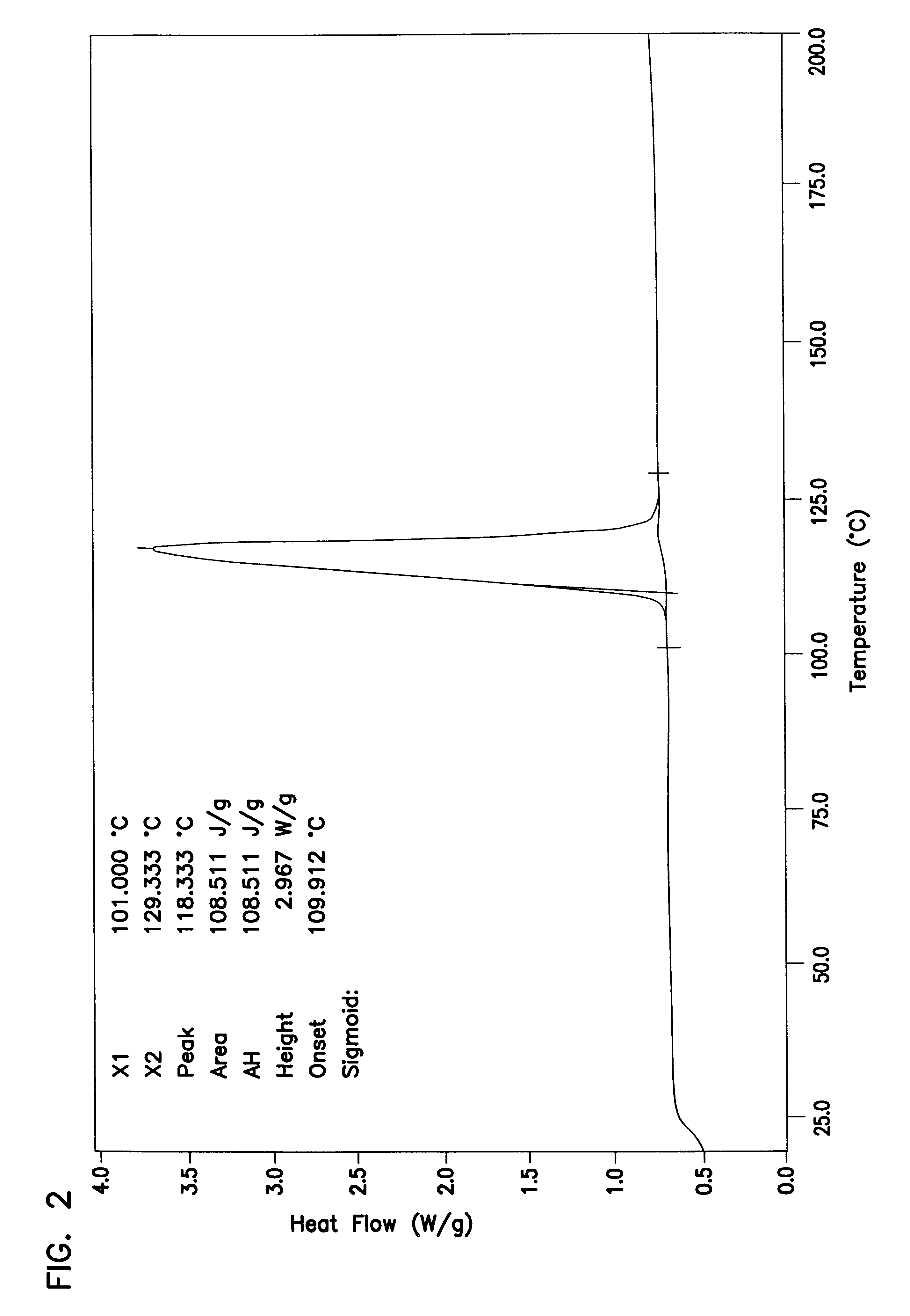
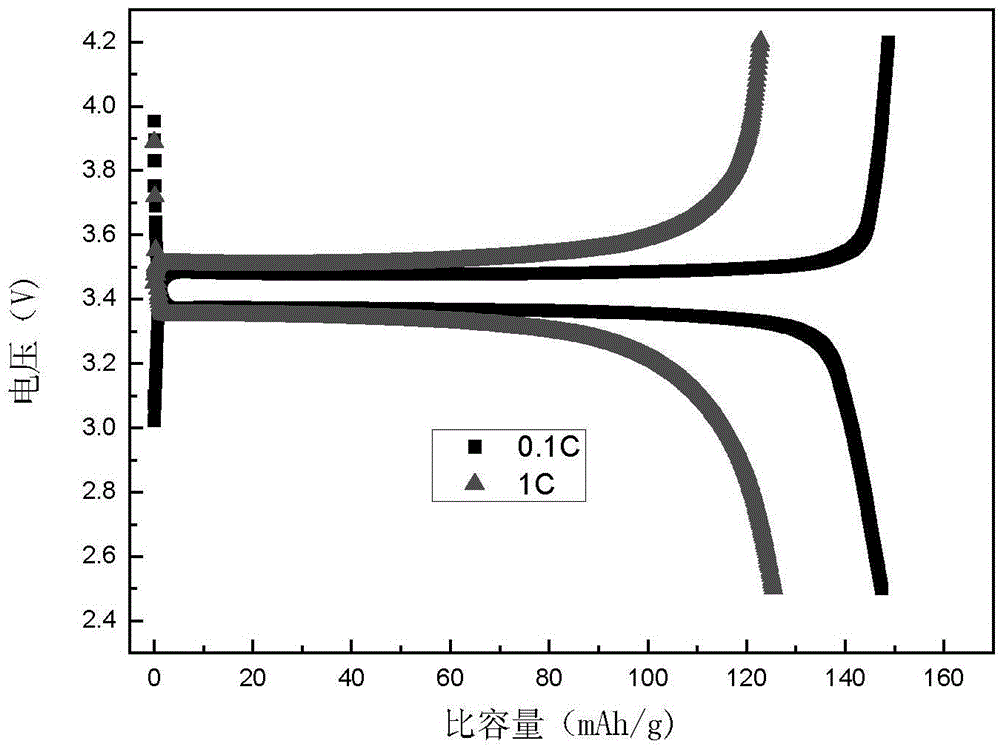
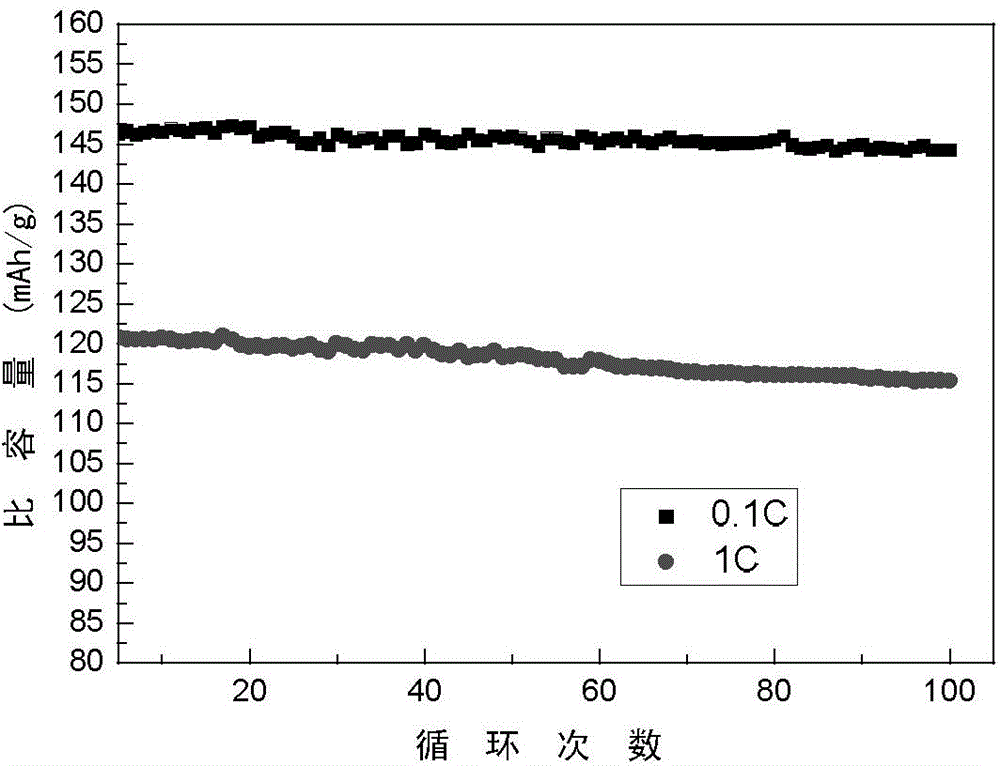
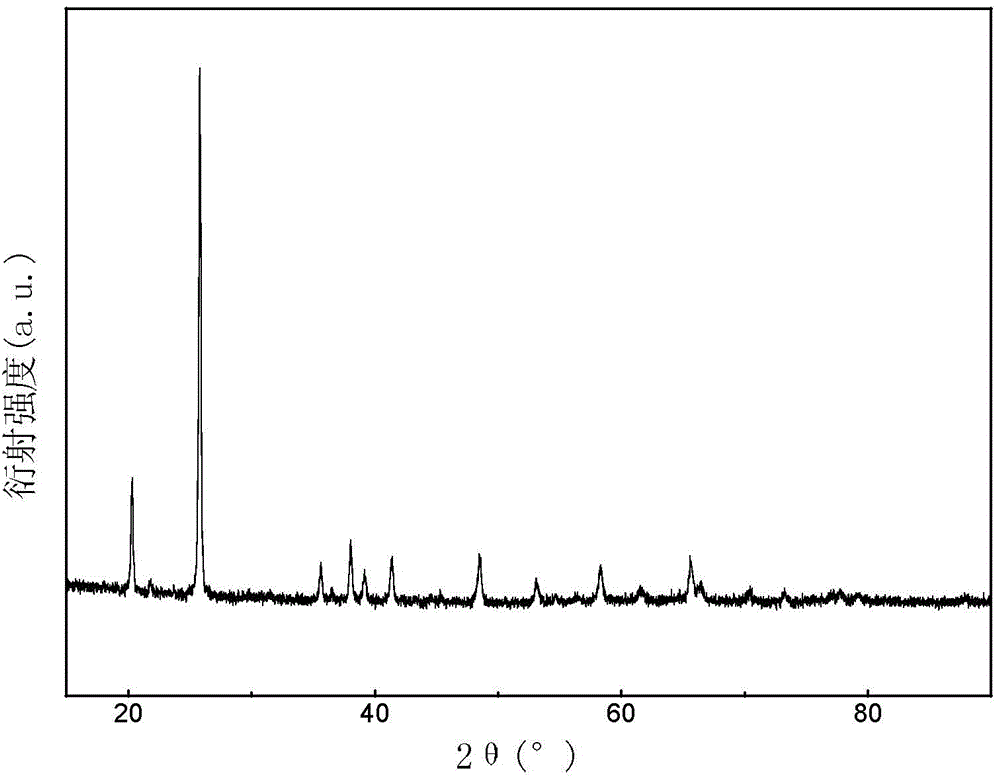
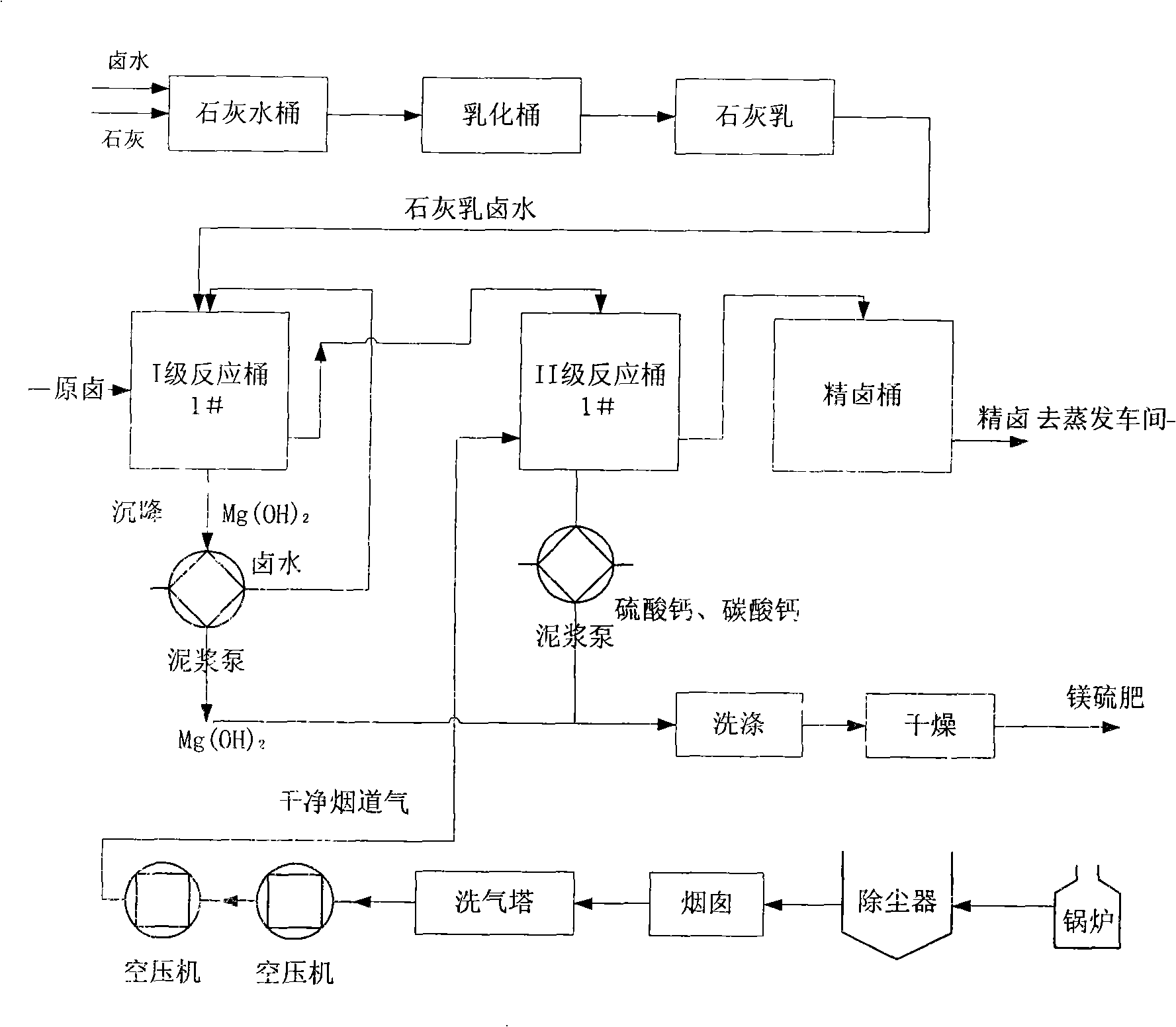
Patents
Literature
14852 results about "Sodium carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium carbonate, Na₂CO₃, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na₂CO₃ and its various hydrates. All forms are white, water-soluble salts. All forms have a strongly alkaline taste and give moderately alkaline solutions in water. Historically it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.
Smokeless tobacco
InactiveUS20080173317A1Improve barrier propertiesObstruct passageTobacco preparationTobacco treatmentSodium bicarbonateEngineering
A smokeless tobacco product is provided. A water-permeable pouch containing a tobacco formulation and configured for insertion into the mouth of a user of that product is provided. The tobacco formulation includes granular tobacco and a buffer comprised of sodium carbonate and sodium bicarbonate. An outer packaging material enveloping the pouch is provided and is sealed so as to allow a controlled environment to be maintained within.
Owner:R J REYNOLDS TOBACCO COMPANY
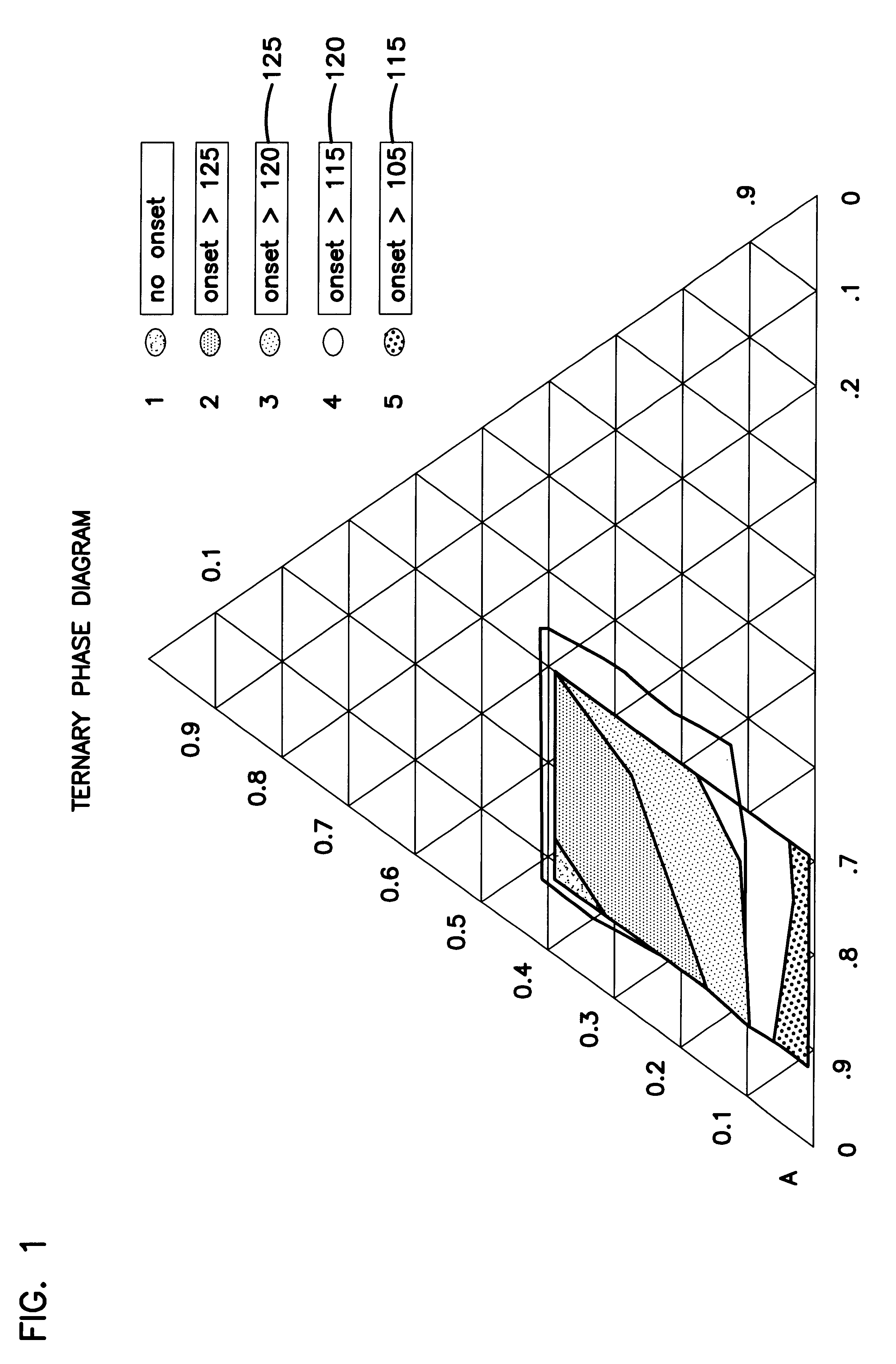
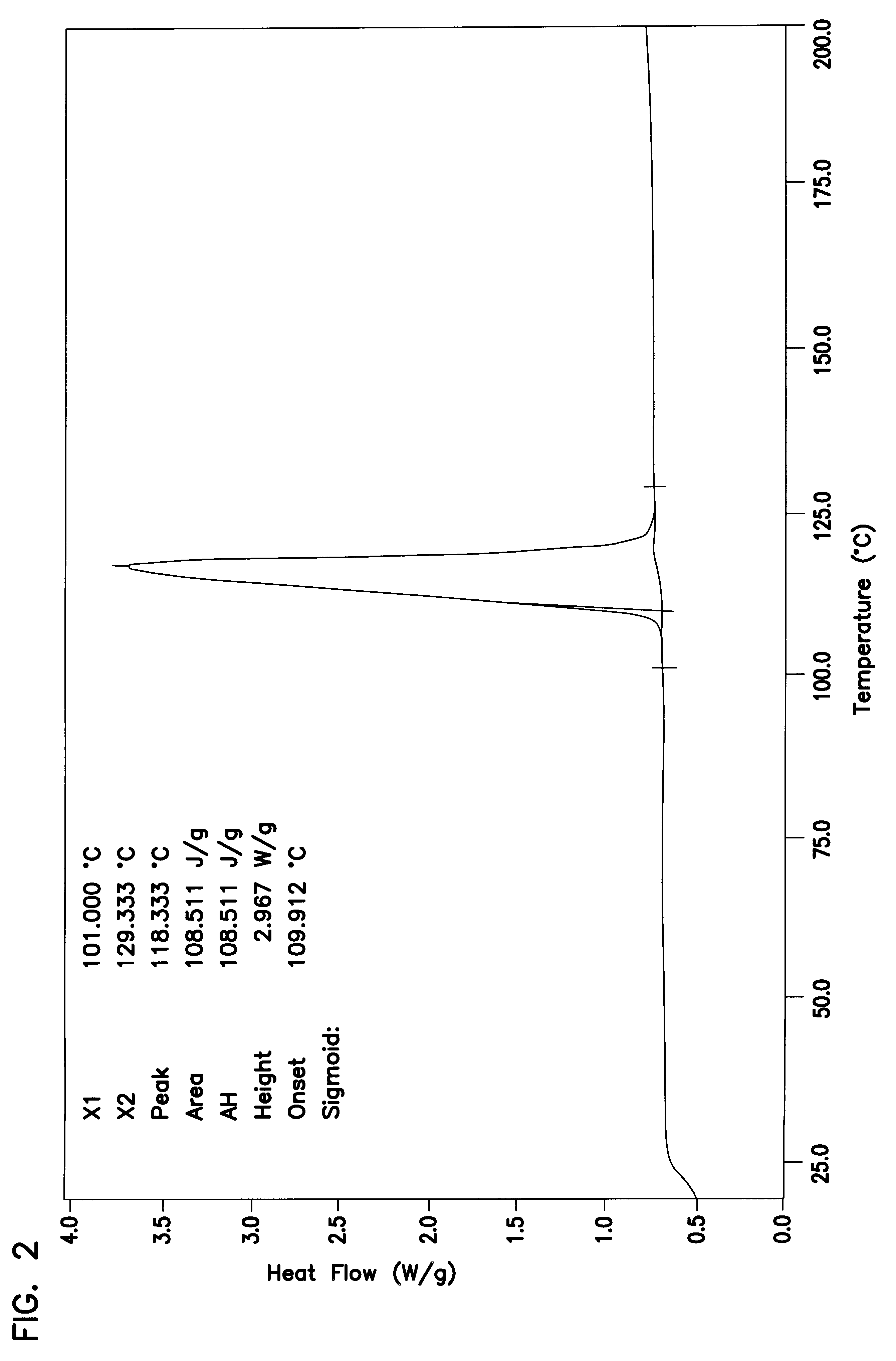
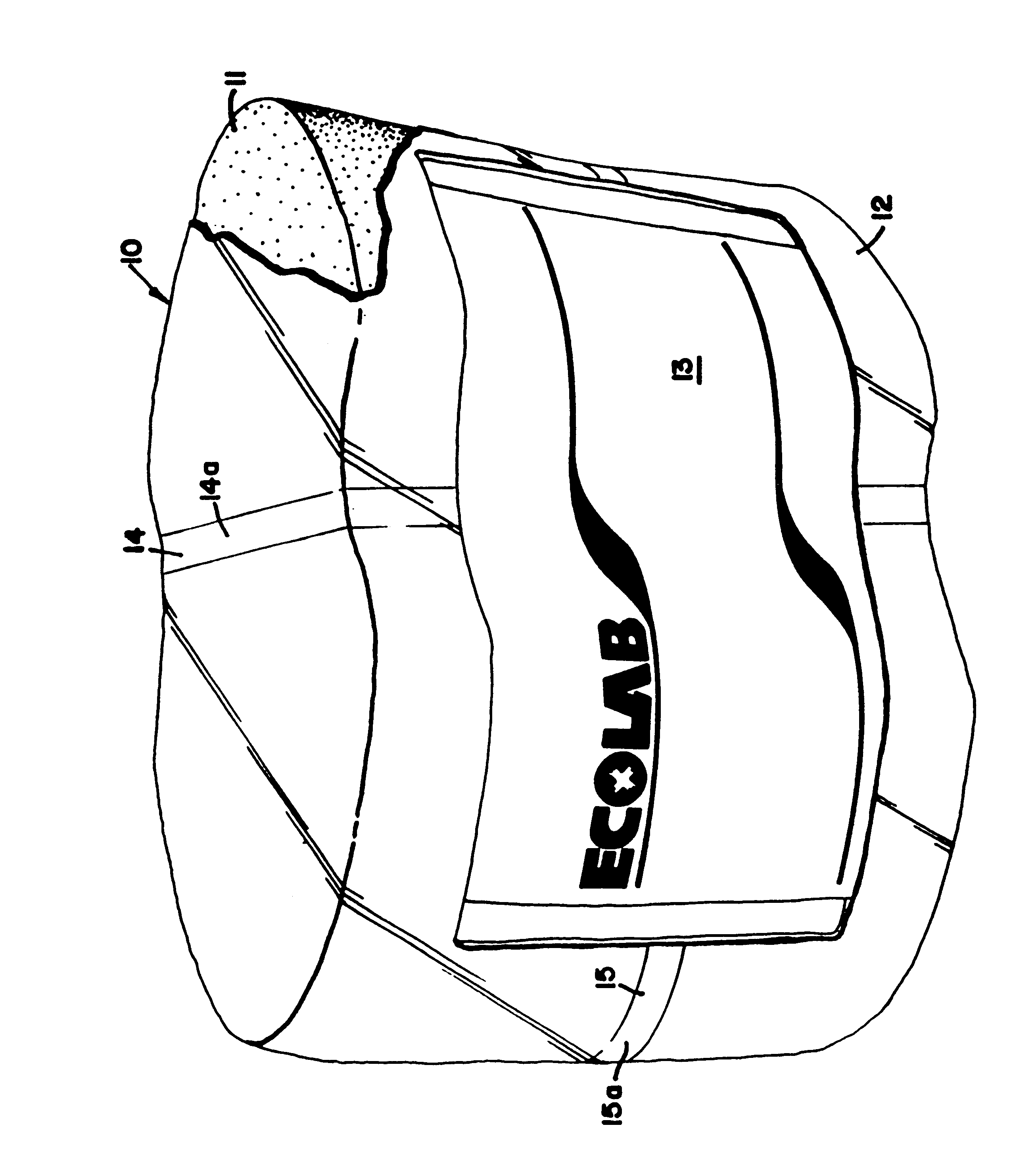
Stable solid block detergent composition
InactiveUS6177392B1Fit tightlyEasy to cleanInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAlkalinityIndustrial setting
The dimensionally stable alkaline solid block warewashing detergent uses an E-form binder forming a solid comprising a sodium carbonate source of alkalinity, a sequestrant, a surfactant package and other optional material. The solid block is dimensionally stable and highly effective in removing soil from the surfaces of dishware in the institutional and industrial environment. The E-form hydrate comprises an organic phosphonate and a hydrated carbonate.
Owner:ECOLAB USA INC
Stable solid block metal protecting warewashing detergent composition
InactiveUS6156715AFit tightlyEasy to cleanInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAlkalinityIndustrial setting
The dimensionally stable alkaline solid block warewashing detergent uses an E-form binder forming a solid comprising a sodium carbonate source of alkalinity, a metal corrosion protecting alkali metal silicate composition, a sequestrant, a surfactant package and other optional material. The solid block is dimensionally stable and highly effective in removing soil from the surfaces of dishware in the institutional and industrial environment. The E-form hydrate comprises an organic phosphonate and a hydrated carbonate.
Owner:ECOLAB USA INC
Method for preparing lithium cobaltate by directly using invalid lithium ion battery
InactiveCN102030375AReduce dispersionHigh purityCell electrodesCobalt compoundsElectrical batteryPotassium hydroxide
The invention provides a method for preparing lithium cobaltate by directly using an invalid lithium ion battery. The method comprises the following steps: crushing the invalid lithium ion battery or scraps generated when a lithium cobaltate battery is produced by a mechanical crusher at normal temperature; adding water and one or more of acetic acid, sulfuric acid, hydrochloric acid or nitric acid to produce mixed aqueous solution of the battery scraps and acid; filling the mixed aqueous solution into a hermetic pressure reactor, and controlling the temperature in the reactor to be between 50 and 150 DEG C; introducing or adding one leaching additive of sulfur dioxide or hydrogen, or adding hydrazine hydrate; stirring and leaching, cooling, and filtering; adding one precipitator of sodium carbonate, potassium carbonate and ammonium carbonate, or adding composite precipitator consisting of one of the sodium carbonate, the potassium carbonate and the ammonium carbonate and one of sodium hydroxide and potassium hydroxide to obtain mixture of lithium carbonate, cobalt carbonate and cobalt hydroxide; drying and calcining at high temperature to produce a lithium cobaltate product. The method is particularly suitable for the treatment scale of medium-sized and small enterprises, and is an effective method for directly materializing cobalt secondary resources.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Stable solid block metal protecting warewashing detergent composition
InactiveUS6410495B1Easy to cleanImprove decontamination abilityInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAlkalinityIndustrial setting
Owner:ECOLAB USA INC
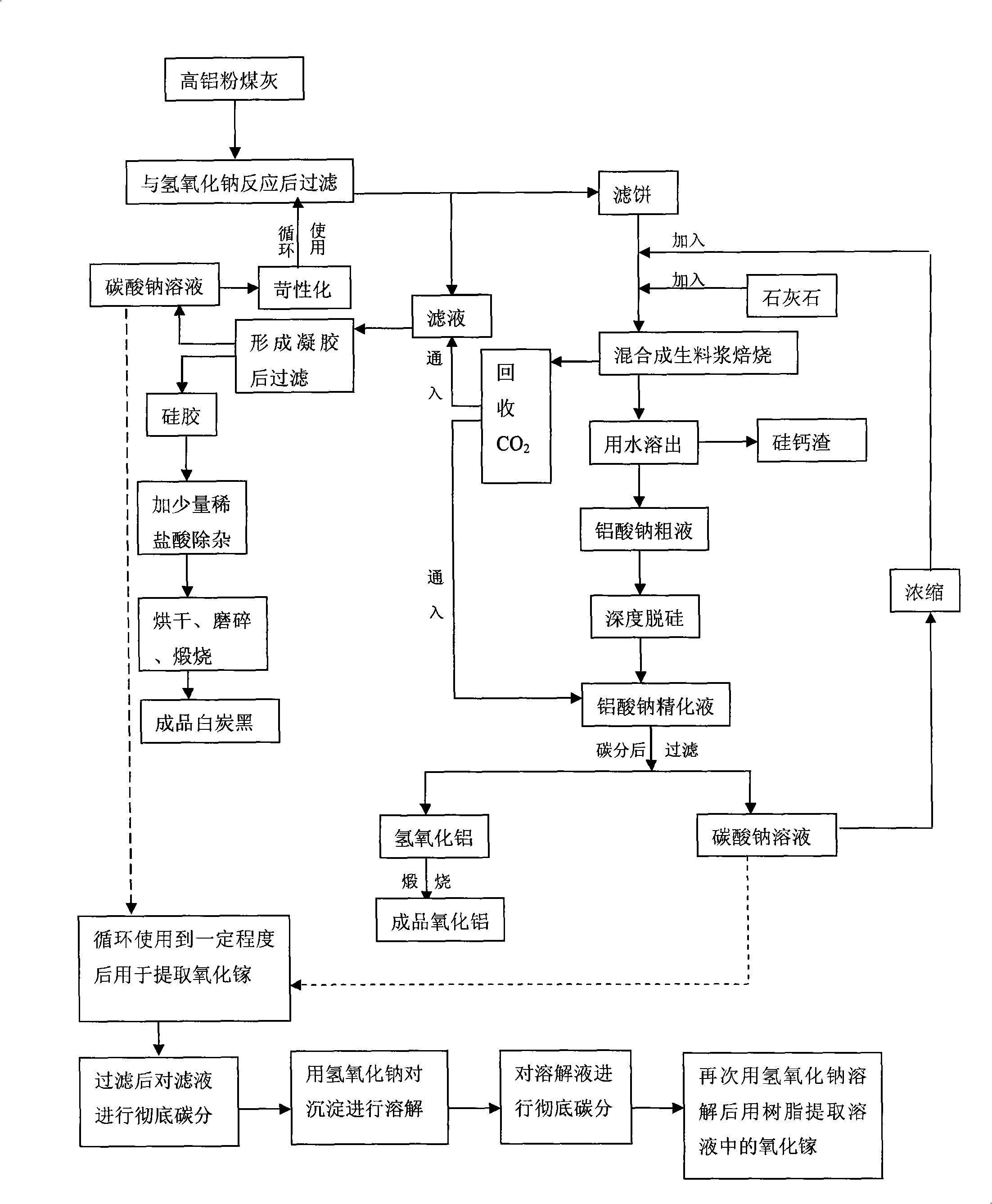
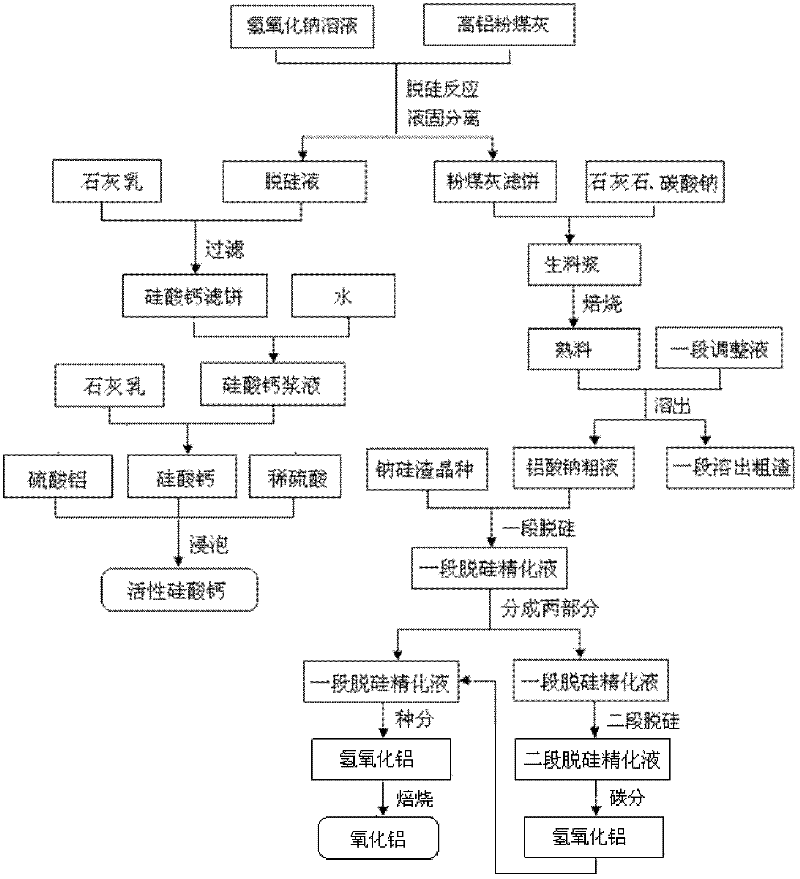
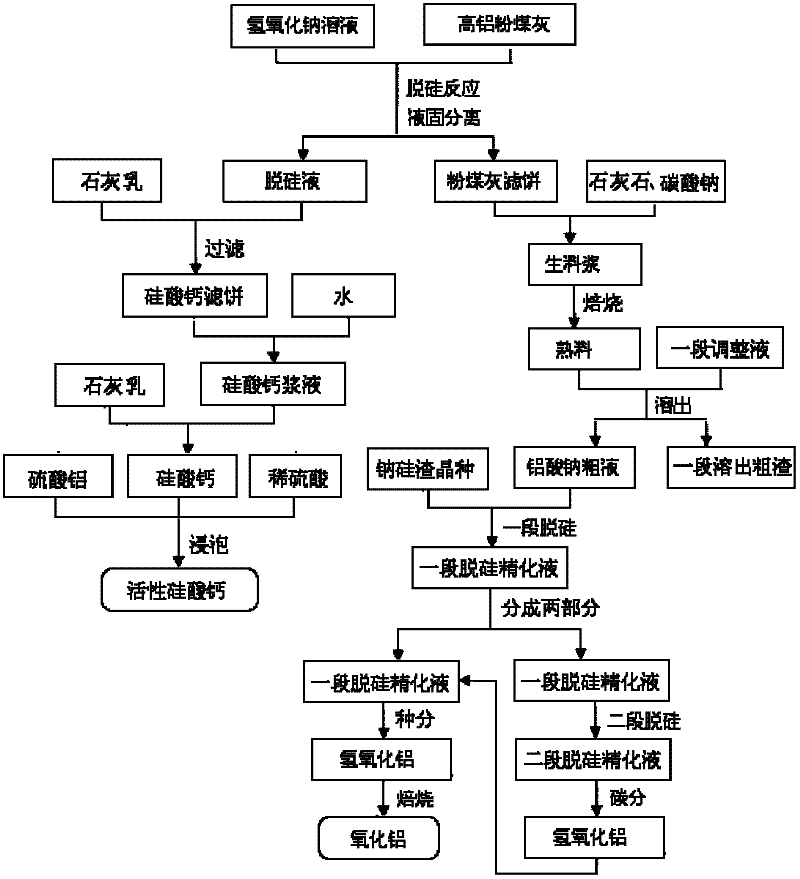
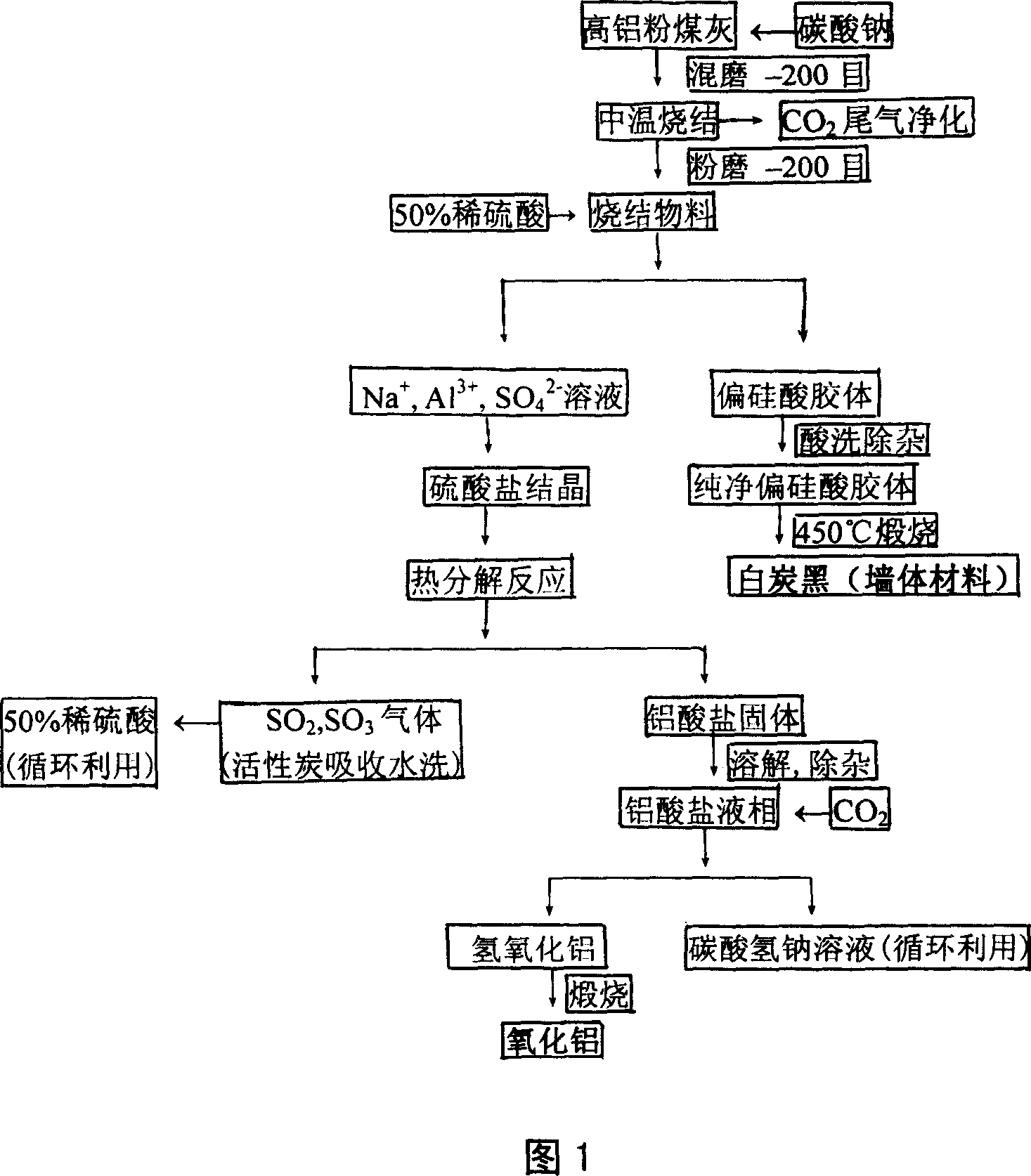
Process for abstracting earth silicon, oxide of alumina and gallium oxide from high-alumina flying ash
ActiveCN101284668AReduce the total massLow firing temperatureGallium/indium/thallium compoundsSilicon oxidesChemical industryFiltration
A method for extracting silicon dioxide, alumina and gallium oxide from high-alumina fly ash relates to the technology fields of environmental mineralogy and material, chemical industry and metallurgy. The method comprises the main steps as follows: causing the high-alumina fly ash to react with sodium hydroxide solution; filtering the solution; introducing CO2 to the filtrate for full gelation; cleaning, purifying, drying, grinding and calcining the silica gel after gel filtration to obtain finished white carbon black; adding limestone and a sodium carbonate solution into the filter mass after the reaction and filtration of the high-alumina fly ash and the sodium hydroxide solution; ball grinding the mixture into raw slurry; dissolving out the clinker obtained by baking the raw slurry; subjecting the filtrate to deep desiliconization to obtain sodium aluminate extraction liquid; filtrating the sodium aluminate extraction liquid after subjecting the sodium aluminate extraction liquid to carbon dioxide decomposition; baking the aluminum hydroxide after washing the filter mass to form the aluminum hydroxide product; and extracting the gallium oxide from the carbon dioxide decomposition mother solution and desiliconized solution. The method has the advantages of low material price, simple operating procedures, low investment, low production cost, low energy consumption and less slag.
Owner:TSINGHUA UNIV +1
Stable solid block detergent composition
InactiveUS6583094B1Easy to cleanImprove decontamination abilityInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAlkalinityIndustrial setting
The dimensionally stable alkaline solid block warewashing detergent uses an E-form binder forming a solid comprising a sodium carbonate source of alkalinity, a sequestrant, a surfactant package and other optional material. The solid block is dimensionally stable and highly effective in removing soil from the surfaces of dishware in the institutional and industrial environment. The E-form hydrate comprises an organic phosphonate and a hydrated carbonate.
Owner:ECOLAB USA INC
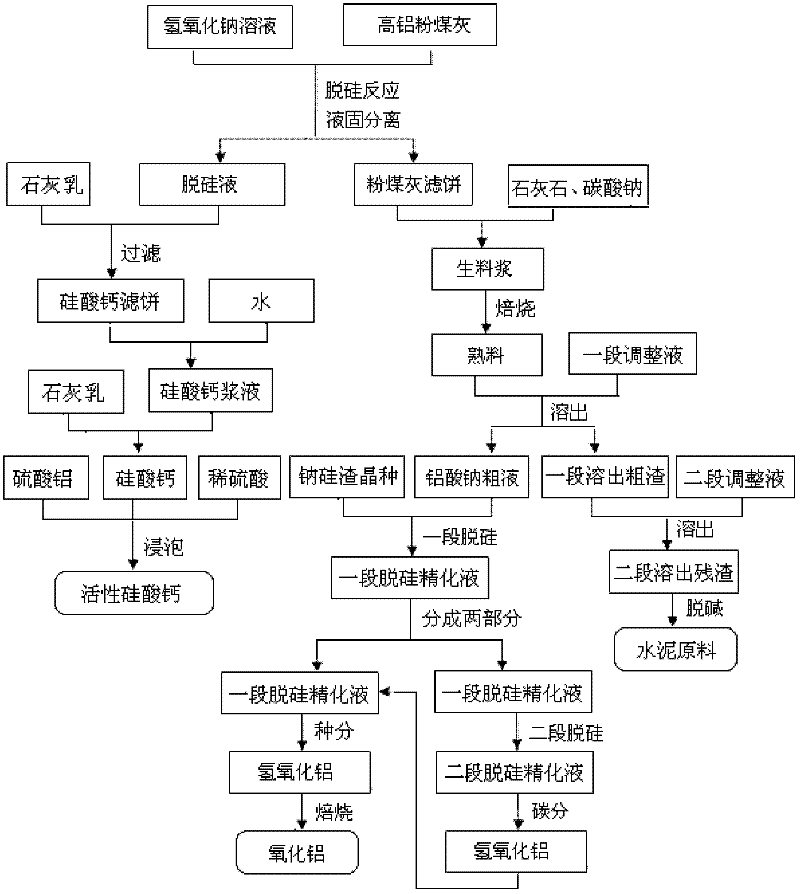
Method for producing aluminum oxide and co-producing active calcium silicate through high-alumina fly ash
ActiveCN102249253AExtraction is effective and cheapIncrease Al-Si RatioAlkaline-earth metal silicatesAluminium oxide/hydroxide preparationCalcium silicateSodium aluminate
The invention provides a method for producing aluminum oxide and co-producing active calcium silicate through high-alumina fly ash. The method comprises the following steps that: the high-alumina fly ash firstly reacts with a sodium hydroxide solution to carry out pre-desilication to obtain a liquid-phase desiliconized solution and a solid-phase desiliconized fly ash; lime cream is added to the liquid-phase desiliconized solution to carry out a causticization reaction, the resulting solid phase is active calcium silicate which is prepared through carrying out filter pressing, flash evaporation and drying to obtain the finished product; limestone and a sodium carbonate solution are added to the desiliconized fly ash to blend qualified raw slurry, then the blend qualified raw slurry is subjected to baking into the clinker, the liquid phase generated from dissolution of the clinker is a crude solution of sodium aluminate; the crude solution of the sodium aluminate is subjected to processes of first-stage deep desilication, second-stage deep desilication, carbonation, seed precipitation, baking and the like to obtain the metallurgical grade aluminum oxide meeting requirements. According to the present invention, the defects in the prior art are overcome; purposes of less material flow and small amount of slaggling are achieved; energy consumption, material consumption and production cost are relative low; extraction rate of the aluminum oxide is high; the calcium silicate with high added value is co-produced; the method provided by the present invention can be widely applicable for the field of chemical engineering.
Owner:INNER MONGOLIA DATANG INT RENEWABLE RESOURCES DEV
Acidified attapulgite clay
Owner:XUYI BOTU ATTAPULGITE CLAY HIGH TECH DEV
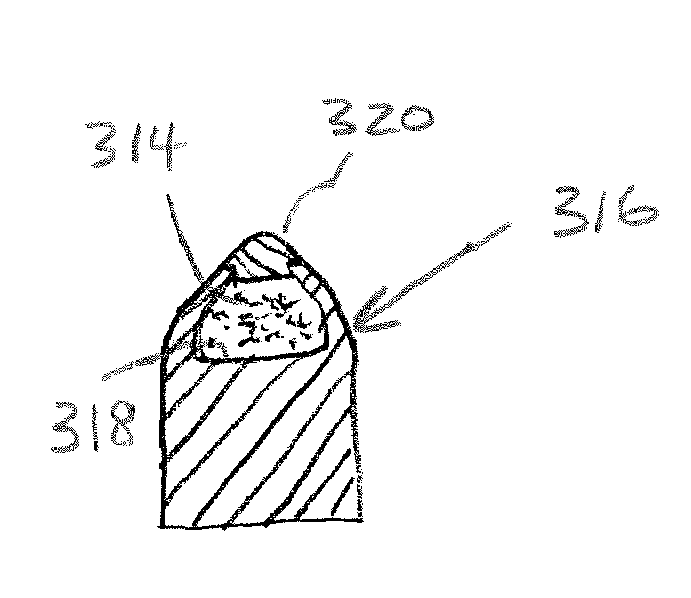
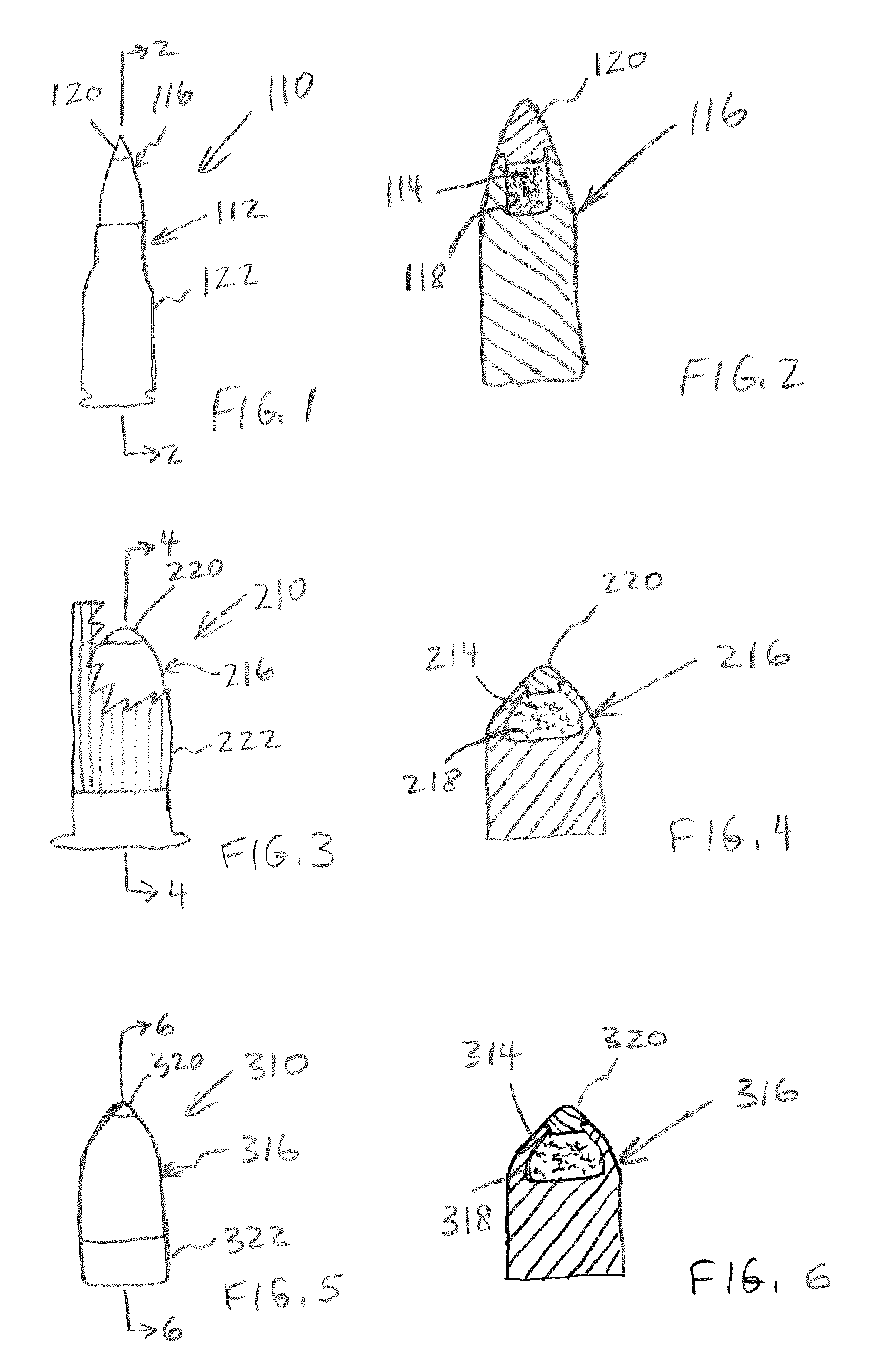
Firearm ammunition for tracking wounded prey
InactiveUS20060278116A1Easy for to track and findIncrease awarenessAmmunition projectilesArrowsChemical reactionBiological stain
A firearm ammunition device includes a cartridge with a projectile that is loaded with a tracer agent. When the projectile is fired and strikes a game animal, the impact causes the release and dispersal of the tracer agent from the projectile. The dispersed tracer agent identifies an enhanced-visibility trail of the fleeing animal. In example embodiments, the cartridge is provided by a centerfire cartridge, a shotgun cartridge, and a muzzleloading cartridge. The tracer agent preferably comprises one or more compounds that produce visible light to the naked eye and / or under a black light source. Example tracer agents include biological stains that produce visible light upon contact with blood, luminol or another chemiluminescent compound that releases light by a chemical reaction such as may occur when contacting blood, a basic salt such as sodium carbonate or another effervescence-inducing agent, a phosphorescent compound, or a fluorescent compound.
Owner:HUNT C TIMOTHY
Anti-emulsification water-soluble metal washing agent
The invention relates to an anti-emulsification water-soluble metal washing agent. Every 100 parts of the anti-emulsification water-soluble metal washing agent include the following components according to parts by weight: 3-7 non-ionic surfactant, 3-7 bi-ion active agent, 1-5 chelator, 1-5 rust preventive, 5-10 inorganic builder and the balance water, wherein the non-ionic surfactant is any one of fatty amine polyoxypropylene ether, alkylphenol ether and fatty amine polyoxyethylene alkyl ether ammonium sulfate, the bi-ion active agent is any one of alkyl dimethylin acetic acid betaine, lauramidopropyl betaine and cocamidopropyl betaine, the chelator is any one of sodium citrate, ethylenediaminetetraacetic acid tetrasodium salt and nitrilotriacetic acid sodium salt, the rust preventive is any one of sodium borate, sodium nitrite, sodium benzoate and long carbon chain carboxylic acid amine, and the inorganic builder is any one of trisodium phosphate, sodium metasillcate, sodium carbonate, sodium bicarbonate and sodium hydroxide. The anti-emulsification water-soluble metal washing agent has the advantage of higher cleaning capacity and reutilization capacity.
Owner:NANJING KERUN LUBRICANTS
Stainless steel cleaning agent
InactiveCN101135056AImprove cleaning efficiencySimple cleaning processPhosphoric acidCleansing Agents
The stainless steel detergent for washing stainless steel product is prepared with acids, basic salts, surfactant, assistant, stabilizer and water, and through stirring and reaction at normal temperature. Specifically, it consists of sodium carbonate, sodium tripolyphosphate, tartaric acid, citric acid, hydrofluoric acid, nitric acid, phosphoric aicd, JFC, OP-10, triethanolamine, urotropin, trisodium phosphate, acetic acid, alcohol and water in certain weight proportion. It has the features of simple preparation process, environment friendship, high cleaning efficiency, low cleaning cost, etc.
Owner:吴铭鑫
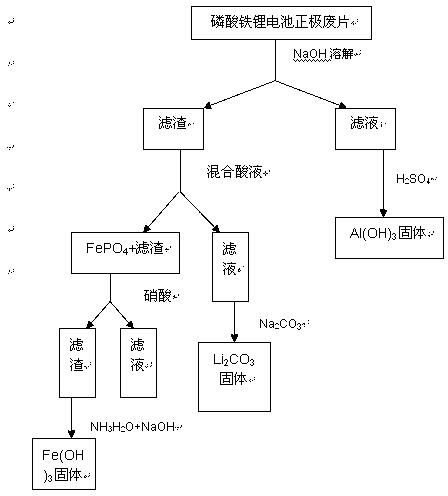
Method for recovering waste lithium iron phosphate battery positive pieces
ActiveCN103280610AReduce pollutionWaste accumulators reclaimingProcess efficiency improvementPhosphoric acidImpurity
The invention discloses a method for recovering Al, Fe and Li in waste lithium iron phosphate battery positive pieces through an acid-alkali leaching process. The method comprises the following steps: dismounting the lithium iron phosphate battery positive pieces, dissolving the positive pieces by using an alkali, filtering, and dissolving obtained filter residues by a mixed acid solution for making Fe exist in an iron phosphate precipitate form and be separated from impurities comprising carbon black and the like and a lithium-containing solution; adding the lithium-containing solution to a 95DEG C saturated sodium carbonate solution for precipitation to obtain lithium carbonate; and adding an acid to obtain an iron-containing precipitate for leaching iron ions, and adding an alkaline solution to obtain Fe(OH)3. Al, Fe and Li in the waste lithium iron phosphate battery positive pieces are simply and effectively recovered through using low-concentration acids and alkalis and routine chemicals, and raw materials having economic benefits are recovered through using simple and effective equipment and the method.
Owner:STATE GRID JIANGXI ELECTRIC POWER CO LTD RES INST +1
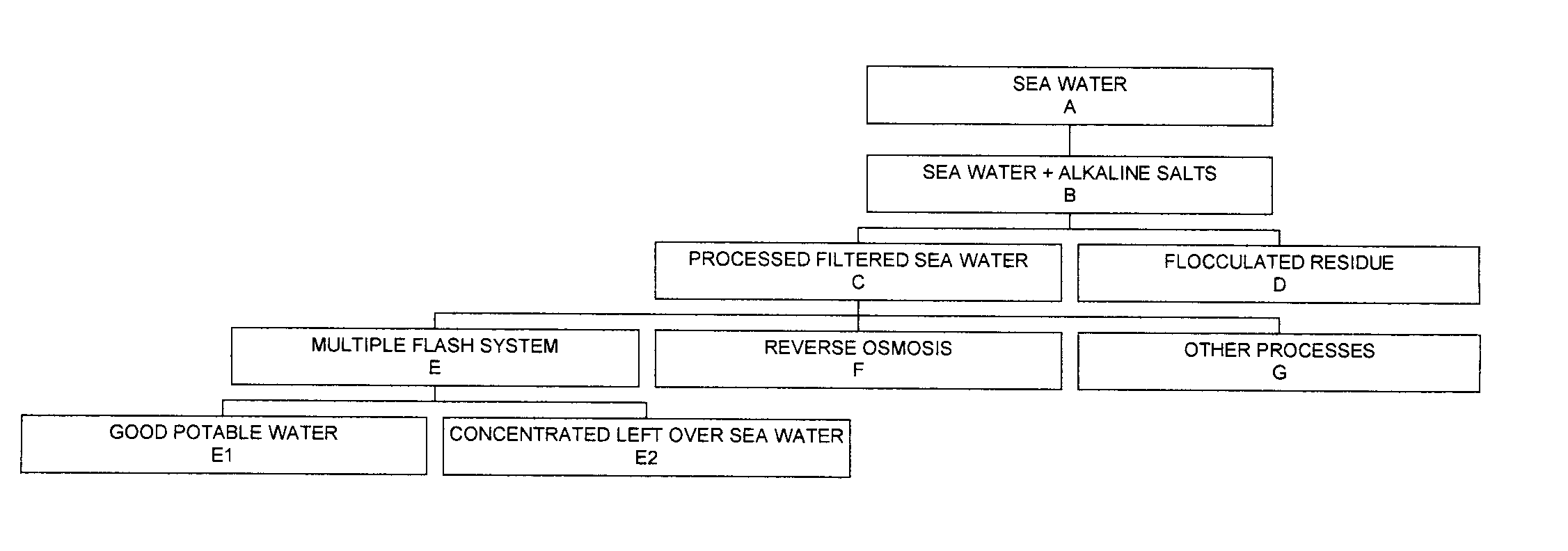
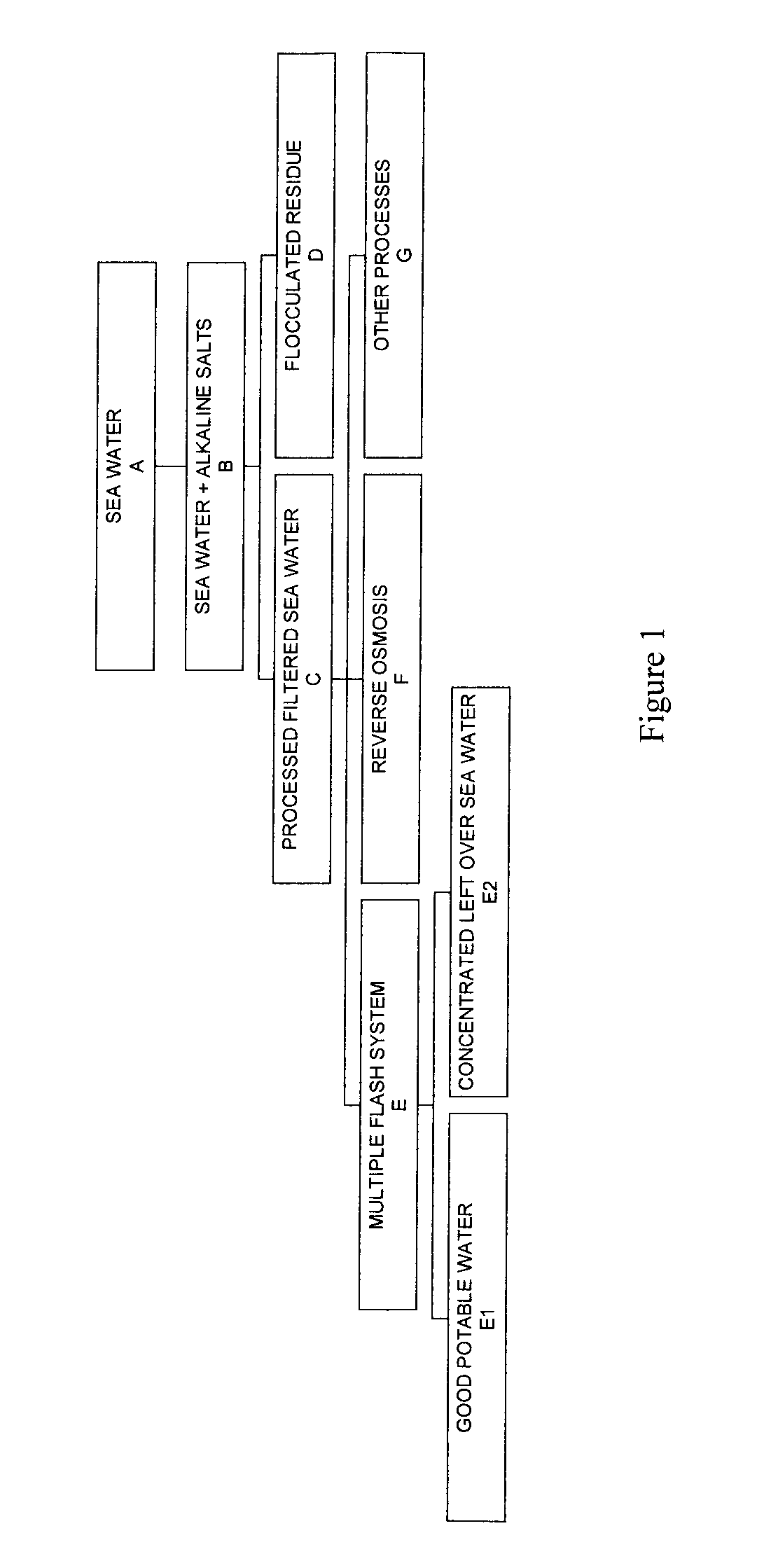
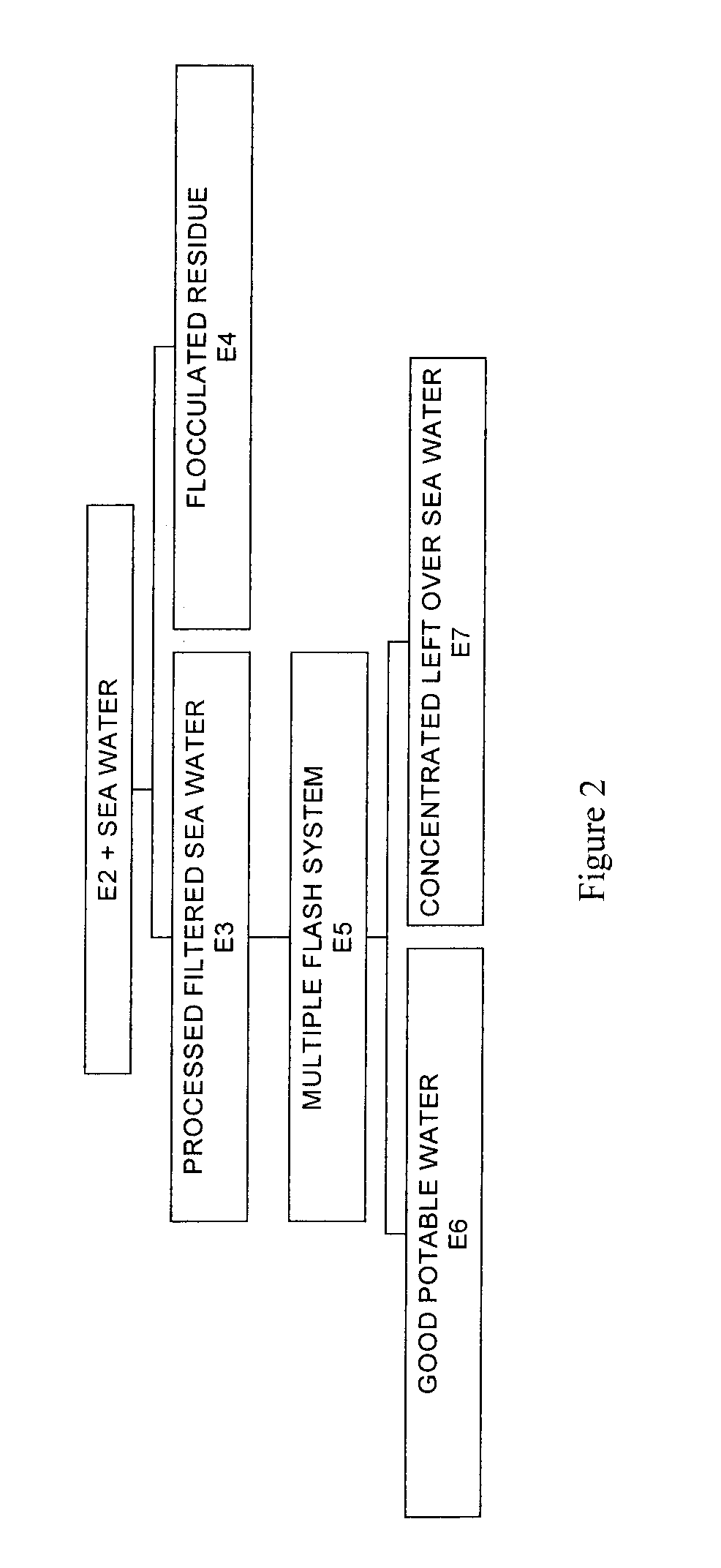
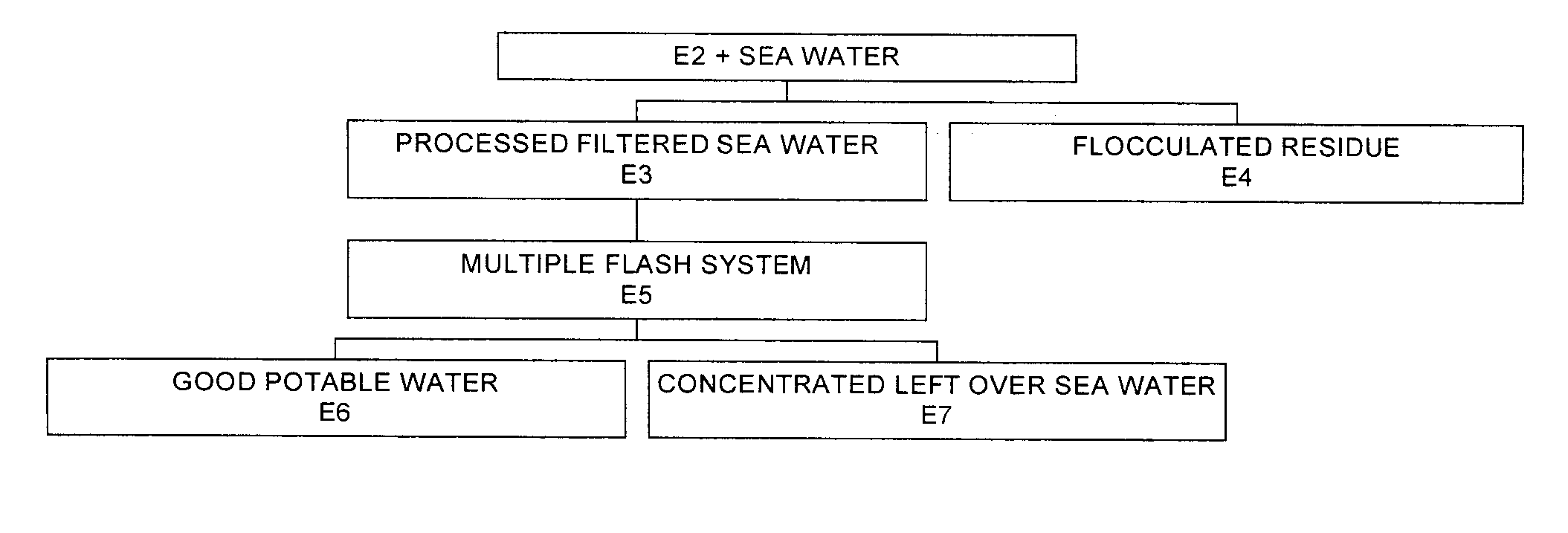
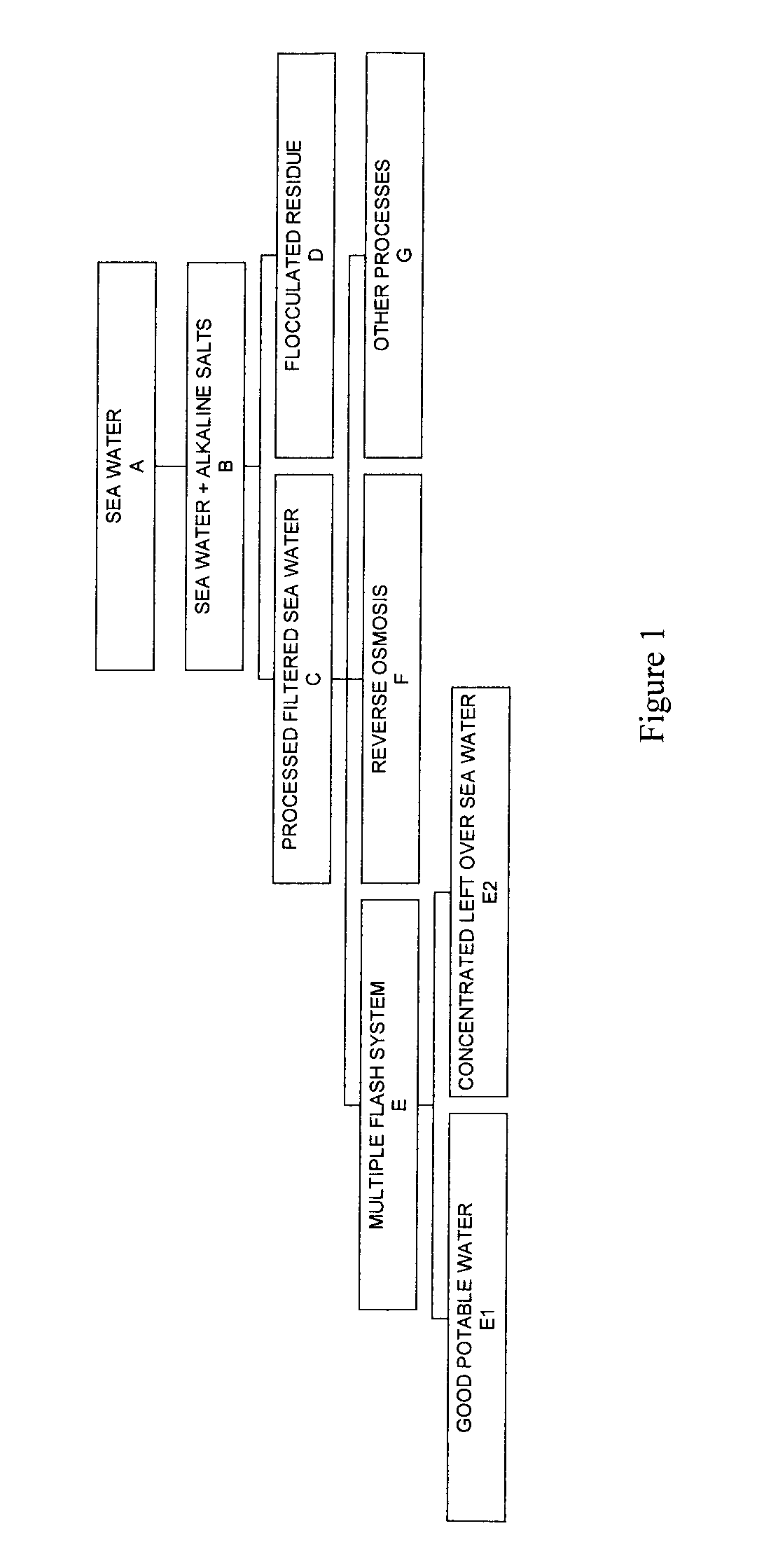
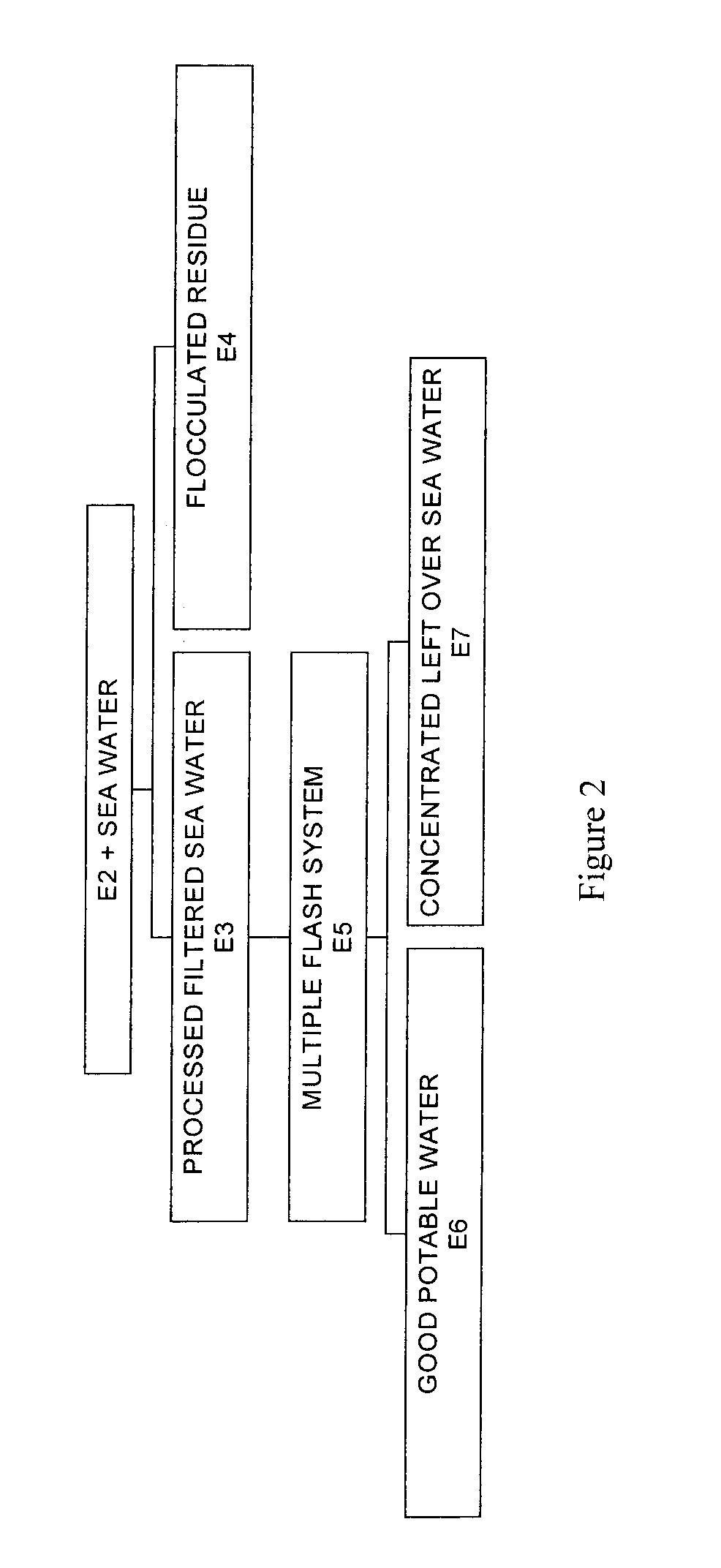
Process for pre-treating and desalinating sea water
ActiveUS20050098499A1Reduce maintenanceExtend equipment lifeGeneral water supply conservationSeawater treatmentCalcium bicarbonatePotassium hydroxide
Water containing dissolved salts, such as calcium sulfate, calcium chloride, magnesium sulfate, magnesium chloride, sodium carbonate, sodium chloride, sodium sulfate, calcium bicarbonate, and mixtures thereof, is treated to reduce the concentration of those salts. About 0.1 to about 60 g / L of sodium hydroxide, sodium carbonate, potassium hydroxide, potassium carbonate, calcium hydroxide, calcium carbonate, aluminum hydroxide, aluminum sulfate, aluminum potassium sulfate, and mixtures thereof is added to the water, whereby a precipitate forms in the water. The precipitate is separated from said water and the water is desalinated using reverse osmosis, flash evaporation, or another method. The process is preferably performed by first adding calcium oxide or calcium hydroxide, separating the precipitate that forms, then adding sodium hydroxide and sodium carbonate to form a second precipitate.
Owner:HUSSAIN MOHAMMED AZAM
Technology for recovering water resources and salt from coking wastewater in coal chemical industry
ActiveCN105502782ANo secondary hazardous waste generatedLow running costMultistage water/sewage treatmentAlkali metal chloridesChemical treatmentAdvanced oxidation process
A technology for recovering water resources and salt from coking wastewater in the coal chemical industry comprises the following steps: the wastewater is subjected to defluorination chemical treatment and subjected to sodium carbonate softening and precipitating treatment simultaneously, an advanced oxidation process is used for TOC (total organic carbon) degradation, a multi-medium and activated carbon filter is used for filtering separation, ultrafiltration is performed, nanofiltration membrane separation is performed, calcium and magnesium ions are separated, the calcium and magnesium ions in water produced through nanofiltration are lower than 2 mg / L, CaF2 crystallization scaling is hard to form, and nanofiltration passing liquid and nanofiltration strong brine are obtained; the nanofiltration passing liquid and the nanofiltration strong brine are treated respectively. Fluoride ions, hardness and organic carbon in the wastewater are removed, separation of multivalent salt and monovalent salt as well as concentration and evaporative crystallization of the salt is realized, more than 98% of the water resources is recovered, more than 95% of the salt resources are recovered, secondary hazardous waste is not produced, the system operation cost is reduced, and the problem about resource recovery and the environmental problem are solved finally.
Owner:湖南湘牛环保实业有限公司
Method for recovering tungsten trioxide and ammonium metavanadate from selective catalytic reduction (SCR) denitration catalyst
ActiveCN102557142AEmission reductionTungsten oxides/hydroxidesDispersed particle separationWarm waterAmmonium paratungstate
The invention relates to a method for recovering tungsten trioxide and ammonium metavanadate from a selective catalytic reduction (SCR) denitration catalyst. The method comprises the following steps of: crushing the SCR denitration catalyst, sieving to obtain catalyst powder, mixing with sodium carbonate, and stirring fully and uniformly; putting the mixed powder into a sintering furnace, and sintering to obtain a sintered material; keeping temperature for 1 hour, and sieving to obtain sintered material powder; pouring warm water, so that Na2WO4 and NaVO3 in the sintered material powder are dissolved fully, filtering, and removing precipitates to obtain a mixed solution of Na2WO4 and NaVO3; regulating the pH value to be 6.5-7.5, adding an ammonium bicarbonate solution or an ammonium chloride solution, and precipitating ammonium metavanadate precipitate; filtering, washing by using a diluted ammonium bicarbonate solution for 2 to 3 times, washing by using 30 percent ethanol for 1 to 2 times, and drying to obtain an ammonium metavanadate finished product; and converting the Na2WO4 in the residual solution into ammonium paratungstate, evaporating the residual solution to obtain ammonium paratungstate crystals, and calcining to obtain the tungsten trioxide. By the method, the ammonium metavanadate and the tungsten trioxide can be recovered, and the discharge of pollutants is reduced.
Owner:江苏万德环保科技有限公司
Process for pre-treating and desalinating sea water
ActiveUS7198722B2Extend equipment lifeReduce maintenanceGeneral water supply conservationSeawater treatmentCalcium bicarbonateReverse osmosis
Owner:HUSSAIN MOHAMMED AZAM
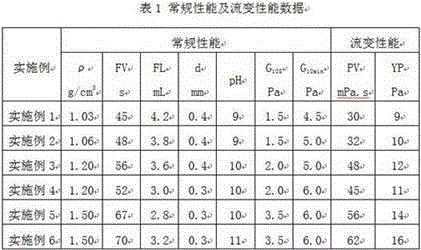
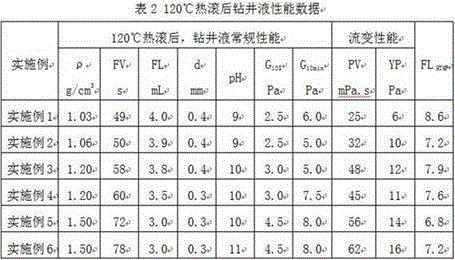
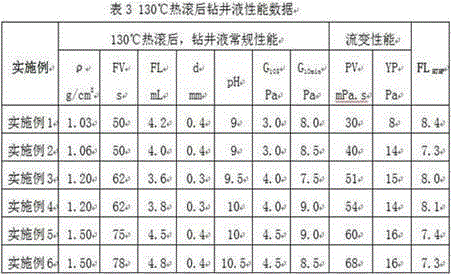
Strong-inhibition anticaving drilling fluid and application thereof
InactiveCN105038737AReduce fluid lossAdjust the flow patternDrilling compositionOrganic chemistryPetroleum engineering
The invention discloses a strong-inhibition anticaving drilling fluid and application thereof. The strong-inhibition anticaving drilling fluid consists of 1000 parts of water, 20 to 60 parts of bentonite for drilling fluid, 5 to 20 parts of sodium carbonate, 5 to 20 parts of alkali, 10 to 20 parts of coating agent, 5 to 20 parts of flow pattern regulator, 5 to 20 parts of shale inhibitor, 10 to 30 parts of borehole stabilizer, 10 to 30 parts of plugging agent, 5 to 30 parts of lubricating agent, 5 to 40 parts of filtrate reducer, 1 to 10 parts of pH regulator, and a proper amount of salts and barite added according to the requirement. The strong-inhibition anticaving drilling fluid has the remarkable characteristics of properties of stabilizing a borehole, protecting an oil layer, improving the drilling speed, and preventing a balled bit and the like from approaching to oil-based drilling fluid.
Owner:北京中科天启油气技术有限公司
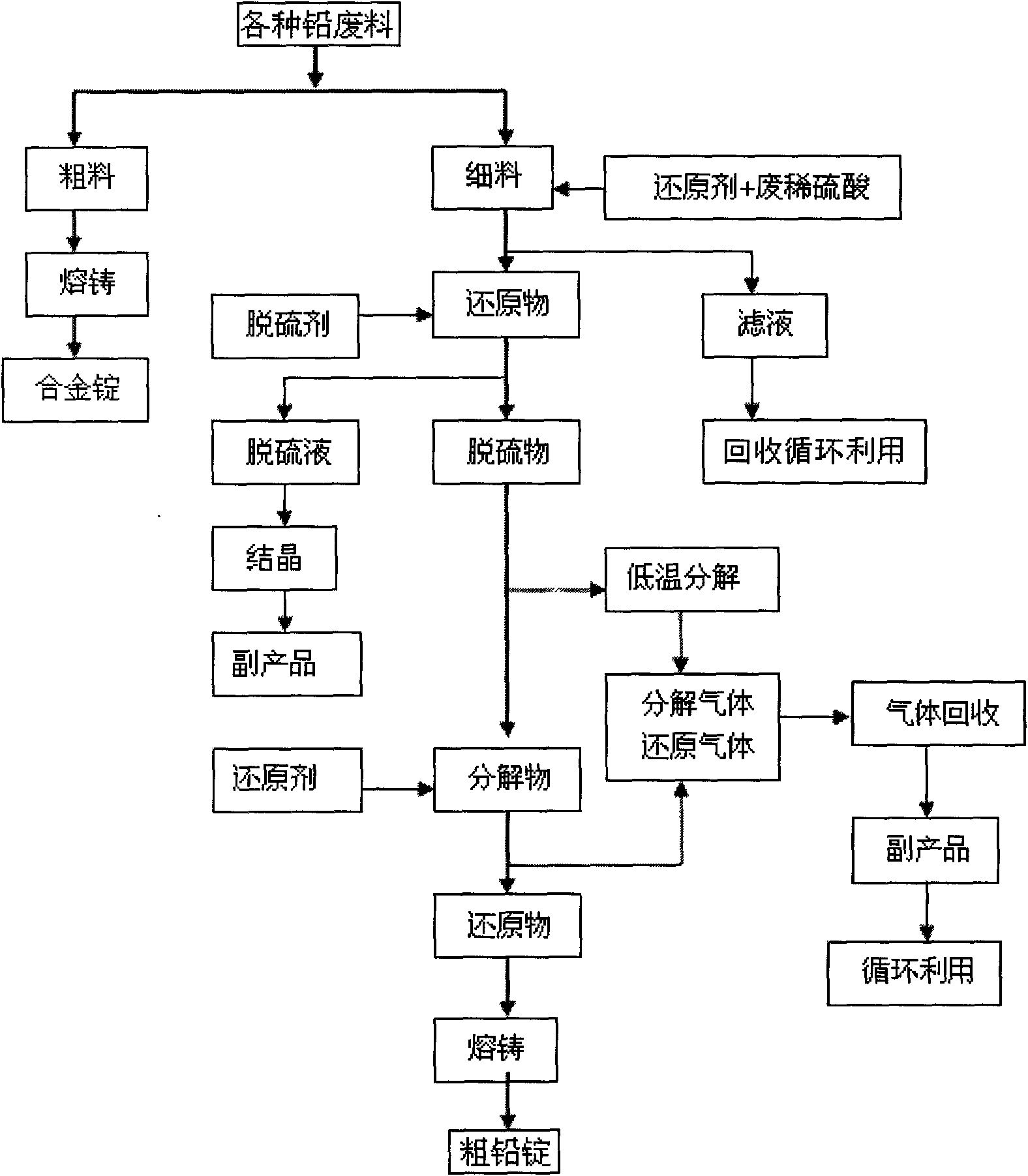
Waste lead recovering method for lead-acid storage batteries
InactiveCN101608264AAvoid harmLower decomposition temperaturePhotography auxillary processesProcess efficiency improvementLead dioxideEngineering
The invention discloses a waste lead recovering method for lead-acid storage batteries. The method comprises the following steps: fine stuff such as diachylon and the like are added in a reaction kettle with a stirring device; reducing agent (FeSO4) and dilute sulfuric acid are simultaneously added; stirring reaction is carried out at the temperature of 50-60 DEG C for 50-70 minutes so as to reduce lead dioxide into lead sulfate; the lead sulfate is added into the reaction kettle with the stirring device; water is simultaneously added into the reaction kettle for size mixing; then sodium carbonate is added; desulfuration is carried out at the temperature of 50-60 DEG C so as to obtain solid lead carbonate; the lead carbonate is put into a smelting furnace and then decomposed at the temperature of 320-350 DEG C so as to obtain lead oxide; and reducing agent (carbon) is added into the smelting furnace to reduce the lead oxide into metal lead at the temperature of 700-800 DEG C. The method recovers the lead by means of the combination of the wet and the dry processes, thereby avoiding the harm to the environment caused by lead dust, lead vapor, lead skim, sulfur dioxide gas, and the like by adopting fire smelting. The method has the advantages of high lead recovery rate, low energy consumption and no environment pollution.
Owner:张天任
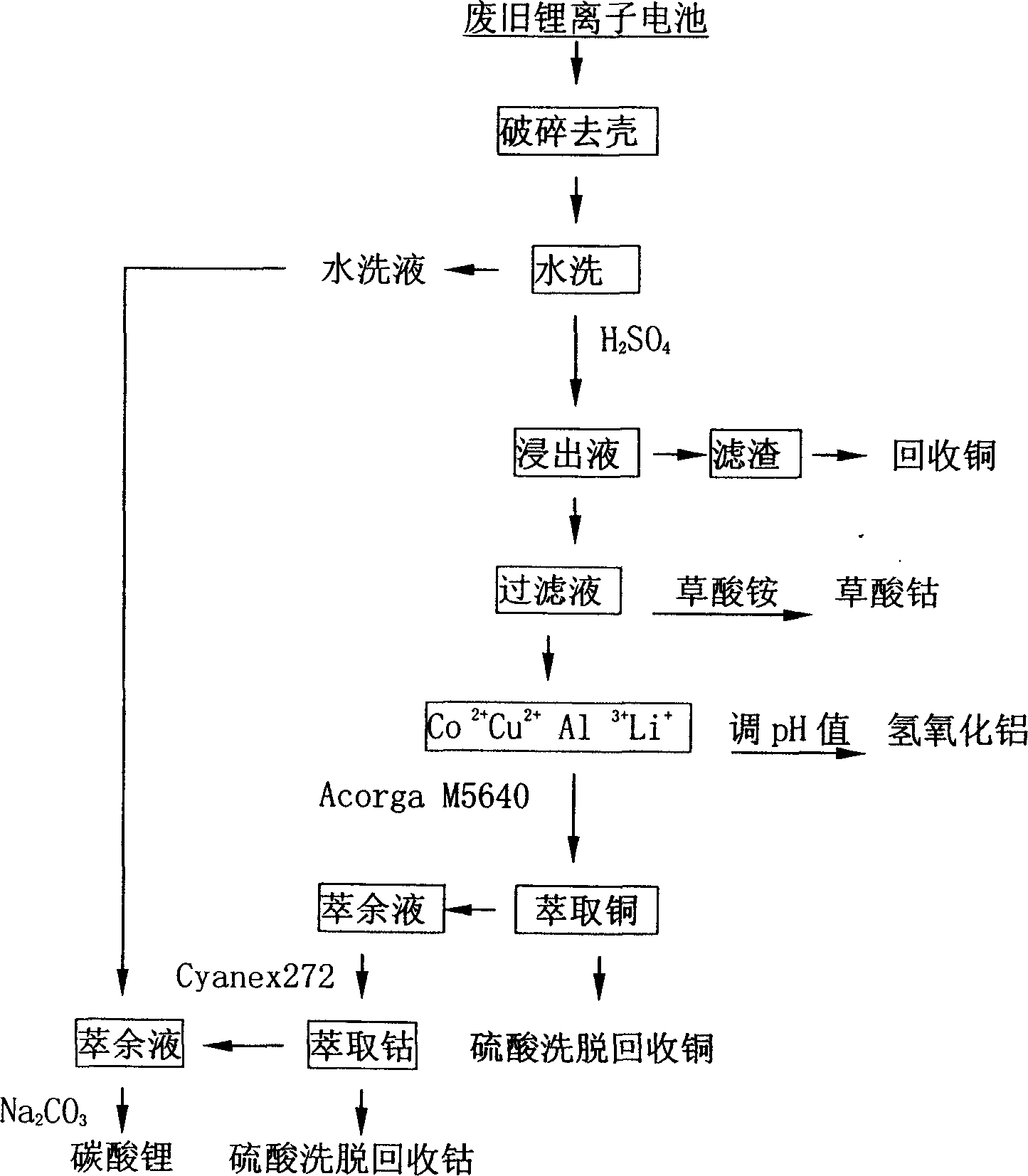
Recovery and treatment method for waster lithium ion cell
InactiveCN1601805AAvoid it happening againEasy to recycleSolid waste disposalWaste accumulators reclaimingSolventCobalt
The method for recycling and processing worn-out lithium ion battery includes steps: (1) taking out pack of worn-out battery; (2) using crushing facilities to open up case of battery, and segregation through magnetic method; (3) dissolving wastes of pole core of battery by using acid, and separating out most of cobalt through ammonium oxalate depositing method; (4) cobalt and copper deposited in residual liquid is extracted through solvent extraction method. Adding sodium carbonate generates deposition to retrieve lithium. Advantages are: simple technique, on solving pollution of worn-out batteries, and reclaining resources at same time.
Owner:SOUTH CHINA NORMAL UNIVERSITY
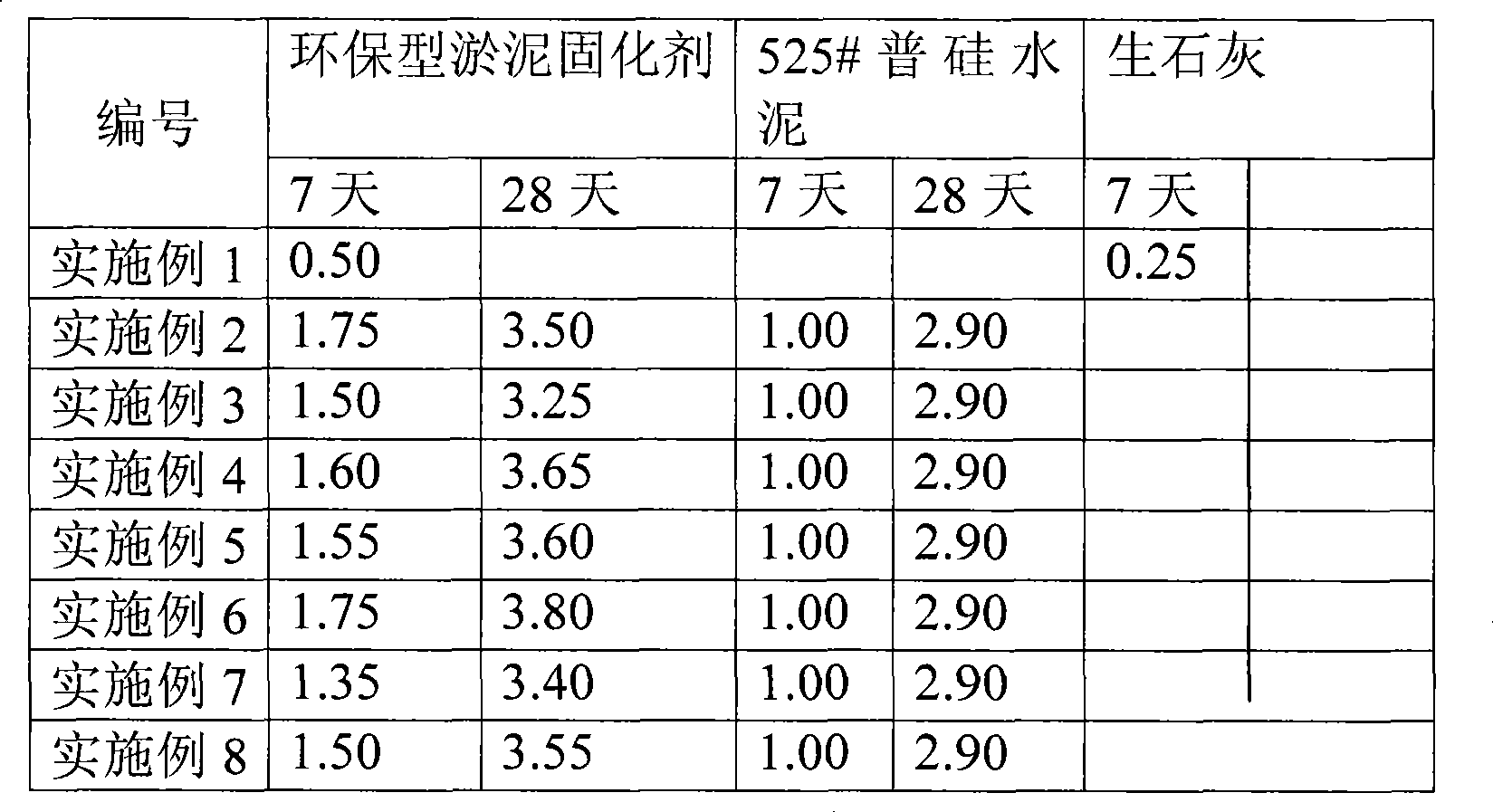
Environment-friendly type sludge firming agent
InactiveCN101381194AImprove curing abilityImprove curing effectSludge treatment by de-watering/drying/thickeningRoad engineeringSlag
The invention provides an environment-friendly silt curing agent, which is manufactured through the following steps: one or two among fly ash, calcium sulfate, sodium sulfate, sodium carbonate and potassium carbonate, one or two among slag, slag combination, potassium hydroxide, calcium oxide, sodium silicate or silicon dioxide, one or two among carbide slag, lime or gypsum, as well as one or two among triethanol amine surfactant, calcium lignosulfonate or sodium lignosulfonate form a plurality of optimal compound formulations according to respective attributes, are optimized, compounded, ground till the Brinell specific surface areas are between 300 and 900 m2 / kg respectively and then mixed, wherein particle sizes are between 0.00040 and 0.5 mm. As a large amount of waste is utilized, the curing agent saves raw materials, solves the problems about waste discharge and environmental pollution, controls waste through waste, and has important significance to environmental protection. The invention aims to provide the environment-friendly silt curing agent which has strong adaptability to a plurality of types of silt and soil, is good in curing effect, good in durability after curing and capable of utilizing industrial waste, and can be widely applied to fill engineering, embanking or embankment reinforcement engineering, road engineering and other fields.
Owner:天津渤海环保工程有限公司 +1
Firearm ammunition for tracking wounded prey
InactiveUS7426888B2Easy for to track and findIncrease awarenessAmmunition projectilesArrowsChemical reactionBiological stain
A firearm ammunition device includes a cartridge with a projectile that is loaded with a tracer agent. When the projectile is fired and strikes a game animal, the impact causes the release and dispersal of the tracer agent from the projectile. The dispersed tracer agent identifies an enhanced-visibility trail of the fleeing animal. In example embodiments, the cartridge is provided by a centerfire cartridge, a shotgun cartridge, and a muzzleloading cartridge. The tracer agent preferably comprises one or more compounds that produce visible light to the naked eye and / or under a black light source. Example tracer agents include biological stains that produce visible light upon contact with blood, luminol or another chemiluminescent compound that releases light by a chemical reaction such as may occur when contacting blood, a basic salt such as sodium carbonate or another effervescence-inducing agent, a phosphorescent compound, or a fluorescent compound.
Owner:HUNT C TIMOTHY
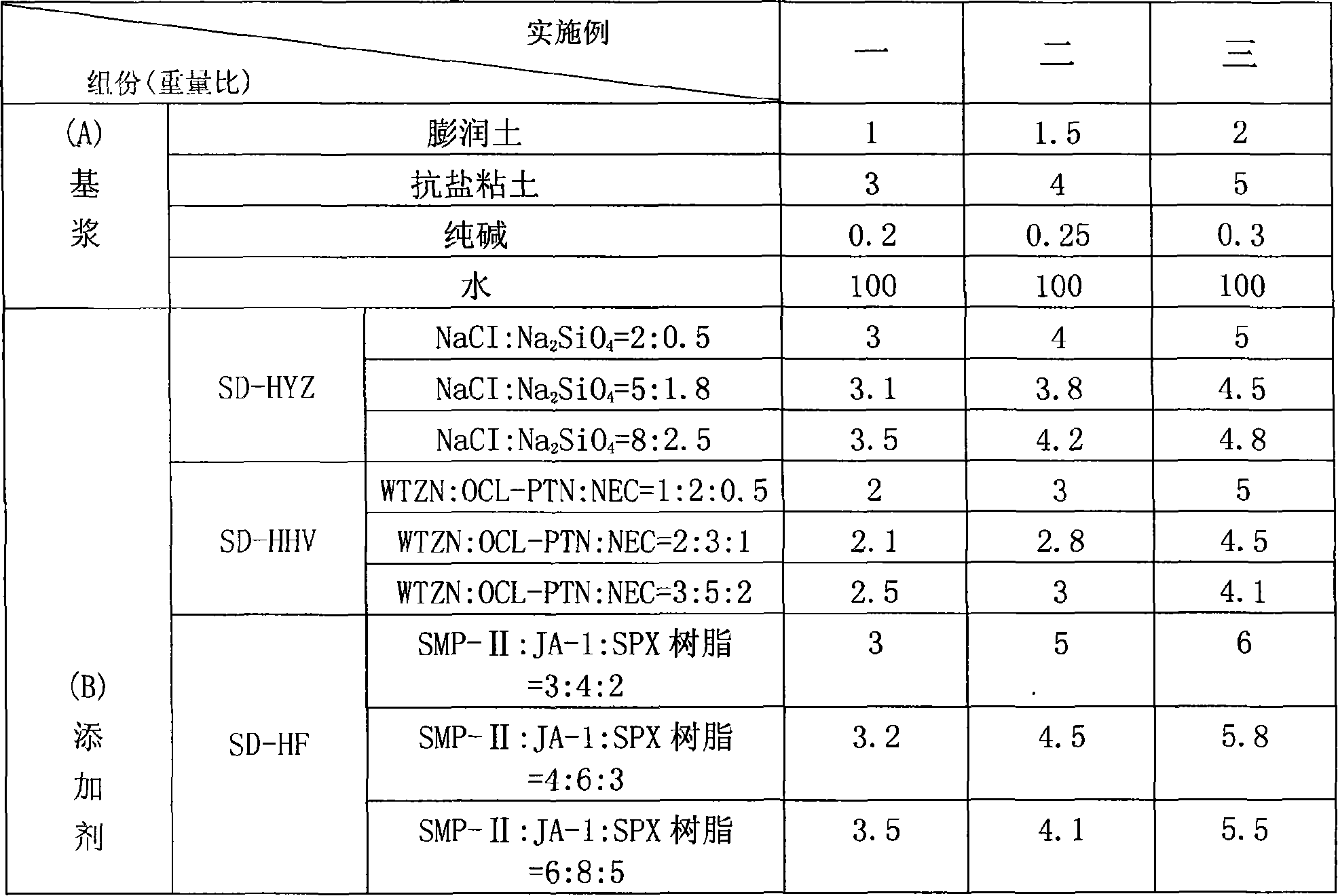
High temperature resistant circulating micro-foam drilling fluid or completion fluid
ActiveCN101148579AImprove stabilityHigh temperature resistanceDrilling compositionFoaming agentWell drilling
The present invention discloses one kind of high temperature recyclable microbubble drilling and completing fluid, which includes basic slurry comprising bentonite 1-2 (in weight portions, the same below), salt tolerant clay 3-5, sodium carbonate 0.2-0.3 and water 100; and additives comprising high temperature inhibitor 3-5, high temperature tackifier 2-5, high temperature filter loss reducing agent 3-6, high temperature salt resisting filter loss reducing tackifier 1-3, flow form regulator 1-3, high temperature foaming agent 2-5 and high temperature foam stabilizer 1-2.5. The high temperature recyclable microbubble drilling and completing fluid has excellent high temperature performance, high carrying, suspending capacity in low pressure deep well, and functions of inhibiting the hydrating expansion of mudstone and protecting oil and gas reservoir, is suitable for different types of high temperature stratum. It is applied in well drilling and well completing construction for low pressure and low permeation stratum.
Owner:DONGYING TAIER GASOLINE TECH
Clean production technique for preparation of aluminium oxide and white carbon black by using high-alumina coal ash
InactiveCN101041450ATake advantage ofWide variety of sourcesSilicon oxidesAluminium oxides/hydroxidesSodium bicarbonateSilica gel
The invention discloses a clean producing craft of aluminum oxide and white carbon black comprehensively with high-alumina coal ash, which comprises the following steps: making sodium carbonate as raw material; disintegrating high-alumina coal ash under medium-temperature; generating acid dissoluble aluminosilicate materials; using dilute sulphuric acid to acid dip; separating aluminum oxide and silica in the high-alumina coal ash; further treating aluminous liquid part; generating hydrated alumina deposition; finishing gamma-alumina or alpha- alumina product through calcining; washing, purifying, drying; calcining the silica gel part; getting white carbon black, silica aerogel, ultra-fine silica, porous silica and so on inorganic silicide.
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
Method for directly roasting and processing spent lithium ion batteries and recycling valuable metals
InactiveCN101519726AImprove leaching rateEasily brokenProcess efficiency improvementCobaltSodium sulfate
The invention relates to a method for directly roasting and treating spent lithium ion batteries and recycling valuable metals, in particular to a method for recycling and treating spent lithium ion batteries using lithium cobalt oxide as an anode material. The method comprises the following steps: firstly, remove organic binder on an organic diaphragm material and an electrode material in the batteries by roasting at a temperature of 500 DEG C to 850 DEG C; crushing and mixing the roasted battery material with sodium sulfate (or potassium sulfate) and concentrated sulfuric acid before size mixing; carrying out secondary heat treatment in an electric stove at a temperature of 350 DEG C to 600 DEG C to convert metals in the spent lithium ion batteries, such as cobalt, copper, lithium, and the like into easily water-soluble sulfate which is leached by water or a dilute sulphuric acid solution; then, using an organic extracting agent to respectively extract the cobalt and the copper from a leaching solution and obtain a cobalt product and a copper product; using sodium carbonate to precipitate the metal lithium from the leaching solution after the cobalt and the copper are removed; and enabling the leaching solution to return treatment heat so as to secondarily treat materials. The invention has a metal leaching rate higher than 99.5 percent and a metal recovery rate higher than 99 percent.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Process for preparing biological diesel oil from waste animal and plant oil
InactiveCN1382762AEliminate emissionsSimple production processLiquid hydrocarbon mixture productionBio-feedstockOil and greaseBiodiesel
A process for preparing biologic diesel oil from waste plant or animal oil includes alcoholysis and esterifying reactions under the existance of acidic catalyst, separating out the excessive non-product part to obtain coarse product, adding saturated equeous solution of edible salt containing sodium carbonate (10%), neutralizing reaction, adding industrial sodium carbonat4e, heating distillation and collecting the gas-phase fraction at 22-320 deg.C to obtain biologic diesel oil. Its advantages are high performance no pollution, and simple preparing process.
Owner:叶活动 +1
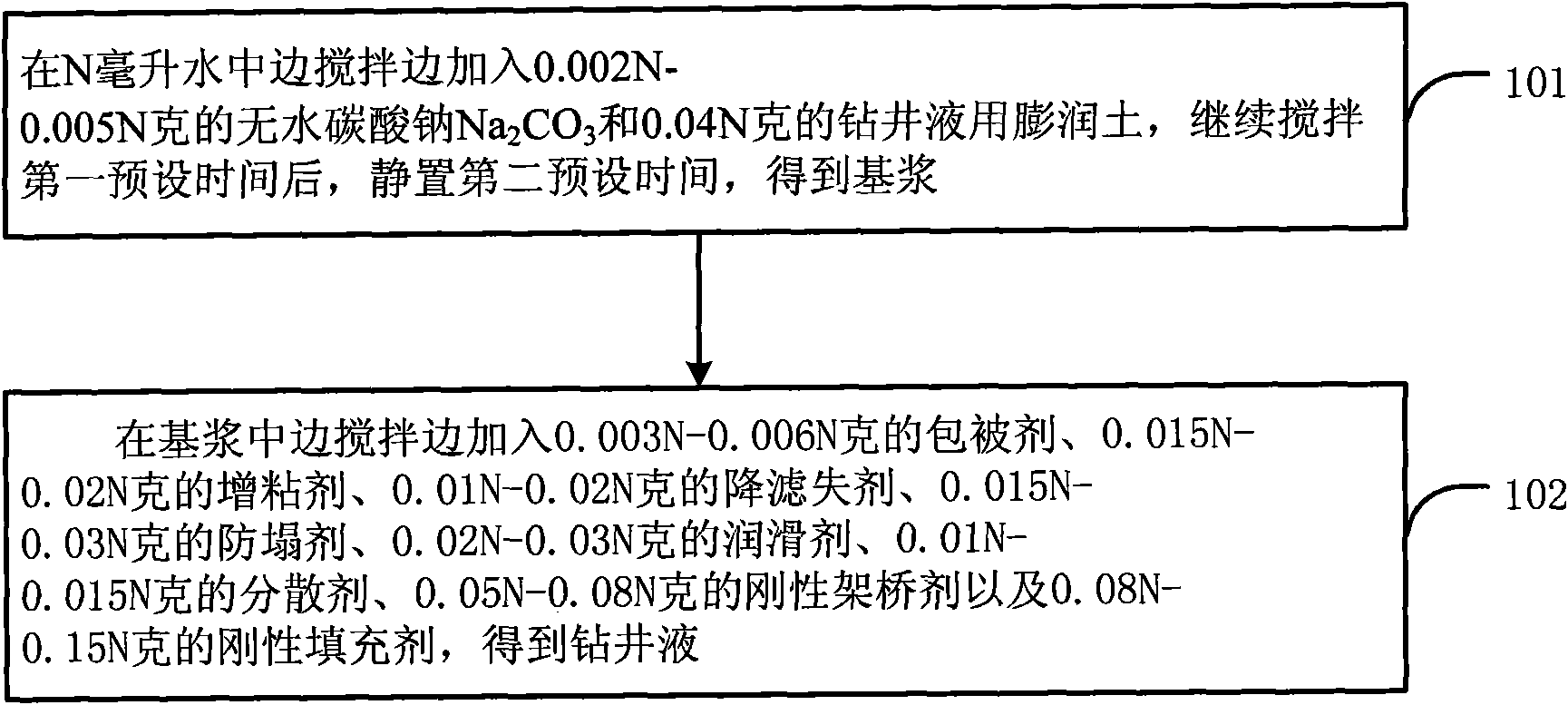
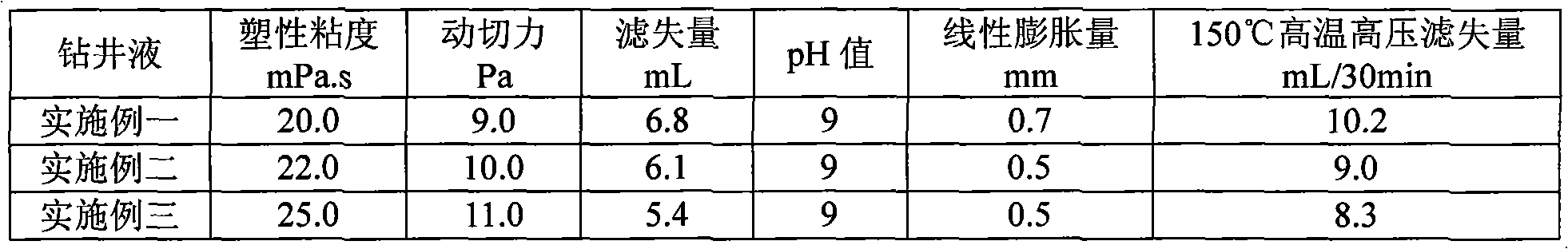
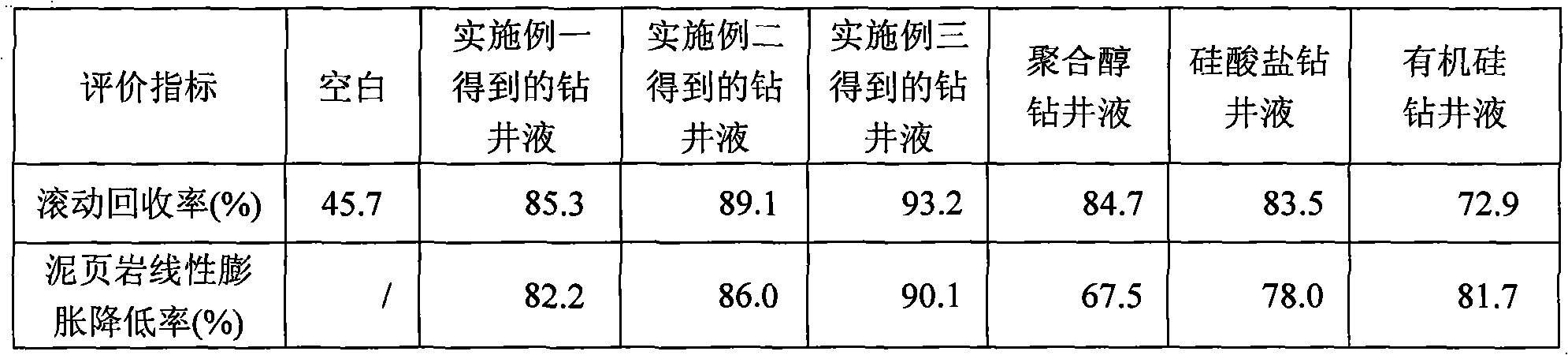
Drilling fluid and preparation method thereof
The invention discloses a drilling fluid and a preparation method thereof. The drilling fluid comprises Nml of water, wherein N is a positive number, 0.002N-0.005N gram of anhydrous sodium carbonate Na2CO3, 0.04N gram of bentonite for the drilling fluid, 0.003N-0.006N gram of coating agent, 0.015N-0.02N gram of tackifier, 0.01N-0.02N gram of filtrate reducer, 0.015N-0.03N gram of anti-collapse agent, 0.02N-0.03N gram of lubricant, 0.01N-0.015N gram of dispersing agent, 0.05N-0.08N gram of rigid bridging agent and 0.08N-0.15N gram of rigid filler. The drilling fluid of the invention is applicable to a loss formation and has obvious leakage stoppage effect, a leak-proof function at the same time and obvious inhibiting effect on a formation which is easy to disperse and collapse.
Owner:CHINA UNITED COALBED METHANE NAT ENGRES CENT +1
Method for recycling battery-grade iron phosphate in lithium iron phosphate battery and preparing lithium iron phosphate positive material by utilizing waste lithium ion phosphate battery
ActiveCN104953200AAchieve synthesisRealize the recovery of added valueWaste accumulators reclaimingBattery recyclingPhosphatePhosphoric acid
The invention relates to a method for recycling battery-grade iron phosphate in a lithium iron phosphate battery and preparing a lithium iron phosphate positive material by utilizing a waste lithium ion phosphate battery, and relates to a method for recycling a battery and preparing the battery positive material by utilizing the waste battery recycled material, solving the problems of the traditional method for recycling the LiFePO4 lithium ion battery positive electrode that the purity of the obtained element or substances is low and the obtained element or substances cannot be used for preparing the LiFePO4 lithium ion battery positive electrode. The method comprises the steps: I, crushing a positive pole piece, and carrying out heat treatment; II, dissolving the crushed positive pole piece in an acid solution; III, charging a surface active agent; IV, charging an alkaline solution, thereby obtaining a battery-grade iron phosphate; V, charging sodium carbonate to obtain a lithium carbonate; VI, mixing iron phosphate, lithium carbonate and a carbon source reduction agent; and VII, calcining. In the process for recycling the battery-grade iron phosphate in the lithium iron phosphate battery and preparing the lithium iron phosphate positive material by utilizing the waste lithium iron phosphate battery, no secondary pollution is produced, and the comprehensive and high-added-value recycling of the waste lithium iron phosphate battery can be realized.
Owner:HARBIN INST OF TECH
Technological process for purifying bittern
InactiveCN101289200AReduce purification costsImprove purification qualityAlkali metal chloridesSlurryCalcium carbonate precipitation
The invention provides a brine purification technical method which comprises that: a first step is that limewater is added into brine so that reaction happens to calcium hydroxide in the limewater and magnesian ion in the brine to generate magnesium hydrate sedimentation, the magnesian ion in the brine is removed, excessive calcium hydroxide is used for causticizing the sodium sulfate in the brine into sodium hydroxide; a second step is that flue gas is put into the brine after the magnesian ion is removed for carrying out reaction between carbon dioxide in the flue gas and the sodium hydroxide causticized in the brine to generate sodium carbonate which reacts with calcium ion in the brine to generate calcium carbonate sedimentation, the calcium ion in the brine is removed; a third step is that slurry produced in the first step and the second step is collected; and a fourth step is that refined brine after being treated by the first step and the second step is recycled. The technical method can reduce the pollution to environment, can save energy, and can reduce the consumption of raw brine and the purification cost of the brine at the same time, thereby conforming to the requirements of the strategy of sustainable development.
Owner:CHINA NATIONAL SALT INDUSTRY CORPORATION +1
Method for handling oil refining alkaline residue
InactiveCN1485409AGood entrainment effectEmission complianceWater/sewage treatment by heatingHydrocarbon oils refiningAlcoholEvaporation
A method of processing alkali wastes in oil refinement comprises: evaporating alkali wastes in oil refinement containing an evaporation accelerating agent, the evaporated gas-phrase condensate is recycled, introducing the concentrated alkali wastes into a burning oven, and producing sodium carbonate and sodium sulfate at 750í½950degree C. The evaporation accelerating agent comprises: 30í½85úÑ polymer of polyoxypropylen-polyoxyethylene, 5í½23úÑlow-carbon alcohol, 5í½55úÑ water.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com