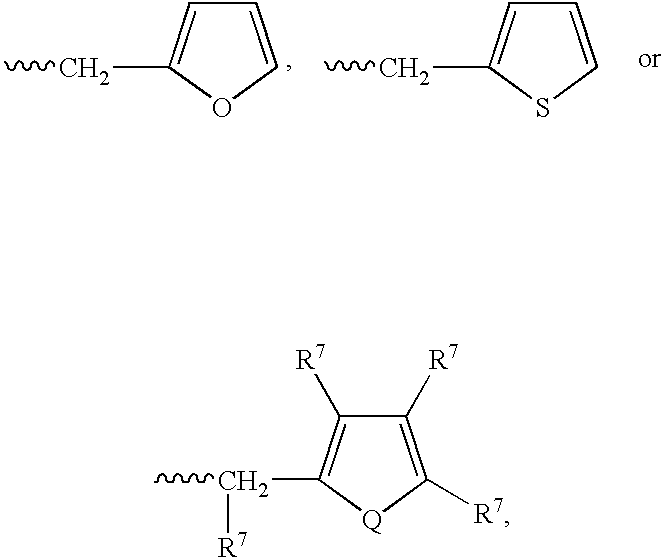
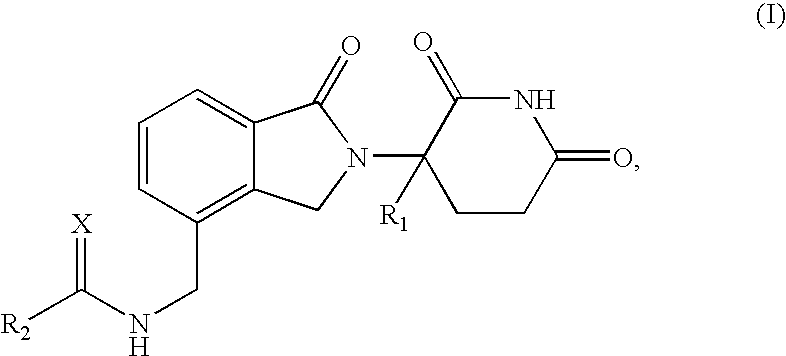
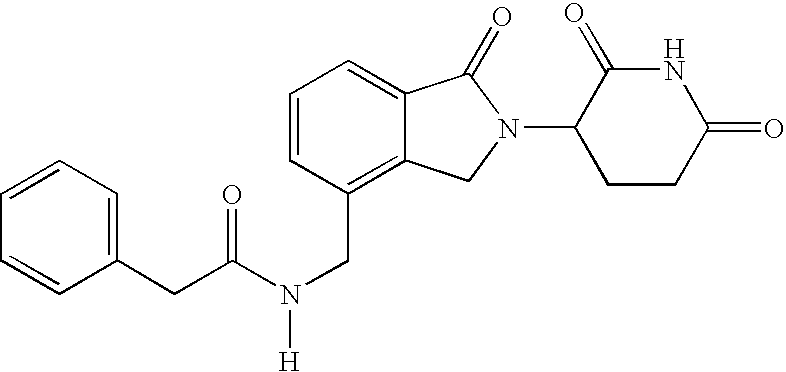
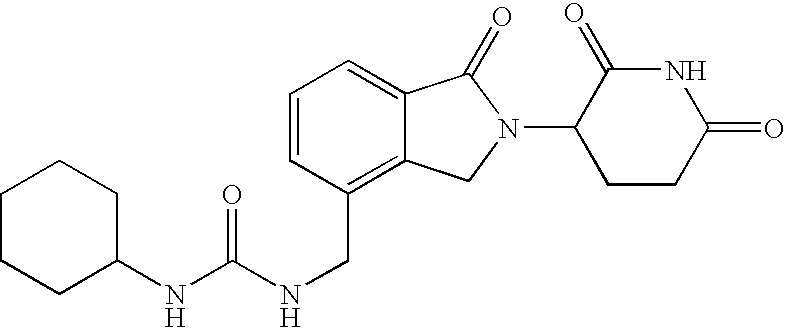
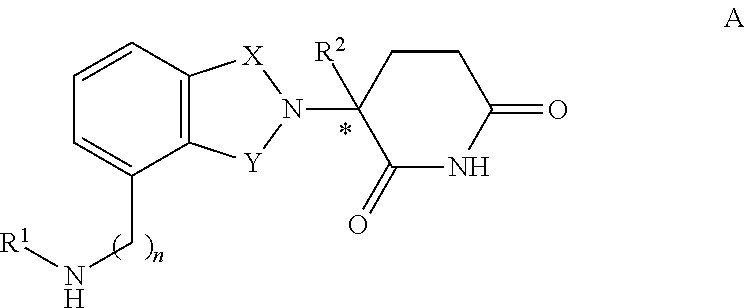
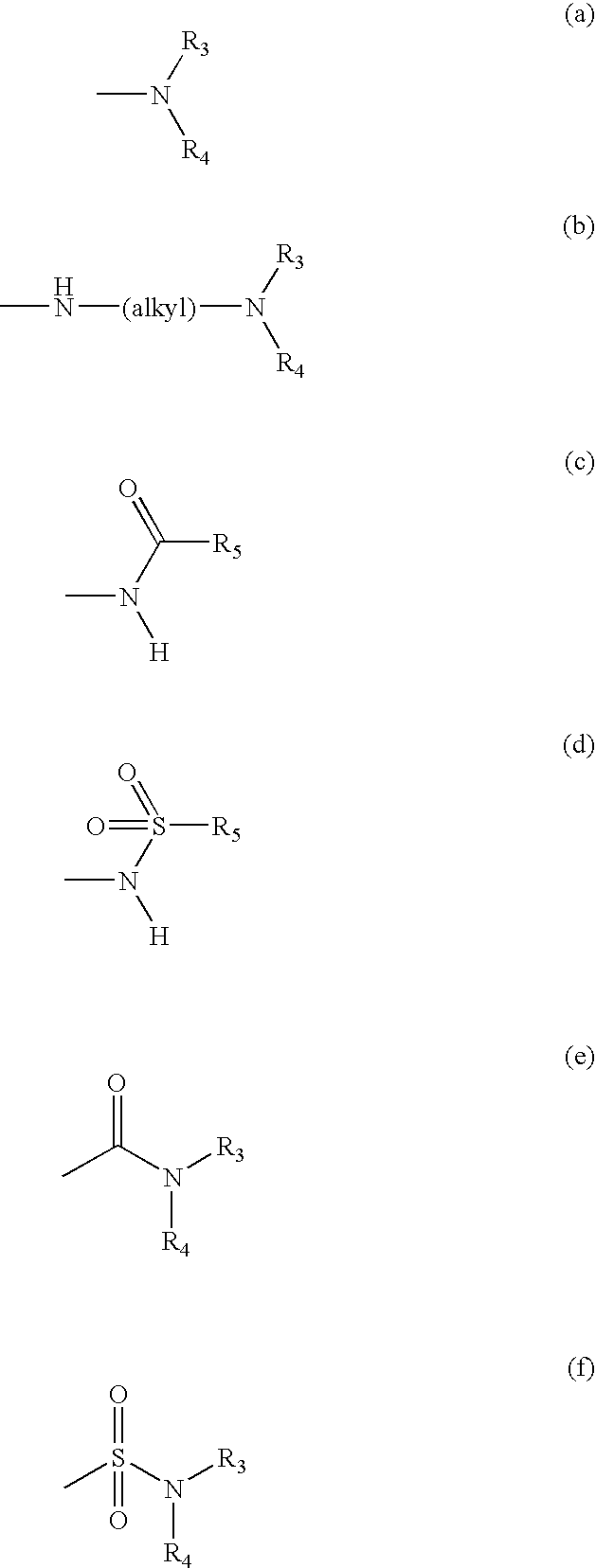
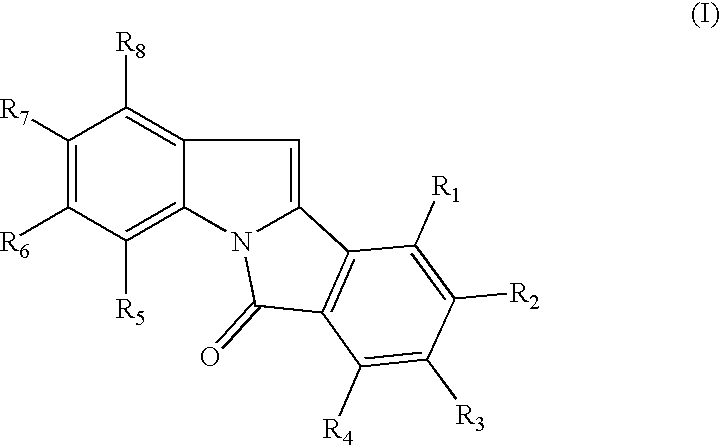
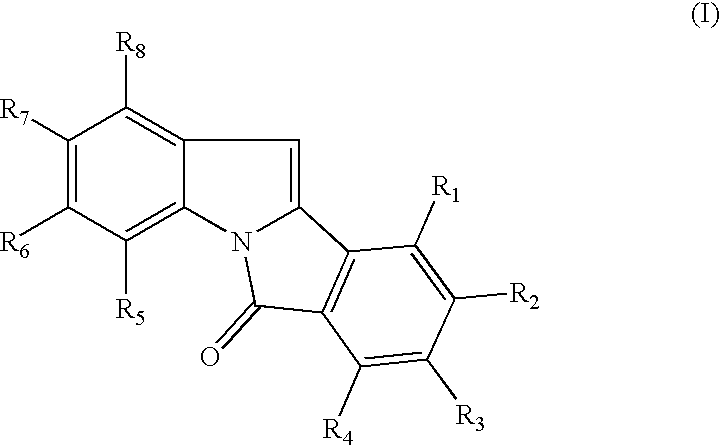
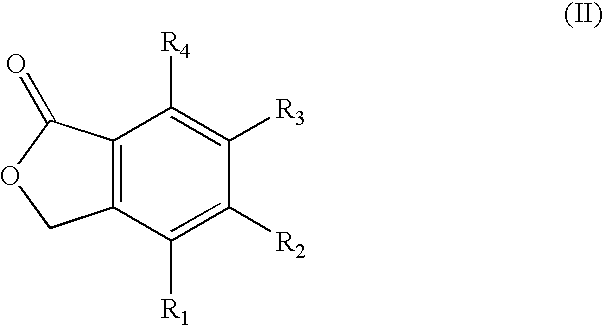
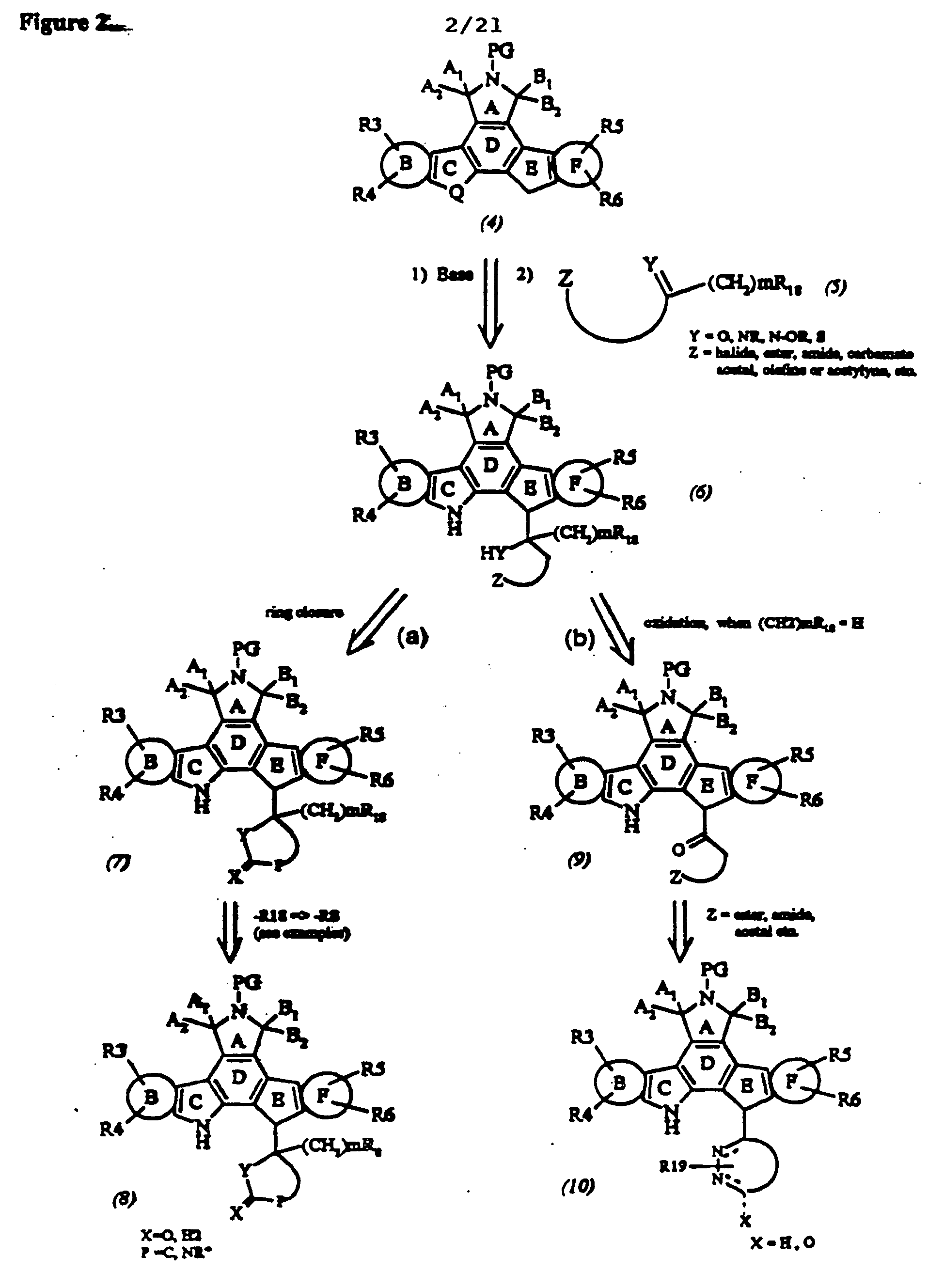
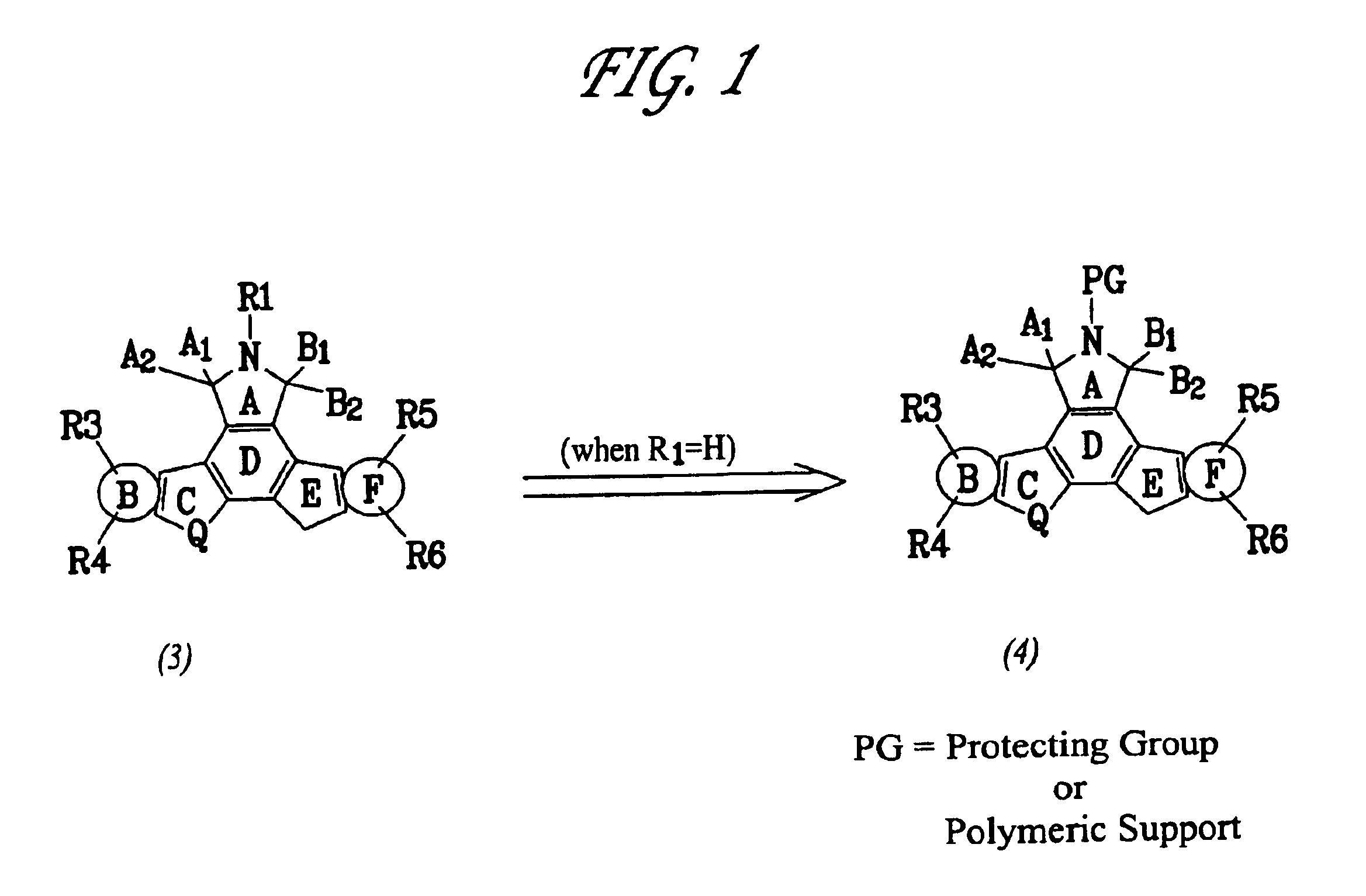
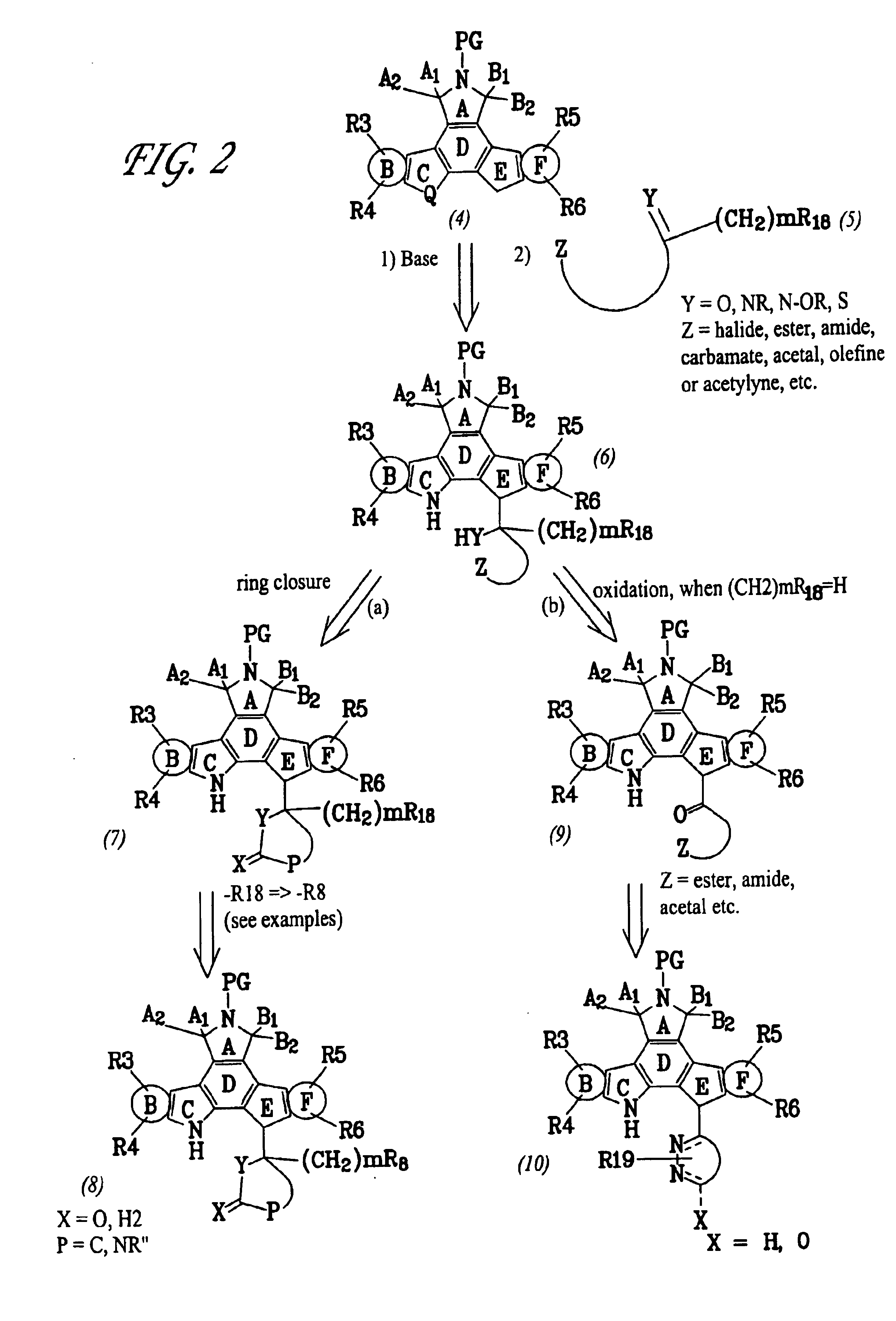
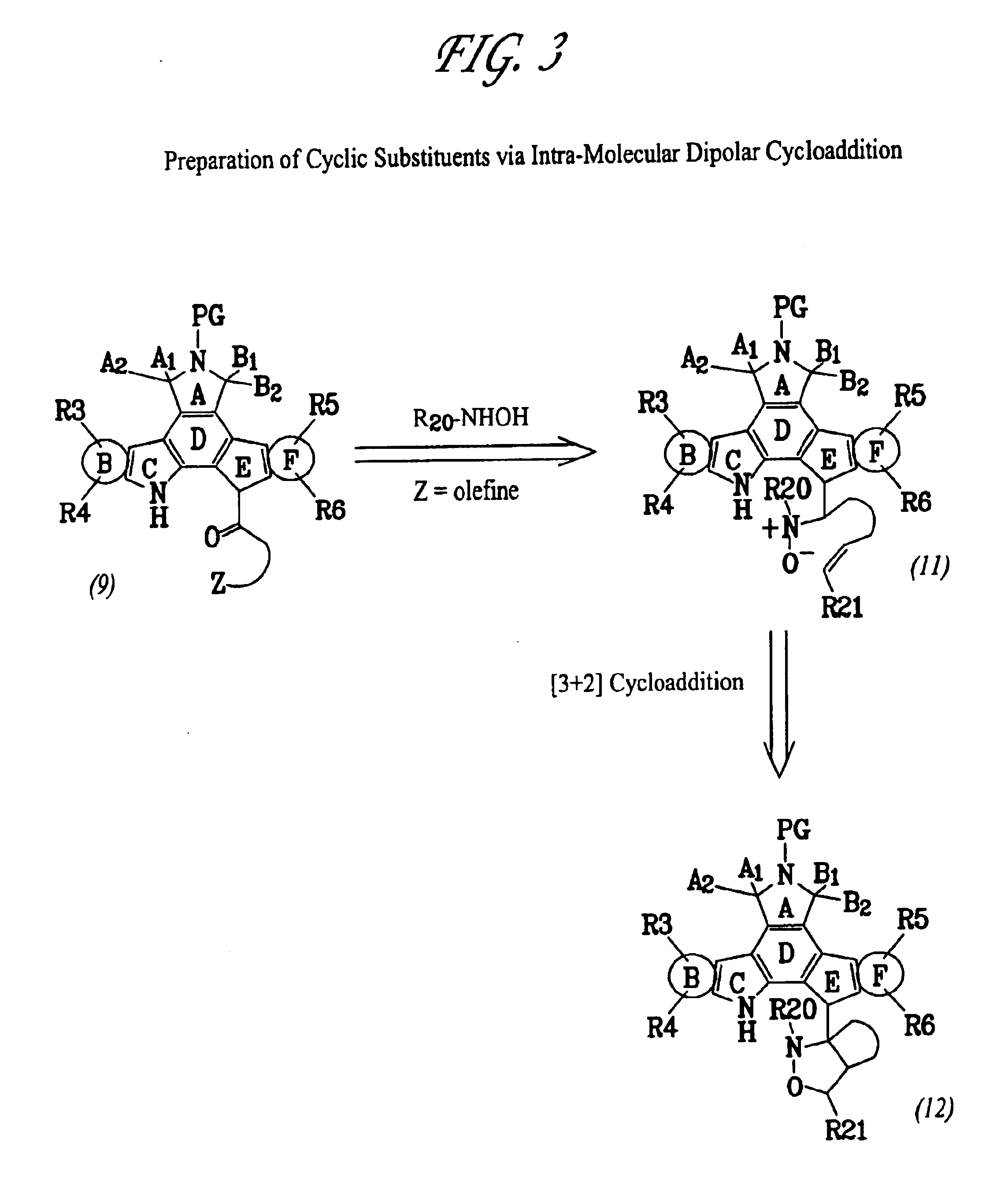
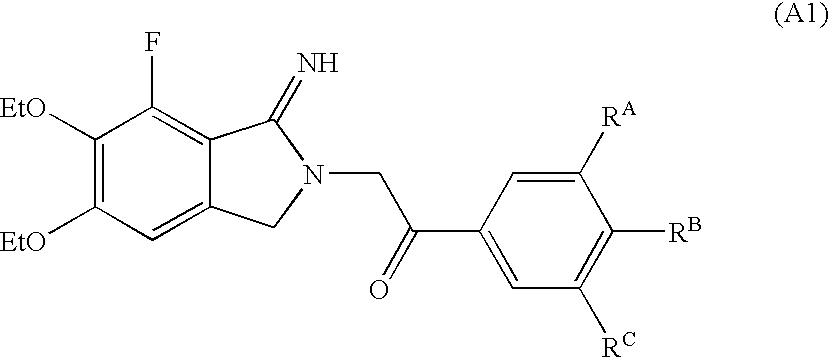
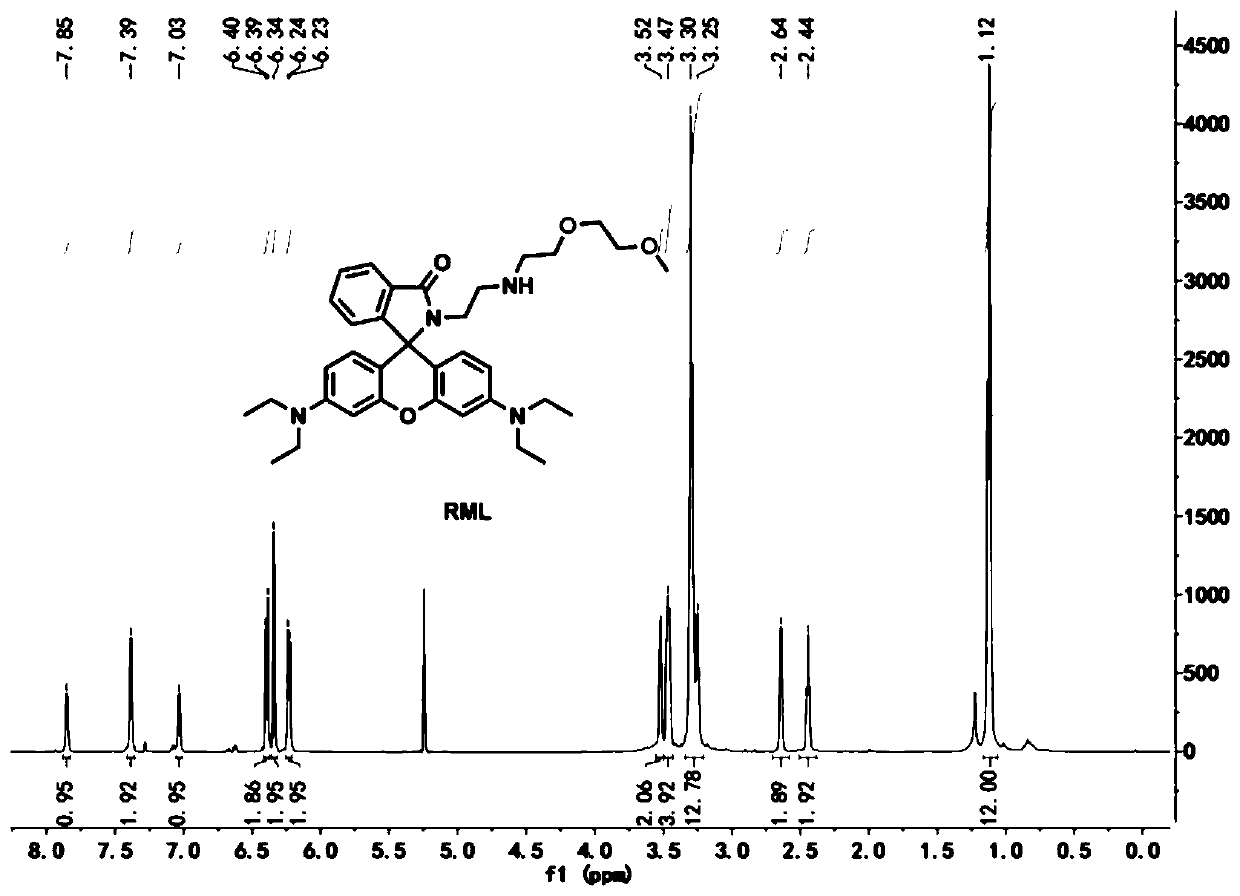
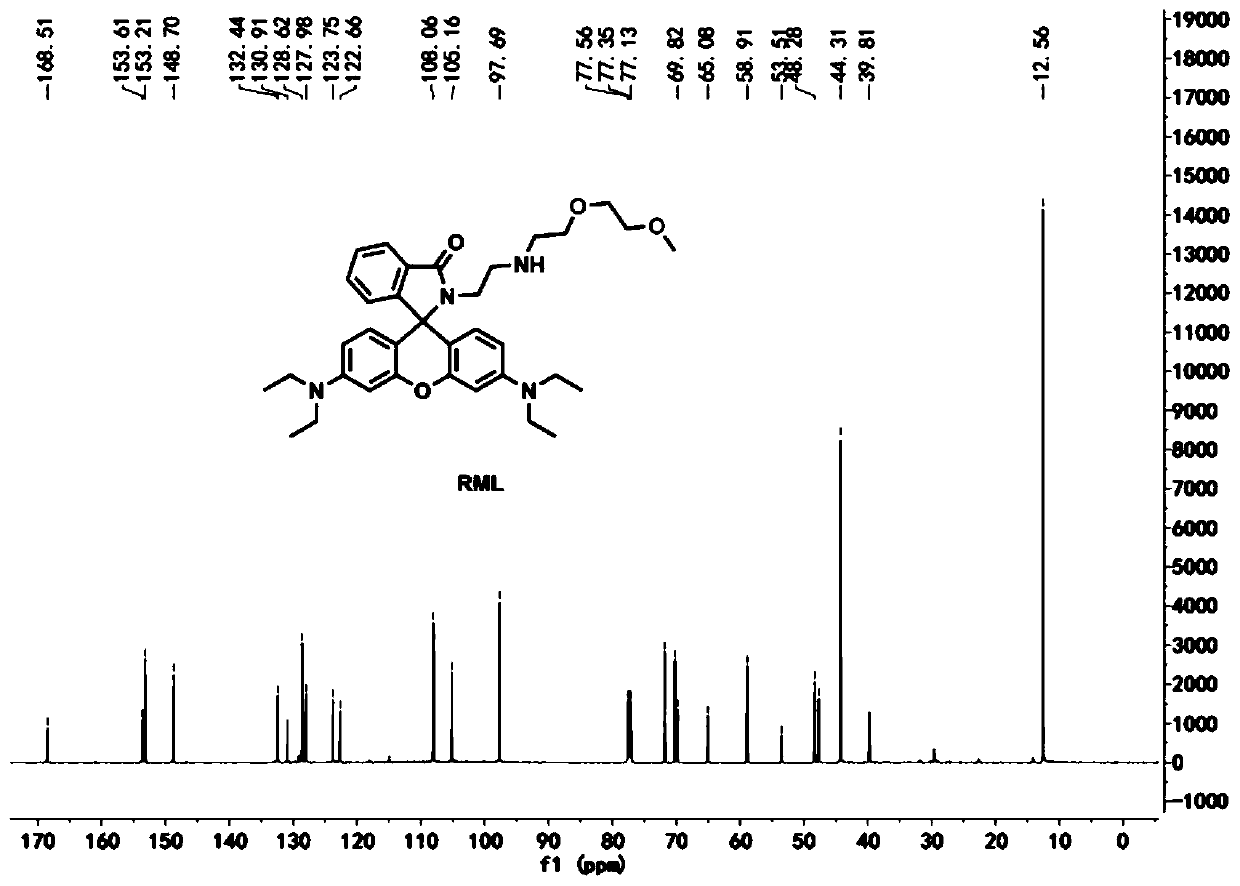
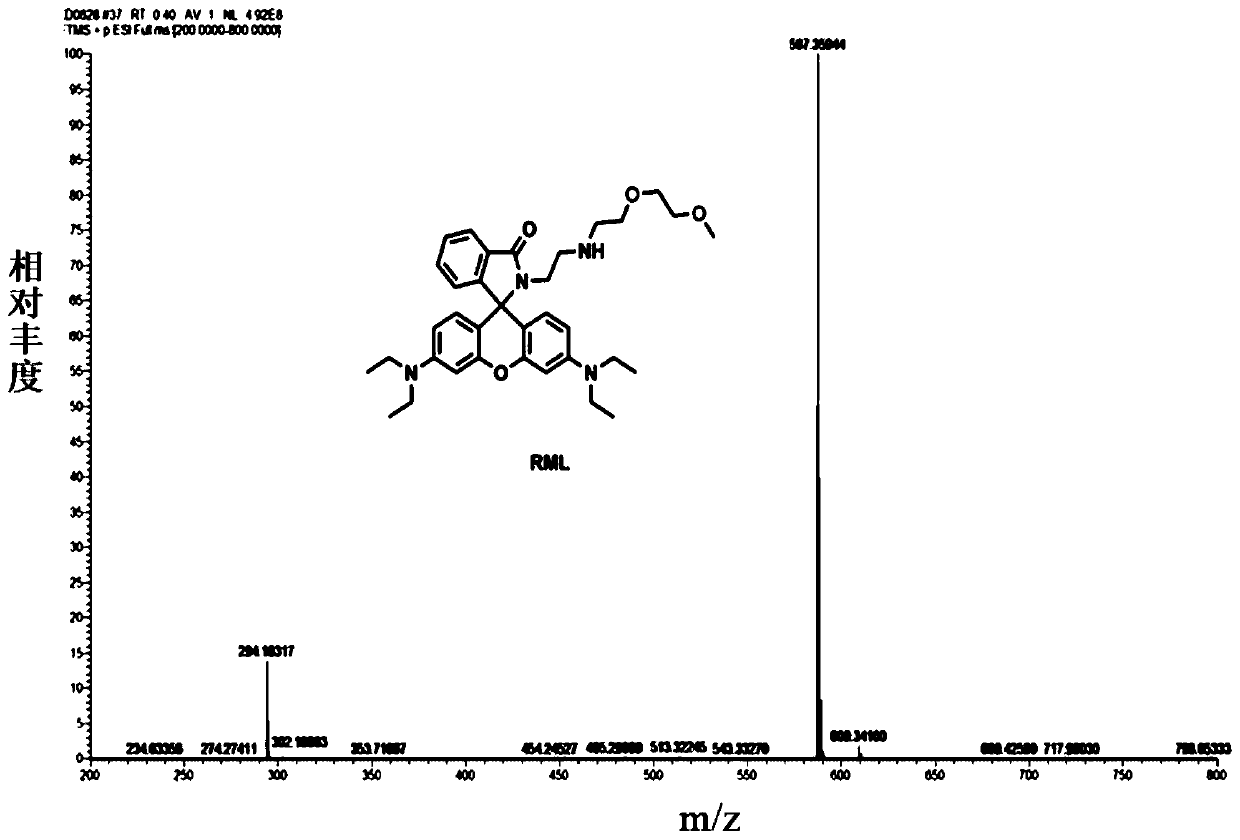
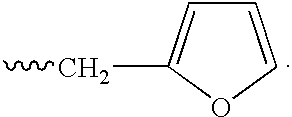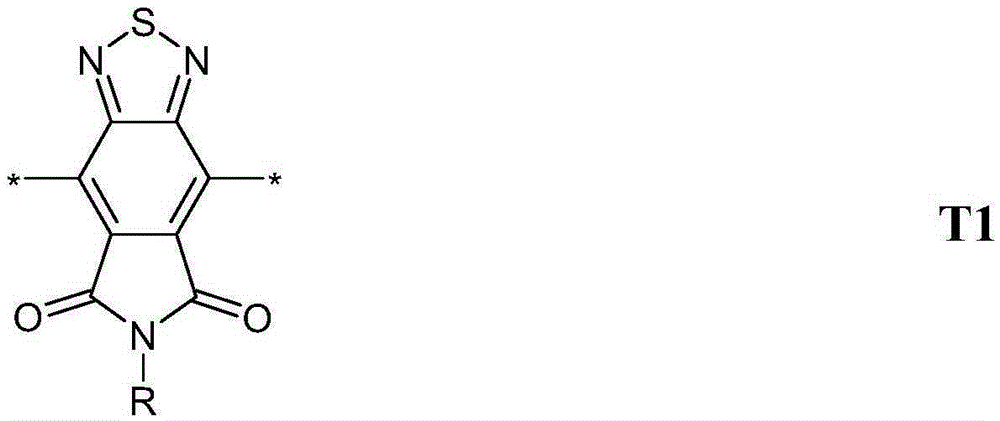Patents
Literature
176 results about "Isoindole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isoindole in heterocyclic chemistry is a benzo-fused pyrrole. The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical literature, but substituted derivatives are useful commercially and occur naturally. Isoindoles units occur in phthalocyanines, an important family of dyes. Some alkaloids containing isoindole have been isolated and characterized.
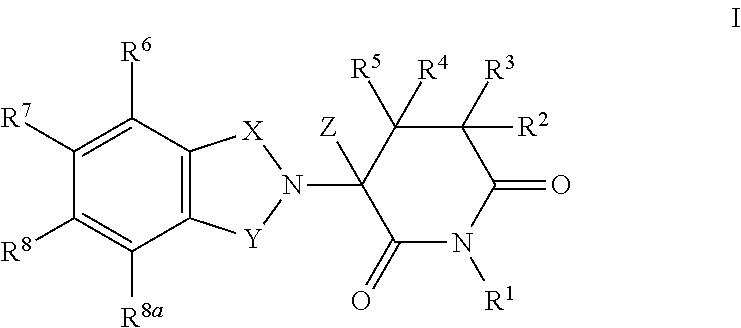
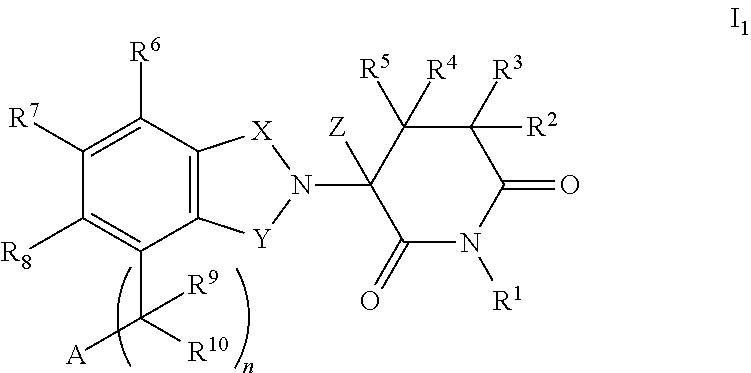
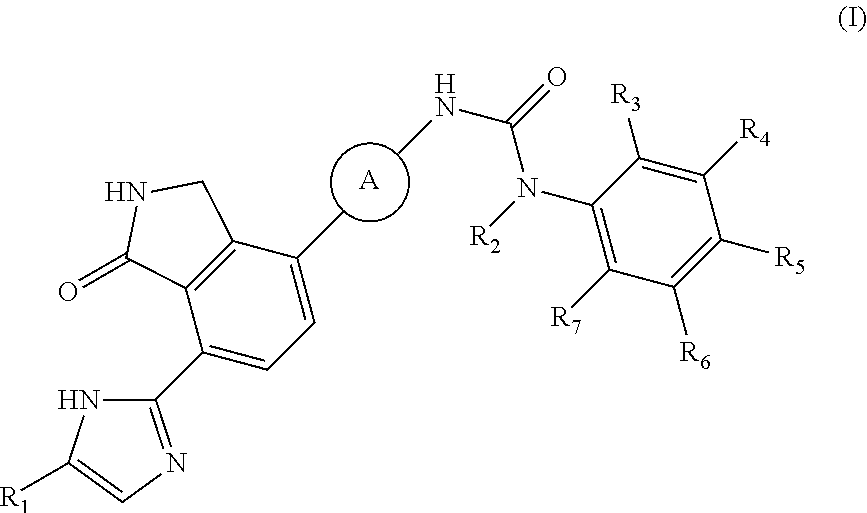
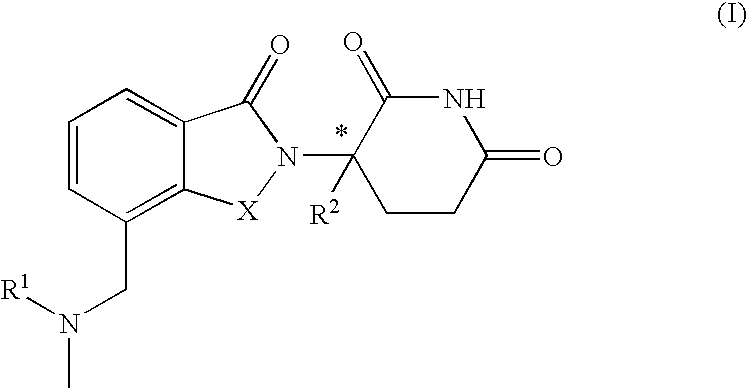
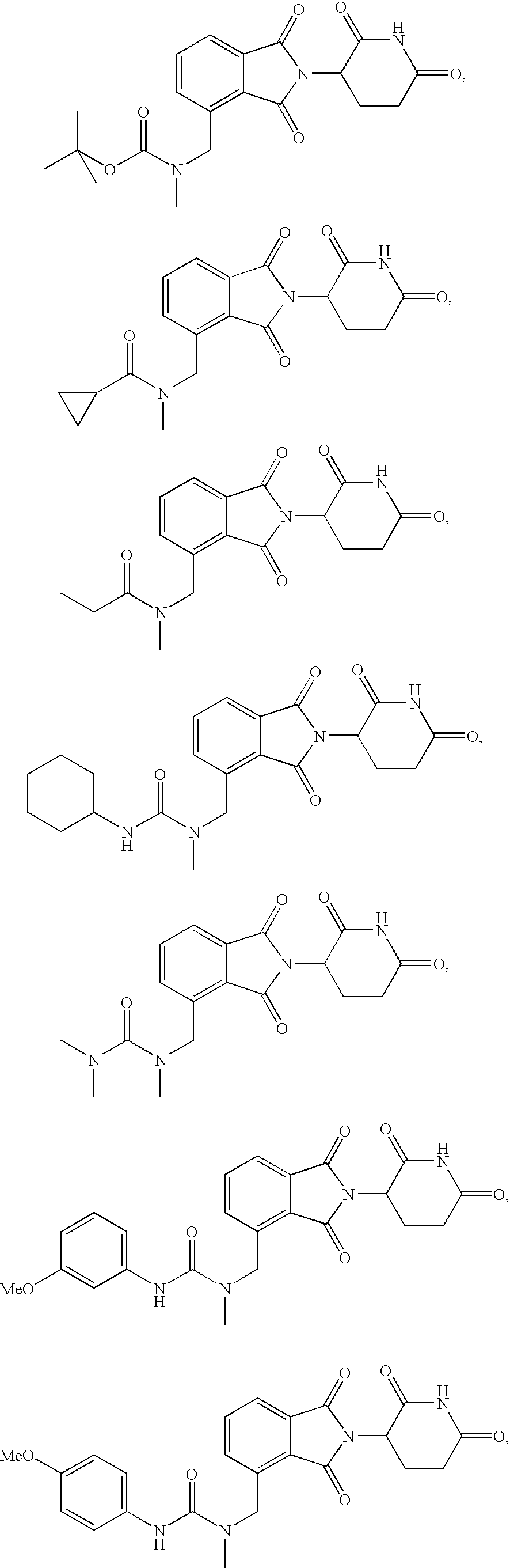
Isoindole-imide compounds, compositions, and uses thereof
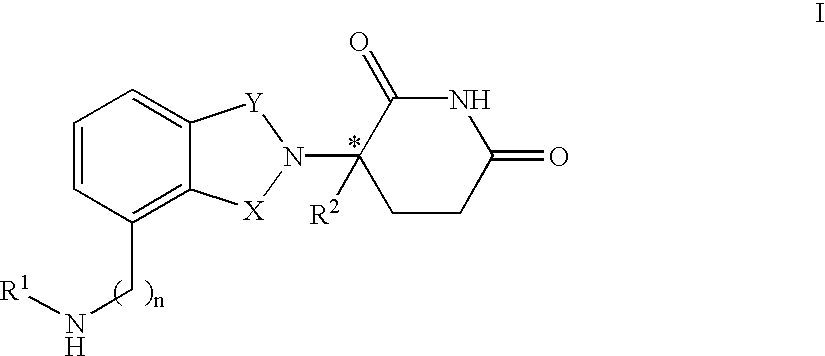
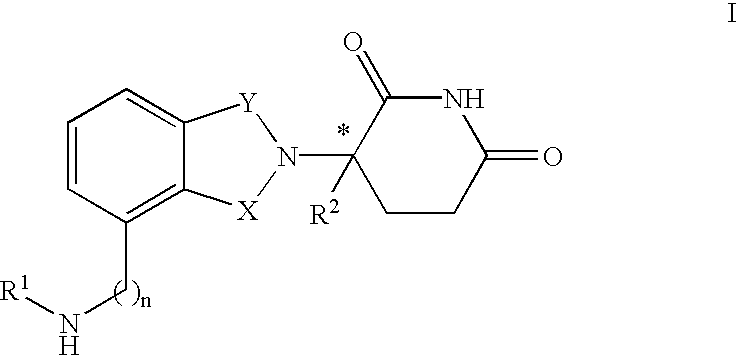
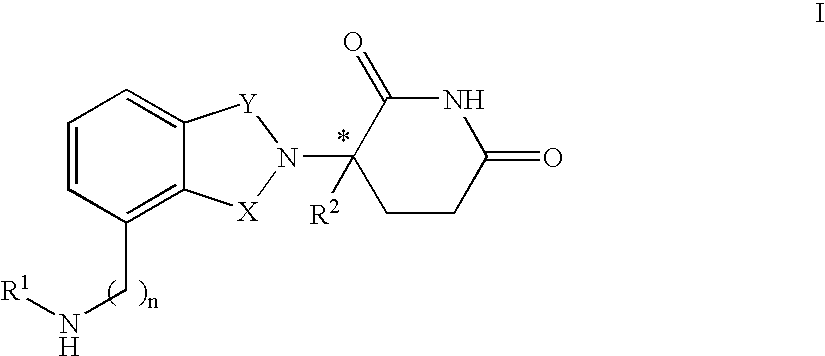
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
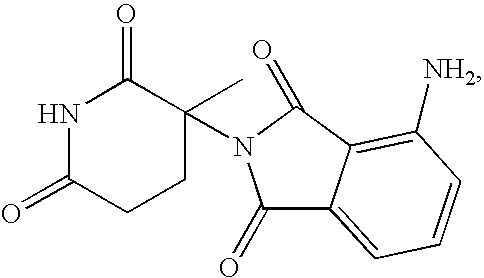
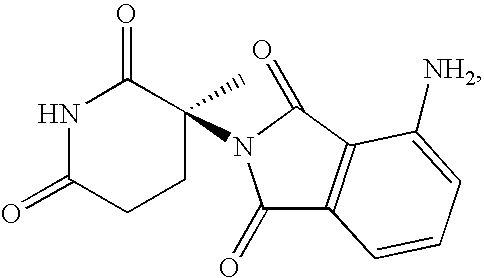
Methods and compositions using 4-amino-2-(3-methyl-2,6-dioxopiperidin-3-yl)-isoindole-1,3-dione
This invention relates to racemic and stereomerically pure 4-amino-2-(3-methyl-2,6-dioxopiperidin-3-yl)-isoindole-1,3-dione, and prodrugs, salts and solvates thereof. Synthesis, methods of use, and pharmaceutical compositions of racemic and stereomerically pure 4-amino-2-(3-methyl-2,6-dioxo-piperidine-3-yl)-isoindole-1,3-dione, and prodrugs, salts and solvates thereof, are disclosed.
Owner:MULLER GEORGE W +1
Fluoroalkoxy-substituted 1,3-dihydro-isoindolyl compounds and their pharmaceutical uses
InactiveUS20040204448A1Subject is at riskReduce probabilityAntibacterial agentsBiocideEnantiomerDiastereomer
Owner:AMGEN INC
Isoindole-imide compounds and compositions comprising and methods of using the same
Owner:CELGENE CORP
2,6-dioxo-3-deutero-piperdin-3-yl-isoindoline compounds
The present application describes 2-(2′,6′-dioxo-3′-deutero-piperidin-3′-yl)isoindoles, deuterated derivatives thereof, stereoisomers thereof, pharmaceutically acceptable salt forms thereof, and methods of treating using the same.
Owner:DEUTERX
2,3-dihydro-isoindole-1-on derivative as BTK kinase suppressant, and pharmaceutical composition including same
The present invention provides a compound selected from the group consisting of a compound of formula (I), pharmaceutically acceptable salts, esters, prodrugs, hydrates, solvates and isomers thereof; a use of the compound for the treatment, relief or prevention of diseases caused by abnormal or uncontrolled activation of protein kinase, and a use of the compound for the manufacture of a medicament for the treatment, relief or prevention of the diseases; a pharmaceutical composition comprising the compound as an active ingredient; and a method for the treatment, relief or prevention of the diseases using the compound. The inventive compound is useful for the treatment, relief or prevention of diseases caused by abnormal or uncontrolled activation of protein kinase.
Owner:CRYSTAL GENOMICS INC
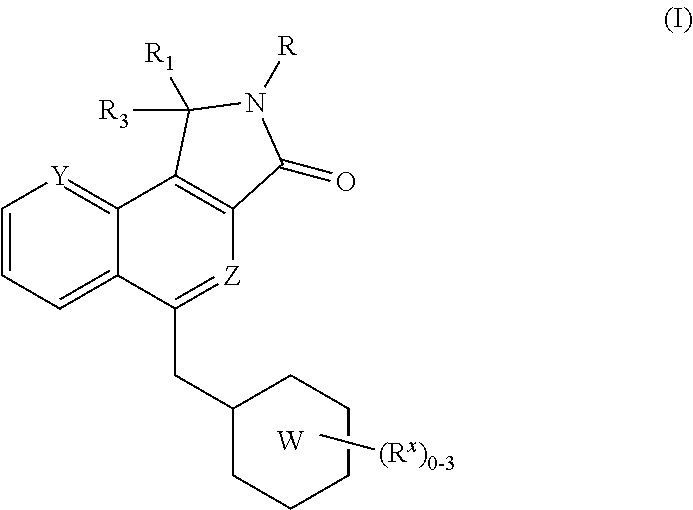
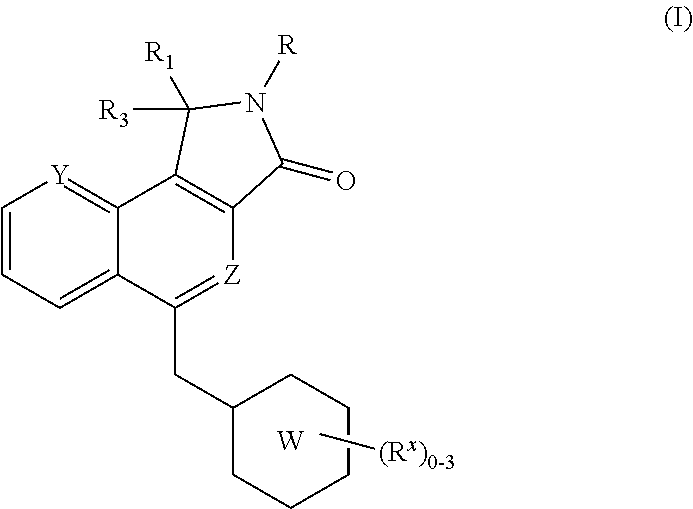
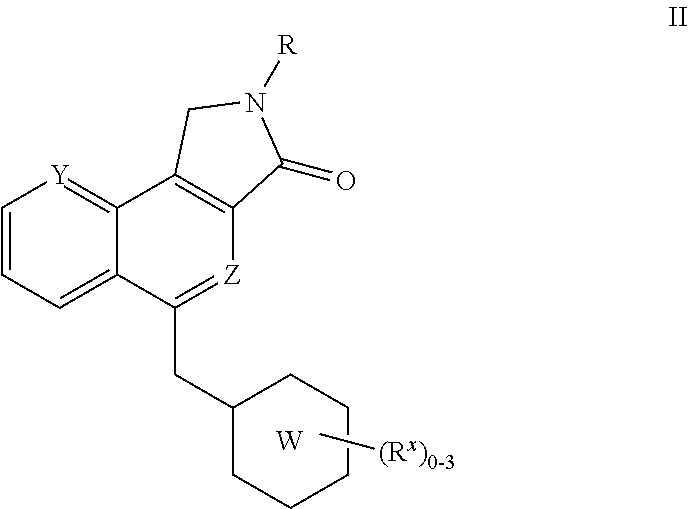
Substituted Dihydroisoindolones As Allosteric Modulators of Glucokinase
The present invention relates to compounds of Formula (I), methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating glucokinase mediated disorders. More particularly, the compounds of the present invention are glucokinase modulators useful for treating disorders including, but not limited to, type II diabetes.
Owner:JANSSEN PHARMA NV
Organic element for electroluminescent devices
ActiveUS20050123798A1Group 8/9/10/18 element organic compoundsSolid-state devicesIridiumEffect light
Disclosed is an electroluminescent device comprising a light-emitting layer containing a light emitting phosphorescent material that contains an organometallic complex comprising iridium and an indole compound with an unsubstituted phenyl ring or comprising Ir, Rh, Os, Ru, Pt, and Pd and an isoindole compound. The invention further comprises compositions of certain such complexes as well as a display or area lighting devices and a process for emitting light. The organometallic materials function as useful phosphorescent light emitting materials in electroluminescent devices.
Owner:GLOBAL OLED TECH
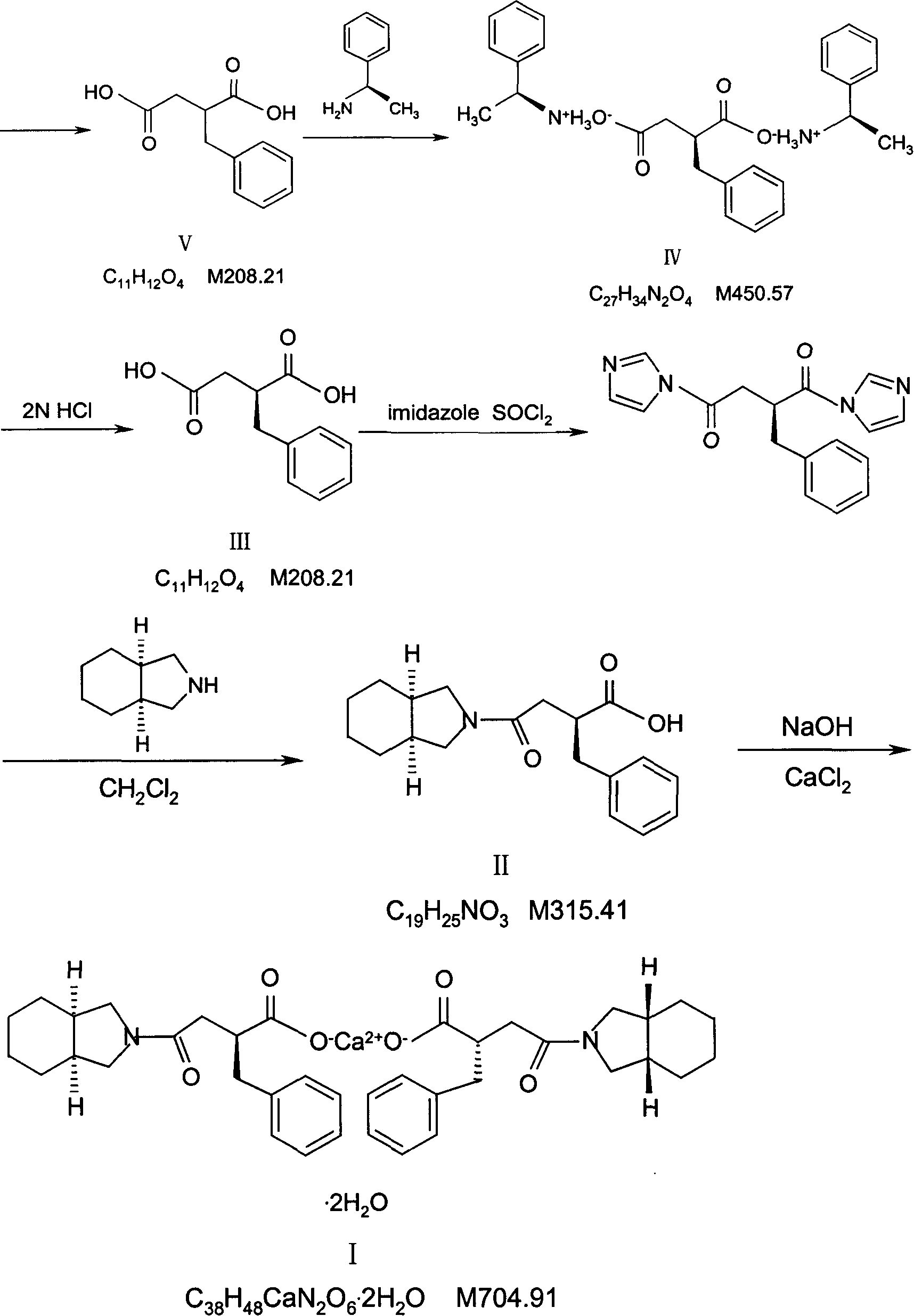
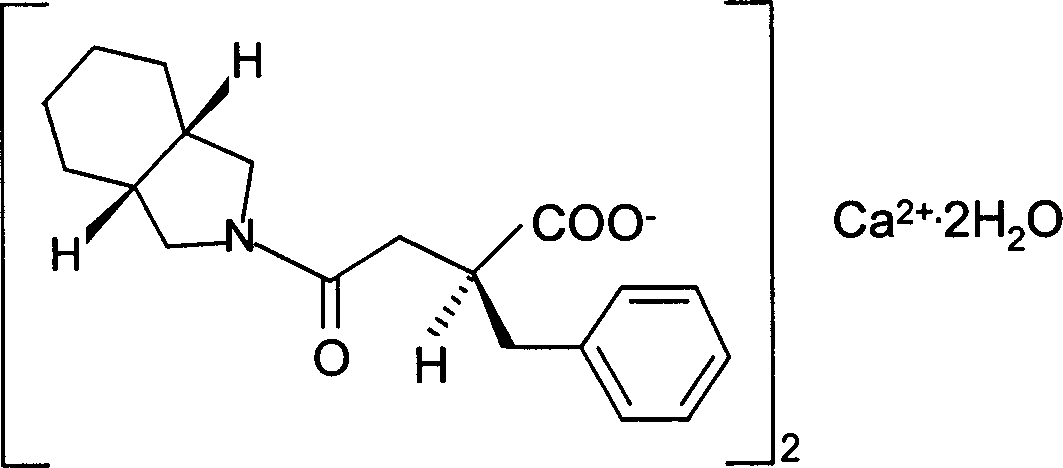
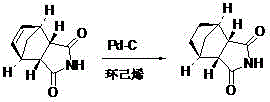
Preparation of mitiglinide calcium and its quality control method
InactiveCN1844096AImprove accuracyHigh sensitivityOrganic active ingredientsOrganic chemistryPropanoic acidMalonate
The invention relates to a preparation and quality control method of Mitiglinide Calcium comprising steps: 1,synthesis of cis- cyclohexyl-1,2-dimethylacid imide; 2,synthesis of cis-hexa-hydrogen isoindole; 3,synthesis of alpha-benzyldiethyl malonate; 4,synthesis of benzylsuccinic acid; 5,S- benzylsuccinic acid methylbenzylamine salt; 6,synthesis of s- benzylsuccinic acid; 7,synthesis of (2S)-2- benzyl-3-(cis-hexa-hydrogen isoindole-2- carbonyl) propanoic acid; 8,synthesis of Mitiglinide Calcium; 9,purity of Mitiglinide Calcium. This invention also contains the method for quality control of Mitiglinide Calcium comprising steps: watching deseription, messureing specific rotation, authenticating, checking, content messureing for Mitiglinide Calcium.
Owner:天津汉康医药生物技术有限公司
Methods for treating inflammatory conditions or inhibiting JNK
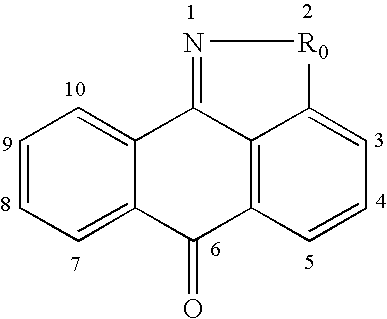
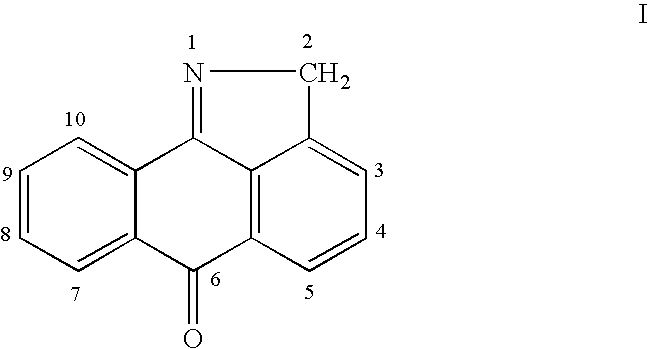
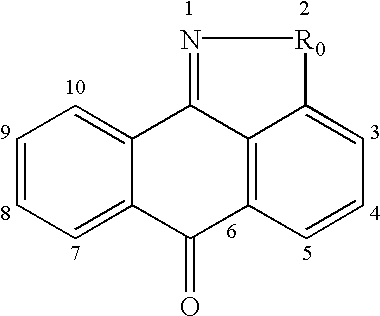
This invention is generally directed to methods for treating or preventing a disease or disorder comprising administering to a patient in need thereof an effective amount of a Jun N-terminal kinase (JNK) inhibitor, such as an isothiazoloanthrone, isoxazoloanthrone, isoindolanthrone, or derivative thereof having the general formula: and pharmaceutically acceptable salts thereof, wherein Ro is -CH2-, -SO-, -O-, -SO2-, or -S-.
Owner:SIGNAL PHARMA LLC
Isoindoloindolone compounds
Compound of formula (I):wherein:R1, R2, R3, R4, R5, R6 and R8, which may be identical or different, each represents hydrogen, alkyl, arylalkyl, hydroxy, alkoxy, arylalkoxy, acyloxy, arylcarbonyloxy, carboxyalkyl or carboxy,R7 represents hydrogen, hydroxy, alkoxy, arylalkoxy, acyloxy or arylcarbonyloxy group,or one of R1 to R8, together with another of R1 to R8 adjacent to it, forms an alkylenedioxy,its optical isomers, and addition salts thereof with a pharmaceutically acceptable acid or base.
Owner:LES LAB SERVIER
Conjugated polymers
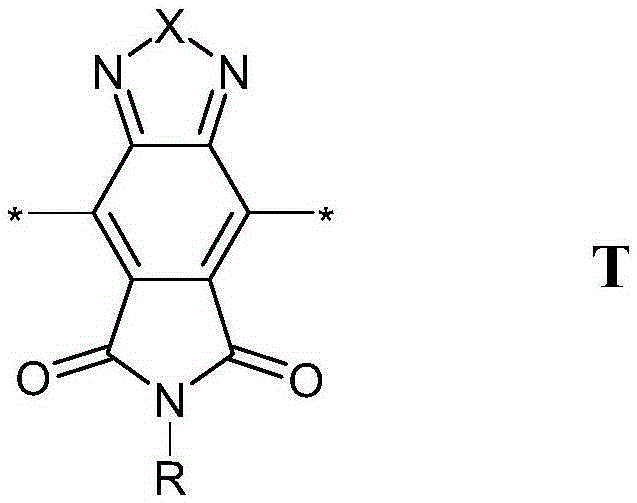
The invention relates to novel conjugated polymers containing one or more [1,2,5]Thiadiazolo[3,4-e]isoindole-5,7-dione (TID) repeating units, to methods for their preparation and educts or intermediates used therein, to polymer blends, mixtures and formulations containing them, to the use of the polymers, polymer blends, mixtures and formulations as organic semiconductors in, or for the preparation of, organic electronic (OE) devices, especially organic photovoltaic (OPV) devices and organic photodetectors (OPD), and to OE, OPV and OPD devices comprising, or being prepared from, these polymers, polymer blends, mixtures or formulations.
Owner:RAYNERGY TEK INC
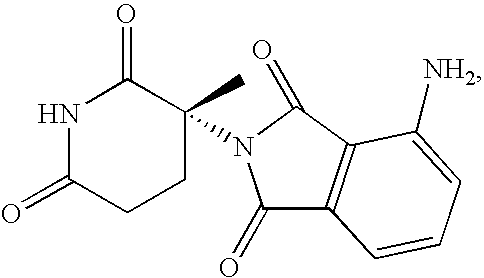
Crystal IV of 3-(substituted dihydroisoindolinone-2-yl)-2,6-piperidinediketone and medicinal composite thereof
ActiveCN101817813ANot suitable for industrial scale parallel productionImprove stabilityOrganic active ingredientsOrganic chemistryDiketoneKetone
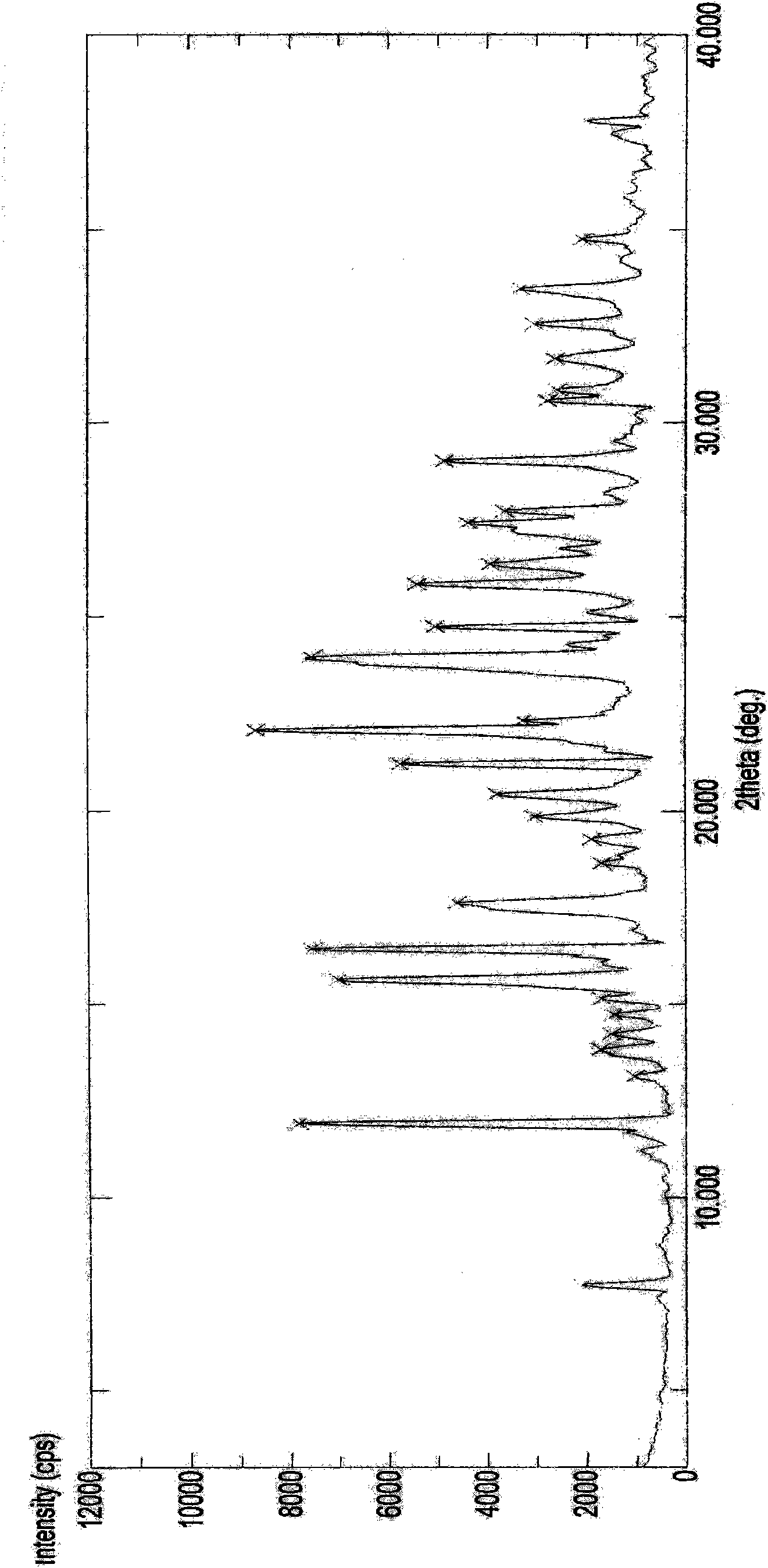
The invention discloses a crystal IV of 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindole-2-yl) piperidine-2,6-diketone. 2theta expressed by degrees has diffraction peaks between 7.7+ / -0.2 and 11.9+ / -0.2 in the X-ray diffraction pattern of the crystal. Moreover, the invention also discloses a preparation method and a medicinal composite of the crystal.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Isothiazoloanthrones, isoxazoloanthrones, isoindolanthrones and derivatives thereof as JNK inhibitors and compositions and methods related thereto
Isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof having the general formula: and pharmaceutically acceptable salts thereof, wherein R0 is -CH2-, -SO-, -O-, -SO2-, or -S-; compositions comprising the isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof; and methods for treating or preventing a disorder alleviated by inhibiting Jun N-terminal kinase (JNK) by administering the isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof are described herein.
Owner:SIGNAL PHARMA LLC
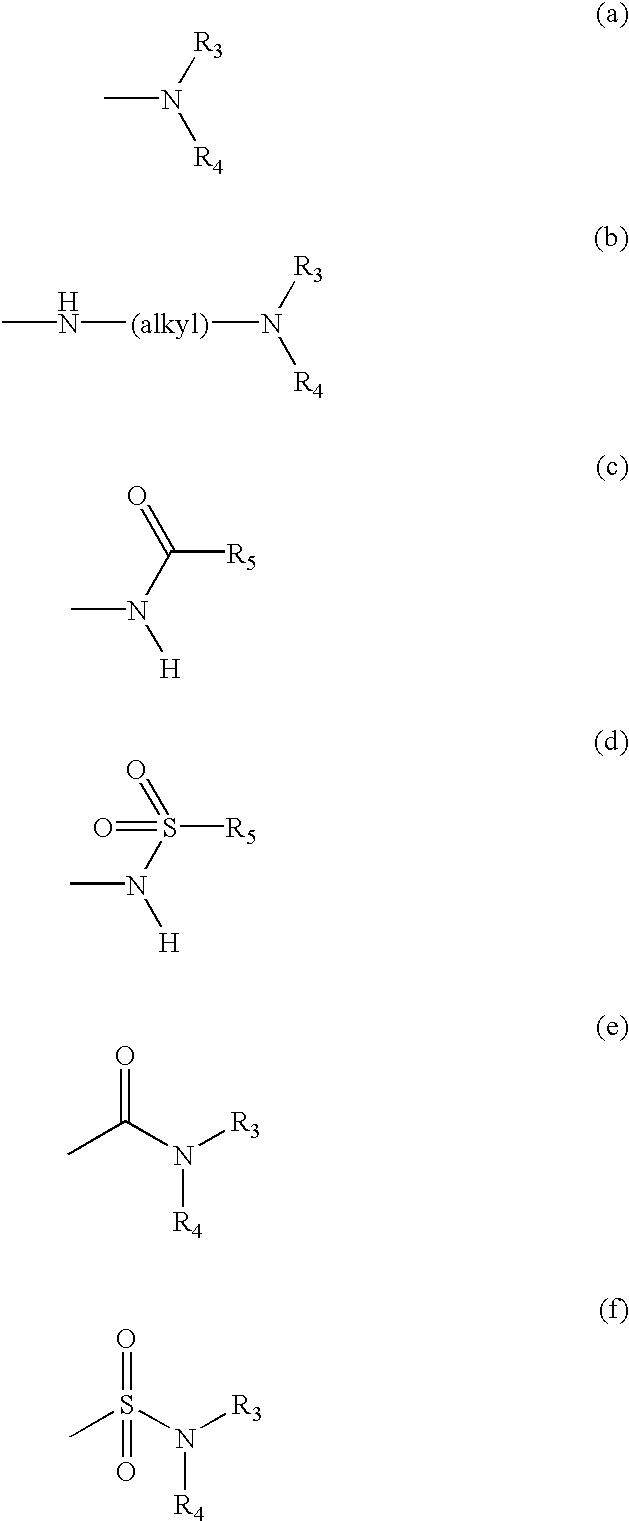
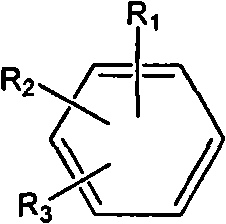
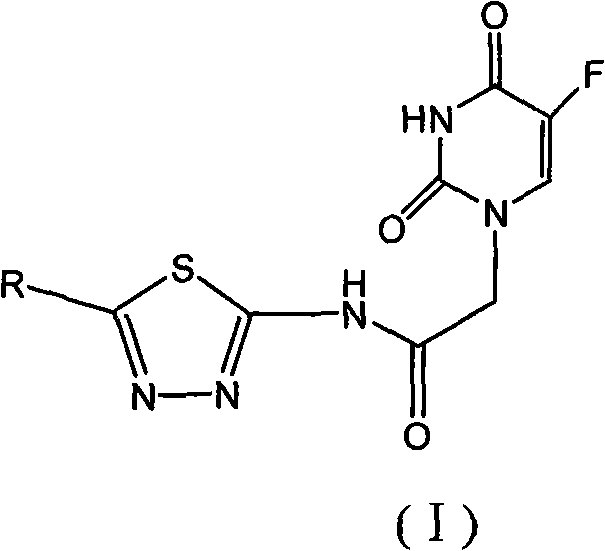
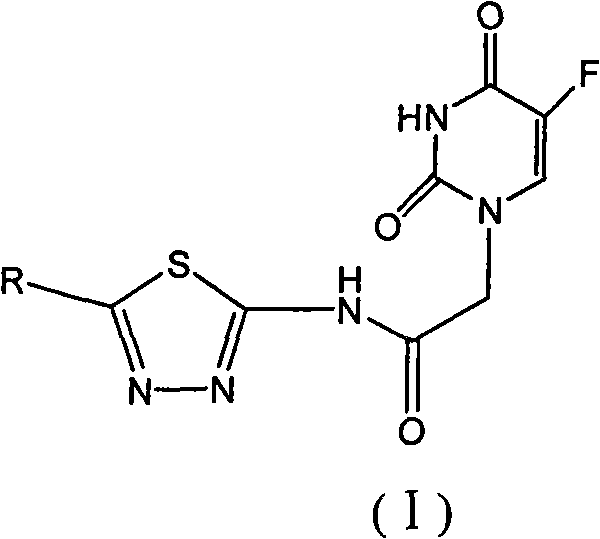
1,3,4-thiadiazole fluorouracil compound as well as preparation method and application thereof
The invention discloses a 1,3,4-thiadiazole fluorouracil compound as well as a preparation method and application thereof. The compound has a general formula I, wherein R is substituted phenyl, aromatic heterocycle or aryloxy alkyl; the substituent group R1 in tri-substituted phenyl is hydrogen, nitryl, alkoxyl, halogen atom, alkyl, substituted alkyl or phenoxyl, R2 is hydrogen, nitryl, alkoxyl, halogen atom, alkyl, substituted alkyl or phenoxyl, and R3 is hydrogen, nitryl, alkoxyl, halogen atom, alkyl, substituted alkyl or phenoxyl; and the aromatic heterocycle is pyridine, thiofuran, furan, indole or isoindole. The invention has less compound consumption, good insecticidal effect, simple process method, low cost and wide market prospect.
Owner:NANJING UNIV OF TECH
Isoindolone m1 receptor positive allosteric modulators
The present invention is directed to isoindolone compounds of formula (I) which are M1 receptor positive allosteric modulators and that are useful in the treatment of diseases in which the M1 receptor is involved, such as Alzheimer's disease, schizophrenia, pain or sleep disorders. The invention is also directed to pharmaceutical compositions comprising the compounds, and to the use of the compounds and compositions in the treatment of diseases mediated by the M1 receptor.
Owner:MERCK SHARP & DOHME LLC
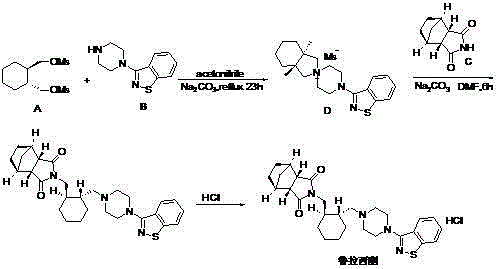
Preparation method of lurasidone
The invention provides a preparation method of lurasidone. On the basis of the existing preparation method of lurasidone, a one-pot method is adopted to replace the method including multiple steps and obtain a target product once. The preparation method comprises the following steps: adding 3-(1-piperazinyl)-1,2-benzisothiazole in toluene, stirring to dissolve; adding (1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane and an inorganic alkali, heating and carrying out reflux reaction for 12-36 hours; adding (3alpha R,4S,7R,7alpha S)4,7-methano-1H-isoindole-1,3(2H)-dione; heating and refluxing; recycling toluene at reduced pressure; adding ethyl acetate in the residue, stirring to dissolve, washing for 2-3 times with 5% hydrochloric acid, separating out the organic layers, drying for 20-120 minutes, filtering to remove the drying agent, concentrating the obtained ethyl acetate solution, dropwise adding concentrated hydrochloric acid, precipitating the solid, and performing suction filtration to obtain crude lurasidone; and refining crude lurasidone to obtain pure lurasidone. By adopting the preparation method of lurasidone, the solvent can be recycled conveniently and the method is simple in operation.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Cyclic substituted fused pyrrolocarbazoles and isoindolones
InactiveUS20060128780A1Enhancing trophic activity of trophic factorTherapy is simpleBiocideSenses disorderCombinatorial chemistryCarbazole
Owner:CEPHALON INC
Cyclic substituted fused pyrrolocarbazoles and isoindolones
InactiveUS6841567B1Enhancing trophic factor-induced activityTherapy is simpleBiocideSenses disorderCombinatorial chemistryCarbazole
Owner:CEPHALON INC
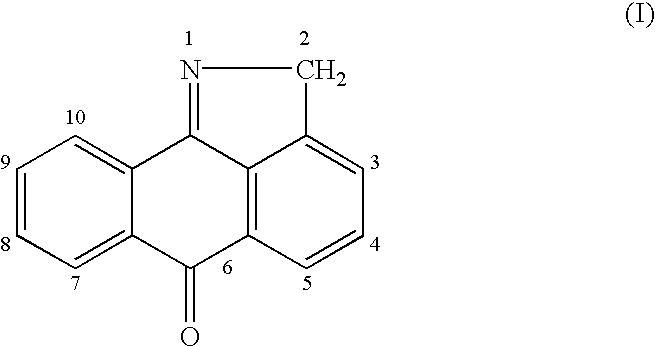
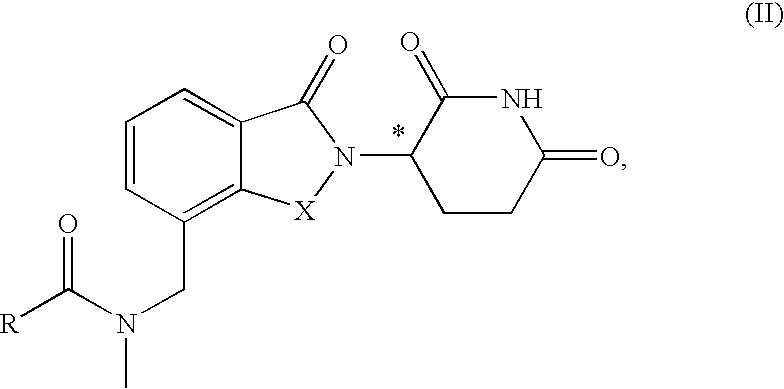
N-methylaminomethyl isoindole compounds and compositions comprising and methods of using the same
This invention relates to N-methylaminomethyl isoindole compounds. Pharmaceutical compositions comprising the compounds and methods for treating, preventing and managing various disorders are also disclosed.
Owner:CELGENE CORP
Pachysandra terminalis alkaloid compound for resisting tumor metastasis
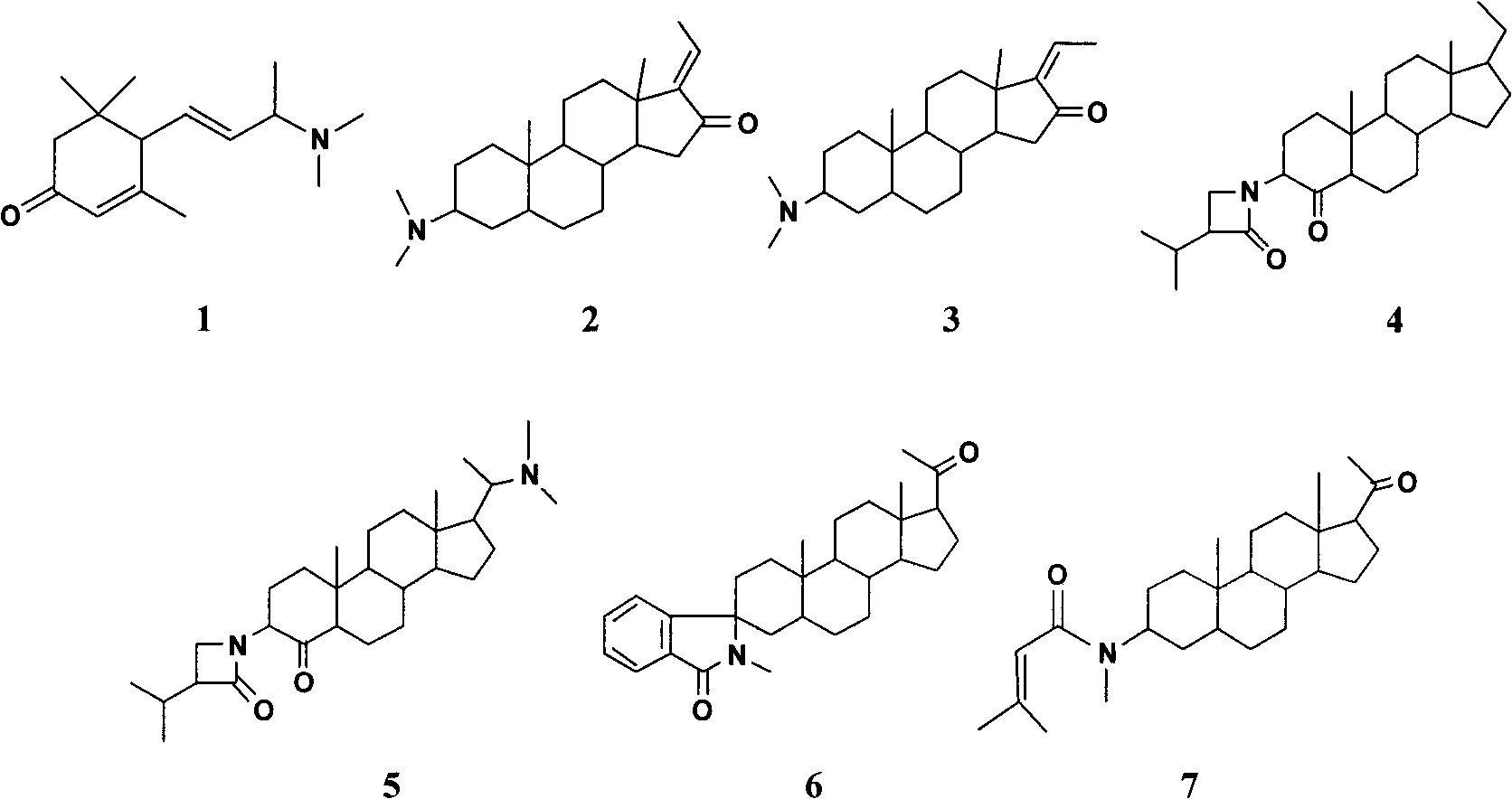
InactiveCN101822657ALow toxicityStrong anti-tumor metastasis effectSteroidsAmine active ingredientsLymphatic SpreadFungating tumour
the invention relates to a pachysandra terminalis alkaloid compound for resisting tumor metastasis (as shown in the figure), which comprises 9-dimethylamino-4,7-megastigmadien-3-one (1), E-salignone (2), Z-salignone (3), 3beta-(3'alpha-isopropyl)-lactam-5alpha-pregn-4-one (4), pachystermine A (5), 3-spiro-(3'-oxo-isoindole)-5alpha-pregn-20-one (6) and 3beta-(methylsenecioylamino)-5alpha-pregn-20-one(7). The invention has a strong effect on resisting the chemotaxis migration of breast cancer cells, and can be used for preparing medicaments for resisting tumor metastasis.
Owner:TIANJIN MEDICAL UNIV
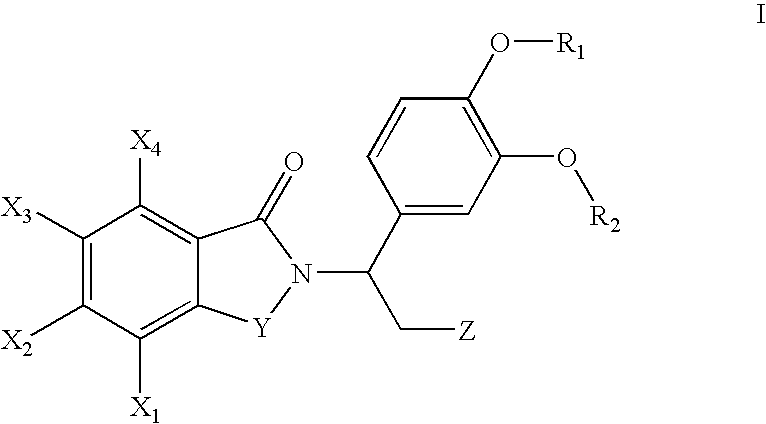
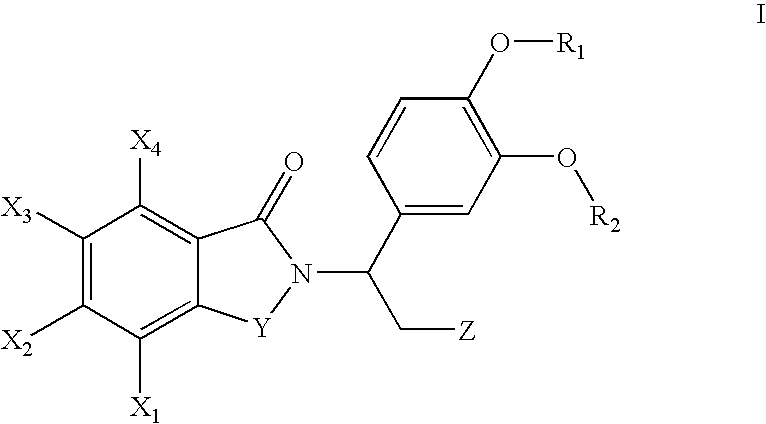
Pharmaceutical compounds
InactiveUS8383619B2Avoid painReduce and even painOrganic active ingredientsOrganic chemistryAcetic acidHydrogen
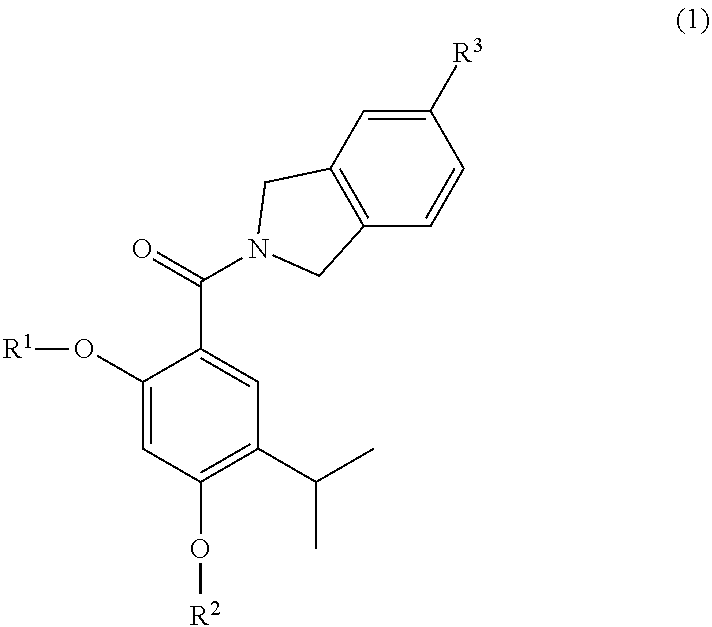
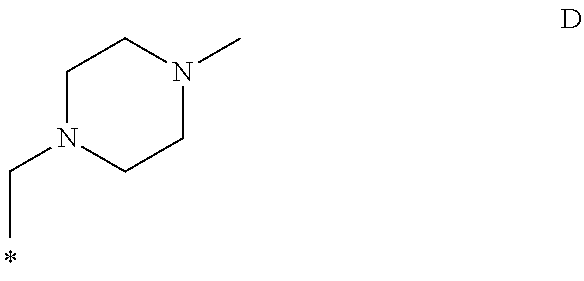
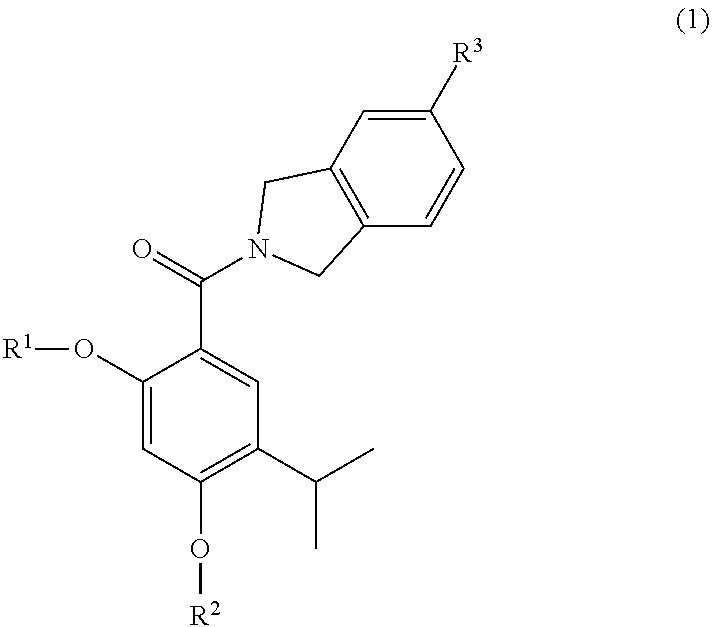
The invention provides a compound of the formula (1):or a salt, solvate, N-oxide or tautomer thereof;wherein either R1 is R1a and R2 is R2a; or R1 is R1b and R2 is R2b; provided that in each case at least one of R1 and R2 is other than hydrogen;R1a and R2a are the same or different and each is selected from hydrogen, C1-4 alkyl, C2-4 alkenyl and C2-4 alkynyl wherein the C1-4 alkyl is optionally substituted by C1-2 alkoxy;R1b and R2b are the same or different and are selected from hydrogen, C(O)NR4R5, C(O)R6 and C(O)OR6 where R6 is C1-4 alkyl, R4 and R5 are both C1-4 alkyl, or NR4R5 forms a 4 to 7 membered saturated heterocyclic ring optionally containing a second heteroatom ring member selected from O, N or S and oxidized forms of N and S, the heterocyclic ring being optionally substituted by one or two C1-4 alkyl groups and / or one or two oxo groups; andR3 is a group D:wherein the asterisk denotes the point of attachment to the isoindoline ring;but excluding acetic acid 5-acetoxy-4-isopropyl-2-[5-(4-methyl-piperazin-1-ylmethyl)-1,3-dihydro-isoindole-2-carbonyl]-phenyl ester.
Owner:ASTEX THERAPEUTICS LTD
Crosslinkable host materials
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
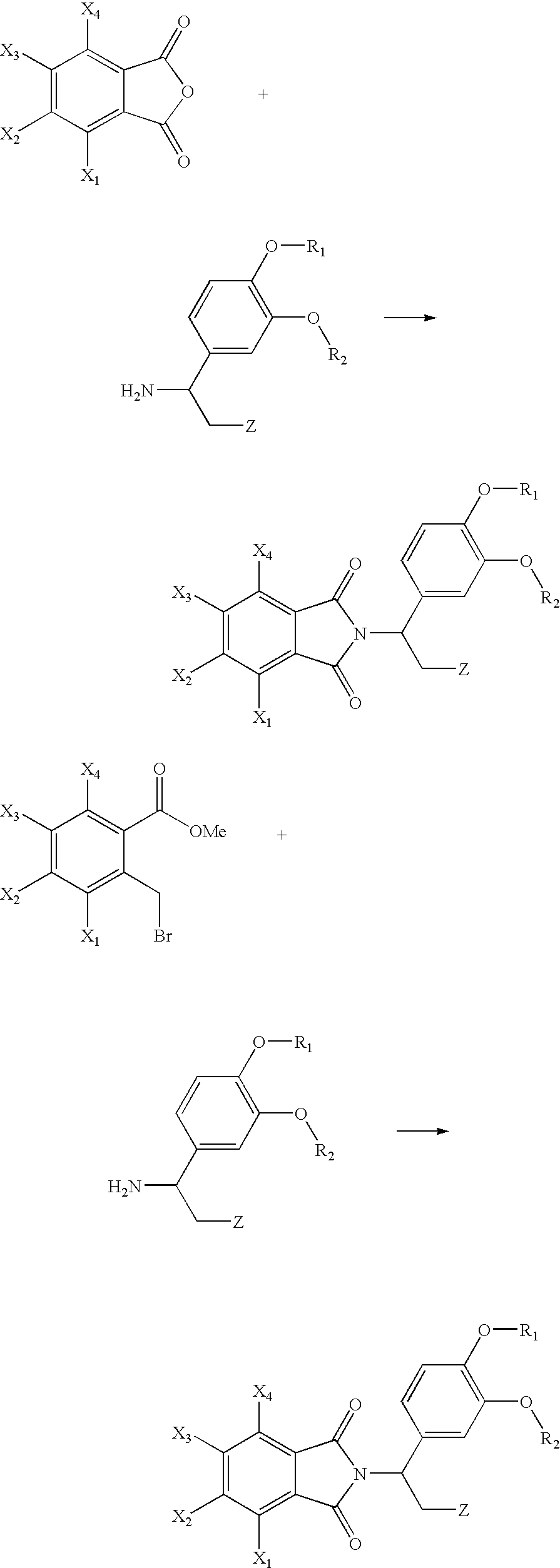
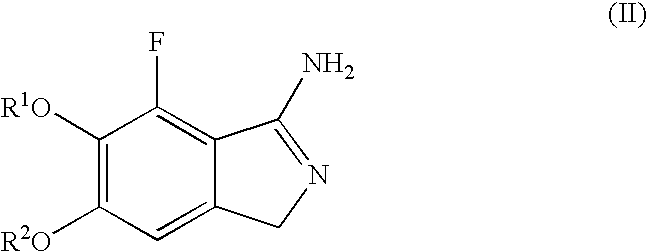
Methods For Producing Isoindole Derivatives
InactiveUS20080214834A1High regional selectivityReduce the risk of fireOrganic chemistrySolventAlkyl
The present invention relates to a method for producing an isoindole derivative (compound (II)) with the following general formula (II):(wherein R1 and R2 each independently represents a C1-6 alkyl group) or a salt thereof, comprising the step of cyclizing, in a solvent, compound (I) with the following general formula (I):(wherein R1 and R2 have the same meanings as R1 and R2 in formula (II) above) or a salt thereof, or their hydrate or solvate in the presence of a base (Step 1).
Owner:EISIA R&D MANAGEMENT CO LTD
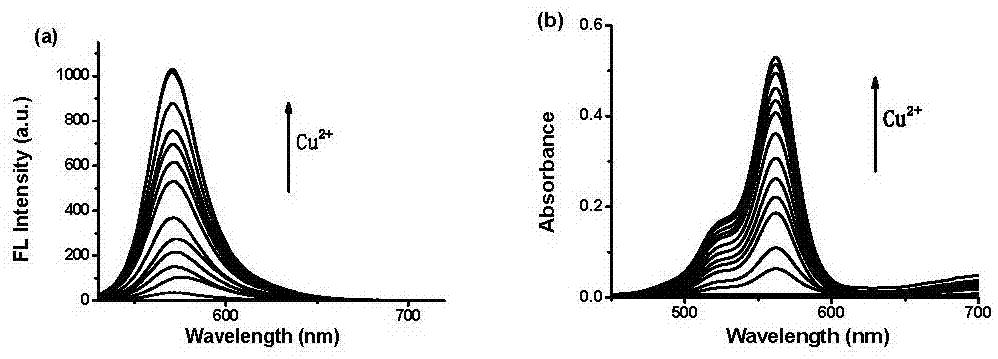
Fluorescent probe for detecting bivalent copper ions, and preparation method and application of fluorescent probe
InactiveCN107235985AEasy to identifyGood choiceOrganic chemistryFluorescence/phosphorescenceChromatographic separationMethylene Dichloride
The invention relates to a fluorescent molecular probe for detecting Cu<2+> in the field of fine chemical engineering, in particular to a preparation method of a Cu<2+> fluorescent molecular probe based on rhodamine dyes and an application of the fluorescent molecular probe to biocellular imaging and water system copper ion detection. A preparation method of a compound I includes the steps: adding 1 part of (E)-2-((3', 6'-bis (diethylamino)-3-oxaspiro [isoindazole-1, 9'-xanthene]-2-alkene) imine) ethylal and 6 parts of hydrazine hydrate into a reaction vessel, heating and stirring mixture, performing rotary evaporation to remove ethyl alcohol after reaction, and adding 50 parts of methylene dichloride and 150 parts of saturated sodium chloride solution; separating mixture by the aid of a separating funnel, drying a organic phase by the aid of anhydrous sodium sulfate, performing spin drying on solvents, and separating coarse products by a column chromatography to obtain the compound I. The preparation method is mild in reaction condition, less in synthesis step and high in product yield, and raw materials are low in cost and easily acquired.
Owner:SHANXI DATONG UNIV
Lysosome targeted pH fluorescent probe for monitoring cell autophagy as well as preparation and application thereof
InactiveCN110951483AHas commercial application valueWith visual monitoringOrganic chemistryFluorescence/phosphorescenceFluoProbesLysosomal targeting
The invention relates to the technical field of pH fluorescent probes and particularly relates to a lysosome targeted pH fluorescent probe for monitoring cell autophagy as well as preparation and application of the lysosome targeted pH fluorescent probe. The preparation method comprises the following steps of dissolving 2-(2-aminoethyl)-3',6'-bis(diethylamino) spiro [isoindole-1,9'-xanthan]-3-ketone and 2-(2-methoxyethoxy)4-methyl benzene sulfonic acid ethyl ester in N, N-dimethylformamide, and carrying out heating reflux to obtain a crude product; and removing a solvent from the crude product, and separating through a silica gel column to obtain a pure product. Cytotoxicity tests show that the probe has almost no toxic or side effect on cells, a cell co-localization experiment determinesthat the probe can specifically target a cell lysosome, and a laser confocal microimaging experiment shows that the probe has good cell membrane permeability and can perform high-sensitivity detectionon pH change in the lysosome. The probe provided by the invention can monitor the autophagy process of the cells by detecting the change of pH in the lysosome.
Owner:SHANXI UNIV
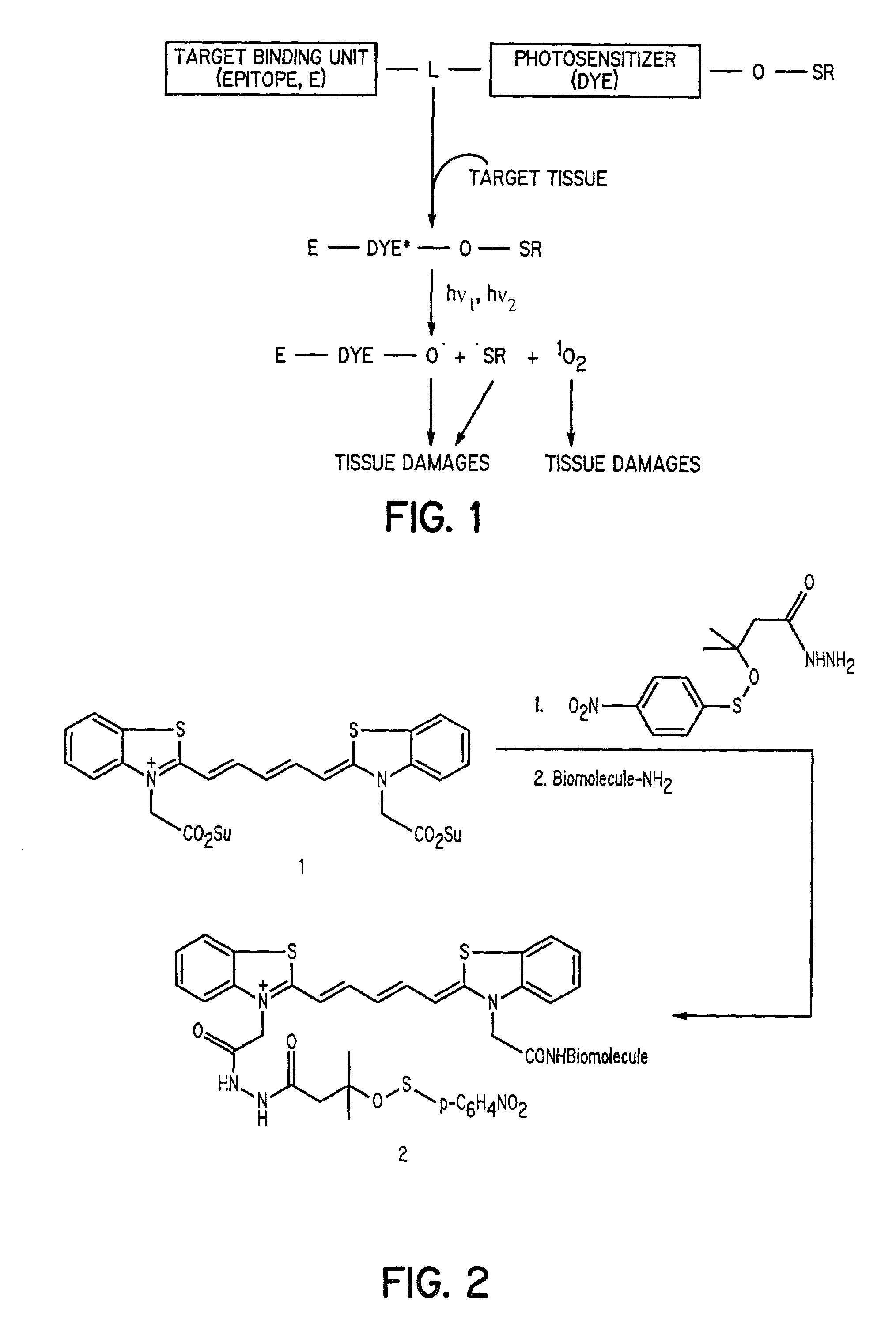
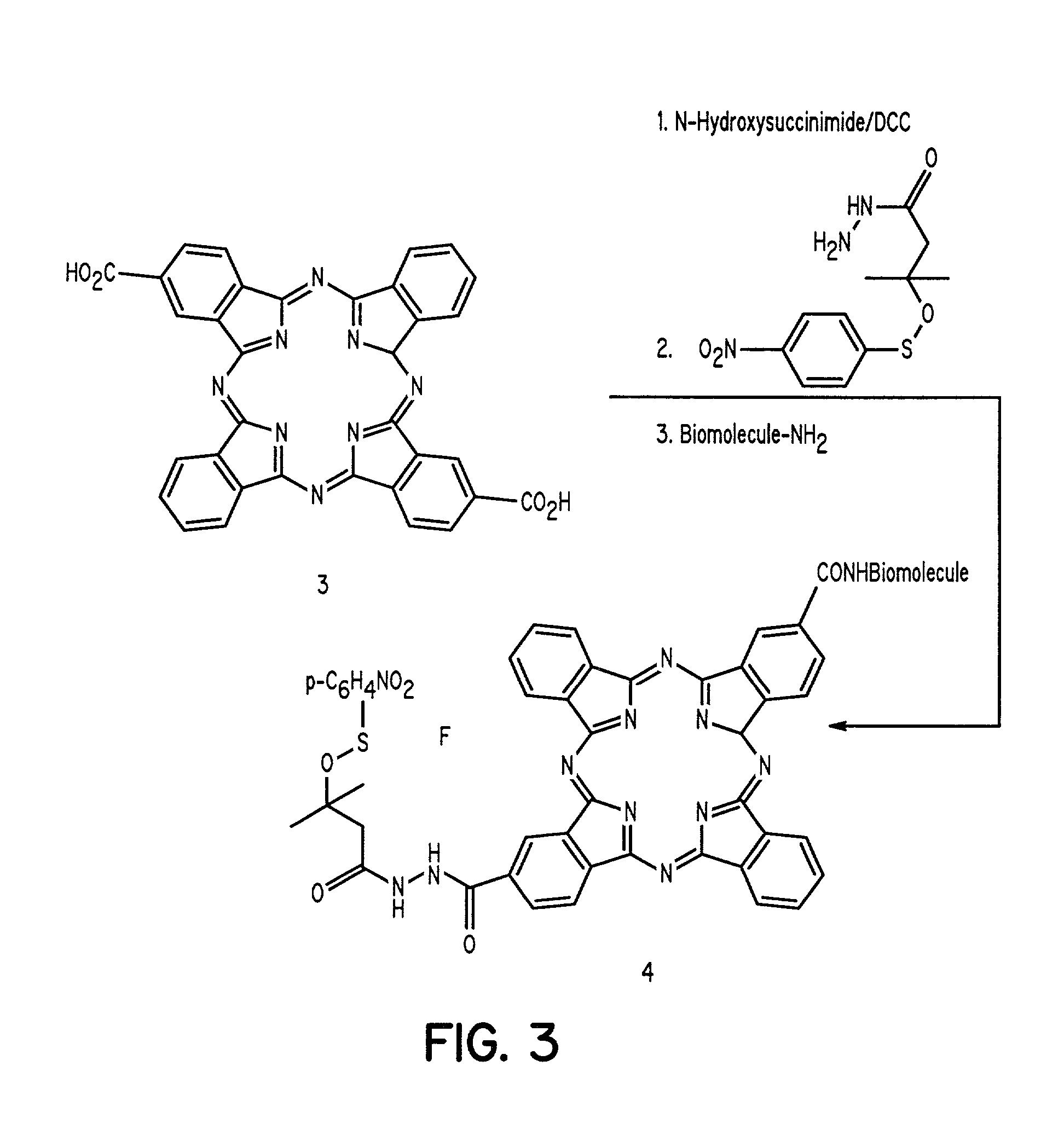
Cyanine-sulfenates for dual phototherapy
Dye-sulfenate derivatives and their bioconjugates for dual phototherapy of tumors and other lesions. The compounds comprise sulfenates having the formula, t,0080where E is selected from the group consisting of somatostatin receptor binding molecules, heat sensitive bacterioendotoxin receptor binding molecules, neurotensin receptor binding molecules, bombesin receptor binding molecules, cholecystekinin receptor binding molecules, steroid receptor binding molecules, and carbohydrate receptor binding molecules, and dihydoxyindolecarboxylic acid; L and X are independently selected from the group consisting of —(R5)NOC—, —(R5)NOCCH2O—, —(R5)NOCCH2CH2O—, —OCN(R5)—, —HNC(═S)NH—, and HNC(═O)NH—; DYE is an aromatic or a heteroaromatic radical derived from the group consisting of cyanines, indocyanines, phthalocyanines, rhodamines, phenoxazines, phenothiazines, phenoselenazines, fluoresceins, porphyrins, benzoporphyrins, squaraines, corrins, croconiums, azo dyes, methine dyes, indolenium dyes, crellins, and hypocrellins; R1 to R5 are independently selected from the group comprising hydrogen, C1-C10 alkyl, C5-C10 aryl, C1-C10 polyhydroxyalkyl, and C1-C10 polyalkoxyalkyl; and Ar is an aromatic or heteroaromatic radical derived from the group consisting of benzenes, naphthalenes, naphthoquinones, diphenylmethanes, fluorenes, anthracenes, anthraquinones, phenanthrenes, tetracenes, naphthacenediones, pyridines, quinolines, isoquinolines, indoles, isoindoles, pyrroles, imidiazoles, oxazoles, thiazoles, pyrazoles, pyrazines, purines, benzimidazoles, furans, benzofurans, dibenzofurans, carbazoles, acridines, acridones, phenanthridines, thiophenes, benzothiophenes, dibenzothiophenes, xanthenes, xanthones, flavones, coumarins, and anthacylines. The compounds are designed to produce both Type 1 and Type 2 phototherapeutic effects at once using a dual wavelength light source that will produce singlet oxygen and free radicals at the lesion of interest.
Owner:MEDIBEACON
Isoindole compounds and uses thereof
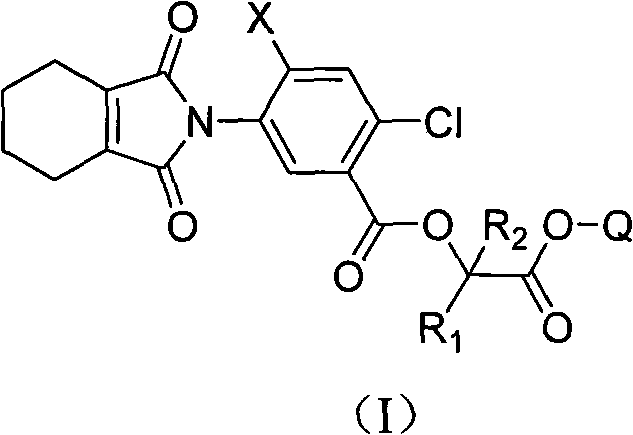
The invention discloses an isonindole compound containing 2-substituent acetic acid unsaturated ester with novel structure as shown in general formula (I), in the formula: X is chosen from H or F; R1 is chosen from H or C1-C6 alkyl; R2 is chosen from H or C1-C6 alkyl; Q is chosen from substitutional alkenyl or alkynyl; and a stereoisomer thereof. The compound with the general formula (I) has postemergence herbicide activity and is safe to corn, wheat, and rice.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-α in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com