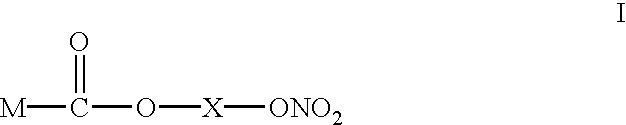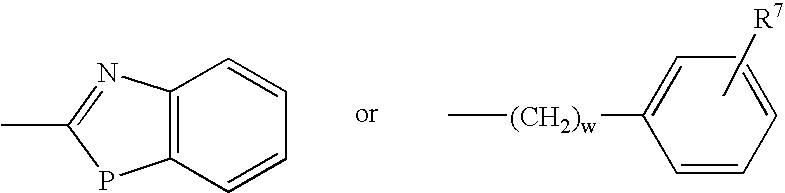Patents
Literature
419 results about "Proton-pump inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Proton-pump inhibitors (PPIs) are a group of medications whose main action is a pronounced and long-lasting reduction of stomach acid production. Within the class of medications, there is no clear evidence that one agent works better than another.
Combination of proton pump inhibitor, buffering agent, and nonsteroidal anti-inflammatory drug
InactiveUS20050249806A1Preventing gastric acid related disorderReduce riskBiocideSenses disorderNonsteroidal Antiinflammatory Drugs/NSAIDsBuffering agent
Pharmaceutical compositions comprising a proton pump inhibitor, one or more buffering agent and a nonsteroidal anti-inflammatory drug are described. Methods are described for treating gastric acid related disorders and treating inflammatory disorders, using pharmaceutical compositions comprising a proton pump inhibitor, a buffering agent, and a nonsteroidal anti-inflammatory drug.
Owner:SANTARUS
Pharmaceutical compositions comprising substituted benzimidazoles and methods of using same
InactiveUS20050054682A1Improve stabilityDecreased time to therapeutic effectBiocideDispersion deliveryBuffering agentPharmacology
The present invention is directed to, inter alia, pharmaceutical compositions comprising at least one proton pump inhibitor and at least one buffering agent. Compositions of the invention are useful in treating, inter alia, gastric acid related disorders.
Owner:UNIVERSITY OF MISSOURI
Oral pharmaceutical composition with delayed release of active ingredient for pantoprazole
An oral pharmaceutical composition comprises an acid-labile irreversible proton pump inhibitor in pellet or tablet form, wherein the irreversible proton pump inhibitor is at least partly in slow-release form. On combined administration with an anti-microbially-active ingredient, the composition is distinguished by imparting an enhanced action of rapid onset against disorders caused by Helicobacter.
Owner:TAKEDA GMBH
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20040048896A1Improve pharmacological activityGood effectBiocideOrganic chemistryDosage formBenzimidazole
Disclosed herein are compositions and methods for treating gastric acid disorders employing pharmaceutical compositions comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier.
Owner:UNIVERSITY OF MISSOURI
Gastric acid secretion inhibiting composition
InactiveUS20080031941A1Quick and lasting reliefAntibacterial agentsBiocideHelicobacter pyloriGastric ph
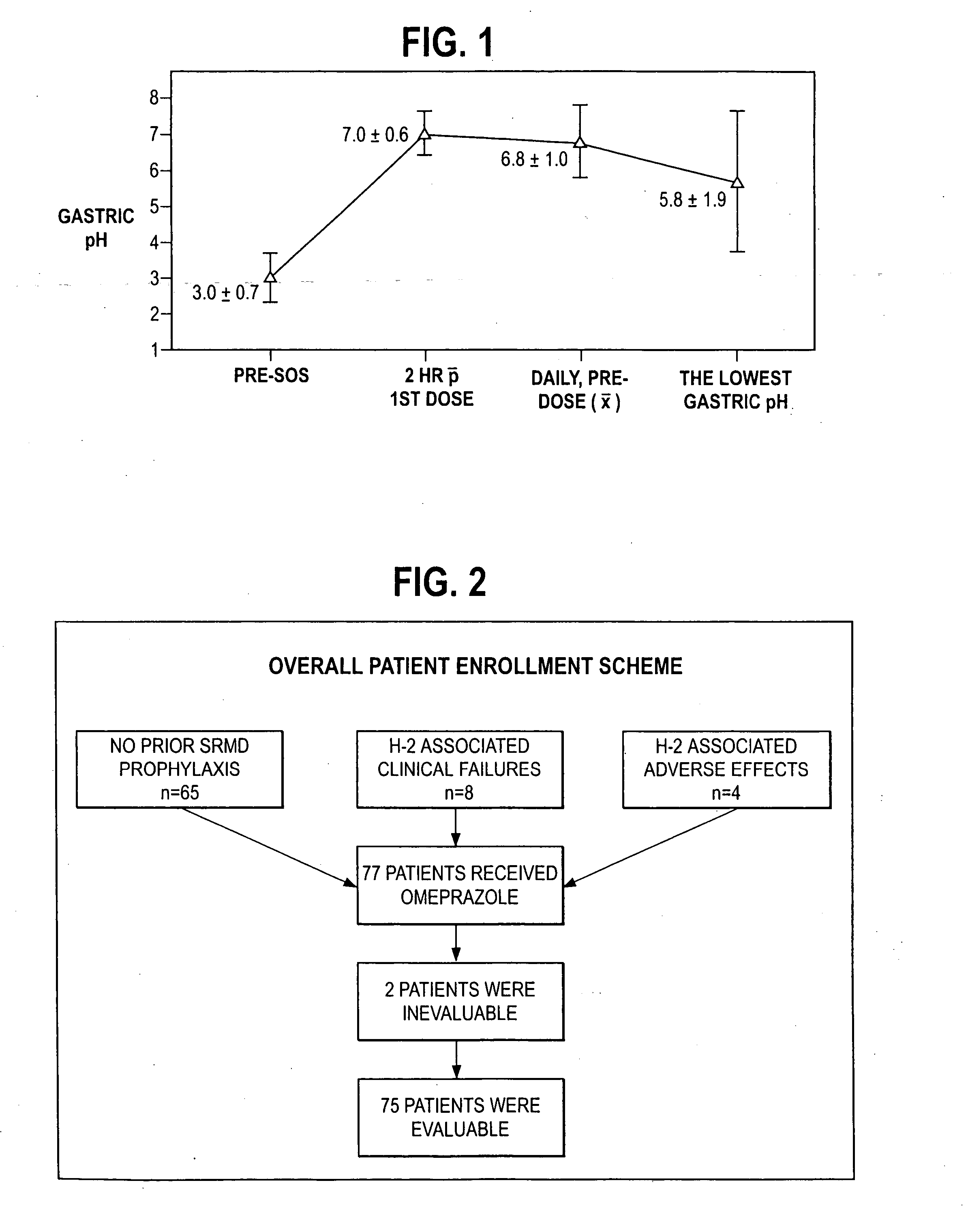
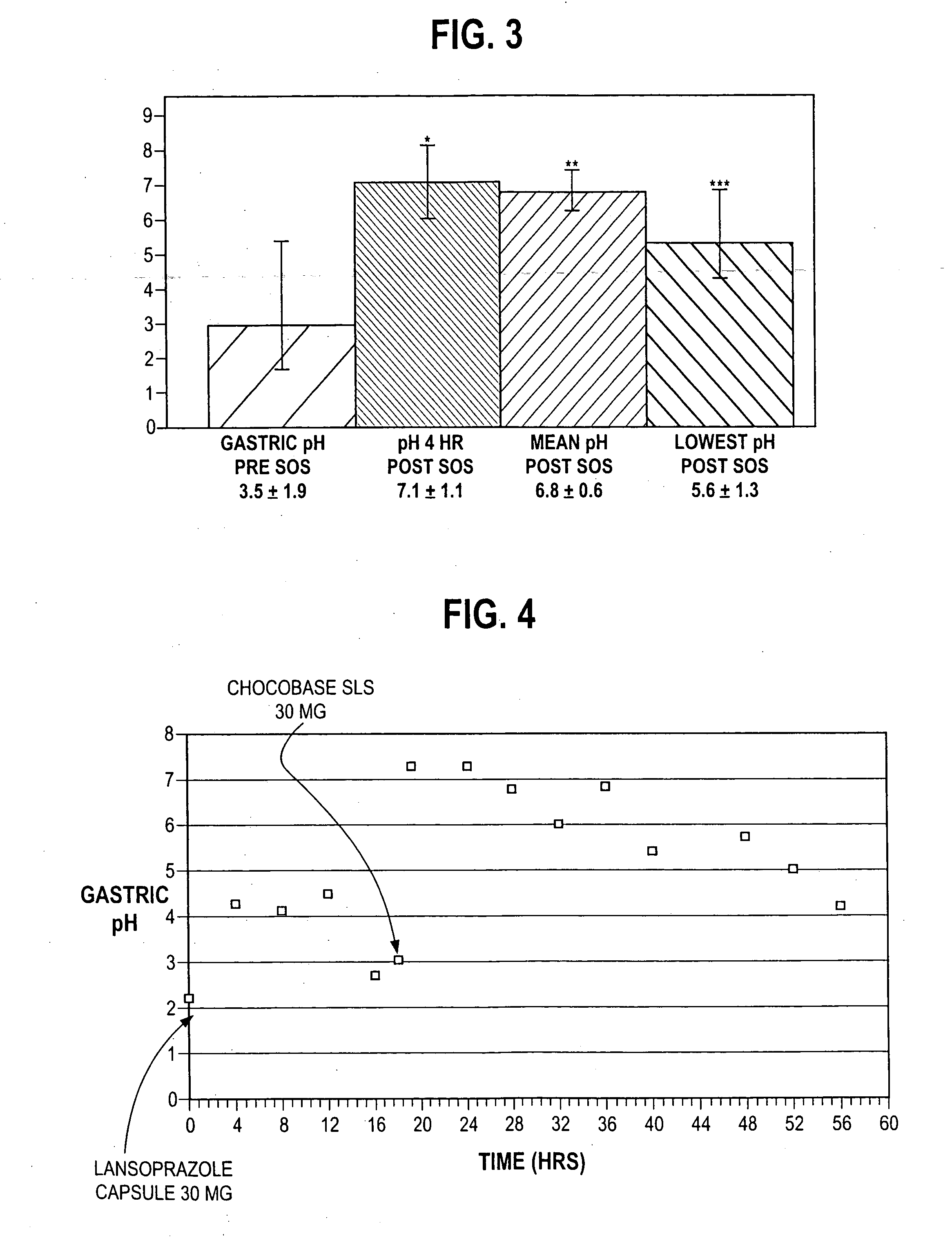
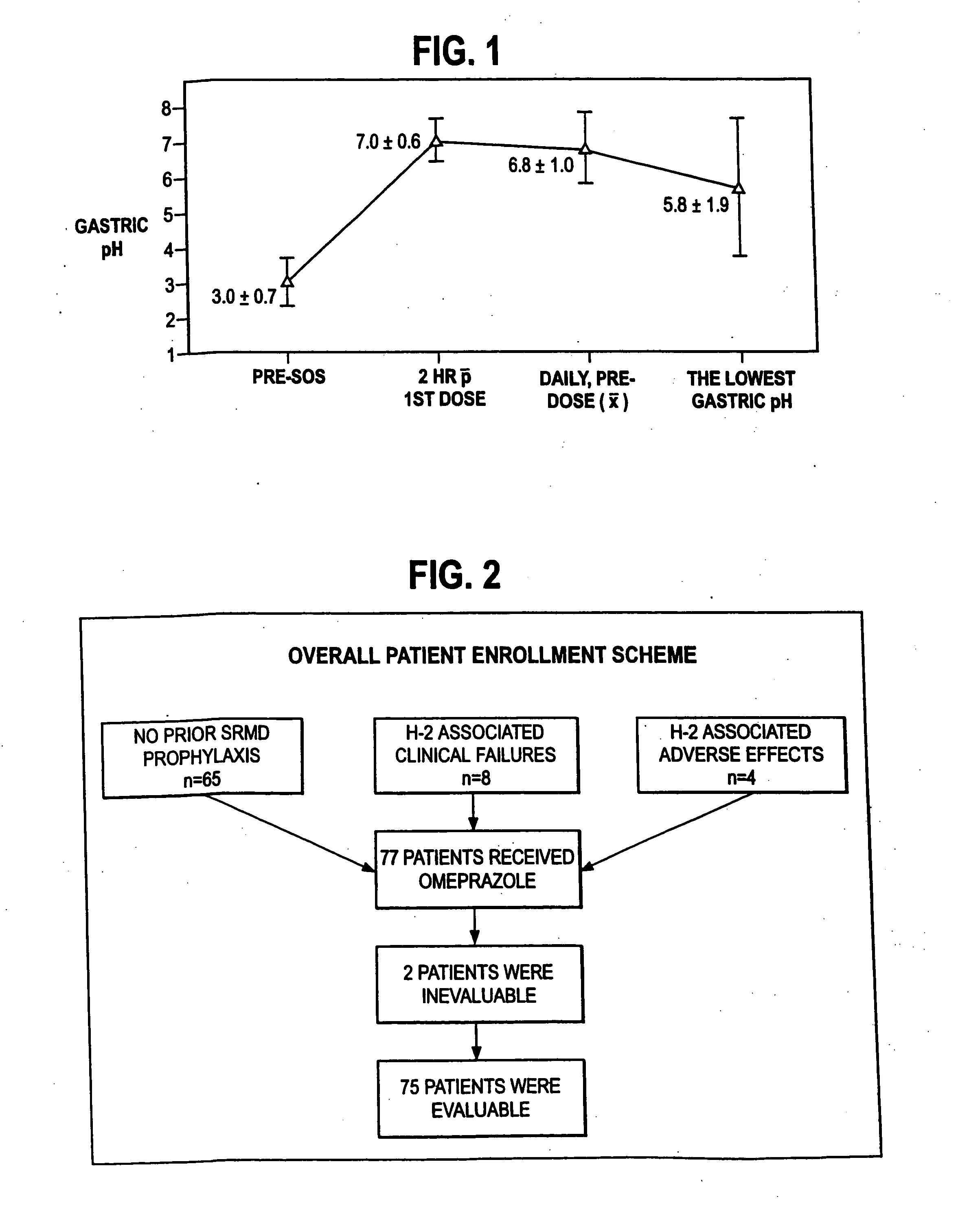
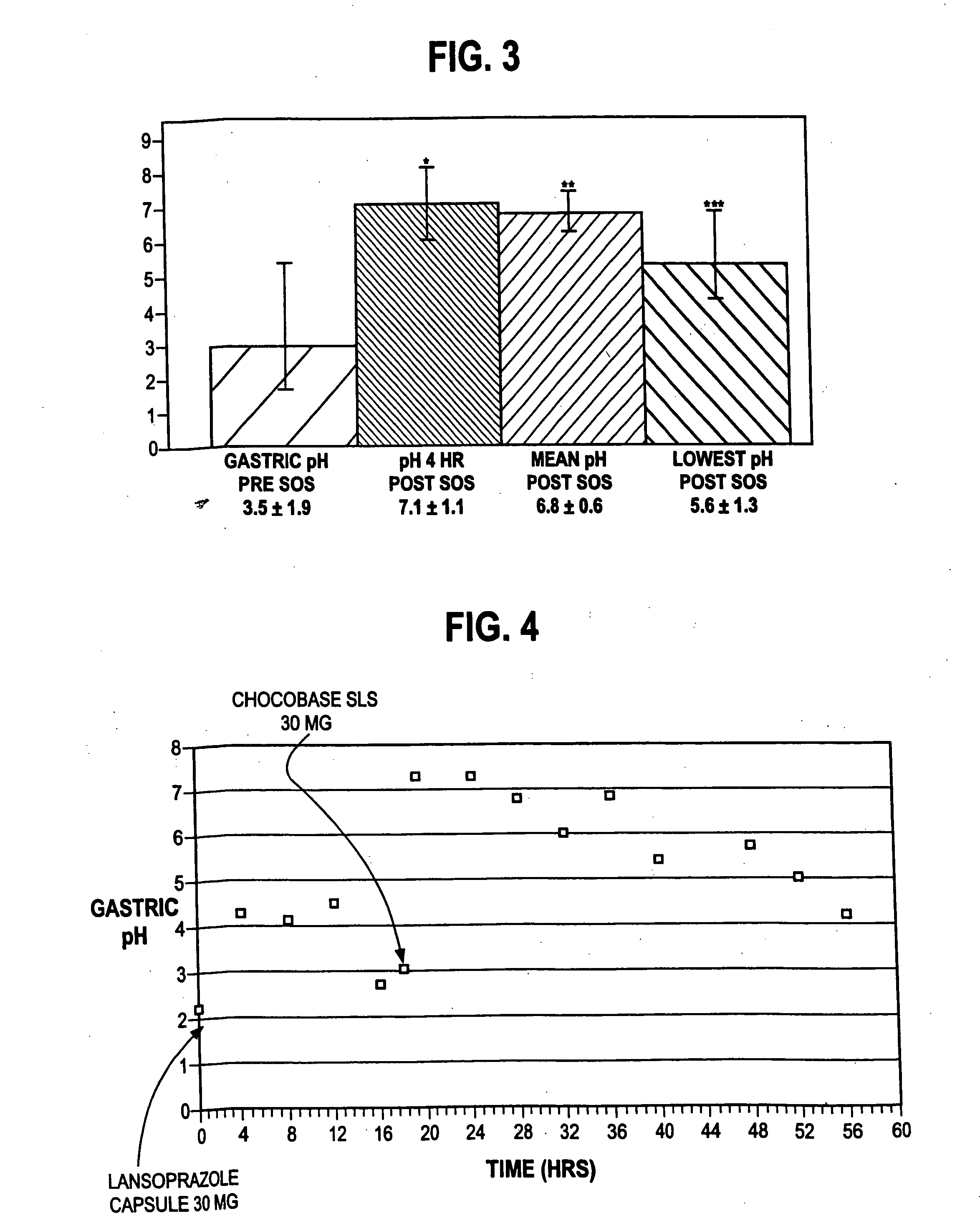
An oral pharmaceutical dosage form comprises pharmacologically effective amounts of an acid susceptible proton pump inhibitor or a salt thereof, an H2 receptor antagonist or a salt thereof and a pharmaceutically acceptable carrier. The dosage form is capable of raising gastric pH to above 4 within two hours after administration and to keep it at that level for at least 4 hours. Also disclosed is a method of manufacture of the dosage form, its use in treating dyspepsia and infection by Helicobacter pylori, and a method of treating disorders associated with gastric acid secretion.
Owner:OREXO AB
Pharmaceutical formulations containing a non-steroidal antiinflammatory drug and a proton pump inhibitor
InactiveUS6869615B2Decrease risk of development and exacerbationGood curative effectPowder deliveryAntipyreticSide effectDepressant
An oral solid dosage form includes a therapeutically effective amount of an NSAID and a proton pump inhibitor in an amount effective to inhibit or prevent gastrointestinal side effects normally associated with the NSAID. Also disclosed is a method of treating a human patient in need of antiinflammatory, analgesic and / or antipyretic therapy, comprising orally administering to the patient an oral pharmaceutical dosage form which includes a therapeutically effective amount of an NSAID and an amount of a proton pump inhibitor effective to substantially inhibit gastrointestinal side effects of the NSAID. The invention is further related to a method of prophylactically treating a human patient who is on a therapy known to have significant gastrointestinal side effects or is about to begin such a therapy, via concurrent administration of an NSAID and a proton pump inhibitor in a combination (single) oral dosage form.
Owner:ANDRX LABS
Oral administration form for an acid liable active proton pump inhibitor
Novel administration form for acid-labile active compounds are described. The novel administration forms have no enteric layers and are suitable for oral administration.
Owner:TAKEDA GMBH
Stable solid preparations
It is intended to provide a process for producing unstable amorphous benzimidazole compounds having a proton pump inhibitor function, and stable solid preparations for medicinal use containing these compounds which are produced by blending such an amorphous benzimidazole compound with a nontoxic base such as a basic inorganic salt, forming an intermediate coating layer on the layer containing the active ingredient and further forming an enteric coating layer or a release-controlling coating layer.
Owner:TAKEDA PHARMA CO LTD
Pulsatile gastric retentive dosage forms
Dosage forms for delayed and pulsed release of therapeutic agents into the stomach are described. The dosage forms are gastric retentive dosage forms that achieve release of the therapeutic agent into the stomach and upper gastrointestinal tract subsequent to administration of the dosage form. The dosage forms find particular use in administration of acid-labile active agents such as proton pump inhibitors, and in treating gastric acid secretion such as gastro-esophageal reflux disease (GERD) and nocturnal acid breakthrough (NAB).
Owner:DEPOMED SYST INC
Controlled-release compositions comprising a proton pump inhibitor
The present invention relates to pharmaceutical compositions, and methods of preparing such compositions, comprising one or more populations of controlled-release particles comprising one or more proton pump inhibitors. The present invention also relates to pharmaceutical dosage forms, including orally disintegrating tablets, tablets, capsules, and methods for their preparation.
Owner:APTALIS PHARMA +1
Solid dosage form for acid-labile active ingredient
InactiveUS20050281876A1Prevent and inhibit acid degradationPreserve bioavailabilityPowder deliveryMedical devicesBULK ACTIVE INGREDIENTSolid Dose Form
The present invention relates to solid, orally administrable dosage forms for acid-labile actives having at least one molded insert or core containing an acid-labile active ingredient, such as a proton pump inhibitor that is surrounded by barrier layer that is subsequently coated with an enteric layer. The present invention also relates to a dosage form that combines the barrier coated active ingredient containing insert with a second active ingredient.
Owner:MCNEIL PPC INC
Combination pain medication
InactiveUS20060177504A1Eliminate side effectsReduce riskBiocideSalicyclic acid active ingredientsSide effectMortality rate
This patent is an evolution of previous combination medication patents. Previous combination patents such as U.S. Pat. No. 6,613,354 which is a combination of an NSAID and Proton Pump Inhibitor. Thus the previous patents have covered gastrointestinal prophylaxis but none has covered both gastrointestinal and cardiovascular prophylaxis. This is likely because the cardiovascular side effects of NSAIDs were only recently discovered. This patent thus represents a leap in safety in a class of medication that is used by millions of Americans on a daily basis. This combination would thus decrease morbidity and mortality.
Owner:SUNDHARADAS RENJIT
Substituted benzimidazole dosage forms and method of using same
InactiveUS7399772B2Easy to prepareImprove pharmacological activityBiocidePowder deliveryEffervescent PowderPharmaceutical formulation
The present invention relates to pharmaceutical preparations comprising substituted benzimidazole proton pump inhibitors. There is provided a liquid or solid pharmaceutical composition consisting of a proton pump inhibitor and at least one buffering agent. Also provided is a pharmaceutical composition further comprising a parietal cell activator, an anti-foaming agent, a flavoring agent and combinations thereof; a method for treating acid-related gastrointestinal disorders by administering a solid pharmaceutical composition; and, a kit for the preparation of a liquid oral pharmaceutical composition. Dosage forms include: liquid, powder, tablet, capsule, effervescent powder, effervescent tablet, pellets, and granules.
Owner:UNIVERSITY OF MISSOURI
Dosage form for treating gastrointestinal disorders
InactiveUS20060165797A1Quick reliefPrevent relapseBiocideDigestive systemGastrointestinal disorderSecreted substance
The present invention is directed to drug dosage forms that can be used to treat diseases characterized by abnormal gastric acid secretion. The dosage forms have a core containing a proton pump inhibitor surrounded by an enteric coating or multiple particles containing proton pump inhibitor, each particle being surrounded by an enteric coating. The enteric coating delays the release of drug until the surrounding pH has risen. The tablets also include an outer coating that contains either a proton pump inhibitor or an H2 blocker. The outer coating is designed to rapidly dissolve in a patient's stomach.
Owner:POZEN INC
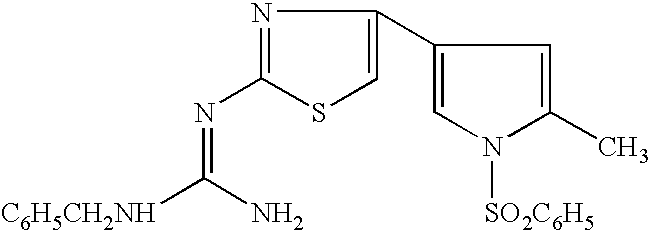
Proton Pump Inhibitors
ActiveUS20080139639A1Improve isolationEasy to purifyBiocideOrganic chemistryAcyl groupPyrazolylchalcone
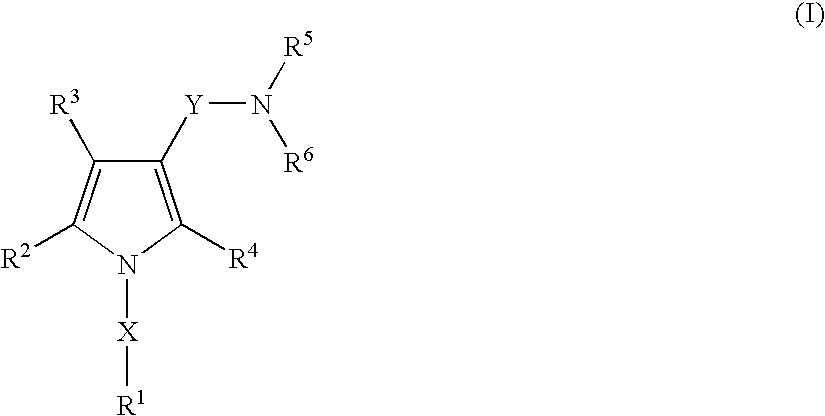
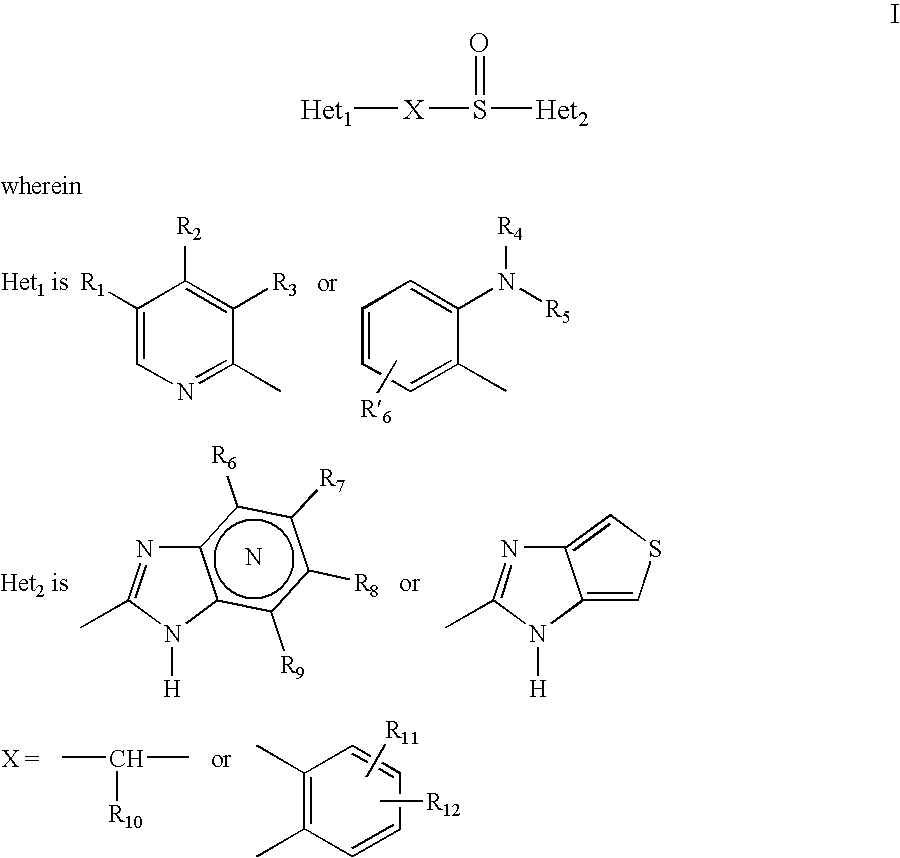
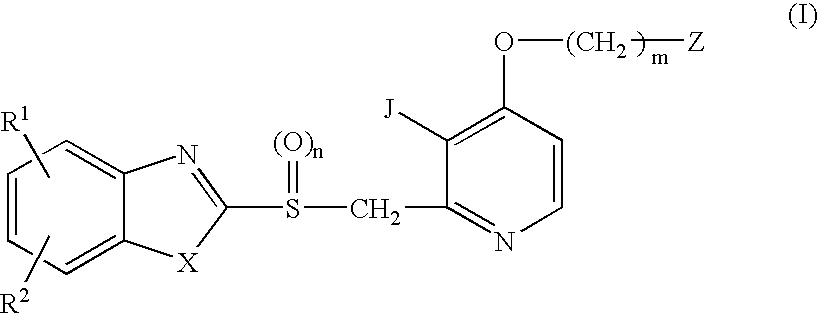
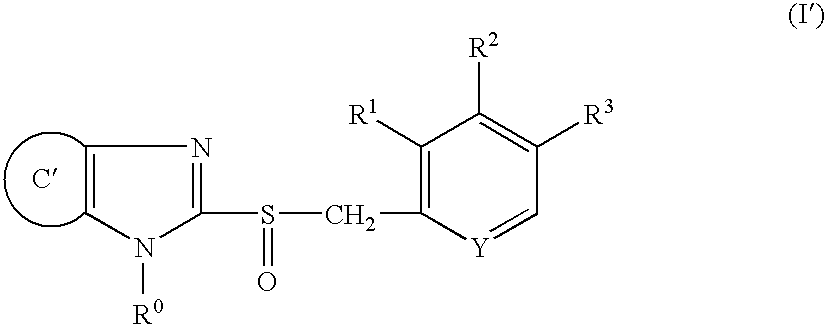
A proton pump inhibitor containing a compound represented by the formula (I)wherein X and Y are the same or different and each is a bond or a spacer having 1 to 20 carbon atoms in the main chain, R1 is an optionally substituted hydrocarbon group or an optionally substituted heterocyclic group, R2, R3 and R4 are the same or different and each is a hydrogen atom, an optionally substituted hydrocarbon group, an optionally substituted thienyl group, an optionally substituted benzo[b]thienyl group, an optionally substituted furyl group, an optionally substituted pyridyl group, an optionally substituted pyrazolyl group, an optionally substituted pyrimidinyl group, an acyl group, a halogen atom, a cyano group or a nitro group, R5 and R6 are the same or different and each is a hydrogen atom or an optionally substituted hydrocarbon group, which has a superior proton pump action and shows an antiulcer activity and the like after conversion to a proton pump inhibitor in the body, or a salt thereof. or a prodrug thereof is provided.
Owner:TAKEDA PHARMA CO LTD
Use of compounds as antibacterial agents
InactiveUS6593339B1Antibacterial agentsSalicyclic acid active ingredientsHelicobacterAntibacterial agent
The present invention discloses a new use of NO-releasing NSAIDs, especially NO-releasing NSAIDs of the formula I, or a pharmaceutically acceptable salt or enantiomer thereof, for the manufacture of a medicament for the treatment of bacterial infections, especially caused or mediated by Helicobacter pylon.Disclosed is also the new use of a NO-releasing NSAID in combination with an acid susceptible proton pump inhibitor for the treatment of bacterial infections.
Owner:ASTRAZENECA AB
Solid Dosage Form Comprising Proton Pump Inhibitor and Suspension Made Thereof
InactiveUS20080020053A1Stable levelConvenient coatingAntibacterial agentsPowder deliveryBULK ACTIVE INGREDIENTSolid Dose Form
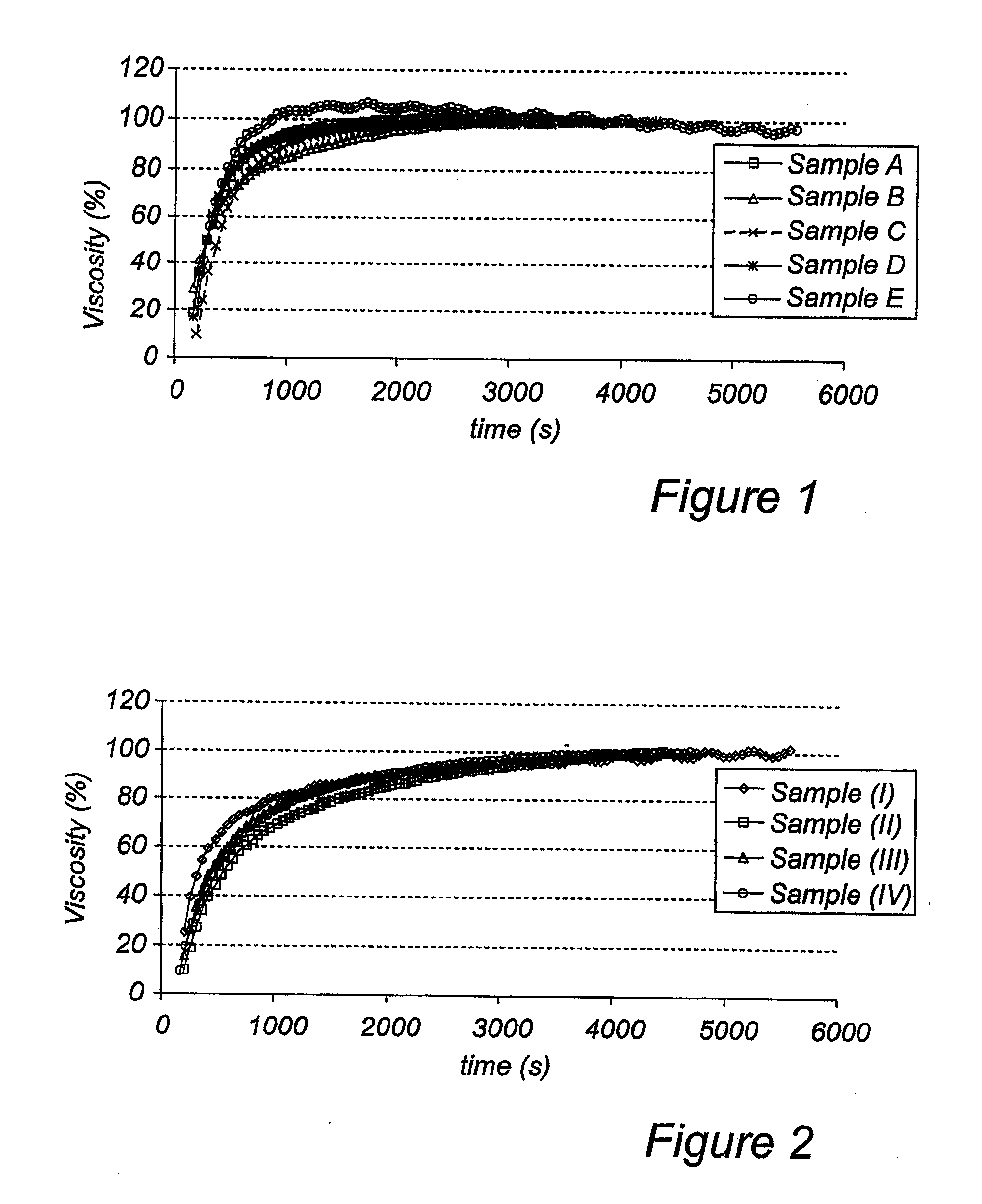
A solid rapidly gelling oral pharmaceutical dosage form, as well as aqueous suspensions prepared thereof, comprising an acid sensitive proton pump inhibitor as active ingredient distributed in a multitude of enteric coated pellets and a suspension modifying granulate comprising a rapidly dissolving diluent granulated together with a gelling agent chosen among xanthan gums, and an acidic pH-regulating agent and a binder. The suspension modifying granulate is rapidly disintegrating and gelling when suspended in an aqueous medium and thus forming a homogenous stable and robust suspension having a reproducible and stable viscosity. Furthermore the invention relates to an improved process for its manufacture and the use of such formulation in medical treatment including prevention of gastrointestinal disorders in humans.
Owner:ASTRAZENECA AB
Novel substituted benzimidazole dosage forms and method of using same
Disclosed herein are methods, kits, combinations, and compositions for treating gastric acid disorders employing pharmaceutical compositions comprising a proton pump inhibiting agent (PPI) and a buffering agent in a pharmaceutically acceptable carrier.
Owner:UNIVERSITY OF MISSOURI
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
Pharmaceutical formulatins useful for inhibiting acid secretion and methods for making and using them
InactiveUS20050037070A1Extended shelf lifeEnhance processing and performancePowder deliveryBiocideSecretionSecreted substance
In one general aspect of the present invention, pharmaceutical formulations comprising both a proton pump inhibitor microencapsulated with a material that enhances the shelf-life of the pharmaceutical composition and one or more antacid are described. In another general aspect of the present invention, pharmaceutical formulations comprising both a proton pump inhibitor microencapsulated with a taste-masking material and one or more antacid are described.
Owner:SANTARUS
Combination of proton pump inhibitor and sleep aid
InactiveUS20050244517A1Extended shelf lifeSimple compositionBiocideDigestive systemDiseaseBuffering agent
Pharmaceutical compositions comprising a proton pump inhibitor, one or more buffering agent and a sleep aid are described. Methods are described for treating gastric acid related disorders and inducing sleep, using pharmaceutical compositions comprising a proton pump inhibitor, a buffering agent, and a sleep aid.
Owner:SANTARUS
New Combination Dosage Form
InactiveUS20070122470A1Salicyclic acid active ingredientsBiocideGastrointestinal complicationsSalicylic acid
The present invention relates to an oral pharmaceutical preparation for use in the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid. The present preparation comprises a fixed oral dosage form comprising a proton pump inhibitor in combination with acetyl salicylic acid. Furthermore, the present invention refers to a method for the manufacture thereof and the use thereof in medicine. The present invention also relates to a specific combination comprising esomeprazole, or an alkaline salt thereof or a hydrated form of any one of them, and acetyl salicylic acid for use as a medicament for the prevention of thromboembolic vascular events, such as myocardial infarction or stroke, and for the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid.
Owner:ASTRAZENECA AB
Composition and methods for inhibiting gastric acid secretion
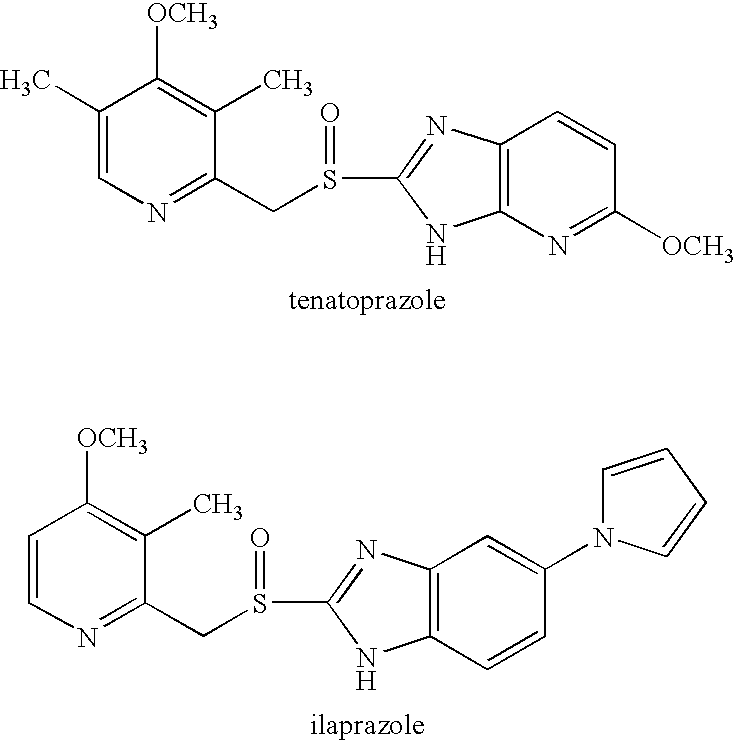
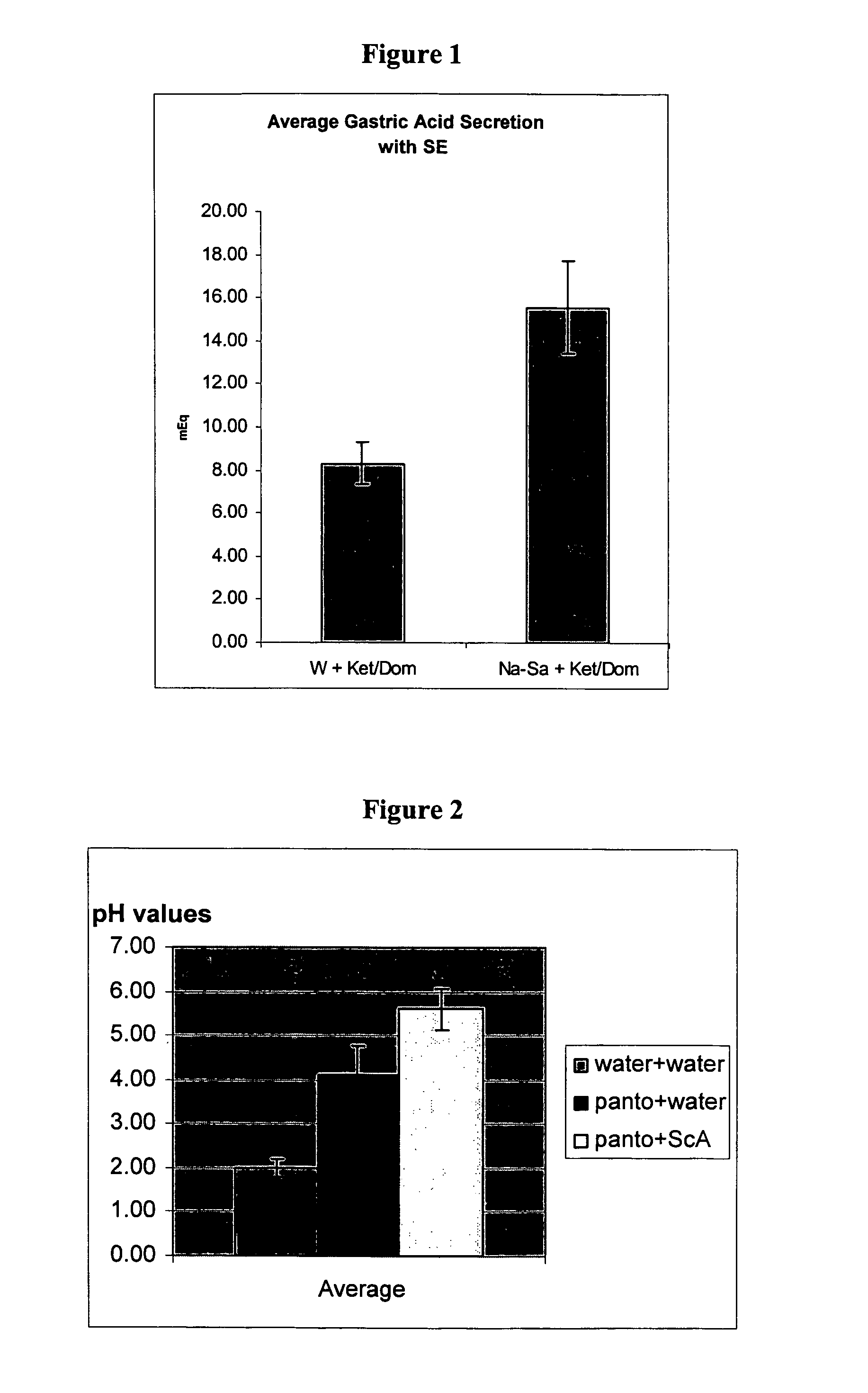
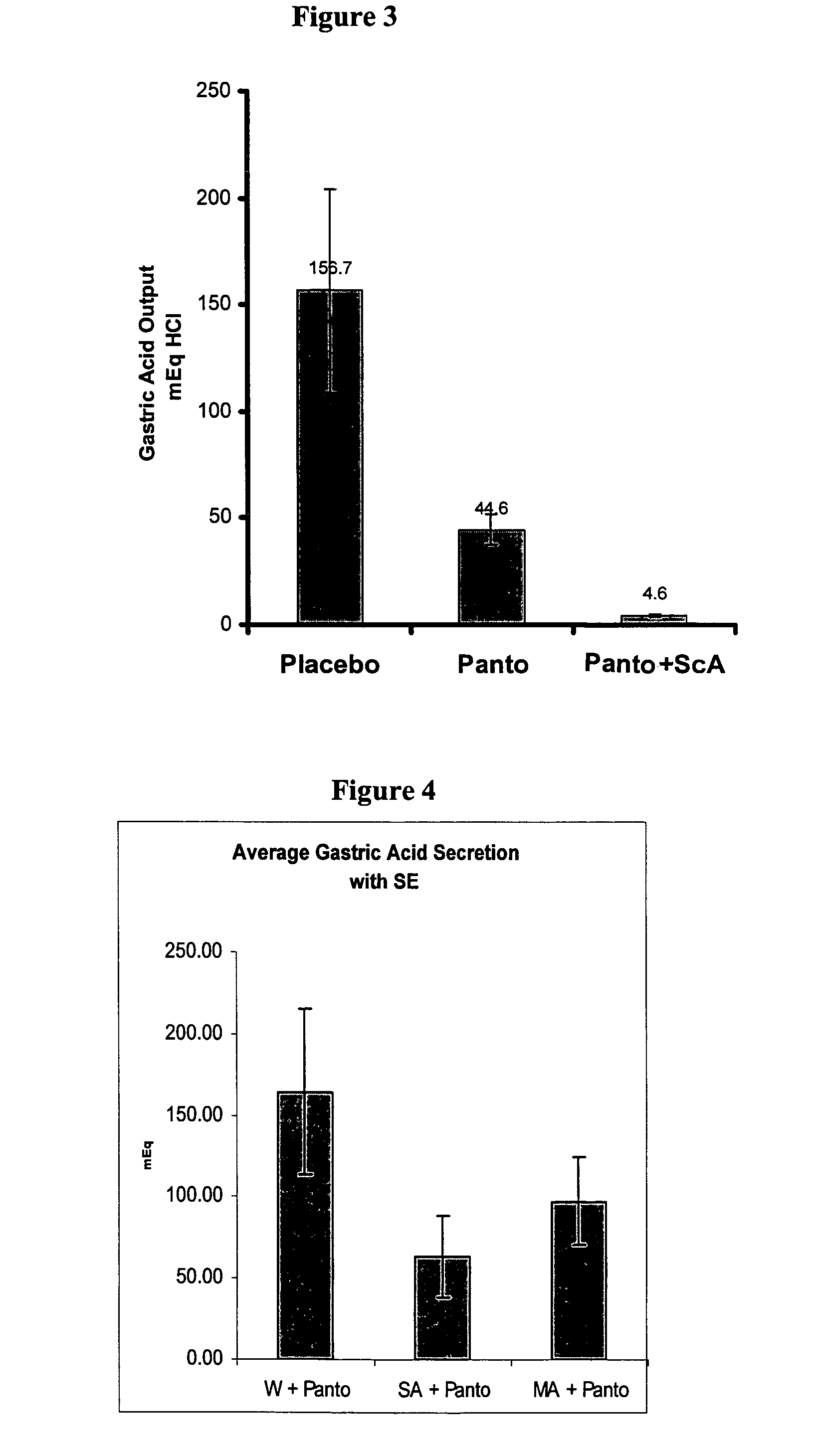
The present invention is related to oral compositions comprising an irreversible gastric H+ / K+-ATPase proton pump inhibitor (PPI) as a gastric acid secretion inhibitor and succinc acid as a parietal cell activator in the gastric lumen. The compositions of the present invention are capable of enhancing the anti-acid activity of PPI in the stomach. The present invention further relates to a method of using such compositions to reduce gastric acid secretion in a mammal.
Owner:VECTA
Oral pharmaceutical dosage forms comprising a proton pump inhibitor and a NSAID
InactiveUS7488497B2Simplify regimenPatient compliance is goodAntibacterial agentsOrganic chemistrySide effectEnteric coating
An oral pharmaceutical dosage form comprising an acid susceptible proton pump inhibitor and one or more NSAIDs in a fixed formulation, wherein the proton pump inhibitor is protected by an enteric coating layer. The fixed formulation is in the form of an enteric coating layered tablet, a capsule or a multiple unit tableted dosage form. The multiple unit dosage forms are most preferred. The new fixed formulation is especially useful in the treatment of gastrointestinal side-effects associated with NSAID treatment.
Owner:ASTRAZENECA AB
Methods for Treating Diabetes
InactiveUS20090042781A1Reduce loss rateBiocidePeptide/protein ingredientsInsulin secretionIncreasing insulin
Owner:NOVO NORDISK AS
Methods using proton pump inhibitors
The invention provides methods of treating and preventing asthma, laryngitis, symptomatic gastroesophageal reflux disease, pregnancy-induced gastroesophageal reflux disease, noncardiac chest pains, coughing, apnea, dyspepsia, inflammatory bowel disease, irritable bowel syndrome, gastritis, stress ulcers, bleeding peptic ulcers, acute gastrointestinal bleeding, infectious enteritis, collagenous colitis, lymphocytic colitis, chronic diarrhea in immunocompromised patients, esophageal ulcers in immunocompromised patients, idiopathic gastric acid hypersecretion, gastroparesis, gastrointestinal motility disorders, Zollinger-Ellison syndrome, short bowel syndrome, emesis, regurgitation, early satiety, chronic sore throat, abdominal pain, abdominal bloating, nausea, sour stomach, diarrhea, constipation, bacterial infections, refractory ulcers, gastrointestinal disorders induced by NSAIDs, Barrett's esophagus, gastrointestinal disorders caused by steroids, gastrointestinal disorders induced by cholinergic compounds, and fungal or viral-induced ulcers in the gastrointestinal tract by administering a therapeutically effective amount of at least one proton pump to a patient in need thereof. The invention also provides on demand relief of symptoms associated with gastroesophageal reflux disease (GERD), and provides relief from symptoms caused by the consumption of excessive amounts of food and / or alcohol by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof. The invention also provides methods for treating parasitic infections, such as malaria, by administering a therapeutically effective amount of at least one proton pump inhibitor to a patient in need thereof.
Owner:EISAI CO LTD
Enteric-coated pellet preparation of proton pump inhibitor and preparation method thereof
ActiveCN102119927AEvenly dispersedAvoid degradationOrganic active ingredientsDigestive systemWater solubleBioavailability
The invention relate to an enteric-coated pellet preparation of proton pump inhibitor and a preparation method thereof. The enteric-coated pellet preparation of proton pump inhibitor provided by the invention is composed of a blank pellet core, a drug-loaded layer, isolating layers (I) and (II) and an enteric-coated layer, wherein both the drug-loaded layer and the isolating layer (I) contain water soluble inorganic bases, and the base used in the drug-loaded layer contains sodium hydroxide and another water soluble inorganic base which can form buffer with sodium hydroxide in a water solution and the pH of which is in an alkaline environment of 11-12 (excluding 11). The enteric-coated pellet of proton pump inhibitor provided by the invention successfully solves the technical problems of the existing enteric-coated pellet preparation of proton pump inhibitor, and is a superior preparation with high drug loading efficiency, good anti-acid effect, high release rate, good repeatability, high bioavailability and good stability.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Packaging system
InactiveUS20070184078A1Small article dispensingMicrobiological testing/measurementNon steroidal anti inflammatoryProton-pump inhibitor
Owner:ANDRX
Controlled release composition
ActiveUS20060177509A1Reduce the amount requiredIncrease release rateDigestive systemPill deliveryControl releaseMedicine
The present invention provides a controlled release composition showing release of an active ingredient (proton pump inhibitor) controlled in two or more steps at different release rates, which contains 1) a release-controlled part A capable of controlling release of the active ingredient to occur at a predetermined rate, 2) a release-controlled part B capable of controlling release of the active ingredient to occur at a predetermined rate lower than the release rate of the release-controlled part A, and where necessary, 3) a release-controlled part C capable of controlling release of the active ingredient to occur at a predetermined rate faster than the release rate of the release-controlled part B, wherein the release of the active ingredient from the release-controlled part B precedes the release of the active ingredient from the release-controlled part A (when release-controlled part C is contained, the release of the active ingredient from the release-controlled part C precedes the release of the active ingredient from the release-controlled part B).
Owner:TAKEDA PHARMA CO LTD
Proton pump inhibitors
Novel thiadiazole compounds are provided, which are effective as proton pumps inhibitors, useful in treating peptic ulcers by inhibition of the proton pump enzyme H+ / K+-ATPase. The compounds are 3-substituted 1,2,4-thiadiazolo [4,5- alpha ]benzimidazole and 3-substituted imidazo[1,2-d]-1,2,4-thiadiazoles corresponding to the general formula: where X and Z either represent an optionally substituted benzene ring fused to the diazole nucleus, or represent a variety of independent chemical groupings (hydrogen, lower alkyl, halo, etc.) and Y is selected from a wide range, e.g. heterocyclics and carbonyl groups.
Owner:KARIMIAN KHASHAYAR +5
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com