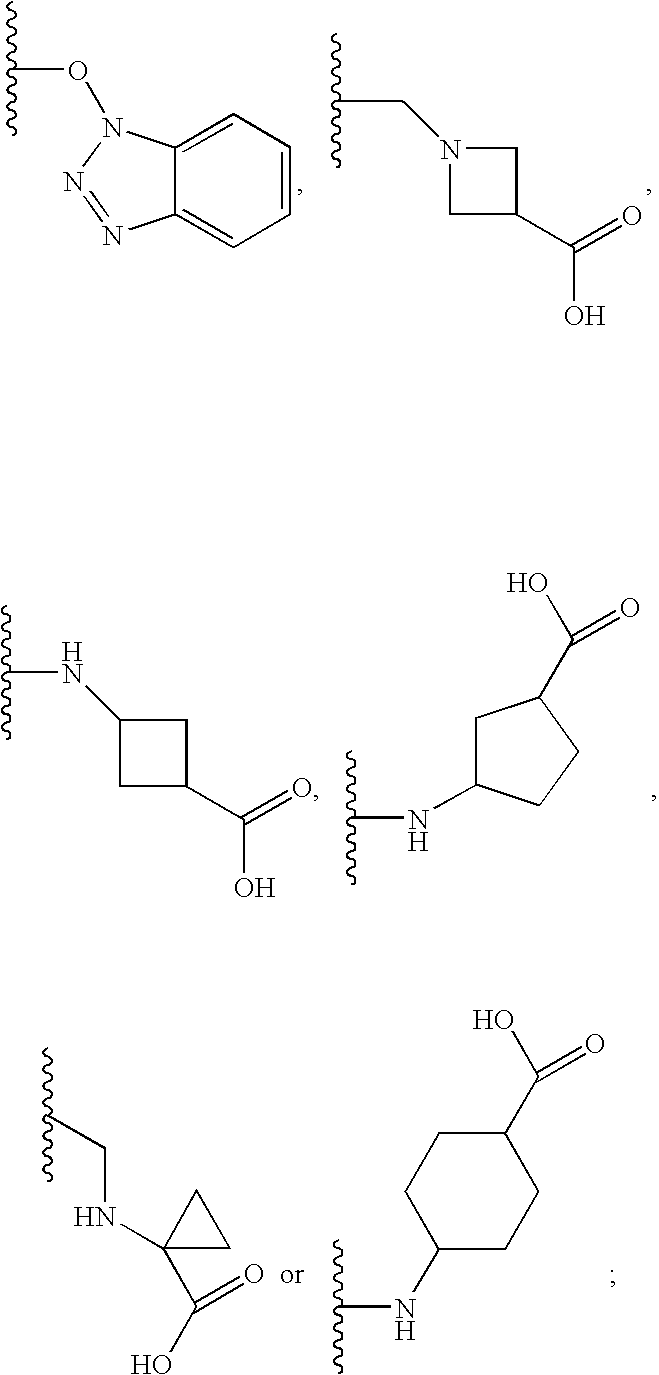
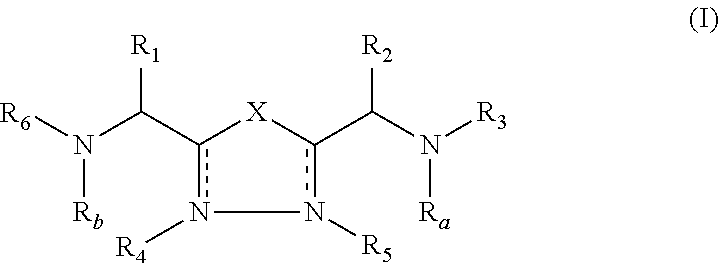
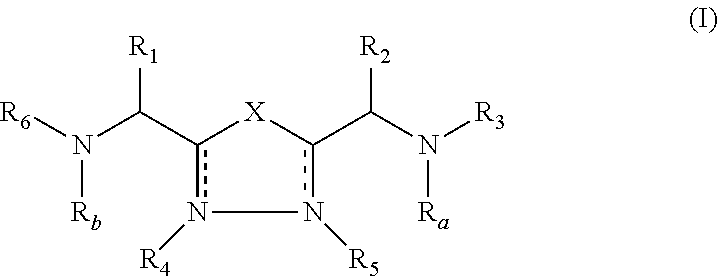
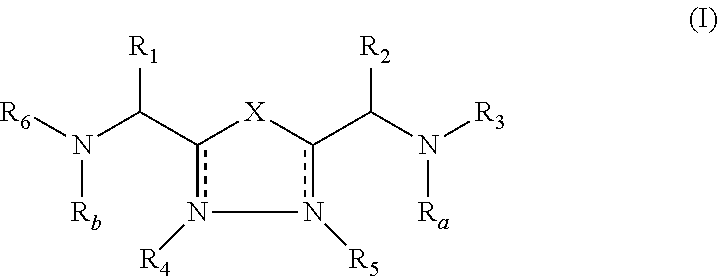
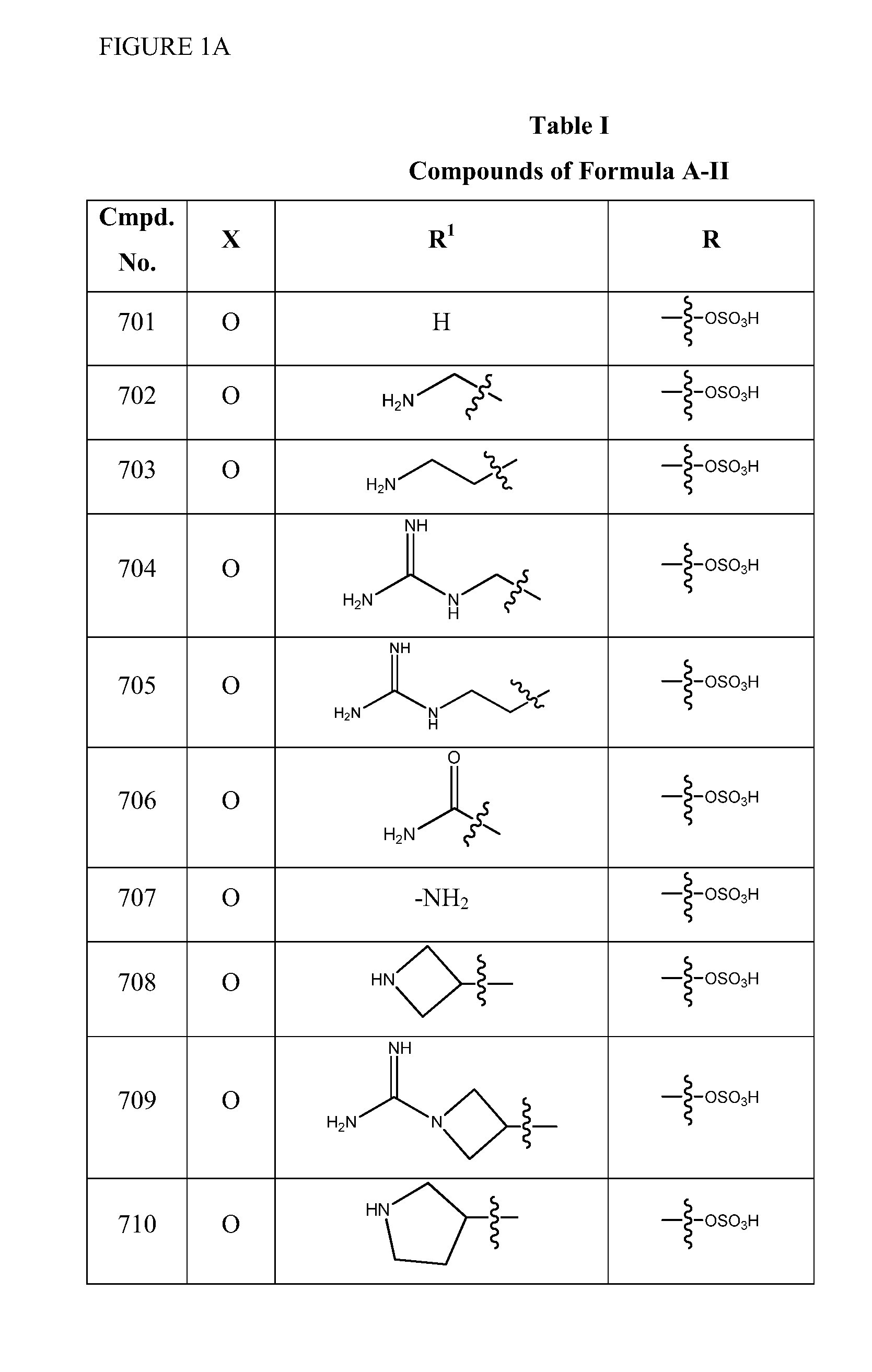
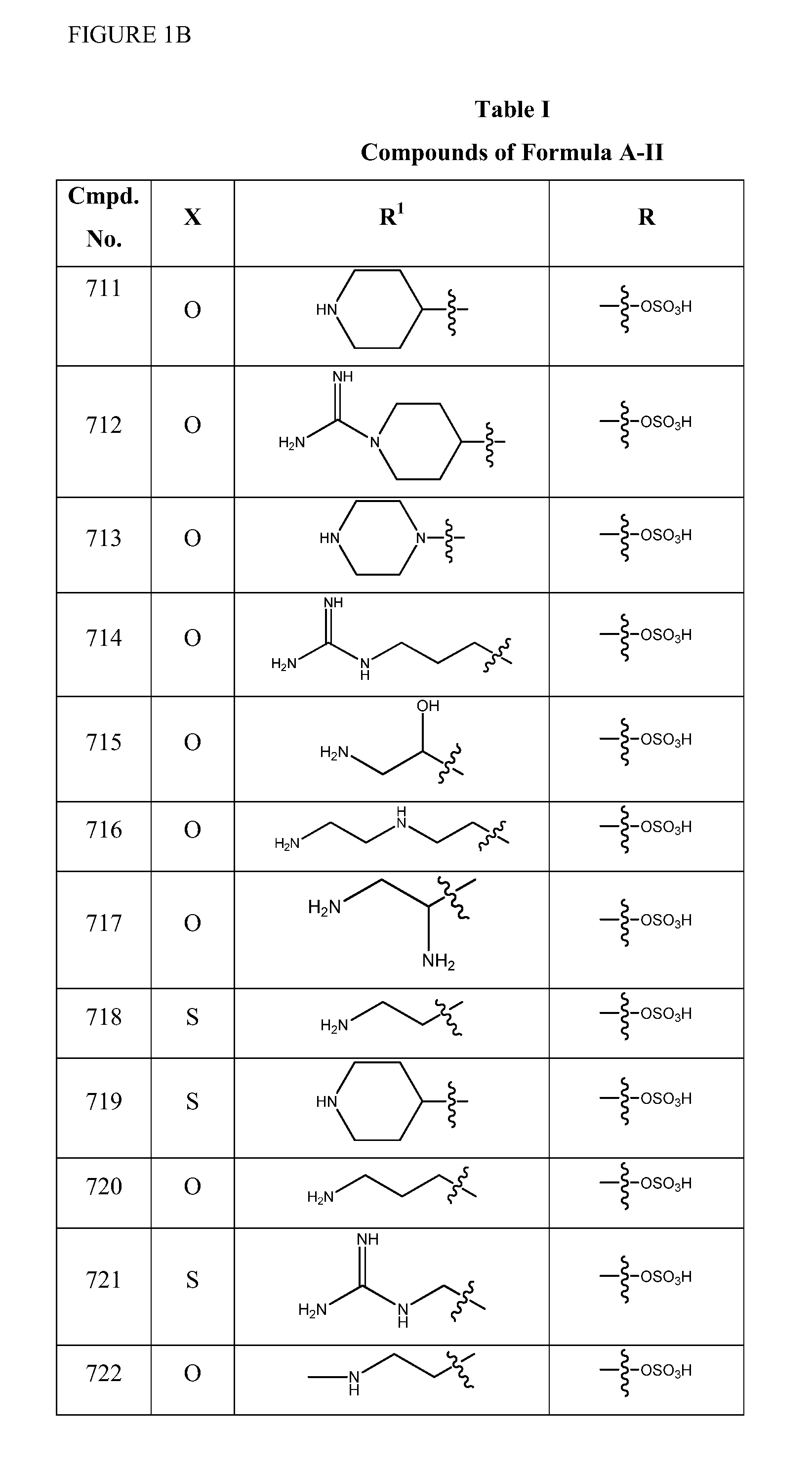
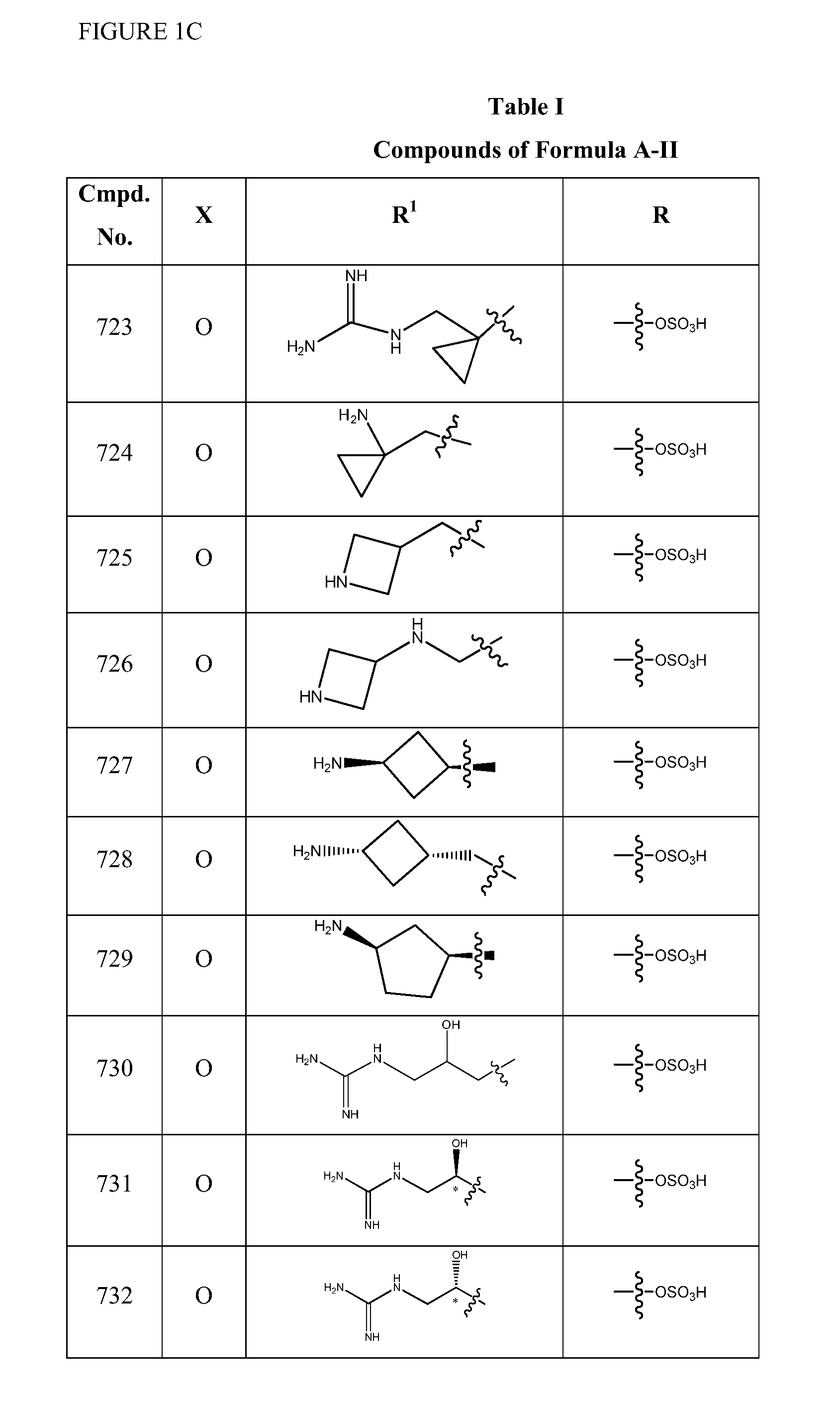
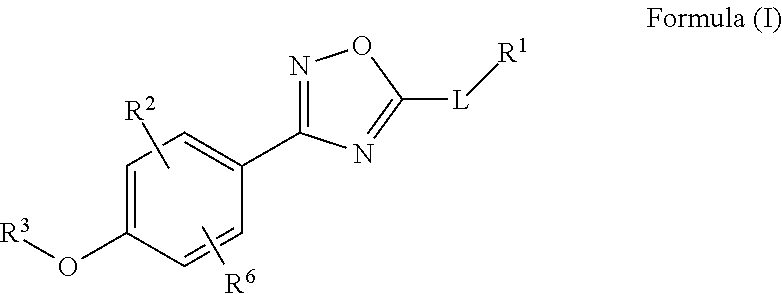
Patents
Literature
1020 results about "Oxadiazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxadiazoles are a class of heterocyclic aromatic chemical compound of the azole family; with the molecular formula C₂H₂N₂O. 1,2,4-Oxadiazole, 1,2,5-oxadiazole, and 1,3,4-oxadiazole are all known and appear in a variety of pharmaceutical drugs including raltegravir, butalamine, fasiplon, oxolamine, and pleconaril. The 1,2,3-isomer is unstable and ring-opens to form the diazoketone tautomer; however, it does exist within the unusual sydnone motif.
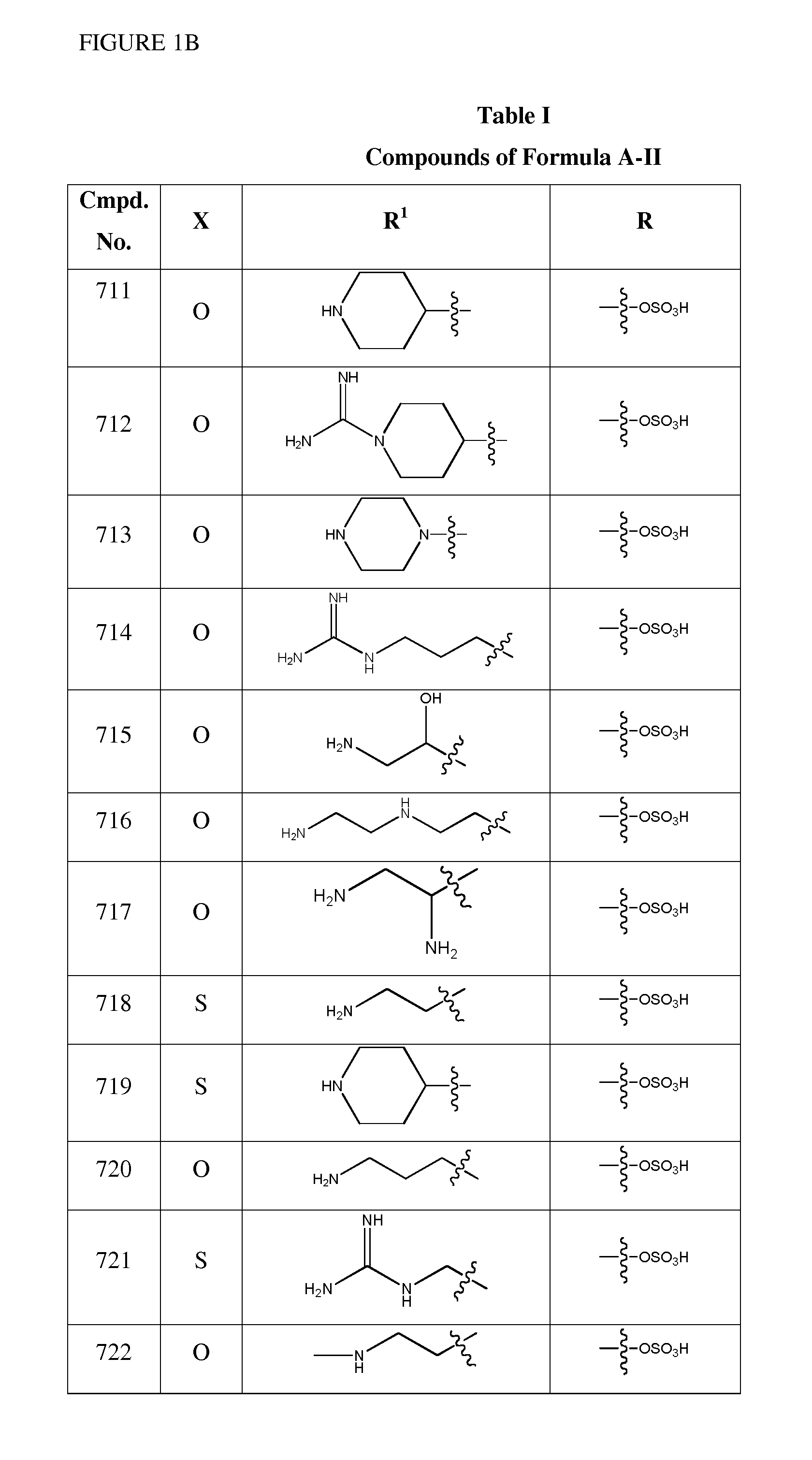
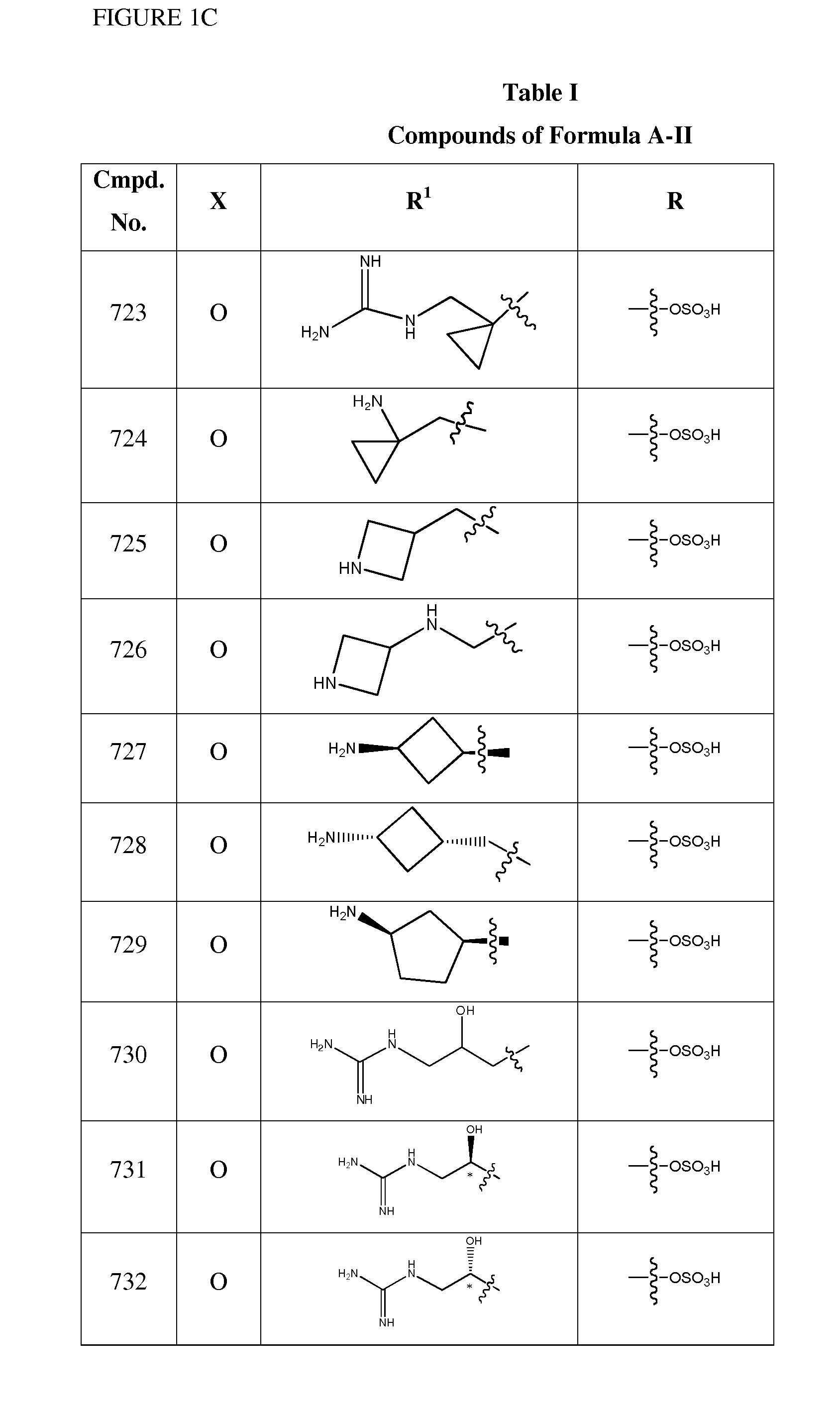
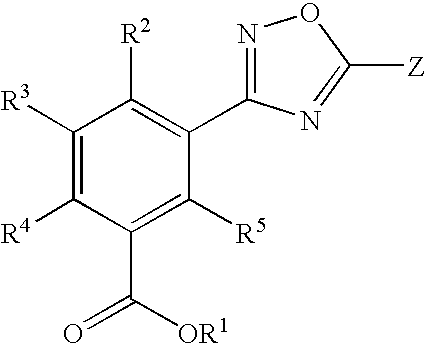
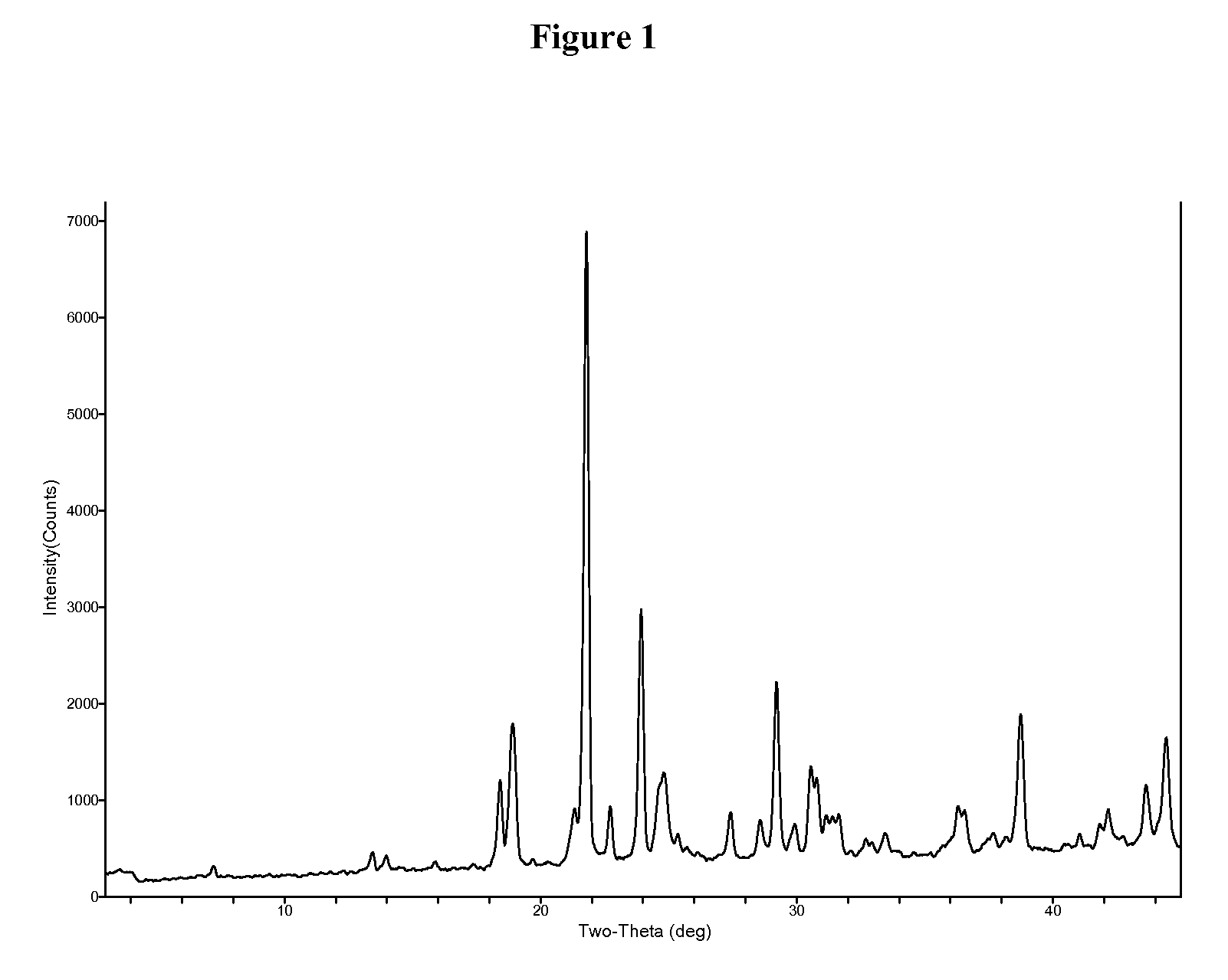
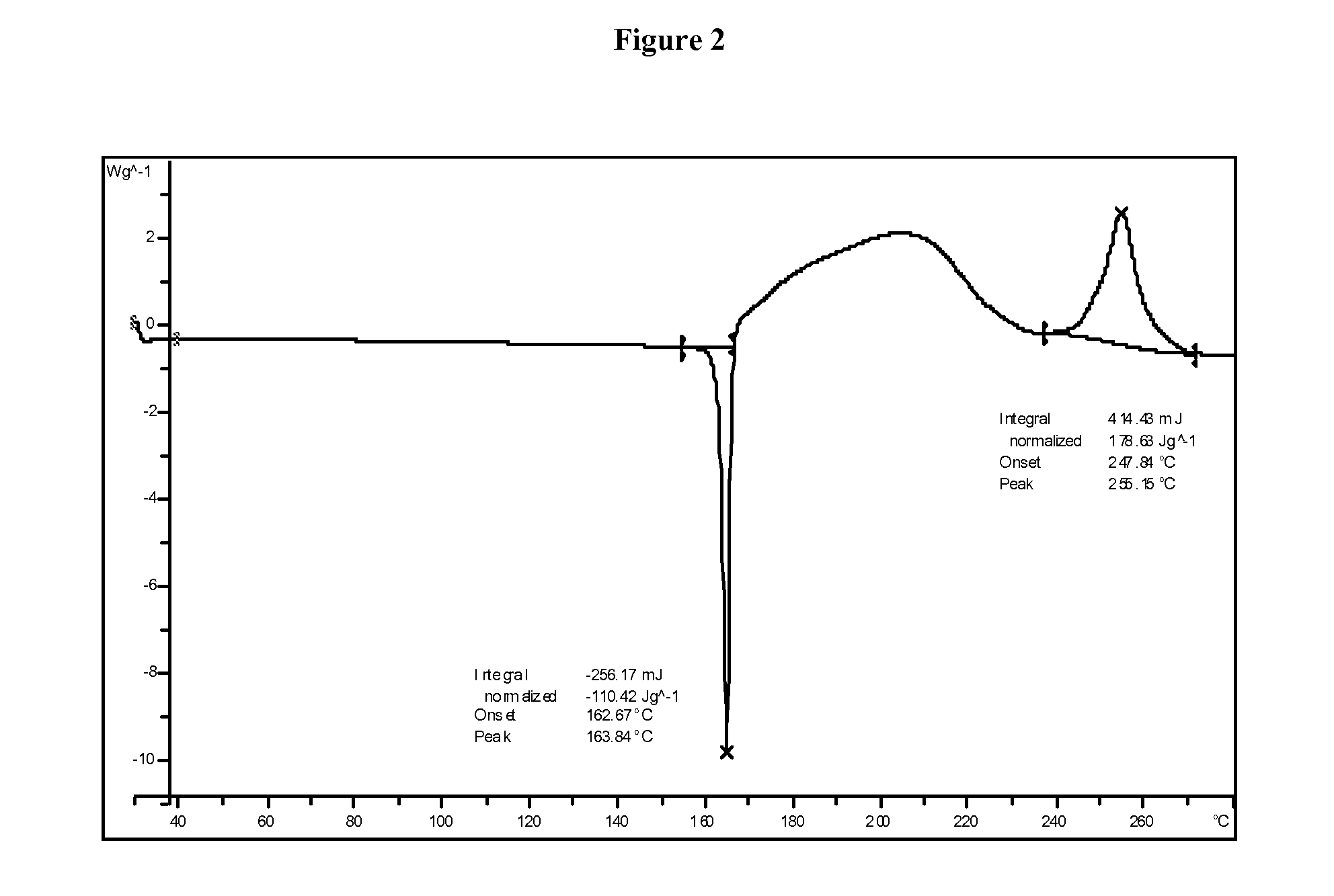
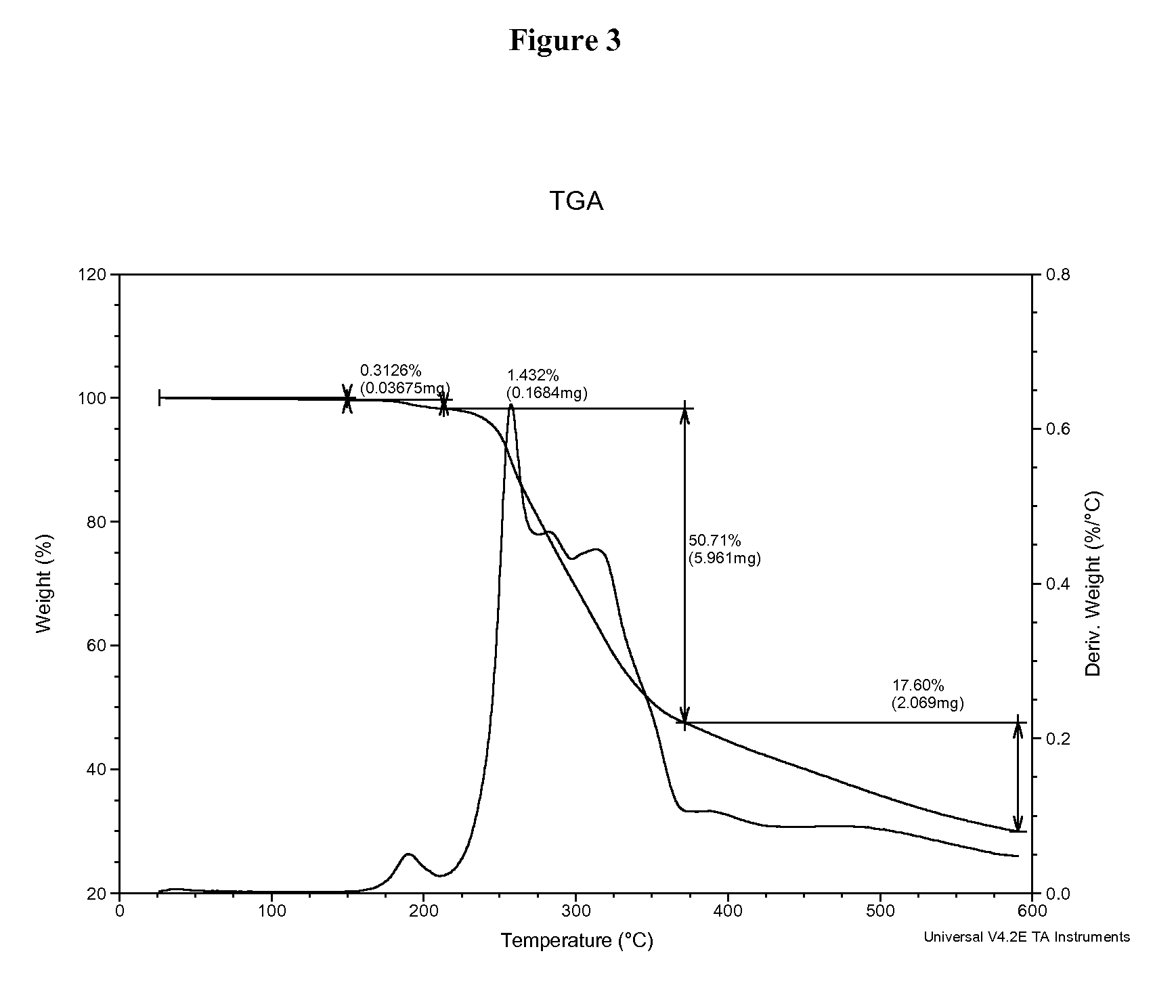
1,2,4-Oxadiazole benzoic acid compounds and their use for nonsense suppression and the treatment of disease
ActiveUS20050164973A1Promote readthroughDecreased amount of producedBiocideSenses disorderBenzoic acidMRNA Decay
Novel 1,2,4-oxadiazole benzoic acid compounds, methods of using and pharmaceutical compositions comprising an 1,2,4-oxadiazole benzoic acid derivative are disclosed. The methods include methods of treating or preventing a disease ameliorated by modulation of premature translation termination or nonsense-mediated mRNA decay, or ameliorating one or more symptoms associated therewith.
Owner:PTC THERAPEUTICS INC
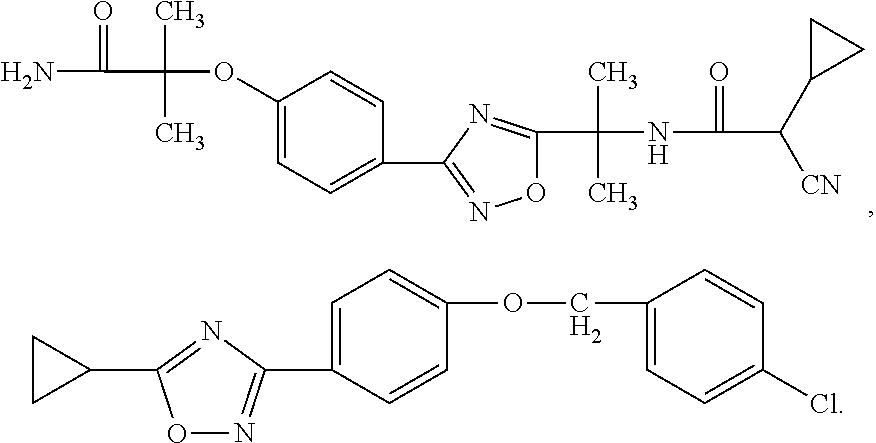
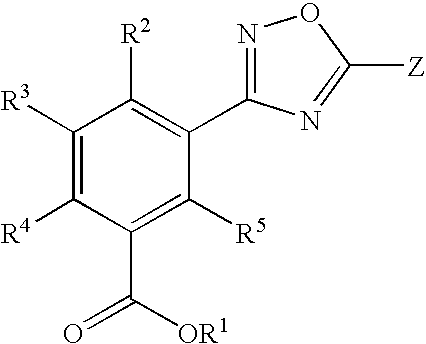
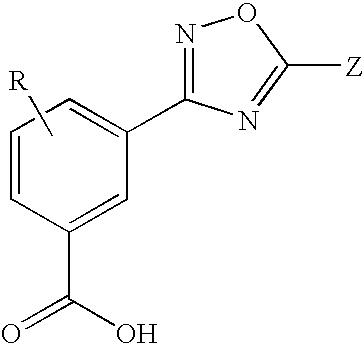
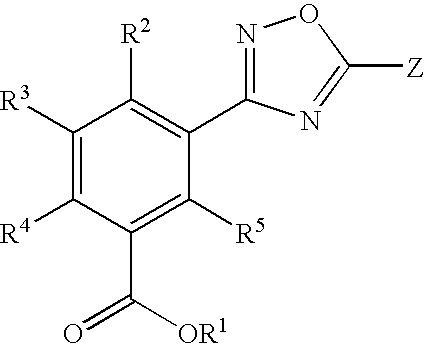
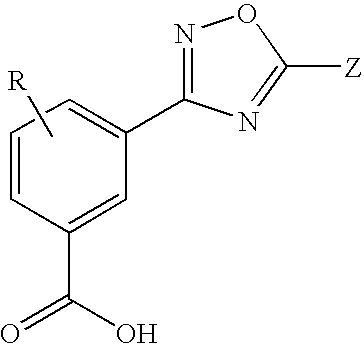
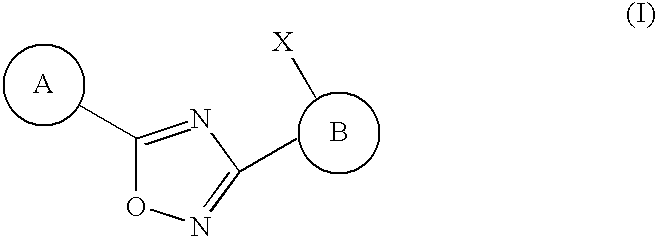
Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof
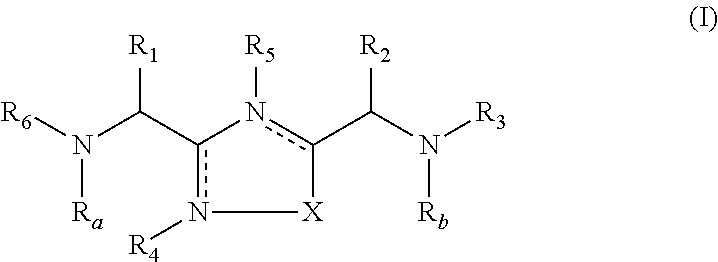
The present invention is directed to substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs thereof, represented by the Formula I: wherein Ar1, Ar3, A, B and D are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
1,2,5-oxadiazoles as inhibitors of indoleamine 2,3-dioxygenase
ActiveUS20100015178A1Prevent immunosuppressionUseful in preparationBiocideSenses disorderDiseaseDioxygenase
The present invention is directed to 1,2,5-oxadiazole derivatives, and compositions of the same, which are inhibitors of indoleamine 2,3-dioxygenase and are useful in the treatment of cancer and other disorders, and to the processes and intermediates for making such 1,2,5-oxadiazole derivatives.
Owner:INCYTE
1,2,4-oxadiazole benzoic acid compounds and their use for nonsense suppression and the treatment of disease
ActiveUS20040204461A1Eradication ameliorationMinimizing spreadBiocideSenses disorderBenzoic acidMRNA Decay
Novel 1,2,4-oxadiazole benzoic acid compounds, methods of using and pharmaceutical compositions comprising an 1,2,4-oxadiazole benzoic acid derivative are disclosed. The methods include methods of treating or preventing a disease ameliorated by modulation of premature translation termination or nonsense-mediated mRNA decay, or ameliorating one or more symptoms associated therewith.
Owner:PTC THERAPEUTICS INC
Gsk-3betainhibitor
InactiveUS20100069381A1Superior GSK-3 specific inhibitory activityImprove solubilityAntibacterial agentsBiocideDiseaseDitazole
For the purpose of providing a GSK-3β inhibitor containing an oxadiazole compound or a salt thereof or a prodrug thereof useful as an agent for the prophylaxis or treatment of a GSK-3β-related pathology or disease, the present invention provides a GSK-3β inhibitor containing a compound represented by the formula (I):wherein each symbol is as defined in the specification, or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
BENZAMIDE mGluR5 POSITIVE ALLOSTERIC MODULATORS AND METHODS OF MAKING AND USING SAME
InactiveUS20090042855A1Increase awarenessPotentiate metabotropic glutamate receptor activityBiocideNervous disorderAllosteric modulatorMetabotropic glutamate receptor
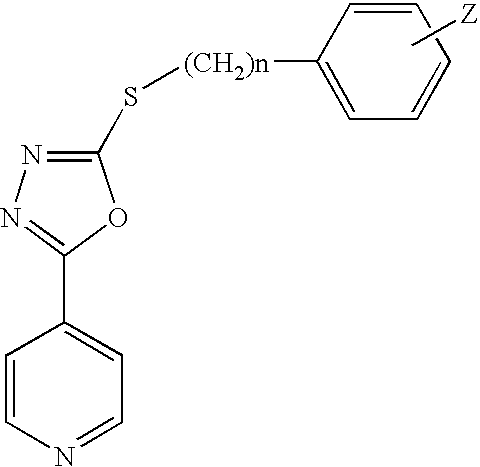
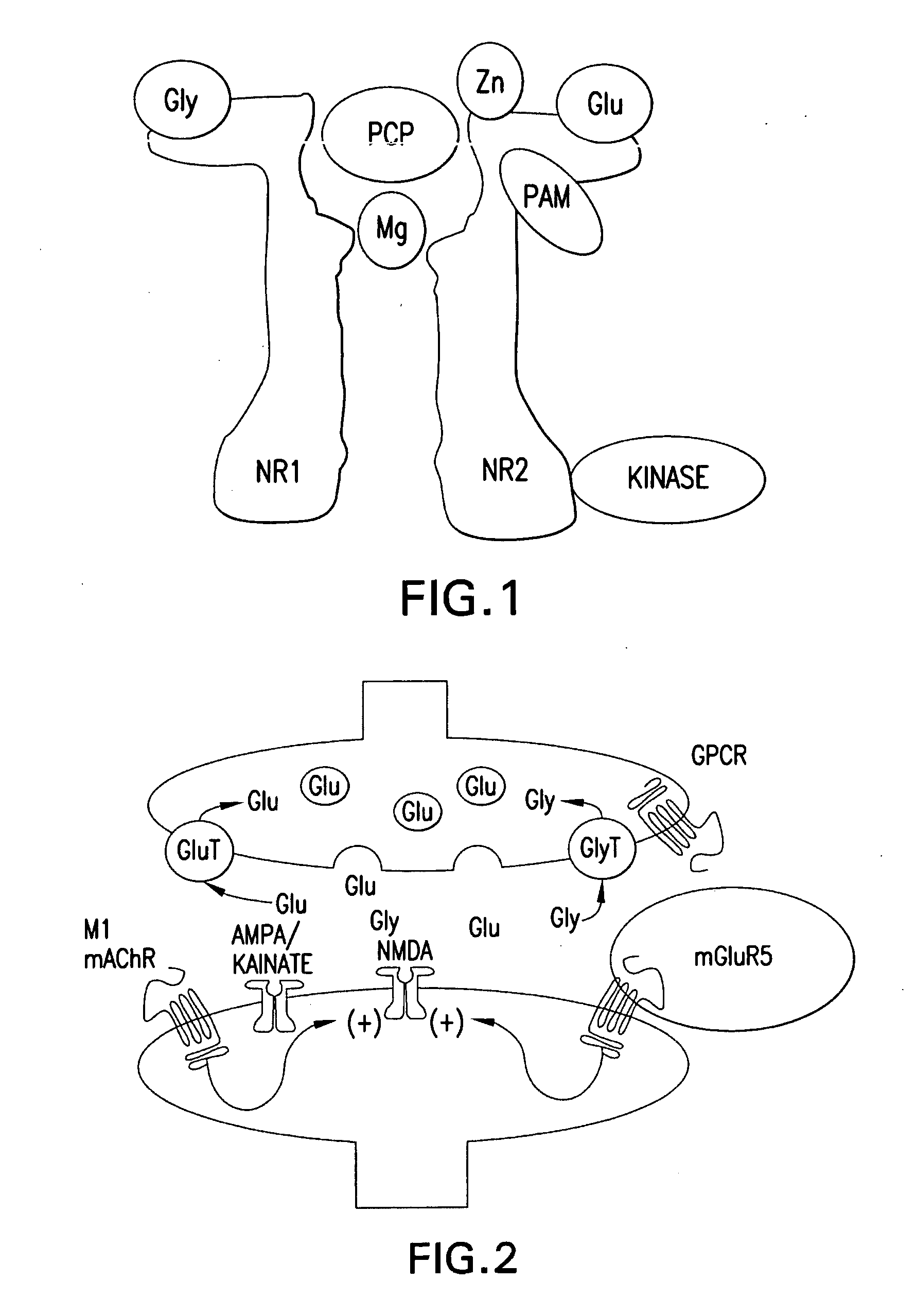
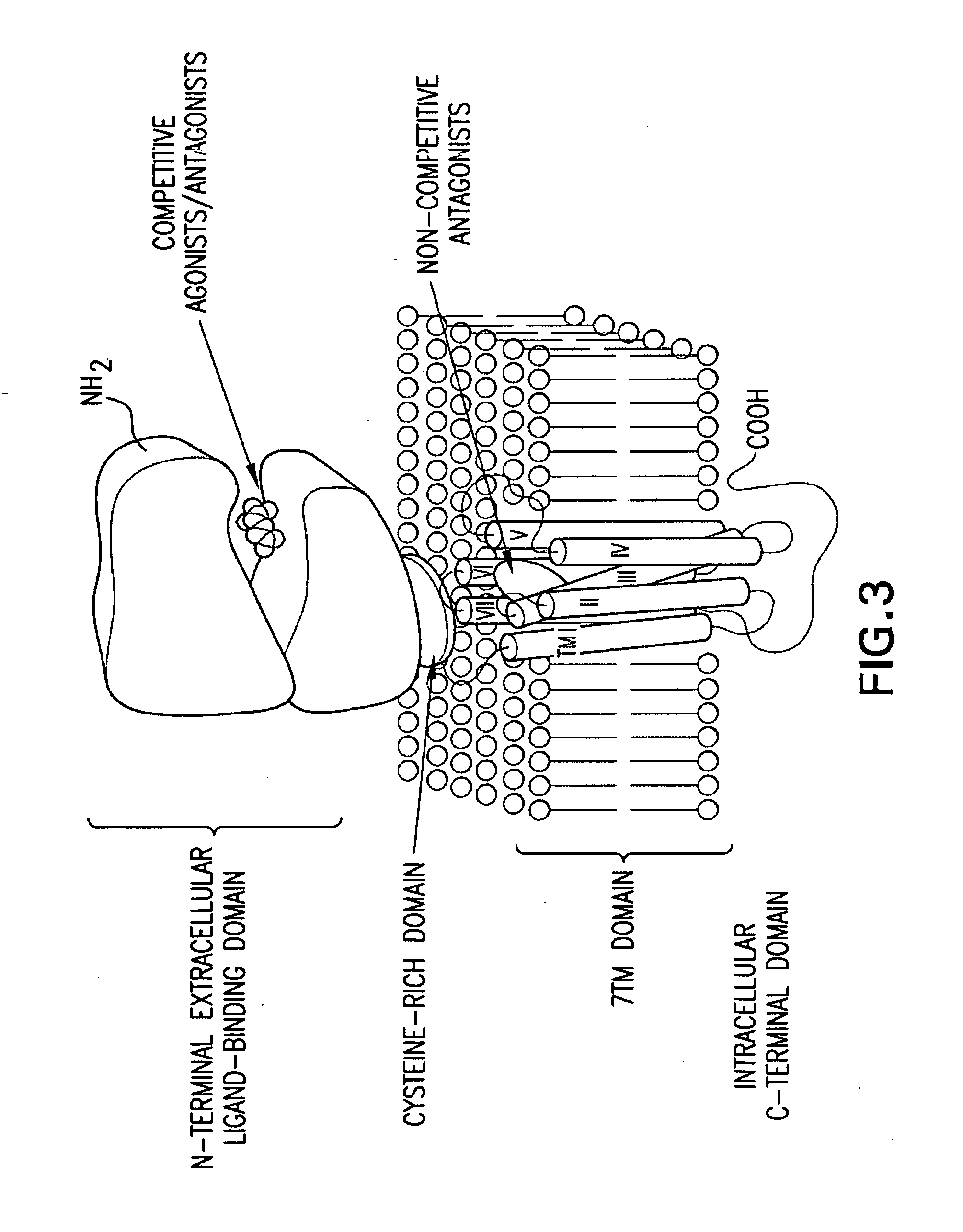
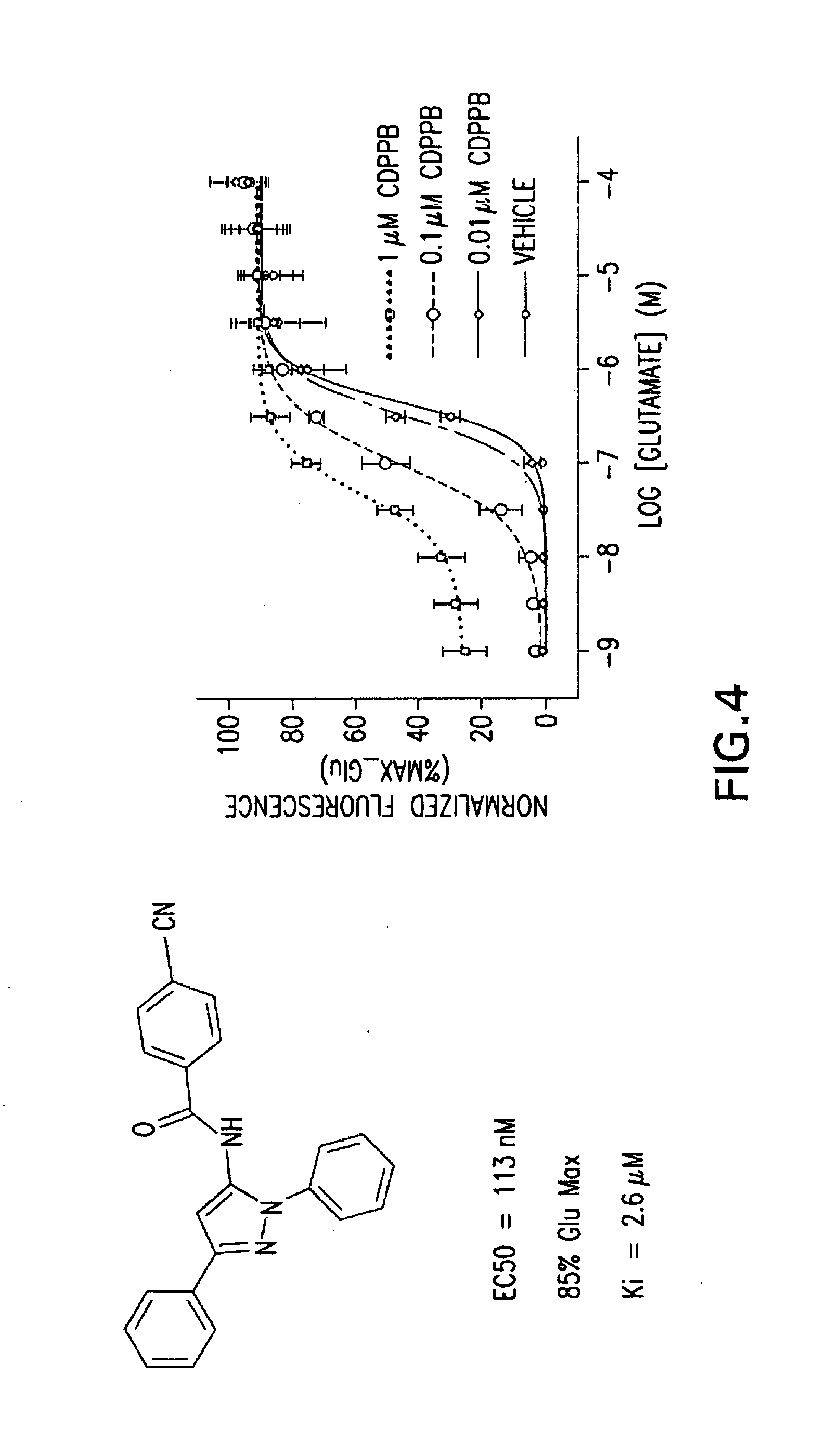
In one aspect, the invention relates to compounds, including phenylethynylbenzamide derivatives, cycloalkylethynylbenzamide derivatives, styrylbenzamide derivatives, 4-(3-phenyl-1,2,4-oxadiazol-5-yl)benzamide derivatives, 4-(pyridinylethynyl)benzamide derivatives, and N1-phenylterephthalamide derivatives, which are useful as positive allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5); synthetic methods for making the compounds; pharmaceutical compositions comprising the compounds; and methods of treating neurological and psychiatric disorders associated with glutamate dysfunction using the compounds and compositions. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
Owner:VANDERBILT UNIV
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
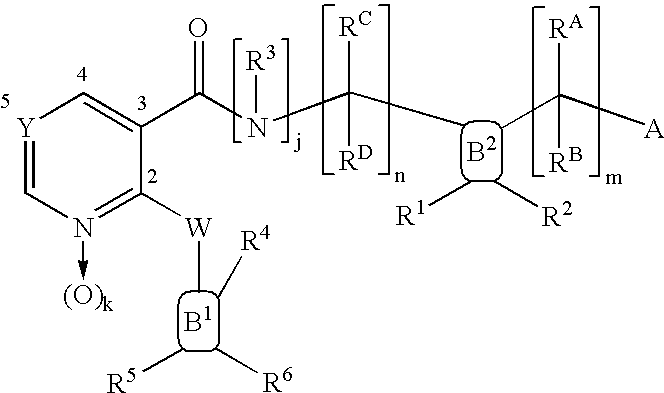
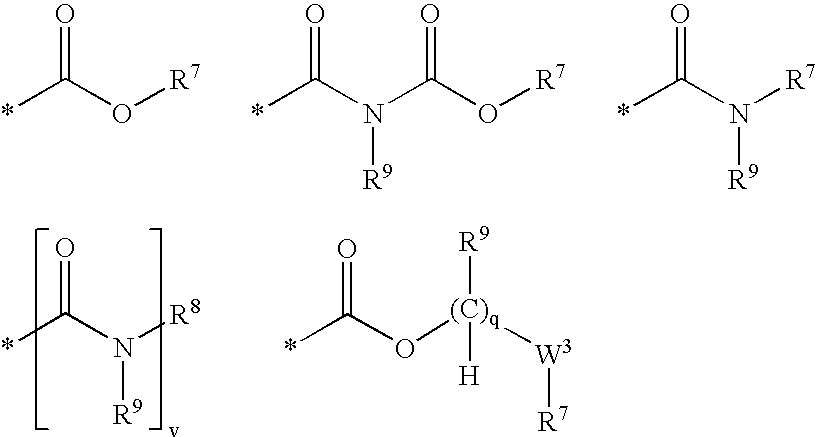
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Substituted-pent-4-ynoic acids
InactiveUS6037367AImprove the level ofSuppressing inappropriate activationBiocideOrganic chemistryMethyl groupCarbon atom
PCT No. PCT / US96 / 11613 Sec. 371 Date Sep. 14, 1998 Sec. 102(e) Date Sep. 14, 1998 PCT Filed Jul. 12, 1996 PCT Pub. No. WO97 / 03945 PCT Pub. Date Feb. 6, 1997Compounds of formula (I) wherein: R1 is -(CR4R5)nC(O)O(CR4R5)mR6, -(CR4R5)nC(O)NR4(CR4R5)mR6, (CR4R5)nO(CR4R5)mR6, or -(CR4R5)rR6: W is alkynyl or 2 carbon atoms; R3 is H or R7; Z is C(O)R13, (CH2)0-1C(O)OR13, (CH2)0-1C(O)NR10R13, (CH2)0-1C(R8R8)OR8, -NHC(O)R7, (CH2)0-1NR10R13, NH[C(O)C(O)OR8], CH2NH[C(O)CNR10R13], CH2S(O)qR7, CH[S(O)qR7]2, dithiolane, (tetrazol-5-yl), thiazol-2-yl, [1,2,4]thiadiazol-5-yl, [1,3,4]oxadiazol-2-yl, imidazol-2-yl, oxazol-2-yl, or (3- or 5-oxadiazolyl[1,2,4]; R7 is -(CR4R5)qR11 or C1-6 alkyl wherein the R11 or C1-6 alkyl group is unsubstituted or substituted one or more times by methyl or ethyl unsubstituted or substituted by 1-3 fluorines, -NR8R10, -CO2R8, -O(CH2qR8, -NR8C(O)R8 or R12; or the pharmaceutically acceptable salts thereof.
Owner:SMITHKLINE BECKMAN CORP
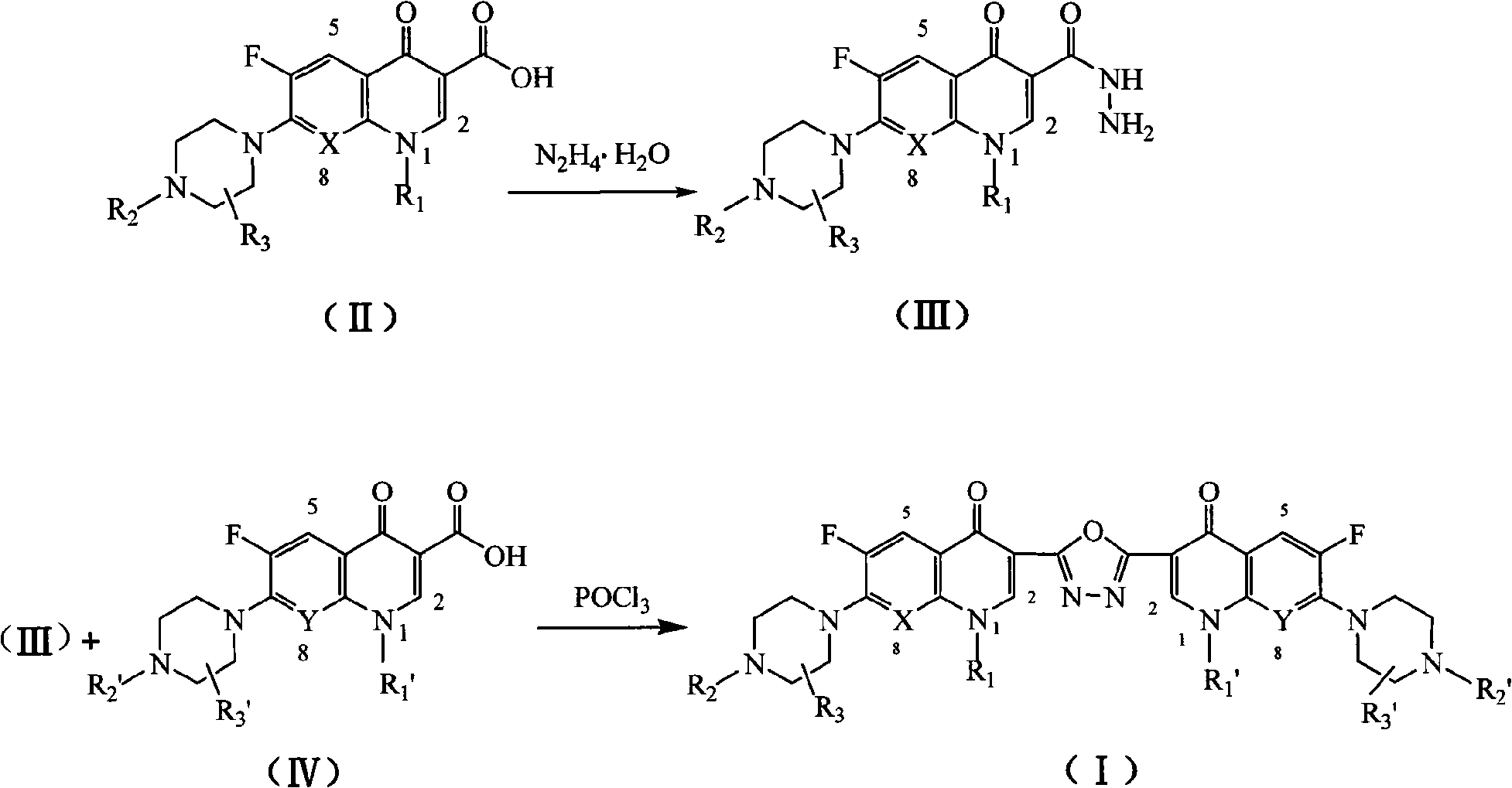
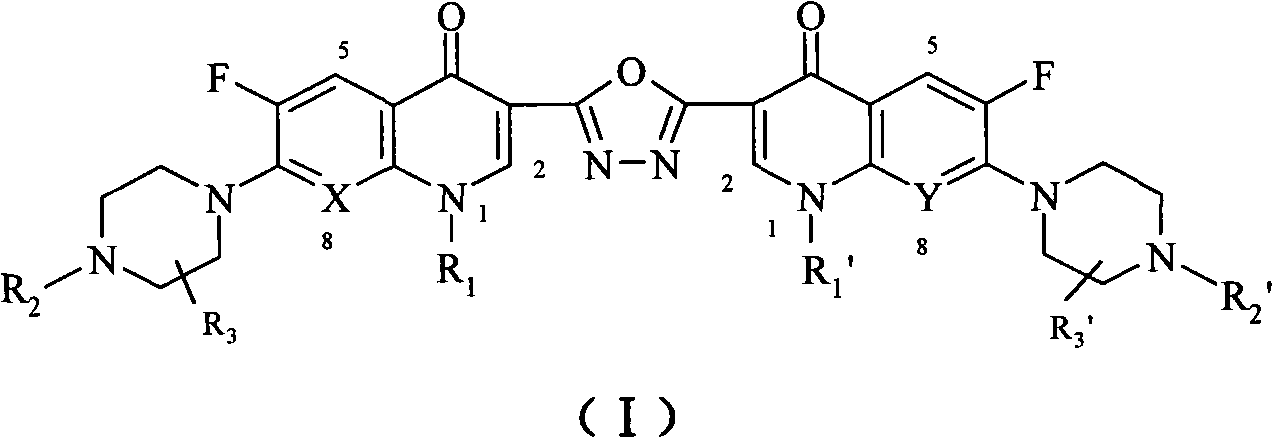
C3/C3 fluoroquinolone dimmer derivative using oxadiazole as connection chain as well as preparation method and application thereof
InactiveCN101643471AStrong cytotoxicityStrong growth inhibitory activityOrganic active ingredientsOrganic chemistryDimmerAryl radical
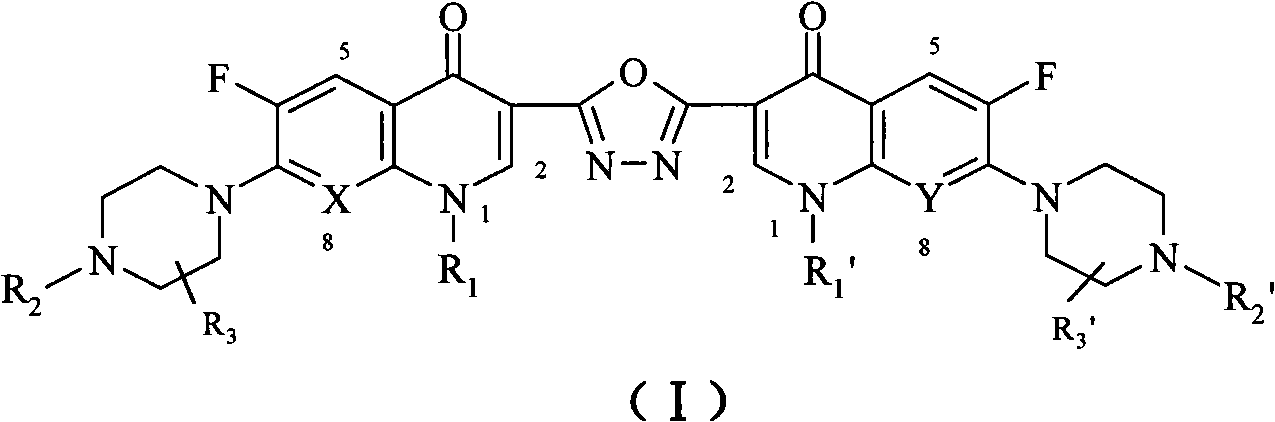
The invention discloses a C3 / C3 fluoroquinolone dimmer derivative using oxadiazole as a connection chain as well as a preparation method and an application thereof. The C3 / C3 fluoroquinolone dimmer derivative using oxadiazole as a connection chain is a compound which has the following structural general formula (I), wherein R1 and R1' are independently selected from H, (C1-C10) alkyl, (C3)-(C10) cycloalkyl, (C1)-(C10) haloalkyl and substituted aryl group or substituted heterocyclic aryl group; R2 and R2' are independently selected from H, (C1-C7)alkyl, (C3)-(C7) cycloalkyl, substituted aryl group, substituted heterocyclic aryl group and hydrocarbon acyl or sulfonyl; R3 and R3' are independently selected from H, (C1-C5) alkyl, (C3)-(C5) cycloalkyl, substituted aryl radical or substitutionalheterocyclic aryl radical; and X and Y are independently selected from CH, N or carbon atoms connected with halogen, alkyl, oxyl, sulfenyl, amino, substituted amino, substituted aryl radical or substituted heterocyclic aryl radical. The C3 / C3 fluoroquinolone dimmer derivative using oxadiazole as a connection chain can be used for preparing medicaments for treating tumors and preventing microbialinfection diseases.
Owner:河南省健康伟业生物医药研究股份有限公司
MEK inhibiting compounds
InactiveUS20050004186A1Inhibit phosphorylationAntibacterial agentsBiocidePercent Diameter StenosisImmunomodulations
This invention provides substituted Phenyl-(2-[1,3,4]thiadiazol-2-yl-phenyl)-amine and (2-[1,3,4]Oxadiazol-2-yl-phenyl)phenyl-amine compounds which act as inhibitors of MAPK / ERK Kinase (“MEK”) enzymes and pharmaceutical compositions and methods for their use in immunomodulation and in the treatment and alleviation of inflammation, and proliferative diseases such as cancer and restenosis.
Owner:PFIZER INC
3,5-Aryl, heteroaryl or cycloalkyl substituted-1,2,4-oxadiazoles as s1p receptor agonists
The present invention encompasses compounds of Formula I: (I) as well as the pharmaceutically acceptable salts thereof. The compounds are useful for treating immune mediated diseases and conditions, such as bone marrow, organ and tissue transplant rejection. Pharmaceutical compositions and methods of use are included.
Owner:MERCK & CO INC
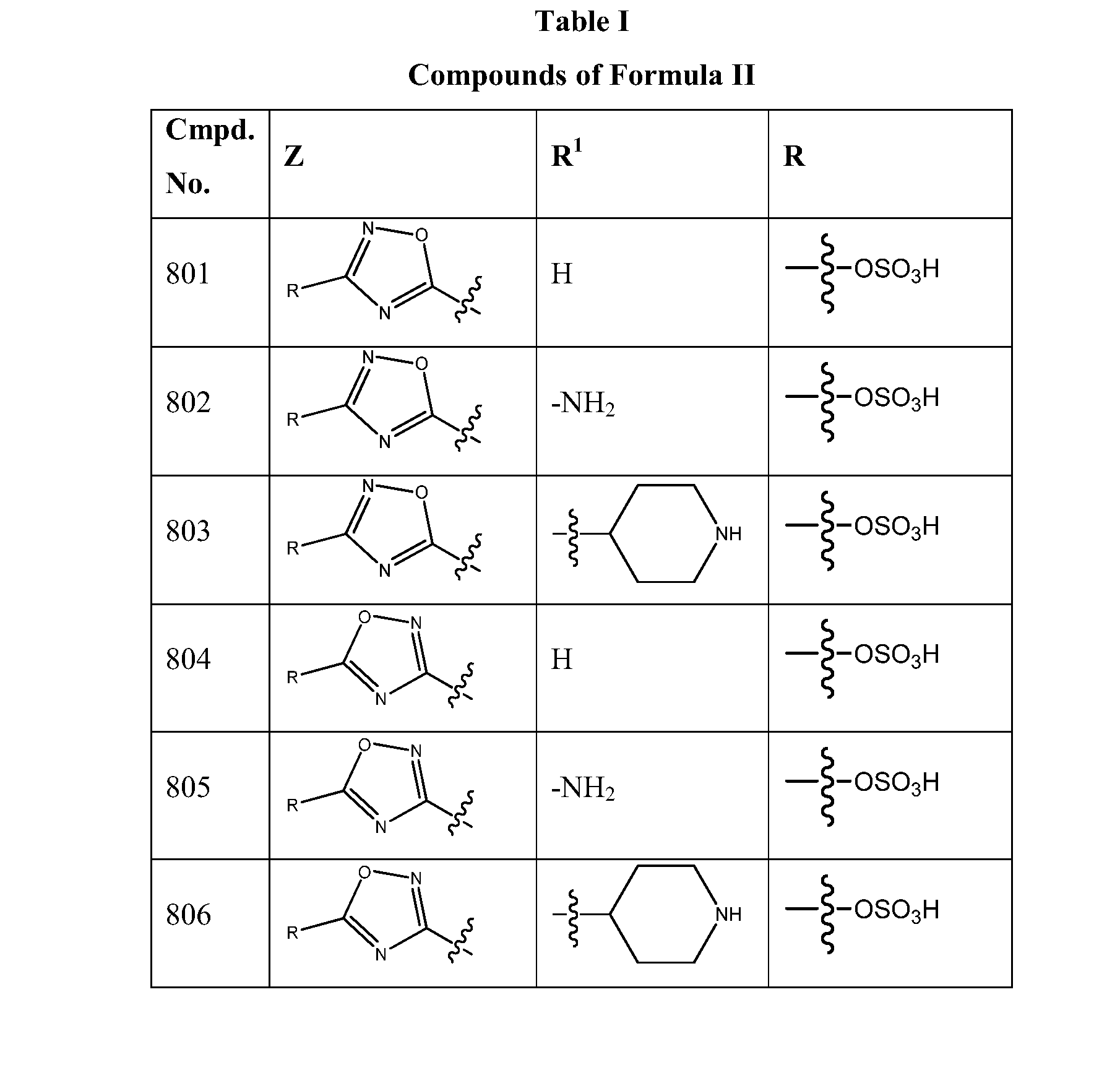
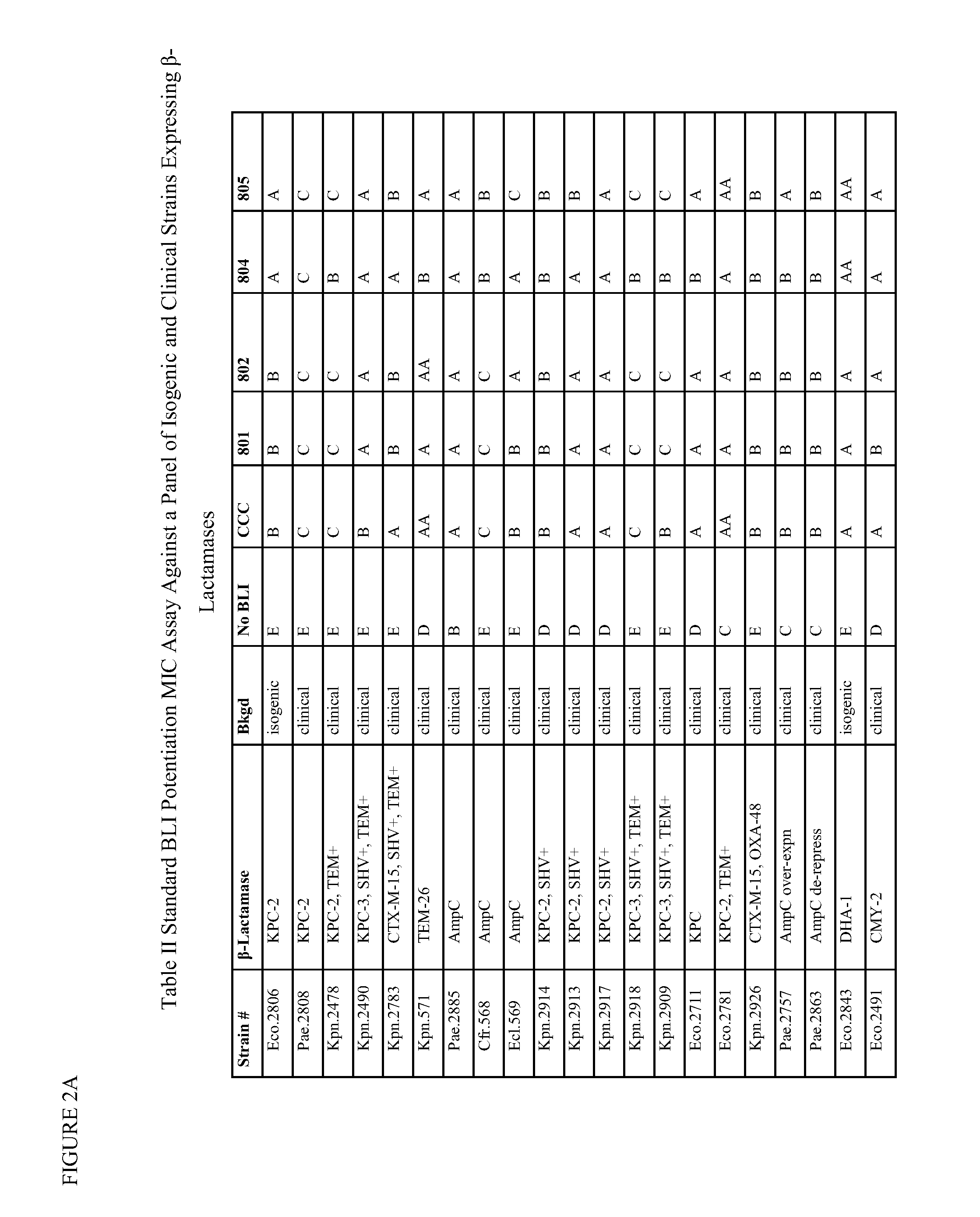
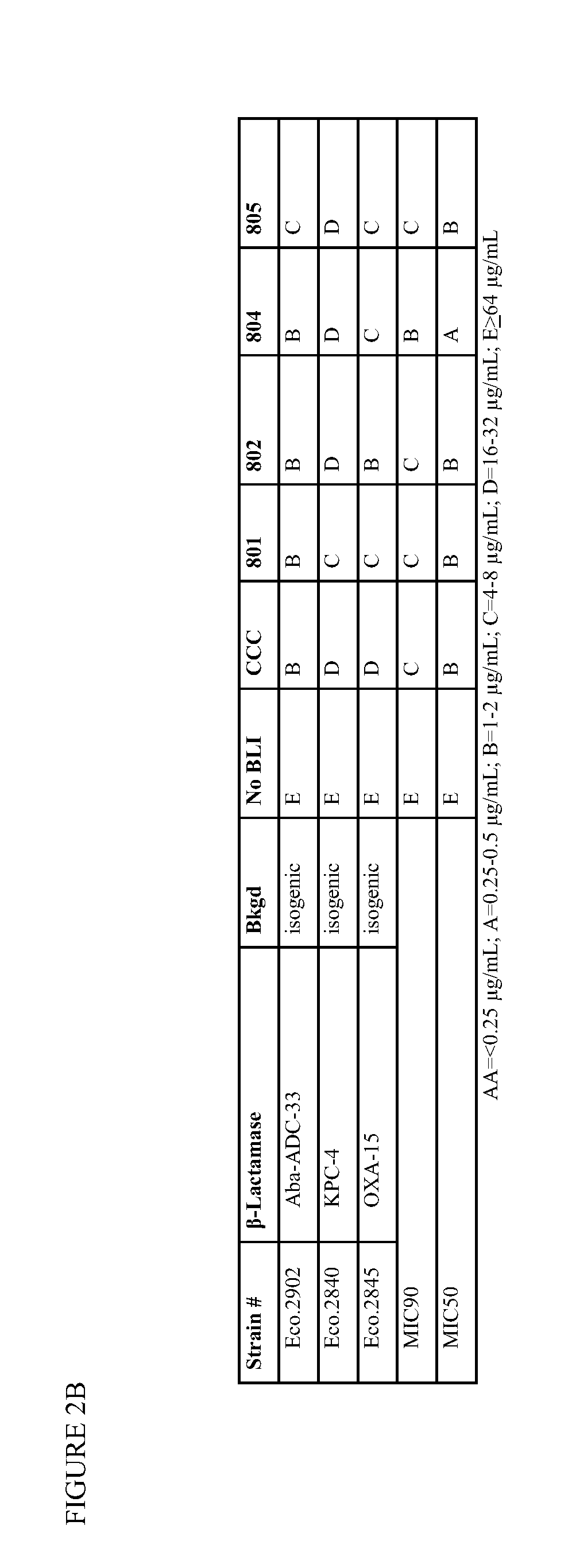
1,2,4-oxadiazole and 1,2,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
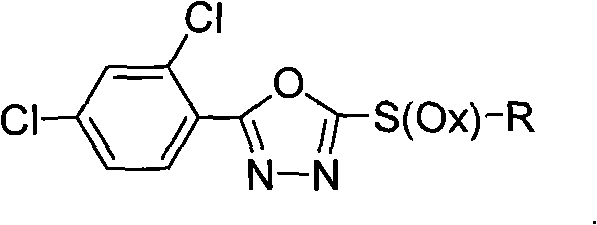
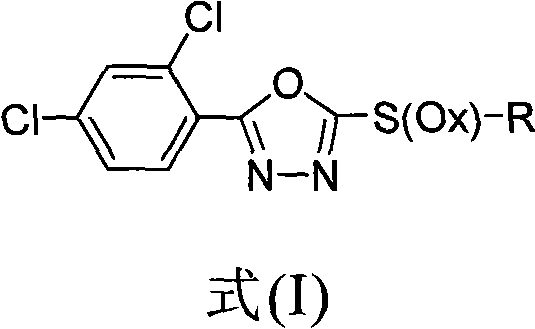
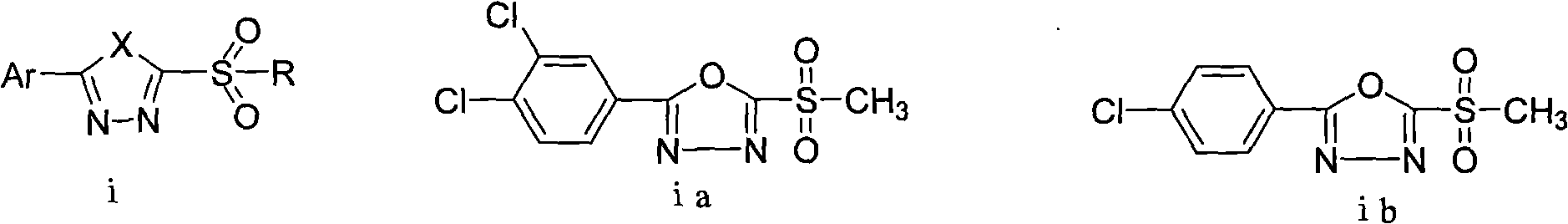
2-substituent-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole derivative, synthetic method and application thereof
InactiveCN101812034AGrowth inhibitionEnhanced inhibitory effectBiocideOrganic chemistryChemical industryDisease
The invention discloses a 2-substituent-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole derivative, a synthetic method and application thereof, belonging to the field of chemical industry and pesticides. The compound is expressed in the following general formula in the specification, wherein X is 0 or 2, and R is as defined in the specification. In the synthetic method, 2,4-dichlorophenyl formic acid is used as an initial raw material for synthesizing sulfoether or sulfone compounds; biological activity screening is carried out; and a part of the screened compounds has better inhibiting activity and better application prospect for various plant diseases and particularly for soil-borne diseases.
Owner:GUIZHOU UNIV
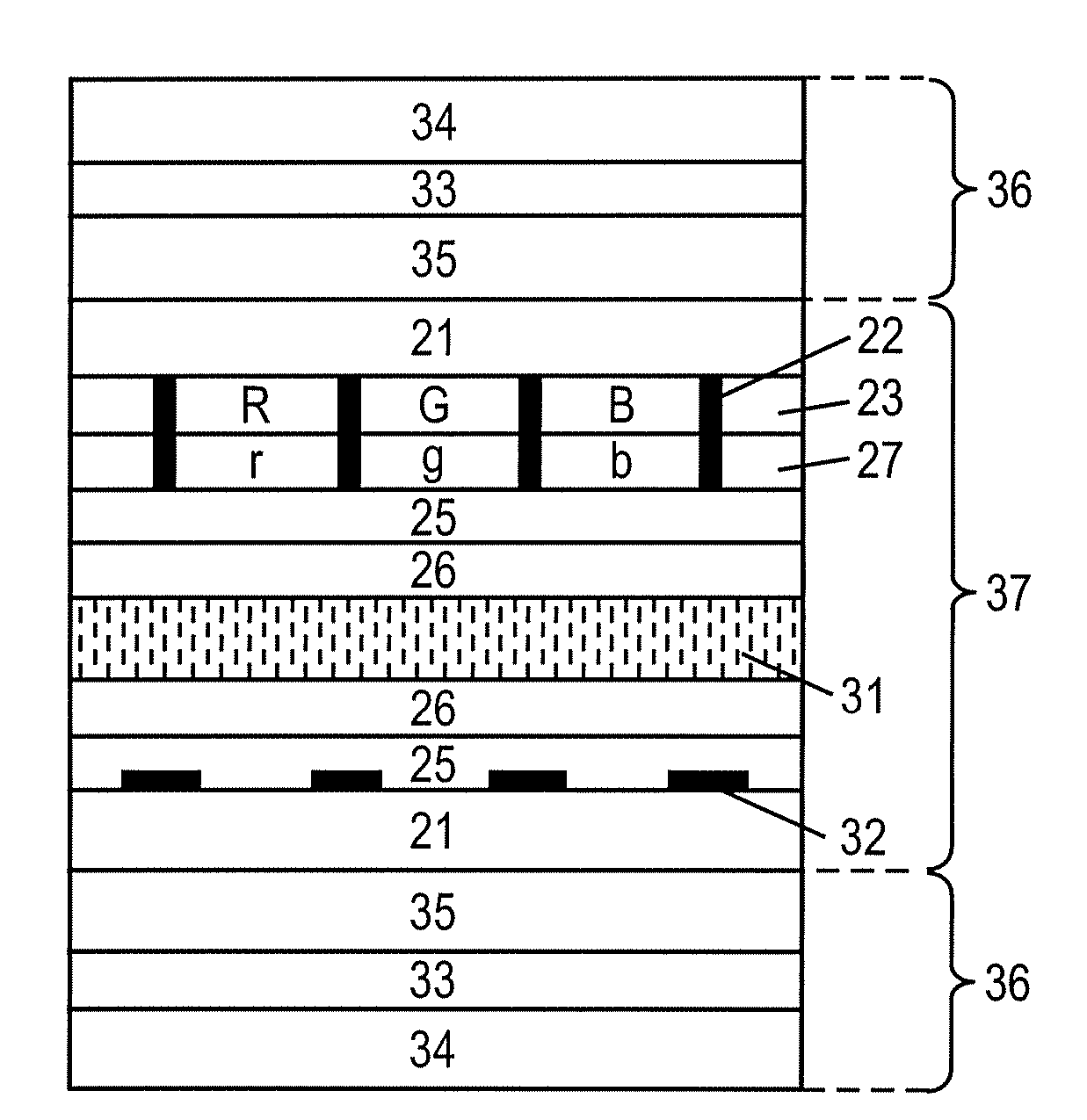
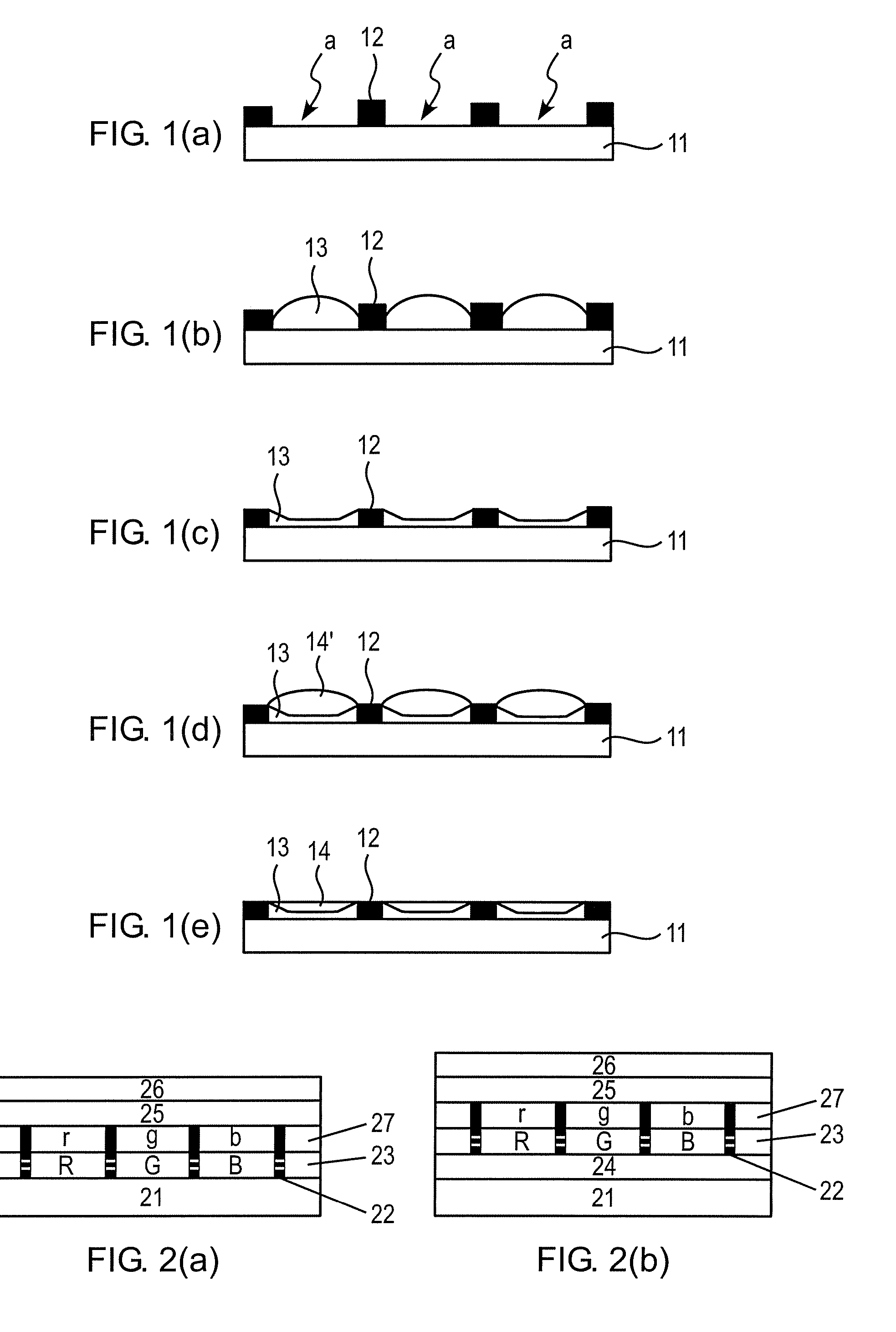
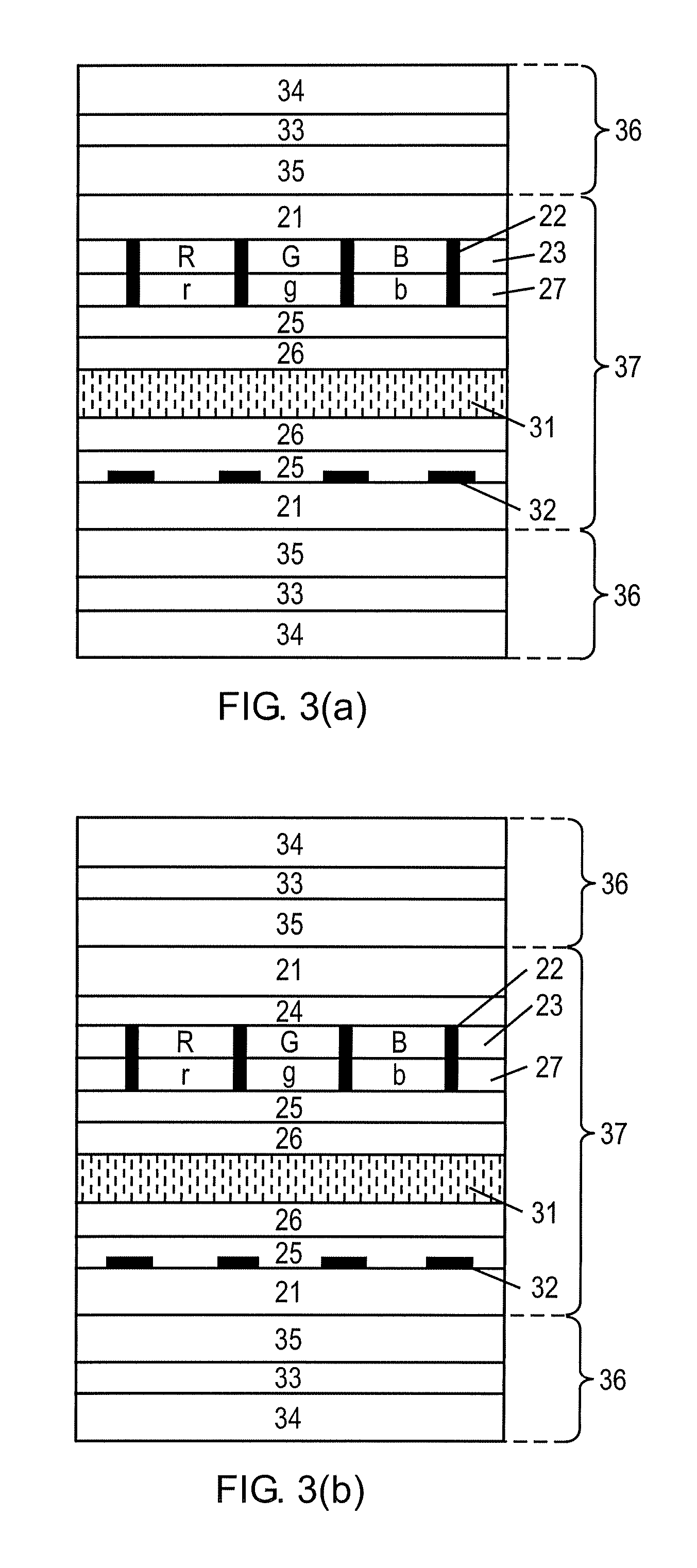
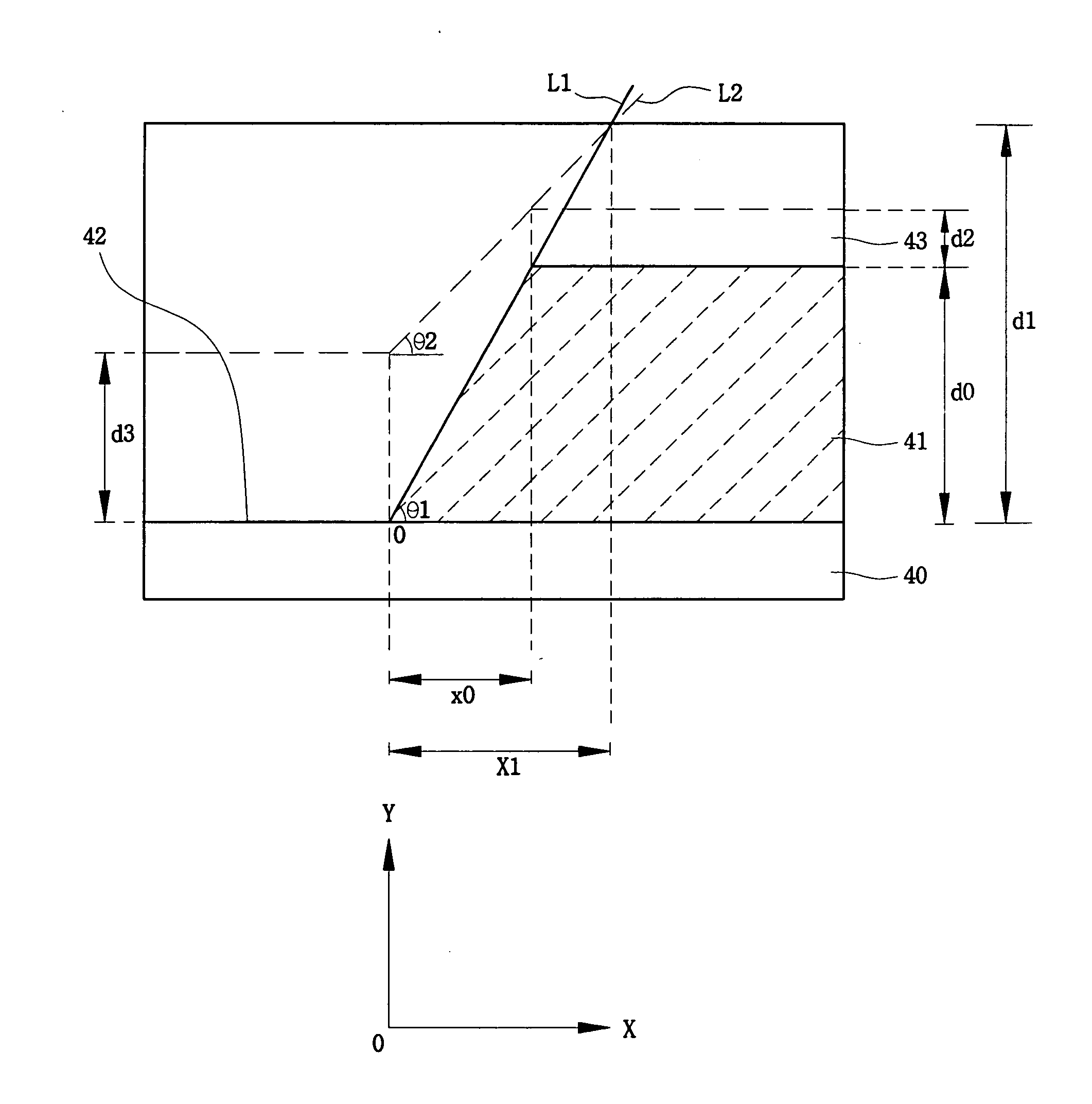
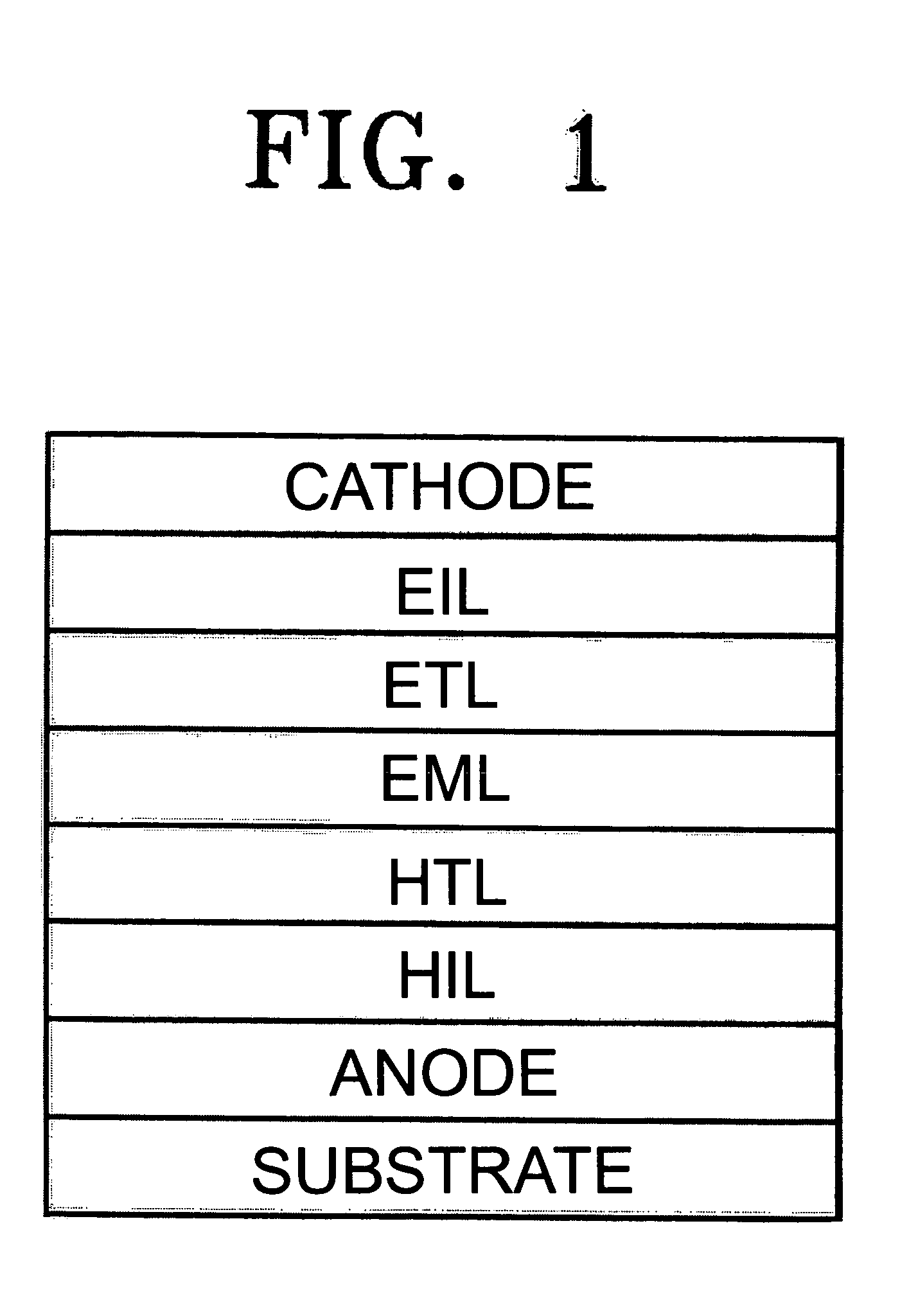
Flat panel display
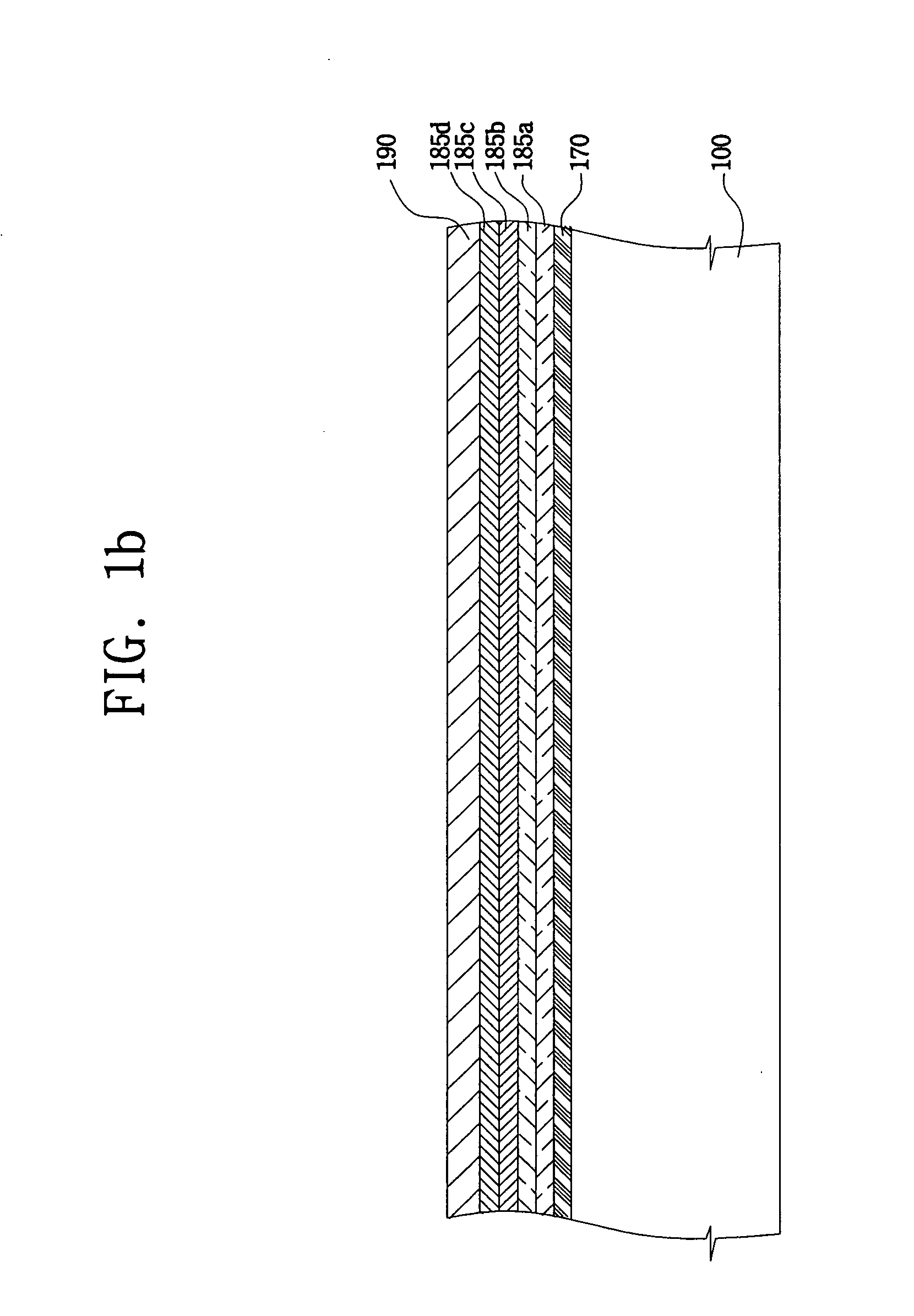
ActiveUS20050116240A1Avoid pinholesImprove picture qualityDischarge tube luminescnet screensElectroluminescent light sourcesOrganic layerDisplay device
The present invention discloses an organic light emitting device for preventing element defects and improving picture quality by reducing a taper angle of a substrate surface. The flat panel display of the present invention comprises, an insulating substrate, a lower layer formed on the insulating substrate and having a first step and a first taper angle with respect to the substrate surface, and an upper layer formed on the insulating substrate and for reducing the taper angle of the lower layer. The upper layer has a second taper angle smaller than the first taper angle of the lower layer. The upper layer is a conductive layer that may be applied by a wet coating method, has a charge transporting capability, and is selected from at least one of a small-molecule organic layer including a carbazole-based, arylamine-based, hydrazone-based, stilbene-based, oxadiazole-based, starburst-based derivatives, and a polymer organic layer including PEDOT, PANI, carbazole-based, arylamine-based, perylene-based, pyrrole-based, oxadiazole-based derivatives.
Owner:SAMSUNG DISPLAY CO LTD
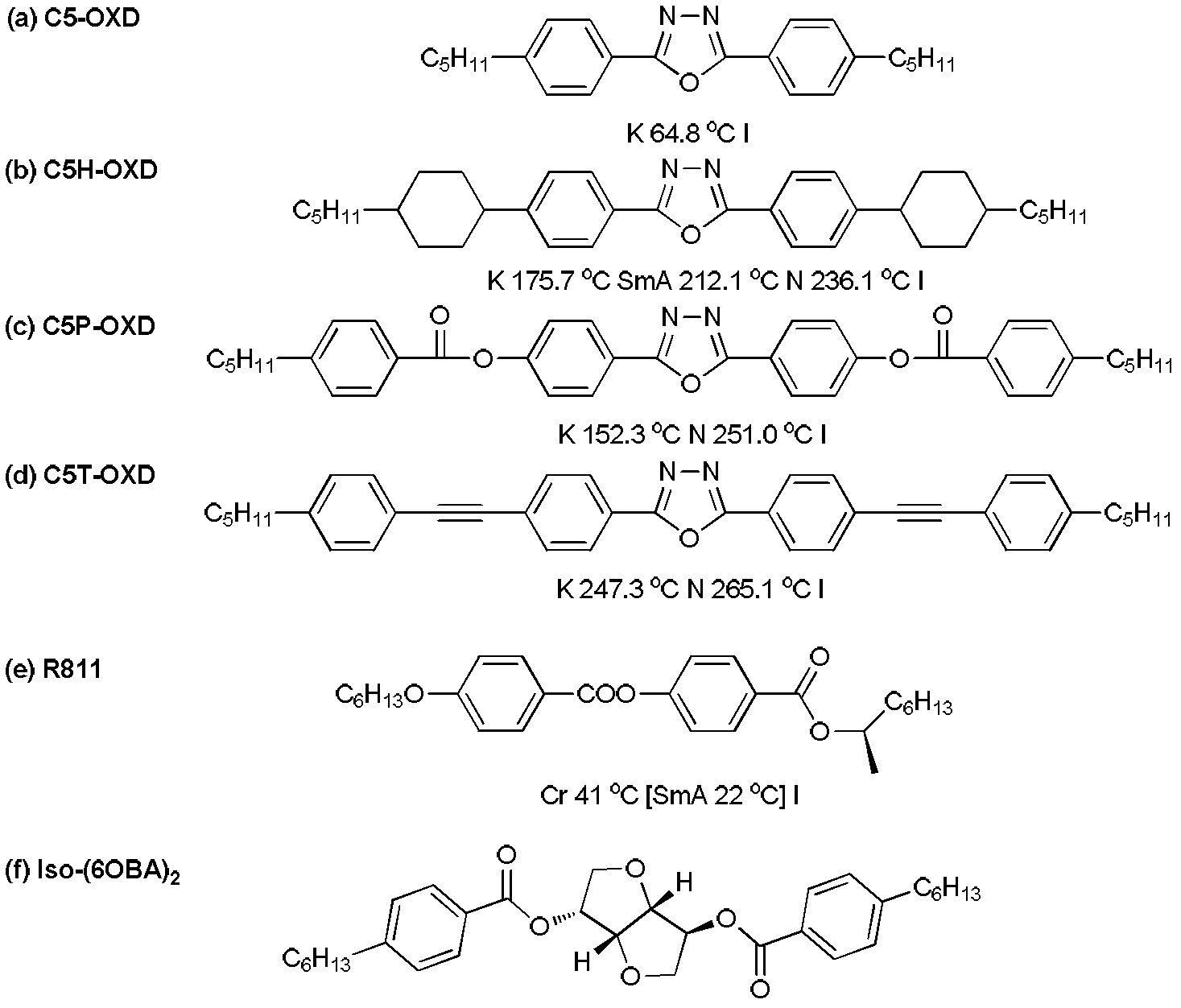
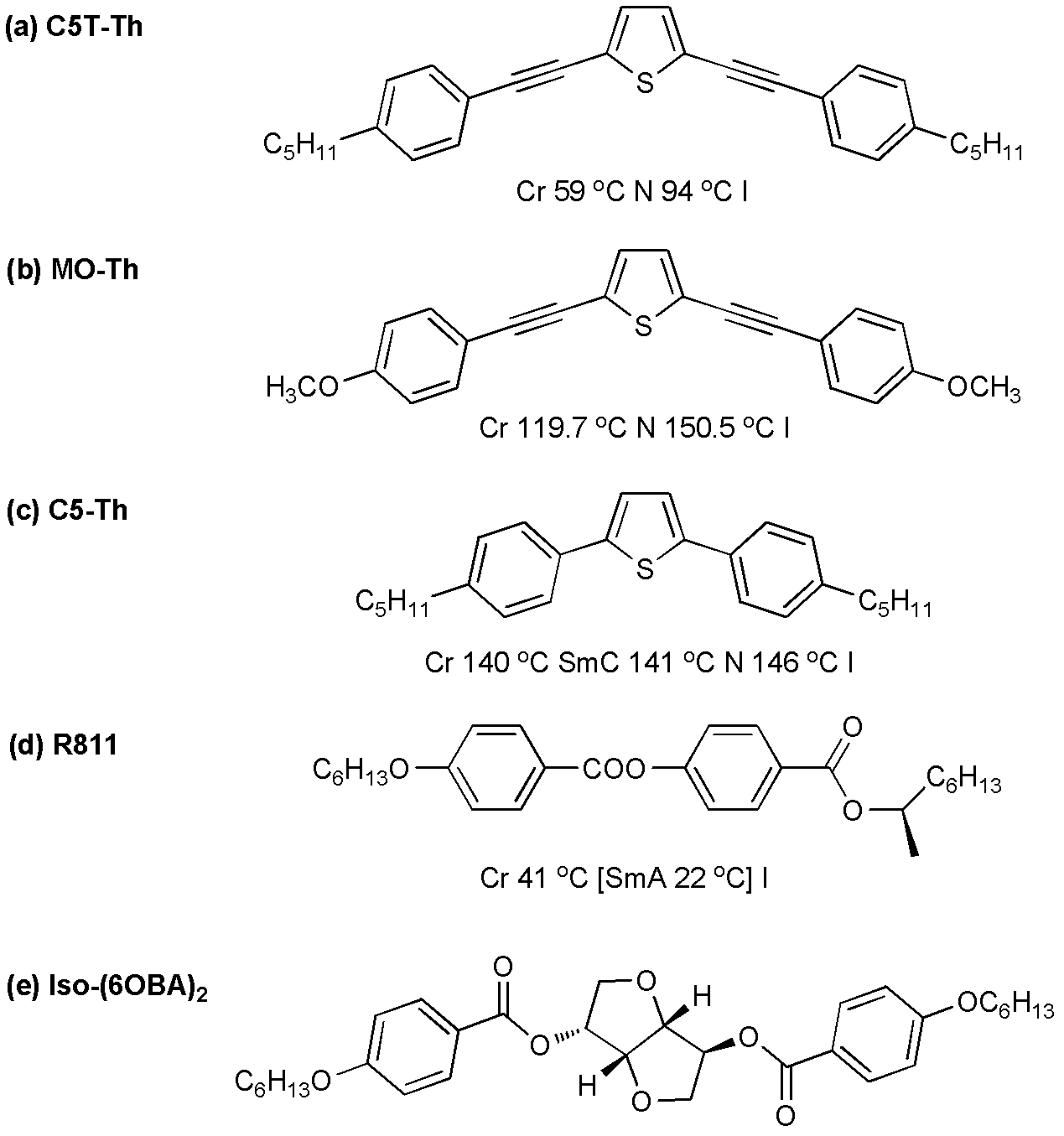
Preparation method for wide-temperature blue-phase liquid crystal composite material
The invention provides a preparation method for a wide-temperature blue-phase liquid crystal composite material, comprising the following steps of: adding bent liquid crystal molecules such as oxadiazoles and thiophenes in a micro-molecular nematic mixed crystal according to a certain ratio, then adding one or more chiral compounds in a proper amount and having a helical twisting power (HTP) of 8-100 mum<-1>, so as to induce and broaden the temperature range of a blue-phase liquid crystal, wherein the temperature range can achieve 5-30 DEG C. The preparation method for a wide-temperature blue-phase liquid crystal composite material provided by the invention is simple in preparation and remarkable in effect; and the prepared system is good in stability, low in viscosity, and fast in the speed of a response to an electric field.
Owner:UNIV OF SCI & TECH BEIJING
Organic electroluminescent device
InactiveUS20050287393A1Improve emission efficiencyEnhanced lifetime characteristicDischarge tube luminescnet screensElectroluminescent light sourcesDopantCarbazole
An organic EL device which has a light emission layer between a pair of electrodes. The light emission layer is formed of a phosphorescent dopant and a host including (i) a carbazole compound and (ii) one or more selected from an oxadiazole compound, a phenanthroline compound, a triazine compound, and a triazole compound. Since the host has both hole transport property and electron transport property, the organic EL device has enhanced emission efficiency and lifetime characteristics even in the absence of a hole-blocking layer.
Owner:SAMSUNG SDI CO LTD
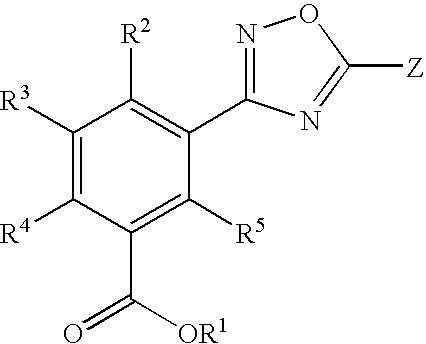
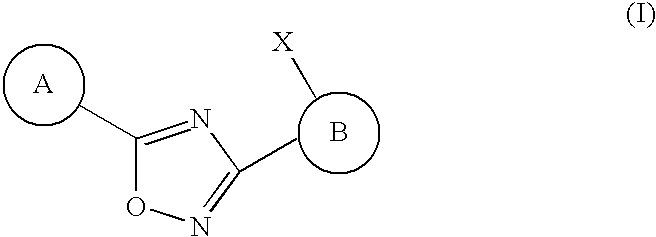
3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof
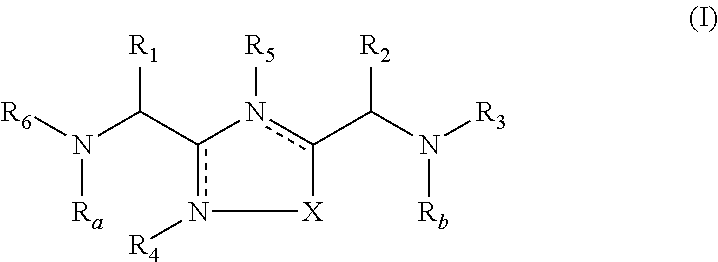
Disclosed are 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs thereof, represented by the Formula I:wherein Ar1, R2, A, B and D are defined herein. The present invention relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
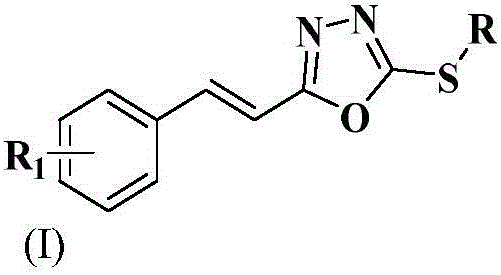
Styryl-containing 1,3,4-oxadiazole thioether compound, as well as preparation method and application thereof
InactiveCN106674147ASimple methodGreat development and application valueBiocideOrganic chemistryHydrogenBromine
The invention discloses a styryl-containing 1,3,4-oxadiazole thioether compound. The structural general formula (I) is as follows: R1 is hydrogen, 4-methoxyl, 4-chlorine, 4-fluorine or 4-bromine; R is methyl, ethyl, benzyl, 2,5-dichlorobenzyl, 4-fluorobenzyl, 4-hlorobenzyl, 2-methylbenzyl, 4-nitrobenzyl, 1,1,2-trifluoro-butenyl or the like. The styryl-containing 1,3,4-oxadiazole thioether compound has activities of killing citrus nematode and meloidogyne incognita chitwood. (The formula (I) is as shown in the description.).
Owner:GUIZHOU UNIV
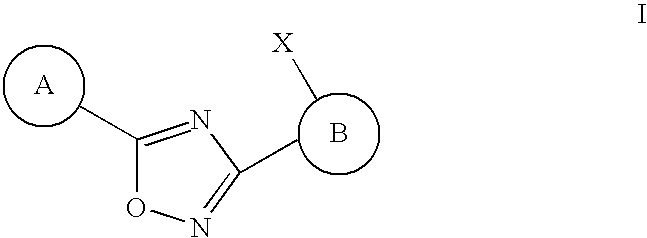
3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof
Disclosed are 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs thereof, represented by the Formula I:wherein Ar1, R2, A, B and D are defined herein. The present invention relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC
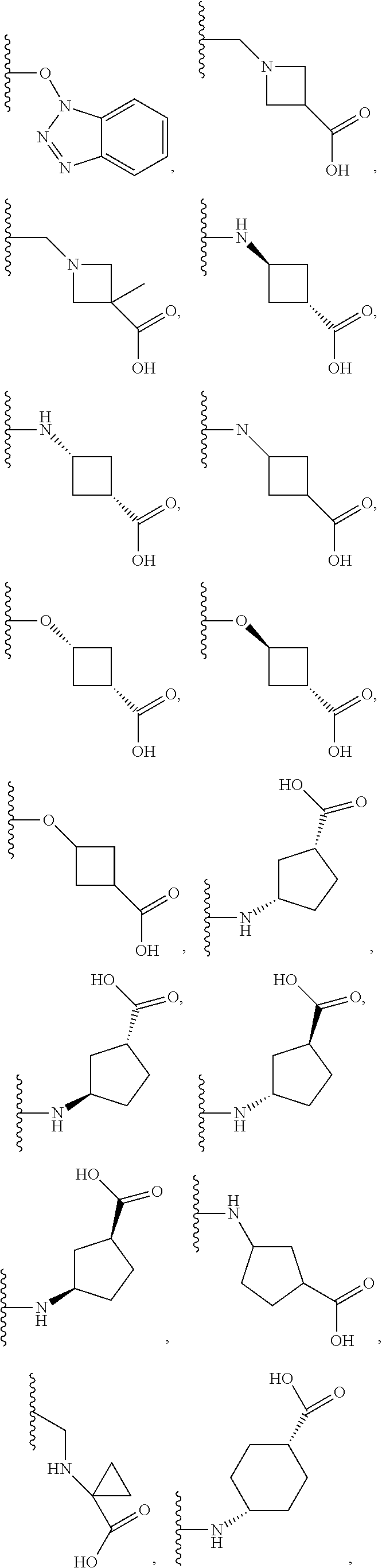
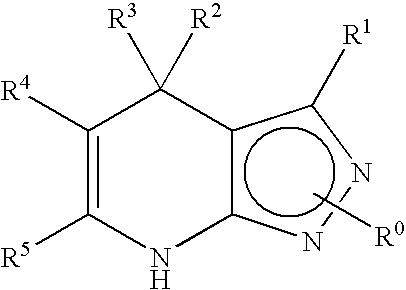
Dihydro-1h-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives which act as s1p1 agonists
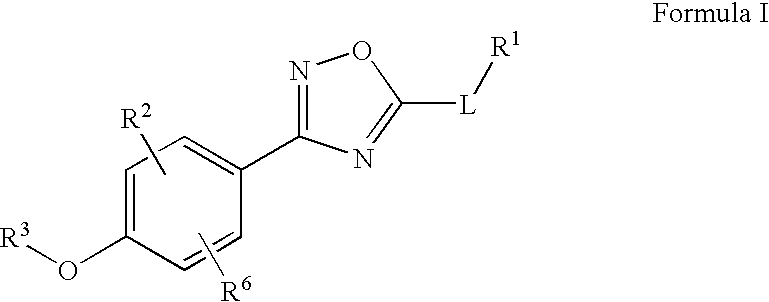
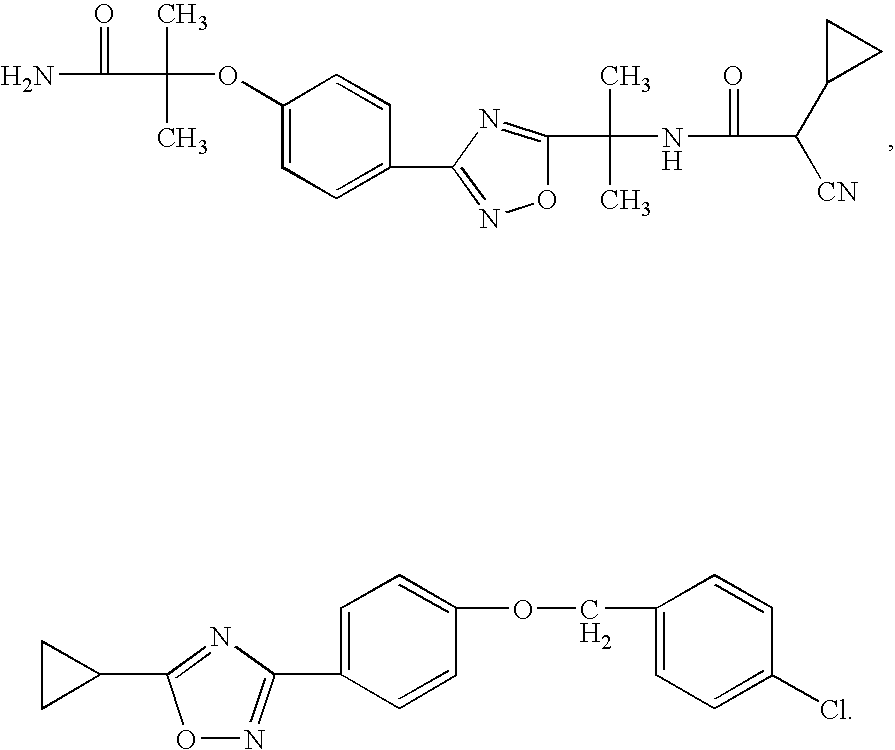
The present invention relates to certain (1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives of Formula (Ia) and pharmaceutically acceptable salts thereof, which exhibit useful pharmacological properties, for example, as agonists of the S1P1 receptor. Also provided by the present invention are pharmaceutical compositions containing compounds of the invention, and methods of using the compounds and compositions of the invention in the treatment of S1P1 associated disorders, for example, psoriasis, rheumatoid arthritis, Crohn's disease, transplant rejection, multiple sclerosis, systemic lupus erythematosus, ulcerative colitis, type I diabetes, sepsis, myocardial infarction, ischemic stroke, acne, microbial infections or diseases and viral infections or diseases.
Owner:ARENA PHARMA
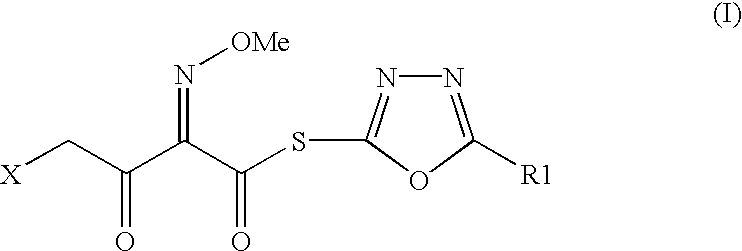
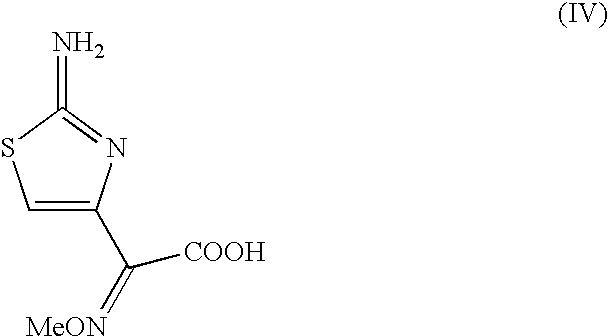
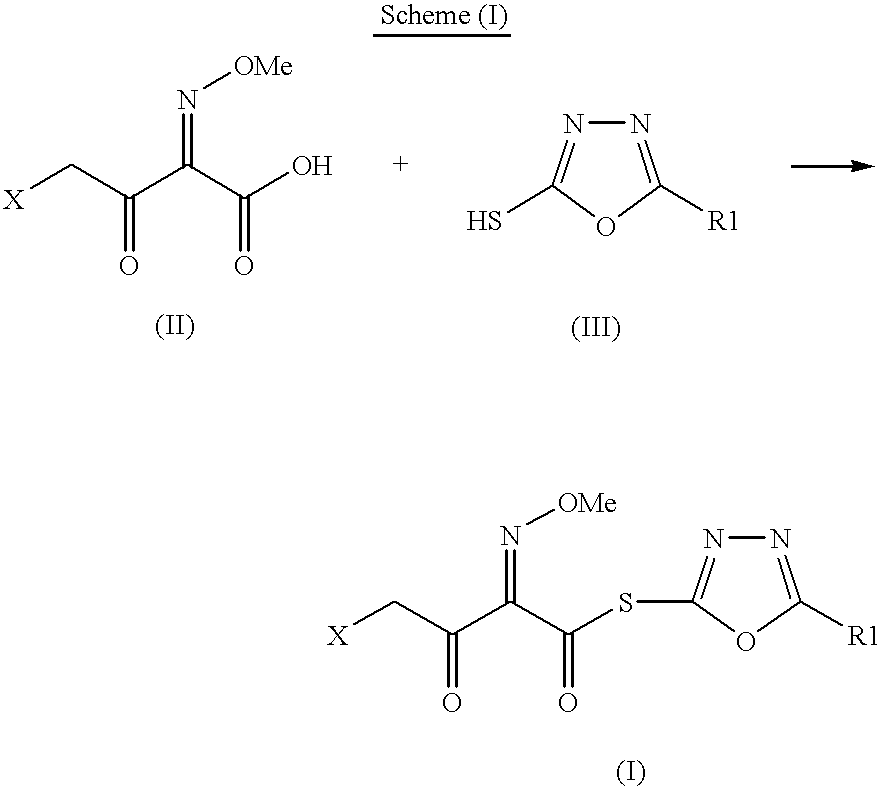
Preparation of new intermediates and their use in manufacturing of cephalosporin compounds
The present invention provides new thioester derivatives of 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (I), also, the invention provides a method by which the said thioester derivatives can be prepared by reacting 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (II) with 2-mercapto-5-substituted-1,3,4-oxadiazoles of the general formula (III) in a solvent, in the presence of DMF / POCl3 and in presence of an organic base and if desired the so obtained thioester derivatives so obtained are reacted with 7-amino-cephem carboxylic acids of the general formula (V) to produce condensed products which are insitu reacted with thiourea to get cephalosporin antibiotic compounds having the general formula (VI).
Owner:ORCHID CHEM &PHARMA LIMITED INDIA
1,2,4-oxadiazole and thiadiazole compounds as immunomodulators
ActiveUS20180044303A1Suppress and inhibit programmed cell death (PD1) signaling pathwaySuppress and/or inhibit the programmed cell death 1 (PD1) signaling pathwayAntibacterial agentsOrganic active ingredientsPD-L1Thiadiazoles
The present invention relates to 1,2,4-oxadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE ONCOLOGY LTD
Oxadiazole compounds
ActiveUS7834039B2Reduce in quantityGood effectBiocideSenses disorderDiseaseG protein-coupled receptor
Novel oxadiazole compounds, pharmaceutical compositions containing such compounds and the use of those compounds or compositions as agonists or antagonists of the S1P family of G protein-coupled receptors for treating diseases associated with modulation of S1P family receptor activity, in particular by affording a beneficial immunosuppressive effect are disclosed.
Owner:ABBVIE INC
1,3,4-oxadiazole and thiadiazole compounds as immunomodulators
InactiveUS20180044304A1Suppress and inhibit programmed cell death (PD-1) signaling pathwaySuppress and/or inhibit the programmed cell death 1 (PD-1) signaling pathwayOrganic active ingredientsOrganic chemistryPD-L1Thiadiazoles
The present invention relates to 1,3,4-oxadiazole and thiadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE DISCOVERY TECH
1,3,4-oxadiazole and 1,3,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
Novel Oxadiazole Compounds
InactiveUS20110207704A1Reduce in quantityImprove immunosuppressionBiocideNervous disorderG protein-coupled receptorAgonist
Novel oxadiazole compounds, pharmaceutical compositions containing such compounds and the use of those compounds or compositions as agonists or antagonists of the S1P family of G protein-coupled receptors for treating diseases associated with modulation of S1P family receptor activity, in particular by affording a beneficial immunosuppressive effect are disclosed.
Owner:ABBOTT LAB INC
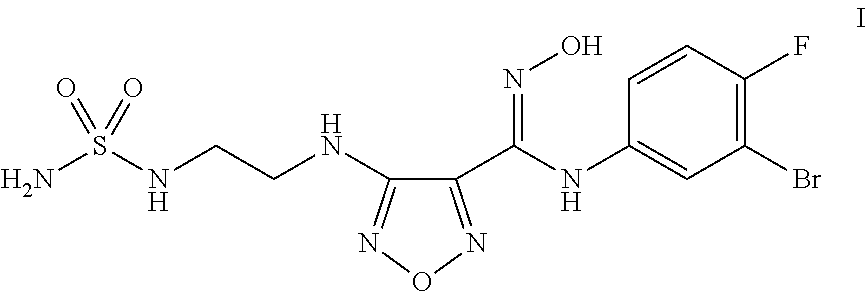
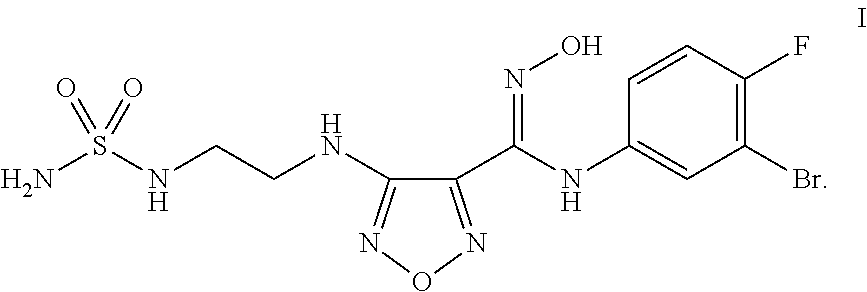
Process for the synthesis of an indoleamine 2,3-dioxygenase inhibitor
ActiveUS20150133674A1Organic active ingredientsCarbamic acid derivatives preparationDioxygenaseStereochemistry
The present application is directed to processes and intermediates for making 4-({2-[(aminosulfonyl)amino]ethyl}amino)-N-(3-bromo-4-fluorophenyl)-N′-hydroxy-1,2,5-oxadiazole-3-carboximidamide, which is an inhibitor of indoleamine 2,3-dioxygenase, useful in the treatment of cancer and other disorders.
Owner:INCYTE
Optically anisotropic film, method of producing the same, and liquid crystal display device using the same
InactiveUS20090087590A1Suppress fluctuationsIncrease production capacityLiquid crystal compositionsOrganic chemistryArylLiquid-crystal display
Disclosed is an optically anisotropic film comprising at least one compound having a partial structure represented by formula (1): where, each of R1, R2 and R3 independently represent a substituent; X represents a divalent linking group; “A” represents —COO—, —OCO—, or a substituted or non-substituted phenylene group, oxadiazole group or alkynylene group; Z represents a substituted or non-substituted alkyl group or aryl group; each of n1, n2 and n3 represents an integer of 0 to 4; and each of l, m and n represents an integer of 0 to 4.
Owner:FUJIFILM CORP
Gas generant and manufacturing method thereof
InactiveUS20050257866A1Improve solubilityPromote aggregationExplosive working-up apparatusPressure gas generationTriazole antifungalsTetrazole
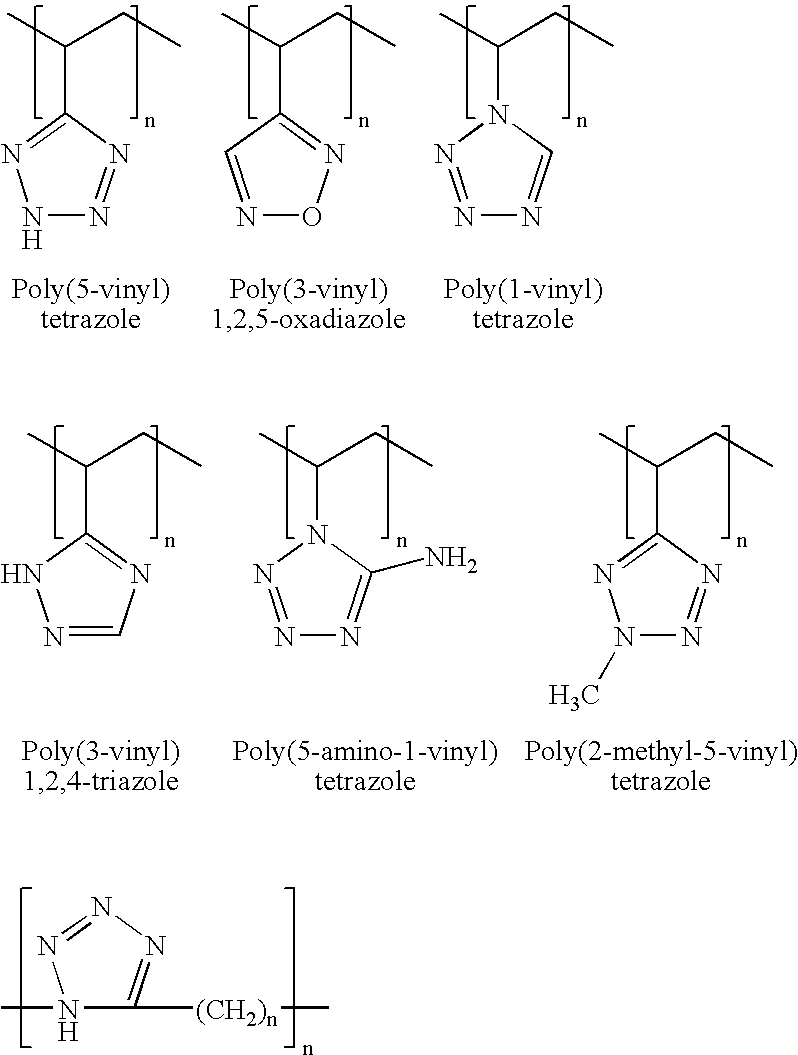
The present invention generally relates to gas generant compositions for inflators of occupant restraint systems, for example. An extrudable pyrotechnic composition includes polyvinylazoles for use within an airbag gas generator. The fuel may be selected from exemplary polyvinylazoles including 5-amino-1-vinyltetrazole, poly(5-vinyltetrazole), poly(2-methyl-5-vinyl) tetrazole, poly(1-vinyl) tetrazole, poly(3-vinyl) 1,2,5 oxadiazole, and poly(3-vinyl) 1,2,4-triazole. An oxidizer is combined with the fuel and preferably contains phase stabilized ammonium nitrate. A novel method of forming the compositions is also presented wherein the various constituents are wetted and / or dissolved, and then cured within the polyvinylazole matrix thereby forming a more intimate combination within the gas generant composition. A vehicle occupant protection system 180, and other gas generating systems, incorporate the compositions of the present invention.
Owner:AUTOMOTIVE SYST LAB
1,3,4-oxadiazole and 1,3,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/768e38c5-0ca4-463d-aa96-15225b7f63ef/US07041685-20060509-C00001.png)
![Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/768e38c5-0ca4-463d-aa96-15225b7f63ef/US07041685-20060509-C00002.png)
![Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof Substituted 3-aryl-5-aryl-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/768e38c5-0ca4-463d-aa96-15225b7f63ef/US07041685-20060509-C00003.png)

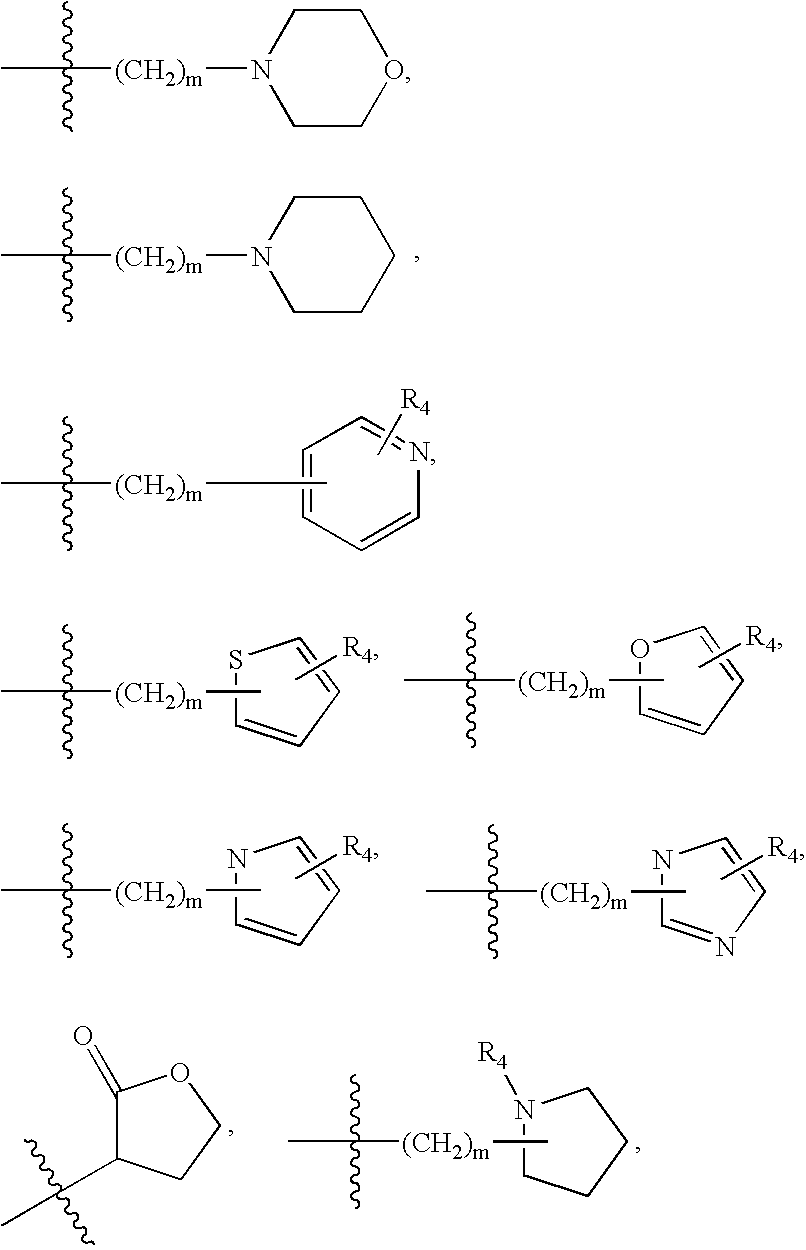
![3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/48af0cf9-bcee-443c-b772-e23fd87dddd1/US07317029-20080108-C00001.png)
![3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/48af0cf9-bcee-443c-b772-e23fd87dddd1/US07317029-20080108-C00002.png)
![3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/48af0cf9-bcee-443c-b772-e23fd87dddd1/US07317029-20080108-C00003.png)
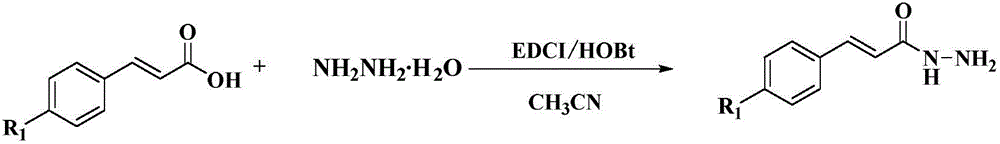
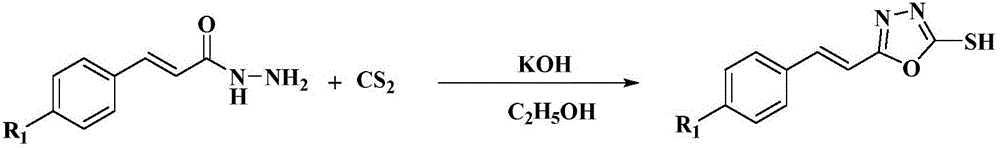
![3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/439e030b-234e-4cd7-9537-32fb6c0d143d/US07144876-20061205-C00001.png)
![3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/439e030b-234e-4cd7-9537-32fb6c0d143d/US07144876-20061205-C00002.png)
![3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof 3,5-Disubstituted-[1,2,4]-oxadiazoles and analogs as activators of caspases and inducers of apoptosis and the use thereof](https://images-eureka.patsnap.com/patent_img/439e030b-234e-4cd7-9537-32fb6c0d143d/US07144876-20061205-C00003.png)
![Dihydro-1h-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives which act as s1p1 agonists Dihydro-1h-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives which act as s1p1 agonists](https://images-eureka.patsnap.com/patent_img/46052328-f670-4632-9214-3658d5fe9461/US20100292233A1-20101118-D00000.png)
![Dihydro-1h-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives which act as s1p1 agonists Dihydro-1h-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives which act as s1p1 agonists](https://images-eureka.patsnap.com/patent_img/46052328-f670-4632-9214-3658d5fe9461/US20100292233A1-20101118-D00001.png)
![Dihydro-1h-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives which act as s1p1 agonists Dihydro-1h-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivatives which act as s1p1 agonists](https://images-eureka.patsnap.com/patent_img/46052328-f670-4632-9214-3658d5fe9461/US20100292233A1-20101118-D00002.png)