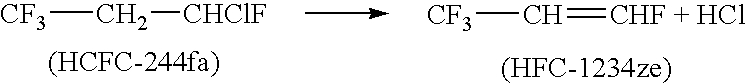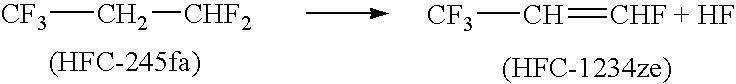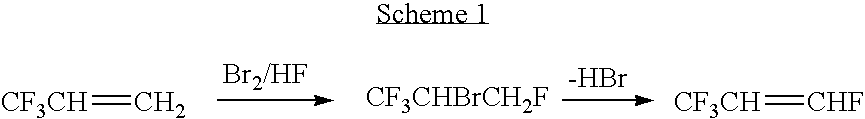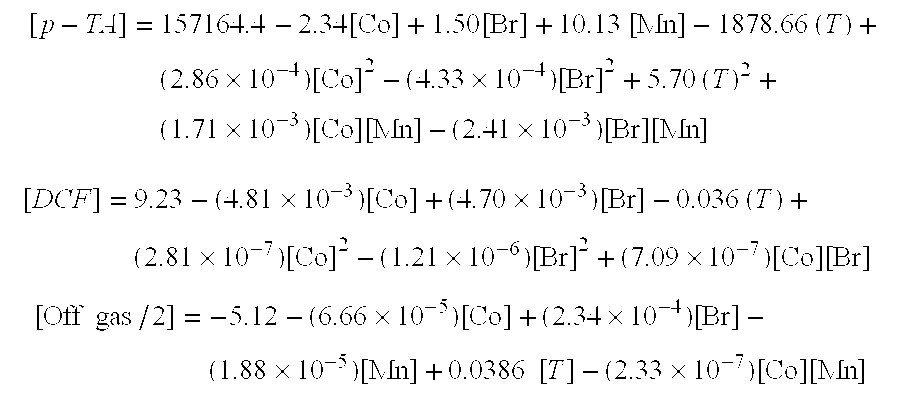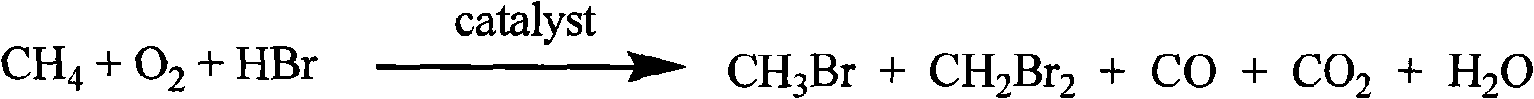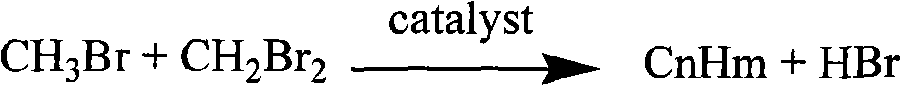Patents
Literature
8244 results about "Bromine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bromine is a chemical element with symbol Br and atomic number 35. It is the third-lightest halogen, and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas. Its properties are thus intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek βρῶμος ("stench"), referencing its sharp and disagreeable smell.
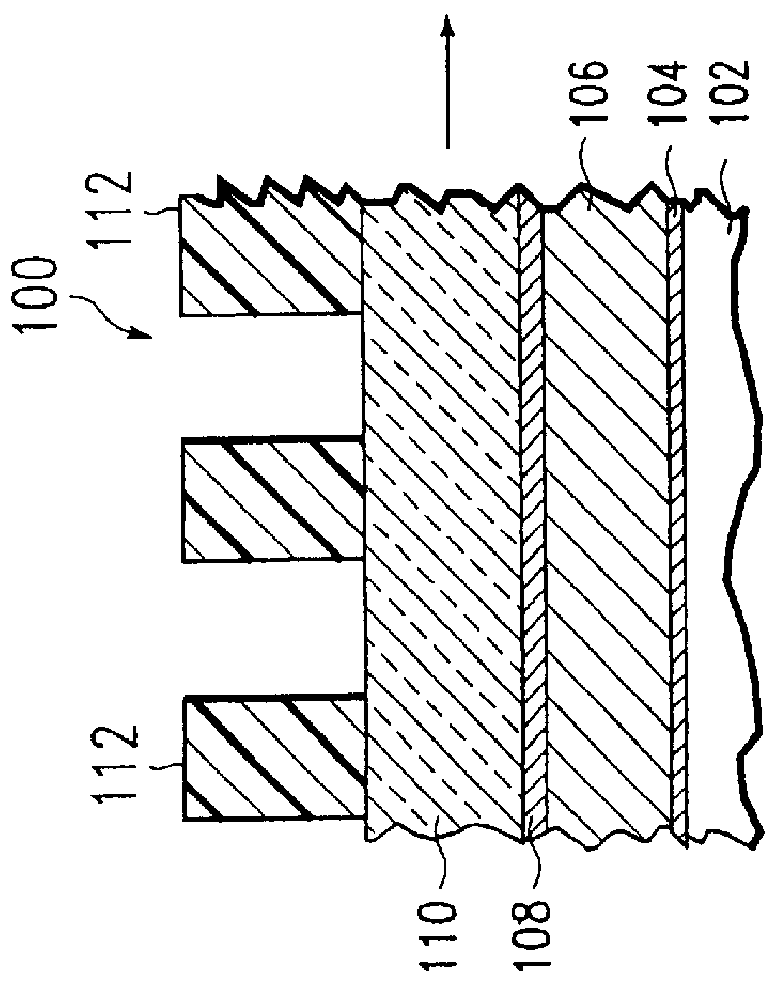
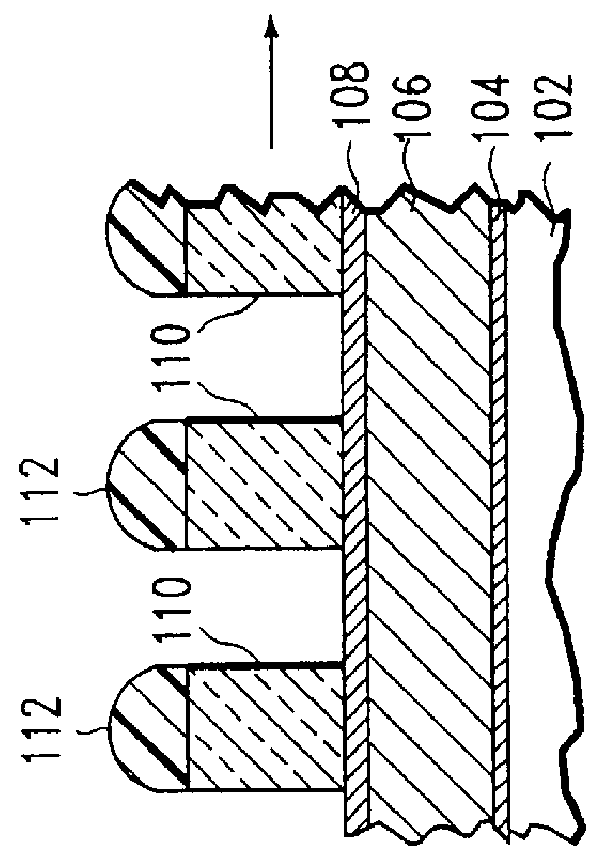
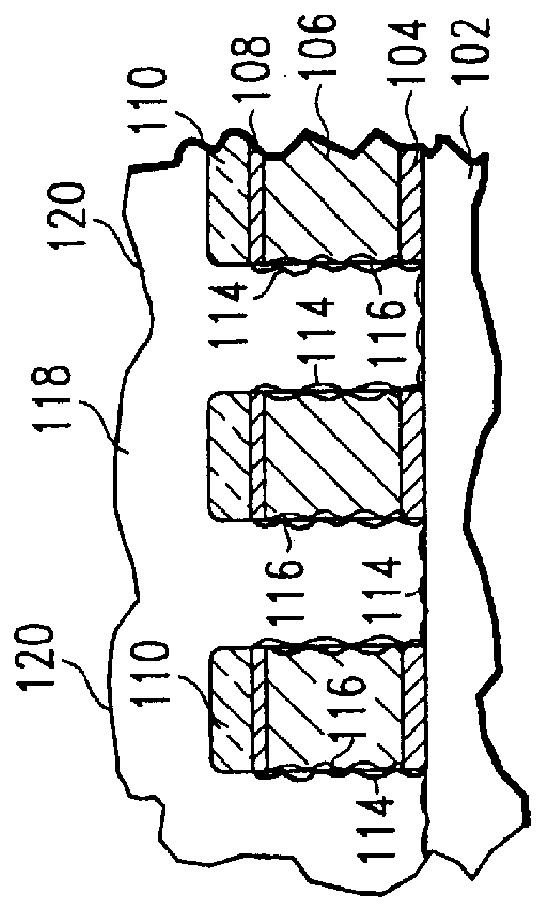
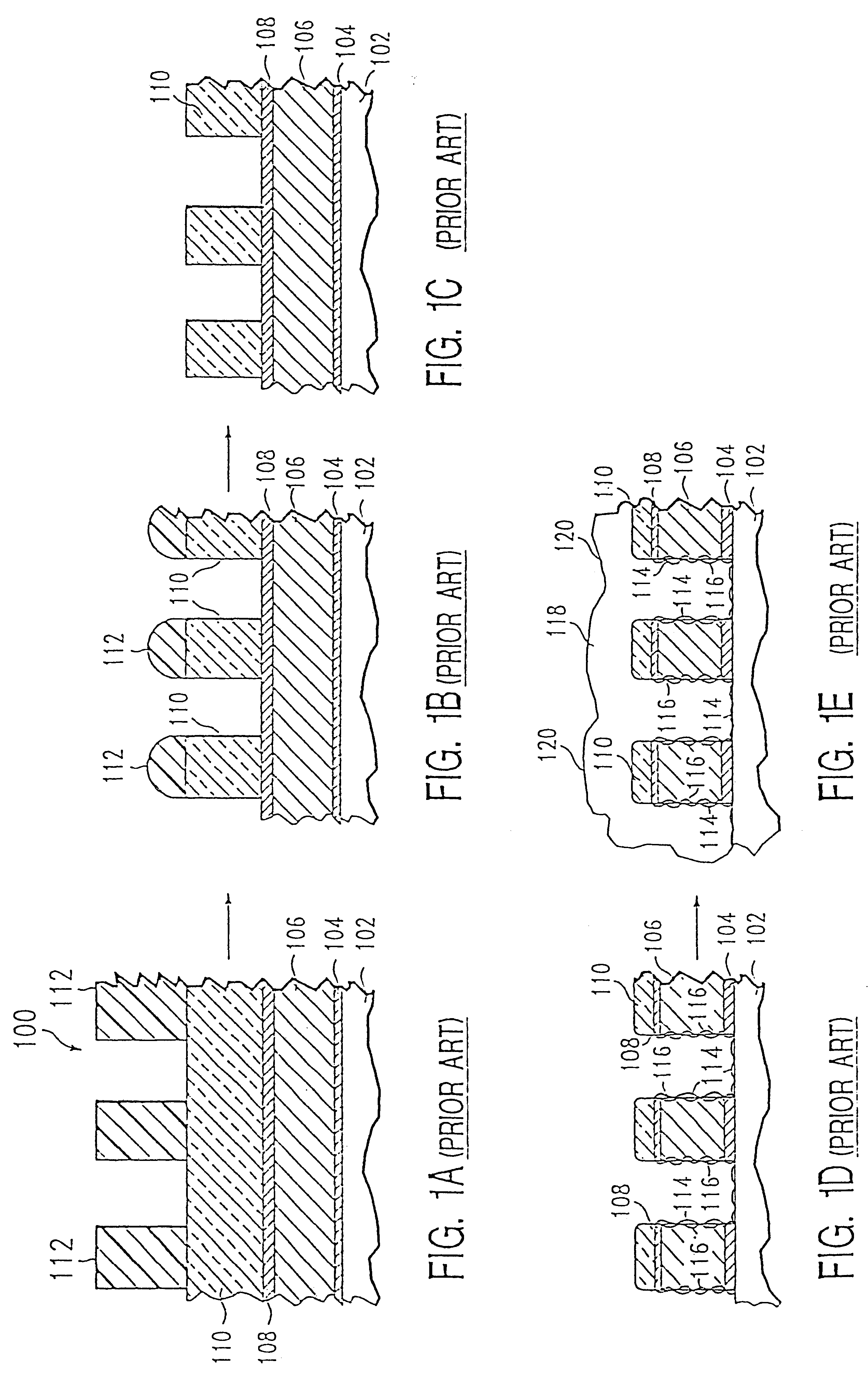
Method of etching patterned layers useful as masking during subsequent etching or for damascene structures
InactiveUS6080529APhotomechanical apparatusSemiconductor/solid-state device manufacturingConductive polymerOrganic base
A first embodiment of the present invention pertains to a method of patterning a semiconductor device conductive feature while permitting easy removal of any residual masking layer which remains after completion of the etching process. A multi-layered masking structure is used which includes a layer of high-temperature organic-based masking material overlaid by either a patterned layer of inorganic masking material or by a layer of patterned high-temperature imageable organic masking material. The inorganic masking material is used to transfer a pattern to the high-temperature organic-based masking material and is then removed. The high-temperature organic-based masking material is used to transfer the pattern and then may be removed if desired. This method is also useful in the pattern etching of aluminum, even though aluminum can be etched at lower temperatures. A second embodiment of the present invention pertains to a specialized etch chemistry useful in the patterning of organic polymeric layers such as low k dielectrics, or other organic polymeric interfacial layers. This etch chemistry is useful for mask opening during the etch of a conductive layer or is useful in etching damascene structures where a metal fill layer is applied over the surface of a patterned organic-based dielectric layer. The etch chemistry provides for the use of etchant plasma species which minimize oxygen, fluorine, chlorine, and bromine content.
Owner:APPLIED MATERIALS INC
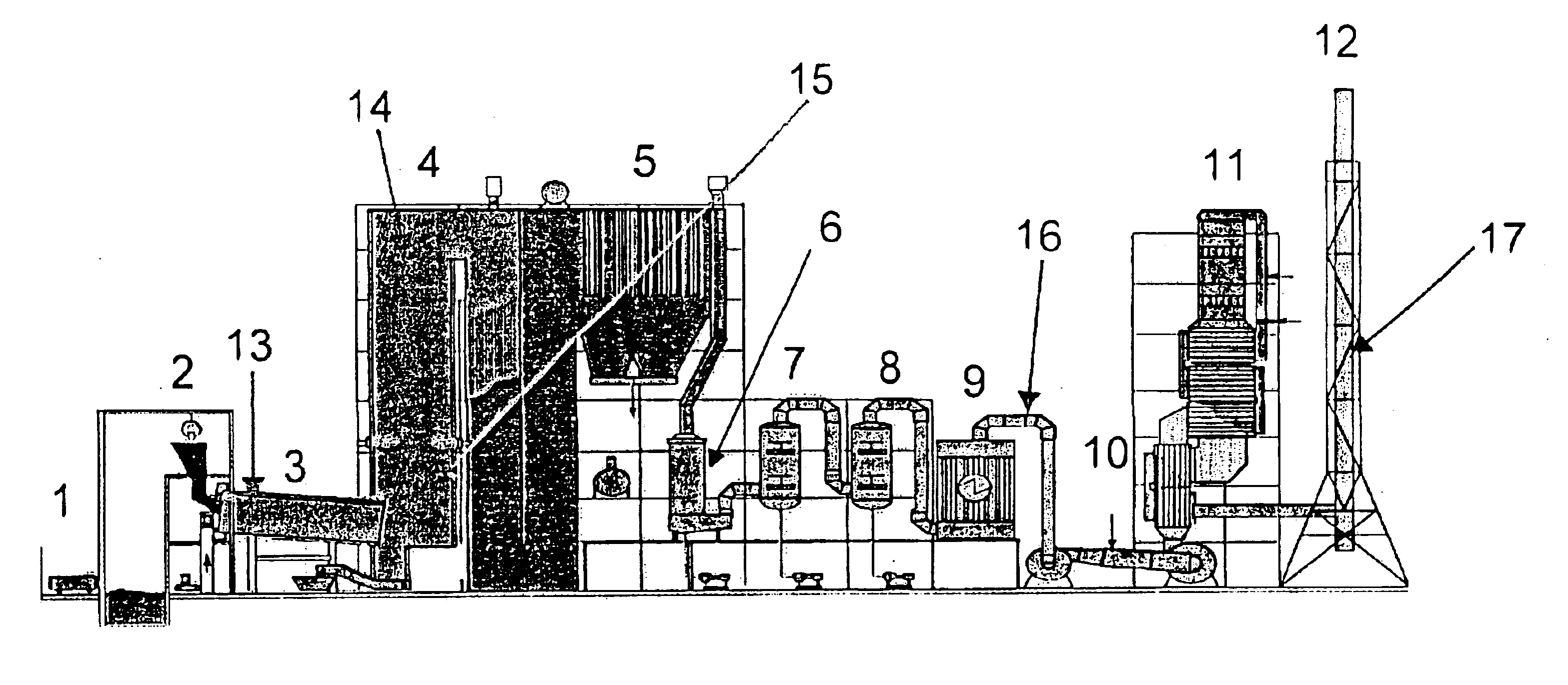
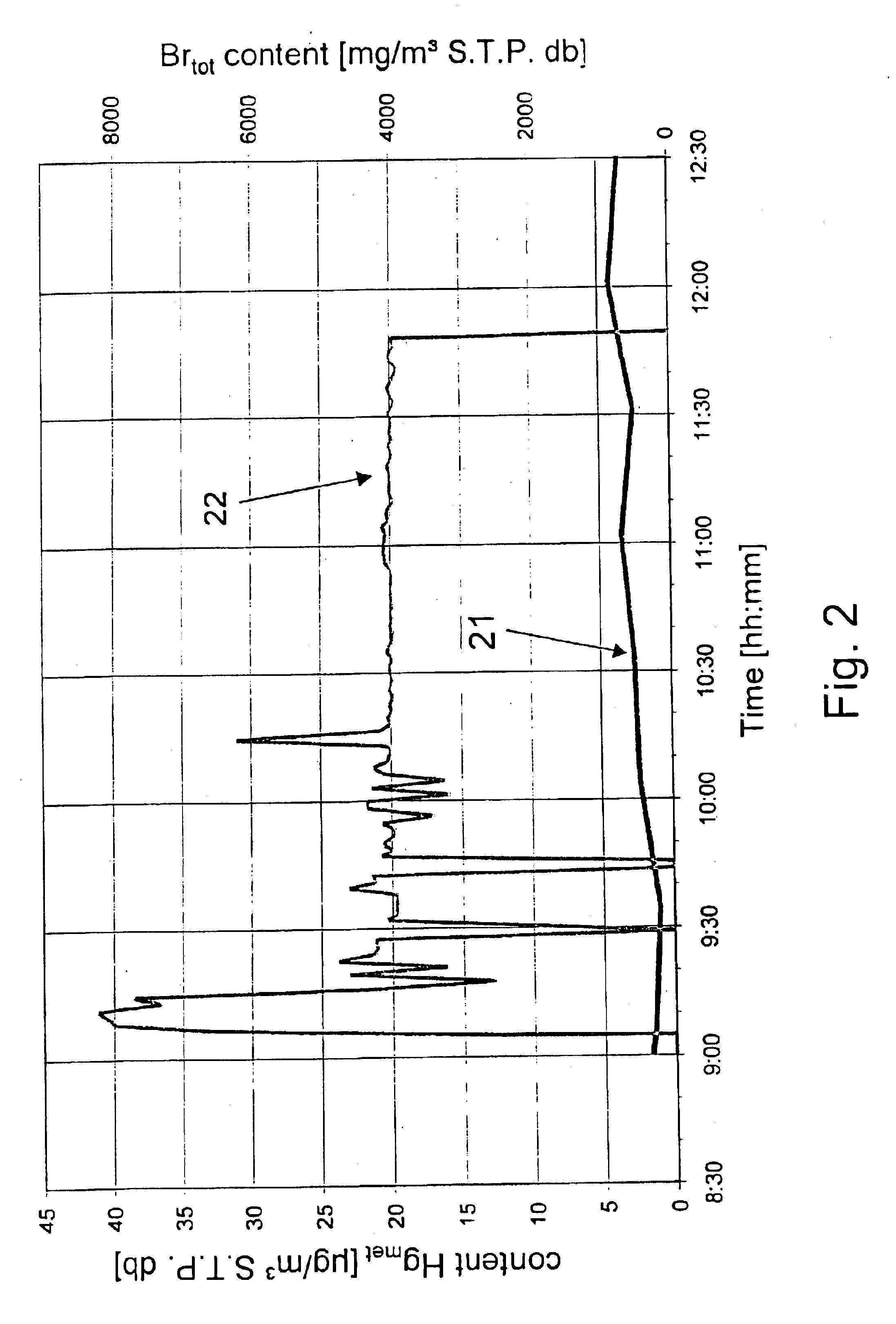
Process for removing mercury from flue gases
InactiveUS6878358B2Reduce operating costsSmall amountUsing liquid separation agentChemical/physical processesPower stationCombustion
Process for removing mercury from flue gases of high-temperature plants, in particular power stations and waste incineration plants in which a bromine compound is fed to the multistage furnace and / or the flue gas in a plant section downstream of the furnace, the temperature during contact of the bromine compound with the flue gas being at least 500° C., preferably at least 800° C. The combustion is carried out in the presence of a sulphur compound, in particular sulphur dioxide. Subsequently to the furnace, the flue gas is subjected to an optional multistage cleanup for removing mercury from the flue gas, which cleanup comprises a wet scrubber and / or a dry cleanup.
Owner:BROMERC
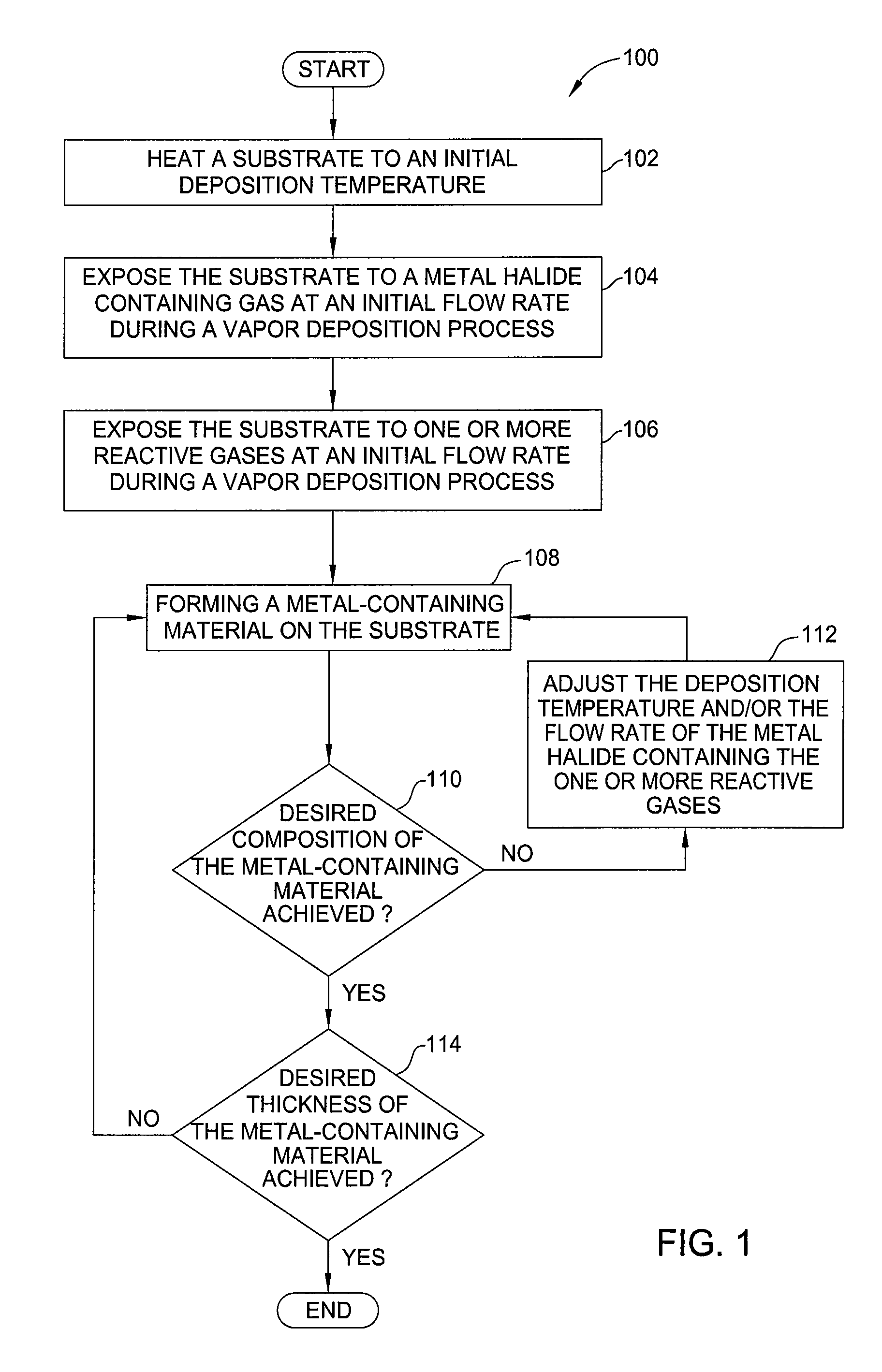
Nmos metal gate materials, manufacturing methods, and equipment using CVD and ald processes with metal based precursors
ActiveUS20110263115A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseMetallic materials
Embodiments of the invention generally provide methods for depositing metal-containing materials and compositions thereof. The methods include deposition processes that form metal, metal carbide, metal silicide, metal nitride, and metal carbide derivatives by a vapor deposition process, including thermal decomposition, CVD, pulsed-CVD, or ALD. In one embodiment, a method for processing a substrate is provided which includes depositing a dielectric material having a dielectric constant greater than 10, forming a feature definition in the dielectric material, depositing a work function material conformally on the sidewalls and bottom of the feature definition, and depositing a metal gate fill material on the work function material to fill the feature definition, wherein the work function material is deposited by reacting at least one metal-halide precursor having the formula MXY, wherein M is tantalum, hafnium, titanium, and lanthanum, X is a halide selected from the group of fluorine, chlorine, bromine, or iodine, and y is from 3 to 5.
Owner:APPLIED MATERIALS INC
Method of fabricating a gate structure of a field effect transistor having a metal-containing gate electrode
InactiveUS20050009358A1High selectivitySemiconductor/solid-state device detailsSolid-state devicesBromineTitanium nitride
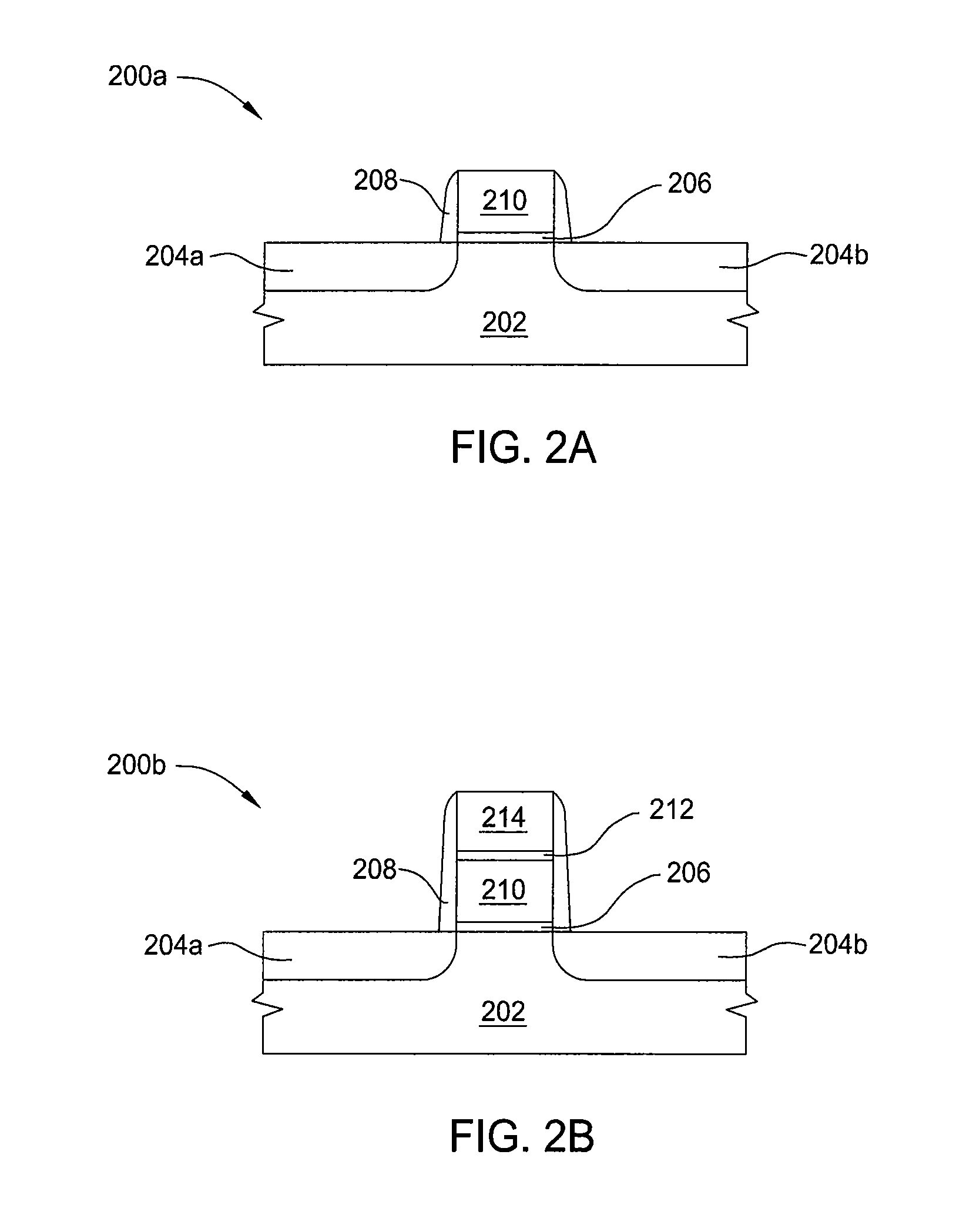
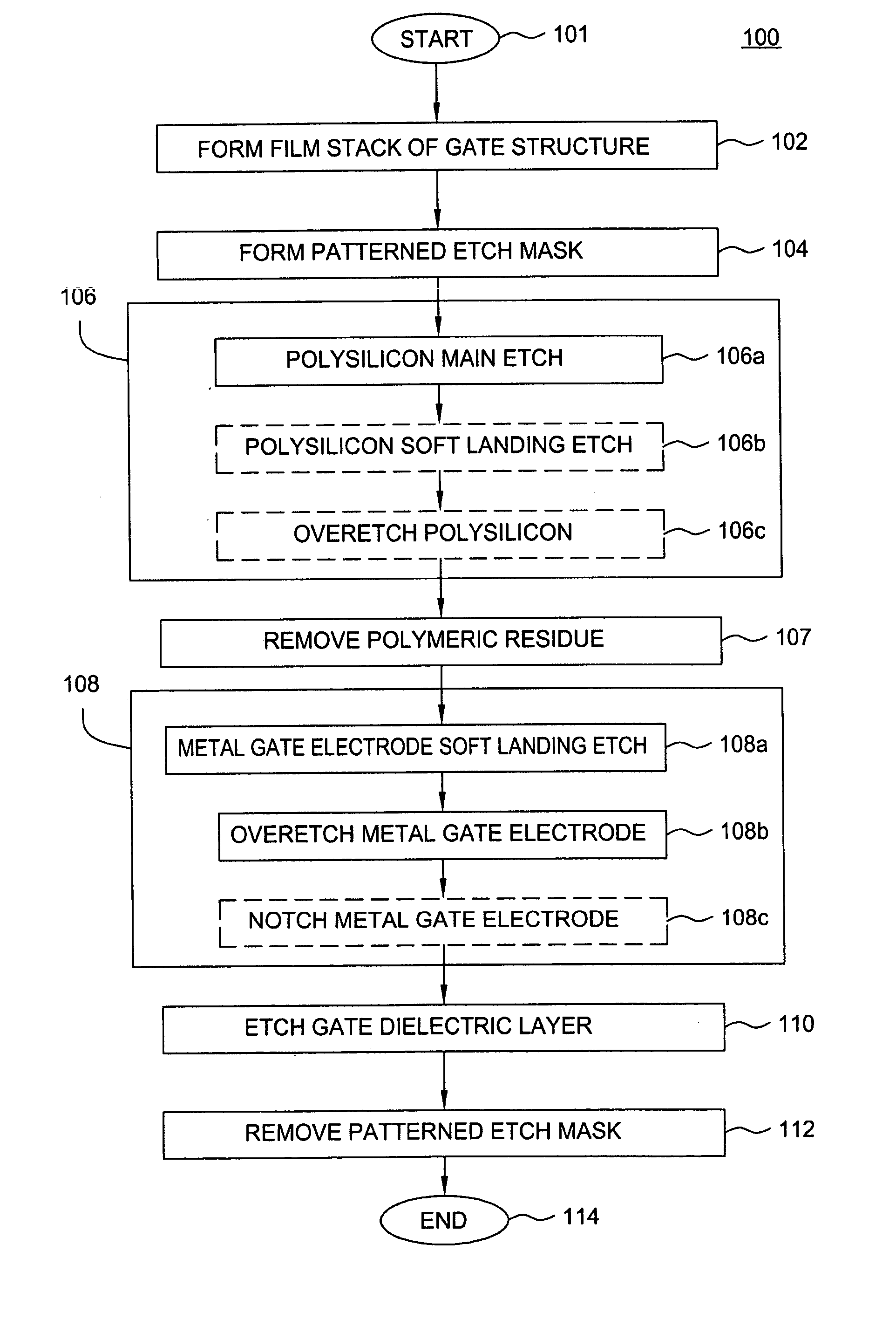
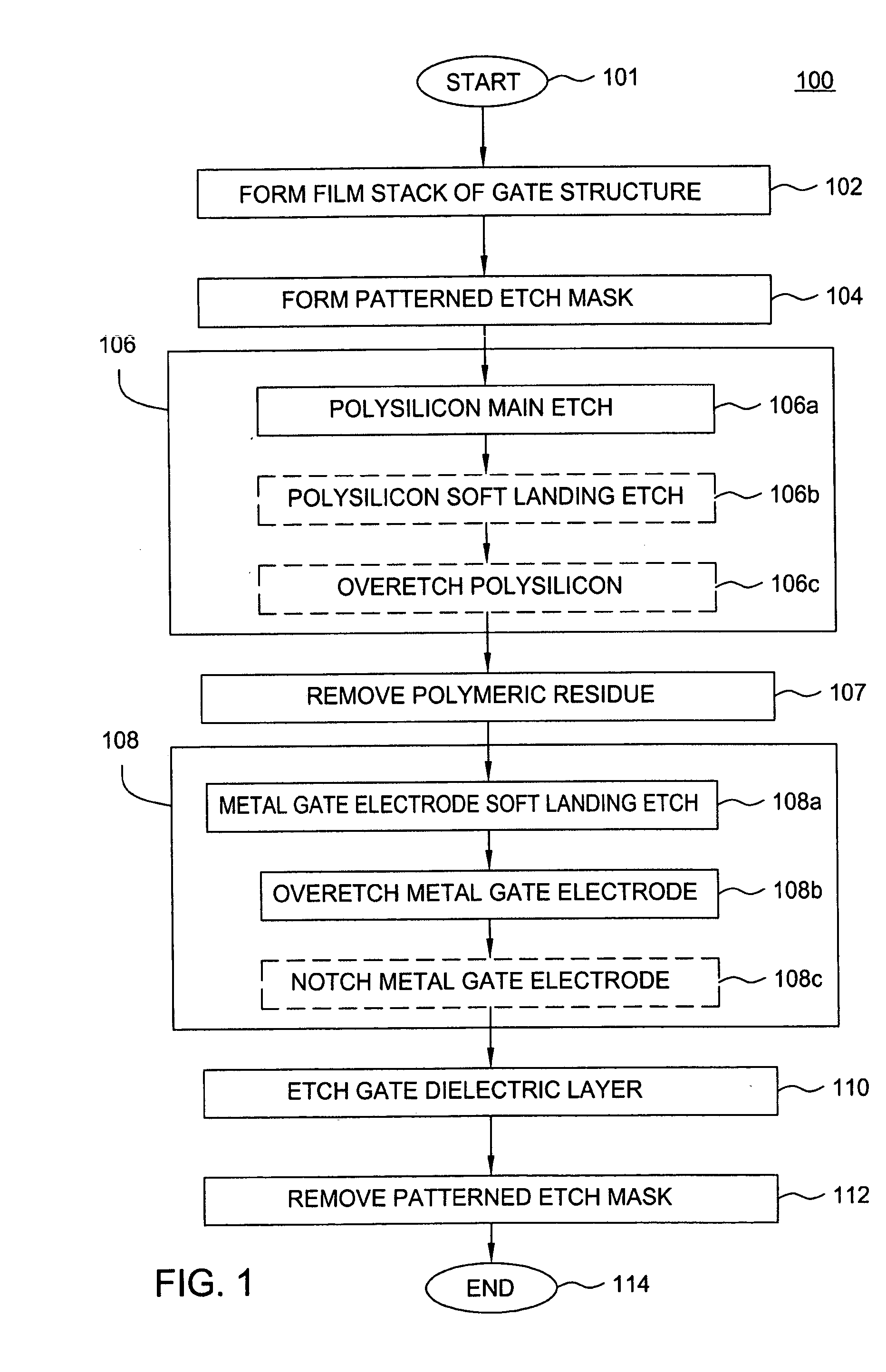
A method of etching metals and / or metal-containing compounds using a plasma comprising a bromine-containing gas. In one embodiment, the method is used during fabrication of a gate structure of a field effect transistor having a titanium nitride gate electrode, an ultra-thin (about 10 to 20 Angstroms) silicon dioxide gate dielectric, and a polysilicon upper contact. In a further embodiment, the gate electrode is selectively notched to a pre-determined width.
Owner:APPLIED MATERIALS INC
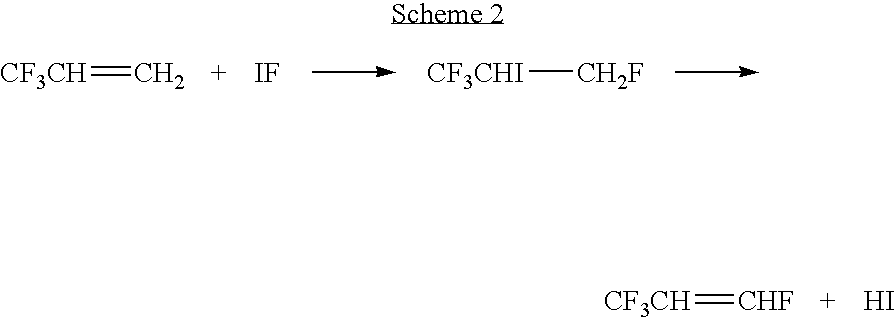
Process for producing fluoropropenes
InactiveUS7230146B2Simplifies isolationPreparation by hydrogen halide split-offPreparation by halogen halide additionHalogenHydrogen
Dehydrohalogenation processes for the preparation of fluoropropenes from corresponding halopropanes, in which the fluoropropenes have the formula CF3CY═CXNHP, wherein X and Y are independently hydrogen or a halogen selected from fluorine, chlorine, bromine and iodine; and N and P are independently integers equal to 0, 1 or 2, provided that (N+P)=2.
Owner:HONEYWELL INT INC
Zirconium oxide and hafnium oxide etching using halogen containing chemicals
ActiveUS20050164479A1Effective meanSemiconductor/solid-state device manufacturingSemiconductor devicesGate dielectricHafnium
A method is described for selectively etching a high k dielectric layer that is preferably a hafnium or zirconium oxide, silicate, nitride, or oxynitride with a selectivity of greater than 2:1 relative to silicon oxide, polysilicon, or silicon. The plasma etch chemistry is comprised of one or more halogen containing gases such as CF4, CHF3, CH2F2, CH3F, C4F8, C4F6, C5F6, BCl3, Br2, HF, HCl, HBr, HI, and NF3 and leaves no etch residues. An inert gas or an inert gas and oxidant gas may be added to the halogen containing gas. In one embodiment, a high k gate dielectric layer is removed on portions of an active area in a MOS transistor. Alternatively, the high k dielectric layer is used in a capacitor between two conducting layers and is selectively removed from portions of an ILD layer.
Owner:TAIWAN SEMICON MFG CO LTD
Method for anisotropic plasma-chemical dry etching of silicon nitride layers using a gas mixture containing fluorine
InactiveUS6569773B1Improve uniformityHigh atomic weightSemiconductor/solid-state device manufacturingSilicon oxideOxygen
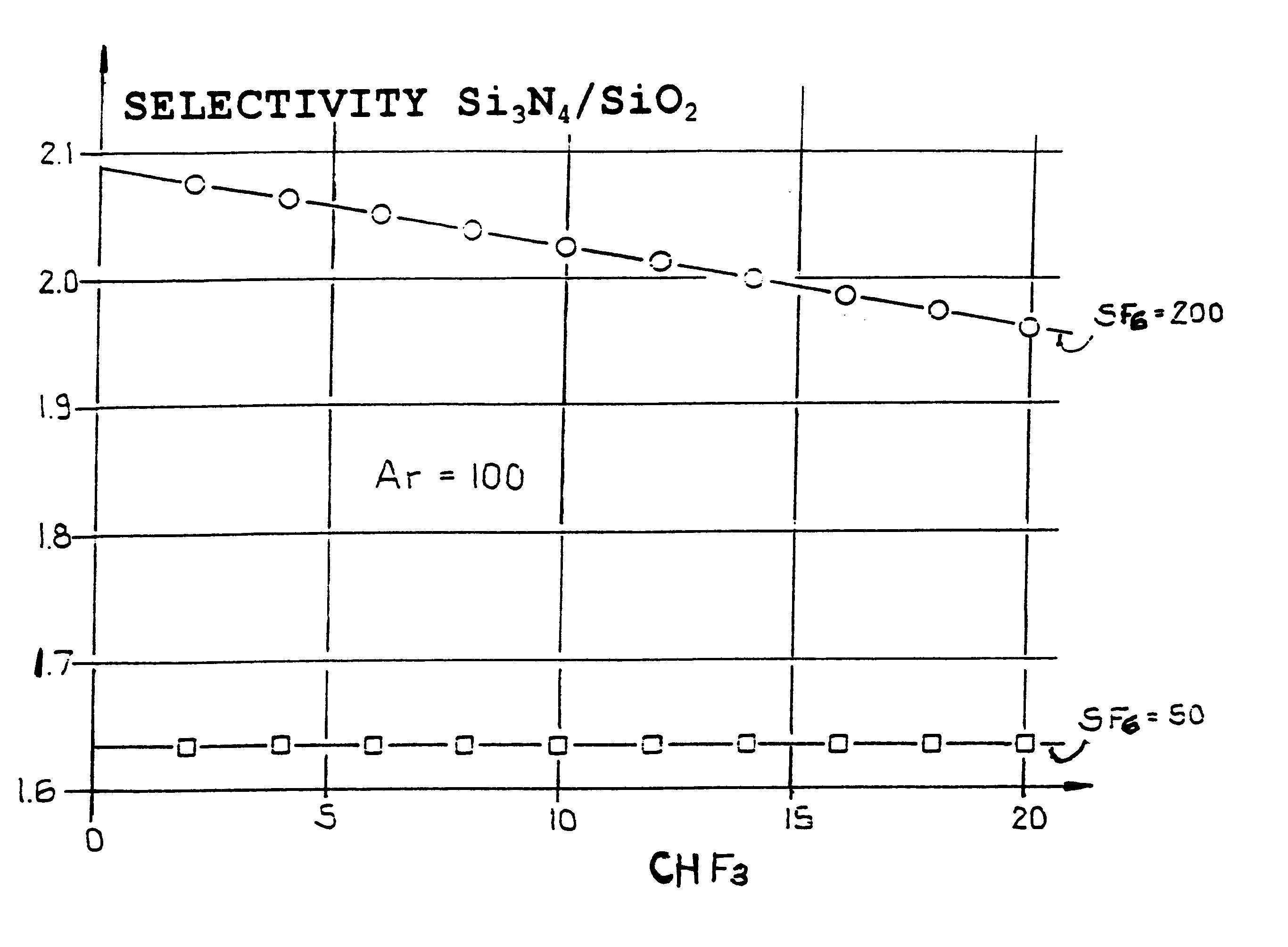
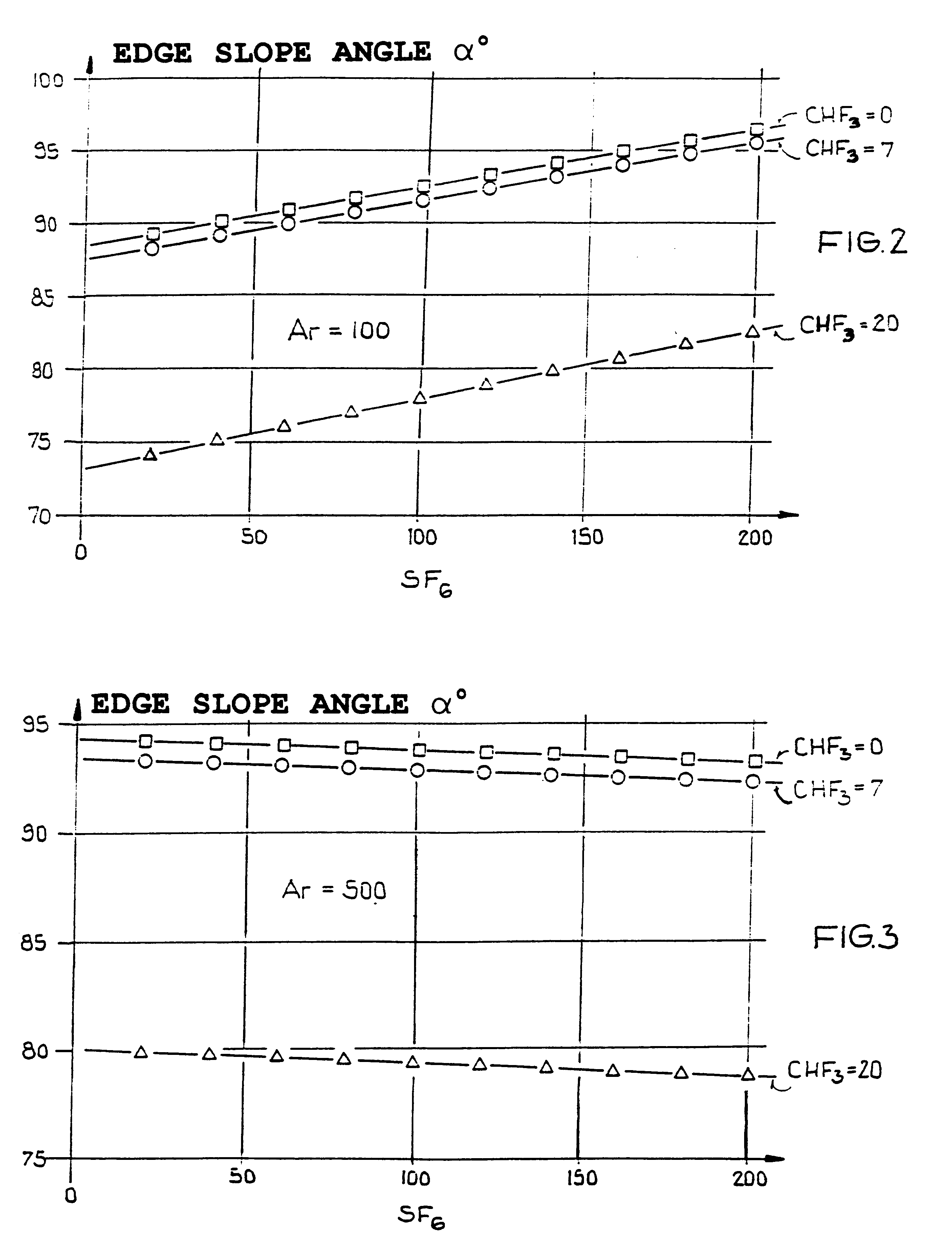
An etching gas mixture containing CHF3, SF6 and a non-oxidizing gas such as Ar is used as an etching gas mixture for the anisotropic plasma-chemical dry-etching of a silicon nitride layer differentially or selectively relative to a silicon oxide layer. The gas mixture does not contain oxygen, chlorine, bromine, iodine or halides in addition to the above mentioned constituents, so that the process can be carried out in reactor systems equipped with oxidizable electrodes. By adjusting the gas flow rates or composition ratios of CHF3, SF6, and argon in the etching gas mixture, it is possible to adjust the resulting etching selectivity of silicon nitride relative to silicon oxide, and the particular edge slope angle of the etched edge of the remaining silicon nitride layer. A high etch rate for the silicon nitride is simultaneously achieved.
Owner:ATMEL CORP +1
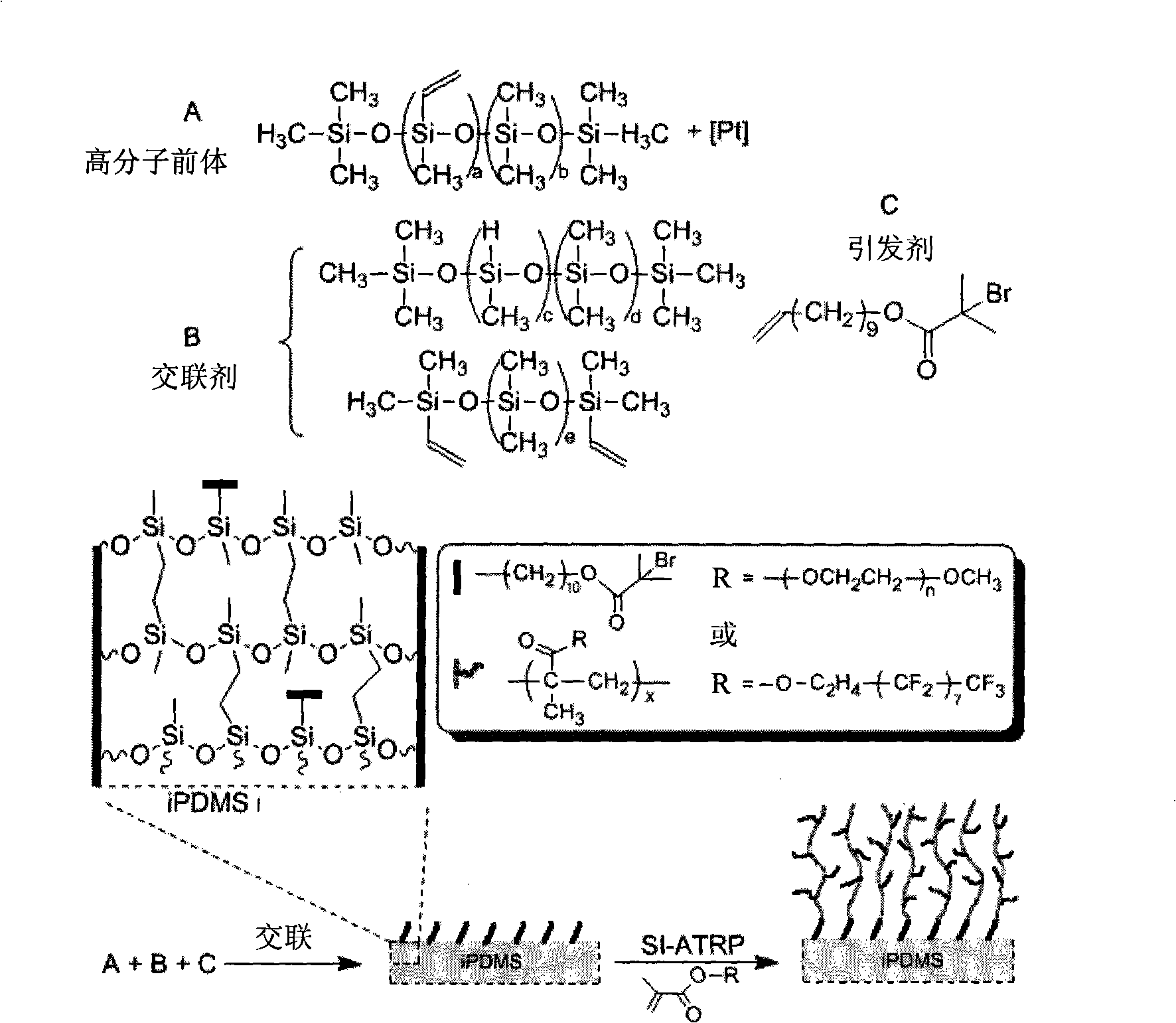
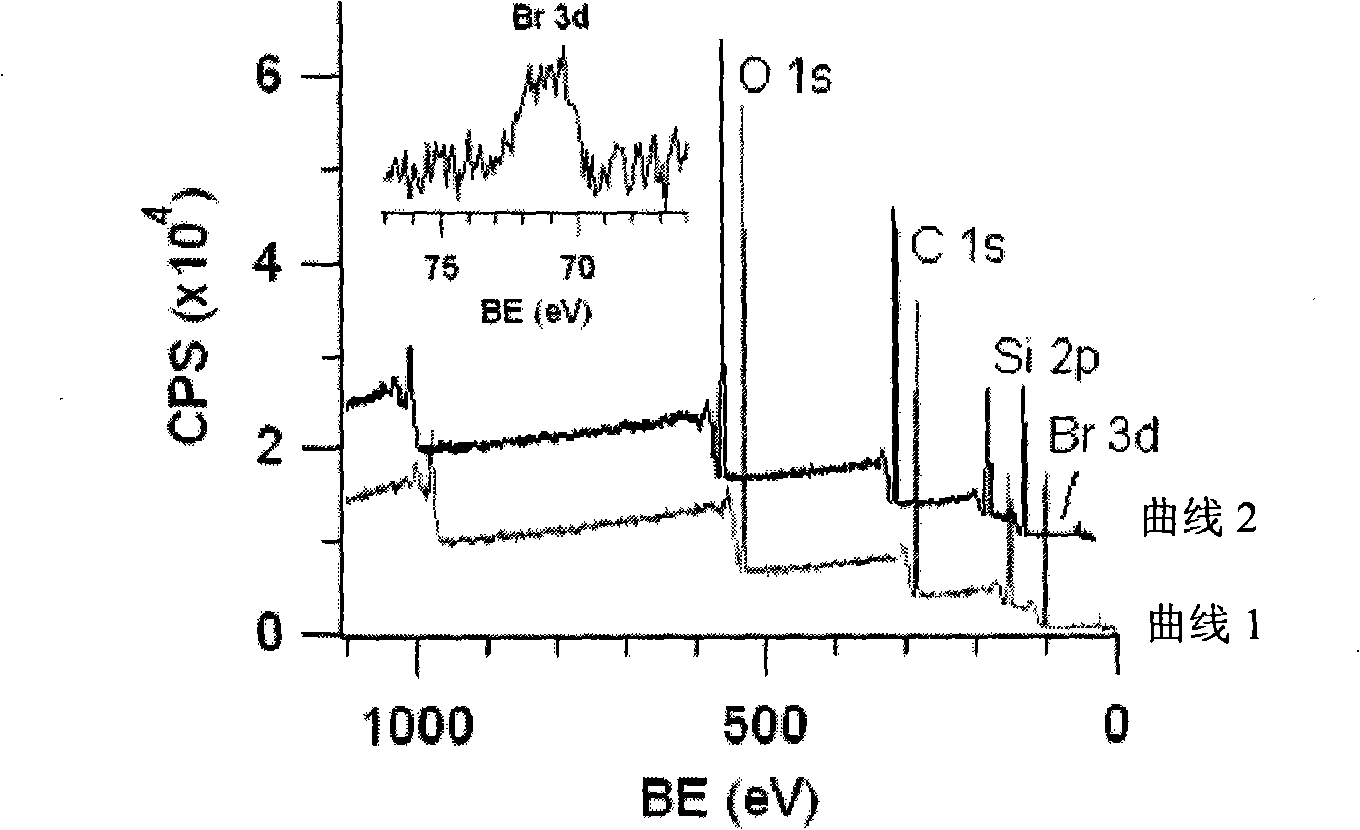
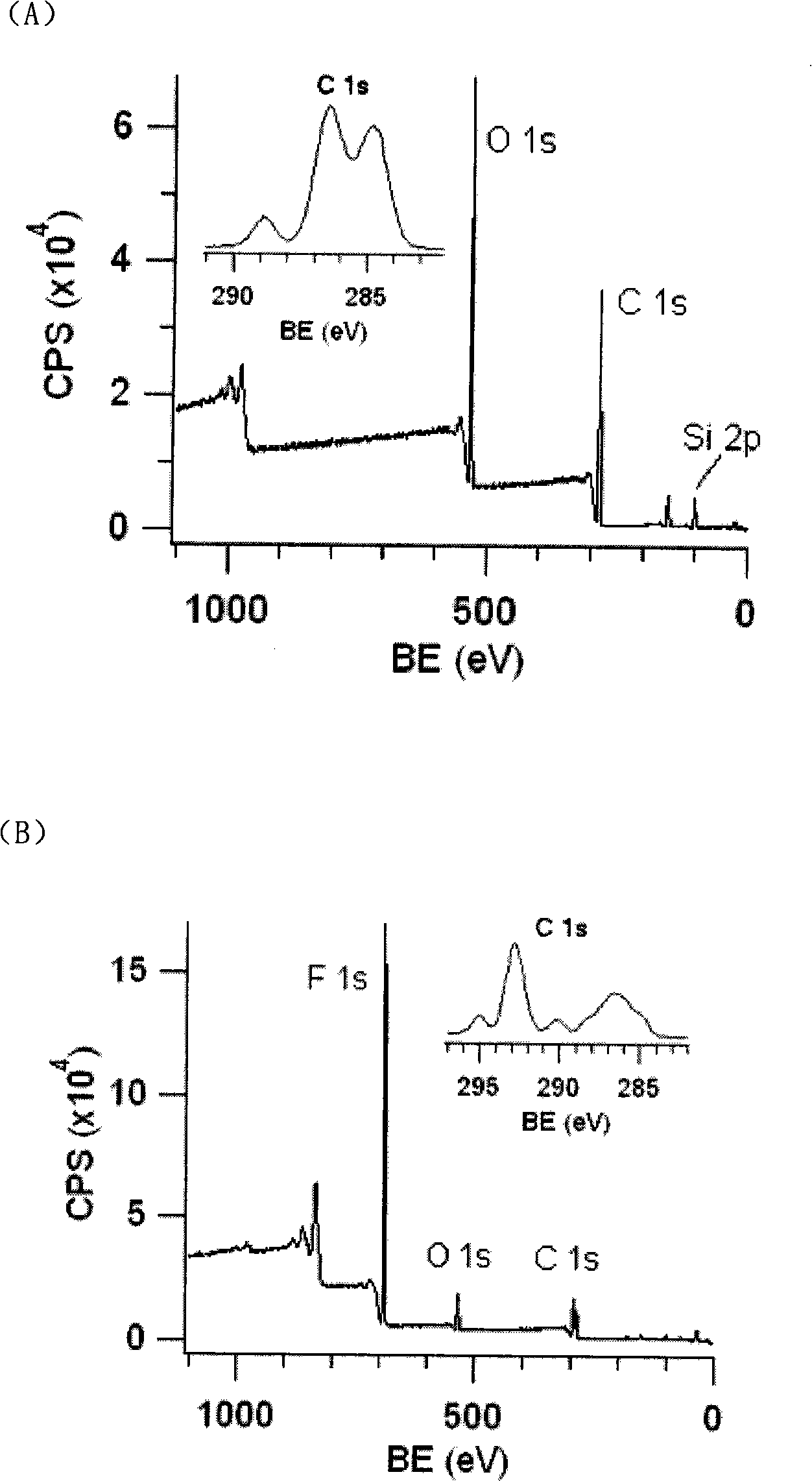
Polydimethylsiloxane with initiator on surface and its preparation method and use
The invention relates to polydimethylsiloxane which is provided with an initiator at the surface, and is the silicon-hydrogen bonding cross-linked polydimethylsiloxane, of which the surface contains 0.01 to 1At percent 2-bromine-2-methyl propioric acid 10-undecene ester. The material is formed by mixing a polymer precursor, a cross-liner and the initiator with olefinic end in the weight ratio of 10: 1: 4-0.01 and placing for 6 to 24 hours. The polydimethylsiloxane with the initiator at the surface which is provided by the invention further modifies a function layer on the surface by triggering the polymerization reaction on the surface, so the polydimethylsiloxane can be applied on biocompatible, organic solvent compatible and thermal sensitive materials. The invention uses the simple and convenient method to realize the universal, permanent, diverse and functional surface modified polydimethylsiloxane.
Owner:SUZHOU SIJU BIOMATERIALS
Precursors and processes for low temperature selective epitaxial growth
InactiveUS20090087967A1Practical and cost-effective SEGReduce processingDecorative surface effectsSemiconductor/solid-state device manufacturingBromineGas phase
This invention generally relates to low temperature epitaxy. More specifically, this invention relates to processes for achieving low temperature selective epitaxial growth by chemical vapor deposition of source precursors containing Si or Ge in the presence of bromine or iodine, compositions containing precursors and brominated or iodinated compounds suitable for achieving selective epitaxial growth using the processes, epitaxial layers made using the processes, devices and other types of structures made using the processes, and processes for cleaning epitaxy reactor chambers using a bromine etchant source.
Owner:TODD MICHAEL A
Processes for synthesis of tetrafluoropropene
ActiveUS7189884B2Preparation by hydrogen halide split-offPreparation by halogen halide additionBromineCombinatorial chemistry
Disclosed in one embodiment is a process for the synthesis of 1,3,3,3-tetrafluoropropene that comprises (a) reacting a compound of formula (I) X1X2 with a compound of formula (II) CF3CH═CH2 to produce a reaction product comprising a compound of formula (III) CF3CHX1CH2X2, wherein X1 and X2 are each independently selected from the group consisting of hydrogen, chlorine, bromine and iodine, provided that X1 and X2 are not both hydrogen; (b) when X2 in formula (III) is not fluorine, fluorinating the compound of formula (III) to produce a reaction product comprising a compound of formula (III) wherein X1 is as described above and X2 is fluorine; and (c) exposing said compound of formula (III) to reaction conditions effective to convert said compound to 1,3,3,3-tetrafluoropropene.
Owner:HONEYWELL INT INC
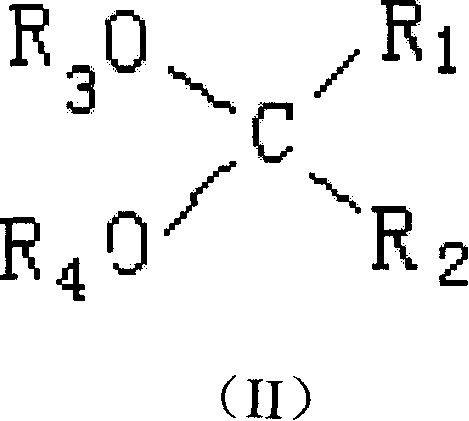
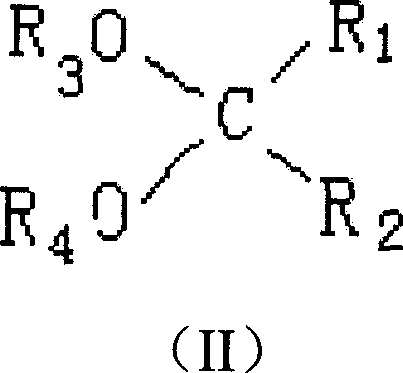
Adduct of magnesium halides, preparation method, and application
This invention provides a kind of magnesium halide adducts, whose general chemical formula is MgX2-mROH-nE-pH2O (I), wherein, X is Cl or Br; R is C1-C12 alkyl, C3-C10 cycloalkyl or C6-C10 aryl; E is dialkoxy hydrocarbon compound as shown in formula (II); m is 1-5; n is 0.005-1.0; p is 0-0.8. In formula (II), R1, R2, R3 and R4 are the same or different, and are H or C1-C10 linear or branched alkyl, C3-C10 cycloalkyl, C6-C10 aryl, C7-C10 alkylaryl or arylalkyl; two or more groups of R1 and R2 can bond into one or several fused rings; H is aryl or alkylaryl or H in benzene ring of arylalkyl can be substituted by halogen.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method of pattern etching a low K dielectric layer
InactiveUS6331380B1Photomechanical apparatusSemiconductor/solid-state device manufacturingOrganic baseOxygen
A first embodiment of the present invention pertains to a method of patterning a semiconductor device conductive feature while permitting easy removal of any residual masking layer which remains after completion of the etching process. A multi-layered masking structure is used which includes a layer of high-temperature organic-based masking material overlaid by either a patterned layer of inorganic masking material or by a layer of patterned high-temperature imageable organic masking material. The inorganic masking material is used to transfer a pattern to the high-temperature organic-based masking material and is then removed. The high-temperature organic-based masking material is used to transfer the pattern and then may be removed if desired. This method is also useful in the pattern etching of aluminum, even though aluminum can be etched at lower temperatures. A second embodiment of the present invention pertains to a specialized etch chemistry useful in the patterning of organic polymeric layers such as low k dielectrics, or other organic polymeric interfacial layers. This etch chemistry is useful for mask opening during the etch of a conductive layer or is useful in etching damascene structures where a metal fill layer is applied over the surface of a patterned organic-based dielectric layer. The etch chemistry provides for the use of etchant plasma species which minimize oxygen, fluorine, chlorine, and bromine content.
Owner:APPLIED MATERIALS INC
Blue phosphorescent compound and organic electroluminescent device using the same
Disclosed are a blue phosphorescent compound with a high color purity and a high efficiency, and an organic electroluminescent device using the same. The blue phosphorescent compound is represented by the following Formula:wherein R1 to R5 are each independently hydrogen (H), fluorine (F), chlorine (Cl), bromine (Br), a cyano group, a C1 to C6 alkyl group, a C1 to C6 alkoxy group, a C6 to C20 substituted or unsubstituted aromatic group, a C5 to C20 substituted or unsubstituted heterocyclic group, a C1 to C6 amine group, a C6 to C20 aromatic-substituted amine group, or a C5 to C20 heterocycle-substituted amine group, X is selected from nitrogen (N), oxygen (O), phosphorous (P) and sulfur (S) atoms, at least one of A1, A2, A3 and A4 is nitrogen (N), and the remaining are selected from hydrogen (H)-substituted carbon, and alkyl or alkoxy-substituted carbon, L is a monodentate or bidentate ligand and n is 1 to 3.
Owner:LG DISPLAY CO LTD
Analyte injection system
InactiveUS20050133370A1Increase in sizeShort amount of timeSludge treatmentVolume/mass flow measurementGlutaric acidAntibody conjugate
This invention provides methods and devices for spatially separating at least first and second components in a sample which in one exemplary embodiment comprises introducing the first and second components into a first microfluidic channel of a microfluidic device in a carrier fluid comprising a spacer electrolyte solution and stacking the first and second components by isotachophoresis between a leading electrolyte solution and a trailing electrolyte solution, wherein the spacer electrolyte solution comprises ions which have an intermediate mobility in an electric field between the mobility of the ions present in the leading and trailing electrolyte solutions and wherein the spacer electrolyte solution comprises at least one of the following spacer ions MOPS, MES, Nonanoic acid, D-Glucuronic acid, Acetylsalicyclic acid, 4-Ethoxybenzoic acid, Glutaric acid, 3-Phenylpropionic acid, Phenoxyacetic acid, Cysteine, hippuric acid, p-hydroxyphenylacetic acid, isopropylmalonic acid, itaconic acid, citraconic acid, 3,5-dimethylbenzoic acid, 2,3-dimethylbenzoic acid, p-hydroxycinnamic acid, and 5-br-2,4-dihydroxybenzoic acid, and wherein the first component comprises a DNA-antibody conjugate and the second component comprises a complex of the DNA-antibody conjugate and an analyte.
Owner:WAKO PURE CHEMICAL INDUSTRIES +1
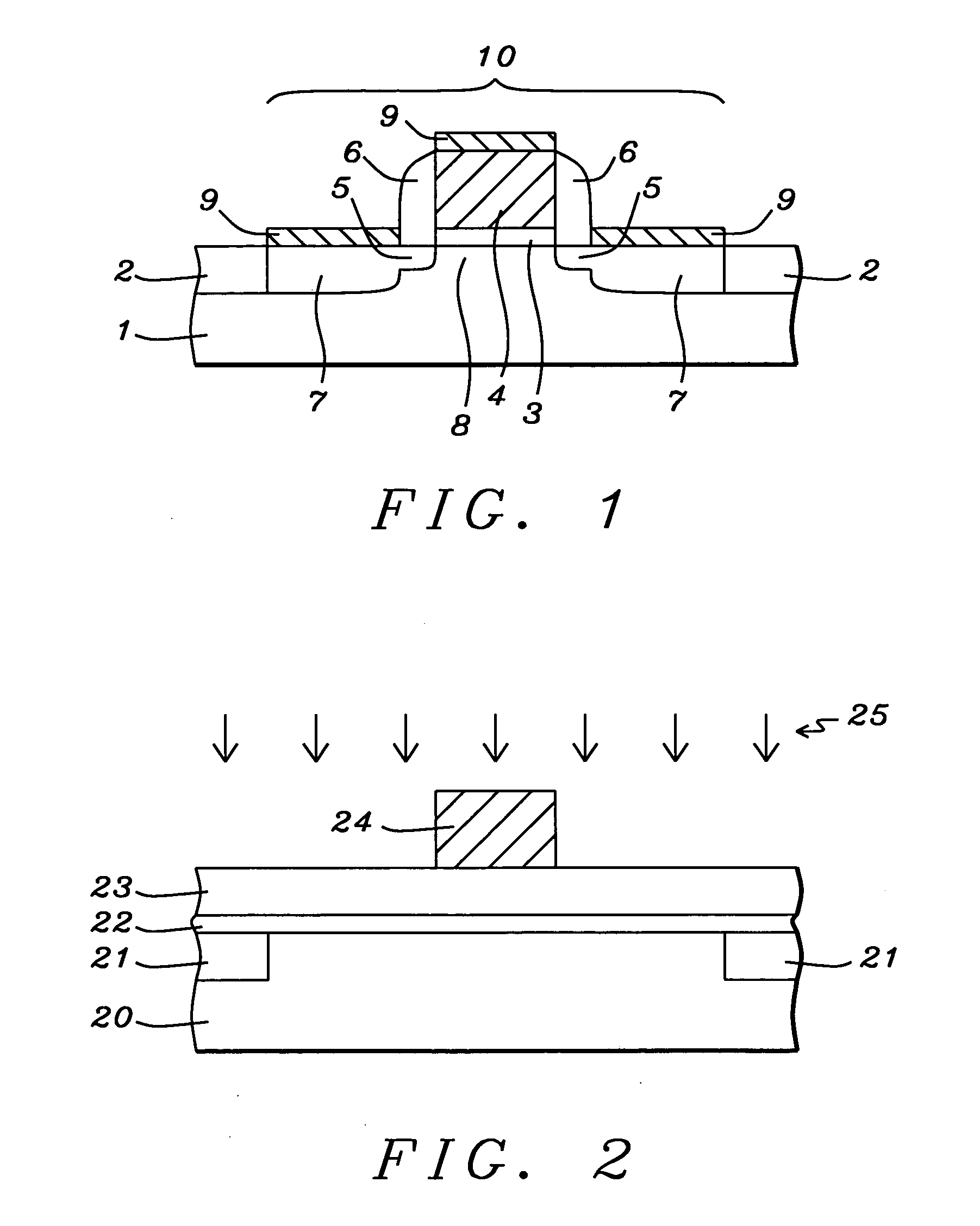
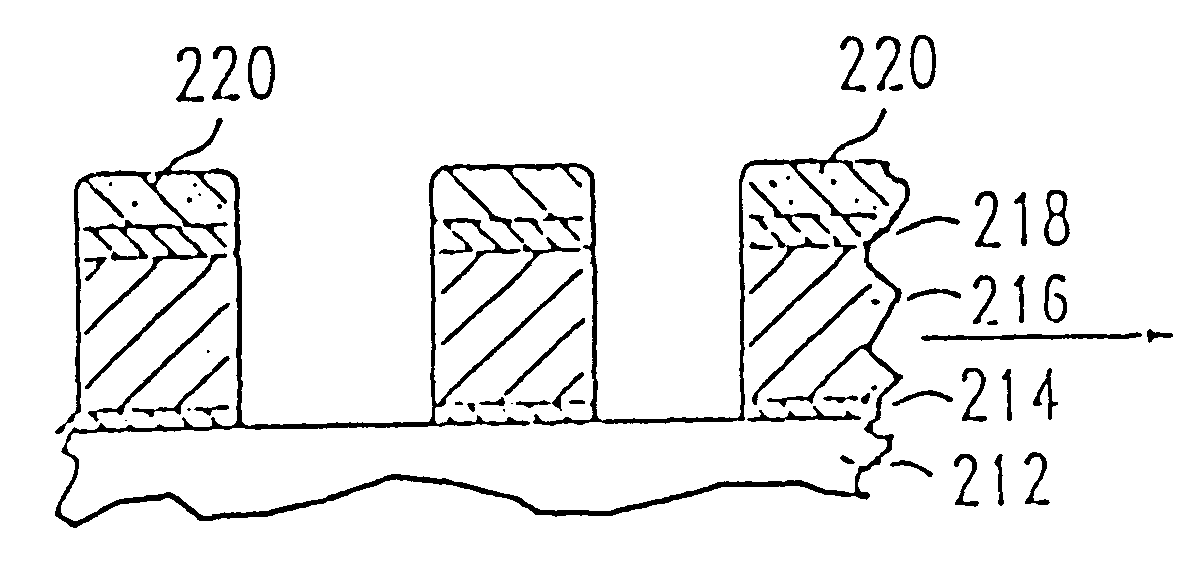
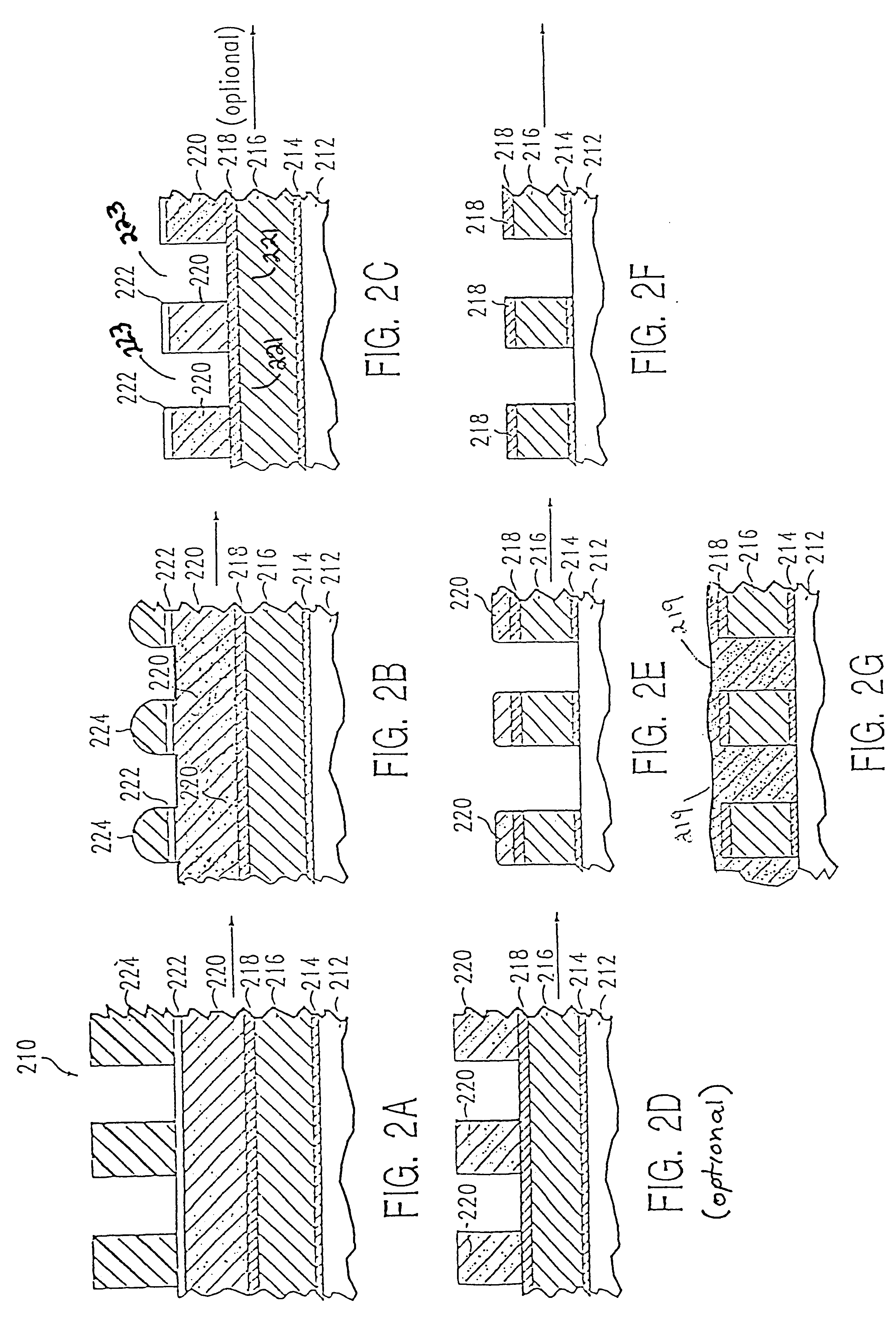
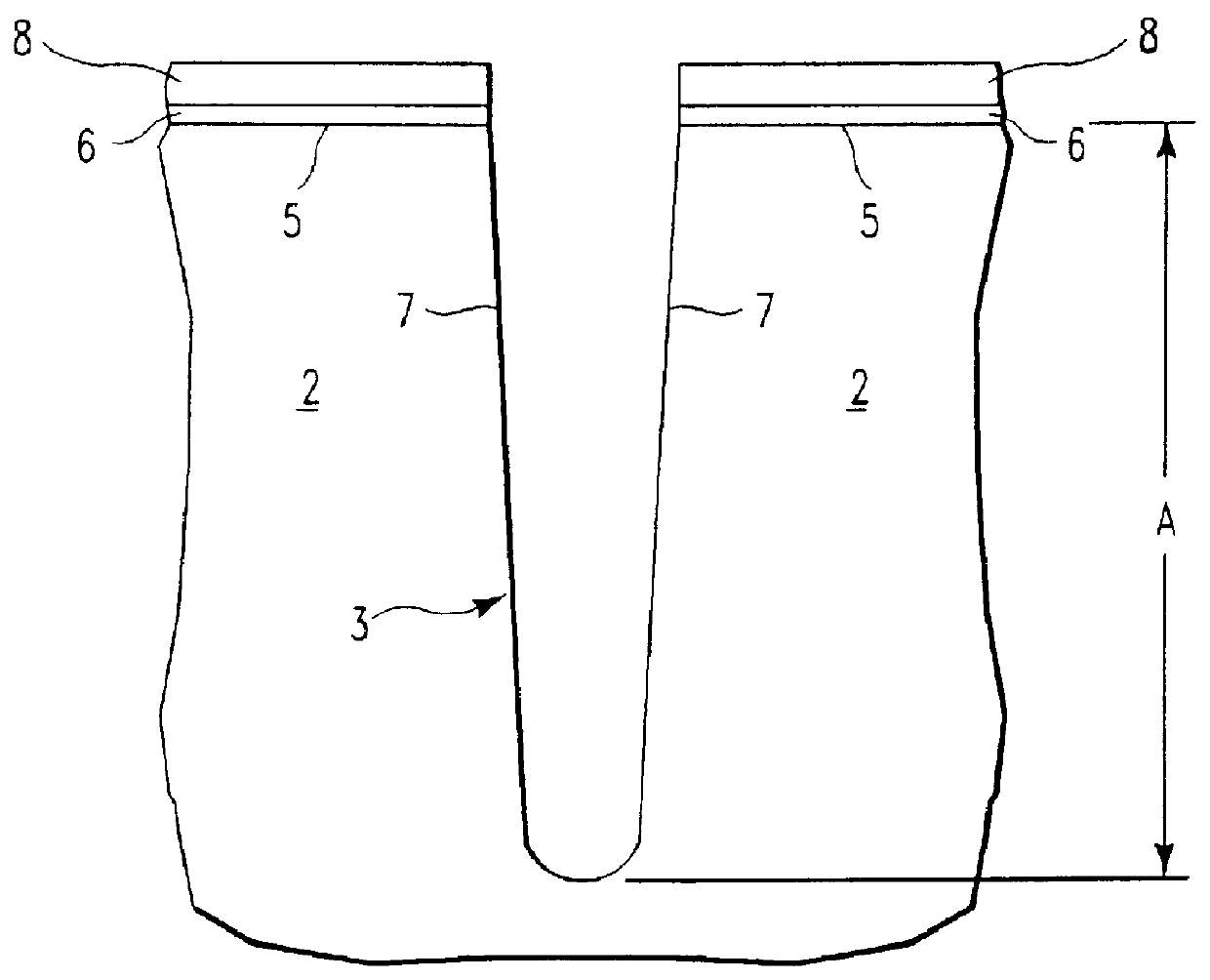
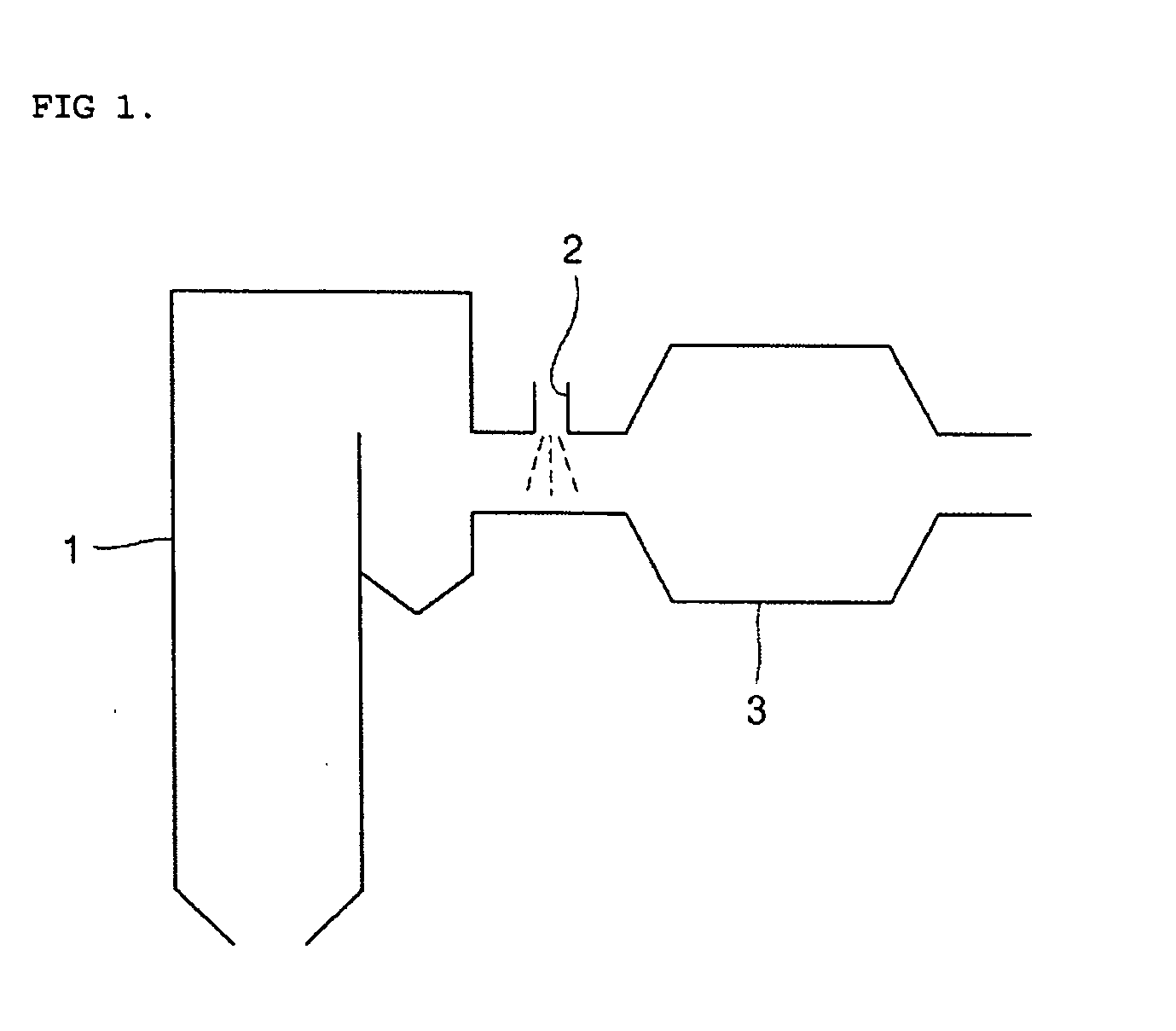
Process for control of the shape of the etch front in the etching of polysilicon
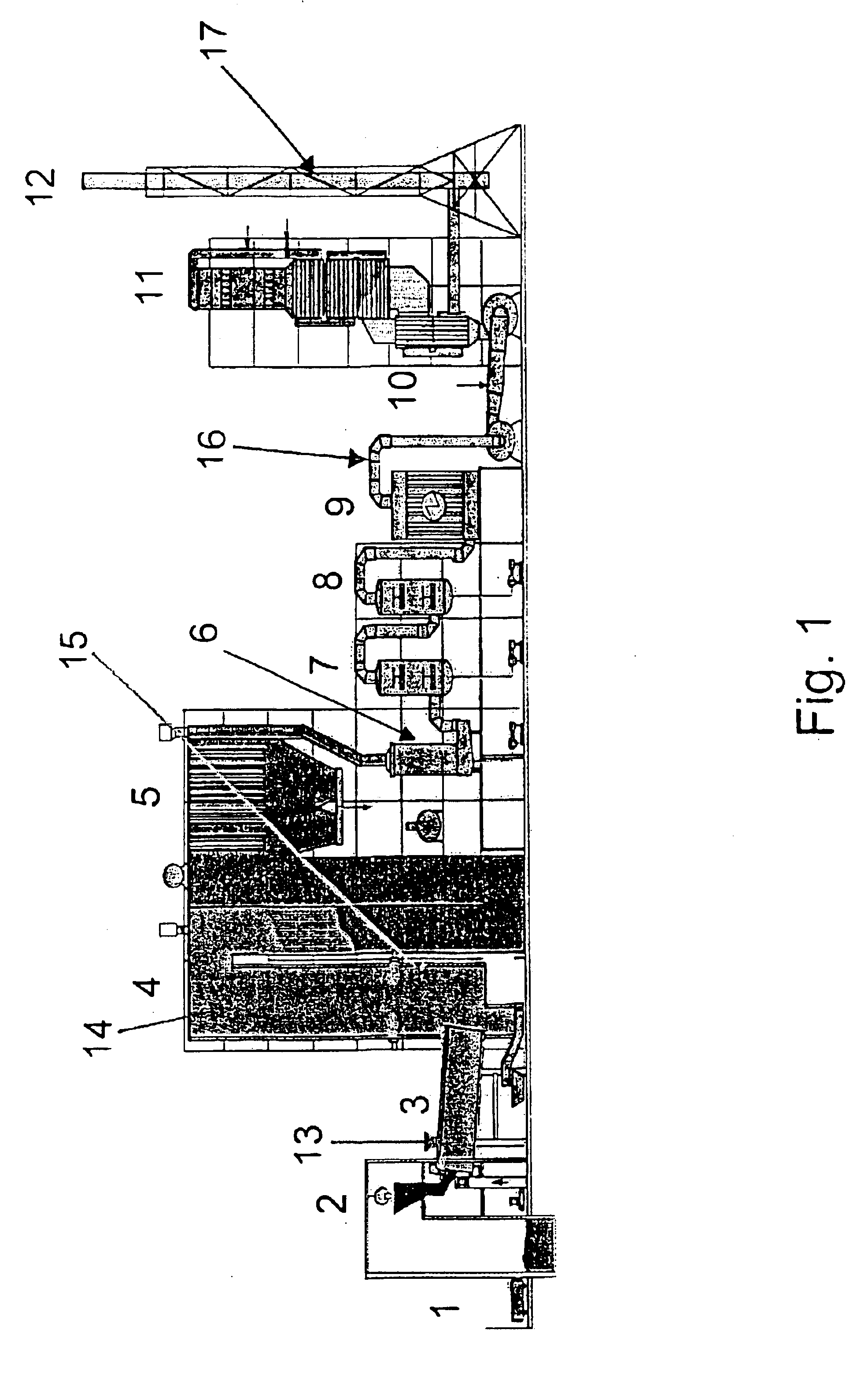
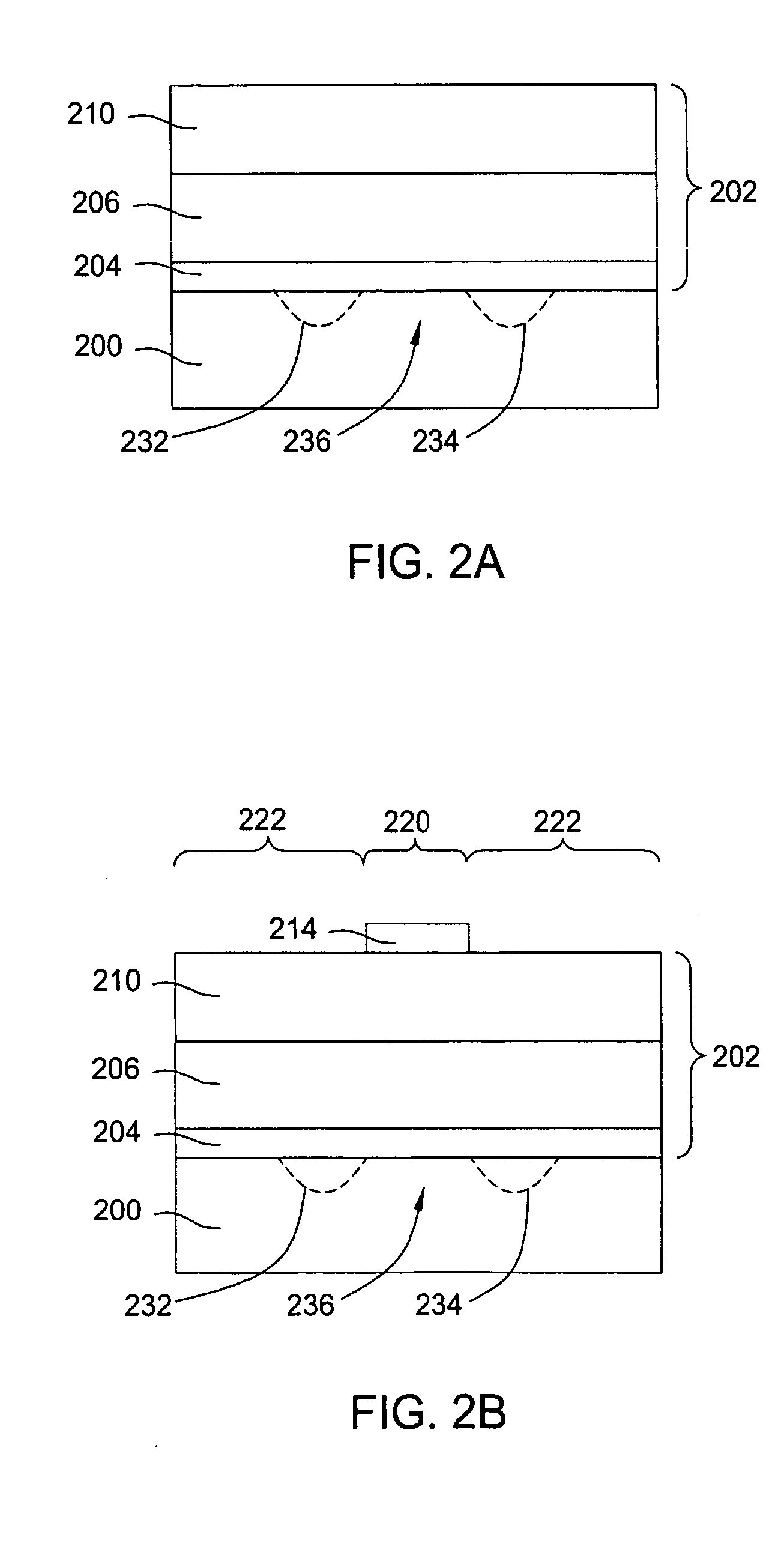
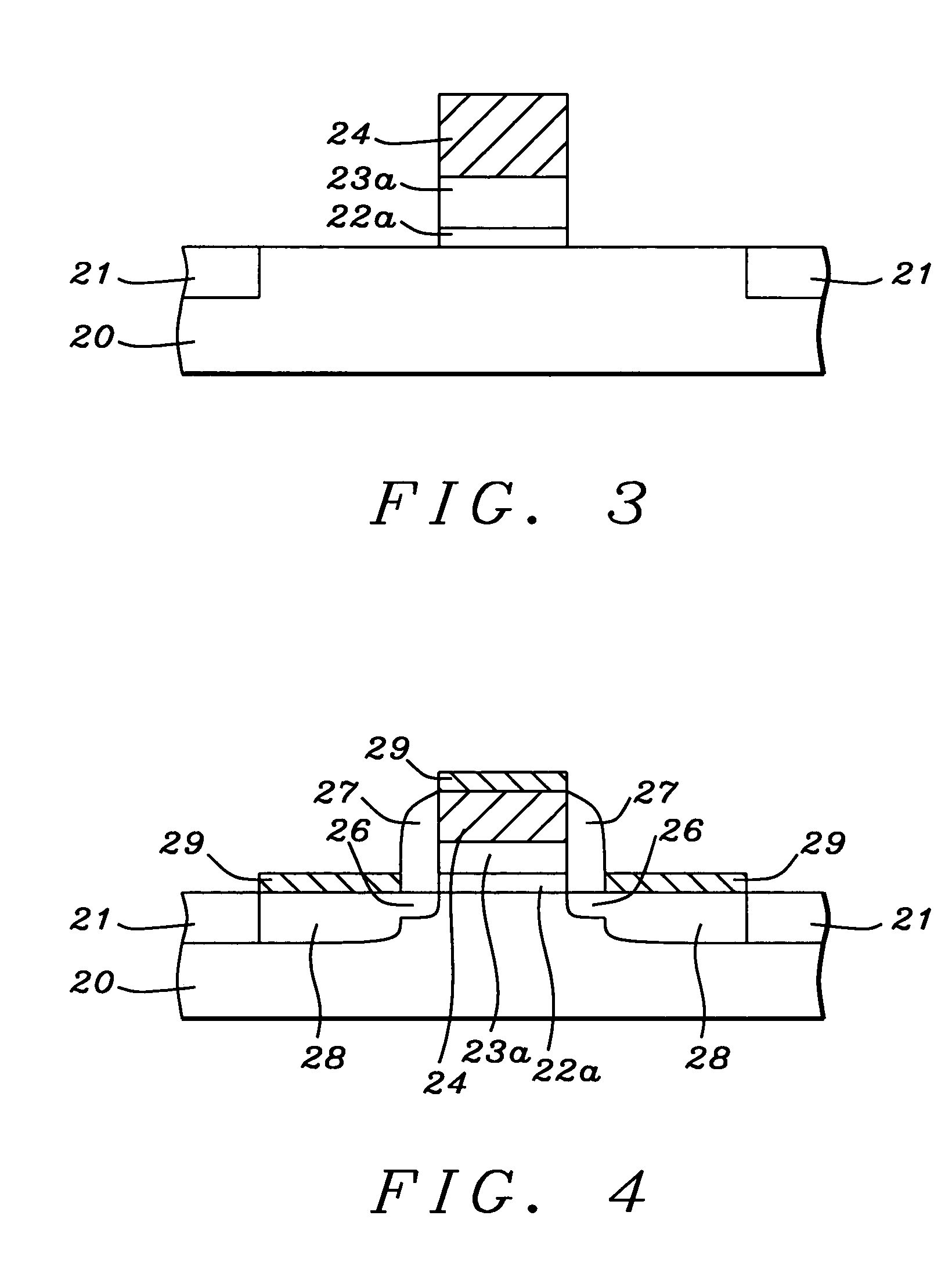
The present disclosure pertains to our discovery that the use of a particular combination of etchant gases results in the formation of a substantially flat etch front for polysilicon etching applications. In general, the process of the invention is useful for controlling the shape of the etch front during the etchback of polysilicon. Typically, the process comprises isotropically etching the polysilicon using a plasma produced from a plasma source gas comprising a particular combination of reactive species which selectively etch polysilicon. The plasma source gas comprises from about 80% to about 95% by volume of a fluorine-comprising gas, and from about 5% to about 20% by volume of an additive gas selected from a group consisting of a bromine-comprising gas, a chlorine-comprising gas, an iodine-comprising gas, or a combination thereof. One preferred mixture is SF6, Cl2 and HBr. A preferred method of the invention, used to perform recess etchback of a polysilicon-filled trench in a substrate, comprises the following steps: a) providing a trench 3 formed in a semiconductor structure, wherein the structure includes a substrate 2, at least one gate dielectric layer 6 overlying a surface of the substrate, and at least one etch barrier layer 8 overlying the gate dielectric layer; b) forming a conformal dielectric film 10 overlying the etch barrier layer and the sidewall and bottom of the trench; c) filling the trench with a layer of polysilicon 12 which overlies the conformal dielectric film; and d) isotropically etching the polysilicon back to a predetermined depth within the trench using a plasma produced from the invention plasma source gas. Also disclosed herein is a method of forming a trench capacitor in a single-crystal silicon substrate, the trench capacitor including a dielectric collar and a buried strap.
Owner:APPLIED MATERIALS INC
Pharmaceutical compositions and treatment methods
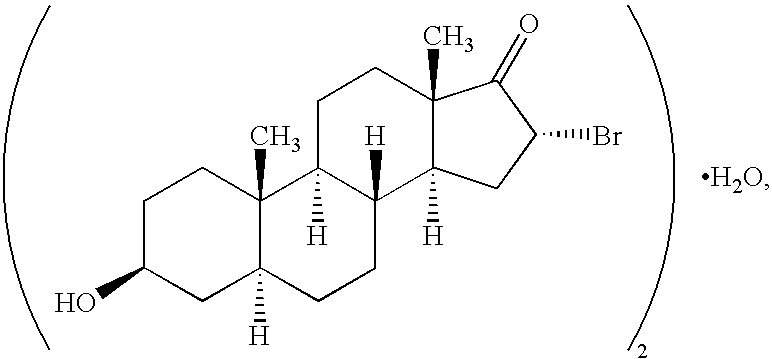
InactiveUS6667299B1Efficient transportReduce yieldAntibacterial agentsOrganic active ingredientsRegimenKetone
The invention provides compositions comprising, 16alpha-bromo-3beta-hydroxy-5alpha-androstan-17-one hemihydrate and one or more excipients, typically wherein the composition comprises less than about 3% water. The compositions are useful to make improved pharmaceutical formulations. The invention also provides methods of intermittent dosing of steroid compounds such as analogs of 16alpha-bromo-3beta-hydroxy-5alpha-androstan-17-one and compositions useful in such dosing regimens. The invention further provides compositions and methods to inhibit pathogen (viral) replication, ameliorate symptoms associated with immune dysregulation and to modulate immune responses in a subject using certain steroids and steroid analogs. The invention also provides methods to make and use these immunomodulatory compositions and formulations.
Owner:NEURMEDIX +2
Chemical vapor deposition method using a catalyst on a substrate surface
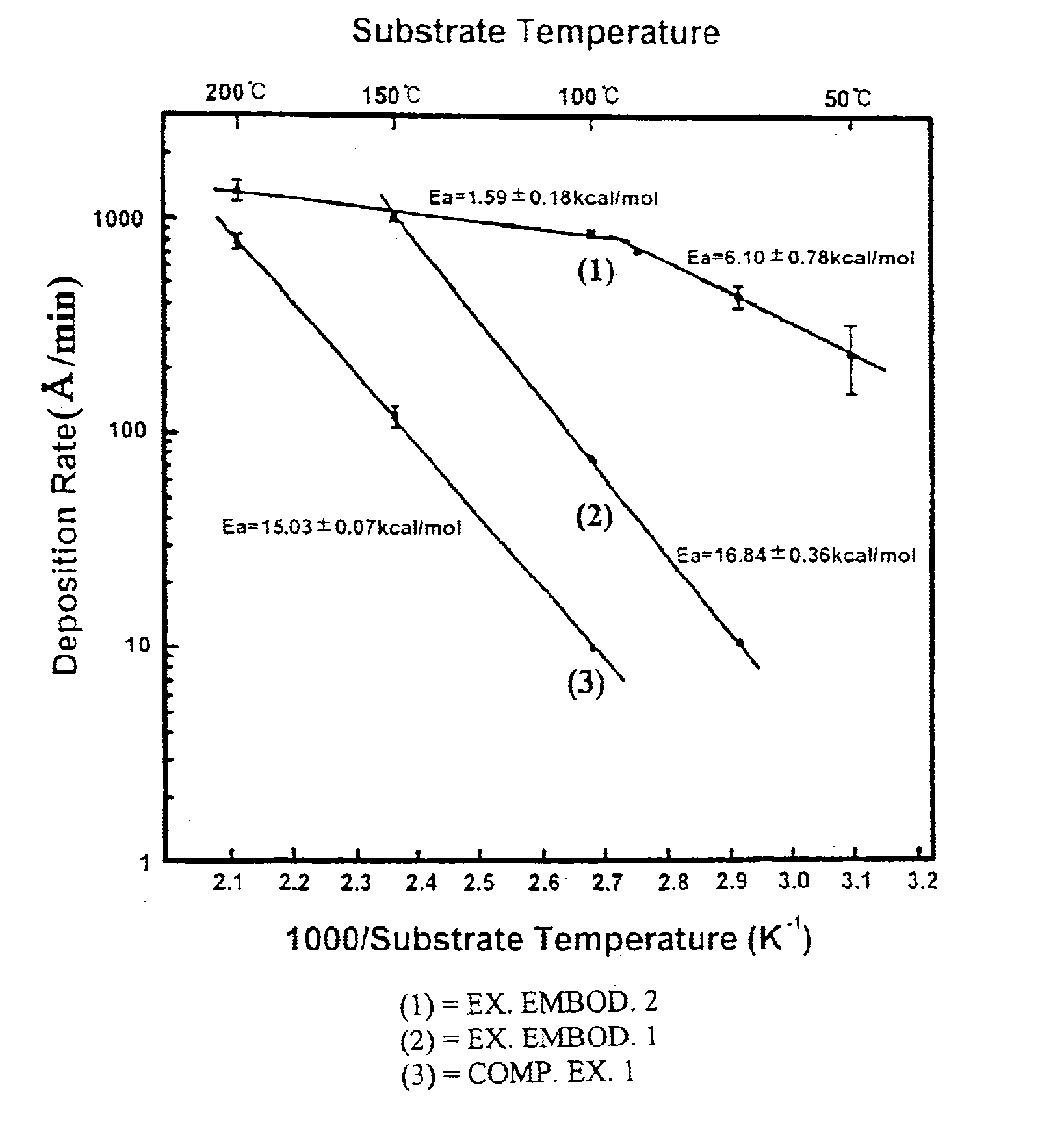
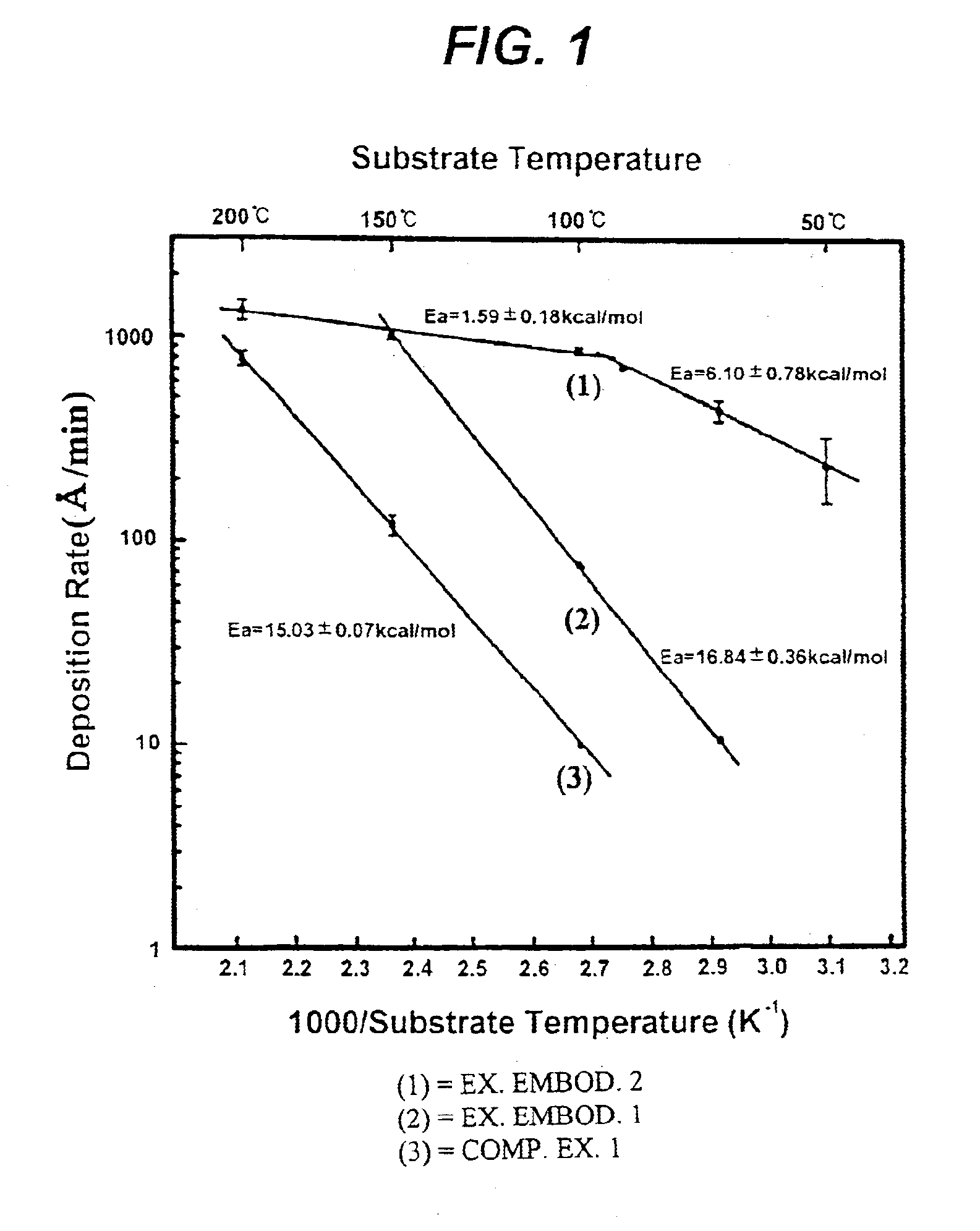
InactiveUS6623799B1Increase deposition rateIncrease response rateOrganic-compounds/hydrides/coordination-complexes catalystsSemiconductor/solid-state device manufacturingBromineGas phase
A method of chemically depositing a copper film in which a bromine or iodine-containing catalyst component is employed to enhance the deposition rate. The present invention is characterized in that the catalyst component floats on the film surface during the film formation. Accordingly, a film deposition having superior step coverage and high deposition rate is obtained.
Owner:ASM KOREA LTD
Reducing mercury emissions from the burning of coal
Processes and compositions are provided for decreasing emissions of mercury upon combustion of fuels such as coal. Various sorbent compositions are provided that contain components that reduce the level of mercury and / or sulfur emitted into the atmosphere upon burning of coal. In various embodiments, the sorbent compositions are added directly to the fuel before combustion; are added partially to the fuel before combustion and partially into the flue gas post combustion zone; or are added completely into the flue gas post combustion zone. In preferred embodiments, the sorbent compositions comprise a source of halogen and preferably a source of calcium. Among the halogens, iodine and bromine are preferred. In various embodiments, inorganic bromides make up a part of the sorbent compositions.
Owner:NOX II LTD
Processes for producing terephthalic acid
InactiveUS20060205977A1Organic compound preparationCarboxylic preparation by oxidationSolventBromine
Processes for producing terephthalic acid are disclosed, the processes comprising combining in a reaction medium p-xylene, a solvent comprising water and one or more saturated organic acids having from 2-4 carbon atoms, and an oxygen-containing gas, at a temperature from about 135° C. to about 165° C., in the presence of a catalyst composition comprising cobalt atoms and manganese atoms, with bromine atoms provided as a promoter. The amount of cobalt used may be from about 1,800 ppm to about 6,000 ppm, with respect to the total weight of the liquid in the reaction medium, and the weight ratio of cobalt to manganese may be from about 40 to about 400. The processes according to the invention produce terephthalic acid as a precipitated reaction product, with typically no more than about 10 ppm 2,6-dicarboxyfluorenone produced, with respect to the weight of the terephthalic acid produced, or no more than about 20 ppm 2,6-dicarboxyfluorenone, with respect to the total weight of the reaction medium, per batch or upon one pass through a reactor. The terephthalic acid so produced may be further purified by one or more additional oxidation reactions, without the need for expensive hydrogenation purification processes.
Owner:EASTMAN CHEM CO
Method for continuously preparing regenerated cellulose fibre
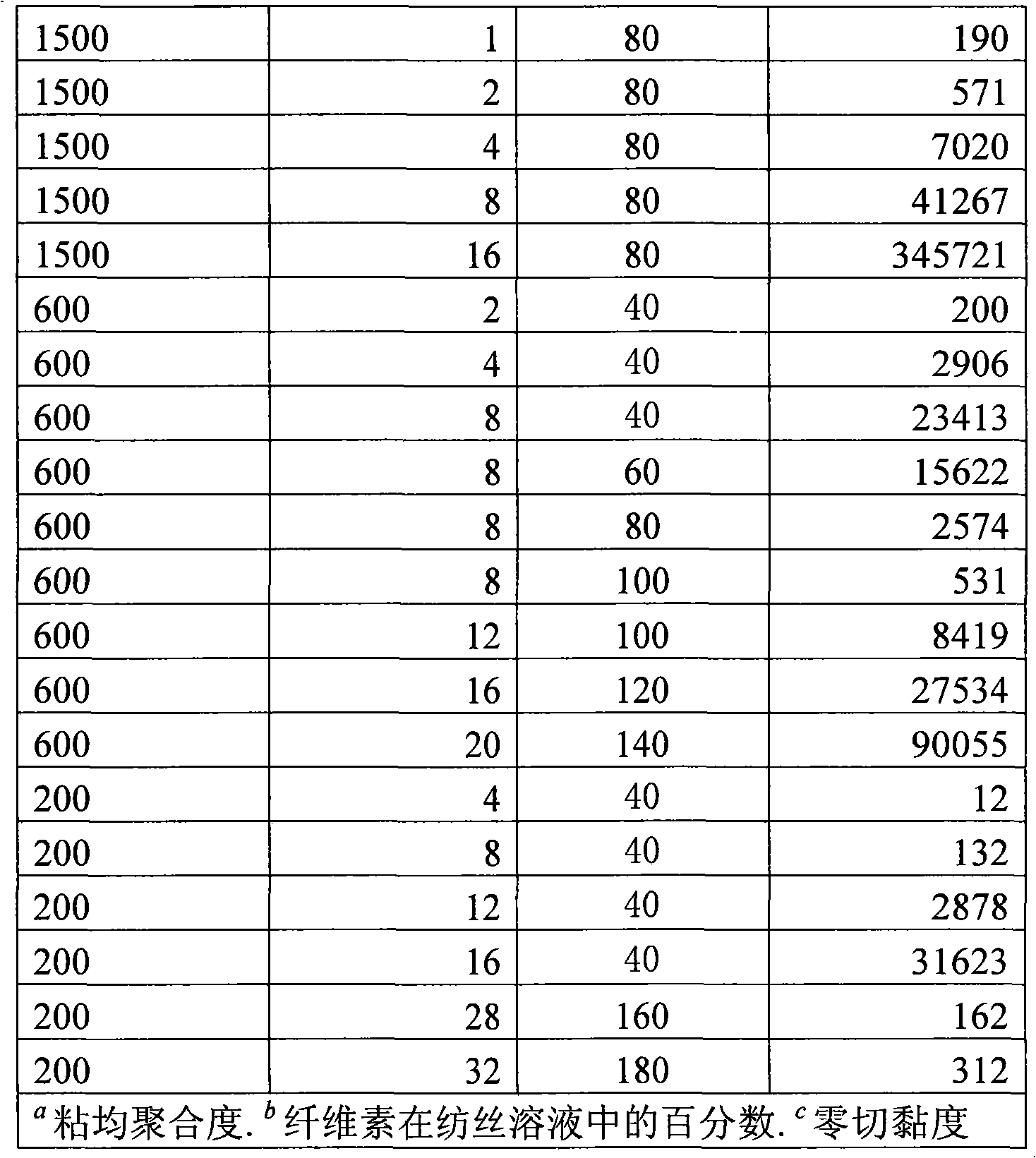
InactiveCN101328626AReduce manufacturing costReduce the temperatureArtificial filament recoveryFibre treatmentPolymer scienceTetrafluoroborate
The invention discloses a method for continuously preparing regenerated cellulose fibers through the solvent method, comprising the following steps that: a cellulose raw material is dissolved into an ion liquid to prepare a spinning liquid; gel type regenerated cellulose fibers are obtained through spinning; and the regenerated cellulose fibers are obtained through cleaning, rear draft and drying, wherein, the ion liquid is selected from one or a plurality among the following ion liquids: a). an ion liquid with 1, 3-dialkyl imidazole as a cation and formiate radical, radical vinegar or propionate radical as an anion; and b). an ion liquid with 1-R1-3-R2- dialkyl imidazole as the cation and chlorine, bromine, iodine, formiate radical, radical vinegar, sulfate radical, nitrate radical, tetrafluoroborate radical, thiocyanate radical, hexafluorophosphate radical, p-toluenesulfonate radical or trifluoromethanesulfonic acid radical as the anion. The method has the advantages of wide technological range, mild temperature condition, adequate pressure, quick spinning speed and so on, can prepare the regenerated cellulose fibers with superior performance and complete specifications, and has low production cost, high production efficiency and wide application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
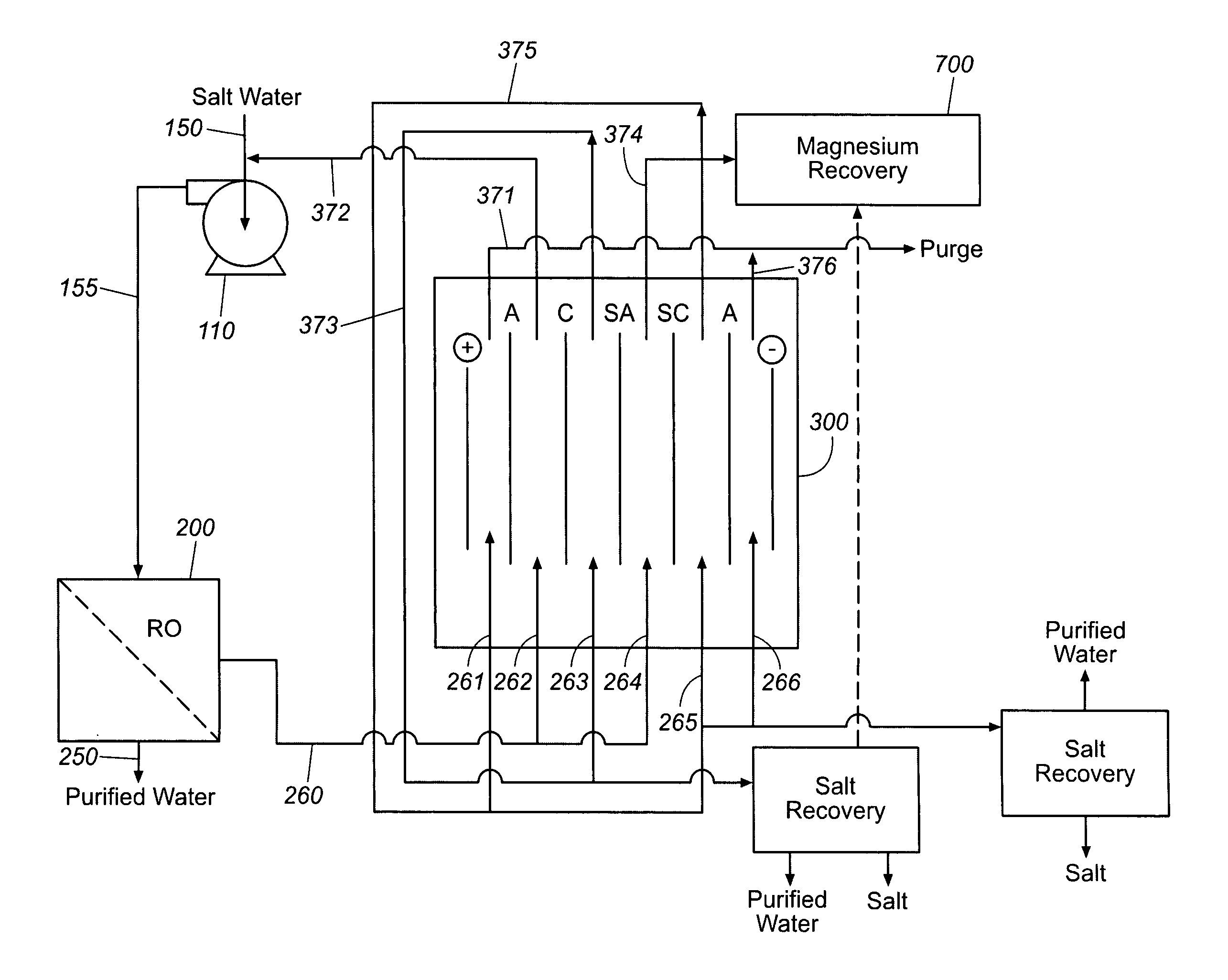
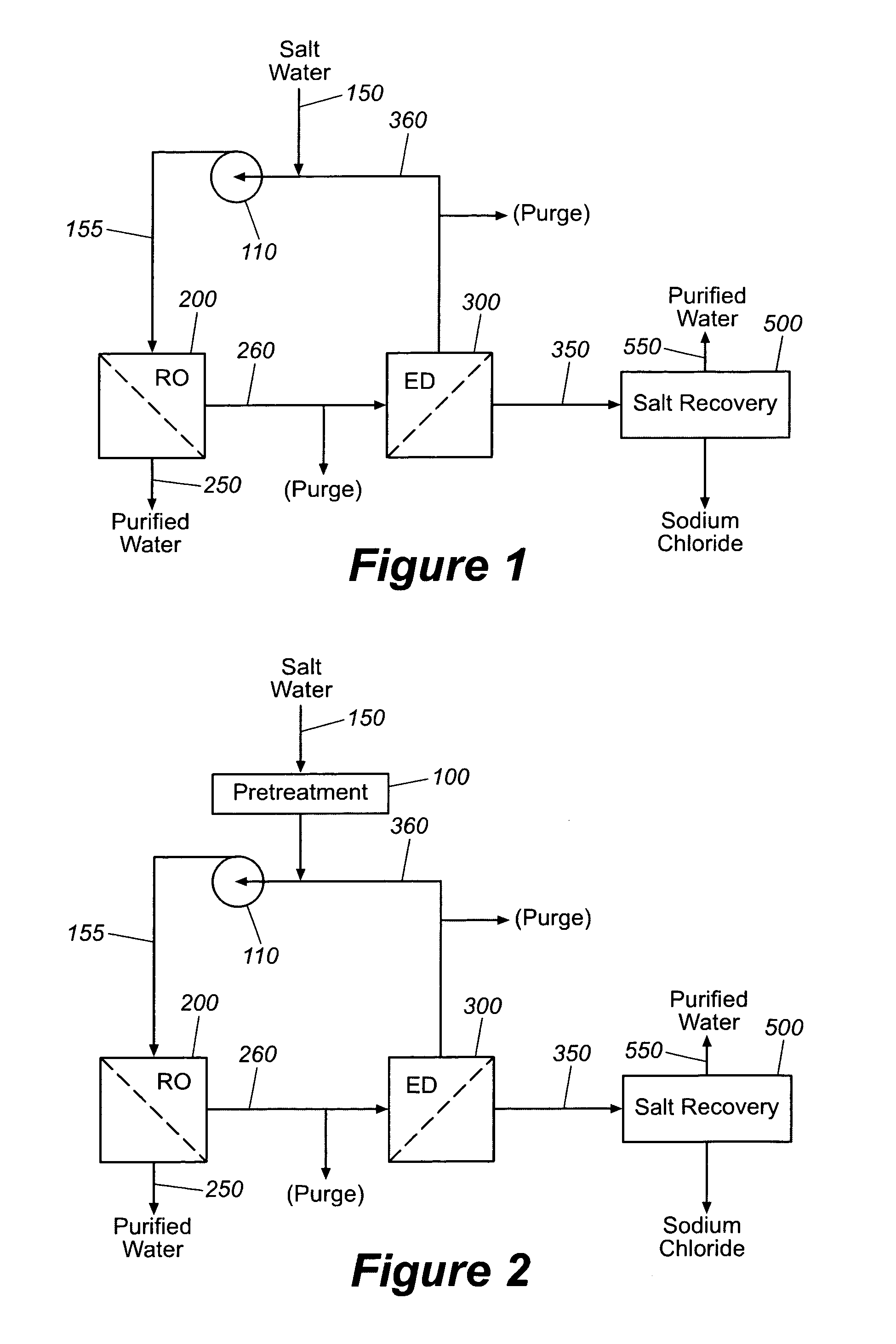
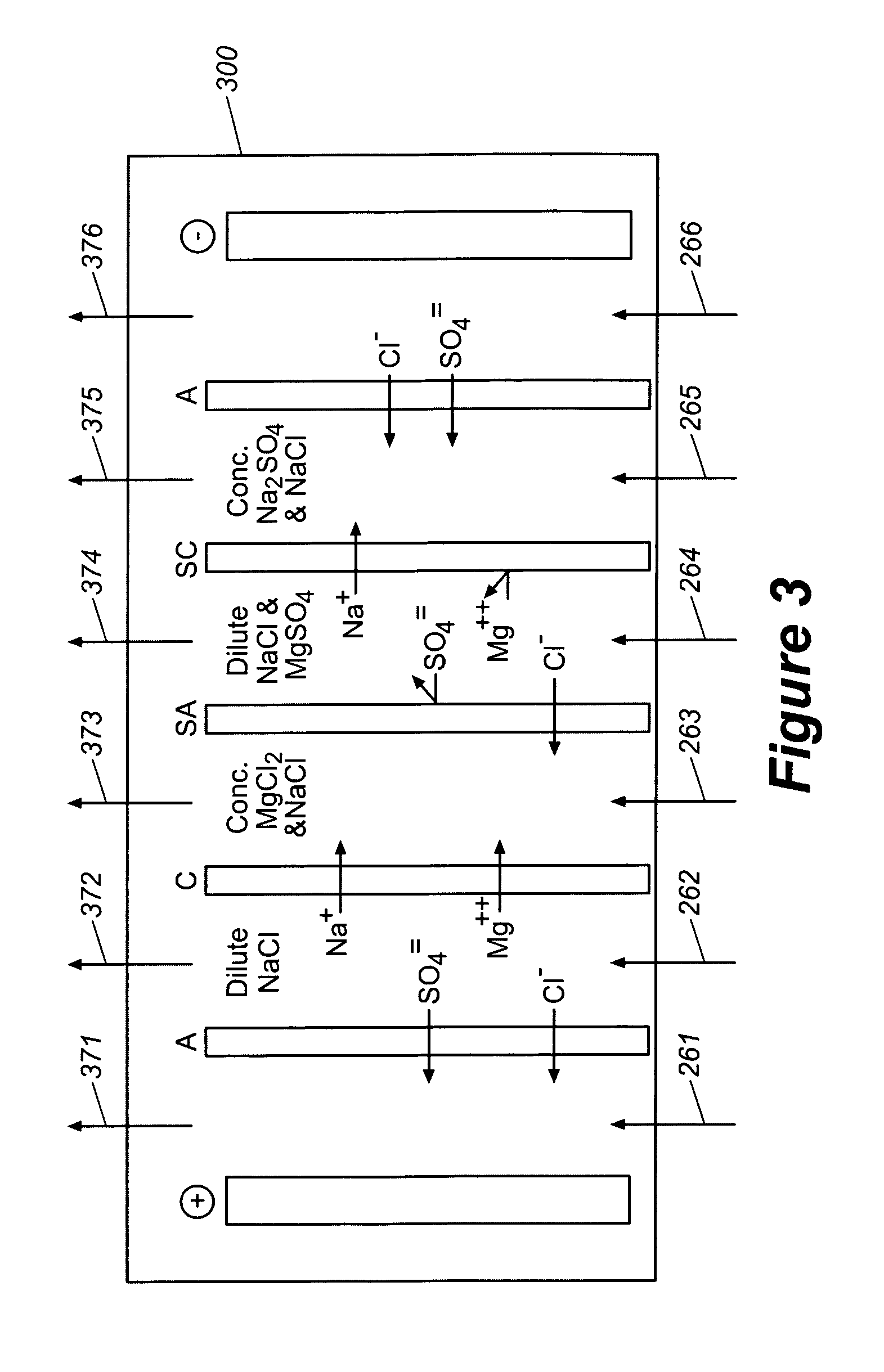
Production of purified water and high value chemicals from salt water
ActiveUS7083730B2Effective recoveryElectrolysis componentsSolvent extractionReverse osmosisHigh pressure
Sodium chloride and purified water are recovered by treating salt water that contains sodium chloride with an integrated reverse osmosis and electrodialysis system, which includes an efficiency-enhancing feature that is one or more of the following: the use of univalent anion and univalent cation selective membranes in the electrodialysis unit; the addition of a nanofiltration unit to process the diluate from the electrodialysis unit; or operation of the electrodialysis unit at an elevated pressure. Magnesium and bromine can optionally be produced when the salt water contains these materials.
Owner:SOUTH CAROLINA UNIV OF
Reducing mercury emissions from the burning of coal
Processes and compositions are provided for decreasing emissions of mercury upon combustion of fuels such as coal. Various sorbent compositions are provided that contain components that reduce the level of mercury and / or sulfur emitted into the atmosphere upon burning of coal. In various embodiments, the sorbent compositions are added directly to the fuel before combustion; are added partially to the fuel before combustion and partially into the flue gas post combustion zone; or are added completely into the flue gas post combustion zone. In preferred embodiments, the sorbent compositions comprise a source of halogen and preferably a source of calcium. Among the halogens, iodine and bromine are preferred. In various embodiments, inorganic bromides make up a part of the sorbent compositions.
Owner:NOX II LTD
Process for synthesis of fluorinated olefins
ActiveUS20090099396A1Preparation by dehalogenationPreparation by hydrogen halide split-offHydrogenBromine
Disclosed is a process for the synthesis of fluorinated olefins, and in particularly preferred embodiments tetrafluorinated olefins having F on an unsaturated, non-terminal carbon, such as 2,3,3,3-tetrafluoropropene. The preferred processes of the present invention in accordance with one embodiment generally comprise:(a) reacting a compound of formula (I)X1X2 (I)with a compound of formula (II)CX1X2X3CX1═CX1X2 (II)to produce a reaction product comprising a compound of formula (III)CF3CHX1CH2X2 (III), and(b) exposing said compound of formula (III) to reaction conditions effective to convert said compound of formula (III) to a compound of formula (IV)CF3CZ=CH2 (IV)wherein X1, X2, and X3 are each independently selected from the group consisting of hydrogen, chlorine, bromine, fluorine and iodine, provided that X1 and X2 in formula (I) are not both hydrogen and Z is Cl, I, Br, or F.
Owner:HONEYWELL INT INC
Bromomethane prepared by bromine oxidation of methane and catalyst for conversing the bromomethane into hydrocarbon
The invention discloses a catalyst which is used when methane is transformed into hydrobromic ether through the bromine oxidation reaction, and the catalyst which is used when preparing heavy hydrocarbons by using the hydrobromic ether further, and belongs to the technology field of the catalyst and the preparation method thereof. The first type of catalyst which is used when the methane is transformed into the hydrobromic ether through the bromine oxidation reaction comprises the preparation step that water-soluble metallic compound precursors such as Rh, Ru, Cu, Zn, Ag, Ce, V, W, Cd, Mo, Mn, Cr, La, etc. are mixed with a silica precursor, the mixture is processed through hydrolyzation, drying and roasting, and then the catalyst is obtained; the first type of catalyst can lead definite masses of reactant composed of the methane, HBr, H2O and oxygen sources (that is O2, air or oxygen-enriched air) to perform the catalytic bromine oxidation reaction, to generate target products such as CH3Br, CH2Br2, etc. The second type of catalyst, which is used when preparing the heavy hydrocarbons by using the hydrobromic ether further, includes the preparation step that molecular sieves such as HZSM-5, HY, H Beta, 3A, 4A, 5A, 13X, etc. load metallic compounds composed of Zn and Mg, so that the catalyst is obtained; the second type of catalyst can lead the methane bromination products composed of the CH3Br and the CH2Br2 to further react so as to generate the heavy hydrocarbons containing C3 to C13 and HBr, wherein, the HBr is used as a circular reaction medium.
Owner:MICROVAST POWER SYST CO LTD
Sorbent for removal of trace hazardous air pollutants from combustion flue gas and preparation method thereof
InactiveUS20070179056A1Low costLow raw material costGas treatmentOther chemical processesSorbentToxic industrial waste
Disclosed is a sorbent for the removal of mercury from combustion flue gas and a preparation method thereof. The sorbent includes an activated heavy oil heavy ash impregnated with 0.1-30% by weight of any chemical substance selected from sulfur, iodine, bromine and chlorine. The sorbent is prepared in an economical manner using heavy oil fly ash, industrial waste generated from heavy oil-fired boilers, and has excellent sorption performance for mercury, so that a low concentration of mercury contained in combustion flue gas discharged from large-scale boilers can be removed by injection of a small amount of the sorbent. Thus, the invention can prevent a reduction in the recycling rate of coal fly ash in coal-fired power plants and minimize operation cost.
Owner:KOREA ELECTRIC POWER CORP
Polyalpha-Olefin Compositions and Processes to Produce the Same
ActiveUS20090005279A1Hydrocarbon by hydrogenationHydrocarbons from unsaturated hydrocarbon additionBromine numberPolymer science
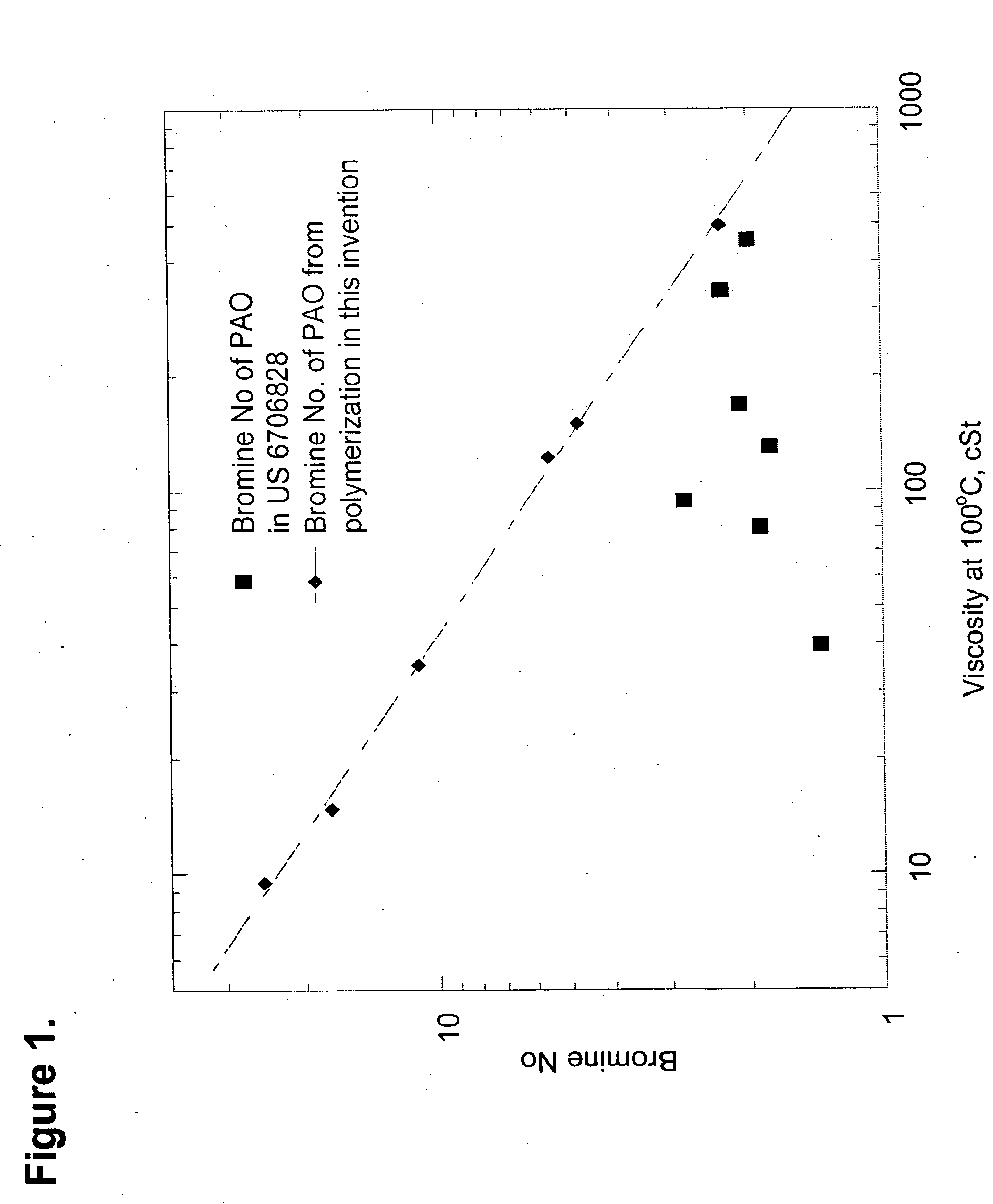
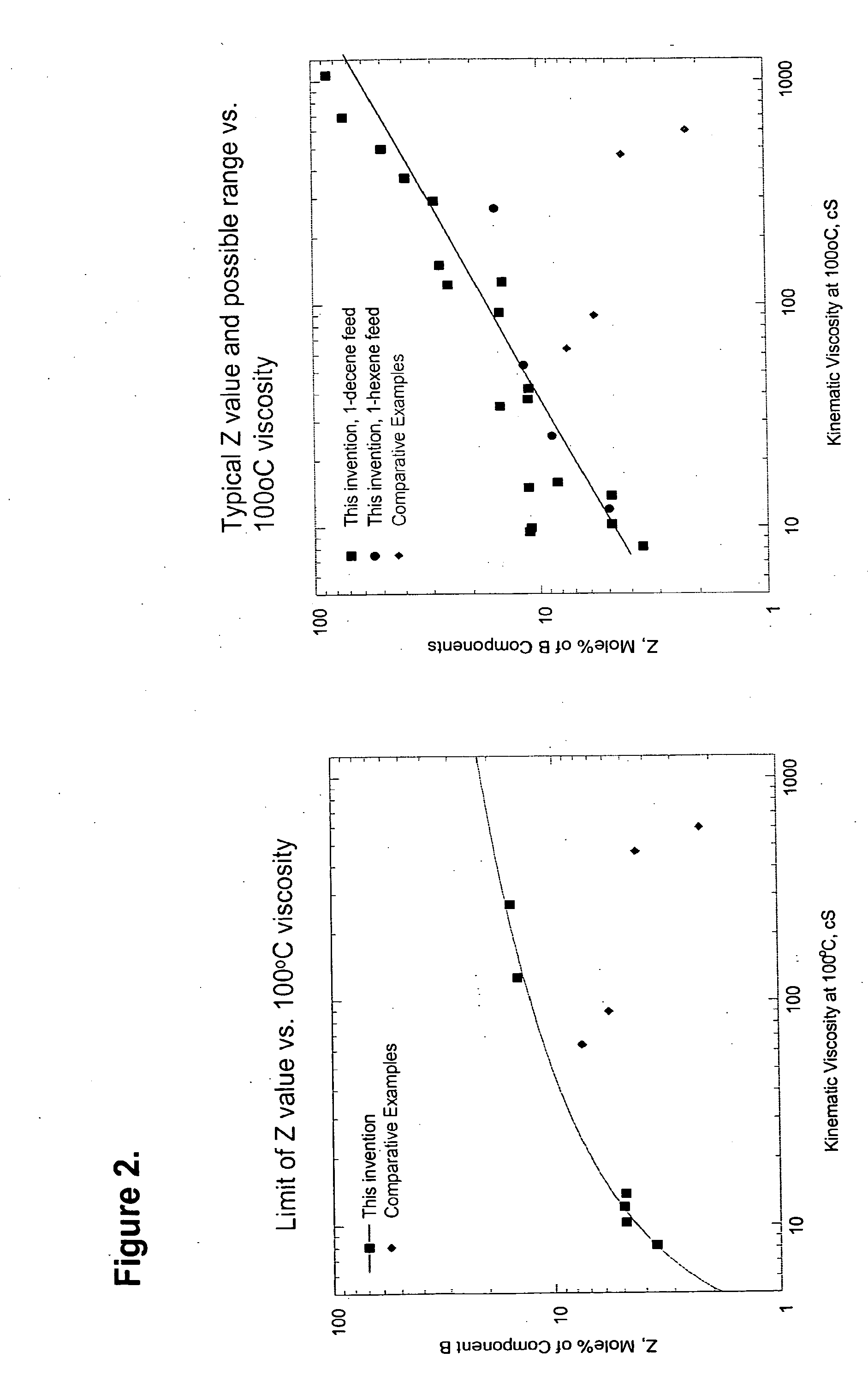
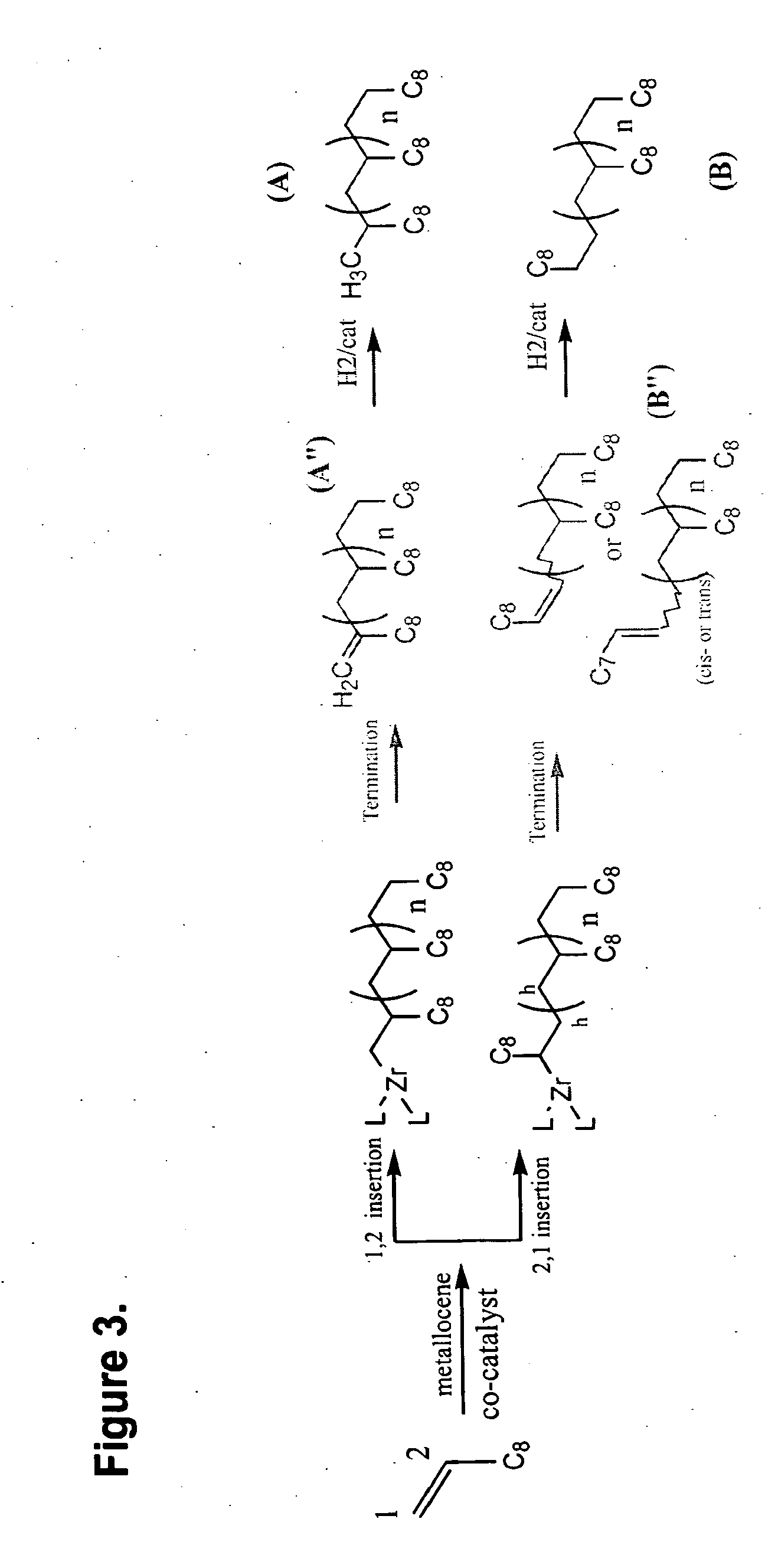
This invention relates to a polyalpha-olefin (and hydrogenated analogs thereof) comprising more than 50 mole % of one or more C5 to C24 alpha-olefin monomers where the polyalpha-olefin has: a) 40 mole % or more of mm triads, b) a Bromine number of Y or greater, where Y is equal to 89.92*(V)′°5863, where V is the Kinematic Viscosity of the polyalpha-olefin measured at 100° C. in cSt, and c) 1,2 disubstituted olefins present at 7 mole % or more, preferably having Z mole % or more of units represented by the formula: where j, k and m are each, independently, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, or 22, n is an integer from 1 to 350, and where Z=8.420*Log(V)−4.048, where V is the kinematic viscosity of the polyalpha-olefin measured at 1000 C in cSt This invention also relates to process to produce such polyalpha-olefins.
Owner:EXXONMOBIL CHEM PAT INC
Method of producing 2,5-furandicarboxylic acid
Provided is a method of producing FDCA by which high-purity FDCA can be produced in high yield. 2,5-furandicarboxylic acid is produced by: bringing 5-hydroxymethylfurfural into contact with an oxidant in an organic acid solvent in the presence of bromine and a metal catalyst; and allowing 5-hydroxymethylfurfural and the oxidant to react with each other while removing water produced by the reaction.
Owner:CANON KK
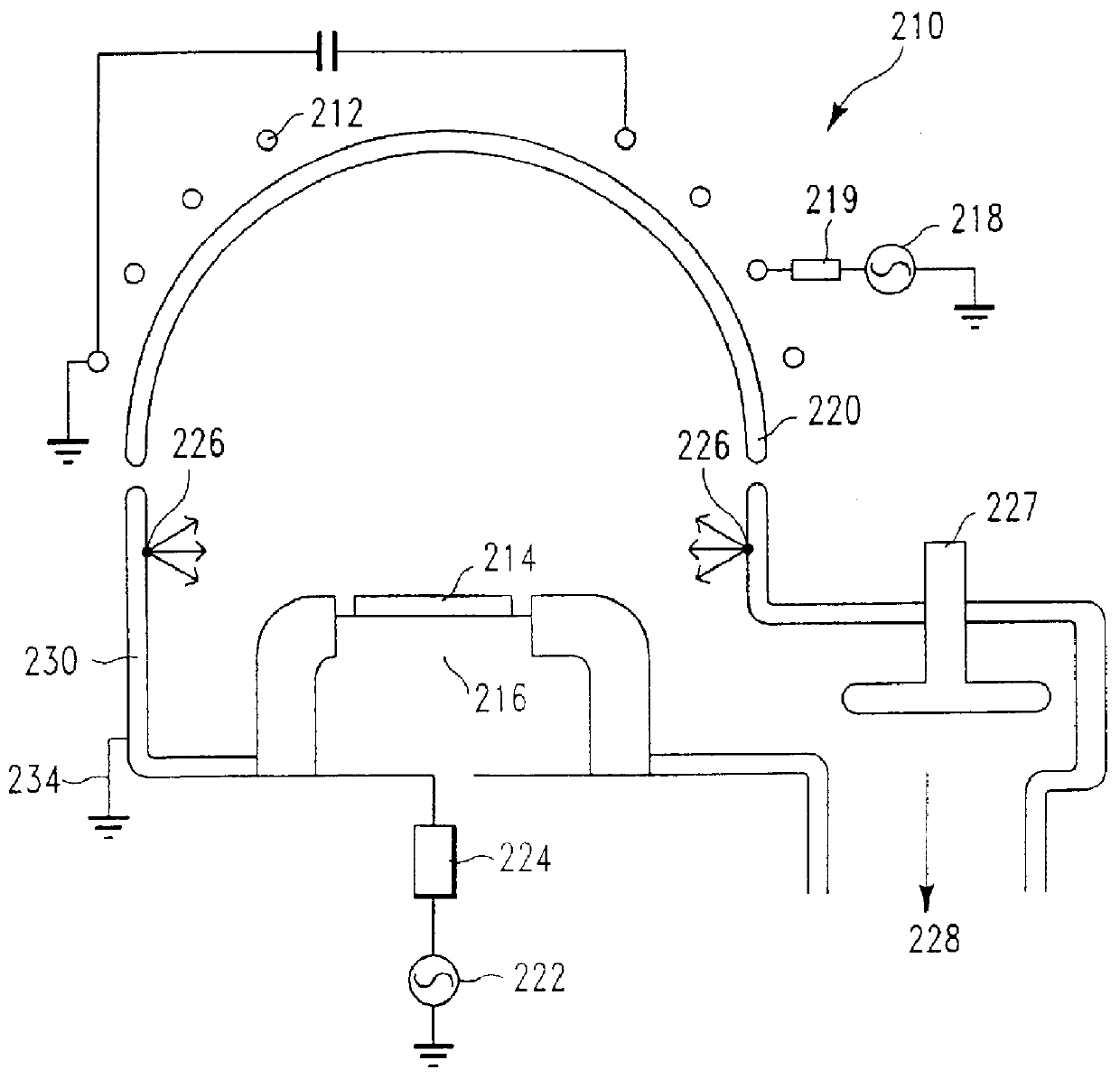
In-situ pre-coating of plasma etch chamber for improved productivity and chamber condition control
A semiconductor processing chamber having a silicon containing pre-coat is provided. The chamber includes a top electrode in communication with a power supply and a processing chamber defined within a base, a sidewall extending from the base, and a top disposed on the sidewall. The processing chamber has an outlet enabling removal of fluids within the processing chamber and includes a substrate support where an outer surface of the substrate support coated with the removable silicon containing coating, wherein the silicon containing coating is a compound consisting essentially of silicon and one of bromine and chlorine. The chamber includes an inner surface defined by the base, the sidewall and the top, where the inner surface is coated with a removable silicon containing coating.
Owner:LAM RES CORP
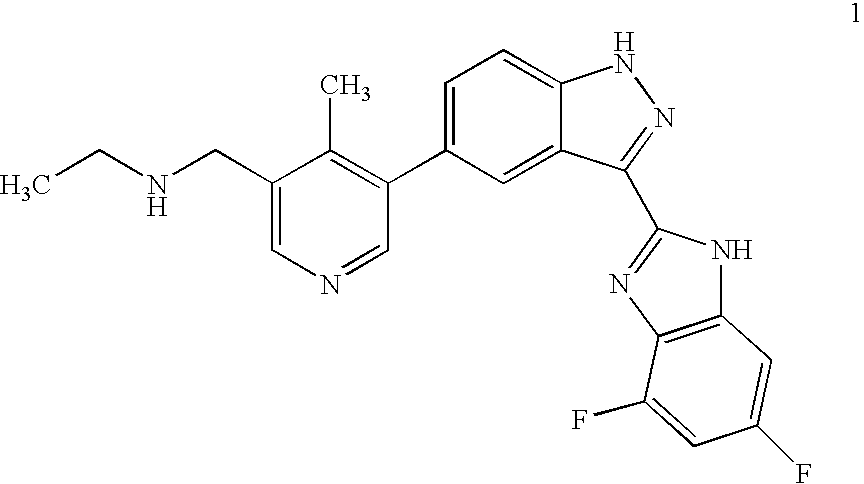
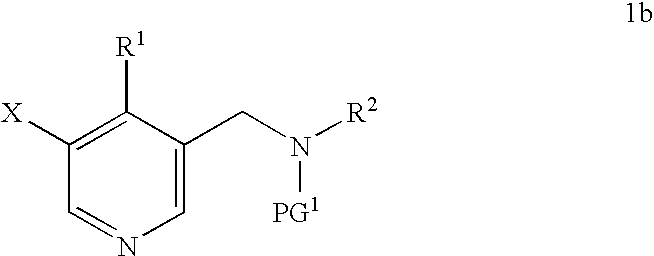
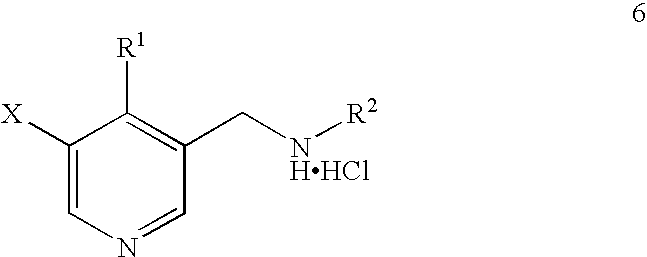
Synthesis of 5-bromo-4-methyl-pyridin-3-ylmethyl)-ethyl-carbamic acid tert-butyl ester
Owner:AGOURON PHARMA INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com