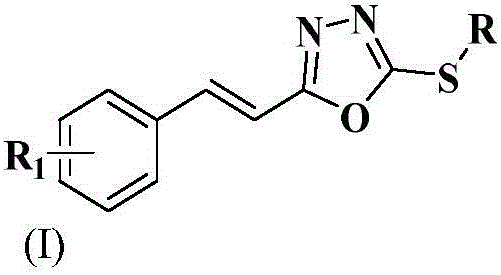
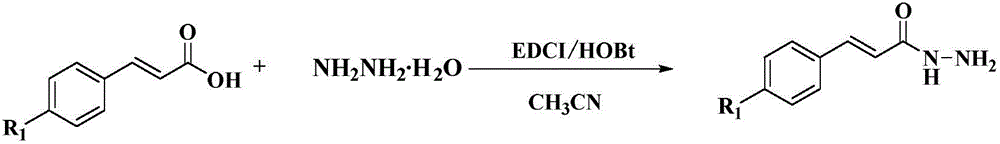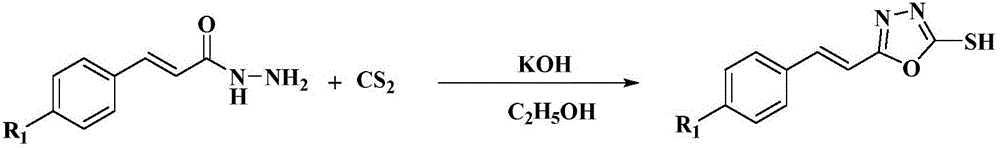Styryl-containing 1,3,4-oxadiazole thioether compound, as well as preparation method and application thereof
A technology based on oxadiazole sulfide and styrene, which is applied in the chemical industry to achieve the effect of great development and application value and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1, the synthesis of (E)-2-methylthio-5-styryl-1,3,4-oxadiazole (the compound number is a):
[0049] (1) Synthesis of (E)-cinnamic acid hydrazide:
[0050]Add (6.00g, 40.50mmol) cinnamic acid to a 100mL three-necked flask, add 80mL acetonitrile as a solvent, stir until uniform at room temperature, then take (6.57g, 48.60mmol) HOBt and add it to a three-necked flask, and weigh (9.23g , 48.60mmol) EDCI was added to the reaction system portion by portion, and the reaction system changed from white turbidity to clear transparent liquid, followed by a large amount of white solid, which was an active intermediate salt. The reaction progress was tracked by TLC. The developer was ethyl acetate (v):petroleum ether (v)=10:1, and the cinnamic acid was completely consumed after 2 hours of reaction. Keeping the reaction temperature at 5° C., 80% hydrazine hydrate (5.07 g, 80.99 mmol) solution was slowly added dropwise to the system, and the dropwise addition was completed wi...
Embodiment 2
[0055] Example 2, the synthesis of (E)-2-ethylthio-5-styryl-1,3,4-oxadiazole (the compound number is b):
[0056] (1) with embodiment 1 (1) step;
[0057] (2) with embodiment 1 (2) step;
[0058] (3) Add (0.30g, 1.47mmol) 5-styryl-1,3,4-oxadiazole-2-thiol and (0.10g, 2.45mmol) KOH in sequence to a 50mL three-necked flask, and then add 20mL of water As a solvent, stir at room temperature until all mercaptans are dissolved. Then weigh (0.23g, 2.45mmol) diethyl sulfate and dissolve it in 2mL of absolute ethanol, and slowly drop it into the above reaction system in a constant pressure funnel. After the completion of the reaction, suction filtration was performed directly, and the filter cake was recrystallized with absolute ethanol to obtain a white solid with a yield of 80.6%.
Embodiment 3
[0059] Embodiment 3, the synthesis of (E)-2-styryl-5-((3,4,4-trifluoro-3-en-1-yl)sulfur)-1,3,4-oxadiazole ( Compound number is c):
[0060] (1) with embodiment 1 (1) step;
[0061] (2) with embodiment 1 (2) step;
[0062] (3) Add (0.3g, 1.47mmol) (E)-5-styryl-1,3,4-oxadiazole-2-thiol and (0.27g, 2.00mmol) potassium carbonate successively to the reactor, Use 20mL of acetonitrile as solvent, then take 0.02g of 1,1,2-trifluoro-4-bromo-1-butene, and heat to reflux for 4h to complete the reaction. After the reaction was completed, 5 mL of distilled water was added to the system, the mixed solution was extracted with 3×20 mL of dichloromethane, the organic phase was dried with anhydrous magnesium sulfate, the solvent was removed by distillation under reduced pressure, and recrystallized from absolute ethanol to obtain a light yellow solid, which was collected The rate is 81.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com