Antimicrobial preservatives to achieve multi-dose formulation using beta-cyclodextrins for liquid dosage forms
一种环糊精、防腐剂的技术,应用在环糊精和防腐剂的药物组合物,开发防腐的API组合物领域,能够解决抗菌效力丧失等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
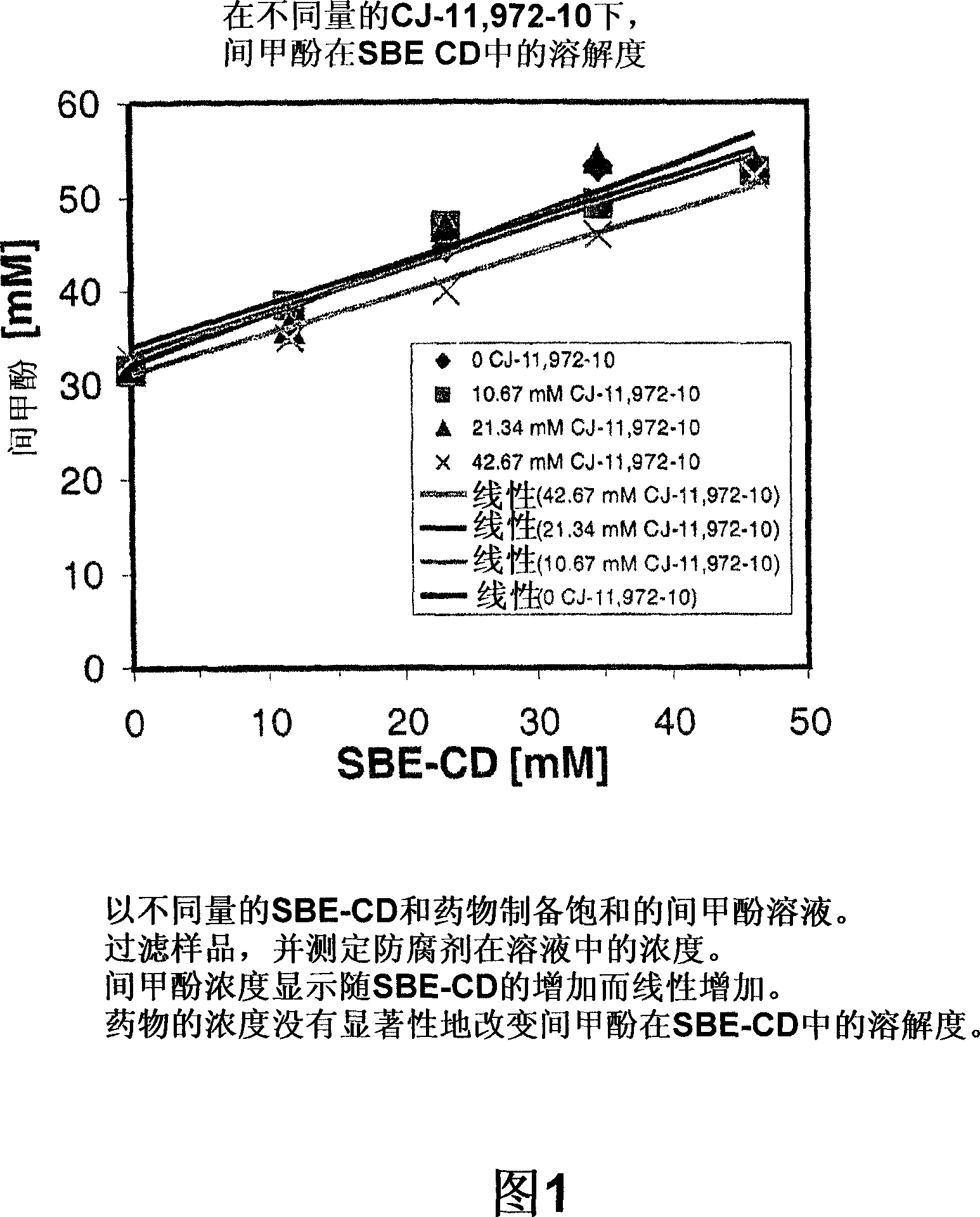
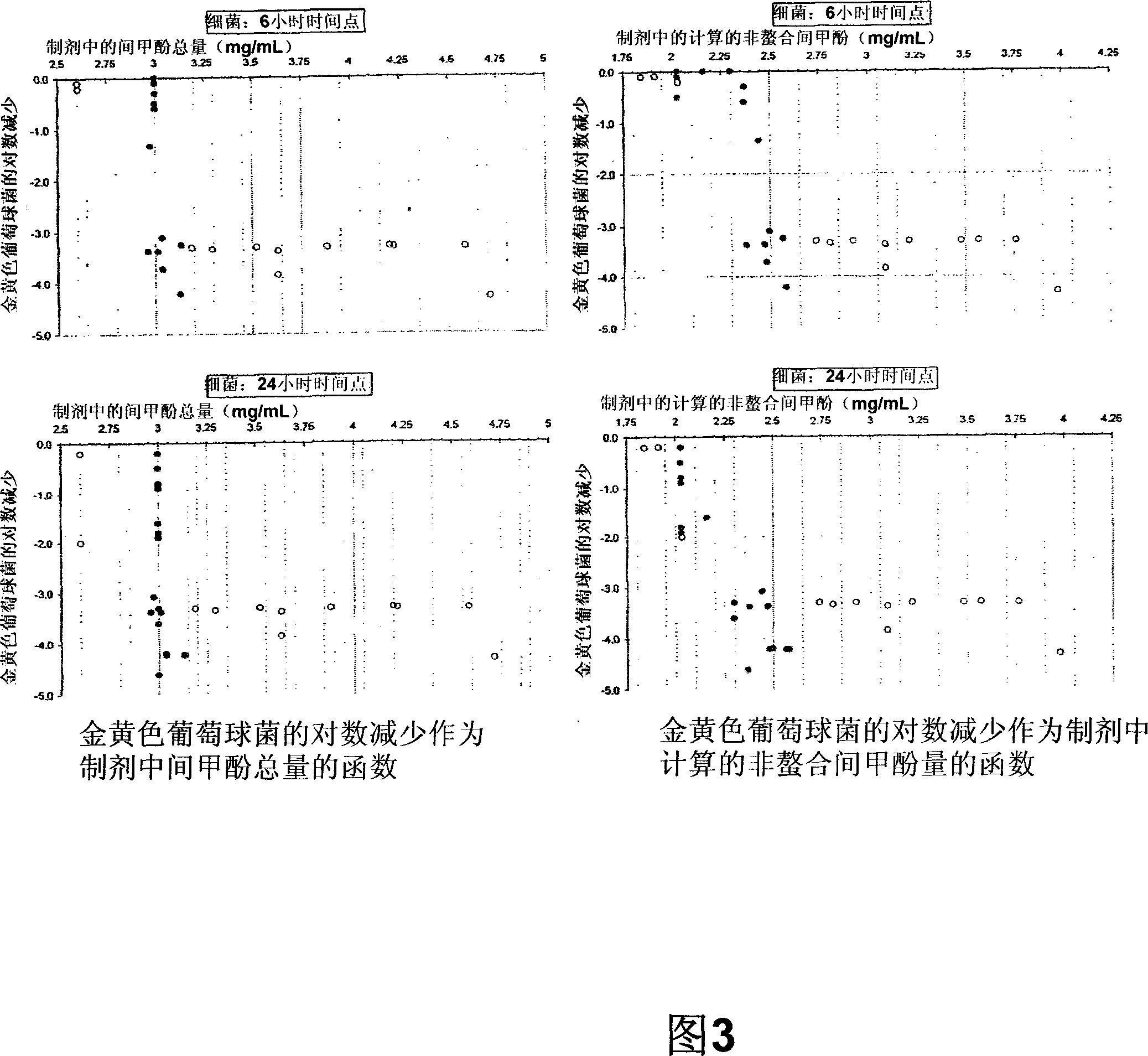
[0147] preparation of formulations . Three different assay formulations were prepared, consisting of a single component control; a binary system containing drug or m-cresol, and SBE-CD; or a ternary system containing drug, m-cresol, and SBE-CD. Formulations were prepared in various ratios and concentrations at room temperature 24 hours prior to assay to confirm equilibrium binding. Formulations were prepared by first dissolving SBE-CD at the appropriate concentration, then adding the drug or m-cresol and dissolving it in the cyclodextrin solution.
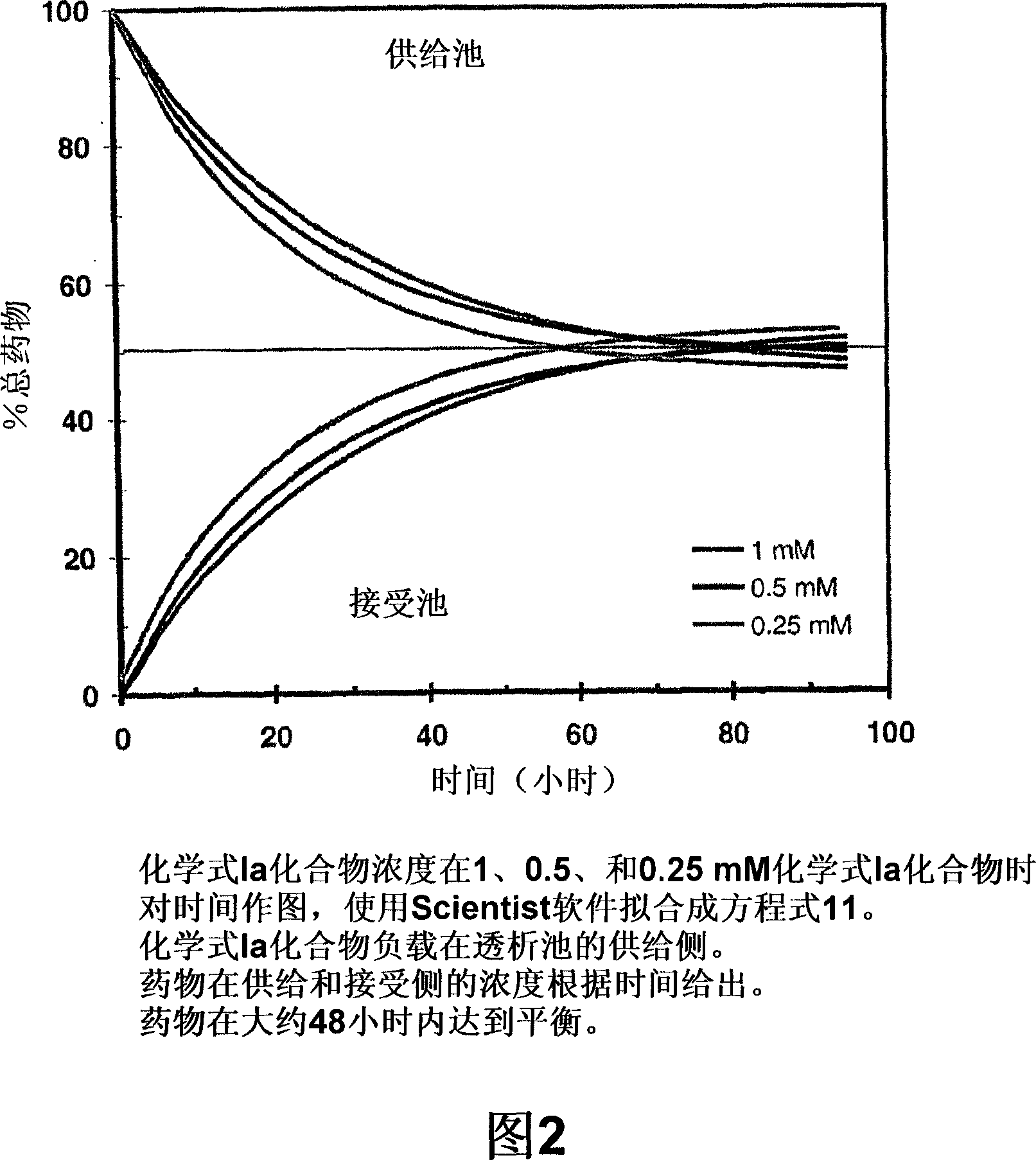
[0148] Dialysis .1 mL of compound or control formulation was loaded on the supply side of the membrane. The receiving side was loaded with 1 mL of sodium citrate (pH 4.4) to maintain ion balance across the chamber. At various time points, 50 [mu]L aliquots were withdrawn from both the donor and recipient sides of the equilibrated dialysis chamber and analyzed using HPLC. Concentration (mM) data for each side ligand at differe...
Embodiment approach
[0211] A. A pharmaceutical composition comprising a therapeutically effective amount of an active pharmaceutical ingredient, β-cyclodextrin, a pharmaceutically acceptable preservative, a pharmaceutically acceptable carrier, and an optional pharmaceutically acceptable excipient, wherein the The preservatives described above demonstrate pharmaceutically acceptable antimicrobial preservative efficacy.
[0212] B. The pharmaceutical composition according to preferred embodiment A, wherein said β-cyclodextrin is 2-hydroxypropyl-β-cyclodextrin or sulfobutyl ether-β-cyclodextrin.
[0213] C. The pharmaceutical composition according to preferred embodiment B, wherein the preservative is selected from the group consisting of sodium ethylmercury thiosalicylate, propylene glycol, phenol, or m-cresol, or a combination thereof.
[0214] D. The pharmaceutical composition according to preferred embodiment B or C, wherein the binding value of the preservative to the cyclodextrin is less than ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com