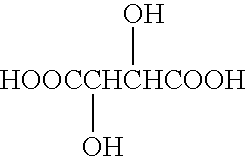
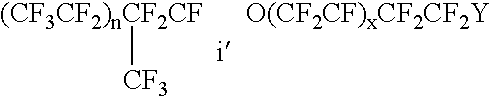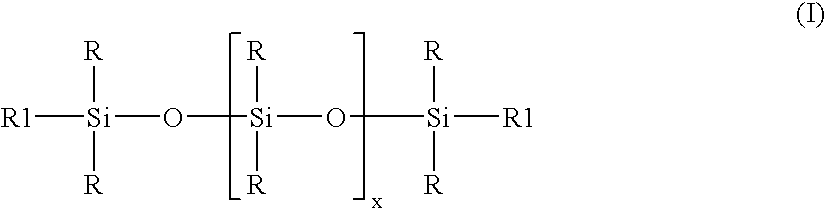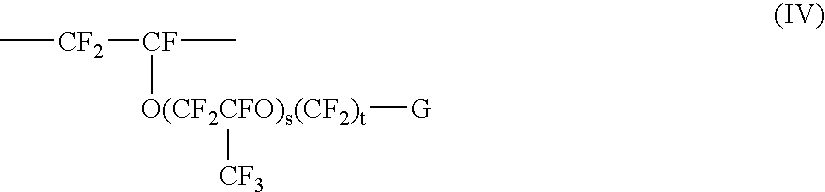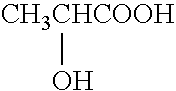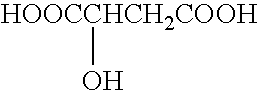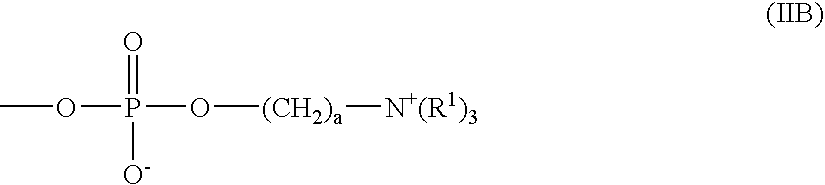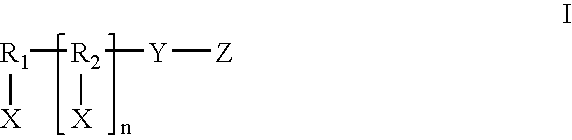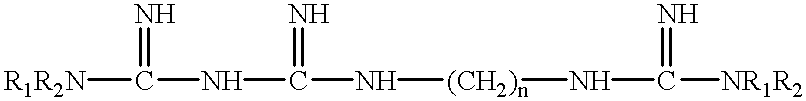Patents
Literature
1724 results about "Non ionic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of nonionic. : not ionic; especially. : not dependent on a surface-active anion for effect. nonionic surfactants.
High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same
InactiveUS7014866B2Satisfactory bioavailabilitySatisfactory dissolutionPowder deliveryBiocideHigh dosesNelfinavir mesylate
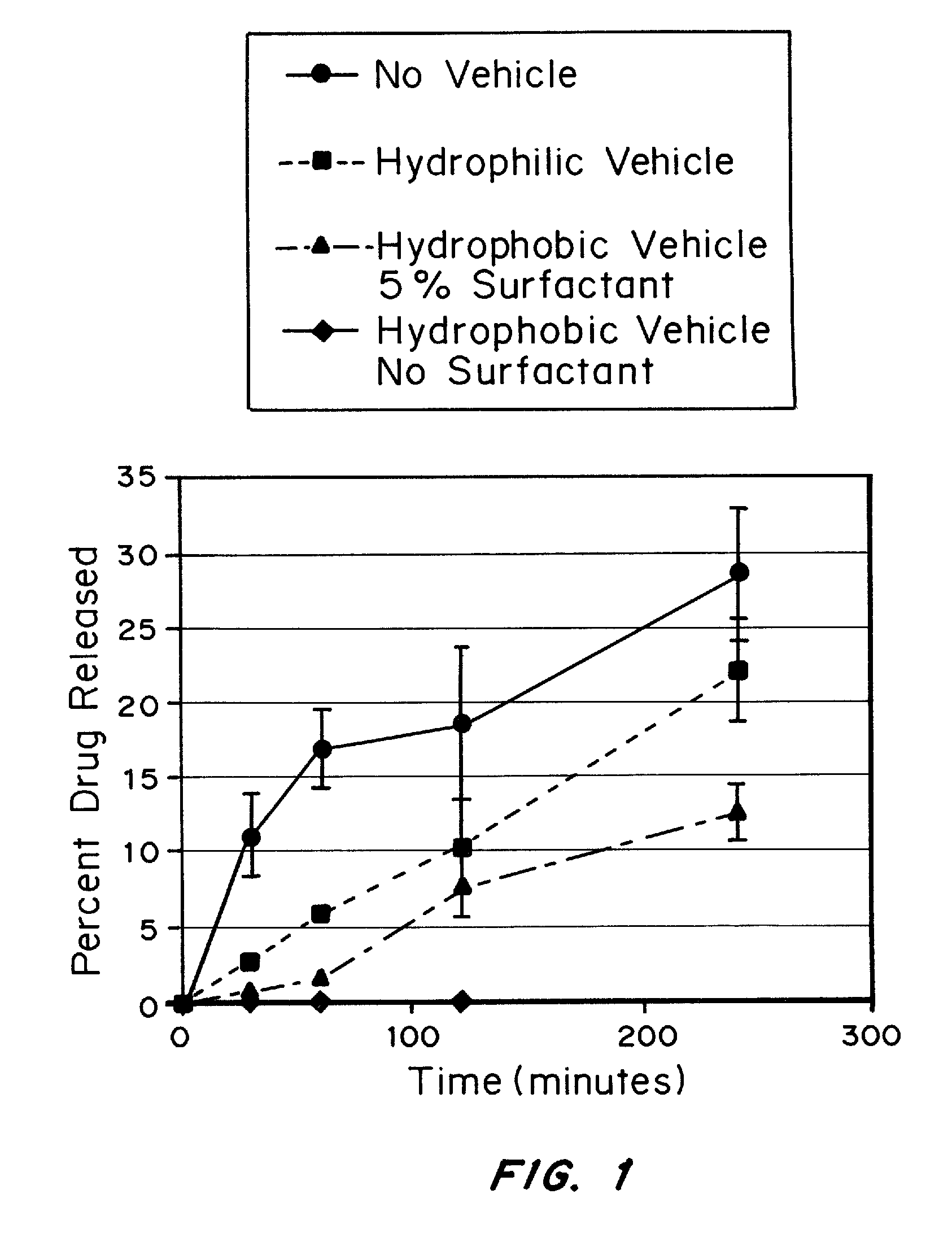
A solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate is provided comprising amorphous nelfinavir mesylate in an amount of from about 400 mg to about 700 mg calculated as nelfinavir base, and a pharmaceutically acceptable water soluble, non-ionic synthetic block copolymer of ethylene oxide and propylene oxide, the copolymer having a melting point of at least about 45° C. and an HLB value at 25° C. of from about 18 to about 29, wherein the copolymer is present from about 40% to about 65% by weight of the nelfinavir mesylate. A hot melt granulation process for making the dosage form is provided.
Owner:F HOFFMANN LA ROCHE & CO AG
Oleaginous pharmaceutical and cosmetic foam
ActiveUS20050031547A1Pleasant and easy to spreadPatient compliance is goodAntibacterial agentsCosmetic preparationsActive agentNon ionic
The invention relates to stable oleaginous cosmetic or therapeutic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent selected from a hydrophobic solvent, a silicone oil, an emollient, a co-solvent, and mixtures thereof, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition; at least one gelling agent at a concentration of about 0.1% to about 5% by weight of the total composition; a therapeutically effective amount of at least one active agent; and at least one liquefied or compressed gas propellant, at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Oleaginous pharmaceutical and cosmetic foam
InactiveUS20070292461A1Pleasant and easy to spreadPatient compliance is goodCosmetic preparationsMetabolism disorderActive agentPolyethylene glycol
The invention relates to stable pharmaceutical or cosmetic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent comprising polyethylene glycol (PEG) or PEG derivative and mixtures thereof, or comprising propylene glycol, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Compositions for removing stains from dental surfaces and methods of making and using the same
InactiveUS20050025721A1Sufficient timeCosmetic preparationsContainers for annular articlesChemistryAbrasive agent
Stain-removing oral compositions, such as gum compositions are herein provided. The compositions include a chelating agent and a surfactant. The surfactant includes a fatty acid salt and at least one component selected from nonionic and anionic surfactants. The fatty acid salt may have at least one hydroxyl functionality. The oral compositions may optionally include an abrasive agent.
Owner:INTERCONTINENTAL GREAT BRANDS
Fluoropolymer dispersion containing no or little low molecular weight fluorinated surfactant
ActiveUS6861466B2Good film formingTransportation and packagingFibre treatmentPolymer scienceRoom temperature
In an aspect of the invention, a fluoropolymer dispersion, preferably a PTFE dispersion, is provided that comprises fluoropolymer particles having an average particle size of 10 to 400 nm dispersed in water whereby the dispersion has an amount of solids between 35 and 70% by weight. The dispersion is free of fluorinated surfactant having a molecular weight of less than 1000 g / mol (hereinafter called low molecular weight fluorinated surfactant) or contains the low molecular weight fluorinated surfactant in an amount of not more than 0.05% by weight based on the total weight solids of the dispersion. The dispersion further comprises a non-ionic non-fluorinated surfactant or mixture of non-ionic non-fluorinated surfactants and one or more non-fluorinated anionic surfactants. Through the use of a non-fluorinated anionic surfactant, a dispersion is obtained that has a low viscosity at room temperature (20° C.). The dispersion is further free of aromatic group containing non-ionic surfactants and is accordingly environmentally more friendly and can yield coatings that are less susceptible of discoloration. The amount and nature of the non-ionic non-fluorinated surfactant or mixture of non-ionic non-fluorinated surfactants is selected such that the Viscosity Transition Temperature (VTT) (measured as set forth in the examples) of the fluoropolymer dispersion is at least 26, preferably at least 28° C. In a further aspect of the invention, a method is provided to obtain the aforementioned dispersion.
Owner:3M INNOVATIVE PROPERTIES CO
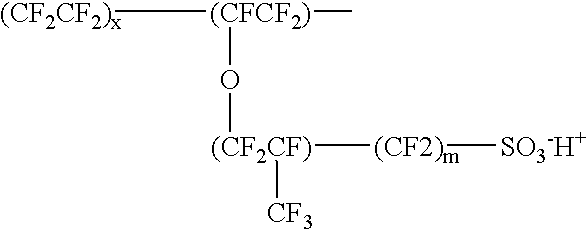
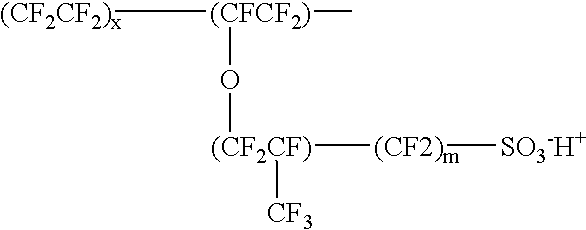
Proton-selective conducting membranes
InactiveUS20020127474A1Easy to operateSelective operationIon-exchanger regenerationSolid electrolyte cellsHydrophobic polymerProton
A membrane comprising: (a) a hydrophobic matrix polymer, and (b) a hydrophilic non-ionic polymer, wherein the hydrophobic polymer and the hydrophilic polymer are disposed so as to form a dense selectively proton-conducting membrane. The microstructure of such a membrane can be tailored to specific functionality requirements, such as proton conductivity vs. proton selectivity, and selectivity to particular species.
Owner:E C R ELECTRO CHEM RES
Manufacture of stable low particle size organopolysiloxane emuslion
ActiveUS20070276087A1Rapid emulsificationReduce polymerization timeMaterial nanotechnologyOther chemical processesPolymer scienceEmulsion
Stable high viscosity organopolysiloxane emulsions with particle sizes up to 150 nanometer may be made in a simple and cost-effective manner employing a standard homogenizer, and optional subsequent polymerization of the organopolysiloxan at controlled temperature. A combination of non-ionic emulsifier together with an at least one anionic emulsifier is employed, having an HLB value 12-15, while maintaining a temperature up to 50° C.
Owner:WACKER CHEM GMBH
Oleaginous pharmaceutical and cosmetic foam
InactiveUS20080063607A1Pleasant and easy to spreadPatient compliance is goodBiocideCosmetic preparationsActive agentPolyethylene glycol
The invention relates to stable pharmaceutical or cosmetic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent comprising polyethylene glycol (PEG) or PEG derivative and mixtures thereof, or comprising propylene glycol, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
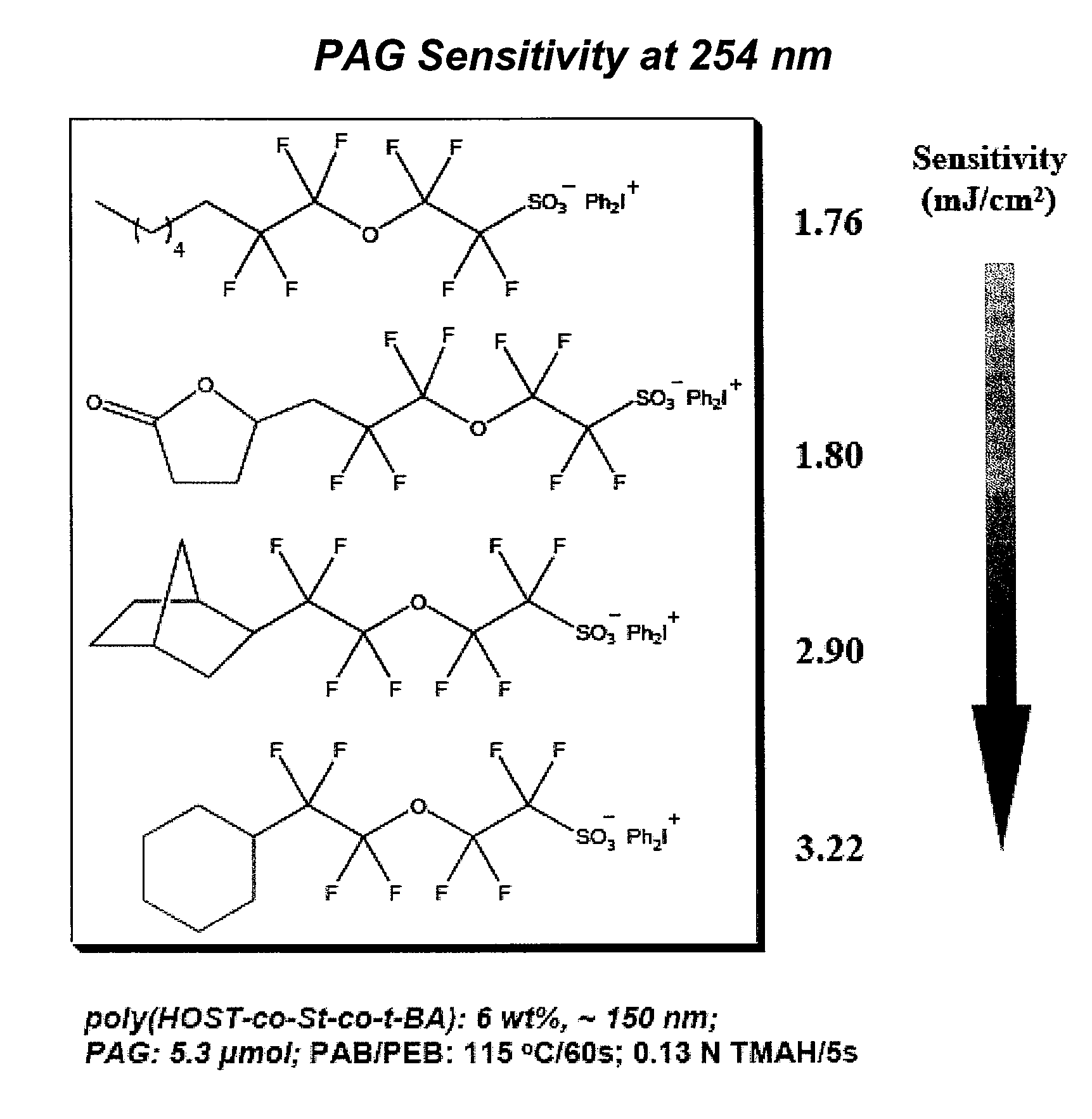
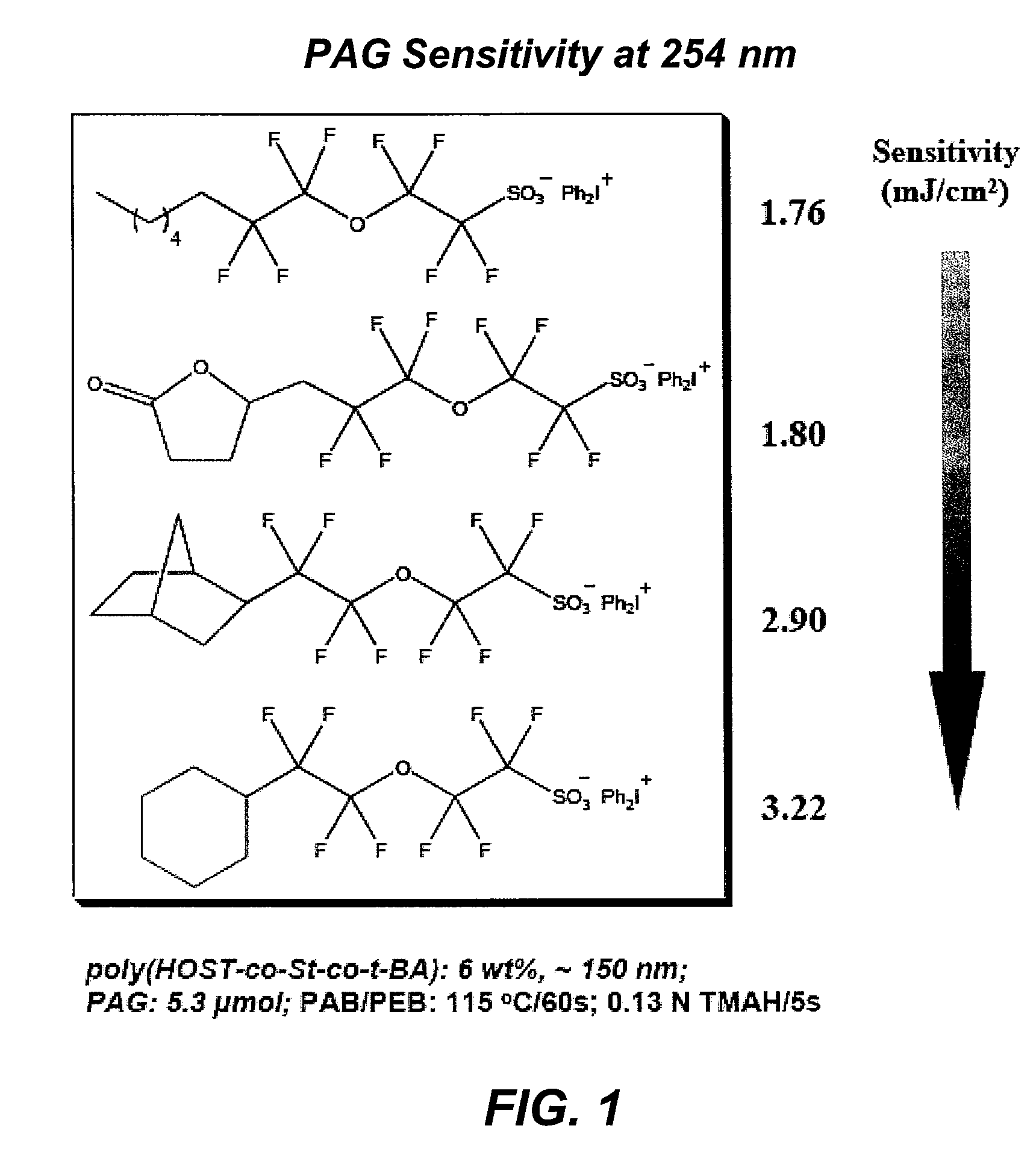
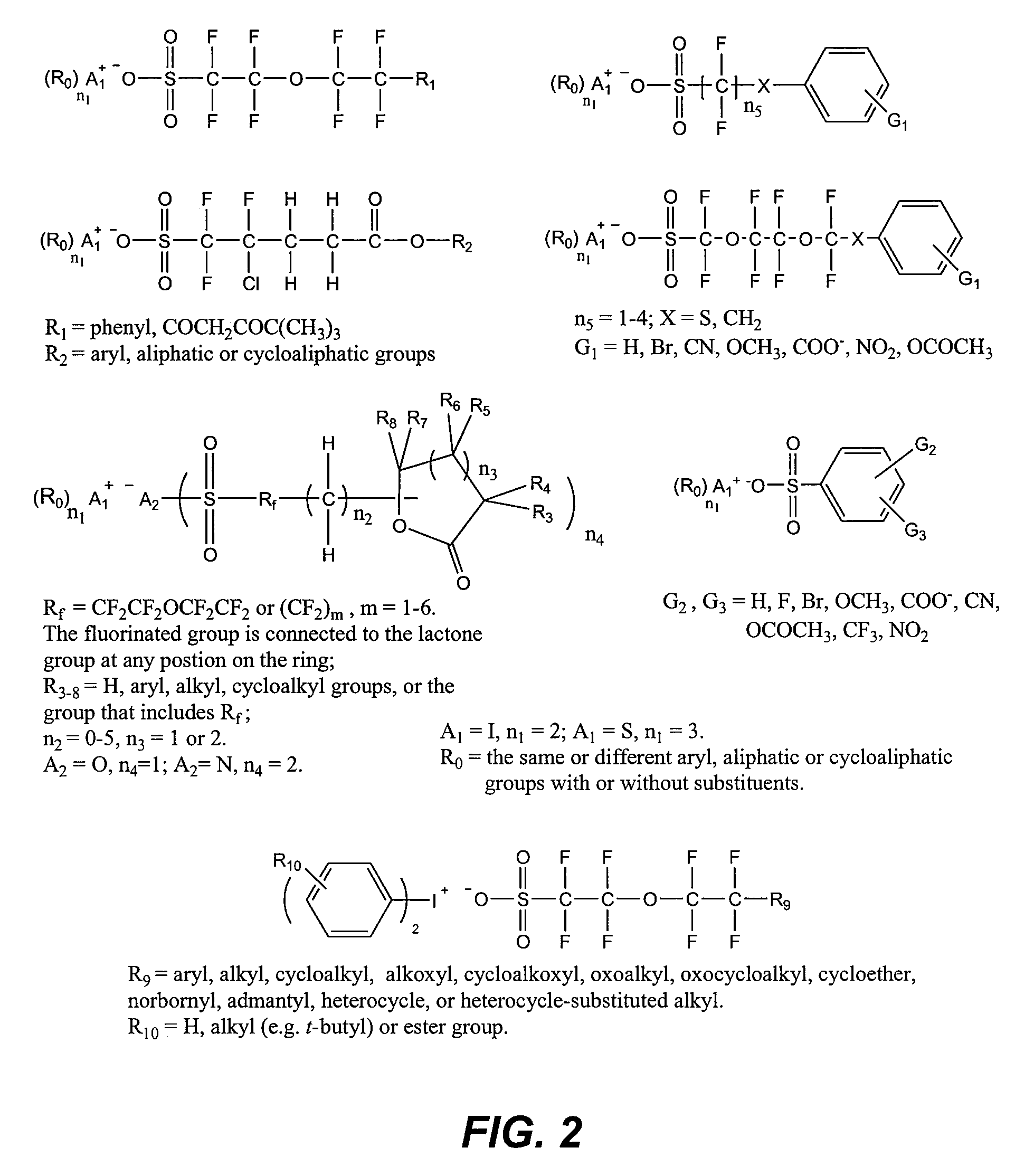
Photoacid generator compounds and compositions
InactiveUS7824839B2Appropriate mobilityHomogeneous distribution of in resistOrganic chemistryOrganic compound preparationResistMicrofabrication
The invention provides various ionic and non-ionic photoacid generator compounds. Photoresist compositions that include the novel ionic and non-ionic photoacid generator compounds are also provided. The invention further provides methods of making and using the photoacid generator compounds and photoresist compositions disclosed herein. The compounds and compositions are useful as photoactive components in chemically amplified resist compositions for various microfabrication applications.
Owner:CORNELL RES FOUNDATION INC
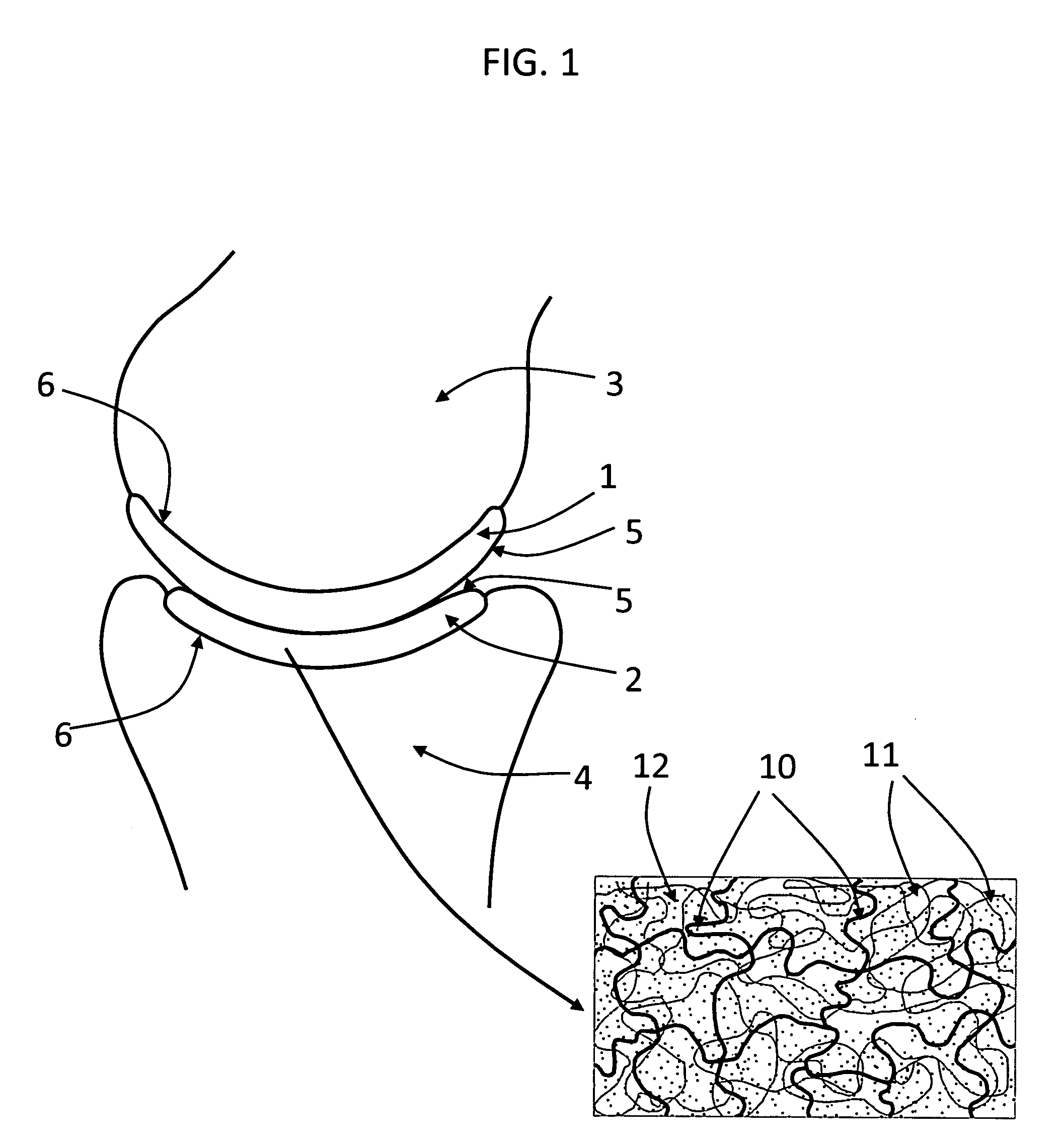
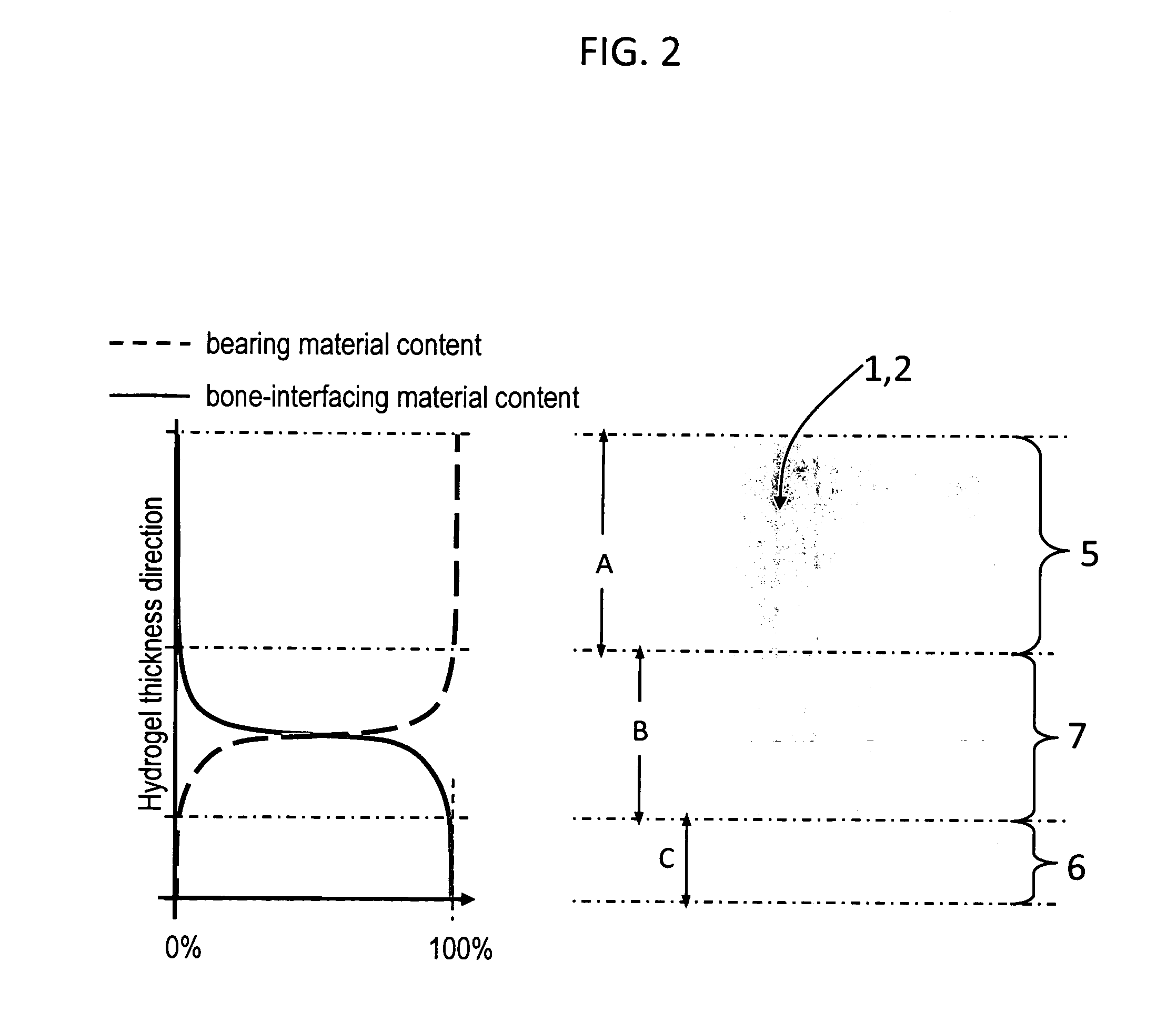
Hydrogel arthroplasty device
InactiveUS20090088846A1Stimulate bone cell growthEasy adhesionFinger jointsPowder deliveryCross-linkNeutral ph
An arthroplasty device is provided having an interpenetrating polymer network (IPN) hydrogel that is strain-hardened by swelling and adapted to be held in place in a joint by conforming to a bone geometry. The strain-hardened IPN hydrogel is based on two different networks: (1) a non-silicone network of preformed hydrophilic non-ionic telechelic macromonomers chemically cross-linked by polymerization of its end-groups, and (2) a non-silicone network of ionizable monomers. The second network was polymerized and chemically cross-linked in the presence of the first network and has formed physical cross-links with the first network. Within the IPN, the degree of chemical cross-linking in the second network is less than in the first network. An aqueous salt solution (neutral pH) is used to ionize and swell the second network. The swelling of the second network is constrained by the first network resulting in an increase in effective physical cross-links within the IPN.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
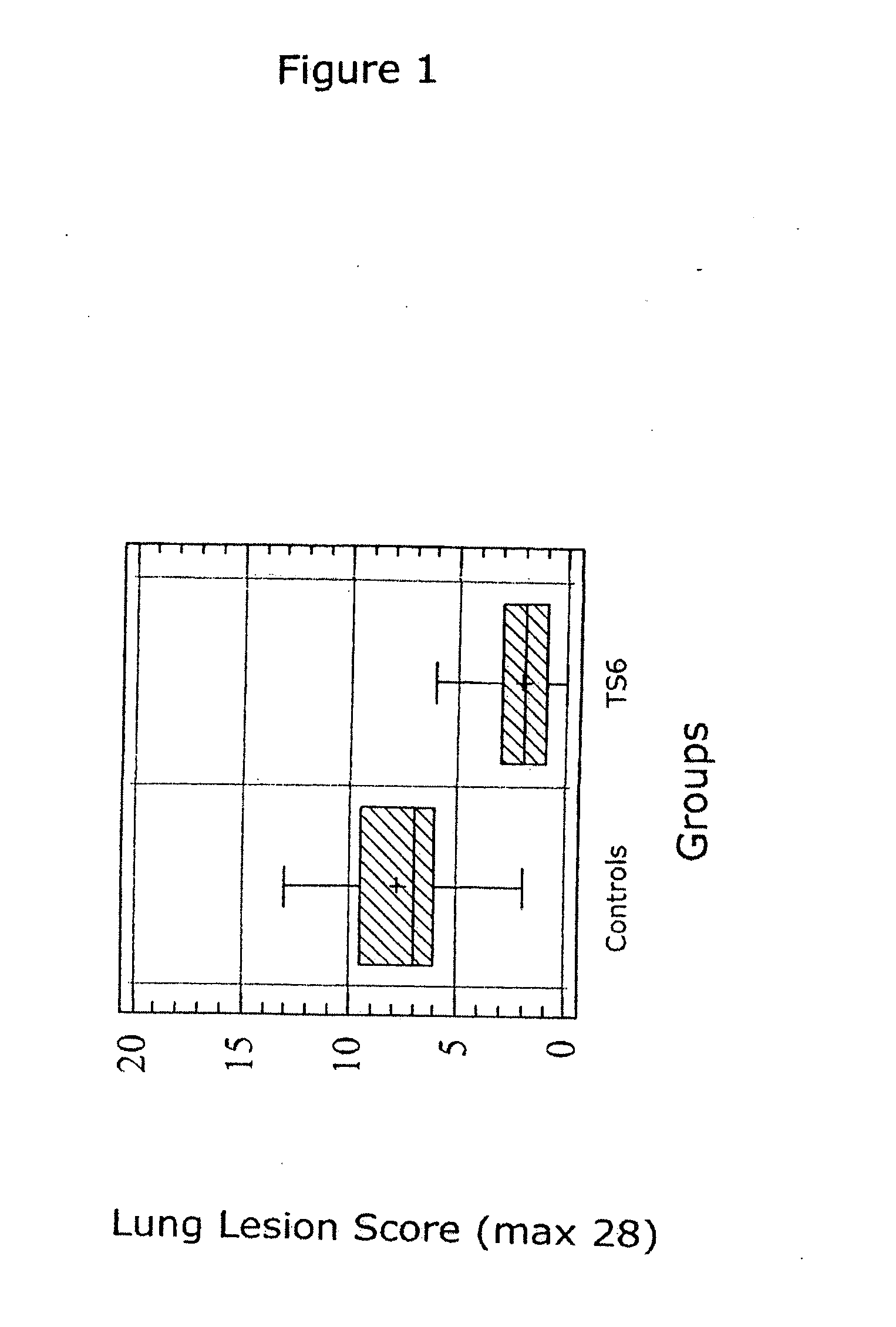
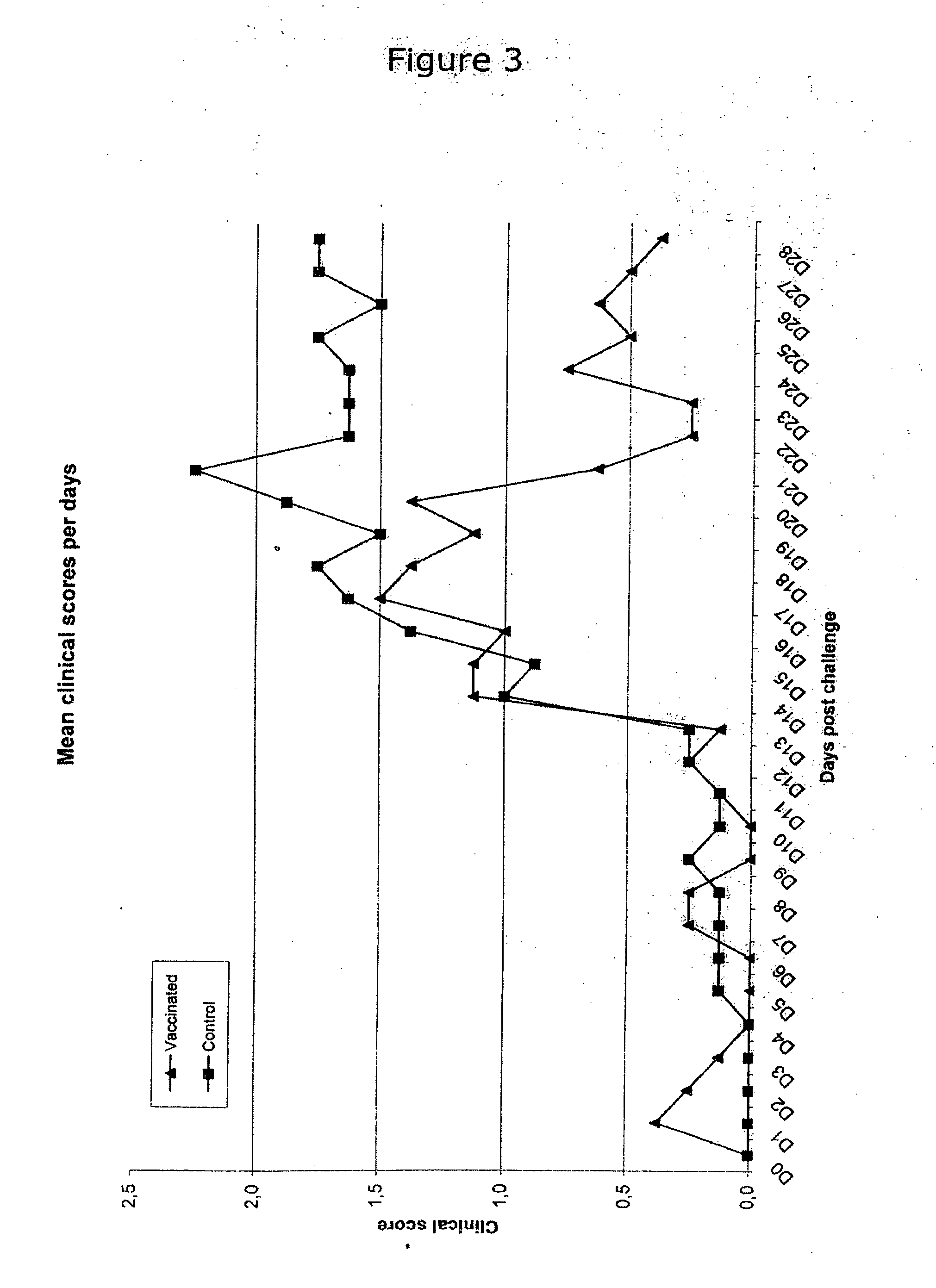
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
Microfluidic surfaces
ActiveUS20020125135A1Easy to createStrong interactionSludge treatmentVolume/mass flow measurementPlanar substrateNon ionic
A microfluidic device comprising a set of one or more, preferably more than 5, covered microchannel structures manufactured in the surface of a planar substrate. The device is characterized in that a part surface of at least one of the microchannel structures has a coat exposing a non-ionic hydrophilic polymer. The non-ionic hydrophilic polymer is preferably attached covalently directly to the part surface or to a polymer skeleton that is attached to the surface.
Owner:GYROS
Fluoropolymer dispersions containing no or little low molecular weight fluorinated surfactant
Owner:3M INNOVATIVE PROPERTIES CO
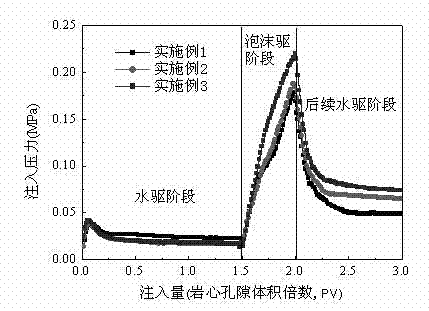
Foaming agent suitable for oil-field development
ActiveCN102504788AHigh production costReduce manufacturing costDrilling compositionActive agentEngineering
The invention provides a foaming agent suitable for oil-field development. The foaming agent suitable for oil-field development comprises the following components by weight percent: 0.05-1.0% of anionic surfactant, 0.05-1.0% of amphoteric surfactant, 0-1.0% of nonionic surfactant, 0.01-1.0% of foam stabilizer, 0-0.8% of inorganic salt and the balance water. The composition and proportioning of the foaming agent can be adjusted at any time according to the actual situations of different application fields of oil-field development, the foaming agent is convenient in production, has wide applicability and remarkable economic benefit; and the foaming agent has good biodegradability and can not pollute the environment or damage the formation.
Owner:PETROCHINA CO LTD +1
Compositions and methods using microspheres and non-ionic contrast agents
ActiveUS20060251582A1Minimizing side-effectsImprove load effectAntibacterial agentsPowder deliveryAbnormal tissue growthEmbolization Therapy
The present invention relates to compositions and methods for treating diseases and disorders including cancer and various other angiogenic-dependent diseases, vascular malfunctions, arteriovenous malformations (AVM), hemorrhagic processes and treatment of pain, in particular tumor-related pain by drug delivery and / or therapeutic embolization using microspheres. More particularly the invention relates to microspheres containing non-ionic contrast agents, to compositions comprising these microspheres, as well as methods for preparing and using such compositions for embolization therapy. The invention further relates to compositions and methods using detectable microspheres for targeted drug delivery, irrespective of whether embolization is also needed.
Owner:BIOSPHERE MEDICAL SA (FR)
Processes for removing cells and cell debris from tissue and tissue constructs used in transplantation and tissue reconstruction
InactiveUS20070123700A1Improve inflammatory responseImprove responseHeart valvesDead animal preservationPresent methodFresh Tissue
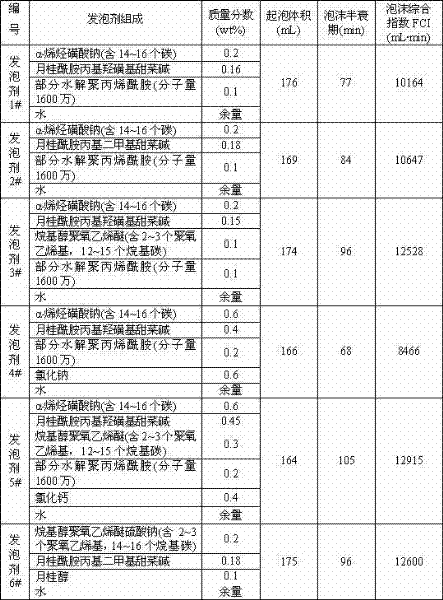
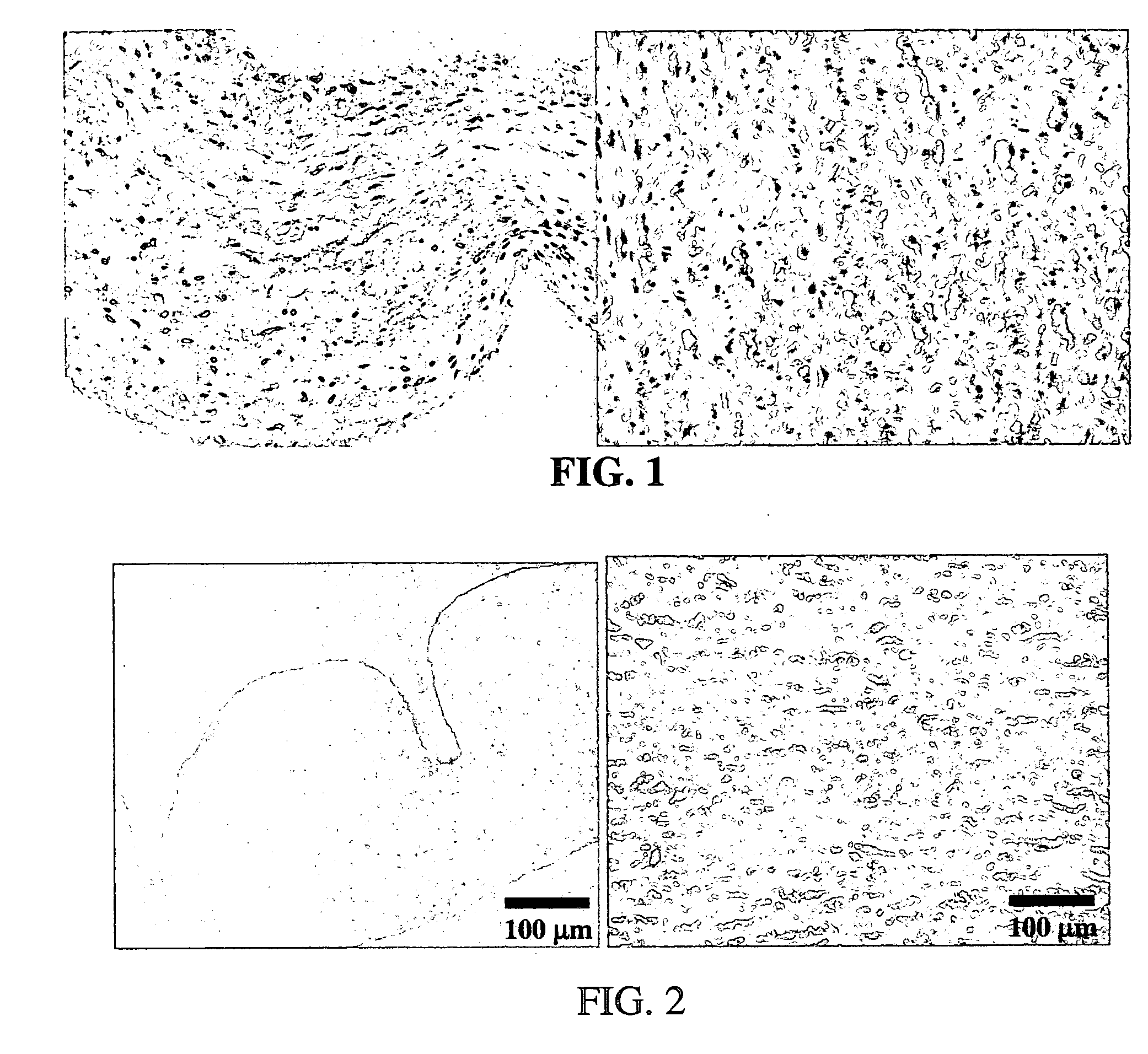
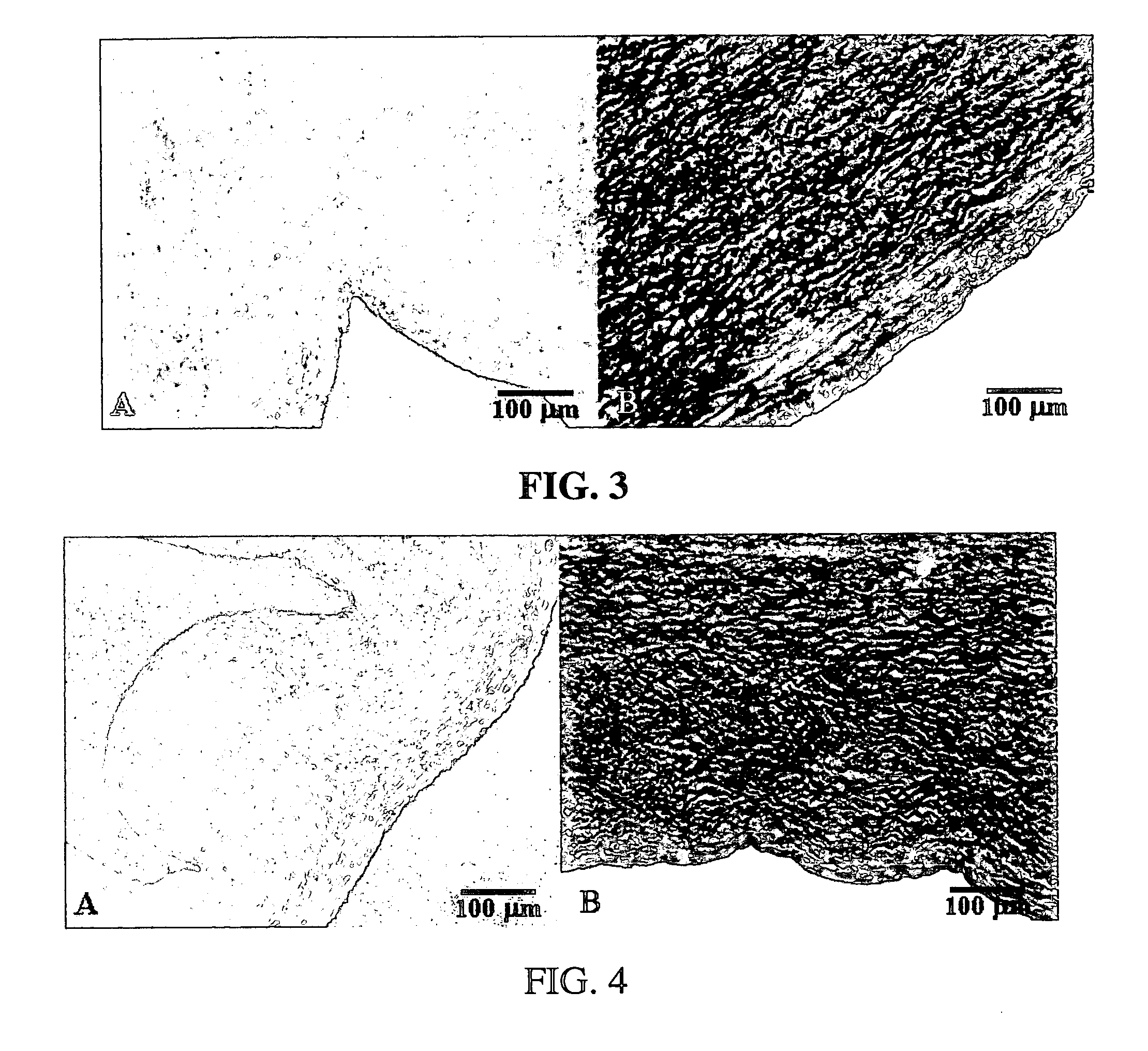
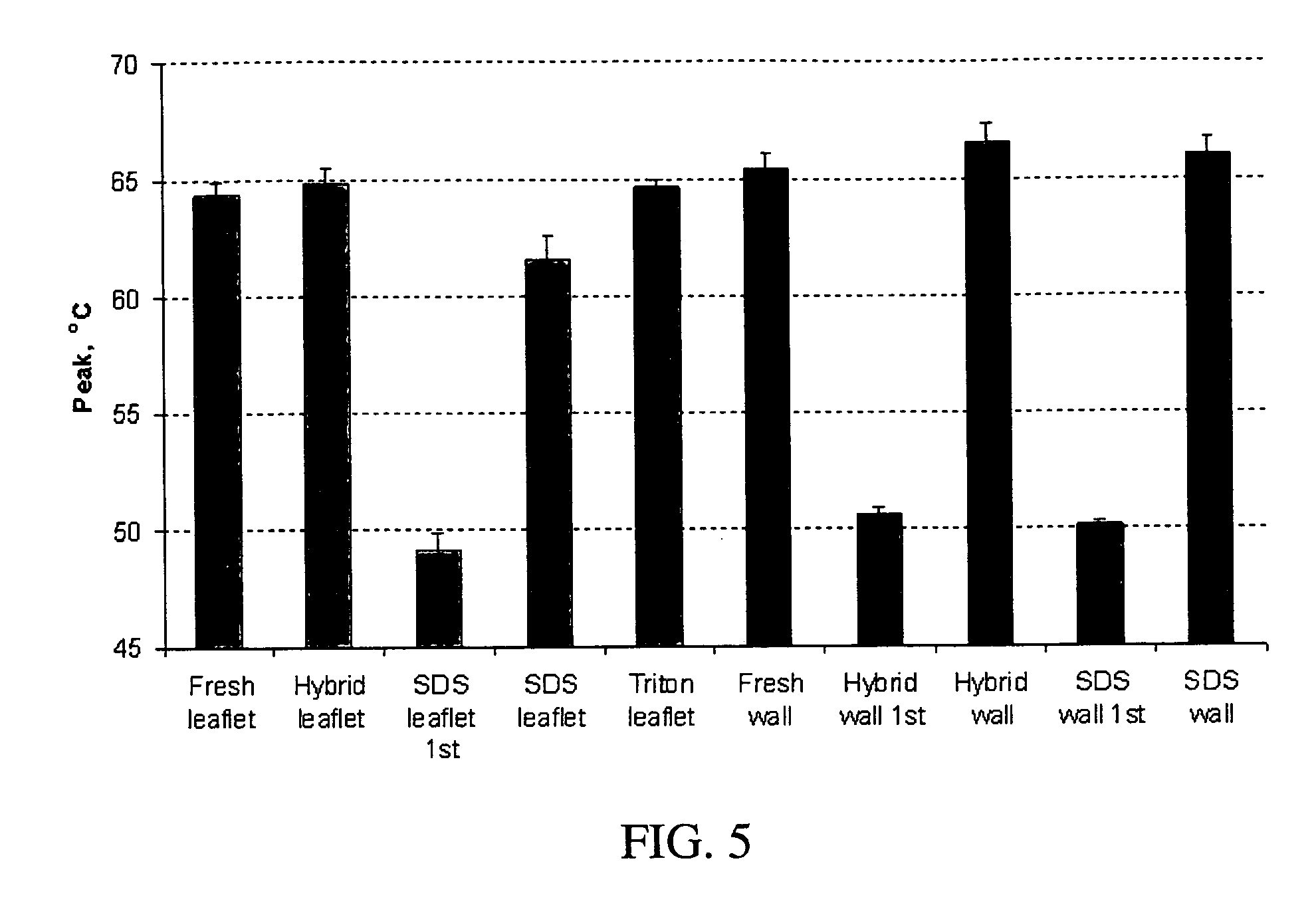
Methods for decellularizing mammalian tissue for use in transplantation and tissue engineering. The invention includes methods for simultaneous application of an ionic detergent and a nonionic detergent for a long time period, which may exceed five days. One method utilizes SDS as the ionic detergent and Triton-X 100 as the nonionic detergent. A long rinse step follows, which may also exceed five days in length. This long duration, simultaneous extraction with two detergents produced tissue showing stress-strain curves and DSC data similar to that of fresh, unprocessed tissue. The processed tissue is largely devoid of cells, has the underlying structure essentially intact, and also shows a significantly improved inflammatory response relative to fresh tissue, even without glutaraldehyde fixation. Significantly reduced in situ calcification has also been demonstrated relative to glutaraldehyde fixed tissue. Applicants believe the ionic and non-ionic detergents may act synergistically to bind protein to the ionic detergent and may remove an ionic detergent-protein complex from the tissue using the non-ionic detergent. The present methods find one exemplary use in decellularizing porcine heart valve leaflet and wall tissue for use in transplantation.
Owner:UEDA YUICHIRO +1
Topical Therapeutic Delivery System
The present invention relates to an oil-in-water emulsion topical delivery system comprising an oil phase; an aqueous phase; phenoxyethanol; an effective exfoliatingamount of a hydrophobic hydroxycarboxylic acid; a non-ionic emulsifier having an HLB of from about 7 to about 10; and at least one skin-supporting ordermatopharmaceutically active agent.
Owner:MURAD HOWARD
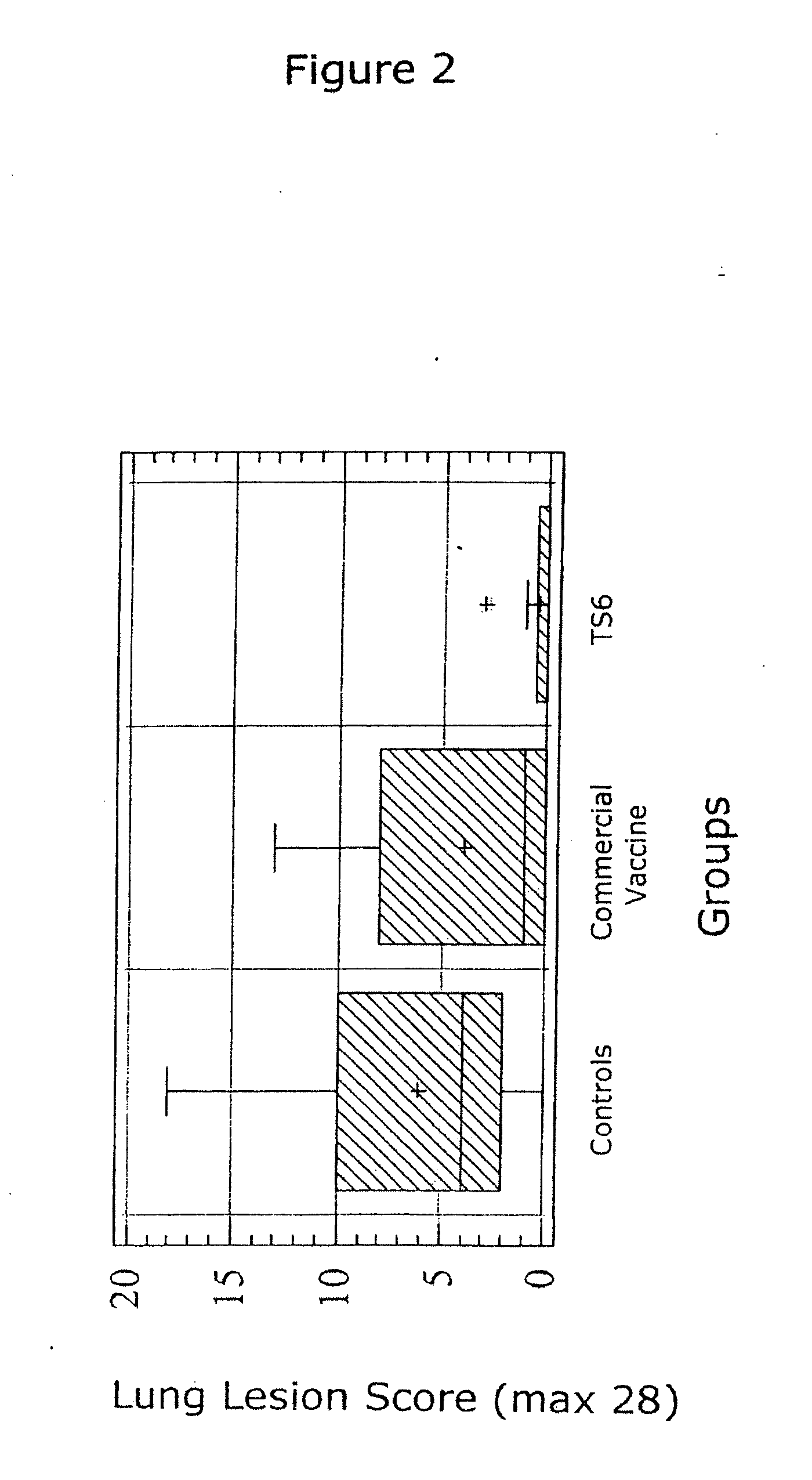
Novel vaccine formulations
ActiveUS20060233831A1Improve stabilityStable and safe and easily administrableAntibacterial agentsBiocideAdjuvantNon ionic
The present invention relates to oil-in-water emulsions, their use as adjuvants, and pharmaceutical, immunologic, or vaccine compositions that may comprise the same. In one embodiment, the oil-in-water (O / W) emulsion may comprise an aqueous solution containing an immunogen, a mineral oil, a non-ionic lipophilic ethoxylated fatty alcohol and a non-ionic hydrophilic surfactant. In another embodiment, the oil-in-water (O / W) emulsion may comprise an aqueous solution containing an immunogen, a non-ionic lipophilic surfactant, a mineral oil and a non-ionic hydrophilic ethoxylated fatty alcohol. The present invention also encompasses a method of making a vaccine composition using the adjuvant of the instant invention, the vaccine composition so obtained and methods of use.
Owner:MERIAL INC
Ophthalmic Solutions
InactiveUS20080314767A1Avoid pollutionLens cleaning compositionsPackage sterilisationOsmolar ConcentrationNon ionic
Disclosed are ophthalmic solutions such as packaging solutions for storing ophthalmic devices and lens care solutions for cleaning, disinfecting, rinsing and / or storing ophthalmic devices. The ophthalmic solutions contain at least a polymerization product obtained from a monomeric mixture comprising (a) a monomer bearing a center of permanent positive charge and (b) a non-ionic ethylenically unsaturated monomer, wherein the solution has an osmolality of at least about 200 mOsm / kg, and a pH of about 4 to about 9.
Owner:BAUSCH & LOMB INC
Slurry compositions and CMP methods using the same
InactiveUS20050130428A1Reduce and eliminate excessive removalOther chemical processesSemiconductor/solid-state device manufacturingArylSlurry
The exemplary embodiments of the present invention providing new slurry compositions suitable for use in processes involving the chemical mechanical polishing (CMP) of a polysilicon layer. The slurry compositions include one or more non-ionic polymeric surfactants that will selectively form a passivation layer on an exposed polysilicon surface in order to suppress the polysilicon removal rate relative to silicon oxide and silicon nitride and improve the planarity of the polished substrate. Exemplary surfactants include alkyl and aryl alcohols of ethylene oxide (EO) and propylene oxide (PO) block copolymers and may be present in the slurry compositions in an amount of up to about 5 wt %, although much smaller concentrations may be effective. Other slurry additives may include viscosity modifiers, pH modifiers, dispersion agents, chelating agents, and amine or imine surfactants suitable for modifying the relative removal rates of silicon nitride and silicon oxide.
Owner:SAMSUNG ELECTRONICS CO LTD
Hydrophilic polyproylene melt additives
InactiveUS20120077886A1Stable and durable hydrophilicityLow costBiocideCosmetic preparationsPolymer scienceThermosetting polymer
Melt additive ionic and non-ionic surfactants to impart stable durable hydrophilicity to thermoplastic polymers or blends thereof.
Owner:3M INNOVATIVE PROPERTIES CO
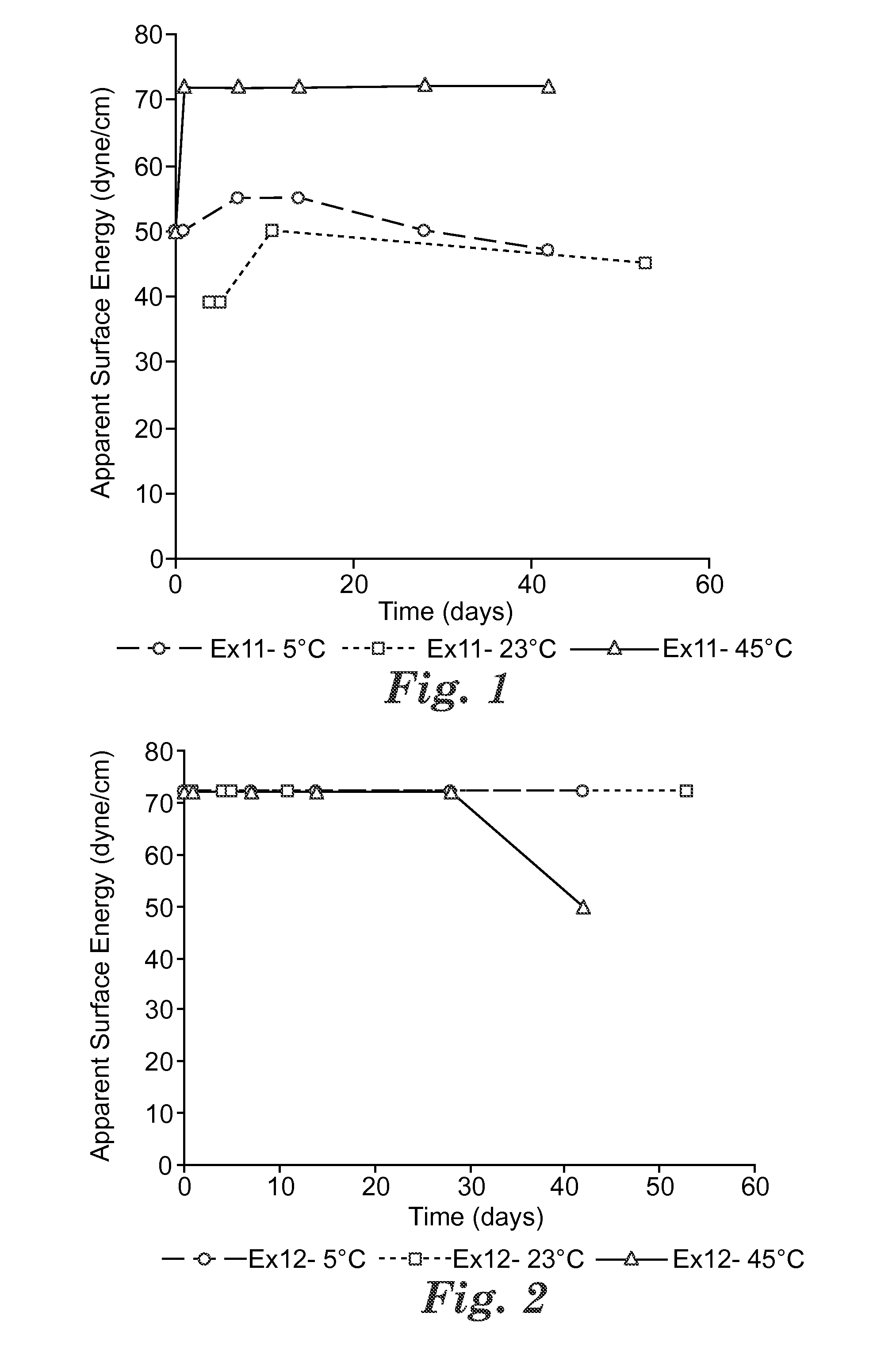
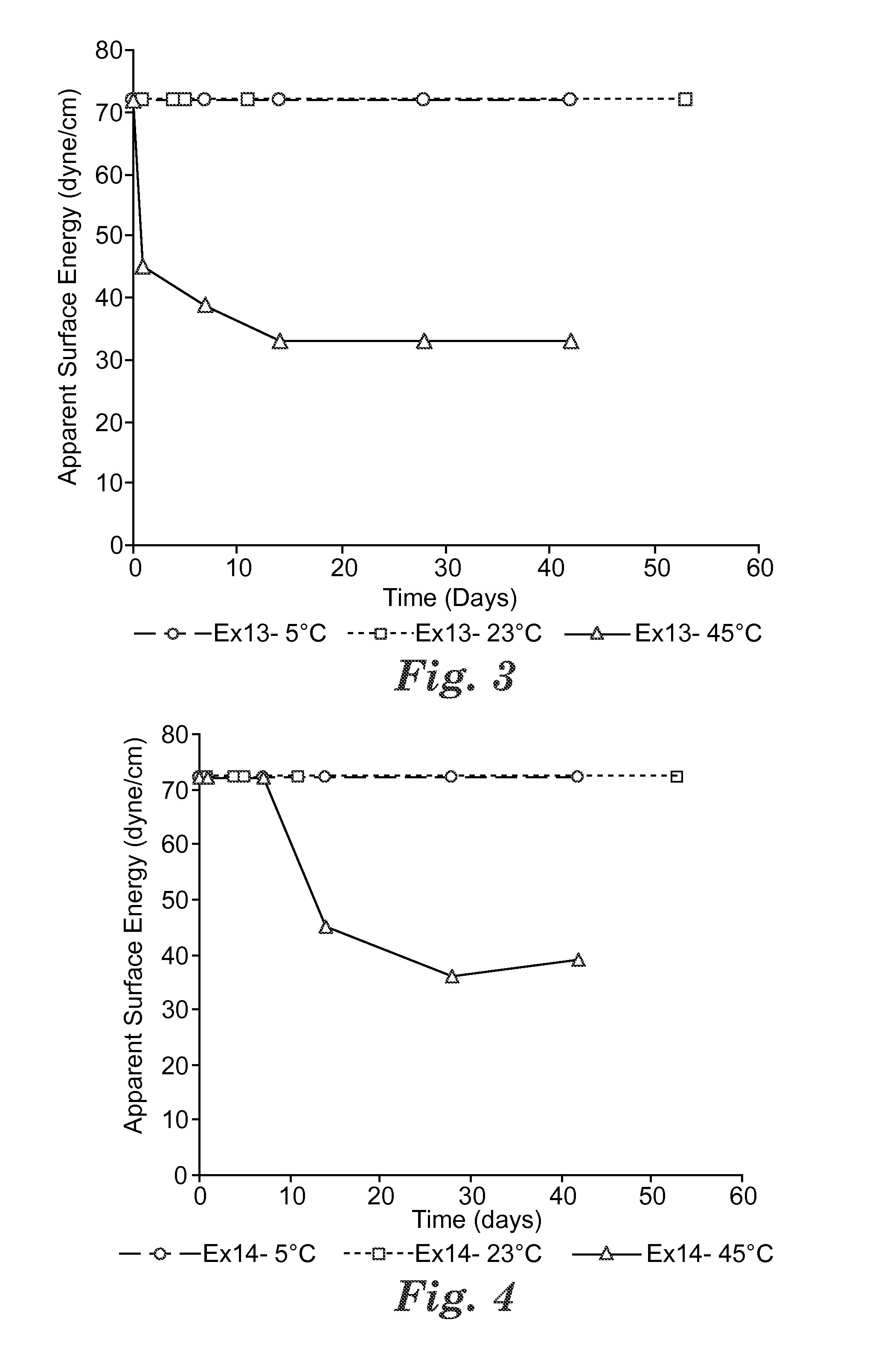
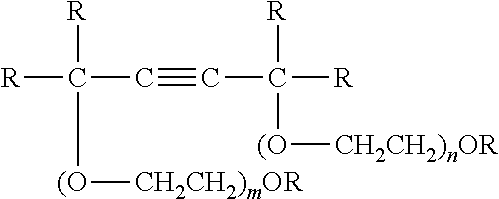
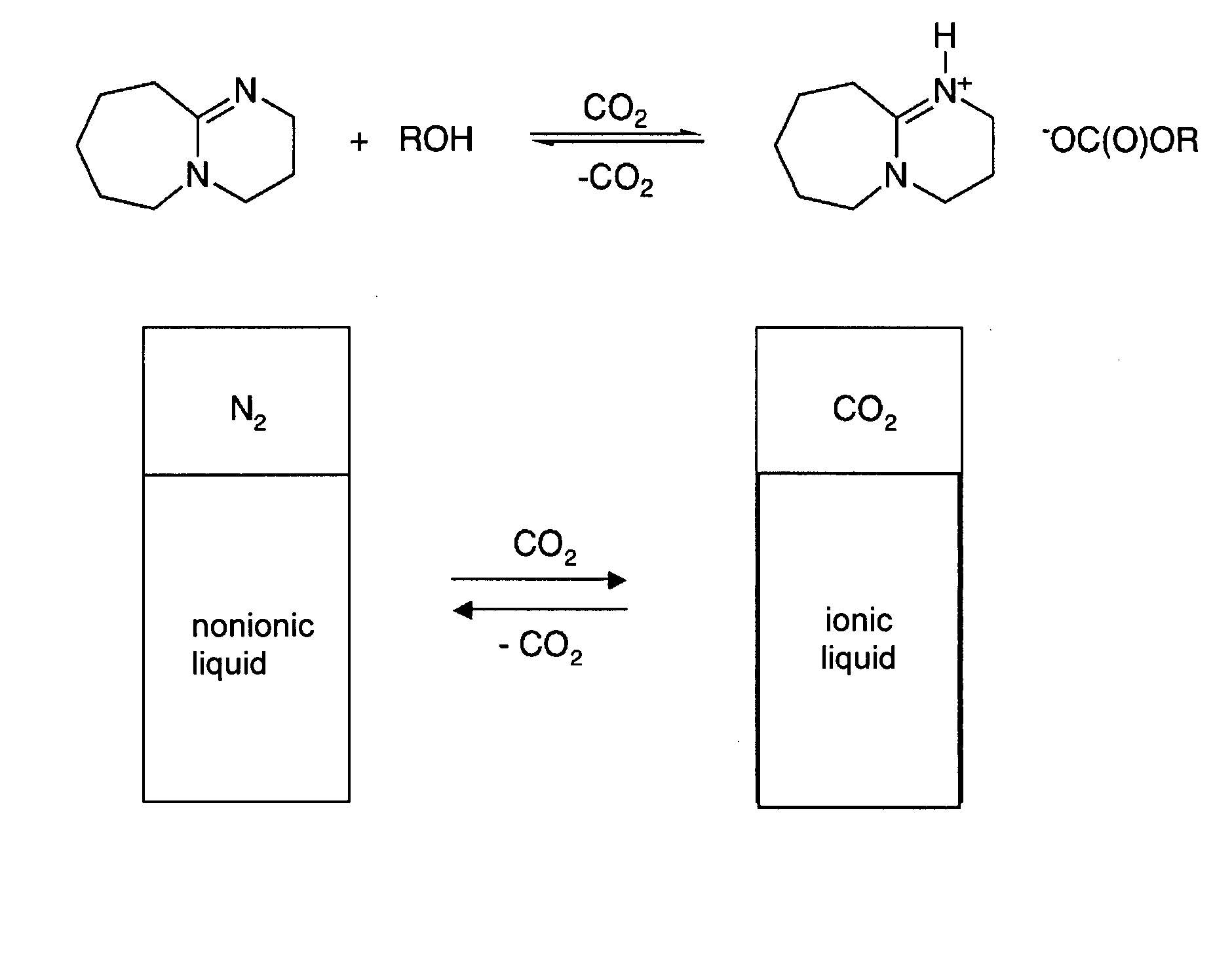
Switchable solvents and methods of use thereof
InactiveUS20080058549A1Low viscosityOrganic compounds purification/separation/stabilisationSolvent extractionAlcoholNon ionic
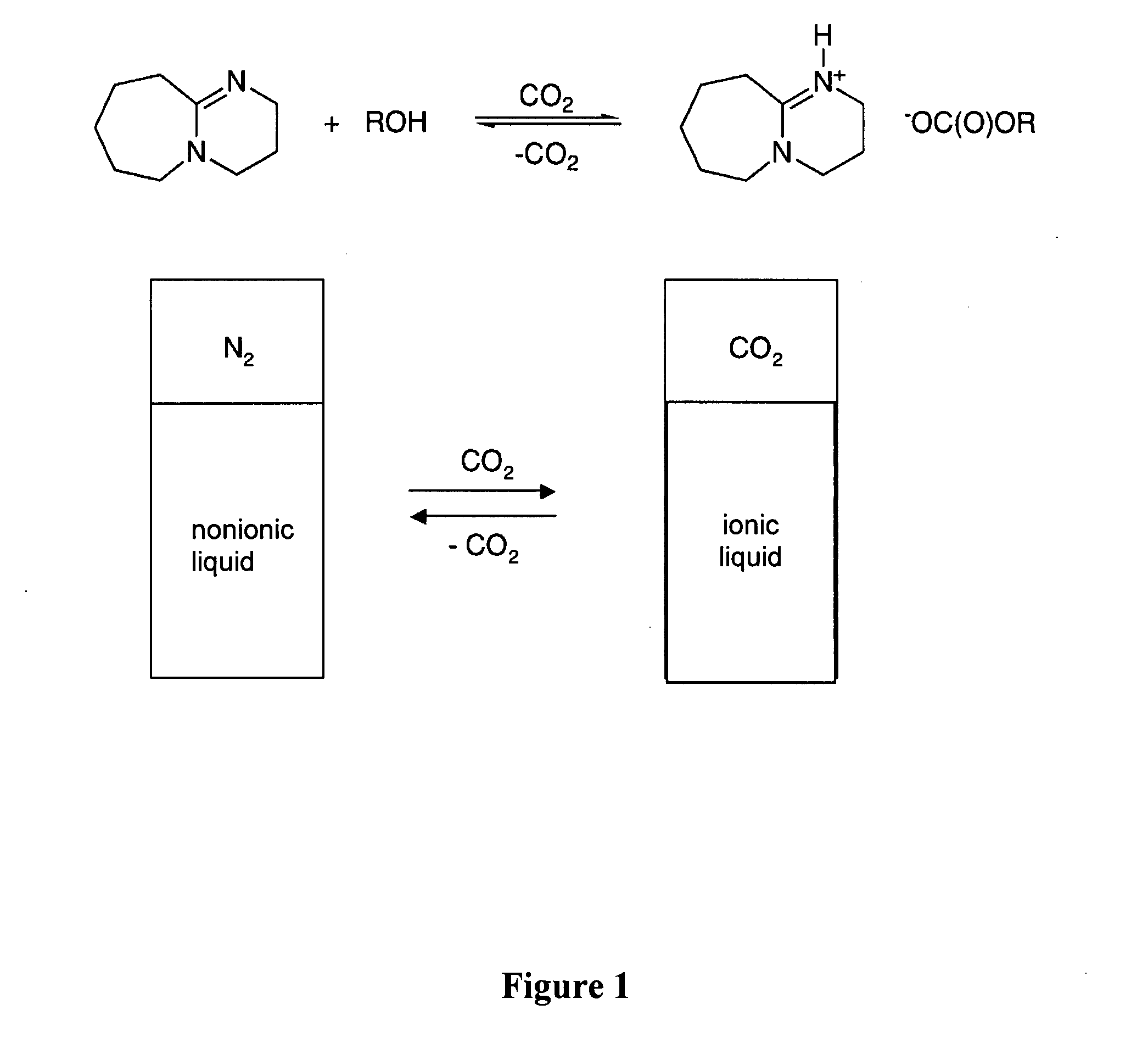
A solvent that reversibly converts from a nonionic liquid mixture to an ionic liquid upon contact with a selected trigger, e.g., contact with CO2, is described. In preferred embodiments, the ionic solvent is readily converted back to the nonionic liquid mixture. The nonionic liquid mixture includes an amidine or guanidine or both, and water, alcohol, or a combination thereof. Single component amine solvents that reversibly convert between ionic and non-ionic states are also described. Some embodiments require increased pressure to convert; others convert at 1 atmosphere.
Owner:GEORGIA TECH RES CORP +1
Anti-emulsification water-soluble metal washing agent
The invention relates to an anti-emulsification water-soluble metal washing agent. Every 100 parts of the anti-emulsification water-soluble metal washing agent include the following components according to parts by weight: 3-7 non-ionic surfactant, 3-7 bi-ion active agent, 1-5 chelator, 1-5 rust preventive, 5-10 inorganic builder and the balance water, wherein the non-ionic surfactant is any one of fatty amine polyoxypropylene ether, alkylphenol ether and fatty amine polyoxyethylene alkyl ether ammonium sulfate, the bi-ion active agent is any one of alkyl dimethylin acetic acid betaine, lauramidopropyl betaine and cocamidopropyl betaine, the chelator is any one of sodium citrate, ethylenediaminetetraacetic acid tetrasodium salt and nitrilotriacetic acid sodium salt, the rust preventive is any one of sodium borate, sodium nitrite, sodium benzoate and long carbon chain carboxylic acid amine, and the inorganic builder is any one of trisodium phosphate, sodium metasillcate, sodium carbonate, sodium bicarbonate and sodium hydroxide. The anti-emulsification water-soluble metal washing agent has the advantage of higher cleaning capacity and reutilization capacity.
Owner:NANJING KERUN LUBRICANTS
Non-ionic non-aqueous vehicles for topical and oral administration of carrier-complexed active agents
InactiveUS20070036843A1Control releaseLow costOrganic active ingredientsPharmaceutical non-active ingredientsActive agentNon ionic
An improved controlled release composition for non-parenteral administration of active agents and other therapeutics, particularly for oral or topical administration, has been developed. The composition is made by dispersing a complex formed of an active agent bound to an ion-exchange resin or to another form of resin or carrier, in a non-ionic non-aqueous (“NINA”) vehicle. The complexes are optionally coated with one or more layers of coating material to provide a controlled pattern of release of active agent from the carrier. Replacing the usual aqueous vehicle with a NINA vehicle, such as an oil or an ointment, allows the active agent-carrier complexes, with or without coatings, to be both orally and topically administered. The compositions can be formulated as powders, liquids, liquid suspensions, gels, capsules, soft gelatin capsules, tablets, chewable tablets, topical ointments, lotions, pourable or pumpable fluids, semisolid, crushable tablets, and unit-of-use sachets or capsules for reconstitution or direct application. The combination of multiple active agents is possible with this system, in which one or more active agents are bound to particles and one or more active agents are dissolved or dispersed in the NINA vehicle. This allows the combination of two or more active agents, which are otherwise incompatible, into a single dosage form.
Owner:COLLEGIUM PHARMA INC
Formulations and methods for delivery of growth factor analogs
Formulations, kits and methods for bone or cartilage repair, including treatment of osteogenic defects, including formulations of synthetic heparin-binding growth factor analogs, non-ionic polymers, gelling agents and calcium-containing agents.
Owner:BROOKHAVEN SCI ASSOCS +1
Stable fragrance microcapsule suspension and process for using same
InactiveUS20050227907A1Improve performanceReduce diffuseCosmetic preparationsToilet preparationsDeodorantNon ionic
Described is a stable initial impact and continuous impact fragrance and / or benefit agent-imparting aqueous suspension of microencapsulated fragrance and / or benefit agent, e.g., malodour counteractant suspended in a non-confined fragrance-containing and / or benefit agent-containing liquid phase oil-in-water emulsion. On storage, the viscosity of the suspension undergoes a minimal increase over an extended period of time thereby avoiding undesirable agitation resistance during the blending of the suspension with other materials. The suspension is thus useful for imparting a benefit or an aroma to a consumable material such as a liquid anionic, cationic, non-ionic or zwitterionic detergent, a shampoo, a bodywash, liquid soaps, hair conditioners, skin lotions, anti-perspirants, deodorants or liquid fabric softener and / or conditioner compositions. Also described is a process for preparing such stable suspensions and apparatus for carrying out such process.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
High molecular weight associative amphoteric polymers and uses thereof
High molecular weight associative amphoteric polymers for increasing the viscosity of aqueous solutions, comprise: at lease one cationic monomer derived from acrylamide bearing at least one hydrophobic chain of 8 to 30 carbon atoms; 1 to 99.9 mole % of at least one anionic monomer; and 1 to 99 mole % of one or several non-ionic water-soluble monomers. Aqueous solutions containing said polymers find uses in industry, in particular the oil, paper, water treatment, mining, cosmetics, textile, detergency industries and generally in all industrial techniques using thickened solutions.
Owner:S P C M SA
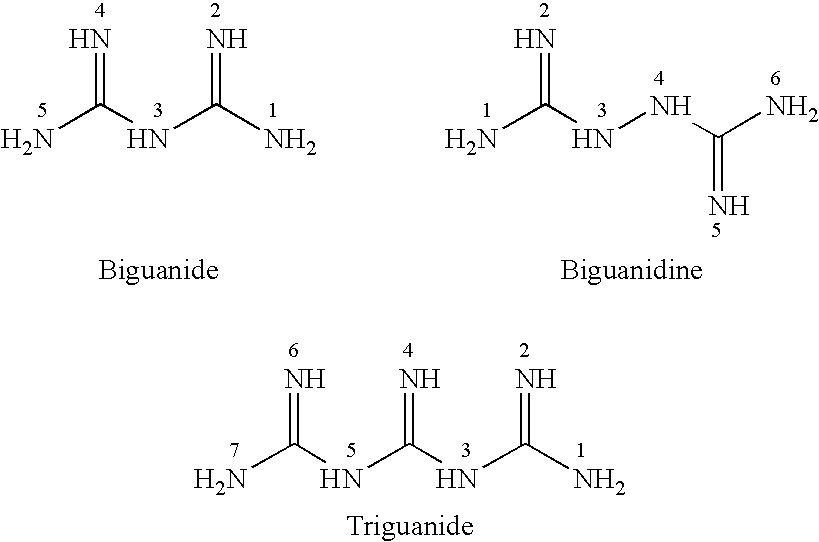
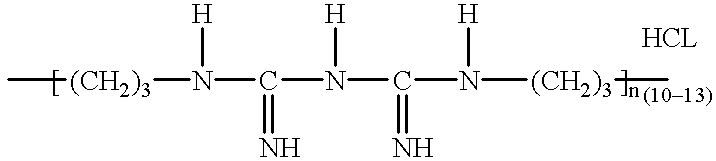
Fast acting disinfectant and cleaner containing a polymeric biguanide
InactiveUS6303557B1Improve effectivenessImprove efficiencyBiocideOrganic detergent compounding agentsSolventNon ionic
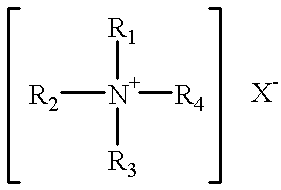
A cleaning and biocidal composition in liquid form comprising a solvent, a polymeric biguanide, a single quaternary ammonium salt, a sequestrant, and at least one surfactant. The composition comprising the solvent including water, the polymeric biguanide including a polyhexamethylene biguanide hydrochloride, the quaternary ammonium salt including a didecyldimethyl ammonium chloride, the sequestrant including an amino acid chelating agent selected from the group consisting of: ethylenediaminetetraacetic acid, nitrilotriacetic acid, tetrasodium ethylenediaminetetraacetic acid, or mixtures thereof, the surfactant including a non-ionic surfactant and an amphoteric surfactant.
Owner:JOHNSONDIVERSEY INC
Adjuvant and Vaccine Compositions
InactiveUS20100226932A1Small sizeSnake antigen ingredientsInorganic non-active ingredientsSterolEmulsion
Abstract Compositions comprising an emulsion and aluminum salt nano- / micro-particles surface stabilized with at least one surfactant are useful as immunological adjuvants. The emulsion of these compositions comprises at least one oil; at least one surfactant; a plurality of surfactant vesicles; optionally at least one sterol; and an aqueous phase. The present invention also provides vaccines comprising one or more antigens combined with the emulsion and surface stabilized aluminum salt particles of the present invention, or one or more antigens combined with non-ionic surfactant vesicles.
Owner:NOVAVAX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com