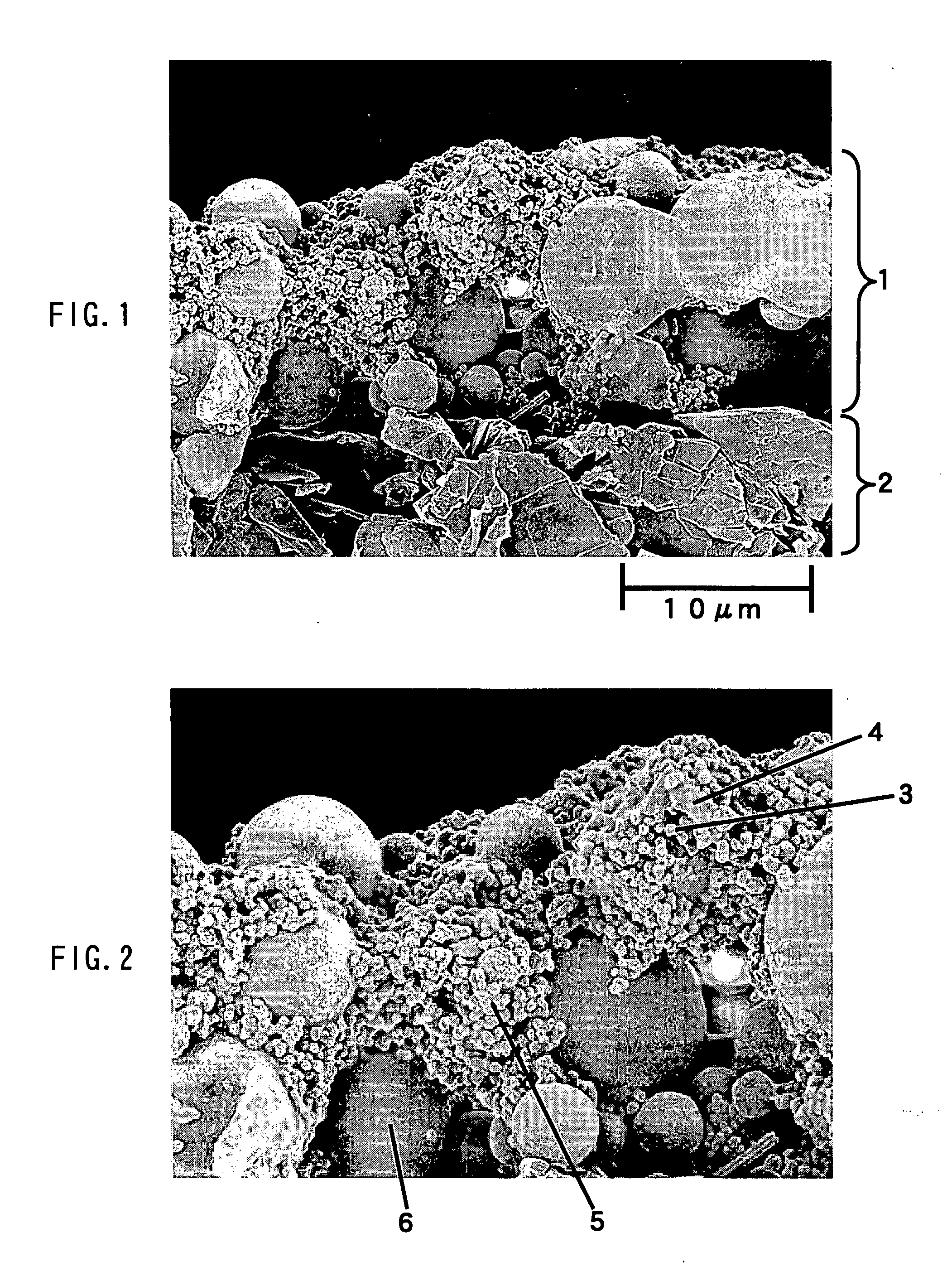
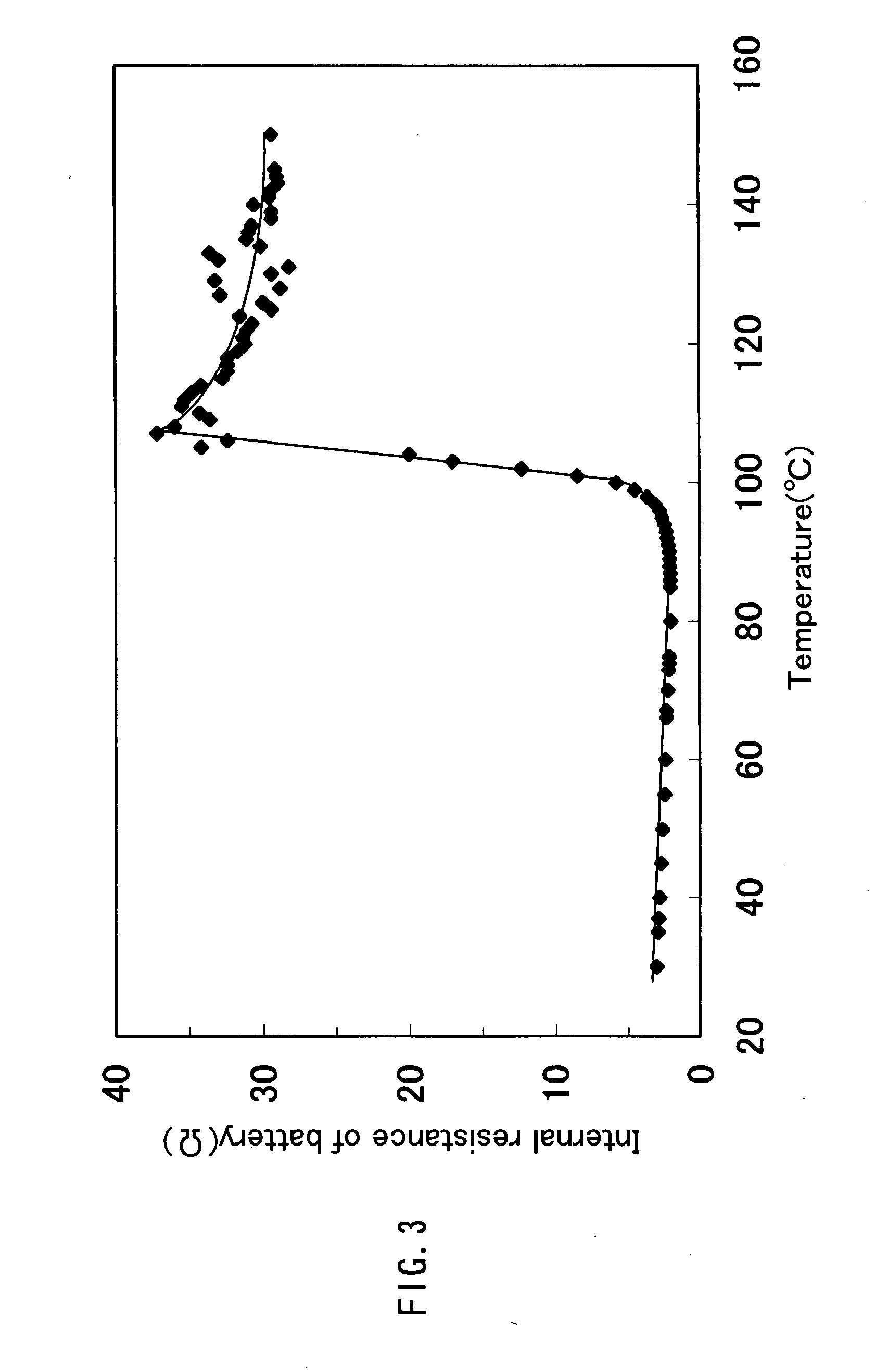
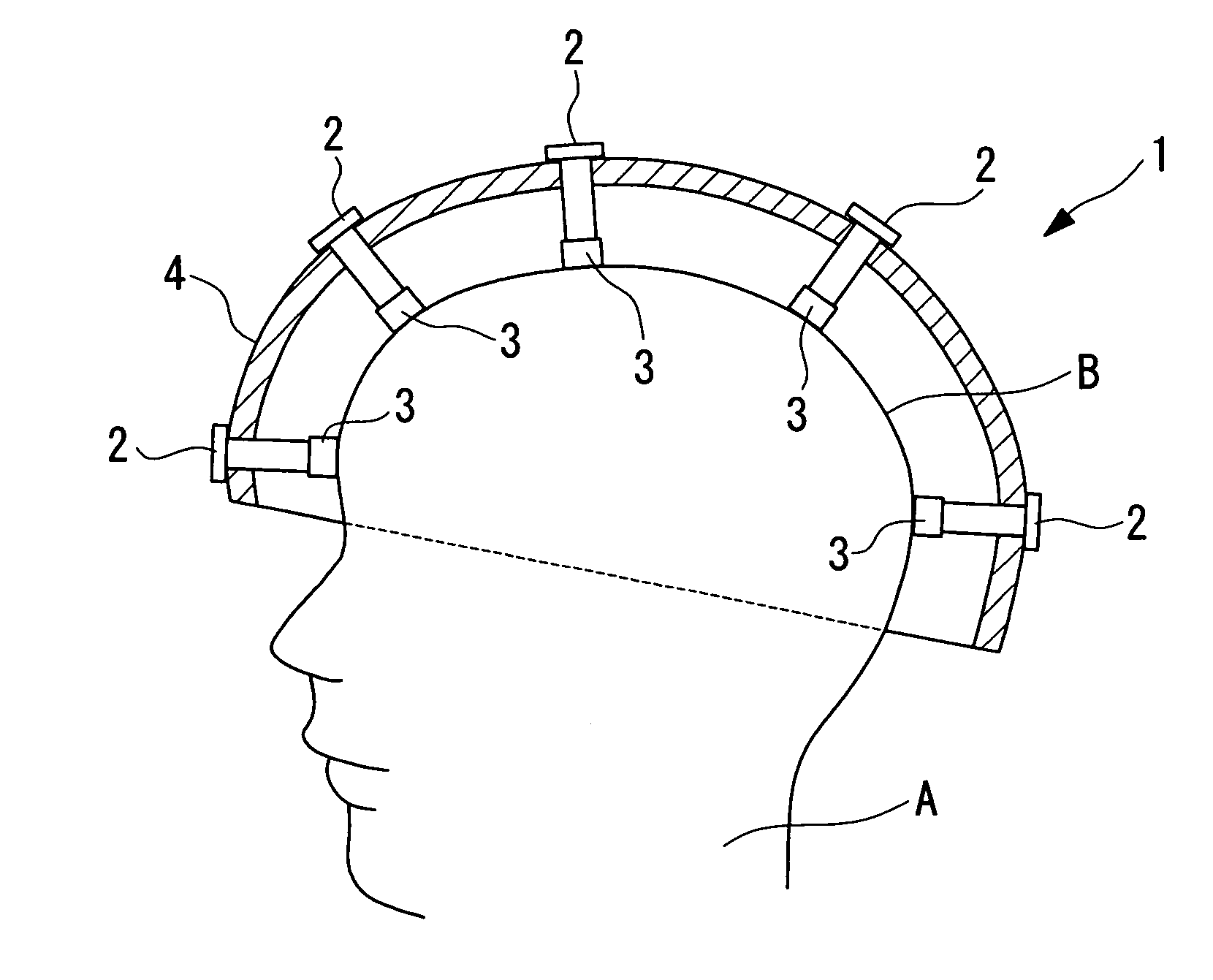
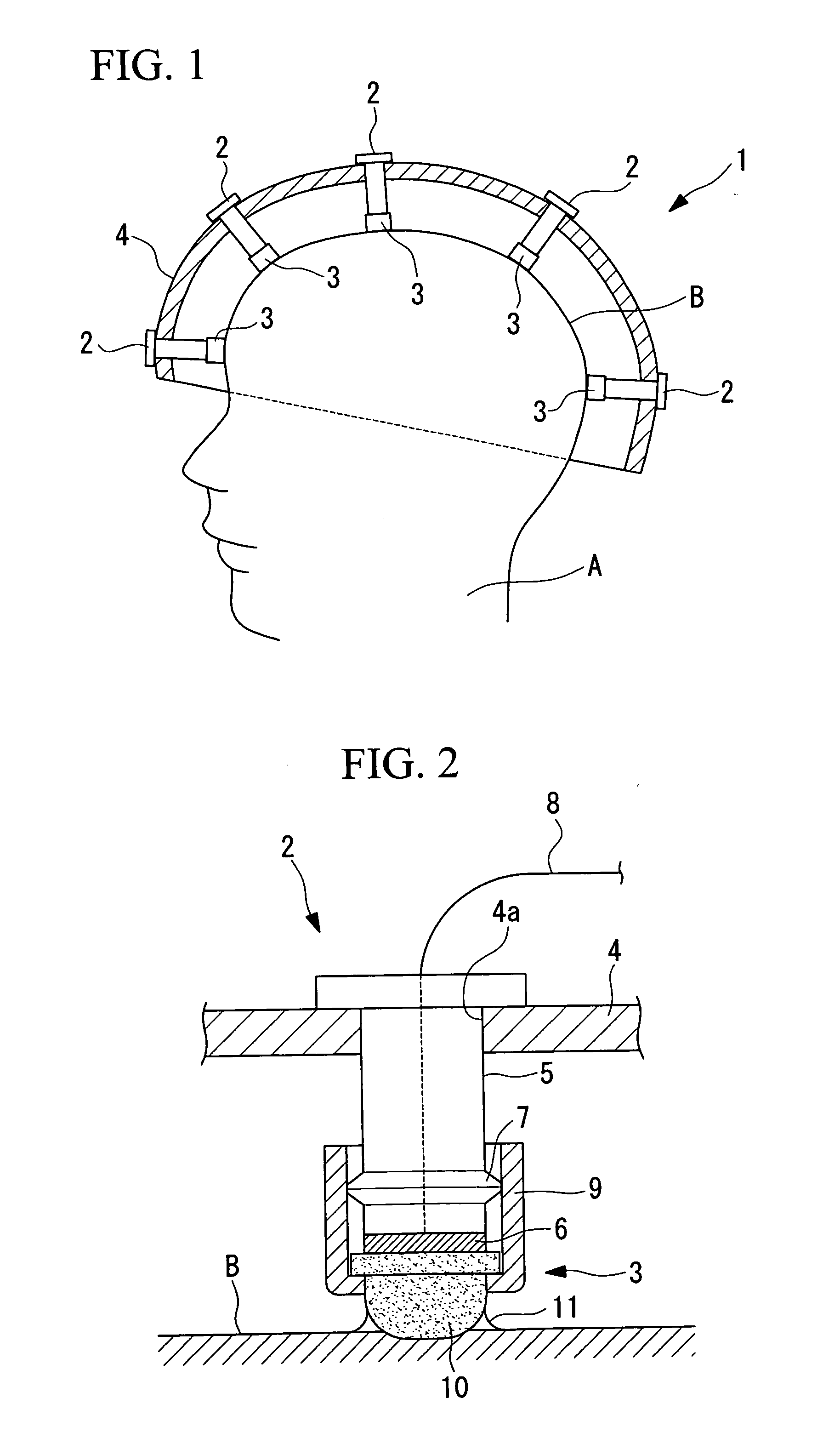
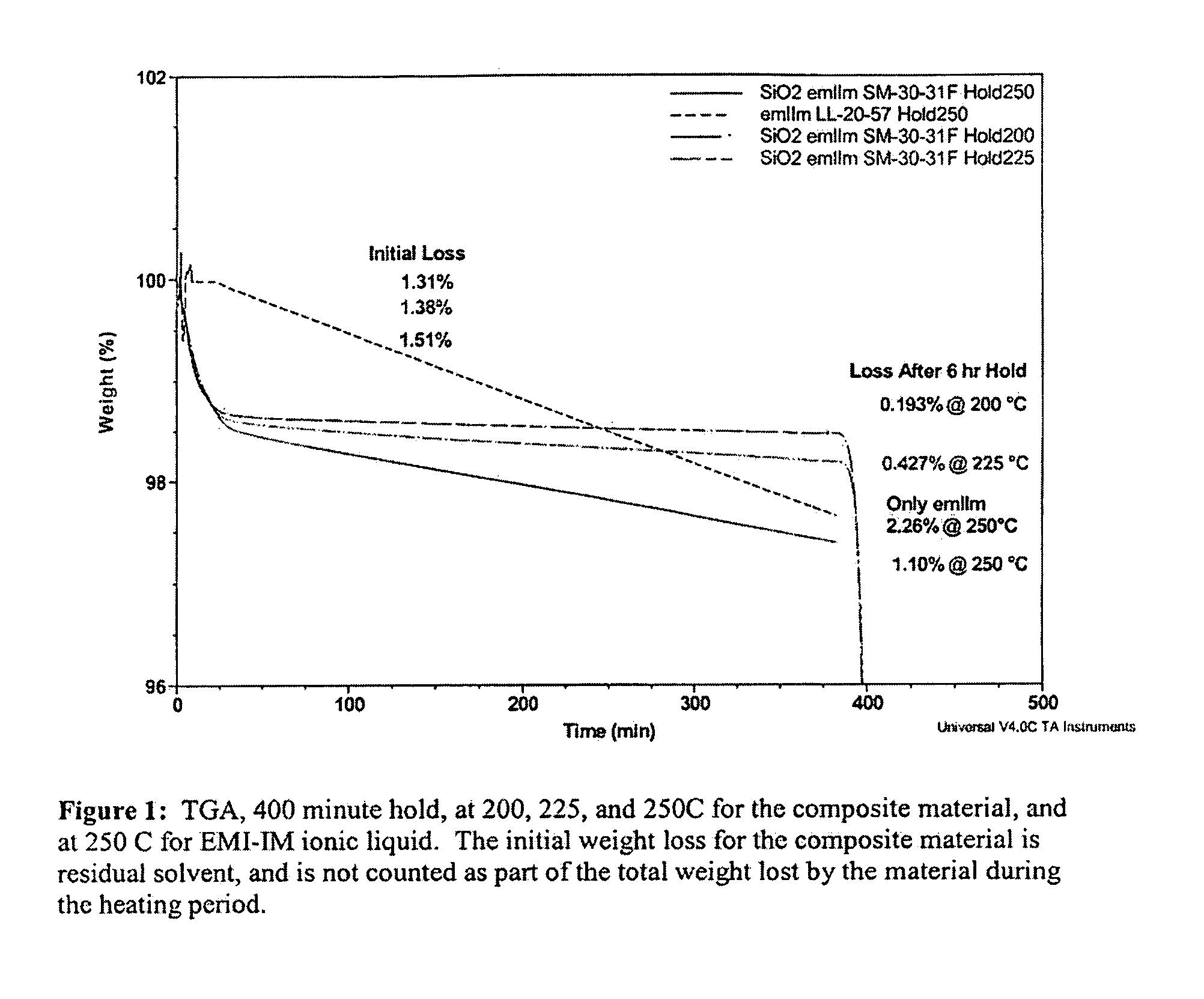
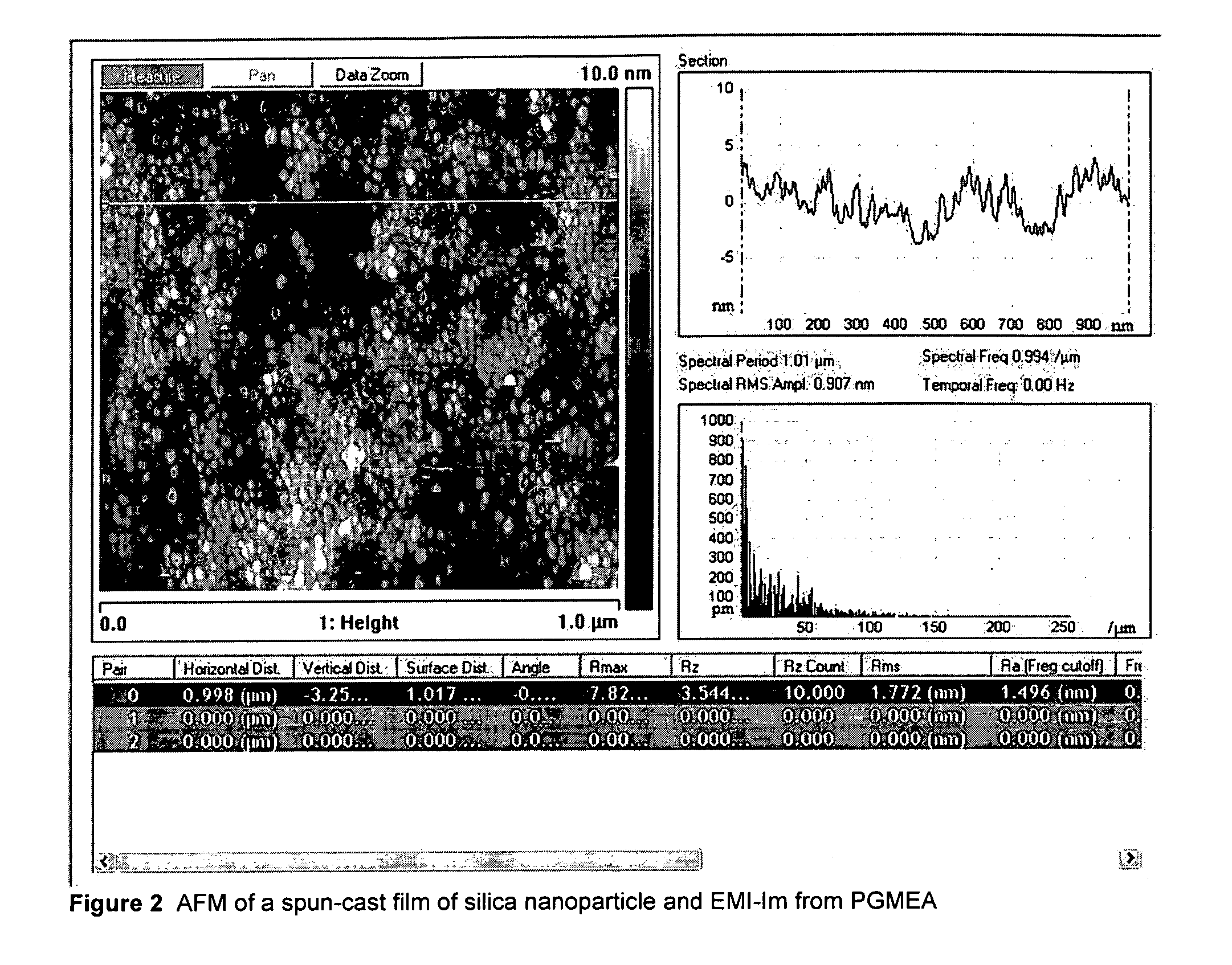
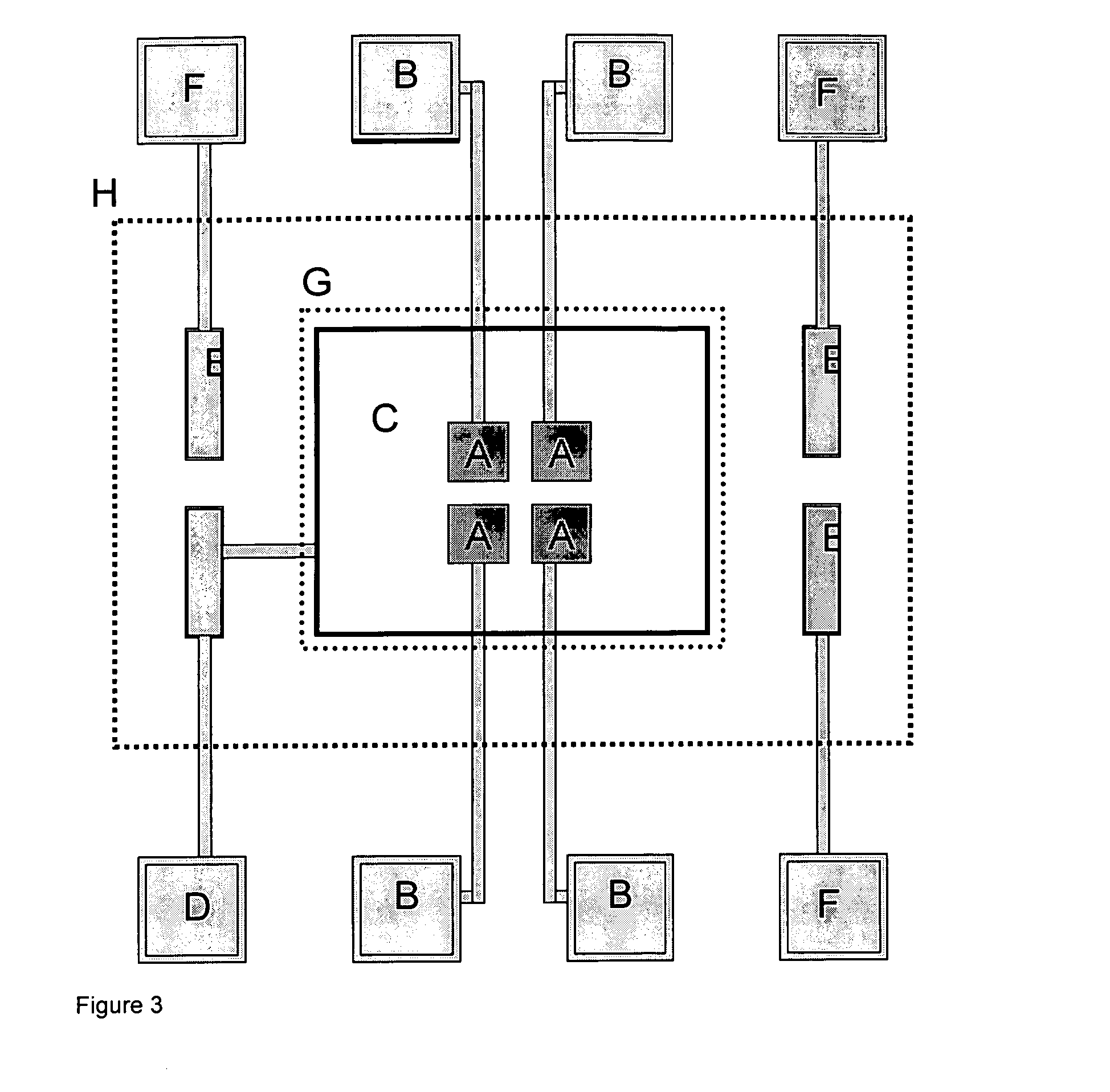
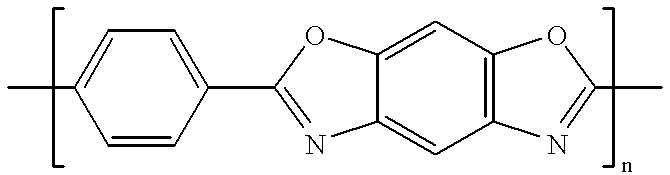
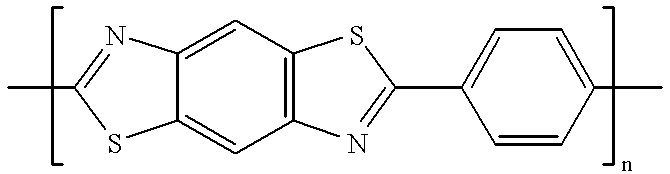
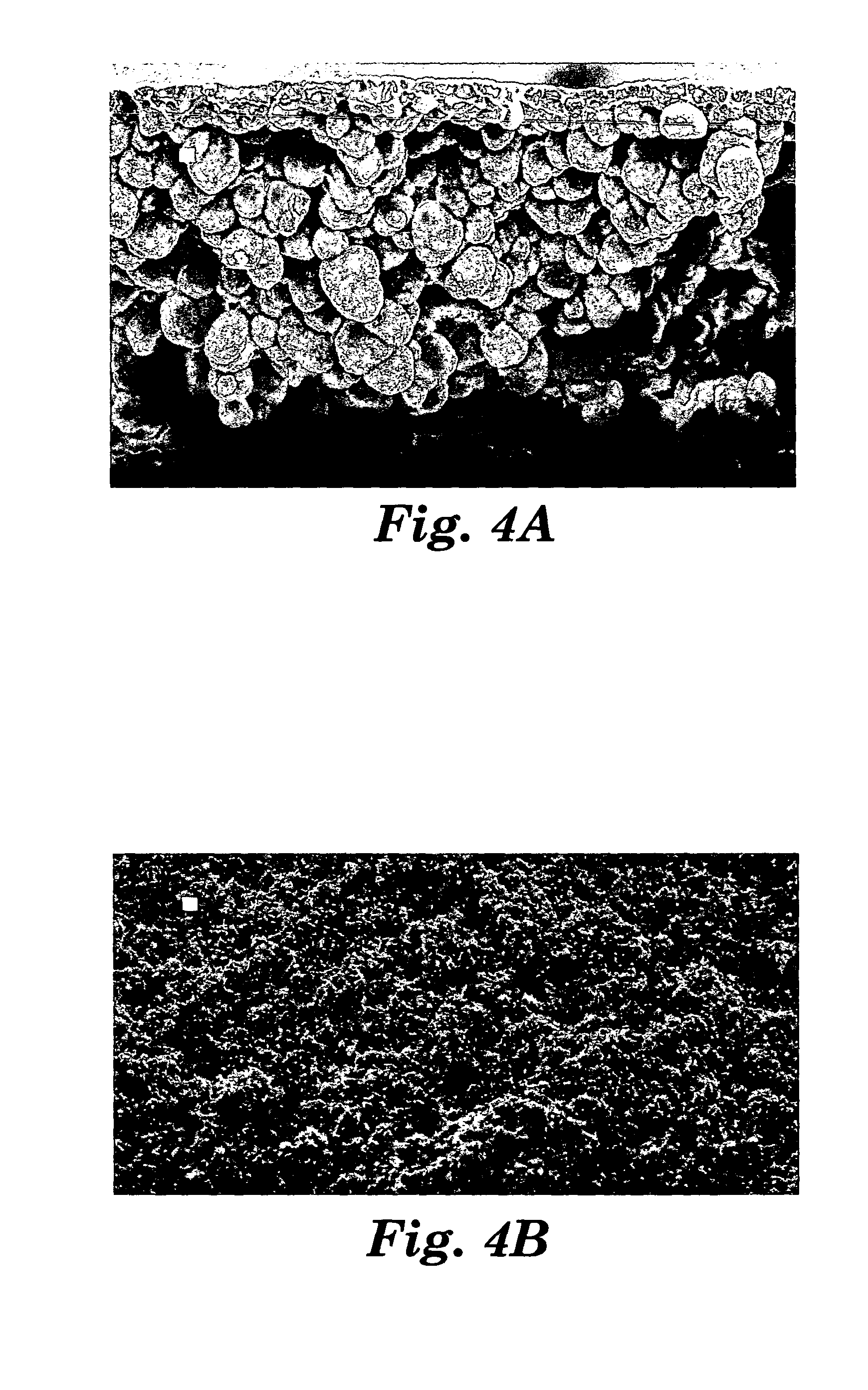
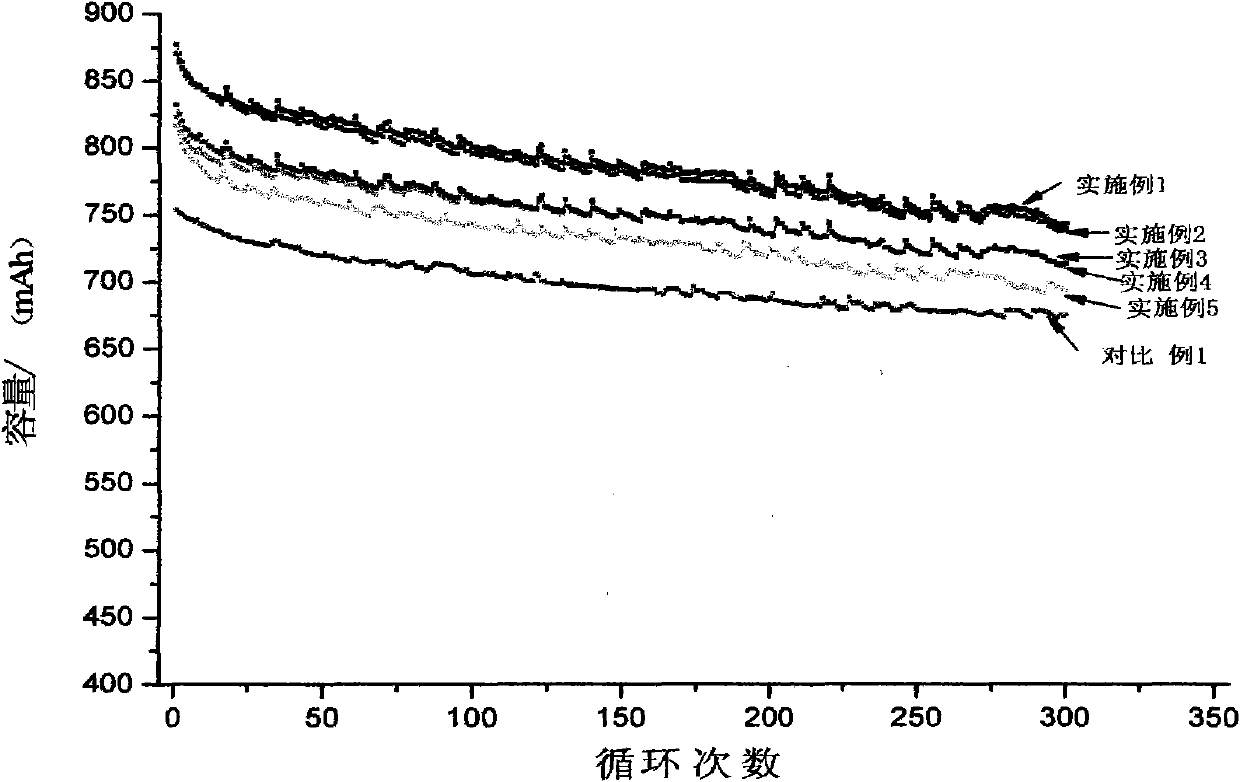
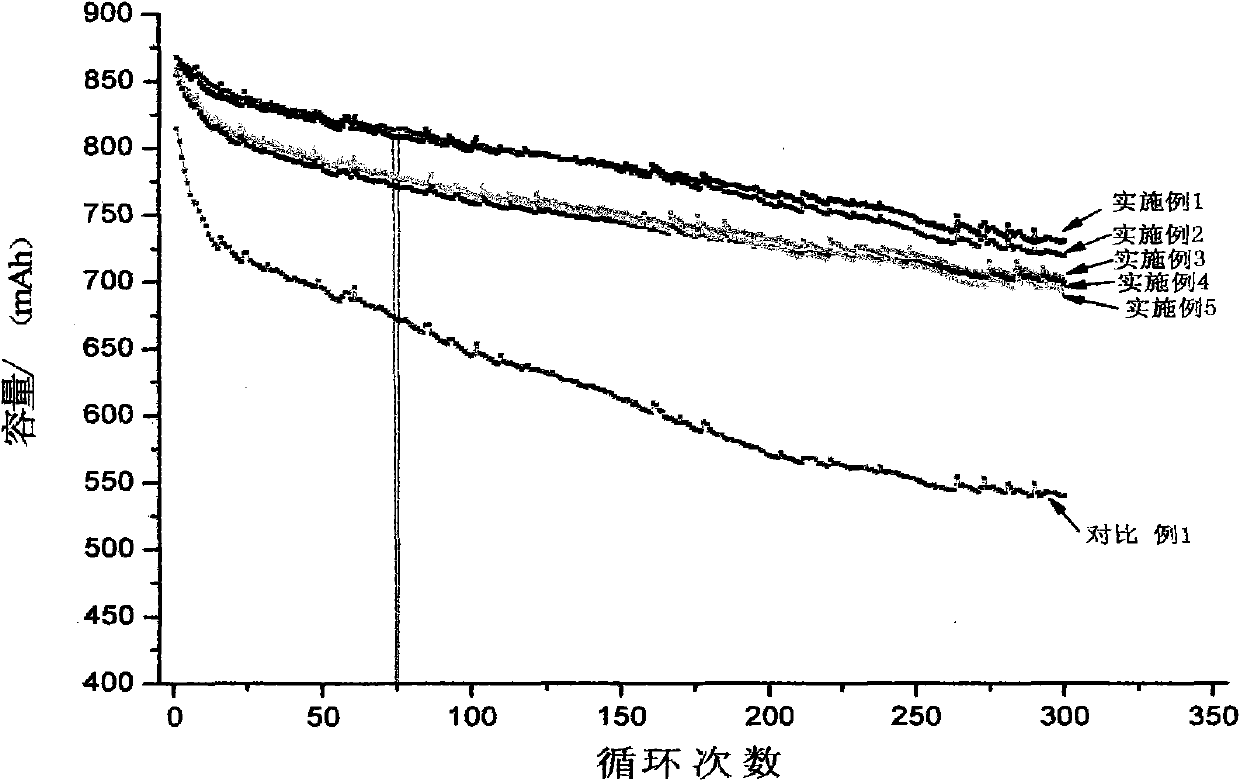
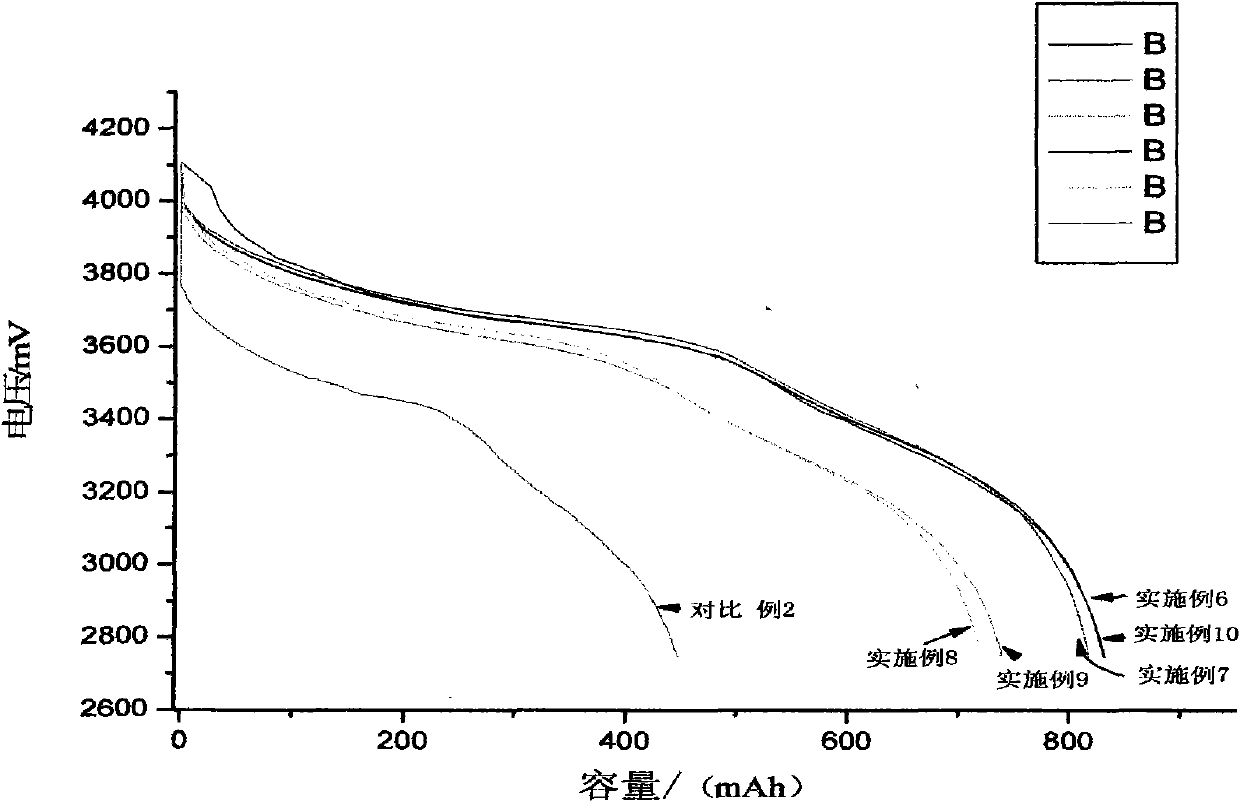
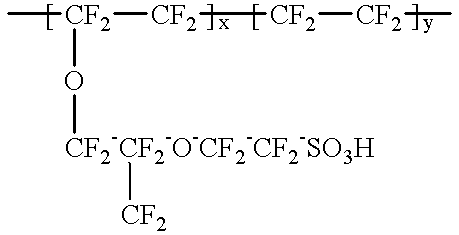Patents
Literature
35799 results about "Electrolyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An electrolyte is a substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water. The dissolved electrolyte separates into cations and anions, which disperse uniformly through the solvent. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. This includes most soluble salts, acids, and bases. Some gases, such as hydrogen chloride, under conditions of high temperature or low pressure can also function as electrolytes. Electrolyte solutions can also result from the dissolution of some biological (e.g., DNA, polypeptides) and synthetic polymers (e.g., polystyrene sulfonate), termed "polyelectrolytes", which contain charged functional groups. A substance that dissociates into ions in solution acquires the capacity to conduct electricity. Sodium, potassium, chloride, calcium, magnesium, and phosphate are examples of electrolytes.
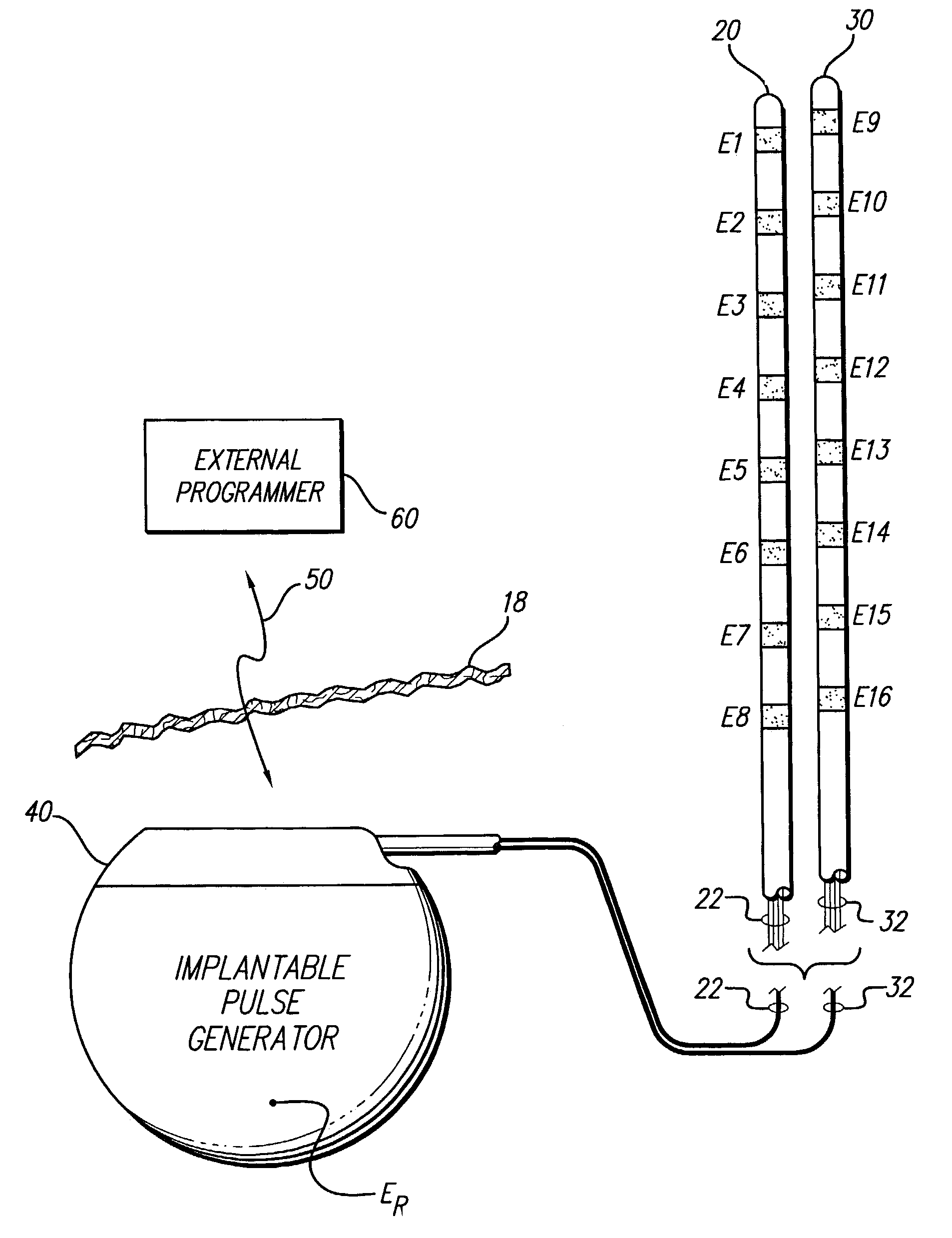
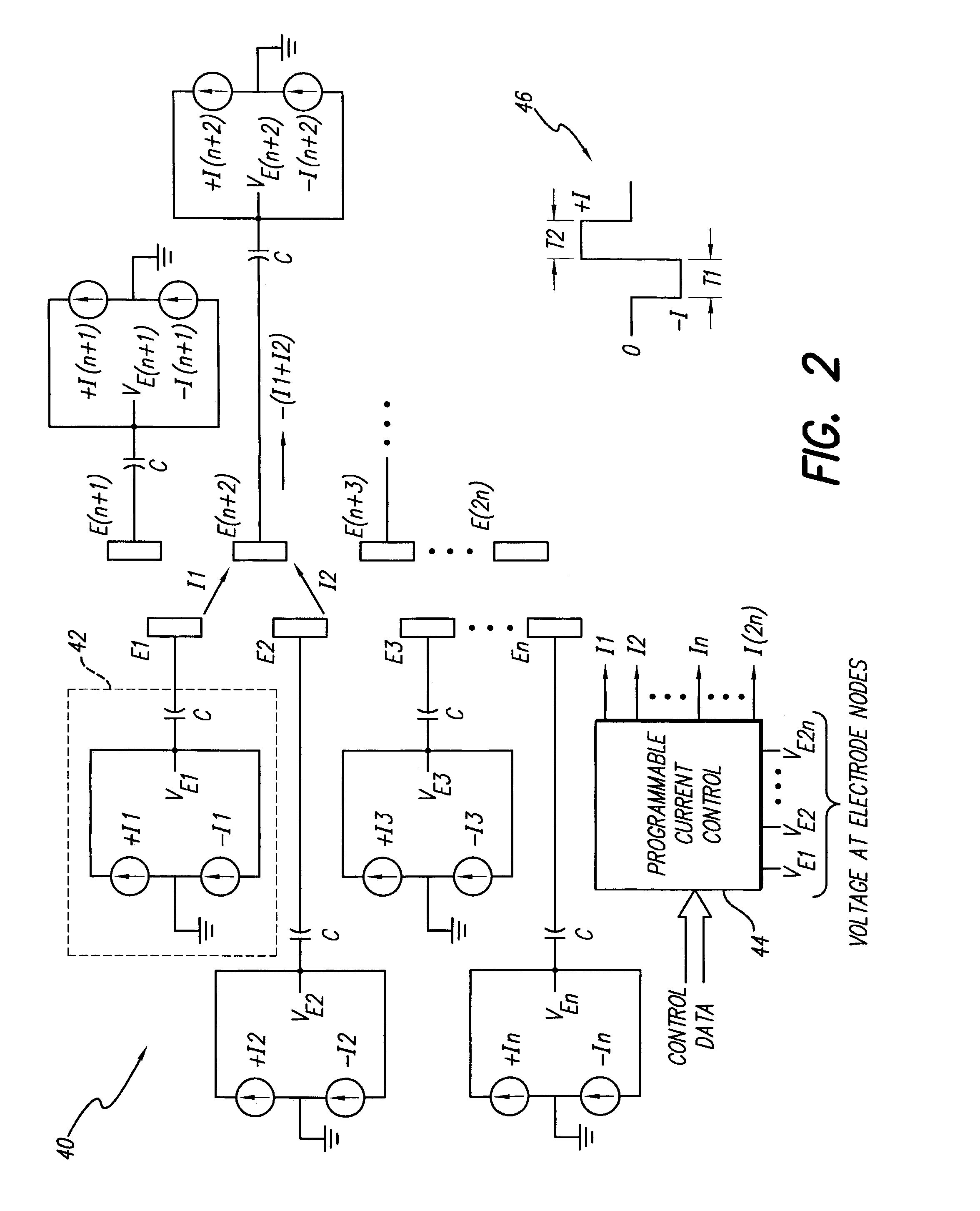
Apparatus and method for determining the relative position and orientation of neurostimulation leads
ActiveUS6993384B2Sure easySpinal electrodesDiagnostic recording/measuringPotential measurementSpinal column
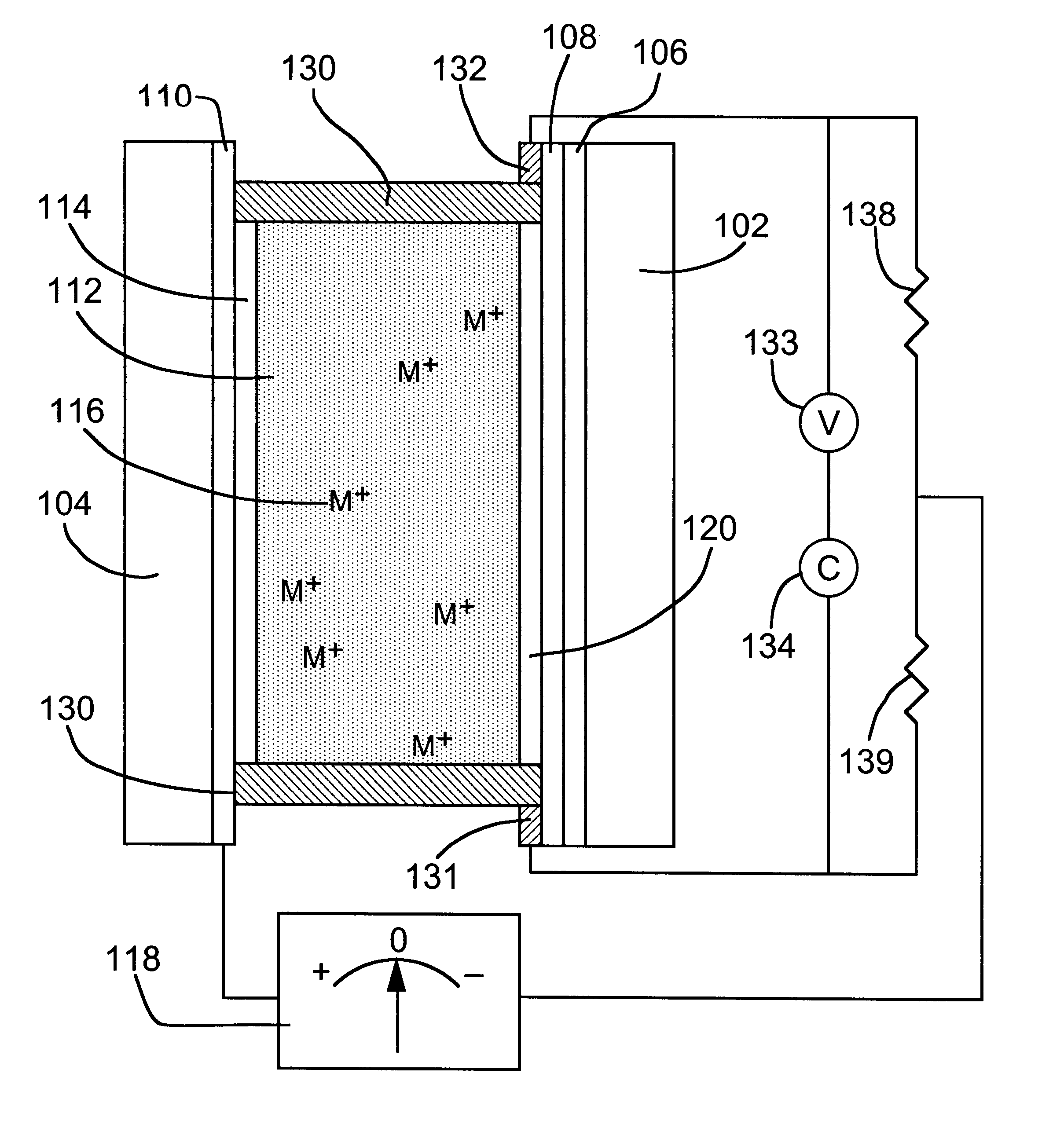
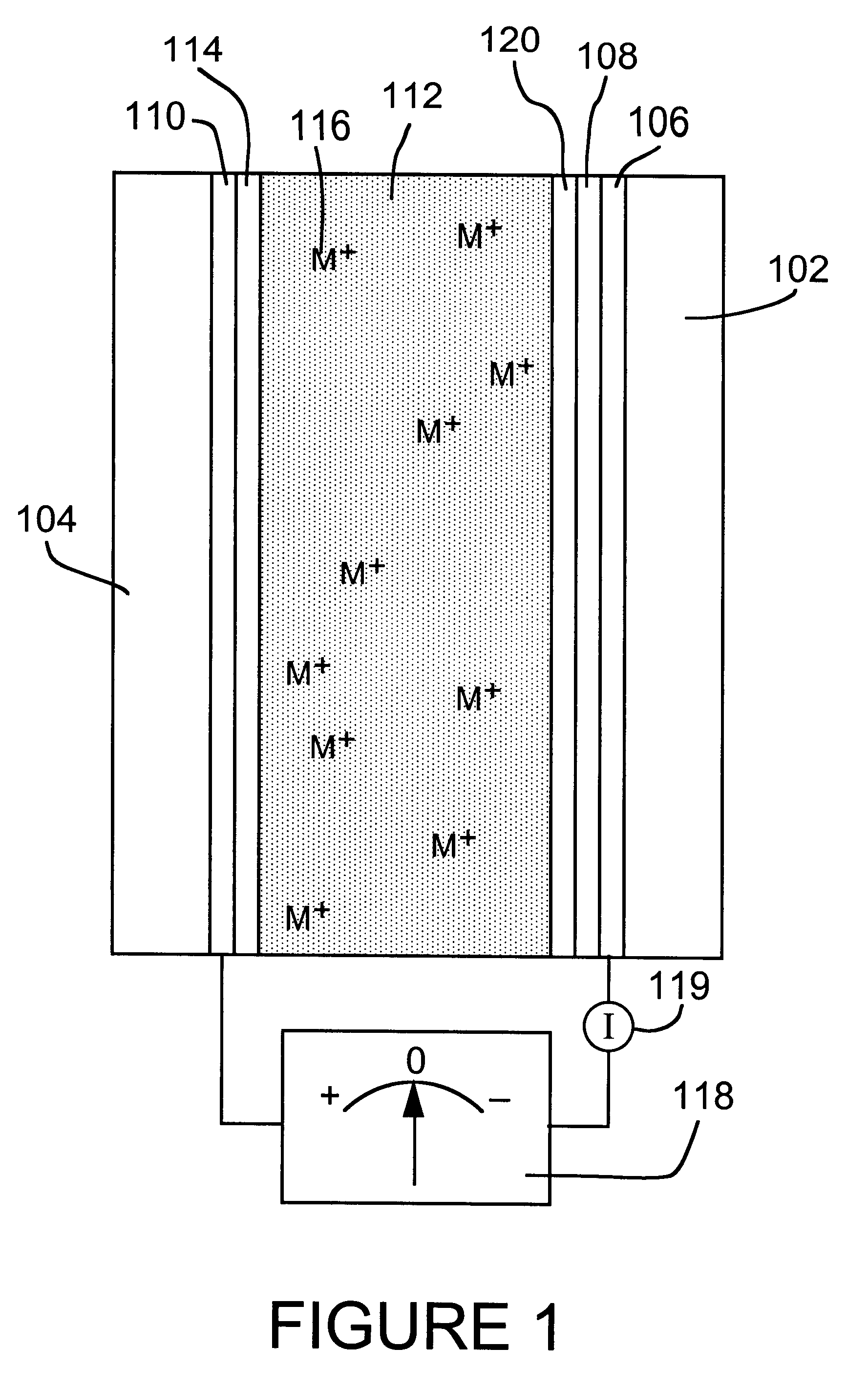
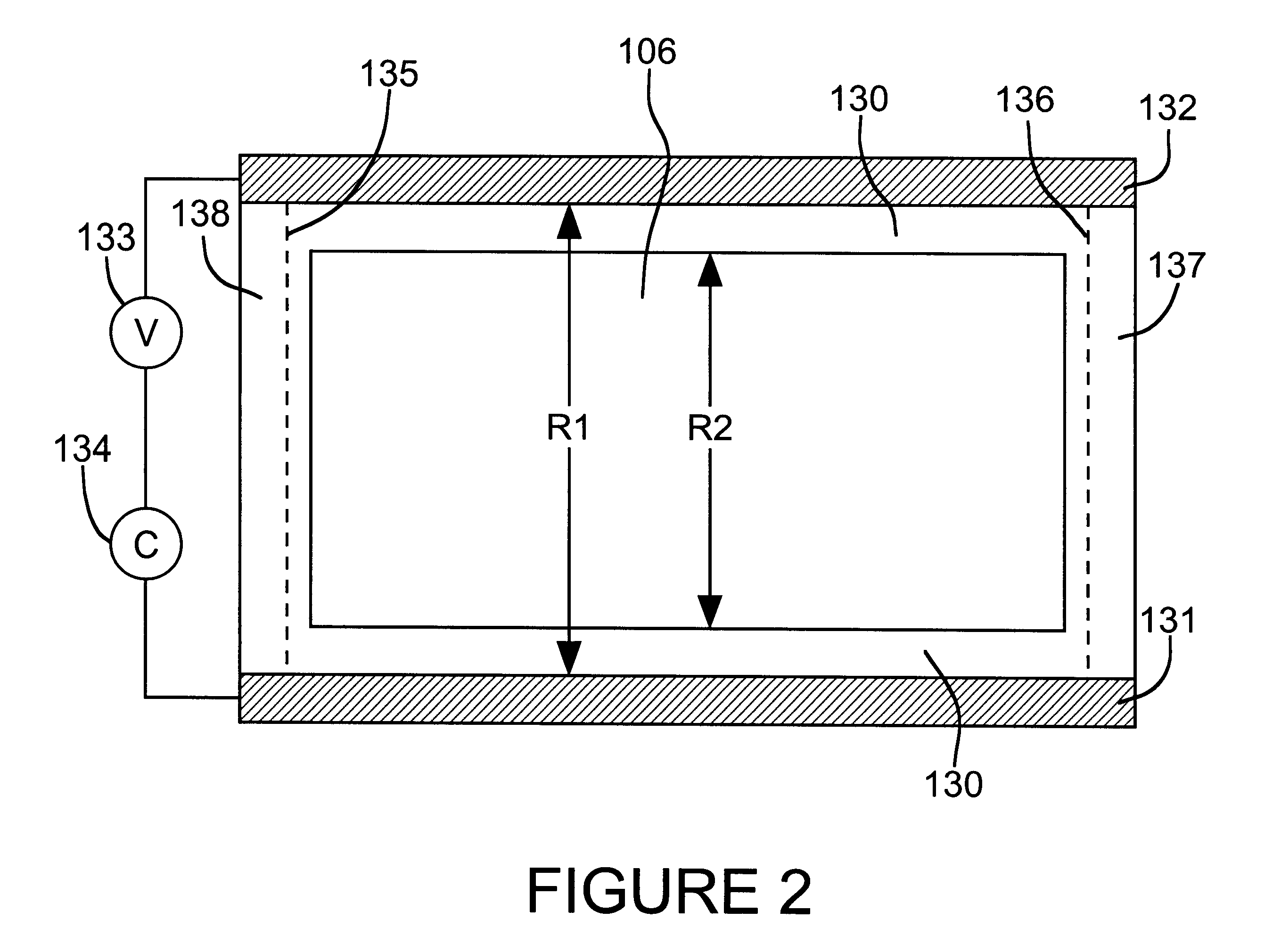
Interelectrode impedance or electric field potential measurements are used to determine the relative orientation of one lead to other leads in the spinal column or other body / tissue location. Interelectrode impedance is determined by measuring impedance vectors. The value of the impedance vector is due primarily to the electrode-electrolyte interface, and the bulk impedance between the electrodes. The bulk impedance between the electrodes is, in turn, made up of (1) the impedance of the tissue adjacent to the electrodes, and (2) the impedance of the tissue between the electrodes. In one embodiment, the present invention makes both monopolar and bipolar impedance measurements, and then corrects the bipolar impedance measurements using the monopolar measurements to eliminate the effect of the impedance of the tissue adjacent the electrodes. The orientation and position of the leads may be inferred from the relative minima of the corrected bipolar impedance values. These corrected impedance values may also be mapped and stored to facilitate a comparison with subsequent corrected impedance measurement values. Such comparison allows a determination to be made as to whether the lead position and / or orientation has changed appreciably over time. In another embodiment, one or more electrodes are stimulated and the resulting electric field potential on the non-stimulated electrodes is measured. Such field potential measurements provide an indication of the relative orientation of the electrodes. Once known, the relative orientation may be used to track lead migration, to setup stimulation configurations and parameters for nominal stimulation and / or navigation. Also, such measurements allow automatic adjustment of stimulation energy to a previously-defined optimal potential field in the case of lead migration or postural changes.
Owner:BOSTON SCI NEUROMODULATION CORP
Programmable conductor memory cell structure
In programmable conductor memory cells, metal ions precipitate out of a glass electrolyte element in response to an applied electric field in one direction only, causing a conductive pathway to grow from cathode to anode. The amount of conductive pathway growth, and therefore the programming, depends, in part, on the availability of metal ions. It is important that the metal ions come only from the solid solution of the memory cell body. If additional metal ions are supplied from other sources, such as the sidewall edge at the anode interface, the amount of metal ions may not be directly related to the strength of the electric field, and the programming will not respond consistently from cell to cell. The embodiments described herein provide new and novel structures that block interface diffusion paths for metal ions, leaving diffusion from the bulk glass electrolyte as the only supply of metal ions for conductive pathway formation.
Owner:OVONYX MEMORY TECH LLC
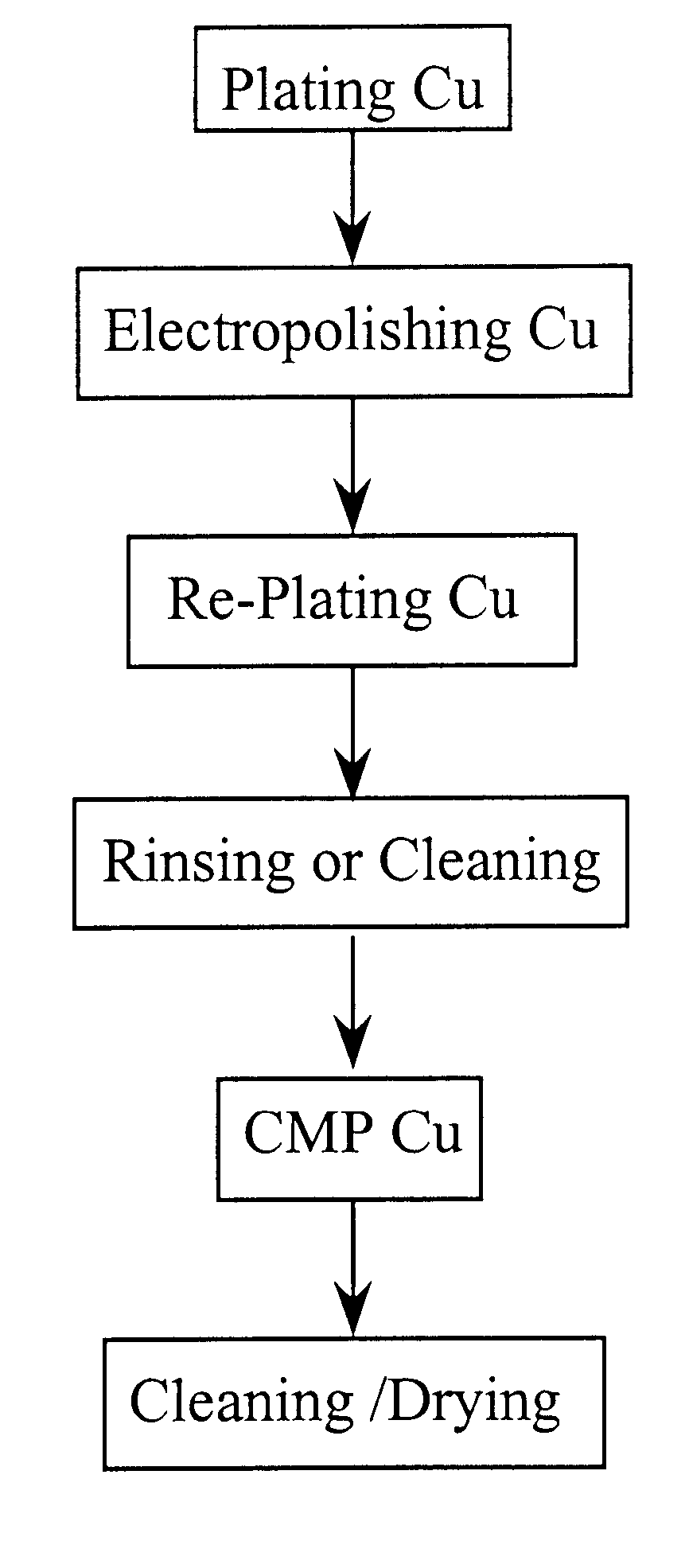
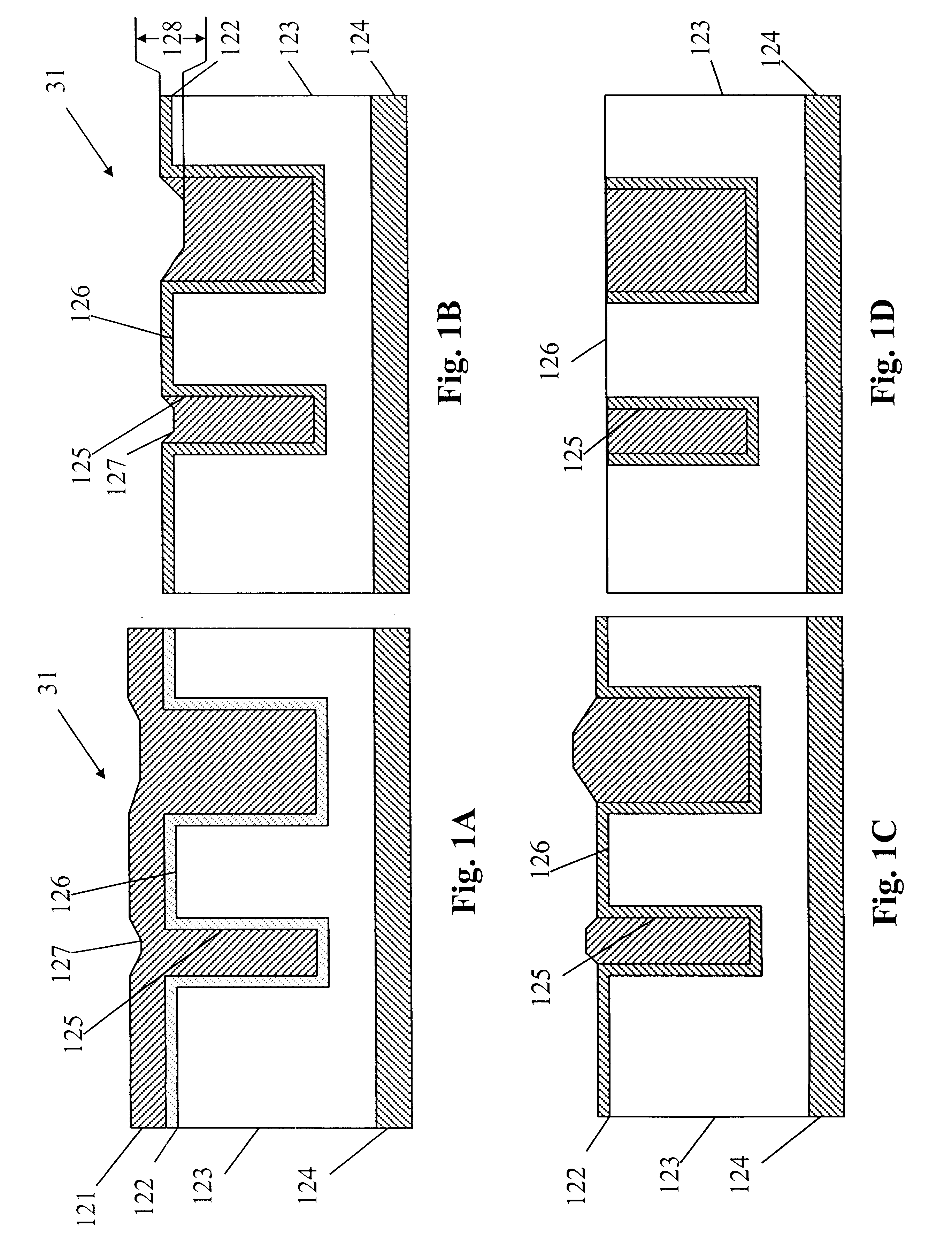
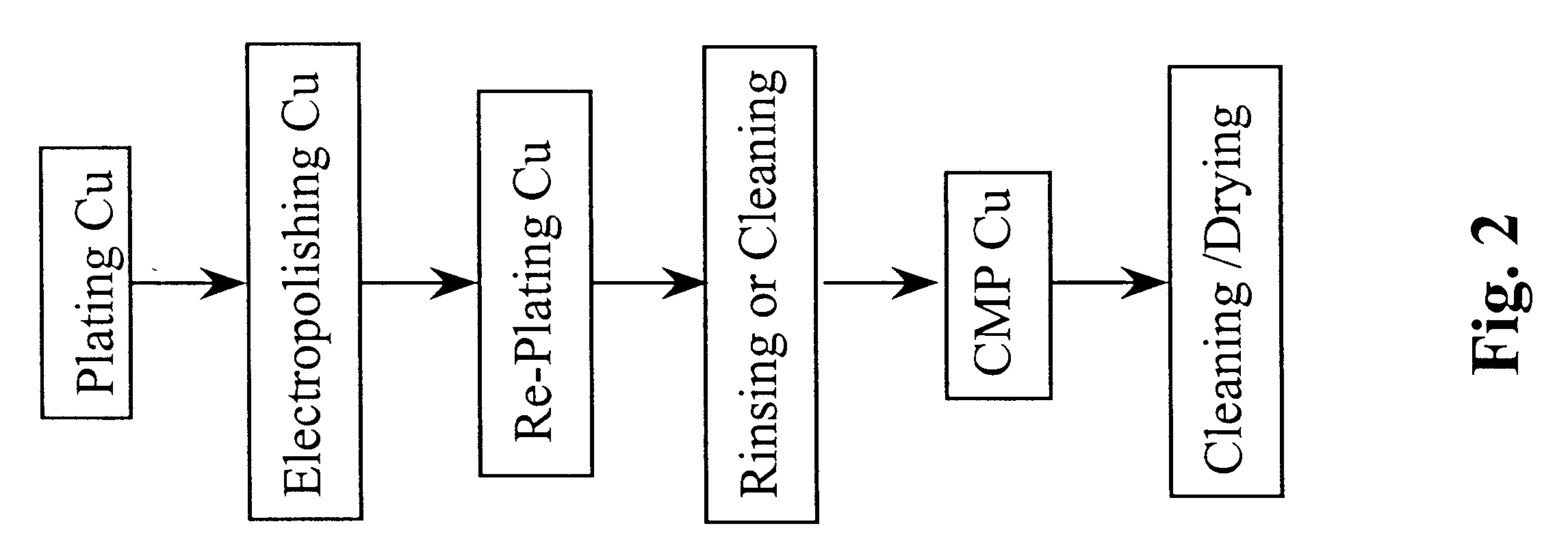
Methods and apparatus for electropolishing metal interconnections on semiconductor devices
InactiveUS6395152B1CellsSemiconductor/solid-state device manufacturingMetal interconnectSemiconductor
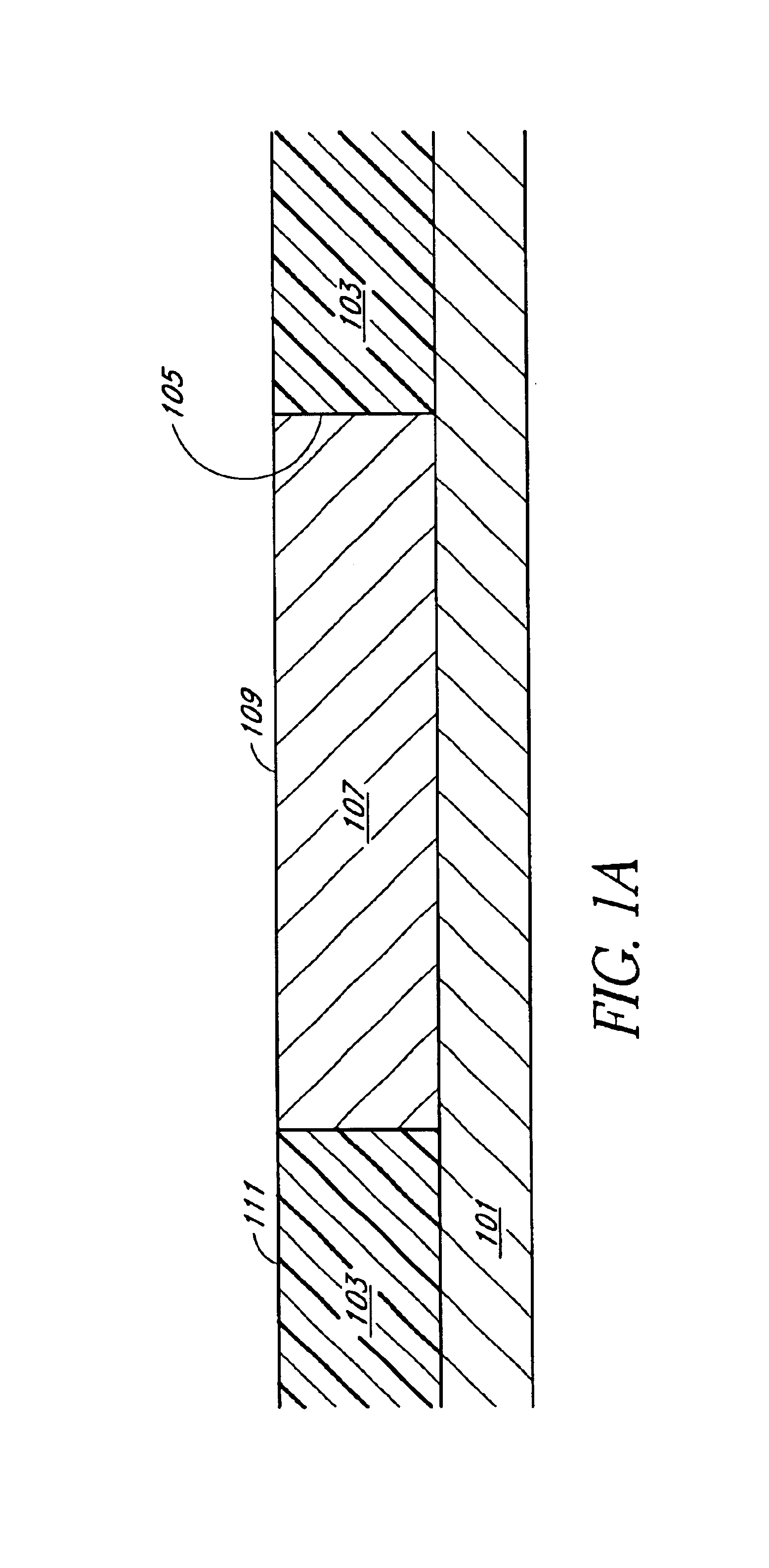
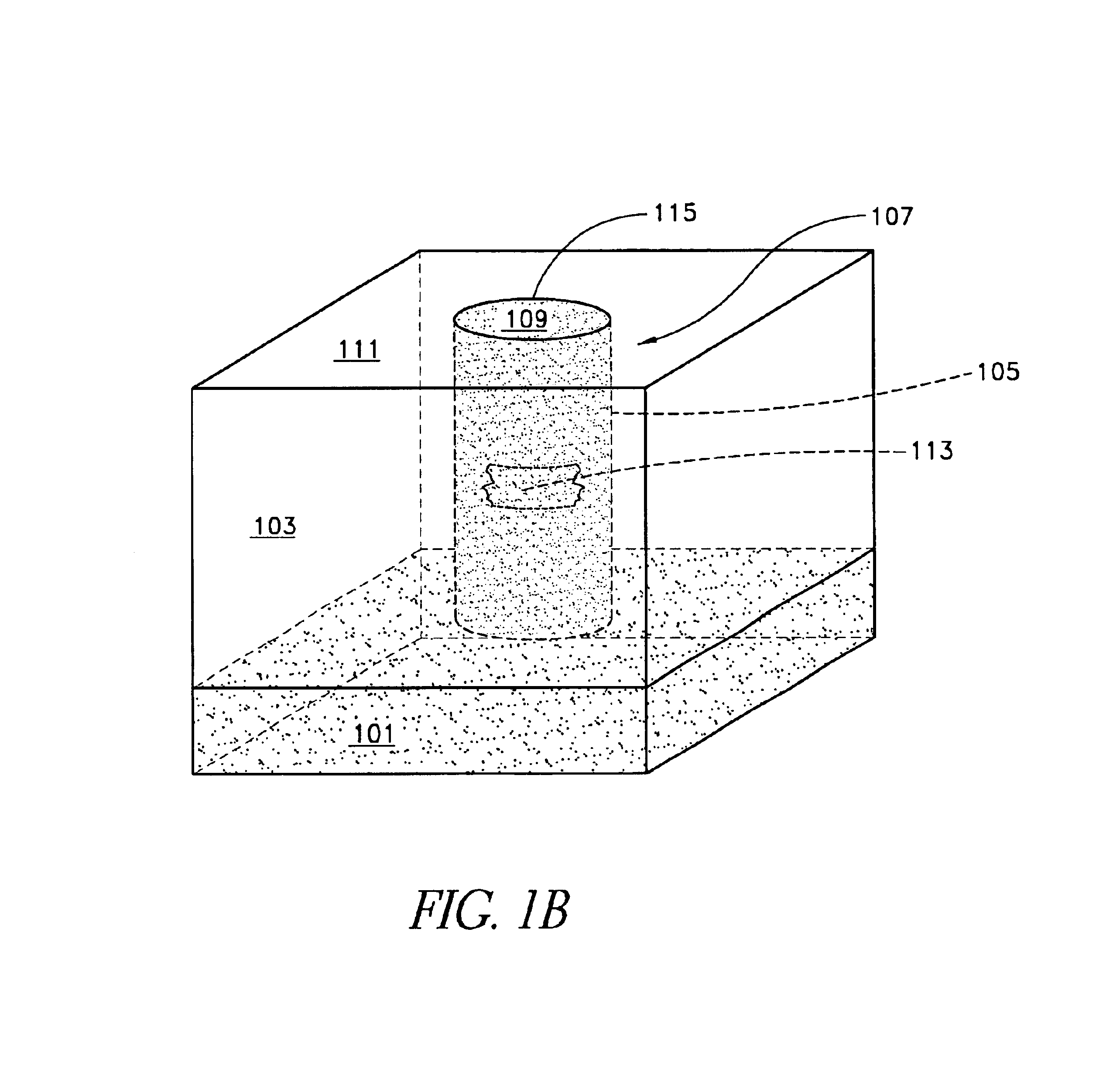
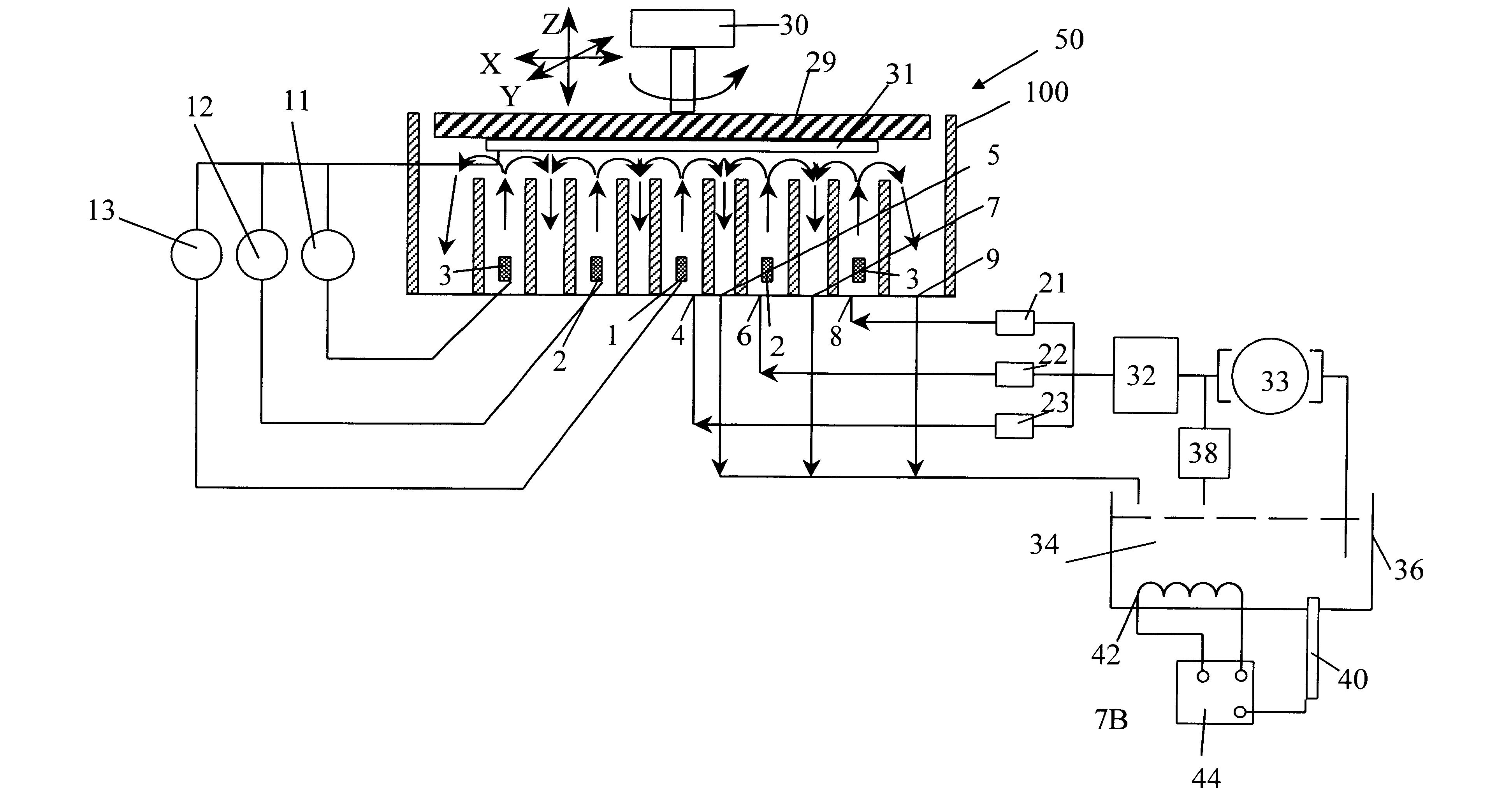
An electropolishing apparatus for polishing a metal layer formed on a wafer (31) includes an electrolyte (34), a polishing receptacle (100), a wafer chuck (29), a fluid inlet (5, 7, 9), and at least one cathode (1, 2, 3). The wafer chuck (29) holds and positions the wafer (31) within the polishing receptacle (100). The electrolyte (34) is delivered through the fluid inlet (5, 7, 9) into the polishing receptacle (100). The cathode (1, 2, 3) then applies an electropolishing current to the electrolyte to electropolish the wafer (31). In accordance with one aspect of the present invention, discrete portions of the wafer (31) can be electropolished to enhance the uniformity of the electropolished wafer.
Owner:ACM RES
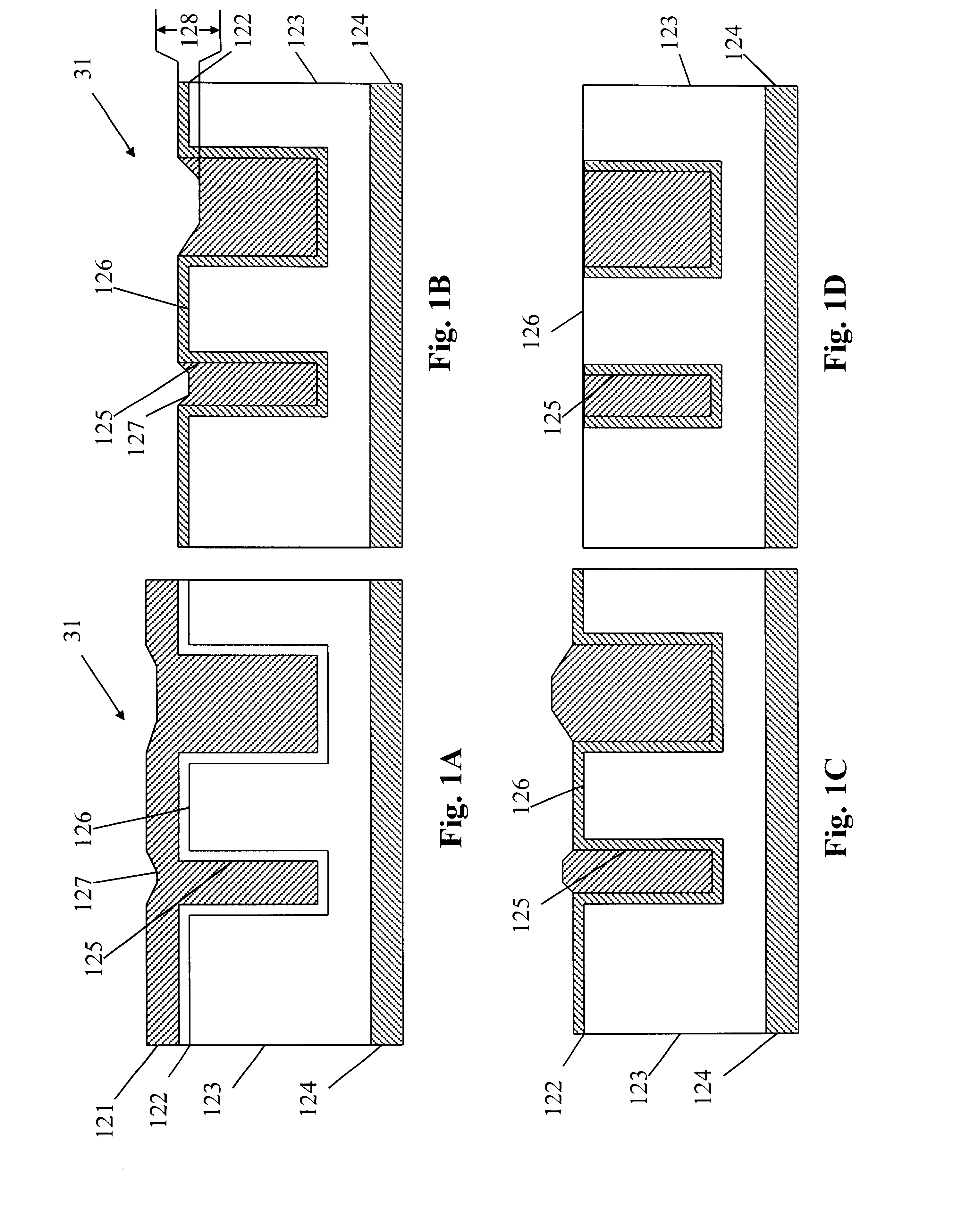
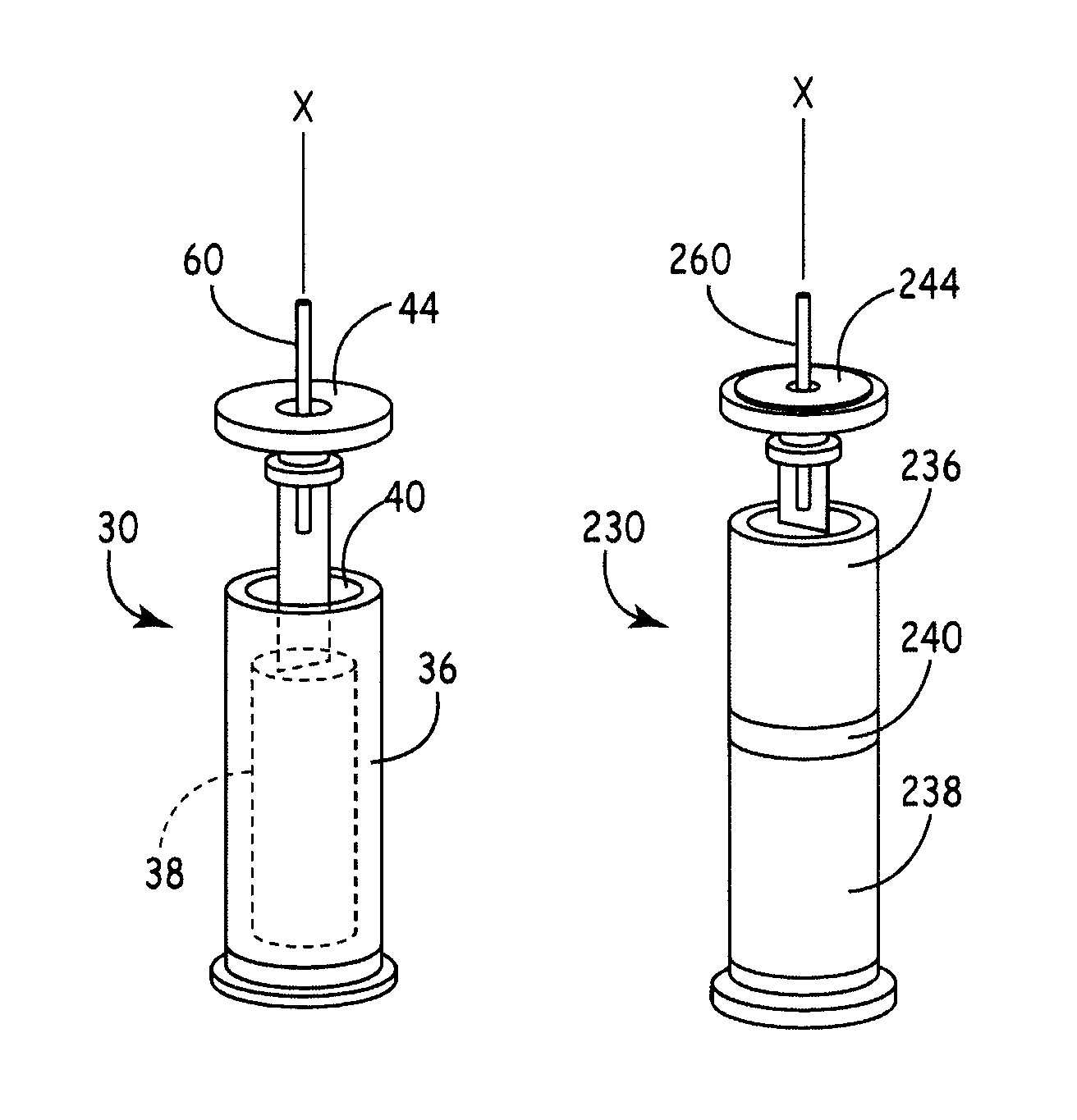
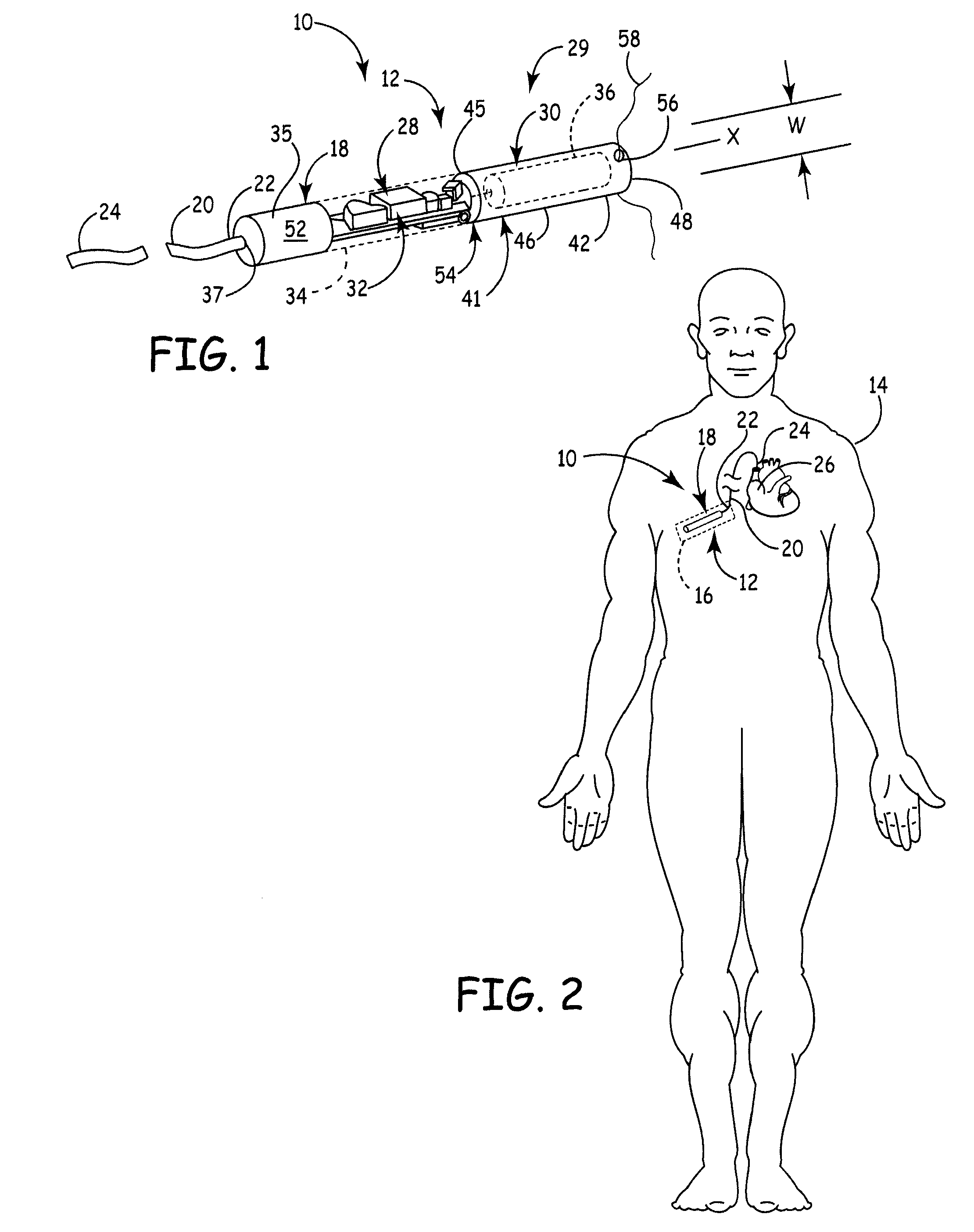
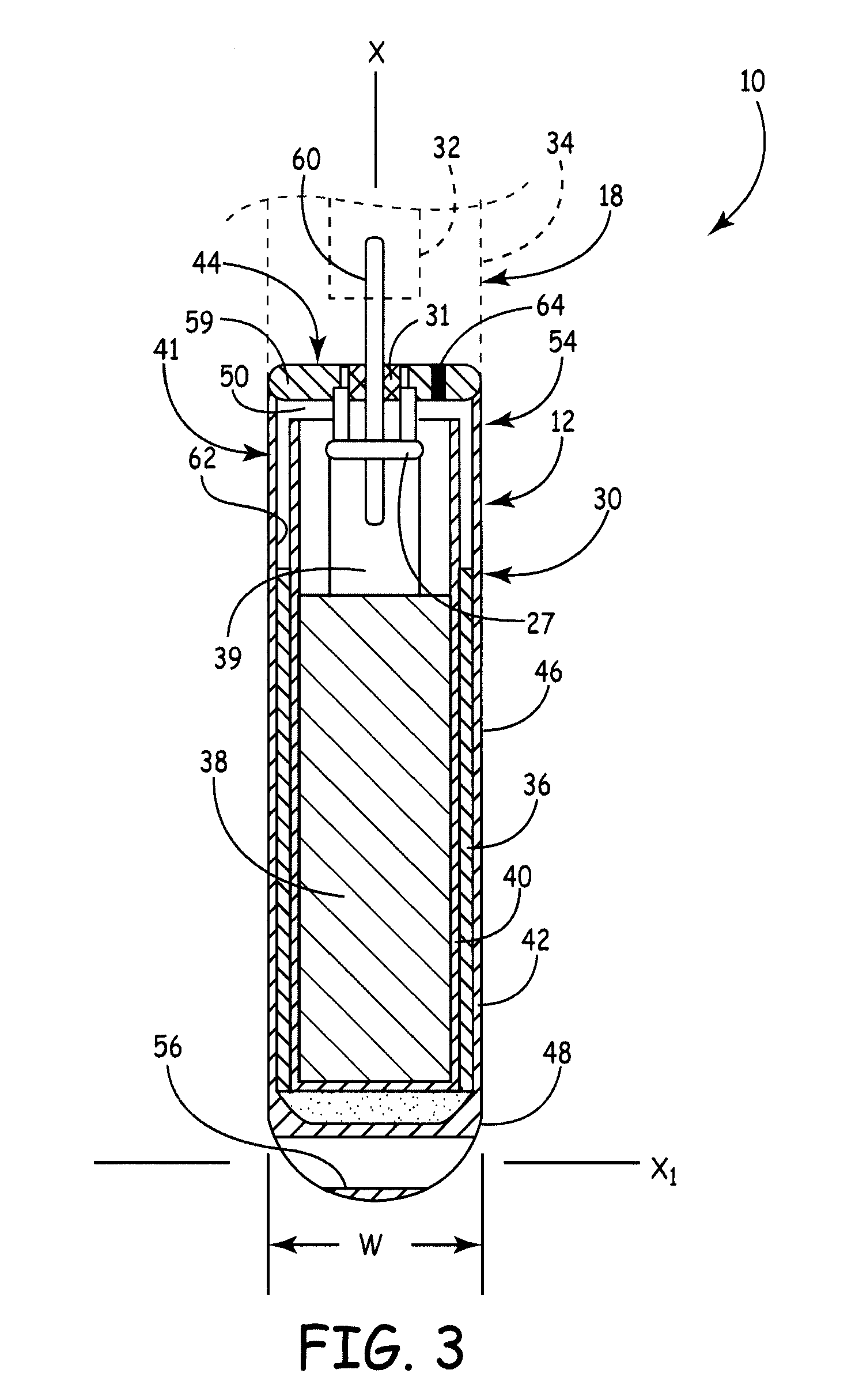
Elongate battery for implantable medical device
A battery assembly for a medical device includes an elongate cathode, an elongate anode, an electrolyte, and an elongate housing assembly encapsulating the cathode, the anode, and the electrolyte. The battery assembly also includes a first electrode that is exposed from and electrically insulated from the housing assembly. One of the anode and the cathode is electrically coupled to the first electrode, and the other of the anode and the cathode is electrically coupled to the housing assembly. One of the cathode and the anode includes a first portion and a second portion disposed in spaced relationship from the first portion. The other of the cathode and the anode is disposed between the first and second portions.
Owner:MEDTRONIC INC
Memory using mixed valence conductive oxides
A memory using a mixed valence conductive oxides. The memory includes a mixed valence conductive oxide that is less conductive in its oxygen deficient state and a mixed electronic ionic conductor that is an electrolyte to oxygen and promotes an electric field effective to cause oxygen ionic motion.
Owner:UNITY SEMICON
Composite solid polymer electrolyte membranes
InactiveUS7550216B2Improve performanceLow costElectrolyte holding meansMembranesPolymer electrolytesFuel cells
Owner:FOSTER-MILLER
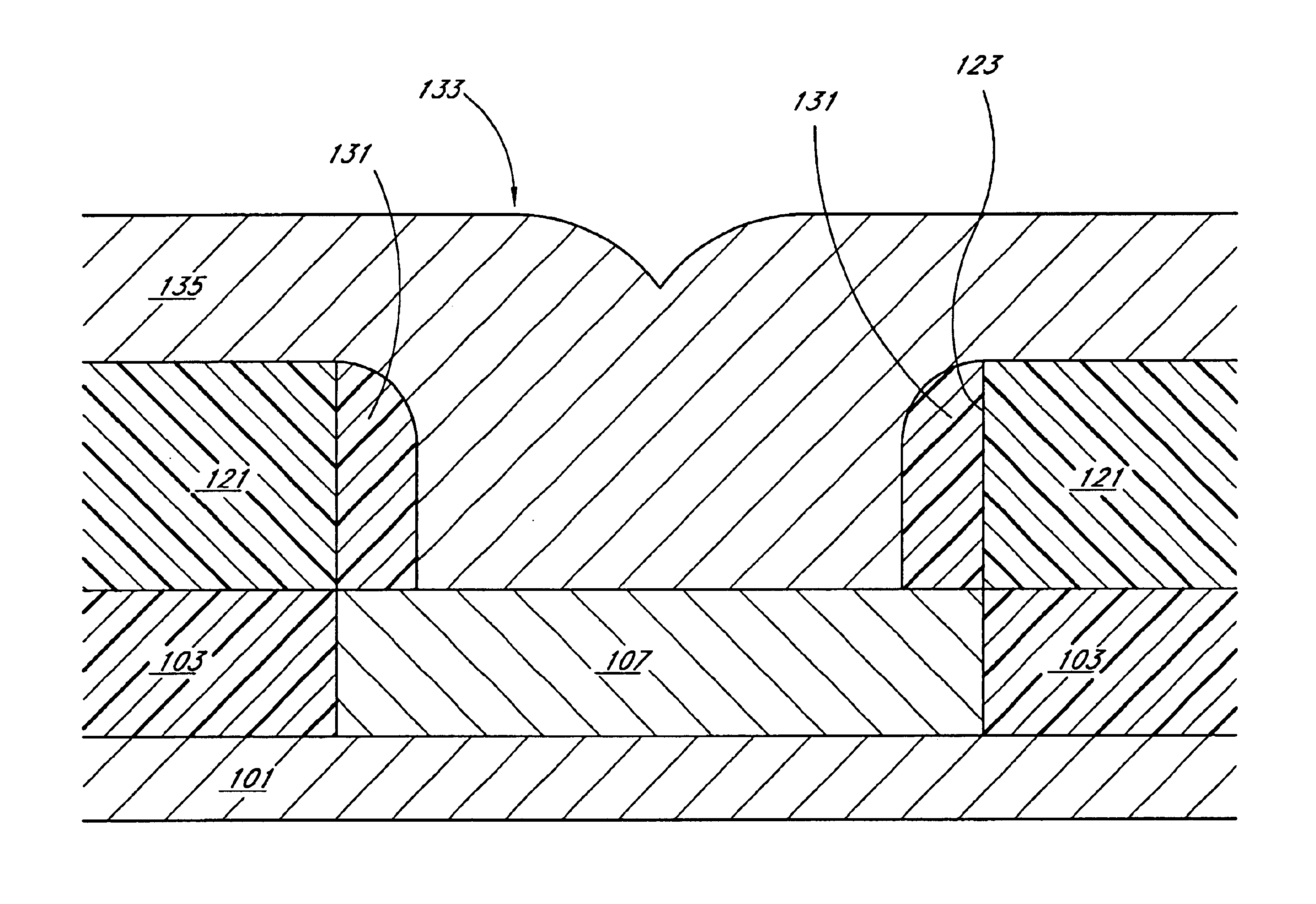
Semiconductor device
InactiveUS20050045919A1Increase the areaFine granularitySemiconductor/solid-state device detailsSolid-state devicesDevice materialEngineering
A programmable semiconductor device has a switch element in an interconnection layer, wherein in at least one of the inside of a via, interconnecting a wire of a first interconnection layer and a wire of a second interconnection layer, a contact part of the via with the wire of the first interconnection layer and a contact part of the via with the wire of the second interconnection layer, there is provided a variable electrical conductivity member, such as a member of an electrolyte material. The via is used as a variable electrical conductivity type switch element or as a variable resistance device having a contact part with the wire of the first interconnection layer as a first terminal and having a contact part with the wire of the second interconnection layer as a second terminal. By varying the electrical conductivity of the switch element, the state of connection of the via with the wire of the first interconnection layer and the state of connection of the via with the wire of the second interconnection layer may be variably set to a shorted state, an open-circuited state or to an intermediate state A two-state switch element includes an ion conductor for conducting metal ions interposed between the first and second electrodes. The second electrode is formed of a material lower in reactivity than the first electrode. The electrical conductivity across the first and second electrodes is changed by the oxidation-reduction reaction of the metal ions. There are provided first and second transistors of opposite polarities, connected to the first electrode, and third and fourth transistors of opposite polarities, connected to the second electrode.
Owner:NEC CORP
Electrode materials with high surface conductivity
InactiveUS6855273B2Electrode manufacturing processesDouble layer capacitorsSurface conductivityIon exchange
The present invention concerns electrode materials capable of redox reactions by electrons and alkaline ions exchange with an electrolyte. The applications are in the field of primary (batteries) or secondary electrochemical generators, super capacitors and light modulating system of the super capacitor type.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Method for electropolishing metal on semiconductor devices
An electropolishing apparatus for polishing a metal layer formed on a wafer (31) includes an electrolyte (34), a polishing receptacle (100), a wafer chuck (29), a fluid inlet (5, 7, 9), and at least one cathode (1, 2, 3). The wafer chuck (29) holds and positions the wafer (31) within the polishing receptacle (100). The electrolyte (34) is delivered through the fluid inlet (5, 7, 9) into the polishing receptacle (100). The cathode (1, 2, 3) then applies an electropolishing current to the electrolyte to electropolish the wafer (31). In accordance with one aspect of the present invention, discrete portions of the wafer (31) can be electropolished to enhance the uniformity of the electropolished wafer.
Owner:ACM RES
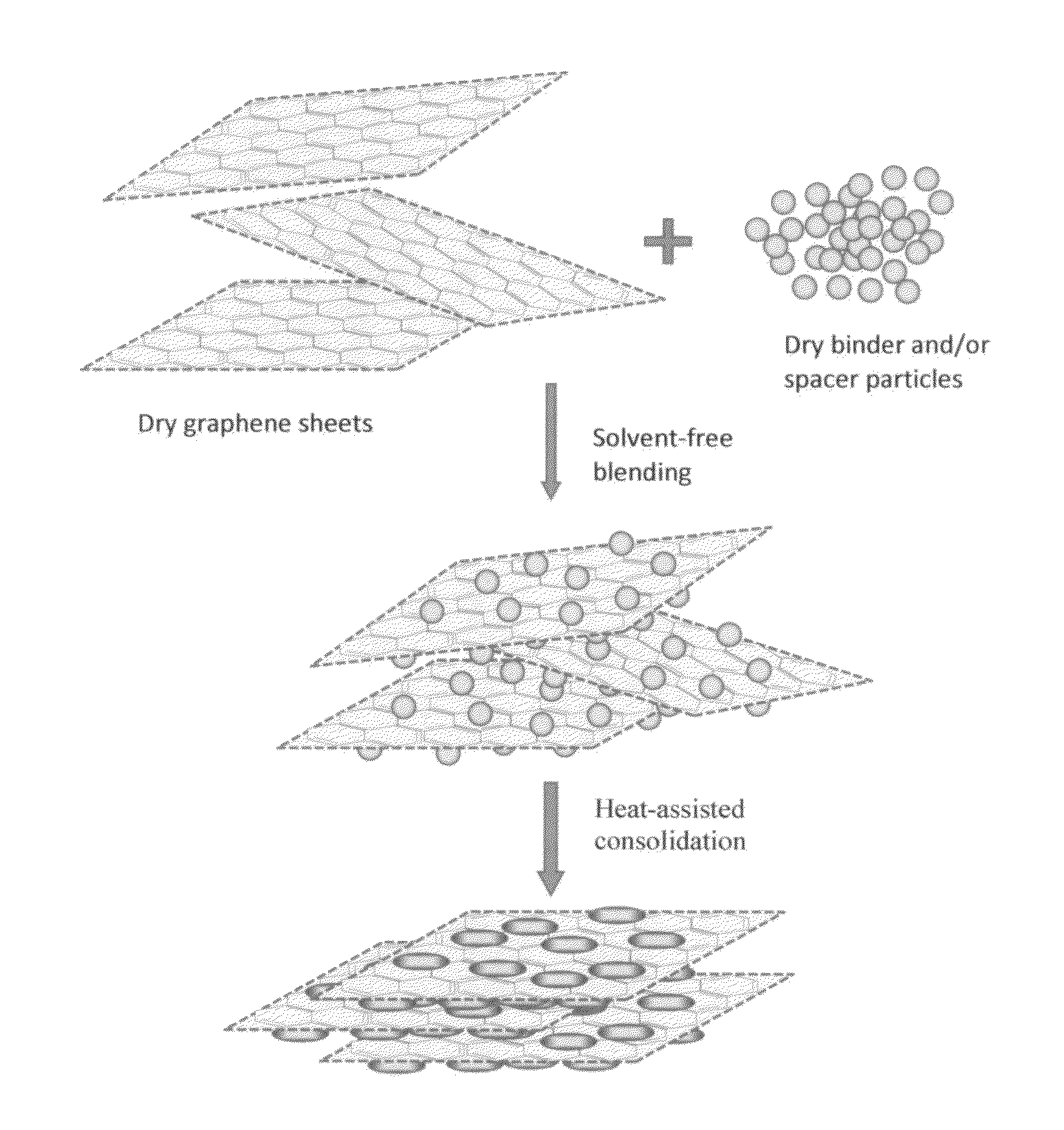
Solvent-free process based graphene electrode for energy storage devices
PendingUS20140030590A1Inexpensive and durable and highly reliableHigh capacitanceMaterial nanotechnologyHybrid capacitor electrodesGraphene flakeSolvent free
Disclosed is an electrode for an electrochemical energy storage device, the electrode comprising a self-supporting layer of a mixture of graphene sheets and spacer particles and / or binder particles, wherein the electrode is prepared without using water, solvent, or liquid chemical. The graphene electrode prepared by the solvent-free process exhibits many desirable features and advantages as compared to the corresponding electrode prepared by a known wet process. These advantages include a higher electrode specific surface area, higher energy storage capacity, improved or higher packing density or tap density, lower amount of binder required, lower internal electrode resistance, more consistent and uniform dispersion of graphene sheets and binder, reduction or elimination of undesirable effect of electrolyte oxidation or decomposition due to the presence of water, solvent, or chemical, etc.
Owner:GLOBAL GRAPHENE GRP INC
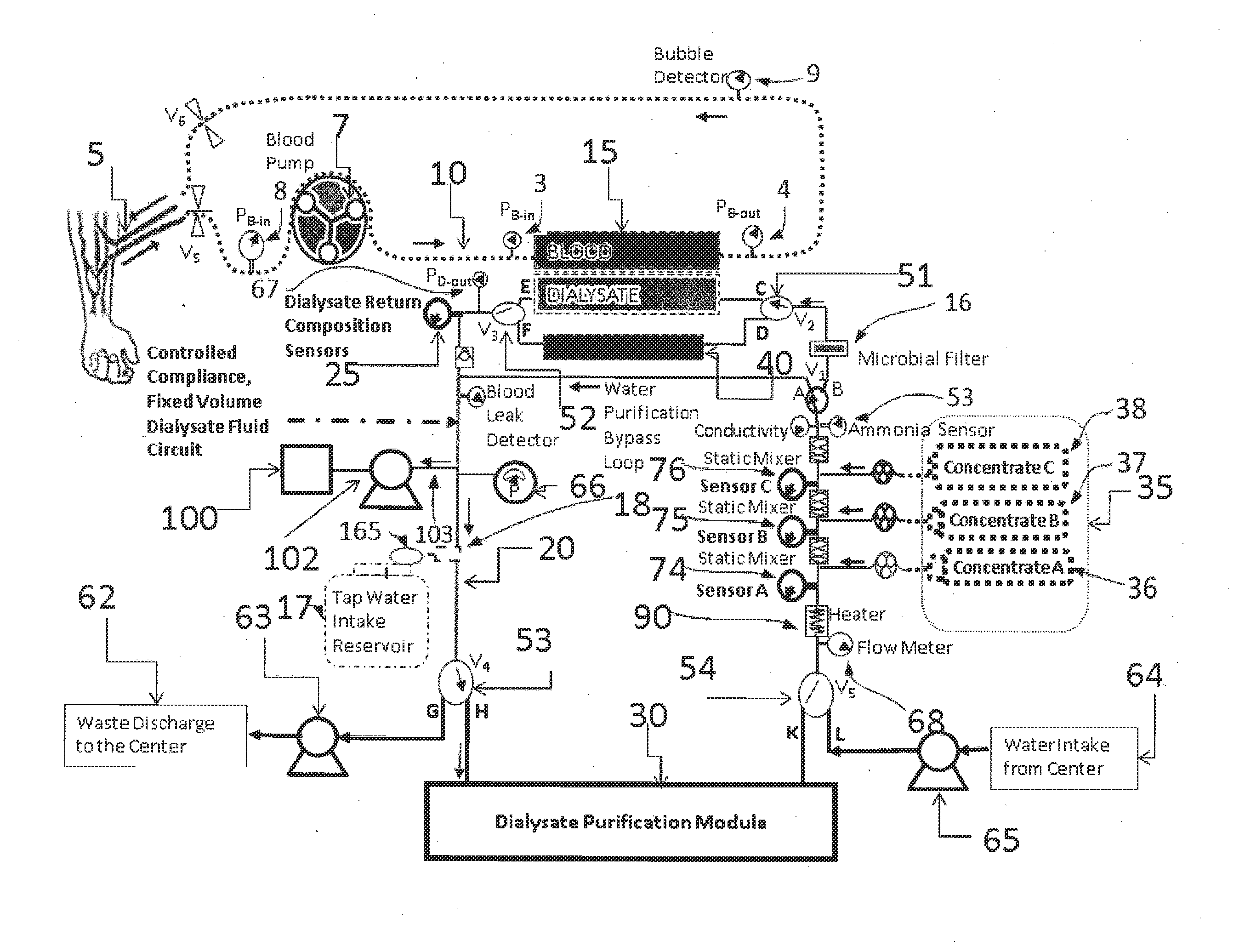
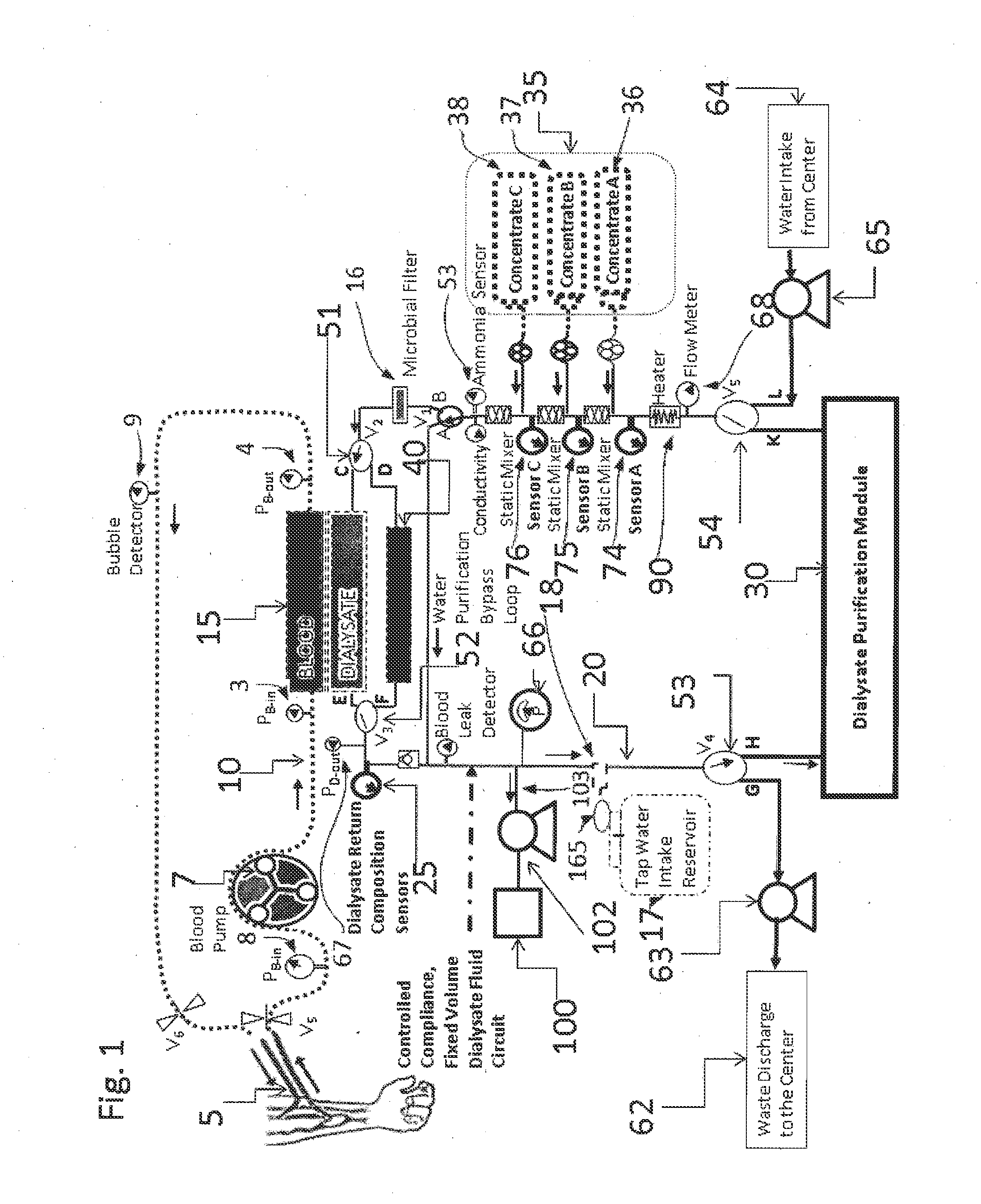
Systems and methods for performing peritoneal dialysis
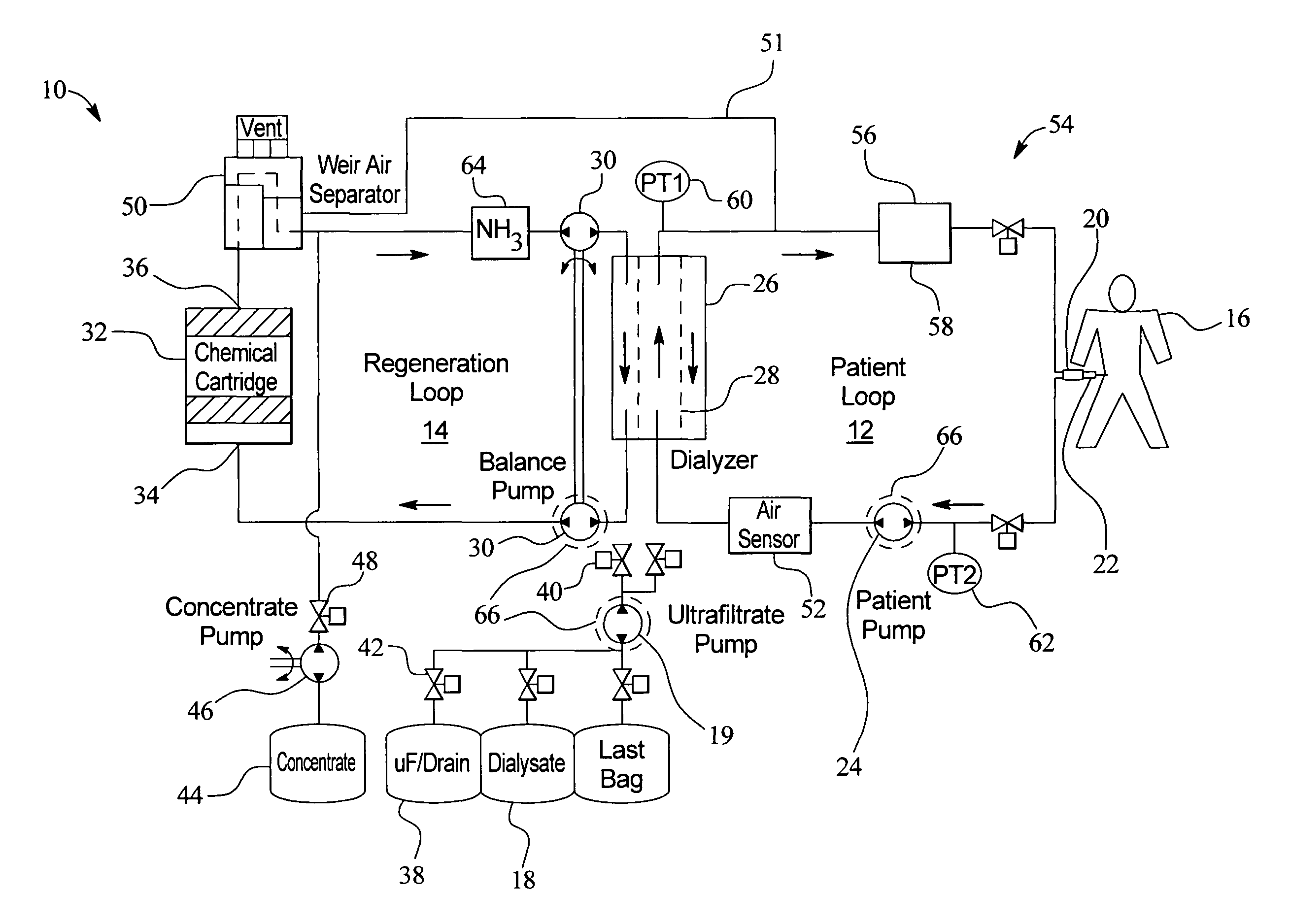
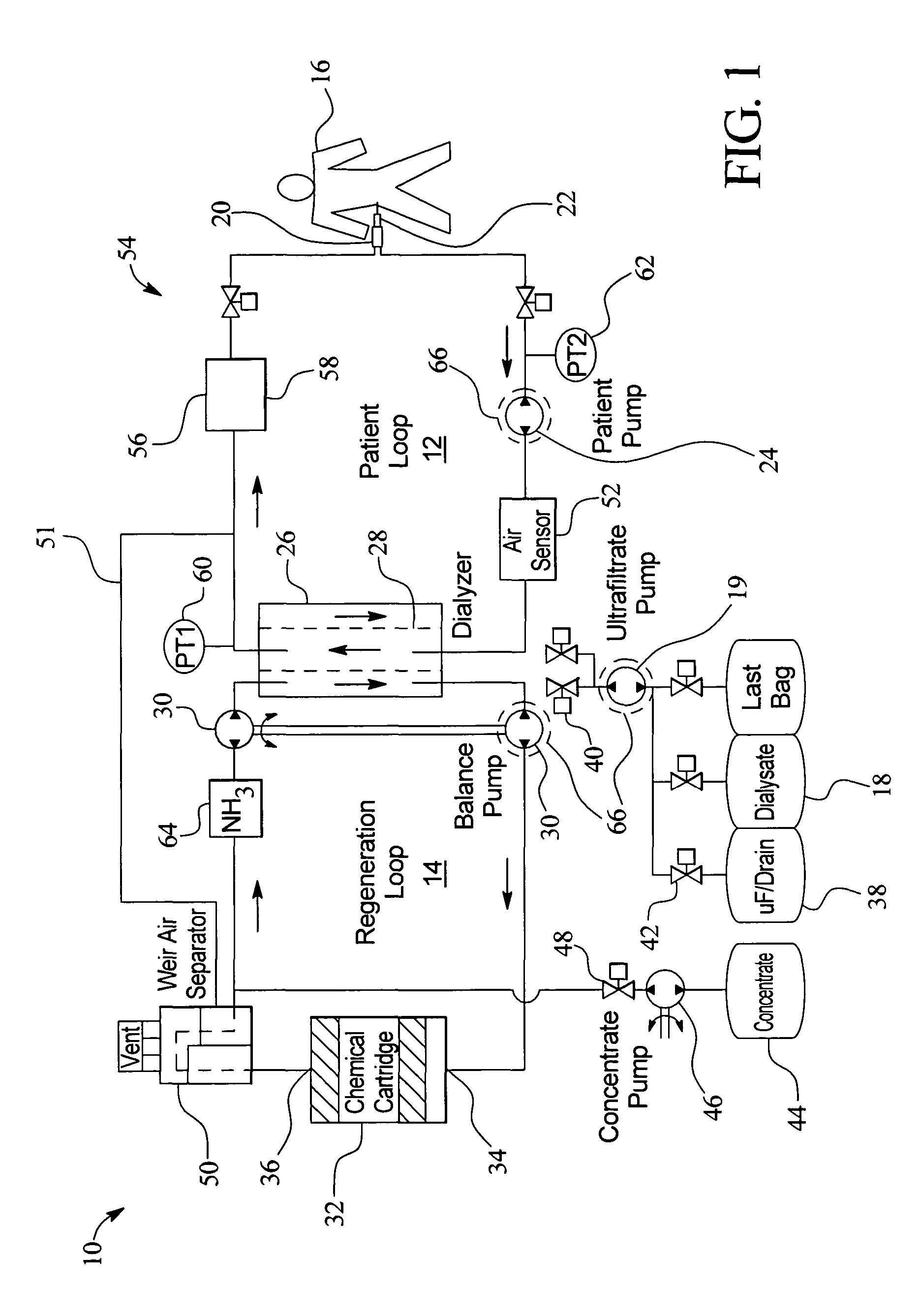
ActiveUS7867214B2Strengthen the systemImprove methodSolvent extractionIon-exchanger regenerationMetabolic wasteSorbent
In a peritoneal dialysis embodiment of the present invention, spent dialysate from the patient's peritoneal cavity passes, along a patient loop, through a dialyzer having a membrane that separates waste components from the spent dialysate, wherein the patient loop returns fresh dialysate to the patient's peritoneal cavity. The waste components are carried away in a second regeneration loop to a regeneration unit or sorbent cartridge, which absorbs the waste components. The regeneration unit removes undesirable components in the dialysate that were removed from the patient loop by the dialyzer, for example, excess water (ultrafiltrate or UF), toxins and metabolic wastes. Desirable components can be added to the dialysate by the system, such as glucose and electrolytes. The additives assist in maintaining the proper osmotic gradients in the patient to perform dialysis and provide the necessary compounds to the patient.
Owner:BAXTER INT INC
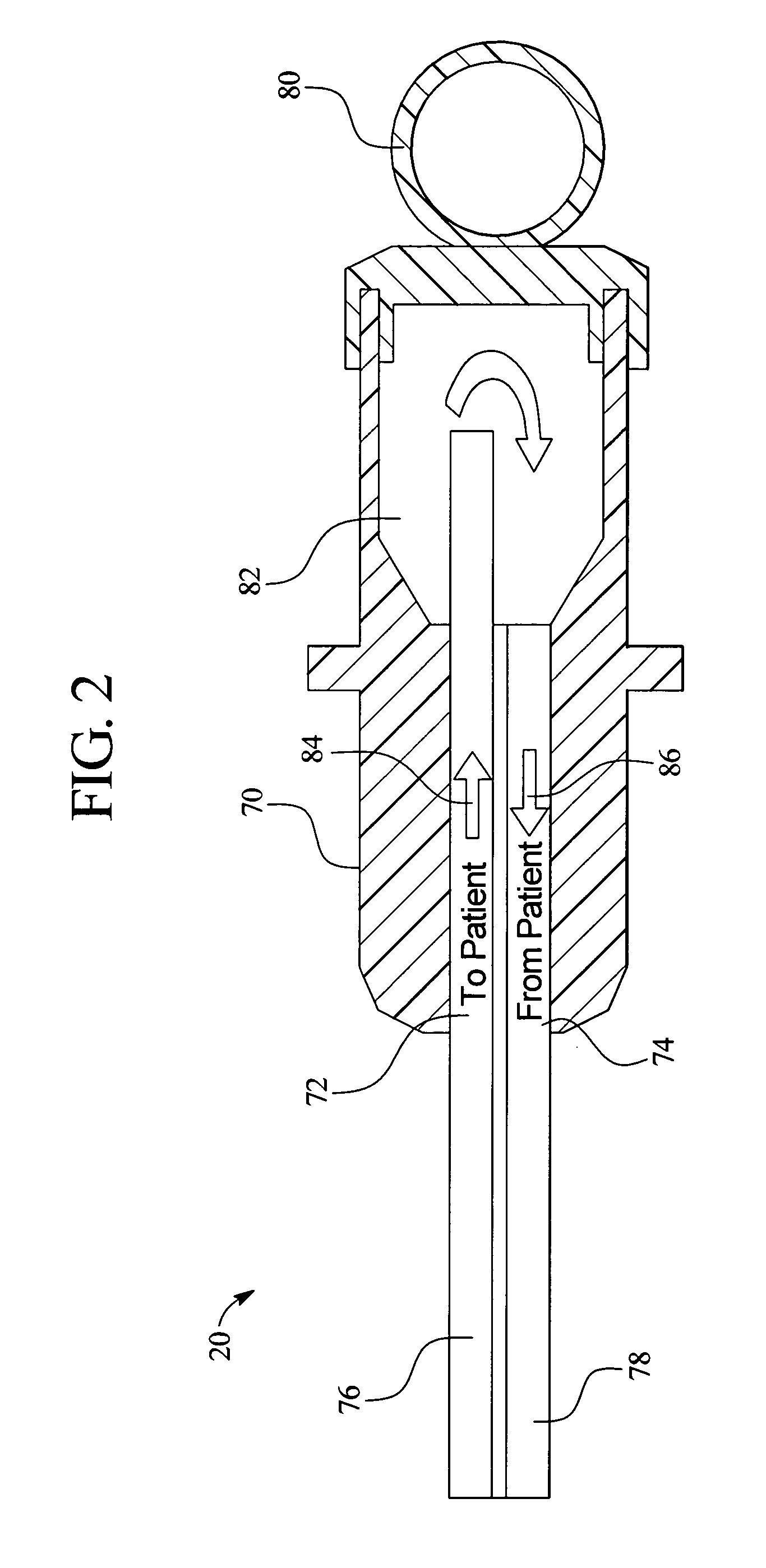
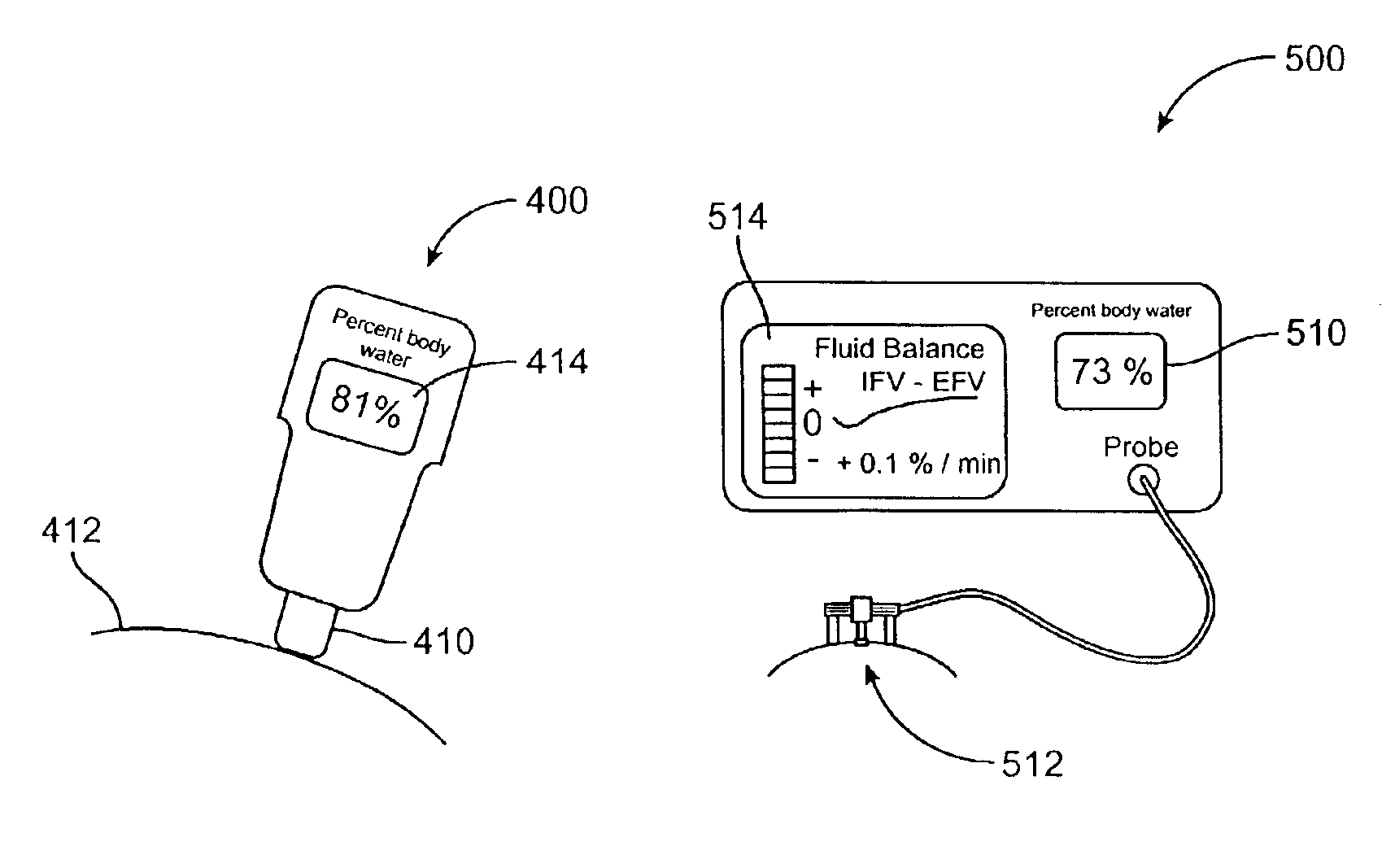
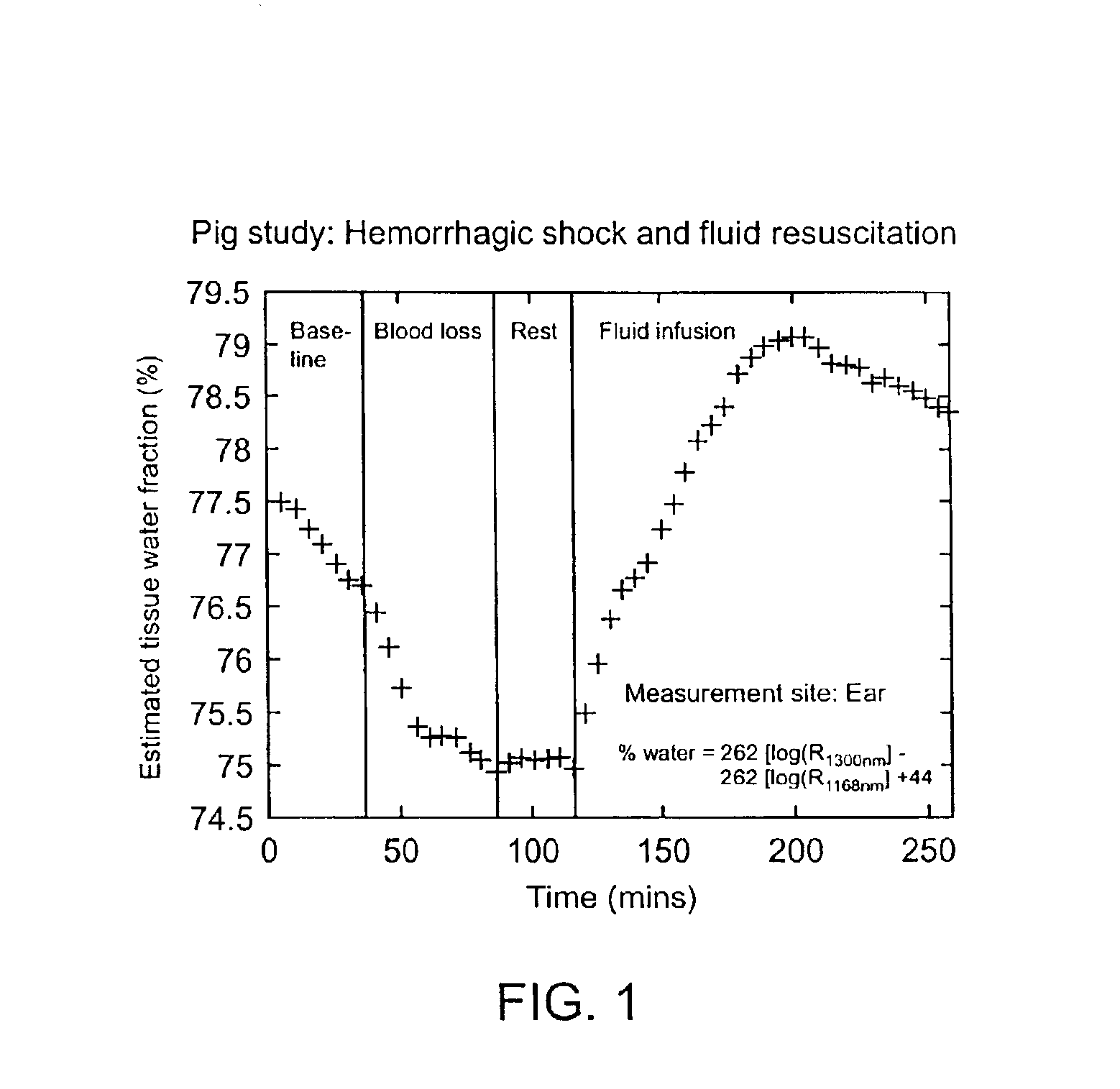
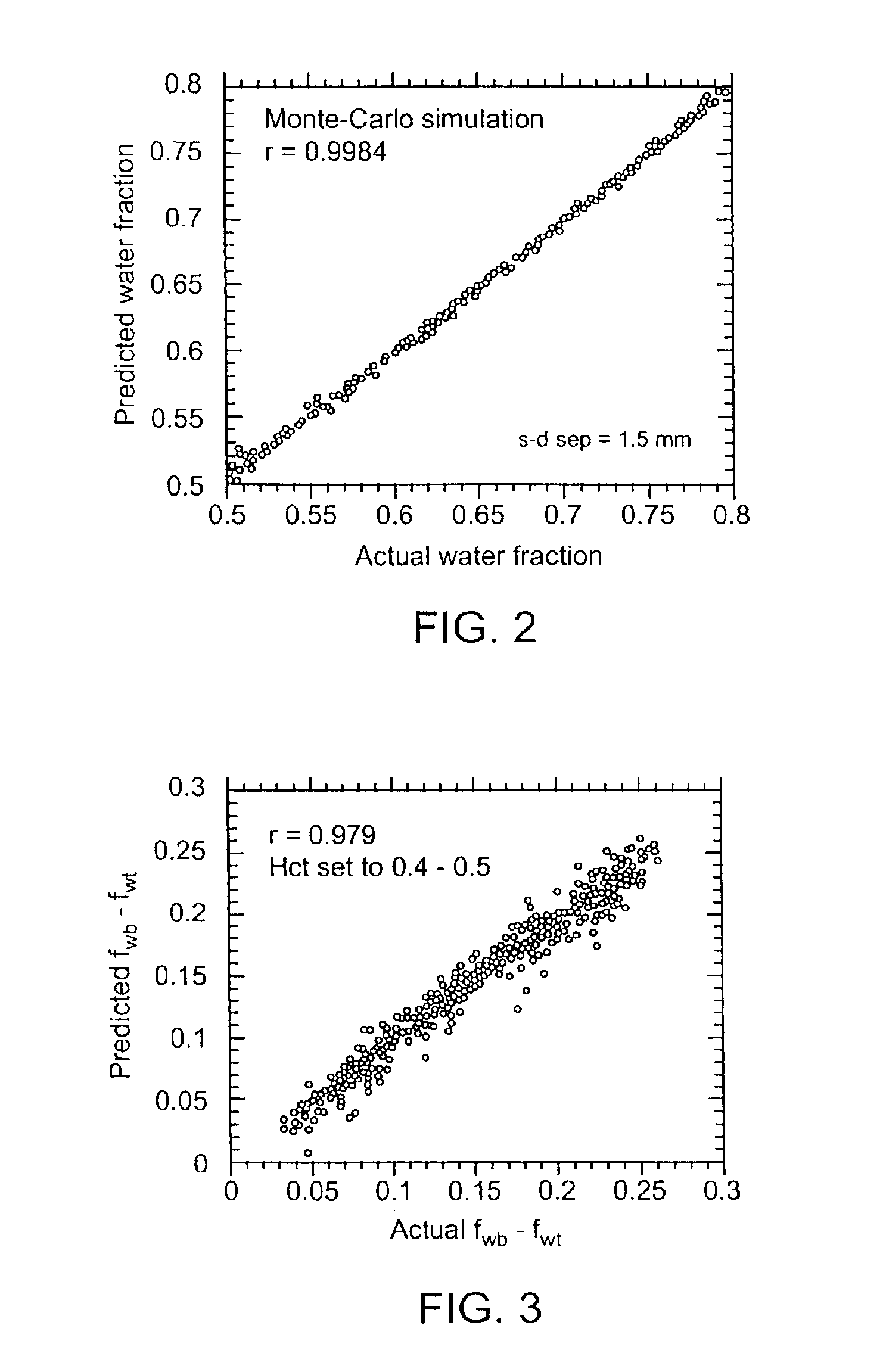
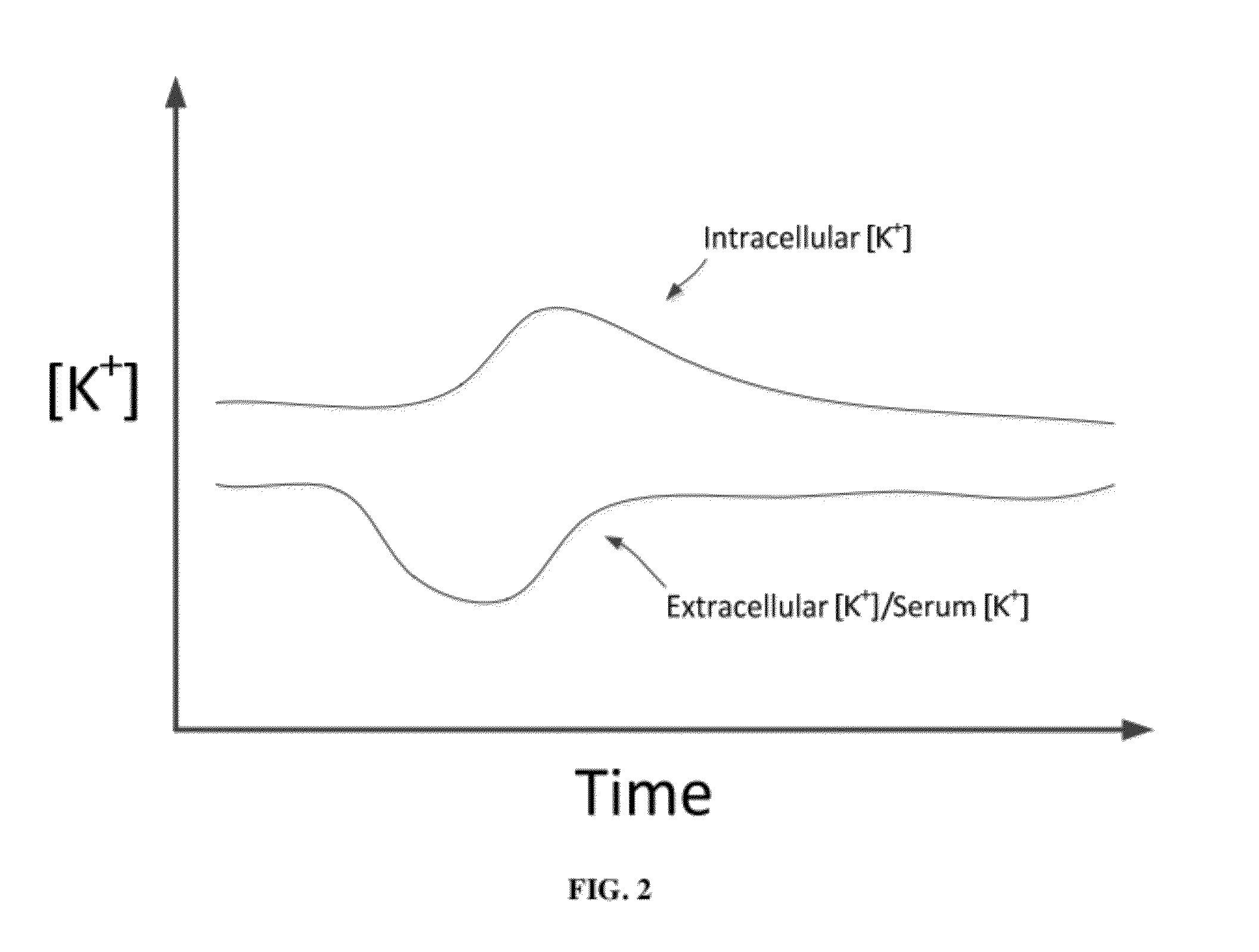
Device and method for monitoring body fluid and electrolyte disorders
InactiveUS7236811B2Improve measurement reliabilityDiagnostics using lightDiagnostics using pressureLipid formationTissue compartment
Owner:COVIDIEN LP
Multimodal dialysis system
ActiveUS20120273354A1Rate of fluid is decreasedReduce probabilitySludge treatmentIon-exchanger regenerationClinical settingsHaemodialysis machine
A dialysis device for operation in multiple modes and for maintaining a known gradient of potassium ion or other electrolyte between the blood of a patient and a dialysate fluid is described. The dialysis device is capable of being used for hemodialysis or peritoneal dialysis, and the dialysis device is capable of operation with a dialysate purification unit outside of a clinical setting or with a supply of water that can be supplied in a clinical setting. The dialysis device has a composition sensor containing a potassium-sensitive electrode for measuring a potassium ion concentration in one or more of the patient's blood and the dialysate fluid and an infusate pump operated to adjust a potassium ion concentration in the dialysate fluid based at least in part on data from the composition sensor.
Owner:MOZARC MEDICAL US LLC
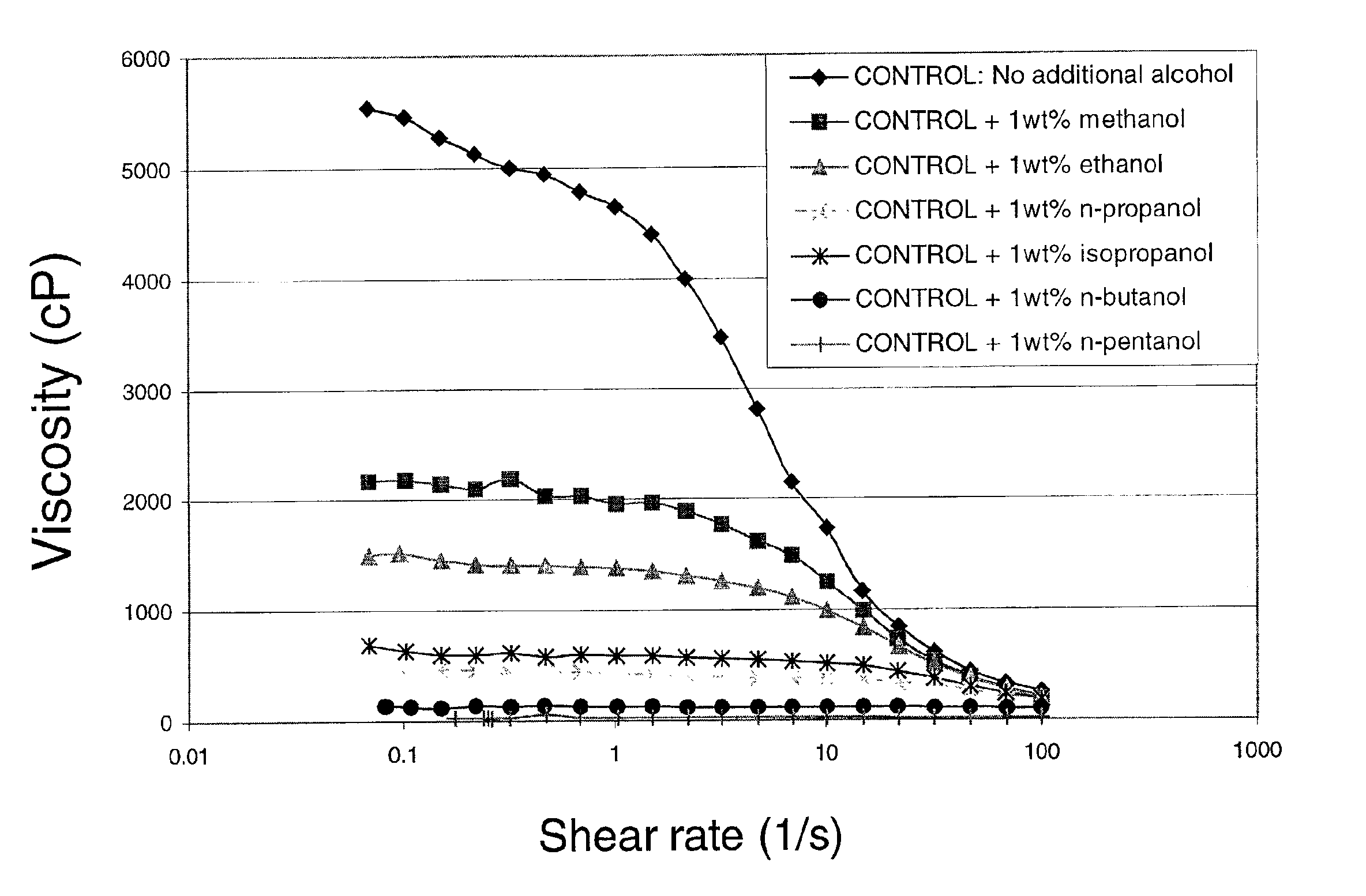
Viscosity reduction of viscoelastic surfactant based fluids
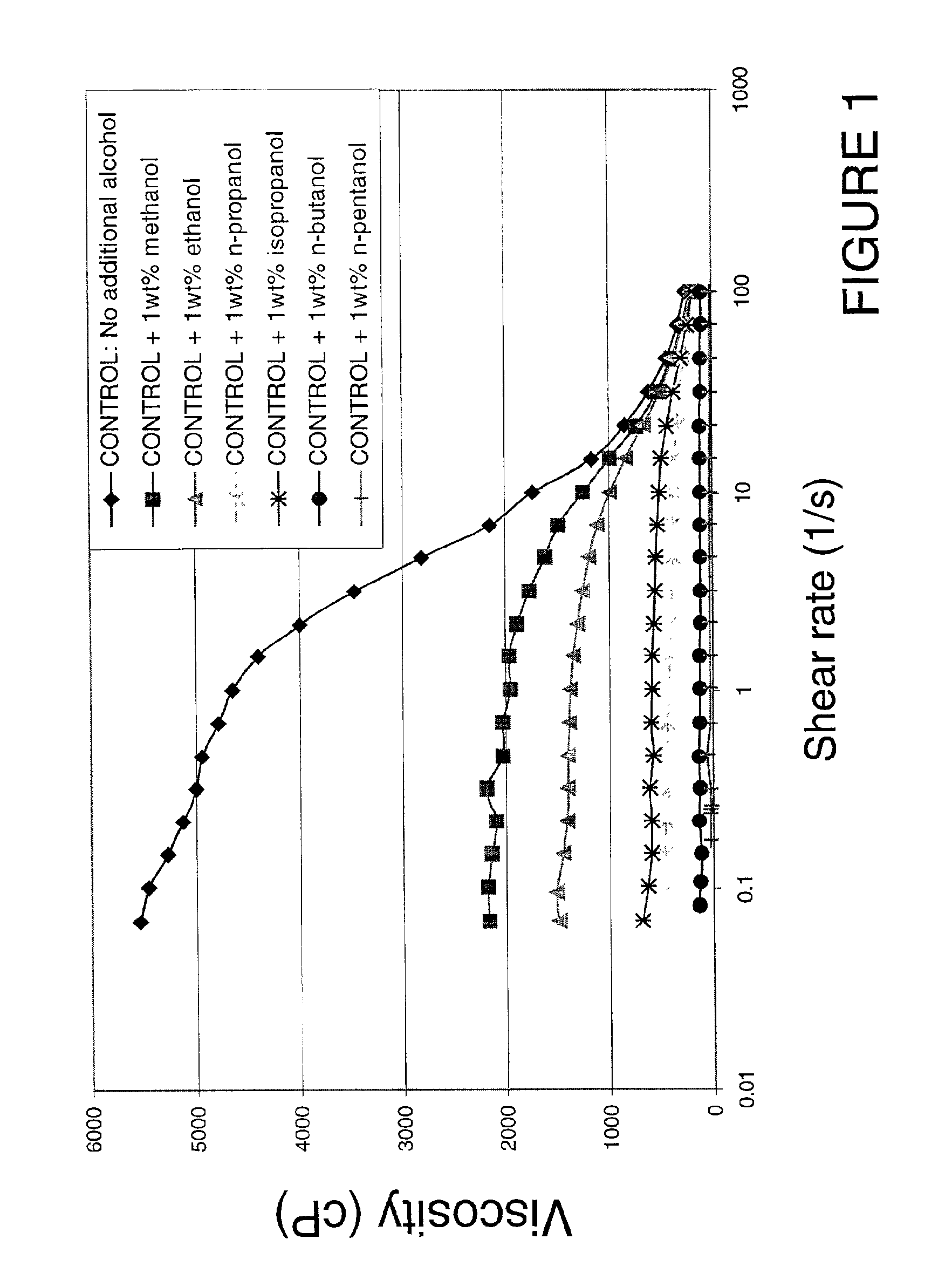
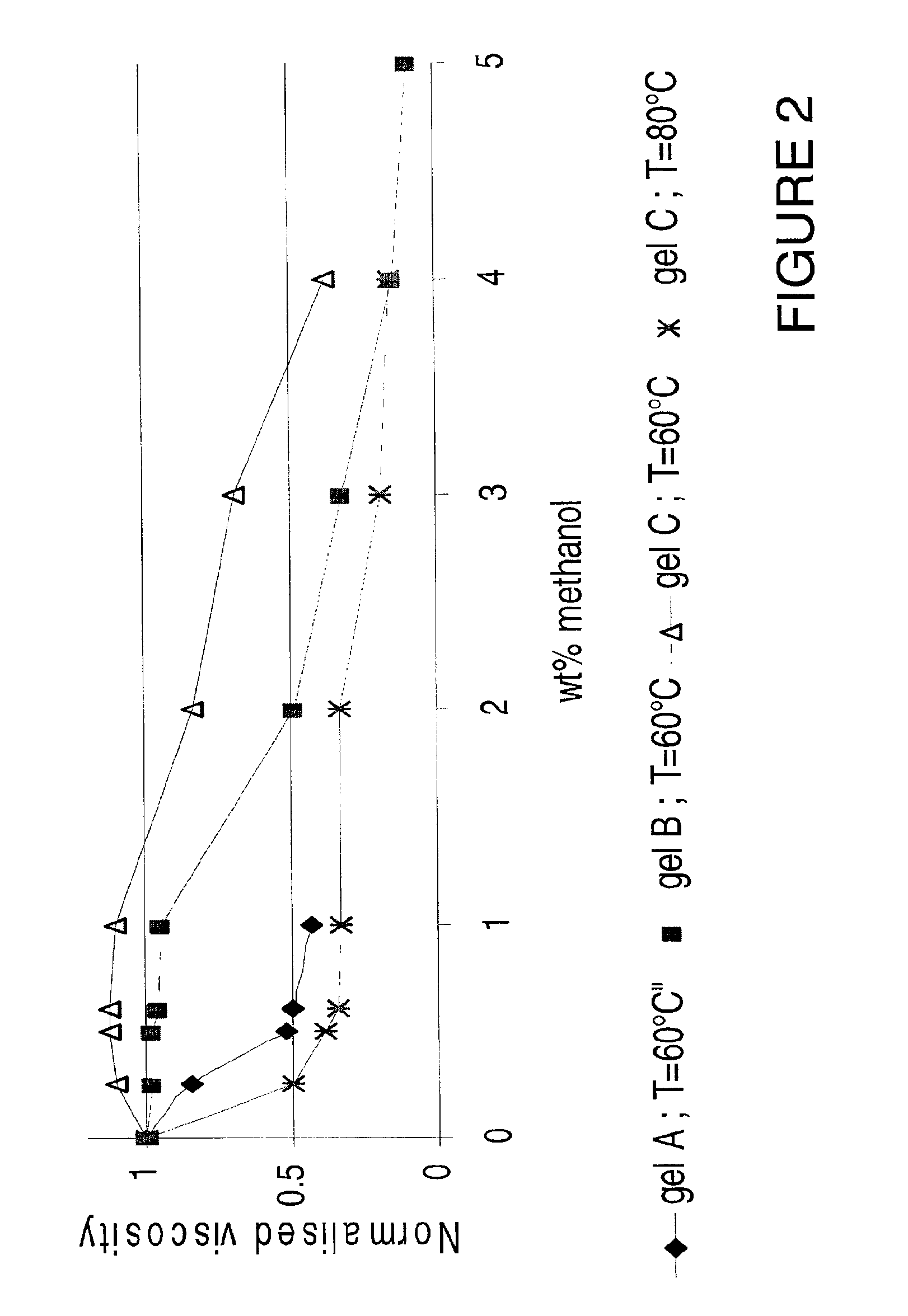
InactiveUS6881709B2Improve efficiencyImproves and optimizes conditionOther chemical processesFluid removalAlcoholSolid particle
Methods and compositions are disclosed for controlled addition of components that decrease the viscosity of the viscoelastic surfactant fluids or for controlled changes in the electrolyte concentration or composition of the viscoelastic surfactant fluids. One aspect of the invention relates to the use of internal breakers with a delayed activation. Another aspect of the invention relates to the use of precursors that release a breaking system such as alcohol by a process such as melting, slow dissolution, reaction with a compound present in the fluid or added to the fluid during or after the step of injecting, rupture of an encapsulating coating and de-adsorption of a breaking agent absorbed into solid particles. In another aspect of the invention, alcohols are included in a pad to reduce the low-shear viscosity and reduce the resistance to flow of the treatment fluids during a desired phase of the treatment.
Owner:SCHLUMBERGER TECH CORP
Thermostat incorporating thin film carbon dioxide sensor and environmental control system
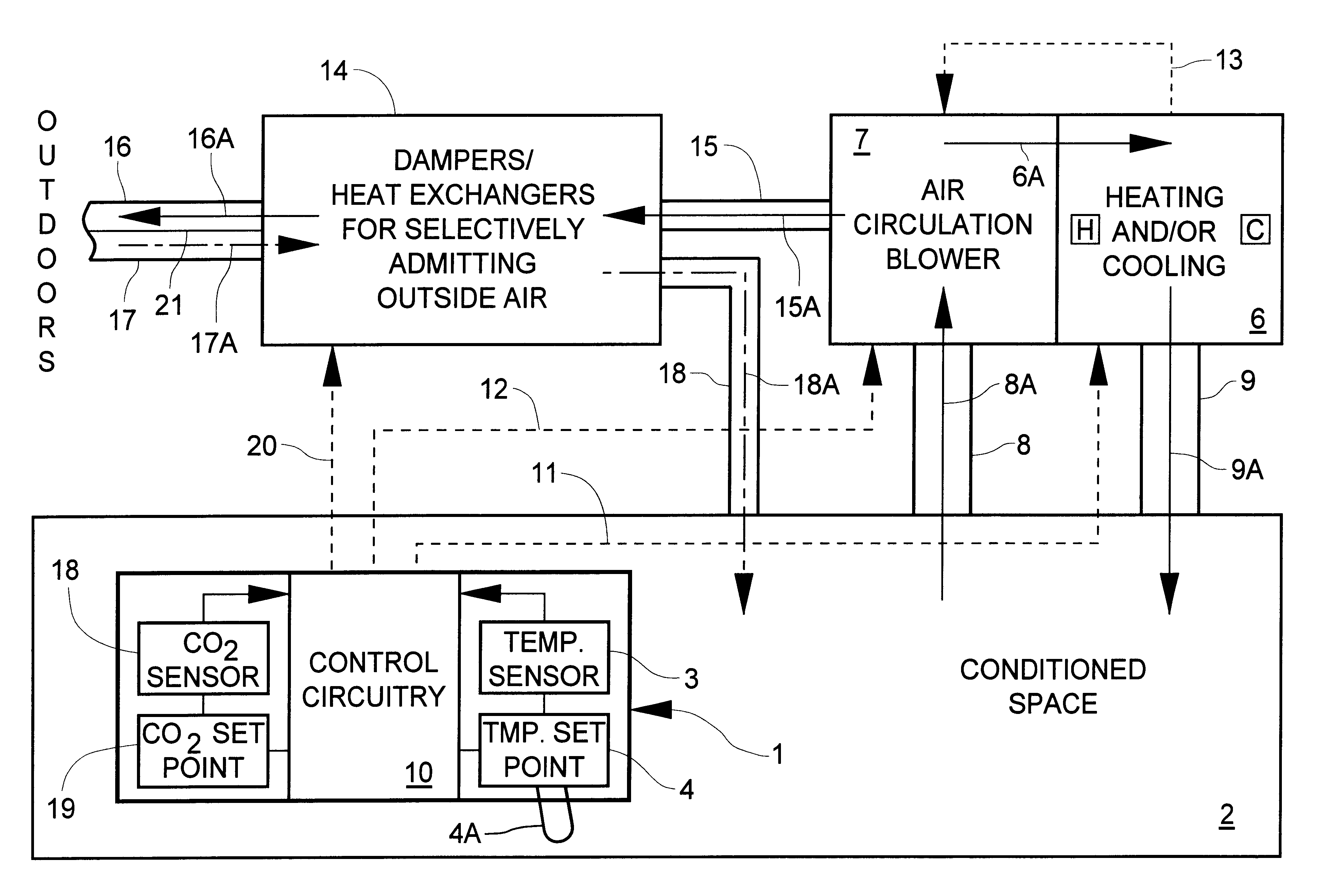
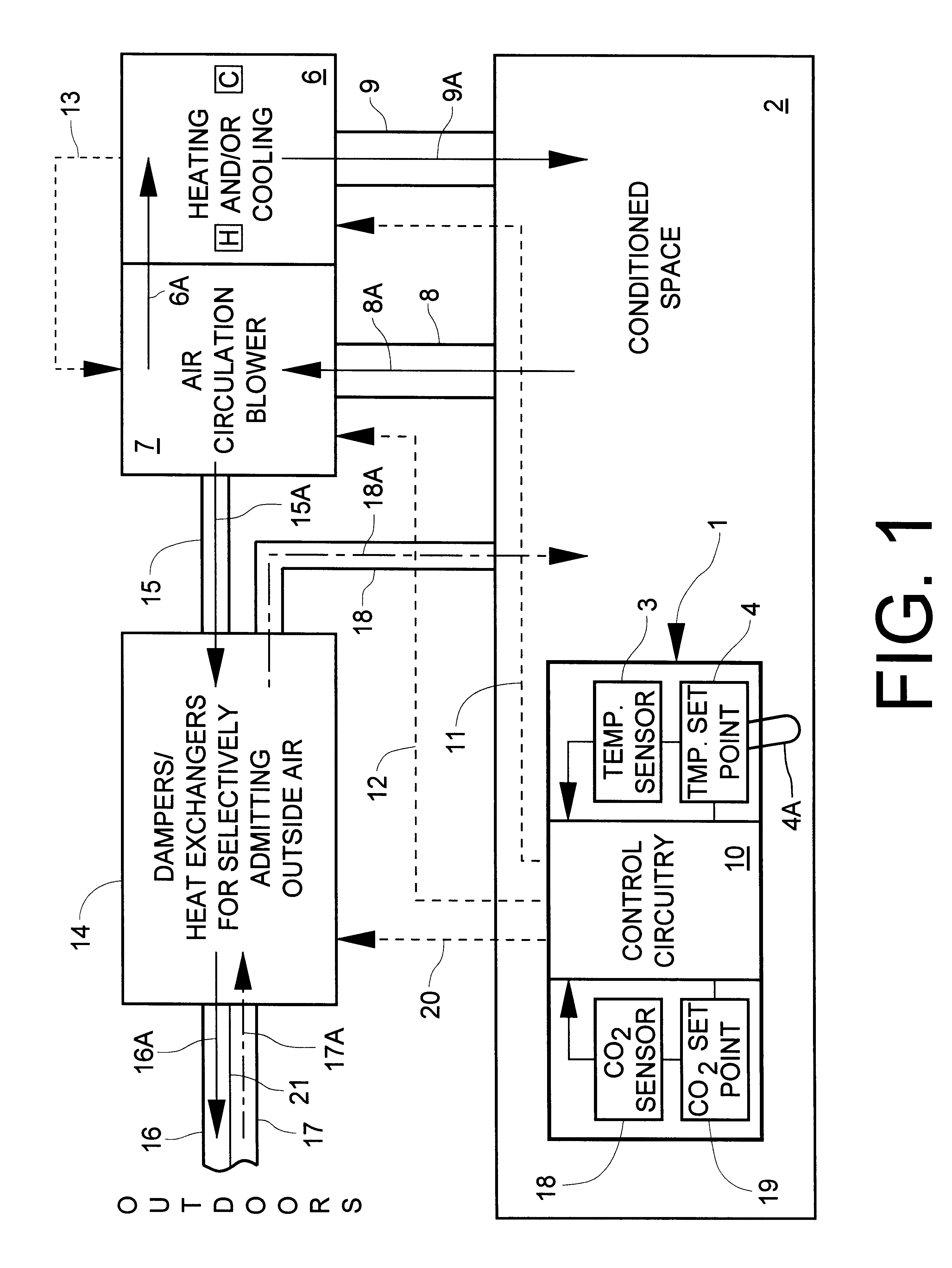
InactiveUS6398118B1Sufficiently compactSimple enoughMechanical apparatusSpace heating and ventilation safety systemsThermostatEngineering
A system for monitoring and modifying the quality and temperature of air within a conditioned space includes a blower unit, a damper unit for selectively admitting outside air into the conditioned space, a temperature moderating unit and a control unit. The control unit includes a thermostat and conventional temperature control apparatus for selectively activating the temperature moderating unit to maintain the desired temperature in the conditioned space. The control unit also incorporates CO2 concentration measuring and control apparatus which includes a small CO2 sensor. The CO2 sensor includes a cathode, an anode and a solid electrolyte disposed intermediate and electrically in contact with each of the cathode and the anode to effect a primary electrical cell. A heater and a heater thermostat serve to maintain the temperature of the cell at about 250° C. The cathode and anode materials and the chemical composition of the electrolyte are further selected such that the voltage generated across the heated cell varies in accordance with the CO2 concentration. CO2 concentration modifying apparatus is responsive to sensing a first predetermined CO2 concentration for turning on the blower unit and to sensing a second, higher, predetermined CO2 concentration for actuating the damper unit to admit outside air.
Owner:ROSEN HOWARD B +1
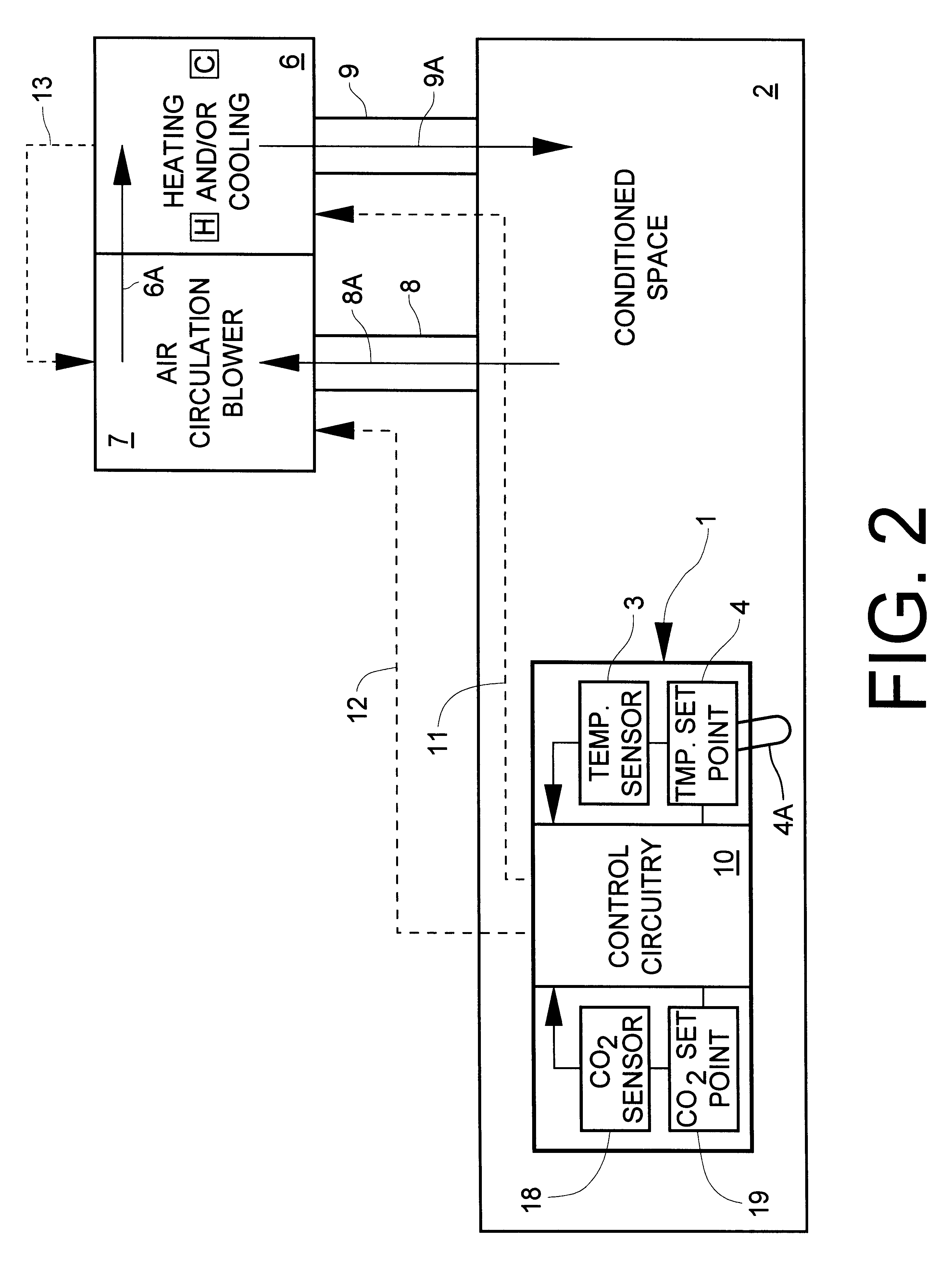
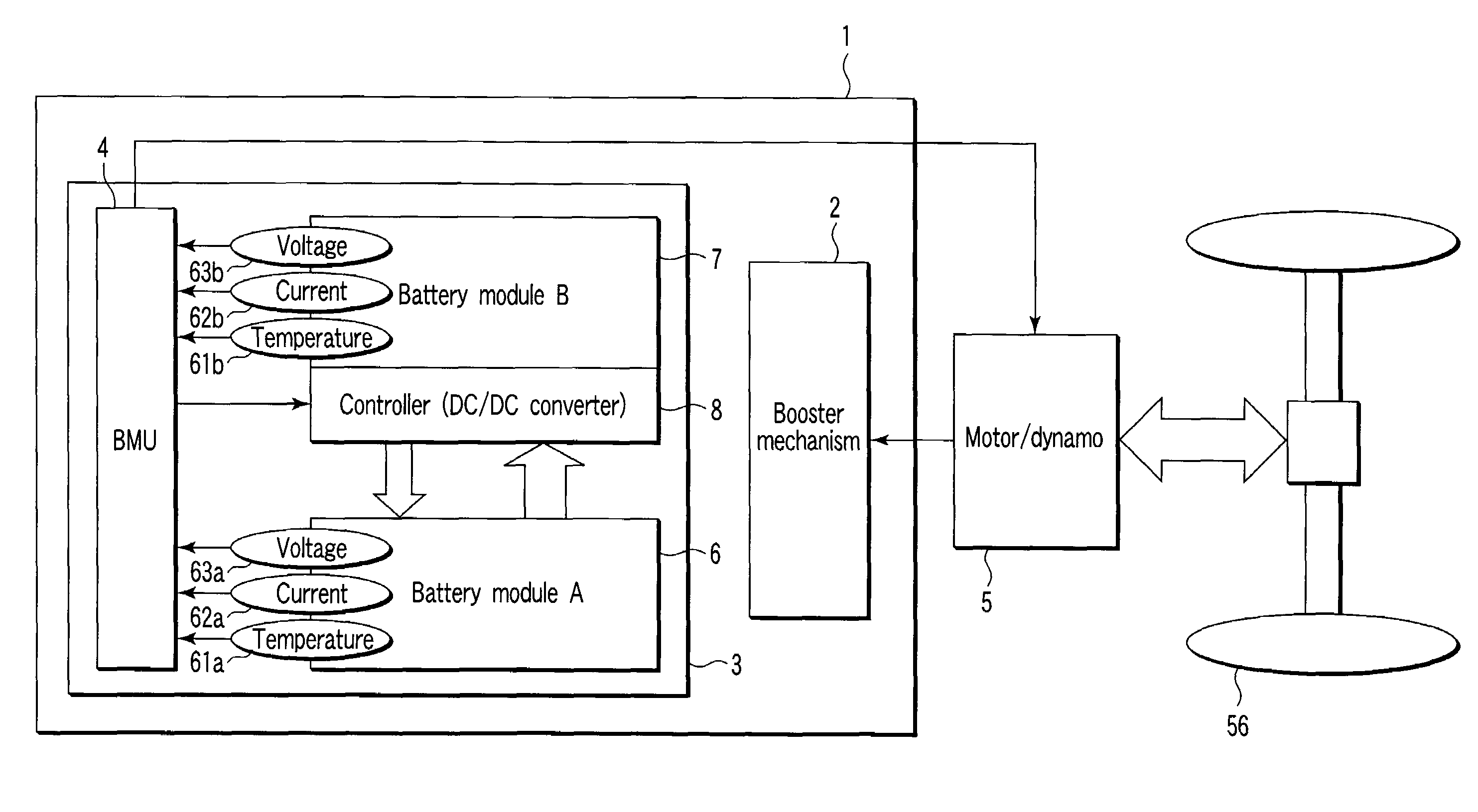
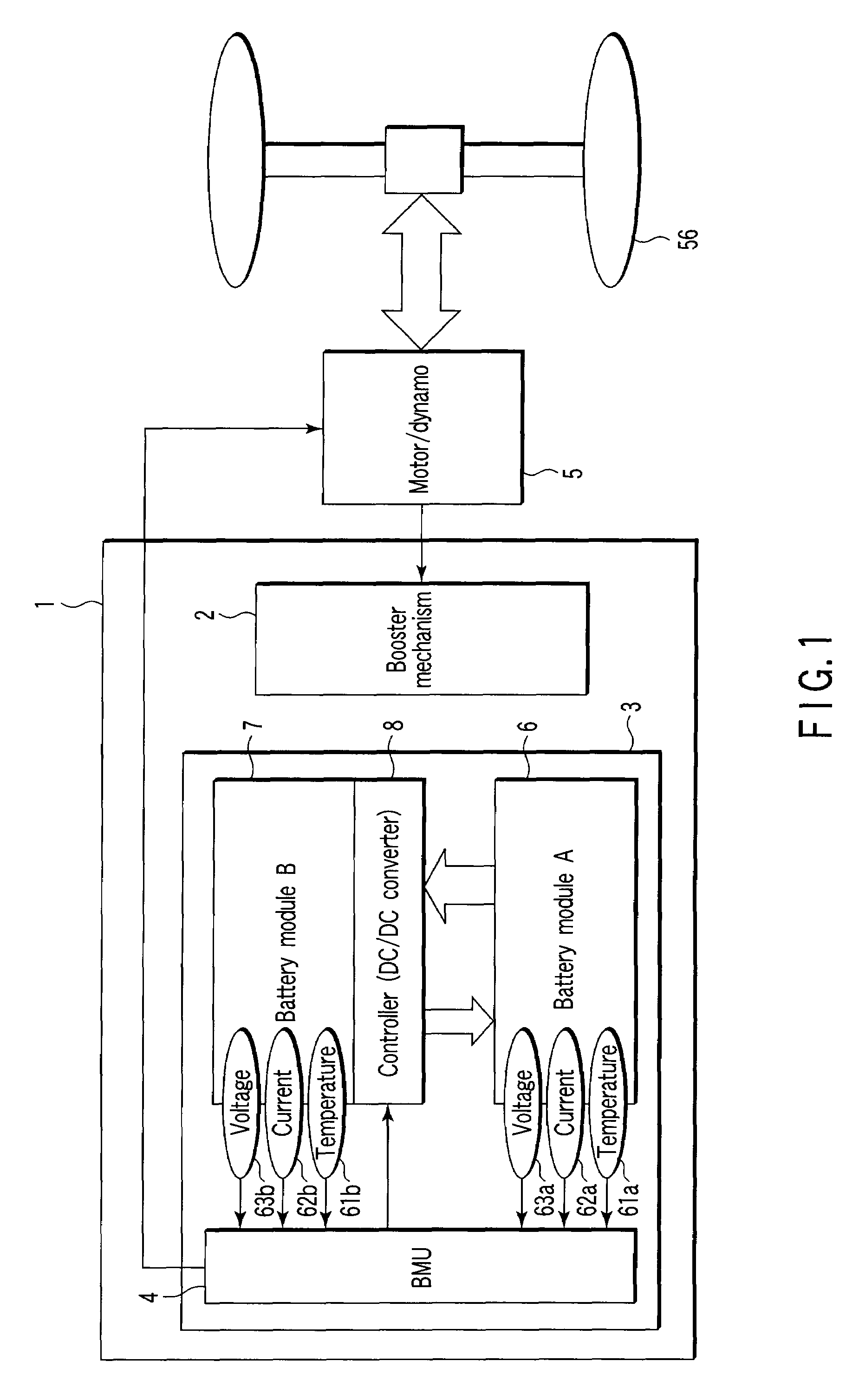
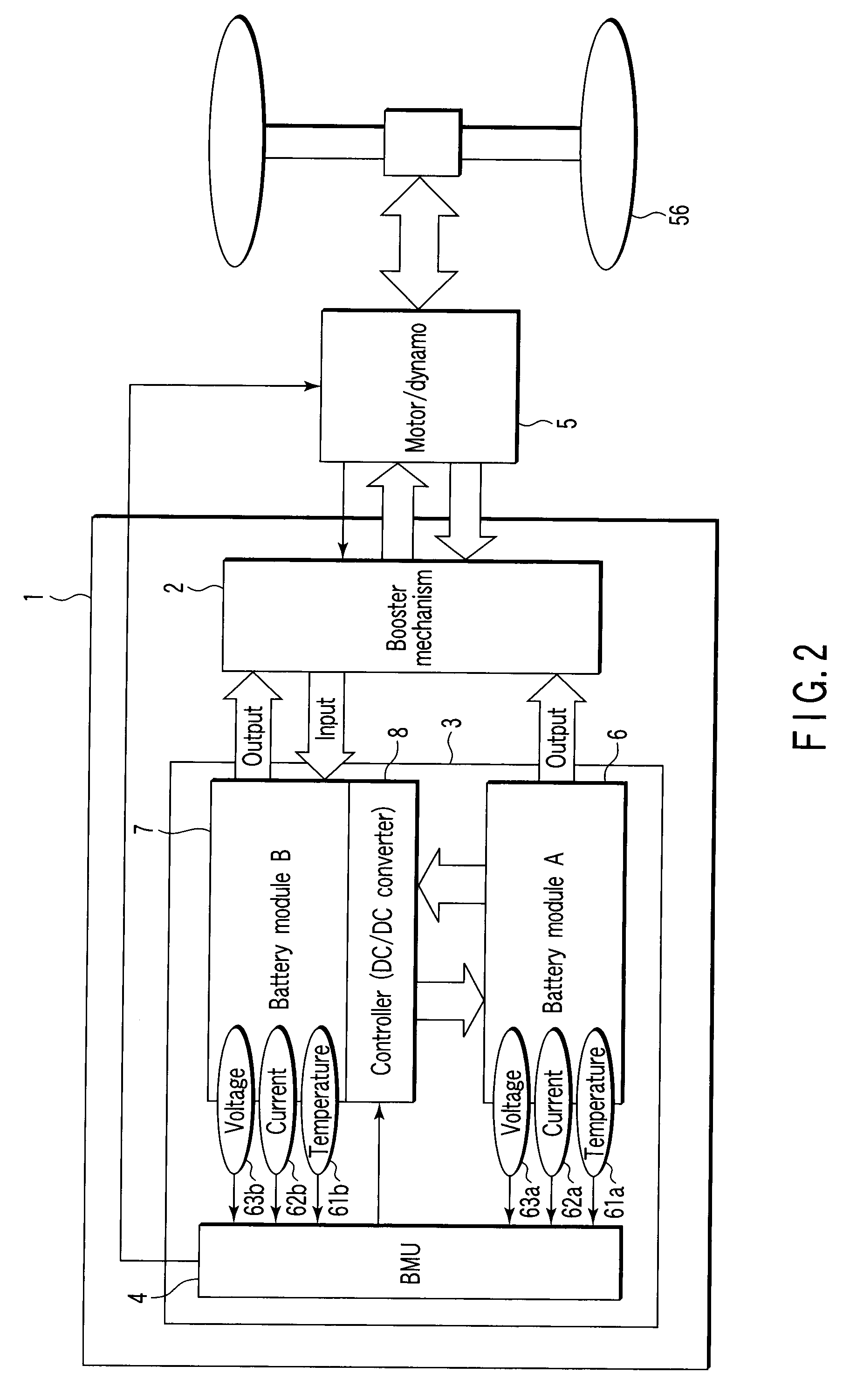
Storage battery system, on-vehicle power supply system, vehicle and method for charging storage battery system
ActiveUS20070284159A1Efficient chargingKeep for a long timeBatteries circuit arrangementsRailway vehiclesLithiumElectrical battery
A storage battery system includes a battery module A with a first nonaqueous electrolyte battery including a negative-electrode material which has an average grain size of 2 μm or more and is used to occlude and discharge lithium ions, a battery module B with a second nonaqueous electrolyte battery set at a lithium-ion-occluding potential of 0.4V (vs.Li / Li) or more, and including a negative-electrode material which has an average grain size of primary particles of 1 μm or less and is used to occlude lithium ions, and a controller configured to intermittently connect the module A to the module B to intermittently supply power from the module A to the module B to set a charge state and a discharge depth of the second nonaqueous electrolyte battery within a range of 10 to 90%, when no power is supplied to the module B at least from an outside.
Owner:KK TOSHIBA
Reversible electrochemical mirror (REM) state monitoring
Reversible electrochemical mirror (REM) devices typically comprise a conductive oxide mirror electrode that is substantially transparent to radiation of some wavelengths, a counter electrode that may also be substantially transparent, and an electrolyte that contains ions of an electrodepositable metal. A voltage applied between the two electrodes causes electrodeposition of a mirror deposit on the mirror electrode and dissolution of the mirror deposit on the counter electrode, and these processes are reversed when the polarity of the applied voltage is changed. Such REM devices provide precise control over the reflection and transmission of radiation and can be used for a variety of applications, including smart windows and automatically adjusting automotive mirrors. According to the present invention, measurements of the sheet resistance of the mirror electrode in a REM device are correlated with the thickness of electrodeposited mirror metal and can be used to monitor the reflectance of the device. Sheet resistance measurements can be performed while the mirror state of the device is being switched if adequate isolation between the measurement and switching circuits is provided. This can be accomplished by use of external resistors or more sophisticated circuitry, or by taking advantage of the relatively high sheet resistance of the mirror electrode itself. Monitoring the reflectance of REM devices according to this invention provides significant cost and performance advantages.
Owner:TELEDYNE SCI & IMAGING
Separator for Electrochemical Device, and Electrochemical Device
ActiveUS20070264577A1Improve securityCell electrodesSecondary cellsPhysical chemistryElectrochemistry
An electrochemical device having excellent safety at high temperature is provided by using a separator for an electrochemical device, which is made of a porous film comprising: a porous base (5) having a heat-resistant temperature of 150° C. or higher and including filler particles (3); at least one kind of shutdown resin (6) selected from the group consisting of resin A that has a melting point in a range of 80° C. to 130° C. and resin B that absorbs an electrolyte and swells due to heating, and the swelling degree is increased as the temperature rises; and a binder (4).
Owner:MAXELL HLDG LTD
Electrode Apparatus For Detecting Brain Waves And Package
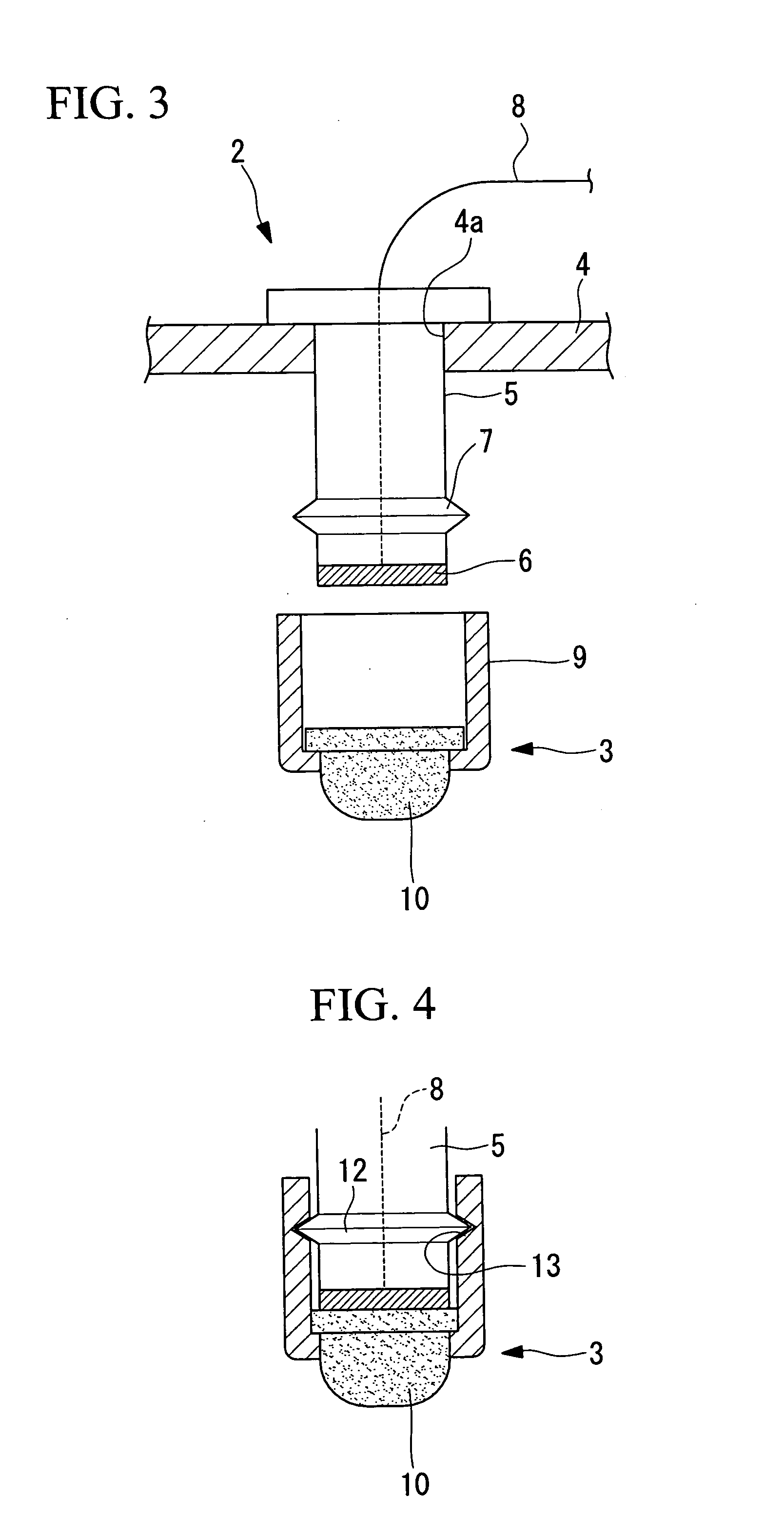
The labor involved in cleaning for each examination is reduced, the examination can be carried out hygienically, and the convenience of use is improved. An electrode apparatus for detecting brain waves which is arranged in contact with the scalp and which detects brain wave signals is provided. The electrode apparatus for detecting brain waves comprises a rod-shaped electrode apparatus main body having an electrode disposed at the tip thereof, a cap which is mountable on the tip of the electrode apparatus main body and which has an elastic member which contains an electrolyte and which is disposed making close contact so as to cover the electrode, and a connection means which detachably connects the cap to the electrode apparatus main body.
Owner:OLYMPUS CORP +1
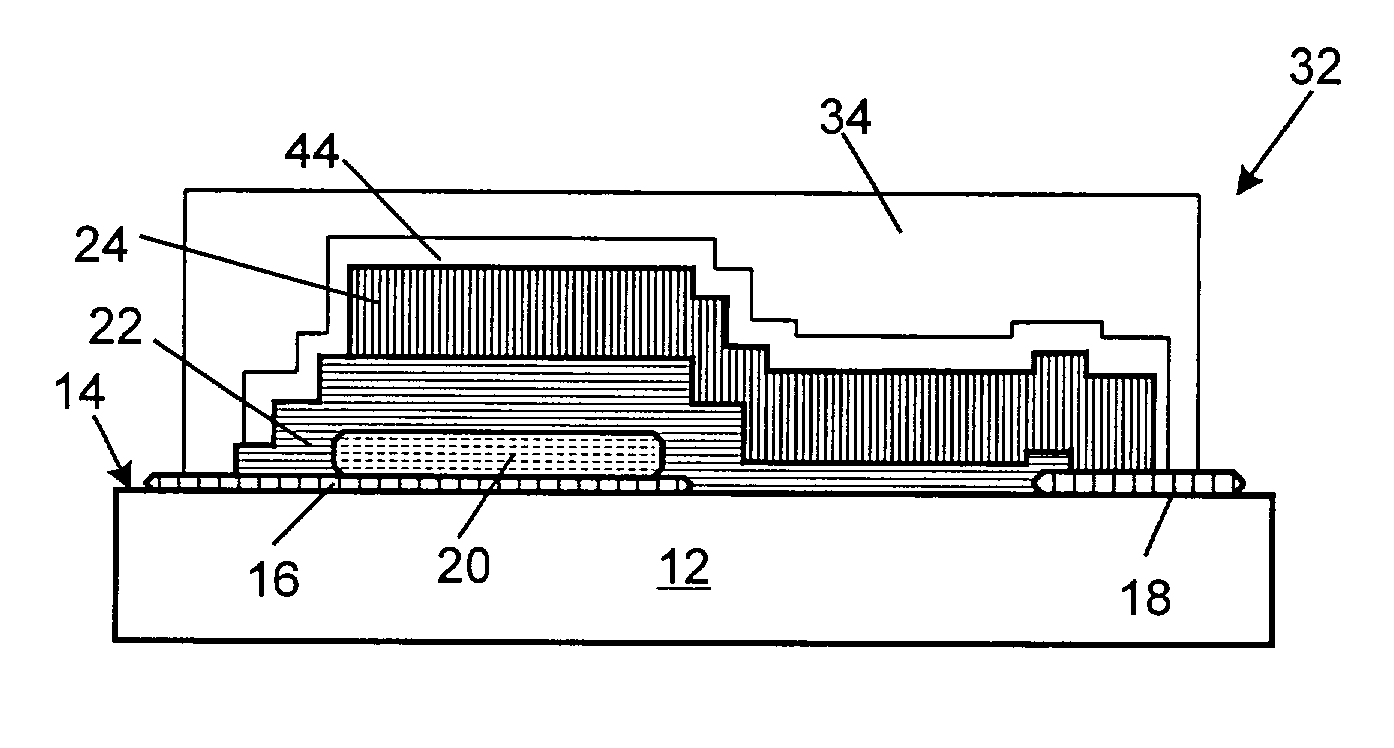
Flat capacitor for an implantable medical device
InactiveUS6699265B1Liquid electrolytic capacitorsCapacitor terminalsCapacitanceSemipermeable membrane
One embodiment includes a capacitor having a first anode stack having a first number of anode foils, a second anode stack having a second number of anode foils, where the first number of anode foils is different than the second number of anode foils. Another aspect provides a capacitor having a case having a curved interior surface, and first, second, and third capacitor modules that confront the curved interior surface of the case. One aspect provides a capacitor having one or more anodes and a cathode structure comprising a plurality of integrally connected cathode plates, the cathode structure having a serpentine shape, interweaving under and over each of the one or more anodes. One aspect provides a feedthrough assembly having an electrically conductive member dimensioned to extend at least partially through a feedthrough hole of a case of the capacitor, the conductive member having a passage therethrough. One aspect provides a capacitor having a first stack of capacitive elements a second stack of capacitive elements, wherein the first and second stacks are enclosed in separate compartments of a capacitor case that electrically isolate the electrolytes of each stack from one another. One aspect provides a capacitor case including a portion having opposing interior and exterior surfaces, with the portion having a hole; and a semi-permeable membrane adjacent the hole to regulate passage of fluids through the hole.
Owner:CARDIAC PACEMAKERS INC
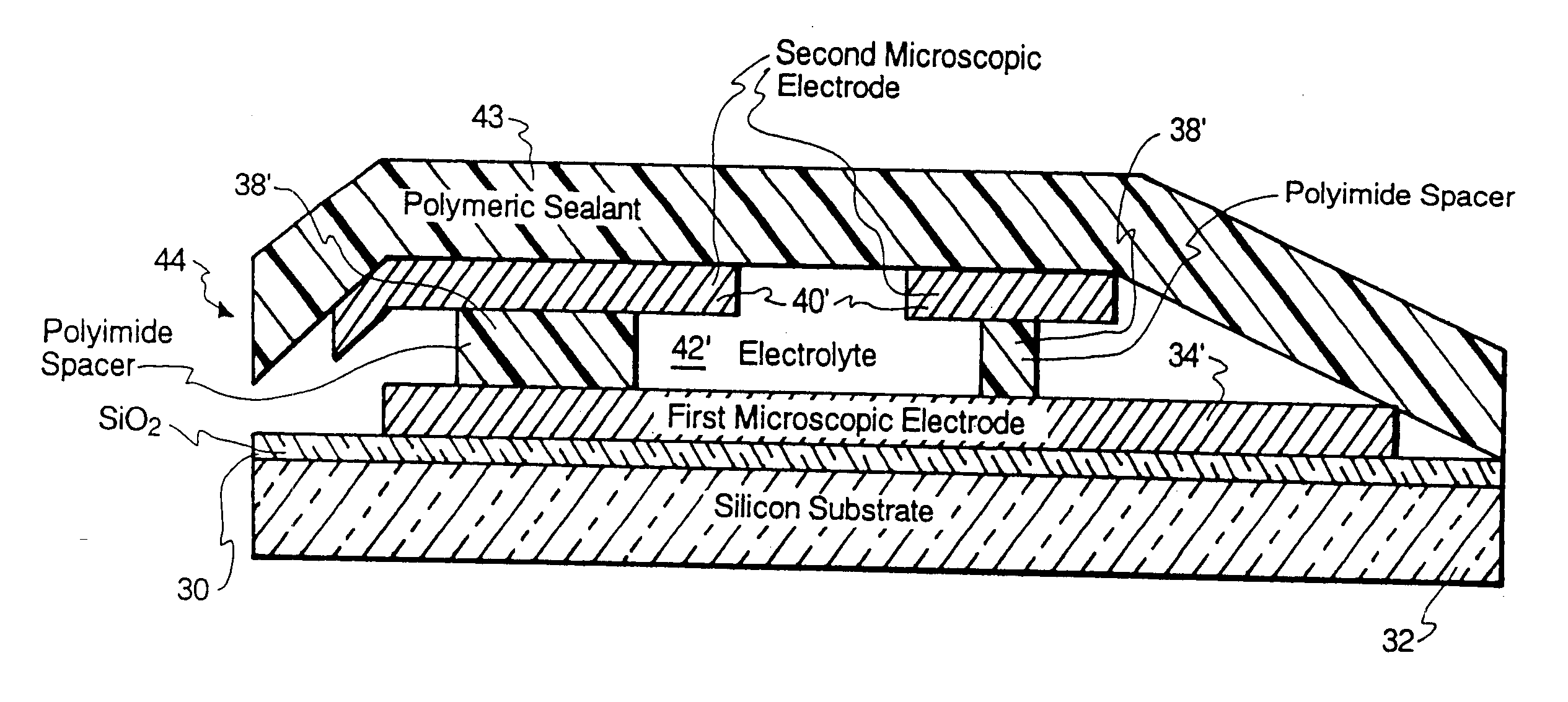
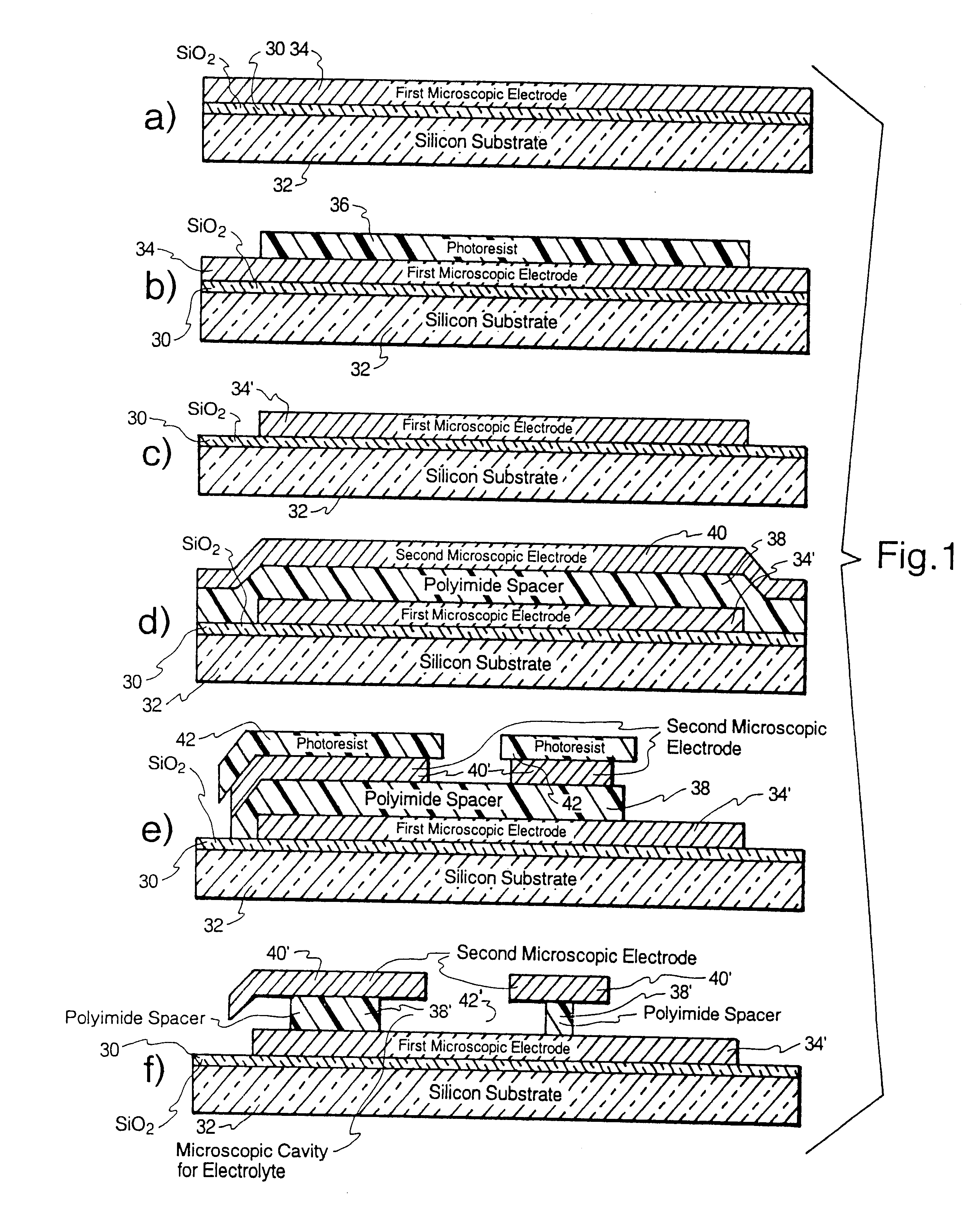
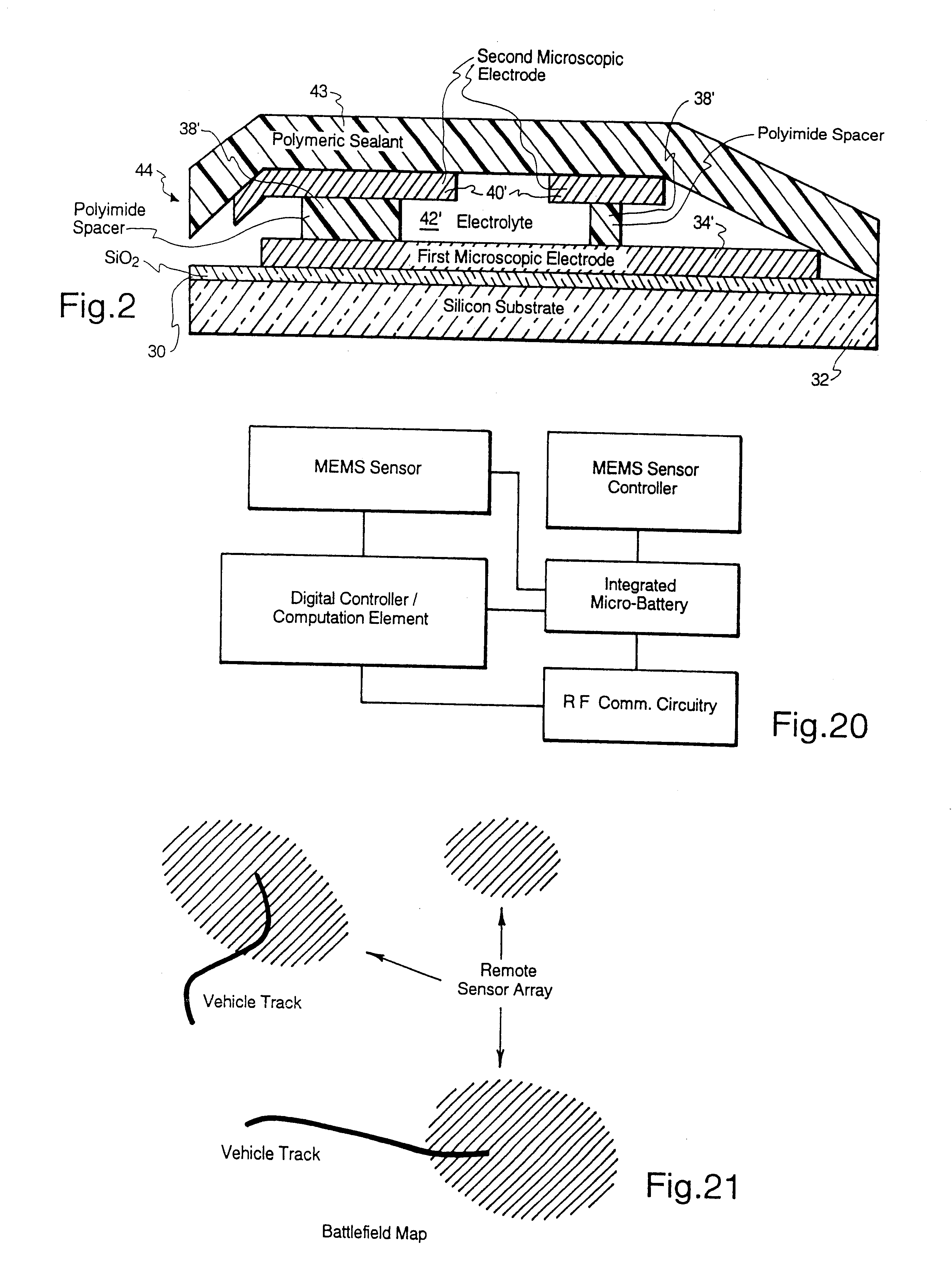
Microscopic batteries for MEMS systems
InactiveUS6610440B1Reduce power lossIncrease powerBatteries circuit arrangementsFinal product manufactureElectricityMicrofabrication
Microscopic batteries, integratable or integrated with microelectromechanical systems or other microscopic circuits, including a MEMS microcircuit, and methods of microfabrication of such microscopic batteries are disclosed, among which comprise closed system microscopic batteries for internal storage of electricity using interval reactants only, which comprise microscopic electrodes, electrolyte and reservoir for the electrolyte.
Owner:BIPOLAR TECH
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
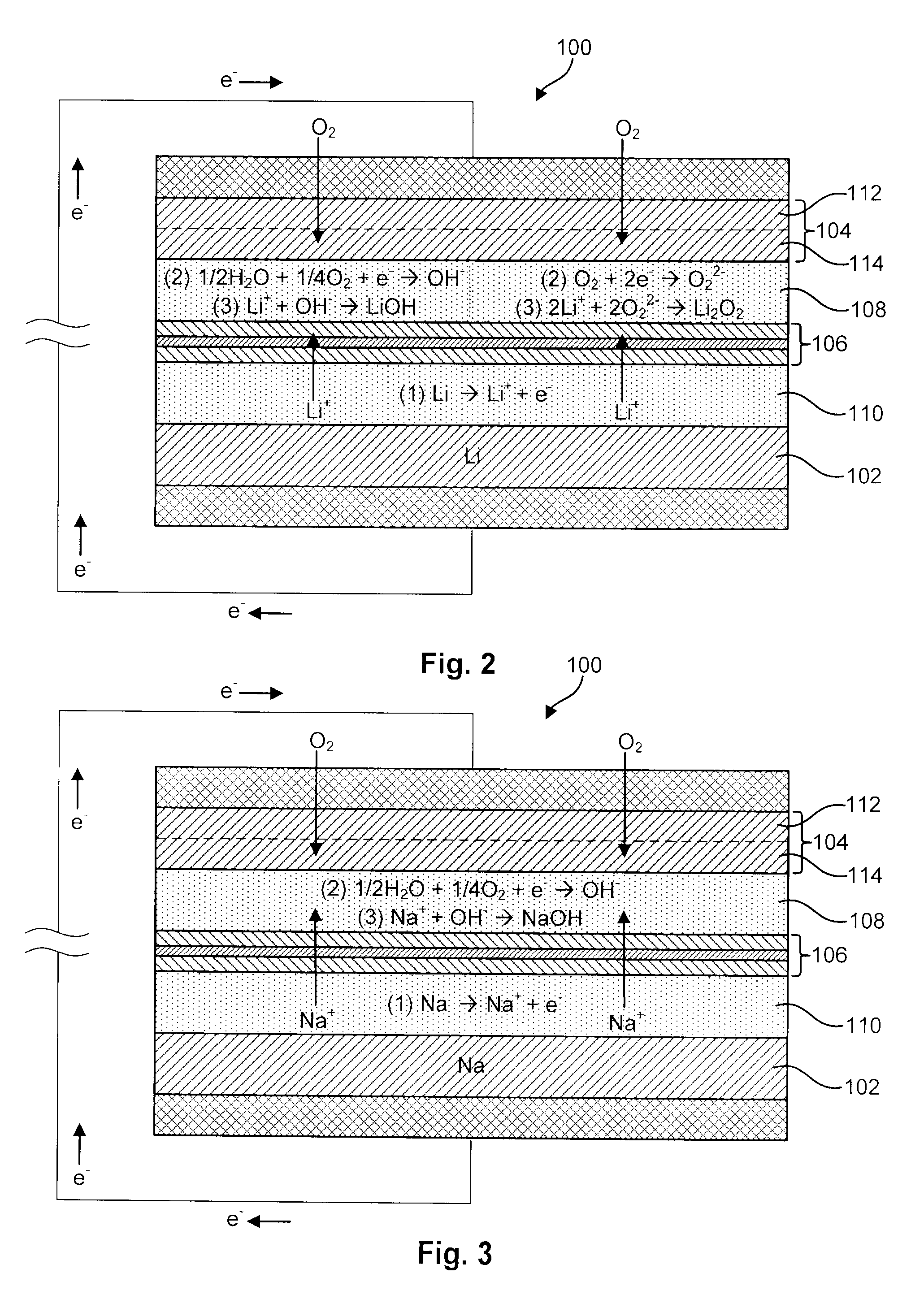
Advanced Metal-Air Battery Having a Ceramic Membrane Electrolyte Background of the Invention
ActiveUS20080268327A1Reduce oxygenReduce layeringFuel and primary cellsSolid electrolytesOxygenCeramic membrane
A metal-air battery is disclosed in one embodiment of the invention as including a cathode to reduce oxygen molecules and an alkali-metal-containing anode to oxidize the alkali metal (e.g., Li, Na, and K) contained therein to produce alkali-metal ions. An aqueous catholyte is placed in ionic communication with the cathode to store reaction products generated by reacting the alkali-metal ions with the oxygen containing anions. These reaction products are stored as solutes dissolved in the aqueous catholyte. An ion-selective membrane is interposed between the alkali-metal containing anode and the aqueous catholyte. The ion-selective membrane is designed to be conductive to the alkali-metal ions while being impermeable to the aqueous catholyte.
Owner:FIELD UPGRADING USA INC
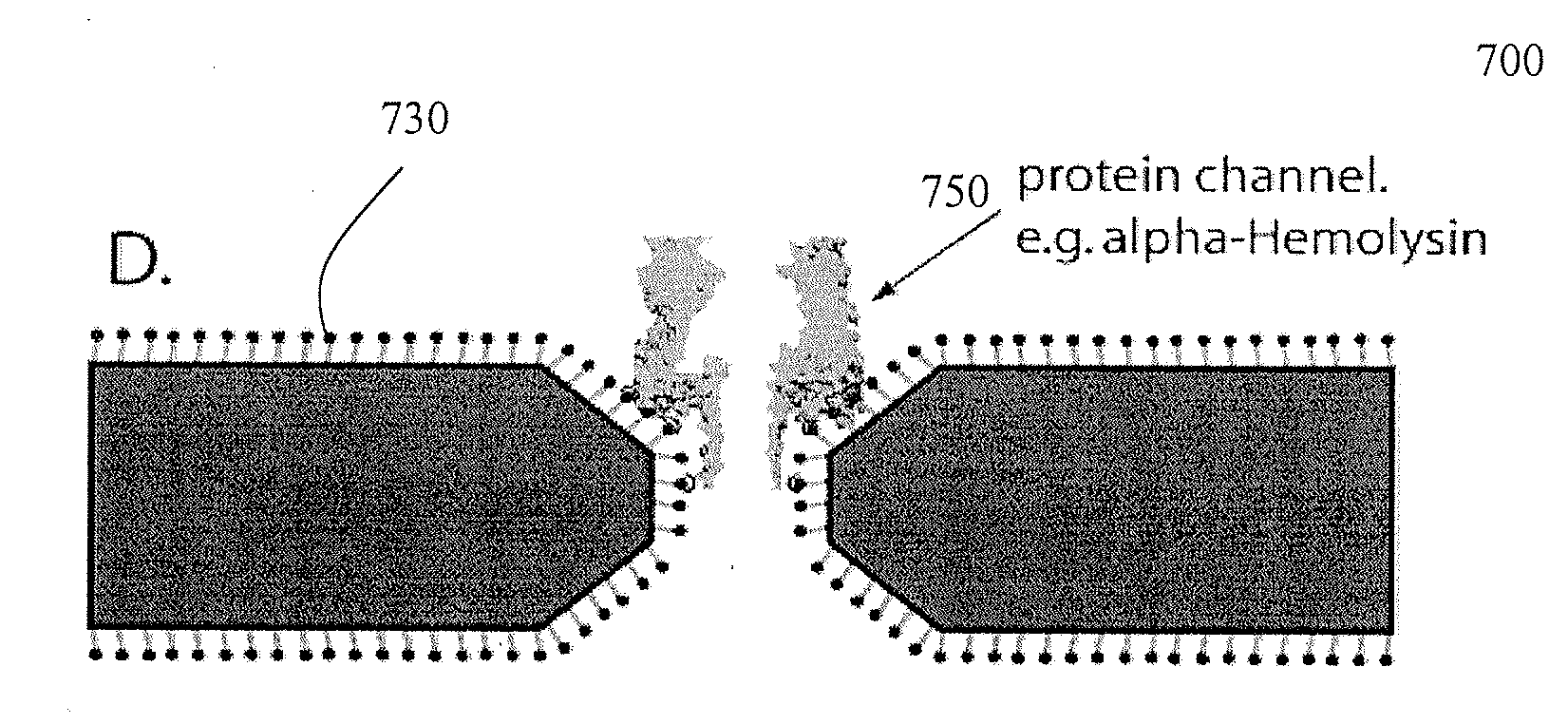
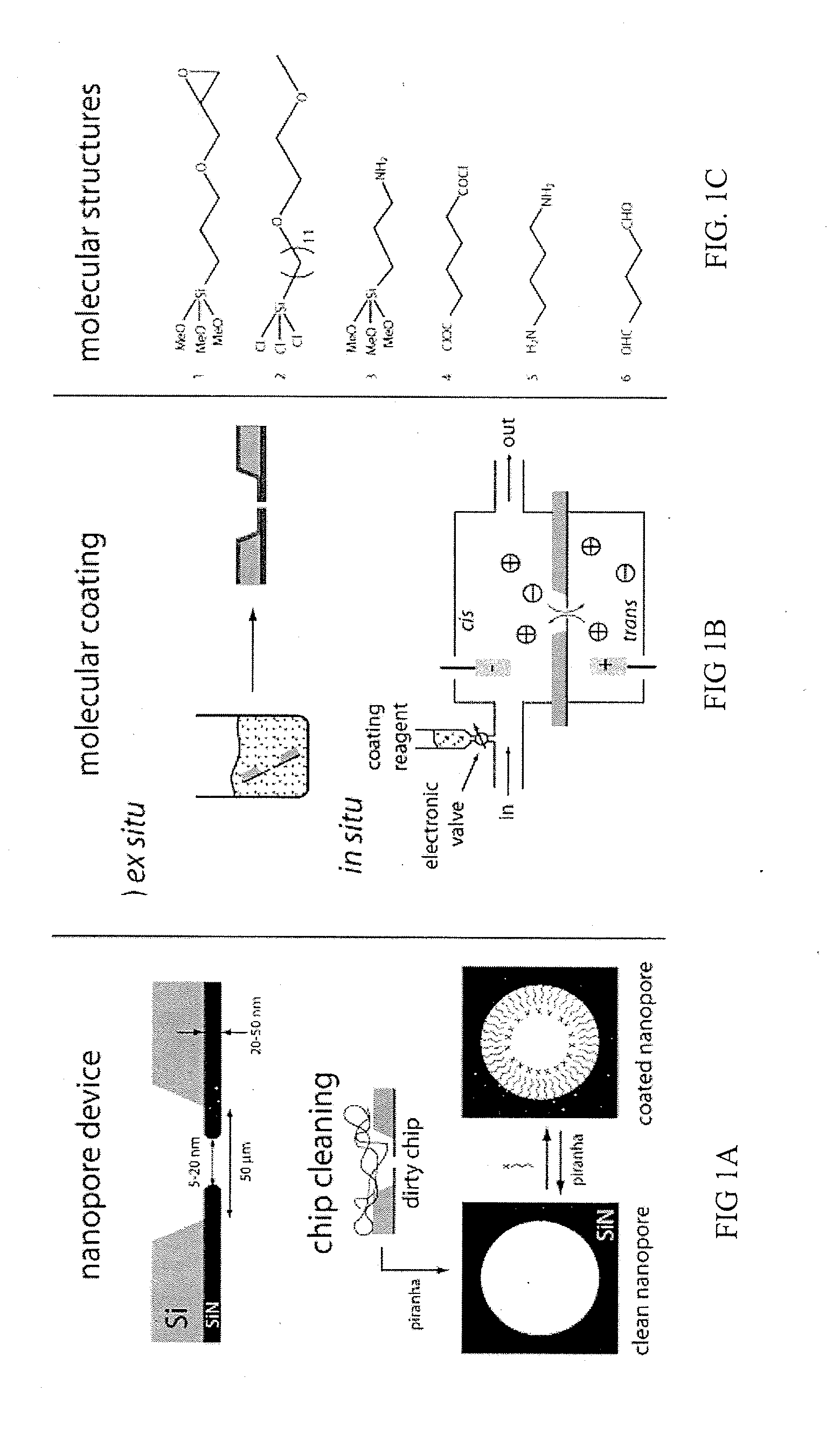
Chemical functionalization of solid-state nanopores and nanopore arrays and applications thereof
ActiveUS20110053284A1Avoid stickingChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansBiopolymerElectron
Chemical functionalization of solid-state nanopores and nanopore arrays and applications thereof. Nanopores are extremely sensitive single-molecule sensors. Recently, electron beams have been used to fabricate synthetic nanopores in thin solid-state membranes with sub-nanometer resolution. A new class of chemically modified nanopore sensors are provided with two approaches for monolayer coating of nanopores by: (1) self-assembly from solution, in which nanopores −10 nm diameter can be reproducibly coated, and (2) self-assembly under voltage-driven electrolyte flow, in which 5 nm nanopores may be coated. Applications of chemically modified nanopore are provided including: the detection of biopolymers such as DNA and RNA; immobilizing enzymes or other proteins for detection or for generating chemical gradients; and localized pH sensing.
Owner:TRUSTEES OF BOSTON UNIV
Liquid Composite Compositions Using Non-Volatile Liquids and Nanoparticles and Uses Thereof
InactiveUS20080209876A1Reduce CTEImprove performanceElectrolytic capacitorsCell electrodesOrganic solventNanoparticle
A solvent composition comprising an organic solvent; dispersed nanoparticles; and a non-volatile electrolyte.
Owner:ESIONIC
Composite solid polymer elecrolyte membranes
InactiveUS20020045085A1Optimize swellingOptimize fuel crossover resistanceElectrolyte holding meansFinal product manufacturePolymer electrolytesPolymer science
The present invention relates to composite solid polymer electrolyte membranes (SPEMs) which include a porous polymer substrate interpenetrated with an ion-conducting material. SPEMs of the present invention are useful in electrochemical applications, including fuel cells and electrodialysis.
Owner:FOSTER-MILLER
Microporous PVDF films
Shaped microporous articles are produced from polyvinylidene fluoride (PVDF) and nucleating agents using thermally induced phase separation (TIPS) processes. The shaped microporous article is oriented in at least one direction at a stretch ratio of at least approximately 1.1 to 1.0. The shaped article may also comprise a diluent, glyceryl triacetate. The shaped microporous article may also have the micropores filled with a sufficient quantity of ion conducting electrolyte to allow the membrane to function as an ion conductive membrane. The method of making a microporous article comprises the steps of melt blending polyvinylidene fluoride, nucleating agent and glyceryl triacetate; forming a shaped article of the mixture; cooling the shaped article to cause crystallization of the polyvinylidene fluoride and phase separation of the polyvinylidene fluoride and glyceryl triacetate; and stretching the shaped article in at least one direction at a stretch ratio of at least approximately 1.1 to 1.0.
Owner:3M INNOVATIVE PROPERTIES CO
High-capacity lithium-ion electrolyte, battery and preparation method of battery
ActiveCN101771167AIncrease capacityLow costFinal product manufactureCell electrodesElectrolytic agentPhysical chemistry
The invention relates to a high-capacity lithium-ion electrolyte, a battery and a preparation method of a battery, in particular to the high-capacity lithium-ion electrolyte and the battery using the high-capacity lithium-ion electrolyte and the preparation method of the battery. The electrolyte disclosed by the invention comprises lithium salt and non-aqueous organic solvent, and also consists of the following components in weight percent in terms of the total weight of the electrolyte: 0.5-7% of film-forming additive, 0-15% of flame-retardant additive, 2-10% of antiovefill additive, 0.01-2% of stabilizer and 0.01-1% of wetting agent; the electrolyte can enable the anode with high Ni content to work stably, and reduce the battery cost; the high-capacity lithium-ion battery can perform high ratio capacity and excellent safety and high temperature property and cyclic life fully due to the addition and synergetic functions of various functional additives.
Owner:JIUJIANG TINCI ADVANCED MATERIALS CO LTD
Ceramic material and process for producing the same
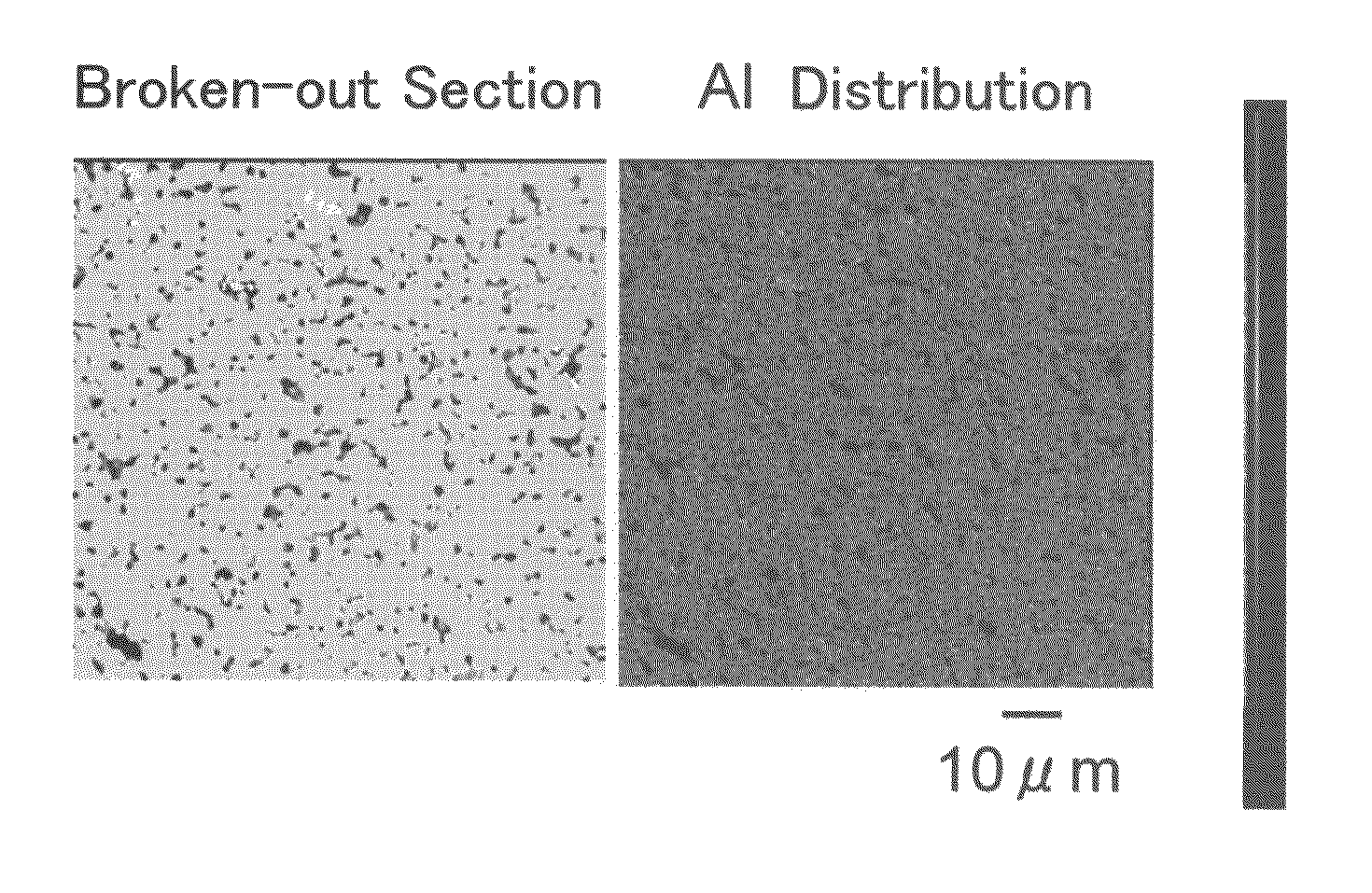
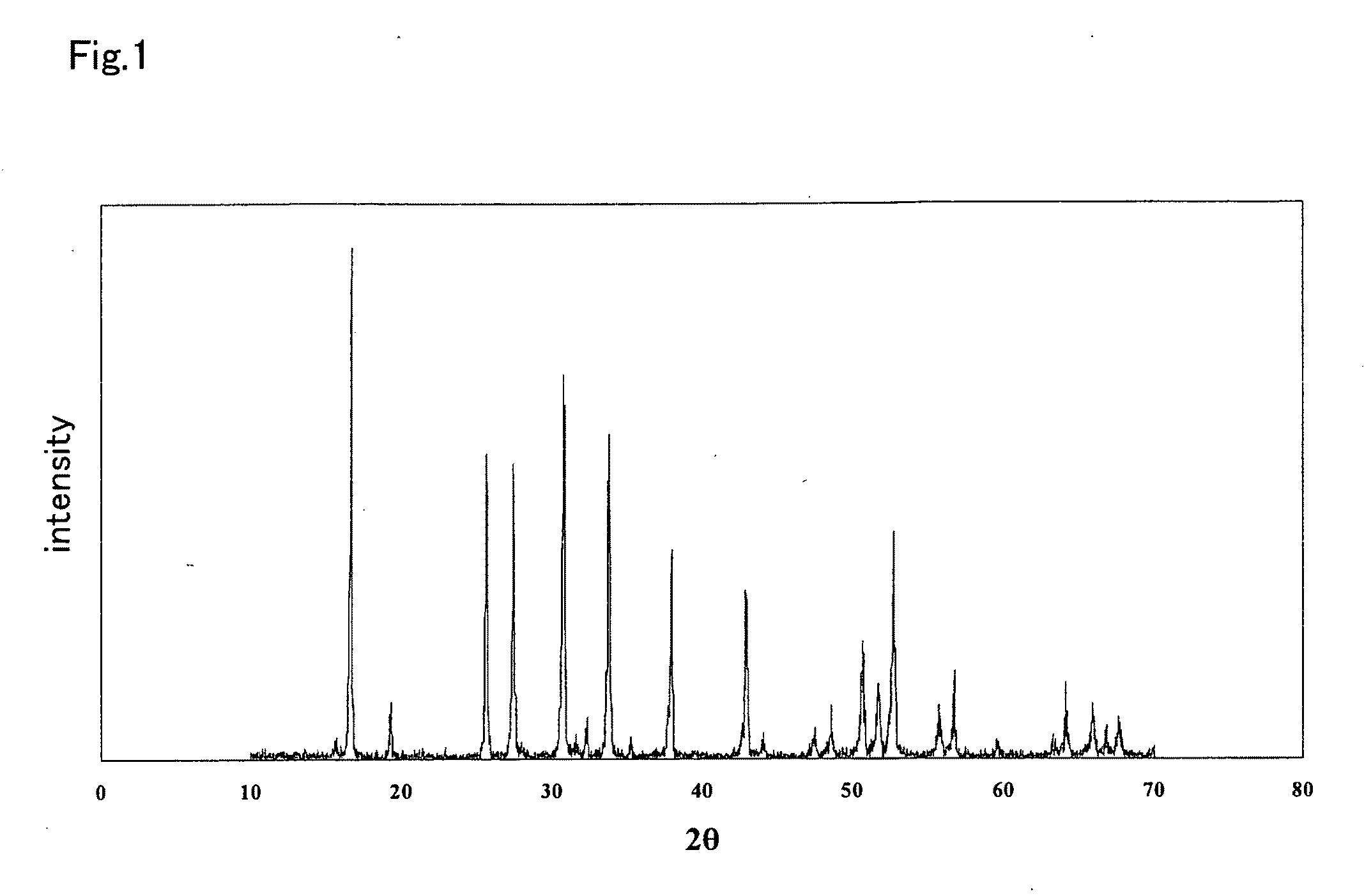
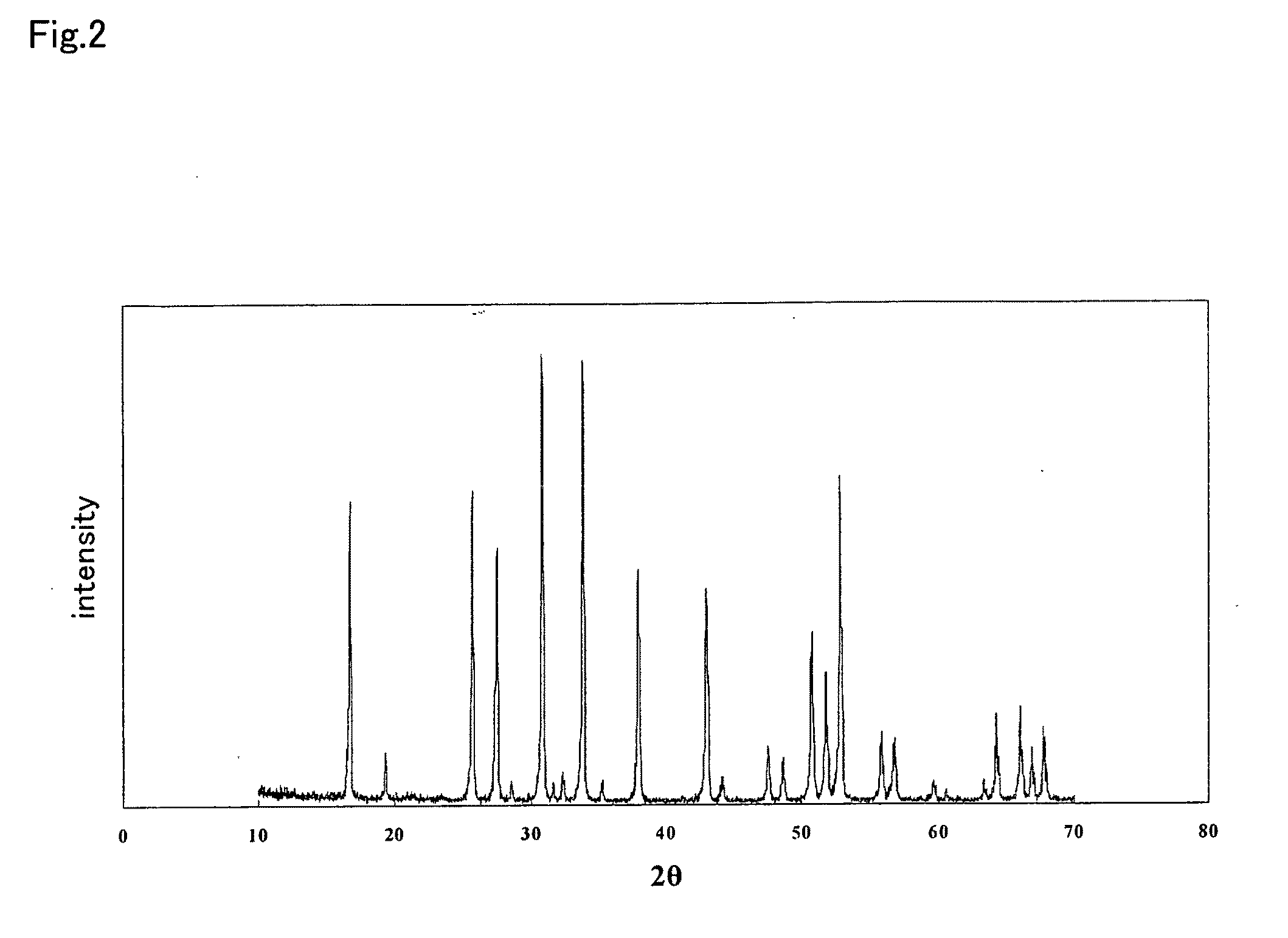
ActiveUS20100047696A1Compactness sufficientOvercome lack of conductivitySolid electrolyte cellsCapacitor electrolytes/absorbentsLithiumCrystal structure
A ceramic material that can exhibit sufficient compactness and lithium (Li) conductivity to enable the use thereof as a solid electrolyte material for a lithium secondary battery and the like is provided. The ceramic material contains aluminum (Al) and has a garnet-type crystal structure or a garnet-like crystal structure containing lithium (Li), lanthanum (La), zirconium (Zr) and oxygen (O).
Owner:NGK INSULATORS LTD
Long life thin film battery and method therefor
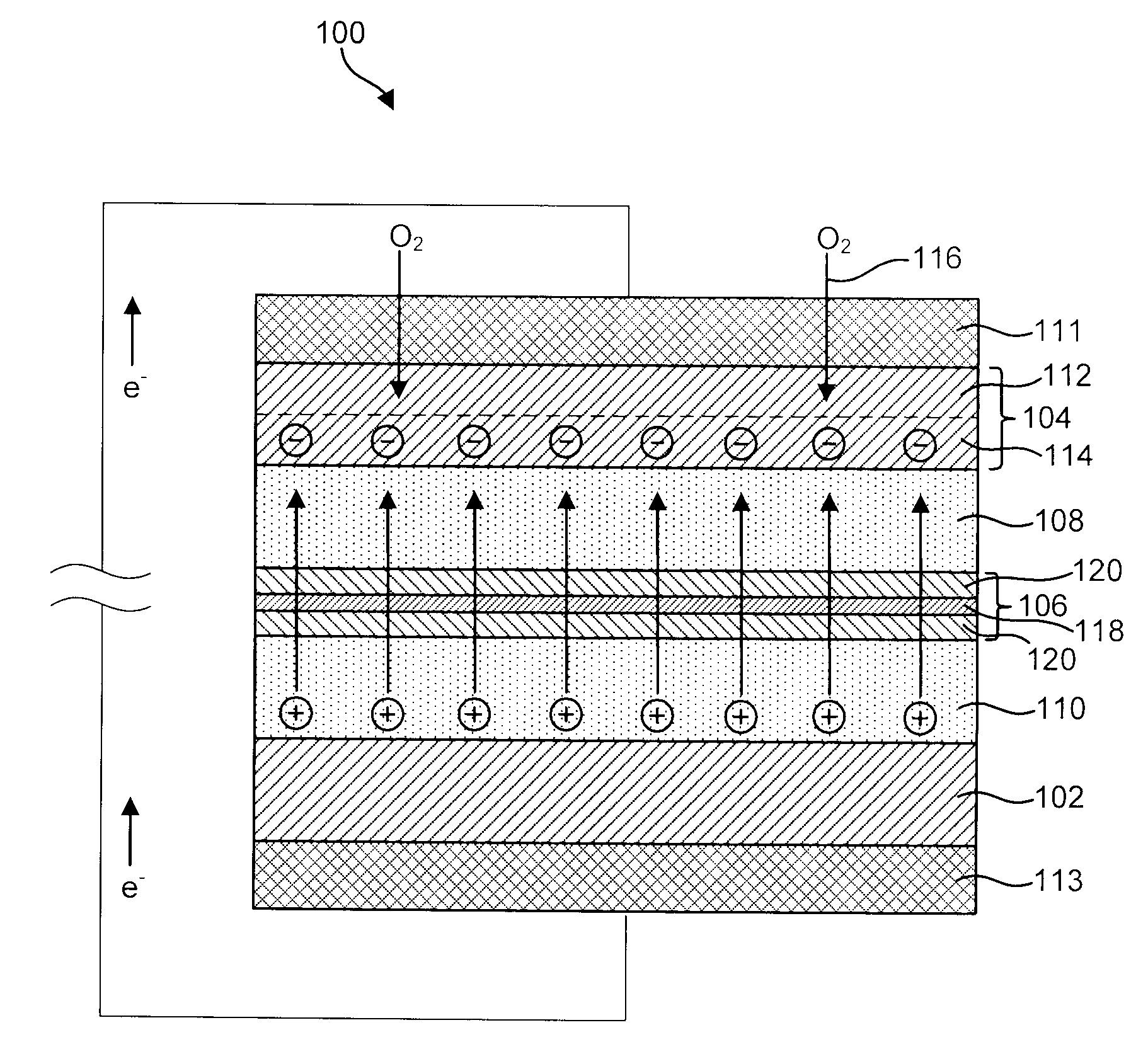
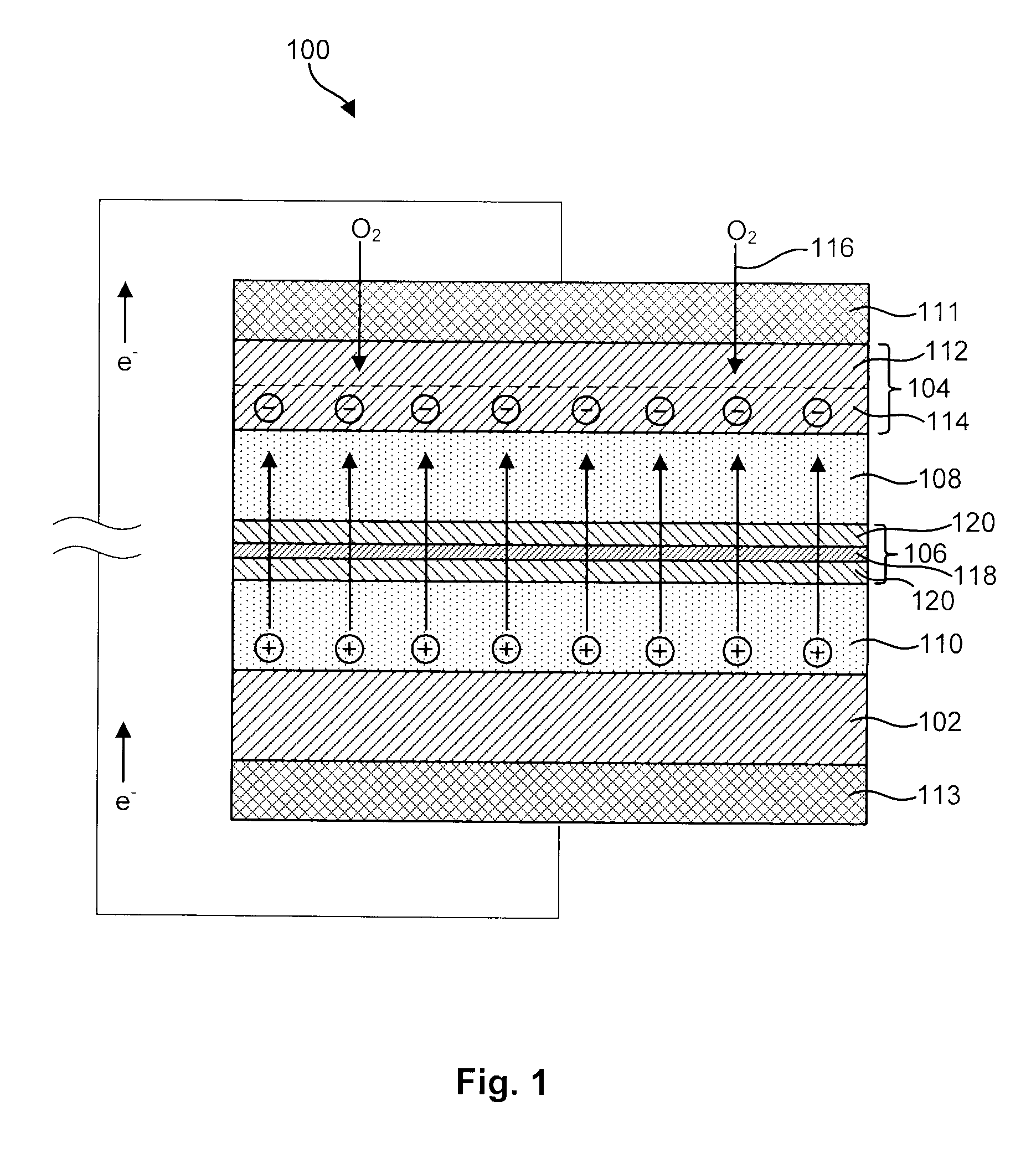
InactiveUS6994933B1Extend battery lifeSmall-sized cells cases/jacketsLarge-sized cells cases/jacketsOxygen fluxSurface roughness
A thin film battery including an anode layer, a cathode layer and a solid electrolyte layer. The battery also includes, a planarization layer applied to the thin film battery. The planarization layer has a surface roughness of no more than about 1.0 nanometers root mean square and a flatness no larger than about 0.005 cm / inch. A barrier layer is applied to the planarization layer. The barrier layer is provided by one or more layers of material selected from the group consisting of polymeric materials, metals and ceramic materials. The planarization layer and barrier layer are sufficient to reduce oxygen flux through the barrier layer to the anode layer to no more than about 1.6 μmol / m2-day, and H2O flux through the barrier layer to the anode layer to less than about 3.3 μmol / m2-day thereby improving the life of the thin film battery.
Owner:OAK RIDGE MICRO ENERGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com