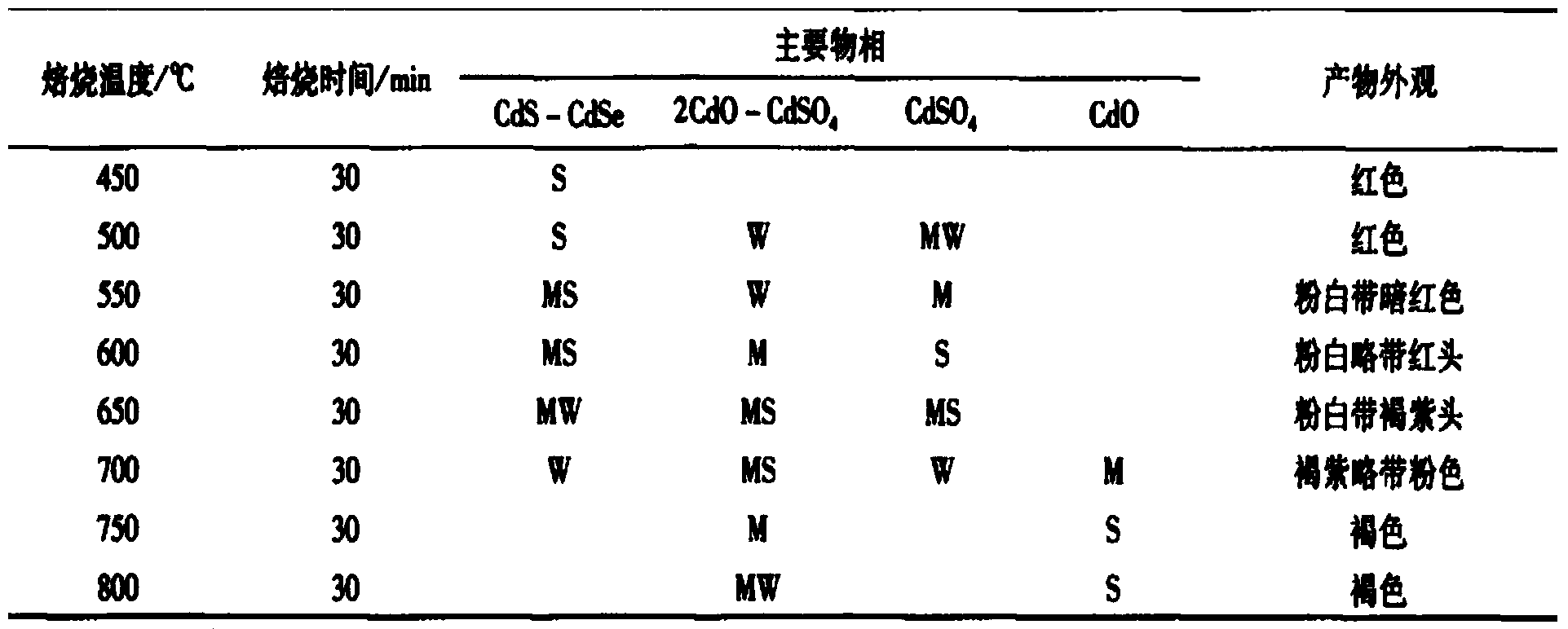
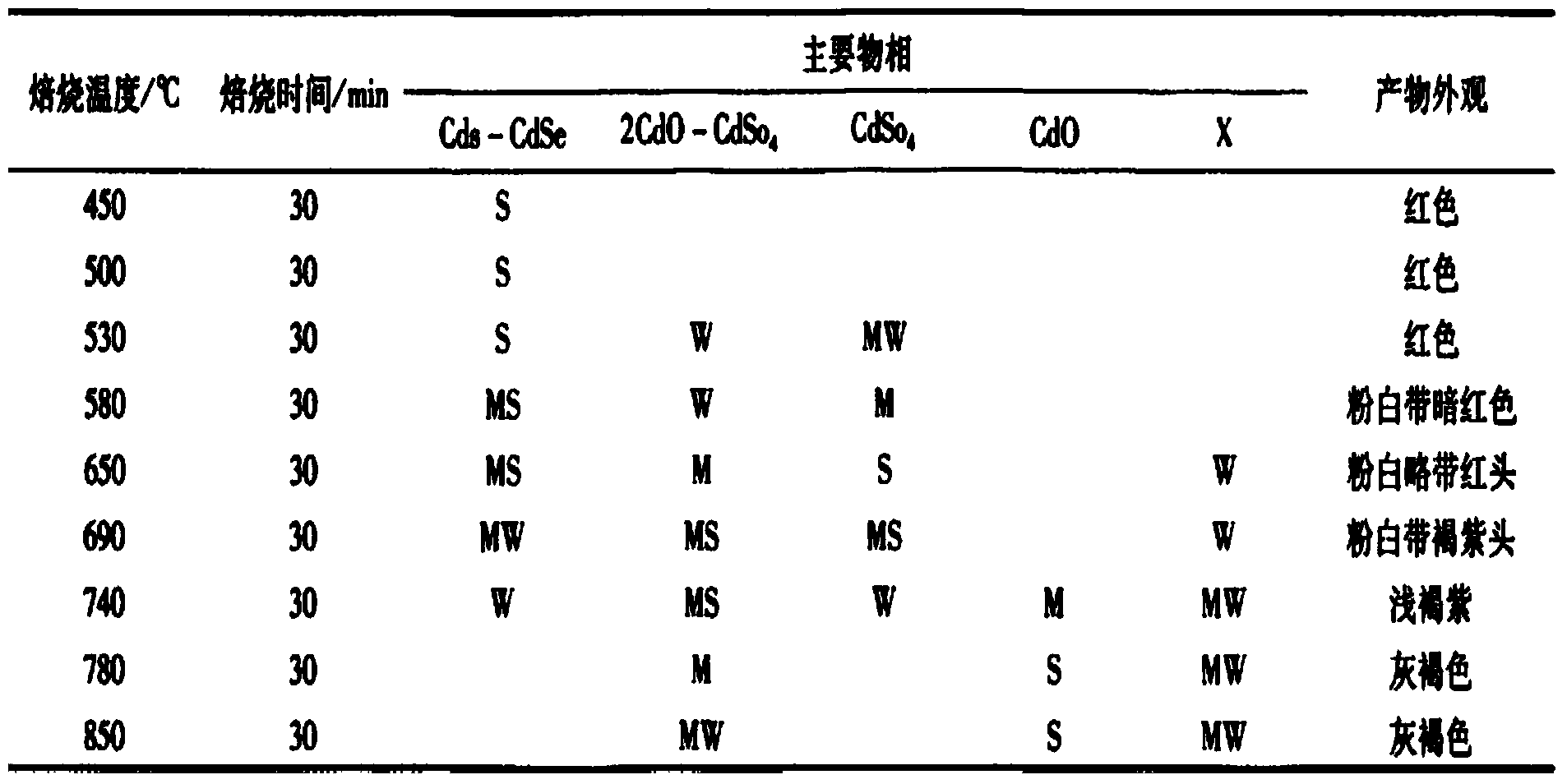
Patents
Literature
169 results about "Lead carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lead(II) carbonate is the chemical compound PbCO₃. It is a white solid with several practical uses, despite its toxicity. It occurs naturally as the mineral cerussite.
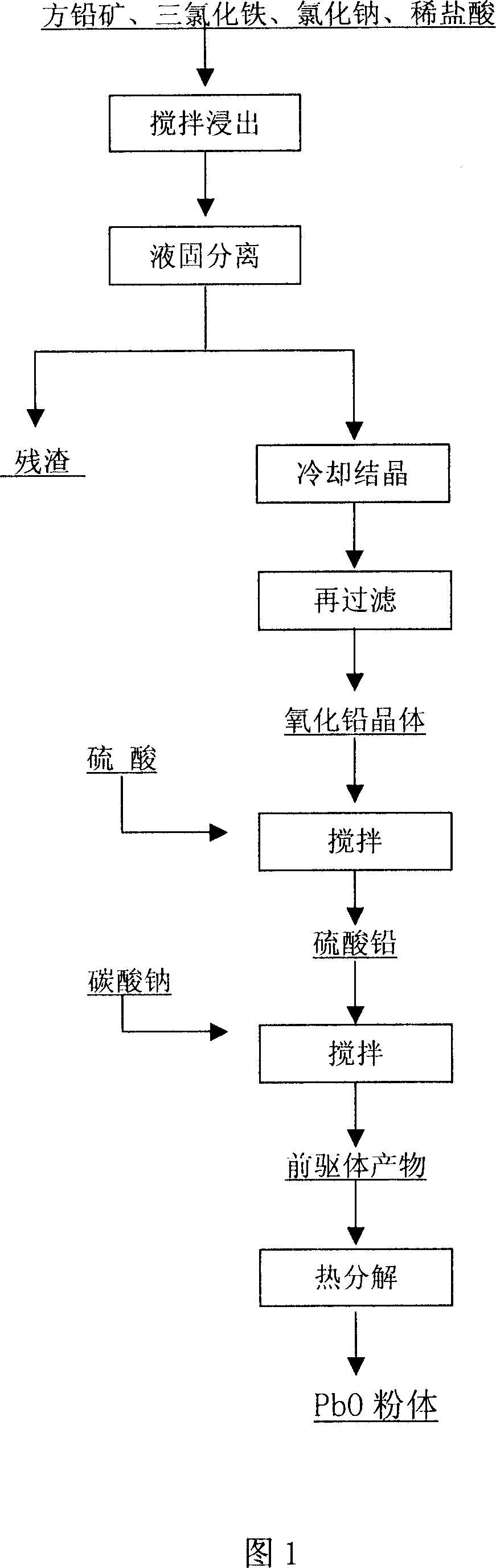
Waste lead recovering method for lead-acid storage batteries
InactiveCN101608264AAvoid harmLower decomposition temperaturePhotography auxillary processesProcess efficiency improvementLead dioxideEngineering
The invention discloses a waste lead recovering method for lead-acid storage batteries. The method comprises the following steps: fine stuff such as diachylon and the like are added in a reaction kettle with a stirring device; reducing agent (FeSO4) and dilute sulfuric acid are simultaneously added; stirring reaction is carried out at the temperature of 50-60 DEG C for 50-70 minutes so as to reduce lead dioxide into lead sulfate; the lead sulfate is added into the reaction kettle with the stirring device; water is simultaneously added into the reaction kettle for size mixing; then sodium carbonate is added; desulfuration is carried out at the temperature of 50-60 DEG C so as to obtain solid lead carbonate; the lead carbonate is put into a smelting furnace and then decomposed at the temperature of 320-350 DEG C so as to obtain lead oxide; and reducing agent (carbon) is added into the smelting furnace to reduce the lead oxide into metal lead at the temperature of 700-800 DEG C. The method recovers the lead by means of the combination of the wet and the dry processes, thereby avoiding the harm to the environment caused by lead dust, lead vapor, lead skim, sulfur dioxide gas, and the like by adopting fire smelting. The method has the advantages of high lead recovery rate, low energy consumption and no environment pollution.
Owner:张天任
Carbon dioxide sensor
InactiveUS20040158410A1Gas analyser construction detailsSpecial data processing applicationsIndiumAlkaline earth metal
A carbon oxide sensor having high sensitivity capable of reduction of influence of humidity is provided. A detection electrode and a counter electrode are provided on one side of an electrolyte, respectively. The detection electrode contains a metal oxide and a metal carbonate including plural components. The metal carbonate preferably contains a first component of alkali metal carbonate and a second component of at least one element selected from the group consisting of alkali earth metal carbonate, transition metal carbonate, zinc carbonate, cadmium carbonate, indium carbonate, lead carbonate and bismuth carbonate. Specifically, it is preferable to contain the first component including Li2CO3 and the second component including at least one element selected from the group consisting of BaCO3, CaCO3 and SrCO3.
Owner:TDK CORPARATION
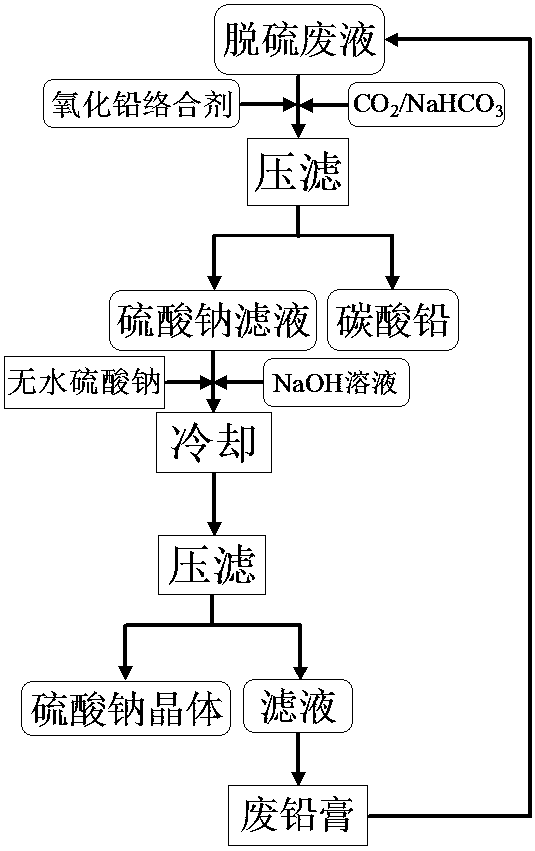
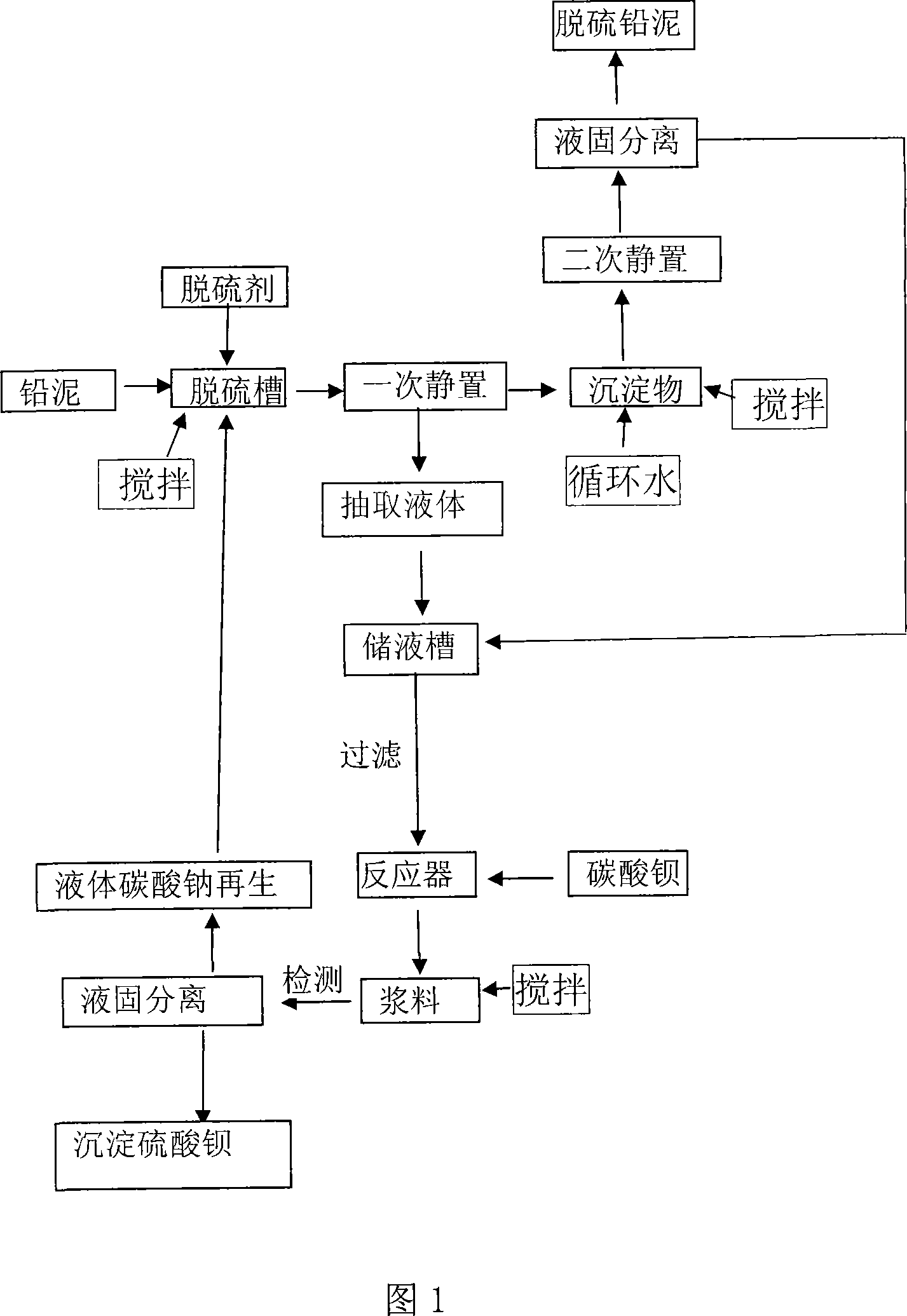
Method of recovering sodium sulfate from lead-bearing desulfurized waste liquid
ActiveCN103771459AStrong complexing abilityEliminate secondary pollutionAlkali metal sulfite/sulfate purificationLead carbonateLead oxide
The invention discloses a method of recovering sodium sulfate from a lead-bearing desulfurized waste liquid. The method is characterized by comprising the following steps: after adding a complexing agent into the lead plaster-bearing desulfurized waste liquid, forming a lead carbonate precipitate of lead complex ions in the liquor by adopting carbon dioxide or sodium hydrogen carbonate, and then carrying out solid-liquid separation to obtain a sodium sulfate filtrate and the lead carbonate precipitate; adding anhydrous sodium sulfate and sodium hydroxide into the sodium sulfate filtrate obtained by separating lead carbonate, and cooling at low temperature to separate out sodium sulfate crystals; and returning the filtrate without the sodium sulfate crystals again for desulfurization of lead oxide-bearing wastes.
Owner:BEIJING UNIV OF CHEM TECH
Ultrafine lead oxide prepared from desulfurated lead plaster by means of three-stage process and method thereof
InactiveCN103374658ALead volatility is smallReduce soot rateReclaiming serviceable partsLead oxidesLead carbonateLead oxide
The invention discloses ultrafine lead oxide prepared from desulfurated lead plaster by means of three-stage process and a method for preparing the ultrafine lead oxide. The method comprises the following steps of: carrying out a desulfurization and lead plaster acid leaching process by causing the desulfurated lead plaster to react with acid, simultaneously adding a reducing agent, and carrying out solid-liquid separation after the reaction is ended to obtain a plumbic acid containing solution; carrying out a lead carbonate preparation process by causing the plumbic acid containing solution to react with sodium carbonate, carrying out solid-liquid separation, washing and drying to obtain the lead carbonate; carrying out a roasting process by preparing the ultrafine lead carbonate by roasting the lead carbonate, wherein the ultrafine lead oxide can be PbO, Pb3O4, or the mixture of the PbO and the Pb3O4, the mean particle granularity of the ultrafine lead oxide is less than 2 mu m, and the nanocrystalline grain size is less than 500nm. Compared with the prior art, the preparation method of the ultrafine lead oxide disclosed by the invention has the following beneficial effects: the active material of the ultrafine lead oxide powder compound for the production of a storage battery enterprise can be directly prepared, lead volatilize quantity is small, the ash rate is low, the direct lead yield is high, the energy consumption is low, pollutants of sulfur dioxide and the like are not generated, and the like.
Owner:湖北金洋冶金股份有限公司 +1
Method for recycling lead oxide from lead plaster of waste lead-acid storage battery
ActiveCN107460339AImprove economyAchieve a reversible cycleLead monoxideWaste accumulators reclaimingEnvironmental resistanceLead dioxide
The invention relates to the technical field of clean recycling of waste batteries and discloses a method for recycling lead oxide from lead plaster of a waste lead-acid storage battery. The method comprises the following steps that (1) pre-desalination is conducted, specifically, the lead plaster of the waste lead-acid storage battery is added with alkali liquor to be subjected to pre-desalination, so that lead acid, lead acetate, lead nitrate, lead perchlorate or lead carbonate in the lead plaster is removed; (2) dissolution is conducted, specifically, a complexing agent solution is added into the lead plaster subjected to pre-desalination, all PbO in the lead plaster reacts with a complexing agent to generate lead complex ions, and a lead-bearing solution and filter residues are obtained; (3) dissociation is conducted, specifically, the pH value of the lead-bearing solution is adjusted by adding a dissociation agent solution, so that the lead complex ions are dissociated, and the PbO and a mixed solution containing the complexing agent and a dissociation agent are obtained; and (4) separating is conducted, specifically, a lead oxide product is obtained after solid-liquid separation and washing. According to the method, the technological condition is mild, the technological process is environmentally friendly, the technological procedure is simple, energy consumption is small, the cost is low, the lead oxide recycling rate is high, the purity is high, and the method has very high industrial application value.
Owner:CHAOWEI POWER CO LTD
Comprehensive recycling method of electric steel making lead and zinc-contained dust
ActiveCN107460327AWide variety of sourcesLow priceRotary drum furnacesProcess efficiency improvementSteelmakingCarbonate
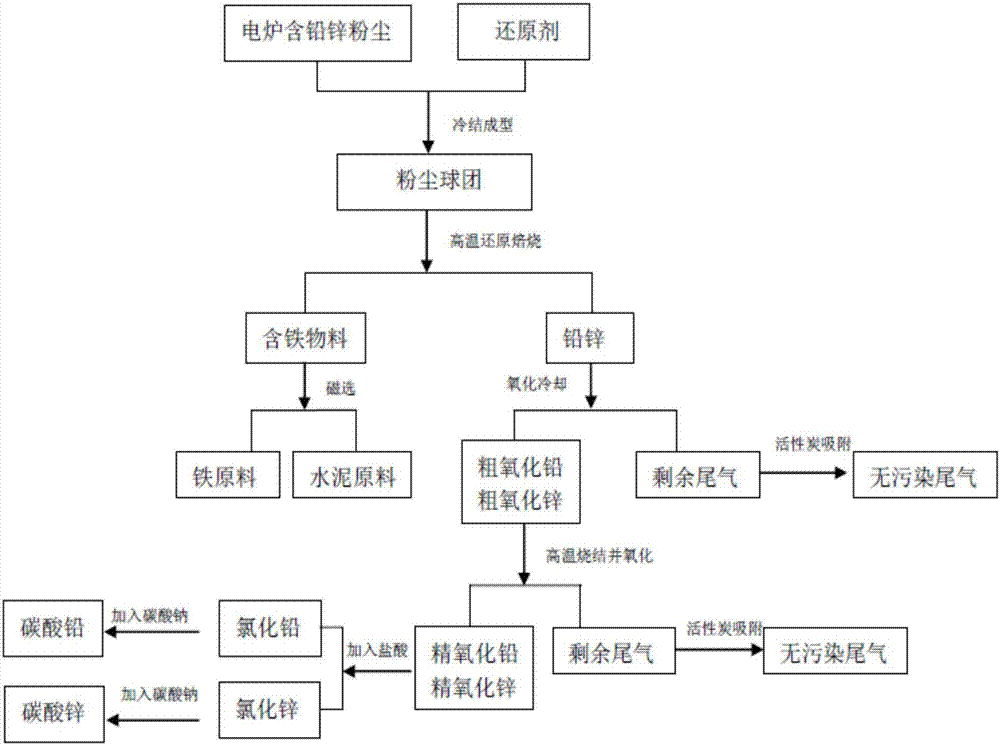
The invention provides a comprehensive recycling method of electric steel making lead and zinc-contained dust. The comprehensive recycling method comprises the following steps: lead and zinc-contained dust is prepared as pellets for reduction roasting at high temperature; lead and zinc are reduced as simple substances for volatilization in a gas-state form; iron-contained materials are formed to molten blocks, and are separated from lead and zinc; lead and zinc-contained gas and dust are collected, and are oxidized and cooled to obtain rough oxidized lead powder and rough oxidized zinc powder; the iron-contained materials without lead and zinc are collected for quick cooling, crushing and magnetic separation to obtain iron raw materials; the rough oxidized lead powder and the rough oxidized zinc powder are put in a rotary kiln again for high-temperature sintering, and are oxidized and cooled to obtain high-purity finish oxidized lead powder and finish oxidized zinc powder; the finish oxidized lead powder and the finish oxidized zinc powder are firstly reacted with hydrochloric acid to obtain lead chloride and zinc chloride; and lead chloride and zinc chloride are reacted with sodium carbonate to finally obtain lead carbonate and zinc carbonate. The comprehensive recycling method is simple in process, high in lead and zinc recovery rate and high overall resource recycling rate.
Owner:CHONGQING UNIVERSITY OF SCIENCE AND TECHNOLOGY +1
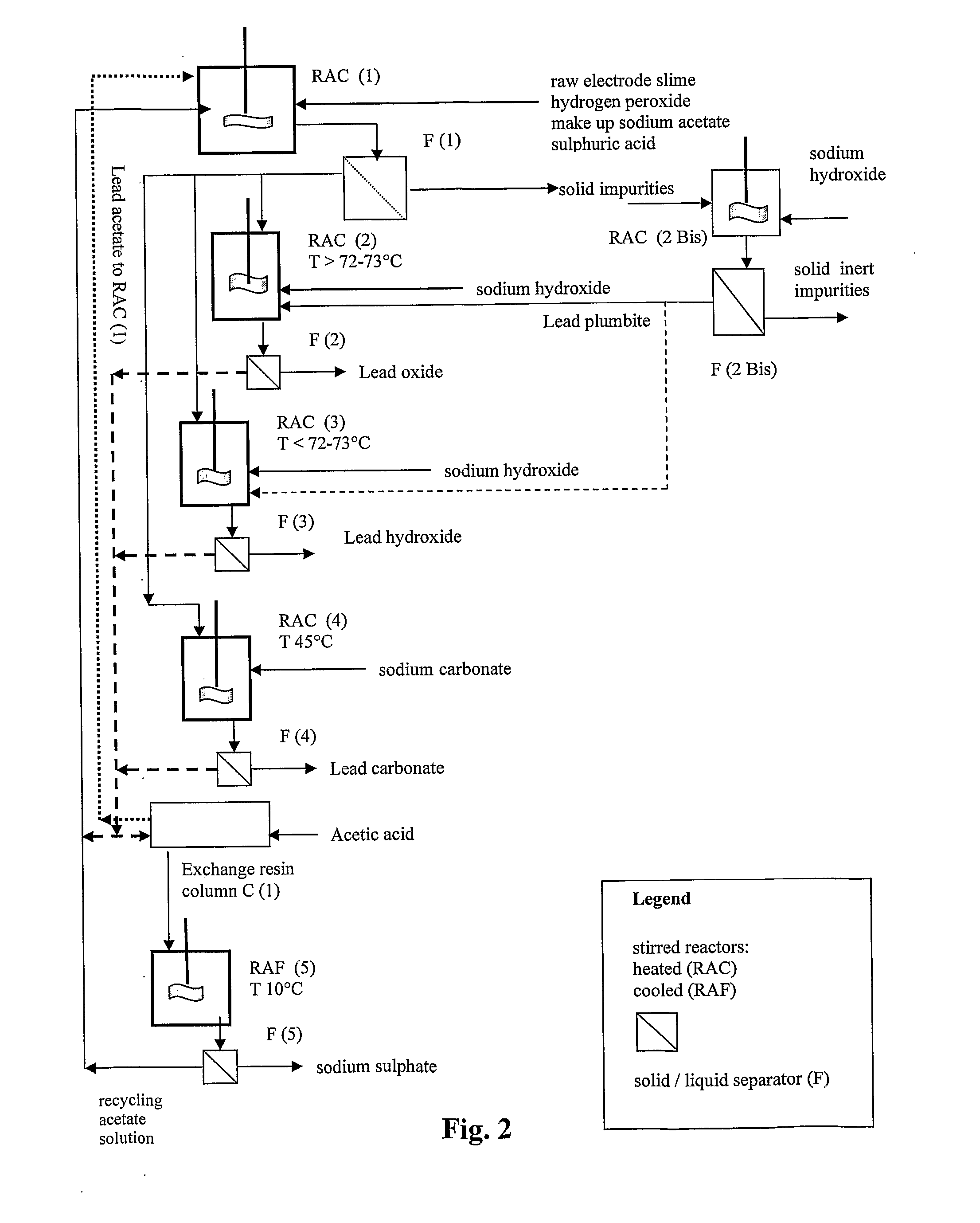
Reclaiming of lead in form of high purity lead compound from recovered electrode paste slime of dismissed lead batteries and/or of lead minerals
InactiveUS20120186397A1Reduce recycling costsEffective simplificationFinal product manufactureWaste accumulators reclaimingInorganic lead compoundSolid phases
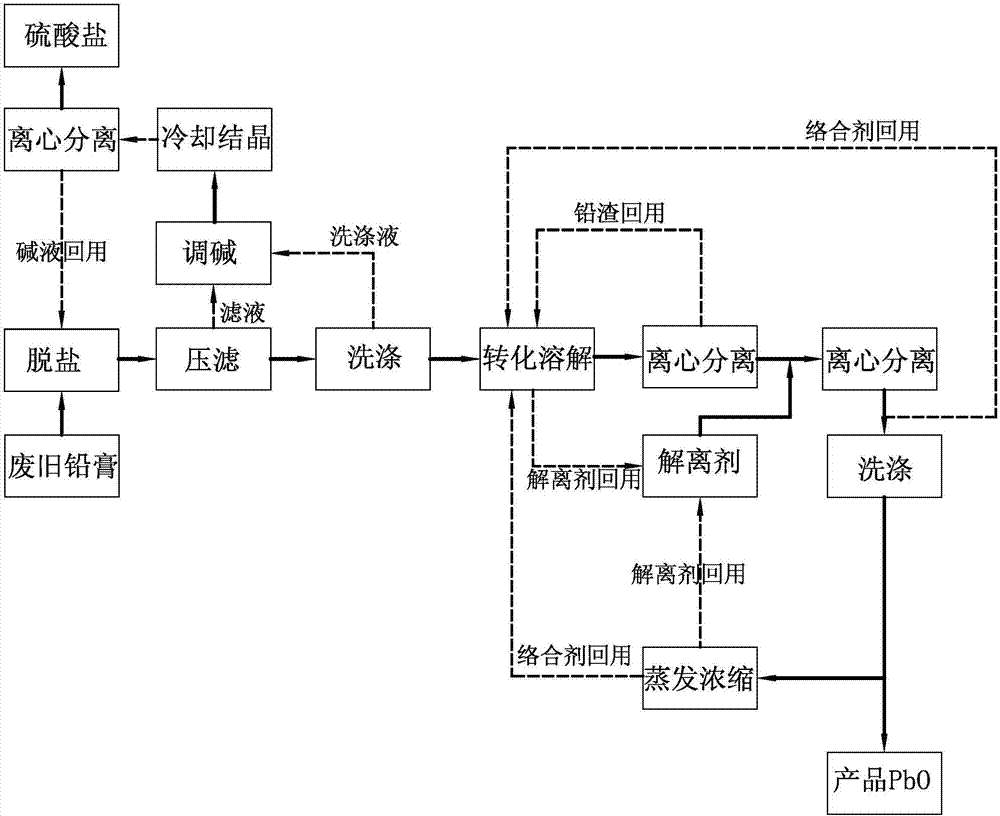
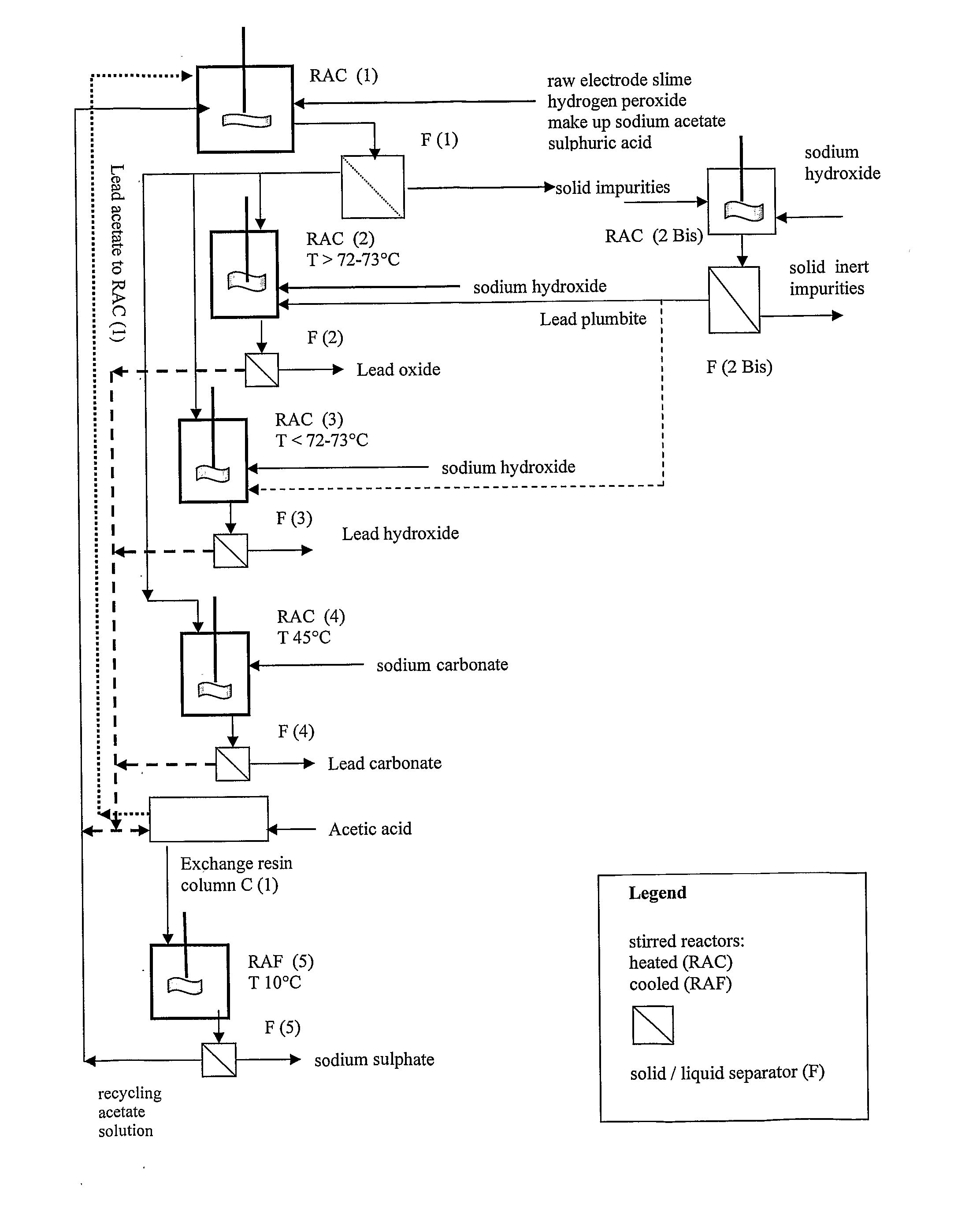
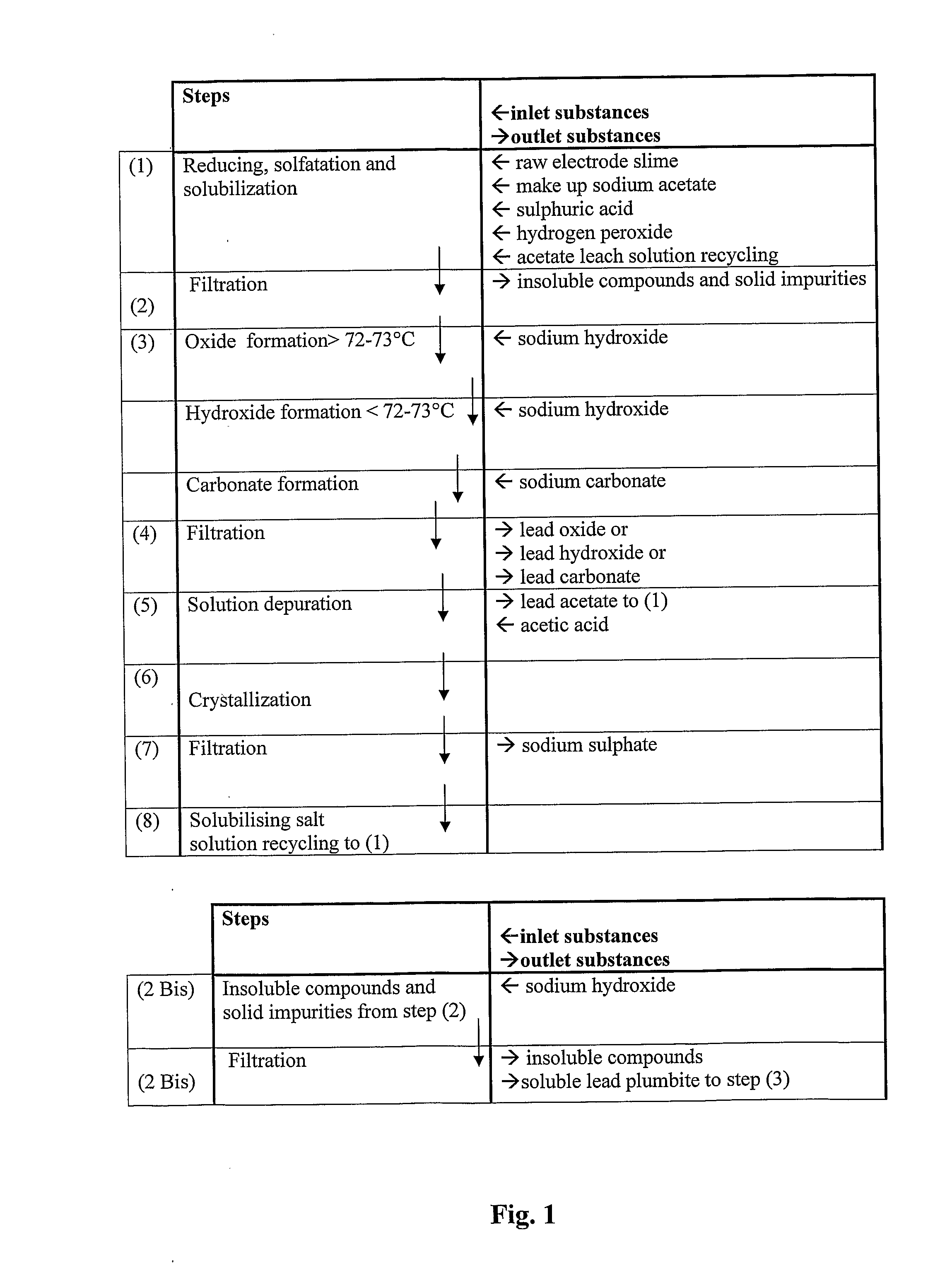
An all-wet process for reclaiming the lead content of impure electrode paste or slime from discarded lead batteries and / or lead minerals, in form of high purity lead compound, comprises a) suspending the impure lead containing material in a lead sulphate dissolving aqueous solution of a salt belonging to the group composed of the acetates of sodium, potassium and ammonium; b) adding to the suspension sulphuric acid in an amount sufficient to convert all lead oxides to lead sulphate soluble in the acetate salt solution and slowly adding to the suspension either hydrogen peroxide or a sulphite or bubbling sulphurous anhydride through it, in a measure adapted to reduce any lead dioxide to lead oxide converted eventually to soluble lead sulphate by the sulphuric acid; c) separating a limpid acetate salt solution containing dissolved lead sulphate from a solid phase residue including all undissolved compounds and impurities; d) adding to the separated solution of lead sulphate either carbonate or hydroxide of the same cation of the acetate salt of the lead sulphate dissolving solution for precipitating highly pure lead carbonate / oxycarbonate or lead oxide or hydroxide, respectively, while forming sulphate of the cation, soluble in the acetate salt solution; and e) separating the precipitated high purity lead compound from the acetate salt solution now containing also sulphate of the same cation of the acetate salt. The acetate salt solution containing also sulphate of the same cation of the acetate salt separated from the precipitated compound of lead is re-cycled to step a) and the content of sulphate of the same cation in the solution is maintained below saturation limit by continuously or periodically cooling at least a portion of the solution separated from the precipitated lead compound to cause selective crystallization of sulphate salt of the same cation of the acetate salt and removing it as a by-product. Optionally, the separated solid phase comprising insoluble compounds of lead and / or undissolved concretions of lead compounds is treated in hot concentrated hydroxide of the same cation of the selected acetate salt and converting these compounds of lead and / or undissolved concretions of lead compounds to soluble plumbites, and the separated lead containing alkaline liquor may be added to the limpid acetate solution for precipitating all reclaimable lead in form of high purity lead oxide or hydroxide.
Owner:MILLBROOK LEAD RECYCLING TECH
Purple pearly luster optical variable anti-counterfeiting ink
ActiveCN101333353AIncrease craft anti-counterfeiting functionNot easy to imitateInksLead carbonatePrinting ink
Disclosed is violet pearlescent anti-forgery phototropic printing ink, containing anti-forgery dye violet pearl luster, printing ink vehicle, filler, flatting agent, wetting agent, foam killer and solvent. The violet pearlescent anti-forgery ink is characterized in that the weight percentages of the ingredients are as follow: 5-30g green-turn-violet anti-forgery printing ink, 20-40g printing ink vehicle acrylics, 5-20g filler lead carbonate, 0.3-3g flatting agent, 0.3-3g wetting agent, 0.5-3g foam killer, and 40-60g solvent n-propyl. The invention is characterized in that: 1, the phototropic printing ink is hard to forge and is high in fidelity, thus improving actual anti-forgery value; 2,the violet pearlescent phototropic oil, added with violet lead carbonate, supplements the color system of changing from blue to violet, which conventional printing ink fails to realize; 3, the violet pearlescent phototropic oil, added with violet lead carbonate, is enlarged in granule and increased in solid content; on the basis of the same consumption, more printings can be produced, thus improving output and improving the texture of the printings.
Owner:CHANGDE JINPENG PRINTING
Desulfurization and transformation method for waste lead accumulator regenerative lead
InactiveCN101078059ASolve the real problemThe effect is positive and obviousProcess efficiency improvementCarbonate preparationLead carbonateChloride
A kind of desulfitation transformation method to regenerate Pb with waste chloride accumulator, it contains a procedure to break waste chloride accumulator, the liquid and the solid in the Pb mud fluid after breaking will be separated, and wet lead slime can be gained. The wet lead slime will be put into a desulfurizer. And sodium carbonate will be also added as desulfurating agent, then they will be fully agitated, they will fully react. The liquid and solid will be separated after stewing, The sediment lead carbonate and liquid sodium sulfate can be gained, the later will be put into a reactor, and barium carbonate will also be put in, then they will be agitated, and reacted fully, they will be stew, and then the liquid and solid of them will be separated, the sediment barium sulfate and liquid sodium carbonate can be gained, and the later will be delivered into the desulfurizer to recycle as desulfurating agent. This patent translates the reaction between sodium sulfate and barium carbonate in the remained liquid, recycle the desulfurater sodium carbonate. It not only resolves the problem how to deal with the sodium sulfate in the desulfitation transformation technology, but also resolves the regeneration and the recycle of desulfurater sodium carbonate.
Owner:上海飞轮有色冶炼厂
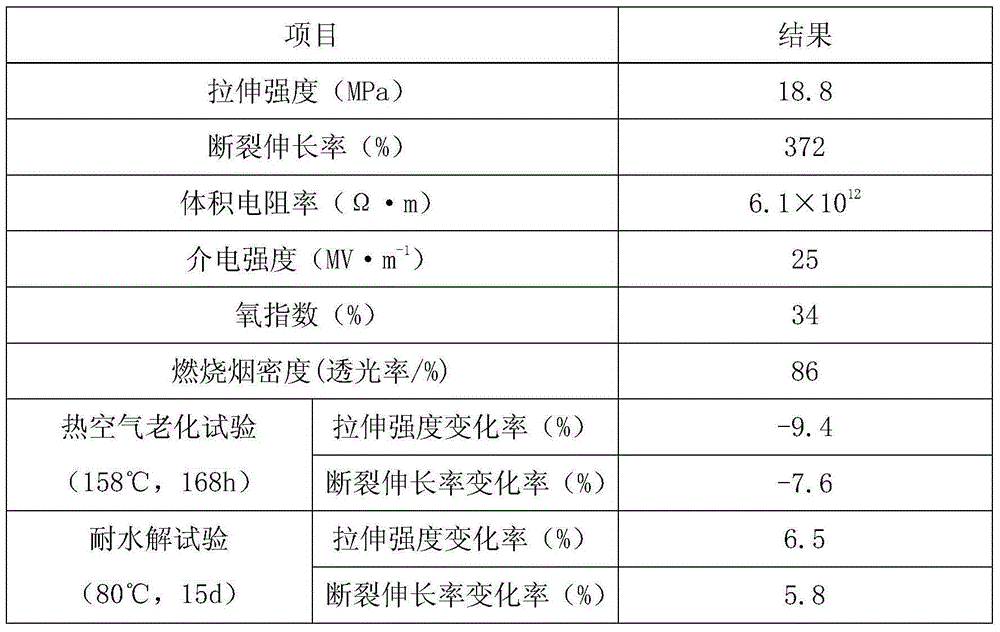
Hydrolysis-resistant cable material for automobile and preparation method of hydrolysis-resistant cable material
InactiveCN105037960AImprove hydrolysis resistanceAvoid hydrolysisRubber insulatorsFire retardantTert butyl
The invention discloses a hydrolysis-resistant cable material for an automobile and a preparation method of the hydrolysis-resistant cable material. The hydrolysis-resistant cable material is prepared from the following raw materials, by weight part: 45-60 of ethylene propylene diene monomer rubber, 22-34 of tetrafluoroethylene-propylene rubber, 9-14 of rectorite powder, 5-10 of C5 hydrogenated petroleum resin, 2-3 of tert-butyl-hydroperoxide, 1.5-2.5 of basic lead carbonate, 3-5 of polyparadinitrosobenzene, 4-8 of dibutyl sebacate, 2-3 of magnesium oxide, 1-2 of zinc stearate, 3-6 of disproportionated rosin, 2.5-4.5 of N-ethyl-p-toluenesulfonamide, 12-17 of a composite flame retardant agent, 5-10 of spherical silicon powder, 1-2 of an accelerant PPD, 1-2 of an accelerant ZBPD, and 1-2 of an antioxidant 2246. The prepared cable material has excellent hydrolysis resistance, so that the electric wires and cables of the automobile can be effectively prevented against hydrolysis. The cable material can maintain excellent properties after continuously used in high pressure and high temperature, and has good electrical insulation property, mechanical strength, corrosion resistance, abrasive resistance, and thermal resistance.
Owner:ANHUI CHUNHUI INSTR CABLE GROUP
Desulfurization process for waste material containing lead sulfate and circulation method of desulfurization mother liquor thereof
ActiveCN106916952AProcess energy saving and environmental protectionMild process conditionsProcess efficiency improvementLead carbonateSulfate
The invention relates to a desulfurization process for a waste material containing lead sulfate and a circulation method of desulfurization mother liquor thereof, belonging to the technical field of comprehensive utilization of solid waste materials containing lead. The desulfurization process comprises the steps: dissolving the waste material containing the lead sulfate with a compound leaching agent, thus dissolving lead sulfate solid, and obtaining filter liquor dissolved with the lead sulfate and unreacted filter residues after solid-liquid separation, wherein the compound leaching agent comprises an A and B conjugate solution, A is one or more of ammonia and amine substances, and b is ammonium salt; adding a carburizing agent providing carbonate into the lead sulfate filter liquor, and obtaining lead carbonate precipitation and deleading mother liquor after solid-liquid separation; adding a desulfurizing agent into the deleading mother liquor, and obtaining sulfate and the desulfurization mother liquor after solid-liquid separation. The desulfurization process industriously achieves the requirement of atom economy reaction.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing highly oxidized lead powder from waste battery lead plaster
ActiveCN103436702AHarm reductionReduce manufacturing costRed leadWaste accumulators reclaimingLead carbonateCooking & baking
The invention discloses a method for preparing highly oxidized lead powder from a waste battery lead plaster, which comprises the following steps: (1) grinding a waste lead plaster into powder; (2) adding a sulfuric acid solution and reducer into the lead plaster powder, sufficiently reacting, separating to remove the liquid, washing the solid material to neutral with water, and baking; (3) adding desulfurizer into the solid material obtained in the step (2), sufficiently reacting, separating to remove the liquid, washing the solid material to neutral with water, and baking; (4) adding an acid solution into the solid material obtained in the step (3), sufficiently reacting, removing the insoluble impurities to obtain a lead-containing solution, adding carbonate into the solution, sufficiently reacting, removing the liquid, washing the solid with water, and baking to obtain a pure lead carbonate solid; and (5) calcining the lead carbonate solid obtained in the step (4) in a muffle furnace to obtain the highly oxidized lead powder. The method has the advantages of low preparation cost, simple technical process and high lead recovery rate and quality, and can obviously lower energy consumption and reduce environmental pollution.
Owner:YANGZHOU APOLLO BATTERY
Method for preparing tribasic lead sulfate by using ash of copper converter
InactiveCN101519729ASimple processImprove the operating environmentProcess efficiency improvementLead sulfatesPregnant leach solutionLead carbonate
The invention relates to a method for preparing tribasic lead sulfate by using ash of a copper converter, which comprises the step: (1) the ash of the copper converter is leached by dilute sulphuric acid to obtain leach liquor containing copper and zinc and lixiviated residues containing lead and bismuth. The method is characterized by also comprising the steps: (2) sodium chloride is added into the leached residues containing lead and bismuth, and the leached residues containing lead and bismuth are leached by concentrated sulfuric acid to obtain leach liquor containing bismuth and chloride leached residues; (3) the chloride leached residues are converted by ammonium carbonate to obtain ammonium carbonate converting residues; (4) the ammonium carbonate converting residues are dissolved by nitric acid; (5) the ammonium carbonate converting residue solution dissolved by the nitric acid is deposited by sulphuric acid; (6) the formed lead sulfate deposition and sodium hydroxide reacts to synthesize tribasic lead sulfate. The method adopts a wet process, lead in the ash is transformed into lead carbonate through sectioning leaching, and then, the lead carbonate is dissolved by nitric acid, deposited by sulphuric acid and added with sodium hydroxide to synthesize the tribasic lead sulfate. The technical process is simpler, and has good operating environment due to the adoption of the wet process.
Owner:JINCHUAN GROUP LIMITED
Method of preparing tribasic lead sulphate using lead mud in waste lead battery
A process for preparing lead trihydroxy sulfate from the lead mud of used lead accumulator includes such steps as reaction between said lead mud and sodium carbonate, desulfurizing to obtain lead carbonate, filtering, decoloring the filtrate, evaporating, crystallizing to obtain sodium sulfate as by-product, dissolving the filtered dregs in nitric acid, reacting on sulfuric acid to obtain the deposit of pure lead sulfate, reacting on sodium hydroxide to obtain lead trihydroxy sulfate, filtering, drying and pulverizing.
Owner:HUNAN UNIV
Method of directly producing ultra-fine lead oxide powder from galena concentrate
InactiveCN1920065AExpand sourceReduce contentLead monoxideProcess efficiency improvementLead carbonateLead oxide
The invention relates the method for producing super fine lead oxide powder from blue lead finished ore. The method comprises the following steps: using blue lead finished ore as raw material, leaching, crystallizing, getting PbCl2 crystal, adding sulfuric acid into PbCl2 crystal to get PbSO4 powder, carrying out chemical precipitation method for PbSO4 powder, getting the mixture of PbCO3 and 2PbCO3.Pb(OH)2, carrying out thermal decomposition, and getting the ultra-fine and high-purity beta-PbO. The method has the advantages of reducing the cost, extending the origin of raw materials, and reducing the impurity. The product has high activity, and the particle mean size of the products is 4.0-5.0 mum.
Owner:CENT SOUTH UNIV
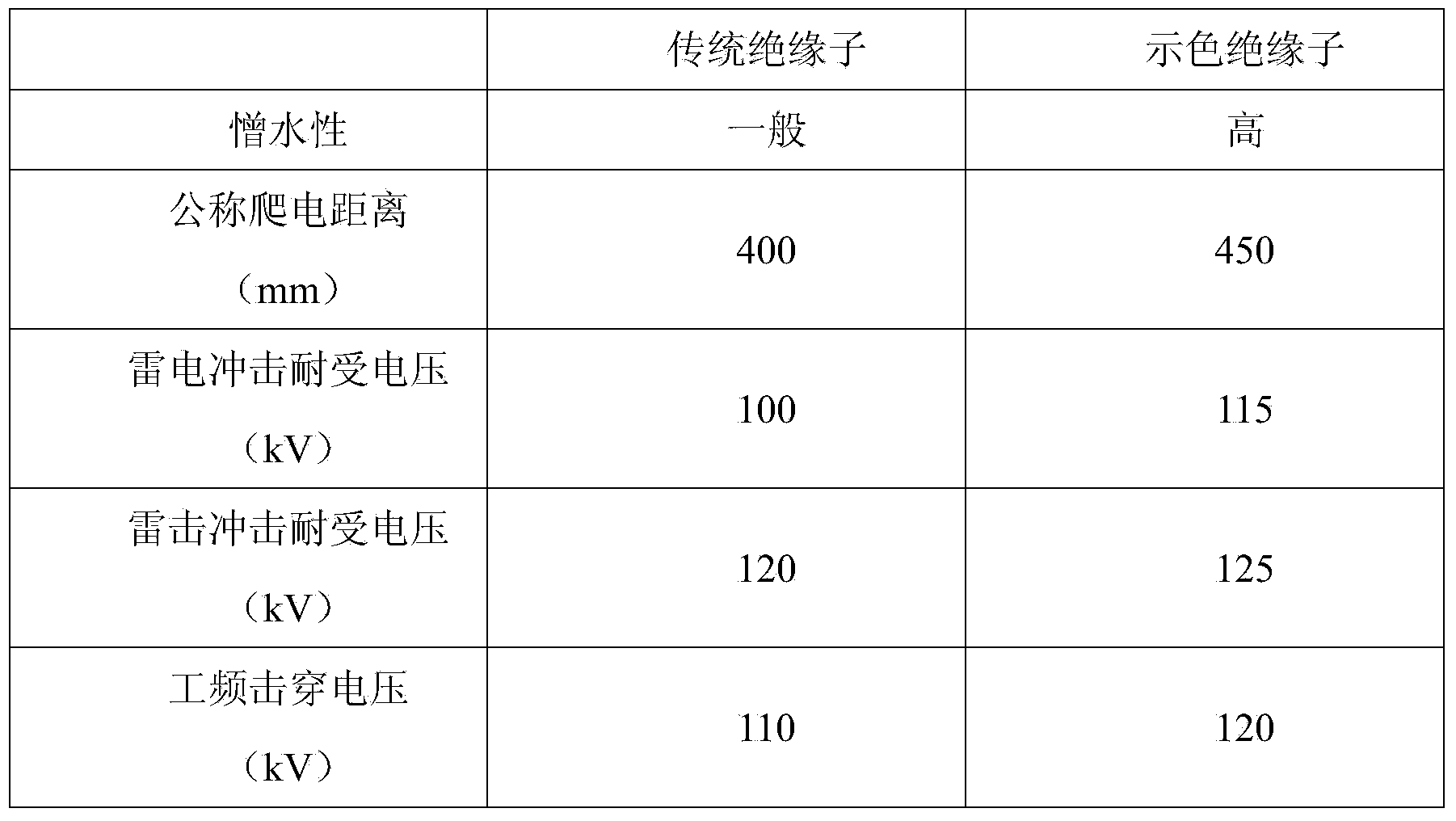
Visual allochroic insulator coating and preparation method thereof
InactiveCN103709936AReduce labor intensityEasy maintenanceInsulatorsThermosensitive paintsLead carbonateSilicon rubber
The invention discloses a visual allochroic insulator coating and a preparation method thereof. The visual allochroic insulator coating is prepared from the following raw materials in parts by weight: 40-50 parts of silicon rubber, 4-7 parts of phthalocyanine blue, 1-6 parts of calcium carbonate, 0.1-1.0 part of lead carbonate, 1-3 parts of talcum powder, 15-20 parts of cadmium red, 10-15 parts of greenish orchid, 1-3 parts of titanium dioxide, 1-5 parts of barium metaborate, 1-3 parts of dispersing assistant and 1-3 parts of binder. By using a visual rubber insulator containing the visual allochroic insulator coating disclosed by the invention, the flashover position can be obviously seen under the condition of not climbing on the rod, and the flashover origin can be judged.
Owner:BEIJING DINGYI TONGYUAN SCI & TECH DEV CO LTD
Novel technology for colouring on glaze during ceramic painting
The invention relates to a novel technology for colouring on glaze during ceramic painting. The novel technology for colouring on glaze during ceramic painting comprises the following steps: adopting a 18# white material, namely selecting 50% by weight of feldspar glaze, 10% by weight of quartz powder, 20% by weight of boric acid and 20% by weight of lead carbonate, mixing and placing the mixture into a ceramic crucible, melting to be bright and transparent at the temperature of 1300 DEG C, cooling into a glass fusion cake, smashing and grinding for late use; by adopting a guide pipe metal pen as a colouring tool, mixing the prepared white material with camphor oil equivalent to 60% by weight of the weight of the white material, grinding manually, then sequentially adding 20% by weight of pine distillate oil and 20% by weight of frankincense oil, and grinding into liquid paste; colouring and roasting, namely painting various glazes, colouring and roasting at the temperature of 790-820 DEG C. The novel technology for colouring on glaze during ceramic painting has the beneficial effects that high-grade ceramic or present ceramic can be prepared; the 18# white material has the advantages of high brightness, no cracking and no strict requirement on roasting atmosphere; uniform and regular lines, easy control and high production rate can be guaranteed; thickness of lines can be graded.
Owner:叶丽虹
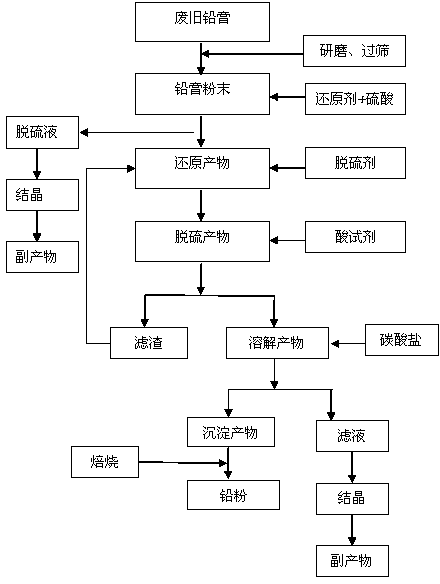
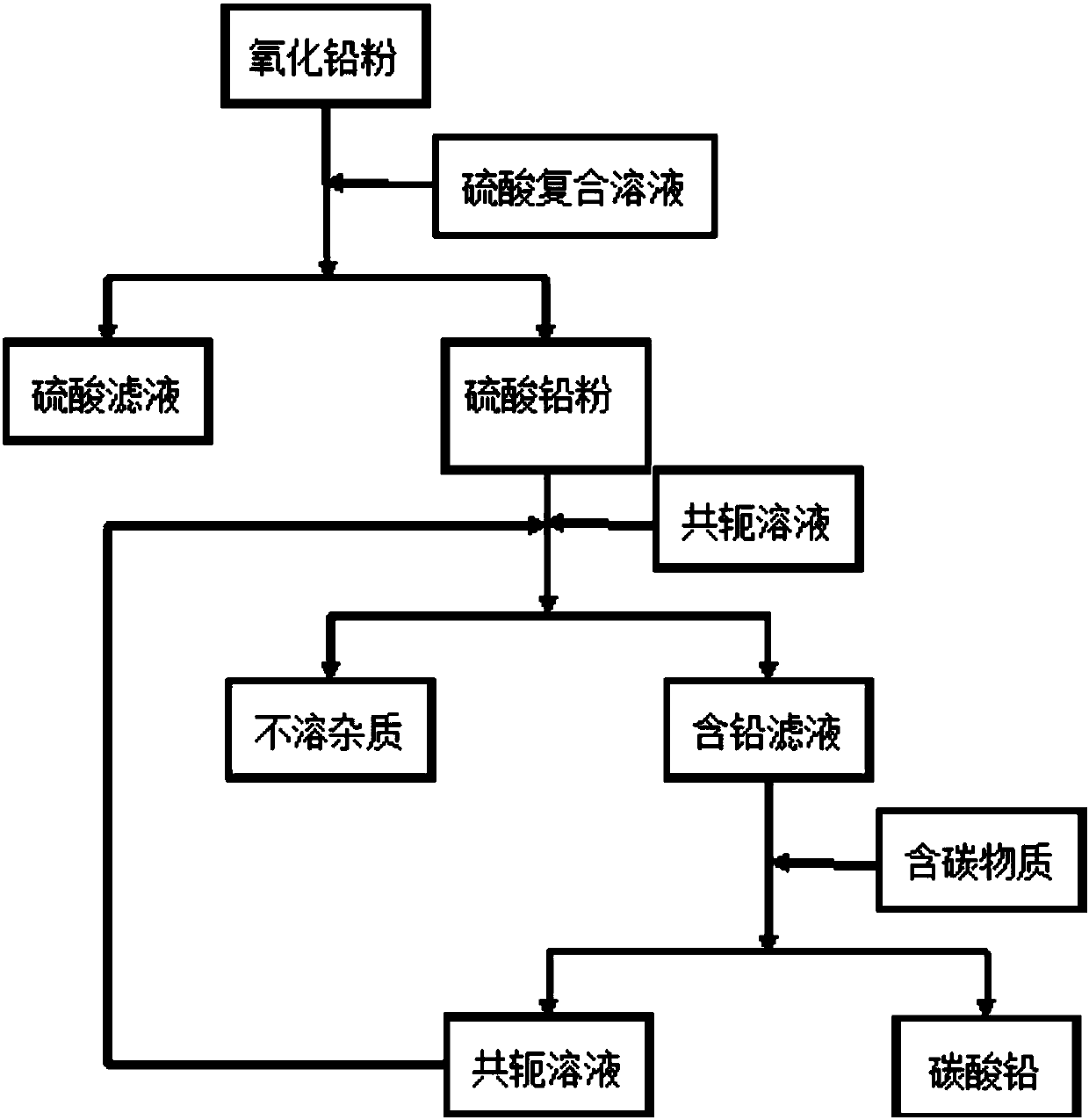
Method for preparing lead carbonate from lead oxide waste
The invention discloses a method for preparing lead carbonate from lead oxide waste and belongs to the field of recycling waste containing lead oxide. The method comprises the following steps: (1) utilizing a sulfuric acid composite solution to convert lead oxide waste into a lead sulfate crude product; (2) dissolving the lead sulfate crude product in the step (1) by an X-Y conjugate solution to dissolve lead sulfate in the crude product and filtering to obtain lead sulfate filtrate and insoluble impurities; (3) adding carbon containing matter into the lead sulfate filtrate obtained in the step (2) and separating to obtain the lead carbonate. The method disclosed by the invention can be widely applied to varieties of sources such as waste lead-acid cell lead plaster, waste generated in lead-acid cell production and lead containing waste in a smelting process and has the advantages of high lead recovery rate, cyclic utilization of a mother solution and the like.
Owner:北京绿色引领环保科技研究院有限公司
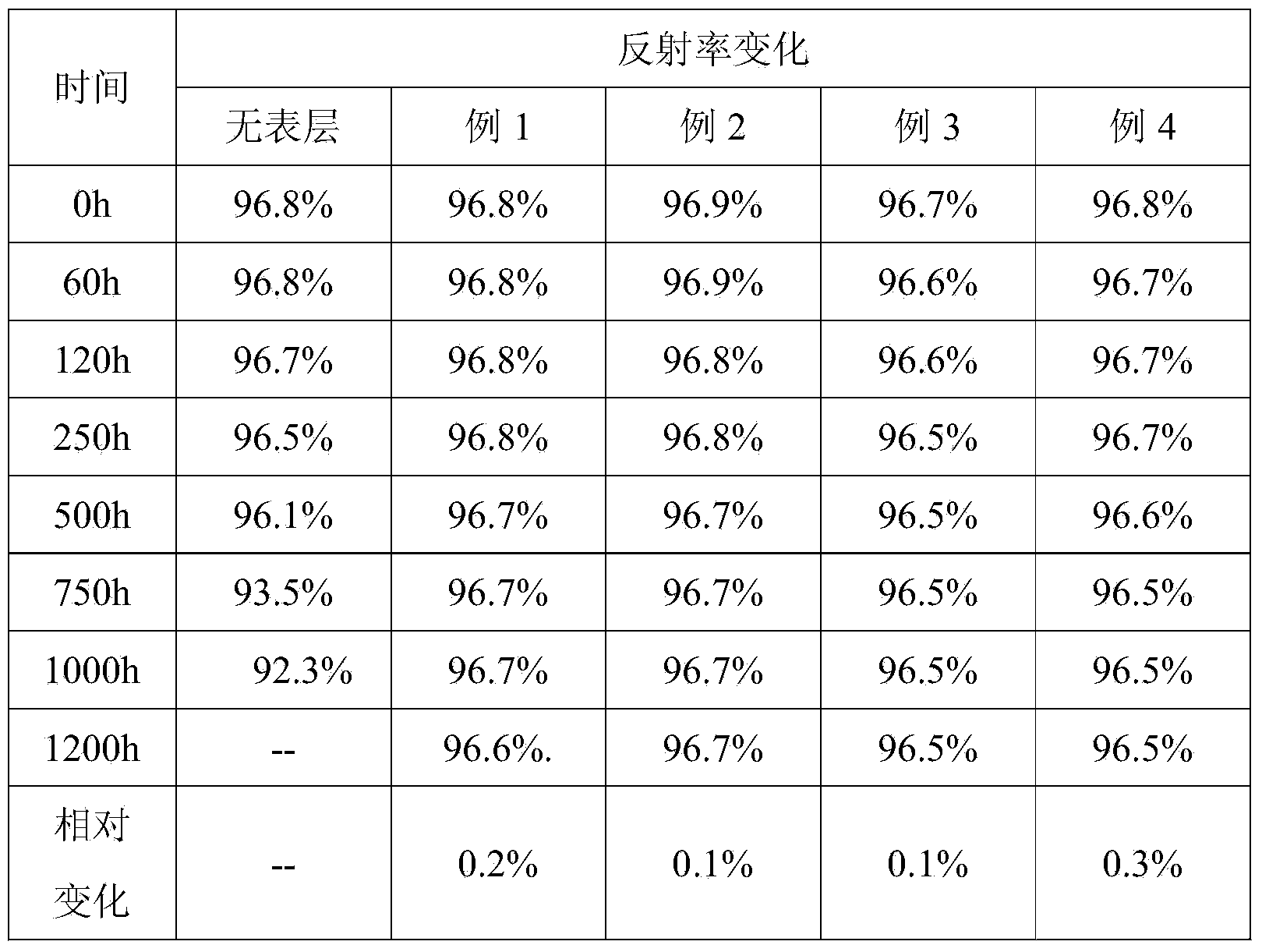
Reflecting film
ActiveCN103777261APrevent water degradationReflective stabilizationMirrorsSynthetic resin layered productsPolyethylene vinyl acetatePolyester
The invention discloses a reflecting film of a three-layer structure. The surface layers on the two sides are graft-modified polymers, and the core layer is the mixture of aliphatic polyester and inorganic particles. The graft-modified polymers are graft-modified polycarbonate or polyacrylic acid or polyethylene vinyl acetate or polystyrene or polypropylene or polyethylene; graft-modified perssad is acrylic acid or acrylonitrile or glycidyl ester or maleic anhydride; the aliphatic polyester is polylactic acid or polycaprolactone or poly hydroxy alkyl acid esters; the inorganic particles are one or two or more of calcium carbonate, titanium dioxide, zirconium oxide, zinc oxide, zinc sulfide, lead carbonate and barium sulfate. The graft-modified polymers on the surface layers play a supporting role, the film is prevented from being cracked and folded, the aliphatic polyester is prevented from absorbing water and being degraded due to the high water vapor barrier property, and the film reflecting performance is stable.
Owner:ZHANGJIAGANG KANGDE XIN OPTRONICS MATERIAL
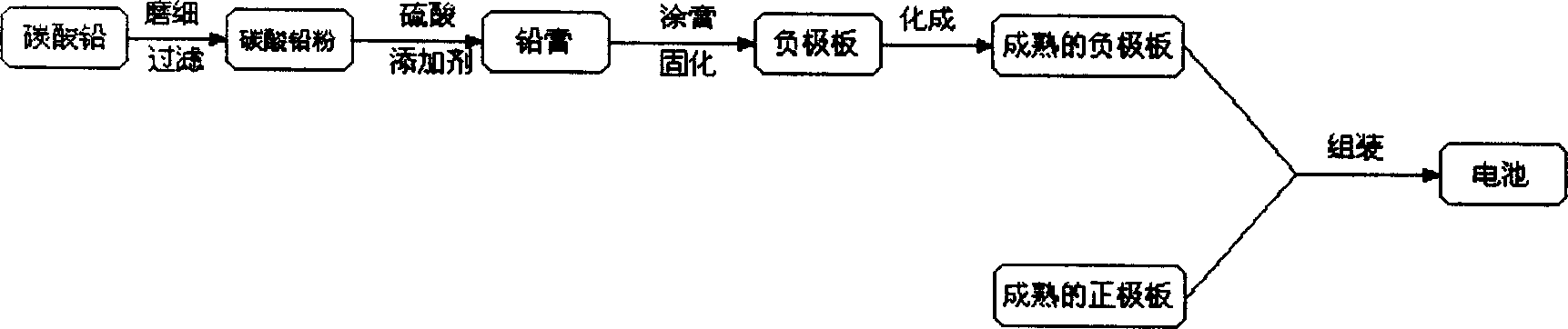
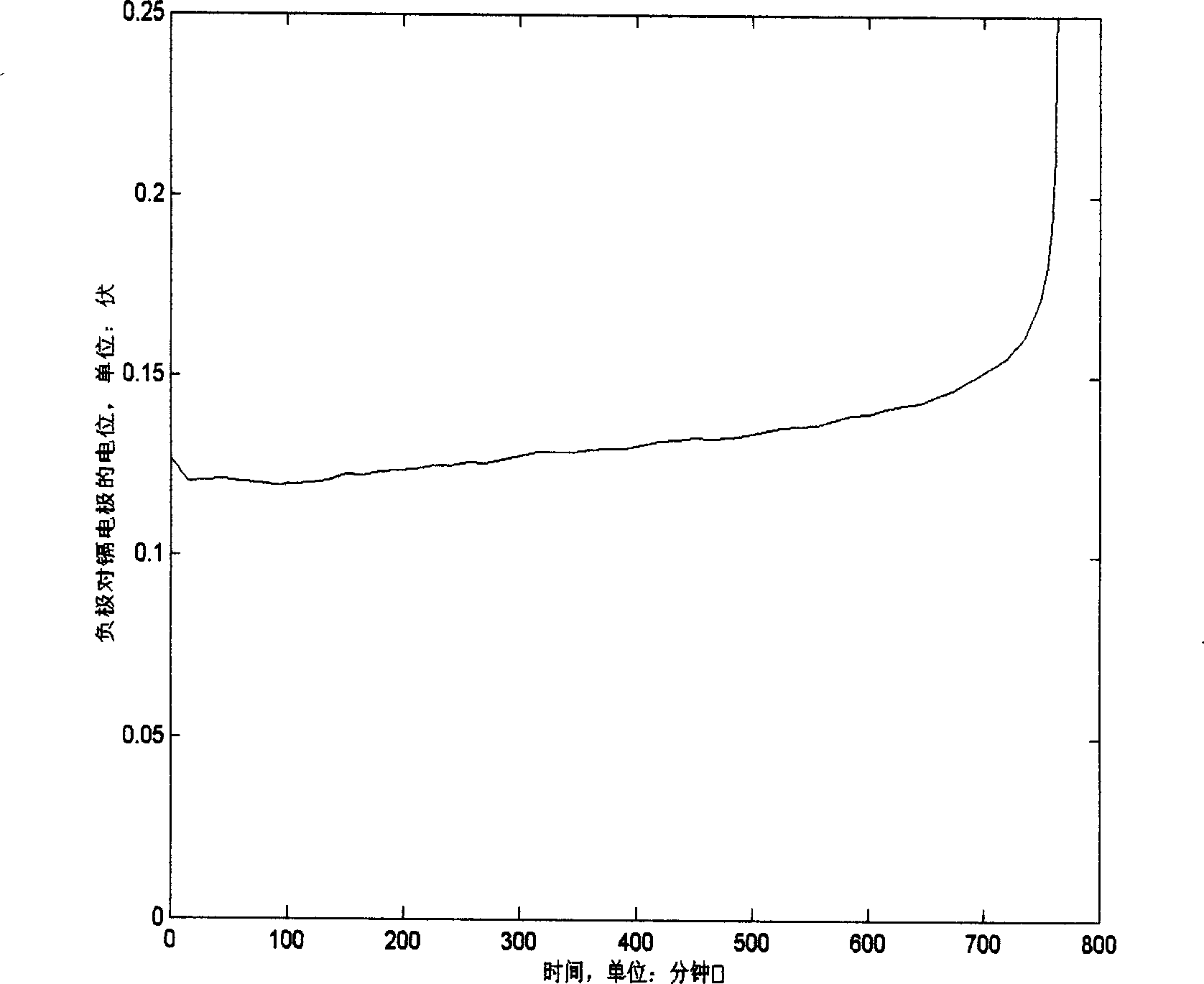
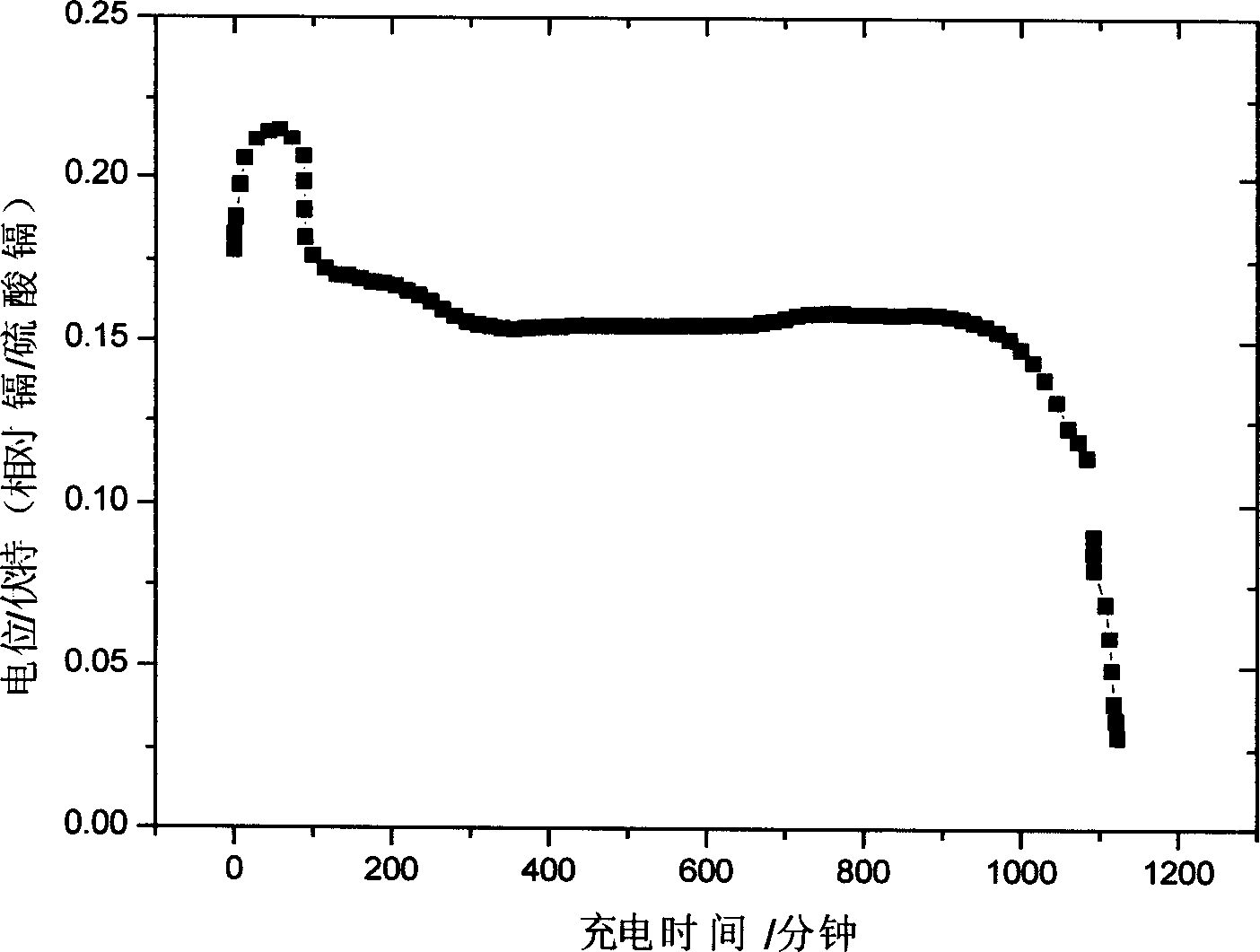
Method for preparing lead-acid battery negative pole
InactiveCN1426122AReduce production linksIncrease profitLead-acid accumulator electrodesElectricityLead carbonate
The present invention relates to the perparation process of lead-acid accumulator negative pole with lead carbaonate as material. It includes the following steps: grinding and sieving lead carbonate; imxing with additives and reaction with sulfuric acid to produce lead paste; painting the lead paste to Pb-Sb or Pb-Ca alloy slab lattice; setting the slab lattice into sodium sulfate and / or sulfuric acid solution for constant current compounding to obtain the negative pole board. Compared with traditional preparation process, the present invention has less steps, lower power consumption, high material utilizatino and thus lower production cost. The production process may be combined with the recovery of waste lead-acid accumulator to reuse material and reduce pollution.
Owner:WUHAN UNIV
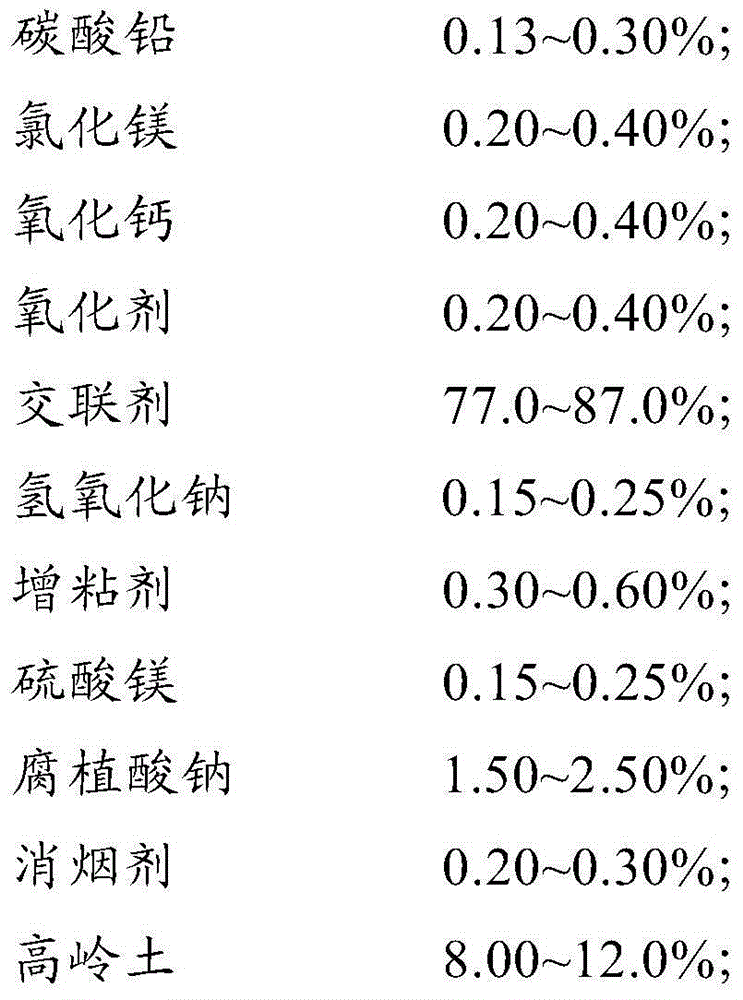
Cleaning briquette coal binder
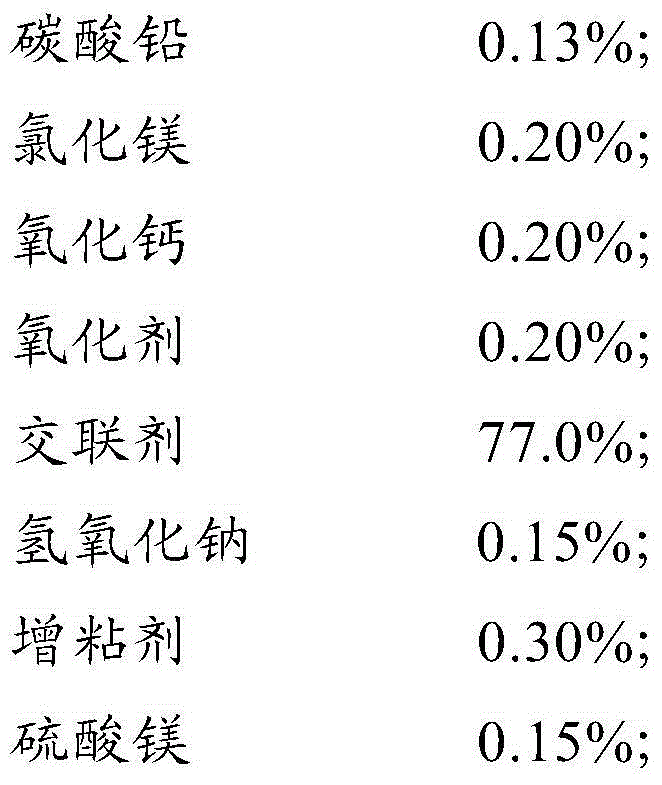
The invention discloses a cleaning briquette coal binder. The binder comprises the following raw materials: 0.13-0.30% of lead carbonate, 0.20-0.40% of magnesium chloride, 0.20-0.40% of calcium oxide, 0.20-0.40% of an oxidizing agent, 77.0-87.0% of a crosslinking agent, 0.15%-0.25% of sodium hydroxide, 0.30-0.60% of a tackifier, 0.15%-0.25% of magnesium sulfate, 1.50-2.50% of sodium humate, 0.20-0.30% of a smoke suppressant and 8.00-12.0% of kaolin. The briquette coal binder disclosed by the invention has the effects of suppressing smoke, preventing moisture, reducing dust, fixing sulfur, inhibiting nitrogen and the like, and is developed in line with the national situation of energy conservation, emission reduction and haze treatment according to China coal energy actual application and the characteristics of the haze formation cause in recent years; the binder has prominent energy conservation and emission reduction effects and an oxidative combustion-promoting effect; and since the binder is fully burnt, the energy conservation effect is achieved.
Owner:李晓雷
Method for comprehensively recycling carbon, iron, aluminum, zinc and lead from blast furnace gas sludge
ActiveCN109811132AAvoid problems such as consumptionReduce consumptionProcess efficiency improvementZinc hydroxideFerric hydroxide
The invention discloses a method for comprehensively recycling carbon, iron, aluminum, zinc and lead from blast furnace gas sludge. The method comprises the steps that blast furnace gas sludge is subjected to drying, crushing, grinding, multi-stage flotation, pickling and suction filtration to recover carbon at first; tailings with carbon recovered are leached in acid, oxidized and filtered to obtain a filtrate and filter residue; then the pH value of the obtained filtrate is adjusted, and iron hydroxide, zinc hydroxide and aluminum hydroxide are precipitated in steps, and respectively calcined to obtain iron concentrate, zinc concentrate and aluminum concentrate; and a saturated ammonium acetate solution is added into the filtered filter residue, stirred and filtered, a sodium carbonate solution is added into the filtrate, filtering is carried out to obtain basic lead carbonate precipitation, and roasting is carried out to obtain lead concentrate. The involved recycling process easy to operate, carbon, iron, zinc, aluminum and lead in the gas sludge can be comprehensively recycled, recycling of various valuable elements is achieved, pollution to the environment is reduced, and themethod has important economic and environmental benefits and suitable for being applied and popularized.
Owner:WUHAN UNIV OF TECH
Method for alkaline leaching of waste residue containing zinc ferrite or lean zinc ore
InactiveCN102534208AOvercome insoluble problemsRealize low temperature direct wet smeltingProcess efficiency improvementLead carbonateElectrolysis
The invention relates to a method for alkaline leaching of waste residue containing zinc ferrite or lean zinc ore. A conversion agent and a raw material are added into a stirred mill reactor simultaneously, the mass ratio of the raw material to a ball milling medium is 1:10-30, and the mass ratio of the conversion agent to the zinc ferrite contained in the raw material is 0.2-1:1; stirring speed is 400-800 r / min, activation conversion reaction time is 1-3h, and the zinc ferrite in the raw material is converted into zinc sulfide; aqueous alkali with alkali concentration of 2-8 mol / L and lead carbonate are added into the stirred mill reactor to be subjected to mechanical agitation leaching for 1-2 h, and solid and liquid are separated through filtering; metal zinc is extracted from a leaching agent through a traditional electrolysis method, and the leaching agent is circulated in next leaching process; leaching residue obtained through filter is converted through sodium carbonate solution to recycle lead in the leaching residue, lead carbonate is produced, and then the lead carbonate is returned to a leaching process to be used repeatedly. The leaching residue after lead conversion can be used as a blast-furnace iron-making raw material, and resource recycling is achieved. The method solves the problem that the zinc ferrite cannot dissolve in the aqueous alkali at the low temperature basically and is low in cost, easy to control and free of environment pollution.
Owner:SHANGHAI SECOND POLYTECHNIC UNIVERSITY
Recycling process of waste battery lead
ActiveCN105925807ARealize repeated recyclingHigh purityPhotography auxillary processesWater/sewage treatment bu osmosis/dialysisElectrolysisLEAD TETROXIDE
The invention relates to the technical field of battery recycling equipment, in particular to a recycling process of waste battery lead. The recycling process is completed according to the following steps that a lead storage battery is mechanically cut off and broken and then is washed and screened at the same time by a rotary screen washing machine through an alkaline solution, plastic, waste electrolytes and lead bullion are screened out, and the waste electrolytes are conveyed to an ultrafiltration membrane processing system to be processed; lead carbonate is subjected to electromagnetic induction heating, sufficient oxygen is led in, the electromagnetic induction frequency ranges from 8 KHz to 60 KHz, the control temperature ranges from 330 DEG C to 460 DEG C, heat preservation is performed for 5 h to 6 h, and completely-oxidized lead tetroxide will be obtained; and in the electrolysis period, an electromagnetic induction magnetic field is applied at the bottom of an electrolytic tank, the magnetic induction intensity is 800 mT, and national standard number 1 lead is obtained. The recycling process has the beneficial effects that refining of the lead is achieved through a method that electrolysis is used in cooperation with electromagnetism, the purity of the lead is greatly improved, and repeated recycling of precious metal is also achieved; and the recycling process is simple, machining is easy, the recycling process is suitable for large-scale recycling, and therefore the recycling cost is reduced.
Owner:GUANGDONG NEW ENVIRONMENTAL PROTECTION TECH CO LTD +1
Method for recovering lead from waste lead-acid storage batteries
The invention discloses a method for recovering lead from waste lead-acid storage batteries. The method comprises the following steps: discharging waste acid of waste lead-acid storage batteries, breaking and sieving the waste lead-acid storage batteries, leaching obtained lead slime by virtue of nitric acid oxidation, carrying out a sulfuric acid reaction on obtained leachate, and carrying out a sodium carbonate reaction on obtained lead sulfate to obtain lead carbonate, decomposing the lead carbonate in a smelting furnace, adding pulverized coal, sodium carbonate and quartz sand to obtained lead oxide, and carrying out a high-temperature reaction to obtain refined lead. By leaching the lead slime by virtue of nitric acid oxidation and then preparing the lead sulfate by virtue of the sodium sulfate, the method disclosed by the invention can be used for effectively improving the recovery rate of lead in the lead-acid storage batteries to more than 96%; and by converting the lead sulfate into lead carbonate by virtue of sodium carbonate and preparing metallic lead from the lead carbonate, the method can be used for effectively solving the problems of high energy consumption, environmental pollution due to the emission of sulfur dioxide and the like in the case that the lead sulfate is directly decomposed.
Owner:ZUNYI JINSHI METAL ALLOY
Method for detecting hydrogen sulfide (H2S), carbon dioxide (CO2) and hydrogen cyanide (HCN) in acid gas
InactiveCN103293151AQuick checkEasy to operateMaterial analysis by observing effect on chemical indicatorPhenolphthaleinDehydrogenation
The invention provides a method for detecting hydrogen sulfide (H2S), carbon dioxide (CO2) and hydrogen cyanide (HCN) in acid gas. The method comprises the following steps: combining a mixing solution of potassium hydroxide and a phenolphthalein indicator which are contained in different flasks in a volumetric flask; and adding pure water until reaching the scale, wherein potassium carbonate is generated by the CO2 adsorbed by the potassium hydroxide and reacts with calcium chloride to generate a calcium carbonate precipitation; the calcium carbonate precipitation is dissolved and titrated by using hydrochloric acid and a sodium hydroxide solution respectively, so that the content of the CO2 is obtained. The H2S adsorbed by the potassium hydroxide can generate potassium sulphide which reacts with a zinc acetate solution, so that a flocculent yellow precipitation is generated; an excess amount of standard iodine solution is added under the acid condition; the back titration is performed by using sodium thiosulfate; the content of the H2S is computed after blanking. After the HCN is adsorbed by potassium hydroxide, lead carbonate solid powder and a rhodanine indicator are added; the content of the HCN is computed after blanking. The method is rapid in detection speed and strong in operability; the precision and accuracy of the method can completely meet the requirement of a coking desulfurization and dehydrogenation process. Thus, the accurate detection data for the coking desulfurization and dehydrogenation process is provided.
Owner:ANGANG STEEL CO LTD
Pyrogenic attack comprehensive recovery process for lead and silver slag leached from bismuth oxide slag
The invention relates to a pyrogenic attack comprehensive recovery process for lead and silver slag leached from bismuth oxide slag and belongs to the field of metallurgy. In the method, anthracite serves as a reducing agent and industrial sodium carbonate serves as a slag forming constituent to convert lead chloride in the lead and silver slag leached from the bismuth oxide slag into lead carbonate; the lead carbonate is decomposed into lead oxide; lead is reclaimed by the principle that carbon in the anthracite and the lead oxide are subjected to reduction reaction to generate elementary lead; because of the sodium chloride generated in the reaction, the melting point of the slag is reduced and then the slag and the lead are more thoroughly separated; and the silver and the lead in the lead and silver slag leached from the bismuth oxide slag are reclaimed together by the principle that the lead is a good collector of silver and silver can be dissolved in liquid lead in a molecular state. At the same time, the other impurity metals, such as stibium, bismuth and the like in the lead and silver slag, are also subjected to the similar reaction and then are reduced into a lead and silver alloy.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
Waste lead-acid storage battery spent acid recycling method
ActiveCN107959074AAffect cycle lifeReduce loadMultistage water/sewage treatmentWaste accumulators reclaimingUltrafiltrationLead sulfate
The invention discloses a waste lead-acid storage battery spent acid recycling method. Most impurities in spent acid solution are filtered by a physical filtering method, colloidal substances and large-particle suspended matters are removed by a flocculant mode, ultrafine barium sulfate particle suspended matters and lignosulfonate remain, lignin and lead carbonate ultrafiltration lead sulfate particles are decomposed by an electrolytic process, and sulfuric acid electrolyte can be recycled again. Recycled sulfuric acid is treated by the process, the impurities in the electrolyte can be screened and removed step by step, filtering efficiency is improved, expanding agents such as barium sulfate and lignosulfonate in the electrolyte are completely removed, and the influence of entering of the impurities in positive active materials on the cycle life of a battery is avoided.
Owner:CHAOWEI POWER CO LTD
Desulphurization method for lead plaster of waste lead-acid storage battery
InactiveCN107394300AWide range of usesThe desulfurization reaction continuesWaste accumulators reclaimingProcess efficiency improvementLead carbonateAmmonium carbonate
The invention discloses a desulphurization method for lead plaster of a waste lead-acid storage battery, and relates to the technical field of desulphurization. The method disclosed by the invention comprises the steps of: carrying out wet desulphurization on the lead plaster by using ammonium carbonate as a desulphurization agent; cycling and stirring a mixture of the lead plaster, water for diluting the lead plaster and the ammonium carbonate between a desulphurization tank and a forced desulphuriser; feeding the desulphurized lead plaster into a smelting system; reusing desulphurization liquid as liquid for diluting the lead plaster; and enabling the reused secondary desulphurization liquid to enter an evaporative crystallization system and outputting ammonium sulfate. According to the method disclosed by the invention, after the desulphurization liquid is reused, salt concentration of the desulphurization liquid is improved, and thus, cost of enabling the desulphurization liquid to enter the evaporative crystallization system is reduced; according to the invention, the ammonium carbonate is used as the desulphurization agent, so that the produced ammonium sulfate has use value; and according to the invention, in the desulphurizing process, the forced desulphuriser which is of a special structure is used, and newly generated lead carbonate particles coating the surface of lead sulphate can be ground and removed, so that the desulphurizing process is guaranteed to be successfully carried out, desulphurizing efficiency is improved, and environment pollution is reduced.
Owner:HUBEI CHUKAI METALLURGY
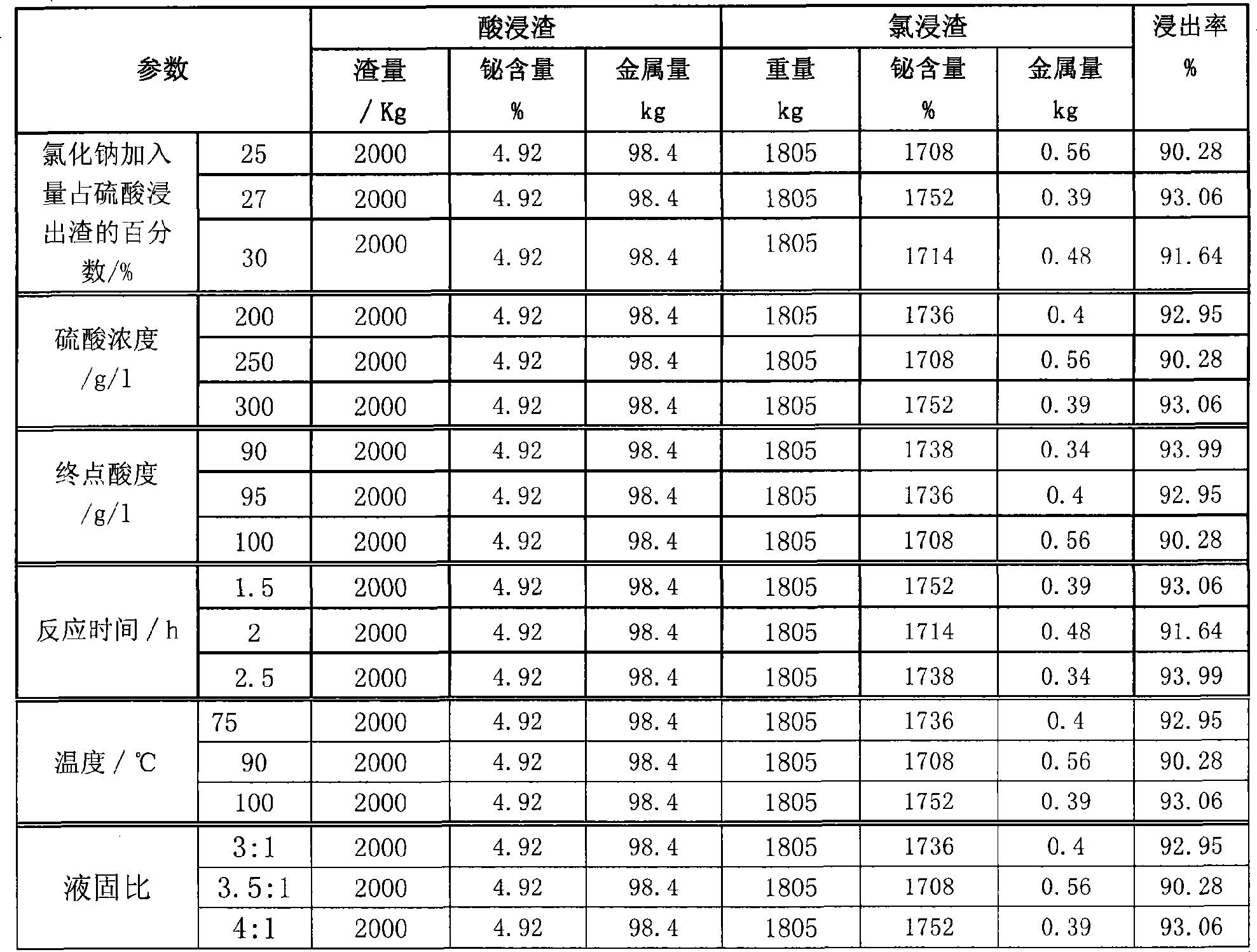
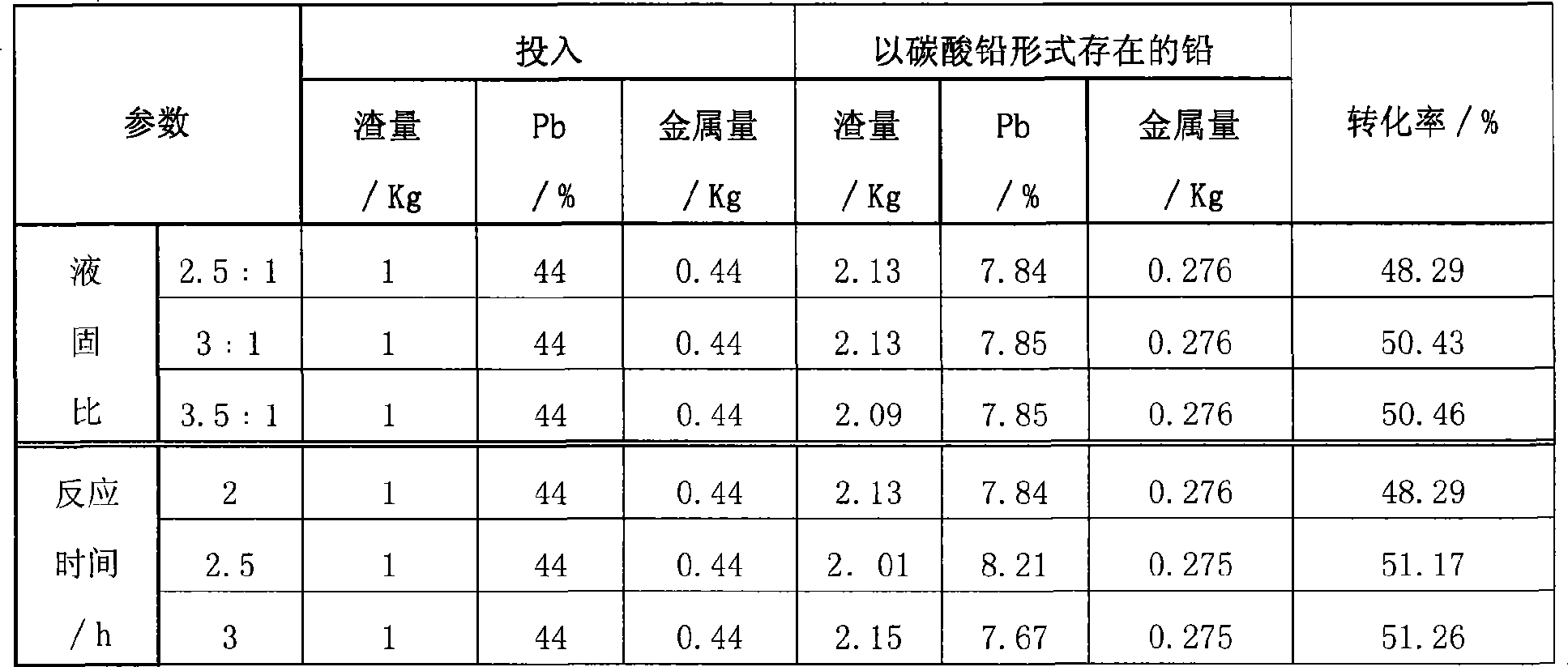
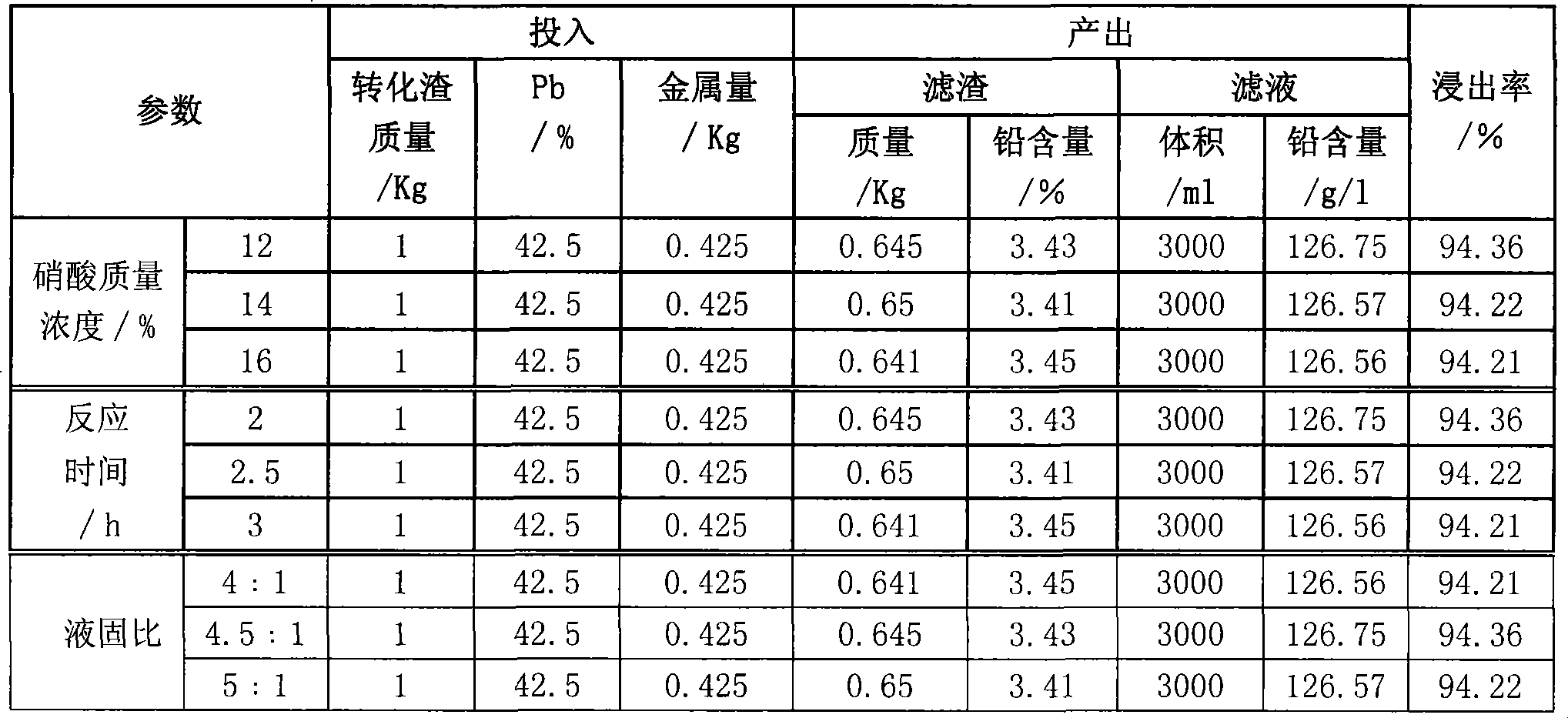
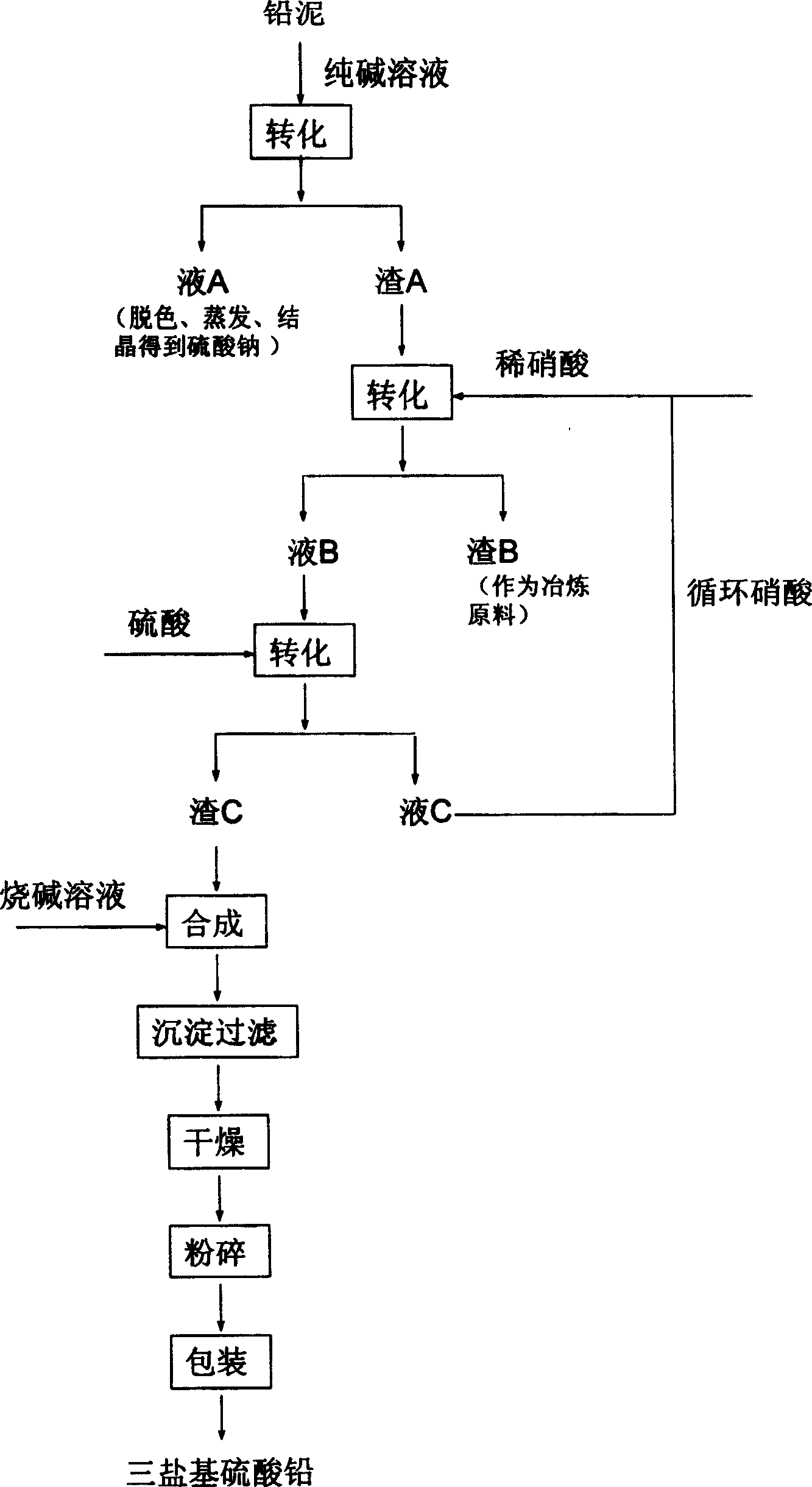
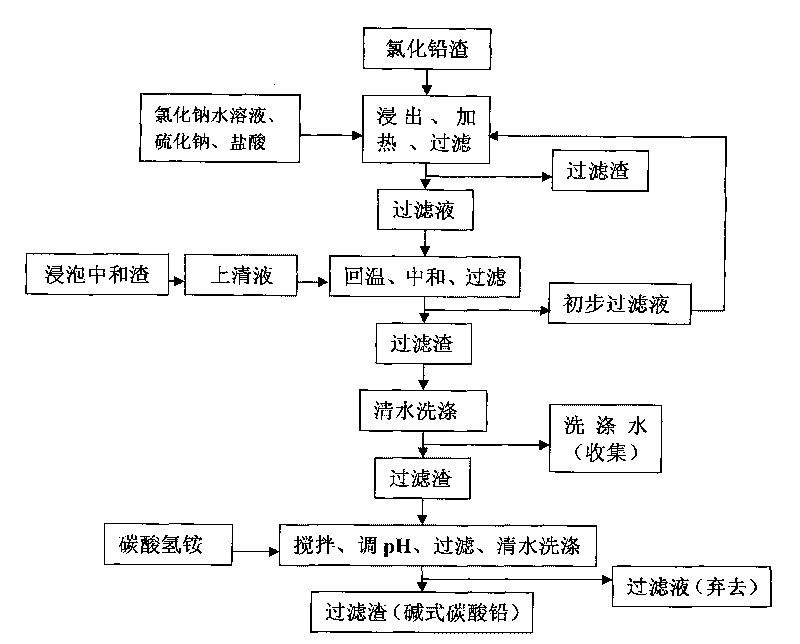
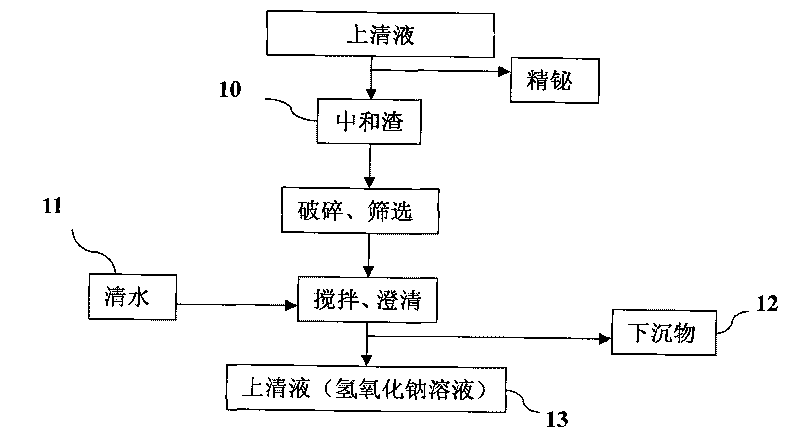
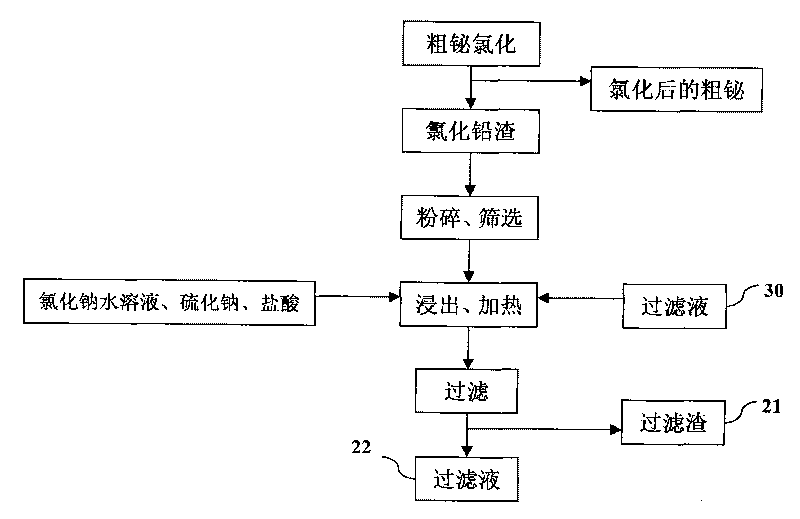
Process for producing basic lead carbonate
InactiveCN101723440AOptimizing the smelting production processEasy to handleSolid waste disposalLead carbonatesLead carbonateLead bismuth
The invention relates to a process for producing basic lead carbonate, which comprises the following steps: (1) soaking and neutralizing slag to obtain solution of sodium hydroxide, (2) leaching lead chloride slag and filtering, (3) alkali neutralization, filtering and washing, and (4) carbonate conversion, crystallization, filtering and washing. The process uses residual slag of fire refining bismuth as a raw material, optimizes smelting production technology of blast furnace smelting, electrolysis of lead-bismuth alloy, and fire refining to ensure that smelting slag of bismuth can be better treated and utilized, and saves resources. In the process of the invention, lead circulates in a closed system so as to reduce environmental pollution.
Owner:JIANGXI RARE EARTH & RARE METALS TUNGSTEN GRP HLDG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com