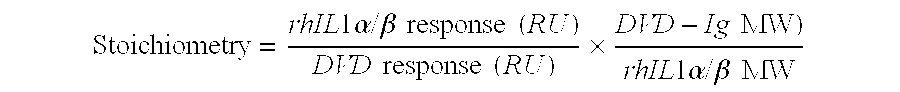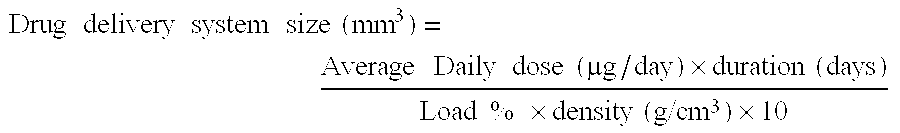Patents
Literature
29688 results about "Inflammation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Conditions characterized by inflammation including allergy and autoimmunity.
Avenanthramide-containing compositions
Methods and compositions for treating or preventing a skin condition, an inflammation, an irritation or an allergy associated with an ectoparasitic infection or infestation on an animal. The methods involve applying to the skin of the animal a pharmaceutical composition that contains a therapeutically effective amount of one or more than one avenanthramide, an optional ecto and / or endo-parasiticidal agent, and a pharmaceutically acceptable diluent or carrier
Owner:CEAPRO
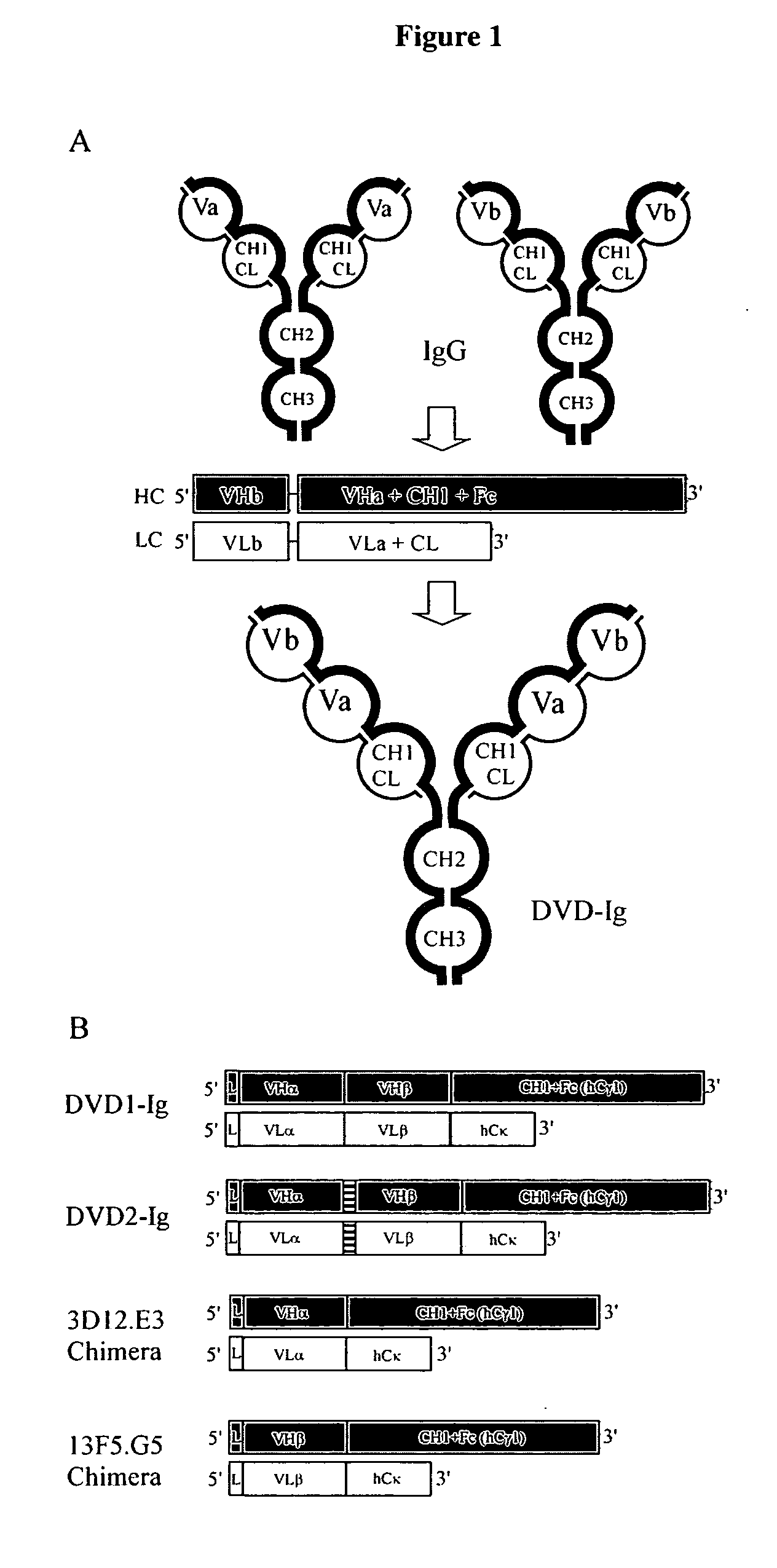
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
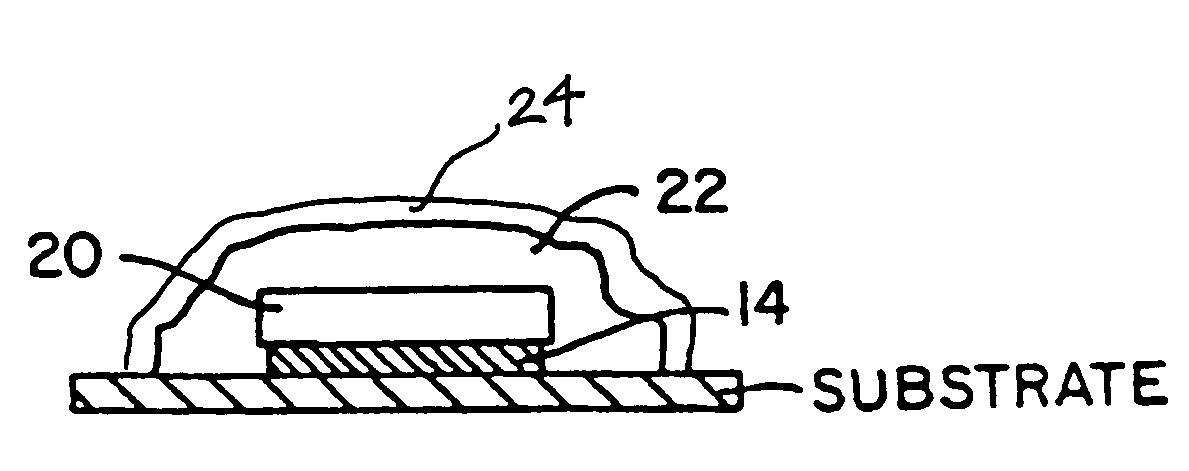
Anti-inflammatory biosensor for reduced biofouling and enhanced sensor performance
A biosensor including an external surface, and an accessory material in close proximity to the external surface. The accessory material includes a coating containing a hydrophilic material and / or a fiber modified to deliver a therapeutic agent. The biosensor modifies a biological response to the biosensor upon contact with a tissue, such as upon implantation into the skin of a subject, thereby reducing biofouling, inflammation and other undesirable tissue responses that interfere with biosensor performance. The biosensor can be any biocompatible sensor, suitable for short- or long-term use. Preferably, the biosensor is an enzymatic or electrochemical sensor, such as a glucose sensor. Also provided are a method of producing a biosensor and a method of delivering a biologically active substance to a subject.
Owner:MEDTRONIC MIMIMED INC
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Device and method for attenuating an immune response
ActiveUS20050075701A1Good flexibilityMore levelsSpinal electrodesImplantable neurostimulatorsNervous systemNeuron
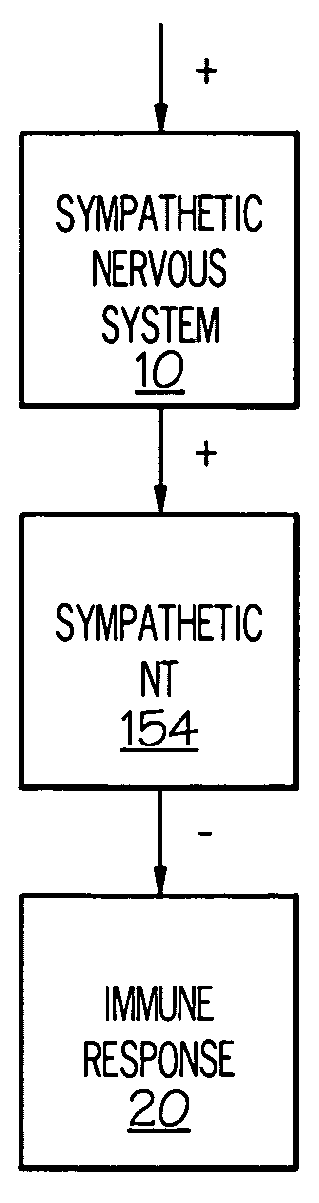
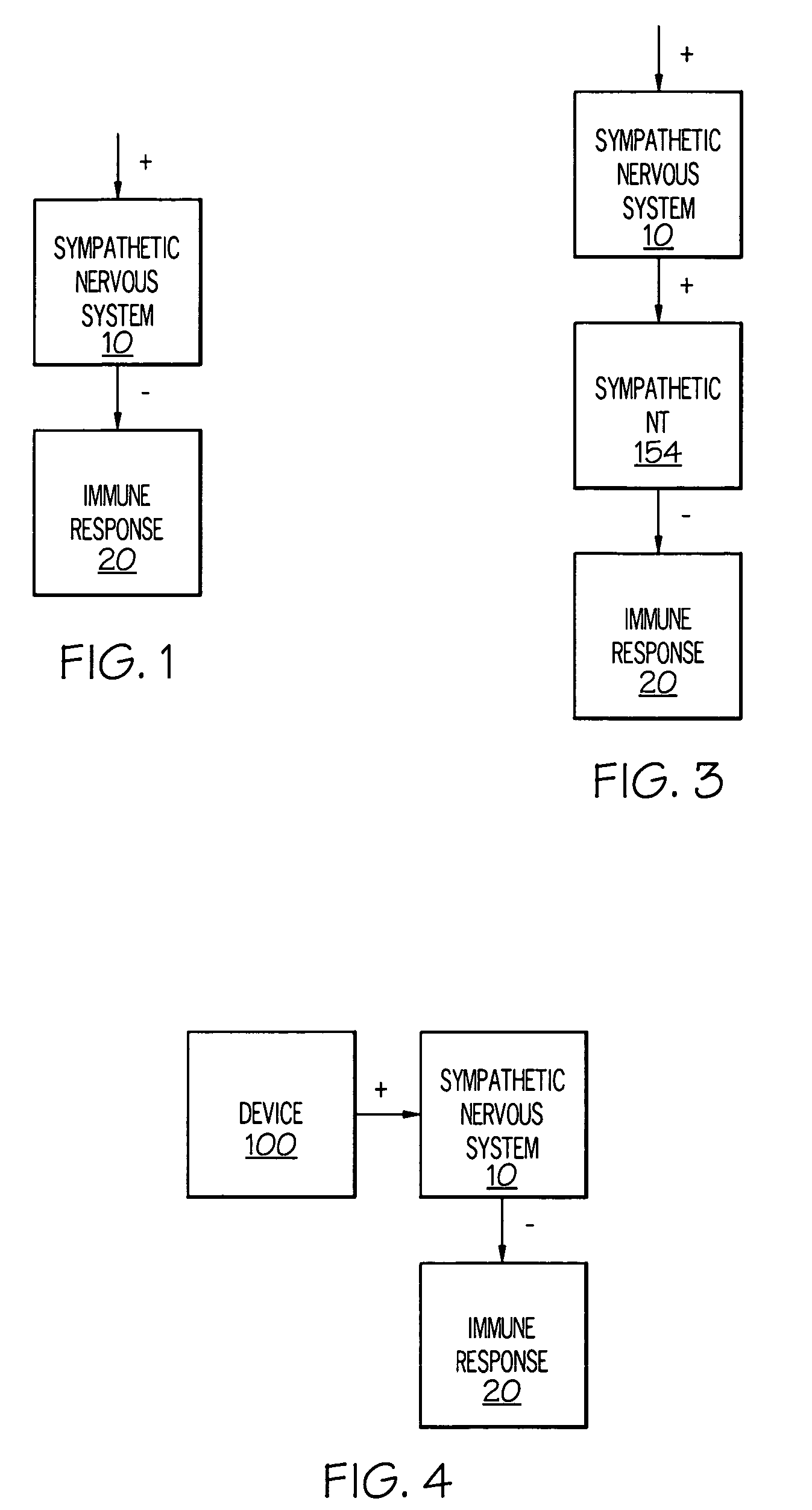
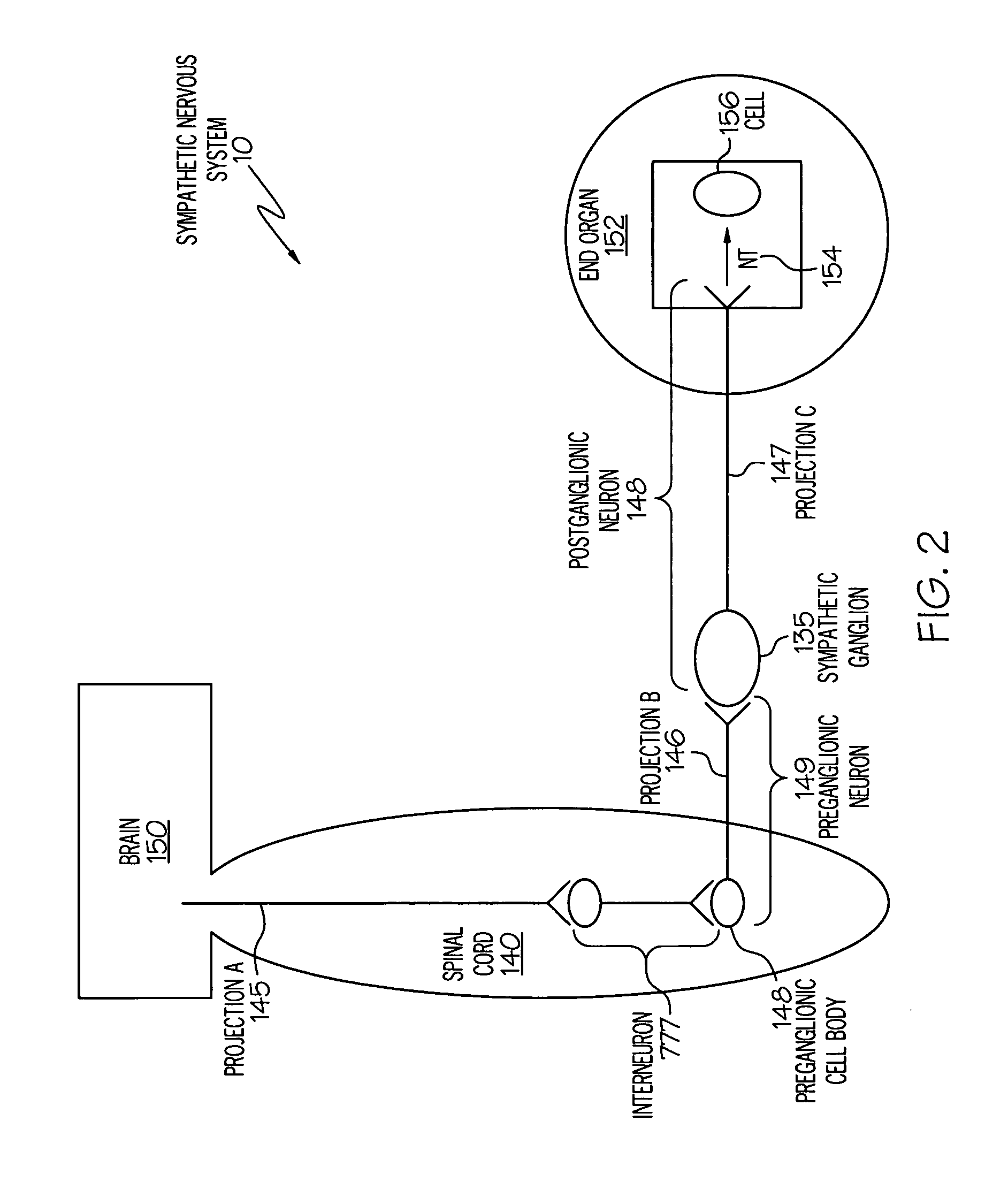
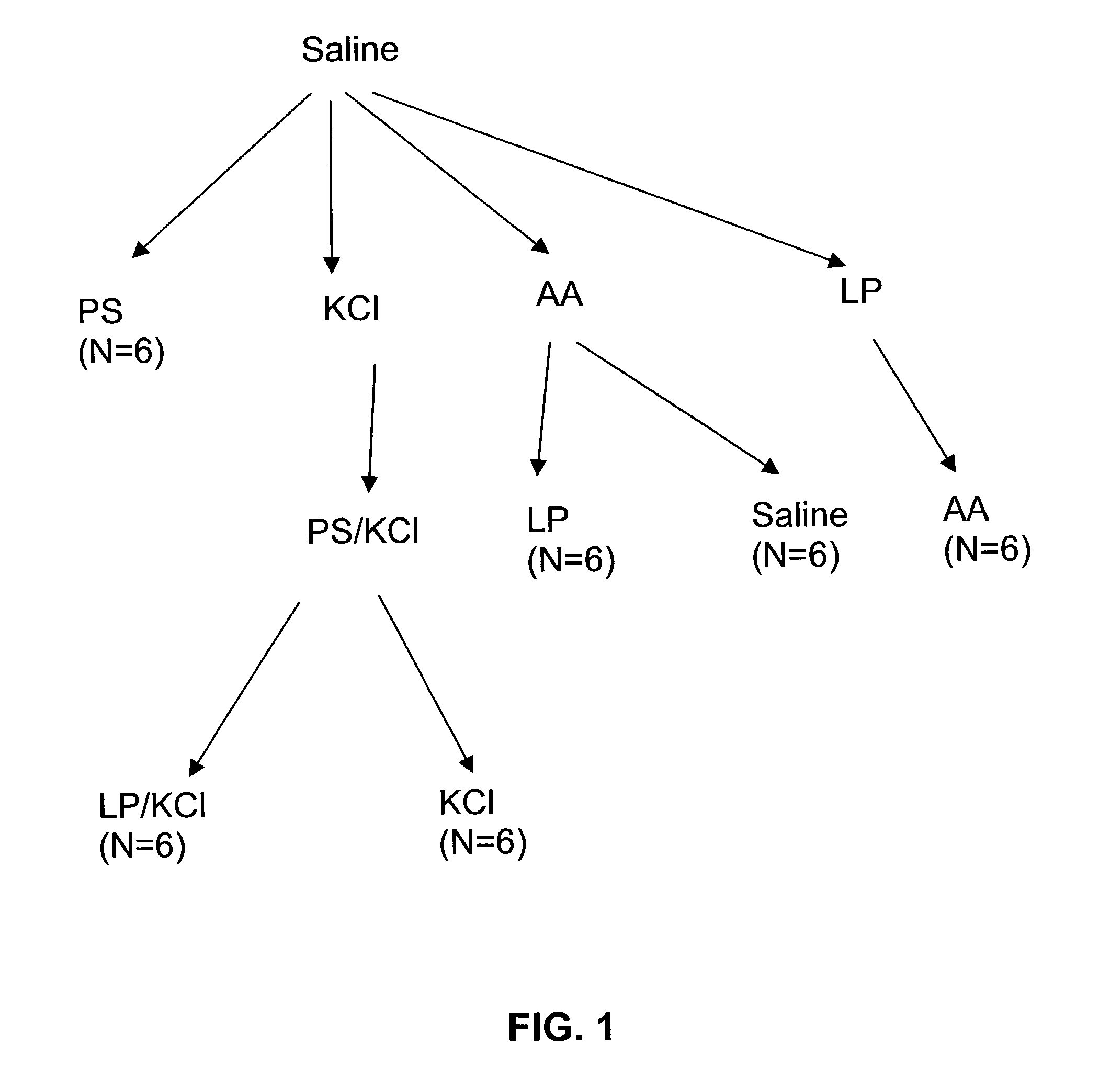
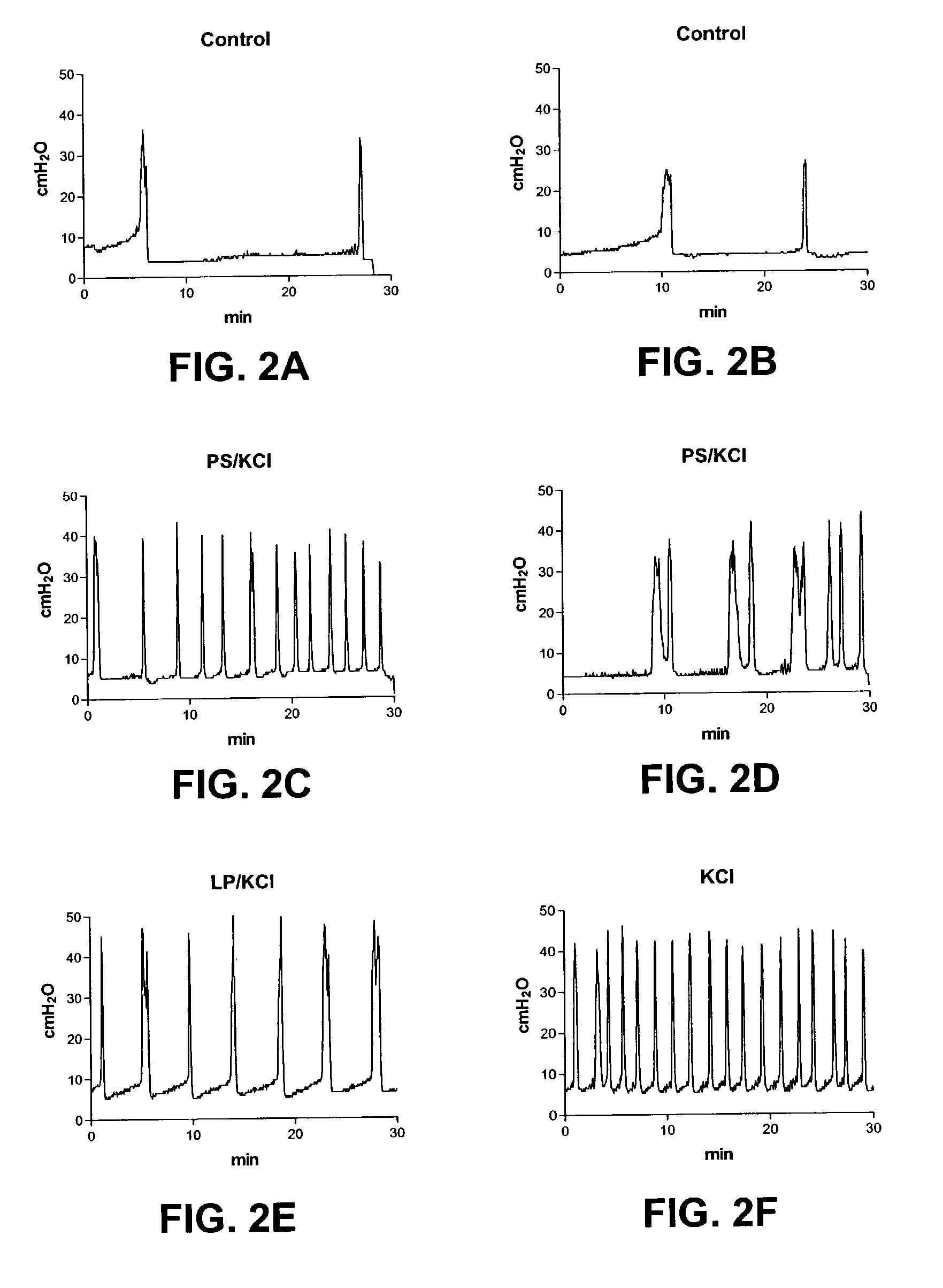
Stimulation of one or more neurons of the sympathetic nervous system, including the splenic nerve, to attenuate an immune response, including an inflammatory immune response, is discussed. Devices and systems to stimulate the sympathetic nervous system to attenuate an immune response are also discussed. Devices discussed include pulse generators and drug pumps. Systems are described as optionally having one or more sensors and operator instructions. In specific examples, stimulation of the splenic nerve of pigs with a pulse generator is shown to be safe and effective in attenuating a lipopolysaccharide-induced immune response.
Owner:MEDTRONIC INC
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Application of lipid vehicles and use for drug delivery
InactiveUS7063860B2Reduce and prevent antibody-mediated resistanceIncrease stimulationBiocideAntipyreticAnticarcinogenCapsaicin
The present invention relates to compositions and methods for the administration of lipid-based vehicles to treat various disorders, including bladder inflammation, infection, dysfunction, and cancer. In various aspects, the compositions and methods of the invention are useful for prolonged delivery of drugs, e.g., antibiotics, pain treatments, and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system, and other organs or body systems. In particular, the present invention relates to liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin, and toxins, such as botulinum toxin, for the treatment of bladder conditions, including pain, inflammation, incontinence, and voiding dysfunction. Further related are methods of using these vehicles alone or in conjunction with antibodies, e.g., uroplakin antibodies, to improve duration of liposome attachment, and provide a long-term intravesical drug delivery platform. The present invention specifically relates to antibody-coated liposomes that are useful for targeting specific receptors for drug, peptide, polypeptide, or nucleic acid delivery. In one particular aspect, the present invention relates to liposomes coated with antibodies against nerve growth factor (NGF) receptor and containing NGF antisense nucleic acids, which are used as a treatment for neurogenic bladder dysfunction.
Owner:UNIVERSITY OF PITTSBURGH
Methods and compositions for reducing or eliminating post-surgical adhesion formation
The present invention relates to a method for reducing adhesions associated with post-operative surgery. The present method comprises administering or affixing a polymeric composition preferably comprising chain extended, coupled or crosslinked polyester / poly(oxyalkylene) ABA triblocks or AB diblocks having favorable EO / LA ratios to a site in the body which has been subjected to trauma, e.g. by surgery, excision or inflammatory disease. In the present invention, the polymeric material provides a barrier to prevent or reduce the extent of adhesions forming.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
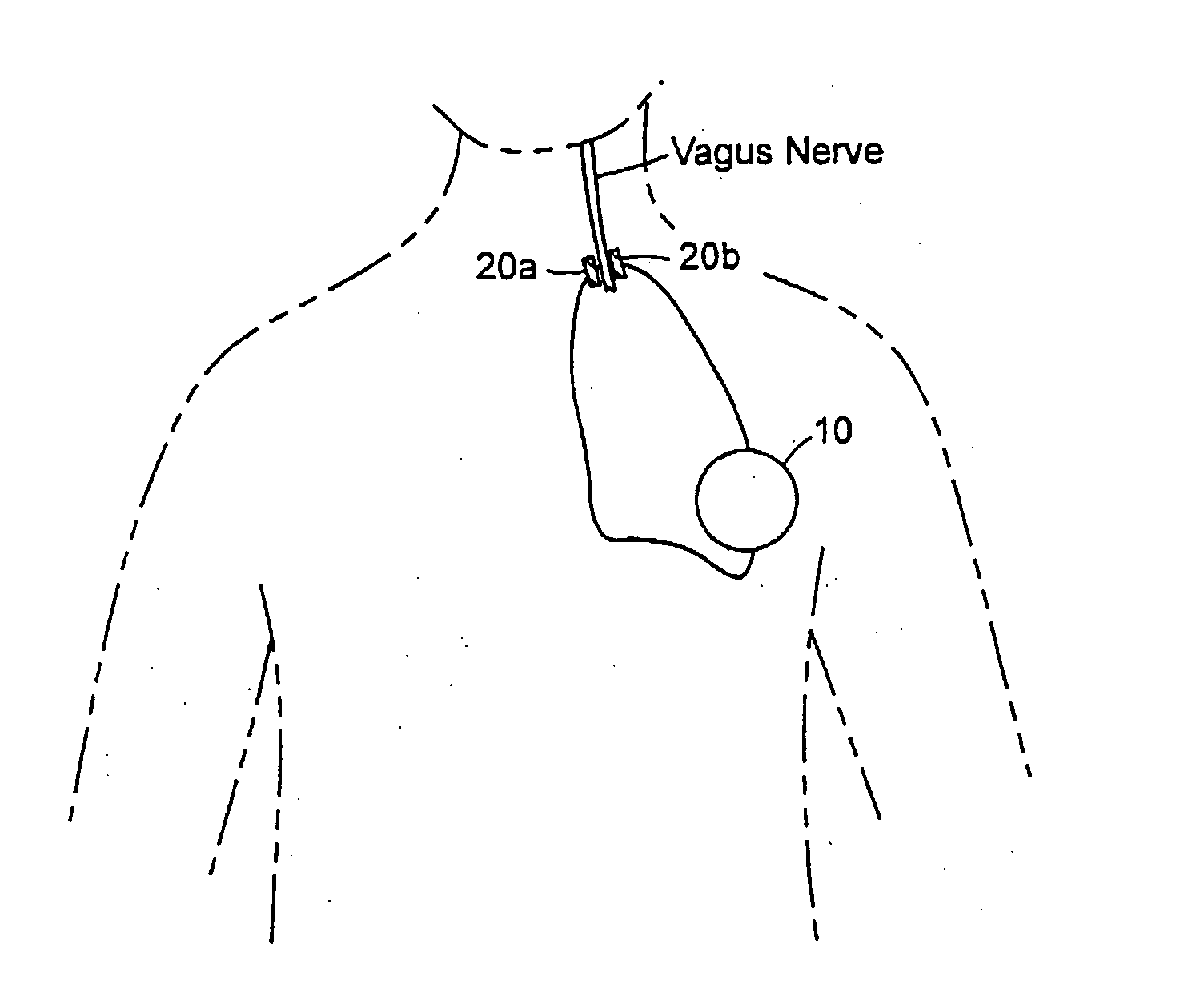
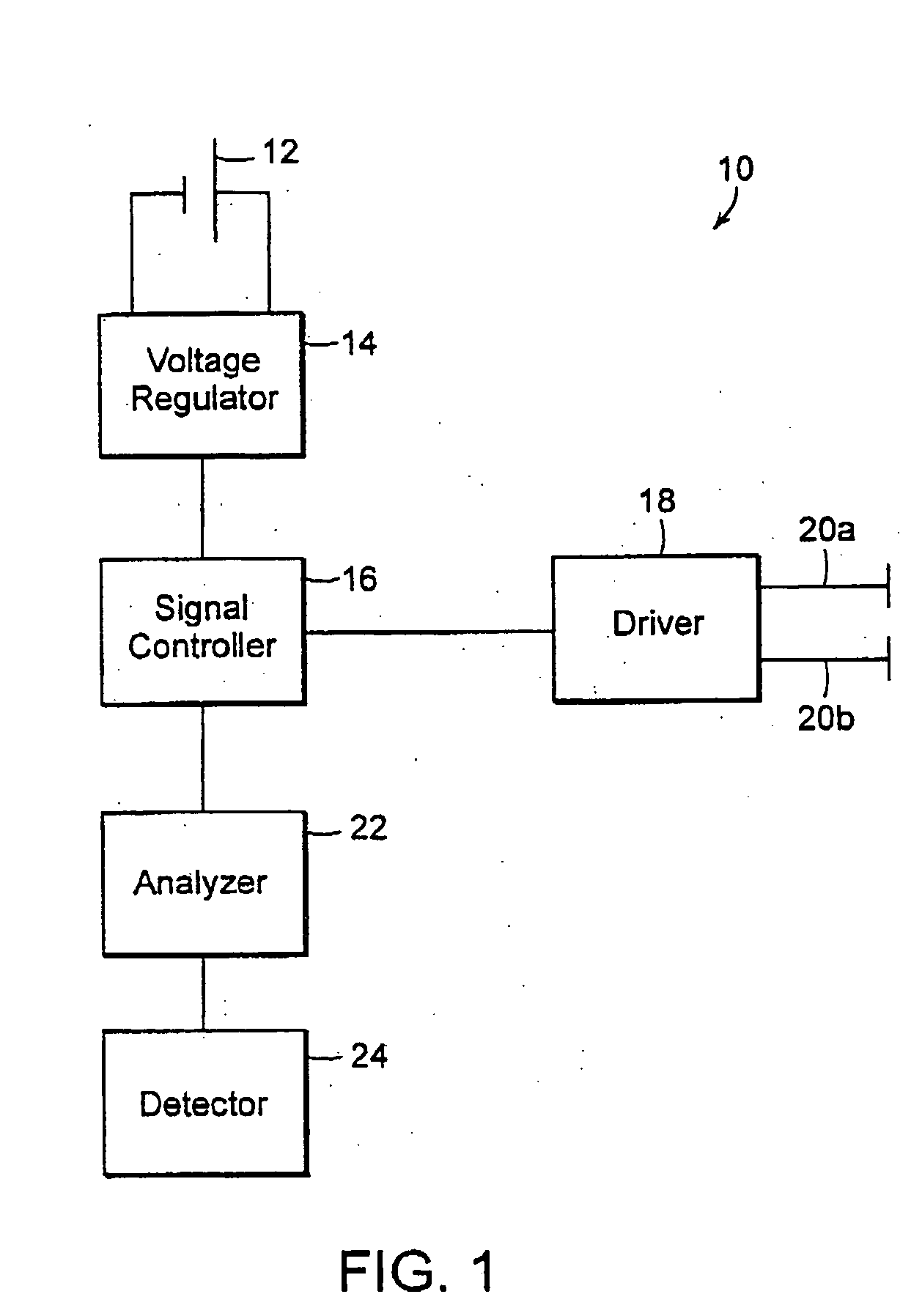
Treating inflammatory disorders by electrical vagus nerve stimulation
A method and an apparatus for treating a patient suffering from, or at risk for, a condition mediated by the inflammatory cytokine cascade, by electrically stimulating vagus nerve activity in an amount sufficient to inhibit the inflammatory cytokine cascade.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Sensors for detecting substances indicative of stroke, ischemia, infection or inflammation
ActiveUS20080176271A1Thickness minimizationTransport of glucose to the sensor is not altered over timeStentsMicrobiological testing/measurementMetaboliteNitric oxide
A system is disclosed that extracts bodily fluid to a reaction chamber for monitoring a substance or property of the patient fluid. In one embodiment, a pump is used to advance the sample of bodily fluid through a filter to produce a filtrate. Another pump advances filtrate into the reaction chamber, while another pump advances reactant into the reaction chamber. A sensor in communication with the reaction chamber determines a concentration of nitric oxide or one of its metabolic products. Methods are also disclosed.
Owner:SILVER JAMES H
Methods and drug delivery systems for the treatment of orofacial diseases
This invention relates to methods of treating various orofacial diseases involving inflammation, infection and / or pain, using intratissue controlled release drug delivery systems. More particularly, the invention relates to methods for localized or targeted administration of a sustained release formulation of an agent such as an anti-inflammatory agent to a specified tissue location within the orofacial environment.
Owner:HALLUX
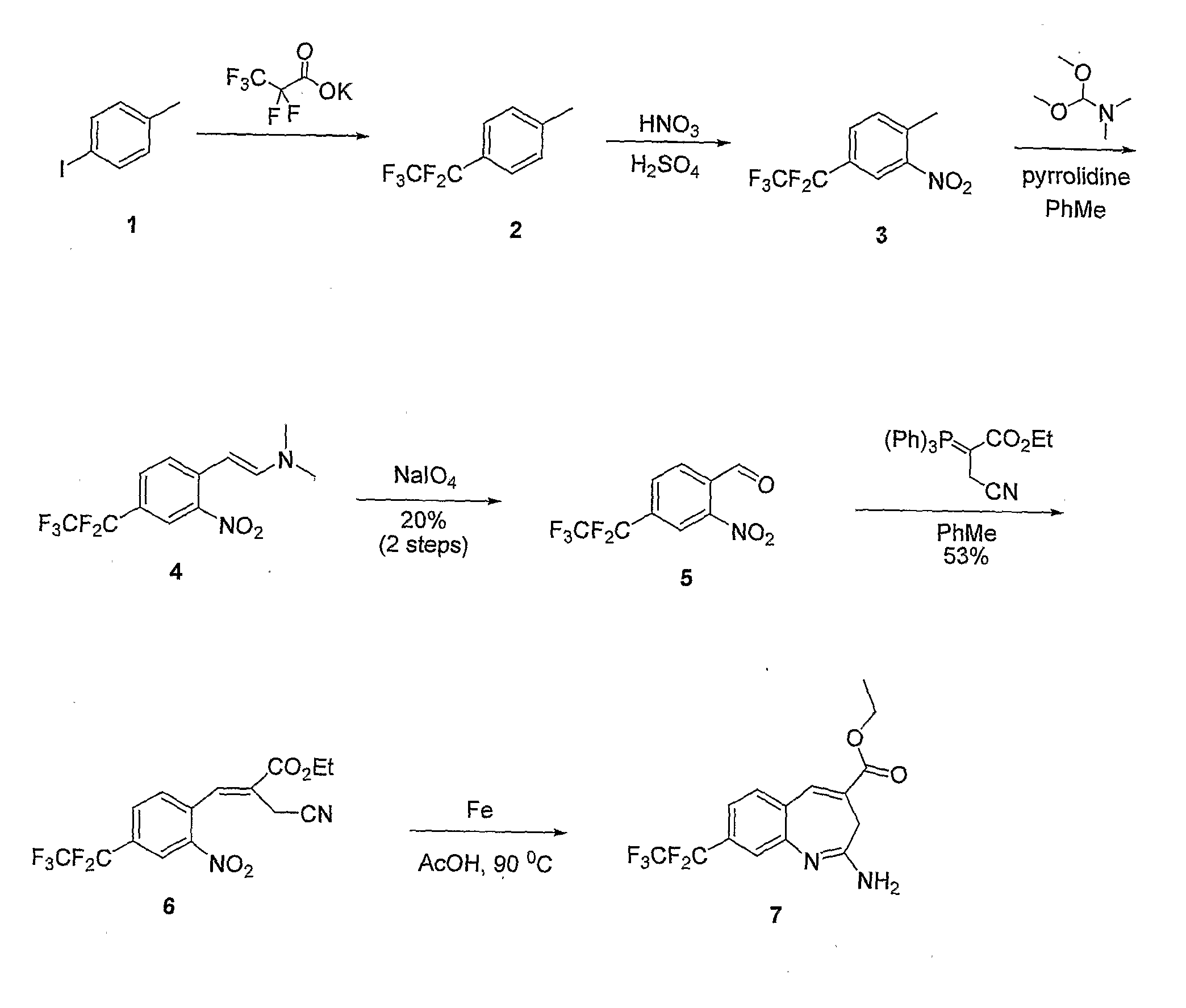
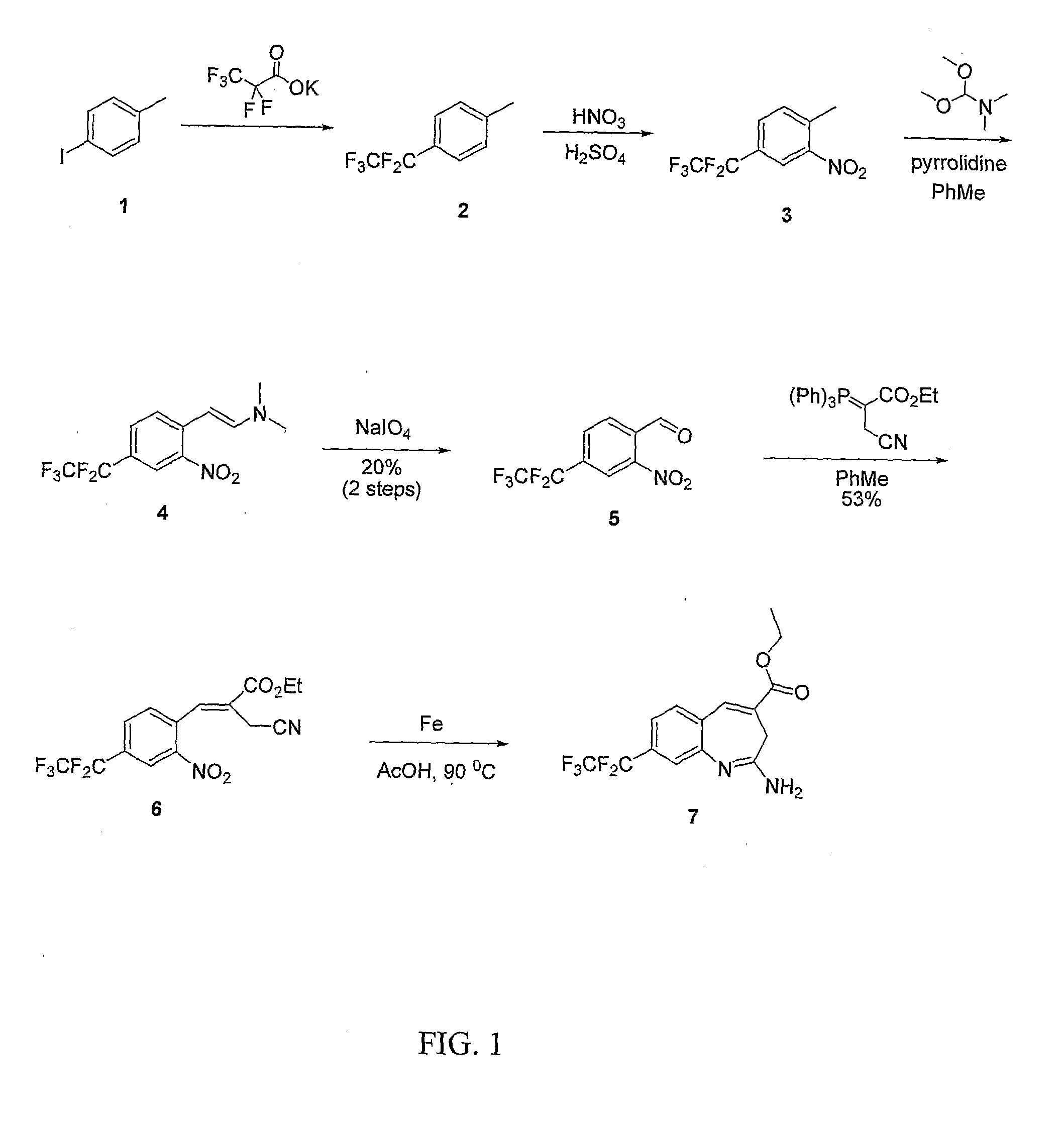
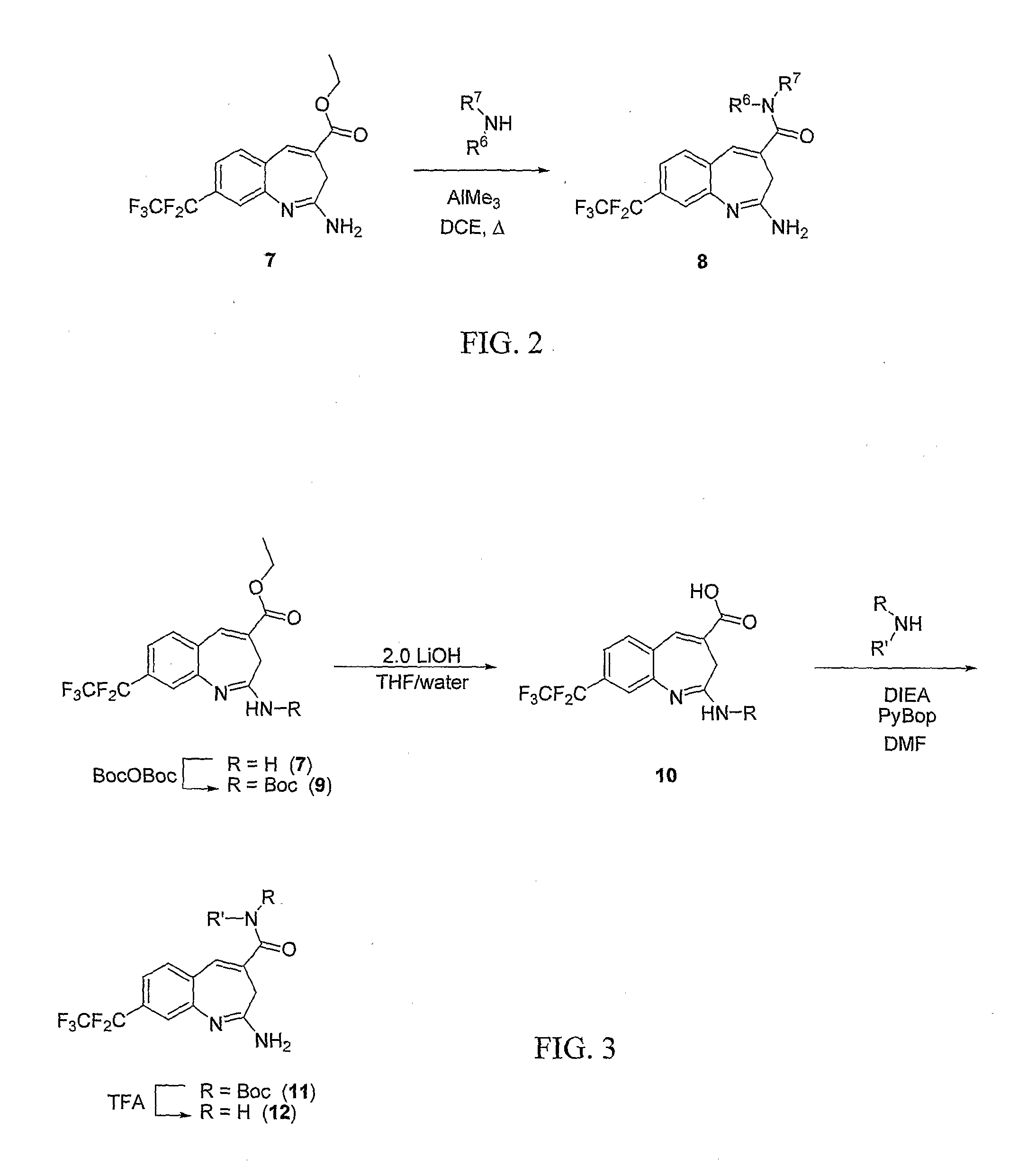
8-Substituted Benzoazepines as Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in the treatment of autoimmunity, inflammation allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer and immunodeficiency.
Owner:ARRAY BIOPHARMA
Implant to be implanted in bone tissue or in bone tissue supplemented with bone substitute material
InactiveUS6921264B2High softening temperatureLow softening temperatureDental implantsInternal osteosythesisBone tissueGrowth promoting
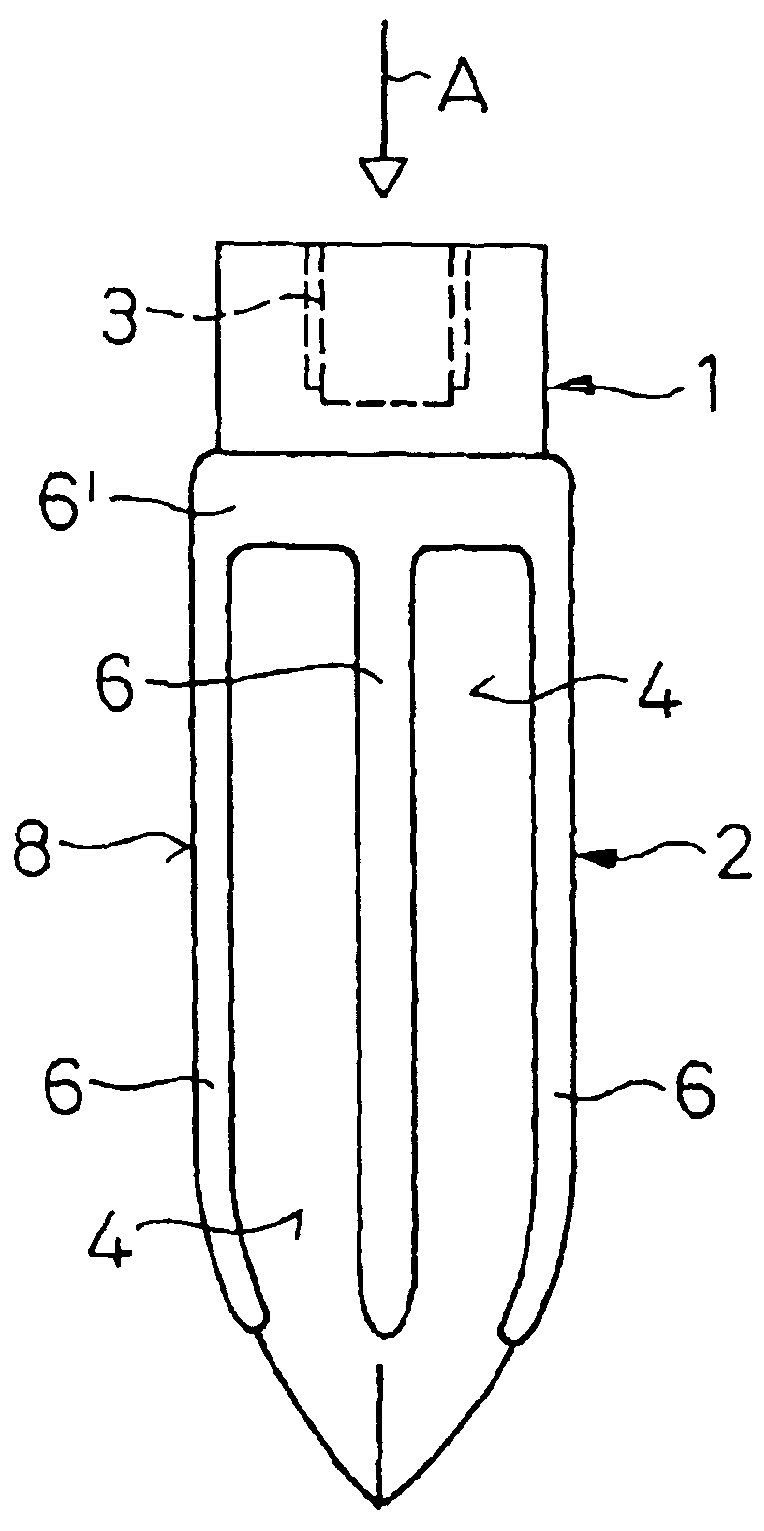
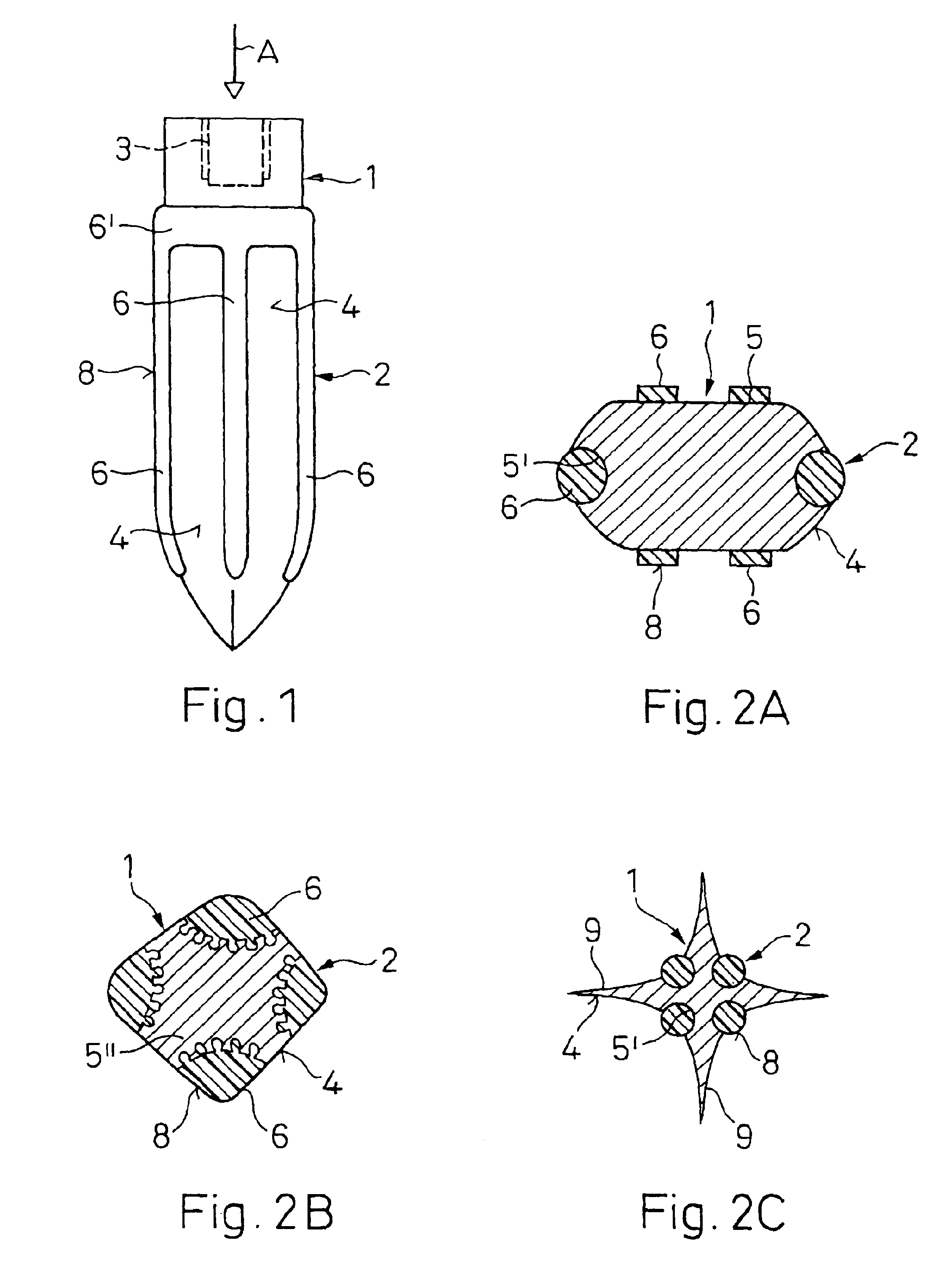
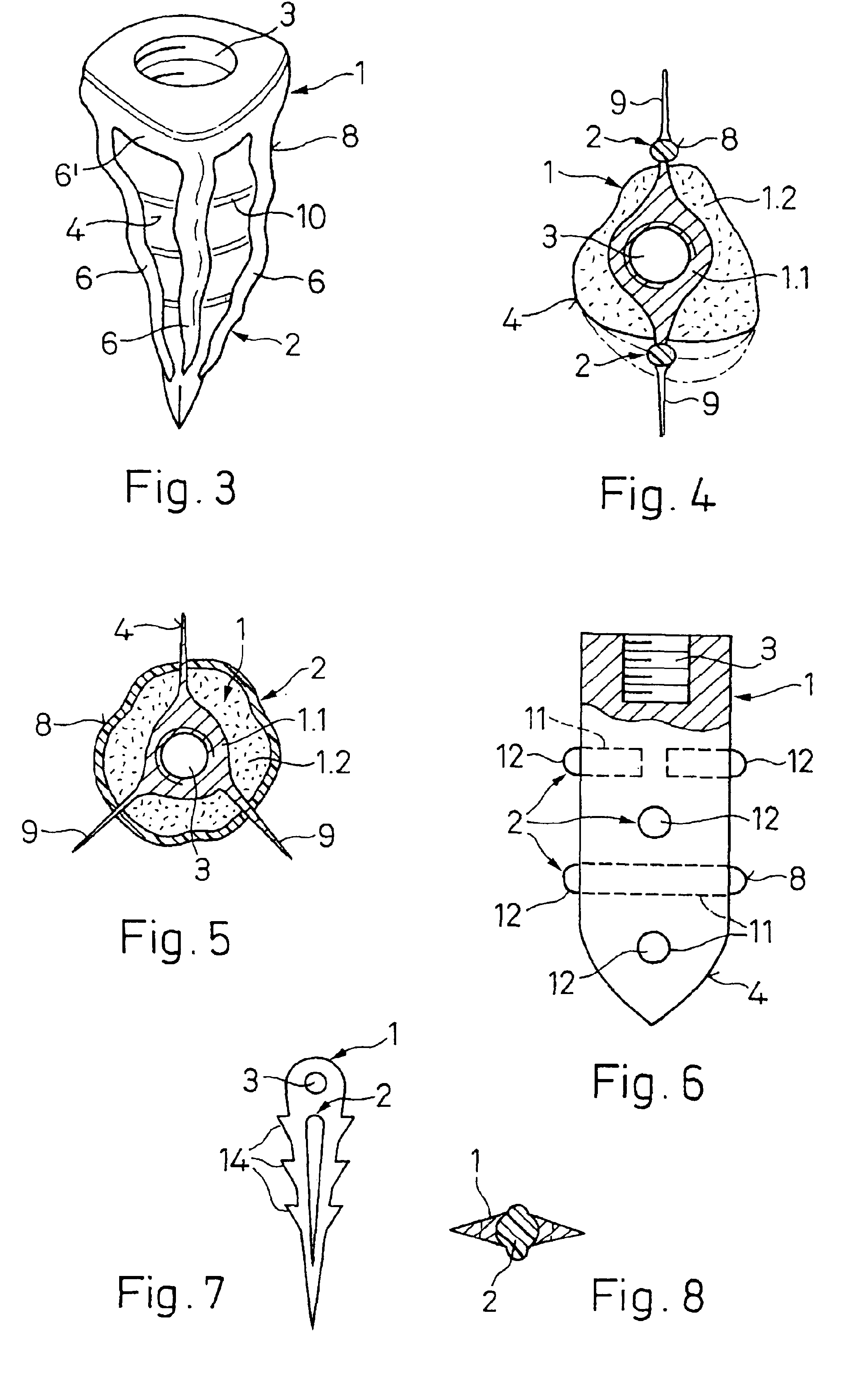
An implant (1) to be implanted in bone tissue, e.g. a dental implant or an implant for an orthopedic application, comprises surface regions (4) of a first type which have e.g. osseo-integrative, inflammation-inhibiting, infection-combating and / or growth-promoting properties, and surface regions (8) of a second type which consist of a material being liquefiable by mechanical oscillation. The implant is positioned in an opening of e.g. a jawbone and then mechanical oscillations, e.g. ultrasound is applied to it while it is pressed against the bone. The liquefiable material is such liquefied at least partly and is pressed into unevennesses and pores of the surrounding bone tissue where after resolidification it forms a positive-fit connection between the implant and the bone tissue. The surface regions of the two types are arranged and dimensioned such that, during implantation, the liquefied material does not flow or flows only to a clinically irrelevant degree over the surface regions of the first type such enabling the biologically integrative properties of these surface regions to start acting directly after implantation. The implant achieves with the help of the named positive fit a very good (primary) stability, i.e. it can be loaded immediately after implantation. By this, negative effects of non-loading are prevented and relative movements between implant and bone tissue are reduced to physiological measures and therefore have an osseo-integration promoting effect.
Owner:WOODWELDING
Small molecule toll-like receptor (TLR) antagonists
The invention provides methods and compositions useful for modulating signaling through Toll-like receptors. The methods involve contacting a TLR-expressing cell with a small molecule having a core structure including at least two rings. Certain of the compounds are 4-primary amino quinolines. Many of the compounds and methods are useful specifically for inhibiting immune stimulation involving at least one of TLR9, TLR8, TLR7, and TLR3. The methods may have use in the treatment of autoimmunity, inflammation, allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer, and immunodeficiency.
Owner:COLEY PHARMA GMBH +1
Degraded agonist antibody
InactiveUS20040242847A1Excellent antigen-binding propertyExcellent agonist activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAntiendomysial antibodies
The invention relates to a modified antibody which contains two or more H chain V regions and two or more L chain V regions of monoclonal antibody and can transduce a signal into cells by crosslinking a cell surface molecule(s) to thereby serve as an agonist. The modified antibody can be used as a signal transduction agonist and, therefore, useful as a preventive and / or remedy for various diseases such as cancer, inflammation, hormone disorders and blood diseases.
Owner:CHUGAI PHARMA CO LTD
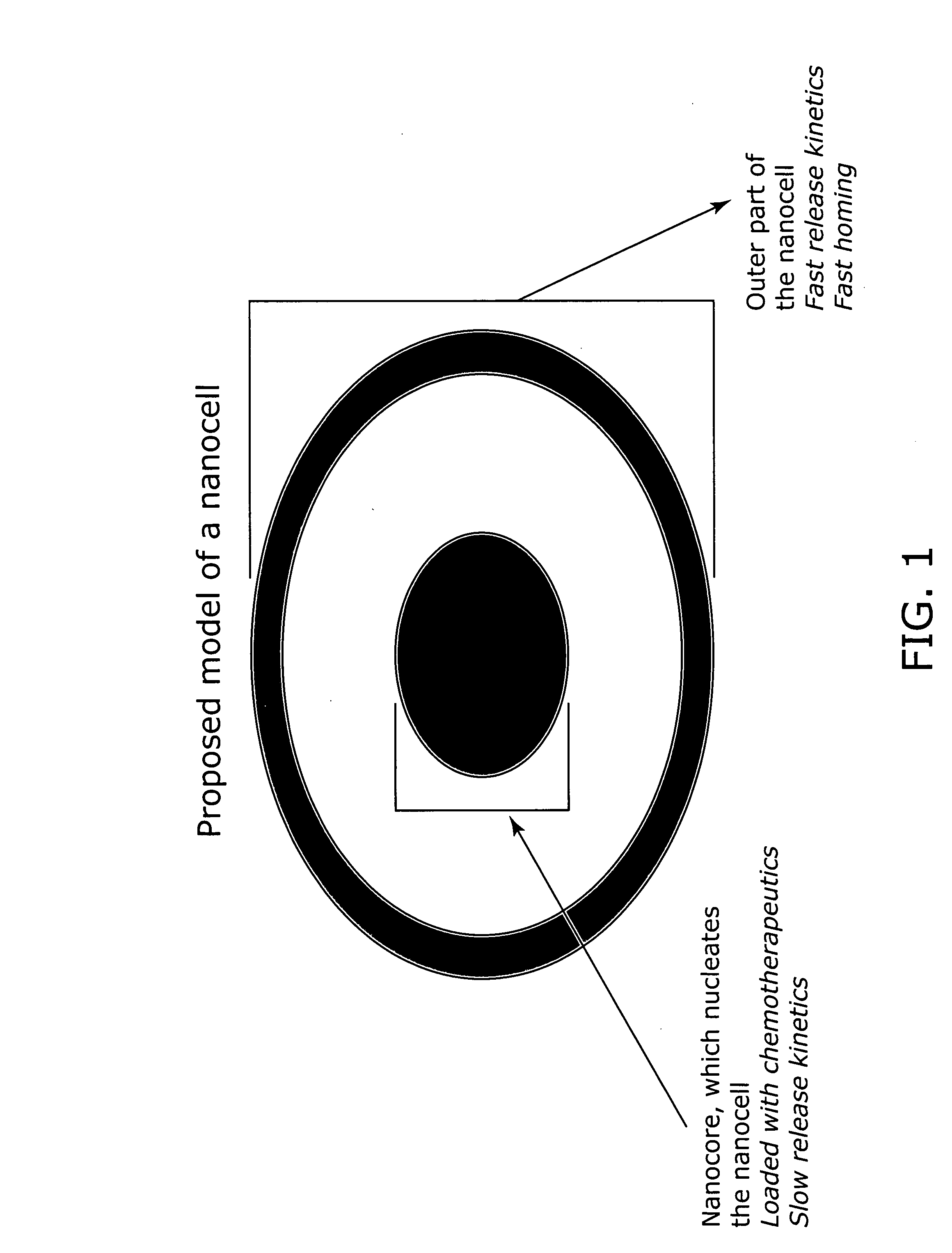
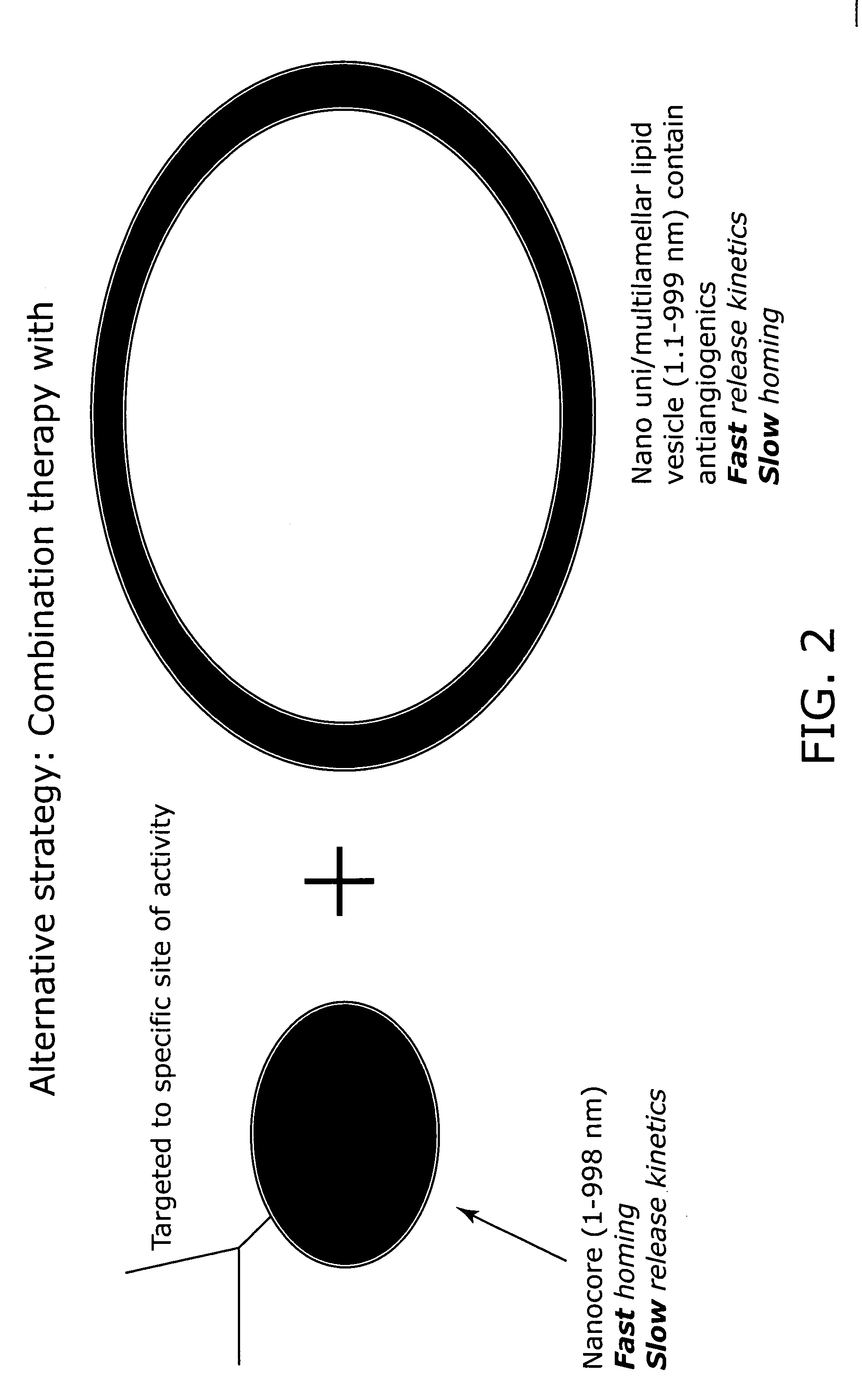
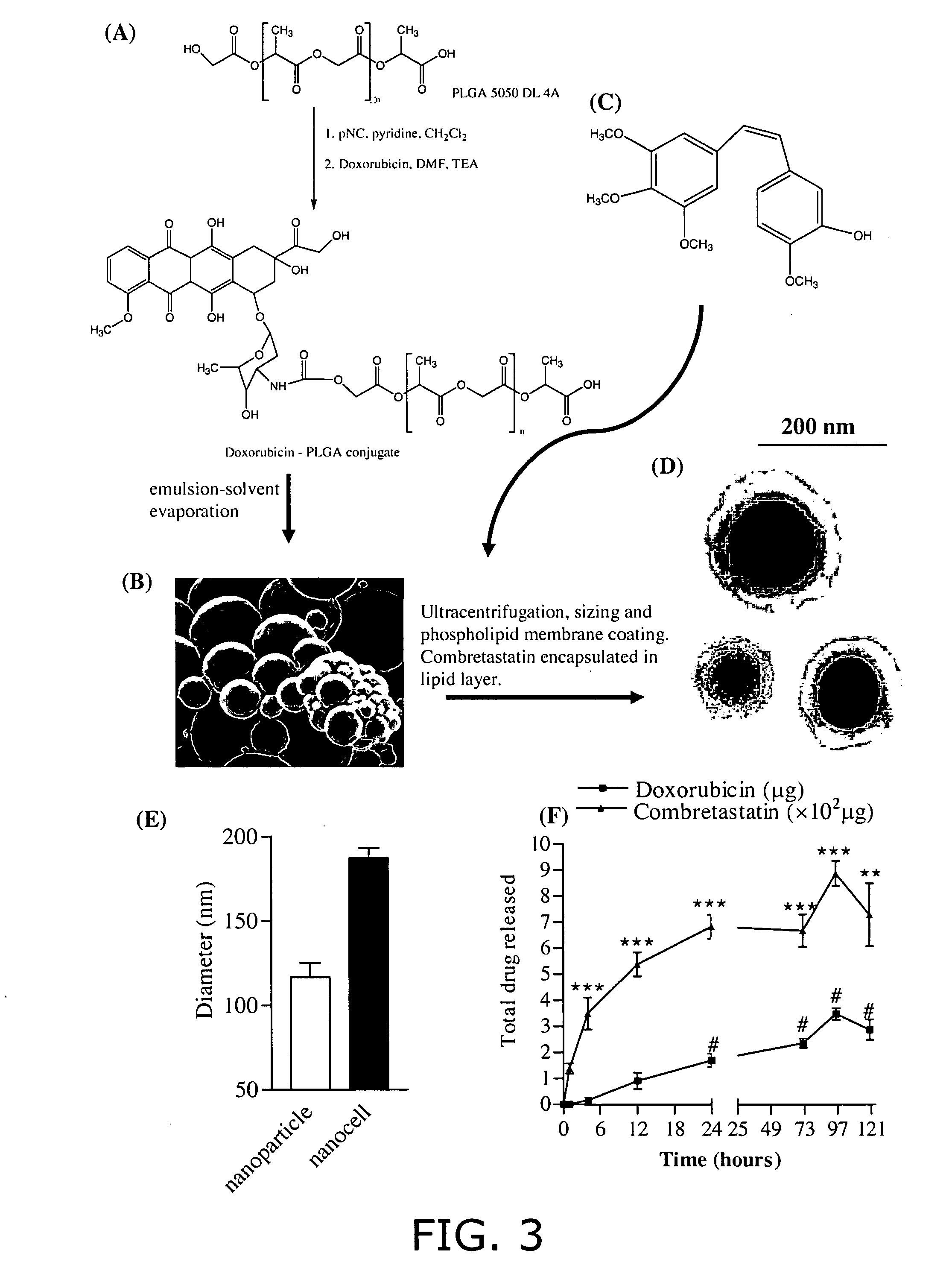
Nanocell drug delivery system
InactiveUS20050266067A1Avoid flowIncreased toxicityAntibacterial agentsOrganic active ingredientsLipid formationAntigen
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
Nanoparticle Mediated Ultrasound Therapy and Diagnostic Imaging
InactiveUS20080045865A1Enhanced and localized and targeted hyperthermiaMinimizing collateral damageUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyDiseaseTissue repair
The present invention relates to systems and methods for localized delivery of heat, useful for localized imaging and treatment of a biological material. The systems and methods of the invention can be utilized for localized treatment of cancer, inflammation or other disorders involving over proliferation of tissue, and for tissue repair. The method comprises exposing nanoparticles to electromagnetic radiation under conditions wherein the nanoparticles generate microbubbles which emit heat when exposed to ultrasonic radiation.
Owner:KISLEV HANOCH
Method and tools for oral hygiene
InactiveUS6561808B2Increase motivation for treatmentImprove adhesionCosmetic preparationsImpression capsOral diseasePhotosensitizer
A method and material for self-cleaning of the teeth and mouth using a source of light in the visible range in conjunction with a photosensitive oral hygiene composition possessing a broad absorption spectrum in the visible range. The invention selectively eliminates harmful bacteria by use of a photosensitive agent and a light source. The present invention involves the use of a light-providing dental device to activate a photosensitive agent and destroy harmful bacteria in the oral cavity. It prevents or deters oral diseases, inflammations, and infections.
Owner:BIOLITEC UNTERNEHMENSBETEILLIGUNGS II AG
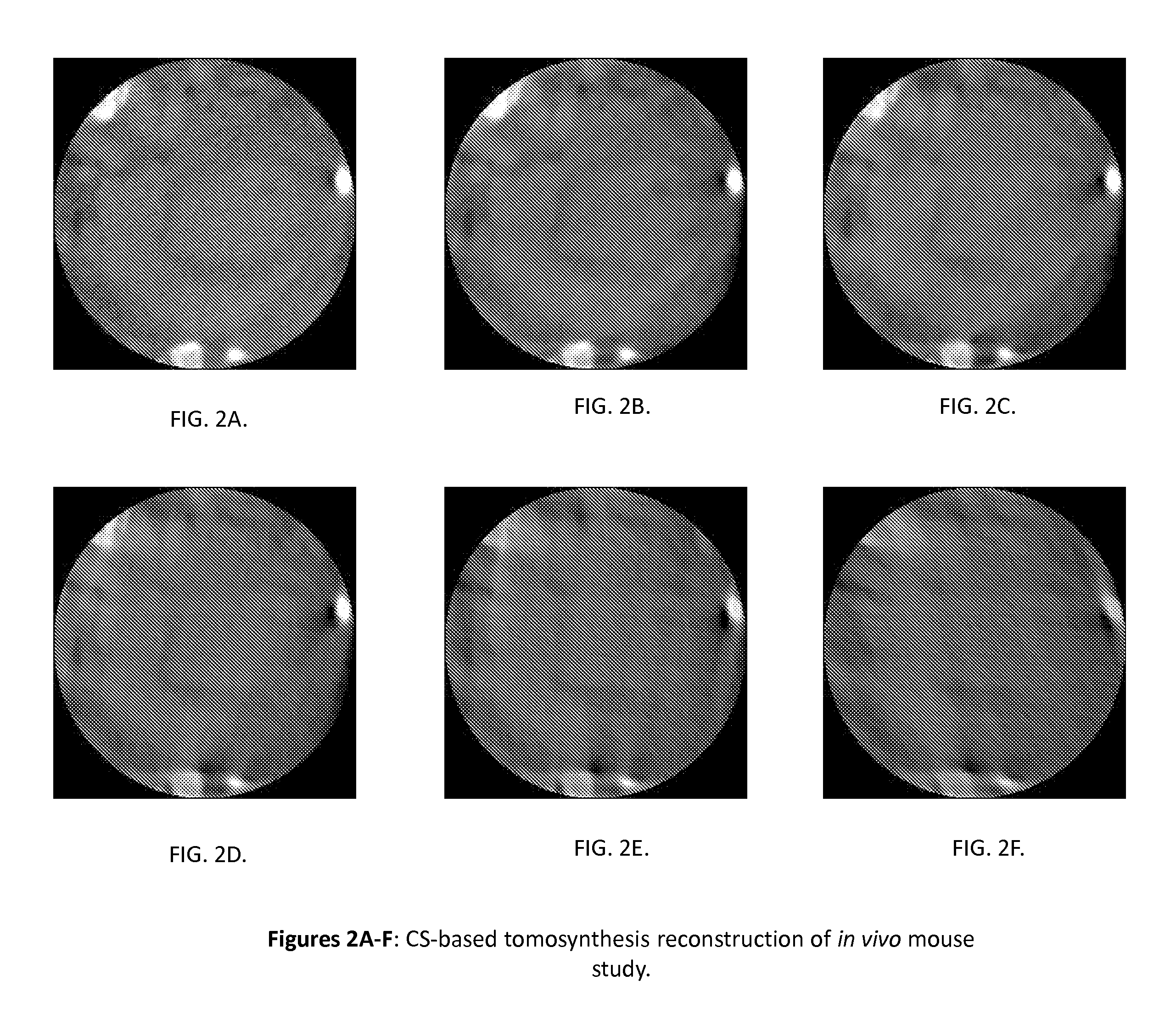
Tomography-Based and MRI-Based Imaging Systems
InactiveUS20110142316A1Comparable image qualityImproved temporalReconstruction from projectionCharacter and pattern recognitionTomosynthesisElastography
Tomography limitations in vivo due to incomplete, inconsistent and intricate measurements require solution of inverse problems. The new strategies disclosed in this application are capable of providing faster data acquisition, higher image quality, lower radiation dose, greater flexibility, and lower system cost. Such benefits can be used to advance research in cardiovascular diseases, regenerative medicine, inflammation, and nanotechnology. The present invention relates to the field of medical imaging. More particularly, embodiments of the invention relate to methods, systems, and devices for imaging, including tomography-based and MRI-based applications. For example, included in embodiments of the invention are compressive sampling based tomosynthesis methods, which have great potential to reduce the overall x-ray radiation dose for a patient. To name a few, compressive sensing based carbon nano-tube based interior tomosynthesis systems, tomography-based dynamic cardiac elastography systems, cardiac elastodynamic biomarkers from interior MR imaging, exact and stable interior ROI reconstructions for radial MRI, and interior reconstruction based ultrafast tomography systems are provided.
Owner:WANG GE +5
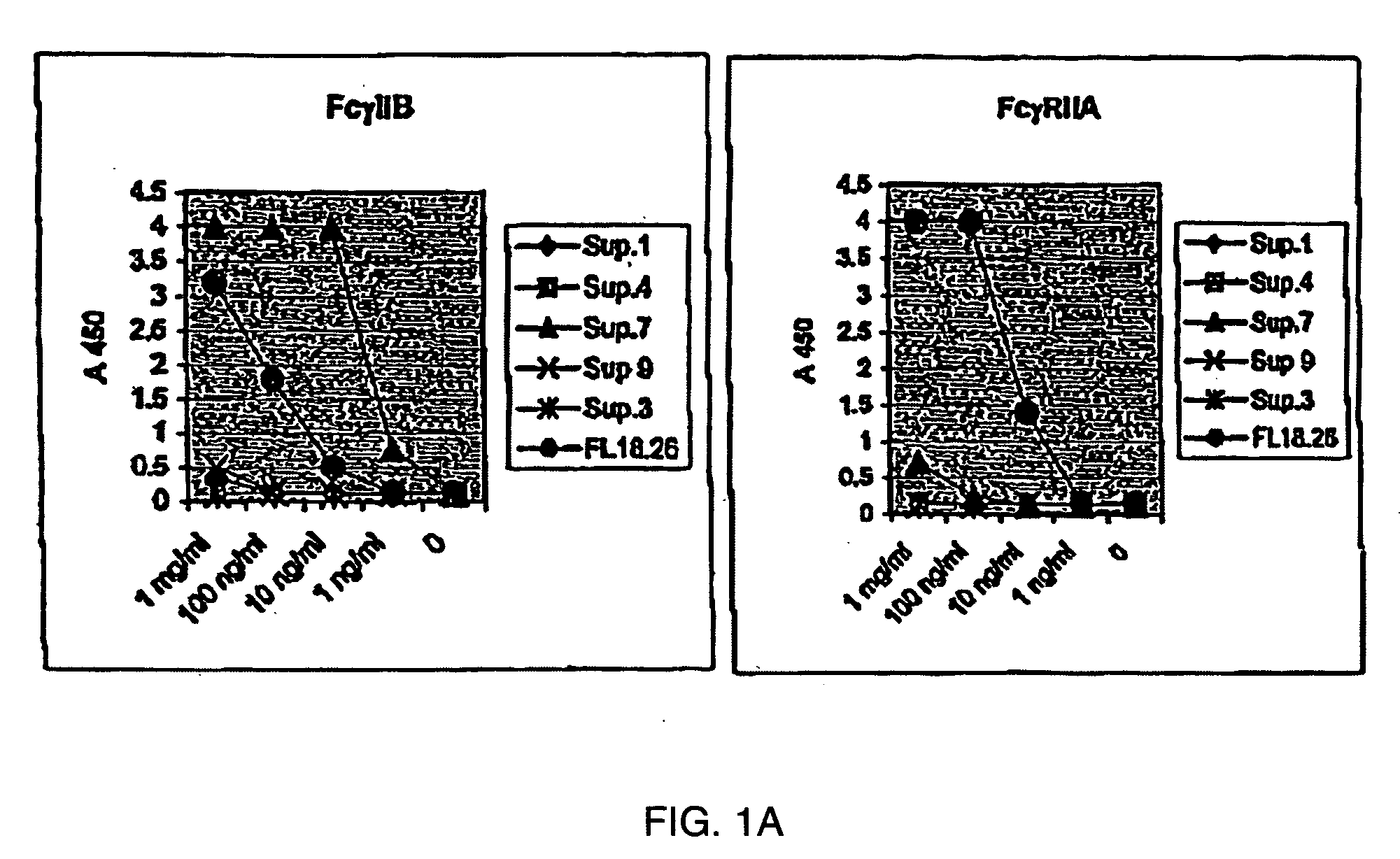
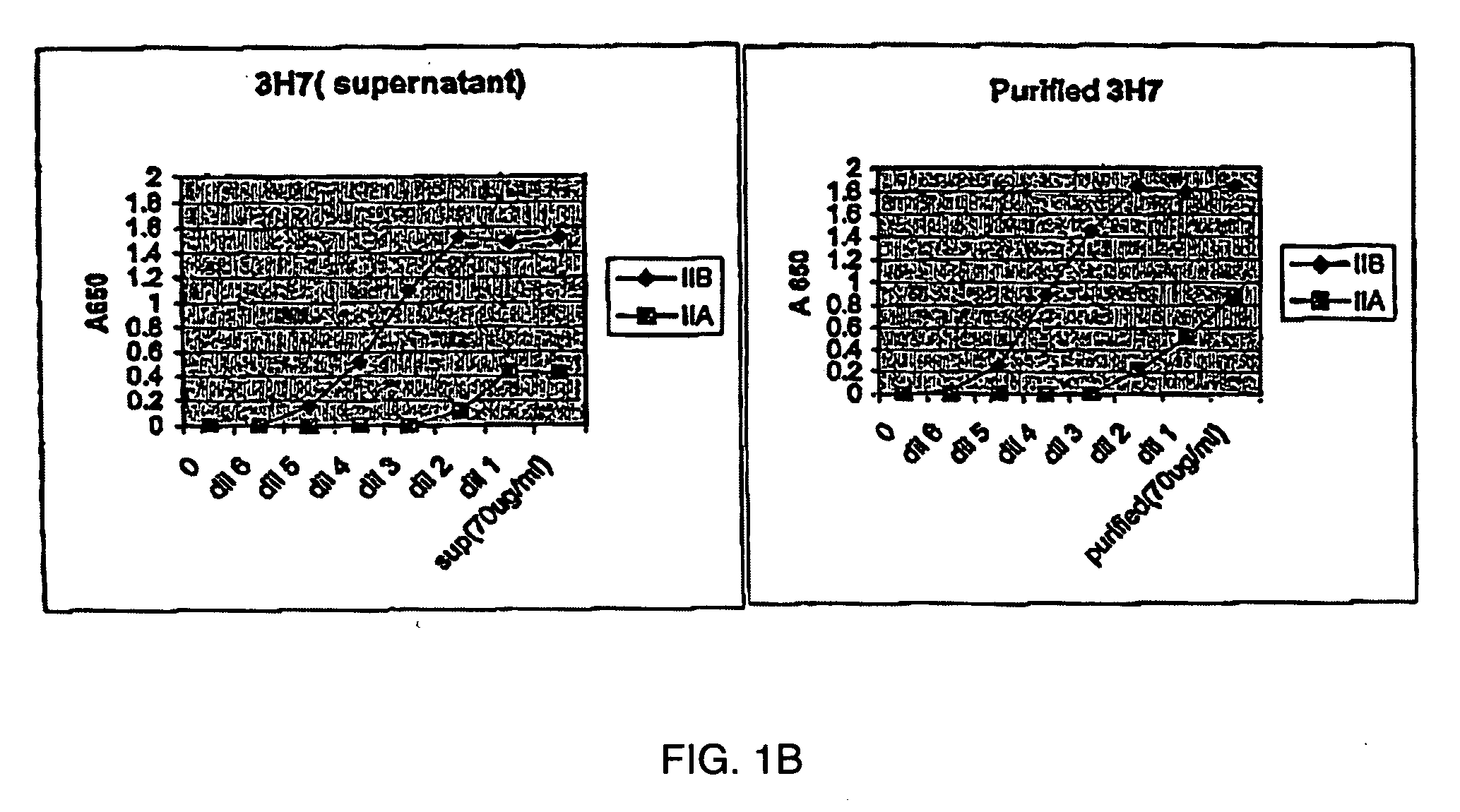
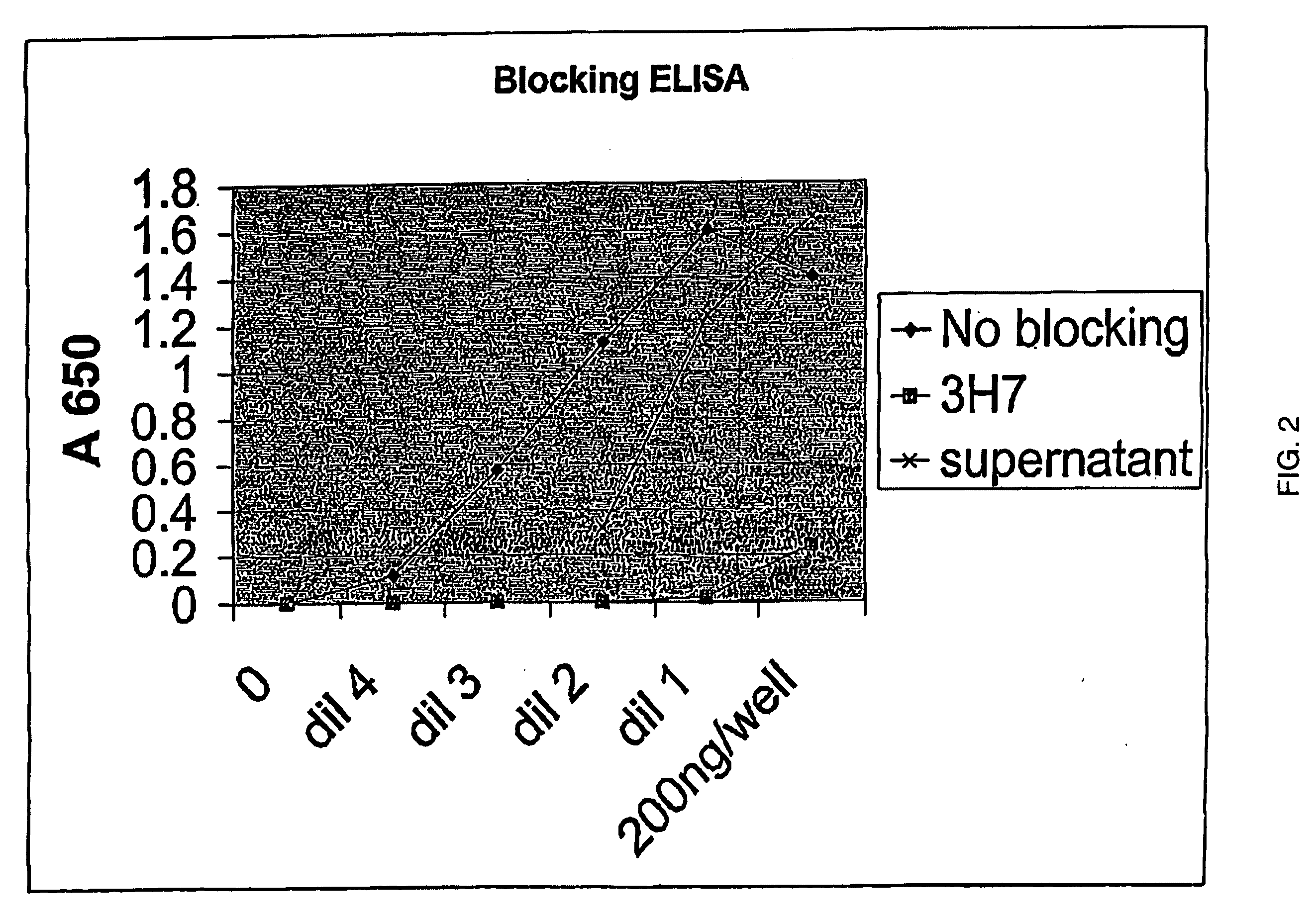
Fcgamma-RIIB-specific antibodies and methods of use thereof
InactiveUS20050260213A1Good curative effectAvoid managementHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen Binding FragmentTherapeutic effect
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
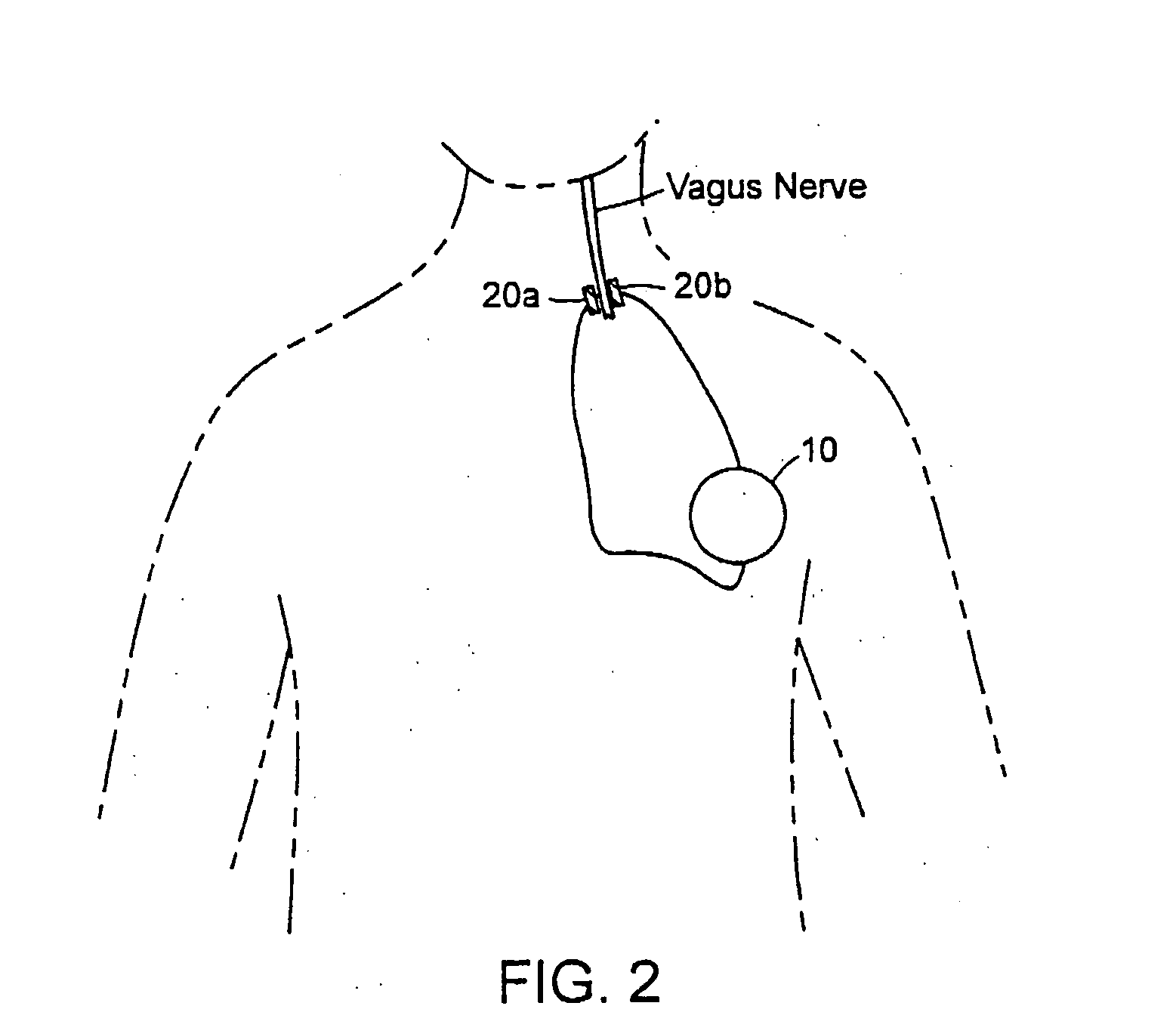
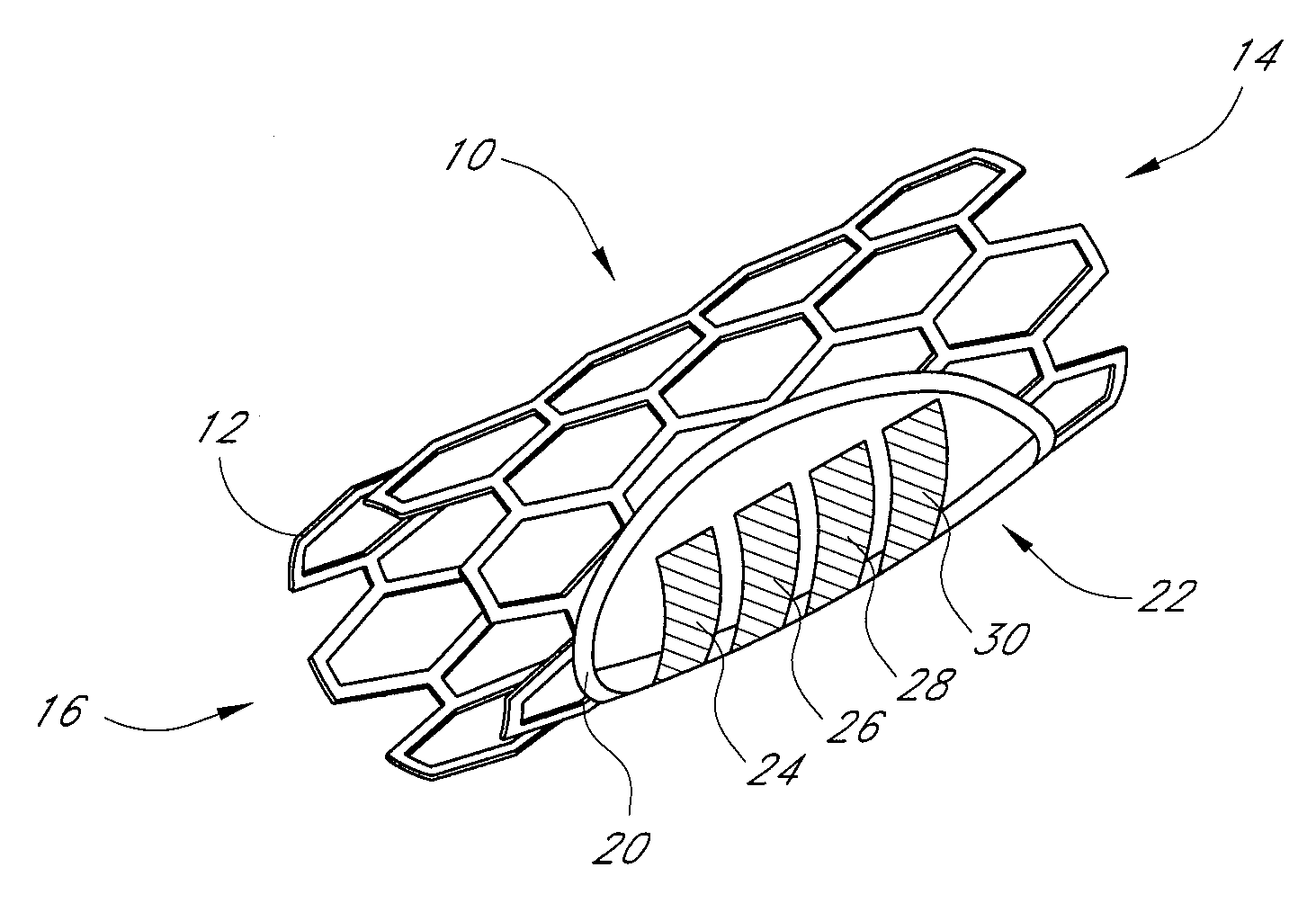
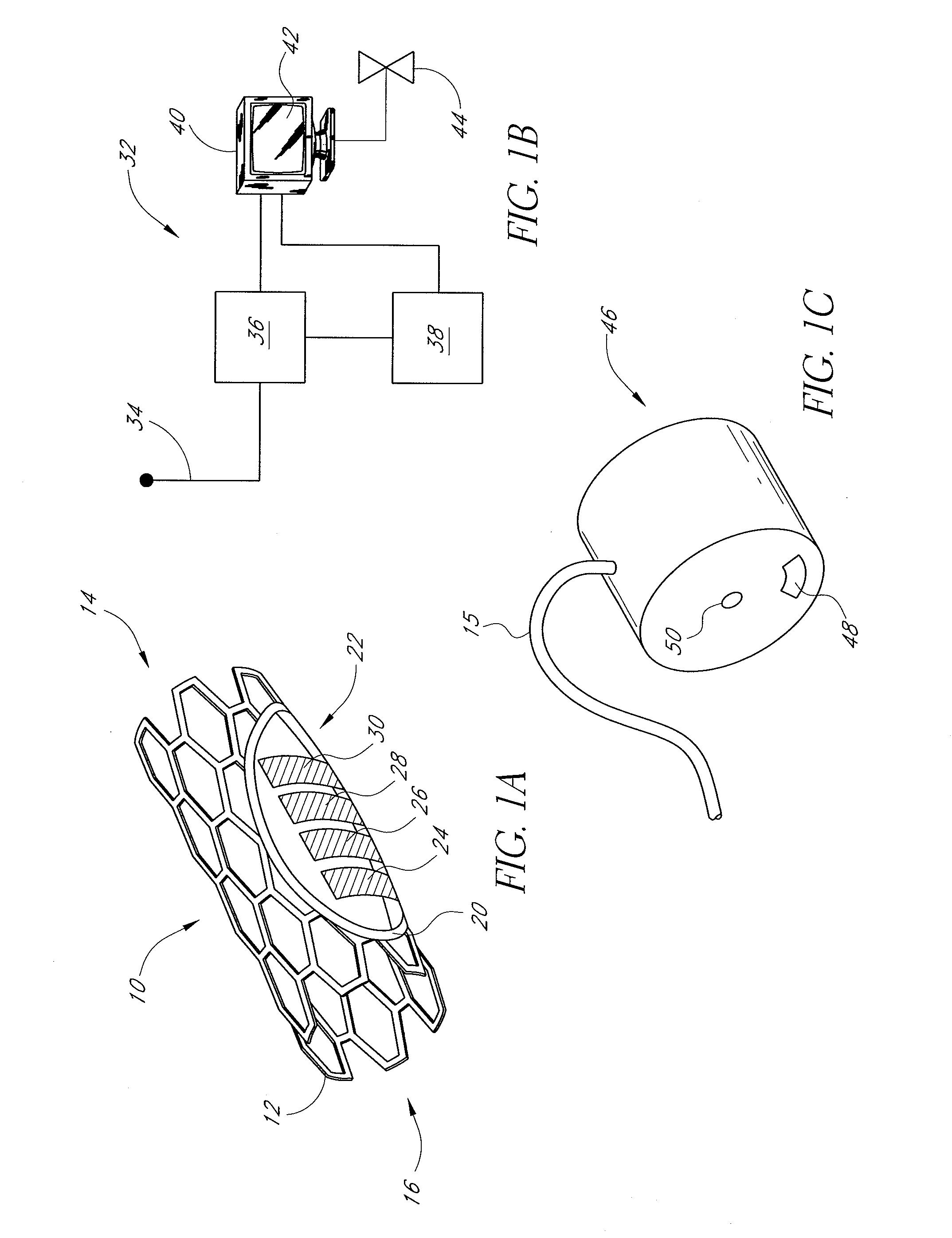
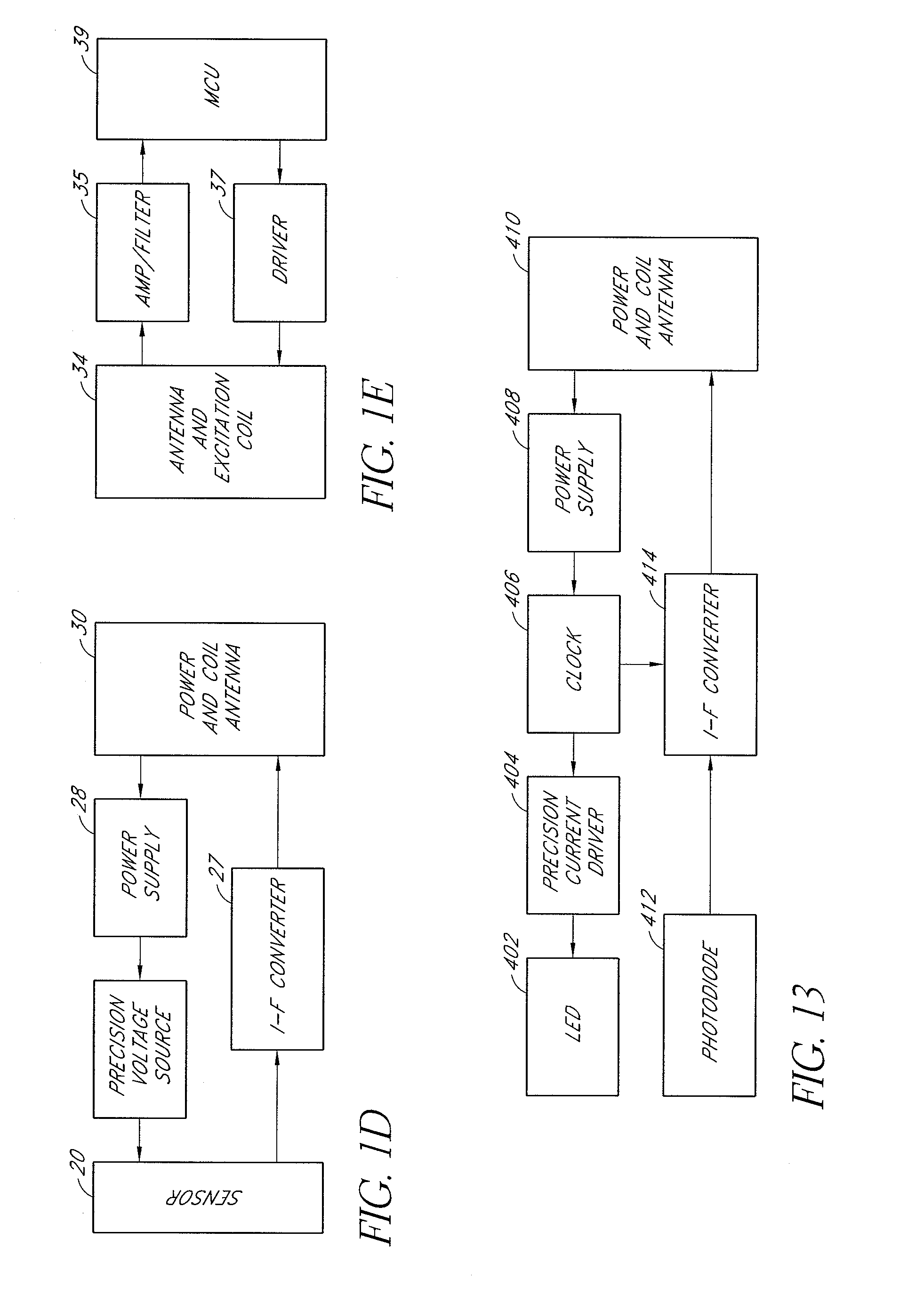
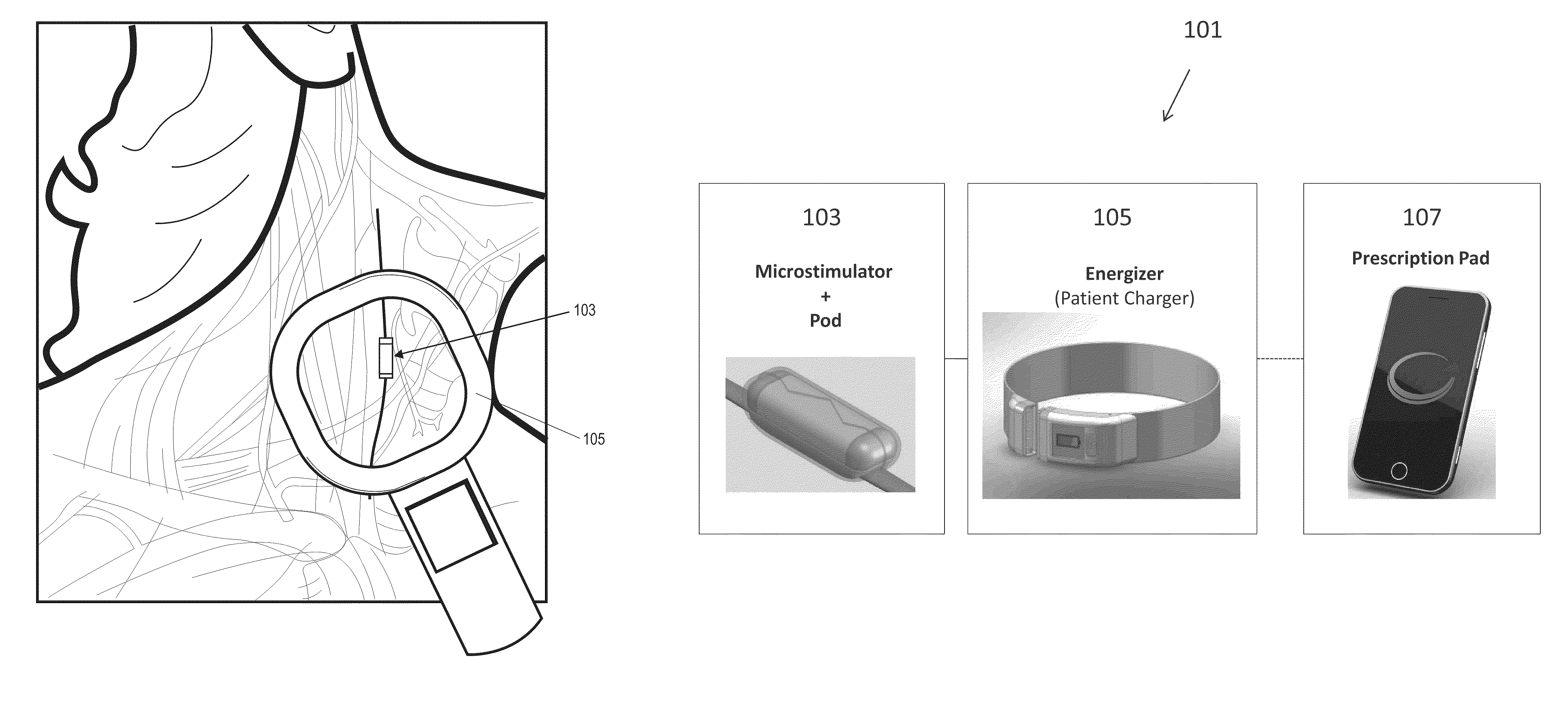
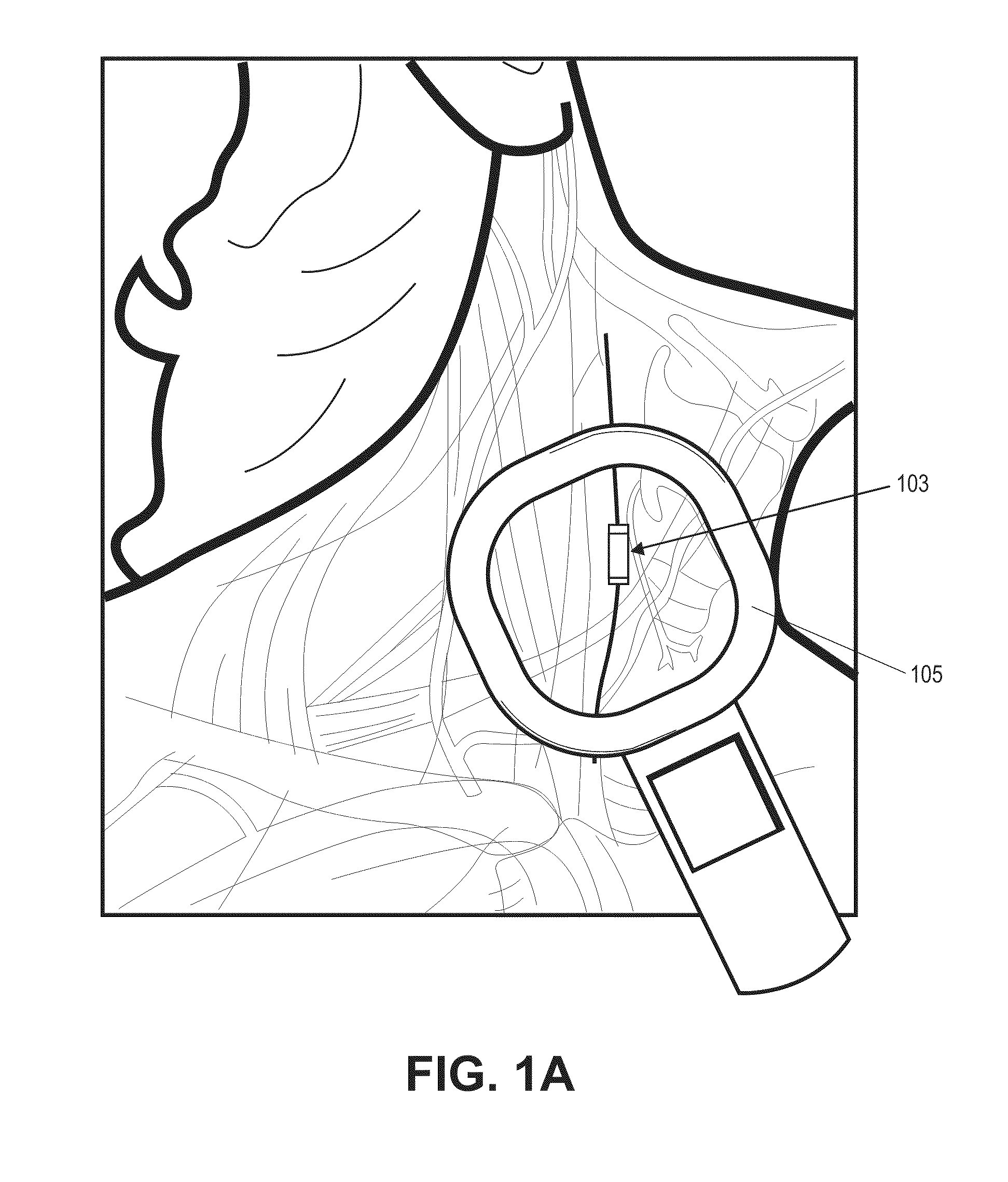
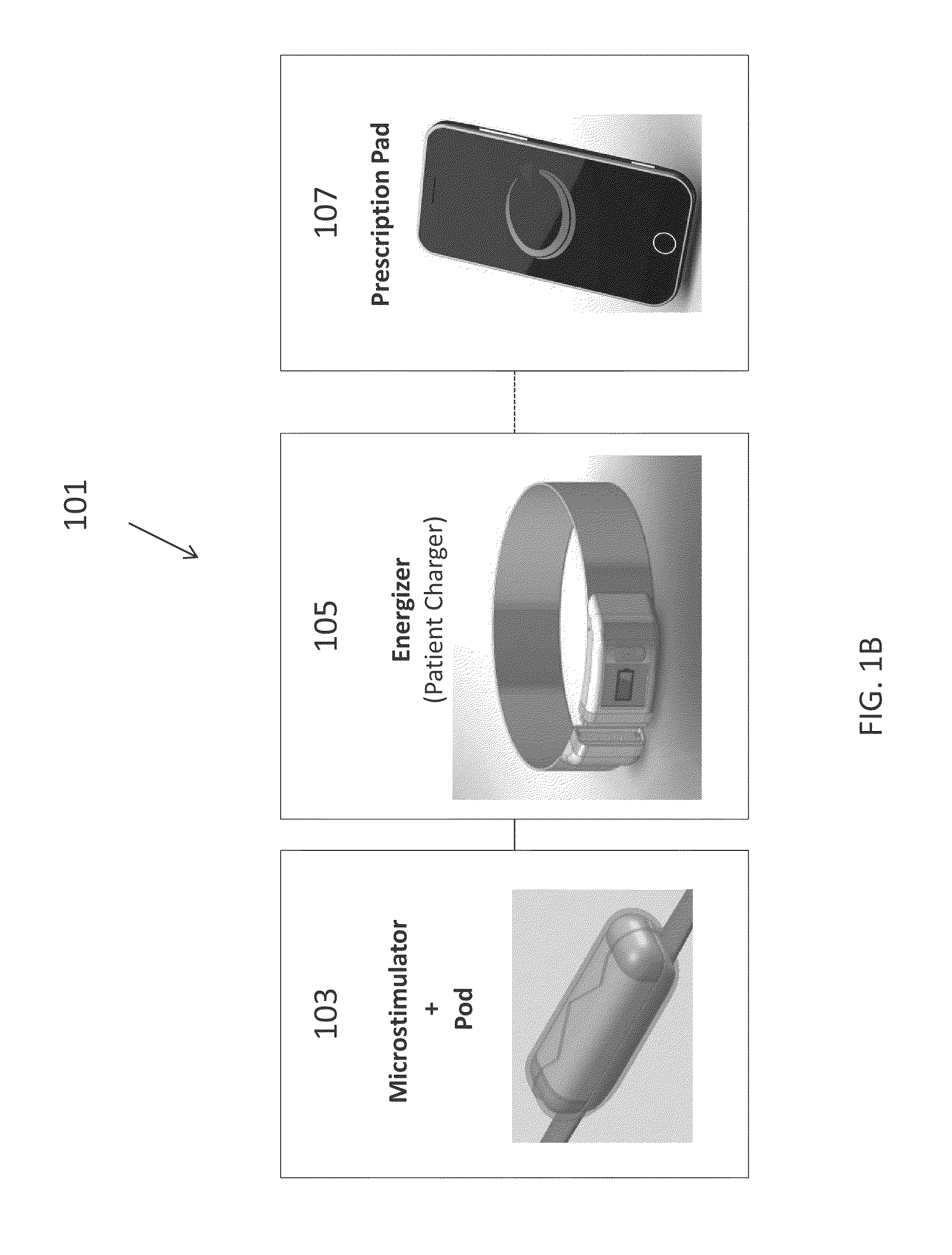
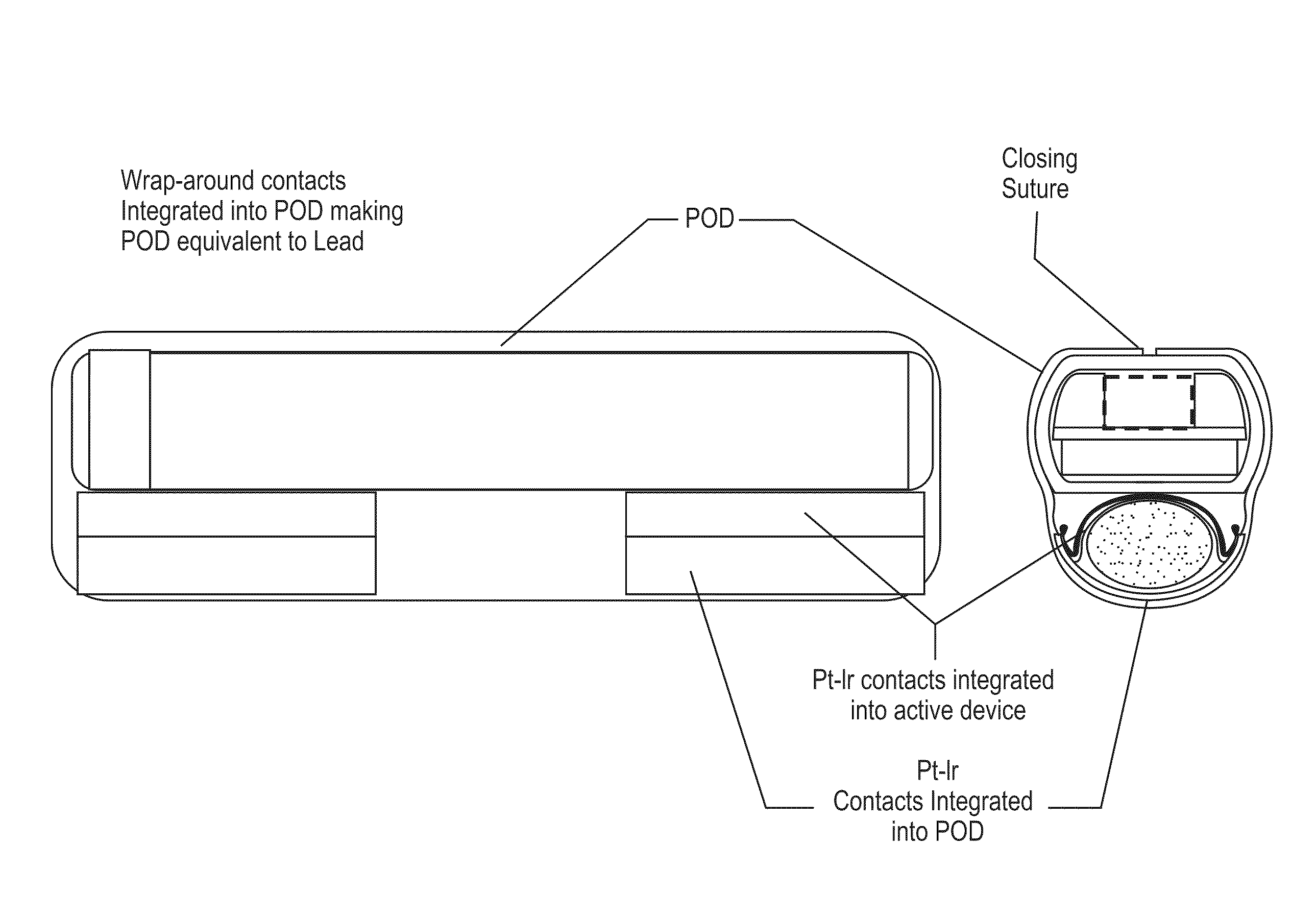
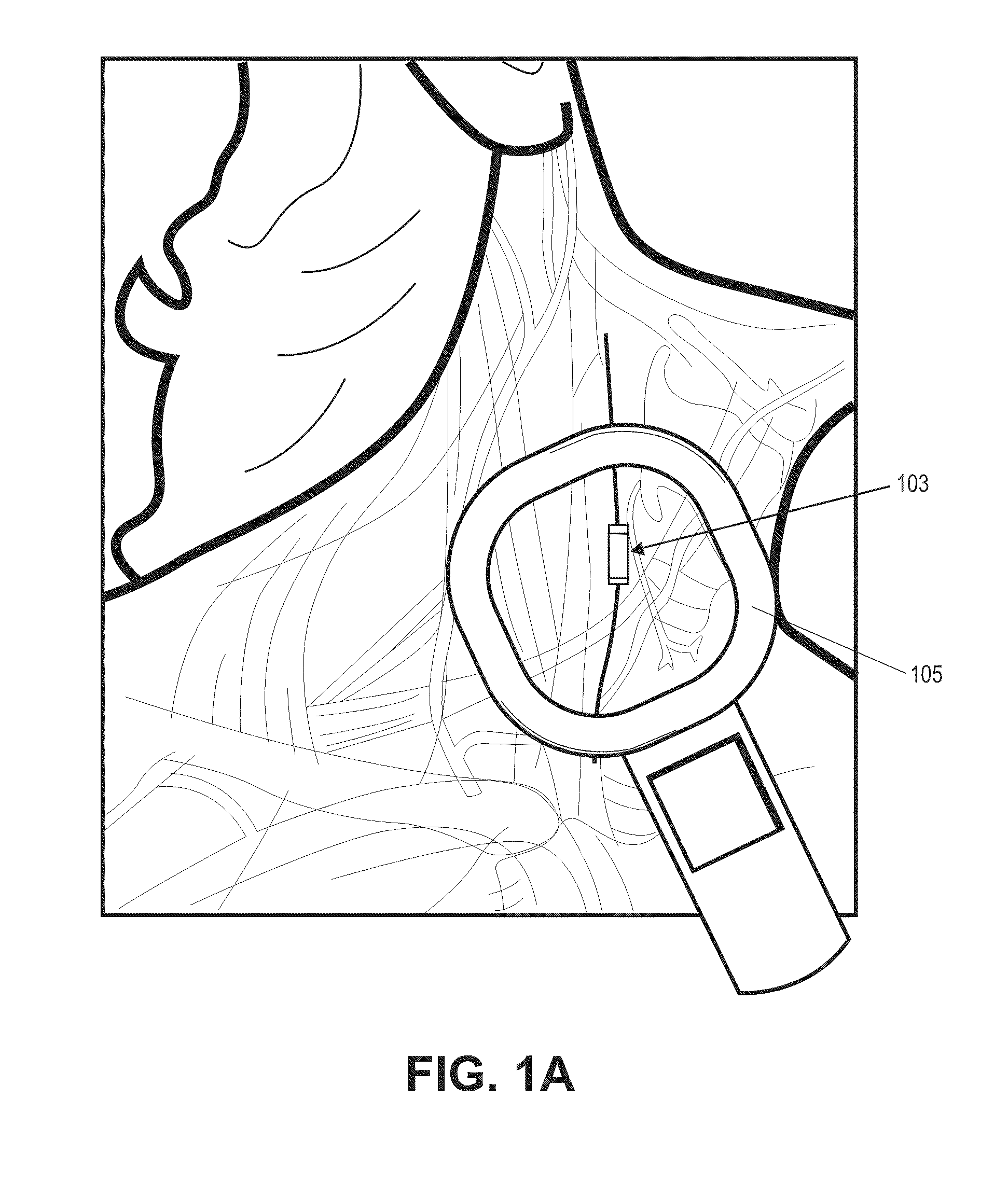
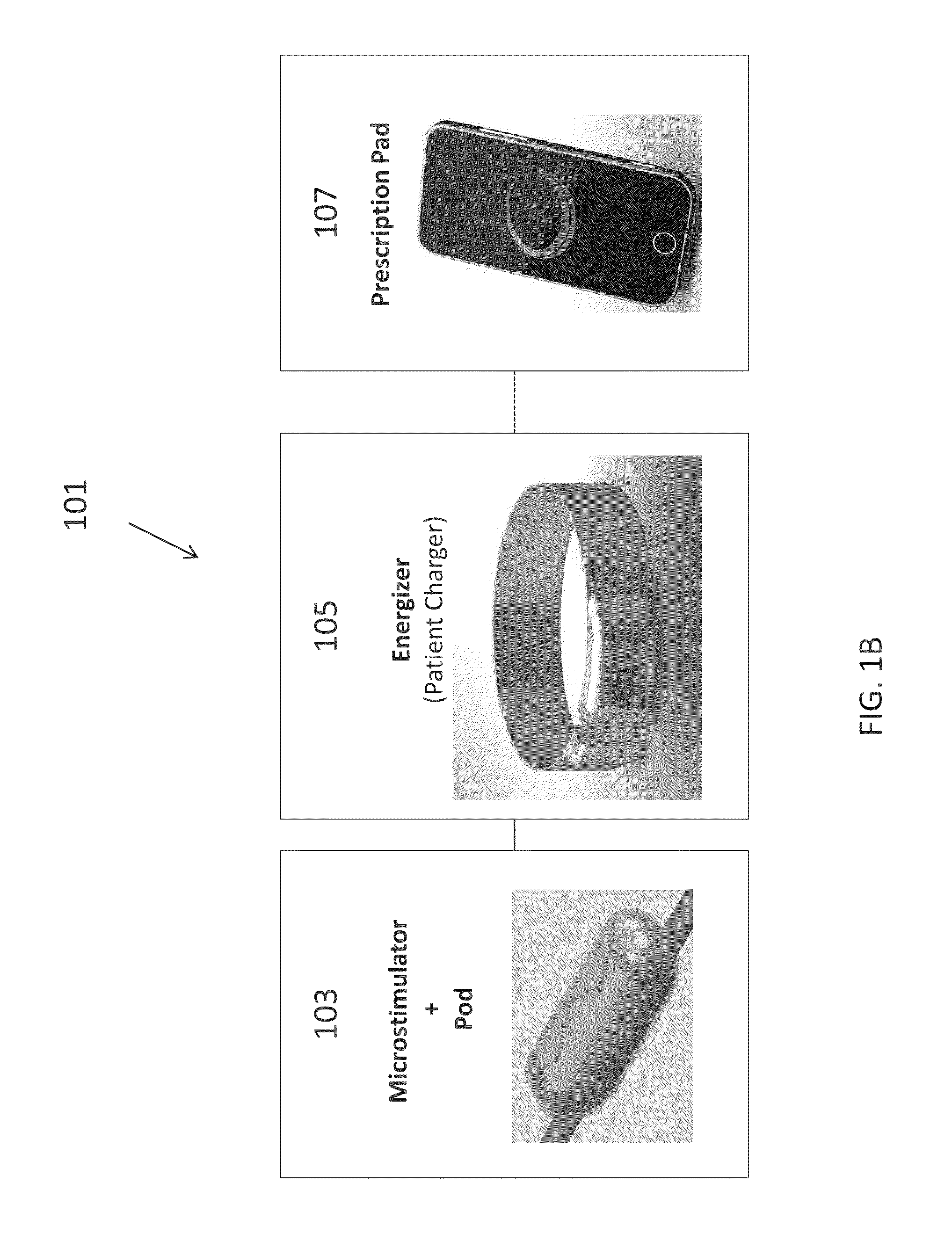
Neural stimulation devices and systems for treatment of chronic inflammation
ActiveUS8612002B2Precise positioningUnwanted stimulationSpinal electrodesImplantable neurostimulatorsDose deliveryMedicine
A system for treating chronic inflammation may include an implantable microstimulator, a wearable charger, and optionally an external controller. The implantable microstimulator may be implemented as a leadless neurostimulator implantable in communication with a cervical region of a vagus nerve. The microstimulator can address several types of stimulation including regular dose delivery. The wearable charger may be worn around the subject's neck to rapidly (<10 minutes per week) charge an implanted microstimulator. The external controller may be configured as a prescription pad that controls the dosing and activity of the microstimulator.
Owner:SETPOINT MEDICAL CORP
Chondroprotective/restorative compositions and methods of use thereof
The instant invention provides a method of treating or preventing osteoarthritis, joint effusion, joint inflammation and pain, synovitis, lameness, post operative arthroscopic surgery, deterioration of proper joint function including joint mobility, the reduction or inhibition of metabolic activity of chondrocytes, the activity of enzymes that degrade cartilage, the reduction or inhibition of the production of Hyaluronic acid, said method comprising orally administering to a mammalian species a therapeutically effective amount of Hyaluronic Acid or pharmaceutically acceptable salts thereof. Additionally, compositions containing hyaluronic acid; chondroitin sulfate, and glucosamine sulfate in a paste formulation are also disclosed which can be administered on their own or can be used as a feed additive.
Owner:PIERCE SCOTT W
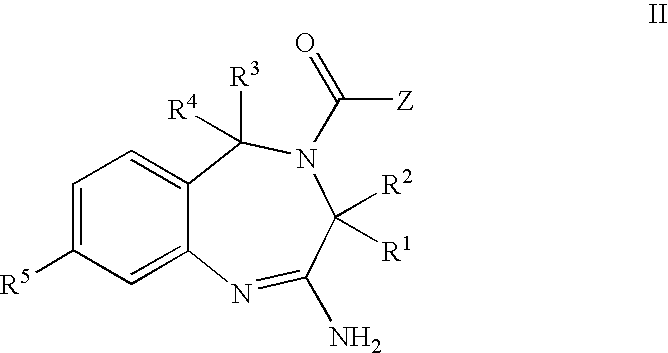
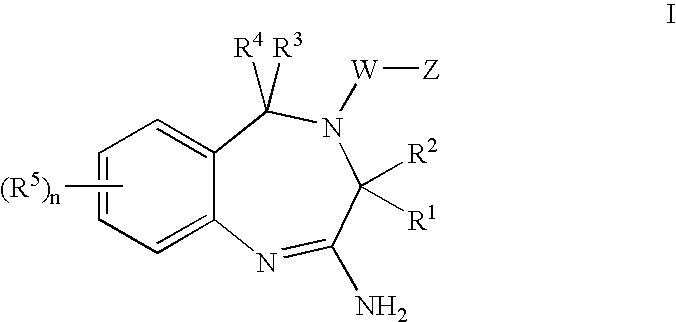
Aminodiazepines as Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation signaling through the Toll-like receptor TLR8. The compositions and methods have use in the treatment of autoimmunity, inflammation allergy, asthma, graft rejection, graft versus host disease, infection, sepsis, cancer and immunodeficiency.
Owner:ARRAY BIOPHARMA
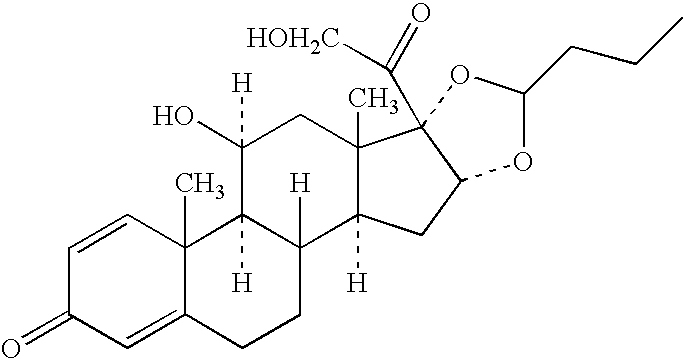
Sterilized nanoparticulate glucocorticosteroid formulations
InactiveUS20070178051A1Readily heat sterilizedImprove the heating effectOrganic active ingredientsPowder deliveryPediatric patientMicroparticle
The invention is directed sterile to compositions of glucocorticosteroids useful in the prophylaxis and chronic treatment of asthma and other allergic and inflammatory conditions in adults and pediatric patients.
Owner:ELAN PHRMA INT LTD
Topically Bioavailable Acne and Rosacea Treatment Compositions
InactiveUS20040156873A1Reduce stimulationSynergistic superior anti-acneBiocideCosmetic preparationsAdditive ingredientIrritation
The present invention relates to acne and rosacea compositions by a six-prong synergistic combination treatment strategy that includes (1) control of excess sebum production, (2) control of undesirable bacteria or mites, (3) control of inflammation, (4) enhanced desquamation of follicular infundibulum cells, (5) reduction of irritation from anti-acne or rosacea compositions themselves, and (6) enhancement of the topical bioavailability of anti-acne and rosacea compositions. This is achieved by a synergistic combination of commonly utilized topical anti-acne and rosacea ingredients with a topical bioavailability enhancement composition, which results in enhanced anti-acne and rosacea action from such ingredients. Moreover, additional inclusion of an anti-inflammatory composition, and also a vascular micro-circulation enhancement composition, further results in synergistic superior anti-acne and rosacea benefits from such compositions. The present invention discloses additional surprising synergistic combinations for the control of acne and rosacea that are suitable for a variety of delivery systems and packaging forms.
Owner:GUPTA SHYAM K
Antibodies that specifically bind to TIM3
ActiveUS8841418B2Promote growthReduce inflammationHeavy metal active ingredientsPeptide/protein ingredientsCancer cellAntibody
Provided herein are antibodies specific for TIM3 that can be used to detect cancer cells, in particular, cancer stem cells. The antibodies can also be used in therapeutic compositions for treating cancer and reducing inflammation.
Owner:ONK THERAPEUTICS LTD
Inhibitors of human phosphatidyl-inositol 3-kinase delta
InactiveUS6949535B2Inhibit growthPrevent proliferationAntibacterial agentsBiocideDiseaseLeukocyte function
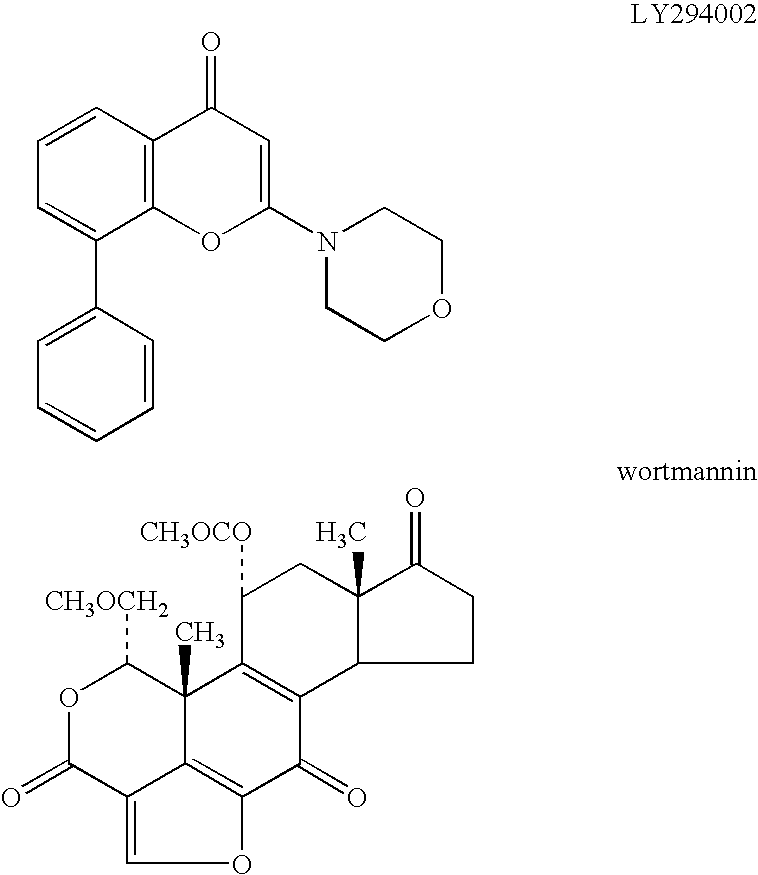
Methods of inhibiting phosphatidylinositol 3-kinase delta isoform (PI3Kδ) activity, and methods of treating diseases, such as disorders of immunity and inflammation, in which PI3Kδ plays a role in leukocyte function are disclosed. Preferably, the methods employ active agents that selectively inhibit PI3Kδ, while not significantly inhibiting activity of other PI3K isoforms. Compounds are provided that inhibit PI3Kδ activity, including compounds that selectively inhibit PI3Kδ activity. Methods of using PI3Kδ inhibitory compounds to inhibit cancer cell growth or proliferation are also provided. Accordingly, the invention provides methods of using PI3Kδ inhibitory compounds to inhibit PI3Kδ-mediated processes in vitro and in vivo.
Owner:ICOS CORP
Neural stimulation devices and systems for treatment of chronic inflammation
ActiveUS20110190849A1Reduce communication errorsLimited amountSpinal electrodesImplantable neurostimulatorsMedicineDose delivery
A system for treating chronic inflammation may include an implantable microstimulator, a wearable charger, and optionally an external controller. The implantable microstimulator may be implemented as a leadless neurostimulator implantable in communication with a cervical region of a vagus nerve. The microstimulator can address several types of stimulation including regular dose delivery. The wearable charger may be worn around the subject's neck to rapidly (<10 minutes per week) charge an implanted microstimulator. The external controller may be configured as a prescription pad that controls the dosing and activity of the microstimulator.
Owner:SETPOINT MEDICAL CORP
6,6-Bicyclic ring substituted heterobicyclic protein kinase inhibitors
ActiveUS20060235031A1Treatment and/or prevention of hyperproliferative diseasesBiocideSenses disorderDiseasePTK Inhibitors
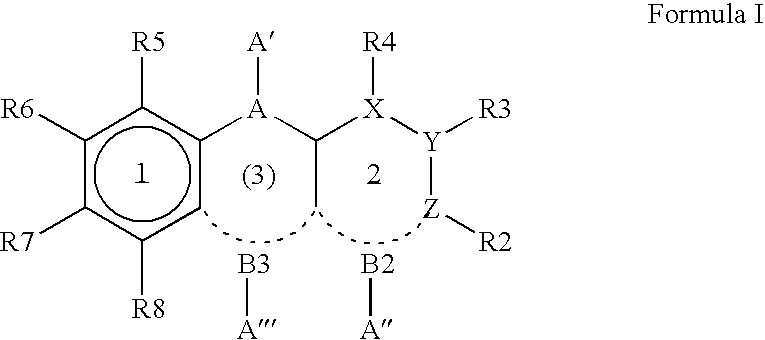
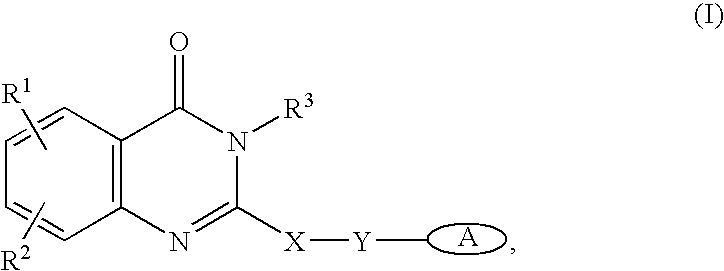
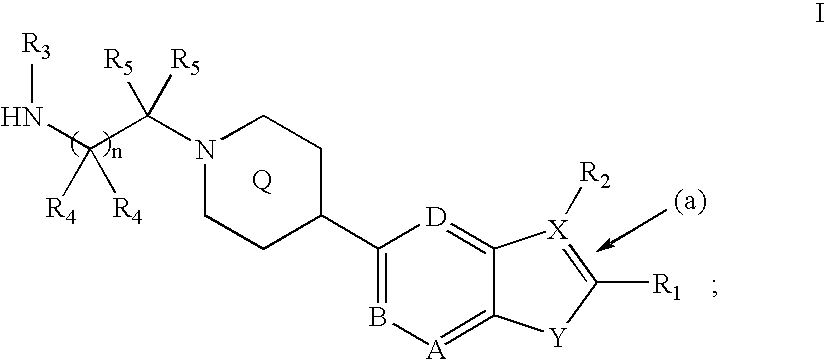
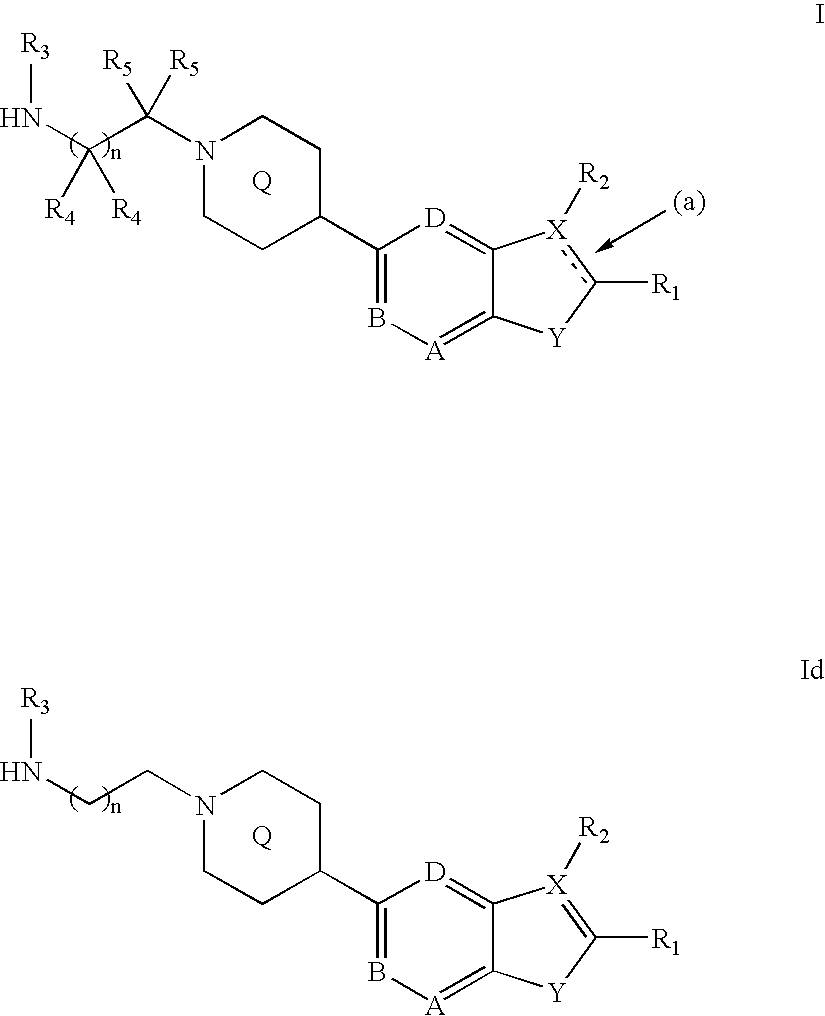
Compounds of the formula and pharmaceutically acceptable salts thereof, wherein X1, X2, X3, X4, X5, X6, X7, R1, and Q1 are defined herein, inhibit the IGF-1R enzyme and are useful for the treatment and / or prevention of hyperproliferative diseases such as cancer, inflammation, psoriasis, allergy / asthma, disease and conditions of the immune system, disease and conditions of the central nervous system.
Owner:ACERTA PHARMA BV
Heterocyclic inhibitors of protein arginine methyl transferases
InactiveUS20060235037A1BiocideOrganic chemistryProtein-arginine methyltransferaseCompound (substance)
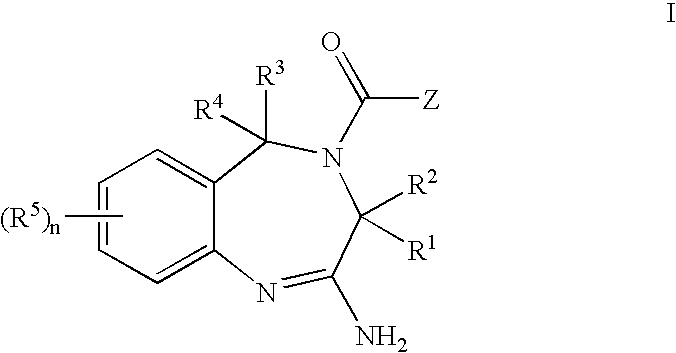
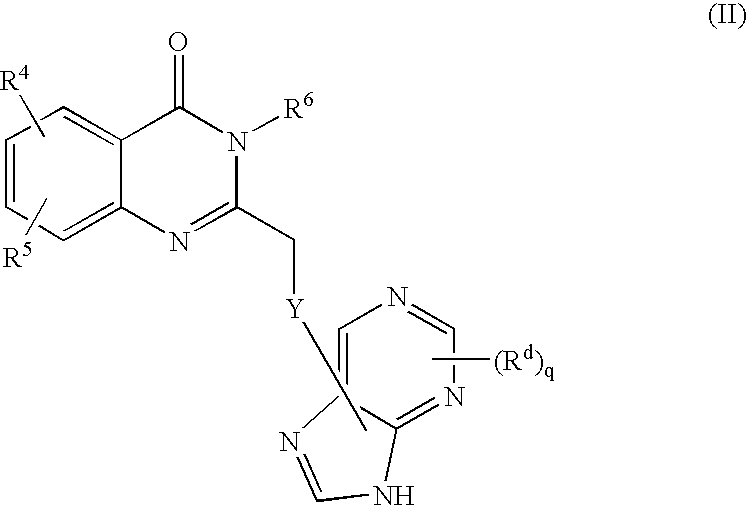
A compound of formula I, or a stereoisomer, a tautomer, a pharmaceutically acceptable salt or solvate thereof, methods of using such compounds in the treatment of hyperproliferative, inflammatory, infectious, and immunoregulatory disorders and diseases; and to pharmaceutical compositions containing such compounds.
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com