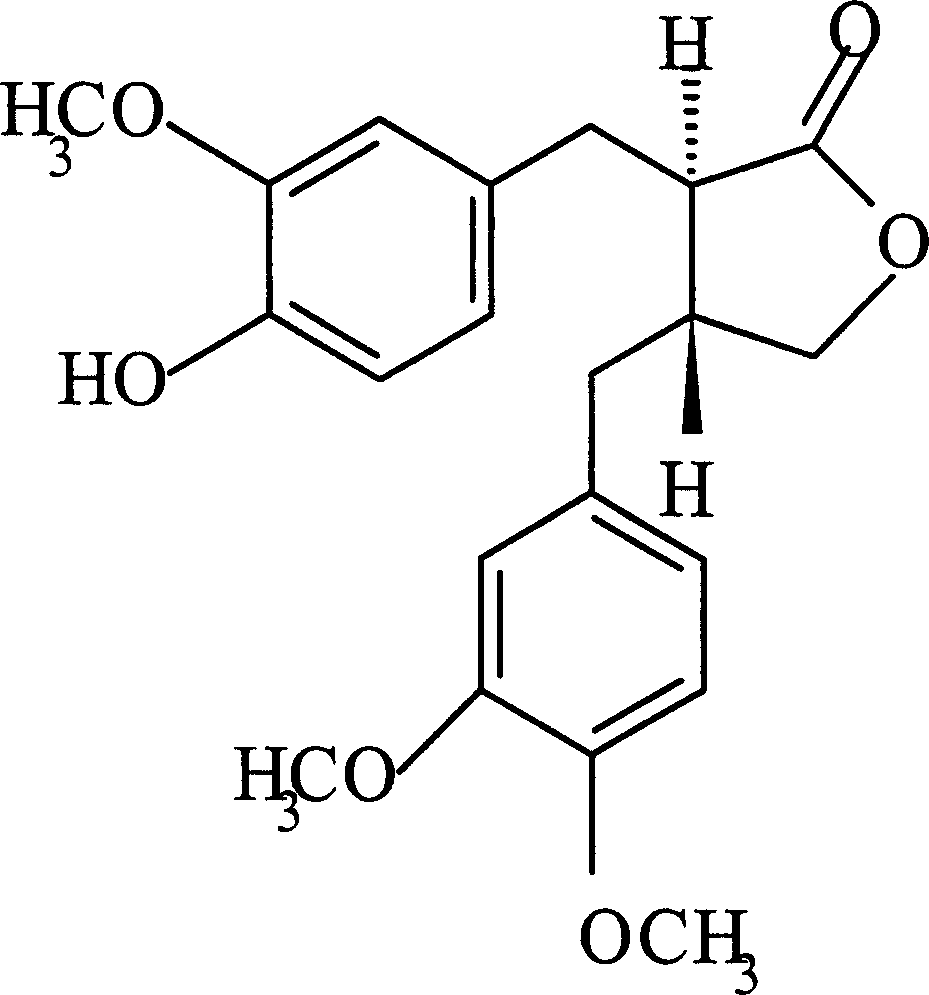
Patents
Literature
519results about How to "Improve pharmacological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20040048896A1Improve pharmacological activityGood effectBiocideOrganic chemistryDosage formBenzimidazole
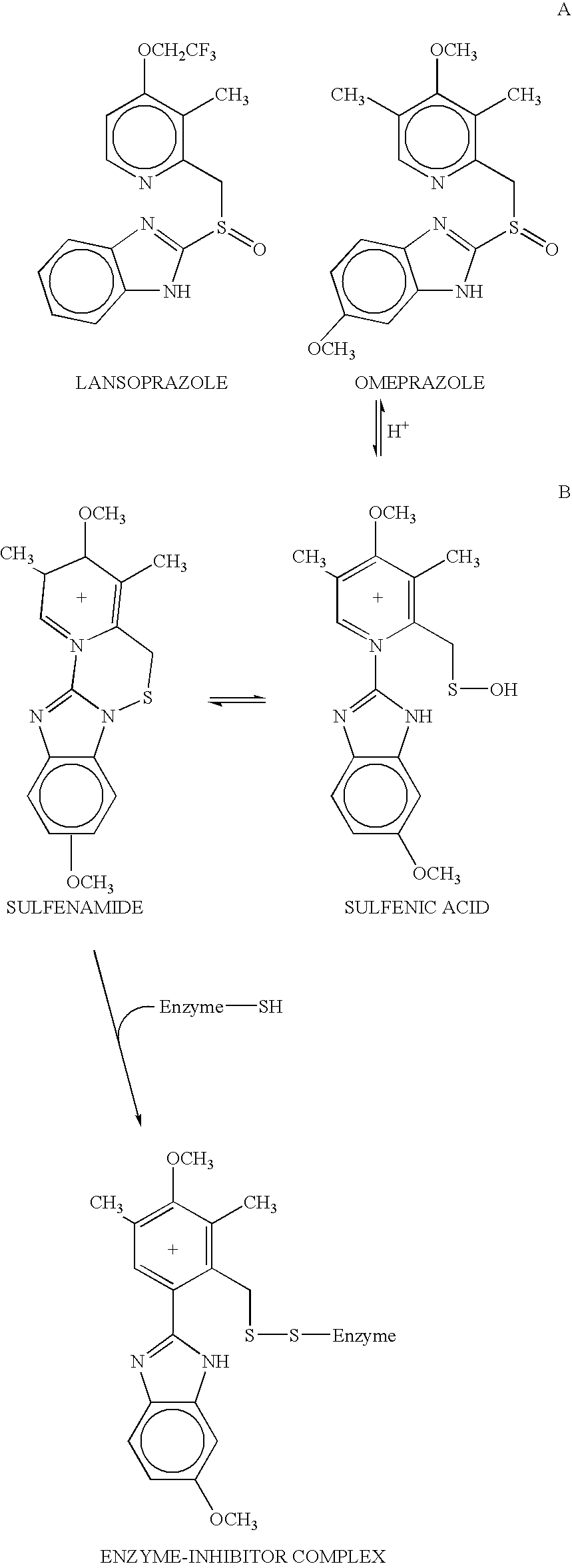
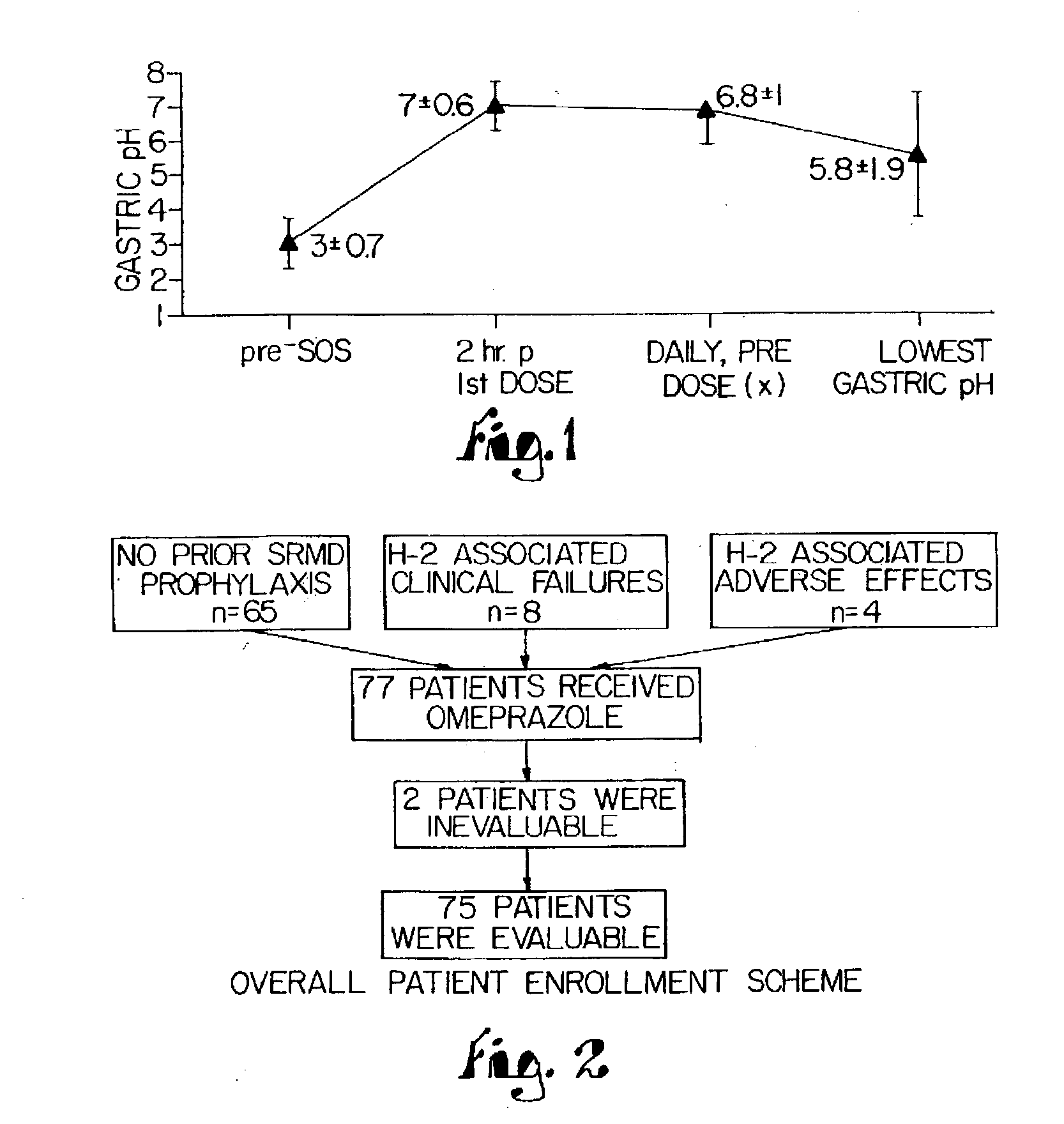
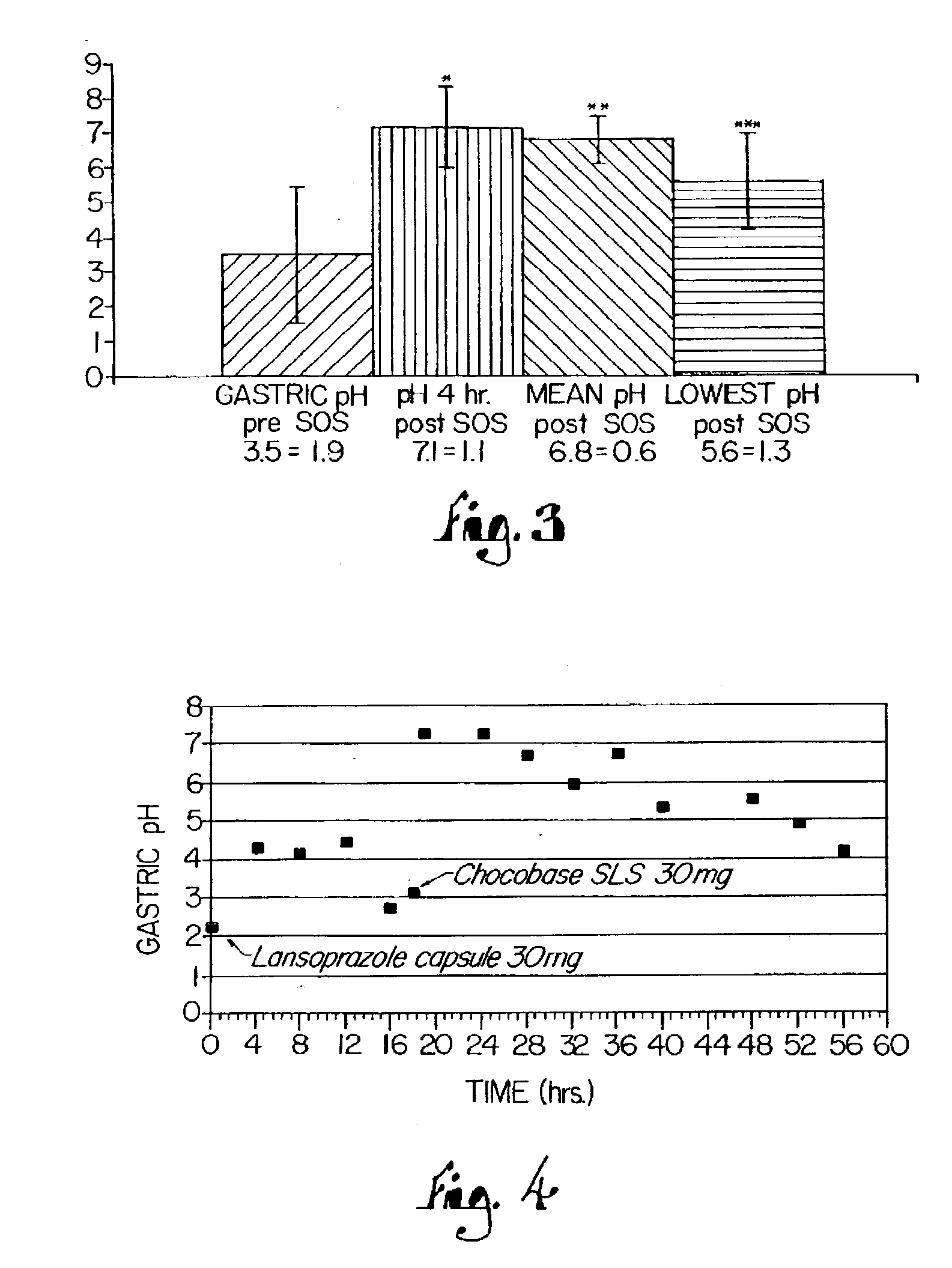
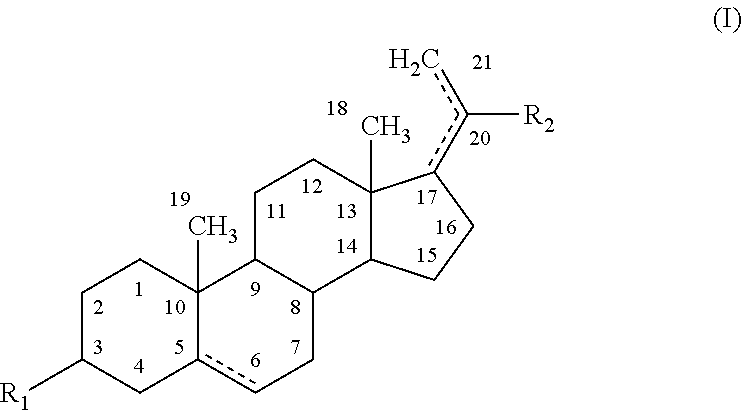
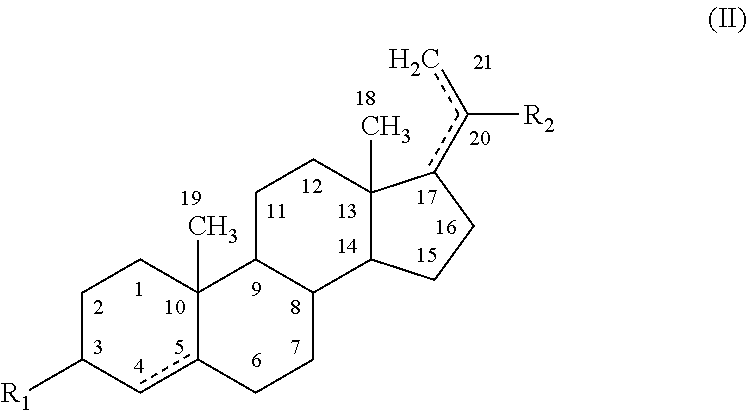
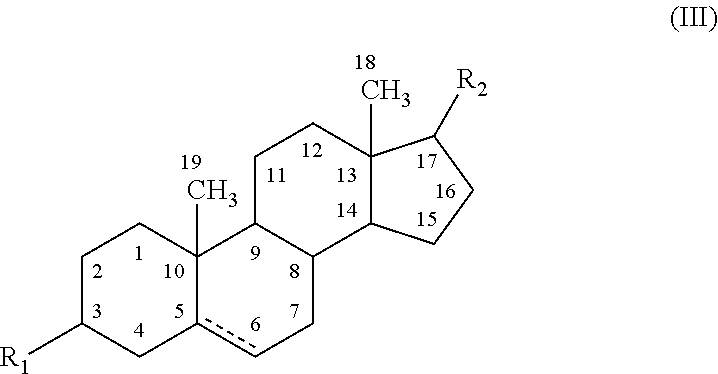
Disclosed herein are compositions and methods for treating gastric acid disorders employing pharmaceutical compositions comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier.
Owner:UNIVERSITY OF MISSOURI
Conjugated Neuroactive Steroid Compositions And Methods Of Use
The present disclosure provides modified neuroactive steroids. The modified neuroactive steroids may comprise, consist of, or consist essentially of a therapeutic agent and / or a modifying moiety. The modified neuroactive steroid can have modified characteristics as compared to native neuroactive steroids that do not include a modifying moiety and / or therapeutic agent. The modified neuroactive steroid may be, for example, modified pregnenolone, pregnenolone metabolites, allopregnanolone, and / or allopregnanolone metabolites. The modified neuroactive steroids can be used to treat, prevent and / or ameliorating a phenotypic state of interest in a subject.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Substituted benzimidazole dosage forms and method of using same
InactiveUS7399772B2Easy to prepareImprove pharmacological activityBiocidePowder deliveryEffervescent PowderPharmaceutical formulation
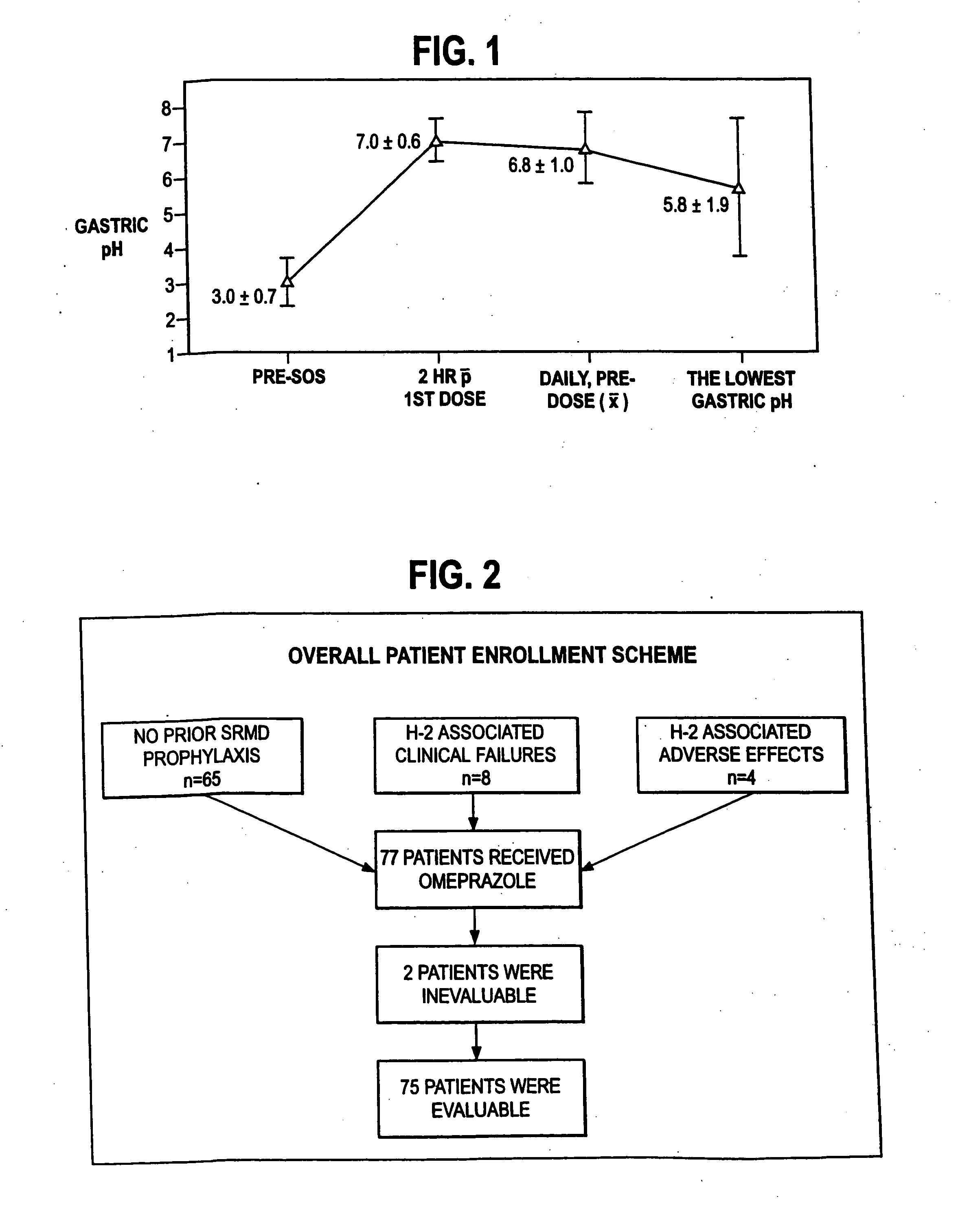
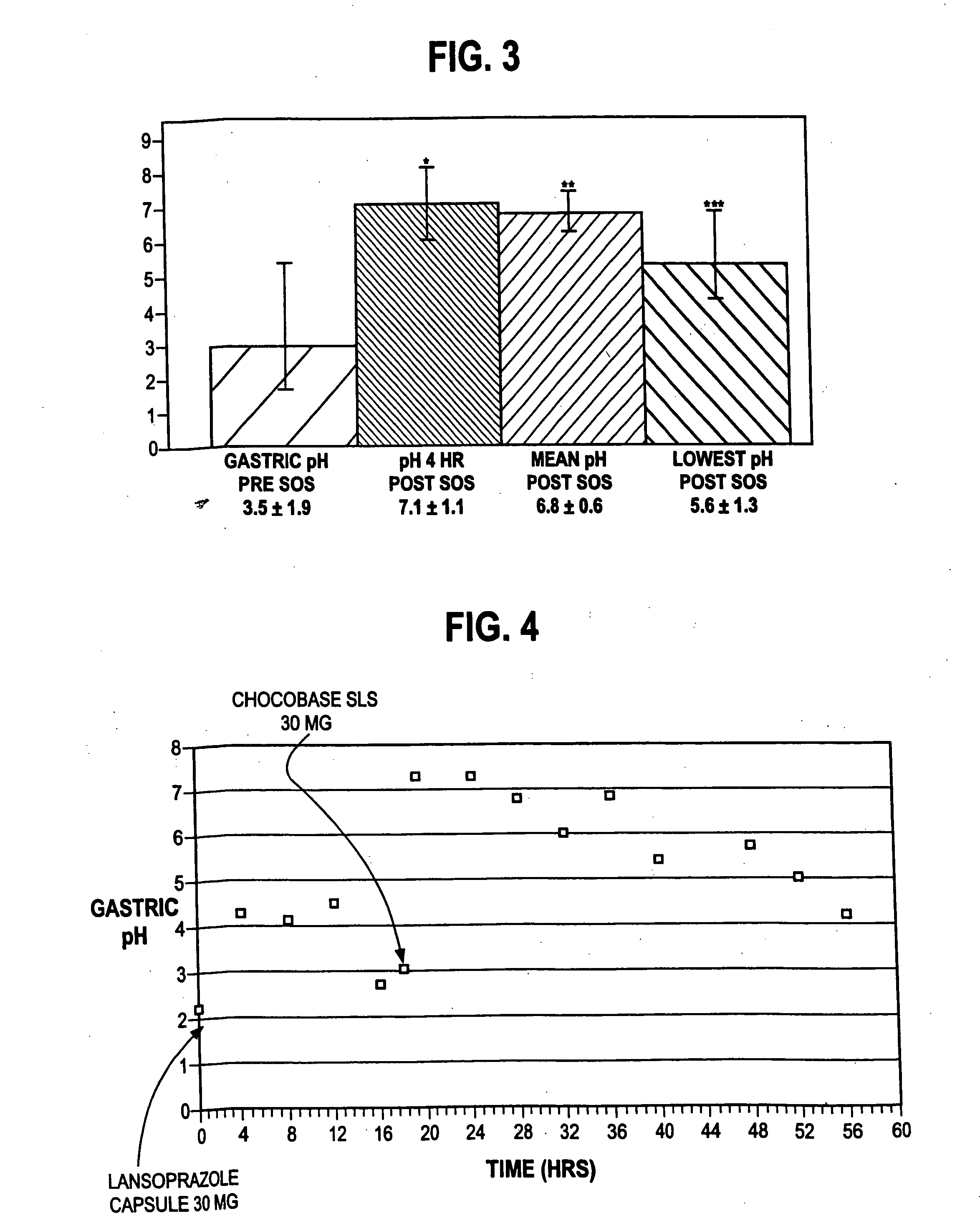
The present invention relates to pharmaceutical preparations comprising substituted benzimidazole proton pump inhibitors. There is provided a liquid or solid pharmaceutical composition consisting of a proton pump inhibitor and at least one buffering agent. Also provided is a pharmaceutical composition further comprising a parietal cell activator, an anti-foaming agent, a flavoring agent and combinations thereof; a method for treating acid-related gastrointestinal disorders by administering a solid pharmaceutical composition; and, a kit for the preparation of a liquid oral pharmaceutical composition. Dosage forms include: liquid, powder, tablet, capsule, effervescent powder, effervescent tablet, pellets, and granules.
Owner:UNIVERSITY OF MISSOURI
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
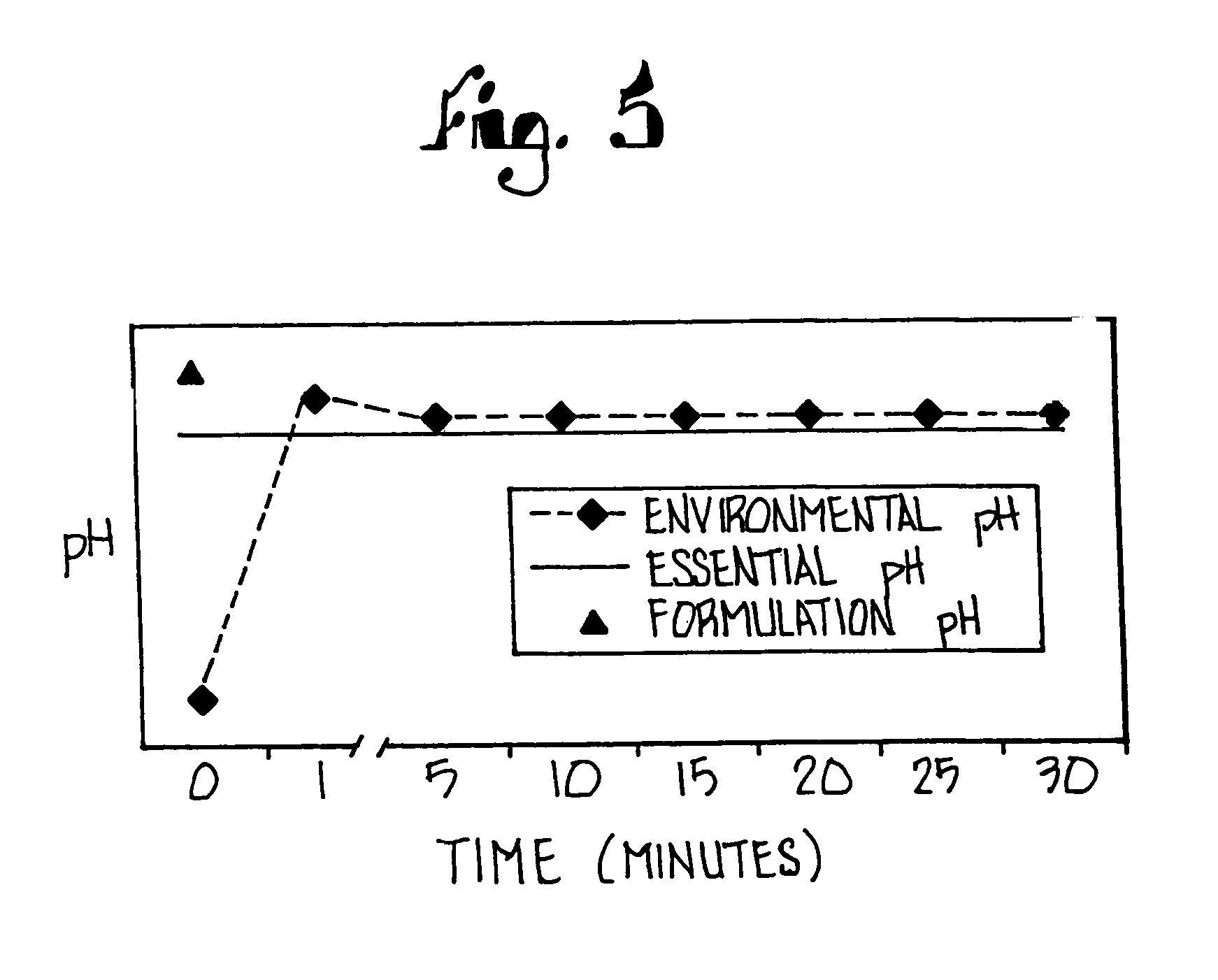
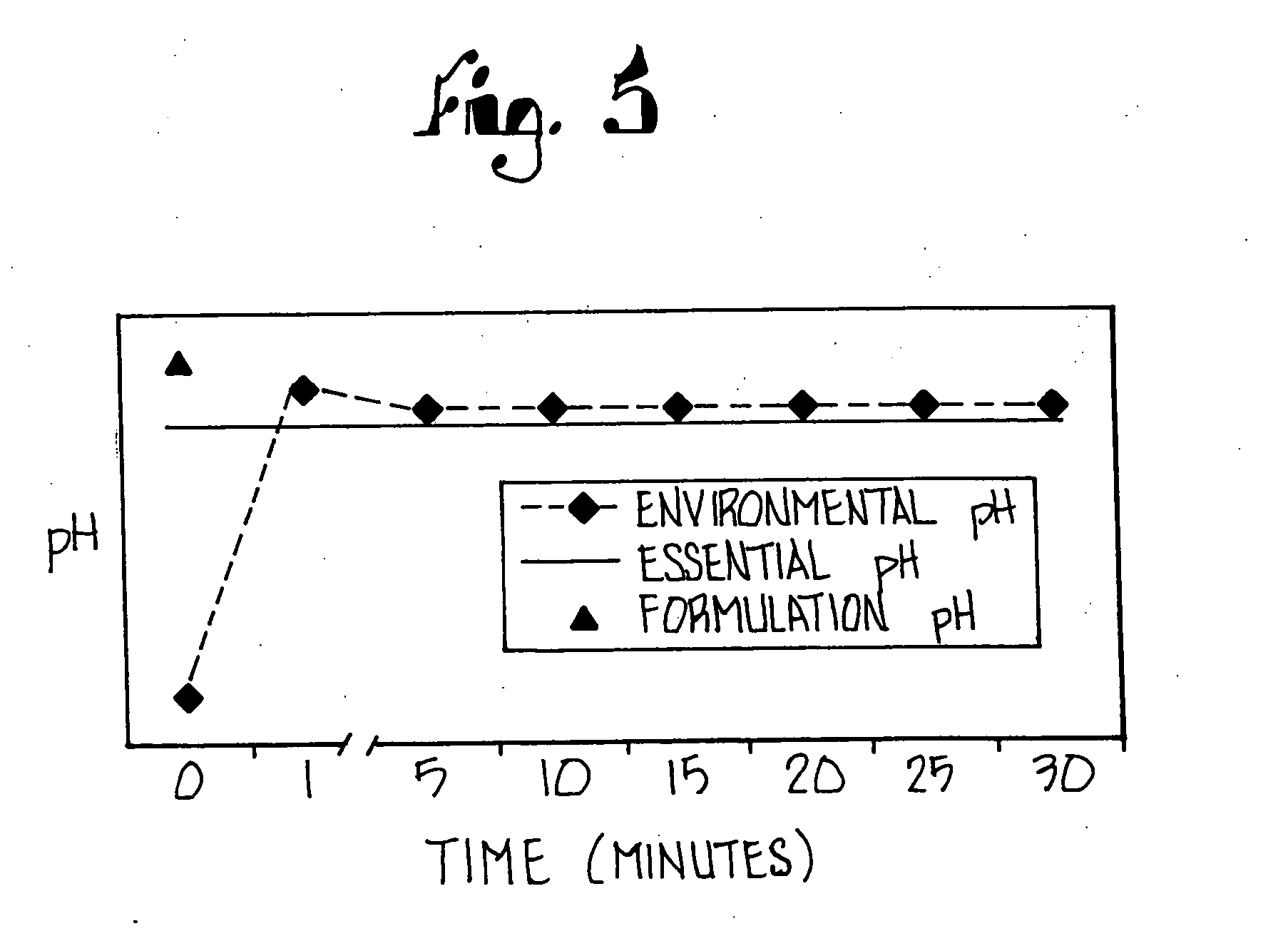
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
Pharmacological activities of Curcuma longa extracts
InactiveUS7220438B2Increase proliferative activityHigh activityBiocideDrug compositionsCurcuma longa extractWater soluble
This invention concerns to a topical pharmaceutical composition comprising an water soluble Curcuma extract, and suitable excipients for said topical administration; the process for obtaining said pharmaceutical compositions; the use of different Curcuma extracts as photosensitizing agents for the treatment of proliferative diseases; and the use of Curcuma extract or curcuminoids in combination with a radiation for the treatment of proliferative diseases on eukaryote cells.
Owner:ASAC COMPANIA DE BIOTECHA E INVESTIGACION
Ester derivatives
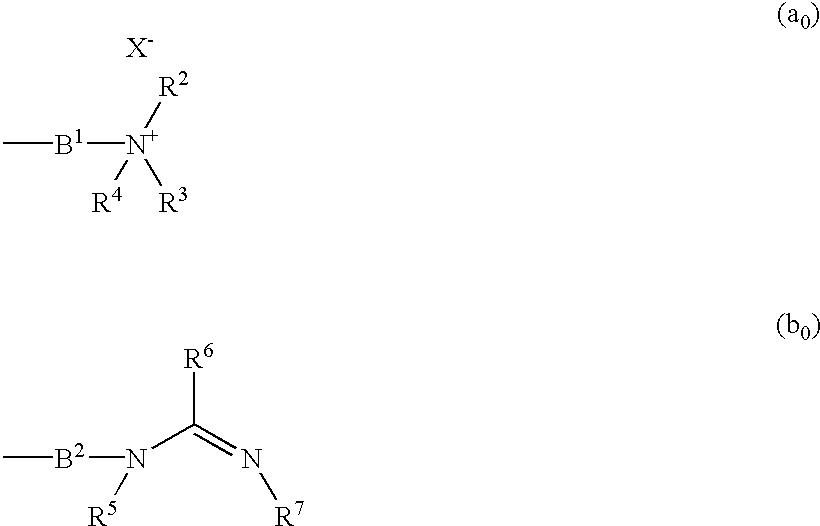
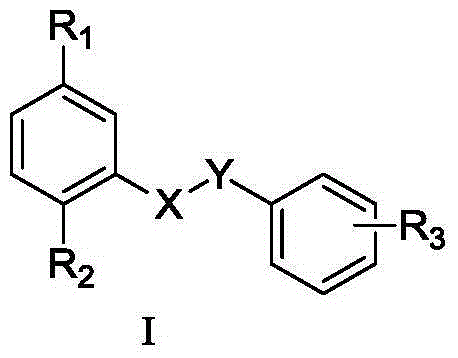
This invention relates to compounds which exhibit selective muscarinic M3 receptor antagonism, have little side effects, are suitable for inhalation therapy and are useful as treating agents of respiratory system diseases, of the general formula (I); [in which A signifies a group expressed by a formula (a0) or (b0); Ar signifies optionally substituted aryl or heteroaryl; B1 and B2 signify aliphatic hydrocarbon; R1 signifies fluorine-substituted cycloalkyl; R2, R3 and R4 signify lower alkyl, single bond or alkylene bonded to B1, or R2 and R3 are united to signify alkylene; R5 and R7 signify hydrogen, lower alkyl, or a single bond or alkylene bonded to B2; R6 signifies hydrogen, lower alkyl or a group expressed as —N(R8)R9; and X− signifies an anion].
Owner:MSD KK
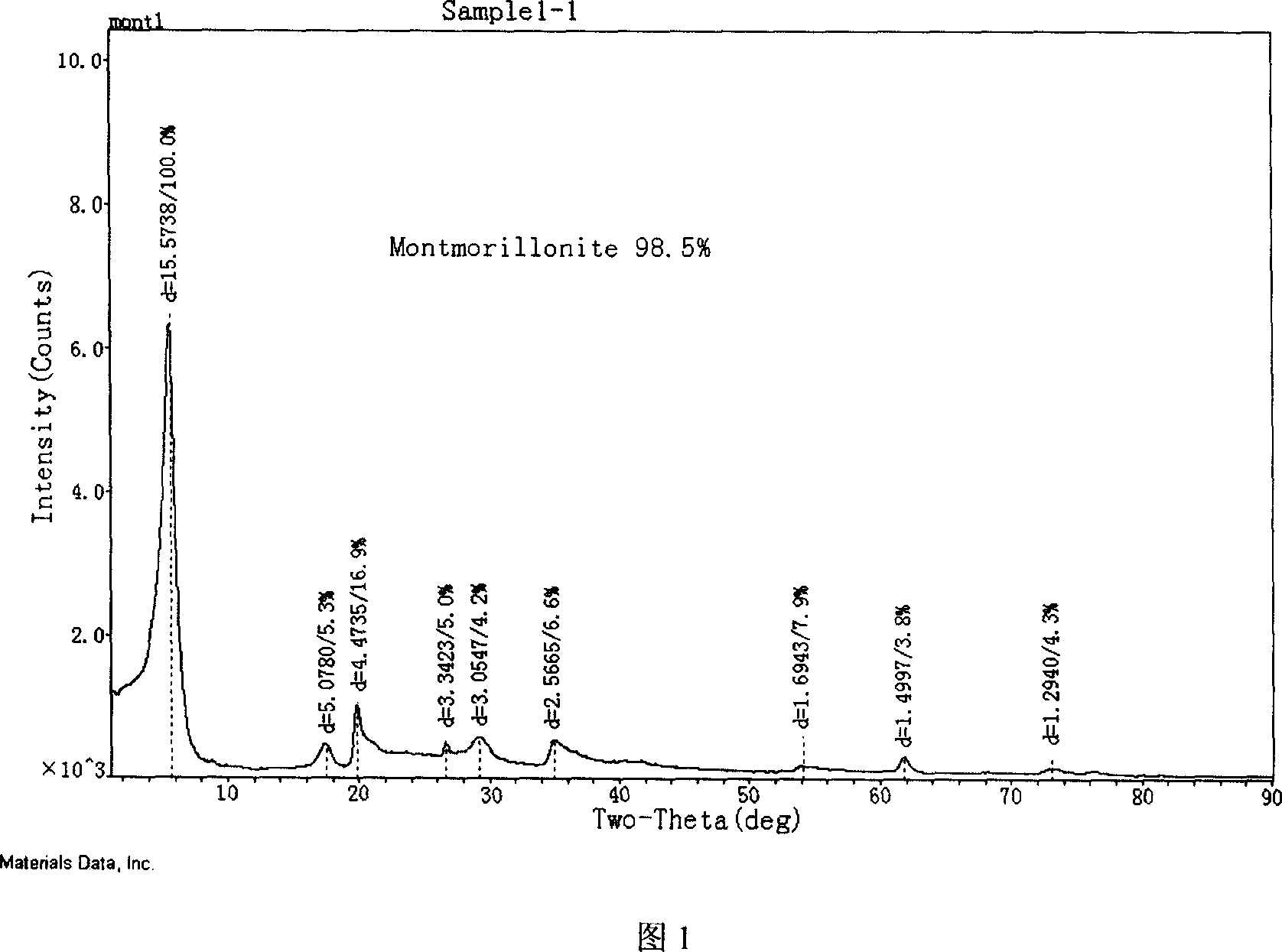
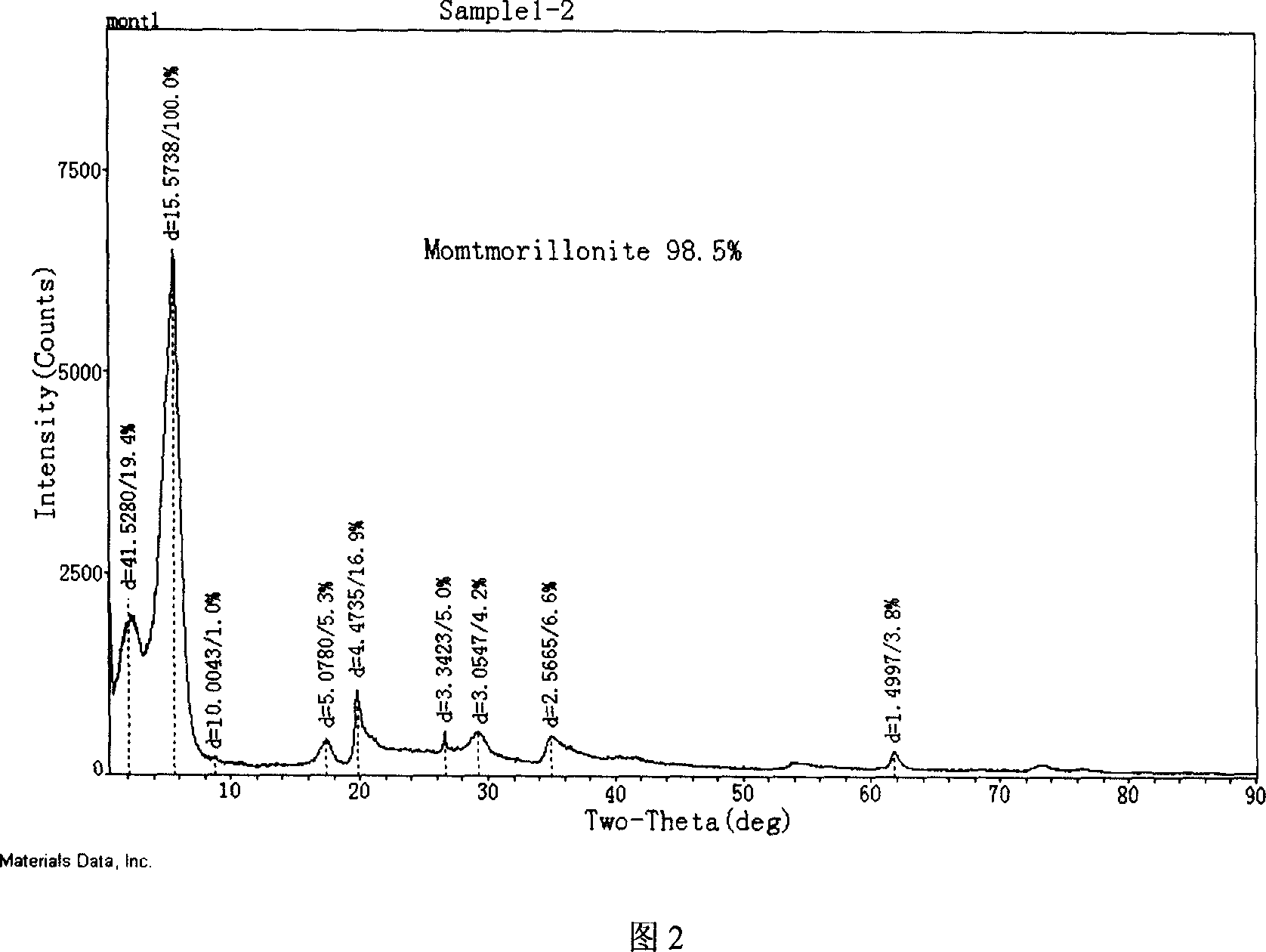
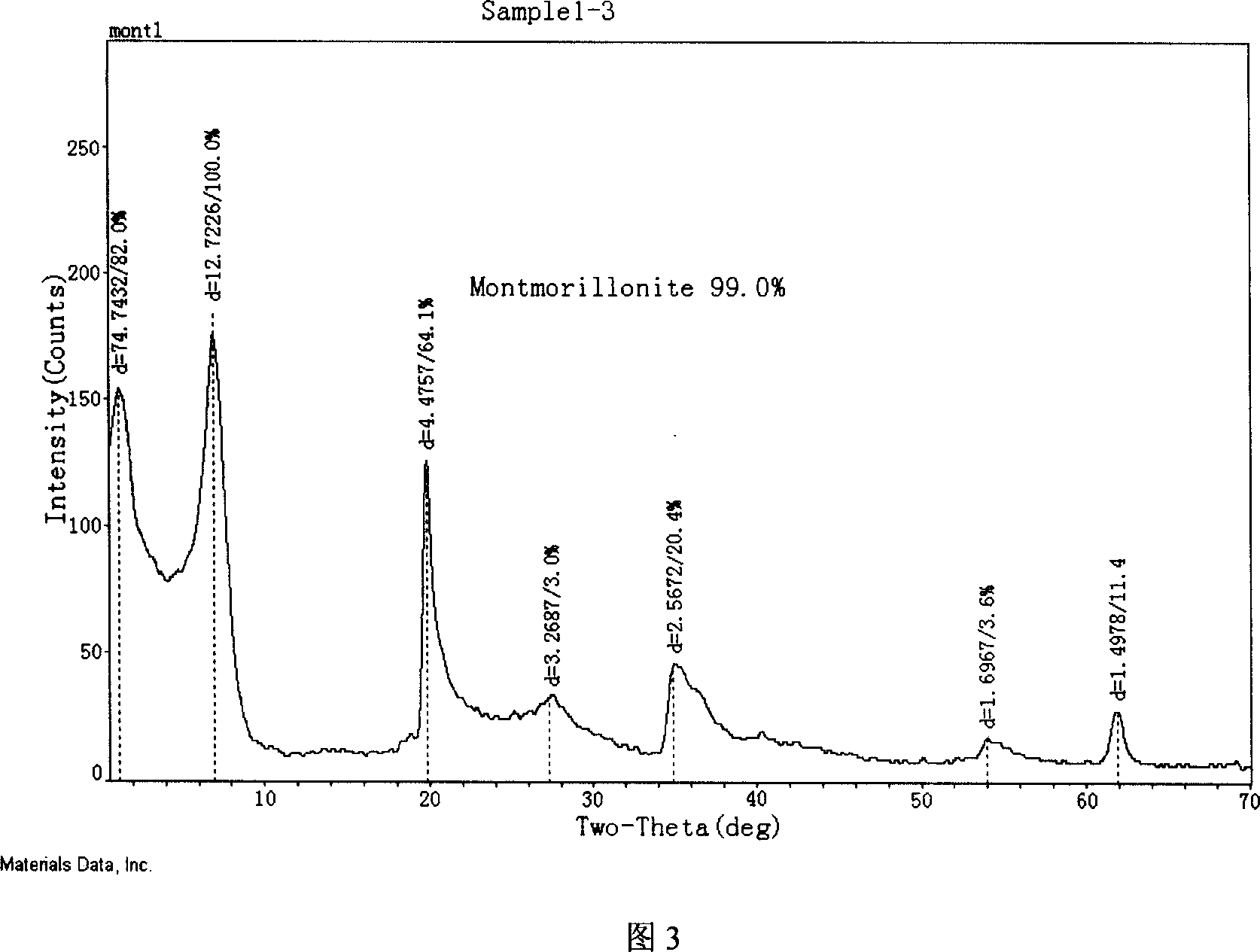
Modified montmorillonite, preparation method and application
InactiveCN1957948AIncrease exposureIncrease inhomogeneityNervous disorderAluminium silicatesChemistryAtrophic gastritis
A modified montmorillonite with exposed and increased electric charges on its end face and between layers, lower ordered structure in C axis and improved curative effect for the reversible therapy of chronic atrophic gastritis is disclosed. Its preparing process is also disclosed.
Owner:ZHEJIANG HAILISHENG PHARM CO LTD
An arctium fruit extract, its preparation method and application
InactiveCN1864705AImprove pharmacological activitySmall side effectsMetabolism disorderUrinary disorderPL ExtractAdditive ingredient
The invention relates to a burdock fruit extract, its preparing process and use thereof, wherein the extract comprises 51-80 wt% of total lignanoids compound with burdock aglycone as the main ingredient and burdock glycosides, and 20-49 wt% of other constituents.
Owner:SHANGHAI NUOREN BIOMEDICAL TECH
Apocynum extract and extracting method thereof
InactiveCN1634325ARetain pharmacological activityImprove use valueNervous disorderMetabolism disorderHyperosideHydrolysis
The invention relates toa an Apocynum extract and extracting method, which consists of, (1) determining the extract with hyperin as the representing composition, the content of flavones in the extract is 35-90%, (2) after the complete hydrolysis of the extract, the content of meletin compound is 30-80%, (3) the content of hyperin in the extract is 15-55%.
Owner:李青山
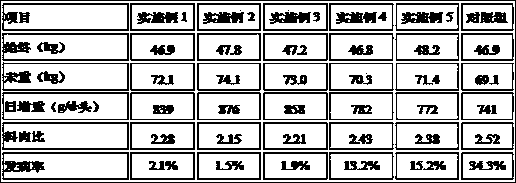
Safe and efficient biological feed additive for pigs
ActiveCN103549217AImprove pharmacological activityPromote healthy growthAnimal feeding stuffBiotechnologyOfficinalis
The invention provides a safe and efficient biological feed additive for pigs and belongs to the field of feed additives. The additive is composed of fermented Chinese herbal medicine powder, an aloe polysaccharide, a complex enzyme preparation, an acanthopanax senticosus extract, radix polygonati officinalis powder, medlar powder, pericarpium citri reticulatae and a carrier, wherein the carrier is composed of degreased rice bran, peanut shell powder and bentonite. According to the additive, the safe and efficient biological feed additive is good for the utilization rate of the feed by live pigs, the utilization value of the feed is improved and the immunity of the live pigs can also be enhanced; the safe and efficient biological feed additive for the pigs has very important meanings on the utilization of antibiotics and the like and has a very good market application prospect.
Owner:荆门双胞胎饲料有限公司
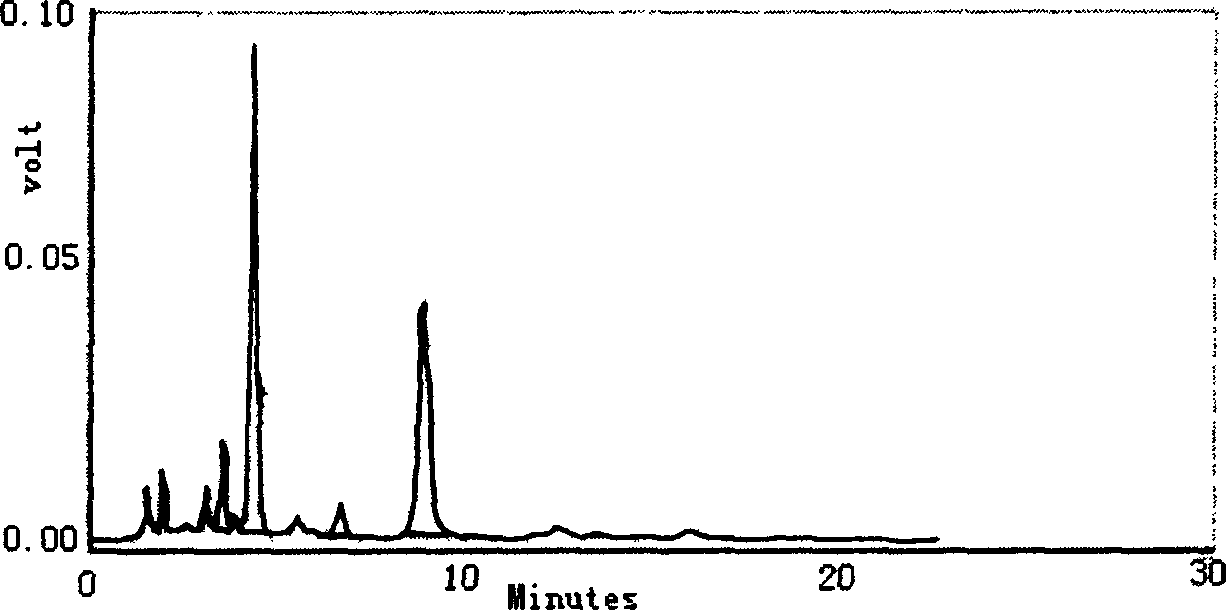
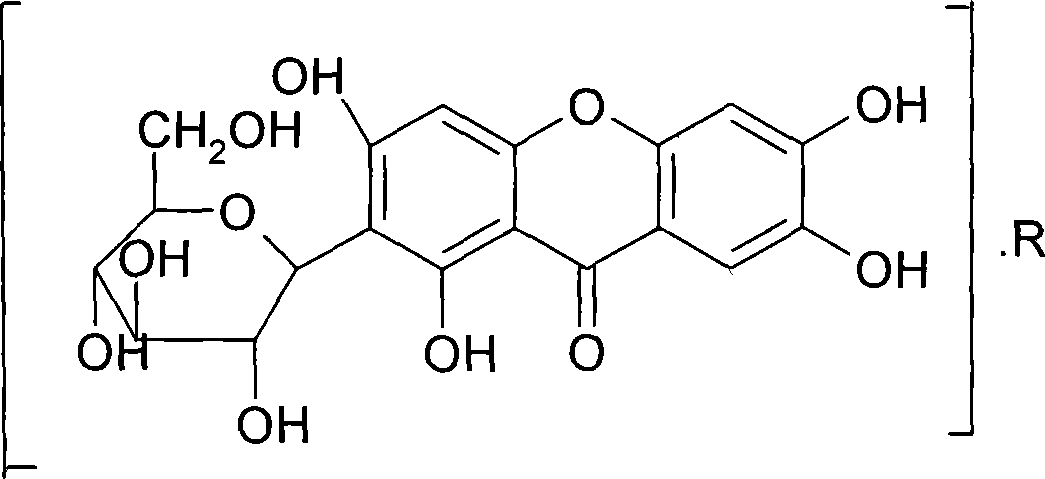
Mangiferin salt and method of preparing the same and use thereof
ActiveCN101108869ASolve solubilityImprove pharmacological activityOrganic active ingredientsSugar derivativesDiseaseSolubility
The invention provides a mangiferin salt, in particular to a salt formed in the basic amino acid, organic amine and inorganic alkaline matter capable of being accepted by the mangiferin and medicine. The salt can solve the dissolubility, improve the pharmacological activity and be used for curing or curing auxiliary the diseases related closely to the oxidative damage. The general formula of the structure refers to the drawing, wherein, R is the basic amino acid, organic amine or inorganic alkaline metallic ion.
Owner:CHANGZHOU DEZE MEDICAL SCI CO LTD
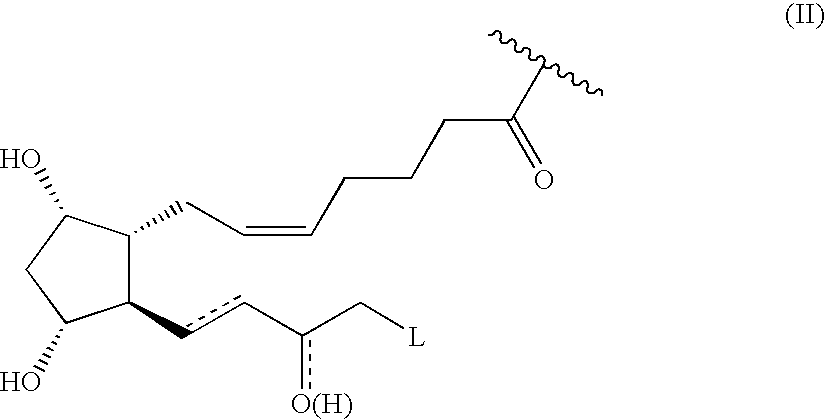
Prostaglandin derivatives
ActiveUS7273946B2Enhance the imageEliminate orBiocideSenses disorderIntra ocular pressureTolerability
Prostaglandin nitroderivatives having improved pharmacological activity and enhanced tolerability are described. They can be employed for the treatment of glaucoma and ocular hypertension.
Owner:NICOX SA
Amide compounds, preparation method and medical use thereof
ActiveCN105175284AExcellent logPExcellent membrane permeabilityCarboxylic acid nitrile preparationOrganic compound preparationPharmaceutical drugCombinatorial chemistry
The invention relates to the field of pharmaceutical chemistry, particularly a group of amide compounds (I) and a preparation method therefor and use thereof. Pharmacodynamic experiments prove that the compounds of the invention can be used for preparing medicines for treating leukemia. The formula (I) is shown in the description.
Owner:CHINA PHARM UNIV
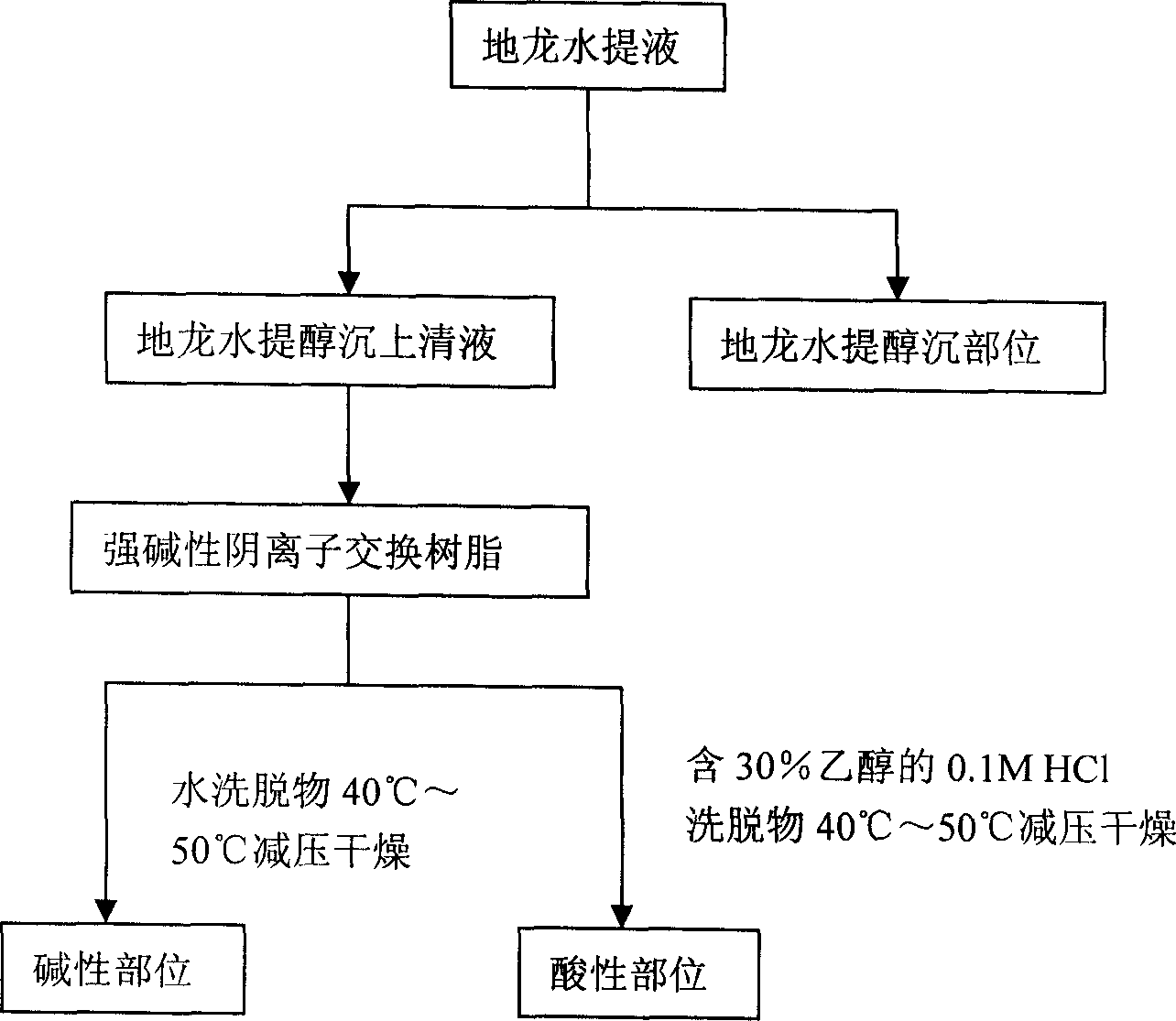
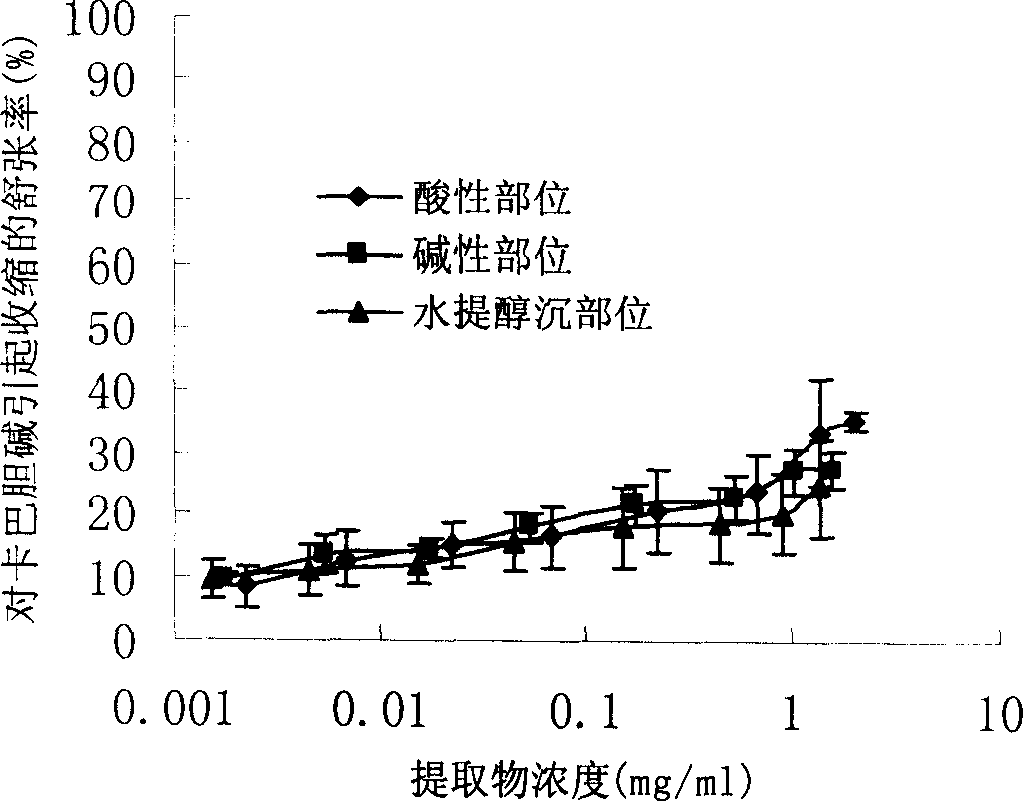
Earthworm acidic-part medicine for treating cough asthma disease and preparing method
InactiveCN1850118AEasy to acceptIncreased content of anti-asthma active ingredientsRespiratory disorderLeech/worm material medical ingredientsDiseasePurine
The present invention relates to an earthworm acid region medicine for curing cough and asthma diseases and its preparation method. Said earthworm acid region medicine contains fatty acid, amino acid and purine compound, and can be used for curing the diseases of asthma and chronic bronchitis, etc. Its preparation method includes the following steps: using strong base anion-exchange resin to adsorb aqueous extract of earthworm, then using dilute hydrochloric acid or alcohol-containing dilute hydrochloric acid to make elution.
Owner:SHANGHAI JIAO TONG UNIV
Mutant polypeptide having effector function
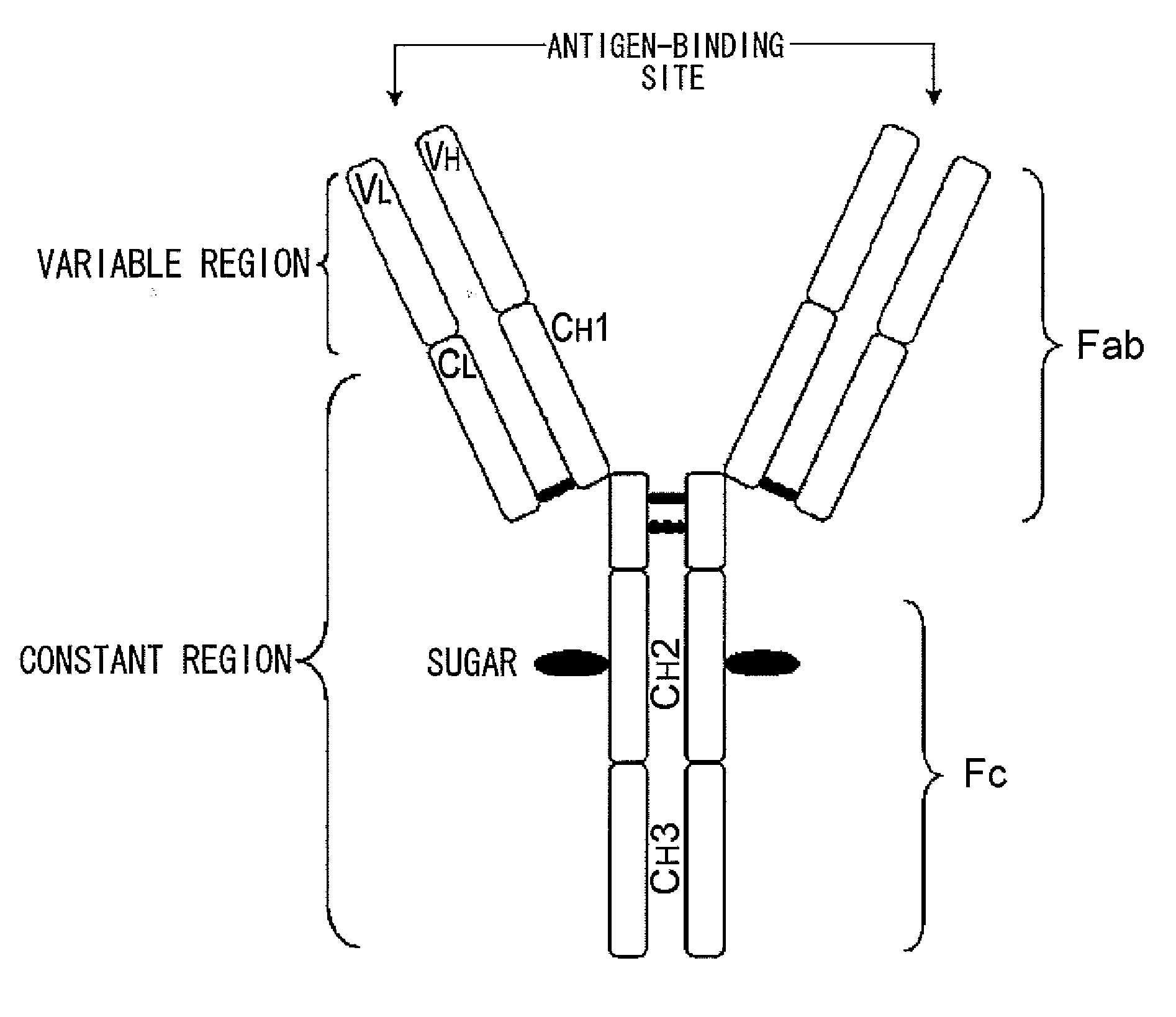
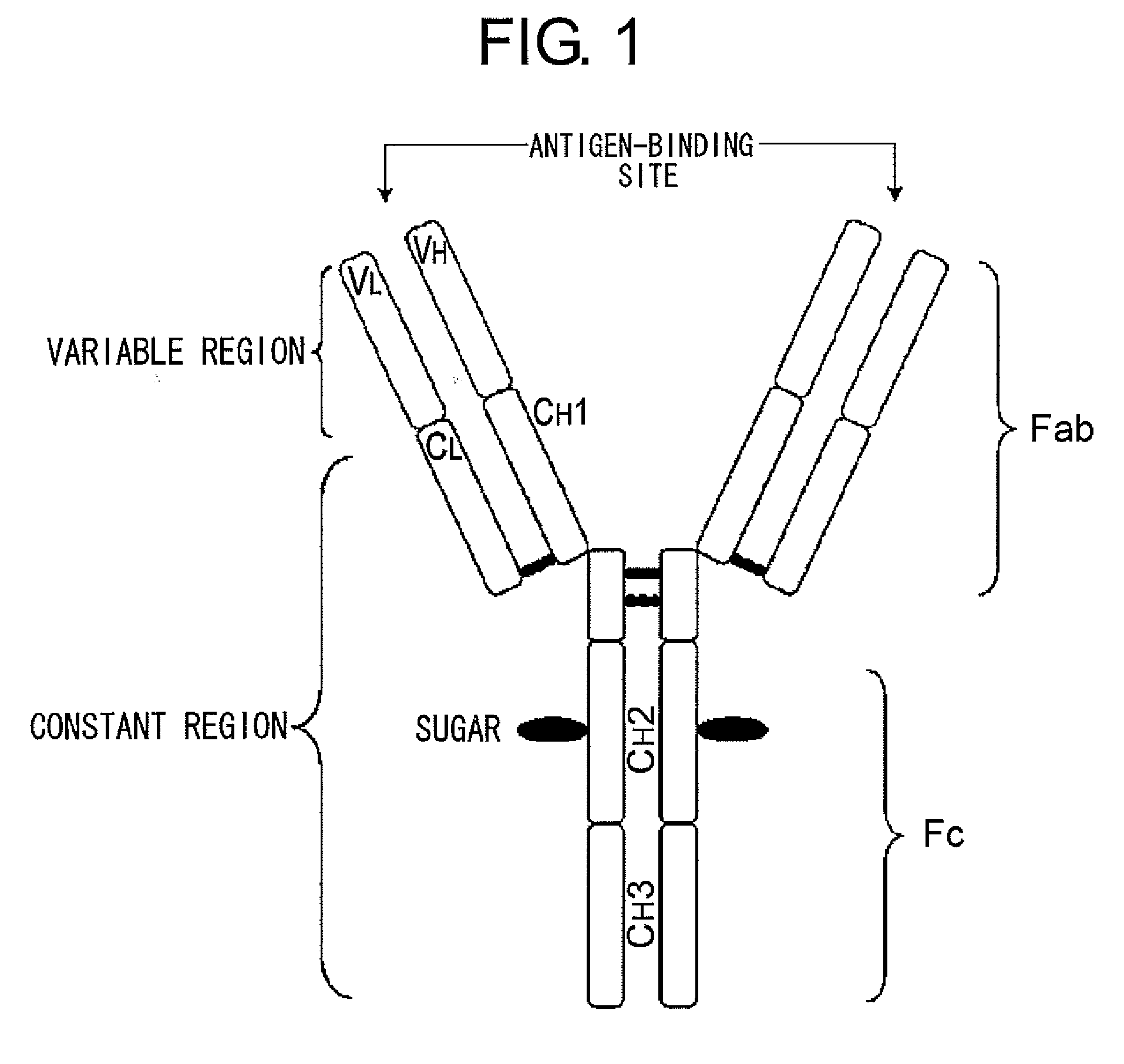
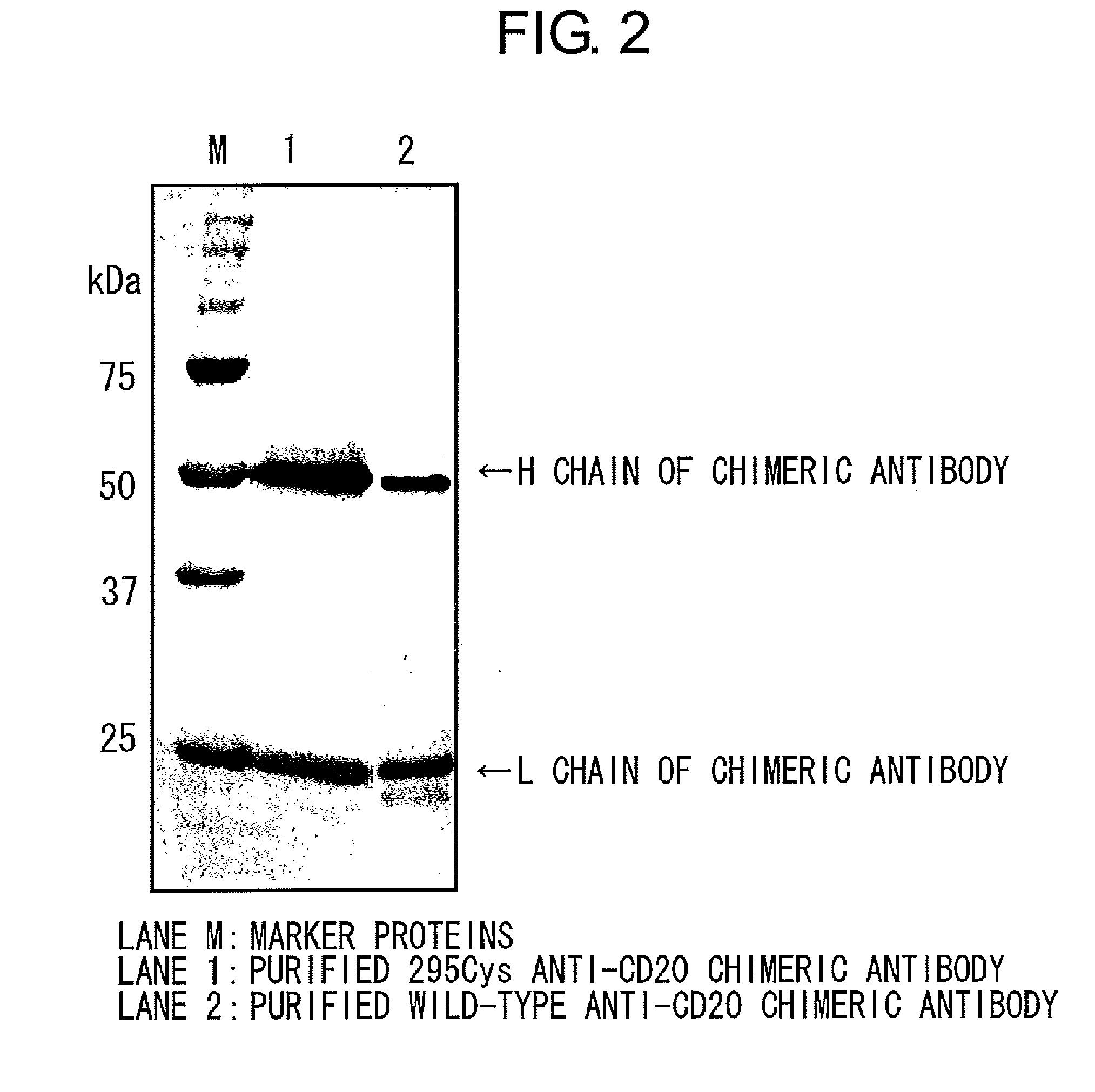
InactiveUS20100080794A1Enhanced effector functionLess side effectsAntipyreticAnalgesicsHuman cellSugar
Mutant polypeptides of the present invention contain an Fc region in which a cysteine residue is substituted for the second amino acid from the glycosylation site to the N terminal side in the Fc region. The Fc region is preferably a human IgG Fc region. The mutant polypeptides of the present invention may also contain an N-linked sugar chain at the glycosylation site in Fc region. Furthermore, a polypeptide domain other than the Fc region of the mutant polypeptides of the present invention may be a polypeptide molecule that recognizes a human cell surface molecule.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD +1
Green pig feed additive capable of substituting antibiotic
ActiveCN103750072AImprove growth performanceImprove immunityFood processingAnimal feeding stuffRHODIOLA ROSEA ROOTFeed additive
The invention discloses a green pig feed additive capable of substituting antibiotic, and belongs to the field of feed additives. The additive comprises fermented Chinese herbal medicine powder, a rhodiola rosea extract, folium artemisiae argyi powder, radix glehniae powder, folium cortex eucommiae powder, a honeysuckle stem, centella asiatica powder, platycodon grandiflorum powder and dandelion powder. The additive has the characteristics of no toxicity, no residue and no tolerance, can effectively improve the immunity of a pig, can improve the disease resistance of the pig, can reduce the incidence of the pig, can improve the growth property of the pig, can increase a breeding benefit, can substitute the antibiotic in a daily ration of the pug, and is broad in application prospect.
Owner:宜昌双胞胎饲料有限公司
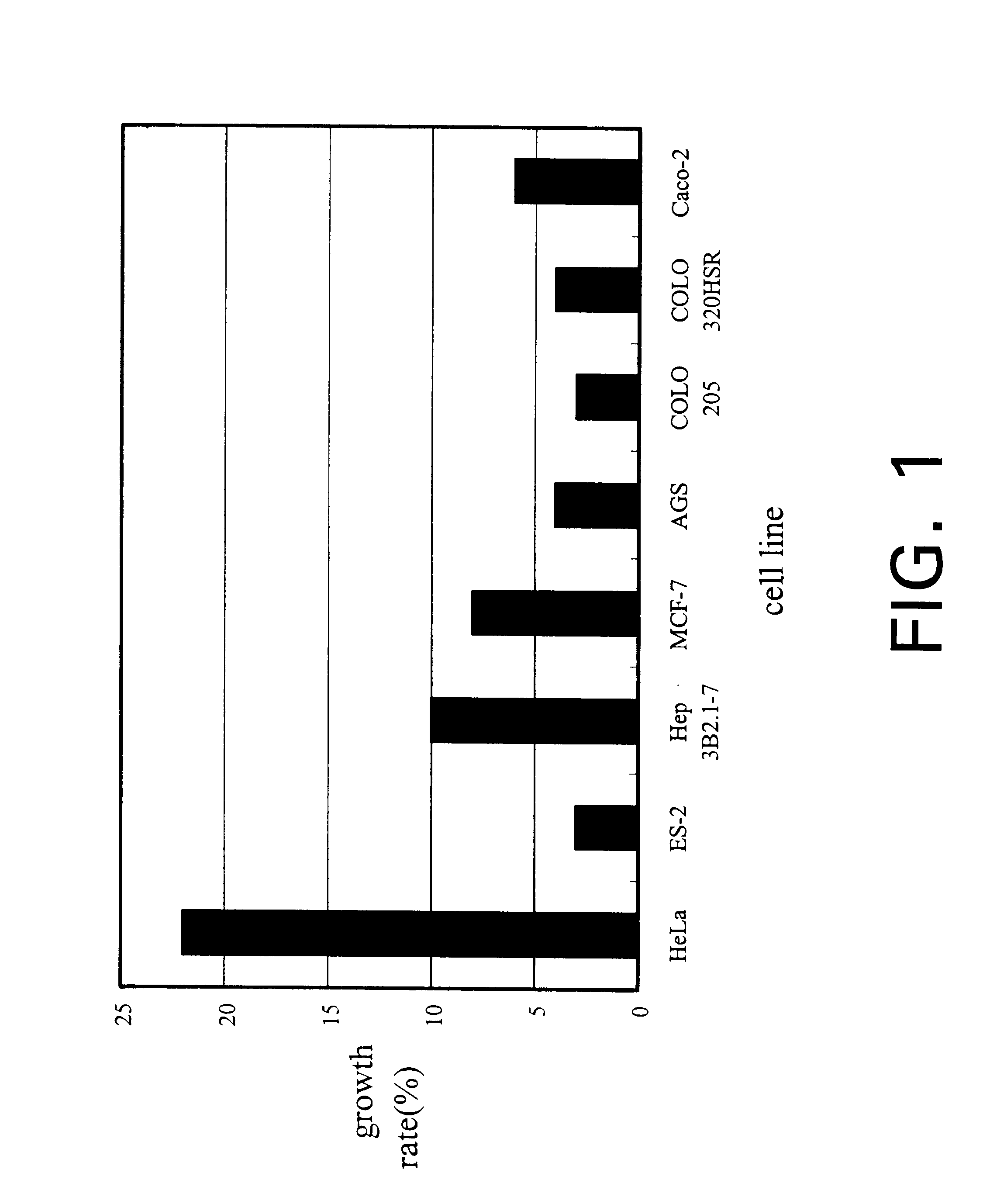
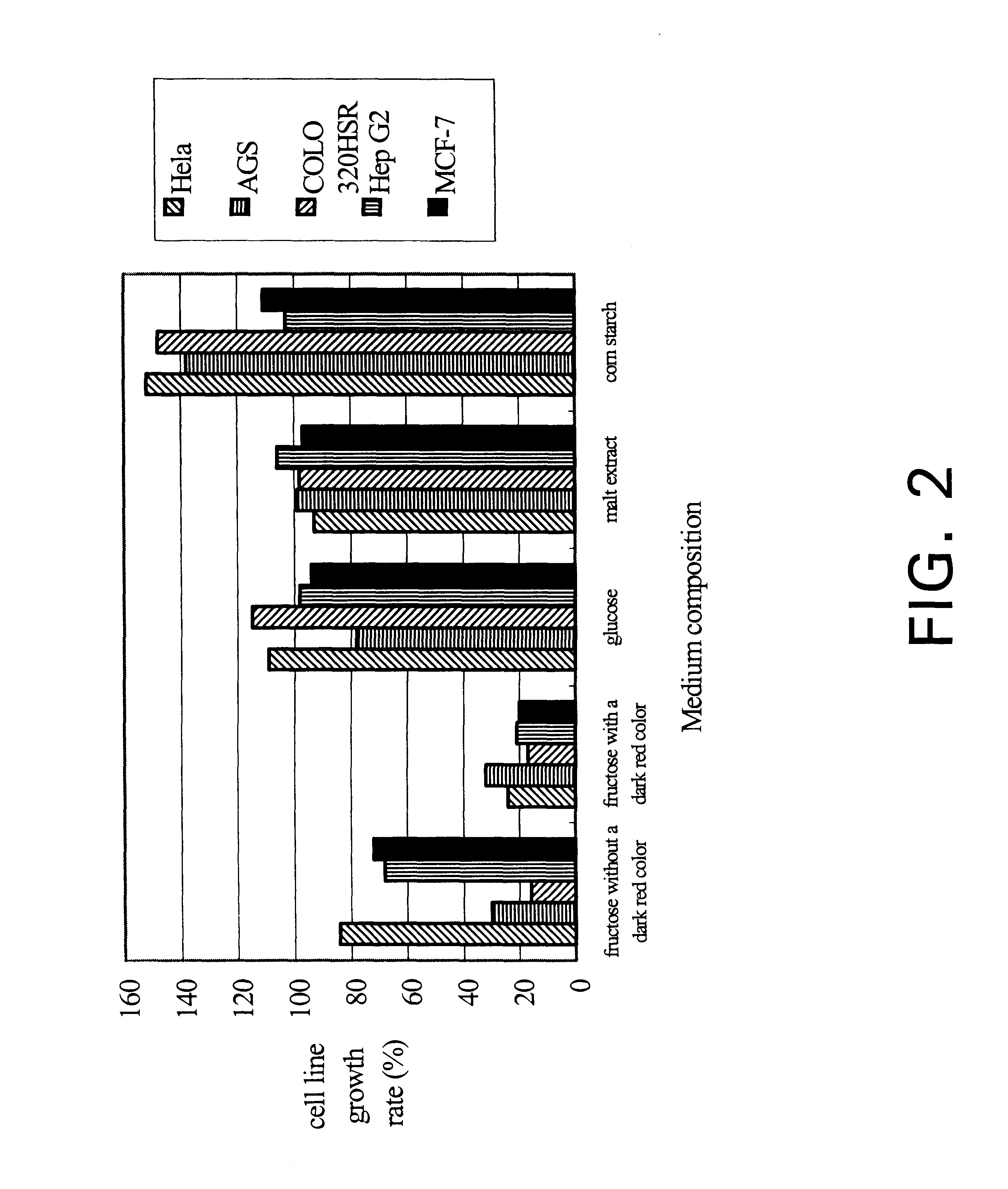
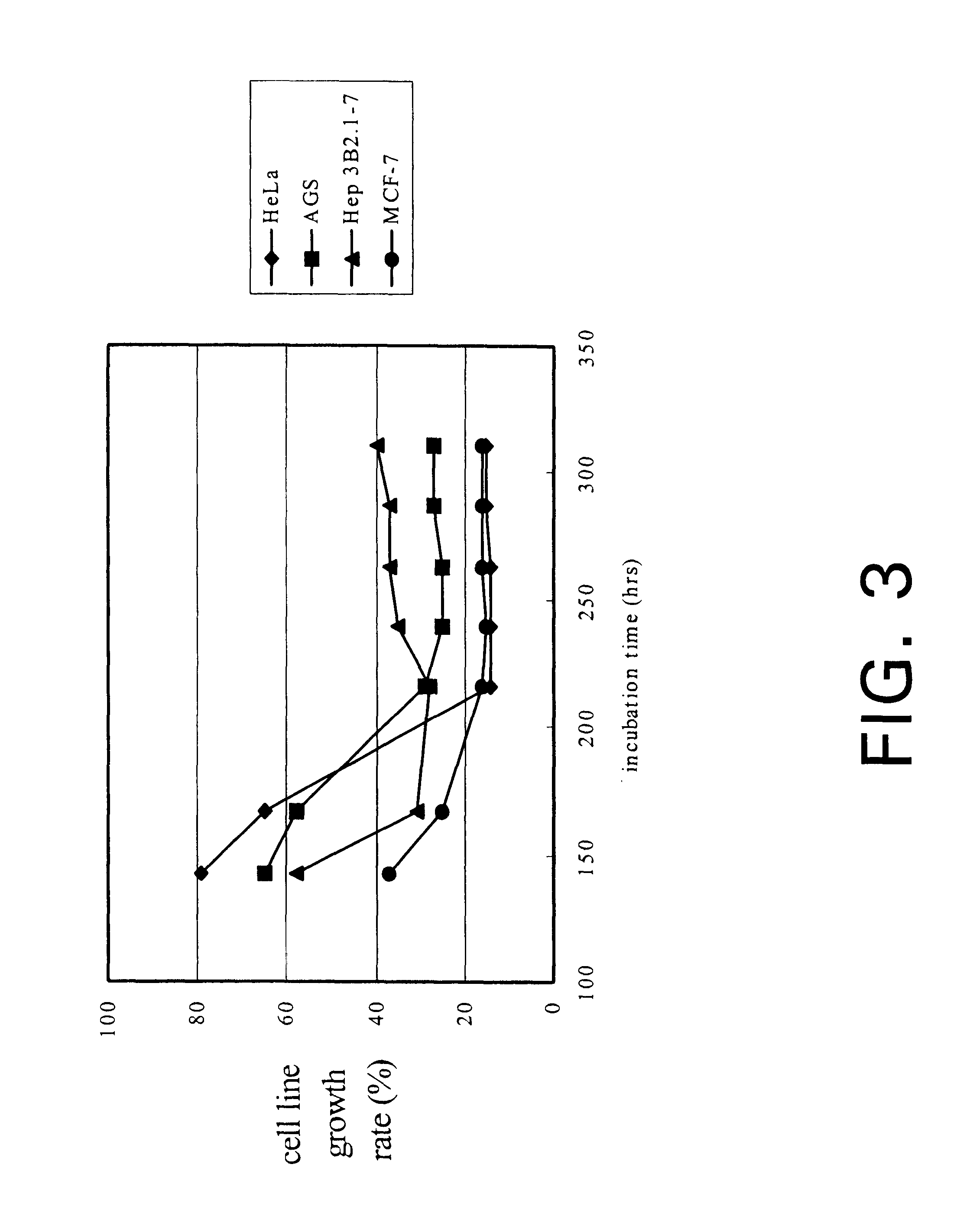
Isolate of Antrodia camphorata process for producing a culture of the same and product obtained thereby
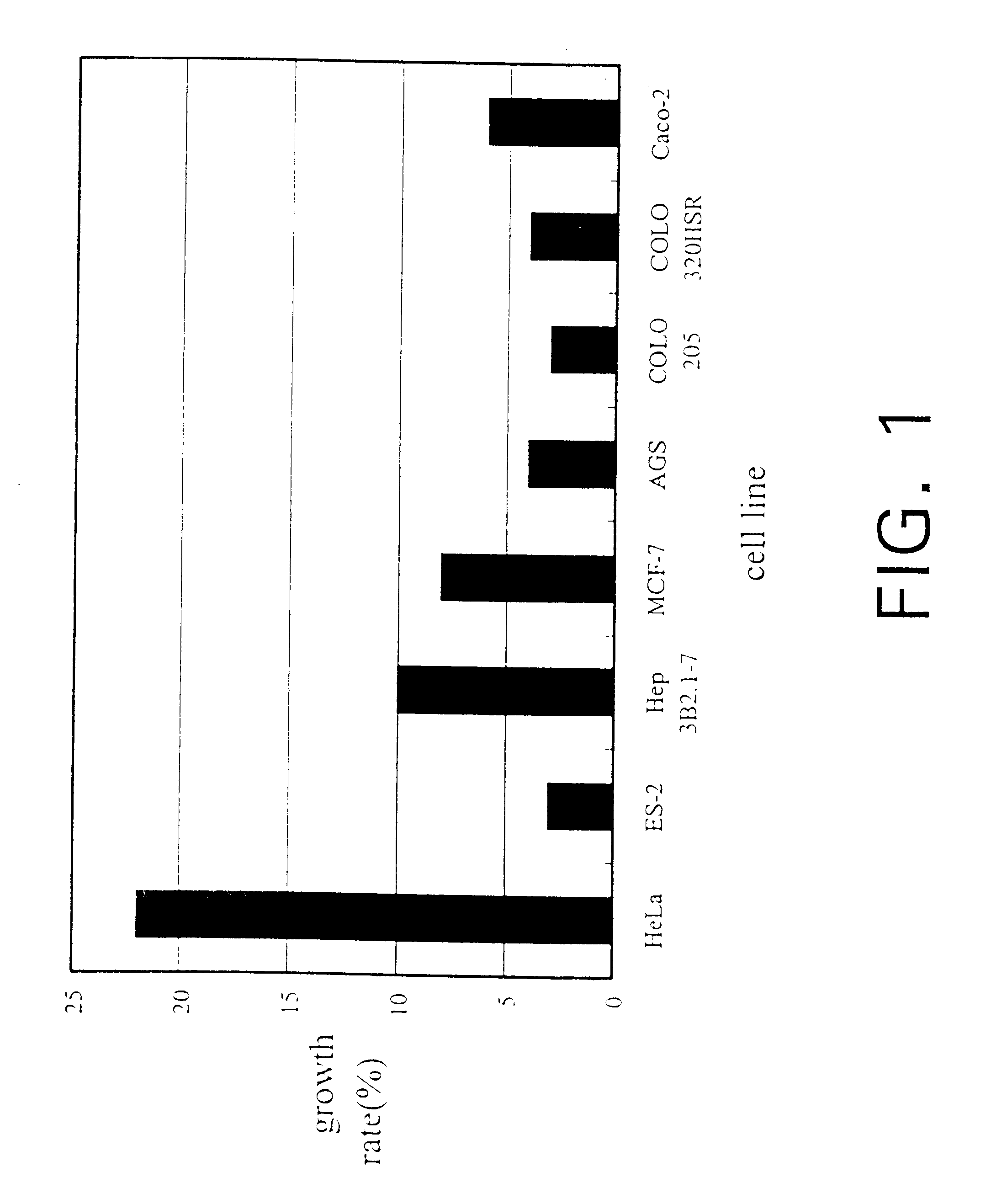
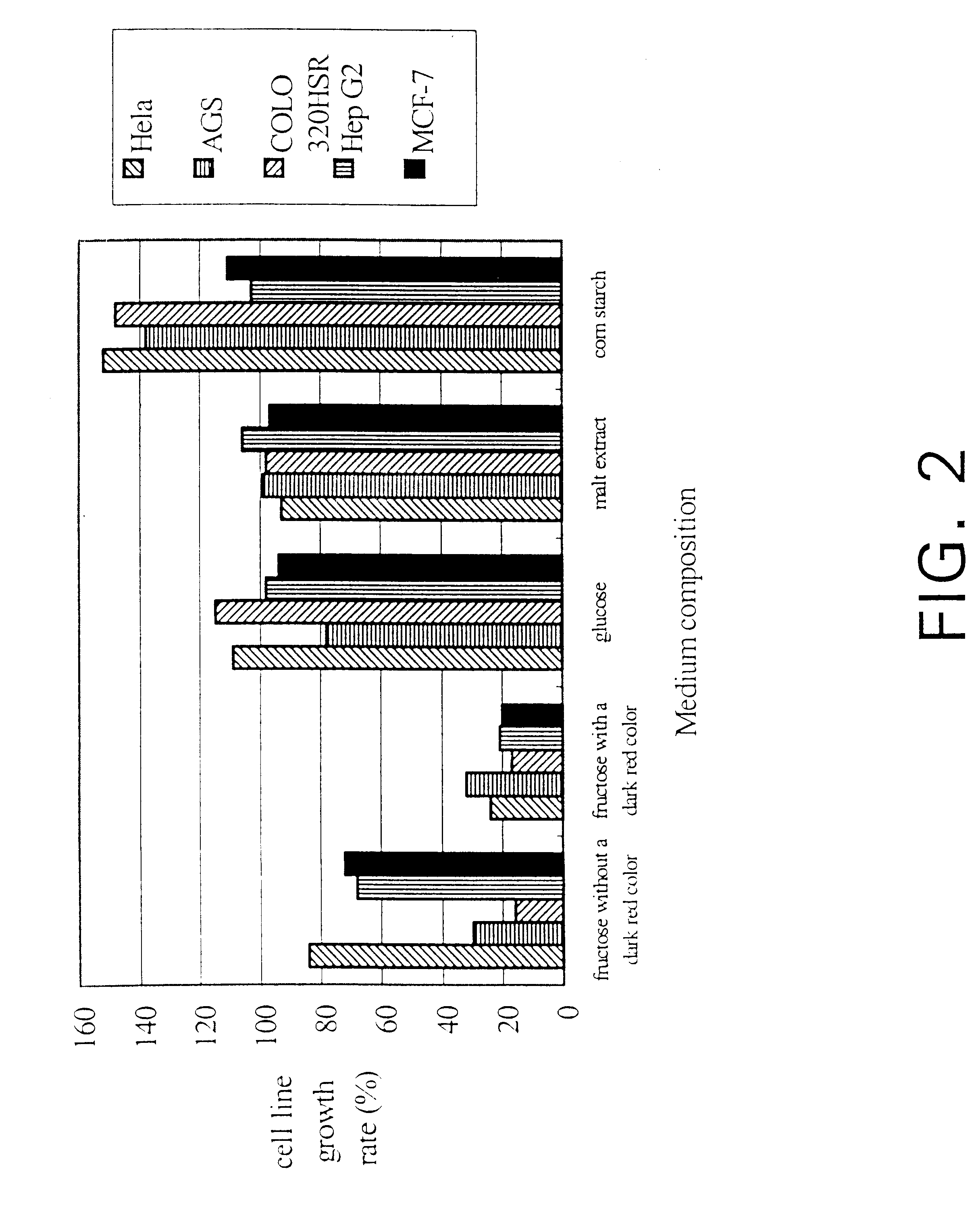
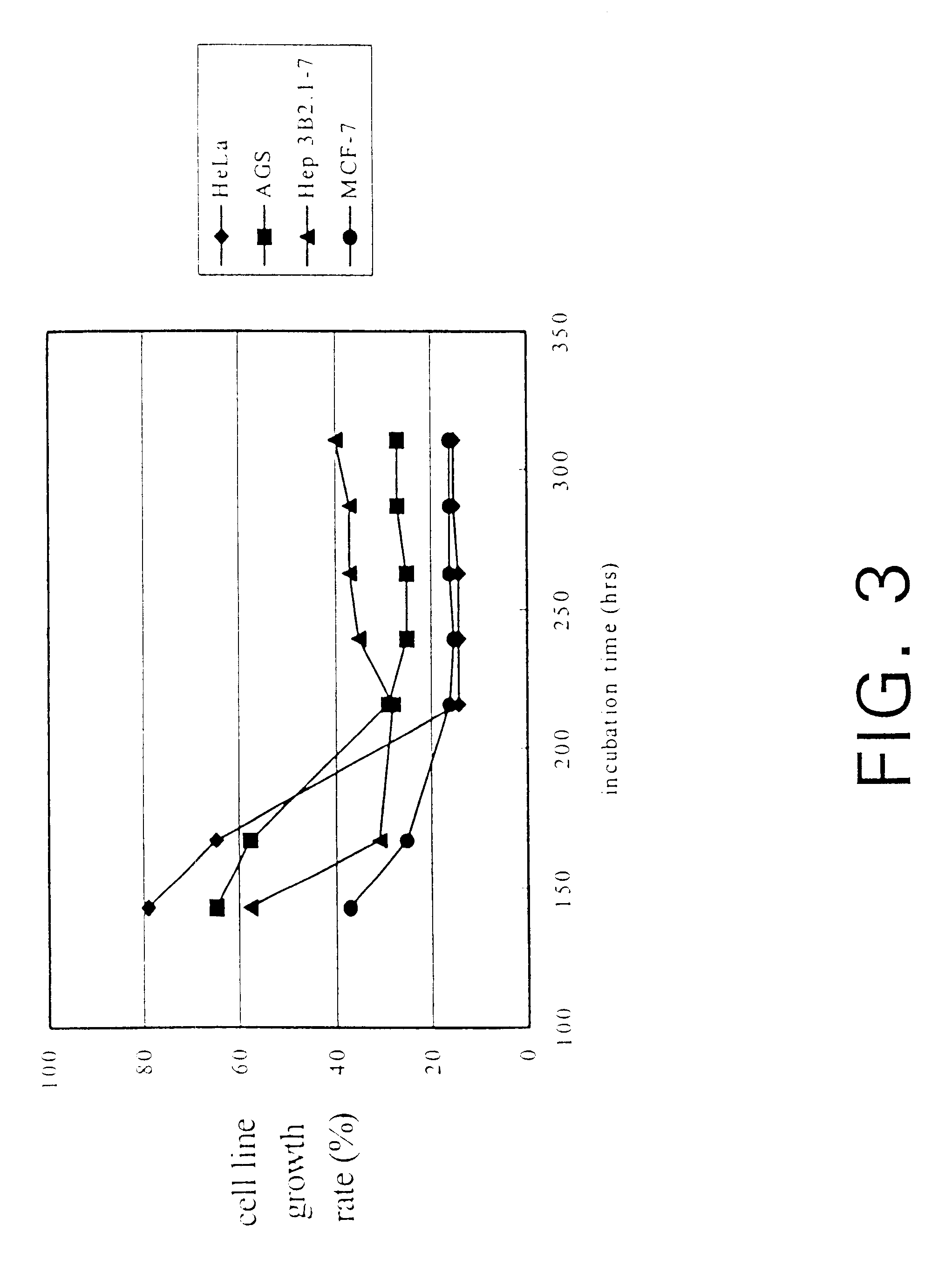
InactiveUS6391615B1Improve pharmacological activityPromote growthFungiEnzymologyBiotechnologyFructose
The present invention relates a process for culturing isolates of Antrodia camphorata to provide a product useful in medical and nourishment fields. The present invention also relates to a novel isolate of Antrodia camphorata capable of growing in a suitable artificial medium, while exhibiting desired pharmacological activities, in particular anti-tumor activity. The utilization of potato dextrose broth and the synthetic medium containing fructose as major carbon source leads to a significant increase in the pharmacological activity of the cultures of A. camphorata.
Owner:COUNCIL OF AGRICULTURE
Isolate of antrodia camphorata, process for producing a culture of the same and product obtained thereby
InactiveUS6355475B1Improve pharmacological activityPromote growthFungiEnzymologyBiotechnologyFructose
The present invention relates a process for culturing isolates of Antrodia camphorata to provide a product useful in medical and nourishment fields. The present invention also relates to a novel isolate of Antrodia camphorata capable of growing in a suitable artificial medium, while exhibiting desired pharmacological activities, in particular anti-tumor activity. The utilization of potato dextrose broth and the synthetic medium containing fructose as major carbon source leads to a significant increase in the pharmacological activity of the cultures of A. camphorata.
Owner:COUNCIL OF AGRICULTURE
Levo-isovaleryl spiramycin I and preparation, preparation method and application thereof
ActiveCN102229634AImprove antibacterial propertiesImprove pharmacological activityAntibacterial agentsOrganic active ingredientsDiseaseFreeze-drying
The invention relates to levo-isovaleryl spiramycin I and a preparation, a preparation method and application thereof. The preparation consists of the levo-isovaleryl spiramycin I and pharmaceutically acceptable carriers and / or excipient, wherein the purity of the levo-isovaleryl spiramycin I is over 90 weight percent, preferably over 95 weight percent and further preferably over 98 weight percent. The levo-isovaleryl spiramycin I has good antimicrobial activity; the preparation of the levo-isovaleryl spiramycin I comprises water injection, powder injection or freeze dried powder injection; the preparation fills the blank of the single component preparation of the isovaleryl spiramycin I at present market, and provides a new path with quick response for treating anti-infective diseases; and the production process for the preparation of single component of the isovaleryl spiramycin I is stable, standard and easily controlled in quality and suitable for large-scale industrialized production.
Owner:SHENYANG TONGLIAN GRP CO LTD
No-donating corticosteroid with improved pharmacokinetic, anti-inflammatory and vasodilatory properties
InactiveUS20090088411A1Improve pharmacological activityEliminate side effectsOrganic active ingredientsRespiratory disorderDiseaseMedicine
There are herein provided methods of treatment and nitric oxide donating compositions of matter for the treatment of respiratory diseases and associated conditions.
Owner:TOPIGEN PHARMA
Preparation method of chrysin amino acid derivative
InactiveCN106632193AThe killing effect is remarkableHigh anticancer activityOrganic active ingredientsOrganic chemistrySolubilityLife activity
Owner:NANHUA UNIV
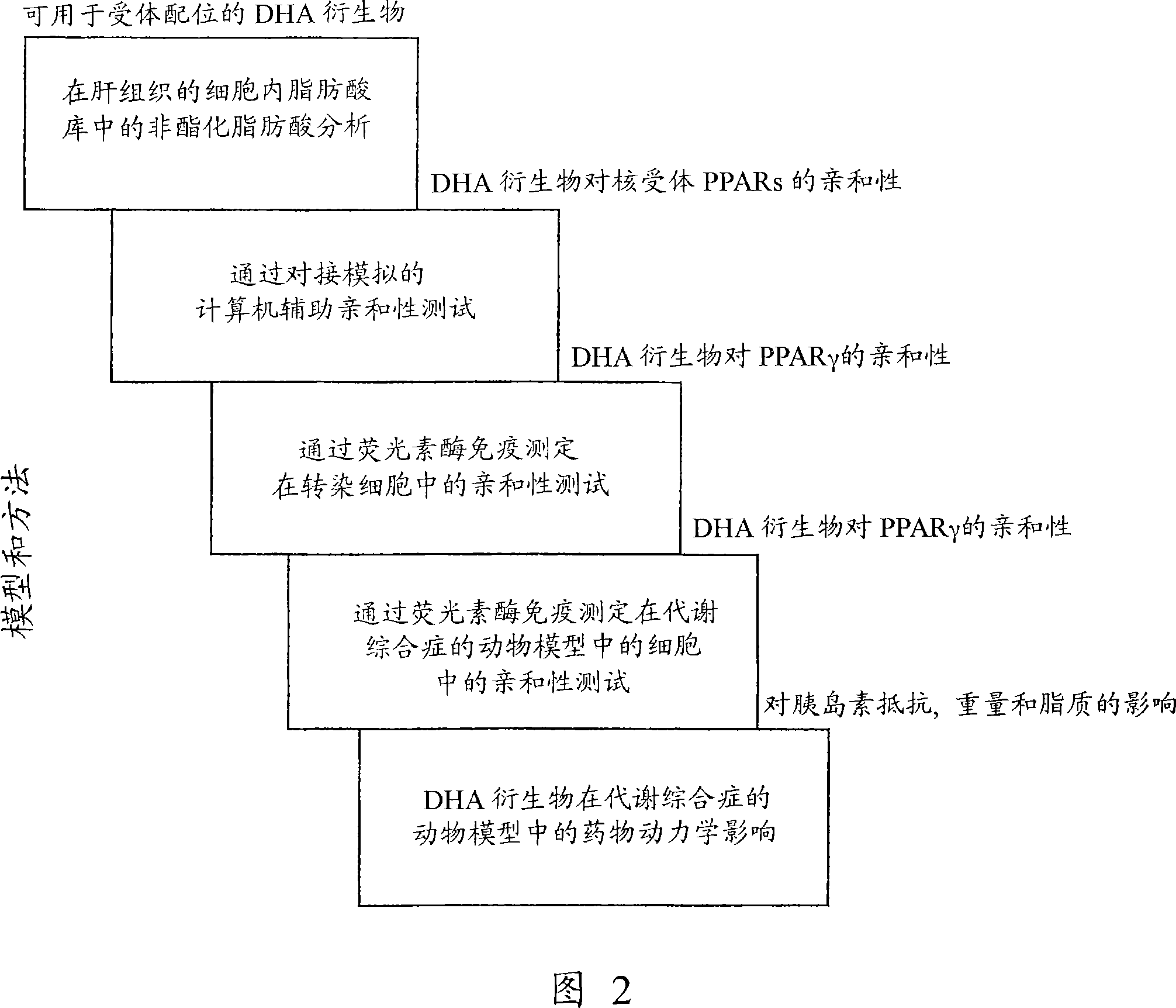
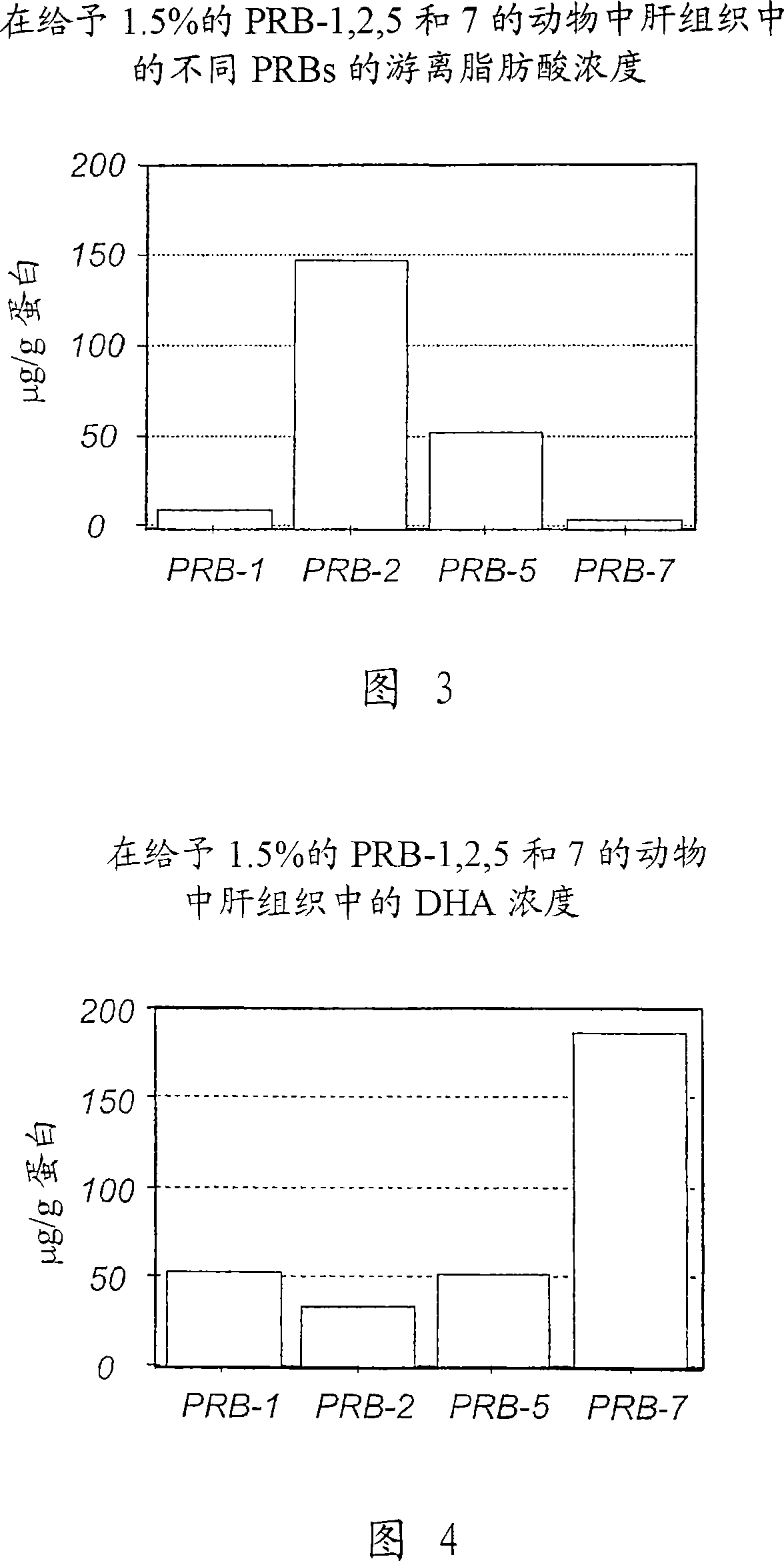
New DHA derivatives and their use as medicaments
InactiveCN101213281AImprove pharmacological activityPrevent obesityOrganic active ingredientsOrganic chemistryDocosahexaenoic acidCarboxylic salt
Compounds of formula (I); wherein - R1 and R2 are the same or different and may be selected from the group consisting of a hydrogen atom, a hydroxy group, an alkyl group, a halogen atom, an alkoxy group, an acyloxy group, an acyl group, an alkenyl group, an alkynyl group, an aryl group, an alkylthio group, an alkoxycarbonyl group, an alkylsulfmyl group, an alkylsulfonyl group, an amino group, and an alkylamino group; and - X represents a carboxylic acid group, a carboxylate group, or a carboxamide group; or any pharmaceutically acceptable salt, solvate, complex or pro-drug thereof, with the provisos that the compound of formula (I) is not (all-Z)-4,7,10,13,16,19- docosahexaenoic acid (DHA), alpha-methyl DHA, alpha-methyl DHA methyl ester, alpha-methyl DHA ethyl ester or alpha-hydroxy DHA ethyl ester, are disclosed. A fatty acid composition and a pharmaceutical composition comprising such compounds are also disclosed. The use of such compounds as medicaments, in particular for the treatment of diabetes type 2, is also disclosed.
Owner:PRONOVA BIOPHARMA NORGE
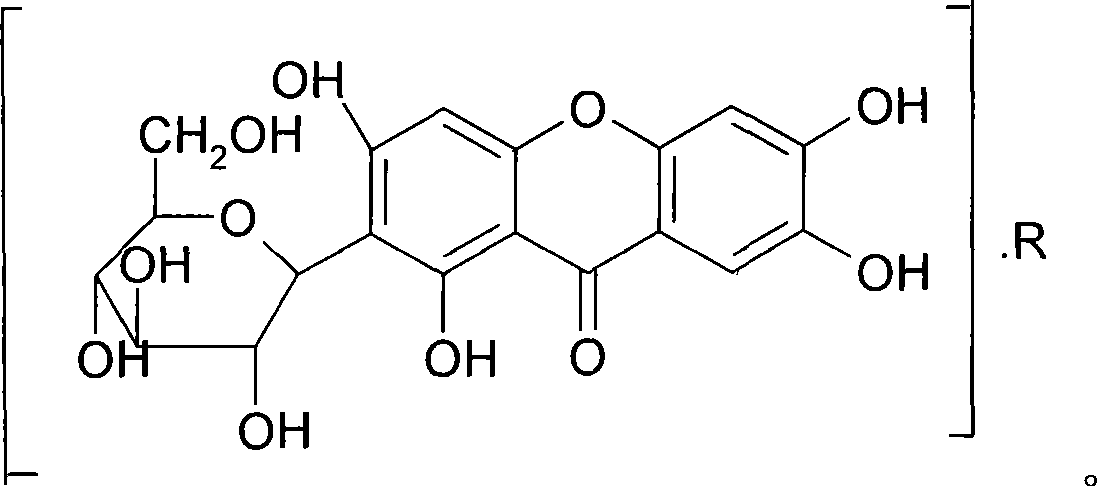
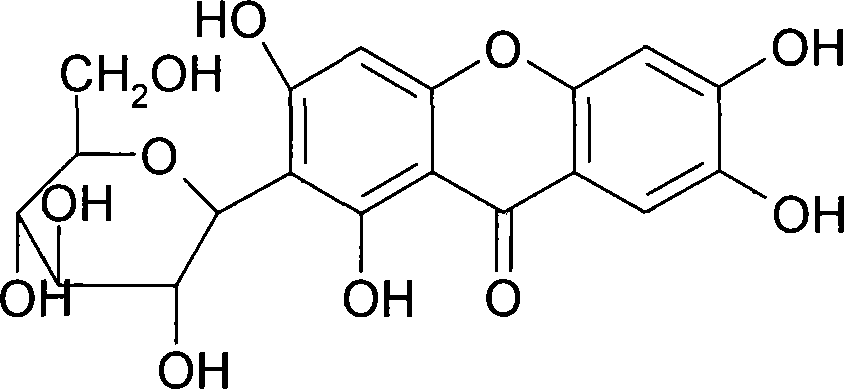
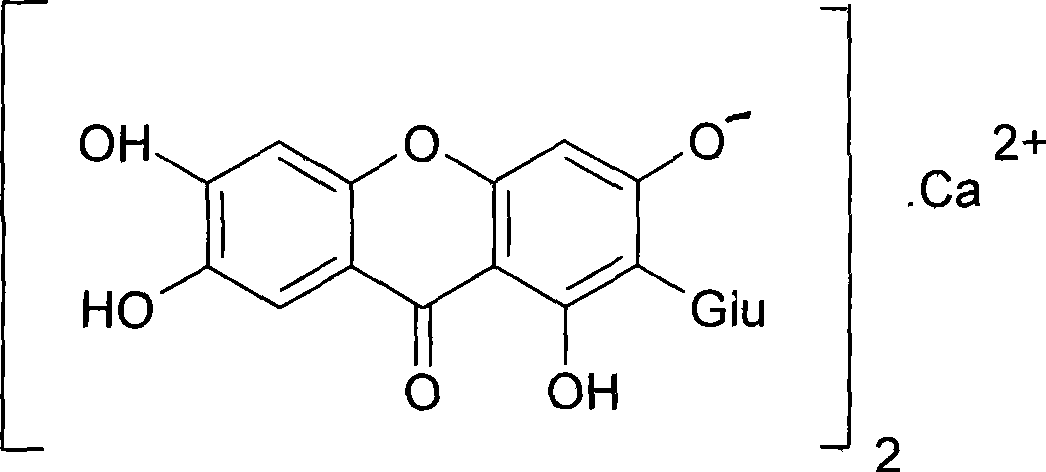
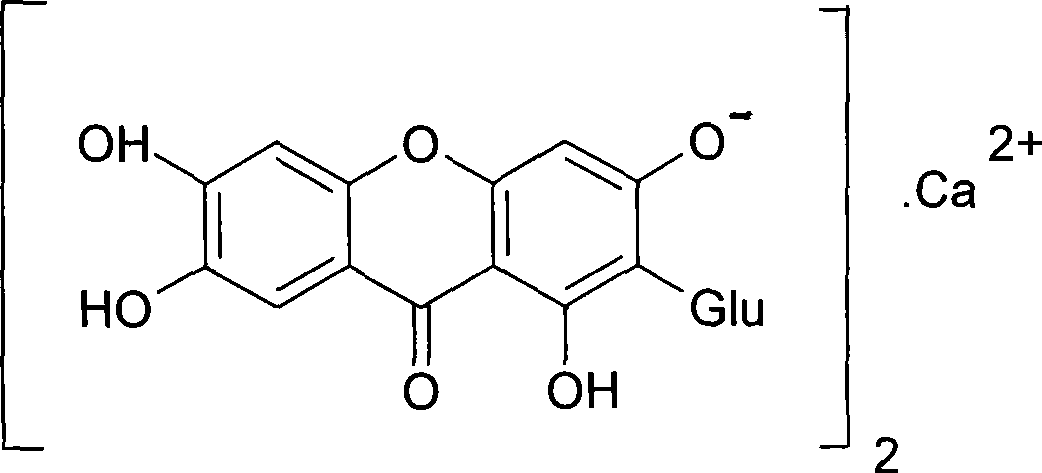
Use of mangiferin calcium salt as peroxisome proliferator-activated receptor agonist
InactiveCN101461819AImprove pharmacological activityOrganic active ingredientsDrug compositionsAgonistMangiferin
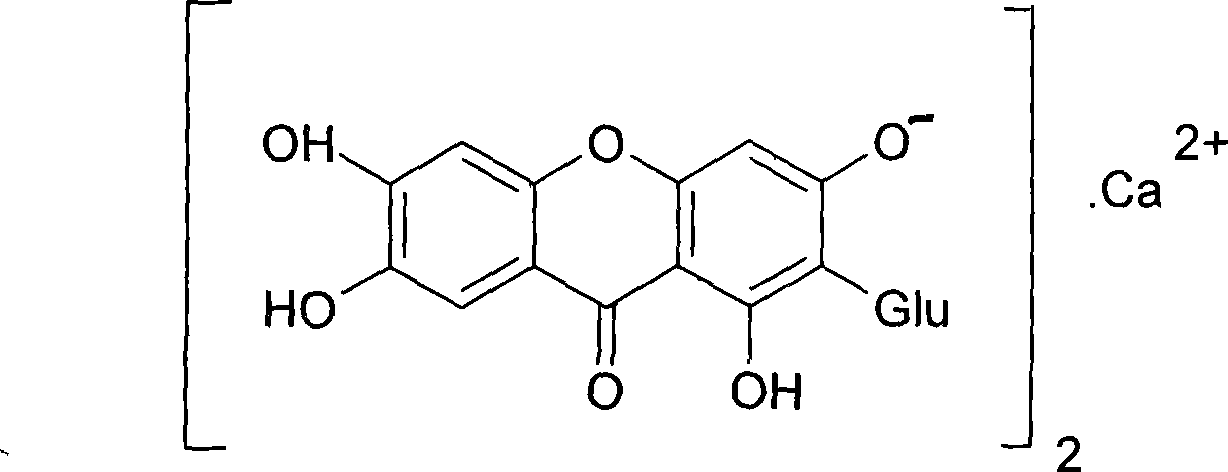
The invention provides use of a mangiferin calcium salt as a PPARs excitant. The structural formula of the mangiferin calcium salt is as shown on the right.
Owner:HAINAN DEZE DRUG RES
Method for purifying gibberellin GA3 and preparing water-soluble pulvis of gibberellin GA3
InactiveCN102246756AImprove finenessPromote precipitationBiocidePlant growth regulatorsAcetic acidEthyl ester
The invention provides a method for purifying gibberellin GA3 and preparing water-soluble pulvis of gibberellin GA3. The invention brings forward control of purity and particle size of gibberellin GA3 and utilization of complex formulation to prepare water-soluble pulvis of gibberellin GA3. According to the invention, a mixed solvent of tetrahydrofuran / ethyl acetate / water is used for recrystallization of a crude product of gibberellin GA3, which enables a reasonable process and simple operation and enables purity of the product in one operation to be more than 98.5% and yield of the product to be more than 90%; the technology of complex formulation is employed and gibberellin GA3 is made to be rapidly dissolved in a water phase and to form a stable disperse phase through using of accessories like an acidic buffer reagent, a dispersant and a surfactant, thereby enabling gibberellin GA3 to be easier to store and transport and more convenient to use and drug effects and the utilization rate to be improved.
Owner:JIANGXI LIFENG BIOLOGICAL TECH
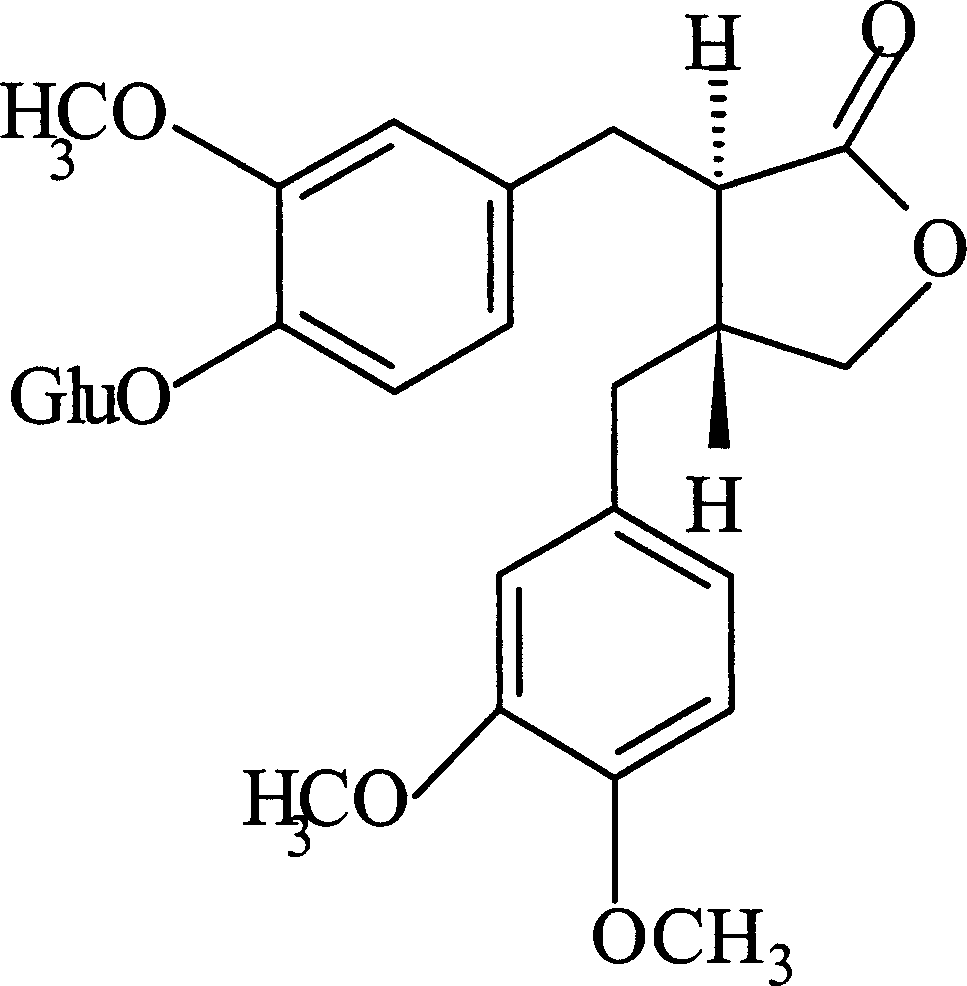
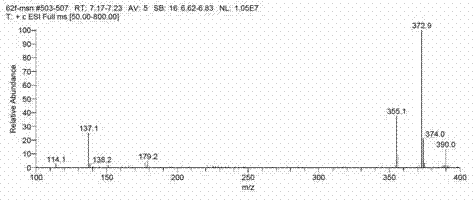
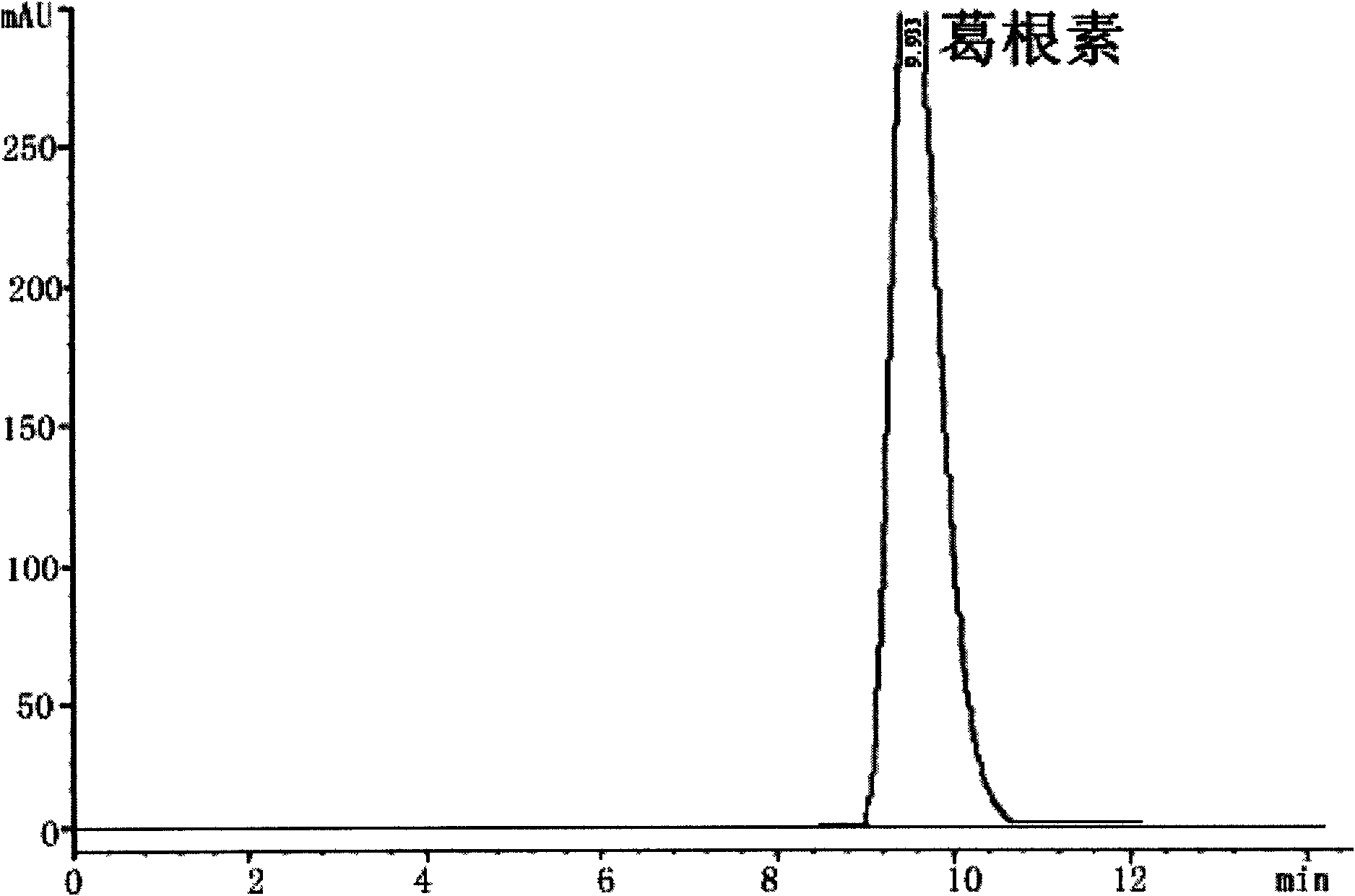
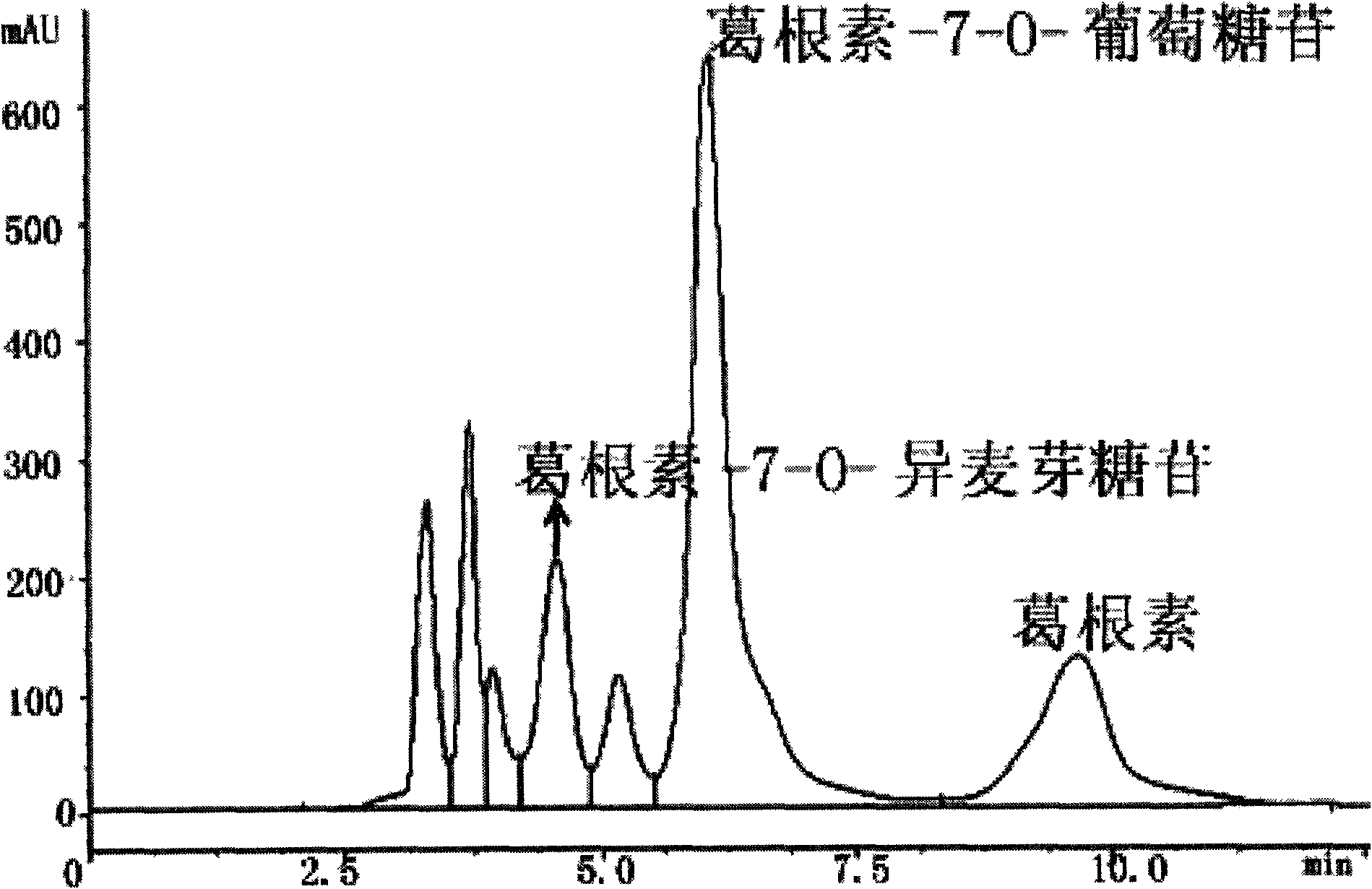
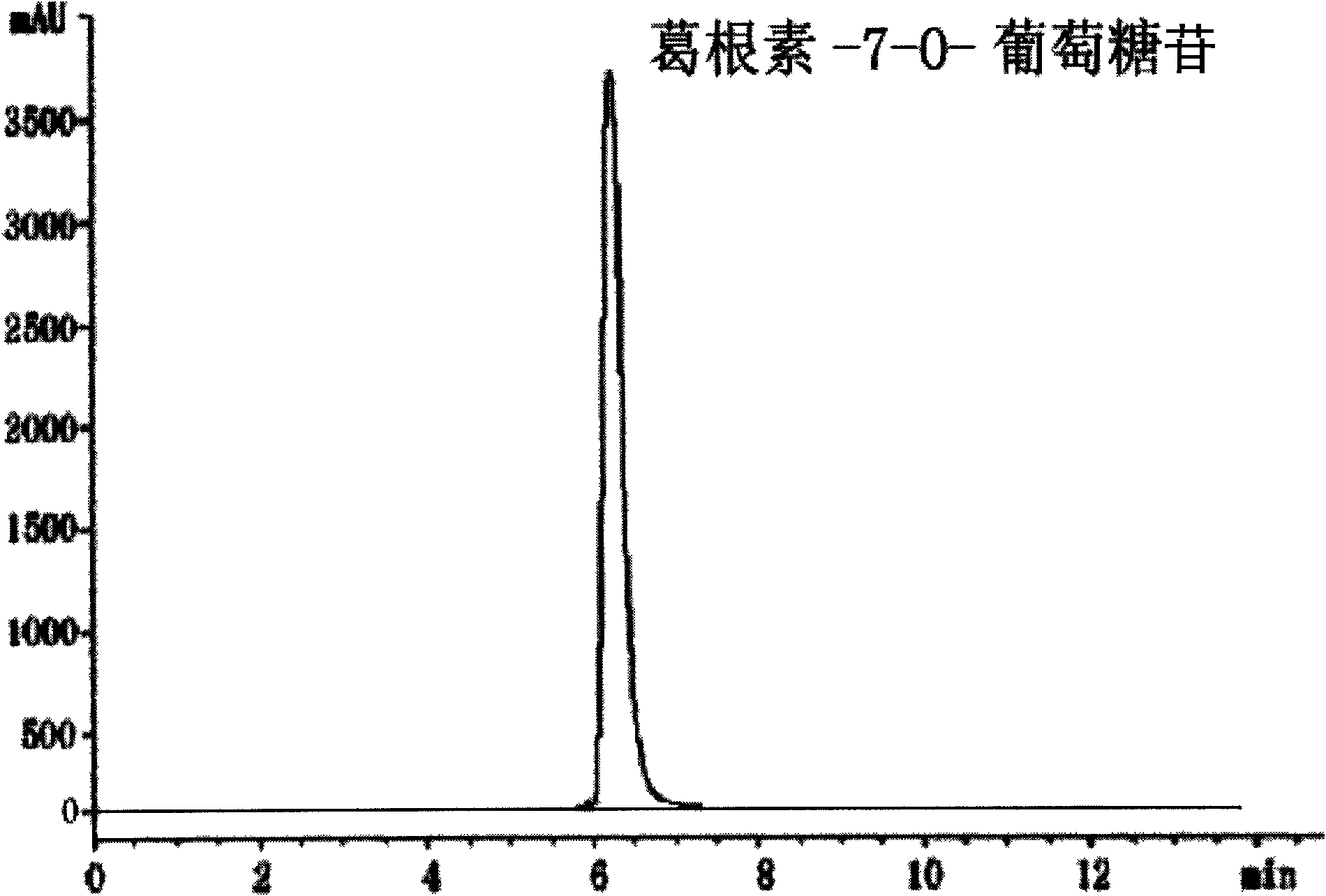
Puerarin glycosylation derivative, medicine compound, preparation method and application thereof
InactiveCN101723997AImprove physical and chemical propertiesImprove mechanical propertiesOrganic active ingredientsSugar derivativesSolubilityOrganic base
The invention discloses a puerarin glycosylation derivative, a medicine compound thereof, a preparation method and application thereof, in particular to the puerarin glycosylation derivative of Formula (I) or a medical acceptable salt thereof, wherein R is a monose base (hexose or pentose) or an oligose base (connecting 2 to 5 hexose or pentose ). The medical acceptable salt is compounded salt of inorganic base or organic base. The puerarin glycosylation derivative or the medical acceptable salt thereof of the invention has better stability and water-solubility, promotes the bioavailability and enhances the healing effect.
Owner:NANJING NORMAL UNIVERSITY +2
Method for obtaining anhydroicaritin from icariin by adopting naringinase
InactiveCN104561178AImprove pharmacological activityImprove conversion rateFermentationIcariinAqueous solution
The invention relates to a method for converting icariin to anhydroicaritin by biological enzyme reaction so as to improve the biological activity. Under the action of naringinase, the reaction is performed in an ethanol water solution with the concentration of 30%-70%. The reaction temperature is 40-70 DEG C, and the reaction time is 1-30h. A glycosyl group on a hydroxyl group of icariin is cut off, and then icariin is converted to anhydroicaritin with higher pharmacological activity. By adopting the method provided by the invention, the processability of icariin is improved, and meanwhile, the actual application range can be expanded.
Owner:山东大学(威海)
L-isovalerylspiramycin iii, its preparation, preparation method and application
ActiveCN102260308AImprove antibacterial propertiesImprove pharmacological activityAntibacterial agentsOrganic active ingredientsFreeze-dryingAntibacterial activity
The invention relates to levorotatory isovaleryl spiramycin III, and also relates to a preparation, a preparation method and application thereof. The preparation consists of the levorotatory isovaleryl spiramycin III and a pharmaceutically acceptable carrier and / or auxiliary materials, and the purity of the levorotatory isovaleryl spiramycin III is over 90 weight percent, preferably over 95 weight percent and further preferably over 98 weight percent. The levorotatory isovaleryl spiramycin III has good antibacterial activity; the preparation of the levorotatory isovaleryl spiramycin III comprises water injection for injection, powder injection for injection and freeze-dried powder injection, fills up a blank of a single-component preparation of the isovaleryl spiramycin III in the current market and provides a new quick-response way for treating infectious diseases. The single-component preparation of the isovaleryl spiramycin III has the advantages of stable production process, easily controlled quality standard, and suitability for large-scale industrial production.
Owner:SHENYANG TONGLIAN GRP CO LTD
Pichia kudriavzevii ZJPH0802 and application thereof in preparation of curcumin derivatives
ActiveCN102965290AImprove pharmacological activityImprove biological activityFungiMicroorganism based processesChemistryDrug biotransformation
The invention discloses Pichia kudriavzevii ZJPH0802 and an application thereof in preparation of curcumin derivatives. The strain is collected in China Center for Type Culture Collection (CCTCC), the address is Wuhan University, Wuhan, China, the collection data is September 24, 2012, and the collection number is CCTCC M 2012373. The invention provides a new strain, namely the Pichia kudriavzevii ZJPH0802 for preparing the curcumin derivatives by biotransformation, and structural modification can be performed on curcumin by utilizing a strain resting cell transformation method for obtaining the corresponding derivatives; the pharmacological or biological activity of structurally modified matters of the curcumin can be improved to different extents relative to a curcumin substrate before modification so as to be conductive to development of new pharmaceutical preparations. The technology for obtaining the curcumin derivatives by adopting the biotransformation method, disclosed by the invention, is simpler and environment-friendly, a biological catalyst is a microbial thallus, and the Pichia kudriavzevii ZJPH0802 further has the advantages of self-fermentation and production, stable quality and low cost.
Owner:菏泽建数智能科技有限公司
Application of glycosylated puerarin derivate and its combination for preventing and treating cardiovascular and cerebrovascular disease
InactiveCN101585858AImprove physical and chemical propertiesImprove mechanical propertiesOrganic active ingredientsSugar derivativesDiseasePuerarin
The invention relates to a glycosylated puerarin derivate shown in the general formula (I) and its pharmaceutically acceptable salt. In the formula, R is described as the text. A preparation method and a medicament combination containing the compound shown in the general formula (I) of the effective dose are also provided. The combination is used for treating and preventing the cardiovascular and cerebrovascular diseases, diabetic nephropathy and osteoporosis.
Owner:NANJING NORMAL UNIVERSITY +1
Chinese wolfberry glycopeptide, preparation method and applications thereof
ActiveCN107021995AImprove pharmacological activityPeptide/protein ingredientsPeptide preparation methodsFlocculationLycium barbarum fruit
The present invention discloses a Chinese wolfberry glycopeptide, a preparation method and applications thereof, wherein the molecular weight distribution of the Chinese wolfberry glycopeptide is that the Chinese wolfberry glycopeptide having the molecular weight of 1000-10000 Da accounts for 50-85%, the protein content is 20-35%, the neutral polysaccharide content is 20-35%, and the uronic acid content is 5-20%. According to the present invention, the heating flocculation method is used to remove the partial insoluble impurities in the preparation method of the present invention and replaces the application of a large amount of ethanol to precipitate in the traditional extraction method, such that the production safety is improved, the ethanol recovery step is avoided, the production cost is reduced, the activity of the obtained Chinese wolfberry glycopeptide is not changed, and the god production promotion prospect is provided.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com