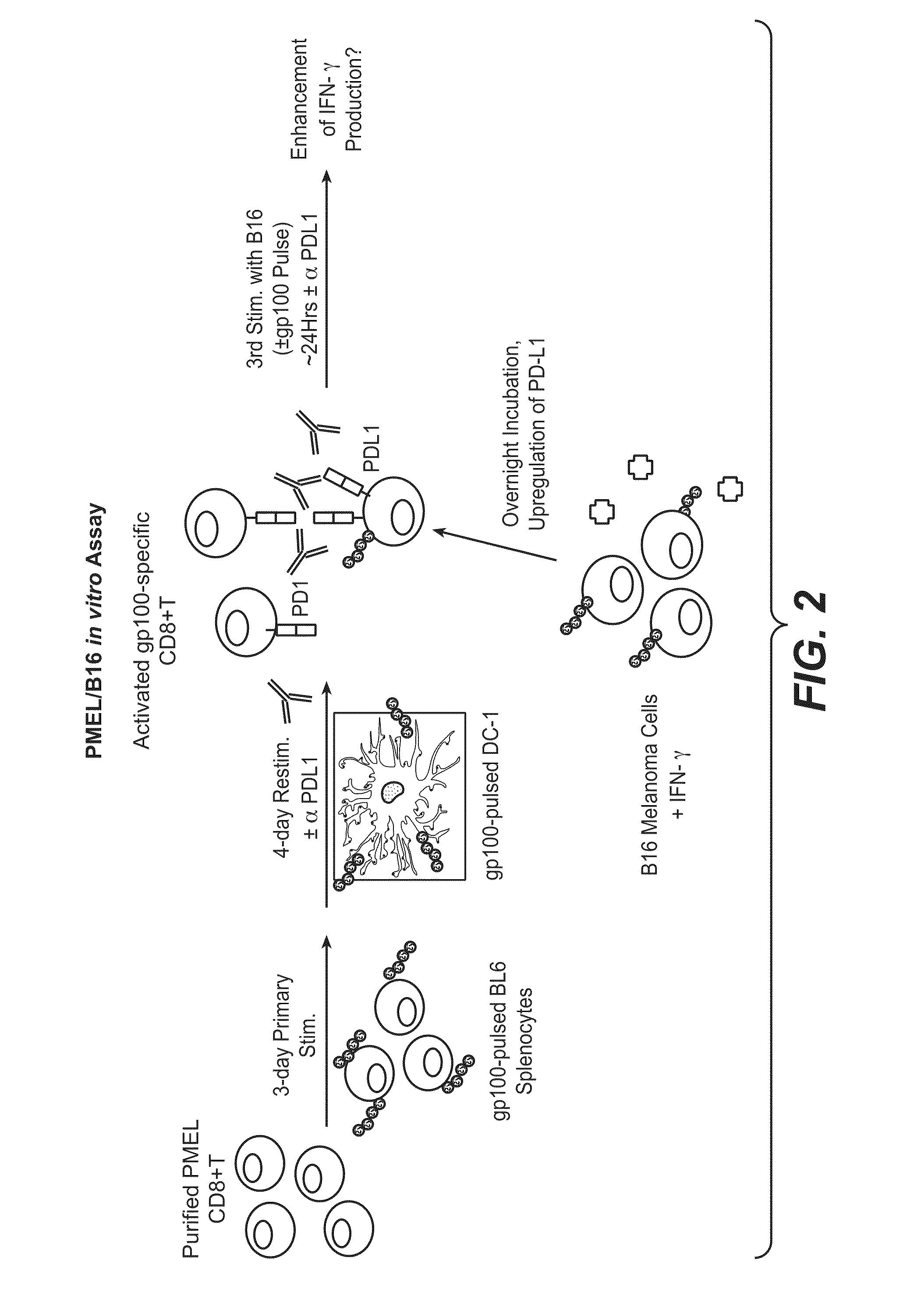
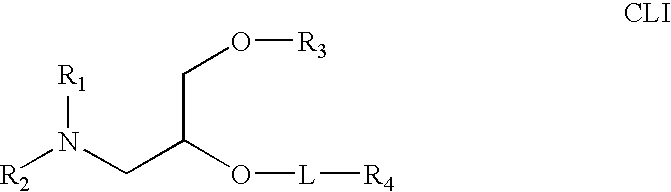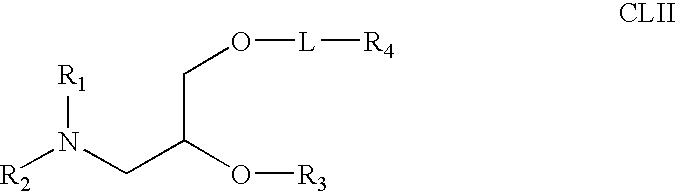Patents
Literature
16241results about "Antimycotics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anti-PD-L1 antibodies, compositions and articles of manufacture
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
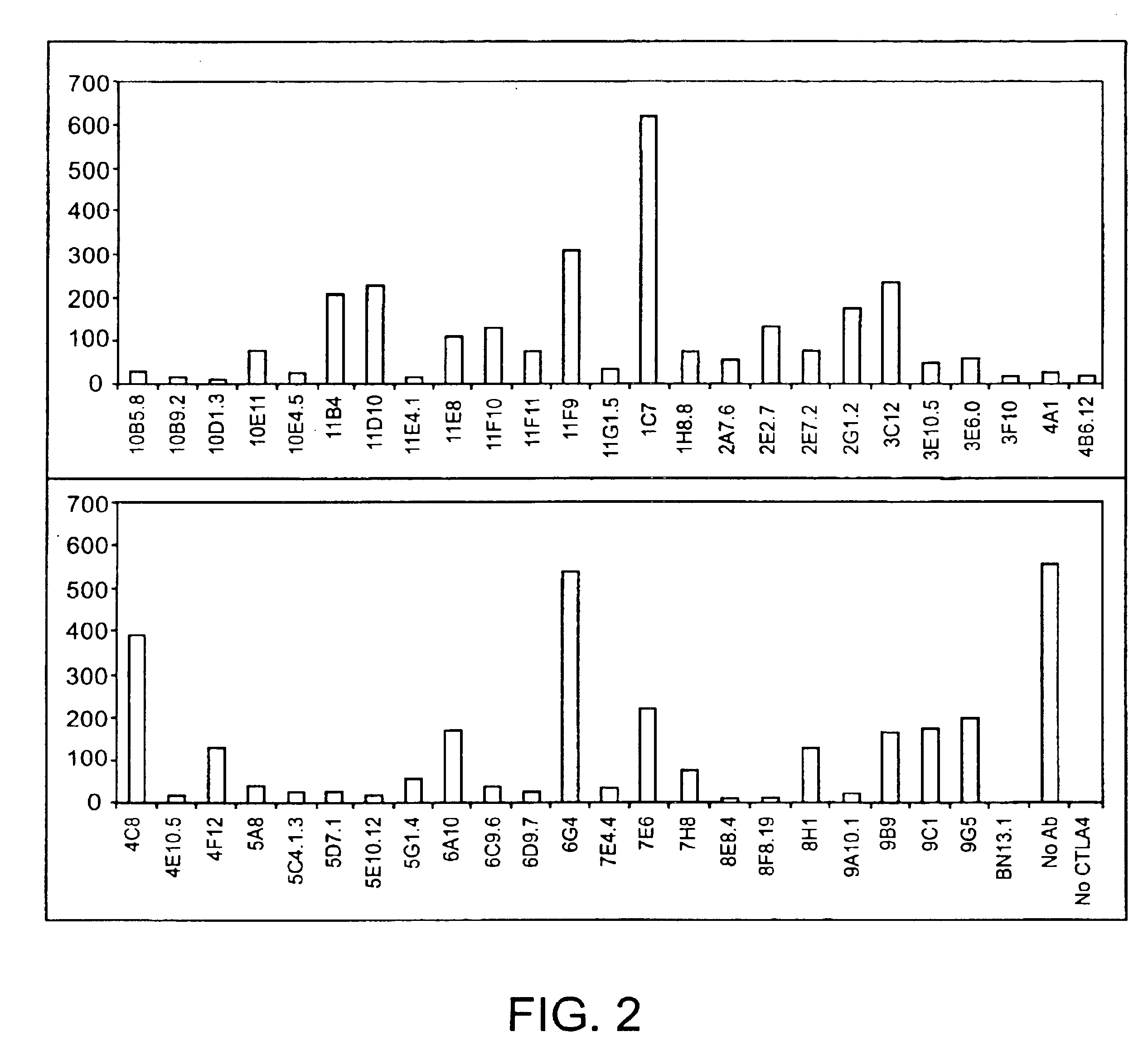
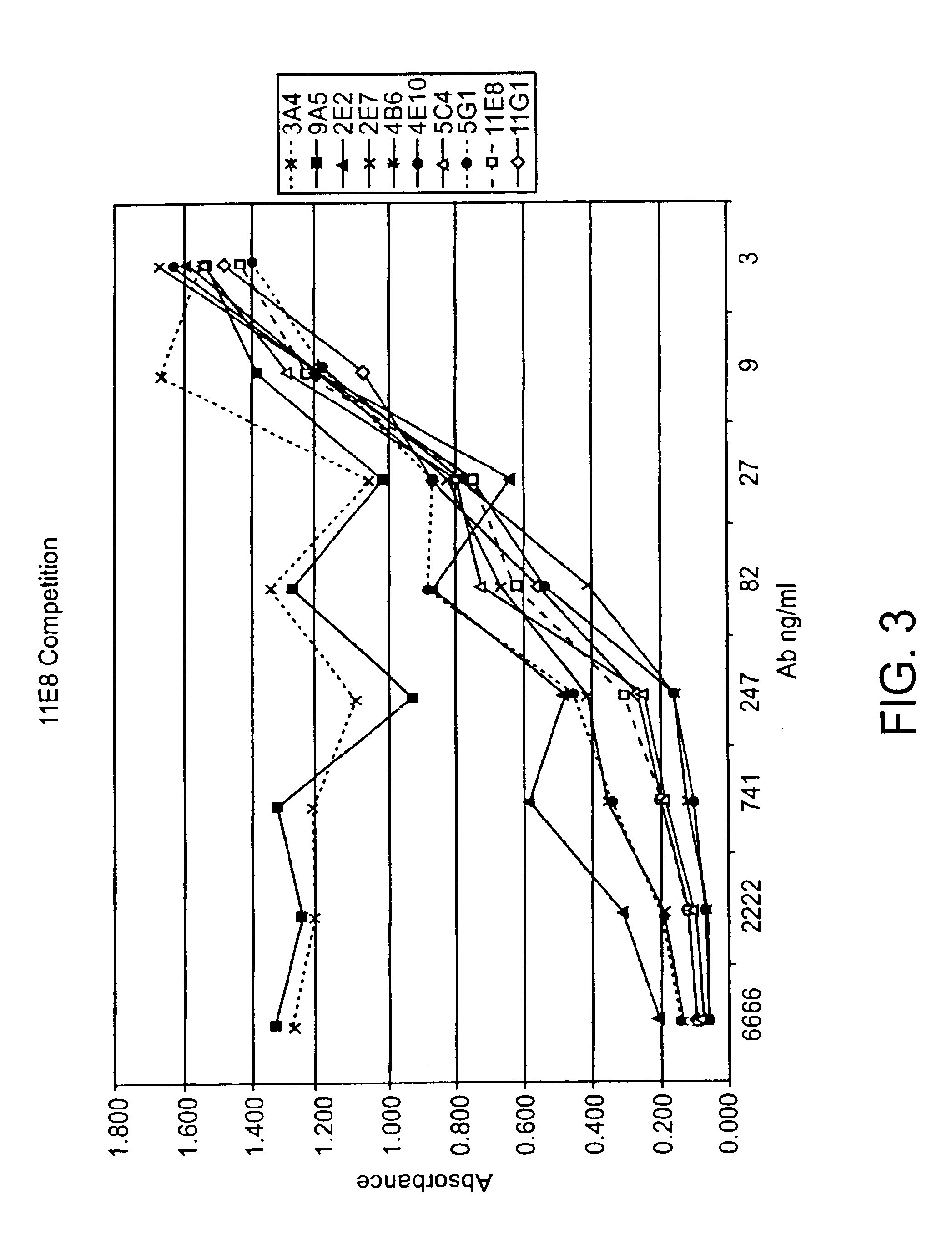
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
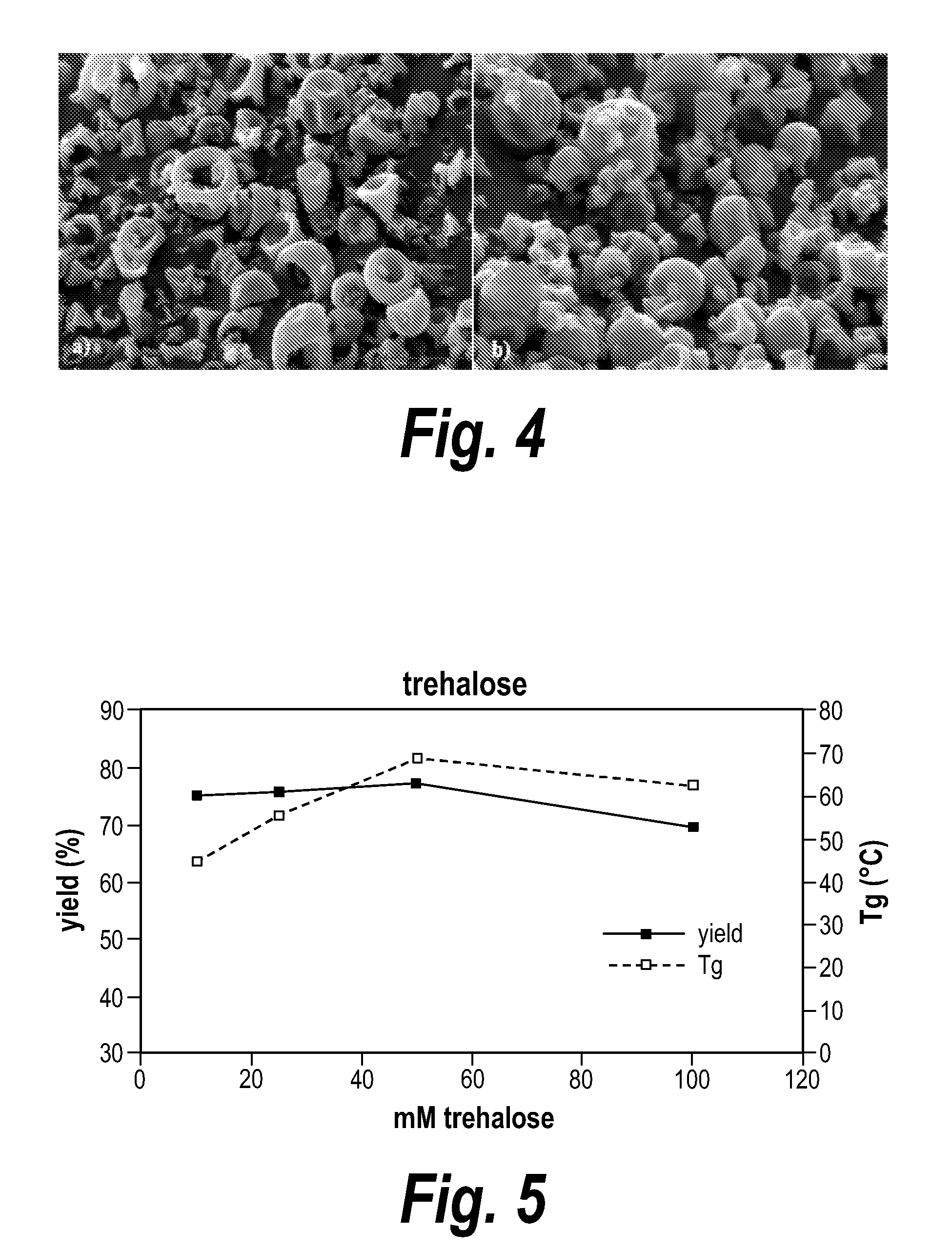
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114649A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsT cellInfective disorder
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
PD-1 Antibodies and PD-L1 Antibodies and Uses Thereof
ActiveUS20120039906A1Reduced activityStrong cytotoxicityAntibacterial agentsAnimal cellsPD-L1Antibody
Owner:INST JEAN PAOLI & IRENE CALMETTES +2
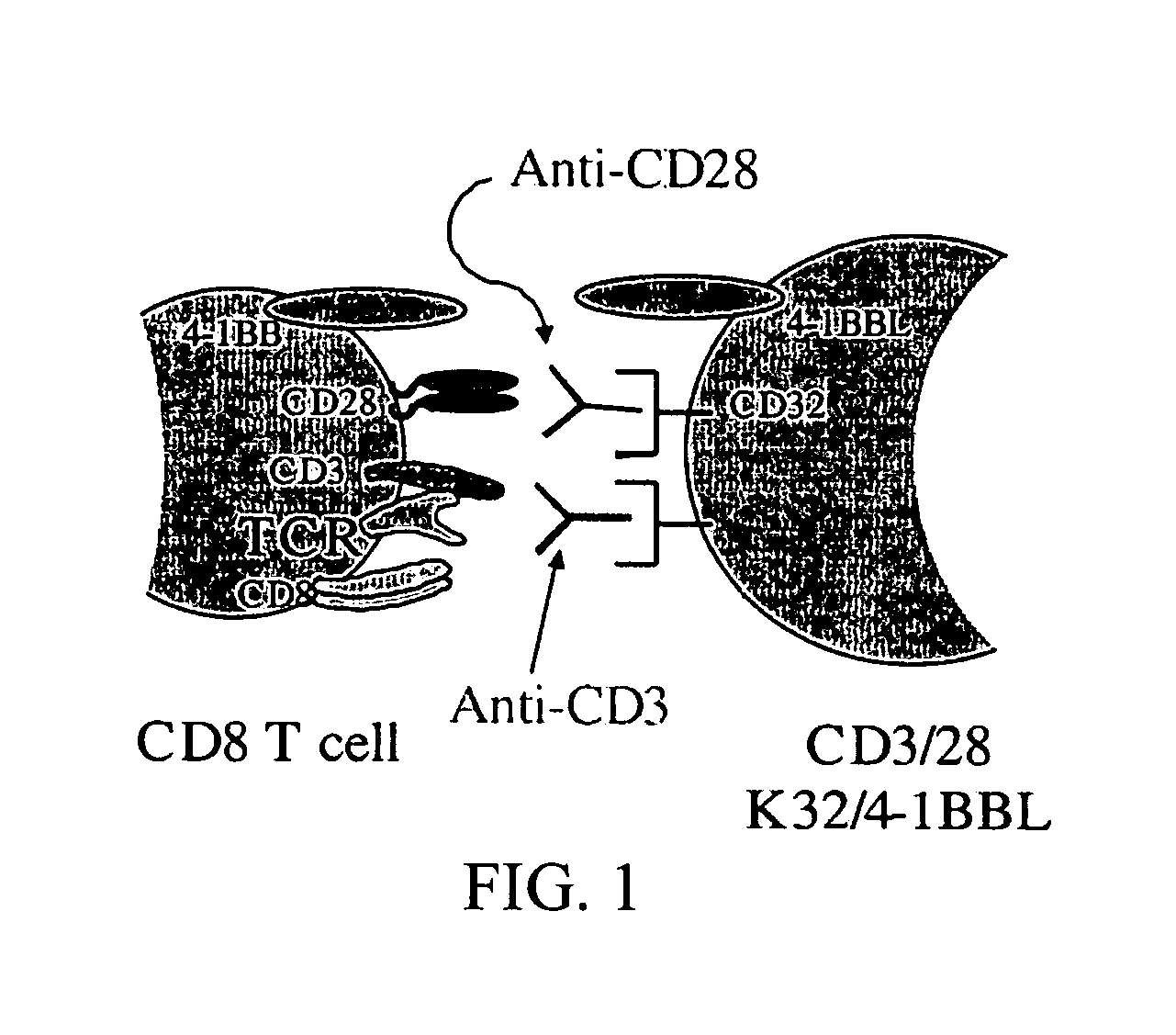
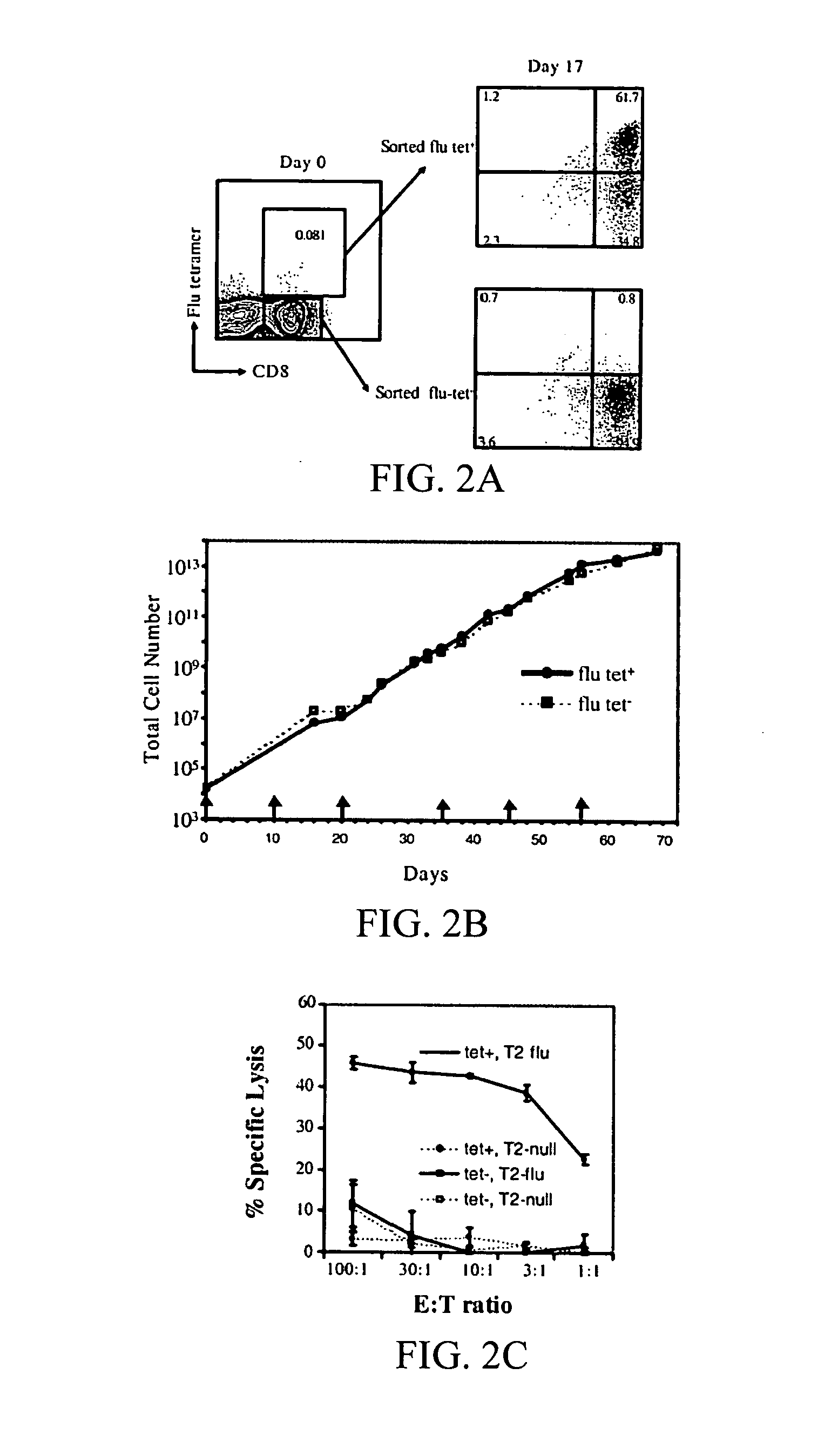
Novel artificial antigen presenting cells and uses therefor
The invention relates to novel artificial antigen presenting cells (aAPCs). The aAPC comprises at least one stimulatory ligand and at least one co-stimulatory ligand where the ligands each specifically bind with a cognate molecule on a T cell of interest, thereby mediating expansion of the T cell. The aAPC of the invention can further comprise additional molecules useful for expanding a T cell of interest. The aAPC of the invention can be used as an “off the shelf” APC that can be readily designed to expand a T cell of interest. Also, the aAPC of the invention can be used identify the stimulatory, co-stimulatory, and any other factors that mediate growth and expansion of a T cell of interest. Thus, the present invention provides powerful tools for development of novel therapeutics where activation and expansion of a T cell can provide a benefit.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Polyvalent protein complex
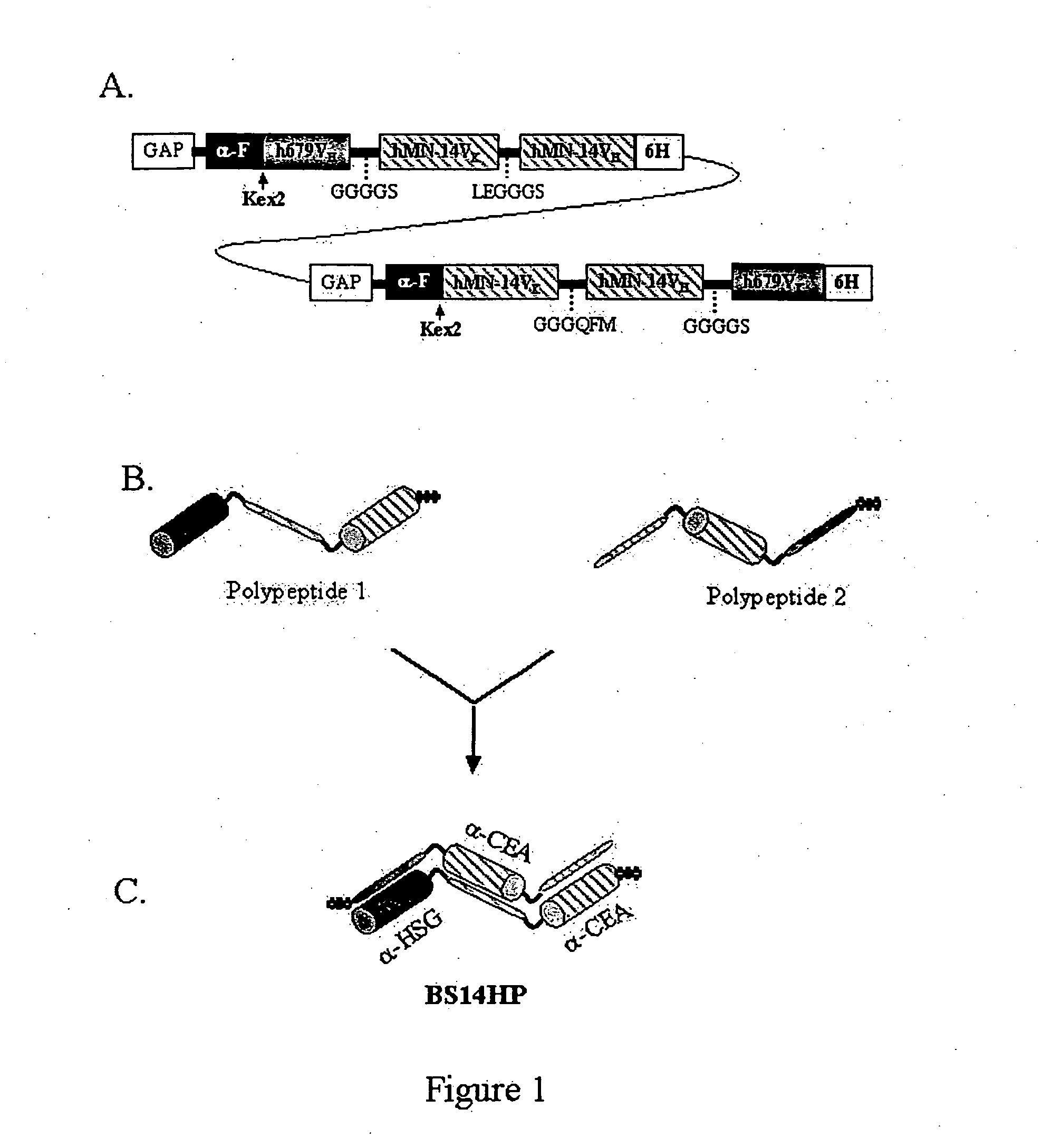
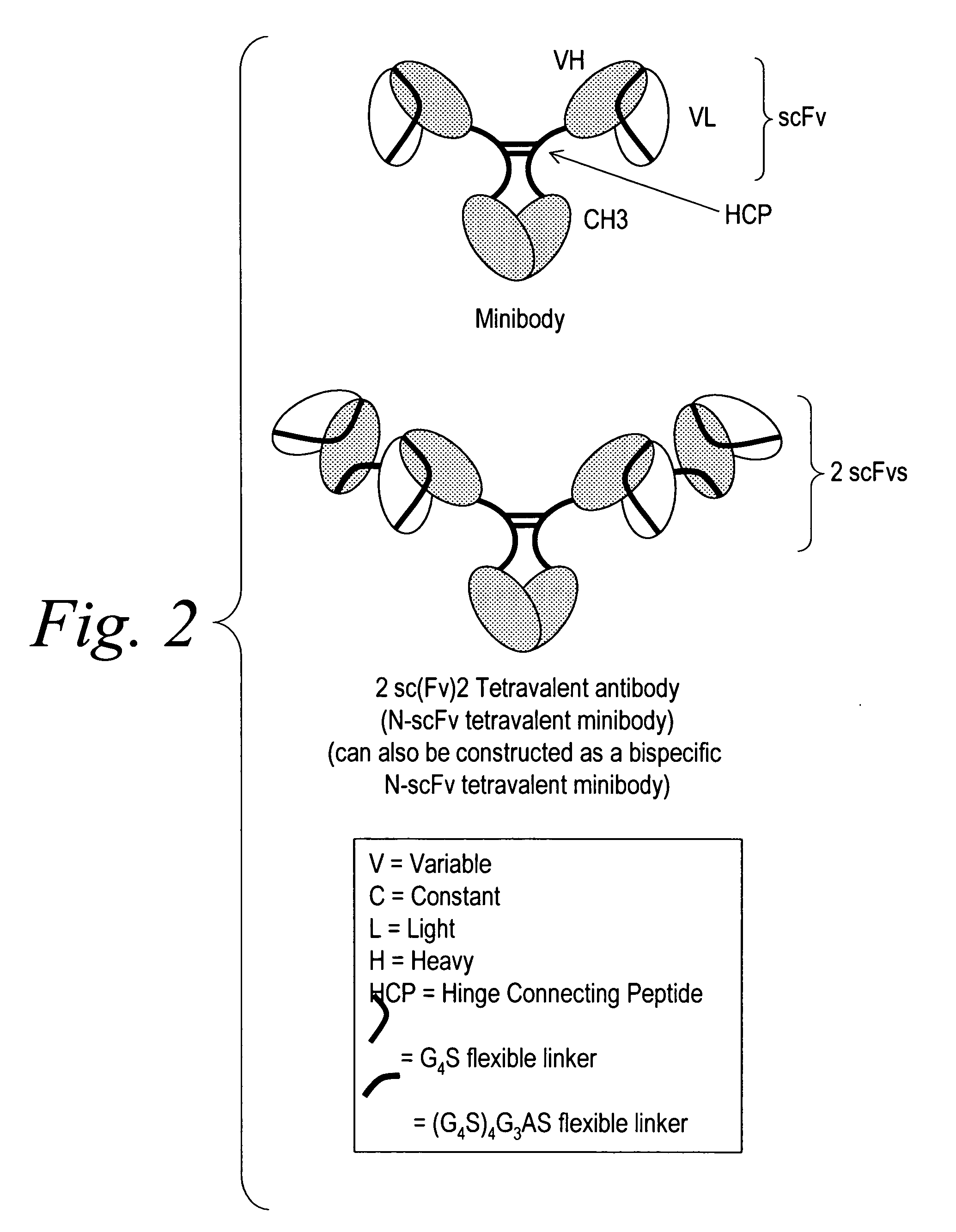
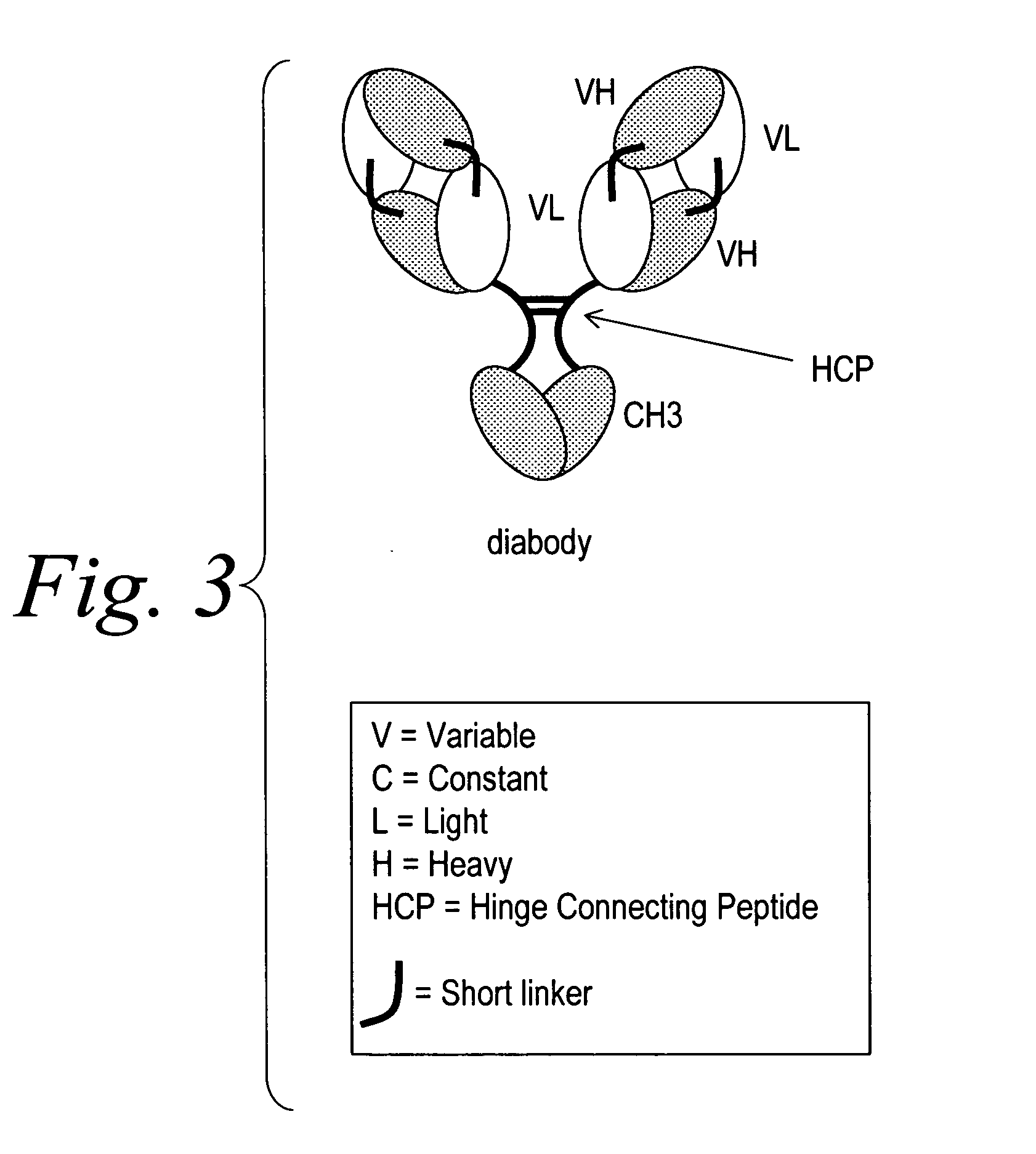
The invention provides for a polyvalent protein complex (PPC) comprising two polypeptide chains generally arranged laterally to one another. Each polypeptide chain typically comprises 3 or 4 “v-regions”, which comprise amino acid sequences capable of forming an antigen binding site when matched with a corresponding v-region on the opposite polypeptide chain. Up to about 6 “v-regions” can be used on each polypeptide chain. The v-regions of each polypeptide chain are connected linearly to one another and may be connected by interspersed linking regions. When arranged in the form of the PPC, the v-regions on each polypeptide chain form individual antigen binding sites.
Owner:IBC PHARMACEUTICALS INC
Stably tethered structures of defined compositions with multiple functions or binding specificities
Owner:IBC PHARMACEUTICALS INC
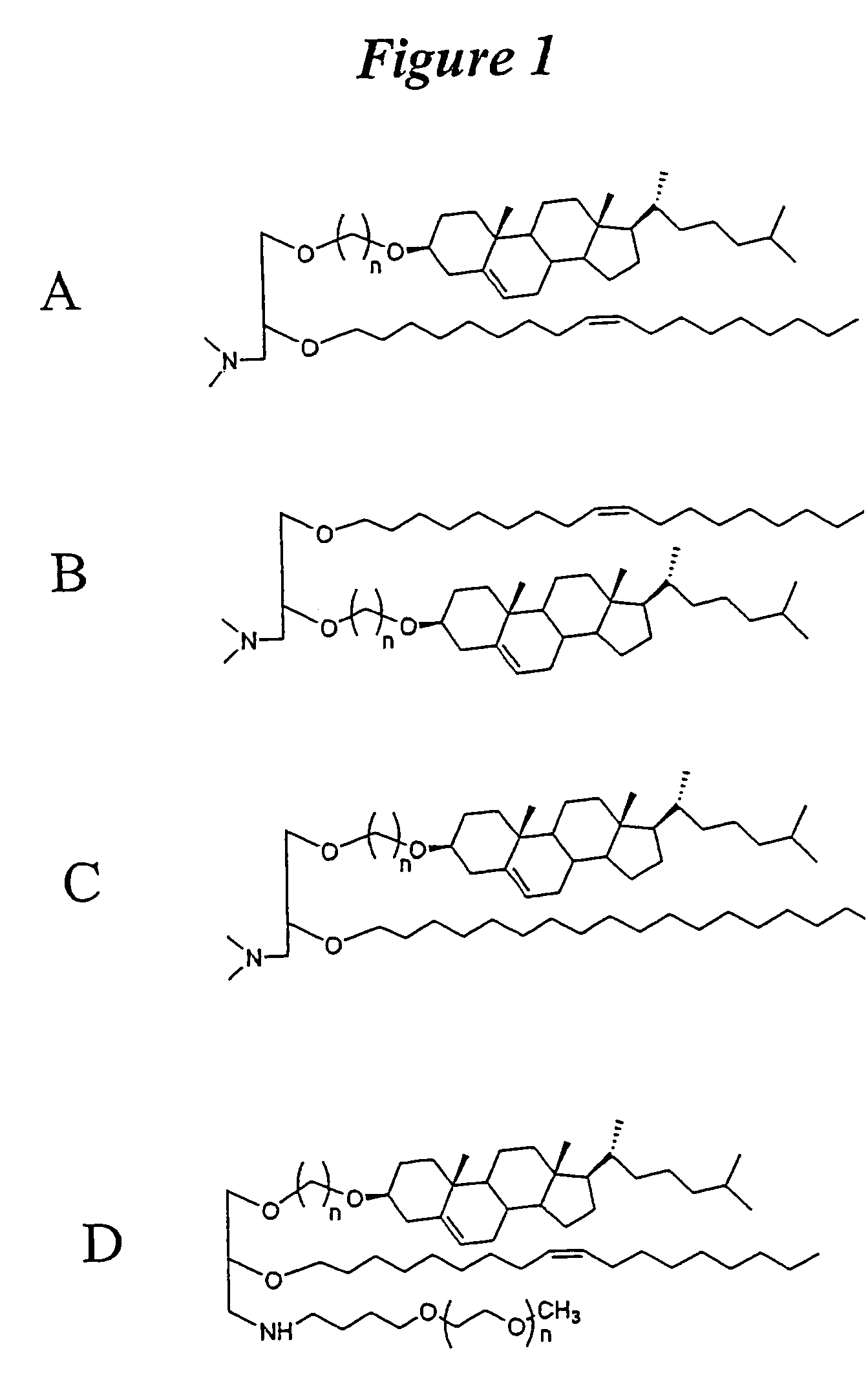
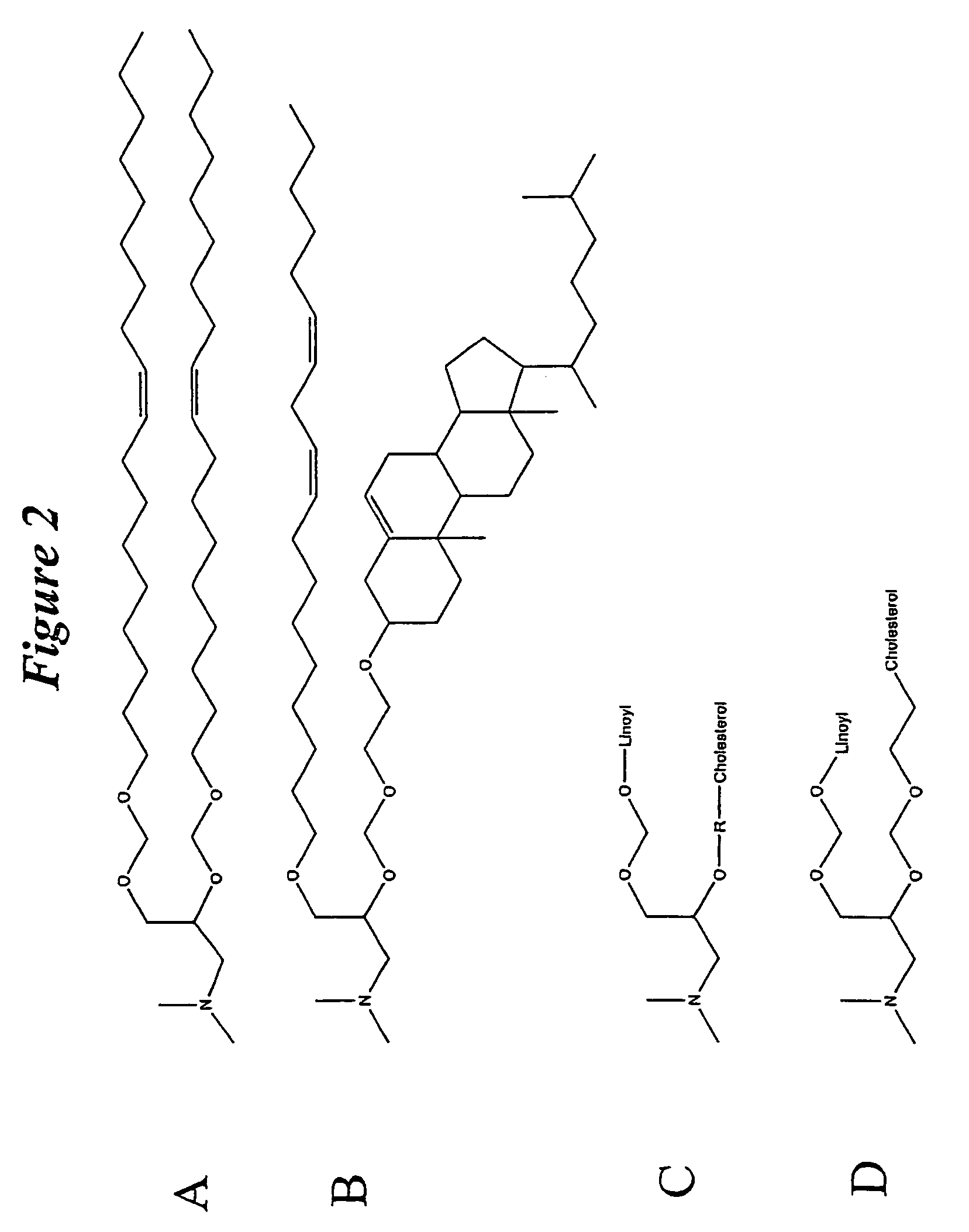
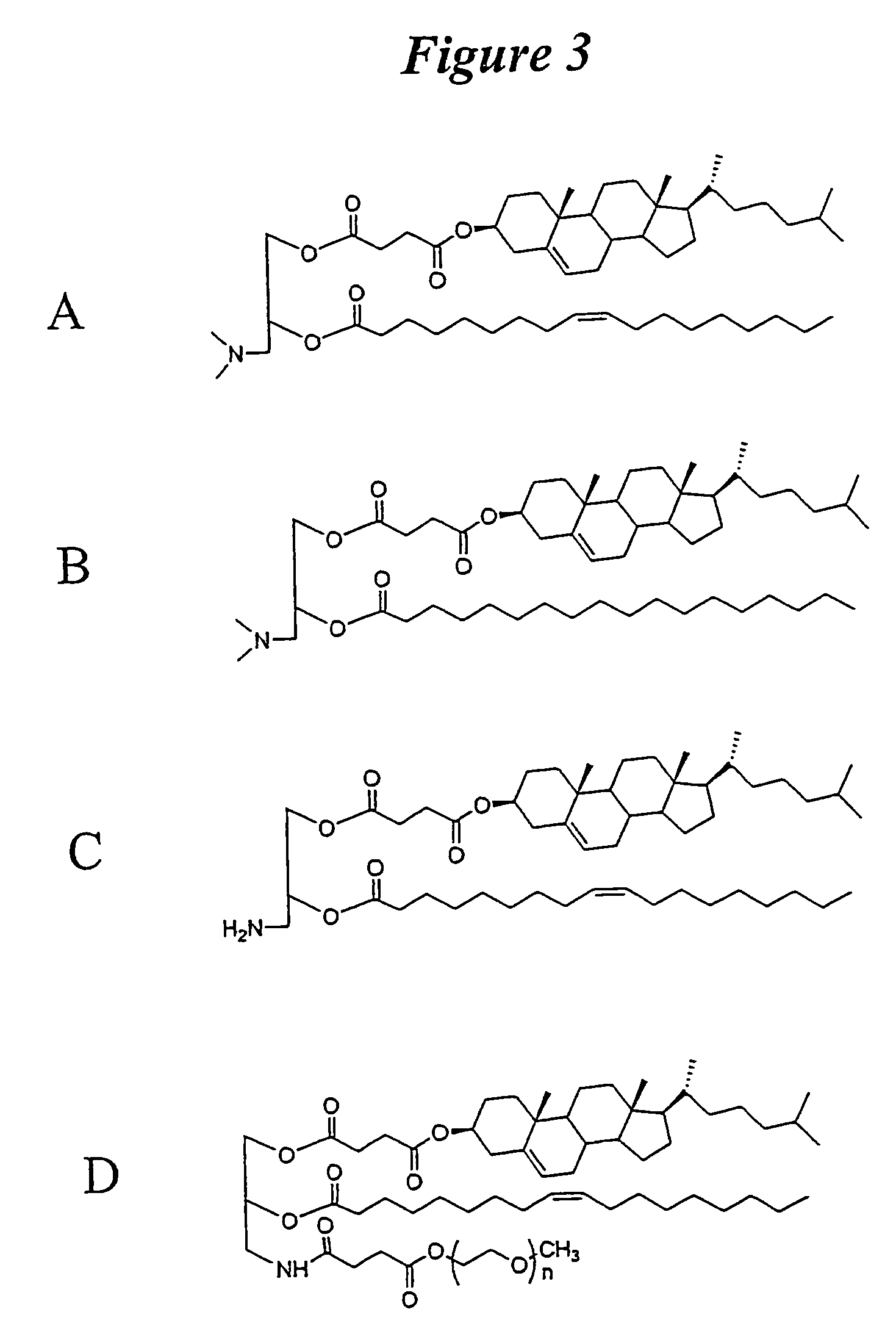
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
ActiveUS7404969B2Reduce deliveryAntibacterial agentsOrganic active ingredientsLipid formationMolecular composition
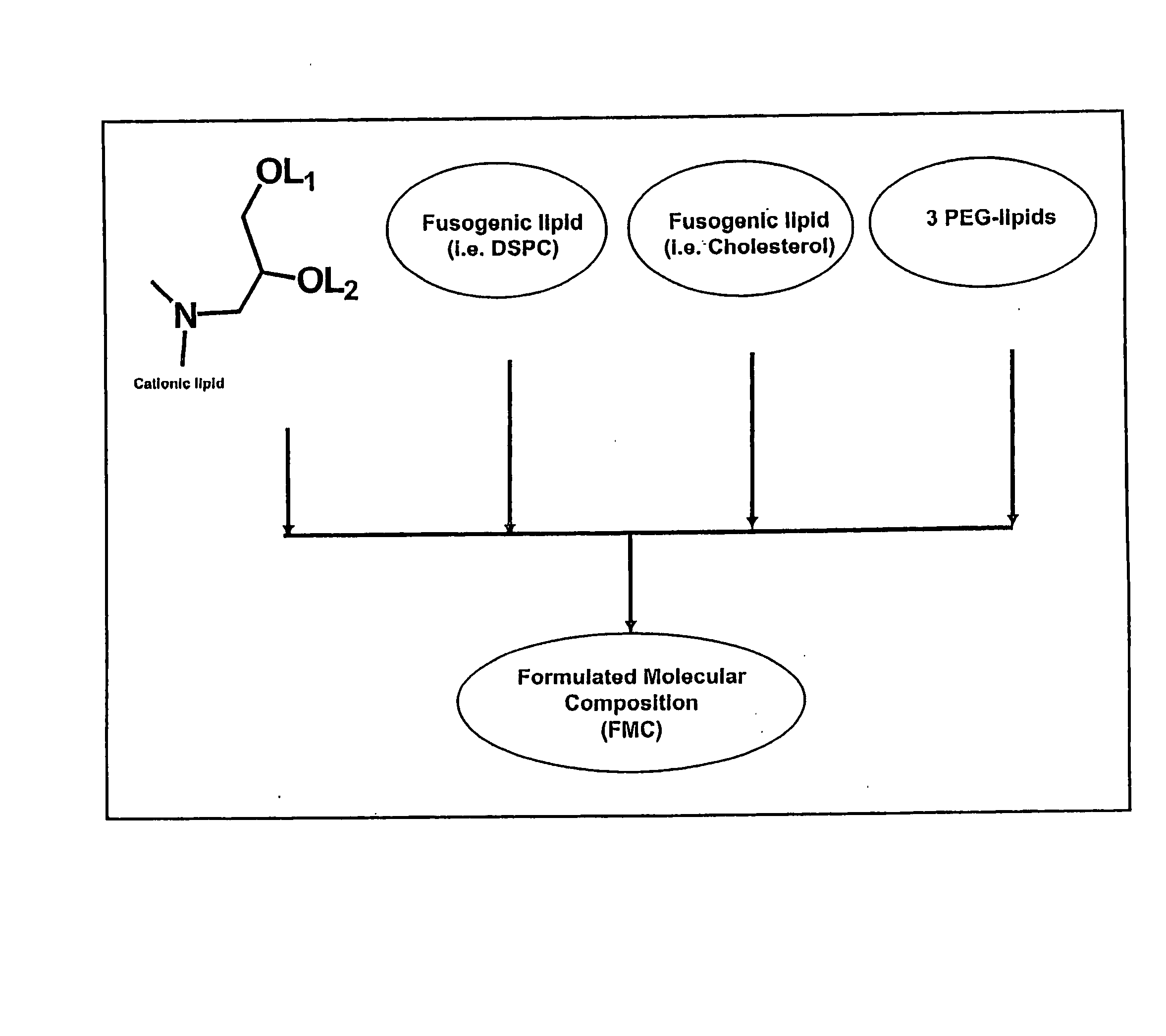
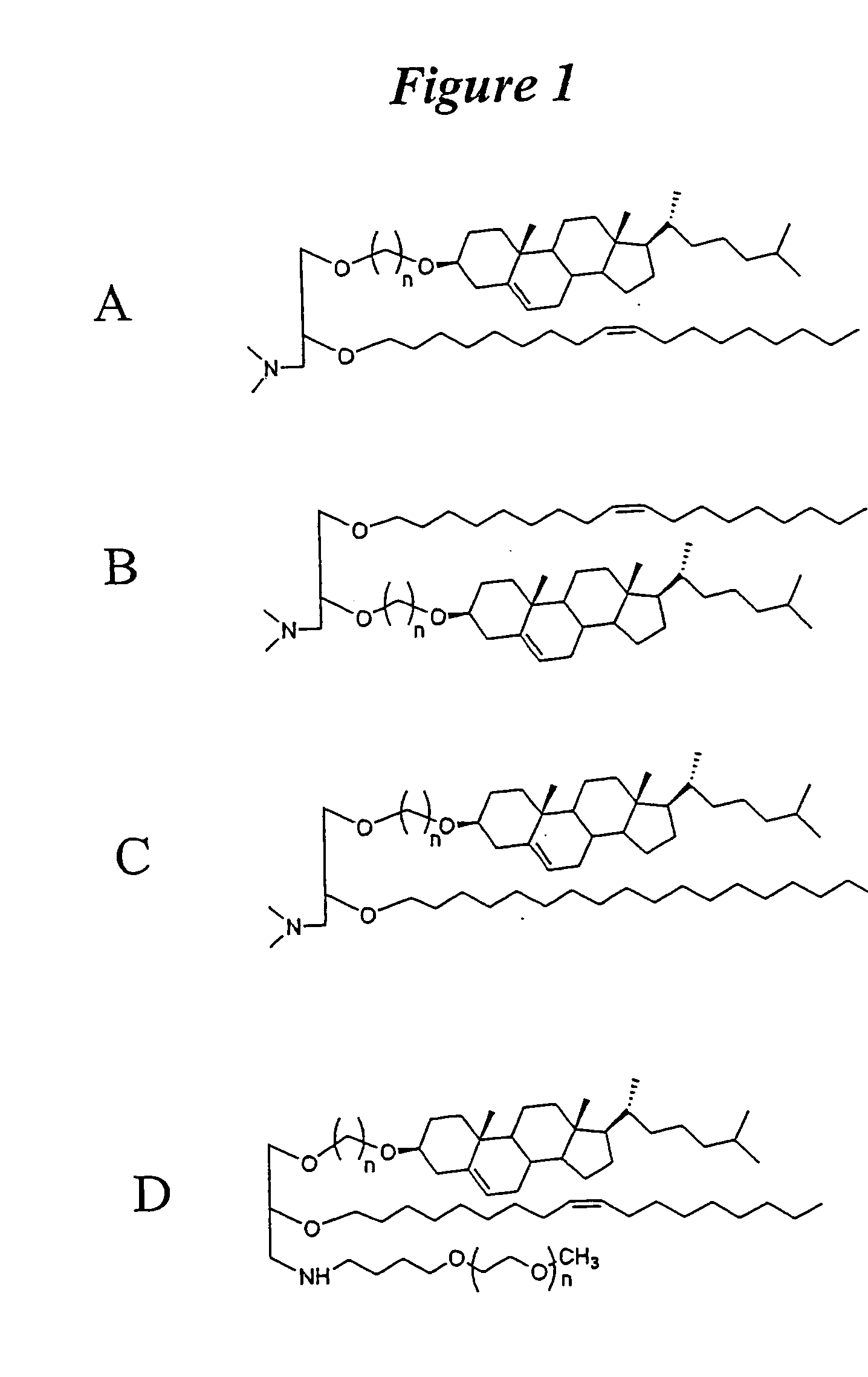
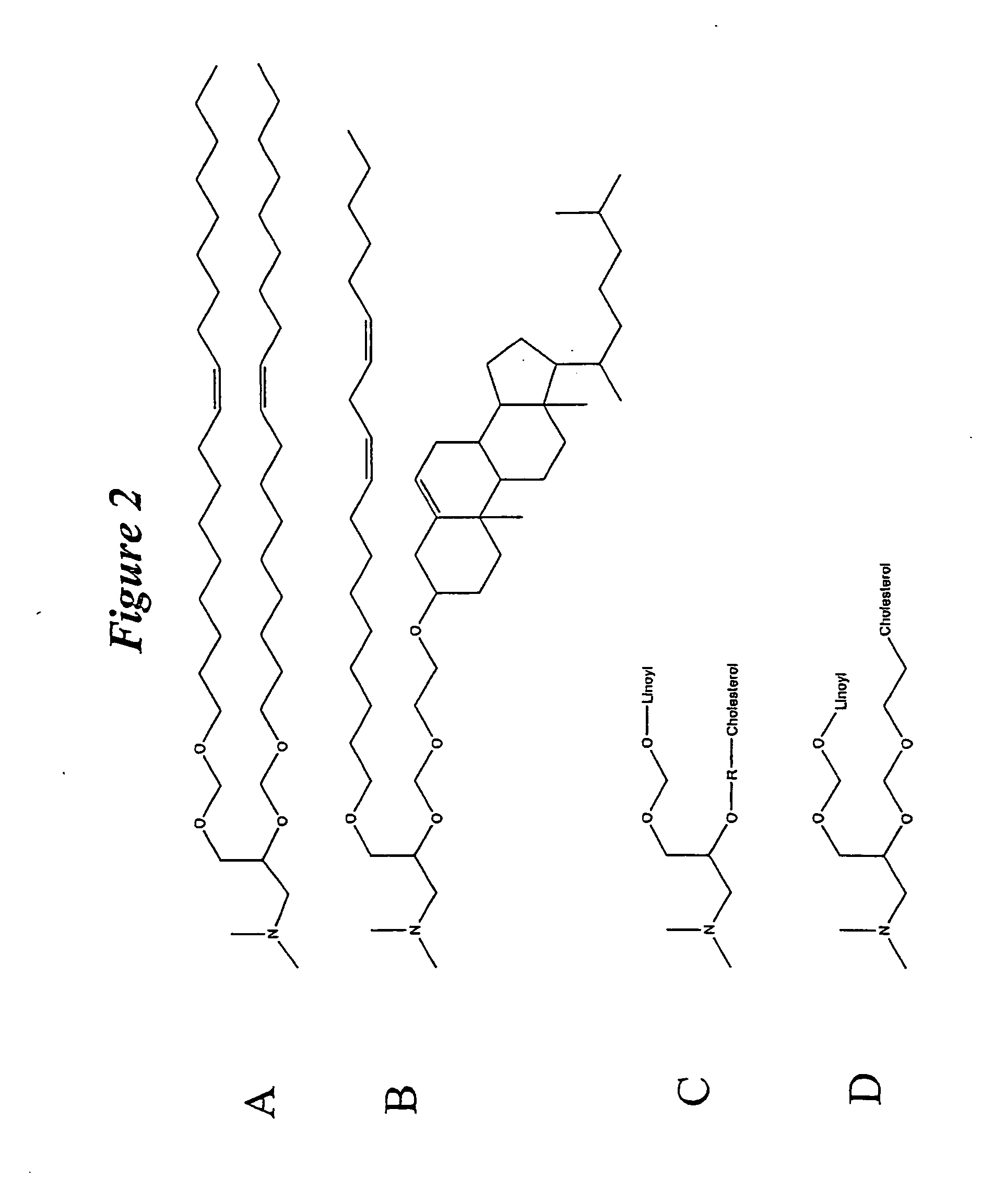
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver short interfering nucleic acid (siNA). The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
Glucocorticoid blocking agents for increasing blood-brain barrier permeability stan-261con
InactiveUS20050124533A1Improve breathabilityIncrease volumeAntibacterial agentsBiocideBlood brain barrier permeabilityDisease cause
Glucocorticoid blockers, including glucocorticoid receptor antagonists, are effective to prevent glucocorticoid-induced decrease in permeability of the blood-brain barrier and to increase the permeability of the blood-brain barrier. Administration of glucocorticoid blockers, including glucocorticoid receptor antagonists, concomitant with administration of drugs for treating diseases of the central nervous system increases delivery of such drugs into the central nervous system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
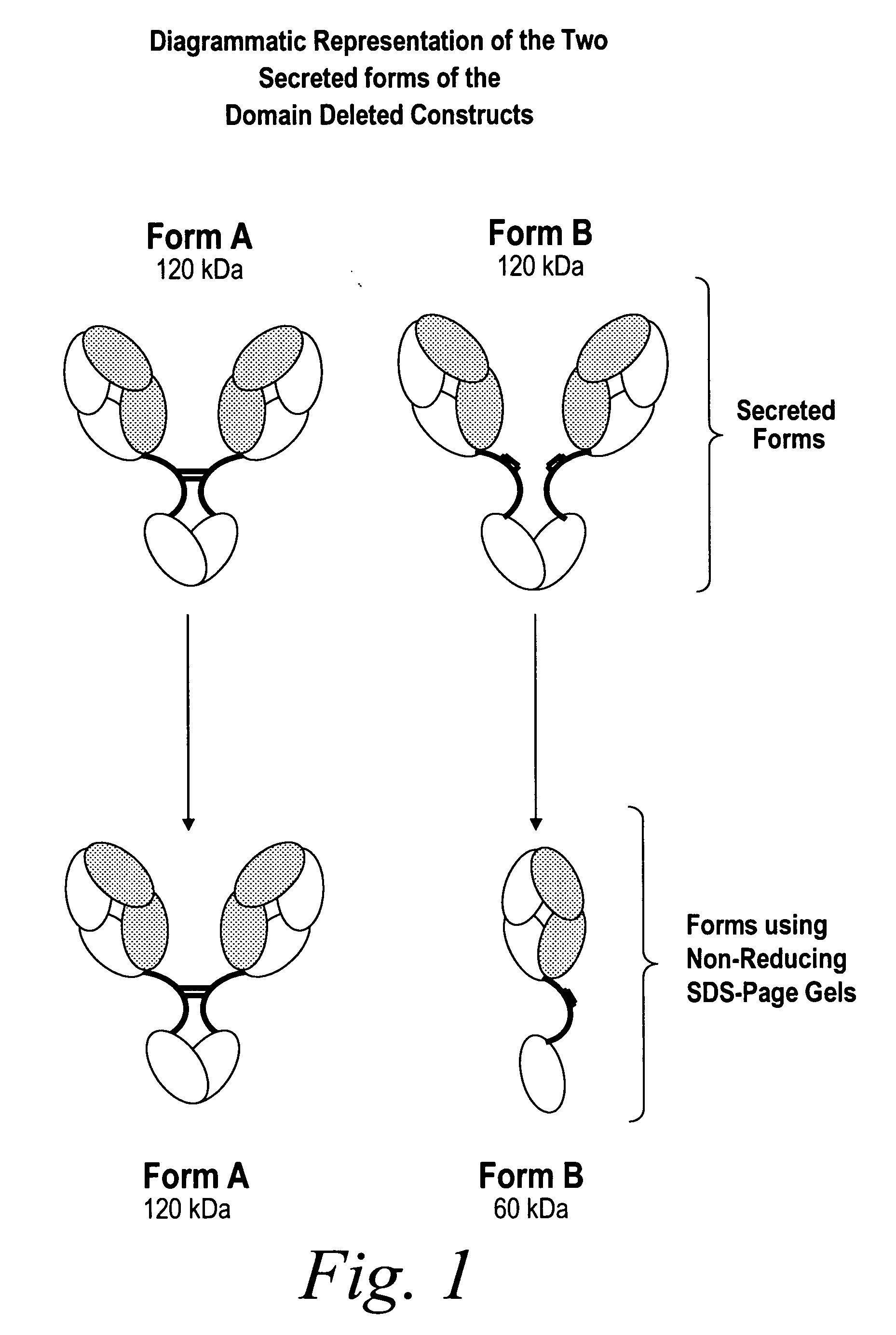
Modified binding molecules comprising connecting peptides
InactiveUS20050163782A1Well formedIncreased formationAntibacterial agentsSenses disorderDisulfide LinkagePeptide
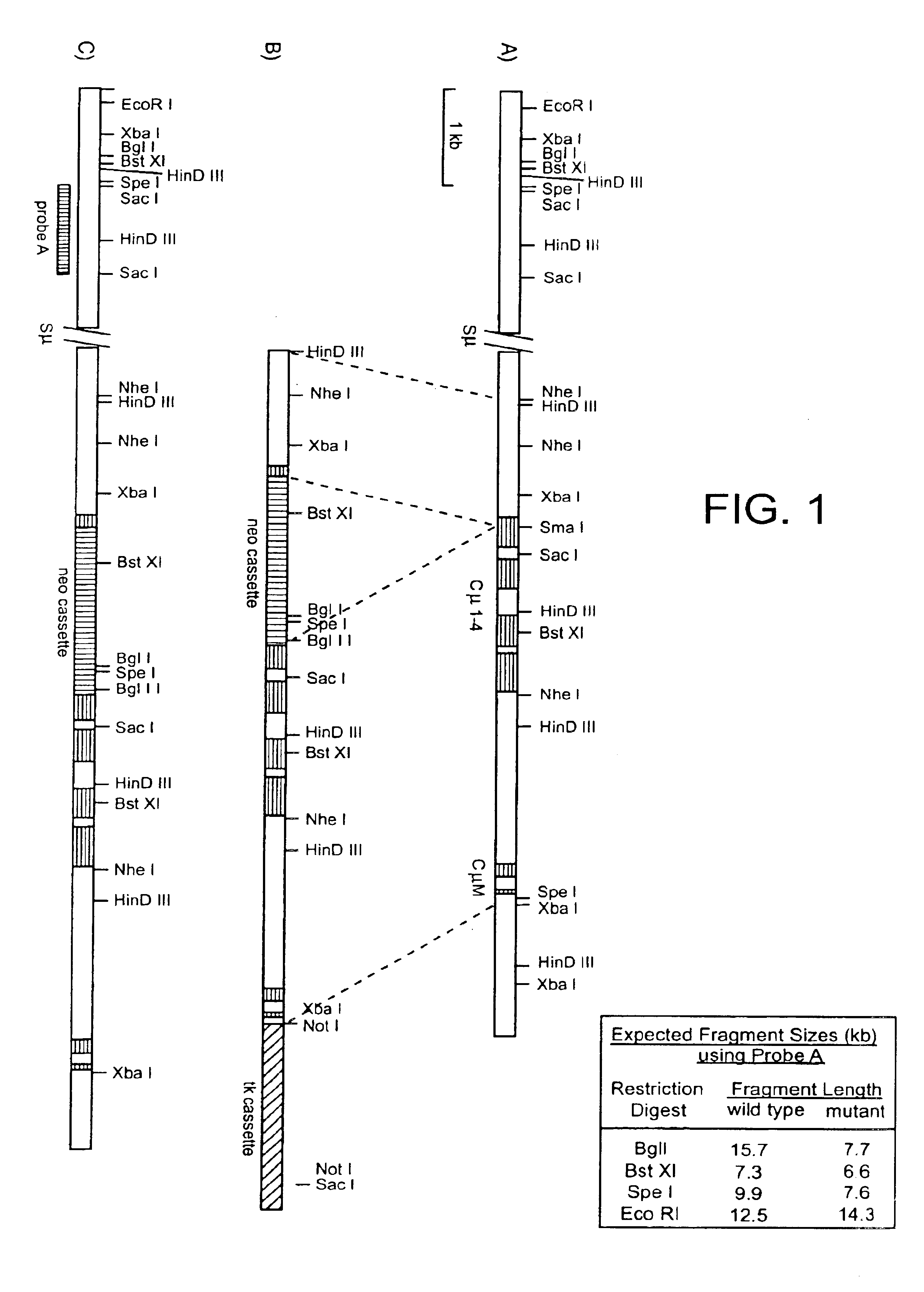
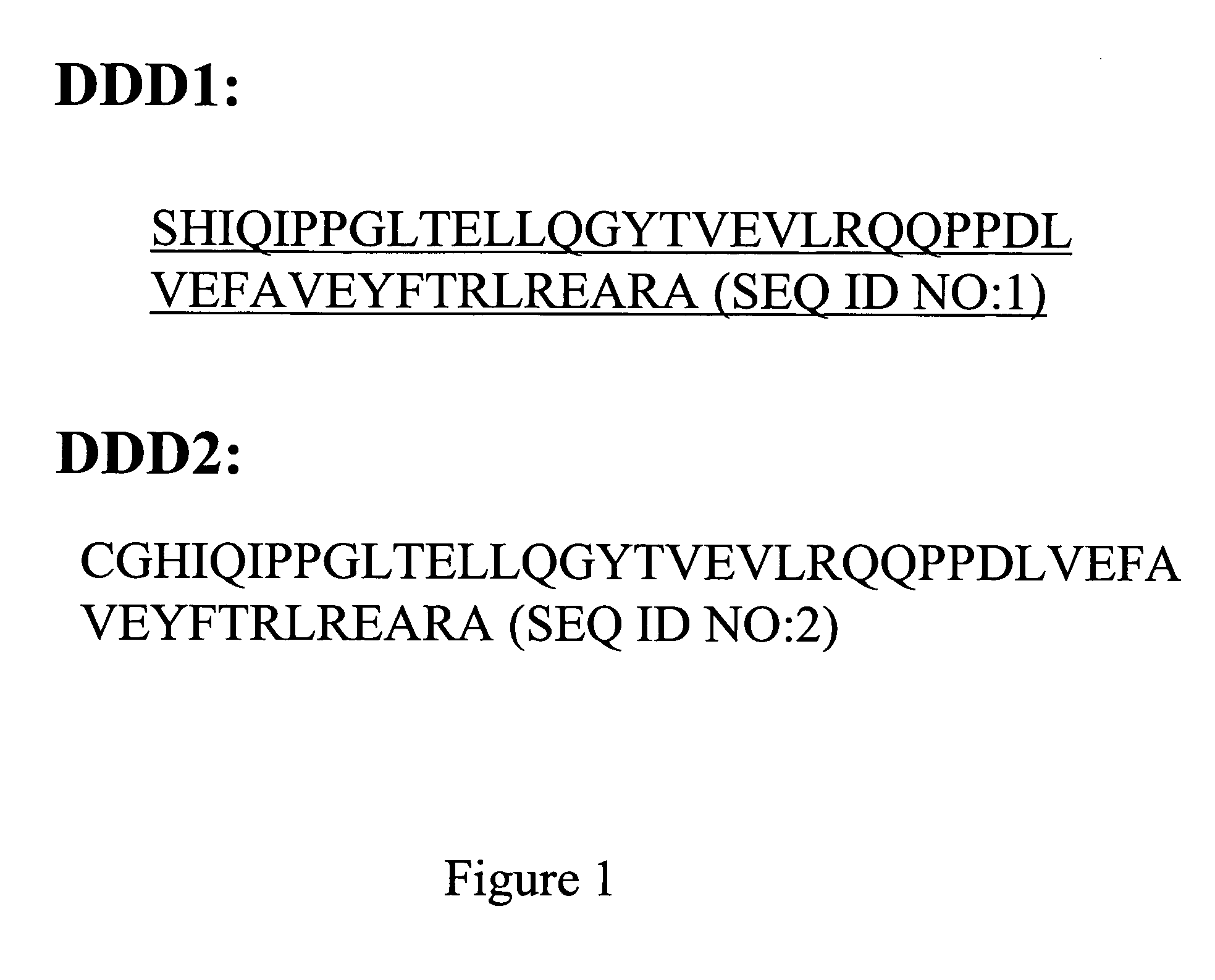
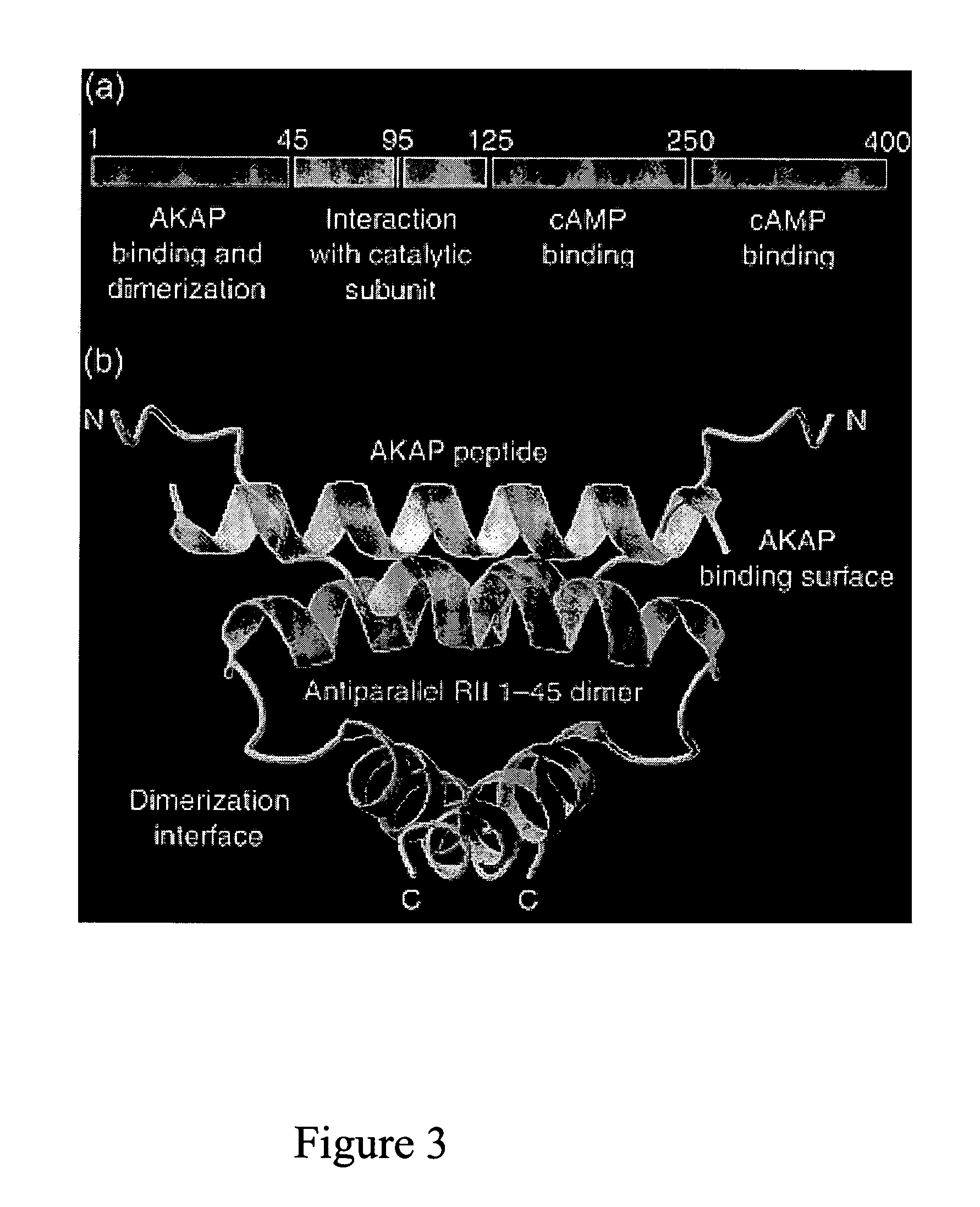
The instant invention describes methods of separating or preferentially synthesizing dimers which are linked via at least one interchain disulfide linkage from dimers which are not linked via at least one interchain disulfide linkage from a mixture comprising the two types of polypeptide dimers. These forms can be separated from each other using hydrophobic interaction chromatography. In addition, the invention pertains to connecting peptides that result in the preferential biosynthesis of dimers that are linked via at least one interchain disulfide linkage or that are not linked via at least one interchain disulfide linkage. The invention also pertains to compositions in which a majority of the dimers are linked via at least one interchain disulfide linkage or are not linked via at least one interchain disulfide linkage. The invention still further pertains to novel binding molecules, e.g., comprising connecting peptides of the invention.
Owner:BIOGEN MA INC
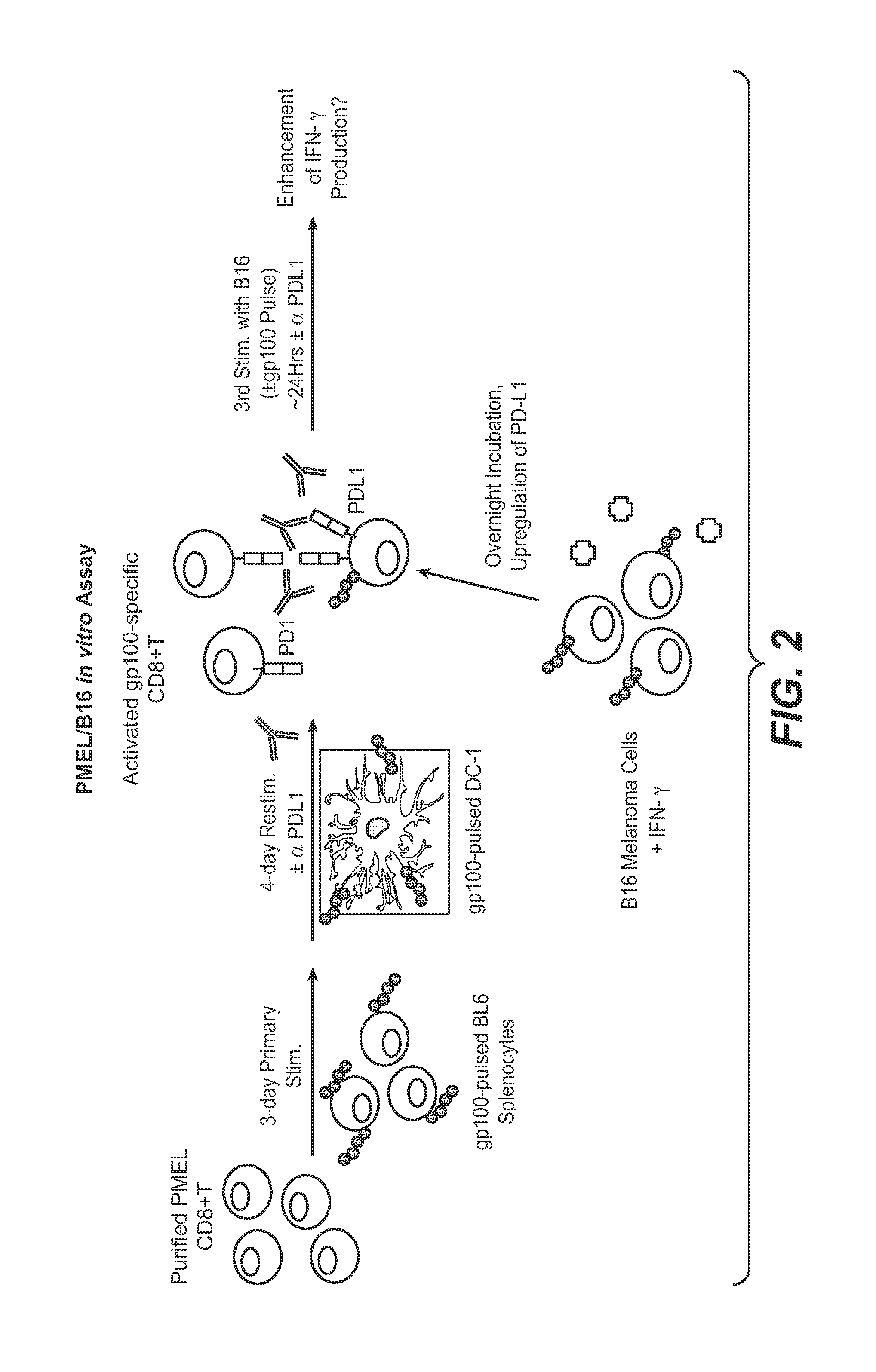
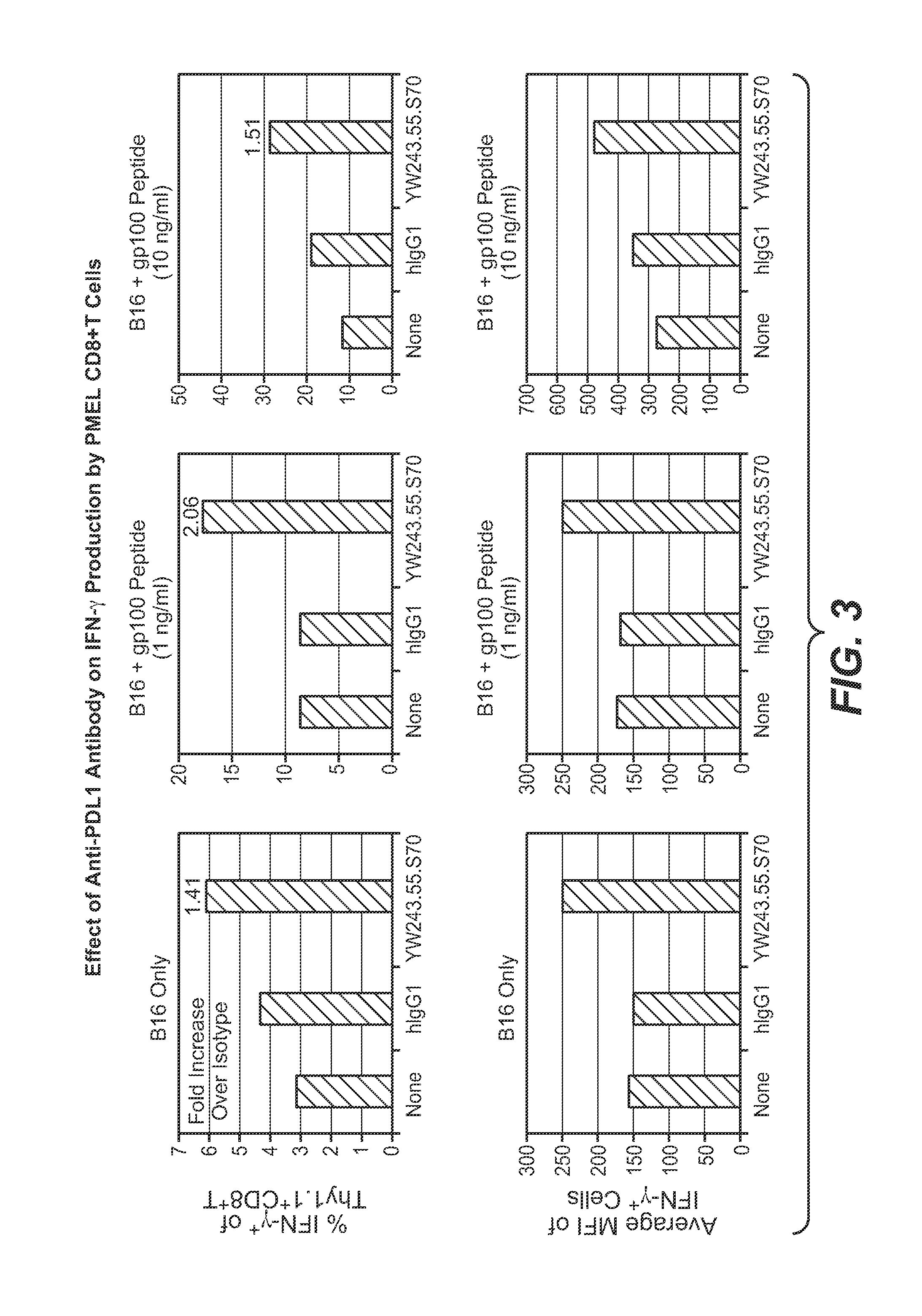
Anti-pd-l1 antibodies and their use to enhance t-cell function
ActiveUS20100203056A1Reduce level of pathogenChronic infectionAntibacterial agentsOrganic active ingredientsT-cell dysfunctionPD-L1
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
Methods and compositions using immunomodulatory compounds for treatment and management of cancers and other diseases
ActiveUS20040029832A1Prevent proliferationAntibacterial agentsBiocideSide effectBiologically-Based Therapy
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
N-acetyl aldosamines, n-acetylamino acids and related n-acetyl compounds and their topical use
Compositions comprising N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl compounds are useful to alleviate or improve various cosmetic conditions and dermatological disorders, including changes or damage to skin, nail and hair associated with intrinsic aging and / or extrinsic aging, as well as changes or damage caused by extrinsic factors. N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl composition may further comprise a cosmetic, pharmaceutical or other topical agent to enhance or create synergetic effects.
Owner:TRISTRATA TECH
Modified polynucleotides for the production of secreted proteins
Owner:MODERNATX INC
Cationic antiseptic compositions and methods of use
ActiveUS20060051385A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideAmmonium compoundsCetylpyridinium
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including a cationic antiseptic such as biguanides and bisbiguanides such as chlorhexidine and its various salts including but not limited to the digluconate, diacetate, dimethosulfate, and dilactate salts; polymeric quaternary ammonium compounds such as polyhexamethylenebiguanide; silver and various silver complexes; small molecule quaternary ammonium compounds such as benzalkoium chloride and alkyl substituted derivatives; di-long chain alkyl (C8-C18) quaternary ammonium compounds; cetylpyridinium halides and their derivatives; benzethonium chloride and its alkyl substituted derivatives; and octenidine. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
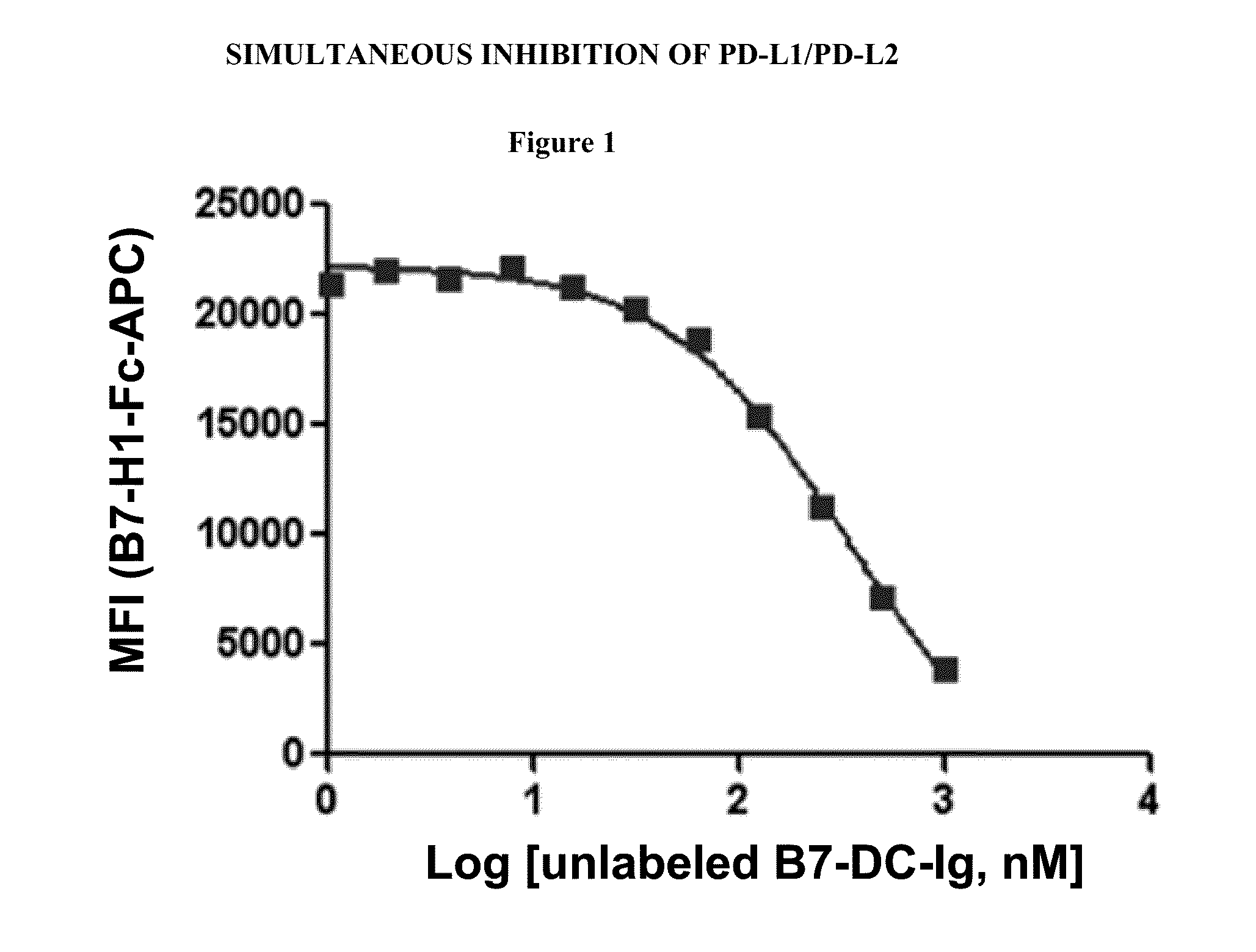
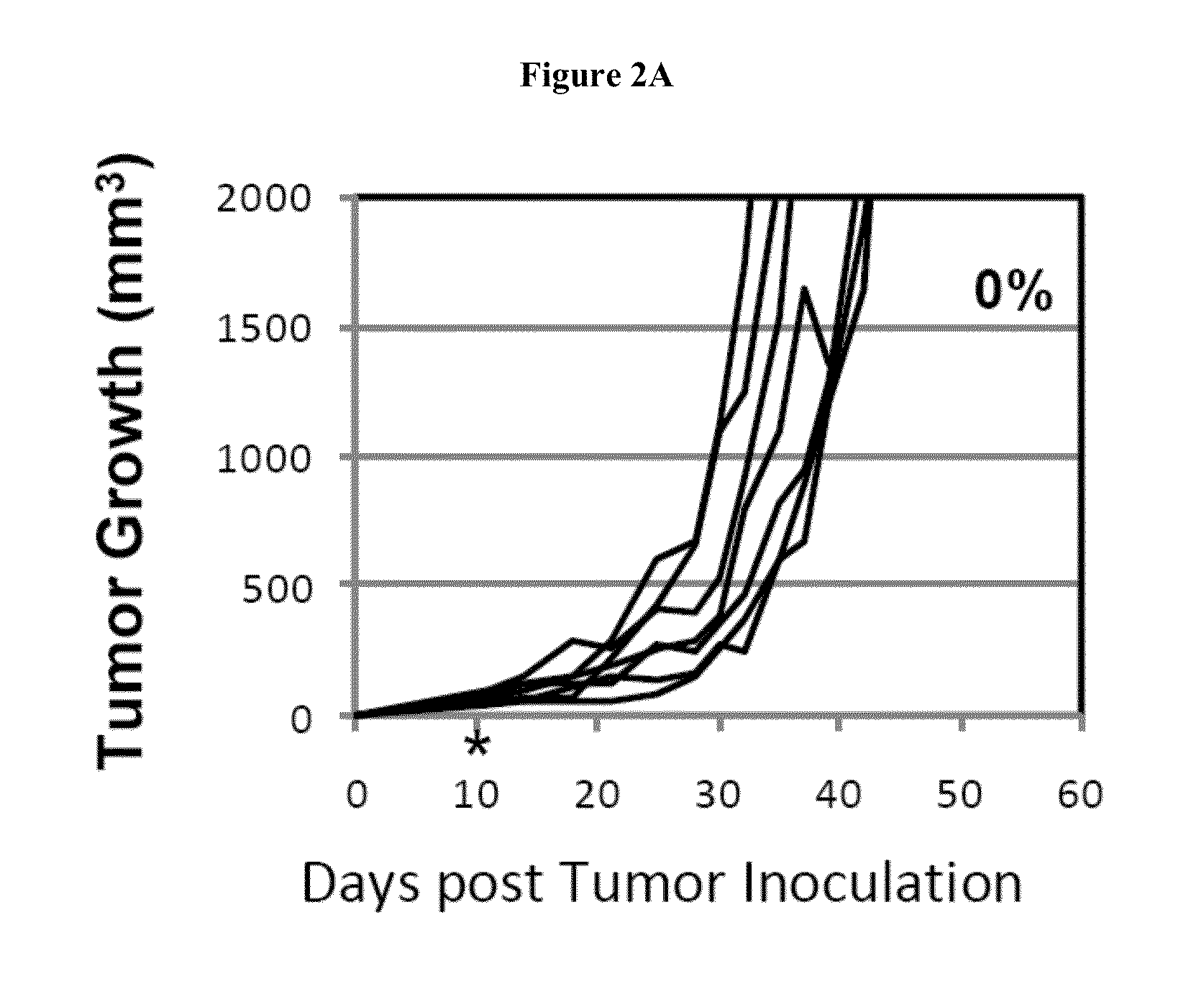
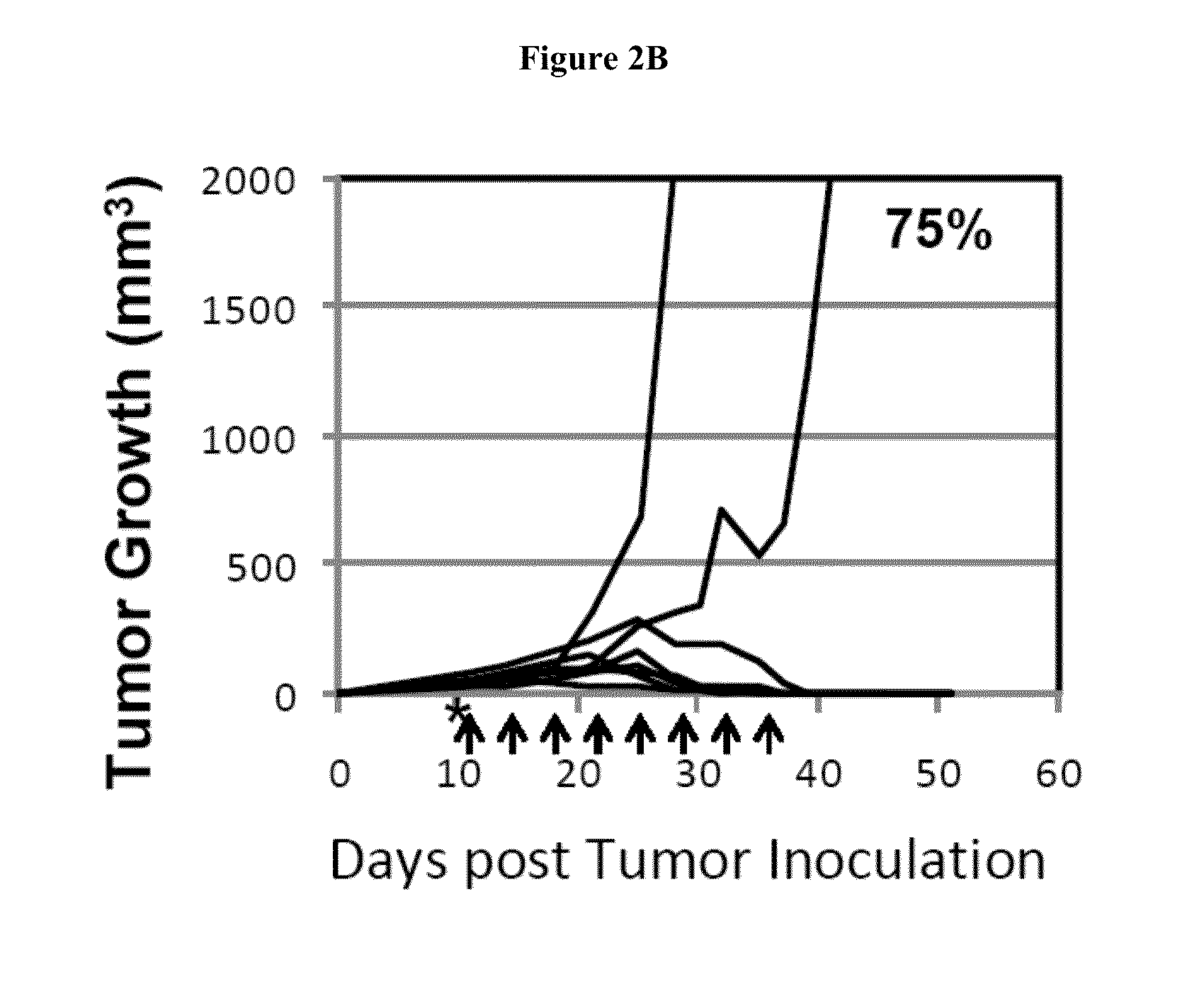
Simultaneous inhibition of pd-l1/pd-l2
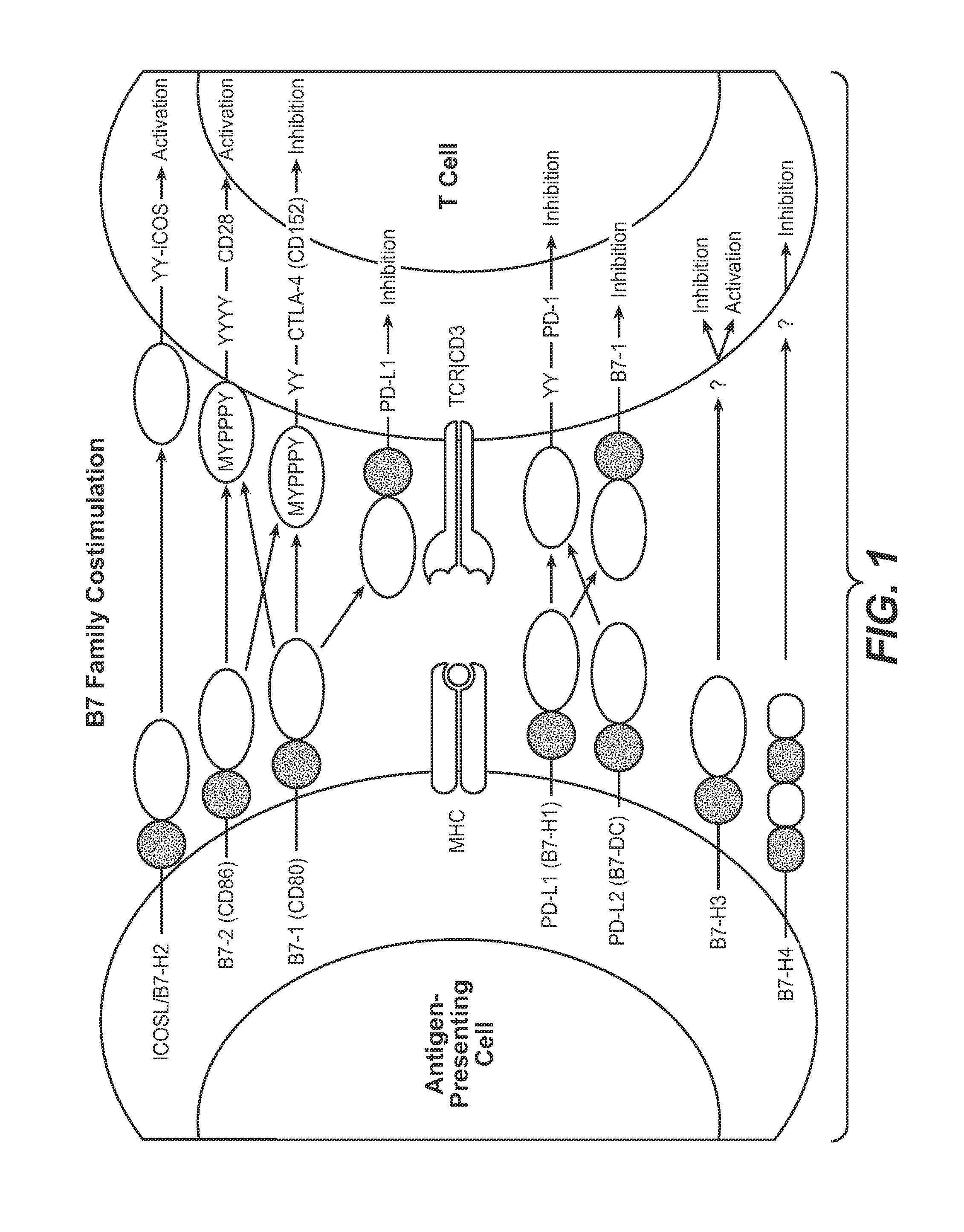
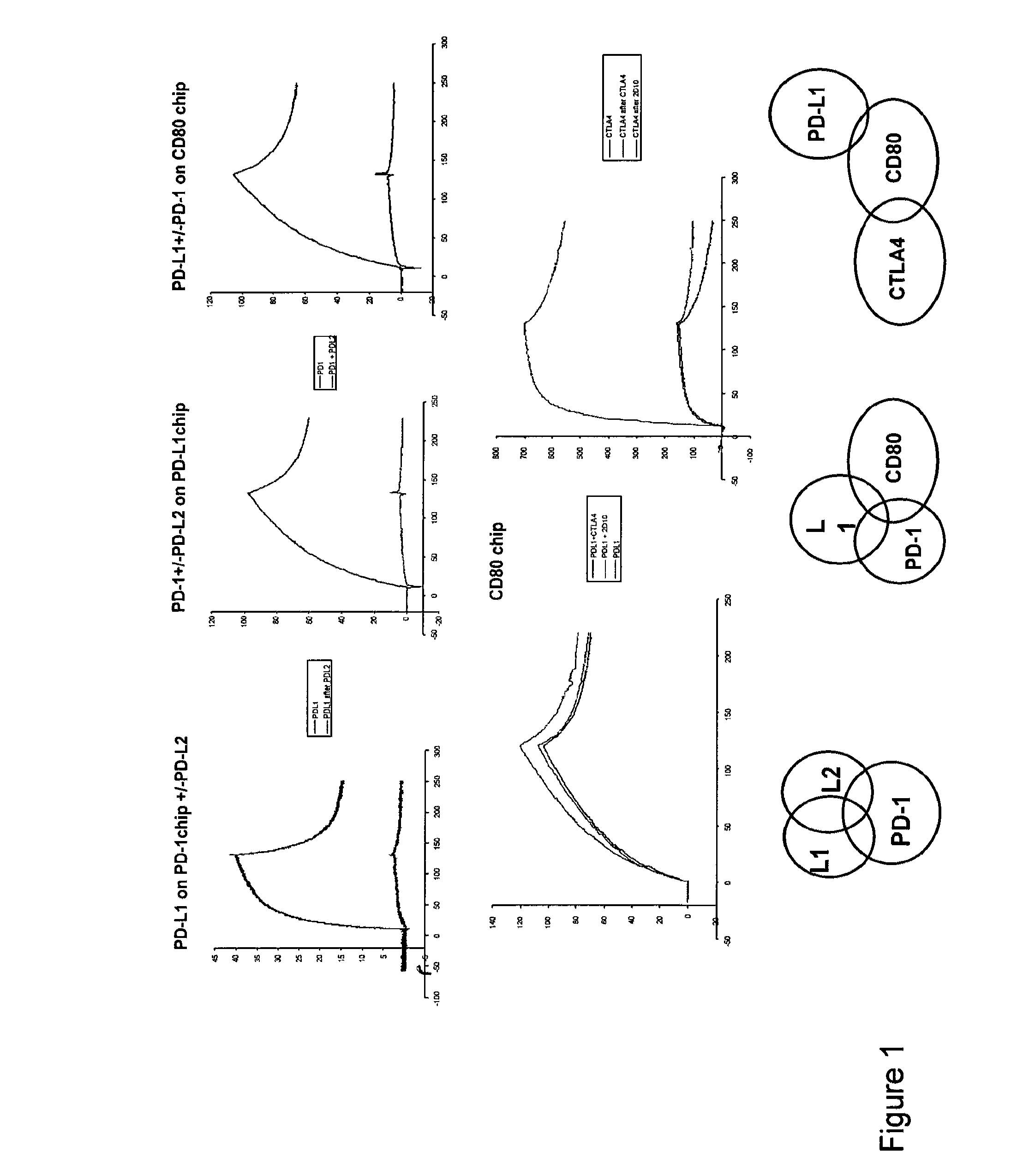
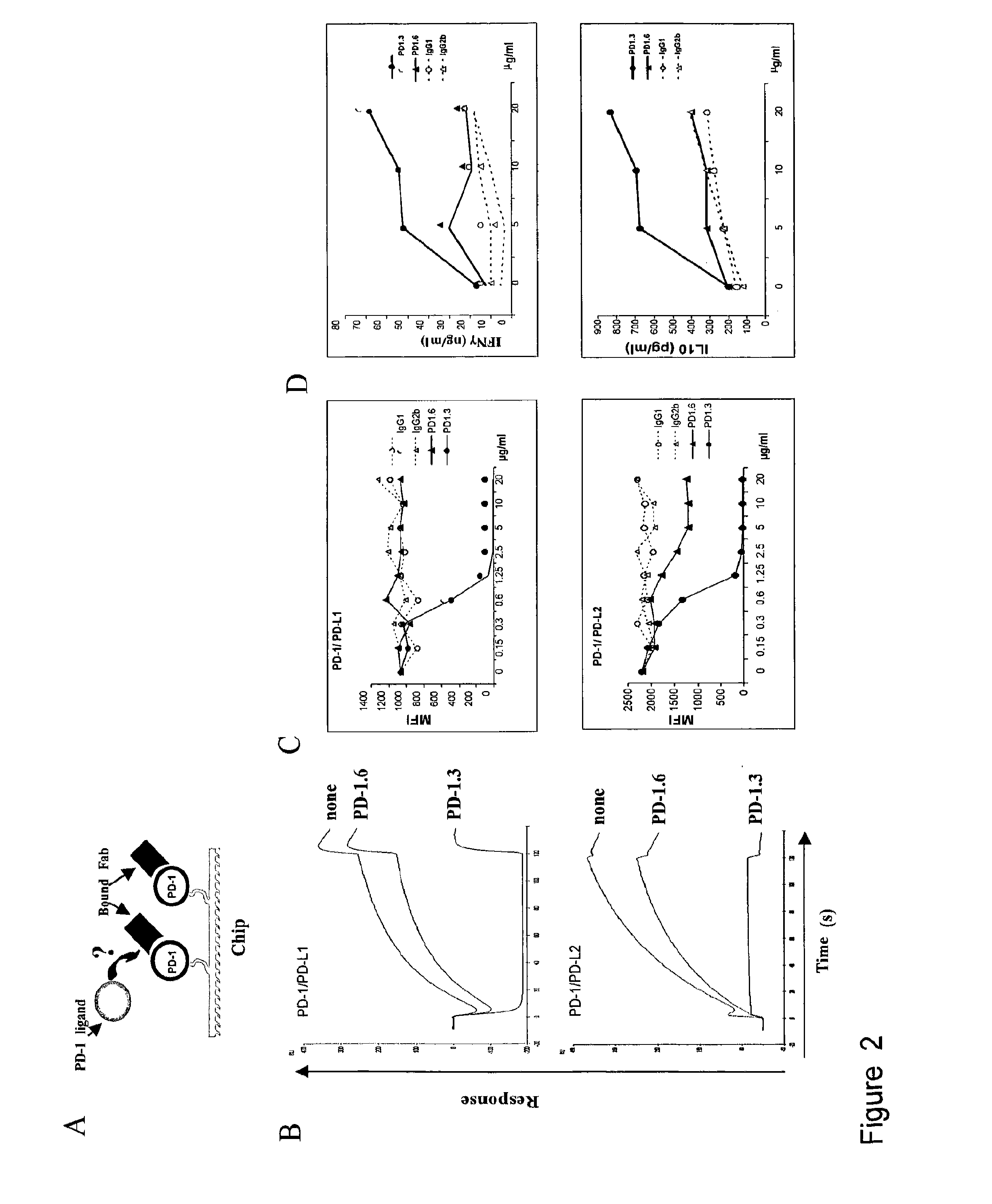
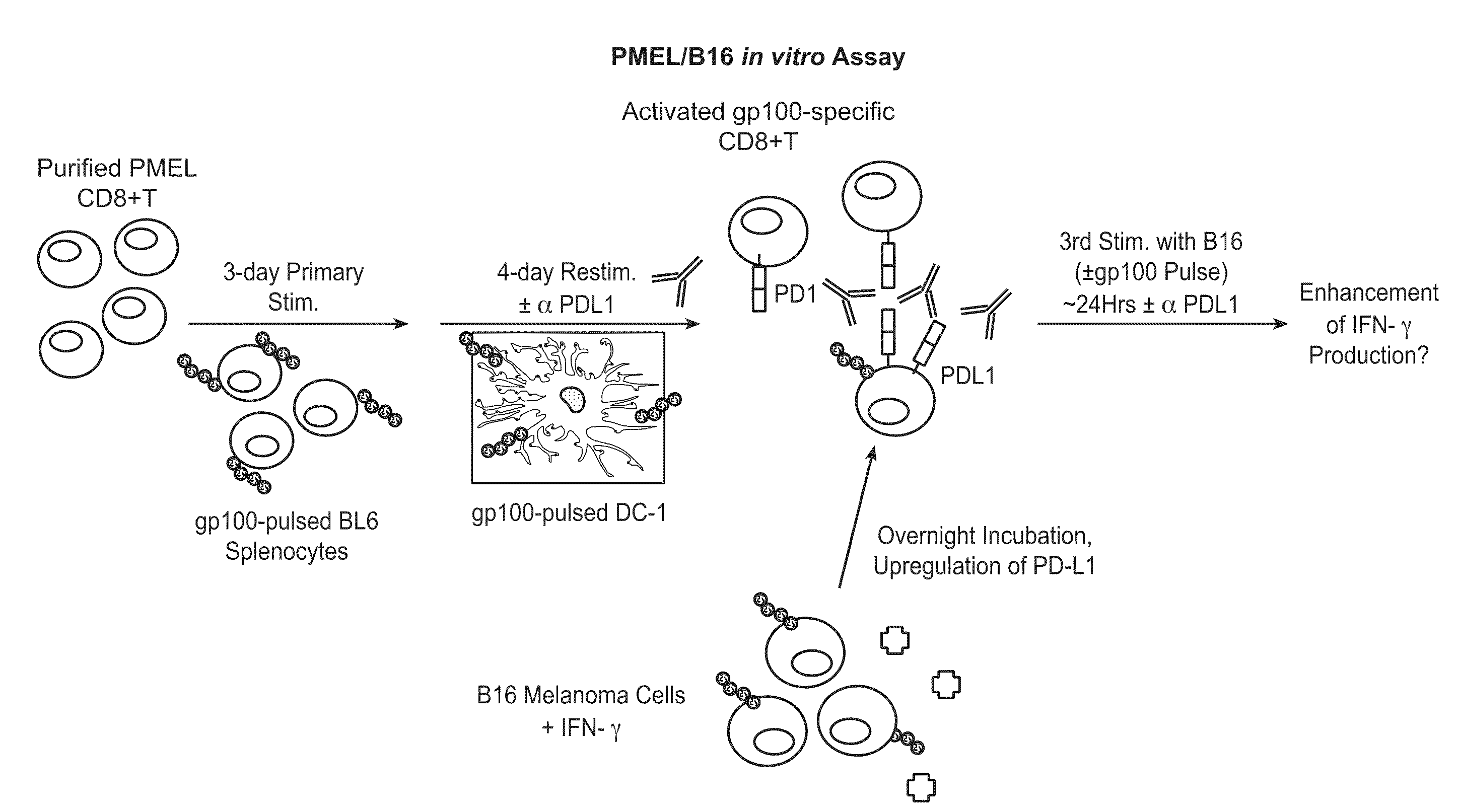
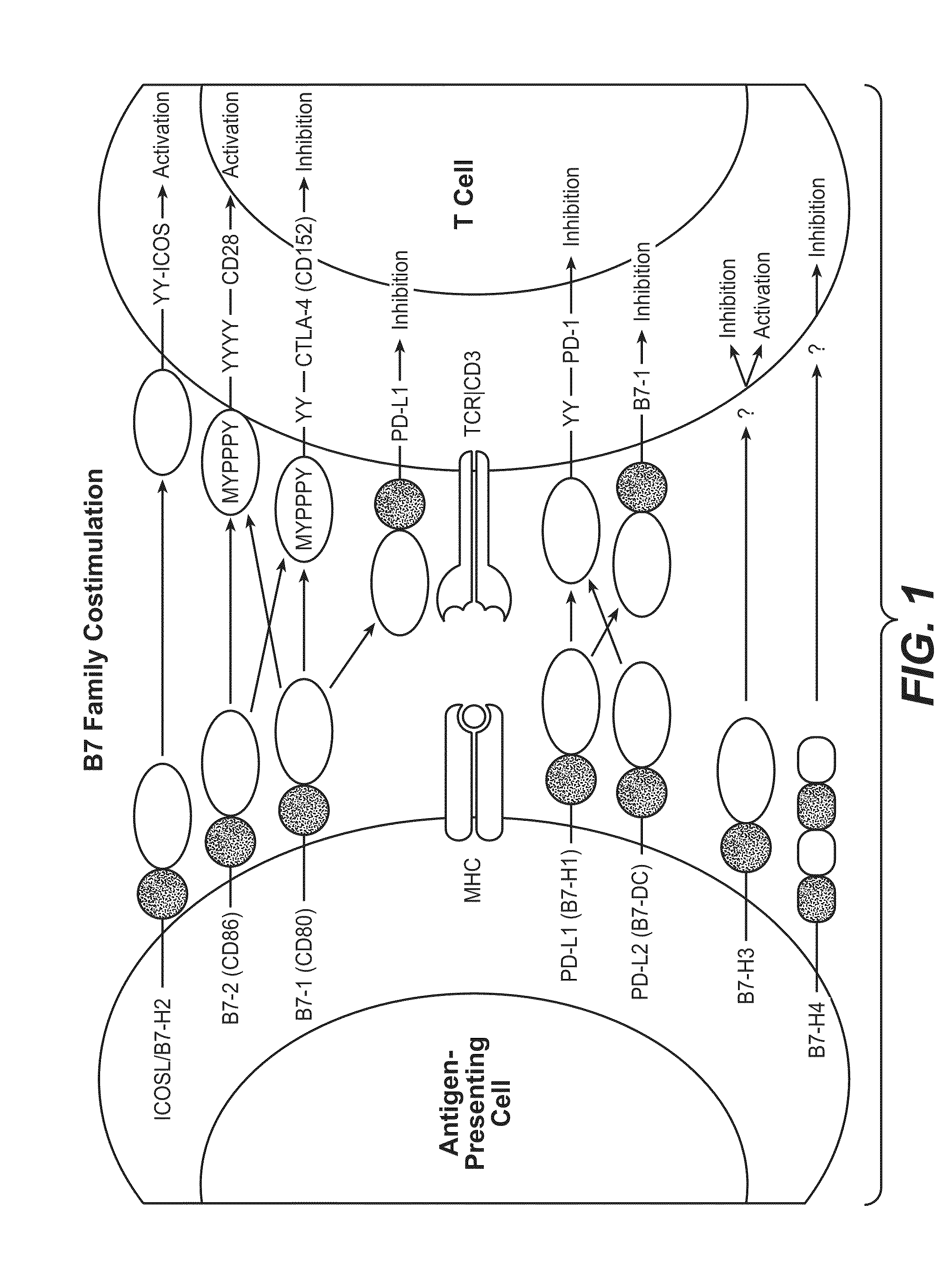
InactiveUS20130017199A1Increase frequencyIncrease percentageAntibacterial agentsAntimycoticsDiseaseDendritic cell
Methods and compositions for treating an infection or disease that results from (1) failure to elicit rapid T cell mediated responses, (2) induction of T cell exhaustion, T cell anergy or both, or (3) failure to activate monocytes, macrophages, dendritic cells and / or other APCs, for example, as required to kill intracellular pathogens. The method and compositions solve the problem of undesired T cell inhibition by simultaneously inhibiting the PD-1 ligands, PD-L1 and PD-L2. The immune response can be modulated by providing antagonists which bind with different affinity, by varying the dosage of agent which is administered, by intermittent dosing over a regime, and combinations thereof, that provides for dissociation of agent from the molecule to which it is bound prior to being administered again. In some cases it may be particularly desirable to stimulate the immune system, then remove the stimulation.
Owner:AMPLIMMUNE
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
ActiveUS20080020058A1Improves various propertyImprove the immunityAntibacterial agentsPowder deliveryLipid formationCholesterol
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. The invention also features compositions, and methods of use for the study, diagnosis, and treatment of traits, diseases and conditions that respond to the modulation of gene expression and / or activity in a subject or organism. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver biologically active molecules, such as antibodies (e.g., monoclonal, chimeric, humanized etc.), cholesterol, hormones, antivirals, peptides, proteins, chemotherapeutics, small molecules, vitamins, co-factors, nucleosides, nucleotides, oligonucleotides, enzymatic nucleic acids, antisense nucleic acids, triplex forming oligonucleotides, 2,5-A chimeras, dsRNA, allozymes, aptamers, decoys and analogs thereof, and small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), short hairpin RNA (shRNA), and RNAi inhibitor molecules, to relevant cells and / or tissues, such as in a subject or organism. Such novel cationic lipids, microparticles, nanoparticles and transfection agents are useful, for example, in providing compositions to prevent, inhibit, or treat diseases, conditions, or traits in a cell, subject or organism. The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. The invention also features compositions, and methods of use for the study, diagnosis, and treatment of traits, diseases and conditions that respond to the modulation of gene expression and / or activity in a subject or organism. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver biologically active molecules, such as antibodies (e.g., monoclonal, chimeric, humanized etc.), cholesterol, hormones, antivirals, peptides, proteins, chemotherapeutics, small molecules, vitamins, co-factors, nucleosides, nucleotides, oligonucleotides, enzymatic nucleic acids, antisense nucleic acids, triplex forming oligonucleotides, 2,5-A chimeras, dsRNA, allozymes, aptamers, decoys and analogs thereof, and small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules, to relevant cells and / or tissues, such as in a subject or organism. Such novel cationic lipids, microparticles, nanoparticles and transfection agents are useful, for example, in providing compositions to prevent, inhibit, or treat diseases, conditions, or traits in a cell, subject or organism. The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
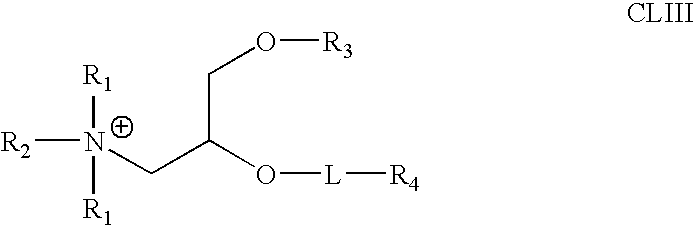
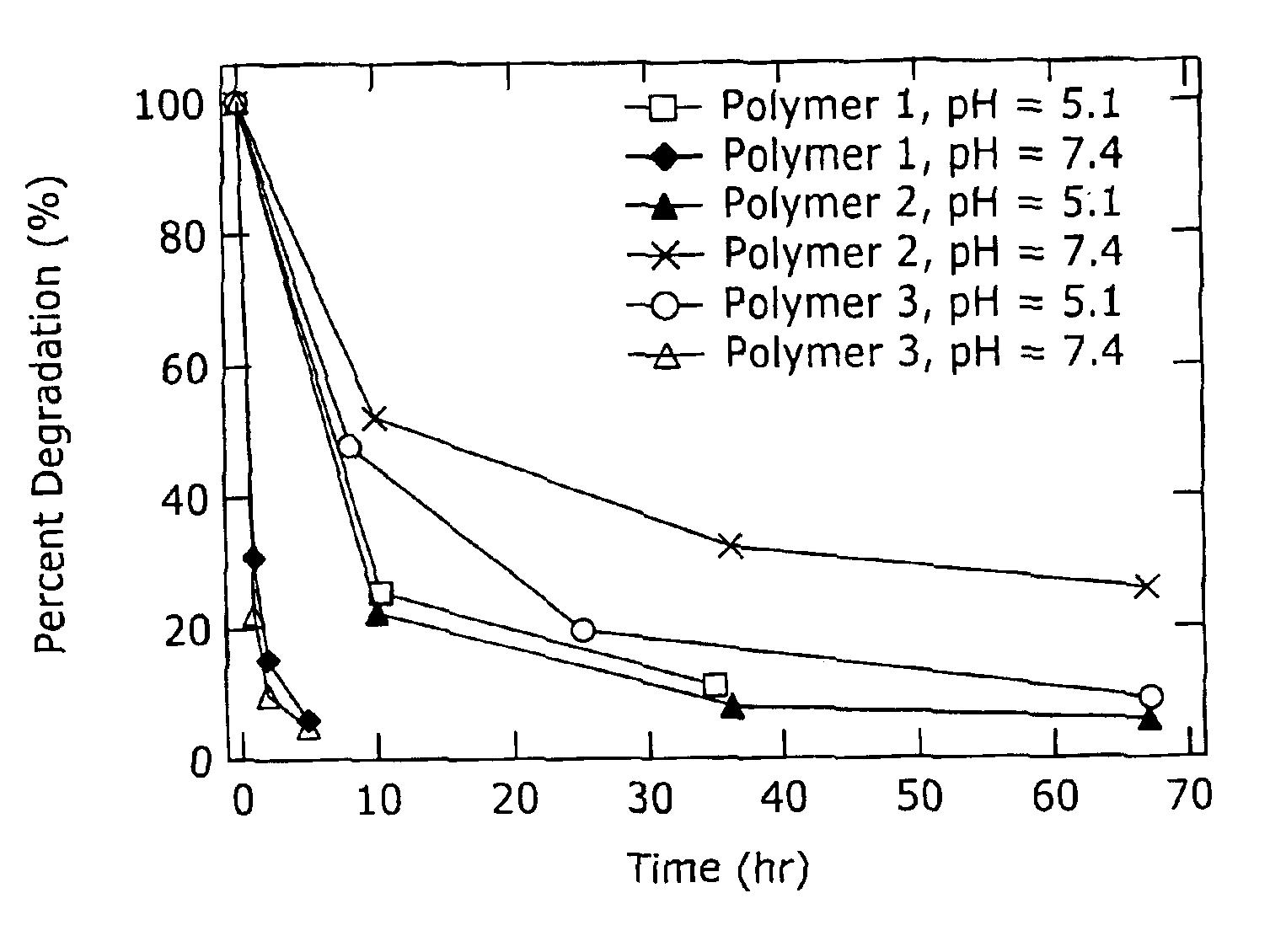
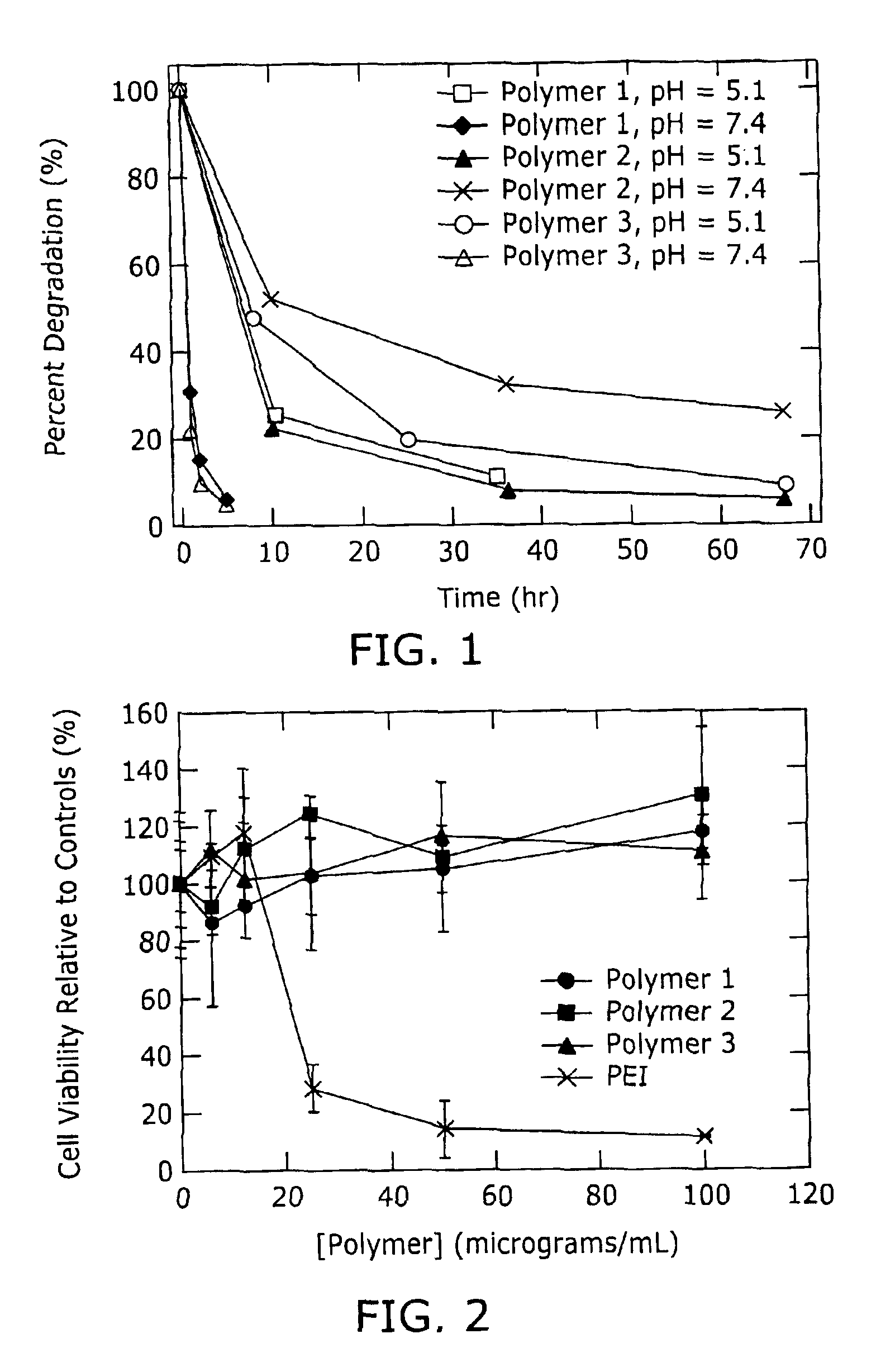
Biodegradable poly(β-amino esters) and uses thereof
Poly(β-amino esters) prepared from the conjugate addition of bis(secondary amines) or primary amines to a bis(acrylate ester) are described. Methods of preparing these polymers from commercially available starting materials are also provided. These tertiary amine-containing polymers are preferably biodegradable and biocompatible and may be used in a variety of drug delivery systems. Given the poly(amine) nature of these polymers, they are particularly suited for the delivery of polynucleotides. Nanoparticles containing polymer / polynucleotide complexes have been prepared. The inventive polymers may also be used to encapsulate other agents to be delivered. They are particularly useful in delivering labile agents given their ability to buffer the pH of their surroundings.
Owner:MASSACHUSETTS INST OF TECH
Methods and compositions for the prevention and treatment of atherosclerosis, restenosis and related disorders
Methods and compositions for the prevention and treatment of all forms of atherosclerosis are described. Administration of compounds such as thalidomide, its analogs, hydrolysis products, metabolites, derivatives and precursors as well as additional compounds capable of inhibiting tumor necrosis factor alpha (TNF-alpha) are used in the invention. Also disclosed is the coating of prosthetic devices, such as stents, with the compounds of the invention for the prevention and / or treatment of restenosis.
Owner:CELGENE CORP
Nanoemulsion compositions having anti-inflammatory activity
InactiveUS20070036831A1Minimizing microbial resistanceMinimize ToxicityAntibacterial agentsBiocidePathogenic microorganismDisease
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, at least one anti-inflammatory agent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Methods and compositions for the inhibition of biofilms on medical devices
Embodiments of the invention provide compositions which are effective to inhibit the development of biofilms on a surface of a medical device having the composition applied thereto, to medical devices having the composition applied to a surface thereof and to methods for using the compositions to coat medical devices.
Owner:MEDTRONIC MIMIMED INC
Powdered protein compositions and methods of making same
InactiveUS20090226530A1Easy to moveWide concentration rangeAntibacterial agentsPowder deliveryBiotechnologyProtein composition
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Compositions and methods for mucosal delivery
Mucosal surface-coat-forming film dosage units containing a water-soluble hydrocolloid, an effective dose of an active agent and a mucosal adhesion enhancer; wherein the active agent is encapsulated within a polymer which is chemically or physically distinct from the hydocolloid; wherein the mucosal adhesion enhancer is a starch graft copolymer; wherein the film exhibits a dry tack value of less than 3.5 g, a wet tack of greater than 35 g, a gelation temperature that is greater than 70° C. for a 2% polymer solution, a dry film thickness of less than 20 mil, a water content of 0.5 to 10%, a tensile strength greater than 1500 psi, a modulus in the range of 35,000 to 300,000 psi, a % elongation of less than 20%, a tear probagation resistance of 0.001 to 1 N, and a dissolution time in the range of 1 to 600 seconds upon application to an oral mucosal surface.
Owner:THALLIUM HLDG CO LLC
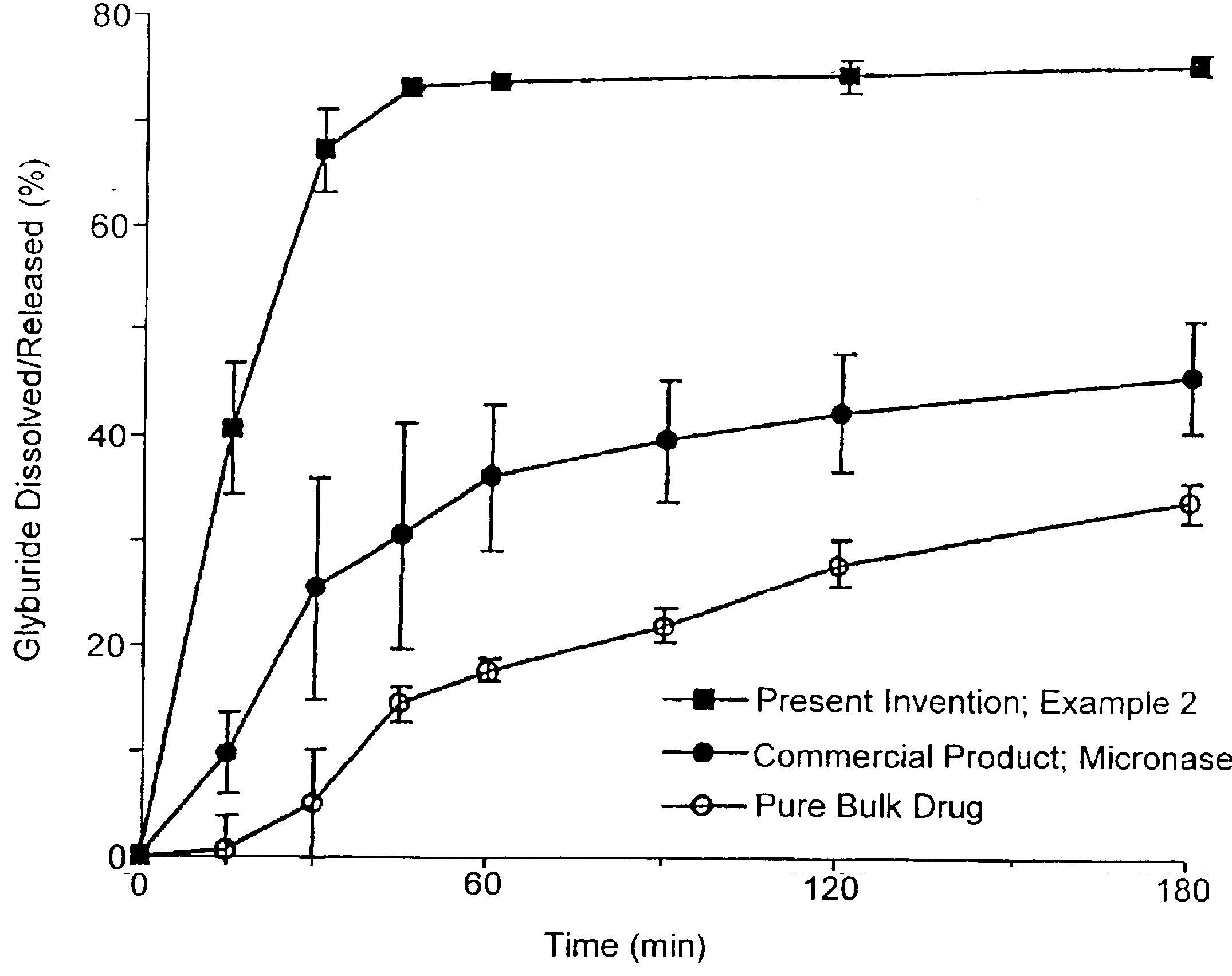
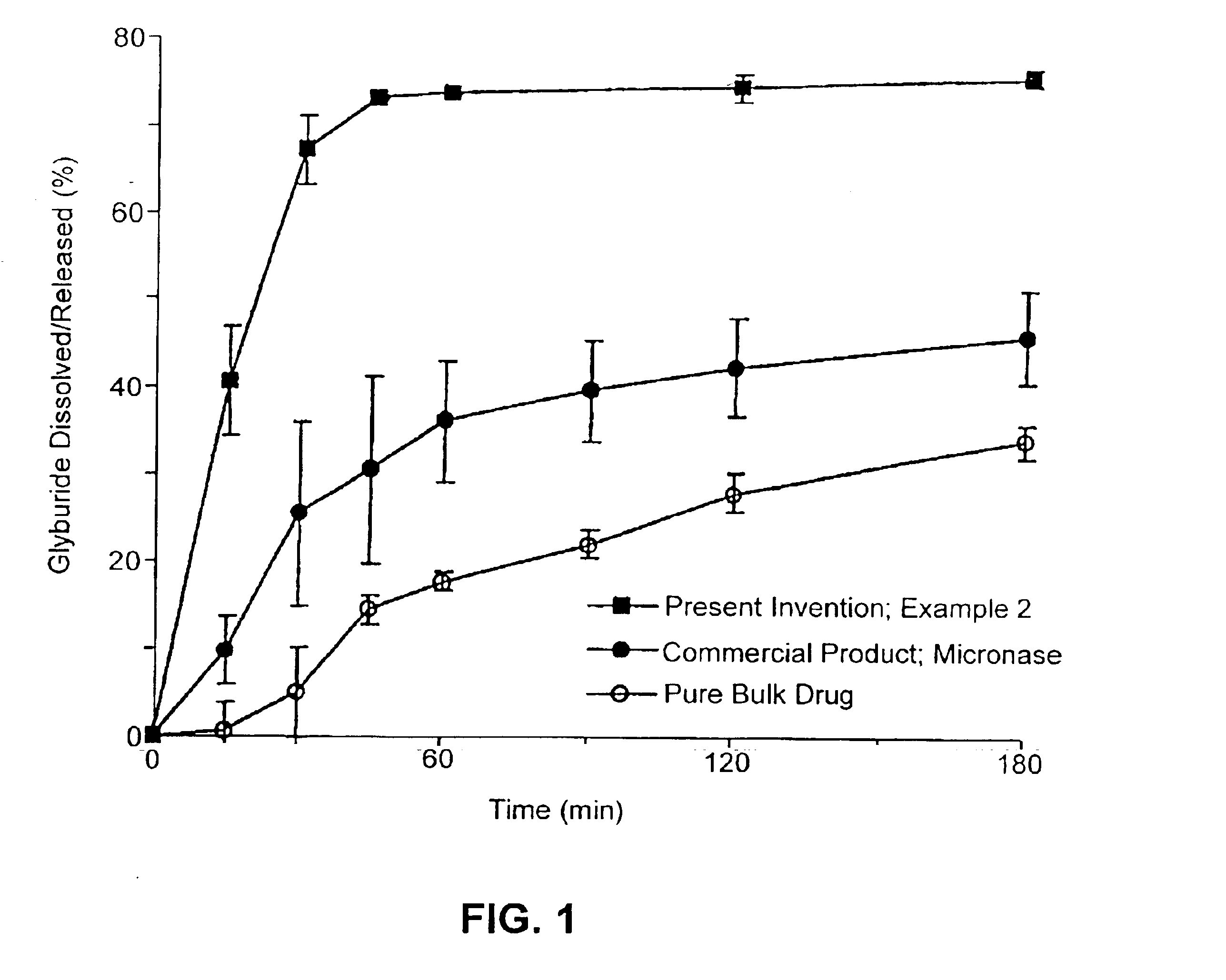
Methods of spray-drying a drug and a hydrophobic amino acid
InactiveUS6372258B1High level of stabilityEasy to manufacturePowder deliveryOrganic active ingredientsMoistureParticle-size distribution
According to the subject invention, dispersible dry powder pharmaceutical-based compositions are provided, including methods for their manufacture and dry powder dispersion devices. A dispersible dry powder pharmaceutical-based composition is one having a moisture content of less than about 10% by weight (% w) water, usually below about 5% w and preferably less than about 3% w; a particle size of about 1.0-5.0 mum mass median diameter (MMD), usually 1.0-4.0 mum MMD, and preferably 1.0-3.0 mum MMD; a delivered dose of about >30%, usually >40%, preferably >50%, and most preferred >60%; and an aerosol particle size distribution of about 1.0-5.0 mum mass median aerodynamic diameter (MMAD), usually 1.5-4.5 mum MMAD, and preferably 1.5-4.0 MMAD. Such composition are of pharmaceutical grade purity.
Owner:NOVARTIS FARMA
Oleaginous pharmaceutical and cosmetic foam
ActiveUS20050031547A1Pleasant and easy to spreadPatient compliance is goodAntibacterial agentsCosmetic preparationsActive agentNon ionic
The invention relates to stable oleaginous cosmetic or therapeutic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent selected from a hydrophobic solvent, a silicone oil, an emollient, a co-solvent, and mixtures thereof, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition; at least one gelling agent at a concentration of about 0.1% to about 5% by weight of the total composition; a therapeutically effective amount of at least one active agent; and at least one liquefied or compressed gas propellant, at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
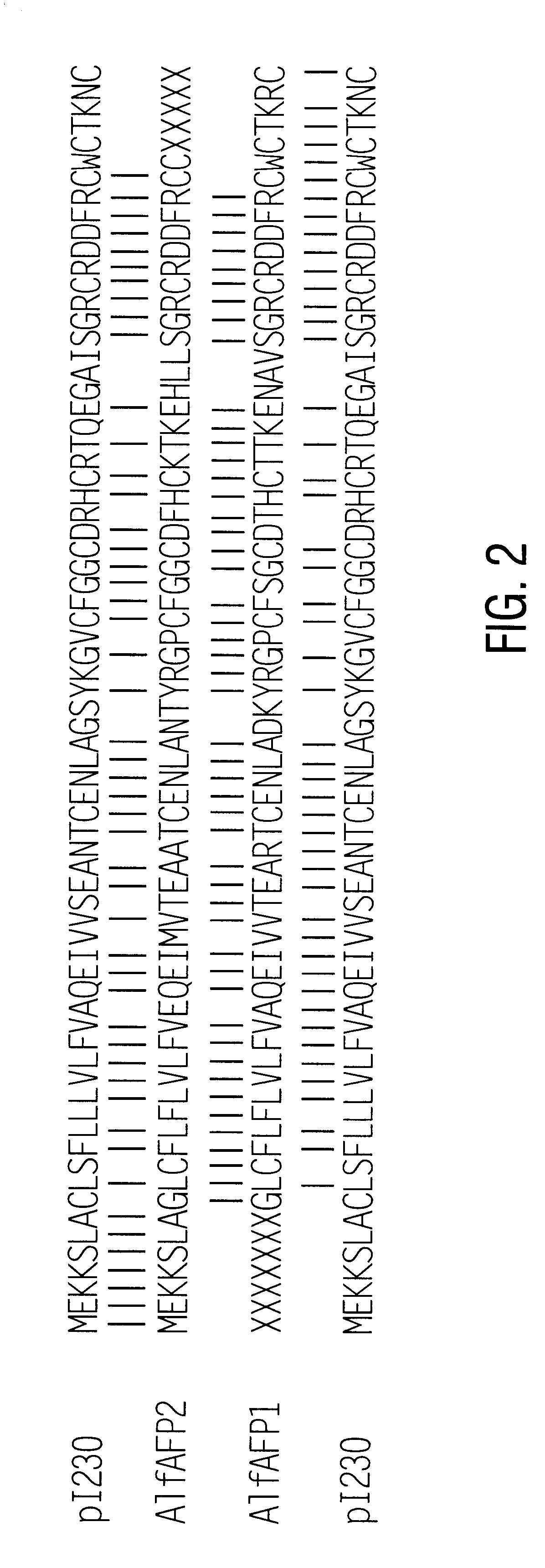
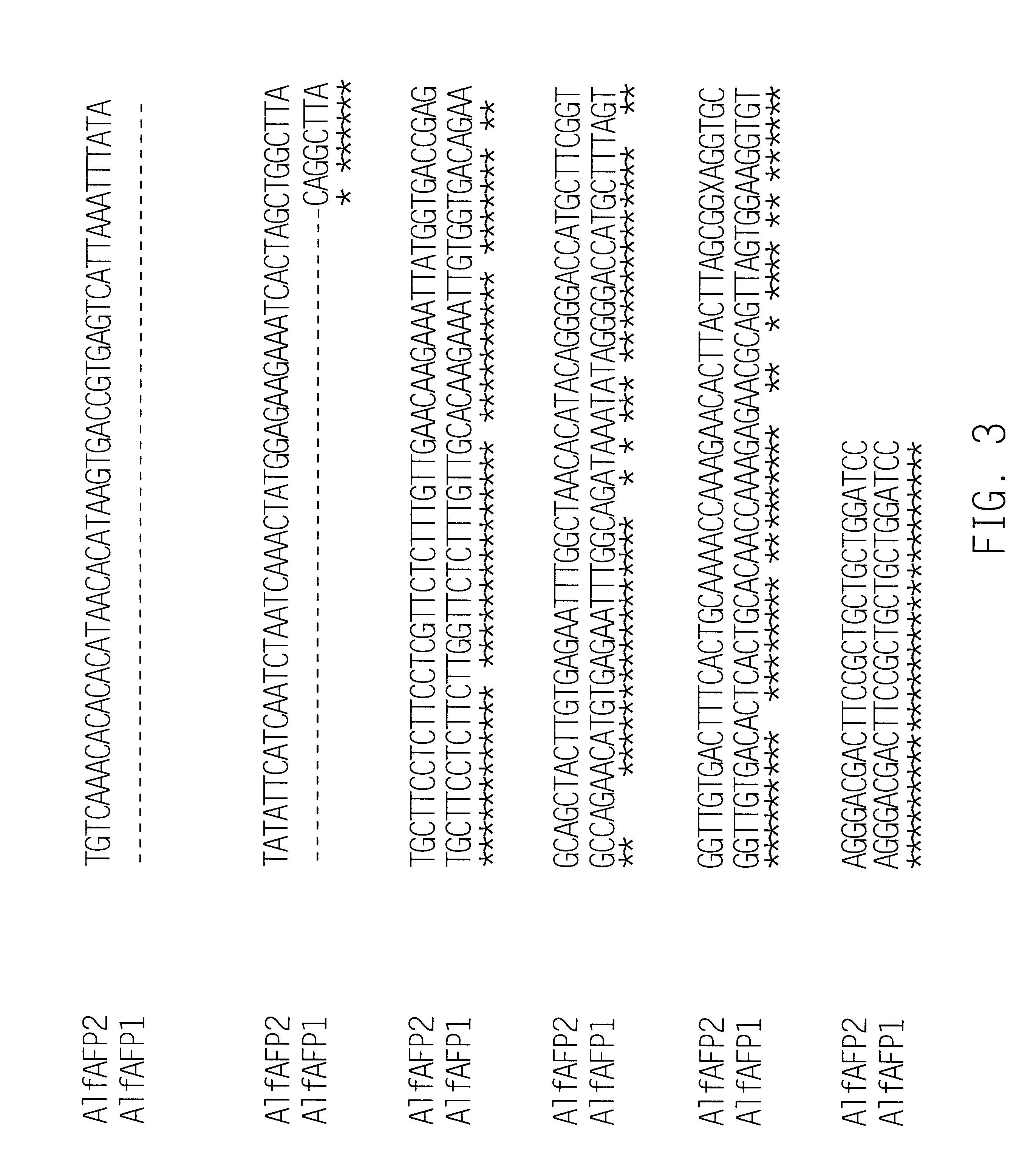
Antifungal polypeptide from alfalfa and methods for controlling plant pathogenic fungi
InactiveUS6316407B1Convenient labelingGood reproducibilityBiocideAntimycoticsPathogenic fungusNigella sativa
Antifungal polypeptides, isolated from Medicago plants, are shown to control fungal damage to plants. The polypeptides can be formulated into compositions useful in controlling undesired fungi.
Owner:MONSANTO TECH LLC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com