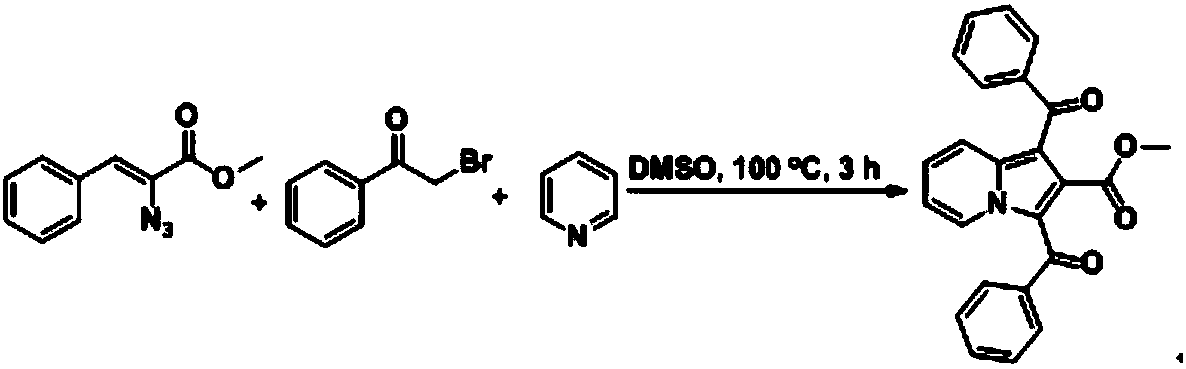Patents
Literature
122 results about "Indolizine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Indolizine (Chemical formula C₈H₇N) is a heterocyclic aromatic organic compound that is an isomer of indole. The saturated analog indolizidine forms the structural core of a variety of alkaloids such as swainsonine.
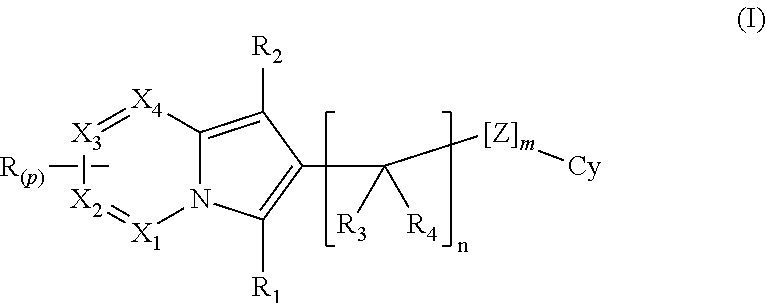
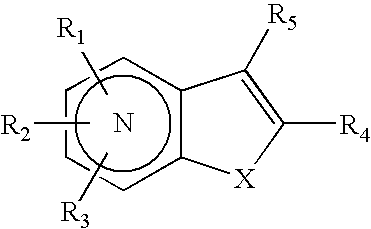
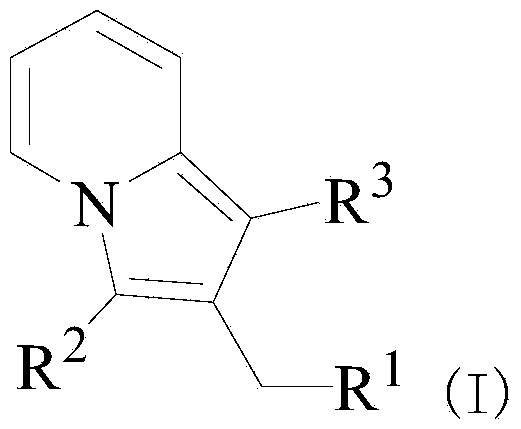
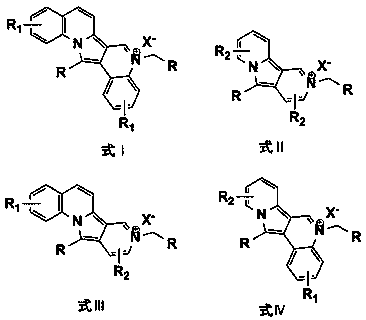
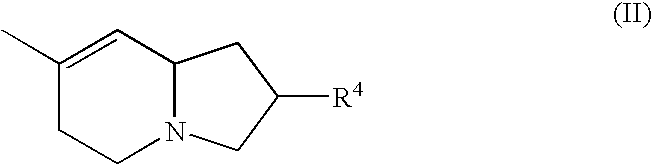
(AZA)indolizine derivative and pharmaceutical use thereof
InactiveUS20130217878A1Inhibit productionExcellent xanthine oxidase inhibitory activityOrganic active ingredientsOrganic chemistryDiseaseXanthine
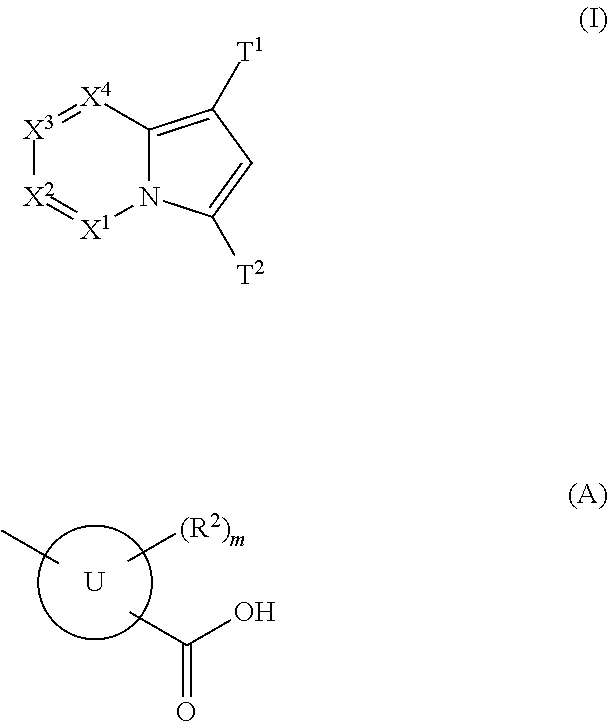
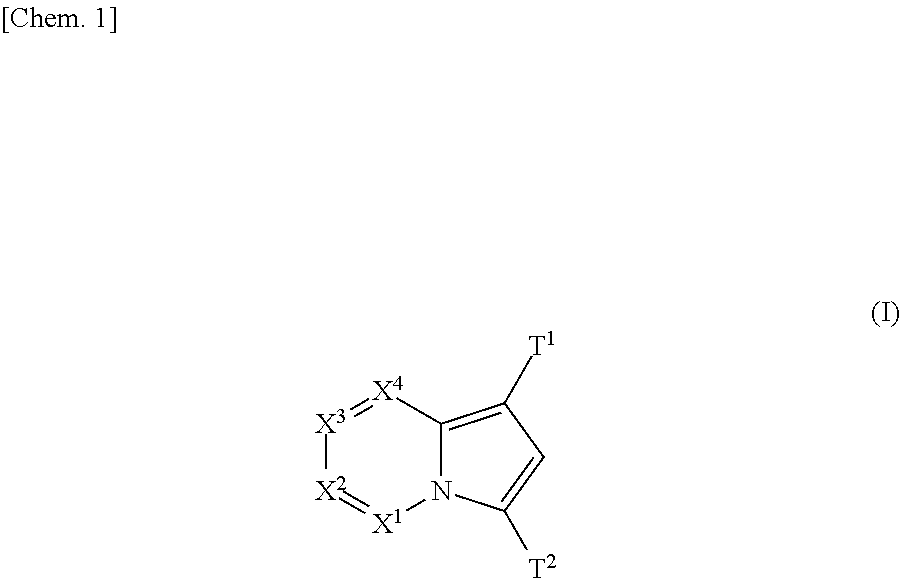
(Aza)indolizine derivatives represented by Formula (I) having xanthine oxidase inhibitory activities and useful as agents for the prevention or treatment of a disease associated with abnormality of serum uric acid level, prodrugs thereof, salts thereof or the like. In Formula (I), 0 to 2 of X1, X2, X3 and X4 are a nitrogen atom and the others are CR1; one of T1 and T2 represents cyano and the other represents a group represented by Formula (A), with the proviso that when T1 is cyano, at least one of X1 to X4 is a nitrogen atom; R1 independently represents a hydrogen atom, a halogen atom, a hydroxy group, C1-6 alkyl, C1-6 alkoxy or the like; ring U represents a benzene ring or the like; m represents integral number from 0 to 2; R2 independently represents a fluorine atom, a hydroxy group or the like.
Owner:KISSEI PHARMA
Indolizine compound with antitumor activity and derivative of indolizine compound
The invention relates to an indolizine compound with the antitumor activity and a derivative of the indolizine compound. The indolizine compound and the derivative thereof are shown in the following description and have the inhibition effect to various cancers and tumors.
Owner:GUANGDONG PHARMA UNIV
Indolizine derivatives as phoshoinositide 3-kinases inhibitors
Owner:CHIESI FARM SPA
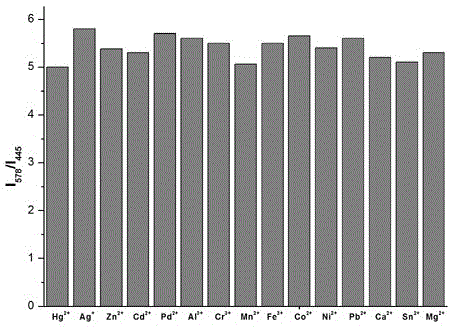
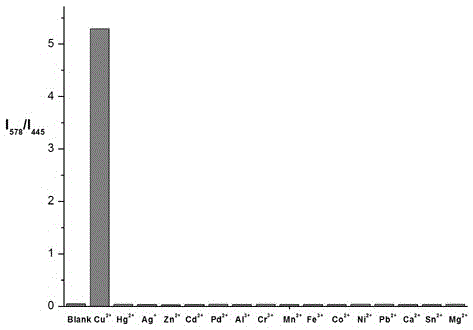
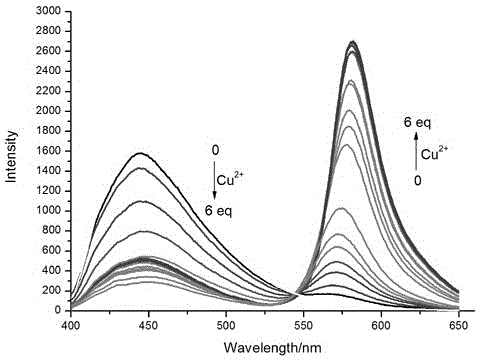
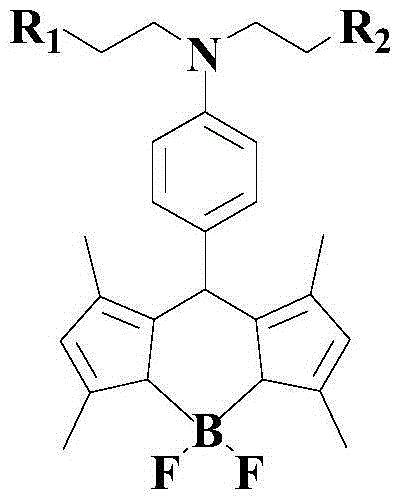
Indolizine-rhodamine hydrazide type cupric ion ratiometric fluorescent probe and application thereof
InactiveCN106632406ADecreased fluorescence intensityHigh fluorescence intensityOrganic chemistryFluorescence/phosphorescenceCupric IonHydrazide
The invention discloses an indolizine-rhodamine hydrazide derivative type Cu<2+> ratiometric fluorescent probe. The probe is indolizine substituted rhodamine hydrazide, and the chemical formula of the probe is as shown in formula (1). The fluorescent probe has unique fluorescent selectivity to Cu<2+> in an ethanol solution and is high in sensitivity, high in resistance to interference of other ions and promising in application prospect.
Owner:TAISHAN MEDICAL UNIV
Substituted indolizine-like compounds and methods of use
Selected novel substituted indolizine-like compounds are effective for treatment of diseases, such as TNF-α, IL-1β, IL-6 and / or IL-8 mediated diseases, and other maladies, such as cancer, pain and diabetes. The invention encompasses novel compounds, analogs, prodrugs and pharmaceutically acceptable salts thereof, pharmaceutical compositions and methods for treatment of diseases and other maladies or conditions involving inflammation, cancer, pain, diabetes and the like. The subject invention also relates to processes for making such compounds as well as to intermediates useful in such processes.
Owner:AMGEN INC
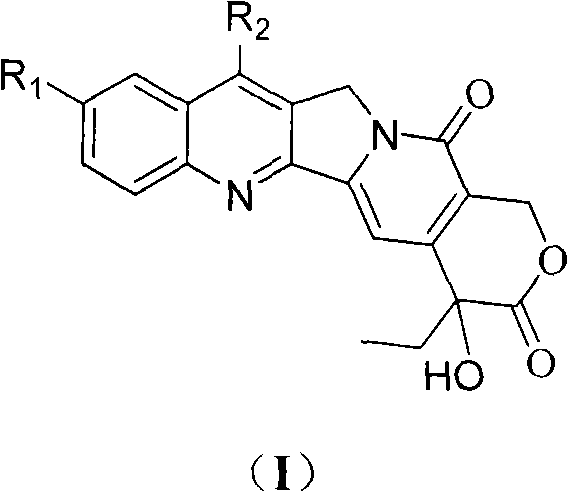
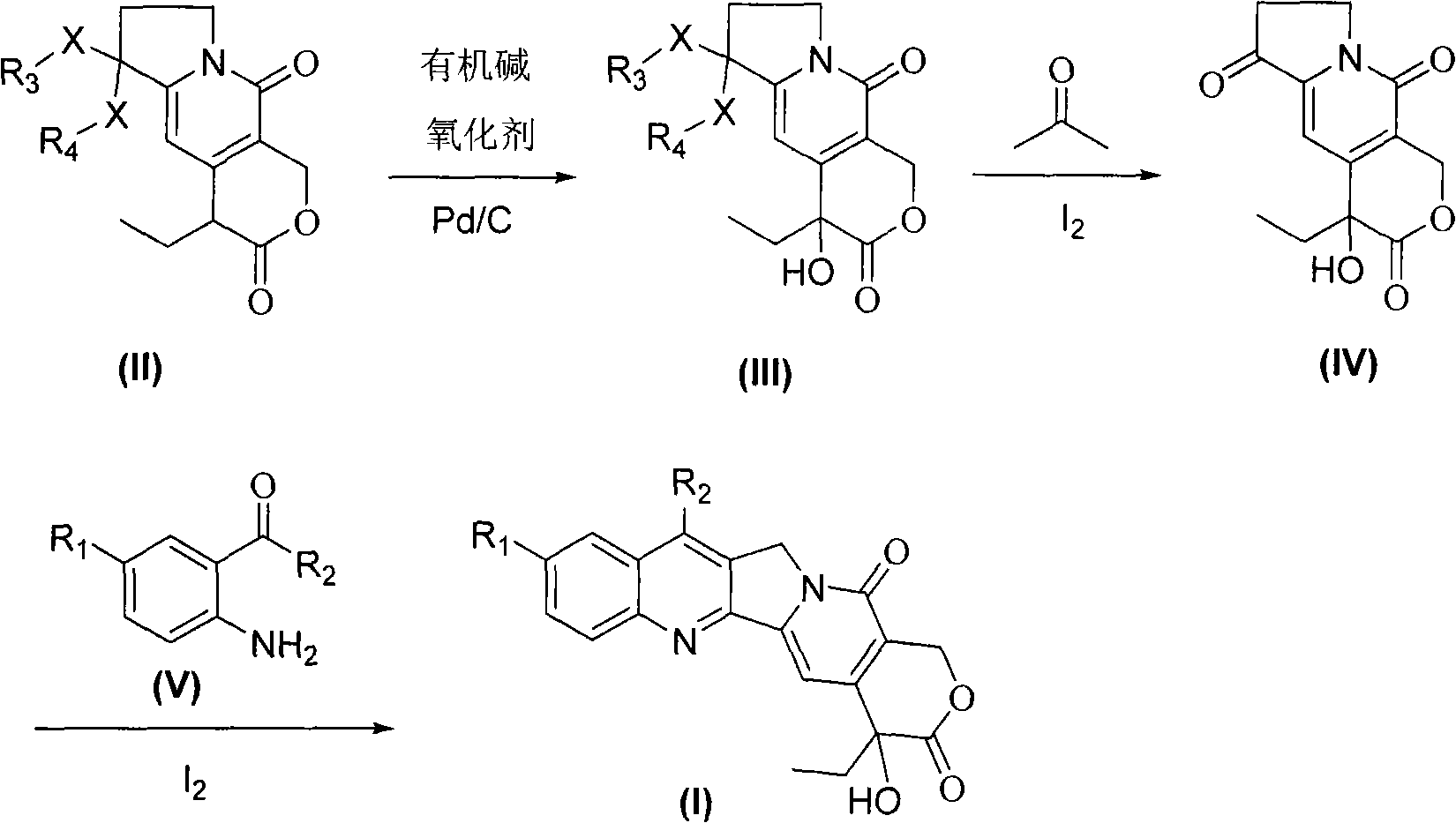
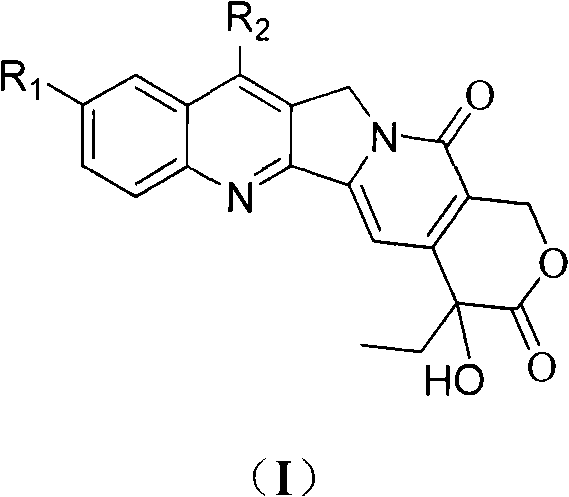
Process for the Manufacturing of 7-Ethyl-10-Hydroxy Camptothecin
The invention discloses the preparation method of 7-ethyl-10-hydroxycamptothecin from 4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione and 1-(2-amino-5 -hydroxyphenyl)-propan-1-one using higher reaction temperature and faster heating to that temperature.
Owner:FERMION
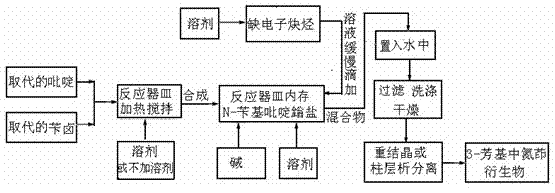
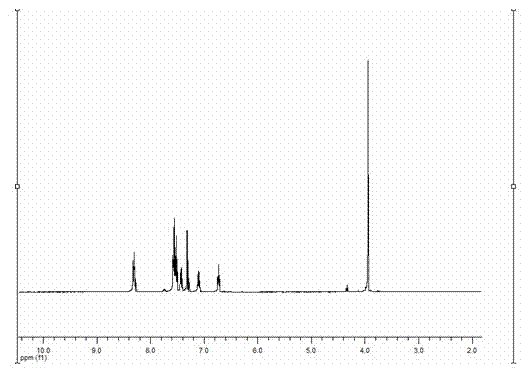
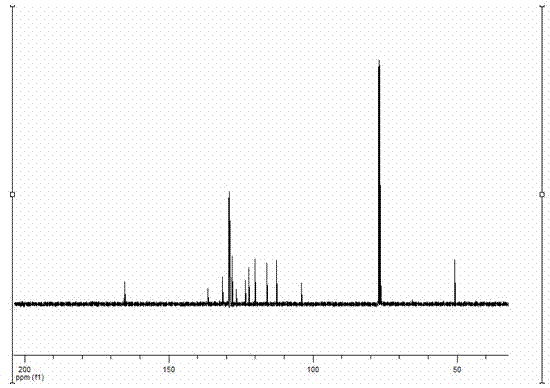
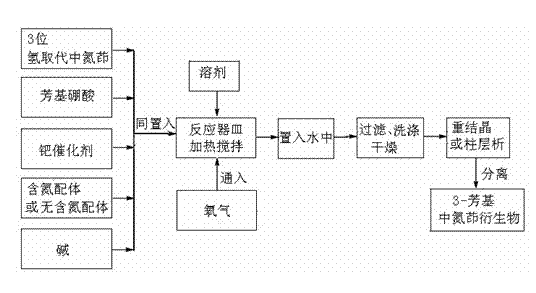
Simple preparation method of 3-aryl indolizine derivative
The invention provides a simple preparation method of a 3-aryl indolizine derivative. The method comprises the following step of: reacting N-benzylpyridine onium salt directly prepared from a pyridine derivative and benzyl halide with electron-deficient alkyne under the existence of alkali to prepare the 3-aryl indolizine derivative. All raw materials used in the simple preparation method can be directly purchased, and are relatively low in price and easy to obtain; any catalyst, ligand and oxidant do not need to be used in the process flow, the process flow is simple, and the cost is obviously reduced; the problem that transition metal in target products exceeds standards is avoided; the whole process is insensitive to air or moisture, and the normal operation can be carried out under loose reaction conditions without polluting the environment; and the simple preparation method can be used for providing the 3-aryl indolizine derivative with abundant sources and low price for the preparation of related products in the fields of biology, pesticides and medicine.
Owner:常熟紫金知识产权服务有限公司
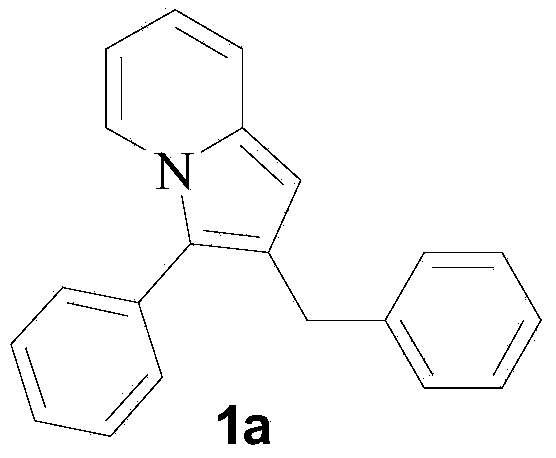
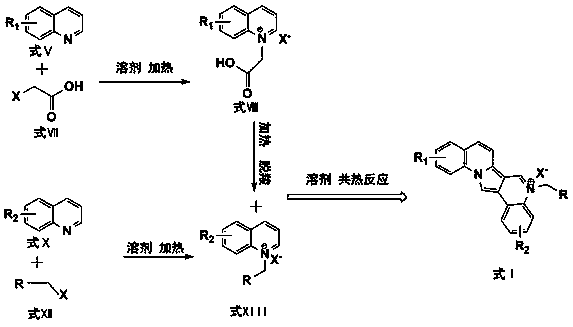
Purrocoline derivative and synthetic method and application thereof
InactiveCN103641827ANovel structureReduced responseOrganic active ingredientsOrganic chemistrySynthesis methodsTriflic acid
The invention discloses a purrocoline derivative and a synthetic method and application thereof. The purrocoline derivative with a novel structure can be prepared by reacting propargyl alcohol and a 2-alkyl pyridine compound by taking samarium trifluoromethanesulfonate as a catalyst under the condition without a solvent; the reaction consumption and the chemical contamination are reduced, the cost is reduced and the operation is simplified; the obtained purrocoline derivative has better activity on a gastric cancer cell strain MGC80-3. The structural formula of the purrocoline derivative is shown in a formula (I) in the specification, wherein R<1> is phenyl, 4-methoxyphenyl, 4-fluorophenyl and 2-chlorphenyl or thienyl, R<2> is phenyl, and R<3> is H, phenyl, methyl, a nitrile group or an ethyl acetate group.
Owner:GUANGXI NORMAL UNIV
Acidification corrosion inhibitor based on interpolymer indolizine derivative as well as preparation method and application thereof
InactiveCN108440527AEfficient corrosion inhibitionGood corrosion inhibitionOrganic chemistryDrilling compositionAcetic acidQuaternary ammonium cation
The invention discloses an acidification corrosion inhibitor based on an interpolymer indolizine derivative as well as a preparation method and application thereof. The acidification corrosion inhibitor contains the interpolymer indolizine derivative; the interpolymer indolizine derivative is prepared by carrying out decarboxylation on heterocyclic alkali including (substituted) quinoline, (substituted) pyridine and the like, and carboxymethyl heterocyclic alkali quaternary ammonium salt obtained by alpha-haloacetic acid, and then carrying out intermolecular addition polymerization reaction onquaternary ammonium salt of the heterocyclic alkali including the (substituted) quinoline, the (substituted) pyridine and the like. The acidification corrosion inhibitor disclosed by the invention has relatively good corrosion inhibition performance under the condition that common corrosion inhibition synergists including alkynol and the like do not need to be compounded; the use amount of the acidification corrosion inhibitor is less and the acidification corrosion inhibitor can reach, even be better than the requirements of an acidification corrosion inhibitor performance testing method andfirst-grade to third-grade standards in evaluation indexes SY / T 5405-1996 when being independently used.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Substituted indolizines and derivatives as CNS agents
Compounds of formula 1 or pharmaceutically acceptable salts thereof are provided: which are agonists or partial agonists of the 2C subtype of brain serotonin receptors. The compounds, and compositions containing the compounds, can be used to treat a variety of central nervous system disorders such as schizophrenia.
Owner:WYETH LLC
Crosslinkable host materials
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
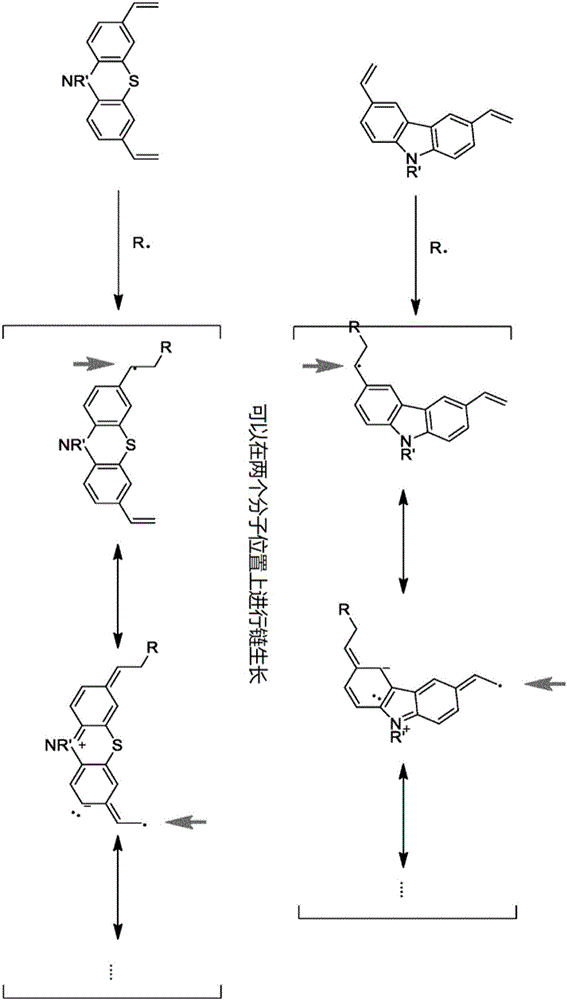
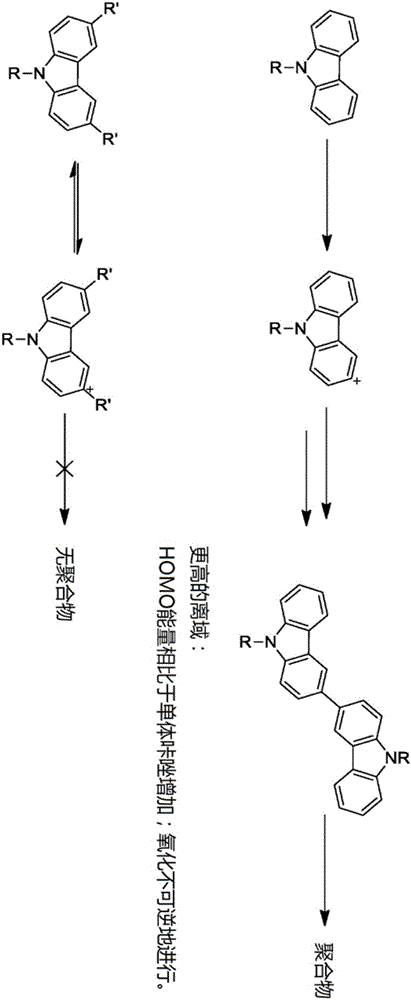
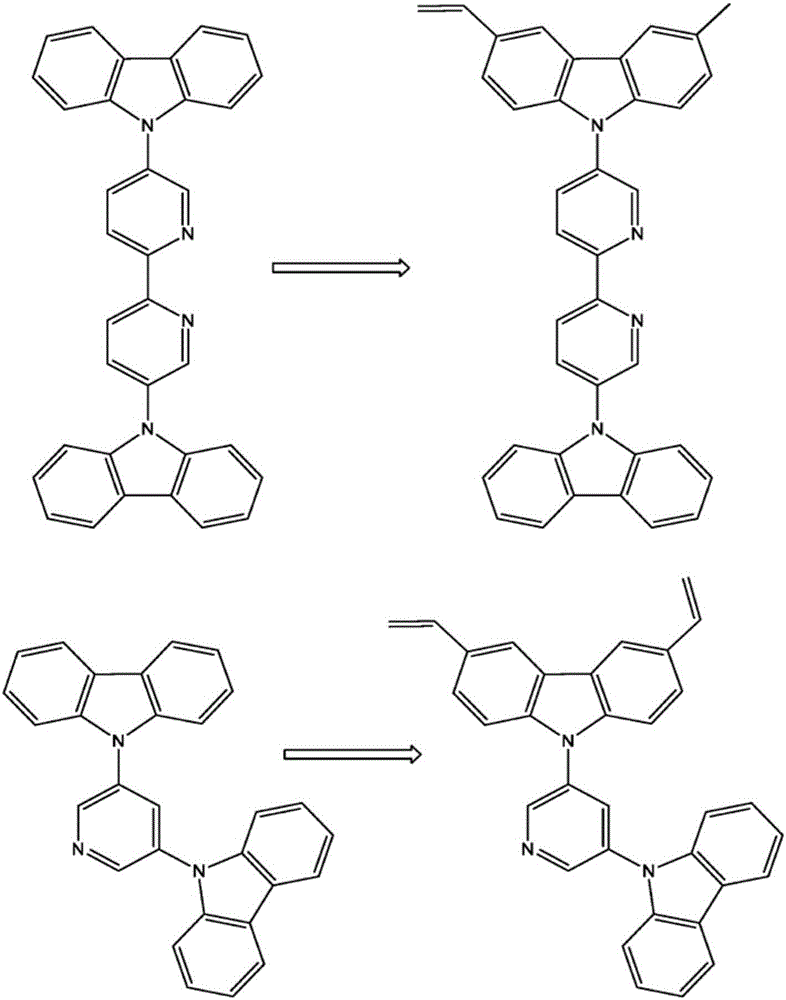
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
Acidizing corrosion inhibitor based on dimer indolizine derivative and preparation method and application of acidizing corrosion inhibitor
The invention discloses an acidizing corrosion inhibitor based on a dimer indolizine derivative and a preparation method and application of the acidizing corrosion inhibitor. The acidizing corrosion inhibitor contains the dimer indolizine derivative; and the dimer indolizine derivative is mainly obtained by further intermolecular dimerization of active alpha-cetyl trimethyl ammonium chloride of heterocyclic alkali such as (substituted) quinolone and (substituted) pyridine under alkali acceleration. The acidizing corrosion inhibitor has good corrosion inhibition under the condition that a common corrosion inhibition synergist such as alkynol does not need to be compounded, the use amount is small, and when the acidizing corrosion inhibitor is used independently, requirements of first-levelto third-level standard in an acidizing corrosion inhibitor performance testing method and an evaluation index SY / T 5405-1996 can be met and are even higher.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
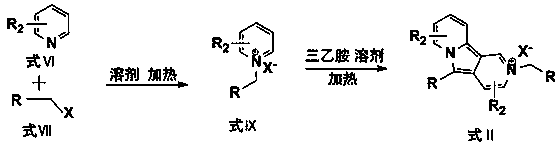
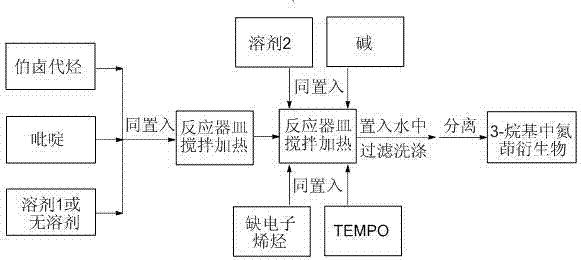
Preparation method of 3-alkyl indolizine derivative
ActiveCN106928222ANo pollution in the processReduce manufacturing costOrganic chemistryPyridiniumPyridine
The invention discloses a preparation method of a 3-alkyl indolizine derivative. The method comprises the steps that N-alkyl pyridinium salt directedly prepared from a pyridine derivative and primary halogenated hydrocarbon is reacted with electron-poor alkene in the presence of alkali and 2,2,6,6-tetramethylpiperidinooxy (TEMPO), and the 3-alkyl indolizine derivative is prepared. The preparation method aims to develop the brand new preparation raw materials of the 3-alkyl indolizine derivative and the corresponding technology method so as to simply and easily synthesize the 3-alkyl indolizine derivative in an environmentally friendly mode at the low production cost under loose reaction conditions.
Owner:HUAIYIN TEACHERS COLLEGE
Bicyclic unsaturated tertiary amine compounds
Owner:SANKYO CO LTD
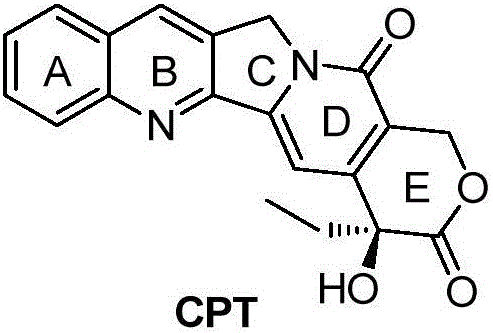
Camptothecin and method for preparing analogues thereof
InactiveCN101824038AMild reaction conditionsHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsAlkali freeOrganic base
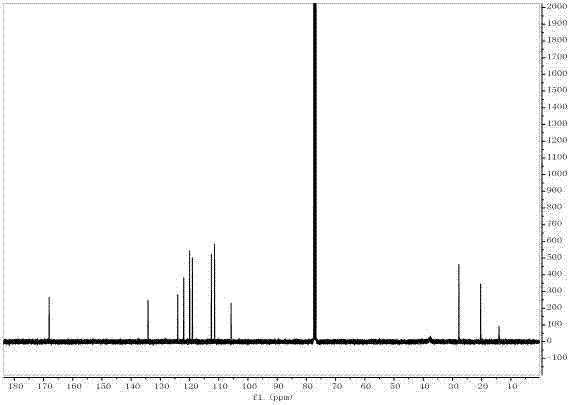
The invention relates to camptothecin and a method for preparing analogues of the camptothecin, and belongs to the field of organic chemistry. The method comprises the following steps of: carrying out palladium / carbon catalytic oxidation on 3,10-dioxo-4-ethyl-6,6-disubstituted alkoxy (alkylthio)-1,4,7,8-tetrahydropyrane (3,4-f) indolizine serving as a starting material in the presence of catalytic amount of organic base or under an alkali-free condition to generate 3,10-dioxo-4-ethyl-6,6-disubstituted alkoxy (alkylthio)-1,4,7,8-tetralin-hydroxy-pyran(3,4-f) indolizine; and carrying out molecular iodine-catalyzed ketal deprotection and carrying out Friedlander condensation of substituted o-aminobenzaldehyde (o-aminobenzophenone) and the 3,0-dioxo-4-ethyl-6,6-disubstituted alkoxy (alkylthio)-1,4,7,8-tetralin-hydroxy-pyran(3,4-f) indolizine so as to obtain the camptothecin and the analogues thereof. In the invention, the catalyst palladium / carbon can be recycled for three times without reducing catalytic effect, and the whole synthetic route has the advantages of mild reaction conditions, simple operation, environmental friendliness, high yield and quality of products, and suitability for industrial production. In the formula, R1 refers to H, OH and OCH3, and R2 refers to H, and C1 to C5 alkyl.
Owner:FUDAN UNIV
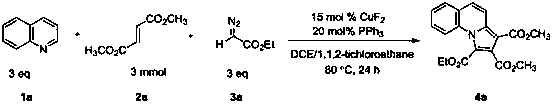
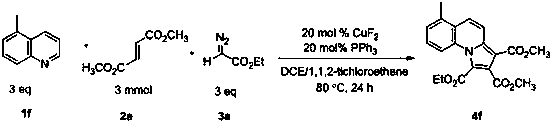
Indolizine derivative and preparation method thereof
The invention discloses an indolizine derivative and a preparation method thereof. A cheap copper compound is taken as a catalyst, a pyridine derivative, an olefinic derivative and a heavy nitrogen derivative are taken as reactants, and the catalyst and the reactants are subjected to cyclization reaction in a mixed solvent to obtain a product indolizine. The catalyst prepared with the method disclosed by the invention is cheap, can be easily obtained, is high in reaction activity, is moderate in reaction conditions, only needs to be carried out in the air, has a wide primer applicable range and is convenient in aftertreatment, and a final product can be obtained through simple column chromatography after reaction is finished. A target product has the advantages of high yield, simple preparation process, environment protection and wide raw material resource. The indolizine derivative conforms to the requirement and the direction of modern green chemistry development and has a potential industrial application value.
Owner:SUZHOU UNIV
Novel pH-responsive fluorescent molecular probe and preparation method thereof
InactiveCN103980885AOpen up new usesOrganic chemistryFluorescence/phosphorescenceFluorescenceNitrogen
The invention relates to a pH-responsive fluorescent molecular probe with a chemical name, namely, 5-(2-phenyl-pyrrolo[2,1,5-cd]indolizine-5-yl)-1,3,4-oxadiazole-2-thiophenol, as well as a preparation method and application thereof. According to the preparation method, a mercapto group is adopted as an identifying group and an indolizine group is adopted as a signal group for the first time to successfully prepare the compound. By virtue of the fluorescent probe molecules, a sensitive identification of H<+1> under different pH values is realized, the types of the pH-responsive fluorescent molecular probe are enriched, and a novel use of the mercapto functional group is opened up. The pH-responsive fluorescent molecular probe has very broad industrial application prospects and provides valuable reference to make a scientific research.
Owner:NANJING NORMAL UNIVERSITY
Synthetic process of O-3-chloro-2-propenyl hydroxylamine
ActiveCN105348139ARaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationCarboxylic compound preparationHydroxylamineOrganic synthesis
The invention belongs to the technical field of organic synthesis, in particular relates to a synthetic process of O-3-chloro-2-propenyl hydroxylamine. The synthetic process comprises the following steps of (1) successively adding phthalic acid and thionyl chloride into a reactor, performing a temperature rising reaction, distilling and removing excessive thionyl chloride to obtain o-phthaloyl chloride; (2) dissolving hydroxylamine hydrochloride into dichloromethane, dropwise adding the o-phthaloyl chloride, continually stirring and reacting after dropwise adding, distilling and removing the dichloromethane after reaction completion, so as to obtain N-hydroxyl phathalicimide; (3) dissolving the N-hydroxyl phathalicimide into a sodium hydroxide aqueous solution, dropwise adding trans-1,3-dichloropropene for reaction, after reaction completion, filtering to obtain 2-(3-chloroallyloxy)-isopropyl indolizine-1,3-dione; (4) adding the 2-(3-chloroallyloxy)-isopropyl indolizine-1,3-dione into a hydrochloric acid solution for hydrolysis, filtering, collecting organic phases, and the O-3-chloro-2-propenyl hydroxylamine is obtained. The synthetic process provided by the invention has the advantages that raw materials are low in price and easy to obtain, the reaction condition is mild and the yield is high.
Owner:SHANDONG KAISHENG NEW MATERIALS
Method for preparing 3-aryl purrocoline derivative
ActiveCN103087064ASimple processReduce manufacturing costOrganic chemistryPtru catalystPalladium catalyst
Owner:常熟紫金知识产权服务有限公司
Synthesizing method of indole and indolizine ketone compounds
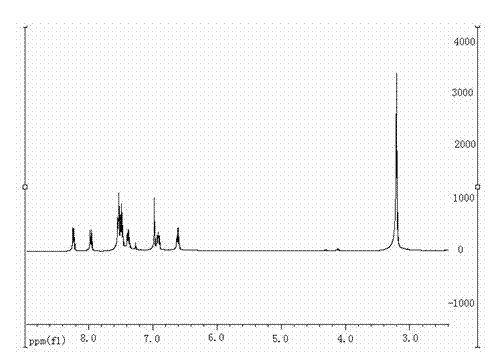
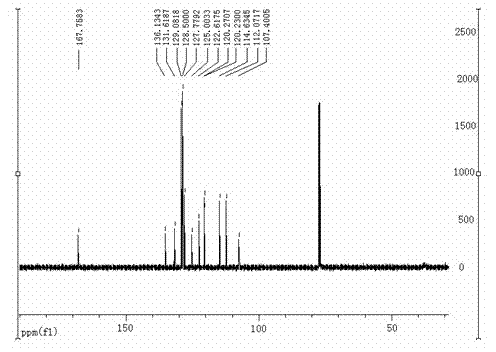
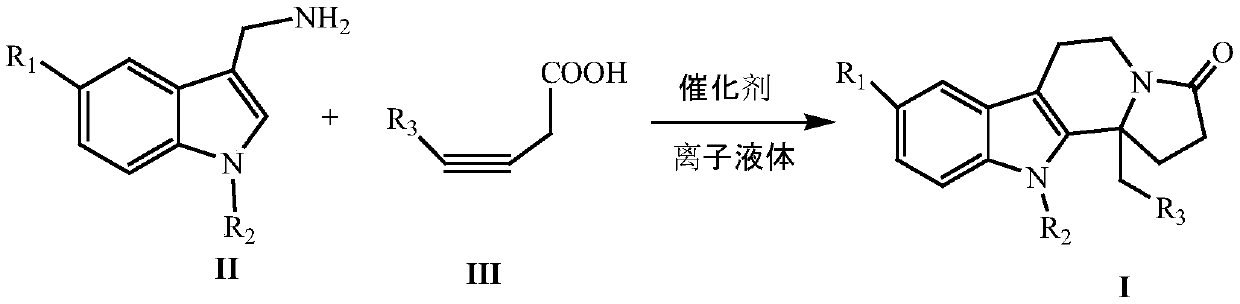
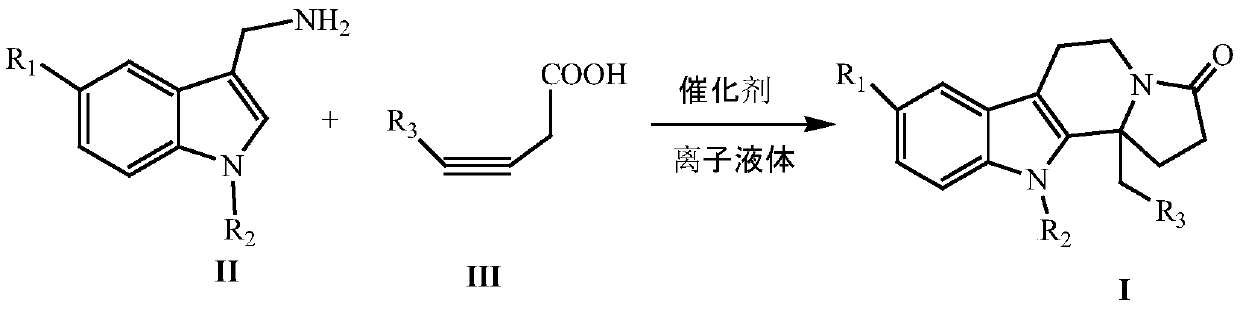
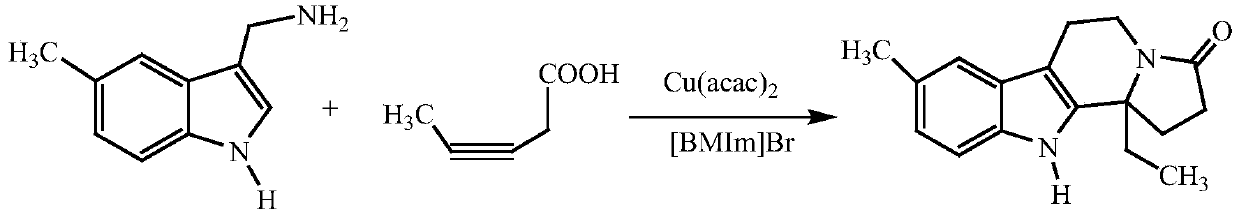
InactiveCN109824667AWide substrate adaptabilityHigh synthesis efficiencyOrganic chemistryKetoneIonic liquid
The invention discloses a synthesizing method of indole and indolizine ketone compounds. Chemical equation of the synthesizing method is shown in the description, wherein R1 and R2 represent groups onaromatic rings and nitrogen atoms of 3-indole methylamine compounds respectively, R3 represents groups on triple bonds of 3-tetrolic acid compounds, a catalyst is acetylacetone metal complex, and thegeneral formula of the catalyst is M(acac)2; ionic liquid is [BMIm]X, [PMIm]X, [HMIm]X, [BMMIm]X, [BMMIm]BF4 and [EMIm]BF4. The synthesizing method of the indole and indolizine ketone compounds is novel, easy and efficient.
Owner:HUAIHAI INST OF TECH
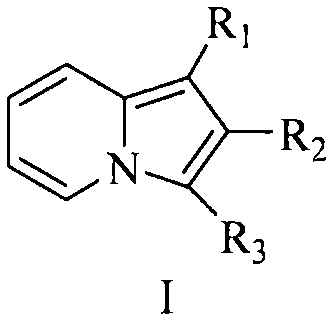
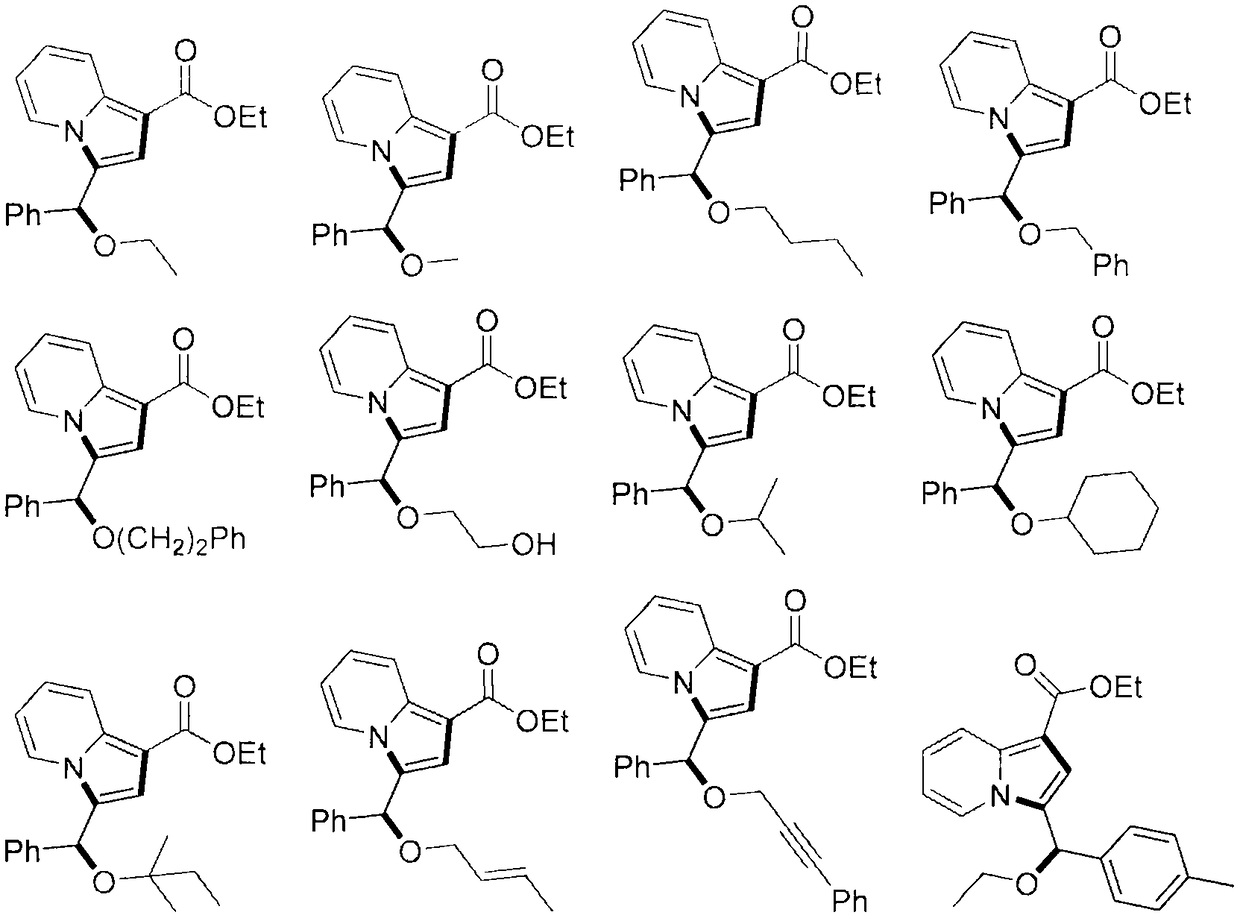
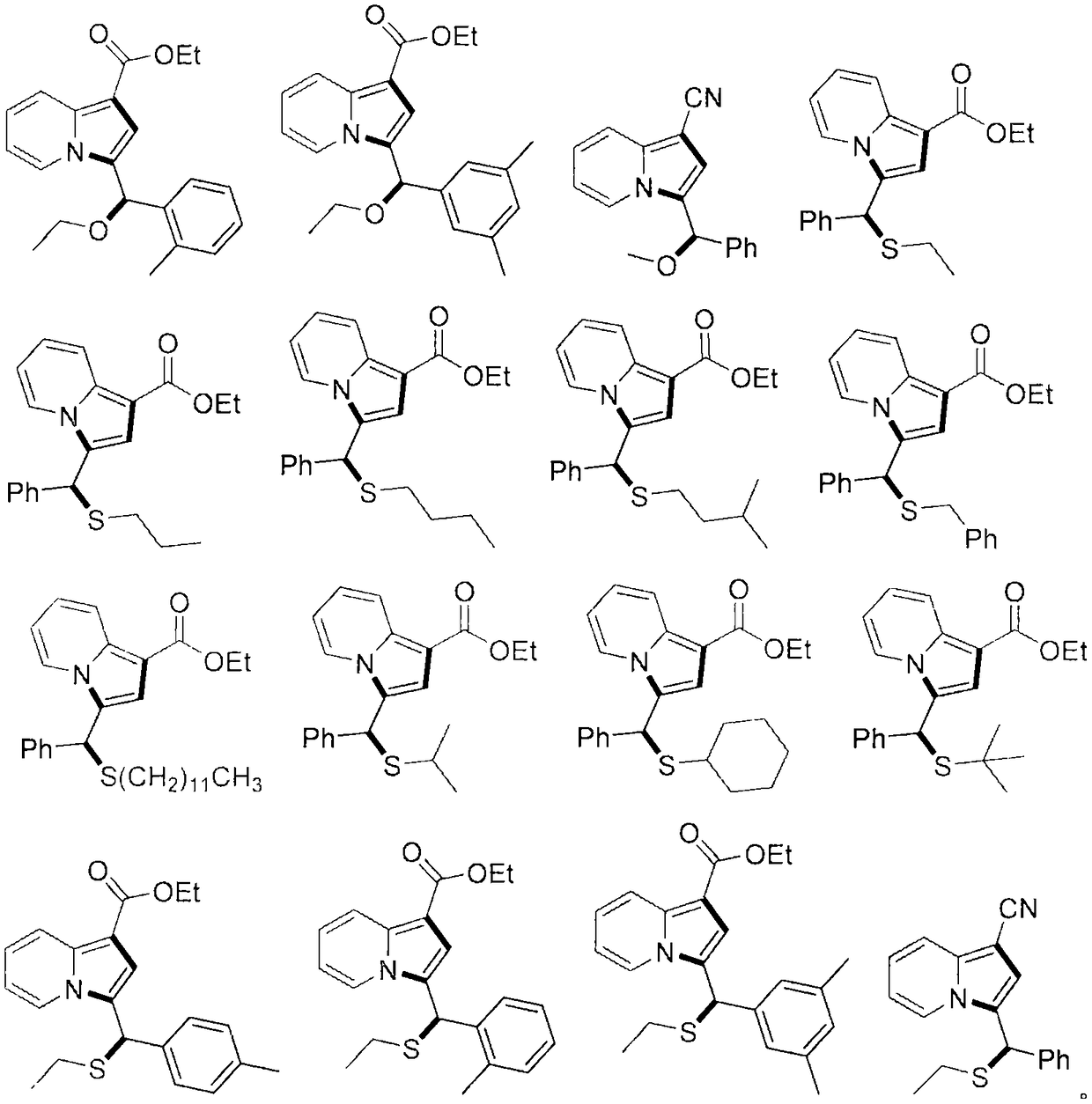
Indolizine derivatives, method for preparing same, and therapeutic compositions comprising same
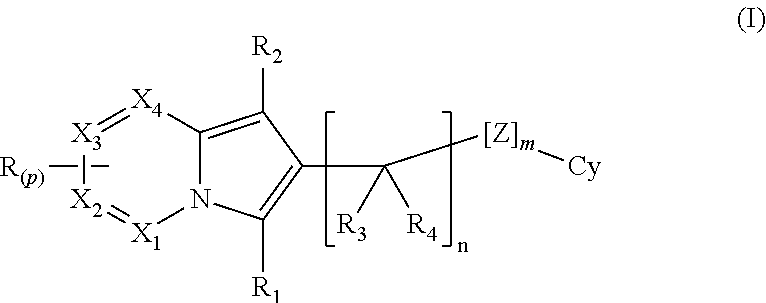
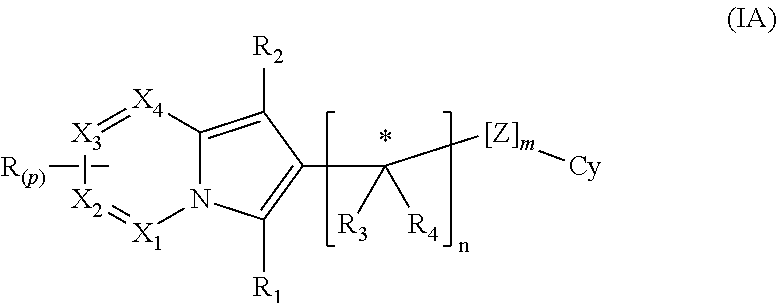
The present invention is directed to compounds of formula (I):described as novel indolizine derivatives and to a method for their preparation along with pharmaceutical compositions thereof whose substituents are as described in the specification.
Owner:SANOFI SA
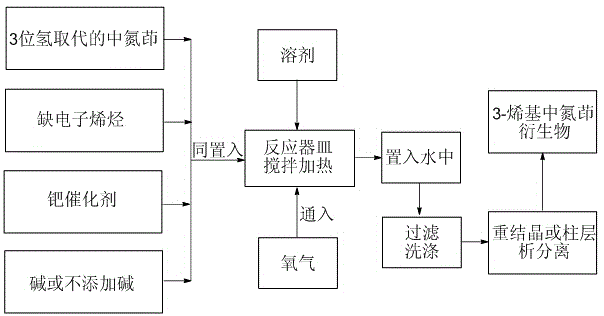
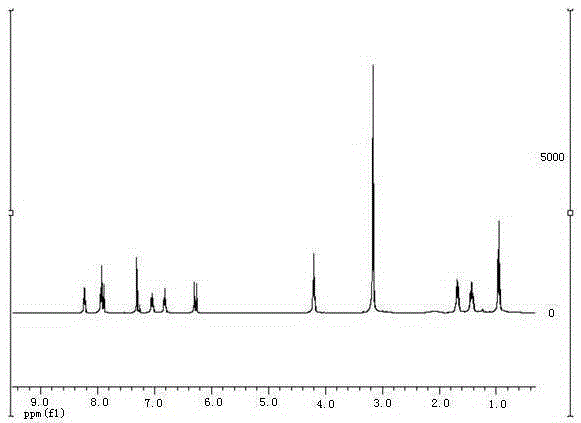
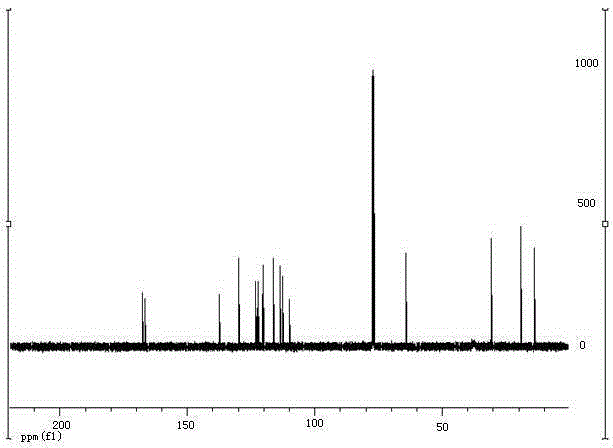
Green preparation method for 3-alkenyl indolizine derivative
InactiveCN105348280ALower synthesis costNo pollution in the processOrganic chemistryPtru catalystPalladium catalyst
The present invention discloses a green preparation method for a 3-alkenyl indolizine derivative. The method comprises the following steps: starting from indolizine substituted by 3-bit hydrogen; under the condition that a catalytic amount of a palladium catalyst and alkali exists or the alkali is not added, using oxygen from the air as a unique oxidant to react with electron deficient olefin; and preparing to obtain the 3-alkenyl indolizine derivative. The present invention aims to synthesize the 3-alkenyl indolizine derivative in a simple and environmental friendly way by a low production cost under a loose reaction condition by developing the oxidant that can be used in preparation of the 3-alkenyl indolizine derivative and a corresponding process.
Owner:SOUTHEAST UNIV
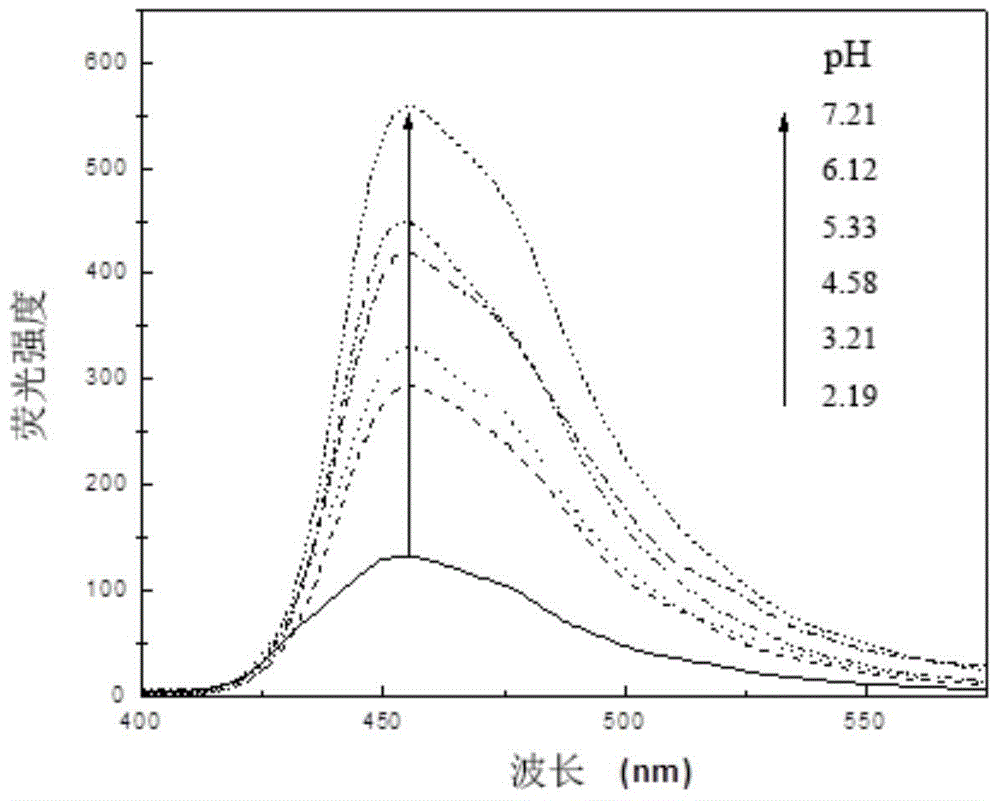
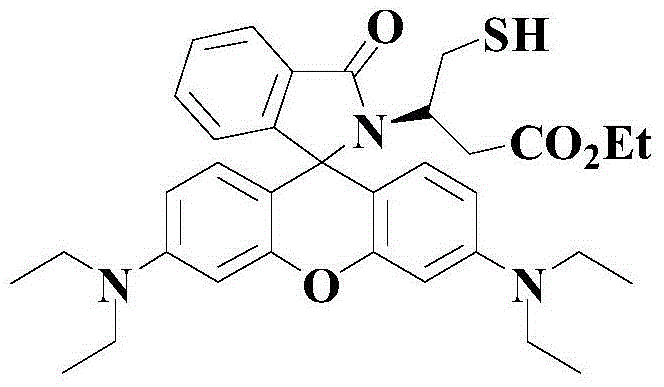
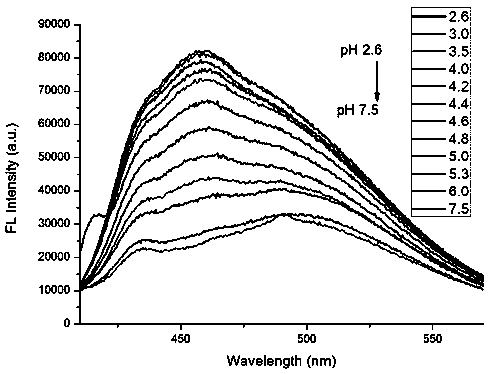
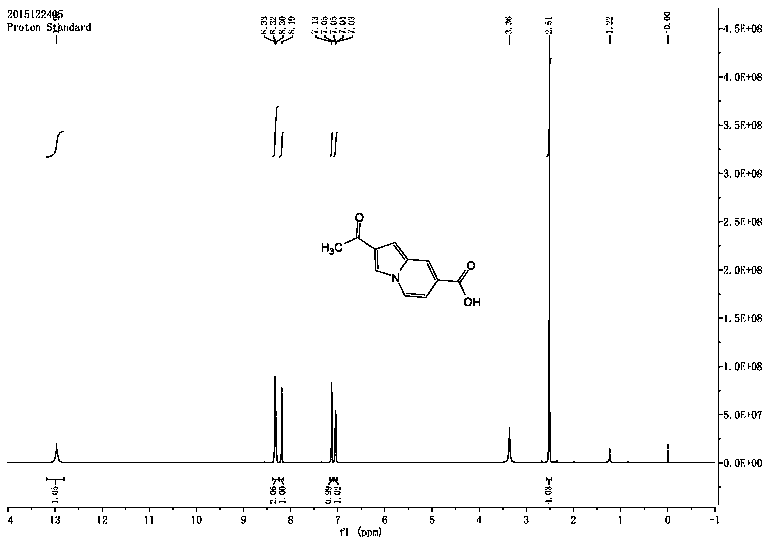
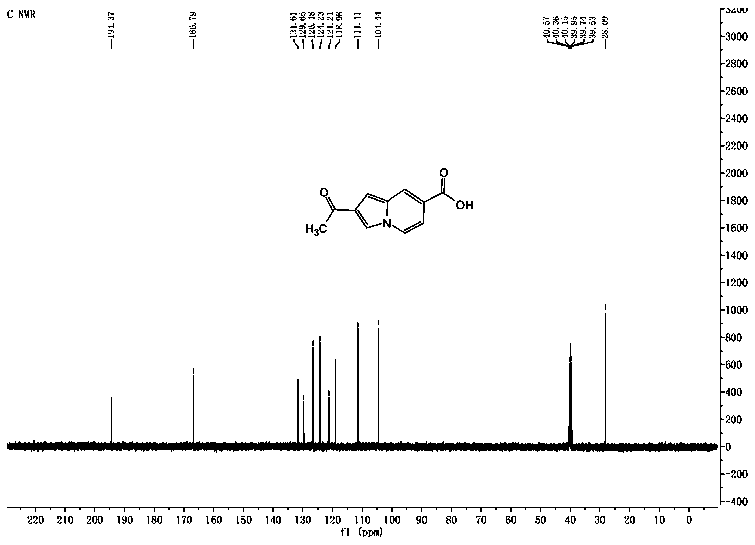
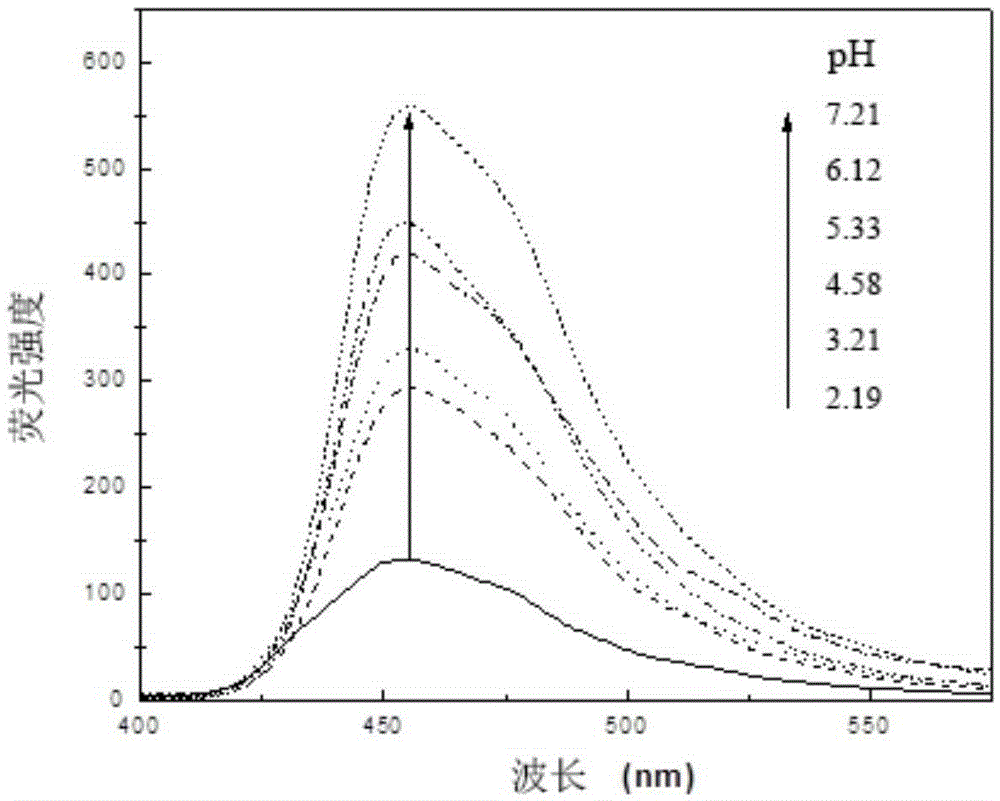
A kind of indolizine carboxylic acid pH fluorescent probe and its application
The invention discloses an indolizine carboxylic acid pH fluorescent probe, the probe is 2-acetyl indolizine-7-carboxylic acid, and its chemical structural formula is shown in formula (1). The fluorescent probe of the present invention has unique fluorescence selectivity and higher sensitivity to pH in an aqueous solution of dimethyl sulfoxide. Compared with existing probes, it has a narrow pH corresponding range and is not subject to background interference. It is suitable for It has significant advantages such as pH detection in strongly acidic environments, and has great application prospects.
Owner:TAISHAN MEDICAL UNIV
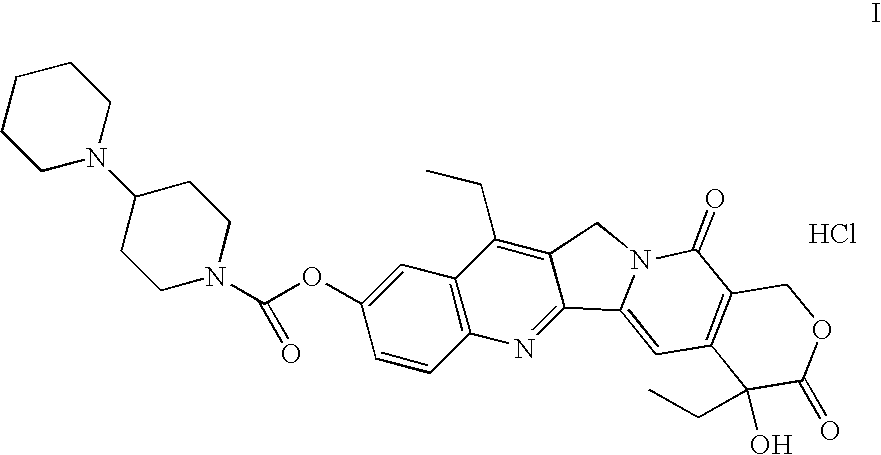
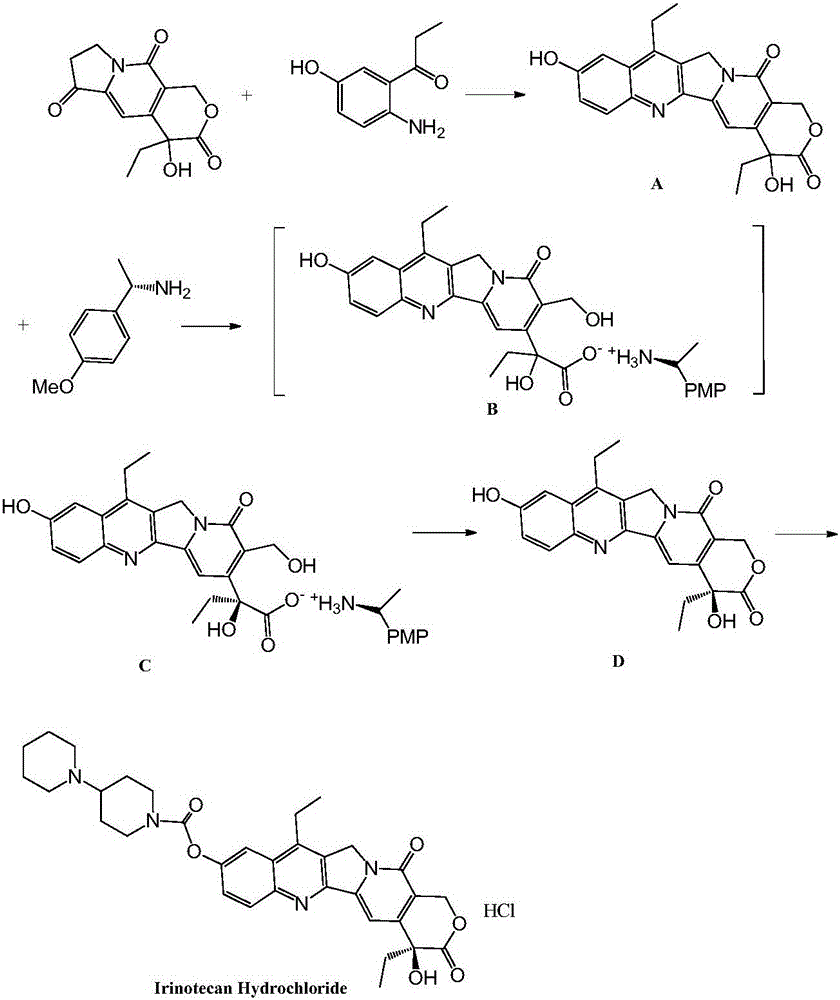
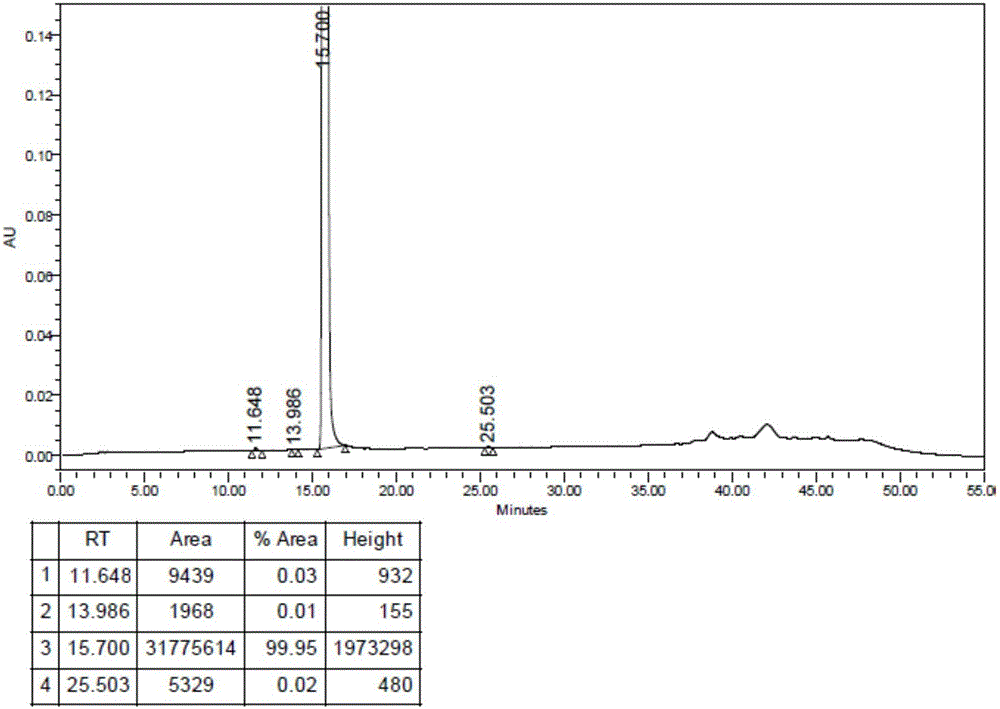
Preparation method of irinotecan hydrochloride
ActiveCN106632368AGood split effectLow isomer contentOptically-active compound separationOrganic racemisationOrganic acidChiral amine
The invention discloses a preparation method of irinotecan hydrochloride. The preparation method comprises following steps: 1, chemical raw materials of racemic indolizine derivatives are taken as initial raw materials, ring closing reaction and alkali ring-opening reaction are carried out so as to obtain an organic acid intermediate; 2, a chiral amine and the organic acid intermediate are subjected to salt forming resolution so as to obtain a chiral intermediate; 3, the chiral intermediate is subjected to acid ring-opening reaction so as to remove the chiral amine; 4, esterification with a piperidine derivative, and salt forming are carried out so as to obtain the target product irinotecan hydrochloride. The preparation method is used for total synthesis of irinotecan hydrochloride, large scale production is realized, reaction conditions are mild, the preparation method is easy to control, product purity is 98% or higher, and the largest individual impurity content is lower than 0.1%.
Owner:SHANGHAI JINHE BIO TECH
A novel pH-responsive fluorescent molecular probe and its preparation method
InactiveCN103980885BOpen up new usesOrganic chemistryFluorescence/phosphorescenceFluorescenceNitrogen
The invention relates to a pH-responsive fluorescent molecular probe with a chemical name, namely, 5-(2-phenyl-pyrrolo[2,1,5-cd]indolizine-5-yl)-1,3,4-oxadiazole-2-thiophenol, as well as a preparation method and application thereof. According to the preparation method, a mercapto group is adopted as an identifying group and an indolizine group is adopted as a signal group for the first time to successfully prepare the compound. By virtue of the fluorescent probe molecules, a sensitive identification of H<+1> under different pH values is realized, the types of the pH-responsive fluorescent molecular probe are enriched, and a novel use of the mercapto functional group is opened up. The pH-responsive fluorescent molecular probe has very broad industrial application prospects and provides valuable reference to make a scientific research.
Owner:NANJING NORMAL UNIVERSITY
A method for synthesizing 1-halogen-2-aryl indolizine compounds
InactiveCN102295644AConvenient and effective synthesisMild reaction conditionsOrganic chemistryPyridiniumPtru catalyst
The invention relates to a method for synthesizing a 1-halo-2-aryl indolizine compound. According to the method, the 1-halo-2-aryl indolizine compound can be conveniently and effectively synthesized by using brominated N-ethyoxyl carbonyl methyl pyridinium salt (or chlorinated N-cyan methyl pyridinium salt) and 1,1-dibromo-2-arylethylene (or 1-halo-2-arylacetylene) as substrates under the action of alkali. Compared with the existing methods, the method provided by the invention has the advantages that the range of adaptable substrates is wide, reaction conditions are mild, no catalyst is needed, and reaction efficiency is high and is simple to operate, thus the method has an important application value.
Owner:TONGJI UNIV
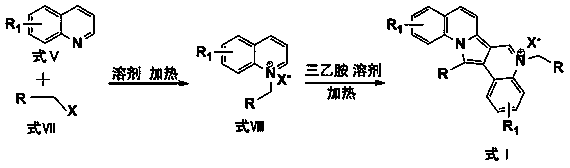
6-cyano-1,1(1,3-subpropyldioxy)-7-[1'-(carbalkoxy)-propyl]-5-oxo-delta6(8)-tetrahydro indolizine compound preparation method
InactiveCN101007807AMild reaction conditionsSimple and fast operationOrganic chemistryOrganic solventMethyl group
The invention belongs to organic chemistry field, which in detail relates to a process of preparing 6- cyano-1, 1 (1, 3- dioxypropylidene)-7- [1' (alkoxycarbonyl)- propyl ]- 5- oxo-Delt6 (8)- tetrahydrochysene indolizine (I). The 6- cyano-1, 1 (1, 3- dioxypropylidene)-7- [ (carbomethoxyl group)- methyl ]- 5- oxo-Delt6 (8)- tetrahydrochysene indolizine (II) and haloethane reacts with each other for ethylization with existence of alkali and organic solution with or without phase transversion catalyst, and produces mentioned product. The invention is characterized by temperatate reaction condition, easy operation, low cost, high-purity product and suitability for industrial production.
Owner:FUDAN UNIV
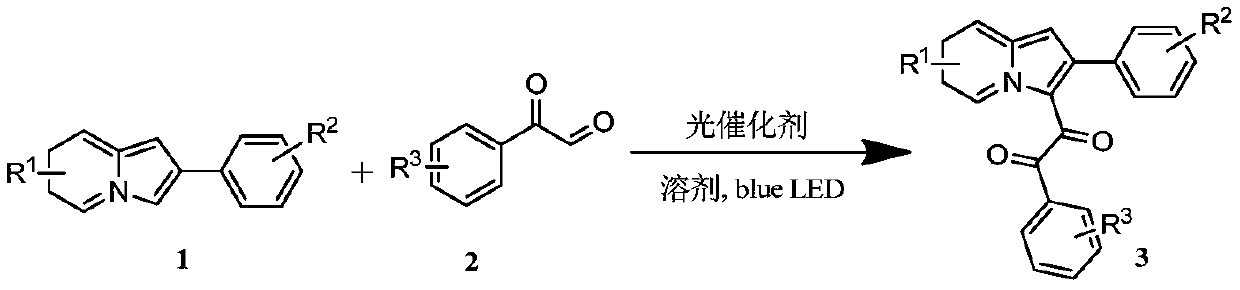
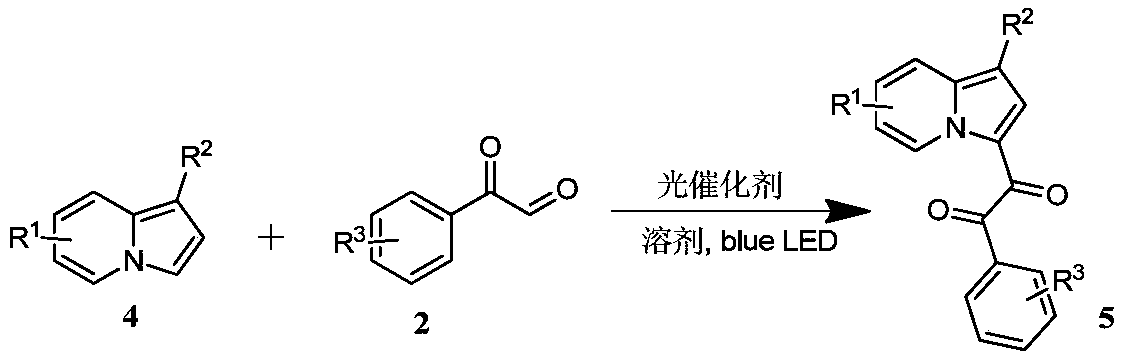
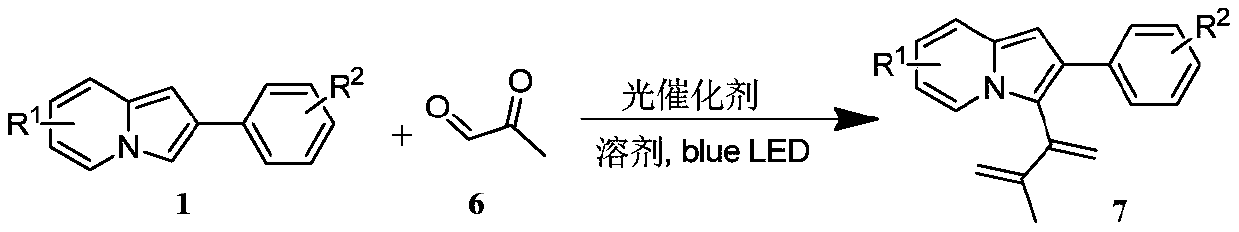
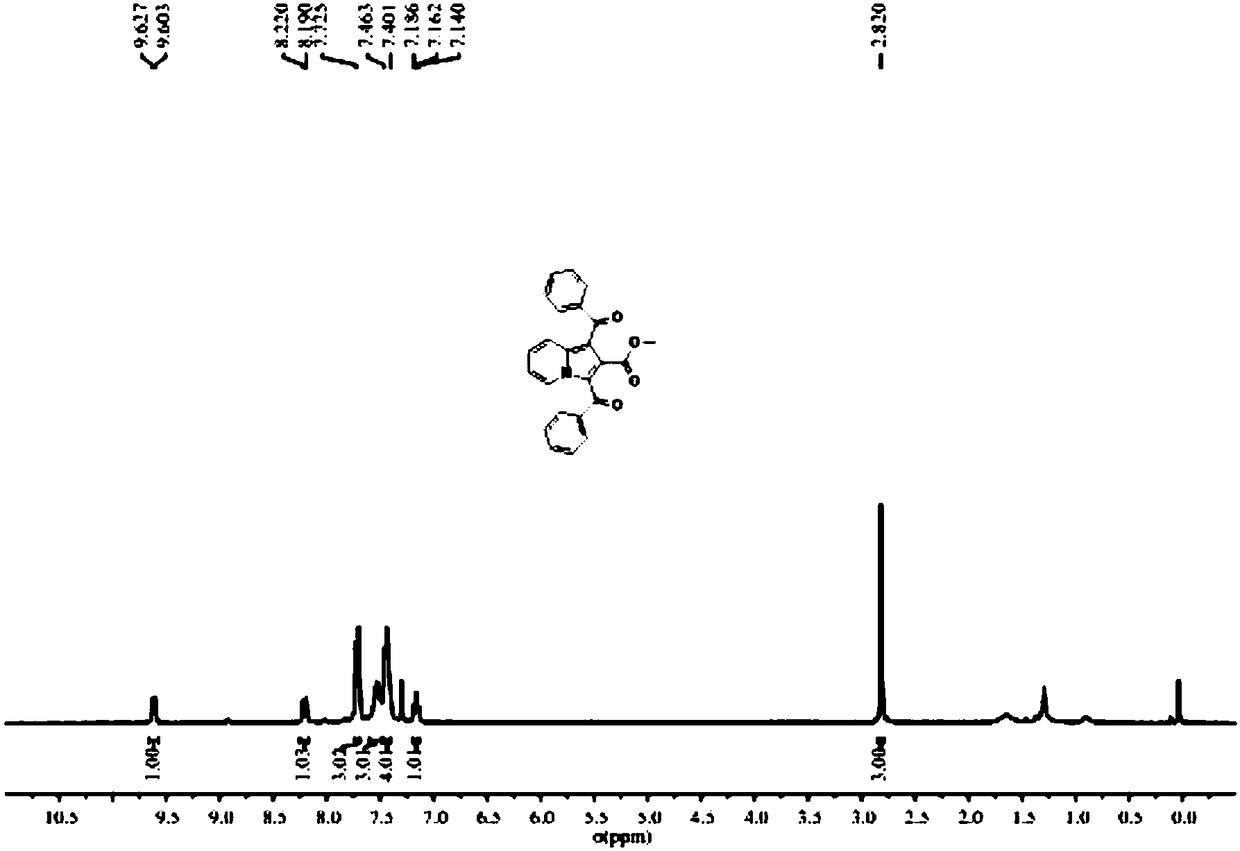
Preparation method of indolizine cyclo-1, 2-diketone with fluorescence activity and derivative thereof
ActiveCN111269228AHigh regional selectivityEasy to operateOrganic chemistryLuminescent compositionsOrganic synthesisCatalytic oxidation
The invention belongs to the field of organic synthesis, and particularly relates to a preparation method of indolizine cyclo-1, 2-diketone with fluorescence activity and a derivative thereof. The indolizine cyclo-1, 2-diketone is prepared by adopting a new metal-free photocatalytic oxidation method, and the indolizine cyclo-1, 2-diketone is synthesized from commercially available indolizine, 2-oxo-2-phenylacetaldehyde, rose bengal and other basic raw materials, wherein the groups R1, R2 and R3 are alkyl, an electron withdrawing group or an electron donating group; rose bengal can also be replaced with a photocatalyst eosin Y, eosin B, rhodamine 6G or fluorescein; the solvent dimethyl sulfoxide can also be replaced with an organic solvent N, N-dimethylformamide, acetonitrile or dichloromethane. The method has the characteristics of easily available starting materials, wide substrate application range, economy, high efficiency, mild conditions, environmental friendliness and high yield.
Owner:山东中新科农生物科技有限公司
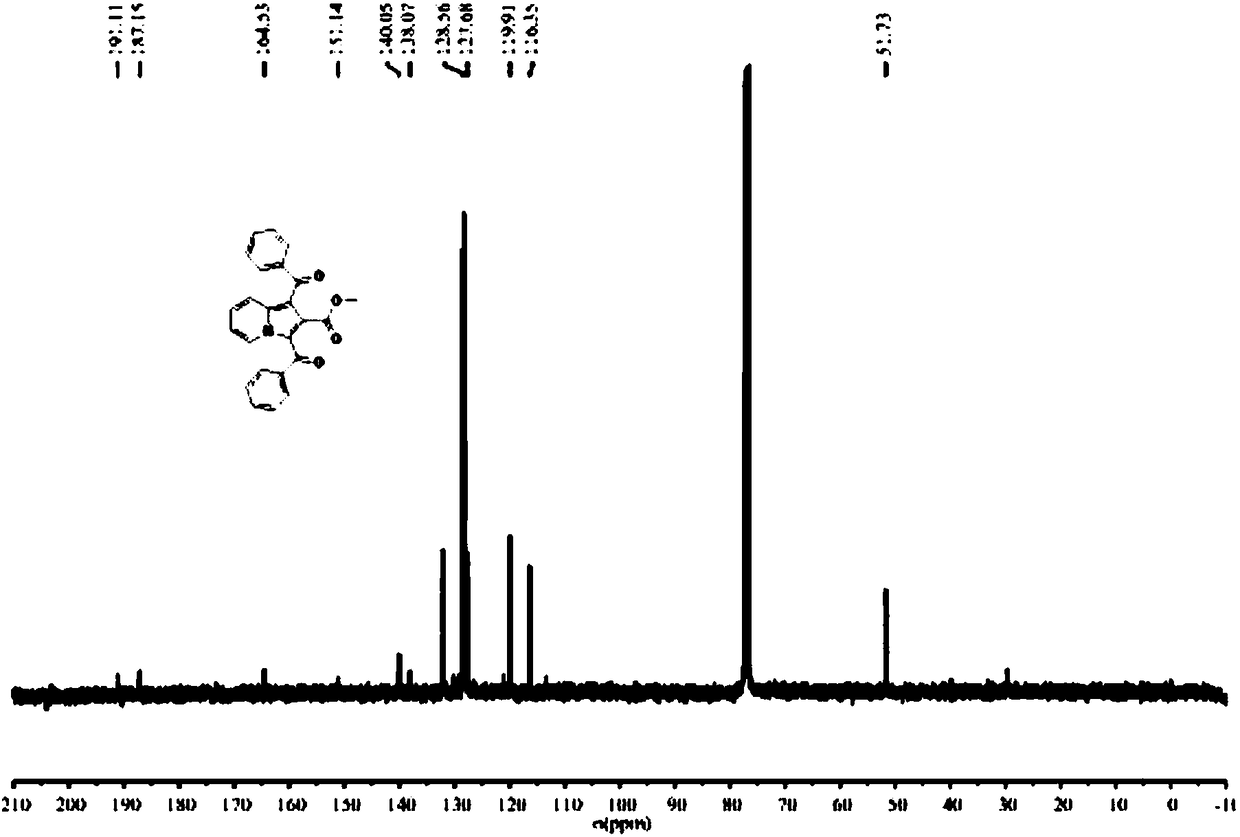
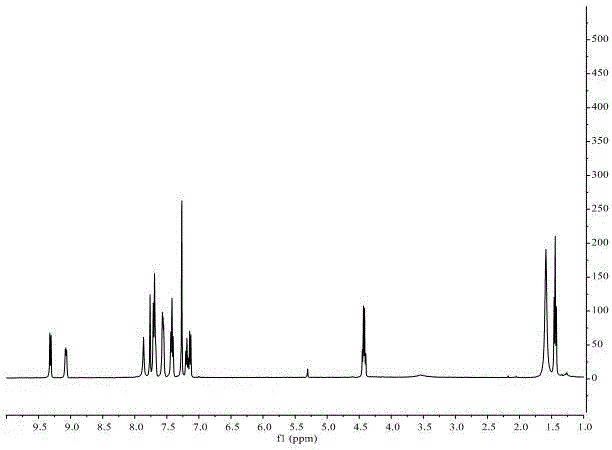
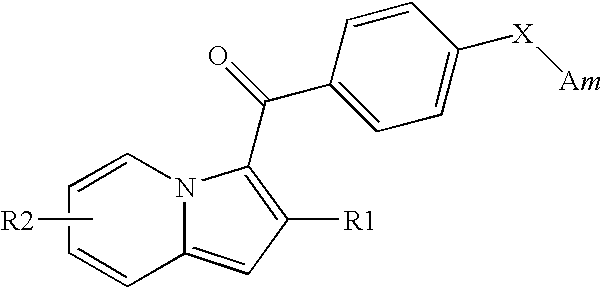
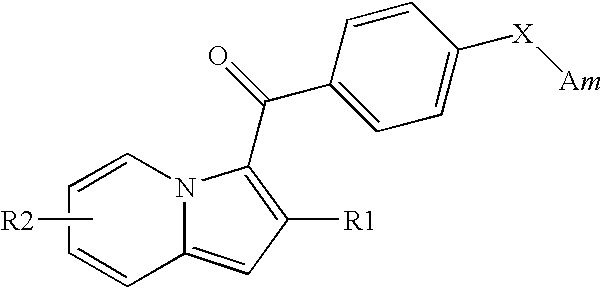
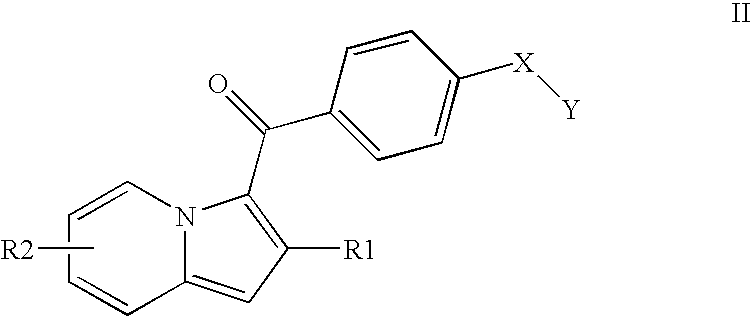
1,2,3-trisubstituted indolizine derivative and preparation method thereof
The present invention relates to an indolizine derivative, and specifically discloses a 1,2,3-trisubstituted indolizine derivative and a preparation method thereof, wherein the chemical structure formula of the indolizine derivative is I as shown in the specification. The 1,2,3-trisubstituted indolizine derivative has benzoyl groups at both 1 and 3 positions, and an ester group at 2-position, namely, the 1,2,3-trisubstituted indolizine derivative has substituents containing carbonyl groups, benzene rings and the ester group, and further modification and processing of the product structure arefacilitated by the chemical nature of the benzoyl groups and the ester group, and more possibilities are provided for the construction of more complex indolizine derivatives. Moreover, the preparationmethod of the 1,2,3-trisubstituted indolizine derivative does not require addition of a metal catalyst, does not require inert gas protection, and has the advantages of easy-to-obtain reaction raw materials, high reaction yield, and mild reaction conditions.
Owner:ANHUI NORMAL UNIV
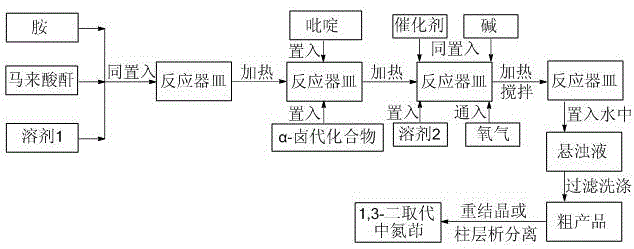
Method for preparing 1,3-disubstituted indolizine derivative
The invention discloses a method for preparing a 1,3-disubstituted indolizine derivative. The method comprises the following steps: by taking amine, maleic anhydride, pyridine and an alpha-halogenated compound as raw materials, copper salt as a catalyst and oxygen as an oxidant, preparing the 1,3-disubstituted indolizine derivative in the presence of alkali. According to the method, a process of a one-pot method is adopted, reaction is implemented in one same reactor, no any intermediate needs to be purified, and the synthesis efficiency is greatly improved; only a catalytic amount of a copper catalyst and the environment-friendly oxidant oxygen are used in the process, so that a post treatment process is relatively simple, and the environment pollution risk can be avoided; the whole process is not sensitive to air and humidity, and normal operation can be implanted under a loose reaction condition; and related products can be prepared for fields such as biologics, pesticides and medicines, and the 1,3-disubstituted indolizine derivative which is rich in resource and relatively low in price can be provided.
Owner:HUAIYIN TEACHERS COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
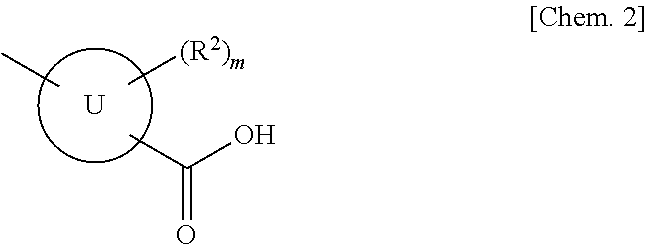
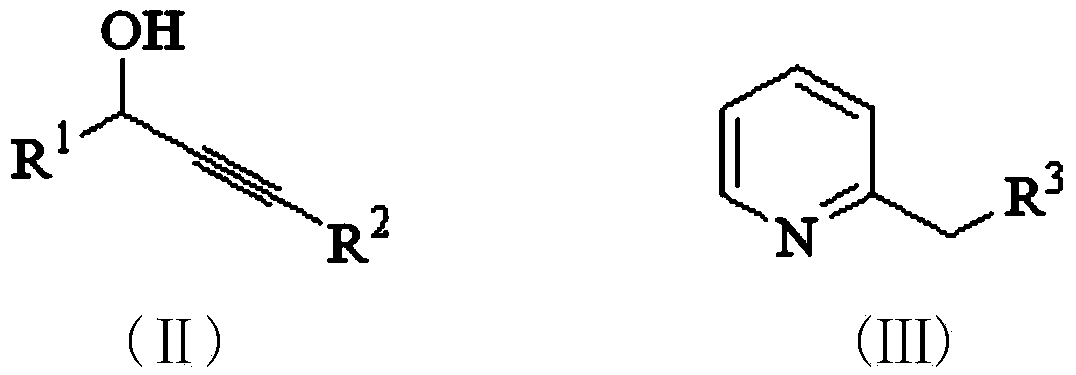



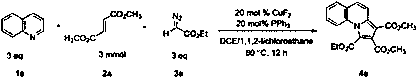
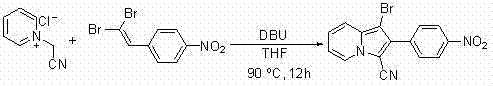

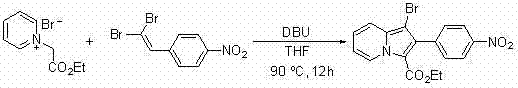
![6-cyano-1,1(1,3-subpropyldioxy)-7-[1'-(carbalkoxy)-propyl]-5-oxo-delta6(8)-tetrahydro indolizine compound preparation method 6-cyano-1,1(1,3-subpropyldioxy)-7-[1'-(carbalkoxy)-propyl]-5-oxo-delta6(8)-tetrahydro indolizine compound preparation method](https://images-eureka.patsnap.com/patent_img/32748908-971f-4684-9d63-05fd0bab7b36/A2007100363110002C1.PNG)
![6-cyano-1,1(1,3-subpropyldioxy)-7-[1'-(carbalkoxy)-propyl]-5-oxo-delta6(8)-tetrahydro indolizine compound preparation method 6-cyano-1,1(1,3-subpropyldioxy)-7-[1'-(carbalkoxy)-propyl]-5-oxo-delta6(8)-tetrahydro indolizine compound preparation method](https://images-eureka.patsnap.com/patent_img/32748908-971f-4684-9d63-05fd0bab7b36/A2007100363110002C2.PNG)
![6-cyano-1,1(1,3-subpropyldioxy)-7-[1'-(carbalkoxy)-propyl]-5-oxo-delta6(8)-tetrahydro indolizine compound preparation method 6-cyano-1,1(1,3-subpropyldioxy)-7-[1'-(carbalkoxy)-propyl]-5-oxo-delta6(8)-tetrahydro indolizine compound preparation method](https://images-eureka.patsnap.com/patent_img/32748908-971f-4684-9d63-05fd0bab7b36/A20071003631100041.PNG)