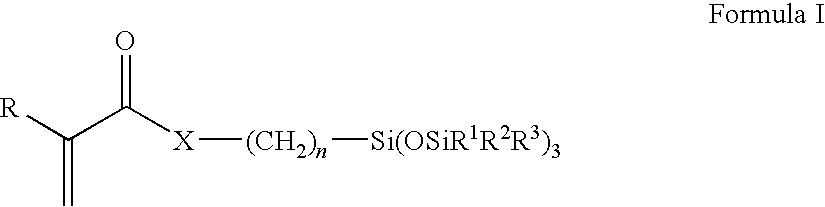Patents
Literature
10681 results about "Diluent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A diluent (also referred to as a filler, dilutant or thinner) is a diluting agent. Certain fluids are too viscous to be pumped easily or too dense to flow from one particular point to the other. This can be problematic, because it might not be economically feasible to transport such fluids in this state. To ease this restricted movement, diluents are added. This decreases the viscosity of the fluids, thereby also decreasing the pumping/transportation costs.
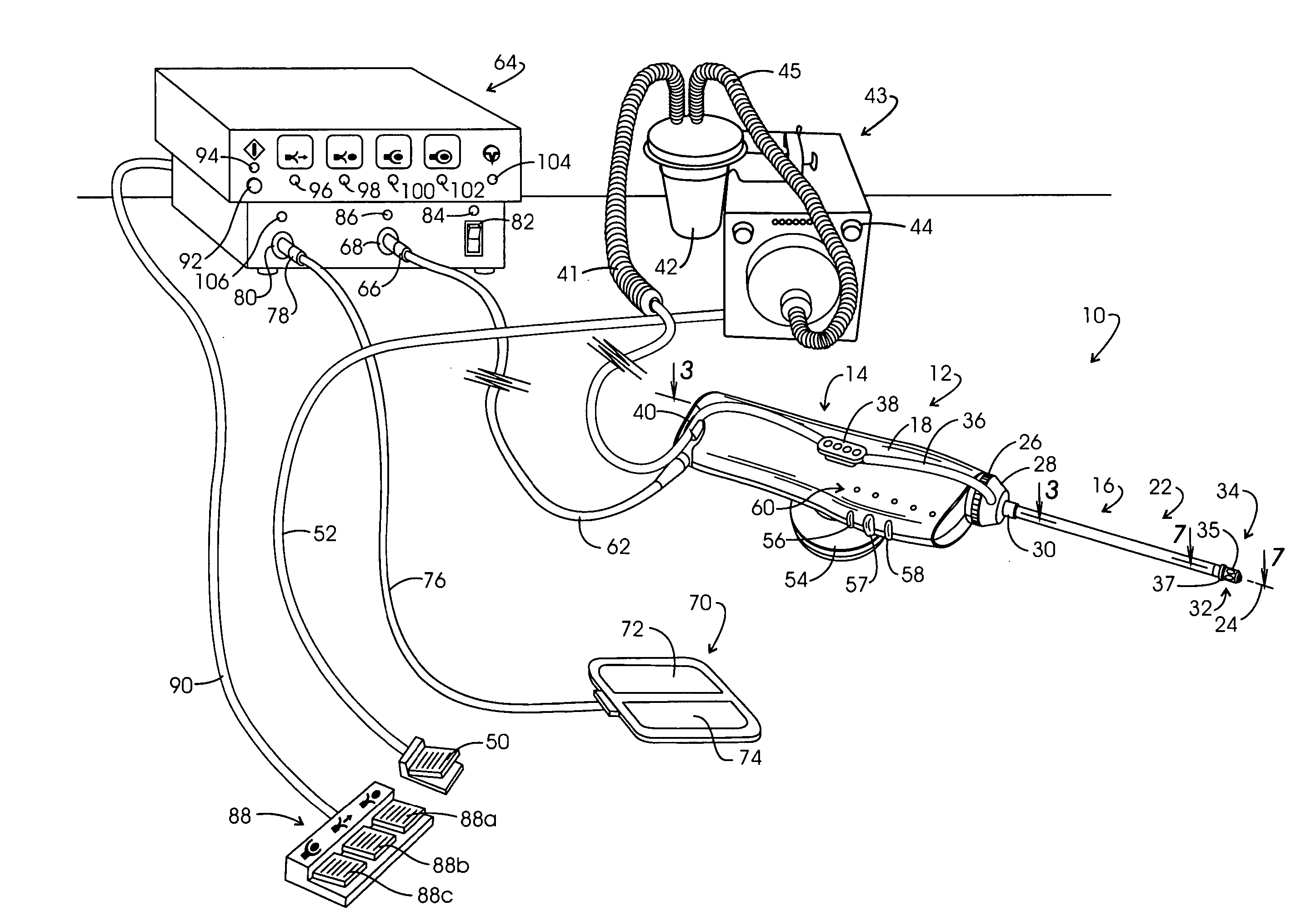
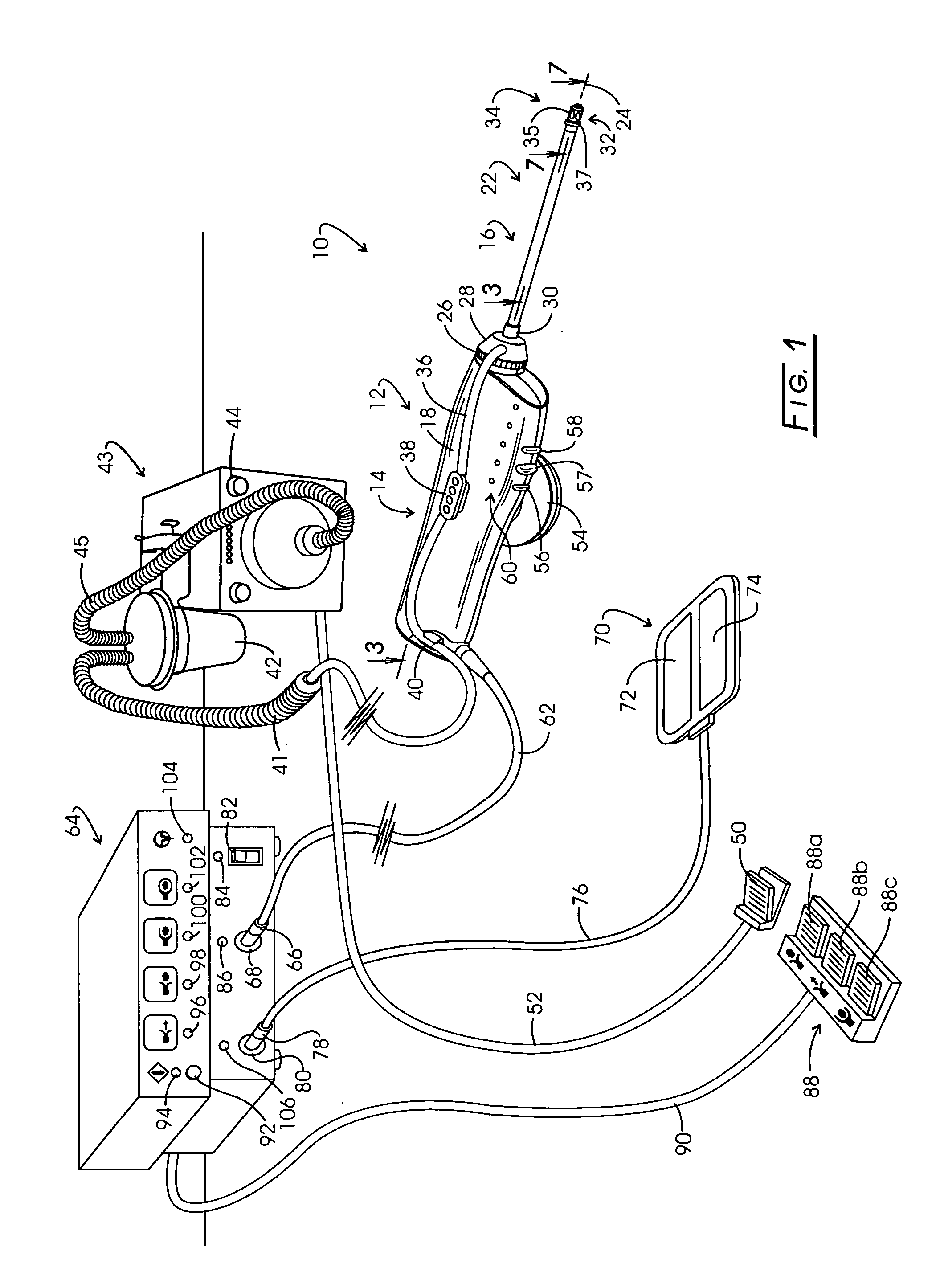
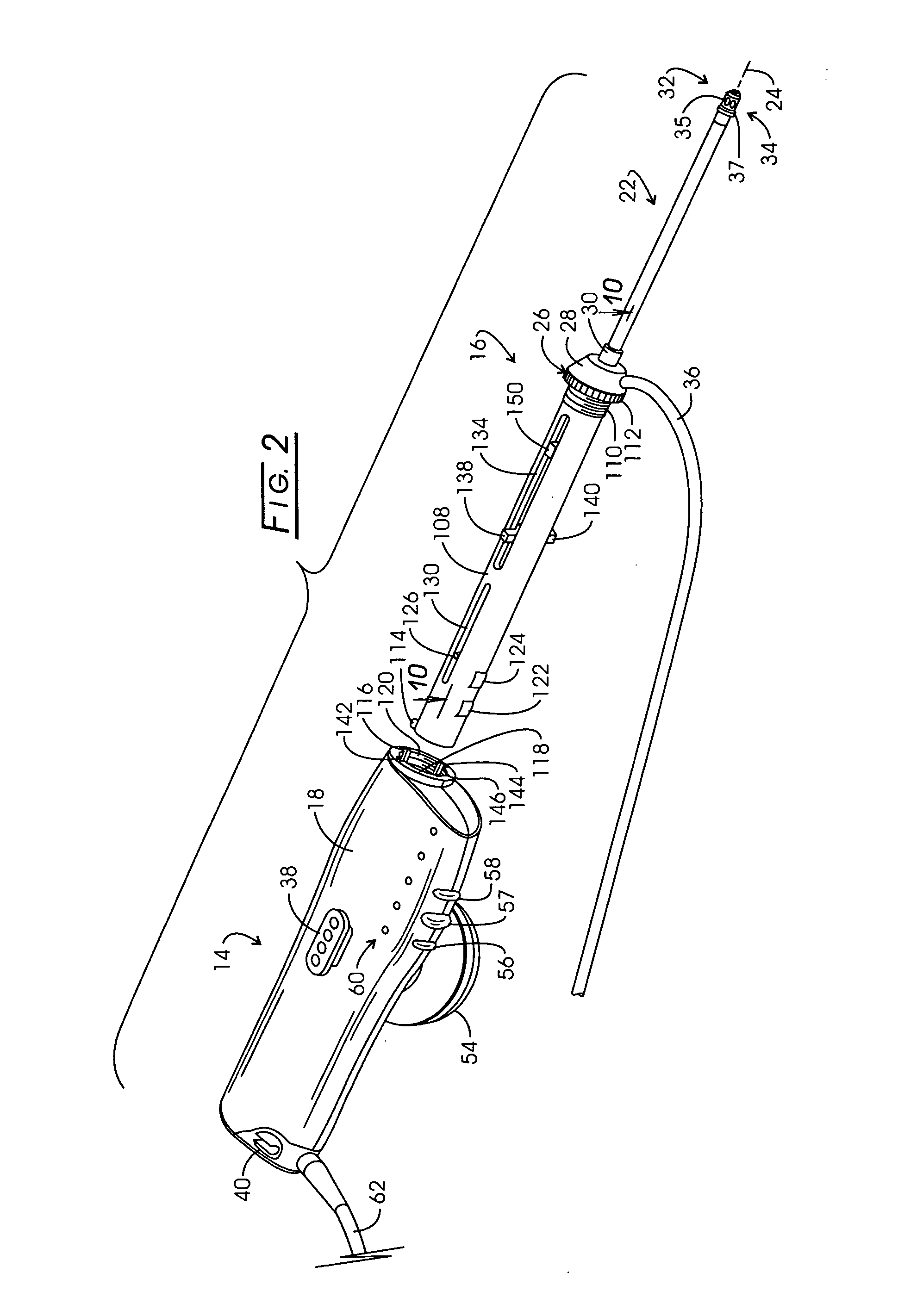
Electrosurgery with infiltration anesthesia
InactiveUS20050267455A1Reliable formationAnaesthesiaSurgical instruments for heatingElectrical resistance and conductanceElectrosurgery
Method for carrying out the recovery of an intact volume of tissue wherein a delivery cannula tip is positioned in confronting adjacency with the volume of tissue to be recovered. The electrosurgical generator employed to form an arc at a capture component extending from the tip is configured having a resistance-power profile which permits recovery of the specimen without excessive thermal artifact while providing sufficient power to sustain a cutting arc. For the recovery procedure, a local anesthetic employing a diluent which exhibits a higher resistivity is utilized and the method for deploying the capture component involves an intermittent formation of a cutting arc with capture component actuation interspersed with pauses of duration effective to evacuate any accumulation or pockets of local anesthetic solution encountered by the cutting electrodes.
Owner:INTACT MEDICAL
Avenanthramide-containing compositions
Methods and compositions for treating or preventing a skin condition, an inflammation, an irritation or an allergy associated with an ectoparasitic infection or infestation on an animal. The methods involve applying to the skin of the animal a pharmaceutical composition that contains a therapeutically effective amount of one or more than one avenanthramide, an optional ecto and / or endo-parasiticidal agent, and a pharmaceutically acceptable diluent or carrier
Owner:CEAPRO
Electrosurgery with infiltration anesthesia
InactiveUS7004174B2AnaesthesiaVaccination/ovulation diagnosticsElectrical resistance and conductanceElectrosurgery
Method for carrying out the recovery of an intact volume of tissue wherein a delivery cannula tip is positioned in confronting adjacency with the volume of tissue to be recovered. The electrosurgical generator employed to form an arc at a capture component extending from the tip is configured having a resistance-power profile which permits recovery of the specimen without excessive thermal artifact while providing sufficient power to sustain a cutting arc. For the recovery procedure, a local anesthetic employing a diluent which exhibits a higher resistivity is utilized and the method for deploying the capture component involves an intermittent formation of a cutting arc with capture component actuation interspersed with pauses of duration effective to evacuate any accumulation or pockets of local anesthetic solution encountered by the cutting electrodes.
Owner:COVIDIEN AG
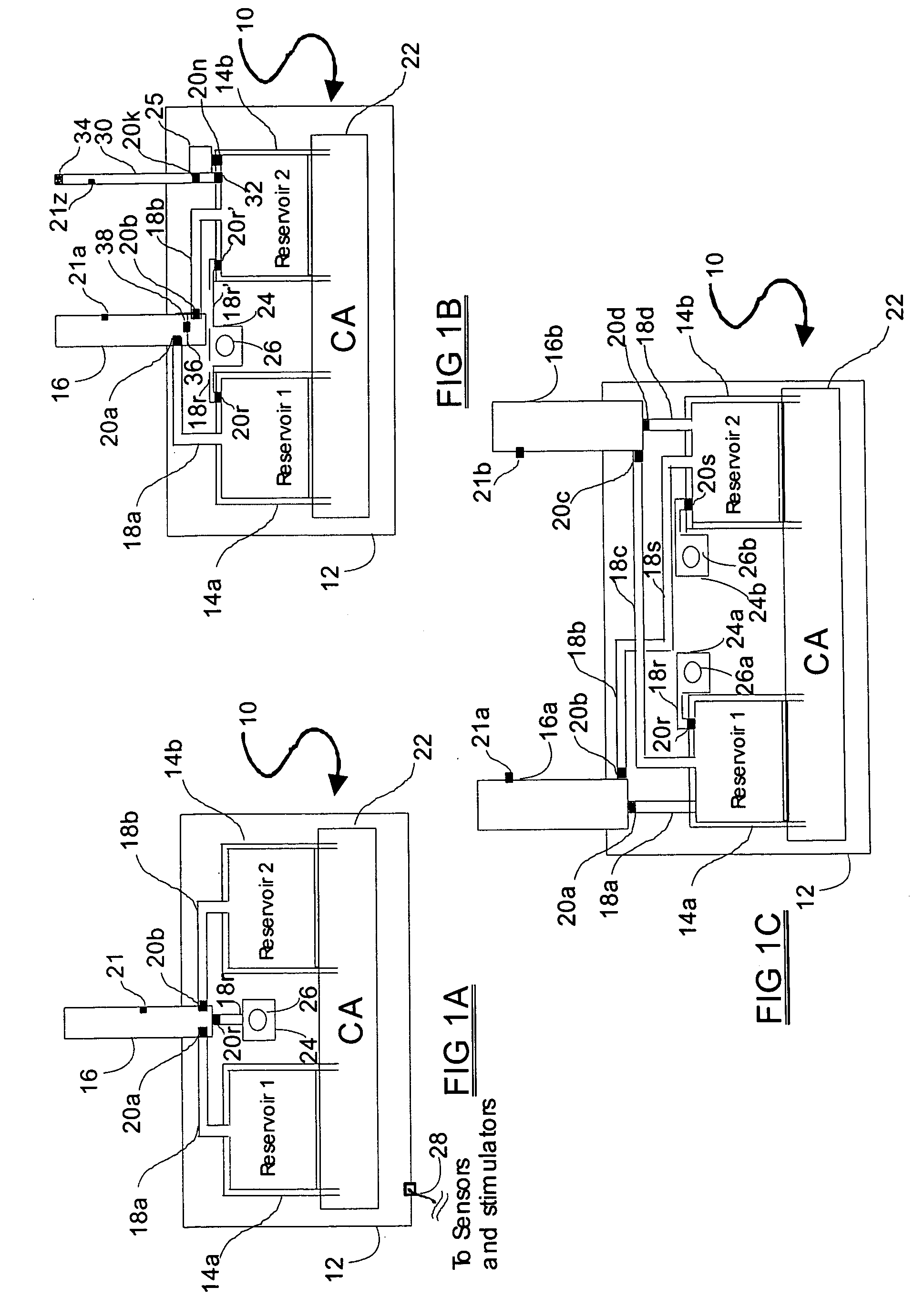
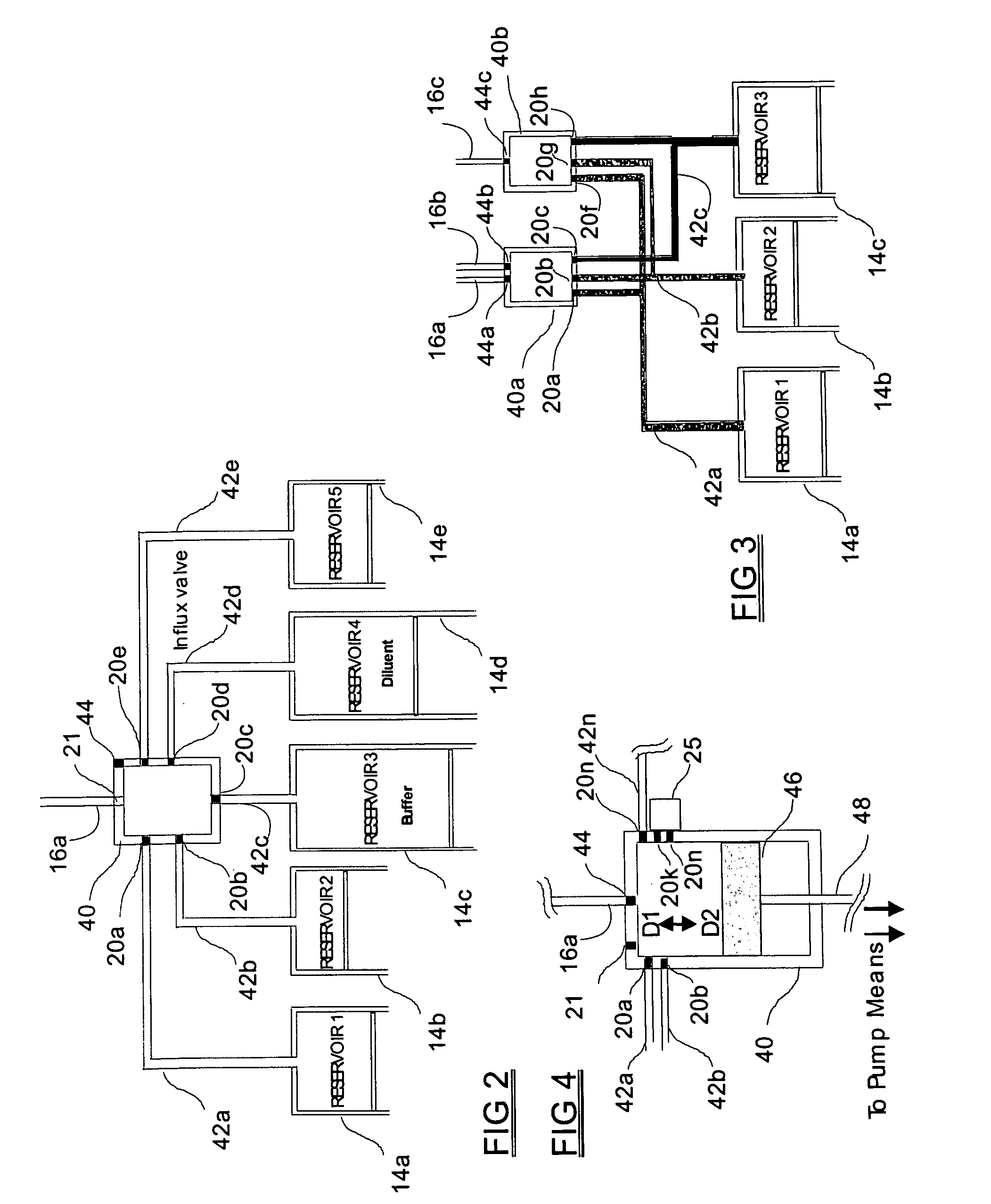
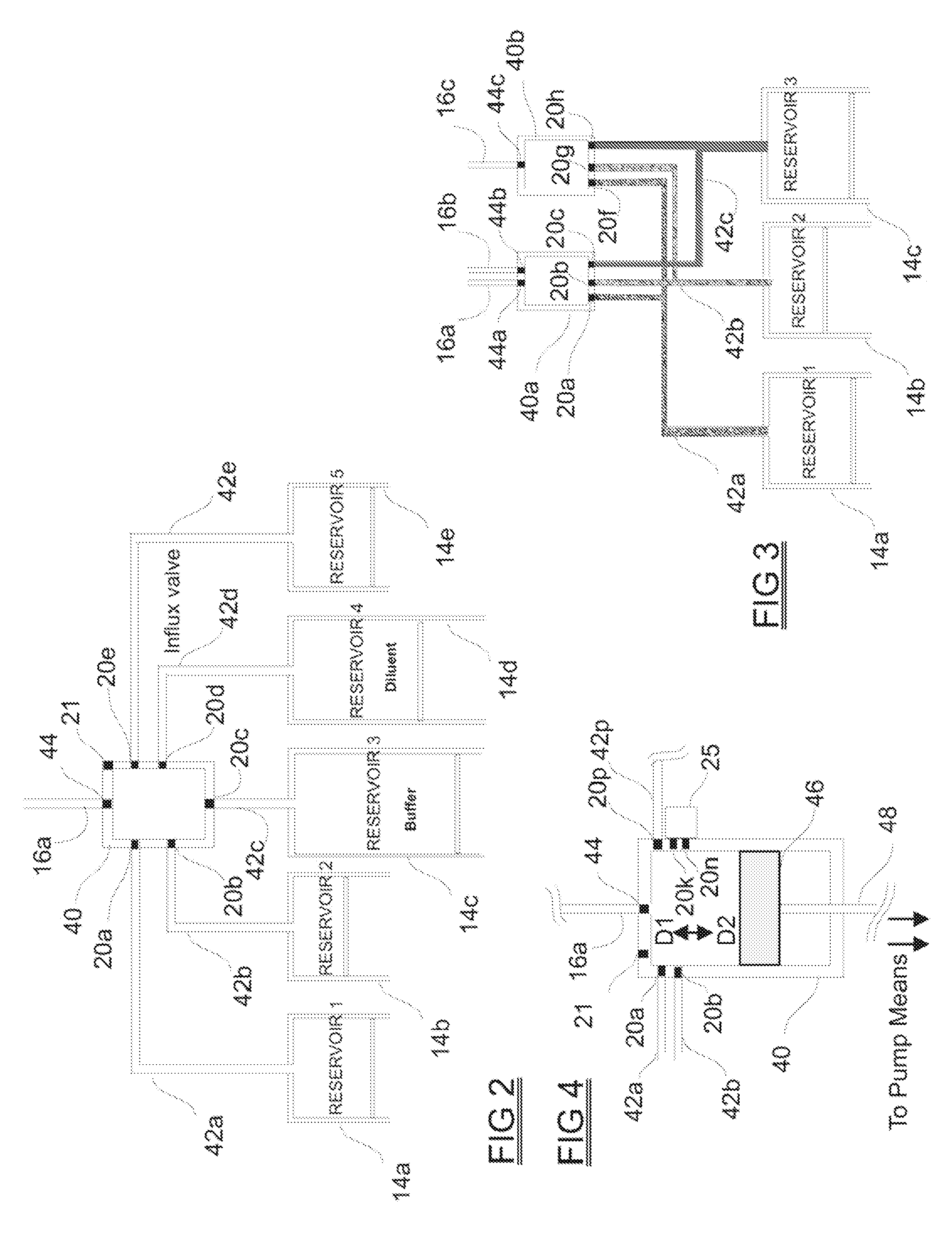
Programmable medical drug delivery systems and methods for delivery of multiple fluids and concentrations
InactiveUS20050277912A1Extension of timeEasy to fillMulti-lumen catheterDrug and medicationsControl mannerDelivery system
A drug delivery system provides for mixing various drugs in an optimally controlled manner, for using flow controllers to guide multiple drugs into a single or into multiple catheters, for enabling a single lumen catheter to treat a specific region with several drugs, for allowing for dilution of a concentrated drug in order to both increase the time between refilling and also for providing any concentration of a drug that might be desired, for using a buffer fluid to deliver exact amounts of several drugs from the same catheter or to separate several drugs within a single catheter, for using external fluid present in the human body either as a diluent or buffer fluid, and for providing for a drug testing / filler apparatus to be used prior to implant to ensure proper function and easy means of filling multiple reservoirs with different fluids, and also after implant for refilling operations. The drug delivery system (DDS) can perform both bolus and continuous delivery of substances, and enable the measured delivery of any one of several drugs to one or more distal locations at independently programmable rates. New types of catheter systems and uses therefore are also described. Catheter hub assemblies allowing for easy replacement of drug delivery systems offer advantages when replacing drug delivery systems. New methods for using the DDS in the promotion of healthy pregnancy and treatment of a developing fetus are also possible.
Owner:JOHN SASHA
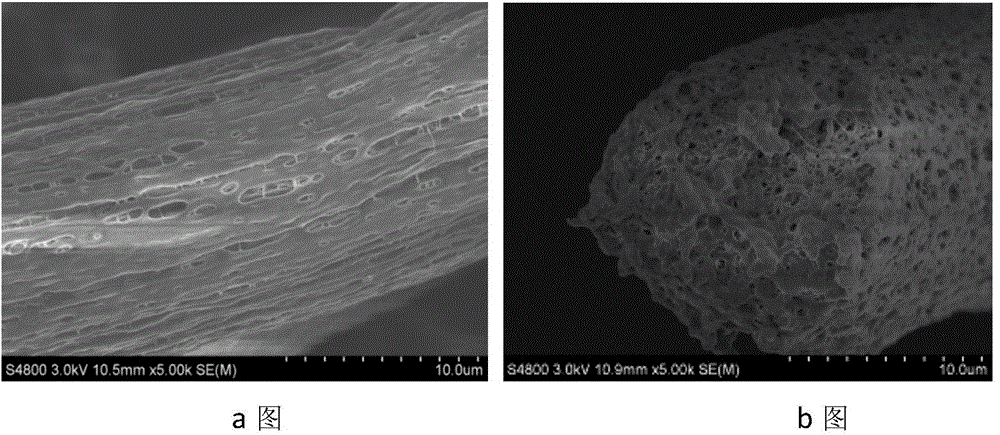
Preparation method of porous fiber non-woven fabric
ActiveCN103981635AReduce melt viscosityReduce degradationFilament forming substance formingMelt spinning methodsDiluentNonwoven fabric
The invention relates to a preparation method of a porous fiber non-woven fabric. The aim of the preparation method is to improve the product performance of the conventional non-woven fabric, so that the non-woven fabric meets the requirements on high-precision and high-performance filter. The technical scheme is that the preparation method of the porous fiber non-woven fabric comprises the following steps in sequence: (1) uniformly mixing a polymer and a diluent to obtain a blend with 10 to 60 percent of polymer; (2) melting and extruding the blend in the step (1) by adopting a screw extruder granulator, and directly cooling and granulating in air; (3) producing master batches in the step (2) by melt-down equipment to obtain a primary non-woven fabric; (4) extracting to remove the diluent from the primary non-woven fabric in the step (3), performing pore-forming on fibers in the non-woven fabric, and drying to obtain the porous fiber non-wave fabric; (5) recovering mixed waste liquid of the diluent and an extraction agent for reuse.
Owner:浙江省轻工业品质量检验研究院
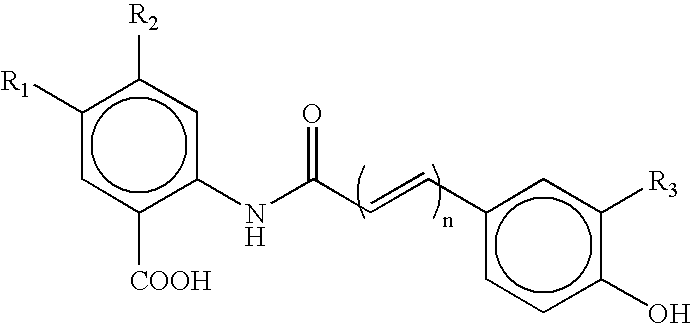
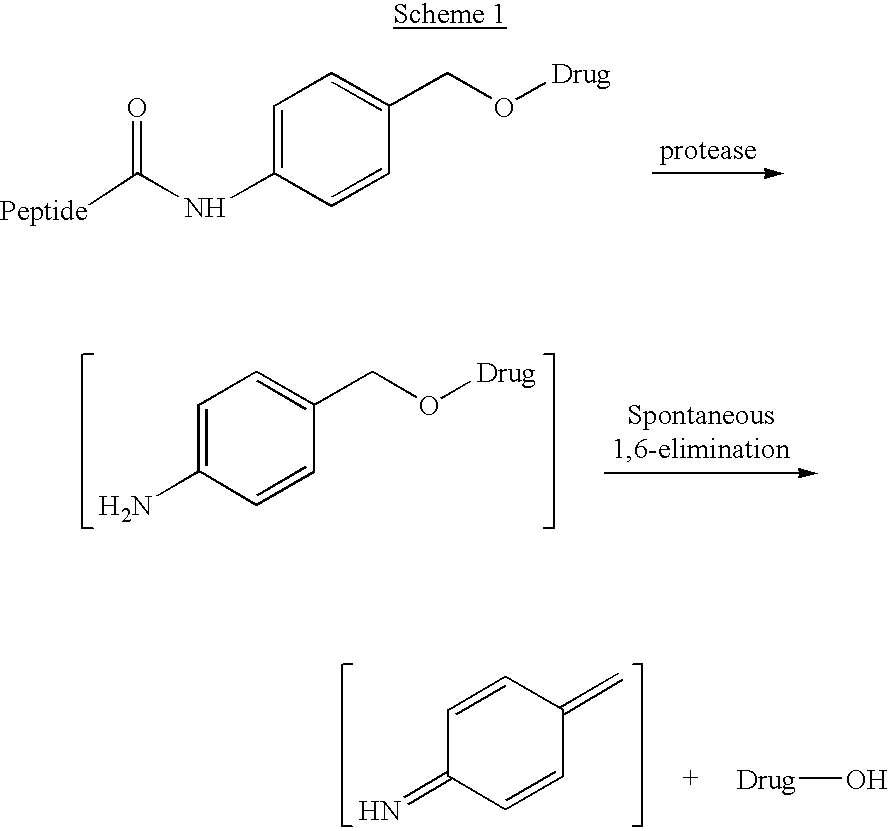
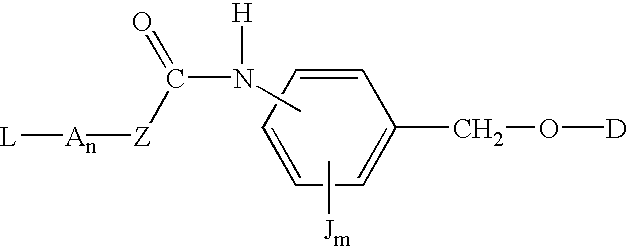
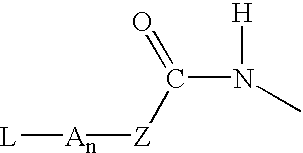
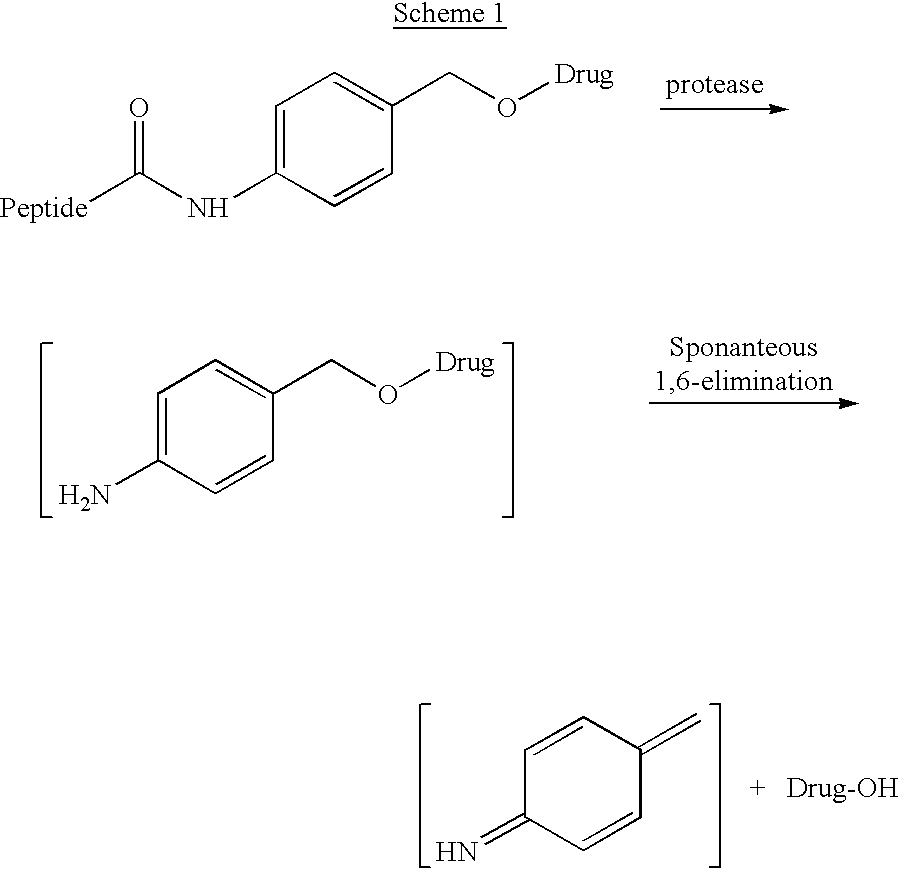
P-amidobenzylethers in drug delivery agents
InactiveUS20030130189A1Decrease in drug activityImprove stabilityTetrapeptide ingredientsTripeptide ingredientsEtherDiluent
Compounds of the formulas LAn-Z-X-WwD and BZ-X-WwD wherein: D is a drug moiety; L is a ligand; B is a blocking group; A is an optional acyl unit; Z is an amino acid or a peptide; X is an aminobenzyl ether self-immolative spacer group; W is an optional second self-immolative group; n is an integer of 0 or 1; and w is an integer of 0 or 1, and compositions of said compounds with pharmaceutically acceptable carrier, diluent and / or excipient, and methods of delivery the drug D via the compounds.
Owner:SEAGEN INC
Plant extracts for the preparation of pharmaceutical compositions for the treatment of hepatitis
The use of extracts from the plant Hypericum perforatum in the preparation of pharmaceutical compositions for the treatment of hepatitis C, chronic hepatitis C and related viruses, said pharmaceutical compositions comprising at least one extract of the plant Hypericum perforatum and optionally in addition, one or more pharmaceutically acceptable inactive components, selected from, carriers, coatings, diluents and adjuvants.
Owner:LAVIE DAVID +1
Lyophilization process and products obtained thereby
ActiveUS20070116729A1Dissolve fastSuitable for useBiocidePowder deliveryHigh concentrationFreeze-drying
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
p-Amidobenzylethers in drug delivery agents
Compounds of the formulaeLAn-Z-X—WwD and BZ-X—WwDwherein: D is a drug moiety; L is a ligand; B is a blocking group; A is an optional acyl unit; Z is an amino acid or a peptide residue; X is an aminobenzyl ether self-immolative spacer group; W is an optional second self-immolative group; n is an integer of 0 or 1; and w is an integer of 0 or 1, and compositions of said compounds with pharmaceutically acceptable carrier, diluent and / or excipient, and methods of delivery the drug D via the compounds.
Owner:SEAGEN INC
Protein formulation
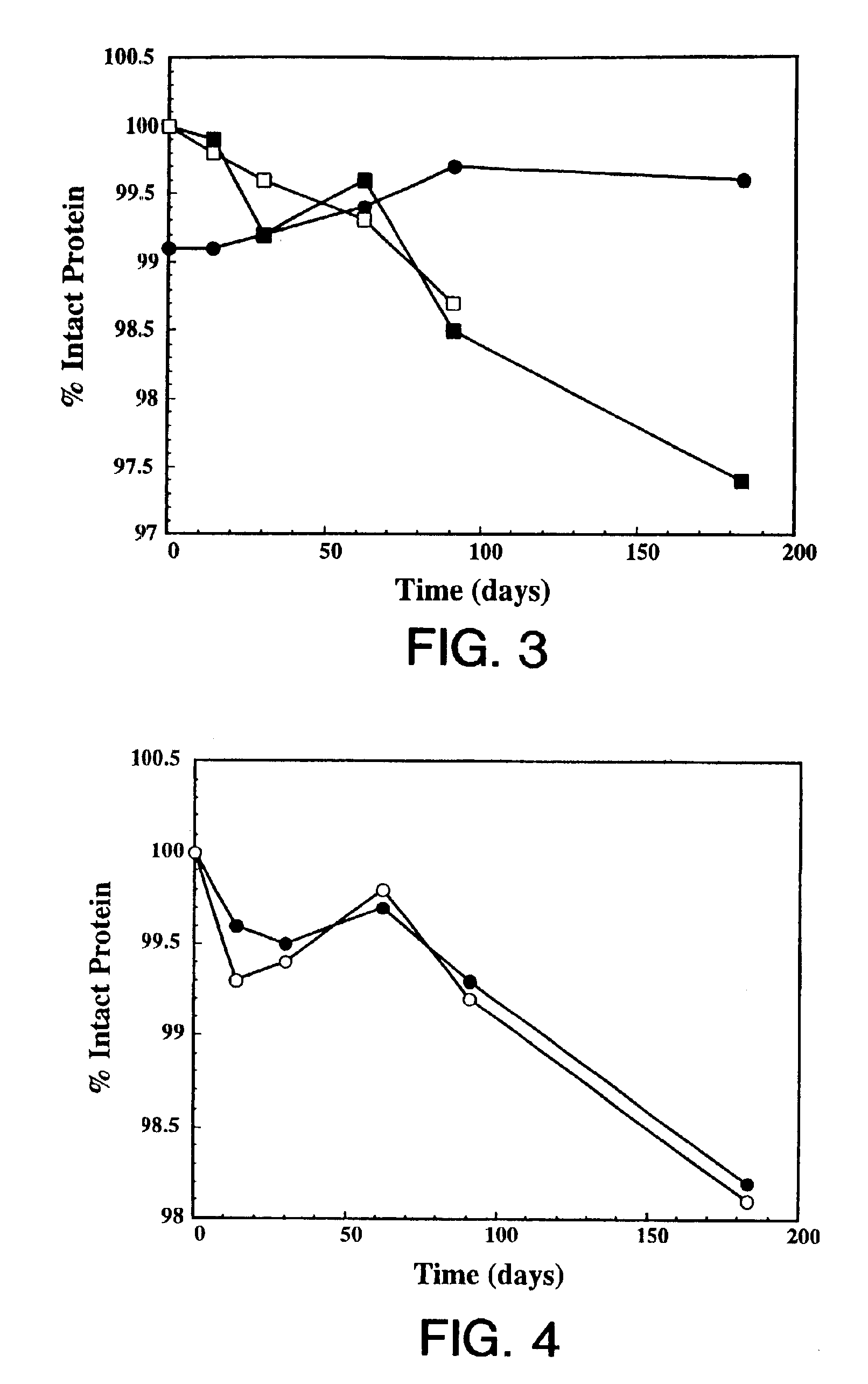
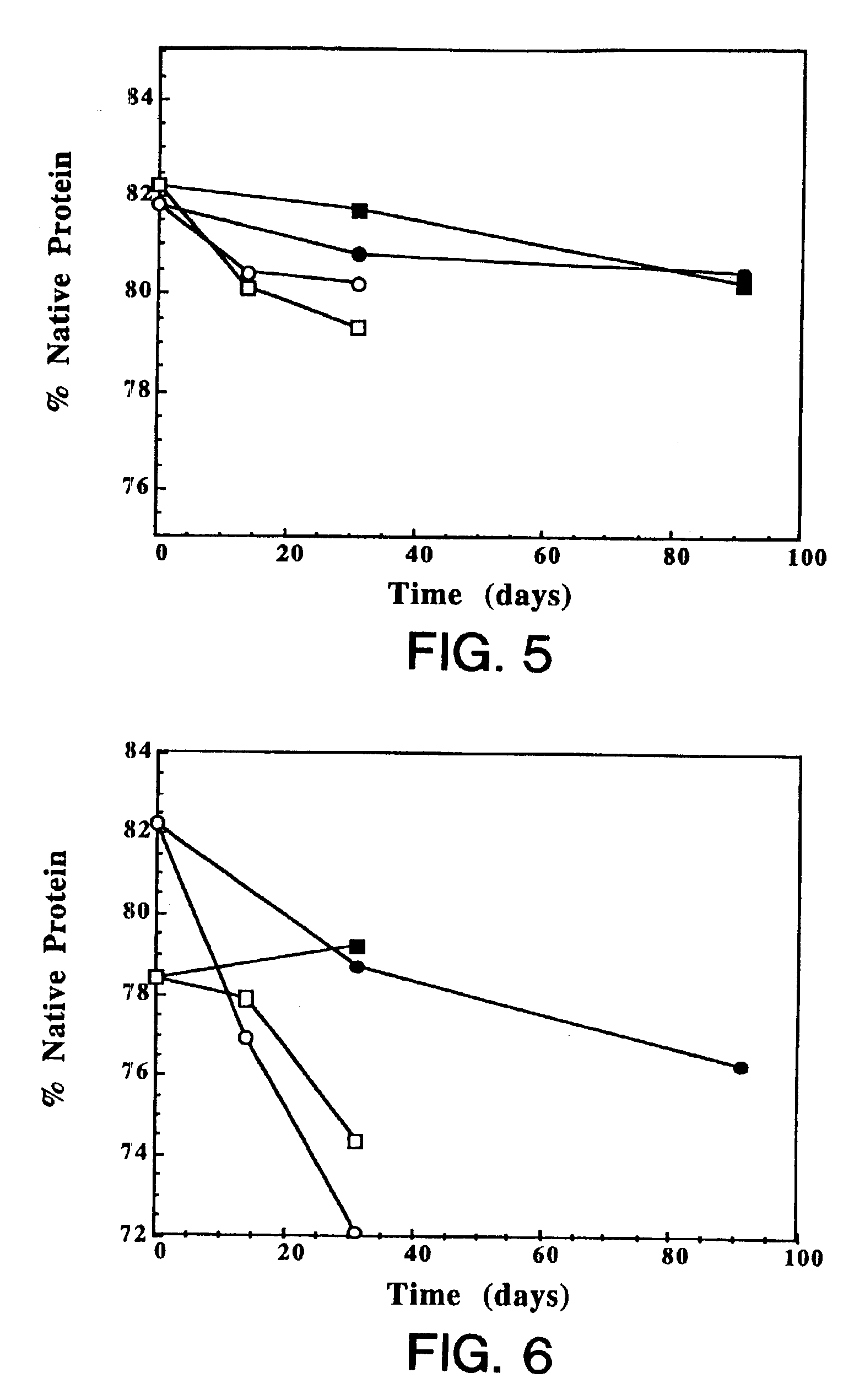
InactiveUS7060268B2Reduce aggregationReduce formation of particulateBiocideOrganic active ingredientsDiluentHigh protein
A stable lyophilized protein formulation is described which can be reconstituted with a suitable diluent to generate a high protein concentration reconstituted formulation which is suitable for subcutaneous administration. For example, anti-IgE and anti-HER2 antibody formulations have been prepared by lyophilizing these antibodies in the presence of a lyoprotectant. The lyophilized mixture thus formed is reconstituted to a high protein concentration without apparent loss of stability of the protein.
Owner:GENENTECH INC
Programmable medical drug delivery systems and methods for delivery of multiple fluids and concentrations
InactiveUS7811279B2Extension of timeEasy to fillMulti-lumen catheterDrug and medicationsControl mannerDelivery system
A drug delivery system provides for mixing various drugs in an optimally controlled manner, for using flow controllers to guide multiple drugs into a single or into multiple catheters, for enabling a single lumen catheter to treat a specific region with several drugs, for allowing for dilution of a concentrated drug in order to both increase the time between refilling and also for providing any concentration of a drug that might be desired, for using a buffer fluid to deliver exact amounts of several drugs from the same catheter or to separate several drugs within a single catheter, for using external fluid present in the human body either as a diluent or buffer fluid, and for providing for a drug testing / filler apparatus to be used prior to implant to ensure proper function and easy means of filling multiple reservoirs with different fluids, and also after implant for refilling operations. The drug delivery system (DDS) can perform both bolus and continuous delivery of substances, and enable the measured delivery of any one of several drugs to one or more distal locations at independently programmable rates. New types of catheter systems and uses therefore are also described. Catheter hub assemblies allowing for easy replacement of drug delivery systems offer advantages when replacing drug delivery systems. New methods for using the DDS in the promotion of healthy pregnancy and treatment of a developing fetus are also possible.
Owner:JOHN SASHA
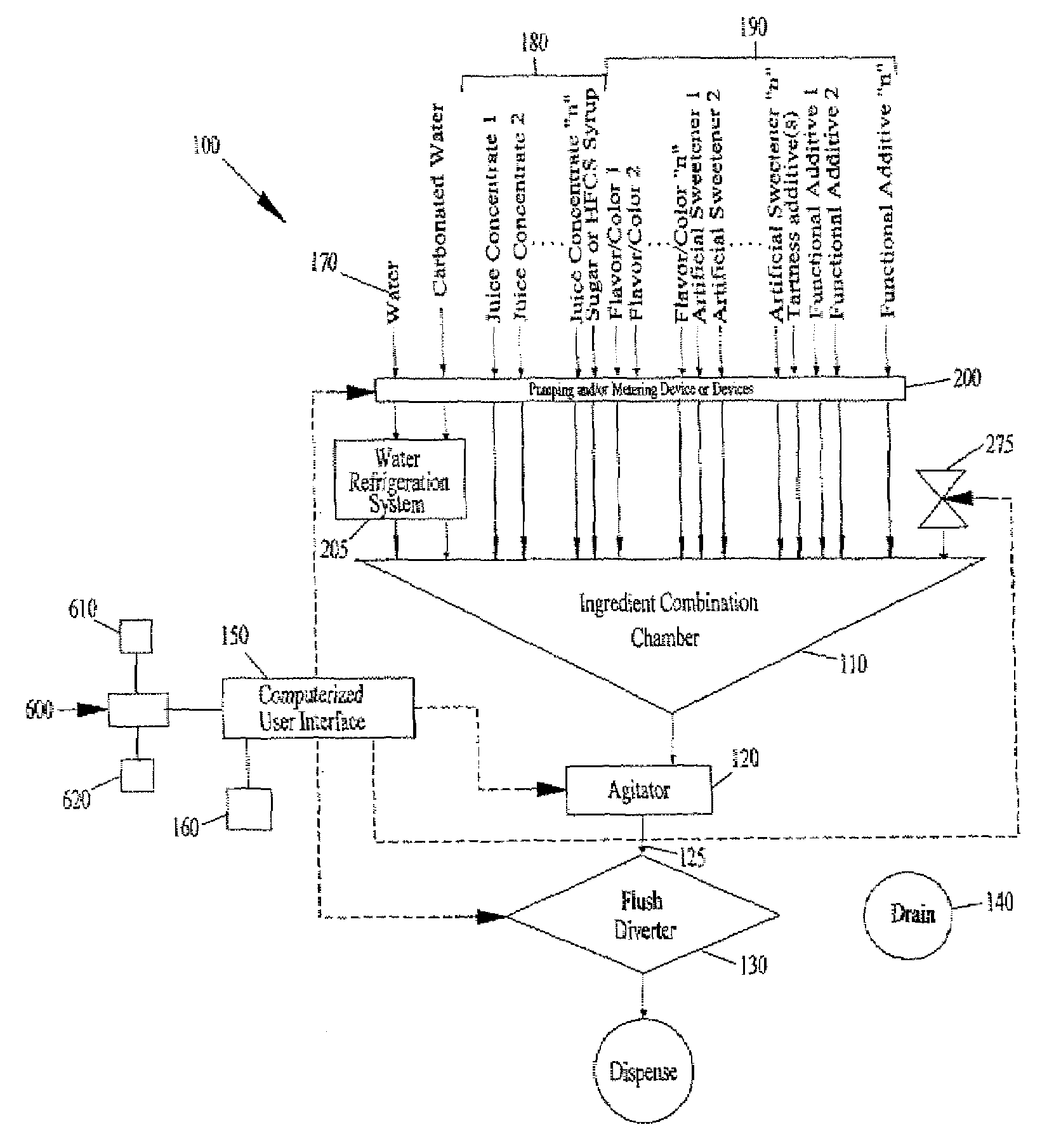
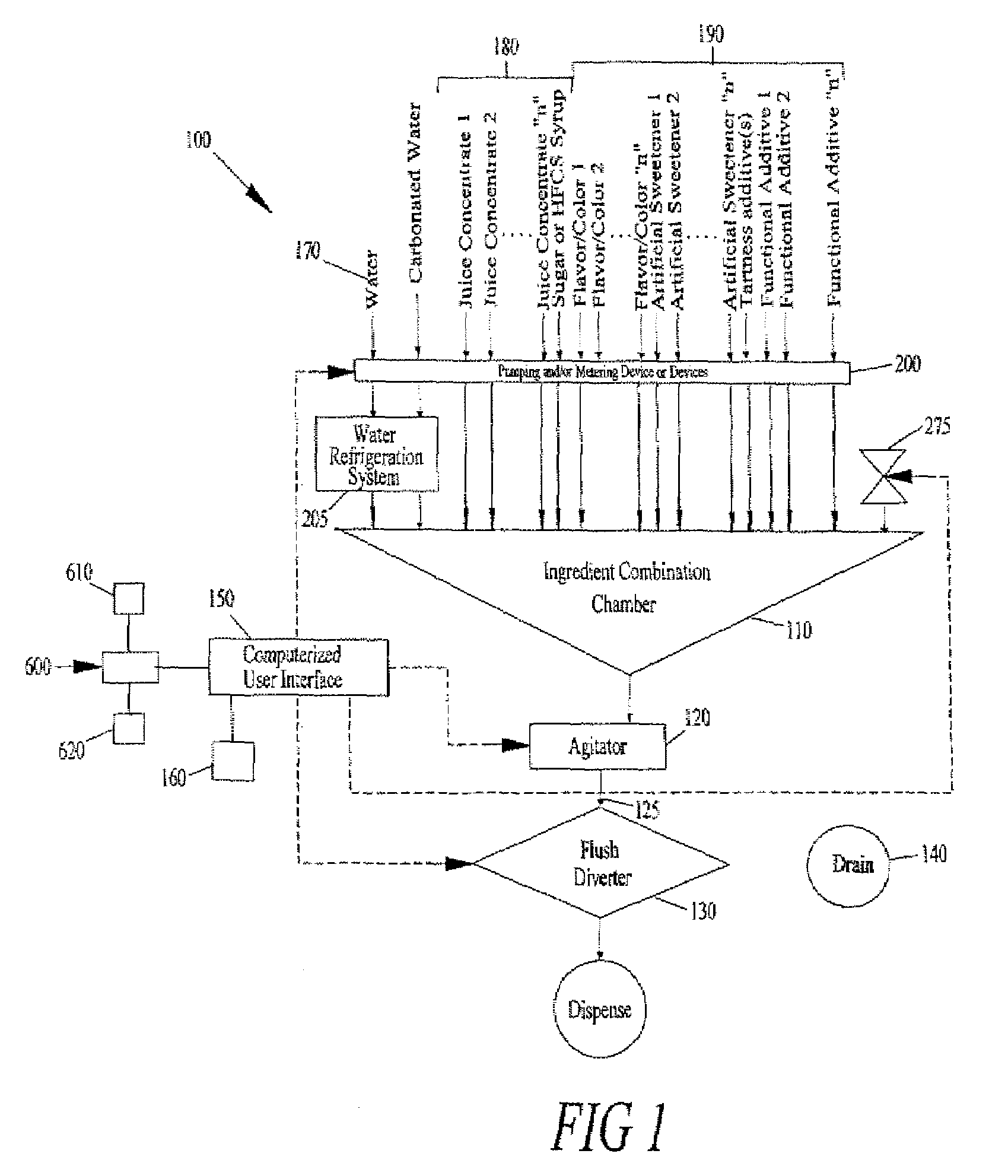
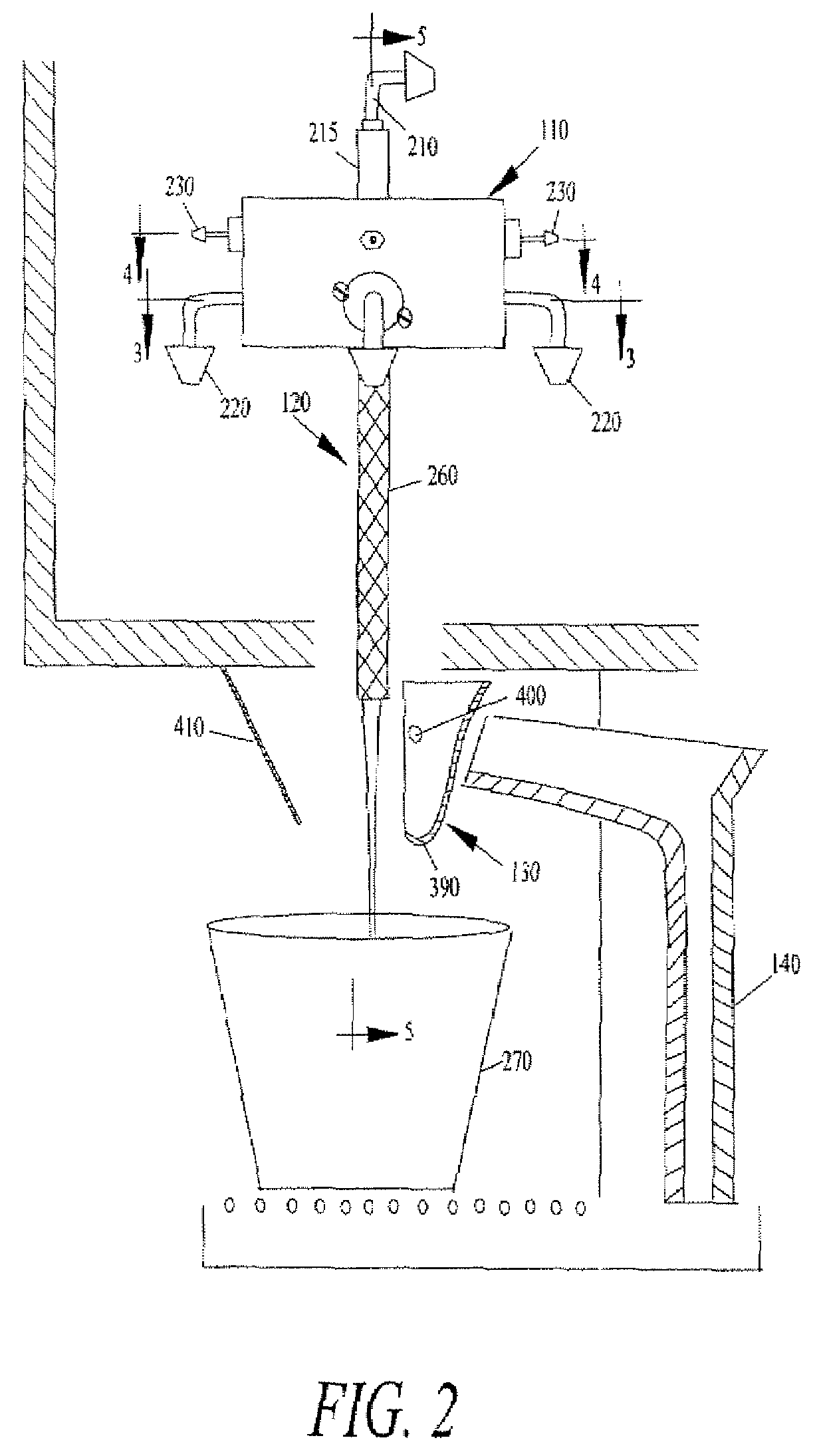
Juice Dispensing System
ActiveUS20070205220A1Liquid flow controllersLiquid transferring devicesFruit juiceAdditive ingredient
The present application describes a product mixing device. The product mixing device includes an ingredient combination chamber and means for agitation positioned about the ingredient combination chamber. The ingredient combination chamber includes a diluent inlet, a number of macro-ingredient inlets, a number of micro-ingredient inlets, and an outlet.
Owner:THE COCA-COLA CO
Liquid analysis cartridge
InactiveUS6852284B1Rapidly and effectively reconstituteEasy to reorganizeFlow mixersTransportation and packagingShell moldingDiluent
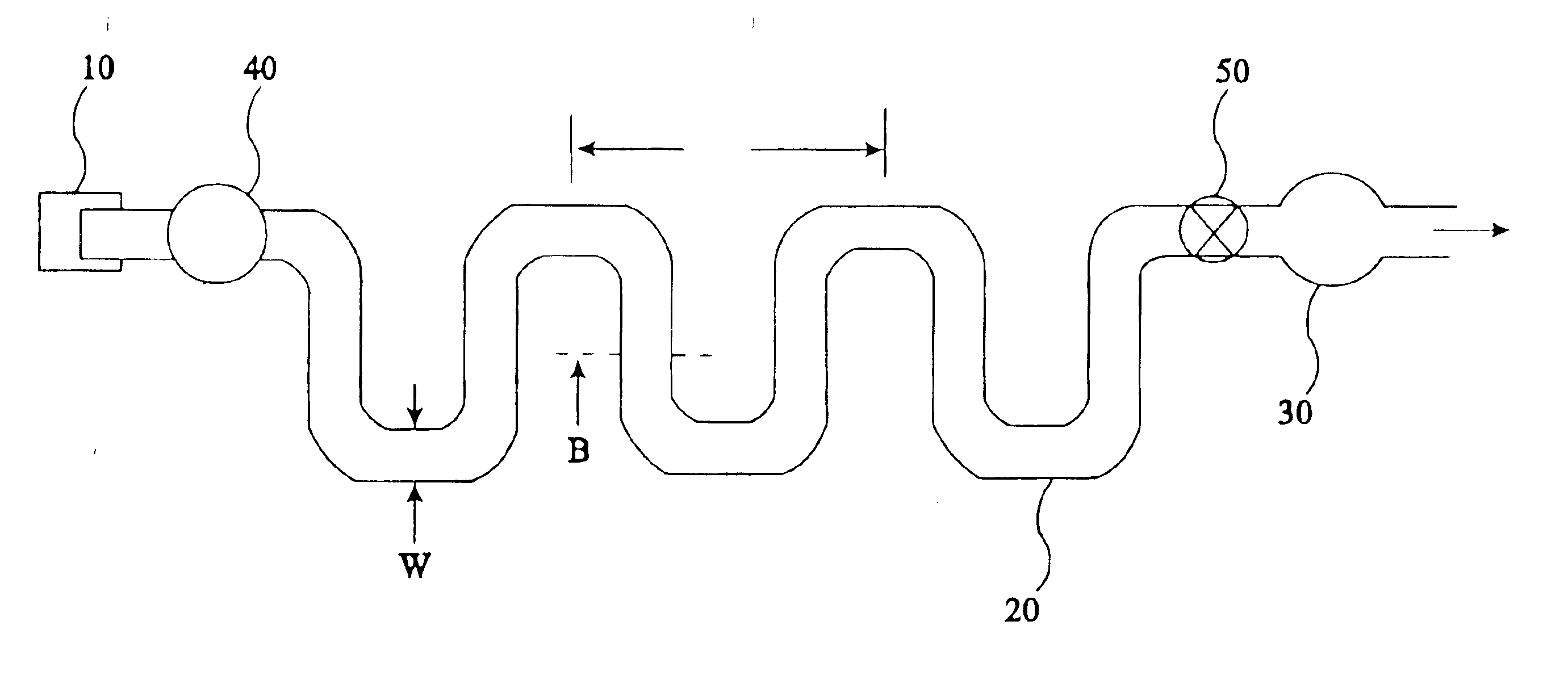
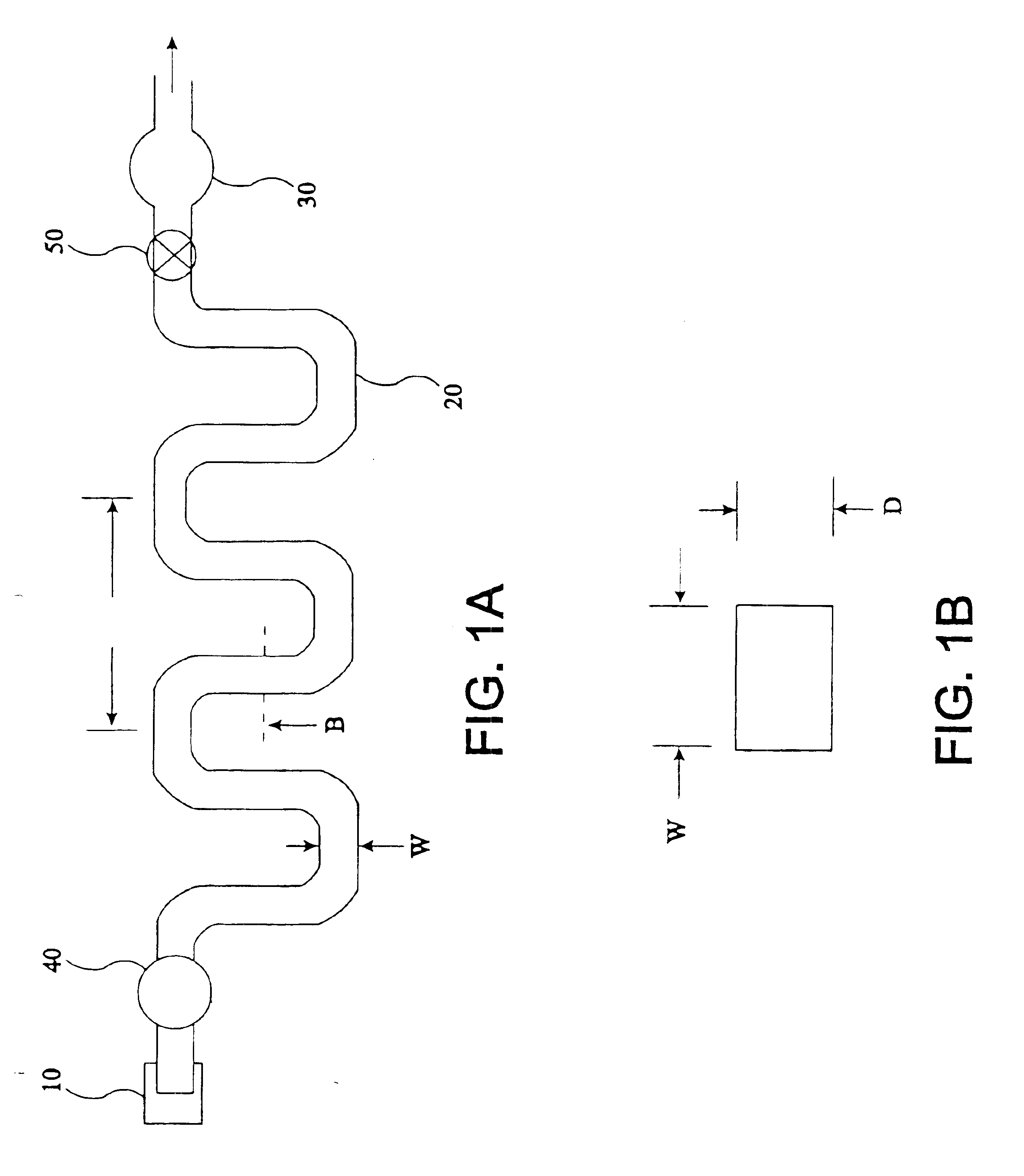
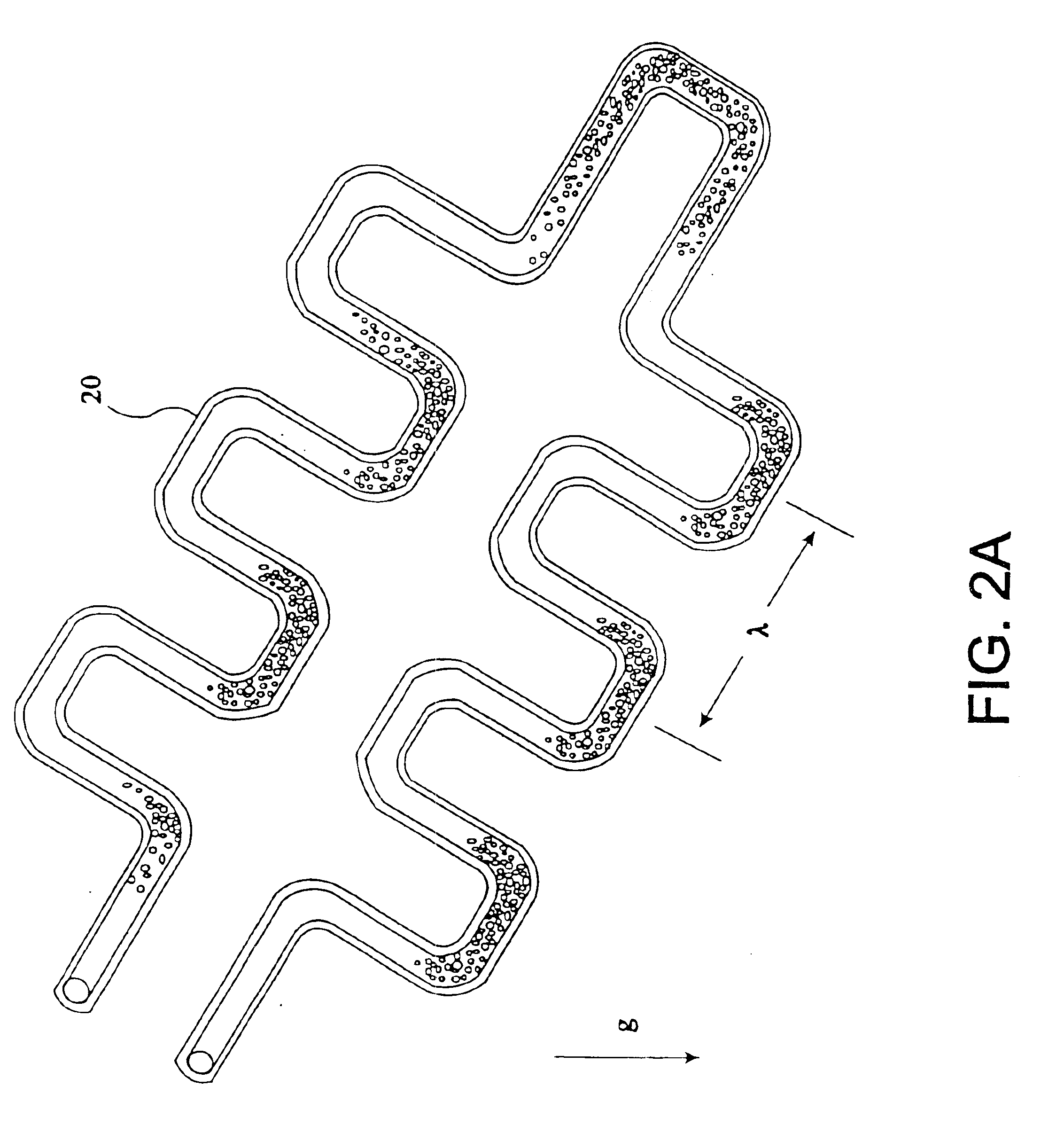
The present invention provides an apparatus and method for storing a particle-containing liquid. The storage apparatus comprises a microfluidic convoluted flow channel having a plurality of article capture regions. The storage channel is preferably an isotropic spatially periodic channel. Sedimented particles can be resuspended following storage. This invention further provides a microfluidic analysis cartridge having a convoluted storage channel therein. The sample analysis can use optical, electrical, pressure sensitive, or flow sensitive detection. A plurality of analysis channels can be included in a single cartridge. The analysis channels can be joined to reagent inlets for diluents, indicators or lysing agents. A mixing channel can be positioned between the reagent inlet and the analysis region to allow mixing and reaction of the reagent. The cartridge can include additional valves and pumps for flow management. The analysis cartridge can be a self-contained disposable cartridge having an integral waste storage container. This invention further provides a sheath flow assembly. The sheath flow assembly includes a sample channel and first and second sheath fluid channels positioned on either side of and converging with the sample channel. The assembly also includes upper and lower sheath fluid chambers positioned above and below and converging with the sample channel. The flow cartridges of this invention can be formed by molding, machining or etching. In a preferred embodiment they are laminated. This invention further provides a method of fabricating a laminated microfluidic flow device. In the method, flow elements are formed in rigid sheets and abutting surfaces of the sheets are bonded together.
Owner:UNIV OF WASHINGTON
Method for reducing emissions from evaporative emissions control systems
InactiveUS6540815B1Loss in working capacityEmission reductionGas treatmentNon-fuel substance addition to fuelHigh concentrationSorbent
Disclosed is a method for sharply reducing diurnal breathing loss emissions from automotive evaporative emissions control systems by providing multiple layers, or stages, of adsorbents. On the fuel source-side of an emissions control system canister, high working capacity carbons are preferred in a first canister (adsorb) region. In subsequent canister region(s) on the vent-side, the preferred adsorbent should exhibit a flat or flattened adsorption isotherm on a volumetric basis and relatively lower capacity for high concentration vapors as compared with the fuel source-side adsorbent. Multiple approaches are described for attaining the preferred properties for the vent-side canister region. One approach is to use a filler and / or voidages as a volumetric diluent for flattening an adsorption isotherm. Another approach is to employ an adsorbent with the desired adsorption isotherm properties and to process it into an appropriate shape or form without necessarily requiring any special provision for dilution. The improved combination of high working capacity carbons on the fuel source-side and preferred lower working capacity adsorbent on the vent-side provides substantially lower diurnal breathing emissions without a significant loss in working capacity or increase in flow restriction compared with known adsorbents used in canister configurations for automotive emissions control systems.
Owner:INGEVITY SOUTH CAROLINA
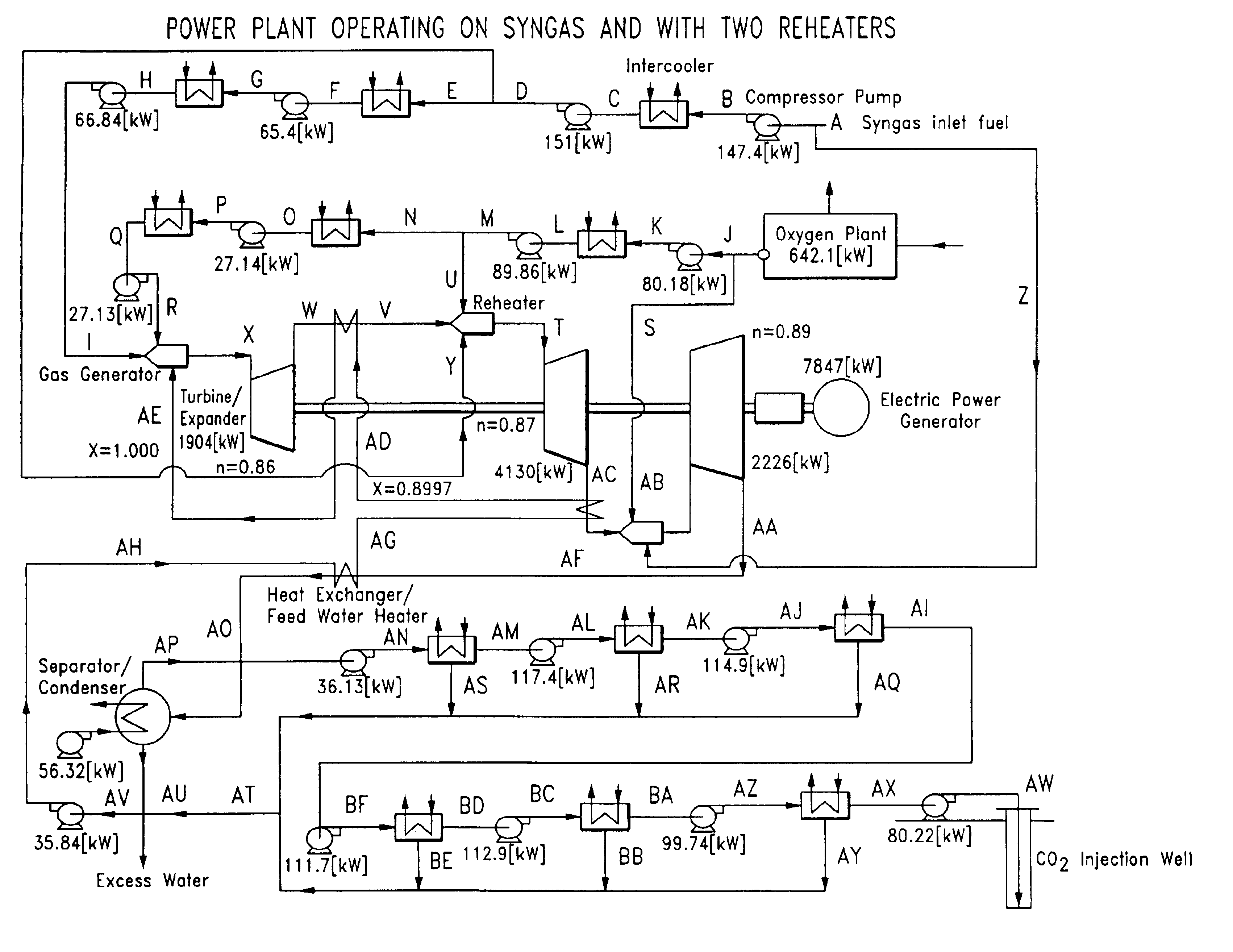
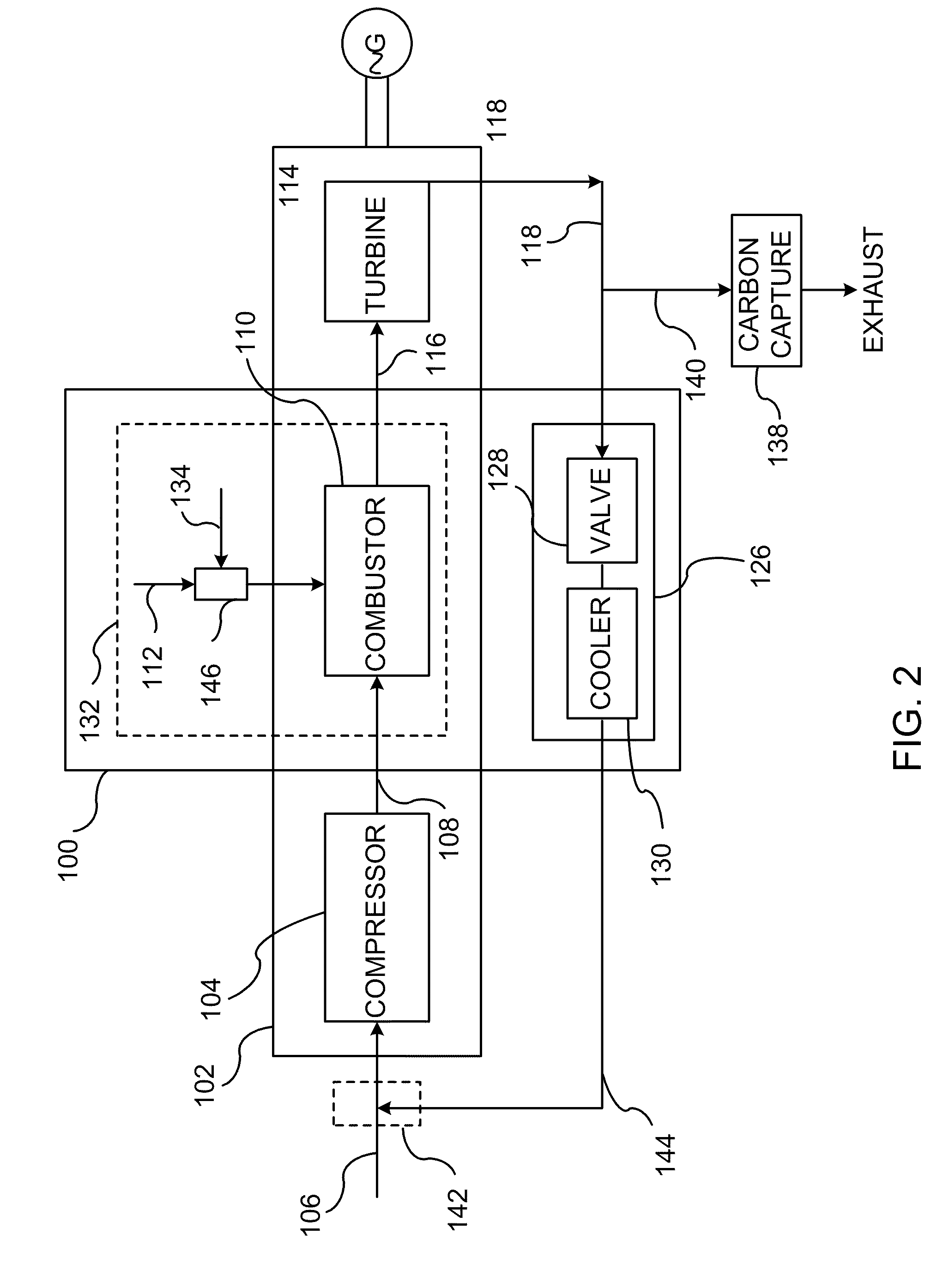
Low pollution power generation system with ion transfer membrane air separation
InactiveUS6945029B2Nitrogen oxideReduce electricity demandSolidificationLiquefactionPollutionCombustion products
A low or no pollution power generation system is provided. The system has an air separator to collect oxygen. A gas generator is provided with inputs for the oxygen and a hydrocarbon fuel. The fuel and oxygen are combusted within the gas generator, forming water and carbon dioxide. Water or other diluents are also delivered into the gas generator to control temperature of the combustion products. The combustion products are then expanded through at least one turbine or other expander to deliver output power. The combustion products are then passed through a separator where the steam is condensed. A portion of the water is discharged and the remainder is routed back to the gas generator as diluent. The carbon dioxide can be conditioned for sequestration. The system can be optimized by adding multiple expanders, reheaters and water diluent preheaters, and by preheating air for an ion transfer membrane oxygen separation.
Owner:CLEAN ENERGY SYST
Silicone hydrogels comprising desirable water content and oxygen permeability
The present invention relates to a process comprising the steps of reacting a reactive mixture comprising at least one silicone-containing component, at least one hydrophilic component, and at least one diluent to form an ophthalmic device having an advancing contact angle of less than about 80°; and contacting the ophthalmic device with an aqueous extraction solution at an elevated extraction temperature, wherein said at least one diluent has a boiling point at least about 10° higher than said extraction temperature.
Owner:JOHNSON & JOHNSON VISION CARE INC
Silicone hydrogels comprising n-vinyl amides and hydroxyalkyl (meth)acrylates or (meth)acrylamides
The present invention relates to a process comprising the steps of reacting a reactive mixture comprising at least one silicone-containing component, at least one hydrophilic component, and at least one diluent to form an ophthalmic device having an advancing contact angle of less than about 80°; and contacting the ophthalmic device with an aqueous extraction solution at an elevated extraction temperature, wherein said at least one diluent has a boiling point at least about 10° higher than said extraction temperature.
Owner:JOHNSON & JOHNSON VISION CARE INC
System for managing inventory of bulk liquids
ActiveUS20100322822A1Read intuitivelyReduce laborPreparing sample for investigationMaterial analysis by optical meansHigh ratePipette
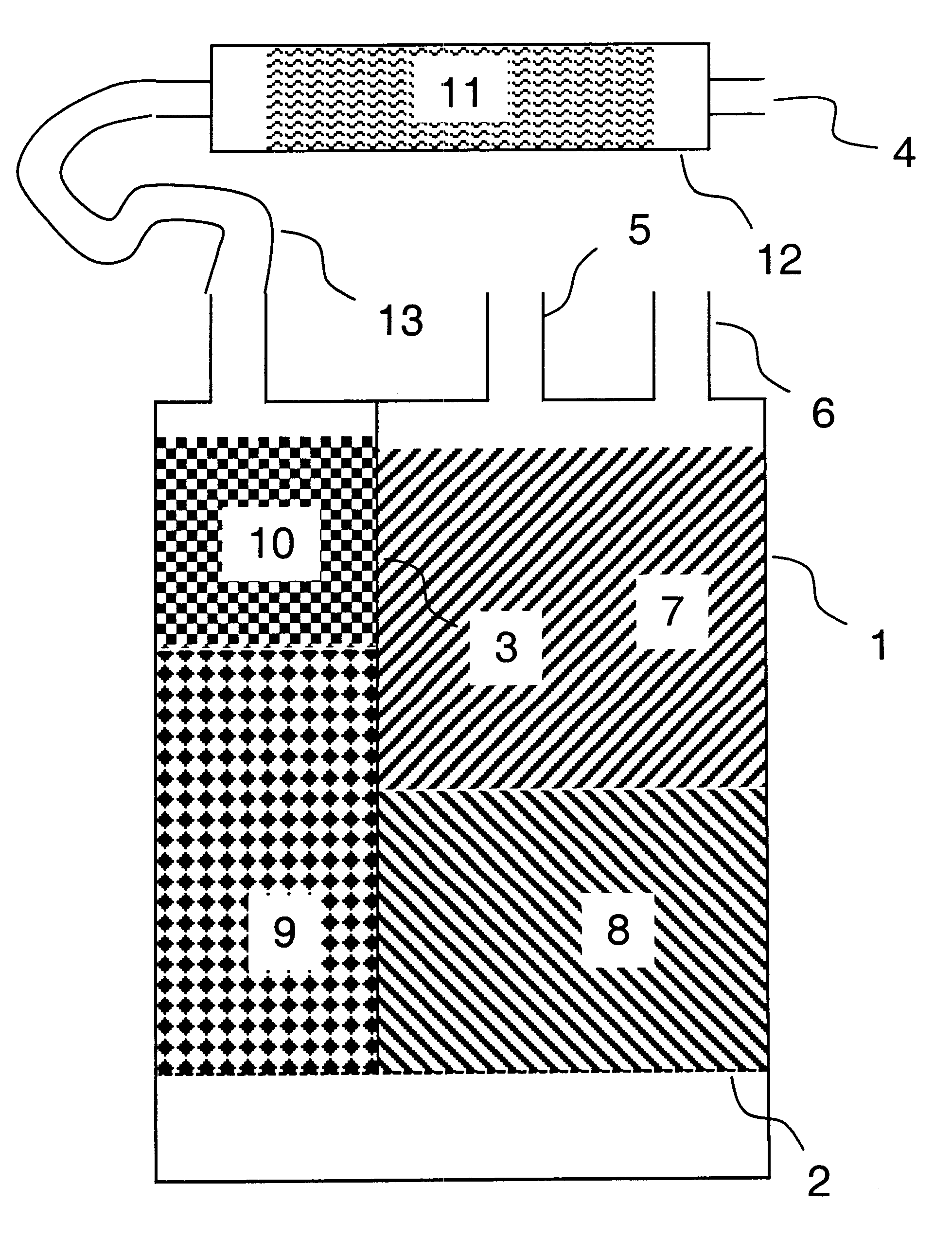
A system for managing bulk liquids for an automated clinical analyzer. The system comprises (a) at least one local reservoir for storing a bulk liquid for impending use, (b) at least one container for holding a bulk liquid before the liquid is transferred to a local reservoir, and (c) a controller for monitoring the level of a bulk liquid in a local reservoir. The local reservoir for storing a bulk liquid for impending use can be a trough. The use of troughs for storing a reagent, a diluent, or some other treating agent for impending use enables an aspirating / dispensing device having a plurality of pipettes to aspirate and dispense the reagent, diluent, or other treating agent at a high rate of throughput. The controller can monitor the level of a liquid in (a) a local reservoir for storing a bulk liquid for imminent use and the level of liquid in a (b) container for holding a bulk liquid before the liquid is transferred to a local reservoir. In the laboratory automation system described herein, the container for holding a bulk liquid before the liquid is transferred to a local reservoir can be a bottle. Other desirable features in the system include, but are not limited to, pump(s), valves, liquid level sensors.
Owner:ABBOTT LAB INC
Ionic silicone hydrogels
The present invention relates to a process comprising the steps of reacting a reactive mixture comprising at least one silicone-containing component, at least one hydrophilic component, and at least one diluent to form an ophthalmic device having an advancing contact angle of less than about 80°; and contacting the ophthalmic device with an aqueous extraction solution at an elevated extraction temperature, wherein said at least one diluent has a boiling point at least about 10° higher than said extraction temperature.
Owner:JOHNSON & JOHNSON VISION CARE INC
Process to produce low viscosity poly-alpha-olefins
ActiveUS20070043248A1Hydrocarbon by hydrogenationHydrocarbons from unsaturated hydrocarbon additionPolyolefinAlpha-olefin
This invention relates to a process to produce a polyalpha-olefin comprising: 1) contacting one or more alpha-olefin monomers having 3 to 24 carbon atoms with an unbridged substituted bis cyclopentadienyl transition metal compound having: 1) at least one non-isoolefin substitution on both cyclopentadientyl rings, or 2) at least two substitutions on at least one cyclopentadienyl ring, a non-coordinating anion activator, and optionally an alkyl-aluminum compound, where the molar ratio of transition metal compound to activator is 10:1 to 0.1:1, and if the alkyl aluminum compound is present then the molar ratio of alkyl aluminum compound to transition metal compound is 1:4 to 4000:1, under polymerization conditions wherein: i) hydrogen is present at a partial pressure of 0.1 to 50 psi, based upon the total pressure of the reactor or the concentration of the hydrogen is from 1 to 10,000 ppm or less by weight; ii) wherein the alpha-olefin monomer(s) having 3 to 24 carbon atoms are present at 10 volume % or more based upon the total volume of the catalyst / activator / alkylaluminum compound solutions, monomers, and any diluents or solvents present in the reaction; iii) the residence time of the reaction is at least 5 minutes; iv) the productivity of the process is at least 43,000 grams of total product per gram of transition metal compound; v) the process is continuous or semi-continuous, and vi) the temperature in the reaction zone does not rise by more than 10° C. during the reaction; and vii) ethylene is not present at more than 30 volume % of the monomers entering the reaction zone; and 2) obtaining a polyalpha-olefin (PAO), optionally hydrogenating the PAO, wherein the PAO comprises at least 50 mole % of a C3 to C24 alpha-olefin monomer, and wherein the PAO has a kinematic viscosity at 100° C. of 20 cSt or less.
Owner:EXXONMOBIL CHEM PAT INC
Microporous PVDF films
Shaped microporous articles are produced from polyvinylidene fluoride (PVDF) and nucleating agents using thermally induced phase separation (TIPS) processes. The shaped microporous article is oriented in at least one direction at a stretch ratio of at least approximately 1.1 to 1.0. The shaped article may also comprise a diluent, glyceryl triacetate. The shaped microporous article may also have the micropores filled with a sufficient quantity of ion conducting electrolyte to allow the membrane to function as an ion conductive membrane. The method of making a microporous article comprises the steps of melt blending polyvinylidene fluoride, nucleating agent and glyceryl triacetate; forming a shaped article of the mixture; cooling the shaped article to cause crystallization of the polyvinylidene fluoride and phase separation of the polyvinylidene fluoride and glyceryl triacetate; and stretching the shaped article in at least one direction at a stretch ratio of at least approximately 1.1 to 1.0.
Owner:3M INNOVATIVE PROPERTIES CO
Microporous PVDF films and method of manufacturing
Shaped microporous articles are produced from polyvinylidene fluoride (PVDF) and nucleating agents using thermally induced phase separation (TIPS) processes. The shaped microporous article is oriented in at least one direction at a stretch ratio of at least approximately 1.1 to 1.0. The shaped article may also comprise a diluent, glyceryl triacetate. The shaped microporous article may also have the micropores filled with a sufficient quantity of ion conducting electrolyte to allow the membrane to function as an ion conductive membrane. The method of making a microporous article comprises the steps of melt blending polyvinylidene fluoride, nucleating agent and glyceryl triacetate; forming a shaped article of the mixture; cooling the shaped article to cause crystallization of the polyvinylidene fluoride and phase separation of the polyvinylidene fluoride and glyceryl triacetate; and stretching the shaped article in at least one direction at a stretch ratio of at least approximately 1.1 to 1.0.
Owner:3M INNOVATIVE PROPERTIES CO
Process for the production of ultrafine powders of metal oxides
InactiveUS6503475B1Low costHigh yield rateAlkaline earth titanatesMaterial nanotechnologyDiluentBiological activation
A process for the production of ultrafine powders that includes subjecting a mixture of precursor metal compound and a non-reactant diluent phase to mechanical milling whereby the process of mechanical activation reduces the microstructure of the mixture to the form of nano-sized grains of the metal compound uniformly dispersed in the diluent phase. The process also includes heat treating the mixture of nano-sized grains of the metal compound uniformly dispersed in the diluent phase to convert the nano-sized grains of the metal compound into a metal oxide phase. The process further includes removing the diluent phase such that the nano-sized grains of the metal oxide phase are left behind in the form of an ultrafine powder.
Owner:SAMSUNG CORNING PRECISION MATERIALS CO LTD +1
Forming clear, wettable silicone hydrogel articles without surface treatments
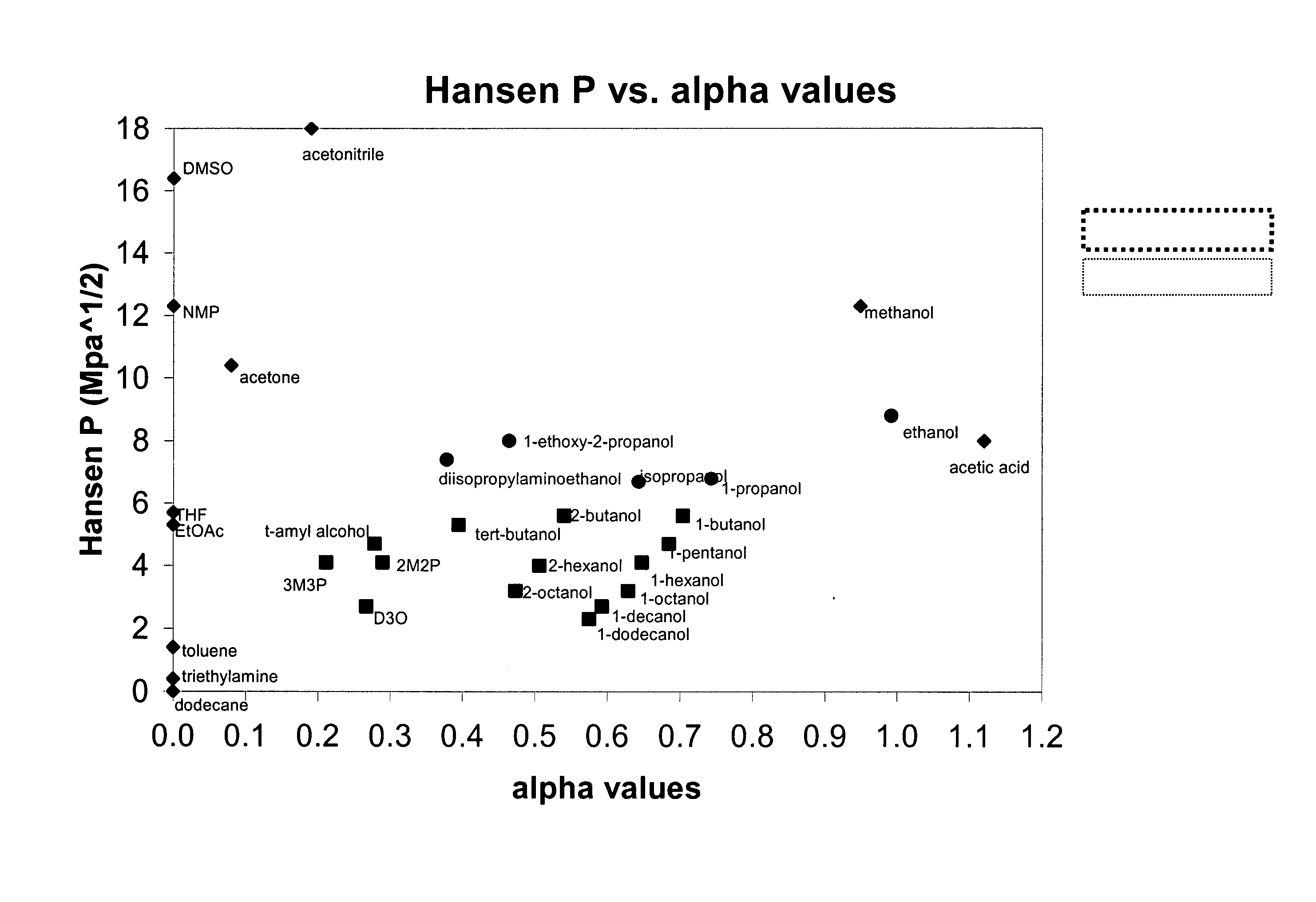
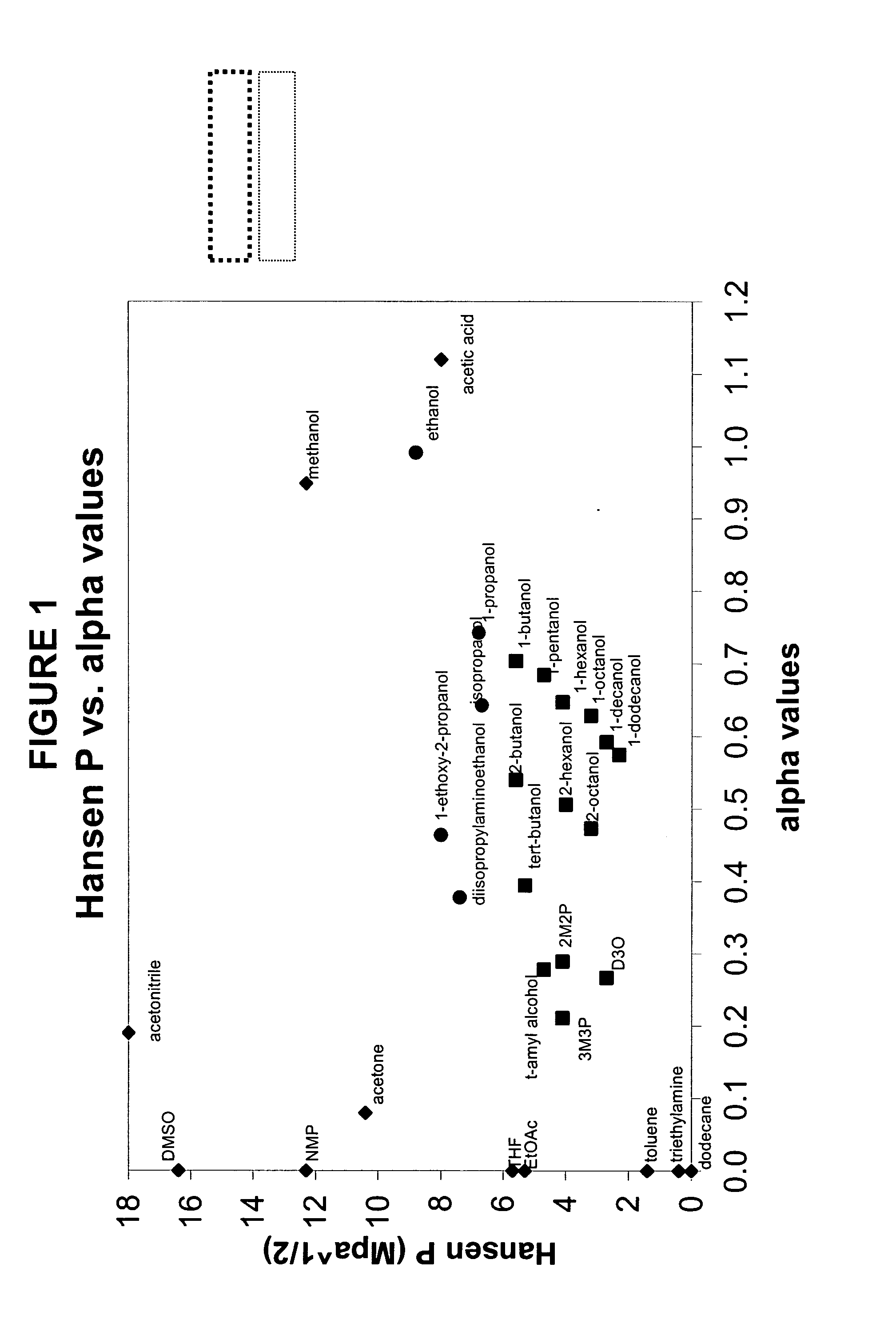
The present invention is a composition, which may be used to form contact lenses, comprising at least one silicone containing component, at least one hydrophilic component, at least one high molecular weight hydrophilic polymer and at least one diluent with an alpha value of about 0.5 to about 1 and a Hansen solubility parameter of less than about 10.
Owner:JOHNSON & JOHNSON VISION CARE INC
System and method of improving emission performance of a gas turbine
InactiveUS20110138766A1Improve emission effectReduce oxygen concentrationEngine fuctionsGas turbine plantsHigh pressureOxygen
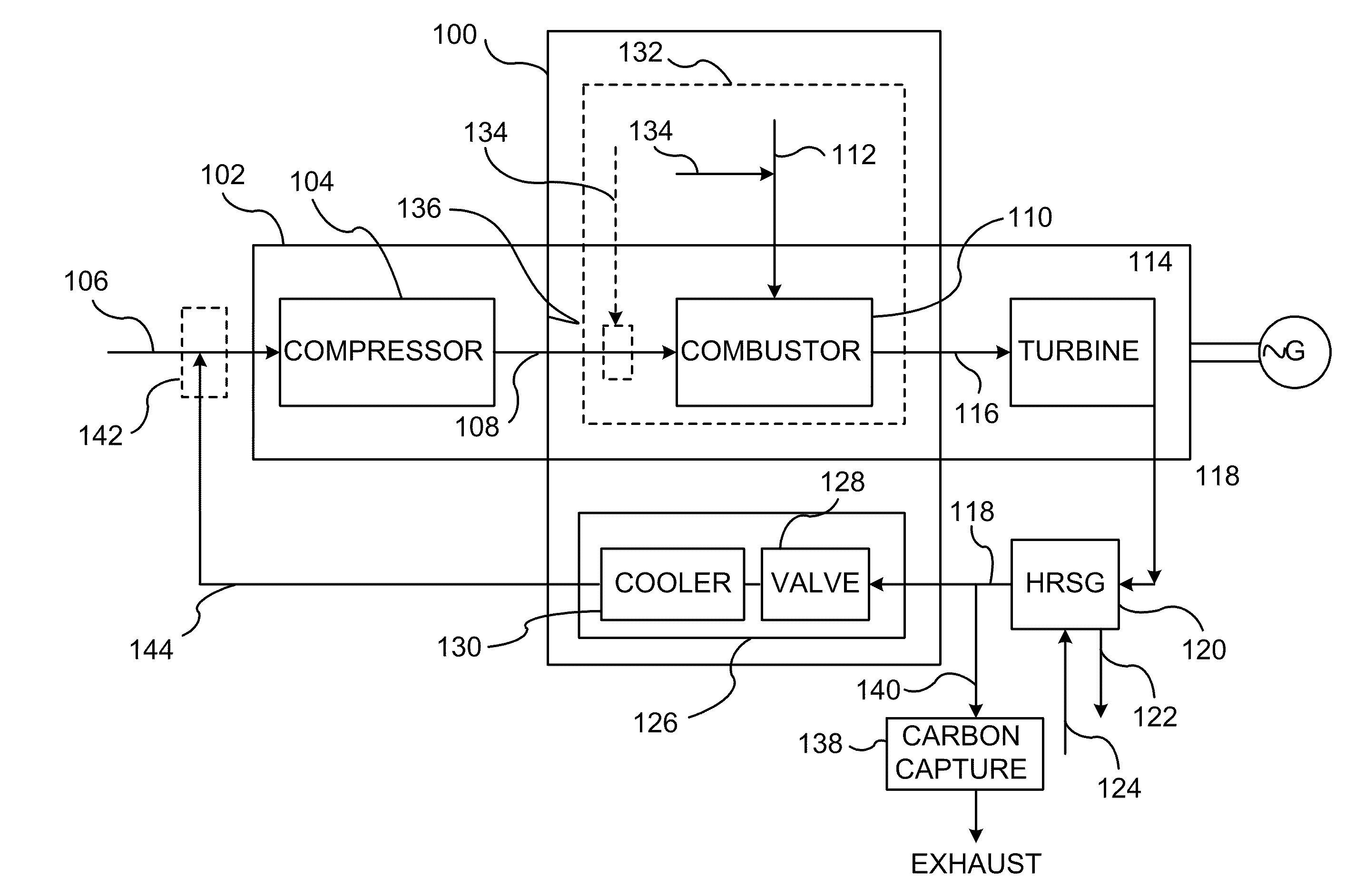
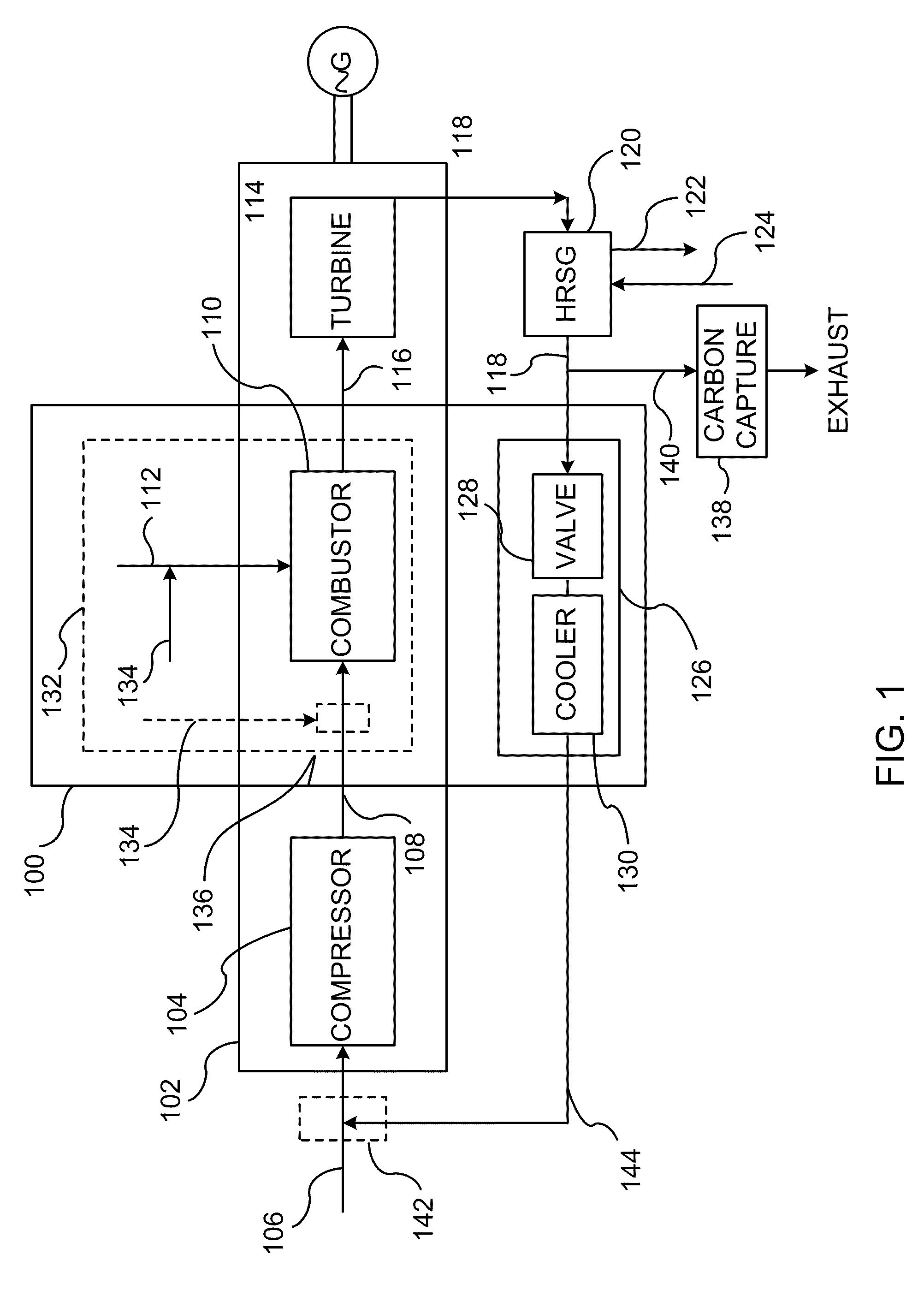
A method of improving emission performance of a gas turbine is provided. The method includes recirculating a portion of an exhaust gas stream to a compressor of the gas turbine via an exhaust gas recirculating system, to reduce concentration of oxygen in a high pressure feed oxidant stream into a combustor of the gas turbine. The method further includes adding diluent to at least one of a fuel stream directed to the combustor or a low pressure feed oxidant stream directed to the compressor, to reduce concentration of oxides of nitrogen (NOx) and increase concentration of carbon dioxide in a resultant exhaust gas stream.
Owner:GENERAL ELECTRIC CO
System, method, and computer program product for handling, mixing, dispensing, and injecting radiopharmaceutical agents
InactiveUS20050277833A1Reduce processingReduce manual handlingInfusion syringesMedical devicesDiluentEngineering
The present invention is directed to a system, method, and computer program product for handling, mixing, dispensing and / or injecting a mixture into an individual during a medical procedure. The present invention provides one or more mixing devices, containers, and dispensing devices to facilitate the handling, mixing, dispensing, and / or injecting of a mixture containing, for example, pharmaceutical agents and / or radiopharmaceutical agents. The present invention also provides a mixing device capable of diluting a radiopharmaceutical agent with, for instance, a diluent, for altering a radiation dose emitted by the radiopharmaceutical agent.
Owner:ACIST MEDICAL SYST
Polymerization processes
Owner:EXXONMOBIL CHEM PAT INC
Rapid acting drug delivery compositions
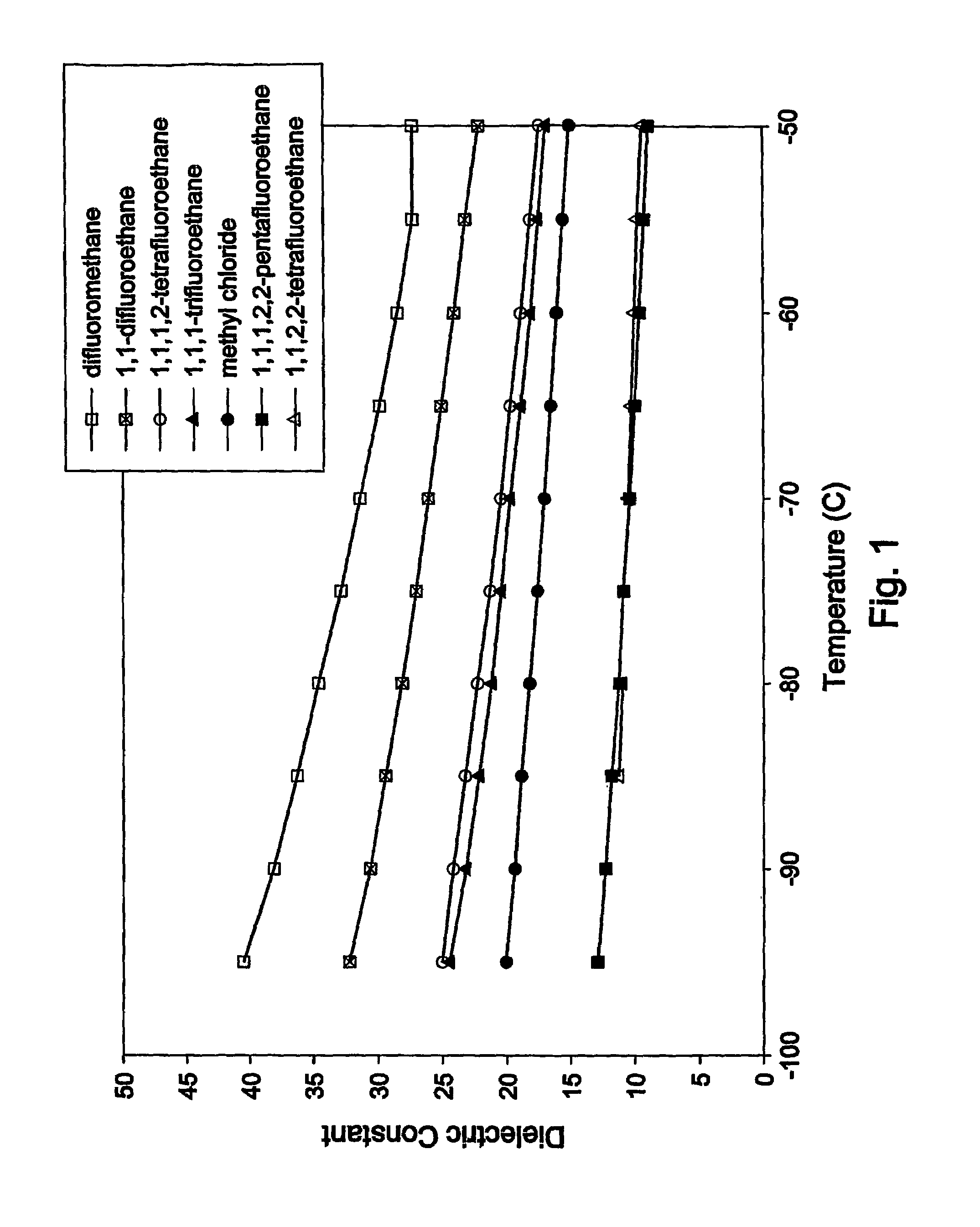
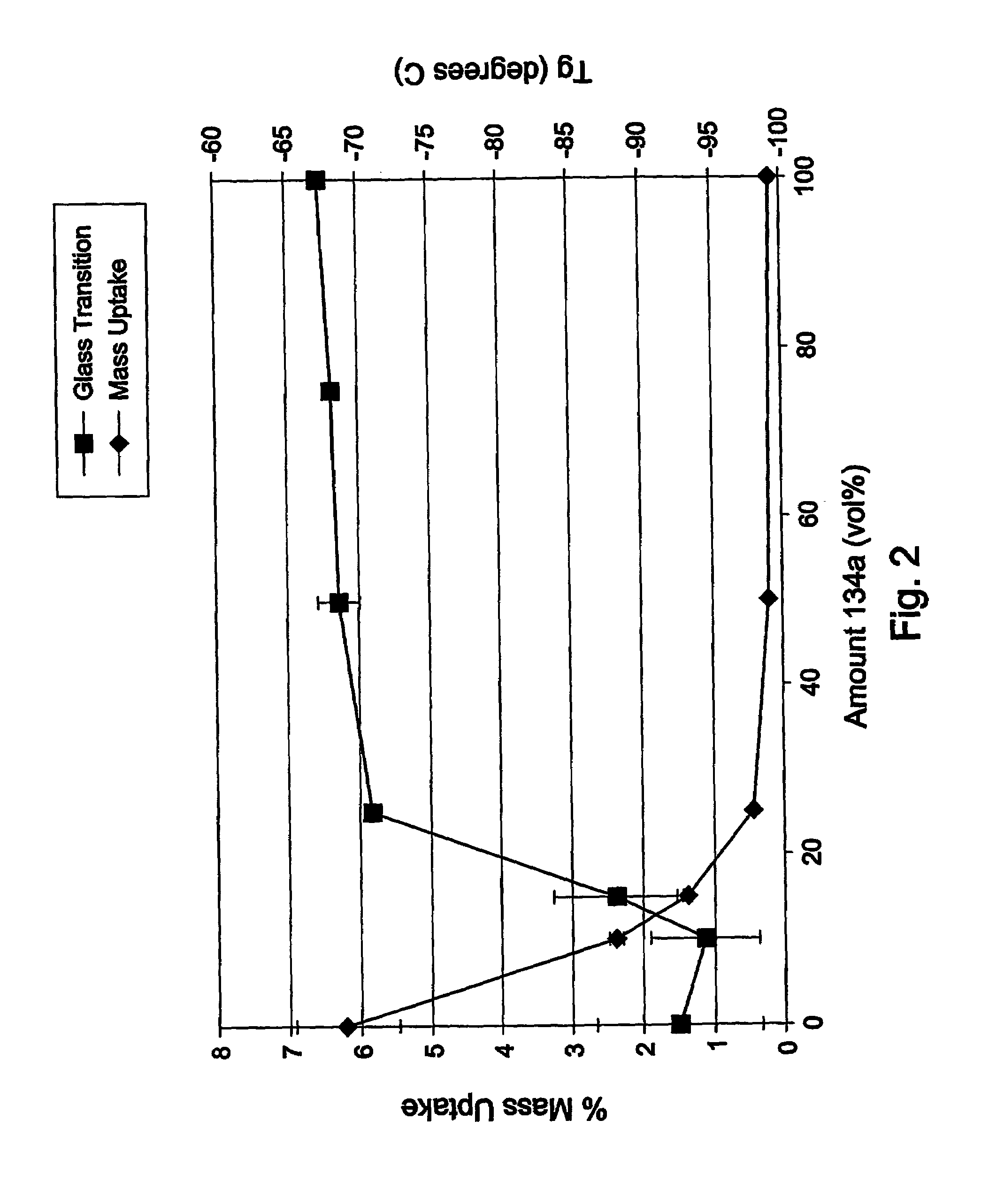
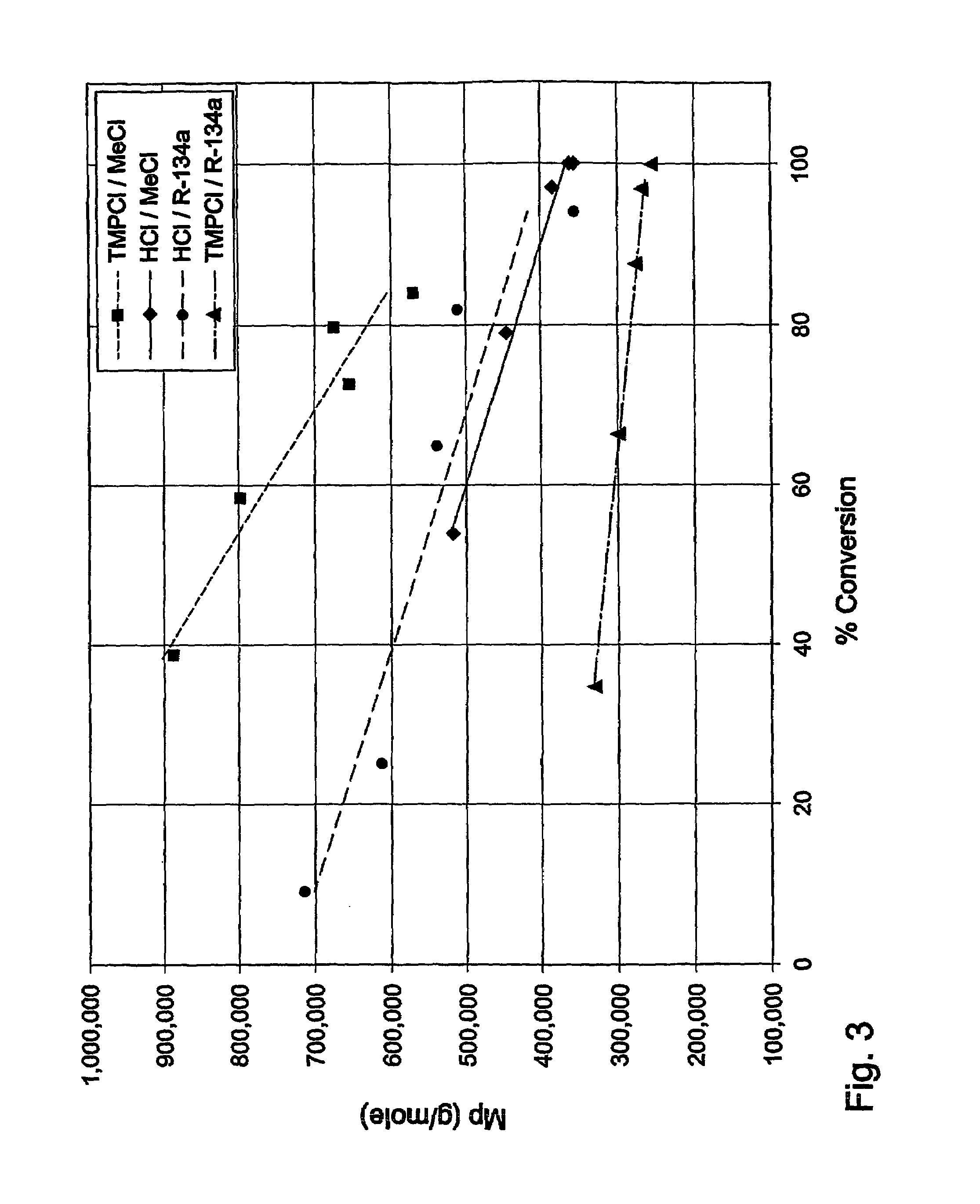
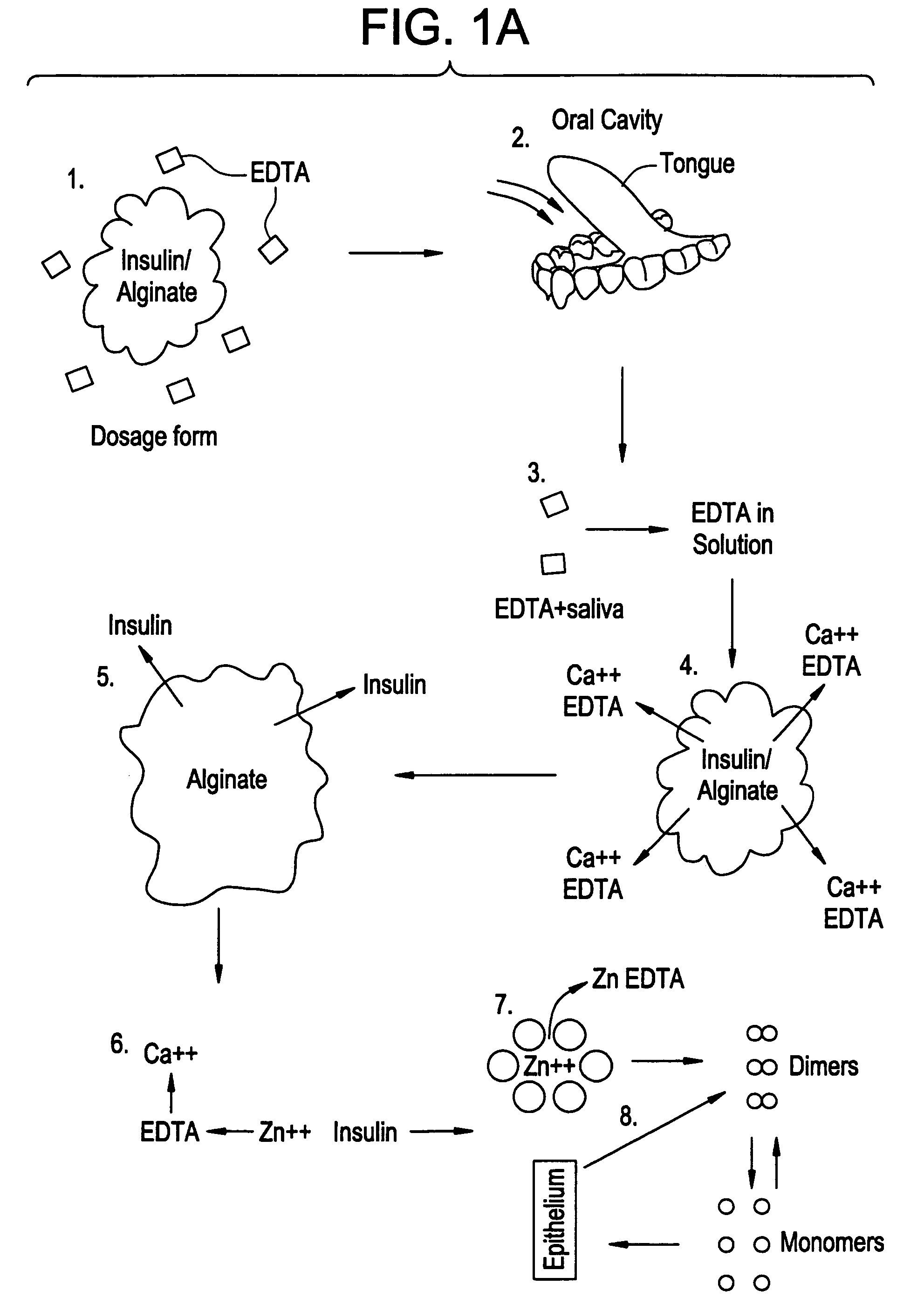
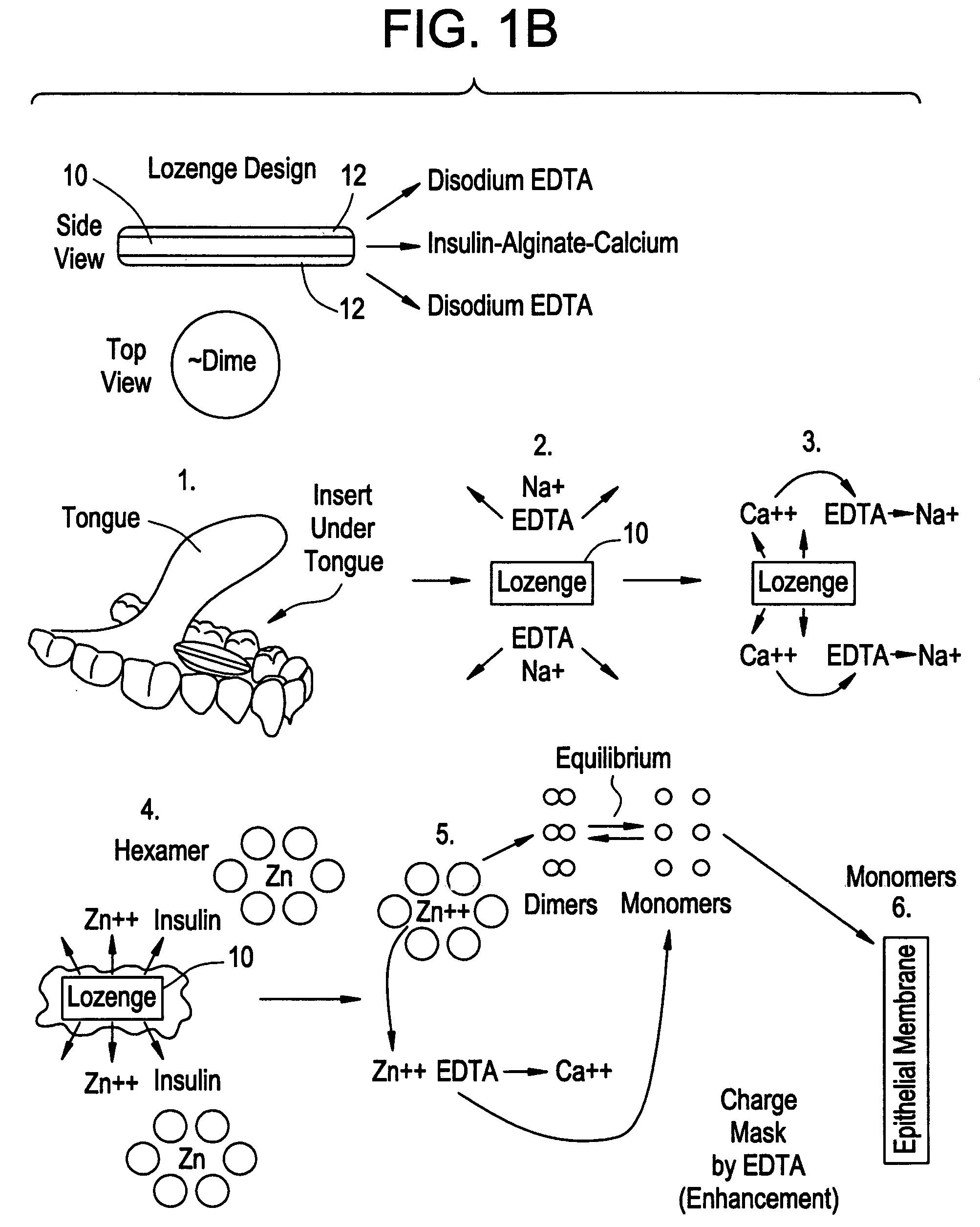
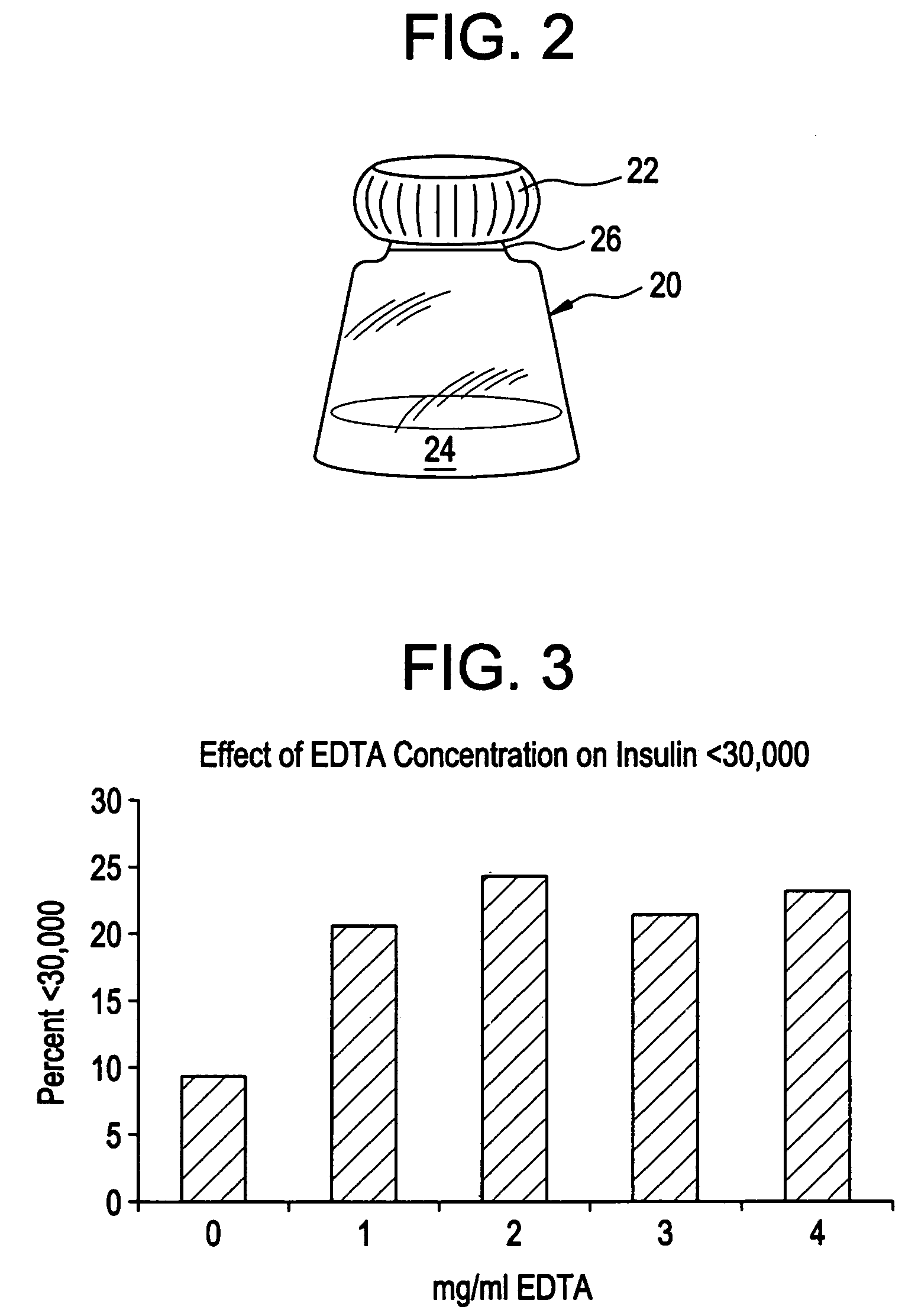
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Process for producing polyolefins
InactiveUS6355741B1Reduce amountReduce the amount requiredChemical/physical/physico-chemical stationary reactorsChemical recyclingPolyolefinDiluent
A process for producing polyolefins having a bimodal molecular weight distribution, the process comprising producing a first polyolefin fraction in the presence of a catalyst in a first loop reactor, and producing a second polyolefin fraction in the presence of the catalyst in a second loop reactor which is serially connected to and downstream of the first loop reactor, the first and second polyolefin fractions being blended in the second loop reactor to form a polyolefin having a bimodal molecular weight distribution, at least the first loop reactor containing a diluent under supercritical conditions which is circulated around the loop of the reactor, and wherein at least the first loop reactor is provided with a fluff concentrating device communicating with the loop and in which polyolefin fluff of the first fraction is concentrated in the supercritical diluent, and polyolefin fluff of the first polyolefin fraction is transferred together with an amount of supercritical diluent from the fluff concentrating device of the first loop reactor into the second loop reactor.
Owner:FINA RES SA
Compositions and methods for the treatment of disease
InactiveUS20040209805A1Efficient transferAvoid cross contaminationOrganic active ingredientsPeptide/protein ingredientsAdjuvantMedicine
The present invention relates to pharmaceutical compositions for the treatment and / or prophylaxis of disease associated with fibrosis in a vertebrate, said composition comprising at least one activin antagonist, and optionally a pharmaceutically acceptable carrier, adjuvant and / or diluent. The invention also relates to methods of treatment of disease associated with fibrosis in a vertebrate, as well as methods for diagnosing such conditions, and kits therefor.
Owner:MONASH UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com