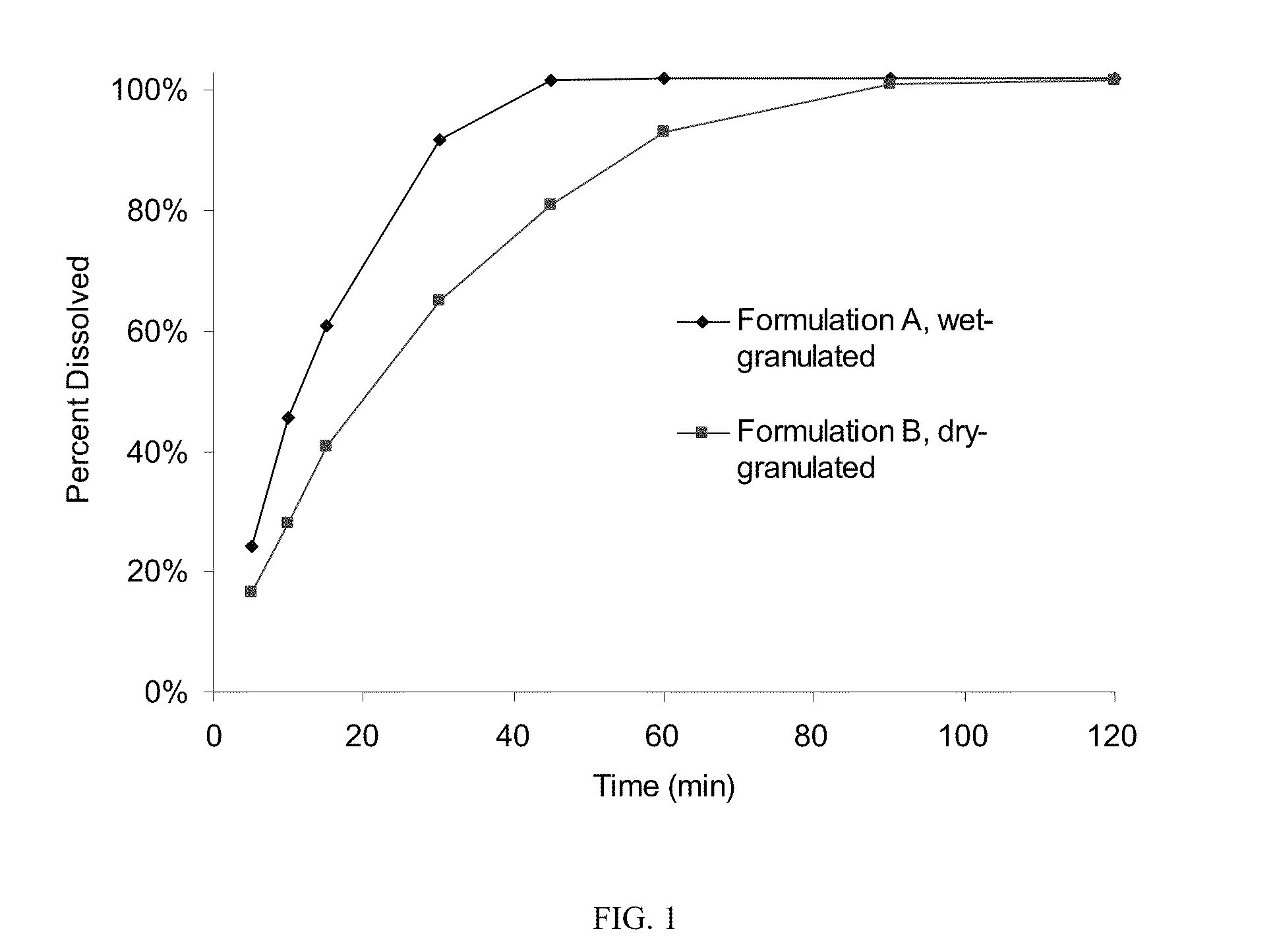
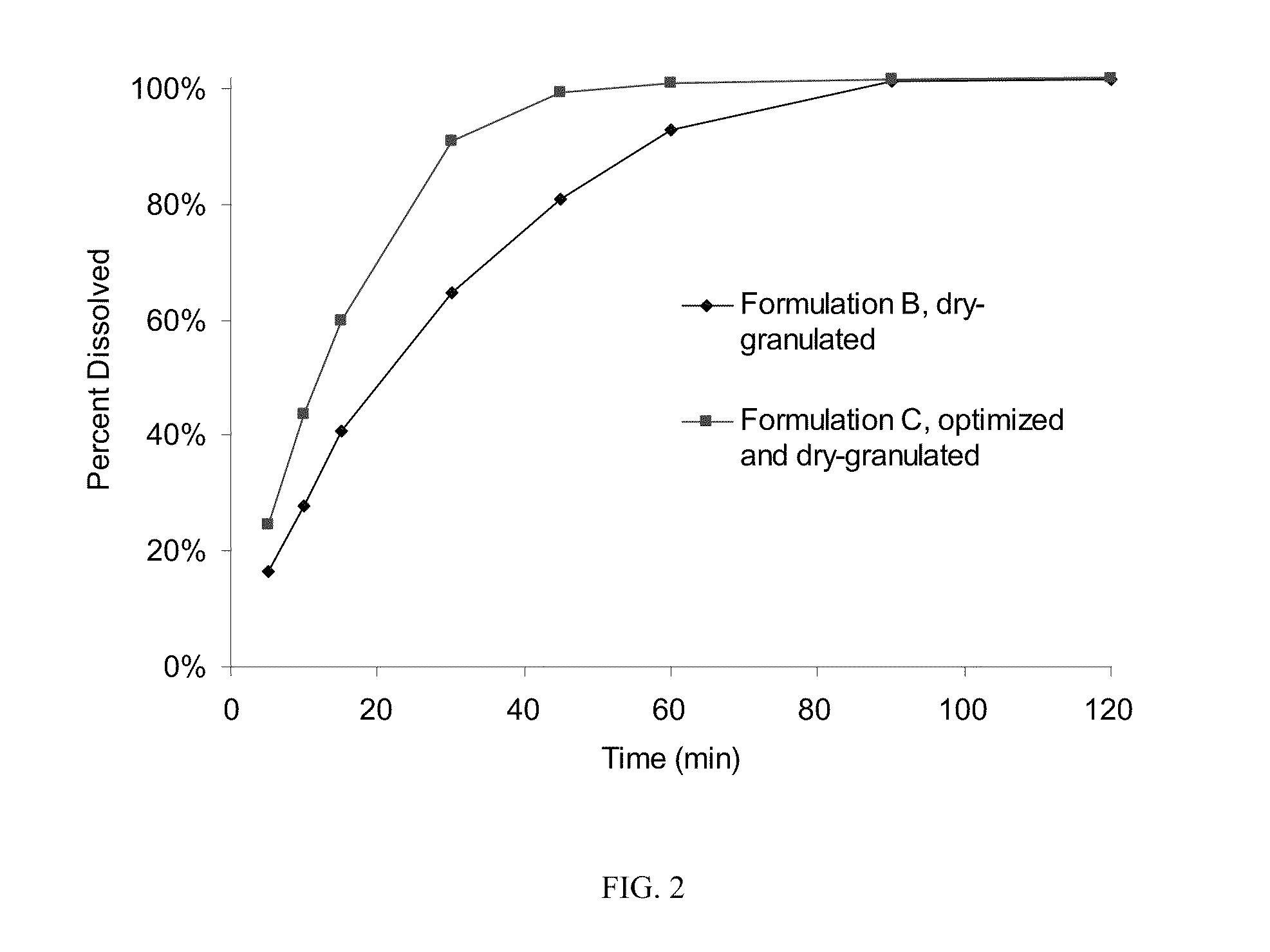
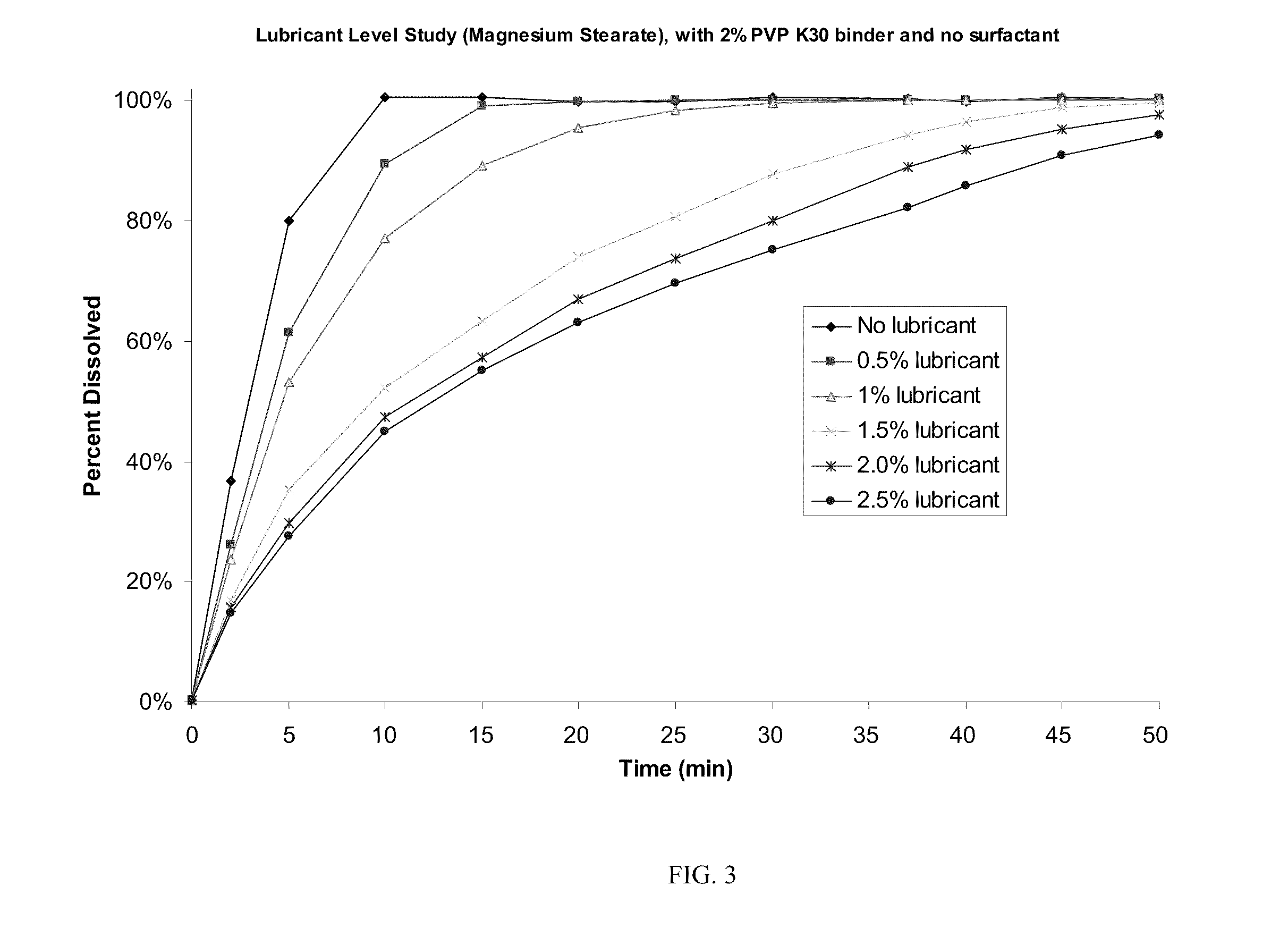
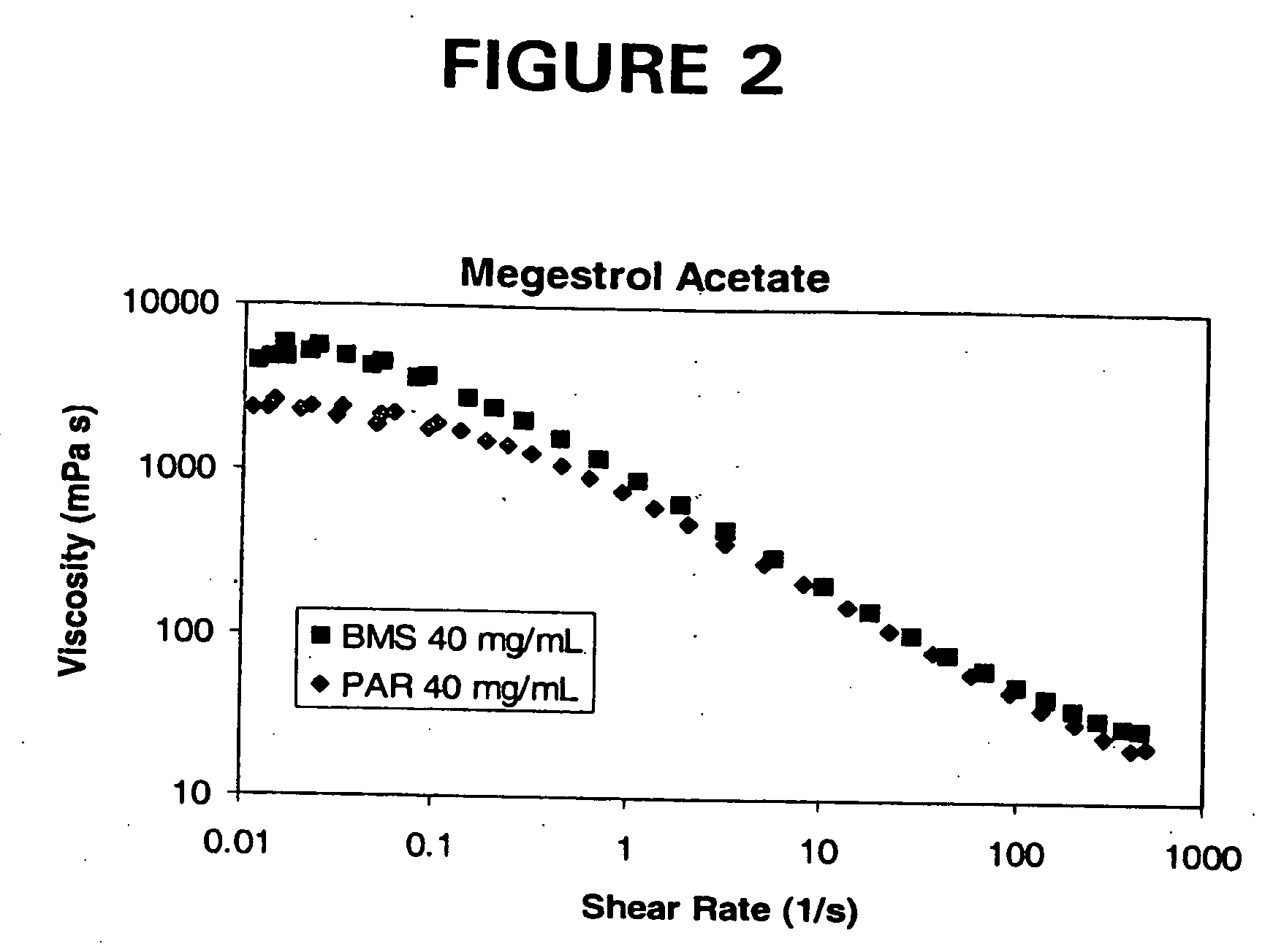
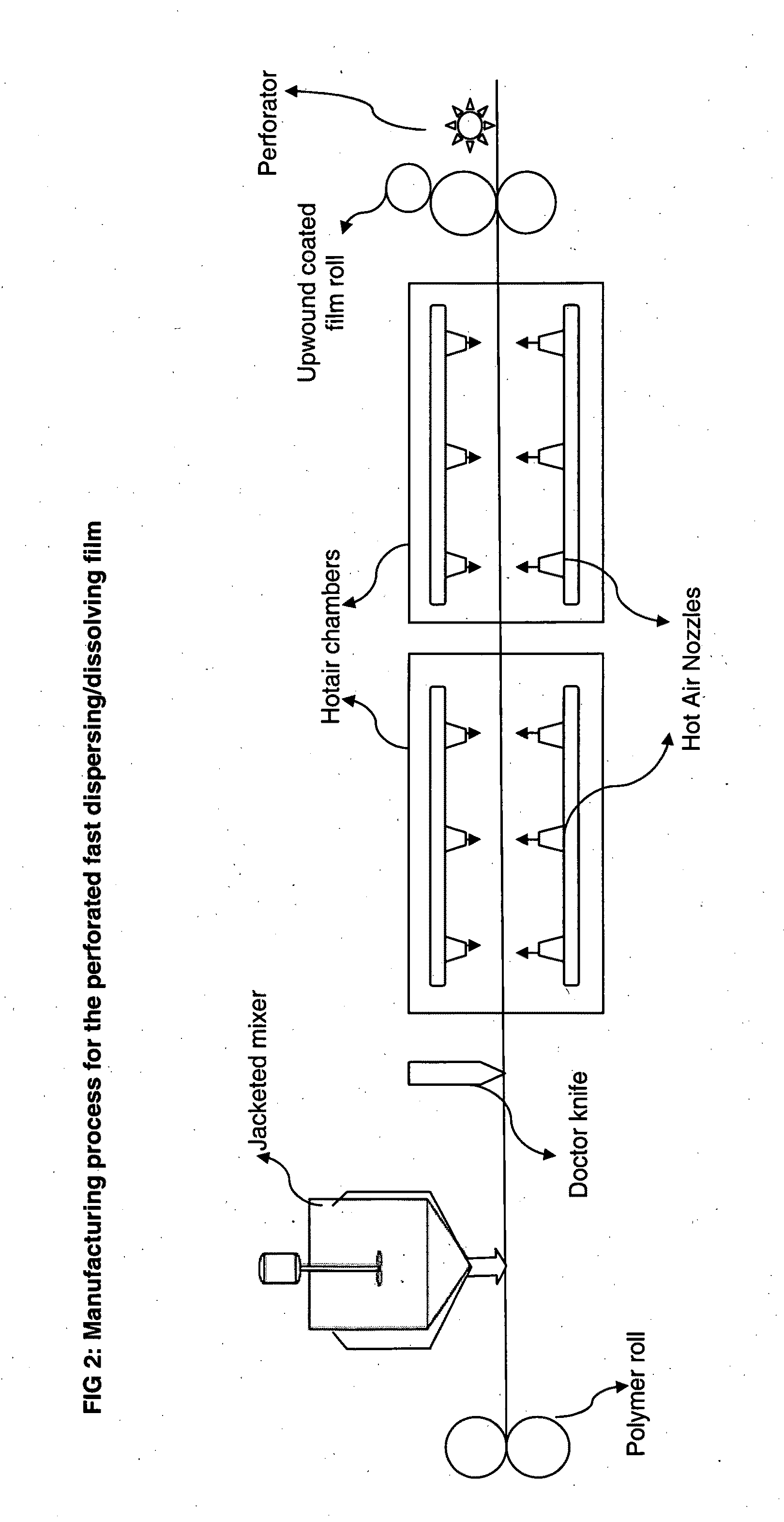
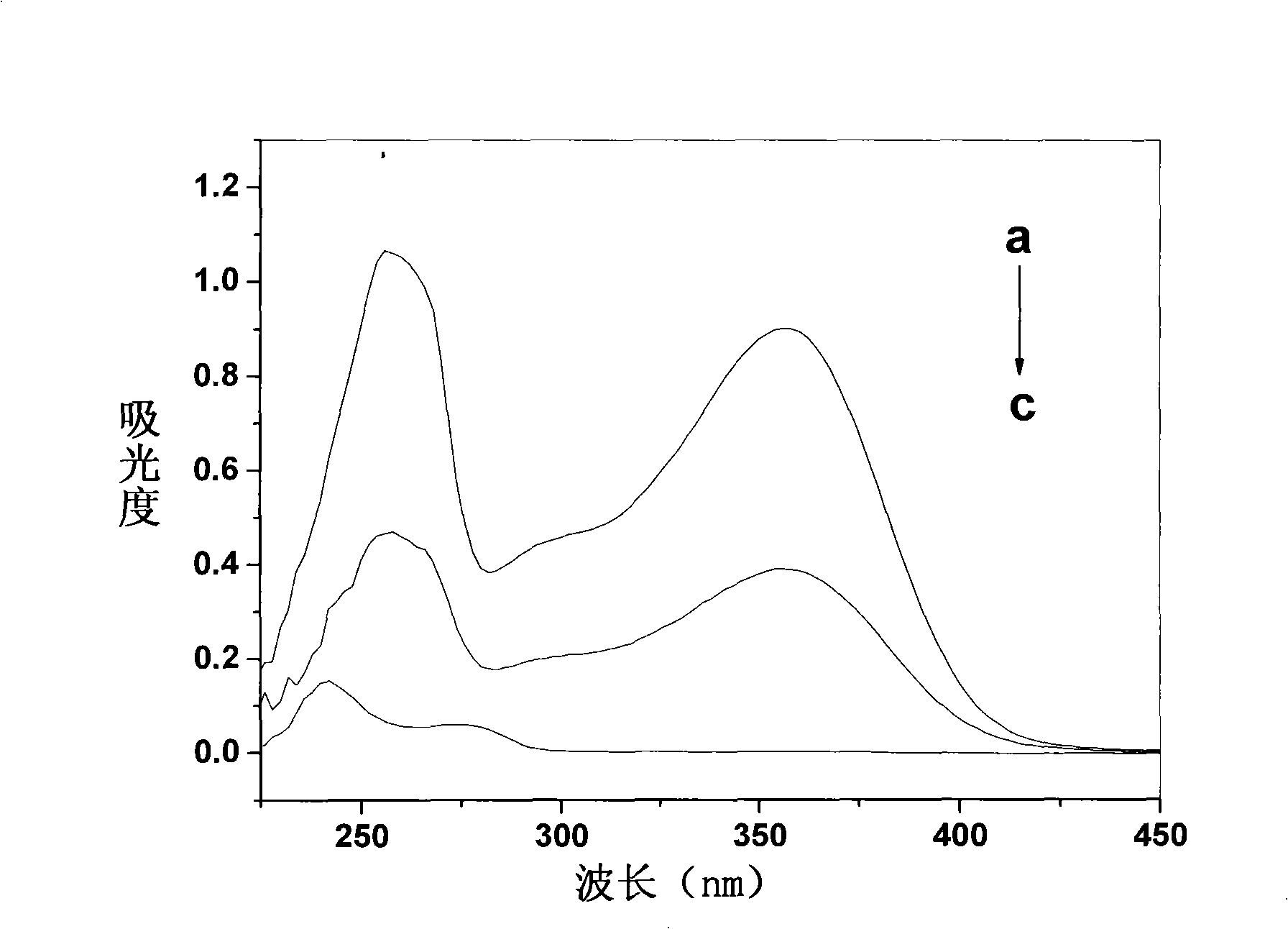
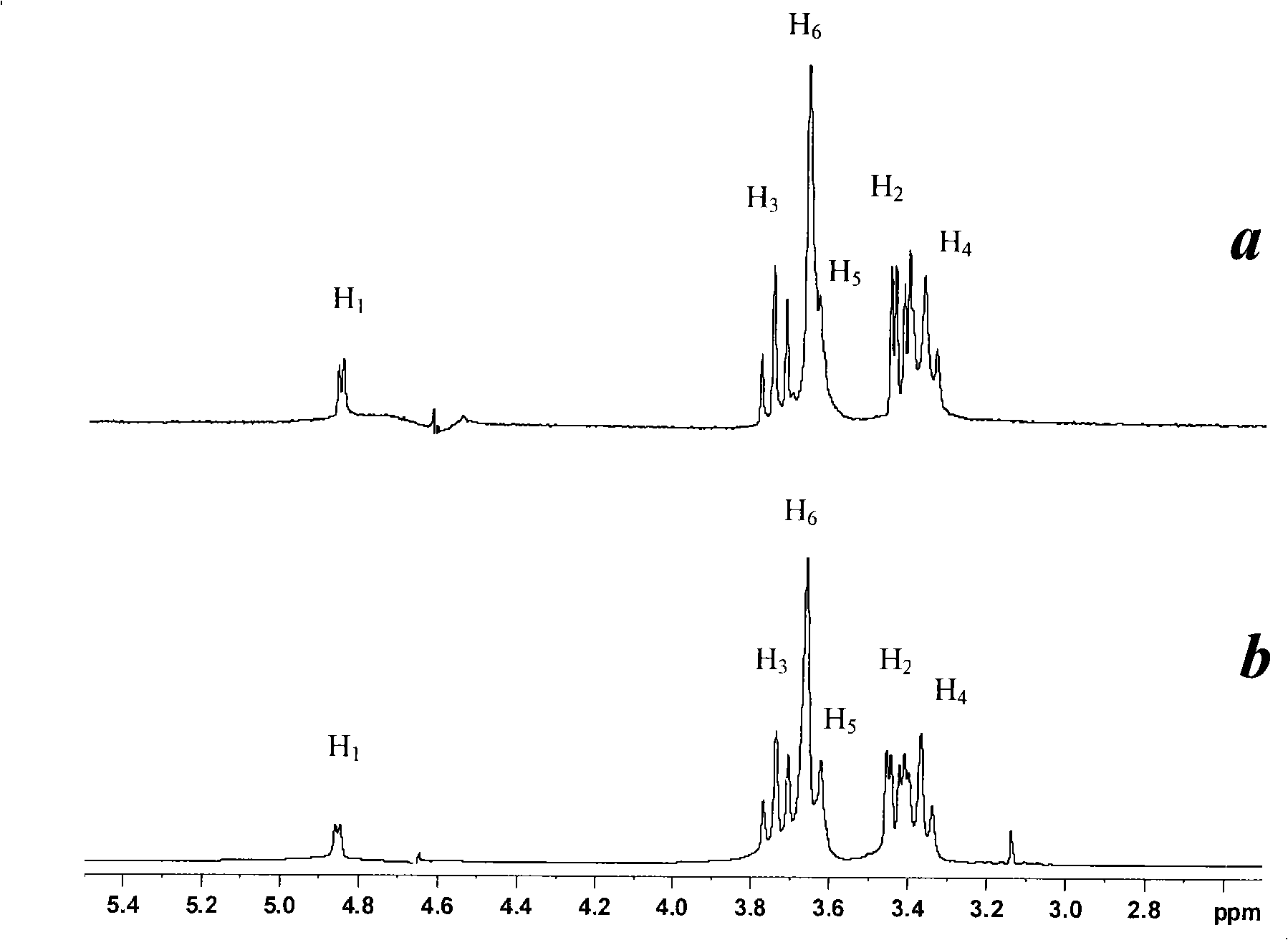
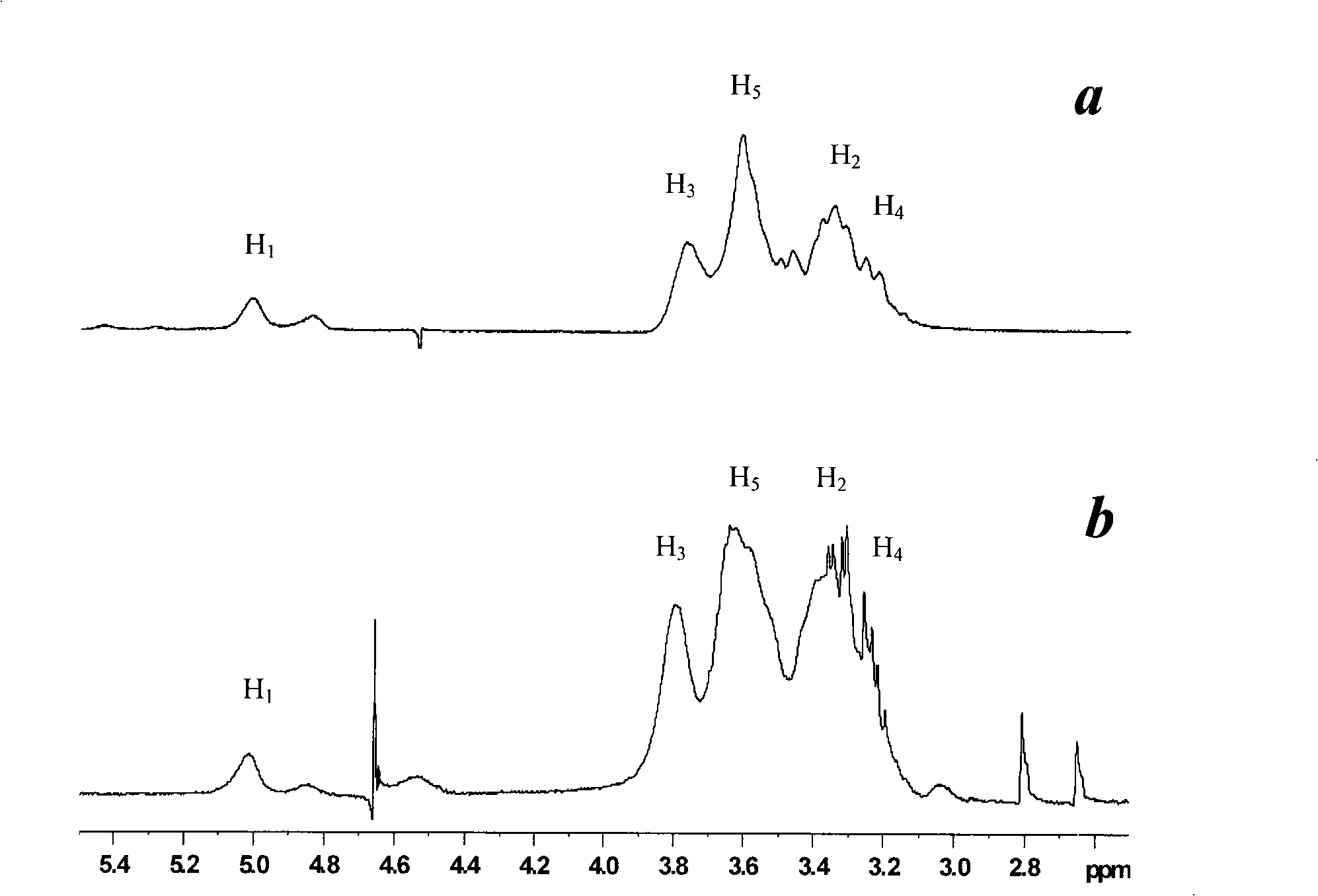
Patents
Literature
140 results about "Liquid Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liquid dosage compositions of stable nanoparticulate active agents
The present invention relates to liquid dosage compositions of stable nanoparticulate active agents. The liquid dosage compositions of the invention include osmotically active crystal growth inhibitors that stabilize the nanoparticulate active agents against crystal and particle size growth of the active agent.
Owner:ELAN PHRMA INT LTD
Immediate release formulations and dosage forms of gamma-hydroxybutyrate
ActiveUS20110111027A1Reduce brittlenessDissolve fastBiocideNervous disorderOral medicationHigh weight
The present invention provides a solid immediate release dosage form adapted for oral administration of GHB. The solid immediate release dosage form includes an immediate release formulation comprising a relatively high weight-percentage of GHB with a bioavailability similar to that of a liquid GHB dosage form.
Owner:JAZZ PHARMA
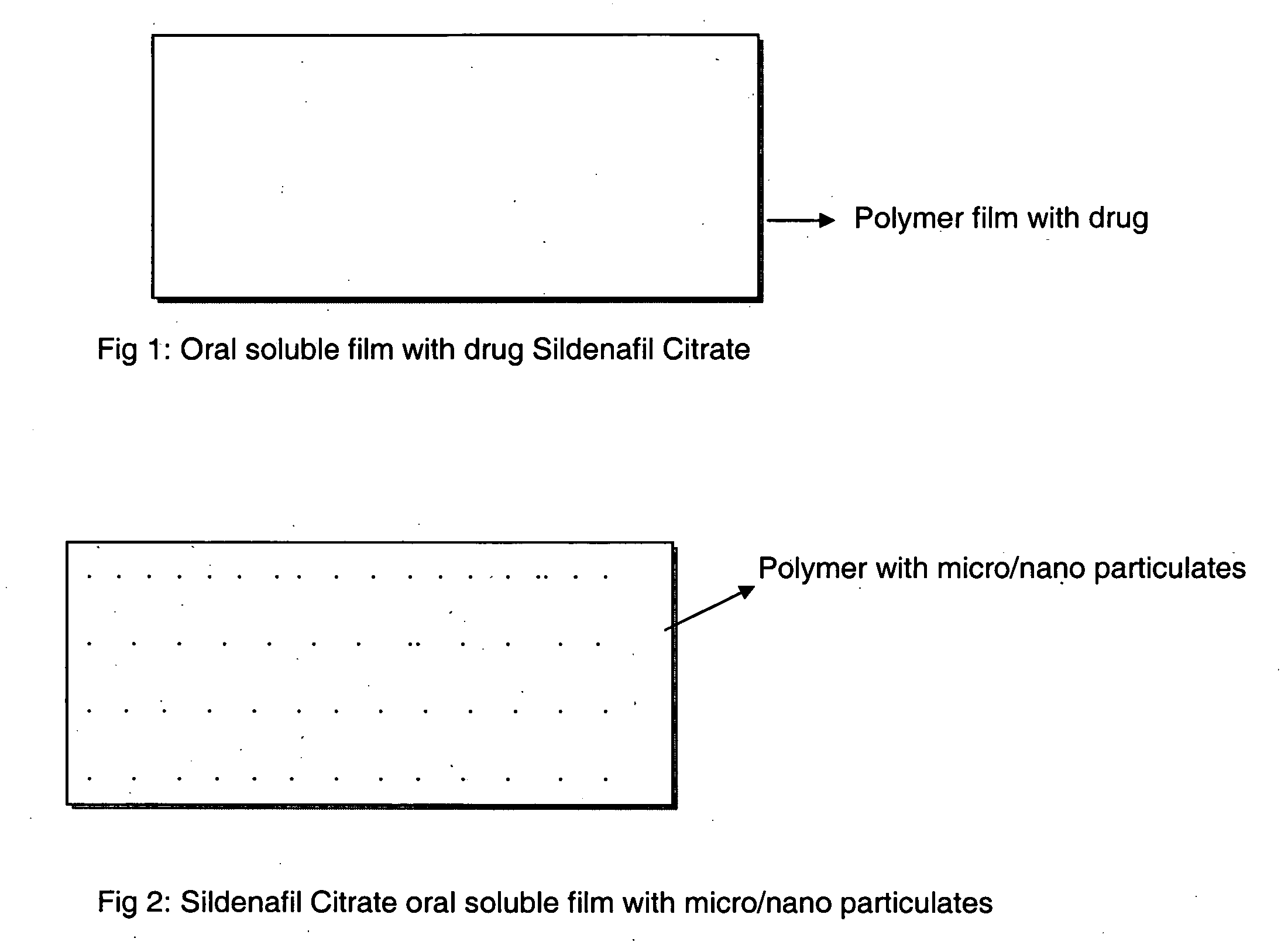
Oral fast dissolving films for erectile dysfunction bioactive agents
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
Pharmaceutical formulation for sulfur-containing drugs in liquid dosage forms
InactiveUS20070134277A1Improve stabilityMask odorBiocidePeptide/protein ingredientsActive agentFood flavor
The pharmaceutical formulations of the invention for masking the odor from the sulfur-containing active agent comprise at least one sulfur-containing active agent, an effective amount of at least one flavoring agent. Any flavoring agent or combinations of flavoring agents may be used in the pharmaceutical formulation of the invention. The flavoring agent may be natural flavors, natural fruit flavors, artificial flavors, and mixtures thereof. The pharmaceutical formulation may contain an artificial sweetener, a natural sweetener or mixtures thereof. The pharmaceutical formulations are provided in liquid dosage form or a dry powder dosage form for reconstitution in water. Stabilizer added as one of expicients can extend the stability of the pharmaceutical formulation liquid dosage form for a period of at least 30 days when the formulation is stored below room temperature. The pharmaceutical formulations of the invention are palatable and particularly useful for the administration of sulfur-containing drugs to very small children that are in need of such medications. Methods of forming a liquid dosage form of pharmaceutical formulation by adding water to the dry powder form, methods to prepare an odor-masking pharmaceutical formulation and methods for treating lead poisoning or Wilson's disease using the odor-masking pharmaceutical formulation are also provided.
Owner:CHILDRENS MEDICAL CENT CORP
Taste masked pharmaceutical compositions
InactiveUS20060182796A1Fast absorptionQuick releaseDispersion deliveryPill deliveryOral medicationBULK ACTIVE INGREDIENT
A pharmaceutical composition for oral administration containing a pharmaceutically active ingredient coated with an amount of a polymer combination of an enteric polymer and an ammonio methacrylate copolymer to effectively mask the taste of the medicament. In a preferred embodiment, the ratio of the enteric polymer to the ammonio methacrylate copolymer is about 40:60 to about 90:10, preferably about 60:40, by weight of polymer. The pharmaceutical coating composition is soluble in the acidic environment of the stomach, which generally has a pH value of about 1.0 to 3.0, but relatively insoluble at higher pH values of the mouth. The coatings provide for rapid release and absorption of the drug after it passes through the mouth, and is particularly desirable in the case of liquid dosage forms.
Owner:ABRIKA PHARMA
Liquid dosage forms having enteric properties of delayed and then sustained release
Owner:EASTMAN CHEM CO
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20050042304A1Easy to prepareImprove pharmacological activityPowder deliveryBiocideATPaseOral suspensions
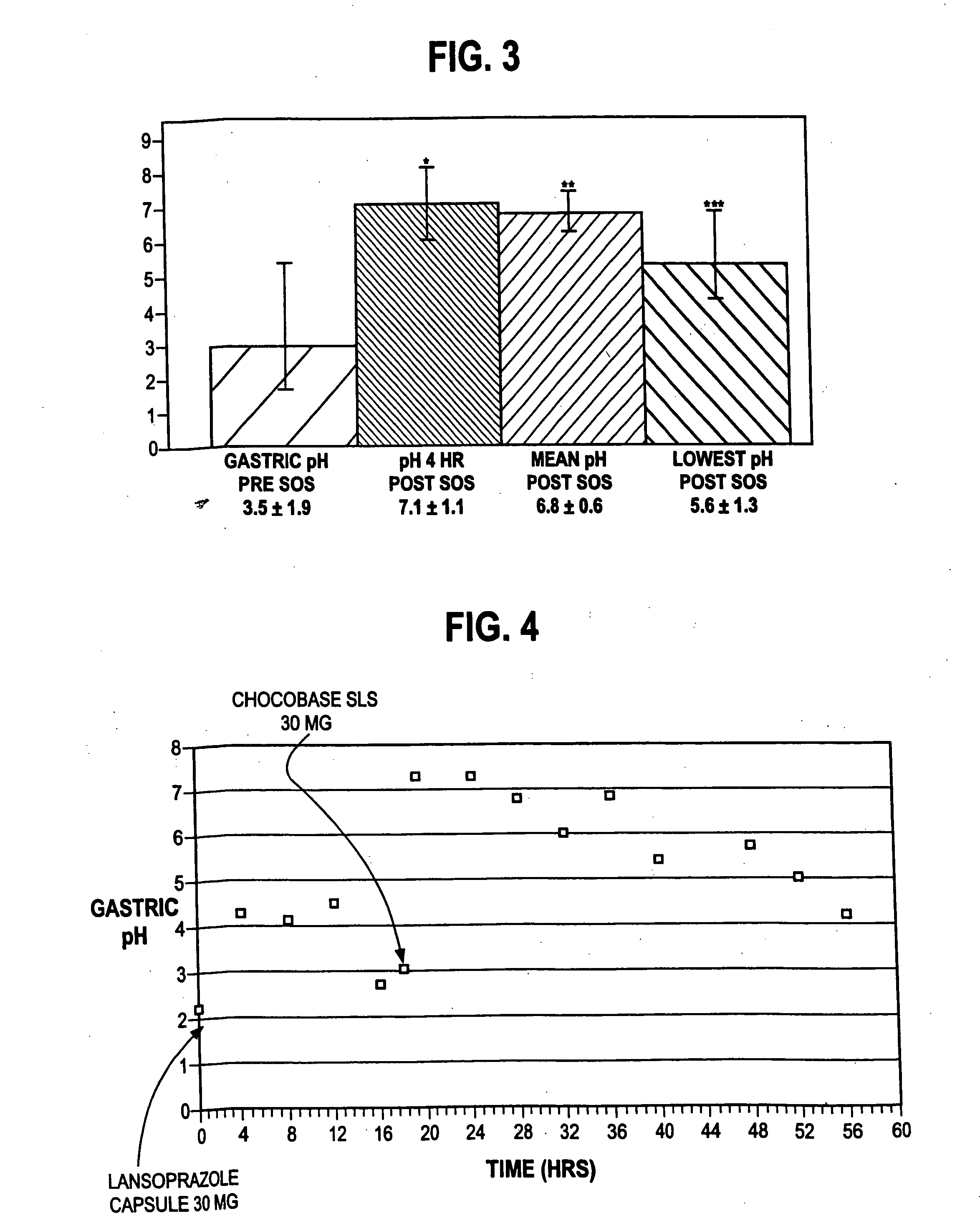
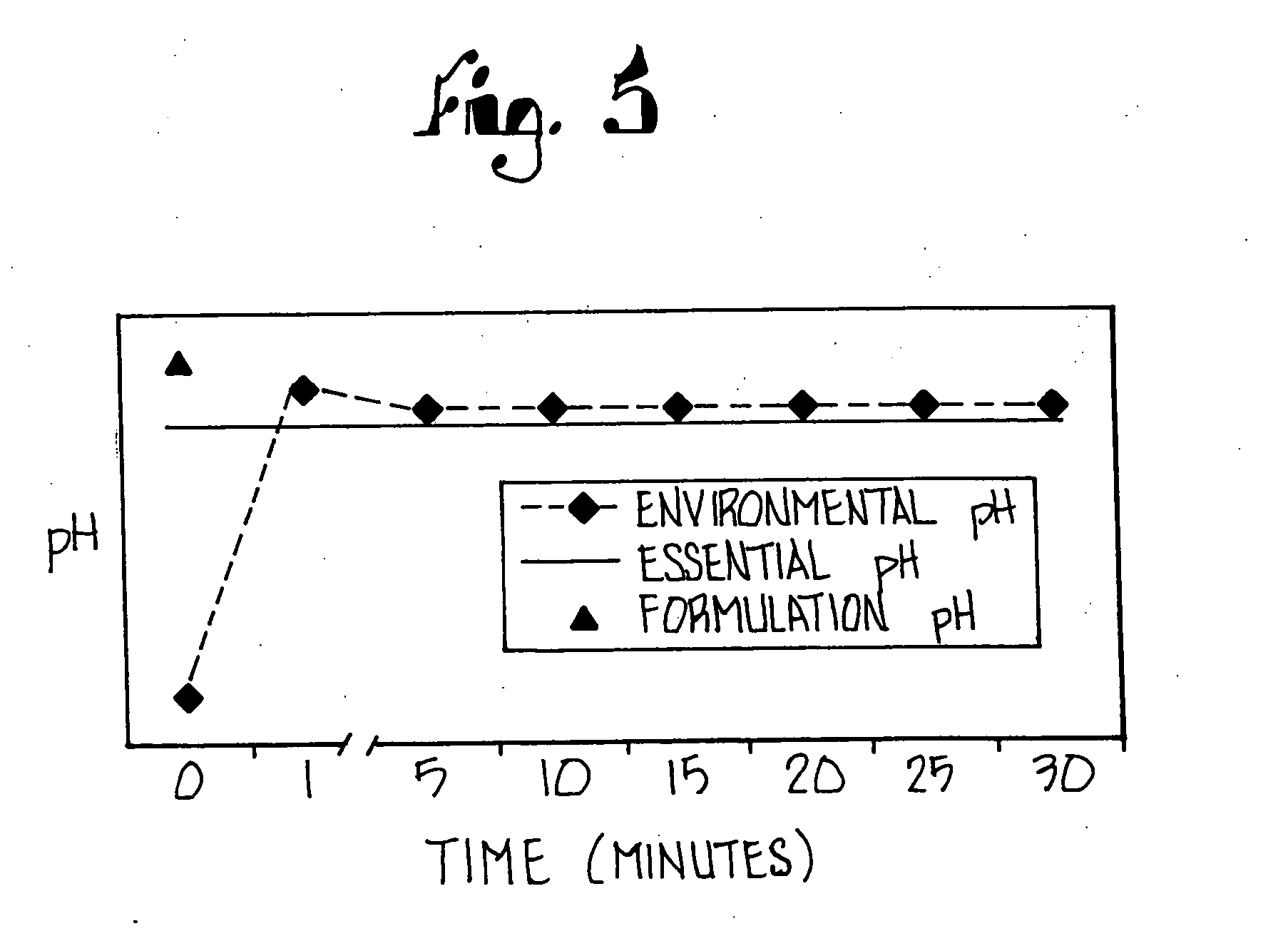
A method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier. The present invention provides an oral solution / suspension comprising a proton pump inhibitor and at least one buffering agent. The PPI can be any substituted benzimidazole compound having H+,K+-ATPase inhibiting activity and being unstable to acid. Omeprazole and lansoprazole are the preferred PPIs for use in oral suspensions in concentrations of at least greater than 1.2 mg / ml and 0.3 mg, respectively. The liquid oral compositions can be further comprised of parietal cell activators, anti-foaming agents and / or flavoring agents. The inventive compositions can alternatively be formulated as a powder, tablet, suspension tablet, chewable tablet, capsule, effervescent powder, effervescent tablet, pellets and granules. Such dosage forms are advantageously devoid of any enteric coating or delayed or sustained-release delivery mechanisms, and comprise a PPI and at least one buffering agent to protect the PPI against acid degradation. Similar to the liquid dosage form, the dry forms can further include anti-foaming agents, parietal cell activators and flavoring agents. Kits utilizing the inventive dry dosage forms are also disclosed herein to provide for the easy preparation of a liquid composition from the dry forms. In accordance with the present invention, there is further provided a method of treating gastric acid disorders by administering to a patient a pharmaceutical composition comprising a proton pump inhibitor in a pharmaceutically acceptable carrier and at least one buffering agent wherein the administering step comprises providing a patient with a single dose of the composition without requiring further administering of the buffering agent. Additionally, the present invention relates to a method for enhancing the pharmacological activity of an intravenously administered proton pump inhibitor in which at least one parietal cell activator is orally administered to the patient before, during or after the intravenous administration of the proton pump inhibitor.
Owner:UNIVERSITY OF MISSOURI
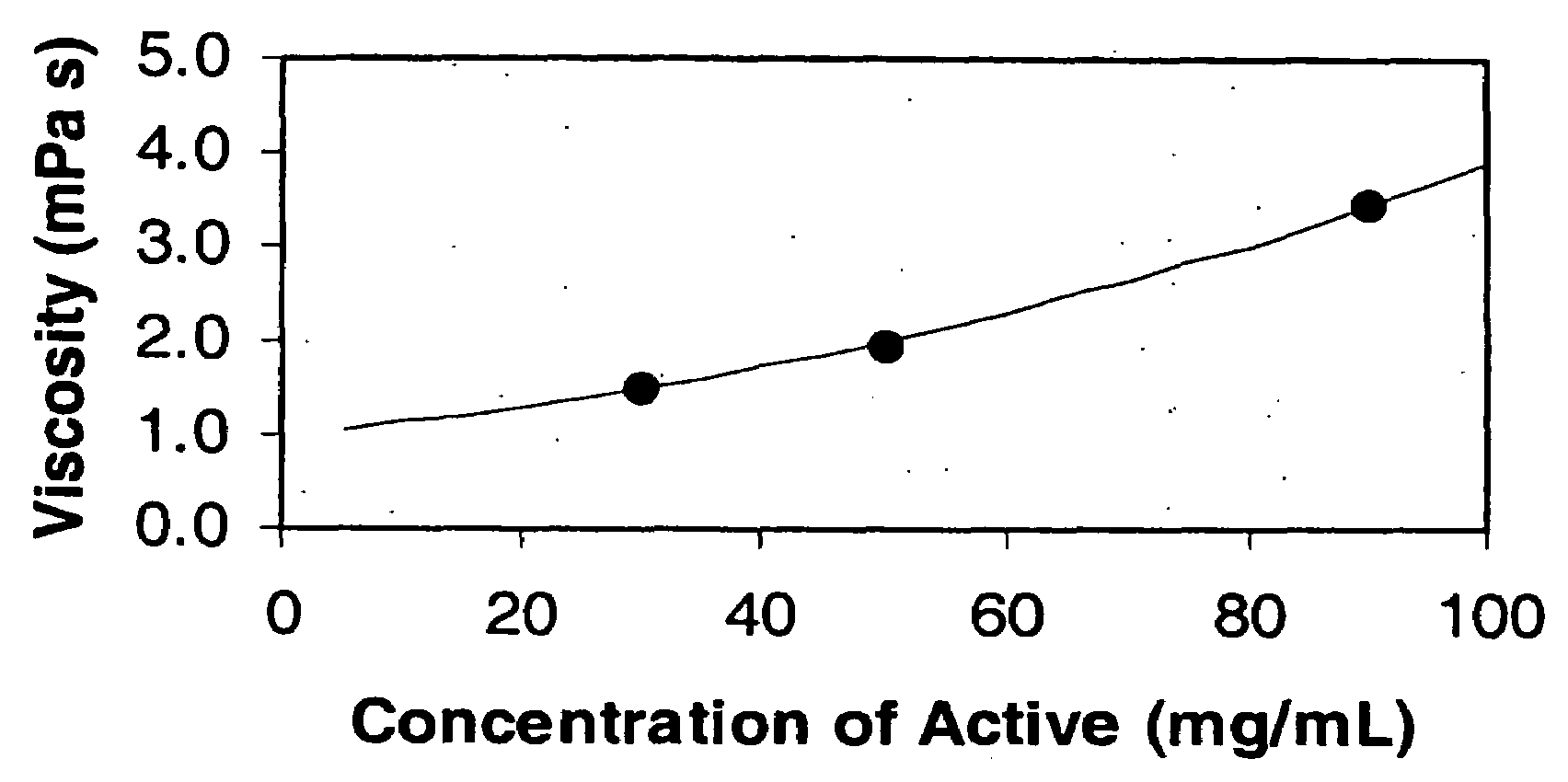
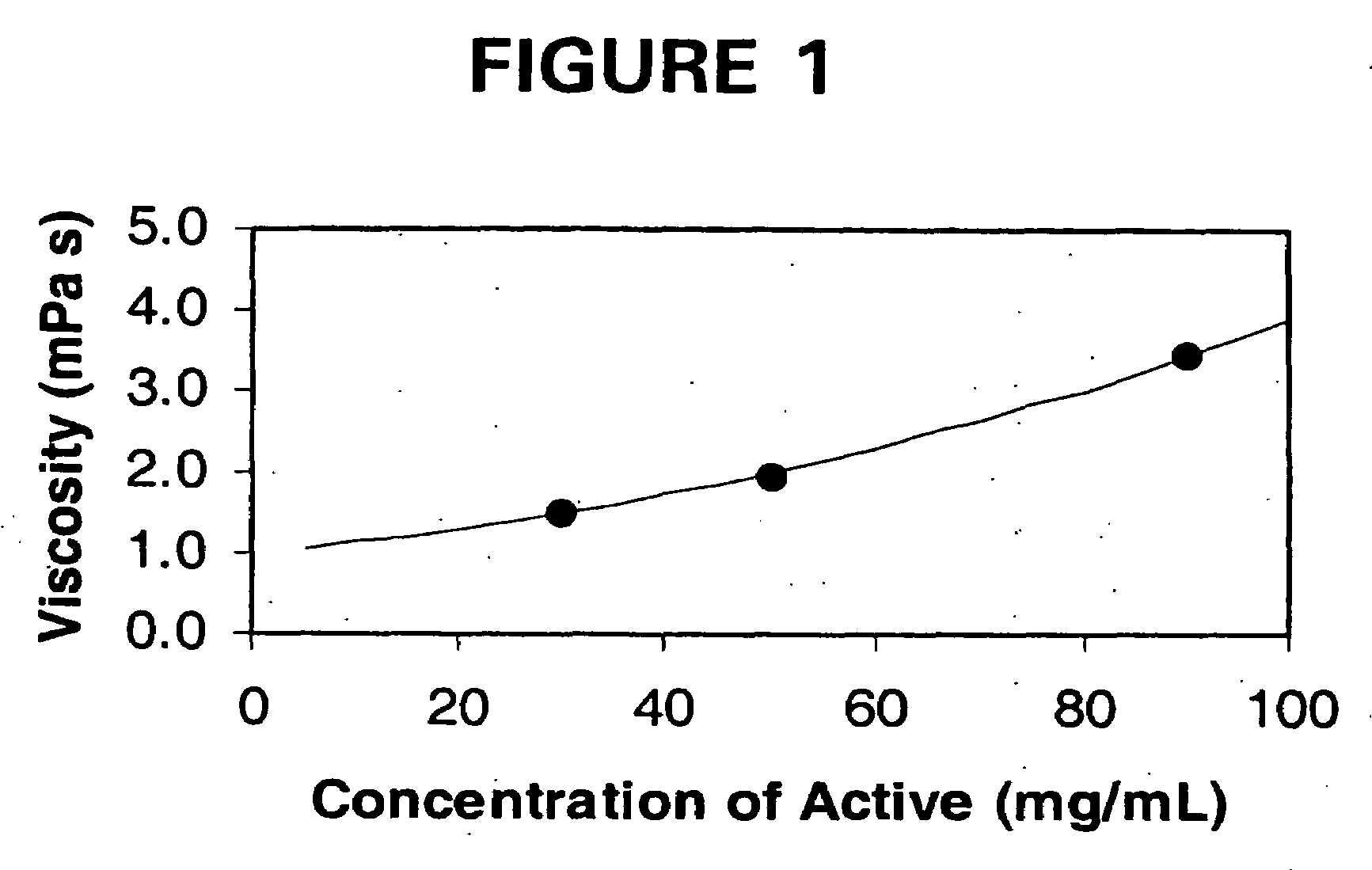
Low viscosity liquid dosage forms
Owner:ALKERMES PHARMA IRELAND LTD
Perforated water soluble polymer based edible films
InactiveUS20090047350A1High drug loadingImprove abilitiesPowder deliveryOrganic active ingredientsSolubilityActive agent
Owner:BANGALORE RAMESH
Rapidly-dispersible tablet and preparation method thereof
InactiveCN107823150AOvercoming stratificationAccurate doseHydrocarbon active ingredientsHydroxy compound active ingredientsWater insolubleActive component
The invention relates to a rapidly-dispersible tablet and a preparation method thereof, in particular to a rapidly-dispersible tablet composition containing no disintegrating agent and a preparation process thereof, which belong to the field of drugs and healthcare products. The invention discloses a rapidly-dispersible tablet. The rapidly-dispersible tablet is prepared from 0.5 to 900 parts by weight of active components, 200 to 900 parts by weight of excipient and 5 to 30 parts by weight of lubricating agents, wherein the active component is a water-insoluble active component. The rapidly-dispersible tablet has the advantages that the rapidly-dispersible tablet composition containing insoluble active component is prepared into the tablet rather than a liquid dosage form, so that a complex process for preparing the insoluble active component into the liquid preparation is not needed, the layering phenomenon of the product liquid during a long-term storage period can also be avoided, and the rapidly-dispersible tablet has accurate dose and stable quality, is convenient to store and carry, can be swallowed and can also be administered after being dissolved.
Owner:北京素维生物科技有限公司
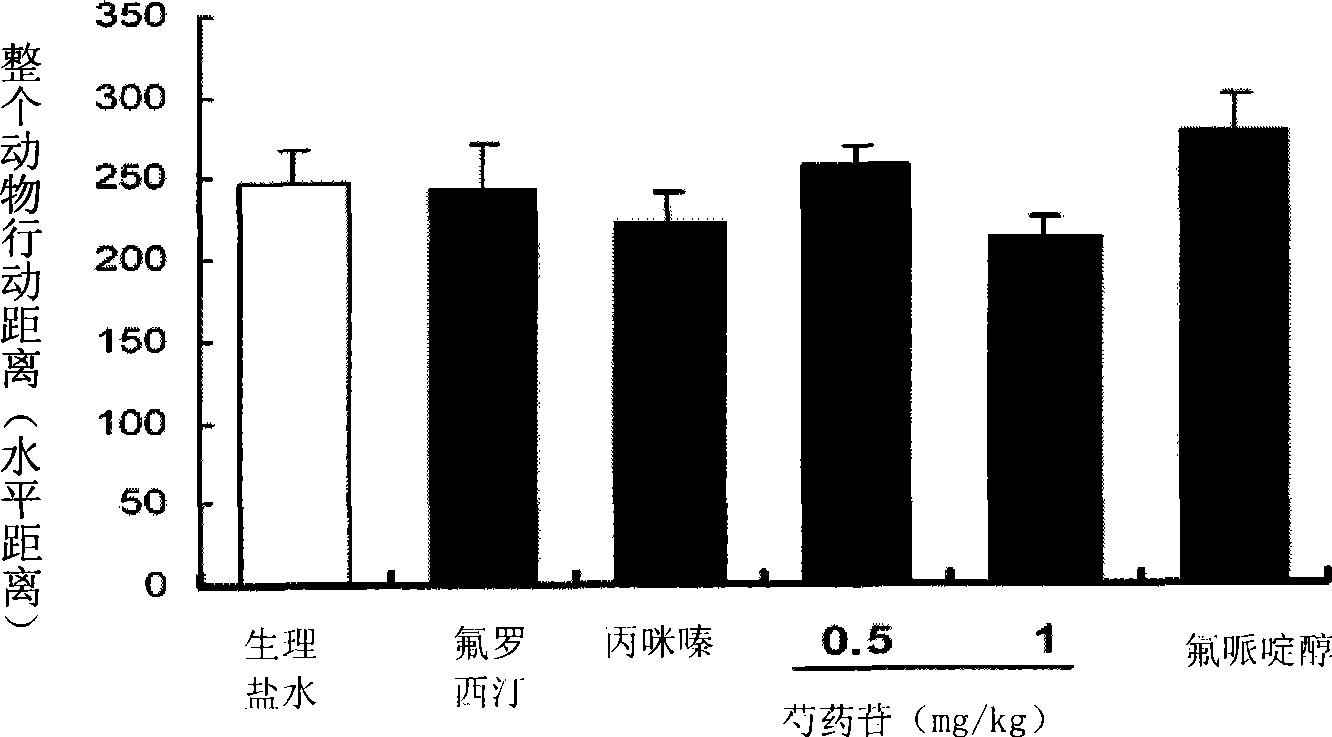
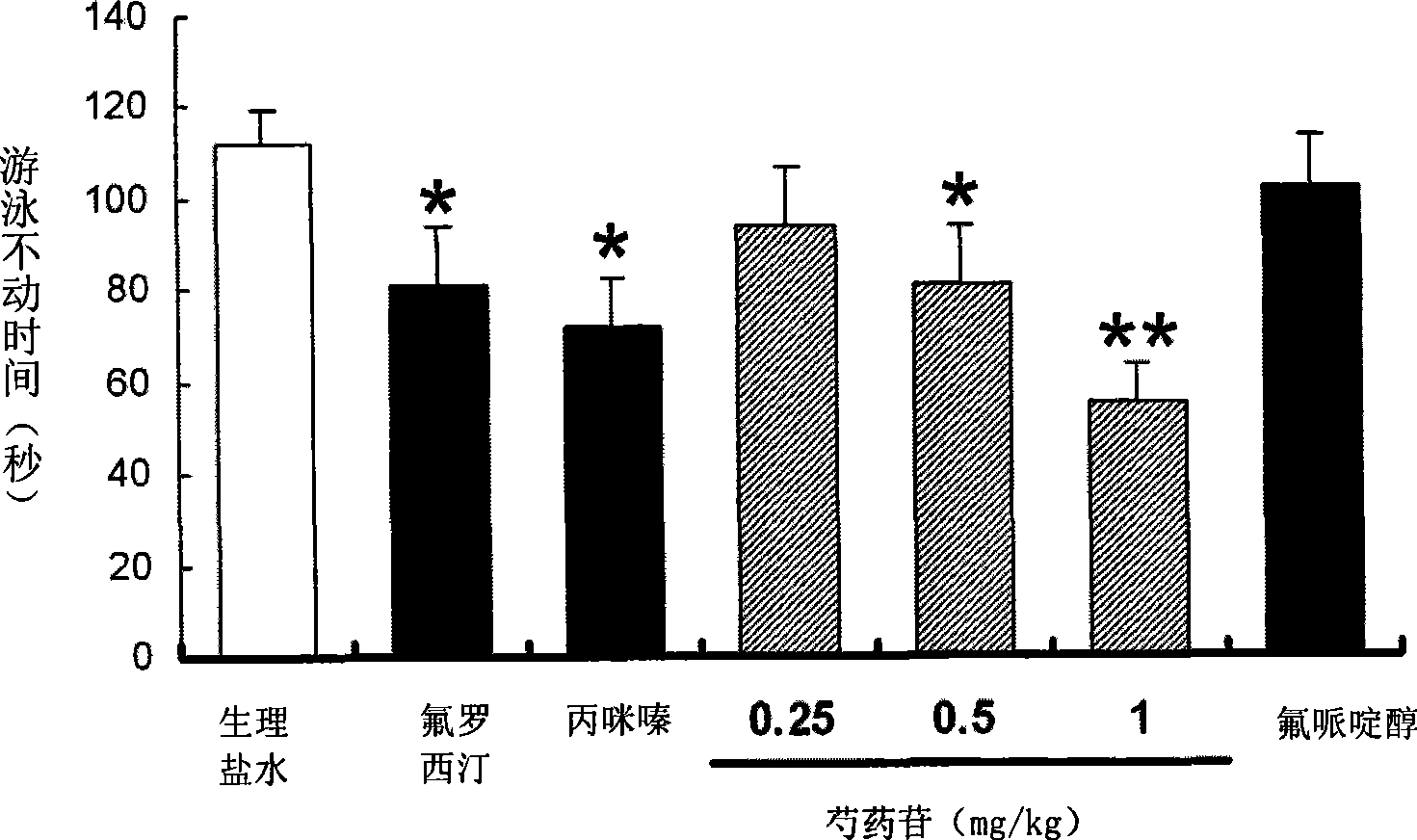
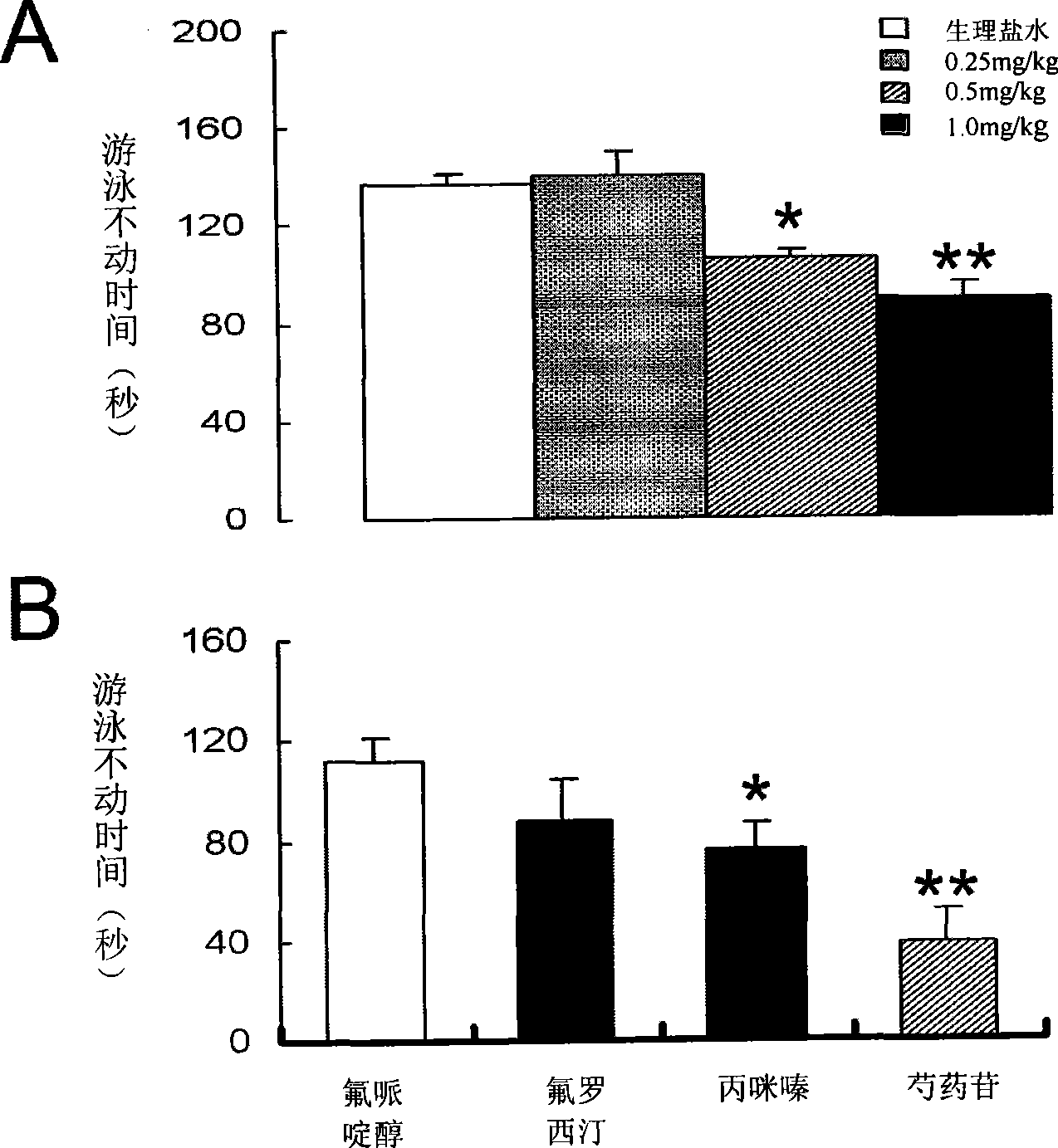
Use of penoniflorin in preparing medicine for preventing and treating depression and medicine composition thereof
InactiveCN101385736ASmall side effectsGood effectOrganic active ingredientsNervous disorderSide effectCurative effect
The invention relates to an application of paeoniflorin in drugs for preventing and treating depression and a pharmaceutical composition thereof, and the pharmaceutical composition contains effective dose of the paeoniflorin and a pharmaceutical acceptable carrier. The pharmaceutical composition can be prepared into various conventional liquid formulations or solid formulations. The paeoniflorin is proved to have better efficacy for the depression and long duration of the efficacy through a rat forced swimming test, a open field test and a new environment feeding inhibition test. The drugs which take the paeoniflorin as an active ingredient and are used for treating and preventing the depression have good effects, long duration, insignificant toxicity and side effect and low price.
Owner:SOUTHERN MEDICAL UNIVERSITY
Liquid dosage form of acetaminophen
The present invention relates to a liquid dosage form comprising pharmaceutically insoluble and unpleasant active drug and liquid excipient base. In particular, the invention relates to a liquid dosage form comprising Acetaminophen and liquid excipient base as a solubilizer.
Owner:AUROBINDO PHARMA LTD
Iron protein succinylate solubilizing method and oral solution preparation thereof
ActiveCN102671189AImprove solubilityLittle side effectsPeptide/protein ingredientsMetabolism disorderCyclodextrinSuccinic acid
The invention discloses an iron-protein succinylate solubilizing method and an oral solution preparation thereof, which are used for solving the problems of difficulty in dissolving iron-protein succinylate into water, unstable solution and the like. In the method, lauryl sodium sulfate or poloxamer or PEG-400 or beta-cyclodextrin is taken as a solubilizing solvent, and 1,2-propylene glycol and the like are taken as latent solvents, so that the water solubility of the iron protein succinylate are increased, and the free iron content is less than 0.1 percent. An iron protein succinylate oral solution prepared by using a process disclosed by the invention is accordant with relevant regulations in the Imported Medicine Registration Standard JX20000298 and oral liquid dosage form items in the second version of the Chinese Pharmacopeia, edition 2010.
Owner:NANJING TEFENG PHARMA +2
Drip-irrigation type salt stress-alleviating and disease-preventing biological bacterial fertilizer and preparation method thereof
InactiveCN103435395APromote germinationPromote growthFertilizer mixturesSodium BentonitePolyvinyl alcohol
The invention relates to a drip-irrigation type salt stress-alleviating and disease-preventing biological bacterial fertilizer and a preparation method thereof. The drip-irrigation type salt stress-relieving and disease-preventing biological bacterial fertilizer comprises nutrition carriers, additives, microelements and functional microorganisms, wherein the nutrition carriers comprise two or more of humic acid, glutamic acid, urea, corn flour, potassium sulphate, potassium chloride and diammonium hydrogen phosphate; the additives comprise two or more of bentonite, sodium carboxymethyl cellulose, sodium alginate an polyvinyl alcohol; the microelements comprise one or two of boric acid an ammonium molybdate; and the functional microorganisms is formed by combining salt stress-alleviating growth-promoting bacteria and disease-preventing growth-promoting bacteria, wherein the salt stress-alleviating growth-promoting bacteria is one or two selected from pseudomonas putida and azotobacter chroococcum; and the disease-preventing growth-promoting bacteria is one or two selected from bacillus subtilis and bacillus megatherium. The biological bacterial fertilizer is in a liquid dosage form, has relatively high effective viable count and relatively long quality guarantee period. The effective viable count of the biological bacterial fertilizer is higher than or equal to 10<10> cfu / mL; and total nutrient content of (N+P2O5+K2O) is higher than or equal to 12%.
Owner:SHIHEZI UNIVERSITY
Pinocembrin and cyclodextrin or cyclodextrin derivative inclusion compound
ActiveCN101537188ALess irritating to blood vesselsDisintegrates quicklyOrganic active ingredientsAntimycoticsSolubilityDisease
The invention discloses a pinocembrin and cyclodextrin or cyclodextrin derivative inclusion compound, wherein cyclodextrin or a cyclodextrin derivative is used to include the pinocembrin. The inclusion compound contains the pinocembrin and the cyclodextrin or cyclodextrin derivative in a molar ratio of 1:1-100. The inclusion compound can improve the water solubility of the pinocembrin and give good play of the therapeutic action of the pinocembrin. The inclusion compound is suitable for the preparation of sold dosage forms and liquid dosage forms required by clinics, including infusion solution, water injection, powder injection, oral solution, syrup, tablets, capsules, granules and dispersible tablets. The inclusion compound can be used for preparing drugs for preventing and\or treating cardiovascular and cerebrovascular diseases, particularly brain stroke, as well as drugs for preventing and\or treating bacterial and\or fungal infections.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Isoquercitrin clathrate and preparation thereof
InactiveCN101301477ASimple manufacturing processHigh inclusion rateOrganic active ingredientsPharmaceutical non-active ingredientsFood additiveSolubility
The present invention provides a clathrate compound formed by isoquercitrin and [beta]-cyclodextrin or derivatives thereof. The weight ratio of the isoquecitrin and the [beta]-cyclodextrin or the derivatives thereof is 1:2 to 20. The preparation method comprises the following steps of: dissolving the [beta]- cyclodextrin or the derivatives thereof into distilled water; putting the isoquercitrin into an organic dissolvant to dissolve; adding the isoquercitrin slowly into the water solution of the [beta]- cyclodextrin or the derivatives thereof, controlling the temperature and stirring; keeping stand, pumping-filtrating or directly condensing, vacuum drying, and obtaining the isoquercitrin clathrate compound. The solubility of the obtained isoquercitrin clathrate compound is significantly improved, and the isoquercitrin clathrate compound can be further developed into multiple solid dosage forms or liquid dosage forms which are suitable for medicines or food additives.
Owner:SHANXI UNIV +1
Oral Transmucosal Pharmaceutical Compositions Including Testosterone and a C-SERM
ActiveUS20160051564A1Improve the level ofRelieve symptomsOrganic active ingredientsBiocideSolid Dose FormMucoadhesion
Formulations for oral transmucosal compositions including a synergistic combination of low doses of testosterone with a clomiphene-like selective estrogen receptor modulator (C-SERM) that are combined with transmucosal absorption enhancers are disclosed. Oral transmucosal compositions can be for fast release or slow release, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Oral transmucosal compositions include liquid dosage forms, solid dosage forms, and chewing gums. Further dosage forms include mucoadhesive thin strips, thin films, tablets, patches, and tapes, among others. Other dosage forms are: mucoadhesive liquids such as gel-forming liquids; gel-forming semisolids; and gel-forming powders, among other dosage forms that exhibit mucoadhesive properties, and provide oral transmucosal delivery of testosterone and C-SERM. Oral transmucosal compositions will deliver testosterone and C-SERM directly into the patient's bloodstream, and provide high bioavailability of testosterone and C-SERM; therefore, the required doses are lower.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
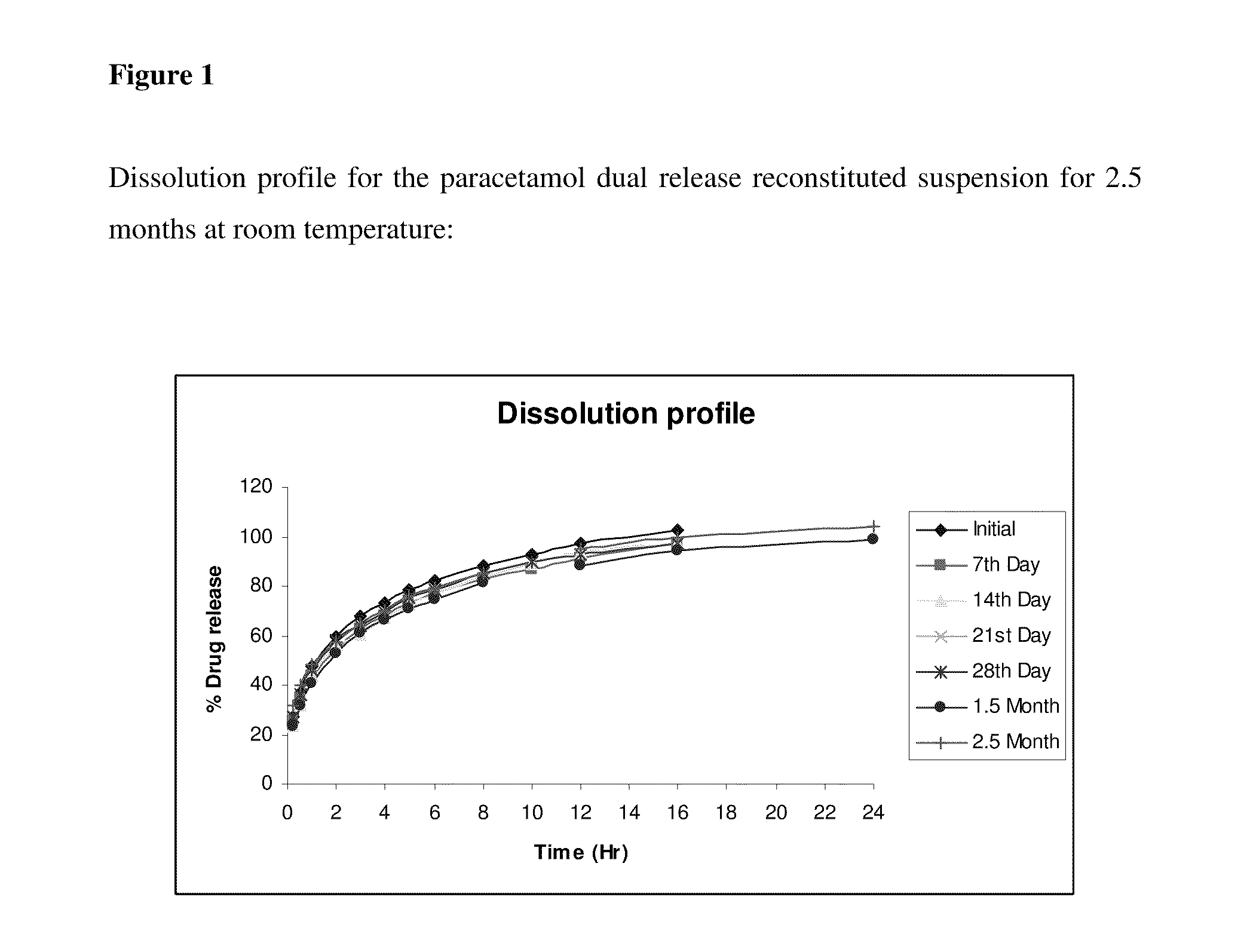
Dual-release pharmaceutical suspension
InactiveUS20110268808A1Stable dissolution profileExtended shelf lifeAntibacterial agentsBiocideDual releaseImmediate release
Orally deliverable dual-release pharmaceutical suspensions, having a first portion comprising an immediate release form of the active in the solution form or granules or suspended form in the vehicle / medium preferably in the solution form and a second portion comprising a sustained-release form of active in the form of microgranules / microparticles suspended in the immediate release fraction of the solulabilised active agent which comprise a core and at least one coat suitable for liquid dosage forms for the administration of the active ingredients, wherein the core comprises at least one active agent(s) or its pharmaceutically acceptable salts, derivatives, isomers, polymorphs, solvates, hydrates, analogues, enantiomers, tautomeric forms or mixtures thereof; optionally at least one water insoluble, and optionally one or more pharmaceutically acceptable excipient(s); and at least one coat comprising at least one pH independent water-insoluble polymer(s) along with one or more pharmaceutically acceptable excipient(s). This coated microparticles and solution of the active agent in the vehicle ensures a dual release profile i.e. immediate release profile as well as predetermined sustained release profile of the active agent and also ensures maintenance of said release profile over time. The present invention can be administered either in the form of ready to use suspension or in the form of powder ready for reconstitution. Further, this invention provides process of preparation of such suspensions and method of using them.
Owner:PANACEA BIOTEC
Liquid dosage forms of isotretinoin
Owner:SUN PHARMA INDS
Fertilizer synergistic additive and preparation thereof
InactiveCN105585371AIncrease the effective concentrationControl formFertilizer mixturesThioureaSorbitan
The invention relates to technology of synergy for fertilizers, and more specifically relates to a fertilizer synergistic additive and a preparation thereof. The solid dosage form synergistic additive comprises the following raw materials in percentage by weight: 55-93.5% of fermentation broth of polyglutamic acid, 0.5-3% of thiourea, 0.5-9% of N-(n-butyl)thiophosphoric triamide, 5-30% of dicyandiamide, and 48-91% of diatomite. Or the liquid dosage form synergistic additive comprises the following raw materials in percentage by weight: 55-93.5% of fermentation broth of polyglutamic acid, 0.5-3% of thiourea, 0.5-9% of N-(n-butyl)thiophosphoric triamide, 5-30% of dicyandiamide, and 0.5-3% of an emulsifier. The fermentation broth contains 3-10% of polyglutamic acid, and the emulsifier is polyoxyethylene sorbitan fatty acid ester. The fertilizer synergistic additive can increase effective concentration of fertilizer nutrients, prolong effective period of the fertilizer, promote crops to absorb and transport nutrients, overcome the problem of insufficient release of the long-acting slow-release fertilizer nutrients at early stage, guarantee efficiency of fertilizer at later period, the validity period of fertilizer nutrients is longer than 120 days, so that long-acting and high-efficiency utilization of fertilizer nutrients are realized.
Owner:辽宁中科生物工程股份有限公司
Medicine for silkworm and its preparation method
InactiveCN1353933AGreat efficacyNo pollution in the processBiocideAnimal repellantsBiotechnologyDisease
The present invention discloses a silkworm medicine for preventing and curing commonly encountered diseases of silkworm and its preparation method. Said invented medicine preparatino contains (by weight portion) 6-8 portions of carbendazim powder and 2-4 portions of thiophanate-methyl powder. The above-menioned materials can be made into powder dosage form. Also, they can be respectively dissolved in dilute acetic acid and ethyl alcohol so as to obtain liquid dosage form. Said invented medicine is low in toxicity, harmless and pollutionless, and possesses the unique killing action for curing white muscardine, grasserie and others.
Owner:唐永琦
Novel formulations and uses for curcuma extracts
InactiveUS20140056828A1High activityNegligible effectCosmetic preparationsBiocideSunscreen agentsCurcuma longa extract
Formulations and uses of extracts of Curcuma longa L. plants for safe use topically, orally, rectally, or vaginally, for example, are provided. A sunscreen containing an extract of Curcuma longa L. is provided, the sunscreen having an absorption that spans the UVA and UVB ranges in a manner that meets updated FDA recommendations without requiring the addition of titanium dioxide. A process of producing the extract is also provided, using an extraction solvent that is at least substantially non-toxic and useful also as a pharmaceutically acceptable carrier in liquid dosage forms. The extraction process also produces a significantly higher yield from a single extraction than the state-of-the-art. Liquid dosage forms can be produced directly from the extraction process without requiring removal of the extraction solvent, reducing complexity and cost of processing over the state-of-the-art. Microemulsions and nanoemulsions are also provided to enhance the bioavailability and stability of the extracts.
Owner:CALIFORNIA NORTHSTATE COLLEGE OF PHARMACY
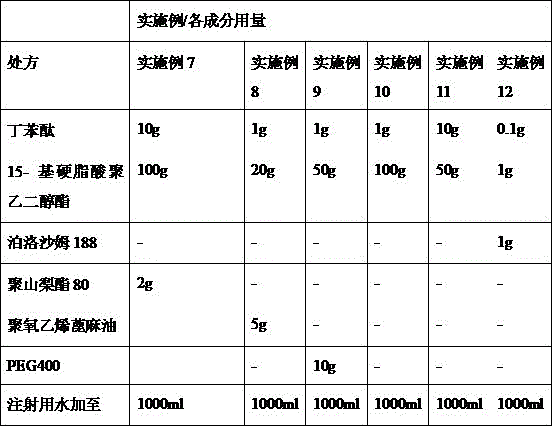
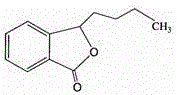
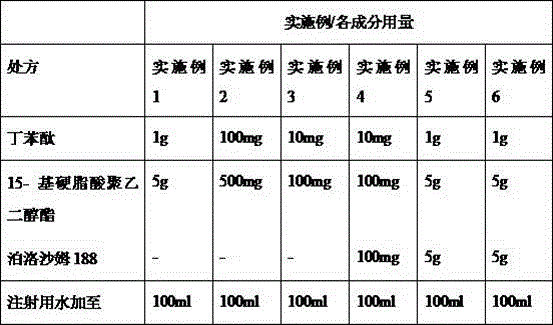
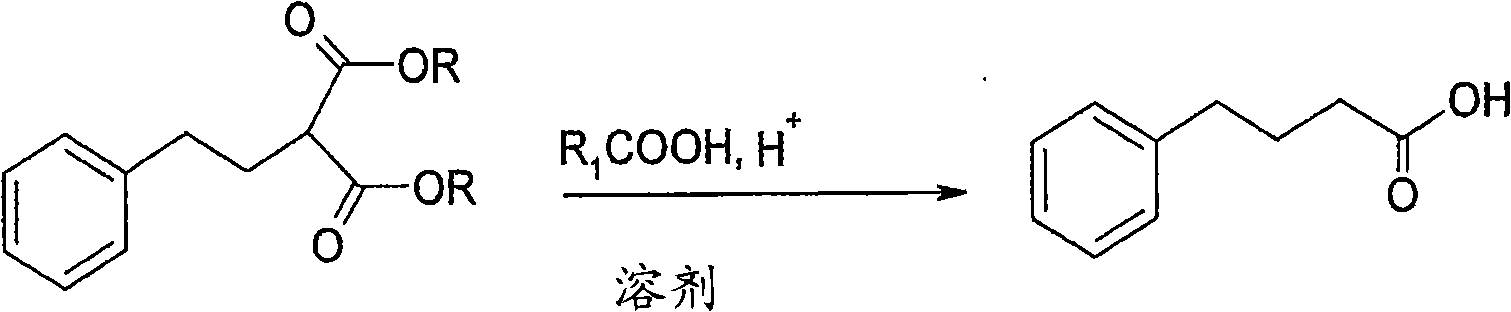
Pharmaceutical composition containing butylphthalide and novel solubilizer
InactiveCN105688220AImprove securityLow hemolytic activityOrganic active ingredientsPowder deliverySolubilityButylphthalide
The invention relates to the field of medicine, in particular to a pharmaceutical composition containing butylphthalide and a novel solubilizer to improve the water solubility of butylphthalide by means of the novel solubilizer, clinically required solid form, or semisolid form or liquid form of butylphthalide is developed, so that the treatment effect of butylphthalide can be better realized. The composition can be used for preparing various drug forms such as tablets, capsules, particles, powder, ointment, cream, gel, infusion, squirt cut, powder filling and oral liquid. Compared with the prior art, the pharmaceutical composition is better in safety performance and water solubility of butylphthalide.
Owner:SICHUAN MANSAISI MEDICINE TECH CO LTD
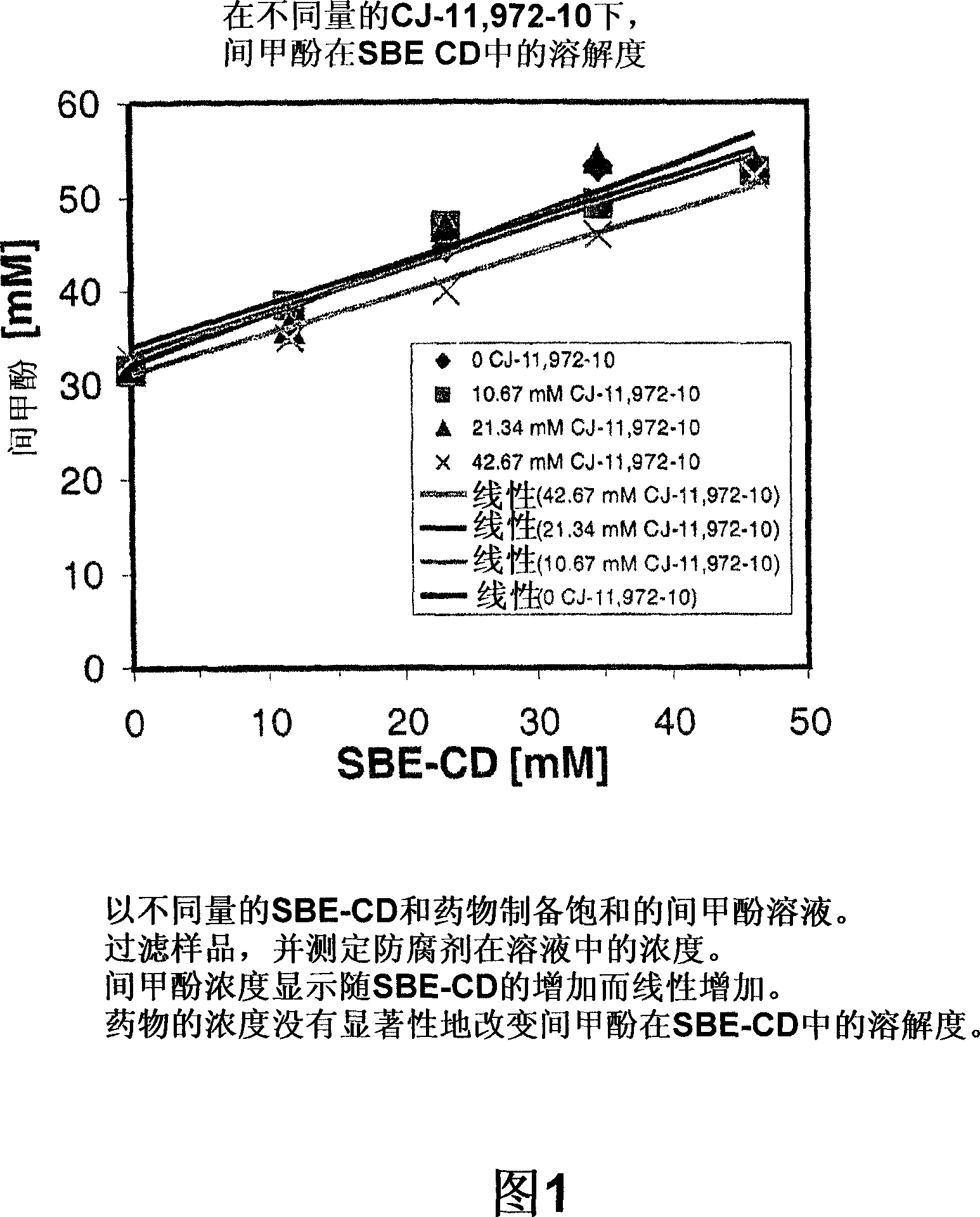
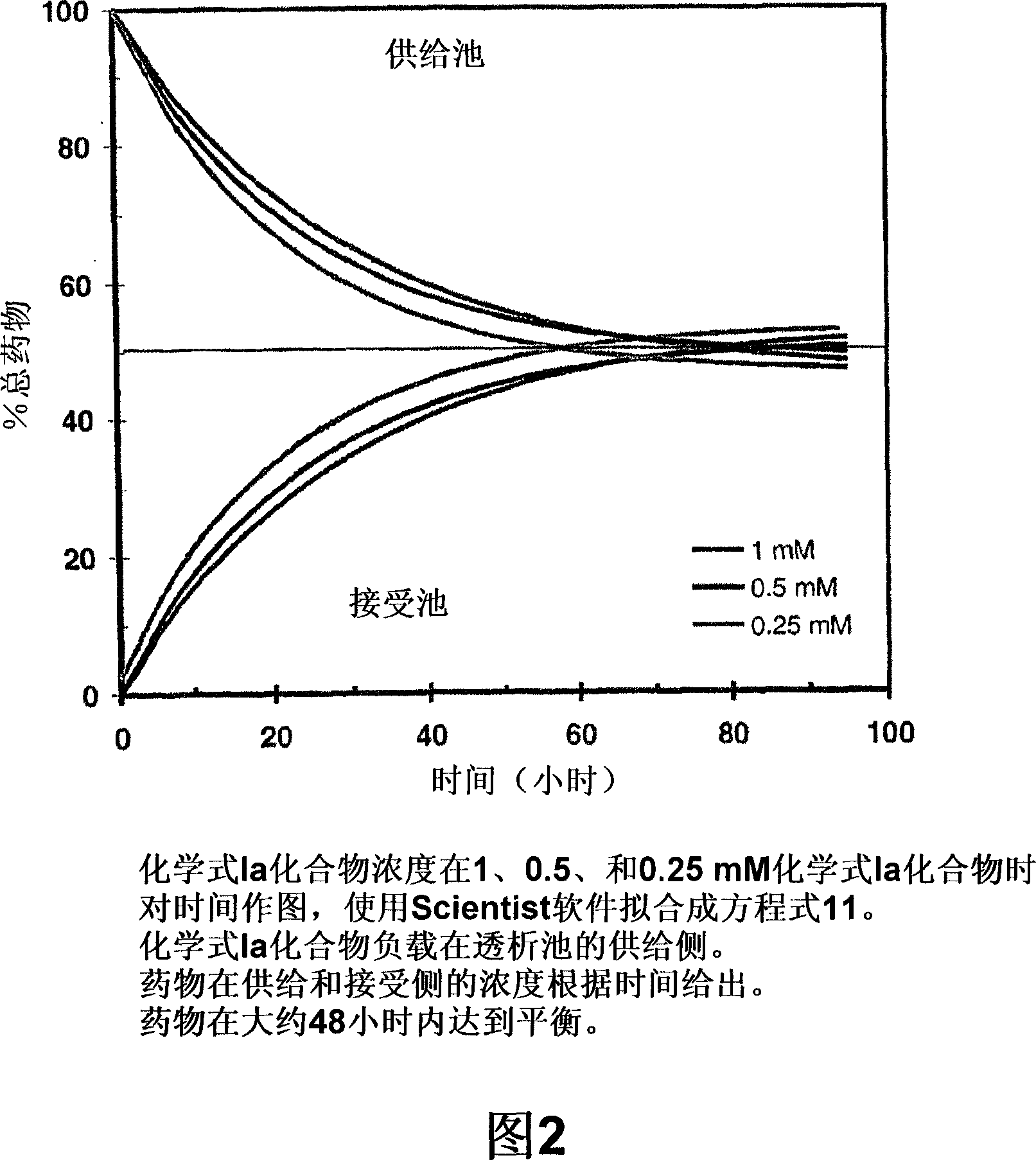
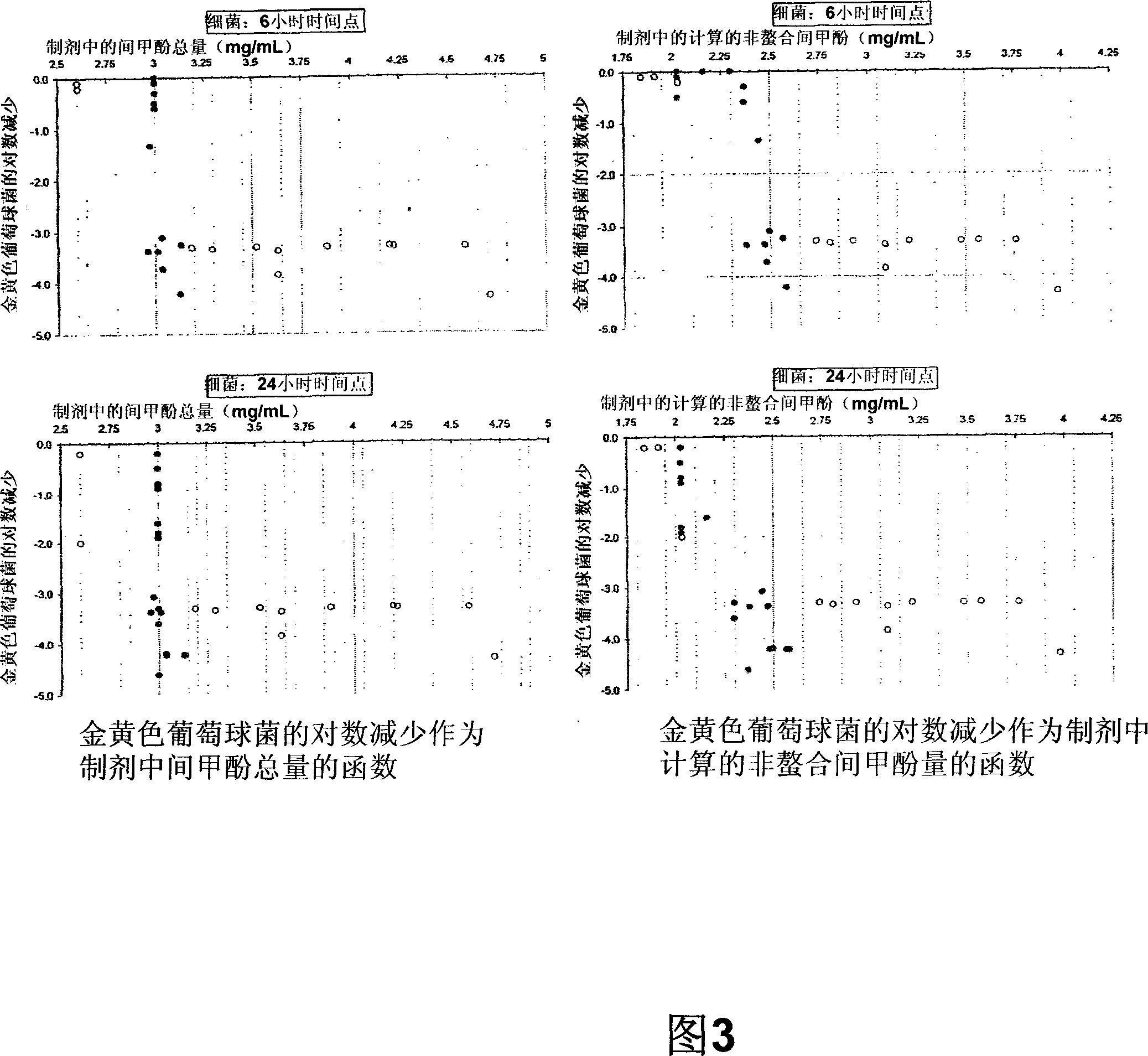
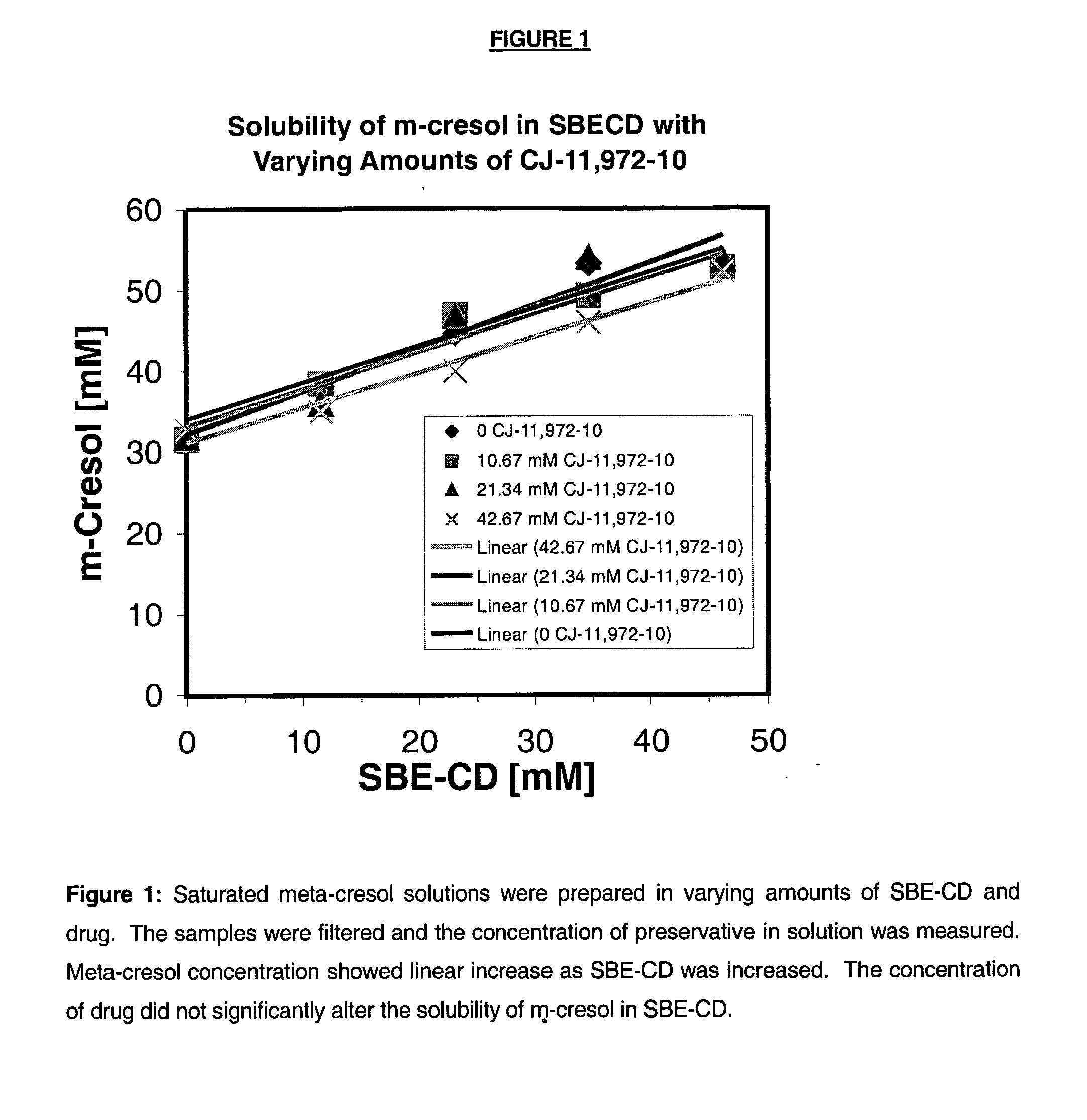
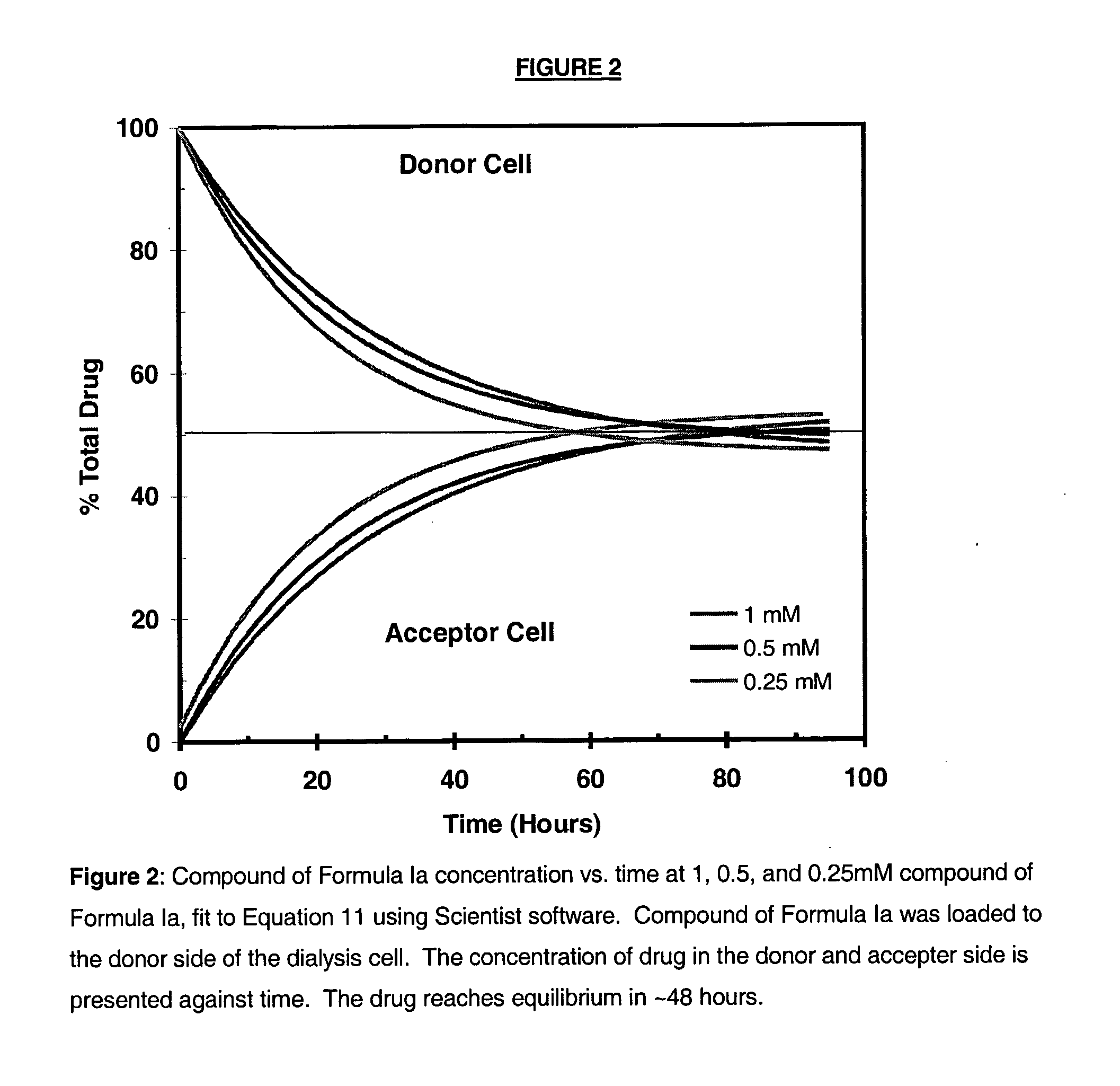
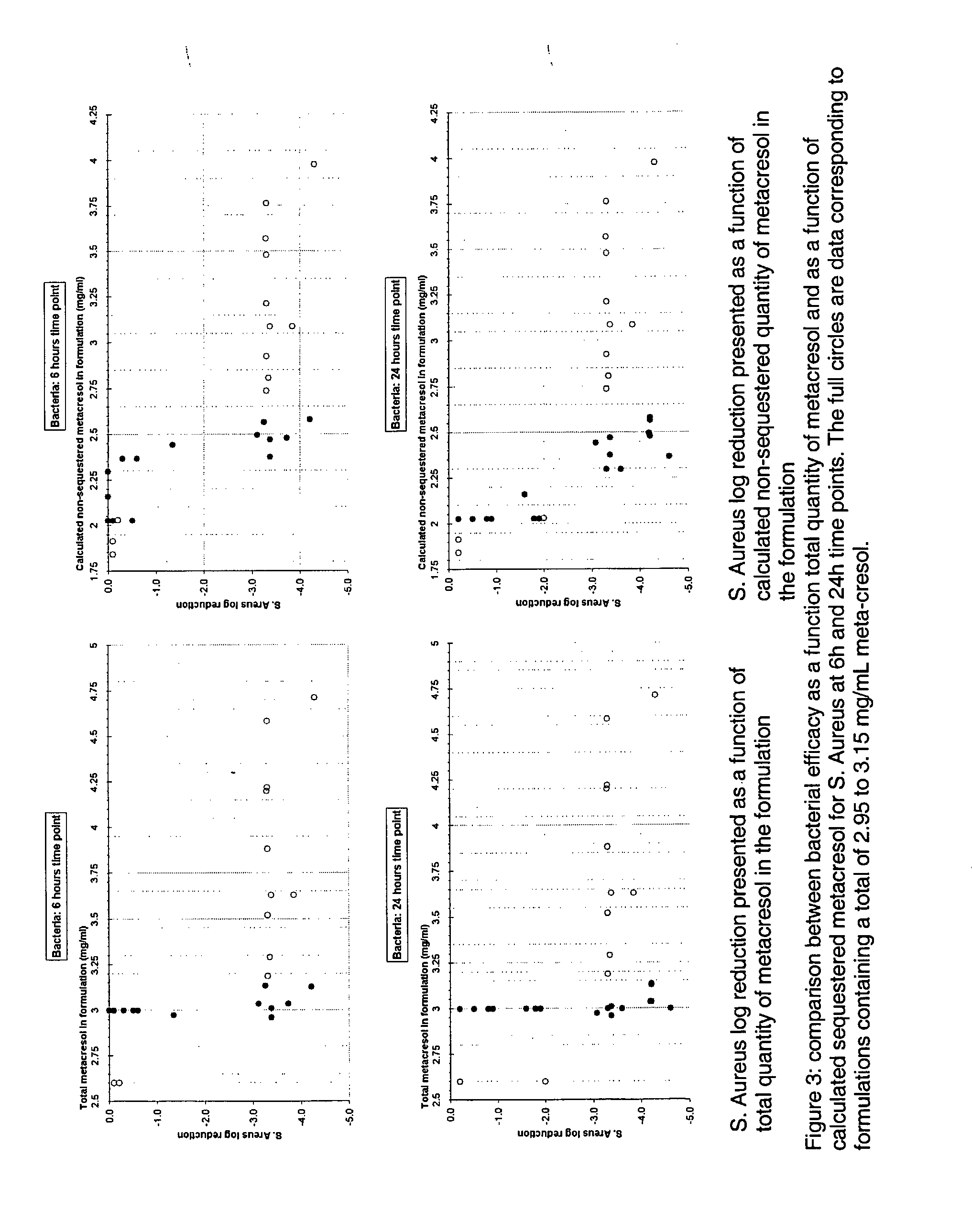
Antimicrobial preservatives to achieve multi-dose formulation using beta-cyclodextrins for liquid dosage forms
The present invention is directed to pharmaceutical compositions containing a therapeutically effective amount of an Active Pharmaceutical Ingredient (''API''), a pharmaceutically acceptable cyclodextrin and a pharmaceutically acceptable preservative. The invention is also directed to pharmaceutical compositions of the compounds of Formula (I) wherein R<2> is selected from the group consisting of methyl, ethyl, isopropyl, sec-butyl and tert-butyl and a pharmaceutically acceptable cyclodextrin and preservative. Formula (I): In particular, the invention is directed to pharmaceutical compositions of the compound of Formula la, and a pharmaceutically acceptable cyclodextrin and a preservative.
Owner:PFIZER PROD INC
Evening primrose oil liquid capsule capable reducing blood sugar, blood fat and weight and preparation method thereof
InactiveCN101336943AFast absorptionFully absorbedMetabolism disorderCapsule deliverySide effectHard Capsule
The invention relates to an evening primrose oil liquid capsule for reducing blood sugar, blood lipid and body weight, and a preparation method thereof. The inventive capsule has the the following advantages that: (1) the evening primrose oil which is in a transparent liquid state is used as a principal drug component and filled in hard capsules, so that the principal drug component is administered in form of a solid dosage form and absorbed in form of a liquid dosage form, thereby improving bioavailability; (2) a proper antioxidant such as vitamin E, lecithin, propyl gallate, etc. is added to ensure the stability of the principal drug component; (3) the mouthfeel is remarkably improved, so as to improve compliance of patients; (4) the content of the capsule is liquid which has fast absorption rate and short stomach retention time, so as to reduce the occurrence of adverse effects; (5) the production process is simple and the production cycle is short, so as to reduce cost; and (6) the inventive capsule has transparent liquid content, stable properties, esthetic appearance, good patient compliance, and wide market of applications.
Owner:TIANJIN DANXI TCM INST
Process for preparation of liquid dosage form containing sodium 4-phenylbutyrate
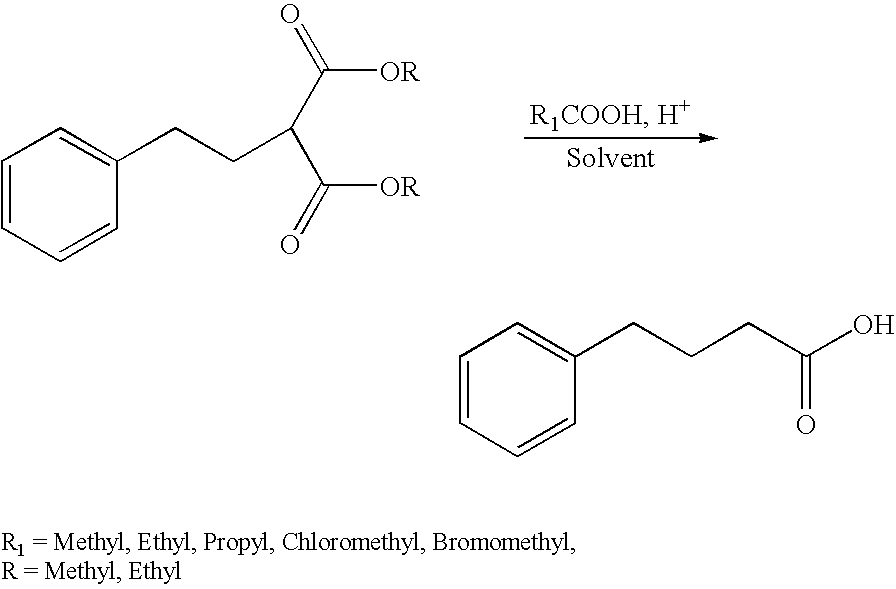
InactiveUS20070004805A1Easy to manageMore palatableBiocideDispersion deliveryPHENYLBUTYRIC ACIDChemistry
A process for preparing a stable aqueous dosage form of sodium 4-phenylbutyrate, including such dosage forms in a highly concentrated solution, as well as methods for making 4-phenylbutyrate and 4-phenylbutyric acid, and for using 4-phenylbutyrate. The stable aqueous dosage forms do not freeze at 0° C.
Owner:NAVINTA
Antimicrobial preservatives to achieve multi-dose formulation using beta-cyclodextrins for liquid dosage forms
ActiveUS20070155697A1Reduced binding valueEffective antimicrobial effectivenessAntibacterial agentsBiocidePreservativeAdditive ingredient
The present invention is directed to pharmaceutical compositions containing a therapeutically effective amount of an Active Pharmaceutical Ingredient (“API”), a pharmaceutically acceptable cyclodextrin and a pharmaceutically acceptable preservative. The invention is also directed to pharmaceutical compositions of the compounds of Formula (I) wherein R2 is selected from the group consisting of methyl, ethyl, isopropyl, sec-butyl and tert-butyl and a pharmaceutically acceptable cyclodextrin and preservative. Formula (I): In particular, the invention is directed to pharmaceutical compositions of the compound of Formula 1a, and a pharmaceutically acceptable cyclodextrin and a preservative.
Owner:ZOETIS SERVICE LLC
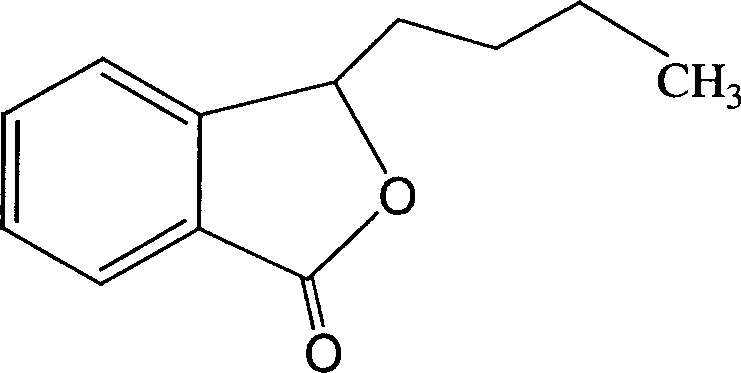
Butylbenzene phthalein cyclodextrin or cyclodextrin derivative clathrate, its preparation method and application
The present invention relates to butylbenzene phthalein cyclodextrin or cyclodextrin derivative clathrate, its preparation method and application. The described butylbenzene phthalein is mixed-rotatory butylbenzene phthalein and levo-butylbenzene phthalein. In order to raise water solubility of butylbenzene phthalein and develop solid dosage form and liquid dosage form required for clinical application said invention adopts cyclodextrin or cyclodextrin derivative to include the butylbenzene phthalein. Said clatherate contains butylbenzene phthalein and cyclodextrin or cyclodextrin derivative, the mole ratio of both them is 1:1-10. The cyclodextrin or cyclodextrin derivative optimally selects hydroxypropyl-beta-cyclodextrin. Said clathrate can be used for preparing various dosage forms of injection, oral preparation, tablet, capsule and others.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Cadotril particles
InactiveUS20160120834A1Avoid incompatibilityReduce degradationBiocidePowder deliveryMedicineLiquid Dosage Form
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com